User login
Improving Ototoxicity Monitoring with Cisplatin Therapy at VA Northeast Ohio Healthcare System (VANOHS), An Interdisciplinary Team Approach
BACKGROUND: Platinum-based chemotherapy is very effective in treating a variety of cancer types however, it has the potential to cause dose limiting ototoxicity that may result in permanent hearing loss. Studies have shown that hearing loss can affect quality of life by interfering with relationships and degrading communication. Early detection of hearing loss assists the oncologist in determining drug dosing and selecting the appropriate treatment regimens. It also allows the audiologist the opportunity for early intervention with rehabilitative measures. At our facility, Veterans starting cisplatin did not consistently have an audiology consult placed or a baseline audiogram completed prior to initiating treatment. A literature review was conducted, and an interdisciplinary team was formed with key stakeholders from medical oncology, audiology, pharmacy, and nursing.
RESULTS: The initial data review from January 1, 2016 to August 8, 2018 (n=85) showed only 17 Veterans (20%) had an audiology consult placed prior to initiating treatment. The target timeframe determined by the audiology department for baseline audiogram completion was eight weeks prior to or up to 24 hours post initial cisplatin administration. Following these guidelines, only seven (8%) of the 17 audiology consults were placed and completed within the recommended timeframe. Our goal was to increase the number of Veterans receiving audiograms prior to cisplatin administration from 8% to 100% by January 1, 2020.
INTERVENTIONS: enhanced provider education for early identification of Veterans starting cisplatin, creation of an email group for increased communication between nursing and audiology, trialing a portable audiometer in the outpatient infusion clinic, and adding a quick order set to the audiology consult on all cisplatin templates. A post-intervention data review from January 1, 2020 to April 30, 2020 (n=17) demonstrated all 17 (100%) Veterans had an audiology consult placed prior to the first dose of cisplatin. The data review also showed that 17 out of 17 Veterans (100%) had an audiogram completed within the target timeframe. This quality improvement project is aimed at maintaining quality of life for our Veterans throughout their cancer journey.
BACKGROUND: Platinum-based chemotherapy is very effective in treating a variety of cancer types however, it has the potential to cause dose limiting ototoxicity that may result in permanent hearing loss. Studies have shown that hearing loss can affect quality of life by interfering with relationships and degrading communication. Early detection of hearing loss assists the oncologist in determining drug dosing and selecting the appropriate treatment regimens. It also allows the audiologist the opportunity for early intervention with rehabilitative measures. At our facility, Veterans starting cisplatin did not consistently have an audiology consult placed or a baseline audiogram completed prior to initiating treatment. A literature review was conducted, and an interdisciplinary team was formed with key stakeholders from medical oncology, audiology, pharmacy, and nursing.
RESULTS: The initial data review from January 1, 2016 to August 8, 2018 (n=85) showed only 17 Veterans (20%) had an audiology consult placed prior to initiating treatment. The target timeframe determined by the audiology department for baseline audiogram completion was eight weeks prior to or up to 24 hours post initial cisplatin administration. Following these guidelines, only seven (8%) of the 17 audiology consults were placed and completed within the recommended timeframe. Our goal was to increase the number of Veterans receiving audiograms prior to cisplatin administration from 8% to 100% by January 1, 2020.
INTERVENTIONS: enhanced provider education for early identification of Veterans starting cisplatin, creation of an email group for increased communication between nursing and audiology, trialing a portable audiometer in the outpatient infusion clinic, and adding a quick order set to the audiology consult on all cisplatin templates. A post-intervention data review from January 1, 2020 to April 30, 2020 (n=17) demonstrated all 17 (100%) Veterans had an audiology consult placed prior to the first dose of cisplatin. The data review also showed that 17 out of 17 Veterans (100%) had an audiogram completed within the target timeframe. This quality improvement project is aimed at maintaining quality of life for our Veterans throughout their cancer journey.
BACKGROUND: Platinum-based chemotherapy is very effective in treating a variety of cancer types however, it has the potential to cause dose limiting ototoxicity that may result in permanent hearing loss. Studies have shown that hearing loss can affect quality of life by interfering with relationships and degrading communication. Early detection of hearing loss assists the oncologist in determining drug dosing and selecting the appropriate treatment regimens. It also allows the audiologist the opportunity for early intervention with rehabilitative measures. At our facility, Veterans starting cisplatin did not consistently have an audiology consult placed or a baseline audiogram completed prior to initiating treatment. A literature review was conducted, and an interdisciplinary team was formed with key stakeholders from medical oncology, audiology, pharmacy, and nursing.
RESULTS: The initial data review from January 1, 2016 to August 8, 2018 (n=85) showed only 17 Veterans (20%) had an audiology consult placed prior to initiating treatment. The target timeframe determined by the audiology department for baseline audiogram completion was eight weeks prior to or up to 24 hours post initial cisplatin administration. Following these guidelines, only seven (8%) of the 17 audiology consults were placed and completed within the recommended timeframe. Our goal was to increase the number of Veterans receiving audiograms prior to cisplatin administration from 8% to 100% by January 1, 2020.
INTERVENTIONS: enhanced provider education for early identification of Veterans starting cisplatin, creation of an email group for increased communication between nursing and audiology, trialing a portable audiometer in the outpatient infusion clinic, and adding a quick order set to the audiology consult on all cisplatin templates. A post-intervention data review from January 1, 2020 to April 30, 2020 (n=17) demonstrated all 17 (100%) Veterans had an audiology consult placed prior to the first dose of cisplatin. The data review also showed that 17 out of 17 Veterans (100%) had an audiogram completed within the target timeframe. This quality improvement project is aimed at maintaining quality of life for our Veterans throughout their cancer journey.
Distress Screening and Management in an Outpatient VA Cancer Clinic: A Pilot Project Involving Ambulatory Patients Across the Disease Trajectory (FULL)
A diagnosis of cancer, its treatment, and surveillance are fraught with distress. Distress is defined by the National Comprehensive Cancer Network® (NCCN®) as “a multifactorial unpleasant emotional experience of a psychological (cognitive, behavioral, emotional), social, and/or spiritual nature that may interfere with the ability to cope effectively with cancer, its physical symptoms, and its treatment.”1 Distress is known to occur at any point along the cancer-disease trajectory: during diagnosis, during treatment, at the end of treatment, at pivotal treatment decision points, from survivorship through to end of life.2 The severity of the distress can range from “common normal feelings of vulnerability, sadness, and fears to problems that can become disabling, such as depression, anxiety, panic, social isolation, and existential and spiritual crisis.”1 Most important, the impact of distress has been associated with reduced quality of life (QOL) and potentially reduced survival.3,4
About 33% of all persons with cancer experience severe distress.5,6 As a result of the prevalence and severity of distress, the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Distress Management recommend that all patients with cancer should be screened for distress, using a standardized tool, at their initial visit, at appropriate intervals, and as clinically indicated.1 The time line for longitudinal screening of “appropriate intervals” has not been firmly established.2 However, it is well recognized that appropriate intervals include times of vulnerability such as remission, recurrence, termination of treatment, and progression.1,7 Despite efforts to improve distress screening and intervention, many institutions struggle to adhere to the NCCN Guidelines®.8,9
In 2012, the American College of Surgeons Commission on Cancer (ACoS CoC) identified distress screening as an essential accreditation standard by 2015.10 The standard mandates that patients be screened a minimum of 1 time at a “pivotal” medical visit (such as time of diagnosis, transitions in cancer treatment, recurrence, completion of cancer treatment, and progression of disease). In practice, most institutions typically screen at diagnosis.2 According to the ACoS CoC, 41 VAMCs are accredited sites that will be impacted by the implementation of this standard.10
Distress Screening Tools
A major challenge and barrier to integrating distress screening in cancer clinics is the lack of consensus on the best measurement tool in a busy ambulatory clinic. Although a number of screening tools are available for measuring cancer-related distress, they vary in efficacy and feasibility. According to Zabora and Macmurray, the perfect screening instrument for distress in persons with cancer does not exist.6 Brief screening tools demonstrate high sensitivity in identifying very distressed patients but lack specificity, resulting in false positives.8,11 More extensive screening instruments, such as the Hospital Anxiety and Depression Scale (HADS), the Brief Symptom Inventory (BSI)-18, and the Psycho-Oncology Screening Tool (POST), have lower rates of false positives but may be more burdensome for providers, especially when considering copyright and cost.6
Ambulatory cancer care requires a rapid screening method with high sensitivity and minimal burden.12 The NCCN Distress Thermometer (DT) has face validity and allows for rapid screening; however, its psychometric properties are not as robust as other instru ments, such as the Center for Epidemiological Studies Depression Scale, the Hospital Anxiety and Depression Scale, Psychological Distress Inventory, or Brief Symptom Inventory.13 Although the DT has been shown to identify clinically significant anxiety, it is not as sensitive in identifying depression.4
The NCCN DT has 2 parts to the screening: (1) an overall distressintensity score within the past week, including the current day; and (2) an accompanying problem list, grouped into 5 categories, addressing QOL domains.14 The quantitative score ranges from 0 (no distress) to 10 (extreme distress). The problem list complements the quantitative score by providing information about the source of distress and can help to tailor the intervention (Figure 1). Access to the NCCN Guideline and DT is free for clinical and personal use.
According to the NCCN Guideline, scores of ≥ 4 require distress-management intervention.1 Mild distress (score < 4) usually can be managed by the primary oncology team.15 However, if the patient’s score is moderate (4-7) or severe (8-10), urgent intervention is necessary. Depending on the source of the distress, the patient should be seen by the appropriate discipline. For patients with practical problems, such as transportation, finances, and housing issues, a referral to social work is needed. For those with distress related to mental health issues, psychology, psychiatry, or social work may be appropriate.
Patients with distressing physical symptoms should be seen by the physician or advanced practice registered nurse (APRN) from the oncology or palliative care team. With limited psychosocial resources available at many cancer clinics, identification and triage for those with the highest levels of distress are critical.5 Triage must incorporate both the total distress score and the components of the distress so that the appropriate disciplines are accessed for the plan of care. More than one discipline may be needed to address multifactorial distress.
Despite strong recommendations from NCCN, ACoS, and many other professional and accrediting agencies, numerous cancer programs face challenges implementing routine screening. This article reports on a large, inner city ambulatory clinic’s pilot project to distress screen all patients at every appointment in the Cancer Center of Excellence (CoE) at Louis Stokes Cleveland VAMC (LSCVAMC) between May 2012 and May 2014 and to provide immediate intervention from the appropriate discipline for patients scoring ≥ 4 on a 0 to 10 DT scale. Results of the screenings, feasibility of screening in an ambulatory VA cancer clinic, and impact on psychosocial resources are presented.
Center of Excellence Project
The LSCVAMC CoE Cancer Care Clinic began as a 3-year grant-funded project from the VA Offices of Specialty Care and Academic Affiliations with 2 major objectives: (1) to deliver quality patient-centered cancer care as measured by implementation of a process for distress screening and management, and development and implementation of a survivorship care plan for patients who have completed cancer treatment; and (2) to provide interprofessional education for the interdisciplinary health care professionals who participate in the clinic as part of their training experience.
Patients in this unique CoE cancer clinic have sameday access to all members of the interdisciplinary and interprofessional team. The ambulatory cancer care CoE team was originally composed of a surgical oncologist, a medical oncologist, a clinical nurse specialist (CNS) patient navigator, a nurse practitioner (NP) in survivorship care, a registered nurse (RN), a psychologist, and an oncology social worker. The project’s patient population included patients with a cancer concern (positive family history and suspicious scans) or a diagnosis of breast cancer, melanoma, sarcoma, or hematologic malignancies. The patient population for the project was based on the CoE team expertise and feasibility of implementation, with plans to roll out the model of care for all patients with any cancer diagnosis across the VAMC at the completion of the project.
The CoE made distress screening and management the leading priority for quality patient-centered care at the start of the project. The purpose of this emphasis on distress screening was to develop a process at LSCVAMC that would meet the 2015 CoC standards and to teach health care professional trainees (NP students, residents, social work students, and fellows in psychology and medical oncology) about distress screening and intervention.
A plan-do-act model of quality improvement (QI) was used to support the development and implementation of the distress-screening process. At the beginning of the project, the institutional review board (IRB) reviewed the protocol and determined that informed consent was not necessary because a QI project for a new standard of care did not require IRB approval. The CoE team met for about 4 months to develop a policy and procedure for the process, based on evidence from national guidelines, a review of the literature, and a discussion of the benefits and burdens of implementation within the current practice.
Limiting initial implementation to a single clinic day made the process more manageable. Descriptive methods analyzed the incidence and percentage of overall distress in this veteran population and quantified the incidences and percentages of each DT component. Feedback from patients and staff offered information on the feasibility of and satisfaction with the process.
From May 2012 to May 2014, all patients who attended the Monday outpatient CoE clinic with a diagnosis of cancer or a cancer concern were given the NCCN, 2.2013 DT instrument by the registration desk clerk at the time they registered for their clinic appointment. 16 Veterans who had difficulty filling out the DT or who had diminished capacity were assisted in completing the instrument by a designated family member and/or the clinic RN.
The completed instrument was evaluated by the CNS patient navigator, and any patient with a score ≥ 4 received an automatic referral to the behavioral health psychologist, social worker, NP, or all team members and their trainees, depending on the areas of distress (eg, practical, family, and emotional problems, spiritual/religious concerns, and/or physical problems) endorsed by the patient.
A psychiatrist was not embedded into the team but worked closely with the team’s oncology psychologist. The psychologist communicated directly with the psychiatrist, and the plan was shared with the team through the electronic medical record (EMR). The appropriate team member(s) and trainee(s) saw the patient at the visit to address needs in real time. Access to palliative care support and spiritual care was readily available if needed.
Distress screenings were recorded in a templated note in the patient’s EMR, which allowed the team to follow the distress scores on an individual basis across the cancer disease trajectory and to assess response to interventions. Multiple screenings of individuals resulted from the fact that many of the patients were seen monthly or every 3/6/9 months, depending on their disease and treatment status. Because levels of distress can fluctuate, distress was assessed at every visit to determine whether an intervention was needed at that visit. Once distress screenings were recorded in the patient’s EMR, the DT instrument was de-identified and given to the CoE research consultant to enter into a database file for analysis.
Trainees were educated about the use of the DT at time of diagnosis and across the disease trajectory. The 4-week CoE curriculum included 2 weeks of conference time to teach about the roles of psychologist, oncology social worker, and survivorship NP in assessing and initiating interventions to address the multidimensional components of the DT. Trainees working with a veteran who was distressed participated in the assessment(s) and intervention(s) for all components of distress that were endorsed.
Results
A total of 866 distress screenings were performed during the first 2 years of the project. Since all patients were screened at all visits, the 866 distress screenings reflect multiple screenings for 445 unique patients. Of the 866 screenings, 290 (33%) had distress scores of ≥ 4, meeting the criteria for intervention. Screenings reflected patient visits at any point in the disease trajectory. Because this was a new standard of care QI project rather than a research project, additional data, such as diagnosis or staging, were not collected, and IRB approval was not needed.
Because the NCCN Guideline recommendation for intervention is a score of ≥ 4, the descriptive statistics focused on those with moderate-to-severe distress. However, there were numerous occasions when the veteran would report a score of 0 to 3 and still endorse a number of the problems on the DT. The CNS and RN on the team discussed these findings with the appropriate discipline. For example, if the veteran reported a score of 1 but endorsed all 6 components on the emotional problem list, the nurses discussed the patient with the social worker or psychologist to determine whether behavioral health intervention was needed.
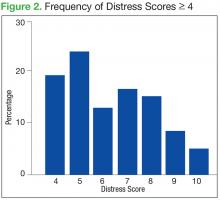
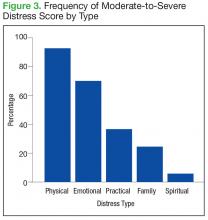
The mean distress score for the 290 screenings ≥ 4 was 6.3 on a 0 to 10 scale; median was 6.0 and mode 5.0. Two hundred ten of these screenings (72%) were categorized as moderate distress (4-7), and 80 patients (28%) reported severe distress (Figure 2). If the veteran left a box empty on the problem list, itwas recorded as missing. The frequency that patients reported each type of distress are reported in Figure 3.
The incidence of each component of distress, from those screenings with a score of ≥ 4 is described below, along with case study examples for each component. Team members involved in patient interventions provided these case studies to demonstrate clinical examples of the veteran’s distress from the problem list on the DT.
Practical/Family Distress
Practical issues were reported in 38% of the screenings (109/290). Intervention for moderate-to-severe distress associated with practical problems and family issues was provided by the team social worker. The social worker frequently addressed transportationrelated distress. Providing transportation was essential for adherence to clinic appointments, follow-up testing, treatments, and ultimately, disease management. Housing was also a problem for many veterans. It is critical that patients have access to electricity, heating, food, and water to be able to safely undergo adjuvant therapy. Thus, treatments decisions could be impacted by the veterans’ housing and transportation issues; immediate access to social work support is essential for quality cancer care.
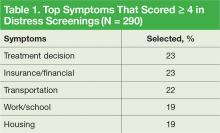
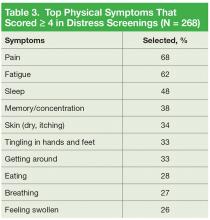
Twenty-six percent of patients that were screened expressed concerns with practical and/or family problems (75/289). Issues of domestic violence, difficulties dealing with a significant other, and concerns about children were referred to social work (Table 1).
