User login
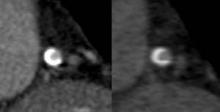
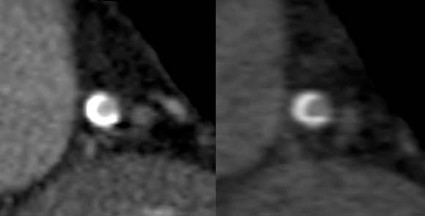
CTA Lacks Accuracy for Assessing In-Stent Restenosis
BALTIMORE – CT angiography is not accurate enough to assess in-stent restenosis, compared with invasive coronary angiography, based on the results of a meta-analysis.
In 11 studies with data on the patient level (804 patients with 1,295 stents), the pooled sensitivity of CT was 94% and the specificity was 92%. In six studies using 64-row or greater CT, the pooled sensitivity of CT was 94% and specificity of CT was 94%. However, the difference compared with all 11 patient-level studies was not significant.
They also looked at the influence of stent size on CT accuracy in eight studies with 1,246 stents. Sensitivity was 81% for stents less than 3 mm and 92% for stents at least 3 mm in size. Specificity was 84% for stents less than 3 mm and 98% for stents at least 3 mm in size.
"In other words, the type of stent and the characteristics that the patient brings with him or her is much more important than the type of scanner that you have sitting there," said Dr. Marc Dewey of Charité – Universitätsmedizin Berlin. The findings were presented at the annual meeting of the Society of Cardiovascular Computed Tomography.
The researchers included prospective studies using at least 12-row CT scanners. Patients were assessed for 50% diameter in-stent restenosis as clinically relevant. All patients had to have coronary angiography.
The researchers included several databases: MEDLINE, EMBASE, ISI’s Web of Science (now Thomson Reuters Web of Knowledge) and the Cochrane Library. Search terms included "computed tomography," "coronary angiography," and "stent." Overall, they identified 33 studies with stent-level data.
Based on likelihood ratios, "we couldn’t conclude that CT was reliable in ruling in and ruling out coronary stent restenosis," said Dr. Dewey.
The investigators also looked at nondiagnostic studies. There were 30 studies with information on the stent level and 17 studies with information on the patient level. They used a missing data technique to assess for less-than-perfect reporting on the study level. The nondiagnostic rate on the patient level was 6% on average, based on the reported results, but the confidence intervals went up to more than 13%. "So almost every six or seven patients would be nondiagnostic."
"Coronary stent imaging by CT is slightly better using 64-row CT and above, though this was not significant. On the other hand, patients with stents that were at least 3 mm or larger could be significantly better assessed. Specificity in particular was significantly improved," said Dr. Dewey.
On the basis of these findings, CT should not be recommended for the assessment of coronary stent restenosis.
The study was funded by the German Research Foundation. The investigators did not report whether they have any significant conflicts of interest.
BALTIMORE – CT angiography is not accurate enough to assess in-stent restenosis, compared with invasive coronary angiography, based on the results of a meta-analysis.
In 11 studies with data on the patient level (804 patients with 1,295 stents), the pooled sensitivity of CT was 94% and the specificity was 92%. In six studies using 64-row or greater CT, the pooled sensitivity of CT was 94% and specificity of CT was 94%. However, the difference compared with all 11 patient-level studies was not significant.
They also looked at the influence of stent size on CT accuracy in eight studies with 1,246 stents. Sensitivity was 81% for stents less than 3 mm and 92% for stents at least 3 mm in size. Specificity was 84% for stents less than 3 mm and 98% for stents at least 3 mm in size.
"In other words, the type of stent and the characteristics that the patient brings with him or her is much more important than the type of scanner that you have sitting there," said Dr. Marc Dewey of Charité – Universitätsmedizin Berlin. The findings were presented at the annual meeting of the Society of Cardiovascular Computed Tomography.
The researchers included prospective studies using at least 12-row CT scanners. Patients were assessed for 50% diameter in-stent restenosis as clinically relevant. All patients had to have coronary angiography.
The researchers included several databases: MEDLINE, EMBASE, ISI’s Web of Science (now Thomson Reuters Web of Knowledge) and the Cochrane Library. Search terms included "computed tomography," "coronary angiography," and "stent." Overall, they identified 33 studies with stent-level data.
Based on likelihood ratios, "we couldn’t conclude that CT was reliable in ruling in and ruling out coronary stent restenosis," said Dr. Dewey.
The investigators also looked at nondiagnostic studies. There were 30 studies with information on the stent level and 17 studies with information on the patient level. They used a missing data technique to assess for less-than-perfect reporting on the study level. The nondiagnostic rate on the patient level was 6% on average, based on the reported results, but the confidence intervals went up to more than 13%. "So almost every six or seven patients would be nondiagnostic."
"Coronary stent imaging by CT is slightly better using 64-row CT and above, though this was not significant. On the other hand, patients with stents that were at least 3 mm or larger could be significantly better assessed. Specificity in particular was significantly improved," said Dr. Dewey.
On the basis of these findings, CT should not be recommended for the assessment of coronary stent restenosis.
The study was funded by the German Research Foundation. The investigators did not report whether they have any significant conflicts of interest.
BALTIMORE – CT angiography is not accurate enough to assess in-stent restenosis, compared with invasive coronary angiography, based on the results of a meta-analysis.
In 11 studies with data on the patient level (804 patients with 1,295 stents), the pooled sensitivity of CT was 94% and the specificity was 92%. In six studies using 64-row or greater CT, the pooled sensitivity of CT was 94% and specificity of CT was 94%. However, the difference compared with all 11 patient-level studies was not significant.
They also looked at the influence of stent size on CT accuracy in eight studies with 1,246 stents. Sensitivity was 81% for stents less than 3 mm and 92% for stents at least 3 mm in size. Specificity was 84% for stents less than 3 mm and 98% for stents at least 3 mm in size.
"In other words, the type of stent and the characteristics that the patient brings with him or her is much more important than the type of scanner that you have sitting there," said Dr. Marc Dewey of Charité – Universitätsmedizin Berlin. The findings were presented at the annual meeting of the Society of Cardiovascular Computed Tomography.
The researchers included prospective studies using at least 12-row CT scanners. Patients were assessed for 50% diameter in-stent restenosis as clinically relevant. All patients had to have coronary angiography.
The researchers included several databases: MEDLINE, EMBASE, ISI’s Web of Science (now Thomson Reuters Web of Knowledge) and the Cochrane Library. Search terms included "computed tomography," "coronary angiography," and "stent." Overall, they identified 33 studies with stent-level data.
Based on likelihood ratios, "we couldn’t conclude that CT was reliable in ruling in and ruling out coronary stent restenosis," said Dr. Dewey.
The investigators also looked at nondiagnostic studies. There were 30 studies with information on the stent level and 17 studies with information on the patient level. They used a missing data technique to assess for less-than-perfect reporting on the study level. The nondiagnostic rate on the patient level was 6% on average, based on the reported results, but the confidence intervals went up to more than 13%. "So almost every six or seven patients would be nondiagnostic."
"Coronary stent imaging by CT is slightly better using 64-row CT and above, though this was not significant. On the other hand, patients with stents that were at least 3 mm or larger could be significantly better assessed. Specificity in particular was significantly improved," said Dr. Dewey.
