User login
VHA Practice Guideline Recommendations for Diffuse Gliomas (FULL)
Over the past few decades, our understanding of the molecular underpinning of primary neoplasms of the central nervous system (CNS) has progressed substantially. Thanks in large part to this expansion in our knowledge base, the World Health Organization (WHO) has recently updated its classification of tumors of the CNS.1 One of the key elements of this update was the inclusion of molecular diagnostic criteria for the classification of infiltrating gliomas. While the previous classification system was based upon histologic subtypes of the tumor (astrocytoma, oligodendroglioma, and oligoastrocytoma), the revised classification system incorporates molecular testing to establish the genetic characteristics of the tumor to reach a final integrated diagnosis.
In this article, we present 3 cases to highlight some of these recent changes in the WHO diagnostic categories of primary CNS tumors and to illustrate the role of specific molecular tests in reaching a final integrated diagnosis. We then propose a clinical practice guideline for the Veterans Health Administration (VHA) that recommends use of molecular testing for veterans as part of the diagnostic workup of primary CNS neoplasms.
Purpose
In 2013 the VHA National Director of Pathology & Laboratory Medicine Services (P&LMS) chartered a national molecular genetics pathology workgroup (MGPW) that was charged with 4 specific tasks: (1) Provide recommendations about the effective use of molecular genetic testing for veterans; (2) Promote increased quality and availability of molecular testing within the VHA; (3) Encourage internal referral testing; and (4) Create an organizational structure and policies for molecular genetic testing and laboratory developed tests. The workgroup is currently composed of 4 subcommittees: genetic medicine, hematopathology, pharmacogenomics, and molecular oncology. The molecular oncology subcommittee is focused upon molecular genetic testing for solid tumors.
This article is intended to be the first of several publications from the molecular oncology subcommittee of the MGPW that address some of the aforementioned tasks. Similar to the recent publication from the hematopathology subcommittee of the MGPW, this article focuses on CNS neoplasms.2
Scope of Problem
The incidence of tumors of the CNS in the US population varies among age groups. It is the most common solid tumor in children aged < 14 years and represents a significant cause of mortality across all age groups.3 Of CNS tumors, diffuse gliomas comprise about 20% of the tumors and more than 70% of the primary malignant CNS tumors.3 Analysis of the VA Central Cancer Registry data from 2010 to 2014 identified 1,186 veterans (about 237 veterans per year) who were diagnosed with diffuse gliomas. (Lynch, Kulich, Colman, unpublished data, February 2018). While the majority (nearly 80%) of these cases were glioblastomas (GBMs), unfortunately a majority of these cases did not undergo molecular testing (Lynch, Kulich, Colman, unpublished data, February 2018).
Although this low rate of testing may be in part reflective of the period from which these data were gleaned (ie, prior to the WHO release of their updated the classification of tumors of the CNS), it is important to raise VA practitioners’ awareness of these recent changes to ensure that veterans receive the proper diagnosis and treatment for their disease. Thus, while the number of veterans diagnosed with diffuse gliomas within the VHA is relatively small in comparison to other malignancies, such as prostatic adenocarcinomas and lung carcinomas, the majority of diffuse gliomas do not seem to be receiving the molecular testing that would be necessary for (1) appropriate classification under the recently revised WHO recommendations; and (2) making important treatment decisions.
Case Presentations
Case 1. A veteran of the Gulf War presented with a 3-month history of possible narcoleptic events associated with a motor vehicle accident. Magnetic resonance imaging (MRI) revealed a large left frontal mass lesion with minimal surrounding edema without appreciable contrast enhancement (Figures 1A, 1B, and 1C).
Neither mitotic figures nor endothelial proliferation were identified. Immunohistochemical stains revealed a lack of R132H mutant IDH1 protein expression, a loss of nuclear staining for ATRX protein within a substantial number of cells, and a clonal pattern of p53 protein overexpression (Figures 1E, 1F, and 1G). The lesion demonstrated diffuse glial fibrillary acidic protein (GFAP) immunoreactivity and a low proliferation index (as determined by Ki-67 staining; estimated at less than 5%) (Figures 1H and 1I).
Based upon these results, an initial morphologic diagnosis of diffuse glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. While fluorescence in situ hybridization (FISH) studies were negative for 1p/19q codeletion, pyrosequencing analysis revealed the presence of a c.394C>T (R132C) mutation of the IDH1 gene (Figure 1J). The University of Pittsburgh Medical Center’s GlioSeq targeted next-generation sequence (NGS) analysis confirmed the presence of the c.394C > T mutation in IDH1 gene.4 Based upon this additional information, a final integrated morphologic and molecular diagnosis of diffuse astrocytoma, IDH-mutant was rendered.
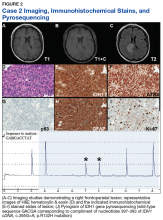
Case 2. A Vietnam War veteran presented with a 6-week history of new onset falls with associated left lower extremity weakness. A MRI revealed a right frontoparietal mass lesion with surrounding edema without appreciable contrast enhancement (Figures 2A, 2B, and 2C).
Immunohistochemical stains revealed R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, the lack of a clonal pattern of p53 protein overexpression, diffuse GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 20% (Figures 2E, 2F, 2G, 2H and 2I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were positive for 1p/19q codeletion, and pyrosequencing analysis confirmed the immunohistochemical findings of a c.395G>A (R132H) mutation of the IDH1 gene (Figure 2J). GlioSeq targeted NGS analysis confirmed the presence of the c.395G>A mutation in the IDH1 gene, a mutation in the telomerase reverse transcriptase (TERT) promoter, and possible decreased copy number of the CIC (chromosome 1p) and FUBP1 (chromosome 19q) genes.
A final integrated morphologic and molecular diagnosis of anaplastic oligodendroglioma, IDH-mutant and 1p/19q-codeleted was rendered based on the additional information. With this final diagnosis, methylation analysis of the MGMT gene promoter, which was performed for prognostic and predictive purposes, was identified in this case.5,6
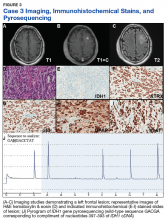
Case 3. A veteran of the Vietnam War presented with a new onset seizure. A MRI revealed a focally contrast-enhancing mass with surrounding edema within the left frontal lobe (Figures 3A, 3B, and 3C).
Hematoxylin and eosin (H&E) stained sections following formalin fixation and paraffin embedding demonstrated similar findings (Figure 3D), and while mitotic figures were readily identified, areas of necrosis were not identified and endothelial proliferation was not a prominent feature. Immunohistochemical stains revealed no evidence of R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, a clonal pattern of p53 protein overexpression, patchy GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 50% (Figures 3E, 3F, 3G, 3H, and 3I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and the tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were negative for EGFR gene amplification and 1p/19q codeletion, although a gain of the long arm of chromosome 1 was detected. Pyrosequencing analysis for mutations in codon 132 of the IDH1 gene revealed no mutations (Figure 3J). GlioSeq targeted NGS analysis identified mutations within the NF1, TP53, and PIK3CA genes without evidence of mutations in the IDH1, IDH2, ATRX, H3F3A, or EGFR genes or the TERT promoter. Based upon this additional information, a final integrated morphologic and molecular diagnosis of GBM, IDH wild-type was issued. The MGMT gene promoter was negative for methylation, a finding that has prognostic and predictive impact with regard to treatment with temazolamide.7-9
New Diffuse Glioma Classification
Since the issuance of the previous edition of the WHO classification of CNS tumors in 2007, several sentinel discoveries have been made that have advanced our understanding of the underlying biology of primary CNS neoplasms. Since a detailed review of these findings is beyond the scope and purpose of this manuscript and salient reviews on the topic can be found elsewhere, we will focus on the molecular findings that have been incorporated into the recently revised WHO classification.10 The importance of providing such information for proper patient management is illustrated by the recent acknowledgement by the American Academy of Neurology that molecular testing of brain tumors is a specific area in which there is a need for quality improvement.11 Therefore, it is critical that these underlying molecular abnormalities are identified to allow for proper classification and treatment of diffuse gliomas in the veteran population.
As noted previously, based on VA cancer registry data, diffuse gliomas are the most commonly encountered primary CNS cancers in the veteran population. Several of the aforementioned seminal discoveries have been incorporated into the updated classification of diffuse gliomas. While the recently updated WHO classification allows for the assignment of “not otherwise specified (NOS)” diagnostic designation, this category must be limited to cases where there is insufficient data to allow for a more precise classification due to sample limitations and not simply due to a failure of VA pathology laboratories to pursue the appropriate diagnostic testing.
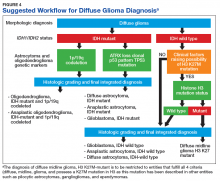
Figure 4 presents the recommended diagnostic workflow for the workup of diffuse gliomas. As illustrated in the above cases, a variety of different methodologies, including immunohistochemical, FISH, loss of heterozygosity analysis, traditional and NGS may be applied when elucidating the status of molecular events at critical diagnostic branch points.
Diagnostic Uses of Molecular Testing
While the case studies in this article demonstrate the use of ancillary testing and provide a suggested strategy for properly subclassifying diffuse gliomas, inherent in this strategy is the assumption that, based upon the initial clinical and pathologic information available, one can accurately categorize the lesion as a diffuse glioma. In reality, such a distinction is not always a straightforward endeavor. It is well recognized that a proportion of low-grade, typically radiologically circumscribed, CNS neoplasms, such as pilocytic astrocytomas and glioneuronal tumors, may infiltrate the surrounding brain parenchyma. In addition, many of these low-grade CNS neoplasms also may have growth patterns that are shared with diffuse gliomas, a diagnostic challenge that often can be further hampered by the inherent limitations involved in obtaining adequate samples for diagnosis from the CNS.
Although there are limitations and caveats, molecular diagnostic testing may be invaluable in properly classifying CNS tumors in such situations. The finding of mutations in the IDH1 or IDH2 genes has been shown to be very valuable in distinguishing low-grade diffuse glioma from both nonneoplastic and low-grade circumscribed neuroepithelial neoplasms that may exhibit growth patterns that can mimic those of diffuse gliomas.15-17 Conversely, finding abnormalities in the BRAF gene in a brain neoplasm that has a low-grade morphology suggests that the lesion may represent one of these low-grade lesions such as a pleomorphic xanthoastrocytoma, pilocytic astrocytoma, or mixed neuronal-glial tumor as opposed to a diffuse glioma.18,19
Depending upon the environment in which one practices, small biopsy specimens may be prevalent, and unfortunately, it is not uncommon to obtain a biopsy that exhibits a histologic growth pattern that is discordant from what one would predict based on the clinical context and imaging findings. Molecular testing may be useful in resolving discordances in such situations. If a biopsy of a ring-enhancing lesion demonstrates a diffuse glioma that doesn’t meet WHO grade IV criteria, applying methodologies that look for genetic features commonly encountered in high-grade astrocytomas may identify genetic abnormalities that suggest a more aggressive lesion than is indicated by the histologic findings. The presence of genetic abnormalities such as homozygous deletion of the CDKN2A gene, TERT promoter mutation, loss of heterozygosity of chromosome 10q and/or phosphatase and tensin homolog (PTEN) mutations, EGFR gene amplification or the presence of the EGFR variant III are a few findings that would suggest the aforementioned sample may represent an undersampling of a higher grade diffuse astrocytoma, which would be important information to convey to the treating clinicians.20-26
Testing In the VA
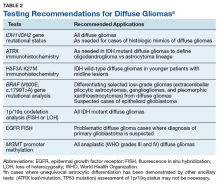
The goals of the MPWG include promoting increased quality and availability of genetic testing within the VHA as well as encouraging internal referral testing. An informal survey of the chiefs of VA Pathology and Laboratory Medicine Services was conducted in November of 2017 in an attempt to identify internal VA pathology laboratories currently conducting testing that may be of use in the workup of diffuse gliomas (Table 1).
The VA currently offers NGS panels for patients with advanced-stage malignancies under the auspices of the Precision Oncology Program, whose reports provide both (1) mutational analyses for genes such as TP53, ATRX, NF1, BRAF, PTEN, TERT IDH1, and IDH2 that may be useful in the proper classifying of high-grade diffuse gliomas; and (2) information regarding clinical trials for which the veteran may be eligible for based on their glioma’s mutational profile. Interested VA providers should visit tinyurl.com/precisiononcology/ for more information about this program. Finally, although internal testing within VA laboratories is recommended to allow for the development of more cost-effective testing, testing may be performed through many nationally contracted reference laboratories.
Conclusion
In light of the recent progress made in our understanding of the molecular events of gliomagenesis, the way we diagnose diffuse gliomas within the CNS has undergone a major paradigm shift. While histology still plays a critical role in the process, we believe that additional ancillary testing is a requirement for all diffuse gliomas diagnosed within VA pathology laboratories. In the context of recently encountered cases, we have provided a recommended workflow highlighting the testing that can be performed to allow for the proper diagnosis of our veterans with diffuse gliomas (Figure 4).
Unless limited by the amount of tissue available for such tests, ancillary testing must be performed on all diffuse gliomas diagnosed within the VA system to ensure proper diagnosis and treatment of our veterans with diffuse gliomas.
