User login
Transition From Lichen Sclerosus to Squamous Cell Carcinoma in a Single Tissue Section
To the Editor:
Lichen sclerosus (LS) is a chronic inflammatory disorder of unknown etiology that most commonly affects the anogenital region. Progressive sclerosis results in scarring with distortion of the normal epithelial architecture.1,2 The lifetime risk for developing squamous cell carcinoma (SCC) as a complication of long-standing LS has been estimated as 4% to 6%.3,4 However, there is no general agreement concerning the exact relationship between anogenital LS and SCC.1 The coexistence of histologic findings of LS, vulvar intraepithelial neoplasia (VIN), and SCC in the same tissue is rare. We report a case of VIN and SCC developing in a region of preexisting LS.
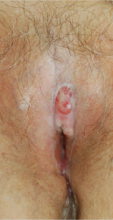
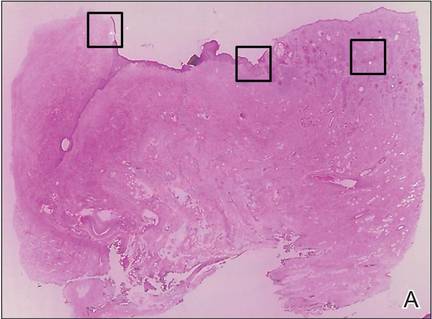
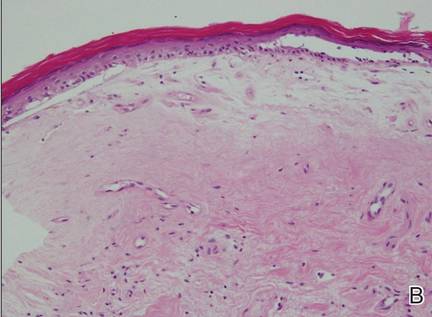
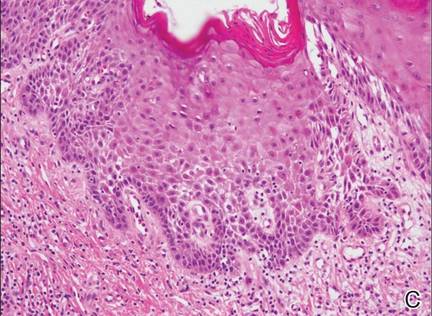
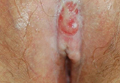
A 76-year-old woman presented with a 7-mm nodule on the clitoris that was surrounded by a pearly white, smooth, glistening area (Figure 1). The patient reported pain and tenderness associated with the nodule. No regional lymphadenopathy was evident. We performed an excisional biopsy of the entire nodule and a small part of the whitish patch (Figure 2A). On histologic examination, the presence of hyperkeratosis, epidermal atrophy, a swollen dermal collagen bundle, and prominent edema was consistent with LS (Figure 2B). The presence of dysplastic changes with mild disturbance of the epithelial architecture as well as acanthosis and dyskeratosis in the same tissue confirmed VIN (Figure 2C). Dermal invasion and transition to SCC were seen in the part of the tissue verified as VIN. The presence of dermal tumor nests and an irregular border between the epidermis and dermis pointed to the existence of fully developed SCC (Figure 2D). To prevent the recurrence of SCC, the patient returned for follow-up periodically. There was no recurrence within 6 months after excision.