On the basis of these findings, CT should not be recommended for the assessment of coronary stent restenosis.
The study was funded by the German Research Foundation. The investigators did not report whether they have any significant conflicts of interest.
FROM THE ANNUAL MEETING OF THE SOCIETY OF CARDIOVASCULAR COMPUTED TOMOGRAPHY
Major Finding: In 11 studies with data on the patient level (804 patients with 1,295 stents), the pooled sensitivity of CT was 94% and the specificity was 92%, compared with coronary angiography. In six studies using 64-row or greater CT, the pooled sensitivity of CT was 94% and specificity of CT was 94%.
Data Source: A meta-analysis of 33 prospective studies in which at least 12-row CT scanners were used. Patients were assessed for 50% diameter in-stent restenosis as clinically relevant. All patients had to have coronary angiography.
Disclosures: The study was funded by the German Research Foundation. The investigators did not report whether they have any significant conflicts of interest.
Hospital Volume Not Equal to Quality?
SAN FRANCISCO - Hospital procedure volume, which is commonly used as a proxy measure for hospital quality, is not significantly associated with in-hospital mortality for four common surgical procedures, based on a rigorous statistical analysis of data from the Nationwide Inpatient Sample.
Furthermore, "no identifiable threshold values exist for hospital procedure volume at which mortality risk significantly increased. Mortality risk was primarily attributable to patient-level risk factors," said Dr. Damian J. LaPar of the University of Virginia, Charlottesville.
He and his colleagues examined the relative strength of association between hospital volume and mortality vs. other modeled variables by comparing model covariate likelihood ratios for four high-risk procedures: pancreatic resection, abdominal aortic aneurysm (AAA) repair, esophageal resection, and coronary artery bypass graft (CABG).
Using data from the Nationwide Inpatient Sample in 2008, they obtained weighted discharge records for 261,142 patients: 19,194 patients who had pancreatic resection, 15,266 who had AAA repair, 4,764 who had esophageal resection, and 222,122 who had CABG. The primary outcome of interest was the estimated risk-adjusted effect of hospital procedure volume on mortality (in-hospital death). Comorbid disease was assessed based on Agency for Healthcare Research and Quality (AHRQ) comorbidity categories.
"In all four models, hospital volume was associated with the lowest statistical strength of association with mortality," compared with all other factors, Dr. LaPar said at the annual meeting of the American Surgical Association. Alternatively, other operation and patient-related risk factors -- including elective vs. nonelective status, age, sex, hypertension, weight loss, heart failure, chronic obstructive pulmonary disease, liver disease, and renal failure -- had higher strengths of association with mortality.
Dr. LaPar noted that procedure volume is an attractive metric for regulatory bodies to use as a predictor of outcomes; it is easy to measure and intuitive in nature. In addition, higher-volume hospitals are more likely to have established system-based processes and the infrastructure in place to improve patient outcomes.
The Leapfrog Group and the AHRQ both have adopted procedure volume as a quality indicator for the four high-risk surgical procedures. Arbitrarily defined volume thresholds have been adopted as a metric of quality for these procedures. However, many previous statistical methods that are used to define these thresholds have drawn criticism in the recent surgical literature. In many former series, volume is represented as arbitrarily defined categories, rather than as a continuous variable. Furthermore, there has been little attempt to rigorously assess and compare statistical model performance; to assess the relative strength of the association of procedure volume with other outcome predictors; and to utilize hierarchical, multilevel, statistical modeling techniques for complex, multicenter patient samples.
Dr. LaPar and his colleagues used hierarchical general linear modeling and created separate models for each procedure, which were adjusted for patient and operative factors as potential confounders. Patient factors included age, sex, and comorbid disease. Operative factors included procedure volume and elective/nonelective status. All model covariates were selected a priori.
The researchers used hospital volume as a continuous variable with restricted cubic spline regression, which uses all data points to estimate the shape of the association between hospital volume and mortality, and is considered to be the best way to visually identify threshold values. They also assessed the relative strength of association between hospital volume and mortality, compared with other factors (likelihood ratio). Model performance was assessed by looking at discrimination, calibration, and predictive capacity.
AAA repair was associated with the greatest in-hospital death. Patients having AAA repair had the greatest burden of comorbid disease, including peripheral vascular disease, chronic obstructive pulmonary disease, and renal failure.
Patient age was 60 years or greater. Most procedures were elective.
Dr. LaPar noted that the study did not investigate the impact of surgeon volume, nor did it adjust for surgical risk factors such as tumor type/stage, pulmonary function, performance status, surgical technique, preoperative medications, and neoadjuvant therapy. They were also unable to assess the effects of hospital volume on long-term survival, resource utilization, and readmission.
The findings have several implications. Previous reports using conventional modeling techniques may have overestimated the significance of hospital volume as a predictor of mortality. "However, these data do not intend to declare that hospital volume is irrelevant, but rather that hospital procedure volume may be a surrogate for other unidentified institutional factors that influence quality," said Dr. LaPar. "Most importantly, these data do not support the current policy of using hospital procedure volume as a proxy measure for quality."
Invited discussant Dr. Edward Livingston praised the group's rigorous statistical analysis of the association between hospital procedure volume and quality of care (mortality). He noted that earlier papers showed a statistical association between procedure volume and mortality. "Where the volume outcome research efforts took a left turn is that, instead of trying to understand what it was about volume that's associated with outcomes, there have been 2 decades of papers published looking at and reconfirming a statistical association between procedure volume and outcomes. Procedure volume itself does not translate into better outcomes. It is the things associated with procedure volume, such as surgeon experience, better functioning [operating room teams, and the like]. We really haven't looked into those causative factors."
If the causative factors could be identified, "then we could take the experience of high-volume centers and translate that to everybody else, so everybody could have good outcomes," he said.
According to Dr. Livingston, of the University of Texas, Dallas, previous studies relied on statistical modeling of the mortality relationship. "Those models are only as good as the model can represent the data," he said, and very few have been rigorously assessed to see how well they describe the phenomenon that they're trying to describe. This paper "should serve as the template for what everyone should do when they're performing volume outcome studies or any kind of regression analysis," he added.
The authors reported that they have no financial disclosures.
Procedural volume and its relationship to outcome has enormous implications, not just for patients undergoing surgical treatments, but in planning future surgical services. This study is important in respect of the large data set analysed and the finding that volume was only weakly associated with mortality compared to other factors such as patient co-morbidity. This raises questions about the current trend across the developed world to move towards high volume surgical units.
As impressive as the study initially appears there are some weaknesses that need to be considered. We of course only know about patients who were operated on and know nothing about patients declined surgery. There are studies that indicate patients are more likely to be declined appropriate surgery in units with less experience and lower volumes. Also of course many low volume units will refer high risk or complex cases to larger more experienced centres. It may be expected that some of this effect would be picked up in the Agency for Health Care Research and Quality comorbidities category (AHRQ) but the most usual reason for transfer to a regional unit is because of surgical complexity rather than patient co-morbidity.
This study also does not allow for any clinical governance measures that may be in force. Most if not all units with outcomes worse than average will take measures to address the problem ranging from ceasing undertaking the procedure to better case selection or improved team structure. At the time this study was conducted it is inconceivable to assume that no such measures were in action. It is incorrect then to conclude that this is an unselected study of the effects of volume. In the United Kingdom following concerns raised about outcomes from aortic surgery prospectively collected national data showed a very strong association with volume and outcome. The highest volume units had mortality 2.5 times lower than the lowest volume units (Outcomes after elective repair of infra-renal abdominal aortic aneurysm, March 2012; www.vascularsociety.org).
