User login
Clinical Progress Note: Vascular Access Appropriateness Guidance for Pediatric Hospitalists
Hospitalized pediatric patients often require vascular access for necessary therapies, such as antibiotics. However, vascular access devices (VADs) are also associated with harm, ranging from insertion complications to life-threatening bloodstream infections or thrombosis.1 Pediatric hospitalists often guide VAD placement. There is a paucity of evidence to guide VAD selection based on the relative benefits and risks.2 The Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics (miniMAGIC)2 offers the first set of standards. Like its adult predecessor guideline (MAGIC) published in 2015, it provides guidance on appropriate VAD selection based on current evidence and expertise from a multidisciplinary panel.2 The guideline informs device selection, device characteristics, and insertion technique for the pediatric population (term neonates to adolescents) and across a wide range of clinical indications.2 This review highlights key recommendations for pediatric hospitalists to help their decision-making.
METHODS USED IN PREPARING THE GUIDELINE
miniMAGIC was developed using the RAND/UCLA Appropriateness Method, a method proven to reduce inappropriate (ie, overused or underused) healthcare interventions.3 It combines rigorous evidence review with multidisciplinary expert opinion on real-world clinical scenarios to provide recommendations about an intervention’s appropriateness.3 This is particularly useful for clinical scenarios that lack high-quality evidence to guide decision-making. The RAND/UCLA method deems an intervention appropriate if the benefits outweigh the risks by a wide enough margin that proceeding is worthwhile, and it does not take cost into account.2 The method design consists of five phases: (1) defining the scope and key terms, (2) reviewing and synthesizing the literature, (3) selecting an expert panel, (4) developing case scenarios, and (5) conducting two rounds of appropriateness ratings by the expert panel for each clinical scenario.3 The guideline’s scope included term neonates (aged 0-30 days), infants (aged 31 days-1 year), children (aged 1-12 years), and adolescents (aged 12-18 years). Infants receiving care in the neonatal intensive care unit or special care nursery were excluded. Other specialized populations addressed based on setting or diagnosis were general hospitalized patients and patients with congenital cardiac disease, critical illness, oncologic and hematologic conditions, and long-term VAD-dependent conditions.3
A total of 133 studies or clinical practice guidelines (CPGs) met the eligibility criteria for the systematic review.4 Although the systematic review was conducted per the RAND/UCLA method using two independent reviewers who evaluated the methodologic quality, transparency, and relevancy of each article, there was no formal assessment of evidence quality. The recommendations were based primarily on observational studies and CPGs because there were few randomized controlled trials or systematic reviews on VAD selection for pediatric patients in the literature. Pediatric evidence was limited for certain scenarios or populations (eg, term neonates, midline catheters, difficult venous access, long-term VAD), so adult and/or neonatal evidence was included when applicable.
The panel included 14 pediatric clinical experts from cardiology, vascular access, critical care, hematology/oncology, emergency medicine, general surgery, hospital medicine, anesthesia, interventional radiology, pharmacology, and infectious diseases. The panel also included nonvoting panel members such as the panel facilitators, a methodologist, and a patient representative.
RESULTS OF THE CLINICAL REVIEW
We review four common clinical scenarios encountered by pediatric hospitalists and summarize key recommendations (Table
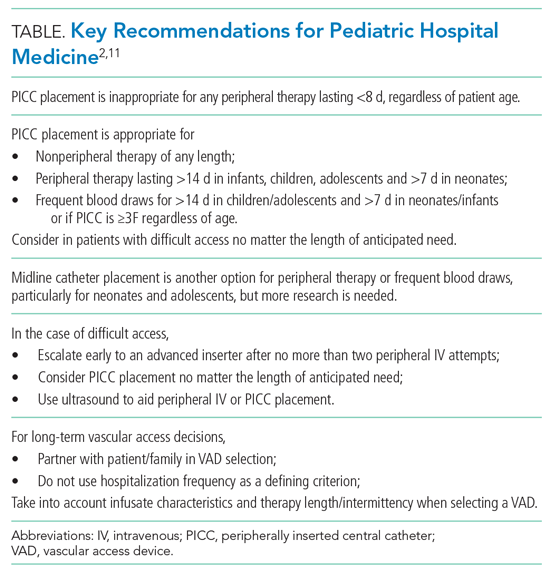
Peripherally Inserted Central Catheter
Patients may require peripherally inserted central catheters (PICCs) to facilitate a longer duration of intravenous (IV) therapy, such as delivery of antibiotics, or frequent blood draws. The need for prolonged vascular access is decreasing, as studies show many infections in children previously treated with prolonged IV antibiotics can be safely and equally effectively managed with early transition to oral therapy.5-8 These studies highlight the higher rate of complications and risks associated with PICCs, including thrombosis, infection, and mechanical issues, as well as the added healthcare utilization needed to evaluate and manage the complications. PICC-associated complication rates also increase with duration.4
However, there are some clinical scenarios that still warrant prolonged therapy and/or access; PICC recommendations are summarized in the Appendix Figure. The miniMAGIC panel deemed PICC lines appropriate for any nonperipheral therapy of any length. For peripherally compatible therapy, the panel rated PICC placement as inappropriate for therapy lasting less than 8 days, regardless of patient age. PICC placement in infants, children, and adolescents was rated appropriate for therapy with a duration exceeding 14 days, but the panel was uncertain about therapy expected to last between 8 and 14 days. Recognizing the additional challenges with maintaining peripheral IV catheter access in neonates, PICCs were deemed appropriate for neonates needing peripheral therapy lasting longer than 7 days.
The panel rated PICC placement appropriate for frequent blood draws (defined as more than one time per day) for more than 7 days in neonates or infants and more than 14 days in children and adolescents. But regardless of patient age, the PICC caliber must be at least 3F.
The miniMAGIC panel found that a single lumen is appropriate in most cases, highlighting that multilumen catheters increase the risk for infection, occlusion, and venous thrombosis.4 Multilumen catheters were rated as inappropriate in the case of reserving a lumen for blood products and blood sampling. When reserving a lumen for lipids and parenteral nutrition (PN), the panel was uncertain given the lack of evidence regarding the risks/benefits of the complications associated with the infusions themselves versus those of the device. Regardless, collaboration with a pharmacist and vascular access specialist is recommended to aid in choosing the most appropriate device characteristics.
Midline Catheters
Midline catheters are inserted in a peripheral vein, but the catheter tip terminates in the proximal extremity. Compared with peripheral IV catheters, midline catheters last longer and have lower rates of phlebitis. In addition, midline catheter placement does not require sedation or fluoroscopy and has lower rates of infection compared with PICC lines.9 Although there is good evidence in adults, and multiple panelists reported success in using midline catheters in various age groups, the evidence for their safe and efficacious use in pediatrics is limited, particularly for infants. Midline catheters were rated as appropriate for peripheral therapy lasting less than 8 days in neonates and less than 15 days in children and adolescents. Use in infants was deemed uncertain based on lack of published evidence. Midline catheters were also rated as appropriate for frequent blood draws of less than 8 days in neonates and less than 15 days in adolescents, but uncertain for children and infants.
Difficult Access and Insertion Procedure
The panel rated three or more attempts for peripheral IV catheter insertion by a single clinician as inappropriate and recommended early escalation to a more experienced inserter after 0 to 2 attempts by a single provider. The goal is to preserve insertion sites and reduce patient discomfort. If a patient loses access when only 1 day of therapy remains, the provider should transition to oral or intramuscular therapy when appropriate, particularly if there are no advanced insertion staff available or after two or more attempts at re-insertion are unsuccessful. There is high-quality evidence that supports vessel visualization (primarily ultrasound) with peripheral IV catheter and PICC placement.2 In the case of two or more unsuccessful attempts at peripheral IV catheter placement by an advanced inserter using technology assistance (ultrasound), PICC placement is considered appropriate by the panel to avoid delays in treatment and limit patient discomfort associated with repeat attempts.
Long-term Vascular Access
Children with medical complexity or chronic illness may require long-term (>2 months) or very-long-term (>1 year) vascular access. Common themes for VAD selection in this heterogeneous population include a focus on vessel preservation and complication prevention.2 The panel strongly recommended that clinicians partner with the patient and caregivers in the decision-making process. Shared decision-making is necessary to meet both the short-term and evolving needs of the of the patient and family. The panel also believed the frequency of hospitalization should not be used as a criterion for VAD selection since acute hospitalization is an unreliable proxy for disease severity in pediatric chronic disease conditions.2 Rather, the infusate characteristics and length/intermittency of therapy should be primary influencers of VAD selection. In general, the panel rated cuffed tunneled central VADs (CVADs) as appropriate for all age groups for long-term PN, long-term continuous infusions, and long-term intermittent therapies. For continuous non-PN infusions, appropriate ratings were given to PICCs for infants and children and total implanted venous devices (TIVDs) in children and adolescents. For intermittent (but at least daily) access, TIVDs and PICC lines were both rated as appropriate for children and adolescents but uncertain for neonates and infants. Peripheral devices were deemed inappropriate for all long-term complex therapies. For children and adolescents needing intermittent, regular peripheral treatments (eg, steroids or antibiotics), peripheral IVs and TIVDs were rated appropriate for short duration (<7 days) therapies. PICCs and midlines for this indication were uncertain because of the lack of evidence. For medium-duration intermittent therapies (8-14 days), PICCs, tunneled cuffed CVADs, and TIVDs were rated as appropriate. A recently released mobile application can help guide the clinician through many varied clinical scenarios and indications.10
LIMITATIONS AND GAPS
The guideline recommendations were more often reliant on clinical practice guidelines and expert panel opinion given the lack of high-quality pediatric evidence for most scenarios. The panel members were from the United States and Australia, so the recommendations may not be generalizable to care systems in other countries. Although the panel included experts from many specialties that care for patient populations needing VADs, not all subspecialty populations were considered, particularly those with long-term vascular access–dependent conditions who may be commonly hospitalized. Scenarios with disagreement or uncertainty highlight gaps in need of future study (eg, midline catheter use and device selection for blood draws).
CONCLUSIONS AND APPLICATION
miniMAGIC is the first appropriateness guideline to help standardize the safe use of VADs in children. Although some gaps remain, the authors intend it to be a living document that will need revisions as new evidence is published. A mobile health application facilitates use of the recommendations, providing quick, point-of-care decision support based on clinical features.10 Pediatric hospitalists should collaborate with their institutions to examine their current VAD use in hospitalized children and identify opportunities for practice change and standardization. Use of these recommendations may help hospitalists improve the care of hospitalized children by decreasing unnecessary PICC placement and better partner with patients and caregivers to limit discomfort surrounding VAD placement.
