User login
Treating comorbid posttraumatic stress disorder and cardiovascular disease
Mr. S, 64, has a history of posttraumatic stress disorder (PTSD), which has been well controlled for the past 15 years with cognitive-processing therapy and fluoxetine, 40 mg/d. However, over the past 6 weeks, Mr. S has experienced increased hypervigilance, nightmares, and flashbacks. He states that his primary care provider recommended an adjustment in pharmacotherapy to address this exacerbation of symptoms. Previous medication trials include sertraline, 200 mg/d, discontinued due to lack of perceived efficacy, and venlafaxine, 150 mg/d, discontinued due to increased blood pressure.
Mr. S’s medical history includes hypertension, dyslipidemia, and myocardial infarction (MI) 5 years ago. His family history includes sudden cardiac death (mother and father) and major depressive disorder (sister). His blood pressure is currently uncontrolled on lisinopril, 5 mg/d, and metoprolol succinate, 50 mg/d. Today, serial blood pressure readings measured approximately 180/90 mm Hg, with a pulse 50-60 beats per minute.
What is the next step in treating Mr. S’s hypertension and PTSD symptoms? Is there any evidence to support concomitant therapy?
PTSD is characterized by emotional and behavioral symptoms following exposure to a traumatic event. Its 12-month prevalence in the United States is estimated at 3.5%. Diagnostic criteria necessitate the presence of intrusive symptoms, persistent effortful avoidance of distressing trauma-related stimuli, negative cognitions or mood, and alterations in arousal and reactivity. PTSD negatively impacts social and occupational functioning.1
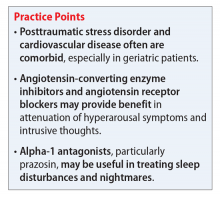
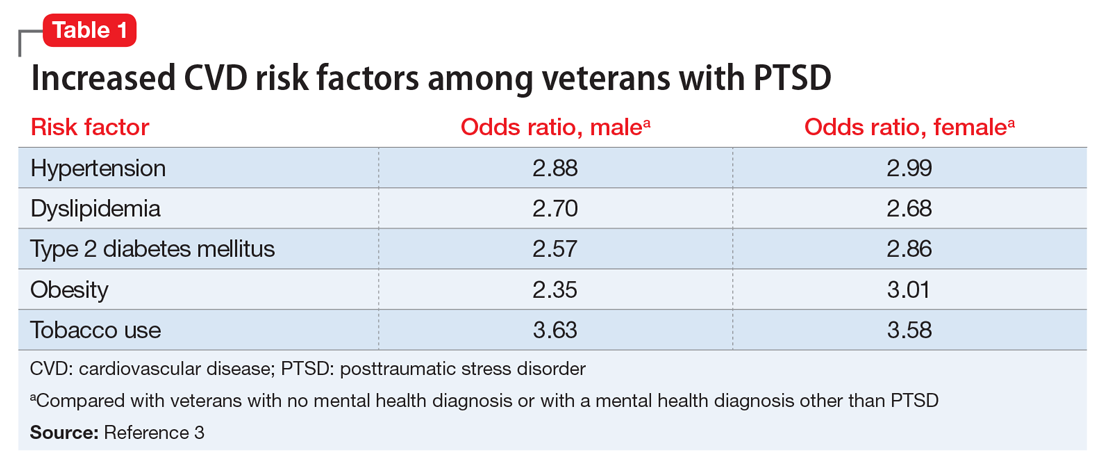
Studies have revealed a correlation between the presence of psychosocial factors, such as depression and anxiety, and the occurrence of cardiovascular events. The mechanism appears to consist of a behavioral component (eg, poor diet, tobacco use) and a direct pathophysiologic component (eg, excessive sympathetic nervous system activation) (Table 13).4 Management of concomitant PTSD and CVD presents a challenge to clinicians.