Case Study
Ms. S. is a veteran aged 71 years with recently diagnosed breast cancer. She is being seen in the clinic for a postoperative visit following partial mastectomy and is anticipating beginning radiation therapy within the next 3 weeks. She reports a distress score of 7 and identifies concerns about work and transportation to the clinic as the sources of distress. The social worker meets with the patient and learns that she fears losing her job because of the daily travel time to and from radiation and that she cannot afford to travel 65 miles daily to LSCVAMC for radiation. The social worker listens to her concerns and assists her with a plan for short-term disability and VA housing during her radiation therapy treatments. Ms. S. was able to complete radiation at LSCVAMC with temporary housing and to return to work after therapy.
Emotional Distress
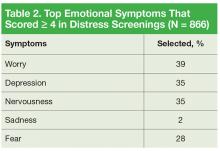
Patients who identified that their moderate-to-severe distress was related to emotional problems received same-day intervention from a psychologist skilled in providing emotional support, cognitive behavioral strategies, and assessing the need for referral to either a psychiatrist or oncology social worker. Seventy-one percent of patients reported emotional problems, such as worry, depression, and nervousness (Table 2).
Case Study
Mr. K. is a veteran aged 71 years with a new diagnosis of breast cancer. He lives on his own but has family and a few friends nearby. He reports that he doesn’t like to share his problems with others and has not told anyone of his new diagnosis. Mr. K. rates his distress a 7 and endorses worry, fear, and depression. At a treatment-planning visit, he agrees to see the psychologist for help in dealing with his distress. Treatment involves a mastectomy followed by hormonal therapy.
Mr. K. was scared about having cancer; some of his veteran colleagues have developed cancer recently, and 2 have died. He told the psychologist that he feels worthless and that this disease just makes him more of a burden on society. He has had thoughts of taking his life so that he doesn’t have to deal with cancer, but he does not have a plan. The team formulated a plan to address his anxiety and depression. Mr. K. started a serotonin reuptake inhibitor, and he met with the VA psychiatrist weekly to help develop coping strategies. The team’s psychologist worked closely with Mr. K.’s psychiatrist, and he successfully completed surgery and chemotherapy. He is now being seen in survivorship clinic, continuing care with the team and his psychologist.
Spiritual Distress
Although spiritual/religious concerns are part of DT screening, it is only a single item on the DT. Just 8% of patients (21/276) reported moderate-to-severe spiritual distress. However, there was access to a chaplain at LSCVAMC.
Case Study
Mr. H., a 63-year-old veteran with stage IV melanoma, was seen in the clinic for severe pain in his left hip and ribs (8 on a 10-point scale); he was unresponsive to escalating doses of oxycodone. During the visit, he reported that his distress level is a 10, and in addition to identifying pain as a source of distress, he indicated that he has spiritual distress. When questioned further about spiritual distress, Mr. H. reported that he deserves this pain since he caused so many others pain during his time in Vietnam. The chaplain was contacted, and the patient was seen in clinic at this visit. The chaplain gave him the opportunity to share his feelings of guilt. The importance of spiritual care when the patient is experiencing “total pain” is essential to pain management. Within 3 days, his pain score decreased to an acceptable level of 3 with no additional pharmacologic intervention.
Physical Distress
Physical problems associated with the distress scores were addressed by the surgical and medical oncologists and the APRNs (CNS patient navigator and survivorship NP). When the clinic opened, the team used the Memorial Symptom Assessment Scale to assess physical and psychological symptoms.16 However, patients reported experiencing distress at having to complete 2 tools that had a great deal of overlap. The team determined that the DT could be used as the sole screening tool for all QOL domains.
It is important to note that 92% of patients with moderate-to-severe distress reported physical symptoms as a source of distress (Table 3).
Case Study
Ms. L. is a Vietnam War veteran aged 64 years who was seen in the survivorship clinic. She was diagnosed with estrogen-receptor (ER) and progesterone-receptor (PR) breast cancer 1 year previously, had a lumpectomy followed by radiation therapy, and was on hormonal therapy. She recorded her distress score as a 6 and indicated that multiple physical symptoms were her major concern. She had difficulty with insomnia, fatigue, and hot flashes. The survivorship NP talked with Ms. L. about her symptoms and made nonpharmacologic recommendations for improving sleep, provided an exercise plan for fatigue, and initiated venlafaxine to manage the hot flashes. Ms. L. continued to be seen by the team in survivorship clinic, and during her 3-month follow-up visit, she reported improvement in sleep as the hot flashes diminished.
Multifactoral Distress
Many patients endorsed ≥ 1 component of distress. This required a team approach to intervene for the multifactorial nature of the distress.
Case Study
Mr. K. is a veteran aged 82 years who had been a farmer most of his life. He was cared for at the VA for an advancedstage squamous cell skin cancer of his scalp, which he had allowed to go untreated. The cancer has completely eroded beneath his scalp, and he wore a hat to cover the foul-smelling wound. He lived in rural Ohio with his wife of 55 years; 3 adult daughters lived in the Cleveland area. His daughters served as primary caregivers when Mr. K. came to Cleveland for daily radiation and weekly chemotherapy treatments. He had not been away from his wife since the war and misses her terribly, returning home only on weekends during the 6-week course of radiation.
His primary goal was to return home in time to harvest his farm’s produce 2 months later. He was aware that he has < 6 months to live but wanted chemotherapy and radiation to control the growth of the cancer. During this visit to the ambulatory clinic, he reported a distress score of 5 andidentified family concerns (eg, living away from his wife most of every week) and endorsed emotional concerns of fear, worry, and sadness, and reported pain, fatigue, insomnia, and constipation as physical concerns.
Mr. K. received support from the social worker, the psychologist, and the APRN for symptom management during this visit. The social worker was able to advocate for limited palliative radiation therapy treatments rather than a 6-week course; the psychologist spent 45 minutes talking with him about his fears of a painful death, worries about his wife, and sadness at not being alive for another planting season. The APRN recommended both pharmacologic and nonpharmacologic interventions for his fatigue and insomnia and initiated a pain and bowel pharmacologic regimen. The team respected Mr. K.’s wish to reconsider hospice care at the following visit after he had talked with his wife. Mr. K. died peacefully in his home with his wife and family just before the start of planting season.
Clinical Implications
Distress screening and intervention is essential for quality cancer care. While a great deal of controversy exists about the best time to screen for distress, the LSCVAMC CoE has taken on the challenge of screening and intervening in real time at every patient visit across the disease trajectory. The model of distress screening all veterans at CoE clinic visits has been rolled out to other cancer clinics at LSCVAMC.
Distress screening at each visit is not time intensive. Patients are willing to fill out the instrument while waiting for their clinic visit, and most patients find that it takes less than 5 minutes to complete. The major challenge for institutions considering screening with each visit is not the screening but access to appropriate providers able to provide timely intervention. The success of this model results, in part, because the clinic RN assesses the responses to the DT and refers to the appropriate discipline, utilizing precious resources of social work and psychology appropriately. The VA system is already committed to improving the psychosocial well-being of veterans and has established social work and psychology resources specifically for the cancer clinics.
Many patients reported to the authors that they might not have been able or willing to return to LSCVAMC to see the behavioral health specialists on another day. In addition, scheduling behavioral health appointments at another time would not allow for attending to the distress in real time. Also, from a systems standpoint, it would have been an added cost to the VA and/or the veteran for transportation for additional appointments on different days.
Finally, although the impact of the CoE project on health professional trainees has been reported elsewhere, the distress screening and intervention process were valued as being very positive for all trainees who participated in CoE clinic.17 The trainees were able to stay with the patient for the entire clinic visit, including the visits made by disciplines other than their own. For example, the family medicine residents stayed with the patient they examined to observe the distress assessments and interventions offered by the social worker and/or psychologist for the patients who scored ≥ 4 on the DT.
At the end of their rotations in the CoE, trainees reported an increased awareness of the importance of distress screening in a cancer clinic. Many were not aware of the NCCN guidelines and the ACoS CoC mandate for distress screening as a standard of cancer care. Interdisciplinary trainees rated the CoE curriculum and the conference teaching/learning sessions on distress management highly. However, observing the role of the social worker and psychologist were the most valuable to trainees, regardless of the area of practice they enter.
Conclusion
Addressing practical, psychosocial, physical, and spiritual needs will help decrease distress, support patients’ ability to tolerate treatment, and improve veterans’ QOL across the cancer-disease trajectory. Screening all patients at an outpatient cancer clinic at LSCVAMC is feasible and does not seem to be a burden for patients or providers. This pilot project has become standard of care across the LSCVAMC cancer clinics, demonstrating its sustainability.
Screening with the DT provides information about the intensity of the distress and the components contributing to the distress. The most important aspect of the screening is assessing the components of the distress and providing real-time intervention from the appropriate discipline. It is critical that the oncology team refer to the appropriate discipline based on the source of the distress rather than on only the intensity. Findings from this project indicate that physical symptoms are frequently the source of distress and may not require behavioral health intervention. However, for patients with psychosocial needs, rapid access to behavioral health care services is critical for quality veteran-centered cancer care.
Since 2015, all VA cancer centers are required to have implemented distress screening. According to the CoC, at least 1 screening must be done on every patient.10 Many institutions have begun to screen at diagnosis, but it is well known that there are many points along the cancer trajectory when patients may experience an increase in distress. Simple screening with the DT at every cancer clinic visit helps identify the veterans’ needs at any point along the disease spectrum.
At LSCVAMC, the CoE was designed as an interdisciplinary cancer clinic. With the conclusion of funding in FY 2015, the clinic has continued to function. The rollout into other clinics has continued with movement toward use of formal consult requests and continual, real-time evaluation of the process. Work on accurate, timely identification of new cancer patients and identifying pivotal cancer visits is underway. The LSCVAMC is committed to improving care and access to its veterans with cancer to ensure appropriate and adequate services across the cancer trajectory.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Holand JC, Jacobsen PB, Anderson A, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Distress Management 2.2016. © 2014 National Comprehensive Cancer Network, Inc. https://www.nccn.org/professionals/physician_gls/pdf/distress.pdf. Updated July 25, 2016. Accessed January 13, 2017.
2. Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol. 2012;30(11):1160-1177.
3. Hamer M, Chida Y, Molloy G. Psychological distress and cancer mortality. J Psychosom Res. 2009;66(3):255-258.
4. Mitchell AJ. Short screening tools for cancer-related distress: a review and diagnostic validity meta-analysis. J Natl Compr Canc Netw. 2013;8(4):487-494.
5. Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19-28.
6. Zabora JR, Macmurray L. The history of psychosocial screening among cancer patients. J Psychosoc Oncol. 2012;30(6):625-635.
7. Pirl WF, Fann JR, Greer JA, et al. Recommendations for the implementation of distress screening programs in cancer centers: report from the American Psychosocial Oncology Society (APOS), Association of Oncology Social Work (AOSW), and Oncology Nursing Society (ONS) joint task force. Cancer. 2014;120(91):2946-2954.
8. Parry C, Padgett LS, Zebrack B. Now what? Toward an integrated research and practice agenda in distress screening. J Psychosoc Oncol. 2012;30(6):715-727.
9. Wagner LI, Spiegel D, Pearman T. Using the science of psychosocial care to implement the new American College of Surgeons Commission on Cancer distress screening standard. J Natl Compr Canc Netw. 2013;11(2):214-221.
10. American College of Surgeons Commision on Cancer. https://www.facs.org/quality-programs/cancer/coc Published 1996. Updated January 19, 2017. Accessed April 16, 2016.
11. Rohan EA. Removing the stress from selecting instruments: arming social workers to take leadership in routine distress screening implementation. J Psychosoc Oncol. 2012;30(6):667-678.
12. Merport A, Bober SL, Grose A, Recklitis CJ. Can the distress thermometer (DT) identify significant psychological distress in long-term cancer survivors? A comparison with the Brief Symptom Inventory-18 (BSI-18). Support Care Cancer. 2012;20(1):195-198.
13. Carlson LE, Bultz BD. Cancer distress screening: Needs, models, and methods. J Psychosom Res. 2003;55(5):403-409.
14. Holland JC, Alici Y. Management of distress in cancer patients. J Support Oncol. 2010:8(1):4-12.
15. Jacobsen P, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103(7):1494-1502.
16. Portenoy R, Thaler HT, Korblith AB, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A(9):1326-1336.
17. Arfons L, Mazanec P, Smith J, et al. Training health care professionals in interprofessional collaborative cancer care. Health Interprof Pract. 2015;2(3):eP1073.
A diagnosis of cancer, its treatment, and surveillance are fraught with distress. Distress is defined by the National Comprehensive Cancer Network® (NCCN®) as “a multifactorial unpleasant emotional experience of a psychological (cognitive, behavioral, emotional), social, and/or spiritual nature that may interfere with the ability to cope effectively with cancer, its physical symptoms, and its treatment.”1 Distress is known to occur at any point along the cancer-disease trajectory: during diagnosis, during treatment, at the end of treatment, at pivotal treatment decision points, from survivorship through to end of life.2 The severity of the distress can range from “common normal feelings of vulnerability, sadness, and fears to problems that can become disabling, such as depression, anxiety, panic, social isolation, and existential and spiritual crisis.”1 Most important, the impact of distress has been associated with reduced quality of life (QOL) and potentially reduced survival.3,4
About 33% of all persons with cancer experience severe distress.5,6 As a result of the prevalence and severity of distress, the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Distress Management recommend that all patients with cancer should be screened for distress, using a standardized tool, at their initial visit, at appropriate intervals, and as clinically indicated.1 The time line for longitudinal screening of “appropriate intervals” has not been firmly established.2 However, it is well recognized that appropriate intervals include times of vulnerability such as remission, recurrence, termination of treatment, and progression.1,7 Despite efforts to improve distress screening and intervention, many institutions struggle to adhere to the NCCN Guidelines®.8,9
In 2012, the American College of Surgeons Commission on Cancer (ACoS CoC) identified distress screening as an essential accreditation standard by 2015.10 The standard mandates that patients be screened a minimum of 1 time at a “pivotal” medical visit (such as time of diagnosis, transitions in cancer treatment, recurrence, completion of cancer treatment, and progression of disease). In practice, most institutions typically screen at diagnosis.2 According to the ACoS CoC, 41 VAMCs are accredited sites that will be impacted by the implementation of this standard.10
Distress Screening Tools
A major challenge and barrier to integrating distress screening in cancer clinics is the lack of consensus on the best measurement tool in a busy ambulatory clinic. Although a number of screening tools are available for measuring cancer-related distress, they vary in efficacy and feasibility. According to Zabora and Macmurray, the perfect screening instrument for distress in persons with cancer does not exist.6 Brief screening tools demonstrate high sensitivity in identifying very distressed patients but lack specificity, resulting in false positives.8,11 More extensive screening instruments, such as the Hospital Anxiety and Depression Scale (HADS), the Brief Symptom Inventory (BSI)-18, and the Psycho-Oncology Screening Tool (POST), have lower rates of false positives but may be more burdensome for providers, especially when considering copyright and cost.6
Ambulatory cancer care requires a rapid screening method with high sensitivity and minimal burden.12 The NCCN Distress Thermometer (DT) has face validity and allows for rapid screening; however, its psychometric properties are not as robust as other instru ments, such as the Center for Epidemiological Studies Depression Scale, the Hospital Anxiety and Depression Scale, Psychological Distress Inventory, or Brief Symptom Inventory.13 Although the DT has been shown to identify clinically significant anxiety, it is not as sensitive in identifying depression.4
The NCCN DT has 2 parts to the screening: (1) an overall distressintensity score within the past week, including the current day; and (2) an accompanying problem list, grouped into 5 categories, addressing QOL domains.14 The quantitative score ranges from 0 (no distress) to 10 (extreme distress). The problem list complements the quantitative score by providing information about the source of distress and can help to tailor the intervention (Figure 1). Access to the NCCN Guideline and DT is free for clinical and personal use.
According to the NCCN Guideline, scores of ≥ 4 require distress-management intervention.1 Mild distress (score < 4) usually can be managed by the primary oncology team.15 However, if the patient’s score is moderate (4-7) or severe (8-10), urgent intervention is necessary. Depending on the source of the distress, the patient should be seen by the appropriate discipline. For patients with practical problems, such as transportation, finances, and housing issues, a referral to social work is needed. For those with distress related to mental health issues, psychology, psychiatry, or social work may be appropriate.
Patients with distressing physical symptoms should be seen by the physician or advanced practice registered nurse (APRN) from the oncology or palliative care team. With limited psychosocial resources available at many cancer clinics, identification and triage for those with the highest levels of distress are critical.5 Triage must incorporate both the total distress score and the components of the distress so that the appropriate disciplines are accessed for the plan of care. More than one discipline may be needed to address multifactorial distress.
Despite strong recommendations from NCCN, ACoS, and many other professional and accrediting agencies, numerous cancer programs face challenges implementing routine screening. This article reports on a large, inner city ambulatory clinic’s pilot project to distress screen all patients at every appointment in the Cancer Center of Excellence (CoE) at Louis Stokes Cleveland VAMC (LSCVAMC) between May 2012 and May 2014 and to provide immediate intervention from the appropriate discipline for patients scoring ≥ 4 on a 0 to 10 DT scale. Results of the screenings, feasibility of screening in an ambulatory VA cancer clinic, and impact on psychosocial resources are presented.
