User login
Precision and Accuracy of Identification of Anatomical Surface Landmarks by 30 Expert Hip Arthroscopists
Take-Home Points
- Surface landmarks are routinely used for physical examination and surgical technique.
- Common surface landmarks used in establishing arthroscopic portals may be more difficult to accurately identify than previously thought.
- The greater trochanter was the surface landmark most precisely identified by expert examiners.
- Ultrasound examination identified landmarks varied from landmarks identified by palpation alone.
Anatomical surface landmarks about the hip and lower abdomen are often referenced when placing arthroscopic portals and office-based injections.1-3 However, the degree to which these landmarks can be reproducibly identified using only visual inspection and palpation is unknown.
Safe access to the hip joint and surrounding structures during hip arthroscopy has been a focus in the orthopedic literature. Authors have described anatomical relationships of recommended portals to neurovascular and other anatomical structures.4-6 This information has been reported in millimeters to centimeters of safety based on cadaver dissection studies.4-7We conducted a study to assess expert hip arthroscopists’ ability to identify, using only physical examination techniques, the anatomical structures used for reference when creating safe starting points for arthroscopic access. We hypothesized that variance in examiner-identified points would exceed safe distances from neurovascular structures for the most commonly used hip arthroscopic portals. The volunteer in this study provided written informed consent for print and electronic publication of this article.
Methods
In this study, we prospectively assessed 30 expert hip arthroscopic surgeons’ ability to identify commonly referenced surface landmarks on the adult male hip, using only inspection and manual palpation. Surgeons were defined as experts on the basis of their status as hip arthroscopy instructors at the Orthopaedic Learning Center (Rosemont, IL) for the Arthroscopy Association of North America and industry-sponsored hip arthroscopy education faculty (Arthrex). Five surface landmarks were selected for their relevance to publications on safe portal placement2-5: anterior superior iliac spine (ASIS), tip of greater trochanter (GT), rectus origin (RO), superficial inguinal ring (SIR), and psoas tendon (PT).
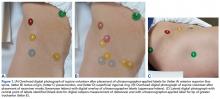
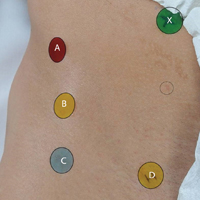
A healthy adult male volunteer was placed supine on an examination table and exposed distally from the mid abdomen, with the perineum and the genital area covered bikini-style. An expert musculoskeletal ultrasonographer used a handheld musculoskeletal ultrasound transducer (Sonosite) to identify the 5 landmarks. Short- and long-axis images of each structure were obtained. The examiner applied a round (1 cm in diameter), uniquely colored adhesive label to the skin over each location. A professional photographer using a Canon digital camera and fixed mounts made precise overhead and lateral images. The positional integrity and scale of these images were confirmed with referral to constant anatomical skin features. Images were archived for analysis (Figure 1A).
After the ultrasonographer’s labels were removed, each of the 30 expert hip arthroscopic surgeons identified the structures by static physical examination (inspection and palpation only) and applied the same colored labels to the skin.
Imaging software (Adobe Photoshop Creative Suite 5.1) was used to superimpose the digital images of the examiner labels on those of the ultrasound-verified anatomical labels (Figure 1C). Measurements were then taken with digital calipers to determine average distance from ultrasound label; accuracy within 10 mm of verified ultrasound label; true average location (TAL) determined by 95% confidence interval (CI); and interobserver variability calculated by 95% prediction interval, which determined the probability of where an additional examiner data point would lie.
In the second arm of the study, examiner data were compared with previously published data on arthroscopic portal safety.
Results
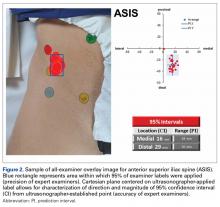
Average absolute distance from examiner labels to ultrasonographer labels was 31 mm for ASIS, 24 mm for GT, 26 mm for RO, 19 mm for SIR, and 35 mm for PT (Figure 2).
Of the 30 surgeons, 1 (3%) came within 10 mm of the ultrasound for ASIS, 1 (3%) for GT, 4 (13%) for RO, 5 (17%) for SIR, and 1 (3%) for PT (Table 1).
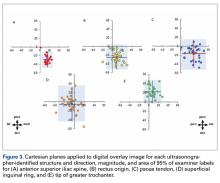
TAL as determined by CI was 16 mm medial and 29 mm inferior for ASIS; 8 mm anterior and 22 mm superior for GT; 10 mm medial and 25 mm inferior for RO; 5 mm lateral and 5 mm inferior for SIR; and 28 mm medial and 16 mm inferior for PT (Figure 3, Table 2). Interobserver variability determined by prediction interval had a range of 18 mm medial to lateral × 36 mm proximal to distal for ASIS; 33 mm anterior to posterior × 48 mm superior to inferior for GT; 41 mm medial to distal × 54 mm proximal to distal for RO; 51 mm medial to lateral × 74 mm proximal to distal for SIR; and 49 mm medial to distal × 61 mm proximal to distal for PT.
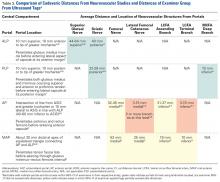
Given the difference between examiner data (direction and distance from ultrasound labels) and published data (distance to significant neurovascular structures), inaccurate identification of surface landmarks has the potential to lead to AP and MAP damage (Table 3). The examiner GT and ASIS surface landmarks used for AP overlapped directly with the safe distances for the lateral femoral cutaneous nerve and the terminal branch of the lateral circumflex femoral artery.
Discussion
Others have investigated examiners’ use of palpation, compared with ultrasound, to identify common shoulder and knee structures.8-10 In a 2011 systematic review, Gilliland and colleagues11 confirmed that accuracy was improved with use of ultrasound (vs palpation) for injections in the shoulder, hip, knee, wrist, and ankle. Given the scarcity of data in this setting, we conducted the present study to assess the precision and accuracy of expert arthroscopists in identifying common surface landmarks. We hypothesized that physical examination and ultrasound examination would differ significantly in precisely and accurately identifying these landmarks.
Working with a standard awake volunteer, our test group of examiners was consistently inaccurate when they accepted ultrasonographer-placed labels as the ideal. Precision within the group, however, trended toward close agreement; examiners consistently placed labels in the same direction and approximate magnitude away from ultrasonographer labels. This suggests that a discrepancy between the ultrasonographic surface structure definitions taught to ultrasonographers and the manually identified definitions taught to surgeons for arthroscopy (training bias) can generate differences in landmark identification.
Given reported low rates of complications in the creation of standard surface anatomy portals, more data is needed to correlate whether safe distance guidelines best apply to the points identified by hip experts or the points identified by ultrasonographers. In a 2013 systematic review, Harris and colleagues8 found a 7.5% overall complication rate, with temporary neuropraxia 1 of the 2 most common complications. Whether adding ultrasound to physical examination for the creation of some or all portals will reduce the incidence of these problems is unknown. Regardless of the anatomical area referenced by experts for portal creation, the tight grouping of examiner marks in our study supports a consensus regarding the location of the landmarks studied.
In our study of the use of surface anatomical landmarks for the creation of portals, we analyzed 4 previously described locations: ALP, AP, PLP, and MAP. ALP, AP, and PLP directly reference at least 1 surface anatomical structure; AP references 2 anatomical structures (ASIS, GT); and MAP indirectly references ASIS and GT and directly references ALP and AP. In cadaveric and radiographic studies, 7 neurovascular structures have been described in proximity to ALP, AP, MAP, and PLP: superior gluteal nerve, sciatic nerve, femoral nerve, lateral femoral cutaneous nerve, lateral circumflex femoral artery, and medial circumflex femoral artery.5,6 Our results showed that use of surface anatomy in AP and MAP creation most likely places structures at risk, given the overlap of examiner CIs and the previously published cadaveric5,6 and radiographic7 data.
Hua and colleagues12 confirmed the feasibility of using ultrasound for the creation of hip arthroscopy portals. More data is needed to assess how the standard palpation-and-fluoroscopy method described by Byrd3 compares with an ultrasound-guided technique in safety and cost. However, data from our study should not be used to justify a demand for ultrasound during arthroscopy portal establishment, as limitations do not permit such a recommendation.
With diagnostic injection remaining a mainstay of differential diagnosis and treatment about the hip,1 the data presented here suggest a potential for ultrasound in enhancing outcomes. There is evidence supporting the role of image guidance in improving palpation accuracy in the area of the biceps tendon in the forearm.10 Potentially, identification and treatment of specific extra-articular structures surrounding the hip could be made safer with more routine use of ultrasound.
Limitations
This study had several limitations. The surgeons were limited to palpation and static examination of a body in its natural state. Hip arthroscopic portals typically are created under traction and after a standard perineal post is placed for hip arthroscopy. In addition, in an awake injection setting, the clinician may receive patient feedback in the form of limb movement or speech. To what degree palpation or ultrasound will be affected in these scenarios is unknown.
Another limitation is the lack of serial examination by each examiner—intrarater variability could not be gauged. In addition, with only 1 ultrasonographic examination performed, there is the potential that adding ultrasonographic examinations, or having an examiner perform serial physical examinations, could better define the precision of each component. Given the practical limitations of our volunteer’s time and the schedules of 30 expert arthroscopists, we kept the chosen study design for its single setting.
Conclusion
Visual inspection and manual palpation are standard means of identifying common surface anatomical landmarks for the creation of arthroscopy portals and the placement of injections. Our study results showed variance in landmark identification between expert examiners and an ultrasonographer. The degree of variance exceeded established neurovascular safe zones, particularly for AP and MAP. This new evidence calls for further investigation into the best, safest means of performing hip arthroscopic techniques and injection-based interventions.
Am J Orthop. 2017;46(1):E65-E70. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Byrd JW, Potts EA, Allison RK, Jones KS. Ultrasound-guided hip injections: a comparative study with fluoroscopy-guided injections. Arthroscopy. 2014;30(1):42-46.
2. Dienst M, Seil R, Kohn DM. Safe arthroscopic access to the central compartment of the hip. Arthroscopy. 2005;21(12):1510-1514.