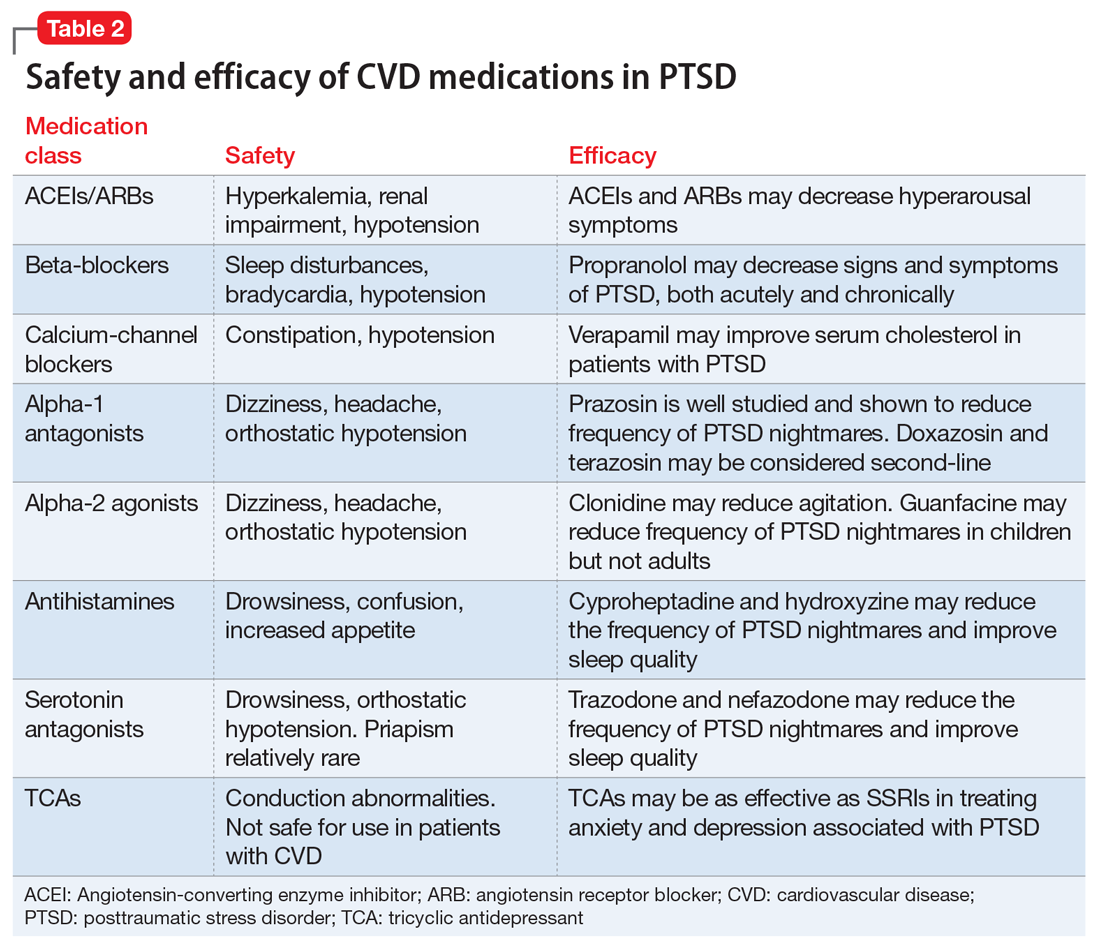
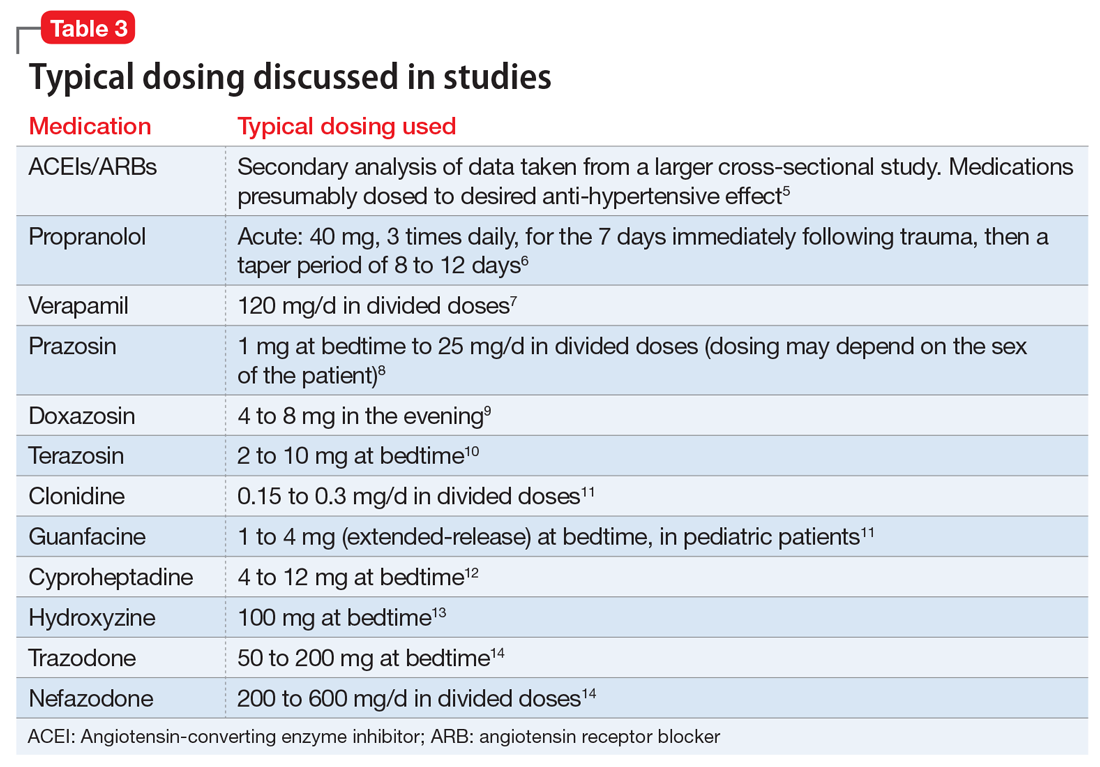
This article summarizes the evidence for the use of CVD medications in treating PTSD (Table 2) and how to apply these principles in patient care (Table 35-14).