Center of Excellence Project
The LSCVAMC CoE Cancer Care Clinic began as a 3-year grant-funded project from the VA Offices of Specialty Care and Academic Affiliations with 2 major objectives: (1) to deliver quality patient-centered cancer care as measured by implementation of a process for distress screening and management, and development and implementation of a survivorship care plan for patients who have completed cancer treatment; and (2) to provide interprofessional education for the interdisciplinary health care professionals who participate in the clinic as part of their training experience.
Patients in this unique CoE cancer clinic have sameday access to all members of the interdisciplinary and interprofessional team. The ambulatory cancer care CoE team was originally composed of a surgical oncologist, a medical oncologist, a clinical nurse specialist (CNS) patient navigator, a nurse practitioner (NP) in survivorship care, a registered nurse (RN), a psychologist, and an oncology social worker. The project’s patient population included patients with a cancer concern (positive family history and suspicious scans) or a diagnosis of breast cancer, melanoma, sarcoma, or hematologic malignancies. The patient population for the project was based on the CoE team expertise and feasibility of implementation, with plans to roll out the model of care for all patients with any cancer diagnosis across the VAMC at the completion of the project.
The CoE made distress screening and management the leading priority for quality patient-centered care at the start of the project. The purpose of this emphasis on distress screening was to develop a process at LSCVAMC that would meet the 2015 CoC standards and to teach health care professional trainees (NP students, residents, social work students, and fellows in psychology and medical oncology) about distress screening and intervention.
A plan-do-act model of quality improvement (QI) was used to support the development and implementation of the distress-screening process. At the beginning of the project, the institutional review board (IRB) reviewed the protocol and determined that informed consent was not necessary because a QI project for a new standard of care did not require IRB approval. The CoE team met for about 4 months to develop a policy and procedure for the process, based on evidence from national guidelines, a review of the literature, and a discussion of the benefits and burdens of implementation within the current practice.
Limiting initial implementation to a single clinic day made the process more manageable. Descriptive methods analyzed the incidence and percentage of overall distress in this veteran population and quantified the incidences and percentages of each DT component. Feedback from patients and staff offered information on the feasibility of and satisfaction with the process.
From May 2012 to May 2014, all patients who attended the Monday outpatient CoE clinic with a diagnosis of cancer or a cancer concern were given the NCCN, 2.2013 DT instrument by the registration desk clerk at the time they registered for their clinic appointment. 16 Veterans who had difficulty filling out the DT or who had diminished capacity were assisted in completing the instrument by a designated family member and/or the clinic RN.
The completed instrument was evaluated by the CNS patient navigator, and any patient with a score ≥ 4 received an automatic referral to the behavioral health psychologist, social worker, NP, or all team members and their trainees, depending on the areas of distress (eg, practical, family, and emotional problems, spiritual/religious concerns, and/or physical problems) endorsed by the patient.
A psychiatrist was not embedded into the team but worked closely with the team’s oncology psychologist. The psychologist communicated directly with the psychiatrist, and the plan was shared with the team through the electronic medical record (EMR). The appropriate team member(s) and trainee(s) saw the patient at the visit to address needs in real time. Access to palliative care support and spiritual care was readily available if needed.
Distress screenings were recorded in a templated note in the patient’s EMR, which allowed the team to follow the distress scores on an individual basis across the cancer disease trajectory and to assess response to interventions. Multiple screenings of individuals resulted from the fact that many of the patients were seen monthly or every 3/6/9 months, depending on their disease and treatment status. Because levels of distress can fluctuate, distress was assessed at every visit to determine whether an intervention was needed at that visit. Once distress screenings were recorded in the patient’s EMR, the DT instrument was de-identified and given to the CoE research consultant to enter into a database file for analysis.
Trainees were educated about the use of the DT at time of diagnosis and across the disease trajectory. The 4-week CoE curriculum included 2 weeks of conference time to teach about the roles of psychologist, oncology social worker, and survivorship NP in assessing and initiating interventions to address the multidimensional components of the DT. Trainees working with a veteran who was distressed participated in the assessment(s) and intervention(s) for all components of distress that were endorsed.
Results
A total of 866 distress screenings were performed during the first 2 years of the project. Since all patients were screened at all visits, the 866 distress screenings reflect multiple screenings for 445 unique patients. Of the 866 screenings, 290 (33%) had distress scores of ≥ 4, meeting the criteria for intervention. Screenings reflected patient visits at any point in the disease trajectory. Because this was a new standard of care QI project rather than a research project, additional data, such as diagnosis or staging, were not collected, and IRB approval was not needed.
Because the NCCN Guideline recommendation for intervention is a score of ≥ 4, the descriptive statistics focused on those with moderate-to-severe distress. However, there were numerous occasions when the veteran would report a score of 0 to 3 and still endorse a number of the problems on the DT. The CNS and RN on the team discussed these findings with the appropriate discipline. For example, if the veteran reported a score of 1 but endorsed all 6 components on the emotional problem list, the nurses discussed the patient with the social worker or psychologist to determine whether behavioral health intervention was needed.
The mean distress score for the 290 screenings ≥ 4 was 6.3 on a 0 to 10 scale; median was 6.0 and mode 5.0. Two hundred ten of these screenings (72%) were categorized as moderate distress (4-7), and 80 patients (28%) reported severe distress (Figure 2). If the veteran left a box empty on the problem list, itwas recorded as missing. The frequency that patients reported each type of distress are reported in Figure 3.
The incidence of each component of distress, from those screenings with a score of ≥ 4 is described below, along with case study examples for each component. Team members involved in patient interventions provided these case studies to demonstrate clinical examples of the veteran’s distress from the problem list on the DT.
Practical/Family Distress
Practical issues were reported in 38% of the screenings (109/290). Intervention for moderate-to-severe distress associated with practical problems and family issues was provided by the team social worker. The social worker frequently addressed transportationrelated distress. Providing transportation was essential for adherence to clinic appointments, follow-up testing, treatments, and ultimately, disease management. Housing was also a problem for many veterans. It is critical that patients have access to electricity, heating, food, and water to be able to safely undergo adjuvant therapy. Thus, treatments decisions could be impacted by the veterans’ housing and transportation issues; immediate access to social work support is essential for quality cancer care.
Twenty-six percent of patients that were screened expressed concerns with practical and/or family problems (75/289). Issues of domestic violence, difficulties dealing with a significant other, and concerns about children were referred to social work (Table 1).
Case Study
Ms. S. is a veteran aged 71 years with recently diagnosed breast cancer. She is being seen in the clinic for a postoperative visit following partial mastectomy and is anticipating beginning radiation therapy within the next 3 weeks. She reports a distress score of 7 and identifies concerns about work and transportation to the clinic as the sources of distress. The social worker meets with the patient and learns that she fears losing her job because of the daily travel time to and from radiation and that she cannot afford to travel 65 miles daily to LSCVAMC for radiation. The social worker listens to her concerns and assists her with a plan for short-term disability and VA housing during her radiation therapy treatments. Ms. S. was able to complete radiation at LSCVAMC with temporary housing and to return to work after therapy.
Emotional Distress
Patients who identified that their moderate-to-severe distress was related to emotional problems received same-day intervention from a psychologist skilled in providing emotional support, cognitive behavioral strategies, and assessing the need for referral to either a psychiatrist or oncology social worker. Seventy-one percent of patients reported emotional problems, such as worry, depression, and nervousness (Table 2).
Case Study
Mr. K. is a veteran aged 71 years with a new diagnosis of breast cancer. He lives on his own but has family and a few friends nearby. He reports that he doesn’t like to share his problems with others and has not told anyone of his new diagnosis. Mr. K. rates his distress a 7 and endorses worry, fear, and depression. At a treatment-planning visit, he agrees to see the psychologist for help in dealing with his distress. Treatment involves a mastectomy followed by hormonal therapy.
Mr. K. was scared about having cancer; some of his veteran colleagues have developed cancer recently, and 2 have died. He told the psychologist that he feels worthless and that this disease just makes him more of a burden on society. He has had thoughts of taking his life so that he doesn’t have to deal with cancer, but he does not have a plan. The team formulated a plan to address his anxiety and depression. Mr. K. started a serotonin reuptake inhibitor, and he met with the VA psychiatrist weekly to help develop coping strategies. The team’s psychologist worked closely with Mr. K.’s psychiatrist, and he successfully completed surgery and chemotherapy. He is now being seen in survivorship clinic, continuing care with the team and his psychologist.
Spiritual Distress
Although spiritual/religious concerns are part of DT screening, it is only a single item on the DT. Just 8% of patients (21/276) reported moderate-to-severe spiritual distress. However, there was access to a chaplain at LSCVAMC.
Case Study
Mr. H., a 63-year-old veteran with stage IV melanoma, was seen in the clinic for severe pain in his left hip and ribs (8 on a 10-point scale); he was unresponsive to escalating doses of oxycodone. During the visit, he reported that his distress level is a 10, and in addition to identifying pain as a source of distress, he indicated that he has spiritual distress. When questioned further about spiritual distress, Mr. H. reported that he deserves this pain since he caused so many others pain during his time in Vietnam. The chaplain was contacted, and the patient was seen in clinic at this visit. The chaplain gave him the opportunity to share his feelings of guilt. The importance of spiritual care when the patient is experiencing “total pain” is essential to pain management. Within 3 days, his pain score decreased to an acceptable level of 3 with no additional pharmacologic intervention.
Physical Distress
Physical problems associated with the distress scores were addressed by the surgical and medical oncologists and the APRNs (CNS patient navigator and survivorship NP). When the clinic opened, the team used the Memorial Symptom Assessment Scale to assess physical and psychological symptoms.16 However, patients reported experiencing distress at having to complete 2 tools that had a great deal of overlap. The team determined that the DT could be used as the sole screening tool for all QOL domains.
It is important to note that 92% of patients with moderate-to-severe distress reported physical symptoms as a source of distress (Table 3).
Case Study
Ms. L. is a Vietnam War veteran aged 64 years who was seen in the survivorship clinic. She was diagnosed with estrogen-receptor (ER) and progesterone-receptor (PR) breast cancer 1 year previously, had a lumpectomy followed by radiation therapy, and was on hormonal therapy. She recorded her distress score as a 6 and indicated that multiple physical symptoms were her major concern. She had difficulty with insomnia, fatigue, and hot flashes. The survivorship NP talked with Ms. L. about her symptoms and made nonpharmacologic recommendations for improving sleep, provided an exercise plan for fatigue, and initiated venlafaxine to manage the hot flashes. Ms. L. continued to be seen by the team in survivorship clinic, and during her 3-month follow-up visit, she reported improvement in sleep as the hot flashes diminished.
Multifactoral Distress
Many patients endorsed ≥ 1 component of distress. This required a team approach to intervene for the multifactorial nature of the distress.
Case Study
Mr. K. is a veteran aged 82 years who had been a farmer most of his life. He was cared for at the VA for an advancedstage squamous cell skin cancer of his scalp, which he had allowed to go untreated. The cancer has completely eroded beneath his scalp, and he wore a hat to cover the foul-smelling wound. He lived in rural Ohio with his wife of 55 years; 3 adult daughters lived in the Cleveland area. His daughters served as primary caregivers when Mr. K. came to Cleveland for daily radiation and weekly chemotherapy treatments. He had not been away from his wife since the war and misses her terribly, returning home only on weekends during the 6-week course of radiation.
His primary goal was to return home in time to harvest his farm’s produce 2 months later. He was aware that he has < 6 months to live but wanted chemotherapy and radiation to control the growth of the cancer. During this visit to the ambulatory clinic, he reported a distress score of 5 andidentified family concerns (eg, living away from his wife most of every week) and endorsed emotional concerns of fear, worry, and sadness, and reported pain, fatigue, insomnia, and constipation as physical concerns.
Mr. K. received support from the social worker, the psychologist, and the APRN for symptom management during this visit. The social worker was able to advocate for limited palliative radiation therapy treatments rather than a 6-week course; the psychologist spent 45 minutes talking with him about his fears of a painful death, worries about his wife, and sadness at not being alive for another planting season. The APRN recommended both pharmacologic and nonpharmacologic interventions for his fatigue and insomnia and initiated a pain and bowel pharmacologic regimen. The team respected Mr. K.’s wish to reconsider hospice care at the following visit after he had talked with his wife. Mr. K. died peacefully in his home with his wife and family just before the start of planting season.
Clinical Implications
Distress screening and intervention is essential for quality cancer care. While a great deal of controversy exists about the best time to screen for distress, the LSCVAMC CoE has taken on the challenge of screening and intervening in real time at every patient visit across the disease trajectory. The model of distress screening all veterans at CoE clinic visits has been rolled out to other cancer clinics at LSCVAMC.
Distress screening at each visit is not time intensive. Patients are willing to fill out the instrument while waiting for their clinic visit, and most patients find that it takes less than 5 minutes to complete. The major challenge for institutions considering screening with each visit is not the screening but access to appropriate providers able to provide timely intervention. The success of this model results, in part, because the clinic RN assesses the responses to the DT and refers to the appropriate discipline, utilizing precious resources of social work and psychology appropriately. The VA system is already committed to improving the psychosocial well-being of veterans and has established social work and psychology resources specifically for the cancer clinics.
Many patients reported to the authors that they might not have been able or willing to return to LSCVAMC to see the behavioral health specialists on another day. In addition, scheduling behavioral health appointments at another time would not allow for attending to the distress in real time. Also, from a systems standpoint, it would have been an added cost to the VA and/or the veteran for transportation for additional appointments on different days.
Finally, although the impact of the CoE project on health professional trainees has been reported elsewhere, the distress screening and intervention process were valued as being very positive for all trainees who participated in CoE clinic.17 The trainees were able to stay with the patient for the entire clinic visit, including the visits made by disciplines other than their own. For example, the family medicine residents stayed with the patient they examined to observe the distress assessments and interventions offered by the social worker and/or psychologist for the patients who scored ≥ 4 on the DT.
At the end of their rotations in the CoE, trainees reported an increased awareness of the importance of distress screening in a cancer clinic. Many were not aware of the NCCN guidelines and the ACoS CoC mandate for distress screening as a standard of cancer care. Interdisciplinary trainees rated the CoE curriculum and the conference teaching/learning sessions on distress management highly. However, observing the role of the social worker and psychologist were the most valuable to trainees, regardless of the area of practice they enter.
Conclusion
Addressing practical, psychosocial, physical, and spiritual needs will help decrease distress, support patients’ ability to tolerate treatment, and improve veterans’ QOL across the cancer-disease trajectory. Screening all patients at an outpatient cancer clinic at LSCVAMC is feasible and does not seem to be a burden for patients or providers. This pilot project has become standard of care across the LSCVAMC cancer clinics, demonstrating its sustainability.
Screening with the DT provides information about the intensity of the distress and the components contributing to the distress. The most important aspect of the screening is assessing the components of the distress and providing real-time intervention from the appropriate discipline. It is critical that the oncology team refer to the appropriate discipline based on the source of the distress rather than on only the intensity. Findings from this project indicate that physical symptoms are frequently the source of distress and may not require behavioral health intervention. However, for patients with psychosocial needs, rapid access to behavioral health care services is critical for quality veteran-centered cancer care.
Since 2015, all VA cancer centers are required to have implemented distress screening. According to the CoC, at least 1 screening must be done on every patient.10 Many institutions have begun to screen at diagnosis, but it is well known that there are many points along the cancer trajectory when patients may experience an increase in distress. Simple screening with the DT at every cancer clinic visit helps identify the veterans’ needs at any point along the disease spectrum.
At LSCVAMC, the CoE was designed as an interdisciplinary cancer clinic. With the conclusion of funding in FY 2015, the clinic has continued to function. The rollout into other clinics has continued with movement toward use of formal consult requests and continual, real-time evaluation of the process. Work on accurate, timely identification of new cancer patients and identifying pivotal cancer visits is underway. The LSCVAMC is committed to improving care and access to its veterans with cancer to ensure appropriate and adequate services across the cancer trajectory.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
A diagnosis of cancer, its treatment, and surveillance are fraught with distress. Distress is defined by the National Comprehensive Cancer Network® (NCCN®) as “a multifactorial unpleasant emotional experience of a psychological (cognitive, behavioral, emotional), social, and/or spiritual nature that may interfere with the ability to cope effectively with cancer, its physical symptoms, and its treatment.”1 Distress is known to occur at any point along the cancer-disease trajectory: during diagnosis, during treatment, at the end of treatment, at pivotal treatment decision points, from survivorship through to end of life.2 The severity of the distress can range from “common normal feelings of vulnerability, sadness, and fears to problems that can become disabling, such as depression, anxiety, panic, social isolation, and existential and spiritual crisis.”1 Most important, the impact of distress has been associated with reduced quality of life (QOL) and potentially reduced survival.3,4
About 33% of all persons with cancer experience severe distress.5,6 As a result of the prevalence and severity of distress, the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Distress Management recommend that all patients with cancer should be screened for distress, using a standardized tool, at their initial visit, at appropriate intervals, and as clinically indicated.1 The time line for longitudinal screening of “appropriate intervals” has not been firmly established.2 However, it is well recognized that appropriate intervals include times of vulnerability such as remission, recurrence, termination of treatment, and progression.1,7 Despite efforts to improve distress screening and intervention, many institutions struggle to adhere to the NCCN Guidelines®.8,9
In 2012, the American College of Surgeons Commission on Cancer (ACoS CoC) identified distress screening as an essential accreditation standard by 2015.10 The standard mandates that patients be screened a minimum of 1 time at a “pivotal” medical visit (such as time of diagnosis, transitions in cancer treatment, recurrence, completion of cancer treatment, and progression of disease). In practice, most institutions typically screen at diagnosis.2 According to the ACoS CoC, 41 VAMCs are accredited sites that will be impacted by the implementation of this standard.10
Distress Screening Tools
A major challenge and barrier to integrating distress screening in cancer clinics is the lack of consensus on the best measurement tool in a busy ambulatory clinic. Although a number of screening tools are available for measuring cancer-related distress, they vary in efficacy and feasibility. According to Zabora and Macmurray, the perfect screening instrument for distress in persons with cancer does not exist.6 Brief screening tools demonstrate high sensitivity in identifying very distressed patients but lack specificity, resulting in false positives.8,11 More extensive screening instruments, such as the Hospital Anxiety and Depression Scale (HADS), the Brief Symptom Inventory (BSI)-18, and the Psycho-Oncology Screening Tool (POST), have lower rates of false positives but may be more burdensome for providers, especially when considering copyright and cost.6
Ambulatory cancer care requires a rapid screening method with high sensitivity and minimal burden.12 The NCCN Distress Thermometer (DT) has face validity and allows for rapid screening; however, its psychometric properties are not as robust as other instru ments, such as the Center for Epidemiological Studies Depression Scale, the Hospital Anxiety and Depression Scale, Psychological Distress Inventory, or Brief Symptom Inventory.13 Although the DT has been shown to identify clinically significant anxiety, it is not as sensitive in identifying depression.4
The NCCN DT has 2 parts to the screening: (1) an overall distressintensity score within the past week, including the current day; and (2) an accompanying problem list, grouped into 5 categories, addressing QOL domains.14 The quantitative score ranges from 0 (no distress) to 10 (extreme distress). The problem list complements the quantitative score by providing information about the source of distress and can help to tailor the intervention (Figure 1). Access to the NCCN Guideline and DT is free for clinical and personal use.
