User login
Extensive scarring alopecia and widespread rash
A 23-year-old woman with systemic lupus erythematosus (SLE) and a history of poor adherence to recommended treatment presented with a widespread pruritic rash and diffuse hair loss. The rash had rapidly progressed following sun exposure during the summer. The patient cited her mental health status (anxiety, depression), socioeconomic factors, and challenges with prescription insurance coverage as reasons for nonadherence to treatment.
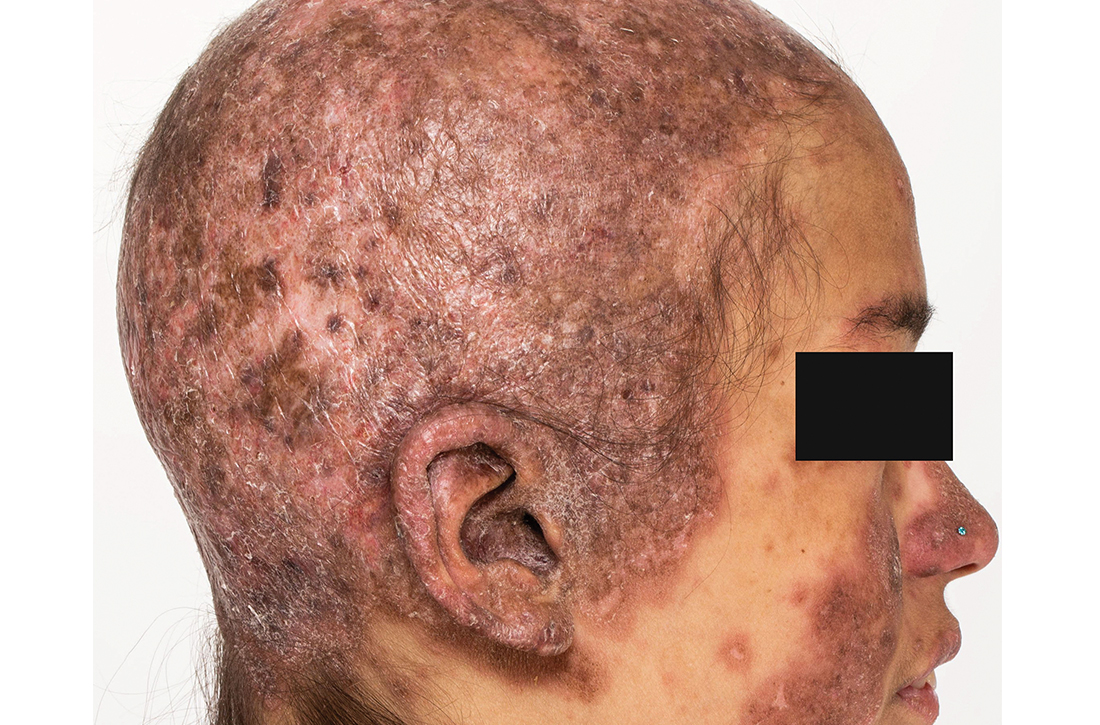
Clinical examination revealed diffuse scarring alopecia and abnormal pigmentation of the scalp (FIGURE 1A), as well as large, red-brown, scaly, atrophic plaques on the face, ears, extremities, back, and buttocks (FIGURES 1B and 1C).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Generalized chronic cutaneouslupus erythematosus
The clinical features of our patient were most consistent with generalized chronic cutaneous lupus erythematosus (CCLE), which is 1 of 3 subtypes of cutaneous lupus erythematosus (CLE). The other 2 are acute and subacute cutaneous lupus erythematosus (ACLE and SCLE, respectively). CCLE is further divided into 3 distinct entities: discoid lupus erythematosus (DLE), chilblain lupus erythematosus, and lupus erythematosus panniculitis.
Distinguishing between the different forms of cutaneous lupus can be challenging; diagnosis is based on differences in clinical features and duration of skin changes, as well as biopsy and lab results.1 The clinical features of our patient were most consistent with DLE, based on the scarring alopecia with scaly atrophic plaques, dyspigmentation, and exacerbation following sun exposure.
DLE is the most common form of CCLE and frequently manifests in a localized, photosensitive distribution involving the scalp, ears, and/or face.2 Less commonly, it can demonstrate a more generalized distribution involving the trunk and/or extremities (reported incidence of 1.04 per 100,000 people).3 Longstanding DLE lesions commonly exhibit scarring and dyspigmentation. DLE occurs in approximately 15% to 30% of SLE patients,4 whereas about 10% of patients with DLE will progress to SLE.3
Positive antinuclear antibodies (ANA) are found in 54% of patients with CCLE, compared to 74% and 81% of patients with SCLE and ACLE, respectively.5 Thus, a negative ANA should not rule out the possibility of CLE.