ACEIs, ARBs, beta blockers, and calcium channel blockers
Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) inhibit the renin-angiotensin system: ACEIs prevent formation of angiotensin II, a potent vasoconstrictor, and ARBs prevent interaction between angiotensin II and its receptor. In one study, patients were recruited from a large public hospital serving primarily a highly traumatized, low-income population. Patients taking an ACEI or ARB who had experienced at least 1 traumatic event exhibited significantly decreased hyperarousal symptoms and decreased intrusive thoughts on the PTSD Symptom Scale and Clinician Administered PTSD Scale.5 Other studies have reported that blockade of angiotensin II AT1 receptors may result in decreased stress, anxiety, and inflammation.15
Evidence supports the use of the centrally acting, beta-adrenergic antagonist propranolol for decreasing the physiologic reactivity to acute trauma. Emotional arousal enhances the consolidation of emotional experiences into long-term memories via the adrenal stress hormones epinephrine and corticosterone. The amygdala mediates these stress hormones and releases norepinephrine, which subsequently activates noradrenergic receptors essential for memory enhancement. Several studies have reported that patients who received propranolol within several hours of a traumatic event experienced fewer physiologic signs of PTSD at follow-up 1 month later.16 Moreover, researchers have hypothesized that chronic treatment with propranolol may be effective in decreasing hyperarousal symptoms in patients with chronic PTSD by reducing tonically elevated norepinephrine signaling.6
Chronic elevation of noradrenergic activity may induce lipoprotein lipase and suppress low-density lipoprotein (LDL) receptor activity, which in turn elevates serum cholesterol levels. The results of one study suggested that verapamil, a non-dihydropyridine calcium channel blocker, significantly improves serum cholesterol levels in patients with PTSD by increasing LDL receptor activity and decreasing norepinephrine release.7
Alpha-1 and alpha-2 antagonists
Alpha-1 antagonists relax vascular smooth muscle by blocking norepinephrine stimulation at postsynaptic α-1-adrenergic receptors. They frequently are prescribed for hypertension and benign prostatic hypertrophy. One α-1 antagonist in particular, prazosin, appears especially useful in treating sleep disturbances, which occur in up to 90% of patients with PTSD.17 Because of its relatively greater lipophilicity, prazosin crosses the blood–brain barrier and acts centrally to reduce the fight-or-flight and hyperarousal reactions related to nightmares caused by PTSD.18 Common adverse effects include dizziness and orthostatic hypotension. These usually can be mitigated with titration to effective dose. In a study of active-duty soldiers who returned from Iraq and Afghanistan, Raskind et al8 found that prazosin doses up to 25 mg/d in men and 12 mg/d in women were tolerated with weekly adjustments and blood pressure monitoring.
Other α-1 antagonists have shown efficacy in a limited number of trials and may be considered second-line treatment of PTSD hyperarousal symptoms. Doxazosin has a longer half-life compared with prazosin (22 hours vs 3 hours) and may be useful in treating daytime hyperarousal with once-daily dosing. However, its hydrophilicity prevents it from crossing the blood–brain barrier to the same degree as prazosin.19 Terazosin also has a longer half-life (12 hours) and reaches peak plasma concentration in 1 hour. It undergoes minimal first-pass metabolism, leaving almost the entire circulating dose in the parent form, but clinical data are limited to only a small case report.10
Alpha-2 agonists inhibit sympathetic outflow in the CNS, which ultimately relaxes vascular smooth muscle like α-1 antagonists. Clonidine exhibits sedative properties, which derive from its nonspecific binding to α-2a-, -2b-, and -2c-adrenergic receptors. Several case studies have described a reduction in agitation in PTSD patients with the use of clonidine, likely through the induction of sleep and relaxation. Guanfacine, on the other hand, selectively binds to the α-2a-adrenergic receptor and therefore lacks the sedative properties of clonidine. Several placebo-controlled trials showed no alleviation of PTSD symptoms in adults with the use of guanfacine.11 However, case reports and open-label trials have suggested that guanfacine may reduce trauma-induced nightmares in pediatric patients. Further investigation is needed to clarify the potential use of guanfacine in pediatric PTSD.19
Antihistamines and antidepressants
Several second-line pharmacologic agents may be useful in patients with PTSD who are already taking cardiovascular medication. A limited number of studies have demonstrated reduced frequency of PTSD nightmares with the histamine-1 antagonists cyproheptadine and hydroxyzine, both of which exhibit minor anti-serotonergic properties.12,13 Likewise, the serotonin antagonists nefazodone and trazodone have been shown to reduce the frequency of PTSD nightmares, as well as improve overall sleep quality.14 Nefazodone should be considered an option only after treatment failure of multiple other medications, because it is associated with a small, but significant, risk of life-threatening hepatotoxicity.20
Tricyclic antidepressants (TCAs) may reduce anxiety and depression associated with PTSD to the same degree as SSRIs.21 However, their effect on PTSD-associated sleep disturbances is much less pronounced than other available medications.14 TCAs should be avoided in patients with CVD because they may exacerbate cardiac conduction abnormalities. This is especially true for those recovering from acute MI.22
CASE CONTINUED
Mr. S is started on prazosin, 1 mg at bedtime, titrated weekly to 6 mg at bedtime with regular blood pressure monitoring because of the risk of orthostatic hypotension. Although the frequency of his nightmares decreases to 1 or 2 per month, he still experiences flashbacks at the same frequency and intensity as before. Prazosin, 1 mg every morning, is added, titrated weekly to 4 mg every morning. This combination of morning and bedtime dosing leads to resolution of both nightmares and flashbacks along with a significant reduction in hyperarousal. Lisinopril is increased from 5 to 10 mg/d to address Mr. S’s uncontrolled hypertension; this change also could have contributed to the reduction in hyperarousal. CPT and fluoxetine are continued.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Laslett LJ, Alagona P Jr, Clark BA 3rd, et al. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. 2012;60(suppl 25):S1-S49.