According to the NCCN Guideline, scores of ≥ 4 require distress-management intervention.1 Mild distress (score < 4) usually can be managed by the primary oncology team.15 However, if the patient’s score is moderate (4-7) or severe (8-10), urgent intervention is necessary. Depending on the source of the distress, the patient should be seen by the appropriate discipline. For patients with practical problems, such as transportation, finances, and housing issues, a referral to social work is needed. For those with distress related to mental health issues, psychology, psychiatry, or social work may be appropriate.
Patients with distressing physical symptoms should be seen by the physician or advanced practice registered nurse (APRN) from the oncology or palliative care team. With limited psychosocial resources available at many cancer clinics, identification and triage for those with the highest levels of distress are critical.5 Triage must incorporate both the total distress score and the components of the distress so that the appropriate disciplines are accessed for the plan of care. More than one discipline may be needed to address multifactorial distress.
Despite strong recommendations from NCCN, ACoS, and many other professional and accrediting agencies, numerous cancer programs face challenges implementing routine screening. This article reports on a large, inner city ambulatory clinic’s pilot project to distress screen all patients at every appointment in the Cancer Center of Excellence (CoE) at Louis Stokes Cleveland VAMC (LSCVAMC) between May 2012 and May 2014 and to provide immediate intervention from the appropriate discipline for patients scoring ≥ 4 on a 0 to 10 DT scale. Results of the screenings, feasibility of screening in an ambulatory VA cancer clinic, and impact on psychosocial resources are presented.
Center of Excellence Project
The LSCVAMC CoE Cancer Care Clinic began as a 3-year grant-funded project from the VA Offices of Specialty Care and Academic Affiliations with 2 major objectives: (1) to deliver quality patient-centered cancer care as measured by implementation of a process for distress screening and management, and development and implementation of a survivorship care plan for patients who have completed cancer treatment; and (2) to provide interprofessional education for the interdisciplinary health care professionals who participate in the clinic as part of their training experience.
Patients in this unique CoE cancer clinic have sameday access to all members of the interdisciplinary and interprofessional team. The ambulatory cancer care CoE team was originally composed of a surgical oncologist, a medical oncologist, a clinical nurse specialist (CNS) patient navigator, a nurse practitioner (NP) in survivorship care, a registered nurse (RN), a psychologist, and an oncology social worker. The project’s patient population included patients with a cancer concern (positive family history and suspicious scans) or a diagnosis of breast cancer, melanoma, sarcoma, or hematologic malignancies. The patient population for the project was based on the CoE team expertise and feasibility of implementation, with plans to roll out the model of care for all patients with any cancer diagnosis across the VAMC at the completion of the project.
The CoE made distress screening and management the leading priority for quality patient-centered care at the start of the project. The purpose of this emphasis on distress screening was to develop a process at LSCVAMC that would meet the 2015 CoC standards and to teach health care professional trainees (NP students, residents, social work students, and fellows in psychology and medical oncology) about distress screening and intervention.
A plan-do-act model of quality improvement (QI) was used to support the development and implementation of the distress-screening process. At the beginning of the project, the institutional review board (IRB) reviewed the protocol and determined that informed consent was not necessary because a QI project for a new standard of care did not require IRB approval. The CoE team met for about 4 months to develop a policy and procedure for the process, based on evidence from national guidelines, a review of the literature, and a discussion of the benefits and burdens of implementation within the current practice.
Limiting initial implementation to a single clinic day made the process more manageable. Descriptive methods analyzed the incidence and percentage of overall distress in this veteran population and quantified the incidences and percentages of each DT component. Feedback from patients and staff offered information on the feasibility of and satisfaction with the process.
From May 2012 to May 2014, all patients who attended the Monday outpatient CoE clinic with a diagnosis of cancer or a cancer concern were given the NCCN, 2.2013 DT instrument by the registration desk clerk at the time they registered for their clinic appointment. 16 Veterans who had difficulty filling out the DT or who had diminished capacity were assisted in completing the instrument by a designated family member and/or the clinic RN.
The completed instrument was evaluated by the CNS patient navigator, and any patient with a score ≥ 4 received an automatic referral to the behavioral health psychologist, social worker, NP, or all team members and their trainees, depending on the areas of distress (eg, practical, family, and emotional problems, spiritual/religious concerns, and/or physical problems) endorsed by the patient.
A psychiatrist was not embedded into the team but worked closely with the team’s oncology psychologist. The psychologist communicated directly with the psychiatrist, and the plan was shared with the team through the electronic medical record (EMR). The appropriate team member(s) and trainee(s) saw the patient at the visit to address needs in real time. Access to palliative care support and spiritual care was readily available if needed.
Distress screenings were recorded in a templated note in the patient’s EMR, which allowed the team to follow the distress scores on an individual basis across the cancer disease trajectory and to assess response to interventions. Multiple screenings of individuals resulted from the fact that many of the patients were seen monthly or every 3/6/9 months, depending on their disease and treatment status. Because levels of distress can fluctuate, distress was assessed at every visit to determine whether an intervention was needed at that visit. Once distress screenings were recorded in the patient’s EMR, the DT instrument was de-identified and given to the CoE research consultant to enter into a database file for analysis.
Trainees were educated about the use of the DT at time of diagnosis and across the disease trajectory. The 4-week CoE curriculum included 2 weeks of conference time to teach about the roles of psychologist, oncology social worker, and survivorship NP in assessing and initiating interventions to address the multidimensional components of the DT. Trainees working with a veteran who was distressed participated in the assessment(s) and intervention(s) for all components of distress that were endorsed.
Results
A total of 866 distress screenings were performed during the first 2 years of the project. Since all patients were screened at all visits, the 866 distress screenings reflect multiple screenings for 445 unique patients. Of the 866 screenings, 290 (33%) had distress scores of ≥ 4, meeting the criteria for intervention. Screenings reflected patient visits at any point in the disease trajectory. Because this was a new standard of care QI project rather than a research project, additional data, such as diagnosis or staging, were not collected, and IRB approval was not needed.
Because the NCCN Guideline recommendation for intervention is a score of ≥ 4, the descriptive statistics focused on those with moderate-to-severe distress. However, there were numerous occasions when the veteran would report a score of 0 to 3 and still endorse a number of the problems on the DT. The CNS and RN on the team discussed these findings with the appropriate discipline. For example, if the veteran reported a score of 1 but endorsed all 6 components on the emotional problem list, the nurses discussed the patient with the social worker or psychologist to determine whether behavioral health intervention was needed.
The mean distress score for the 290 screenings ≥ 4 was 6.3 on a 0 to 10 scale; median was 6.0 and mode 5.0. Two hundred ten of these screenings (72%) were categorized as moderate distress (4-7), and 80 patients (28%) reported severe distress (Figure 2). If the veteran left a box empty on the problem list, itwas recorded as missing. The frequency that patients reported each type of distress are reported in Figure 3.
The incidence of each component of distress, from those screenings with a score of ≥ 4 is described below, along with case study examples for each component. Team members involved in patient interventions provided these case studies to demonstrate clinical examples of the veteran’s distress from the problem list on the DT.
Practical/Family Distress
Practical issues were reported in 38% of the screenings (109/290). Intervention for moderate-to-severe distress associated with practical problems and family issues was provided by the team social worker. The social worker frequently addressed transportationrelated distress. Providing transportation was essential for adherence to clinic appointments, follow-up testing, treatments, and ultimately, disease management. Housing was also a problem for many veterans. It is critical that patients have access to electricity, heating, food, and water to be able to safely undergo adjuvant therapy. Thus, treatments decisions could be impacted by the veterans’ housing and transportation issues; immediate access to social work support is essential for quality cancer care.
Twenty-six percent of patients that were screened expressed concerns with practical and/or family problems (75/289). Issues of domestic violence, difficulties dealing with a significant other, and concerns about children were referred to social work (Table 1).
Case Study
Ms. S. is a veteran aged 71 years with recently diagnosed breast cancer. She is being seen in the clinic for a postoperative visit following partial mastectomy and is anticipating beginning radiation therapy within the next 3 weeks. She reports a distress score of 7 and identifies concerns about work and transportation to the clinic as the sources of distress. The social worker meets with the patient and learns that she fears losing her job because of the daily travel time to and from radiation and that she cannot afford to travel 65 miles daily to LSCVAMC for radiation. The social worker listens to her concerns and assists her with a plan for short-term disability and VA housing during her radiation therapy treatments. Ms. S. was able to complete radiation at LSCVAMC with temporary housing and to return to work after therapy.
Emotional Distress
Patients who identified that their moderate-to-severe distress was related to emotional problems received same-day intervention from a psychologist skilled in providing emotional support, cognitive behavioral strategies, and assessing the need for referral to either a psychiatrist or oncology social worker. Seventy-one percent of patients reported emotional problems, such as worry, depression, and nervousness (Table 2).
Case Study
Mr. K. is a veteran aged 71 years with a new diagnosis of breast cancer. He lives on his own but has family and a few friends nearby. He reports that he doesn’t like to share his problems with others and has not told anyone of his new diagnosis. Mr. K. rates his distress a 7 and endorses worry, fear, and depression. At a treatment-planning visit, he agrees to see the psychologist for help in dealing with his distress. Treatment involves a mastectomy followed by hormonal therapy.
Mr. K. was scared about having cancer; some of his veteran colleagues have developed cancer recently, and 2 have died. He told the psychologist that he feels worthless and that this disease just makes him more of a burden on society. He has had thoughts of taking his life so that he doesn’t have to deal with cancer, but he does not have a plan. The team formulated a plan to address his anxiety and depression. Mr. K. started a serotonin reuptake inhibitor, and he met with the VA psychiatrist weekly to help develop coping strategies. The team’s psychologist worked closely with Mr. K.’s psychiatrist, and he successfully completed surgery and chemotherapy. He is now being seen in survivorship clinic, continuing care with the team and his psychologist.
Spiritual Distress
Although spiritual/religious concerns are part of DT screening, it is only a single item on the DT. Just 8% of patients (21/276) reported moderate-to-severe spiritual distress. However, there was access to a chaplain at LSCVAMC.
Case Study
Mr. H., a 63-year-old veteran with stage IV melanoma, was seen in the clinic for severe pain in his left hip and ribs (8 on a 10-point scale); he was unresponsive to escalating doses of oxycodone. During the visit, he reported that his distress level is a 10, and in addition to identifying pain as a source of distress, he indicated that he has spiritual distress. When questioned further about spiritual distress, Mr. H. reported that he deserves this pain since he caused so many others pain during his time in Vietnam. The chaplain was contacted, and the patient was seen in clinic at this visit. The chaplain gave him the opportunity to share his feelings of guilt. The importance of spiritual care when the patient is experiencing “total pain” is essential to pain management. Within 3 days, his pain score decreased to an acceptable level of 3 with no additional pharmacologic intervention.
Physical Distress
Physical problems associated with the distress scores were addressed by the surgical and medical oncologists and the APRNs (CNS patient navigator and survivorship NP). When the clinic opened, the team used the Memorial Symptom Assessment Scale to assess physical and psychological symptoms.16 However, patients reported experiencing distress at having to complete 2 tools that had a great deal of overlap. The team determined that the DT could be used as the sole screening tool for all QOL domains.
It is important to note that 92% of patients with moderate-to-severe distress reported physical symptoms as a source of distress (Table 3).
Case Study
Ms. L. is a Vietnam War veteran aged 64 years who was seen in the survivorship clinic. She was diagnosed with estrogen-receptor (ER) and progesterone-receptor (PR) breast cancer 1 year previously, had a lumpectomy followed by radiation therapy, and was on hormonal therapy. She recorded her distress score as a 6 and indicated that multiple physical symptoms were her major concern. She had difficulty with insomnia, fatigue, and hot flashes. The survivorship NP talked with Ms. L. about her symptoms and made nonpharmacologic recommendations for improving sleep, provided an exercise plan for fatigue, and initiated venlafaxine to manage the hot flashes. Ms. L. continued to be seen by the team in survivorship clinic, and during her 3-month follow-up visit, she reported improvement in sleep as the hot flashes diminished.
Multifactoral Distress
Many patients endorsed ≥ 1 component of distress. This required a team approach to intervene for the multifactorial nature of the distress.
Case Study
Mr. K. is a veteran aged 82 years who had been a farmer most of his life. He was cared for at the VA for an advancedstage squamous cell skin cancer of his scalp, which he had allowed to go untreated. The cancer has completely eroded beneath his scalp, and he wore a hat to cover the foul-smelling wound. He lived in rural Ohio with his wife of 55 years; 3 adult daughters lived in the Cleveland area. His daughters served as primary caregivers when Mr. K. came to Cleveland for daily radiation and weekly chemotherapy treatments. He had not been away from his wife since the war and misses her terribly, returning home only on weekends during the 6-week course of radiation.
His primary goal was to return home in time to harvest his farm’s produce 2 months later. He was aware that he has < 6 months to live but wanted chemotherapy and radiation to control the growth of the cancer. During this visit to the ambulatory clinic, he reported a distress score of 5 andidentified family concerns (eg, living away from his wife most of every week) and endorsed emotional concerns of fear, worry, and sadness, and reported pain, fatigue, insomnia, and constipation as physical concerns.
Mr. K. received support from the social worker, the psychologist, and the APRN for symptom management during this visit. The social worker was able to advocate for limited palliative radiation therapy treatments rather than a 6-week course; the psychologist spent 45 minutes talking with him about his fears of a painful death, worries about his wife, and sadness at not being alive for another planting season. The APRN recommended both pharmacologic and nonpharmacologic interventions for his fatigue and insomnia and initiated a pain and bowel pharmacologic regimen. The team respected Mr. K.’s wish to reconsider hospice care at the following visit after he had talked with his wife. Mr. K. died peacefully in his home with his wife and family just before the start of planting season.
Clinical Implications
Distress screening and intervention is essential for quality cancer care. While a great deal of controversy exists about the best time to screen for distress, the LSCVAMC CoE has taken on the challenge of screening and intervening in real time at every patient visit across the disease trajectory. The model of distress screening all veterans at CoE clinic visits has been rolled out to other cancer clinics at LSCVAMC.
Distress screening at each visit is not time intensive. Patients are willing to fill out the instrument while waiting for their clinic visit, and most patients find that it takes less than 5 minutes to complete. The major challenge for institutions considering screening with each visit is not the screening but access to appropriate providers able to provide timely intervention. The success of this model results, in part, because the clinic RN assesses the responses to the DT and refers to the appropriate discipline, utilizing precious resources of social work and psychology appropriately. The VA system is already committed to improving the psychosocial well-being of veterans and has established social work and psychology resources specifically for the cancer clinics.
Many patients reported to the authors that they might not have been able or willing to return to LSCVAMC to see the behavioral health specialists on another day. In addition, scheduling behavioral health appointments at another time would not allow for attending to the distress in real time. Also, from a systems standpoint, it would have been an added cost to the VA and/or the veteran for transportation for additional appointments on different days.
Finally, although the impact of the CoE project on health professional trainees has been reported elsewhere, the distress screening and intervention process were valued as being very positive for all trainees who participated in CoE clinic.17 The trainees were able to stay with the patient for the entire clinic visit, including the visits made by disciplines other than their own. For example, the family medicine residents stayed with the patient they examined to observe the distress assessments and interventions offered by the social worker and/or psychologist for the patients who scored ≥ 4 on the DT.
At the end of their rotations in the CoE, trainees reported an increased awareness of the importance of distress screening in a cancer clinic. Many were not aware of the NCCN guidelines and the ACoS CoC mandate for distress screening as a standard of cancer care. Interdisciplinary trainees rated the CoE curriculum and the conference teaching/learning sessions on distress management highly. However, observing the role of the social worker and psychologist were the most valuable to trainees, regardless of the area of practice they enter.