Comprehensive lab work and biopsy could expose a systemic origin
While our patient already had a diagnosis of SLE, many patients will present with no prior history of autoimmune connective tissue disease, and, in that case, the objective should be to confirm the diagnosis and evaluate for systemic involvement. This includes a thorough review of systems; skin biopsy; complete blood count; liver function tests; urinalysis; and measurement of creatinine, inflammatory markers, ANA, extractable nuclear antigens, double-stranded DNA, complement levels (C3, C4, total), and antiphospholipid antibodies.6
Continue to: Biopsy
Biopsy features of DLE include vacuolar interface dermatitis, basement membrane zone thickening, follicular plugging, superficial and deep perivascular and periadnexal lymphohistiocytic inflammation with plasma cells, and increased mucin deposition. Direct immunofluorescence biopsy may show a continuous granular immunoglobulin (Ig) G/IgA/IgM and C3 band at the basement membrane zone.
Abnormal serologic tests may support the diagnosis of SLE based on American College of Rheumatology criteria and could suggest additional organ involvement or associated conditions, such as lupus nephritis or antiphospholipid syndrome (respectively). Currently, no clear consensus exists on monitoring patients with cutaneous lupus for systemic disease.
A gamut of skin-changing conditions should be considered
The differential diagnosis in this case includes SCLE, dermatitis, tinea corporis, cutaneous drug eruptions, and graft-versus-host disease (GVHD).
SCLE classically manifests with annular or psoriasiform lesions on the sun-exposed areas of the upper trunk (eg, the chest, neck, and upper extremities), while the central face and scalp are typically spared. Differentiating between generalized DLE and SCLE may be the most difficult, given similarities in the associated skin changes.
Dermatitis (atopic or contact) manifests as pruritic erythematous eczematous plaques, most commonly involving the flexural areas in atopic dermatitis and an exposure-dependent distribution pattern in contact dermatitis. The patient may have a history of atopy.
Continue to: Tinea corporis
Tinea corporis will manifest with annular scaly patches or plaques and may demonstrate erythematous papules around hair follicles in Majocchi granuloma. A positive potassium hydroxide exam demonstrating fungal hyphae confirms the diagnosis.
Cutaneous drug eruptions can have various morphologies and timing of onset. Certain photosensitive drug reactions can be triggered or exacerbated with sun exposure. Therefore, it is necessary to obtain a thorough medication history, including any new medications that were started within the past 4 to 6 weeks, although onset can be delayed beyond this timeframe.
GVHD is a complication that more commonly follows allogeneic hematopoietic stem cell transplants, although it may be seen following solid-organ transplantation or transfusion of nonirradiated blood. Chronic GVHD has an onset ≥ 100 days after transplant and is divided into nonsclerotic (lichenoid, atopic dermatitis-like, psoriasiform, poikilodermatous) and sclerotic morphologies.
Successful Tx requires adherence but may not prevent flare-ups
First-line treatment options for severe and widespread skin manifestations of CLE include photoprotection, smoking cessation, topical corticosteroids, hydroxychloroquine, and systemic corticosteroids. Second-line treatments include chloroquine, methotrexate, or mycophenolate mofetil; thalidomide or lenalidomide may be considered for patients with refractory disease.7,8
With successful treatment and photoprotection, patients may achieve significant skin clearing. Occasional flares, especially during warmer months, may occur if they are not diligent about photoprotection. Systemic treatments will also improve the patient’s systemic symptoms if the patient has concomitant SLE.
Our patient was advised to use topical steroids and to restart hydroxychloroquine 300 mg/d and mycophenolate mofetil 1000 mg/d (a regimen with which she had previously been nonadherent). The patient followed up with her family physician for assessment of her other medical issues. No new interventions for her mental health were initiated during this visit, as the severity of her depression was considered mild. She was referred to a case manager to navigate multiple medical appointments and prescription insurance coverage issues. The patient’s dose of mycophenolate mofetil was increased gradually to 3 g/d, and the patient experienced improvement in both her cutaneous lesions and systemic symptoms.
