User login
Acquired Epidermodysplasia Verruciformis Occurring in a Renal Transplant Recipient
Acquired epidermodysplasia verruciformis (EDV) is a rare disorder occurring in patients with depressed cellular immunity, particularly individuals with human immunodeficiency virus (HIV). Rare cases of acquired EDV have been reported in stem cell or solid organ transplant recipients. Weakened cellular immunity predisposes the patient to human papillomavirus (HPV) infections, with 92% of renal transplant recipients developing warts within 5 years posttransplantation.1 Specific EDV-HPV subtypes have been isolated from lesions in several immunosuppressed individuals, with HPV-5 and HPV-8 being the most commonly isolated subtypes.2,3 Herein, we present the clinical findings of a renal transplant recipient who presented for evaluation of multiple skin lesions characteristic of EDV 5 years following transplantation and initiation of immunosuppressive therapy. Additionally, we review the current diagnostic findings, management, and treatment of acquired EDV.
A 44-year-old white woman presented for evaluation of several pruritic cutaneous lesions that had developed on the chest and neck of 1 month’s duration. The patient had been on the immunosuppressant medications cyclosporine and mycophenolate mofetil for more than 5 years following renal transplantation 7 years prior to the current presentation. She also was on low-dose prednisone for chronic systemic lupus erythematosus. Her family history was negative for any pertinent skin conditions.
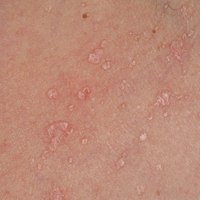
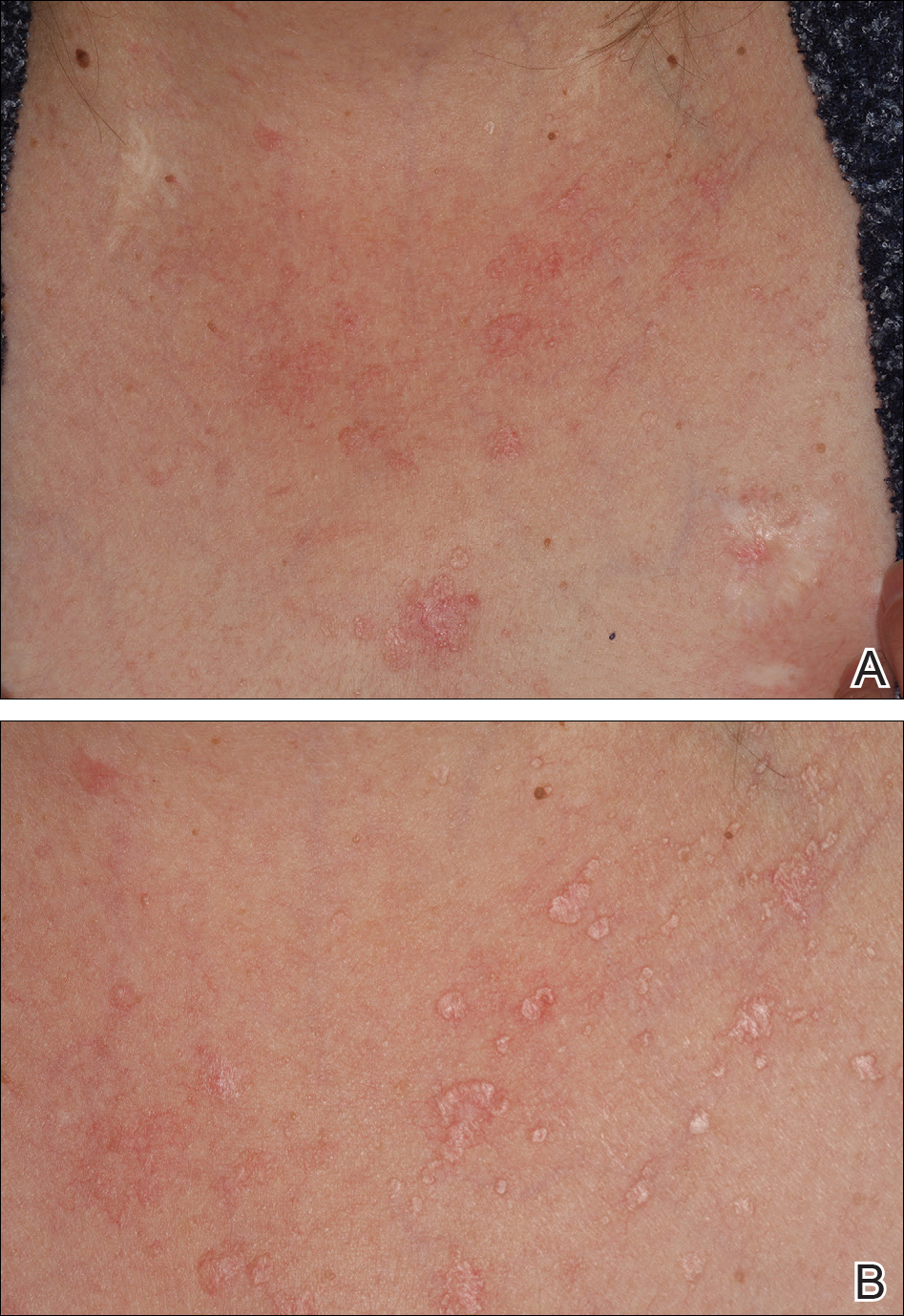
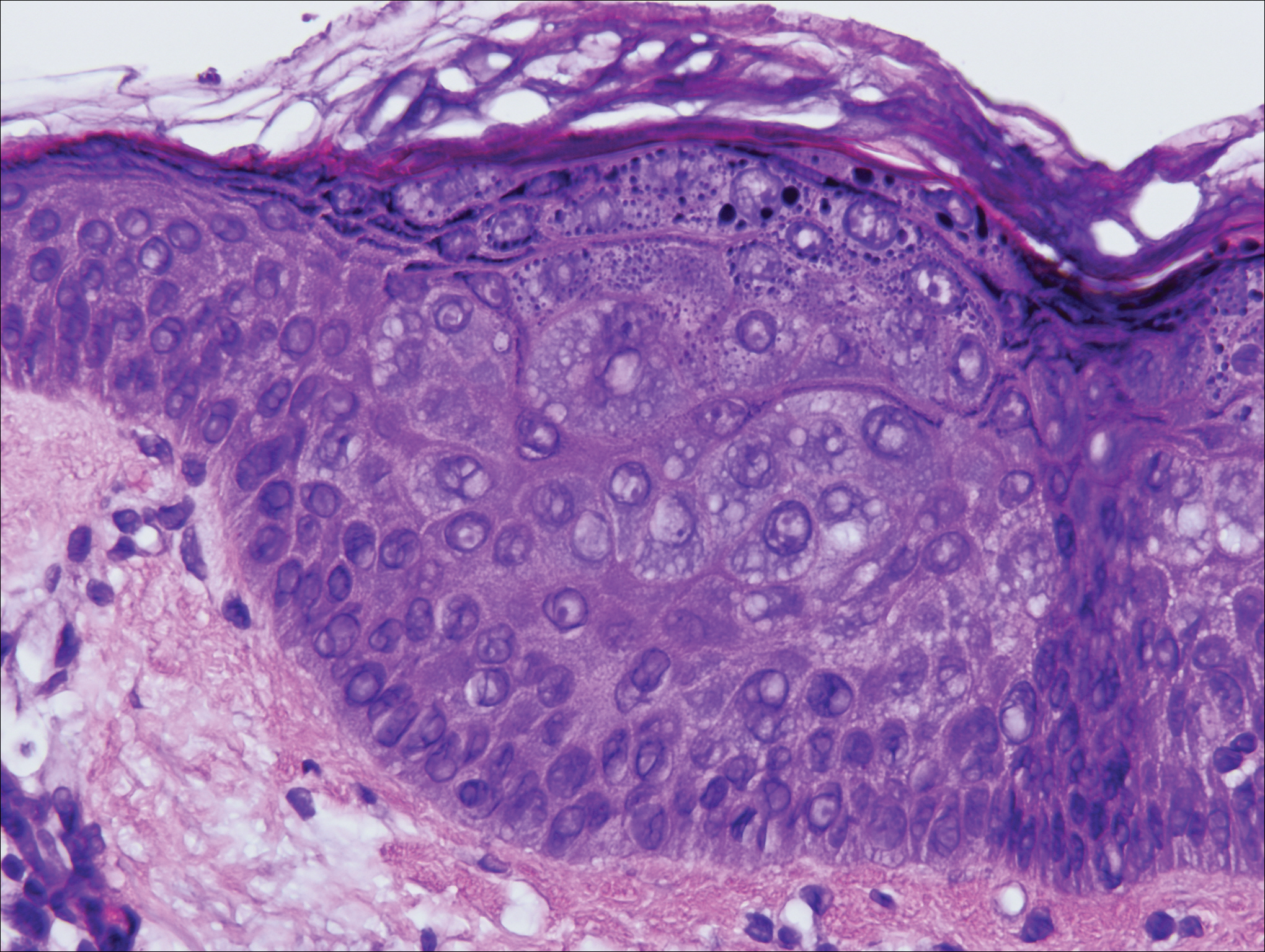
On physical examination the patient exhibited several grouped 0.5-cm, shiny, pink lichenoid macules located on the upper mid chest, anterior neck, and left leg clinically resembling the lesions of pityriasis versicolor (Figure 1). A shave biopsy was taken from one of the newest lesions on the left leg. Histopathology revealed viral epidermal cytopathic changes, blue cytoplasm, and coarse hypergranulosis characteristic of EDV (Figure 2). A diagnosis of acquired EDV was made based on the clinical and histopathologic findings.
The patient’s skin lesions became more widespread despite several different treatment regimens, including cryosurgery; tazarotene cream 0.05% nightly; imiquimod cream 5% once weekly; and intermittent short courses of 5-fluorouracil cream 5%, which provided the best response. At her most recent clinic visit 8 years after initial presentation, she continued to have more widespread lesions on the trunk, arms, and legs, but no evidence of malignant transformation.