1. Ullman AJ, Marsh N, Mihala G, Cooke M, Rickard CM. Complications of central venous access devices: a systematic review. Pediatrics. 2015;136(5):e1331-e1344. https://doi.org/10.1542/peds.2015-1507
2. Ullman AJ, Bernstein SJ, Brown E, et al. The Michigan appropriateness guide for intravenous catheters in pediatrics: miniMAGIC. Pediatrics. 2020;145(Suppl 3):S269-S284. https://doi.org/10.1542/peds.2019-3474I
3. Ullman AJ, Chopra V, Brown E, et al. Developing appropriateness criteria for pediatric vascular access. Pediatrics. 2020;145(Suppl 3):S233-S242. https://doi.org/10.1542/peds.2019-3474G
4. Paterson RS, Chopra V, Brown E, et al. Selection and insertion of vascular access devices in pediatrics: a systematic review. Pediatrics. 2020;145(Suppl 3):S243-S268. https://doi.org/10.1542/peds.2019-3474H
5. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomeyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
6. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e20161692. https://doi.org/10.1542/peds.2016-1692
7. Rangel SJ, Anderson BR, Srivastava R, et al. Intravenous versus oral antibiotics for the prevention of treatment failure in children with complicated appendicitis: has the abandonment of peripherally inserted catheters been justified? Ann Surg. 2017;266(2):361-368. https://doi.org/10.1097/SLA.0000000000001923
8. Desai S, Aronson PL, Shabanova V, et al. Parenteral antibiotic therapy duration in young infants with bacteremic urinary tract infections. Pediatrics. 2019;144(3):e20183844. https://doi.org/10.1542/peds.2018-3844
9. Anderson J, Greenwell A, Louderback J, Polivka BJ, Herron Behr J. Comparison of outcomes of extended dwell/midline peripheral intravenous catheters and peripherally inserted central catheters in children. J Assoc Vasc Access. 2016;21(3):158-164. https://doi.org/10.1016/j.java.2016.03.007
10. miniMAGIC: the Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics app. Version 1.0.0. Alliance for Vascular Access Teaching and Research.
11. Shaughnessy EE, Morton K, Shah SS. Vascular access in hospitalized children. Pediatrics. 2020;145(Suppl 3):S298-S299. https://doi.org/10.1542/peds.2019-3474P
Hospitalized pediatric patients often require vascular access for necessary therapies, such as antibiotics. However, vascular access devices (VADs) are also associated with harm, ranging from insertion complications to life-threatening bloodstream infections or thrombosis.1 Pediatric hospitalists often guide VAD placement. There is a paucity of evidence to guide VAD selection based on the relative benefits and risks.2 The Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics (miniMAGIC)2 offers the first set of standards. Like its adult predecessor guideline (MAGIC) published in 2015, it provides guidance on appropriate VAD selection based on current evidence and expertise from a multidisciplinary panel.2 The guideline informs device selection, device characteristics, and insertion technique for the pediatric population (term neonates to adolescents) and across a wide range of clinical indications.2 This review highlights key recommendations for pediatric hospitalists to help their decision-making.
METHODS USED IN PREPARING THE GUIDELINE
miniMAGIC was developed using the RAND/UCLA Appropriateness Method, a method proven to reduce inappropriate (ie, overused or underused) healthcare interventions.3 It combines rigorous evidence review with multidisciplinary expert opinion on real-world clinical scenarios to provide recommendations about an intervention’s appropriateness.3 This is particularly useful for clinical scenarios that lack high-quality evidence to guide decision-making. The RAND/UCLA method deems an intervention appropriate if the benefits outweigh the risks by a wide enough margin that proceeding is worthwhile, and it does not take cost into account.2 The method design consists of five phases: (1) defining the scope and key terms, (2) reviewing and synthesizing the literature, (3) selecting an expert panel, (4) developing case scenarios, and (5) conducting two rounds of appropriateness ratings by the expert panel for each clinical scenario.3 The guideline’s scope included term neonates (aged 0-30 days), infants (aged 31 days-1 year), children (aged 1-12 years), and adolescents (aged 12-18 years). Infants receiving care in the neonatal intensive care unit or special care nursery were excluded. Other specialized populations addressed based on setting or diagnosis were general hospitalized patients and patients with congenital cardiac disease, critical illness, oncologic and hematologic conditions, and long-term VAD-dependent conditions.3
A total of 133 studies or clinical practice guidelines (CPGs) met the eligibility criteria for the systematic review.4 Although the systematic review was conducted per the RAND/UCLA method using two independent reviewers who evaluated the methodologic quality, transparency, and relevancy of each article, there was no formal assessment of evidence quality. The recommendations were based primarily on observational studies and CPGs because there were few randomized controlled trials or systematic reviews on VAD selection for pediatric patients in the literature. Pediatric evidence was limited for certain scenarios or populations (eg, term neonates, midline catheters, difficult venous access, long-term VAD), so adult and/or neonatal evidence was included when applicable.
The panel included 14 pediatric clinical experts from cardiology, vascular access, critical care, hematology/oncology, emergency medicine, general surgery, hospital medicine, anesthesia, interventional radiology, pharmacology, and infectious diseases. The panel also included nonvoting panel members such as the panel facilitators, a methodologist, and a patient representative.
RESULTS OF THE CLINICAL REVIEW
We review four common clinical scenarios encountered by pediatric hospitalists and summarize key recommendations (Table

Peripherally Inserted Central Catheter
Patients may require peripherally inserted central catheters (PICCs) to facilitate a longer duration of intravenous (IV) therapy, such as delivery of antibiotics, or frequent blood draws. The need for prolonged vascular access is decreasing, as studies show many infections in children previously treated with prolonged IV antibiotics can be safely and equally effectively managed with early transition to oral therapy.5-8 These studies highlight the higher rate of complications and risks associated with PICCs, including thrombosis, infection, and mechanical issues, as well as the added healthcare utilization needed to evaluate and manage the complications. PICC-associated complication rates also increase with duration.4
However, there are some clinical scenarios that still warrant prolonged therapy and/or access; PICC recommendations are summarized in the Appendix Figure. The miniMAGIC panel deemed PICC lines appropriate for any nonperipheral therapy of any length. For peripherally compatible therapy, the panel rated PICC placement as inappropriate for therapy lasting less than 8 days, regardless of patient age. PICC placement in infants, children, and adolescents was rated appropriate for therapy with a duration exceeding 14 days, but the panel was uncertain about therapy expected to last between 8 and 14 days. Recognizing the additional challenges with maintaining peripheral IV catheter access in neonates, PICCs were deemed appropriate for neonates needing peripheral therapy lasting longer than 7 days.
The panel rated PICC placement appropriate for frequent blood draws (defined as more than one time per day) for more than 7 days in neonates or infants and more than 14 days in children and adolescents. But regardless of patient age, the PICC caliber must be at least 3F.
The miniMAGIC panel found that a single lumen is appropriate in most cases, highlighting that multilumen catheters increase the risk for infection, occlusion, and venous thrombosis.4 Multilumen catheters were rated as inappropriate in the case of reserving a lumen for blood products and blood sampling. When reserving a lumen for lipids and parenteral nutrition (PN), the panel was uncertain given the lack of evidence regarding the risks/benefits of the complications associated with the infusions themselves versus those of the device. Regardless, collaboration with a pharmacist and vascular access specialist is recommended to aid in choosing the most appropriate device characteristics.
Midline Catheters
Midline catheters are inserted in a peripheral vein, but the catheter tip terminates in the proximal extremity. Compared with peripheral IV catheters, midline catheters last longer and have lower rates of phlebitis. In addition, midline catheter placement does not require sedation or fluoroscopy and has lower rates of infection compared with PICC lines.9 Although there is good evidence in adults, and multiple panelists reported success in using midline catheters in various age groups, the evidence for their safe and efficacious use in pediatrics is limited, particularly for infants. Midline catheters were rated as appropriate for peripheral therapy lasting less than 8 days in neonates and less than 15 days in children and adolescents. Use in infants was deemed uncertain based on lack of published evidence. Midline catheters were also rated as appropriate for frequent blood draws of less than 8 days in neonates and less than 15 days in adolescents, but uncertain for children and infants.
Difficult Access and Insertion Procedure
The panel rated three or more attempts for peripheral IV catheter insertion by a single clinician as inappropriate and recommended early escalation to a more experienced inserter after 0 to 2 attempts by a single provider. The goal is to preserve insertion sites and reduce patient discomfort. If a patient loses access when only 1 day of therapy remains, the provider should transition to oral or intramuscular therapy when appropriate, particularly if there are no advanced insertion staff available or after two or more attempts at re-insertion are unsuccessful. There is high-quality evidence that supports vessel visualization (primarily ultrasound) with peripheral IV catheter and PICC placement.2 In the case of two or more unsuccessful attempts at peripheral IV catheter placement by an advanced inserter using technology assistance (ultrasound), PICC placement is considered appropriate by the panel to avoid delays in treatment and limit patient discomfort associated with repeat attempts.
Long-term Vascular Access
Children with medical complexity or chronic illness may require long-term (>2 months) or very-long-term (>1 year) vascular access. Common themes for VAD selection in this heterogeneous population include a focus on vessel preservation and complication prevention.2 The panel strongly recommended that clinicians partner with the patient and caregivers in the decision-making process. Shared decision-making is necessary to meet both the short-term and evolving needs of the of the patient and family. The panel also believed the frequency of hospitalization should not be used as a criterion for VAD selection since acute hospitalization is an unreliable proxy for disease severity in pediatric chronic disease conditions.2 Rather, the infusate characteristics and length/intermittency of therapy should be primary influencers of VAD selection. In general, the panel rated cuffed tunneled central VADs (CVADs) as appropriate for all age groups for long-term PN, long-term continuous infusions, and long-term intermittent therapies. For continuous non-PN infusions, appropriate ratings were given to PICCs for infants and children and total implanted venous devices (TIVDs) in children and adolescents. For intermittent (but at least daily) access, TIVDs and PICC lines were both rated as appropriate for children and adolescents but uncertain for neonates and infants. Peripheral devices were deemed inappropriate for all long-term complex therapies. For children and adolescents needing intermittent, regular peripheral treatments (eg, steroids or antibiotics), peripheral IVs and TIVDs were rated appropriate for short duration (<7 days) therapies. PICCs and midlines for this indication were uncertain because of the lack of evidence. For medium-duration intermittent therapies (8-14 days), PICCs, tunneled cuffed CVADs, and TIVDs were rated as appropriate. A recently released mobile application can help guide the clinician through many varied clinical scenarios and indications.10
LIMITATIONS AND GAPS
The guideline recommendations were more often reliant on clinical practice guidelines and expert panel opinion given the lack of high-quality pediatric evidence for most scenarios. The panel members were from the United States and Australia, so the recommendations may not be generalizable to care systems in other countries. Although the panel included experts from many specialties that care for patient populations needing VADs, not all subspecialty populations were considered, particularly those with long-term vascular access–dependent conditions who may be commonly hospitalized. Scenarios with disagreement or uncertainty highlight gaps in need of future study (eg, midline catheter use and device selection for blood draws).
CONCLUSIONS AND APPLICATION
miniMAGIC is the first appropriateness guideline to help standardize the safe use of VADs in children. Although some gaps remain, the authors intend it to be a living document that will need revisions as new evidence is published. A mobile health application facilitates use of the recommendations, providing quick, point-of-care decision support based on clinical features.10 Pediatric hospitalists should collaborate with their institutions to examine their current VAD use in hospitalized children and identify opportunities for practice change and standardization. Use of these recommendations may help hospitalists improve the care of hospitalized children by decreasing unnecessary PICC placement and better partner with patients and caregivers to limit discomfort surrounding VAD placement.