3. Byrd JW. Hip arthroscopy, the supine approach: technique and anatomy of the intraarticular and peripheral compartments. Tech Orthop. 2005;20(1):17-31.
4. Bond JL, Knutson ZA, Ebert A, Guanche CA. The 23-point arthroscopic examination of the hip: basic setup, portal placement, and surgical technique. Arthroscopy. 2009;25(4):416-429.
5. Roberson WJ, Kelly BT. The safe zone for hip arthroscopy: a cadaveric assessment of central, peripheral, and lateral compartment portal placement. Arthroscopy. 2008;24(9):1019-1026.
6. Byrd JW, Pappas JN, Pedley MJ. Hip arthroscopy: an anatomic study of portal placement and relationship to the extra-articular structures. Arthroscopy. 1995;11(4):418-423.
7. Watson JN, Bohnenkamp F, El-Bitar Y, Moretti V, Domb BG. Variability in locations of hip neurovascular structures and their proximity to hip arthroscopic portals. Arthroscopy. 2014;30(4):462-467.
8. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
9. Jacobson JA, Bedi A, Sekiya JK, Blankenbaker DG. Evaluation of the painful athletic hip: imaging options and imaging-guided injections. AJR Am J Roentgenol. 2012;199(3):516-524.
10. Gazzillo GP, Finnoff JT, Hall MM, Sayeed YA, Smith J. Accuracy of palpating the long head of the biceps tendon: an ultrasonographic study. PM R. 2011;3(11):1035-1040.
11. Gilliland CA, Salazar LD, Borchers JR. Ultrasound versus anatomic guidance for intra-articular and periarticular injection: a systematic review. Phys Sportsmed. 2011;39(3):121-131.
12. Hua Y, Yang Y, Chen S, et al. Ultrasound-guided establishment of hip arthroscopy portals. Arthroscopy. 2009;25(12):1491-1495.
Take-Home Points
- Surface landmarks are routinely used for physical examination and surgical technique.
- Common surface landmarks used in establishing arthroscopic portals may be more difficult to accurately identify than previously thought.
- The greater trochanter was the surface landmark most precisely identified by expert examiners.
- Ultrasound examination identified landmarks varied from landmarks identified by palpation alone.
Anatomical surface landmarks about the hip and lower abdomen are often referenced when placing arthroscopic portals and office-based injections.1-3 However, the degree to which these landmarks can be reproducibly identified using only visual inspection and palpation is unknown.
Safe access to the hip joint and surrounding structures during hip arthroscopy has been a focus in the orthopedic literature. Authors have described anatomical relationships of recommended portals to neurovascular and other anatomical structures.4-6 This information has been reported in millimeters to centimeters of safety based on cadaver dissection studies.4-7We conducted a study to assess expert hip arthroscopists’ ability to identify, using only physical examination techniques, the anatomical structures used for reference when creating safe starting points for arthroscopic access. We hypothesized that variance in examiner-identified points would exceed safe distances from neurovascular structures for the most commonly used hip arthroscopic portals. The volunteer in this study provided written informed consent for print and electronic publication of this article.
Methods
In this study, we prospectively assessed 30 expert hip arthroscopic surgeons’ ability to identify commonly referenced surface landmarks on the adult male hip, using only inspection and manual palpation. Surgeons were defined as experts on the basis of their status as hip arthroscopy instructors at the Orthopaedic Learning Center (Rosemont, IL) for the Arthroscopy Association of North America and industry-sponsored hip arthroscopy education faculty (Arthrex). Five surface landmarks were selected for their relevance to publications on safe portal placement2-5: anterior superior iliac spine (ASIS), tip of greater trochanter (GT), rectus origin (RO), superficial inguinal ring (SIR), and psoas tendon (PT).
A healthy adult male volunteer was placed supine on an examination table and exposed distally from the mid abdomen, with the perineum and the genital area covered bikini-style. An expert musculoskeletal ultrasonographer used a handheld musculoskeletal ultrasound transducer (Sonosite) to identify the 5 landmarks. Short- and long-axis images of each structure were obtained. The examiner applied a round (1 cm in diameter), uniquely colored adhesive label to the skin over each location. A professional photographer using a Canon digital camera and fixed mounts made precise overhead and lateral images. The positional integrity and scale of these images were confirmed with referral to constant anatomical skin features. Images were archived for analysis (Figure 1A).
After the ultrasonographer’s labels were removed, each of the 30 expert hip arthroscopic surgeons identified the structures by static physical examination (inspection and palpation only) and applied the same colored labels to the skin.
Imaging software (Adobe Photoshop Creative Suite 5.1) was used to superimpose the digital images of the examiner labels on those of the ultrasound-verified anatomical labels (Figure 1C). Measurements were then taken with digital calipers to determine average distance from ultrasound label; accuracy within 10 mm of verified ultrasound label; true average location (TAL) determined by 95% confidence interval (CI); and interobserver variability calculated by 95% prediction interval, which determined the probability of where an additional examiner data point would lie.
In the second arm of the study, examiner data were compared with previously published data on arthroscopic portal safety.
Results
Average absolute distance from examiner labels to ultrasonographer labels was 31 mm for ASIS, 24 mm for GT, 26 mm for RO, 19 mm for SIR, and 35 mm for PT (Figure 2).
Of the 30 surgeons, 1 (3%) came within 10 mm of the ultrasound for ASIS, 1 (3%) for GT, 4 (13%) for RO, 5 (17%) for SIR, and 1 (3%) for PT (Table 1).
TAL as determined by CI was 16 mm medial and 29 mm inferior for ASIS; 8 mm anterior and 22 mm superior for GT; 10 mm medial and 25 mm inferior for RO; 5 mm lateral and 5 mm inferior for SIR; and 28 mm medial and 16 mm inferior for PT (Figure 3, Table 2). Interobserver variability determined by prediction interval had a range of 18 mm medial to lateral × 36 mm proximal to distal for ASIS; 33 mm anterior to posterior × 48 mm superior to inferior for GT; 41 mm medial to distal × 54 mm proximal to distal for RO; 51 mm medial to lateral × 74 mm proximal to distal for SIR; and 49 mm medial to distal × 61 mm proximal to distal for PT.
Given the difference between examiner data (direction and distance from ultrasound labels) and published data (distance to significant neurovascular structures), inaccurate identification of surface landmarks has the potential to lead to AP and MAP damage (Table 3). The examiner GT and ASIS surface landmarks used for AP overlapped directly with the safe distances for the lateral femoral cutaneous nerve and the terminal branch of the lateral circumflex femoral artery.
Discussion
Others have investigated examiners’ use of palpation, compared with ultrasound, to identify common shoulder and knee structures.8-10 In a 2011 systematic review, Gilliland and colleagues11 confirmed that accuracy was improved with use of ultrasound (vs palpation) for injections in the shoulder, hip, knee, wrist, and ankle. Given the scarcity of data in this setting, we conducted the present study to assess the precision and accuracy of expert arthroscopists in identifying common surface landmarks. We hypothesized that physical examination and ultrasound examination would differ significantly in precisely and accurately identifying these landmarks.
Working with a standard awake volunteer, our test group of examiners was consistently inaccurate when they accepted ultrasonographer-placed labels as the ideal. Precision within the group, however, trended toward close agreement; examiners consistently placed labels in the same direction and approximate magnitude away from ultrasonographer labels. This suggests that a discrepancy between the ultrasonographic surface structure definitions taught to ultrasonographers and the manually identified definitions taught to surgeons for arthroscopy (training bias) can generate differences in landmark identification.
Given reported low rates of complications in the creation of standard surface anatomy portals, more data is needed to correlate whether safe distance guidelines best apply to the points identified by hip experts or the points identified by ultrasonographers. In a 2013 systematic review, Harris and colleagues8 found a 7.5% overall complication rate, with temporary neuropraxia 1 of the 2 most common complications. Whether adding ultrasound to physical examination for the creation of some or all portals will reduce the incidence of these problems is unknown. Regardless of the anatomical area referenced by experts for portal creation, the tight grouping of examiner marks in our study supports a consensus regarding the location of the landmarks studied.
In our study of the use of surface anatomical landmarks for the creation of portals, we analyzed 4 previously described locations: ALP, AP, PLP, and MAP. ALP, AP, and PLP directly reference at least 1 surface anatomical structure; AP references 2 anatomical structures (ASIS, GT); and MAP indirectly references ASIS and GT and directly references ALP and AP. In cadaveric and radiographic studies, 7 neurovascular structures have been described in proximity to ALP, AP, MAP, and PLP: superior gluteal nerve, sciatic nerve, femoral nerve, lateral femoral cutaneous nerve, lateral circumflex femoral artery, and medial circumflex femoral artery.5,6 Our results showed that use of surface anatomy in AP and MAP creation most likely places structures at risk, given the overlap of examiner CIs and the previously published cadaveric5,6 and radiographic7 data.
Hua and colleagues12 confirmed the feasibility of using ultrasound for the creation of hip arthroscopy portals. More data is needed to assess how the standard palpation-and-fluoroscopy method described by Byrd3 compares with an ultrasound-guided technique in safety and cost. However, data from our study should not be used to justify a demand for ultrasound during arthroscopy portal establishment, as limitations do not permit such a recommendation.
With diagnostic injection remaining a mainstay of differential diagnosis and treatment about the hip,1 the data presented here suggest a potential for ultrasound in enhancing outcomes. There is evidence supporting the role of image guidance in improving palpation accuracy in the area of the biceps tendon in the forearm.10 Potentially, identification and treatment of specific extra-articular structures surrounding the hip could be made safer with more routine use of ultrasound.
Limitations
This study had several limitations. The surgeons were limited to palpation and static examination of a body in its natural state. Hip arthroscopic portals typically are created under traction and after a standard perineal post is placed for hip arthroscopy. In addition, in an awake injection setting, the clinician may receive patient feedback in the form of limb movement or speech. To what degree palpation or ultrasound will be affected in these scenarios is unknown.
Another limitation is the lack of serial examination by each examiner—intrarater variability could not be gauged. In addition, with only 1 ultrasonographic examination performed, there is the potential that adding ultrasonographic examinations, or having an examiner perform serial physical examinations, could better define the precision of each component. Given the practical limitations of our volunteer’s time and the schedules of 30 expert arthroscopists, we kept the chosen study design for its single setting.
