User login
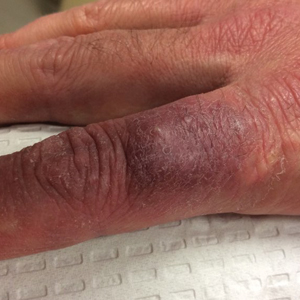
Dusky Pink Nodular Plaque on the Finger
The Diagnosis: Majocchi Granuloma
Majocchi granuloma (MG) is a dermatophytic infection that reveals hyphal elements within the cornified cells of follicles and most commonly is caused by Trichophyton rubrum. However, occasionally other Trichophyton, Trichosporon, and Aspergillus species are involved.1
There typically are 2 forms of MG: (1) the small perifollicular papular form that usually is localized to the dermis and occurs in immunocompetent individuals, and (2) a deep form featuring subcutaneous plaques and nodules that generally occur on the hair-bearing surfaces in immunosuppressed hosts.2 Majocchi granuloma also commonly occurs from the use of potent topical steroids on unsuspected tinea.3
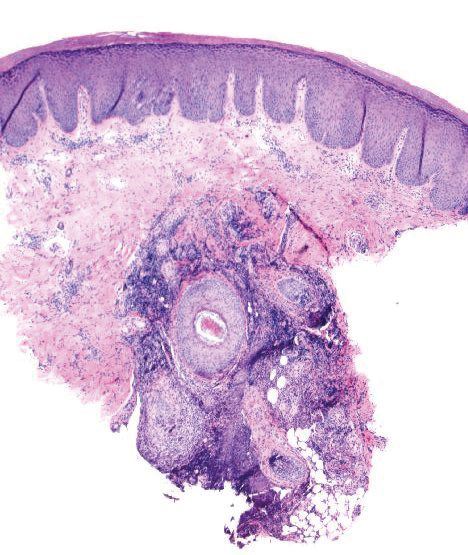
Histopathologically, MG generally presents as granulomatous inflammation with perifollicular neutrophilic infiltration. This polymorphonuclear cell infiltrate was visible clinically as a single pustule overlying the nodular plaque, a clue appreciable only on close inspection. Histopathologic examination revealed segmented branching filaments present within cornified elements of a follicle (Figure). Notably, potassium hydroxide (KOH) preparations are unreliable diagnostic aids in MG, as evidenced by the 2 negative KOH preparations in this case. According to Chou and Hsu,4 because KOH preparation can only detect fungi located in the stratum corneum, the result may be negative for MG due to deeper invasion of the fungi into the dermal follicular component. In fact, KOH preparations of MG may reveal no hyphae in 23.3% of cases.2
The initiating factor in MG is not entirely known but is thought to be physical trauma that either directly or indirectly leads to follicle disruption and passive introduction of the organism into the dermis (eg, traumatic implantation via gardening or other recreational activities).2 Other proposed mechanisms include the presentation of the membrane-associated ATP-binding cassette transporter on the surface of T rubrum.1 Dermatophytes evade the host immune system through a variety of mechanisms: (1) cell wall glycoproteins, (2) release of anti-inflammatory cytokines, and (3) generation of immunosuppressive regulatory T cells.1
Collectively, the clinical and histopathologic findings distinguish MG from other cutaneous conditions. Sporotrichosis, a granulomatous infection caused by Sporothrix schenckii, typically is found in tropical regions of the world and often is associated with floriculture.5 Sporotrichosis initially presents in a subcutaneous papulonodular form, but unlike MG, it later ulcerates and progresses along adjacent lymphatic chains.5 Pathology of sporotrichosis exhibits pseudoepitheliomatous hyperplasia with granulomas, possible foci of suppuration, and yeastlike forms called cigar bodies. Chromoblastomycosis clinically is defined by tumorlike lesions on the skin including verrucous, nodular, or scarlike plaques and typically is associated with traumatic injury and implantation of the microorganism. Histologically, chromoblastomycosis demonstrates pseudoepitheliomatous hyperplasia with granulomas and characteristic darkly pigmented, thick-walled sclerotic cells called Medlar bodies.6Mycobacterium marinum is one cause of nontuberculous mycobacterial skin infections in humans. Clinically, M marinum is associated with improper hygiene techniques and contact with fish tanks and other aqueous environments. Mycobacterium marinum can present histopathologically as early neutrophilic infiltration or late dermal granulomatous inflammation.7 Acid-fast bacilli typically are scant, leaving the diagnosis best secured via polymerase chain reaction assay. Nodular Kaposi sarcoma (KS) can present as a dusky nodular plaque on an acral surface but typically is seen in patients with underlying human immunodeficiency virus/AIDS or other immunosuppressive conditions. The pathology for KS shows a proliferation of human herpes virus 8-positive spindle cells with slitlike spaces containing red blood cells instead of granulomatous inflammation.
Treatment regimens with topical corticosteroids can exacerbate the infection due to local suppression of cell-mediated immunity.8 In these scenarios, fungal infection is suspected, and systemic antifungals such as ketoconazole; itraconazole; or terbinafine, which has become the mainstay, are prescribed. Resolution of the infection with these medications usually is seen after 4 weeks.2
A diagnosis of MG can be elusive and often may take multiple visits. Clinicians should note that MG could demonstrate repeated false-negative KOH preparations; therefore, these tests should not be relied on as the sole determination of a diagnosis. Although chromoblastomycosis, sporotrichosis, nodular KS, and infection with M marinum may all present as nodular plaques with granulomatous pathology, a follicular pustule may be a clinical clue to MG, as its mimics typically lack folliculocentric neutrophils.
- Tirado-Sánchez A, Ponce-Olivera RM, Bonifaz A. Majocchi's granuloma (dermatophytic granuloma): updated therapeutic options. Curr Fungal Infect Rep. 2015;9:204-212.
- Ilkit M, Durdu M, Karakas¸ M. Majocchi's granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50:449-457.
- Schwartz RA, Janniger CK. Majocchi granuloma. Medscape website. https://emedicine.medscape.com/article/1092601-overview. Updated May 14, 2019. Accessed April 13, 2020.
- Chou WY, Hsu CJ. A case report of Majocchi's granuloma associated with combined therapy of topical steroids and adalimumab. Medicine (Baltimore). 2016;95:E2245.
- Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633-654.
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280.
- Slany M, Jezek P, Bodnarova M. Fish tank granuloma caused by Mycobacterium marinum in two aquarists: two case reports. Biomed Res Int. 2013;2013:161329.
- Coondoo A, Phiske M, Verma S, et al. Side-effects of topical steroids: a long overdue revisit. Indian Dermatol Online J. 2014;5:416-425.
The Diagnosis: Majocchi Granuloma
Majocchi granuloma (MG) is a dermatophytic infection that reveals hyphal elements within the cornified cells of follicles and most commonly is caused by Trichophyton rubrum. However, occasionally other Trichophyton, Trichosporon, and Aspergillus species are involved.1
There typically are 2 forms of MG: (1) the small perifollicular papular form that usually is localized to the dermis and occurs in immunocompetent individuals, and (2) a deep form featuring subcutaneous plaques and nodules that generally occur on the hair-bearing surfaces in immunosuppressed hosts.2 Majocchi granuloma also commonly occurs from the use of potent topical steroids on unsuspected tinea.3
Histopathologically, MG generally presents as granulomatous inflammation with perifollicular neutrophilic infiltration. This polymorphonuclear cell infiltrate was visible clinically as a single pustule overlying the nodular plaque, a clue appreciable only on close inspection. Histopathologic examination revealed segmented branching filaments present within cornified elements of a follicle (Figure). Notably, potassium hydroxide (KOH) preparations are unreliable diagnostic aids in MG, as evidenced by the 2 negative KOH preparations in this case. According to Chou and Hsu,4 because KOH preparation can only detect fungi located in the stratum corneum, the result may be negative for MG due to deeper invasion of the fungi into the dermal follicular component. In fact, KOH preparations of MG may reveal no hyphae in 23.3% of cases.2

The initiating factor in MG is not entirely known but is thought to be physical trauma that either directly or indirectly leads to follicle disruption and passive introduction of the organism into the dermis (eg, traumatic implantation via gardening or other recreational activities).2 Other proposed mechanisms include the presentation of the membrane-associated ATP-binding cassette transporter on the surface of T rubrum.1 Dermatophytes evade the host immune system through a variety of mechanisms: (1) cell wall glycoproteins, (2) release of anti-inflammatory cytokines, and (3) generation of immunosuppressive regulatory T cells.1
Collectively, the clinical and histopathologic findings distinguish MG from other cutaneous conditions. Sporotrichosis, a granulomatous infection caused by Sporothrix schenckii, typically is found in tropical regions of the world and often is associated with floriculture.5 Sporotrichosis initially presents in a subcutaneous papulonodular form, but unlike MG, it later ulcerates and progresses along adjacent lymphatic chains.5 Pathology of sporotrichosis exhibits pseudoepitheliomatous hyperplasia with granulomas, possible foci of suppuration, and yeastlike forms called cigar bodies. Chromoblastomycosis clinically is defined by tumorlike lesions on the skin including verrucous, nodular, or scarlike plaques and typically is associated with traumatic injury and implantation of the microorganism. Histologically, chromoblastomycosis demonstrates pseudoepitheliomatous hyperplasia with granulomas and characteristic darkly pigmented, thick-walled sclerotic cells called Medlar bodies.6Mycobacterium marinum is one cause of nontuberculous mycobacterial skin infections in humans. Clinically, M marinum is associated with improper hygiene techniques and contact with fish tanks and other aqueous environments. Mycobacterium marinum can present histopathologically as early neutrophilic infiltration or late dermal granulomatous inflammation.7 Acid-fast bacilli typically are scant, leaving the diagnosis best secured via polymerase chain reaction assay. Nodular Kaposi sarcoma (KS) can present as a dusky nodular plaque on an acral surface but typically is seen in patients with underlying human immunodeficiency virus/AIDS or other immunosuppressive conditions. The pathology for KS shows a proliferation of human herpes virus 8-positive spindle cells with slitlike spaces containing red blood cells instead of granulomatous inflammation.
Treatment regimens with topical corticosteroids can exacerbate the infection due to local suppression of cell-mediated immunity.8 In these scenarios, fungal infection is suspected, and systemic antifungals such as ketoconazole; itraconazole; or terbinafine, which has become the mainstay, are prescribed. Resolution of the infection with these medications usually is seen after 4 weeks.2
A diagnosis of MG can be elusive and often may take multiple visits. Clinicians should note that MG could demonstrate repeated false-negative KOH preparations; therefore, these tests should not be relied on as the sole determination of a diagnosis. Although chromoblastomycosis, sporotrichosis, nodular KS, and infection with M marinum may all present as nodular plaques with granulomatous pathology, a follicular pustule may be a clinical clue to MG, as its mimics typically lack folliculocentric neutrophils.
The Diagnosis: Majocchi Granuloma
Majocchi granuloma (MG) is a dermatophytic infection that reveals hyphal elements within the cornified cells of follicles and most commonly is caused by Trichophyton rubrum. However, occasionally other Trichophyton, Trichosporon, and Aspergillus species are involved.1
There typically are 2 forms of MG: (1) the small perifollicular papular form that usually is localized to the dermis and occurs in immunocompetent individuals, and (2) a deep form featuring subcutaneous plaques and nodules that generally occur on the hair-bearing surfaces in immunosuppressed hosts.2 Majocchi granuloma also commonly occurs from the use of potent topical steroids on unsuspected tinea.3
Histopathologically, MG generally presents as granulomatous inflammation with perifollicular neutrophilic infiltration. This polymorphonuclear cell infiltrate was visible clinically as a single pustule overlying the nodular plaque, a clue appreciable only on close inspection. Histopathologic examination revealed segmented branching filaments present within cornified elements of a follicle (Figure). Notably, potassium hydroxide (KOH) preparations are unreliable diagnostic aids in MG, as evidenced by the 2 negative KOH preparations in this case. According to Chou and Hsu,4 because KOH preparation can only detect fungi located in the stratum corneum, the result may be negative for MG due to deeper invasion of the fungi into the dermal follicular component. In fact, KOH preparations of MG may reveal no hyphae in 23.3% of cases.2

The initiating factor in MG is not entirely known but is thought to be physical trauma that either directly or indirectly leads to follicle disruption and passive introduction of the organism into the dermis (eg, traumatic implantation via gardening or other recreational activities).2 Other proposed mechanisms include the presentation of the membrane-associated ATP-binding cassette transporter on the surface of T rubrum.1 Dermatophytes evade the host immune system through a variety of mechanisms: (1) cell wall glycoproteins, (2) release of anti-inflammatory cytokines, and (3) generation of immunosuppressive regulatory T cells.1
Collectively, the clinical and histopathologic findings distinguish MG from other cutaneous conditions. Sporotrichosis, a granulomatous infection caused by Sporothrix schenckii, typically is found in tropical regions of the world and often is associated with floriculture.5 Sporotrichosis initially presents in a subcutaneous papulonodular form, but unlike MG, it later ulcerates and progresses along adjacent lymphatic chains.5 Pathology of sporotrichosis exhibits pseudoepitheliomatous hyperplasia with granulomas, possible foci of suppuration, and yeastlike forms called cigar bodies. Chromoblastomycosis clinically is defined by tumorlike lesions on the skin including verrucous, nodular, or scarlike plaques and typically is associated with traumatic injury and implantation of the microorganism. Histologically, chromoblastomycosis demonstrates pseudoepitheliomatous hyperplasia with granulomas and characteristic darkly pigmented, thick-walled sclerotic cells called Medlar bodies.6Mycobacterium marinum is one cause of nontuberculous mycobacterial skin infections in humans. Clinically, M marinum is associated with improper hygiene techniques and contact with fish tanks and other aqueous environments. Mycobacterium marinum can present histopathologically as early neutrophilic infiltration or late dermal granulomatous inflammation.7 Acid-fast bacilli typically are scant, leaving the diagnosis best secured via polymerase chain reaction assay. Nodular Kaposi sarcoma (KS) can present as a dusky nodular plaque on an acral surface but typically is seen in patients with underlying human immunodeficiency virus/AIDS or other immunosuppressive conditions. The pathology for KS shows a proliferation of human herpes virus 8-positive spindle cells with slitlike spaces containing red blood cells instead of granulomatous inflammation.
