User login
Fungating Mass on the Abdominal Wall
The Diagnosis: Basal Cell Carcinoma
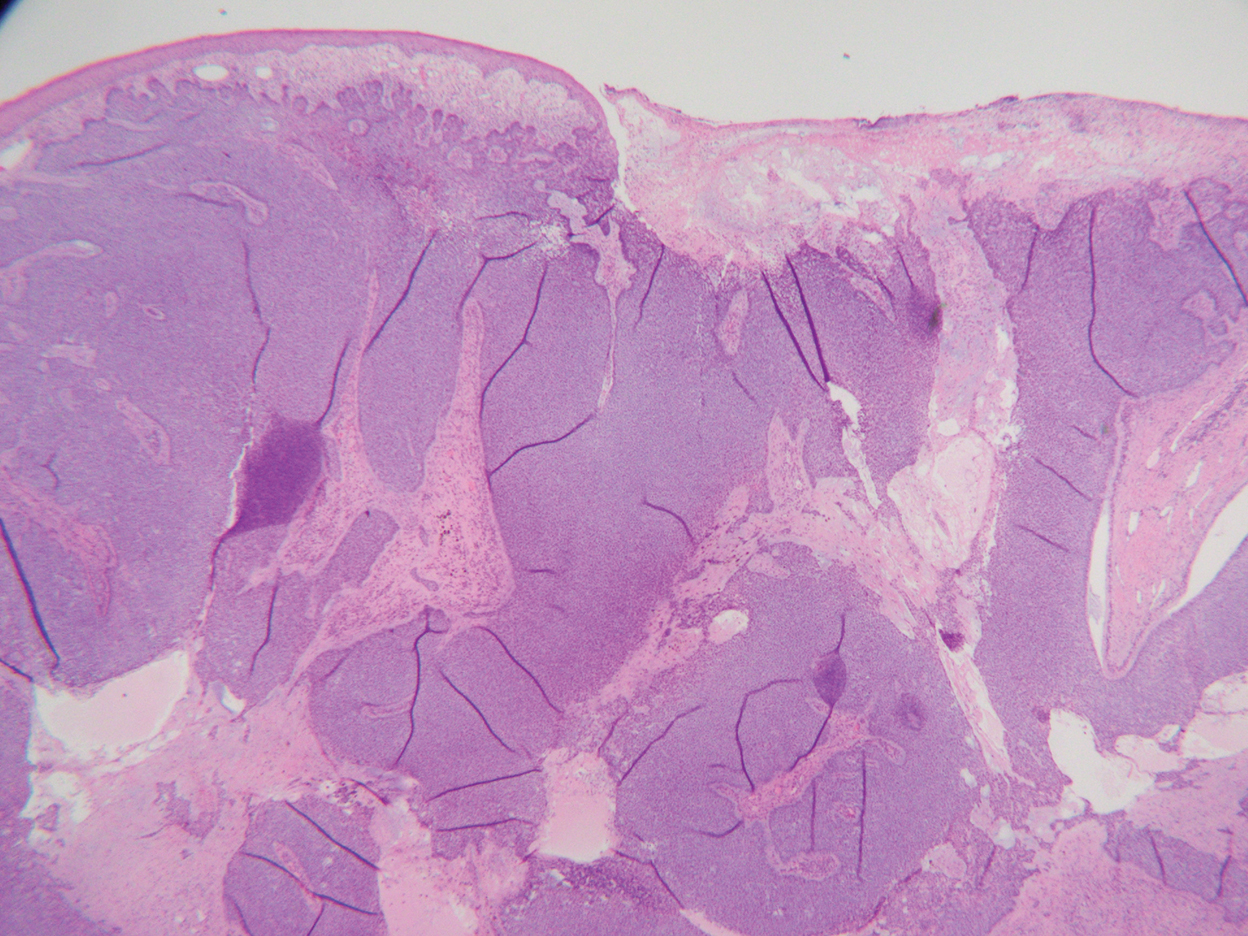
Histopathology was consistent with fungating basal cell carcinoma (BCC). The nodules were comprised of syncytial basaloid cells with high nuclear to cytoplasmic ratios, numerous mitotic figures, fibromyxoid stroma, and peripheral nuclear palisading (Figure). Fortunately, no perineural or lymphovascular invasion was identified, and the margins of the specimen were negative. Despite the high-risk nature of giant BCC, the mass was solitary without notable local invasion, leaving it amendable to surgery. On follow-up, the patient has remained recurrence free, and her hemoglobin level has since stabilized.
Skin cancer is the most common malignancy worldwide, and BCC accounts for more than 80% of nonmelanoma skin cancers in the United States. The incidence is on the rise due to the aging population and increasing cumulative skin exposure.1 Risk factors include both individual physical characteristics and environmental exposures. Individuals with lighter skin tones, red and blonde hair, and blue and green eyes are at an increased risk.2 UV radiation exposure is the most important cause of BCC.3 Chronic immunosuppression and exposure to arsenic, ionizing radiation, and psoralen plus UVA radiation also have been linked to the development of BCC.4-6 Basal cell carcinomas most commonly arise on sun-exposed areas such as the face, though more than 10% of cases appear on the trunk.7 Lesions characteristically remain localized, and growth rate is variable; however, when left untreated, BCCs have the potential to become locally destructive and difficult to treat.