Acknowledgments
The authors thank Dr. Craig M. Horbinski (Feinberg School of Medicine, Northwestern University) and Dr. Geoffrey H. Murdoch (University of Pittsburgh) for their constructive criticism of the manuscript. We also thank the following individuals for past service as members of the molecular oncology subcommittee of the MGPW: Dr. George Ansstas (Washington University School of Medicine), Dr. Osssama Hemadeh (Bay Pines VA Health Care System), Dr. James Herman (VA Pittsburgh Healthcare System), and Dr. Ryan Phan (formerly of the VA Greater Los Angeles Healthcare System) as well as the members of the Veterans Administration pathology and laboratory medicine service molecular genetics pathology workgroup.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
Dr. Kulich is the Acting Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System and member of the Division of Neuropathology at University of Pittsburgh Department of Pathology, Dr. Duvvuri is an Otolaryngologist at VA Pittsburgh Healthcare System, and Dr. Passero is the Section Chief of Hematology\Oncology at VA Pittsburgh Healthcare System in Pennsylvania. Dr. Becker is an Oncologist at VA-New York Harbor Healthcare System. Dr. Dacic is a Pathologist at University of Pittsburgh Department of Pathology in Pennsylvania. Dr. Ehsan is Chief of Pathology and Laboratory Medicine Services at the South Texas Veterans Healthcare System in San Antonio. Dr. Gutkin is the former Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System. Dr. Hou is a Pathologist at St. Louis VA Medical Center in Missouri. Dr. Icardi is the VA National Director of Pathology and Laboratory Medicine Services. Dr. Lyle is a Pathologist at Bay Pine Health Care System in Florida. Dr. Lynch is an Investigator at VA Salt Lake Health Care System Informatics and Computing Infrastructure. Dr. Montgomery is an Oncologist at VA Puget Sound Health Care System, in Seattle, Washington. Dr. Przygodzki is the Director of Genomic Medicine Implementation and Associate Director of Genomic Medicine for the VA. Dr. Colman is a Neuro-Oncologist at George E. Wahlen VA Medical Center and the Director of Medical Neuro-Oncology at the Huntsman Cancer Institute, Salt Lake City, Utah.
Correspondence: Dr. Kulich (scott.kulich@va.gov)
1. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803-820.
2. Wang-Rodriguez J, Yunes A, Phan R, et al. The challenges of precision medicine and new advances in molecular diagnostic testing in hematolymphoid malignancies: impact on the VHA. Fed Pract. 2017;34(suppl 5):S38-S49.
3. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(suppl 5):v1-v88.
4. Nikiforova MN, Wald AI, Melan MA, et al. Targeted next-generation sequencing panel (GlioSeq) provides comprehensive genetic profiling of central nervous system tumors. Neuro Oncol. 2016;18(3)379-387.
5. Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90(19):1473-1479.
6. van den Bent MJ, Erdem-Eraslan L, Idbaih A, et al. MGMT-STP27 methylation status as predictive marker for response to PCV in anaplastic oligodendrogliomas and oligoastrocytomas. A report from EORTC study 26951. Clin Cancer Res. 2013;19(19):5513-5522.
7. Stupp R, Hegi ME, Mason WP, et al; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-466.
8. Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916-926.
9. van den Bent MJ, Kros JM. Predictive and prognostic markers in neuro-oncology. J Neuropathol Exp Neurol. 2007;66(12):1074-1081.
10. Chen R, Smith-Cohn M, Cohen AL, Colman H. Glioma subclassifications and their clinical significance. Neurotherapeutics. 2017;14(2):284-297.
11. Jordan JT, Sanders AE, Armstrong T, et al. Quality improvement in neurology: neuro-oncology quality measurement set. Neurology. 2018;90(14):652-658.
12. Chen L, Voronovich Z, Clark K, et al. Predicting the likelihood of an isocitrate dehydrogenase 1 or 2 mutation in diagnoses of infiltrative glioma. Neuro Oncol. 2014;16(11):1478-1483.
13. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997-1003.
14. Wick W, Platten M, Meisner C, et al; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707-715.
15. Horbinski C, Kofler J, Kelly LM, Murdoch GH, Nikiforova MN. Diagnostic use of IDH1/2 mutation analysis in routine clinical testing of formalin-fixed, paraffin-embedded glioma tissues. J Neuropathol Exp Neurol. 2009;68(12):1319-1325.
16. Camelo-Piragua S, Jansen M, Ganguly A, Kim JC, Louis DN, Nutt CL. Mutant IDH1-specific immunohistochemistry distinguishes diffuse astrocytoma from astrocytosis. Acta Neuropathol. 2010;119(4):509-511.
17. Horbinski C, Kofler J, Yeaney G, et al. Isocitrate dehydrogenase 1 analysis differentiates gangliogliomas from infiltrative gliomas. Brain Pathol. 2011;21(5):564-574.
18. Berghoff AS, Preusser M. BRAF alterations in brain tumours: molecular pathology and therapeutic opportunities. Curr Opin Neurol. 2014;27(6):689-696.
19. Korshunov A, Meyer J, Capper D, et al. Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol. 2009;118(3):401-405.
20. Fuller CE, Schmidt RE, Roth KA, et al. Clinical utility of fluorescence in situ hybridization (FISH) in morphologically ambiguous gliomas with hybrid oligodendroglial/astrocytic features. J Neuropathol Exp Neurol. 2003;62(11):1118-1128.
21. Horbinski C. Practical molecular diagnostics in neuropathology: making a tough job a little easier. Semin Diagn Pathol. 2010;27(2):105-113.
22. Fuller GN, Bigner SH. Amplified cellular oncogenes in neoplasms of the human central nervous system. Mutat Res. 1992;276(3):299-306.
23. Brennan CW, Verhaak RG, McKenna A, et al; TCGA Research Network. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462-477.
24. Aldape K, Zadeh G, Mansouri S, Reifenberger G, von Deimling A. Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129(6):829-848.
25. Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110(15):6021-6026.
26. Nikiforova MN, Hamilton RL. Molecular diagnostics of gliomas. Arch Pathol Lab Med. 2011;135(5):558-568.
Over the past few decades, our understanding of the molecular underpinning of primary neoplasms of the central nervous system (CNS) has progressed substantially. Thanks in large part to this expansion in our knowledge base, the World Health Organization (WHO) has recently updated its classification of tumors of the CNS.1 One of the key elements of this update was the inclusion of molecular diagnostic criteria for the classification of infiltrating gliomas. While the previous classification system was based upon histologic subtypes of the tumor (astrocytoma, oligodendroglioma, and oligoastrocytoma), the revised classification system incorporates molecular testing to establish the genetic characteristics of the tumor to reach a final integrated diagnosis.
In this article, we present 3 cases to highlight some of these recent changes in the WHO diagnostic categories of primary CNS tumors and to illustrate the role of specific molecular tests in reaching a final integrated diagnosis. We then propose a clinical practice guideline for the Veterans Health Administration (VHA) that recommends use of molecular testing for veterans as part of the diagnostic workup of primary CNS neoplasms.
Purpose
In 2013 the VHA National Director of Pathology & Laboratory Medicine Services (P&LMS) chartered a national molecular genetics pathology workgroup (MGPW) that was charged with 4 specific tasks: (1) Provide recommendations about the effective use of molecular genetic testing for veterans; (2) Promote increased quality and availability of molecular testing within the VHA; (3) Encourage internal referral testing; and (4) Create an organizational structure and policies for molecular genetic testing and laboratory developed tests. The workgroup is currently composed of 4 subcommittees: genetic medicine, hematopathology, pharmacogenomics, and molecular oncology. The molecular oncology subcommittee is focused upon molecular genetic testing for solid tumors.
This article is intended to be the first of several publications from the molecular oncology subcommittee of the MGPW that address some of the aforementioned tasks. Similar to the recent publication from the hematopathology subcommittee of the MGPW, this article focuses on CNS neoplasms.2
Scope of Problem
The incidence of tumors of the CNS in the US population varies among age groups. It is the most common solid tumor in children aged < 14 years and represents a significant cause of mortality across all age groups.3 Of CNS tumors, diffuse gliomas comprise about 20% of the tumors and more than 70% of the primary malignant CNS tumors.3 Analysis of the VA Central Cancer Registry data from 2010 to 2014 identified 1,186 veterans (about 237 veterans per year) who were diagnosed with diffuse gliomas. (Lynch, Kulich, Colman, unpublished data, February 2018). While the majority (nearly 80%) of these cases were glioblastomas (GBMs), unfortunately a majority of these cases did not undergo molecular testing (Lynch, Kulich, Colman, unpublished data, February 2018).
Although this low rate of testing may be in part reflective of the period from which these data were gleaned (ie, prior to the WHO release of their updated the classification of tumors of the CNS), it is important to raise VA practitioners’ awareness of these recent changes to ensure that veterans receive the proper diagnosis and treatment for their disease. Thus, while the number of veterans diagnosed with diffuse gliomas within the VHA is relatively small in comparison to other malignancies, such as prostatic adenocarcinomas and lung carcinomas, the majority of diffuse gliomas do not seem to be receiving the molecular testing that would be necessary for (1) appropriate classification under the recently revised WHO recommendations; and (2) making important treatment decisions.
Case Presentations
Case 1. A veteran of the Gulf War presented with a 3-month history of possible narcoleptic events associated with a motor vehicle accident. Magnetic resonance imaging (MRI) revealed a large left frontal mass lesion with minimal surrounding edema without appreciable contrast enhancement (Figures 1A, 1B, and 1C).
Neither mitotic figures nor endothelial proliferation were identified. Immunohistochemical stains revealed a lack of R132H mutant IDH1 protein expression, a loss of nuclear staining for ATRX protein within a substantial number of cells, and a clonal pattern of p53 protein overexpression (Figures 1E, 1F, and 1G). The lesion demonstrated diffuse glial fibrillary acidic protein (GFAP) immunoreactivity and a low proliferation index (as determined by Ki-67 staining; estimated at less than 5%) (Figures 1H and 1I).
Based upon these results, an initial morphologic diagnosis of diffuse glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. While fluorescence in situ hybridization (FISH) studies were negative for 1p/19q codeletion, pyrosequencing analysis revealed the presence of a c.394C>T (R132C) mutation of the IDH1 gene (Figure 1J). The University of Pittsburgh Medical Center’s GlioSeq targeted next-generation sequence (NGS) analysis confirmed the presence of the c.394C > T mutation in IDH1 gene.4 Based upon this additional information, a final integrated morphologic and molecular diagnosis of diffuse astrocytoma, IDH-mutant was rendered.
Case 2. A Vietnam War veteran presented with a 6-week history of new onset falls with associated left lower extremity weakness. A MRI revealed a right frontoparietal mass lesion with surrounding edema without appreciable contrast enhancement (Figures 2A, 2B, and 2C).
Immunohistochemical stains revealed R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, the lack of a clonal pattern of p53 protein overexpression, diffuse GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 20% (Figures 2E, 2F, 2G, 2H and 2I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were positive for 1p/19q codeletion, and pyrosequencing analysis confirmed the immunohistochemical findings of a c.395G>A (R132H) mutation of the IDH1 gene (Figure 2J). GlioSeq targeted NGS analysis confirmed the presence of the c.395G>A mutation in the IDH1 gene, a mutation in the telomerase reverse transcriptase (TERT) promoter, and possible decreased copy number of the CIC (chromosome 1p) and FUBP1 (chromosome 19q) genes.
A final integrated morphologic and molecular diagnosis of anaplastic oligodendroglioma, IDH-mutant and 1p/19q-codeleted was rendered based on the additional information. With this final diagnosis, methylation analysis of the MGMT gene promoter, which was performed for prognostic and predictive purposes, was identified in this case.5,6
Case 3. A veteran of the Vietnam War presented with a new onset seizure. A MRI revealed a focally contrast-enhancing mass with surrounding edema within the left frontal lobe (Figures 3A, 3B, and 3C).
Hematoxylin and eosin (H&E) stained sections following formalin fixation and paraffin embedding demonstrated similar findings (Figure 3D), and while mitotic figures were readily identified, areas of necrosis were not identified and endothelial proliferation was not a prominent feature. Immunohistochemical stains revealed no evidence of R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, a clonal pattern of p53 protein overexpression, patchy GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 50% (Figures 3E, 3F, 3G, 3H, and 3I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and the tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were negative for EGFR gene amplification and 1p/19q codeletion, although a gain of the long arm of chromosome 1 was detected. Pyrosequencing analysis for mutations in codon 132 of the IDH1 gene revealed no mutations (Figure 3J). GlioSeq targeted NGS analysis identified mutations within the NF1, TP53, and PIK3CA genes without evidence of mutations in the IDH1, IDH2, ATRX, H3F3A, or EGFR genes or the TERT promoter. Based upon this additional information, a final integrated morphologic and molecular diagnosis of GBM, IDH wild-type was issued. The MGMT gene promoter was negative for methylation, a finding that has prognostic and predictive impact with regard to treatment with temazolamide.7-9
New Diffuse Glioma Classification
Since the issuance of the previous edition of the WHO classification of CNS tumors in 2007, several sentinel discoveries have been made that have advanced our understanding of the underlying biology of primary CNS neoplasms. Since a detailed review of these findings is beyond the scope and purpose of this manuscript and salient reviews on the topic can be found elsewhere, we will focus on the molecular findings that have been incorporated into the recently revised WHO classification.10 The importance of providing such information for proper patient management is illustrated by the recent acknowledgement by the American Academy of Neurology that molecular testing of brain tumors is a specific area in which there is a need for quality improvement.11 Therefore, it is critical that these underlying molecular abnormalities are identified to allow for proper classification and treatment of diffuse gliomas in the veteran population.
As noted previously, based on VA cancer registry data, diffuse gliomas are the most commonly encountered primary CNS cancers in the veteran population. Several of the aforementioned seminal discoveries have been incorporated into the updated classification of diffuse gliomas. While the recently updated WHO classification allows for the assignment of “not otherwise specified (NOS)” diagnostic designation, this category must be limited to cases where there is insufficient data to allow for a more precise classification due to sample limitations and not simply due to a failure of VA pathology laboratories to pursue the appropriate diagnostic testing.
Figure 4 presents the recommended diagnostic workflow for the workup of diffuse gliomas. As illustrated in the above cases, a variety of different methodologies, including immunohistochemical, FISH, loss of heterozygosity analysis, traditional and NGS may be applied when elucidating the status of molecular events at critical diagnostic branch points.
Diagnostic Uses of Molecular Testing
While the case studies in this article demonstrate the use of ancillary testing and provide a suggested strategy for properly subclassifying diffuse gliomas, inherent in this strategy is the assumption that, based upon the initial clinical and pathologic information available, one can accurately categorize the lesion as a diffuse glioma. In reality, such a distinction is not always a straightforward endeavor. It is well recognized that a proportion of low-grade, typically radiologically circumscribed, CNS neoplasms, such as pilocytic astrocytomas and glioneuronal tumors, may infiltrate the surrounding brain parenchyma. In addition, many of these low-grade CNS neoplasms also may have growth patterns that are shared with diffuse gliomas, a diagnostic challenge that often can be further hampered by the inherent limitations involved in obtaining adequate samples for diagnosis from the CNS.