Treatment regimens with topical corticosteroids can exacerbate the infection due to local suppression of cell-mediated immunity.8 In these scenarios, fungal infection is suspected, and systemic antifungals such as ketoconazole; itraconazole; or terbinafine, which has become the mainstay, are prescribed. Resolution of the infection with these medications usually is seen after 4 weeks.2
A diagnosis of MG can be elusive and often may take multiple visits. Clinicians should note that MG could demonstrate repeated false-negative KOH preparations; therefore, these tests should not be relied on as the sole determination of a diagnosis. Although chromoblastomycosis, sporotrichosis, nodular KS, and infection with M marinum may all present as nodular plaques with granulomatous pathology, a follicular pustule may be a clinical clue to MG, as its mimics typically lack folliculocentric neutrophils.
- Tirado-Sánchez A, Ponce-Olivera RM, Bonifaz A. Majocchi's granuloma (dermatophytic granuloma): updated therapeutic options. Curr Fungal Infect Rep. 2015;9:204-212.
- Ilkit M, Durdu M, Karakas¸ M. Majocchi's granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50:449-457.
- Schwartz RA, Janniger CK. Majocchi granuloma. Medscape website. https://emedicine.medscape.com/article/1092601-overview. Updated May 14, 2019. Accessed April 13, 2020.
- Chou WY, Hsu CJ. A case report of Majocchi's granuloma associated with combined therapy of topical steroids and adalimumab. Medicine (Baltimore). 2016;95:E2245.
- Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633-654.
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280.
- Slany M, Jezek P, Bodnarova M. Fish tank granuloma caused by Mycobacterium marinum in two aquarists: two case reports. Biomed Res Int. 2013;2013:161329.
- Coondoo A, Phiske M, Verma S, et al. Side-effects of topical steroids: a long overdue revisit. Indian Dermatol Online J. 2014;5:416-425.
- Tirado-Sánchez A, Ponce-Olivera RM, Bonifaz A. Majocchi's granuloma (dermatophytic granuloma): updated therapeutic options. Curr Fungal Infect Rep. 2015;9:204-212.
- Ilkit M, Durdu M, Karakas¸ M. Majocchi's granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50:449-457.
- Schwartz RA, Janniger CK. Majocchi granuloma. Medscape website. https://emedicine.medscape.com/article/1092601-overview. Updated May 14, 2019. Accessed April 13, 2020.
- Chou WY, Hsu CJ. A case report of Majocchi's granuloma associated with combined therapy of topical steroids and adalimumab. Medicine (Baltimore). 2016;95:E2245.
- Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633-654.
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280.
- Slany M, Jezek P, Bodnarova M. Fish tank granuloma caused by Mycobacterium marinum in two aquarists: two case reports. Biomed Res Int. 2013;2013:161329.
- Coondoo A, Phiske M, Verma S, et al. Side-effects of topical steroids: a long overdue revisit. Indian Dermatol Online J. 2014;5:416-425.
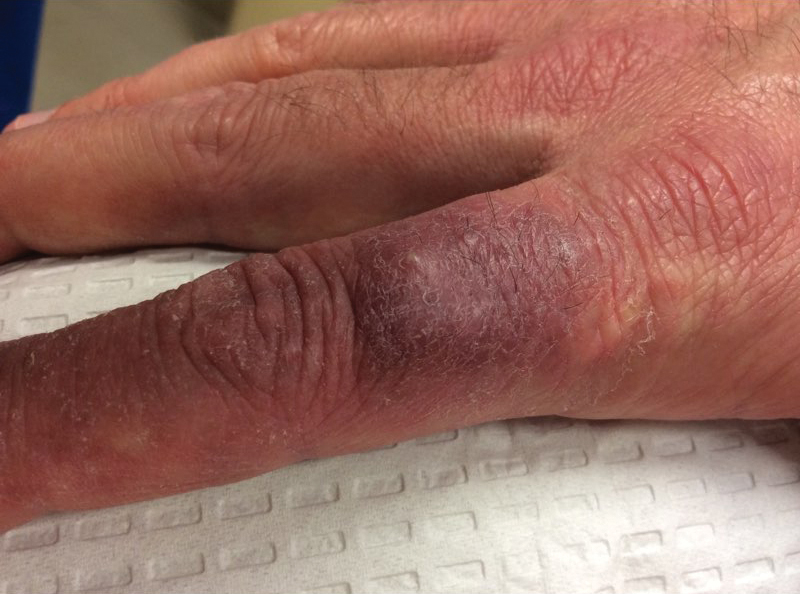
A 38-year-old man presented with a persistent pruritic nodular plaque on the proximal right index finger of 4 months' duration. He reported pruning roses in the garden but denied any trauma. The patient previously had been treated by another clinician with fluocinonide cream 0.05%, clobetasol cream 0.05%, intramuscular methylprednisolone 40 mg, and oral doxycycline hyclate 100 mg with no improvement. Two potassium hydroxide preparations were performed as well as a bacterial culture and sensitivity, with all results returning as negative. Physical examination revealed a 2-cm pink to purple, scaly, nodular plaque on the right index finger. A punch biopsy was obtained for histopathology with hematoxylin and eosin stain.
Primary Cutaneous Mycobacterium avium Complex Infection Following Squamous Cell Carcinoma Excision
Case Report
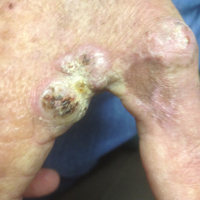
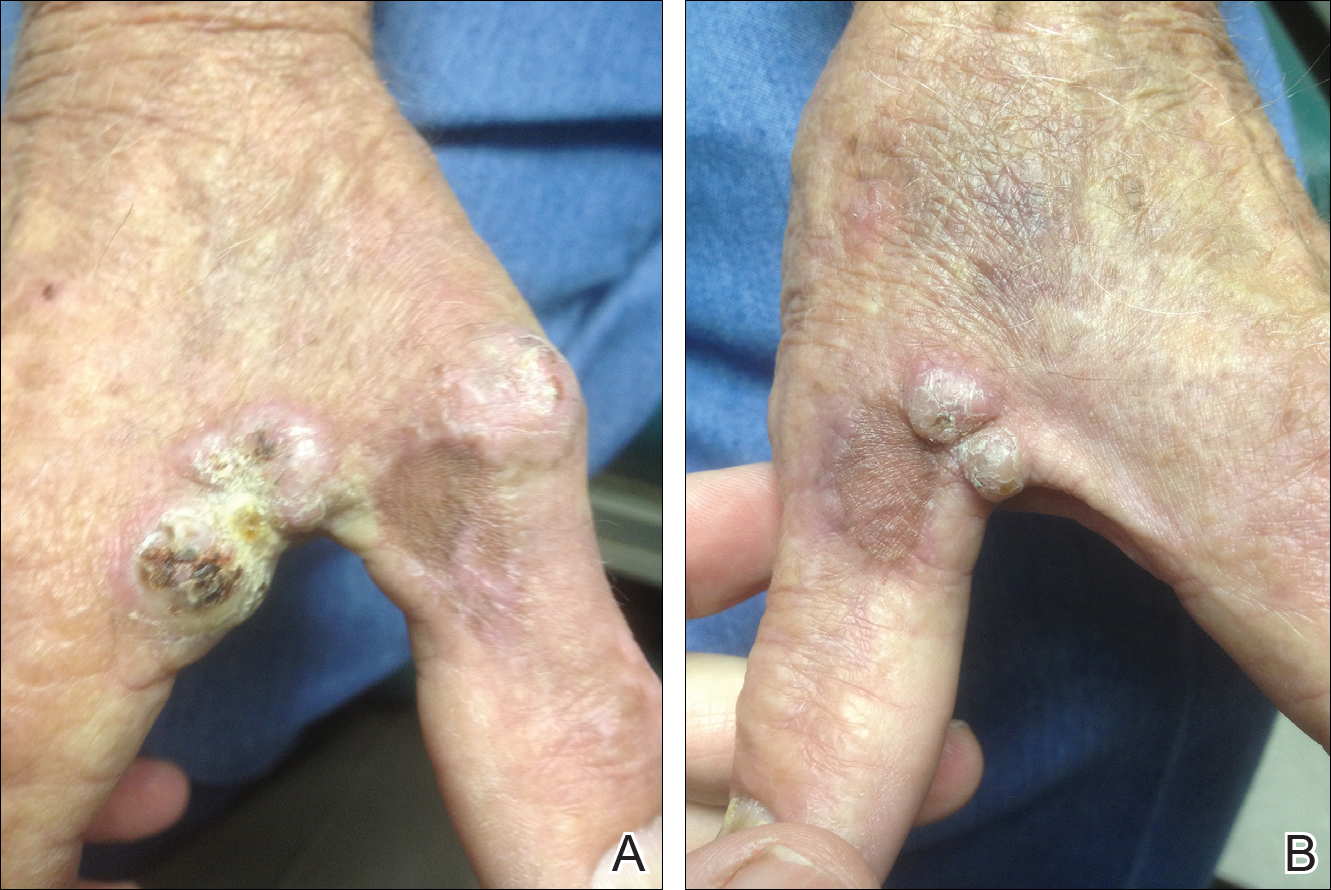
A 78-year-old man presented for evaluation of 4 painful keratotic nodules that had appeared on the dorsal aspect of the right thumb, the first web space of the right hand, and the first web space of the left hand. The nodules developed in pericicatricial skin following Mohs micrographic surgery to the affected areas for treatment of invasive squamous cell carcinomas (SCCs) 2 months prior. The patient had worked in lawn maintenance for decades and continued to garden on an avocational basis. He denied exposure to angling or aquariums.
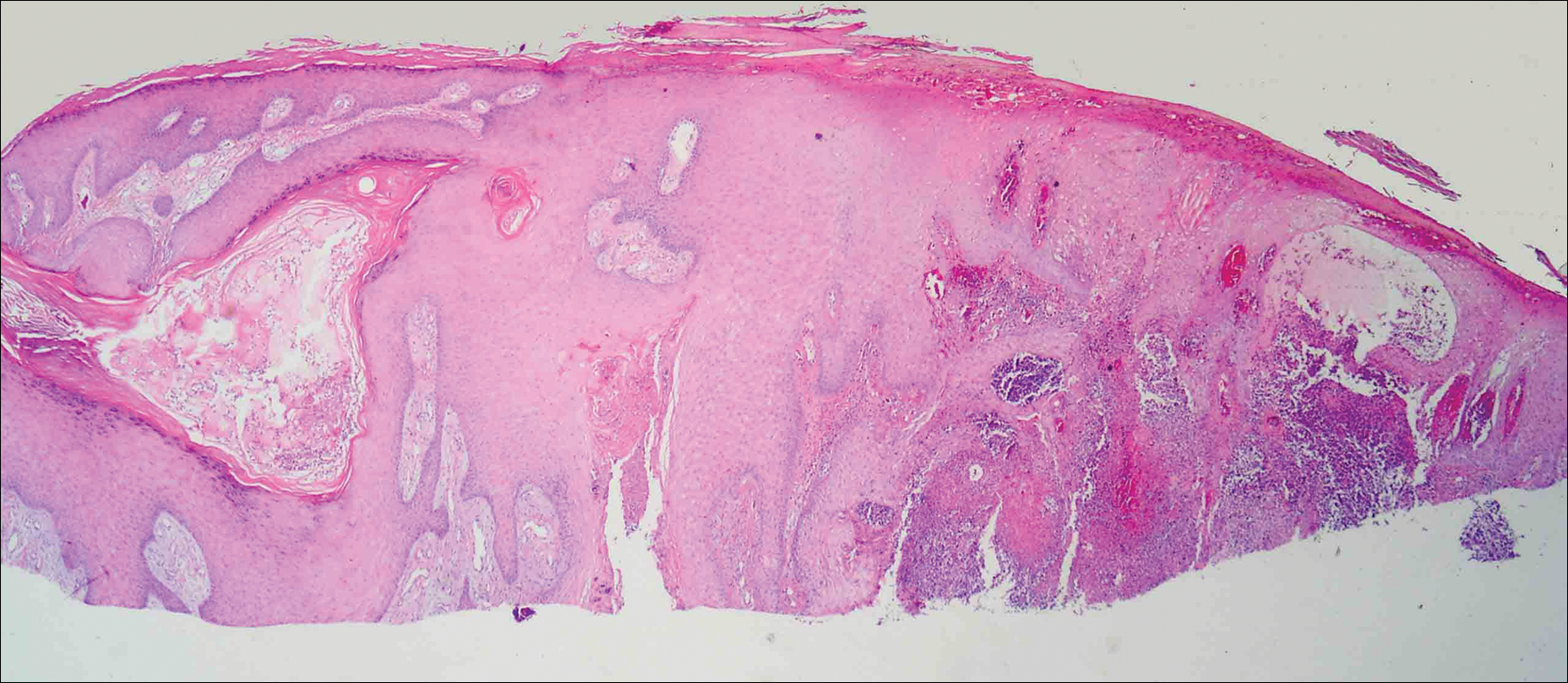
On physical examination the lesions appeared as firm, dusky-violaceous, crusted nodules (Figure 1). Brown patches of hyperpigmentation or characteristic cornlike elevations of the palm were not present to implicate arsenic exposure. Extensive sun damage to the face, neck, forearms, and dorsal aspect of the hands was noted. Epitrochlear lymphadenopathy or lymphangitic streaking were not appreciated. Routine hematologic parameters including leukocyte count were normal, except for chronic thrombocytopenia. Computerized tomography of the abdomen demonstrated no hepatosplenomegaly or enlarged lymph nodes. Hematoxylin and eosin staining of biopsy specimens from the right thumb showed irregular squamous epithelial hyperplasia with an impetiginized scale crust and pustular tissue reaction, including suppurative abscess formation in the dermis (Figure 2). Initial acid-fast staining performed on the biopsy from the right thumb was negative for microorganisms. Given the concerning histologic features indicating infection, a tissue culture was performed. Subsequent growth on Lowenstein-Jensen culture medium confirmed infection with Mycobacterium avium complex (MAC). The patient was started on clarithromycin 500 mg twice daily in accordance with laboratory susceptibilities, and the cutaneous nodules improved. Unfortunately, the patient died 6 months later secondary to cardiac arrest.
