User login
“Non‐Heart‐Beating” Organ Donation
In April 2003 the Health Resources and Services Administration of the U.S. Department of Health and Human Services (DHHS) announced the formation of the Organ Donation Breakthrough Collaborative (ODBC).1 The Organ Donation Breakthrough Collaborative created 58 national donation service areas (DSAs) to organize the transplant community across the United States. Each of the 58 organ procurement organizations (OPOs) is joined to a regional transplant center or centers and donor hospitals to form a DSA. The ODBC's goal is to achieve a cadaveric organ donation rate of 75% or higher from hospitals within each DSA.2
A requirement for organ donation from patients facing imminent or cardiac death has been introduced to increase the supply of transplantable organs and shorten the waiting time for transplantation candidates.35 This type of organ donation represents a significant source of organs required for future expansion of transplantation practice in the United States. The requirement for donation in imminent or cardiac death is implemented through the collaboration of the Advisory Committee on Organ Transplantation of the DHHS (Table 1), the Centers for Medicare & Medicaid Services (CMS), and the Joint Commission on Accreditation of Healthcare Organizations (JCAHO).3, 57 The organ donor pool of those facing imminent or cardiac death has also been expanded to include neurologically intact patients who may not fulfill brain death criteria before withdrawal of life support.4, 8, 9
| State law (year)* | |
|---|---|
| |
| Uniform Anatomical Gift Act (1968) | Any 18‐year‐old with a sound mind may donate his or her body after death to be used for medical research or as a source of transplantable tissues and organs and barring others from overriding a donor's decision to make an anatomical gift. |
| Amendment (1987) | Minors who can apply for a driver's license are empowered to make anatomical gifts, but either parent can revoke the gift if the minor dies before the age of 18. |
| Revision (2006) | Declaration of a gift does not require any witnesses. |
| Amendment (2007) | Document of a gift or donor registry is sufficient for the removal of organs, which means an OPO does not need consent of the spouse or the family. |
| Enables an OPO to gain access to documents of gifts in donor registries, medical records, and records of a state motor vehicle department. | |
| Facilitates donations by expanding the list of those who may make an anatomical gift for another individual who has not declared a preference for or against donation. | |
| Permits removal of organs by medical personnel without explicit consent from a potential donor or from a relative of the donor, so long as the appropriate medical personnel or authorities have made a reasonable effort to discover any objection by the donor or the donor's family. | |
| Require hospitals to notify an OPO or third party designated by the OPO of an individual whose death is imminent or who has died in the hospital in order to increase donation opportunity. | |
| Gives an OPO the right to inspect a patient's medical records. | |
| Measures necessary to ensure the medical suitability of a part not be withdrawn while an examination is being made to determine whether an individual who has been referred to OPO has a part that could be the subject of an anatomical gift. If, following such an examination, it is determined by the OPO that the individual has a part that could be the subject of an anatomical gift, the individual is a prospective donor under this act unless the individual had signed a refusal. | |
| Forbids the buying and selling of organs. | |
| Measures necessary to ensure the medical suitability of an organ for transplantation or therapy may not be withheld or withdrawn from a prospective donor who has an advance health‐care directive or declaration unless the directive or declaration expressly provides to the contrary. The section presumes that for prospective donors the desire to save lives by making an anatomical gift trumps the desire to have life support systems withheld or withdrawn. Individuals who desire to overcome this presumption can do so by express language in their advance health‐care directive or declaration. | |
| Uniform Determination of Death Act (1981) | An individual who has sustained either (1) irreversible cessation of circulatory and respiratory functions, or (2) irreversible cessation of all functions of the entire brain including the brain stem is dead. A determination of death must be made in accordance with accepted medical standards. |
| Federal laws | |
| National Organ Transplant Act (1984) | Calls for a unified transplant network to be operated by a private, nonprofit organization under federal contract and the establishment of a Task Force in Organ Procurement and Transplantation and an Organ Procurement and Transplantation Registry. |
| Public Health and Welfare Act Title 42 USC (1999) | |
| Section 273 | Establishes guidelines to be a qualified OPO that can receive federal grants. |
| Section 274 | Establishes the OPTN & Scientific Registry for Transplantation. |
| Federal regulations | |
| Title Code 42 CFR Part 121 (1999) | Lists regulations of the OPTN final rule. |
| Explains the OPTN structure. | |
| Lists the policy that the OPTN board of directors is responsible for developing. | |
| Explains rules that an OPTN member must obey when including a person on the organ waiting list. | |
| Describes the requirements and tests for determining the suitability of donated organs. | |
| Explains how the OPTN identifies an organ recipient, allocates the organ, and transports it to the recipient. | |
| Describes how the board of directors should develop allocation policies to guarantee they are both efficient and just and allocation performance goals to ensure the best possible use and most equitable allocation of organs. | |
| Lists designated transplant program requirements. | |
| Describes how the HHS conducts reviews and evaluations and enforces rules. | |
| Describes the recording and reporting requirements of the various groups involved in the transplantation process. | |
| Establishes the Advisory Committee on Organ Transplantation (ACOT), which advises the HHS secretary on organ donation, procurement, allocation, and transplantation The HHS secretary may ask for ACOT's opinion of proposed OPTN policies. | |
| Title Code 42 CFR Parts 413, 441, 486 and 498 (2006) | Lists regulations of the OPO final rule. |
| Establishes new conditions for coverage for OPOs that include multiple new outcome and process performance measures based on organ donor potential and other related factors in each donation service area of qualified OPOs. | |
The President's Council on Bioethics independently evaluated the issues surrounding deceased organ donation and procurement.10 The President's Council on Bioethics has expressed major concerns about several issues pertinent to cardiac or imminent death organ donation that have not been addressed explicitly by the bodies that have made recommendations for reforming or expanding that type of organ donation in the United States. The debate on organ procurement in imminent or cardiac death has come to the forefront because of doubts about its ethical appropriateness and acceptance within the medical profession and the community at large. This review focuses on the serious issues related to organ procurement from patients facing imminent or cardiac death.
Organ Procurement and the Dead Donor Rule
Organ procurement is only permitted when the donor is already dead (ie, the dead donor rule), and the act of organ recovery cannot have been the immediate act to cause that death.10, 11 There are 2 criteria for death as defined in the Uniform Determination of Death Act (UDDA; Table 1): an individual who has sustained irreversible cessation of either (1) circulatory and respiratory functions or (2) all functions of the entire brain, including the brain stem, is considered dead and this determination of death must have been made in accordance with accepted medical standards.12 When organs are procured from an individual in whom all brain function has ceased but normal cardiac pump activity is continuing, it is referred to as heart‐beating organ donation. Organ procurement after cessation of cardiac pump activity and cardiorespiratory functions is referred to as non‐heart‐beating organ donation (NHBOD). Organ procurement from an individual in imminent or cardiac death is considered NHBOD.
Non‐heart‐beating organ donors can be neurologically intact and do not fulfill the brain death criterion prior to cessation of cardiac pump activity. In response to this dilemma, the University of Pittsburgh Medical Centre developed a protocol for donation of organs that permitted their procurement from patients who were pulseless and apneic for 2 minutes and did not fulfill brain criteria and who had previously given consent for organ donation.13 Because it is uncertain if cessation of cardiorespiratory function is irreversible after only a short time, the Institute of Medicine (IOM) extended the time required for pulselessness and apnea from 2 to 5 minutes before permitting organ procurement.14 Waiting longer than 5 minutes for the determination of death would compromise the quality of procured organs because of warm ischemia time and would influence the functioning of grafts in transplant recipients.
One of the pivotal assumptions for NHBOD acceptance is that 5 minutes of pulselessness and apnea eliminates the possibility that the procurement process itself could be the cause of death and fulfills the dead donor rule.14, 15 The cardiorespiratory death criteria were derived from observations that did not evaluate delayed autoresuscitation (spontaneous return of circulation) or simultaneous cessation of brain electrical activity (as recorded in brain death).1618 The death criteria applied for organ procurement must also comply with the irreversibility requirement of the UDDA.11, 12
The true incidence, temporal characteristics, and predictors of autoresuscitation in humans remain unknown because of underreporting in the literature. However, there have been case reports of autoresuscitation with return of neurologic function (also called the Lazarus phenomenon) after 10 minutes of cardiac asystole.19, 20 Maleck et al. and Adhiyaman et al. described autoresuscitation 5 minutes or longer after cardiorespiratory arrest in 44% and 50% of the published case reports, respectively.19, 20 Although cardiac asystole leads to the loss of arterial pulse pressure, circulatory arterial mean pressure is maintained in diastole by arteriolar vasomotor tone. The relaxation (diastole) phase systemic arterial to venous pressure gradient provides the perfusion pressure for vital organs and the spontaneous return of circulation after circulatory arrest.21 It is likely that autoresuscitation occurs because of the persistence of circulatory vasomotor tone after cessation of cardiac function. The time course of systemic vascular tone after circulatory arrest has not been well characterized in humans. However, the IOM criteria did not account for the incidence of delayed autoresuscitation in humans even though the Maastricht protocol (developed by the University of Zurich, Zurich, Switzerland) acknowledged this phenomenon and required at least 10 minutes to elapse after cardiorespiratory arrest before starting organ procurement.22 The 10‐minute waiting time did not compromise the quality of the organs procured.
The cardiorespiratory death criteria for organ procurement also ignore cardiac electrical activity (such as pulseless electrical activity or ventricular fibrillation) on an electrocardiogram. Research with cardiac ultrasonography and indwelling arterial catheters confirms that pulseless cardiac electrical activity can be associated with cardiac mechanical contractions, although these contractions are too weak to be detected by blood pressure monitoring.23 The presence of cardiac electrical activity on an electrocardiogram can also increase the likelihood that delayed autoresuscitation will occur.19, 20 Furthermore, whether there is brain electrical activity or neurologic function when cardiac electrical activity is still observed on an electrocardiogram remains unknown.24 It can be argued that donors who have already suffered severe neurologic injury cannot have meaningful neurologic function at the time of cardiorespiratory death. However, the presence of brain activity becomes relevant for organ donors with intact neurologic and brain function prior to cardiorespiratory arrest when only cardiorespiratory criteria for organ procurement are being used.4, 8, 9
In situ circulatory support with extracorporeal perfusion in organ donors has also refuted that cardiorespiratory arrest for 5 minutes fulfills the UDDA requirement because reversibility can occur during the procurement process. In situ extracorporeal perfusion is initiated 5 minutes after cardiorespiratory arrest of donors in order to preserve organs for procurement.25 Coronary and cerebral reperfusion can lead to the return of cardiac and neurologic functions (also called reanimation) of donors during the procurement process. Mechanical occlusion of the aortic arch and pharmacological agents are required to suppress donor reanimation during organ procurement.26 Martin et al. documented that in situ extracorporeal perfusion returned full neurologic and cardiac function 25 minute after cardiorespiratory arrest that occurred outside the hospital.27 Similar observations of full neurologic recovery and survival to hospital discharge were reported after in situ extracorporeal perfusion for in‐hospital cardiorespiratory arrest.28 These observations confirm that the time required for irreversible loss of neurologic function after cessation of circulation is much longer than the 25 minutes of cardiorespiratory arrest required to begin the process of organ procurement in NHBOD.
