Article
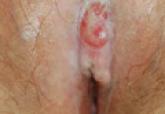
Transition From Lichen Sclerosus to Squamous Cell Carcinoma in a Single Tissue Section
- Author:
- Joung Soo Kim, MD, PhD
- Min Won Lee, MD
- Jun Oh Paek, MD
Article
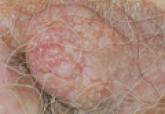
Nodular Extramammary Paget Disease With Fibroepitheliomatous Hyperplasia
- Author:
- Joung Soo Kim, MD, PhD
- Myeong Gil Jeong, MD
Extramammary Paget disease (EMPD) is a rare skin condition usually found in the anogenital region. Histologically, EMPD may be associated with...
Article
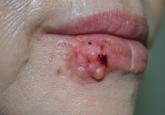
An Amelanotic Malignant Melanoma of the Lip: Unusual Shape and Atypical Location
- Author:
- Hyun Chul Park, MD
- Ho Song Kang, MD
- Joung Soo Kim, MD, PhD
Amelanotic malignant melanoma (AMM) is characterized by little or no visible pigment. The diagnosis of AMM is a challenge for clinicians because...
