User login
Genetic testing and the future of cerebral palsy malpractice cases
CASE Mixed CP diagnosed at age 6 months
After learning that the statute of limitations was to run out in the near future, the parents of a 17-year-old with cerebral palsy (CP) initiated a lawsuit. At the time of her pregnancy, the mother (G2P2002) was age 39 and first sought prenatal care at 14 weeks.
Her past medical history was largely noncontributory to her current pregnancy, except for that she had hypothyroidism that was being treated with levothyroxine. She also had a history of asthma, but had had no acute episodes for years. During the course of the pregnancy there was evidence of polyhydramnios; her initial thyroid studies were abnormal (thyroid-stimulating hormone levels, 7.1 mIU/L), in part due to lack of adherence with prescribed medications. She was noted to have elevated blood pressure (BP) 150/100 mm Hg but no proteinuria, with BP monitoring during her last trimester.
The patient went into labor at 40 3/7 weeks, after spontaneous rupture of membranes. In labor and delivery she was placed on a monitor, and irregular contractions were noted. The initial vaginal examination was noted as 1-cm cervical dilation, 90% effaced, and station zero. The obstetrician evaluated the patient and ordered Pitocin augmentation. The next vaginal exam several hours later noted 3-cm dilation and 100% effacement. The Pitocin was continued. Several early decelerations, moderate variability, and better contraction pattern was noted. Eight hours into the Pitocin, there were repetitive late decelerations; the obstetrician was not notified. The nursing staff proceeded with vaginal examination, and the patient was fully dilated at station +1. Again, the doctor was not informed of the patient’s status. At 10 hours post-Pitocin initiation, the patient felt the urge to push. The obstetrician was notified, and he promptly arrived to the unit and patient’s bedside. His decision was to use forceps for the delivery, feeling this would be the most expedient way to proceed, although cesarean delivery (CD) was a definite consideration. Forceps were applied, and as the nursing staff noted,” the doctor really had to pull to deliver the head.” A male baby, 8 lb 8 oz, was delivered. A second-degree tear was noted and easily repaired following delivery of the placenta. Apgar scores were 5 and 7 at 1 and 5 minutes after birth, respectively.
The patient’s postpartum course was uneventful. The patient and baby were discharged on the third day postpartum.
As the child was evaluated by the pediatrician, the mother noted at 6 months that the child’s head lagged behind when he was picked up. He appeared stiff at times and floppy at other times according to the parents. As the child progressed he had problems with hand-to-mouth coordination, and when he would crawl he seemed to “scoot his butt,” as they stated.
The child was tested and a diagnosis of mixed cerebral palsy was made, implying a combination of spastic CP and dyskinetic CP. He is wheelchair bound. The parents filed a lawsuit against the obstetrician and the hospital, focused on hypoxic-ischemic encephalopathy (HIE) due to labor and delivery management being below the standard of care. They claimed that the obstetrician should have been informed by the hospital staff during the course of labor, and the obstetrician should have been more proactive in monitoring the deteriorating circumstances. This included performing a CD based on “the Category III fetal heart tracing.”
At trial, the plaintiff expert argued that failure of nursing staff to properly communicate with the obstetrician led to mismanagement. Furthermore, the obstetrician used poor judgement (ie, below the standard of care) in not performing a CD. The defense expert argued that, overall, the fetal heart tracing was Category II, and the events occurred in utero, in part reflected by the mother having polyhydramnios and hypothyroidism that was not well controlled due to lack of adherence with prescribed medications. The child in his wheelchair was brought into the courtroom. The trial went on for more than 1 week, and the jury deliberated for several hours. (Note: This case is a composite of several different events and claims.)
Continue to: WHAT’S THE VERDICT?
WHAT’S THE VERDICT?
The jury returns a verdict for the defense.
Should anything have been done differently in this trial?
Medical considerations
Cerebral palsy is a neurodevelopmental disorder affecting 1 in 500 children.1 Other prevalence data (from a European study) indicate an incidence of 1.3–1.9 cases per 1,000 livebirths.1 The controversy continues with respect to the disorder’s etiology, especially when the infant’s magnetic resonance imaging (MRI) does not identify specific pathology. The finger is then pointed at HIE and thus the fault of the obstetrician and labor and delivery staff. In reality, HIE accounts for less than 10% of all cases of CP.2 Overall, CP is a condition focused on progressive motor impairments, many times associated with specific MRI findings.3 In addition, “MRI-negative” CP is a more vague diagnosis as discussed among neurologists.
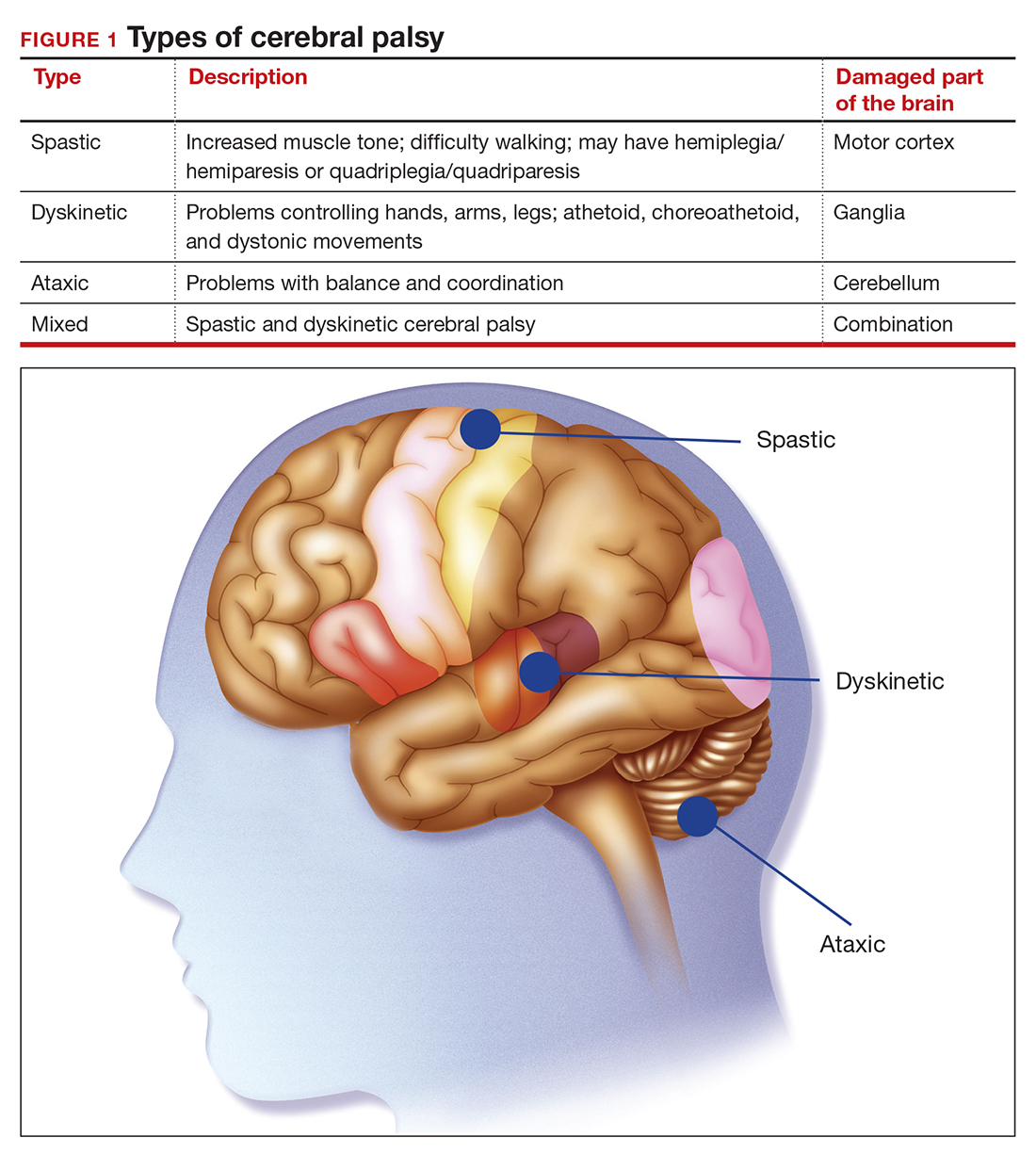
The International Consensus Definition of CP is “a group of permanent disorders of the development of movement and posture, causing activity limitations, that are attributed to nonprogressive disturbances that occurred in the developing fetal or infant brain.”4 The International Cerebral Palsy Genomics Consortium have provided a consensus statement that defines CP based upon clinical type as opposed to etiology.5 Many times, however, ascribing an HIE cause to CP is “barking up the wrong tree,” in that we now know there are clear cut genetic causes of CP, and etiology attributed to perinatal causes, in reality, are genetic in up to 80% of cases.3 Types of CP are addressed in FIGURE 1. Overall, the pathophysiology of the disorder remains unknown. Some affected children have intellectual disabilities, as well as visual, hearing, and/or speech impairment.
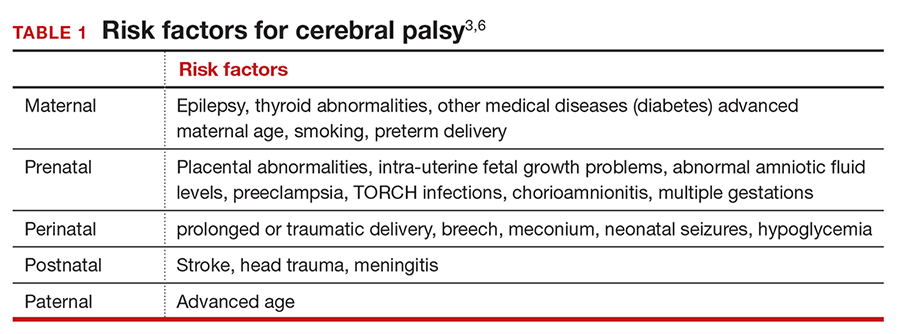
A number of risk factors have been associated with CP (TABLE 1),3,6 which contribute to cell death in the brain or altered maturation of neurons and glia, resulting in abnormal white matter tracts and smaller central nervous system (CNS) volume or cerebellar hypoxia.6 One very important aspect of assessment for CP is specific gene mutations, which may vary in part dependent upon the presence or absence of environmental factors (insults).1 Mutations can lead to profound adverse effects with resultant CNS ischemia and neuromotor disability. In fact, genetics play a major role in determining the etiology of CP.1 Of interest, animal models who are subject to HIE induction have CNS effects resulting in permanent motor impairment.7
DNA sequencing
The DNA story continues to unfold with the concept that DNA variants alter susceptibility to environmental influences. These insults are, for example, thrombosis or hemorrhage, all of which affect motor function.1 Duplications or deletions of portions of a chromosome, related to copy number variants (CNVs) as well as advances in human-genome sequencing, can identify a single gene mutation leading to CP.1 Microdeletions, microduplications, and single nucleotide variants (SNVs) are to be included in genetic-related problems causing CP.3
A number of candidate genes have been considered and include “de novo heterozygous mutations in known Online Mendelian Inheritance (OMIM).” TIBA1A and SCN8A genes are highly associated with CP.8 Genetic assessment, as it evolves and more recently with the advent of exome sequencing, appears to provide a new and unprecedented level of understanding of CP. Specifically, exome sequencing provides a diagnostic tool with which to identify the prevalence of pathogenic and pathogenic variants (the latter encompassing genomic variants) with CP.9 A retrospective study assessed a cohort of patients with CP and noted that 32.7% of the pediatric-aged patients who underwent exome sequencing had pathogenic and pathogenic variants in the sequencing.9 Thus, we have a tool to identify underlying genetic pathogenesis with CP. This theoretically can change the outcome of lawsuits initiated for CP that ascribe an HIE etiology. Clinicians need to stay tuned as the genetic repertoire continues to unfold.
Continue to: Legal considerations...
Legal considerations
Although CP is not a common event, it has been a major factor in the total malpractice payments for ObGyns, neonatologists, and related medical disciplines. That is because the per-event liability can be staggering. Some law firms provide a “checklist” for plaintiffs early on in assessing a potential case (FIGURE 2).10

The financial risks and incentives
To understand what the current settlements and verdicts are in birth-related CP cases, a search of Lexis files revealed the reported outcomes of cases in 2019 and 2020 (FIGURE 3). Taking into account that the pandemic limited legal activity, 23 unduplicated cases were described with a reported settlement or verdict. Four cases resulted in verdicts for the injured patients, with the mean of these awards substantially higher than the settlements ($88.3 million vs $11.1 million, respectively).
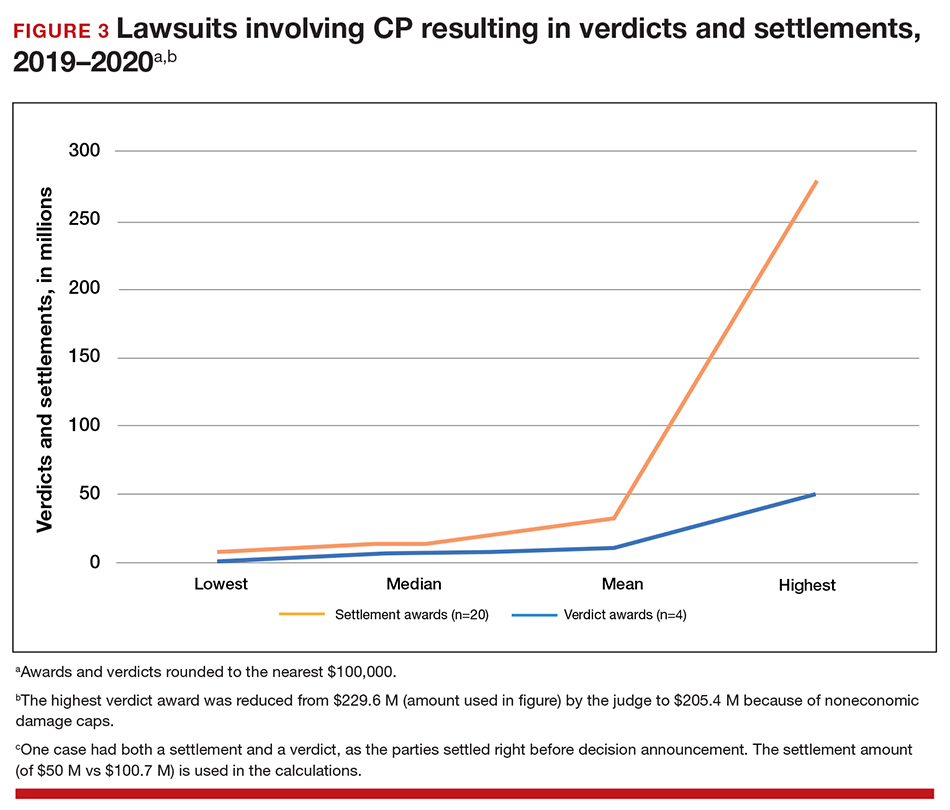
These numbers are a glimpse at some of the very high settlements and verdicts that are common in CP cases. Notably, these are not a random sample of CP cases, but only those with the amount of the verdict or settlement reported. Potentially tried cases that may have been simply abandoned or dismissed are not reported. Furthermore, most settlements include confidentiality clauses, which may preclude the release of the financial value of the settlement. Cases in which the defense won (for example, a jury verdict in favor of the physician) are not included.
The high monetary awards in some CP cases are indirectly backed by Google search results for “cerebral palsy and liability” or “cerebral palsy and malpractice.” A very large number of results for law firms seeking clients with CP injuries is produced. Some of the websites note that only 10% (or 20% on some sites) of CP cases are caused by medical negligence, offering a “free legal case review” and a phone number for callers to “ask a legal question.” In the fine print one site notes that, “if you request any information you may receive a phone call or email from a partner law firm.”11 US physicians may be interested to note that a recent study of CP-based malpractice cases in China found that, although nearly 90% of the claims resulted in compensation, the mean damage award was $73,500.12 This was compared with a mean actual loss to the family of $128,200.
The interest by law firms in CP cases may be generated in part by the opportunity to assist a settlement or judgement that may be in the tens of millions of dollars. It is financially sensible to take a substantial risk on a contingency fee in a CP case compared with many other malpractice areas or claims where the likely damages are much lower. In addition, the vast majority of the damages in CP cases are for economic damages (cost of care and treatment and lost earning capacity), not noneconomic damages (pain and suffering). Therefore, the cap on noneconomic damages available in many states would not reduce the damages by a significant percentage.
CP cases are a significant part of the malpractice costs for ObGyns. Nearly one-third of obstetric claims are for neurologic injuries, including CP.13,14 These cases are often very complex and difficult, meaning that, in addition to the payments to the injured, there are considerable litigation costs associated with defending the cases. Perhaps as much as 60% of malpractice costs in obstetrics are in some way related to CP claims.15,16
Continue to: Negligence...
Negligence
Malpractice cases require not only damages (which clearly there are with CP) but also negligence and causation. (A more complete discussion of the elements of professional liability are included in a recent “What’s the Verdict?” column within
Several areas of negligence are common in CP related to delivery, including failure to monitor properly or ignoring, or not responding to, fetal heart rate (FHR) monitoring.18,19 For FHR monitoring, the claim is that problems can lead to asphyxia, resulting in HIE. Electronic fetal monitoring (EFM) has been an especially contentious matter. On one hand, the evidence of its efficacy is doubtful, but it has remained a standard practice, and it is often a centerpiece of delivery.20 Attorney Thomas Sartwelle has been prolific in suggesting that it not only has created legal problems for physicians but also results in unnecessary cesarean deliveries (CDs), which carry attendant risks for mother and infant.21 (It should be noted that other attorneys have expressed quite different views.22) He has argued that experts relying on EFM should be excluded from testifying because the technology is not based on sufficient science to meet the standard criteria used to determine the admissibility of expert witness (the Daubert standard).23 This argument is a difficult one so long as EFM is standard practice. Other claims of negligence include improper use of instruments at delivery, resulting in physical damage to the baby’s head, neck, or shoulders or internal hemorrhage. In addition, failure to deal with neonatal infection may be the basis for negligence.24
Causation
The question of whether or not the negligence (no matter how bad it was) caused the CP still needs to be addressed. Because a number of factors may cause CP, it has often been difficult to determine for any individual what the cause, or contributing causes, were. This fact would ordinarily work to the advantage of defendant-physicians and hospitals because the plaintiff in a malpractice case must prove by a preponderance of the evidence that the defendant’s negligence caused the CP. “Caused” is a term of art in the law; at the most basic level it means that the harm would not have occurred (or would have been less severe) but for the negligence.
In most CP cases the real cause is unknowable. It is, therefore, important to understand the difference between the certainty required in negligence cases and the certainty required in scientific studies (eg, 95% confidence). Negligence and causation in civil cases (including malpractice) must only be demonstrated by a preponderance of the evidence, which means “more likely than not.” For recovery in malpractice cases, states may require only that negligence be a “substantial factor.”
The theory that this lack of knowledge means that the plaintiff cannot prove causation, however, does not always hold.25 The following is what a jury might see: a child who will have a lifetime of medical, social, and financial burdens. Clear negligent practice by the physician coupled with severe injury can create considerable sympathy for the family. Then there are experts on both sides claiming that it is reasonably certain, in their opinions, that the injury was/was not caused by the negligence of the physician and health care team. The plaintiff’s witnesses will start eliminating other causes of CP in a form of differential diagnosis, stating that the remaining possibilities of causation clearly point to malpractice as the cause of CP. At some point, the elimination of alternative explanations for CP makes malpractice more likely than not to be a substantial factor in causing CP. On the other hand, the defense witnesses will stress that CP occurs most often without any negligence, and that, in this case, there are remaining, perhaps unknown, possible causes that are more likely than malpractice.
In this trial mix, it is not unthinkable that a jury or judge might find the plaintiff’s opinions more appealing. As a practical matter, and contrary to the technical rules, the burden of proof can seem to shift. The defendant clinician may, in effect, have to prove that the CP was caused by something other than the clinician’s negligence.
The role of insurance in award amounts
One reason that malpractice insurance companies settle CP cases for millions of dollars is that they face the possibility of judgements in the tens of millions. We saw even more than $100 million, in the 2019-2020 CP cases reported above. Another risk for malpractice insurance companies is that, if they do not settle, they may have liability beyond the policy limits. (Policy limits are the maximum an insurance policy is obligated to pay for any occurrence, or the total for all claims for the time covered by the premium.) For example, assume a malpractice policy has a $5 million policy limit covering Dr. Defendant, who has been sued for CP resulting from malpractice. There was apparently negligence during delivery in monitoring the fetus, but on the issue of causation the best estimate is that there is a 75% probability a jury would find no causal link between the negligence and the CP. If there is liability, damages would likely range from $5 to $25 million. Assume that the plaintiff has signaled it would settle for the policy limits ($5 million). Based purely on the odds and the policy limits, the insurance company should go to trial as opposed to settling for $5 million. That is because the physician personally (as opposed to the insurance company) is responsible for that part of a verdict that exceeds $5 million.
To prevent just such abuse (or bad faith), in most states, if the insurance company declines to settle the case for $5 million, it may become liable for the excess verdict above the policy limits. One reason that the cases that result in a verdict on damages—the 4 cases reported above for 2019‒2020—are interesting is that they help establish the risk of failing to settle a CP case.
Genetic understanding of causation
Given the importance of defendant-clinicians to be able to find a cause other than negligence to explain CP, the recent research of Moreno-De-Luca and colleagues may be especially meaningful.9 Using exome sequencing, the researchers found that 32.7% of pediatric-aged CP patients had pathogenic variance in the sequencing. In theory, this might mean that for about one-third of the CP plaintiffs, there may be genomic (rather than malpractice) explanations for CP, which might ultimately result in fewer cases of CP.
As significant as these findings are, caution is warranted. As the authors note, “this was an observational study and a causal relationship between detected gene variants and phenotypes in participants was not definitively established.”9 Until the causal relationship is established, it is not clear how much influence such a study would have in CP malpractice cases. Another caveat is that, at most, the genetic variants accounted for less than a third of CP cases studied, leaving many cases in which the cause remains unknown. In those cases in which a genomic association was not found, the case may be stronger for the “malpractice was the cause” claim. The follow-up research will likely shed light on some of these issues. Of course, if the genetic research demonstrates that in some proportion of cases there are genetic factors that contribute to the probability of CP, then the search will be for other triggering elements, which could possibly include poor care (that might well be a substantial factor for malpractice). Therefore, the preliminary genetic research likely represents only a part of the CP puzzle in malpractice cases.
Continue to: Why the opening case outcome was for the defense...
Why the opening case outcome was for the defense
Juries, of course, do not write opinions, so the basis for the jury’s decision in the example case is somewhat speculative. It seems most likely that causation had not been established. That is, the plaintiff-patient did not demonstrate that any malpractice was the likely, or substantial contributing, cause of the CP. The case illustrates several important issues.
Statute of limitations. This issue is common in CP cases because the condition may not be diagnosed for some time after birth. The statute of limitations can vary by state for medical malpractice cases “from 2 years to 22 years.”26 Many states begin with a 2-year statute but extend it if the injury or harm is not discovered. The extension is sometimes referred to as a statute of repose because, after that time, there is no extension even if the harm is discovered only later. In some states the statute does not run until the plaintiff is at or near the time of majority (usually age 18).27
Establishing negligence. The information provided about the presented case is mixed on the question of negligence, both regarding the hospital (through its nursing staff) for not properly contacting the obstetrician over the 10 hours, or the physician for inadequate monitoring. In addition, the reference to “really had to pull to deliver the head” may be the basis for claiming excessive, and potentially harmful use of force, which may have caused injury. In addition, the question remains whether the combination of these factors, including the Category III fetal heart tracing, made a cesarean delivery the appropriate standard of care.
Addressing causation. Assuming negligence, there is still a question of causation. It is far from clear that what the clinician did, or did not do, in terms of monitoring caused the CP injury. There is, however, no alternative causation that appeared in the case record, and this may be because of dueling expert witnesses.
The plaintiff sued both the obstetrician and the hospital, which is common among CP cases. While the legal interest of the two parties are aligned in some areas (causation), they may be in conflict in others (the failure of the hospital staff to keep the obstetrician informed). These potential conflicts are not for the clinicians to try to work out on their own. There is the potential for their actions to be misunderstood. When such a case is filed or threatened, the obstetrician should immediately discuss these matters with their attorney. In malpractice cases, malpractice insurance companies often select the attorneys who are experienced in such conflicts. If clinicians are not entirely comfortable that the appointed attorney is representing their interest and preserving a relationship with the hospital or other institution, however, they may engage their own legal counsel to protect their interests.
Practical considerations for avoiding malpractice claims
Good practices for avoiding malpractice claims apply with special force as it relates to CP.28,29
Uphold practice standards and good patient records. The causation element of these legal cases will remain problematic in the foreseeable future. But causation does not matter if negligent practice is not demonstrated. Therefore, maintaining best practices and continuous efforts at quality assurance and following all relevant professional practice guidelines is a good start. More than good intentions, it is essential that policies are implemented and reviewed. Among the areas of ongoing concern is the failure to monitor patients sufficiently. The long period of labor—where perhaps no physician is present for many hours—can introduce problems, as laypersons may have the impression that medical personnel were not on top of the situation.
Maintaining excellent records is also key for clinicians. The more complete the record, the fewer opportunities there are for faulty memories of parties and caregivers to fill in the gaps (especially when causation is so difficult to establish). Under absolutely no circumstances should records be changed or modified to eliminate damaging or an otherwise unfortunate notation. Few things are as harmful to credibility as discovered record tampering.
Inform patients of what is to come. Expectations are an important part of patient satisfaction. While not unduly frightening pregnant patients or eliminating reassurance, the informed consent process and patient counseling should be opportunities to avoid unreasonable expectations.
Stay alert to early genetic counseling, which is becoming increasingly available and important. Maintaining currency with what early testing can be done will become a critical part of ObGyn practice. For CP cases, in the near future, genetic testing may become part of determining causation. In the longer term, it will be part of counseling women and couples in deciding whether to have children, or potentially to end a pregnancy.
Expect the unexpected, and plan for it. Sometimes things just go wrong—there is a bad outcome, mistakes are made, patients are upset. It is important that any practice or institution have a clear plan for when such things happen. Some organizations have used apologies when appropriate,30 others have more complex plans for dealing with bad outcomes.31 Implement developed plans when they are needed. Individual practitioners also should consult with their attorney, who is familiar with their practice and who can help them maintain adherence to legal requirements and good legal problem prevention. ●
During a trial, all parties generally present evidence on negligence, causation, and damages. They do so without knowing whether a jury will find negligence and causation. The question of what the damages should be in cerebral palsy (CP) cases is also quite complex and expensive, but neither the defense nor the plaintiff can afford to ignore it. Past economic damages are relatively easy to calculate. Damages, for instance, includes medical care (pharmaceuticals and supplies, tests and procedures) and personal care (physical, occupational, and psychological therapy; long-term care; special educational costs; assistive equipment; and home modifications) that would have been avoided if it were not for CP. Future and personal care costs are more speculative, and must be estimated with the help of experts. In addition to future costs for the medical and personal care suggested above, depending on the state, the cost of lost future earnings (or earning capacity) may be additional economic damages. The cost of such intensive care, over a lifetime, accounts for many of the large verdicts and settlements.
Noneconomic damages are also available for such things as pain and suffering and diminished quality of life, both past and future. A number of states cap these noneconomic damages.
The wide range of damages correctly suggests that experts from several disciplines must be engaged to cover the damages landscape. This fact accounts for some of the costs of litigating these cases, and also for why damage calculations can be so complex.
- Fahey M, Macleenan A, Kretzschmar D, et al. The genetic basis of cerebral palsy. Dev Med Child Neurol. 2017;59:462-469. doi: 10.1111/dmcn.13363.
- Ellenberg J, Nelson, K. The association of cerebral palsy with birth asphyxia: a definitional quagmire. Dev Med Child Neurol. 2013;55:210-216. doi: 10.1111/dmcn.12016.
- Emrick L, DiCarlo S. The expanding role of genetics in cerebral palsy. Phys Med Rehabil Clin N Am. 2020;31:15-24. doi: 10.1016/j.pmr.2019.09.006.
- Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy [published correction appears in Dev Med Child Neurol. 2007;49:480]. Dev Med Child Neuro. 2007;109(suppl):8-14.
- MacLenan A, Lewis S, Moreno-DeLuca A, et al. Genetic or other causation should not change the clinical diagnosis of cerebral palsy. J Child Neurol. 2019;34:472-476. doi: 10.1177/0883073819840449.
- Lewis S, Shetty S, Wilson B, et al. Insights from genetic studies of cerebral palsy. Front Neurol. 2021;11:1-10. doi: 10.3389/fneur.2020.625428.
- Derick M, Drobyshevsky A, Ji X. A model of cerebral palsy from fetal hypoxia-ischemia. Stroke. 2007;38:731-735. doi: 10.1161/01.STR.0000251445.94697.64.
- McMichael G, Bainbridge M, Haan E, et al. Whole exome sequencing points to considerable genetic heterogeneity of cerebral palsy. Mol Psychiatry. 2015;20:176-182. doi: 10.1038/mp.2014.189.
- Moreno-DeLuca A, Milan F, Pesacreta D, et al. Molecular diagnostic yield of exome sequencing in patients with cerebral palsy. JAMA. 2021;325:467-475. doi: 10.1001/jama.2020.26148.
- Helping disabled children across Maryland & throughout the U.S. The Law Firm of Michael H. Bereston, Inc. website. https://www.berestonlaw.com/birth-injury/. Accessed April 26, 2021.
- Cerebral palsy lawsuits explained. Cerebral Palsy Guide website. https://www.cerebralpalsyguide.com/legal/. Accessed March 22, 2021.
- Zhou L, Li H, Li C, et al. Risk management and provider liabilities in infantile cerebral palsy based on malpractice litigation cases. J Forensic Leg Med. 2019;61:82-88. doi: 10.1016/j.jflm.2018.11.010.
- Cavanaugh MA. Bad cures for bad babies: policy challenges to the statutory removal of the common law claim for birth-related neurological injuries. Case West Res L Rev. 1992;43:1299-1346.
- Kain ZN, Caldwell-Andrews AA. What pediatricians should know about child-related malpractice payments in the United States. Pediatrics. 2006;118:464-468. doi: 10.1542/peds.2005-3112.
- Tabarrok A, Agan A. Medical malpractice awards, insurance, and negligence: which are related? Manhattan Institute Policy Research. Civil Justice Report; 2006. https://media4.manhattan-institute.org/pdf/cjr_10.pdf. Accessed April 27, 2021.
- Freeman AD, Freeman JM. No-fault cerebral palsy insurance: an alternative to the obstetrical malpractice lottery. J Health Politics Policy Law. 1989;14:707-718. doi: 10.1215/03616878-14-4-707.
- Sanfilippo JS, Smith SR. Is there liability if you don’t test for BRCA? OBG Manag. 2021;33:39-46. doi: 10.12788/obgm.0077.
- Fanaroff JM, Goldsmith JP. The most common patient safety issues resulting in legal action against neonatologists. Semin Perinatol. 2019;43:151181-1-9. doi: 10.1053/j.semperi.2019.08.010.
- Sartwelle TP, Johnston, JC. Cerebral palsy litigation: change course or abandon ship. J Child Neurol. 2015;30:828-841. doi: 10.1177/0883073814543306.
- Roth LM. The Business of Birth. NYU Press: New York, NY; 2021.
- Sartwelle TP. Electronic fetal monitoring: a bridge too far. J Legal Med. 2012;33:313-379. doi: 10.1080/01947648.2012.714321.
- Reiter JM, Walsh RS, Thomas EG. Best practices in birth injury litigation: timing hypoxic-ischemic fetal brain injury. Michigan Bar J. 2018;97:42-44.
- Sartwelle TP. Defending a neurologic birth injury: asphyxia neonatorum redux. J Legal Med. 2009;30:181-247. doi: 10.1080/01947640902936522.
- Daubert v Merrell Dow Pharm, Inc. 509 U.S. 579 (1993).
- Jha S. The factors making Americans litigious. J Am College Radiology. 2019;17:551-553. doi: 10.1016/j.jacr.2019.10.011.
- Salvi S, Pritchard PC. Statute of limitations on cerebral palsy cases. Personal Injury Lawyers website. https://www.salvilaw.com/birth-injury-lawyers/cerebral-palsy/time-limits/. Accessed March 24, 2021.
- Wharton R. Cerebral palsy statute of limitations. Cerebral Palsy Guidance website. October 16, 2020. https://www.cerebralpalsyguidance.com/cerebral-palsy-lawyer/statute-of-limitations/. Accessed March 24, 2021.
- Kassim PJ, Ushiro S, Najid KM. Compensating cerebral palsy cases: problems in court litigation and the no-fault alternative. Med Law. 2015;34:335-355.
- Williams D. Practice patterns to decrease the risk of malpractice suit. Clin Obstet Gynecol. 2008;51:680-687. doi: 10.1097/GRF.0b013e3181899bc7.
- McMichael BJ, Van Horn RL, Viscusi WK. “Sorry” is never enough: how state apology laws fail to reduce medical malpractice liability risk. Stanford Law Rev. 2019;71:341-409.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221. doi: 10.7326/0003-4819-153-4-201008170-00002.

CASE Mixed CP diagnosed at age 6 months
After learning that the statute of limitations was to run out in the near future, the parents of a 17-year-old with cerebral palsy (CP) initiated a lawsuit. At the time of her pregnancy, the mother (G2P2002) was age 39 and first sought prenatal care at 14 weeks.
Her past medical history was largely noncontributory to her current pregnancy, except for that she had hypothyroidism that was being treated with levothyroxine. She also had a history of asthma, but had had no acute episodes for years. During the course of the pregnancy there was evidence of polyhydramnios; her initial thyroid studies were abnormal (thyroid-stimulating hormone levels, 7.1 mIU/L), in part due to lack of adherence with prescribed medications. She was noted to have elevated blood pressure (BP) 150/100 mm Hg but no proteinuria, with BP monitoring during her last trimester.
The patient went into labor at 40 3/7 weeks, after spontaneous rupture of membranes. In labor and delivery she was placed on a monitor, and irregular contractions were noted. The initial vaginal examination was noted as 1-cm cervical dilation, 90% effaced, and station zero. The obstetrician evaluated the patient and ordered Pitocin augmentation. The next vaginal exam several hours later noted 3-cm dilation and 100% effacement. The Pitocin was continued. Several early decelerations, moderate variability, and better contraction pattern was noted. Eight hours into the Pitocin, there were repetitive late decelerations; the obstetrician was not notified. The nursing staff proceeded with vaginal examination, and the patient was fully dilated at station +1. Again, the doctor was not informed of the patient’s status. At 10 hours post-Pitocin initiation, the patient felt the urge to push. The obstetrician was notified, and he promptly arrived to the unit and patient’s bedside. His decision was to use forceps for the delivery, feeling this would be the most expedient way to proceed, although cesarean delivery (CD) was a definite consideration. Forceps were applied, and as the nursing staff noted,” the doctor really had to pull to deliver the head.” A male baby, 8 lb 8 oz, was delivered. A second-degree tear was noted and easily repaired following delivery of the placenta. Apgar scores were 5 and 7 at 1 and 5 minutes after birth, respectively.
The patient’s postpartum course was uneventful. The patient and baby were discharged on the third day postpartum.
As the child was evaluated by the pediatrician, the mother noted at 6 months that the child’s head lagged behind when he was picked up. He appeared stiff at times and floppy at other times according to the parents. As the child progressed he had problems with hand-to-mouth coordination, and when he would crawl he seemed to “scoot his butt,” as they stated.
The child was tested and a diagnosis of mixed cerebral palsy was made, implying a combination of spastic CP and dyskinetic CP. He is wheelchair bound. The parents filed a lawsuit against the obstetrician and the hospital, focused on hypoxic-ischemic encephalopathy (HIE) due to labor and delivery management being below the standard of care. They claimed that the obstetrician should have been informed by the hospital staff during the course of labor, and the obstetrician should have been more proactive in monitoring the deteriorating circumstances. This included performing a CD based on “the Category III fetal heart tracing.”
At trial, the plaintiff expert argued that failure of nursing staff to properly communicate with the obstetrician led to mismanagement. Furthermore, the obstetrician used poor judgement (ie, below the standard of care) in not performing a CD. The defense expert argued that, overall, the fetal heart tracing was Category II, and the events occurred in utero, in part reflected by the mother having polyhydramnios and hypothyroidism that was not well controlled due to lack of adherence with prescribed medications. The child in his wheelchair was brought into the courtroom. The trial went on for more than 1 week, and the jury deliberated for several hours. (Note: This case is a composite of several different events and claims.)
Continue to: WHAT’S THE VERDICT?
WHAT’S THE VERDICT?
The jury returns a verdict for the defense.
Should anything have been done differently in this trial?
Medical considerations
Cerebral palsy is a neurodevelopmental disorder affecting 1 in 500 children.1 Other prevalence data (from a European study) indicate an incidence of 1.3–1.9 cases per 1,000 livebirths.1 The controversy continues with respect to the disorder’s etiology, especially when the infant’s magnetic resonance imaging (MRI) does not identify specific pathology. The finger is then pointed at HIE and thus the fault of the obstetrician and labor and delivery staff. In reality, HIE accounts for less than 10% of all cases of CP.2 Overall, CP is a condition focused on progressive motor impairments, many times associated with specific MRI findings.3 In addition, “MRI-negative” CP is a more vague diagnosis as discussed among neurologists.
The International Consensus Definition of CP is “a group of permanent disorders of the development of movement and posture, causing activity limitations, that are attributed to nonprogressive disturbances that occurred in the developing fetal or infant brain.”4 The International Cerebral Palsy Genomics Consortium have provided a consensus statement that defines CP based upon clinical type as opposed to etiology.5 Many times, however, ascribing an HIE cause to CP is “barking up the wrong tree,” in that we now know there are clear cut genetic causes of CP, and etiology attributed to perinatal causes, in reality, are genetic in up to 80% of cases.3 Types of CP are addressed in FIGURE 1. Overall, the pathophysiology of the disorder remains unknown. Some affected children have intellectual disabilities, as well as visual, hearing, and/or speech impairment.

A number of risk factors have been associated with CP (TABLE 1),3,6 which contribute to cell death in the brain or altered maturation of neurons and glia, resulting in abnormal white matter tracts and smaller central nervous system (CNS) volume or cerebellar hypoxia.6 One very important aspect of assessment for CP is specific gene mutations, which may vary in part dependent upon the presence or absence of environmental factors (insults).1 Mutations can lead to profound adverse effects with resultant CNS ischemia and neuromotor disability. In fact, genetics play a major role in determining the etiology of CP.1 Of interest, animal models who are subject to HIE induction have CNS effects resulting in permanent motor impairment.7

DNA sequencing
The DNA story continues to unfold with the concept that DNA variants alter susceptibility to environmental influences. These insults are, for example, thrombosis or hemorrhage, all of which affect motor function.1 Duplications or deletions of portions of a chromosome, related to copy number variants (CNVs) as well as advances in human-genome sequencing, can identify a single gene mutation leading to CP.1 Microdeletions, microduplications, and single nucleotide variants (SNVs) are to be included in genetic-related problems causing CP.3
A number of candidate genes have been considered and include “de novo heterozygous mutations in known Online Mendelian Inheritance (OMIM).” TIBA1A and SCN8A genes are highly associated with CP.8 Genetic assessment, as it evolves and more recently with the advent of exome sequencing, appears to provide a new and unprecedented level of understanding of CP. Specifically, exome sequencing provides a diagnostic tool with which to identify the prevalence of pathogenic and pathogenic variants (the latter encompassing genomic variants) with CP.9 A retrospective study assessed a cohort of patients with CP and noted that 32.7% of the pediatric-aged patients who underwent exome sequencing had pathogenic and pathogenic variants in the sequencing.9 Thus, we have a tool to identify underlying genetic pathogenesis with CP. This theoretically can change the outcome of lawsuits initiated for CP that ascribe an HIE etiology. Clinicians need to stay tuned as the genetic repertoire continues to unfold.
Continue to: Legal considerations...
Legal considerations
Although CP is not a common event, it has been a major factor in the total malpractice payments for ObGyns, neonatologists, and related medical disciplines. That is because the per-event liability can be staggering. Some law firms provide a “checklist” for plaintiffs early on in assessing a potential case (FIGURE 2).10

The financial risks and incentives
To understand what the current settlements and verdicts are in birth-related CP cases, a search of Lexis files revealed the reported outcomes of cases in 2019 and 2020 (FIGURE 3). Taking into account that the pandemic limited legal activity, 23 unduplicated cases were described with a reported settlement or verdict. Four cases resulted in verdicts for the injured patients, with the mean of these awards substantially higher than the settlements ($88.3 million vs $11.1 million, respectively).

These numbers are a glimpse at some of the very high settlements and verdicts that are common in CP cases. Notably, these are not a random sample of CP cases, but only those with the amount of the verdict or settlement reported. Potentially tried cases that may have been simply abandoned or dismissed are not reported. Furthermore, most settlements include confidentiality clauses, which may preclude the release of the financial value of the settlement. Cases in which the defense won (for example, a jury verdict in favor of the physician) are not included.
The high monetary awards in some CP cases are indirectly backed by Google search results for “cerebral palsy and liability” or “cerebral palsy and malpractice.” A very large number of results for law firms seeking clients with CP injuries is produced. Some of the websites note that only 10% (or 20% on some sites) of CP cases are caused by medical negligence, offering a “free legal case review” and a phone number for callers to “ask a legal question.” In the fine print one site notes that, “if you request any information you may receive a phone call or email from a partner law firm.”11 US physicians may be interested to note that a recent study of CP-based malpractice cases in China found that, although nearly 90% of the claims resulted in compensation, the mean damage award was $73,500.12 This was compared with a mean actual loss to the family of $128,200.
The interest by law firms in CP cases may be generated in part by the opportunity to assist a settlement or judgement that may be in the tens of millions of dollars. It is financially sensible to take a substantial risk on a contingency fee in a CP case compared with many other malpractice areas or claims where the likely damages are much lower. In addition, the vast majority of the damages in CP cases are for economic damages (cost of care and treatment and lost earning capacity), not noneconomic damages (pain and suffering). Therefore, the cap on noneconomic damages available in many states would not reduce the damages by a significant percentage.
CP cases are a significant part of the malpractice costs for ObGyns. Nearly one-third of obstetric claims are for neurologic injuries, including CP.13,14 These cases are often very complex and difficult, meaning that, in addition to the payments to the injured, there are considerable litigation costs associated with defending the cases. Perhaps as much as 60% of malpractice costs in obstetrics are in some way related to CP claims.15,16
Continue to: Negligence...
Negligence
Malpractice cases require not only damages (which clearly there are with CP) but also negligence and causation. (A more complete discussion of the elements of professional liability are included in a recent “What’s the Verdict?” column within
Several areas of negligence are common in CP related to delivery, including failure to monitor properly or ignoring, or not responding to, fetal heart rate (FHR) monitoring.18,19 For FHR monitoring, the claim is that problems can lead to asphyxia, resulting in HIE. Electronic fetal monitoring (EFM) has been an especially contentious matter. On one hand, the evidence of its efficacy is doubtful, but it has remained a standard practice, and it is often a centerpiece of delivery.20 Attorney Thomas Sartwelle has been prolific in suggesting that it not only has created legal problems for physicians but also results in unnecessary cesarean deliveries (CDs), which carry attendant risks for mother and infant.21 (It should be noted that other attorneys have expressed quite different views.22) He has argued that experts relying on EFM should be excluded from testifying because the technology is not based on sufficient science to meet the standard criteria used to determine the admissibility of expert witness (the Daubert standard).23 This argument is a difficult one so long as EFM is standard practice. Other claims of negligence include improper use of instruments at delivery, resulting in physical damage to the baby’s head, neck, or shoulders or internal hemorrhage. In addition, failure to deal with neonatal infection may be the basis for negligence.24
Causation
The question of whether or not the negligence (no matter how bad it was) caused the CP still needs to be addressed. Because a number of factors may cause CP, it has often been difficult to determine for any individual what the cause, or contributing causes, were. This fact would ordinarily work to the advantage of defendant-physicians and hospitals because the plaintiff in a malpractice case must prove by a preponderance of the evidence that the defendant’s negligence caused the CP. “Caused” is a term of art in the law; at the most basic level it means that the harm would not have occurred (or would have been less severe) but for the negligence.
In most CP cases the real cause is unknowable. It is, therefore, important to understand the difference between the certainty required in negligence cases and the certainty required in scientific studies (eg, 95% confidence). Negligence and causation in civil cases (including malpractice) must only be demonstrated by a preponderance of the evidence, which means “more likely than not.” For recovery in malpractice cases, states may require only that negligence be a “substantial factor.”
The theory that this lack of knowledge means that the plaintiff cannot prove causation, however, does not always hold.25 The following is what a jury might see: a child who will have a lifetime of medical, social, and financial burdens. Clear negligent practice by the physician coupled with severe injury can create considerable sympathy for the family. Then there are experts on both sides claiming that it is reasonably certain, in their opinions, that the injury was/was not caused by the negligence of the physician and health care team. The plaintiff’s witnesses will start eliminating other causes of CP in a form of differential diagnosis, stating that the remaining possibilities of causation clearly point to malpractice as the cause of CP. At some point, the elimination of alternative explanations for CP makes malpractice more likely than not to be a substantial factor in causing CP. On the other hand, the defense witnesses will stress that CP occurs most often without any negligence, and that, in this case, there are remaining, perhaps unknown, possible causes that are more likely than malpractice.
In this trial mix, it is not unthinkable that a jury or judge might find the plaintiff’s opinions more appealing. As a practical matter, and contrary to the technical rules, the burden of proof can seem to shift. The defendant clinician may, in effect, have to prove that the CP was caused by something other than the clinician’s negligence.
The role of insurance in award amounts
One reason that malpractice insurance companies settle CP cases for millions of dollars is that they face the possibility of judgements in the tens of millions. We saw even more than $100 million, in the 2019-2020 CP cases reported above. Another risk for malpractice insurance companies is that, if they do not settle, they may have liability beyond the policy limits. (Policy limits are the maximum an insurance policy is obligated to pay for any occurrence, or the total for all claims for the time covered by the premium.) For example, assume a malpractice policy has a $5 million policy limit covering Dr. Defendant, who has been sued for CP resulting from malpractice. There was apparently negligence during delivery in monitoring the fetus, but on the issue of causation the best estimate is that there is a 75% probability a jury would find no causal link between the negligence and the CP. If there is liability, damages would likely range from $5 to $25 million. Assume that the plaintiff has signaled it would settle for the policy limits ($5 million). Based purely on the odds and the policy limits, the insurance company should go to trial as opposed to settling for $5 million. That is because the physician personally (as opposed to the insurance company) is responsible for that part of a verdict that exceeds $5 million.
To prevent just such abuse (or bad faith), in most states, if the insurance company declines to settle the case for $5 million, it may become liable for the excess verdict above the policy limits. One reason that the cases that result in a verdict on damages—the 4 cases reported above for 2019‒2020—are interesting is that they help establish the risk of failing to settle a CP case.
Genetic understanding of causation
Given the importance of defendant-clinicians to be able to find a cause other than negligence to explain CP, the recent research of Moreno-De-Luca and colleagues may be especially meaningful.9 Using exome sequencing, the researchers found that 32.7% of pediatric-aged CP patients had pathogenic variance in the sequencing. In theory, this might mean that for about one-third of the CP plaintiffs, there may be genomic (rather than malpractice) explanations for CP, which might ultimately result in fewer cases of CP.
As significant as these findings are, caution is warranted. As the authors note, “this was an observational study and a causal relationship between detected gene variants and phenotypes in participants was not definitively established.”9 Until the causal relationship is established, it is not clear how much influence such a study would have in CP malpractice cases. Another caveat is that, at most, the genetic variants accounted for less than a third of CP cases studied, leaving many cases in which the cause remains unknown. In those cases in which a genomic association was not found, the case may be stronger for the “malpractice was the cause” claim. The follow-up research will likely shed light on some of these issues. Of course, if the genetic research demonstrates that in some proportion of cases there are genetic factors that contribute to the probability of CP, then the search will be for other triggering elements, which could possibly include poor care (that might well be a substantial factor for malpractice). Therefore, the preliminary genetic research likely represents only a part of the CP puzzle in malpractice cases.
Continue to: Why the opening case outcome was for the defense...
Why the opening case outcome was for the defense
Juries, of course, do not write opinions, so the basis for the jury’s decision in the example case is somewhat speculative. It seems most likely that causation had not been established. That is, the plaintiff-patient did not demonstrate that any malpractice was the likely, or substantial contributing, cause of the CP. The case illustrates several important issues.
Statute of limitations. This issue is common in CP cases because the condition may not be diagnosed for some time after birth. The statute of limitations can vary by state for medical malpractice cases “from 2 years to 22 years.”26 Many states begin with a 2-year statute but extend it if the injury or harm is not discovered. The extension is sometimes referred to as a statute of repose because, after that time, there is no extension even if the harm is discovered only later. In some states the statute does not run until the plaintiff is at or near the time of majority (usually age 18).27
Establishing negligence. The information provided about the presented case is mixed on the question of negligence, both regarding the hospital (through its nursing staff) for not properly contacting the obstetrician over the 10 hours, or the physician for inadequate monitoring. In addition, the reference to “really had to pull to deliver the head” may be the basis for claiming excessive, and potentially harmful use of force, which may have caused injury. In addition, the question remains whether the combination of these factors, including the Category III fetal heart tracing, made a cesarean delivery the appropriate standard of care.
Addressing causation. Assuming negligence, there is still a question of causation. It is far from clear that what the clinician did, or did not do, in terms of monitoring caused the CP injury. There is, however, no alternative causation that appeared in the case record, and this may be because of dueling expert witnesses.
The plaintiff sued both the obstetrician and the hospital, which is common among CP cases. While the legal interest of the two parties are aligned in some areas (causation), they may be in conflict in others (the failure of the hospital staff to keep the obstetrician informed). These potential conflicts are not for the clinicians to try to work out on their own. There is the potential for their actions to be misunderstood. When such a case is filed or threatened, the obstetrician should immediately discuss these matters with their attorney. In malpractice cases, malpractice insurance companies often select the attorneys who are experienced in such conflicts. If clinicians are not entirely comfortable that the appointed attorney is representing their interest and preserving a relationship with the hospital or other institution, however, they may engage their own legal counsel to protect their interests.
Practical considerations for avoiding malpractice claims
Good practices for avoiding malpractice claims apply with special force as it relates to CP.28,29
Uphold practice standards and good patient records. The causation element of these legal cases will remain problematic in the foreseeable future. But causation does not matter if negligent practice is not demonstrated. Therefore, maintaining best practices and continuous efforts at quality assurance and following all relevant professional practice guidelines is a good start. More than good intentions, it is essential that policies are implemented and reviewed. Among the areas of ongoing concern is the failure to monitor patients sufficiently. The long period of labor—where perhaps no physician is present for many hours—can introduce problems, as laypersons may have the impression that medical personnel were not on top of the situation.
Maintaining excellent records is also key for clinicians. The more complete the record, the fewer opportunities there are for faulty memories of parties and caregivers to fill in the gaps (especially when causation is so difficult to establish). Under absolutely no circumstances should records be changed or modified to eliminate damaging or an otherwise unfortunate notation. Few things are as harmful to credibility as discovered record tampering.
Inform patients of what is to come. Expectations are an important part of patient satisfaction. While not unduly frightening pregnant patients or eliminating reassurance, the informed consent process and patient counseling should be opportunities to avoid unreasonable expectations.
Stay alert to early genetic counseling, which is becoming increasingly available and important. Maintaining currency with what early testing can be done will become a critical part of ObGyn practice. For CP cases, in the near future, genetic testing may become part of determining causation. In the longer term, it will be part of counseling women and couples in deciding whether to have children, or potentially to end a pregnancy.
Expect the unexpected, and plan for it. Sometimes things just go wrong—there is a bad outcome, mistakes are made, patients are upset. It is important that any practice or institution have a clear plan for when such things happen. Some organizations have used apologies when appropriate,30 others have more complex plans for dealing with bad outcomes.31 Implement developed plans when they are needed. Individual practitioners also should consult with their attorney, who is familiar with their practice and who can help them maintain adherence to legal requirements and good legal problem prevention. ●
During a trial, all parties generally present evidence on negligence, causation, and damages. They do so without knowing whether a jury will find negligence and causation. The question of what the damages should be in cerebral palsy (CP) cases is also quite complex and expensive, but neither the defense nor the plaintiff can afford to ignore it. Past economic damages are relatively easy to calculate. Damages, for instance, includes medical care (pharmaceuticals and supplies, tests and procedures) and personal care (physical, occupational, and psychological therapy; long-term care; special educational costs; assistive equipment; and home modifications) that would have been avoided if it were not for CP. Future and personal care costs are more speculative, and must be estimated with the help of experts. In addition to future costs for the medical and personal care suggested above, depending on the state, the cost of lost future earnings (or earning capacity) may be additional economic damages. The cost of such intensive care, over a lifetime, accounts for many of the large verdicts and settlements.
Noneconomic damages are also available for such things as pain and suffering and diminished quality of life, both past and future. A number of states cap these noneconomic damages.
The wide range of damages correctly suggests that experts from several disciplines must be engaged to cover the damages landscape. This fact accounts for some of the costs of litigating these cases, and also for why damage calculations can be so complex.

CASE Mixed CP diagnosed at age 6 months
After learning that the statute of limitations was to run out in the near future, the parents of a 17-year-old with cerebral palsy (CP) initiated a lawsuit. At the time of her pregnancy, the mother (G2P2002) was age 39 and first sought prenatal care at 14 weeks.
Her past medical history was largely noncontributory to her current pregnancy, except for that she had hypothyroidism that was being treated with levothyroxine. She also had a history of asthma, but had had no acute episodes for years. During the course of the pregnancy there was evidence of polyhydramnios; her initial thyroid studies were abnormal (thyroid-stimulating hormone levels, 7.1 mIU/L), in part due to lack of adherence with prescribed medications. She was noted to have elevated blood pressure (BP) 150/100 mm Hg but no proteinuria, with BP monitoring during her last trimester.
The patient went into labor at 40 3/7 weeks, after spontaneous rupture of membranes. In labor and delivery she was placed on a monitor, and irregular contractions were noted. The initial vaginal examination was noted as 1-cm cervical dilation, 90% effaced, and station zero. The obstetrician evaluated the patient and ordered Pitocin augmentation. The next vaginal exam several hours later noted 3-cm dilation and 100% effacement. The Pitocin was continued. Several early decelerations, moderate variability, and better contraction pattern was noted. Eight hours into the Pitocin, there were repetitive late decelerations; the obstetrician was not notified. The nursing staff proceeded with vaginal examination, and the patient was fully dilated at station +1. Again, the doctor was not informed of the patient’s status. At 10 hours post-Pitocin initiation, the patient felt the urge to push. The obstetrician was notified, and he promptly arrived to the unit and patient’s bedside. His decision was to use forceps for the delivery, feeling this would be the most expedient way to proceed, although cesarean delivery (CD) was a definite consideration. Forceps were applied, and as the nursing staff noted,” the doctor really had to pull to deliver the head.” A male baby, 8 lb 8 oz, was delivered. A second-degree tear was noted and easily repaired following delivery of the placenta. Apgar scores were 5 and 7 at 1 and 5 minutes after birth, respectively.
The patient’s postpartum course was uneventful. The patient and baby were discharged on the third day postpartum.
As the child was evaluated by the pediatrician, the mother noted at 6 months that the child’s head lagged behind when he was picked up. He appeared stiff at times and floppy at other times according to the parents. As the child progressed he had problems with hand-to-mouth coordination, and when he would crawl he seemed to “scoot his butt,” as they stated.
The child was tested and a diagnosis of mixed cerebral palsy was made, implying a combination of spastic CP and dyskinetic CP. He is wheelchair bound. The parents filed a lawsuit against the obstetrician and the hospital, focused on hypoxic-ischemic encephalopathy (HIE) due to labor and delivery management being below the standard of care. They claimed that the obstetrician should have been informed by the hospital staff during the course of labor, and the obstetrician should have been more proactive in monitoring the deteriorating circumstances. This included performing a CD based on “the Category III fetal heart tracing.”
At trial, the plaintiff expert argued that failure of nursing staff to properly communicate with the obstetrician led to mismanagement. Furthermore, the obstetrician used poor judgement (ie, below the standard of care) in not performing a CD. The defense expert argued that, overall, the fetal heart tracing was Category II, and the events occurred in utero, in part reflected by the mother having polyhydramnios and hypothyroidism that was not well controlled due to lack of adherence with prescribed medications. The child in his wheelchair was brought into the courtroom. The trial went on for more than 1 week, and the jury deliberated for several hours. (Note: This case is a composite of several different events and claims.)
Continue to: WHAT’S THE VERDICT?
WHAT’S THE VERDICT?
The jury returns a verdict for the defense.
Should anything have been done differently in this trial?
Medical considerations
Cerebral palsy is a neurodevelopmental disorder affecting 1 in 500 children.1 Other prevalence data (from a European study) indicate an incidence of 1.3–1.9 cases per 1,000 livebirths.1 The controversy continues with respect to the disorder’s etiology, especially when the infant’s magnetic resonance imaging (MRI) does not identify specific pathology. The finger is then pointed at HIE and thus the fault of the obstetrician and labor and delivery staff. In reality, HIE accounts for less than 10% of all cases of CP.2 Overall, CP is a condition focused on progressive motor impairments, many times associated with specific MRI findings.3 In addition, “MRI-negative” CP is a more vague diagnosis as discussed among neurologists.
The International Consensus Definition of CP is “a group of permanent disorders of the development of movement and posture, causing activity limitations, that are attributed to nonprogressive disturbances that occurred in the developing fetal or infant brain.”4 The International Cerebral Palsy Genomics Consortium have provided a consensus statement that defines CP based upon clinical type as opposed to etiology.5 Many times, however, ascribing an HIE cause to CP is “barking up the wrong tree,” in that we now know there are clear cut genetic causes of CP, and etiology attributed to perinatal causes, in reality, are genetic in up to 80% of cases.3 Types of CP are addressed in FIGURE 1. Overall, the pathophysiology of the disorder remains unknown. Some affected children have intellectual disabilities, as well as visual, hearing, and/or speech impairment.

A number of risk factors have been associated with CP (TABLE 1),3,6 which contribute to cell death in the brain or altered maturation of neurons and glia, resulting in abnormal white matter tracts and smaller central nervous system (CNS) volume or cerebellar hypoxia.6 One very important aspect of assessment for CP is specific gene mutations, which may vary in part dependent upon the presence or absence of environmental factors (insults).1 Mutations can lead to profound adverse effects with resultant CNS ischemia and neuromotor disability. In fact, genetics play a major role in determining the etiology of CP.1 Of interest, animal models who are subject to HIE induction have CNS effects resulting in permanent motor impairment.7

DNA sequencing
The DNA story continues to unfold with the concept that DNA variants alter susceptibility to environmental influences. These insults are, for example, thrombosis or hemorrhage, all of which affect motor function.1 Duplications or deletions of portions of a chromosome, related to copy number variants (CNVs) as well as advances in human-genome sequencing, can identify a single gene mutation leading to CP.1 Microdeletions, microduplications, and single nucleotide variants (SNVs) are to be included in genetic-related problems causing CP.3
A number of candidate genes have been considered and include “de novo heterozygous mutations in known Online Mendelian Inheritance (OMIM).” TIBA1A and SCN8A genes are highly associated with CP.8 Genetic assessment, as it evolves and more recently with the advent of exome sequencing, appears to provide a new and unprecedented level of understanding of CP. Specifically, exome sequencing provides a diagnostic tool with which to identify the prevalence of pathogenic and pathogenic variants (the latter encompassing genomic variants) with CP.9 A retrospective study assessed a cohort of patients with CP and noted that 32.7% of the pediatric-aged patients who underwent exome sequencing had pathogenic and pathogenic variants in the sequencing.9 Thus, we have a tool to identify underlying genetic pathogenesis with CP. This theoretically can change the outcome of lawsuits initiated for CP that ascribe an HIE etiology. Clinicians need to stay tuned as the genetic repertoire continues to unfold.
Continue to: Legal considerations...
Legal considerations
Although CP is not a common event, it has been a major factor in the total malpractice payments for ObGyns, neonatologists, and related medical disciplines. That is because the per-event liability can be staggering. Some law firms provide a “checklist” for plaintiffs early on in assessing a potential case (FIGURE 2).10

The financial risks and incentives
To understand what the current settlements and verdicts are in birth-related CP cases, a search of Lexis files revealed the reported outcomes of cases in 2019 and 2020 (FIGURE 3). Taking into account that the pandemic limited legal activity, 23 unduplicated cases were described with a reported settlement or verdict. Four cases resulted in verdicts for the injured patients, with the mean of these awards substantially higher than the settlements ($88.3 million vs $11.1 million, respectively).