The authors conclude that volume is a poor indicator of quality of health care. This may be correct, but it is hard to suggest that based on this study as the authors did not measure factors such as length of hospital stay, re-admission rates, costs or patient experience of their care. In a review of cardiovascular services in London, not only did unit volume for AAA repair influence mortality it had a strong effect on efficiency. While patient outcome must be the main factor in planning service configurations, other issues such as cost and manpower need also to be considered.
Finally there are some practical issues. Small volume units can get good results. A unit that does 5 aortic aneurysm repairs in a year with no mortality will look very good. However, if they have a single mortality they will become one of the worst performing units overnight. Performance of units can only be assessed on large numbers. If a small volume unit is underperforming it may take a long time to identify that. There cannot be a surgical team in the world that is not aware that the more they work together to undertake complex surgical interventions the better they function across the board. So while the results of the study need to be considered carefully they do fly in the face of what most surgeons know in their hearts.
Dr. C. P. Shearman is Professor of Vascular Surgery, University of Southampton, United Kingdom, and an associate medical editor of Vascular Specialist.
Procedural volume and its relationship to outcome has enormous implications, not just for patients undergoing surgical treatments, but in planning future surgical services. This study is important in respect of the large data set analysed and the finding that volume was only weakly associated with mortality compared to other factors such as patient co-morbidity. This raises questions about the current trend across the developed world to move towards high volume surgical units.
As impressive as the study initially appears there are some weaknesses that need to be considered. We of course only know about patients who were operated on and know nothing about patients declined surgery. There are studies that indicate patients are more likely to be declined appropriate surgery in units with less experience and lower volumes. Also of course many low volume units will refer high risk or complex cases to larger more experienced centres. It may be expected that some of this effect would be picked up in the Agency for Health Care Research and Quality comorbidities category (AHRQ) but the most usual reason for transfer to a regional unit is because of surgical complexity rather than patient co-morbidity.
This study also does not allow for any clinical governance measures that may be in force. Most if not all units with outcomes worse than average will take measures to address the problem ranging from ceasing undertaking the procedure to better case selection or improved team structure. At the time this study was conducted it is inconceivable to assume that no such measures were in action. It is incorrect then to conclude that this is an unselected study of the effects of volume. In the United Kingdom following concerns raised about outcomes from aortic surgery prospectively collected national data showed a very strong association with volume and outcome. The highest volume units had mortality 2.5 times lower than the lowest volume units (Outcomes after elective repair of infra-renal abdominal aortic aneurysm, March 2012; www.vascularsociety.org).
The authors conclude that volume is a poor indicator of quality of health care. This may be correct, but it is hard to suggest that based on this study as the authors did not measure factors such as length of hospital stay, re-admission rates, costs or patient experience of their care. In a review of cardiovascular services in London, not only did unit volume for AAA repair influence mortality it had a strong effect on efficiency. While patient outcome must be the main factor in planning service configurations, other issues such as cost and manpower need also to be considered.
Finally there are some practical issues. Small volume units can get good results. A unit that does 5 aortic aneurysm repairs in a year with no mortality will look very good. However, if they have a single mortality they will become one of the worst performing units overnight. Performance of units can only be assessed on large numbers. If a small volume unit is underperforming it may take a long time to identify that. There cannot be a surgical team in the world that is not aware that the more they work together to undertake complex surgical interventions the better they function across the board. So while the results of the study need to be considered carefully they do fly in the face of what most surgeons know in their hearts.
Dr. C. P. Shearman is Professor of Vascular Surgery, University of Southampton, United Kingdom, and an associate medical editor of Vascular Specialist.
Procedural volume and its relationship to outcome has enormous implications, not just for patients undergoing surgical treatments, but in planning future surgical services. This study is important in respect of the large data set analysed and the finding that volume was only weakly associated with mortality compared to other factors such as patient co-morbidity. This raises questions about the current trend across the developed world to move towards high volume surgical units.
As impressive as the study initially appears there are some weaknesses that need to be considered. We of course only know about patients who were operated on and know nothing about patients declined surgery. There are studies that indicate patients are more likely to be declined appropriate surgery in units with less experience and lower volumes. Also of course many low volume units will refer high risk or complex cases to larger more experienced centres. It may be expected that some of this effect would be picked up in the Agency for Health Care Research and Quality comorbidities category (AHRQ) but the most usual reason for transfer to a regional unit is because of surgical complexity rather than patient co-morbidity.
This study also does not allow for any clinical governance measures that may be in force. Most if not all units with outcomes worse than average will take measures to address the problem ranging from ceasing undertaking the procedure to better case selection or improved team structure. At the time this study was conducted it is inconceivable to assume that no such measures were in action. It is incorrect then to conclude that this is an unselected study of the effects of volume. In the United Kingdom following concerns raised about outcomes from aortic surgery prospectively collected national data showed a very strong association with volume and outcome. The highest volume units had mortality 2.5 times lower than the lowest volume units (Outcomes after elective repair of infra-renal abdominal aortic aneurysm, March 2012; www.vascularsociety.org).
The authors conclude that volume is a poor indicator of quality of health care. This may be correct, but it is hard to suggest that based on this study as the authors did not measure factors such as length of hospital stay, re-admission rates, costs or patient experience of their care. In a review of cardiovascular services in London, not only did unit volume for AAA repair influence mortality it had a strong effect on efficiency. While patient outcome must be the main factor in planning service configurations, other issues such as cost and manpower need also to be considered.
Finally there are some practical issues. Small volume units can get good results. A unit that does 5 aortic aneurysm repairs in a year with no mortality will look very good. However, if they have a single mortality they will become one of the worst performing units overnight. Performance of units can only be assessed on large numbers. If a small volume unit is underperforming it may take a long time to identify that. There cannot be a surgical team in the world that is not aware that the more they work together to undertake complex surgical interventions the better they function across the board. So while the results of the study need to be considered carefully they do fly in the face of what most surgeons know in their hearts.
Dr. C. P. Shearman is Professor of Vascular Surgery, University of Southampton, United Kingdom, and an associate medical editor of Vascular Specialist.
SAN FRANCISCO - Hospital procedure volume, which is commonly used as a proxy measure for hospital quality, is not significantly associated with in-hospital mortality for four common surgical procedures, based on a rigorous statistical analysis of data from the Nationwide Inpatient Sample.
Furthermore, "no identifiable threshold values exist for hospital procedure volume at which mortality risk significantly increased. Mortality risk was primarily attributable to patient-level risk factors," said Dr. Damian J. LaPar of the University of Virginia, Charlottesville.
He and his colleagues examined the relative strength of association between hospital volume and mortality vs. other modeled variables by comparing model covariate likelihood ratios for four high-risk procedures: pancreatic resection, abdominal aortic aneurysm (AAA) repair, esophageal resection, and coronary artery bypass graft (CABG).
Using data from the Nationwide Inpatient Sample in 2008, they obtained weighted discharge records for 261,142 patients: 19,194 patients who had pancreatic resection, 15,266 who had AAA repair, 4,764 who had esophageal resection, and 222,122 who had CABG. The primary outcome of interest was the estimated risk-adjusted effect of hospital procedure volume on mortality (in-hospital death). Comorbid disease was assessed based on Agency for Healthcare Research and Quality (AHRQ) comorbidity categories.