Conclusion
Visual inspection and manual palpation are standard means of identifying common surface anatomical landmarks for the creation of arthroscopy portals and the placement of injections. Our study results showed variance in landmark identification between expert examiners and an ultrasonographer. The degree of variance exceeded established neurovascular safe zones, particularly for AP and MAP. This new evidence calls for further investigation into the best, safest means of performing hip arthroscopic techniques and injection-based interventions.
Am J Orthop. 2017;46(1):E65-E70. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Surface landmarks are routinely used for physical examination and surgical technique.
- Common surface landmarks used in establishing arthroscopic portals may be more difficult to accurately identify than previously thought.
- The greater trochanter was the surface landmark most precisely identified by expert examiners.
- Ultrasound examination identified landmarks varied from landmarks identified by palpation alone.
Anatomical surface landmarks about the hip and lower abdomen are often referenced when placing arthroscopic portals and office-based injections.1-3 However, the degree to which these landmarks can be reproducibly identified using only visual inspection and palpation is unknown.
Safe access to the hip joint and surrounding structures during hip arthroscopy has been a focus in the orthopedic literature. Authors have described anatomical relationships of recommended portals to neurovascular and other anatomical structures.4-6 This information has been reported in millimeters to centimeters of safety based on cadaver dissection studies.4-7We conducted a study to assess expert hip arthroscopists’ ability to identify, using only physical examination techniques, the anatomical structures used for reference when creating safe starting points for arthroscopic access. We hypothesized that variance in examiner-identified points would exceed safe distances from neurovascular structures for the most commonly used hip arthroscopic portals. The volunteer in this study provided written informed consent for print and electronic publication of this article.
Methods
In this study, we prospectively assessed 30 expert hip arthroscopic surgeons’ ability to identify commonly referenced surface landmarks on the adult male hip, using only inspection and manual palpation. Surgeons were defined as experts on the basis of their status as hip arthroscopy instructors at the Orthopaedic Learning Center (Rosemont, IL) for the Arthroscopy Association of North America and industry-sponsored hip arthroscopy education faculty (Arthrex). Five surface landmarks were selected for their relevance to publications on safe portal placement2-5: anterior superior iliac spine (ASIS), tip of greater trochanter (GT), rectus origin (RO), superficial inguinal ring (SIR), and psoas tendon (PT).
A healthy adult male volunteer was placed supine on an examination table and exposed distally from the mid abdomen, with the perineum and the genital area covered bikini-style. An expert musculoskeletal ultrasonographer used a handheld musculoskeletal ultrasound transducer (Sonosite) to identify the 5 landmarks. Short- and long-axis images of each structure were obtained. The examiner applied a round (1 cm in diameter), uniquely colored adhesive label to the skin over each location. A professional photographer using a Canon digital camera and fixed mounts made precise overhead and lateral images. The positional integrity and scale of these images were confirmed with referral to constant anatomical skin features. Images were archived for analysis (Figure 1A).
After the ultrasonographer’s labels were removed, each of the 30 expert hip arthroscopic surgeons identified the structures by static physical examination (inspection and palpation only) and applied the same colored labels to the skin.
Imaging software (Adobe Photoshop Creative Suite 5.1) was used to superimpose the digital images of the examiner labels on those of the ultrasound-verified anatomical labels (Figure 1C). Measurements were then taken with digital calipers to determine average distance from ultrasound label; accuracy within 10 mm of verified ultrasound label; true average location (TAL) determined by 95% confidence interval (CI); and interobserver variability calculated by 95% prediction interval, which determined the probability of where an additional examiner data point would lie.
In the second arm of the study, examiner data were compared with previously published data on arthroscopic portal safety.
Results
Average absolute distance from examiner labels to ultrasonographer labels was 31 mm for ASIS, 24 mm for GT, 26 mm for RO, 19 mm for SIR, and 35 mm for PT (Figure 2).
Of the 30 surgeons, 1 (3%) came within 10 mm of the ultrasound for ASIS, 1 (3%) for GT, 4 (13%) for RO, 5 (17%) for SIR, and 1 (3%) for PT (Table 1).
TAL as determined by CI was 16 mm medial and 29 mm inferior for ASIS; 8 mm anterior and 22 mm superior for GT; 10 mm medial and 25 mm inferior for RO; 5 mm lateral and 5 mm inferior for SIR; and 28 mm medial and 16 mm inferior for PT (Figure 3, Table 2). Interobserver variability determined by prediction interval had a range of 18 mm medial to lateral × 36 mm proximal to distal for ASIS; 33 mm anterior to posterior × 48 mm superior to inferior for GT; 41 mm medial to distal × 54 mm proximal to distal for RO; 51 mm medial to lateral × 74 mm proximal to distal for SIR; and 49 mm medial to distal × 61 mm proximal to distal for PT.
Given the difference between examiner data (direction and distance from ultrasound labels) and published data (distance to significant neurovascular structures), inaccurate identification of surface landmarks has the potential to lead to AP and MAP damage (Table 3). The examiner GT and ASIS surface landmarks used for AP overlapped directly with the safe distances for the lateral femoral cutaneous nerve and the terminal branch of the lateral circumflex femoral artery.
Discussion
Others have investigated examiners’ use of palpation, compared with ultrasound, to identify common shoulder and knee structures.8-10 In a 2011 systematic review, Gilliland and colleagues11 confirmed that accuracy was improved with use of ultrasound (vs palpation) for injections in the shoulder, hip, knee, wrist, and ankle. Given the scarcity of data in this setting, we conducted the present study to assess the precision and accuracy of expert arthroscopists in identifying common surface landmarks. We hypothesized that physical examination and ultrasound examination would differ significantly in precisely and accurately identifying these landmarks.
Working with a standard awake volunteer, our test group of examiners was consistently inaccurate when they accepted ultrasonographer-placed labels as the ideal. Precision within the group, however, trended toward close agreement; examiners consistently placed labels in the same direction and approximate magnitude away from ultrasonographer labels. This suggests that a discrepancy between the ultrasonographic surface structure definitions taught to ultrasonographers and the manually identified definitions taught to surgeons for arthroscopy (training bias) can generate differences in landmark identification.
Given reported low rates of complications in the creation of standard surface anatomy portals, more data is needed to correlate whether safe distance guidelines best apply to the points identified by hip experts or the points identified by ultrasonographers. In a 2013 systematic review, Harris and colleagues8 found a 7.5% overall complication rate, with temporary neuropraxia 1 of the 2 most common complications. Whether adding ultrasound to physical examination for the creation of some or all portals will reduce the incidence of these problems is unknown. Regardless of the anatomical area referenced by experts for portal creation, the tight grouping of examiner marks in our study supports a consensus regarding the location of the landmarks studied.
In our study of the use of surface anatomical landmarks for the creation of portals, we analyzed 4 previously described locations: ALP, AP, PLP, and MAP. ALP, AP, and PLP directly reference at least 1 surface anatomical structure; AP references 2 anatomical structures (ASIS, GT); and MAP indirectly references ASIS and GT and directly references ALP and AP. In cadaveric and radiographic studies, 7 neurovascular structures have been described in proximity to ALP, AP, MAP, and PLP: superior gluteal nerve, sciatic nerve, femoral nerve, lateral femoral cutaneous nerve, lateral circumflex femoral artery, and medial circumflex femoral artery.5,6 Our results showed that use of surface anatomy in AP and MAP creation most likely places structures at risk, given the overlap of examiner CIs and the previously published cadaveric5,6 and radiographic7 data.
Hua and colleagues12 confirmed the feasibility of using ultrasound for the creation of hip arthroscopy portals. More data is needed to assess how the standard palpation-and-fluoroscopy method described by Byrd3 compares with an ultrasound-guided technique in safety and cost. However, data from our study should not be used to justify a demand for ultrasound during arthroscopy portal establishment, as limitations do not permit such a recommendation.
With diagnostic injection remaining a mainstay of differential diagnosis and treatment about the hip,1 the data presented here suggest a potential for ultrasound in enhancing outcomes. There is evidence supporting the role of image guidance in improving palpation accuracy in the area of the biceps tendon in the forearm.10 Potentially, identification and treatment of specific extra-articular structures surrounding the hip could be made safer with more routine use of ultrasound.
Limitations
This study had several limitations. The surgeons were limited to palpation and static examination of a body in its natural state. Hip arthroscopic portals typically are created under traction and after a standard perineal post is placed for hip arthroscopy. In addition, in an awake injection setting, the clinician may receive patient feedback in the form of limb movement or speech. To what degree palpation or ultrasound will be affected in these scenarios is unknown.
Another limitation is the lack of serial examination by each examiner—intrarater variability could not be gauged. In addition, with only 1 ultrasonographic examination performed, there is the potential that adding ultrasonographic examinations, or having an examiner perform serial physical examinations, could better define the precision of each component. Given the practical limitations of our volunteer’s time and the schedules of 30 expert arthroscopists, we kept the chosen study design for its single setting.
Conclusion
Visual inspection and manual palpation are standard means of identifying common surface anatomical landmarks for the creation of arthroscopy portals and the placement of injections. Our study results showed variance in landmark identification between expert examiners and an ultrasonographer. The degree of variance exceeded established neurovascular safe zones, particularly for AP and MAP. This new evidence calls for further investigation into the best, safest means of performing hip arthroscopic techniques and injection-based interventions.
Am J Orthop. 2017;46(1):E65-E70. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Byrd JW, Potts EA, Allison RK, Jones KS. Ultrasound-guided hip injections: a comparative study with fluoroscopy-guided injections. Arthroscopy. 2014;30(1):42-46.
2. Dienst M, Seil R, Kohn DM. Safe arthroscopic access to the central compartment of the hip. Arthroscopy. 2005;21(12):1510-1514.
3. Byrd JW. Hip arthroscopy, the supine approach: technique and anatomy of the intraarticular and peripheral compartments. Tech Orthop. 2005;20(1):17-31.
4. Bond JL, Knutson ZA, Ebert A, Guanche CA. The 23-point arthroscopic examination of the hip: basic setup, portal placement, and surgical technique. Arthroscopy. 2009;25(4):416-429.