The incidence of donor reanimation during procurement is unknown because its reporting violates the dead donor rule and can create legal concerns.11 It can be argued that reanimation of organ donors is irrelevant because it does not mean survival. However, the occurrence of reanimation invalidates the premise that the cardiorespiratory criteria for organ procurement comply with the uniform determination of death. Others have accepted the inaccuracy of these criteria for determining death for procurement of organs from deceased donors and proposed abandoning the dead donor rule in order to permit recovery of transplantable organs before death.29
In the face of the uncertainty in determining death and in response to a media and marketing campaign by organ procurement organizations (OPOs) to promote public enrollment in deceased organ donation, the transplant community renamed NHBOD cardiac death organ donation.30, 31 The use of the term cardiac death is scientifically inaccurate and perhaps misleading. This term is used to denote the cessation of circulation and cardiac pump activity. The term cardiac death can be misinterpreted as meaning the heart has irreversibly ceased at the time of procurement, thus contradicting the scientific evidence for spontaneous resumption of cardiac function and autoresuscitation.19, 20 Alternatively, the use of this term can falsely imply that neurologic activity or brain stem function has ceased irreversibly after loss of cardiac activity, when scientific evidence suggests that brain stem function can remain after cardiac arrest.32
Consent for Organ Donation
Several state laws and federal regulations have been enacted to ensure that organ donation and transplantation practice comply with the ethical and legal standards of society (Table 1). The current regulations require hospitals across the United States to provde regional OPOs with early notification of all patients whose deaths are imminent before life support has been withdrawn so that discussion of organ donation with surrogate decision makers can be initiated independently and consent obtained.3, 5, 9 The Organ Donation Breakthrough Collaborative has set a goal of each OPO accomplishing a target organ donation rate of 75% or higher at local hospitals within an assigned donation service area (DSA).1, 2, 5 The financial and administrative incentives for the OPO to achieve that target organ donation rate have introduced undisclosed conflict within the donation consent process.33 Self‐serving bias of OPOs can influence whether pertinent information necessary for surrogate decision makers to provide informed consent is disclosed.34 As an example of this bias, alternative options for care and palliation may be discussed with surrogate decision makers with less enthusiasm than are the benefits and altruistic notion of organ donation. In obtaining donation consent, OPOs often avoid disclosing details of perimortem interventions performed on donors that are required for successful procurement of transplantable organs.10, 34, 35 After receiving consent for organ donation, OPO staffs also assume the responsibility of planning donor medical care and treatment pathways essential for maintaining organ viability and of preparing for subsequent procurement.5, 36 In essence, the care of the dying patient is guided by a team whose primary interest is the preservation of organs until procurement has been accomplished.
The Uniform Anatomical Gift Act (UAGA) assigns explicit priority to the donor's expressed intent so that consent for organ donation becomes irrevocable and does not require the consent or concurrence of any person after the donor's death (Table 1).9, 37 The donor's authorization to donate, recorded on an organ donor card, the individual's driver's license, or a donor registry, becomes a legally binding advance directive. The UAGA amendment enables OPOs to procure organs without family consent and in certain instances after family refusal to donate.37
Other consent options for organ donation from deceased donors have been proposed to maximize OPO recovery of transplantable organs in the United States (Table 2).8 The IOM has considered presumed consent for organ donation as a favorable option.8 Presumed consent means the default option is consent to donation, that if an individual has not expressly rejected donation, that individual is considered to have consented to organ donation. Legislative enactment of presumed consent enables OPOs to avoid the potential for surrogates to deny consent for donation, thus increasing the pool of future organ donors. The revised UAGA replaces nondonation with the intent to donate organs as the default option. In the default option, all measures necessary to ensure the medical suitability of an organ for transplantation can not be withheld or withdrawn until the OPO has determined medical suitability of the individual as a prospective donor (Table 1). The default option overrides the expression of intent in a declaration or advance health‐care directives not to have life prolonged by withholding or withdrawing life support system unless the individual has expressed refusal of donation (Tables 1 and 2). The revised UAGA presumes that for prospective donors the desire to save lives by making an anatomical gift trumps the desire to have life support systems withheld or withdrawn. Mandated consent (or conscription) has also been proposed for recovery of cadaveric organs (Table 2).38 Under mandated consent, consent for organ donation is automatic from all deceased individuals; therefore, OPOs would not require or request consent because removal of all needed transplantable cadaveric organs would be compulsory. OPO staffs would no longer have to discuss organ procurement from potential donors with family members or other surrogates. An alternative form of donation consent is mandated choice, which requires each individual to decide in advance either to agree to organ donation or to refuse it. Mandated choice is the IOM's least favorite option because it would require extensive public informational programs on organ donation to facilitate individual choices and decision making (Table 2).8
| Type | Description |
|---|---|
| Requested (expressed) consent | An individual is asked to voluntarily agree to organ donation. |
| Presumed (implied) consent | Unless an individual has expressly refused to donate organs, the default option is agreement to donate organs. |
| Mandated consent (conscription) | An individual is not required to decide on organ donation before death, and there is an automatic right to procure organs from any and all deceased individuals. |
| Mandated choice | An individual must choose between 2 options before death: agreement or refusal to donate organs. |
End‐of‐Life Care
Quality of end‐of‐life (EOL) care for an organ donor, as for any individual whose treatment is being withdrawn, is considered the highest priority of care and must not be compromised by the donation process. Yet no studies have investigated the impact of organ donation on the quality of EOL care in NHBOD.35 Previous reports have criticized the quality of EOL care offered to dying patients in intensive care units (ICUs).39, 40 Many of these patients are undergoing withdrawal of life support in anticipation of death and are considered candidates for NHBOD. The Robert Wood Johnson Foundation (RWJF) Critical Care End‐Of‐Life Peer Workgroup developed 53 EOL quality indicators to standardize and measure the quality of EOL care.41 These quality indicators, organized in 7 domains, focus on delivering patient‐ and family‐centered care and facilitating a good death experience in the ICU (Table 3).
| Domains of comprehensive quality indicators of EOLC (n = 53)41 | Abbreviated quality indicators of EOLC (n = 18)46 |
|---|---|
| |
| Patient and family‐centered decision making (n = 13) | |
| Recognize the patient and family as the unit of care | |
| Assess the patient's and family's decision‐making style and preferences | |
| Address conflicts in decision making within the family | |
| Assess, together with appropriate clinical consultants, the patient's capacity to participate in decision making about treatment and document assessment | Assessment of the patient's decisional capacity |
| Initiate advance care planning with the patient and family | |
| Clarify and document the status of the patient's advance directive | Documentation of the presence and, if present, contents of advance directives |
| Identify the healthcare proxy or surrogate decision maker | Documentation of a surrogate decision maker within 24 hours of admission |
| Clarify and document resuscitation orders | |
| Assure patients and families that decision making by the health care team will incorporate their preferences | |
| Follow ethical and legal guidelines for patients who lack both capacity to make decisions and a surrogate decision maker | |
| Establish and document clear, realistic, and appropriate goals of care in consultation with the patient and family | Documentation of the goals of care |
| Help the patient and family assess the benefits and burdens of alternative treatment choices as the patient's condition changes | |
| Forgo life‐sustaining treatments in a way that ensures patient and family preferences are elicited and respected | |
| Communication within the team and with patients and families (n = 10) | |
| Meet as interdisciplinary team to discuss the patient's condition, clarify goals of treatment, and identify the patient's and family's needs and preferences | Documentation of a timely interdisciplinary clinicianfamily conference |
| Address conflicts among the clinical team before meeting with the patient and/or family | |
| Utilize expert clinical, ethical, and spiritual consultants when appropriate | |
| Recognize the adaptations in communication strategy required for patients and families according to the chronic versus acute nature of the illness, cultural and spiritual differences, and other influences | |
| Meet with the patient and/or family on a regular basis to review patient's status and to answer questions | Documentation of timely physician communication with the family |
| Communicate all information to the patient and family, including distressing news, in a clear, sensitive, unhurried manner and in an appropriate setting | |
| Clarify the patient's and family's understanding of the patient's condition and goals of care at the beginning and end of each meeting | |
| Designate primary clinical liaison(s) who will communicate with the family daily | |
| Identify a family member who will serve as the contact person for the family | |
| Prepare the patient and family for the dying process | |
| Continuity of care (n = 3) | |
| Maximize continuity of care across clinicians, consultants, and settings | |
| Orient new clinicians to the status of the patient and family | Transmission of key information with transfer of the patient out of the ICU |
| Policy for continuity of nursing services | |
| Prepare the patient and/or family for a change of clinician(s) and introduce new clinicians | |
| Emotional and practical support for patients and families (n = 8) | |
| Elicit and attend to the needs of the dying person and his/her family | |
| Distribute written material (booklet) for families that includes orientation to the ICU environment and open visitation guidelines, logistical information (nearby hotels, banks, restaurants, directions), listings of financial consultation services, and bereavement programs and resources | Open visitation policy for family members |
| Facilitate strengthening of patientfamily relationships and communication | |
| Maximize privacy of the patient and family | |
| Value and support the patient's and family's cultural traditions | |
| Arrange for social support for patients who do not have family or friends | Documentation that psychosocial support has been offered |
| Distribute written material (booklet) containing essential logistical information and listings of financial consultation services and bereavement support programs/resources | |
| Support family members through the patient's death and their bereavement | |
| Symptom management and comfort care (n = 10) | |
| Emphasize the comprehensive comfort care that will be provided to the patient rather than the removal of life‐sustaining treatments | |
| Institute and use uniform quantitative symptom assessment scales appropriate for communicative and noncommunicative patients on a routine basis | Documentation of pain assessment |
| Documentation of respiratory distress assessment | |
| Standardize and follow best clinical practices for symptom management | Protocol for analgesia/sedation in terminal withdrawal of mechanical ventilation |
| Appropriate medications available during withdrawal of mechanical ventilation | |
| Use nonpharmacologic as well as pharmacologic measures to maximize comfort as appropriate and desired by the patient and family | |
| Reassess and document symptoms following interventions | Documentation of pain management |
| Documentation of respiratory distress management | |
| Know and follow best clinical practices for withdrawing life‐sustaining treatments to avoid patient and family distress | |
| Eliminate unnecessary tests and procedures (laboratory work, weights, routine vital signs) and only maintain intravenous catheters for symptom management when life support is being withdrawn | |
| Minimize noxious stimuli (monitors, strong lights) | |
| Attend to patient's appearance and hygiene | |
| Ensure family's and/or clinician's presence so the patient is not dying alone | |
| Spiritual support for patients and families (n = 3) | |
| Assess and document spiritual needs of the patient and family on an ongoing basis | Documentation that spiritual support was offered |
| Encourage access to spiritual resources | |
| Elicit and facilitate spiritual and cultural practices that the patient and family find comforting | |
| Emotional and organizational support for ICU clinicians (n = 6) | |
| Support health care team colleagues caring for dying patients | Opportunity to review experience of caring for dying patients by ICU clinicians |
| Adjust nursing staff and medical rotation schedules to maximize continuity of care providers for dying patients | |
| Communicate regularly with interdisciplinary team about goals of care | |
| Establish a staff support group, based on the input and needs of ICU staff and experienced group facilitators, and integrate meeting times into the routine of the ICU | |
| Enlist palliative care experts, pastoral care representatives, and other consultants to teach and model aspects of EOLC | |
| Facilitate rituals for the staff to mark the death of patients | |
| Strategies to improve EOLC and provide a good death42, 43 | |
| EOL care quality monitoring | |
| Making environmental changes to promote dying with dignity | |
| Managing patients' pain and discomfort | |
| Knowing and following patients' wishes for end‐of‐life care | |
| Bereavement program or service | |
| Regular meetings of senior ICU physician and nurse with patients' families | |
| Training of ICU clinicians in end‐of‐life communication skills | |
| Role modeling and supervision of trainees by clinicians experienced in end‐of‐life care | |
| Formal mechanism for emotional support of staff caring for dying patients | |
| Access to palliative care consultants | |
| Training of ICU clinicians in symptom management | |
| Scheduling staff to promote continuity of care for dying patients | |
| Formal system for scaled assessment and charting of patients' symptoms | |
| Method to help resolve differences about appropriate care goals | |
| Resources to accommodate diversity among patients/families at the end of life | |
| Access to clinical ethics consultants | |
| Regular pastoral care visits to the ICU | |