3. Cohen BE, Marmar C, Ren L, et al. Association of cardiovascular risk factors with mental health diagnoses in Iraq and Afghanistan war veterans using VA health care. JAMA. 2009;302(5):489-492.
4. Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99(16):2192-2217.
5. Khoury NM, Marvar PJ, Gillespie CF, et al. The renin-angiotensin pathway in posttraumatic stress disorder: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with fewer traumatic stress symptoms. J Clin Psychiatry. 2012;73(6):849-855.
6. Giustino TF, Fitzgerald PJ, Maren S. Revisiting propranolol and PTSD: memory erasure or extinction enhancement? Neurobiol Learn Mem. 2016;130:26-33.
7. Ansari MA, Ahmed S. Calcium channel blocker verapamil: a new intervention for high cholesterol levels in patients with PTSD. Turk Jem. 2007;11:93-97.
8. Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371-373.
9. De Jong J, Wauben P, Huijbrechts I, et al. Doxazosin treatment for posttraumatic stress disorder. J Clin Psychopharmacol. 2010;30(1):84-85.
10. Nirmalani-Gandhy A, Sanchez D, Catalano G. Terazosin for the treatment of trauma-related nightmares: a report of four cases. Clin Neuropharmacol. 2015;38(3):109-111.
11. Belkin MR, Schwartz TL. Alpha-2 receptor agonists for the treatment of posttraumatic stress disorder. Drugs Context. 2015;4:212286. doi: 10.7573/dic.212286.
12. Gupta S, Popli A, Bathurst E, et al. Efficacy of cyproheptadine for nightmares associated with posttraumatic stress disorder. Compr Psychiatry. 1998;39(3):160-164.
13. Ahmadpanah M, Sabzeiee P, Hosseini SM, et al. Comparing the effect of prazosin and hydroxyzine on sleep quality in patients suffering from posttraumatic stress disorder. Neuropsychobiology. 2014;69(4):235-242.
14. Maher MJ, Rego SA, Asnis GM. Sleep disturbances in patients with post-traumatic stress disorder: epidemiology, impact and approaches to management. CNS Drugs. 2006;20(7):567-590.
15. Saavedra JM, Sánchez-Lemus E, Benicky J. Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation, and ischemia: therapeutic implications. Psychoneuroendocrinology. 2011;36(1):1-18.
16. McGaugh JL. Making lasting memories: remembering the significant. Proc Natl Acad Sci U S A. 2013;110(suppl 2):10402-10407.
17. Writer BW, Meyer EG, Schillerstrom JE. Prazosin for military combat-related PTSD nightmares: a critical review. J Neuropsychiatry Clin Neurosci. 2014;26(1):24-33.
18. Kung S, Espinel Z, Lapid MI. Treatment of nightmares with prazosin: a systematic review. Mayo Clin Proc. 2012;87(9):890-900.
19. Arnsten AF, Raskind MA, Taylor FB, et al. The effects of stress exposure on prefrontal cortex: translating basic research into successful treatments for post-traumatic stress disorder. Neurobiol Stress. 2015;1:89-99.
20. Serzone [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2003.
21. Puetz TW, Youngstedt SD, Herring MP. Effects of pharmacotherapy on combat-related PTSD, anxiety, and depression: a systematic review and meta-regression analysis. PLoS One. 2015;10(5):e0126529. doi: 10.1371/journal. pone.0126529.
22. Glassman AH. Cardiovascular effects of tricyclic antidepressants. Annu Rev Med. 1984;35:503-511.
Mr. S, 64, has a history of posttraumatic stress disorder (PTSD), which has been well controlled for the past 15 years with cognitive-processing therapy and fluoxetine, 40 mg/d. However, over the past 6 weeks, Mr. S has experienced increased hypervigilance, nightmares, and flashbacks. He states that his primary care provider recommended an adjustment in pharmacotherapy to address this exacerbation of symptoms. Previous medication trials include sertraline, 200 mg/d, discontinued due to lack of perceived efficacy, and venlafaxine, 150 mg/d, discontinued due to increased blood pressure.
Mr. S’s medical history includes hypertension, dyslipidemia, and myocardial infarction (MI) 5 years ago. His family history includes sudden cardiac death (mother and father) and major depressive disorder (sister). His blood pressure is currently uncontrolled on lisinopril, 5 mg/d, and metoprolol succinate, 50 mg/d. Today, serial blood pressure readings measured approximately 180/90 mm Hg, with a pulse 50-60 beats per minute.
What is the next step in treating Mr. S’s hypertension and PTSD symptoms? Is there any evidence to support concomitant therapy?
PTSD is characterized by emotional and behavioral symptoms following exposure to a traumatic event. Its 12-month prevalence in the United States is estimated at 3.5%. Diagnostic criteria necessitate the presence of intrusive symptoms, persistent effortful avoidance of distressing trauma-related stimuli, negative cognitions or mood, and alterations in arousal and reactivity. PTSD negatively impacts social and occupational functioning.1
Studies have revealed a correlation between the presence of psychosocial factors, such as depression and anxiety, and the occurrence of cardiovascular events. The mechanism appears to consist of a behavioral component (eg, poor diet, tobacco use) and a direct pathophysiologic component (eg, excessive sympathetic nervous system activation) (Table 13).4 Management of concomitant PTSD and CVD presents a challenge to clinicians.