Comment
The genus Mycobacterium comprises more than 130 described bacteria, including the precipitants of tuberculosis and leprosy. Mycobacterium avium complex--an umbrella term for M avium, Mycobacterium intracellulare, and other close relatives--is a member of the genus that maintains a low pathogenicity for healthy individuals.1,2 Nonetheless, MAC accounts for more than 70% of cases of nontuberculous mycobacterial disease in the United States.3Mycobacterium avium complex typically acts as a respiratory pathogen, but infection may manifest with lymphadenitis, osteomyelitis, hepatosplenomegaly, or skin involvement. Disseminated MAC infection can occur in patients with defective immune systems, including those with conditions such as AIDS or hairy cell leukemia and those undergoing immunosuppressive therapy.1,4 Although uncommon, cutaneous infection with MAC occurs via 3 possible mechanisms: (1) primary inoculation, (2) lymphogenous extension, or (3) hematologic dissemination.4 According to a PubMed search of articles indexed for MEDLINE using the terms primary cutaneous Mycobacterium avium complex and MAC skin infection, only 11 known cases of primary cutaneous MAC infection have been reported in the English-language literature,4-14 the most recent being a report by Landriscina et al.11
A Runyon group III bacillus, MAC is a slow-growing nonchromogen that is ubiquitous in nature.15 It has been isolated from soil, water, house dust, vegetables, eggs, and milk. According to Reed et al,3 occupational exposure to soil is an independent risk factor for MAC infection, with individuals reporting more than 6 years of cumulative participation in lawn and landscaping services, farming, or other occupations involving substantial exposure to dirt or dust most likely to be MAC-positive. Cutaneous MAC infection may be associated with water exposure, as Sugita et al2 described one familial outbreak of cutaneous MAC infection linked to use of a circulating, constantly heated bathwater system. With respect to US geography, individuals living in rural areas of the South seem most prone to MAC infection.3
Primary cutaneous infection with MAC occurs after a breach in the skin surface, though this fact may not be elicited by history. Modes of entry include minor abrasions after falling,1 small wounds,2 traumatic inoculation,15 and intramuscular injection.16 Clinically, cutaneous lesions of MAC are protean. In the literature, clinical presentation is described as a polymorphous appearance with scaling plaques, verrucous nodules, crusted ulcers, inflammatory nodules, dermatitis, panniculitis, draining sinuses, ecthymatous lesions, sporotrichoid growth patterns, or rosacealike papulopustules.1,15,17 Lesions may affect the arms and legs, trunk, buttocks, and face.18
The differential diagnosis of MAC infection includes lupus vulgaris, Mycobacterium marinum infection (also known as swimming pool granuloma), sporotrichosis, nocardiosis, sarcoidosis, neutrophilic dermatosis, pyoderma gangrenosum, and cutaneous blastomycosis. Given its rarity and variability, diagnosis of MAC infection requires a high index of suspicion. Cutaneous MAC infection should be considered if a nodule, plaque, or ulcer fails to respond to conventional treatment, especially in patients with a history of environmental exposure and possible injury to the skin.
We report a rare case of primary cutaneous MAC infection arising in SCC excision sites in a patient without known immune deficiency. This presentation may have occurred for several reasons. First, the surgical excision sites coupled with the substantial occupational and recreational exposure to soil experienced by our patient may have served as portals for infection. Although SCCs are common on the hands, Mohs micrographic surgery is not always performed for excision; in our patient's case, this approach allowed for maximum tissue conservation and preserved manual function given the number and location of the lesions. Second, despite an overtly intact immune system, our patient may have harbored an occult immune deficiency, predisposing him to dermatologic infection with a microorganism of low intrinsic virulence and recurrent malignant neoplasms. This presentation may have been the first clinical indication of subtle immune compromise. For example, inadequate proinflammatory cytokines may contribute to both mycobacterial and malignant disease. A potential risk of inhibition of tumor necrosis factor α is the unmasking of tuberculosis or lymphoma.19,20 Likewise, IFN-γ is vital in suppressing mycobacteria and malignancy. Yonekura et al21 found that IFN-γ induces apoptosis in oral SCC lines. It follows that a paucity of IFN-γ could allow neoplastic growth. Normal function of IFN-γ prompts microbicidal activity in macrophages and stimulates granuloma formation, both of which combat mycobacterial infection.19 A final postulation is that a simmering cutaneous MAC infection precipitated neoplastic degeneration into SCC, much the same way that the human papillomavirus has been correlated in the carcinogenesis of cervical cancer. As an intracellular microbe, MAC could cause the genetic machinery of skin cells to go awry. Kullavanijaya et al18 described a patient with cutaneous MAC in association with cervical cancer.
Conclusion
This association of primary cutaneous MAC infection and cutaneous malignancy in a reportedly immunocompetent patient is rare. Cancer patients, as noted by Feld et al,22 are 3 times more likely to develop infections with mycobacteria, with SCC, lymphoma, and leukemia being most commonly indicated. A specific immune deficit in the IFN-γ receptor is known to confer a selective predisposition to mycobacterial infection.23,24 Toyoda et al25 outlined the case of a pediatric patient with IFN-γ receptor 2 deficiency who presented with disseminated MAC infection and later succumbed to multiple SCCs of the hands and face. The authors' assertion was that inherited disorders of IFN-γ-mediated immunity may be associated with SCCs.25 Unfortunately, our patient died before more specific immunological testing could be conducted. This case highlights the remarkable singularity of primary cutaneous MAC infection in association with multiple SCCs with seemingly intact immune status and offers some intriguing hypotheses regarding its occurrence.
- Hong BK, Kumar C, Marottoli RA. "MAC" attack. Am J Med. 2009;122:1096-1098.
- Sugita Y, Ishii N, Katsuno M, et al. Familial cluster of cutaneous Mycobacterium avium infection resulting from use of a circulating, constantly heated bath water system. Br J Dermatol. 2000;142:789-793.
- Reed C, von Reyn CF, Chamblee S, et al. Environmental risk factors for infection with Mycobacterium avium complex [published online May 4, 2006]. Am J Epidemiol. 2006;164:32-40.
- Ichiki Y, Hirose M, Akiyama T, et al. Skin infection caused by Mycobacterium avium. Br J Dermatol. 1997;136:260-263.
- Aboutalebi A, Shen A, Katta R, et al. Primary cutaneous infection by Mycobacterium avium: a case report and literature review. Cutis. 2012;89:175-179.
- Nassar D, Ortonne N, Grégoire-Krikorian B, et al. Chronic granulomatous Mycobacterium avium skin pseudotumor. Lancet Infect Dis. 2009;9:136.
- Escalonilla P, Esteban J, Soriano ML, et al. Cutaneous manifestations of infection by nontuberculous mycobacteria. Clin Exp Dermatol. 1998;23:214-221.
- Lugo-Janer G, Cruz A, Sanchez JL. Disseminated cutaneous infection caused by Mycobacterium avium complex. Arch Dermatol. 1990;126:1108-1110.
- Schmidt JD, Yeager H Jr, Smith EB, et al. Cutaneous infection due to a Runyon group 3 atypical Mycobacterium. Am Rev Respir Dis. 1972;106:469-471.
- Carlos C, Tang YW, Adler DJ, et al. Mycobacterial infection identified with broad-range PCR amplification and suspension array identification. J Clin Pathol. 2012;39:795-797.
- Landriscina A, Musaev T, Amin B, et al. A surprising case of Mycobacterium avium complex skin infection in an immunocompetent patient. J Drugs Dermatol. 2014;13:1491-1493.
- Zhou L, Wang HS, Feng SY, et al. Cutaneous Mycobacterium intracellulare infection in an immunocompetent person. Acta Derm Venereol. 2013;93:711-714.
- Cox S, Strausbaugh L. Chronic cutaneous infection caused by Mycobacterium intracellulare. Arch Dermatol. 1981;117:794-796.
- Sachs M, Fraimow HF, Staros EB, et al. Mycobacterium intracellulare soft tissue infection. J Am Acad Dermatol. 1992;27:1019-1021.
- Jogi R, Tyring SK. Therapy of nontuberculous mycobacterial infections. Dermatol Ther. 2004;17:491-498.
- Meadows JR, Carter R, Katner HP. Cutaneous Mycobacterium avium complex infection at an intramuscular injection site in a patient with AIDS. Clin Infect Dis. 1997;24:1273-1274.
- Kayal JD, McCall CO. Sporotrichoid cutaneous Mycobacterium avium complex infection. J Am Acad Dermatol. 2002;47(5 suppl):S249-S250.
- Kullavanijaya P, Sirimachan S, Surarak S. Primary cutaneous infection with Mycobacterium avium intracellulare complex resembling lupus vulgaris. Br J Dermatol. 1997;136:264-266.
- Netea MG, Kullberg BJ, Van der Meer JW. Proinflammatory cytokines in the treatment of bacterial and fungal infections. BioDrugs. 2004;18:9-22.
- Dommasch E, Gelfand JM. Is there truly a risk of lymphoma from biologic therapies? Dermatol Ther. 2009;22:418-430.
- Yonekura N, Yokota S, Yonekura K, et al. Interferon-γ downregulates Hsp27 expression and suppresses the negative regulation of cell death in oral squamous cell carcinoma lines. Cell Death Differ. 2003;10:313-322.
- Feld R, Bodey GP, Groschel D. Mycobacteriosis in patients with malignant disease. Arch Intern Med. 1976;136:67-70.
- Dorman S, Picard C, Lammas D, et al. Clinical features of dominant and recessive interferon γ receptor 1 deficiencies. Lancet. 2004;364:2113-2121.
- Storgaard M, Varming K, Herlin T, et al. Novel mutation in the interferon-γ receptor gene and susceptibility to mycobacterial infections. Scand J Immunol. 2006;64:137-139.
- Toyoda H, Ido M, Nakanishi K, et al. Multiple cutaneous squamous cell carcinomas in a patient with interferon γ receptor 2 (IFNγR2) deficiency [published online June 18, 2010]. J Med Genet. 2010;47:631-634.
Case Report
A 78-year-old man presented for evaluation of 4 painful keratotic nodules that had appeared on the dorsal aspect of the right thumb, the first web space of the right hand, and the first web space of the left hand. The nodules developed in pericicatricial skin following Mohs micrographic surgery to the affected areas for treatment of invasive squamous cell carcinomas (SCCs) 2 months prior. The patient had worked in lawn maintenance for decades and continued to garden on an avocational basis. He denied exposure to angling or aquariums.