Conclusion
Addressing practical, psychosocial, physical, and spiritual needs will help decrease distress, support patients’ ability to tolerate treatment, and improve veterans’ QOL across the cancer-disease trajectory. Screening all patients at an outpatient cancer clinic at LSCVAMC is feasible and does not seem to be a burden for patients or providers. This pilot project has become standard of care across the LSCVAMC cancer clinics, demonstrating its sustainability.
Screening with the DT provides information about the intensity of the distress and the components contributing to the distress. The most important aspect of the screening is assessing the components of the distress and providing real-time intervention from the appropriate discipline. It is critical that the oncology team refer to the appropriate discipline based on the source of the distress rather than on only the intensity. Findings from this project indicate that physical symptoms are frequently the source of distress and may not require behavioral health intervention. However, for patients with psychosocial needs, rapid access to behavioral health care services is critical for quality veteran-centered cancer care.
Since 2015, all VA cancer centers are required to have implemented distress screening. According to the CoC, at least 1 screening must be done on every patient.10 Many institutions have begun to screen at diagnosis, but it is well known that there are many points along the cancer trajectory when patients may experience an increase in distress. Simple screening with the DT at every cancer clinic visit helps identify the veterans’ needs at any point along the disease spectrum.
At LSCVAMC, the CoE was designed as an interdisciplinary cancer clinic. With the conclusion of funding in FY 2015, the clinic has continued to function. The rollout into other clinics has continued with movement toward use of formal consult requests and continual, real-time evaluation of the process. Work on accurate, timely identification of new cancer patients and identifying pivotal cancer visits is underway. The LSCVAMC is committed to improving care and access to its veterans with cancer to ensure appropriate and adequate services across the cancer trajectory.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Holand JC, Jacobsen PB, Anderson A, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Distress Management 2.2016. © 2014 National Comprehensive Cancer Network, Inc. https://www.nccn.org/professionals/physician_gls/pdf/distress.pdf. Updated July 25, 2016. Accessed January 13, 2017.
2. Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol. 2012;30(11):1160-1177.
3. Hamer M, Chida Y, Molloy G. Psychological distress and cancer mortality. J Psychosom Res. 2009;66(3):255-258.
4. Mitchell AJ. Short screening tools for cancer-related distress: a review and diagnostic validity meta-analysis. J Natl Compr Canc Netw. 2013;8(4):487-494.
5. Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19-28.
6. Zabora JR, Macmurray L. The history of psychosocial screening among cancer patients. J Psychosoc Oncol. 2012;30(6):625-635.
7. Pirl WF, Fann JR, Greer JA, et al. Recommendations for the implementation of distress screening programs in cancer centers: report from the American Psychosocial Oncology Society (APOS), Association of Oncology Social Work (AOSW), and Oncology Nursing Society (ONS) joint task force. Cancer. 2014;120(91):2946-2954.
8. Parry C, Padgett LS, Zebrack B. Now what? Toward an integrated research and practice agenda in distress screening. J Psychosoc Oncol. 2012;30(6):715-727.
9. Wagner LI, Spiegel D, Pearman T. Using the science of psychosocial care to implement the new American College of Surgeons Commission on Cancer distress screening standard. J Natl Compr Canc Netw. 2013;11(2):214-221.
10. American College of Surgeons Commision on Cancer. https://www.facs.org/quality-programs/cancer/coc Published 1996. Updated January 19, 2017. Accessed April 16, 2016.
11. Rohan EA. Removing the stress from selecting instruments: arming social workers to take leadership in routine distress screening implementation. J Psychosoc Oncol. 2012;30(6):667-678.
12. Merport A, Bober SL, Grose A, Recklitis CJ. Can the distress thermometer (DT) identify significant psychological distress in long-term cancer survivors? A comparison with the Brief Symptom Inventory-18 (BSI-18). Support Care Cancer. 2012;20(1):195-198.
13. Carlson LE, Bultz BD. Cancer distress screening: Needs, models, and methods. J Psychosom Res. 2003;55(5):403-409.
14. Holland JC, Alici Y. Management of distress in cancer patients. J Support Oncol. 2010:8(1):4-12.
15. Jacobsen P, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103(7):1494-1502.
16. Portenoy R, Thaler HT, Korblith AB, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A(9):1326-1336.
17. Arfons L, Mazanec P, Smith J, et al. Training health care professionals in interprofessional collaborative cancer care. Health Interprof Pract. 2015;2(3):eP1073.
1. Holand JC, Jacobsen PB, Anderson A, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Distress Management 2.2016. © 2014 National Comprehensive Cancer Network, Inc. https://www.nccn.org/professionals/physician_gls/pdf/distress.pdf. Updated July 25, 2016. Accessed January 13, 2017.
2. Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol. 2012;30(11):1160-1177.
3. Hamer M, Chida Y, Molloy G. Psychological distress and cancer mortality. J Psychosom Res. 2009;66(3):255-258.
4. Mitchell AJ. Short screening tools for cancer-related distress: a review and diagnostic validity meta-analysis. J Natl Compr Canc Netw. 2013;8(4):487-494.
5. Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19-28.
6. Zabora JR, Macmurray L. The history of psychosocial screening among cancer patients. J Psychosoc Oncol. 2012;30(6):625-635.
7. Pirl WF, Fann JR, Greer JA, et al. Recommendations for the implementation of distress screening programs in cancer centers: report from the American Psychosocial Oncology Society (APOS), Association of Oncology Social Work (AOSW), and Oncology Nursing Society (ONS) joint task force. Cancer. 2014;120(91):2946-2954.
8. Parry C, Padgett LS, Zebrack B. Now what? Toward an integrated research and practice agenda in distress screening. J Psychosoc Oncol. 2012;30(6):715-727.
9. Wagner LI, Spiegel D, Pearman T. Using the science of psychosocial care to implement the new American College of Surgeons Commission on Cancer distress screening standard. J Natl Compr Canc Netw. 2013;11(2):214-221.
10. American College of Surgeons Commision on Cancer. https://www.facs.org/quality-programs/cancer/coc Published 1996. Updated January 19, 2017. Accessed April 16, 2016.
11. Rohan EA. Removing the stress from selecting instruments: arming social workers to take leadership in routine distress screening implementation. J Psychosoc Oncol. 2012;30(6):667-678.
12. Merport A, Bober SL, Grose A, Recklitis CJ. Can the distress thermometer (DT) identify significant psychological distress in long-term cancer survivors? A comparison with the Brief Symptom Inventory-18 (BSI-18). Support Care Cancer. 2012;20(1):195-198.
13. Carlson LE, Bultz BD. Cancer distress screening: Needs, models, and methods. J Psychosom Res. 2003;55(5):403-409.
14. Holland JC, Alici Y. Management of distress in cancer patients. J Support Oncol. 2010:8(1):4-12.
15. Jacobsen P, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103(7):1494-1502.
16. Portenoy R, Thaler HT, Korblith AB, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A(9):1326-1336.
17. Arfons L, Mazanec P, Smith J, et al. Training health care professionals in interprofessional collaborative cancer care. Health Interprof Pract. 2015;2(3):eP1073.
Development and Implementation of a Veterans’ Cancer Survivorship Program
The aging of the U.S. population has led to an increase in the number of patients diagnosed with cancer each year. Fortunately, advances in screening, detection, and treatments have contributed to an improvement in cancer survival rates during the past few decades. More than 1.6 million new cases of cancer are expected to be diagnosed in 2014. It is estimated that there are currently 14 million cancer survivors, and the number of survivors by 2022 is expected to be 18 million.1,2
The growing number of cancer survivors is exceeding the ability of the cancer care system to meet the demand.3 Many primary care providers (PCPs) lack the confidence to provide cancer surveillance for survivors, but at the same time, patients and physicians continue to expect that PCPs will play a substantial role in general preventive health and in treating other medical problems.4 These conditions make it critical that at a minimum, survivorship care is integrated between oncology and primary care teams through a systematic, coordinated plan.5 This integration is especially important for the vulnerable population of veterans who are cancer survivors, as they have additional survivorship needs.
The purpose of this article is to assist other VA health care providers in establishing a cancer survivorship program to address the unique needs of veterans not only during active treatment, but after their initial treatment is completed. Described are the unique needs of veterans who are cancer survivors and the development and implementation of a cancer survivorship program at a large metropolitan VAMC, which is grounded in VA and national guidelines and evidence-based cancer care. Lessons learned and recommendations for other VA programs seeking to improve coordination of care for veteran cancer survivors are presented.
Cancer Survivorship
The Institute of Medicine (IOM) report, From Cancer Patient to Cancer Survivor: Lost in Transition, identified the importance of providing quality survivorship care to those “living with, through, and beyond a diagnosis of cancer.”6,7 The period of survivorship extends from the time of diagnosis, through treatment, long-term survival, and end-of-life.8,9 Although there are several definitions of cancer survivor, the most widely accepted definition is one who has been diagnosed with cancer, regardless of their position on the disease trajectory.8
The complex needs of cancer survivors encompass physical, psychological, social, and spiritual concerns across the disease trajectory.3 Cancer survivors who are also veterans have additional needs and risk factors related to their service that can make survivorship care more challenging.10 Veterans tend to be older compared with the age of the general population, have more comorbid conditions, and many have combat-related posttraumatic stress disorder (PTSD), all of which can complicate the survivorship experience.11
The first challenge for veteran cancer survivors is in the term cancer survivor, which may take on a different meaning for a veteran when compared with a civilian. For some civilians and veterans, survivor is a constant reminder of having had cancer. There are some veterans who prefer not to be called survivors, because they do not feel worthy of this terminology. They believe they have not struggled enough to self-identify as a survivor and that survivorship is “something to be earned, following a physically grueling experience.”12
The meaning of the word survivor may even be culturally linked to the population of veterans who have survived a life-threatening combat experience. More research is needed to understand the veteran cancer survivorship experience. The meaning of survivorship must be explored with each veteran, as it may influence his or her adherence to a survivorship plan of care.
Veterans make up a unique subset of cancer survivors, in part because of risk factors associated with their service. Many veterans developed cancer as a result of their military exposure to toxic chemicals and radiation. To date, VA recognizes that chronic B-cell leukemias, Hodgkin disease, multiple myeloma, non-Hodgkin lymphomas, prostate cancer, respiratory cancers, and soft tissue sarcomas are all presumptive diseases related to Agent Orange exposure.13 There are other substances also presumed to increase the risk of certain cancers in veterans who have had ionizing radiation exposure.14 There is still much to learn regarding veterans who served during the Gulf War, Operation Enduring Freedom, and Operation Iraqi Freedom.15,16
In a comparison of VA data files with U.S. SEER data files from 2007, researchers identified differences in characteristics between veteran cancer survivors and civilian cancer survivors.17 In addition to increased exposure risks, the veteran cancer survivor population is older than the general cancer survivorship population and is mostly male.17 Veterans’ comorbid conditions, such as type 2 diabetes, ischemic heart disease, Parkinson disease, and peripheral neuropathy, which may be service related, complicate survivorship.17 These characteristics (age, gender, exposure risks, and comorbid conditions) influence the type of cancer diagnosed and treatment options, and they may ultimately impact survivorship needs
(Table 1).
The prevalence of mental health issues in the veteran population is significant.18 Posttraumatic stress disorder affects 7% to 8% of the general population at some point during their lifetime and as many as 16% of those returning from military deployment.19 In a predominantly
male veteran study correlating combat PTSD with cancerrelated PTSD, about half the participants (n = 170) met PTSD Criterion A, viewing their cancer as a traumatic experience.20 Posttraumatic stress disorder, depression, anxiety, and addictive disease all must be addressed in the survivorship plan of care.
Poor mental health has been linked to increased morbidity and mortality and can limit the veteran’s ability to participate in health promotion and medical care.21 Distress related to cancer is well recognized in the civilian population.22,23 Veterans are at risk for moderate-tosevere disabling distress, especially when the cancer is associated with their military service. Vietnam veterans who have a diagnosis of cancer report that they have already served their time and are now serving it again, having to wage a battle on cancer and undergo difficult treatments and associated adverse effects (AEs).24 It is important to note, however, that some veterans have developed strong coping skills, which gives them strength and resilience for the survivorship experience.25
Other factors also contribute to veterans’ unique survivorship needs. Many veterans have limited social and/or economic resources, making it difficult to receive cancer treatment and follow recommendations for a healthful lifestyle as a cancer survivor. Demographics from the VA have illustrated that many veterans have a limited support system (65% do not have a spouse), and many have low incomes.26 Although veterans comprise about 11% of the general population, they make up 26% of the homeless population.26 It is estimated that 260,000 veterans are homeless at some time during the course of a year, and of these, 45% have mental health issues and 70% have substance abuse problems.27 Basic needs such as housing, running water, heat and electricity, and nutrition must be met in order to prevent infection during treatment, maximize the benefit, and reduce the risks associated with treatment. Transportation issues can make it challenging to travel to medical centers for cancer surveillance following treatment.
Models of Care
As defined in the aforementioned IOM report, multiple models of survivorship care have surfaced over the years.6 Much that was originally seen and implemented in adult cancer survivorship was known from pediatric cancer care. Early models that surfaced included shared care models, nurse-led models, and tertiary survivorship clinics. Each model has its strengths and disadvantages.
The shared care model of survivorship involves a sharing of the responsibility for the survivor among different specialties, potentially at different facilities, and the primary care team. Typically, the PCP refers the patient to the oncologist when cancer is suspected or diagnosed. The primary care team continues to provide routine health maintenance and manages other health problems while the oncology team provides cancer care. The patient is transitioned back to the primary care team with a survivorship care plan (SCP) at 1 to 2 years after completion of cancer therapy or at the discretion of the oncology team.28 For
this model to work, the PCP must be willing to take on this responsibility, and there must be a coordinated effort for seamless communication between teams, which can be potentially challenging.
Nurse-led programs emerged in the pediatric populations. Pediatric nurse-led clinics assume care of the patient after active treatment to manage long-term AEs of cancer treatments, symptom management, care planning, and education. A comprehensive review of the literature identified that “nurse-led follow-up services are acceptable, appropriate, and effective.”6 Barriers to this model of care include a shortage of trained oncology nurses and a preference for physician follow-up by some cancer survivors who want the security of their oncologist for ongoing, long-term care.6
Survivorship follow-up clinics, a tertiary model of care, have been implemented at some larger academic centers. These clinics focus on cancer survivorship and are often separate from other routine health care visits. Typically, these clinics include multiple specialties and are often disease-specific. These types of clinics pose a different set of challenges regarding duplication of services and reimbursement issues.
As of yet, no model has been proven more effective than the others. Each institution and patient population may not lend themselves to a one-size-fits-all model. There may be different models of care needed, based on patient population. Regardless of the model selected, individualized survivorship care plans are an essential component of quality cancer survivorship care.
Addessing Survivorship Care
In 2009, 5 interdisciplinary leaders in VA cancer care (Ellen Ballard, RN; David Haggstrom, MD, MAS; Veronica Reis, PhD; Mark Detzer, PhD; and Tina Gill, MA) attended a breakout session on psychosocial oncology at the Association of VA Hematology and Oncology (AVAHO) meeting in Minneapolis, Minnesota, and most members of this team participated in the 2009-2012 VHA Cancer Care Collaboratives to improve the timeliness and quality of care for veterans who were cancer patients. Dr. Haggstrom and Ms. Ballard developed a SharePoint site for the Survivorship Special Interest Group (SIG) members through the Loma Linda VAMC in California. The SIG workgroup then built the Cancer Survivorship Toolkit, composed of
5 critical tools (Figure).
In July 2012, the VA Cancer Survivorship Toolkit content was disseminated at AVAHO and launched behind the VA firewall. It subsequently received accolades from the national program director for VHA Oncology and was listed on the American College of Surgeons Commission on Cancer (CoC) Best Practices website. The toolkit is accessible to all VA programs, and suggestions for new content can be submitted directly on the site (Figure).
The development of a SCP began in late 2011 when SIG members collected examples of SCPs from leading organizations. The members compared this content with the IOM recommendations for SCPs and developed a template. The template was programmed for the VHA computerized patient record system (CPRS) and placed on the internal VA toolkit website. The template included the treatment summary and care plan. The treatment summary portion included the diagnosis and tumor characteristics, diagnostic tests used, dates and types of treatment, chemoprevention or maintenance treatments, supportive services required, the surveillance plan, and signs of recurrence. The care plan portion provided information on the likely course of recovery and a checklist for common long-term AEs in the areas of psychological distress, financial and practical effects, and physical effects. Also included was information about referral, health behaviors, late effects that may develop, contact information, and general resource information.
The computer applications coordinator at any VA can download the template from the toolkit onto their CPRS, and the template can then be brought into any progress note. Individual sites may also edit the template to suit specific needs. The SCP can be completed by any clinician with the appropriate clinical competencies. To date, > 50 sites have downloaded the SCP template for use.
Cancer Survivorship Clinic
At the Louis Stokes Cleveland (LSC) VAMC, a nurse-led model of a cancer survivorship clinic was established with an expert nurse practitioner (NP). A major catalyst for the development of this clinic was the receipt of a Specialty Care Education Center of Excellence, funded by the Offices of Specialty Care and Academic Affiliations. A priority of this project was the implementation of survivorship care for every veteran with a cancer diagnosis. A system redesign was implemented to deliver quality, cost-effective, patient-centered cancer care within an interprofessional, team-based practice. This clinic is imbedded within an interdisciplinary clinic setting where the NP works in close collaboration with the medical and surgical oncologists as well as providers from mental health, social work, nutrition, physical therapy, and others.
