Article
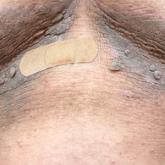
Hyperpigmented Flexural Plaques, Hypohidrosis, and Hypotrichosis
- Author:
- India A. Loyd, MD, MPH
- Amanda S. Weissman, MD
- Jarad Levin, MD
A 61-year-old woman with a history of hypohidrosis and deafness presented with a pruritic rash on the neck and antecubital fossae of several years...
Article
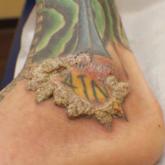
Verruciform Plaques Within a Tattoo of an HIV-Positive Patient
- Author:
- Jarad Levin, MD
- Thomas Stasko, MD
- Daniel Tinker, MD
- Katelin Harrell, MD
- Henry Haskell, MD
A 40-year-old man with a medical history of human immunodeficiency virus infection managed with highly active antiretroviral therapy, psoriasis,...
Article
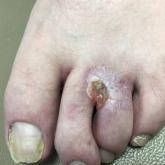
Nonhealing Eroded Plaque on an Interdigital Web Space of the Foot
- Author:
- Thomas Stasko, MD
- Jarad Levin, MD
- Ngoc Nguyen, MD
- Nancy Dawson, MD
- A. Neil Crowson, MD
A 53-year-old man with a history of numerous basal cell carcinomas and odontogenic keratocysts presented with a nonhealing erosion between the...