5. Roberson WJ, Kelly BT. The safe zone for hip arthroscopy: a cadaveric assessment of central, peripheral, and lateral compartment portal placement. Arthroscopy. 2008;24(9):1019-1026.
6. Byrd JW, Pappas JN, Pedley MJ. Hip arthroscopy: an anatomic study of portal placement and relationship to the extra-articular structures. Arthroscopy. 1995;11(4):418-423.
7. Watson JN, Bohnenkamp F, El-Bitar Y, Moretti V, Domb BG. Variability in locations of hip neurovascular structures and their proximity to hip arthroscopic portals. Arthroscopy. 2014;30(4):462-467.
8. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
9. Jacobson JA, Bedi A, Sekiya JK, Blankenbaker DG. Evaluation of the painful athletic hip: imaging options and imaging-guided injections. AJR Am J Roentgenol. 2012;199(3):516-524.
10. Gazzillo GP, Finnoff JT, Hall MM, Sayeed YA, Smith J. Accuracy of palpating the long head of the biceps tendon: an ultrasonographic study. PM R. 2011;3(11):1035-1040.
11. Gilliland CA, Salazar LD, Borchers JR. Ultrasound versus anatomic guidance for intra-articular and periarticular injection: a systematic review. Phys Sportsmed. 2011;39(3):121-131.
12. Hua Y, Yang Y, Chen S, et al. Ultrasound-guided establishment of hip arthroscopy portals. Arthroscopy. 2009;25(12):1491-1495.
1. Byrd JW, Potts EA, Allison RK, Jones KS. Ultrasound-guided hip injections: a comparative study with fluoroscopy-guided injections. Arthroscopy. 2014;30(1):42-46.
2. Dienst M, Seil R, Kohn DM. Safe arthroscopic access to the central compartment of the hip. Arthroscopy. 2005;21(12):1510-1514.
3. Byrd JW. Hip arthroscopy, the supine approach: technique and anatomy of the intraarticular and peripheral compartments. Tech Orthop. 2005;20(1):17-31.
4. Bond JL, Knutson ZA, Ebert A, Guanche CA. The 23-point arthroscopic examination of the hip: basic setup, portal placement, and surgical technique. Arthroscopy. 2009;25(4):416-429.
5. Roberson WJ, Kelly BT. The safe zone for hip arthroscopy: a cadaveric assessment of central, peripheral, and lateral compartment portal placement. Arthroscopy. 2008;24(9):1019-1026.
6. Byrd JW, Pappas JN, Pedley MJ. Hip arthroscopy: an anatomic study of portal placement and relationship to the extra-articular structures. Arthroscopy. 1995;11(4):418-423.
7. Watson JN, Bohnenkamp F, El-Bitar Y, Moretti V, Domb BG. Variability in locations of hip neurovascular structures and their proximity to hip arthroscopic portals. Arthroscopy. 2014;30(4):462-467.
8. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
9. Jacobson JA, Bedi A, Sekiya JK, Blankenbaker DG. Evaluation of the painful athletic hip: imaging options and imaging-guided injections. AJR Am J Roentgenol. 2012;199(3):516-524.
10. Gazzillo GP, Finnoff JT, Hall MM, Sayeed YA, Smith J. Accuracy of palpating the long head of the biceps tendon: an ultrasonographic study. PM R. 2011;3(11):1035-1040.
11. Gilliland CA, Salazar LD, Borchers JR. Ultrasound versus anatomic guidance for intra-articular and periarticular injection: a systematic review. Phys Sportsmed. 2011;39(3):121-131.
12. Hua Y, Yang Y, Chen S, et al. Ultrasound-guided establishment of hip arthroscopy portals. Arthroscopy. 2009;25(12):1491-1495.
Multicenter Outcomes After Hip Arthroscopy: Epidemiology (MASH Study Group). What Are We Seeing in the Office, and Who Are We Choosing to Treat?
Take-Home Points
- MASH is a multicenter arthroscopic study of the hip that features a large prospective database of 10 separate institutions in the United States.
- The mean patient demographic was age 34.6 years, BMI 25.9 kg/m2, 62.8% females, and 97% white.
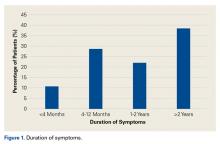
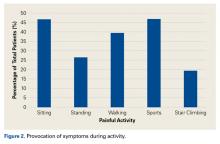
- Most patients had anterior or groin pain, but 17.6% had lateral hip pain, 13.8% had posterior hip pain, and 2.9% had low back or sacral pain.
- Patients typically had pain for about 1 year that was worsened with athletic activity as well as sitting.
- The most common surgical procedures that were performed included labral surgery in 64.7%, femoroplasty in 49.9%, acetabuloplasty in 33.3%, and chondroplasty in 31.1%
Arthroscopic surgery of the hip has been growing over the past decade, with drastically increasing rates of arthroscopic hip procedures and increased education and interest in orthopedic trainees.1-3 The rise of this minimally invasive surgical technique may be attributed to expanding knowledge of surgical management of morphologic hip disorders as a means of hip preservation. Many arthroscopic techniques have been developed to treat intra-articular hip joint pathologies, including femoroacetabular impingement (FAI), labral tears, and cartilage damage.4-11 These hip pathologies are widely recognized as painful limitations to activities of daily living and sports as well as early indicators of hip osteoarthritis.12,13 Limited evidence suggests that arthroscopic treatment of these intra-articular hip joint pathologies preserves the hip from osteoarthritis and progression to total hip arthroplasty.13-15
FAI is the most common etiology of pathologies related to arthroscopic surgery of the hip, including both labral tears and cartilage damage.4,7,14 FAI is a morphologic bone disorder characterized by impingement of the femur and the acetabulum on flexion or rotation. The etiology of FAI is not completely understood, but evidence suggests that stress to the proximal femoral physis during skeletal growth increases the risk of developing femoral head and neck deformations leading to cam-type FAI.15-17 Understanding the characteristics of the patient population in which FAI occurs may shed light on the processes of intra-articular damage, such as labral tears and cartilage damage.
In the present study, we collected epidemiologic data, including demographics, pathologic entities treated, patient-reported measures of disease, and surgical treatment preferences, on a hip pathology population that elected to undergo arthroscopic surgery. These data are important in gaining a better understanding of the population and environment in which hip arthroscopy is performed across multiple centers throughout the United States and may help guide clinical practice and research to advance hip arthroscopy.
Methods
The Multicenter Arthroscopic Study of the Hip (MASH) Study Group conducts multicenter clinical studies in arthroscopic hip preservation surgery. Patients are enrolled in this large prospective longitudinal study at 10 sites nationwide by 10 fellowship-trained hip arthroscopists. Institutional Review Board approval was obtained from all institutions before patient enrollment. After enrollment, we collected comprehensive patient data, including demographics, common symptoms and their duration, provocative activities, patient-reported outcome measures (modified Harris Hip Score, International Hip Outcome Tool, 12-item Short Form Health Survey, visual analog scale pain rating, Hip Outcome Score), physical examination findings, imaging findings, diagnoses, surgical findings, and surgical procedures.
All study participants were patients undergoing arthroscopic hip surgery by one of the members of the MASH Study Group. Patients with incomplete preoperative information (needed for data analysis) were excluded. Data analysis was performed with SPSS Statistics Version 21.0 (SPSS Inc.) to obtain descriptive statistics of the quantitative data and frequencies of the nominal data.
Results
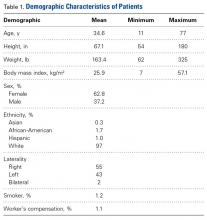
Between January 2014 and November 2016, we enrolled 1738 patients (647 male, 1091 female) in the study. Table 1 lists the demographics of the population.
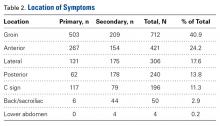
Regarding symptom location, 40.9% of patients described pain in the groin region, 24.2% in the anterior hip region, and 11.3% in a C-sign distribution (Table 2).
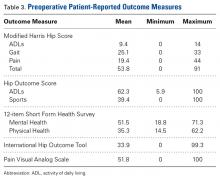
Table 3 lists the results of the patient-reported outcome measures.
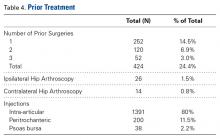
Of the 1738 patients enrolled, 424 (24.4%) had prior surgery related to current symptoms, 252 (14.5%) had 1 previous surgery, 120 (6.9%) had 2 previous surgeries, and 52 (3%) had 3 previous surgeries. Twenty-six patients (1.5%) had a previous revision hip arthroscopy on the ipsilateral side, and 14 (0.8%) had a previous hip arthroscopy on the contralateral side. Before surgery, 80% of patients received an intra-articular injection of corticosteroid and lidocaine. The peritrochanteric region was injected in 11.5% of patients and the psoas bursa in 2.2% (Table 4).
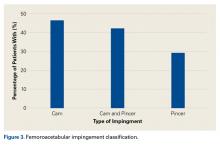
Of the 1011 patients who had magnetic resonance imaging (MRI) performed, 943 (93.3%) had abnormal acetabular labrum findings, and 163 (17.1%) had acetabular articular damage. According to radiographic evaluation, 953 patients had abnormal hip joint morphology consistent with FAI. Figure 3 shows the FAI classification percentages.
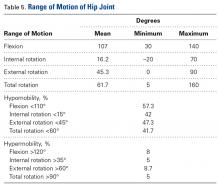
On clinical examination, 1079 patients (62.1%) had a positive anterior impingement sign. The subspine impingement sign was positive in 447 patients (25.7%), and the trochanteric pain sign was positive in 400 (23%). Table 5 lists range-of-motion values for flexion and hip rotation from 90° of flexion.
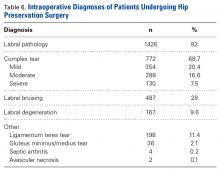
As seen in Table 6, labral pathology was the most common diagnosis (1426/1738 patients, 82%).
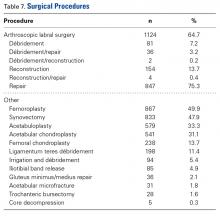
As seen in Table 7, the most common procedure was femoroplasty (867/1738, 49.9%).