Comment
Epidermodysplasia verruciformis was first recognized as an inherited condition, most commonly inherited in an autosomal-dominant fashion; however, X-linked recessive cases have been reported.4,5 Patients with the inherited forms of this condition are prone to recurrent HPV infections secondary to a missense mutation in the epidermodysplasia verruciformis 1 and 2 genes, EVER1 and EVER2, on the EV1 locus located on chromosome 17q25.6 Because of this mutation, the patient’s cellular immunity becomes weakened. Cellular presentation of the EDV-HPV antigen to T lymphocytes becomes impaired, thereby inhibiting the body’s ability to successfully clear itself of the virus.5,6 The most commonly isolated EDV-HPV subtypes are HPV-5 and HPV-8, but HPV types 9, 12, 14, 15, 17, 19, 20, 21, 22, 23, 24, 25, and 50 also have been associated with EDV.1,3,7
Patients who have suppressed cellular immunity, such as transplant recipients on long-term immunosuppressant medications and individuals with HIV, graft-vs-host disease, systemic lupus erythematosus, and hematologic malignancies, are susceptible to EDV, as well as patients with atopic dermatitis being treated with topical calcineurin inhibitors.2,3,8-15 These patients acquire depressed cellular immunity and become increasingly susceptible to infections with the EDV-HPV subtypes. When clinical and histopathologic findings are consistent with EDV, a diagnosis of acquired EDV is given, which was further confirmed in a study conducted by Harwood et al.16 They found immunocompromised patients carry more EDV-HPV subtypes in skin lesions analyzed by polymerase chain reaction than immunocompetent individuals.16 Additionally, there is a positive correlation between the length of immunosuppression and the development of HPV lesions, with a majority of patients developing lesions within 5 years following initial immunosuppression.1,7,10,17
Epidermodysplasia verruciformis commonly presents with multiple hypopigmented to red macules that may coalesce into patches with a fine scale, clinically resembling the lesions of pityriasis versicolor.2,3,8-15 Epidermodysplasia verruciformis also may present as multiple flesh-colored, flat-topped, verrucous papules that clinically resemble the lesions of verruca plana on sun-exposed areas such as the face, arms, and legs.9 The characteristic histopathologic findings are enlarged keratinocytes with perinuclear halos and blue-gray cytoplasm as well as hypergranulosis.18 Immunocompromised hosts infected with EDV-HPV histologically tend to display more severe dysplasia than immunocompetent individuals.19 The differential diagnosis includes pityriasis versicolor, squamous cell carcinoma (SCC), and verruca plana. Tissue cultures and potassium hydroxide scrapings for microorganisms should be negative.
The specific EDV-HPV strains 5, 8, and 41 carry the highest oncogenic potential, with more than 60% of inherited EDV patients developing SCC by the fourth and fifth decades of life.16 Unlike inherited EDV, the clinical course of acquired EDV is less well known; however, UV light is thought to act synergistically with the EDV-HPV in oncogenic transformation of the lesions, as most of the SCCs develop on sun-exposed areas, and darker-skinned patients seem to have a decreased risk for malignant transformation of EDV lesions.4,9,20,21 Preventative measures such as strict sun protection and annual surveillance of lesions can help to prevent oncogenic progression of the lesions; however, several single- and multiple-agent regimens have been used in the treatment of EDV with variable results. Topical imiquimod, 5-fluorouracil, tretinoin, and tazarotene have been used with variable success. Acitretin alone and in combination with interferon alfa-2a also has been used.22,23 Highly active antiretroviral therapy in patients with HIV has effectively decreased the number of lesions in a subset of patients.24 We (anecdotal) and others25 also have had success using photodynamic therapy. Squamous cell carcinoma arising in patients with EDV can be managed by excision or by Mohs micrographic surgery.
Conclusion
We report a rare case of acquired EDV in a solid organ transplant recipient. Epidermodysplasia verruciformis can be acquired in immunosuppressed patients such as ours, and these patients should be followed closely due to the potential for malignant transformation. More studies regarding the anticipated clinical course of skin lesions in patients with acquired EDV are needed to better predict the time frame for malignant transformation.
- Dyall-Smith D, Trowell H, Dyall-Smith ML. Benign human papillomavirus infection in renal transplant recipients. Int J Dermatol. 1991;30:785-789.
- Lutzner MA, Orth G, Dutronquay V, et al. Detection of human papillomavirus type 5 DNA in skin cancers of an immunosuppressed renal allograft recipient. Lancet. 1983;2:422-424.
- Lutzner M, Croissant O, Ducasse MF, et al. A potentially oncogenic human papillomavirus (HPV-5) found in two renal allograft recipients. J Invest Dermatol. 1980;75:353-356.
- Androphy EJ, Dvoretzky I, Lowy DR. X-linked inheritance of epidermodysplasia verruciformis. genetic and virologic studies of a kindred. Arch Dermatol. 1985;121:864-868.
- Lutzner MA. Epidermodysplasia verruciformis. an autosomal recessive disease characterized by viral warts and skin cancer. a model for viral oncogenesis. Bull Cancer. 1978;65:169-182.
- Ramoz N, Rueda LA, Bouadjar B, et al. Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat Genet. 2002;32:579-581.