1. Petty AJ, Floyd L, Henderson C, et al. Cutaneous lupus erythematosus: progress and challenges. Curr Allergy Asthma Rep. 2020;20:12. doi: 10.1007/s11882-020-00906-8
2. Kuhn A, Landmann A. The classification and diagnosis of cutaneous lupus erythematosus. J Autoimmun. 2014;48-49:14-19. doi: 10.1016/j.jaut.2014.01.021
3. Durosaro O, Davis MDP, Reed KB, et al. Incidence of cutaneous lupus erythematosus, 1965-2005: a population-based study. Arch Dermatol. 2009;145:249-253. doi: 10.1001/archdermatol.2009.21
4. Merola JF. Overview of cutaneous lupus erythematosus. UpToDate. Updated September 19, 2021. Accessed February 17, 2022. www.uptodate.com/contents/overview-of-cutaneous-lupus-erythematosus
5. Biazar C, Sigges J, Patsinakidis N, et al. Cutaneous lupus erythematosus: first multicenter database analysis of 1002 patients from the European Society of Cutaneous Lupus Erythematosus (EUSCLE). Autoimmun Rev. 2013;12:444-454. doi: 10.1016/j.autrev.2012.08.019
6. O’Brien JC, Chong BF. not just skin deep: systemic disease involvement in patients with cutaneous lupus. J Investig Dermatol Symp Proc. 2017;18:S69-S74. doi: 10.1016/j.jisp.2016.09.001
7. Kuhn A, Ruland V, Bonsmann G. Cutaneous lupus erythematosus: update of therapeutic options part I. J Am Acad Dermatol. 2011;65:e179-e193. doi: 10.1016/j.jaad.2010.06.018
8. Kindle SA, Wetter DA, Davis MDP, et al. Lenalidomide treatment of cutaneous lupus erythematosus: the Mayo Clinic experience. Int J Dermatol. 2016;55:e431-e439. doi: 10.1111/ijd.13226
A 23-year-old woman with systemic lupus erythematosus (SLE) and a history of poor adherence to recommended treatment presented with a widespread pruritic rash and diffuse hair loss. The rash had rapidly progressed following sun exposure during the summer. The patient cited her mental health status (anxiety, depression), socioeconomic factors, and challenges with prescription insurance coverage as reasons for nonadherence to treatment.
Clinical examination revealed diffuse scarring alopecia and abnormal pigmentation of the scalp (FIGURE 1A), as well as large, red-brown, scaly, atrophic plaques on the face, ears, extremities, back, and buttocks (FIGURES 1B and 1C).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Generalized chronic cutaneouslupus erythematosus
The clinical features of our patient were most consistent with generalized chronic cutaneous lupus erythematosus (CCLE), which is 1 of 3 subtypes of cutaneous lupus erythematosus (CLE). The other 2 are acute and subacute cutaneous lupus erythematosus (ACLE and SCLE, respectively). CCLE is further divided into 3 distinct entities: discoid lupus erythematosus (DLE), chilblain lupus erythematosus, and lupus erythematosus panniculitis.
Distinguishing between the different forms of cutaneous lupus can be challenging; diagnosis is based on differences in clinical features and duration of skin changes, as well as biopsy and lab results.1 The clinical features of our patient were most consistent with DLE, based on the scarring alopecia with scaly atrophic plaques, dyspigmentation, and exacerbation following sun exposure.
DLE is the most common form of CCLE and frequently manifests in a localized, photosensitive distribution involving the scalp, ears, and/or face.2 Less commonly, it can demonstrate a more generalized distribution involving the trunk and/or extremities (reported incidence of 1.04 per 100,000 people).3 Longstanding DLE lesions commonly exhibit scarring and dyspigmentation. DLE occurs in approximately 15% to 30% of SLE patients,4 whereas about 10% of patients with DLE will progress to SLE.3
Positive antinuclear antibodies (ANA) are found in 54% of patients with CCLE, compared to 74% and 81% of patients with SCLE and ACLE, respectively.5 Thus, a negative ANA should not rule out the possibility of CLE.
Comprehensive lab work and biopsy could expose a systemic origin
While our patient already had a diagnosis of SLE, many patients will present with no prior history of autoimmune connective tissue disease, and, in that case, the objective should be to confirm the diagnosis and evaluate for systemic involvement. This includes a thorough review of systems; skin biopsy; complete blood count; liver function tests; urinalysis; and measurement of creatinine, inflammatory markers, ANA, extractable nuclear antigens, double-stranded DNA, complement levels (C3, C4, total), and antiphospholipid antibodies.6
Continue to: Biopsy
Biopsy features of DLE include vacuolar interface dermatitis, basement membrane zone thickening, follicular plugging, superficial and deep perivascular and periadnexal lymphohistiocytic inflammation with plasma cells, and increased mucin deposition. Direct immunofluorescence biopsy may show a continuous granular immunoglobulin (Ig) G/IgA/IgM and C3 band at the basement membrane zone.