These numbers are a glimpse at some of the very high settlements and verdicts that are common in CP cases. Notably, these are not a random sample of CP cases, but only those with the amount of the verdict or settlement reported. Potentially tried cases that may have been simply abandoned or dismissed are not reported. Furthermore, most settlements include confidentiality clauses, which may preclude the release of the financial value of the settlement. Cases in which the defense won (for example, a jury verdict in favor of the physician) are not included.
The high monetary awards in some CP cases are indirectly backed by Google search results for “cerebral palsy and liability” or “cerebral palsy and malpractice.” A very large number of results for law firms seeking clients with CP injuries is produced. Some of the websites note that only 10% (or 20% on some sites) of CP cases are caused by medical negligence, offering a “free legal case review” and a phone number for callers to “ask a legal question.” In the fine print one site notes that, “if you request any information you may receive a phone call or email from a partner law firm.”11 US physicians may be interested to note that a recent study of CP-based malpractice cases in China found that, although nearly 90% of the claims resulted in compensation, the mean damage award was $73,500.12 This was compared with a mean actual loss to the family of $128,200.
The interest by law firms in CP cases may be generated in part by the opportunity to assist a settlement or judgement that may be in the tens of millions of dollars. It is financially sensible to take a substantial risk on a contingency fee in a CP case compared with many other malpractice areas or claims where the likely damages are much lower. In addition, the vast majority of the damages in CP cases are for economic damages (cost of care and treatment and lost earning capacity), not noneconomic damages (pain and suffering). Therefore, the cap on noneconomic damages available in many states would not reduce the damages by a significant percentage.
CP cases are a significant part of the malpractice costs for ObGyns. Nearly one-third of obstetric claims are for neurologic injuries, including CP.13,14 These cases are often very complex and difficult, meaning that, in addition to the payments to the injured, there are considerable litigation costs associated with defending the cases. Perhaps as much as 60% of malpractice costs in obstetrics are in some way related to CP claims.15,16
Continue to: Negligence...
Negligence
Malpractice cases require not only damages (which clearly there are with CP) but also negligence and causation. (A more complete discussion of the elements of professional liability are included in a recent “What’s the Verdict?” column within
Several areas of negligence are common in CP related to delivery, including failure to monitor properly or ignoring, or not responding to, fetal heart rate (FHR) monitoring.18,19 For FHR monitoring, the claim is that problems can lead to asphyxia, resulting in HIE. Electronic fetal monitoring (EFM) has been an especially contentious matter. On one hand, the evidence of its efficacy is doubtful, but it has remained a standard practice, and it is often a centerpiece of delivery.20 Attorney Thomas Sartwelle has been prolific in suggesting that it not only has created legal problems for physicians but also results in unnecessary cesarean deliveries (CDs), which carry attendant risks for mother and infant.21 (It should be noted that other attorneys have expressed quite different views.22) He has argued that experts relying on EFM should be excluded from testifying because the technology is not based on sufficient science to meet the standard criteria used to determine the admissibility of expert witness (the Daubert standard).23 This argument is a difficult one so long as EFM is standard practice. Other claims of negligence include improper use of instruments at delivery, resulting in physical damage to the baby’s head, neck, or shoulders or internal hemorrhage. In addition, failure to deal with neonatal infection may be the basis for negligence.24
Causation
The question of whether or not the negligence (no matter how bad it was) caused the CP still needs to be addressed. Because a number of factors may cause CP, it has often been difficult to determine for any individual what the cause, or contributing causes, were. This fact would ordinarily work to the advantage of defendant-physicians and hospitals because the plaintiff in a malpractice case must prove by a preponderance of the evidence that the defendant’s negligence caused the CP. “Caused” is a term of art in the law; at the most basic level it means that the harm would not have occurred (or would have been less severe) but for the negligence.
In most CP cases the real cause is unknowable. It is, therefore, important to understand the difference between the certainty required in negligence cases and the certainty required in scientific studies (eg, 95% confidence). Negligence and causation in civil cases (including malpractice) must only be demonstrated by a preponderance of the evidence, which means “more likely than not.” For recovery in malpractice cases, states may require only that negligence be a “substantial factor.”
The theory that this lack of knowledge means that the plaintiff cannot prove causation, however, does not always hold.25 The following is what a jury might see: a child who will have a lifetime of medical, social, and financial burdens. Clear negligent practice by the physician coupled with severe injury can create considerable sympathy for the family. Then there are experts on both sides claiming that it is reasonably certain, in their opinions, that the injury was/was not caused by the negligence of the physician and health care team. The plaintiff’s witnesses will start eliminating other causes of CP in a form of differential diagnosis, stating that the remaining possibilities of causation clearly point to malpractice as the cause of CP. At some point, the elimination of alternative explanations for CP makes malpractice more likely than not to be a substantial factor in causing CP. On the other hand, the defense witnesses will stress that CP occurs most often without any negligence, and that, in this case, there are remaining, perhaps unknown, possible causes that are more likely than malpractice.
In this trial mix, it is not unthinkable that a jury or judge might find the plaintiff’s opinions more appealing. As a practical matter, and contrary to the technical rules, the burden of proof can seem to shift. The defendant clinician may, in effect, have to prove that the CP was caused by something other than the clinician’s negligence.
The role of insurance in award amounts
One reason that malpractice insurance companies settle CP cases for millions of dollars is that they face the possibility of judgements in the tens of millions. We saw even more than $100 million, in the 2019-2020 CP cases reported above. Another risk for malpractice insurance companies is that, if they do not settle, they may have liability beyond the policy limits. (Policy limits are the maximum an insurance policy is obligated to pay for any occurrence, or the total for all claims for the time covered by the premium.) For example, assume a malpractice policy has a $5 million policy limit covering Dr. Defendant, who has been sued for CP resulting from malpractice. There was apparently negligence during delivery in monitoring the fetus, but on the issue of causation the best estimate is that there is a 75% probability a jury would find no causal link between the negligence and the CP. If there is liability, damages would likely range from $5 to $25 million. Assume that the plaintiff has signaled it would settle for the policy limits ($5 million). Based purely on the odds and the policy limits, the insurance company should go to trial as opposed to settling for $5 million. That is because the physician personally (as opposed to the insurance company) is responsible for that part of a verdict that exceeds $5 million.
To prevent just such abuse (or bad faith), in most states, if the insurance company declines to settle the case for $5 million, it may become liable for the excess verdict above the policy limits. One reason that the cases that result in a verdict on damages—the 4 cases reported above for 2019‒2020—are interesting is that they help establish the risk of failing to settle a CP case.
Genetic understanding of causation
Given the importance of defendant-clinicians to be able to find a cause other than negligence to explain CP, the recent research of Moreno-De-Luca and colleagues may be especially meaningful.9 Using exome sequencing, the researchers found that 32.7% of pediatric-aged CP patients had pathogenic variance in the sequencing. In theory, this might mean that for about one-third of the CP plaintiffs, there may be genomic (rather than malpractice) explanations for CP, which might ultimately result in fewer cases of CP.
As significant as these findings are, caution is warranted. As the authors note, “this was an observational study and a causal relationship between detected gene variants and phenotypes in participants was not definitively established.”9 Until the causal relationship is established, it is not clear how much influence such a study would have in CP malpractice cases. Another caveat is that, at most, the genetic variants accounted for less than a third of CP cases studied, leaving many cases in which the cause remains unknown. In those cases in which a genomic association was not found, the case may be stronger for the “malpractice was the cause” claim. The follow-up research will likely shed light on some of these issues. Of course, if the genetic research demonstrates that in some proportion of cases there are genetic factors that contribute to the probability of CP, then the search will be for other triggering elements, which could possibly include poor care (that might well be a substantial factor for malpractice). Therefore, the preliminary genetic research likely represents only a part of the CP puzzle in malpractice cases.
Continue to: Why the opening case outcome was for the defense...
Why the opening case outcome was for the defense
Juries, of course, do not write opinions, so the basis for the jury’s decision in the example case is somewhat speculative. It seems most likely that causation had not been established. That is, the plaintiff-patient did not demonstrate that any malpractice was the likely, or substantial contributing, cause of the CP. The case illustrates several important issues.
Statute of limitations. This issue is common in CP cases because the condition may not be diagnosed for some time after birth. The statute of limitations can vary by state for medical malpractice cases “from 2 years to 22 years.”26 Many states begin with a 2-year statute but extend it if the injury or harm is not discovered. The extension is sometimes referred to as a statute of repose because, after that time, there is no extension even if the harm is discovered only later. In some states the statute does not run until the plaintiff is at or near the time of majority (usually age 18).27
Establishing negligence. The information provided about the presented case is mixed on the question of negligence, both regarding the hospital (through its nursing staff) for not properly contacting the obstetrician over the 10 hours, or the physician for inadequate monitoring. In addition, the reference to “really had to pull to deliver the head” may be the basis for claiming excessive, and potentially harmful use of force, which may have caused injury. In addition, the question remains whether the combination of these factors, including the Category III fetal heart tracing, made a cesarean delivery the appropriate standard of care.
Addressing causation. Assuming negligence, there is still a question of causation. It is far from clear that what the clinician did, or did not do, in terms of monitoring caused the CP injury. There is, however, no alternative causation that appeared in the case record, and this may be because of dueling expert witnesses.
The plaintiff sued both the obstetrician and the hospital, which is common among CP cases. While the legal interest of the two parties are aligned in some areas (causation), they may be in conflict in others (the failure of the hospital staff to keep the obstetrician informed). These potential conflicts are not for the clinicians to try to work out on their own. There is the potential for their actions to be misunderstood. When such a case is filed or threatened, the obstetrician should immediately discuss these matters with their attorney. In malpractice cases, malpractice insurance companies often select the attorneys who are experienced in such conflicts. If clinicians are not entirely comfortable that the appointed attorney is representing their interest and preserving a relationship with the hospital or other institution, however, they may engage their own legal counsel to protect their interests.
Practical considerations for avoiding malpractice claims
Good practices for avoiding malpractice claims apply with special force as it relates to CP.28,29
Uphold practice standards and good patient records. The causation element of these legal cases will remain problematic in the foreseeable future. But causation does not matter if negligent practice is not demonstrated. Therefore, maintaining best practices and continuous efforts at quality assurance and following all relevant professional practice guidelines is a good start. More than good intentions, it is essential that policies are implemented and reviewed. Among the areas of ongoing concern is the failure to monitor patients sufficiently. The long period of labor—where perhaps no physician is present for many hours—can introduce problems, as laypersons may have the impression that medical personnel were not on top of the situation.
Maintaining excellent records is also key for clinicians. The more complete the record, the fewer opportunities there are for faulty memories of parties and caregivers to fill in the gaps (especially when causation is so difficult to establish). Under absolutely no circumstances should records be changed or modified to eliminate damaging or an otherwise unfortunate notation. Few things are as harmful to credibility as discovered record tampering.
Inform patients of what is to come. Expectations are an important part of patient satisfaction. While not unduly frightening pregnant patients or eliminating reassurance, the informed consent process and patient counseling should be opportunities to avoid unreasonable expectations.
Stay alert to early genetic counseling, which is becoming increasingly available and important. Maintaining currency with what early testing can be done will become a critical part of ObGyn practice. For CP cases, in the near future, genetic testing may become part of determining causation. In the longer term, it will be part of counseling women and couples in deciding whether to have children, or potentially to end a pregnancy.
Expect the unexpected, and plan for it. Sometimes things just go wrong—there is a bad outcome, mistakes are made, patients are upset. It is important that any practice or institution have a clear plan for when such things happen. Some organizations have used apologies when appropriate,30 others have more complex plans for dealing with bad outcomes.31 Implement developed plans when they are needed. Individual practitioners also should consult with their attorney, who is familiar with their practice and who can help them maintain adherence to legal requirements and good legal problem prevention. ●
During a trial, all parties generally present evidence on negligence, causation, and damages. They do so without knowing whether a jury will find negligence and causation. The question of what the damages should be in cerebral palsy (CP) cases is also quite complex and expensive, but neither the defense nor the plaintiff can afford to ignore it. Past economic damages are relatively easy to calculate. Damages, for instance, includes medical care (pharmaceuticals and supplies, tests and procedures) and personal care (physical, occupational, and psychological therapy; long-term care; special educational costs; assistive equipment; and home modifications) that would have been avoided if it were not for CP. Future and personal care costs are more speculative, and must be estimated with the help of experts. In addition to future costs for the medical and personal care suggested above, depending on the state, the cost of lost future earnings (or earning capacity) may be additional economic damages. The cost of such intensive care, over a lifetime, accounts for many of the large verdicts and settlements.
Noneconomic damages are also available for such things as pain and suffering and diminished quality of life, both past and future. A number of states cap these noneconomic damages.
The wide range of damages correctly suggests that experts from several disciplines must be engaged to cover the damages landscape. This fact accounts for some of the costs of litigating these cases, and also for why damage calculations can be so complex.
- Fahey M, Macleenan A, Kretzschmar D, et al. The genetic basis of cerebral palsy. Dev Med Child Neurol. 2017;59:462-469. doi: 10.1111/dmcn.13363.
- Ellenberg J, Nelson, K. The association of cerebral palsy with birth asphyxia: a definitional quagmire. Dev Med Child Neurol. 2013;55:210-216. doi: 10.1111/dmcn.12016.
- Emrick L, DiCarlo S. The expanding role of genetics in cerebral palsy. Phys Med Rehabil Clin N Am. 2020;31:15-24. doi: 10.1016/j.pmr.2019.09.006.
- Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy [published correction appears in Dev Med Child Neurol. 2007;49:480]. Dev Med Child Neuro. 2007;109(suppl):8-14.
- MacLenan A, Lewis S, Moreno-DeLuca A, et al. Genetic or other causation should not change the clinical diagnosis of cerebral palsy. J Child Neurol. 2019;34:472-476. doi: 10.1177/0883073819840449.
- Lewis S, Shetty S, Wilson B, et al. Insights from genetic studies of cerebral palsy. Front Neurol. 2021;11:1-10. doi: 10.3389/fneur.2020.625428.
- Derick M, Drobyshevsky A, Ji X. A model of cerebral palsy from fetal hypoxia-ischemia. Stroke. 2007;38:731-735. doi: 10.1161/01.STR.0000251445.94697.64.
- McMichael G, Bainbridge M, Haan E, et al. Whole exome sequencing points to considerable genetic heterogeneity of cerebral palsy. Mol Psychiatry. 2015;20:176-182. doi: 10.1038/mp.2014.189.
- Moreno-DeLuca A, Milan F, Pesacreta D, et al. Molecular diagnostic yield of exome sequencing in patients with cerebral palsy. JAMA. 2021;325:467-475. doi: 10.1001/jama.2020.26148.
- Helping disabled children across Maryland & throughout the U.S. The Law Firm of Michael H. Bereston, Inc. website. https://www.berestonlaw.com/birth-injury/. Accessed April 26, 2021.
- Cerebral palsy lawsuits explained. Cerebral Palsy Guide website. https://www.cerebralpalsyguide.com/legal/. Accessed March 22, 2021.
- Zhou L, Li H, Li C, et al. Risk management and provider liabilities in infantile cerebral palsy based on malpractice litigation cases. J Forensic Leg Med. 2019;61:82-88. doi: 10.1016/j.jflm.2018.11.010.
- Cavanaugh MA. Bad cures for bad babies: policy challenges to the statutory removal of the common law claim for birth-related neurological injuries. Case West Res L Rev. 1992;43:1299-1346.
- Kain ZN, Caldwell-Andrews AA. What pediatricians should know about child-related malpractice payments in the United States. Pediatrics. 2006;118:464-468. doi: 10.1542/peds.2005-3112.
- Tabarrok A, Agan A. Medical malpractice awards, insurance, and negligence: which are related? Manhattan Institute Policy Research. Civil Justice Report; 2006. https://media4.manhattan-institute.org/pdf/cjr_10.pdf. Accessed April 27, 2021.
- Freeman AD, Freeman JM. No-fault cerebral palsy insurance: an alternative to the obstetrical malpractice lottery. J Health Politics Policy Law. 1989;14:707-718. doi: 10.1215/03616878-14-4-707.
- Sanfilippo JS, Smith SR. Is there liability if you don’t test for BRCA? OBG Manag. 2021;33:39-46. doi: 10.12788/obgm.0077.
- Fanaroff JM, Goldsmith JP. The most common patient safety issues resulting in legal action against neonatologists. Semin Perinatol. 2019;43:151181-1-9. doi: 10.1053/j.semperi.2019.08.010.
- Sartwelle TP, Johnston, JC. Cerebral palsy litigation: change course or abandon ship. J Child Neurol. 2015;30:828-841. doi: 10.1177/0883073814543306.
- Roth LM. The Business of Birth. NYU Press: New York, NY; 2021.
- Sartwelle TP. Electronic fetal monitoring: a bridge too far. J Legal Med. 2012;33:313-379. doi: 10.1080/01947648.2012.714321.
- Reiter JM, Walsh RS, Thomas EG. Best practices in birth injury litigation: timing hypoxic-ischemic fetal brain injury. Michigan Bar J. 2018;97:42-44.
- Sartwelle TP. Defending a neurologic birth injury: asphyxia neonatorum redux. J Legal Med. 2009;30:181-247. doi: 10.1080/01947640902936522.
- Daubert v Merrell Dow Pharm, Inc. 509 U.S. 579 (1993).
- Jha S. The factors making Americans litigious. J Am College Radiology. 2019;17:551-553. doi: 10.1016/j.jacr.2019.10.011.
- Salvi S, Pritchard PC. Statute of limitations on cerebral palsy cases. Personal Injury Lawyers website. https://www.salvilaw.com/birth-injury-lawyers/cerebral-palsy/time-limits/. Accessed March 24, 2021.
- Wharton R. Cerebral palsy statute of limitations. Cerebral Palsy Guidance website. October 16, 2020. https://www.cerebralpalsyguidance.com/cerebral-palsy-lawyer/statute-of-limitations/. Accessed March 24, 2021.
- Kassim PJ, Ushiro S, Najid KM. Compensating cerebral palsy cases: problems in court litigation and the no-fault alternative. Med Law. 2015;34:335-355.
- Williams D. Practice patterns to decrease the risk of malpractice suit. Clin Obstet Gynecol. 2008;51:680-687. doi: 10.1097/GRF.0b013e3181899bc7.
- McMichael BJ, Van Horn RL, Viscusi WK. “Sorry” is never enough: how state apology laws fail to reduce medical malpractice liability risk. Stanford Law Rev. 2019;71:341-409.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221. doi: 10.7326/0003-4819-153-4-201008170-00002.
- Fahey M, Macleenan A, Kretzschmar D, et al. The genetic basis of cerebral palsy. Dev Med Child Neurol. 2017;59:462-469. doi: 10.1111/dmcn.13363.
- Ellenberg J, Nelson, K. The association of cerebral palsy with birth asphyxia: a definitional quagmire. Dev Med Child Neurol. 2013;55:210-216. doi: 10.1111/dmcn.12016.
- Emrick L, DiCarlo S. The expanding role of genetics in cerebral palsy. Phys Med Rehabil Clin N Am. 2020;31:15-24. doi: 10.1016/j.pmr.2019.09.006.
- Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy [published correction appears in Dev Med Child Neurol. 2007;49:480]. Dev Med Child Neuro. 2007;109(suppl):8-14.
- MacLenan A, Lewis S, Moreno-DeLuca A, et al. Genetic or other causation should not change the clinical diagnosis of cerebral palsy. J Child Neurol. 2019;34:472-476. doi: 10.1177/0883073819840449.
- Lewis S, Shetty S, Wilson B, et al. Insights from genetic studies of cerebral palsy. Front Neurol. 2021;11:1-10. doi: 10.3389/fneur.2020.625428.
- Derick M, Drobyshevsky A, Ji X. A model of cerebral palsy from fetal hypoxia-ischemia. Stroke. 2007;38:731-735. doi: 10.1161/01.STR.0000251445.94697.64.
- McMichael G, Bainbridge M, Haan E, et al. Whole exome sequencing points to considerable genetic heterogeneity of cerebral palsy. Mol Psychiatry. 2015;20:176-182. doi: 10.1038/mp.2014.189.
- Moreno-DeLuca A, Milan F, Pesacreta D, et al. Molecular diagnostic yield of exome sequencing in patients with cerebral palsy. JAMA. 2021;325:467-475. doi: 10.1001/jama.2020.26148.
- Helping disabled children across Maryland & throughout the U.S. The Law Firm of Michael H. Bereston, Inc. website. https://www.berestonlaw.com/birth-injury/. Accessed April 26, 2021.
- Cerebral palsy lawsuits explained. Cerebral Palsy Guide website. https://www.cerebralpalsyguide.com/legal/. Accessed March 22, 2021.
- Zhou L, Li H, Li C, et al. Risk management and provider liabilities in infantile cerebral palsy based on malpractice litigation cases. J Forensic Leg Med. 2019;61:82-88. doi: 10.1016/j.jflm.2018.11.010.
- Cavanaugh MA. Bad cures for bad babies: policy challenges to the statutory removal of the common law claim for birth-related neurological injuries. Case West Res L Rev. 1992;43:1299-1346.
- Kain ZN, Caldwell-Andrews AA. What pediatricians should know about child-related malpractice payments in the United States. Pediatrics. 2006;118:464-468. doi: 10.1542/peds.2005-3112.
- Tabarrok A, Agan A. Medical malpractice awards, insurance, and negligence: which are related? Manhattan Institute Policy Research. Civil Justice Report; 2006. https://media4.manhattan-institute.org/pdf/cjr_10.pdf. Accessed April 27, 2021.
- Freeman AD, Freeman JM. No-fault cerebral palsy insurance: an alternative to the obstetrical malpractice lottery. J Health Politics Policy Law. 1989;14:707-718. doi: 10.1215/03616878-14-4-707.
- Sanfilippo JS, Smith SR. Is there liability if you don’t test for BRCA? OBG Manag. 2021;33:39-46. doi: 10.12788/obgm.0077.
- Fanaroff JM, Goldsmith JP. The most common patient safety issues resulting in legal action against neonatologists. Semin Perinatol. 2019;43:151181-1-9. doi: 10.1053/j.semperi.2019.08.010.
- Sartwelle TP, Johnston, JC. Cerebral palsy litigation: change course or abandon ship. J Child Neurol. 2015;30:828-841. doi: 10.1177/0883073814543306.
- Roth LM. The Business of Birth. NYU Press: New York, NY; 2021.
- Sartwelle TP. Electronic fetal monitoring: a bridge too far. J Legal Med. 2012;33:313-379. doi: 10.1080/01947648.2012.714321.
- Reiter JM, Walsh RS, Thomas EG. Best practices in birth injury litigation: timing hypoxic-ischemic fetal brain injury. Michigan Bar J. 2018;97:42-44.
- Sartwelle TP. Defending a neurologic birth injury: asphyxia neonatorum redux. J Legal Med. 2009;30:181-247. doi: 10.1080/01947640902936522.
- Daubert v Merrell Dow Pharm, Inc. 509 U.S. 579 (1993).
- Jha S. The factors making Americans litigious. J Am College Radiology. 2019;17:551-553. doi: 10.1016/j.jacr.2019.10.011.
- Salvi S, Pritchard PC. Statute of limitations on cerebral palsy cases. Personal Injury Lawyers website. https://www.salvilaw.com/birth-injury-lawyers/cerebral-palsy/time-limits/. Accessed March 24, 2021.
- Wharton R. Cerebral palsy statute of limitations. Cerebral Palsy Guidance website. October 16, 2020. https://www.cerebralpalsyguidance.com/cerebral-palsy-lawyer/statute-of-limitations/. Accessed March 24, 2021.
- Kassim PJ, Ushiro S, Najid KM. Compensating cerebral palsy cases: problems in court litigation and the no-fault alternative. Med Law. 2015;34:335-355.
- Williams D. Practice patterns to decrease the risk of malpractice suit. Clin Obstet Gynecol. 2008;51:680-687. doi: 10.1097/GRF.0b013e3181899bc7.
- McMichael BJ, Van Horn RL, Viscusi WK. “Sorry” is never enough: how state apology laws fail to reduce medical malpractice liability risk. Stanford Law Rev. 2019;71:341-409.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221. doi: 10.7326/0003-4819-153-4-201008170-00002.
Is there liability if you don’t test for BRCA?
CASE Young woman with family history of breast cancer detects lump
Two weeks after noting a lump on her breast when her cat happened to jump on her in that spot, a 28-year-old woman (G0) went to her primary care provider. She was referred to her gynecologist; breast imaging, ultrasonography, and mammography were obtained, with microcalcifications noted. A fine needle aspiration diagnosed intraductal malignancy. The surgical breast tissue specimen was estrogen receptor (ER)- and progestogen receptor (PR)-positive and HER2-negative. Other tumor markers were obtained, including carcinoembryonic antigen, and tissue polypeptide specific antigen, p53, cathepsin D, cyclin E, and nestin, but results were not available.
With regard to family history, the woman’s mother and maternal grandmother had a history of breast cancer. The patient and her family underwent gene testing. The patient was found to be BRCA1- and BRCA2-positive; her mother was BRCA1-positive, an older sister was BRCA2-positive, and her grandmother was not tested.
The question arose in light of her family history as to why she was not tested for BRCA and appropriately counseled by her gynecologist prior to the cancer diagnosis. Litigation was initiated. While the case did not go forward regarding litigation, it is indeed a case in point. (Please note that this is a hypothetical case. It is based on a composite of several cases.)
Medical considerations
Breast cancer is the most common type of cancer affecting women in the Western world.1 Advances in clinical testing for gene mutations have escalated and allowed for identification of patients at increased risk for breast and ovarian cancer. Along with these advances come professional liability risk. After looking at the medical considerations for BRCA1 and 2 testing, we will consider a number of important legal issues. In the view of some commentators, the failure to diagnose genetic mutations in patients predisposed to cancer is “poised to become the next wave of medical professional liability lawsuits.”2
BRCA1 and BRCA2 genes provide tumor suppressor proteins, and assessment for mutations is recommended for individuals at high risk for breast and/or ovarian cancer; mutations in BRCA genes cause DNA damage, which increases the chance of developing cancer. The other way to look at it is, BRCA1 and 2 are tumor suppressor genes that are integrally involved with DNA damage control. Once there is a mutation, it adversely affects the beneficial effects of the gene. Mutations in these genes account for 5% to 10% of all hereditary breast cancers.3 Of note, men with BRCA2 are at increased risk for prostate cancer.
A patient who presents to her gynecologist stating that there is a family history of breast cancer, without knowledge of genetic components, presents a challenge (and a medicolegal risk) for the provider to assess. Prediction models have been used to determine specific patient risk for carrying a genetic mutation with resultant breast cancer development.4 Risk prediction models do not appear to be a good answer to predicting who is more likely to develop breast or ovarian cancer, however. A Mayo model may assist (FIGURE).5 Clinicians should also be aware of other models of risk assessment, including the Gail Model (TABLE 1).6
Continue to: Guidelines for genetic testing...
Guidelines for genetic testing
The American College of Obstetricians and Gynecologists states that patient medical history and family history are paramount in obtaining information regarding risk for breast and ovarian cancer. First- and second-degree relatives are allocated to this category. Information regarding age of diagnosis, maternal and paternal lineage, and ethnic background can imply a need for genetic testing (TABLE 2).7,8 A number of genetics national organizations have participated in recommendations and include the American College of Medical Genetics and Genomics, the National Society for Genetic Counselors, and the Society of Gynecologic Oncology.7
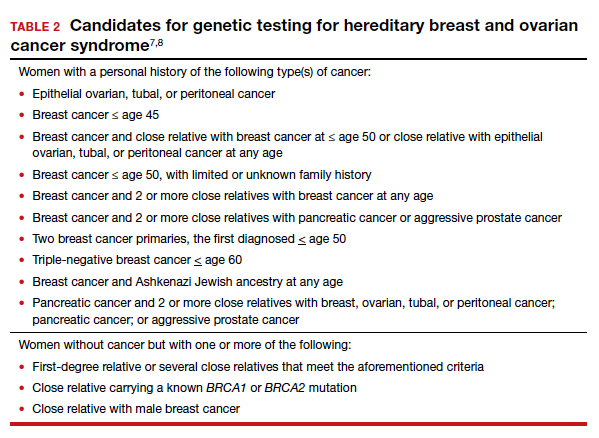
The question always surfaces, could the clinical outcome of the cancer when diagnosed have been changed if screening were undertaken, with earlier diagnosis, or prevented with prophylactic mastectomy, and changed the end result. In addition, it is well known that breast augmentation mammoplasty alters the ability to accurately evaluate mammograms. Patients considering this type of plastic surgery, ideally, should be counselled accordingly.9
Bottom line, we as clinicians must be cognizant of both ACOG and United States Preventive Services Task Force (USPSTF) recommendations regarding screening and gene testing for women considered high risk for breast cancer based on family history.7
Legal considerations
The case presented demonstrates that the discovery of the BRCA1 and BRCA2 genes, and reliable tests for determining the existence of the genes, brought with them legal issues as well as medical advantages. We look at professional liability (malpractice) questions this technology raises, and then consider the outcome of the hypothetical case. (BRCA is used here to apply broadly—not only to BRCA1 and 2 but also to PALB2, CHEK2, and similar genetic abnormalities.)
To date, the most visible BRCA legal issues covered in cases and law reviews have focused more on patent law than malpractice. The most important of these was a decision of the US Supreme Court in Association for Molecular Pathology v Myriad Genetics.10 The US Patent Office was granting patents to companies finding useful, naturally occurring segments of human DNA, and had granted Myriad several patents on BRCA1 and BRCA2 genes. This patent policy had the potential to seriously interfere with broad scientific use of these genes.11 Fortunately, the Supreme Court stepped in and unanimously invalidated such patents. It held that a “naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated.” The Court noted, “Finding the location of the BRCA1 and BRCA2 genes does not render the genes patent eligible ‘new . . . composition[s] of matter.’”8 The Court did allow the patenting of tests for specific gene structures, and artificial changes in naturally occurring genes.
Malpractice and BRCA
While the BRCA patent wars have lingered, the potential for a significant increase in BRCA-related malpractice cases is of increasing concern. Like most malpractice liability, these new claims are based on very old principles of negligence.12 To prevail, the plaintiff (ordinarily, an injured patient) must demonstrate 4 things:
- A duty. That is, the physician owed a duty to the injured party. Usually (but not always) that requires a professional relationship between the physician and the person injured.
- A breach of that duty. Malpractice liability is based on the fact that the physician did something that a reasonably careful physician (generally, of the same specialty) would not have done, or that the physician failed to do something that a reasonable physician would have done. This usually means that the profession itself sees what the physician did (or did not do) as medically inappropriate. In medical malpractice cases, that is ordinarily measured by what the usual or common practice is among prudent physicians. In rare circumstances, courts have found the standard practice of a profession to be negligent. Where, for example, it was custom for a professional not to give an eye pressure test to anyone under age 40, a court found that common standard to be inappropriate.13 In the words of Judge Learned Hand (speaking about a different case), “a whole calling may have unduly lagged in the adoption of new and available devices. It never may set its own tests.”14 Underlying negligence is a cost-benefit analysis (discussed below).
- Damages. There must have been some damage that courts recognize, usually loss of money or opportunity to work, the cost of care, pain and suffering, or loss of enjoyment/quality of life. In malpractice, many states now recognize the “loss of chance” or the “loss of a chance.” That means, if a “physician negligently fails to diagnose a curable disease, and the patient is harmed by the disease, the physician should be liable for causing the ‘loss of a chance of a cure.’”15 (Delay in diagnosis is the most common reason for claims in breast cancer care.)16
- Causation. The breach of duty (negligence) must have caused the damages. The causation must have been reasonably close. If a driver drives through a stop sign, or a physician misreads a test, and someone is injured but there is no connection between the negligence and the injury, there is not tort liability.
The 4 elements of malpractice just described are raised in some way in the possible liability associated with BRCA testing. We next look at the ways in which liability may arise from that testing (or lack of it).
Underlying much of the following discussion is the “cost-benefit” consideration noted above. This concept is that the total cost (financial and health) of testing should be compared with the value of the benefits of testing, taking into account the probabilities that the testing will result in better health outcomes. BRCA testing, for example, is essentially cost-free in terms of physical risk. Its financial cost, while not trivial, is not great, and it is commonly covered by health insurance.17 In terms of benefits, the testing has the potential for providing critical information in making treatment decisions for a meaningful percentage of patients and their families. There are many ways of analyzing the liability risks of genetic malpractice,7,18 and the following is intended to discuss some of the greatest risks related to BRCA testing.
Continue to: Areas of liability...
Areas of liability
The failure to recommend a test. The circumstances in which BRCA testing should be undertaken are set out by professional organizations (noted above). These recommendations are not static, however. They change from time to time. Given the potential harm caused by the failure to test in relevant circumstances, malpractice liability is certainly a possibility when the failure to recommend a test to a patient results in a cancer that might have been prevented had the genetic problem been identified in a timely manner. The circumstances in which testing should be considered continue to change, placing an obligation on clinicians to stay well informed of changing genetic understandings. Another risk is that one specialist may assume that it is the job of another specialist to order the test. Whatever the cause of the failure to test, or unnecessary delay in testing, it appears to be the primary basis for BRCA liability.
The failure to properly interpret a test. Any test that is misinterpreted may lead to harm for the patient. A false negative, of course, may mean that preventive treatment that could have been undertaken will be foregone, as a “loss of a chance.” On the other hand, a false positive can lead to radical, unnecessary surgery or treatment. If a misinterpretation occurred because of carelessness by the testing organization, or confusion by a practitioner, there is a likelihood of negligence.19
A different form of “misinterpretation” could be reasonable—and not negligent. Advances in scientific-medical understanding may result in the outcome of tests being reconsidered and changed. That has been the case with genetic testing and breast cancer. The availability of multiple breast cancer SNPs (single nucleotide polymorphisms), and combining this information with other risk factors for example, results in a polygenic risk score that may be at odds with the level of risk from earlier testing.20,21 This naturally leads to the question of when later, updated testing should be recommended to look for a better current interpretation.22,23
The failure to act on BRCA test results. Testing is of no value, of course, if the results are not used properly. Test results or analyses that are not sent to the proper physicians, or are somehow ignored when properly directed, is a “never” event—it should never happen. It almost always would be considered negligence, and if the patient were injured, could lead to liability. Amazingly, one study found that, in genetic testing liability cases, nearly 20% of the claims arose from failure to return test results to patients.24 In addition, when a patient is found to be BRCA-positive, there is an obligation to discuss the options for dealing with the increased risk associated with the gene mutation(s), as well as to recommend the prudent course of action or to refer the patient to someone who will have that discussion.
Informed consent to the patient. BRCA testing requires informed consent. The physical risks of the testing process are minimal, of course, but it carries a number of other emotional and family risks. The informed consent process is an invitation to an honest discussion between clinicians and patients. It should be an opportunity to discuss what the testing is, and is not, and what the test may mean for treatment. It may also be an opportunity to discuss the implications for other members of the patient’s family (noted below).
One element of informed consent is a discussion of the consequences of failure to consent, or to undertake one of the alternatives. In the case of BRCA testing, this is especially important in cases in which a patient expresses a hesitancy to be tested with an “I’d rather not know philosophy.” Although clinicians should not practice law, some patient concerns about discrimination may be addressed by the protection that the federal Genetic Information Nondiscrimination Act (GINA) and other laws provide (which prohibit insurance and employment discrimination based on genetic information). A good source of information about GINA and related nondiscrimination laws is provided by the National Human Genome Research Institute.25 In addition, the National Institutes of Health has a website that may be helpful to many patients26 (and a much more complex site for health professionals).27 At the same time, courts have resisted plaintiffs/patients who have tried to use informed consent as a way of suing for failure to offer genetic testing.28,29
The failure to refer. In some cases, a patient should be formally referred for genetics consultation. The considerations here are similar to other circumstances in modern, fast developing medical practice that require special sensitivity to those occasions in which a patient will benefit from additional expertise. It is a principle that the AMA Council on Ethical and Judicial Affairs has expressed this way: “In the absence of adequate expertise in pretest and posttest counseling, a physician should refer the patient to an appropriate specialist.”30 The failure to refer, when that deviates from acceptable practice, may result in liability.
Informing others. BRCA testing is an area of medicine in which results may be of great significance not only to the patient but also to the patient’s family.31 Physicians should counsel patients on the importance of informing relatives about relevant results and “should make themselves available to assist patients in communicating with relatives to discuss opportunities for counseling and testing, as appropriate.”30 The question may arise, however, of whether in some circumstances physicians should go a step further in ensuring relatives receive important information regarding their loved one’s health.32 The law has been reluctant to impose liability to “third parties” (someone not a patient). Duties usually arise through the physician-patient relationship. There are exceptions. Perhaps the best known has been the obligation of mental health professionals to take action to protect third parties from patients who have made believable threats against identifiable victims.33 There are indications that some courts could find, in extreme circumstances, a “duty to warn” nonpatients in some instances where it is essential to inform third parties that they should receive a specific form of genetic testing.34,35 Such a duty would, of course, have to protect the privacy rights of the patient to the maximum extent possible. A general duty of this type has not been established widely, but may be part of the future.
Continue to: Was there liability in our example case?...
Was there liability in our example case?
The hypothetical case provided above suggests that there could be liability. Routine medical history by the primary care physician would have produced the fact that the patient’s mother, sister, and maternal grandmother had breast cancer. That would clearly have put her in a category of those who should have received genetic testing. Yet, she was not tested until after her cancer was found. From the limited facts we have, it appears that this timeline of events would have been outside accepted practice—and negligent. The case was not pursued by the patient, however, and this may represent the current state of liability for BRCA issues.
The extent of liability seems to be significant
Our discussion of liability suggests that there is significant potential for BRCA testing negligence within practice, and that the damages in these cases could be substantial. Yet the predicted “tsunami” of malpractice lawsuits related to genetic testing has not appeared.36,37 One study of cases in the United States (through 2016) found a “slowly rising tide” of liability cases instead of a tsunami,24 as the number of claims made was low. On the other hand, the payments where damages were awarded were an order of magnitude larger than other malpractice cases—a mean of $5.3 million and median of $2 million. This is compared with mean values in the range of $275,000 to $600,000 in other areas of malpractice.
The majority of the genetic malpractice cases involve prenatal and newborn testing, and diagnosis/susceptibility/pharmacogenomic accounting for about 25% of cases. In terms of type of errors claimed, approximately 50% were diagnostic-interpretation errors, 30% failure to offer testing, nearly 20% failure to return test results to the patients, and a few remaining cases of failure to properly treat in light of genetic testing.24
Despite a few very large payments, however, the fact remains that there is a surprisingly low number of genetics malpractice cases. Gary Marchant and colleagues suggest that several reasons may account for this:
- the clinical implementation of genetic science has been slower than expected
- the lack of expertise of many physicians in genetic science
- expert witnesses have sometimes been hard to find
- the lack of understanding by plaintiffs’ attorneys of genetic malpractice
- potential plaintiffs’ lack of understanding of the nature of genetic testing and the harms resulting from genetic negligence.17,24,37
The tide is slowly coming in
By all appearances, there is every reason to think that genetic malpractice will be increasing, and that the recent past of much higher damages per claim paid in the genetics area will be part of that tide. The National Human Genome Research LawSeq project has suggested a number of useful ways of dealing with the liability issues.18 In addition to the BRCA issues that we have considered in this article for ObGyns, there are other critical issues of prenatal and newborn genetic testing.38 But those are topics for another day. ●
- Sevilla C, Moatti JP, Reynier CJ, et al. Testing for BRCA1 mutations: a cost-effective analysis. Europ J Human Genetics. 2002;10:599-606.
- Cotton V, Kirkpatrick D. Failure to recommend genetic counseling in breast cancer: is the next wave of medical professional liability lawsuits? Contemp OB/GYN. June 1, 2017.
- Suryavanshi M, Kumar D, Panigrahi M, et al. Detection of false positive mutations in BRCA gene by next generation sequencing. Fam Cancer. 2017;16:311-317.
- Black L, Knoppers B, Avard D, et al. Legal liability and the uncertain nature of risk prediction: the case of breast cancer risk prediction models. Public Health Genomics. 2012;15:335-340.
- McClintock A, Gollab A, Laya M. Breast cancer risk assessment, a step-wise approach for primary care physicians on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275.
- National Cancer Institute. The Breast Cancer Risk Assessment Tool. https://bcrisktool.cancer.gov/. Accessed February 25, 2021.
- Neff J, Richardson G, Phelps J. Legal liabilities associated with hereditary breast and ovarian cancers. J Reprod Med. 2020;65:227-230.
- American College of Obstetricians and Gynecologists. Practice Bulletin No 182: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130:e110-e126.
- Sá dos Reis C, Gremion I, and Meystre NR. Study of breast implants mammography examinations for identification of suitable image quality criteria. Insights Imaging. 2020;11:3.
- Association for Molecular Pathology v Myriad Genetics, 569 U.S. 576 (2013).
- Smith SR. The Supreme Court 2012-2013: dogs, DNA, and DOMA. Register Rep. 2013;39(Fall):26-33.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Helling v Carey, 83 Wn.2d 514, 519 P.2d 981 (1974).
- The T.J. Hooper, 60 F.2d 737, 740 (2d Cir.1932), cert. denied 287 U.S. 662 (1932).
- Fischer DA. Tort recovery for loss of a chance. Wake Forest L Rev. 2001;36:605-655.
- Murphy BL, Ray-Zack MD, Reddy PN, et al. Breast cancer litigation in the 21st century. Ann Surg Oncol. 2018;25:2939- 2947.
- Prince AE. Prevention for those who can pay: insurance reimbursement of genetic-based preventive interventions in the liminal state between health and disease. J Law Biosci. 2015;2:365-395.
- Marchant G, Barnes M, Evans JP, et al; LawSeq Liability Task Force. From genetics to genomics: facing the liability implications in clinical care. J Law Med Ethics. 2020;48:11-43.
- Complaint, Held v Ambry Genetics Corp., No. 15-CV-8683, 2015 WL 6750024 (S.D.N.Y. Nov. 4, 2015); Order of Dismissal, Held v Ambry Genetics Corp., No. 15-CV-8683, (S.D.N.Y. Dec. 6, 2016).
- Pederson HJ. Breast cancer risk assessment and treatment: current concepts in genetics and genomics. Contemp OB/ GYN. 2017; 62:A1-A4.
- Pederson HJ. Who needs breast cancer genetics testing? OBG Manag. 2018;30:34-39.
- Roberts JL, Foulkes A. Genetic duties. William Mary L Rev. 2020;62:143-212.
- Thorogood A, Cook-Deegan R, Knoppers B. Public variant databases: liability? Genet Med. 2017;19:838–841.
- Marchant G, Lindor R. Genomic malpractice: an emerging tide or gentle ripple? Food Drug Law J. 2018;73:1-37.
- National Human Genome Research Institute. Genetic discrimination. https://www.genome.gov/about-genomics /policy-issues/Genetic-Discrimination. Updated September 16, 2020. Accessed February 25, 2021.
- National Cancer Institute. BRCA mutations: cancer risk and genetic testing. https://www.cancer.gov/about-cancer /causes-prevention/genetics/brca-fact-sheet. Reviewed November 19, 2020. Accessed February 25, 2021.
- National Cancer Institute. Genetics of breast and gynecologic cancers (PDQ®)–Health Professional Version. https://www .cancer.gov/types/breast/hp/breast-ovarian-genetics-pdq. Updated February 12, 2021. Accessed February 25, 2021.
- Reed v Campagnolo, 630 A.2d 1145, 1152–54 (Md. 1993).
- Munro v Regents of Univ. of Cal.,263 Cal. Rptr. 878, 885, 988 (1989).
- AMA Council on Ethical and Judicial Affairs. AMA Code of Medical Ethics’ opinions on genetic testing. Opinion 2.131. 2009;11:683-685. https://journalofethics.ama-assn .org/article/ama-code-medical-ethics-opinions-genetictesting/2009-09.
- Gilbar R, Barnoy S. Disclosing genetic test results to the patient’ relatives: how does the law influence clinical practice? J Law Technol Policy. 2019;125-168.
- Song K. Warning third parties of genetic risks in the era of personalized medicine. U.C. Davis L Rev. 2016;49:1987-2018.
- Tarasoff v Regents of the University of California, 551 P.2d 334, 131 Cal. Rptr. 14 (Cal. 1976).
- Safer v Estate of Pack, 677 A.2d 1188 (N.J. App. 1996), cert. denied, 683 A.2d 1163 (N.J. 1996).
- Pate v Threlkel, 661 So.2d 278 (Fla. 1995).
- Rothstein MA. Liability issues in pharmacogenomics. Louisiana L Rev. 2005;66:117-124.
- Marchant G, Lindor R. Personalized medicine and genetic malpractice. Genet Med. 2013;15:921-922.
- Westbrook M. Transforming the physician’s standard of care in the context of whole genome sequencing technologies: finding guidance in best practice standards. Saint Louis U J Health Law Policy. 2015;9:111-148.
CASE Young woman with family history of breast cancer detects lump
Two weeks after noting a lump on her breast when her cat happened to jump on her in that spot, a 28-year-old woman (G0) went to her primary care provider. She was referred to her gynecologist; breast imaging, ultrasonography, and mammography were obtained, with microcalcifications noted. A fine needle aspiration diagnosed intraductal malignancy. The surgical breast tissue specimen was estrogen receptor (ER)- and progestogen receptor (PR)-positive and HER2-negative. Other tumor markers were obtained, including carcinoembryonic antigen, and tissue polypeptide specific antigen, p53, cathepsin D, cyclin E, and nestin, but results were not available.
With regard to family history, the woman’s mother and maternal grandmother had a history of breast cancer. The patient and her family underwent gene testing. The patient was found to be BRCA1- and BRCA2-positive; her mother was BRCA1-positive, an older sister was BRCA2-positive, and her grandmother was not tested.
The question arose in light of her family history as to why she was not tested for BRCA and appropriately counseled by her gynecologist prior to the cancer diagnosis. Litigation was initiated. While the case did not go forward regarding litigation, it is indeed a case in point. (Please note that this is a hypothetical case. It is based on a composite of several cases.)
Medical considerations
Breast cancer is the most common type of cancer affecting women in the Western world.1 Advances in clinical testing for gene mutations have escalated and allowed for identification of patients at increased risk for breast and ovarian cancer. Along with these advances come professional liability risk. After looking at the medical considerations for BRCA1 and 2 testing, we will consider a number of important legal issues. In the view of some commentators, the failure to diagnose genetic mutations in patients predisposed to cancer is “poised to become the next wave of medical professional liability lawsuits.”2
BRCA1 and BRCA2 genes provide tumor suppressor proteins, and assessment for mutations is recommended for individuals at high risk for breast and/or ovarian cancer; mutations in BRCA genes cause DNA damage, which increases the chance of developing cancer. The other way to look at it is, BRCA1 and 2 are tumor suppressor genes that are integrally involved with DNA damage control. Once there is a mutation, it adversely affects the beneficial effects of the gene. Mutations in these genes account for 5% to 10% of all hereditary breast cancers.3 Of note, men with BRCA2 are at increased risk for prostate cancer.
A patient who presents to her gynecologist stating that there is a family history of breast cancer, without knowledge of genetic components, presents a challenge (and a medicolegal risk) for the provider to assess. Prediction models have been used to determine specific patient risk for carrying a genetic mutation with resultant breast cancer development.4 Risk prediction models do not appear to be a good answer to predicting who is more likely to develop breast or ovarian cancer, however. A Mayo model may assist (FIGURE).5 Clinicians should also be aware of other models of risk assessment, including the Gail Model (TABLE 1).6


Continue to: Guidelines for genetic testing...
Guidelines for genetic testing
The American College of Obstetricians and Gynecologists states that patient medical history and family history are paramount in obtaining information regarding risk for breast and ovarian cancer. First- and second-degree relatives are allocated to this category. Information regarding age of diagnosis, maternal and paternal lineage, and ethnic background can imply a need for genetic testing (TABLE 2).7,8 A number of genetics national organizations have participated in recommendations and include the American College of Medical Genetics and Genomics, the National Society for Genetic Counselors, and the Society of Gynecologic Oncology.7

The question always surfaces, could the clinical outcome of the cancer when diagnosed have been changed if screening were undertaken, with earlier diagnosis, or prevented with prophylactic mastectomy, and changed the end result. In addition, it is well known that breast augmentation mammoplasty alters the ability to accurately evaluate mammograms. Patients considering this type of plastic surgery, ideally, should be counselled accordingly.9
Bottom line, we as clinicians must be cognizant of both ACOG and United States Preventive Services Task Force (USPSTF) recommendations regarding screening and gene testing for women considered high risk for breast cancer based on family history.7
Legal considerations
The case presented demonstrates that the discovery of the BRCA1 and BRCA2 genes, and reliable tests for determining the existence of the genes, brought with them legal issues as well as medical advantages. We look at professional liability (malpractice) questions this technology raises, and then consider the outcome of the hypothetical case. (BRCA is used here to apply broadly—not only to BRCA1 and 2 but also to PALB2, CHEK2, and similar genetic abnormalities.)
To date, the most visible BRCA legal issues covered in cases and law reviews have focused more on patent law than malpractice. The most important of these was a decision of the US Supreme Court in Association for Molecular Pathology v Myriad Genetics.10 The US Patent Office was granting patents to companies finding useful, naturally occurring segments of human DNA, and had granted Myriad several patents on BRCA1 and BRCA2 genes. This patent policy had the potential to seriously interfere with broad scientific use of these genes.11 Fortunately, the Supreme Court stepped in and unanimously invalidated such patents. It held that a “naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated.” The Court noted, “Finding the location of the BRCA1 and BRCA2 genes does not render the genes patent eligible ‘new . . . composition[s] of matter.’”8 The Court did allow the patenting of tests for specific gene structures, and artificial changes in naturally occurring genes.
Malpractice and BRCA
While the BRCA patent wars have lingered, the potential for a significant increase in BRCA-related malpractice cases is of increasing concern. Like most malpractice liability, these new claims are based on very old principles of negligence.12 To prevail, the plaintiff (ordinarily, an injured patient) must demonstrate 4 things:
- A duty. That is, the physician owed a duty to the injured party. Usually (but not always) that requires a professional relationship between the physician and the person injured.
- A breach of that duty. Malpractice liability is based on the fact that the physician did something that a reasonably careful physician (generally, of the same specialty) would not have done, or that the physician failed to do something that a reasonable physician would have done. This usually means that the profession itself sees what the physician did (or did not do) as medically inappropriate. In medical malpractice cases, that is ordinarily measured by what the usual or common practice is among prudent physicians. In rare circumstances, courts have found the standard practice of a profession to be negligent. Where, for example, it was custom for a professional not to give an eye pressure test to anyone under age 40, a court found that common standard to be inappropriate.13 In the words of Judge Learned Hand (speaking about a different case), “a whole calling may have unduly lagged in the adoption of new and available devices. It never may set its own tests.”14 Underlying negligence is a cost-benefit analysis (discussed below).
- Damages. There must have been some damage that courts recognize, usually loss of money or opportunity to work, the cost of care, pain and suffering, or loss of enjoyment/quality of life. In malpractice, many states now recognize the “loss of chance” or the “loss of a chance.” That means, if a “physician negligently fails to diagnose a curable disease, and the patient is harmed by the disease, the physician should be liable for causing the ‘loss of a chance of a cure.’”15 (Delay in diagnosis is the most common reason for claims in breast cancer care.)16
- Causation. The breach of duty (negligence) must have caused the damages. The causation must have been reasonably close. If a driver drives through a stop sign, or a physician misreads a test, and someone is injured but there is no connection between the negligence and the injury, there is not tort liability.
The 4 elements of malpractice just described are raised in some way in the possible liability associated with BRCA testing. We next look at the ways in which liability may arise from that testing (or lack of it).
Underlying much of the following discussion is the “cost-benefit” consideration noted above. This concept is that the total cost (financial and health) of testing should be compared with the value of the benefits of testing, taking into account the probabilities that the testing will result in better health outcomes. BRCA testing, for example, is essentially cost-free in terms of physical risk. Its financial cost, while not trivial, is not great, and it is commonly covered by health insurance.17 In terms of benefits, the testing has the potential for providing critical information in making treatment decisions for a meaningful percentage of patients and their families. There are many ways of analyzing the liability risks of genetic malpractice,7,18 and the following is intended to discuss some of the greatest risks related to BRCA testing.
Continue to: Areas of liability...
Areas of liability
The failure to recommend a test. The circumstances in which BRCA testing should be undertaken are set out by professional organizations (noted above). These recommendations are not static, however. They change from time to time. Given the potential harm caused by the failure to test in relevant circumstances, malpractice liability is certainly a possibility when the failure to recommend a test to a patient results in a cancer that might have been prevented had the genetic problem been identified in a timely manner. The circumstances in which testing should be considered continue to change, placing an obligation on clinicians to stay well informed of changing genetic understandings. Another risk is that one specialist may assume that it is the job of another specialist to order the test. Whatever the cause of the failure to test, or unnecessary delay in testing, it appears to be the primary basis for BRCA liability.
The failure to properly interpret a test. Any test that is misinterpreted may lead to harm for the patient. A false negative, of course, may mean that preventive treatment that could have been undertaken will be foregone, as a “loss of a chance.” On the other hand, a false positive can lead to radical, unnecessary surgery or treatment. If a misinterpretation occurred because of carelessness by the testing organization, or confusion by a practitioner, there is a likelihood of negligence.19
A different form of “misinterpretation” could be reasonable—and not negligent. Advances in scientific-medical understanding may result in the outcome of tests being reconsidered and changed. That has been the case with genetic testing and breast cancer. The availability of multiple breast cancer SNPs (single nucleotide polymorphisms), and combining this information with other risk factors for example, results in a polygenic risk score that may be at odds with the level of risk from earlier testing.20,21 This naturally leads to the question of when later, updated testing should be recommended to look for a better current interpretation.22,23
The failure to act on BRCA test results. Testing is of no value, of course, if the results are not used properly. Test results or analyses that are not sent to the proper physicians, or are somehow ignored when properly directed, is a “never” event—it should never happen. It almost always would be considered negligence, and if the patient were injured, could lead to liability. Amazingly, one study found that, in genetic testing liability cases, nearly 20% of the claims arose from failure to return test results to patients.24 In addition, when a patient is found to be BRCA-positive, there is an obligation to discuss the options for dealing with the increased risk associated with the gene mutation(s), as well as to recommend the prudent course of action or to refer the patient to someone who will have that discussion.
Informed consent to the patient. BRCA testing requires informed consent. The physical risks of the testing process are minimal, of course, but it carries a number of other emotional and family risks. The informed consent process is an invitation to an honest discussion between clinicians and patients. It should be an opportunity to discuss what the testing is, and is not, and what the test may mean for treatment. It may also be an opportunity to discuss the implications for other members of the patient’s family (noted below).
One element of informed consent is a discussion of the consequences of failure to consent, or to undertake one of the alternatives. In the case of BRCA testing, this is especially important in cases in which a patient expresses a hesitancy to be tested with an “I’d rather not know philosophy.” Although clinicians should not practice law, some patient concerns about discrimination may be addressed by the protection that the federal Genetic Information Nondiscrimination Act (GINA) and other laws provide (which prohibit insurance and employment discrimination based on genetic information). A good source of information about GINA and related nondiscrimination laws is provided by the National Human Genome Research Institute.25 In addition, the National Institutes of Health has a website that may be helpful to many patients26 (and a much more complex site for health professionals).27 At the same time, courts have resisted plaintiffs/patients who have tried to use informed consent as a way of suing for failure to offer genetic testing.28,29
The failure to refer. In some cases, a patient should be formally referred for genetics consultation. The considerations here are similar to other circumstances in modern, fast developing medical practice that require special sensitivity to those occasions in which a patient will benefit from additional expertise. It is a principle that the AMA Council on Ethical and Judicial Affairs has expressed this way: “In the absence of adequate expertise in pretest and posttest counseling, a physician should refer the patient to an appropriate specialist.”30 The failure to refer, when that deviates from acceptable practice, may result in liability.
Informing others. BRCA testing is an area of medicine in which results may be of great significance not only to the patient but also to the patient’s family.31 Physicians should counsel patients on the importance of informing relatives about relevant results and “should make themselves available to assist patients in communicating with relatives to discuss opportunities for counseling and testing, as appropriate.”30 The question may arise, however, of whether in some circumstances physicians should go a step further in ensuring relatives receive important information regarding their loved one’s health.32 The law has been reluctant to impose liability to “third parties” (someone not a patient). Duties usually arise through the physician-patient relationship. There are exceptions. Perhaps the best known has been the obligation of mental health professionals to take action to protect third parties from patients who have made believable threats against identifiable victims.33 There are indications that some courts could find, in extreme circumstances, a “duty to warn” nonpatients in some instances where it is essential to inform third parties that they should receive a specific form of genetic testing.34,35 Such a duty would, of course, have to protect the privacy rights of the patient to the maximum extent possible. A general duty of this type has not been established widely, but may be part of the future.
Continue to: Was there liability in our example case?...
Was there liability in our example case?
The hypothetical case provided above suggests that there could be liability. Routine medical history by the primary care physician would have produced the fact that the patient’s mother, sister, and maternal grandmother had breast cancer. That would clearly have put her in a category of those who should have received genetic testing. Yet, she was not tested until after her cancer was found. From the limited facts we have, it appears that this timeline of events would have been outside accepted practice—and negligent. The case was not pursued by the patient, however, and this may represent the current state of liability for BRCA issues.
The extent of liability seems to be significant
Our discussion of liability suggests that there is significant potential for BRCA testing negligence within practice, and that the damages in these cases could be substantial. Yet the predicted “tsunami” of malpractice lawsuits related to genetic testing has not appeared.36,37 One study of cases in the United States (through 2016) found a “slowly rising tide” of liability cases instead of a tsunami,24 as the number of claims made was low. On the other hand, the payments where damages were awarded were an order of magnitude larger than other malpractice cases—a mean of $5.3 million and median of $2 million. This is compared with mean values in the range of $275,000 to $600,000 in other areas of malpractice.
The majority of the genetic malpractice cases involve prenatal and newborn testing, and diagnosis/susceptibility/pharmacogenomic accounting for about 25% of cases. In terms of type of errors claimed, approximately 50% were diagnostic-interpretation errors, 30% failure to offer testing, nearly 20% failure to return test results to the patients, and a few remaining cases of failure to properly treat in light of genetic testing.24
Despite a few very large payments, however, the fact remains that there is a surprisingly low number of genetics malpractice cases. Gary Marchant and colleagues suggest that several reasons may account for this:
- the clinical implementation of genetic science has been slower than expected
- the lack of expertise of many physicians in genetic science
- expert witnesses have sometimes been hard to find
- the lack of understanding by plaintiffs’ attorneys of genetic malpractice
- potential plaintiffs’ lack of understanding of the nature of genetic testing and the harms resulting from genetic negligence.17,24,37
The tide is slowly coming in
By all appearances, there is every reason to think that genetic malpractice will be increasing, and that the recent past of much higher damages per claim paid in the genetics area will be part of that tide. The National Human Genome Research LawSeq project has suggested a number of useful ways of dealing with the liability issues.18 In addition to the BRCA issues that we have considered in this article for ObGyns, there are other critical issues of prenatal and newborn genetic testing.38 But those are topics for another day. ●
CASE Young woman with family history of breast cancer detects lump
Two weeks after noting a lump on her breast when her cat happened to jump on her in that spot, a 28-year-old woman (G0) went to her primary care provider. She was referred to her gynecologist; breast imaging, ultrasonography, and mammography were obtained, with microcalcifications noted. A fine needle aspiration diagnosed intraductal malignancy. The surgical breast tissue specimen was estrogen receptor (ER)- and progestogen receptor (PR)-positive and HER2-negative. Other tumor markers were obtained, including carcinoembryonic antigen, and tissue polypeptide specific antigen, p53, cathepsin D, cyclin E, and nestin, but results were not available.
With regard to family history, the woman’s mother and maternal grandmother had a history of breast cancer. The patient and her family underwent gene testing. The patient was found to be BRCA1- and BRCA2-positive; her mother was BRCA1-positive, an older sister was BRCA2-positive, and her grandmother was not tested.
The question arose in light of her family history as to why she was not tested for BRCA and appropriately counseled by her gynecologist prior to the cancer diagnosis. Litigation was initiated. While the case did not go forward regarding litigation, it is indeed a case in point. (Please note that this is a hypothetical case. It is based on a composite of several cases.)
Medical considerations
Breast cancer is the most common type of cancer affecting women in the Western world.1 Advances in clinical testing for gene mutations have escalated and allowed for identification of patients at increased risk for breast and ovarian cancer. Along with these advances come professional liability risk. After looking at the medical considerations for BRCA1 and 2 testing, we will consider a number of important legal issues. In the view of some commentators, the failure to diagnose genetic mutations in patients predisposed to cancer is “poised to become the next wave of medical professional liability lawsuits.”2
BRCA1 and BRCA2 genes provide tumor suppressor proteins, and assessment for mutations is recommended for individuals at high risk for breast and/or ovarian cancer; mutations in BRCA genes cause DNA damage, which increases the chance of developing cancer. The other way to look at it is, BRCA1 and 2 are tumor suppressor genes that are integrally involved with DNA damage control. Once there is a mutation, it adversely affects the beneficial effects of the gene. Mutations in these genes account for 5% to 10% of all hereditary breast cancers.3 Of note, men with BRCA2 are at increased risk for prostate cancer.
A patient who presents to her gynecologist stating that there is a family history of breast cancer, without knowledge of genetic components, presents a challenge (and a medicolegal risk) for the provider to assess. Prediction models have been used to determine specific patient risk for carrying a genetic mutation with resultant breast cancer development.4 Risk prediction models do not appear to be a good answer to predicting who is more likely to develop breast or ovarian cancer, however. A Mayo model may assist (FIGURE).5 Clinicians should also be aware of other models of risk assessment, including the Gail Model (TABLE 1).6


Continue to: Guidelines for genetic testing...
Guidelines for genetic testing
The American College of Obstetricians and Gynecologists states that patient medical history and family history are paramount in obtaining information regarding risk for breast and ovarian cancer. First- and second-degree relatives are allocated to this category. Information regarding age of diagnosis, maternal and paternal lineage, and ethnic background can imply a need for genetic testing (TABLE 2).7,8 A number of genetics national organizations have participated in recommendations and include the American College of Medical Genetics and Genomics, the National Society for Genetic Counselors, and the Society of Gynecologic Oncology.7

The question always surfaces, could the clinical outcome of the cancer when diagnosed have been changed if screening were undertaken, with earlier diagnosis, or prevented with prophylactic mastectomy, and changed the end result. In addition, it is well known that breast augmentation mammoplasty alters the ability to accurately evaluate mammograms. Patients considering this type of plastic surgery, ideally, should be counselled accordingly.9
Bottom line, we as clinicians must be cognizant of both ACOG and United States Preventive Services Task Force (USPSTF) recommendations regarding screening and gene testing for women considered high risk for breast cancer based on family history.7
Legal considerations
The case presented demonstrates that the discovery of the BRCA1 and BRCA2 genes, and reliable tests for determining the existence of the genes, brought with them legal issues as well as medical advantages. We look at professional liability (malpractice) questions this technology raises, and then consider the outcome of the hypothetical case. (BRCA is used here to apply broadly—not only to BRCA1 and 2 but also to PALB2, CHEK2, and similar genetic abnormalities.)
To date, the most visible BRCA legal issues covered in cases and law reviews have focused more on patent law than malpractice. The most important of these was a decision of the US Supreme Court in Association for Molecular Pathology v Myriad Genetics.10 The US Patent Office was granting patents to companies finding useful, naturally occurring segments of human DNA, and had granted Myriad several patents on BRCA1 and BRCA2 genes. This patent policy had the potential to seriously interfere with broad scientific use of these genes.11 Fortunately, the Supreme Court stepped in and unanimously invalidated such patents. It held that a “naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated.” The Court noted, “Finding the location of the BRCA1 and BRCA2 genes does not render the genes patent eligible ‘new . . . composition[s] of matter.’”8 The Court did allow the patenting of tests for specific gene structures, and artificial changes in naturally occurring genes.
Malpractice and BRCA
While the BRCA patent wars have lingered, the potential for a significant increase in BRCA-related malpractice cases is of increasing concern. Like most malpractice liability, these new claims are based on very old principles of negligence.12 To prevail, the plaintiff (ordinarily, an injured patient) must demonstrate 4 things:
- A duty. That is, the physician owed a duty to the injured party. Usually (but not always) that requires a professional relationship between the physician and the person injured.
- A breach of that duty. Malpractice liability is based on the fact that the physician did something that a reasonably careful physician (generally, of the same specialty) would not have done, or that the physician failed to do something that a reasonable physician would have done. This usually means that the profession itself sees what the physician did (or did not do) as medically inappropriate. In medical malpractice cases, that is ordinarily measured by what the usual or common practice is among prudent physicians. In rare circumstances, courts have found the standard practice of a profession to be negligent. Where, for example, it was custom for a professional not to give an eye pressure test to anyone under age 40, a court found that common standard to be inappropriate.13 In the words of Judge Learned Hand (speaking about a different case), “a whole calling may have unduly lagged in the adoption of new and available devices. It never may set its own tests.”14 Underlying negligence is a cost-benefit analysis (discussed below).
- Damages. There must have been some damage that courts recognize, usually loss of money or opportunity to work, the cost of care, pain and suffering, or loss of enjoyment/quality of life. In malpractice, many states now recognize the “loss of chance” or the “loss of a chance.” That means, if a “physician negligently fails to diagnose a curable disease, and the patient is harmed by the disease, the physician should be liable for causing the ‘loss of a chance of a cure.’”15 (Delay in diagnosis is the most common reason for claims in breast cancer care.)16
- Causation. The breach of duty (negligence) must have caused the damages. The causation must have been reasonably close. If a driver drives through a stop sign, or a physician misreads a test, and someone is injured but there is no connection between the negligence and the injury, there is not tort liability.
The 4 elements of malpractice just described are raised in some way in the possible liability associated with BRCA testing. We next look at the ways in which liability may arise from that testing (or lack of it).
Underlying much of the following discussion is the “cost-benefit” consideration noted above. This concept is that the total cost (financial and health) of testing should be compared with the value of the benefits of testing, taking into account the probabilities that the testing will result in better health outcomes. BRCA testing, for example, is essentially cost-free in terms of physical risk. Its financial cost, while not trivial, is not great, and it is commonly covered by health insurance.17 In terms of benefits, the testing has the potential for providing critical information in making treatment decisions for a meaningful percentage of patients and their families. There are many ways of analyzing the liability risks of genetic malpractice,7,18 and the following is intended to discuss some of the greatest risks related to BRCA testing.
Continue to: Areas of liability...
Areas of liability
The failure to recommend a test. The circumstances in which BRCA testing should be undertaken are set out by professional organizations (noted above). These recommendations are not static, however. They change from time to time. Given the potential harm caused by the failure to test in relevant circumstances, malpractice liability is certainly a possibility when the failure to recommend a test to a patient results in a cancer that might have been prevented had the genetic problem been identified in a timely manner. The circumstances in which testing should be considered continue to change, placing an obligation on clinicians to stay well informed of changing genetic understandings. Another risk is that one specialist may assume that it is the job of another specialist to order the test. Whatever the cause of the failure to test, or unnecessary delay in testing, it appears to be the primary basis for BRCA liability.
The failure to properly interpret a test. Any test that is misinterpreted may lead to harm for the patient. A false negative, of course, may mean that preventive treatment that could have been undertaken will be foregone, as a “loss of a chance.” On the other hand, a false positive can lead to radical, unnecessary surgery or treatment. If a misinterpretation occurred because of carelessness by the testing organization, or confusion by a practitioner, there is a likelihood of negligence.19
A different form of “misinterpretation” could be reasonable—and not negligent. Advances in scientific-medical understanding may result in the outcome of tests being reconsidered and changed. That has been the case with genetic testing and breast cancer. The availability of multiple breast cancer SNPs (single nucleotide polymorphisms), and combining this information with other risk factors for example, results in a polygenic risk score that may be at odds with the level of risk from earlier testing.20,21 This naturally leads to the question of when later, updated testing should be recommended to look for a better current interpretation.22,23
The failure to act on BRCA test results. Testing is of no value, of course, if the results are not used properly. Test results or analyses that are not sent to the proper physicians, or are somehow ignored when properly directed, is a “never” event—it should never happen. It almost always would be considered negligence, and if the patient were injured, could lead to liability. Amazingly, one study found that, in genetic testing liability cases, nearly 20% of the claims arose from failure to return test results to patients.24 In addition, when a patient is found to be BRCA-positive, there is an obligation to discuss the options for dealing with the increased risk associated with the gene mutation(s), as well as to recommend the prudent course of action or to refer the patient to someone who will have that discussion.
Informed consent to the patient. BRCA testing requires informed consent. The physical risks of the testing process are minimal, of course, but it carries a number of other emotional and family risks. The informed consent process is an invitation to an honest discussion between clinicians and patients. It should be an opportunity to discuss what the testing is, and is not, and what the test may mean for treatment. It may also be an opportunity to discuss the implications for other members of the patient’s family (noted below).
One element of informed consent is a discussion of the consequences of failure to consent, or to undertake one of the alternatives. In the case of BRCA testing, this is especially important in cases in which a patient expresses a hesitancy to be tested with an “I’d rather not know philosophy.” Although clinicians should not practice law, some patient concerns about discrimination may be addressed by the protection that the federal Genetic Information Nondiscrimination Act (GINA) and other laws provide (which prohibit insurance and employment discrimination based on genetic information). A good source of information about GINA and related nondiscrimination laws is provided by the National Human Genome Research Institute.25 In addition, the National Institutes of Health has a website that may be helpful to many patients26 (and a much more complex site for health professionals).27 At the same time, courts have resisted plaintiffs/patients who have tried to use informed consent as a way of suing for failure to offer genetic testing.28,29
The failure to refer. In some cases, a patient should be formally referred for genetics consultation. The considerations here are similar to other circumstances in modern, fast developing medical practice that require special sensitivity to those occasions in which a patient will benefit from additional expertise. It is a principle that the AMA Council on Ethical and Judicial Affairs has expressed this way: “In the absence of adequate expertise in pretest and posttest counseling, a physician should refer the patient to an appropriate specialist.”30 The failure to refer, when that deviates from acceptable practice, may result in liability.
Informing others. BRCA testing is an area of medicine in which results may be of great significance not only to the patient but also to the patient’s family.31 Physicians should counsel patients on the importance of informing relatives about relevant results and “should make themselves available to assist patients in communicating with relatives to discuss opportunities for counseling and testing, as appropriate.”30 The question may arise, however, of whether in some circumstances physicians should go a step further in ensuring relatives receive important information regarding their loved one’s health.32 The law has been reluctant to impose liability to “third parties” (someone not a patient). Duties usually arise through the physician-patient relationship. There are exceptions. Perhaps the best known has been the obligation of mental health professionals to take action to protect third parties from patients who have made believable threats against identifiable victims.33 There are indications that some courts could find, in extreme circumstances, a “duty to warn” nonpatients in some instances where it is essential to inform third parties that they should receive a specific form of genetic testing.34,35 Such a duty would, of course, have to protect the privacy rights of the patient to the maximum extent possible. A general duty of this type has not been established widely, but may be part of the future.
Continue to: Was there liability in our example case?...
Was there liability in our example case?
The hypothetical case provided above suggests that there could be liability. Routine medical history by the primary care physician would have produced the fact that the patient’s mother, sister, and maternal grandmother had breast cancer. That would clearly have put her in a category of those who should have received genetic testing. Yet, she was not tested until after her cancer was found. From the limited facts we have, it appears that this timeline of events would have been outside accepted practice—and negligent. The case was not pursued by the patient, however, and this may represent the current state of liability for BRCA issues.
The extent of liability seems to be significant
Our discussion of liability suggests that there is significant potential for BRCA testing negligence within practice, and that the damages in these cases could be substantial. Yet the predicted “tsunami” of malpractice lawsuits related to genetic testing has not appeared.36,37 One study of cases in the United States (through 2016) found a “slowly rising tide” of liability cases instead of a tsunami,24 as the number of claims made was low. On the other hand, the payments where damages were awarded were an order of magnitude larger than other malpractice cases—a mean of $5.3 million and median of $2 million. This is compared with mean values in the range of $275,000 to $600,000 in other areas of malpractice.
The majority of the genetic malpractice cases involve prenatal and newborn testing, and diagnosis/susceptibility/pharmacogenomic accounting for about 25% of cases. In terms of type of errors claimed, approximately 50% were diagnostic-interpretation errors, 30% failure to offer testing, nearly 20% failure to return test results to the patients, and a few remaining cases of failure to properly treat in light of genetic testing.24
Despite a few very large payments, however, the fact remains that there is a surprisingly low number of genetics malpractice cases. Gary Marchant and colleagues suggest that several reasons may account for this:
- the clinical implementation of genetic science has been slower than expected
- the lack of expertise of many physicians in genetic science
- expert witnesses have sometimes been hard to find
- the lack of understanding by plaintiffs’ attorneys of genetic malpractice
- potential plaintiffs’ lack of understanding of the nature of genetic testing and the harms resulting from genetic negligence.17,24,37
The tide is slowly coming in
By all appearances, there is every reason to think that genetic malpractice will be increasing, and that the recent past of much higher damages per claim paid in the genetics area will be part of that tide. The National Human Genome Research LawSeq project has suggested a number of useful ways of dealing with the liability issues.18 In addition to the BRCA issues that we have considered in this article for ObGyns, there are other critical issues of prenatal and newborn genetic testing.38 But those are topics for another day. ●
- Sevilla C, Moatti JP, Reynier CJ, et al. Testing for BRCA1 mutations: a cost-effective analysis. Europ J Human Genetics. 2002;10:599-606.
- Cotton V, Kirkpatrick D. Failure to recommend genetic counseling in breast cancer: is the next wave of medical professional liability lawsuits? Contemp OB/GYN. June 1, 2017.
- Suryavanshi M, Kumar D, Panigrahi M, et al. Detection of false positive mutations in BRCA gene by next generation sequencing. Fam Cancer. 2017;16:311-317.
- Black L, Knoppers B, Avard D, et al. Legal liability and the uncertain nature of risk prediction: the case of breast cancer risk prediction models. Public Health Genomics. 2012;15:335-340.
- McClintock A, Gollab A, Laya M. Breast cancer risk assessment, a step-wise approach for primary care physicians on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275.
- National Cancer Institute. The Breast Cancer Risk Assessment Tool. https://bcrisktool.cancer.gov/. Accessed February 25, 2021.
- Neff J, Richardson G, Phelps J. Legal liabilities associated with hereditary breast and ovarian cancers. J Reprod Med. 2020;65:227-230.
- American College of Obstetricians and Gynecologists. Practice Bulletin No 182: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130:e110-e126.
- Sá dos Reis C, Gremion I, and Meystre NR. Study of breast implants mammography examinations for identification of suitable image quality criteria. Insights Imaging. 2020;11:3.
- Association for Molecular Pathology v Myriad Genetics, 569 U.S. 576 (2013).
- Smith SR. The Supreme Court 2012-2013: dogs, DNA, and DOMA. Register Rep. 2013;39(Fall):26-33.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Helling v Carey, 83 Wn.2d 514, 519 P.2d 981 (1974).
- The T.J. Hooper, 60 F.2d 737, 740 (2d Cir.1932), cert. denied 287 U.S. 662 (1932).
- Fischer DA. Tort recovery for loss of a chance. Wake Forest L Rev. 2001;36:605-655.
- Murphy BL, Ray-Zack MD, Reddy PN, et al. Breast cancer litigation in the 21st century. Ann Surg Oncol. 2018;25:2939- 2947.
- Prince AE. Prevention for those who can pay: insurance reimbursement of genetic-based preventive interventions in the liminal state between health and disease. J Law Biosci. 2015;2:365-395.
- Marchant G, Barnes M, Evans JP, et al; LawSeq Liability Task Force. From genetics to genomics: facing the liability implications in clinical care. J Law Med Ethics. 2020;48:11-43.
- Complaint, Held v Ambry Genetics Corp., No. 15-CV-8683, 2015 WL 6750024 (S.D.N.Y. Nov. 4, 2015); Order of Dismissal, Held v Ambry Genetics Corp., No. 15-CV-8683, (S.D.N.Y. Dec. 6, 2016).
- Pederson HJ. Breast cancer risk assessment and treatment: current concepts in genetics and genomics. Contemp OB/ GYN. 2017; 62:A1-A4.
- Pederson HJ. Who needs breast cancer genetics testing? OBG Manag. 2018;30:34-39.
- Roberts JL, Foulkes A. Genetic duties. William Mary L Rev. 2020;62:143-212.
- Thorogood A, Cook-Deegan R, Knoppers B. Public variant databases: liability? Genet Med. 2017;19:838–841.
- Marchant G, Lindor R. Genomic malpractice: an emerging tide or gentle ripple? Food Drug Law J. 2018;73:1-37.
- National Human Genome Research Institute. Genetic discrimination. https://www.genome.gov/about-genomics /policy-issues/Genetic-Discrimination. Updated September 16, 2020. Accessed February 25, 2021.
- National Cancer Institute. BRCA mutations: cancer risk and genetic testing. https://www.cancer.gov/about-cancer /causes-prevention/genetics/brca-fact-sheet. Reviewed November 19, 2020. Accessed February 25, 2021.
- National Cancer Institute. Genetics of breast and gynecologic cancers (PDQ®)–Health Professional Version. https://www .cancer.gov/types/breast/hp/breast-ovarian-genetics-pdq. Updated February 12, 2021. Accessed February 25, 2021.
- Reed v Campagnolo, 630 A.2d 1145, 1152–54 (Md. 1993).
- Munro v Regents of Univ. of Cal.,263 Cal. Rptr. 878, 885, 988 (1989).
- AMA Council on Ethical and Judicial Affairs. AMA Code of Medical Ethics’ opinions on genetic testing. Opinion 2.131. 2009;11:683-685. https://journalofethics.ama-assn .org/article/ama-code-medical-ethics-opinions-genetictesting/2009-09.
- Gilbar R, Barnoy S. Disclosing genetic test results to the patient’ relatives: how does the law influence clinical practice? J Law Technol Policy. 2019;125-168.
- Song K. Warning third parties of genetic risks in the era of personalized medicine. U.C. Davis L Rev. 2016;49:1987-2018.
- Tarasoff v Regents of the University of California, 551 P.2d 334, 131 Cal. Rptr. 14 (Cal. 1976).
- Safer v Estate of Pack, 677 A.2d 1188 (N.J. App. 1996), cert. denied, 683 A.2d 1163 (N.J. 1996).
- Pate v Threlkel, 661 So.2d 278 (Fla. 1995).
- Rothstein MA. Liability issues in pharmacogenomics. Louisiana L Rev. 2005;66:117-124.
- Marchant G, Lindor R. Personalized medicine and genetic malpractice. Genet Med. 2013;15:921-922.
- Westbrook M. Transforming the physician’s standard of care in the context of whole genome sequencing technologies: finding guidance in best practice standards. Saint Louis U J Health Law Policy. 2015;9:111-148.
- Sevilla C, Moatti JP, Reynier CJ, et al. Testing for BRCA1 mutations: a cost-effective analysis. Europ J Human Genetics. 2002;10:599-606.
- Cotton V, Kirkpatrick D. Failure to recommend genetic counseling in breast cancer: is the next wave of medical professional liability lawsuits? Contemp OB/GYN. June 1, 2017.
- Suryavanshi M, Kumar D, Panigrahi M, et al. Detection of false positive mutations in BRCA gene by next generation sequencing. Fam Cancer. 2017;16:311-317.
- Black L, Knoppers B, Avard D, et al. Legal liability and the uncertain nature of risk prediction: the case of breast cancer risk prediction models. Public Health Genomics. 2012;15:335-340.
- McClintock A, Gollab A, Laya M. Breast cancer risk assessment, a step-wise approach for primary care physicians on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275.
- National Cancer Institute. The Breast Cancer Risk Assessment Tool. https://bcrisktool.cancer.gov/. Accessed February 25, 2021.
- Neff J, Richardson G, Phelps J. Legal liabilities associated with hereditary breast and ovarian cancers. J Reprod Med. 2020;65:227-230.
- American College of Obstetricians and Gynecologists. Practice Bulletin No 182: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130:e110-e126.
- Sá dos Reis C, Gremion I, and Meystre NR. Study of breast implants mammography examinations for identification of suitable image quality criteria. Insights Imaging. 2020;11:3.
- Association for Molecular Pathology v Myriad Genetics, 569 U.S. 576 (2013).
- Smith SR. The Supreme Court 2012-2013: dogs, DNA, and DOMA. Register Rep. 2013;39(Fall):26-33.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Helling v Carey, 83 Wn.2d 514, 519 P.2d 981 (1974).
- The T.J. Hooper, 60 F.2d 737, 740 (2d Cir.1932), cert. denied 287 U.S. 662 (1932).
- Fischer DA. Tort recovery for loss of a chance. Wake Forest L Rev. 2001;36:605-655.
- Murphy BL, Ray-Zack MD, Reddy PN, et al. Breast cancer litigation in the 21st century. Ann Surg Oncol. 2018;25:2939- 2947.
- Prince AE. Prevention for those who can pay: insurance reimbursement of genetic-based preventive interventions in the liminal state between health and disease. J Law Biosci. 2015;2:365-395.
- Marchant G, Barnes M, Evans JP, et al; LawSeq Liability Task Force. From genetics to genomics: facing the liability implications in clinical care. J Law Med Ethics. 2020;48:11-43.
- Complaint, Held v Ambry Genetics Corp., No. 15-CV-8683, 2015 WL 6750024 (S.D.N.Y. Nov. 4, 2015); Order of Dismissal, Held v Ambry Genetics Corp., No. 15-CV-8683, (S.D.N.Y. Dec. 6, 2016).
- Pederson HJ. Breast cancer risk assessment and treatment: current concepts in genetics and genomics. Contemp OB/ GYN. 2017; 62:A1-A4.
- Pederson HJ. Who needs breast cancer genetics testing? OBG Manag. 2018;30:34-39.
- Roberts JL, Foulkes A. Genetic duties. William Mary L Rev. 2020;62:143-212.
- Thorogood A, Cook-Deegan R, Knoppers B. Public variant databases: liability? Genet Med. 2017;19:838–841.
- Marchant G, Lindor R. Genomic malpractice: an emerging tide or gentle ripple? Food Drug Law J. 2018;73:1-37.
- National Human Genome Research Institute. Genetic discrimination. https://www.genome.gov/about-genomics /policy-issues/Genetic-Discrimination. Updated September 16, 2020. Accessed February 25, 2021.
- National Cancer Institute. BRCA mutations: cancer risk and genetic testing. https://www.cancer.gov/about-cancer /causes-prevention/genetics/brca-fact-sheet. Reviewed November 19, 2020. Accessed February 25, 2021.
- National Cancer Institute. Genetics of breast and gynecologic cancers (PDQ®)–Health Professional Version. https://www .cancer.gov/types/breast/hp/breast-ovarian-genetics-pdq. Updated February 12, 2021. Accessed February 25, 2021.
- Reed v Campagnolo, 630 A.2d 1145, 1152–54 (Md. 1993).
- Munro v Regents of Univ. of Cal.,263 Cal. Rptr. 878, 885, 988 (1989).
- AMA Council on Ethical and Judicial Affairs. AMA Code of Medical Ethics’ opinions on genetic testing. Opinion 2.131. 2009;11:683-685. https://journalofethics.ama-assn .org/article/ama-code-medical-ethics-opinions-genetictesting/2009-09.
- Gilbar R, Barnoy S. Disclosing genetic test results to the patient’ relatives: how does the law influence clinical practice? J Law Technol Policy. 2019;125-168.
- Song K. Warning third parties of genetic risks in the era of personalized medicine. U.C. Davis L Rev. 2016;49:1987-2018.
- Tarasoff v Regents of the University of California, 551 P.2d 334, 131 Cal. Rptr. 14 (Cal. 1976).
- Safer v Estate of Pack, 677 A.2d 1188 (N.J. App. 1996), cert. denied, 683 A.2d 1163 (N.J. 1996).
- Pate v Threlkel, 661 So.2d 278 (Fla. 1995).
- Rothstein MA. Liability issues in pharmacogenomics. Louisiana L Rev. 2005;66:117-124.
- Marchant G, Lindor R. Personalized medicine and genetic malpractice. Genet Med. 2013;15:921-922.
- Westbrook M. Transforming the physician’s standard of care in the context of whole genome sequencing technologies: finding guidance in best practice standards. Saint Louis U J Health Law Policy. 2015;9:111-148.
Home pregnancy tests—Is ectopic always on your mind?
CASE Unidentified ectopic pregnancy leads to rupture*
A 33-year-old woman (G1 P0010) with 2 positive home pregnancy tests presents to the emergency department (ED) reporting intermittent vaginal bleeding for 3 days. Her last menstrual period was 10 weeks ago, but she reports that her menses are always irregular. She has a history of asymptomatic chlamydia, as well as spontaneous abortion 2 years prior. At present, she denies abdominal pain or vaginal discharge.
Upon examination her vital signs are: temperature, 98.3 °F; pulse, 112 bpm, with a resting rate of 16 bpm; blood pressure (BP), 142/91 mm Hg; pulse O2, 99%; height, 4’ 3”; weight, 115 lb. Her labs are: hemoglobin, 12.1 g/dL; hematocrit, 38%; serum human chorionic gonadotropin (hCG) 236 mIU/mL. Upon pelvic examination, no active bleeding is noted. She agrees to be followed up by her gynecologist and is given a prescription for serum hCG in 2 days. She is instructed to return to the ED should she have pain or increased vaginal bleeding.
Three days later, the patient follows up with her gynecologist reporting mild cramping. She notes having had an episode of heavy vaginal bleeding and a “weakly positive” home pregnancy test. Transvaginal ultrasonography notes endometrial thickness 0.59 mm and unremarkable adnexa. A urine pregnancy test performed in the office is positive; urinalysis is positive for nitrites. With the bleeding slowed, the gynecologist’s overall impression is that the patient has undergone complete spontaneous abortion. She prescribes Macrobid for the urinary tract infection. She does not obtain the ED-prescribed serum HCG levels, as she feels, since complete spontaneous abortion has occurred there is no need to obtain a follow-up serum HCG.
Five days later, the patient returns to the ED reporting abdominal pain after eating. Fever and productive cough of 2 days are noted. The patient states that she had a recent miscarriage. The overall impression of the patient’s condition is bronchitis, and it is noted on the patient’s record, “unlikely ectopic pregnancy and pregnancy test may be false positive,” hence a pregnancy test is not ordered. Examination reveals mild suprapubic tenderness with no rebound; no pelvic exam is performed. The patient is instructed to follow up with a health care clinic within a week, and to return to the ED with severe abdominal pain, higher fever, or any new concerning symptoms. A Zithromax Z-pak is prescribed.
Four days later, the patient is brought by ambulance to the ED of the local major medical center with severe abdominal pain involving the right lower quadrant. She states that she had a miscarriage 3 weeks prior and was recently treated for bronchitis. She has dizziness when standing. Her vital signs are: temperature, 97.8 °F; heart rate, 95 bpm; BP, 72/48 mm Hg; pulse O2, 100%. She reports her abdominal pain to be 6/10.
The patient is given a Lactated Ringer’s bolus of 1,000 mL for a hypotensive episode. Computed tomography is obtained and notes, “low attenuation in the left adnexa with a dilated fallopian tube.” A large heterogeneous collection of fluid in the pelvis is noted with active extravasation, consistent with an “acute bleed.”
The patient is brought to the operating room with a diagnosis of probable ruptured ectopic pregnancy. Intraoperatively she is noted to have a right ruptured ectopic and left tubo-ovarian abscess. The surgeon proceeds with right salpingectomy and left salpingo-oophorectomy. Three liters of hemoperitoneum is found.
She is followed postoperatively with serum hCG until levels are negative. Her postoperative course is uneventful. Her only future option for pregnancy is through assisted reproductive technology (ART) with in vitro fertilization (IVF). The patient sues the gynecologist and second ED physician for presumed inappropriate assessment for ectopic pregnancy.
*The “facts” of this case are a composite, drawn from several cases to illustrate medical and legal issues. The statement of facts should be considered hypothetical.
Continue to: WHAT’S THE VERDICT?...
WHAT’S THE VERDICT?
A defense verdict is returned.
Medical considerations
The incidence of ectopic pregnancy is 2% of all pregnancies, with a higher incidence (about 4%) among infertility patients.1 Up to 10% of ectopic pregnancies have no symptoms.2
Clinical presentations. Classic signs of ectopic pregnancy include:
- abdominal pain
- vaginal bleeding
- late menses (often noted).
A recent case of ectopic pregnancy presenting with chest pain was reported.3 Clinicians must never lose site of the fact that ectopic pregnancy is the most common cause of maternal mortality in the first trimester, with an incidence of 1% to 10% of all first-trimester deaths.4
Risk factors include pelvic inflammatory disease, as demonstrated in the opening case. “The silent epidemic of chlamydia” comes to mind, and tobacco smoking can adversely affect tubal cilia, as can pelvic adhesions and/or prior tubal surgery. All of these factors can predispose a patient to ectopic pregnancy; in addition, intrauterine devices, endometriosis, tubal ligation (or ligation reversal), all can set the stage for an ectopic pregnancy.5 Appropriate serum hCG monitoring during early pregnancy can assist in sorting out pregnancies of unknown location (PUL; FIGURE). First trimester ultrasonography, at 5 weeks gestation, usually identifies early intrauterine gestation.