- Rüdlinger R, Smith IW, Bunney MH, et al. Human papillomavirus infections in a group of renal transplant recipients. Br J Dermatol. 1986;115:681-692.
- Kawai K, Egawa N, Kiyono T, et al. Epidermodysplasia-verruciformis-like eruption associated with gamma-papillomavirus infection in a patient with adult T-cell leukemia. Dermatology. 2009;219:274-278.
- Barr BB, Benton EC, McLaren K, et al. Human papilloma virus infection and skin cancer in renal allograft recipients. Lancet. 1989;1:124-129.
- Tanigaki T, Kanda R, Sato K. Epidermodysplasia verruciformis (L-L, 1922) in a patient with systemic lupus erythematosus. Arch Dermatol Res. 1986;278:247-248.
- Holmes C, Chong AH, Tabrizi SN, et al. Epidermodysplasia verruciformis-like syndrome in association with systemic lupus erythematosus. Australas J Dermatol. 2009;50:44-47.
- Gross G, Ellinger K, Roussaki A, et al. Epidermodysplasia verruciformis in a patient with Hodgkin’s disease: characterization of a new papillomavirus type and interferon treatment. J Invest Dermatol. 1988;91:43-48.
- Fernandez KH, Rady P, Tyring S, et al. Acquired epidermodysplasia verruciformis in a child with atopic dermatitis [published online September 3, 2012]. Pediatr Dermatol. 2014;31:400-402.
- Hultgren TL, Srinivasan SK, DiMaio DJ. Epidermodysplasia verruciformis occurring in a patient with human immunodeficiency virus: a case report. Cutis. 2007;79:307-311.
- Kunishige JH, Hymes SR, Madkan V, et al. Epidermodysplasia verruciformis in the setting of graft-versus-host disease. J Am Acad Dermatol. 2007;57(5 suppl):S78-S80.
- Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289-297.
- Moloney FJ, Keane S, O’Kelly P, et al. The impact of skin disease following renal transplantation on quality of life. Br J Dermatol. 2005;153:574-578.
- Tanigaki T, Endo H. A case of epidermodysplasia verruciformis (Lewandowsky-Lutz, 1922) with skin cancer: histopathology of malignant cutaneous changes. Dermatologica. 1984;169:97-101.
- Morrison C, Eliezri Y, Magro C, et al. The histologic spectrum of epidermodysplasia verruciformis in transplant and AIDS patients. J Cutan Pathol. 2002;29:480-489.
- Majewski S, Jabło´nska S. Epidermodysplasia verruciformis as a model of human papillomavirus-induced genetic cancer of the skin. Arch Dermatol. 1995;131:1312-1318.
- Jacyk WK, De Villiers EM. Epidermodysplasia verruciformis in Africans. Int J Dermatol. 1993;32:806-810.
- Gubinelli E, Posteraro P, Cocuroccia B, et al. Epidermodysplasia verruciformis with multiple mucosal carcinomas treated with pegylated interferon alfa and acitretin. J Dermatolog Treat. 2003;14:184-188.
- Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a. J Am Acad Dermatol. 2001;45:296-299.
- Haas N, Fuchs PG, Hermes B, et al. Remission of epidermodysplasia verruciformis-like skin eruption after highly active antiretroviral therapy in a human immunodeficiency virus-positive patient. Br J Dermatol. 2001;145:669-670.
- Karrer S, Szeimies RM, Abels C, et al. Epidermo-dysplasia verruciformis treated using topical 5-aminolaevulinic acid photodynamic therapy. Br J Dermatol. 1999;140:935-938.
Acquired epidermodysplasia verruciformis (EDV) is a rare disorder occurring in patients with depressed cellular immunity, particularly individuals with human immunodeficiency virus (HIV). Rare cases of acquired EDV have been reported in stem cell or solid organ transplant recipients. Weakened cellular immunity predisposes the patient to human papillomavirus (HPV) infections, with 92% of renal transplant recipients developing warts within 5 years posttransplantation.1 Specific EDV-HPV subtypes have been isolated from lesions in several immunosuppressed individuals, with HPV-5 and HPV-8 being the most commonly isolated subtypes.2,3 Herein, we present the clinical findings of a renal transplant recipient who presented for evaluation of multiple skin lesions characteristic of EDV 5 years following transplantation and initiation of immunosuppressive therapy. Additionally, we review the current diagnostic findings, management, and treatment of acquired EDV.
A 44-year-old white woman presented for evaluation of several pruritic cutaneous lesions that had developed on the chest and neck of 1 month’s duration. The patient had been on the immunosuppressant medications cyclosporine and mycophenolate mofetil for more than 5 years following renal transplantation 7 years prior to the current presentation. She also was on low-dose prednisone for chronic systemic lupus erythematosus. Her family history was negative for any pertinent skin conditions.
On physical examination the patient exhibited several grouped 0.5-cm, shiny, pink lichenoid macules located on the upper mid chest, anterior neck, and left leg clinically resembling the lesions of pityriasis versicolor (Figure 1). A shave biopsy was taken from one of the newest lesions on the left leg. Histopathology revealed viral epidermal cytopathic changes, blue cytoplasm, and coarse hypergranulosis characteristic of EDV (Figure 2). A diagnosis of acquired EDV was made based on the clinical and histopathologic findings.