In a subsequent U.S. survey, the Critical Care Peer Workgroup of the Promoting Excellence in End‐of‐Life Care Project reported that more than 75% of ICUs were still not monitoring the quality of EOL care.42 The survey also identified multiple barriers to optimal EOL care found in most ICUs. The study group proposed several strategies to overcome these barriers and improve the quality of EOL care (Table 3).42, 43 It can also be inferred from the survey findings that most ICUs are unprepared and lack the necessary tools to appropriately inform patients and families of the trade‐off in EOL care for NHBOD. The President's Council for Bioethics has also warned that NHBOD can transform EOL care from a peaceful dignified death into a profanely high‐tech death experience for donors' families.10
Several aspects of medical care that are neither palliative nor beneficial are performed for donor management for NHBOD and can explain the feared transformation of the death experience. The revised UAGA reaffirms that all measures necessary to ensure the medical suitability of an organ for transplantation cannot be withheld or withdrawn from the prospective donor and overrides the expression of intent by a prospective donor in either a declaration or advance health‐care directive not to have life prolonged by use of life support systems (Table 1).9 The 2007 amendment to revised UAGA section 21 recognizes the conflict between all measures necessary to ensure organs viability for transplantation and appropriate EOL care and requires the attending physician and OPO to resolve the conflict with the prospective donor or surrogate decision‐maker.9
OPOs apply donor management critical pathways to potential organ donors in order to maintain organ viability for successful execution of organ procurement.36 The University of Wisconsin developed a protocol and an evaluation tool to determine the eligibility of potential candidates for NHBOD.44 The protocol entails temporary discontinuation of mechanical ventilation for a trial of spontaneous respiration lasting up to 10 minutes to determine the likelihood of cardiorespiratory death within 60‐90 minutes of the withdrawal of life support. Those patients predicted by the University of Wisconsin evaluation tool to survive a longer time are not candidates for NHBOD and are transferred to palliative care. Those patients who meet the necessary criteria of the University of Wisconsin evaluation tool become candidates for NHBOD and undergo additional antemortem testing, invasive vascular instrumentation, and infusion of medications essential for organ preservation.36 The instrumentation and medications used for organ preservation can also expedite death on withdrawal of life support.45 Other interventions (such as circulatory support with invasive and noninvasive devices, extracorporeal perfusion and oxygenation, endotracheal reintubation, mechanical ventilation, and bronchoscopy) are performed when cardiorespiratory death is pronounced in order to maintain organ viability and can inadvertently reanimate the donor during the procurement process.26
The process of obtaining donation consent and subsequent donor management protocols for NHBOD deviate from more than 60% of the RWJF quality indicators recommended for optimal EOL care.35, 36, 41 Therefore, NHBOD can have a profound impact on the quality of EOL care. There has been a recent proposal to abbreviate the original RWJF quality indicators to include 14 of the 53 (26%) original quality indicators described for optimal EOL care in the ICU (Table 3).46 Many of the quality indicators expected to be negatively affected by NHBOD are not included in the proposal for an abbreviated list. There has been a concern that the application of an abbreviated rather than a comprehensive metrics for EOL care can portray an incomplete assessment and perhaps misinform donors and their families about the potential trade‐off in EOL care. The President's Council on Bioethics has emphasized that comprehensive evaluation of the quality of EOL care is an ethical imperative so that families can decide if the trade‐off is acceptable for organ donation.10 Deciding to donate organs at the end of life can be stressful for many families, and therefore they must be fully informed of the possible consequences. Posttraumatic stress disorders, anxiety, depression, and decreased quality of life have been reported in the deceased's family members who shared in stressful EOL decisions.47 Posttraumatic stress disorders have been reported in family members of deceased organ donors.48 Organ‐focused behavior by professionals requesting consent for organ donation and ambivalent decision making by family members appeared to increase the risk of relatives of deceased donors subsequently developing traumatic memories and stress disorders. The processes required for successful accomplishment of donation consent and subsequent organ recovery can interfere with many of the interventions that lessen the burden of bereavement of relatives of ICU decedents.49
The variability in decision making by health care providers about medical futility and EOL care has been given as a reason for concern about the implementation of NHBOD.50 The variability of EOL practice raises the possibility of conflicted decision making on medical futility within institutions that have transplant programs.50 Ethical conflicts and moral distress have been reported among health care providers who were directly involved in organ procurement in NHBOD.51 The pressure to recover transplantable organs from NHBOD candidates has been associated with health care professionals' perception of euthanasia and premature determination of medical futility and withdrawal of life support. The long‐term psychological impact of NHBOD practice on caregivers, health care providers, and professionals remains unknown.
CONCLUSIONS
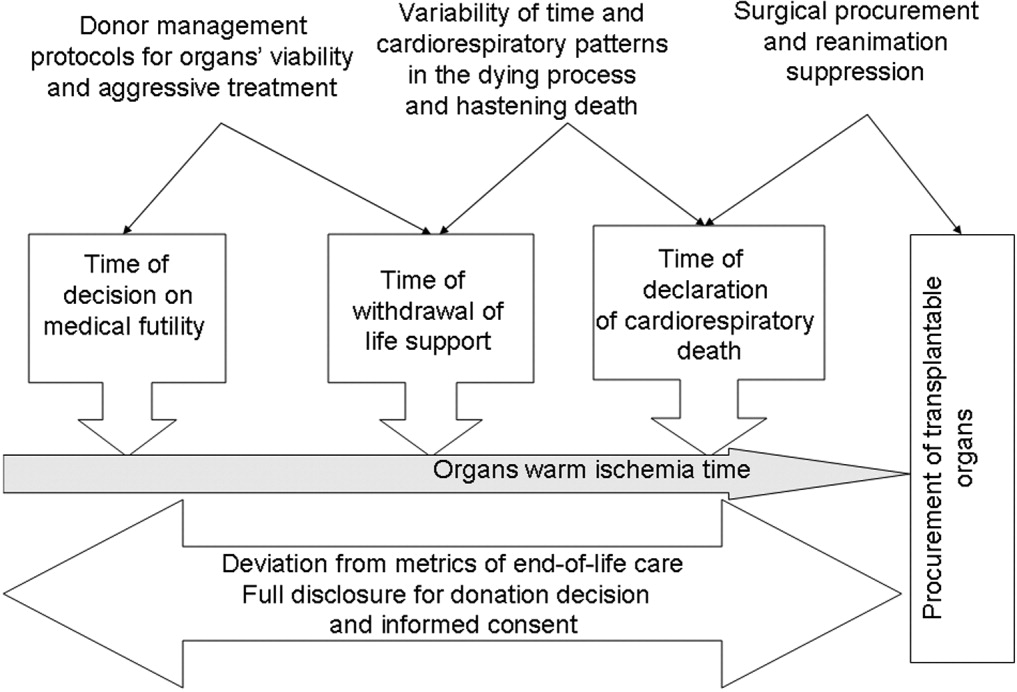
In conclusion, NHBOD influences medical care at critical time points to maximize the procurement of transplantable organs and minimize their warm ischemia time with negative consequences on the EOL care for the prospective donors and their families (see Figure 1).
Mandatory implementation of NHBOD in the face of difficulties surrounding the quality of EOL care for donors raises concern across the medical profession and community. There is a need for better scientific validation of the timing of organ procurement to ensure that organ recovery is not the irreversible event defining death in NHBOD. The desire of OPOs or their affiliates to maximize recovery of transplantable organs introduces self‐serving bias into obtaining consent for organ donation and violates the basic tenet of true informed consent. The use of comprehensive quality indicators of EOL care will help to determine the impact of NHBOD on donors, families, caregivers, and health care providers.
- ,,, et al.Organ donation and utilization, 1995‐2004: Entering the collaborative era.Am J Transplant.2006;6:1101–1110.
- U.S. Department of Health and Human Servcies—Organ Donation and Transplant Breakthrough Collaborative. Measurement strategy: Organ Donation and Transplant Breakthrough Collaborative. Available at: http://www.organdonationnow.org/. Accessed December 15,2006.
- U.S. Department of Health and Human Services Advisory Committee on Organ Transplantation. Consensus Recommendations to the HHS Secretary. Available at: http://www.organdonor.gov/research/acot.htm. Accessed January 30,2007.
- ,,, et al.Report of a National Conference on Donation after Cardiac Death.Am J Transplant.2006;6:281–291.
- Centers for Medicare and Medicaid Services, Department of Health and Human Services.Medicare and Medicaid Programs. Conditions for coverage for organ procurement organizations (OPOs); final rule.Federal Register.2006;71:30981–31054.
- [JCAHO] Joint Commission on Accreditation of Healthcare Organizations. Health care at the crossroads: strategies for narrowing the organ donation gap and protecting patients. Available at: http://www.jointcommission.org/PublicPolicy/organ_donation.htm. Accessed January 30,2007.
- [JCAHO] Joint Commission on Accreditation of Healthcare Organizations.Revisions to Standard LD.3.110.Jt Comm Perspect.2006;26:7.
- Committee on Increasing Rates of Organ Donation‐Board on Health Sciences Policy‐Institute of Medicine.Organ Donation: Opportunities for Action.Washington, DC:National Academies Press;2006.
- National Conference of Commissioners on Uniform State Laws. Revised Uniform Anatomical Gift Act (2006) and Amendment to Section 21 (2007). http://www.law.upenn.edu/bll/ulc/uaga/2006final.htm and http://www.anatomicalgiftact.org/DesktopDefault.aspx?tabindex=0
In April 2003 the Health Resources and Services Administration of the U.S. Department of Health and Human Services (DHHS) announced the formation of the Organ Donation Breakthrough Collaborative (ODBC).1 The Organ Donation Breakthrough Collaborative created 58 national donation service areas (DSAs) to organize the transplant community across the United States. Each of the 58 organ procurement organizations (OPOs) is joined to a regional transplant center or centers and donor hospitals to form a DSA. The ODBC's goal is to achieve a cadaveric organ donation rate of 75% or higher from hospitals within each DSA.2
A requirement for organ donation from patients facing imminent or cardiac death has been introduced to increase the supply of transplantable organs and shorten the waiting time for transplantation candidates.35 This type of organ donation represents a significant source of organs required for future expansion of transplantation practice in the United States. The requirement for donation in imminent or cardiac death is implemented through the collaboration of the Advisory Committee on Organ Transplantation of the DHHS (Table 1), the Centers for Medicare & Medicaid Services (CMS), and the Joint Commission on Accreditation of Healthcare Organizations (JCAHO).3, 57 The organ donor pool of those facing imminent or cardiac death has also been expanded to include neurologically intact patients who may not fulfill brain death criteria before withdrawal of life support.4, 8, 9
| State law (year)* | |
|---|---|
| |
| Uniform Anatomical Gift Act (1968) | Any 18‐year‐old with a sound mind may donate his or her body after death to be used for medical research or as a source of transplantable tissues and organs and barring others from overriding a donor's decision to make an anatomical gift. |
| Amendment (1987) | Minors who can apply for a driver's license are empowered to make anatomical gifts, but either parent can revoke the gift if the minor dies before the age of 18. |
| Revision (2006) | Declaration of a gift does not require any witnesses. |
| Amendment (2007) | Document of a gift or donor registry is sufficient for the removal of organs, which means an OPO does not need consent of the spouse or the family. |
| Enables an OPO to gain access to documents of gifts in donor registries, medical records, and records of a state motor vehicle department. | |
| Facilitates donations by expanding the list of those who may make an anatomical gift for another individual who has not declared a preference for or against donation. | |
| Permits removal of organs by medical personnel without explicit consent from a potential donor or from a relative of the donor, so long as the appropriate medical personnel or authorities have made a reasonable effort to discover any objection by the donor or the donor's family. | |
| Require hospitals to notify an OPO or third party designated by the OPO of an individual whose death is imminent or who has died in the hospital in order to increase donation opportunity. | |
| Gives an OPO the right to inspect a patient's medical records. | |
| Measures necessary to ensure the medical suitability of a part not be withdrawn while an examination is being made to determine whether an individual who has been referred to OPO has a part that could be the subject of an anatomical gift. If, following such an examination, it is determined by the OPO that the individual has a part that could be the subject of an anatomical gift, the individual is a prospective donor under this act unless the individual had signed a refusal. | |
| Forbids the buying and selling of organs. | |
| Measures necessary to ensure the medical suitability of an organ for transplantation or therapy may not be withheld or withdrawn from a prospective donor who has an advance health‐care directive or declaration unless the directive or declaration expressly provides to the contrary. The section presumes that for prospective donors the desire to save lives by making an anatomical gift trumps the desire to have life support systems withheld or withdrawn. Individuals who desire to overcome this presumption can do so by express language in their advance health‐care directive or declaration. | |
| Uniform Determination of Death Act (1981) | An individual who has sustained either (1) irreversible cessation of circulatory and respiratory functions, or (2) irreversible cessation of all functions of the entire brain including the brain stem is dead. A determination of death must be made in accordance with accepted medical standards. |
| Federal laws | |
| National Organ Transplant Act (1984) | Calls for a unified transplant network to be operated by a private, nonprofit organization under federal contract and the establishment of a Task Force in Organ Procurement and Transplantation and an Organ Procurement and Transplantation Registry. |
| Public Health and Welfare Act Title 42 USC (1999) | |
| Section 273 | Establishes guidelines to be a qualified OPO that can receive federal grants. |
| Section 274 | Establishes the OPTN & Scientific Registry for Transplantation. |
| Federal regulations | |
| Title Code 42 CFR Part 121 (1999) | Lists regulations of the OPTN final rule. |
| Explains the OPTN structure. | |
| Lists the policy that the OPTN board of directors is responsible for developing. | |
| Explains rules that an OPTN member must obey when including a person on the organ waiting list. | |
| Describes the requirements and tests for determining the suitability of donated organs. | |
| Explains how the OPTN identifies an organ recipient, allocates the organ, and transports it to the recipient. | |
| Describes how the board of directors should develop allocation policies to guarantee they are both efficient and just and allocation performance goals to ensure the best possible use and most equitable allocation of organs. | |
| Lists designated transplant program requirements. | |
| Describes how the HHS conducts reviews and evaluations and enforces rules. | |
| Describes the recording and reporting requirements of the various groups involved in the transplantation process. | |
| Establishes the Advisory Committee on Organ Transplantation (ACOT), which advises the HHS secretary on organ donation, procurement, allocation, and transplantation The HHS secretary may ask for ACOT's opinion of proposed OPTN policies. | |
| Title Code 42 CFR Parts 413, 441, 486 and 498 (2006) | Lists regulations of the OPO final rule. |
| Establishes new conditions for coverage for OPOs that include multiple new outcome and process performance measures based on organ donor potential and other related factors in each donation service area of qualified OPOs. | |
The President's Council on Bioethics independently evaluated the issues surrounding deceased organ donation and procurement.10 The President's Council on Bioethics has expressed major concerns about several issues pertinent to cardiac or imminent death organ donation that have not been addressed explicitly by the bodies that have made recommendations for reforming or expanding that type of organ donation in the United States. The debate on organ procurement in imminent or cardiac death has come to the forefront because of doubts about its ethical appropriateness and acceptance within the medical profession and the community at large. This review focuses on the serious issues related to organ procurement from patients facing imminent or cardiac death.