Discussion
In this study, we collected epidemiologic data (demographics, pathologic entities treated, patient-reported measures of disease, surgical treatment preferences) from a large multicenter population of hip pathology patients who elected to undergo arthroscopic surgery. Our results showed these patients were most commonly younger to middle-aged white females with pain primarily in the groin region. Most had pain for at least 1 year, and it was commonly exacerbated by sitting and athletics. Patients reported clinically significant pain and functional limitation, which showed evidence of affecting general physical and mental health. It was not uncommon for patients to have undergone another, related surgery and nonoperative treatments, including intra-articular injection and/or physical therapy, before surgery. There was a high incidence of abnormal hip morphology suggestive of a cam lesion, but the incidence of arthritic changes on radiographs was relatively low. Labral tear was the most common diagnosis, and most often it was addressed with repair. Many patients underwent femoroplasty, acetabuloplasty, and chondroplasty in addition to labral repair.
According to patient-reported outcome measures administered before surgery, 40% to 65% of patients seeking hip preservation surgery reported functional deficits and pain—which falls within the range of results from other multicenter studies on the epidemiology of FAI.18,19 There was, however, a high amount of variability in individual scores on the functional and pain measures; some patients rated their functional ability very high. These findings were supported by the general health forms measuring global physical and mental health. Mean Physical Health and Mental Health scores on the 12-item Short Form Health Survey indicated that patients seeking hip preservation surgery thought their hip condition affected their general well-being. This finding is consistent with research on FAI,18 hip arthritis,20 and total hip arthroplasty.19Our results further showed that hip arthroscopists commonly prescribed alternative treatment measures ahead of surgery. Before elective surgery, 80% of patients received an intra-articular injection, underwent physical therapy, or both. This could suggest a high failure rate for patients who chose conservative treatment approaches for hip-related pathology. However, our study was limited in that it may have included patients who had improved significantly with conservative measures and decided to forgo arthroscopic hip surgery. Although conservative treatment often is recommended in an effort to potentially avoid surgery, there is a lack of research evaluating the efficacy of nonoperative care.21,22Analysis of diagnostic imaging and clinical examination findings revealed some unique characteristics of patients undergoing elective hip preservation surgery. MRI showed labral pathology in an overwhelming majority of these patients, but few had evidence of articular damage. Previous research has found a 67% rate of arthritic changes on diagnostic imaging, but our rate was much lower (17%).23 Radiograph evaluation confirmed the pattern: More than 90% of our patients had Tönnis grade 0 osteoarthritis. Tönnis grade 1 or 2 osteoarthritis is a predictor of acetabular cartilage degeneration,23 and long-term studies have related these osteoarthritic changes to poorer hip arthroscopy outcomes.24 Thus, the lower incidence of osteoarthritis in our study population may reflect current evidence-based practice and a contemporary approach to patient selection.
Most of our patients had isolated cam-type FAI as opposed to pincer-type FAI or a combination of cam and pincer—contrary to research findings that combination cam–pincer FAI is most prevalent.25,26 Our results are more consistent with more recent research findings of a higher incidence of isolated cam lesion, particularly in female patients, and combination cam–pincer in male patients.18,27,28 Similar distributions of surgical procedures and diagnoses exist between the present study and other multicenter evaluations of the epidemiologic characteristics of patients with hip pathology.18Our study had several limitations. First, the population consisted entirely of patients who sought evaluation by a hip arthroscopy specialist and underwent elective surgery. Therefore, the data cannot be applied to a more general orthopedic population or to patients who consult other medical specialists. Second, the population, which was 97% white and had small percentages of African-American, Latino, and Asian patients, lacked ethnic diversity. This finding is consistent with recent epidemiologic research in which ethnicity was identified as a factor in patterns of hip disease.13,29,30 Access to specialists, however, was likely affected by multiple other factors. Fourth, the validity and the reliability of the imaging modalities used have been questioned.31-33 There is controversy regarding ideal imaging modalities for assessment of articular cartilage damage31,32 and FAI. However, the modalities that we used to determine diagnoses in this study are well supported26 and represent common practice patterns.
Am J Orthop. 2017;46(1):35-41. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cvetanovich GL, Chalmers PN, Levy DM, et al. Hip arthroscopy surgical volume trends and 30-day postoperative complications. Arthroscopy. 2016;32(7):1286-1292.
2. Peters CL, Aoki SK, Erickson JA, Anderson LA, Anderson AE. Early experience with a comprehensive hip preservation service intended to improve clinical care, education, and academic productivity. Clin Orthop Relat Res. 2012;470(12):3446-3452.
3. Siebenrock KA, Peters CL. ABJS Carl T. Brighton workshop on hip preservation surgery: editorial comment. Clin Orthop Relat Res. 2012;470(12):3281-3283.
4. Parvizi J, Leunig M, Ganz R. Femoroacetabular impingement. J Am Acad Orthop Surg. 2007;15(9):561-570.
5. Poh SY, Hube R, Dienst M. Arthroscopic treatment of femoroacetabular pincer impingement. Oper Orthop Traumatol. 2015;27(6):536-552.
6. Javed A, O’Donnell JM. Arthroscopic femoral osteochondroplasty for cam femoroacetabular impingement in patients over 60 years of age. J Bone Joint Surg Br. 2011;93(3):326-331.
7. Groh MM, Herrera J. A comprehensive review of hip labral tears. Curr Rev Musculoskelet Med. 2009;2(2):105-117.
8. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
9. White BJ, Herzog MM. Labral reconstruction: when to perform and how. Front Surg. 2015;2:27.
10. Yen YM, Kocher MS. Chondral lesions of the hip: microfracture and chondroplasty. Sports Med Arthrosc. 2010;18(2):83-89.
11. Jordan MA, Van Thiel GS, Chahal J, Nho SJ. Operative treatment of chondral defects in the hip joint: a systematic review. Curr Rev Musculoskelet Med. 2012;5(3):244-253.
12. Griffin DW, Kinnard MJ, Formby PM, McCabe MP, Anderson TD. Outcomes of hip arthroscopy in the older adult: a systematic review of the literature [published online October 18, 2016]. Am J Sports Med. doi:10.1177/0363546516667915.
13. Ganz R, Leunig M, Leunig-Ganz K, Harris WH. The etiology of osteoarthritis of the hip. Clin Orthop Relat Res. 2008;466(2):264-272.
14. Kaya M, Suzuki T, Emori M, Yamashita T. Hip morphology influences the pattern of articular cartilage damage. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):2016-2023.
15. Beck M, Leunig M, Parvizi J, Boutier V, Wyss D, Ganz R. Anterior femoroacetabular impingement: part II. Midterm results of surgical treatment. Clin Orthop Relat Res. 2004;(418):67-73.
16. Byrd JT. Hip arthroscopy in athletes. Oper Tech Sports Med. 2005;13(1):24-36.
17. Werner BC, Gaudiani MA, Ranawat AS. The etiology and arthroscopic surgical management of cam lesions. Clin Sports Med. 2016;35(3):391-404.
18. Clohisy JC, Baca G, Beaulé PE, et al; ANCHOR Study Group. Descriptive epidemiology of femoroacetabular impingement: a North American cohort of patients undergoing surgery. Am J Sports Med. 2013;41(6):1348-1356.
19. Shia DS, Clohisy JC, Schinsky MF, Martell JM, Maloney WJ. THA with highly cross-linked polyethylene in patients 50 years or younger. Clin Orthop Relat Res. 2009;467(8):2059-2065.
20. Gandhi SK, Salmon JW, Zhao SZ, Lambert BL, Gore PR, Conrad K. Psychometric evaluation of the 12-item Short-Form Health Survey (SF-12) in osteoarthritis and rheumatoid arthritis clinical trials. Clin Ther. 2001;23(7):1080-1098.
21. Loudon JK, Reiman MP. Conservative management of femoroacetabular impingement (FAI) in the long distance runner. Phys Ther Sport. 2014;15(2):82-90.
22. Wall PD, Fernandez M, Griffin DR, Foster NE. Nonoperative treatment for femoroacetabular impingement: a systematic review of the literature. PM R. 2013;5(5):418-426.
23. Nepple JJ, Carlisle JC, Nunley RM, Clohisy JC. Clinical and radiographic predictors of intra-articular hip disease in arthroscopy. Am J Sports Med. 2011;39(2):296-303.
24. McCormick F, Nwachukwu BU, Alpaugh K, Martin SD. Predictors of hip arthroscopy outcomes for labral tears at minimum 2-year follow-up: the influence of age and arthritis. Arthroscopy. 2012;28(10):1359-1364.
25. Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87(7):1012-1018.
26. Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis—what the radiologist should know. AJR Am J Roentgenol. 2007;188(6):1540-1552.
27. Kapron AL, Peters CL, Aoki SK, et al. The prevalence of radiographic findings of structural hip deformities in female collegiate athletes. Am J Sports Med. 2015;43(6):1324-1330.
28. Lee WY, Kang C, Hwang DS, Jeon JH, Zheng L. Descriptive epidemiology of symptomatic femoroacetabular impingement in young athlete: single center study. Hip Pelvis. 2016;28(1):29-34.
29. Dudda M, Kim YJ, Zhang Y, et al. Morphologic differences between the hips of Chinese women and white women: could they account for the ethnic difference in the prevalence of hip osteoarthritis? Arthritis Rheum. 2011;63(10):2992-2999.
30. Solomon L, Beighton P. Osteoarthrosis of the hip and its relationship to pre-existing in an African population. J Bone Joint Surg Br. 1973;55(1):216-217.
31. Keeney JA, Peelle MW, Jackson J, Rubin D, Maloney WJ, Clohisy JC. Magnetic resonance arthrography versus arthroscopy in the evaluation of articular hip pathology. Clin Orthop Relat Res. 2004;(429):163-169.
32. Schmid MR, Nötzli HP, Zanetti M, Wyss TF, Hodler J. Cartilage lesions in the hip: diagnostic effectiveness of MR arthrography. Radiology. 2003;226(2):382-386.