This article summarizes the evidence for the use of CVD medications in treating PTSD (Table 2) and how to apply these principles in patient care (Table 35-14).
ACEIs, ARBs, beta blockers, and calcium channel blockers
Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) inhibit the renin-angiotensin system: ACEIs prevent formation of angiotensin II, a potent vasoconstrictor, and ARBs prevent interaction between angiotensin II and its receptor. In one study, patients were recruited from a large public hospital serving primarily a highly traumatized, low-income population. Patients taking an ACEI or ARB who had experienced at least 1 traumatic event exhibited significantly decreased hyperarousal symptoms and decreased intrusive thoughts on the PTSD Symptom Scale and Clinician Administered PTSD Scale.5 Other studies have reported that blockade of angiotensin II AT1 receptors may result in decreased stress, anxiety, and inflammation.15
Evidence supports the use of the centrally acting, beta-adrenergic antagonist propranolol for decreasing the physiologic reactivity to acute trauma. Emotional arousal enhances the consolidation of emotional experiences into long-term memories via the adrenal stress hormones epinephrine and corticosterone. The amygdala mediates these stress hormones and releases norepinephrine, which subsequently activates noradrenergic receptors essential for memory enhancement. Several studies have reported that patients who received propranolol within several hours of a traumatic event experienced fewer physiologic signs of PTSD at follow-up 1 month later.16 Moreover, researchers have hypothesized that chronic treatment with propranolol may be effective in decreasing hyperarousal symptoms in patients with chronic PTSD by reducing tonically elevated norepinephrine signaling.6
Chronic elevation of noradrenergic activity may induce lipoprotein lipase and suppress low-density lipoprotein (LDL) receptor activity, which in turn elevates serum cholesterol levels. The results of one study suggested that verapamil, a non-dihydropyridine calcium channel blocker, significantly improves serum cholesterol levels in patients with PTSD by increasing LDL receptor activity and decreasing norepinephrine release.7
Alpha-1 and alpha-2 antagonists
Alpha-1 antagonists relax vascular smooth muscle by blocking norepinephrine stimulation at postsynaptic α-1-adrenergic receptors. They frequently are prescribed for hypertension and benign prostatic hypertrophy. One α-1 antagonist in particular, prazosin, appears especially useful in treating sleep disturbances, which occur in up to 90% of patients with PTSD.17 Because of its relatively greater lipophilicity, prazosin crosses the blood–brain barrier and acts centrally to reduce the fight-or-flight and hyperarousal reactions related to nightmares caused by PTSD.18 Common adverse effects include dizziness and orthostatic hypotension. These usually can be mitigated with titration to effective dose. In a study of active-duty soldiers who returned from Iraq and Afghanistan, Raskind et al8 found that prazosin doses up to 25 mg/d in men and 12 mg/d in women were tolerated with weekly adjustments and blood pressure monitoring.
Other α-1 antagonists have shown efficacy in a limited number of trials and may be considered second-line treatment of PTSD hyperarousal symptoms. Doxazosin has a longer half-life compared with prazosin (22 hours vs 3 hours) and may be useful in treating daytime hyperarousal with once-daily dosing. However, its hydrophilicity prevents it from crossing the blood–brain barrier to the same degree as prazosin.19 Terazosin also has a longer half-life (12 hours) and reaches peak plasma concentration in 1 hour. It undergoes minimal first-pass metabolism, leaving almost the entire circulating dose in the parent form, but clinical data are limited to only a small case report.10
Alpha-2 agonists inhibit sympathetic outflow in the CNS, which ultimately relaxes vascular smooth muscle like α-1 antagonists. Clonidine exhibits sedative properties, which derive from its nonspecific binding to α-2a-, -2b-, and -2c-adrenergic receptors. Several case studies have described a reduction in agitation in PTSD patients with the use of clonidine, likely through the induction of sleep and relaxation. Guanfacine, on the other hand, selectively binds to the α-2a-adrenergic receptor and therefore lacks the sedative properties of clonidine. Several placebo-controlled trials showed no alleviation of PTSD symptoms in adults with the use of guanfacine.11 However, case reports and open-label trials have suggested that guanfacine may reduce trauma-induced nightmares in pediatric patients. Further investigation is needed to clarify the potential use of guanfacine in pediatric PTSD.19
Antihistamines and antidepressants
Several second-line pharmacologic agents may be useful in patients with PTSD who are already taking cardiovascular medication. A limited number of studies have demonstrated reduced frequency of PTSD nightmares with the histamine-1 antagonists cyproheptadine and hydroxyzine, both of which exhibit minor anti-serotonergic properties.12,13 Likewise, the serotonin antagonists nefazodone and trazodone have been shown to reduce the frequency of PTSD nightmares, as well as improve overall sleep quality.14 Nefazodone should be considered an option only after treatment failure of multiple other medications, because it is associated with a small, but significant, risk of life-threatening hepatotoxicity.20
Tricyclic antidepressants (TCAs) may reduce anxiety and depression associated with PTSD to the same degree as SSRIs.21 However, their effect on PTSD-associated sleep disturbances is much less pronounced than other available medications.14 TCAs should be avoided in patients with CVD because they may exacerbate cardiac conduction abnormalities. This is especially true for those recovering from acute MI.22
CASE CONTINUED
Mr. S is started on prazosin, 1 mg at bedtime, titrated weekly to 6 mg at bedtime with regular blood pressure monitoring because of the risk of orthostatic hypotension. Although the frequency of his nightmares decreases to 1 or 2 per month, he still experiences flashbacks at the same frequency and intensity as before. Prazosin, 1 mg every morning, is added, titrated weekly to 4 mg every morning. This combination of morning and bedtime dosing leads to resolution of both nightmares and flashbacks along with a significant reduction in hyperarousal. Lisinopril is increased from 5 to 10 mg/d to address Mr. S’s uncontrolled hypertension; this change also could have contributed to the reduction in hyperarousal. CPT and fluoxetine are continued.