On physical examination the lesions appeared as firm, dusky-violaceous, crusted nodules (Figure 1). Brown patches of hyperpigmentation or characteristic cornlike elevations of the palm were not present to implicate arsenic exposure. Extensive sun damage to the face, neck, forearms, and dorsal aspect of the hands was noted. Epitrochlear lymphadenopathy or lymphangitic streaking were not appreciated. Routine hematologic parameters including leukocyte count were normal, except for chronic thrombocytopenia. Computerized tomography of the abdomen demonstrated no hepatosplenomegaly or enlarged lymph nodes. Hematoxylin and eosin staining of biopsy specimens from the right thumb showed irregular squamous epithelial hyperplasia with an impetiginized scale crust and pustular tissue reaction, including suppurative abscess formation in the dermis (Figure 2). Initial acid-fast staining performed on the biopsy from the right thumb was negative for microorganisms. Given the concerning histologic features indicating infection, a tissue culture was performed. Subsequent growth on Lowenstein-Jensen culture medium confirmed infection with Mycobacterium avium complex (MAC). The patient was started on clarithromycin 500 mg twice daily in accordance with laboratory susceptibilities, and the cutaneous nodules improved. Unfortunately, the patient died 6 months later secondary to cardiac arrest.


Comment
The genus Mycobacterium comprises more than 130 described bacteria, including the precipitants of tuberculosis and leprosy. Mycobacterium avium complex--an umbrella term for M avium, Mycobacterium intracellulare, and other close relatives--is a member of the genus that maintains a low pathogenicity for healthy individuals.1,2 Nonetheless, MAC accounts for more than 70% of cases of nontuberculous mycobacterial disease in the United States.3Mycobacterium avium complex typically acts as a respiratory pathogen, but infection may manifest with lymphadenitis, osteomyelitis, hepatosplenomegaly, or skin involvement. Disseminated MAC infection can occur in patients with defective immune systems, including those with conditions such as AIDS or hairy cell leukemia and those undergoing immunosuppressive therapy.1,4 Although uncommon, cutaneous infection with MAC occurs via 3 possible mechanisms: (1) primary inoculation, (2) lymphogenous extension, or (3) hematologic dissemination.4 According to a PubMed search of articles indexed for MEDLINE using the terms primary cutaneous Mycobacterium avium complex and MAC skin infection, only 11 known cases of primary cutaneous MAC infection have been reported in the English-language literature,4-14 the most recent being a report by Landriscina et al.11
A Runyon group III bacillus, MAC is a slow-growing nonchromogen that is ubiquitous in nature.15 It has been isolated from soil, water, house dust, vegetables, eggs, and milk. According to Reed et al,3 occupational exposure to soil is an independent risk factor for MAC infection, with individuals reporting more than 6 years of cumulative participation in lawn and landscaping services, farming, or other occupations involving substantial exposure to dirt or dust most likely to be MAC-positive. Cutaneous MAC infection may be associated with water exposure, as Sugita et al2 described one familial outbreak of cutaneous MAC infection linked to use of a circulating, constantly heated bathwater system. With respect to US geography, individuals living in rural areas of the South seem most prone to MAC infection.3
Primary cutaneous infection with MAC occurs after a breach in the skin surface, though this fact may not be elicited by history. Modes of entry include minor abrasions after falling,1 small wounds,2 traumatic inoculation,15 and intramuscular injection.16 Clinically, cutaneous lesions of MAC are protean. In the literature, clinical presentation is described as a polymorphous appearance with scaling plaques, verrucous nodules, crusted ulcers, inflammatory nodules, dermatitis, panniculitis, draining sinuses, ecthymatous lesions, sporotrichoid growth patterns, or rosacealike papulopustules.1,15,17 Lesions may affect the arms and legs, trunk, buttocks, and face.18
The differential diagnosis of MAC infection includes lupus vulgaris, Mycobacterium marinum infection (also known as swimming pool granuloma), sporotrichosis, nocardiosis, sarcoidosis, neutrophilic dermatosis, pyoderma gangrenosum, and cutaneous blastomycosis. Given its rarity and variability, diagnosis of MAC infection requires a high index of suspicion. Cutaneous MAC infection should be considered if a nodule, plaque, or ulcer fails to respond to conventional treatment, especially in patients with a history of environmental exposure and possible injury to the skin.
We report a rare case of primary cutaneous MAC infection arising in SCC excision sites in a patient without known immune deficiency. This presentation may have occurred for several reasons. First, the surgical excision sites coupled with the substantial occupational and recreational exposure to soil experienced by our patient may have served as portals for infection. Although SCCs are common on the hands, Mohs micrographic surgery is not always performed for excision; in our patient's case, this approach allowed for maximum tissue conservation and preserved manual function given the number and location of the lesions. Second, despite an overtly intact immune system, our patient may have harbored an occult immune deficiency, predisposing him to dermatologic infection with a microorganism of low intrinsic virulence and recurrent malignant neoplasms. This presentation may have been the first clinical indication of subtle immune compromise. For example, inadequate proinflammatory cytokines may contribute to both mycobacterial and malignant disease. A potential risk of inhibition of tumor necrosis factor α is the unmasking of tuberculosis or lymphoma.19,20 Likewise, IFN-γ is vital in suppressing mycobacteria and malignancy. Yonekura et al21 found that IFN-γ induces apoptosis in oral SCC lines. It follows that a paucity of IFN-γ could allow neoplastic growth. Normal function of IFN-γ prompts microbicidal activity in macrophages and stimulates granuloma formation, both of which combat mycobacterial infection.19 A final postulation is that a simmering cutaneous MAC infection precipitated neoplastic degeneration into SCC, much the same way that the human papillomavirus has been correlated in the carcinogenesis of cervical cancer. As an intracellular microbe, MAC could cause the genetic machinery of skin cells to go awry. Kullavanijaya et al18 described a patient with cutaneous MAC in association with cervical cancer.
Conclusion
This association of primary cutaneous MAC infection and cutaneous malignancy in a reportedly immunocompetent patient is rare. Cancer patients, as noted by Feld et al,22 are 3 times more likely to develop infections with mycobacteria, with SCC, lymphoma, and leukemia being most commonly indicated. A specific immune deficit in the IFN-γ receptor is known to confer a selective predisposition to mycobacterial infection.23,24 Toyoda et al25 outlined the case of a pediatric patient with IFN-γ receptor 2 deficiency who presented with disseminated MAC infection and later succumbed to multiple SCCs of the hands and face. The authors' assertion was that inherited disorders of IFN-γ-mediated immunity may be associated with SCCs.25 Unfortunately, our patient died before more specific immunological testing could be conducted. This case highlights the remarkable singularity of primary cutaneous MAC infection in association with multiple SCCs with seemingly intact immune status and offers some intriguing hypotheses regarding its occurrence.
Case Report
A 78-year-old man presented for evaluation of 4 painful keratotic nodules that had appeared on the dorsal aspect of the right thumb, the first web space of the right hand, and the first web space of the left hand. The nodules developed in pericicatricial skin following Mohs micrographic surgery to the affected areas for treatment of invasive squamous cell carcinomas (SCCs) 2 months prior. The patient had worked in lawn maintenance for decades and continued to garden on an avocational basis. He denied exposure to angling or aquariums.
On physical examination the lesions appeared as firm, dusky-violaceous, crusted nodules (Figure 1). Brown patches of hyperpigmentation or characteristic cornlike elevations of the palm were not present to implicate arsenic exposure. Extensive sun damage to the face, neck, forearms, and dorsal aspect of the hands was noted. Epitrochlear lymphadenopathy or lymphangitic streaking were not appreciated. Routine hematologic parameters including leukocyte count were normal, except for chronic thrombocytopenia. Computerized tomography of the abdomen demonstrated no hepatosplenomegaly or enlarged lymph nodes. Hematoxylin and eosin staining of biopsy specimens from the right thumb showed irregular squamous epithelial hyperplasia with an impetiginized scale crust and pustular tissue reaction, including suppurative abscess formation in the dermis (Figure 2). Initial acid-fast staining performed on the biopsy from the right thumb was negative for microorganisms. Given the concerning histologic features indicating infection, a tissue culture was performed. Subsequent growth on Lowenstein-Jensen culture medium confirmed infection with Mycobacterium avium complex (MAC). The patient was started on clarithromycin 500 mg twice daily in accordance with laboratory susceptibilities, and the cutaneous nodules improved. Unfortunately, the patient died 6 months later secondary to cardiac arrest.


Comment
The genus Mycobacterium comprises more than 130 described bacteria, including the precipitants of tuberculosis and leprosy. Mycobacterium avium complex--an umbrella term for M avium, Mycobacterium intracellulare, and other close relatives--is a member of the genus that maintains a low pathogenicity for healthy individuals.1,2 Nonetheless, MAC accounts for more than 70% of cases of nontuberculous mycobacterial disease in the United States.3Mycobacterium avium complex typically acts as a respiratory pathogen, but infection may manifest with lymphadenitis, osteomyelitis, hepatosplenomegaly, or skin involvement. Disseminated MAC infection can occur in patients with defective immune systems, including those with conditions such as AIDS or hairy cell leukemia and those undergoing immunosuppressive therapy.1,4 Although uncommon, cutaneous infection with MAC occurs via 3 possible mechanisms: (1) primary inoculation, (2) lymphogenous extension, or (3) hematologic dissemination.4 According to a PubMed search of articles indexed for MEDLINE using the terms primary cutaneous Mycobacterium avium complex and MAC skin infection, only 11 known cases of primary cutaneous MAC infection have been reported in the English-language literature,4-14 the most recent being a report by Landriscina et al.11
A Runyon group III bacillus, MAC is a slow-growing nonchromogen that is ubiquitous in nature.15 It has been isolated from soil, water, house dust, vegetables, eggs, and milk. According to Reed et al,3 occupational exposure to soil is an independent risk factor for MAC infection, with individuals reporting more than 6 years of cumulative participation in lawn and landscaping services, farming, or other occupations involving substantial exposure to dirt or dust most likely to be MAC-positive. Cutaneous MAC infection may be associated with water exposure, as Sugita et al2 described one familial outbreak of cutaneous MAC infection linked to use of a circulating, constantly heated bathwater system. With respect to US geography, individuals living in rural areas of the South seem most prone to MAC infection.3
Primary cutaneous infection with MAC occurs after a breach in the skin surface, though this fact may not be elicited by history. Modes of entry include minor abrasions after falling,1 small wounds,2 traumatic inoculation,15 and intramuscular injection.16 Clinically, cutaneous lesions of MAC are protean. In the literature, clinical presentation is described as a polymorphous appearance with scaling plaques, verrucous nodules, crusted ulcers, inflammatory nodules, dermatitis, panniculitis, draining sinuses, ecthymatous lesions, sporotrichoid growth patterns, or rosacealike papulopustules.1,15,17 Lesions may affect the arms and legs, trunk, buttocks, and face.18
The differential diagnosis of MAC infection includes lupus vulgaris, Mycobacterium marinum infection (also known as swimming pool granuloma), sporotrichosis, nocardiosis, sarcoidosis, neutrophilic dermatosis, pyoderma gangrenosum, and cutaneous blastomycosis. Given its rarity and variability, diagnosis of MAC infection requires a high index of suspicion. Cutaneous MAC infection should be considered if a nodule, plaque, or ulcer fails to respond to conventional treatment, especially in patients with a history of environmental exposure and possible injury to the skin.
We report a rare case of primary cutaneous MAC infection arising in SCC excision sites in a patient without known immune deficiency. This presentation may have occurred for several reasons. First, the surgical excision sites coupled with the substantial occupational and recreational exposure to soil experienced by our patient may have served as portals for infection. Although SCCs are common on the hands, Mohs micrographic surgery is not always performed for excision; in our patient's case, this approach allowed for maximum tissue conservation and preserved manual function given the number and location of the lesions. Second, despite an overtly intact immune system, our patient may have harbored an occult immune deficiency, predisposing him to dermatologic infection with a microorganism of low intrinsic virulence and recurrent malignant neoplasms. This presentation may have been the first clinical indication of subtle immune compromise. For example, inadequate proinflammatory cytokines may contribute to both mycobacterial and malignant disease. A potential risk of inhibition of tumor necrosis factor α is the unmasking of tuberculosis or lymphoma.19,20 Likewise, IFN-γ is vital in suppressing mycobacteria and malignancy. Yonekura et al21 found that IFN-γ induces apoptosis in oral SCC lines. It follows that a paucity of IFN-γ could allow neoplastic growth. Normal function of IFN-γ prompts microbicidal activity in macrophages and stimulates granuloma formation, both of which combat mycobacterial infection.19 A final postulation is that a simmering cutaneous MAC infection precipitated neoplastic degeneration into SCC, much the same way that the human papillomavirus has been correlated in the carcinogenesis of cervical cancer. As an intracellular microbe, MAC could cause the genetic machinery of skin cells to go awry. Kullavanijaya et al18 described a patient with cutaneous MAC in association with cervical cancer.
Conclusion
This association of primary cutaneous MAC infection and cutaneous malignancy in a reportedly immunocompetent patient is rare. Cancer patients, as noted by Feld et al,22 are 3 times more likely to develop infections with mycobacteria, with SCC, lymphoma, and leukemia being most commonly indicated. A specific immune deficit in the IFN-γ receptor is known to confer a selective predisposition to mycobacterial infection.23,24 Toyoda et al25 outlined the case of a pediatric patient with IFN-γ receptor 2 deficiency who presented with disseminated MAC infection and later succumbed to multiple SCCs of the hands and face. The authors' assertion was that inherited disorders of IFN-γ-mediated immunity may be associated with SCCs.25 Unfortunately, our patient died before more specific immunological testing could be conducted. This case highlights the remarkable singularity of primary cutaneous MAC infection in association with multiple SCCs with seemingly intact immune status and offers some intriguing hypotheses regarding its occurrence.
