User login
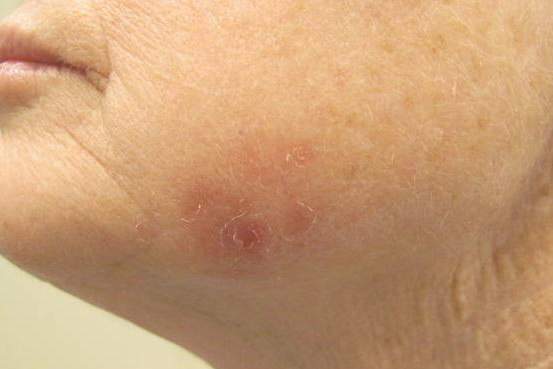
Erythematous Scaly Patch on the Jawline
The Diagnosis: Amelanotic Melanoma In Situ
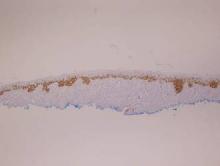
Histopathology revealed a broad asymmetric melanocytic proliferation at the dermoepidermal junction, consisting both of singly dispersed cells as well as randomly positioned nests (Figure 1). The single cells demonstrated junctional confluence and extension along adnexal structures highlighted by melan-A stain (Figure 2). The melanocytes were markedly atypical with enlarged and hyperchromatic nuclei containing multiple nucleoli. No dermal involvement was seen. There was papillary dermal fibrosis and an active host lymphocytic response. Based on these findings, a diagnosis of amelanotic melanoma in situ was made.
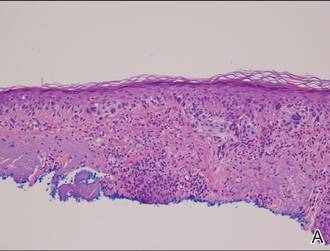 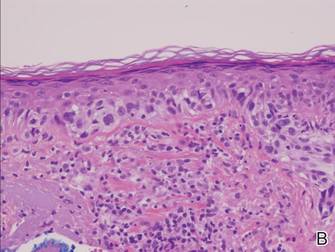 |
Figure 1. Histopathology revealed confluence of atypical melanocytes at the dermoepidermal junction and pagetoid scatter of melanocytes to the spinous layer (A)(H&E, original magnification ×4). Higher-power magnification highlighted the atypia of the individual melanocytes (B)(H&E, original magnification ×10). |
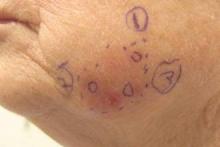
Subsequent scouting punch biopsies at the superior, anterior, and posterior aspects of the lesion were performed (Figure 3). All 3 revealed a similar nested and single cell proliferation at the dermoepidermal junction, confirming residual amelanotic melanoma in situ. The patient was referred to the otolaryngology department and underwent wide local excision with 5-mm margins and reconstructive repair.
Amelanotic melanoma comprises 2% to 8% of cutaneous melanomas. It is more common in fair-skinned elderly women with an average age of diagnosis of 61.8 years. Because features typically associated with melanoma such as asymmetry, border irregularity, and color variegation often are absent, amelanotic melanoma represents a notable diagnostic challenge for clinicians. Lesions can present nonspecifically as erythematous macules, papules, patches, or plaques and can have associated pruritus and scale.1,2
Clinical misdiagnoses for amelanotic melanoma include Bowen disease, basal cell carcinoma, actinic keratosis, lichenoid keratosis, intradermal nevus, dermatofibroma, inflamed seborrheic keratosis, nummular dermatitis, pyogenic granuloma, and granuloma annulare.1-6 There have been few case reports of amelanotic melanoma in situ, with most being the lentigo maligna variant that were initially clinically diagnosed as superficial basal cell carcinoma, Bowen disease, or dermatitis.7,8 In one case report, an amelanotic lentigo maligna was incidentally discovered after performing a mapping shave biopsy on what was normal-appearing skin.9
Dermoscopic evidence of vascular structures in lesions, including the presence of dotted vessels, milky red areas, and/or serpentine (linear irregular) vessels, may be the only clues to suggest amelanotic melanoma before biopsy. However, these findings are nonspecific and can be seen in other benign and malignant skin conditions.2
Complete surgical excision is the standard treatment of amelanotic melanoma in situ given its potential for invasion. However, the lack of pigment can make margins difficult to define. Because of its ability to detect disease beyond visual margins, Mohs micrographic surgery may have better cure rates than conventional excision.4 Prognosis for amelanotic melanoma is the same as other melanomas of equal thickness and location, though delay in diagnosis can adversely affect outcomes. Furthermore, amelanotic melanoma in situ can rapidly progress to invasive melanoma.3,5 Thus it is important to maintain clinical suspicion for amelanotic melanoma in fair-skinned elderly women presenting with a persistent or recurring erythematous scaly lesion on sun-exposed skin.