Imaging. With regard to pelvic sonography, the earliest sign of an intrauterine pregnancy (IUP) is a sac eccentrically located in the decidua.6 As the IUP progresses, it becomes equated with a “double decidual sign,” with double rings of tissue around the sac.6 If the pregnancy is located in an adnexal mass, it is frequently inhomogeneous or noncystic in appearance (ie, “the blob” sign); the positive predictive value (PPV) is 96%.2 The PPV of transvaginal ultrasound is 80%, as paratubal, paraovarian, ovarian cyst, and hydrosalpinx can affect the interpretation.7
Heterotopic pregnancy includes an intrauterine gestation and an ectopic pregnancy. This presentation includes the presence of a “pseudosac” in the endometrial cavity plus an extrauterine gestation. Heterotopic pregnancies have become somewhat more common as ART/IVF has unfolded, especially prior to the predominance of single embryo transfer.
Managing ectopic pregnancy
For cases of early pregnancy complicated by intermittent bleeding and/or pain, monitoring with serum hCG levels at 48-hour intervals to distinguish a viable IUP from an abnormal IUP or an ectopic is appropriate. The “discriminatory zone” collates serum hCG levels with findings on ultrasonography. Specific lower limits of serum hCG levels are not clear cut, with recommendations of 3,500 mIU/mL to provide sonographic evidence of an intrauterine gestation “to avoid misdiagnosis and possible interruption of intrauterine pregnancy,” as conveyed in the American College of Obstetricians and Gynecologists 2018 practice bulletin.8 Serum progesterone levels also have been suggested to complement hCG levels; a progesterone level of <20 nmol/L is consistent with an abnormal pregnancy, whereas levels >25 nmol/L are suggestive of a viable pregnancy.2 Inhibin A levels also have been suggested to be helpful, but they are not an ideal monitoring tool.
While most ectopic pregnancies are located in the fallopian tube, other locations also can be abdominal or ovarian. In addition, cesarean scar ectopic pregnancy can occur and often is associated with delay in diagnosis and greater morbidity due to such delay.9 With regard to ovarian ectopic, Spiegelberg criteria are established for diagnosis (TABLE 1).10
Appropriate management of an ectopic pregnancy is dependent upon the gestational age, serum hCG levels, and imaging findings, as well as the patient’s symptoms and exam findings. Treatment is established in large part on a case-by-case basis and includes, for early pregnancy, expectant management and use of methotrexate (TABLE 2).11 Dilation and curettage may be used to identify the pregnancy’s location when the serum hCG level is below 2,000 mIU/mL and there is no evidence of an IUP on ultrasound. Surgical treatment can include minimally invasive salpingostomy or salpingectomy and, depending on circumstance, laparotomy may be indicated.

Fertility following ectopic pregnancy varies and is affected by location, treatment, predisposing factors, total number of ectopic pregnancies, and other factors. Ectopic pregnancy, although rare, also can occur with use of IVF. Humans are not unique with regard to ectopic pregnancies, as they also occur in sheep.12
Continue to: Legal perspective...
Legal perspective
Lawsuits related to ectopic pregnancy are not a new phenomenon. In fact, in 1897, a physician in Ohio who misdiagnosed an “extrauterine pregnancy” as appendicitis was the center of a malpractice lawsuit.13 Unrecognized or mishandled ectopic pregnancy can result in serious injuries—in the range of 1% to 10% (see above) of maternal deaths are related to ectopic pregnancy.14 Ectopic pregnancy cases, therefore, have been the subject of substantial litigation over the years. An informal, noncomprehensive review of malpractice lawsuits brought from 2000 to 2019, found more than 300 ectopic pregnancy cases. Given the large number of malpractice claims against ObGyns,15 ectopic pregnancy cases are only a small portion of all ObGyn malpractice cases.16
A common claim: negligent diagnosis or treatment
The most common basis for lawsuits in cases of ectopic pregnancy is the clinician’s negligent failure to properly diagnose the ectopic nature of the pregnancy. There are also a number of cases claiming negligent treatment of an identified ectopic pregnancy. Not every missed diagnosis, or unsuccessful treatment, leads to liability, of course. It is only when a diagnosis or treatment fails to meet the standard of care within the profession that there should be liability. That standard of care is generally defined by what a reasonably prudent physician would do under the circumstances. Expert witnesses, who are familiar with the standard of practice within the specialty, are usually necessary to establish what that practice is. Both the plaintiff and the defense obtain experts, the former to prove what the standard of care is and that the standard was not met in the case at hand. The defense experts are usually arguing that the standard of care was met.17 Inadequate diagnosis of ectopic pregnancy or other condition may arise from a failure to take a sufficient history, conduct an appropriately thorough physical examination, recognize any of the symptoms that would suggest it is present, use and conduct ultrasound correctly, or follow-up appropriately with additional testing.18
A malpractice claim of negligent treatment can involve any the following circumstances19:
- failure to establish an appropriate treatment plan
- prescribing inappropriate medications for the patient (eg, methotrexate, when it is contraindicated)
- delivering the wrong medication or the wrong amount of the right medication
- performing a procedure badly
- undertaking a new treatment without adequate instruction and preparation.
Given the nature and risks of ectopic pregnancy, ongoing, frequent contact with the patient is essential from the point at which the condition is suspected. The greater the risk of harm (probability or consequence), the more careful any professional ought to be. Because ectopic pregnancy is not an uncommon occurrence, and because it can have devastating effects, including death, a reasonably prudent practitioner would be especially aware of the clinical presentations discussed above.20 In the opening case, the treatment plan was not well documented.
Negligence must lead to patient harm. In addition to negligence (proving that the physician did not act in accordance with the standard of care), to prevail in a malpractice case, the plaintiff-patient must prove that the negligence caused the injury, or worsened it. If the failure to make a diagnosis would not have made any difference in a harm the patient suffered, there are no damages and no liability. Suppose, for example, that a physician negligently failed to diagnose ectopic pregnancy, but performed surgery expecting to find the misdiagnosed condition. In the course of the surgery, however, the surgeon discovered and appropriately treated the ectopic pregnancy. (A version of this happened in the old 19th century case mentioned above.) The negligence of the physician did not cause harm, so there are no damages and no liability.
Continue to: Informed consent is vital...
Informed consent is vital
A part of malpractice is informed consent (or the absence of it)—issues that can arise in any medical care.21 It is wise to pay particular attention in cases where the nature of the illness is unknown, and where there are significant uncertainties and the nature of testing and treatment may change substantially over a period of a few days or few weeks. As always, informed consent should include a discussion of what process or procedure is proposed, its risks and benefits, alternative approaches that might be available, and the risk of doing nothing. Frequently, the uncertainty of ectopic pregnancy complicates the informed consent process.22
Because communication with the patient is an essential function of informed consent, the consent process should productively be used in PUL and similar cases to inform the patient about the uncertainty, and the testing and (nonsurgical) treatment that will occur. This is an opportunity to reinforce the message that the patient must maintain ongoing communication with the physician’s office about changes in her condition, and appear for each appointment scheduled. If more invasive procedures—notably surgery—become required, a separate consent process should be completed, because the risks and considerations are now meaningfully different than when treatment began. As a general matter, any possible treatment that may result in infertility or reduced reproductive capacity should specifically be included in the consent process.
In the hypothetical case, the gynecologist failed to obtain a follow-up serum hCG level. In addition, the record did not reflect ectopic pregnancy in the differential diagnosis. As noted above, the patient had predisposing factors for an ectopic pregnancy. The physician should have acknowledged the history of sexually transmitted disease predisposing her to an ectopic pregnancy. Monitoring of serum hCG levels until they are negative is appropriate with ectopic, or presumed ectopic, pregnancy management. Appropriate monitoring did not occur in this case. Each of these errors (following up on serum hCG levels and the inadequacy of notations about the possibility of ectopic pregnancy) seem inconsistent with the usual standard of care. Furthermore, as a result of the outcome, the only future option for the patient to pursue pregnancy was IVF.
Other legal issues
There are a number of other legal issues that are associated with the topic of ectopic pregnancy. There is evidence, for example, that Catholic and non-Catholic hospitals treat ectopic pregnancies differently,23 which may reflect different views on taking a life or the use of methotrexate and its association with abortion.24 In addition, the possibility of an increase in future ectopic pregnancies is one of the “risks” of abortion that pro-life organizations have pushed to see included in abortion informed consent.25 This has led some commentators to conclude that some Catholic hospitals violate federal law in managing ectopic pregnancy. There is also evidence of “overwhelming rates of medical misinformation on pregnancy center websites, including a link between abortion and ectopic pregnancy.”26
The fact that cesarean deliveries are related to an increased risk for ectopic pregnancy (because of the risk of cesarean scar ectopic pregnancy) also has been cited as information that should play a role in the consent process for cesarean delivery.27 In terms of liability, failed tubal ligation leads to a 33% risk of ectopic pregnancy.28 The risk of ectopic pregnancy is also commonly included in surrogacy contracts.29
Why the outcome was for the defense
The opening hypothetical case illustrates some of the uncertainties of medical malpractice cases. As noted, there appeared a deviation from the usual standard of care, particularly the failure to follow up on the serum hCG level. The weakness in the medical record, failing to note the possibility of ectopic pregnancy, also was probably an error but, apparently, the court felt that this did not result in any harm to the patient.
The question arises of how there would be a defense verdict in light of the failure to track consecutive serum hCG levels. A speculative explanation is that there are many uncertainties in most lawsuits. Procedural problems may result in a case being limited, expert witnesses are essential to both the plaintiff and defense, with the quality of their review and testimony possibly uneven. Judges and juries may rely on one expert witness rather than another, juries vary, and the quality of advocacy differs. Any of these situations can contribute to the unpredictability of the outcome of a case. In the case above, the liability was somewhat uncertain, and the various other factors tipped in favor of a defense verdict. ●
- Centers for Disease Control and Prevention. Ectopic pregnancy—United States, 1990‒1992. MMWR Morb Mortal Wkly Rep. 1995;44:46-48.
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2012;20:250-261.
- Dichter E, Espinosa J, Baird J, Lucerna A. An unusual emergency department case: ruptured ectopic pregnancy presenting as chest pain. World J Emerg Med. 2017;8:71-73.
- Cecchino GN, Araujo E, Elito J. Methotrexate for ectopic pregnancy: when and how. Arch Gynecol Obstet. 2014;290:417- 423.
- Barnhart KT, Sammel MD, Cracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic firsttrimester pregnancies. Fertil Steril. 2006;86:36-43.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Barnhart KT, Fay CA, Suescum M, et al. Clinical factors affecting the accuracy of ultrasonography in symptomatic first-trimester pregnancy. Obstet Gynecol. 2011;117:299-306.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 193: tubal ectopic pregnancy. Obstet Gynecol. 2018;131:e91-e103.
- Bouyer J, Coste J, Fernandez H, et al. Sites of ectopic pregnancy: a 10-year population-based study of 1800 cases. Hum Reprod. 2002;17:3224-3230.
- Spiegelberg O. Zur casuistic der ovarial schwangerschaft. Arch Gynecol. 1978;13:73.
- OB Hospitalist Group. Methotrexate use for ectopic pregnancies guidelines. https://www.obhg.com/wp-content /uploads/2020/01/Methotrexate-Use-for-EctopicPregnancies_2016-updates.pdf. Accessed December 10, 2020.
- Brozos C, Kargiannis I, Kiossis E, et al. Ectopic pregnancy through a caesarean scar in a ewe. N Z Vet J. 2013;61:373-375.
- Tucker v. Gillette, 12 Ohio Cir. Dec. 401 (Cir. Ct. 1901).
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130:366-373.
- Matthews LR, Alvi FA, Milad MP. Reproductive surgery malpractice patterns. Fertil Steril. 2016;106:e42-e43.
- Kim B. The impact of malpractice risk on the use of obstetrics procedures. J Legal Studies. 2006;36:S79-S120.
- Abinader R, Warsof S. Complications involving obstetrical ultrasound. In: Warsof S, Shwayder JM, eds. Legal Concepts and Best Practices in Obstetrics: The Nuts and Bolts Guide to Mitigating Risk. 2019;45-48.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Shwayder JM. IUP diagnosed and treated as ectopic: How bad can it get? Contemporary OB/GYN. 2019;64:49-46.
- Kaplan AI. Should this ectopic pregnancy have been diagnosed earlier? Contemporary OB/GYN. 2017;62:53.
- American College of Obstetricians and Gynecologists Committee on Ethics. Committee opinion 439: informed consent. Reaffirmed 2015. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2009/08 /informed-consent. Accessed December 9, 2020.
- Shwayder JM. Liability in ob/gyn ultrasound. Contemporary OB/GYN. 2017;62:32-49.
- Fisher LN. Institutional religious exemptions: a balancing approach. BYU Law Review. 2014;415-444.
- Makdisi J. Aquinas’s prohibition of killing reconsidered. J Catholic Legal Stud. 2019:57:67-128.
- Franzonello A. Remarks of Anna Franzonello. Alb Law J Sci Tech. 2012;23:519-530.
- Malcolm HE. Pregnancy centers and the limits of mandated disclosure. Columbia Law Rev. 2019;119:1133-1168.
- Kukura E. Contested care: the limitations of evidencebased maternity care reform. Berkeley J Gender Law Justice. 2016;31:241-298.
- Donley G. Contraceptive equity: curing the sex discrimination in the ACA’s mandate. Alabama Law Rev. 2019;71:499-560.
- Berk H. Savvy surrogates and rock star parents: compensation provisions, contracting practices, and the value of womb work. Law Social Inquiry. 2020;45:398-431.
CASE Unidentified ectopic pregnancy leads to rupture*
A 33-year-old woman (G1 P0010) with 2 positive home pregnancy tests presents to the emergency department (ED) reporting intermittent vaginal bleeding for 3 days. Her last menstrual period was 10 weeks ago, but she reports that her menses are always irregular. She has a history of asymptomatic chlamydia, as well as spontaneous abortion 2 years prior. At present, she denies abdominal pain or vaginal discharge.
Upon examination her vital signs are: temperature, 98.3 °F; pulse, 112 bpm, with a resting rate of 16 bpm; blood pressure (BP), 142/91 mm Hg; pulse O2, 99%; height, 4’ 3”; weight, 115 lb. Her labs are: hemoglobin, 12.1 g/dL; hematocrit, 38%; serum human chorionic gonadotropin (hCG) 236 mIU/mL. Upon pelvic examination, no active bleeding is noted. She agrees to be followed up by her gynecologist and is given a prescription for serum hCG in 2 days. She is instructed to return to the ED should she have pain or increased vaginal bleeding.
Three days later, the patient follows up with her gynecologist reporting mild cramping. She notes having had an episode of heavy vaginal bleeding and a “weakly positive” home pregnancy test. Transvaginal ultrasonography notes endometrial thickness 0.59 mm and unremarkable adnexa. A urine pregnancy test performed in the office is positive; urinalysis is positive for nitrites. With the bleeding slowed, the gynecologist’s overall impression is that the patient has undergone complete spontaneous abortion. She prescribes Macrobid for the urinary tract infection. She does not obtain the ED-prescribed serum HCG levels, as she feels, since complete spontaneous abortion has occurred there is no need to obtain a follow-up serum HCG.
Five days later, the patient returns to the ED reporting abdominal pain after eating. Fever and productive cough of 2 days are noted. The patient states that she had a recent miscarriage. The overall impression of the patient’s condition is bronchitis, and it is noted on the patient’s record, “unlikely ectopic pregnancy and pregnancy test may be false positive,” hence a pregnancy test is not ordered. Examination reveals mild suprapubic tenderness with no rebound; no pelvic exam is performed. The patient is instructed to follow up with a health care clinic within a week, and to return to the ED with severe abdominal pain, higher fever, or any new concerning symptoms. A Zithromax Z-pak is prescribed.
Four days later, the patient is brought by ambulance to the ED of the local major medical center with severe abdominal pain involving the right lower quadrant. She states that she had a miscarriage 3 weeks prior and was recently treated for bronchitis. She has dizziness when standing. Her vital signs are: temperature, 97.8 °F; heart rate, 95 bpm; BP, 72/48 mm Hg; pulse O2, 100%. She reports her abdominal pain to be 6/10.
The patient is given a Lactated Ringer’s bolus of 1,000 mL for a hypotensive episode. Computed tomography is obtained and notes, “low attenuation in the left adnexa with a dilated fallopian tube.” A large heterogeneous collection of fluid in the pelvis is noted with active extravasation, consistent with an “acute bleed.”
The patient is brought to the operating room with a diagnosis of probable ruptured ectopic pregnancy. Intraoperatively she is noted to have a right ruptured ectopic and left tubo-ovarian abscess. The surgeon proceeds with right salpingectomy and left salpingo-oophorectomy. Three liters of hemoperitoneum is found.
She is followed postoperatively with serum hCG until levels are negative. Her postoperative course is uneventful. Her only future option for pregnancy is through assisted reproductive technology (ART) with in vitro fertilization (IVF). The patient sues the gynecologist and second ED physician for presumed inappropriate assessment for ectopic pregnancy.
*The “facts” of this case are a composite, drawn from several cases to illustrate medical and legal issues. The statement of facts should be considered hypothetical.
Continue to: WHAT’S THE VERDICT?...
WHAT’S THE VERDICT?
A defense verdict is returned.
Medical considerations
The incidence of ectopic pregnancy is 2% of all pregnancies, with a higher incidence (about 4%) among infertility patients.1 Up to 10% of ectopic pregnancies have no symptoms.2
Clinical presentations. Classic signs of ectopic pregnancy include:
- abdominal pain
- vaginal bleeding
- late menses (often noted).
A recent case of ectopic pregnancy presenting with chest pain was reported.3 Clinicians must never lose site of the fact that ectopic pregnancy is the most common cause of maternal mortality in the first trimester, with an incidence of 1% to 10% of all first-trimester deaths.4
Risk factors include pelvic inflammatory disease, as demonstrated in the opening case. “The silent epidemic of chlamydia” comes to mind, and tobacco smoking can adversely affect tubal cilia, as can pelvic adhesions and/or prior tubal surgery. All of these factors can predispose a patient to ectopic pregnancy; in addition, intrauterine devices, endometriosis, tubal ligation (or ligation reversal), all can set the stage for an ectopic pregnancy.5 Appropriate serum hCG monitoring during early pregnancy can assist in sorting out pregnancies of unknown location (PUL; FIGURE). First trimester ultrasonography, at 5 weeks gestation, usually identifies early intrauterine gestation.

Imaging. With regard to pelvic sonography, the earliest sign of an intrauterine pregnancy (IUP) is a sac eccentrically located in the decidua.6 As the IUP progresses, it becomes equated with a “double decidual sign,” with double rings of tissue around the sac.6 If the pregnancy is located in an adnexal mass, it is frequently inhomogeneous or noncystic in appearance (ie, “the blob” sign); the positive predictive value (PPV) is 96%.2 The PPV of transvaginal ultrasound is 80%, as paratubal, paraovarian, ovarian cyst, and hydrosalpinx can affect the interpretation.7
Heterotopic pregnancy includes an intrauterine gestation and an ectopic pregnancy. This presentation includes the presence of a “pseudosac” in the endometrial cavity plus an extrauterine gestation. Heterotopic pregnancies have become somewhat more common as ART/IVF has unfolded, especially prior to the predominance of single embryo transfer.
Managing ectopic pregnancy
For cases of early pregnancy complicated by intermittent bleeding and/or pain, monitoring with serum hCG levels at 48-hour intervals to distinguish a viable IUP from an abnormal IUP or an ectopic is appropriate. The “discriminatory zone” collates serum hCG levels with findings on ultrasonography. Specific lower limits of serum hCG levels are not clear cut, with recommendations of 3,500 mIU/mL to provide sonographic evidence of an intrauterine gestation “to avoid misdiagnosis and possible interruption of intrauterine pregnancy,” as conveyed in the American College of Obstetricians and Gynecologists 2018 practice bulletin.8 Serum progesterone levels also have been suggested to complement hCG levels; a progesterone level of <20 nmol/L is consistent with an abnormal pregnancy, whereas levels >25 nmol/L are suggestive of a viable pregnancy.2 Inhibin A levels also have been suggested to be helpful, but they are not an ideal monitoring tool.
While most ectopic pregnancies are located in the fallopian tube, other locations also can be abdominal or ovarian. In addition, cesarean scar ectopic pregnancy can occur and often is associated with delay in diagnosis and greater morbidity due to such delay.9 With regard to ovarian ectopic, Spiegelberg criteria are established for diagnosis (TABLE 1).10
Appropriate management of an ectopic pregnancy is dependent upon the gestational age, serum hCG levels, and imaging findings, as well as the patient’s symptoms and exam findings. Treatment is established in large part on a case-by-case basis and includes, for early pregnancy, expectant management and use of methotrexate (TABLE 2).11 Dilation and curettage may be used to identify the pregnancy’s location when the serum hCG level is below 2,000 mIU/mL and there is no evidence of an IUP on ultrasound. Surgical treatment can include minimally invasive salpingostomy or salpingectomy and, depending on circumstance, laparotomy may be indicated.

Fertility following ectopic pregnancy varies and is affected by location, treatment, predisposing factors, total number of ectopic pregnancies, and other factors. Ectopic pregnancy, although rare, also can occur with use of IVF. Humans are not unique with regard to ectopic pregnancies, as they also occur in sheep.12
Continue to: Legal perspective...
Legal perspective
Lawsuits related to ectopic pregnancy are not a new phenomenon. In fact, in 1897, a physician in Ohio who misdiagnosed an “extrauterine pregnancy” as appendicitis was the center of a malpractice lawsuit.13 Unrecognized or mishandled ectopic pregnancy can result in serious injuries—in the range of 1% to 10% (see above) of maternal deaths are related to ectopic pregnancy.14 Ectopic pregnancy cases, therefore, have been the subject of substantial litigation over the years. An informal, noncomprehensive review of malpractice lawsuits brought from 2000 to 2019, found more than 300 ectopic pregnancy cases. Given the large number of malpractice claims against ObGyns,15 ectopic pregnancy cases are only a small portion of all ObGyn malpractice cases.16
A common claim: negligent diagnosis or treatment
The most common basis for lawsuits in cases of ectopic pregnancy is the clinician’s negligent failure to properly diagnose the ectopic nature of the pregnancy. There are also a number of cases claiming negligent treatment of an identified ectopic pregnancy. Not every missed diagnosis, or unsuccessful treatment, leads to liability, of course. It is only when a diagnosis or treatment fails to meet the standard of care within the profession that there should be liability. That standard of care is generally defined by what a reasonably prudent physician would do under the circumstances. Expert witnesses, who are familiar with the standard of practice within the specialty, are usually necessary to establish what that practice is. Both the plaintiff and the defense obtain experts, the former to prove what the standard of care is and that the standard was not met in the case at hand. The defense experts are usually arguing that the standard of care was met.17 Inadequate diagnosis of ectopic pregnancy or other condition may arise from a failure to take a sufficient history, conduct an appropriately thorough physical examination, recognize any of the symptoms that would suggest it is present, use and conduct ultrasound correctly, or follow-up appropriately with additional testing.18
A malpractice claim of negligent treatment can involve any the following circumstances19:
- failure to establish an appropriate treatment plan
- prescribing inappropriate medications for the patient (eg, methotrexate, when it is contraindicated)
- delivering the wrong medication or the wrong amount of the right medication
- performing a procedure badly
- undertaking a new treatment without adequate instruction and preparation.
Given the nature and risks of ectopic pregnancy, ongoing, frequent contact with the patient is essential from the point at which the condition is suspected. The greater the risk of harm (probability or consequence), the more careful any professional ought to be. Because ectopic pregnancy is not an uncommon occurrence, and because it can have devastating effects, including death, a reasonably prudent practitioner would be especially aware of the clinical presentations discussed above.20 In the opening case, the treatment plan was not well documented.
Negligence must lead to patient harm. In addition to negligence (proving that the physician did not act in accordance with the standard of care), to prevail in a malpractice case, the plaintiff-patient must prove that the negligence caused the injury, or worsened it. If the failure to make a diagnosis would not have made any difference in a harm the patient suffered, there are no damages and no liability. Suppose, for example, that a physician negligently failed to diagnose ectopic pregnancy, but performed surgery expecting to find the misdiagnosed condition. In the course of the surgery, however, the surgeon discovered and appropriately treated the ectopic pregnancy. (A version of this happened in the old 19th century case mentioned above.) The negligence of the physician did not cause harm, so there are no damages and no liability.
Continue to: Informed consent is vital...
Informed consent is vital
A part of malpractice is informed consent (or the absence of it)—issues that can arise in any medical care.21 It is wise to pay particular attention in cases where the nature of the illness is unknown, and where there are significant uncertainties and the nature of testing and treatment may change substantially over a period of a few days or few weeks. As always, informed consent should include a discussion of what process or procedure is proposed, its risks and benefits, alternative approaches that might be available, and the risk of doing nothing. Frequently, the uncertainty of ectopic pregnancy complicates the informed consent process.22
Because communication with the patient is an essential function of informed consent, the consent process should productively be used in PUL and similar cases to inform the patient about the uncertainty, and the testing and (nonsurgical) treatment that will occur. This is an opportunity to reinforce the message that the patient must maintain ongoing communication with the physician’s office about changes in her condition, and appear for each appointment scheduled. If more invasive procedures—notably surgery—become required, a separate consent process should be completed, because the risks and considerations are now meaningfully different than when treatment began. As a general matter, any possible treatment that may result in infertility or reduced reproductive capacity should specifically be included in the consent process.
In the hypothetical case, the gynecologist failed to obtain a follow-up serum hCG level. In addition, the record did not reflect ectopic pregnancy in the differential diagnosis. As noted above, the patient had predisposing factors for an ectopic pregnancy. The physician should have acknowledged the history of sexually transmitted disease predisposing her to an ectopic pregnancy. Monitoring of serum hCG levels until they are negative is appropriate with ectopic, or presumed ectopic, pregnancy management. Appropriate monitoring did not occur in this case. Each of these errors (following up on serum hCG levels and the inadequacy of notations about the possibility of ectopic pregnancy) seem inconsistent with the usual standard of care. Furthermore, as a result of the outcome, the only future option for the patient to pursue pregnancy was IVF.
Other legal issues
There are a number of other legal issues that are associated with the topic of ectopic pregnancy. There is evidence, for example, that Catholic and non-Catholic hospitals treat ectopic pregnancies differently,23 which may reflect different views on taking a life or the use of methotrexate and its association with abortion.24 In addition, the possibility of an increase in future ectopic pregnancies is one of the “risks” of abortion that pro-life organizations have pushed to see included in abortion informed consent.25 This has led some commentators to conclude that some Catholic hospitals violate federal law in managing ectopic pregnancy. There is also evidence of “overwhelming rates of medical misinformation on pregnancy center websites, including a link between abortion and ectopic pregnancy.”26
The fact that cesarean deliveries are related to an increased risk for ectopic pregnancy (because of the risk of cesarean scar ectopic pregnancy) also has been cited as information that should play a role in the consent process for cesarean delivery.27 In terms of liability, failed tubal ligation leads to a 33% risk of ectopic pregnancy.28 The risk of ectopic pregnancy is also commonly included in surrogacy contracts.29
Why the outcome was for the defense
The opening hypothetical case illustrates some of the uncertainties of medical malpractice cases. As noted, there appeared a deviation from the usual standard of care, particularly the failure to follow up on the serum hCG level. The weakness in the medical record, failing to note the possibility of ectopic pregnancy, also was probably an error but, apparently, the court felt that this did not result in any harm to the patient.
The question arises of how there would be a defense verdict in light of the failure to track consecutive serum hCG levels. A speculative explanation is that there are many uncertainties in most lawsuits. Procedural problems may result in a case being limited, expert witnesses are essential to both the plaintiff and defense, with the quality of their review and testimony possibly uneven. Judges and juries may rely on one expert witness rather than another, juries vary, and the quality of advocacy differs. Any of these situations can contribute to the unpredictability of the outcome of a case. In the case above, the liability was somewhat uncertain, and the various other factors tipped in favor of a defense verdict. ●
CASE Unidentified ectopic pregnancy leads to rupture*
A 33-year-old woman (G1 P0010) with 2 positive home pregnancy tests presents to the emergency department (ED) reporting intermittent vaginal bleeding for 3 days. Her last menstrual period was 10 weeks ago, but she reports that her menses are always irregular. She has a history of asymptomatic chlamydia, as well as spontaneous abortion 2 years prior. At present, she denies abdominal pain or vaginal discharge.
Upon examination her vital signs are: temperature, 98.3 °F; pulse, 112 bpm, with a resting rate of 16 bpm; blood pressure (BP), 142/91 mm Hg; pulse O2, 99%; height, 4’ 3”; weight, 115 lb. Her labs are: hemoglobin, 12.1 g/dL; hematocrit, 38%; serum human chorionic gonadotropin (hCG) 236 mIU/mL. Upon pelvic examination, no active bleeding is noted. She agrees to be followed up by her gynecologist and is given a prescription for serum hCG in 2 days. She is instructed to return to the ED should she have pain or increased vaginal bleeding.
Three days later, the patient follows up with her gynecologist reporting mild cramping. She notes having had an episode of heavy vaginal bleeding and a “weakly positive” home pregnancy test. Transvaginal ultrasonography notes endometrial thickness 0.59 mm and unremarkable adnexa. A urine pregnancy test performed in the office is positive; urinalysis is positive for nitrites. With the bleeding slowed, the gynecologist’s overall impression is that the patient has undergone complete spontaneous abortion. She prescribes Macrobid for the urinary tract infection. She does not obtain the ED-prescribed serum HCG levels, as she feels, since complete spontaneous abortion has occurred there is no need to obtain a follow-up serum HCG.
Five days later, the patient returns to the ED reporting abdominal pain after eating. Fever and productive cough of 2 days are noted. The patient states that she had a recent miscarriage. The overall impression of the patient’s condition is bronchitis, and it is noted on the patient’s record, “unlikely ectopic pregnancy and pregnancy test may be false positive,” hence a pregnancy test is not ordered. Examination reveals mild suprapubic tenderness with no rebound; no pelvic exam is performed. The patient is instructed to follow up with a health care clinic within a week, and to return to the ED with severe abdominal pain, higher fever, or any new concerning symptoms. A Zithromax Z-pak is prescribed.
Four days later, the patient is brought by ambulance to the ED of the local major medical center with severe abdominal pain involving the right lower quadrant. She states that she had a miscarriage 3 weeks prior and was recently treated for bronchitis. She has dizziness when standing. Her vital signs are: temperature, 97.8 °F; heart rate, 95 bpm; BP, 72/48 mm Hg; pulse O2, 100%. She reports her abdominal pain to be 6/10.
The patient is given a Lactated Ringer’s bolus of 1,000 mL for a hypotensive episode. Computed tomography is obtained and notes, “low attenuation in the left adnexa with a dilated fallopian tube.” A large heterogeneous collection of fluid in the pelvis is noted with active extravasation, consistent with an “acute bleed.”
The patient is brought to the operating room with a diagnosis of probable ruptured ectopic pregnancy. Intraoperatively she is noted to have a right ruptured ectopic and left tubo-ovarian abscess. The surgeon proceeds with right salpingectomy and left salpingo-oophorectomy. Three liters of hemoperitoneum is found.
She is followed postoperatively with serum hCG until levels are negative. Her postoperative course is uneventful. Her only future option for pregnancy is through assisted reproductive technology (ART) with in vitro fertilization (IVF). The patient sues the gynecologist and second ED physician for presumed inappropriate assessment for ectopic pregnancy.
*The “facts” of this case are a composite, drawn from several cases to illustrate medical and legal issues. The statement of facts should be considered hypothetical.
Continue to: WHAT’S THE VERDICT?...
WHAT’S THE VERDICT?
A defense verdict is returned.
Medical considerations
The incidence of ectopic pregnancy is 2% of all pregnancies, with a higher incidence (about 4%) among infertility patients.1 Up to 10% of ectopic pregnancies have no symptoms.2
Clinical presentations. Classic signs of ectopic pregnancy include:
- abdominal pain
- vaginal bleeding
- late menses (often noted).
A recent case of ectopic pregnancy presenting with chest pain was reported.3 Clinicians must never lose site of the fact that ectopic pregnancy is the most common cause of maternal mortality in the first trimester, with an incidence of 1% to 10% of all first-trimester deaths.4
Risk factors include pelvic inflammatory disease, as demonstrated in the opening case. “The silent epidemic of chlamydia” comes to mind, and tobacco smoking can adversely affect tubal cilia, as can pelvic adhesions and/or prior tubal surgery. All of these factors can predispose a patient to ectopic pregnancy; in addition, intrauterine devices, endometriosis, tubal ligation (or ligation reversal), all can set the stage for an ectopic pregnancy.5 Appropriate serum hCG monitoring during early pregnancy can assist in sorting out pregnancies of unknown location (PUL; FIGURE). First trimester ultrasonography, at 5 weeks gestation, usually identifies early intrauterine gestation.

Imaging. With regard to pelvic sonography, the earliest sign of an intrauterine pregnancy (IUP) is a sac eccentrically located in the decidua.6 As the IUP progresses, it becomes equated with a “double decidual sign,” with double rings of tissue around the sac.6 If the pregnancy is located in an adnexal mass, it is frequently inhomogeneous or noncystic in appearance (ie, “the blob” sign); the positive predictive value (PPV) is 96%.2 The PPV of transvaginal ultrasound is 80%, as paratubal, paraovarian, ovarian cyst, and hydrosalpinx can affect the interpretation.7
Heterotopic pregnancy includes an intrauterine gestation and an ectopic pregnancy. This presentation includes the presence of a “pseudosac” in the endometrial cavity plus an extrauterine gestation. Heterotopic pregnancies have become somewhat more common as ART/IVF has unfolded, especially prior to the predominance of single embryo transfer.
Managing ectopic pregnancy
For cases of early pregnancy complicated by intermittent bleeding and/or pain, monitoring with serum hCG levels at 48-hour intervals to distinguish a viable IUP from an abnormal IUP or an ectopic is appropriate. The “discriminatory zone” collates serum hCG levels with findings on ultrasonography. Specific lower limits of serum hCG levels are not clear cut, with recommendations of 3,500 mIU/mL to provide sonographic evidence of an intrauterine gestation “to avoid misdiagnosis and possible interruption of intrauterine pregnancy,” as conveyed in the American College of Obstetricians and Gynecologists 2018 practice bulletin.8 Serum progesterone levels also have been suggested to complement hCG levels; a progesterone level of <20 nmol/L is consistent with an abnormal pregnancy, whereas levels >25 nmol/L are suggestive of a viable pregnancy.2 Inhibin A levels also have been suggested to be helpful, but they are not an ideal monitoring tool.
While most ectopic pregnancies are located in the fallopian tube, other locations also can be abdominal or ovarian. In addition, cesarean scar ectopic pregnancy can occur and often is associated with delay in diagnosis and greater morbidity due to such delay.9 With regard to ovarian ectopic, Spiegelberg criteria are established for diagnosis (TABLE 1).10
Appropriate management of an ectopic pregnancy is dependent upon the gestational age, serum hCG levels, and imaging findings, as well as the patient’s symptoms and exam findings. Treatment is established in large part on a case-by-case basis and includes, for early pregnancy, expectant management and use of methotrexate (TABLE 2).11 Dilation and curettage may be used to identify the pregnancy’s location when the serum hCG level is below 2,000 mIU/mL and there is no evidence of an IUP on ultrasound. Surgical treatment can include minimally invasive salpingostomy or salpingectomy and, depending on circumstance, laparotomy may be indicated.