Advanced BCCs are tumors that penetrate deeply into the skin. They often are not amenable to traditional therapy and/ or metastasize. Those that grow to a diameter greater than 5 cm, as in our patient, are known as giant BCCs. Only 0.5% to 1% of BCCs are giant BCCs8 ; they typically are more aggressive in nature with higher rates of local recurrence and metastasis. Individuals who develop giant BCCs either have had a delay in access to medical care or a history of BCC that was inadequately managed.9,10 During the COVID-19 pandemic, patient access to health care was substantially impacted during lockdowns. As in our patient, skin neoplasms and other medical conditions may present in later stages due to medical neglect.11,12 Metastasis is rare, even in advanced BCCs. A review of the literature from 1984 estimated that the incidence of metastasis of BCCs is 1 in 1000 to 35,000. Metastasis portends a poor prognosis with a median overall survival of 8 to 14 months.13 An updated review in 2013 found similar outcomes.14
The choice of management for BCCs depends on the risk for recurrence as well as individual patient factors. Characteristics such as tumor size, location, histology, whether it is a primary or recurrent lesion, and the presence of chronic skin disease determine the recurrence rate.15 The management of advanced BCCs often requires a multidisciplinary approach, as these neoplasms may not be amenable to local therapy without causing substantial morbidity. Mohs micrographic surgery is the treatment of choice for BCCs at high risk for recurrence.16 Standard surgical excision with postoperative margin assessment is acceptable when Mohs micrographic surgery is not available.17 Radiation therapy is an alternative for patients who are not candidates for surgery.18
Recently, improved understanding of the molecular pathogenesis of BCCs has led to the development of novel systemic therapies. The Hedgehog signaling pathway has been found to play a critical role in the development of most BCCs.19 Vismodegib and sonidegib are small-molecule inhibitors of the Hedgehog signaling pathway approved for the treatment of locally advanced and metastatic BCCs that are not amenable to surgery or radiation. Approximately 50% of advanced BCCs respond to these therapies; however, long-term treatment may be limited by intolerable side effects and the development of resistance.20 Basal cell carcinomas that spread to lymph nodes or distant sites are treated with traditional systemic therapy. Historically, conventional cytotoxic chemotherapies, such as platinum-containing regimens, were employed with limited benefit and notable morbidity.21
The differential diagnosis for our patient included several other cutaneous neoplasms. Squamous cell carcinoma is the second most common type of skin cancer. Similar to BCC, it can reach a substantial size if left untreated. Risk factors include chronic inflammation, exposure to radiation or chemical carcinogens, burns, human papillomavirus, and other chronic infections. Giant squamous cell carcinomas have high malignant potential and require imaging to assess the extent of invasion and for metastasis. Surgery typically is necessary for both staging and treatment. Adjuvant therapy also may be necessary.22,23
Internal malignant neoplasms rarely present as cutaneous metastases. Breast cancer, melanoma, and cancers of the upper respiratory tract most frequently metastasize to the skin. Although colorectal cancer (CRC) rarely metastasizes to the skin, it is an important cause of cutaneous metastasis due to its high incidence in the general population. When it does spread to the skin, CRC preferentially affects the abdominal wall. Lesions typically resemble the primary tumor but may appear anaplastic. The occurrence of cutaneous metastasis suggests latestage disease and carries a poor prognosis.24
Merkel cell carcinoma and melanoma are aggressive skin cancers with high mortality rates. The former is rarer but more lethal. Merkel cell carcinomas typically occur in elderly white men on sun-exposed areas of the skin. Tumors present as asymptomatic, rapidly expanding, blue-red, firm nodules. Immunosuppression and UV light exposure are notable risk factors.25 Of the 4 major subtypes of cutaneous melanoma, superficial spreading is the most common, followed by nodular, lentigo maligna, and acral lentiginous.26 Superficial spreading melanoma characteristically presents as an expanding asymmetric macule or thin plaque with irregular borders and variation in size and color (black, brown, or red). Nodular melanoma usually presents as symmetric in shape and color (amelanotic, black, or brown). Early recognition by both the patient and clinician is essential in preventing tumor growth and progression.27
Our patient’s presentation was highly concerning for cutaneous metastasis given her history of CRC. Furthermore, the finding of severe anemia was atypical for skin cancer and more characteristic of the prior malignancy. Imaging revealed a locally confined mass with no evidence of extension, lymph node involvement, or additional lesions. The diagnosis was clinched with histopathologic examination.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Lear JT, Tan BB, Smith AG, et al. Risk factors for basal cell carcinoma in the UK: case-control study in 806 patients. J R Soc Med. 1997; 90:371-374.
- Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer: I. basal cell carcinoma. Arch Dermatol. 1995;131:157-163.
- Guo HR, Yu HS, Hu H, et al. Arsenic in drinking water and skin cancers: cell-type specificity (Taiwan, ROC). Cancer Causes Control. 2001;12:909-916.
- Lichter MD, Karagas MR, Mott LA, et al; The New Hampshire Skin Cancer Study Group. Therapeutic ionizing radiation and the incidence of basal cell carcinoma and squamous cell carcinoma. Arch Dermatol. 2000;136:1007-1011.
- Nijsten TEC, Stern RS. The increased risk of skin cancer is persistent after discontinuation of psoralen plus ultraviolet A: a cohort study. J Invest Dermatol. 2003;121:252-258.
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41-47.
- Gualdi G, Monari P, Calzavara‐Pinton P, et al. When basal cell carcinomas became giant: an Italian multicenter study. Int J Dermatol. 2020;59:377-382.
- Randle HW, Roenigk RK, Brodland DG. Giant basal cell carcinoma (T3). who is at risk? Cancer. 1993;72:1624-1630.
- Archontaki M, Stavrianos SD, Korkolis DP, et al. Giant basal cell carcinoma: clinicopathological analysis of 51 cases and review of the literature. Anticancer Res. 2009;29:2655-2663.
- Shifat Ahmed SAK, Ajisola M, Azeem K, et al. Impact of the societal response to COVID-19 on access to healthcare for non-COVID-19 health issues in slum communities of Bangladesh, Kenya, Nigeria and Pakistan: results of pre-COVID and COVID-19 lockdown ssstakeholder engagements. BMJ Glob Health. 2020;5:E003042.
- Gomolin T, Cline A, Handler MZ. The danger of neglecting melanoma during the COVID-19 pandemic. J Dermatolog Treat. 2020;31:444-445.
- von Domarus H, Stevens PJ. Metastatic basal cell carcinoma. report of five cases and review of 170 cases in the literature. J Am Acad Dermatol. 1984;10:1043-1060.
- Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149:615-616.
- Bøgelund FS, Philipsen PA, Gniadecki R. Factors affecting the recurrence rate of basal cell carcinoma. Acta Derm Venereol. 2007;87:330-334.
- Mosterd K, Krekels GAM, Nieman FH, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008;9:1149-1156.
- Wetzig T, Woitek M, Eichhorn K, et al. Surgical excision of basal cell carcinoma with complete margin control: outcome at 5-year follow-up. Dermatology. 2010;220:363-369.
- Silverman MK, Kopf AW, Gladstein AH, et al. Recurrence rates of treated basal cell carcinomas. part 4: X-ray therapy. J Dermatol Surg Oncol. 1992;18:549-554.
- Tanese K, Emoto K, Kubota N, et al. Immunohistochemical visualization of the signature of activated Hedgehog signaling pathway in cutaneous epithelial tumors. J Dermatol. 2018;45:1181-1186.
- Basset-Séguin N, Hauschild A, Kunstfeld R, et al. Vismodegib in patients with advanced basal cell carcinoma: primary analysis of STEVIE, an international, open-label trial. Eur J Cancer. 2017;86:334-348.
- Carneiro BA, Watkin WG, Mehta UK, et al. Metastatic basal cell carcinoma: complete response to chemotherapy and associated pure red cell aplasia. Cancer Invest. 2006;24:396-400.
- Misiakos EP, Damaskou V, Koumarianou A, et al. A giant squamous cell carcinoma of the skin of the thoracic wall: a case report and review of the literature. J Med Case Rep. 2017;11:136.
- Wollina U, Bayyoud Y, Krönert C, et al. Giant epithelial malignancies (basal cell carcinoma, squamous cell carcinoma): a series of 20 tumors from a single center. J Cutan Aesthet Surg. 2012;5:12-19.
- Bittencourt MJS, Imbiriba AA, Oliveira OA, et al. Cutaneous metastasis of colorectal cancer. An Bras Dermatol. 2018;93:884-886.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
- Buettner PG, Leiter U, Eigentler TK, et al. Development of prognostic factors and survival in cutaneous melanoma over 25 years: an analysis of the Central Malignant Melanoma Registry of the German Dermatological Society. Cancer. 2005;103:616-624.
- Klebanov N, Gunasekera N, Lin WM, et al. The clinical spectrum of cutaneous melanoma morphology. J Am Acad Dermatol. 2019; 80:178-188.e3.
The Diagnosis: Basal Cell Carcinoma
Histopathology was consistent with fungating basal cell carcinoma (BCC). The nodules were comprised of syncytial basaloid cells with high nuclear to cytoplasmic ratios, numerous mitotic figures, fibromyxoid stroma, and peripheral nuclear palisading (Figure). Fortunately, no perineural or lymphovascular invasion was identified, and the margins of the specimen were negative. Despite the high-risk nature of giant BCC, the mass was solitary without notable local invasion, leaving it amendable to surgery. On follow-up, the patient has remained recurrence free, and her hemoglobin level has since stabilized.