Although there are limitations and caveats, molecular diagnostic testing may be invaluable in properly classifying CNS tumors in such situations. The finding of mutations in the IDH1 or IDH2 genes has been shown to be very valuable in distinguishing low-grade diffuse glioma from both nonneoplastic and low-grade circumscribed neuroepithelial neoplasms that may exhibit growth patterns that can mimic those of diffuse gliomas.15-17 Conversely, finding abnormalities in the BRAF gene in a brain neoplasm that has a low-grade morphology suggests that the lesion may represent one of these low-grade lesions such as a pleomorphic xanthoastrocytoma, pilocytic astrocytoma, or mixed neuronal-glial tumor as opposed to a diffuse glioma.18,19
Depending upon the environment in which one practices, small biopsy specimens may be prevalent, and unfortunately, it is not uncommon to obtain a biopsy that exhibits a histologic growth pattern that is discordant from what one would predict based on the clinical context and imaging findings. Molecular testing may be useful in resolving discordances in such situations. If a biopsy of a ring-enhancing lesion demonstrates a diffuse glioma that doesn’t meet WHO grade IV criteria, applying methodologies that look for genetic features commonly encountered in high-grade astrocytomas may identify genetic abnormalities that suggest a more aggressive lesion than is indicated by the histologic findings. The presence of genetic abnormalities such as homozygous deletion of the CDKN2A gene, TERT promoter mutation, loss of heterozygosity of chromosome 10q and/or phosphatase and tensin homolog (PTEN) mutations, EGFR gene amplification or the presence of the EGFR variant III are a few findings that would suggest the aforementioned sample may represent an undersampling of a higher grade diffuse astrocytoma, which would be important information to convey to the treating clinicians.20-26
Testing In the VA
The goals of the MPWG include promoting increased quality and availability of genetic testing within the VHA as well as encouraging internal referral testing. An informal survey of the chiefs of VA Pathology and Laboratory Medicine Services was conducted in November of 2017 in an attempt to identify internal VA pathology laboratories currently conducting testing that may be of use in the workup of diffuse gliomas (Table 1).
The VA currently offers NGS panels for patients with advanced-stage malignancies under the auspices of the Precision Oncology Program, whose reports provide both (1) mutational analyses for genes such as TP53, ATRX, NF1, BRAF, PTEN, TERT IDH1, and IDH2 that may be useful in the proper classifying of high-grade diffuse gliomas; and (2) information regarding clinical trials for which the veteran may be eligible for based on their glioma’s mutational profile. Interested VA providers should visit tinyurl.com/precisiononcology/ for more information about this program. Finally, although internal testing within VA laboratories is recommended to allow for the development of more cost-effective testing, testing may be performed through many nationally contracted reference laboratories.
Conclusion
In light of the recent progress made in our understanding of the molecular events of gliomagenesis, the way we diagnose diffuse gliomas within the CNS has undergone a major paradigm shift. While histology still plays a critical role in the process, we believe that additional ancillary testing is a requirement for all diffuse gliomas diagnosed within VA pathology laboratories. In the context of recently encountered cases, we have provided a recommended workflow highlighting the testing that can be performed to allow for the proper diagnosis of our veterans with diffuse gliomas (Figure 4).
Unless limited by the amount of tissue available for such tests, ancillary testing must be performed on all diffuse gliomas diagnosed within the VA system to ensure proper diagnosis and treatment of our veterans with diffuse gliomas.

Acknowledgments
The authors thank Dr. Craig M. Horbinski (Feinberg School of Medicine, Northwestern University) and Dr. Geoffrey H. Murdoch (University of Pittsburgh) for their constructive criticism of the manuscript. We also thank the following individuals for past service as members of the molecular oncology subcommittee of the MGPW: Dr. George Ansstas (Washington University School of Medicine), Dr. Osssama Hemadeh (Bay Pines VA Health Care System), Dr. James Herman (VA Pittsburgh Healthcare System), and Dr. Ryan Phan (formerly of the VA Greater Los Angeles Healthcare System) as well as the members of the Veterans Administration pathology and laboratory medicine service molecular genetics pathology workgroup.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
Dr. Kulich is the Acting Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System and member of the Division of Neuropathology at University of Pittsburgh Department of Pathology, Dr. Duvvuri is an Otolaryngologist at VA Pittsburgh Healthcare System, and Dr. Passero is the Section Chief of Hematology\Oncology at VA Pittsburgh Healthcare System in Pennsylvania. Dr. Becker is an Oncologist at VA-New York Harbor Healthcare System. Dr. Dacic is a Pathologist at University of Pittsburgh Department of Pathology in Pennsylvania. Dr. Ehsan is Chief of Pathology and Laboratory Medicine Services at the South Texas Veterans Healthcare System in San Antonio. Dr. Gutkin is the former Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System. Dr. Hou is a Pathologist at St. Louis VA Medical Center in Missouri. Dr. Icardi is the VA National Director of Pathology and Laboratory Medicine Services. Dr. Lyle is a Pathologist at Bay Pine Health Care System in Florida. Dr. Lynch is an Investigator at VA Salt Lake Health Care System Informatics and Computing Infrastructure. Dr. Montgomery is an Oncologist at VA Puget Sound Health Care System, in Seattle, Washington. Dr. Przygodzki is the Director of Genomic Medicine Implementation and Associate Director of Genomic Medicine for the VA. Dr. Colman is a Neuro-Oncologist at George E. Wahlen VA Medical Center and the Director of Medical Neuro-Oncology at the Huntsman Cancer Institute, Salt Lake City, Utah.
Correspondence: Dr. Kulich (scott.kulich@va.gov)
Over the past few decades, our understanding of the molecular underpinning of primary neoplasms of the central nervous system (CNS) has progressed substantially. Thanks in large part to this expansion in our knowledge base, the World Health Organization (WHO) has recently updated its classification of tumors of the CNS.1 One of the key elements of this update was the inclusion of molecular diagnostic criteria for the classification of infiltrating gliomas. While the previous classification system was based upon histologic subtypes of the tumor (astrocytoma, oligodendroglioma, and oligoastrocytoma), the revised classification system incorporates molecular testing to establish the genetic characteristics of the tumor to reach a final integrated diagnosis.
In this article, we present 3 cases to highlight some of these recent changes in the WHO diagnostic categories of primary CNS tumors and to illustrate the role of specific molecular tests in reaching a final integrated diagnosis. We then propose a clinical practice guideline for the Veterans Health Administration (VHA) that recommends use of molecular testing for veterans as part of the diagnostic workup of primary CNS neoplasms.
Purpose
In 2013 the VHA National Director of Pathology & Laboratory Medicine Services (P&LMS) chartered a national molecular genetics pathology workgroup (MGPW) that was charged with 4 specific tasks: (1) Provide recommendations about the effective use of molecular genetic testing for veterans; (2) Promote increased quality and availability of molecular testing within the VHA; (3) Encourage internal referral testing; and (4) Create an organizational structure and policies for molecular genetic testing and laboratory developed tests. The workgroup is currently composed of 4 subcommittees: genetic medicine, hematopathology, pharmacogenomics, and molecular oncology. The molecular oncology subcommittee is focused upon molecular genetic testing for solid tumors.
This article is intended to be the first of several publications from the molecular oncology subcommittee of the MGPW that address some of the aforementioned tasks. Similar to the recent publication from the hematopathology subcommittee of the MGPW, this article focuses on CNS neoplasms.2
Scope of Problem
The incidence of tumors of the CNS in the US population varies among age groups. It is the most common solid tumor in children aged < 14 years and represents a significant cause of mortality across all age groups.3 Of CNS tumors, diffuse gliomas comprise about 20% of the tumors and more than 70% of the primary malignant CNS tumors.3 Analysis of the VA Central Cancer Registry data from 2010 to 2014 identified 1,186 veterans (about 237 veterans per year) who were diagnosed with diffuse gliomas. (Lynch, Kulich, Colman, unpublished data, February 2018). While the majority (nearly 80%) of these cases were glioblastomas (GBMs), unfortunately a majority of these cases did not undergo molecular testing (Lynch, Kulich, Colman, unpublished data, February 2018).
Although this low rate of testing may be in part reflective of the period from which these data were gleaned (ie, prior to the WHO release of their updated the classification of tumors of the CNS), it is important to raise VA practitioners’ awareness of these recent changes to ensure that veterans receive the proper diagnosis and treatment for their disease. Thus, while the number of veterans diagnosed with diffuse gliomas within the VHA is relatively small in comparison to other malignancies, such as prostatic adenocarcinomas and lung carcinomas, the majority of diffuse gliomas do not seem to be receiving the molecular testing that would be necessary for (1) appropriate classification under the recently revised WHO recommendations; and (2) making important treatment decisions.
Case Presentations
Case 1. A veteran of the Gulf War presented with a 3-month history of possible narcoleptic events associated with a motor vehicle accident. Magnetic resonance imaging (MRI) revealed a large left frontal mass lesion with minimal surrounding edema without appreciable contrast enhancement (Figures 1A, 1B, and 1C).
Neither mitotic figures nor endothelial proliferation were identified. Immunohistochemical stains revealed a lack of R132H mutant IDH1 protein expression, a loss of nuclear staining for ATRX protein within a substantial number of cells, and a clonal pattern of p53 protein overexpression (Figures 1E, 1F, and 1G). The lesion demonstrated diffuse glial fibrillary acidic protein (GFAP) immunoreactivity and a low proliferation index (as determined by Ki-67 staining; estimated at less than 5%) (Figures 1H and 1I).
Based upon these results, an initial morphologic diagnosis of diffuse glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. While fluorescence in situ hybridization (FISH) studies were negative for 1p/19q codeletion, pyrosequencing analysis revealed the presence of a c.394C>T (R132C) mutation of the IDH1 gene (Figure 1J). The University of Pittsburgh Medical Center’s GlioSeq targeted next-generation sequence (NGS) analysis confirmed the presence of the c.394C > T mutation in IDH1 gene.4 Based upon this additional information, a final integrated morphologic and molecular diagnosis of diffuse astrocytoma, IDH-mutant was rendered.
Case 2. A Vietnam War veteran presented with a 6-week history of new onset falls with associated left lower extremity weakness. A MRI revealed a right frontoparietal mass lesion with surrounding edema without appreciable contrast enhancement (Figures 2A, 2B, and 2C).
Immunohistochemical stains revealed R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, the lack of a clonal pattern of p53 protein overexpression, diffuse GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 20% (Figures 2E, 2F, 2G, 2H and 2I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were positive for 1p/19q codeletion, and pyrosequencing analysis confirmed the immunohistochemical findings of a c.395G>A (R132H) mutation of the IDH1 gene (Figure 2J). GlioSeq targeted NGS analysis confirmed the presence of the c.395G>A mutation in the IDH1 gene, a mutation in the telomerase reverse transcriptase (TERT) promoter, and possible decreased copy number of the CIC (chromosome 1p) and FUBP1 (chromosome 19q) genes.
A final integrated morphologic and molecular diagnosis of anaplastic oligodendroglioma, IDH-mutant and 1p/19q-codeleted was rendered based on the additional information. With this final diagnosis, methylation analysis of the MGMT gene promoter, which was performed for prognostic and predictive purposes, was identified in this case.5,6
Case 3. A veteran of the Vietnam War presented with a new onset seizure. A MRI revealed a focally contrast-enhancing mass with surrounding edema within the left frontal lobe (Figures 3A, 3B, and 3C).
Hematoxylin and eosin (H&E) stained sections following formalin fixation and paraffin embedding demonstrated similar findings (Figure 3D), and while mitotic figures were readily identified, areas of necrosis were not identified and endothelial proliferation was not a prominent feature. Immunohistochemical stains revealed no evidence of R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, a clonal pattern of p53 protein overexpression, patchy GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 50% (Figures 3E, 3F, 3G, 3H, and 3I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and the tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were negative for EGFR gene amplification and 1p/19q codeletion, although a gain of the long arm of chromosome 1 was detected. Pyrosequencing analysis for mutations in codon 132 of the IDH1 gene revealed no mutations (Figure 3J). GlioSeq targeted NGS analysis identified mutations within the NF1, TP53, and PIK3CA genes without evidence of mutations in the IDH1, IDH2, ATRX, H3F3A, or EGFR genes or the TERT promoter. Based upon this additional information, a final integrated morphologic and molecular diagnosis of GBM, IDH wild-type was issued. The MGMT gene promoter was negative for methylation, a finding that has prognostic and predictive impact with regard to treatment with temazolamide.7-9
New Diffuse Glioma Classification
Since the issuance of the previous edition of the WHO classification of CNS tumors in 2007, several sentinel discoveries have been made that have advanced our understanding of the underlying biology of primary CNS neoplasms. Since a detailed review of these findings is beyond the scope and purpose of this manuscript and salient reviews on the topic can be found elsewhere, we will focus on the molecular findings that have been incorporated into the recently revised WHO classification.10 The importance of providing such information for proper patient management is illustrated by the recent acknowledgement by the American Academy of Neurology that molecular testing of brain tumors is a specific area in which there is a need for quality improvement.11 Therefore, it is critical that these underlying molecular abnormalities are identified to allow for proper classification and treatment of diffuse gliomas in the veteran population.
As noted previously, based on VA cancer registry data, diffuse gliomas are the most commonly encountered primary CNS cancers in the veteran population. Several of the aforementioned seminal discoveries have been incorporated into the updated classification of diffuse gliomas. While the recently updated WHO classification allows for the assignment of “not otherwise specified (NOS)” diagnostic designation, this category must be limited to cases where there is insufficient data to allow for a more precise classification due to sample limitations and not simply due to a failure of VA pathology laboratories to pursue the appropriate diagnostic testing.
Figure 4 presents the recommended diagnostic workflow for the workup of diffuse gliomas. As illustrated in the above cases, a variety of different methodologies, including immunohistochemical, FISH, loss of heterozygosity analysis, traditional and NGS may be applied when elucidating the status of molecular events at critical diagnostic branch points.