33. Chevillotte CJ, Ali MH, Trousdale RT, Pagnano MW. Variability in hip range of motion on clinical examination. J Arthroplasty. 2009;24(5):693-697.
Take-Home Points
- MASH is a multicenter arthroscopic study of the hip that features a large prospective database of 10 separate institutions in the United States.
- The mean patient demographic was age 34.6 years, BMI 25.9 kg/m2, 62.8% females, and 97% white.
- Most patients had anterior or groin pain, but 17.6% had lateral hip pain, 13.8% had posterior hip pain, and 2.9% had low back or sacral pain.
- Patients typically had pain for about 1 year that was worsened with athletic activity as well as sitting.
- The most common surgical procedures that were performed included labral surgery in 64.7%, femoroplasty in 49.9%, acetabuloplasty in 33.3%, and chondroplasty in 31.1%
Arthroscopic surgery of the hip has been growing over the past decade, with drastically increasing rates of arthroscopic hip procedures and increased education and interest in orthopedic trainees.1-3 The rise of this minimally invasive surgical technique may be attributed to expanding knowledge of surgical management of morphologic hip disorders as a means of hip preservation. Many arthroscopic techniques have been developed to treat intra-articular hip joint pathologies, including femoroacetabular impingement (FAI), labral tears, and cartilage damage.4-11 These hip pathologies are widely recognized as painful limitations to activities of daily living and sports as well as early indicators of hip osteoarthritis.12,13 Limited evidence suggests that arthroscopic treatment of these intra-articular hip joint pathologies preserves the hip from osteoarthritis and progression to total hip arthroplasty.13-15
FAI is the most common etiology of pathologies related to arthroscopic surgery of the hip, including both labral tears and cartilage damage.4,7,14 FAI is a morphologic bone disorder characterized by impingement of the femur and the acetabulum on flexion or rotation. The etiology of FAI is not completely understood, but evidence suggests that stress to the proximal femoral physis during skeletal growth increases the risk of developing femoral head and neck deformations leading to cam-type FAI.15-17 Understanding the characteristics of the patient population in which FAI occurs may shed light on the processes of intra-articular damage, such as labral tears and cartilage damage.
In the present study, we collected epidemiologic data, including demographics, pathologic entities treated, patient-reported measures of disease, and surgical treatment preferences, on a hip pathology population that elected to undergo arthroscopic surgery. These data are important in gaining a better understanding of the population and environment in which hip arthroscopy is performed across multiple centers throughout the United States and may help guide clinical practice and research to advance hip arthroscopy.
Methods
The Multicenter Arthroscopic Study of the Hip (MASH) Study Group conducts multicenter clinical studies in arthroscopic hip preservation surgery. Patients are enrolled in this large prospective longitudinal study at 10 sites nationwide by 10 fellowship-trained hip arthroscopists. Institutional Review Board approval was obtained from all institutions before patient enrollment. After enrollment, we collected comprehensive patient data, including demographics, common symptoms and their duration, provocative activities, patient-reported outcome measures (modified Harris Hip Score, International Hip Outcome Tool, 12-item Short Form Health Survey, visual analog scale pain rating, Hip Outcome Score), physical examination findings, imaging findings, diagnoses, surgical findings, and surgical procedures.
All study participants were patients undergoing arthroscopic hip surgery by one of the members of the MASH Study Group. Patients with incomplete preoperative information (needed for data analysis) were excluded. Data analysis was performed with SPSS Statistics Version 21.0 (SPSS Inc.) to obtain descriptive statistics of the quantitative data and frequencies of the nominal data.
Results
Between January 2014 and November 2016, we enrolled 1738 patients (647 male, 1091 female) in the study. Table 1 lists the demographics of the population.
Regarding symptom location, 40.9% of patients described pain in the groin region, 24.2% in the anterior hip region, and 11.3% in a C-sign distribution (Table 2).
Table 3 lists the results of the patient-reported outcome measures.
Of the 1738 patients enrolled, 424 (24.4%) had prior surgery related to current symptoms, 252 (14.5%) had 1 previous surgery, 120 (6.9%) had 2 previous surgeries, and 52 (3%) had 3 previous surgeries. Twenty-six patients (1.5%) had a previous revision hip arthroscopy on the ipsilateral side, and 14 (0.8%) had a previous hip arthroscopy on the contralateral side. Before surgery, 80% of patients received an intra-articular injection of corticosteroid and lidocaine. The peritrochanteric region was injected in 11.5% of patients and the psoas bursa in 2.2% (Table 4).
Of the 1011 patients who had magnetic resonance imaging (MRI) performed, 943 (93.3%) had abnormal acetabular labrum findings, and 163 (17.1%) had acetabular articular damage. According to radiographic evaluation, 953 patients had abnormal hip joint morphology consistent with FAI. Figure 3 shows the FAI classification percentages.
On clinical examination, 1079 patients (62.1%) had a positive anterior impingement sign. The subspine impingement sign was positive in 447 patients (25.7%), and the trochanteric pain sign was positive in 400 (23%). Table 5 lists range-of-motion values for flexion and hip rotation from 90° of flexion.
As seen in Table 6, labral pathology was the most common diagnosis (1426/1738 patients, 82%).
As seen in Table 7, the most common procedure was femoroplasty (867/1738, 49.9%).
Discussion
In this study, we collected epidemiologic data (demographics, pathologic entities treated, patient-reported measures of disease, surgical treatment preferences) from a large multicenter population of hip pathology patients who elected to undergo arthroscopic surgery. Our results showed these patients were most commonly younger to middle-aged white females with pain primarily in the groin region. Most had pain for at least 1 year, and it was commonly exacerbated by sitting and athletics. Patients reported clinically significant pain and functional limitation, which showed evidence of affecting general physical and mental health. It was not uncommon for patients to have undergone another, related surgery and nonoperative treatments, including intra-articular injection and/or physical therapy, before surgery. There was a high incidence of abnormal hip morphology suggestive of a cam lesion, but the incidence of arthritic changes on radiographs was relatively low. Labral tear was the most common diagnosis, and most often it was addressed with repair. Many patients underwent femoroplasty, acetabuloplasty, and chondroplasty in addition to labral repair.
According to patient-reported outcome measures administered before surgery, 40% to 65% of patients seeking hip preservation surgery reported functional deficits and pain—which falls within the range of results from other multicenter studies on the epidemiology of FAI.18,19 There was, however, a high amount of variability in individual scores on the functional and pain measures; some patients rated their functional ability very high. These findings were supported by the general health forms measuring global physical and mental health. Mean Physical Health and Mental Health scores on the 12-item Short Form Health Survey indicated that patients seeking hip preservation surgery thought their hip condition affected their general well-being. This finding is consistent with research on FAI,18 hip arthritis,20 and total hip arthroplasty.19Our results further showed that hip arthroscopists commonly prescribed alternative treatment measures ahead of surgery. Before elective surgery, 80% of patients received an intra-articular injection, underwent physical therapy, or both. This could suggest a high failure rate for patients who chose conservative treatment approaches for hip-related pathology. However, our study was limited in that it may have included patients who had improved significantly with conservative measures and decided to forgo arthroscopic hip surgery. Although conservative treatment often is recommended in an effort to potentially avoid surgery, there is a lack of research evaluating the efficacy of nonoperative care.21,22Analysis of diagnostic imaging and clinical examination findings revealed some unique characteristics of patients undergoing elective hip preservation surgery. MRI showed labral pathology in an overwhelming majority of these patients, but few had evidence of articular damage. Previous research has found a 67% rate of arthritic changes on diagnostic imaging, but our rate was much lower (17%).23 Radiograph evaluation confirmed the pattern: More than 90% of our patients had Tönnis grade 0 osteoarthritis. Tönnis grade 1 or 2 osteoarthritis is a predictor of acetabular cartilage degeneration,23 and long-term studies have related these osteoarthritic changes to poorer hip arthroscopy outcomes.24 Thus, the lower incidence of osteoarthritis in our study population may reflect current evidence-based practice and a contemporary approach to patient selection.
Most of our patients had isolated cam-type FAI as opposed to pincer-type FAI or a combination of cam and pincer—contrary to research findings that combination cam–pincer FAI is most prevalent.25,26 Our results are more consistent with more recent research findings of a higher incidence of isolated cam lesion, particularly in female patients, and combination cam–pincer in male patients.18,27,28 Similar distributions of surgical procedures and diagnoses exist between the present study and other multicenter evaluations of the epidemiologic characteristics of patients with hip pathology.18Our study had several limitations. First, the population consisted entirely of patients who sought evaluation by a hip arthroscopy specialist and underwent elective surgery. Therefore, the data cannot be applied to a more general orthopedic population or to patients who consult other medical specialists. Second, the population, which was 97% white and had small percentages of African-American, Latino, and Asian patients, lacked ethnic diversity. This finding is consistent with recent epidemiologic research in which ethnicity was identified as a factor in patterns of hip disease.13,29,30 Access to specialists, however, was likely affected by multiple other factors. Fourth, the validity and the reliability of the imaging modalities used have been questioned.31-33 There is controversy regarding ideal imaging modalities for assessment of articular cartilage damage31,32 and FAI. However, the modalities that we used to determine diagnoses in this study are well supported26 and represent common practice patterns.
Am J Orthop. 2017;46(1):35-41. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- MASH is a multicenter arthroscopic study of the hip that features a large prospective database of 10 separate institutions in the United States.
- The mean patient demographic was age 34.6 years, BMI 25.9 kg/m2, 62.8% females, and 97% white.
- Most patients had anterior or groin pain, but 17.6% had lateral hip pain, 13.8% had posterior hip pain, and 2.9% had low back or sacral pain.
- Patients typically had pain for about 1 year that was worsened with athletic activity as well as sitting.