Skin cancer is the most common malignancy worldwide, and BCC accounts for more than 80% of nonmelanoma skin cancers in the United States. The incidence is on the rise due to the aging population and increasing cumulative skin exposure.1 Risk factors include both individual physical characteristics and environmental exposures. Individuals with lighter skin tones, red and blonde hair, and blue and green eyes are at an increased risk.2 UV radiation exposure is the most important cause of BCC.3 Chronic immunosuppression and exposure to arsenic, ionizing radiation, and psoralen plus UVA radiation also have been linked to the development of BCC.4-6 Basal cell carcinomas most commonly arise on sun-exposed areas such as the face, though more than 10% of cases appear on the trunk.7 Lesions characteristically remain localized, and growth rate is variable; however, when left untreated, BCCs have the potential to become locally destructive and difficult to treat.
Advanced BCCs are tumors that penetrate deeply into the skin. They often are not amenable to traditional therapy and/ or metastasize. Those that grow to a diameter greater than 5 cm, as in our patient, are known as giant BCCs. Only 0.5% to 1% of BCCs are giant BCCs8 ; they typically are more aggressive in nature with higher rates of local recurrence and metastasis. Individuals who develop giant BCCs either have had a delay in access to medical care or a history of BCC that was inadequately managed.9,10 During the COVID-19 pandemic, patient access to health care was substantially impacted during lockdowns. As in our patient, skin neoplasms and other medical conditions may present in later stages due to medical neglect.11,12 Metastasis is rare, even in advanced BCCs. A review of the literature from 1984 estimated that the incidence of metastasis of BCCs is 1 in 1000 to 35,000. Metastasis portends a poor prognosis with a median overall survival of 8 to 14 months.13 An updated review in 2013 found similar outcomes.14
The choice of management for BCCs depends on the risk for recurrence as well as individual patient factors. Characteristics such as tumor size, location, histology, whether it is a primary or recurrent lesion, and the presence of chronic skin disease determine the recurrence rate.15 The management of advanced BCCs often requires a multidisciplinary approach, as these neoplasms may not be amenable to local therapy without causing substantial morbidity. Mohs micrographic surgery is the treatment of choice for BCCs at high risk for recurrence.16 Standard surgical excision with postoperative margin assessment is acceptable when Mohs micrographic surgery is not available.17 Radiation therapy is an alternative for patients who are not candidates for surgery.18
Recently, improved understanding of the molecular pathogenesis of BCCs has led to the development of novel systemic therapies. The Hedgehog signaling pathway has been found to play a critical role in the development of most BCCs.19 Vismodegib and sonidegib are small-molecule inhibitors of the Hedgehog signaling pathway approved for the treatment of locally advanced and metastatic BCCs that are not amenable to surgery or radiation. Approximately 50% of advanced BCCs respond to these therapies; however, long-term treatment may be limited by intolerable side effects and the development of resistance.20 Basal cell carcinomas that spread to lymph nodes or distant sites are treated with traditional systemic therapy. Historically, conventional cytotoxic chemotherapies, such as platinum-containing regimens, were employed with limited benefit and notable morbidity.21
The differential diagnosis for our patient included several other cutaneous neoplasms. Squamous cell carcinoma is the second most common type of skin cancer. Similar to BCC, it can reach a substantial size if left untreated. Risk factors include chronic inflammation, exposure to radiation or chemical carcinogens, burns, human papillomavirus, and other chronic infections. Giant squamous cell carcinomas have high malignant potential and require imaging to assess the extent of invasion and for metastasis. Surgery typically is necessary for both staging and treatment. Adjuvant therapy also may be necessary.22,23
Internal malignant neoplasms rarely present as cutaneous metastases. Breast cancer, melanoma, and cancers of the upper respiratory tract most frequently metastasize to the skin. Although colorectal cancer (CRC) rarely metastasizes to the skin, it is an important cause of cutaneous metastasis due to its high incidence in the general population. When it does spread to the skin, CRC preferentially affects the abdominal wall. Lesions typically resemble the primary tumor but may appear anaplastic. The occurrence of cutaneous metastasis suggests latestage disease and carries a poor prognosis.24
Merkel cell carcinoma and melanoma are aggressive skin cancers with high mortality rates. The former is rarer but more lethal. Merkel cell carcinomas typically occur in elderly white men on sun-exposed areas of the skin. Tumors present as asymptomatic, rapidly expanding, blue-red, firm nodules. Immunosuppression and UV light exposure are notable risk factors.25 Of the 4 major subtypes of cutaneous melanoma, superficial spreading is the most common, followed by nodular, lentigo maligna, and acral lentiginous.26 Superficial spreading melanoma characteristically presents as an expanding asymmetric macule or thin plaque with irregular borders and variation in size and color (black, brown, or red). Nodular melanoma usually presents as symmetric in shape and color (amelanotic, black, or brown). Early recognition by both the patient and clinician is essential in preventing tumor growth and progression.27
Our patient’s presentation was highly concerning for cutaneous metastasis given her history of CRC. Furthermore, the finding of severe anemia was atypical for skin cancer and more characteristic of the prior malignancy. Imaging revealed a locally confined mass with no evidence of extension, lymph node involvement, or additional lesions. The diagnosis was clinched with histopathologic examination.