Hospitalized pediatric patients often require vascular access for necessary therapies, such as antibiotics. However, vascular access devices (VADs) are also associated with harm, ranging from insertion complications to life-threatening bloodstream infections or thrombosis.1 Pediatric hospitalists often guide VAD placement. There is a paucity of evidence to guide VAD selection based on the relative benefits and risks.2 The Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics (miniMAGIC)2 offers the first set of standards. Like its adult predecessor guideline (MAGIC) published in 2015, it provides guidance on appropriate VAD selection based on current evidence and expertise from a multidisciplinary panel.2 The guideline informs device selection, device characteristics, and insertion technique for the pediatric population (term neonates to adolescents) and across a wide range of clinical indications.2 This review highlights key recommendations for pediatric hospitalists to help their decision-making.
METHODS USED IN PREPARING THE GUIDELINE
miniMAGIC was developed using the RAND/UCLA Appropriateness Method, a method proven to reduce inappropriate (ie, overused or underused) healthcare interventions.3 It combines rigorous evidence review with multidisciplinary expert opinion on real-world clinical scenarios to provide recommendations about an intervention’s appropriateness.3 This is particularly useful for clinical scenarios that lack high-quality evidence to guide decision-making. The RAND/UCLA method deems an intervention appropriate if the benefits outweigh the risks by a wide enough margin that proceeding is worthwhile, and it does not take cost into account.2 The method design consists of five phases: (1) defining the scope and key terms, (2) reviewing and synthesizing the literature, (3) selecting an expert panel, (4) developing case scenarios, and (5) conducting two rounds of appropriateness ratings by the expert panel for each clinical scenario.3 The guideline’s scope included term neonates (aged 0-30 days), infants (aged 31 days-1 year), children (aged 1-12 years), and adolescents (aged 12-18 years). Infants receiving care in the neonatal intensive care unit or special care nursery were excluded. Other specialized populations addressed based on setting or diagnosis were general hospitalized patients and patients with congenital cardiac disease, critical illness, oncologic and hematologic conditions, and long-term VAD-dependent conditions.3
A total of 133 studies or clinical practice guidelines (CPGs) met the eligibility criteria for the systematic review.4 Although the systematic review was conducted per the RAND/UCLA method using two independent reviewers who evaluated the methodologic quality, transparency, and relevancy of each article, there was no formal assessment of evidence quality. The recommendations were based primarily on observational studies and CPGs because there were few randomized controlled trials or systematic reviews on VAD selection for pediatric patients in the literature. Pediatric evidence was limited for certain scenarios or populations (eg, term neonates, midline catheters, difficult venous access, long-term VAD), so adult and/or neonatal evidence was included when applicable.
The panel included 14 pediatric clinical experts from cardiology, vascular access, critical care, hematology/oncology, emergency medicine, general surgery, hospital medicine, anesthesia, interventional radiology, pharmacology, and infectious diseases. The panel also included nonvoting panel members such as the panel facilitators, a methodologist, and a patient representative.
RESULTS OF THE CLINICAL REVIEW
We review four common clinical scenarios encountered by pediatric hospitalists and summarize key recommendations (Table

Peripherally Inserted Central Catheter
Patients may require peripherally inserted central catheters (PICCs) to facilitate a longer duration of intravenous (IV) therapy, such as delivery of antibiotics, or frequent blood draws. The need for prolonged vascular access is decreasing, as studies show many infections in children previously treated with prolonged IV antibiotics can be safely and equally effectively managed with early transition to oral therapy.5-8 These studies highlight the higher rate of complications and risks associated with PICCs, including thrombosis, infection, and mechanical issues, as well as the added healthcare utilization needed to evaluate and manage the complications. PICC-associated complication rates also increase with duration.4
However, there are some clinical scenarios that still warrant prolonged therapy and/or access; PICC recommendations are summarized in the Appendix Figure. The miniMAGIC panel deemed PICC lines appropriate for any nonperipheral therapy of any length. For peripherally compatible therapy, the panel rated PICC placement as inappropriate for therapy lasting less than 8 days, regardless of patient age. PICC placement in infants, children, and adolescents was rated appropriate for therapy with a duration exceeding 14 days, but the panel was uncertain about therapy expected to last between 8 and 14 days. Recognizing the additional challenges with maintaining peripheral IV catheter access in neonates, PICCs were deemed appropriate for neonates needing peripheral therapy lasting longer than 7 days.
The panel rated PICC placement appropriate for frequent blood draws (defined as more than one time per day) for more than 7 days in neonates or infants and more than 14 days in children and adolescents. But regardless of patient age, the PICC caliber must be at least 3F.
The miniMAGIC panel found that a single lumen is appropriate in most cases, highlighting that multilumen catheters increase the risk for infection, occlusion, and venous thrombosis.4 Multilumen catheters were rated as inappropriate in the case of reserving a lumen for blood products and blood sampling. When reserving a lumen for lipids and parenteral nutrition (PN), the panel was uncertain given the lack of evidence regarding the risks/benefits of the complications associated with the infusions themselves versus those of the device. Regardless, collaboration with a pharmacist and vascular access specialist is recommended to aid in choosing the most appropriate device characteristics.
Midline Catheters
Midline catheters are inserted in a peripheral vein, but the catheter tip terminates in the proximal extremity. Compared with peripheral IV catheters, midline catheters last longer and have lower rates of phlebitis. In addition, midline catheter placement does not require sedation or fluoroscopy and has lower rates of infection compared with PICC lines.9 Although there is good evidence in adults, and multiple panelists reported success in using midline catheters in various age groups, the evidence for their safe and efficacious use in pediatrics is limited, particularly for infants. Midline catheters were rated as appropriate for peripheral therapy lasting less than 8 days in neonates and less than 15 days in children and adolescents. Use in infants was deemed uncertain based on lack of published evidence. Midline catheters were also rated as appropriate for frequent blood draws of less than 8 days in neonates and less than 15 days in adolescents, but uncertain for children and infants.
Difficult Access and Insertion Procedure
The panel rated three or more attempts for peripheral IV catheter insertion by a single clinician as inappropriate and recommended early escalation to a more experienced inserter after 0 to 2 attempts by a single provider. The goal is to preserve insertion sites and reduce patient discomfort. If a patient loses access when only 1 day of therapy remains, the provider should transition to oral or intramuscular therapy when appropriate, particularly if there are no advanced insertion staff available or after two or more attempts at re-insertion are unsuccessful. There is high-quality evidence that supports vessel visualization (primarily ultrasound) with peripheral IV catheter and PICC placement.2 In the case of two or more unsuccessful attempts at peripheral IV catheter placement by an advanced inserter using technology assistance (ultrasound), PICC placement is considered appropriate by the panel to avoid delays in treatment and limit patient discomfort associated with repeat attempts.
Long-term Vascular Access
Children with medical complexity or chronic illness may require long-term (>2 months) or very-long-term (>1 year) vascular access. Common themes for VAD selection in this heterogeneous population include a focus on vessel preservation and complication prevention.2 The panel strongly recommended that clinicians partner with the patient and caregivers in the decision-making process. Shared decision-making is necessary to meet both the short-term and evolving needs of the of the patient and family. The panel also believed the frequency of hospitalization should not be used as a criterion for VAD selection since acute hospitalization is an unreliable proxy for disease severity in pediatric chronic disease conditions.2 Rather, the infusate characteristics and length/intermittency of therapy should be primary influencers of VAD selection. In general, the panel rated cuffed tunneled central VADs (CVADs) as appropriate for all age groups for long-term PN, long-term continuous infusions, and long-term intermittent therapies. For continuous non-PN infusions, appropriate ratings were given to PICCs for infants and children and total implanted venous devices (TIVDs) in children and adolescents. For intermittent (but at least daily) access, TIVDs and PICC lines were both rated as appropriate for children and adolescents but uncertain for neonates and infants. Peripheral devices were deemed inappropriate for all long-term complex therapies. For children and adolescents needing intermittent, regular peripheral treatments (eg, steroids or antibiotics), peripheral IVs and TIVDs were rated appropriate for short duration (<7 days) therapies. PICCs and midlines for this indication were uncertain because of the lack of evidence. For medium-duration intermittent therapies (8-14 days), PICCs, tunneled cuffed CVADs, and TIVDs were rated as appropriate. A recently released mobile application can help guide the clinician through many varied clinical scenarios and indications.10
LIMITATIONS AND GAPS
The guideline recommendations were more often reliant on clinical practice guidelines and expert panel opinion given the lack of high-quality pediatric evidence for most scenarios. The panel members were from the United States and Australia, so the recommendations may not be generalizable to care systems in other countries. Although the panel included experts from many specialties that care for patient populations needing VADs, not all subspecialty populations were considered, particularly those with long-term vascular access–dependent conditions who may be commonly hospitalized. Scenarios with disagreement or uncertainty highlight gaps in need of future study (eg, midline catheter use and device selection for blood draws).
CONCLUSIONS AND APPLICATION
miniMAGIC is the first appropriateness guideline to help standardize the safe use of VADs in children. Although some gaps remain, the authors intend it to be a living document that will need revisions as new evidence is published. A mobile health application facilitates use of the recommendations, providing quick, point-of-care decision support based on clinical features.10 Pediatric hospitalists should collaborate with their institutions to examine their current VAD use in hospitalized children and identify opportunities for practice change and standardization. Use of these recommendations may help hospitalists improve the care of hospitalized children by decreasing unnecessary PICC placement and better partner with patients and caregivers to limit discomfort surrounding VAD placement.
1. Ullman AJ, Marsh N, Mihala G, Cooke M, Rickard CM. Complications of central venous access devices: a systematic review. Pediatrics. 2015;136(5):e1331-e1344. https://doi.org/10.1542/peds.2015-1507
2. Ullman AJ, Bernstein SJ, Brown E, et al. The Michigan appropriateness guide for intravenous catheters in pediatrics: miniMAGIC. Pediatrics. 2020;145(Suppl 3):S269-S284. https://doi.org/10.1542/peds.2019-3474I
3. Ullman AJ, Chopra V, Brown E, et al. Developing appropriateness criteria for pediatric vascular access. Pediatrics. 2020;145(Suppl 3):S233-S242. https://doi.org/10.1542/peds.2019-3474G
4. Paterson RS, Chopra V, Brown E, et al. Selection and insertion of vascular access devices in pediatrics: a systematic review. Pediatrics. 2020;145(Suppl 3):S243-S268. https://doi.org/10.1542/peds.2019-3474H
5. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomeyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
6. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e20161692. https://doi.org/10.1542/peds.2016-1692
7. Rangel SJ, Anderson BR, Srivastava R, et al. Intravenous versus oral antibiotics for the prevention of treatment failure in children with complicated appendicitis: has the abandonment of peripherally inserted catheters been justified? Ann Surg. 2017;266(2):361-368. https://doi.org/10.1097/SLA.0000000000001923
8. Desai S, Aronson PL, Shabanova V, et al. Parenteral antibiotic therapy duration in young infants with bacteremic urinary tract infections. Pediatrics. 2019;144(3):e20183844. https://doi.org/10.1542/peds.2018-3844
9. Anderson J, Greenwell A, Louderback J, Polivka BJ, Herron Behr J. Comparison of outcomes of extended dwell/midline peripheral intravenous catheters and peripherally inserted central catheters in children. J Assoc Vasc Access. 2016;21(3):158-164. https://doi.org/10.1016/j.java.2016.03.007
10. miniMAGIC: the Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics app. Version 1.0.0. Alliance for Vascular Access Teaching and Research.