- The most common surgical procedures that were performed included labral surgery in 64.7%, femoroplasty in 49.9%, acetabuloplasty in 33.3%, and chondroplasty in 31.1%
Arthroscopic surgery of the hip has been growing over the past decade, with drastically increasing rates of arthroscopic hip procedures and increased education and interest in orthopedic trainees.1-3 The rise of this minimally invasive surgical technique may be attributed to expanding knowledge of surgical management of morphologic hip disorders as a means of hip preservation. Many arthroscopic techniques have been developed to treat intra-articular hip joint pathologies, including femoroacetabular impingement (FAI), labral tears, and cartilage damage.4-11 These hip pathologies are widely recognized as painful limitations to activities of daily living and sports as well as early indicators of hip osteoarthritis.12,13 Limited evidence suggests that arthroscopic treatment of these intra-articular hip joint pathologies preserves the hip from osteoarthritis and progression to total hip arthroplasty.13-15
FAI is the most common etiology of pathologies related to arthroscopic surgery of the hip, including both labral tears and cartilage damage.4,7,14 FAI is a morphologic bone disorder characterized by impingement of the femur and the acetabulum on flexion or rotation. The etiology of FAI is not completely understood, but evidence suggests that stress to the proximal femoral physis during skeletal growth increases the risk of developing femoral head and neck deformations leading to cam-type FAI.15-17 Understanding the characteristics of the patient population in which FAI occurs may shed light on the processes of intra-articular damage, such as labral tears and cartilage damage.
In the present study, we collected epidemiologic data, including demographics, pathologic entities treated, patient-reported measures of disease, and surgical treatment preferences, on a hip pathology population that elected to undergo arthroscopic surgery. These data are important in gaining a better understanding of the population and environment in which hip arthroscopy is performed across multiple centers throughout the United States and may help guide clinical practice and research to advance hip arthroscopy.
Methods
The Multicenter Arthroscopic Study of the Hip (MASH) Study Group conducts multicenter clinical studies in arthroscopic hip preservation surgery. Patients are enrolled in this large prospective longitudinal study at 10 sites nationwide by 10 fellowship-trained hip arthroscopists. Institutional Review Board approval was obtained from all institutions before patient enrollment. After enrollment, we collected comprehensive patient data, including demographics, common symptoms and their duration, provocative activities, patient-reported outcome measures (modified Harris Hip Score, International Hip Outcome Tool, 12-item Short Form Health Survey, visual analog scale pain rating, Hip Outcome Score), physical examination findings, imaging findings, diagnoses, surgical findings, and surgical procedures.
All study participants were patients undergoing arthroscopic hip surgery by one of the members of the MASH Study Group. Patients with incomplete preoperative information (needed for data analysis) were excluded. Data analysis was performed with SPSS Statistics Version 21.0 (SPSS Inc.) to obtain descriptive statistics of the quantitative data and frequencies of the nominal data.
Results
Between January 2014 and November 2016, we enrolled 1738 patients (647 male, 1091 female) in the study. Table 1 lists the demographics of the population.
Regarding symptom location, 40.9% of patients described pain in the groin region, 24.2% in the anterior hip region, and 11.3% in a C-sign distribution (Table 2).
Table 3 lists the results of the patient-reported outcome measures.
Of the 1738 patients enrolled, 424 (24.4%) had prior surgery related to current symptoms, 252 (14.5%) had 1 previous surgery, 120 (6.9%) had 2 previous surgeries, and 52 (3%) had 3 previous surgeries. Twenty-six patients (1.5%) had a previous revision hip arthroscopy on the ipsilateral side, and 14 (0.8%) had a previous hip arthroscopy on the contralateral side. Before surgery, 80% of patients received an intra-articular injection of corticosteroid and lidocaine. The peritrochanteric region was injected in 11.5% of patients and the psoas bursa in 2.2% (Table 4).
Of the 1011 patients who had magnetic resonance imaging (MRI) performed, 943 (93.3%) had abnormal acetabular labrum findings, and 163 (17.1%) had acetabular articular damage. According to radiographic evaluation, 953 patients had abnormal hip joint morphology consistent with FAI. Figure 3 shows the FAI classification percentages.
On clinical examination, 1079 patients (62.1%) had a positive anterior impingement sign. The subspine impingement sign was positive in 447 patients (25.7%), and the trochanteric pain sign was positive in 400 (23%). Table 5 lists range-of-motion values for flexion and hip rotation from 90° of flexion.
As seen in Table 6, labral pathology was the most common diagnosis (1426/1738 patients, 82%).
As seen in Table 7, the most common procedure was femoroplasty (867/1738, 49.9%).
Discussion
In this study, we collected epidemiologic data (demographics, pathologic entities treated, patient-reported measures of disease, surgical treatment preferences) from a large multicenter population of hip pathology patients who elected to undergo arthroscopic surgery. Our results showed these patients were most commonly younger to middle-aged white females with pain primarily in the groin region. Most had pain for at least 1 year, and it was commonly exacerbated by sitting and athletics. Patients reported clinically significant pain and functional limitation, which showed evidence of affecting general physical and mental health. It was not uncommon for patients to have undergone another, related surgery and nonoperative treatments, including intra-articular injection and/or physical therapy, before surgery. There was a high incidence of abnormal hip morphology suggestive of a cam lesion, but the incidence of arthritic changes on radiographs was relatively low. Labral tear was the most common diagnosis, and most often it was addressed with repair. Many patients underwent femoroplasty, acetabuloplasty, and chondroplasty in addition to labral repair.
According to patient-reported outcome measures administered before surgery, 40% to 65% of patients seeking hip preservation surgery reported functional deficits and pain—which falls within the range of results from other multicenter studies on the epidemiology of FAI.18,19 There was, however, a high amount of variability in individual scores on the functional and pain measures; some patients rated their functional ability very high. These findings were supported by the general health forms measuring global physical and mental health. Mean Physical Health and Mental Health scores on the 12-item Short Form Health Survey indicated that patients seeking hip preservation surgery thought their hip condition affected their general well-being. This finding is consistent with research on FAI,18 hip arthritis,20 and total hip arthroplasty.19Our results further showed that hip arthroscopists commonly prescribed alternative treatment measures ahead of surgery. Before elective surgery, 80% of patients received an intra-articular injection, underwent physical therapy, or both. This could suggest a high failure rate for patients who chose conservative treatment approaches for hip-related pathology. However, our study was limited in that it may have included patients who had improved significantly with conservative measures and decided to forgo arthroscopic hip surgery. Although conservative treatment often is recommended in an effort to potentially avoid surgery, there is a lack of research evaluating the efficacy of nonoperative care.21,22Analysis of diagnostic imaging and clinical examination findings revealed some unique characteristics of patients undergoing elective hip preservation surgery. MRI showed labral pathology in an overwhelming majority of these patients, but few had evidence of articular damage. Previous research has found a 67% rate of arthritic changes on diagnostic imaging, but our rate was much lower (17%).23 Radiograph evaluation confirmed the pattern: More than 90% of our patients had Tönnis grade 0 osteoarthritis. Tönnis grade 1 or 2 osteoarthritis is a predictor of acetabular cartilage degeneration,23 and long-term studies have related these osteoarthritic changes to poorer hip arthroscopy outcomes.24 Thus, the lower incidence of osteoarthritis in our study population may reflect current evidence-based practice and a contemporary approach to patient selection.
Most of our patients had isolated cam-type FAI as opposed to pincer-type FAI or a combination of cam and pincer—contrary to research findings that combination cam–pincer FAI is most prevalent.25,26 Our results are more consistent with more recent research findings of a higher incidence of isolated cam lesion, particularly in female patients, and combination cam–pincer in male patients.18,27,28 Similar distributions of surgical procedures and diagnoses exist between the present study and other multicenter evaluations of the epidemiologic characteristics of patients with hip pathology.18Our study had several limitations. First, the population consisted entirely of patients who sought evaluation by a hip arthroscopy specialist and underwent elective surgery. Therefore, the data cannot be applied to a more general orthopedic population or to patients who consult other medical specialists. Second, the population, which was 97% white and had small percentages of African-American, Latino, and Asian patients, lacked ethnic diversity. This finding is consistent with recent epidemiologic research in which ethnicity was identified as a factor in patterns of hip disease.13,29,30 Access to specialists, however, was likely affected by multiple other factors. Fourth, the validity and the reliability of the imaging modalities used have been questioned.31-33 There is controversy regarding ideal imaging modalities for assessment of articular cartilage damage31,32 and FAI. However, the modalities that we used to determine diagnoses in this study are well supported26 and represent common practice patterns.
Am J Orthop. 2017;46(1):35-41. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cvetanovich GL, Chalmers PN, Levy DM, et al. Hip arthroscopy surgical volume trends and 30-day postoperative complications. Arthroscopy. 2016;32(7):1286-1292.
2. Peters CL, Aoki SK, Erickson JA, Anderson LA, Anderson AE. Early experience with a comprehensive hip preservation service intended to improve clinical care, education, and academic productivity. Clin Orthop Relat Res. 2012;470(12):3446-3452.
3. Siebenrock KA, Peters CL. ABJS Carl T. Brighton workshop on hip preservation surgery: editorial comment. Clin Orthop Relat Res. 2012;470(12):3281-3283.
4. Parvizi J, Leunig M, Ganz R. Femoroacetabular impingement. J Am Acad Orthop Surg. 2007;15(9):561-570.
5. Poh SY, Hube R, Dienst M. Arthroscopic treatment of femoroacetabular pincer impingement. Oper Orthop Traumatol. 2015;27(6):536-552.
6. Javed A, O’Donnell JM. Arthroscopic femoral osteochondroplasty for cam femoroacetabular impingement in patients over 60 years of age. J Bone Joint Surg Br. 2011;93(3):326-331.
7. Groh MM, Herrera J. A comprehensive review of hip labral tears. Curr Rev Musculoskelet Med. 2009;2(2):105-117.
8. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
9. White BJ, Herzog MM. Labral reconstruction: when to perform and how. Front Surg. 2015;2:27.
10. Yen YM, Kocher MS. Chondral lesions of the hip: microfracture and chondroplasty. Sports Med Arthrosc. 2010;18(2):83-89.
11. Jordan MA, Van Thiel GS, Chahal J, Nho SJ. Operative treatment of chondral defects in the hip joint: a systematic review. Curr Rev Musculoskelet Med. 2012;5(3):244-253.
12. Griffin DW, Kinnard MJ, Formby PM, McCabe MP, Anderson TD. Outcomes of hip arthroscopy in the older adult: a systematic review of the literature [published online October 18, 2016]. Am J Sports Med. doi:10.1177/0363546516667915.
