Article
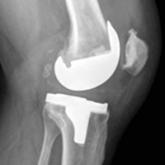
Plesiomonas shigelloides Periprosthetic Knee Infection After Consumption of Raw Oysters
- Author:
- Joshua W. Hustedt, MD, MHS
- Sarim Ahmed, MD
Periprosthetic infections are a leading cause of morbidity after total joint arthroplasty. Common pathogens include Staphylococcus aureus...