Fertility following ectopic pregnancy varies and is affected by location, treatment, predisposing factors, total number of ectopic pregnancies, and other factors. Ectopic pregnancy, although rare, also can occur with use of IVF. Humans are not unique with regard to ectopic pregnancies, as they also occur in sheep.12
Continue to: Legal perspective...
Legal perspective
Lawsuits related to ectopic pregnancy are not a new phenomenon. In fact, in 1897, a physician in Ohio who misdiagnosed an “extrauterine pregnancy” as appendicitis was the center of a malpractice lawsuit.13 Unrecognized or mishandled ectopic pregnancy can result in serious injuries—in the range of 1% to 10% (see above) of maternal deaths are related to ectopic pregnancy.14 Ectopic pregnancy cases, therefore, have been the subject of substantial litigation over the years. An informal, noncomprehensive review of malpractice lawsuits brought from 2000 to 2019, found more than 300 ectopic pregnancy cases. Given the large number of malpractice claims against ObGyns,15 ectopic pregnancy cases are only a small portion of all ObGyn malpractice cases.16
A common claim: negligent diagnosis or treatment
The most common basis for lawsuits in cases of ectopic pregnancy is the clinician’s negligent failure to properly diagnose the ectopic nature of the pregnancy. There are also a number of cases claiming negligent treatment of an identified ectopic pregnancy. Not every missed diagnosis, or unsuccessful treatment, leads to liability, of course. It is only when a diagnosis or treatment fails to meet the standard of care within the profession that there should be liability. That standard of care is generally defined by what a reasonably prudent physician would do under the circumstances. Expert witnesses, who are familiar with the standard of practice within the specialty, are usually necessary to establish what that practice is. Both the plaintiff and the defense obtain experts, the former to prove what the standard of care is and that the standard was not met in the case at hand. The defense experts are usually arguing that the standard of care was met.17 Inadequate diagnosis of ectopic pregnancy or other condition may arise from a failure to take a sufficient history, conduct an appropriately thorough physical examination, recognize any of the symptoms that would suggest it is present, use and conduct ultrasound correctly, or follow-up appropriately with additional testing.18
A malpractice claim of negligent treatment can involve any the following circumstances19:
- failure to establish an appropriate treatment plan
- prescribing inappropriate medications for the patient (eg, methotrexate, when it is contraindicated)
- delivering the wrong medication or the wrong amount of the right medication
- performing a procedure badly
- undertaking a new treatment without adequate instruction and preparation.
Given the nature and risks of ectopic pregnancy, ongoing, frequent contact with the patient is essential from the point at which the condition is suspected. The greater the risk of harm (probability or consequence), the more careful any professional ought to be. Because ectopic pregnancy is not an uncommon occurrence, and because it can have devastating effects, including death, a reasonably prudent practitioner would be especially aware of the clinical presentations discussed above.20 In the opening case, the treatment plan was not well documented.
Negligence must lead to patient harm. In addition to negligence (proving that the physician did not act in accordance with the standard of care), to prevail in a malpractice case, the plaintiff-patient must prove that the negligence caused the injury, or worsened it. If the failure to make a diagnosis would not have made any difference in a harm the patient suffered, there are no damages and no liability. Suppose, for example, that a physician negligently failed to diagnose ectopic pregnancy, but performed surgery expecting to find the misdiagnosed condition. In the course of the surgery, however, the surgeon discovered and appropriately treated the ectopic pregnancy. (A version of this happened in the old 19th century case mentioned above.) The negligence of the physician did not cause harm, so there are no damages and no liability.
Continue to: Informed consent is vital...
Informed consent is vital
A part of malpractice is informed consent (or the absence of it)—issues that can arise in any medical care.21 It is wise to pay particular attention in cases where the nature of the illness is unknown, and where there are significant uncertainties and the nature of testing and treatment may change substantially over a period of a few days or few weeks. As always, informed consent should include a discussion of what process or procedure is proposed, its risks and benefits, alternative approaches that might be available, and the risk of doing nothing. Frequently, the uncertainty of ectopic pregnancy complicates the informed consent process.22
Because communication with the patient is an essential function of informed consent, the consent process should productively be used in PUL and similar cases to inform the patient about the uncertainty, and the testing and (nonsurgical) treatment that will occur. This is an opportunity to reinforce the message that the patient must maintain ongoing communication with the physician’s office about changes in her condition, and appear for each appointment scheduled. If more invasive procedures—notably surgery—become required, a separate consent process should be completed, because the risks and considerations are now meaningfully different than when treatment began. As a general matter, any possible treatment that may result in infertility or reduced reproductive capacity should specifically be included in the consent process.
In the hypothetical case, the gynecologist failed to obtain a follow-up serum hCG level. In addition, the record did not reflect ectopic pregnancy in the differential diagnosis. As noted above, the patient had predisposing factors for an ectopic pregnancy. The physician should have acknowledged the history of sexually transmitted disease predisposing her to an ectopic pregnancy. Monitoring of serum hCG levels until they are negative is appropriate with ectopic, or presumed ectopic, pregnancy management. Appropriate monitoring did not occur in this case. Each of these errors (following up on serum hCG levels and the inadequacy of notations about the possibility of ectopic pregnancy) seem inconsistent with the usual standard of care. Furthermore, as a result of the outcome, the only future option for the patient to pursue pregnancy was IVF.
Other legal issues
There are a number of other legal issues that are associated with the topic of ectopic pregnancy. There is evidence, for example, that Catholic and non-Catholic hospitals treat ectopic pregnancies differently,23 which may reflect different views on taking a life or the use of methotrexate and its association with abortion.24 In addition, the possibility of an increase in future ectopic pregnancies is one of the “risks” of abortion that pro-life organizations have pushed to see included in abortion informed consent.25 This has led some commentators to conclude that some Catholic hospitals violate federal law in managing ectopic pregnancy. There is also evidence of “overwhelming rates of medical misinformation on pregnancy center websites, including a link between abortion and ectopic pregnancy.”26
The fact that cesarean deliveries are related to an increased risk for ectopic pregnancy (because of the risk of cesarean scar ectopic pregnancy) also has been cited as information that should play a role in the consent process for cesarean delivery.27 In terms of liability, failed tubal ligation leads to a 33% risk of ectopic pregnancy.28 The risk of ectopic pregnancy is also commonly included in surrogacy contracts.29
Why the outcome was for the defense
The opening hypothetical case illustrates some of the uncertainties of medical malpractice cases. As noted, there appeared a deviation from the usual standard of care, particularly the failure to follow up on the serum hCG level. The weakness in the medical record, failing to note the possibility of ectopic pregnancy, also was probably an error but, apparently, the court felt that this did not result in any harm to the patient.
The question arises of how there would be a defense verdict in light of the failure to track consecutive serum hCG levels. A speculative explanation is that there are many uncertainties in most lawsuits. Procedural problems may result in a case being limited, expert witnesses are essential to both the plaintiff and defense, with the quality of their review and testimony possibly uneven. Judges and juries may rely on one expert witness rather than another, juries vary, and the quality of advocacy differs. Any of these situations can contribute to the unpredictability of the outcome of a case. In the case above, the liability was somewhat uncertain, and the various other factors tipped in favor of a defense verdict. ●
- Centers for Disease Control and Prevention. Ectopic pregnancy—United States, 1990‒1992. MMWR Morb Mortal Wkly Rep. 1995;44:46-48.
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2012;20:250-261.
- Dichter E, Espinosa J, Baird J, Lucerna A. An unusual emergency department case: ruptured ectopic pregnancy presenting as chest pain. World J Emerg Med. 2017;8:71-73.
- Cecchino GN, Araujo E, Elito J. Methotrexate for ectopic pregnancy: when and how. Arch Gynecol Obstet. 2014;290:417- 423.
- Barnhart KT, Sammel MD, Cracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic firsttrimester pregnancies. Fertil Steril. 2006;86:36-43.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Barnhart KT, Fay CA, Suescum M, et al. Clinical factors affecting the accuracy of ultrasonography in symptomatic first-trimester pregnancy. Obstet Gynecol. 2011;117:299-306.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 193: tubal ectopic pregnancy. Obstet Gynecol. 2018;131:e91-e103.
- Bouyer J, Coste J, Fernandez H, et al. Sites of ectopic pregnancy: a 10-year population-based study of 1800 cases. Hum Reprod. 2002;17:3224-3230.
- Spiegelberg O. Zur casuistic der ovarial schwangerschaft. Arch Gynecol. 1978;13:73.
- OB Hospitalist Group. Methotrexate use for ectopic pregnancies guidelines. https://www.obhg.com/wp-content /uploads/2020/01/Methotrexate-Use-for-EctopicPregnancies_2016-updates.pdf. Accessed December 10, 2020.
- Brozos C, Kargiannis I, Kiossis E, et al. Ectopic pregnancy through a caesarean scar in a ewe. N Z Vet J. 2013;61:373-375.
- Tucker v. Gillette, 12 Ohio Cir. Dec. 401 (Cir. Ct. 1901).
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130:366-373.
- Matthews LR, Alvi FA, Milad MP. Reproductive surgery malpractice patterns. Fertil Steril. 2016;106:e42-e43.
- Kim B. The impact of malpractice risk on the use of obstetrics procedures. J Legal Studies. 2006;36:S79-S120.
- Abinader R, Warsof S. Complications involving obstetrical ultrasound. In: Warsof S, Shwayder JM, eds. Legal Concepts and Best Practices in Obstetrics: The Nuts and Bolts Guide to Mitigating Risk. 2019;45-48.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Shwayder JM. IUP diagnosed and treated as ectopic: How bad can it get? Contemporary OB/GYN. 2019;64:49-46.
- Kaplan AI. Should this ectopic pregnancy have been diagnosed earlier? Contemporary OB/GYN. 2017;62:53.
- American College of Obstetricians and Gynecologists Committee on Ethics. Committee opinion 439: informed consent. Reaffirmed 2015. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2009/08 /informed-consent. Accessed December 9, 2020.
- Shwayder JM. Liability in ob/gyn ultrasound. Contemporary OB/GYN. 2017;62:32-49.
- Fisher LN. Institutional religious exemptions: a balancing approach. BYU Law Review. 2014;415-444.
- Makdisi J. Aquinas’s prohibition of killing reconsidered. J Catholic Legal Stud. 2019:57:67-128.
- Franzonello A. Remarks of Anna Franzonello. Alb Law J Sci Tech. 2012;23:519-530.
- Malcolm HE. Pregnancy centers and the limits of mandated disclosure. Columbia Law Rev. 2019;119:1133-1168.
- Kukura E. Contested care: the limitations of evidencebased maternity care reform. Berkeley J Gender Law Justice. 2016;31:241-298.
- Donley G. Contraceptive equity: curing the sex discrimination in the ACA’s mandate. Alabama Law Rev. 2019;71:499-560.
- Berk H. Savvy surrogates and rock star parents: compensation provisions, contracting practices, and the value of womb work. Law Social Inquiry. 2020;45:398-431.
- Centers for Disease Control and Prevention. Ectopic pregnancy—United States, 1990‒1992. MMWR Morb Mortal Wkly Rep. 1995;44:46-48.
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2012;20:250-261.
- Dichter E, Espinosa J, Baird J, Lucerna A. An unusual emergency department case: ruptured ectopic pregnancy presenting as chest pain. World J Emerg Med. 2017;8:71-73.
- Cecchino GN, Araujo E, Elito J. Methotrexate for ectopic pregnancy: when and how. Arch Gynecol Obstet. 2014;290:417- 423.
- Barnhart KT, Sammel MD, Cracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic firsttrimester pregnancies. Fertil Steril. 2006;86:36-43.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Barnhart KT, Fay CA, Suescum M, et al. Clinical factors affecting the accuracy of ultrasonography in symptomatic first-trimester pregnancy. Obstet Gynecol. 2011;117:299-306.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 193: tubal ectopic pregnancy. Obstet Gynecol. 2018;131:e91-e103.
- Bouyer J, Coste J, Fernandez H, et al. Sites of ectopic pregnancy: a 10-year population-based study of 1800 cases. Hum Reprod. 2002;17:3224-3230.
- Spiegelberg O. Zur casuistic der ovarial schwangerschaft. Arch Gynecol. 1978;13:73.
- OB Hospitalist Group. Methotrexate use for ectopic pregnancies guidelines. https://www.obhg.com/wp-content /uploads/2020/01/Methotrexate-Use-for-EctopicPregnancies_2016-updates.pdf. Accessed December 10, 2020.
- Brozos C, Kargiannis I, Kiossis E, et al. Ectopic pregnancy through a caesarean scar in a ewe. N Z Vet J. 2013;61:373-375.
- Tucker v. Gillette, 12 Ohio Cir. Dec. 401 (Cir. Ct. 1901).
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130:366-373.
- Matthews LR, Alvi FA, Milad MP. Reproductive surgery malpractice patterns. Fertil Steril. 2016;106:e42-e43.
- Kim B. The impact of malpractice risk on the use of obstetrics procedures. J Legal Studies. 2006;36:S79-S120.
- Abinader R, Warsof S. Complications involving obstetrical ultrasound. In: Warsof S, Shwayder JM, eds. Legal Concepts and Best Practices in Obstetrics: The Nuts and Bolts Guide to Mitigating Risk. 2019;45-48.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Shwayder JM. IUP diagnosed and treated as ectopic: How bad can it get? Contemporary OB/GYN. 2019;64:49-46.
- Kaplan AI. Should this ectopic pregnancy have been diagnosed earlier? Contemporary OB/GYN. 2017;62:53.
- American College of Obstetricians and Gynecologists Committee on Ethics. Committee opinion 439: informed consent. Reaffirmed 2015. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2009/08 /informed-consent. Accessed December 9, 2020.
- Shwayder JM. Liability in ob/gyn ultrasound. Contemporary OB/GYN. 2017;62:32-49.
- Fisher LN. Institutional religious exemptions: a balancing approach. BYU Law Review. 2014;415-444.
- Makdisi J. Aquinas’s prohibition of killing reconsidered. J Catholic Legal Stud. 2019:57:67-128.
- Franzonello A. Remarks of Anna Franzonello. Alb Law J Sci Tech. 2012;23:519-530.
- Malcolm HE. Pregnancy centers and the limits of mandated disclosure. Columbia Law Rev. 2019;119:1133-1168.
- Kukura E. Contested care: the limitations of evidencebased maternity care reform. Berkeley J Gender Law Justice. 2016;31:241-298.
- Donley G. Contraceptive equity: curing the sex discrimination in the ACA’s mandate. Alabama Law Rev. 2019;71:499-560.
- Berk H. Savvy surrogates and rock star parents: compensation provisions, contracting practices, and the value of womb work. Law Social Inquiry. 2020;45:398-431.
The latest US Supreme Court decisions on contraception, transgender discrimination, more
The 2019-2020 term of the US Supreme Court was remarkable by any standard. An extraordinary number of important cases made it “a buffet of blockbusters.”1
We look first at several cases that will be of particular interest to ObGyns. Then we look briefly at a number of other important cases that affect the medical profession as a whole and the direction of the country (see “Other significant US Supreme Court decisions”), and finally we conclude with an analysis of this term and a forecast for the next.
We chose cases in which specialty organizations, such as the American College of Obstetricians and Gynecologists (ACOG), or organized medicine (the American Medical Association [AMA], the Association of American Medical Colleges [AAMC], or the American Hospital Association [AHA]), took a special interest by filing “amicus curiae” (friend of the court) briefs with the Supreme Court. These briefs are filed by an organization or person who is not a party to the case but who may have important information to convey to the Court. Because these briefs represent a significant commitment of money, time, and effort, they are usually not undertaken lightly.
Decisions concerning abortion
June v Russo
Decided June 29, 2020, June v Russo involved a Louisiana statute that required abortion providers have “active admitting privileges at a hospital” within 30 miles of where the abortion is performed.2 The Court decided a case in 2016 (from Texas) that involved almost the same statutory provision, so it might seem like an easy ruling.3 But Justice Kennedy (the deciding vote in 2016) has been replaced by Justice Gorsuch, so the outcome was uncertain. It was a difficult case, with a total of 5 opinions covering 138 pages and a “surprise” from the Chief Justice.
The Court, in a 5-4 decision, struck down the Louisiana law, but there was no majority opinion. Four justices in the plurality emphasized that the Louisiana law (like the Texas law) substantially burdened the right to abortion without any corresponding benefit to the health of the women seeking abortions. (Under earlier Court precedents, “undue burdens” on abortion are unconstitutional.4) Justice Breyer noted that the state could not present even one example in which a woman would have had better treatment if her doctor had admitting privileges. For a variety of reasons, admitting privileges were cumbersome for abortion providers to obtain; therefore, enforcing the law had little or no benefit, but significant risk of reduced availability of abortion services.
In June v Russo, Chief Justice Roberts literally became the “swing vote”—the fifth vote to strike down the Louisiana law. In 2016, he had voted the other way—to uphold essentially the same law (in Texas) that he struck down here. He attributed his switch to precedent (the general obligation of courts to follow prior decisions). He disagreed with the earlier decision, but felt bound by it.
This should be the end of the abortion provider “hospital privileges requirements” that a number of states have passed. States seeking to nibble away at abortion rights will undoubtedly look elsewhere. Beyond that, it is difficult, from this case, to discern the future of abortion rights.
ACOG was the lead in amicus briefs urging the Court to strike down the Louisiana law. ACOG (with others) was one of only a handful of organizations filing a brief urging the Court to agree to hear the case.5 When the Court did agree to hear the case (“granted certiorari”), ACOG and a number of other medical organizations filed a formal amicus brief on the merits of the case.6 The brief made 2 arguments: First, that this case was essentially decided in Whole Woman’s Health in 2016 (the Texas case) and, second, that “an admitting privileges requirement is not medically necessary” and “clinicians who provide abortions are unable to obtain admitting privileges for reasons unrelated to their ability to safely and competently perform abortions.” Justice Breyer cited the ACOG brief twice.
The American Association of Pro-Life Obstetricians and Gynecologists also filed an amicus brief.7 The brief was directed solely at arguing that ACOG was not presenting reliable science. It summarized, “The American College of Obstetricians and Gynecologists has always presented itself to the Court as a source of objective medical knowledge. However, when it comes to abortion, the College today is primarily a pro-abortion political advocacy organization.” That brief concluded that the “Court should read ACOG’s amicus brief not as an authoritative recitation of settled science, but as a partisan advocacy paper on behalf of a mere subset of American obstetricians and gynecologists.”
The Association of American Physicians and Surgeons (which should not be confused with the “National Board of Physicians and Surgeons”) also filed an amicus brief. The brief argued, “Abortion, like other outpatient surgical procedures, sometimes results in patient hospitalization. Requiring abortion providers to maintain admitting privileges will improve communication between physicians in the transfer of patients to the hospital and allow them to participate in the care of their patients while in the hospital, in line with their ethical duty to ensure their patients’ continuity of care.”8
Continue to: Ultrasonography requirement for abortion...
Ultrasonography requirement for abortion
In another abortion case, the Court was asked to review a Kentucky abortion statute requiring that an ultrasound image be shown to the woman as part of informed consent for an abortion.9 ACOG filed an amicus brief in favor of a review, but the Court declined to hear the case.10,11
Contraception considerations
The Affordable Care Act (ACA) has an ambiguous provision regarding no-cost “preventive care and screenings” for women. The ACA does not, however, specify contraceptive coverage.12 Several departments and the Health Resources and Services Administration (collectively referred to as “HRSA”) interpreted the provision to include contraception, but from the start there were religious objections. HRSA eventually provided an exemption regarding contraception for employers (nonprofits and for-profits with no publicly traded components) that had “sincerely held moral” objections to providing forms of contraceptive coverage. That regulation was again before the Court this term in Little Sisters of the Poor Saints Peter and Paul Home v Pennsylvania.13
In a 7-2 decision, the Court held that the ACA gave HRSA authority to adopt regulations related to the undefined term “preventive care.” Therefore, it found that HRSA could exempt those with religious objections from participation in providing contraceptive coverage. ACOG and other medical groups filed an amicus brief arguing that contraception is an essential preventive service. “Contraception not only helps to prevent unintended pregnancy, but also helps to protect the health and well-being of women and their children.”14 It was cited only by Justice Ginsburg in her dissent.15
Deferred Action for Childhood Arrivals (DACA)
The AAMC, ACOG, AMA, and many other organizations filed an amicus brief16 in Department of Homeland Security v Regents of University of California.17 The case raised the question of whether a decision to end the DACA program followed the appropriate administrative procedures. In 2012, the Obama administration issued a “memorandum” establishing DACA (without congressional approval or formal rulemaking). A lower court decision barring implementation of DACA was upheld by the Supreme Court in 2016 on a 4-4 vote.18 In 2017, the Trump administration moved to end DACA.
In a 5-4 decision, the Court held that the explanation for ending DACA was inadequate, and violated the Administrative Procedures Act, so DACA could continue until the administration redid the repeal, following the proper procedures. The decision of the Court dealt solely with the process by which the rescission took place—there was general agreement that the administration had the right to rescind it if the procedure (with legitimate reasons) was proper.
The brief for the medical groups argued that the failure of the regulation to consider “reliance interests” would have especially difficult consequences in the medical fields. It noted, “At this moment, an estimated 27,000 health care workers and support staff depend on DACA for their authorization to work in the United States. Among those 27,000 are nurses, dentists, pharmacists, physician assistants, home health aides, technicians, and others. The number also includes nearly 200 medical students, medical residents, and physicians who depend on DACA for their eligibility to practice medicine.”16 The brief was not cited by the Court, but the reliance interest the brief spoke about was an important part of the case.
Continue to: Employment discrimination against gay and transgender employees...
Employment discrimination against gay and transgender employees
Federal law (“Title VII”) makes it illegal for an employer to “discriminate against any individual because of race, color, religion, sex, or national origin.”19 The question this term was whether discrimination based on sexual orientation or sexual identity is within the statute’s meaning of “sex.” By a 6-3 majority, the Court held that Title VII applies both to orientation and identity. (This was an interpretation of the statute, not a broad constitutional ruling.)
The majority reasoned that “it is impossible to discriminate against a person for being homosexual or transgender without discriminating against that individual based on sex. Consider, for example, an employer with 2 employees, both of whom are attracted to men.” If the employer fires the gay employee, “the employer discriminates against him for traits or actions it tolerates in his female colleague.”20
AMA and a number of other medical organizations filed an amicus brief in the case.21 The core of the argument of the brief was, “Employment discrimination against transgender people frustrates the treatment of gender dysphoria by preventing transgender individuals from living openly in accordance with their true gender identity and impeding access to needed medical care. Experiencing discrimination in one of the most important aspects of adult life—employment—makes it nearly impossible to live in full congruence with one’s gender identity. The fear of facing such discrimination alone can prompt transgender individuals to hide their gender identity, directly thwarting the goal of social transition…. Lack of treatment, in turn, increases the rate of negative mental health outcomes, substance abuse, and suicide.” The brief was not cited in the opinions in the case.
This decision is likely to have great impact on many aspects of American life. In the employment area, it is now a matter of course that employers may not discriminate based on orientation or identity in any employment decisions including hiring, firing, compensation, fringe benefits, etc. Harassment based on identity or orientation may similarly be an employment law violation. The decision also likely means that giving employment preferences to gay employees would now be as illegal as would be giving preferences to straight employees. (Limited exceptions, notably to some religious organization employees, are not included in anti-discrimination laws.)22
The importance of the decision goes well beyond employment, however. More than 100 federal statutes are in place that prohibit “discrimination because of sex.” It is now likely that these statutes will be interpreted as prohibiting discrimination related to sexual orientation and identification.
Additional cases of interest
HIV/AIDS International Program
A major US program fighting HIV/AIDS worldwide—the United States Leadership Against HIV/AIDS, Tuberculosis, and Malaria Act (aka the Leadership Act)—has provided billions of dollars to agencies abroad.23 Nongovernmental organizations (NGOs) receiving funds under the program must agree to have a “policy explicitly opposing prostitution and sex trafficking” (known as the “Policy Requirement”). Some grant recipients in foreign countries, generally affiliates of US NGOs, do not want to have such a policy and challenged the policy requirement as a violation of First Amendment right of free speech. The Court held that it is a well-settled principle that “foreign citizens outside US territory do not possess rights under the US Constitution.”24 Nor do organizations become entitled to such rights as a result of an affiliation with US organizations. This decision means that foreign organizations are free not to have the required policies, but they will be ineligible for funds under the Leadership Act.
Continue to: ACA government debts edition...
ACA government debts edition
The ACA was before the Court, yet again. To encourage private insurers to participate in online health insurance exchanges, the ACA provided that the federal government would share in insurance company losses for 3 years.25 The Act, however, did not appropriate any money for these “risk corridors,” and insurance companies lost $12 billion.
Congress (after the 2010 election) prohibited any appropriated funds from being used to pay insurance companies for their risk corridor losses. Four insurance companies sued the United States, seeking reimbursements for their losses. This term the Court held that the government must pay for their losses under the ACA.26 The Court said that Congress could have expressly repealed the risk corridor obligation (in the appropriation bill), but instead had only prohibited the expenditure of the money, which the Court said did not amount to an implied repeal of the obligation. We will see that ACA will be back before the Court again next term in California v Texas (discussed below).
Child custody and international abduction
The Hague Convention on the Civil Aspects of International Child Abduction (to which the United States is a party) provides that the courts of the country where the child has “habitual residence” have jurisdiction to decide custody.27 If a parent takes the child to another country, that country is obligated to return the child to the country of “habitual residence.”
This term the Court was called upon to define “habitual residence.” The Court held that determining habitual residence depends on the “totality of the circumstances,” and that “locating a child’s home is a fact-driven inquiry,” and that “courts must be sensitive to the unique circumstances of the case and informed by common sense.”28 An exception to the Convention’s obligation to return a child to the country of habitual residence is where “there is a grave risk that [the] return would expose the child to physical or psychological harm or otherwise place the child in an intolerable situation.”29 Who the parent is can affect many aspects of legal authority over the child, including consent to medical care, and the right to receive information concerning care.
Analysis of the term
The term began October 7, 2019, and adjourned July 9, 2020, somewhat later than usual because of coronavirus disease 2019 (COVID-19). During the term, the Court decided 60 cases, including 53 “signed” merit opinions after oral argument—the lowest number of decided cases in many years.30 Of those 60 cases, 22 (35%) were unanimous, and 13 (22%) resulted in a 5-4 split.30 Ten-year averages are 48% unanimous and 20% with 5-4 decisions.30
Chief Justice Roberts was the central focus of the term. He presided over the impeachment trial of President Trump in the Senate early in the term. He also presided over the Court’s accommodations of the COVID-19 pandemic. He is the “median,” or “swing,” justice. He was in the majority in 12 of the 13 cases with 5-4 decisions.30 He was in the majority in 97% of all cases and in 95% of “divided cases”—the highest of any of the justices this term.30 In some of the most critical decisions, Chief Justice Roberts sided with the “liberal” wing, including on cases concerning abortion, gay and transgender employment, DACA, and 2 Presidential subpoena cases. More often (in 9 of the 5-4 decisions), however, he sided with the more conservative justices.30 Justice Kavanaugh agreed with Chief Justice Roberts most often (in 93% of all cases).30 Among the others, these justices agreed with each other 90% or more of the time: Justices Ginsburg and Breyer (93%), Justices Alito and Thomas (92%), and Justices Breyer and Kagan (90%).30
Continue to: COVID-19 and the Court...
COVID-19 and the Court
Some of the biggest news of the term came not from the law, but from medicine in the form of COVID-19. The Court was in the process of preparing a final period of important arguments when, on March 16, it announced that it was postponing further arguments. The Court rescheduled 10 oral arguments that were held by telephone (other cases were held over to the next term). The phone arguments, during the first 2 weeks of May, necessitated a change in format. Each justice was called on (in order of seniority) by the Chief Justice to ask questions. This was in contrast to the free-for-all questions that usually characterize in-person arguments. These arguments were broadcast live—something that had never been done before. Public access was, on balance, a good thing. There were a couple failures to unmute, and there was “the flush heard round the world” in the middle of one argument, but otherwise the arguments went off with few hitches.31
Looking ahead
By the end of the term, no justice had announced an intention to retire from the Court. On September 18, however, Justice Ruth Bader Ginsburg passed away. In 2009, she had been diagnosed with early-stage pancreatic cancer. This term she had been hospitalized twice, and at the end of the term, she announced a recurrence of pancreatic cancer, which was being treated with chemotherapy. See “RBG: The woman, the legacy” for a tribute to this remarkable woman, lawyer, and justice.
Justice Ginsburg’s death, occurring in the middle of a presidential campaign, ignited a political firestorm concerning her successor. The outcome of selecting and confirming her successor and the political fallout were not immediately apparent. Justice Ginsburg was confirmed just 7 weeks after her nomination by President Clinton, by a vote of 96-3. But those days of Senate consensus are not the current norm.
The next term (called the “October 2020 Term”) will begin on October 5, 2020. The Court will begin with 8 justices and, depending on the nomination process, may operate with 8 justices for some time. When there is a “tie” vote in the Court, the lower court decision is upheld. The Court has been short-handed several times in the past and, with few exceptions, has managed the cases successfully.
The Court has announced that initial arguments will be telephonic. It already has taken a number of cases. The constitutionality of the individual mandate (coverage) in the ACA will once again be before the Court, and that already has produced a flood of amicus briefs from health-related organizations.32 Among other upcoming issues are cases related to state regulation of pharmacy benefit managers, gay rights and foster care, sentencing of juveniles to life in prison without the possibility of parole, a face-off between Google and Oracle on software copyrights, and arbitration. In addition, some of the issues we saw this term will reappear, with more on robocalls, religious freedom and Catholic charities, and immigration and removal cases.
Ruth Bader Ginsburg, as a law student, law professor, lawyer, judge, and justice, was a leading advocate for the rights of women. There were only a few women in law school when she attended, but she graduated tied for first in her class. Although she found it difficult to be hired as a lawyer, as a law professor and lawyer she helped map a strategy to expand legal rights for women, arguing 6 cases before the Supreme Court and winning 5 of them. She served as a federal appeals court judge and then was appointed to the Supreme Court in 1993. She was the second woman to serve on the Court.
As a justice, she was known during much of her tenure on the Court as the leader of the liberal justices, although her jurisprudence was more complex than that simple statement. She was always a strong advocate for the rights of women (and equal rights of men) during her time on the Court. She was a very clear writer; her opinions were direct and easy to understand. She was also fast—she routinely had the record of announcing opinions faster than any of the other current justices. She was 87 when she passed away, having served on the Court for 27 years.
Justice Ginsburg was also something of a cultural phenomenon. In later years she was sometimes known as “the Notorious RBG.” Books, movies, songs, and even workout videos were made about her. In groups she seemed almost shy, but she was thoughtful, kind, and funny (sometimes wickedly so). The outpouring of affection and sympathy at her death was a symbol of the place she held in America. She loved the opera, a passion she shared with her friend, Justice Antonin Scalia. Despite their considerable disagreements on legal matters, Justices Ginsburg and Scalia were close friends. They attended opera with one another, and their families usually spent New Year’s Eves together. They were the 2 most recent justices to pass away while serving on the Court.
The Court heard and ruled on a large number of other significant cases that will have consequences for many years to come. Highlights include:
- In 2 cases involving subpoenas for the President’s personal records, the Court suggested some balance between “nobody is above the law” and not unnecessarily hectoring or interfering with fulfilling the office of President. The Court held that Congress may subpoena a President’s personal and family records, while the President is still in office.1 It instructed lower courts to assess whether the papers are necessary, the subpoena is limited in scope, there is legitimate legislative purpose, whether the burden it imposes on the President is reasonable, and whether the subpoena would unduly interfere with the ability to do the work required as President.
- Similarly, local (state) grand juries may subpoena such personal records, but the President will have the opportunity to raise specific objections to the subpoenas—undue burden, bad faith, or overbreadth. In addition, the respect owed to the office should inform the conduct regarding the subpoena.2
- The Court upheld a federal law that prohibits most robocalls.3 It struck down an amendment that allowed robocalls made to collect debts owed to or guaranteed by the federal government.
- The Court held that a single-director federal agency, whose director cannot be removed by the President (at will), violates the Constitution.4 The Consumer Financial Protection Bureau (created by the Dodd-Frank law) has such a single, no-removal director and that will have to be modified.
- The Court held that the eastern half of Oklahoma (including Tulsa) is part of a Creek Nation reservation.5 This was a question of criminal law jurisdiction, not property ownership. The practical effect is that for crimes involving Native Americans, serious crimes will have to be tried in federal court, while lesser crimes may be tried in tribal courts.
- The Court determined that it was unconstitutional for a state program providing tuition assistance to parents who send their children to private schools, to prohibit students attending religious private schools from participating in the program. That is a burden on the “free exercise” of religion.6
- The Court considered whether there can be civil liability for damages caused by a federal official in the United States harming a foreign national in another country. In this case, a border patrol agent standing in the US shot and killed a Mexican juvenile who was just across the border in Mexico.7 The issue was whether the parents of the Mexican national could sue the US officials for damages. The Court declined to expand liability to include those injured outside the US. Ultimately, the Court was reluctant to impose liability because this liability is not authorized by Congress.
- In a COVID-19 religion case, the Court refused to stop the enforcement of a governor’s COVID-19 order that allowed churches to operate with <100 attendees or 25% occupancy (whichever was lower).8 Meanwhile, businesses, malls, and stores were allowed to reopen without these stringent limitations. The church objected that greater burdens were placed on religion than secular activity. The Court denied the church’s request for an injunction.
- The Court unanimously held that a state may punish or remove a “faithless elector.” Electors cast votes on behalf of their states in the Electoral College—where Presidents are technically selected. Electors are generally pledged to vote for the winner of a state’s vote for President. A few have violated that pledge and voted for someone else. As a practical matter, that could cause real disruption, and the Court upheld state laws that take action against these “faithless” electors.9
- Several days after the Court had officially adjourned for the term, it received several petitions to delay the execution of federal prisoners. One case was based on the method of execution (use of pentobarbital),10 and another was based on the claim that a prisoner had become so mentally incompetent that it was improper to execute him.11 The Court turned down these appeals, allowing the executions to proceed. These were the first federal government executions in 17 years.
References
- Trump v Mazars USA, LLP, 140 S. Ct. 2019 (2020).
- Trump v Vance, 140 S. Ct. 2412 (2020).
- Barr v American Association of Political Consultants, Inc, 140 S. Ct. 2335 (2020).
- Seila Law LLC v Consumer Financial Protection Bureau, 140 S. Ct. 2183 (2020).
- McGirt v Oklahoma, 140 S. Ct. 2452 (2020).
- Espinoza v Montana Department of Revenue, 140 S. Ct. 2246 (2020).
- Hernández v Mesa, 140 S. Ct. 735, 206 L. Ed. 2d 29 (2020).
- South Bay United Pentecostal Church v Newsom, 140 S. Ct. 1613, 207 L. Ed. 2d 154 (2020).
- Chiafalo v Washington, 140 S. Ct. 2316 (2020).
- Barr v Lee, ____ S. Ct. ____ (2020).
- Barr v Purkey, ____ S. Ct. ____ (2020).
1. Liptak A. In a term full of major cases, the Supreme Court tacked to the center. The New York Times. July 10, 2020.
2. June Medical Services LLC v Russo, 591 US 140 S. Ct. 2103, 2112 (2020).
3. Whole Woman’s Health v Hellerstedt, 579 US ___ (2016).
4. Planned Parenthood of Southeastern PA v Casey, 505 US 833, 874 (1992).
5. Brief of the American College of Obstetricians and Gynecologists, the American Academy of Family Physicians, the American Academy of Pediatrics, the American College of Nurse-Midwives, the American College of Osteopathic Obstetricians and Gynecologists, the American College of Physicians, the American Society for Reproductive Medicine, the National Association of Nurse Practitioners in Women’s Health, the North American Society for Pediatric and Adolescent Gynecology, and the Society for Maternal-Fetal Medicine Amici Curiae In Support of Petitioners, June Medical Services v Russo. May 20, 2019. https://www.supremecourt.gov/DocketPDF/18/18-1323/100434/20190520175434029_18-1323%20ACOG%20et%20al.%20cert.%20amicus%20brief.pdf. Accessed August 31, 2020.
6. Brief of Amici Curiae American College of Obstetricians and Gynecologists, American Medical Association, American Academy of Family Physicians, American Academy of Nursing, American Academy of Pediatrics, American College of Nurse-Midwives, American College of Osteopathic Obstetricians and Gynecologists, American College of Physicians, American Osteopathic Association, American Public Health Association, American Society for Reproductive Medicine, North American Society for Pediatric and Adolescent Gynecology, Society for Maternal-Fetal Medicine, and the Society of Ob/Gyn Hospitalists, In Support of June Medical Services, June Medical Services v Russo. December 2, 2019. https://www.supremecourt.gov/DocketPDF/18/18-1323/124091/20191202145531124_18-1323%2018-1460%20tsac%20American%20College%20of%20Obstetricians%20and%20
Gynecologists%20et%20al.pdf [https://perma.cc/8T8V-4D6S]. Accessed August 31, 2020.
7. Brief of Amicus Curiae American Association of Pro-Life Obstetricians and Gynecologists In Support of [Russo] Louisiana Department of Health and Hospitals, June Medical Services v Russo. December 27, 2019. https://www.supremecourt.gov/DocketPDF/18/18-1323/126927/20191227154424488_AAPLOG%20Amicus%20Brief.pdf. Accessed August 31, 2020.
8. Brief of Association of American Physicians and Surgeons as Amicus Curiae in Support of Respondent–Cross-Petitioner, June Medical Services v Russo 2. December 27, 2019. https://www.supremecourt.gov/DocketPDF/18/18-1323/126828/20191227104605915_18-1323%20-1460%20bsac%20AAPS--PDFA.pdf. Accessed August 31, 2020.
9. Ky. Rev. Stat. § 311.727(2).
10. Brief for the American College of Obstetricians and Gynecologists, the American Medical Association, the North American Society for Pediatric and Adolescent Gynecology, the American College of Osteopathic Obstetricians and Gynecologists, and the American Academy of Family Physicians Amici Curiae Supporting Petitioners, EMW Women’s Surgical Center v Meier. October 28, 2019. https://www.supremecourt.gov/DocketPDF/19/19-417/120550/20191028184956458_19-417%20ACOG%20et%20al.%20-%20cert.%20amicus%20brief.pdf. Accessed August 31, 2020.
11. EMW Women’s Surgical Center, PSC v Meier, 140 S. Ct. 655 (2019).
12. Codified at 26 U. S. C. §5000A(f )(2); §§4980H(a), (c)(2) requires employers to provide women with “preventive care and screenings” without “any cost sharing requirements.”
13. Little Sisters of the Poor Saints Peter and Paul Home v Pennsylvania, 140 S. Ct. 2367 (2020).
14. Brief of Amici Curiae American College of Obstetricians and Gynecologists, American Nurses Association, American Academy of Nursing, Physicians for Reproductive Health, and Nurses for Sexual and Reproductive Health, In Support of Respondents and Affirmance, Little Sisters of the Poor Saints Peter and Paul Home v Pennsylvania. April 8, 2020. https://www.supremecourt.gov/DocketPDF/19/19-431/141177/20200408152340136_19-431%20and%2019-454%20Amici%20Curiae.pdf. Accessed August 31, 2020.
15. Little Sisters of the Poor Saints Peter and Paul Home v Pennsylvania, 140 S. Ct. 2367, 2400–12 (2020).
16. Brief for the Association of American Medical Colleges (and more than 30 other organizations, including the American Medical Association, and the American College of Obstetricians and Gynecologists) Amici Curiae, In Support of Respondents, Department of Homeland Security v Regents of University of California. October 4, 2019. https://www.supremecourt.gov/DocketPDF/18/18-587/118129/20191004130646281_Brief%20for%20AAMC%20et%20al%20
Supporting%20Respondents.pdf. Accessed August 31, 2020.
17. Department of Homeland Security v Regents of The University of California, 140 S. Ct. 1891 (2020).
18. United States v Texas, 136 S. Ct. 2271 (2016).
19. Title VII of the Civil Rights Act of 1964, 42 U.S.C §2000e–2(a)(1).
20. Bostock v Clayton County, 140 S. Ct. 1731, 1741 (2020).
21. Brief of the American Medical Association, the American College of Physicians, and 14 additional medical, mental health, and health care organizations as Amici Curiae In Support of the Employees, Bostock v Clayton County. July 3, 2019. https://www.supremecourt.gov/DocketPDF/17/17-1618/107177/20190703172548842_Amicus%20Brief.pdf. Accessed August 31, 2020.
22. Our Lady of Guadalupe School v Morrissey-Berru, 140 S. Ct. 2049 (2020).
23. United States Leadership Against HIV/AIDS, Tuberculosis, and Malaria Act (“the Leadership Act”), 22 U. S. C. §7601 et seq.
24. Agency for International Development v Alliance for Open Society, 140 S. Ct. 2082, 2086 (2020).
25. 42 U.S.C. §1342, §18063.
26. Maine Community Health Options v United States, 140 S. Ct. 1308 (2020).
27. Hague Convention on the Civil Aspects of International Child Abduction (Hague Convention or Convention), implemented in the United States by the International Child Abduction Remedies Act, 22 U. S. C. §9001 et seq.
28. Monasky v Taglieri, 140 S. Ct. 719 (2020).
29. Monasky v Taglieri, 140 S. Ct. 719, 723, 729 (2020).
30. Feldman A. Final stat pack for October term 2019 (upated). July 10, 2020. https://www.scotusblog.com/2020/07/final-stat-pack-for-october-term-2019/. Accessed August 31, 2020.
31. Hejmanowski D. Flush heard around the world. Delaware Gazette. May 8, 2020. https://www.delgazette.com/opinion/columns/83610/flush-heard-around-the-world. Accessed August 31, 2020.
32. SCOTUSblog.com. California v Texas. https://www.scotusblog.com/case-files/cases/california-v-texas/. Accessed August 31, 2020.
The 2019-2020 term of the US Supreme Court was remarkable by any standard. An extraordinary number of important cases made it “a buffet of blockbusters.”1
We look first at several cases that will be of particular interest to ObGyns. Then we look briefly at a number of other important cases that affect the medical profession as a whole and the direction of the country (see “Other significant US Supreme Court decisions”), and finally we conclude with an analysis of this term and a forecast for the next.
We chose cases in which specialty organizations, such as the American College of Obstetricians and Gynecologists (ACOG), or organized medicine (the American Medical Association [AMA], the Association of American Medical Colleges [AAMC], or the American Hospital Association [AHA]), took a special interest by filing “amicus curiae” (friend of the court) briefs with the Supreme Court. These briefs are filed by an organization or person who is not a party to the case but who may have important information to convey to the Court. Because these briefs represent a significant commitment of money, time, and effort, they are usually not undertaken lightly.
Decisions concerning abortion
June v Russo
Decided June 29, 2020, June v Russo involved a Louisiana statute that required abortion providers have “active admitting privileges at a hospital” within 30 miles of where the abortion is performed.2 The Court decided a case in 2016 (from Texas) that involved almost the same statutory provision, so it might seem like an easy ruling.3 But Justice Kennedy (the deciding vote in 2016) has been replaced by Justice Gorsuch, so the outcome was uncertain. It was a difficult case, with a total of 5 opinions covering 138 pages and a “surprise” from the Chief Justice.
The Court, in a 5-4 decision, struck down the Louisiana law, but there was no majority opinion. Four justices in the plurality emphasized that the Louisiana law (like the Texas law) substantially burdened the right to abortion without any corresponding benefit to the health of the women seeking abortions. (Under earlier Court precedents, “undue burdens” on abortion are unconstitutional.4) Justice Breyer noted that the state could not present even one example in which a woman would have had better treatment if her doctor had admitting privileges. For a variety of reasons, admitting privileges were cumbersome for abortion providers to obtain; therefore, enforcing the law had little or no benefit, but significant risk of reduced availability of abortion services.
In June v Russo, Chief Justice Roberts literally became the “swing vote”—the fifth vote to strike down the Louisiana law. In 2016, he had voted the other way—to uphold essentially the same law (in Texas) that he struck down here. He attributed his switch to precedent (the general obligation of courts to follow prior decisions). He disagreed with the earlier decision, but felt bound by it.
This should be the end of the abortion provider “hospital privileges requirements” that a number of states have passed. States seeking to nibble away at abortion rights will undoubtedly look elsewhere. Beyond that, it is difficult, from this case, to discern the future of abortion rights.
ACOG was the lead in amicus briefs urging the Court to strike down the Louisiana law. ACOG (with others) was one of only a handful of organizations filing a brief urging the Court to agree to hear the case.5 When the Court did agree to hear the case (“granted certiorari”), ACOG and a number of other medical organizations filed a formal amicus brief on the merits of the case.6 The brief made 2 arguments: First, that this case was essentially decided in Whole Woman’s Health in 2016 (the Texas case) and, second, that “an admitting privileges requirement is not medically necessary” and “clinicians who provide abortions are unable to obtain admitting privileges for reasons unrelated to their ability to safely and competently perform abortions.” Justice Breyer cited the ACOG brief twice.
The American Association of Pro-Life Obstetricians and Gynecologists also filed an amicus brief.7 The brief was directed solely at arguing that ACOG was not presenting reliable science. It summarized, “The American College of Obstetricians and Gynecologists has always presented itself to the Court as a source of objective medical knowledge. However, when it comes to abortion, the College today is primarily a pro-abortion political advocacy organization.” That brief concluded that the “Court should read ACOG’s amicus brief not as an authoritative recitation of settled science, but as a partisan advocacy paper on behalf of a mere subset of American obstetricians and gynecologists.”
The Association of American Physicians and Surgeons (which should not be confused with the “National Board of Physicians and Surgeons”) also filed an amicus brief. The brief argued, “Abortion, like other outpatient surgical procedures, sometimes results in patient hospitalization. Requiring abortion providers to maintain admitting privileges will improve communication between physicians in the transfer of patients to the hospital and allow them to participate in the care of their patients while in the hospital, in line with their ethical duty to ensure their patients’ continuity of care.”8
Continue to: Ultrasonography requirement for abortion...
Ultrasonography requirement for abortion
In another abortion case, the Court was asked to review a Kentucky abortion statute requiring that an ultrasound image be shown to the woman as part of informed consent for an abortion.9 ACOG filed an amicus brief in favor of a review, but the Court declined to hear the case.10,11
Contraception considerations
The Affordable Care Act (ACA) has an ambiguous provision regarding no-cost “preventive care and screenings” for women. The ACA does not, however, specify contraceptive coverage.12 Several departments and the Health Resources and Services Administration (collectively referred to as “HRSA”) interpreted the provision to include contraception, but from the start there were religious objections. HRSA eventually provided an exemption regarding contraception for employers (nonprofits and for-profits with no publicly traded components) that had “sincerely held moral” objections to providing forms of contraceptive coverage. That regulation was again before the Court this term in Little Sisters of the Poor Saints Peter and Paul Home v Pennsylvania.13
In a 7-2 decision, the Court held that the ACA gave HRSA authority to adopt regulations related to the undefined term “preventive care.” Therefore, it found that HRSA could exempt those with religious objections from participation in providing contraceptive coverage. ACOG and other medical groups filed an amicus brief arguing that contraception is an essential preventive service. “Contraception not only helps to prevent unintended pregnancy, but also helps to protect the health and well-being of women and their children.”14 It was cited only by Justice Ginsburg in her dissent.15
Deferred Action for Childhood Arrivals (DACA)
The AAMC, ACOG, AMA, and many other organizations filed an amicus brief16 in Department of Homeland Security v Regents of University of California.17 The case raised the question of whether a decision to end the DACA program followed the appropriate administrative procedures. In 2012, the Obama administration issued a “memorandum” establishing DACA (without congressional approval or formal rulemaking). A lower court decision barring implementation of DACA was upheld by the Supreme Court in 2016 on a 4-4 vote.18 In 2017, the Trump administration moved to end DACA.
In a 5-4 decision, the Court held that the explanation for ending DACA was inadequate, and violated the Administrative Procedures Act, so DACA could continue until the administration redid the repeal, following the proper procedures. The decision of the Court dealt solely with the process by which the rescission took place—there was general agreement that the administration had the right to rescind it if the procedure (with legitimate reasons) was proper.
The brief for the medical groups argued that the failure of the regulation to consider “reliance interests” would have especially difficult consequences in the medical fields. It noted, “At this moment, an estimated 27,000 health care workers and support staff depend on DACA for their authorization to work in the United States. Among those 27,000 are nurses, dentists, pharmacists, physician assistants, home health aides, technicians, and others. The number also includes nearly 200 medical students, medical residents, and physicians who depend on DACA for their eligibility to practice medicine.”16 The brief was not cited by the Court, but the reliance interest the brief spoke about was an important part of the case.
Continue to: Employment discrimination against gay and transgender employees...
Employment discrimination against gay and transgender employees
Federal law (“Title VII”) makes it illegal for an employer to “discriminate against any individual because of race, color, religion, sex, or national origin.”19 The question this term was whether discrimination based on sexual orientation or sexual identity is within the statute’s meaning of “sex.” By a 6-3 majority, the Court held that Title VII applies both to orientation and identity. (This was an interpretation of the statute, not a broad constitutional ruling.)
The majority reasoned that “it is impossible to discriminate against a person for being homosexual or transgender without discriminating against that individual based on sex. Consider, for example, an employer with 2 employees, both of whom are attracted to men.” If the employer fires the gay employee, “the employer discriminates against him for traits or actions it tolerates in his female colleague.”20
AMA and a number of other medical organizations filed an amicus brief in the case.21 The core of the argument of the brief was, “Employment discrimination against transgender people frustrates the treatment of gender dysphoria by preventing transgender individuals from living openly in accordance with their true gender identity and impeding access to needed medical care. Experiencing discrimination in one of the most important aspects of adult life—employment—makes it nearly impossible to live in full congruence with one’s gender identity. The fear of facing such discrimination alone can prompt transgender individuals to hide their gender identity, directly thwarting the goal of social transition…. Lack of treatment, in turn, increases the rate of negative mental health outcomes, substance abuse, and suicide.” The brief was not cited in the opinions in the case.
This decision is likely to have great impact on many aspects of American life. In the employment area, it is now a matter of course that employers may not discriminate based on orientation or identity in any employment decisions including hiring, firing, compensation, fringe benefits, etc. Harassment based on identity or orientation may similarly be an employment law violation. The decision also likely means that giving employment preferences to gay employees would now be as illegal as would be giving preferences to straight employees. (Limited exceptions, notably to some religious organization employees, are not included in anti-discrimination laws.)22
The importance of the decision goes well beyond employment, however. More than 100 federal statutes are in place that prohibit “discrimination because of sex.” It is now likely that these statutes will be interpreted as prohibiting discrimination related to sexual orientation and identification.
Additional cases of interest
HIV/AIDS International Program
A major US program fighting HIV/AIDS worldwide—the United States Leadership Against HIV/AIDS, Tuberculosis, and Malaria Act (aka the Leadership Act)—has provided billions of dollars to agencies abroad.23 Nongovernmental organizations (NGOs) receiving funds under the program must agree to have a “policy explicitly opposing prostitution and sex trafficking” (known as the “Policy Requirement”). Some grant recipients in foreign countries, generally affiliates of US NGOs, do not want to have such a policy and challenged the policy requirement as a violation of First Amendment right of free speech. The Court held that it is a well-settled principle that “foreign citizens outside US territory do not possess rights under the US Constitution.”24 Nor do organizations become entitled to such rights as a result of an affiliation with US organizations. This decision means that foreign organizations are free not to have the required policies, but they will be ineligible for funds under the Leadership Act.
Continue to: ACA government debts edition...
ACA government debts edition
The ACA was before the Court, yet again. To encourage private insurers to participate in online health insurance exchanges, the ACA provided that the federal government would share in insurance company losses for 3 years.25 The Act, however, did not appropriate any money for these “risk corridors,” and insurance companies lost $12 billion.
Congress (after the 2010 election) prohibited any appropriated funds from being used to pay insurance companies for their risk corridor losses. Four insurance companies sued the United States, seeking reimbursements for their losses. This term the Court held that the government must pay for their losses under the ACA.26 The Court said that Congress could have expressly repealed the risk corridor obligation (in the appropriation bill), but instead had only prohibited the expenditure of the money, which the Court said did not amount to an implied repeal of the obligation. We will see that ACA will be back before the Court again next term in California v Texas (discussed below).
Child custody and international abduction
The Hague Convention on the Civil Aspects of International Child Abduction (to which the United States is a party) provides that the courts of the country where the child has “habitual residence” have jurisdiction to decide custody.27 If a parent takes the child to another country, that country is obligated to return the child to the country of “habitual residence.”
This term the Court was called upon to define “habitual residence.” The Court held that determining habitual residence depends on the “totality of the circumstances,” and that “locating a child’s home is a fact-driven inquiry,” and that “courts must be sensitive to the unique circumstances of the case and informed by common sense.”28 An exception to the Convention’s obligation to return a child to the country of habitual residence is where “there is a grave risk that [the] return would expose the child to physical or psychological harm or otherwise place the child in an intolerable situation.”29 Who the parent is can affect many aspects of legal authority over the child, including consent to medical care, and the right to receive information concerning care.
Analysis of the term
The term began October 7, 2019, and adjourned July 9, 2020, somewhat later than usual because of coronavirus disease 2019 (COVID-19). During the term, the Court decided 60 cases, including 53 “signed” merit opinions after oral argument—the lowest number of decided cases in many years.30 Of those 60 cases, 22 (35%) were unanimous, and 13 (22%) resulted in a 5-4 split.30 Ten-year averages are 48% unanimous and 20% with 5-4 decisions.30
Chief Justice Roberts was the central focus of the term. He presided over the impeachment trial of President Trump in the Senate early in the term. He also presided over the Court’s accommodations of the COVID-19 pandemic. He is the “median,” or “swing,” justice. He was in the majority in 12 of the 13 cases with 5-4 decisions.30 He was in the majority in 97% of all cases and in 95% of “divided cases”—the highest of any of the justices this term.30 In some of the most critical decisions, Chief Justice Roberts sided with the “liberal” wing, including on cases concerning abortion, gay and transgender employment, DACA, and 2 Presidential subpoena cases. More often (in 9 of the 5-4 decisions), however, he sided with the more conservative justices.30 Justice Kavanaugh agreed with Chief Justice Roberts most often (in 93% of all cases).30 Among the others, these justices agreed with each other 90% or more of the time: Justices Ginsburg and Breyer (93%), Justices Alito and Thomas (92%), and Justices Breyer and Kagan (90%).30
Continue to: COVID-19 and the Court...
COVID-19 and the Court
Some of the biggest news of the term came not from the law, but from medicine in the form of COVID-19. The Court was in the process of preparing a final period of important arguments when, on March 16, it announced that it was postponing further arguments. The Court rescheduled 10 oral arguments that were held by telephone (other cases were held over to the next term). The phone arguments, during the first 2 weeks of May, necessitated a change in format. Each justice was called on (in order of seniority) by the Chief Justice to ask questions. This was in contrast to the free-for-all questions that usually characterize in-person arguments. These arguments were broadcast live—something that had never been done before. Public access was, on balance, a good thing. There were a couple failures to unmute, and there was “the flush heard round the world” in the middle of one argument, but otherwise the arguments went off with few hitches.31
Looking ahead
By the end of the term, no justice had announced an intention to retire from the Court. On September 18, however, Justice Ruth Bader Ginsburg passed away. In 2009, she had been diagnosed with early-stage pancreatic cancer. This term she had been hospitalized twice, and at the end of the term, she announced a recurrence of pancreatic cancer, which was being treated with chemotherapy. See “RBG: The woman, the legacy” for a tribute to this remarkable woman, lawyer, and justice.
Justice Ginsburg’s death, occurring in the middle of a presidential campaign, ignited a political firestorm concerning her successor. The outcome of selecting and confirming her successor and the political fallout were not immediately apparent. Justice Ginsburg was confirmed just 7 weeks after her nomination by President Clinton, by a vote of 96-3. But those days of Senate consensus are not the current norm.
The next term (called the “October 2020 Term”) will begin on October 5, 2020. The Court will begin with 8 justices and, depending on the nomination process, may operate with 8 justices for some time. When there is a “tie” vote in the Court, the lower court decision is upheld. The Court has been short-handed several times in the past and, with few exceptions, has managed the cases successfully.
The Court has announced that initial arguments will be telephonic. It already has taken a number of cases. The constitutionality of the individual mandate (coverage) in the ACA will once again be before the Court, and that already has produced a flood of amicus briefs from health-related organizations.32 Among other upcoming issues are cases related to state regulation of pharmacy benefit managers, gay rights and foster care, sentencing of juveniles to life in prison without the possibility of parole, a face-off between Google and Oracle on software copyrights, and arbitration. In addition, some of the issues we saw this term will reappear, with more on robocalls, religious freedom and Catholic charities, and immigration and removal cases.
Ruth Bader Ginsburg, as a law student, law professor, lawyer, judge, and justice, was a leading advocate for the rights of women. There were only a few women in law school when she attended, but she graduated tied for first in her class. Although she found it difficult to be hired as a lawyer, as a law professor and lawyer she helped map a strategy to expand legal rights for women, arguing 6 cases before the Supreme Court and winning 5 of them. She served as a federal appeals court judge and then was appointed to the Supreme Court in 1993. She was the second woman to serve on the Court.
As a justice, she was known during much of her tenure on the Court as the leader of the liberal justices, although her jurisprudence was more complex than that simple statement. She was always a strong advocate for the rights of women (and equal rights of men) during her time on the Court. She was a very clear writer; her opinions were direct and easy to understand. She was also fast—she routinely had the record of announcing opinions faster than any of the other current justices. She was 87 when she passed away, having served on the Court for 27 years.
Justice Ginsburg was also something of a cultural phenomenon. In later years she was sometimes known as “the Notorious RBG.” Books, movies, songs, and even workout videos were made about her. In groups she seemed almost shy, but she was thoughtful, kind, and funny (sometimes wickedly so). The outpouring of affection and sympathy at her death was a symbol of the place she held in America. She loved the opera, a passion she shared with her friend, Justice Antonin Scalia. Despite their considerable disagreements on legal matters, Justices Ginsburg and Scalia were close friends. They attended opera with one another, and their families usually spent New Year’s Eves together. They were the 2 most recent justices to pass away while serving on the Court.
The Court heard and ruled on a large number of other significant cases that will have consequences for many years to come. Highlights include:
- In 2 cases involving subpoenas for the President’s personal records, the Court suggested some balance between “nobody is above the law” and not unnecessarily hectoring or interfering with fulfilling the office of President. The Court held that Congress may subpoena a President’s personal and family records, while the President is still in office.1 It instructed lower courts to assess whether the papers are necessary, the subpoena is limited in scope, there is legitimate legislative purpose, whether the burden it imposes on the President is reasonable, and whether the subpoena would unduly interfere with the ability to do the work required as President.
- Similarly, local (state) grand juries may subpoena such personal records, but the President will have the opportunity to raise specific objections to the subpoenas—undue burden, bad faith, or overbreadth. In addition, the respect owed to the office should inform the conduct regarding the subpoena.2
- The Court upheld a federal law that prohibits most robocalls.3 It struck down an amendment that allowed robocalls made to collect debts owed to or guaranteed by the federal government.
- The Court held that a single-director federal agency, whose director cannot be removed by the President (at will), violates the Constitution.4 The Consumer Financial Protection Bureau (created by the Dodd-Frank law) has such a single, no-removal director and that will have to be modified.
- The Court held that the eastern half of Oklahoma (including Tulsa) is part of a Creek Nation reservation.5 This was a question of criminal law jurisdiction, not property ownership. The practical effect is that for crimes involving Native Americans, serious crimes will have to be tried in federal court, while lesser crimes may be tried in tribal courts.
- The Court determined that it was unconstitutional for a state program providing tuition assistance to parents who send their children to private schools, to prohibit students attending religious private schools from participating in the program. That is a burden on the “free exercise” of religion.6
- The Court considered whether there can be civil liability for damages caused by a federal official in the United States harming a foreign national in another country. In this case, a border patrol agent standing in the US shot and killed a Mexican juvenile who was just across the border in Mexico.7 The issue was whether the parents of the Mexican national could sue the US officials for damages. The Court declined to expand liability to include those injured outside the US. Ultimately, the Court was reluctant to impose liability because this liability is not authorized by Congress.
- In a COVID-19 religion case, the Court refused to stop the enforcement of a governor’s COVID-19 order that allowed churches to operate with <100 attendees or 25% occupancy (whichever was lower).8 Meanwhile, businesses, malls, and stores were allowed to reopen without these stringent limitations. The church objected that greater burdens were placed on religion than secular activity. The Court denied the church’s request for an injunction.
- The Court unanimously held that a state may punish or remove a “faithless elector.” Electors cast votes on behalf of their states in the Electoral College—where Presidents are technically selected. Electors are generally pledged to vote for the winner of a state’s vote for President. A few have violated that pledge and voted for someone else. As a practical matter, that could cause real disruption, and the Court upheld state laws that take action against these “faithless” electors.9
- Several days after the Court had officially adjourned for the term, it received several petitions to delay the execution of federal prisoners. One case was based on the method of execution (use of pentobarbital),10 and another was based on the claim that a prisoner had become so mentally incompetent that it was improper to execute him.11 The Court turned down these appeals, allowing the executions to proceed. These were the first federal government executions in 17 years.
References
- Trump v Mazars USA, LLP, 140 S. Ct. 2019 (2020).
- Trump v Vance, 140 S. Ct. 2412 (2020).
- Barr v American Association of Political Consultants, Inc, 140 S. Ct. 2335 (2020).
- Seila Law LLC v Consumer Financial Protection Bureau, 140 S. Ct. 2183 (2020).
- McGirt v Oklahoma, 140 S. Ct. 2452 (2020).
- Espinoza v Montana Department of Revenue, 140 S. Ct. 2246 (2020).
- Hernández v Mesa, 140 S. Ct. 735, 206 L. Ed. 2d 29 (2020).
- South Bay United Pentecostal Church v Newsom, 140 S. Ct. 1613, 207 L. Ed. 2d 154 (2020).
- Chiafalo v Washington, 140 S. Ct. 2316 (2020).
- Barr v Lee, ____ S. Ct. ____ (2020).
- Barr v Purkey, ____ S. Ct. ____ (2020).
The 2019-2020 term of the US Supreme Court was remarkable by any standard. An extraordinary number of important cases made it “a buffet of blockbusters.”1
We look first at several cases that will be of particular interest to ObGyns. Then we look briefly at a number of other important cases that affect the medical profession as a whole and the direction of the country (see “Other significant US Supreme Court decisions”), and finally we conclude with an analysis of this term and a forecast for the next.
We chose cases in which specialty organizations, such as the American College of Obstetricians and Gynecologists (ACOG), or organized medicine (the American Medical Association [AMA], the Association of American Medical Colleges [AAMC], or the American Hospital Association [AHA]), took a special interest by filing “amicus curiae” (friend of the court) briefs with the Supreme Court. These briefs are filed by an organization or person who is not a party to the case but who may have important information to convey to the Court. Because these briefs represent a significant commitment of money, time, and effort, they are usually not undertaken lightly.
Decisions concerning abortion
June v Russo
Decided June 29, 2020, June v Russo involved a Louisiana statute that required abortion providers have “active admitting privileges at a hospital” within 30 miles of where the abortion is performed.2 The Court decided a case in 2016 (from Texas) that involved almost the same statutory provision, so it might seem like an easy ruling.3 But Justice Kennedy (the deciding vote in 2016) has been replaced by Justice Gorsuch, so the outcome was uncertain. It was a difficult case, with a total of 5 opinions covering 138 pages and a “surprise” from the Chief Justice.
The Court, in a 5-4 decision, struck down the Louisiana law, but there was no majority opinion. Four justices in the plurality emphasized that the Louisiana law (like the Texas law) substantially burdened the right to abortion without any corresponding benefit to the health of the women seeking abortions. (Under earlier Court precedents, “undue burdens” on abortion are unconstitutional.4) Justice Breyer noted that the state could not present even one example in which a woman would have had better treatment if her doctor had admitting privileges. For a variety of reasons, admitting privileges were cumbersome for abortion providers to obtain; therefore, enforcing the law had little or no benefit, but significant risk of reduced availability of abortion services.
In June v Russo, Chief Justice Roberts literally became the “swing vote”—the fifth vote to strike down the Louisiana law. In 2016, he had voted the other way—to uphold essentially the same law (in Texas) that he struck down here. He attributed his switch to precedent (the general obligation of courts to follow prior decisions). He disagreed with the earlier decision, but felt bound by it.
This should be the end of the abortion provider “hospital privileges requirements” that a number of states have passed. States seeking to nibble away at abortion rights will undoubtedly look elsewhere. Beyond that, it is difficult, from this case, to discern the future of abortion rights.
ACOG was the lead in amicus briefs urging the Court to strike down the Louisiana law. ACOG (with others) was one of only a handful of organizations filing a brief urging the Court to agree to hear the case.5 When the Court did agree to hear the case (“granted certiorari”), ACOG and a number of other medical organizations filed a formal amicus brief on the merits of the case.6 The brief made 2 arguments: First, that this case was essentially decided in Whole Woman’s Health in 2016 (the Texas case) and, second, that “an admitting privileges requirement is not medically necessary” and “clinicians who provide abortions are unable to obtain admitting privileges for reasons unrelated to their ability to safely and competently perform abortions.” Justice Breyer cited the ACOG brief twice.
The American Association of Pro-Life Obstetricians and Gynecologists also filed an amicus brief.7 The brief was directed solely at arguing that ACOG was not presenting reliable science. It summarized, “The American College of Obstetricians and Gynecologists has always presented itself to the Court as a source of objective medical knowledge. However, when it comes to abortion, the College today is primarily a pro-abortion political advocacy organization.” That brief concluded that the “Court should read ACOG’s amicus brief not as an authoritative recitation of settled science, but as a partisan advocacy paper on behalf of a mere subset of American obstetricians and gynecologists.”
The Association of American Physicians and Surgeons (which should not be confused with the “National Board of Physicians and Surgeons”) also filed an amicus brief. The brief argued, “Abortion, like other outpatient surgical procedures, sometimes results in patient hospitalization. Requiring abortion providers to maintain admitting privileges will improve communication between physicians in the transfer of patients to the hospital and allow them to participate in the care of their patients while in the hospital, in line with their ethical duty to ensure their patients’ continuity of care.”8
Continue to: Ultrasonography requirement for abortion...
Ultrasonography requirement for abortion
In another abortion case, the Court was asked to review a Kentucky abortion statute requiring that an ultrasound image be shown to the woman as part of informed consent for an abortion.9 ACOG filed an amicus brief in favor of a review, but the Court declined to hear the case.10,11
Contraception considerations
The Affordable Care Act (ACA) has an ambiguous provision regarding no-cost “preventive care and screenings” for women. The ACA does not, however, specify contraceptive coverage.12 Several departments and the Health Resources and Services Administration (collectively referred to as “HRSA”) interpreted the provision to include contraception, but from the start there were religious objections. HRSA eventually provided an exemption regarding contraception for employers (nonprofits and for-profits with no publicly traded components) that had “sincerely held moral” objections to providing forms of contraceptive coverage. That regulation was again before the Court this term in Little Sisters of the Poor Saints Peter and Paul Home v Pennsylvania.13
In a 7-2 decision, the Court held that the ACA gave HRSA authority to adopt regulations related to the undefined term “preventive care.” Therefore, it found that HRSA could exempt those with religious objections from participation in providing contraceptive coverage. ACOG and other medical groups filed an amicus brief arguing that contraception is an essential preventive service. “Contraception not only helps to prevent unintended pregnancy, but also helps to protect the health and well-being of women and their children.”14 It was cited only by Justice Ginsburg in her dissent.15
Deferred Action for Childhood Arrivals (DACA)
The AAMC, ACOG, AMA, and many other organizations filed an amicus brief16 in Department of Homeland Security v Regents of University of California.17 The case raised the question of whether a decision to end the DACA program followed the appropriate administrative procedures. In 2012, the Obama administration issued a “memorandum” establishing DACA (without congressional approval or formal rulemaking). A lower court decision barring implementation of DACA was upheld by the Supreme Court in 2016 on a 4-4 vote.18 In 2017, the Trump administration moved to end DACA.
In a 5-4 decision, the Court held that the explanation for ending DACA was inadequate, and violated the Administrative Procedures Act, so DACA could continue until the administration redid the repeal, following the proper procedures. The decision of the Court dealt solely with the process by which the rescission took place—there was general agreement that the administration had the right to rescind it if the procedure (with legitimate reasons) was proper.
The brief for the medical groups argued that the failure of the regulation to consider “reliance interests” would have especially difficult consequences in the medical fields. It noted, “At this moment, an estimated 27,000 health care workers and support staff depend on DACA for their authorization to work in the United States. Among those 27,000 are nurses, dentists, pharmacists, physician assistants, home health aides, technicians, and others. The number also includes nearly 200 medical students, medical residents, and physicians who depend on DACA for their eligibility to practice medicine.”16 The brief was not cited by the Court, but the reliance interest the brief spoke about was an important part of the case.
Continue to: Employment discrimination against gay and transgender employees...
Employment discrimination against gay and transgender employees
Federal law (“Title VII”) makes it illegal for an employer to “discriminate against any individual because of race, color, religion, sex, or national origin.”19 The question this term was whether discrimination based on sexual orientation or sexual identity is within the statute’s meaning of “sex.” By a 6-3 majority, the Court held that Title VII applies both to orientation and identity. (This was an interpretation of the statute, not a broad constitutional ruling.)
The majority reasoned that “it is impossible to discriminate against a person for being homosexual or transgender without discriminating against that individual based on sex. Consider, for example, an employer with 2 employees, both of whom are attracted to men.” If the employer fires the gay employee, “the employer discriminates against him for traits or actions it tolerates in his female colleague.”20
AMA and a number of other medical organizations filed an amicus brief in the case.21 The core of the argument of the brief was, “Employment discrimination against transgender people frustrates the treatment of gender dysphoria by preventing transgender individuals from living openly in accordance with their true gender identity and impeding access to needed medical care. Experiencing discrimination in one of the most important aspects of adult life—employment—makes it nearly impossible to live in full congruence with one’s gender identity. The fear of facing such discrimination alone can prompt transgender individuals to hide their gender identity, directly thwarting the goal of social transition…. Lack of treatment, in turn, increases the rate of negative mental health outcomes, substance abuse, and suicide.” The brief was not cited in the opinions in the case.
This decision is likely to have great impact on many aspects of American life. In the employment area, it is now a matter of course that employers may not discriminate based on orientation or identity in any employment decisions including hiring, firing, compensation, fringe benefits, etc. Harassment based on identity or orientation may similarly be an employment law violation. The decision also likely means that giving employment preferences to gay employees would now be as illegal as would be giving preferences to straight employees. (Limited exceptions, notably to some religious organization employees, are not included in anti-discrimination laws.)22
The importance of the decision goes well beyond employment, however. More than 100 federal statutes are in place that prohibit “discrimination because of sex.” It is now likely that these statutes will be interpreted as prohibiting discrimination related to sexual orientation and identification.
Additional cases of interest
HIV/AIDS International Program
A major US program fighting HIV/AIDS worldwide—the United States Leadership Against HIV/AIDS, Tuberculosis, and Malaria Act (aka the Leadership Act)—has provided billions of dollars to agencies abroad.23 Nongovernmental organizations (NGOs) receiving funds under the program must agree to have a “policy explicitly opposing prostitution and sex trafficking” (known as the “Policy Requirement”). Some grant recipients in foreign countries, generally affiliates of US NGOs, do not want to have such a policy and challenged the policy requirement as a violation of First Amendment right of free speech. The Court held that it is a well-settled principle that “foreign citizens outside US territory do not possess rights under the US Constitution.”24 Nor do organizations become entitled to such rights as a result of an affiliation with US organizations. This decision means that foreign organizations are free not to have the required policies, but they will be ineligible for funds under the Leadership Act.
Continue to: ACA government debts edition...
ACA government debts edition
The ACA was before the Court, yet again. To encourage private insurers to participate in online health insurance exchanges, the ACA provided that the federal government would share in insurance company losses for 3 years.25 The Act, however, did not appropriate any money for these “risk corridors,” and insurance companies lost $12 billion.
Congress (after the 2010 election) prohibited any appropriated funds from being used to pay insurance companies for their risk corridor losses. Four insurance companies sued the United States, seeking reimbursements for their losses. This term the Court held that the government must pay for their losses under the ACA.26 The Court said that Congress could have expressly repealed the risk corridor obligation (in the appropriation bill), but instead had only prohibited the expenditure of the money, which the Court said did not amount to an implied repeal of the obligation. We will see that ACA will be back before the Court again next term in California v Texas (discussed below).
Child custody and international abduction
The Hague Convention on the Civil Aspects of International Child Abduction (to which the United States is a party) provides that the courts of the country where the child has “habitual residence” have jurisdiction to decide custody.27 If a parent takes the child to another country, that country is obligated to return the child to the country of “habitual residence.”
This term the Court was called upon to define “habitual residence.” The Court held that determining habitual residence depends on the “totality of the circumstances,” and that “locating a child’s home is a fact-driven inquiry,” and that “courts must be sensitive to the unique circumstances of the case and informed by common sense.”28 An exception to the Convention’s obligation to return a child to the country of habitual residence is where “there is a grave risk that [the] return would expose the child to physical or psychological harm or otherwise place the child in an intolerable situation.”29 Who the parent is can affect many aspects of legal authority over the child, including consent to medical care, and the right to receive information concerning care.
Analysis of the term
The term began October 7, 2019, and adjourned July 9, 2020, somewhat later than usual because of coronavirus disease 2019 (COVID-19). During the term, the Court decided 60 cases, including 53 “signed” merit opinions after oral argument—the lowest number of decided cases in many years.30 Of those 60 cases, 22 (35%) were unanimous, and 13 (22%) resulted in a 5-4 split.30 Ten-year averages are 48% unanimous and 20% with 5-4 decisions.30
Chief Justice Roberts was the central focus of the term. He presided over the impeachment trial of President Trump in the Senate early in the term. He also presided over the Court’s accommodations of the COVID-19 pandemic. He is the “median,” or “swing,” justice. He was in the majority in 12 of the 13 cases with 5-4 decisions.30 He was in the majority in 97% of all cases and in 95% of “divided cases”—the highest of any of the justices this term.30 In some of the most critical decisions, Chief Justice Roberts sided with the “liberal” wing, including on cases concerning abortion, gay and transgender employment, DACA, and 2 Presidential subpoena cases. More often (in 9 of the 5-4 decisions), however, he sided with the more conservative justices.30 Justice Kavanaugh agreed with Chief Justice Roberts most often (in 93% of all cases).30 Among the others, these justices agreed with each other 90% or more of the time: Justices Ginsburg and Breyer (93%), Justices Alito and Thomas (92%), and Justices Breyer and Kagan (90%).30
Continue to: COVID-19 and the Court...
COVID-19 and the Court
Some of the biggest news of the term came not from the law, but from medicine in the form of COVID-19. The Court was in the process of preparing a final period of important arguments when, on March 16, it announced that it was postponing further arguments. The Court rescheduled 10 oral arguments that were held by telephone (other cases were held over to the next term). The phone arguments, during the first 2 weeks of May, necessitated a change in format. Each justice was called on (in order of seniority) by the Chief Justice to ask questions. This was in contrast to the free-for-all questions that usually characterize in-person arguments. These arguments were broadcast live—something that had never been done before. Public access was, on balance, a good thing. There were a couple failures to unmute, and there was “the flush heard round the world” in the middle of one argument, but otherwise the arguments went off with few hitches.31
Looking ahead
By the end of the term, no justice had announced an intention to retire from the Court. On September 18, however, Justice Ruth Bader Ginsburg passed away. In 2009, she had been diagnosed with early-stage pancreatic cancer. This term she had been hospitalized twice, and at the end of the term, she announced a recurrence of pancreatic cancer, which was being treated with chemotherapy. See “RBG: The woman, the legacy” for a tribute to this remarkable woman, lawyer, and justice.
Justice Ginsburg’s death, occurring in the middle of a presidential campaign, ignited a political firestorm concerning her successor. The outcome of selecting and confirming her successor and the political fallout were not immediately apparent. Justice Ginsburg was confirmed just 7 weeks after her nomination by President Clinton, by a vote of 96-3. But those days of Senate consensus are not the current norm.
The next term (called the “October 2020 Term”) will begin on October 5, 2020. The Court will begin with 8 justices and, depending on the nomination process, may operate with 8 justices for some time. When there is a “tie” vote in the Court, the lower court decision is upheld. The Court has been short-handed several times in the past and, with few exceptions, has managed the cases successfully.
The Court has announced that initial arguments will be telephonic. It already has taken a number of cases. The constitutionality of the individual mandate (coverage) in the ACA will once again be before the Court, and that already has produced a flood of amicus briefs from health-related organizations.32 Among other upcoming issues are cases related to state regulation of pharmacy benefit managers, gay rights and foster care, sentencing of juveniles to life in prison without the possibility of parole, a face-off between Google and Oracle on software copyrights, and arbitration. In addition, some of the issues we saw this term will reappear, with more on robocalls, religious freedom and Catholic charities, and immigration and removal cases.
Ruth Bader Ginsburg, as a law student, law professor, lawyer, judge, and justice, was a leading advocate for the rights of women. There were only a few women in law school when she attended, but she graduated tied for first in her class. Although she found it difficult to be hired as a lawyer, as a law professor and lawyer she helped map a strategy to expand legal rights for women, arguing 6 cases before the Supreme Court and winning 5 of them. She served as a federal appeals court judge and then was appointed to the Supreme Court in 1993. She was the second woman to serve on the Court.
As a justice, she was known during much of her tenure on the Court as the leader of the liberal justices, although her jurisprudence was more complex than that simple statement. She was always a strong advocate for the rights of women (and equal rights of men) during her time on the Court. She was a very clear writer; her opinions were direct and easy to understand. She was also fast—she routinely had the record of announcing opinions faster than any of the other current justices. She was 87 when she passed away, having served on the Court for 27 years.
Justice Ginsburg was also something of a cultural phenomenon. In later years she was sometimes known as “the Notorious RBG.” Books, movies, songs, and even workout videos were made about her. In groups she seemed almost shy, but she was thoughtful, kind, and funny (sometimes wickedly so). The outpouring of affection and sympathy at her death was a symbol of the place she held in America. She loved the opera, a passion she shared with her friend, Justice Antonin Scalia. Despite their considerable disagreements on legal matters, Justices Ginsburg and Scalia were close friends. They attended opera with one another, and their families usually spent New Year’s Eves together. They were the 2 most recent justices to pass away while serving on the Court.
The Court heard and ruled on a large number of other significant cases that will have consequences for many years to come. Highlights include:
- In 2 cases involving subpoenas for the President’s personal records, the Court suggested some balance between “nobody is above the law” and not unnecessarily hectoring or interfering with fulfilling the office of President. The Court held that Congress may subpoena a President’s personal and family records, while the President is still in office.1 It instructed lower courts to assess whether the papers are necessary, the subpoena is limited in scope, there is legitimate legislative purpose, whether the burden it imposes on the President is reasonable, and whether the subpoena would unduly interfere with the ability to do the work required as President.
- Similarly, local (state) grand juries may subpoena such personal records, but the President will have the opportunity to raise specific objections to the subpoenas—undue burden, bad faith, or overbreadth. In addition, the respect owed to the office should inform the conduct regarding the subpoena.2
- The Court upheld a federal law that prohibits most robocalls.3 It struck down an amendment that allowed robocalls made to collect debts owed to or guaranteed by the federal government.
- The Court held that a single-director federal agency, whose director cannot be removed by the President (at will), violates the Constitution.4 The Consumer Financial Protection Bureau (created by the Dodd-Frank law) has such a single, no-removal director and that will have to be modified.
- The Court held that the eastern half of Oklahoma (including Tulsa) is part of a Creek Nation reservation.5 This was a question of criminal law jurisdiction, not property ownership. The practical effect is that for crimes involving Native Americans, serious crimes will have to be tried in federal court, while lesser crimes may be tried in tribal courts.
- The Court determined that it was unconstitutional for a state program providing tuition assistance to parents who send their children to private schools, to prohibit students attending religious private schools from participating in the program. That is a burden on the “free exercise” of religion.6
- The Court considered whether there can be civil liability for damages caused by a federal official in the United States harming a foreign national in another country. In this case, a border patrol agent standing in the US shot and killed a Mexican juvenile who was just across the border in Mexico.7 The issue was whether the parents of the Mexican national could sue the US officials for damages. The Court declined to expand liability to include those injured outside the US. Ultimately, the Court was reluctant to impose liability because this liability is not authorized by Congress.
- In a COVID-19 religion case, the Court refused to stop the enforcement of a governor’s COVID-19 order that allowed churches to operate with <100 attendees or 25% occupancy (whichever was lower).8 Meanwhile, businesses, malls, and stores were allowed to reopen without these stringent limitations. The church objected that greater burdens were placed on religion than secular activity. The Court denied the church’s request for an injunction.
- The Court unanimously held that a state may punish or remove a “faithless elector.” Electors cast votes on behalf of their states in the Electoral College—where Presidents are technically selected. Electors are generally pledged to vote for the winner of a state’s vote for President. A few have violated that pledge and voted for someone else. As a practical matter, that could cause real disruption, and the Court upheld state laws that take action against these “faithless” electors.9
- Several days after the Court had officially adjourned for the term, it received several petitions to delay the execution of federal prisoners. One case was based on the method of execution (use of pentobarbital),10 and another was based on the claim that a prisoner had become so mentally incompetent that it was improper to execute him.11 The Court turned down these appeals, allowing the executions to proceed. These were the first federal government executions in 17 years.
References
- Trump v Mazars USA, LLP, 140 S. Ct. 2019 (2020).
- Trump v Vance, 140 S. Ct. 2412 (2020).
- Barr v American Association of Political Consultants, Inc, 140 S. Ct. 2335 (2020).
- Seila Law LLC v Consumer Financial Protection Bureau, 140 S. Ct. 2183 (2020).
- McGirt v Oklahoma, 140 S. Ct. 2452 (2020).
- Espinoza v Montana Department of Revenue, 140 S. Ct. 2246 (2020).
- Hernández v Mesa, 140 S. Ct. 735, 206 L. Ed. 2d 29 (2020).
- South Bay United Pentecostal Church v Newsom, 140 S. Ct. 1613, 207 L. Ed. 2d 154 (2020).
- Chiafalo v Washington, 140 S. Ct. 2316 (2020).
- Barr v Lee, ____ S. Ct. ____ (2020).
- Barr v Purkey, ____ S. Ct. ____ (2020).
1. Liptak A. In a term full of major cases, the Supreme Court tacked to the center. The New York Times. July 10, 2020.
2. June Medical Services LLC v Russo, 591 US 140 S. Ct. 2103, 2112 (2020).
3. Whole Woman’s Health v Hellerstedt, 579 US ___ (2016).
4. Planned Parenthood of Southeastern PA v Casey, 505 US 833, 874 (1992).
5. Brief of the American College of Obstetricians and Gynecologists, the American Academy of Family Physicians, the American Academy of Pediatrics, the American College of Nurse-Midwives, the American College of Osteopathic Obstetricians and Gynecologists, the American College of Physicians, the American Society for Reproductive Medicine, the National Association of Nurse Practitioners in Women’s Health, the North American Society for Pediatric and Adolescent Gynecology, and the Society for Maternal-Fetal Medicine Amici Curiae In Support of Petitioners, June Medical Services v Russo. May 20, 2019. https://www.supremecourt.gov/DocketPDF/18/18-1323/100434/20190520175434029_18-1323%20ACOG%20et%20al.%20cert.%20amicus%20brief.pdf. Accessed August 31, 2020.
6. Brief of Amici Curiae American College of Obstetricians and Gynecologists, American Medical Association, American Academy of Family Physicians, American Academy of Nursing, American Academy of Pediatrics, American College of Nurse-Midwives, American College of Osteopathic Obstetricians and Gynecologists, American College of Physicians, American Osteopathic Association, American Public Health Association, American Society for Reproductive Medicine, North American Society for Pediatric and Adolescent Gynecology, Society for Maternal-Fetal Medicine, and the Society of Ob/Gyn Hospitalists, In Support of June Medical Services, June Medical Services v Russo. December 2, 2019. https://www.supremecourt.gov/DocketPDF/18/18-1323/124091/20191202145531124_18-1323%2018-1460%20tsac%20American%20College%20of%20Obstetricians%20and%20
Gynecologists%20et%20al.pdf [https://perma.cc/8T8V-4D6S]. Accessed August 31, 2020.
7. Brief of Amicus Curiae American Association of Pro-Life Obstetricians and Gynecologists In Support of [Russo] Louisiana Department of Health and Hospitals, June Medical Services v Russo. December 27, 2019. https://www.supremecourt.gov/DocketPDF/18/18-1323/126927/20191227154424488_AAPLOG%20Amicus%20Brief.pdf. Accessed August 31, 2020.
8. Brief of Association of American Physicians and Surgeons as Amicus Curiae in Support of Respondent–Cross-Petitioner, June Medical Services v Russo 2. December 27, 2019. https://www.supremecourt.gov/DocketPDF/18/18-1323/126828/20191227104605915_18-1323%20-1460%20bsac%20AAPS--PDFA.pdf. Accessed August 31, 2020.
9. Ky. Rev. Stat. § 311.727(2).
10. Brief for the American College of Obstetricians and Gynecologists, the American Medical Association, the North American Society for Pediatric and Adolescent Gynecology, the American College of Osteopathic Obstetricians and Gynecologists, and the American Academy of Family Physicians Amici Curiae Supporting Petitioners, EMW Women’s Surgical Center v Meier. October 28, 2019. https://www.supremecourt.gov/DocketPDF/19/19-417/120550/20191028184956458_19-417%20ACOG%20et%20al.%20-%20cert.%20amicus%20brief.pdf. Accessed August 31, 2020.
11. EMW Women’s Surgical Center, PSC v Meier, 140 S. Ct. 655 (2019).
12. Codified at 26 U. S. C. §5000A(f )(2); §§4980H(a), (c)(2) requires employers to provide women with “preventive care and screenings” without “any cost sharing requirements.”
13. Little Sisters of the Poor Saints Peter and Paul Home v Pennsylvania, 140 S. Ct. 2367 (2020).
14. Brief of Amici Curiae American College of Obstetricians and Gynecologists, American Nurses Association, American Academy of Nursing, Physicians for Reproductive Health, and Nurses for Sexual and Reproductive Health, In Support of Respondents and Affirmance, Little Sisters of the Poor Saints Peter and Paul Home v Pennsylvania. April 8, 2020. https://www.supremecourt.gov/DocketPDF/19/19-431/141177/20200408152340136_19-431%20and%2019-454%20Amici%20Curiae.pdf. Accessed August 31, 2020.
15. Little Sisters of the Poor Saints Peter and Paul Home v Pennsylvania, 140 S. Ct. 2367, 2400–12 (2020).
16. Brief for the Association of American Medical Colleges (and more than 30 other organizations, including the American Medical Association, and the American College of Obstetricians and Gynecologists) Amici Curiae, In Support of Respondents, Department of Homeland Security v Regents of University of California. October 4, 2019. https://www.supremecourt.gov/DocketPDF/18/18-587/118129/20191004130646281_Brief%20for%20AAMC%20et%20al%20
Supporting%20Respondents.pdf. Accessed August 31, 2020.
17. Department of Homeland Security v Regents of The University of California, 140 S. Ct. 1891 (2020).
18. United States v Texas, 136 S. Ct. 2271 (2016).
19. Title VII of the Civil Rights Act of 1964, 42 U.S.C §2000e–2(a)(1).
20. Bostock v Clayton County, 140 S. Ct. 1731, 1741 (2020).
21. Brief of the American Medical Association, the American College of Physicians, and 14 additional medical, mental health, and health care organizations as Amici Curiae In Support of the Employees, Bostock v Clayton County. July 3, 2019. https://www.supremecourt.gov/DocketPDF/17/17-1618/107177/20190703172548842_Amicus%20Brief.pdf. Accessed August 31, 2020.
22. Our Lady of Guadalupe School v Morrissey-Berru, 140 S. Ct. 2049 (2020).
23. United States Leadership Against HIV/AIDS, Tuberculosis, and Malaria Act (“the Leadership Act”), 22 U. S. C. §7601 et seq.
24. Agency for International Development v Alliance for Open Society, 140 S. Ct. 2082, 2086 (2020).
25. 42 U.S.C. §1342, §18063.
26. Maine Community Health Options v United States, 140 S. Ct. 1308 (2020).
27. Hague Convention on the Civil Aspects of International Child Abduction (Hague Convention or Convention), implemented in the United States by the International Child Abduction Remedies Act, 22 U. S. C. §9001 et seq.
28. Monasky v Taglieri, 140 S. Ct. 719 (2020).
29. Monasky v Taglieri, 140 S. Ct. 719, 723, 729 (2020).
30. Feldman A. Final stat pack for October term 2019 (upated). July 10, 2020. https://www.scotusblog.com/2020/07/final-stat-pack-for-october-term-2019/. Accessed August 31, 2020.
31. Hejmanowski D. Flush heard around the world. Delaware Gazette. May 8, 2020. https://www.delgazette.com/opinion/columns/83610/flush-heard-around-the-world. Accessed August 31, 2020.
32. SCOTUSblog.com. California v Texas. https://www.scotusblog.com/case-files/cases/california-v-texas/. Accessed August 31, 2020.
1. Liptak A. In a term full of major cases, the Supreme Court tacked to the center. The New York Times. July 10, 2020.
2. June Medical Services LLC v Russo, 591 US 140 S. Ct. 2103, 2112 (2020).
3. Whole Woman’s Health v Hellerstedt, 579 US ___ (2016).
4. Planned Parenthood of Southeastern PA v Casey, 505 US 833, 874 (1992).
5. Brief of the American College of Obstetricians and Gynecologists, the American Academy of Family Physicians, the American Academy of Pediatrics, the American College of Nurse-Midwives, the American College of Osteopathic Obstetricians and Gynecologists, the American College of Physicians, the American Society for Reproductive Medicine, the National Association of Nurse Practitioners in Women’s Health, the North American Society for Pediatric and Adolescent Gynecology, and the Society for Maternal-Fetal Medicine Amici Curiae In Support of Petitioners, June Medical Services v Russo. May 20, 2019. https://www.supremecourt.gov/DocketPDF/18/18-1323/100434/20190520175434029_18-1323%20ACOG%20et%20al.%20cert.%20amicus%20brief.pdf. Accessed August 31, 2020.
6. Brief of Amici Curiae American College of Obstetricians and Gynecologists, American Medical Association, American Academy of Family Physicians, American Academy of Nursing, American Academy of Pediatrics, American College of Nurse-Midwives, American College of Osteopathic Obstetricians and Gynecologists, American College of Physicians, American Osteopathic Association, American Public Health Association, American Society for Reproductive Medicine, North American Society for Pediatric and Adolescent Gynecology, Society for Maternal-Fetal Medicine, and the Society of Ob/Gyn Hospitalists, In Support of June Medical Services, June Medical Services v Russo. December 2, 2019. https://www.supremecourt.gov/DocketPDF/18/18-1323/124091/20191202145531124_18-1323%2018-1460%20tsac%20American%20College%20of%20Obstetricians%20and%20
Gynecologists%20et%20al.pdf [https://perma.cc/8T8V-4D6S]. Accessed August 31, 2020.
7. Brief of Amicus Curiae American Association of Pro-Life Obstetricians and Gynecologists In Support of [Russo] Louisiana Department of Health and Hospitals, June Medical Services v Russo. December 27, 2019. https://www.supremecourt.gov/DocketPDF/18/18-1323/126927/20191227154424488_AAPLOG%20Amicus%20Brief.pdf. Accessed August 31, 2020.
8. Brief of Association of American Physicians and Surgeons as Amicus Curiae in Support of Respondent–Cross-Petitioner, June Medical Services v Russo 2. December 27, 2019. https://www.supremecourt.gov/DocketPDF/18/18-1323/126828/20191227104605915_18-1323%20-1460%20bsac%20AAPS--PDFA.pdf. Accessed August 31, 2020.
9. Ky. Rev. Stat. § 311.727(2).
10. Brief for the American College of Obstetricians and Gynecologists, the American Medical Association, the North American Society for Pediatric and Adolescent Gynecology, the American College of Osteopathic Obstetricians and Gynecologists, and the American Academy of Family Physicians Amici Curiae Supporting Petitioners, EMW Women’s Surgical Center v Meier. October 28, 2019. https://www.supremecourt.gov/DocketPDF/19/19-417/120550/20191028184956458_19-417%20ACOG%20et%20al.%20-%20cert.%20amicus%20brief.pdf. Accessed August 31, 2020.
11. EMW Women’s Surgical Center, PSC v Meier, 140 S. Ct. 655 (2019).
12. Codified at 26 U. S. C. §5000A(f )(2); §§4980H(a), (c)(2) requires employers to provide women with “preventive care and screenings” without “any cost sharing requirements.”
13. Little Sisters of the Poor Saints Peter and Paul Home v Pennsylvania, 140 S. Ct. 2367 (2020).
14. Brief of Amici Curiae American College of Obstetricians and Gynecologists, American Nurses Association, American Academy of Nursing, Physicians for Reproductive Health, and Nurses for Sexual and Reproductive Health, In Support of Respondents and Affirmance, Little Sisters of the Poor Saints Peter and Paul Home v Pennsylvania. April 8, 2020. https://www.supremecourt.gov/DocketPDF/19/19-431/141177/20200408152340136_19-431%20and%2019-454%20Amici%20Curiae.pdf. Accessed August 31, 2020.
15. Little Sisters of the Poor Saints Peter and Paul Home v Pennsylvania, 140 S. Ct. 2367, 2400–12 (2020).
16. Brief for the Association of American Medical Colleges (and more than 30 other organizations, including the American Medical Association, and the American College of Obstetricians and Gynecologists) Amici Curiae, In Support of Respondents, Department of Homeland Security v Regents of University of California. October 4, 2019. https://www.supremecourt.gov/DocketPDF/18/18-587/118129/20191004130646281_Brief%20for%20AAMC%20et%20al%20
Supporting%20Respondents.pdf. Accessed August 31, 2020.
17. Department of Homeland Security v Regents of The University of California, 140 S. Ct. 1891 (2020).
18. United States v Texas, 136 S. Ct. 2271 (2016).
19. Title VII of the Civil Rights Act of 1964, 42 U.S.C §2000e–2(a)(1).
20. Bostock v Clayton County, 140 S. Ct. 1731, 1741 (2020).
21. Brief of the American Medical Association, the American College of Physicians, and 14 additional medical, mental health, and health care organizations as Amici Curiae In Support of the Employees, Bostock v Clayton County. July 3, 2019. https://www.supremecourt.gov/DocketPDF/17/17-1618/107177/20190703172548842_Amicus%20Brief.pdf. Accessed August 31, 2020.
22. Our Lady of Guadalupe School v Morrissey-Berru, 140 S. Ct. 2049 (2020).
23. United States Leadership Against HIV/AIDS, Tuberculosis, and Malaria Act (“the Leadership Act”), 22 U. S. C. §7601 et seq.
24. Agency for International Development v Alliance for Open Society, 140 S. Ct. 2082, 2086 (2020).
25. 42 U.S.C. §1342, §18063.
26. Maine Community Health Options v United States, 140 S. Ct. 1308 (2020).
27. Hague Convention on the Civil Aspects of International Child Abduction (Hague Convention or Convention), implemented in the United States by the International Child Abduction Remedies Act, 22 U. S. C. §9001 et seq.
28. Monasky v Taglieri, 140 S. Ct. 719 (2020).
29. Monasky v Taglieri, 140 S. Ct. 719, 723, 729 (2020).
30. Feldman A. Final stat pack for October term 2019 (upated). July 10, 2020. https://www.scotusblog.com/2020/07/final-stat-pack-for-october-term-2019/. Accessed August 31, 2020.
31. Hejmanowski D. Flush heard around the world. Delaware Gazette. May 8, 2020. https://www.delgazette.com/opinion/columns/83610/flush-heard-around-the-world. Accessed August 31, 2020.
32. SCOTUSblog.com. California v Texas. https://www.scotusblog.com/case-files/cases/california-v-texas/. Accessed August 31, 2020.
The apology in medicine—yes, no, or maybe?
This is the third and final article in a series focusing on malpractice, liability, and reform. In the first article, we looked at the background on malpractice and reasons malpractice rates have been so high—including large verdicts and lawsuit-prone physicians. In the second article we considered recent experience and developments in malpractice exposure, who is sued and why. Finally, in this third article, we focus on apologies, apology laws, and liability.
“I’m sorry”
In childhood we are all taught the basic courtesies: “please” and “thank you,” and “I’m sorry,” when harm has occurred. Should we as adult health care providers fear the consequences of apologizing? Apologies are a way for clinicians to express empathy; they also serve as a tool to reduce medical malpractice claims.1

Apologies, ethics, and care
The American Medical Association takes the position that a physician has an ethical duty to disclose a harmful error to a patient.2,3 Indeed this approach has been an impetus for states to enact apology laws, which we discuss below. As pointed out in this 2013 article title, “Dealing with a medical mistake: Should physicians apologize to patients?”,4 the legal benefits of any apology are an issue. It is a controversial area in medicine still today, including in obstetrics and gynecology.
“Ethical codes for both M.D.s and D.O.s suggest providers should display honesty and empathy following adverse events and errors.”1,3,5 In addition, the American Medical Association states, “a physician should at all times deal honestly and openly with patients.”2 Concerns about liability that may result from truthful disclosure should not affect the physician’s honesty (TABLE). Increasingly, the law has sided with that principle through apology laws.

Some patients sue to get answers to the “What happened?” and “Why did it happen?” questions.6 They also sometimes are motivated by a desire to help ensure that the same injury does not happen to others. Silence on the part of the clinician may be seen as a lack of sympathy or remorse and patients may fear that other patients will be harmed.1
The relationship between physician and patient involves vulnerability and requires trust. When an injury occurs, the relationship can be injured as well. Barriers to apology in part reflect “the culture of medicine” as well as the “inherent psychological difficulties in facing one’s mistakes and apologizing for them.” However, apology by the provider may result in “effective resolution of disputes related to medical error.”7
The patient’s perspective is critical to this type of outcome, of course. A study from the United Kingdom noted that one-third of patients who experience a medical error have a desire to receive an apology or explanation. Furthermore, patients need assurance that a plan of action to prevent such a future occurrence is in place.8 Surveys reflect that patients desire, or even expect, the physician to acknowledge an error.9 We will see that there is evidence that some kinds of apologies tend to diminish blame and make the injured patient less likely to pursue litigation.10 For instance, Dahan and colleagues completed a study that highlights the “act of apology,” which can be seen as a “language art.”11 Medical schools have recognized the importance of the apology and now incorporate training focused on error disclosure and provision of apologies into the curriculum.12
Continue to: Legal issues and medical apologies...
Legal issues and medical apologies
From a legal standpoint, traditionally, an apology from a physician to a patient could be used against a physician in a medical liability (malpractice) case as proof of negligence.
Statements of interest. Such out-of-court statements ordinarily would be “hearsay” and excluded from evidence; there is, however, an exception to this hearsay rule that allows “confessions” or “statements against interest” to be admissible against the party making the statement. The theory is that when a statement is harmful to the person making it, the person likely thought that it was true, and the statement should be admissible at trial. We do not generally go around confessing to things that are not true. Following an auto crash, if one driver jumps out of the car saying, “I am so sorry I hit you. I was using my cell phone and did not see you stop,” the statement is against the interest of the driver and could be used in court.
As a matter of general legal principle, the same issue can arise in medical practice. Suppose a physician says, “I am so sorry for your injury. We made a mistake in interpreting the data from the monitors.” That sounds a lot like not just an apology but a statement against interest. Malpractice cases generally are based on the claim that a “doctor failed to do what a reasonable provider in the same specialty would have done in a similar situation.”13 An apology may be little more than general sympathy (“I’m sorry to tell you that we have not cured the infection. Unfortunately, that will mean more time in the hospital.”), but it can include a confession of error (“I’m sorry we got the x-ray backward and removed the wrong kidney.”). In the latter kind of apology, courts traditionally have found a “statement against interest.”
The legal consequence of a statement against interest is that the statement may be admitted in court. Such statements do not automatically establish negligence, but they can be powerful evidence when presented to a jury.
Courts have struggled with medical apologies. General sympathy or feelings of regret or compassion do not generally rise to the level of an admission that the physician did not use reasonable care under the circumstances and ordinarily are not admissible. (For further details, we refer you to the case of Cobbs v. Grant.14 Even if a physician said to the patient that he “blamed himself for [the patient] being back in the hospital for a second time,…the statement signifies compassion, or at most, a feeling of remorse, for plaintiff’s ordeal.”) On the other hand, in cases in which a physician in an apology referred to a “careless” mistake or even a “negligent” mistake, courts have allowed it admitted at trial as a statement against interest. (A 1946 case, Woronka v. Sewall, is an example.15 In that case, the physician said to the patient, “My God, what a mess…she had a very hard delivery, and it was a burning shame to get [an injury] on top of it, and it was because of negligence when they were upstairs.”) Some of these cases come down to the provider’s use of a single word: fault, careless, or negligence.
The ambiguity over the legal place of medical apologies in medicine led attorneys to urge medical providers to avoid statements that might even remotely be taken as statements against interest, including real apologies. The confusion over the admissibility of medical apologies led state legislatures to adopt apology laws. These laws essentially limit what statements against interest may be introduced in professional liability cases when a provider has issued a responsibility or apologized.
Continue to: Apology statutes...
Apology statutes
Massachusetts was the first state to enact an apology law—in 1986.1 As of 2019, a clear majority of states have some form of apology statute. “Apology laws are gaining traction,” was the first sentence in a 2012 review on the subject by Saitta and colleagues.3 Only a few (5 states) have “strong” statutes that have broad protection for statements of fault, error, and negligence, as well as sympathy. The other 33 states have statutes that only protect against statements of sympathy.4,16 FIGURE 1 is a US map showing the apology laws by state.1
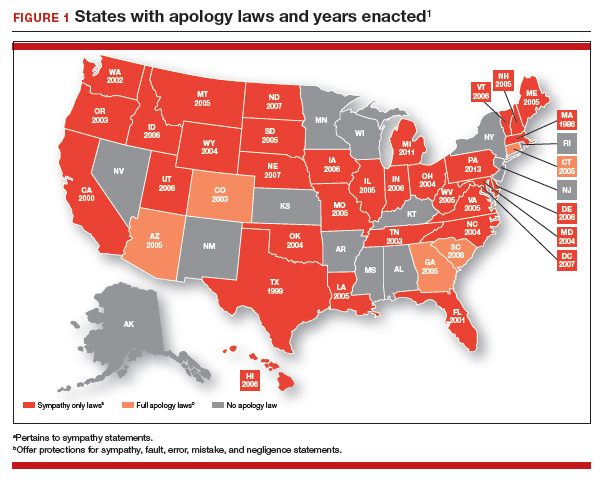
Do apology statutes and apologies reduce liability?
The positive aspects of apology include personal, psychological, and emotional benefits to both the one apologizing and the one receiving the apology. It also may have financial benefits to health care providers.4 The assumption has been, and there has been some evidence for the proposition, that apologies reduce the possibility of malpractice claims. That is one of the reasons that institutions may have formal apology policies. Indeed, there is evidence that apologies reduce financial awards to patients, as manifest in the states of Pennsylvania and Kentucky.4 Apologies appear to reduce patient anger and can open the door to better communication with the provider. There is evidence that some kinds of apologies tend to diminish blame and make the injured patient less likely to pursue litigation.10 The conclusion from these studies might be that honest and open communication serves to decrease the incidence of medical malpractice lawsuit initiation and that honesty is the best policy.
It is important to note the difference, however, between apologies (or institutional apology policies) and apology laws. There is some evidence that apology and institutional apology policies may reduce malpractice claims or losses.17,18 On the other hand, the studies of apology laws have not found that these laws have much impact on malpractice rates. An especially good and thorough study of the effect of apology laws nationwide, using insurance claims data, essentially found little net effect of the apology laws.19,20 One other study could find no evidence that apology statutes reduce defensive medicine (so no reduction in provider concerns over liability).21
It should be noted that most studies on medical apology and its effects on malpractice claims generally have looked at the narrow or limited apology statutes (that do not cover expressions of fault or negligence). Few states have the broader statutes, and it is possible that those broader statutes would be more effective in reducing liability. Removing the disincentives to medical apologies is a good thing, but in and of itself it is probably not a liability game changer.
Continue to: Institutional policy and apology...
Institutional policy and apology
Some institutions have established an “inclusion of apology” strategy for medical errors. These policies appear to have a meaningful effect on reducing medical malpractice costs. These programs commonly include a proactive investigation, disclosure of error, and apologies. Such policies have been studied at the University of Michigan and the Veterans Affairs (VA) Hospital in Lexington, Kentucky. The University of Michigan program resulted in a 60% reduction in compensation costs for medical errors.22 It also cut litigation costs by half.23 The review of the Kentucky VA program also was positive.17 FIGURE 2 illustrates the key features of the Michigan program.24
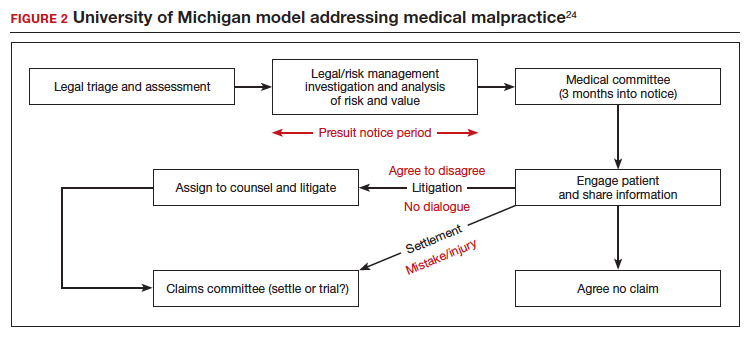
Conclusions: Effective apologies
Our conclusions, first, are that apologies are important from all perspectives: ethical, medical, and legal. On the other hand, all of the attention given in recent years to apology statutes may have been misplaced, at least if they were intended to be malpractice reform.17
Institutional apology and response programs are likely successful because they are thoughtfully put together, generally based on the best understanding of how injured patients respond to apologies and what it takes to be sincere, and communicate that sincerity, in the apology. What is an effective apology?, “The acceptance of responsibility for having caused harm.” It may, for example, mean accepting some financial responsibility for the harm. It is also important that the apology is conveyed in such a way that it includes an element of self-critical expression.25 Although there are many formulations of the elements of an effective apology, one example is, “(1) acknowledging and accepting responsibility for the offense; (2) expressing remorse with forbearance, sincerity, and honesty; (3) explaining the understanding of the offense; and (4) willingness to make reparations.”26
At the other extreme is a medical professional, after a bad event, trying to engage in a half-hearted, awkward, or insincere apology on an ad hoc and poorly planned basis. Worse still, “when victims perceive apologies to be insincere and designed simply to cool them off, they react with more rather than less indignation.”27 Of course, the “forced apology” may be the worst of all. An instance of this was addressed in a New Zealand study in which providers were “forced” to provide a written apology to a couple (Mr. and Mrs. B) and a separate written apology to Baby B when there was failure to discuss vitamin K administration during the antenatal period when it was indicated.28 Rather than emphasizing required apology in such a case, which can seem hollow and disingenuous, emphasis was placed on the apology providing a “positive-physiological” effect for those harmed, and on strategies that “nurture the development of the moral maturity required for authentic apology.”
The great advantage of institutional or practice-wide policies is that they can be developed in the calm of planning, with good foresight and careful consideration. This is much different from having to come up with some approach in the heat of something having gone wrong. Ultimately, however, apologies are not about liability. They are about caring for, respecting, and communicating with those who are harmed. Apologizing is often the right and professional thing to do.
- Afrassiab Z. Why mediation & “sorry” make sense: apology statutes as a catalyst for change in medical malpractice. J Dispute Resolutions. 2019.
- AMA Council on Ethical and Judicial Affairs. AMA code of medical ethics’ opinions on patient safety. Virtual Mentor. 2011;13:626-628.
- Saitta N, Hodge SD. Efficacy of a physician’s words of empathy: an overview of state apology laws. J Am Osteopath Assn. 2012;112:302-306.
- Dealing with a medical mistake: Should physicians apologize to patients? Med Economics. November 10, 2013.
- AOA code of ethics. American Osteopathic Association website. http://www.osteopathic.org/inside-aoa/about /leadershipPages/aos-code-of-ethics.aspx. Accessed January 15, 2020.
- You had me at “I’m sorry”: the impact of physicians’ apologies on medical malpractice litigation. Natl Law Review. November 6, 2018. https://www.natlawreview.com /article/you-had-me-i-m-sorry-impact-physicians-apologiesmedical-malpractice-litigation. Accessed February 6, 2020.
- Robbennolt JK. Apologies and medical error. Clin Orthop Relat Res. 2009;467:376-382.
- Bismark MM. The power of apology. N Z Med J. 2009;122:96-106.
- Witman AB, Park DM, Hardin SB. How do patients want physicians to handle mistakes? A survey of internal medicine patients in an academic setting. Arch Intern Med. 1996;156:2565-2569.
- Lawthers AG, Localio AR, Laird NM, et al. Physicians’ perceptions of the risk of being sued. J Health Polit Policy Law. 1992;17:463-482.
- Dahan S, Ducard D, Caeymaex L. Apology in cases of medical error disclosure: thoughts based on a preliminary study. PLoS One. 2017;12:e0181854.
- Halbach JL, Sullivan LL. Teaching medical students about medical errors and patient safety: evaluation of a required curriculum. Acad Med. 2005;80:600-606.
- Nussbaum L. Trial and error: legislating ADR for medical malpractice reform. 2017. Scholarly Works. https://scholars .law.unlv.edu/facpub/1011. Accessed February 7, 2020.
- Cobbs v. Grant, 8 Cal. 3d 229, 104 Cal. Rptr. 505, 502 P.2d 1 (1972).
- Woronka v. Sewall, 320 Mass. 362, 69 N.E.2d 581 (1946).
- Wei M. Doctors, apologies and the law: an analysis and critique of apology law. J Health Law. 2007;40:107-159.
- Kraman SS, Hamm G. Risk management: extreme honesty may be the best policy. Ann Intern Med. 1999;131:963-967.
- Liebman CB, Hyman CS. Medical error disclosure, mediation skills, and malpractice litigation: a demonstration project in Pennsylvania. 2005. https://perma.cc/7257-99GU. Accessed February 7, 2020.
- McMichael BJ, Van Horn RL, Viscusi WK. “Sorry” is never enough: how state apology laws fail to reduce medical malpractice liability risk. Stanford Law Rev. 2019;71:341-409.
- Ho B, Liu E. What’s an apology worth? Decomposing the effect of apologies on medical malpractice payments using state apology laws. J Empirical Legal Studies. 2011;8:179-199.
- McMichael BJ. The failure of sorry: an empirical evaluation of apology laws, health care, and medical malpractice. Lewis & Clark Law Rev. 2017. https://law.lclark.edu/live/files/27734- lcb224article3mcmichaelpdf. Accessed February 7, 2020.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
- Boothman RC, Blackwell AC, Campbell DA Jr, et al. A better approach to medical malpractice claims? The University of Michigan experience. J Health Life Sci Law. 2009;2:125-159.
- The Michigan model: Medical malpractice and patient safety at Michigan Medicine. University of Michigan website. https:// www.uofmhealth.org/michigan-model-medical-malpracticeand-patient-safety-umhs#summary. Accessed February 7, 2020.
- Mastroianni AC, Mello MM, Sommer S, et al. The flaws in state ‘apology’ and ‘disclosure’ laws dilute their intended impact on malpractice suits. Health Aff (Millwood). 2010;29:1611-1619.
- Davis ER. I’m sorry I’m scared of litigation: evaluating the effectiveness of apology laws. Forum: Tennessee Student Legal J. 2016;3. https://trace.tennessee.edu/forum/vol3/iss1/4/. Accessed February 7, 2020.
- Miller DT. Disrespect and the experience of injustice. Annu Rev Psychol. 2001;52:527-553.
- McLennan S, Walker S, Rich LE. Should health care providers be forced to apologise after things go wrong? J Bioeth Inq. 2014;11:431-435
This is the third and final article in a series focusing on malpractice, liability, and reform. In the first article, we looked at the background on malpractice and reasons malpractice rates have been so high—including large verdicts and lawsuit-prone physicians. In the second article we considered recent experience and developments in malpractice exposure, who is sued and why. Finally, in this third article, we focus on apologies, apology laws, and liability.
“I’m sorry”
In childhood we are all taught the basic courtesies: “please” and “thank you,” and “I’m sorry,” when harm has occurred. Should we as adult health care providers fear the consequences of apologizing? Apologies are a way for clinicians to express empathy; they also serve as a tool to reduce medical malpractice claims.1

Apologies, ethics, and care
The American Medical Association takes the position that a physician has an ethical duty to disclose a harmful error to a patient.2,3 Indeed this approach has been an impetus for states to enact apology laws, which we discuss below. As pointed out in this 2013 article title, “Dealing with a medical mistake: Should physicians apologize to patients?”,4 the legal benefits of any apology are an issue. It is a controversial area in medicine still today, including in obstetrics and gynecology.
“Ethical codes for both M.D.s and D.O.s suggest providers should display honesty and empathy following adverse events and errors.”1,3,5 In addition, the American Medical Association states, “a physician should at all times deal honestly and openly with patients.”2 Concerns about liability that may result from truthful disclosure should not affect the physician’s honesty (TABLE). Increasingly, the law has sided with that principle through apology laws.