Abnormal serologic tests may support the diagnosis of SLE based on American College of Rheumatology criteria and could suggest additional organ involvement or associated conditions, such as lupus nephritis or antiphospholipid syndrome (respectively). Currently, no clear consensus exists on monitoring patients with cutaneous lupus for systemic disease.
A gamut of skin-changing conditions should be considered
The differential diagnosis in this case includes SCLE, dermatitis, tinea corporis, cutaneous drug eruptions, and graft-versus-host disease (GVHD).
SCLE classically manifests with annular or psoriasiform lesions on the sun-exposed areas of the upper trunk (eg, the chest, neck, and upper extremities), while the central face and scalp are typically spared. Differentiating between generalized DLE and SCLE may be the most difficult, given similarities in the associated skin changes.
Dermatitis (atopic or contact) manifests as pruritic erythematous eczematous plaques, most commonly involving the flexural areas in atopic dermatitis and an exposure-dependent distribution pattern in contact dermatitis. The patient may have a history of atopy.
Continue to: Tinea corporis
Tinea corporis will manifest with annular scaly patches or plaques and may demonstrate erythematous papules around hair follicles in Majocchi granuloma. A positive potassium hydroxide exam demonstrating fungal hyphae confirms the diagnosis.
Cutaneous drug eruptions can have various morphologies and timing of onset. Certain photosensitive drug reactions can be triggered or exacerbated with sun exposure. Therefore, it is necessary to obtain a thorough medication history, including any new medications that were started within the past 4 to 6 weeks, although onset can be delayed beyond this timeframe.
GVHD is a complication that more commonly follows allogeneic hematopoietic stem cell transplants, although it may be seen following solid-organ transplantation or transfusion of nonirradiated blood. Chronic GVHD has an onset ≥ 100 days after transplant and is divided into nonsclerotic (lichenoid, atopic dermatitis-like, psoriasiform, poikilodermatous) and sclerotic morphologies.
Successful Tx requires adherence but may not prevent flare-ups
First-line treatment options for severe and widespread skin manifestations of CLE include photoprotection, smoking cessation, topical corticosteroids, hydroxychloroquine, and systemic corticosteroids. Second-line treatments include chloroquine, methotrexate, or mycophenolate mofetil; thalidomide or lenalidomide may be considered for patients with refractory disease.7,8
With successful treatment and photoprotection, patients may achieve significant skin clearing. Occasional flares, especially during warmer months, may occur if they are not diligent about photoprotection. Systemic treatments will also improve the patient’s systemic symptoms if the patient has concomitant SLE.
Our patient was advised to use topical steroids and to restart hydroxychloroquine 300 mg/d and mycophenolate mofetil 1000 mg/d (a regimen with which she had previously been nonadherent). The patient followed up with her family physician for assessment of her other medical issues. No new interventions for her mental health were initiated during this visit, as the severity of her depression was considered mild. She was referred to a case manager to navigate multiple medical appointments and prescription insurance coverage issues. The patient’s dose of mycophenolate mofetil was increased gradually to 3 g/d, and the patient experienced improvement in both her cutaneous lesions and systemic symptoms.
A 23-year-old woman with systemic lupus erythematosus (SLE) and a history of poor adherence to recommended treatment presented with a widespread pruritic rash and diffuse hair loss. The rash had rapidly progressed following sun exposure during the summer. The patient cited her mental health status (anxiety, depression), socioeconomic factors, and challenges with prescription insurance coverage as reasons for nonadherence to treatment.
Clinical examination revealed diffuse scarring alopecia and abnormal pigmentation of the scalp (FIGURE 1A), as well as large, red-brown, scaly, atrophic plaques on the face, ears, extremities, back, and buttocks (FIGURES 1B and 1C).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Generalized chronic cutaneouslupus erythematosus
The clinical features of our patient were most consistent with generalized chronic cutaneous lupus erythematosus (CCLE), which is 1 of 3 subtypes of cutaneous lupus erythematosus (CLE). The other 2 are acute and subacute cutaneous lupus erythematosus (ACLE and SCLE, respectively). CCLE is further divided into 3 distinct entities: discoid lupus erythematosus (DLE), chilblain lupus erythematosus, and lupus erythematosus panniculitis.