"In all four models, hospital volume was associated with the lowest statistical strength of association with mortality," compared with all other factors, Dr. LaPar said at the annual meeting of the American Surgical Association. Alternatively, other operation and patient-related risk factors -- including elective vs. nonelective status, age, sex, hypertension, weight loss, heart failure, chronic obstructive pulmonary disease, liver disease, and renal failure -- had higher strengths of association with mortality.
Dr. LaPar noted that procedure volume is an attractive metric for regulatory bodies to use as a predictor of outcomes; it is easy to measure and intuitive in nature. In addition, higher-volume hospitals are more likely to have established system-based processes and the infrastructure in place to improve patient outcomes.
The Leapfrog Group and the AHRQ both have adopted procedure volume as a quality indicator for the four high-risk surgical procedures. Arbitrarily defined volume thresholds have been adopted as a metric of quality for these procedures. However, many previous statistical methods that are used to define these thresholds have drawn criticism in the recent surgical literature. In many former series, volume is represented as arbitrarily defined categories, rather than as a continuous variable. Furthermore, there has been little attempt to rigorously assess and compare statistical model performance; to assess the relative strength of the association of procedure volume with other outcome predictors; and to utilize hierarchical, multilevel, statistical modeling techniques for complex, multicenter patient samples.
Dr. LaPar and his colleagues used hierarchical general linear modeling and created separate models for each procedure, which were adjusted for patient and operative factors as potential confounders. Patient factors included age, sex, and comorbid disease. Operative factors included procedure volume and elective/nonelective status. All model covariates were selected a priori.
The researchers used hospital volume as a continuous variable with restricted cubic spline regression, which uses all data points to estimate the shape of the association between hospital volume and mortality, and is considered to be the best way to visually identify threshold values. They also assessed the relative strength of association between hospital volume and mortality, compared with other factors (likelihood ratio). Model performance was assessed by looking at discrimination, calibration, and predictive capacity.
AAA repair was associated with the greatest in-hospital death. Patients having AAA repair had the greatest burden of comorbid disease, including peripheral vascular disease, chronic obstructive pulmonary disease, and renal failure.
Patient age was 60 years or greater. Most procedures were elective.
Dr. LaPar noted that the study did not investigate the impact of surgeon volume, nor did it adjust for surgical risk factors such as tumor type/stage, pulmonary function, performance status, surgical technique, preoperative medications, and neoadjuvant therapy. They were also unable to assess the effects of hospital volume on long-term survival, resource utilization, and readmission.
The findings have several implications. Previous reports using conventional modeling techniques may have overestimated the significance of hospital volume as a predictor of mortality. "However, these data do not intend to declare that hospital volume is irrelevant, but rather that hospital procedure volume may be a surrogate for other unidentified institutional factors that influence quality," said Dr. LaPar. "Most importantly, these data do not support the current policy of using hospital procedure volume as a proxy measure for quality."
Invited discussant Dr. Edward Livingston praised the group's rigorous statistical analysis of the association between hospital procedure volume and quality of care (mortality). He noted that earlier papers showed a statistical association between procedure volume and mortality. "Where the volume outcome research efforts took a left turn is that, instead of trying to understand what it was about volume that's associated with outcomes, there have been 2 decades of papers published looking at and reconfirming a statistical association between procedure volume and outcomes. Procedure volume itself does not translate into better outcomes. It is the things associated with procedure volume, such as surgeon experience, better functioning [operating room teams, and the like]. We really haven't looked into those causative factors."
If the causative factors could be identified, "then we could take the experience of high-volume centers and translate that to everybody else, so everybody could have good outcomes," he said.
According to Dr. Livingston, of the University of Texas, Dallas, previous studies relied on statistical modeling of the mortality relationship. "Those models are only as good as the model can represent the data," he said, and very few have been rigorously assessed to see how well they describe the phenomenon that they're trying to describe. This paper "should serve as the template for what everyone should do when they're performing volume outcome studies or any kind of regression analysis," he added.
The authors reported that they have no financial disclosures.
SAN FRANCISCO - Hospital procedure volume, which is commonly used as a proxy measure for hospital quality, is not significantly associated with in-hospital mortality for four common surgical procedures, based on a rigorous statistical analysis of data from the Nationwide Inpatient Sample.
Furthermore, "no identifiable threshold values exist for hospital procedure volume at which mortality risk significantly increased. Mortality risk was primarily attributable to patient-level risk factors," said Dr. Damian J. LaPar of the University of Virginia, Charlottesville.
He and his colleagues examined the relative strength of association between hospital volume and mortality vs. other modeled variables by comparing model covariate likelihood ratios for four high-risk procedures: pancreatic resection, abdominal aortic aneurysm (AAA) repair, esophageal resection, and coronary artery bypass graft (CABG).
Using data from the Nationwide Inpatient Sample in 2008, they obtained weighted discharge records for 261,142 patients: 19,194 patients who had pancreatic resection, 15,266 who had AAA repair, 4,764 who had esophageal resection, and 222,122 who had CABG. The primary outcome of interest was the estimated risk-adjusted effect of hospital procedure volume on mortality (in-hospital death). Comorbid disease was assessed based on Agency for Healthcare Research and Quality (AHRQ) comorbidity categories.
"In all four models, hospital volume was associated with the lowest statistical strength of association with mortality," compared with all other factors, Dr. LaPar said at the annual meeting of the American Surgical Association. Alternatively, other operation and patient-related risk factors -- including elective vs. nonelective status, age, sex, hypertension, weight loss, heart failure, chronic obstructive pulmonary disease, liver disease, and renal failure -- had higher strengths of association with mortality.
Dr. LaPar noted that procedure volume is an attractive metric for regulatory bodies to use as a predictor of outcomes; it is easy to measure and intuitive in nature. In addition, higher-volume hospitals are more likely to have established system-based processes and the infrastructure in place to improve patient outcomes.
The Leapfrog Group and the AHRQ both have adopted procedure volume as a quality indicator for the four high-risk surgical procedures. Arbitrarily defined volume thresholds have been adopted as a metric of quality for these procedures. However, many previous statistical methods that are used to define these thresholds have drawn criticism in the recent surgical literature. In many former series, volume is represented as arbitrarily defined categories, rather than as a continuous variable. Furthermore, there has been little attempt to rigorously assess and compare statistical model performance; to assess the relative strength of the association of procedure volume with other outcome predictors; and to utilize hierarchical, multilevel, statistical modeling techniques for complex, multicenter patient samples.
Dr. LaPar and his colleagues used hierarchical general linear modeling and created separate models for each procedure, which were adjusted for patient and operative factors as potential confounders. Patient factors included age, sex, and comorbid disease. Operative factors included procedure volume and elective/nonelective status. All model covariates were selected a priori.
The researchers used hospital volume as a continuous variable with restricted cubic spline regression, which uses all data points to estimate the shape of the association between hospital volume and mortality, and is considered to be the best way to visually identify threshold values. They also assessed the relative strength of association between hospital volume and mortality, compared with other factors (likelihood ratio). Model performance was assessed by looking at discrimination, calibration, and predictive capacity.