|
|
|
|
| Figure 2. An excisional biopsy showed epidermal thinning on the left side and invasion of the dermis by a tumor nest on the right side (A)(H&E, original magnification ×10). Left, center, and right boxes indicate areas shown in Figures 2B, 2C and 2D, respectively. Hyperkeratosis, epidermal atrophy, a swollen dermal collagen bundle, and prominent edema was evident (B)(H&E, original magnification ×200). Dysplactic changes with mild disturbance of the epithelial architecture accompanied by acanthosis and nuclear atypia were seen (C)(H&E, original magnification ×200). Irregular masses of atypical squamous cells spread downward into the dermis representing squamous cell carcinoma of a well-differentiated type (D)(H&E, original magnification ×200). | |
Although LS is considered a premalignant condition, only a small portion of patients with LS ultimately develop vulvar SCC.5 There are a number of reasons for linking LS with the development of vulvar SCC. First, in the majority of cases of vulvar SCC, LS, squamous cell hyperplasia, or VIN is present in the adjacent epithelium. Lichen sclerosus is found in adjacent regions in up to 62% of vulvar SCC cases.6 Second, patients with LS may develop vulvar SCC, as frequently reported. Third, in a series of LS patients who underwent long-term follow-up, 4% to 6% were reported to have developed vulvar SCC.3,4,7
Lichen sclerosus is an inflammatory dermatosis characterized by clinicopathologic persistence and hypocellular fibrosis.2 Changes in the local environment of the keratinocyte, including chronic inflammation and sclerosis, may be responsible for the promotion of carcinogenesis.8 However, no molecular markers have been proven to identify the LS lesions that are at risk for developing into vulvar SCC.9,10 It has been suggested that VIN is the direct precursor of vulvar SCC.11,12
Histologic diagnosis of VIN is difficult. Its identification is hindered by a high degree of cellular differentiation combined with the absence of widespread architectural disorder, nuclear pleomorphism, and diffuse nuclear atypia.13 The atypia in VIN lesions is strictly confined to the basal and parabasal layers of the epithelium.11 Vulvar intraepithelial neoplasia has seldom been diagnosed as a solitary lesion because it appears to have a short intraepithelial lifetime.
Vulvar SCC can be divided into 2 patterns. The first is found in older women, which is unrelated to human papillomavirus (HPV). This type occurs in a background of LS and/or differentiated VIN. The second is predominantly found in younger women, which is related to high-risk HPV. This type of vulvar SCC frequently is associated with the histologic subtypes of warty and basaloid differentiations and is referred to as undifferentiated VIN. There is no association with LS in these cases.2,14,15
It has been suggested that LS and HPV may not be mutually exclusive but may act as cofactors in SCC pathogenesis.16 Infection with HPV is an early event in the multistep process of vulvar carcinogenesis, and HPV integration into host cell genome seems to be related to the progression of vulvar dysplasia.17 Viral integration generally disrupts the E2 region, resulting in enhanced expression of E6 and E7. E6 and E7 have the ability to bind and inactivate the protein p53 and retinoblastoma protein, which promotes rapid progression through the cell cycle without p53-mediated control of DNA integrity.18 However, the exact influence of HPV in vulvar SCC is uncertain, as divergent prevalence rates have been published.
In our case, histologic examination revealed the characteristic findings of LS, VIN, and SCC in succession. This sequence is evidence of progressive transition from LS to VIN and then to SCC. Consequently, this case suggests that vulvar LS may act as both an initiator and a promoter of carcinogenesis and that VIN may be the direct precursor of vulvar SCC. In conclusion, LS has a considerable risk for malignant transformation and requires continuous follow-up in all patients. Early histological detection of invasive lesions is crucial to reduce the risk for vulvar cancer.
1. Bhattacharjee P, Fatteh SM, Lloyd KL. Squamous cell carcinoma arising in long-standing lichen sclerosus et atrophicus. J Am Geriatr Soc. 2004;52:319-320.
2. Funaro D. Lichen sclerosus: a review and practical approach. Dermatol Ther. 2004;17:28-37.
3. Ulrich RH. Lichen sclerosus. In: Wolff K, Goldsmith L, Katz S, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw Hill; 2007:546-550.
4. Heymann WR. Lichen sclerosus. J Am Acad Dermatol. 2007;56:683-684.
5. Cooper SM, Gao XH, Powell JJ, et al. Does treatment of vulvar lichen sclerosus influence its prognosis? Arch Dermatol. 2004;140:702-706.