Distinguishing between the different forms of cutaneous lupus can be challenging; diagnosis is based on differences in clinical features and duration of skin changes, as well as biopsy and lab results.1 The clinical features of our patient were most consistent with DLE, based on the scarring alopecia with scaly atrophic plaques, dyspigmentation, and exacerbation following sun exposure.
DLE is the most common form of CCLE and frequently manifests in a localized, photosensitive distribution involving the scalp, ears, and/or face.2 Less commonly, it can demonstrate a more generalized distribution involving the trunk and/or extremities (reported incidence of 1.04 per 100,000 people).3 Longstanding DLE lesions commonly exhibit scarring and dyspigmentation. DLE occurs in approximately 15% to 30% of SLE patients,4 whereas about 10% of patients with DLE will progress to SLE.3
Positive antinuclear antibodies (ANA) are found in 54% of patients with CCLE, compared to 74% and 81% of patients with SCLE and ACLE, respectively.5 Thus, a negative ANA should not rule out the possibility of CLE.
Comprehensive lab work and biopsy could expose a systemic origin
While our patient already had a diagnosis of SLE, many patients will present with no prior history of autoimmune connective tissue disease, and, in that case, the objective should be to confirm the diagnosis and evaluate for systemic involvement. This includes a thorough review of systems; skin biopsy; complete blood count; liver function tests; urinalysis; and measurement of creatinine, inflammatory markers, ANA, extractable nuclear antigens, double-stranded DNA, complement levels (C3, C4, total), and antiphospholipid antibodies.6
Continue to: Biopsy
Biopsy features of DLE include vacuolar interface dermatitis, basement membrane zone thickening, follicular plugging, superficial and deep perivascular and periadnexal lymphohistiocytic inflammation with plasma cells, and increased mucin deposition. Direct immunofluorescence biopsy may show a continuous granular immunoglobulin (Ig) G/IgA/IgM and C3 band at the basement membrane zone.
Abnormal serologic tests may support the diagnosis of SLE based on American College of Rheumatology criteria and could suggest additional organ involvement or associated conditions, such as lupus nephritis or antiphospholipid syndrome (respectively). Currently, no clear consensus exists on monitoring patients with cutaneous lupus for systemic disease.
A gamut of skin-changing conditions should be considered
The differential diagnosis in this case includes SCLE, dermatitis, tinea corporis, cutaneous drug eruptions, and graft-versus-host disease (GVHD).
SCLE classically manifests with annular or psoriasiform lesions on the sun-exposed areas of the upper trunk (eg, the chest, neck, and upper extremities), while the central face and scalp are typically spared. Differentiating between generalized DLE and SCLE may be the most difficult, given similarities in the associated skin changes.
Dermatitis (atopic or contact) manifests as pruritic erythematous eczematous plaques, most commonly involving the flexural areas in atopic dermatitis and an exposure-dependent distribution pattern in contact dermatitis. The patient may have a history of atopy.
Continue to: Tinea corporis
Tinea corporis will manifest with annular scaly patches or plaques and may demonstrate erythematous papules around hair follicles in Majocchi granuloma. A positive potassium hydroxide exam demonstrating fungal hyphae confirms the diagnosis.
Cutaneous drug eruptions can have various morphologies and timing of onset. Certain photosensitive drug reactions can be triggered or exacerbated with sun exposure. Therefore, it is necessary to obtain a thorough medication history, including any new medications that were started within the past 4 to 6 weeks, although onset can be delayed beyond this timeframe.
GVHD is a complication that more commonly follows allogeneic hematopoietic stem cell transplants, although it may be seen following solid-organ transplantation or transfusion of nonirradiated blood. Chronic GVHD has an onset ≥ 100 days after transplant and is divided into nonsclerotic (lichenoid, atopic dermatitis-like, psoriasiform, poikilodermatous) and sclerotic morphologies.
Successful Tx requires adherence but may not prevent flare-ups
First-line treatment options for severe and widespread skin manifestations of CLE include photoprotection, smoking cessation, topical corticosteroids, hydroxychloroquine, and systemic corticosteroids. Second-line treatments include chloroquine, methotrexate, or mycophenolate mofetil; thalidomide or lenalidomide may be considered for patients with refractory disease.7,8
With successful treatment and photoprotection, patients may achieve significant skin clearing. Occasional flares, especially during warmer months, may occur if they are not diligent about photoprotection. Systemic treatments will also improve the patient’s systemic symptoms if the patient has concomitant SLE.