- Hong BK, Kumar C, Marottoli RA. "MAC" attack. Am J Med. 2009;122:1096-1098.
- Sugita Y, Ishii N, Katsuno M, et al. Familial cluster of cutaneous Mycobacterium avium infection resulting from use of a circulating, constantly heated bath water system. Br J Dermatol. 2000;142:789-793.
- Reed C, von Reyn CF, Chamblee S, et al. Environmental risk factors for infection with Mycobacterium avium complex [published online May 4, 2006]. Am J Epidemiol. 2006;164:32-40.
- Ichiki Y, Hirose M, Akiyama T, et al. Skin infection caused by Mycobacterium avium. Br J Dermatol. 1997;136:260-263.
- Aboutalebi A, Shen A, Katta R, et al. Primary cutaneous infection by Mycobacterium avium: a case report and literature review. Cutis. 2012;89:175-179.
- Nassar D, Ortonne N, Grégoire-Krikorian B, et al. Chronic granulomatous Mycobacterium avium skin pseudotumor. Lancet Infect Dis. 2009;9:136.
- Escalonilla P, Esteban J, Soriano ML, et al. Cutaneous manifestations of infection by nontuberculous mycobacteria. Clin Exp Dermatol. 1998;23:214-221.
- Lugo-Janer G, Cruz A, Sanchez JL. Disseminated cutaneous infection caused by Mycobacterium avium complex. Arch Dermatol. 1990;126:1108-1110.
- Schmidt JD, Yeager H Jr, Smith EB, et al. Cutaneous infection due to a Runyon group 3 atypical Mycobacterium. Am Rev Respir Dis. 1972;106:469-471.
- Carlos C, Tang YW, Adler DJ, et al. Mycobacterial infection identified with broad-range PCR amplification and suspension array identification. J Clin Pathol. 2012;39:795-797.
- Landriscina A, Musaev T, Amin B, et al. A surprising case of Mycobacterium avium complex skin infection in an immunocompetent patient. J Drugs Dermatol. 2014;13:1491-1493.
- Zhou L, Wang HS, Feng SY, et al. Cutaneous Mycobacterium intracellulare infection in an immunocompetent person. Acta Derm Venereol. 2013;93:711-714.
- Cox S, Strausbaugh L. Chronic cutaneous infection caused by Mycobacterium intracellulare. Arch Dermatol. 1981;117:794-796.
- Sachs M, Fraimow HF, Staros EB, et al. Mycobacterium intracellulare soft tissue infection. J Am Acad Dermatol. 1992;27:1019-1021.
- Jogi R, Tyring SK. Therapy of nontuberculous mycobacterial infections. Dermatol Ther. 2004;17:491-498.
- Meadows JR, Carter R, Katner HP. Cutaneous Mycobacterium avium complex infection at an intramuscular injection site in a patient with AIDS. Clin Infect Dis. 1997;24:1273-1274.
- Kayal JD, McCall CO. Sporotrichoid cutaneous Mycobacterium avium complex infection. J Am Acad Dermatol. 2002;47(5 suppl):S249-S250.
- Kullavanijaya P, Sirimachan S, Surarak S. Primary cutaneous infection with Mycobacterium avium intracellulare complex resembling lupus vulgaris. Br J Dermatol. 1997;136:264-266.
- Netea MG, Kullberg BJ, Van der Meer JW. Proinflammatory cytokines in the treatment of bacterial and fungal infections. BioDrugs. 2004;18:9-22.
- Dommasch E, Gelfand JM. Is there truly a risk of lymphoma from biologic therapies? Dermatol Ther. 2009;22:418-430.
- Yonekura N, Yokota S, Yonekura K, et al. Interferon-γ downregulates Hsp27 expression and suppresses the negative regulation of cell death in oral squamous cell carcinoma lines. Cell Death Differ. 2003;10:313-322.
- Feld R, Bodey GP, Groschel D. Mycobacteriosis in patients with malignant disease. Arch Intern Med. 1976;136:67-70.
- Dorman S, Picard C, Lammas D, et al. Clinical features of dominant and recessive interferon γ receptor 1 deficiencies. Lancet. 2004;364:2113-2121.
- Storgaard M, Varming K, Herlin T, et al. Novel mutation in the interferon-γ receptor gene and susceptibility to mycobacterial infections. Scand J Immunol. 2006;64:137-139.
- Toyoda H, Ido M, Nakanishi K, et al. Multiple cutaneous squamous cell carcinomas in a patient with interferon γ receptor 2 (IFNγR2) deficiency [published online June 18, 2010]. J Med Genet. 2010;47:631-634.
- Hong BK, Kumar C, Marottoli RA. "MAC" attack. Am J Med. 2009;122:1096-1098.
- Sugita Y, Ishii N, Katsuno M, et al. Familial cluster of cutaneous Mycobacterium avium infection resulting from use of a circulating, constantly heated bath water system. Br J Dermatol. 2000;142:789-793.
- Reed C, von Reyn CF, Chamblee S, et al. Environmental risk factors for infection with Mycobacterium avium complex [published online May 4, 2006]. Am J Epidemiol. 2006;164:32-40.
- Ichiki Y, Hirose M, Akiyama T, et al. Skin infection caused by Mycobacterium avium. Br J Dermatol. 1997;136:260-263.
- Aboutalebi A, Shen A, Katta R, et al. Primary cutaneous infection by Mycobacterium avium: a case report and literature review. Cutis. 2012;89:175-179.
- Nassar D, Ortonne N, Grégoire-Krikorian B, et al. Chronic granulomatous Mycobacterium avium skin pseudotumor. Lancet Infect Dis. 2009;9:136.
- Escalonilla P, Esteban J, Soriano ML, et al. Cutaneous manifestations of infection by nontuberculous mycobacteria. Clin Exp Dermatol. 1998;23:214-221.
- Lugo-Janer G, Cruz A, Sanchez JL. Disseminated cutaneous infection caused by Mycobacterium avium complex. Arch Dermatol. 1990;126:1108-1110.
- Schmidt JD, Yeager H Jr, Smith EB, et al. Cutaneous infection due to a Runyon group 3 atypical Mycobacterium. Am Rev Respir Dis. 1972;106:469-471.
- Carlos C, Tang YW, Adler DJ, et al. Mycobacterial infection identified with broad-range PCR amplification and suspension array identification. J Clin Pathol. 2012;39:795-797.
- Landriscina A, Musaev T, Amin B, et al. A surprising case of Mycobacterium avium complex skin infection in an immunocompetent patient. J Drugs Dermatol. 2014;13:1491-1493.
- Zhou L, Wang HS, Feng SY, et al. Cutaneous Mycobacterium intracellulare infection in an immunocompetent person. Acta Derm Venereol. 2013;93:711-714.
- Cox S, Strausbaugh L. Chronic cutaneous infection caused by Mycobacterium intracellulare. Arch Dermatol. 1981;117:794-796.
- Sachs M, Fraimow HF, Staros EB, et al. Mycobacterium intracellulare soft tissue infection. J Am Acad Dermatol. 1992;27:1019-1021.
- Jogi R, Tyring SK. Therapy of nontuberculous mycobacterial infections. Dermatol Ther. 2004;17:491-498.
- Meadows JR, Carter R, Katner HP. Cutaneous Mycobacterium avium complex infection at an intramuscular injection site in a patient with AIDS. Clin Infect Dis. 1997;24:1273-1274.
- Kayal JD, McCall CO. Sporotrichoid cutaneous Mycobacterium avium complex infection. J Am Acad Dermatol. 2002;47(5 suppl):S249-S250.
- Kullavanijaya P, Sirimachan S, Surarak S. Primary cutaneous infection with Mycobacterium avium intracellulare complex resembling lupus vulgaris. Br J Dermatol. 1997;136:264-266.
- Netea MG, Kullberg BJ, Van der Meer JW. Proinflammatory cytokines in the treatment of bacterial and fungal infections. BioDrugs. 2004;18:9-22.
- Dommasch E, Gelfand JM. Is there truly a risk of lymphoma from biologic therapies? Dermatol Ther. 2009;22:418-430.
- Yonekura N, Yokota S, Yonekura K, et al. Interferon-γ downregulates Hsp27 expression and suppresses the negative regulation of cell death in oral squamous cell carcinoma lines. Cell Death Differ. 2003;10:313-322.
- Feld R, Bodey GP, Groschel D. Mycobacteriosis in patients with malignant disease. Arch Intern Med. 1976;136:67-70.
- Dorman S, Picard C, Lammas D, et al. Clinical features of dominant and recessive interferon γ receptor 1 deficiencies. Lancet. 2004;364:2113-2121.
- Storgaard M, Varming K, Herlin T, et al. Novel mutation in the interferon-γ receptor gene and susceptibility to mycobacterial infections. Scand J Immunol. 2006;64:137-139.
- Toyoda H, Ido M, Nakanishi K, et al. Multiple cutaneous squamous cell carcinomas in a patient with interferon γ receptor 2 (IFNγR2) deficiency [published online June 18, 2010]. J Med Genet. 2010;47:631-634.
Practice Points
- Mycobacterium avium complex (MAC) is a ubiquitous bacterium that commonly infects the lungs and less commonly infects the skin.
- Clinically, cutaneous MAC infection is polymorphous and may present as a nodule, plaque, or ulcer.
- Standard treatment of primary cutaneous MAC includes systemic antibiotics with or without surgical excision.
Recurrent Varicella in an Immunocompetent Woman
Case Report
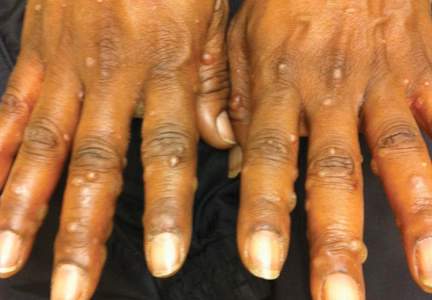
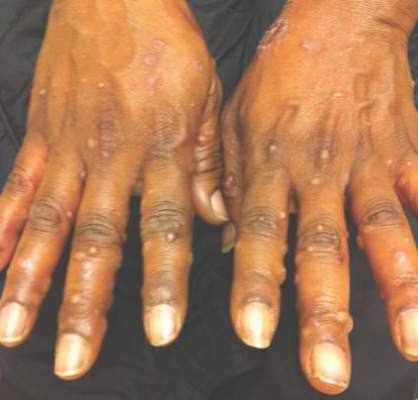
A 52-year-old black woman presented to our dermatology clinic for evaluation of a generalized pruritic rash of 5 days’ duration. The eruption had started on the trunk and subsequently spread to the face, legs, and arms, including the dorsal surfaces of the hands (Figure 1). The patient reported that she had developed a similar rash 4 years prior. She recalled no sick contacts but had occupational exposure to many people as a food service worker. Two days prior, the referring physician had initiated treatment with oral acyclovir 400 mg every 6 hours. The patient was in otherwise good health and reported no fever, chills, diaphoresis, or fatigue. She did not recall any recent insect bites, and a review of systems was negative.
The patient’s medical history was remarkable for 2 cases of varicella: the first, which occurred at 5 years of age, was diagnosed by a pediatrician and manifested as diffuse papules, vesicles, and crusts with concurrent mild fever. The infection followed a typical clinical course and resolved without complications after 1 week. The second case of varicella was diagnosed clinically at our dermatology clinic approximately 4 years prior to the current presentation and manifested as widespread pruritic lesions that were too numerous to count. Given her history of varicella in childhood, a punch biopsy specimen was taken from a lesion on the left trunk and a dermatopathologist confirmed the diagnosis of a herpesvirus infection. The second infection also resolved without sequelae after 12 days. Her medical history was otherwise unremarkable, revealing no exceptional sinopulmonary or gastrointestinal infections. The patient was not currently taking any medications or supplements and reported no known drug allergies.
Physical examination at the current presentation revealed a well-nourished, afebrile woman with vesicles and papules on the hands, arms, and legs along with vesicular and crusted papules in various stages of healing distributed on the chest, abdomen, and back. Lesions on the legs and feet were present but scant. The eruption was not confined to a single dermatome. No lesions were noted on the palms, soles, or oral mucosa and no epitrochlear, axillary, or supraclavicular lymphadenopathy was noted.
Initial laboratory values were obtained. A complete blood count demonstrated a normal leukocyte number of 5700 cells/μL (reference range, 4500–11,000 cells/μL) and mild anemia with a hemoglobin level of 10.3 g/dL (reference range, 14.0–17.5 g/dL). Monocytes were mildly elevated at 11% (reference range, 1%–9%). Serologic tests showed positive titers for varicella-zoster virus (VZV) IgM at 1.64 (negative, <0.91) and VZV IgG at 1.72 (negative, <0.91), indicating current and past VZV infection, respectively. Antibodies against herpes simplex virus (HSV) types 1 and 2 were negative for IgM and positive for IgG at >5.00 (negative, <0.90), indicating a remote HSV infection. Furthermore, results from a culture of a lesion on the left hand were negative for HSV.