13. Ganz R, Leunig M, Leunig-Ganz K, Harris WH. The etiology of osteoarthritis of the hip. Clin Orthop Relat Res. 2008;466(2):264-272.
14. Kaya M, Suzuki T, Emori M, Yamashita T. Hip morphology influences the pattern of articular cartilage damage. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):2016-2023.
15. Beck M, Leunig M, Parvizi J, Boutier V, Wyss D, Ganz R. Anterior femoroacetabular impingement: part II. Midterm results of surgical treatment. Clin Orthop Relat Res. 2004;(418):67-73.
16. Byrd JT. Hip arthroscopy in athletes. Oper Tech Sports Med. 2005;13(1):24-36.
17. Werner BC, Gaudiani MA, Ranawat AS. The etiology and arthroscopic surgical management of cam lesions. Clin Sports Med. 2016;35(3):391-404.
18. Clohisy JC, Baca G, Beaulé PE, et al; ANCHOR Study Group. Descriptive epidemiology of femoroacetabular impingement: a North American cohort of patients undergoing surgery. Am J Sports Med. 2013;41(6):1348-1356.
19. Shia DS, Clohisy JC, Schinsky MF, Martell JM, Maloney WJ. THA with highly cross-linked polyethylene in patients 50 years or younger. Clin Orthop Relat Res. 2009;467(8):2059-2065.
20. Gandhi SK, Salmon JW, Zhao SZ, Lambert BL, Gore PR, Conrad K. Psychometric evaluation of the 12-item Short-Form Health Survey (SF-12) in osteoarthritis and rheumatoid arthritis clinical trials. Clin Ther. 2001;23(7):1080-1098.
21. Loudon JK, Reiman MP. Conservative management of femoroacetabular impingement (FAI) in the long distance runner. Phys Ther Sport. 2014;15(2):82-90.
22. Wall PD, Fernandez M, Griffin DR, Foster NE. Nonoperative treatment for femoroacetabular impingement: a systematic review of the literature. PM R. 2013;5(5):418-426.
23. Nepple JJ, Carlisle JC, Nunley RM, Clohisy JC. Clinical and radiographic predictors of intra-articular hip disease in arthroscopy. Am J Sports Med. 2011;39(2):296-303.
24. McCormick F, Nwachukwu BU, Alpaugh K, Martin SD. Predictors of hip arthroscopy outcomes for labral tears at minimum 2-year follow-up: the influence of age and arthritis. Arthroscopy. 2012;28(10):1359-1364.
25. Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87(7):1012-1018.
26. Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis—what the radiologist should know. AJR Am J Roentgenol. 2007;188(6):1540-1552.
27. Kapron AL, Peters CL, Aoki SK, et al. The prevalence of radiographic findings of structural hip deformities in female collegiate athletes. Am J Sports Med. 2015;43(6):1324-1330.
28. Lee WY, Kang C, Hwang DS, Jeon JH, Zheng L. Descriptive epidemiology of symptomatic femoroacetabular impingement in young athlete: single center study. Hip Pelvis. 2016;28(1):29-34.
29. Dudda M, Kim YJ, Zhang Y, et al. Morphologic differences between the hips of Chinese women and white women: could they account for the ethnic difference in the prevalence of hip osteoarthritis? Arthritis Rheum. 2011;63(10):2992-2999.
30. Solomon L, Beighton P. Osteoarthrosis of the hip and its relationship to pre-existing in an African population. J Bone Joint Surg Br. 1973;55(1):216-217.
31. Keeney JA, Peelle MW, Jackson J, Rubin D, Maloney WJ, Clohisy JC. Magnetic resonance arthrography versus arthroscopy in the evaluation of articular hip pathology. Clin Orthop Relat Res. 2004;(429):163-169.
32. Schmid MR, Nötzli HP, Zanetti M, Wyss TF, Hodler J. Cartilage lesions in the hip: diagnostic effectiveness of MR arthrography. Radiology. 2003;226(2):382-386.
33. Chevillotte CJ, Ali MH, Trousdale RT, Pagnano MW. Variability in hip range of motion on clinical examination. J Arthroplasty. 2009;24(5):693-697.
1. Cvetanovich GL, Chalmers PN, Levy DM, et al. Hip arthroscopy surgical volume trends and 30-day postoperative complications. Arthroscopy. 2016;32(7):1286-1292.
2. Peters CL, Aoki SK, Erickson JA, Anderson LA, Anderson AE. Early experience with a comprehensive hip preservation service intended to improve clinical care, education, and academic productivity. Clin Orthop Relat Res. 2012;470(12):3446-3452.
3. Siebenrock KA, Peters CL. ABJS Carl T. Brighton workshop on hip preservation surgery: editorial comment. Clin Orthop Relat Res. 2012;470(12):3281-3283.
4. Parvizi J, Leunig M, Ganz R. Femoroacetabular impingement. J Am Acad Orthop Surg. 2007;15(9):561-570.
5. Poh SY, Hube R, Dienst M. Arthroscopic treatment of femoroacetabular pincer impingement. Oper Orthop Traumatol. 2015;27(6):536-552.
6. Javed A, O’Donnell JM. Arthroscopic femoral osteochondroplasty for cam femoroacetabular impingement in patients over 60 years of age. J Bone Joint Surg Br. 2011;93(3):326-331.
7. Groh MM, Herrera J. A comprehensive review of hip labral tears. Curr Rev Musculoskelet Med. 2009;2(2):105-117.
8. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
9. White BJ, Herzog MM. Labral reconstruction: when to perform and how. Front Surg. 2015;2:27.
10. Yen YM, Kocher MS. Chondral lesions of the hip: microfracture and chondroplasty. Sports Med Arthrosc. 2010;18(2):83-89.
11. Jordan MA, Van Thiel GS, Chahal J, Nho SJ. Operative treatment of chondral defects in the hip joint: a systematic review. Curr Rev Musculoskelet Med. 2012;5(3):244-253.
12. Griffin DW, Kinnard MJ, Formby PM, McCabe MP, Anderson TD. Outcomes of hip arthroscopy in the older adult: a systematic review of the literature [published online October 18, 2016]. Am J Sports Med. doi:10.1177/0363546516667915.
13. Ganz R, Leunig M, Leunig-Ganz K, Harris WH. The etiology of osteoarthritis of the hip. Clin Orthop Relat Res. 2008;466(2):264-272.
14. Kaya M, Suzuki T, Emori M, Yamashita T. Hip morphology influences the pattern of articular cartilage damage. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):2016-2023.
15. Beck M, Leunig M, Parvizi J, Boutier V, Wyss D, Ganz R. Anterior femoroacetabular impingement: part II. Midterm results of surgical treatment. Clin Orthop Relat Res. 2004;(418):67-73.
16. Byrd JT. Hip arthroscopy in athletes. Oper Tech Sports Med. 2005;13(1):24-36.
17. Werner BC, Gaudiani MA, Ranawat AS. The etiology and arthroscopic surgical management of cam lesions. Clin Sports Med. 2016;35(3):391-404.
18. Clohisy JC, Baca G, Beaulé PE, et al; ANCHOR Study Group. Descriptive epidemiology of femoroacetabular impingement: a North American cohort of patients undergoing surgery. Am J Sports Med. 2013;41(6):1348-1356.
19. Shia DS, Clohisy JC, Schinsky MF, Martell JM, Maloney WJ. THA with highly cross-linked polyethylene in patients 50 years or younger. Clin Orthop Relat Res. 2009;467(8):2059-2065.
20. Gandhi SK, Salmon JW, Zhao SZ, Lambert BL, Gore PR, Conrad K. Psychometric evaluation of the 12-item Short-Form Health Survey (SF-12) in osteoarthritis and rheumatoid arthritis clinical trials. Clin Ther. 2001;23(7):1080-1098.
21. Loudon JK, Reiman MP. Conservative management of femoroacetabular impingement (FAI) in the long distance runner. Phys Ther Sport. 2014;15(2):82-90.
22. Wall PD, Fernandez M, Griffin DR, Foster NE. Nonoperative treatment for femoroacetabular impingement: a systematic review of the literature. PM R. 2013;5(5):418-426.
23. Nepple JJ, Carlisle JC, Nunley RM, Clohisy JC. Clinical and radiographic predictors of intra-articular hip disease in arthroscopy. Am J Sports Med. 2011;39(2):296-303.
24. McCormick F, Nwachukwu BU, Alpaugh K, Martin SD. Predictors of hip arthroscopy outcomes for labral tears at minimum 2-year follow-up: the influence of age and arthritis. Arthroscopy. 2012;28(10):1359-1364.
25. Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87(7):1012-1018.
26. Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis—what the radiologist should know. AJR Am J Roentgenol. 2007;188(6):1540-1552.
27. Kapron AL, Peters CL, Aoki SK, et al. The prevalence of radiographic findings of structural hip deformities in female collegiate athletes. Am J Sports Med. 2015;43(6):1324-1330.
28. Lee WY, Kang C, Hwang DS, Jeon JH, Zheng L. Descriptive epidemiology of symptomatic femoroacetabular impingement in young athlete: single center study. Hip Pelvis. 2016;28(1):29-34.
29. Dudda M, Kim YJ, Zhang Y, et al. Morphologic differences between the hips of Chinese women and white women: could they account for the ethnic difference in the prevalence of hip osteoarthritis? Arthritis Rheum. 2011;63(10):2992-2999.
30. Solomon L, Beighton P. Osteoarthrosis of the hip and its relationship to pre-existing in an African population. J Bone Joint Surg Br. 1973;55(1):216-217.
31. Keeney JA, Peelle MW, Jackson J, Rubin D, Maloney WJ, Clohisy JC. Magnetic resonance arthrography versus arthroscopy in the evaluation of articular hip pathology. Clin Orthop Relat Res. 2004;(429):163-169.
32. Schmid MR, Nötzli HP, Zanetti M, Wyss TF, Hodler J. Cartilage lesions in the hip: diagnostic effectiveness of MR arthrography. Radiology. 2003;226(2):382-386.
33. Chevillotte CJ, Ali MH, Trousdale RT, Pagnano MW. Variability in hip range of motion on clinical examination. J Arthroplasty. 2009;24(5):693-697.