Our patient was advised to use topical steroids and to restart hydroxychloroquine 300 mg/d and mycophenolate mofetil 1000 mg/d (a regimen with which she had previously been nonadherent). The patient followed up with her family physician for assessment of her other medical issues. No new interventions for her mental health were initiated during this visit, as the severity of her depression was considered mild. She was referred to a case manager to navigate multiple medical appointments and prescription insurance coverage issues. The patient’s dose of mycophenolate mofetil was increased gradually to 3 g/d, and the patient experienced improvement in both her cutaneous lesions and systemic symptoms.
1. Petty AJ, Floyd L, Henderson C, et al. Cutaneous lupus erythematosus: progress and challenges. Curr Allergy Asthma Rep. 2020;20:12. doi: 10.1007/s11882-020-00906-8
2. Kuhn A, Landmann A. The classification and diagnosis of cutaneous lupus erythematosus. J Autoimmun. 2014;48-49:14-19. doi: 10.1016/j.jaut.2014.01.021
3. Durosaro O, Davis MDP, Reed KB, et al. Incidence of cutaneous lupus erythematosus, 1965-2005: a population-based study. Arch Dermatol. 2009;145:249-253. doi: 10.1001/archdermatol.2009.21
4. Merola JF. Overview of cutaneous lupus erythematosus. UpToDate. Updated September 19, 2021. Accessed February 17, 2022. www.uptodate.com/contents/overview-of-cutaneous-lupus-erythematosus
5. Biazar C, Sigges J, Patsinakidis N, et al. Cutaneous lupus erythematosus: first multicenter database analysis of 1002 patients from the European Society of Cutaneous Lupus Erythematosus (EUSCLE). Autoimmun Rev. 2013;12:444-454. doi: 10.1016/j.autrev.2012.08.019
6. O’Brien JC, Chong BF. not just skin deep: systemic disease involvement in patients with cutaneous lupus. J Investig Dermatol Symp Proc. 2017;18:S69-S74. doi: 10.1016/j.jisp.2016.09.001
7. Kuhn A, Ruland V, Bonsmann G. Cutaneous lupus erythematosus: update of therapeutic options part I. J Am Acad Dermatol. 2011;65:e179-e193. doi: 10.1016/j.jaad.2010.06.018
8. Kindle SA, Wetter DA, Davis MDP, et al. Lenalidomide treatment of cutaneous lupus erythematosus: the Mayo Clinic experience. Int J Dermatol. 2016;55:e431-e439. doi: 10.1111/ijd.13226
1. Petty AJ, Floyd L, Henderson C, et al. Cutaneous lupus erythematosus: progress and challenges. Curr Allergy Asthma Rep. 2020;20:12. doi: 10.1007/s11882-020-00906-8
2. Kuhn A, Landmann A. The classification and diagnosis of cutaneous lupus erythematosus. J Autoimmun. 2014;48-49:14-19. doi: 10.1016/j.jaut.2014.01.021
3. Durosaro O, Davis MDP, Reed KB, et al. Incidence of cutaneous lupus erythematosus, 1965-2005: a population-based study. Arch Dermatol. 2009;145:249-253. doi: 10.1001/archdermatol.2009.21
4. Merola JF. Overview of cutaneous lupus erythematosus. UpToDate. Updated September 19, 2021. Accessed February 17, 2022. www.uptodate.com/contents/overview-of-cutaneous-lupus-erythematosus
5. Biazar C, Sigges J, Patsinakidis N, et al. Cutaneous lupus erythematosus: first multicenter database analysis of 1002 patients from the European Society of Cutaneous Lupus Erythematosus (EUSCLE). Autoimmun Rev. 2013;12:444-454. doi: 10.1016/j.autrev.2012.08.019
6. O’Brien JC, Chong BF. not just skin deep: systemic disease involvement in patients with cutaneous lupus. J Investig Dermatol Symp Proc. 2017;18:S69-S74. doi: 10.1016/j.jisp.2016.09.001
7. Kuhn A, Ruland V, Bonsmann G. Cutaneous lupus erythematosus: update of therapeutic options part I. J Am Acad Dermatol. 2011;65:e179-e193. doi: 10.1016/j.jaad.2010.06.018
8. Kindle SA, Wetter DA, Davis MDP, et al. Lenalidomide treatment of cutaneous lupus erythematosus: the Mayo Clinic experience. Int J Dermatol. 2016;55:e431-e439. doi: 10.1111/ijd.13226