The patient’s skin lesions became more widespread despite several different treatment regimens, including cryosurgery; tazarotene cream 0.05% nightly; imiquimod cream 5% once weekly; and intermittent short courses of 5-fluorouracil cream 5%, which provided the best response. At her most recent clinic visit 8 years after initial presentation, she continued to have more widespread lesions on the trunk, arms, and legs, but no evidence of malignant transformation.
Comment
Epidermodysplasia verruciformis was first recognized as an inherited condition, most commonly inherited in an autosomal-dominant fashion; however, X-linked recessive cases have been reported.4,5 Patients with the inherited forms of this condition are prone to recurrent HPV infections secondary to a missense mutation in the epidermodysplasia verruciformis 1 and 2 genes, EVER1 and EVER2, on the EV1 locus located on chromosome 17q25.6 Because of this mutation, the patient’s cellular immunity becomes weakened. Cellular presentation of the EDV-HPV antigen to T lymphocytes becomes impaired, thereby inhibiting the body’s ability to successfully clear itself of the virus.5,6 The most commonly isolated EDV-HPV subtypes are HPV-5 and HPV-8, but HPV types 9, 12, 14, 15, 17, 19, 20, 21, 22, 23, 24, 25, and 50 also have been associated with EDV.1,3,7
Patients who have suppressed cellular immunity, such as transplant recipients on long-term immunosuppressant medications and individuals with HIV, graft-vs-host disease, systemic lupus erythematosus, and hematologic malignancies, are susceptible to EDV, as well as patients with atopic dermatitis being treated with topical calcineurin inhibitors.2,3,8-15 These patients acquire depressed cellular immunity and become increasingly susceptible to infections with the EDV-HPV subtypes. When clinical and histopathologic findings are consistent with EDV, a diagnosis of acquired EDV is given, which was further confirmed in a study conducted by Harwood et al.16 They found immunocompromised patients carry more EDV-HPV subtypes in skin lesions analyzed by polymerase chain reaction than immunocompetent individuals.16 Additionally, there is a positive correlation between the length of immunosuppression and the development of HPV lesions, with a majority of patients developing lesions within 5 years following initial immunosuppression.1,7,10,17
Epidermodysplasia verruciformis commonly presents with multiple hypopigmented to red macules that may coalesce into patches with a fine scale, clinically resembling the lesions of pityriasis versicolor.2,3,8-15 Epidermodysplasia verruciformis also may present as multiple flesh-colored, flat-topped, verrucous papules that clinically resemble the lesions of verruca plana on sun-exposed areas such as the face, arms, and legs.9 The characteristic histopathologic findings are enlarged keratinocytes with perinuclear halos and blue-gray cytoplasm as well as hypergranulosis.18 Immunocompromised hosts infected with EDV-HPV histologically tend to display more severe dysplasia than immunocompetent individuals.19 The differential diagnosis includes pityriasis versicolor, squamous cell carcinoma (SCC), and verruca plana. Tissue cultures and potassium hydroxide scrapings for microorganisms should be negative.
The specific EDV-HPV strains 5, 8, and 41 carry the highest oncogenic potential, with more than 60% of inherited EDV patients developing SCC by the fourth and fifth decades of life.16 Unlike inherited EDV, the clinical course of acquired EDV is less well known; however, UV light is thought to act synergistically with the EDV-HPV in oncogenic transformation of the lesions, as most of the SCCs develop on sun-exposed areas, and darker-skinned patients seem to have a decreased risk for malignant transformation of EDV lesions.4,9,20,21 Preventative measures such as strict sun protection and annual surveillance of lesions can help to prevent oncogenic progression of the lesions; however, several single- and multiple-agent regimens have been used in the treatment of EDV with variable results. Topical imiquimod, 5-fluorouracil, tretinoin, and tazarotene have been used with variable success. Acitretin alone and in combination with interferon alfa-2a also has been used.22,23 Highly active antiretroviral therapy in patients with HIV has effectively decreased the number of lesions in a subset of patients.24 We (anecdotal) and others25 also have had success using photodynamic therapy. Squamous cell carcinoma arising in patients with EDV can be managed by excision or by Mohs micrographic surgery.
Conclusion
We report a rare case of acquired EDV in a solid organ transplant recipient. Epidermodysplasia verruciformis can be acquired in immunosuppressed patients such as ours, and these patients should be followed closely due to the potential for malignant transformation. More studies regarding the anticipated clinical course of skin lesions in patients with acquired EDV are needed to better predict the time frame for malignant transformation.