- Rahbari H, Nabai H, Mehregan AH, et al. Amelanotic lentigo maligna melanoma: a diagnostic conundrum— presentation of four new cases. Cancer. 1996;77:2052-2057.
- Jaimes N, Braun RP, Thomas L, et al. Clinical and dermoscopic characteristics of amelanotic melanomas that are not of the nodular subtype. J Eur Acad Dermatol Venereol. 2012;26:591-596.
- Koch SE, Lange JR. Amelanotic melanoma: the great masquerader. J Am Acad Dermatol. 2000;42:731-734.
- Conrad N, Jackson B, Goldberg L. Amelanotic lentigo maligna melanoma: a unique case presentation. Dermatol Surg. 1999;25:408-411.
- Cliff S, Otter M, Holden CA. Amelanotic lentigo maligna melanoma of the face: a case report and review of the literature. Clin Exp Dermatol. 1997;22:177-179.
- Dalton SR, Fillman EP, Altman CE, et al. Atypical junctional melanocytic proliferations in benign lichenoid keratosis. Hum Pathol. 2003;34:706-709.
- Paver K, Stewart M, Kossard S, et al. Amelanotic lentigo maligna. Australas J Dermatol. 1981;22:106-108.
- Lewis JE. Lentigo maligna presenting as an eczematous lesion. Cutis. 1987;40:357-359.
- Perera E, Mellick N, Teng P, et al. A clinically invisible melanoma. Australas J Dermatol. 2014;55:e58-e59.
The Diagnosis: Amelanotic Melanoma In Situ
Histopathology revealed a broad asymmetric melanocytic proliferation at the dermoepidermal junction, consisting both of singly dispersed cells as well as randomly positioned nests (Figure 1). The single cells demonstrated junctional confluence and extension along adnexal structures highlighted by melan-A stain (Figure 2). The melanocytes were markedly atypical with enlarged and hyperchromatic nuclei containing multiple nucleoli. No dermal involvement was seen. There was papillary dermal fibrosis and an active host lymphocytic response. Based on these findings, a diagnosis of amelanotic melanoma in situ was made.
  |
Figure 1. Histopathology revealed confluence of atypical melanocytes at the dermoepidermal junction and pagetoid scatter of melanocytes to the spinous layer (A)(H&E, original magnification ×4). Higher-power magnification highlighted the atypia of the individual melanocytes (B)(H&E, original magnification ×10). |
Subsequent scouting punch biopsies at the superior, anterior, and posterior aspects of the lesion were performed (Figure 3). All 3 revealed a similar nested and single cell proliferation at the dermoepidermal junction, confirming residual amelanotic melanoma in situ. The patient was referred to the otolaryngology department and underwent wide local excision with 5-mm margins and reconstructive repair.