Diagnostic Uses of Molecular Testing
While the case studies in this article demonstrate the use of ancillary testing and provide a suggested strategy for properly subclassifying diffuse gliomas, inherent in this strategy is the assumption that, based upon the initial clinical and pathologic information available, one can accurately categorize the lesion as a diffuse glioma. In reality, such a distinction is not always a straightforward endeavor. It is well recognized that a proportion of low-grade, typically radiologically circumscribed, CNS neoplasms, such as pilocytic astrocytomas and glioneuronal tumors, may infiltrate the surrounding brain parenchyma. In addition, many of these low-grade CNS neoplasms also may have growth patterns that are shared with diffuse gliomas, a diagnostic challenge that often can be further hampered by the inherent limitations involved in obtaining adequate samples for diagnosis from the CNS.
Although there are limitations and caveats, molecular diagnostic testing may be invaluable in properly classifying CNS tumors in such situations. The finding of mutations in the IDH1 or IDH2 genes has been shown to be very valuable in distinguishing low-grade diffuse glioma from both nonneoplastic and low-grade circumscribed neuroepithelial neoplasms that may exhibit growth patterns that can mimic those of diffuse gliomas.15-17 Conversely, finding abnormalities in the BRAF gene in a brain neoplasm that has a low-grade morphology suggests that the lesion may represent one of these low-grade lesions such as a pleomorphic xanthoastrocytoma, pilocytic astrocytoma, or mixed neuronal-glial tumor as opposed to a diffuse glioma.18,19
Depending upon the environment in which one practices, small biopsy specimens may be prevalent, and unfortunately, it is not uncommon to obtain a biopsy that exhibits a histologic growth pattern that is discordant from what one would predict based on the clinical context and imaging findings. Molecular testing may be useful in resolving discordances in such situations. If a biopsy of a ring-enhancing lesion demonstrates a diffuse glioma that doesn’t meet WHO grade IV criteria, applying methodologies that look for genetic features commonly encountered in high-grade astrocytomas may identify genetic abnormalities that suggest a more aggressive lesion than is indicated by the histologic findings. The presence of genetic abnormalities such as homozygous deletion of the CDKN2A gene, TERT promoter mutation, loss of heterozygosity of chromosome 10q and/or phosphatase and tensin homolog (PTEN) mutations, EGFR gene amplification or the presence of the EGFR variant III are a few findings that would suggest the aforementioned sample may represent an undersampling of a higher grade diffuse astrocytoma, which would be important information to convey to the treating clinicians.20-26
Testing In the VA
The goals of the MPWG include promoting increased quality and availability of genetic testing within the VHA as well as encouraging internal referral testing. An informal survey of the chiefs of VA Pathology and Laboratory Medicine Services was conducted in November of 2017 in an attempt to identify internal VA pathology laboratories currently conducting testing that may be of use in the workup of diffuse gliomas (Table 1).
The VA currently offers NGS panels for patients with advanced-stage malignancies under the auspices of the Precision Oncology Program, whose reports provide both (1) mutational analyses for genes such as TP53, ATRX, NF1, BRAF, PTEN, TERT IDH1, and IDH2 that may be useful in the proper classifying of high-grade diffuse gliomas; and (2) information regarding clinical trials for which the veteran may be eligible for based on their glioma’s mutational profile. Interested VA providers should visit tinyurl.com/precisiononcology/ for more information about this program. Finally, although internal testing within VA laboratories is recommended to allow for the development of more cost-effective testing, testing may be performed through many nationally contracted reference laboratories.
Conclusion
In light of the recent progress made in our understanding of the molecular events of gliomagenesis, the way we diagnose diffuse gliomas within the CNS has undergone a major paradigm shift. While histology still plays a critical role in the process, we believe that additional ancillary testing is a requirement for all diffuse gliomas diagnosed within VA pathology laboratories. In the context of recently encountered cases, we have provided a recommended workflow highlighting the testing that can be performed to allow for the proper diagnosis of our veterans with diffuse gliomas (Figure 4).
Unless limited by the amount of tissue available for such tests, ancillary testing must be performed on all diffuse gliomas diagnosed within the VA system to ensure proper diagnosis and treatment of our veterans with diffuse gliomas.

Acknowledgments
The authors thank Dr. Craig M. Horbinski (Feinberg School of Medicine, Northwestern University) and Dr. Geoffrey H. Murdoch (University of Pittsburgh) for their constructive criticism of the manuscript. We also thank the following individuals for past service as members of the molecular oncology subcommittee of the MGPW: Dr. George Ansstas (Washington University School of Medicine), Dr. Osssama Hemadeh (Bay Pines VA Health Care System), Dr. James Herman (VA Pittsburgh Healthcare System), and Dr. Ryan Phan (formerly of the VA Greater Los Angeles Healthcare System) as well as the members of the Veterans Administration pathology and laboratory medicine service molecular genetics pathology workgroup.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
Dr. Kulich is the Acting Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System and member of the Division of Neuropathology at University of Pittsburgh Department of Pathology, Dr. Duvvuri is an Otolaryngologist at VA Pittsburgh Healthcare System, and Dr. Passero is the Section Chief of Hematology\Oncology at VA Pittsburgh Healthcare System in Pennsylvania. Dr. Becker is an Oncologist at VA-New York Harbor Healthcare System. Dr. Dacic is a Pathologist at University of Pittsburgh Department of Pathology in Pennsylvania. Dr. Ehsan is Chief of Pathology and Laboratory Medicine Services at the South Texas Veterans Healthcare System in San Antonio. Dr. Gutkin is the former Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System. Dr. Hou is a Pathologist at St. Louis VA Medical Center in Missouri. Dr. Icardi is the VA National Director of Pathology and Laboratory Medicine Services. Dr. Lyle is a Pathologist at Bay Pine Health Care System in Florida. Dr. Lynch is an Investigator at VA Salt Lake Health Care System Informatics and Computing Infrastructure. Dr. Montgomery is an Oncologist at VA Puget Sound Health Care System, in Seattle, Washington. Dr. Przygodzki is the Director of Genomic Medicine Implementation and Associate Director of Genomic Medicine for the VA. Dr. Colman is a Neuro-Oncologist at George E. Wahlen VA Medical Center and the Director of Medical Neuro-Oncology at the Huntsman Cancer Institute, Salt Lake City, Utah.
Correspondence: Dr. Kulich (scott.kulich@va.gov)
1. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803-820.
2. Wang-Rodriguez J, Yunes A, Phan R, et al. The challenges of precision medicine and new advances in molecular diagnostic testing in hematolymphoid malignancies: impact on the VHA. Fed Pract. 2017;34(suppl 5):S38-S49.
3. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(suppl 5):v1-v88.
4. Nikiforova MN, Wald AI, Melan MA, et al. Targeted next-generation sequencing panel (GlioSeq) provides comprehensive genetic profiling of central nervous system tumors. Neuro Oncol. 2016;18(3)379-387.
5. Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90(19):1473-1479.
6. van den Bent MJ, Erdem-Eraslan L, Idbaih A, et al. MGMT-STP27 methylation status as predictive marker for response to PCV in anaplastic oligodendrogliomas and oligoastrocytomas. A report from EORTC study 26951. Clin Cancer Res. 2013;19(19):5513-5522.
7. Stupp R, Hegi ME, Mason WP, et al; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-466.
8. Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916-926.
9. van den Bent MJ, Kros JM. Predictive and prognostic markers in neuro-oncology. J Neuropathol Exp Neurol. 2007;66(12):1074-1081.
10. Chen R, Smith-Cohn M, Cohen AL, Colman H. Glioma subclassifications and their clinical significance. Neurotherapeutics. 2017;14(2):284-297.
11. Jordan JT, Sanders AE, Armstrong T, et al. Quality improvement in neurology: neuro-oncology quality measurement set. Neurology. 2018;90(14):652-658.
12. Chen L, Voronovich Z, Clark K, et al. Predicting the likelihood of an isocitrate dehydrogenase 1 or 2 mutation in diagnoses of infiltrative glioma. Neuro Oncol. 2014;16(11):1478-1483.
13. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997-1003.
14. Wick W, Platten M, Meisner C, et al; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707-715.
15. Horbinski C, Kofler J, Kelly LM, Murdoch GH, Nikiforova MN. Diagnostic use of IDH1/2 mutation analysis in routine clinical testing of formalin-fixed, paraffin-embedded glioma tissues. J Neuropathol Exp Neurol. 2009;68(12):1319-1325.
16. Camelo-Piragua S, Jansen M, Ganguly A, Kim JC, Louis DN, Nutt CL. Mutant IDH1-specific immunohistochemistry distinguishes diffuse astrocytoma from astrocytosis. Acta Neuropathol. 2010;119(4):509-511.
17. Horbinski C, Kofler J, Yeaney G, et al. Isocitrate dehydrogenase 1 analysis differentiates gangliogliomas from infiltrative gliomas. Brain Pathol. 2011;21(5):564-574.
18. Berghoff AS, Preusser M. BRAF alterations in brain tumours: molecular pathology and therapeutic opportunities. Curr Opin Neurol. 2014;27(6):689-696.
19. Korshunov A, Meyer J, Capper D, et al. Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol. 2009;118(3):401-405.
20. Fuller CE, Schmidt RE, Roth KA, et al. Clinical utility of fluorescence in situ hybridization (FISH) in morphologically ambiguous gliomas with hybrid oligodendroglial/astrocytic features. J Neuropathol Exp Neurol. 2003;62(11):1118-1128.
21. Horbinski C. Practical molecular diagnostics in neuropathology: making a tough job a little easier. Semin Diagn Pathol. 2010;27(2):105-113.
22. Fuller GN, Bigner SH. Amplified cellular oncogenes in neoplasms of the human central nervous system. Mutat Res. 1992;276(3):299-306.
23. Brennan CW, Verhaak RG, McKenna A, et al; TCGA Research Network. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462-477.
24. Aldape K, Zadeh G, Mansouri S, Reifenberger G, von Deimling A. Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129(6):829-848.
25. Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110(15):6021-6026.
26. Nikiforova MN, Hamilton RL. Molecular diagnostics of gliomas. Arch Pathol Lab Med. 2011;135(5):558-568.
1. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803-820.
2. Wang-Rodriguez J, Yunes A, Phan R, et al. The challenges of precision medicine and new advances in molecular diagnostic testing in hematolymphoid malignancies: impact on the VHA. Fed Pract. 2017;34(suppl 5):S38-S49.
3. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(suppl 5):v1-v88.
4. Nikiforova MN, Wald AI, Melan MA, et al. Targeted next-generation sequencing panel (GlioSeq) provides comprehensive genetic profiling of central nervous system tumors. Neuro Oncol. 2016;18(3)379-387.
5. Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90(19):1473-1479.
6. van den Bent MJ, Erdem-Eraslan L, Idbaih A, et al. MGMT-STP27 methylation status as predictive marker for response to PCV in anaplastic oligodendrogliomas and oligoastrocytomas. A report from EORTC study 26951. Clin Cancer Res. 2013;19(19):5513-5522.
7. Stupp R, Hegi ME, Mason WP, et al; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-466.
8. Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916-926.
9. van den Bent MJ, Kros JM. Predictive and prognostic markers in neuro-oncology. J Neuropathol Exp Neurol. 2007;66(12):1074-1081.
10. Chen R, Smith-Cohn M, Cohen AL, Colman H. Glioma subclassifications and their clinical significance. Neurotherapeutics. 2017;14(2):284-297.
11. Jordan JT, Sanders AE, Armstrong T, et al. Quality improvement in neurology: neuro-oncology quality measurement set. Neurology. 2018;90(14):652-658.
12. Chen L, Voronovich Z, Clark K, et al. Predicting the likelihood of an isocitrate dehydrogenase 1 or 2 mutation in diagnoses of infiltrative glioma. Neuro Oncol. 2014;16(11):1478-1483.
13. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997-1003.
14. Wick W, Platten M, Meisner C, et al; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707-715.
15. Horbinski C, Kofler J, Kelly LM, Murdoch GH, Nikiforova MN. Diagnostic use of IDH1/2 mutation analysis in routine clinical testing of formalin-fixed, paraffin-embedded glioma tissues. J Neuropathol Exp Neurol. 2009;68(12):1319-1325.
16. Camelo-Piragua S, Jansen M, Ganguly A, Kim JC, Louis DN, Nutt CL. Mutant IDH1-specific immunohistochemistry distinguishes diffuse astrocytoma from astrocytosis. Acta Neuropathol. 2010;119(4):509-511.
17. Horbinski C, Kofler J, Yeaney G, et al. Isocitrate dehydrogenase 1 analysis differentiates gangliogliomas from infiltrative gliomas. Brain Pathol. 2011;21(5):564-574.
18. Berghoff AS, Preusser M. BRAF alterations in brain tumours: molecular pathology and therapeutic opportunities. Curr Opin Neurol. 2014;27(6):689-696.
19. Korshunov A, Meyer J, Capper D, et al. Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol. 2009;118(3):401-405.
20. Fuller CE, Schmidt RE, Roth KA, et al. Clinical utility of fluorescence in situ hybridization (FISH) in morphologically ambiguous gliomas with hybrid oligodendroglial/astrocytic features. J Neuropathol Exp Neurol. 2003;62(11):1118-1128.
21. Horbinski C. Practical molecular diagnostics in neuropathology: making a tough job a little easier. Semin Diagn Pathol. 2010;27(2):105-113.
22. Fuller GN, Bigner SH. Amplified cellular oncogenes in neoplasms of the human central nervous system. Mutat Res. 1992;276(3):299-306.
23. Brennan CW, Verhaak RG, McKenna A, et al; TCGA Research Network. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462-477.
24. Aldape K, Zadeh G, Mansouri S, Reifenberger G, von Deimling A. Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129(6):829-848.
25. Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110(15):6021-6026.
26. Nikiforova MN, Hamilton RL. Molecular diagnostics of gliomas. Arch Pathol Lab Med. 2011;135(5):558-568.
The Use of Immuno-Oncology Treatments in the VA (FULL)
The following is a lightly edited transcript of a teleconference discussion recorded in April 2018.
