Quiz
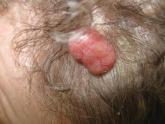
Lobular-Appearing Nodule on the Scalp
- Author:
- E. Eugene Bain Iii, MD
- Melissa Hoffman, BS
- Ilene L. Rothman, MD
A 79-year-old woman presented with a lesion on the left side of the scalp of several years’ duration that had slowly increased in size. Despite...
Article
Hyperpigmented patches on the neck, shoulder, and back
- Author:
- Jason M. Rizzo, BS
- Lesley C. Loss, MD
- Ilene L. Rothman, MD
A 17-year-old boy has had asymptomatic, unilateral hyperpigmented patches since birth. What is the diagnosis?
