Article
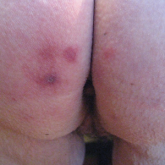
Ice Pack–Induced Perniosis: A Rare and Underrecognized Association
- Author:
- Donna Tran, DO
- Jessica Riley, DO
- Anny Xiao, DO
- Shirlene Jay, MD
- Paul Shitabata, MD
- Navid Nami, DO
Perniosis, or chilblain, is characterized by skin lesions that occur as an abnormal reaction to exposure to cold and damp conditions. Ice pack–...
Article
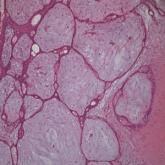
Concomitant Fibrofolliculoma and Trichodiscoma on the Abdomen
- Author:
- Jessica Riley, DO
- Leela Athalye, DO
- Donna Tran, DO
- Stephanie Fogelson, MD
- Paul Shitabata, MD
Fibrofolliculoma and trichodiscoma are adnexal tumors that arise from or around hair follicles and are two of the many characteristic features of...