Some patients sue to get answers to the “What happened?” and “Why did it happen?” questions.6 They also sometimes are motivated by a desire to help ensure that the same injury does not happen to others. Silence on the part of the clinician may be seen as a lack of sympathy or remorse and patients may fear that other patients will be harmed.1
The relationship between physician and patient involves vulnerability and requires trust. When an injury occurs, the relationship can be injured as well. Barriers to apology in part reflect “the culture of medicine” as well as the “inherent psychological difficulties in facing one’s mistakes and apologizing for them.” However, apology by the provider may result in “effective resolution of disputes related to medical error.”7
The patient’s perspective is critical to this type of outcome, of course. A study from the United Kingdom noted that one-third of patients who experience a medical error have a desire to receive an apology or explanation. Furthermore, patients need assurance that a plan of action to prevent such a future occurrence is in place.8 Surveys reflect that patients desire, or even expect, the physician to acknowledge an error.9 We will see that there is evidence that some kinds of apologies tend to diminish blame and make the injured patient less likely to pursue litigation.10 For instance, Dahan and colleagues completed a study that highlights the “act of apology,” which can be seen as a “language art.”11 Medical schools have recognized the importance of the apology and now incorporate training focused on error disclosure and provision of apologies into the curriculum.12
Continue to: Legal issues and medical apologies...
Legal issues and medical apologies
From a legal standpoint, traditionally, an apology from a physician to a patient could be used against a physician in a medical liability (malpractice) case as proof of negligence.
Statements of interest. Such out-of-court statements ordinarily would be “hearsay” and excluded from evidence; there is, however, an exception to this hearsay rule that allows “confessions” or “statements against interest” to be admissible against the party making the statement. The theory is that when a statement is harmful to the person making it, the person likely thought that it was true, and the statement should be admissible at trial. We do not generally go around confessing to things that are not true. Following an auto crash, if one driver jumps out of the car saying, “I am so sorry I hit you. I was using my cell phone and did not see you stop,” the statement is against the interest of the driver and could be used in court.
As a matter of general legal principle, the same issue can arise in medical practice. Suppose a physician says, “I am so sorry for your injury. We made a mistake in interpreting the data from the monitors.” That sounds a lot like not just an apology but a statement against interest. Malpractice cases generally are based on the claim that a “doctor failed to do what a reasonable provider in the same specialty would have done in a similar situation.”13 An apology may be little more than general sympathy (“I’m sorry to tell you that we have not cured the infection. Unfortunately, that will mean more time in the hospital.”), but it can include a confession of error (“I’m sorry we got the x-ray backward and removed the wrong kidney.”). In the latter kind of apology, courts traditionally have found a “statement against interest.”
The legal consequence of a statement against interest is that the statement may be admitted in court. Such statements do not automatically establish negligence, but they can be powerful evidence when presented to a jury.
Courts have struggled with medical apologies. General sympathy or feelings of regret or compassion do not generally rise to the level of an admission that the physician did not use reasonable care under the circumstances and ordinarily are not admissible. (For further details, we refer you to the case of Cobbs v. Grant.14 Even if a physician said to the patient that he “blamed himself for [the patient] being back in the hospital for a second time,…the statement signifies compassion, or at most, a feeling of remorse, for plaintiff’s ordeal.”) On the other hand, in cases in which a physician in an apology referred to a “careless” mistake or even a “negligent” mistake, courts have allowed it admitted at trial as a statement against interest. (A 1946 case, Woronka v. Sewall, is an example.15 In that case, the physician said to the patient, “My God, what a mess…she had a very hard delivery, and it was a burning shame to get [an injury] on top of it, and it was because of negligence when they were upstairs.”) Some of these cases come down to the provider’s use of a single word: fault, careless, or negligence.
The ambiguity over the legal place of medical apologies in medicine led attorneys to urge medical providers to avoid statements that might even remotely be taken as statements against interest, including real apologies. The confusion over the admissibility of medical apologies led state legislatures to adopt apology laws. These laws essentially limit what statements against interest may be introduced in professional liability cases when a provider has issued a responsibility or apologized.
Continue to: Apology statutes...
Apology statutes
Massachusetts was the first state to enact an apology law—in 1986.1 As of 2019, a clear majority of states have some form of apology statute. “Apology laws are gaining traction,” was the first sentence in a 2012 review on the subject by Saitta and colleagues.3 Only a few (5 states) have “strong” statutes that have broad protection for statements of fault, error, and negligence, as well as sympathy. The other 33 states have statutes that only protect against statements of sympathy.4,16 FIGURE 1 is a US map showing the apology laws by state.1

Do apology statutes and apologies reduce liability?
The positive aspects of apology include personal, psychological, and emotional benefits to both the one apologizing and the one receiving the apology. It also may have financial benefits to health care providers.4 The assumption has been, and there has been some evidence for the proposition, that apologies reduce the possibility of malpractice claims. That is one of the reasons that institutions may have formal apology policies. Indeed, there is evidence that apologies reduce financial awards to patients, as manifest in the states of Pennsylvania and Kentucky.4 Apologies appear to reduce patient anger and can open the door to better communication with the provider. There is evidence that some kinds of apologies tend to diminish blame and make the injured patient less likely to pursue litigation.10 The conclusion from these studies might be that honest and open communication serves to decrease the incidence of medical malpractice lawsuit initiation and that honesty is the best policy.
It is important to note the difference, however, between apologies (or institutional apology policies) and apology laws. There is some evidence that apology and institutional apology policies may reduce malpractice claims or losses.17,18 On the other hand, the studies of apology laws have not found that these laws have much impact on malpractice rates. An especially good and thorough study of the effect of apology laws nationwide, using insurance claims data, essentially found little net effect of the apology laws.19,20 One other study could find no evidence that apology statutes reduce defensive medicine (so no reduction in provider concerns over liability).21
It should be noted that most studies on medical apology and its effects on malpractice claims generally have looked at the narrow or limited apology statutes (that do not cover expressions of fault or negligence). Few states have the broader statutes, and it is possible that those broader statutes would be more effective in reducing liability. Removing the disincentives to medical apologies is a good thing, but in and of itself it is probably not a liability game changer.
Continue to: Institutional policy and apology...
Institutional policy and apology
Some institutions have established an “inclusion of apology” strategy for medical errors. These policies appear to have a meaningful effect on reducing medical malpractice costs. These programs commonly include a proactive investigation, disclosure of error, and apologies. Such policies have been studied at the University of Michigan and the Veterans Affairs (VA) Hospital in Lexington, Kentucky. The University of Michigan program resulted in a 60% reduction in compensation costs for medical errors.22 It also cut litigation costs by half.23 The review of the Kentucky VA program also was positive.17 FIGURE 2 illustrates the key features of the Michigan program.24

Conclusions: Effective apologies
Our conclusions, first, are that apologies are important from all perspectives: ethical, medical, and legal. On the other hand, all of the attention given in recent years to apology statutes may have been misplaced, at least if they were intended to be malpractice reform.17
Institutional apology and response programs are likely successful because they are thoughtfully put together, generally based on the best understanding of how injured patients respond to apologies and what it takes to be sincere, and communicate that sincerity, in the apology. What is an effective apology?, “The acceptance of responsibility for having caused harm.” It may, for example, mean accepting some financial responsibility for the harm. It is also important that the apology is conveyed in such a way that it includes an element of self-critical expression.25 Although there are many formulations of the elements of an effective apology, one example is, “(1) acknowledging and accepting responsibility for the offense; (2) expressing remorse with forbearance, sincerity, and honesty; (3) explaining the understanding of the offense; and (4) willingness to make reparations.”26
At the other extreme is a medical professional, after a bad event, trying to engage in a half-hearted, awkward, or insincere apology on an ad hoc and poorly planned basis. Worse still, “when victims perceive apologies to be insincere and designed simply to cool them off, they react with more rather than less indignation.”27 Of course, the “forced apology” may be the worst of all. An instance of this was addressed in a New Zealand study in which providers were “forced” to provide a written apology to a couple (Mr. and Mrs. B) and a separate written apology to Baby B when there was failure to discuss vitamin K administration during the antenatal period when it was indicated.28 Rather than emphasizing required apology in such a case, which can seem hollow and disingenuous, emphasis was placed on the apology providing a “positive-physiological” effect for those harmed, and on strategies that “nurture the development of the moral maturity required for authentic apology.”
The great advantage of institutional or practice-wide policies is that they can be developed in the calm of planning, with good foresight and careful consideration. This is much different from having to come up with some approach in the heat of something having gone wrong. Ultimately, however, apologies are not about liability. They are about caring for, respecting, and communicating with those who are harmed. Apologizing is often the right and professional thing to do.
This is the third and final article in a series focusing on malpractice, liability, and reform. In the first article, we looked at the background on malpractice and reasons malpractice rates have been so high—including large verdicts and lawsuit-prone physicians. In the second article we considered recent experience and developments in malpractice exposure, who is sued and why. Finally, in this third article, we focus on apologies, apology laws, and liability.
“I’m sorry”
In childhood we are all taught the basic courtesies: “please” and “thank you,” and “I’m sorry,” when harm has occurred. Should we as adult health care providers fear the consequences of apologizing? Apologies are a way for clinicians to express empathy; they also serve as a tool to reduce medical malpractice claims.1

Apologies, ethics, and care
The American Medical Association takes the position that a physician has an ethical duty to disclose a harmful error to a patient.2,3 Indeed this approach has been an impetus for states to enact apology laws, which we discuss below. As pointed out in this 2013 article title, “Dealing with a medical mistake: Should physicians apologize to patients?”,4 the legal benefits of any apology are an issue. It is a controversial area in medicine still today, including in obstetrics and gynecology.
“Ethical codes for both M.D.s and D.O.s suggest providers should display honesty and empathy following adverse events and errors.”1,3,5 In addition, the American Medical Association states, “a physician should at all times deal honestly and openly with patients.”2 Concerns about liability that may result from truthful disclosure should not affect the physician’s honesty (TABLE). Increasingly, the law has sided with that principle through apology laws.

Some patients sue to get answers to the “What happened?” and “Why did it happen?” questions.6 They also sometimes are motivated by a desire to help ensure that the same injury does not happen to others. Silence on the part of the clinician may be seen as a lack of sympathy or remorse and patients may fear that other patients will be harmed.1
The relationship between physician and patient involves vulnerability and requires trust. When an injury occurs, the relationship can be injured as well. Barriers to apology in part reflect “the culture of medicine” as well as the “inherent psychological difficulties in facing one’s mistakes and apologizing for them.” However, apology by the provider may result in “effective resolution of disputes related to medical error.”7
The patient’s perspective is critical to this type of outcome, of course. A study from the United Kingdom noted that one-third of patients who experience a medical error have a desire to receive an apology or explanation. Furthermore, patients need assurance that a plan of action to prevent such a future occurrence is in place.8 Surveys reflect that patients desire, or even expect, the physician to acknowledge an error.9 We will see that there is evidence that some kinds of apologies tend to diminish blame and make the injured patient less likely to pursue litigation.10 For instance, Dahan and colleagues completed a study that highlights the “act of apology,” which can be seen as a “language art.”11 Medical schools have recognized the importance of the apology and now incorporate training focused on error disclosure and provision of apologies into the curriculum.12
Continue to: Legal issues and medical apologies...
Legal issues and medical apologies
From a legal standpoint, traditionally, an apology from a physician to a patient could be used against a physician in a medical liability (malpractice) case as proof of negligence.
Statements of interest. Such out-of-court statements ordinarily would be “hearsay” and excluded from evidence; there is, however, an exception to this hearsay rule that allows “confessions” or “statements against interest” to be admissible against the party making the statement. The theory is that when a statement is harmful to the person making it, the person likely thought that it was true, and the statement should be admissible at trial. We do not generally go around confessing to things that are not true. Following an auto crash, if one driver jumps out of the car saying, “I am so sorry I hit you. I was using my cell phone and did not see you stop,” the statement is against the interest of the driver and could be used in court.
As a matter of general legal principle, the same issue can arise in medical practice. Suppose a physician says, “I am so sorry for your injury. We made a mistake in interpreting the data from the monitors.” That sounds a lot like not just an apology but a statement against interest. Malpractice cases generally are based on the claim that a “doctor failed to do what a reasonable provider in the same specialty would have done in a similar situation.”13 An apology may be little more than general sympathy (“I’m sorry to tell you that we have not cured the infection. Unfortunately, that will mean more time in the hospital.”), but it can include a confession of error (“I’m sorry we got the x-ray backward and removed the wrong kidney.”). In the latter kind of apology, courts traditionally have found a “statement against interest.”
The legal consequence of a statement against interest is that the statement may be admitted in court. Such statements do not automatically establish negligence, but they can be powerful evidence when presented to a jury.
Courts have struggled with medical apologies. General sympathy or feelings of regret or compassion do not generally rise to the level of an admission that the physician did not use reasonable care under the circumstances and ordinarily are not admissible. (For further details, we refer you to the case of Cobbs v. Grant.14 Even if a physician said to the patient that he “blamed himself for [the patient] being back in the hospital for a second time,…the statement signifies compassion, or at most, a feeling of remorse, for plaintiff’s ordeal.”) On the other hand, in cases in which a physician in an apology referred to a “careless” mistake or even a “negligent” mistake, courts have allowed it admitted at trial as a statement against interest. (A 1946 case, Woronka v. Sewall, is an example.15 In that case, the physician said to the patient, “My God, what a mess…she had a very hard delivery, and it was a burning shame to get [an injury] on top of it, and it was because of negligence when they were upstairs.”) Some of these cases come down to the provider’s use of a single word: fault, careless, or negligence.
The ambiguity over the legal place of medical apologies in medicine led attorneys to urge medical providers to avoid statements that might even remotely be taken as statements against interest, including real apologies. The confusion over the admissibility of medical apologies led state legislatures to adopt apology laws. These laws essentially limit what statements against interest may be introduced in professional liability cases when a provider has issued a responsibility or apologized.
Continue to: Apology statutes...
Apology statutes
Massachusetts was the first state to enact an apology law—in 1986.1 As of 2019, a clear majority of states have some form of apology statute. “Apology laws are gaining traction,” was the first sentence in a 2012 review on the subject by Saitta and colleagues.3 Only a few (5 states) have “strong” statutes that have broad protection for statements of fault, error, and negligence, as well as sympathy. The other 33 states have statutes that only protect against statements of sympathy.4,16 FIGURE 1 is a US map showing the apology laws by state.1

Do apology statutes and apologies reduce liability?
The positive aspects of apology include personal, psychological, and emotional benefits to both the one apologizing and the one receiving the apology. It also may have financial benefits to health care providers.4 The assumption has been, and there has been some evidence for the proposition, that apologies reduce the possibility of malpractice claims. That is one of the reasons that institutions may have formal apology policies. Indeed, there is evidence that apologies reduce financial awards to patients, as manifest in the states of Pennsylvania and Kentucky.4 Apologies appear to reduce patient anger and can open the door to better communication with the provider. There is evidence that some kinds of apologies tend to diminish blame and make the injured patient less likely to pursue litigation.10 The conclusion from these studies might be that honest and open communication serves to decrease the incidence of medical malpractice lawsuit initiation and that honesty is the best policy.
It is important to note the difference, however, between apologies (or institutional apology policies) and apology laws. There is some evidence that apology and institutional apology policies may reduce malpractice claims or losses.17,18 On the other hand, the studies of apology laws have not found that these laws have much impact on malpractice rates. An especially good and thorough study of the effect of apology laws nationwide, using insurance claims data, essentially found little net effect of the apology laws.19,20 One other study could find no evidence that apology statutes reduce defensive medicine (so no reduction in provider concerns over liability).21
It should be noted that most studies on medical apology and its effects on malpractice claims generally have looked at the narrow or limited apology statutes (that do not cover expressions of fault or negligence). Few states have the broader statutes, and it is possible that those broader statutes would be more effective in reducing liability. Removing the disincentives to medical apologies is a good thing, but in and of itself it is probably not a liability game changer.
Continue to: Institutional policy and apology...
Institutional policy and apology
Some institutions have established an “inclusion of apology” strategy for medical errors. These policies appear to have a meaningful effect on reducing medical malpractice costs. These programs commonly include a proactive investigation, disclosure of error, and apologies. Such policies have been studied at the University of Michigan and the Veterans Affairs (VA) Hospital in Lexington, Kentucky. The University of Michigan program resulted in a 60% reduction in compensation costs for medical errors.22 It also cut litigation costs by half.23 The review of the Kentucky VA program also was positive.17 FIGURE 2 illustrates the key features of the Michigan program.24

Conclusions: Effective apologies
Our conclusions, first, are that apologies are important from all perspectives: ethical, medical, and legal. On the other hand, all of the attention given in recent years to apology statutes may have been misplaced, at least if they were intended to be malpractice reform.17
Institutional apology and response programs are likely successful because they are thoughtfully put together, generally based on the best understanding of how injured patients respond to apologies and what it takes to be sincere, and communicate that sincerity, in the apology. What is an effective apology?, “The acceptance of responsibility for having caused harm.” It may, for example, mean accepting some financial responsibility for the harm. It is also important that the apology is conveyed in such a way that it includes an element of self-critical expression.25 Although there are many formulations of the elements of an effective apology, one example is, “(1) acknowledging and accepting responsibility for the offense; (2) expressing remorse with forbearance, sincerity, and honesty; (3) explaining the understanding of the offense; and (4) willingness to make reparations.”26
At the other extreme is a medical professional, after a bad event, trying to engage in a half-hearted, awkward, or insincere apology on an ad hoc and poorly planned basis. Worse still, “when victims perceive apologies to be insincere and designed simply to cool them off, they react with more rather than less indignation.”27 Of course, the “forced apology” may be the worst of all. An instance of this was addressed in a New Zealand study in which providers were “forced” to provide a written apology to a couple (Mr. and Mrs. B) and a separate written apology to Baby B when there was failure to discuss vitamin K administration during the antenatal period when it was indicated.28 Rather than emphasizing required apology in such a case, which can seem hollow and disingenuous, emphasis was placed on the apology providing a “positive-physiological” effect for those harmed, and on strategies that “nurture the development of the moral maturity required for authentic apology.”
The great advantage of institutional or practice-wide policies is that they can be developed in the calm of planning, with good foresight and careful consideration. This is much different from having to come up with some approach in the heat of something having gone wrong. Ultimately, however, apologies are not about liability. They are about caring for, respecting, and communicating with those who are harmed. Apologizing is often the right and professional thing to do.
- Afrassiab Z. Why mediation & “sorry” make sense: apology statutes as a catalyst for change in medical malpractice. J Dispute Resolutions. 2019.
- AMA Council on Ethical and Judicial Affairs. AMA code of medical ethics’ opinions on patient safety. Virtual Mentor. 2011;13:626-628.
- Saitta N, Hodge SD. Efficacy of a physician’s words of empathy: an overview of state apology laws. J Am Osteopath Assn. 2012;112:302-306.
- Dealing with a medical mistake: Should physicians apologize to patients? Med Economics. November 10, 2013.
- AOA code of ethics. American Osteopathic Association website. http://www.osteopathic.org/inside-aoa/about /leadershipPages/aos-code-of-ethics.aspx. Accessed January 15, 2020.
- You had me at “I’m sorry”: the impact of physicians’ apologies on medical malpractice litigation. Natl Law Review. November 6, 2018. https://www.natlawreview.com /article/you-had-me-i-m-sorry-impact-physicians-apologiesmedical-malpractice-litigation. Accessed February 6, 2020.
- Robbennolt JK. Apologies and medical error. Clin Orthop Relat Res. 2009;467:376-382.
- Bismark MM. The power of apology. N Z Med J. 2009;122:96-106.
- Witman AB, Park DM, Hardin SB. How do patients want physicians to handle mistakes? A survey of internal medicine patients in an academic setting. Arch Intern Med. 1996;156:2565-2569.
- Lawthers AG, Localio AR, Laird NM, et al. Physicians’ perceptions of the risk of being sued. J Health Polit Policy Law. 1992;17:463-482.
- Dahan S, Ducard D, Caeymaex L. Apology in cases of medical error disclosure: thoughts based on a preliminary study. PLoS One. 2017;12:e0181854.
- Halbach JL, Sullivan LL. Teaching medical students about medical errors and patient safety: evaluation of a required curriculum. Acad Med. 2005;80:600-606.
- Nussbaum L. Trial and error: legislating ADR for medical malpractice reform. 2017. Scholarly Works. https://scholars .law.unlv.edu/facpub/1011. Accessed February 7, 2020.
- Cobbs v. Grant, 8 Cal. 3d 229, 104 Cal. Rptr. 505, 502 P.2d 1 (1972).
- Woronka v. Sewall, 320 Mass. 362, 69 N.E.2d 581 (1946).
- Wei M. Doctors, apologies and the law: an analysis and critique of apology law. J Health Law. 2007;40:107-159.
- Kraman SS, Hamm G. Risk management: extreme honesty may be the best policy. Ann Intern Med. 1999;131:963-967.
- Liebman CB, Hyman CS. Medical error disclosure, mediation skills, and malpractice litigation: a demonstration project in Pennsylvania. 2005. https://perma.cc/7257-99GU. Accessed February 7, 2020.
- McMichael BJ, Van Horn RL, Viscusi WK. “Sorry” is never enough: how state apology laws fail to reduce medical malpractice liability risk. Stanford Law Rev. 2019;71:341-409.
- Ho B, Liu E. What’s an apology worth? Decomposing the effect of apologies on medical malpractice payments using state apology laws. J Empirical Legal Studies. 2011;8:179-199.
- McMichael BJ. The failure of sorry: an empirical evaluation of apology laws, health care, and medical malpractice. Lewis & Clark Law Rev. 2017. https://law.lclark.edu/live/files/27734- lcb224article3mcmichaelpdf. Accessed February 7, 2020.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
- Boothman RC, Blackwell AC, Campbell DA Jr, et al. A better approach to medical malpractice claims? The University of Michigan experience. J Health Life Sci Law. 2009;2:125-159.
- The Michigan model: Medical malpractice and patient safety at Michigan Medicine. University of Michigan website. https:// www.uofmhealth.org/michigan-model-medical-malpracticeand-patient-safety-umhs#summary. Accessed February 7, 2020.
- Mastroianni AC, Mello MM, Sommer S, et al. The flaws in state ‘apology’ and ‘disclosure’ laws dilute their intended impact on malpractice suits. Health Aff (Millwood). 2010;29:1611-1619.
- Davis ER. I’m sorry I’m scared of litigation: evaluating the effectiveness of apology laws. Forum: Tennessee Student Legal J. 2016;3. https://trace.tennessee.edu/forum/vol3/iss1/4/. Accessed February 7, 2020.
- Miller DT. Disrespect and the experience of injustice. Annu Rev Psychol. 2001;52:527-553.
- McLennan S, Walker S, Rich LE. Should health care providers be forced to apologise after things go wrong? J Bioeth Inq. 2014;11:431-435
- Afrassiab Z. Why mediation & “sorry” make sense: apology statutes as a catalyst for change in medical malpractice. J Dispute Resolutions. 2019.
- AMA Council on Ethical and Judicial Affairs. AMA code of medical ethics’ opinions on patient safety. Virtual Mentor. 2011;13:626-628.
- Saitta N, Hodge SD. Efficacy of a physician’s words of empathy: an overview of state apology laws. J Am Osteopath Assn. 2012;112:302-306.
- Dealing with a medical mistake: Should physicians apologize to patients? Med Economics. November 10, 2013.
- AOA code of ethics. American Osteopathic Association website. http://www.osteopathic.org/inside-aoa/about /leadershipPages/aos-code-of-ethics.aspx. Accessed January 15, 2020.
- You had me at “I’m sorry”: the impact of physicians’ apologies on medical malpractice litigation. Natl Law Review. November 6, 2018. https://www.natlawreview.com /article/you-had-me-i-m-sorry-impact-physicians-apologiesmedical-malpractice-litigation. Accessed February 6, 2020.
- Robbennolt JK. Apologies and medical error. Clin Orthop Relat Res. 2009;467:376-382.
- Bismark MM. The power of apology. N Z Med J. 2009;122:96-106.
- Witman AB, Park DM, Hardin SB. How do patients want physicians to handle mistakes? A survey of internal medicine patients in an academic setting. Arch Intern Med. 1996;156:2565-2569.
- Lawthers AG, Localio AR, Laird NM, et al. Physicians’ perceptions of the risk of being sued. J Health Polit Policy Law. 1992;17:463-482.
- Dahan S, Ducard D, Caeymaex L. Apology in cases of medical error disclosure: thoughts based on a preliminary study. PLoS One. 2017;12:e0181854.
- Halbach JL, Sullivan LL. Teaching medical students about medical errors and patient safety: evaluation of a required curriculum. Acad Med. 2005;80:600-606.
- Nussbaum L. Trial and error: legislating ADR for medical malpractice reform. 2017. Scholarly Works. https://scholars .law.unlv.edu/facpub/1011. Accessed February 7, 2020.
- Cobbs v. Grant, 8 Cal. 3d 229, 104 Cal. Rptr. 505, 502 P.2d 1 (1972).
- Woronka v. Sewall, 320 Mass. 362, 69 N.E.2d 581 (1946).
- Wei M. Doctors, apologies and the law: an analysis and critique of apology law. J Health Law. 2007;40:107-159.
- Kraman SS, Hamm G. Risk management: extreme honesty may be the best policy. Ann Intern Med. 1999;131:963-967.
- Liebman CB, Hyman CS. Medical error disclosure, mediation skills, and malpractice litigation: a demonstration project in Pennsylvania. 2005. https://perma.cc/7257-99GU. Accessed February 7, 2020.
- McMichael BJ, Van Horn RL, Viscusi WK. “Sorry” is never enough: how state apology laws fail to reduce medical malpractice liability risk. Stanford Law Rev. 2019;71:341-409.
- Ho B, Liu E. What’s an apology worth? Decomposing the effect of apologies on medical malpractice payments using state apology laws. J Empirical Legal Studies. 2011;8:179-199.
- McMichael BJ. The failure of sorry: an empirical evaluation of apology laws, health care, and medical malpractice. Lewis & Clark Law Rev. 2017. https://law.lclark.edu/live/files/27734- lcb224article3mcmichaelpdf. Accessed February 7, 2020.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
- Boothman RC, Blackwell AC, Campbell DA Jr, et al. A better approach to medical malpractice claims? The University of Michigan experience. J Health Life Sci Law. 2009;2:125-159.
- The Michigan model: Medical malpractice and patient safety at Michigan Medicine. University of Michigan website. https:// www.uofmhealth.org/michigan-model-medical-malpracticeand-patient-safety-umhs#summary. Accessed February 7, 2020.
- Mastroianni AC, Mello MM, Sommer S, et al. The flaws in state ‘apology’ and ‘disclosure’ laws dilute their intended impact on malpractice suits. Health Aff (Millwood). 2010;29:1611-1619.
- Davis ER. I’m sorry I’m scared of litigation: evaluating the effectiveness of apology laws. Forum: Tennessee Student Legal J. 2016;3. https://trace.tennessee.edu/forum/vol3/iss1/4/. Accessed February 7, 2020.
- Miller DT. Disrespect and the experience of injustice. Annu Rev Psychol. 2001;52:527-553.
- McLennan S, Walker S, Rich LE. Should health care providers be forced to apologise after things go wrong? J Bioeth Inq. 2014;11:431-435
ObGyn malpractice liability risk: 2020 developments and probabilities
In this second in a series of 3 articles discussing medical malpractice and the ObGyn we look at the reasons for malpractice claims and liability, what happens to malpractice claims, and the direction and future of medical malpractice. The first article dealt with 2 sources of major malpractice damages: the “big verdict” and physicians with multiple malpractice paid claims. Next month we look at the place of apology in medicine, in cases in which error, including negligence, may have caused a patient injury.
CASE 1 Long-term brachial plexus injury
Right upper extremity injury occurs in the neonate at delivery with sequela of long-term brachial plexus injury (which is diagnosed around 6 months of age). Physical therapy and orthopedic assessment are rendered. Despite continued treatment, discrepancy in arm lengths (ie, affected side arm is noticeably shorter than opposite side) remains. The child cannot play basketball with his older brother and is the victim of ridicule, the plaintiff’s attorney emphasizes. He is unable to properly pronate or supinate the affected arm.
The defendant ObGyn maintains that there was “no shoulder dystocia [at delivery] and the shoulder did not get obstructed in the pelvis; shoulder was delivered 15 seconds after delivery of the head.” The nursing staff testifies that if shoulder dystocia had been the problem they would have launched upon a series of procedures to address such, in accord with the delivering obstetrician. The defense expert witness testifies that a brachial plexus injury can happen without shoulder dystocia.
A defense verdict is rendered by the Florida jury.1
CASE 2 Shoulder dystocia
During delivery, the obstetrician notes a shoulder dystocia (“turtle sign”). After initial attempts to release the shoulder were unsuccessful, the physician applies traction several times to the head of the child, and the baby is delivered. There is permanent injury to the right brachial plexus. The defendant ObGyn says that traction was necessary to dislodge the shoulder, and that the injury was the result of the forces of labor (not the traction). The expert witness for the plaintiff testifies that the medical standard of care did not permit traction under these circumstances, and that the traction was the likely cause of the injury.
The Virginia jury awards $2.32 million in damages.2
Note: The above vignettes are drawn from actual cases but are only outlines of those cases and are not complete descriptions of the claims in the cases. Because the information comes from informal sources, not formal court records, the facts may be inaccurate and incomplete. They should be viewed as illustrations only.

The trend in malpractice
It has been clear for many years that medical malpractice claims are not randomly or evenly distributed among physicians. Notably, the variation among specialties has, and continues to be, substantial (FIGURE 1).3 Recent data suggest that, although paid claims per “1,000 physician-years” averages 14 paid claims per 1,000 physician years, it ranges from 4 or 5 in 1,000 (psychiatry and pediatrics) to 53 and 49 claims per 1,000 (neurology and plastic surgery, respectively). Obstetrics and gynecology has the fourth highest rate at 42.5 paid claims per 1,000 physician years.4 (These data are for the years 1992–2014.)
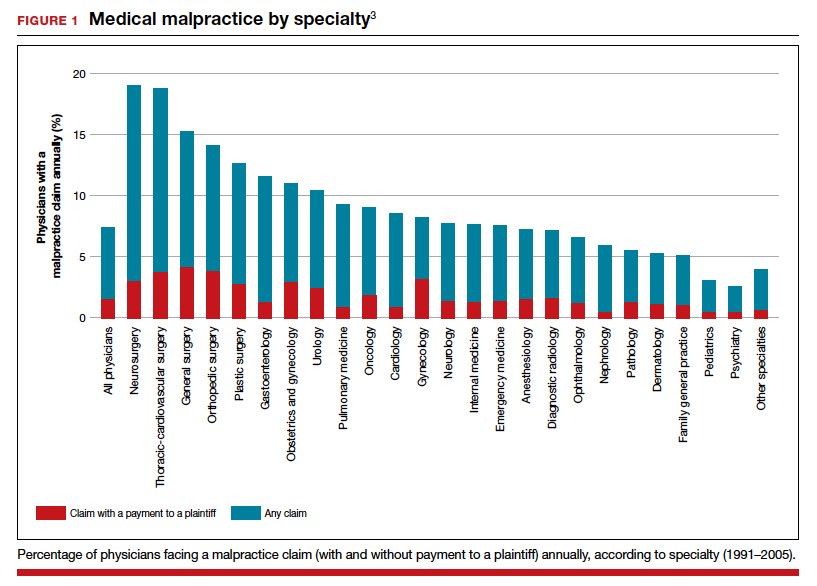
Continue to: The number of ObGyn paid malpractice claims has decreased over time...
The number of ObGyn paid malpractice claims has decreased over time. Although large verdicts and physicians with multiple paid malpractice claims receive a good deal of attention (as we noted in part 1 of our series), in fact, paid medical malpractice claims have trended downward in recent decades.5 When the data above are disaggregated by 5-year periods, for example, in obstetrics and gynecology, there has been a consistent reduction in paid malpractice claims from 1992 to 2014. Paid claims went from 58 per 1,000 physician-years in 1992–1996 to 25 per 1,000 in 2009–2014 (FIGURE 2).4,6 In short, the rate dropped by half over approximately 20 years.4
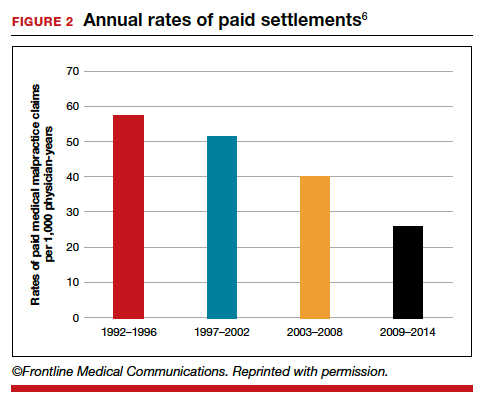
It is reasonable to expect that such a decline in the cost of malpractice insurance premiums would follow. Robert L. Barbieri, MD, who practices in Boston, Massachusetts, in his excellent recent editorial in OBG M
Why have malpractice payouts declined overall?
Have medical errors declined?
It would be wonderful if the reduction in malpractice claims represented a significant decrease in medical errors. Attention to medical errors was driven by the first widely noticed study of medical error deaths. The Institute of Medicine (IOM) study in 2000, put the number of deaths annually at 44,000 to 98,000.8 There have been many efforts to reduce such errors, and it is possible that those efforts have indeed reduced errors somewhat.4 Barbieri provided a helpful digest of many of the error-reduction suggestions for ObGyn practice (TABLE 1).6 But the number of medical errors remains high. More recent studies have suggested that the IOM’s reported number of injuries may have been low.9 In 2013, one study suggested that 210,000 deaths annually were “associated with preventable harm” in hospitals. Because of how the data were gathered the authors estimated that the actual number of preventable deaths was closer to 400,000 annually. Serious harm to patients was estimated at 10 to 20 times the IOM rate.9
Therefore, a dramatic reduction in preventable medical errors does not appear to explain the reduction in malpractice claims. Some portion of it may be explained by malpractice reforms—see "The medical reform factor" section below.
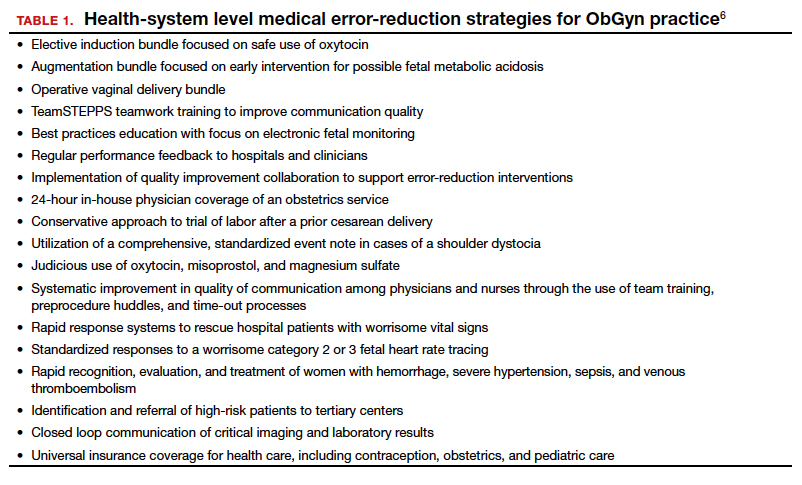
The collective accountability factor
The way malpractice claims are paid (FIGURE 4),10 reported, and handled may explain some of the apparent reduction in overall paid claims. Perhaps the advent of “collective accountability,” in which patient care is rendered by teams and responsibility accepted at a team level, can alleviate a significant amount of individual physician medical malpractice claims.11 This “enterprise liability” may shift the burden of medical error from physicians to health care organizations.12 Collective accountability may, therefore, focus on institutional responsibility rather than individual physician negligence.11,13 Institutions frequently hire multiple specialists and cover their medical malpractice costs as well as stand to be named in suits.
Continue to: The institutional involvement in malpractice cases also may affect...
The institutional involvement in malpractice cases also may affect apparent malpractice rates in another way. The National Practitioner Data Bank, which is the source of information for many malpractice studies, only requires reporting about individual physicians, not institutions.14 If, therefore, claims are settled on behalf of an institution, without implicating the physician, the number of physician malpractice cases may appear to decline without any real change in malpractice rates.14 In addition, institutions have taken the lead in informal resolution of injuries that occur in the institution, and these programs may reduce the direct malpractice claims against physicians. (These “disclosure, apology, and offer,” and similar programs, are discussed in the upcoming third part of this series.)
The medical reform factor
As noted, annual rates paid for medical malpractice in our specialty are trending downward. Many commentators look to malpractice reforms as the reason for the drop in malpractice rates.15-17 Because medical malpractice is essentially a matter of state law, the medical malpractice reform has occurred primarily at the state level.18 There have been many different reforms tried—limits on expert witnesses, review panels, and a variety of procedural limitations.19 Perhaps the most effective reform has been caps being placed on noneconomic damages (generally pain and suffering).20 These caps vary by state (FIGURE 5)21,22 and, of course, affect the “big verdict” cases. (As we saw in the second case scenario above, Virginia is an example of a state with a cap on malpractice awards.) They also have the secondary effect of reducing the number of malpractice cases. They make malpractice cases less attractive to some attorneys because they reduce the opportunity of large contingency fees from large verdicts. (Virtually all medical malpractice cases in the United States are tried on a contingency-fee basis, meaning that the plaintiff does not pay the attorney handling the case but rather the attorney takes a percentage of any recovery—typically in the neighborhood of 35%.) The reform process continues, although, presently, there is less pressure to act on the malpractice crisis.
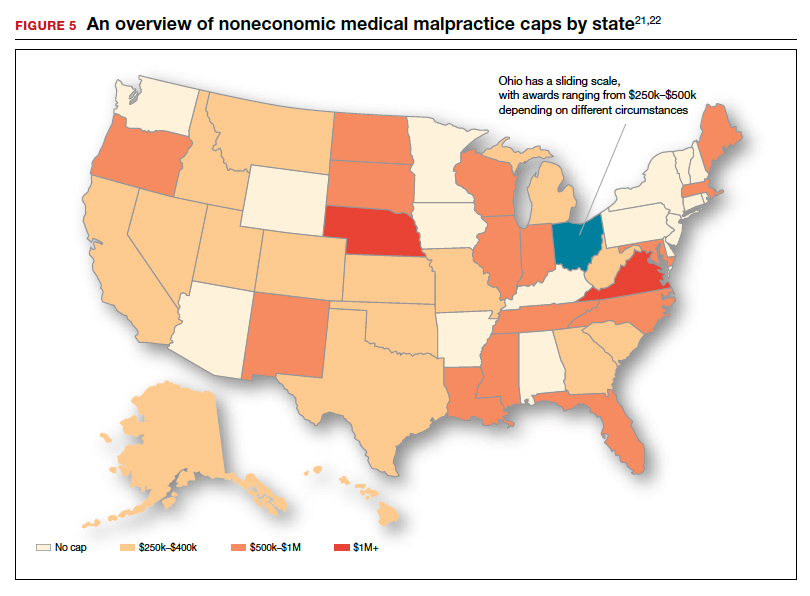
Medical malpractice cases are emotional and costly
Another reason for the relatively low rate of paid claims is that medical malpractice cases are difficult, emotionally challenging, time consuming, and expensive to pursue.23 They typically drag on for years, require extensive and expensive expert consultants as well as witnesses, and face stiff defense (compared with many other torts). The settlement of medical malpractice cases, for example, is less likely than other kinds of personal injury cases.
The contingency-fee basis does mean that injured patients do not have to pay attorney fees up front; however, plaintiffs may have to pay substantial costs along the way. The other side of this coin is that lawyers can be reluctant to take malpractice cases in which the damages are likely to be small, or where the legal uncertainty reduces the odds of achieving any damages. Thus, many potential malpractice cases are never filed.
A word of caution
The news of a reduction in malpractice paid claims may not be permanent. The numbers can conceivably be cyclical, and political reforms achieved can be changed. In addition, new technology will likely bring new kinds of malpractice claims. That appears to be the case, for example, with electronic health records (EHRs). One insurer reports that EHR malpractice claims have increased over the last 8 years.24 The most common injury in these claims was death (25%), as well as a magnitude of less serious injuries. EHR-related claims result from system failures, copy-paste inaccuracies, faulty drop-down menu use, and uncorrected “auto-populated” fields. Obstetrics is tied for fifth on the list of 14 specialties with claims related to EHRs, and gynecology is tied for eighth place.24
Continue to: A federal court ruled that a hospital that changed from...
A federal court ruled that a hospital that changed from paper records to EHRs for test results had a duty to “‘implement a reasonable procedure during the transition phase’ to ensure the timely delivery of test results” to health care providers.25 We will address this in a future “What’s the Verdict?”.
Rates of harm, malpractice cases, and the disposition of cases
There are many surprises when looking at medical malpractice claims data generally. The first surprise is how few claims are filed relative to the number of error-related injuries. Given the estimate of 210,000 to 400,000 deaths “associated with preventable harm” in hospitals, plus 10 to 20 times that number of serious injuries, it would be reasonable to expect claims of many hundreds of thousands per year. Compare the probability of a malpractice claim from an error-related injury, for example, with the probability of other personal injuries—eg, of traffic deaths associated with preventable harm.
The second key observation is how many of the claims filed are not successful—even when there was evidence in the record of errors associated with the injury. Studies slice the data in different ways but collectively suggest that only a small proportion of malpractice claims filed (a claim is generally regarded as some written demand for compensation for injuries) result in payments, either through settlement or by trial. A 2006 study by Studdert and colleagues determined that 63% of formal malpractice claims filed did involve injuries resulting from errors.26 The study found that in 16% of the claims (not injuries) there was no payment even though there was error. In 10% of the claims there was payment, even in the absence of error.
Overall, in this study, 56% of the claims received some compensation.26 That is higher than a more recent study by Jena and others, which found only 22% of claims resulted in compensation.3
How malpractice claims are decided is also interesting. Jena and colleagues found that only 55% of claims resulted in litigation.27 Presumably, the other 45% may have resulted in the plaintiff dropping the case, or in some form of settlement. Of the claims that were litigated, 54% were dismissed by the court, and another 35% were settled before a trial verdict. The cases that went to trial (about 10%), overwhelmingly (80%) resulted in verdicts for the defense.3,27 A different study found that only 9% of cases went to trial, and 87% were a defense verdict.28 The high level of defense verdicts may suggest that malpractice defense lawyers, and their client physicians, do a good job of assessing cases they are likely to lose, and settling them before trial.
ObGyns generally have larger numbers of claims and among the largest payment amounts when there is payment. Fewer of their cases are dismissed by the courts, so more go to trial. At trial, however, ObGyns prevail at a remarkably high rate.27 As for the probability of payment of a malpractice claim for ObGyns, one study suggested that there is approximately a 16% annual probability of a claim being filed, but only a 3% annual probability of a payment being made (suggesting about a 20% probability of payment per claim).3
Continue to: The purposes and effects of the medical malpractice system...
The purposes and effects of the medical malpractice system
The essential goals of tort law (including medical malpractice) include compensation for those who are injured and deterrence of future injuries (TABLE 2). What are the overall effects to the medical malpractice system? Unfortunately, the answer is that the law delivers disappointing results at best. It has a fairly high error rate. Many people who deserve some compensation for their injuries never seek compensation, and many deserving injured patients fail in efforts to receive compensation. At the same time, a few of the injured receive huge recoveries (even windfalls), and at least a small fraction receive compensation when there was no medical error. In addition to the high error rate, the system is inefficient and very expensive. Both defendants (through their insurance carriers) and plaintiffs spend a lot of money, years of time, and untold emotional pain dealing with these cases. The system also exacts high emotional and personal costs on plaintiffs and defendants.

Malpractice reform has not really addressed these issues—it has generally been focused on ways to reduce the cost of malpractice insurance. The most effective reform in reducing rates—caps—has had the effect of compensating the most seriously injured as though they were more modestly injured, and dissuading attorneys from taking the cases of those less seriously injured.
The medical and legal professions exist to help patients (the public). It does not seem that we have arrived at a system that does that very fairly or efficiently when a patient is injured because of preventable medical error.
The two vignettes described at the beginning, with similar injuries (shoulder dystocia), had disparate outcomes. In one there was a defense verdict and in the other a verdict for the plaintiffs of more than $2 million. The differences explain a number of important elements related to malpractice claims. (We have only very abbreviated and incomplete descriptions of the cases, so this discussion necessarily assumes facts and jumps to conclusions that may not be entirely consistent with the actual cases.)
These vignettes are unusual in that they went to trial. As we have noted, only a small percentage of malpractice cases are tried. And the verdict for the plaintiff-patient (in the second case) is unusual among those cases that go to trial, where plaintiffs seldom prevail.
From the facts we have, one significant difference in the 2 cases is that the plaintiff’s expert witness specifically testified in the second case that the “medical standard of care did not permit traction under these circumstances.” That is an essential element of a successful plaintiff’s malpractice case. In this case, the expert could also draw a connection between that breach of standard of care and harm to the child. In the case without liability, the nursing staff was able to testify that there was no shoulder dystocia because if there had been such an injury, they would have immediately launched into special action, which did not happen. By contrast, in the liability case, there seemed to be critical gaps in the medical record.
It is also important to remember that these cases were tried in different states, with different laws. The juries and judges in the 2 cases were different. Finally, the quality of the attorneys representing the plaintiffs and defendants were different. We mention these factors to point out that medical malpractice is not an exact science. It depends on many human elements that make the outcome of cases somewhat unpredictable. This unpredictability is one reason why parties and attorneys like to settle cases.
Watch for the third and final article in this series next month, as we are going to look at “apology in medicine and a proactive response” to communication regarding a complication.
- Shoulder dystocia—Florida defense verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(1):18.
- Shoulder dystocia improperly managed--$2.320 million Virginia verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(2):13.
- Jena AB, Seabury S, Lakdawalla D, et al. Malpractice risk according to physician specialty. N Engl J Med. 2011;365:629-636.
- Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177:710-718.
- Lowes R. Malpractice premiums trail inflation for some physicians. Medscape. December 16, 2016. https://www.medscape.com/viewarticle/873422. Accessed January 10, 2020.
- Barbieri RL. Good news for ObGyns: medical liability claims resulting in payment are decreasing! OBG Manag. 2019;31:10-13.
- Guardado JR. Medical professional liability insurance premiums: an overview of the market from 2008 to 2017. AMA Policy Research Perspectives, 2018. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/government/advocacy/policy-research-perspective-liability-insurance-premiums.pdf. Accessed January 10, 2020.
- Institute of Medicine Committee on Quality Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
- James JT. A new, evidence-based estimate of patient harms associated with hospital care. J Patient Saf. 2013;9:122-128. https://journals.lww.com/journalpatientsafety/Fulltext/
2013/09000/A_New,_Evidence_based_Estimate_of_Patient_
Harms.2.aspx. Accessed January 10, 2020. - Public Citizen Congress Watch. The great medical malpractice hoax: NPDB data continue to show medical liability system produces rational outcomes. January 2007. https://www.citizen.org/wp-content/uploads/npdb_report_
final.pdf. Accessed January 23, 2020. - Bell SK, Delbanco T, Anderson-Shaw L, et al. Accountability for medical error: moving beyond blame to advocacy. Chest. 2011;140:519-526.
- Ramanathan T. Legal mechanisms supporting accountable care principles. Am J Public Health. 2014;104:2048-2051.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
- National Practitioner Data Bank web site. What you must report to the NPDB. https://www.npdb.hrsa.gov/hcorg/whatYouMustReport
ToTheDataBank.jsp. Accessed January 10, 2020. - Bovbjerg RR. Malpractice crisis and reform. Clin Perinatol. 2005;32:203-233, viii-ix.
- Viscusi WK. Medical malpractice reform: what works and what doesn't. Denver Law Rev. 2019;96:775-791. https://static1.squarespace.com/static/5cb79f7efd6793296c0eb738 /t/5d5f4ffabd6c5400011a12f6/1566527483118/Vol96_Issue4_Viscusi_
FINAL.pdf. Accessed January 10, 2020. - National Conference of State Legislatures. Medical malpractice reform. Health Cost Containment and Efficiencies: NCSL Briefs for State Legislators. 2011;(16). http://www.ncsl.org/research/health/medical-malpractice-reform-health-cost-brief.aspx. Accessed January 10, 2020.
- Kass JS, Rose RV. Medical malpractice reform: historical approaches, alternative models, and communication and resolution programs. AMA J Ethics. 2016;18:299-310.
- Boehm G. Debunking medical malpractice myths: unraveling the false premises behind "tort reform". Yale J Health Policy Law Ethics. 2005;5:357-369.
- Hellinger FJ, Encinosa WE. The impact of state laws limiting malpractice damage awards on health care expenditures. Am J Public Health. 2006;96:1375-1381.
- Perry G. Medical malpractice caps by state [infographic]. January 3, 2013. https://www.business2community.com/infographics/medical-malpractice-caps-by-state-infographic-0368345. Accessed January 23, 2020.
- Goguen D. State-by-state medical malpractice damages caps. An in-depth look at state laws limiting compensation for medical malpractice plaintiffs. https://www.nolo.com/legal-encyclopedia/state-state-medical-malpractice-damages-caps.html. Accessed January 23, 2020.
- Berlin L. Medical errors, malpractice, and defensive medicine: an ill-fated triad. Diagnosis (Berl). 2017;4:133-139.
- Ranum D. Electronic health records continue to lead to medical malpractice suits. The Doctors Company. August 2019. https://www.thedoctors.com/articles/electronic-health-records-continue-to-lead-to-medical-malpractice-suits/. Accessed January 10, 2020.
- Mangalmurti SS, Murtagh L, Mello MM. Medical malpractice liability in the age of electronic health records. N Engl J Med. 2010;363:2060-2067.
- Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024-2033.
- Jena AB, Chandra A, Lakdawalla D, et al. Outcomes of medical malpractice litigation against US physicians. Arch Intern Med. 2012;172:892-894.
- Glaser LM, Alvi FA, Milad MP. Trends in malpractice claims for obstetric and gynecologic procedures, 2005 through 2014. Am J Obstet Gynecol. 2017;217:340.e1-340.e6.
In this second in a series of 3 articles discussing medical malpractice and the ObGyn we look at the reasons for malpractice claims and liability, what happens to malpractice claims, and the direction and future of medical malpractice. The first article dealt with 2 sources of major malpractice damages: the “big verdict” and physicians with multiple malpractice paid claims. Next month we look at the place of apology in medicine, in cases in which error, including negligence, may have caused a patient injury.
CASE 1 Long-term brachial plexus injury
Right upper extremity injury occurs in the neonate at delivery with sequela of long-term brachial plexus injury (which is diagnosed around 6 months of age). Physical therapy and orthopedic assessment are rendered. Despite continued treatment, discrepancy in arm lengths (ie, affected side arm is noticeably shorter than opposite side) remains. The child cannot play basketball with his older brother and is the victim of ridicule, the plaintiff’s attorney emphasizes. He is unable to properly pronate or supinate the affected arm.
The defendant ObGyn maintains that there was “no shoulder dystocia [at delivery] and the shoulder did not get obstructed in the pelvis; shoulder was delivered 15 seconds after delivery of the head.” The nursing staff testifies that if shoulder dystocia had been the problem they would have launched upon a series of procedures to address such, in accord with the delivering obstetrician. The defense expert witness testifies that a brachial plexus injury can happen without shoulder dystocia.
A defense verdict is rendered by the Florida jury.1
CASE 2 Shoulder dystocia
During delivery, the obstetrician notes a shoulder dystocia (“turtle sign”). After initial attempts to release the shoulder were unsuccessful, the physician applies traction several times to the head of the child, and the baby is delivered. There is permanent injury to the right brachial plexus. The defendant ObGyn says that traction was necessary to dislodge the shoulder, and that the injury was the result of the forces of labor (not the traction). The expert witness for the plaintiff testifies that the medical standard of care did not permit traction under these circumstances, and that the traction was the likely cause of the injury.
The Virginia jury awards $2.32 million in damages.2
Note: The above vignettes are drawn from actual cases but are only outlines of those cases and are not complete descriptions of the claims in the cases. Because the information comes from informal sources, not formal court records, the facts may be inaccurate and incomplete. They should be viewed as illustrations only.

The trend in malpractice
It has been clear for many years that medical malpractice claims are not randomly or evenly distributed among physicians. Notably, the variation among specialties has, and continues to be, substantial (FIGURE 1).3 Recent data suggest that, although paid claims per “1,000 physician-years” averages 14 paid claims per 1,000 physician years, it ranges from 4 or 5 in 1,000 (psychiatry and pediatrics) to 53 and 49 claims per 1,000 (neurology and plastic surgery, respectively). Obstetrics and gynecology has the fourth highest rate at 42.5 paid claims per 1,000 physician years.4 (These data are for the years 1992–2014.)

Continue to: The number of ObGyn paid malpractice claims has decreased over time...
The number of ObGyn paid malpractice claims has decreased over time. Although large verdicts and physicians with multiple paid malpractice claims receive a good deal of attention (as we noted in part 1 of our series), in fact, paid medical malpractice claims have trended downward in recent decades.5 When the data above are disaggregated by 5-year periods, for example, in obstetrics and gynecology, there has been a consistent reduction in paid malpractice claims from 1992 to 2014. Paid claims went from 58 per 1,000 physician-years in 1992–1996 to 25 per 1,000 in 2009–2014 (FIGURE 2).4,6 In short, the rate dropped by half over approximately 20 years.4

It is reasonable to expect that such a decline in the cost of malpractice insurance premiums would follow. Robert L. Barbieri, MD, who practices in Boston, Massachusetts, in his excellent recent editorial in OBG M

Why have malpractice payouts declined overall?
Have medical errors declined?
It would be wonderful if the reduction in malpractice claims represented a significant decrease in medical errors. Attention to medical errors was driven by the first widely noticed study of medical error deaths. The Institute of Medicine (IOM) study in 2000, put the number of deaths annually at 44,000 to 98,000.8 There have been many efforts to reduce such errors, and it is possible that those efforts have indeed reduced errors somewhat.4 Barbieri provided a helpful digest of many of the error-reduction suggestions for ObGyn practice (TABLE 1).6 But the number of medical errors remains high. More recent studies have suggested that the IOM’s reported number of injuries may have been low.9 In 2013, one study suggested that 210,000 deaths annually were “associated with preventable harm” in hospitals. Because of how the data were gathered the authors estimated that the actual number of preventable deaths was closer to 400,000 annually. Serious harm to patients was estimated at 10 to 20 times the IOM rate.9
Therefore, a dramatic reduction in preventable medical errors does not appear to explain the reduction in malpractice claims. Some portion of it may be explained by malpractice reforms—see "The medical reform factor" section below.

The collective accountability factor
The way malpractice claims are paid (FIGURE 4),10 reported, and handled may explain some of the apparent reduction in overall paid claims. Perhaps the advent of “collective accountability,” in which patient care is rendered by teams and responsibility accepted at a team level, can alleviate a significant amount of individual physician medical malpractice claims.11 This “enterprise liability” may shift the burden of medical error from physicians to health care organizations.12 Collective accountability may, therefore, focus on institutional responsibility rather than individual physician negligence.11,13 Institutions frequently hire multiple specialists and cover their medical malpractice costs as well as stand to be named in suits.

Continue to: The institutional involvement in malpractice cases also may affect...
The institutional involvement in malpractice cases also may affect apparent malpractice rates in another way. The National Practitioner Data Bank, which is the source of information for many malpractice studies, only requires reporting about individual physicians, not institutions.14 If, therefore, claims are settled on behalf of an institution, without implicating the physician, the number of physician malpractice cases may appear to decline without any real change in malpractice rates.14 In addition, institutions have taken the lead in informal resolution of injuries that occur in the institution, and these programs may reduce the direct malpractice claims against physicians. (These “disclosure, apology, and offer,” and similar programs, are discussed in the upcoming third part of this series.)
The medical reform factor
As noted, annual rates paid for medical malpractice in our specialty are trending downward. Many commentators look to malpractice reforms as the reason for the drop in malpractice rates.15-17 Because medical malpractice is essentially a matter of state law, the medical malpractice reform has occurred primarily at the state level.18 There have been many different reforms tried—limits on expert witnesses, review panels, and a variety of procedural limitations.19 Perhaps the most effective reform has been caps being placed on noneconomic damages (generally pain and suffering).20 These caps vary by state (FIGURE 5)21,22 and, of course, affect the “big verdict” cases. (As we saw in the second case scenario above, Virginia is an example of a state with a cap on malpractice awards.) They also have the secondary effect of reducing the number of malpractice cases. They make malpractice cases less attractive to some attorneys because they reduce the opportunity of large contingency fees from large verdicts. (Virtually all medical malpractice cases in the United States are tried on a contingency-fee basis, meaning that the plaintiff does not pay the attorney handling the case but rather the attorney takes a percentage of any recovery—typically in the neighborhood of 35%.) The reform process continues, although, presently, there is less pressure to act on the malpractice crisis.

Medical malpractice cases are emotional and costly
Another reason for the relatively low rate of paid claims is that medical malpractice cases are difficult, emotionally challenging, time consuming, and expensive to pursue.23 They typically drag on for years, require extensive and expensive expert consultants as well as witnesses, and face stiff defense (compared with many other torts). The settlement of medical malpractice cases, for example, is less likely than other kinds of personal injury cases.
The contingency-fee basis does mean that injured patients do not have to pay attorney fees up front; however, plaintiffs may have to pay substantial costs along the way. The other side of this coin is that lawyers can be reluctant to take malpractice cases in which the damages are likely to be small, or where the legal uncertainty reduces the odds of achieving any damages. Thus, many potential malpractice cases are never filed.
A word of caution
The news of a reduction in malpractice paid claims may not be permanent. The numbers can conceivably be cyclical, and political reforms achieved can be changed. In addition, new technology will likely bring new kinds of malpractice claims. That appears to be the case, for example, with electronic health records (EHRs). One insurer reports that EHR malpractice claims have increased over the last 8 years.24 The most common injury in these claims was death (25%), as well as a magnitude of less serious injuries. EHR-related claims result from system failures, copy-paste inaccuracies, faulty drop-down menu use, and uncorrected “auto-populated” fields. Obstetrics is tied for fifth on the list of 14 specialties with claims related to EHRs, and gynecology is tied for eighth place.24
Continue to: A federal court ruled that a hospital that changed from...
A federal court ruled that a hospital that changed from paper records to EHRs for test results had a duty to “‘implement a reasonable procedure during the transition phase’ to ensure the timely delivery of test results” to health care providers.25 We will address this in a future “What’s the Verdict?”.
Rates of harm, malpractice cases, and the disposition of cases
There are many surprises when looking at medical malpractice claims data generally. The first surprise is how few claims are filed relative to the number of error-related injuries. Given the estimate of 210,000 to 400,000 deaths “associated with preventable harm” in hospitals, plus 10 to 20 times that number of serious injuries, it would be reasonable to expect claims of many hundreds of thousands per year. Compare the probability of a malpractice claim from an error-related injury, for example, with the probability of other personal injuries—eg, of traffic deaths associated with preventable harm.
The second key observation is how many of the claims filed are not successful—even when there was evidence in the record of errors associated with the injury. Studies slice the data in different ways but collectively suggest that only a small proportion of malpractice claims filed (a claim is generally regarded as some written demand for compensation for injuries) result in payments, either through settlement or by trial. A 2006 study by Studdert and colleagues determined that 63% of formal malpractice claims filed did involve injuries resulting from errors.26 The study found that in 16% of the claims (not injuries) there was no payment even though there was error. In 10% of the claims there was payment, even in the absence of error.
Overall, in this study, 56% of the claims received some compensation.26 That is higher than a more recent study by Jena and others, which found only 22% of claims resulted in compensation.3
How malpractice claims are decided is also interesting. Jena and colleagues found that only 55% of claims resulted in litigation.27 Presumably, the other 45% may have resulted in the plaintiff dropping the case, or in some form of settlement. Of the claims that were litigated, 54% were dismissed by the court, and another 35% were settled before a trial verdict. The cases that went to trial (about 10%), overwhelmingly (80%) resulted in verdicts for the defense.3,27 A different study found that only 9% of cases went to trial, and 87% were a defense verdict.28 The high level of defense verdicts may suggest that malpractice defense lawyers, and their client physicians, do a good job of assessing cases they are likely to lose, and settling them before trial.
ObGyns generally have larger numbers of claims and among the largest payment amounts when there is payment. Fewer of their cases are dismissed by the courts, so more go to trial. At trial, however, ObGyns prevail at a remarkably high rate.27 As for the probability of payment of a malpractice claim for ObGyns, one study suggested that there is approximately a 16% annual probability of a claim being filed, but only a 3% annual probability of a payment being made (suggesting about a 20% probability of payment per claim).3
Continue to: The purposes and effects of the medical malpractice system...
The purposes and effects of the medical malpractice system
The essential goals of tort law (including medical malpractice) include compensation for those who are injured and deterrence of future injuries (TABLE 2). What are the overall effects to the medical malpractice system? Unfortunately, the answer is that the law delivers disappointing results at best. It has a fairly high error rate. Many people who deserve some compensation for their injuries never seek compensation, and many deserving injured patients fail in efforts to receive compensation. At the same time, a few of the injured receive huge recoveries (even windfalls), and at least a small fraction receive compensation when there was no medical error. In addition to the high error rate, the system is inefficient and very expensive. Both defendants (through their insurance carriers) and plaintiffs spend a lot of money, years of time, and untold emotional pain dealing with these cases. The system also exacts high emotional and personal costs on plaintiffs and defendants.

Malpractice reform has not really addressed these issues—it has generally been focused on ways to reduce the cost of malpractice insurance. The most effective reform in reducing rates—caps—has had the effect of compensating the most seriously injured as though they were more modestly injured, and dissuading attorneys from taking the cases of those less seriously injured.
The medical and legal professions exist to help patients (the public). It does not seem that we have arrived at a system that does that very fairly or efficiently when a patient is injured because of preventable medical error.
The two vignettes described at the beginning, with similar injuries (shoulder dystocia), had disparate outcomes. In one there was a defense verdict and in the other a verdict for the plaintiffs of more than $2 million. The differences explain a number of important elements related to malpractice claims. (We have only very abbreviated and incomplete descriptions of the cases, so this discussion necessarily assumes facts and jumps to conclusions that may not be entirely consistent with the actual cases.)
These vignettes are unusual in that they went to trial. As we have noted, only a small percentage of malpractice cases are tried. And the verdict for the plaintiff-patient (in the second case) is unusual among those cases that go to trial, where plaintiffs seldom prevail.
From the facts we have, one significant difference in the 2 cases is that the plaintiff’s expert witness specifically testified in the second case that the “medical standard of care did not permit traction under these circumstances.” That is an essential element of a successful plaintiff’s malpractice case. In this case, the expert could also draw a connection between that breach of standard of care and harm to the child. In the case without liability, the nursing staff was able to testify that there was no shoulder dystocia because if there had been such an injury, they would have immediately launched into special action, which did not happen. By contrast, in the liability case, there seemed to be critical gaps in the medical record.
It is also important to remember that these cases were tried in different states, with different laws. The juries and judges in the 2 cases were different. Finally, the quality of the attorneys representing the plaintiffs and defendants were different. We mention these factors to point out that medical malpractice is not an exact science. It depends on many human elements that make the outcome of cases somewhat unpredictable. This unpredictability is one reason why parties and attorneys like to settle cases.
Watch for the third and final article in this series next month, as we are going to look at “apology in medicine and a proactive response” to communication regarding a complication.
In this second in a series of 3 articles discussing medical malpractice and the ObGyn we look at the reasons for malpractice claims and liability, what happens to malpractice claims, and the direction and future of medical malpractice. The first article dealt with 2 sources of major malpractice damages: the “big verdict” and physicians with multiple malpractice paid claims. Next month we look at the place of apology in medicine, in cases in which error, including negligence, may have caused a patient injury.
CASE 1 Long-term brachial plexus injury
Right upper extremity injury occurs in the neonate at delivery with sequela of long-term brachial plexus injury (which is diagnosed around 6 months of age). Physical therapy and orthopedic assessment are rendered. Despite continued treatment, discrepancy in arm lengths (ie, affected side arm is noticeably shorter than opposite side) remains. The child cannot play basketball with his older brother and is the victim of ridicule, the plaintiff’s attorney emphasizes. He is unable to properly pronate or supinate the affected arm.
The defendant ObGyn maintains that there was “no shoulder dystocia [at delivery] and the shoulder did not get obstructed in the pelvis; shoulder was delivered 15 seconds after delivery of the head.” The nursing staff testifies that if shoulder dystocia had been the problem they would have launched upon a series of procedures to address such, in accord with the delivering obstetrician. The defense expert witness testifies that a brachial plexus injury can happen without shoulder dystocia.
A defense verdict is rendered by the Florida jury.1
CASE 2 Shoulder dystocia
During delivery, the obstetrician notes a shoulder dystocia (“turtle sign”). After initial attempts to release the shoulder were unsuccessful, the physician applies traction several times to the head of the child, and the baby is delivered. There is permanent injury to the right brachial plexus. The defendant ObGyn says that traction was necessary to dislodge the shoulder, and that the injury was the result of the forces of labor (not the traction). The expert witness for the plaintiff testifies that the medical standard of care did not permit traction under these circumstances, and that the traction was the likely cause of the injury.
The Virginia jury awards $2.32 million in damages.2
Note: The above vignettes are drawn from actual cases but are only outlines of those cases and are not complete descriptions of the claims in the cases. Because the information comes from informal sources, not formal court records, the facts may be inaccurate and incomplete. They should be viewed as illustrations only.

The trend in malpractice
It has been clear for many years that medical malpractice claims are not randomly or evenly distributed among physicians. Notably, the variation among specialties has, and continues to be, substantial (FIGURE 1).3 Recent data suggest that, although paid claims per “1,000 physician-years” averages 14 paid claims per 1,000 physician years, it ranges from 4 or 5 in 1,000 (psychiatry and pediatrics) to 53 and 49 claims per 1,000 (neurology and plastic surgery, respectively). Obstetrics and gynecology has the fourth highest rate at 42.5 paid claims per 1,000 physician years.4 (These data are for the years 1992–2014.)

Continue to: The number of ObGyn paid malpractice claims has decreased over time...
The number of ObGyn paid malpractice claims has decreased over time. Although large verdicts and physicians with multiple paid malpractice claims receive a good deal of attention (as we noted in part 1 of our series), in fact, paid medical malpractice claims have trended downward in recent decades.5 When the data above are disaggregated by 5-year periods, for example, in obstetrics and gynecology, there has been a consistent reduction in paid malpractice claims from 1992 to 2014. Paid claims went from 58 per 1,000 physician-years in 1992–1996 to 25 per 1,000 in 2009–2014 (FIGURE 2).4,6 In short, the rate dropped by half over approximately 20 years.4

It is reasonable to expect that such a decline in the cost of malpractice insurance premiums would follow. Robert L. Barbieri, MD, who practices in Boston, Massachusetts, in his excellent recent editorial in OBG M

Why have malpractice payouts declined overall?
Have medical errors declined?
It would be wonderful if the reduction in malpractice claims represented a significant decrease in medical errors. Attention to medical errors was driven by the first widely noticed study of medical error deaths. The Institute of Medicine (IOM) study in 2000, put the number of deaths annually at 44,000 to 98,000.8 There have been many efforts to reduce such errors, and it is possible that those efforts have indeed reduced errors somewhat.4 Barbieri provided a helpful digest of many of the error-reduction suggestions for ObGyn practice (TABLE 1).6 But the number of medical errors remains high. More recent studies have suggested that the IOM’s reported number of injuries may have been low.9 In 2013, one study suggested that 210,000 deaths annually were “associated with preventable harm” in hospitals. Because of how the data were gathered the authors estimated that the actual number of preventable deaths was closer to 400,000 annually. Serious harm to patients was estimated at 10 to 20 times the IOM rate.9
Therefore, a dramatic reduction in preventable medical errors does not appear to explain the reduction in malpractice claims. Some portion of it may be explained by malpractice reforms—see "The medical reform factor" section below.

The collective accountability factor
The way malpractice claims are paid (FIGURE 4),10 reported, and handled may explain some of the apparent reduction in overall paid claims. Perhaps the advent of “collective accountability,” in which patient care is rendered by teams and responsibility accepted at a team level, can alleviate a significant amount of individual physician medical malpractice claims.11 This “enterprise liability” may shift the burden of medical error from physicians to health care organizations.12 Collective accountability may, therefore, focus on institutional responsibility rather than individual physician negligence.11,13 Institutions frequently hire multiple specialists and cover their medical malpractice costs as well as stand to be named in suits.

Continue to: The institutional involvement in malpractice cases also may affect...
The institutional involvement in malpractice cases also may affect apparent malpractice rates in another way. The National Practitioner Data Bank, which is the source of information for many malpractice studies, only requires reporting about individual physicians, not institutions.14 If, therefore, claims are settled on behalf of an institution, without implicating the physician, the number of physician malpractice cases may appear to decline without any real change in malpractice rates.14 In addition, institutions have taken the lead in informal resolution of injuries that occur in the institution, and these programs may reduce the direct malpractice claims against physicians. (These “disclosure, apology, and offer,” and similar programs, are discussed in the upcoming third part of this series.)
The medical reform factor
As noted, annual rates paid for medical malpractice in our specialty are trending downward. Many commentators look to malpractice reforms as the reason for the drop in malpractice rates.15-17 Because medical malpractice is essentially a matter of state law, the medical malpractice reform has occurred primarily at the state level.18 There have been many different reforms tried—limits on expert witnesses, review panels, and a variety of procedural limitations.19 Perhaps the most effective reform has been caps being placed on noneconomic damages (generally pain and suffering).20 These caps vary by state (FIGURE 5)21,22 and, of course, affect the “big verdict” cases. (As we saw in the second case scenario above, Virginia is an example of a state with a cap on malpractice awards.) They also have the secondary effect of reducing the number of malpractice cases. They make malpractice cases less attractive to some attorneys because they reduce the opportunity of large contingency fees from large verdicts. (Virtually all medical malpractice cases in the United States are tried on a contingency-fee basis, meaning that the plaintiff does not pay the attorney handling the case but rather the attorney takes a percentage of any recovery—typically in the neighborhood of 35%.) The reform process continues, although, presently, there is less pressure to act on the malpractice crisis.

Medical malpractice cases are emotional and costly
Another reason for the relatively low rate of paid claims is that medical malpractice cases are difficult, emotionally challenging, time consuming, and expensive to pursue.23 They typically drag on for years, require extensive and expensive expert consultants as well as witnesses, and face stiff defense (compared with many other torts). The settlement of medical malpractice cases, for example, is less likely than other kinds of personal injury cases.
The contingency-fee basis does mean that injured patients do not have to pay attorney fees up front; however, plaintiffs may have to pay substantial costs along the way. The other side of this coin is that lawyers can be reluctant to take malpractice cases in which the damages are likely to be small, or where the legal uncertainty reduces the odds of achieving any damages. Thus, many potential malpractice cases are never filed.
A word of caution
The news of a reduction in malpractice paid claims may not be permanent. The numbers can conceivably be cyclical, and political reforms achieved can be changed. In addition, new technology will likely bring new kinds of malpractice claims. That appears to be the case, for example, with electronic health records (EHRs). One insurer reports that EHR malpractice claims have increased over the last 8 years.24 The most common injury in these claims was death (25%), as well as a magnitude of less serious injuries. EHR-related claims result from system failures, copy-paste inaccuracies, faulty drop-down menu use, and uncorrected “auto-populated” fields. Obstetrics is tied for fifth on the list of 14 specialties with claims related to EHRs, and gynecology is tied for eighth place.24
Continue to: A federal court ruled that a hospital that changed from...
A federal court ruled that a hospital that changed from paper records to EHRs for test results had a duty to “‘implement a reasonable procedure during the transition phase’ to ensure the timely delivery of test results” to health care providers.25 We will address this in a future “What’s the Verdict?”.
Rates of harm, malpractice cases, and the disposition of cases
There are many surprises when looking at medical malpractice claims data generally. The first surprise is how few claims are filed relative to the number of error-related injuries. Given the estimate of 210,000 to 400,000 deaths “associated with preventable harm” in hospitals, plus 10 to 20 times that number of serious injuries, it would be reasonable to expect claims of many hundreds of thousands per year. Compare the probability of a malpractice claim from an error-related injury, for example, with the probability of other personal injuries—eg, of traffic deaths associated with preventable harm.
The second key observation is how many of the claims filed are not successful—even when there was evidence in the record of errors associated with the injury. Studies slice the data in different ways but collectively suggest that only a small proportion of malpractice claims filed (a claim is generally regarded as some written demand for compensation for injuries) result in payments, either through settlement or by trial. A 2006 study by Studdert and colleagues determined that 63% of formal malpractice claims filed did involve injuries resulting from errors.26 The study found that in 16% of the claims (not injuries) there was no payment even though there was error. In 10% of the claims there was payment, even in the absence of error.
Overall, in this study, 56% of the claims received some compensation.26 That is higher than a more recent study by Jena and others, which found only 22% of claims resulted in compensation.3
How malpractice claims are decided is also interesting. Jena and colleagues found that only 55% of claims resulted in litigation.27 Presumably, the other 45% may have resulted in the plaintiff dropping the case, or in some form of settlement. Of the claims that were litigated, 54% were dismissed by the court, and another 35% were settled before a trial verdict. The cases that went to trial (about 10%), overwhelmingly (80%) resulted in verdicts for the defense.3,27 A different study found that only 9% of cases went to trial, and 87% were a defense verdict.28 The high level of defense verdicts may suggest that malpractice defense lawyers, and their client physicians, do a good job of assessing cases they are likely to lose, and settling them before trial.
ObGyns generally have larger numbers of claims and among the largest payment amounts when there is payment. Fewer of their cases are dismissed by the courts, so more go to trial. At trial, however, ObGyns prevail at a remarkably high rate.27 As for the probability of payment of a malpractice claim for ObGyns, one study suggested that there is approximately a 16% annual probability of a claim being filed, but only a 3% annual probability of a payment being made (suggesting about a 20% probability of payment per claim).3
Continue to: The purposes and effects of the medical malpractice system...
The purposes and effects of the medical malpractice system
The essential goals of tort law (including medical malpractice) include compensation for those who are injured and deterrence of future injuries (TABLE 2). What are the overall effects to the medical malpractice system? Unfortunately, the answer is that the law delivers disappointing results at best. It has a fairly high error rate. Many people who deserve some compensation for their injuries never seek compensation, and many deserving injured patients fail in efforts to receive compensation. At the same time, a few of the injured receive huge recoveries (even windfalls), and at least a small fraction receive compensation when there was no medical error. In addition to the high error rate, the system is inefficient and very expensive. Both defendants (through their insurance carriers) and plaintiffs spend a lot of money, years of time, and untold emotional pain dealing with these cases. The system also exacts high emotional and personal costs on plaintiffs and defendants.