11. Shaughnessy EE, Morton K, Shah SS. Vascular access in hospitalized children. Pediatrics. 2020;145(Suppl 3):S298-S299. https://doi.org/10.1542/peds.2019-3474P
1. Ullman AJ, Marsh N, Mihala G, Cooke M, Rickard CM. Complications of central venous access devices: a systematic review. Pediatrics. 2015;136(5):e1331-e1344. https://doi.org/10.1542/peds.2015-1507
2. Ullman AJ, Bernstein SJ, Brown E, et al. The Michigan appropriateness guide for intravenous catheters in pediatrics: miniMAGIC. Pediatrics. 2020;145(Suppl 3):S269-S284. https://doi.org/10.1542/peds.2019-3474I
3. Ullman AJ, Chopra V, Brown E, et al. Developing appropriateness criteria for pediatric vascular access. Pediatrics. 2020;145(Suppl 3):S233-S242. https://doi.org/10.1542/peds.2019-3474G
4. Paterson RS, Chopra V, Brown E, et al. Selection and insertion of vascular access devices in pediatrics: a systematic review. Pediatrics. 2020;145(Suppl 3):S243-S268. https://doi.org/10.1542/peds.2019-3474H
5. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomeyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
6. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e20161692. https://doi.org/10.1542/peds.2016-1692
7. Rangel SJ, Anderson BR, Srivastava R, et al. Intravenous versus oral antibiotics for the prevention of treatment failure in children with complicated appendicitis: has the abandonment of peripherally inserted catheters been justified? Ann Surg. 2017;266(2):361-368. https://doi.org/10.1097/SLA.0000000000001923
8. Desai S, Aronson PL, Shabanova V, et al. Parenteral antibiotic therapy duration in young infants with bacteremic urinary tract infections. Pediatrics. 2019;144(3):e20183844. https://doi.org/10.1542/peds.2018-3844
9. Anderson J, Greenwell A, Louderback J, Polivka BJ, Herron Behr J. Comparison of outcomes of extended dwell/midline peripheral intravenous catheters and peripherally inserted central catheters in children. J Assoc Vasc Access. 2016;21(3):158-164. https://doi.org/10.1016/j.java.2016.03.007
10. miniMAGIC: the Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics app. Version 1.0.0. Alliance for Vascular Access Teaching and Research.
11. Shaughnessy EE, Morton K, Shah SS. Vascular access in hospitalized children. Pediatrics. 2020;145(Suppl 3):S298-S299. https://doi.org/10.1542/peds.2019-3474P
© 2021 Society of Hospital Medicine
Pediatric Hospital Medicine Management, Staffing, and Well-being in the Face of COVID-19
MANAGEMENT AND COMMUNICATION
Establish a Command Team
We benefit from having an existing divisional leadership structure comprising the director, medical directors of our clinical service lines, directors of education and community integration, and associate directors of clinical operations, research, and quality. This established team provides us broad representation of team member expertise and ideas. We maintain our weekly leadership team meeting through video chat and have added daily 30-minute virtual huddles to provide updates from our respective areas and discuss logistical challenges and planning. We use ad hoc phone meetings with relevant team members to address issues of immediate concern.
In the absence of a formal leadership team structure, establish a command team comprising representative leaders of your varied groups (eg, clinical operations, quality improvement, education, research, and business).
Collaborate With Institutional Response
Align divisional command team actions with the institutional response. Our clinical operations leader serves as our primary representative on the institutional emergency preparedness team. This participation allows bidirectional communication, both for institutional updates to be shared with division members and division-specific initiatives to be shared with institutional leadership to facilitate learning across the system.
In conjunction with hospital leadership, our division created a special isolation unit (SIU) to isolate patients positive for COVID-19 and persons under investigation. The institutional emergency preparedness team highlighted the need for such a unit, and our divisional leadership team developed the physician staffing model and medical care delivery system. We collaborated with key stakeholders, including nurses, respiratory therapists, other patient care services members, and subspecialists. The SIU leadership, which includes representatives from hospital medicine, nursing, respiratory therapy, and hospital operations, holds regular phone huddles to provide support and enlist resources based on identified gaps, which allows the frontline SIU physicians to focus on patient care. The calls initially occurred twice daily, but we transitioned to a once-daily schedule after routines were established and resources were procured.
Communicate With Everyone
Frequent communication with the clinical staff is paramount given the rapidly evolving operational changes and medical management recommendations. The divisional leadership team provides frequent email updates to the attending physicians on clinical shifts to communicate clinical updates, send reminders to conserve personal protective equipment (PPE), and share links to COVID-19 resources.
We use our weekly divisional meetings, now held virtually, to provide updates and to allow staff to ask questions and provide input. These meetings routinely include our nonclinical staff, such as administrative assistants and research coordinators, to ensure all team members’ voices are heard and skill sets are utilized. Our divisional infrastructure promotes dialogue and transparency, which is key to our division’s culture. Applying a learning health network approach has allowed us to generate new ideas, accelerate improvement, and encourage everyone to be a part of our community focused on improving outcomes.6 We continue to leverage this approach in our pandemic response.
One idea generated from this approach prompted us to create a centralized communication forum, using Microsoft Teams, to serve as a repository for the most up-to-date information related to COVID-19, the SIU, and general information, including links to divisional and institutional resources.
Maintain Nonclinical Operations
Nonclinical staff are working remotely. The business director and research director hold daily calls with the administrative staff and research coordinators, respectively, to discuss workload and to reallocate responsibilities as needed. This approach allows the business, administrative, and research support teams to function efficiently and redistribute work as the nonclinical priorities shift to meet divisional needs.
STAFFING
Establish a Backup Pool
We anticipate needing a larger pool of backup providers in the event of ill or quarantined staff or in case of increased patient volumes. The latter may be less likely for pediatric patients based on early studies3-5 but could occur if our free-standing children’s hospital expands to include the care of adult patients. We asked physicians to volunteer for backup shifts to augment our existing “jeopardy” backup system with a greater request to those with a lower clinical full-time equivalence. Each day, two backup shift positions are filled by volunteers, with additional positions added on days when medicine-pediatrics providers are scheduled for shifts in case they are needed at the university (adult) hospital.
Minimize Staffing to Reserve Pool
We monitor census closely on all service lines, including our consult service lines and secondary inpatient site, with plans to dissolve unnecessary consult services and combine medical teams, when feasible, to reduce the risk of staff exposure and maintain reserves. For example, after elective procedures were canceled, we reduced physician staffing of our surgical comanagement service to the minimal necessary coverage. We assign nonpatient-facing clinical duties to physicians who are called off their shift, in quarantine, or mildly ill to help off-load the clinical burden. Such duties include accepting direct admission phone calls, triaging patient care calls, entering orders remotely, and assisting with care coordination needs.
Anticipate Adult Care Needs
Our pediatric institution admits select groups of adult patients with congenital or complex healthcare needs who require specialized care. Hospitalists board certified in both pediatrics and internal medicine provide consultative services to many of these patients. Anticipating that these physicians may be needed in adult facilities, we plan to dissolve this consult service and utilize our reserve pool of providers to cover their pediatric shifts if needed. Additionally, if our hospital expands coverage for adult patients, these medicine-pediatrics providers will be instrumental in coordinating that expanded effort and will serve as leaders for teams of physicians and advanced practice providers with limited or no adult medicine training.
Special Isolation Unit
Logistic planning for our SIU evolved over the first few patients with rapid-cycle feedback and learning with each admission. This feedback was facilitated with our twice-daily huddle calls, which involved all key stakeholders, including nursing and respiratory therapy representatives. For division physician staffing, higher-risk team members are excluded from working on this unit. Because the SIU was developed to care for all patients positive for COVID-19 and persons under investigation, subspecialty patients not typically cared for by Hospital Medicine at our institution are being admitted to this unit. Therefore, subspecialty divisions assign attending physicians to provide consultative services to the SIU. These consultants use the unit’s telemedicine capabilities, when feasible, to limit staff exposure and conserve PPE. Our hospital medicine leaders in the SIU proactively worked with subspecialty divisions that are anticipated to have more admissions given their at-risk patient populations, such as pulmonary medicine, cardiology, and oncology. They specifically developed staffing plans for these patients if the SIU census becomes unsustainable under Hospital Medicine alone.
STAFF WELL-BEING
Healthcare workers are experiencing numerous stressors at work and home during this tumultuous time. Our workforce is at risk of developing emotional distress and mental health concerns. A cross-sectional survey of more than 1,200 healthcare workers in China who cared for COVID-19 patients found that many experienced symptoms of psychological distress (71%), as well as depression (51%), anxiety (44%), and insomnia (34%).7 Hospital medicine groups should consider methods to support their staff to mitigate stressors and promote self-care.
Anticipate Childcare Issues
When we were faced with impending school and daycare closures, we surveyed our division to assess childcare needs (Table) and share resources. We created a system of emergency childcare coverage options by connecting parents with similarly aged children and who lived in geographic proximity. This approach to childcare contingency planning was shared with and adopted by other divisions within the institution.
Build Support Measures
To support each other during this particularly stressful time, we divided division members into groups or “support pods,” each facilitated by a leadership team member. Group text messages and weekly phone or video chats have promoted connectivity and peer support.
Promote Self-care
The divisional leadership team provides food and drink for staff on clinical shifts. We also collated self-care resources to share via a central repository. These resources include ideas for meditation, home education for children, parenting, exercise, faith communities, entertainment, methods to support our local community through volunteerism and donations, and mental health resources, as well as online links to these resources.
Adult health systems will be disproportionately affected as this pandemic evolves. Pediatric hospitalists have the unique opportunity to support the response efforts by maintaining teams that are flexible and adaptable to evolving community needs. To do this, team leaders need to promote transparency, share learnings, and leverage the diverse skills of team members to ensure we are ready to meet the challenges of the moment.
1. World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report - 51. [Situation Report]. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19. Accessed March 26, 2020.
2. Centers for Disease Control and Prevention. Interim Guidance for Healthcare Facilities: Preparing for Community Transmission of COVID-19 in the United States. 2020. https://www.cdc.gov/coronavirus/2019-ncov/healthcare-facilities/guidance-hcf.html. Accessed March 27, 2020.
3. Dong Y, Mo X, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020. https://doi.org/10.1542/peds.2020-0702.
4. Cruz A, Zeichner S. COVID-19 in children: initial characterization of pediatric disease. Pediatrics. 2020;e20200834. https://doi.org/10.1542/peds.2020-0834.
5. Wu Z, McGoogan J. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020. https://doi.org/10.1001/jama.2020.2648.
6. James M Anderson Center of Health Systems Excellence. The Power of Learning Networks. https://www.cincinnatichildrens.org/research/divisions/j/anderson-center/learning-networks. Accessed April 2, 2020.
7. Lai J, Ma S, Wang Y, et al. Factors Associated With Mental Health Outcomes Among Health Care Workers Exposed to Coronavirus Disease 2019. JAMA Netw Open. 2020;3(3):e203976. https://doi.org/10.1001/jamanetworkopen.2020.3976.