After consultation with the Department of Infectious Diseases, further laboratory studies were performed. The absolute lymphocyte number was within normal range at 1600 cells/μL (reference range, 850–3900 cells/μL). Likewise, CD4+ T lymphocytes were normal at 618 cells/μL (reference range, 490–1740 cells/μL) or 39% of total lymphocytes (reference range, 30%–61%). Screening results were negative for human immunodeficiency virus types 1 and 2. Immunoglobulin subtype analysis revealed slightly elevated IgG at 1709 mg/dL (reference range, 723–1685 mg/dL), elevated IgA at 487 mg/dL (reference range, 65–382 mg/dL), and normal IgM at 238 mg/dL (reference range, 63–277 mg/dL).
Consistent with the clinical presentation and serologic studies, recurrent varicella was accepted as the most plausible diagnosis. Over the next 2 weeks, the eruption resolved with postinflammatory hyperpigmentation (Figure 2). The patient returned to work without further incident.
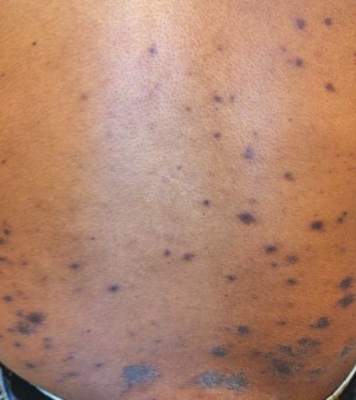
Comment
As denoted by its hyphenated name, VZV infection can cause 2 distinct disease processes.1,2 Varicella, the generalized initial exanthem known as chickenpox, appears predominantly in childhood. With resolution of this primary infection, the virus lies dormant in sensory ganglia, persisting in neurons. Stress, advanced age, and/or compromised immunity may reactivate latent VZV. This secondary expression is known as herpes zoster (shingles), a unilateral eruption of lesions localized to a single dermatome.
In most cases, morphology of the varicella eruption confirms the diagnosis. Lesions evolve through stages from macules and papules to vesicles and pustules and then to crusts. This evolution typically takes 24 to 48 hours.2 The varicella eruption contains an admixture of elements from each stage simultaneously. Crusts usually resolve over an average of 14 days. Serologically, IgM is measurable as early as 1 to 2 days after appearance of the eruption.3,4 Next to appear are IgG antibodies, which generally remain detectable for life. With more than 90% of the US population being seropositive for VZV,5 diagnosis and management of varicella and herpes zoster usually are straightforward; however, there have been unusual variations on this classic sequence of pathogenesis.
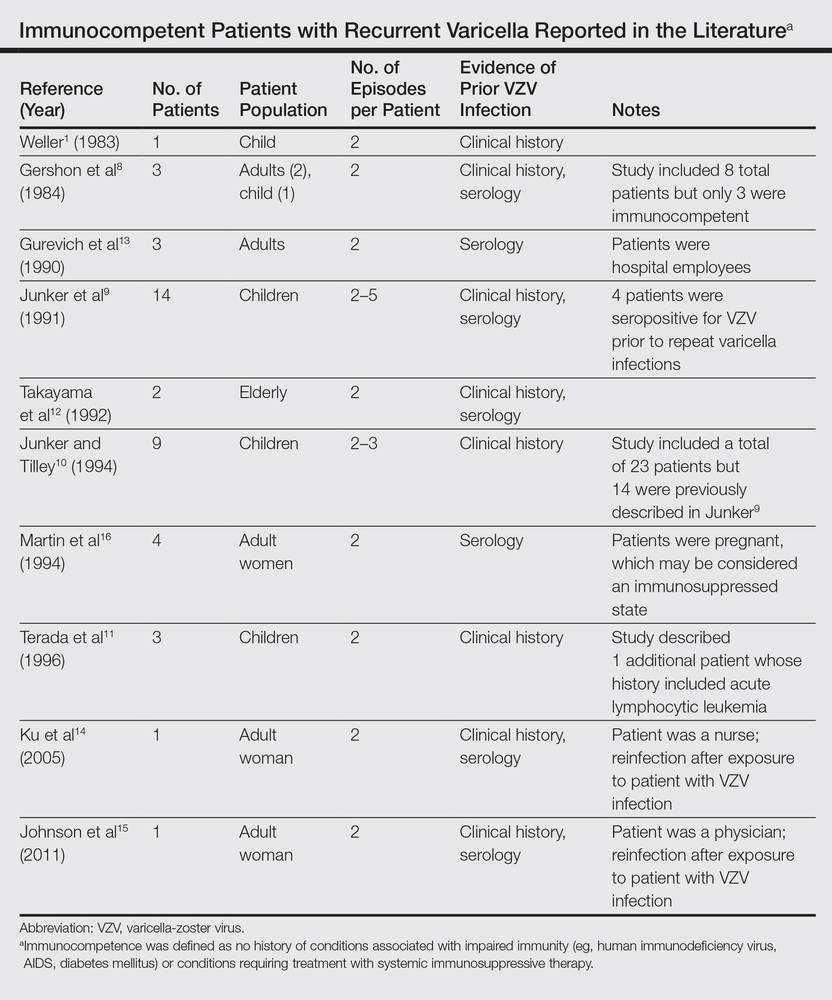
In disseminated zoster, the clinical presentation includes more than 20 lesions outside the dermatome primarily affected.6 Another permutation of VZV infection is zoster sine herpete, which causes the characteristic dermatomal pain of herpes zoster but without the rash.7 Occasionally, 2 cases of chickenpox occur in the same person, usually indicating an underlying immune deficiency. Recurrent varicella in those with intact immunity is purportedly rare. A PubMed search of articles indexed for MEDLINE using the search terms recurrent varicella, chickenpox reinfection, and immunocompetent revealed 41 cases of recurrent varicella in immunocompetent patients in the English language literature occurring among children,1,8-11 adults,8 the elderly,12 health care workers,13-15 and pregnant women16 (Table).
 |
Surveillance studies, however, have challenged the apparent rarity of recurrent varicella, asserting that varicella may recur more frequently than is generally recognized.17,18 Hall et al17 described 9947 cases of varicella, with nearly 6.9% reporting prior varicella infection. Another surveillance report by Marin et al18 evaluating data from 1047 adults with varicella noted that 21% of participants reported prior VZV infections. Both of these studies defined varicella by clinical parameters as a condition with acute onset of generalized maculopapulovesicular rash without other known cause. Although laboratory confirmation of VZV infection was not documented in either study, a history of varicella is considered a reliable indicator of immunity. In fact, studies show that a history of varicella is associated with serologic evidence of immunity 97% to 100% of the time.19,20
Immunity against VZV in humans is not well understood. Although both humoral and cellular factors play a role, cell-mediated immunity may be more important in suppressing primary infection and defending against reinfection. Varicella is more likely to disseminate in lymphopenic patients,21,22 while its course is uninfluenced by hypogammaglobulinemia.1,23 One study of simian varicella virus, which demonstrates 75% genetic homology with VZV, noted that simian varicella virus–infected rhesus macaques without CD4+ T lymphocyte response experienced higher viral loads, prolonged viremia, and disseminated varicella.24 The loss of CD20+ B lymphocytes did not intensify the severity of varicella in the primate model. It is accepted, however, that waning humoral immunity and lower antibody levels correlate with varicella recurrence.25 Ethnicity may impact immunoglobulin persistence. One investigation postulated that individuals with darker skin types experience reduced viral shedding and therefore less antigenic boosting from secondary VZV infections, as they may less readily maintain protective levels of VZV-specific immunoglobulins.25 This phenomenon may have contributed to the 3 episodes of varicella in our patient.
Virulence factors that are intrinsic to VZV may also prompt reinfection. Although taxonomy is still in flux, 3 to 5 major genotypes of VZV have been recognized to date, categorized into European (Dumas), Japanese (Oka), and mosaic clades.26-28 In one study population, approximately 80% of the VZV strains isolated in the United States were of the European variety.26 It is unclear whether infection with one strain of VZV affords immunoprotection against the other strains. Interestingly, one report documented recurrent herpes zoster caused by 2 distinct VZV strains in the same individual.29 Since subtypes of VZV vary geographically, it is possible that increasing global travel may correlate with increased incidence and reporting of varicella reinfection, particularly in cosmopolitan centers. In patients with recurrent varicella, a careful investigation of their international travel history may be necessary.
1. Weller T. Varicella and herpes zoster: changing concepts of the natural history, control, and importance of a not-so-benign virus. N Engl J Med. 1983;309:1362-1368.
2. Heininger U, Seward JF. Varicella. Lancet. 2006;368:1365-1376.
3. Krah DL. Assays for antibodies to varicella-zoster virus. Infect Dis Clin North Am. 1996;10:507-527.
4. Oladepo DK, Klapper PE, Percival D, et al. Serological diagnosis of varicella-zoster virus in sera with antibody-capture enzyme-linked immunosorbent assay of IgM. J Virol Meth. 2000;84:169-173.
5. Kilgore PE, Kruszon-Moran D, Seward JF, et al. Varicella in Americans from NHANES III: implications for control through routine immunizations. J Med Virol. 2003;70:S111-S118.
6. Gupta S, Jain A, Gardiner C, et al. A rare case of disseminated cutaneous zoster in an immunocompetent patient. BMC Fam Pract. 2005;6:50.
7. Lewis GW. Zoster sine herpete. Br Med J. 1958;8:418-421.
8. Gershon AA, Steinberg SP, Gelb L. Clinical reinfection with varicella-zoster virus. J Infect Dis. 1984;149:137-142.
9. Junker AK, Angus E, Thomas EE. Recurrent varicella-zoster virus infections in apparently immunocompetent children. Pediatr Infect Dis J. 1991;10:569-575.
10. Junker AK, Tilley P. Varicella-zoster virus antibody avidity and IgG-subclass patterns in children with recurrent chickenpox. J Med Virol. 1994;43:119-124.
11. Terada K, Kawano S, Shimada Y, et al. Recurrent chickenpox after natural infection. Ped Infect Dis J. 1996;15:179-181.
12. Takayama N, Takayama M, Negishi M. Clinical varicella-zoster virus reinfection observed in two advanced-age persons [article in Japanese]. Kansenshogaku Zasshi. 1992;66:1373-1377.
13. Gurevich I, Jensen L, Kalter R, et al. Chickenpox in apparently “immune” hospital workers. Infect Control Hosp Epidemiol. 1990;11:510, 512.
14. Ku C, Liu Y, Christiani DC. Case report: occupationally related recurrent varicella (chickenpox) in a hospital nurse. Environ Health Perspect. 2005;113:1373-1375.
15. Johnson JA, Bloch KC, Dang BN. Varicella reinfection in a seropositive physician following occupational exposure to localized zoster. Clin Infect Dis. 2011;52:907-909.
16. Martin KA, Junker AK, Thomas EE, et al. Occurrence of chickenpox during pregnancy in women seropositive for varicella-zoster virus. J Infect Dis. 1994;170:991-995.
17. Hall S, Maupin T, Seward J, et al. Second varicella infections: are they more common than previously thought? Pediatrics. 2002;109:1068-1073.
18. Marin M, Watson TL, Chaves SS, et al. Varicella among adults: data from an active surveillance project, 1995-2005. J Infect Dis. 2008;197(Suppl 2):S94-S100.
19. Perella DM, Fiks A, Spain CV. Validity of reported varicella history as a marker for varicella-zoster virus immunity. Paper presented at: Pediatric Academic Societies Annual Meeting; 2005; Washington, DC.
20. Ferson MJ, Bell SM, Robertson PW. Determination and importance of varicella immune status of nursing staff in a children’s hospital. J Hosp Infect. 1990;15:347-351.
21. Arvin AM, Pollard RB, Rasmussen LE, et al. Selective impairment of lymphocyte reactivity to varicella-zoster virus antigen among untreated patients with lymphoma. J Infect Dis. 1978;137:531-540.
22. Feldman S, Hughes WT, Daniel CB. Varicella in children with cancer: seventy-seven cases. Pediatrics. 1975;56:388-397.
23. Arvin AM. Varicella-zoster virus. Clin Microbiol Rev. 1996;9:361-381.
24. Haberthur K, Engelmann F, Park B, et al. CD4 T cell immunity is critical for the control of simian varicella virus infection in nonhuman primate model of VZV infection. PLoS Pathog. 2011;7:e1002367.
25. Ayres KL, Talukder Y, Breuer J. Humoral immunity following chickenpox is influenced by geography and ethnicity. J Infect. 2010;61:244-251.
26. Loparev VN, Gonzalez A, Deleon-Carnes M, et al. Global identification of three major genotypes of varicella-zoster virus: longitudinal clustering and strategies for genotyping. J Virol. 2004;78:8349-8358.
27. Parker SP, Breuer J, Taha Y, et al. Genotyping of varicella-zoster virus and the discrimination of Oka vaccine strains by TaqMan real-time PCR. J Clin Microbiol. 2006;44:3911-3914.
28. Loparev VN, Rubtcova EN, Bostik V, et al. Identification of five major and two minor genotypes of varicella-zoster virus strains: a practical two-amplicon approach used to genotype clinical isolates in Australia and New Zealand. J Virol. 2007;81:12758-12765.