Organ Procurement and the Dead Donor Rule
Organ procurement is only permitted when the donor is already dead (ie, the dead donor rule), and the act of organ recovery cannot have been the immediate act to cause that death.10, 11 There are 2 criteria for death as defined in the Uniform Determination of Death Act (UDDA; Table 1): an individual who has sustained irreversible cessation of either (1) circulatory and respiratory functions or (2) all functions of the entire brain, including the brain stem, is considered dead and this determination of death must have been made in accordance with accepted medical standards.12 When organs are procured from an individual in whom all brain function has ceased but normal cardiac pump activity is continuing, it is referred to as heart‐beating organ donation. Organ procurement after cessation of cardiac pump activity and cardiorespiratory functions is referred to as non‐heart‐beating organ donation (NHBOD). Organ procurement from an individual in imminent or cardiac death is considered NHBOD.
Non‐heart‐beating organ donors can be neurologically intact and do not fulfill the brain death criterion prior to cessation of cardiac pump activity. In response to this dilemma, the University of Pittsburgh Medical Centre developed a protocol for donation of organs that permitted their procurement from patients who were pulseless and apneic for 2 minutes and did not fulfill brain criteria and who had previously given consent for organ donation.13 Because it is uncertain if cessation of cardiorespiratory function is irreversible after only a short time, the Institute of Medicine (IOM) extended the time required for pulselessness and apnea from 2 to 5 minutes before permitting organ procurement.14 Waiting longer than 5 minutes for the determination of death would compromise the quality of procured organs because of warm ischemia time and would influence the functioning of grafts in transplant recipients.
One of the pivotal assumptions for NHBOD acceptance is that 5 minutes of pulselessness and apnea eliminates the possibility that the procurement process itself could be the cause of death and fulfills the dead donor rule.14, 15 The cardiorespiratory death criteria were derived from observations that did not evaluate delayed autoresuscitation (spontaneous return of circulation) or simultaneous cessation of brain electrical activity (as recorded in brain death).1618 The death criteria applied for organ procurement must also comply with the irreversibility requirement of the UDDA.11, 12
The true incidence, temporal characteristics, and predictors of autoresuscitation in humans remain unknown because of underreporting in the literature. However, there have been case reports of autoresuscitation with return of neurologic function (also called the Lazarus phenomenon) after 10 minutes of cardiac asystole.19, 20 Maleck et al. and Adhiyaman et al. described autoresuscitation 5 minutes or longer after cardiorespiratory arrest in 44% and 50% of the published case reports, respectively.19, 20 Although cardiac asystole leads to the loss of arterial pulse pressure, circulatory arterial mean pressure is maintained in diastole by arteriolar vasomotor tone. The relaxation (diastole) phase systemic arterial to venous pressure gradient provides the perfusion pressure for vital organs and the spontaneous return of circulation after circulatory arrest.21 It is likely that autoresuscitation occurs because of the persistence of circulatory vasomotor tone after cessation of cardiac function. The time course of systemic vascular tone after circulatory arrest has not been well characterized in humans. However, the IOM criteria did not account for the incidence of delayed autoresuscitation in humans even though the Maastricht protocol (developed by the University of Zurich, Zurich, Switzerland) acknowledged this phenomenon and required at least 10 minutes to elapse after cardiorespiratory arrest before starting organ procurement.22 The 10‐minute waiting time did not compromise the quality of the organs procured.
The cardiorespiratory death criteria for organ procurement also ignore cardiac electrical activity (such as pulseless electrical activity or ventricular fibrillation) on an electrocardiogram. Research with cardiac ultrasonography and indwelling arterial catheters confirms that pulseless cardiac electrical activity can be associated with cardiac mechanical contractions, although these contractions are too weak to be detected by blood pressure monitoring.23 The presence of cardiac electrical activity on an electrocardiogram can also increase the likelihood that delayed autoresuscitation will occur.19, 20 Furthermore, whether there is brain electrical activity or neurologic function when cardiac electrical activity is still observed on an electrocardiogram remains unknown.24 It can be argued that donors who have already suffered severe neurologic injury cannot have meaningful neurologic function at the time of cardiorespiratory death. However, the presence of brain activity becomes relevant for organ donors with intact neurologic and brain function prior to cardiorespiratory arrest when only cardiorespiratory criteria for organ procurement are being used.4, 8, 9
In situ circulatory support with extracorporeal perfusion in organ donors has also refuted that cardiorespiratory arrest for 5 minutes fulfills the UDDA requirement because reversibility can occur during the procurement process. In situ extracorporeal perfusion is initiated 5 minutes after cardiorespiratory arrest of donors in order to preserve organs for procurement.25 Coronary and cerebral reperfusion can lead to the return of cardiac and neurologic functions (also called reanimation) of donors during the procurement process. Mechanical occlusion of the aortic arch and pharmacological agents are required to suppress donor reanimation during organ procurement.26 Martin et al. documented that in situ extracorporeal perfusion returned full neurologic and cardiac function 25 minute after cardiorespiratory arrest that occurred outside the hospital.27 Similar observations of full neurologic recovery and survival to hospital discharge were reported after in situ extracorporeal perfusion for in‐hospital cardiorespiratory arrest.28 These observations confirm that the time required for irreversible loss of neurologic function after cessation of circulation is much longer than the 25 minutes of cardiorespiratory arrest required to begin the process of organ procurement in NHBOD.
The incidence of donor reanimation during procurement is unknown because its reporting violates the dead donor rule and can create legal concerns.11 It can be argued that reanimation of organ donors is irrelevant because it does not mean survival. However, the occurrence of reanimation invalidates the premise that the cardiorespiratory criteria for organ procurement comply with the uniform determination of death. Others have accepted the inaccuracy of these criteria for determining death for procurement of organs from deceased donors and proposed abandoning the dead donor rule in order to permit recovery of transplantable organs before death.29
In the face of the uncertainty in determining death and in response to a media and marketing campaign by organ procurement organizations (OPOs) to promote public enrollment in deceased organ donation, the transplant community renamed NHBOD cardiac death organ donation.30, 31 The use of the term cardiac death is scientifically inaccurate and perhaps misleading. This term is used to denote the cessation of circulation and cardiac pump activity. The term cardiac death can be misinterpreted as meaning the heart has irreversibly ceased at the time of procurement, thus contradicting the scientific evidence for spontaneous resumption of cardiac function and autoresuscitation.19, 20 Alternatively, the use of this term can falsely imply that neurologic activity or brain stem function has ceased irreversibly after loss of cardiac activity, when scientific evidence suggests that brain stem function can remain after cardiac arrest.32
Consent for Organ Donation
Several state laws and federal regulations have been enacted to ensure that organ donation and transplantation practice comply with the ethical and legal standards of society (Table 1). The current regulations require hospitals across the United States to provde regional OPOs with early notification of all patients whose deaths are imminent before life support has been withdrawn so that discussion of organ donation with surrogate decision makers can be initiated independently and consent obtained.3, 5, 9 The Organ Donation Breakthrough Collaborative has set a goal of each OPO accomplishing a target organ donation rate of 75% or higher at local hospitals within an assigned donation service area (DSA).1, 2, 5 The financial and administrative incentives for the OPO to achieve that target organ donation rate have introduced undisclosed conflict within the donation consent process.33 Self‐serving bias of OPOs can influence whether pertinent information necessary for surrogate decision makers to provide informed consent is disclosed.34 As an example of this bias, alternative options for care and palliation may be discussed with surrogate decision makers with less enthusiasm than are the benefits and altruistic notion of organ donation. In obtaining donation consent, OPOs often avoid disclosing details of perimortem interventions performed on donors that are required for successful procurement of transplantable organs.10, 34, 35 After receiving consent for organ donation, OPO staffs also assume the responsibility of planning donor medical care and treatment pathways essential for maintaining organ viability and of preparing for subsequent procurement.5, 36 In essence, the care of the dying patient is guided by a team whose primary interest is the preservation of organs until procurement has been accomplished.
