Article
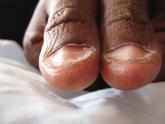
Plummer‐vinson (Patterson‐Kelly) syndrome
- Author:
- Omeed Saghafi, BS
- Matthew Crull, MD
- Ian H. Jenkins, MD
Article

Optimizing prevention of hospital‐acquired venous thromboembolism (VTE): Prospective validation of a VTE risk assessment model
- Author:
- Gregory A. Maynard, MD, MS
- Timothy A. Morris, MD
- Ian H. Jenkins, MD
- Sarah Stone, MD
- Joshua Lee, MD
- Marian Renvall, MS
- Ed Fink, MA
- Robert Schoenhaus, PharmD