6. Kagie MJ, Kenter GG, Hermans J, et al. The relevance of various vulvar epithelial changes in the early detection of squamous cell carcinoma of the vulva. Int J Gynecol Cancer. 1997;7:50-57.
7. Thomas RH, Ridley CM, McGibbon DH, et al. Anogenital lichen sclerosus in women. J R Soc Med. 1996;89:694-698.
8. Walkden V, Chia Y, Wojnarowska F. The association of squamous cell carcinoma of the vulva and lichen sclerosus: implications for follow-up. J Obstet Gynaecol. 1997;17:551-553.
9. Tasker GL, Wojnarowska F. Lichen sclerosus. Clin Exp Dermatol. 2003;28:128-133.
10. Wang SH, Chi CC, Wong YW, et al. Genital verrucous carcinoma is associated with lichen sclerosus: a retrospective study and review of the literature. J Eur Acad Dermatol Venereol. 2010;24:815-819.
11. Hart WR. Vulvar intraepithelial neoplasia: historical aspects and current status. Int J Gynecol Pathol. 2001;20:16-30.
12. van de Nieuwenhof HP, Massuger LF, van der Avoort IA, et al. Vulvar squamous cell carcinoma development after diagnosis of VIN increases with age. Eur J Cancer. 2009;45:851-856.
13. Taube JM, Badger J, Kong CS, et al. Differentiated (simplex) vulvar intraepithelial neoplasia: a case report and review of the literature. Am J Dermatopathol. 2011;33:27-30.
14. Derrick EK, Ridley CM, Kobza-Black A, et al. A clinical study of 23 cases of female anogenital carcinoma. Br J Dermatol. 2000;143:1217-1223.
15. Crum C, McLachlin CM, Tate JE, et al. Pathobiology of vulvar squamous neoplasia. Gynecol Oncol Pathol. 1997;9:63-69.
16. Ansink AC, Krul MRL, De Weger RA, et al. Human papillomavirus, lichen sclerosus, and squamous cell carcinoma of the vulva: detection and prognostic significance. Gynecol Oncol. 1994;52:180-184.
17. Hillemanns P, Wang X. Integration of HPV-16 and HPV-18 DNA in vulvar intraepithelial neoplasia. Gynecol Oncol. 2006;100:276-282.
18. Stoler MH. Human papillomaviruses and cervical neoplasia: a model for carcinogenesis. Int J Gynecol Pathol. 2000;19:16-28.
To the Editor:
Lichen sclerosus (LS) is a chronic inflammatory disorder of unknown etiology that most commonly affects the anogenital region. Progressive sclerosis results in scarring with distortion of the normal epithelial architecture.1,2 The lifetime risk for developing squamous cell carcinoma (SCC) as a complication of long-standing LS has been estimated as 4% to 6%.3,4 However, there is no general agreement concerning the exact relationship between anogenital LS and SCC.1 The coexistence of histologic findings of LS, vulvar intraepithelial neoplasia (VIN), and SCC in the same tissue is rare. We report a case of VIN and SCC developing in a region of preexisting LS.
A 76-year-old woman presented with a 7-mm nodule on the clitoris that was surrounded by a pearly white, smooth, glistening area (Figure 1). The patient reported pain and tenderness associated with the nodule. No regional lymphadenopathy was evident. We performed an excisional biopsy of the entire nodule and a small part of the whitish patch (Figure 2A). On histologic examination, the presence of hyperkeratosis, epidermal atrophy, a swollen dermal collagen bundle, and prominent edema was consistent with LS (Figure 2B). The presence of dysplastic changes with mild disturbance of the epithelial architecture as well as acanthosis and dyskeratosis in the same tissue confirmed VIN (Figure 2C). Dermal invasion and transition to SCC were seen in the part of the tissue verified as VIN. The presence of dermal tumor nests and an irregular border between the epidermis and dermis pointed to the existence of fully developed SCC (Figure 2D). To prevent the recurrence of SCC, the patient returned for follow-up periodically. There was no recurrence within 6 months after excision.