MANAGEMENT AND COMMUNICATION
Establish a Command Team
We benefit from having an existing divisional leadership structure comprising the director, medical directors of our clinical service lines, directors of education and community integration, and associate directors of clinical operations, research, and quality. This established team provides us broad representation of team member expertise and ideas. We maintain our weekly leadership team meeting through video chat and have added daily 30-minute virtual huddles to provide updates from our respective areas and discuss logistical challenges and planning. We use ad hoc phone meetings with relevant team members to address issues of immediate concern.
In the absence of a formal leadership team structure, establish a command team comprising representative leaders of your varied groups (eg, clinical operations, quality improvement, education, research, and business).
Collaborate With Institutional Response
Align divisional command team actions with the institutional response. Our clinical operations leader serves as our primary representative on the institutional emergency preparedness team. This participation allows bidirectional communication, both for institutional updates to be shared with division members and division-specific initiatives to be shared with institutional leadership to facilitate learning across the system.
In conjunction with hospital leadership, our division created a special isolation unit (SIU) to isolate patients positive for COVID-19 and persons under investigation. The institutional emergency preparedness team highlighted the need for such a unit, and our divisional leadership team developed the physician staffing model and medical care delivery system. We collaborated with key stakeholders, including nurses, respiratory therapists, other patient care services members, and subspecialists. The SIU leadership, which includes representatives from hospital medicine, nursing, respiratory therapy, and hospital operations, holds regular phone huddles to provide support and enlist resources based on identified gaps, which allows the frontline SIU physicians to focus on patient care. The calls initially occurred twice daily, but we transitioned to a once-daily schedule after routines were established and resources were procured.
Communicate With Everyone
Frequent communication with the clinical staff is paramount given the rapidly evolving operational changes and medical management recommendations. The divisional leadership team provides frequent email updates to the attending physicians on clinical shifts to communicate clinical updates, send reminders to conserve personal protective equipment (PPE), and share links to COVID-19 resources.
We use our weekly divisional meetings, now held virtually, to provide updates and to allow staff to ask questions and provide input. These meetings routinely include our nonclinical staff, such as administrative assistants and research coordinators, to ensure all team members’ voices are heard and skill sets are utilized. Our divisional infrastructure promotes dialogue and transparency, which is key to our division’s culture. Applying a learning health network approach has allowed us to generate new ideas, accelerate improvement, and encourage everyone to be a part of our community focused on improving outcomes.6 We continue to leverage this approach in our pandemic response.
One idea generated from this approach prompted us to create a centralized communication forum, using Microsoft Teams, to serve as a repository for the most up-to-date information related to COVID-19, the SIU, and general information, including links to divisional and institutional resources.
Maintain Nonclinical Operations
Nonclinical staff are working remotely. The business director and research director hold daily calls with the administrative staff and research coordinators, respectively, to discuss workload and to reallocate responsibilities as needed. This approach allows the business, administrative, and research support teams to function efficiently and redistribute work as the nonclinical priorities shift to meet divisional needs.
STAFFING
Establish a Backup Pool
We anticipate needing a larger pool of backup providers in the event of ill or quarantined staff or in case of increased patient volumes. The latter may be less likely for pediatric patients based on early studies3-5 but could occur if our free-standing children’s hospital expands to include the care of adult patients. We asked physicians to volunteer for backup shifts to augment our existing “jeopardy” backup system with a greater request to those with a lower clinical full-time equivalence. Each day, two backup shift positions are filled by volunteers, with additional positions added on days when medicine-pediatrics providers are scheduled for shifts in case they are needed at the university (adult) hospital.
Minimize Staffing to Reserve Pool
We monitor census closely on all service lines, including our consult service lines and secondary inpatient site, with plans to dissolve unnecessary consult services and combine medical teams, when feasible, to reduce the risk of staff exposure and maintain reserves. For example, after elective procedures were canceled, we reduced physician staffing of our surgical comanagement service to the minimal necessary coverage. We assign nonpatient-facing clinical duties to physicians who are called off their shift, in quarantine, or mildly ill to help off-load the clinical burden. Such duties include accepting direct admission phone calls, triaging patient care calls, entering orders remotely, and assisting with care coordination needs.
Anticipate Adult Care Needs
Our pediatric institution admits select groups of adult patients with congenital or complex healthcare needs who require specialized care. Hospitalists board certified in both pediatrics and internal medicine provide consultative services to many of these patients. Anticipating that these physicians may be needed in adult facilities, we plan to dissolve this consult service and utilize our reserve pool of providers to cover their pediatric shifts if needed. Additionally, if our hospital expands coverage for adult patients, these medicine-pediatrics providers will be instrumental in coordinating that expanded effort and will serve as leaders for teams of physicians and advanced practice providers with limited or no adult medicine training.
Special Isolation Unit
Logistic planning for our SIU evolved over the first few patients with rapid-cycle feedback and learning with each admission. This feedback was facilitated with our twice-daily huddle calls, which involved all key stakeholders, including nursing and respiratory therapy representatives. For division physician staffing, higher-risk team members are excluded from working on this unit. Because the SIU was developed to care for all patients positive for COVID-19 and persons under investigation, subspecialty patients not typically cared for by Hospital Medicine at our institution are being admitted to this unit. Therefore, subspecialty divisions assign attending physicians to provide consultative services to the SIU. These consultants use the unit’s telemedicine capabilities, when feasible, to limit staff exposure and conserve PPE. Our hospital medicine leaders in the SIU proactively worked with subspecialty divisions that are anticipated to have more admissions given their at-risk patient populations, such as pulmonary medicine, cardiology, and oncology. They specifically developed staffing plans for these patients if the SIU census becomes unsustainable under Hospital Medicine alone.
STAFF WELL-BEING
Healthcare workers are experiencing numerous stressors at work and home during this tumultuous time. Our workforce is at risk of developing emotional distress and mental health concerns. A cross-sectional survey of more than 1,200 healthcare workers in China who cared for COVID-19 patients found that many experienced symptoms of psychological distress (71%), as well as depression (51%), anxiety (44%), and insomnia (34%).7 Hospital medicine groups should consider methods to support their staff to mitigate stressors and promote self-care.
Anticipate Childcare Issues
When we were faced with impending school and daycare closures, we surveyed our division to assess childcare needs (Table) and share resources. We created a system of emergency childcare coverage options by connecting parents with similarly aged children and who lived in geographic proximity. This approach to childcare contingency planning was shared with and adopted by other divisions within the institution.
Build Support Measures
To support each other during this particularly stressful time, we divided division members into groups or “support pods,” each facilitated by a leadership team member. Group text messages and weekly phone or video chats have promoted connectivity and peer support.
Promote Self-care
The divisional leadership team provides food and drink for staff on clinical shifts. We also collated self-care resources to share via a central repository. These resources include ideas for meditation, home education for children, parenting, exercise, faith communities, entertainment, methods to support our local community through volunteerism and donations, and mental health resources, as well as online links to these resources.
Adult health systems will be disproportionately affected as this pandemic evolves. Pediatric hospitalists have the unique opportunity to support the response efforts by maintaining teams that are flexible and adaptable to evolving community needs. To do this, team leaders need to promote transparency, share learnings, and leverage the diverse skills of team members to ensure we are ready to meet the challenges of the moment.
MANAGEMENT AND COMMUNICATION
Establish a Command Team
We benefit from having an existing divisional leadership structure comprising the director, medical directors of our clinical service lines, directors of education and community integration, and associate directors of clinical operations, research, and quality. This established team provides us broad representation of team member expertise and ideas. We maintain our weekly leadership team meeting through video chat and have added daily 30-minute virtual huddles to provide updates from our respective areas and discuss logistical challenges and planning. We use ad hoc phone meetings with relevant team members to address issues of immediate concern.
In the absence of a formal leadership team structure, establish a command team comprising representative leaders of your varied groups (eg, clinical operations, quality improvement, education, research, and business).
Collaborate With Institutional Response
Align divisional command team actions with the institutional response. Our clinical operations leader serves as our primary representative on the institutional emergency preparedness team. This participation allows bidirectional communication, both for institutional updates to be shared with division members and division-specific initiatives to be shared with institutional leadership to facilitate learning across the system.
In conjunction with hospital leadership, our division created a special isolation unit (SIU) to isolate patients positive for COVID-19 and persons under investigation. The institutional emergency preparedness team highlighted the need for such a unit, and our divisional leadership team developed the physician staffing model and medical care delivery system. We collaborated with key stakeholders, including nurses, respiratory therapists, other patient care services members, and subspecialists. The SIU leadership, which includes representatives from hospital medicine, nursing, respiratory therapy, and hospital operations, holds regular phone huddles to provide support and enlist resources based on identified gaps, which allows the frontline SIU physicians to focus on patient care. The calls initially occurred twice daily, but we transitioned to a once-daily schedule after routines were established and resources were procured.
Communicate With Everyone
Frequent communication with the clinical staff is paramount given the rapidly evolving operational changes and medical management recommendations. The divisional leadership team provides frequent email updates to the attending physicians on clinical shifts to communicate clinical updates, send reminders to conserve personal protective equipment (PPE), and share links to COVID-19 resources.
We use our weekly divisional meetings, now held virtually, to provide updates and to allow staff to ask questions and provide input. These meetings routinely include our nonclinical staff, such as administrative assistants and research coordinators, to ensure all team members’ voices are heard and skill sets are utilized. Our divisional infrastructure promotes dialogue and transparency, which is key to our division’s culture. Applying a learning health network approach has allowed us to generate new ideas, accelerate improvement, and encourage everyone to be a part of our community focused on improving outcomes.6 We continue to leverage this approach in our pandemic response.
One idea generated from this approach prompted us to create a centralized communication forum, using Microsoft Teams, to serve as a repository for the most up-to-date information related to COVID-19, the SIU, and general information, including links to divisional and institutional resources.
Maintain Nonclinical Operations
Nonclinical staff are working remotely. The business director and research director hold daily calls with the administrative staff and research coordinators, respectively, to discuss workload and to reallocate responsibilities as needed. This approach allows the business, administrative, and research support teams to function efficiently and redistribute work as the nonclinical priorities shift to meet divisional needs.
STAFFING
Establish a Backup Pool
We anticipate needing a larger pool of backup providers in the event of ill or quarantined staff or in case of increased patient volumes. The latter may be less likely for pediatric patients based on early studies3-5 but could occur if our free-standing children’s hospital expands to include the care of adult patients. We asked physicians to volunteer for backup shifts to augment our existing “jeopardy” backup system with a greater request to those with a lower clinical full-time equivalence. Each day, two backup shift positions are filled by volunteers, with additional positions added on days when medicine-pediatrics providers are scheduled for shifts in case they are needed at the university (adult) hospital.
Minimize Staffing to Reserve Pool
We monitor census closely on all service lines, including our consult service lines and secondary inpatient site, with plans to dissolve unnecessary consult services and combine medical teams, when feasible, to reduce the risk of staff exposure and maintain reserves. For example, after elective procedures were canceled, we reduced physician staffing of our surgical comanagement service to the minimal necessary coverage. We assign nonpatient-facing clinical duties to physicians who are called off their shift, in quarantine, or mildly ill to help off-load the clinical burden. Such duties include accepting direct admission phone calls, triaging patient care calls, entering orders remotely, and assisting with care coordination needs.