Suman Kambhampati, MD. Immuno-oncology is a paradigm-shifting treatment approach. It is an easy-to-understand term for both providers and for patients. The underlying principle is that the body’s own immune system is used or stimulated to fight cancer, and there are drugs that clearly have shown huge promise for this, not only in oncology, but also for other diseases. Time will tell whether that really pans out or not, but to begin with, the emphasis has been inoncology, and therefore, the term immunooncology is fitting.
Dr. Kaster. It was encouraging at first, especially when ipilimumab came out, to see the effects on patients with melanoma. Then the KEYNOTE-024 trial came out, and we were able to jump in anduse monoclonal antibodies directed against programmed death 1 (PD-1) in the first line, which is when things got exciting.1 We have a smaller populationin Boise, so PD-1s in lung cancer have had the biggest impact on our patients so far.
Ellen Nason, RN, MSN. Patients are open to immunotherapies.They’re excited about it. And as the other panelists have said, you can start broadly, as the body fights the cancer on its own, to providing more specific details as a patient wants more information. Immuno-oncology is definitely accepted by patients, and they’re very excited about it, especially with all the news about new therapies.
Dr. Kambhampati. For the Department of Veteran Affairs (VA) population, lung cancer has seen significant impact, and now it’s translating into other diseases through more research, trials, and better understanding about how these drugs are used and work.
The paradigm is shifting toward offering these drugs not only in metastatic cancers, but also in the surgically resectable tumors. The 2018 American Association for Cancer Research (AACR) meeting, just concluded. At the meeting several abstracts reported instances where immunooncology drugs are being introduced in the early phases of lung cancer and showing outstanding results. It’s very much possible that we’re going to see less use of traditional chemotherapy in the near future.
Ms. Nason. I primarily work with solid tumors,and the majority of the population I work with have lung cancer. So we’re excited about some of the results that we’ve seen and the lower toxicity involved. Recently, we’ve begun using durvalumab with patients with stage III disease. We have about 5 people now that are using it as a maintenance or consolidative treatment vs just using it for patients with stage IV disease. Hopefully, we’ll see some of the same results describedin the paper published on it.2
Dr. Kaster. Yes, we are incorporating these new changes into care as they're coming out. As Ms. Nason mentioned, we're already using immunotherapies in earlier settings, and we are seeing as much research that could be translated into care soon, like combining immunotherapies
in first-line settings, as we see in the Checkmate-227 study with nivolumab and ipilimumab.3,4 The landscape is going to change dramatically in the next couple of years.
Accessing Testing For First-Line Treatments
Dr. Lynch. There has been an ongoing discussionin the literature on accessing appropriate testing—delays in testing can result in patients who are not able to access the best targeted drugs on a first-line basis. The drug companiesand the VA have become highly sensitized to ensuring that veterans are accessing the appropriate testing. We are expanding the capability of VA labs to do that testing.
Ms. Nason. I want to put in a plug for the VA Precision Oncology Program (POP). It’s about 2 years into its existence, and Neil Spector, MD, is the director. The POP pays for sequencing the tumor samples.
A new sequencing contract will go into effect October 2018 and will include sequencing for hematologic malignancies in addition to the current testing of solid tumors. Patients from New York who have been unable to receive testing through the current vendors used by POP, will be included in the new contract. It is important to note that POP is working closely with the National Pharmacy Benefit Management Service (PBM) to develop a policy for approving off-label use of US Food and Drug Administration-approved targeted therapies based on sequenced data collected on patients tested through POP.
In addition, the leadership of POP is working to leverage the molecular testing results conducted through POP to improve veterans' access to clinical trials, both inside and outside the VA. Within the VA people can access information at tinyurl.com/precisiononcology. There is no reason why any eligible patient with cancer in the VA health care system should not have their tumor tissue sequenced through POP, particularly once the new contract goes into effect.
Dr. Lynch. Fortunately, the cost of next-generation sequencing has come down so much that most VA contracted reference laboratories offer next-generation sequencing, including LabCorp (Burlington,NC), Quest Diagnostics (Secaucus, NJ), Fulgent (Temple City, CA), and academic partners such as Oregon Health Sciences University and University of Washington.
Ms. Nason. At the Durham VAMC, sometimes a lack of tissue has been a barrier, but we now have the ability to send blood (liquid biopsy) for next-generation sequencing. Hopefully that will open up options for veterans with inadequate tissue. Importantly, all VA facilities can request liquid biopsiesthrough POP.
Dr. Lynch. That’s an important point. There have been huge advances in liquid biopsy testing.The VA Salt Lake City Health Care System (VASLCHCS) was in talks with Genomic Health (Redwood City, CA) to do a study as part of clinical operations to look at the concordance between the liquid biopsy testing and the precision oncology data. But Genomic Health eventually abandoned its liquid biopsy testing. Currently, the VA is only reimbursing or encouraging liquid biopsy if the tissue is not available or if the veteran has too high a level of comorbidities to undergo tissue biopsy. The main point for the discussion today is that access to testing is a key component of access to all of these advanced drugs.
Dr. Kambhampati. The precision medicine piece will be a game changer—no question about that. Liquid biopsy is very timely. Many patients have difficulty getting rebiopsied, so liquid biopsy is definitely a big, big step forward.
Still, there has not been consistency across the VA as there should be. Perhaps there are a few select centers, including our site in Kansas City, where access to precision medicine is readily available and liquid biopsies are available. We use the PlasmaSELECT test from Personal Genome Diagnostics (Baltimore, MD). We have just added Foundation Medicine (Cambridge, MA) also in hematology. Access to mutational profilingis absolutely a must for precision medicine.
All that being said, the unique issue with immuno-oncology is that it pretty much transcends the mutational profile and perhaps has leveled the playing field, irrespective of the tumor mutation profile or burden. In some solid tumors these immuno-oncology drugs have been shown to work across tumor types and across different mutation types. And there is a hint now in the recent data presented at AACR and in the New England Journalof Medicine showing that the tumor mutational burden is a predictor of pathologic response to at least PD-1 blockade in the resectable stages of lung cancer.1,3 To me, that’s a very important piece of data because that’s something that can be tested and can have a prognostic impact in immuno-oncology, particularly in the early stages of lung cancer and is further proof of the broad value of immunotherapics in targeting tumors irrespective of the precise tumor targets.
Dr. Kaster. Yes, it’s nice to see other options like tumor mutational burden and Lung Immune Prognostic Index being studied.5 It would be nice if we could rely a little more on these, and not PD-L1, which as we all know is a variable and an unreliable target.
Dr. Kambhampati. I agree.
Rural Challenges In A Veterans Population
Dr. Lynch. Providing high-quality cancer care to rural veterans care can be a challenge but it is a VA priority. The VA National Genomic Medicine Services offers better access for rural veterans to germline genetic testing than any other healthcare system in the country. In terms of access to somatic testing and next-generation sequencing, we are working toward providing the same level of cancer care as patients would receive at National Cancer Institute (NCI) cancer centers. The VA oncology leadership has done teleconsults and virtual tumor boards, but for some rural VAMCs, fellowsare leading the clinical care. As we expand use of oral agents for oncology treatment, it will be easier to ensure that rural veterans receive the same standard of care for POP that veterans being cared for at VASLCHCS, Kansas City VAMC, or Durham VAMC get.
Dr. Kambhampati. The Kansas City VAMC in its catchment area includes underserved areas, such as Topeka and Leavenworth, Kansas. What we’ve been able to do here is something that’s unique—Kansas City VAMC is the only standalone VA in the country to be recognized as a primary SWOG (Southwestern Oncology Group) institution, which provides access to many trials, such as the Lung-MAP trial and others. And that has allowed us to use the full expanse of precision medicine without financial barriers. The research has helped us improve the standard of
care for patients across VISN 15.
Dr. Lynch. In precision oncology, the chief of pathology is an important figure in access to advanced care. I’ve worked with Sharad Mathur,MD, of the Kansas City VAMC on many clinical trials. He’s on the Kansas City VAMC Institutional Review Board and the cancer committee and is tuned in to veterans’ access to precision oncology. Kansas City was ordering Foundation One for select patients that met the criteria probably sooner than any other VA and participated in NCI Cooperative Group clinical trials. It is a great example of how veterans are getting access to
the same level of care as are patients who gettreated at NCI partners.
Comorbidities
Dr. Kambhampati. I don’t treat a lot of patients with lung cancer, but I find it easier to use these immuno-oncology drugs than platinums and etoposide. I consider them absolutely nasty chemotherapy drugs now in this era of immuno-oncology and targeted therapy.
Dr. Lynch. The VA is very important in translational lung cancer research and clinical care. It used to be thought that African American patients don’t get epidermal growth factor receptor mutations. And that’s because not enough African American patients with lung cancer were included in the NCI-based clinical trial.There are7,000 veterans who get lung cancer each year, and 20% to 25% of those are African Americans. Prevalence of various mutations and the pharmacogenetics of some of these drugs differ by patient ancestry. Including veterans with lung
cancer in precision oncology clinical trials and clinical care is not just a priority for the VA but a priority for NCI and internationally. I can’t emphasize this enough—veterans with lung cancer should be included in these studies and should be getting the same level of care that our partners are getting at NCI cancer centers. In the VA we’re positioned to do this because of our nationalelectronic health record (EHR) and becauseof our ability to identify patients with specific variants and enroll them in clinical trials.
Ms. Nason. One of the barriers that I find withsome of the patients that I have treated is getting them to a trial. If the trial isn’t available locally, specifically there are socioeconomic and distance issues that are hard to overcome.
Dr. Kaster. For smaller medical centers, getting patients to clinical trials can be difficult. The Boise VAMC is putting together a proposal now to justify hiring a research pharmacist in order to get trials atour site. The goal is to offer trial participation to our patients who otherwise might not be able to participate while offsetting some of the costs of immunotherapy. We are trying to make what could be a negative into a positive.
Measuring Success
Dr. Kambhampati. Unfortunately, we do not have any calculators to incorporate the quality of lives saved to the society. I know there are clearmetrics in transplant and in hematology, but unfortunately, there are no established metrics in solid tumor treatment that allow us to predict the cost savings to the health care system or to society or the benefit to the society. I don’t use any such predictive models or metrics in my decision making. These decisions are made based on existing evidence, and the existing evidence overwhelmingly supports use of immuno-oncology in certain types of solid tumors and in a select group of hematologic malignancies.
Dr. Kaster. This is where you can get more bang for your buck with an oncology pharmacist these days. A pharmacist can make a minor dosing change that will allow the same benefit for the patient, but could equal tens of thousands of dollars in cost-benefit for the VA. They can also be the second set of eyes when adjudicating a nonformulary request to ensure that a patient will benefit.
Dr. Lynch. Inappropriate prescribing is far more expensive than appropriate treatment. And the care for veterans whose long-term health outcomes could be improved by the new immunotherapies. It’s cheaper for veterans to be healthy and live longer than it is to take care of them in
their last 6 weeks of life. Unfortunately, there are not a lot of studies that have demonstrated that empirically, but I think it’s important to do those studies.
Role of Pharmacists
Dr. Lynch. I was at a meeting recently talking about how to improve veteran access to clinical trials. Francesca Cunningham, PharmD, director of the VA Center for Medication Safety of the VA Pharmacy Benefit Management Service (PBM) described the commitment that pharmacy has in taking a leadership role in the integration of precision medicine. Linking veterans’ tumor mutation status and pharmacogenetic variants to pharmacy databases is the best way to ensure treatment is informed by genetics. We have to be realistic about what we’re asking community oncologists to do. With the onset of precision oncology, 10 cancers have become really 100 cancers. In the prior model of care, it was the oncologist, maybe in collaboration with a pathologist, but it was mostly oncologists who determined care.
And in the evolution of precision oncology, Ithink that it’s become an interdisciplinary adventure. Pharmacy is going to play an increasinglyimportant role in precision medicine around all of the molecular alterations, even immuno-oncology regardless of molecular status in which the VA has an advantage. We’re not talking about some community pharmacist. We’re talking about a national health care system where there’s a national EHR, where there’s national PBM systems. So my thoughts on this aspect is that it’s an intricate multidisciplinary team who can ensure that veteran sget the best care possible: the best most cost-effective care possible.
Dr. Kaster. As an oncology pharmacist, I have to second that.
Ms. Nason. As Dr. Kaster said earlier, having a dedicated oncology pharmacist is tremendouslybeneficial. The oncology/hematology pharmacists are following the patients closely and notice when dose adjustments need to be made, optimizing the drug benefit and providing additional safety. Not to mention the cost benefit that can be realized with appropriate adjustment and the expertise they bring to managing possible interactionsand pharmacodynamics.
Dr. Kambhampati. To brag about the Kansas City VAMC program, we have published in Federal Practitioner our best practices showing the collaboration between a pharmacist and providers.6 And we have used several examples of cost savings, which have basically helped us build the research program, and several examples of dual monitoring oral chemotherapy monitoring. And we have created these templates within the EHR that allow everyone to get a quick snapshot of where things are, what needs to be done, and what needs to be monitored.
Now, we are taking it a step further to determine when to stop chemotherapy or when to stop treatments. For example, for chronic myeloid leukemia (CML), there are good data onstopping tyrosine kinase inhibitors.7 And that alone, if implemented across the VA, could bring
in huge cost savings, which perhaps could be put into investments in immuno-oncology or other efforts. We have several examples here that we have published, and we continue to increaseand strengthen our collaboration withour oncology pharmacist. We are very lucky and privileged to have a dedicated oncology pharmacistfor clinics and for research.
Dr. Lynch. The example of CML is perfect, because precision oncology has increased the complexity of care substantially. The VA is wellpositioned to be a leader in this area when care becomes this complex because of its ability to measure access to testing, to translate the results
of testing to pharmacy, to have pharmacists take the lead on prescribing, to have pathologists take the lead on molecular alterations, and to have oncologists take the lead on delivering the cancer care to the patients.