|
|
|
|
| Figure 2. An excisional biopsy showed epidermal thinning on the left side and invasion of the dermis by a tumor nest on the right side (A)(H&E, original magnification ×10). Left, center, and right boxes indicate areas shown in Figures 2B, 2C and 2D, respectively. Hyperkeratosis, epidermal atrophy, a swollen dermal collagen bundle, and prominent edema was evident (B)(H&E, original magnification ×200). Dysplactic changes with mild disturbance of the epithelial architecture accompanied by acanthosis and nuclear atypia were seen (C)(H&E, original magnification ×200). Irregular masses of atypical squamous cells spread downward into the dermis representing squamous cell carcinoma of a well-differentiated type (D)(H&E, original magnification ×200). | |
Although LS is considered a premalignant condition, only a small portion of patients with LS ultimately develop vulvar SCC.5 There are a number of reasons for linking LS with the development of vulvar SCC. First, in the majority of cases of vulvar SCC, LS, squamous cell hyperplasia, or VIN is present in the adjacent epithelium. Lichen sclerosus is found in adjacent regions in up to 62% of vulvar SCC cases.6 Second, patients with LS may develop vulvar SCC, as frequently reported. Third, in a series of LS patients who underwent long-term follow-up, 4% to 6% were reported to have developed vulvar SCC.3,4,7
Lichen sclerosus is an inflammatory dermatosis characterized by clinicopathologic persistence and hypocellular fibrosis.2 Changes in the local environment of the keratinocyte, including chronic inflammation and sclerosis, may be responsible for the promotion of carcinogenesis.8 However, no molecular markers have been proven to identify the LS lesions that are at risk for developing into vulvar SCC.9,10 It has been suggested that VIN is the direct precursor of vulvar SCC.11,12
Histologic diagnosis of VIN is difficult. Its identification is hindered by a high degree of cellular differentiation combined with the absence of widespread architectural disorder, nuclear pleomorphism, and diffuse nuclear atypia.13 The atypia in VIN lesions is strictly confined to the basal and parabasal layers of the epithelium.11 Vulvar intraepithelial neoplasia has seldom been diagnosed as a solitary lesion because it appears to have a short intraepithelial lifetime.
Vulvar SCC can be divided into 2 patterns. The first is found in older women, which is unrelated to human papillomavirus (HPV). This type occurs in a background of LS and/or differentiated VIN. The second is predominantly found in younger women, which is related to high-risk HPV. This type of vulvar SCC frequently is associated with the histologic subtypes of warty and basaloid differentiations and is referred to as undifferentiated VIN. There is no association with LS in these cases.2,14,15
It has been suggested that LS and HPV may not be mutually exclusive but may act as cofactors in SCC pathogenesis.16 Infection with HPV is an early event in the multistep process of vulvar carcinogenesis, and HPV integration into host cell genome seems to be related to the progression of vulvar dysplasia.17 Viral integration generally disrupts the E2 region, resulting in enhanced expression of E6 and E7. E6 and E7 have the ability to bind and inactivate the protein p53 and retinoblastoma protein, which promotes rapid progression through the cell cycle without p53-mediated control of DNA integrity.18 However, the exact influence of HPV in vulvar SCC is uncertain, as divergent prevalence rates have been published.
In our case, histologic examination revealed the characteristic findings of LS, VIN, and SCC in succession. This sequence is evidence of progressive transition from LS to VIN and then to SCC. Consequently, this case suggests that vulvar LS may act as both an initiator and a promoter of carcinogenesis and that VIN may be the direct precursor of vulvar SCC. In conclusion, LS has a considerable risk for malignant transformation and requires continuous follow-up in all patients. Early histological detection of invasive lesions is crucial to reduce the risk for vulvar cancer.