Anticipate Adult Care Needs
Our pediatric institution admits select groups of adult patients with congenital or complex healthcare needs who require specialized care. Hospitalists board certified in both pediatrics and internal medicine provide consultative services to many of these patients. Anticipating that these physicians may be needed in adult facilities, we plan to dissolve this consult service and utilize our reserve pool of providers to cover their pediatric shifts if needed. Additionally, if our hospital expands coverage for adult patients, these medicine-pediatrics providers will be instrumental in coordinating that expanded effort and will serve as leaders for teams of physicians and advanced practice providers with limited or no adult medicine training.
Special Isolation Unit
Logistic planning for our SIU evolved over the first few patients with rapid-cycle feedback and learning with each admission. This feedback was facilitated with our twice-daily huddle calls, which involved all key stakeholders, including nursing and respiratory therapy representatives. For division physician staffing, higher-risk team members are excluded from working on this unit. Because the SIU was developed to care for all patients positive for COVID-19 and persons under investigation, subspecialty patients not typically cared for by Hospital Medicine at our institution are being admitted to this unit. Therefore, subspecialty divisions assign attending physicians to provide consultative services to the SIU. These consultants use the unit’s telemedicine capabilities, when feasible, to limit staff exposure and conserve PPE. Our hospital medicine leaders in the SIU proactively worked with subspecialty divisions that are anticipated to have more admissions given their at-risk patient populations, such as pulmonary medicine, cardiology, and oncology. They specifically developed staffing plans for these patients if the SIU census becomes unsustainable under Hospital Medicine alone.
STAFF WELL-BEING
Healthcare workers are experiencing numerous stressors at work and home during this tumultuous time. Our workforce is at risk of developing emotional distress and mental health concerns. A cross-sectional survey of more than 1,200 healthcare workers in China who cared for COVID-19 patients found that many experienced symptoms of psychological distress (71%), as well as depression (51%), anxiety (44%), and insomnia (34%).7 Hospital medicine groups should consider methods to support their staff to mitigate stressors and promote self-care.
Anticipate Childcare Issues
When we were faced with impending school and daycare closures, we surveyed our division to assess childcare needs (Table) and share resources. We created a system of emergency childcare coverage options by connecting parents with similarly aged children and who lived in geographic proximity. This approach to childcare contingency planning was shared with and adopted by other divisions within the institution.
Build Support Measures
To support each other during this particularly stressful time, we divided division members into groups or “support pods,” each facilitated by a leadership team member. Group text messages and weekly phone or video chats have promoted connectivity and peer support.
Promote Self-care
The divisional leadership team provides food and drink for staff on clinical shifts. We also collated self-care resources to share via a central repository. These resources include ideas for meditation, home education for children, parenting, exercise, faith communities, entertainment, methods to support our local community through volunteerism and donations, and mental health resources, as well as online links to these resources.
Adult health systems will be disproportionately affected as this pandemic evolves. Pediatric hospitalists have the unique opportunity to support the response efforts by maintaining teams that are flexible and adaptable to evolving community needs. To do this, team leaders need to promote transparency, share learnings, and leverage the diverse skills of team members to ensure we are ready to meet the challenges of the moment.
1. World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report - 51. [Situation Report]. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19. Accessed March 26, 2020.
2. Centers for Disease Control and Prevention. Interim Guidance for Healthcare Facilities: Preparing for Community Transmission of COVID-19 in the United States. 2020. https://www.cdc.gov/coronavirus/2019-ncov/healthcare-facilities/guidance-hcf.html. Accessed March 27, 2020.
3. Dong Y, Mo X, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020. https://doi.org/10.1542/peds.2020-0702.
4. Cruz A, Zeichner S. COVID-19 in children: initial characterization of pediatric disease. Pediatrics. 2020;e20200834. https://doi.org/10.1542/peds.2020-0834.
5. Wu Z, McGoogan J. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020. https://doi.org/10.1001/jama.2020.2648.
6. James M Anderson Center of Health Systems Excellence. The Power of Learning Networks. https://www.cincinnatichildrens.org/research/divisions/j/anderson-center/learning-networks. Accessed April 2, 2020.
7. Lai J, Ma S, Wang Y, et al. Factors Associated With Mental Health Outcomes Among Health Care Workers Exposed to Coronavirus Disease 2019. JAMA Netw Open. 2020;3(3):e203976. https://doi.org/10.1001/jamanetworkopen.2020.3976.
1. World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report - 51. [Situation Report]. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19. Accessed March 26, 2020.
2. Centers for Disease Control and Prevention. Interim Guidance for Healthcare Facilities: Preparing for Community Transmission of COVID-19 in the United States. 2020. https://www.cdc.gov/coronavirus/2019-ncov/healthcare-facilities/guidance-hcf.html. Accessed March 27, 2020.
3. Dong Y, Mo X, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020. https://doi.org/10.1542/peds.2020-0702.
4. Cruz A, Zeichner S. COVID-19 in children: initial characterization of pediatric disease. Pediatrics. 2020;e20200834. https://doi.org/10.1542/peds.2020-0834.
5. Wu Z, McGoogan J. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020. https://doi.org/10.1001/jama.2020.2648.
6. James M Anderson Center of Health Systems Excellence. The Power of Learning Networks. https://www.cincinnatichildrens.org/research/divisions/j/anderson-center/learning-networks. Accessed April 2, 2020.
7. Lai J, Ma S, Wang Y, et al. Factors Associated With Mental Health Outcomes Among Health Care Workers Exposed to Coronavirus Disease 2019. JAMA Netw Open. 2020;3(3):e203976. https://doi.org/10.1001/jamanetworkopen.2020.3976.
© 2020 Society of Hospital Medicine
Treatment of Pediatric Venous Thromboembolism
Venous thromboembolism (VTE) occurs uncommonly in pediatrics, affecting 0.07-0.14 per 10,000 children.1,2 Yet, in the last 20 years, the incidence of VTE in hospitalized children has increased dramatically to approximately 58 per 10,000 admissions.3 This increase may be attributed to improved survival of very ill children, better diagnostic imaging modalities, and heightened awareness by managing physicians.3 Randomized controlled trials are lacking in pediatric thrombosis, and clinical care is based on extrapolation of adult data and expert consensus guidelines.4,5 In 2014, the American Society of Hematology (ASH) sought to develop comprehensive guidelines on thrombosis. The pediatric VTE treatment guideline is one of six published to date.
RECOMMENDATIONS FOR THE HOSPITALIST
The following are five selected guideline recommendations thought most relevant to pediatric hospitalists. Three focus on the central venous access device (CVAD), since it is the most common risk factor for pediatric VTE.1 Recommendations were graded as “strong” if most providers, patients, and policy makers agreed with the intervention and if it was supported by credible research. Conditional recommendations had less uniform agreement with an emphasis on individualized care and weighing patients’ values and preferences.6
Recommendation 1. It is recommended that pediatric patients receive anticoagulation, versus no anticoagulation, for symptomatic VTE (evidence quality: low certainty; recommendation strength: strong).
There is strong indirect data in adults that symptomatic VTE requires treatment, with limited direct evidence in children. As VTE occurs most commonly in ill, hospitalized children with the potential for VTE to be life threatening, the benefit was felt to justify the strong recommendation despite low-quality evidence.
The primary benefit of anticoagulation in children with symptomatic VTE is the prevention of progressive or recurrent thrombosis with high morbidity and the prevention of life-threatening VTE. The greatest potential harm from the use of anticoagulation, particularly in very ill children, is the risk for major bleeding.4Recommendation 2. Children with asymptomatic VTE can be managed with or without anticoagulation (evidence quality: poor; recommendation strength: conditional).
Recommendation 2. Children with asymptomatic VTE can be managed with or without anticoagulation (evidence quality: poor; recommendation strength: conditional).
The panel focused on the unique features of pediatric VTE related to the heterogeneity in both the site and pathophysiology of VTE in children, such as age, presence of a CVAD, and comorbidities. There is little certainty that treating asymptomatic VTE is beneficial in the same way that treating symptomatic VTE would be in preventing recurrent thrombosis and embolization.
Until better evidence is available to guide care, the primary benefit of this recommendation is individualization of care related to each patient’s risk-benefit profile and parental preferences.
Potential problems with using this recommendation include the cost of anticoagulant drugs and major bleeding if anticoagulation is used. Potential problems with not using anticoagulation would be progressive or recurrent thromboembolism. Close monitoring of children with VTE—regardless of whether anticoagulation is prescribed—is warranted.
Pediatric Patients with Symptomatic CVAD-Related Thrombosis
Recommendations three through five pertain to CVAD-associated thrombosis, so they are reviewed together.
Recommendation 3. No removal of a functioning CVAD is suggested if venous access is still required (evidence quality: low certainty; recommendation strength: conditional).Recommendation 4. It is recommended to remove a nonfunctioning or unneeded CVAD (evidence quality: low certainty; recommendation strength: strong).Recommendation 5. It is suggested to delay CVAD removal until after initiation of anticoagulation (days), rather than immediate removal if the CVAD is nonfunctioning or no longer needed (evidence quality: low certainty; recommendation strength: conditional).
Recommendation 4. It is recommended to remove a nonfunctioning or unneeded CVAD (evidence quality: low certainty; recommendation strength: strong).
Recommendation 5. It is suggested to delay CVAD removal until after initiation of anticoagulation (days), rather than immediate removal if the CVAD is nonfunctioning or no longer needed (evidence quality: low certainty; recommendation strength: conditional).
CVAD is the most common precipitating factor for pediatric VTE, particularly in neonates and older children.1 Based on limited direct and indirect observational studies, there is low evidence of benefit for CVAD removal, but high-quality indirect evidence of harm and high cost, which the panel felt justified the strong recommendation for removing an unneeded or nonfunctioning line. If ongoing care can be safely administered without central access, removing the thrombosis stimulus is recommended. The guideline suggests keeping a functioning CVAD in a patient who requires ongoing venous access and placing high value on avoiding new line insertion when access sites may be limited to avoid the potential thrombogenic effect of new line placement.
In the limited direct and indirect observational studies identified, the optimal timing of CVAD removal is uncertain. Given the potential risk of emboli leading to pulmonary embolism or stroke, prior publications have suggested delaying removal until after three to five days of anticoagulation, particularly in children with known or potential right-to-left shunts.4 The risk of infection and bleeding with anticoagulation prior to CVAD removal was considered small by the panel. This recommendation is primarily based on the panel’s anecdotal experience and first principles, which is a limitation.
CRITIQUE
Methods in Preparing Guideline. The panel included pediatric experts with clinical and research expertise in the guideline topic, including nine hematologists, one intensivist, one cardiologist, one hematology pharmacist, and one anticoagulation nurse practitioner. It also included two methodologists with evidence appraisal and guideline development expertise, as well as two patient representatives.
The panel brainstormed and prioritized questions to be addressed and selected outcomes of interest for each question. The McMaster University GRADE Centre vetted and retained researchers to conduct or update systematic evidence reviews and coordinate the guideline development using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach.6 For each guideline question, the results of systematic reviews were summarized in GRADE Evidence-to-Decision tables. The evidence quality was categorized into four levels ranging from ver
Draft recommendations were made available online for review by stakeholders, including allied organizations, medical professionals, patients, and the public. Revisions were made to address pertinent submitted comments, but the recommendations were not changed. After approval by ASH, the guideline was subjected to peer review by Blood Advances.