The Uniform Anatomical Gift Act (UAGA) assigns explicit priority to the donor's expressed intent so that consent for organ donation becomes irrevocable and does not require the consent or concurrence of any person after the donor's death (Table 1).9, 37 The donor's authorization to donate, recorded on an organ donor card, the individual's driver's license, or a donor registry, becomes a legally binding advance directive. The UAGA amendment enables OPOs to procure organs without family consent and in certain instances after family refusal to donate.37
Other consent options for organ donation from deceased donors have been proposed to maximize OPO recovery of transplantable organs in the United States (Table 2).8 The IOM has considered presumed consent for organ donation as a favorable option.8 Presumed consent means the default option is consent to donation, that if an individual has not expressly rejected donation, that individual is considered to have consented to organ donation. Legislative enactment of presumed consent enables OPOs to avoid the potential for surrogates to deny consent for donation, thus increasing the pool of future organ donors. The revised UAGA replaces nondonation with the intent to donate organs as the default option. In the default option, all measures necessary to ensure the medical suitability of an organ for transplantation can not be withheld or withdrawn until the OPO has determined medical suitability of the individual as a prospective donor (Table 1). The default option overrides the expression of intent in a declaration or advance health‐care directives not to have life prolonged by withholding or withdrawing life support system unless the individual has expressed refusal of donation (Tables 1 and 2). The revised UAGA presumes that for prospective donors the desire to save lives by making an anatomical gift trumps the desire to have life support systems withheld or withdrawn. Mandated consent (or conscription) has also been proposed for recovery of cadaveric organs (Table 2).38 Under mandated consent, consent for organ donation is automatic from all deceased individuals; therefore, OPOs would not require or request consent because removal of all needed transplantable cadaveric organs would be compulsory. OPO staffs would no longer have to discuss organ procurement from potential donors with family members or other surrogates. An alternative form of donation consent is mandated choice, which requires each individual to decide in advance either to agree to organ donation or to refuse it. Mandated choice is the IOM's least favorite option because it would require extensive public informational programs on organ donation to facilitate individual choices and decision making (Table 2).8
| Type | Description |
|---|---|
| Requested (expressed) consent | An individual is asked to voluntarily agree to organ donation. |
| Presumed (implied) consent | Unless an individual has expressly refused to donate organs, the default option is agreement to donate organs. |
| Mandated consent (conscription) | An individual is not required to decide on organ donation before death, and there is an automatic right to procure organs from any and all deceased individuals. |
| Mandated choice | An individual must choose between 2 options before death: agreement or refusal to donate organs. |
End‐of‐Life Care
Quality of end‐of‐life (EOL) care for an organ donor, as for any individual whose treatment is being withdrawn, is considered the highest priority of care and must not be compromised by the donation process. Yet no studies have investigated the impact of organ donation on the quality of EOL care in NHBOD.35 Previous reports have criticized the quality of EOL care offered to dying patients in intensive care units (ICUs).39, 40 Many of these patients are undergoing withdrawal of life support in anticipation of death and are considered candidates for NHBOD. The Robert Wood Johnson Foundation (RWJF) Critical Care End‐Of‐Life Peer Workgroup developed 53 EOL quality indicators to standardize and measure the quality of EOL care.41 These quality indicators, organized in 7 domains, focus on delivering patient‐ and family‐centered care and facilitating a good death experience in the ICU (Table 3).
| Domains of comprehensive quality indicators of EOLC (n = 53)41 | Abbreviated quality indicators of EOLC (n = 18)46 |
|---|---|
| |
| Patient and family‐centered decision making (n = 13) | |
| Recognize the patient and family as the unit of care | |
| Assess the patient's and family's decision‐making style and preferences | |
| Address conflicts in decision making within the family | |
| Assess, together with appropriate clinical consultants, the patient's capacity to participate in decision making about treatment and document assessment | Assessment of the patient's decisional capacity |
| Initiate advance care planning with the patient and family | |
| Clarify and document the status of the patient's advance directive | Documentation of the presence and, if present, contents of advance directives |
| Identify the healthcare proxy or surrogate decision maker | Documentation of a surrogate decision maker within 24 hours of admission |
| Clarify and document resuscitation orders | |
| Assure patients and families that decision making by the health care team will incorporate their preferences | |
| Follow ethical and legal guidelines for patients who lack both capacity to make decisions and a surrogate decision maker | |
| Establish and document clear, realistic, and appropriate goals of care in consultation with the patient and family | Documentation of the goals of care |
| Help the patient and family assess the benefits and burdens of alternative treatment choices as the patient's condition changes | |
| Forgo life‐sustaining treatments in a way that ensures patient and family preferences are elicited and respected | |
| Communication within the team and with patients and families (n = 10) | |
| Meet as interdisciplinary team to discuss the patient's condition, clarify goals of treatment, and identify the patient's and family's needs and preferences | Documentation of a timely interdisciplinary clinicianfamily conference |
| Address conflicts among the clinical team before meeting with the patient and/or family | |
| Utilize expert clinical, ethical, and spiritual consultants when appropriate | |
| Recognize the adaptations in communication strategy required for patients and families according to the chronic versus acute nature of the illness, cultural and spiritual differences, and other influences | |
| Meet with the patient and/or family on a regular basis to review patient's status and to answer questions | Documentation of timely physician communication with the family |
| Communicate all information to the patient and family, including distressing news, in a clear, sensitive, unhurried manner and in an appropriate setting | |
| Clarify the patient's and family's understanding of the patient's condition and goals of care at the beginning and end of each meeting | |
| Designate primary clinical liaison(s) who will communicate with the family daily | |
| Identify a family member who will serve as the contact person for the family | |
| Prepare the patient and family for the dying process | |
| Continuity of care (n = 3) | |
| Maximize continuity of care across clinicians, consultants, and settings | |
| Orient new clinicians to the status of the patient and family | Transmission of key information with transfer of the patient out of the ICU |
| Policy for continuity of nursing services | |
| Prepare the patient and/or family for a change of clinician(s) and introduce new clinicians | |
| Emotional and practical support for patients and families (n = 8) | |
| Elicit and attend to the needs of the dying person and his/her family | |
| Distribute written material (booklet) for families that includes orientation to the ICU environment and open visitation guidelines, logistical information (nearby hotels, banks, restaurants, directions), listings of financial consultation services, and bereavement programs and resources | Open visitation policy for family members |
| Facilitate strengthening of patientfamily relationships and communication | |
| Maximize privacy of the patient and family | |
| Value and support the patient's and family's cultural traditions | |
| Arrange for social support for patients who do not have family or friends | Documentation that psychosocial support has been offered |
| Distribute written material (booklet) containing essential logistical information and listings of financial consultation services and bereavement support programs/resources | |
| Support family members through the patient's death and their bereavement | |
| Symptom management and comfort care (n = 10) | |
| Emphasize the comprehensive comfort care that will be provided to the patient rather than the removal of life‐sustaining treatments | |
| Institute and use uniform quantitative symptom assessment scales appropriate for communicative and noncommunicative patients on a routine basis | Documentation of pain assessment |
| Documentation of respiratory distress assessment | |
| Standardize and follow best clinical practices for symptom management | Protocol for analgesia/sedation in terminal withdrawal of mechanical ventilation |
| Appropriate medications available during withdrawal of mechanical ventilation | |
| Use nonpharmacologic as well as pharmacologic measures to maximize comfort as appropriate and desired by the patient and family | |
| Reassess and document symptoms following interventions | Documentation of pain management |
| Documentation of respiratory distress management | |
| Know and follow best clinical practices for withdrawing life‐sustaining treatments to avoid patient and family distress | |
| Eliminate unnecessary tests and procedures (laboratory work, weights, routine vital signs) and only maintain intravenous catheters for symptom management when life support is being withdrawn | |
| Minimize noxious stimuli (monitors, strong lights) | |
| Attend to patient's appearance and hygiene | |
| Ensure family's and/or clinician's presence so the patient is not dying alone | |
| Spiritual support for patients and families (n = 3) | |
| Assess and document spiritual needs of the patient and family on an ongoing basis | Documentation that spiritual support was offered |
| Encourage access to spiritual resources | |
| Elicit and facilitate spiritual and cultural practices that the patient and family find comforting | |
| Emotional and organizational support for ICU clinicians (n = 6) | |
| Support health care team colleagues caring for dying patients | Opportunity to review experience of caring for dying patients by ICU clinicians |
| Adjust nursing staff and medical rotation schedules to maximize continuity of care providers for dying patients | |
| Communicate regularly with interdisciplinary team about goals of care | |
| Establish a staff support group, based on the input and needs of ICU staff and experienced group facilitators, and integrate meeting times into the routine of the ICU | |
| Enlist palliative care experts, pastoral care representatives, and other consultants to teach and model aspects of EOLC | |
| Facilitate rituals for the staff to mark the death of patients | |
| Strategies to improve EOLC and provide a good death42, 43 | |
| EOL care quality monitoring | |
| Making environmental changes to promote dying with dignity | |
| Managing patients' pain and discomfort | |
| Knowing and following patients' wishes for end‐of‐life care | |
| Bereavement program or service | |
| Regular meetings of senior ICU physician and nurse with patients' families | |
| Training of ICU clinicians in end‐of‐life communication skills | |
| Role modeling and supervision of trainees by clinicians experienced in end‐of‐life care | |
| Formal mechanism for emotional support of staff caring for dying patients | |
| Access to palliative care consultants | |
| Training of ICU clinicians in symptom management | |
| Scheduling staff to promote continuity of care for dying patients | |
| Formal system for scaled assessment and charting of patients' symptoms | |
| Method to help resolve differences about appropriate care goals | |
| Resources to accommodate diversity among patients/families at the end of life | |
| Access to clinical ethics consultants | |
| Regular pastoral care visits to the ICU | |
In a subsequent U.S. survey, the Critical Care Peer Workgroup of the Promoting Excellence in End‐of‐Life Care Project reported that more than 75% of ICUs were still not monitoring the quality of EOL care.42 The survey also identified multiple barriers to optimal EOL care found in most ICUs. The study group proposed several strategies to overcome these barriers and improve the quality of EOL care (Table 3).42, 43 It can also be inferred from the survey findings that most ICUs are unprepared and lack the necessary tools to appropriately inform patients and families of the trade‐off in EOL care for NHBOD. The President's Council for Bioethics has also warned that NHBOD can transform EOL care from a peaceful dignified death into a profanely high‐tech death experience for donors' families.10
Several aspects of medical care that are neither palliative nor beneficial are performed for donor management for NHBOD and can explain the feared transformation of the death experience. The revised UAGA reaffirms that all measures necessary to ensure the medical suitability of an organ for transplantation cannot be withheld or withdrawn from the prospective donor and overrides the expression of intent by a prospective donor in either a declaration or advance health‐care directive not to have life prolonged by use of life support systems (Table 1).9 The 2007 amendment to revised UAGA section 21 recognizes the conflict between all measures necessary to ensure organs viability for transplantation and appropriate EOL care and requires the attending physician and OPO to resolve the conflict with the prospective donor or surrogate decision‐maker.9
OPOs apply donor management critical pathways to potential organ donors in order to maintain organ viability for successful execution of organ procurement.36 The University of Wisconsin developed a protocol and an evaluation tool to determine the eligibility of potential candidates for NHBOD.44 The protocol entails temporary discontinuation of mechanical ventilation for a trial of spontaneous respiration lasting up to 10 minutes to determine the likelihood of cardiorespiratory death within 60‐90 minutes of the withdrawal of life support. Those patients predicted by the University of Wisconsin evaluation tool to survive a longer time are not candidates for NHBOD and are transferred to palliative care. Those patients who meet the necessary criteria of the University of Wisconsin evaluation tool become candidates for NHBOD and undergo additional antemortem testing, invasive vascular instrumentation, and infusion of medications essential for organ preservation.36 The instrumentation and medications used for organ preservation can also expedite death on withdrawal of life support.45 Other interventions (such as circulatory support with invasive and noninvasive devices, extracorporeal perfusion and oxygenation, endotracheal reintubation, mechanical ventilation, and bronchoscopy) are performed when cardiorespiratory death is pronounced in order to maintain organ viability and can inadvertently reanimate the donor during the procurement process.26
The process of obtaining donation consent and subsequent donor management protocols for NHBOD deviate from more than 60% of the RWJF quality indicators recommended for optimal EOL care.35, 36, 41 Therefore, NHBOD can have a profound impact on the quality of EOL care. There has been a recent proposal to abbreviate the original RWJF quality indicators to include 14 of the 53 (26%) original quality indicators described for optimal EOL care in the ICU (Table 3).46 Many of the quality indicators expected to be negatively affected by NHBOD are not included in the proposal for an abbreviated list. There has been a concern that the application of an abbreviated rather than a comprehensive metrics for EOL care can portray an incomplete assessment and perhaps misinform donors and their families about the potential trade‐off in EOL care. The President's Council on Bioethics has emphasized that comprehensive evaluation of the quality of EOL care is an ethical imperative so that families can decide if the trade‐off is acceptable for organ donation.10 Deciding to donate organs at the end of life can be stressful for many families, and therefore they must be fully informed of the possible consequences. Posttraumatic stress disorders, anxiety, depression, and decreased quality of life have been reported in the deceased's family members who shared in stressful EOL decisions.47 Posttraumatic stress disorders have been reported in family members of deceased organ donors.48 Organ‐focused behavior by professionals requesting consent for organ donation and ambivalent decision making by family members appeared to increase the risk of relatives of deceased donors subsequently developing traumatic memories and stress disorders. The processes required for successful accomplishment of donation consent and subsequent organ recovery can interfere with many of the interventions that lessen the burden of bereavement of relatives of ICU decedents.49
The variability in decision making by health care providers about medical futility and EOL care has been given as a reason for concern about the implementation of NHBOD.50 The variability of EOL practice raises the possibility of conflicted decision making on medical futility within institutions that have transplant programs.50 Ethical conflicts and moral distress have been reported among health care providers who were directly involved in organ procurement in NHBOD.51 The pressure to recover transplantable organs from NHBOD candidates has been associated with health care professionals' perception of euthanasia and premature determination of medical futility and withdrawal of life support. The long‐term psychological impact of NHBOD practice on caregivers, health care providers, and professionals remains unknown.