To the Editor:
Lichen sclerosus (LS) is a chronic inflammatory disorder of unknown etiology that most commonly affects the anogenital region. Progressive sclerosis results in scarring with distortion of the normal epithelial architecture.1,2 The lifetime risk for developing squamous cell carcinoma (SCC) as a complication of long-standing LS has been estimated as 4% to 6%.3,4 However, there is no general agreement concerning the exact relationship between anogenital LS and SCC.1 The coexistence of histologic findings of LS, vulvar intraepithelial neoplasia (VIN), and SCC in the same tissue is rare. We report a case of VIN and SCC developing in a region of preexisting LS.
A 76-year-old woman presented with a 7-mm nodule on the clitoris that was surrounded by a pearly white, smooth, glistening area (Figure 1). The patient reported pain and tenderness associated with the nodule. No regional lymphadenopathy was evident. We performed an excisional biopsy of the entire nodule and a small part of the whitish patch (Figure 2A). On histologic examination, the presence of hyperkeratosis, epidermal atrophy, a swollen dermal collagen bundle, and prominent edema was consistent with LS (Figure 2B). The presence of dysplastic changes with mild disturbance of the epithelial architecture as well as acanthosis and dyskeratosis in the same tissue confirmed VIN (Figure 2C). Dermal invasion and transition to SCC were seen in the part of the tissue verified as VIN. The presence of dermal tumor nests and an irregular border between the epidermis and dermis pointed to the existence of fully developed SCC (Figure 2D). To prevent the recurrence of SCC, the patient returned for follow-up periodically. There was no recurrence within 6 months after excision.
|
|
|
|
| Figure 2. An excisional biopsy showed epidermal thinning on the left side and invasion of the dermis by a tumor nest on the right side (A)(H&E, original magnification ×10). Left, center, and right boxes indicate areas shown in Figures 2B, 2C and 2D, respectively. Hyperkeratosis, epidermal atrophy, a swollen dermal collagen bundle, and prominent edema was evident (B)(H&E, original magnification ×200). Dysplactic changes with mild disturbance of the epithelial architecture accompanied by acanthosis and nuclear atypia were seen (C)(H&E, original magnification ×200). Irregular masses of atypical squamous cells spread downward into the dermis representing squamous cell carcinoma of a well-differentiated type (D)(H&E, original magnification ×200). | |
Although LS is considered a premalignant condition, only a small portion of patients with LS ultimately develop vulvar SCC.5 There are a number of reasons for linking LS with the development of vulvar SCC. First, in the majority of cases of vulvar SCC, LS, squamous cell hyperplasia, or VIN is present in the adjacent epithelium. Lichen sclerosus is found in adjacent regions in up to 62% of vulvar SCC cases.6 Second, patients with LS may develop vulvar SCC, as frequently reported. Third, in a series of LS patients who underwent long-term follow-up, 4% to 6% were reported to have developed vulvar SCC.3,4,7
Lichen sclerosus is an inflammatory dermatosis characterized by clinicopathologic persistence and hypocellular fibrosis.2 Changes in the local environment of the keratinocyte, including chronic inflammation and sclerosis, may be responsible for the promotion of carcinogenesis.8 However, no molecular markers have been proven to identify the LS lesions that are at risk for developing into vulvar SCC.9,10 It has been suggested that VIN is the direct precursor of vulvar SCC.11,12
Histologic diagnosis of VIN is difficult. Its identification is hindered by a high degree of cellular differentiation combined with the absence of widespread architectural disorder, nuclear pleomorphism, and diffuse nuclear atypia.13 The atypia in VIN lesions is strictly confined to the basal and parabasal layers of the epithelium.11 Vulvar intraepithelial neoplasia has seldom been diagnosed as a solitary lesion because it appears to have a short intraepithelial lifetime.