Mr. S, 64, has a history of posttraumatic stress disorder (PTSD), which has been well controlled for the past 15 years with cognitive-processing therapy and fluoxetine, 40 mg/d. However, over the past 6 weeks, Mr. S has experienced increased hypervigilance, nightmares, and flashbacks. He states that his primary care provider recommended an adjustment in pharmacotherapy to address this exacerbation of symptoms. Previous medication trials include sertraline, 200 mg/d, discontinued due to lack of perceived efficacy, and venlafaxine, 150 mg/d, discontinued due to increased blood pressure.
Mr. S’s medical history includes hypertension, dyslipidemia, and myocardial infarction (MI) 5 years ago. His family history includes sudden cardiac death (mother and father) and major depressive disorder (sister). His blood pressure is currently uncontrolled on lisinopril, 5 mg/d, and metoprolol succinate, 50 mg/d. Today, serial blood pressure readings measured approximately 180/90 mm Hg, with a pulse 50-60 beats per minute.
What is the next step in treating Mr. S’s hypertension and PTSD symptoms? Is there any evidence to support concomitant therapy?
PTSD is characterized by emotional and behavioral symptoms following exposure to a traumatic event. Its 12-month prevalence in the United States is estimated at 3.5%. Diagnostic criteria necessitate the presence of intrusive symptoms, persistent effortful avoidance of distressing trauma-related stimuli, negative cognitions or mood, and alterations in arousal and reactivity. PTSD negatively impacts social and occupational functioning.1
Studies have revealed a correlation between the presence of psychosocial factors, such as depression and anxiety, and the occurrence of cardiovascular events. The mechanism appears to consist of a behavioral component (eg, poor diet, tobacco use) and a direct pathophysiologic component (eg, excessive sympathetic nervous system activation) (Table 13).4 Management of concomitant PTSD and CVD presents a challenge to clinicians.
This article summarizes the evidence for the use of CVD medications in treating PTSD (Table 2) and how to apply these principles in patient care (Table 35-14).
ACEIs, ARBs, beta blockers, and calcium channel blockers
Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) inhibit the renin-angiotensin system: ACEIs prevent formation of angiotensin II, a potent vasoconstrictor, and ARBs prevent interaction between angiotensin II and its receptor. In one study, patients were recruited from a large public hospital serving primarily a highly traumatized, low-income population. Patients taking an ACEI or ARB who had experienced at least 1 traumatic event exhibited significantly decreased hyperarousal symptoms and decreased intrusive thoughts on the PTSD Symptom Scale and Clinician Administered PTSD Scale.5 Other studies have reported that blockade of angiotensin II AT1 receptors may result in decreased stress, anxiety, and inflammation.15
Evidence supports the use of the centrally acting, beta-adrenergic antagonist propranolol for decreasing the physiologic reactivity to acute trauma. Emotional arousal enhances the consolidation of emotional experiences into long-term memories via the adrenal stress hormones epinephrine and corticosterone. The amygdala mediates these stress hormones and releases norepinephrine, which subsequently activates noradrenergic receptors essential for memory enhancement. Several studies have reported that patients who received propranolol within several hours of a traumatic event experienced fewer physiologic signs of PTSD at follow-up 1 month later.16 Moreover, researchers have hypothesized that chronic treatment with propranolol may be effective in decreasing hyperarousal symptoms in patients with chronic PTSD by reducing tonically elevated norepinephrine signaling.6
Chronic elevation of noradrenergic activity may induce lipoprotein lipase and suppress low-density lipoprotein (LDL) receptor activity, which in turn elevates serum cholesterol levels. The results of one study suggested that verapamil, a non-dihydropyridine calcium channel blocker, significantly improves serum cholesterol levels in patients with PTSD by increasing LDL receptor activity and decreasing norepinephrine release.7
Alpha-1 and alpha-2 antagonists
Alpha-1 antagonists relax vascular smooth muscle by blocking norepinephrine stimulation at postsynaptic α-1-adrenergic receptors. They frequently are prescribed for hypertension and benign prostatic hypertrophy. One α-1 antagonist in particular, prazosin, appears especially useful in treating sleep disturbances, which occur in up to 90% of patients with PTSD.17 Because of its relatively greater lipophilicity, prazosin crosses the blood–brain barrier and acts centrally to reduce the fight-or-flight and hyperarousal reactions related to nightmares caused by PTSD.18 Common adverse effects include dizziness and orthostatic hypotension. These usually can be mitigated with titration to effective dose. In a study of active-duty soldiers who returned from Iraq and Afghanistan, Raskind et al8 found that prazosin doses up to 25 mg/d in men and 12 mg/d in women were tolerated with weekly adjustments and blood pressure monitoring.