29. Taha Y, Scott FT, Parker SP, et al. Reactivation of 2 genetically distinct varicella-zoster viruses in the same individual. Clin Infect Dis. 2006;43:1301-1303.
Case Report
A 52-year-old black woman presented to our dermatology clinic for evaluation of a generalized pruritic rash of 5 days’ duration. The eruption had started on the trunk and subsequently spread to the face, legs, and arms, including the dorsal surfaces of the hands (Figure 1). The patient reported that she had developed a similar rash 4 years prior. She recalled no sick contacts but had occupational exposure to many people as a food service worker. Two days prior, the referring physician had initiated treatment with oral acyclovir 400 mg every 6 hours. The patient was in otherwise good health and reported no fever, chills, diaphoresis, or fatigue. She did not recall any recent insect bites, and a review of systems was negative.

The patient’s medical history was remarkable for 2 cases of varicella: the first, which occurred at 5 years of age, was diagnosed by a pediatrician and manifested as diffuse papules, vesicles, and crusts with concurrent mild fever. The infection followed a typical clinical course and resolved without complications after 1 week. The second case of varicella was diagnosed clinically at our dermatology clinic approximately 4 years prior to the current presentation and manifested as widespread pruritic lesions that were too numerous to count. Given her history of varicella in childhood, a punch biopsy specimen was taken from a lesion on the left trunk and a dermatopathologist confirmed the diagnosis of a herpesvirus infection. The second infection also resolved without sequelae after 12 days. Her medical history was otherwise unremarkable, revealing no exceptional sinopulmonary or gastrointestinal infections. The patient was not currently taking any medications or supplements and reported no known drug allergies.
Physical examination at the current presentation revealed a well-nourished, afebrile woman with vesicles and papules on the hands, arms, and legs along with vesicular and crusted papules in various stages of healing distributed on the chest, abdomen, and back. Lesions on the legs and feet were present but scant. The eruption was not confined to a single dermatome. No lesions were noted on the palms, soles, or oral mucosa and no epitrochlear, axillary, or supraclavicular lymphadenopathy was noted.
Initial laboratory values were obtained. A complete blood count demonstrated a normal leukocyte number of 5700 cells/μL (reference range, 4500–11,000 cells/μL) and mild anemia with a hemoglobin level of 10.3 g/dL (reference range, 14.0–17.5 g/dL). Monocytes were mildly elevated at 11% (reference range, 1%–9%). Serologic tests showed positive titers for varicella-zoster virus (VZV) IgM at 1.64 (negative, <0.91) and VZV IgG at 1.72 (negative, <0.91), indicating current and past VZV infection, respectively. Antibodies against herpes simplex virus (HSV) types 1 and 2 were negative for IgM and positive for IgG at >5.00 (negative, <0.90), indicating a remote HSV infection. Furthermore, results from a culture of a lesion on the left hand were negative for HSV.
After consultation with the Department of Infectious Diseases, further laboratory studies were performed. The absolute lymphocyte number was within normal range at 1600 cells/μL (reference range, 850–3900 cells/μL). Likewise, CD4+ T lymphocytes were normal at 618 cells/μL (reference range, 490–1740 cells/μL) or 39% of total lymphocytes (reference range, 30%–61%). Screening results were negative for human immunodeficiency virus types 1 and 2. Immunoglobulin subtype analysis revealed slightly elevated IgG at 1709 mg/dL (reference range, 723–1685 mg/dL), elevated IgA at 487 mg/dL (reference range, 65–382 mg/dL), and normal IgM at 238 mg/dL (reference range, 63–277 mg/dL).
Consistent with the clinical presentation and serologic studies, recurrent varicella was accepted as the most plausible diagnosis. Over the next 2 weeks, the eruption resolved with postinflammatory hyperpigmentation (Figure 2). The patient returned to work without further incident.

Comment
As denoted by its hyphenated name, VZV infection can cause 2 distinct disease processes.1,2 Varicella, the generalized initial exanthem known as chickenpox, appears predominantly in childhood. With resolution of this primary infection, the virus lies dormant in sensory ganglia, persisting in neurons. Stress, advanced age, and/or compromised immunity may reactivate latent VZV. This secondary expression is known as herpes zoster (shingles), a unilateral eruption of lesions localized to a single dermatome.
In most cases, morphology of the varicella eruption confirms the diagnosis. Lesions evolve through stages from macules and papules to vesicles and pustules and then to crusts. This evolution typically takes 24 to 48 hours.2 The varicella eruption contains an admixture of elements from each stage simultaneously. Crusts usually resolve over an average of 14 days. Serologically, IgM is measurable as early as 1 to 2 days after appearance of the eruption.3,4 Next to appear are IgG antibodies, which generally remain detectable for life. With more than 90% of the US population being seropositive for VZV,5 diagnosis and management of varicella and herpes zoster usually are straightforward; however, there have been unusual variations on this classic sequence of pathogenesis.
In disseminated zoster, the clinical presentation includes more than 20 lesions outside the dermatome primarily affected.6 Another permutation of VZV infection is zoster sine herpete, which causes the characteristic dermatomal pain of herpes zoster but without the rash.7 Occasionally, 2 cases of chickenpox occur in the same person, usually indicating an underlying immune deficiency. Recurrent varicella in those with intact immunity is purportedly rare. A PubMed search of articles indexed for MEDLINE using the search terms recurrent varicella, chickenpox reinfection, and immunocompetent revealed 41 cases of recurrent varicella in immunocompetent patients in the English language literature occurring among children,1,8-11 adults,8 the elderly,12 health care workers,13-15 and pregnant women16 (Table).
 |
Surveillance studies, however, have challenged the apparent rarity of recurrent varicella, asserting that varicella may recur more frequently than is generally recognized.17,18 Hall et al17 described 9947 cases of varicella, with nearly 6.9% reporting prior varicella infection. Another surveillance report by Marin et al18 evaluating data from 1047 adults with varicella noted that 21% of participants reported prior VZV infections. Both of these studies defined varicella by clinical parameters as a condition with acute onset of generalized maculopapulovesicular rash without other known cause. Although laboratory confirmation of VZV infection was not documented in either study, a history of varicella is considered a reliable indicator of immunity. In fact, studies show that a history of varicella is associated with serologic evidence of immunity 97% to 100% of the time.19,20
Immunity against VZV in humans is not well understood. Although both humoral and cellular factors play a role, cell-mediated immunity may be more important in suppressing primary infection and defending against reinfection. Varicella is more likely to disseminate in lymphopenic patients,21,22 while its course is uninfluenced by hypogammaglobulinemia.1,23 One study of simian varicella virus, which demonstrates 75% genetic homology with VZV, noted that simian varicella virus–infected rhesus macaques without CD4+ T lymphocyte response experienced higher viral loads, prolonged viremia, and disseminated varicella.24 The loss of CD20+ B lymphocytes did not intensify the severity of varicella in the primate model. It is accepted, however, that waning humoral immunity and lower antibody levels correlate with varicella recurrence.25 Ethnicity may impact immunoglobulin persistence. One investigation postulated that individuals with darker skin types experience reduced viral shedding and therefore less antigenic boosting from secondary VZV infections, as they may less readily maintain protective levels of VZV-specific immunoglobulins.25 This phenomenon may have contributed to the 3 episodes of varicella in our patient.
Virulence factors that are intrinsic to VZV may also prompt reinfection. Although taxonomy is still in flux, 3 to 5 major genotypes of VZV have been recognized to date, categorized into European (Dumas), Japanese (Oka), and mosaic clades.26-28 In one study population, approximately 80% of the VZV strains isolated in the United States were of the European variety.26 It is unclear whether infection with one strain of VZV affords immunoprotection against the other strains. Interestingly, one report documented recurrent herpes zoster caused by 2 distinct VZV strains in the same individual.29 Since subtypes of VZV vary geographically, it is possible that increasing global travel may correlate with increased incidence and reporting of varicella reinfection, particularly in cosmopolitan centers. In patients with recurrent varicella, a careful investigation of their international travel history may be necessary.
Case Report
A 52-year-old black woman presented to our dermatology clinic for evaluation of a generalized pruritic rash of 5 days’ duration. The eruption had started on the trunk and subsequently spread to the face, legs, and arms, including the dorsal surfaces of the hands (Figure 1). The patient reported that she had developed a similar rash 4 years prior. She recalled no sick contacts but had occupational exposure to many people as a food service worker. Two days prior, the referring physician had initiated treatment with oral acyclovir 400 mg every 6 hours. The patient was in otherwise good health and reported no fever, chills, diaphoresis, or fatigue. She did not recall any recent insect bites, and a review of systems was negative.

The patient’s medical history was remarkable for 2 cases of varicella: the first, which occurred at 5 years of age, was diagnosed by a pediatrician and manifested as diffuse papules, vesicles, and crusts with concurrent mild fever. The infection followed a typical clinical course and resolved without complications after 1 week. The second case of varicella was diagnosed clinically at our dermatology clinic approximately 4 years prior to the current presentation and manifested as widespread pruritic lesions that were too numerous to count. Given her history of varicella in childhood, a punch biopsy specimen was taken from a lesion on the left trunk and a dermatopathologist confirmed the diagnosis of a herpesvirus infection. The second infection also resolved without sequelae after 12 days. Her medical history was otherwise unremarkable, revealing no exceptional sinopulmonary or gastrointestinal infections. The patient was not currently taking any medications or supplements and reported no known drug allergies.
Physical examination at the current presentation revealed a well-nourished, afebrile woman with vesicles and papules on the hands, arms, and legs along with vesicular and crusted papules in various stages of healing distributed on the chest, abdomen, and back. Lesions on the legs and feet were present but scant. The eruption was not confined to a single dermatome. No lesions were noted on the palms, soles, or oral mucosa and no epitrochlear, axillary, or supraclavicular lymphadenopathy was noted.
Initial laboratory values were obtained. A complete blood count demonstrated a normal leukocyte number of 5700 cells/μL (reference range, 4500–11,000 cells/μL) and mild anemia with a hemoglobin level of 10.3 g/dL (reference range, 14.0–17.5 g/dL). Monocytes were mildly elevated at 11% (reference range, 1%–9%). Serologic tests showed positive titers for varicella-zoster virus (VZV) IgM at 1.64 (negative, <0.91) and VZV IgG at 1.72 (negative, <0.91), indicating current and past VZV infection, respectively. Antibodies against herpes simplex virus (HSV) types 1 and 2 were negative for IgM and positive for IgG at >5.00 (negative, <0.90), indicating a remote HSV infection. Furthermore, results from a culture of a lesion on the left hand were negative for HSV.
After consultation with the Department of Infectious Diseases, further laboratory studies were performed. The absolute lymphocyte number was within normal range at 1600 cells/μL (reference range, 850–3900 cells/μL). Likewise, CD4+ T lymphocytes were normal at 618 cells/μL (reference range, 490–1740 cells/μL) or 39% of total lymphocytes (reference range, 30%–61%). Screening results were negative for human immunodeficiency virus types 1 and 2. Immunoglobulin subtype analysis revealed slightly elevated IgG at 1709 mg/dL (reference range, 723–1685 mg/dL), elevated IgA at 487 mg/dL (reference range, 65–382 mg/dL), and normal IgM at 238 mg/dL (reference range, 63–277 mg/dL).
Consistent with the clinical presentation and serologic studies, recurrent varicella was accepted as the most plausible diagnosis. Over the next 2 weeks, the eruption resolved with postinflammatory hyperpigmentation (Figure 2). The patient returned to work without further incident.

Comment
As denoted by its hyphenated name, VZV infection can cause 2 distinct disease processes.1,2 Varicella, the generalized initial exanthem known as chickenpox, appears predominantly in childhood. With resolution of this primary infection, the virus lies dormant in sensory ganglia, persisting in neurons. Stress, advanced age, and/or compromised immunity may reactivate latent VZV. This secondary expression is known as herpes zoster (shingles), a unilateral eruption of lesions localized to a single dermatome.
In most cases, morphology of the varicella eruption confirms the diagnosis. Lesions evolve through stages from macules and papules to vesicles and pustules and then to crusts. This evolution typically takes 24 to 48 hours.2 The varicella eruption contains an admixture of elements from each stage simultaneously. Crusts usually resolve over an average of 14 days. Serologically, IgM is measurable as early as 1 to 2 days after appearance of the eruption.3,4 Next to appear are IgG antibodies, which generally remain detectable for life. With more than 90% of the US population being seropositive for VZV,5 diagnosis and management of varicella and herpes zoster usually are straightforward; however, there have been unusual variations on this classic sequence of pathogenesis.