With hematologic malignancies, adherence in the early stages can result in patients getting offcare sooner, which is cost savings. But that requires access to testing, monitoring that testing, and working in partnership with pharmacy. This is a great story about how the VA is positioned to lead in this area of care.
Dr. Kaster. I would like to put a plug in for advanced practice providers and the use of nurse practitioners (NPs) and physician assistants (PAs).The VA is well positioned because it often has established interdisciplinary teams with these providers, pharmacy, nursing, and often social work, to coordinate the care and manage symptoms outside of oncologist visits.
Dr. Lynch. In the NCI cancer center model, once the patient has become stable, the ongoing careis designated to the NP or PA. Then as soon as there’s a change and it requires reevaluation, the oncologist becomes involved again. That pointabout the oncology treatment team is totally in line
with some of the previous comments.
Areas For Further Investigation
Dr. Kaster. There are so many nuances that we’re finding out all of the time about immunotherapies. A recent study brought up the role of antibiotics in the 30 or possibly 60 days prior to immunotherapy.3 How does that change treatment? Which patients are more likely to benefit from immunotherapies, and which are susceptible to “hyperprogression”? How do we integrate palliative care discussions into the carenow that patients are feeling better on treatment and may be less likely to want to discuss palliative care?
Ms. Nason. I absolutely agree with that, especially keeping palliative care integrated within our services. Our focus is now a little different, in thatwe have more optimistic outcomes in mind, butthere still are symptoms and issues where our colleaguesin palliative care are invaluable.
Dr. Lynch. I third that motion. What I would really like to see come out of this discussion is how veterans are getting access to leading oncology care. We just published an analysis of Medicare data and access to EGFR testing. The result of that analysis showed that testing in the VA was consistent with testing in Medicare.
For palliative care, I think the VA does a better job. And it’s just so discouraging as VA employees and as clinicians treating veterans to see publicationsthat suggest that veterans are getting a lower quality of care and that they would be better if care was privatized or outsourced. It’s just fundamentally not the case.
In CML, we see it. We’ve analyzed the data, in that there’s a far lower number of patients with CML who are included in the registry because patients who are diagnosed outside the VA are incorporated in other cancer registries.8 But as soon as their copays increase for access to targeted drugs, they immediately activate their VA benefits so that theycan get their drugs at the VA. For hematologic malignancies that are diagnosed outside the VA and are captured in other cancer registries, as soon as the drugs become expensive, they start getting their care in the VA. I don’t think there’s beena lot of empirical research that’s shown this, but we have the data to illustrate this trend. I hope thatthere are more publications that show that veterans with cancer are getting really good care inside the VA in the existing VA health care system.
Ms. Nason. It is disheartening to see negativepublicity, knowing that I work with colleagues who are strongly committed to providing up-to-date and relevant oncology care.
Dr. Lynch. As we record this conversation, I am in Rotterdam, Netherlands, in a meeting about genomewide testing. In hematologic malignancies, prostate cancer, and breast cancer, it’s a huge issue. And that is the other area that MVP (Million Veteran Program) is leading the way with the MVP biorepository data. Frankly, there’s no other biorepository that has this many patients, that has so many African Americans, and that has such rich EHR data. So inthat other area, the VA is doing really well.
1. Reck M, Rodríguez-Abreu D, Robinson AG, et al; KEYNOTE-024 Investigators. Pembrolizumab vs chemotherapy for PD-L1-positive non-small cell lung cancer. N Engl J Med. 2016;375(19):1823-1833.
2. Antonia SJ, Villegas A, Daniel D, et al; PACIFIC Investigators. Durvalumab after chemoradiotherapy in stage III non–smallcell lung cancer. N Engl J Med. 2017;377(20):1919-1929.
3. Hellmann MD, Ciuleanu T-E, Pluzansk A, et al. Nivolumab plus ipilimumab in Lung Cancer with a high tumor mutational burden. N Engl J Med. 2018 April 16. [Epub ahead of print.]
4. Motzer RJ, Tannir NM, McDermott DF, et al; CheckMate214 Investigators. Nivolumab plus ipilimumab versus sunitinibin advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290.
5. Derosa L, Hellmann MD, Spaziano M, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small cell
lung cancer. Ann Oncol. 2018 March 30. [Epub ahead of print.]
6. Heinrichs A, Dessars B, El Housni H, et al. Identification of chronic myeloid leukemia patients treated with imatinib who are potentially eligible for treatment discontinuation by assessingreal-life molecular responses on the international scale in a EUTOS-certified lab. Leuk Res. 2018;67:27-31.
7. Keefe S, Kambhampati S, Powers B. An electronic chemotherapy ordering process and template. Fed Pract. 2015;32(suppl 1):21S-25S.
8. Lynch JA, Berse B, Rabb M, et al. Underutilization and disparities in access to EGFR testing among Medicare patients with lung cancer from 2010 - 2013. BMC Cancer. 2018;18(1):306.
The following is a lightly edited transcript of a teleconference discussion recorded in April 2018.
Suman Kambhampati, MD. Immuno-oncology is a paradigm-shifting treatment approach. It is an easy-to-understand term for both providers and for patients. The underlying principle is that the body’s own immune system is used or stimulated to fight cancer, and there are drugs that clearly have shown huge promise for this, not only in oncology, but also for other diseases. Time will tell whether that really pans out or not, but to begin with, the emphasis has been inoncology, and therefore, the term immunooncology is fitting.
Dr. Kaster. It was encouraging at first, especially when ipilimumab came out, to see the effects on patients with melanoma. Then the KEYNOTE-024 trial came out, and we were able to jump in anduse monoclonal antibodies directed against programmed death 1 (PD-1) in the first line, which is when things got exciting.1 We have a smaller populationin Boise, so PD-1s in lung cancer have had the biggest impact on our patients so far.
Ellen Nason, RN, MSN. Patients are open to immunotherapies.They’re excited about it. And as the other panelists have said, you can start broadly, as the body fights the cancer on its own, to providing more specific details as a patient wants more information. Immuno-oncology is definitely accepted by patients, and they’re very excited about it, especially with all the news about new therapies.
Dr. Kambhampati. For the Department of Veteran Affairs (VA) population, lung cancer has seen significant impact, and now it’s translating into other diseases through more research, trials, and better understanding about how these drugs are used and work.
The paradigm is shifting toward offering these drugs not only in metastatic cancers, but also in the surgically resectable tumors. The 2018 American Association for Cancer Research (AACR) meeting, just concluded. At the meeting several abstracts reported instances where immunooncology drugs are being introduced in the early phases of lung cancer and showing outstanding results. It’s very much possible that we’re going to see less use of traditional chemotherapy in the near future.
Ms. Nason. I primarily work with solid tumors,and the majority of the population I work with have lung cancer. So we’re excited about some of the results that we’ve seen and the lower toxicity involved. Recently, we’ve begun using durvalumab with patients with stage III disease. We have about 5 people now that are using it as a maintenance or consolidative treatment vs just using it for patients with stage IV disease. Hopefully, we’ll see some of the same results describedin the paper published on it.2
Dr. Kaster. Yes, we are incorporating these new changes into care as they're coming out. As Ms. Nason mentioned, we're already using immunotherapies in earlier settings, and we are seeing as much research that could be translated into care soon, like combining immunotherapies
in first-line settings, as we see in the Checkmate-227 study with nivolumab and ipilimumab.3,4 The landscape is going to change dramatically in the next couple of years.
Accessing Testing For First-Line Treatments
Dr. Lynch. There has been an ongoing discussionin the literature on accessing appropriate testing—delays in testing can result in patients who are not able to access the best targeted drugs on a first-line basis. The drug companiesand the VA have become highly sensitized to ensuring that veterans are accessing the appropriate testing. We are expanding the capability of VA labs to do that testing.
Ms. Nason. I want to put in a plug for the VA Precision Oncology Program (POP). It’s about 2 years into its existence, and Neil Spector, MD, is the director. The POP pays for sequencing the tumor samples.
A new sequencing contract will go into effect October 2018 and will include sequencing for hematologic malignancies in addition to the current testing of solid tumors. Patients from New York who have been unable to receive testing through the current vendors used by POP, will be included in the new contract. It is important to note that POP is working closely with the National Pharmacy Benefit Management Service (PBM) to develop a policy for approving off-label use of US Food and Drug Administration-approved targeted therapies based on sequenced data collected on patients tested through POP.
In addition, the leadership of POP is working to leverage the molecular testing results conducted through POP to improve veterans' access to clinical trials, both inside and outside the VA. Within the VA people can access information at tinyurl.com/precisiononcology. There is no reason why any eligible patient with cancer in the VA health care system should not have their tumor tissue sequenced through POP, particularly once the new contract goes into effect.
Dr. Lynch. Fortunately, the cost of next-generation sequencing has come down so much that most VA contracted reference laboratories offer next-generation sequencing, including LabCorp (Burlington,NC), Quest Diagnostics (Secaucus, NJ), Fulgent (Temple City, CA), and academic partners such as Oregon Health Sciences University and University of Washington.
Ms. Nason. At the Durham VAMC, sometimes a lack of tissue has been a barrier, but we now have the ability to send blood (liquid biopsy) for next-generation sequencing. Hopefully that will open up options for veterans with inadequate tissue. Importantly, all VA facilities can request liquid biopsiesthrough POP.
Dr. Lynch. That’s an important point. There have been huge advances in liquid biopsy testing.The VA Salt Lake City Health Care System (VASLCHCS) was in talks with Genomic Health (Redwood City, CA) to do a study as part of clinical operations to look at the concordance between the liquid biopsy testing and the precision oncology data. But Genomic Health eventually abandoned its liquid biopsy testing. Currently, the VA is only reimbursing or encouraging liquid biopsy if the tissue is not available or if the veteran has too high a level of comorbidities to undergo tissue biopsy. The main point for the discussion today is that access to testing is a key component of access to all of these advanced drugs.
Dr. Kambhampati. The precision medicine piece will be a game changer—no question about that. Liquid biopsy is very timely. Many patients have difficulty getting rebiopsied, so liquid biopsy is definitely a big, big step forward.
Still, there has not been consistency across the VA as there should be. Perhaps there are a few select centers, including our site in Kansas City, where access to precision medicine is readily available and liquid biopsies are available. We use the PlasmaSELECT test from Personal Genome Diagnostics (Baltimore, MD). We have just added Foundation Medicine (Cambridge, MA) also in hematology. Access to mutational profilingis absolutely a must for precision medicine.
All that being said, the unique issue with immuno-oncology is that it pretty much transcends the mutational profile and perhaps has leveled the playing field, irrespective of the tumor mutation profile or burden. In some solid tumors these immuno-oncology drugs have been shown to work across tumor types and across different mutation types. And there is a hint now in the recent data presented at AACR and in the New England Journalof Medicine showing that the tumor mutational burden is a predictor of pathologic response to at least PD-1 blockade in the resectable stages of lung cancer.1,3 To me, that’s a very important piece of data because that’s something that can be tested and can have a prognostic impact in immuno-oncology, particularly in the early stages of lung cancer and is further proof of the broad value of immunotherapics in targeting tumors irrespective of the precise tumor targets.
Dr. Kaster. Yes, it’s nice to see other options like tumor mutational burden and Lung Immune Prognostic Index being studied.5 It would be nice if we could rely a little more on these, and not PD-L1, which as we all know is a variable and an unreliable target.
Dr. Kambhampati. I agree.
Rural Challenges In A Veterans Population
Dr. Lynch. Providing high-quality cancer care to rural veterans care can be a challenge but it is a VA priority. The VA National Genomic Medicine Services offers better access for rural veterans to germline genetic testing than any other healthcare system in the country. In terms of access to somatic testing and next-generation sequencing, we are working toward providing the same level of cancer care as patients would receive at National Cancer Institute (NCI) cancer centers. The VA oncology leadership has done teleconsults and virtual tumor boards, but for some rural VAMCs, fellowsare leading the clinical care. As we expand use of oral agents for oncology treatment, it will be easier to ensure that rural veterans receive the same standard of care for POP that veterans being cared for at VASLCHCS, Kansas City VAMC, or Durham VAMC get.
Dr. Kambhampati. The Kansas City VAMC in its catchment area includes underserved areas, such as Topeka and Leavenworth, Kansas. What we’ve been able to do here is something that’s unique—Kansas City VAMC is the only standalone VA in the country to be recognized as a primary SWOG (Southwestern Oncology Group) institution, which provides access to many trials, such as the Lung-MAP trial and others. And that has allowed us to use the full expanse of precision medicine without financial barriers. The research has helped us improve the standard of
care for patients across VISN 15.
Dr. Lynch. In precision oncology, the chief of pathology is an important figure in access to advanced care. I’ve worked with Sharad Mathur,MD, of the Kansas City VAMC on many clinical trials. He’s on the Kansas City VAMC Institutional Review Board and the cancer committee and is tuned in to veterans’ access to precision oncology. Kansas City was ordering Foundation One for select patients that met the criteria probably sooner than any other VA and participated in NCI Cooperative Group clinical trials. It is a great example of how veterans are getting access to
the same level of care as are patients who gettreated at NCI partners.
Comorbidities
Dr. Kambhampati. I don’t treat a lot of patients with lung cancer, but I find it easier to use these immuno-oncology drugs than platinums and etoposide. I consider them absolutely nasty chemotherapy drugs now in this era of immuno-oncology and targeted therapy.
Dr. Lynch. The VA is very important in translational lung cancer research and clinical care. It used to be thought that African American patients don’t get epidermal growth factor receptor mutations. And that’s because not enough African American patients with lung cancer were included in the NCI-based clinical trial.There are7,000 veterans who get lung cancer each year, and 20% to 25% of those are African Americans. Prevalence of various mutations and the pharmacogenetics of some of these drugs differ by patient ancestry. Including veterans with lung
cancer in precision oncology clinical trials and clinical care is not just a priority for the VA but a priority for NCI and internationally. I can’t emphasize this enough—veterans with lung cancer should be included in these studies and should be getting the same level of care that our partners are getting at NCI cancer centers. In the VA we’re positioned to do this because of our nationalelectronic health record (EHR) and becauseof our ability to identify patients with specific variants and enroll them in clinical trials.