Malpractice reform has not really addressed these issues—it has generally been focused on ways to reduce the cost of malpractice insurance. The most effective reform in reducing rates—caps—has had the effect of compensating the most seriously injured as though they were more modestly injured, and dissuading attorneys from taking the cases of those less seriously injured.
The medical and legal professions exist to help patients (the public). It does not seem that we have arrived at a system that does that very fairly or efficiently when a patient is injured because of preventable medical error.
The two vignettes described at the beginning, with similar injuries (shoulder dystocia), had disparate outcomes. In one there was a defense verdict and in the other a verdict for the plaintiffs of more than $2 million. The differences explain a number of important elements related to malpractice claims. (We have only very abbreviated and incomplete descriptions of the cases, so this discussion necessarily assumes facts and jumps to conclusions that may not be entirely consistent with the actual cases.)
These vignettes are unusual in that they went to trial. As we have noted, only a small percentage of malpractice cases are tried. And the verdict for the plaintiff-patient (in the second case) is unusual among those cases that go to trial, where plaintiffs seldom prevail.
From the facts we have, one significant difference in the 2 cases is that the plaintiff’s expert witness specifically testified in the second case that the “medical standard of care did not permit traction under these circumstances.” That is an essential element of a successful plaintiff’s malpractice case. In this case, the expert could also draw a connection between that breach of standard of care and harm to the child. In the case without liability, the nursing staff was able to testify that there was no shoulder dystocia because if there had been such an injury, they would have immediately launched into special action, which did not happen. By contrast, in the liability case, there seemed to be critical gaps in the medical record.
It is also important to remember that these cases were tried in different states, with different laws. The juries and judges in the 2 cases were different. Finally, the quality of the attorneys representing the plaintiffs and defendants were different. We mention these factors to point out that medical malpractice is not an exact science. It depends on many human elements that make the outcome of cases somewhat unpredictable. This unpredictability is one reason why parties and attorneys like to settle cases.
Watch for the third and final article in this series next month, as we are going to look at “apology in medicine and a proactive response” to communication regarding a complication.
- Shoulder dystocia—Florida defense verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(1):18.
- Shoulder dystocia improperly managed--$2.320 million Virginia verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(2):13.
- Jena AB, Seabury S, Lakdawalla D, et al. Malpractice risk according to physician specialty. N Engl J Med. 2011;365:629-636.
- Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177:710-718.
- Lowes R. Malpractice premiums trail inflation for some physicians. Medscape. December 16, 2016. https://www.medscape.com/viewarticle/873422. Accessed January 10, 2020.
- Barbieri RL. Good news for ObGyns: medical liability claims resulting in payment are decreasing! OBG Manag. 2019;31:10-13.
- Guardado JR. Medical professional liability insurance premiums: an overview of the market from 2008 to 2017. AMA Policy Research Perspectives, 2018. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/government/advocacy/policy-research-perspective-liability-insurance-premiums.pdf. Accessed January 10, 2020.
- Institute of Medicine Committee on Quality Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
- James JT. A new, evidence-based estimate of patient harms associated with hospital care. J Patient Saf. 2013;9:122-128. https://journals.lww.com/journalpatientsafety/Fulltext/
2013/09000/A_New,_Evidence_based_Estimate_of_Patient_
Harms.2.aspx. Accessed January 10, 2020. - Public Citizen Congress Watch. The great medical malpractice hoax: NPDB data continue to show medical liability system produces rational outcomes. January 2007. https://www.citizen.org/wp-content/uploads/npdb_report_
final.pdf. Accessed January 23, 2020. - Bell SK, Delbanco T, Anderson-Shaw L, et al. Accountability for medical error: moving beyond blame to advocacy. Chest. 2011;140:519-526.
- Ramanathan T. Legal mechanisms supporting accountable care principles. Am J Public Health. 2014;104:2048-2051.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
- National Practitioner Data Bank web site. What you must report to the NPDB. https://www.npdb.hrsa.gov/hcorg/whatYouMustReport
ToTheDataBank.jsp. Accessed January 10, 2020. - Bovbjerg RR. Malpractice crisis and reform. Clin Perinatol. 2005;32:203-233, viii-ix.
- Viscusi WK. Medical malpractice reform: what works and what doesn't. Denver Law Rev. 2019;96:775-791. https://static1.squarespace.com/static/5cb79f7efd6793296c0eb738 /t/5d5f4ffabd6c5400011a12f6/1566527483118/Vol96_Issue4_Viscusi_
FINAL.pdf. Accessed January 10, 2020. - National Conference of State Legislatures. Medical malpractice reform. Health Cost Containment and Efficiencies: NCSL Briefs for State Legislators. 2011;(16). http://www.ncsl.org/research/health/medical-malpractice-reform-health-cost-brief.aspx. Accessed January 10, 2020.
- Kass JS, Rose RV. Medical malpractice reform: historical approaches, alternative models, and communication and resolution programs. AMA J Ethics. 2016;18:299-310.
- Boehm G. Debunking medical malpractice myths: unraveling the false premises behind "tort reform". Yale J Health Policy Law Ethics. 2005;5:357-369.
- Hellinger FJ, Encinosa WE. The impact of state laws limiting malpractice damage awards on health care expenditures. Am J Public Health. 2006;96:1375-1381.
- Perry G. Medical malpractice caps by state [infographic]. January 3, 2013. https://www.business2community.com/infographics/medical-malpractice-caps-by-state-infographic-0368345. Accessed January 23, 2020.
- Goguen D. State-by-state medical malpractice damages caps. An in-depth look at state laws limiting compensation for medical malpractice plaintiffs. https://www.nolo.com/legal-encyclopedia/state-state-medical-malpractice-damages-caps.html. Accessed January 23, 2020.
- Berlin L. Medical errors, malpractice, and defensive medicine: an ill-fated triad. Diagnosis (Berl). 2017;4:133-139.
- Ranum D. Electronic health records continue to lead to medical malpractice suits. The Doctors Company. August 2019. https://www.thedoctors.com/articles/electronic-health-records-continue-to-lead-to-medical-malpractice-suits/. Accessed January 10, 2020.
- Mangalmurti SS, Murtagh L, Mello MM. Medical malpractice liability in the age of electronic health records. N Engl J Med. 2010;363:2060-2067.
- Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024-2033.
- Jena AB, Chandra A, Lakdawalla D, et al. Outcomes of medical malpractice litigation against US physicians. Arch Intern Med. 2012;172:892-894.
- Glaser LM, Alvi FA, Milad MP. Trends in malpractice claims for obstetric and gynecologic procedures, 2005 through 2014. Am J Obstet Gynecol. 2017;217:340.e1-340.e6.
- Shoulder dystocia—Florida defense verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(1):18.
- Shoulder dystocia improperly managed--$2.320 million Virginia verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(2):13.
- Jena AB, Seabury S, Lakdawalla D, et al. Malpractice risk according to physician specialty. N Engl J Med. 2011;365:629-636.
- Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177:710-718.
- Lowes R. Malpractice premiums trail inflation for some physicians. Medscape. December 16, 2016. https://www.medscape.com/viewarticle/873422. Accessed January 10, 2020.
- Barbieri RL. Good news for ObGyns: medical liability claims resulting in payment are decreasing! OBG Manag. 2019;31:10-13.
- Guardado JR. Medical professional liability insurance premiums: an overview of the market from 2008 to 2017. AMA Policy Research Perspectives, 2018. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/government/advocacy/policy-research-perspective-liability-insurance-premiums.pdf. Accessed January 10, 2020.
- Institute of Medicine Committee on Quality Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
- James JT. A new, evidence-based estimate of patient harms associated with hospital care. J Patient Saf. 2013;9:122-128. https://journals.lww.com/journalpatientsafety/Fulltext/
2013/09000/A_New,_Evidence_based_Estimate_of_Patient_
Harms.2.aspx. Accessed January 10, 2020. - Public Citizen Congress Watch. The great medical malpractice hoax: NPDB data continue to show medical liability system produces rational outcomes. January 2007. https://www.citizen.org/wp-content/uploads/npdb_report_
final.pdf. Accessed January 23, 2020. - Bell SK, Delbanco T, Anderson-Shaw L, et al. Accountability for medical error: moving beyond blame to advocacy. Chest. 2011;140:519-526.
- Ramanathan T. Legal mechanisms supporting accountable care principles. Am J Public Health. 2014;104:2048-2051.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
- National Practitioner Data Bank web site. What you must report to the NPDB. https://www.npdb.hrsa.gov/hcorg/whatYouMustReport
ToTheDataBank.jsp. Accessed January 10, 2020. - Bovbjerg RR. Malpractice crisis and reform. Clin Perinatol. 2005;32:203-233, viii-ix.
- Viscusi WK. Medical malpractice reform: what works and what doesn't. Denver Law Rev. 2019;96:775-791. https://static1.squarespace.com/static/5cb79f7efd6793296c0eb738 /t/5d5f4ffabd6c5400011a12f6/1566527483118/Vol96_Issue4_Viscusi_
FINAL.pdf. Accessed January 10, 2020. - National Conference of State Legislatures. Medical malpractice reform. Health Cost Containment and Efficiencies: NCSL Briefs for State Legislators. 2011;(16). http://www.ncsl.org/research/health/medical-malpractice-reform-health-cost-brief.aspx. Accessed January 10, 2020.
- Kass JS, Rose RV. Medical malpractice reform: historical approaches, alternative models, and communication and resolution programs. AMA J Ethics. 2016;18:299-310.
- Boehm G. Debunking medical malpractice myths: unraveling the false premises behind "tort reform". Yale J Health Policy Law Ethics. 2005;5:357-369.
- Hellinger FJ, Encinosa WE. The impact of state laws limiting malpractice damage awards on health care expenditures. Am J Public Health. 2006;96:1375-1381.
- Perry G. Medical malpractice caps by state [infographic]. January 3, 2013. https://www.business2community.com/infographics/medical-malpractice-caps-by-state-infographic-0368345. Accessed January 23, 2020.
- Goguen D. State-by-state medical malpractice damages caps. An in-depth look at state laws limiting compensation for medical malpractice plaintiffs. https://www.nolo.com/legal-encyclopedia/state-state-medical-malpractice-damages-caps.html. Accessed January 23, 2020.
- Berlin L. Medical errors, malpractice, and defensive medicine: an ill-fated triad. Diagnosis (Berl). 2017;4:133-139.
- Ranum D. Electronic health records continue to lead to medical malpractice suits. The Doctors Company. August 2019. https://www.thedoctors.com/articles/electronic-health-records-continue-to-lead-to-medical-malpractice-suits/. Accessed January 10, 2020.
- Mangalmurti SS, Murtagh L, Mello MM. Medical malpractice liability in the age of electronic health records. N Engl J Med. 2010;363:2060-2067.
- Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024-2033.
- Jena AB, Chandra A, Lakdawalla D, et al. Outcomes of medical malpractice litigation against US physicians. Arch Intern Med. 2012;172:892-894.
- Glaser LM, Alvi FA, Milad MP. Trends in malpractice claims for obstetric and gynecologic procedures, 2005 through 2014. Am J Obstet Gynecol. 2017;217:340.e1-340.e6.
Medical malpractice: Its evolution to today’s risk of the “big verdict”
Medical malpractice (more formally, professional liability, but we will use the term malpractice) has been of concern to ObGyns for many years, and for good reasons. This specialty has some of the highest incidents of malpractice claims, some of the largest verdicts, and some of the highest malpractice insurance rates. We look more closely at ObGyn malpractice issues in a 3-part “What’s the Verdict” series over the next few months.
In part 1, we discuss the background on malpractice and reasons why malpractice rates have been so high—including large verdicts and lawsuit-prone physicians. In the second part we will look at recent experience and developments in malpractice exposure—who is sued and why. Finally, in the third part we will consider suggestions for reducing the likelihood of a malpractice lawsuit, with a special focus on recent research regarding apologies.
Two reports of recent trials involving ObGyn care illustrate the risk of “the big verdict.”1,2 (Note that the following vignettes are drawn from actual cases but are outlines of those cases and not complete descriptions of the claims. Because the information does not come from formal court records, the facts may be inaccurate and are incomplete; they should be viewed as illustrations only.)
CASE 1 Delayed delivery, $19M verdict
At 39 weeks’ gestation, a woman was admitted to the hospital in spontaneous labor. Artificial rupture of membranes with clear amniotic fluid was noted. Active contractions occurred for 11 hours. Oxytocin was then initiated, and 17 minutes later, profound fetal bradycardia was detected. There was recurrent evidence of fetal distress with meconium. After a nursing staff change a second nurse restarted oxytocin for a prolonged period. The physician allowed labor to continue despite fetal distress, and performed a cesarean delivery (CD) 4.5 hours later. Five hours postdelivery the neonate was noted to have a pneumothorax, lung damage, and respiratory failure. The infant died at 18 days of age.
The jury felt that there was negligence—failure to timely diagnose fetal distress and failure to timely perform CD, all of which resulted in a verdict for the plaintiff. The jury awarded in excess of $19 million.1
CASE 2 An undiagnosed tumor, $20M verdict
A patient underwent bilateral mastectomy. Following surgery, she reported pain and swelling at the surgical site for 2 years, and the defendant physician “dismissed” her complaint, refusing to evaluate it as the provider felt it was related to scar tissue. Three years after the mastectomies, the patient underwent surgical exploration and removal of 3 ribs and sternum secondary to a desmoid tumor. Surgical mesh and chest reconstruction was required, necessitating long-term opioids and sleeping medications that “will slow her wits, dull her senses and limit activities of daily living.” Of note, discrepancies were found in the medical records maintained by the defendant. (There was, for example, no report in the record of the plaintiff’s pain until late in the process.) The plaintiff based her claim on the fact that her pain and lump were neither evaluated nor discovered until it was too late.
The jury awarded $20 million. The verdict was reduced to $2 million by the court based on state statutory limits on malpractice damages.2,3

Continue to: Medical malpractice: Evolution of a standard of care...
Medical malpractice: Evolution of a standard of care
Medical malpractice is not a modern invention. Some historians trace malpractice to the Code of Hammurabi (2030 BC), through Roman law,4 into English common law.5 It was sufficiently established by 1765 that the classic legal treatise of the century referred to medical malpractice.6,7 Although medical malpractice existed for a long time, actual malpractice cases were relatively rare before the last half of the 20th century.8
Defensive medicine born out of necessity. The number of malpractice cases increased substantially—described as a “geometric increase”—after 1960, with a 300% rise between 1965 and 1970.7,9 This “malpractice maelstrom of the 70s”7 resulted in dramatic increases in malpractice insurance costs and invited the practice of defensive medicine—medically unnecessary or unjustified tests and services.10Although there is controversy about what is defensive medicine and what is reasonably cautious medicine, the practice may account for 3% of total health care spending.11 Mello and others have estimated that there may be a $55 billion annual cost related to the medical malpractice system.12
Several malpractice crises and waves of malpractice or tort reform ensued,13 beginning in the 1970s and extending into the 2000s.11 Malpractice law is primarily a matter of state law, so reform essentially has been at the state level—as we will see in the second part in this series.
Defining a standard of care
Medical malpractice is the application of standard legal principles to medical practice. Those principles generally are torts (intentional torts and negligence), and sometimes contracts.14 Eventually, medical malpractice came to focus primarily on negligence. The legal purposes of imposing negligence liability are compensation (to repay the plaintiff the costs of the harm caused by the defendant) and deterrence (to discourage careless conduct that can harm others.)
Negligence is essentially carelessness that falls below the acceptable standard of care. Negligence may arise, for example, from15:
- doing something (giving a drug to a patient with a known allergy to it)
- not doing something (failing to test for a possible tumor, as in the second case above)
- not giving appropriate informed consent
- failing to conduct an adequate examination
- abandoning a patient
- failing to refer a patient to a specialist (or conduct a consultation).
(In recent years, law reforms directed specifically at medical malpractice have somewhat separated medical malpractice from other tort law.)
In malpractice cases, the core question is whether the provider did (or did not) do something that a reasonably careful physician would have done. It is axiomatic that not all bad outcomes are negligent. Indeed, not all mistakes are negligent—only the mistakes that were unreasonable given all of the circumstances. In the first case above, for example, given all of the facts that preceded it, the delay of the physician for 4.5 hours after the fetal distress started was, as seen by the jury, not just a mistake but an unreasonable mistake. Hence, it was negligent. In the second case, the failure to investigate the pain and swelling in the surgical site for 2 years (or failure to refer the patient to another physician) was seen by the jury as an unreasonable mistake—one that would not have been made by a reasonably careful practitioner.
Continue to: The big verdict...
The big verdict
Everyone—every professional providing service, every manufacturer, every driver—eventually will make an unreasonable mistake (ie, commit negligence). If that negligence results in harming someone else, our standard legal response is that the negligent person should be financially responsible for the harm to the other. So, a driver who fails to stop at a red light and hits another car is responsible for those damages. But the damages may vary—perhaps a banged-up fender, or, in another instance, with the same negligence, perhaps terrible personal injuries that will disable the other driver for life. Thus, the damages can vary for the same level of carelessness. The “big verdict” may therefore fall on someone who was not especially careless.
Big verdicts often involve long-term care. The opening case vignettes illustrate a concern of medical malpractice generally—especially for ObGyn practice—the very high verdict. Very high verdicts generally reflect catastrophic damages that will continue for a long time. Bixenstine and colleagues found, for example, that catastrophic payouts often involved “patient age less than 1 year, quadriplegia, brain damage, or lifelong care.”16 In the case of serious injuries during delivery, for example, the harm to the child may last a lifetime and require years and years of intensive medical services.
Million-dollar-plus payouts are on the rise. The percentage of paid claims (through settlement or trial) that are above $1 million is increasing. These million-dollar cases represent 36% of the total dollars paid in ObGyn malpractice claims, even though they represent only 8% of the number of claims paid.16 The increase in the big verdict cases (above $1 million) suggests that ObGyn practitioners should consider their malpractice policy limits—a million dollars may not be enough.
In big verdict cases, the great harm to the plaintiff is often combined with facts that produce extraordinary sympathy for the plaintiff. Sometimes there is decidedly unsympathetic conduct by the defendant as well. In the second case, for example, the problems with the medical record may have suggested to the jury that the doctor was either trying to hide something or did not care enough about the patient even to note a serious complaint. In a case we reviewed in an earlier “What’s the Verdict” column, a physician left the room for several minutes during a critical time—to take a call from a stockbroker.16-18
The big verdict does not necessarily suggest that the defendant was especially or grossly negligent.16 It was a bad injury that occurred, for instance. On the other hand, the physician with several malpractice judgments may suggest that this is a problem physician.
Physicians facing multiple lawsuits are the exceptions
A number of studies have demonstrated that only a small proportion of physicians are responsible for a disproportionate number of paid medical malpractice claims. (“Paid claims” are those in which the plaintiff receives money from the doctor’s insurance. “Filed claims” are all malpractice lawsuits filed. Many claims are filed, but few are paid.)
ObGyn has high number of paid claims and high risk of claim payment recurrence. Studdert and colleagues found that the probability of future paid malpractice climbed with each past paid claim.19 They also found that 1% of physicians accounted for 32% of all paid claims. The number of paid claims varied by specialty—obstetrics and gynecology accounted for the second largest number of paid claims (13%). The risk of recurrence (more than one paid claim) was highest among 4 surgical specialties and ObGyns (about double the recurrence rate in these specialties compared with internal medicine).19
A minority of physicians responsible for lion share of paid claims. Black and colleagues followed up the Studdert study. Although there were some differences in what they found, the results were very similar.20 For example, they found that having even a single prior paid claim strongly predicted future claims over the next 5 years. They also found that some “outlier” physicians with multiple paid claims “are responsible for a significant share of paid claims.” They specifically found that, even for physicians in high-risk specialties in high-risk states, “bad luck is highly unlikely to explain” multiple claims within 5 years.
Continue to: Both of the studies just mentioned relied on...
Both of the studies just mentioned relied on the National Practitioner Data Bank for information about paid claims. This source has some limitations in capturing claims or payments made by hospitals or other institutions for the actions of its agent-physicians. Some of these limitations were resolved in another recent study that looked at Indiana state insurance and licensing discipline records (over a 41-year period).21 Not surprisingly, this study found that claims paid increase with more severe licensure discipline. On the other hand, although, the “frequent fliers” in terms of malpractice claims made and paid could be identified as a “small number of repeat defendants,” these physicians were not routinely disciplined by the state medical board. This was only a single state study, of course, but it also found that a few physicians accounted for a significant number of the claims. The state board was not taking licensing action against this small group, however.
Should the few bad apples be picked from the orchard?
Collectively, these studies are fairly overwhelming in demonstrating that there are some physicians who are “prone” to malpractice claims (for whom all physicians in the specialty are probably paying higher malpractice rates), but who do not attract the attention of licensing agencies for careful examination. In addition to its self-interest in eliminating physicians prone to malpractice claims and payments, the obligation of professions to protect the public interest suggests that state boards should be more aggressive in pursuing those physicians practicing risky medicine.
This medical malpractice series will continue next month with a look at how to reduce malpractice exposure.
- Delivery delay blamed for baby’s death days later—$19.2 million Illinois verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35:17.
- Failure to identify signs of a growing tumor—$20 million Virginia verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35:18.
- Hellinger FJ, Encinosa WE. The impact of state laws limiting malpractice damage awards on health care expenditures. Am J Public Health. 2006;96:1375-1381.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339–347.
- Everad v. Hopkins, 80 English Reports 1164 (1615).
- Blackstone W. Commentaries on the laws of England. Oxford, England: Clarendon Press; 1768:122.
- Berlin L. Medical errors, malpractice, and defensive medicine: an ill-fated triad. Diagnosis (Berl). 2017;4:133-139.
- DeVille KA. Medical Malpractice in Nineteenth-Century America: Origins and Legacy. New York, NY: NYU Press; 1990.
- Hershey N. The defensive practice of medicine. Myth or reality. Milbank Mem Fund Q. 1972;50:69-98.
- Agarwal R, Gupta A, Gupta S. The impact of tort reform on defensive medicine, quality of care, and physician supply: a systematic review. Health Serv Res. 2019;54:851-859.
- Gerlach J, Abodunde B, Sollosy M, et al. Rethinking the obvious: time for new ideas on medical malpractice tort reform. Health Care Manag (Frederick). 2019;38:109-115.
- Mello MM, Chandra A, Gawande AA, et al. National costs of the medical liability system. Health Aff. 2010;29:1569-1577.
- Bovbjerg RR. Malpractice crisis and reform. Clin Perinatol. 2005;32:203-233, viii-ix.
- Hawkins v. McGee, 84 N.H. 114, 146 A. 641 (1929).
- Gittler GJ, Goldstein EJ. The elements of medical malpractice: an overview. Clin Infect Dis. 1996;23:1152–1155.
- Bixenstine PJ, Shore AD, Mehtsun WT, et al. Catastrophic medical malpractice payouts in the United States. J Healthc Quality. 2014;36:43-53.
- Sanfilippo JS, Smith SR. Lessons from a daunting malpractice event. OBG Manag. 2018;30:41-47.
- Chang D. Miami doctor hit with $33 million judgment in brain-damaged baby suit. Miami Herald. April 28, 2017. http://www.miamiherald.com/news/health-care/ article147506019.html. Accessed December 12, 2019.
- Studdert DM, Bismark MM, Mello MM, et al. Prevalence and characteristics of physicians prone to malpractice claims. N Engl J Med. 2016;374:354-362.
- Black B, Hyman DA, Lerner JY. Physicians with multiple paid medical malpractice claims: Are they outliers or just unlucky? Int Rev Law Econ. 2019;59:146-157.
- Liu J, Hyman DA. Targeting bad doctors: lessons from Indiana, 1975–2015. J Empirical Legal Studies. 2019;16: 248-328.
Medical malpractice (more formally, professional liability, but we will use the term malpractice) has been of concern to ObGyns for many years, and for good reasons. This specialty has some of the highest incidents of malpractice claims, some of the largest verdicts, and some of the highest malpractice insurance rates. We look more closely at ObGyn malpractice issues in a 3-part “What’s the Verdict” series over the next few months.
In part 1, we discuss the background on malpractice and reasons why malpractice rates have been so high—including large verdicts and lawsuit-prone physicians. In the second part we will look at recent experience and developments in malpractice exposure—who is sued and why. Finally, in the third part we will consider suggestions for reducing the likelihood of a malpractice lawsuit, with a special focus on recent research regarding apologies.
Two reports of recent trials involving ObGyn care illustrate the risk of “the big verdict.”1,2 (Note that the following vignettes are drawn from actual cases but are outlines of those cases and not complete descriptions of the claims. Because the information does not come from formal court records, the facts may be inaccurate and are incomplete; they should be viewed as illustrations only.)
CASE 1 Delayed delivery, $19M verdict
At 39 weeks’ gestation, a woman was admitted to the hospital in spontaneous labor. Artificial rupture of membranes with clear amniotic fluid was noted. Active contractions occurred for 11 hours. Oxytocin was then initiated, and 17 minutes later, profound fetal bradycardia was detected. There was recurrent evidence of fetal distress with meconium. After a nursing staff change a second nurse restarted oxytocin for a prolonged period. The physician allowed labor to continue despite fetal distress, and performed a cesarean delivery (CD) 4.5 hours later. Five hours postdelivery the neonate was noted to have a pneumothorax, lung damage, and respiratory failure. The infant died at 18 days of age.
The jury felt that there was negligence—failure to timely diagnose fetal distress and failure to timely perform CD, all of which resulted in a verdict for the plaintiff. The jury awarded in excess of $19 million.1
CASE 2 An undiagnosed tumor, $20M verdict
A patient underwent bilateral mastectomy. Following surgery, she reported pain and swelling at the surgical site for 2 years, and the defendant physician “dismissed” her complaint, refusing to evaluate it as the provider felt it was related to scar tissue. Three years after the mastectomies, the patient underwent surgical exploration and removal of 3 ribs and sternum secondary to a desmoid tumor. Surgical mesh and chest reconstruction was required, necessitating long-term opioids and sleeping medications that “will slow her wits, dull her senses and limit activities of daily living.” Of note, discrepancies were found in the medical records maintained by the defendant. (There was, for example, no report in the record of the plaintiff’s pain until late in the process.) The plaintiff based her claim on the fact that her pain and lump were neither evaluated nor discovered until it was too late.
The jury awarded $20 million. The verdict was reduced to $2 million by the court based on state statutory limits on malpractice damages.2,3

Continue to: Medical malpractice: Evolution of a standard of care...
Medical malpractice: Evolution of a standard of care
Medical malpractice is not a modern invention. Some historians trace malpractice to the Code of Hammurabi (2030 BC), through Roman law,4 into English common law.5 It was sufficiently established by 1765 that the classic legal treatise of the century referred to medical malpractice.6,7 Although medical malpractice existed for a long time, actual malpractice cases were relatively rare before the last half of the 20th century.8
Defensive medicine born out of necessity. The number of malpractice cases increased substantially—described as a “geometric increase”—after 1960, with a 300% rise between 1965 and 1970.7,9 This “malpractice maelstrom of the 70s”7 resulted in dramatic increases in malpractice insurance costs and invited the practice of defensive medicine—medically unnecessary or unjustified tests and services.10Although there is controversy about what is defensive medicine and what is reasonably cautious medicine, the practice may account for 3% of total health care spending.11 Mello and others have estimated that there may be a $55 billion annual cost related to the medical malpractice system.12
Several malpractice crises and waves of malpractice or tort reform ensued,13 beginning in the 1970s and extending into the 2000s.11 Malpractice law is primarily a matter of state law, so reform essentially has been at the state level—as we will see in the second part in this series.
Defining a standard of care
Medical malpractice is the application of standard legal principles to medical practice. Those principles generally are torts (intentional torts and negligence), and sometimes contracts.14 Eventually, medical malpractice came to focus primarily on negligence. The legal purposes of imposing negligence liability are compensation (to repay the plaintiff the costs of the harm caused by the defendant) and deterrence (to discourage careless conduct that can harm others.)
Negligence is essentially carelessness that falls below the acceptable standard of care. Negligence may arise, for example, from15:
- doing something (giving a drug to a patient with a known allergy to it)
- not doing something (failing to test for a possible tumor, as in the second case above)
- not giving appropriate informed consent
- failing to conduct an adequate examination
- abandoning a patient
- failing to refer a patient to a specialist (or conduct a consultation).
(In recent years, law reforms directed specifically at medical malpractice have somewhat separated medical malpractice from other tort law.)
In malpractice cases, the core question is whether the provider did (or did not) do something that a reasonably careful physician would have done. It is axiomatic that not all bad outcomes are negligent. Indeed, not all mistakes are negligent—only the mistakes that were unreasonable given all of the circumstances. In the first case above, for example, given all of the facts that preceded it, the delay of the physician for 4.5 hours after the fetal distress started was, as seen by the jury, not just a mistake but an unreasonable mistake. Hence, it was negligent. In the second case, the failure to investigate the pain and swelling in the surgical site for 2 years (or failure to refer the patient to another physician) was seen by the jury as an unreasonable mistake—one that would not have been made by a reasonably careful practitioner.
Continue to: The big verdict...
The big verdict
Everyone—every professional providing service, every manufacturer, every driver—eventually will make an unreasonable mistake (ie, commit negligence). If that negligence results in harming someone else, our standard legal response is that the negligent person should be financially responsible for the harm to the other. So, a driver who fails to stop at a red light and hits another car is responsible for those damages. But the damages may vary—perhaps a banged-up fender, or, in another instance, with the same negligence, perhaps terrible personal injuries that will disable the other driver for life. Thus, the damages can vary for the same level of carelessness. The “big verdict” may therefore fall on someone who was not especially careless.
Big verdicts often involve long-term care. The opening case vignettes illustrate a concern of medical malpractice generally—especially for ObGyn practice—the very high verdict. Very high verdicts generally reflect catastrophic damages that will continue for a long time. Bixenstine and colleagues found, for example, that catastrophic payouts often involved “patient age less than 1 year, quadriplegia, brain damage, or lifelong care.”16 In the case of serious injuries during delivery, for example, the harm to the child may last a lifetime and require years and years of intensive medical services.
Million-dollar-plus payouts are on the rise. The percentage of paid claims (through settlement or trial) that are above $1 million is increasing. These million-dollar cases represent 36% of the total dollars paid in ObGyn malpractice claims, even though they represent only 8% of the number of claims paid.16 The increase in the big verdict cases (above $1 million) suggests that ObGyn practitioners should consider their malpractice policy limits—a million dollars may not be enough.
In big verdict cases, the great harm to the plaintiff is often combined with facts that produce extraordinary sympathy for the plaintiff. Sometimes there is decidedly unsympathetic conduct by the defendant as well. In the second case, for example, the problems with the medical record may have suggested to the jury that the doctor was either trying to hide something or did not care enough about the patient even to note a serious complaint. In a case we reviewed in an earlier “What’s the Verdict” column, a physician left the room for several minutes during a critical time—to take a call from a stockbroker.16-18
The big verdict does not necessarily suggest that the defendant was especially or grossly negligent.16 It was a bad injury that occurred, for instance. On the other hand, the physician with several malpractice judgments may suggest that this is a problem physician.
Physicians facing multiple lawsuits are the exceptions
A number of studies have demonstrated that only a small proportion of physicians are responsible for a disproportionate number of paid medical malpractice claims. (“Paid claims” are those in which the plaintiff receives money from the doctor’s insurance. “Filed claims” are all malpractice lawsuits filed. Many claims are filed, but few are paid.)
ObGyn has high number of paid claims and high risk of claim payment recurrence. Studdert and colleagues found that the probability of future paid malpractice climbed with each past paid claim.19 They also found that 1% of physicians accounted for 32% of all paid claims. The number of paid claims varied by specialty—obstetrics and gynecology accounted for the second largest number of paid claims (13%). The risk of recurrence (more than one paid claim) was highest among 4 surgical specialties and ObGyns (about double the recurrence rate in these specialties compared with internal medicine).19
A minority of physicians responsible for lion share of paid claims. Black and colleagues followed up the Studdert study. Although there were some differences in what they found, the results were very similar.20 For example, they found that having even a single prior paid claim strongly predicted future claims over the next 5 years. They also found that some “outlier” physicians with multiple paid claims “are responsible for a significant share of paid claims.” They specifically found that, even for physicians in high-risk specialties in high-risk states, “bad luck is highly unlikely to explain” multiple claims within 5 years.
Continue to: Both of the studies just mentioned relied on...
Both of the studies just mentioned relied on the National Practitioner Data Bank for information about paid claims. This source has some limitations in capturing claims or payments made by hospitals or other institutions for the actions of its agent-physicians. Some of these limitations were resolved in another recent study that looked at Indiana state insurance and licensing discipline records (over a 41-year period).21 Not surprisingly, this study found that claims paid increase with more severe licensure discipline. On the other hand, although, the “frequent fliers” in terms of malpractice claims made and paid could be identified as a “small number of repeat defendants,” these physicians were not routinely disciplined by the state medical board. This was only a single state study, of course, but it also found that a few physicians accounted for a significant number of the claims. The state board was not taking licensing action against this small group, however.
Should the few bad apples be picked from the orchard?
Collectively, these studies are fairly overwhelming in demonstrating that there are some physicians who are “prone” to malpractice claims (for whom all physicians in the specialty are probably paying higher malpractice rates), but who do not attract the attention of licensing agencies for careful examination. In addition to its self-interest in eliminating physicians prone to malpractice claims and payments, the obligation of professions to protect the public interest suggests that state boards should be more aggressive in pursuing those physicians practicing risky medicine.
This medical malpractice series will continue next month with a look at how to reduce malpractice exposure.
Medical malpractice (more formally, professional liability, but we will use the term malpractice) has been of concern to ObGyns for many years, and for good reasons. This specialty has some of the highest incidents of malpractice claims, some of the largest verdicts, and some of the highest malpractice insurance rates. We look more closely at ObGyn malpractice issues in a 3-part “What’s the Verdict” series over the next few months.
In part 1, we discuss the background on malpractice and reasons why malpractice rates have been so high—including large verdicts and lawsuit-prone physicians. In the second part we will look at recent experience and developments in malpractice exposure—who is sued and why. Finally, in the third part we will consider suggestions for reducing the likelihood of a malpractice lawsuit, with a special focus on recent research regarding apologies.
Two reports of recent trials involving ObGyn care illustrate the risk of “the big verdict.”1,2 (Note that the following vignettes are drawn from actual cases but are outlines of those cases and not complete descriptions of the claims. Because the information does not come from formal court records, the facts may be inaccurate and are incomplete; they should be viewed as illustrations only.)
CASE 1 Delayed delivery, $19M verdict
At 39 weeks’ gestation, a woman was admitted to the hospital in spontaneous labor. Artificial rupture of membranes with clear amniotic fluid was noted. Active contractions occurred for 11 hours. Oxytocin was then initiated, and 17 minutes later, profound fetal bradycardia was detected. There was recurrent evidence of fetal distress with meconium. After a nursing staff change a second nurse restarted oxytocin for a prolonged period. The physician allowed labor to continue despite fetal distress, and performed a cesarean delivery (CD) 4.5 hours later. Five hours postdelivery the neonate was noted to have a pneumothorax, lung damage, and respiratory failure. The infant died at 18 days of age.
The jury felt that there was negligence—failure to timely diagnose fetal distress and failure to timely perform CD, all of which resulted in a verdict for the plaintiff. The jury awarded in excess of $19 million.1
CASE 2 An undiagnosed tumor, $20M verdict
A patient underwent bilateral mastectomy. Following surgery, she reported pain and swelling at the surgical site for 2 years, and the defendant physician “dismissed” her complaint, refusing to evaluate it as the provider felt it was related to scar tissue. Three years after the mastectomies, the patient underwent surgical exploration and removal of 3 ribs and sternum secondary to a desmoid tumor. Surgical mesh and chest reconstruction was required, necessitating long-term opioids and sleeping medications that “will slow her wits, dull her senses and limit activities of daily living.” Of note, discrepancies were found in the medical records maintained by the defendant. (There was, for example, no report in the record of the plaintiff’s pain until late in the process.) The plaintiff based her claim on the fact that her pain and lump were neither evaluated nor discovered until it was too late.
The jury awarded $20 million. The verdict was reduced to $2 million by the court based on state statutory limits on malpractice damages.2,3

Continue to: Medical malpractice: Evolution of a standard of care...
Medical malpractice: Evolution of a standard of care
Medical malpractice is not a modern invention. Some historians trace malpractice to the Code of Hammurabi (2030 BC), through Roman law,4 into English common law.5 It was sufficiently established by 1765 that the classic legal treatise of the century referred to medical malpractice.6,7 Although medical malpractice existed for a long time, actual malpractice cases were relatively rare before the last half of the 20th century.8
Defensive medicine born out of necessity. The number of malpractice cases increased substantially—described as a “geometric increase”—after 1960, with a 300% rise between 1965 and 1970.7,9 This “malpractice maelstrom of the 70s”7 resulted in dramatic increases in malpractice insurance costs and invited the practice of defensive medicine—medically unnecessary or unjustified tests and services.10Although there is controversy about what is defensive medicine and what is reasonably cautious medicine, the practice may account for 3% of total health care spending.11 Mello and others have estimated that there may be a $55 billion annual cost related to the medical malpractice system.12
Several malpractice crises and waves of malpractice or tort reform ensued,13 beginning in the 1970s and extending into the 2000s.11 Malpractice law is primarily a matter of state law, so reform essentially has been at the state level—as we will see in the second part in this series.
Defining a standard of care
Medical malpractice is the application of standard legal principles to medical practice. Those principles generally are torts (intentional torts and negligence), and sometimes contracts.14 Eventually, medical malpractice came to focus primarily on negligence. The legal purposes of imposing negligence liability are compensation (to repay the plaintiff the costs of the harm caused by the defendant) and deterrence (to discourage careless conduct that can harm others.)
Negligence is essentially carelessness that falls below the acceptable standard of care. Negligence may arise, for example, from15:
- doing something (giving a drug to a patient with a known allergy to it)
- not doing something (failing to test for a possible tumor, as in the second case above)
- not giving appropriate informed consent
- failing to conduct an adequate examination
- abandoning a patient
- failing to refer a patient to a specialist (or conduct a consultation).
(In recent years, law reforms directed specifically at medical malpractice have somewhat separated medical malpractice from other tort law.)
In malpractice cases, the core question is whether the provider did (or did not) do something that a reasonably careful physician would have done. It is axiomatic that not all bad outcomes are negligent. Indeed, not all mistakes are negligent—only the mistakes that were unreasonable given all of the circumstances. In the first case above, for example, given all of the facts that preceded it, the delay of the physician for 4.5 hours after the fetal distress started was, as seen by the jury, not just a mistake but an unreasonable mistake. Hence, it was negligent. In the second case, the failure to investigate the pain and swelling in the surgical site for 2 years (or failure to refer the patient to another physician) was seen by the jury as an unreasonable mistake—one that would not have been made by a reasonably careful practitioner.
Continue to: The big verdict...
The big verdict
Everyone—every professional providing service, every manufacturer, every driver—eventually will make an unreasonable mistake (ie, commit negligence). If that negligence results in harming someone else, our standard legal response is that the negligent person should be financially responsible for the harm to the other. So, a driver who fails to stop at a red light and hits another car is responsible for those damages. But the damages may vary—perhaps a banged-up fender, or, in another instance, with the same negligence, perhaps terrible personal injuries that will disable the other driver for life. Thus, the damages can vary for the same level of carelessness. The “big verdict” may therefore fall on someone who was not especially careless.
Big verdicts often involve long-term care. The opening case vignettes illustrate a concern of medical malpractice generally—especially for ObGyn practice—the very high verdict. Very high verdicts generally reflect catastrophic damages that will continue for a long time. Bixenstine and colleagues found, for example, that catastrophic payouts often involved “patient age less than 1 year, quadriplegia, brain damage, or lifelong care.”16 In the case of serious injuries during delivery, for example, the harm to the child may last a lifetime and require years and years of intensive medical services.
Million-dollar-plus payouts are on the rise. The percentage of paid claims (through settlement or trial) that are above $1 million is increasing. These million-dollar cases represent 36% of the total dollars paid in ObGyn malpractice claims, even though they represent only 8% of the number of claims paid.16 The increase in the big verdict cases (above $1 million) suggests that ObGyn practitioners should consider their malpractice policy limits—a million dollars may not be enough.
In big verdict cases, the great harm to the plaintiff is often combined with facts that produce extraordinary sympathy for the plaintiff. Sometimes there is decidedly unsympathetic conduct by the defendant as well. In the second case, for example, the problems with the medical record may have suggested to the jury that the doctor was either trying to hide something or did not care enough about the patient even to note a serious complaint. In a case we reviewed in an earlier “What’s the Verdict” column, a physician left the room for several minutes during a critical time—to take a call from a stockbroker.16-18
The big verdict does not necessarily suggest that the defendant was especially or grossly negligent.16 It was a bad injury that occurred, for instance. On the other hand, the physician with several malpractice judgments may suggest that this is a problem physician.
Physicians facing multiple lawsuits are the exceptions
A number of studies have demonstrated that only a small proportion of physicians are responsible for a disproportionate number of paid medical malpractice claims. (“Paid claims” are those in which the plaintiff receives money from the doctor’s insurance. “Filed claims” are all malpractice lawsuits filed. Many claims are filed, but few are paid.)
ObGyn has high number of paid claims and high risk of claim payment recurrence. Studdert and colleagues found that the probability of future paid malpractice climbed with each past paid claim.19 They also found that 1% of physicians accounted for 32% of all paid claims. The number of paid claims varied by specialty—obstetrics and gynecology accounted for the second largest number of paid claims (13%). The risk of recurrence (more than one paid claim) was highest among 4 surgical specialties and ObGyns (about double the recurrence rate in these specialties compared with internal medicine).19
A minority of physicians responsible for lion share of paid claims. Black and colleagues followed up the Studdert study. Although there were some differences in what they found, the results were very similar.20 For example, they found that having even a single prior paid claim strongly predicted future claims over the next 5 years. They also found that some “outlier” physicians with multiple paid claims “are responsible for a significant share of paid claims.” They specifically found that, even for physicians in high-risk specialties in high-risk states, “bad luck is highly unlikely to explain” multiple claims within 5 years.
Continue to: Both of the studies just mentioned relied on...
Both of the studies just mentioned relied on the National Practitioner Data Bank for information about paid claims. This source has some limitations in capturing claims or payments made by hospitals or other institutions for the actions of its agent-physicians. Some of these limitations were resolved in another recent study that looked at Indiana state insurance and licensing discipline records (over a 41-year period).21 Not surprisingly, this study found that claims paid increase with more severe licensure discipline. On the other hand, although, the “frequent fliers” in terms of malpractice claims made and paid could be identified as a “small number of repeat defendants,” these physicians were not routinely disciplined by the state medical board. This was only a single state study, of course, but it also found that a few physicians accounted for a significant number of the claims. The state board was not taking licensing action against this small group, however.
Should the few bad apples be picked from the orchard?
Collectively, these studies are fairly overwhelming in demonstrating that there are some physicians who are “prone” to malpractice claims (for whom all physicians in the specialty are probably paying higher malpractice rates), but who do not attract the attention of licensing agencies for careful examination. In addition to its self-interest in eliminating physicians prone to malpractice claims and payments, the obligation of professions to protect the public interest suggests that state boards should be more aggressive in pursuing those physicians practicing risky medicine.
This medical malpractice series will continue next month with a look at how to reduce malpractice exposure.
- Delivery delay blamed for baby’s death days later—$19.2 million Illinois verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35:17.
- Failure to identify signs of a growing tumor—$20 million Virginia verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35:18.
- Hellinger FJ, Encinosa WE. The impact of state laws limiting malpractice damage awards on health care expenditures. Am J Public Health. 2006;96:1375-1381.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339–347.
- Everad v. Hopkins, 80 English Reports 1164 (1615).
- Blackstone W. Commentaries on the laws of England. Oxford, England: Clarendon Press; 1768:122.
- Berlin L. Medical errors, malpractice, and defensive medicine: an ill-fated triad. Diagnosis (Berl). 2017;4:133-139.
- DeVille KA. Medical Malpractice in Nineteenth-Century America: Origins and Legacy. New York, NY: NYU Press; 1990.
- Hershey N. The defensive practice of medicine. Myth or reality. Milbank Mem Fund Q. 1972;50:69-98.
- Agarwal R, Gupta A, Gupta S. The impact of tort reform on defensive medicine, quality of care, and physician supply: a systematic review. Health Serv Res. 2019;54:851-859.
- Gerlach J, Abodunde B, Sollosy M, et al. Rethinking the obvious: time for new ideas on medical malpractice tort reform. Health Care Manag (Frederick). 2019;38:109-115.
- Mello MM, Chandra A, Gawande AA, et al. National costs of the medical liability system. Health Aff. 2010;29:1569-1577.
- Bovbjerg RR. Malpractice crisis and reform. Clin Perinatol. 2005;32:203-233, viii-ix.
- Hawkins v. McGee, 84 N.H. 114, 146 A. 641 (1929).
- Gittler GJ, Goldstein EJ. The elements of medical malpractice: an overview. Clin Infect Dis. 1996;23:1152–1155.
- Bixenstine PJ, Shore AD, Mehtsun WT, et al. Catastrophic medical malpractice payouts in the United States. J Healthc Quality. 2014;36:43-53.
- Sanfilippo JS, Smith SR. Lessons from a daunting malpractice event. OBG Manag. 2018;30:41-47.
- Chang D. Miami doctor hit with $33 million judgment in brain-damaged baby suit. Miami Herald. April 28, 2017. http://www.miamiherald.com/news/health-care/ article147506019.html. Accessed December 12, 2019.
- Studdert DM, Bismark MM, Mello MM, et al. Prevalence and characteristics of physicians prone to malpractice claims. N Engl J Med. 2016;374:354-362.
- Black B, Hyman DA, Lerner JY. Physicians with multiple paid medical malpractice claims: Are they outliers or just unlucky? Int Rev Law Econ. 2019;59:146-157.
- Liu J, Hyman DA. Targeting bad doctors: lessons from Indiana, 1975–2015. J Empirical Legal Studies. 2019;16: 248-328.
- Delivery delay blamed for baby’s death days later—$19.2 million Illinois verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35:17.
- Failure to identify signs of a growing tumor—$20 million Virginia verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35:18.
- Hellinger FJ, Encinosa WE. The impact of state laws limiting malpractice damage awards on health care expenditures. Am J Public Health. 2006;96:1375-1381.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339–347.
- Everad v. Hopkins, 80 English Reports 1164 (1615).
- Blackstone W. Commentaries on the laws of England. Oxford, England: Clarendon Press; 1768:122.
- Berlin L. Medical errors, malpractice, and defensive medicine: an ill-fated triad. Diagnosis (Berl). 2017;4:133-139.
- DeVille KA. Medical Malpractice in Nineteenth-Century America: Origins and Legacy. New York, NY: NYU Press; 1990.
- Hershey N. The defensive practice of medicine. Myth or reality. Milbank Mem Fund Q. 1972;50:69-98.
- Agarwal R, Gupta A, Gupta S. The impact of tort reform on defensive medicine, quality of care, and physician supply: a systematic review. Health Serv Res. 2019;54:851-859.
- Gerlach J, Abodunde B, Sollosy M, et al. Rethinking the obvious: time for new ideas on medical malpractice tort reform. Health Care Manag (Frederick). 2019;38:109-115.
- Mello MM, Chandra A, Gawande AA, et al. National costs of the medical liability system. Health Aff. 2010;29:1569-1577.
- Bovbjerg RR. Malpractice crisis and reform. Clin Perinatol. 2005;32:203-233, viii-ix.
- Hawkins v. McGee, 84 N.H. 114, 146 A. 641 (1929).
- Gittler GJ, Goldstein EJ. The elements of medical malpractice: an overview. Clin Infect Dis. 1996;23:1152–1155.
- Bixenstine PJ, Shore AD, Mehtsun WT, et al. Catastrophic medical malpractice payouts in the United States. J Healthc Quality. 2014;36:43-53.
- Sanfilippo JS, Smith SR. Lessons from a daunting malpractice event. OBG Manag. 2018;30:41-47.
- Chang D. Miami doctor hit with $33 million judgment in brain-damaged baby suit. Miami Herald. April 28, 2017. http://www.miamiherald.com/news/health-care/ article147506019.html. Accessed December 12, 2019.
- Studdert DM, Bismark MM, Mello MM, et al. Prevalence and characteristics of physicians prone to malpractice claims. N Engl J Med. 2016;374:354-362.
- Black B, Hyman DA, Lerner JY. Physicians with multiple paid medical malpractice claims: Are they outliers or just unlucky? Int Rev Law Econ. 2019;59:146-157.
- Liu J, Hyman DA. Targeting bad doctors: lessons from Indiana, 1975–2015. J Empirical Legal Studies. 2019;16: 248-328.
What every ObGyn should know about Supreme Court rulings in the recent term
The most recently concluded term of the US Supreme Court, which began on October 1, 2018, yielded a number of decisions of interest to health care professionals and to ObGyns in particular. Although the term was viewed by some observers as less consequential than other recent terms, a review of the cases decided paints a picture of a more important term than some commentators expected.
When the term began, the Court had only 8 justices—1 short of a full bench: Judge Brett Kavanaugh had not yet been confirmed by the Senate. He was confirmed on October 6, by a 50-48 vote, and Justice Kavanaugh immediately joined the Court and began to hear and decide cases.
Increasingly, important decisions affect medical practice
From the nature of practice (abortion), to payment for service (Medicare reimbursement), resolution of disputes (arbitration), and fraud and abuse (the federal False Claims Act), the decisions of the Court will have an impact on many areas of medical practice. Organized medicine increasingly has recognized the significance of the work of the Court; nowhere has this been more clearly demonstrated than with amicus curiae (friend of the court) briefs filed by medical organizations.
Amicus curiae briefs. These briefs are filed by persons or organizations not a party to a case the Court is hearing. Their legitimate purpose is to inform the Court of 1) special information within the expertise of the amicus (or amici, plural) or 2) consequences of the decision that might not be apparent from arguments made by the parties to the case. Sometimes, the Court cites amicus briefs for having provided important information about the case.
Filing amicus briefs is time-consuming and expensive; organizations do not file them for trivial reasons. Organizations frequently join together to file a joint brief, to share expenses and express to the Court a stronger position.
Three categories of health professionals file amicus briefs in ObGyn-related cases:
- Major national organizations, often representing broad interests of health care professions or institutions (the American Medical Association [AMA], the Association of American Medical Colleges, and the American Hospital Association [AHA]), have filed a number of amicus briefs over the years.
- Specialty boards increasingly file amicus briefs. For example, the American College of Obstetricians and Gynecologists (ACOG) and the American Society for Reproductive Medicine have filed briefs related to abortion issues.
- In reproductive issues, the American Association of Pro-Life Obstetricians and Gynecologists, the American College of Pediatricians, and the Christian Medical & Dental Associations have been active amicus filers—frequently taking positions different than, even inconsistent with, amicus briefs filed by major specialty boards.
Amicus briefs filed by medical associations provide strong clues to what is important to clinicians. We have looked at such briefs to help us identify topics and cases from the just-concluded term that can be of particular interest to you.
Continue to: Surveying the shadow docket...
Surveying the shadow docket. As part of our review of the past term, we also looked at the so-called shadow docket, which includes decisions regarding writs of certiorari (which cases it agrees to hear); stays (usually delaying implementation of a law); or denials of stays. (Persuading the Court to hear a case is not easy: It hears approximately 70 cases per year out of as many as 7,000 applications to be heard.)
Abortion ruling
At stake. A number of states recently enacted a variety of provisions that might make an abortion more difficult to obtain. Some of the cases challenging these restrictions are making their way through lower courts, and one day might be argued before the Supreme Court. However, the Court has not (yet) agreed to hear the substance of many new abortion-related provisions.
Box v Planned Parenthood of Indiana and Kentucky, Inc.
The Court decided only 1 abortion restriction case this term.1 The Indiana law in question included 2 provisions that the Court considered:
Disposal of remains. The law regulated the manner in which abortion providers can dispose of fetal remains (ie, they cannot be treated as “infectious and pathologic waste”).
Motivation for seeking abortion. The Indiana law makes it illegal for an abortion provider to perform an abortion when the provider knows that the mother is seeking that abortion “solely” because of the fetus’s race, sex, diagnosis of Down syndrome, disability, or related characteristics.
Final rulings. The Court held that the disposal-of-remains provision is constitutional. The provision is “rationally related to the state’s interest in proper disposal of fetal remains.”2 Planned Parenthood had not raised the issue of whether the law might impose an undue burden on a woman’s right to obtain an abortion, so the Court did not decide that issue.
The Court did not consider the constitutionality of the part of the law proscribing certain reasons for seeking an otherwise legal abortion; instead, it awaits lower courts’ review of the issue. Justice Clarence Thomas wrote an extensive concurring opinion suggesting that this law is intended to avoid abortion to achieve eugenic goals.3
Key developments from the shadow docket
The Court issued a stay preventing a Louisiana statute that requires physicians who perform abortions to have admitting privileges at a nearby hospital from going into effect, pending the outcome of litigation about that law.4 Four dissenters noted that all 4 physicians who perform abortions in Louisiana have such privileges. Chief Justice Roberts was the fifth vote to grant the stay. This case likely will make its way back to the Court, as will a number of other state laws being adopted. The issue may be back as soon as the term just starting.
The Court is also considering whether to take another Indiana case, Box v Planned Parenthood of Indiana and Kentucky, Inc. (Box II). This case involves an Indiana ultrasonography viewing option as part of the abortion consent process.5
The Court declined to hear cases from Louisiana and Kansas in which the states had cut off Medicaid funding to Planned Parenthood. Lower courts had stopped the implementation of those laws.6 The legal issue was whether private parties, as opposed to the federal government, had standing to bring the case. For now, the decision of the lower courts to stop implementation of the funding cutoff is in effect. There is a split in the Circuit Courts on the issue, however, making it likely that the Supreme Court will have to resolve it sooner or later.
Health care organizations have filed a number of amicus briefs in these and other cases involving new abortion regulations. ACOG and others filed a brief opposing a Louisiana law that requires abortion providers to have admitting privileges at a nearby facility,7 and a brief opposing a similar Oklahoma law.8 The Association of Pro-Life Obstetricians and Gynecologists and others filed amicus curiae briefs in Box II9 and in an Alabama case involving so-called dismemberment abortion.10
Continue to: Medicare payments...
Medicare payments
Azar, Secretary of Health and Human Services v Allina Health Services, et al11
This case drew interest—and many amicus briefs—from health care providers, including the AMA and the AHA.12,13 There was good reason for their interest: First, the case involved more than $3 billion in reimbursements; second, it represented a potentially important precedent about the rights of providers and patients to comment on Medicare reimbursement changes. The question involved the technical calculation of additional payments made to institutions that serve a disproportionate number of low-income patients (known as Medicare Fractions).
At stake. The issue was a statutory requirement for a 60-day public notice and comment period for rules that “change a substantive legal standard” governing the scope of benefits, eligibility, or payment for services.14 In 2014, the Secretary of the Department of Health and Human Services (HHS) in the Obama administration posted a spreadsheet announcing Medicare fractions rates for hospitals (for 2012)—without formal notice or comment regarding the formula used. (The spreadsheet listed what each qualifying institution would receive, but it was based on a formula that, as noted, had not been subject to public notice and comment.) The AMA and AHA briefs emphasized the importance of a notice and comment period, especially when Medicare reimbursement is involved.
Final ruling. The Court held that the HHS process violated the notice and comment provision, thereby invalidating the policy underlying the so-called spreadsheet reimbursement. The decision was significant: This was a careful statutory interpretation of the 60-day notice and comment period, not the reimbursement policy itself. Presumably, had the HHS Secretary provided for sufficient notice and comment, the formula used would have met the requirements for issuing reimbursement formulas.
Key points. Hospitals will collectively receive $3 or $4 billion as a consequence of the ruling. Perhaps more importantly, the decision signals that HHS is going to have to take seriously the requirement that it publish Medicare-related reimbursement policies for the 60-day period.
A number of diverse cases ruled on by the Supreme Court are worth mentioning. The Court:
- allowed the President to move various funds from the US Department of Defense into accounts from which the money could be used to build a portion of a wall along the southern US border.1
- essentially killed the "citizenship question" on the census form. Technically, the Court sent the issue back to the Commerce Department for better justification for including the question (the announced reasons appeared to be pretextual).2
- changed, perhaps substantially, the deference that courts give to federal agencies in interpreting regulations.3
- upheld, in 2 cases, treaty rights of Native Americans to special treatment on Indian Lands4,5; the Court held that treaties ordinarily should be interpreted as the tribe understood them at the time they were signed. (These were 5 to 4 decisions; the split in the Court leaves many unanswered questions.)
- made it easier for landowners to file suit in federal court when they claim that the state has "taken" their property without just compensation.6
- held that a refusal of the US Patent and Trademark Office to register "immoral" or "scandalous" trademarks infringes on the First Amendment. (The petitioner sought to register "FUCT" as a trademark for a line of clothing.)7
- allowed an antitrust case by iPhone users against Apple to go forward. At issue: the claim that Apple monopolizes the retail market for apps by requiring buyers to obtain apps from Apple.8
- held that, if a drunk-driving suspect who has been taken into custody is, or becomes, unconscious, the "reasonable search" provision of the Fourth Amendment generally does not prevent a state from taking a blood specimen without a warrant. (Wisconsin had a specific "implied consent" law, by which someone receiving a driving license consents to a blood draw.9)
- decided numerous capital punishment cases. In many ways, this term seemed to be a "capital term." Issues involved in these cases have split the Court; it is reasonable to expect that the divide will endure through upcoming terms.
References
- Donald J. Trump, President of the United States, et al. v Sierra Club, et al. 588 US 19A60 (2019).
- Department of Commerce et al. v New York et al. 18 996 (2018).
- Kisor v Wilkie, Secretary of Veterans Affairs. 18 15 (2018).
- Washington State Department of Licensing v Cougar Den, Inc. 16 1498 (2018).
- Herrera v Wyoming. 17 532 (2018).
- Knick v Township of Scott, Pennsylvania, et al. 17 647 (2018).
- Iancu, Under Secretary of Commerce for Intellectual Property and Director, Patent and Trademark Office v Brunetti. 18 302 (2018).
- Apple Inc. v Pepper et al. 17 204 (2018).
- Mitchell v Wisconsin. 18 6210 (2018).
Liability under the False Claims Act
The False Claims Act (FCA) protects the federal government from fraudulent claims for payment and for shoddy goods and services. It incentivizes (by a percentage of recovery) private parties to bring cases to enforce the law.15 (Of course, the federal government also enforces the Act.)
At stake. The FCA has been of considerable concern to the AHA, the Association of American Medical Colleges, and other health care organizations—understandably so.16 As the AHA informed the Court in an amicus brief, “The prevalence of [FCA] cases has ballooned over the past three decades.... These suits disproportionately target healthcare entities.... Of the 767 new FCA cases filed in 2018, for example, 506 involved healthcare defendants.”17
Final ruling. The Court considered an ambiguity in the statute of limitations for these actions and the Court unanimously ruled to permit an extended time in which qui tam actions (private actions under the law) can be filed.18
Key points. As long a period as 10 years can pass between the time an FCA violation occurs and an action is brought. This decision is likely to increase the number of FCA actions against health care providers because the case can be filed many years after the conduct that gave rise to the complaint.
Continue to: Registering sex offenders...
Registering sex offenders
The Court upheld the constitutionality of the federal Sex Offender Registration and Notification Act (SORNA).19 Sex offenders must register and periodically report, in person, to law enforcement in every state in which the offender works, studies, or resides.
At stake. The case involved the applicability of SORNA registration obligations to those convicted of sex offenses before SORNA was adopted (pre-Act offenders).20 The court upheld registration requirements for pre-Act offenders.
Former Justice Stevens, the longest-living and third-longest-serving Supreme Court justice, died in July 2019 at 99 years of age. He was appointed to the Court in 1975 by President Ford and served until his retirement in 2010, when he was 90. Stevens had recently published a memoir, The Making of a Justice: Reflections on My First 94 Years.
Stevens's judicial philosophy generally is described as having changed over the course of his 35 years of service: He was viewed as becoming more liberal. He was a justice of enduring kindness and integrity. It is possible to find people who disagree with him, but almost impossible to find anyone who disliked him. He was continuously committed to the law and justice in the United States.
Arbitration
The Court continued its practice of deciding at least one case each term that emphasizes that federal law requires that courts rather strictly enforce agreements to arbitrate (instead of to litigate) future disputes.21 In another case, the Court ruled that there can be “class” or “joint” arbitration only if the agreement to arbitrate a dispute clearly permits such class arbitration.22
Pharma’s liability regarding product risk
The Court somewhat limited the liability of pharmaceutical companies for failing to provide adequate warning about the risk that their products pose. The case against Merck involved 500 patients who took denosumab (Fosamax) and suffered atypical femoral fractures.23
At stake. Because prescribing information (in which warnings are provided) must be approved by the US Food and Drug Administration (FDA), the legal test is: Would the FDA have refused to approve a change in the warning if Merck had “fully informed the FDA of the justifications for the warning” required by state law to avoid liability?24,25 Lower-court judges (not juries) will be expected to apply this test in the future.
The doctor and the death penalty
The Court has established a rule that, when a prisoner facing capital punishment objects to a form of execution because it is too painful, he has to propose an alternative that is reasonably available. In one case,26 a physician, an expert witness for the prisoner, did not answer some essential relative-pain questions (ie, would one procedure be more painful than another?).
At stake. The AMA filed an amicus brief in this case, indicating that it is unethical for physicians to participate in an execution. The brief noted that “testimony used to determine which method of execution would reduce physical suffering would constitute physician participation in capital punishment and would be unethical.”27
The expert witness’s failure to answer the question on relative pain had the unfortunate result of reducing the likelihood that the prisoner would prevail in his request for an alternative method of execution.
Analysis
Despite obvious disagreements about big issues (notably, abortion and the death penalty) the Court maintained a courteous and civil demeanor—something not always seen nowadays in other branches of government. Here are facts about the Court’s term just concluded:
- The Court issued 72 merits opinions (about average).
- Only 39% of decisions were unanimous (compared with the average of 49% in recent terms).
- On the other hand, 26% of decisions were split 5 to 4 (compared with a 10% recent average).
- In those 5 to 4 decisions, Justices were in the majority as follows28: Justice Gorsuch, 65%; Justice Kavanaugh, 61%; Justice Thomas, 60%; Chief Justice Roberts and Justices Ginsburg and Alito, each 55%; Justice Breyer, 50%; and Justices Sotomayor and Kagan each at 45%.
- There were 57 dissenting opinions—up from 48 in the previous term.
- What is referred to as “the liberal-conservative split” might seem more profound than it really is: “Every conservative member of the court at some point voted to form a majority with the liberal justices. And every liberal at least once left behind all of his or her usual voting partners to join the conservatives.”29
Continue to: Last, it was a year of personal health issues for...
Last, it was a year of personal health issues for the Court: Justice Ginsburg had a diagnosis of lung cancer and was absent, following surgery, in January. Of retired Justices, Sandra Day O’Connor suffers from dementia and former Justice John Paul Stevens died.
In closing
The Court has accepted approximately 50 cases for the current term, which began on October 7. The first 2 days of the term were spent on arguments about, first, whether a state can abolish the insanity defense and, second, whether nondiscrimination laws (“based on sex”) prohibit discrimination based on sexual orientation or transgender status. Cases also will deal with Patient Protection and Affordable Care Act payments to providers; the Deferred Action for Childhood Arrivals, or DACA; the death penalty; and international child custody disputes. The Court will be accepting more cases for several months. It promises to be a very interesting term.
- Box v Planned Parenthood of Indiana and Kentucky, Inc. 587 US 18 483 (2019).
- Box v Planned Parenthood of Indiana and Kentucky, Inc., at 2.
- Box v Planned Parenthood of Indiana and Kentucky, Inc., Justice Thomas concurring.
- June Medical Services, LLC, et al. v Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals. 586 US 18A774 (2019).
- Box v Planned Parenthood of Indiana and Kentucky, Inc. Docket 18-1019.
- Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals v Planned Parenthood of Gulf Coast, Inc., et al. 586 US 17 1492 (2018).
- June Medical Services L.L.C., et al., Petitioners, v Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals. No. 18-1323. Brief of Amici Curiae American College of Obstetricians and Gynecologists, American Academy of Family Physicians, American Academy of Pediatrics, American College of Nurse-Midwives, American College of Osteopathic Obstetricians and Gynecologists, American College of Physicians, American Society for Reproductive Medicine, National Association of Nurse Practitioners in Women's Health, North American Society for Pediatric and Adolescent Gynecology, and Society For Maternal-Fetal Medicine, Amicus Curiae in Support of Petitioners. May 2019.
- Planned Parenthood of Kansas & Eastern Oklahoma, et al., Petitioners, v Larry Jegley, et al., Respondents. No. 17-935. Brief Amici Curiae of American College of Obstetricians and Gynecologists and American Public Health Association as Amici Curiae in Support of Petitioners. February 1, 2018.
- Box v Planned Parenthood of Indiana & Kentucky. No. 18-1019. Brief Amici Curiae of American Association of Pro-Life Obstetricians & Gynecologists, American College of Pediatricians, Care Net, Christian Medical Association, Heartbeat International, Inc., and National Institute Of Family & Life Advocates in Support of Petitioners. March 6, 2019.
- Steven T. Marshall, et al., Petitioners, v West Alabama Women's Center, et al., Respondents. No. 18-837. Brief of Amici Curiae American Association of Pro-Life Obstetricians & Gynecologists and American College of Pediatricians, in Support of Petitioners. January 18, 2019.
- Azar, Secretary of Health and Human Services v Allina Health Services, et al. 17 1484 (2018).
- Alex M. Azar, II, Secretary of Health and Human Services, Petitioner, v Allina Health Services, et al., Respondents. Brief of the American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges as Amici Curiae in Support of Respondents. December 2018.
- Alex M. Azar, II, Secretary of Health and Human Services, Petitioner, v Allina Health Services, et al., Respondents. Brief of Amici Curiae American Medical Association and Medical Society of the District of Columbia Amici Curiae in Support of Respondents. December 2018.
- 42 U. S. C. §1395hh. https://uscode.house.gov/view.xhtml?req=(title:42%20section:1395hh%20edition:prelim). Accessed October 22, 2019.
- The False Claims Act: a primer. Washington DC: US Department of Justice. www.justice.gov/sites/default/files/civil/legacy/2011/04/22/C-FRAUDS_FCA_Primer.pdf. Accessed October 18, 2019.
- Universal Health Services, Inc., v United States and Commonwealth of Massachusetts ex rel. Julio Escobar and Carmen Correa. Brief of the American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges Amici Curiae in Support of Petitioner. No. 15-7. January 2016.
- Intermountain Health Care, Inc., et al., Petitioners, v United States ex rel. Gerald Polukoff, et al., Respondents. No. 18-911. Brief of the American Hospital Association and Federation of American Hospitals as Amici Curiae in Support of Petitioners. February 13, 2019.
- Cochise Consultancy, Inc., et al., v United States ex rel. Hunt. 18 315 (2018).
- 34 U.S.C. §20901 et seq. [Chapter 209--Child Protection and Safety.] https://uscode.house.gov/view.xhtml?path=/prelim@title34/subtitle2/chapter209&edition=prelim. Accessed October 17, 2019.
- Gundy v United States. 17 6086 (2018).
- Henry Schein, Inc., et al., v Archer & White Sales, Inc. 17 1272 (2018).
- Lamps Plus, Inc., et al., v Varela. 17 988 (2018).
- Merck Sharp & Dohme Corp. v Albrecht et al. 17 290 (2018).
- Merck Sharp & Dohme Corp. v Albrecht et al. 17 290 (2018) at 13-14.
- Wyeth v Levine, 555 US 555, 571 (2009).
- Russell Bucklew, Petitioner, v Anne L. Precythe, Director, Missouri Department of Corrections, et al., Respondents. 17 8151 (2018).
- Russell Bucklew, Petitioner, v Anne L. Precythe, Director, Missouri Department of Corrections, et al., Respondents. 17 8151 (2018). American Medical Association, Amicus Curiae Brief, in Support of Neither Party. July 23, 2018.
- Final stat pack for October term 2018. SCOTUSblog.com. June 28, 2019. https://www.scotusblog.com/wp-content/uploads/2019/07/StatPack_OT18-7_8_19.pdf. Accessed October 17, 2019.
- Barnes R. They're not 'wonder twins': Gorsuch, Kavanaugh shift the Supreme Court, but their differences are striking. Washington Post, June 28, 2019.
The most recently concluded term of the US Supreme Court, which began on October 1, 2018, yielded a number of decisions of interest to health care professionals and to ObGyns in particular. Although the term was viewed by some observers as less consequential than other recent terms, a review of the cases decided paints a picture of a more important term than some commentators expected.
When the term began, the Court had only 8 justices—1 short of a full bench: Judge Brett Kavanaugh had not yet been confirmed by the Senate. He was confirmed on October 6, by a 50-48 vote, and Justice Kavanaugh immediately joined the Court and began to hear and decide cases.
Increasingly, important decisions affect medical practice
From the nature of practice (abortion), to payment for service (Medicare reimbursement), resolution of disputes (arbitration), and fraud and abuse (the federal False Claims Act), the decisions of the Court will have an impact on many areas of medical practice. Organized medicine increasingly has recognized the significance of the work of the Court; nowhere has this been more clearly demonstrated than with amicus curiae (friend of the court) briefs filed by medical organizations.
Amicus curiae briefs. These briefs are filed by persons or organizations not a party to a case the Court is hearing. Their legitimate purpose is to inform the Court of 1) special information within the expertise of the amicus (or amici, plural) or 2) consequences of the decision that might not be apparent from arguments made by the parties to the case. Sometimes, the Court cites amicus briefs for having provided important information about the case.
Filing amicus briefs is time-consuming and expensive; organizations do not file them for trivial reasons. Organizations frequently join together to file a joint brief, to share expenses and express to the Court a stronger position.
Three categories of health professionals file amicus briefs in ObGyn-related cases:
- Major national organizations, often representing broad interests of health care professions or institutions (the American Medical Association [AMA], the Association of American Medical Colleges, and the American Hospital Association [AHA]), have filed a number of amicus briefs over the years.
- Specialty boards increasingly file amicus briefs. For example, the American College of Obstetricians and Gynecologists (ACOG) and the American Society for Reproductive Medicine have filed briefs related to abortion issues.
- In reproductive issues, the American Association of Pro-Life Obstetricians and Gynecologists, the American College of Pediatricians, and the Christian Medical & Dental Associations have been active amicus filers—frequently taking positions different than, even inconsistent with, amicus briefs filed by major specialty boards.
Amicus briefs filed by medical associations provide strong clues to what is important to clinicians. We have looked at such briefs to help us identify topics and cases from the just-concluded term that can be of particular interest to you.
Continue to: Surveying the shadow docket...
Surveying the shadow docket. As part of our review of the past term, we also looked at the so-called shadow docket, which includes decisions regarding writs of certiorari (which cases it agrees to hear); stays (usually delaying implementation of a law); or denials of stays. (Persuading the Court to hear a case is not easy: It hears approximately 70 cases per year out of as many as 7,000 applications to be heard.)
Abortion ruling
At stake. A number of states recently enacted a variety of provisions that might make an abortion more difficult to obtain. Some of the cases challenging these restrictions are making their way through lower courts, and one day might be argued before the Supreme Court. However, the Court has not (yet) agreed to hear the substance of many new abortion-related provisions.
Box v Planned Parenthood of Indiana and Kentucky, Inc.
The Court decided only 1 abortion restriction case this term.1 The Indiana law in question included 2 provisions that the Court considered:
Disposal of remains. The law regulated the manner in which abortion providers can dispose of fetal remains (ie, they cannot be treated as “infectious and pathologic waste”).
Motivation for seeking abortion. The Indiana law makes it illegal for an abortion provider to perform an abortion when the provider knows that the mother is seeking that abortion “solely” because of the fetus’s race, sex, diagnosis of Down syndrome, disability, or related characteristics.
Final rulings. The Court held that the disposal-of-remains provision is constitutional. The provision is “rationally related to the state’s interest in proper disposal of fetal remains.”2 Planned Parenthood had not raised the issue of whether the law might impose an undue burden on a woman’s right to obtain an abortion, so the Court did not decide that issue.
The Court did not consider the constitutionality of the part of the law proscribing certain reasons for seeking an otherwise legal abortion; instead, it awaits lower courts’ review of the issue. Justice Clarence Thomas wrote an extensive concurring opinion suggesting that this law is intended to avoid abortion to achieve eugenic goals.3
Key developments from the shadow docket
The Court issued a stay preventing a Louisiana statute that requires physicians who perform abortions to have admitting privileges at a nearby hospital from going into effect, pending the outcome of litigation about that law.4 Four dissenters noted that all 4 physicians who perform abortions in Louisiana have such privileges. Chief Justice Roberts was the fifth vote to grant the stay. This case likely will make its way back to the Court, as will a number of other state laws being adopted. The issue may be back as soon as the term just starting.
The Court is also considering whether to take another Indiana case, Box v Planned Parenthood of Indiana and Kentucky, Inc. (Box II). This case involves an Indiana ultrasonography viewing option as part of the abortion consent process.5
The Court declined to hear cases from Louisiana and Kansas in which the states had cut off Medicaid funding to Planned Parenthood. Lower courts had stopped the implementation of those laws.6 The legal issue was whether private parties, as opposed to the federal government, had standing to bring the case. For now, the decision of the lower courts to stop implementation of the funding cutoff is in effect. There is a split in the Circuit Courts on the issue, however, making it likely that the Supreme Court will have to resolve it sooner or later.
Health care organizations have filed a number of amicus briefs in these and other cases involving new abortion regulations. ACOG and others filed a brief opposing a Louisiana law that requires abortion providers to have admitting privileges at a nearby facility,7 and a brief opposing a similar Oklahoma law.8 The Association of Pro-Life Obstetricians and Gynecologists and others filed amicus curiae briefs in Box II9 and in an Alabama case involving so-called dismemberment abortion.10
Continue to: Medicare payments...
Medicare payments
Azar, Secretary of Health and Human Services v Allina Health Services, et al11
This case drew interest—and many amicus briefs—from health care providers, including the AMA and the AHA.12,13 There was good reason for their interest: First, the case involved more than $3 billion in reimbursements; second, it represented a potentially important precedent about the rights of providers and patients to comment on Medicare reimbursement changes. The question involved the technical calculation of additional payments made to institutions that serve a disproportionate number of low-income patients (known as Medicare Fractions).
At stake. The issue was a statutory requirement for a 60-day public notice and comment period for rules that “change a substantive legal standard” governing the scope of benefits, eligibility, or payment for services.14 In 2014, the Secretary of the Department of Health and Human Services (HHS) in the Obama administration posted a spreadsheet announcing Medicare fractions rates for hospitals (for 2012)—without formal notice or comment regarding the formula used. (The spreadsheet listed what each qualifying institution would receive, but it was based on a formula that, as noted, had not been subject to public notice and comment.) The AMA and AHA briefs emphasized the importance of a notice and comment period, especially when Medicare reimbursement is involved.
Final ruling. The Court held that the HHS process violated the notice and comment provision, thereby invalidating the policy underlying the so-called spreadsheet reimbursement. The decision was significant: This was a careful statutory interpretation of the 60-day notice and comment period, not the reimbursement policy itself. Presumably, had the HHS Secretary provided for sufficient notice and comment, the formula used would have met the requirements for issuing reimbursement formulas.
Key points. Hospitals will collectively receive $3 or $4 billion as a consequence of the ruling. Perhaps more importantly, the decision signals that HHS is going to have to take seriously the requirement that it publish Medicare-related reimbursement policies for the 60-day period.
A number of diverse cases ruled on by the Supreme Court are worth mentioning. The Court:
- allowed the President to move various funds from the US Department of Defense into accounts from which the money could be used to build a portion of a wall along the southern US border.1
- essentially killed the "citizenship question" on the census form. Technically, the Court sent the issue back to the Commerce Department for better justification for including the question (the announced reasons appeared to be pretextual).2
- changed, perhaps substantially, the deference that courts give to federal agencies in interpreting regulations.3
- upheld, in 2 cases, treaty rights of Native Americans to special treatment on Indian Lands4,5; the Court held that treaties ordinarily should be interpreted as the tribe understood them at the time they were signed. (These were 5 to 4 decisions; the split in the Court leaves many unanswered questions.)
- made it easier for landowners to file suit in federal court when they claim that the state has "taken" their property without just compensation.6
- held that a refusal of the US Patent and Trademark Office to register "immoral" or "scandalous" trademarks infringes on the First Amendment. (The petitioner sought to register "FUCT" as a trademark for a line of clothing.)7
- allowed an antitrust case by iPhone users against Apple to go forward. At issue: the claim that Apple monopolizes the retail market for apps by requiring buyers to obtain apps from Apple.8
- held that, if a drunk-driving suspect who has been taken into custody is, or becomes, unconscious, the "reasonable search" provision of the Fourth Amendment generally does not prevent a state from taking a blood specimen without a warrant. (Wisconsin had a specific "implied consent" law, by which someone receiving a driving license consents to a blood draw.9)
- decided numerous capital punishment cases. In many ways, this term seemed to be a "capital term." Issues involved in these cases have split the Court; it is reasonable to expect that the divide will endure through upcoming terms.
References
- Donald J. Trump, President of the United States, et al. v Sierra Club, et al. 588 US 19A60 (2019).
- Department of Commerce et al. v New York et al. 18 996 (2018).
- Kisor v Wilkie, Secretary of Veterans Affairs. 18 15 (2018).
- Washington State Department of Licensing v Cougar Den, Inc. 16 1498 (2018).
- Herrera v Wyoming. 17 532 (2018).
- Knick v Township of Scott, Pennsylvania, et al. 17 647 (2018).
- Iancu, Under Secretary of Commerce for Intellectual Property and Director, Patent and Trademark Office v Brunetti. 18 302 (2018).
- Apple Inc. v Pepper et al. 17 204 (2018).
- Mitchell v Wisconsin. 18 6210 (2018).
Liability under the False Claims Act
The False Claims Act (FCA) protects the federal government from fraudulent claims for payment and for shoddy goods and services. It incentivizes (by a percentage of recovery) private parties to bring cases to enforce the law.15 (Of course, the federal government also enforces the Act.)
At stake. The FCA has been of considerable concern to the AHA, the Association of American Medical Colleges, and other health care organizations—understandably so.16 As the AHA informed the Court in an amicus brief, “The prevalence of [FCA] cases has ballooned over the past three decades.... These suits disproportionately target healthcare entities.... Of the 767 new FCA cases filed in 2018, for example, 506 involved healthcare defendants.”17
Final ruling. The Court considered an ambiguity in the statute of limitations for these actions and the Court unanimously ruled to permit an extended time in which qui tam actions (private actions under the law) can be filed.18
Key points. As long a period as 10 years can pass between the time an FCA violation occurs and an action is brought. This decision is likely to increase the number of FCA actions against health care providers because the case can be filed many years after the conduct that gave rise to the complaint.
Continue to: Registering sex offenders...
Registering sex offenders
The Court upheld the constitutionality of the federal Sex Offender Registration and Notification Act (SORNA).19 Sex offenders must register and periodically report, in person, to law enforcement in every state in which the offender works, studies, or resides.
At stake. The case involved the applicability of SORNA registration obligations to those convicted of sex offenses before SORNA was adopted (pre-Act offenders).20 The court upheld registration requirements for pre-Act offenders.
Former Justice Stevens, the longest-living and third-longest-serving Supreme Court justice, died in July 2019 at 99 years of age. He was appointed to the Court in 1975 by President Ford and served until his retirement in 2010, when he was 90. Stevens had recently published a memoir, The Making of a Justice: Reflections on My First 94 Years.
Stevens's judicial philosophy generally is described as having changed over the course of his 35 years of service: He was viewed as becoming more liberal. He was a justice of enduring kindness and integrity. It is possible to find people who disagree with him, but almost impossible to find anyone who disliked him. He was continuously committed to the law and justice in the United States.
Arbitration
The Court continued its practice of deciding at least one case each term that emphasizes that federal law requires that courts rather strictly enforce agreements to arbitrate (instead of to litigate) future disputes.21 In another case, the Court ruled that there can be “class” or “joint” arbitration only if the agreement to arbitrate a dispute clearly permits such class arbitration.22
Pharma’s liability regarding product risk
The Court somewhat limited the liability of pharmaceutical companies for failing to provide adequate warning about the risk that their products pose. The case against Merck involved 500 patients who took denosumab (Fosamax) and suffered atypical femoral fractures.23
At stake. Because prescribing information (in which warnings are provided) must be approved by the US Food and Drug Administration (FDA), the legal test is: Would the FDA have refused to approve a change in the warning if Merck had “fully informed the FDA of the justifications for the warning” required by state law to avoid liability?24,25 Lower-court judges (not juries) will be expected to apply this test in the future.
The doctor and the death penalty
The Court has established a rule that, when a prisoner facing capital punishment objects to a form of execution because it is too painful, he has to propose an alternative that is reasonably available. In one case,26 a physician, an expert witness for the prisoner, did not answer some essential relative-pain questions (ie, would one procedure be more painful than another?).
At stake. The AMA filed an amicus brief in this case, indicating that it is unethical for physicians to participate in an execution. The brief noted that “testimony used to determine which method of execution would reduce physical suffering would constitute physician participation in capital punishment and would be unethical.”27
The expert witness’s failure to answer the question on relative pain had the unfortunate result of reducing the likelihood that the prisoner would prevail in his request for an alternative method of execution.
Analysis
Despite obvious disagreements about big issues (notably, abortion and the death penalty) the Court maintained a courteous and civil demeanor—something not always seen nowadays in other branches of government. Here are facts about the Court’s term just concluded:
- The Court issued 72 merits opinions (about average).
- Only 39% of decisions were unanimous (compared with the average of 49% in recent terms).
- On the other hand, 26% of decisions were split 5 to 4 (compared with a 10% recent average).
- In those 5 to 4 decisions, Justices were in the majority as follows28: Justice Gorsuch, 65%; Justice Kavanaugh, 61%; Justice Thomas, 60%; Chief Justice Roberts and Justices Ginsburg and Alito, each 55%; Justice Breyer, 50%; and Justices Sotomayor and Kagan each at 45%.
- There were 57 dissenting opinions—up from 48 in the previous term.
- What is referred to as “the liberal-conservative split” might seem more profound than it really is: “Every conservative member of the court at some point voted to form a majority with the liberal justices. And every liberal at least once left behind all of his or her usual voting partners to join the conservatives.”29
Continue to: Last, it was a year of personal health issues for...
Last, it was a year of personal health issues for the Court: Justice Ginsburg had a diagnosis of lung cancer and was absent, following surgery, in January. Of retired Justices, Sandra Day O’Connor suffers from dementia and former Justice John Paul Stevens died.
In closing
The Court has accepted approximately 50 cases for the current term, which began on October 7. The first 2 days of the term were spent on arguments about, first, whether a state can abolish the insanity defense and, second, whether nondiscrimination laws (“based on sex”) prohibit discrimination based on sexual orientation or transgender status. Cases also will deal with Patient Protection and Affordable Care Act payments to providers; the Deferred Action for Childhood Arrivals, or DACA; the death penalty; and international child custody disputes. The Court will be accepting more cases for several months. It promises to be a very interesting term.
The most recently concluded term of the US Supreme Court, which began on October 1, 2018, yielded a number of decisions of interest to health care professionals and to ObGyns in particular. Although the term was viewed by some observers as less consequential than other recent terms, a review of the cases decided paints a picture of a more important term than some commentators expected.
When the term began, the Court had only 8 justices—1 short of a full bench: Judge Brett Kavanaugh had not yet been confirmed by the Senate. He was confirmed on October 6, by a 50-48 vote, and Justice Kavanaugh immediately joined the Court and began to hear and decide cases.
Increasingly, important decisions affect medical practice
From the nature of practice (abortion), to payment for service (Medicare reimbursement), resolution of disputes (arbitration), and fraud and abuse (the federal False Claims Act), the decisions of the Court will have an impact on many areas of medical practice. Organized medicine increasingly has recognized the significance of the work of the Court; nowhere has this been more clearly demonstrated than with amicus curiae (friend of the court) briefs filed by medical organizations.
Amicus curiae briefs. These briefs are filed by persons or organizations not a party to a case the Court is hearing. Their legitimate purpose is to inform the Court of 1) special information within the expertise of the amicus (or amici, plural) or 2) consequences of the decision that might not be apparent from arguments made by the parties to the case. Sometimes, the Court cites amicus briefs for having provided important information about the case.
Filing amicus briefs is time-consuming and expensive; organizations do not file them for trivial reasons. Organizations frequently join together to file a joint brief, to share expenses and express to the Court a stronger position.
Three categories of health professionals file amicus briefs in ObGyn-related cases:
- Major national organizations, often representing broad interests of health care professions or institutions (the American Medical Association [AMA], the Association of American Medical Colleges, and the American Hospital Association [AHA]), have filed a number of amicus briefs over the years.
- Specialty boards increasingly file amicus briefs. For example, the American College of Obstetricians and Gynecologists (ACOG) and the American Society for Reproductive Medicine have filed briefs related to abortion issues.
- In reproductive issues, the American Association of Pro-Life Obstetricians and Gynecologists, the American College of Pediatricians, and the Christian Medical & Dental Associations have been active amicus filers—frequently taking positions different than, even inconsistent with, amicus briefs filed by major specialty boards.
Amicus briefs filed by medical associations provide strong clues to what is important to clinicians. We have looked at such briefs to help us identify topics and cases from the just-concluded term that can be of particular interest to you.
Continue to: Surveying the shadow docket...
Surveying the shadow docket. As part of our review of the past term, we also looked at the so-called shadow docket, which includes decisions regarding writs of certiorari (which cases it agrees to hear); stays (usually delaying implementation of a law); or denials of stays. (Persuading the Court to hear a case is not easy: It hears approximately 70 cases per year out of as many as 7,000 applications to be heard.)
Abortion ruling
At stake. A number of states recently enacted a variety of provisions that might make an abortion more difficult to obtain. Some of the cases challenging these restrictions are making their way through lower courts, and one day might be argued before the Supreme Court. However, the Court has not (yet) agreed to hear the substance of many new abortion-related provisions.
Box v Planned Parenthood of Indiana and Kentucky, Inc.
The Court decided only 1 abortion restriction case this term.1 The Indiana law in question included 2 provisions that the Court considered:
Disposal of remains. The law regulated the manner in which abortion providers can dispose of fetal remains (ie, they cannot be treated as “infectious and pathologic waste”).
Motivation for seeking abortion. The Indiana law makes it illegal for an abortion provider to perform an abortion when the provider knows that the mother is seeking that abortion “solely” because of the fetus’s race, sex, diagnosis of Down syndrome, disability, or related characteristics.
Final rulings. The Court held that the disposal-of-remains provision is constitutional. The provision is “rationally related to the state’s interest in proper disposal of fetal remains.”2 Planned Parenthood had not raised the issue of whether the law might impose an undue burden on a woman’s right to obtain an abortion, so the Court did not decide that issue.
The Court did not consider the constitutionality of the part of the law proscribing certain reasons for seeking an otherwise legal abortion; instead, it awaits lower courts’ review of the issue. Justice Clarence Thomas wrote an extensive concurring opinion suggesting that this law is intended to avoid abortion to achieve eugenic goals.3
Key developments from the shadow docket
The Court issued a stay preventing a Louisiana statute that requires physicians who perform abortions to have admitting privileges at a nearby hospital from going into effect, pending the outcome of litigation about that law.4 Four dissenters noted that all 4 physicians who perform abortions in Louisiana have such privileges. Chief Justice Roberts was the fifth vote to grant the stay. This case likely will make its way back to the Court, as will a number of other state laws being adopted. The issue may be back as soon as the term just starting.
The Court is also considering whether to take another Indiana case, Box v Planned Parenthood of Indiana and Kentucky, Inc. (Box II). This case involves an Indiana ultrasonography viewing option as part of the abortion consent process.5
The Court declined to hear cases from Louisiana and Kansas in which the states had cut off Medicaid funding to Planned Parenthood. Lower courts had stopped the implementation of those laws.6 The legal issue was whether private parties, as opposed to the federal government, had standing to bring the case. For now, the decision of the lower courts to stop implementation of the funding cutoff is in effect. There is a split in the Circuit Courts on the issue, however, making it likely that the Supreme Court will have to resolve it sooner or later.
Health care organizations have filed a number of amicus briefs in these and other cases involving new abortion regulations. ACOG and others filed a brief opposing a Louisiana law that requires abortion providers to have admitting privileges at a nearby facility,7 and a brief opposing a similar Oklahoma law.8 The Association of Pro-Life Obstetricians and Gynecologists and others filed amicus curiae briefs in Box II9 and in an Alabama case involving so-called dismemberment abortion.10
Continue to: Medicare payments...
Medicare payments
Azar, Secretary of Health and Human Services v Allina Health Services, et al11
This case drew interest—and many amicus briefs—from health care providers, including the AMA and the AHA.12,13 There was good reason for their interest: First, the case involved more than $3 billion in reimbursements; second, it represented a potentially important precedent about the rights of providers and patients to comment on Medicare reimbursement changes. The question involved the technical calculation of additional payments made to institutions that serve a disproportionate number of low-income patients (known as Medicare Fractions).
At stake. The issue was a statutory requirement for a 60-day public notice and comment period for rules that “change a substantive legal standard” governing the scope of benefits, eligibility, or payment for services.14 In 2014, the Secretary of the Department of Health and Human Services (HHS) in the Obama administration posted a spreadsheet announcing Medicare fractions rates for hospitals (for 2012)—without formal notice or comment regarding the formula used. (The spreadsheet listed what each qualifying institution would receive, but it was based on a formula that, as noted, had not been subject to public notice and comment.) The AMA and AHA briefs emphasized the importance of a notice and comment period, especially when Medicare reimbursement is involved.
Final ruling. The Court held that the HHS process violated the notice and comment provision, thereby invalidating the policy underlying the so-called spreadsheet reimbursement. The decision was significant: This was a careful statutory interpretation of the 60-day notice and comment period, not the reimbursement policy itself. Presumably, had the HHS Secretary provided for sufficient notice and comment, the formula used would have met the requirements for issuing reimbursement formulas.
Key points. Hospitals will collectively receive $3 or $4 billion as a consequence of the ruling. Perhaps more importantly, the decision signals that HHS is going to have to take seriously the requirement that it publish Medicare-related reimbursement policies for the 60-day period.
A number of diverse cases ruled on by the Supreme Court are worth mentioning. The Court:
- allowed the President to move various funds from the US Department of Defense into accounts from which the money could be used to build a portion of a wall along the southern US border.1
- essentially killed the "citizenship question" on the census form. Technically, the Court sent the issue back to the Commerce Department for better justification for including the question (the announced reasons appeared to be pretextual).2
- changed, perhaps substantially, the deference that courts give to federal agencies in interpreting regulations.3
- upheld, in 2 cases, treaty rights of Native Americans to special treatment on Indian Lands4,5; the Court held that treaties ordinarily should be interpreted as the tribe understood them at the time they were signed. (These were 5 to 4 decisions; the split in the Court leaves many unanswered questions.)
- made it easier for landowners to file suit in federal court when they claim that the state has "taken" their property without just compensation.6
- held that a refusal of the US Patent and Trademark Office to register "immoral" or "scandalous" trademarks infringes on the First Amendment. (The petitioner sought to register "FUCT" as a trademark for a line of clothing.)7
- allowed an antitrust case by iPhone users against Apple to go forward. At issue: the claim that Apple monopolizes the retail market for apps by requiring buyers to obtain apps from Apple.8
- held that, if a drunk-driving suspect who has been taken into custody is, or becomes, unconscious, the "reasonable search" provision of the Fourth Amendment generally does not prevent a state from taking a blood specimen without a warrant. (Wisconsin had a specific "implied consent" law, by which someone receiving a driving license consents to a blood draw.9)
- decided numerous capital punishment cases. In many ways, this term seemed to be a "capital term." Issues involved in these cases have split the Court; it is reasonable to expect that the divide will endure through upcoming terms.
References
- Donald J. Trump, President of the United States, et al. v Sierra Club, et al. 588 US 19A60 (2019).
- Department of Commerce et al. v New York et al. 18 996 (2018).
- Kisor v Wilkie, Secretary of Veterans Affairs. 18 15 (2018).
- Washington State Department of Licensing v Cougar Den, Inc. 16 1498 (2018).
- Herrera v Wyoming. 17 532 (2018).
- Knick v Township of Scott, Pennsylvania, et al. 17 647 (2018).
- Iancu, Under Secretary of Commerce for Intellectual Property and Director, Patent and Trademark Office v Brunetti. 18 302 (2018).
- Apple Inc. v Pepper et al. 17 204 (2018).
- Mitchell v Wisconsin. 18 6210 (2018).
Liability under the False Claims Act
The False Claims Act (FCA) protects the federal government from fraudulent claims for payment and for shoddy goods and services. It incentivizes (by a percentage of recovery) private parties to bring cases to enforce the law.15 (Of course, the federal government also enforces the Act.)
At stake. The FCA has been of considerable concern to the AHA, the Association of American Medical Colleges, and other health care organizations—understandably so.16 As the AHA informed the Court in an amicus brief, “The prevalence of [FCA] cases has ballooned over the past three decades.... These suits disproportionately target healthcare entities.... Of the 767 new FCA cases filed in 2018, for example, 506 involved healthcare defendants.”17
Final ruling. The Court considered an ambiguity in the statute of limitations for these actions and the Court unanimously ruled to permit an extended time in which qui tam actions (private actions under the law) can be filed.18
Key points. As long a period as 10 years can pass between the time an FCA violation occurs and an action is brought. This decision is likely to increase the number of FCA actions against health care providers because the case can be filed many years after the conduct that gave rise to the complaint.
Continue to: Registering sex offenders...
Registering sex offenders
The Court upheld the constitutionality of the federal Sex Offender Registration and Notification Act (SORNA).19 Sex offenders must register and periodically report, in person, to law enforcement in every state in which the offender works, studies, or resides.
At stake. The case involved the applicability of SORNA registration obligations to those convicted of sex offenses before SORNA was adopted (pre-Act offenders).20 The court upheld registration requirements for pre-Act offenders.
Former Justice Stevens, the longest-living and third-longest-serving Supreme Court justice, died in July 2019 at 99 years of age. He was appointed to the Court in 1975 by President Ford and served until his retirement in 2010, when he was 90. Stevens had recently published a memoir, The Making of a Justice: Reflections on My First 94 Years.
Stevens's judicial philosophy generally is described as having changed over the course of his 35 years of service: He was viewed as becoming more liberal. He was a justice of enduring kindness and integrity. It is possible to find people who disagree with him, but almost impossible to find anyone who disliked him. He was continuously committed to the law and justice in the United States.
Arbitration
The Court continued its practice of deciding at least one case each term that emphasizes that federal law requires that courts rather strictly enforce agreements to arbitrate (instead of to litigate) future disputes.21 In another case, the Court ruled that there can be “class” or “joint” arbitration only if the agreement to arbitrate a dispute clearly permits such class arbitration.22
Pharma’s liability regarding product risk
The Court somewhat limited the liability of pharmaceutical companies for failing to provide adequate warning about the risk that their products pose. The case against Merck involved 500 patients who took denosumab (Fosamax) and suffered atypical femoral fractures.23
At stake. Because prescribing information (in which warnings are provided) must be approved by the US Food and Drug Administration (FDA), the legal test is: Would the FDA have refused to approve a change in the warning if Merck had “fully informed the FDA of the justifications for the warning” required by state law to avoid liability?24,25 Lower-court judges (not juries) will be expected to apply this test in the future.
The doctor and the death penalty
The Court has established a rule that, when a prisoner facing capital punishment objects to a form of execution because it is too painful, he has to propose an alternative that is reasonably available. In one case,26 a physician, an expert witness for the prisoner, did not answer some essential relative-pain questions (ie, would one procedure be more painful than another?).
At stake. The AMA filed an amicus brief in this case, indicating that it is unethical for physicians to participate in an execution. The brief noted that “testimony used to determine which method of execution would reduce physical suffering would constitute physician participation in capital punishment and would be unethical.”27
The expert witness’s failure to answer the question on relative pain had the unfortunate result of reducing the likelihood that the prisoner would prevail in his request for an alternative method of execution.
Analysis
Despite obvious disagreements about big issues (notably, abortion and the death penalty) the Court maintained a courteous and civil demeanor—something not always seen nowadays in other branches of government. Here are facts about the Court’s term just concluded:
- The Court issued 72 merits opinions (about average).
- Only 39% of decisions were unanimous (compared with the average of 49% in recent terms).
- On the other hand, 26% of decisions were split 5 to 4 (compared with a 10% recent average).
- In those 5 to 4 decisions, Justices were in the majority as follows28: Justice Gorsuch, 65%; Justice Kavanaugh, 61%; Justice Thomas, 60%; Chief Justice Roberts and Justices Ginsburg and Alito, each 55%; Justice Breyer, 50%; and Justices Sotomayor and Kagan each at 45%.
- There were 57 dissenting opinions—up from 48 in the previous term.
- What is referred to as “the liberal-conservative split” might seem more profound than it really is: “Every conservative member of the court at some point voted to form a majority with the liberal justices. And every liberal at least once left behind all of his or her usual voting partners to join the conservatives.”29
Continue to: Last, it was a year of personal health issues for...
Last, it was a year of personal health issues for the Court: Justice Ginsburg had a diagnosis of lung cancer and was absent, following surgery, in January. Of retired Justices, Sandra Day O’Connor suffers from dementia and former Justice John Paul Stevens died.
In closing
The Court has accepted approximately 50 cases for the current term, which began on October 7. The first 2 days of the term were spent on arguments about, first, whether a state can abolish the insanity defense and, second, whether nondiscrimination laws (“based on sex”) prohibit discrimination based on sexual orientation or transgender status. Cases also will deal with Patient Protection and Affordable Care Act payments to providers; the Deferred Action for Childhood Arrivals, or DACA; the death penalty; and international child custody disputes. The Court will be accepting more cases for several months. It promises to be a very interesting term.
- Box v Planned Parenthood of Indiana and Kentucky, Inc. 587 US 18 483 (2019).
- Box v Planned Parenthood of Indiana and Kentucky, Inc., at 2.
- Box v Planned Parenthood of Indiana and Kentucky, Inc., Justice Thomas concurring.
- June Medical Services, LLC, et al. v Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals. 586 US 18A774 (2019).
- Box v Planned Parenthood of Indiana and Kentucky, Inc. Docket 18-1019.
- Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals v Planned Parenthood of Gulf Coast, Inc., et al. 586 US 17 1492 (2018).
- June Medical Services L.L.C., et al., Petitioners, v Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals. No. 18-1323. Brief of Amici Curiae American College of Obstetricians and Gynecologists, American Academy of Family Physicians, American Academy of Pediatrics, American College of Nurse-Midwives, American College of Osteopathic Obstetricians and Gynecologists, American College of Physicians, American Society for Reproductive Medicine, National Association of Nurse Practitioners in Women's Health, North American Society for Pediatric and Adolescent Gynecology, and Society For Maternal-Fetal Medicine, Amicus Curiae in Support of Petitioners. May 2019.
- Planned Parenthood of Kansas & Eastern Oklahoma, et al., Petitioners, v Larry Jegley, et al., Respondents. No. 17-935. Brief Amici Curiae of American College of Obstetricians and Gynecologists and American Public Health Association as Amici Curiae in Support of Petitioners. February 1, 2018.
- Box v Planned Parenthood of Indiana & Kentucky. No. 18-1019. Brief Amici Curiae of American Association of Pro-Life Obstetricians & Gynecologists, American College of Pediatricians, Care Net, Christian Medical Association, Heartbeat International, Inc., and National Institute Of Family & Life Advocates in Support of Petitioners. March 6, 2019.
- Steven T. Marshall, et al., Petitioners, v West Alabama Women's Center, et al., Respondents. No. 18-837. Brief of Amici Curiae American Association of Pro-Life Obstetricians & Gynecologists and American College of Pediatricians, in Support of Petitioners. January 18, 2019.
- Azar, Secretary of Health and Human Services v Allina Health Services, et al. 17 1484 (2018).
- Alex M. Azar, II, Secretary of Health and Human Services, Petitioner, v Allina Health Services, et al., Respondents. Brief of the American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges as Amici Curiae in Support of Respondents. December 2018.
- Alex M. Azar, II, Secretary of Health and Human Services, Petitioner, v Allina Health Services, et al., Respondents. Brief of Amici Curiae American Medical Association and Medical Society of the District of Columbia Amici Curiae in Support of Respondents. December 2018.
- 42 U. S. C. §1395hh. https://uscode.house.gov/view.xhtml?req=(title:42%20section:1395hh%20edition:prelim). Accessed October 22, 2019.
- The False Claims Act: a primer. Washington DC: US Department of Justice. www.justice.gov/sites/default/files/civil/legacy/2011/04/22/C-FRAUDS_FCA_Primer.pdf. Accessed October 18, 2019.
- Universal Health Services, Inc., v United States and Commonwealth of Massachusetts ex rel. Julio Escobar and Carmen Correa. Brief of the American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges Amici Curiae in Support of Petitioner. No. 15-7. January 2016.
- Intermountain Health Care, Inc., et al., Petitioners, v United States ex rel. Gerald Polukoff, et al., Respondents. No. 18-911. Brief of the American Hospital Association and Federation of American Hospitals as Amici Curiae in Support of Petitioners. February 13, 2019.
- Cochise Consultancy, Inc., et al., v United States ex rel. Hunt. 18 315 (2018).
- 34 U.S.C. §20901 et seq. [Chapter 209--Child Protection and Safety.] https://uscode.house.gov/view.xhtml?path=/prelim@title34/subtitle2/chapter209&edition=prelim. Accessed October 17, 2019.
- Gundy v United States. 17 6086 (2018).
- Henry Schein, Inc., et al., v Archer & White Sales, Inc. 17 1272 (2018).
- Lamps Plus, Inc., et al., v Varela. 17 988 (2018).
- Merck Sharp & Dohme Corp. v Albrecht et al. 17 290 (2018).
- Merck Sharp & Dohme Corp. v Albrecht et al. 17 290 (2018) at 13-14.
- Wyeth v Levine, 555 US 555, 571 (2009).
- Russell Bucklew, Petitioner, v Anne L. Precythe, Director, Missouri Department of Corrections, et al., Respondents. 17 8151 (2018).
- Russell Bucklew, Petitioner, v Anne L. Precythe, Director, Missouri Department of Corrections, et al., Respondents. 17 8151 (2018). American Medical Association, Amicus Curiae Brief, in Support of Neither Party. July 23, 2018.
- Final stat pack for October term 2018. SCOTUSblog.com. June 28, 2019. https://www.scotusblog.com/wp-content/uploads/2019/07/StatPack_OT18-7_8_19.pdf. Accessed October 17, 2019.
- Barnes R. They're not 'wonder twins': Gorsuch, Kavanaugh shift the Supreme Court, but their differences are striking. Washington Post, June 28, 2019.
- Box v Planned Parenthood of Indiana and Kentucky, Inc. 587 US 18 483 (2019).
- Box v Planned Parenthood of Indiana and Kentucky, Inc., at 2.
- Box v Planned Parenthood of Indiana and Kentucky, Inc., Justice Thomas concurring.
- June Medical Services, LLC, et al. v Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals. 586 US 18A774 (2019).
- Box v Planned Parenthood of Indiana and Kentucky, Inc. Docket 18-1019.
- Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals v Planned Parenthood of Gulf Coast, Inc., et al. 586 US 17 1492 (2018).
- June Medical Services L.L.C., et al., Petitioners, v Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals. No. 18-1323. Brief of Amici Curiae American College of Obstetricians and Gynecologists, American Academy of Family Physicians, American Academy of Pediatrics, American College of Nurse-Midwives, American College of Osteopathic Obstetricians and Gynecologists, American College of Physicians, American Society for Reproductive Medicine, National Association of Nurse Practitioners in Women's Health, North American Society for Pediatric and Adolescent Gynecology, and Society For Maternal-Fetal Medicine, Amicus Curiae in Support of Petitioners. May 2019.
- Planned Parenthood of Kansas & Eastern Oklahoma, et al., Petitioners, v Larry Jegley, et al., Respondents. No. 17-935. Brief Amici Curiae of American College of Obstetricians and Gynecologists and American Public Health Association as Amici Curiae in Support of Petitioners. February 1, 2018.
- Box v Planned Parenthood of Indiana & Kentucky. No. 18-1019. Brief Amici Curiae of American Association of Pro-Life Obstetricians & Gynecologists, American College of Pediatricians, Care Net, Christian Medical Association, Heartbeat International, Inc., and National Institute Of Family & Life Advocates in Support of Petitioners. March 6, 2019.
- Steven T. Marshall, et al., Petitioners, v West Alabama Women's Center, et al., Respondents. No. 18-837. Brief of Amici Curiae American Association of Pro-Life Obstetricians & Gynecologists and American College of Pediatricians, in Support of Petitioners. January 18, 2019.
- Azar, Secretary of Health and Human Services v Allina Health Services, et al. 17 1484 (2018).
- Alex M. Azar, II, Secretary of Health and Human Services, Petitioner, v Allina Health Services, et al., Respondents. Brief of the American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges as Amici Curiae in Support of Respondents. December 2018.
- Alex M. Azar, II, Secretary of Health and Human Services, Petitioner, v Allina Health Services, et al., Respondents. Brief of Amici Curiae American Medical Association and Medical Society of the District of Columbia Amici Curiae in Support of Respondents. December 2018.
- 42 U. S. C. §1395hh. https://uscode.house.gov/view.xhtml?req=(title:42%20section:1395hh%20edition:prelim). Accessed October 22, 2019.
- The False Claims Act: a primer. Washington DC: US Department of Justice. www.justice.gov/sites/default/files/civil/legacy/2011/04/22/C-FRAUDS_FCA_Primer.pdf. Accessed October 18, 2019.
- Universal Health Services, Inc., v United States and Commonwealth of Massachusetts ex rel. Julio Escobar and Carmen Correa. Brief of the American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges Amici Curiae in Support of Petitioner. No. 15-7. January 2016.
- Intermountain Health Care, Inc., et al., Petitioners, v United States ex rel. Gerald Polukoff, et al., Respondents. No. 18-911. Brief of the American Hospital Association and Federation of American Hospitals as Amici Curiae in Support of Petitioners. February 13, 2019.
- Cochise Consultancy, Inc., et al., v United States ex rel. Hunt. 18 315 (2018).
- 34 U.S.C. §20901 et seq. [Chapter 209--Child Protection and Safety.] https://uscode.house.gov/view.xhtml?path=/prelim@title34/subtitle2/chapter209&edition=prelim. Accessed October 17, 2019.
- Gundy v United States. 17 6086 (2018).
- Henry Schein, Inc., et al., v Archer & White Sales, Inc. 17 1272 (2018).
- Lamps Plus, Inc., et al., v Varela. 17 988 (2018).
- Merck Sharp & Dohme Corp. v Albrecht et al. 17 290 (2018).
- Merck Sharp & Dohme Corp. v Albrecht et al. 17 290 (2018) at 13-14.
- Wyeth v Levine, 555 US 555, 571 (2009).
- Russell Bucklew, Petitioner, v Anne L. Precythe, Director, Missouri Department of Corrections, et al., Respondents. 17 8151 (2018).
- Russell Bucklew, Petitioner, v Anne L. Precythe, Director, Missouri Department of Corrections, et al., Respondents. 17 8151 (2018). American Medical Association, Amicus Curiae Brief, in Support of Neither Party. July 23, 2018.
- Final stat pack for October term 2018. SCOTUSblog.com. June 28, 2019. https://www.scotusblog.com/wp-content/uploads/2019/07/StatPack_OT18-7_8_19.pdf. Accessed October 17, 2019.
- Barnes R. They're not 'wonder twins': Gorsuch, Kavanaugh shift the Supreme Court, but their differences are striking. Washington Post, June 28, 2019.
The mesh mess, enmeshed in controversy
CASE Complications with mesh placement for SUI
A 47-year-old woman (G4 P3013) presents 5 months posthysterectomy with evidence of urinary tract infection (UTI). Escherichia coli is isolated, and she responds to antibiotic therapy.
Her surgical history includes a mini-sling procedure using a needleless device and mesh placement in order to correct progressive worsening of loss of urine when coughing and sneezing. She also reported slight pelvic pain, dysuria, and urgency upon urination at that time. After subsequent development of pelvic organ prolapse (POP), she underwent the vaginal hysterectomy.
Following her UTI treatment, a host of problems occur for the patient, including pelvic pain and dyspareunia. Her male partner reports “feeling something during sex,” especially at the anterior vaginal wall. A plain radiograph of the abdomen identifies a 2 cm x 2 cm stone over the vaginal mesh. In consultation with female pelvic medicine and reconstructive surgery subspecialists, lithotripsy is performed, with the stone fragmented. The patient remains symptomatic, however.
The mesh is noted to be eroding through the vaginal wall. An attempt is made to excise the mesh, initially via transuretheral resection, then through a laparoscopic approach. Due to the mesh being embedded in the tissue, however, an open approach is undertaken. Extensive excision of the mesh and stone fragments is performed. Postoperatively, the patient reports “dry vagina,” with no other genitourinary complaints.
The patient sues. She sues the mesh manufacturer. She also seeks to sue the gynecologist who placed the sling and vaginal mesh (as she says she was not informed of “all the risks” of vaginal mesh placement. She is part of a class action lawsuit, along with thousands of other women.
WHAT’S THE VERDICT?
The device manufacturer settled out of court with the class action suit. (The gynecologist was never formally a defendant because the patient/plaintiff was advised to “drop the physician from the suit.”) The attorneys representing the class action received 40% of the award plus presented costs for the representation. The class as a whole received a little more than 50% of the negotiated award. The patient in this case received $60,000.
Medical background
Stress urinary incontinence (SUI) is a prevalent condition; it affects 35% of women.1 Overall, 80% of women aged 80 or younger will undergo some form of surgery for POP during their lifetime.2 The pathophysiology of SUI includes urethral hypermobility and intrinsic sphincter deficiency.3
Surgical correction for urinary incontinence: A timeline
Use of the gracilis muscle flap to surgically correct urinary incontinence was introduced in 1907. This technique has been replaced by today’s more common Burch procedure, which was first described in 1961. Surgical mesh use dates back to the 1950s, when it was primarily used for abdominal hernia repair. Tension-free tape was introduced in 1995.4-6
Continue to: In the late 1990s the US Food and Drug Administration...
In the late 1990s the US Food and Drug Administration (FDA) permitted use of the first transvaginal meshes, which were designed to treat SUI—the midurethral sling. These mesh slings were so successful that similar meshes were developed to treat POP.7 Almost immediately there were problems with the new POP devices, and 3 years later Boston Scientific recalled its device.8 Nonetheless, the FDA cleared more than 150 devices using surgical mesh for urogynecologic indications (FIGURE).9