Other α-1 antagonists have shown efficacy in a limited number of trials and may be considered second-line treatment of PTSD hyperarousal symptoms. Doxazosin has a longer half-life compared with prazosin (22 hours vs 3 hours) and may be useful in treating daytime hyperarousal with once-daily dosing. However, its hydrophilicity prevents it from crossing the blood–brain barrier to the same degree as prazosin.19 Terazosin also has a longer half-life (12 hours) and reaches peak plasma concentration in 1 hour. It undergoes minimal first-pass metabolism, leaving almost the entire circulating dose in the parent form, but clinical data are limited to only a small case report.10
Alpha-2 agonists inhibit sympathetic outflow in the CNS, which ultimately relaxes vascular smooth muscle like α-1 antagonists. Clonidine exhibits sedative properties, which derive from its nonspecific binding to α-2a-, -2b-, and -2c-adrenergic receptors. Several case studies have described a reduction in agitation in PTSD patients with the use of clonidine, likely through the induction of sleep and relaxation. Guanfacine, on the other hand, selectively binds to the α-2a-adrenergic receptor and therefore lacks the sedative properties of clonidine. Several placebo-controlled trials showed no alleviation of PTSD symptoms in adults with the use of guanfacine.11 However, case reports and open-label trials have suggested that guanfacine may reduce trauma-induced nightmares in pediatric patients. Further investigation is needed to clarify the potential use of guanfacine in pediatric PTSD.19
Antihistamines and antidepressants
Several second-line pharmacologic agents may be useful in patients with PTSD who are already taking cardiovascular medication. A limited number of studies have demonstrated reduced frequency of PTSD nightmares with the histamine-1 antagonists cyproheptadine and hydroxyzine, both of which exhibit minor anti-serotonergic properties.12,13 Likewise, the serotonin antagonists nefazodone and trazodone have been shown to reduce the frequency of PTSD nightmares, as well as improve overall sleep quality.14 Nefazodone should be considered an option only after treatment failure of multiple other medications, because it is associated with a small, but significant, risk of life-threatening hepatotoxicity.20
Tricyclic antidepressants (TCAs) may reduce anxiety and depression associated with PTSD to the same degree as SSRIs.21 However, their effect on PTSD-associated sleep disturbances is much less pronounced than other available medications.14 TCAs should be avoided in patients with CVD because they may exacerbate cardiac conduction abnormalities. This is especially true for those recovering from acute MI.22
CASE CONTINUED
Mr. S is started on prazosin, 1 mg at bedtime, titrated weekly to 6 mg at bedtime with regular blood pressure monitoring because of the risk of orthostatic hypotension. Although the frequency of his nightmares decreases to 1 or 2 per month, he still experiences flashbacks at the same frequency and intensity as before. Prazosin, 1 mg every morning, is added, titrated weekly to 4 mg every morning. This combination of morning and bedtime dosing leads to resolution of both nightmares and flashbacks along with a significant reduction in hyperarousal. Lisinopril is increased from 5 to 10 mg/d to address Mr. S’s uncontrolled hypertension; this change also could have contributed to the reduction in hyperarousal. CPT and fluoxetine are continued.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Laslett LJ, Alagona P Jr, Clark BA 3rd, et al. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. 2012;60(suppl 25):S1-S49.
3. Cohen BE, Marmar C, Ren L, et al. Association of cardiovascular risk factors with mental health diagnoses in Iraq and Afghanistan war veterans using VA health care. JAMA. 2009;302(5):489-492.
4. Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99(16):2192-2217.
5. Khoury NM, Marvar PJ, Gillespie CF, et al. The renin-angiotensin pathway in posttraumatic stress disorder: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with fewer traumatic stress symptoms. J Clin Psychiatry. 2012;73(6):849-855.
6. Giustino TF, Fitzgerald PJ, Maren S. Revisiting propranolol and PTSD: memory erasure or extinction enhancement? Neurobiol Learn Mem. 2016;130:26-33.
7. Ansari MA, Ahmed S. Calcium channel blocker verapamil: a new intervention for high cholesterol levels in patients with PTSD. Turk Jem. 2007;11:93-97.
8. Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371-373.
9. De Jong J, Wauben P, Huijbrechts I, et al. Doxazosin treatment for posttraumatic stress disorder. J Clin Psychopharmacol. 2010;30(1):84-85.
10. Nirmalani-Gandhy A, Sanchez D, Catalano G. Terazosin for the treatment of trauma-related nightmares: a report of four cases. Clin Neuropharmacol. 2015;38(3):109-111.
11. Belkin MR, Schwartz TL. Alpha-2 receptor agonists for the treatment of posttraumatic stress disorder. Drugs Context. 2015;4:212286. doi: 10.7573/dic.212286.
12. Gupta S, Popli A, Bathurst E, et al. Efficacy of cyproheptadine for nightmares associated with posttraumatic stress disorder. Compr Psychiatry. 1998;39(3):160-164.