The Diagnosis: Basal Cell Carcinoma
Histopathology was consistent with fungating basal cell carcinoma (BCC). The nodules were comprised of syncytial basaloid cells with high nuclear to cytoplasmic ratios, numerous mitotic figures, fibromyxoid stroma, and peripheral nuclear palisading (Figure). Fortunately, no perineural or lymphovascular invasion was identified, and the margins of the specimen were negative. Despite the high-risk nature of giant BCC, the mass was solitary without notable local invasion, leaving it amendable to surgery. On follow-up, the patient has remained recurrence free, and her hemoglobin level has since stabilized.
Skin cancer is the most common malignancy worldwide, and BCC accounts for more than 80% of nonmelanoma skin cancers in the United States. The incidence is on the rise due to the aging population and increasing cumulative skin exposure.1 Risk factors include both individual physical characteristics and environmental exposures. Individuals with lighter skin tones, red and blonde hair, and blue and green eyes are at an increased risk.2 UV radiation exposure is the most important cause of BCC.3 Chronic immunosuppression and exposure to arsenic, ionizing radiation, and psoralen plus UVA radiation also have been linked to the development of BCC.4-6 Basal cell carcinomas most commonly arise on sun-exposed areas such as the face, though more than 10% of cases appear on the trunk.7 Lesions characteristically remain localized, and growth rate is variable; however, when left untreated, BCCs have the potential to become locally destructive and difficult to treat.
Advanced BCCs are tumors that penetrate deeply into the skin. They often are not amenable to traditional therapy and/ or metastasize. Those that grow to a diameter greater than 5 cm, as in our patient, are known as giant BCCs. Only 0.5% to 1% of BCCs are giant BCCs8 ; they typically are more aggressive in nature with higher rates of local recurrence and metastasis. Individuals who develop giant BCCs either have had a delay in access to medical care or a history of BCC that was inadequately managed.9,10 During the COVID-19 pandemic, patient access to health care was substantially impacted during lockdowns. As in our patient, skin neoplasms and other medical conditions may present in later stages due to medical neglect.11,12 Metastasis is rare, even in advanced BCCs. A review of the literature from 1984 estimated that the incidence of metastasis of BCCs is 1 in 1000 to 35,000. Metastasis portends a poor prognosis with a median overall survival of 8 to 14 months.13 An updated review in 2013 found similar outcomes.14
The choice of management for BCCs depends on the risk for recurrence as well as individual patient factors. Characteristics such as tumor size, location, histology, whether it is a primary or recurrent lesion, and the presence of chronic skin disease determine the recurrence rate.15 The management of advanced BCCs often requires a multidisciplinary approach, as these neoplasms may not be amenable to local therapy without causing substantial morbidity. Mohs micrographic surgery is the treatment of choice for BCCs at high risk for recurrence.16 Standard surgical excision with postoperative margin assessment is acceptable when Mohs micrographic surgery is not available.17 Radiation therapy is an alternative for patients who are not candidates for surgery.18
Recently, improved understanding of the molecular pathogenesis of BCCs has led to the development of novel systemic therapies. The Hedgehog signaling pathway has been found to play a critical role in the development of most BCCs.19 Vismodegib and sonidegib are small-molecule inhibitors of the Hedgehog signaling pathway approved for the treatment of locally advanced and metastatic BCCs that are not amenable to surgery or radiation. Approximately 50% of advanced BCCs respond to these therapies; however, long-term treatment may be limited by intolerable side effects and the development of resistance.20 Basal cell carcinomas that spread to lymph nodes or distant sites are treated with traditional systemic therapy. Historically, conventional cytotoxic chemotherapies, such as platinum-containing regimens, were employed with limited benefit and notable morbidity.21
The differential diagnosis for our patient included several other cutaneous neoplasms. Squamous cell carcinoma is the second most common type of skin cancer. Similar to BCC, it can reach a substantial size if left untreated. Risk factors include chronic inflammation, exposure to radiation or chemical carcinogens, burns, human papillomavirus, and other chronic infections. Giant squamous cell carcinomas have high malignant potential and require imaging to assess the extent of invasion and for metastasis. Surgery typically is necessary for both staging and treatment. Adjuvant therapy also may be necessary.22,23