Vulvar SCC can be divided into 2 patterns. The first is found in older women, which is unrelated to human papillomavirus (HPV). This type occurs in a background of LS and/or differentiated VIN. The second is predominantly found in younger women, which is related to high-risk HPV. This type of vulvar SCC frequently is associated with the histologic subtypes of warty and basaloid differentiations and is referred to as undifferentiated VIN. There is no association with LS in these cases.2,14,15
It has been suggested that LS and HPV may not be mutually exclusive but may act as cofactors in SCC pathogenesis.16 Infection with HPV is an early event in the multistep process of vulvar carcinogenesis, and HPV integration into host cell genome seems to be related to the progression of vulvar dysplasia.17 Viral integration generally disrupts the E2 region, resulting in enhanced expression of E6 and E7. E6 and E7 have the ability to bind and inactivate the protein p53 and retinoblastoma protein, which promotes rapid progression through the cell cycle without p53-mediated control of DNA integrity.18 However, the exact influence of HPV in vulvar SCC is uncertain, as divergent prevalence rates have been published.
In our case, histologic examination revealed the characteristic findings of LS, VIN, and SCC in succession. This sequence is evidence of progressive transition from LS to VIN and then to SCC. Consequently, this case suggests that vulvar LS may act as both an initiator and a promoter of carcinogenesis and that VIN may be the direct precursor of vulvar SCC. In conclusion, LS has a considerable risk for malignant transformation and requires continuous follow-up in all patients. Early histological detection of invasive lesions is crucial to reduce the risk for vulvar cancer.
1. Bhattacharjee P, Fatteh SM, Lloyd KL. Squamous cell carcinoma arising in long-standing lichen sclerosus et atrophicus. J Am Geriatr Soc. 2004;52:319-320.
2. Funaro D. Lichen sclerosus: a review and practical approach. Dermatol Ther. 2004;17:28-37.
3. Ulrich RH. Lichen sclerosus. In: Wolff K, Goldsmith L, Katz S, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw Hill; 2007:546-550.
4. Heymann WR. Lichen sclerosus. J Am Acad Dermatol. 2007;56:683-684.
5. Cooper SM, Gao XH, Powell JJ, et al. Does treatment of vulvar lichen sclerosus influence its prognosis? Arch Dermatol. 2004;140:702-706.
6. Kagie MJ, Kenter GG, Hermans J, et al. The relevance of various vulvar epithelial changes in the early detection of squamous cell carcinoma of the vulva. Int J Gynecol Cancer. 1997;7:50-57.
7. Thomas RH, Ridley CM, McGibbon DH, et al. Anogenital lichen sclerosus in women. J R Soc Med. 1996;89:694-698.
8. Walkden V, Chia Y, Wojnarowska F. The association of squamous cell carcinoma of the vulva and lichen sclerosus: implications for follow-up. J Obstet Gynaecol. 1997;17:551-553.
9. Tasker GL, Wojnarowska F. Lichen sclerosus. Clin Exp Dermatol. 2003;28:128-133.
10. Wang SH, Chi CC, Wong YW, et al. Genital verrucous carcinoma is associated with lichen sclerosus: a retrospective study and review of the literature. J Eur Acad Dermatol Venereol. 2010;24:815-819.
11. Hart WR. Vulvar intraepithelial neoplasia: historical aspects and current status. Int J Gynecol Pathol. 2001;20:16-30.
12. van de Nieuwenhof HP, Massuger LF, van der Avoort IA, et al. Vulvar squamous cell carcinoma development after diagnosis of VIN increases with age. Eur J Cancer. 2009;45:851-856.
13. Taube JM, Badger J, Kong CS, et al. Differentiated (simplex) vulvar intraepithelial neoplasia: a case report and review of the literature. Am J Dermatopathol. 2011;33:27-30.
14. Derrick EK, Ridley CM, Kobza-Black A, et al. A clinical study of 23 cases of female anogenital carcinoma. Br J Dermatol. 2000;143:1217-1223.
15. Crum C, McLachlin CM, Tate JE, et al. Pathobiology of vulvar squamous neoplasia. Gynecol Oncol Pathol. 1997;9:63-69.
16. Ansink AC, Krul MRL, De Weger RA, et al. Human papillomavirus, lichen sclerosus, and squamous cell carcinoma of the vulva: detection and prognostic significance. Gynecol Oncol. 1994;52:180-184.