Acquired epidermodysplasia verruciformis (EDV) is a rare disorder occurring in patients with depressed cellular immunity, particularly individuals with human immunodeficiency virus (HIV). Rare cases of acquired EDV have been reported in stem cell or solid organ transplant recipients. Weakened cellular immunity predisposes the patient to human papillomavirus (HPV) infections, with 92% of renal transplant recipients developing warts within 5 years posttransplantation.1 Specific EDV-HPV subtypes have been isolated from lesions in several immunosuppressed individuals, with HPV-5 and HPV-8 being the most commonly isolated subtypes.2,3 Herein, we present the clinical findings of a renal transplant recipient who presented for evaluation of multiple skin lesions characteristic of EDV 5 years following transplantation and initiation of immunosuppressive therapy. Additionally, we review the current diagnostic findings, management, and treatment of acquired EDV.
A 44-year-old white woman presented for evaluation of several pruritic cutaneous lesions that had developed on the chest and neck of 1 month’s duration. The patient had been on the immunosuppressant medications cyclosporine and mycophenolate mofetil for more than 5 years following renal transplantation 7 years prior to the current presentation. She also was on low-dose prednisone for chronic systemic lupus erythematosus. Her family history was negative for any pertinent skin conditions.
On physical examination the patient exhibited several grouped 0.5-cm, shiny, pink lichenoid macules located on the upper mid chest, anterior neck, and left leg clinically resembling the lesions of pityriasis versicolor (Figure 1). A shave biopsy was taken from one of the newest lesions on the left leg. Histopathology revealed viral epidermal cytopathic changes, blue cytoplasm, and coarse hypergranulosis characteristic of EDV (Figure 2). A diagnosis of acquired EDV was made based on the clinical and histopathologic findings.


The patient’s skin lesions became more widespread despite several different treatment regimens, including cryosurgery; tazarotene cream 0.05% nightly; imiquimod cream 5% once weekly; and intermittent short courses of 5-fluorouracil cream 5%, which provided the best response. At her most recent clinic visit 8 years after initial presentation, she continued to have more widespread lesions on the trunk, arms, and legs, but no evidence of malignant transformation.
Comment
Epidermodysplasia verruciformis was first recognized as an inherited condition, most commonly inherited in an autosomal-dominant fashion; however, X-linked recessive cases have been reported.4,5 Patients with the inherited forms of this condition are prone to recurrent HPV infections secondary to a missense mutation in the epidermodysplasia verruciformis 1 and 2 genes, EVER1 and EVER2, on the EV1 locus located on chromosome 17q25.6 Because of this mutation, the patient’s cellular immunity becomes weakened. Cellular presentation of the EDV-HPV antigen to T lymphocytes becomes impaired, thereby inhibiting the body’s ability to successfully clear itself of the virus.5,6 The most commonly isolated EDV-HPV subtypes are HPV-5 and HPV-8, but HPV types 9, 12, 14, 15, 17, 19, 20, 21, 22, 23, 24, 25, and 50 also have been associated with EDV.1,3,7
Patients who have suppressed cellular immunity, such as transplant recipients on long-term immunosuppressant medications and individuals with HIV, graft-vs-host disease, systemic lupus erythematosus, and hematologic malignancies, are susceptible to EDV, as well as patients with atopic dermatitis being treated with topical calcineurin inhibitors.2,3,8-15 These patients acquire depressed cellular immunity and become increasingly susceptible to infections with the EDV-HPV subtypes. When clinical and histopathologic findings are consistent with EDV, a diagnosis of acquired EDV is given, which was further confirmed in a study conducted by Harwood et al.16 They found immunocompromised patients carry more EDV-HPV subtypes in skin lesions analyzed by polymerase chain reaction than immunocompetent individuals.16 Additionally, there is a positive correlation between the length of immunosuppression and the development of HPV lesions, with a majority of patients developing lesions within 5 years following initial immunosuppression.1,7,10,17
Epidermodysplasia verruciformis commonly presents with multiple hypopigmented to red macules that may coalesce into patches with a fine scale, clinically resembling the lesions of pityriasis versicolor.2,3,8-15 Epidermodysplasia verruciformis also may present as multiple flesh-colored, flat-topped, verrucous papules that clinically resemble the lesions of verruca plana on sun-exposed areas such as the face, arms, and legs.9 The characteristic histopathologic findings are enlarged keratinocytes with perinuclear halos and blue-gray cytoplasm as well as hypergranulosis.18 Immunocompromised hosts infected with EDV-HPV histologically tend to display more severe dysplasia than immunocompetent individuals.19 The differential diagnosis includes pityriasis versicolor, squamous cell carcinoma (SCC), and verruca plana. Tissue cultures and potassium hydroxide scrapings for microorganisms should be negative.
The specific EDV-HPV strains 5, 8, and 41 carry the highest oncogenic potential, with more than 60% of inherited EDV patients developing SCC by the fourth and fifth decades of life.16 Unlike inherited EDV, the clinical course of acquired EDV is less well known; however, UV light is thought to act synergistically with the EDV-HPV in oncogenic transformation of the lesions, as most of the SCCs develop on sun-exposed areas, and darker-skinned patients seem to have a decreased risk for malignant transformation of EDV lesions.4,9,20,21 Preventative measures such as strict sun protection and annual surveillance of lesions can help to prevent oncogenic progression of the lesions; however, several single- and multiple-agent regimens have been used in the treatment of EDV with variable results. Topical imiquimod, 5-fluorouracil, tretinoin, and tazarotene have been used with variable success. Acitretin alone and in combination with interferon alfa-2a also has been used.22,23 Highly active antiretroviral therapy in patients with HIV has effectively decreased the number of lesions in a subset of patients.24 We (anecdotal) and others25 also have had success using photodynamic therapy. Squamous cell carcinoma arising in patients with EDV can be managed by excision or by Mohs micrographic surgery.