Amelanotic melanoma comprises 2% to 8% of cutaneous melanomas. It is more common in fair-skinned elderly women with an average age of diagnosis of 61.8 years. Because features typically associated with melanoma such as asymmetry, border irregularity, and color variegation often are absent, amelanotic melanoma represents a notable diagnostic challenge for clinicians. Lesions can present nonspecifically as erythematous macules, papules, patches, or plaques and can have associated pruritus and scale.1,2
Clinical misdiagnoses for amelanotic melanoma include Bowen disease, basal cell carcinoma, actinic keratosis, lichenoid keratosis, intradermal nevus, dermatofibroma, inflamed seborrheic keratosis, nummular dermatitis, pyogenic granuloma, and granuloma annulare.1-6 There have been few case reports of amelanotic melanoma in situ, with most being the lentigo maligna variant that were initially clinically diagnosed as superficial basal cell carcinoma, Bowen disease, or dermatitis.7,8 In one case report, an amelanotic lentigo maligna was incidentally discovered after performing a mapping shave biopsy on what was normal-appearing skin.9
Dermoscopic evidence of vascular structures in lesions, including the presence of dotted vessels, milky red areas, and/or serpentine (linear irregular) vessels, may be the only clues to suggest amelanotic melanoma before biopsy. However, these findings are nonspecific and can be seen in other benign and malignant skin conditions.2
Complete surgical excision is the standard treatment of amelanotic melanoma in situ given its potential for invasion. However, the lack of pigment can make margins difficult to define. Because of its ability to detect disease beyond visual margins, Mohs micrographic surgery may have better cure rates than conventional excision.4 Prognosis for amelanotic melanoma is the same as other melanomas of equal thickness and location, though delay in diagnosis can adversely affect outcomes. Furthermore, amelanotic melanoma in situ can rapidly progress to invasive melanoma.3,5 Thus it is important to maintain clinical suspicion for amelanotic melanoma in fair-skinned elderly women presenting with a persistent or recurring erythematous scaly lesion on sun-exposed skin.
The Diagnosis: Amelanotic Melanoma In Situ
Histopathology revealed a broad asymmetric melanocytic proliferation at the dermoepidermal junction, consisting both of singly dispersed cells as well as randomly positioned nests (Figure 1). The single cells demonstrated junctional confluence and extension along adnexal structures highlighted by melan-A stain (Figure 2). The melanocytes were markedly atypical with enlarged and hyperchromatic nuclei containing multiple nucleoli. No dermal involvement was seen. There was papillary dermal fibrosis and an active host lymphocytic response. Based on these findings, a diagnosis of amelanotic melanoma in situ was made.
  |
Figure 1. Histopathology revealed confluence of atypical melanocytes at the dermoepidermal junction and pagetoid scatter of melanocytes to the spinous layer (A)(H&E, original magnification ×4). Higher-power magnification highlighted the atypia of the individual melanocytes (B)(H&E, original magnification ×10). |
Subsequent scouting punch biopsies at the superior, anterior, and posterior aspects of the lesion were performed (Figure 3). All 3 revealed a similar nested and single cell proliferation at the dermoepidermal junction, confirming residual amelanotic melanoma in situ. The patient was referred to the otolaryngology department and underwent wide local excision with 5-mm margins and reconstructive repair.
Amelanotic melanoma comprises 2% to 8% of cutaneous melanomas. It is more common in fair-skinned elderly women with an average age of diagnosis of 61.8 years. Because features typically associated with melanoma such as asymmetry, border irregularity, and color variegation often are absent, amelanotic melanoma represents a notable diagnostic challenge for clinicians. Lesions can present nonspecifically as erythematous macules, papules, patches, or plaques and can have associated pruritus and scale.1,2
Clinical misdiagnoses for amelanotic melanoma include Bowen disease, basal cell carcinoma, actinic keratosis, lichenoid keratosis, intradermal nevus, dermatofibroma, inflamed seborrheic keratosis, nummular dermatitis, pyogenic granuloma, and granuloma annulare.1-6 There have been few case reports of amelanotic melanoma in situ, with most being the lentigo maligna variant that were initially clinically diagnosed as superficial basal cell carcinoma, Bowen disease, or dermatitis.7,8 In one case report, an amelanotic lentigo maligna was incidentally discovered after performing a mapping shave biopsy on what was normal-appearing skin.9
Dermoscopic evidence of vascular structures in lesions, including the presence of dotted vessels, milky red areas, and/or serpentine (linear irregular) vessels, may be the only clues to suggest amelanotic melanoma before biopsy. However, these findings are nonspecific and can be seen in other benign and malignant skin conditions.2
Complete surgical excision is the standard treatment of amelanotic melanoma in situ given its potential for invasion. However, the lack of pigment can make margins difficult to define. Because of its ability to detect disease beyond visual margins, Mohs micrographic surgery may have better cure rates than conventional excision.4 Prognosis for amelanotic melanoma is the same as other melanomas of equal thickness and location, though delay in diagnosis can adversely affect outcomes. Furthermore, amelanotic melanoma in situ can rapidly progress to invasive melanoma.3,5 Thus it is important to maintain clinical suspicion for amelanotic melanoma in fair-skinned elderly women presenting with a persistent or recurring erythematous scaly lesion on sun-exposed skin.