Sources of Potential Conflict of Interest or Bias. The guideline was developed and funded by ASH. All participants’ conflicts of interest were managed according to ASH policies based on recommendations of the Institute of Medicine and the Guideline International Network. A majority of the guideline panel had no conflicts. During deliberations, panelists with direct financial interests were recused from making judgments about relevant recommendations. The McMaster University-affiliated researchers had no conflicts.Generalizability. While this guideline included 30 recommendations, the ones highlighted apply to the most commonly seen pediatric VTE cases in hospital medicine. ASH emphasized that these guidelines should not be construed as the standard of care, but as a guide to help clinicians make treatment decisions for children with VTE and to enable them to individualize care when needed. The greatest limitation of this guideline is the lack of strong direct supporting evidence in pediatric VTE management.
Generalizability. While this guideline included 30 recommendations, the ones highlighted apply to the most commonly seen pediatric VTE cases in hospital medicine. ASH emphasized that these guidelines should not be construed as the standard of care, but as a guide to help clinicians make treatment decisions for children with VTE and to enable them to individualize care when needed. The greatest limitation of this guideline is the lack of strong direct supporting evidence in pediatric VTE management.
AREAS IN NEED OF FUTURE STUDY
Although there is increasing interest in pediatric VTE prevention and risk assessment,7 there is currently limited evidence on the best ways to mitigate VTE risk or anticoagulation-associated major bleeding in hospitalized children. The relatively low incidence of VTE in children makes large randomized controlled trials difficult, but several are ongoing. The Evaluation of the Duration of Therapy for Thrombosis in Children (Kids-DOTT) multicenter, randomized trial will inform care on the optimal duration of anticoagulation in children with a transient provoking factor,8 and several phase III studies are investigating the safety and efficacy of direct oral anticoagulants in children (NCT02234843, NCT02464969, NCT01895777, NCT02234843). These and future trials will better inform therapy in pediatric VTE.
Disclosures
The authors have no financial relationships or conflicts of interest relevant to this article to disclose.
Funding
No funding was secured for this study.
1. Andrew M, David M, Adams M, et al. Venous thromboembolic complications (VTE) in children: first analyses of the Canadian registry of VTE. Blood. 1994;83(5):1251-1257. PubMed
2. van Ommen CH, Heijboer H, Buller HR, Hirasing RA, Heijmans HS, Peters M. Venous thromboembolism in childhood: a prospective two-year registry in the Netherlands. J Pediatr. 2001;139(5):676-681. https://doi.org/10.1067/mpd.2001.118192.
3. Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124(4):1001-1008. https://doi.org/10.1542/peds.2009-0768.
4. Monagle P, Chan AK, Goldenberg NA, et al. Antithrombotic therapy in neonates and children: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2):e737S-e801S. https://doi.org/10.1378/chest.11-2308.
5. Monagle P, Cuello CA, Augustine C, et al. American Society of Hematology 2018 Guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. 2018;2(22):3292-3316. https://doi.org/10.1182/bloodadvances.2018024786.
6. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. https://doi.org/10.1136/bmj.39489.470347.AD.
7. Faustino EV, Raffini LJ. Prevention of hospital-acquired venous thromboembolism in children: a review of published guidelines. Front Pediatr. 2017;5(9):1597-605. https://doi.org/10.3389/fped.2017.00009.8. Goldenberg NA, Abshire T, Blatchford PJ, et al. Multicenter randomized controlled trial on Duration of Therapy for Thrombosis in Children and Young Adults (the Kids-DOTT trial): pilot/feasibility phase findings. J Thromb Haemost. 2015;13(9):1597-1605. https://doi.org/10.1111/jth.13038.
Venous thromboembolism (VTE) occurs uncommonly in pediatrics, affecting 0.07-0.14 per 10,000 children.1,2 Yet, in the last 20 years, the incidence of VTE in hospitalized children has increased dramatically to approximately 58 per 10,000 admissions.3 This increase may be attributed to improved survival of very ill children, better diagnostic imaging modalities, and heightened awareness by managing physicians.3 Randomized controlled trials are lacking in pediatric thrombosis, and clinical care is based on extrapolation of adult data and expert consensus guidelines.4,5 In 2014, the American Society of Hematology (ASH) sought to develop comprehensive guidelines on thrombosis. The pediatric VTE treatment guideline is one of six published to date.
RECOMMENDATIONS FOR THE HOSPITALIST
The following are five selected guideline recommendations thought most relevant to pediatric hospitalists. Three focus on the central venous access device (CVAD), since it is the most common risk factor for pediatric VTE.1 Recommendations were graded as “strong” if most providers, patients, and policy makers agreed with the intervention and if it was supported by credible research. Conditional recommendations had less uniform agreement with an emphasis on individualized care and weighing patients’ values and preferences.6
Recommendation 1. It is recommended that pediatric patients receive anticoagulation, versus no anticoagulation, for symptomatic VTE (evidence quality: low certainty; recommendation strength: strong).
There is strong indirect data in adults that symptomatic VTE requires treatment, with limited direct evidence in children. As VTE occurs most commonly in ill, hospitalized children with the potential for VTE to be life threatening, the benefit was felt to justify the strong recommendation despite low-quality evidence.
The primary benefit of anticoagulation in children with symptomatic VTE is the prevention of progressive or recurrent thrombosis with high morbidity and the prevention of life-threatening VTE. The greatest potential harm from the use of anticoagulation, particularly in very ill children, is the risk for major bleeding.4Recommendation 2. Children with asymptomatic VTE can be managed with or without anticoagulation (evidence quality: poor; recommendation strength: conditional).
Recommendation 2. Children with asymptomatic VTE can be managed with or without anticoagulation (evidence quality: poor; recommendation strength: conditional).
The panel focused on the unique features of pediatric VTE related to the heterogeneity in both the site and pathophysiology of VTE in children, such as age, presence of a CVAD, and comorbidities. There is little certainty that treating asymptomatic VTE is beneficial in the same way that treating symptomatic VTE would be in preventing recurrent thrombosis and embolization.
Until better evidence is available to guide care, the primary benefit of this recommendation is individualization of care related to each patient’s risk-benefit profile and parental preferences.
Potential problems with using this recommendation include the cost of anticoagulant drugs and major bleeding if anticoagulation is used. Potential problems with not using anticoagulation would be progressive or recurrent thromboembolism. Close monitoring of children with VTE—regardless of whether anticoagulation is prescribed—is warranted.
Pediatric Patients with Symptomatic CVAD-Related Thrombosis
Recommendations three through five pertain to CVAD-associated thrombosis, so they are reviewed together.
Recommendation 3. No removal of a functioning CVAD is suggested if venous access is still required (evidence quality: low certainty; recommendation strength: conditional).Recommendation 4. It is recommended to remove a nonfunctioning or unneeded CVAD (evidence quality: low certainty; recommendation strength: strong).Recommendation 5. It is suggested to delay CVAD removal until after initiation of anticoagulation (days), rather than immediate removal if the CVAD is nonfunctioning or no longer needed (evidence quality: low certainty; recommendation strength: conditional).
Recommendation 4. It is recommended to remove a nonfunctioning or unneeded CVAD (evidence quality: low certainty; recommendation strength: strong).
Recommendation 5. It is suggested to delay CVAD removal until after initiation of anticoagulation (days), rather than immediate removal if the CVAD is nonfunctioning or no longer needed (evidence quality: low certainty; recommendation strength: conditional).
CVAD is the most common precipitating factor for pediatric VTE, particularly in neonates and older children.1 Based on limited direct and indirect observational studies, there is low evidence of benefit for CVAD removal, but high-quality indirect evidence of harm and high cost, which the panel felt justified the strong recommendation for removing an unneeded or nonfunctioning line. If ongoing care can be safely administered without central access, removing the thrombosis stimulus is recommended. The guideline suggests keeping a functioning CVAD in a patient who requires ongoing venous access and placing high value on avoiding new line insertion when access sites may be limited to avoid the potential thrombogenic effect of new line placement.
In the limited direct and indirect observational studies identified, the optimal timing of CVAD removal is uncertain. Given the potential risk of emboli leading to pulmonary embolism or stroke, prior publications have suggested delaying removal until after three to five days of anticoagulation, particularly in children with known or potential right-to-left shunts.4 The risk of infection and bleeding with anticoagulation prior to CVAD removal was considered small by the panel. This recommendation is primarily based on the panel’s anecdotal experience and first principles, which is a limitation.
CRITIQUE
Methods in Preparing Guideline. The panel included pediatric experts with clinical and research expertise in the guideline topic, including nine hematologists, one intensivist, one cardiologist, one hematology pharmacist, and one anticoagulation nurse practitioner. It also included two methodologists with evidence appraisal and guideline development expertise, as well as two patient representatives.
The panel brainstormed and prioritized questions to be addressed and selected outcomes of interest for each question. The McMaster University GRADE Centre vetted and retained researchers to conduct or update systematic evidence reviews and coordinate the guideline development using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach.6 For each guideline question, the results of systematic reviews were summarized in GRADE Evidence-to-Decision tables. The evidence quality was categorized into four levels ranging from ver
Draft recommendations were made available online for review by stakeholders, including allied organizations, medical professionals, patients, and the public. Revisions were made to address pertinent submitted comments, but the recommendations were not changed. After approval by ASH, the guideline was subjected to peer review by Blood Advances.
Sources of Potential Conflict of Interest or Bias. The guideline was developed and funded by ASH. All participants’ conflicts of interest were managed according to ASH policies based on recommendations of the Institute of Medicine and the Guideline International Network. A majority of the guideline panel had no conflicts. During deliberations, panelists with direct financial interests were recused from making judgments about relevant recommendations. The McMaster University-affiliated researchers had no conflicts.Generalizability. While this guideline included 30 recommendations, the ones highlighted apply to the most commonly seen pediatric VTE cases in hospital medicine. ASH emphasized that these guidelines should not be construed as the standard of care, but as a guide to help clinicians make treatment decisions for children with VTE and to enable them to individualize care when needed. The greatest limitation of this guideline is the lack of strong direct supporting evidence in pediatric VTE management.
Generalizability. While this guideline included 30 recommendations, the ones highlighted apply to the most commonly seen pediatric VTE cases in hospital medicine. ASH emphasized that these guidelines should not be construed as the standard of care, but as a guide to help clinicians make treatment decisions for children with VTE and to enable them to individualize care when needed. The greatest limitation of this guideline is the lack of strong direct supporting evidence in pediatric VTE management.
AREAS IN NEED OF FUTURE STUDY
Although there is increasing interest in pediatric VTE prevention and risk assessment,7 there is currently limited evidence on the best ways to mitigate VTE risk or anticoagulation-associated major bleeding in hospitalized children. The relatively low incidence of VTE in children makes large randomized controlled trials difficult, but several are ongoing. The Evaluation of the Duration of Therapy for Thrombosis in Children (Kids-DOTT) multicenter, randomized trial will inform care on the optimal duration of anticoagulation in children with a transient provoking factor,8 and several phase III studies are investigating the safety and efficacy of direct oral anticoagulants in children (NCT02234843, NCT02464969, NCT01895777, NCT02234843). These and future trials will better inform therapy in pediatric VTE.