Conclusion
We report a rare case of acquired EDV in a solid organ transplant recipient. Epidermodysplasia verruciformis can be acquired in immunosuppressed patients such as ours, and these patients should be followed closely due to the potential for malignant transformation. More studies regarding the anticipated clinical course of skin lesions in patients with acquired EDV are needed to better predict the time frame for malignant transformation.
- Dyall-Smith D, Trowell H, Dyall-Smith ML. Benign human papillomavirus infection in renal transplant recipients. Int J Dermatol. 1991;30:785-789.
- Lutzner MA, Orth G, Dutronquay V, et al. Detection of human papillomavirus type 5 DNA in skin cancers of an immunosuppressed renal allograft recipient. Lancet. 1983;2:422-424.
- Lutzner M, Croissant O, Ducasse MF, et al. A potentially oncogenic human papillomavirus (HPV-5) found in two renal allograft recipients. J Invest Dermatol. 1980;75:353-356.
- Androphy EJ, Dvoretzky I, Lowy DR. X-linked inheritance of epidermodysplasia verruciformis. genetic and virologic studies of a kindred. Arch Dermatol. 1985;121:864-868.
- Lutzner MA. Epidermodysplasia verruciformis. an autosomal recessive disease characterized by viral warts and skin cancer. a model for viral oncogenesis. Bull Cancer. 1978;65:169-182.
- Ramoz N, Rueda LA, Bouadjar B, et al. Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat Genet. 2002;32:579-581.
- Rüdlinger R, Smith IW, Bunney MH, et al. Human papillomavirus infections in a group of renal transplant recipients. Br J Dermatol. 1986;115:681-692.
- Kawai K, Egawa N, Kiyono T, et al. Epidermodysplasia-verruciformis-like eruption associated with gamma-papillomavirus infection in a patient with adult T-cell leukemia. Dermatology. 2009;219:274-278.
- Barr BB, Benton EC, McLaren K, et al. Human papilloma virus infection and skin cancer in renal allograft recipients. Lancet. 1989;1:124-129.
- Tanigaki T, Kanda R, Sato K. Epidermodysplasia verruciformis (L-L, 1922) in a patient with systemic lupus erythematosus. Arch Dermatol Res. 1986;278:247-248.
- Holmes C, Chong AH, Tabrizi SN, et al. Epidermodysplasia verruciformis-like syndrome in association with systemic lupus erythematosus. Australas J Dermatol. 2009;50:44-47.
- Gross G, Ellinger K, Roussaki A, et al. Epidermodysplasia verruciformis in a patient with Hodgkin’s disease: characterization of a new papillomavirus type and interferon treatment. J Invest Dermatol. 1988;91:43-48.
- Fernandez KH, Rady P, Tyring S, et al. Acquired epidermodysplasia verruciformis in a child with atopic dermatitis [published online September 3, 2012]. Pediatr Dermatol. 2014;31:400-402.
- Hultgren TL, Srinivasan SK, DiMaio DJ. Epidermodysplasia verruciformis occurring in a patient with human immunodeficiency virus: a case report. Cutis. 2007;79:307-311.
- Kunishige JH, Hymes SR, Madkan V, et al. Epidermodysplasia verruciformis in the setting of graft-versus-host disease. J Am Acad Dermatol. 2007;57(5 suppl):S78-S80.
- Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289-297.
- Moloney FJ, Keane S, O’Kelly P, et al. The impact of skin disease following renal transplantation on quality of life. Br J Dermatol. 2005;153:574-578.
- Tanigaki T, Endo H. A case of epidermodysplasia verruciformis (Lewandowsky-Lutz, 1922) with skin cancer: histopathology of malignant cutaneous changes. Dermatologica. 1984;169:97-101.
- Morrison C, Eliezri Y, Magro C, et al. The histologic spectrum of epidermodysplasia verruciformis in transplant and AIDS patients. J Cutan Pathol. 2002;29:480-489.
- Majewski S, Jabło´nska S. Epidermodysplasia verruciformis as a model of human papillomavirus-induced genetic cancer of the skin. Arch Dermatol. 1995;131:1312-1318.
- Jacyk WK, De Villiers EM. Epidermodysplasia verruciformis in Africans. Int J Dermatol. 1993;32:806-810.
- Gubinelli E, Posteraro P, Cocuroccia B, et al. Epidermodysplasia verruciformis with multiple mucosal carcinomas treated with pegylated interferon alfa and acitretin. J Dermatolog Treat. 2003;14:184-188.
- Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a. J Am Acad Dermatol. 2001;45:296-299.
- Haas N, Fuchs PG, Hermes B, et al. Remission of epidermodysplasia verruciformis-like skin eruption after highly active antiretroviral therapy in a human immunodeficiency virus-positive patient. Br J Dermatol. 2001;145:669-670.