CONCLUSIONS
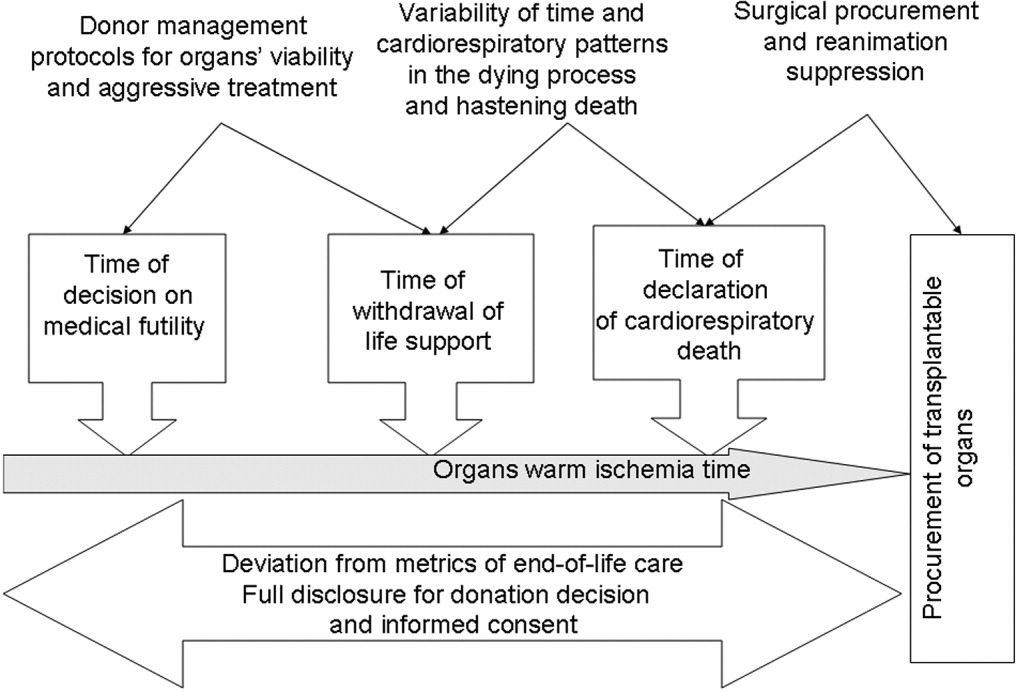
In conclusion, NHBOD influences medical care at critical time points to maximize the procurement of transplantable organs and minimize their warm ischemia time with negative consequences on the EOL care for the prospective donors and their families (see Figure 1).

Mandatory implementation of NHBOD in the face of difficulties surrounding the quality of EOL care for donors raises concern across the medical profession and community. There is a need for better scientific validation of the timing of organ procurement to ensure that organ recovery is not the irreversible event defining death in NHBOD. The desire of OPOs or their affiliates to maximize recovery of transplantable organs introduces self‐serving bias into obtaining consent for organ donation and violates the basic tenet of true informed consent. The use of comprehensive quality indicators of EOL care will help to determine the impact of NHBOD on donors, families, caregivers, and health care providers.
In April 2003 the Health Resources and Services Administration of the U.S. Department of Health and Human Services (DHHS) announced the formation of the Organ Donation Breakthrough Collaborative (ODBC).1 The Organ Donation Breakthrough Collaborative created 58 national donation service areas (DSAs) to organize the transplant community across the United States. Each of the 58 organ procurement organizations (OPOs) is joined to a regional transplant center or centers and donor hospitals to form a DSA. The ODBC's goal is to achieve a cadaveric organ donation rate of 75% or higher from hospitals within each DSA.2
A requirement for organ donation from patients facing imminent or cardiac death has been introduced to increase the supply of transplantable organs and shorten the waiting time for transplantation candidates.35 This type of organ donation represents a significant source of organs required for future expansion of transplantation practice in the United States. The requirement for donation in imminent or cardiac death is implemented through the collaboration of the Advisory Committee on Organ Transplantation of the DHHS (Table 1), the Centers for Medicare & Medicaid Services (CMS), and the Joint Commission on Accreditation of Healthcare Organizations (JCAHO).3, 57 The organ donor pool of those facing imminent or cardiac death has also been expanded to include neurologically intact patients who may not fulfill brain death criteria before withdrawal of life support.4, 8, 9
| State law (year)* | |
|---|---|
| |
| Uniform Anatomical Gift Act (1968) | Any 18‐year‐old with a sound mind may donate his or her body after death to be used for medical research or as a source of transplantable tissues and organs and barring others from overriding a donor's decision to make an anatomical gift. |
| Amendment (1987) | Minors who can apply for a driver's license are empowered to make anatomical gifts, but either parent can revoke the gift if the minor dies before the age of 18. |
| Revision (2006) | Declaration of a gift does not require any witnesses. |
| Amendment (2007) | Document of a gift or donor registry is sufficient for the removal of organs, which means an OPO does not need consent of the spouse or the family. |
| Enables an OPO to gain access to documents of gifts in donor registries, medical records, and records of a state motor vehicle department. | |
| Facilitates donations by expanding the list of those who may make an anatomical gift for another individual who has not declared a preference for or against donation. | |
| Permits removal of organs by medical personnel without explicit consent from a potential donor or from a relative of the donor, so long as the appropriate medical personnel or authorities have made a reasonable effort to discover any objection by the donor or the donor's family. | |
| Require hospitals to notify an OPO or third party designated by the OPO of an individual whose death is imminent or who has died in the hospital in order to increase donation opportunity. | |
| Gives an OPO the right to inspect a patient's medical records. | |
| Measures necessary to ensure the medical suitability of a part not be withdrawn while an examination is being made to determine whether an individual who has been referred to OPO has a part that could be the subject of an anatomical gift. If, following such an examination, it is determined by the OPO that the individual has a part that could be the subject of an anatomical gift, the individual is a prospective donor under this act unless the individual had signed a refusal. | |
| Forbids the buying and selling of organs. | |
| Measures necessary to ensure the medical suitability of an organ for transplantation or therapy may not be withheld or withdrawn from a prospective donor who has an advance health‐care directive or declaration unless the directive or declaration expressly provides to the contrary. The section presumes that for prospective donors the desire to save lives by making an anatomical gift trumps the desire to have life support systems withheld or withdrawn. Individuals who desire to overcome this presumption can do so by express language in their advance health‐care directive or declaration. | |
| Uniform Determination of Death Act (1981) | An individual who has sustained either (1) irreversible cessation of circulatory and respiratory functions, or (2) irreversible cessation of all functions of the entire brain including the brain stem is dead. A determination of death must be made in accordance with accepted medical standards. |
| Federal laws | |
| National Organ Transplant Act (1984) | Calls for a unified transplant network to be operated by a private, nonprofit organization under federal contract and the establishment of a Task Force in Organ Procurement and Transplantation and an Organ Procurement and Transplantation Registry. |
| Public Health and Welfare Act Title 42 USC (1999) | |
| Section 273 | Establishes guidelines to be a qualified OPO that can receive federal grants. |
| Section 274 | Establishes the OPTN & Scientific Registry for Transplantation. |
| Federal regulations | |
| Title Code 42 CFR Part 121 (1999) | Lists regulations of the OPTN final rule. |
| Explains the OPTN structure. | |
| Lists the policy that the OPTN board of directors is responsible for developing. | |
| Explains rules that an OPTN member must obey when including a person on the organ waiting list. | |
| Describes the requirements and tests for determining the suitability of donated organs. | |
| Explains how the OPTN identifies an organ recipient, allocates the organ, and transports it to the recipient. | |
| Describes how the board of directors should develop allocation policies to guarantee they are both efficient and just and allocation performance goals to ensure the best possible use and most equitable allocation of organs. | |
| Lists designated transplant program requirements. | |
| Describes how the HHS conducts reviews and evaluations and enforces rules. | |
| Describes the recording and reporting requirements of the various groups involved in the transplantation process. | |
| Establishes the Advisory Committee on Organ Transplantation (ACOT), which advises the HHS secretary on organ donation, procurement, allocation, and transplantation The HHS secretary may ask for ACOT's opinion of proposed OPTN policies. | |
| Title Code 42 CFR Parts 413, 441, 486 and 498 (2006) | Lists regulations of the OPO final rule. |
| Establishes new conditions for coverage for OPOs that include multiple new outcome and process performance measures based on organ donor potential and other related factors in each donation service area of qualified OPOs. | |
The President's Council on Bioethics independently evaluated the issues surrounding deceased organ donation and procurement.10 The President's Council on Bioethics has expressed major concerns about several issues pertinent to cardiac or imminent death organ donation that have not been addressed explicitly by the bodies that have made recommendations for reforming or expanding that type of organ donation in the United States. The debate on organ procurement in imminent or cardiac death has come to the forefront because of doubts about its ethical appropriateness and acceptance within the medical profession and the community at large. This review focuses on the serious issues related to organ procurement from patients facing imminent or cardiac death.
Organ Procurement and the Dead Donor Rule
Organ procurement is only permitted when the donor is already dead (ie, the dead donor rule), and the act of organ recovery cannot have been the immediate act to cause that death.10, 11 There are 2 criteria for death as defined in the Uniform Determination of Death Act (UDDA; Table 1): an individual who has sustained irreversible cessation of either (1) circulatory and respiratory functions or (2) all functions of the entire brain, including the brain stem, is considered dead and this determination of death must have been made in accordance with accepted medical standards.12 When organs are procured from an individual in whom all brain function has ceased but normal cardiac pump activity is continuing, it is referred to as heart‐beating organ donation. Organ procurement after cessation of cardiac pump activity and cardiorespiratory functions is referred to as non‐heart‐beating organ donation (NHBOD). Organ procurement from an individual in imminent or cardiac death is considered NHBOD.
Non‐heart‐beating organ donors can be neurologically intact and do not fulfill the brain death criterion prior to cessation of cardiac pump activity. In response to this dilemma, the University of Pittsburgh Medical Centre developed a protocol for donation of organs that permitted their procurement from patients who were pulseless and apneic for 2 minutes and did not fulfill brain criteria and who had previously given consent for organ donation.13 Because it is uncertain if cessation of cardiorespiratory function is irreversible after only a short time, the Institute of Medicine (IOM) extended the time required for pulselessness and apnea from 2 to 5 minutes before permitting organ procurement.14 Waiting longer than 5 minutes for the determination of death would compromise the quality of procured organs because of warm ischemia time and would influence the functioning of grafts in transplant recipients.
One of the pivotal assumptions for NHBOD acceptance is that 5 minutes of pulselessness and apnea eliminates the possibility that the procurement process itself could be the cause of death and fulfills the dead donor rule.14, 15 The cardiorespiratory death criteria were derived from observations that did not evaluate delayed autoresuscitation (spontaneous return of circulation) or simultaneous cessation of brain electrical activity (as recorded in brain death).1618 The death criteria applied for organ procurement must also comply with the irreversibility requirement of the UDDA.11, 12
The true incidence, temporal characteristics, and predictors of autoresuscitation in humans remain unknown because of underreporting in the literature. However, there have been case reports of autoresuscitation with return of neurologic function (also called the Lazarus phenomenon) after 10 minutes of cardiac asystole.19, 20 Maleck et al. and Adhiyaman et al. described autoresuscitation 5 minutes or longer after cardiorespiratory arrest in 44% and 50% of the published case reports, respectively.19, 20 Although cardiac asystole leads to the loss of arterial pulse pressure, circulatory arterial mean pressure is maintained in diastole by arteriolar vasomotor tone. The relaxation (diastole) phase systemic arterial to venous pressure gradient provides the perfusion pressure for vital organs and the spontaneous return of circulation after circulatory arrest.21 It is likely that autoresuscitation occurs because of the persistence of circulatory vasomotor tone after cessation of cardiac function. The time course of systemic vascular tone after circulatory arrest has not been well characterized in humans. However, the IOM criteria did not account for the incidence of delayed autoresuscitation in humans even though the Maastricht protocol (developed by the University of Zurich, Zurich, Switzerland) acknowledged this phenomenon and required at least 10 minutes to elapse after cardiorespiratory arrest before starting organ procurement.22 The 10‐minute waiting time did not compromise the quality of the organs procured.