17. Hillemanns P, Wang X. Integration of HPV-16 and HPV-18 DNA in vulvar intraepithelial neoplasia. Gynecol Oncol. 2006;100:276-282.
18. Stoler MH. Human papillomaviruses and cervical neoplasia: a model for carcinogenesis. Int J Gynecol Pathol. 2000;19:16-28.
1. Bhattacharjee P, Fatteh SM, Lloyd KL. Squamous cell carcinoma arising in long-standing lichen sclerosus et atrophicus. J Am Geriatr Soc. 2004;52:319-320.
2. Funaro D. Lichen sclerosus: a review and practical approach. Dermatol Ther. 2004;17:28-37.
3. Ulrich RH. Lichen sclerosus. In: Wolff K, Goldsmith L, Katz S, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw Hill; 2007:546-550.
4. Heymann WR. Lichen sclerosus. J Am Acad Dermatol. 2007;56:683-684.
5. Cooper SM, Gao XH, Powell JJ, et al. Does treatment of vulvar lichen sclerosus influence its prognosis? Arch Dermatol. 2004;140:702-706.
6. Kagie MJ, Kenter GG, Hermans J, et al. The relevance of various vulvar epithelial changes in the early detection of squamous cell carcinoma of the vulva. Int J Gynecol Cancer. 1997;7:50-57.
7. Thomas RH, Ridley CM, McGibbon DH, et al. Anogenital lichen sclerosus in women. J R Soc Med. 1996;89:694-698.
8. Walkden V, Chia Y, Wojnarowska F. The association of squamous cell carcinoma of the vulva and lichen sclerosus: implications for follow-up. J Obstet Gynaecol. 1997;17:551-553.
9. Tasker GL, Wojnarowska F. Lichen sclerosus. Clin Exp Dermatol. 2003;28:128-133.
10. Wang SH, Chi CC, Wong YW, et al. Genital verrucous carcinoma is associated with lichen sclerosus: a retrospective study and review of the literature. J Eur Acad Dermatol Venereol. 2010;24:815-819.
11. Hart WR. Vulvar intraepithelial neoplasia: historical aspects and current status. Int J Gynecol Pathol. 2001;20:16-30.
12. van de Nieuwenhof HP, Massuger LF, van der Avoort IA, et al. Vulvar squamous cell carcinoma development after diagnosis of VIN increases with age. Eur J Cancer. 2009;45:851-856.
13. Taube JM, Badger J, Kong CS, et al. Differentiated (simplex) vulvar intraepithelial neoplasia: a case report and review of the literature. Am J Dermatopathol. 2011;33:27-30.
14. Derrick EK, Ridley CM, Kobza-Black A, et al. A clinical study of 23 cases of female anogenital carcinoma. Br J Dermatol. 2000;143:1217-1223.
15. Crum C, McLachlin CM, Tate JE, et al. Pathobiology of vulvar squamous neoplasia. Gynecol Oncol Pathol. 1997;9:63-69.
16. Ansink AC, Krul MRL, De Weger RA, et al. Human papillomavirus, lichen sclerosus, and squamous cell carcinoma of the vulva: detection and prognostic significance. Gynecol Oncol. 1994;52:180-184.
17. Hillemanns P, Wang X. Integration of HPV-16 and HPV-18 DNA in vulvar intraepithelial neoplasia. Gynecol Oncol. 2006;100:276-282.
18. Stoler MH. Human papillomaviruses and cervical neoplasia: a model for carcinogenesis. Int J Gynecol Pathol. 2000;19:16-28.
Practice Points
- Lichen sclerosus has a considerable risk for malignant transformation and requires continuous follow-up in all patients.
- Early histological detection of invasive lesions is crucial to reduce the risk for vulvar cancer.