Ms. Nason. One of the barriers that I find withsome of the patients that I have treated is getting them to a trial. If the trial isn’t available locally, specifically there are socioeconomic and distance issues that are hard to overcome.
Dr. Kaster. For smaller medical centers, getting patients to clinical trials can be difficult. The Boise VAMC is putting together a proposal now to justify hiring a research pharmacist in order to get trials atour site. The goal is to offer trial participation to our patients who otherwise might not be able to participate while offsetting some of the costs of immunotherapy. We are trying to make what could be a negative into a positive.
Measuring Success
Dr. Kambhampati. Unfortunately, we do not have any calculators to incorporate the quality of lives saved to the society. I know there are clearmetrics in transplant and in hematology, but unfortunately, there are no established metrics in solid tumor treatment that allow us to predict the cost savings to the health care system or to society or the benefit to the society. I don’t use any such predictive models or metrics in my decision making. These decisions are made based on existing evidence, and the existing evidence overwhelmingly supports use of immuno-oncology in certain types of solid tumors and in a select group of hematologic malignancies.
Dr. Kaster. This is where you can get more bang for your buck with an oncology pharmacist these days. A pharmacist can make a minor dosing change that will allow the same benefit for the patient, but could equal tens of thousands of dollars in cost-benefit for the VA. They can also be the second set of eyes when adjudicating a nonformulary request to ensure that a patient will benefit.
Dr. Lynch. Inappropriate prescribing is far more expensive than appropriate treatment. And the care for veterans whose long-term health outcomes could be improved by the new immunotherapies. It’s cheaper for veterans to be healthy and live longer than it is to take care of them in
their last 6 weeks of life. Unfortunately, there are not a lot of studies that have demonstrated that empirically, but I think it’s important to do those studies.
Role of Pharmacists
Dr. Lynch. I was at a meeting recently talking about how to improve veteran access to clinical trials. Francesca Cunningham, PharmD, director of the VA Center for Medication Safety of the VA Pharmacy Benefit Management Service (PBM) described the commitment that pharmacy has in taking a leadership role in the integration of precision medicine. Linking veterans’ tumor mutation status and pharmacogenetic variants to pharmacy databases is the best way to ensure treatment is informed by genetics. We have to be realistic about what we’re asking community oncologists to do. With the onset of precision oncology, 10 cancers have become really 100 cancers. In the prior model of care, it was the oncologist, maybe in collaboration with a pathologist, but it was mostly oncologists who determined care.
And in the evolution of precision oncology, Ithink that it’s become an interdisciplinary adventure. Pharmacy is going to play an increasinglyimportant role in precision medicine around all of the molecular alterations, even immuno-oncology regardless of molecular status in which the VA has an advantage. We’re not talking about some community pharmacist. We’re talking about a national health care system where there’s a national EHR, where there’s national PBM systems. So my thoughts on this aspect is that it’s an intricate multidisciplinary team who can ensure that veteran sget the best care possible: the best most cost-effective care possible.
Dr. Kaster. As an oncology pharmacist, I have to second that.
Ms. Nason. As Dr. Kaster said earlier, having a dedicated oncology pharmacist is tremendouslybeneficial. The oncology/hematology pharmacists are following the patients closely and notice when dose adjustments need to be made, optimizing the drug benefit and providing additional safety. Not to mention the cost benefit that can be realized with appropriate adjustment and the expertise they bring to managing possible interactionsand pharmacodynamics.
Dr. Kambhampati. To brag about the Kansas City VAMC program, we have published in Federal Practitioner our best practices showing the collaboration between a pharmacist and providers.6 And we have used several examples of cost savings, which have basically helped us build the research program, and several examples of dual monitoring oral chemotherapy monitoring. And we have created these templates within the EHR that allow everyone to get a quick snapshot of where things are, what needs to be done, and what needs to be monitored.
Now, we are taking it a step further to determine when to stop chemotherapy or when to stop treatments. For example, for chronic myeloid leukemia (CML), there are good data onstopping tyrosine kinase inhibitors.7 And that alone, if implemented across the VA, could bring
in huge cost savings, which perhaps could be put into investments in immuno-oncology or other efforts. We have several examples here that we have published, and we continue to increaseand strengthen our collaboration withour oncology pharmacist. We are very lucky and privileged to have a dedicated oncology pharmacistfor clinics and for research.
Dr. Lynch. The example of CML is perfect, because precision oncology has increased the complexity of care substantially. The VA is wellpositioned to be a leader in this area when care becomes this complex because of its ability to measure access to testing, to translate the results
of testing to pharmacy, to have pharmacists take the lead on prescribing, to have pathologists take the lead on molecular alterations, and to have oncologists take the lead on delivering the cancer care to the patients.
With hematologic malignancies, adherence in the early stages can result in patients getting offcare sooner, which is cost savings. But that requires access to testing, monitoring that testing, and working in partnership with pharmacy. This is a great story about how the VA is positioned to lead in this area of care.
Dr. Kaster. I would like to put a plug in for advanced practice providers and the use of nurse practitioners (NPs) and physician assistants (PAs).The VA is well positioned because it often has established interdisciplinary teams with these providers, pharmacy, nursing, and often social work, to coordinate the care and manage symptoms outside of oncologist visits.
Dr. Lynch. In the NCI cancer center model, once the patient has become stable, the ongoing careis designated to the NP or PA. Then as soon as there’s a change and it requires reevaluation, the oncologist becomes involved again. That pointabout the oncology treatment team is totally in line
with some of the previous comments.
Areas For Further Investigation
Dr. Kaster. There are so many nuances that we’re finding out all of the time about immunotherapies. A recent study brought up the role of antibiotics in the 30 or possibly 60 days prior to immunotherapy.3 How does that change treatment? Which patients are more likely to benefit from immunotherapies, and which are susceptible to “hyperprogression”? How do we integrate palliative care discussions into the carenow that patients are feeling better on treatment and may be less likely to want to discuss palliative care?
Ms. Nason. I absolutely agree with that, especially keeping palliative care integrated within our services. Our focus is now a little different, in thatwe have more optimistic outcomes in mind, butthere still are symptoms and issues where our colleaguesin palliative care are invaluable.
Dr. Lynch. I third that motion. What I would really like to see come out of this discussion is how veterans are getting access to leading oncology care. We just published an analysis of Medicare data and access to EGFR testing. The result of that analysis showed that testing in the VA was consistent with testing in Medicare.
For palliative care, I think the VA does a better job. And it’s just so discouraging as VA employees and as clinicians treating veterans to see publicationsthat suggest that veterans are getting a lower quality of care and that they would be better if care was privatized or outsourced. It’s just fundamentally not the case.
In CML, we see it. We’ve analyzed the data, in that there’s a far lower number of patients with CML who are included in the registry because patients who are diagnosed outside the VA are incorporated in other cancer registries.8 But as soon as their copays increase for access to targeted drugs, they immediately activate their VA benefits so that theycan get their drugs at the VA. For hematologic malignancies that are diagnosed outside the VA and are captured in other cancer registries, as soon as the drugs become expensive, they start getting their care in the VA. I don’t think there’s beena lot of empirical research that’s shown this, but we have the data to illustrate this trend. I hope thatthere are more publications that show that veterans with cancer are getting really good care inside the VA in the existing VA health care system.
Ms. Nason. It is disheartening to see negativepublicity, knowing that I work with colleagues who are strongly committed to providing up-to-date and relevant oncology care.
Dr. Lynch. As we record this conversation, I am in Rotterdam, Netherlands, in a meeting about genomewide testing. In hematologic malignancies, prostate cancer, and breast cancer, it’s a huge issue. And that is the other area that MVP (Million Veteran Program) is leading the way with the MVP biorepository data. Frankly, there’s no other biorepository that has this many patients, that has so many African Americans, and that has such rich EHR data. So inthat other area, the VA is doing really well.
The following is a lightly edited transcript of a teleconference discussion recorded in April 2018.
Suman Kambhampati, MD. Immuno-oncology is a paradigm-shifting treatment approach. It is an easy-to-understand term for both providers and for patients. The underlying principle is that the body’s own immune system is used or stimulated to fight cancer, and there are drugs that clearly have shown huge promise for this, not only in oncology, but also for other diseases. Time will tell whether that really pans out or not, but to begin with, the emphasis has been inoncology, and therefore, the term immunooncology is fitting.
Dr. Kaster. It was encouraging at first, especially when ipilimumab came out, to see the effects on patients with melanoma. Then the KEYNOTE-024 trial came out, and we were able to jump in anduse monoclonal antibodies directed against programmed death 1 (PD-1) in the first line, which is when things got exciting.1 We have a smaller populationin Boise, so PD-1s in lung cancer have had the biggest impact on our patients so far.
Ellen Nason, RN, MSN. Patients are open to immunotherapies.They’re excited about it. And as the other panelists have said, you can start broadly, as the body fights the cancer on its own, to providing more specific details as a patient wants more information. Immuno-oncology is definitely accepted by patients, and they’re very excited about it, especially with all the news about new therapies.
Dr. Kambhampati. For the Department of Veteran Affairs (VA) population, lung cancer has seen significant impact, and now it’s translating into other diseases through more research, trials, and better understanding about how these drugs are used and work.
The paradigm is shifting toward offering these drugs not only in metastatic cancers, but also in the surgically resectable tumors. The 2018 American Association for Cancer Research (AACR) meeting, just concluded. At the meeting several abstracts reported instances where immunooncology drugs are being introduced in the early phases of lung cancer and showing outstanding results. It’s very much possible that we’re going to see less use of traditional chemotherapy in the near future.
Ms. Nason. I primarily work with solid tumors,and the majority of the population I work with have lung cancer. So we’re excited about some of the results that we’ve seen and the lower toxicity involved. Recently, we’ve begun using durvalumab with patients with stage III disease. We have about 5 people now that are using it as a maintenance or consolidative treatment vs just using it for patients with stage IV disease. Hopefully, we’ll see some of the same results describedin the paper published on it.2
Dr. Kaster. Yes, we are incorporating these new changes into care as they're coming out. As Ms. Nason mentioned, we're already using immunotherapies in earlier settings, and we are seeing as much research that could be translated into care soon, like combining immunotherapies
in first-line settings, as we see in the Checkmate-227 study with nivolumab and ipilimumab.3,4 The landscape is going to change dramatically in the next couple of years.
Accessing Testing For First-Line Treatments
Dr. Lynch. There has been an ongoing discussionin the literature on accessing appropriate testing—delays in testing can result in patients who are not able to access the best targeted drugs on a first-line basis. The drug companiesand the VA have become highly sensitized to ensuring that veterans are accessing the appropriate testing. We are expanding the capability of VA labs to do that testing.
Ms. Nason. I want to put in a plug for the VA Precision Oncology Program (POP). It’s about 2 years into its existence, and Neil Spector, MD, is the director. The POP pays for sequencing the tumor samples.
A new sequencing contract will go into effect October 2018 and will include sequencing for hematologic malignancies in addition to the current testing of solid tumors. Patients from New York who have been unable to receive testing through the current vendors used by POP, will be included in the new contract. It is important to note that POP is working closely with the National Pharmacy Benefit Management Service (PBM) to develop a policy for approving off-label use of US Food and Drug Administration-approved targeted therapies based on sequenced data collected on patients tested through POP.
In addition, the leadership of POP is working to leverage the molecular testing results conducted through POP to improve veterans' access to clinical trials, both inside and outside the VA. Within the VA people can access information at tinyurl.com/precisiononcology. There is no reason why any eligible patient with cancer in the VA health care system should not have their tumor tissue sequenced through POP, particularly once the new contract goes into effect.
Dr. Lynch. Fortunately, the cost of next-generation sequencing has come down so much that most VA contracted reference laboratories offer next-generation sequencing, including LabCorp (Burlington,NC), Quest Diagnostics (Secaucus, NJ), Fulgent (Temple City, CA), and academic partners such as Oregon Health Sciences University and University of Washington.
Ms. Nason. At the Durham VAMC, sometimes a lack of tissue has been a barrier, but we now have the ability to send blood (liquid biopsy) for next-generation sequencing. Hopefully that will open up options for veterans with inadequate tissue. Importantly, all VA facilities can request liquid biopsiesthrough POP.
Dr. Lynch. That’s an important point. There have been huge advances in liquid biopsy testing.The VA Salt Lake City Health Care System (VASLCHCS) was in talks with Genomic Health (Redwood City, CA) to do a study as part of clinical operations to look at the concordance between the liquid biopsy testing and the precision oncology data. But Genomic Health eventually abandoned its liquid biopsy testing. Currently, the VA is only reimbursing or encouraging liquid biopsy if the tissue is not available or if the veteran has too high a level of comorbidities to undergo tissue biopsy. The main point for the discussion today is that access to testing is a key component of access to all of these advanced drugs.
Dr. Kambhampati. The precision medicine piece will be a game changer—no question about that. Liquid biopsy is very timely. Many patients have difficulty getting rebiopsied, so liquid biopsy is definitely a big, big step forward.
Still, there has not been consistency across the VA as there should be. Perhaps there are a few select centers, including our site in Kansas City, where access to precision medicine is readily available and liquid biopsies are available. We use the PlasmaSELECT test from Personal Genome Diagnostics (Baltimore, MD). We have just added Foundation Medicine (Cambridge, MA) also in hematology. Access to mutational profilingis absolutely a must for precision medicine.
All that being said, the unique issue with immuno-oncology is that it pretty much transcends the mutational profile and perhaps has leveled the playing field, irrespective of the tumor mutation profile or burden. In some solid tumors these immuno-oncology drugs have been shown to work across tumor types and across different mutation types. And there is a hint now in the recent data presented at AACR and in the New England Journalof Medicine showing that the tumor mutational burden is a predictor of pathologic response to at least PD-1 blockade in the resectable stages of lung cancer.1,3 To me, that’s a very important piece of data because that’s something that can be tested and can have a prognostic impact in immuno-oncology, particularly in the early stages of lung cancer and is further proof of the broad value of immunotherapics in targeting tumors irrespective of the precise tumor targets.