AAA repair was associated with the greatest in-hospital death. Patients having AAA repair had the greatest burden of comorbid disease, including peripheral vascular disease, chronic obstructive pulmonary disease, and renal failure.
Patient age was 60 years or greater. Most procedures were elective.
Dr. LaPar noted that the study did not investigate the impact of surgeon volume, nor did it adjust for surgical risk factors such as tumor type/stage, pulmonary function, performance status, surgical technique, preoperative medications, and neoadjuvant therapy. They were also unable to assess the effects of hospital volume on long-term survival, resource utilization, and readmission.
The findings have several implications. Previous reports using conventional modeling techniques may have overestimated the significance of hospital volume as a predictor of mortality. "However, these data do not intend to declare that hospital volume is irrelevant, but rather that hospital procedure volume may be a surrogate for other unidentified institutional factors that influence quality," said Dr. LaPar. "Most importantly, these data do not support the current policy of using hospital procedure volume as a proxy measure for quality."
Invited discussant Dr. Edward Livingston praised the group's rigorous statistical analysis of the association between hospital procedure volume and quality of care (mortality). He noted that earlier papers showed a statistical association between procedure volume and mortality. "Where the volume outcome research efforts took a left turn is that, instead of trying to understand what it was about volume that's associated with outcomes, there have been 2 decades of papers published looking at and reconfirming a statistical association between procedure volume and outcomes. Procedure volume itself does not translate into better outcomes. It is the things associated with procedure volume, such as surgeon experience, better functioning [operating room teams, and the like]. We really haven't looked into those causative factors."
If the causative factors could be identified, "then we could take the experience of high-volume centers and translate that to everybody else, so everybody could have good outcomes," he said.
According to Dr. Livingston, of the University of Texas, Dallas, previous studies relied on statistical modeling of the mortality relationship. "Those models are only as good as the model can represent the data," he said, and very few have been rigorously assessed to see how well they describe the phenomenon that they're trying to describe. This paper "should serve as the template for what everyone should do when they're performing volume outcome studies or any kind of regression analysis," he added.
The authors reported that they have no financial disclosures.
Major Finding: Hospital procedure volume is not significantly associated with in-hospital mortality for four common surgical procedures.
Data Source: The researchers used data from the NIS in 2008. They obtained weighted-discharge records for 19,194 patients who had pancreatic resection, 15,266 who had AAA repair, 4,764 who had esophageal resection, and 222,122 who had CABG. The primary outcome of interest was the estimated risk-adjusted effect of hospital procedure volume on mortality (in-hospital death).
Disclosures: The authors reported that they have no financial disclosures.
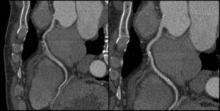
Cardiovascular CT Poised to Take Imaging Center Stage
New technologies that solve some old problems could make cardiovascular CT the go-to imaging tool to assess both anatomy and function.
Cardiovascular CT imaging has been a welcome tool in patient assessment due to its noninvasive nature, ease, and quickness. Likewise, cardiovascular CT angiography (CCTA) has provided noninvasive information about vessel stenoses, with lower costs and radiation exposure compared with invasive angiography.
Perhaps the biggest criticism about cardiovascular CT is that, while it provides very good anatomic detail, functional information – a crucial piece to the overall picture – is not discernable.
"Historically, coronary CT angiography has been an extremely anatomic method for documenting the severity of coronary artery stenoses or luminal compromise," said Dr. James K. Min, immediate past president of SCCT. This has left CTA open to the criticism that the more useful evaluation of coronary artery disease is not only to document high-grade stenoses – as with coronary CT angiography – but also to determine the physiologic significance of those stenoses.
However, at this year’s meeting of the Society of Cardiovascular Computed Tomography in Baltimore, "we saw the emergence of two robust methods for physiologic assessment of coronary disease by cardiac CT," he said. These are fractional flow reserve CT and CT-based myocardial perfusion imaging.
Evidence regarding these methods was then bolstered at the annual congress of the European Society of Cardiology in Munich, with the presentation of two imaging trials. The DeFACTO study, comparing CT-based FFR with FFR obtained by conventional angiography, did not show diagnostic superiority of CT-based FFR over angiography, but it did enhance diagnoses compared with CT without FFR. The CORE320 trial, assessing the diagnostic accuracy CT-based myocardial perfusion imaging in patients with suspected coronary artery disease, showed that CT-based myocardial perfusion imaging significantly increased the diagnostic accuracy of CTA alone to delineate flow-limiting disease.
Cardiovascular CT also has been hamstrung by a number of technical limitations. Calcium blooming artifacts make it difficult to get an accurate measurement of calcium lesion components and lumen size and motion artifacts also limit the clarity of CT images, leaving some vessels simply unreadable.
Two new technological advances – dual-energy CT and intelligent motion correction using snapshot freeze – are already changing practice, according to leaders at this year’s annual meeting of the SCCT.
CT Fractional Flow Reserve
Fractional flow reserve (FFR) is defined as the maximal blood flow through a diseased artery to the blood flow in the hypothetical case that this artery is normal. CT-based FFR relies on the use of some complicated math and modeling to calculate FFR from CT data and actually traces its roots back to the air flow modeling that goes into designing airplane wings.
All of the data needed can be gleaned from a single-phase static CT image. Geometry can be extracted from CCTA anatomic data. Boundary conditions can also be determined. Resting coronary blood flow can be calculated from myocardial mass. Mean blood pressure can be estimated from brachial artery pressure. Coronary microcirculatory resistance can be derived from morphometry data. Lastly, fluid properties include the viscosity and density of blood. "Putting it all together, we can come out with a calculated assessment of things like coronary flow and FFR ... now, we can do anatomy and function," said Dr. Matthew J. Budoff, director of cardiology at the University of California at Los Angeles School of Medicine in Torrance.
Until now, FFR was determined through invasive coronary angiography, which was the only method for specific determination of coronary artery lesions (lesion-specific ischemia). Values of 0.80 and lower or 0.75 and lower are considered diagnostic of lesion-specific ischemia. "It tells us whether there is a physiologic significance of the lesion," Dr. Budoff said.
At the same time, stenosis seen on CCTA has been an unreliable predictor of lesion-specific ischemia in trials. "Just seeing that anatomical stenosis doesn’t mean it’s functionally significant," said Dr. Budoff. CCTA results in a lot of false positives when it comes to physiologic significance.
FFR estimates throughout the entire coronary tree. "Because it relies on the entire coronary anatomy, it’s actually less sensitive to artifacts and less sensitive to things like calcification than individual segment assessment."
The first trial of FFR-CT was in the Diagnosis of Ischemia-Causing Stenoses Obtained via Noninvasive Fractional Flow Reserve (DISCOVER-FLOW) trial. In the study, the researchers compared the accuracy of CCTA with FFR-CT (invasive FFR served as the reference).
The results showed that the coronary stenoses that cause ischemia can be identified noninvasively with computer analysis of CCTA to construct FFR for specific lesions (Eur. Heart J. Cardiovasc. Imaging 2012 Jul 15 [doi: 10.1093/ehjci/jes130]).
The upshot of this study is that "when we think it’s a high-grade stenosis [with FFR-CT], it is a physiologically significant stenosis," Dr. Budoff said. "So we’ve dramatically improved the ability of CT to correlate with the most invasive and probably the most definitive way, currently, of assessing physiologic significance, and that’s FFR."