The cardiorespiratory death criteria for organ procurement also ignore cardiac electrical activity (such as pulseless electrical activity or ventricular fibrillation) on an electrocardiogram. Research with cardiac ultrasonography and indwelling arterial catheters confirms that pulseless cardiac electrical activity can be associated with cardiac mechanical contractions, although these contractions are too weak to be detected by blood pressure monitoring.23 The presence of cardiac electrical activity on an electrocardiogram can also increase the likelihood that delayed autoresuscitation will occur.19, 20 Furthermore, whether there is brain electrical activity or neurologic function when cardiac electrical activity is still observed on an electrocardiogram remains unknown.24 It can be argued that donors who have already suffered severe neurologic injury cannot have meaningful neurologic function at the time of cardiorespiratory death. However, the presence of brain activity becomes relevant for organ donors with intact neurologic and brain function prior to cardiorespiratory arrest when only cardiorespiratory criteria for organ procurement are being used.4, 8, 9
In situ circulatory support with extracorporeal perfusion in organ donors has also refuted that cardiorespiratory arrest for 5 minutes fulfills the UDDA requirement because reversibility can occur during the procurement process. In situ extracorporeal perfusion is initiated 5 minutes after cardiorespiratory arrest of donors in order to preserve organs for procurement.25 Coronary and cerebral reperfusion can lead to the return of cardiac and neurologic functions (also called reanimation) of donors during the procurement process. Mechanical occlusion of the aortic arch and pharmacological agents are required to suppress donor reanimation during organ procurement.26 Martin et al. documented that in situ extracorporeal perfusion returned full neurologic and cardiac function 25 minute after cardiorespiratory arrest that occurred outside the hospital.27 Similar observations of full neurologic recovery and survival to hospital discharge were reported after in situ extracorporeal perfusion for in‐hospital cardiorespiratory arrest.28 These observations confirm that the time required for irreversible loss of neurologic function after cessation of circulation is much longer than the 25 minutes of cardiorespiratory arrest required to begin the process of organ procurement in NHBOD.
The incidence of donor reanimation during procurement is unknown because its reporting violates the dead donor rule and can create legal concerns.11 It can be argued that reanimation of organ donors is irrelevant because it does not mean survival. However, the occurrence of reanimation invalidates the premise that the cardiorespiratory criteria for organ procurement comply with the uniform determination of death. Others have accepted the inaccuracy of these criteria for determining death for procurement of organs from deceased donors and proposed abandoning the dead donor rule in order to permit recovery of transplantable organs before death.29
In the face of the uncertainty in determining death and in response to a media and marketing campaign by organ procurement organizations (OPOs) to promote public enrollment in deceased organ donation, the transplant community renamed NHBOD cardiac death organ donation.30, 31 The use of the term cardiac death is scientifically inaccurate and perhaps misleading. This term is used to denote the cessation of circulation and cardiac pump activity. The term cardiac death can be misinterpreted as meaning the heart has irreversibly ceased at the time of procurement, thus contradicting the scientific evidence for spontaneous resumption of cardiac function and autoresuscitation.19, 20 Alternatively, the use of this term can falsely imply that neurologic activity or brain stem function has ceased irreversibly after loss of cardiac activity, when scientific evidence suggests that brain stem function can remain after cardiac arrest.32
Consent for Organ Donation
Several state laws and federal regulations have been enacted to ensure that organ donation and transplantation practice comply with the ethical and legal standards of society (Table 1). The current regulations require hospitals across the United States to provde regional OPOs with early notification of all patients whose deaths are imminent before life support has been withdrawn so that discussion of organ donation with surrogate decision makers can be initiated independently and consent obtained.3, 5, 9 The Organ Donation Breakthrough Collaborative has set a goal of each OPO accomplishing a target organ donation rate of 75% or higher at local hospitals within an assigned donation service area (DSA).1, 2, 5 The financial and administrative incentives for the OPO to achieve that target organ donation rate have introduced undisclosed conflict within the donation consent process.33 Self‐serving bias of OPOs can influence whether pertinent information necessary for surrogate decision makers to provide informed consent is disclosed.34 As an example of this bias, alternative options for care and palliation may be discussed with surrogate decision makers with less enthusiasm than are the benefits and altruistic notion of organ donation. In obtaining donation consent, OPOs often avoid disclosing details of perimortem interventions performed on donors that are required for successful procurement of transplantable organs.10, 34, 35 After receiving consent for organ donation, OPO staffs also assume the responsibility of planning donor medical care and treatment pathways essential for maintaining organ viability and of preparing for subsequent procurement.5, 36 In essence, the care of the dying patient is guided by a team whose primary interest is the preservation of organs until procurement has been accomplished.
The Uniform Anatomical Gift Act (UAGA) assigns explicit priority to the donor's expressed intent so that consent for organ donation becomes irrevocable and does not require the consent or concurrence of any person after the donor's death (Table 1).9, 37 The donor's authorization to donate, recorded on an organ donor card, the individual's driver's license, or a donor registry, becomes a legally binding advance directive. The UAGA amendment enables OPOs to procure organs without family consent and in certain instances after family refusal to donate.37
Other consent options for organ donation from deceased donors have been proposed to maximize OPO recovery of transplantable organs in the United States (Table 2).8 The IOM has considered presumed consent for organ donation as a favorable option.8 Presumed consent means the default option is consent to donation, that if an individual has not expressly rejected donation, that individual is considered to have consented to organ donation. Legislative enactment of presumed consent enables OPOs to avoid the potential for surrogates to deny consent for donation, thus increasing the pool of future organ donors. The revised UAGA replaces nondonation with the intent to donate organs as the default option. In the default option, all measures necessary to ensure the medical suitability of an organ for transplantation can not be withheld or withdrawn until the OPO has determined medical suitability of the individual as a prospective donor (Table 1). The default option overrides the expression of intent in a declaration or advance health‐care directives not to have life prolonged by withholding or withdrawing life support system unless the individual has expressed refusal of donation (Tables 1 and 2). The revised UAGA presumes that for prospective donors the desire to save lives by making an anatomical gift trumps the desire to have life support systems withheld or withdrawn. Mandated consent (or conscription) has also been proposed for recovery of cadaveric organs (Table 2).38 Under mandated consent, consent for organ donation is automatic from all deceased individuals; therefore, OPOs would not require or request consent because removal of all needed transplantable cadaveric organs would be compulsory. OPO staffs would no longer have to discuss organ procurement from potential donors with family members or other surrogates. An alternative form of donation consent is mandated choice, which requires each individual to decide in advance either to agree to organ donation or to refuse it. Mandated choice is the IOM's least favorite option because it would require extensive public informational programs on organ donation to facilitate individual choices and decision making (Table 2).8
| Type | Description |
|---|---|
| Requested (expressed) consent | An individual is asked to voluntarily agree to organ donation. |
| Presumed (implied) consent | Unless an individual has expressly refused to donate organs, the default option is agreement to donate organs. |
| Mandated consent (conscription) | An individual is not required to decide on organ donation before death, and there is an automatic right to procure organs from any and all deceased individuals. |
| Mandated choice | An individual must choose between 2 options before death: agreement or refusal to donate organs. |
End‐of‐Life Care
Quality of end‐of‐life (EOL) care for an organ donor, as for any individual whose treatment is being withdrawn, is considered the highest priority of care and must not be compromised by the donation process. Yet no studies have investigated the impact of organ donation on the quality of EOL care in NHBOD.35 Previous reports have criticized the quality of EOL care offered to dying patients in intensive care units (ICUs).39, 40 Many of these patients are undergoing withdrawal of life support in anticipation of death and are considered candidates for NHBOD. The Robert Wood Johnson Foundation (RWJF) Critical Care End‐Of‐Life Peer Workgroup developed 53 EOL quality indicators to standardize and measure the quality of EOL care.41 These quality indicators, organized in 7 domains, focus on delivering patient‐ and family‐centered care and facilitating a good death experience in the ICU (Table 3).