Mesh complications
Managing complications from intravesical mesh is a clinically challenging problem. Bladder perforation, stone formation, and penetration through the vagina can occur. Bladder-related complications can manifest as recurrent UTIs and obstructive urinary symptoms, especially in association with stone formation. From the gynecologic perspective, the more common complications with mesh utilization are pelvic pain, groin pain, dyspareunia, contracture and scarring of mesh, and narrowing of the vaginal canal.10 Mesh erosion problems will occur in an estimated 10% to 25% of transvaginal mesh POP implants.11
In 2008, a comparison of transvaginal mesh to native tissue repair (suture-based) or other (biologic) grafts was published.12 The bottom line: there is insufficient evidence to suggest that transvaginal mesh significantly improves outcomes for both posterior and apical defects.
Legal background
Mesh used for surgical purposes is a medical device, which legally is a product—a special product to be sure, but a product nonetheless. Products are subject to product liability rules. Mesh is also subject to an FDA regulatory system. We will briefly discuss products liability and the regulation of devices, both of which have played important roles in mesh-related injuries.
Products liability
As a general matter, defective products subject their manufacturer and seller to liability. There are several legal theories regarding product liability: negligence (in which the defect was caused through carelessness), breach of warranty or guarantee (in addition to express warranties, there are a number of implied warranties for products, including that it is fit for its intended purpose), and strict liability (there was a defect in the product, but it may not have been because of negligence). The product may be defective in the way it was designed, manufactured, or packaged, or it may be defective because adequate instructions and warning were not given to consumers.
Of course, not every product involved in an injury is defective—most automobile accidents, for example, are not the result of any defect in the automobile. In medicine, almost no product (device or pharmaceutical) is entirely safe. In some ways they are unavoidably unsafe and bound to cause some injuries. But when injuries are caused by a defect in the product (design or manufacturing defect or failure to warn), then there may be products liability. Most products liability cases arise under state law.
FDA’s device regulations
Both drugs and medical devices are subject to FDA review and ordinarily require some form of FDA clearance before they may be marketed. In the case of devices, the FDA has 3 classes, with an increase in risk to the user from Class I to III. Various levels of FDA review are required before marketing of the device is permitted, again with the intensity of review increasing from I to III as follows:
- Class I devices pose the least risk, have the least regulation, and are subject to general controls (ie, manufacturing and marketing practices).
- Class II devices pose slightly higher risks and are subject to special controls in addition to the criteria for Class I.
- Class III devices pose the most risk to patients and require premarket approval (scientific review and studies are required to ensure efficacy and safety).13
Continue to: There are a number of limits on manufacturer liability for defective devices...
There are a number of limits on manufacturer liability for defective devices. For Class III devices, the thorough FDA review of the safety of a device may limit the ability of an injured patient to sue based on the state product liability laws.14 For the most part, this “preemption” of state law has not played a major role in mesh litigation because they were initially classified as Class II devices which did not require or include a detailed FDA review.15
The duty to warn of the dangers and risk of medical devices means that manufacturers (or sellers) of devices are obligated to inform health care providers and other medical personnel of the risks. Unlike other manufacturers, device manufacturers do not have to directly warn consumers—because physicians deal directly with patients and prescribe the devices. Therefore, the health care providers, rather than the manufacturers, are obligated to inform the patient.16 This is known as the learned intermediary rule. Manufacturers may still be liable for failure to warn if they do not convey to health care providers proper warnings.
Manufacturers and sellers are not the only entities that may be subject to liability caused by medical devices. Hospitals or other entities that stock and care for devices are responsible for maintaining the safety and functionality of devices in their care.
Health care providers also may be responsible for injuries from medical devices. Generally, that liability is based on negligence. Negligence may relate to selecting an improper device, installing or using it incorrectly, or failing to give the patient adequate information (or informed consent) about the device and alternatives to it.17
A look at the mesh mess
There are a lot of distressing problems and professional disappointments in dissecting the “mesh mess,” including a failure of the FDA to regulate effectively, the extended sale and promotion of intrinsic sphincter deficiency mesh products, the improper use of mesh by physicians even after the risks were known, and, in some instances, the taking advantage of injured patients by attorneys and businesses.18 A lot of finger pointing also has occurred.19 We will recount some of the lowlights of this unfortunate tale.
Continue to: The FDA, in the 1990s, classified the first POP and SUI mesh...
The FDA, in the 1990s, classified the first POP and SUI mesh as Class II after deciding these products were “substantially equivalent” to older surgical meshes. This, of course, proved not to be the case.20 The FDA started receiving thousands of reports of adverse events and, in 2008, warned physicians to be vigilant for adverse events from the mesh. The FDA’s notification recommendations regarding mesh included the following13:
- Obtain specialized training for each mesh implantation technique, and be cognizant of risks.
- Be vigilant for potential adverse events from mesh, including erosion and infection.
- Be observant for complications associated with tools of transvaginal placement (ie, bowel, bladder, and vessel perforation).
- Inform patients that implantation of mesh is permanent and complications may require additional surgery for correction.
- Be aware that complications may affect quality of life—eg, pain with intercourse, scarring, and vaginal wall narrowing (POP repair).
- Provide patients with written copy of patient labeling from the surgical mesh manufacturer.
In 2011, the FDA issued a formal warning to providers that transvaginal mesh posed meaningful risks beyond nonmesh surgery. The FDA’s bulletin draws attention to how the mesh is placed more so than the material per se.19,21 Mesh was a Class II device for sacrocolpopexy or midurethral sling and, similarly, the transvaginal kit was also a Class II device. Overall, use of mesh midurethral slings has been well received as treatment for SUI. The FDA also accepted it for POP, however, but with increasingly strong warnings. The FDA’s 2011 communication stated, “This update is to inform you that serious complications associated with surgical mesh for transvaginal repair of POP are not rare….Furthermore, it is not clear that transvaginal POP repair with mesh is more effective than traditional non-mesh repair in all patients with POP and it may expose patients to greater risk.”7,13
In 2014 the FDA proposed reclassifying mesh to a Class III device, which would require that manufacturers obtain approval, based on safety and effectiveness, before selling mesh. Not until 2016 did the FDA actually reclassify the mess as Class III. Of course, during this time, mesh manufacturers were well aware of the substantial problems the products were causing.13
After serious problems with mesh became well known, and especially after FDA warnings, the use of mesh other than as indicated by the FDA was increasingly risky from a legal (as well as a health) standpoint. As long as mesh was still on the market, of course, it was available for use. But use of mesh for POP procedures without good indications in a way that was contrary to the FDA warnings might well be negligent.
Changes to informed consent
The FDA warnings also should have changed the informed consent for the use of mesh.22 Informed consent commonly consists of the following:
- informing the patient of the proposed procedure
- describing risks (and benefits) of the proposed process
- explaining reasonable alternatives
- noting the risks of taking no action.
Information that is material to a decision should be disclosed. If mesh were going to be used, after the problems of mesh were known and identified by the FDA (other than midurethral slings as treatment of SUI), the risks should have been clearly identified for patients, with alternatives outlined. The American College of Obstetricians and Gynecologists Committee on Ethics has 8 fundamental concepts with regard to informed consent that are worth keeping in mind23:
- Obtaining informed consent for medical treatment and research is an ethical requirement.
- The process expresses respect for the patient as a person.
- It protects patients against unwanted treatment and allows patients’ active involvement in medical planning and care.
- Communication is of paramount importance.
- Informed consent is a process and not a signature on a form.
- A commitment to informed consent and to provision of medical benefit to the patient are linked to provision of care.
- If obtaining informed consent is impossible, a designated surrogate should be identified representing the patient’s best interests.
- Knowledge on the part of the provider regarding state and federal requirements is necessary.
Continue to: Lawsuits line up...
Lawsuits line up
The widespread use of a product with a significant percentage of injuries and eventually with warnings about injuries from use sounds like the formula for a lot of lawsuits. This certainly has happened. A large number of suits—both class actions and individual actions—were filed as a result of mesh injuries.24 These suits were overwhelmingly against the manufacturer, although some included physicians.7 Device makers are more attractive defendants for several reasons. First, they have very deep pockets. In addition, jurors are generally much less sympathetic to large companies than to doctors. Large class actions meant that there were many different patients among the plaintiffs, and medical malpractice claims in most states have a number of trial difficulties not present in other product liability cases. Common defendants have included Johnson & Johnson, Boston Scientific, and Medtronic.
Some of the cases resulted in very large damage awards against manufacturers based on various kinds of product(s) liability. Many other cases were settled or tried with relatively small damages. There were, in addition, a number of instances in which the manufacturers were not liable. Of the 32 plaintiffs who have gone to trial thus far, 24 have obtained verdicts totaling $345 million ($14 million average). The cases that have settled have been for much less—perhaps $60,000 on average. A number of cases remain unresolved. To date, the estimate is that 100,000 women have received almost $8 billion from 7 device manufacturers to resolve claims.25
Some state attorneys general have gotten into the process as well. Attorneys general from California, Kentucky, Mississippi, and Washington have filed lawsuits against Johnson & Johnson, claiming that they deceived doctors and patients about the risks of their pelvic mesh. The states claim that marketing and instructional literature should have contained more information about the risks. Some physicians in these states have expressed concern that these lawsuit risks may do more harm than good because the suits conflate mesh used to treat incontinence with the more risky mesh for POP.26
The “ugly” of class action lawsuits
We have discussed both the sad (the injuries to patients) and the bad (the slow regulatory response and continuing injuries). (The ethics of the marketing by the manufacturers might also be raised as the bad.27) Next, let’s look briefly at the ugly.
Some of the patients affected by mesh injuries have been victimized a second time by medical “lenders” and some of their attorneys. Press reports describe patients with modest awards paying 40% in attorney fees (on the high side for personal injury settlements) plus extravagant costs—leaving modest amounts of actual recovery.25
Worse still, a process of “medical lending” has arisen in mesh cases.28 Medical lenders may contact mesh victims offering to pay up front for surgery to remove mesh, and then place a lien against the settlement for repayment at a much higher rate. They might pay the surgeon $2,500 for the surgery, but place a lien on the settlement amount for $60,000.29,30 In addition, there are allegations that lawyers may recruit the doctors to overstate the injuries or do unnecessary removal surgery because that will likely up the award.31 A quick Google search indicates dozens of offers of cash now for your mesh lawsuit (transvaginal and hernia repair).
The patient in our hypothetical case at the beginning had a fairly typical experience. She was a member of a class filing and received a modest settlement. The attorneys representing the class were allowed by the court to charge substantial attorneys’ fees and costs. The patient had the good sense to avoid medical lenders, although other members of the class did use medical lenders and are now filing complaints about the way they were treated by these lenders.
- Maintain surgical skills and be open to new technology. Medical practice requires constant updating and use of new and improved technology as it comes along. By definition, new technology often requires new skills and understanding. A significant portion of surgeons using mesh indicated that they had not read the instructions for use, or had done so only once.1 CME programs that include surgical education remain of particular value.
- Whether new technology or old, it is essential to keep up to date on all FDA bulletins pertinent to devices and pharmaceuticals that you use and prescribe. For example, in 2016 and 2018 the FDA warned that the use of a very old class of drugs (fluoroquinolones) should be limited. It advised "that the serious side effects associated with fluoroquinolones generally outweigh the benefits for patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infections who have other treatment options. For patients with these conditions, fluoroquinolones should be reserved for those who do not have alternative treatment options."2 Continued, unnecessary prescriptions for fluoroquinolones would put a physician at some legal risk whether or not the physician had paid any attention to the warning.
- Informed consent is a very important legal and medical process. Take it seriously, and make sure the patient has the information necessary to make informed decisions about treatment. Document the process and the information provided. In some cases consider directing patients to appropriate literature or websites of the manufacturers.
- As to the use of mesh, if not following FDA advice, it is important to document the reason for this and to document the informed consent especially carefully.
- Follow patients after mesh placement for a minimum of 1 year and emphasize to patients they should convey signs and symptoms of complications from initial placement.3 High-risk patients should be of particular concern and be monitored very closely.
References
- Kirkpatrick G, Faber KD, Fromer DL. Transvaginal mesh placement and the instructions for use: a survey of North American urologists. J Urol. https://doi.org/10.1016/j.urpr.2018.05.004.
- FDA Drug Safety Communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. July 26, 2016. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed June 19, 2019.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Maral I, Ozkardeş H, Peşkircioğlu L, et al. Prevalence of stress urinary incontinence in both sexes at or after age 15 years: a cross-sectional study. J Urol. 2001;165:408-412.
- Olsen AL, Smith VJ, Bergstrom JO, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
- Chang J, Lee D. Midurethral slings in the mesh litigation era. Transl Androl Urol. 2017;6(suppl 2): S68-S75.
- Mattingly R, ed. TeLinde's Operative Gynecology, 5th edition. Lippincott, William, and Wilkins: Philadelphia, PA; 1997.
- Burch J. Urethrovaginal fixation to Cooper's ligament for correction of stress incontinence, cystocele, and prolapse. Am J Obstet Gynecol. 1961;81:281-290.
- Ulmsten U, Falconer C, Johnson P, et al. A multicenter study of tension-free vaginal tape (TVT) for surgical treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:210-213.
- Kuhlmann-Capek MJ, Kilic GS, Shah AB, et al. Enmeshed in controversy: use of vaginal mesh in the current medicolegal environment. Female Pelvic Med Reconstr Surg. 2015;21:241-243.
- Powell SF. Changing our minds: reforming the FDA medical device reclassification process. Food Drug Law J. 2018;73:177-209.
- US Food and Drug Administration. Surgical Mesh for Treatment of Women with Pelvic Organ Prolapse and Stress Urinary Incontinence. September 2011. https://www.thesenatorsfirm.com/documents/OBS.pdf. Accessed June 19, 2019.
- Maher C, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;(4):CD004014.
- Ganj FA, Ibeanu OA, Bedestani A, Nolan TE, Chesson RR. Complications of transvaginal monofilament polypropylene mesh in pelvic organ prolapse repair. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:919-925.
- Sung VW, Rogers RG, Schaffer JI, et al. Graft use in transvaginal pelvic organ prolapse repair: a systematic review. Obstet Gynecol. 2008;112:1131-1142.
- FDA public health notification: serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. October 20, 2008. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm061976.htm. Accessed February 14, 2019.
- Riegel v. Medtronic, 552 U.S. 312 (2008).
- Whitney DW. Guide to preemption of state-law claims against Class III PMA medical devices. Food Drug Law J. 2010;65:113-139.
- Alam P, Iglesia CB. Informed consent for reconstructive pelvic surgery. Obstet Gynecol Clin North Am. 2016;43:131-139.
- Nosti PA, Iglesia CB. Medicolegal issues surrounding devices and mesh for surgical treatment of prolapse and incontinence. Clin Obstet Gynecol. 2013;56:221-228.
- Shepherd CG. Transvaginal mesh litigation: a new opportunity to resolve mass medical device failure claims. Tennessee Law Rev. 2012;80:3:477-94.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Cohn JA, Timbrook Brown E, Kowalik CG, et al. The mesh controversy. F1000Research website. https://f1000research.com/articles/5-2423/v1. Accessed June 17, 2019.
- Obstetrics and Gynecology Devices Panel Meeting, February 12, 2019. US Food and Drug Administration website. https://www.fda.gov/media/122867/download. Accessed June 19, 2019.
- Mucowski SJ, Jurnalov C, Phelps JY. Use of vaginal mesh in the face of recent FDA warnings and litigation. Am J Obstet Gynecol. 2010;203:103.e1-e4.
- American College of Obstetricians and Gynecologists Committee on Ethics. ACOG Committee Opinion No. 439: informed consent. Obstet Gynecol. 2009;114(2 pt 1):401-408.
- Souders CP, Eilber KS, McClelland L, et al. The truth behind transvaginal mesh litigation: devices, timelines, and provider characteristics. Female Pelvic Med Reconstr Surg. 2018;24:21-25.
- Goldstein M. As pelvic mesh settlements near $8 billion, women question lawyers' fees. New York Times. February 1, 2019. https://www.nytimes.com/2019/02/01/business/pelvic-mesh-settlements-lawyers.html. Accessed June 19, 2019.
- Johnson G. Surgeons fear pelvic mesh lawsuits will spook patients. Associated Press News. January 10, 2019. https://www.apnews.com/25777c3c33e3489283b1dc2ebdde6b55. Accessed June 19, 2019.
- Clarke RN. Medical device marketing and the ethics of vaginal mesh kit marketing. In The Innovation and Evolution of Medical Devices. New York, NY: Springer; 2019:103-123.
- Top 5 drug and medical device developments of 2018. Law 360. January 1, 2019. Accessed through LexisNexis.
- Frankel A, Dye J. The Lien Machine. New breed of investor profits by financing surgeries for desperate women patients. Reuters. August 18, 2015. https://www.reuters.com/investigates/special-report/usa-litigation-mesh/. Accessed June 19, 2019.
- Sullivan T. New report looks at intersection of "medical lending" and pelvic mesh lawsuits. Policy & Medicine. May 5, 2018. https://www.policymed.com/2015/08/medical-lending-and-pelvic-mesh-litigation.html. Accessed June 19, 2019.
- Goldstein M, Sliver-Greensberg J. How profiteers lure women into often-unneeded surgery. New York Times. April 14, 2018. https://www.nytimes.com/2018/04/14/business/vaginal-mesh-surgery-lawsuits-financing.html. Accessed June 19, 2019.
CASE Complications with mesh placement for SUI
A 47-year-old woman (G4 P3013) presents 5 months posthysterectomy with evidence of urinary tract infection (UTI). Escherichia coli is isolated, and she responds to antibiotic therapy.
Her surgical history includes a mini-sling procedure using a needleless device and mesh placement in order to correct progressive worsening of loss of urine when coughing and sneezing. She also reported slight pelvic pain, dysuria, and urgency upon urination at that time. After subsequent development of pelvic organ prolapse (POP), she underwent the vaginal hysterectomy.
Following her UTI treatment, a host of problems occur for the patient, including pelvic pain and dyspareunia. Her male partner reports “feeling something during sex,” especially at the anterior vaginal wall. A plain radiograph of the abdomen identifies a 2 cm x 2 cm stone over the vaginal mesh. In consultation with female pelvic medicine and reconstructive surgery subspecialists, lithotripsy is performed, with the stone fragmented. The patient remains symptomatic, however.
The mesh is noted to be eroding through the vaginal wall. An attempt is made to excise the mesh, initially via transuretheral resection, then through a laparoscopic approach. Due to the mesh being embedded in the tissue, however, an open approach is undertaken. Extensive excision of the mesh and stone fragments is performed. Postoperatively, the patient reports “dry vagina,” with no other genitourinary complaints.
The patient sues. She sues the mesh manufacturer. She also seeks to sue the gynecologist who placed the sling and vaginal mesh (as she says she was not informed of “all the risks” of vaginal mesh placement. She is part of a class action lawsuit, along with thousands of other women.
WHAT’S THE VERDICT?
The device manufacturer settled out of court with the class action suit. (The gynecologist was never formally a defendant because the patient/plaintiff was advised to “drop the physician from the suit.”) The attorneys representing the class action received 40% of the award plus presented costs for the representation. The class as a whole received a little more than 50% of the negotiated award. The patient in this case received $60,000.
Medical background
Stress urinary incontinence (SUI) is a prevalent condition; it affects 35% of women.1 Overall, 80% of women aged 80 or younger will undergo some form of surgery for POP during their lifetime.2 The pathophysiology of SUI includes urethral hypermobility and intrinsic sphincter deficiency.3
Surgical correction for urinary incontinence: A timeline
Use of the gracilis muscle flap to surgically correct urinary incontinence was introduced in 1907. This technique has been replaced by today’s more common Burch procedure, which was first described in 1961. Surgical mesh use dates back to the 1950s, when it was primarily used for abdominal hernia repair. Tension-free tape was introduced in 1995.4-6
Continue to: In the late 1990s the US Food and Drug Administration...
In the late 1990s the US Food and Drug Administration (FDA) permitted use of the first transvaginal meshes, which were designed to treat SUI—the midurethral sling. These mesh slings were so successful that similar meshes were developed to treat POP.7 Almost immediately there were problems with the new POP devices, and 3 years later Boston Scientific recalled its device.8 Nonetheless, the FDA cleared more than 150 devices using surgical mesh for urogynecologic indications (FIGURE).9

Mesh complications
Managing complications from intravesical mesh is a clinically challenging problem. Bladder perforation, stone formation, and penetration through the vagina can occur. Bladder-related complications can manifest as recurrent UTIs and obstructive urinary symptoms, especially in association with stone formation. From the gynecologic perspective, the more common complications with mesh utilization are pelvic pain, groin pain, dyspareunia, contracture and scarring of mesh, and narrowing of the vaginal canal.10 Mesh erosion problems will occur in an estimated 10% to 25% of transvaginal mesh POP implants.11
In 2008, a comparison of transvaginal mesh to native tissue repair (suture-based) or other (biologic) grafts was published.12 The bottom line: there is insufficient evidence to suggest that transvaginal mesh significantly improves outcomes for both posterior and apical defects.
Legal background
Mesh used for surgical purposes is a medical device, which legally is a product—a special product to be sure, but a product nonetheless. Products are subject to product liability rules. Mesh is also subject to an FDA regulatory system. We will briefly discuss products liability and the regulation of devices, both of which have played important roles in mesh-related injuries.
Products liability
As a general matter, defective products subject their manufacturer and seller to liability. There are several legal theories regarding product liability: negligence (in which the defect was caused through carelessness), breach of warranty or guarantee (in addition to express warranties, there are a number of implied warranties for products, including that it is fit for its intended purpose), and strict liability (there was a defect in the product, but it may not have been because of negligence). The product may be defective in the way it was designed, manufactured, or packaged, or it may be defective because adequate instructions and warning were not given to consumers.
Of course, not every product involved in an injury is defective—most automobile accidents, for example, are not the result of any defect in the automobile. In medicine, almost no product (device or pharmaceutical) is entirely safe. In some ways they are unavoidably unsafe and bound to cause some injuries. But when injuries are caused by a defect in the product (design or manufacturing defect or failure to warn), then there may be products liability. Most products liability cases arise under state law.
FDA’s device regulations
Both drugs and medical devices are subject to FDA review and ordinarily require some form of FDA clearance before they may be marketed. In the case of devices, the FDA has 3 classes, with an increase in risk to the user from Class I to III. Various levels of FDA review are required before marketing of the device is permitted, again with the intensity of review increasing from I to III as follows:
- Class I devices pose the least risk, have the least regulation, and are subject to general controls (ie, manufacturing and marketing practices).
- Class II devices pose slightly higher risks and are subject to special controls in addition to the criteria for Class I.
- Class III devices pose the most risk to patients and require premarket approval (scientific review and studies are required to ensure efficacy and safety).13
Continue to: There are a number of limits on manufacturer liability for defective devices...
There are a number of limits on manufacturer liability for defective devices. For Class III devices, the thorough FDA review of the safety of a device may limit the ability of an injured patient to sue based on the state product liability laws.14 For the most part, this “preemption” of state law has not played a major role in mesh litigation because they were initially classified as Class II devices which did not require or include a detailed FDA review.15
The duty to warn of the dangers and risk of medical devices means that manufacturers (or sellers) of devices are obligated to inform health care providers and other medical personnel of the risks. Unlike other manufacturers, device manufacturers do not have to directly warn consumers—because physicians deal directly with patients and prescribe the devices. Therefore, the health care providers, rather than the manufacturers, are obligated to inform the patient.16 This is known as the learned intermediary rule. Manufacturers may still be liable for failure to warn if they do not convey to health care providers proper warnings.
Manufacturers and sellers are not the only entities that may be subject to liability caused by medical devices. Hospitals or other entities that stock and care for devices are responsible for maintaining the safety and functionality of devices in their care.
Health care providers also may be responsible for injuries from medical devices. Generally, that liability is based on negligence. Negligence may relate to selecting an improper device, installing or using it incorrectly, or failing to give the patient adequate information (or informed consent) about the device and alternatives to it.17
A look at the mesh mess
There are a lot of distressing problems and professional disappointments in dissecting the “mesh mess,” including a failure of the FDA to regulate effectively, the extended sale and promotion of intrinsic sphincter deficiency mesh products, the improper use of mesh by physicians even after the risks were known, and, in some instances, the taking advantage of injured patients by attorneys and businesses.18 A lot of finger pointing also has occurred.19 We will recount some of the lowlights of this unfortunate tale.
Continue to: The FDA, in the 1990s, classified the first POP and SUI mesh...
The FDA, in the 1990s, classified the first POP and SUI mesh as Class II after deciding these products were “substantially equivalent” to older surgical meshes. This, of course, proved not to be the case.20 The FDA started receiving thousands of reports of adverse events and, in 2008, warned physicians to be vigilant for adverse events from the mesh. The FDA’s notification recommendations regarding mesh included the following13:
- Obtain specialized training for each mesh implantation technique, and be cognizant of risks.
- Be vigilant for potential adverse events from mesh, including erosion and infection.
- Be observant for complications associated with tools of transvaginal placement (ie, bowel, bladder, and vessel perforation).
- Inform patients that implantation of mesh is permanent and complications may require additional surgery for correction.
- Be aware that complications may affect quality of life—eg, pain with intercourse, scarring, and vaginal wall narrowing (POP repair).
- Provide patients with written copy of patient labeling from the surgical mesh manufacturer.
In 2011, the FDA issued a formal warning to providers that transvaginal mesh posed meaningful risks beyond nonmesh surgery. The FDA’s bulletin draws attention to how the mesh is placed more so than the material per se.19,21 Mesh was a Class II device for sacrocolpopexy or midurethral sling and, similarly, the transvaginal kit was also a Class II device. Overall, use of mesh midurethral slings has been well received as treatment for SUI. The FDA also accepted it for POP, however, but with increasingly strong warnings. The FDA’s 2011 communication stated, “This update is to inform you that serious complications associated with surgical mesh for transvaginal repair of POP are not rare….Furthermore, it is not clear that transvaginal POP repair with mesh is more effective than traditional non-mesh repair in all patients with POP and it may expose patients to greater risk.”7,13
In 2014 the FDA proposed reclassifying mesh to a Class III device, which would require that manufacturers obtain approval, based on safety and effectiveness, before selling mesh. Not until 2016 did the FDA actually reclassify the mess as Class III. Of course, during this time, mesh manufacturers were well aware of the substantial problems the products were causing.13
After serious problems with mesh became well known, and especially after FDA warnings, the use of mesh other than as indicated by the FDA was increasingly risky from a legal (as well as a health) standpoint. As long as mesh was still on the market, of course, it was available for use. But use of mesh for POP procedures without good indications in a way that was contrary to the FDA warnings might well be negligent.
Changes to informed consent
The FDA warnings also should have changed the informed consent for the use of mesh.22 Informed consent commonly consists of the following:
- informing the patient of the proposed procedure
- describing risks (and benefits) of the proposed process
- explaining reasonable alternatives
- noting the risks of taking no action.
Information that is material to a decision should be disclosed. If mesh were going to be used, after the problems of mesh were known and identified by the FDA (other than midurethral slings as treatment of SUI), the risks should have been clearly identified for patients, with alternatives outlined. The American College of Obstetricians and Gynecologists Committee on Ethics has 8 fundamental concepts with regard to informed consent that are worth keeping in mind23:
- Obtaining informed consent for medical treatment and research is an ethical requirement.
- The process expresses respect for the patient as a person.
- It protects patients against unwanted treatment and allows patients’ active involvement in medical planning and care.
- Communication is of paramount importance.
- Informed consent is a process and not a signature on a form.
- A commitment to informed consent and to provision of medical benefit to the patient are linked to provision of care.
- If obtaining informed consent is impossible, a designated surrogate should be identified representing the patient’s best interests.
- Knowledge on the part of the provider regarding state and federal requirements is necessary.
Continue to: Lawsuits line up...
Lawsuits line up
The widespread use of a product with a significant percentage of injuries and eventually with warnings about injuries from use sounds like the formula for a lot of lawsuits. This certainly has happened. A large number of suits—both class actions and individual actions—were filed as a result of mesh injuries.24 These suits were overwhelmingly against the manufacturer, although some included physicians.7 Device makers are more attractive defendants for several reasons. First, they have very deep pockets. In addition, jurors are generally much less sympathetic to large companies than to doctors. Large class actions meant that there were many different patients among the plaintiffs, and medical malpractice claims in most states have a number of trial difficulties not present in other product liability cases. Common defendants have included Johnson & Johnson, Boston Scientific, and Medtronic.
Some of the cases resulted in very large damage awards against manufacturers based on various kinds of product(s) liability. Many other cases were settled or tried with relatively small damages. There were, in addition, a number of instances in which the manufacturers were not liable. Of the 32 plaintiffs who have gone to trial thus far, 24 have obtained verdicts totaling $345 million ($14 million average). The cases that have settled have been for much less—perhaps $60,000 on average. A number of cases remain unresolved. To date, the estimate is that 100,000 women have received almost $8 billion from 7 device manufacturers to resolve claims.25
Some state attorneys general have gotten into the process as well. Attorneys general from California, Kentucky, Mississippi, and Washington have filed lawsuits against Johnson & Johnson, claiming that they deceived doctors and patients about the risks of their pelvic mesh. The states claim that marketing and instructional literature should have contained more information about the risks. Some physicians in these states have expressed concern that these lawsuit risks may do more harm than good because the suits conflate mesh used to treat incontinence with the more risky mesh for POP.26
The “ugly” of class action lawsuits
We have discussed both the sad (the injuries to patients) and the bad (the slow regulatory response and continuing injuries). (The ethics of the marketing by the manufacturers might also be raised as the bad.27) Next, let’s look briefly at the ugly.
Some of the patients affected by mesh injuries have been victimized a second time by medical “lenders” and some of their attorneys. Press reports describe patients with modest awards paying 40% in attorney fees (on the high side for personal injury settlements) plus extravagant costs—leaving modest amounts of actual recovery.25
Worse still, a process of “medical lending” has arisen in mesh cases.28 Medical lenders may contact mesh victims offering to pay up front for surgery to remove mesh, and then place a lien against the settlement for repayment at a much higher rate. They might pay the surgeon $2,500 for the surgery, but place a lien on the settlement amount for $60,000.29,30 In addition, there are allegations that lawyers may recruit the doctors to overstate the injuries or do unnecessary removal surgery because that will likely up the award.31 A quick Google search indicates dozens of offers of cash now for your mesh lawsuit (transvaginal and hernia repair).
The patient in our hypothetical case at the beginning had a fairly typical experience. She was a member of a class filing and received a modest settlement. The attorneys representing the class were allowed by the court to charge substantial attorneys’ fees and costs. The patient had the good sense to avoid medical lenders, although other members of the class did use medical lenders and are now filing complaints about the way they were treated by these lenders.
- Maintain surgical skills and be open to new technology. Medical practice requires constant updating and use of new and improved technology as it comes along. By definition, new technology often requires new skills and understanding. A significant portion of surgeons using mesh indicated that they had not read the instructions for use, or had done so only once.1 CME programs that include surgical education remain of particular value.
- Whether new technology or old, it is essential to keep up to date on all FDA bulletins pertinent to devices and pharmaceuticals that you use and prescribe. For example, in 2016 and 2018 the FDA warned that the use of a very old class of drugs (fluoroquinolones) should be limited. It advised "that the serious side effects associated with fluoroquinolones generally outweigh the benefits for patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infections who have other treatment options. For patients with these conditions, fluoroquinolones should be reserved for those who do not have alternative treatment options."2 Continued, unnecessary prescriptions for fluoroquinolones would put a physician at some legal risk whether or not the physician had paid any attention to the warning.
- Informed consent is a very important legal and medical process. Take it seriously, and make sure the patient has the information necessary to make informed decisions about treatment. Document the process and the information provided. In some cases consider directing patients to appropriate literature or websites of the manufacturers.
- As to the use of mesh, if not following FDA advice, it is important to document the reason for this and to document the informed consent especially carefully.
- Follow patients after mesh placement for a minimum of 1 year and emphasize to patients they should convey signs and symptoms of complications from initial placement.3 High-risk patients should be of particular concern and be monitored very closely.
References
- Kirkpatrick G, Faber KD, Fromer DL. Transvaginal mesh placement and the instructions for use: a survey of North American urologists. J Urol. https://doi.org/10.1016/j.urpr.2018.05.004.
- FDA Drug Safety Communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. July 26, 2016. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed June 19, 2019.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
CASE Complications with mesh placement for SUI
A 47-year-old woman (G4 P3013) presents 5 months posthysterectomy with evidence of urinary tract infection (UTI). Escherichia coli is isolated, and she responds to antibiotic therapy.
Her surgical history includes a mini-sling procedure using a needleless device and mesh placement in order to correct progressive worsening of loss of urine when coughing and sneezing. She also reported slight pelvic pain, dysuria, and urgency upon urination at that time. After subsequent development of pelvic organ prolapse (POP), she underwent the vaginal hysterectomy.
Following her UTI treatment, a host of problems occur for the patient, including pelvic pain and dyspareunia. Her male partner reports “feeling something during sex,” especially at the anterior vaginal wall. A plain radiograph of the abdomen identifies a 2 cm x 2 cm stone over the vaginal mesh. In consultation with female pelvic medicine and reconstructive surgery subspecialists, lithotripsy is performed, with the stone fragmented. The patient remains symptomatic, however.
The mesh is noted to be eroding through the vaginal wall. An attempt is made to excise the mesh, initially via transuretheral resection, then through a laparoscopic approach. Due to the mesh being embedded in the tissue, however, an open approach is undertaken. Extensive excision of the mesh and stone fragments is performed. Postoperatively, the patient reports “dry vagina,” with no other genitourinary complaints.
The patient sues. She sues the mesh manufacturer. She also seeks to sue the gynecologist who placed the sling and vaginal mesh (as she says she was not informed of “all the risks” of vaginal mesh placement. She is part of a class action lawsuit, along with thousands of other women.
WHAT’S THE VERDICT?
The device manufacturer settled out of court with the class action suit. (The gynecologist was never formally a defendant because the patient/plaintiff was advised to “drop the physician from the suit.”) The attorneys representing the class action received 40% of the award plus presented costs for the representation. The class as a whole received a little more than 50% of the negotiated award. The patient in this case received $60,000.
Medical background
Stress urinary incontinence (SUI) is a prevalent condition; it affects 35% of women.1 Overall, 80% of women aged 80 or younger will undergo some form of surgery for POP during their lifetime.2 The pathophysiology of SUI includes urethral hypermobility and intrinsic sphincter deficiency.3
Surgical correction for urinary incontinence: A timeline
Use of the gracilis muscle flap to surgically correct urinary incontinence was introduced in 1907. This technique has been replaced by today’s more common Burch procedure, which was first described in 1961. Surgical mesh use dates back to the 1950s, when it was primarily used for abdominal hernia repair. Tension-free tape was introduced in 1995.4-6
Continue to: In the late 1990s the US Food and Drug Administration...
In the late 1990s the US Food and Drug Administration (FDA) permitted use of the first transvaginal meshes, which were designed to treat SUI—the midurethral sling. These mesh slings were so successful that similar meshes were developed to treat POP.7 Almost immediately there were problems with the new POP devices, and 3 years later Boston Scientific recalled its device.8 Nonetheless, the FDA cleared more than 150 devices using surgical mesh for urogynecologic indications (FIGURE).9