Internal malignant neoplasms rarely present as cutaneous metastases. Breast cancer, melanoma, and cancers of the upper respiratory tract most frequently metastasize to the skin. Although colorectal cancer (CRC) rarely metastasizes to the skin, it is an important cause of cutaneous metastasis due to its high incidence in the general population. When it does spread to the skin, CRC preferentially affects the abdominal wall. Lesions typically resemble the primary tumor but may appear anaplastic. The occurrence of cutaneous metastasis suggests latestage disease and carries a poor prognosis.24
Merkel cell carcinoma and melanoma are aggressive skin cancers with high mortality rates. The former is rarer but more lethal. Merkel cell carcinomas typically occur in elderly white men on sun-exposed areas of the skin. Tumors present as asymptomatic, rapidly expanding, blue-red, firm nodules. Immunosuppression and UV light exposure are notable risk factors.25 Of the 4 major subtypes of cutaneous melanoma, superficial spreading is the most common, followed by nodular, lentigo maligna, and acral lentiginous.26 Superficial spreading melanoma characteristically presents as an expanding asymmetric macule or thin plaque with irregular borders and variation in size and color (black, brown, or red). Nodular melanoma usually presents as symmetric in shape and color (amelanotic, black, or brown). Early recognition by both the patient and clinician is essential in preventing tumor growth and progression.27
Our patient’s presentation was highly concerning for cutaneous metastasis given her history of CRC. Furthermore, the finding of severe anemia was atypical for skin cancer and more characteristic of the prior malignancy. Imaging revealed a locally confined mass with no evidence of extension, lymph node involvement, or additional lesions. The diagnosis was clinched with histopathologic examination.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Lear JT, Tan BB, Smith AG, et al. Risk factors for basal cell carcinoma in the UK: case-control study in 806 patients. J R Soc Med. 1997; 90:371-374.
- Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer: I. basal cell carcinoma. Arch Dermatol. 1995;131:157-163.
- Guo HR, Yu HS, Hu H, et al. Arsenic in drinking water and skin cancers: cell-type specificity (Taiwan, ROC). Cancer Causes Control. 2001;12:909-916.
- Lichter MD, Karagas MR, Mott LA, et al; The New Hampshire Skin Cancer Study Group. Therapeutic ionizing radiation and the incidence of basal cell carcinoma and squamous cell carcinoma. Arch Dermatol. 2000;136:1007-1011.
- Nijsten TEC, Stern RS. The increased risk of skin cancer is persistent after discontinuation of psoralen plus ultraviolet A: a cohort study. J Invest Dermatol. 2003;121:252-258.
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41-47.
- Gualdi G, Monari P, Calzavara‐Pinton P, et al. When basal cell carcinomas became giant: an Italian multicenter study. Int J Dermatol. 2020;59:377-382.
- Randle HW, Roenigk RK, Brodland DG. Giant basal cell carcinoma (T3). who is at risk? Cancer. 1993;72:1624-1630.
- Archontaki M, Stavrianos SD, Korkolis DP, et al. Giant basal cell carcinoma: clinicopathological analysis of 51 cases and review of the literature. Anticancer Res. 2009;29:2655-2663.
- Shifat Ahmed SAK, Ajisola M, Azeem K, et al. Impact of the societal response to COVID-19 on access to healthcare for non-COVID-19 health issues in slum communities of Bangladesh, Kenya, Nigeria and Pakistan: results of pre-COVID and COVID-19 lockdown ssstakeholder engagements. BMJ Glob Health. 2020;5:E003042.
- Gomolin T, Cline A, Handler MZ. The danger of neglecting melanoma during the COVID-19 pandemic. J Dermatolog Treat. 2020;31:444-445.
- von Domarus H, Stevens PJ. Metastatic basal cell carcinoma. report of five cases and review of 170 cases in the literature. J Am Acad Dermatol. 1984;10:1043-1060.
- Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149:615-616.
- Bøgelund FS, Philipsen PA, Gniadecki R. Factors affecting the recurrence rate of basal cell carcinoma. Acta Derm Venereol. 2007;87:330-334.
- Mosterd K, Krekels GAM, Nieman FH, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008;9:1149-1156.
- Wetzig T, Woitek M, Eichhorn K, et al. Surgical excision of basal cell carcinoma with complete margin control: outcome at 5-year follow-up. Dermatology. 2010;220:363-369.
- Silverman MK, Kopf AW, Gladstein AH, et al. Recurrence rates of treated basal cell carcinomas. part 4: X-ray therapy. J Dermatol Surg Oncol. 1992;18:549-554.
- Tanese K, Emoto K, Kubota N, et al. Immunohistochemical visualization of the signature of activated Hedgehog signaling pathway in cutaneous epithelial tumors. J Dermatol. 2018;45:1181-1186.
- Basset-Séguin N, Hauschild A, Kunstfeld R, et al. Vismodegib in patients with advanced basal cell carcinoma: primary analysis of STEVIE, an international, open-label trial. Eur J Cancer. 2017;86:334-348.