| Domains of comprehensive quality indicators of EOLC (n = 53)41 | Abbreviated quality indicators of EOLC (n = 18)46 |
|---|---|
| |
| Patient and family‐centered decision making (n = 13) | |
| Recognize the patient and family as the unit of care | |
| Assess the patient's and family's decision‐making style and preferences | |
| Address conflicts in decision making within the family | |
| Assess, together with appropriate clinical consultants, the patient's capacity to participate in decision making about treatment and document assessment | Assessment of the patient's decisional capacity |
| Initiate advance care planning with the patient and family | |
| Clarify and document the status of the patient's advance directive | Documentation of the presence and, if present, contents of advance directives |
| Identify the healthcare proxy or surrogate decision maker | Documentation of a surrogate decision maker within 24 hours of admission |
| Clarify and document resuscitation orders | |
| Assure patients and families that decision making by the health care team will incorporate their preferences | |
| Follow ethical and legal guidelines for patients who lack both capacity to make decisions and a surrogate decision maker | |
| Establish and document clear, realistic, and appropriate goals of care in consultation with the patient and family | Documentation of the goals of care |
| Help the patient and family assess the benefits and burdens of alternative treatment choices as the patient's condition changes | |
| Forgo life‐sustaining treatments in a way that ensures patient and family preferences are elicited and respected | |
| Communication within the team and with patients and families (n = 10) | |
| Meet as interdisciplinary team to discuss the patient's condition, clarify goals of treatment, and identify the patient's and family's needs and preferences | Documentation of a timely interdisciplinary clinicianfamily conference |
| Address conflicts among the clinical team before meeting with the patient and/or family | |
| Utilize expert clinical, ethical, and spiritual consultants when appropriate | |
| Recognize the adaptations in communication strategy required for patients and families according to the chronic versus acute nature of the illness, cultural and spiritual differences, and other influences | |
| Meet with the patient and/or family on a regular basis to review patient's status and to answer questions | Documentation of timely physician communication with the family |
| Communicate all information to the patient and family, including distressing news, in a clear, sensitive, unhurried manner and in an appropriate setting | |
| Clarify the patient's and family's understanding of the patient's condition and goals of care at the beginning and end of each meeting | |
| Designate primary clinical liaison(s) who will communicate with the family daily | |
| Identify a family member who will serve as the contact person for the family | |
| Prepare the patient and family for the dying process | |
| Continuity of care (n = 3) | |
| Maximize continuity of care across clinicians, consultants, and settings | |
| Orient new clinicians to the status of the patient and family | Transmission of key information with transfer of the patient out of the ICU |
| Policy for continuity of nursing services | |
| Prepare the patient and/or family for a change of clinician(s) and introduce new clinicians | |
| Emotional and practical support for patients and families (n = 8) | |
| Elicit and attend to the needs of the dying person and his/her family | |
| Distribute written material (booklet) for families that includes orientation to the ICU environment and open visitation guidelines, logistical information (nearby hotels, banks, restaurants, directions), listings of financial consultation services, and bereavement programs and resources | Open visitation policy for family members |
| Facilitate strengthening of patientfamily relationships and communication | |
| Maximize privacy of the patient and family | |
| Value and support the patient's and family's cultural traditions | |
| Arrange for social support for patients who do not have family or friends | Documentation that psychosocial support has been offered |
| Distribute written material (booklet) containing essential logistical information and listings of financial consultation services and bereavement support programs/resources | |
| Support family members through the patient's death and their bereavement | |
| Symptom management and comfort care (n = 10) | |
| Emphasize the comprehensive comfort care that will be provided to the patient rather than the removal of life‐sustaining treatments | |
| Institute and use uniform quantitative symptom assessment scales appropriate for communicative and noncommunicative patients on a routine basis | Documentation of pain assessment |
| Documentation of respiratory distress assessment | |
| Standardize and follow best clinical practices for symptom management | Protocol for analgesia/sedation in terminal withdrawal of mechanical ventilation |
| Appropriate medications available during withdrawal of mechanical ventilation | |
| Use nonpharmacologic as well as pharmacologic measures to maximize comfort as appropriate and desired by the patient and family | |
| Reassess and document symptoms following interventions | Documentation of pain management |
| Documentation of respiratory distress management | |
| Know and follow best clinical practices for withdrawing life‐sustaining treatments to avoid patient and family distress | |
| Eliminate unnecessary tests and procedures (laboratory work, weights, routine vital signs) and only maintain intravenous catheters for symptom management when life support is being withdrawn | |
| Minimize noxious stimuli (monitors, strong lights) | |
| Attend to patient's appearance and hygiene | |
| Ensure family's and/or clinician's presence so the patient is not dying alone | |
| Spiritual support for patients and families (n = 3) | |
| Assess and document spiritual needs of the patient and family on an ongoing basis | Documentation that spiritual support was offered |
| Encourage access to spiritual resources | |
| Elicit and facilitate spiritual and cultural practices that the patient and family find comforting | |
| Emotional and organizational support for ICU clinicians (n = 6) | |
| Support health care team colleagues caring for dying patients | Opportunity to review experience of caring for dying patients by ICU clinicians |
| Adjust nursing staff and medical rotation schedules to maximize continuity of care providers for dying patients | |
| Communicate regularly with interdisciplinary team about goals of care | |
| Establish a staff support group, based on the input and needs of ICU staff and experienced group facilitators, and integrate meeting times into the routine of the ICU | |
| Enlist palliative care experts, pastoral care representatives, and other consultants to teach and model aspects of EOLC | |
| Facilitate rituals for the staff to mark the death of patients | |
| Strategies to improve EOLC and provide a good death42, 43 | |
| EOL care quality monitoring | |
| Making environmental changes to promote dying with dignity | |
| Managing patients' pain and discomfort | |
| Knowing and following patients' wishes for end‐of‐life care | |
| Bereavement program or service | |
| Regular meetings of senior ICU physician and nurse with patients' families | |
| Training of ICU clinicians in end‐of‐life communication skills | |
| Role modeling and supervision of trainees by clinicians experienced in end‐of‐life care | |
| Formal mechanism for emotional support of staff caring for dying patients | |
| Access to palliative care consultants | |
| Training of ICU clinicians in symptom management | |
| Scheduling staff to promote continuity of care for dying patients | |
| Formal system for scaled assessment and charting of patients' symptoms | |
| Method to help resolve differences about appropriate care goals | |
| Resources to accommodate diversity among patients/families at the end of life | |
| Access to clinical ethics consultants | |
| Regular pastoral care visits to the ICU | |
In a subsequent U.S. survey, the Critical Care Peer Workgroup of the Promoting Excellence in End‐of‐Life Care Project reported that more than 75% of ICUs were still not monitoring the quality of EOL care.42 The survey also identified multiple barriers to optimal EOL care found in most ICUs. The study group proposed several strategies to overcome these barriers and improve the quality of EOL care (Table 3).42, 43 It can also be inferred from the survey findings that most ICUs are unprepared and lack the necessary tools to appropriately inform patients and families of the trade‐off in EOL care for NHBOD. The President's Council for Bioethics has also warned that NHBOD can transform EOL care from a peaceful dignified death into a profanely high‐tech death experience for donors' families.10
Several aspects of medical care that are neither palliative nor beneficial are performed for donor management for NHBOD and can explain the feared transformation of the death experience. The revised UAGA reaffirms that all measures necessary to ensure the medical suitability of an organ for transplantation cannot be withheld or withdrawn from the prospective donor and overrides the expression of intent by a prospective donor in either a declaration or advance health‐care directive not to have life prolonged by use of life support systems (Table 1).9 The 2007 amendment to revised UAGA section 21 recognizes the conflict between all measures necessary to ensure organs viability for transplantation and appropriate EOL care and requires the attending physician and OPO to resolve the conflict with the prospective donor or surrogate decision‐maker.9
OPOs apply donor management critical pathways to potential organ donors in order to maintain organ viability for successful execution of organ procurement.36 The University of Wisconsin developed a protocol and an evaluation tool to determine the eligibility of potential candidates for NHBOD.44 The protocol entails temporary discontinuation of mechanical ventilation for a trial of spontaneous respiration lasting up to 10 minutes to determine the likelihood of cardiorespiratory death within 60‐90 minutes of the withdrawal of life support. Those patients predicted by the University of Wisconsin evaluation tool to survive a longer time are not candidates for NHBOD and are transferred to palliative care. Those patients who meet the necessary criteria of the University of Wisconsin evaluation tool become candidates for NHBOD and undergo additional antemortem testing, invasive vascular instrumentation, and infusion of medications essential for organ preservation.36 The instrumentation and medications used for organ preservation can also expedite death on withdrawal of life support.45 Other interventions (such as circulatory support with invasive and noninvasive devices, extracorporeal perfusion and oxygenation, endotracheal reintubation, mechanical ventilation, and bronchoscopy) are performed when cardiorespiratory death is pronounced in order to maintain organ viability and can inadvertently reanimate the donor during the procurement process.26
The process of obtaining donation consent and subsequent donor management protocols for NHBOD deviate from more than 60% of the RWJF quality indicators recommended for optimal EOL care.35, 36, 41 Therefore, NHBOD can have a profound impact on the quality of EOL care. There has been a recent proposal to abbreviate the original RWJF quality indicators to include 14 of the 53 (26%) original quality indicators described for optimal EOL care in the ICU (Table 3).46 Many of the quality indicators expected to be negatively affected by NHBOD are not included in the proposal for an abbreviated list. There has been a concern that the application of an abbreviated rather than a comprehensive metrics for EOL care can portray an incomplete assessment and perhaps misinform donors and their families about the potential trade‐off in EOL care. The President's Council on Bioethics has emphasized that comprehensive evaluation of the quality of EOL care is an ethical imperative so that families can decide if the trade‐off is acceptable for organ donation.10 Deciding to donate organs at the end of life can be stressful for many families, and therefore they must be fully informed of the possible consequences. Posttraumatic stress disorders, anxiety, depression, and decreased quality of life have been reported in the deceased's family members who shared in stressful EOL decisions.47 Posttraumatic stress disorders have been reported in family members of deceased organ donors.48 Organ‐focused behavior by professionals requesting consent for organ donation and ambivalent decision making by family members appeared to increase the risk of relatives of deceased donors subsequently developing traumatic memories and stress disorders. The processes required for successful accomplishment of donation consent and subsequent organ recovery can interfere with many of the interventions that lessen the burden of bereavement of relatives of ICU decedents.49
The variability in decision making by health care providers about medical futility and EOL care has been given as a reason for concern about the implementation of NHBOD.50 The variability of EOL practice raises the possibility of conflicted decision making on medical futility within institutions that have transplant programs.50 Ethical conflicts and moral distress have been reported among health care providers who were directly involved in organ procurement in NHBOD.51 The pressure to recover transplantable organs from NHBOD candidates has been associated with health care professionals' perception of euthanasia and premature determination of medical futility and withdrawal of life support. The long‐term psychological impact of NHBOD practice on caregivers, health care providers, and professionals remains unknown.
CONCLUSIONS
In conclusion, NHBOD influences medical care at critical time points to maximize the procurement of transplantable organs and minimize their warm ischemia time with negative consequences on the EOL care for the prospective donors and their families (see Figure 1).

Mandatory implementation of NHBOD in the face of difficulties surrounding the quality of EOL care for donors raises concern across the medical profession and community. There is a need for better scientific validation of the timing of organ procurement to ensure that organ recovery is not the irreversible event defining death in NHBOD. The desire of OPOs or their affiliates to maximize recovery of transplantable organs introduces self‐serving bias into obtaining consent for organ donation and violates the basic tenet of true informed consent. The use of comprehensive quality indicators of EOL care will help to determine the impact of NHBOD on donors, families, caregivers, and health care providers.
- ,,, et al.Organ donation and utilization, 1995‐2004: Entering the collaborative era.Am J Transplant.2006;6:1101–1110.
- U.S. Department of Health and Human Servcies—Organ Donation and Transplant Breakthrough Collaborative. Measurement strategy: Organ Donation and Transplant Breakthrough Collaborative. Available at: http://www.organdonationnow.org/. Accessed December 15,2006.
- U.S. Department of Health and Human Services Advisory Committee on Organ Transplantation. Consensus Recommendations to the HHS Secretary. Available at: http://www.organdonor.gov/research/acot.htm. Accessed January 30,2007.
- ,,, et al.Report of a National Conference on Donation after Cardiac Death.Am J Transplant.2006;6:281–291.
- Centers for Medicare and Medicaid Services, Department of Health and Human Services.Medicare and Medicaid Programs. Conditions for coverage for organ procurement organizations (OPOs); final rule.Federal Register.2006;71:30981–31054.
- [JCAHO] Joint Commission on Accreditation of Healthcare Organizations. Health care at the crossroads: strategies for narrowing the organ donation gap and protecting patients. Available at: http://www.jointcommission.org/PublicPolicy/organ_donation.htm. Accessed January 30,2007.
- [JCAHO] Joint Commission on Accreditation of Healthcare Organizations.Revisions to Standard LD.3.110.Jt Comm Perspect.2006;26:7.
- Committee on Increasing Rates of Organ Donation‐Board on Health Sciences Policy‐Institute of Medicine.Organ Donation: Opportunities for Action.Washington, DC:National Academies Press;2006.
- National Conference of Commissioners on Uniform State Laws. Revised Uniform Anatomical Gift Act (2006) and Amendment to Section 21 (2007). http://www.law.upenn.edu/bll/ulc/uaga/2006final.htm and http://www.anatomicalgiftact.org/DesktopDefault.aspx?tabindex=0
- ,,, et al.Organ donation and utilization, 1995‐2004: Entering the collaborative era.Am J Transplant.2006;6:1101–1110.
- U.S. Department of Health and Human Servcies—Organ Donation and Transplant Breakthrough Collaborative. Measurement strategy: Organ Donation and Transplant Breakthrough Collaborative. Available at: http://www.organdonationnow.org/. Accessed December 15,2006.
- U.S. Department of Health and Human Services Advisory Committee on Organ Transplantation. Consensus Recommendations to the HHS Secretary. Available at: http://www.organdonor.gov/research/acot.htm. Accessed January 30,2007.
- ,,, et al.Report of a National Conference on Donation after Cardiac Death.Am J Transplant.2006;6:281–291.
- Centers for Medicare and Medicaid Services, Department of Health and Human Services.Medicare and Medicaid Programs. Conditions for coverage for organ procurement organizations (OPOs); final rule.Federal Register.2006;71:30981–31054.
- [JCAHO] Joint Commission on Accreditation of Healthcare Organizations. Health care at the crossroads: strategies for narrowing the organ donation gap and protecting patients. Available at: http://www.jointcommission.org/PublicPolicy/organ_donation.htm. Accessed January 30,2007.
- [JCAHO] Joint Commission on Accreditation of Healthcare Organizations.Revisions to Standard LD.3.110.Jt Comm Perspect.2006;26:7.
- Committee on Increasing Rates of Organ Donation‐Board on Health Sciences Policy‐Institute of Medicine.Organ Donation: Opportunities for Action.Washington, DC:National Academies Press;2006.
- National Conference of Commissioners on Uniform State Laws. Revised Uniform Anatomical Gift Act (2006) and Amendment to Section 21 (2007). http://www.law.upenn.edu/bll/ulc/uaga/2006final.htm and http://www.anatomicalgiftact.org/DesktopDefault.aspx?tabindex=0