The first patients to receive survivorship care in this new model from the time of their diagnosis were veterans with breast cancer, sarcoma, melanoma, and lymphomas. Veterans are followed jointly by the NP and the medical and surgical oncologists during active treatment. The NP provides physical symptom assessment and management for patients both during and after treatment.
At the end of active treatment, patient visits are alternated between oncology physicians and the survivorship NP for 5 years. The timeline for follow-up visits is based on National Comprehensive Cancer Network guidelines for each cancer type but then individualized based on patient need.29 During this 5-year time period, patients under active surveillance whose conditions have been stable are seen by the NP. Any concerning symptoms are immediately relayed to the primary oncologist or surgical oncologist, often the same day, and patients can be seen the same day if necessary, to improve coordination and access to services.
A unique focus of the clinic is the integration of health promotion and risk reduction that coincides with the active surveillance plan. This transition of active surveillance patients to the NP-led survivorship clinic not only opens access to newly diagnosed cancer patients to be seen by the oncologist, but also allows for seamless transition and coordination to active surveillance. Within the clinic structure, patients receive patient navigation beginning with a cancer concern; patients also receive screening for psychosocial distress at the time of diagnosis and at every visit. Patient navigation and distress screening are both considered essential elements to survivorship care in the most recent CoC guidelines.30 The survivorship NP keeps the primary care team up-to-date regarding patient care across the disease trajectory by alerting them to updates electronically in the CPRS in real time.
Survivorship Care Plan
A focus of the clinic has also been on the implementation of a formal SCP to be completed 3 months after the conclusion of active treatment. The formal SCP was downloaded from the Cancer Survivorship Toolkit and is composed of a 3-part summary. The 3 parts consist of the treatment summary, the plan for rehabilitation, and the plan for the future. The first section of the SCP is completed by the medical oncologist as a summary of treatment received by the veteran. The summary of treatment section is reviewed and discussed with the veteran survivor at the visit, and the second and third sections are completed during the 3-month follow-up visit with the veteran.
Success and Areas for Improvement
The survivorship clinic has been well received by veterans. Patient satisfaction scores have been overwhelmingly positive. Veterans appreciate and feel comfortable knowing their providers from the beginning of diagnosis along the entire disease trajectory. They know that if problems arise, the survivorship NP has direct access to the medical or surgical oncologist for immediate review.
The difficult challenge for the cancer care providers is to know when is the right time to transition care back to the PCP. Transitions of care often come with high anxiety and a sense of loss for the veteran. The 5-year survival mark is not always the appropriate transition time for some veterans. Those with extensive physical and mental health issues may need continuity of care and continued support from the oncology team.
The SCP has presented challenges in terms of when to complete and who should complete the form. There has also been concern over the length of the summary, how long it will take to complete the document, and which summary template to use. Areas for improvement with the template could potentially be to automate population of the chemotherapy and radiation summaries. Some software packages are available, but they are costly. Another issue with external software is getting it accepted by VHA and incorporated into the CPRS.
Recommendations
Many cancer programs are struggling to provide highquality survivorship care. The CoC, recognizing the challenges programs are having implementing survivorship care, has extended the accreditation requirement for full implementation from 2015 to 2019.31
The following recommendations should be considered for the successful implementation of a new survivorship program:
- Collect information from multiple resources to guide the establishment of the survivorship clinic;
- Become familiar with the IOM From Cancer Patient to Cancer Survivor: Lost in Transition;6
- Understand local issues and barriers specific to your care delivery system;
- Collaborate with key stakeholders from multiple specialties to gain momentum and buy-in;
- Hold regular meetings with stakeholders as well as leadership to identify and remove barriers to the clinic success;
- Join the VA Survivorship SIG to collaborate with other sites who have already started to pilot survivorship programs and discuss barriers to and successes of programs so as to not reinvent the wheel;
- Utilize the Cancer Survivorship Toolkit;
- Download the SCP;
- Establish a close partnership with your local cancer committee; and
- Collect and report data to show effectiveness and need.
All these strategies were vital to the success of the LSCVAMC survivorship program.
Summary
The VA is uniquely positioned to be a leader in highquality, comprehensive, and veteran-centered cancer survivorship care in the years ahead. The close relationship between specialty and primary care allows for smooth continuity of care and easy transitions between oncology and primary care. The comprehensive CPRS allows easy accessibility to information for the entire health care team. The Cancer Survivorship Toolkit provides a template of the survivorship care plan for the veteran and his or her health care providers.
The LSCVAMC is one of many VA institutions implementing quality care for cancer survivors and can serve as a role model for other VA programs initiating the survivorship care process (Table 2).
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. American Cancer Society. Cancer Facts & Figures 2014. Atlanta, GA: American Cancer Society; 2014.
2. Institute of Medicine. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Levit LA, Balough EP, Nass SJ, Ganz PA, eds. Washington, DC: The National Academies Press; 2013.
3. Stricker CT, O’Brien M. Implementing the commission on cancer standards for survivorship care plans. Clin J Oncol Nurs. 2014;(suppl 18):15-22.
4. Cowens-Alvarado R, Sharpe K, Pratt-Chapman M, et al. Advancing survivorship care through the National Cancer Survivorship Resource Center: Developing American Cancer Society guidelines for primary care providers. CA Cancer J Clin. 2013;63(3):147-150.
5. Cheung WY, Neville BA, Cameron DB, Cook EF, Earle CC. Comparisons of patient and physician expectations for cancer survivorship care. J Clin Oncol. 2009;27(15):2489-2495.
6. Institute of Medicine. From Cancer Patient to Cancer Survivor: Lost in Transition. Hewitt M, Greenfield S, Stovall E, eds. Washington, DC: The National Academies Press; 2006.
7. Clark EJ, Stovall EL, Leigh S, Siu AL, Austin DK, Rowland JH, eds. Imperatives for Quality Cancer Care: Access, Advocacy, Action, and Accountability. Silver Spring, MD: National Coalition for Cancer Survivorship; 1996.
8. National Cancer Institute. Survivorship. NCI Dictionary of Cancer Terms Website. http://www.cancer.gov/dictionary?CdrID=445089. Accessed December 3, 2014.
9. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220-241.
10. Moye J, Schuster JL, Latini DM, Naik AD. The future of cancer survivorship care for veterans. Fed Pract. 2010;27(3):36-43.
11. Naik AD, Martin LA, Karel M, et al. Cancer survivor rehabilitation and recovery: Protocol for the Veterans Cancer Rehabilitation Study (Vet-CaRes). BMC Health Serv Res. 2013;13:93.
12. Beehler GP, Rodriques AE, Kay MA, Kiviniemi MT, Steinbrenner L. Lasting impact: Understanding the psychosocial implications of cancer among military veterans. J Psychosoc Oncol. 2013:31(4):430-450.
13. U.S. Department of Veterans Affairs. Veterans’ diseases associated with Agent Orange. Public Health Website. http://www.publichealth.va.gov/exposures/agentorange/conditions/index.asp. Updated December 30, 2013. Accessed December3, 2014.
14. U.S. Department of Veterans Affairs. Radiation. Public Health Website. http://www.publichealth.va.gov/exposures/radiation/index.asp. Updated December 31, 2013. Accessed December 3, 2014.
15. Cohen BE, Gima K, Bertenthal D, Kim S, Marmar CR, Seal KH. Mental health diagnoses and utilization of VA non-mental health medical services among returning Iraq and Afghanistan veterans. J Gen Intern Med. 2010;25(1):18-24.
16. U.S. Department of Veterans Affairs. Gulf War veterans’ illnesses. Public Health Website. http://www.publichealth.va.gov/exposures/gulfwar/index.asp. Updated November 7, 2014. Accessed December 3, 2014.
17. National Cancer Institute. Cancer query systems. Surveillance, Epidemiology, and End Results Program Website. http://seer.cancer.gov/canques/index.html. Accessed December 3, 2014.
18. Suicide in the military: Army-NIH funded study points to risk and protective factors [news release]. Washington, DC: National Institute of Mental Health; March 3, 2014. http://www.nimh.nih.gov/news/science-news/2014/suicide-in-the-military-army-nih-funded-study-points-to-risk-and-protective-factors.shtml. Accessed December 3, 2014.
19. Gates MA, Holowka DW, Vasterling JJ, Keane TM, Marx BP, Rosen RC. Posttraumatic stress disorder in veterans and military personnel: Epidemiology, screening, and case recognition. Psychol Serv. 2012;9(4):361-382.
20. Mulligan EA, Schuster Wachen J, Naik AD, Gosian J, Moye J. Cancer as a criterion a traumatic stressor for veterans: Prevalence and correlates. Psychol Trauma. 2014;6(suppl 1):S73-S81.
21. Musuuza JS, Sherman ME, Knudsen KJ, Sweeney HA, Tyler CV, Koroukian SM. Analyzing excess mortality from cancer among individuals with mental illness. Cancer. 2013;119(13):2469-2476.
22. Zabora J, Macmurray L. The history of psychosocial screening among cancer patients. J Psychosoc Oncol. 2013:30(6):625-635.
23. Holland JC, Andersen B, Breitbart WS, et al. Distress management: Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2010:8(4):448-485.
24. Grassman DL. The Hero Within. St. Petersburg, FL: Vandamere Press; 2012.
25. Jahn AL, Herman L, Schuster J, Naik A, Moye J. Distress and resilience after cancer
in veterans. Res Hum Dev. 2012;9(3):229-247.
26. National Association of Social Workers. Social workers speak on veterans issues June 2009. National Association of Social Workers Website. http://www.naswdc.org/pressroom/2009/Social%20Work%20Veterans%20Fact%20Sheet.pdf. Accessed December 3, 2014.
27. Homeless. U.S. Department of Veterans Affairs Website. http://www.va.gov/homeless. Accessed December 3, 2014.
28. Oeffinger KC, McCabe MS. Models for delivering survivorship care. J Clin Oncol. 2006;24(32):5117-5124.
29. NCCN Guidelines. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site. Accessed December 3, 2014.
30. American College of Surgeons, Commission on Cancer. Cancer program standards 2012, version 1.2.1: Ensuring patient-centered care. https://www.facs.org/~/media/file/quality%20programs/cancer/coc/programstandards2012.ashx. Published January 21, 2014. Accessed December 3, 2014.
31. Accreditation committee clarifications for standard 3.3 survivorship care plan. American College of Surgeons Website. https://www.facs.org/publications/newsletters/coc-source/special-source/standard33. Published September 9, 2014. Accessed December 3, 2014.
The aging of the U.S. population has led to an increase in the number of patients diagnosed with cancer each year. Fortunately, advances in screening, detection, and treatments have contributed to an improvement in cancer survival rates during the past few decades. More than 1.6 million new cases of cancer are expected to be diagnosed in 2014. It is estimated that there are currently 14 million cancer survivors, and the number of survivors by 2022 is expected to be 18 million.1,2
The growing number of cancer survivors is exceeding the ability of the cancer care system to meet the demand.3 Many primary care providers (PCPs) lack the confidence to provide cancer surveillance for survivors, but at the same time, patients and physicians continue to expect that PCPs will play a substantial role in general preventive health and in treating other medical problems.4 These conditions make it critical that at a minimum, survivorship care is integrated between oncology and primary care teams through a systematic, coordinated plan.5 This integration is especially important for the vulnerable population of veterans who are cancer survivors, as they have additional survivorship needs.
The purpose of this article is to assist other VA health care providers in establishing a cancer survivorship program to address the unique needs of veterans not only during active treatment, but after their initial treatment is completed. Described are the unique needs of veterans who are cancer survivors and the development and implementation of a cancer survivorship program at a large metropolitan VAMC, which is grounded in VA and national guidelines and evidence-based cancer care. Lessons learned and recommendations for other VA programs seeking to improve coordination of care for veteran cancer survivors are presented.
Cancer Survivorship
The Institute of Medicine (IOM) report, From Cancer Patient to Cancer Survivor: Lost in Transition, identified the importance of providing quality survivorship care to those “living with, through, and beyond a diagnosis of cancer.”6,7 The period of survivorship extends from the time of diagnosis, through treatment, long-term survival, and end-of-life.8,9 Although there are several definitions of cancer survivor, the most widely accepted definition is one who has been diagnosed with cancer, regardless of their position on the disease trajectory.8
The complex needs of cancer survivors encompass physical, psychological, social, and spiritual concerns across the disease trajectory.3 Cancer survivors who are also veterans have additional needs and risk factors related to their service that can make survivorship care more challenging.10 Veterans tend to be older compared with the age of the general population, have more comorbid conditions, and many have combat-related posttraumatic stress disorder (PTSD), all of which can complicate the survivorship experience.11
The first challenge for veteran cancer survivors is in the term cancer survivor, which may take on a different meaning for a veteran when compared with a civilian. For some civilians and veterans, survivor is a constant reminder of having had cancer. There are some veterans who prefer not to be called survivors, because they do not feel worthy of this terminology. They believe they have not struggled enough to self-identify as a survivor and that survivorship is “something to be earned, following a physically grueling experience.”12
The meaning of the word survivor may even be culturally linked to the population of veterans who have survived a life-threatening combat experience. More research is needed to understand the veteran cancer survivorship experience. The meaning of survivorship must be explored with each veteran, as it may influence his or her adherence to a survivorship plan of care.
Veterans make up a unique subset of cancer survivors, in part because of risk factors associated with their service. Many veterans developed cancer as a result of their military exposure to toxic chemicals and radiation. To date, VA recognizes that chronic B-cell leukemias, Hodgkin disease, multiple myeloma, non-Hodgkin lymphomas, prostate cancer, respiratory cancers, and soft tissue sarcomas are all presumptive diseases related to Agent Orange exposure.13 There are other substances also presumed to increase the risk of certain cancers in veterans who have had ionizing radiation exposure.14 There is still much to learn regarding veterans who served during the Gulf War, Operation Enduring Freedom, and Operation Iraqi Freedom.15,16
In a comparison of VA data files with U.S. SEER data files from 2007, researchers identified differences in characteristics between veteran cancer survivors and civilian cancer survivors.17 In addition to increased exposure risks, the veteran cancer survivor population is older than the general cancer survivorship population and is mostly male.17 Veterans’ comorbid conditions, such as type 2 diabetes, ischemic heart disease, Parkinson disease, and peripheral neuropathy, which may be service related, complicate survivorship.17 These characteristics (age, gender, exposure risks, and comorbid conditions) influence the type of cancer diagnosed and treatment options, and they may ultimately impact survivorship needs
(Table 1).
The prevalence of mental health issues in the veteran population is significant.18 Posttraumatic stress disorder affects 7% to 8% of the general population at some point during their lifetime and as many as 16% of those returning from military deployment.19 In a predominantly
male veteran study correlating combat PTSD with cancerrelated PTSD, about half the participants (n = 170) met PTSD Criterion A, viewing their cancer as a traumatic experience.20 Posttraumatic stress disorder, depression, anxiety, and addictive disease all must be addressed in the survivorship plan of care.
Poor mental health has been linked to increased morbidity and mortality and can limit the veteran’s ability to participate in health promotion and medical care.21 Distress related to cancer is well recognized in the civilian population.22,23 Veterans are at risk for moderate-tosevere disabling distress, especially when the cancer is associated with their military service. Vietnam veterans who have a diagnosis of cancer report that they have already served their time and are now serving it again, having to wage a battle on cancer and undergo difficult treatments and associated adverse effects (AEs).24 It is important to note, however, that some veterans have developed strong coping skills, which gives them strength and resilience for the survivorship experience.25
Other factors also contribute to veterans’ unique survivorship needs. Many veterans have limited social and/or economic resources, making it difficult to receive cancer treatment and follow recommendations for a healthful lifestyle as a cancer survivor. Demographics from the VA have illustrated that many veterans have a limited support system (65% do not have a spouse), and many have low incomes.26 Although veterans comprise about 11% of the general population, they make up 26% of the homeless population.26 It is estimated that 260,000 veterans are homeless at some time during the course of a year, and of these, 45% have mental health issues and 70% have substance abuse problems.27 Basic needs such as housing, running water, heat and electricity, and nutrition must be met in order to prevent infection during treatment, maximize the benefit, and reduce the risks associated with treatment. Transportation issues can make it challenging to travel to medical centers for cancer surveillance following treatment.
Models of Care
As defined in the aforementioned IOM report, multiple models of survivorship care have surfaced over the years.6 Much that was originally seen and implemented in adult cancer survivorship was known from pediatric cancer care. Early models that surfaced included shared care models, nurse-led models, and tertiary survivorship clinics. Each model has its strengths and disadvantages.
The shared care model of survivorship involves a sharing of the responsibility for the survivor among different specialties, potentially at different facilities, and the primary care team. Typically, the PCP refers the patient to the oncologist when cancer is suspected or diagnosed. The primary care team continues to provide routine health maintenance and manages other health problems while the oncology team provides cancer care. The patient is transitioned back to the primary care team with a survivorship care plan (SCP) at 1 to 2 years after completion of cancer therapy or at the discretion of the oncology team.28 For
this model to work, the PCP must be willing to take on this responsibility, and there must be a coordinated effort for seamless communication between teams, which can be potentially challenging.