- Carneiro BA, Watkin WG, Mehta UK, et al. Metastatic basal cell carcinoma: complete response to chemotherapy and associated pure red cell aplasia. Cancer Invest. 2006;24:396-400.
- Misiakos EP, Damaskou V, Koumarianou A, et al. A giant squamous cell carcinoma of the skin of the thoracic wall: a case report and review of the literature. J Med Case Rep. 2017;11:136.
- Wollina U, Bayyoud Y, Krönert C, et al. Giant epithelial malignancies (basal cell carcinoma, squamous cell carcinoma): a series of 20 tumors from a single center. J Cutan Aesthet Surg. 2012;5:12-19.
- Bittencourt MJS, Imbiriba AA, Oliveira OA, et al. Cutaneous metastasis of colorectal cancer. An Bras Dermatol. 2018;93:884-886.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
- Buettner PG, Leiter U, Eigentler TK, et al. Development of prognostic factors and survival in cutaneous melanoma over 25 years: an analysis of the Central Malignant Melanoma Registry of the German Dermatological Society. Cancer. 2005;103:616-624.
- Klebanov N, Gunasekera N, Lin WM, et al. The clinical spectrum of cutaneous melanoma morphology. J Am Acad Dermatol. 2019; 80:178-188.e3.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Lear JT, Tan BB, Smith AG, et al. Risk factors for basal cell carcinoma in the UK: case-control study in 806 patients. J R Soc Med. 1997; 90:371-374.
- Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer: I. basal cell carcinoma. Arch Dermatol. 1995;131:157-163.
- Guo HR, Yu HS, Hu H, et al. Arsenic in drinking water and skin cancers: cell-type specificity (Taiwan, ROC). Cancer Causes Control. 2001;12:909-916.
- Lichter MD, Karagas MR, Mott LA, et al; The New Hampshire Skin Cancer Study Group. Therapeutic ionizing radiation and the incidence of basal cell carcinoma and squamous cell carcinoma. Arch Dermatol. 2000;136:1007-1011.
- Nijsten TEC, Stern RS. The increased risk of skin cancer is persistent after discontinuation of psoralen plus ultraviolet A: a cohort study. J Invest Dermatol. 2003;121:252-258.
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41-47.
- Gualdi G, Monari P, Calzavara‐Pinton P, et al. When basal cell carcinomas became giant: an Italian multicenter study. Int J Dermatol. 2020;59:377-382.
- Randle HW, Roenigk RK, Brodland DG. Giant basal cell carcinoma (T3). who is at risk? Cancer. 1993;72:1624-1630.
- Archontaki M, Stavrianos SD, Korkolis DP, et al. Giant basal cell carcinoma: clinicopathological analysis of 51 cases and review of the literature. Anticancer Res. 2009;29:2655-2663.
- Shifat Ahmed SAK, Ajisola M, Azeem K, et al. Impact of the societal response to COVID-19 on access to healthcare for non-COVID-19 health issues in slum communities of Bangladesh, Kenya, Nigeria and Pakistan: results of pre-COVID and COVID-19 lockdown ssstakeholder engagements. BMJ Glob Health. 2020;5:E003042.
- Gomolin T, Cline A, Handler MZ. The danger of neglecting melanoma during the COVID-19 pandemic. J Dermatolog Treat. 2020;31:444-445.
- von Domarus H, Stevens PJ. Metastatic basal cell carcinoma. report of five cases and review of 170 cases in the literature. J Am Acad Dermatol. 1984;10:1043-1060.
- Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149:615-616.
- Bøgelund FS, Philipsen PA, Gniadecki R. Factors affecting the recurrence rate of basal cell carcinoma. Acta Derm Venereol. 2007;87:330-334.
- Mosterd K, Krekels GAM, Nieman FH, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008;9:1149-1156.
- Wetzig T, Woitek M, Eichhorn K, et al. Surgical excision of basal cell carcinoma with complete margin control: outcome at 5-year follow-up. Dermatology. 2010;220:363-369.
- Silverman MK, Kopf AW, Gladstein AH, et al. Recurrence rates of treated basal cell carcinomas. part 4: X-ray therapy. J Dermatol Surg Oncol. 1992;18:549-554.
- Tanese K, Emoto K, Kubota N, et al. Immunohistochemical visualization of the signature of activated Hedgehog signaling pathway in cutaneous epithelial tumors. J Dermatol. 2018;45:1181-1186.
- Basset-Séguin N, Hauschild A, Kunstfeld R, et al. Vismodegib in patients with advanced basal cell carcinoma: primary analysis of STEVIE, an international, open-label trial. Eur J Cancer. 2017;86:334-348.
- Carneiro BA, Watkin WG, Mehta UK, et al. Metastatic basal cell carcinoma: complete response to chemotherapy and associated pure red cell aplasia. Cancer Invest. 2006;24:396-400.
- Misiakos EP, Damaskou V, Koumarianou A, et al. A giant squamous cell carcinoma of the skin of the thoracic wall: a case report and review of the literature. J Med Case Rep. 2017;11:136.