CT-based fractional flow reserve (FFR) is now a step closer to clinical use with the results of the Determination of FFR by Anatomic CT Angiography (DeFACTO) trial presented at the ESC meeting and simultaneously published (JAMA 2012 Aug. 26 [doi:10.1001/2012.jama.11274]). The addition of CT-based FFR information to CT alone improved diagnostic accuracy of stenoses, compared with invasive angiography and FFR.
"We’ve dramatically improved the ability of CT to correlate with ... FFR," said Dr. Matthew J. Budoff.
Although FFR-CT plus CT narrowly failed to meet the trial’s primary end point – diagnostic accuracy greater than 70% for the lower bound of the 95% confidence interval, the per-patient performance diagnostic accuracy of FFR-CT plus CT was 73% with a 95% CI of 67%-78%.
Nevertheless, the addition of FFR-CT "demonstrated superior diagnostic performance characteristics, as compared with CT stenosis alone in all patients, in all vessels and also in vessels of intermediate stenosis severity," Dr. Min said during a press conference.
Myocardial Perfusion
"The second method of physiologic assessment of coronary disease, which is emerging, is myocardial perfusion imaging – performing a typical stress test with a CT angiogram," said Dr. Min.
This allows the visualization of perfusion/ischemia at specific lesions identified by CTA, providing functional and anatomic information. The data for perfusion images are extracted from a standard CTA scan.
With CT perfusion, a rest CT angiogram is performed to document the coronary artery stenoses that are within the coronary artery bed and also to look at the rest perfusion of the myocardium to determine whether it’s normal or abnormal, according to Dr. Min.
Before or after the rest CTA, a stress CT would be performed via pharmacologic means using a traditional 64-row cardiac CT.
It’s known that patients who have a normal SPECT myocardial perfusion examination have a very low rate of cardiovascular events over the next year, according to Dr. Richard T. George. "However, those patients with an abnormal nuclear scan actually have a quite high event rate over the next year."
In addition, it’s also known that CCTA has great prognostic value "and probably for a longer period than SPECT does, but SPECT myocardial perfusion imaging probably tells you more about the intermediate time period in the future about the event rate in the patient," he said.
Several studies have looked at the additional value of CT perfusion testing.
One of the first studies that compared CCTA and CT perfusion (CTP) with quantitative coronary angiography and SPECT perfusion showed a sensitivity with CTA/CTP of 88% and a specificity of 91% (Circ. Cardiovasc. Imaging 2009;2:174-82). "This study demonstrates some of the additional value of stress CT myocardial perfusion imaging," said Dr. George, assistant professor of medicine at the Heart and Vascular Institute at Johns Hopkins Hospital in Baltimore.
In another study, researchers assessed the additional value of dipyridamole stress myocardial perfusion by 64-row CT in patients with coronary stents (J. Cardiovasc. Comput. Tomogr. 2011;5:449-58). It is often difficult in many of these patients to assess whether in-stent restenosis is present, said Dr. George. The researchers in this study found that the addition of CT myocardial perfusion imaging to CCTA improved accuracy.
In a study targeting reversible ischemia, researchers assessed CT myocardial perfusion imaging with 320-row detector CT in 50 patients with an intermediate- to high-risk for CAD (Circ. Cardiovasc. Imaging 2012;5:333-40).
"The important part of this study is that they actually looked at reversible ischemia. A lot of our studies with CT perfusion imaging just lump all perfusion deficits together." In it, 40% of patients had an abnormal SPECT scan; 90% of those abnormal scans were reversible ischemia," said Dr. George.
In a per-patient analysis of CT perfusion imaging vs. CTA stenosis greater than 50% in the setting of a territorial SPECT myocardial perfusion deficit, sensitivity was 100% and specificity was 81%. The study shows the effectiveness of CT perfusion for assessing lesion-specific ischemia, Dr. George noted.
"What we’re finding is that CT perfusion or stress testing with CT can be as accurate, if not more accurate, than the conventional stress testing methods that we’ve used until this day," Dr. Min said.
"CT perfusion or stress testing with CT can be as accurate, if not more accurate, than the conventional stress testing method," said Dr. James K. Min.
In the Combined Non-invasive Coronary Angiography and Myocardial Perfusion Imaging Using 320 Detector Computed Tomography (Core320) trial, researchers compared the diagnostic accuracy of CTA plus CT-based myocardial perfusion against ICA plus SPECT myocardial perfusion imaging in 381 patients with suspected or diagnosed coronary artery disease with a clinical indication for coronary angiography.
"When we add perfusion [to CTA alone] we gain power to diagnose flow-limiting stenosis," lead author Dr. Joao A.C. Lima said at the ESC meeting. Together, "they have the same power as invasive angiography and SPECT MPI in defining who are the patients who end up going through revascularization," he said.
The area under the receiver operating characteristic curve (ROC) – an effective method of evaluating the performance of diagnostic tests – was 0.79 for CTA-CTP and 0.81 for ICA-SPECT.
"At this point, the [CT perfusion] technology could be used precisely to find the patients who have flow-limiting disease and, therefore, are going to need revascularization," said Dr. Lima, director of cardiovascular imaging at the Johns Hopkins University in Baltimore.
Dual-Energy CT
Dual-energy CT (also known as dual-source CT) "interleaves sets of high- and low-keV images. The energy alternates between two energies (80 keV to 140 keV) every 0.5 msec," said Dr. James P. Earls. The result is two competing sets that can be used simultaneously. The importance of this dual-energy approach is that with higher energies and a software algorithm, calcium blooming is decreased. This means a more accurate size of calcium in lesions and lumen size.
Back-end software produces a range of monochromatic images (images at a single energy). "In terms of calcium reduction, the monochromatic images are probably more important," said Dr. Earls, codirector of the cardiac CT program at Inova Heart and Vascular Institute in Falls Church, Va.
Monochromatic images range from 40 keV to 140 keV. "At 40 keV you’re very close to the k-edge of iodine, so you have significant attenuation of anything that is enhancing ... at about 75 keV you have something that looks akin to a 120-keV image that we would normally get." As the energy increases, "you get away from what we would do on a routine clinical basis."
The ATLANTA I study (Assessment of Tissue Characteristics, Lesion Morphology, and Hemodynamics by Angiography With Fractional Flow Reserve, Intravascular Ultrasound and Virtual Histology, and Noninvasive Computed Tomography in Atherosclerotic Plaques) showed that with conventional single-energy CT calcium, plaque is overestimated and lumen size is therefore underestimated (J. Am. Coll. Cardiol. Intv. 2011;4:198-208 [doi:10.1016/j.jcin.2010.10.008]).
The ATLANTA I study tells us that "when we do routine single-energy CT, calcium plaques are significantly overportrayed." In this study, researchers found that calcified plaque volume is 104% greater than its true volume as determined by intravascular ultrasound. As a result, the minimal luminal diameter is underestimated by 21% and the percentage diameter stenosis is overrepresented by 39%. "Lots of false positives [are] caused by calcified plaque in the area," Dr. Earls observed.
The reason for this is that as energy increases, the calcium blooming decreases. This accounts for the enlargement of the lumen.
Noncalcified plaques don’t show as dramatic an effect, though. Soft plaque size does not change much when seen at different energies, Dr. Earls noted. "Ultimately it may be that we use a combination of monochromatic images and material density images, as we approach this going forward. These are both available every time that you do a scan," said Dr. Earls.