13. Ahmadpanah M, Sabzeiee P, Hosseini SM, et al. Comparing the effect of prazosin and hydroxyzine on sleep quality in patients suffering from posttraumatic stress disorder. Neuropsychobiology. 2014;69(4):235-242.
14. Maher MJ, Rego SA, Asnis GM. Sleep disturbances in patients with post-traumatic stress disorder: epidemiology, impact and approaches to management. CNS Drugs. 2006;20(7):567-590.
15. Saavedra JM, Sánchez-Lemus E, Benicky J. Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation, and ischemia: therapeutic implications. Psychoneuroendocrinology. 2011;36(1):1-18.
16. McGaugh JL. Making lasting memories: remembering the significant. Proc Natl Acad Sci U S A. 2013;110(suppl 2):10402-10407.
17. Writer BW, Meyer EG, Schillerstrom JE. Prazosin for military combat-related PTSD nightmares: a critical review. J Neuropsychiatry Clin Neurosci. 2014;26(1):24-33.
18. Kung S, Espinel Z, Lapid MI. Treatment of nightmares with prazosin: a systematic review. Mayo Clin Proc. 2012;87(9):890-900.
19. Arnsten AF, Raskind MA, Taylor FB, et al. The effects of stress exposure on prefrontal cortex: translating basic research into successful treatments for post-traumatic stress disorder. Neurobiol Stress. 2015;1:89-99.
20. Serzone [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2003.
21. Puetz TW, Youngstedt SD, Herring MP. Effects of pharmacotherapy on combat-related PTSD, anxiety, and depression: a systematic review and meta-regression analysis. PLoS One. 2015;10(5):e0126529. doi: 10.1371/journal. pone.0126529.
22. Glassman AH. Cardiovascular effects of tricyclic antidepressants. Annu Rev Med. 1984;35:503-511.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Laslett LJ, Alagona P Jr, Clark BA 3rd, et al. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. 2012;60(suppl 25):S1-S49.
3. Cohen BE, Marmar C, Ren L, et al. Association of cardiovascular risk factors with mental health diagnoses in Iraq and Afghanistan war veterans using VA health care. JAMA. 2009;302(5):489-492.
4. Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99(16):2192-2217.
5. Khoury NM, Marvar PJ, Gillespie CF, et al. The renin-angiotensin pathway in posttraumatic stress disorder: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with fewer traumatic stress symptoms. J Clin Psychiatry. 2012;73(6):849-855.
6. Giustino TF, Fitzgerald PJ, Maren S. Revisiting propranolol and PTSD: memory erasure or extinction enhancement? Neurobiol Learn Mem. 2016;130:26-33.
7. Ansari MA, Ahmed S. Calcium channel blocker verapamil: a new intervention for high cholesterol levels in patients with PTSD. Turk Jem. 2007;11:93-97.
8. Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371-373.
9. De Jong J, Wauben P, Huijbrechts I, et al. Doxazosin treatment for posttraumatic stress disorder. J Clin Psychopharmacol. 2010;30(1):84-85.
10. Nirmalani-Gandhy A, Sanchez D, Catalano G. Terazosin for the treatment of trauma-related nightmares: a report of four cases. Clin Neuropharmacol. 2015;38(3):109-111.
11. Belkin MR, Schwartz TL. Alpha-2 receptor agonists for the treatment of posttraumatic stress disorder. Drugs Context. 2015;4:212286. doi: 10.7573/dic.212286.
12. Gupta S, Popli A, Bathurst E, et al. Efficacy of cyproheptadine for nightmares associated with posttraumatic stress disorder. Compr Psychiatry. 1998;39(3):160-164.
13. Ahmadpanah M, Sabzeiee P, Hosseini SM, et al. Comparing the effect of prazosin and hydroxyzine on sleep quality in patients suffering from posttraumatic stress disorder. Neuropsychobiology. 2014;69(4):235-242.
14. Maher MJ, Rego SA, Asnis GM. Sleep disturbances in patients with post-traumatic stress disorder: epidemiology, impact and approaches to management. CNS Drugs. 2006;20(7):567-590.
15. Saavedra JM, Sánchez-Lemus E, Benicky J. Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation, and ischemia: therapeutic implications. Psychoneuroendocrinology. 2011;36(1):1-18.
16. McGaugh JL. Making lasting memories: remembering the significant. Proc Natl Acad Sci U S A. 2013;110(suppl 2):10402-10407.
17. Writer BW, Meyer EG, Schillerstrom JE. Prazosin for military combat-related PTSD nightmares: a critical review. J Neuropsychiatry Clin Neurosci. 2014;26(1):24-33.
18. Kung S, Espinel Z, Lapid MI. Treatment of nightmares with prazosin: a systematic review. Mayo Clin Proc. 2012;87(9):890-900.
19. Arnsten AF, Raskind MA, Taylor FB, et al. The effects of stress exposure on prefrontal cortex: translating basic research into successful treatments for post-traumatic stress disorder. Neurobiol Stress. 2015;1:89-99.
20. Serzone [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2003.
21. Puetz TW, Youngstedt SD, Herring MP. Effects of pharmacotherapy on combat-related PTSD, anxiety, and depression: a systematic review and meta-regression analysis. PLoS One. 2015;10(5):e0126529. doi: 10.1371/journal. pone.0126529.
22. Glassman AH. Cardiovascular effects of tricyclic antidepressants. Annu Rev Med. 1984;35:503-511.