In disseminated zoster, the clinical presentation includes more than 20 lesions outside the dermatome primarily affected.6 Another permutation of VZV infection is zoster sine herpete, which causes the characteristic dermatomal pain of herpes zoster but without the rash.7 Occasionally, 2 cases of chickenpox occur in the same person, usually indicating an underlying immune deficiency. Recurrent varicella in those with intact immunity is purportedly rare. A PubMed search of articles indexed for MEDLINE using the search terms recurrent varicella, chickenpox reinfection, and immunocompetent revealed 41 cases of recurrent varicella in immunocompetent patients in the English language literature occurring among children,1,8-11 adults,8 the elderly,12 health care workers,13-15 and pregnant women16 (Table).
 |
Surveillance studies, however, have challenged the apparent rarity of recurrent varicella, asserting that varicella may recur more frequently than is generally recognized.17,18 Hall et al17 described 9947 cases of varicella, with nearly 6.9% reporting prior varicella infection. Another surveillance report by Marin et al18 evaluating data from 1047 adults with varicella noted that 21% of participants reported prior VZV infections. Both of these studies defined varicella by clinical parameters as a condition with acute onset of generalized maculopapulovesicular rash without other known cause. Although laboratory confirmation of VZV infection was not documented in either study, a history of varicella is considered a reliable indicator of immunity. In fact, studies show that a history of varicella is associated with serologic evidence of immunity 97% to 100% of the time.19,20
Immunity against VZV in humans is not well understood. Although both humoral and cellular factors play a role, cell-mediated immunity may be more important in suppressing primary infection and defending against reinfection. Varicella is more likely to disseminate in lymphopenic patients,21,22 while its course is uninfluenced by hypogammaglobulinemia.1,23 One study of simian varicella virus, which demonstrates 75% genetic homology with VZV, noted that simian varicella virus–infected rhesus macaques without CD4+ T lymphocyte response experienced higher viral loads, prolonged viremia, and disseminated varicella.24 The loss of CD20+ B lymphocytes did not intensify the severity of varicella in the primate model. It is accepted, however, that waning humoral immunity and lower antibody levels correlate with varicella recurrence.25 Ethnicity may impact immunoglobulin persistence. One investigation postulated that individuals with darker skin types experience reduced viral shedding and therefore less antigenic boosting from secondary VZV infections, as they may less readily maintain protective levels of VZV-specific immunoglobulins.25 This phenomenon may have contributed to the 3 episodes of varicella in our patient.
Virulence factors that are intrinsic to VZV may also prompt reinfection. Although taxonomy is still in flux, 3 to 5 major genotypes of VZV have been recognized to date, categorized into European (Dumas), Japanese (Oka), and mosaic clades.26-28 In one study population, approximately 80% of the VZV strains isolated in the United States were of the European variety.26 It is unclear whether infection with one strain of VZV affords immunoprotection against the other strains. Interestingly, one report documented recurrent herpes zoster caused by 2 distinct VZV strains in the same individual.29 Since subtypes of VZV vary geographically, it is possible that increasing global travel may correlate with increased incidence and reporting of varicella reinfection, particularly in cosmopolitan centers. In patients with recurrent varicella, a careful investigation of their international travel history may be necessary.
1. Weller T. Varicella and herpes zoster: changing concepts of the natural history, control, and importance of a not-so-benign virus. N Engl J Med. 1983;309:1362-1368.
2. Heininger U, Seward JF. Varicella. Lancet. 2006;368:1365-1376.
3. Krah DL. Assays for antibodies to varicella-zoster virus. Infect Dis Clin North Am. 1996;10:507-527.
4. Oladepo DK, Klapper PE, Percival D, et al. Serological diagnosis of varicella-zoster virus in sera with antibody-capture enzyme-linked immunosorbent assay of IgM. J Virol Meth. 2000;84:169-173.
5. Kilgore PE, Kruszon-Moran D, Seward JF, et al. Varicella in Americans from NHANES III: implications for control through routine immunizations. J Med Virol. 2003;70:S111-S118.
6. Gupta S, Jain A, Gardiner C, et al. A rare case of disseminated cutaneous zoster in an immunocompetent patient. BMC Fam Pract. 2005;6:50.
7. Lewis GW. Zoster sine herpete. Br Med J. 1958;8:418-421.
8. Gershon AA, Steinberg SP, Gelb L. Clinical reinfection with varicella-zoster virus. J Infect Dis. 1984;149:137-142.
9. Junker AK, Angus E, Thomas EE. Recurrent varicella-zoster virus infections in apparently immunocompetent children. Pediatr Infect Dis J. 1991;10:569-575.
10. Junker AK, Tilley P. Varicella-zoster virus antibody avidity and IgG-subclass patterns in children with recurrent chickenpox. J Med Virol. 1994;43:119-124.
11. Terada K, Kawano S, Shimada Y, et al. Recurrent chickenpox after natural infection. Ped Infect Dis J. 1996;15:179-181.
12. Takayama N, Takayama M, Negishi M. Clinical varicella-zoster virus reinfection observed in two advanced-age persons [article in Japanese]. Kansenshogaku Zasshi. 1992;66:1373-1377.
13. Gurevich I, Jensen L, Kalter R, et al. Chickenpox in apparently “immune” hospital workers. Infect Control Hosp Epidemiol. 1990;11:510, 512.
14. Ku C, Liu Y, Christiani DC. Case report: occupationally related recurrent varicella (chickenpox) in a hospital nurse. Environ Health Perspect. 2005;113:1373-1375.
15. Johnson JA, Bloch KC, Dang BN. Varicella reinfection in a seropositive physician following occupational exposure to localized zoster. Clin Infect Dis. 2011;52:907-909.
16. Martin KA, Junker AK, Thomas EE, et al. Occurrence of chickenpox during pregnancy in women seropositive for varicella-zoster virus. J Infect Dis. 1994;170:991-995.
17. Hall S, Maupin T, Seward J, et al. Second varicella infections: are they more common than previously thought? Pediatrics. 2002;109:1068-1073.
18. Marin M, Watson TL, Chaves SS, et al. Varicella among adults: data from an active surveillance project, 1995-2005. J Infect Dis. 2008;197(Suppl 2):S94-S100.
19. Perella DM, Fiks A, Spain CV. Validity of reported varicella history as a marker for varicella-zoster virus immunity. Paper presented at: Pediatric Academic Societies Annual Meeting; 2005; Washington, DC.
20. Ferson MJ, Bell SM, Robertson PW. Determination and importance of varicella immune status of nursing staff in a children’s hospital. J Hosp Infect. 1990;15:347-351.
21. Arvin AM, Pollard RB, Rasmussen LE, et al. Selective impairment of lymphocyte reactivity to varicella-zoster virus antigen among untreated patients with lymphoma. J Infect Dis. 1978;137:531-540.
22. Feldman S, Hughes WT, Daniel CB. Varicella in children with cancer: seventy-seven cases. Pediatrics. 1975;56:388-397.
23. Arvin AM. Varicella-zoster virus. Clin Microbiol Rev. 1996;9:361-381.
24. Haberthur K, Engelmann F, Park B, et al. CD4 T cell immunity is critical for the control of simian varicella virus infection in nonhuman primate model of VZV infection. PLoS Pathog. 2011;7:e1002367.
25. Ayres KL, Talukder Y, Breuer J. Humoral immunity following chickenpox is influenced by geography and ethnicity. J Infect. 2010;61:244-251.
26. Loparev VN, Gonzalez A, Deleon-Carnes M, et al. Global identification of three major genotypes of varicella-zoster virus: longitudinal clustering and strategies for genotyping. J Virol. 2004;78:8349-8358.
27. Parker SP, Breuer J, Taha Y, et al. Genotyping of varicella-zoster virus and the discrimination of Oka vaccine strains by TaqMan real-time PCR. J Clin Microbiol. 2006;44:3911-3914.
28. Loparev VN, Rubtcova EN, Bostik V, et al. Identification of five major and two minor genotypes of varicella-zoster virus strains: a practical two-amplicon approach used to genotype clinical isolates in Australia and New Zealand. J Virol. 2007;81:12758-12765.
29. Taha Y, Scott FT, Parker SP, et al. Reactivation of 2 genetically distinct varicella-zoster viruses in the same individual. Clin Infect Dis. 2006;43:1301-1303.
1. Weller T. Varicella and herpes zoster: changing concepts of the natural history, control, and importance of a not-so-benign virus. N Engl J Med. 1983;309:1362-1368.
2. Heininger U, Seward JF. Varicella. Lancet. 2006;368:1365-1376.
3. Krah DL. Assays for antibodies to varicella-zoster virus. Infect Dis Clin North Am. 1996;10:507-527.
4. Oladepo DK, Klapper PE, Percival D, et al. Serological diagnosis of varicella-zoster virus in sera with antibody-capture enzyme-linked immunosorbent assay of IgM. J Virol Meth. 2000;84:169-173.
5. Kilgore PE, Kruszon-Moran D, Seward JF, et al. Varicella in Americans from NHANES III: implications for control through routine immunizations. J Med Virol. 2003;70:S111-S118.
6. Gupta S, Jain A, Gardiner C, et al. A rare case of disseminated cutaneous zoster in an immunocompetent patient. BMC Fam Pract. 2005;6:50.
7. Lewis GW. Zoster sine herpete. Br Med J. 1958;8:418-421.
8. Gershon AA, Steinberg SP, Gelb L. Clinical reinfection with varicella-zoster virus. J Infect Dis. 1984;149:137-142.
9. Junker AK, Angus E, Thomas EE. Recurrent varicella-zoster virus infections in apparently immunocompetent children. Pediatr Infect Dis J. 1991;10:569-575.
10. Junker AK, Tilley P. Varicella-zoster virus antibody avidity and IgG-subclass patterns in children with recurrent chickenpox. J Med Virol. 1994;43:119-124.
11. Terada K, Kawano S, Shimada Y, et al. Recurrent chickenpox after natural infection. Ped Infect Dis J. 1996;15:179-181.
12. Takayama N, Takayama M, Negishi M. Clinical varicella-zoster virus reinfection observed in two advanced-age persons [article in Japanese]. Kansenshogaku Zasshi. 1992;66:1373-1377.
13. Gurevich I, Jensen L, Kalter R, et al. Chickenpox in apparently “immune” hospital workers. Infect Control Hosp Epidemiol. 1990;11:510, 512.
14. Ku C, Liu Y, Christiani DC. Case report: occupationally related recurrent varicella (chickenpox) in a hospital nurse. Environ Health Perspect. 2005;113:1373-1375.
15. Johnson JA, Bloch KC, Dang BN. Varicella reinfection in a seropositive physician following occupational exposure to localized zoster. Clin Infect Dis. 2011;52:907-909.
16. Martin KA, Junker AK, Thomas EE, et al. Occurrence of chickenpox during pregnancy in women seropositive for varicella-zoster virus. J Infect Dis. 1994;170:991-995.
17. Hall S, Maupin T, Seward J, et al. Second varicella infections: are they more common than previously thought? Pediatrics. 2002;109:1068-1073.
18. Marin M, Watson TL, Chaves SS, et al. Varicella among adults: data from an active surveillance project, 1995-2005. J Infect Dis. 2008;197(Suppl 2):S94-S100.
19. Perella DM, Fiks A, Spain CV. Validity of reported varicella history as a marker for varicella-zoster virus immunity. Paper presented at: Pediatric Academic Societies Annual Meeting; 2005; Washington, DC.
20. Ferson MJ, Bell SM, Robertson PW. Determination and importance of varicella immune status of nursing staff in a children’s hospital. J Hosp Infect. 1990;15:347-351.
21. Arvin AM, Pollard RB, Rasmussen LE, et al. Selective impairment of lymphocyte reactivity to varicella-zoster virus antigen among untreated patients with lymphoma. J Infect Dis. 1978;137:531-540.
22. Feldman S, Hughes WT, Daniel CB. Varicella in children with cancer: seventy-seven cases. Pediatrics. 1975;56:388-397.
23. Arvin AM. Varicella-zoster virus. Clin Microbiol Rev. 1996;9:361-381.
24. Haberthur K, Engelmann F, Park B, et al. CD4 T cell immunity is critical for the control of simian varicella virus infection in nonhuman primate model of VZV infection. PLoS Pathog. 2011;7:e1002367.
25. Ayres KL, Talukder Y, Breuer J. Humoral immunity following chickenpox is influenced by geography and ethnicity. J Infect. 2010;61:244-251.
26. Loparev VN, Gonzalez A, Deleon-Carnes M, et al. Global identification of three major genotypes of varicella-zoster virus: longitudinal clustering and strategies for genotyping. J Virol. 2004;78:8349-8358.
27. Parker SP, Breuer J, Taha Y, et al. Genotyping of varicella-zoster virus and the discrimination of Oka vaccine strains by TaqMan real-time PCR. J Clin Microbiol. 2006;44:3911-3914.
28. Loparev VN, Rubtcova EN, Bostik V, et al. Identification of five major and two minor genotypes of varicella-zoster virus strains: a practical two-amplicon approach used to genotype clinical isolates in Australia and New Zealand. J Virol. 2007;81:12758-12765.
29. Taha Y, Scott FT, Parker SP, et al. Reactivation of 2 genetically distinct varicella-zoster viruses in the same individual. Clin Infect Dis. 2006;43:1301-1303.
Practice Points
- Varicella is a viral exanthem that manifests as generalized and pruritic papules, vesicles, and crusted lesions and is the initial expression of infection with the varicella-zoster virus (VZV).
- Secondary expression of VZV infection is typified by herpes zoster, where painful papules and vesicles are confined within a single dermatome.
- Although contradictory to dogma and public perception, varicella may occur more than once; therefore, patients with a generalized pruritic exanthem should be screened for varicella, even if they report a history of varicella.