Dual-source CT does have limitations, though. There is no retrospective gating; there are limited milliAmpere (electrical charge) presets, and there is no high-resolution mode.
Intelligent Motion Correction
Motion artifacts make it difficult or impossible to evaluate vessels, leaving clinicians to treat the vessels as narrowed by default. A new technology is designed to overcome the problem of motion artifacts in cardiovascular CT.
"The images are acquired in a standard fashion, but instead of reconstructing them in a standard fashion, there is integration of this intelligent motion correction that tracks the motion of the vessel over two or three phases of the cardiac cycle," Dr. Jonathon A. Leipsic said in an interview. "When we acquire the study, we get a couple of phases beyond just the minimal acquisition – which is standard. It sees where the vessel is over a couple of phases and how it looks. Based on the known velocity of the artery, how it appears, and the patient’s known heart rate, [the algorithm] then corrects for the expected motion."
The technology is already changing practice. It is used on Europe and Japan; it is also being used in many centers in the United States. The motion correction algorithm has wide range of applications. "Anywhere there is motion, anyone with a sudden ectopic pace or premature ventricular contraction, anyone with an arrhythmia – all of those patients will benefit from this motion correction," said Dr. Leipsic, chairman of the department of radiology for Providence Health Care and vice chairman of research for the department of radiology at the University of British Columbia, Vancouver.
Importantly, the scan time and radiation dose don’t increase appreciably with the acquisition of vessel velocity data. "It doesn’t change the acquisition. You can just wait and see. If you have a study that has no motion, then you don’t need to use [motion correction]. But if you have a study with motion, then you can use it. You can make that choice after you see the initial data," Dr. Leipsic explained.
Dr. Leipsic was the lead author on one of the first studies to assess the accuracy this of this method (J. Cardiovasc. Comput. Tomography 2012;6:164-71. Epub 2012 Apr 6). In the study, "we looked at a population of convenience patients that happened to be going to the cath lab. We chose very difficult patients – patients undergoing transcatheter valve replacement – who have very high heart rates. We saw a significant improvement in interpretability, overall image quality, and diagnostic accuracy" with this technology. The researchers also noted significant improvements in right coronary evaluation, as well as other coronary territories.
Some have argued that all that is needed to avoid motion artifacts is to rate-control patients. However, "we aggressively rate-controlled patients ... there’s just too much motion," Dr. Leipsic countered. "It’s hard to anticipate some problems – an irregular beat or some irregular rhythm – and having this in your back pocket ... has exciting potential."
Additional trials to assess accuracy are expected, perhaps most prominently the VICTORY trial (Validation of an Intracycle CT Motion Correction Algorithm for Diagnostic Accuracy: A Prospective Multicenter Study). In the study, CCTA will be compared with invasive coronary angiography (ICA) for diagnostic sensitivity and specificity, positive predictive value, negative predictive value, diagnostic accuracy, and positive and negative likelihood ratios.
Coronary segments will be assessed for "significance" of coronary artery luminal diameter obstruction. Individual segments will be graded based on image quality, with the third reader used to achieve consensus. Dr. Leipsic and Dr. James Min are the principal investigators for the study.
For now, the intelligent motion correction algorithm, called SnapShot Freez4e, is available only from GE Healthcare, as part of its Discovery CT750 HD FREEdom Edition, which was granted 510(k) clearance in June. "I think that other companies are going to come up with something similar but I think that each type will be adequately different enough that [these technologies] will require their own validation studies," said Dr. Leipsic.
Siemens is reportedly developing a similar technology.
Dr. Min is a speaker for GE Healthcare. Dr. Budoff receives grant support from HeartFlow. Dr. George has received research support from Toshiba Medical Systems and GE Healthcare, is on the advisory board of GE Healthcare, and is a consultant to ICON Medical Imaging. Dr. Earls is on the speakers bureau for and has research funded by GE Healthcare. Dr. Leipsic is a consultant/speaker for Edwards Lifesciences and GE Healthcare.
New technologies that solve some old problems could make cardiovascular CT the go-to imaging tool to assess both anatomy and function.
Cardiovascular CT imaging has been a welcome tool in patient assessment due to its noninvasive nature, ease, and quickness. Likewise, cardiovascular CT angiography (CCTA) has provided noninvasive information about vessel stenoses, with lower costs and radiation exposure compared with invasive angiography.
Perhaps the biggest criticism about cardiovascular CT is that, while it provides very good anatomic detail, functional information – a crucial piece to the overall picture – is not discernable.
"Historically, coronary CT angiography has been an extremely anatomic method for documenting the severity of coronary artery stenoses or luminal compromise," said Dr. James K. Min, immediate past president of SCCT. This has left CTA open to the criticism that the more useful evaluation of coronary artery disease is not only to document high-grade stenoses – as with coronary CT angiography – but also to determine the physiologic significance of those stenoses.
However, at this year’s meeting of the Society of Cardiovascular Computed Tomography in Baltimore, "we saw the emergence of two robust methods for physiologic assessment of coronary disease by cardiac CT," he said. These are fractional flow reserve CT and CT-based myocardial perfusion imaging.
Evidence regarding these methods was then bolstered at the annual congress of the European Society of Cardiology in Munich, with the presentation of two imaging trials. The DeFACTO study, comparing CT-based FFR with FFR obtained by conventional angiography, did not show diagnostic superiority of CT-based FFR over angiography, but it did enhance diagnoses compared with CT without FFR. The CORE320 trial, assessing the diagnostic accuracy CT-based myocardial perfusion imaging in patients with suspected coronary artery disease, showed that CT-based myocardial perfusion imaging significantly increased the diagnostic accuracy of CTA alone to delineate flow-limiting disease.
Cardiovascular CT also has been hamstrung by a number of technical limitations. Calcium blooming artifacts make it difficult to get an accurate measurement of calcium lesion components and lumen size and motion artifacts also limit the clarity of CT images, leaving some vessels simply unreadable.
Two new technological advances – dual-energy CT and intelligent motion correction using snapshot freeze – are already changing practice, according to leaders at this year’s annual meeting of the SCCT.
CT Fractional Flow Reserve
Fractional flow reserve (FFR) is defined as the maximal blood flow through a diseased artery to the blood flow in the hypothetical case that this artery is normal. CT-based FFR relies on the use of some complicated math and modeling to calculate FFR from CT data and actually traces its roots back to the air flow modeling that goes into designing airplane wings.
All of the data needed can be gleaned from a single-phase static CT image. Geometry can be extracted from CCTA anatomic data. Boundary conditions can also be determined. Resting coronary blood flow can be calculated from myocardial mass. Mean blood pressure can be estimated from brachial artery pressure. Coronary microcirculatory resistance can be derived from morphometry data. Lastly, fluid properties include the viscosity and density of blood. "Putting it all together, we can come out with a calculated assessment of things like coronary flow and FFR ... now, we can do anatomy and function," said Dr. Matthew J. Budoff, director of cardiology at the University of California at Los Angeles School of Medicine in Torrance.
Until now, FFR was determined through invasive coronary angiography, which was the only method for specific determination of coronary artery lesions (lesion-specific ischemia). Values of 0.80 and lower or 0.75 and lower are considered diagnostic of lesion-specific ischemia. "It tells us whether there is a physiologic significance of the lesion," Dr. Budoff said.