Mesh complications
Managing complications from intravesical mesh is a clinically challenging problem. Bladder perforation, stone formation, and penetration through the vagina can occur. Bladder-related complications can manifest as recurrent UTIs and obstructive urinary symptoms, especially in association with stone formation. From the gynecologic perspective, the more common complications with mesh utilization are pelvic pain, groin pain, dyspareunia, contracture and scarring of mesh, and narrowing of the vaginal canal.10 Mesh erosion problems will occur in an estimated 10% to 25% of transvaginal mesh POP implants.11
In 2008, a comparison of transvaginal mesh to native tissue repair (suture-based) or other (biologic) grafts was published.12 The bottom line: there is insufficient evidence to suggest that transvaginal mesh significantly improves outcomes for both posterior and apical defects.
Legal background
Mesh used for surgical purposes is a medical device, which legally is a product—a special product to be sure, but a product nonetheless. Products are subject to product liability rules. Mesh is also subject to an FDA regulatory system. We will briefly discuss products liability and the regulation of devices, both of which have played important roles in mesh-related injuries.
Products liability
As a general matter, defective products subject their manufacturer and seller to liability. There are several legal theories regarding product liability: negligence (in which the defect was caused through carelessness), breach of warranty or guarantee (in addition to express warranties, there are a number of implied warranties for products, including that it is fit for its intended purpose), and strict liability (there was a defect in the product, but it may not have been because of negligence). The product may be defective in the way it was designed, manufactured, or packaged, or it may be defective because adequate instructions and warning were not given to consumers.
Of course, not every product involved in an injury is defective—most automobile accidents, for example, are not the result of any defect in the automobile. In medicine, almost no product (device or pharmaceutical) is entirely safe. In some ways they are unavoidably unsafe and bound to cause some injuries. But when injuries are caused by a defect in the product (design or manufacturing defect or failure to warn), then there may be products liability. Most products liability cases arise under state law.
FDA’s device regulations
Both drugs and medical devices are subject to FDA review and ordinarily require some form of FDA clearance before they may be marketed. In the case of devices, the FDA has 3 classes, with an increase in risk to the user from Class I to III. Various levels of FDA review are required before marketing of the device is permitted, again with the intensity of review increasing from I to III as follows:
- Class I devices pose the least risk, have the least regulation, and are subject to general controls (ie, manufacturing and marketing practices).
- Class II devices pose slightly higher risks and are subject to special controls in addition to the criteria for Class I.
- Class III devices pose the most risk to patients and require premarket approval (scientific review and studies are required to ensure efficacy and safety).13
Continue to: There are a number of limits on manufacturer liability for defective devices...
There are a number of limits on manufacturer liability for defective devices. For Class III devices, the thorough FDA review of the safety of a device may limit the ability of an injured patient to sue based on the state product liability laws.14 For the most part, this “preemption” of state law has not played a major role in mesh litigation because they were initially classified as Class II devices which did not require or include a detailed FDA review.15
The duty to warn of the dangers and risk of medical devices means that manufacturers (or sellers) of devices are obligated to inform health care providers and other medical personnel of the risks. Unlike other manufacturers, device manufacturers do not have to directly warn consumers—because physicians deal directly with patients and prescribe the devices. Therefore, the health care providers, rather than the manufacturers, are obligated to inform the patient.16 This is known as the learned intermediary rule. Manufacturers may still be liable for failure to warn if they do not convey to health care providers proper warnings.
Manufacturers and sellers are not the only entities that may be subject to liability caused by medical devices. Hospitals or other entities that stock and care for devices are responsible for maintaining the safety and functionality of devices in their care.
Health care providers also may be responsible for injuries from medical devices. Generally, that liability is based on negligence. Negligence may relate to selecting an improper device, installing or using it incorrectly, or failing to give the patient adequate information (or informed consent) about the device and alternatives to it.17
A look at the mesh mess
There are a lot of distressing problems and professional disappointments in dissecting the “mesh mess,” including a failure of the FDA to regulate effectively, the extended sale and promotion of intrinsic sphincter deficiency mesh products, the improper use of mesh by physicians even after the risks were known, and, in some instances, the taking advantage of injured patients by attorneys and businesses.18 A lot of finger pointing also has occurred.19 We will recount some of the lowlights of this unfortunate tale.
Continue to: The FDA, in the 1990s, classified the first POP and SUI mesh...
The FDA, in the 1990s, classified the first POP and SUI mesh as Class II after deciding these products were “substantially equivalent” to older surgical meshes. This, of course, proved not to be the case.20 The FDA started receiving thousands of reports of adverse events and, in 2008, warned physicians to be vigilant for adverse events from the mesh. The FDA’s notification recommendations regarding mesh included the following13:
- Obtain specialized training for each mesh implantation technique, and be cognizant of risks.
- Be vigilant for potential adverse events from mesh, including erosion and infection.
- Be observant for complications associated with tools of transvaginal placement (ie, bowel, bladder, and vessel perforation).
- Inform patients that implantation of mesh is permanent and complications may require additional surgery for correction.
- Be aware that complications may affect quality of life—eg, pain with intercourse, scarring, and vaginal wall narrowing (POP repair).
- Provide patients with written copy of patient labeling from the surgical mesh manufacturer.
In 2011, the FDA issued a formal warning to providers that transvaginal mesh posed meaningful risks beyond nonmesh surgery. The FDA’s bulletin draws attention to how the mesh is placed more so than the material per se.19,21 Mesh was a Class II device for sacrocolpopexy or midurethral sling and, similarly, the transvaginal kit was also a Class II device. Overall, use of mesh midurethral slings has been well received as treatment for SUI. The FDA also accepted it for POP, however, but with increasingly strong warnings. The FDA’s 2011 communication stated, “This update is to inform you that serious complications associated with surgical mesh for transvaginal repair of POP are not rare….Furthermore, it is not clear that transvaginal POP repair with mesh is more effective than traditional non-mesh repair in all patients with POP and it may expose patients to greater risk.”7,13
In 2014 the FDA proposed reclassifying mesh to a Class III device, which would require that manufacturers obtain approval, based on safety and effectiveness, before selling mesh. Not until 2016 did the FDA actually reclassify the mess as Class III. Of course, during this time, mesh manufacturers were well aware of the substantial problems the products were causing.13
After serious problems with mesh became well known, and especially after FDA warnings, the use of mesh other than as indicated by the FDA was increasingly risky from a legal (as well as a health) standpoint. As long as mesh was still on the market, of course, it was available for use. But use of mesh for POP procedures without good indications in a way that was contrary to the FDA warnings might well be negligent.
Changes to informed consent
The FDA warnings also should have changed the informed consent for the use of mesh.22 Informed consent commonly consists of the following:
- informing the patient of the proposed procedure
- describing risks (and benefits) of the proposed process
- explaining reasonable alternatives
- noting the risks of taking no action.
Information that is material to a decision should be disclosed. If mesh were going to be used, after the problems of mesh were known and identified by the FDA (other than midurethral slings as treatment of SUI), the risks should have been clearly identified for patients, with alternatives outlined. The American College of Obstetricians and Gynecologists Committee on Ethics has 8 fundamental concepts with regard to informed consent that are worth keeping in mind23:
- Obtaining informed consent for medical treatment and research is an ethical requirement.
- The process expresses respect for the patient as a person.
- It protects patients against unwanted treatment and allows patients’ active involvement in medical planning and care.
- Communication is of paramount importance.
- Informed consent is a process and not a signature on a form.
- A commitment to informed consent and to provision of medical benefit to the patient are linked to provision of care.
- If obtaining informed consent is impossible, a designated surrogate should be identified representing the patient’s best interests.
- Knowledge on the part of the provider regarding state and federal requirements is necessary.
Continue to: Lawsuits line up...
Lawsuits line up
The widespread use of a product with a significant percentage of injuries and eventually with warnings about injuries from use sounds like the formula for a lot of lawsuits. This certainly has happened. A large number of suits—both class actions and individual actions—were filed as a result of mesh injuries.24 These suits were overwhelmingly against the manufacturer, although some included physicians.7 Device makers are more attractive defendants for several reasons. First, they have very deep pockets. In addition, jurors are generally much less sympathetic to large companies than to doctors. Large class actions meant that there were many different patients among the plaintiffs, and medical malpractice claims in most states have a number of trial difficulties not present in other product liability cases. Common defendants have included Johnson & Johnson, Boston Scientific, and Medtronic.
Some of the cases resulted in very large damage awards against manufacturers based on various kinds of product(s) liability. Many other cases were settled or tried with relatively small damages. There were, in addition, a number of instances in which the manufacturers were not liable. Of the 32 plaintiffs who have gone to trial thus far, 24 have obtained verdicts totaling $345 million ($14 million average). The cases that have settled have been for much less—perhaps $60,000 on average. A number of cases remain unresolved. To date, the estimate is that 100,000 women have received almost $8 billion from 7 device manufacturers to resolve claims.25
Some state attorneys general have gotten into the process as well. Attorneys general from California, Kentucky, Mississippi, and Washington have filed lawsuits against Johnson & Johnson, claiming that they deceived doctors and patients about the risks of their pelvic mesh. The states claim that marketing and instructional literature should have contained more information about the risks. Some physicians in these states have expressed concern that these lawsuit risks may do more harm than good because the suits conflate mesh used to treat incontinence with the more risky mesh for POP.26
The “ugly” of class action lawsuits
We have discussed both the sad (the injuries to patients) and the bad (the slow regulatory response and continuing injuries). (The ethics of the marketing by the manufacturers might also be raised as the bad.27) Next, let’s look briefly at the ugly.
Some of the patients affected by mesh injuries have been victimized a second time by medical “lenders” and some of their attorneys. Press reports describe patients with modest awards paying 40% in attorney fees (on the high side for personal injury settlements) plus extravagant costs—leaving modest amounts of actual recovery.25
Worse still, a process of “medical lending” has arisen in mesh cases.28 Medical lenders may contact mesh victims offering to pay up front for surgery to remove mesh, and then place a lien against the settlement for repayment at a much higher rate. They might pay the surgeon $2,500 for the surgery, but place a lien on the settlement amount for $60,000.29,30 In addition, there are allegations that lawyers may recruit the doctors to overstate the injuries or do unnecessary removal surgery because that will likely up the award.31 A quick Google search indicates dozens of offers of cash now for your mesh lawsuit (transvaginal and hernia repair).
The patient in our hypothetical case at the beginning had a fairly typical experience. She was a member of a class filing and received a modest settlement. The attorneys representing the class were allowed by the court to charge substantial attorneys’ fees and costs. The patient had the good sense to avoid medical lenders, although other members of the class did use medical lenders and are now filing complaints about the way they were treated by these lenders.
- Maintain surgical skills and be open to new technology. Medical practice requires constant updating and use of new and improved technology as it comes along. By definition, new technology often requires new skills and understanding. A significant portion of surgeons using mesh indicated that they had not read the instructions for use, or had done so only once.1 CME programs that include surgical education remain of particular value.
- Whether new technology or old, it is essential to keep up to date on all FDA bulletins pertinent to devices and pharmaceuticals that you use and prescribe. For example, in 2016 and 2018 the FDA warned that the use of a very old class of drugs (fluoroquinolones) should be limited. It advised "that the serious side effects associated with fluoroquinolones generally outweigh the benefits for patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infections who have other treatment options. For patients with these conditions, fluoroquinolones should be reserved for those who do not have alternative treatment options."2 Continued, unnecessary prescriptions for fluoroquinolones would put a physician at some legal risk whether or not the physician had paid any attention to the warning.
- Informed consent is a very important legal and medical process. Take it seriously, and make sure the patient has the information necessary to make informed decisions about treatment. Document the process and the information provided. In some cases consider directing patients to appropriate literature or websites of the manufacturers.
- As to the use of mesh, if not following FDA advice, it is important to document the reason for this and to document the informed consent especially carefully.
- Follow patients after mesh placement for a minimum of 1 year and emphasize to patients they should convey signs and symptoms of complications from initial placement.3 High-risk patients should be of particular concern and be monitored very closely.
References
- Kirkpatrick G, Faber KD, Fromer DL. Transvaginal mesh placement and the instructions for use: a survey of North American urologists. J Urol. https://doi.org/10.1016/j.urpr.2018.05.004.
- FDA Drug Safety Communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. July 26, 2016. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed June 19, 2019.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Maral I, Ozkardeş H, Peşkircioğlu L, et al. Prevalence of stress urinary incontinence in both sexes at or after age 15 years: a cross-sectional study. J Urol. 2001;165:408-412.
- Olsen AL, Smith VJ, Bergstrom JO, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
- Chang J, Lee D. Midurethral slings in the mesh litigation era. Transl Androl Urol. 2017;6(suppl 2): S68-S75.
- Mattingly R, ed. TeLinde's Operative Gynecology, 5th edition. Lippincott, William, and Wilkins: Philadelphia, PA; 1997.
- Burch J. Urethrovaginal fixation to Cooper's ligament for correction of stress incontinence, cystocele, and prolapse. Am J Obstet Gynecol. 1961;81:281-290.
- Ulmsten U, Falconer C, Johnson P, et al. A multicenter study of tension-free vaginal tape (TVT) for surgical treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:210-213.
- Kuhlmann-Capek MJ, Kilic GS, Shah AB, et al. Enmeshed in controversy: use of vaginal mesh in the current medicolegal environment. Female Pelvic Med Reconstr Surg. 2015;21:241-243.
- Powell SF. Changing our minds: reforming the FDA medical device reclassification process. Food Drug Law J. 2018;73:177-209.
- US Food and Drug Administration. Surgical Mesh for Treatment of Women with Pelvic Organ Prolapse and Stress Urinary Incontinence. September 2011. https://www.thesenatorsfirm.com/documents/OBS.pdf. Accessed June 19, 2019.
- Maher C, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;(4):CD004014.
- Ganj FA, Ibeanu OA, Bedestani A, Nolan TE, Chesson RR. Complications of transvaginal monofilament polypropylene mesh in pelvic organ prolapse repair. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:919-925.
- Sung VW, Rogers RG, Schaffer JI, et al. Graft use in transvaginal pelvic organ prolapse repair: a systematic review. Obstet Gynecol. 2008;112:1131-1142.
- FDA public health notification: serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. October 20, 2008. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm061976.htm. Accessed February 14, 2019.
- Riegel v. Medtronic, 552 U.S. 312 (2008).
- Whitney DW. Guide to preemption of state-law claims against Class III PMA medical devices. Food Drug Law J. 2010;65:113-139.
- Alam P, Iglesia CB. Informed consent for reconstructive pelvic surgery. Obstet Gynecol Clin North Am. 2016;43:131-139.
- Nosti PA, Iglesia CB. Medicolegal issues surrounding devices and mesh for surgical treatment of prolapse and incontinence. Clin Obstet Gynecol. 2013;56:221-228.
- Shepherd CG. Transvaginal mesh litigation: a new opportunity to resolve mass medical device failure claims. Tennessee Law Rev. 2012;80:3:477-94.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Cohn JA, Timbrook Brown E, Kowalik CG, et al. The mesh controversy. F1000Research website. https://f1000research.com/articles/5-2423/v1. Accessed June 17, 2019.
- Obstetrics and Gynecology Devices Panel Meeting, February 12, 2019. US Food and Drug Administration website. https://www.fda.gov/media/122867/download. Accessed June 19, 2019.
- Mucowski SJ, Jurnalov C, Phelps JY. Use of vaginal mesh in the face of recent FDA warnings and litigation. Am J Obstet Gynecol. 2010;203:103.e1-e4.
- American College of Obstetricians and Gynecologists Committee on Ethics. ACOG Committee Opinion No. 439: informed consent. Obstet Gynecol. 2009;114(2 pt 1):401-408.
- Souders CP, Eilber KS, McClelland L, et al. The truth behind transvaginal mesh litigation: devices, timelines, and provider characteristics. Female Pelvic Med Reconstr Surg. 2018;24:21-25.
- Goldstein M. As pelvic mesh settlements near $8 billion, women question lawyers' fees. New York Times. February 1, 2019. https://www.nytimes.com/2019/02/01/business/pelvic-mesh-settlements-lawyers.html. Accessed June 19, 2019.
- Johnson G. Surgeons fear pelvic mesh lawsuits will spook patients. Associated Press News. January 10, 2019. https://www.apnews.com/25777c3c33e3489283b1dc2ebdde6b55. Accessed June 19, 2019.
- Clarke RN. Medical device marketing and the ethics of vaginal mesh kit marketing. In The Innovation and Evolution of Medical Devices. New York, NY: Springer; 2019:103-123.
- Top 5 drug and medical device developments of 2018. Law 360. January 1, 2019. Accessed through LexisNexis.
- Frankel A, Dye J. The Lien Machine. New breed of investor profits by financing surgeries for desperate women patients. Reuters. August 18, 2015. https://www.reuters.com/investigates/special-report/usa-litigation-mesh/. Accessed June 19, 2019.
- Sullivan T. New report looks at intersection of "medical lending" and pelvic mesh lawsuits. Policy & Medicine. May 5, 2018. https://www.policymed.com/2015/08/medical-lending-and-pelvic-mesh-litigation.html. Accessed June 19, 2019.
- Goldstein M, Sliver-Greensberg J. How profiteers lure women into often-unneeded surgery. New York Times. April 14, 2018. https://www.nytimes.com/2018/04/14/business/vaginal-mesh-surgery-lawsuits-financing.html. Accessed June 19, 2019.
- Maral I, Ozkardeş H, Peşkircioğlu L, et al. Prevalence of stress urinary incontinence in both sexes at or after age 15 years: a cross-sectional study. J Urol. 2001;165:408-412.
- Olsen AL, Smith VJ, Bergstrom JO, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
- Chang J, Lee D. Midurethral slings in the mesh litigation era. Transl Androl Urol. 2017;6(suppl 2): S68-S75.
- Mattingly R, ed. TeLinde's Operative Gynecology, 5th edition. Lippincott, William, and Wilkins: Philadelphia, PA; 1997.
- Burch J. Urethrovaginal fixation to Cooper's ligament for correction of stress incontinence, cystocele, and prolapse. Am J Obstet Gynecol. 1961;81:281-290.
- Ulmsten U, Falconer C, Johnson P, et al. A multicenter study of tension-free vaginal tape (TVT) for surgical treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:210-213.
- Kuhlmann-Capek MJ, Kilic GS, Shah AB, et al. Enmeshed in controversy: use of vaginal mesh in the current medicolegal environment. Female Pelvic Med Reconstr Surg. 2015;21:241-243.
- Powell SF. Changing our minds: reforming the FDA medical device reclassification process. Food Drug Law J. 2018;73:177-209.
- US Food and Drug Administration. Surgical Mesh for Treatment of Women with Pelvic Organ Prolapse and Stress Urinary Incontinence. September 2011. https://www.thesenatorsfirm.com/documents/OBS.pdf. Accessed June 19, 2019.
- Maher C, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;(4):CD004014.
- Ganj FA, Ibeanu OA, Bedestani A, Nolan TE, Chesson RR. Complications of transvaginal monofilament polypropylene mesh in pelvic organ prolapse repair. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:919-925.
- Sung VW, Rogers RG, Schaffer JI, et al. Graft use in transvaginal pelvic organ prolapse repair: a systematic review. Obstet Gynecol. 2008;112:1131-1142.
- FDA public health notification: serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. October 20, 2008. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm061976.htm. Accessed February 14, 2019.
- Riegel v. Medtronic, 552 U.S. 312 (2008).
- Whitney DW. Guide to preemption of state-law claims against Class III PMA medical devices. Food Drug Law J. 2010;65:113-139.
- Alam P, Iglesia CB. Informed consent for reconstructive pelvic surgery. Obstet Gynecol Clin North Am. 2016;43:131-139.
- Nosti PA, Iglesia CB. Medicolegal issues surrounding devices and mesh for surgical treatment of prolapse and incontinence. Clin Obstet Gynecol. 2013;56:221-228.
- Shepherd CG. Transvaginal mesh litigation: a new opportunity to resolve mass medical device failure claims. Tennessee Law Rev. 2012;80:3:477-94.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Cohn JA, Timbrook Brown E, Kowalik CG, et al. The mesh controversy. F1000Research website. https://f1000research.com/articles/5-2423/v1. Accessed June 17, 2019.
- Obstetrics and Gynecology Devices Panel Meeting, February 12, 2019. US Food and Drug Administration website. https://www.fda.gov/media/122867/download. Accessed June 19, 2019.
- Mucowski SJ, Jurnalov C, Phelps JY. Use of vaginal mesh in the face of recent FDA warnings and litigation. Am J Obstet Gynecol. 2010;203:103.e1-e4.
- American College of Obstetricians and Gynecologists Committee on Ethics. ACOG Committee Opinion No. 439: informed consent. Obstet Gynecol. 2009;114(2 pt 1):401-408.
- Souders CP, Eilber KS, McClelland L, et al. The truth behind transvaginal mesh litigation: devices, timelines, and provider characteristics. Female Pelvic Med Reconstr Surg. 2018;24:21-25.
- Goldstein M. As pelvic mesh settlements near $8 billion, women question lawyers' fees. New York Times. February 1, 2019. https://www.nytimes.com/2019/02/01/business/pelvic-mesh-settlements-lawyers.html. Accessed June 19, 2019.
- Johnson G. Surgeons fear pelvic mesh lawsuits will spook patients. Associated Press News. January 10, 2019. https://www.apnews.com/25777c3c33e3489283b1dc2ebdde6b55. Accessed June 19, 2019.
- Clarke RN. Medical device marketing and the ethics of vaginal mesh kit marketing. In The Innovation and Evolution of Medical Devices. New York, NY: Springer; 2019:103-123.
- Top 5 drug and medical device developments of 2018. Law 360. January 1, 2019. Accessed through LexisNexis.
- Frankel A, Dye J. The Lien Machine. New breed of investor profits by financing surgeries for desperate women patients. Reuters. August 18, 2015. https://www.reuters.com/investigates/special-report/usa-litigation-mesh/. Accessed June 19, 2019.
- Sullivan T. New report looks at intersection of "medical lending" and pelvic mesh lawsuits. Policy & Medicine. May 5, 2018. https://www.policymed.com/2015/08/medical-lending-and-pelvic-mesh-litigation.html. Accessed June 19, 2019.
- Goldstein M, Sliver-Greensberg J. How profiteers lure women into often-unneeded surgery. New York Times. April 14, 2018. https://www.nytimes.com/2018/04/14/business/vaginal-mesh-surgery-lawsuits-financing.html. Accessed June 19, 2019.
Fibroids: Patient considerations in medical and surgical management
Uterine fibroids (myomas or leiomyomas) are common and can cause considerable morbidity, including infertility, in reproductive-aged women. In this roundtable discussion, moderated by OBG
Perspectives on a pervasive problem
Joseph S. Sanfilippo, MD, MBA: First let’s discuss the scope of the problem. How prevalent are uterine fibroids, and what are their effects on quality of life?
Linda D. Bradley, MD: Fibroids are extremely prevalent. Depending on age and race, between 60% and 80% of women have them.1 About 50% of women with fibroids have no symptoms2; in symptomatic women, the symptoms may vary based on age. Fibroids are more common in women from the African diaspora, who have earlier onset of symptoms, very large or more numerous fibroids, and more symptomatic fibroids, according to some clinical studies.3 While it is a very common disease state, about half of women with fibroids may not have significant symptoms that warrant anything more than watchful waiting or some minimally invasive options.
Ted L. Anderson, MD, PhD: We probably underestimate the scope because we see people coming in with fibroids only when they have a specific problem. There probably are a lot of asymptomatic women out there that we do not know about.
Case 1: Abnormal uterine bleeding in a young woman desiring pregnancy in the near future
Dr. Sanfilippo: Abnormal uterine bleeding is a common dilemma in my practice. Consider the following case example.
A 24-year-old woman (G1P1) presents with heavy, irregular menses over 6 months’ duration. She is interested in pregnancy, not immediately but in several months. She passes clots, soaks a pad in an hour, and has dysmenorrhea and fatigue. She uses no birth control. She is very distraught, as this bleeding truly has changed her lifestyle.
What is your approach to counseling this patient?
Dr. Bradley: You described a woman whose quality of life is very poor—frequent pad changes, clotting, pain. And she wants to have a child. A patient coming to me with those symptoms does not need to wait 4 to 6 months. I would immediately do some early evaluation.
Dr. Anderson: Sometimes a patient comes to us and already has had an ultrasonography exam. That is helpful, but I am driven by the fact that this patient is interested in pregnancy. I want to look at the uterine cavity and will probably do an office hysteroscopy to see if she has fibroids that distort the uterine cavity. Are there fibroids inside the cavity? To what degree does that possibly play a role? The presence of fibroids does not necessarily mean there is distortion of the cavity, and some evidence suggests that you do not need to do anything about those fibroids.4 Fibroids actually may not be the source of bleeding. We need to keep an open mind when we do the evaluation.
Continue to: Imaging technologies and classification aids...
Imaging technologies and classification aids
Dr. Sanfilippo: Apropos to your comment, is there a role for a sonohysterography in this population?
Dr. Anderson: That is a great technique. Some clinicians prefer to use sonohysterography while others prefer hysteroscopy. I tend to use hysteroscopy, and I have the equipment in the office. Both are great techniques and they answer the same question with respect to cavity evaluation.
Dr. Bradley: We once studied about 150 patients who, on the same day, with 2 separate examiners (one being me), would first undergo saline infusion sonohysterography (SIS) and then hysteroscopy, or vice versa. The sensitivity of identifying an intracavitary lesion is quite good with both. The additional benefit with SIS is that you can look at the adnexa.
In terms of the classification by the International Federation of Gynaecology and Obstetrics (FIGO), sometimes when we do a hysteroscopy, we are not sure how deep a fibroid is—whether it is a type 1 or type 2 or how close it is to the serosa (see illustration, page 26). Are we seeing just the tip of the iceberg? There is a role for imaging, and it is not always an “either/or” situation. There are times, for example, that hysteroscopy will show a type 0. Other times it may not show that, and you look for other things in terms of whether a fibroid abuts the endometrium. The take-home message is that physicians should abandon endometrial biopsy alone and, in this case, not offer a D&C.
In evaluating the endometrium, as gynecologists we should be facile in both technologies. In our workplaces we need to advocate to get trained, to be certified, and to be able to offer both technologies, because sometimes you need both to obtain the right answer.
Dr. Sanfilippo: Let’s talk about the FIGO classification, because it is important to have a communication method not only between physicians but with the patient. If we determine that a fibroid is a type 0, and therefore totally intracavitary, management is different than if the fibroid is a type 1 (less than 50% into the myometrium) or type 2 (more than 50%). What is the role for a classification system such as the FIGO?
Dr. Anderson: I like the FIGO classification system. We can show the patient fibroid classification diagrammatically and she will be able to understand exactly what we are talking about. It’s helpful for patient education and for surgical planning. The approach to a type 0 fibroid is a no-brainer, but with type 1 and more specifically with type 2, where the bulk of the fibroid is intramural and only a portion of that is intracavitary, fibroid size begins to matter a lot in terms of treatment approach.
Sometimes although a fibroid is intracavitary, a laparoscopic rather than hysteroscopic approach is preferred, as long as you can dissect the fibroid away from the endometrium. FIGO classification is very helpful, but I agree with Dr. Bradley that first you need to do a thorough evaluation to make your operative plan.
Continue to: Dr. Sanfilippo...
Dr. Sanfilippo: I encourage residents to go through an orderly sequence of assessment for evaluating abnormal uterine bleeding, including anatomic and endocrinologic factors. The PALM-COEIN classification system is a great mnemonic for use in evaluating abnormal uterine bleeding (TABLE).5 Is there a role for an aid such as PALM-COEIN in your practice?

Dr. Bradley: I totally agree. In 2011, Malcolm Munro and colleagues in the FIGO Working Group on Menstrual Disorders helped us to have a reporting on outcomes by knowing the size, number, and location of fibroids.5 This helps us to look for structural causes and then, to get to the answer, we often use imaging such as ultrasonography or saline infusion, sometimes magnetic resonance imaging (MRI), because other conditions can coexist—endometrial polyps, adenomyosis, and so on.
The PALM-COEIN system helps us to look at 2 things. One is that in addition to structural causes, there can be hematologic causes. While it is rare in a 24-year-old, we all have had the anecdotal patient who came in 6 months ago, had a fibroid, but had a platelet count of 6,000. Second, we have to look at the patient as a whole. My residents, myself, and our fellows look at any bleeding. Does she have a bleeding diathesis, bruising, nose bleeds; has she been anemic, does she have pica? Has she had a blood transfusion, is she on certain medications? We do not want to create a “silo” and think that the patient can have only a fibroid, because then we may miss an opportunity to treat other disease states. She can have a fibroid coexisting with polycystic ovary syndrome (PCOS), for instance. I like to look at everything so we can offer appropriate treatment modalities.
Dr. Sanfilippo: You bring up a very important point. Coagulopathies are more common statistically at the earlier part of a woman’s reproductive age group, soon after menarche, but they also occur toward menopause. We have to be cognizant that a woman can develop a coagulopathy throughout the reproductive years.
Dr. Anderson: You have to look at other medical causes. That is where the PALM-COEIN system can help. It helps you take the blinders off. If you focus on the fibroid and treat the fibroid and the patient still has bleeding, you missed something. You have to consider the whole patient and think of all the nonclassical or nonanatomical things, for example, thyroid disease. The PALM-COEIN helps us to evaluate the patient in a methodical way—every patient every time—so you do not miss something.
The value of MRI
Dr. Sanfilippo: What is the role for MRI, and when do you use it? Is it for only when you do a procedure—laparoscopically, robotically, open—so you have a detailed map of the fibroids?
Dr. Anderson: I love MRI, especially for hysteroscopy. I will print out the MRI image and trace the fibroid because there are things I want to know: exactly how much of the fibroid is inside or outside, where this fibroid is in the uterus, and how much of a normal buffer there is between the edge of that fibroid and the serosa. How aggressive can I be, or how cautious do I need to be, during the resection? Maybe this will be a planned 2-stage resection. MRIs are wonderful for fibroid disease, not only for diagnosis but also for surgical planning and patient counseling.
Dr. Bradley: SIS is also very useful. If the patient has an intracavitary fibroid that is larger than 4.5 to 5 cm and we insert the catheter, however, sometimes you cannot distend the cavity very well. Sometimes large intramural fibroids can compress the cavity, making the procedure difficult in an office setting. You cannot see the limits to help you as a surgical option. Although SIS generally is associated with little pain, some patients may have pain, and some patients cannot tolerate the test.
Continue to: I would order an MRI for surgical planning when...
I would order an MRI for surgical planning when a hysteroscopy is equivocal and if I cannot do an SIS. Also, if a patient who had a hysteroscopic resection with incomplete removal comes to me and is still symptomatic, I want to know the depth of penetration.
Obtaining an MRI may sometimes be difficult at a particular institution, and some clinicians have to go through the hurdles of getting an ultrasound to get certified and approved. We have to be our patient’s advocate and do the peer phone calls; any other specialty would require presurgical planning, and we are no different from other surgeons in that regard.
Dr. Sanfilippo: Yes, that can be a stumbling block. In the operating room, I like to have the images right in front of me, ideally an MRI or an ultrasound scan, as I know how to proceed. Having that visual helps me understand how close the fibroid is to the lining of the uterus.
Tapping into radiologists’ expertise
Dr. Bradley: Every quarter we meet with our radiologists, who are very interested in our MRI and SIS reports. They will describe the count and say how many fibroids—that is very helpful instead of just saying she has a bunch of fibroids—but they also will tell us when there is a type 0, a type 2, a type 7 fibroid. The team looks for adenomyosis and for endometriosis that can coexist.
Dr. Anderson: One caution about reading radiology reports is that often someone will come in with a report from an outside hospital or from a small community hospital that may say, “There is a 2-cm submucosal fibroid.” Some people might be tempted to take this person right to the OR, but you need to look at the images yourself, because in a radiologist’s mind “submucosal” truly means under the mucosa, which in our liturgy would be “intramural.” So we need to make sure that we are talking the same language. You should look at the images yourself.
Dr. Sanfilippo: I totally agree. It is also not unreasonable to speak with the radiologists and educate them about the FIGO classification.
Dr. Bradley: I prefer the word “intracavitary” for fibroids. When I see a typed report without the picture, “submucosal” can mean in the cavity or abutting the endometrium.
Case 2: Woman with heavy bleeding and fibroids seeks nonsurgical treatment
Dr. Sanfilippo: A 39-year-old (G3P3) woman is referred for evaluation for heavy vaginal bleeding, soaking a pad in an hour, which has been going on for months. Her primary ObGyn obtained a pelvic sonogram and noted multiple intramural and subserosal fibroids. A sonohysterogram reveals a submucosal myoma.
The patient is not interested in a hysterectomy. She was treated with birth control pills, with no improvement. She is interested in nonsurgical options. Dr. Bradley, what medical treatments might you offer this patient?
Medical treatment options
Dr. Bradley: If oral contraceptives have not worked, a good option would be tranexamic acid. Years ago our hospital was involved with enrolling patients in the multicenter clinical trial of this drug. The classic patient enrolled had regular, predictable, heavy menstrual cycles with alkaline hematin assay of greater than 80. If the case patient described has regular and predictable heavy bleeding every month at the same time, for the same duration, I would consider the use of tranexamic acid. There are several contraindications for the drug, so those exclusion issues would need to be reviewed. Contraindications include subarachnoid hemorrhage. Cerebral edema and cerebral infarction may be caused by tranexamic acid in such patients. Other contraindications include active intravascular clotting and hypersensitivity.
Continue to: Another option is to see if a progestin-releasing intrauterine system...
Another option is to see if a progestin-releasing intrauterine system (IUS) like the levonorgestrel (LNG) IUS would fit into this patient’s uterine cavity. Like Ted, I want to look into that cavity. I am not sure what “submucosal fibroid” means. If it has not distorted the cavity, or is totally within the uterine cavity, or abuts the endometrial cavity. The LNG-IUS cannot be placed into a uterine cavity that has intracavitary fibroids or sounds to greater than 12 cm. We are not going to put an LNG-IUS in somebody, at least in general, with a globally enlarged uterine cavity. I could ask, do you do that? You do a bimanual exam, and it is 18-weeks in size. I am not sure that I would put it in, but does it meet those criteria? The package insert for the LNG-IUS specifies upper and lower limits of uterine size for placement. I would start with those 2 options (tranexamic acid and LNG-IUS), and also get some more imaging.
Dr. Anderson: I agree with Linda. The submucosal fibroid could be contributing to this patient’s bleeding, but it is not the total contribution. The other fibroids may be completely irrelevant as far as her bleeding is concerned. We may need to deal with that one surgically, which we can do without a hysterectomy, most of the time.
I am a big fan of the LNG-IUS, it has been great in my experience. There are some other treatments available as well, such as gonadotropin–releasing hormone (GnRH) agonists. I tell patients that, while GnRH does work, it is not designed to be long-term therapy. If I have, for example, a 49-year-old patient, I just need to get her to menopause. Longer-term GnRH agonists might be a good option in this case. Otherwise, we could use short-term a GnRH agonist to stop the bleeding for a while so that we can reset the clock and get her started on something like levonorgestrel, tranexamic acid, or one of the other medical therapies. That may be a 2-step combination therapy.
Dr. Sanfilippo: There is a whole category of agents available—selective progesterone receptor modulators (SPRMs), pure progesterone receptor antagonists, ulipristal comes to mind. Clinicians need to know that options are available beyond birth control pills.
Dr. Anderson: As I tell patients, there are also “bridge” options. These are interventional procedures that are not hysterectomy, such as uterine fibroid embolization or endometrial ablation if bleeding is really the problem. We might consider a variety of different approaches. Obviously, we do not typically use fibroid embolization for submucosal fibroids, but it depends on how much of the fibroid is intracavitary and how big it is. Other options are a little more aggressive than medical therapy but they do not involve a hysterectomy.
Pros and cons of uterine artery embolization
Dr. Sanfilippo: If a woman desires future childbearing, is there a role for uterine artery embolization? How would you counsel her about the pros and cons?
Dr. Bradley: At the Cleveland Clinic, we generally do not offer uterine artery embolization if the patient wants a child. While it is an excellent method for treating heavy bleeding and bulk symptoms, the endometrium can be impacted. Patients can develop fistula, adhesions, or concentric narrowing, and changes in anti-Müllerian hormone levels, and there is potential for an Asherman-like syndrome and poor perfusion. I have many hysteroscopic images where the anterior wall of the uterus is nice and pink and the posterior wall is totally pale. The embolic microsphere particles can reach the endometrium—I have seen particles in the endometrium when doing a fibroid resection.
Continue to: A good early study looked at 555 women for almost a year...
A good early study looked at 555 women for almost a year.6 If women became pregnant, they had a higher rate of postpartum hemorrhage; placenta accreta, increta, and percreta; and emergent hysterectomy. It was recommended that these women deliver at a tertiary care center due to higher rates of preterm labor and malposition.
If a patient wants a baby, she should find a gynecologic surgeon who does minimally invasive laparoscopic, robotic, or open surgery, because she is more likely to have a take-home baby with a surgical approach than with embolization. In my experience, there is always going to be a patient who wants to keep her uterus at age 49 and who has every comorbidity. I might offer her the embolization just knowing what the odds of pregnancy are.
Dr. Anderson: I agree with Linda but I take a more liberal approach. Sometimes we do a myomectomy because we are trying to enhance fertility, while other times we do a myomectomy to address fibroid-related symptoms. These patients are having specific symptoms, and we want to leave the embolization option open.
If I have a patient who is 39 and becoming pregnant is not necessarily her goal, but she does not want to have a hysterectomy and if she got pregnant it would be okay, I am going to treat her a little different with respect to fibroid embolization than I would treat someone who is actively trying to have a baby. This goes back to what you were saying, let’s treat the patient, not just the fibroid.
Dr. Bradley: That is so important and sentinel. If she really does not want a hysterectomy but does not want a baby, I will ask, “Would you go through in vitro fertilization? Would you take clomiphene?” If she answers no, then I feel more comfortable, like you, with referring the patient for uterine fibroid embolization. The point is to get the patient with the right team to get the best outcomes.
Surgical approaches, intraoperative agents, and suture technique
Dr. Sanfilippo: Dr. Anderson, tell us about your surgical approaches to fibroids.
Dr. Anderson: At my institution we do have a fellowship in minimally invasive surgery, but I still do a lot of open myomectomies. I have a few guidelines to determine whether I am going to proceed laparoscopically, do a little minilaparotomy incision, or if a gigantic uterus is going to require a big incision. My mantra to my fellows has always been, “minimally invasive is the impact on the patient, not the size of the incision.”
Sometimes, prolonged anesthesia and Trendelenburg create more morbidity than a minilaparotomy. If a patient has 4 or 5 fibroids and most of them are intramural and I cannot see them but I want to be able to feel them, and to get a really good closure of the myometrium, I might choose to do a minilaparotomy. But if it is a case of a solitary fibroid, I would be more inclined to operate laparoscopically.
Continue to: Dr. Bradley...
Dr. Bradley: Our protocol is similar. We use MRI liberally. If patients have 4 or more fibroids and they are larger than 8 cm, most will have open surgery. I do not do robotic or laparoscopic procedures, so my referral source is for the larger myomas. We do not put retractors in; we can make incisions. Even if we do a huge Maylard incision, it is cosmetically wonderful. We use a loading dose of IV tranexamic acid with tranexamic acid throughout the surgery, and misoprostol intravaginally prior to surgery, to control uterine bleeding.
Dr. Sanfilippo: Dr. Anderson, is there a role for agents such as vasopressin, and what about routes of administration?
Dr. Anderson: When I do a laparoscopic or open procedure, I inject vasopressin (dilute 20 U in 100 mL of saline) into the pseudocapsule around the fibroid. I also administer rectal misoprostol (400 µg) just before the patient prep is done, which is amazing in reducing blood loss. There is also a role for a GnRH agonist, not necessarily to reduce the size of the uterus but to reduce blood flow in the pelvis and blood loss. Many different techniques are available. I do not use tourniquets, however. If bleeding does occur, I want to see it so I can fix it—not after I have sewn up the uterus and taken off a tourniquet.
Dr. Bradley: Do you use Floseal hemostatic matrix or any other agent to control bleeding?
Dr. Anderson: I do, for local hemostasis.
Dr. Bradley: Some surgeons will use barbed suture.
Dr. Anderson: I do like barbed sutures. In teaching residents to do myomectomy, it is very beneficial. But I am still a big fan of the good old figure-of-8 stitch because it is compressive and you get a good apposition of the tissue, good hemostasis, and strong closure.
Dr. Sanfilippo: We hope that this conversation will change your management of uterine fibroids. I thank Dr. Bradley and Dr. Anderson for a lively and very informative discussion.
Watch the video: Video roundtable–Fibroids: Patient considerations in medical and surgical management
- Khan AT, Shehmar M, Gupta JK. Uterine fibroids: current perspectives. Int J Womens Health. 2014;6:95-114.
- Divakars H. Asymptomatic uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22:643-654.
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health. 2013;22:807-816.
- Hartmann KE, Velez Edwards DR, Savitz DA, et al. Prospective cohort study of uterine fibroids and miscarriage risk. Am J Epidemiol. 2017;186:1140-1148.
- Munro MG, Critchley HOD, Fraser IS, for the FIGO Menstrual Disorders Working Group. The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertil Steril. 2011;95:2204-2208.
- Pron G, Mocarski E, Bennett J, et al; Ontario UFE Collaborative Group. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
Uterine fibroids (myomas or leiomyomas) are common and can cause considerable morbidity, including infertility, in reproductive-aged women. In this roundtable discussion, moderated by OBG
Perspectives on a pervasive problem
Joseph S. Sanfilippo, MD, MBA: First let’s discuss the scope of the problem. How prevalent are uterine fibroids, and what are their effects on quality of life?
Linda D. Bradley, MD: Fibroids are extremely prevalent. Depending on age and race, between 60% and 80% of women have them.1 About 50% of women with fibroids have no symptoms2; in symptomatic women, the symptoms may vary based on age. Fibroids are more common in women from the African diaspora, who have earlier onset of symptoms, very large or more numerous fibroids, and more symptomatic fibroids, according to some clinical studies.3 While it is a very common disease state, about half of women with fibroids may not have significant symptoms that warrant anything more than watchful waiting or some minimally invasive options.
Ted L. Anderson, MD, PhD: We probably underestimate the scope because we see people coming in with fibroids only when they have a specific problem. There probably are a lot of asymptomatic women out there that we do not know about.
Case 1: Abnormal uterine bleeding in a young woman desiring pregnancy in the near future
Dr. Sanfilippo: Abnormal uterine bleeding is a common dilemma in my practice. Consider the following case example.
A 24-year-old woman (G1P1) presents with heavy, irregular menses over 6 months’ duration. She is interested in pregnancy, not immediately but in several months. She passes clots, soaks a pad in an hour, and has dysmenorrhea and fatigue. She uses no birth control. She is very distraught, as this bleeding truly has changed her lifestyle.
What is your approach to counseling this patient?
Dr. Bradley: You described a woman whose quality of life is very poor—frequent pad changes, clotting, pain. And she wants to have a child. A patient coming to me with those symptoms does not need to wait 4 to 6 months. I would immediately do some early evaluation.
Dr. Anderson: Sometimes a patient comes to us and already has had an ultrasonography exam. That is helpful, but I am driven by the fact that this patient is interested in pregnancy. I want to look at the uterine cavity and will probably do an office hysteroscopy to see if she has fibroids that distort the uterine cavity. Are there fibroids inside the cavity? To what degree does that possibly play a role? The presence of fibroids does not necessarily mean there is distortion of the cavity, and some evidence suggests that you do not need to do anything about those fibroids.4 Fibroids actually may not be the source of bleeding. We need to keep an open mind when we do the evaluation.
Continue to: Imaging technologies and classification aids...
Imaging technologies and classification aids
Dr. Sanfilippo: Apropos to your comment, is there a role for a sonohysterography in this population?
Dr. Anderson: That is a great technique. Some clinicians prefer to use sonohysterography while others prefer hysteroscopy. I tend to use hysteroscopy, and I have the equipment in the office. Both are great techniques and they answer the same question with respect to cavity evaluation.
Dr. Bradley: We once studied about 150 patients who, on the same day, with 2 separate examiners (one being me), would first undergo saline infusion sonohysterography (SIS) and then hysteroscopy, or vice versa. The sensitivity of identifying an intracavitary lesion is quite good with both. The additional benefit with SIS is that you can look at the adnexa.
In terms of the classification by the International Federation of Gynaecology and Obstetrics (FIGO), sometimes when we do a hysteroscopy, we are not sure how deep a fibroid is—whether it is a type 1 or type 2 or how close it is to the serosa (see illustration, page 26). Are we seeing just the tip of the iceberg? There is a role for imaging, and it is not always an “either/or” situation. There are times, for example, that hysteroscopy will show a type 0. Other times it may not show that, and you look for other things in terms of whether a fibroid abuts the endometrium. The take-home message is that physicians should abandon endometrial biopsy alone and, in this case, not offer a D&C.
In evaluating the endometrium, as gynecologists we should be facile in both technologies. In our workplaces we need to advocate to get trained, to be certified, and to be able to offer both technologies, because sometimes you need both to obtain the right answer.
Dr. Sanfilippo: Let’s talk about the FIGO classification, because it is important to have a communication method not only between physicians but with the patient. If we determine that a fibroid is a type 0, and therefore totally intracavitary, management is different than if the fibroid is a type 1 (less than 50% into the myometrium) or type 2 (more than 50%). What is the role for a classification system such as the FIGO?
Dr. Anderson: I like the FIGO classification system. We can show the patient fibroid classification diagrammatically and she will be able to understand exactly what we are talking about. It’s helpful for patient education and for surgical planning. The approach to a type 0 fibroid is a no-brainer, but with type 1 and more specifically with type 2, where the bulk of the fibroid is intramural and only a portion of that is intracavitary, fibroid size begins to matter a lot in terms of treatment approach.
Sometimes although a fibroid is intracavitary, a laparoscopic rather than hysteroscopic approach is preferred, as long as you can dissect the fibroid away from the endometrium. FIGO classification is very helpful, but I agree with Dr. Bradley that first you need to do a thorough evaluation to make your operative plan.
Continue to: Dr. Sanfilippo...
Dr. Sanfilippo: I encourage residents to go through an orderly sequence of assessment for evaluating abnormal uterine bleeding, including anatomic and endocrinologic factors. The PALM-COEIN classification system is a great mnemonic for use in evaluating abnormal uterine bleeding (TABLE).5 Is there a role for an aid such as PALM-COEIN in your practice?

Dr. Bradley: I totally agree. In 2011, Malcolm Munro and colleagues in the FIGO Working Group on Menstrual Disorders helped us to have a reporting on outcomes by knowing the size, number, and location of fibroids.5 This helps us to look for structural causes and then, to get to the answer, we often use imaging such as ultrasonography or saline infusion, sometimes magnetic resonance imaging (MRI), because other conditions can coexist—endometrial polyps, adenomyosis, and so on.
The PALM-COEIN system helps us to look at 2 things. One is that in addition to structural causes, there can be hematologic causes. While it is rare in a 24-year-old, we all have had the anecdotal patient who came in 6 months ago, had a fibroid, but had a platelet count of 6,000. Second, we have to look at the patient as a whole. My residents, myself, and our fellows look at any bleeding. Does she have a bleeding diathesis, bruising, nose bleeds; has she been anemic, does she have pica? Has she had a blood transfusion, is she on certain medications? We do not want to create a “silo” and think that the patient can have only a fibroid, because then we may miss an opportunity to treat other disease states. She can have a fibroid coexisting with polycystic ovary syndrome (PCOS), for instance. I like to look at everything so we can offer appropriate treatment modalities.
Dr. Sanfilippo: You bring up a very important point. Coagulopathies are more common statistically at the earlier part of a woman’s reproductive age group, soon after menarche, but they also occur toward menopause. We have to be cognizant that a woman can develop a coagulopathy throughout the reproductive years.
Dr. Anderson: You have to look at other medical causes. That is where the PALM-COEIN system can help. It helps you take the blinders off. If you focus on the fibroid and treat the fibroid and the patient still has bleeding, you missed something. You have to consider the whole patient and think of all the nonclassical or nonanatomical things, for example, thyroid disease. The PALM-COEIN helps us to evaluate the patient in a methodical way—every patient every time—so you do not miss something.
The value of MRI
Dr. Sanfilippo: What is the role for MRI, and when do you use it? Is it for only when you do a procedure—laparoscopically, robotically, open—so you have a detailed map of the fibroids?
Dr. Anderson: I love MRI, especially for hysteroscopy. I will print out the MRI image and trace the fibroid because there are things I want to know: exactly how much of the fibroid is inside or outside, where this fibroid is in the uterus, and how much of a normal buffer there is between the edge of that fibroid and the serosa. How aggressive can I be, or how cautious do I need to be, during the resection? Maybe this will be a planned 2-stage resection. MRIs are wonderful for fibroid disease, not only for diagnosis but also for surgical planning and patient counseling.
Dr. Bradley: SIS is also very useful. If the patient has an intracavitary fibroid that is larger than 4.5 to 5 cm and we insert the catheter, however, sometimes you cannot distend the cavity very well. Sometimes large intramural fibroids can compress the cavity, making the procedure difficult in an office setting. You cannot see the limits to help you as a surgical option. Although SIS generally is associated with little pain, some patients may have pain, and some patients cannot tolerate the test.
Continue to: I would order an MRI for surgical planning when...
I would order an MRI for surgical planning when a hysteroscopy is equivocal and if I cannot do an SIS. Also, if a patient who had a hysteroscopic resection with incomplete removal comes to me and is still symptomatic, I want to know the depth of penetration.
Obtaining an MRI may sometimes be difficult at a particular institution, and some clinicians have to go through the hurdles of getting an ultrasound to get certified and approved. We have to be our patient’s advocate and do the peer phone calls; any other specialty would require presurgical planning, and we are no different from other surgeons in that regard.
Dr. Sanfilippo: Yes, that can be a stumbling block. In the operating room, I like to have the images right in front of me, ideally an MRI or an ultrasound scan, as I know how to proceed. Having that visual helps me understand how close the fibroid is to the lining of the uterus.
Tapping into radiologists’ expertise
Dr. Bradley: Every quarter we meet with our radiologists, who are very interested in our MRI and SIS reports. They will describe the count and say how many fibroids—that is very helpful instead of just saying she has a bunch of fibroids—but they also will tell us when there is a type 0, a type 2, a type 7 fibroid. The team looks for adenomyosis and for endometriosis that can coexist.
Dr. Anderson: One caution about reading radiology reports is that often someone will come in with a report from an outside hospital or from a small community hospital that may say, “There is a 2-cm submucosal fibroid.” Some people might be tempted to take this person right to the OR, but you need to look at the images yourself, because in a radiologist’s mind “submucosal” truly means under the mucosa, which in our liturgy would be “intramural.” So we need to make sure that we are talking the same language. You should look at the images yourself.
Dr. Sanfilippo: I totally agree. It is also not unreasonable to speak with the radiologists and educate them about the FIGO classification.
Dr. Bradley: I prefer the word “intracavitary” for fibroids. When I see a typed report without the picture, “submucosal” can mean in the cavity or abutting the endometrium.
Case 2: Woman with heavy bleeding and fibroids seeks nonsurgical treatment
Dr. Sanfilippo: A 39-year-old (G3P3) woman is referred for evaluation for heavy vaginal bleeding, soaking a pad in an hour, which has been going on for months. Her primary ObGyn obtained a pelvic sonogram and noted multiple intramural and subserosal fibroids. A sonohysterogram reveals a submucosal myoma.
The patient is not interested in a hysterectomy. She was treated with birth control pills, with no improvement. She is interested in nonsurgical options. Dr. Bradley, what medical treatments might you offer this patient?
Medical treatment options
Dr. Bradley: If oral contraceptives have not worked, a good option would be tranexamic acid. Years ago our hospital was involved with enrolling patients in the multicenter clinical trial of this drug. The classic patient enrolled had regular, predictable, heavy menstrual cycles with alkaline hematin assay of greater than 80. If the case patient described has regular and predictable heavy bleeding every month at the same time, for the same duration, I would consider the use of tranexamic acid. There are several contraindications for the drug, so those exclusion issues would need to be reviewed. Contraindications include subarachnoid hemorrhage. Cerebral edema and cerebral infarction may be caused by tranexamic acid in such patients. Other contraindications include active intravascular clotting and hypersensitivity.
Continue to: Another option is to see if a progestin-releasing intrauterine system...
Another option is to see if a progestin-releasing intrauterine system (IUS) like the levonorgestrel (LNG) IUS would fit into this patient’s uterine cavity. Like Ted, I want to look into that cavity. I am not sure what “submucosal fibroid” means. If it has not distorted the cavity, or is totally within the uterine cavity, or abuts the endometrial cavity. The LNG-IUS cannot be placed into a uterine cavity that has intracavitary fibroids or sounds to greater than 12 cm. We are not going to put an LNG-IUS in somebody, at least in general, with a globally enlarged uterine cavity. I could ask, do you do that? You do a bimanual exam, and it is 18-weeks in size. I am not sure that I would put it in, but does it meet those criteria? The package insert for the LNG-IUS specifies upper and lower limits of uterine size for placement. I would start with those 2 options (tranexamic acid and LNG-IUS), and also get some more imaging.
Dr. Anderson: I agree with Linda. The submucosal fibroid could be contributing to this patient’s bleeding, but it is not the total contribution. The other fibroids may be completely irrelevant as far as her bleeding is concerned. We may need to deal with that one surgically, which we can do without a hysterectomy, most of the time.
I am a big fan of the LNG-IUS, it has been great in my experience. There are some other treatments available as well, such as gonadotropin–releasing hormone (GnRH) agonists. I tell patients that, while GnRH does work, it is not designed to be long-term therapy. If I have, for example, a 49-year-old patient, I just need to get her to menopause. Longer-term GnRH agonists might be a good option in this case. Otherwise, we could use short-term a GnRH agonist to stop the bleeding for a while so that we can reset the clock and get her started on something like levonorgestrel, tranexamic acid, or one of the other medical therapies. That may be a 2-step combination therapy.
Dr. Sanfilippo: There is a whole category of agents available—selective progesterone receptor modulators (SPRMs), pure progesterone receptor antagonists, ulipristal comes to mind. Clinicians need to know that options are available beyond birth control pills.
Dr. Anderson: As I tell patients, there are also “bridge” options. These are interventional procedures that are not hysterectomy, such as uterine fibroid embolization or endometrial ablation if bleeding is really the problem. We might consider a variety of different approaches. Obviously, we do not typically use fibroid embolization for submucosal fibroids, but it depends on how much of the fibroid is intracavitary and how big it is. Other options are a little more aggressive than medical therapy but they do not involve a hysterectomy.
Pros and cons of uterine artery embolization
Dr. Sanfilippo: If a woman desires future childbearing, is there a role for uterine artery embolization? How would you counsel her about the pros and cons?
Dr. Bradley: At the Cleveland Clinic, we generally do not offer uterine artery embolization if the patient wants a child. While it is an excellent method for treating heavy bleeding and bulk symptoms, the endometrium can be impacted. Patients can develop fistula, adhesions, or concentric narrowing, and changes in anti-Müllerian hormone levels, and there is potential for an Asherman-like syndrome and poor perfusion. I have many hysteroscopic images where the anterior wall of the uterus is nice and pink and the posterior wall is totally pale. The embolic microsphere particles can reach the endometrium—I have seen particles in the endometrium when doing a fibroid resection.
Continue to: A good early study looked at 555 women for almost a year...
A good early study looked at 555 women for almost a year.6 If women became pregnant, they had a higher rate of postpartum hemorrhage; placenta accreta, increta, and percreta; and emergent hysterectomy. It was recommended that these women deliver at a tertiary care center due to higher rates of preterm labor and malposition.
If a patient wants a baby, she should find a gynecologic surgeon who does minimally invasive laparoscopic, robotic, or open surgery, because she is more likely to have a take-home baby with a surgical approach than with embolization. In my experience, there is always going to be a patient who wants to keep her uterus at age 49 and who has every comorbidity. I might offer her the embolization just knowing what the odds of pregnancy are.
Dr. Anderson: I agree with Linda but I take a more liberal approach. Sometimes we do a myomectomy because we are trying to enhance fertility, while other times we do a myomectomy to address fibroid-related symptoms. These patients are having specific symptoms, and we want to leave the embolization option open.
If I have a patient who is 39 and becoming pregnant is not necessarily her goal, but she does not want to have a hysterectomy and if she got pregnant it would be okay, I am going to treat her a little different with respect to fibroid embolization than I would treat someone who is actively trying to have a baby. This goes back to what you were saying, let’s treat the patient, not just the fibroid.
Dr. Bradley: That is so important and sentinel. If she really does not want a hysterectomy but does not want a baby, I will ask, “Would you go through in vitro fertilization? Would you take clomiphene?” If she answers no, then I feel more comfortable, like you, with referring the patient for uterine fibroid embolization. The point is to get the patient with the right team to get the best outcomes.
Surgical approaches, intraoperative agents, and suture technique
Dr. Sanfilippo: Dr. Anderson, tell us about your surgical approaches to fibroids.
Dr. Anderson: At my institution we do have a fellowship in minimally invasive surgery, but I still do a lot of open myomectomies. I have a few guidelines to determine whether I am going to proceed laparoscopically, do a little minilaparotomy incision, or if a gigantic uterus is going to require a big incision. My mantra to my fellows has always been, “minimally invasive is the impact on the patient, not the size of the incision.”
Sometimes, prolonged anesthesia and Trendelenburg create more morbidity than a minilaparotomy. If a patient has 4 or 5 fibroids and most of them are intramural and I cannot see them but I want to be able to feel them, and to get a really good closure of the myometrium, I might choose to do a minilaparotomy. But if it is a case of a solitary fibroid, I would be more inclined to operate laparoscopically.
Continue to: Dr. Bradley...
Dr. Bradley: Our protocol is similar. We use MRI liberally. If patients have 4 or more fibroids and they are larger than 8 cm, most will have open surgery. I do not do robotic or laparoscopic procedures, so my referral source is for the larger myomas. We do not put retractors in; we can make incisions. Even if we do a huge Maylard incision, it is cosmetically wonderful. We use a loading dose of IV tranexamic acid with tranexamic acid throughout the surgery, and misoprostol intravaginally prior to surgery, to control uterine bleeding.
Dr. Sanfilippo: Dr. Anderson, is there a role for agents such as vasopressin, and what about routes of administration?
Dr. Anderson: When I do a laparoscopic or open procedure, I inject vasopressin (dilute 20 U in 100 mL of saline) into the pseudocapsule around the fibroid. I also administer rectal misoprostol (400 µg) just before the patient prep is done, which is amazing in reducing blood loss. There is also a role for a GnRH agonist, not necessarily to reduce the size of the uterus but to reduce blood flow in the pelvis and blood loss. Many different techniques are available. I do not use tourniquets, however. If bleeding does occur, I want to see it so I can fix it—not after I have sewn up the uterus and taken off a tourniquet.
Dr. Bradley: Do you use Floseal hemostatic matrix or any other agent to control bleeding?
Dr. Anderson: I do, for local hemostasis.
Dr. Bradley: Some surgeons will use barbed suture.
Dr. Anderson: I do like barbed sutures. In teaching residents to do myomectomy, it is very beneficial. But I am still a big fan of the good old figure-of-8 stitch because it is compressive and you get a good apposition of the tissue, good hemostasis, and strong closure.
Dr. Sanfilippo: We hope that this conversation will change your management of uterine fibroids. I thank Dr. Bradley and Dr. Anderson for a lively and very informative discussion.
Watch the video: Video roundtable–Fibroids: Patient considerations in medical and surgical management
Uterine fibroids (myomas or leiomyomas) are common and can cause considerable morbidity, including infertility, in reproductive-aged women. In this roundtable discussion, moderated by OBG
Perspectives on a pervasive problem
Joseph S. Sanfilippo, MD, MBA: First let’s discuss the scope of the problem. How prevalent are uterine fibroids, and what are their effects on quality of life?
Linda D. Bradley, MD: Fibroids are extremely prevalent. Depending on age and race, between 60% and 80% of women have them.1 About 50% of women with fibroids have no symptoms2; in symptomatic women, the symptoms may vary based on age. Fibroids are more common in women from the African diaspora, who have earlier onset of symptoms, very large or more numerous fibroids, and more symptomatic fibroids, according to some clinical studies.3 While it is a very common disease state, about half of women with fibroids may not have significant symptoms that warrant anything more than watchful waiting or some minimally invasive options.
Ted L. Anderson, MD, PhD: We probably underestimate the scope because we see people coming in with fibroids only when they have a specific problem. There probably are a lot of asymptomatic women out there that we do not know about.
Case 1: Abnormal uterine bleeding in a young woman desiring pregnancy in the near future
Dr. Sanfilippo: Abnormal uterine bleeding is a common dilemma in my practice. Consider the following case example.
A 24-year-old woman (G1P1) presents with heavy, irregular menses over 6 months’ duration. She is interested in pregnancy, not immediately but in several months. She passes clots, soaks a pad in an hour, and has dysmenorrhea and fatigue. She uses no birth control. She is very distraught, as this bleeding truly has changed her lifestyle.
What is your approach to counseling this patient?
Dr. Bradley: You described a woman whose quality of life is very poor—frequent pad changes, clotting, pain. And she wants to have a child. A patient coming to me with those symptoms does not need to wait 4 to 6 months. I would immediately do some early evaluation.
Dr. Anderson: Sometimes a patient comes to us and already has had an ultrasonography exam. That is helpful, but I am driven by the fact that this patient is interested in pregnancy. I want to look at the uterine cavity and will probably do an office hysteroscopy to see if she has fibroids that distort the uterine cavity. Are there fibroids inside the cavity? To what degree does that possibly play a role? The presence of fibroids does not necessarily mean there is distortion of the cavity, and some evidence suggests that you do not need to do anything about those fibroids.4 Fibroids actually may not be the source of bleeding. We need to keep an open mind when we do the evaluation.
Continue to: Imaging technologies and classification aids...
Imaging technologies and classification aids
Dr. Sanfilippo: Apropos to your comment, is there a role for a sonohysterography in this population?
Dr. Anderson: That is a great technique. Some clinicians prefer to use sonohysterography while others prefer hysteroscopy. I tend to use hysteroscopy, and I have the equipment in the office. Both are great techniques and they answer the same question with respect to cavity evaluation.
Dr. Bradley: We once studied about 150 patients who, on the same day, with 2 separate examiners (one being me), would first undergo saline infusion sonohysterography (SIS) and then hysteroscopy, or vice versa. The sensitivity of identifying an intracavitary lesion is quite good with both. The additional benefit with SIS is that you can look at the adnexa.
In terms of the classification by the International Federation of Gynaecology and Obstetrics (FIGO), sometimes when we do a hysteroscopy, we are not sure how deep a fibroid is—whether it is a type 1 or type 2 or how close it is to the serosa (see illustration, page 26). Are we seeing just the tip of the iceberg? There is a role for imaging, and it is not always an “either/or” situation. There are times, for example, that hysteroscopy will show a type 0. Other times it may not show that, and you look for other things in terms of whether a fibroid abuts the endometrium. The take-home message is that physicians should abandon endometrial biopsy alone and, in this case, not offer a D&C.
In evaluating the endometrium, as gynecologists we should be facile in both technologies. In our workplaces we need to advocate to get trained, to be certified, and to be able to offer both technologies, because sometimes you need both to obtain the right answer.
Dr. Sanfilippo: Let’s talk about the FIGO classification, because it is important to have a communication method not only between physicians but with the patient. If we determine that a fibroid is a type 0, and therefore totally intracavitary, management is different than if the fibroid is a type 1 (less than 50% into the myometrium) or type 2 (more than 50%). What is the role for a classification system such as the FIGO?
Dr. Anderson: I like the FIGO classification system. We can show the patient fibroid classification diagrammatically and she will be able to understand exactly what we are talking about. It’s helpful for patient education and for surgical planning. The approach to a type 0 fibroid is a no-brainer, but with type 1 and more specifically with type 2, where the bulk of the fibroid is intramural and only a portion of that is intracavitary, fibroid size begins to matter a lot in terms of treatment approach.
Sometimes although a fibroid is intracavitary, a laparoscopic rather than hysteroscopic approach is preferred, as long as you can dissect the fibroid away from the endometrium. FIGO classification is very helpful, but I agree with Dr. Bradley that first you need to do a thorough evaluation to make your operative plan.
Continue to: Dr. Sanfilippo...
Dr. Sanfilippo: I encourage residents to go through an orderly sequence of assessment for evaluating abnormal uterine bleeding, including anatomic and endocrinologic factors. The PALM-COEIN classification system is a great mnemonic for use in evaluating abnormal uterine bleeding (TABLE).5 Is there a role for an aid such as PALM-COEIN in your practice?

Dr. Bradley: I totally agree. In 2011, Malcolm Munro and colleagues in the FIGO Working Group on Menstrual Disorders helped us to have a reporting on outcomes by knowing the size, number, and location of fibroids.5 This helps us to look for structural causes and then, to get to the answer, we often use imaging such as ultrasonography or saline infusion, sometimes magnetic resonance imaging (MRI), because other conditions can coexist—endometrial polyps, adenomyosis, and so on.
The PALM-COEIN system helps us to look at 2 things. One is that in addition to structural causes, there can be hematologic causes. While it is rare in a 24-year-old, we all have had the anecdotal patient who came in 6 months ago, had a fibroid, but had a platelet count of 6,000. Second, we have to look at the patient as a whole. My residents, myself, and our fellows look at any bleeding. Does she have a bleeding diathesis, bruising, nose bleeds; has she been anemic, does she have pica? Has she had a blood transfusion, is she on certain medications? We do not want to create a “silo” and think that the patient can have only a fibroid, because then we may miss an opportunity to treat other disease states. She can have a fibroid coexisting with polycystic ovary syndrome (PCOS), for instance. I like to look at everything so we can offer appropriate treatment modalities.
Dr. Sanfilippo: You bring up a very important point. Coagulopathies are more common statistically at the earlier part of a woman’s reproductive age group, soon after menarche, but they also occur toward menopause. We have to be cognizant that a woman can develop a coagulopathy throughout the reproductive years.
Dr. Anderson: You have to look at other medical causes. That is where the PALM-COEIN system can help. It helps you take the blinders off. If you focus on the fibroid and treat the fibroid and the patient still has bleeding, you missed something. You have to consider the whole patient and think of all the nonclassical or nonanatomical things, for example, thyroid disease. The PALM-COEIN helps us to evaluate the patient in a methodical way—every patient every time—so you do not miss something.
The value of MRI
Dr. Sanfilippo: What is the role for MRI, and when do you use it? Is it for only when you do a procedure—laparoscopically, robotically, open—so you have a detailed map of the fibroids?
Dr. Anderson: I love MRI, especially for hysteroscopy. I will print out the MRI image and trace the fibroid because there are things I want to know: exactly how much of the fibroid is inside or outside, where this fibroid is in the uterus, and how much of a normal buffer there is between the edge of that fibroid and the serosa. How aggressive can I be, or how cautious do I need to be, during the resection? Maybe this will be a planned 2-stage resection. MRIs are wonderful for fibroid disease, not only for diagnosis but also for surgical planning and patient counseling.
Dr. Bradley: SIS is also very useful. If the patient has an intracavitary fibroid that is larger than 4.5 to 5 cm and we insert the catheter, however, sometimes you cannot distend the cavity very well. Sometimes large intramural fibroids can compress the cavity, making the procedure difficult in an office setting. You cannot see the limits to help you as a surgical option. Although SIS generally is associated with little pain, some patients may have pain, and some patients cannot tolerate the test.
Continue to: I would order an MRI for surgical planning when...
I would order an MRI for surgical planning when a hysteroscopy is equivocal and if I cannot do an SIS. Also, if a patient who had a hysteroscopic resection with incomplete removal comes to me and is still symptomatic, I want to know the depth of penetration.
Obtaining an MRI may sometimes be difficult at a particular institution, and some clinicians have to go through the hurdles of getting an ultrasound to get certified and approved. We have to be our patient’s advocate and do the peer phone calls; any other specialty would require presurgical planning, and we are no different from other surgeons in that regard.
Dr. Sanfilippo: Yes, that can be a stumbling block. In the operating room, I like to have the images right in front of me, ideally an MRI or an ultrasound scan, as I know how to proceed. Having that visual helps me understand how close the fibroid is to the lining of the uterus.
Tapping into radiologists’ expertise
Dr. Bradley: Every quarter we meet with our radiologists, who are very interested in our MRI and SIS reports. They will describe the count and say how many fibroids—that is very helpful instead of just saying she has a bunch of fibroids—but they also will tell us when there is a type 0, a type 2, a type 7 fibroid. The team looks for adenomyosis and for endometriosis that can coexist.
Dr. Anderson: One caution about reading radiology reports is that often someone will come in with a report from an outside hospital or from a small community hospital that may say, “There is a 2-cm submucosal fibroid.” Some people might be tempted to take this person right to the OR, but you need to look at the images yourself, because in a radiologist’s mind “submucosal” truly means under the mucosa, which in our liturgy would be “intramural.” So we need to make sure that we are talking the same language. You should look at the images yourself.
Dr. Sanfilippo: I totally agree. It is also not unreasonable to speak with the radiologists and educate them about the FIGO classification.
Dr. Bradley: I prefer the word “intracavitary” for fibroids. When I see a typed report without the picture, “submucosal” can mean in the cavity or abutting the endometrium.
Case 2: Woman with heavy bleeding and fibroids seeks nonsurgical treatment
Dr. Sanfilippo: A 39-year-old (G3P3) woman is referred for evaluation for heavy vaginal bleeding, soaking a pad in an hour, which has been going on for months. Her primary ObGyn obtained a pelvic sonogram and noted multiple intramural and subserosal fibroids. A sonohysterogram reveals a submucosal myoma.
The patient is not interested in a hysterectomy. She was treated with birth control pills, with no improvement. She is interested in nonsurgical options. Dr. Bradley, what medical treatments might you offer this patient?
Medical treatment options
Dr. Bradley: If oral contraceptives have not worked, a good option would be tranexamic acid. Years ago our hospital was involved with enrolling patients in the multicenter clinical trial of this drug. The classic patient enrolled had regular, predictable, heavy menstrual cycles with alkaline hematin assay of greater than 80. If the case patient described has regular and predictable heavy bleeding every month at the same time, for the same duration, I would consider the use of tranexamic acid. There are several contraindications for the drug, so those exclusion issues would need to be reviewed. Contraindications include subarachnoid hemorrhage. Cerebral edema and cerebral infarction may be caused by tranexamic acid in such patients. Other contraindications include active intravascular clotting and hypersensitivity.
Continue to: Another option is to see if a progestin-releasing intrauterine system...
Another option is to see if a progestin-releasing intrauterine system (IUS) like the levonorgestrel (LNG) IUS would fit into this patient’s uterine cavity. Like Ted, I want to look into that cavity. I am not sure what “submucosal fibroid” means. If it has not distorted the cavity, or is totally within the uterine cavity, or abuts the endometrial cavity. The LNG-IUS cannot be placed into a uterine cavity that has intracavitary fibroids or sounds to greater than 12 cm. We are not going to put an LNG-IUS in somebody, at least in general, with a globally enlarged uterine cavity. I could ask, do you do that? You do a bimanual exam, and it is 18-weeks in size. I am not sure that I would put it in, but does it meet those criteria? The package insert for the LNG-IUS specifies upper and lower limits of uterine size for placement. I would start with those 2 options (tranexamic acid and LNG-IUS), and also get some more imaging.
Dr. Anderson: I agree with Linda. The submucosal fibroid could be contributing to this patient’s bleeding, but it is not the total contribution. The other fibroids may be completely irrelevant as far as her bleeding is concerned. We may need to deal with that one surgically, which we can do without a hysterectomy, most of the time.
I am a big fan of the LNG-IUS, it has been great in my experience. There are some other treatments available as well, such as gonadotropin–releasing hormone (GnRH) agonists. I tell patients that, while GnRH does work, it is not designed to be long-term therapy. If I have, for example, a 49-year-old patient, I just need to get her to menopause. Longer-term GnRH agonists might be a good option in this case. Otherwise, we could use short-term a GnRH agonist to stop the bleeding for a while so that we can reset the clock and get her started on something like levonorgestrel, tranexamic acid, or one of the other medical therapies. That may be a 2-step combination therapy.
Dr. Sanfilippo: There is a whole category of agents available—selective progesterone receptor modulators (SPRMs), pure progesterone receptor antagonists, ulipristal comes to mind. Clinicians need to know that options are available beyond birth control pills.
Dr. Anderson: As I tell patients, there are also “bridge” options. These are interventional procedures that are not hysterectomy, such as uterine fibroid embolization or endometrial ablation if bleeding is really the problem. We might consider a variety of different approaches. Obviously, we do not typically use fibroid embolization for submucosal fibroids, but it depends on how much of the fibroid is intracavitary and how big it is. Other options are a little more aggressive than medical therapy but they do not involve a hysterectomy.
Pros and cons of uterine artery embolization
Dr. Sanfilippo: If a woman desires future childbearing, is there a role for uterine artery embolization? How would you counsel her about the pros and cons?
Dr. Bradley: At the Cleveland Clinic, we generally do not offer uterine artery embolization if the patient wants a child. While it is an excellent method for treating heavy bleeding and bulk symptoms, the endometrium can be impacted. Patients can develop fistula, adhesions, or concentric narrowing, and changes in anti-Müllerian hormone levels, and there is potential for an Asherman-like syndrome and poor perfusion. I have many hysteroscopic images where the anterior wall of the uterus is nice and pink and the posterior wall is totally pale. The embolic microsphere particles can reach the endometrium—I have seen particles in the endometrium when doing a fibroid resection.
Continue to: A good early study looked at 555 women for almost a year...
A good early study looked at 555 women for almost a year.6 If women became pregnant, they had a higher rate of postpartum hemorrhage; placenta accreta, increta, and percreta; and emergent hysterectomy. It was recommended that these women deliver at a tertiary care center due to higher rates of preterm labor and malposition.
If a patient wants a baby, she should find a gynecologic surgeon who does minimally invasive laparoscopic, robotic, or open surgery, because she is more likely to have a take-home baby with a surgical approach than with embolization. In my experience, there is always going to be a patient who wants to keep her uterus at age 49 and who has every comorbidity. I might offer her the embolization just knowing what the odds of pregnancy are.
Dr. Anderson: I agree with Linda but I take a more liberal approach. Sometimes we do a myomectomy because we are trying to enhance fertility, while other times we do a myomectomy to address fibroid-related symptoms. These patients are having specific symptoms, and we want to leave the embolization option open.
If I have a patient who is 39 and becoming pregnant is not necessarily her goal, but she does not want to have a hysterectomy and if she got pregnant it would be okay, I am going to treat her a little different with respect to fibroid embolization than I would treat someone who is actively trying to have a baby. This goes back to what you were saying, let’s treat the patient, not just the fibroid.
Dr. Bradley: That is so important and sentinel. If she really does not want a hysterectomy but does not want a baby, I will ask, “Would you go through in vitro fertilization? Would you take clomiphene?” If she answers no, then I feel more comfortable, like you, with referring the patient for uterine fibroid embolization. The point is to get the patient with the right team to get the best outcomes.
Surgical approaches, intraoperative agents, and suture technique
Dr. Sanfilippo: Dr. Anderson, tell us about your surgical approaches to fibroids.
Dr. Anderson: At my institution we do have a fellowship in minimally invasive surgery, but I still do a lot of open myomectomies. I have a few guidelines to determine whether I am going to proceed laparoscopically, do a little minilaparotomy incision, or if a gigantic uterus is going to require a big incision. My mantra to my fellows has always been, “minimally invasive is the impact on the patient, not the size of the incision.”
Sometimes, prolonged anesthesia and Trendelenburg create more morbidity than a minilaparotomy. If a patient has 4 or 5 fibroids and most of them are intramural and I cannot see them but I want to be able to feel them, and to get a really good closure of the myometrium, I might choose to do a minilaparotomy. But if it is a case of a solitary fibroid, I would be more inclined to operate laparoscopically.
Continue to: Dr. Bradley...
Dr. Bradley: Our protocol is similar. We use MRI liberally. If patients have 4 or more fibroids and they are larger than 8 cm, most will have open surgery. I do not do robotic or laparoscopic procedures, so my referral source is for the larger myomas. We do not put retractors in; we can make incisions. Even if we do a huge Maylard incision, it is cosmetically wonderful. We use a loading dose of IV tranexamic acid with tranexamic acid throughout the surgery, and misoprostol intravaginally prior to surgery, to control uterine bleeding.
Dr. Sanfilippo: Dr. Anderson, is there a role for agents such as vasopressin, and what about routes of administration?
Dr. Anderson: When I do a laparoscopic or open procedure, I inject vasopressin (dilute 20 U in 100 mL of saline) into the pseudocapsule around the fibroid. I also administer rectal misoprostol (400 µg) just before the patient prep is done, which is amazing in reducing blood loss. There is also a role for a GnRH agonist, not necessarily to reduce the size of the uterus but to reduce blood flow in the pelvis and blood loss. Many different techniques are available. I do not use tourniquets, however. If bleeding does occur, I want to see it so I can fix it—not after I have sewn up the uterus and taken off a tourniquet.
Dr. Bradley: Do you use Floseal hemostatic matrix or any other agent to control bleeding?
Dr. Anderson: I do, for local hemostasis.
Dr. Bradley: Some surgeons will use barbed suture.
Dr. Anderson: I do like barbed sutures. In teaching residents to do myomectomy, it is very beneficial. But I am still a big fan of the good old figure-of-8 stitch because it is compressive and you get a good apposition of the tissue, good hemostasis, and strong closure.
Dr. Sanfilippo: We hope that this conversation will change your management of uterine fibroids. I thank Dr. Bradley and Dr. Anderson for a lively and very informative discussion.
Watch the video: Video roundtable–Fibroids: Patient considerations in medical and surgical management
- Khan AT, Shehmar M, Gupta JK. Uterine fibroids: current perspectives. Int J Womens Health. 2014;6:95-114.
- Divakars H. Asymptomatic uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22:643-654.
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health. 2013;22:807-816.
- Hartmann KE, Velez Edwards DR, Savitz DA, et al. Prospective cohort study of uterine fibroids and miscarriage risk. Am J Epidemiol. 2017;186:1140-1148.
- Munro MG, Critchley HOD, Fraser IS, for the FIGO Menstrual Disorders Working Group. The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertil Steril. 2011;95:2204-2208.
- Pron G, Mocarski E, Bennett J, et al; Ontario UFE Collaborative Group. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
- Khan AT, Shehmar M, Gupta JK. Uterine fibroids: current perspectives. Int J Womens Health. 2014;6:95-114.
- Divakars H. Asymptomatic uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22:643-654.
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health. 2013;22:807-816.
- Hartmann KE, Velez Edwards DR, Savitz DA, et al. Prospective cohort study of uterine fibroids and miscarriage risk. Am J Epidemiol. 2017;186:1140-1148.
- Munro MG, Critchley HOD, Fraser IS, for the FIGO Menstrual Disorders Working Group. The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertil Steril. 2011;95:2204-2208.
- Pron G, Mocarski E, Bennett J, et al; Ontario UFE Collaborative Group. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.