- Wollina U, Bayyoud Y, Krönert C, et al. Giant epithelial malignancies (basal cell carcinoma, squamous cell carcinoma): a series of 20 tumors from a single center. J Cutan Aesthet Surg. 2012;5:12-19.
- Bittencourt MJS, Imbiriba AA, Oliveira OA, et al. Cutaneous metastasis of colorectal cancer. An Bras Dermatol. 2018;93:884-886.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
- Buettner PG, Leiter U, Eigentler TK, et al. Development of prognostic factors and survival in cutaneous melanoma over 25 years: an analysis of the Central Malignant Melanoma Registry of the German Dermatological Society. Cancer. 2005;103:616-624.
- Klebanov N, Gunasekera N, Lin WM, et al. The clinical spectrum of cutaneous melanoma morphology. J Am Acad Dermatol. 2019; 80:178-188.e3.
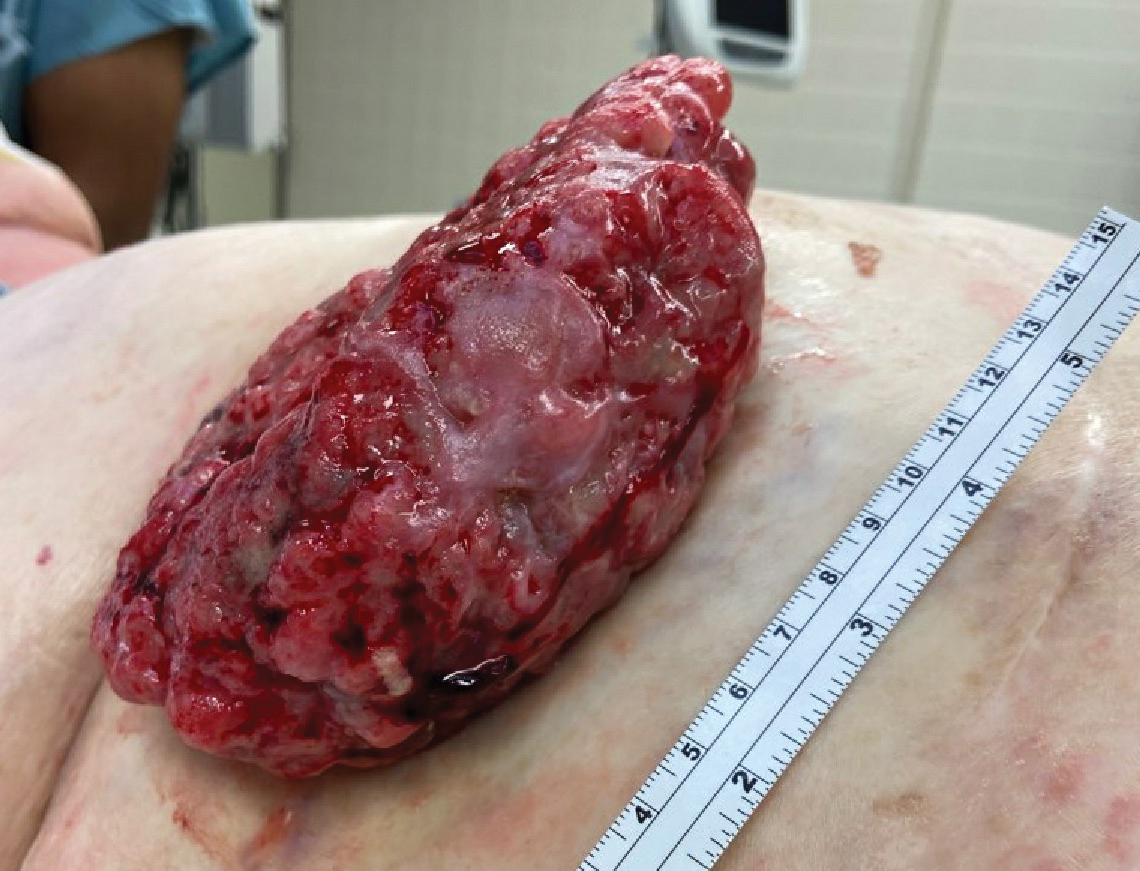
A 77-year-old woman was admitted to the hospital with anemia (hemoglobin, 5.2 g/dL [reference range, 12.0–15.5 g/dL]) and a rapidly growing abdominal wall mass. She had a history of stage IIA colon cancer (T3N0M0) that was treated 5 years prior with a partial colon resection and adjuvant chemotherapy. She initially noticed a red scaly lesion developing around a scar from a prior surgery that had been stable for years. Over the last 2 months, the lesion rapidly expanded and would intermittently bleed. Physical examination revealed a 13×10×4.5-cm, pink-red, nodular, firm mass over the patient’s right upper quadrant. Computed tomography revealed a mass limited to the skin and superficial tissue. General surgery was consulted for excision of the mass.