Nurse-led programs emerged in the pediatric populations. Pediatric nurse-led clinics assume care of the patient after active treatment to manage long-term AEs of cancer treatments, symptom management, care planning, and education. A comprehensive review of the literature identified that “nurse-led follow-up services are acceptable, appropriate, and effective.”6 Barriers to this model of care include a shortage of trained oncology nurses and a preference for physician follow-up by some cancer survivors who want the security of their oncologist for ongoing, long-term care.6
Survivorship follow-up clinics, a tertiary model of care, have been implemented at some larger academic centers. These clinics focus on cancer survivorship and are often separate from other routine health care visits. Typically, these clinics include multiple specialties and are often disease-specific. These types of clinics pose a different set of challenges regarding duplication of services and reimbursement issues.
As of yet, no model has been proven more effective than the others. Each institution and patient population may not lend themselves to a one-size-fits-all model. There may be different models of care needed, based on patient population. Regardless of the model selected, individualized survivorship care plans are an essential component of quality cancer survivorship care.
Addessing Survivorship Care
In 2009, 5 interdisciplinary leaders in VA cancer care (Ellen Ballard, RN; David Haggstrom, MD, MAS; Veronica Reis, PhD; Mark Detzer, PhD; and Tina Gill, MA) attended a breakout session on psychosocial oncology at the Association of VA Hematology and Oncology (AVAHO) meeting in Minneapolis, Minnesota, and most members of this team participated in the 2009-2012 VHA Cancer Care Collaboratives to improve the timeliness and quality of care for veterans who were cancer patients. Dr. Haggstrom and Ms. Ballard developed a SharePoint site for the Survivorship Special Interest Group (SIG) members through the Loma Linda VAMC in California. The SIG workgroup then built the Cancer Survivorship Toolkit, composed of
5 critical tools (Figure).
In July 2012, the VA Cancer Survivorship Toolkit content was disseminated at AVAHO and launched behind the VA firewall. It subsequently received accolades from the national program director for VHA Oncology and was listed on the American College of Surgeons Commission on Cancer (CoC) Best Practices website. The toolkit is accessible to all VA programs, and suggestions for new content can be submitted directly on the site (Figure).
The development of a SCP began in late 2011 when SIG members collected examples of SCPs from leading organizations. The members compared this content with the IOM recommendations for SCPs and developed a template. The template was programmed for the VHA computerized patient record system (CPRS) and placed on the internal VA toolkit website. The template included the treatment summary and care plan. The treatment summary portion included the diagnosis and tumor characteristics, diagnostic tests used, dates and types of treatment, chemoprevention or maintenance treatments, supportive services required, the surveillance plan, and signs of recurrence. The care plan portion provided information on the likely course of recovery and a checklist for common long-term AEs in the areas of psychological distress, financial and practical effects, and physical effects. Also included was information about referral, health behaviors, late effects that may develop, contact information, and general resource information.
The computer applications coordinator at any VA can download the template from the toolkit onto their CPRS, and the template can then be brought into any progress note. Individual sites may also edit the template to suit specific needs. The SCP can be completed by any clinician with the appropriate clinical competencies. To date, > 50 sites have downloaded the SCP template for use.
Cancer Survivorship Clinic
At the Louis Stokes Cleveland (LSC) VAMC, a nurse-led model of a cancer survivorship clinic was established with an expert nurse practitioner (NP). A major catalyst for the development of this clinic was the receipt of a Specialty Care Education Center of Excellence, funded by the Offices of Specialty Care and Academic Affiliations. A priority of this project was the implementation of survivorship care for every veteran with a cancer diagnosis. A system redesign was implemented to deliver quality, cost-effective, patient-centered cancer care within an interprofessional, team-based practice. This clinic is imbedded within an interdisciplinary clinic setting where the NP works in close collaboration with the medical and surgical oncologists as well as providers from mental health, social work, nutrition, physical therapy, and others.
The first patients to receive survivorship care in this new model from the time of their diagnosis were veterans with breast cancer, sarcoma, melanoma, and lymphomas. Veterans are followed jointly by the NP and the medical and surgical oncologists during active treatment. The NP provides physical symptom assessment and management for patients both during and after treatment.
At the end of active treatment, patient visits are alternated between oncology physicians and the survivorship NP for 5 years. The timeline for follow-up visits is based on National Comprehensive Cancer Network guidelines for each cancer type but then individualized based on patient need.29 During this 5-year time period, patients under active surveillance whose conditions have been stable are seen by the NP. Any concerning symptoms are immediately relayed to the primary oncologist or surgical oncologist, often the same day, and patients can be seen the same day if necessary, to improve coordination and access to services.
A unique focus of the clinic is the integration of health promotion and risk reduction that coincides with the active surveillance plan. This transition of active surveillance patients to the NP-led survivorship clinic not only opens access to newly diagnosed cancer patients to be seen by the oncologist, but also allows for seamless transition and coordination to active surveillance. Within the clinic structure, patients receive patient navigation beginning with a cancer concern; patients also receive screening for psychosocial distress at the time of diagnosis and at every visit. Patient navigation and distress screening are both considered essential elements to survivorship care in the most recent CoC guidelines.30 The survivorship NP keeps the primary care team up-to-date regarding patient care across the disease trajectory by alerting them to updates electronically in the CPRS in real time.
Survivorship Care Plan
A focus of the clinic has also been on the implementation of a formal SCP to be completed 3 months after the conclusion of active treatment. The formal SCP was downloaded from the Cancer Survivorship Toolkit and is composed of a 3-part summary. The 3 parts consist of the treatment summary, the plan for rehabilitation, and the plan for the future. The first section of the SCP is completed by the medical oncologist as a summary of treatment received by the veteran. The summary of treatment section is reviewed and discussed with the veteran survivor at the visit, and the second and third sections are completed during the 3-month follow-up visit with the veteran.
Success and Areas for Improvement
The survivorship clinic has been well received by veterans. Patient satisfaction scores have been overwhelmingly positive. Veterans appreciate and feel comfortable knowing their providers from the beginning of diagnosis along the entire disease trajectory. They know that if problems arise, the survivorship NP has direct access to the medical or surgical oncologist for immediate review.
The difficult challenge for the cancer care providers is to know when is the right time to transition care back to the PCP. Transitions of care often come with high anxiety and a sense of loss for the veteran. The 5-year survival mark is not always the appropriate transition time for some veterans. Those with extensive physical and mental health issues may need continuity of care and continued support from the oncology team.
The SCP has presented challenges in terms of when to complete and who should complete the form. There has also been concern over the length of the summary, how long it will take to complete the document, and which summary template to use. Areas for improvement with the template could potentially be to automate population of the chemotherapy and radiation summaries. Some software packages are available, but they are costly. Another issue with external software is getting it accepted by VHA and incorporated into the CPRS.
Recommendations
Many cancer programs are struggling to provide highquality survivorship care. The CoC, recognizing the challenges programs are having implementing survivorship care, has extended the accreditation requirement for full implementation from 2015 to 2019.31
The following recommendations should be considered for the successful implementation of a new survivorship program:
- Collect information from multiple resources to guide the establishment of the survivorship clinic;
- Become familiar with the IOM From Cancer Patient to Cancer Survivor: Lost in Transition;6
- Understand local issues and barriers specific to your care delivery system;
- Collaborate with key stakeholders from multiple specialties to gain momentum and buy-in;
- Hold regular meetings with stakeholders as well as leadership to identify and remove barriers to the clinic success;
- Join the VA Survivorship SIG to collaborate with other sites who have already started to pilot survivorship programs and discuss barriers to and successes of programs so as to not reinvent the wheel;
- Utilize the Cancer Survivorship Toolkit;
- Download the SCP;
- Establish a close partnership with your local cancer committee; and
- Collect and report data to show effectiveness and need.
All these strategies were vital to the success of the LSCVAMC survivorship program.
Summary
The VA is uniquely positioned to be a leader in highquality, comprehensive, and veteran-centered cancer survivorship care in the years ahead. The close relationship between specialty and primary care allows for smooth continuity of care and easy transitions between oncology and primary care. The comprehensive CPRS allows easy accessibility to information for the entire health care team. The Cancer Survivorship Toolkit provides a template of the survivorship care plan for the veteran and his or her health care providers.
The LSCVAMC is one of many VA institutions implementing quality care for cancer survivors and can serve as a role model for other VA programs initiating the survivorship care process (Table 2).
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The aging of the U.S. population has led to an increase in the number of patients diagnosed with cancer each year. Fortunately, advances in screening, detection, and treatments have contributed to an improvement in cancer survival rates during the past few decades. More than 1.6 million new cases of cancer are expected to be diagnosed in 2014. It is estimated that there are currently 14 million cancer survivors, and the number of survivors by 2022 is expected to be 18 million.1,2
The growing number of cancer survivors is exceeding the ability of the cancer care system to meet the demand.3 Many primary care providers (PCPs) lack the confidence to provide cancer surveillance for survivors, but at the same time, patients and physicians continue to expect that PCPs will play a substantial role in general preventive health and in treating other medical problems.4 These conditions make it critical that at a minimum, survivorship care is integrated between oncology and primary care teams through a systematic, coordinated plan.5 This integration is especially important for the vulnerable population of veterans who are cancer survivors, as they have additional survivorship needs.
The purpose of this article is to assist other VA health care providers in establishing a cancer survivorship program to address the unique needs of veterans not only during active treatment, but after their initial treatment is completed. Described are the unique needs of veterans who are cancer survivors and the development and implementation of a cancer survivorship program at a large metropolitan VAMC, which is grounded in VA and national guidelines and evidence-based cancer care. Lessons learned and recommendations for other VA programs seeking to improve coordination of care for veteran cancer survivors are presented.
Cancer Survivorship
The Institute of Medicine (IOM) report, From Cancer Patient to Cancer Survivor: Lost in Transition, identified the importance of providing quality survivorship care to those “living with, through, and beyond a diagnosis of cancer.”6,7 The period of survivorship extends from the time of diagnosis, through treatment, long-term survival, and end-of-life.8,9 Although there are several definitions of cancer survivor, the most widely accepted definition is one who has been diagnosed with cancer, regardless of their position on the disease trajectory.8
The complex needs of cancer survivors encompass physical, psychological, social, and spiritual concerns across the disease trajectory.3 Cancer survivors who are also veterans have additional needs and risk factors related to their service that can make survivorship care more challenging.10 Veterans tend to be older compared with the age of the general population, have more comorbid conditions, and many have combat-related posttraumatic stress disorder (PTSD), all of which can complicate the survivorship experience.11
The first challenge for veteran cancer survivors is in the term cancer survivor, which may take on a different meaning for a veteran when compared with a civilian. For some civilians and veterans, survivor is a constant reminder of having had cancer. There are some veterans who prefer not to be called survivors, because they do not feel worthy of this terminology. They believe they have not struggled enough to self-identify as a survivor and that survivorship is “something to be earned, following a physically grueling experience.”12
The meaning of the word survivor may even be culturally linked to the population of veterans who have survived a life-threatening combat experience. More research is needed to understand the veteran cancer survivorship experience. The meaning of survivorship must be explored with each veteran, as it may influence his or her adherence to a survivorship plan of care.
Veterans make up a unique subset of cancer survivors, in part because of risk factors associated with their service. Many veterans developed cancer as a result of their military exposure to toxic chemicals and radiation. To date, VA recognizes that chronic B-cell leukemias, Hodgkin disease, multiple myeloma, non-Hodgkin lymphomas, prostate cancer, respiratory cancers, and soft tissue sarcomas are all presumptive diseases related to Agent Orange exposure.13 There are other substances also presumed to increase the risk of certain cancers in veterans who have had ionizing radiation exposure.14 There is still much to learn regarding veterans who served during the Gulf War, Operation Enduring Freedom, and Operation Iraqi Freedom.15,16
In a comparison of VA data files with U.S. SEER data files from 2007, researchers identified differences in characteristics between veteran cancer survivors and civilian cancer survivors.17 In addition to increased exposure risks, the veteran cancer survivor population is older than the general cancer survivorship population and is mostly male.17 Veterans’ comorbid conditions, such as type 2 diabetes, ischemic heart disease, Parkinson disease, and peripheral neuropathy, which may be service related, complicate survivorship.17 These characteristics (age, gender, exposure risks, and comorbid conditions) influence the type of cancer diagnosed and treatment options, and they may ultimately impact survivorship needs
(Table 1).
The prevalence of mental health issues in the veteran population is significant.18 Posttraumatic stress disorder affects 7% to 8% of the general population at some point during their lifetime and as many as 16% of those returning from military deployment.19 In a predominantly
male veteran study correlating combat PTSD with cancerrelated PTSD, about half the participants (n = 170) met PTSD Criterion A, viewing their cancer as a traumatic experience.20 Posttraumatic stress disorder, depression, anxiety, and addictive disease all must be addressed in the survivorship plan of care.
Poor mental health has been linked to increased morbidity and mortality and can limit the veteran’s ability to participate in health promotion and medical care.21 Distress related to cancer is well recognized in the civilian population.22,23 Veterans are at risk for moderate-tosevere disabling distress, especially when the cancer is associated with their military service. Vietnam veterans who have a diagnosis of cancer report that they have already served their time and are now serving it again, having to wage a battle on cancer and undergo difficult treatments and associated adverse effects (AEs).24 It is important to note, however, that some veterans have developed strong coping skills, which gives them strength and resilience for the survivorship experience.25
Other factors also contribute to veterans’ unique survivorship needs. Many veterans have limited social and/or economic resources, making it difficult to receive cancer treatment and follow recommendations for a healthful lifestyle as a cancer survivor. Demographics from the VA have illustrated that many veterans have a limited support system (65% do not have a spouse), and many have low incomes.26 Although veterans comprise about 11% of the general population, they make up 26% of the homeless population.26 It is estimated that 260,000 veterans are homeless at some time during the course of a year, and of these, 45% have mental health issues and 70% have substance abuse problems.27 Basic needs such as housing, running water, heat and electricity, and nutrition must be met in order to prevent infection during treatment, maximize the benefit, and reduce the risks associated with treatment. Transportation issues can make it challenging to travel to medical centers for cancer surveillance following treatment.
Models of Care
As defined in the aforementioned IOM report, multiple models of survivorship care have surfaced over the years.6 Much that was originally seen and implemented in adult cancer survivorship was known from pediatric cancer care. Early models that surfaced included shared care models, nurse-led models, and tertiary survivorship clinics. Each model has its strengths and disadvantages.
The shared care model of survivorship involves a sharing of the responsibility for the survivor among different specialties, potentially at different facilities, and the primary care team. Typically, the PCP refers the patient to the oncologist when cancer is suspected or diagnosed. The primary care team continues to provide routine health maintenance and manages other health problems while the oncology team provides cancer care. The patient is transitioned back to the primary care team with a survivorship care plan (SCP) at 1 to 2 years after completion of cancer therapy or at the discretion of the oncology team.28 For
this model to work, the PCP must be willing to take on this responsibility, and there must be a coordinated effort for seamless communication between teams, which can be potentially challenging.
Nurse-led programs emerged in the pediatric populations. Pediatric nurse-led clinics assume care of the patient after active treatment to manage long-term AEs of cancer treatments, symptom management, care planning, and education. A comprehensive review of the literature identified that “nurse-led follow-up services are acceptable, appropriate, and effective.”6 Barriers to this model of care include a shortage of trained oncology nurses and a preference for physician follow-up by some cancer survivors who want the security of their oncologist for ongoing, long-term care.6
Survivorship follow-up clinics, a tertiary model of care, have been implemented at some larger academic centers. These clinics focus on cancer survivorship and are often separate from other routine health care visits. Typically, these clinics include multiple specialties and are often disease-specific. These types of clinics pose a different set of challenges regarding duplication of services and reimbursement issues.
As of yet, no model has been proven more effective than the others. Each institution and patient population may not lend themselves to a one-size-fits-all model. There may be different models of care needed, based on patient population. Regardless of the model selected, individualized survivorship care plans are an essential component of quality cancer survivorship care.
Addessing Survivorship Care
In 2009, 5 interdisciplinary leaders in VA cancer care (Ellen Ballard, RN; David Haggstrom, MD, MAS; Veronica Reis, PhD; Mark Detzer, PhD; and Tina Gill, MA) attended a breakout session on psychosocial oncology at the Association of VA Hematology and Oncology (AVAHO) meeting in Minneapolis, Minnesota, and most members of this team participated in the 2009-2012 VHA Cancer Care Collaboratives to improve the timeliness and quality of care for veterans who were cancer patients. Dr. Haggstrom and Ms. Ballard developed a SharePoint site for the Survivorship Special Interest Group (SIG) members through the Loma Linda VAMC in California. The SIG workgroup then built the Cancer Survivorship Toolkit, composed of
5 critical tools (Figure).
In July 2012, the VA Cancer Survivorship Toolkit content was disseminated at AVAHO and launched behind the VA firewall. It subsequently received accolades from the national program director for VHA Oncology and was listed on the American College of Surgeons Commission on Cancer (CoC) Best Practices website. The toolkit is accessible to all VA programs, and suggestions for new content can be submitted directly on the site (Figure).
The development of a SCP began in late 2011 when SIG members collected examples of SCPs from leading organizations. The members compared this content with the IOM recommendations for SCPs and developed a template. The template was programmed for the VHA computerized patient record system (CPRS) and placed on the internal VA toolkit website. The template included the treatment summary and care plan. The treatment summary portion included the diagnosis and tumor characteristics, diagnostic tests used, dates and types of treatment, chemoprevention or maintenance treatments, supportive services required, the surveillance plan, and signs of recurrence. The care plan portion provided information on the likely course of recovery and a checklist for common long-term AEs in the areas of psychological distress, financial and practical effects, and physical effects. Also included was information about referral, health behaviors, late effects that may develop, contact information, and general resource information.