Disclosures
The authors have no financial relationships or conflicts of interest relevant to this article to disclose.
Funding
No funding was secured for this study.
Venous thromboembolism (VTE) occurs uncommonly in pediatrics, affecting 0.07-0.14 per 10,000 children.1,2 Yet, in the last 20 years, the incidence of VTE in hospitalized children has increased dramatically to approximately 58 per 10,000 admissions.3 This increase may be attributed to improved survival of very ill children, better diagnostic imaging modalities, and heightened awareness by managing physicians.3 Randomized controlled trials are lacking in pediatric thrombosis, and clinical care is based on extrapolation of adult data and expert consensus guidelines.4,5 In 2014, the American Society of Hematology (ASH) sought to develop comprehensive guidelines on thrombosis. The pediatric VTE treatment guideline is one of six published to date.
RECOMMENDATIONS FOR THE HOSPITALIST
The following are five selected guideline recommendations thought most relevant to pediatric hospitalists. Three focus on the central venous access device (CVAD), since it is the most common risk factor for pediatric VTE.1 Recommendations were graded as “strong” if most providers, patients, and policy makers agreed with the intervention and if it was supported by credible research. Conditional recommendations had less uniform agreement with an emphasis on individualized care and weighing patients’ values and preferences.6
Recommendation 1. It is recommended that pediatric patients receive anticoagulation, versus no anticoagulation, for symptomatic VTE (evidence quality: low certainty; recommendation strength: strong).
There is strong indirect data in adults that symptomatic VTE requires treatment, with limited direct evidence in children. As VTE occurs most commonly in ill, hospitalized children with the potential for VTE to be life threatening, the benefit was felt to justify the strong recommendation despite low-quality evidence.
The primary benefit of anticoagulation in children with symptomatic VTE is the prevention of progressive or recurrent thrombosis with high morbidity and the prevention of life-threatening VTE. The greatest potential harm from the use of anticoagulation, particularly in very ill children, is the risk for major bleeding.4Recommendation 2. Children with asymptomatic VTE can be managed with or without anticoagulation (evidence quality: poor; recommendation strength: conditional).
Recommendation 2. Children with asymptomatic VTE can be managed with or without anticoagulation (evidence quality: poor; recommendation strength: conditional).
The panel focused on the unique features of pediatric VTE related to the heterogeneity in both the site and pathophysiology of VTE in children, such as age, presence of a CVAD, and comorbidities. There is little certainty that treating asymptomatic VTE is beneficial in the same way that treating symptomatic VTE would be in preventing recurrent thrombosis and embolization.
Until better evidence is available to guide care, the primary benefit of this recommendation is individualization of care related to each patient’s risk-benefit profile and parental preferences.
Potential problems with using this recommendation include the cost of anticoagulant drugs and major bleeding if anticoagulation is used. Potential problems with not using anticoagulation would be progressive or recurrent thromboembolism. Close monitoring of children with VTE—regardless of whether anticoagulation is prescribed—is warranted.
Pediatric Patients with Symptomatic CVAD-Related Thrombosis
Recommendations three through five pertain to CVAD-associated thrombosis, so they are reviewed together.
Recommendation 3. No removal of a functioning CVAD is suggested if venous access is still required (evidence quality: low certainty; recommendation strength: conditional).Recommendation 4. It is recommended to remove a nonfunctioning or unneeded CVAD (evidence quality: low certainty; recommendation strength: strong).Recommendation 5. It is suggested to delay CVAD removal until after initiation of anticoagulation (days), rather than immediate removal if the CVAD is nonfunctioning or no longer needed (evidence quality: low certainty; recommendation strength: conditional).
Recommendation 4. It is recommended to remove a nonfunctioning or unneeded CVAD (evidence quality: low certainty; recommendation strength: strong).
Recommendation 5. It is suggested to delay CVAD removal until after initiation of anticoagulation (days), rather than immediate removal if the CVAD is nonfunctioning or no longer needed (evidence quality: low certainty; recommendation strength: conditional).
CVAD is the most common precipitating factor for pediatric VTE, particularly in neonates and older children.1 Based on limited direct and indirect observational studies, there is low evidence of benefit for CVAD removal, but high-quality indirect evidence of harm and high cost, which the panel felt justified the strong recommendation for removing an unneeded or nonfunctioning line. If ongoing care can be safely administered without central access, removing the thrombosis stimulus is recommended. The guideline suggests keeping a functioning CVAD in a patient who requires ongoing venous access and placing high value on avoiding new line insertion when access sites may be limited to avoid the potential thrombogenic effect of new line placement.
In the limited direct and indirect observational studies identified, the optimal timing of CVAD removal is uncertain. Given the potential risk of emboli leading to pulmonary embolism or stroke, prior publications have suggested delaying removal until after three to five days of anticoagulation, particularly in children with known or potential right-to-left shunts.4 The risk of infection and bleeding with anticoagulation prior to CVAD removal was considered small by the panel. This recommendation is primarily based on the panel’s anecdotal experience and first principles, which is a limitation.
CRITIQUE
Methods in Preparing Guideline. The panel included pediatric experts with clinical and research expertise in the guideline topic, including nine hematologists, one intensivist, one cardiologist, one hematology pharmacist, and one anticoagulation nurse practitioner. It also included two methodologists with evidence appraisal and guideline development expertise, as well as two patient representatives.
The panel brainstormed and prioritized questions to be addressed and selected outcomes of interest for each question. The McMaster University GRADE Centre vetted and retained researchers to conduct or update systematic evidence reviews and coordinate the guideline development using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach.6 For each guideline question, the results of systematic reviews were summarized in GRADE Evidence-to-Decision tables. The evidence quality was categorized into four levels ranging from ver
Draft recommendations were made available online for review by stakeholders, including allied organizations, medical professionals, patients, and the public. Revisions were made to address pertinent submitted comments, but the recommendations were not changed. After approval by ASH, the guideline was subjected to peer review by Blood Advances.
Sources of Potential Conflict of Interest or Bias. The guideline was developed and funded by ASH. All participants’ conflicts of interest were managed according to ASH policies based on recommendations of the Institute of Medicine and the Guideline International Network. A majority of the guideline panel had no conflicts. During deliberations, panelists with direct financial interests were recused from making judgments about relevant recommendations. The McMaster University-affiliated researchers had no conflicts.Generalizability. While this guideline included 30 recommendations, the ones highlighted apply to the most commonly seen pediatric VTE cases in hospital medicine. ASH emphasized that these guidelines should not be construed as the standard of care, but as a guide to help clinicians make treatment decisions for children with VTE and to enable them to individualize care when needed. The greatest limitation of this guideline is the lack of strong direct supporting evidence in pediatric VTE management.
Generalizability. While this guideline included 30 recommendations, the ones highlighted apply to the most commonly seen pediatric VTE cases in hospital medicine. ASH emphasized that these guidelines should not be construed as the standard of care, but as a guide to help clinicians make treatment decisions for children with VTE and to enable them to individualize care when needed. The greatest limitation of this guideline is the lack of strong direct supporting evidence in pediatric VTE management.
AREAS IN NEED OF FUTURE STUDY
Although there is increasing interest in pediatric VTE prevention and risk assessment,7 there is currently limited evidence on the best ways to mitigate VTE risk or anticoagulation-associated major bleeding in hospitalized children. The relatively low incidence of VTE in children makes large randomized controlled trials difficult, but several are ongoing. The Evaluation of the Duration of Therapy for Thrombosis in Children (Kids-DOTT) multicenter, randomized trial will inform care on the optimal duration of anticoagulation in children with a transient provoking factor,8 and several phase III studies are investigating the safety and efficacy of direct oral anticoagulants in children (NCT02234843, NCT02464969, NCT01895777, NCT02234843). These and future trials will better inform therapy in pediatric VTE.
Disclosures
The authors have no financial relationships or conflicts of interest relevant to this article to disclose.
Funding
No funding was secured for this study.
1. Andrew M, David M, Adams M, et al. Venous thromboembolic complications (VTE) in children: first analyses of the Canadian registry of VTE. Blood. 1994;83(5):1251-1257. PubMed
2. van Ommen CH, Heijboer H, Buller HR, Hirasing RA, Heijmans HS, Peters M. Venous thromboembolism in childhood: a prospective two-year registry in the Netherlands. J Pediatr. 2001;139(5):676-681. https://doi.org/10.1067/mpd.2001.118192.
3. Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124(4):1001-1008. https://doi.org/10.1542/peds.2009-0768.
4. Monagle P, Chan AK, Goldenberg NA, et al. Antithrombotic therapy in neonates and children: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2):e737S-e801S. https://doi.org/10.1378/chest.11-2308.
5. Monagle P, Cuello CA, Augustine C, et al. American Society of Hematology 2018 Guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. 2018;2(22):3292-3316. https://doi.org/10.1182/bloodadvances.2018024786.
6. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. https://doi.org/10.1136/bmj.39489.470347.AD.
7. Faustino EV, Raffini LJ. Prevention of hospital-acquired venous thromboembolism in children: a review of published guidelines. Front Pediatr. 2017;5(9):1597-605. https://doi.org/10.3389/fped.2017.00009.8. Goldenberg NA, Abshire T, Blatchford PJ, et al. Multicenter randomized controlled trial on Duration of Therapy for Thrombosis in Children and Young Adults (the Kids-DOTT trial): pilot/feasibility phase findings. J Thromb Haemost. 2015;13(9):1597-1605. https://doi.org/10.1111/jth.13038.
1. Andrew M, David M, Adams M, et al. Venous thromboembolic complications (VTE) in children: first analyses of the Canadian registry of VTE. Blood. 1994;83(5):1251-1257. PubMed
2. van Ommen CH, Heijboer H, Buller HR, Hirasing RA, Heijmans HS, Peters M. Venous thromboembolism in childhood: a prospective two-year registry in the Netherlands. J Pediatr. 2001;139(5):676-681. https://doi.org/10.1067/mpd.2001.118192.
3. Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124(4):1001-1008. https://doi.org/10.1542/peds.2009-0768.
4. Monagle P, Chan AK, Goldenberg NA, et al. Antithrombotic therapy in neonates and children: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2):e737S-e801S. https://doi.org/10.1378/chest.11-2308.
5. Monagle P, Cuello CA, Augustine C, et al. American Society of Hematology 2018 Guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. 2018;2(22):3292-3316. https://doi.org/10.1182/bloodadvances.2018024786.
6. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. https://doi.org/10.1136/bmj.39489.470347.AD.
7. Faustino EV, Raffini LJ. Prevention of hospital-acquired venous thromboembolism in children: a review of published guidelines. Front Pediatr. 2017;5(9):1597-605. https://doi.org/10.3389/fped.2017.00009.8. Goldenberg NA, Abshire T, Blatchford PJ, et al. Multicenter randomized controlled trial on Duration of Therapy for Thrombosis in Children and Young Adults (the Kids-DOTT trial): pilot/feasibility phase findings. J Thromb Haemost. 2015;13(9):1597-1605. https://doi.org/10.1111/jth.13038.
© 2019 Society of Hospital Medicine