- Rahbari H, Nabai H, Mehregan AH, et al. Amelanotic lentigo maligna melanoma: a diagnostic conundrum— presentation of four new cases. Cancer. 1996;77:2052-2057.
- Jaimes N, Braun RP, Thomas L, et al. Clinical and dermoscopic characteristics of amelanotic melanomas that are not of the nodular subtype. J Eur Acad Dermatol Venereol. 2012;26:591-596.
- Koch SE, Lange JR. Amelanotic melanoma: the great masquerader. J Am Acad Dermatol. 2000;42:731-734.
- Conrad N, Jackson B, Goldberg L. Amelanotic lentigo maligna melanoma: a unique case presentation. Dermatol Surg. 1999;25:408-411.
- Cliff S, Otter M, Holden CA. Amelanotic lentigo maligna melanoma of the face: a case report and review of the literature. Clin Exp Dermatol. 1997;22:177-179.
- Dalton SR, Fillman EP, Altman CE, et al. Atypical junctional melanocytic proliferations in benign lichenoid keratosis. Hum Pathol. 2003;34:706-709.
- Paver K, Stewart M, Kossard S, et al. Amelanotic lentigo maligna. Australas J Dermatol. 1981;22:106-108.
- Lewis JE. Lentigo maligna presenting as an eczematous lesion. Cutis. 1987;40:357-359.
- Perera E, Mellick N, Teng P, et al. A clinically invisible melanoma. Australas J Dermatol. 2014;55:e58-e59.
- Rahbari H, Nabai H, Mehregan AH, et al. Amelanotic lentigo maligna melanoma: a diagnostic conundrum— presentation of four new cases. Cancer. 1996;77:2052-2057.
- Jaimes N, Braun RP, Thomas L, et al. Clinical and dermoscopic characteristics of amelanotic melanomas that are not of the nodular subtype. J Eur Acad Dermatol Venereol. 2012;26:591-596.
- Koch SE, Lange JR. Amelanotic melanoma: the great masquerader. J Am Acad Dermatol. 2000;42:731-734.
- Conrad N, Jackson B, Goldberg L. Amelanotic lentigo maligna melanoma: a unique case presentation. Dermatol Surg. 1999;25:408-411.
- Cliff S, Otter M, Holden CA. Amelanotic lentigo maligna melanoma of the face: a case report and review of the literature. Clin Exp Dermatol. 1997;22:177-179.
- Dalton SR, Fillman EP, Altman CE, et al. Atypical junctional melanocytic proliferations in benign lichenoid keratosis. Hum Pathol. 2003;34:706-709.
- Paver K, Stewart M, Kossard S, et al. Amelanotic lentigo maligna. Australas J Dermatol. 1981;22:106-108.
- Lewis JE. Lentigo maligna presenting as an eczematous lesion. Cutis. 1987;40:357-359.
- Perera E, Mellick N, Teng P, et al. A clinically invisible melanoma. Australas J Dermatol. 2014;55:e58-e59.

Atypia of the individual melanocytes.
Melan-A stain highlighted the density and confluence of melanocytes within the epidermis.
Three scouting punch biopsies were performed along the periphery of the lesion.
A 70-year-old white woman with a history of basal cell carcinoma presented with a 2.7×1.9-cm ill-defined, erythematous, scaly patch along the left side of the jawline. Ten months prior to presentation, the lesion appeared as a grayish macule that was clinically diagnosed as a pigmented actinic keratosis and was treated with cryotherapy with resolution noted at 6-month follow-up. Differential diagnosis of the current lesion included actinic keratosis, lichenoid keratosis, and superficial basal cell carcinoma. A shave biopsy was performed.