The computer applications coordinator at any VA can download the template from the toolkit onto their CPRS, and the template can then be brought into any progress note. Individual sites may also edit the template to suit specific needs. The SCP can be completed by any clinician with the appropriate clinical competencies. To date, > 50 sites have downloaded the SCP template for use.
Cancer Survivorship Clinic
At the Louis Stokes Cleveland (LSC) VAMC, a nurse-led model of a cancer survivorship clinic was established with an expert nurse practitioner (NP). A major catalyst for the development of this clinic was the receipt of a Specialty Care Education Center of Excellence, funded by the Offices of Specialty Care and Academic Affiliations. A priority of this project was the implementation of survivorship care for every veteran with a cancer diagnosis. A system redesign was implemented to deliver quality, cost-effective, patient-centered cancer care within an interprofessional, team-based practice. This clinic is imbedded within an interdisciplinary clinic setting where the NP works in close collaboration with the medical and surgical oncologists as well as providers from mental health, social work, nutrition, physical therapy, and others.
The first patients to receive survivorship care in this new model from the time of their diagnosis were veterans with breast cancer, sarcoma, melanoma, and lymphomas. Veterans are followed jointly by the NP and the medical and surgical oncologists during active treatment. The NP provides physical symptom assessment and management for patients both during and after treatment.
At the end of active treatment, patient visits are alternated between oncology physicians and the survivorship NP for 5 years. The timeline for follow-up visits is based on National Comprehensive Cancer Network guidelines for each cancer type but then individualized based on patient need.29 During this 5-year time period, patients under active surveillance whose conditions have been stable are seen by the NP. Any concerning symptoms are immediately relayed to the primary oncologist or surgical oncologist, often the same day, and patients can be seen the same day if necessary, to improve coordination and access to services.
A unique focus of the clinic is the integration of health promotion and risk reduction that coincides with the active surveillance plan. This transition of active surveillance patients to the NP-led survivorship clinic not only opens access to newly diagnosed cancer patients to be seen by the oncologist, but also allows for seamless transition and coordination to active surveillance. Within the clinic structure, patients receive patient navigation beginning with a cancer concern; patients also receive screening for psychosocial distress at the time of diagnosis and at every visit. Patient navigation and distress screening are both considered essential elements to survivorship care in the most recent CoC guidelines.30 The survivorship NP keeps the primary care team up-to-date regarding patient care across the disease trajectory by alerting them to updates electronically in the CPRS in real time.
Survivorship Care Plan
A focus of the clinic has also been on the implementation of a formal SCP to be completed 3 months after the conclusion of active treatment. The formal SCP was downloaded from the Cancer Survivorship Toolkit and is composed of a 3-part summary. The 3 parts consist of the treatment summary, the plan for rehabilitation, and the plan for the future. The first section of the SCP is completed by the medical oncologist as a summary of treatment received by the veteran. The summary of treatment section is reviewed and discussed with the veteran survivor at the visit, and the second and third sections are completed during the 3-month follow-up visit with the veteran.
Success and Areas for Improvement
The survivorship clinic has been well received by veterans. Patient satisfaction scores have been overwhelmingly positive. Veterans appreciate and feel comfortable knowing their providers from the beginning of diagnosis along the entire disease trajectory. They know that if problems arise, the survivorship NP has direct access to the medical or surgical oncologist for immediate review.
The difficult challenge for the cancer care providers is to know when is the right time to transition care back to the PCP. Transitions of care often come with high anxiety and a sense of loss for the veteran. The 5-year survival mark is not always the appropriate transition time for some veterans. Those with extensive physical and mental health issues may need continuity of care and continued support from the oncology team.
The SCP has presented challenges in terms of when to complete and who should complete the form. There has also been concern over the length of the summary, how long it will take to complete the document, and which summary template to use. Areas for improvement with the template could potentially be to automate population of the chemotherapy and radiation summaries. Some software packages are available, but they are costly. Another issue with external software is getting it accepted by VHA and incorporated into the CPRS.
Recommendations
Many cancer programs are struggling to provide highquality survivorship care. The CoC, recognizing the challenges programs are having implementing survivorship care, has extended the accreditation requirement for full implementation from 2015 to 2019.31
The following recommendations should be considered for the successful implementation of a new survivorship program:
- Collect information from multiple resources to guide the establishment of the survivorship clinic;
- Become familiar with the IOM From Cancer Patient to Cancer Survivor: Lost in Transition;6
- Understand local issues and barriers specific to your care delivery system;
- Collaborate with key stakeholders from multiple specialties to gain momentum and buy-in;
- Hold regular meetings with stakeholders as well as leadership to identify and remove barriers to the clinic success;
- Join the VA Survivorship SIG to collaborate with other sites who have already started to pilot survivorship programs and discuss barriers to and successes of programs so as to not reinvent the wheel;
- Utilize the Cancer Survivorship Toolkit;
- Download the SCP;
- Establish a close partnership with your local cancer committee; and
- Collect and report data to show effectiveness and need.
All these strategies were vital to the success of the LSCVAMC survivorship program.
Summary
The VA is uniquely positioned to be a leader in highquality, comprehensive, and veteran-centered cancer survivorship care in the years ahead. The close relationship between specialty and primary care allows for smooth continuity of care and easy transitions between oncology and primary care. The comprehensive CPRS allows easy accessibility to information for the entire health care team. The Cancer Survivorship Toolkit provides a template of the survivorship care plan for the veteran and his or her health care providers.
The LSCVAMC is one of many VA institutions implementing quality care for cancer survivors and can serve as a role model for other VA programs initiating the survivorship care process (Table 2).
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. American Cancer Society. Cancer Facts & Figures 2014. Atlanta, GA: American Cancer Society; 2014.
2. Institute of Medicine. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Levit LA, Balough EP, Nass SJ, Ganz PA, eds. Washington, DC: The National Academies Press; 2013.
3. Stricker CT, O’Brien M. Implementing the commission on cancer standards for survivorship care plans. Clin J Oncol Nurs. 2014;(suppl 18):15-22.
4. Cowens-Alvarado R, Sharpe K, Pratt-Chapman M, et al. Advancing survivorship care through the National Cancer Survivorship Resource Center: Developing American Cancer Society guidelines for primary care providers. CA Cancer J Clin. 2013;63(3):147-150.
5. Cheung WY, Neville BA, Cameron DB, Cook EF, Earle CC. Comparisons of patient and physician expectations for cancer survivorship care. J Clin Oncol. 2009;27(15):2489-2495.
6. Institute of Medicine. From Cancer Patient to Cancer Survivor: Lost in Transition. Hewitt M, Greenfield S, Stovall E, eds. Washington, DC: The National Academies Press; 2006.
7. Clark EJ, Stovall EL, Leigh S, Siu AL, Austin DK, Rowland JH, eds. Imperatives for Quality Cancer Care: Access, Advocacy, Action, and Accountability. Silver Spring, MD: National Coalition for Cancer Survivorship; 1996.
8. National Cancer Institute. Survivorship. NCI Dictionary of Cancer Terms Website. http://www.cancer.gov/dictionary?CdrID=445089. Accessed December 3, 2014.
9. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220-241.
10. Moye J, Schuster JL, Latini DM, Naik AD. The future of cancer survivorship care for veterans. Fed Pract. 2010;27(3):36-43.
11. Naik AD, Martin LA, Karel M, et al. Cancer survivor rehabilitation and recovery: Protocol for the Veterans Cancer Rehabilitation Study (Vet-CaRes). BMC Health Serv Res. 2013;13:93.
12. Beehler GP, Rodriques AE, Kay MA, Kiviniemi MT, Steinbrenner L. Lasting impact: Understanding the psychosocial implications of cancer among military veterans. J Psychosoc Oncol. 2013:31(4):430-450.
13. U.S. Department of Veterans Affairs. Veterans’ diseases associated with Agent Orange. Public Health Website. http://www.publichealth.va.gov/exposures/agentorange/conditions/index.asp. Updated December 30, 2013. Accessed December3, 2014.
14. U.S. Department of Veterans Affairs. Radiation. Public Health Website. http://www.publichealth.va.gov/exposures/radiation/index.asp. Updated December 31, 2013. Accessed December 3, 2014.
15. Cohen BE, Gima K, Bertenthal D, Kim S, Marmar CR, Seal KH. Mental health diagnoses and utilization of VA non-mental health medical services among returning Iraq and Afghanistan veterans. J Gen Intern Med. 2010;25(1):18-24.
16. U.S. Department of Veterans Affairs. Gulf War veterans’ illnesses. Public Health Website. http://www.publichealth.va.gov/exposures/gulfwar/index.asp. Updated November 7, 2014. Accessed December 3, 2014.
17. National Cancer Institute. Cancer query systems. Surveillance, Epidemiology, and End Results Program Website. http://seer.cancer.gov/canques/index.html. Accessed December 3, 2014.
18. Suicide in the military: Army-NIH funded study points to risk and protective factors [news release]. Washington, DC: National Institute of Mental Health; March 3, 2014. http://www.nimh.nih.gov/news/science-news/2014/suicide-in-the-military-army-nih-funded-study-points-to-risk-and-protective-factors.shtml. Accessed December 3, 2014.
19. Gates MA, Holowka DW, Vasterling JJ, Keane TM, Marx BP, Rosen RC. Posttraumatic stress disorder in veterans and military personnel: Epidemiology, screening, and case recognition. Psychol Serv. 2012;9(4):361-382.
20. Mulligan EA, Schuster Wachen J, Naik AD, Gosian J, Moye J. Cancer as a criterion a traumatic stressor for veterans: Prevalence and correlates. Psychol Trauma. 2014;6(suppl 1):S73-S81.
21. Musuuza JS, Sherman ME, Knudsen KJ, Sweeney HA, Tyler CV, Koroukian SM. Analyzing excess mortality from cancer among individuals with mental illness. Cancer. 2013;119(13):2469-2476.
22. Zabora J, Macmurray L. The history of psychosocial screening among cancer patients. J Psychosoc Oncol. 2013:30(6):625-635.
23. Holland JC, Andersen B, Breitbart WS, et al. Distress management: Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2010:8(4):448-485.
24. Grassman DL. The Hero Within. St. Petersburg, FL: Vandamere Press; 2012.
25. Jahn AL, Herman L, Schuster J, Naik A, Moye J. Distress and resilience after cancer
in veterans. Res Hum Dev. 2012;9(3):229-247.
26. National Association of Social Workers. Social workers speak on veterans issues June 2009. National Association of Social Workers Website. http://www.naswdc.org/pressroom/2009/Social%20Work%20Veterans%20Fact%20Sheet.pdf. Accessed December 3, 2014.
27. Homeless. U.S. Department of Veterans Affairs Website. http://www.va.gov/homeless. Accessed December 3, 2014.
28. Oeffinger KC, McCabe MS. Models for delivering survivorship care. J Clin Oncol. 2006;24(32):5117-5124.
29. NCCN Guidelines. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site. Accessed December 3, 2014.
30. American College of Surgeons, Commission on Cancer. Cancer program standards 2012, version 1.2.1: Ensuring patient-centered care. https://www.facs.org/~/media/file/quality%20programs/cancer/coc/programstandards2012.ashx. Published January 21, 2014. Accessed December 3, 2014.
31. Accreditation committee clarifications for standard 3.3 survivorship care plan. American College of Surgeons Website. https://www.facs.org/publications/newsletters/coc-source/special-source/standard33. Published September 9, 2014. Accessed December 3, 2014.
1. American Cancer Society. Cancer Facts & Figures 2014. Atlanta, GA: American Cancer Society; 2014.
2. Institute of Medicine. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Levit LA, Balough EP, Nass SJ, Ganz PA, eds. Washington, DC: The National Academies Press; 2013.
3. Stricker CT, O’Brien M. Implementing the commission on cancer standards for survivorship care plans. Clin J Oncol Nurs. 2014;(suppl 18):15-22.
4. Cowens-Alvarado R, Sharpe K, Pratt-Chapman M, et al. Advancing survivorship care through the National Cancer Survivorship Resource Center: Developing American Cancer Society guidelines for primary care providers. CA Cancer J Clin. 2013;63(3):147-150.
5. Cheung WY, Neville BA, Cameron DB, Cook EF, Earle CC. Comparisons of patient and physician expectations for cancer survivorship care. J Clin Oncol. 2009;27(15):2489-2495.
6. Institute of Medicine. From Cancer Patient to Cancer Survivor: Lost in Transition. Hewitt M, Greenfield S, Stovall E, eds. Washington, DC: The National Academies Press; 2006.
7. Clark EJ, Stovall EL, Leigh S, Siu AL, Austin DK, Rowland JH, eds. Imperatives for Quality Cancer Care: Access, Advocacy, Action, and Accountability. Silver Spring, MD: National Coalition for Cancer Survivorship; 1996.
8. National Cancer Institute. Survivorship. NCI Dictionary of Cancer Terms Website. http://www.cancer.gov/dictionary?CdrID=445089. Accessed December 3, 2014.
9. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220-241.
10. Moye J, Schuster JL, Latini DM, Naik AD. The future of cancer survivorship care for veterans. Fed Pract. 2010;27(3):36-43.
11. Naik AD, Martin LA, Karel M, et al. Cancer survivor rehabilitation and recovery: Protocol for the Veterans Cancer Rehabilitation Study (Vet-CaRes). BMC Health Serv Res. 2013;13:93.
12. Beehler GP, Rodriques AE, Kay MA, Kiviniemi MT, Steinbrenner L. Lasting impact: Understanding the psychosocial implications of cancer among military veterans. J Psychosoc Oncol. 2013:31(4):430-450.
13. U.S. Department of Veterans Affairs. Veterans’ diseases associated with Agent Orange. Public Health Website. http://www.publichealth.va.gov/exposures/agentorange/conditions/index.asp. Updated December 30, 2013. Accessed December3, 2014.
14. U.S. Department of Veterans Affairs. Radiation. Public Health Website. http://www.publichealth.va.gov/exposures/radiation/index.asp. Updated December 31, 2013. Accessed December 3, 2014.
15. Cohen BE, Gima K, Bertenthal D, Kim S, Marmar CR, Seal KH. Mental health diagnoses and utilization of VA non-mental health medical services among returning Iraq and Afghanistan veterans. J Gen Intern Med. 2010;25(1):18-24.
16. U.S. Department of Veterans Affairs. Gulf War veterans’ illnesses. Public Health Website. http://www.publichealth.va.gov/exposures/gulfwar/index.asp. Updated November 7, 2014. Accessed December 3, 2014.
17. National Cancer Institute. Cancer query systems. Surveillance, Epidemiology, and End Results Program Website. http://seer.cancer.gov/canques/index.html. Accessed December 3, 2014.
18. Suicide in the military: Army-NIH funded study points to risk and protective factors [news release]. Washington, DC: National Institute of Mental Health; March 3, 2014. http://www.nimh.nih.gov/news/science-news/2014/suicide-in-the-military-army-nih-funded-study-points-to-risk-and-protective-factors.shtml. Accessed December 3, 2014.
19. Gates MA, Holowka DW, Vasterling JJ, Keane TM, Marx BP, Rosen RC. Posttraumatic stress disorder in veterans and military personnel: Epidemiology, screening, and case recognition. Psychol Serv. 2012;9(4):361-382.
20. Mulligan EA, Schuster Wachen J, Naik AD, Gosian J, Moye J. Cancer as a criterion a traumatic stressor for veterans: Prevalence and correlates. Psychol Trauma. 2014;6(suppl 1):S73-S81.
21. Musuuza JS, Sherman ME, Knudsen KJ, Sweeney HA, Tyler CV, Koroukian SM. Analyzing excess mortality from cancer among individuals with mental illness. Cancer. 2013;119(13):2469-2476.
22. Zabora J, Macmurray L. The history of psychosocial screening among cancer patients. J Psychosoc Oncol. 2013:30(6):625-635.
23. Holland JC, Andersen B, Breitbart WS, et al. Distress management: Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2010:8(4):448-485.
24. Grassman DL. The Hero Within. St. Petersburg, FL: Vandamere Press; 2012.
25. Jahn AL, Herman L, Schuster J, Naik A, Moye J. Distress and resilience after cancer
in veterans. Res Hum Dev. 2012;9(3):229-247.
26. National Association of Social Workers. Social workers speak on veterans issues June 2009. National Association of Social Workers Website. http://www.naswdc.org/pressroom/2009/Social%20Work%20Veterans%20Fact%20Sheet.pdf. Accessed December 3, 2014.
27. Homeless. U.S. Department of Veterans Affairs Website. http://www.va.gov/homeless. Accessed December 3, 2014.
28. Oeffinger KC, McCabe MS. Models for delivering survivorship care. J Clin Oncol. 2006;24(32):5117-5124.
29. NCCN Guidelines. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site. Accessed December 3, 2014.
30. American College of Surgeons, Commission on Cancer. Cancer program standards 2012, version 1.2.1: Ensuring patient-centered care. https://www.facs.org/~/media/file/quality%20programs/cancer/coc/programstandards2012.ashx. Published January 21, 2014. Accessed December 3, 2014.
31. Accreditation committee clarifications for standard 3.3 survivorship care plan. American College of Surgeons Website. https://www.facs.org/publications/newsletters/coc-source/special-source/standard33. Published September 9, 2014. Accessed December 3, 2014.