- Karrer S, Szeimies RM, Abels C, et al. Epidermo-dysplasia verruciformis treated using topical 5-aminolaevulinic acid photodynamic therapy. Br J Dermatol. 1999;140:935-938.
- Dyall-Smith D, Trowell H, Dyall-Smith ML. Benign human papillomavirus infection in renal transplant recipients. Int J Dermatol. 1991;30:785-789.
- Lutzner MA, Orth G, Dutronquay V, et al. Detection of human papillomavirus type 5 DNA in skin cancers of an immunosuppressed renal allograft recipient. Lancet. 1983;2:422-424.
- Lutzner M, Croissant O, Ducasse MF, et al. A potentially oncogenic human papillomavirus (HPV-5) found in two renal allograft recipients. J Invest Dermatol. 1980;75:353-356.
- Androphy EJ, Dvoretzky I, Lowy DR. X-linked inheritance of epidermodysplasia verruciformis. genetic and virologic studies of a kindred. Arch Dermatol. 1985;121:864-868.
- Lutzner MA. Epidermodysplasia verruciformis. an autosomal recessive disease characterized by viral warts and skin cancer. a model for viral oncogenesis. Bull Cancer. 1978;65:169-182.
- Ramoz N, Rueda LA, Bouadjar B, et al. Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat Genet. 2002;32:579-581.
- Rüdlinger R, Smith IW, Bunney MH, et al. Human papillomavirus infections in a group of renal transplant recipients. Br J Dermatol. 1986;115:681-692.
- Kawai K, Egawa N, Kiyono T, et al. Epidermodysplasia-verruciformis-like eruption associated with gamma-papillomavirus infection in a patient with adult T-cell leukemia. Dermatology. 2009;219:274-278.
- Barr BB, Benton EC, McLaren K, et al. Human papilloma virus infection and skin cancer in renal allograft recipients. Lancet. 1989;1:124-129.
- Tanigaki T, Kanda R, Sato K. Epidermodysplasia verruciformis (L-L, 1922) in a patient with systemic lupus erythematosus. Arch Dermatol Res. 1986;278:247-248.
- Holmes C, Chong AH, Tabrizi SN, et al. Epidermodysplasia verruciformis-like syndrome in association with systemic lupus erythematosus. Australas J Dermatol. 2009;50:44-47.
- Gross G, Ellinger K, Roussaki A, et al. Epidermodysplasia verruciformis in a patient with Hodgkin’s disease: characterization of a new papillomavirus type and interferon treatment. J Invest Dermatol. 1988;91:43-48.
- Fernandez KH, Rady P, Tyring S, et al. Acquired epidermodysplasia verruciformis in a child with atopic dermatitis [published online September 3, 2012]. Pediatr Dermatol. 2014;31:400-402.
- Hultgren TL, Srinivasan SK, DiMaio DJ. Epidermodysplasia verruciformis occurring in a patient with human immunodeficiency virus: a case report. Cutis. 2007;79:307-311.
- Kunishige JH, Hymes SR, Madkan V, et al. Epidermodysplasia verruciformis in the setting of graft-versus-host disease. J Am Acad Dermatol. 2007;57(5 suppl):S78-S80.
- Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289-297.
- Moloney FJ, Keane S, O’Kelly P, et al. The impact of skin disease following renal transplantation on quality of life. Br J Dermatol. 2005;153:574-578.
- Tanigaki T, Endo H. A case of epidermodysplasia verruciformis (Lewandowsky-Lutz, 1922) with skin cancer: histopathology of malignant cutaneous changes. Dermatologica. 1984;169:97-101.
- Morrison C, Eliezri Y, Magro C, et al. The histologic spectrum of epidermodysplasia verruciformis in transplant and AIDS patients. J Cutan Pathol. 2002;29:480-489.
- Majewski S, Jabło´nska S. Epidermodysplasia verruciformis as a model of human papillomavirus-induced genetic cancer of the skin. Arch Dermatol. 1995;131:1312-1318.
- Jacyk WK, De Villiers EM. Epidermodysplasia verruciformis in Africans. Int J Dermatol. 1993;32:806-810.
- Gubinelli E, Posteraro P, Cocuroccia B, et al. Epidermodysplasia verruciformis with multiple mucosal carcinomas treated with pegylated interferon alfa and acitretin. J Dermatolog Treat. 2003;14:184-188.
- Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a. J Am Acad Dermatol. 2001;45:296-299.
- Haas N, Fuchs PG, Hermes B, et al. Remission of epidermodysplasia verruciformis-like skin eruption after highly active antiretroviral therapy in a human immunodeficiency virus-positive patient. Br J Dermatol. 2001;145:669-670.
- Karrer S, Szeimies RM, Abels C, et al. Epidermo-dysplasia verruciformis treated using topical 5-aminolaevulinic acid photodynamic therapy. Br J Dermatol. 1999;140:935-938.
Practice Points
- Acquired epidermodysplasia verruciformis (EDV) is a rare complication of iatrogenic immuno-suppression in the setting of solid organ transplantation.
- Patients with EDV should be counseled to avoid exposure to UV radiation to reduce the risk formalignant transformation.