Dr. Kaster. Yes, it’s nice to see other options like tumor mutational burden and Lung Immune Prognostic Index being studied.5 It would be nice if we could rely a little more on these, and not PD-L1, which as we all know is a variable and an unreliable target.
Dr. Kambhampati. I agree.
Rural Challenges In A Veterans Population
Dr. Lynch. Providing high-quality cancer care to rural veterans care can be a challenge but it is a VA priority. The VA National Genomic Medicine Services offers better access for rural veterans to germline genetic testing than any other healthcare system in the country. In terms of access to somatic testing and next-generation sequencing, we are working toward providing the same level of cancer care as patients would receive at National Cancer Institute (NCI) cancer centers. The VA oncology leadership has done teleconsults and virtual tumor boards, but for some rural VAMCs, fellowsare leading the clinical care. As we expand use of oral agents for oncology treatment, it will be easier to ensure that rural veterans receive the same standard of care for POP that veterans being cared for at VASLCHCS, Kansas City VAMC, or Durham VAMC get.
Dr. Kambhampati. The Kansas City VAMC in its catchment area includes underserved areas, such as Topeka and Leavenworth, Kansas. What we’ve been able to do here is something that’s unique—Kansas City VAMC is the only standalone VA in the country to be recognized as a primary SWOG (Southwestern Oncology Group) institution, which provides access to many trials, such as the Lung-MAP trial and others. And that has allowed us to use the full expanse of precision medicine without financial barriers. The research has helped us improve the standard of
care for patients across VISN 15.
Dr. Lynch. In precision oncology, the chief of pathology is an important figure in access to advanced care. I’ve worked with Sharad Mathur,MD, of the Kansas City VAMC on many clinical trials. He’s on the Kansas City VAMC Institutional Review Board and the cancer committee and is tuned in to veterans’ access to precision oncology. Kansas City was ordering Foundation One for select patients that met the criteria probably sooner than any other VA and participated in NCI Cooperative Group clinical trials. It is a great example of how veterans are getting access to
the same level of care as are patients who gettreated at NCI partners.
Comorbidities
Dr. Kambhampati. I don’t treat a lot of patients with lung cancer, but I find it easier to use these immuno-oncology drugs than platinums and etoposide. I consider them absolutely nasty chemotherapy drugs now in this era of immuno-oncology and targeted therapy.
Dr. Lynch. The VA is very important in translational lung cancer research and clinical care. It used to be thought that African American patients don’t get epidermal growth factor receptor mutations. And that’s because not enough African American patients with lung cancer were included in the NCI-based clinical trial.There are7,000 veterans who get lung cancer each year, and 20% to 25% of those are African Americans. Prevalence of various mutations and the pharmacogenetics of some of these drugs differ by patient ancestry. Including veterans with lung
cancer in precision oncology clinical trials and clinical care is not just a priority for the VA but a priority for NCI and internationally. I can’t emphasize this enough—veterans with lung cancer should be included in these studies and should be getting the same level of care that our partners are getting at NCI cancer centers. In the VA we’re positioned to do this because of our nationalelectronic health record (EHR) and becauseof our ability to identify patients with specific variants and enroll them in clinical trials.
Ms. Nason. One of the barriers that I find withsome of the patients that I have treated is getting them to a trial. If the trial isn’t available locally, specifically there are socioeconomic and distance issues that are hard to overcome.
Dr. Kaster. For smaller medical centers, getting patients to clinical trials can be difficult. The Boise VAMC is putting together a proposal now to justify hiring a research pharmacist in order to get trials atour site. The goal is to offer trial participation to our patients who otherwise might not be able to participate while offsetting some of the costs of immunotherapy. We are trying to make what could be a negative into a positive.
Measuring Success
Dr. Kambhampati. Unfortunately, we do not have any calculators to incorporate the quality of lives saved to the society. I know there are clearmetrics in transplant and in hematology, but unfortunately, there are no established metrics in solid tumor treatment that allow us to predict the cost savings to the health care system or to society or the benefit to the society. I don’t use any such predictive models or metrics in my decision making. These decisions are made based on existing evidence, and the existing evidence overwhelmingly supports use of immuno-oncology in certain types of solid tumors and in a select group of hematologic malignancies.
Dr. Kaster. This is where you can get more bang for your buck with an oncology pharmacist these days. A pharmacist can make a minor dosing change that will allow the same benefit for the patient, but could equal tens of thousands of dollars in cost-benefit for the VA. They can also be the second set of eyes when adjudicating a nonformulary request to ensure that a patient will benefit.
Dr. Lynch. Inappropriate prescribing is far more expensive than appropriate treatment. And the care for veterans whose long-term health outcomes could be improved by the new immunotherapies. It’s cheaper for veterans to be healthy and live longer than it is to take care of them in
their last 6 weeks of life. Unfortunately, there are not a lot of studies that have demonstrated that empirically, but I think it’s important to do those studies.
Role of Pharmacists
Dr. Lynch. I was at a meeting recently talking about how to improve veteran access to clinical trials. Francesca Cunningham, PharmD, director of the VA Center for Medication Safety of the VA Pharmacy Benefit Management Service (PBM) described the commitment that pharmacy has in taking a leadership role in the integration of precision medicine. Linking veterans’ tumor mutation status and pharmacogenetic variants to pharmacy databases is the best way to ensure treatment is informed by genetics. We have to be realistic about what we’re asking community oncologists to do. With the onset of precision oncology, 10 cancers have become really 100 cancers. In the prior model of care, it was the oncologist, maybe in collaboration with a pathologist, but it was mostly oncologists who determined care.
And in the evolution of precision oncology, Ithink that it’s become an interdisciplinary adventure. Pharmacy is going to play an increasinglyimportant role in precision medicine around all of the molecular alterations, even immuno-oncology regardless of molecular status in which the VA has an advantage. We’re not talking about some community pharmacist. We’re talking about a national health care system where there’s a national EHR, where there’s national PBM systems. So my thoughts on this aspect is that it’s an intricate multidisciplinary team who can ensure that veteran sget the best care possible: the best most cost-effective care possible.
Dr. Kaster. As an oncology pharmacist, I have to second that.
Ms. Nason. As Dr. Kaster said earlier, having a dedicated oncology pharmacist is tremendouslybeneficial. The oncology/hematology pharmacists are following the patients closely and notice when dose adjustments need to be made, optimizing the drug benefit and providing additional safety. Not to mention the cost benefit that can be realized with appropriate adjustment and the expertise they bring to managing possible interactionsand pharmacodynamics.
Dr. Kambhampati. To brag about the Kansas City VAMC program, we have published in Federal Practitioner our best practices showing the collaboration between a pharmacist and providers.6 And we have used several examples of cost savings, which have basically helped us build the research program, and several examples of dual monitoring oral chemotherapy monitoring. And we have created these templates within the EHR that allow everyone to get a quick snapshot of where things are, what needs to be done, and what needs to be monitored.
Now, we are taking it a step further to determine when to stop chemotherapy or when to stop treatments. For example, for chronic myeloid leukemia (CML), there are good data onstopping tyrosine kinase inhibitors.7 And that alone, if implemented across the VA, could bring
in huge cost savings, which perhaps could be put into investments in immuno-oncology or other efforts. We have several examples here that we have published, and we continue to increaseand strengthen our collaboration withour oncology pharmacist. We are very lucky and privileged to have a dedicated oncology pharmacistfor clinics and for research.
Dr. Lynch. The example of CML is perfect, because precision oncology has increased the complexity of care substantially. The VA is wellpositioned to be a leader in this area when care becomes this complex because of its ability to measure access to testing, to translate the results
of testing to pharmacy, to have pharmacists take the lead on prescribing, to have pathologists take the lead on molecular alterations, and to have oncologists take the lead on delivering the cancer care to the patients.
With hematologic malignancies, adherence in the early stages can result in patients getting offcare sooner, which is cost savings. But that requires access to testing, monitoring that testing, and working in partnership with pharmacy. This is a great story about how the VA is positioned to lead in this area of care.
Dr. Kaster. I would like to put a plug in for advanced practice providers and the use of nurse practitioners (NPs) and physician assistants (PAs).The VA is well positioned because it often has established interdisciplinary teams with these providers, pharmacy, nursing, and often social work, to coordinate the care and manage symptoms outside of oncologist visits.
Dr. Lynch. In the NCI cancer center model, once the patient has become stable, the ongoing careis designated to the NP or PA. Then as soon as there’s a change and it requires reevaluation, the oncologist becomes involved again. That pointabout the oncology treatment team is totally in line
with some of the previous comments.
Areas For Further Investigation
Dr. Kaster. There are so many nuances that we’re finding out all of the time about immunotherapies. A recent study brought up the role of antibiotics in the 30 or possibly 60 days prior to immunotherapy.3 How does that change treatment? Which patients are more likely to benefit from immunotherapies, and which are susceptible to “hyperprogression”? How do we integrate palliative care discussions into the carenow that patients are feeling better on treatment and may be less likely to want to discuss palliative care?
Ms. Nason. I absolutely agree with that, especially keeping palliative care integrated within our services. Our focus is now a little different, in thatwe have more optimistic outcomes in mind, butthere still are symptoms and issues where our colleaguesin palliative care are invaluable.
Dr. Lynch. I third that motion. What I would really like to see come out of this discussion is how veterans are getting access to leading oncology care. We just published an analysis of Medicare data and access to EGFR testing. The result of that analysis showed that testing in the VA was consistent with testing in Medicare.
For palliative care, I think the VA does a better job. And it’s just so discouraging as VA employees and as clinicians treating veterans to see publicationsthat suggest that veterans are getting a lower quality of care and that they would be better if care was privatized or outsourced. It’s just fundamentally not the case.
In CML, we see it. We’ve analyzed the data, in that there’s a far lower number of patients with CML who are included in the registry because patients who are diagnosed outside the VA are incorporated in other cancer registries.8 But as soon as their copays increase for access to targeted drugs, they immediately activate their VA benefits so that theycan get their drugs at the VA. For hematologic malignancies that are diagnosed outside the VA and are captured in other cancer registries, as soon as the drugs become expensive, they start getting their care in the VA. I don’t think there’s beena lot of empirical research that’s shown this, but we have the data to illustrate this trend. I hope thatthere are more publications that show that veterans with cancer are getting really good care inside the VA in the existing VA health care system.
Ms. Nason. It is disheartening to see negativepublicity, knowing that I work with colleagues who are strongly committed to providing up-to-date and relevant oncology care.
Dr. Lynch. As we record this conversation, I am in Rotterdam, Netherlands, in a meeting about genomewide testing. In hematologic malignancies, prostate cancer, and breast cancer, it’s a huge issue. And that is the other area that MVP (Million Veteran Program) is leading the way with the MVP biorepository data. Frankly, there’s no other biorepository that has this many patients, that has so many African Americans, and that has such rich EHR data. So inthat other area, the VA is doing really well.
1. Reck M, Rodríguez-Abreu D, Robinson AG, et al; KEYNOTE-024 Investigators. Pembrolizumab vs chemotherapy for PD-L1-positive non-small cell lung cancer. N Engl J Med. 2016;375(19):1823-1833.
2. Antonia SJ, Villegas A, Daniel D, et al; PACIFIC Investigators. Durvalumab after chemoradiotherapy in stage III non–smallcell lung cancer. N Engl J Med. 2017;377(20):1919-1929.
3. Hellmann MD, Ciuleanu T-E, Pluzansk A, et al. Nivolumab plus ipilimumab in Lung Cancer with a high tumor mutational burden. N Engl J Med. 2018 April 16. [Epub ahead of print.]
4. Motzer RJ, Tannir NM, McDermott DF, et al; CheckMate214 Investigators. Nivolumab plus ipilimumab versus sunitinibin advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290.
5. Derosa L, Hellmann MD, Spaziano M, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small cell
lung cancer. Ann Oncol. 2018 March 30. [Epub ahead of print.]
6. Heinrichs A, Dessars B, El Housni H, et al. Identification of chronic myeloid leukemia patients treated with imatinib who are potentially eligible for treatment discontinuation by assessingreal-life molecular responses on the international scale in a EUTOS-certified lab. Leuk Res. 2018;67:27-31.
7. Keefe S, Kambhampati S, Powers B. An electronic chemotherapy ordering process and template. Fed Pract. 2015;32(suppl 1):21S-25S.
8. Lynch JA, Berse B, Rabb M, et al. Underutilization and disparities in access to EGFR testing among Medicare patients with lung cancer from 2010 - 2013. BMC Cancer. 2018;18(1):306.
1. Reck M, Rodríguez-Abreu D, Robinson AG, et al; KEYNOTE-024 Investigators. Pembrolizumab vs chemotherapy for PD-L1-positive non-small cell lung cancer. N Engl J Med. 2016;375(19):1823-1833.
2. Antonia SJ, Villegas A, Daniel D, et al; PACIFIC Investigators. Durvalumab after chemoradiotherapy in stage III non–smallcell lung cancer. N Engl J Med. 2017;377(20):1919-1929.
3. Hellmann MD, Ciuleanu T-E, Pluzansk A, et al. Nivolumab plus ipilimumab in Lung Cancer with a high tumor mutational burden. N Engl J Med. 2018 April 16. [Epub ahead of print.]
4. Motzer RJ, Tannir NM, McDermott DF, et al; CheckMate214 Investigators. Nivolumab plus ipilimumab versus sunitinibin advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290.
5. Derosa L, Hellmann MD, Spaziano M, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small cell
lung cancer. Ann Oncol. 2018 March 30. [Epub ahead of print.]
6. Heinrichs A, Dessars B, El Housni H, et al. Identification of chronic myeloid leukemia patients treated with imatinib who are potentially eligible for treatment discontinuation by assessingreal-life molecular responses on the international scale in a EUTOS-certified lab. Leuk Res. 2018;67:27-31.
7. Keefe S, Kambhampati S, Powers B. An electronic chemotherapy ordering process and template. Fed Pract. 2015;32(suppl 1):21S-25S.
8. Lynch JA, Berse B, Rabb M, et al. Underutilization and disparities in access to EGFR testing among Medicare patients with lung cancer from 2010 - 2013. BMC Cancer. 2018;18(1):306.