At the same time, stenosis seen on CCTA has been an unreliable predictor of lesion-specific ischemia in trials. "Just seeing that anatomical stenosis doesn’t mean it’s functionally significant," said Dr. Budoff. CCTA results in a lot of false positives when it comes to physiologic significance.
FFR estimates throughout the entire coronary tree. "Because it relies on the entire coronary anatomy, it’s actually less sensitive to artifacts and less sensitive to things like calcification than individual segment assessment."
The first trial of FFR-CT was in the Diagnosis of Ischemia-Causing Stenoses Obtained via Noninvasive Fractional Flow Reserve (DISCOVER-FLOW) trial. In the study, the researchers compared the accuracy of CCTA with FFR-CT (invasive FFR served as the reference).
The results showed that the coronary stenoses that cause ischemia can be identified noninvasively with computer analysis of CCTA to construct FFR for specific lesions (Eur. Heart J. Cardiovasc. Imaging 2012 Jul 15 [doi: 10.1093/ehjci/jes130]).
The upshot of this study is that "when we think it’s a high-grade stenosis [with FFR-CT], it is a physiologically significant stenosis," Dr. Budoff said. "So we’ve dramatically improved the ability of CT to correlate with the most invasive and probably the most definitive way, currently, of assessing physiologic significance, and that’s FFR."
CT-based fractional flow reserve (FFR) is now a step closer to clinical use with the results of the Determination of FFR by Anatomic CT Angiography (DeFACTO) trial presented at the ESC meeting and simultaneously published (JAMA 2012 Aug. 26 [doi:10.1001/2012.jama.11274]). The addition of CT-based FFR information to CT alone improved diagnostic accuracy of stenoses, compared with invasive angiography and FFR.
"We’ve dramatically improved the ability of CT to correlate with ... FFR," said Dr. Matthew J. Budoff.
Although FFR-CT plus CT narrowly failed to meet the trial’s primary end point – diagnostic accuracy greater than 70% for the lower bound of the 95% confidence interval, the per-patient performance diagnostic accuracy of FFR-CT plus CT was 73% with a 95% CI of 67%-78%.
Nevertheless, the addition of FFR-CT "demonstrated superior diagnostic performance characteristics, as compared with CT stenosis alone in all patients, in all vessels and also in vessels of intermediate stenosis severity," Dr. Min said during a press conference.
Myocardial Perfusion
"The second method of physiologic assessment of coronary disease, which is emerging, is myocardial perfusion imaging – performing a typical stress test with a CT angiogram," said Dr. Min.
This allows the visualization of perfusion/ischemia at specific lesions identified by CTA, providing functional and anatomic information. The data for perfusion images are extracted from a standard CTA scan.
With CT perfusion, a rest CT angiogram is performed to document the coronary artery stenoses that are within the coronary artery bed and also to look at the rest perfusion of the myocardium to determine whether it’s normal or abnormal, according to Dr. Min.
Before or after the rest CTA, a stress CT would be performed via pharmacologic means using a traditional 64-row cardiac CT.
It’s known that patients who have a normal SPECT myocardial perfusion examination have a very low rate of cardiovascular events over the next year, according to Dr. Richard T. George. "However, those patients with an abnormal nuclear scan actually have a quite high event rate over the next year."
In addition, it’s also known that CCTA has great prognostic value "and probably for a longer period than SPECT does, but SPECT myocardial perfusion imaging probably tells you more about the intermediate time period in the future about the event rate in the patient," he said.
Several studies have looked at the additional value of CT perfusion testing.
One of the first studies that compared CCTA and CT perfusion (CTP) with quantitative coronary angiography and SPECT perfusion showed a sensitivity with CTA/CTP of 88% and a specificity of 91% (Circ. Cardiovasc. Imaging 2009;2:174-82). "This study demonstrates some of the additional value of stress CT myocardial perfusion imaging," said Dr. George, assistant professor of medicine at the Heart and Vascular Institute at Johns Hopkins Hospital in Baltimore.
In another study, researchers assessed the additional value of dipyridamole stress myocardial perfusion by 64-row CT in patients with coronary stents (J. Cardiovasc. Comput. Tomogr. 2011;5:449-58). It is often difficult in many of these patients to assess whether in-stent restenosis is present, said Dr. George. The researchers in this study found that the addition of CT myocardial perfusion imaging to CCTA improved accuracy.
In a study targeting reversible ischemia, researchers assessed CT myocardial perfusion imaging with 320-row detector CT in 50 patients with an intermediate- to high-risk for CAD (Circ. Cardiovasc. Imaging 2012;5:333-40).
"The important part of this study is that they actually looked at reversible ischemia. A lot of our studies with CT perfusion imaging just lump all perfusion deficits together." In it, 40% of patients had an abnormal SPECT scan; 90% of those abnormal scans were reversible ischemia," said Dr. George.
In a per-patient analysis of CT perfusion imaging vs. CTA stenosis greater than 50% in the setting of a territorial SPECT myocardial perfusion deficit, sensitivity was 100% and specificity was 81%. The study shows the effectiveness of CT perfusion for assessing lesion-specific ischemia, Dr. George noted.
"What we’re finding is that CT perfusion or stress testing with CT can be as accurate, if not more accurate, than the conventional stress testing methods that we’ve used until this day," Dr. Min said.
"CT perfusion or stress testing with CT can be as accurate, if not more accurate, than the conventional stress testing method," said Dr. James K. Min.
In the Combined Non-invasive Coronary Angiography and Myocardial Perfusion Imaging Using 320 Detector Computed Tomography (Core320) trial, researchers compared the diagnostic accuracy of CTA plus CT-based myocardial perfusion against ICA plus SPECT myocardial perfusion imaging in 381 patients with suspected or diagnosed coronary artery disease with a clinical indication for coronary angiography.
"When we add perfusion [to CTA alone] we gain power to diagnose flow-limiting stenosis," lead author Dr. Joao A.C. Lima said at the ESC meeting. Together, "they have the same power as invasive angiography and SPECT MPI in defining who are the patients who end up going through revascularization," he said.
The area under the receiver operating characteristic curve (ROC) – an effective method of evaluating the performance of diagnostic tests – was 0.79 for CTA-CTP and 0.81 for ICA-SPECT.
"At this point, the [CT perfusion] technology could be used precisely to find the patients who have flow-limiting disease and, therefore, are going to need revascularization," said Dr. Lima, director of cardiovascular imaging at the Johns Hopkins University in Baltimore.
Dual-Energy CT
Dual-energy CT (also known as dual-source CT) "interleaves sets of high- and low-keV images. The energy alternates between two energies (80 keV to 140 keV) every 0.5 msec," said Dr. James P. Earls. The result is two competing sets that can be used simultaneously. The importance of this dual-energy approach is that with higher energies and a software algorithm, calcium blooming is decreased. This means a more accurate size of calcium in lesions and lumen size.
Back-end software produces a range of monochromatic images (images at a single energy). "In terms of calcium reduction, the monochromatic images are probably more important," said Dr. Earls, codirector of the cardiac CT program at Inova Heart and Vascular Institute in Falls Church, Va.
Monochromatic images range from 40 keV to 140 keV. "At 40 keV you’re very close to the k-edge of iodine, so you have significant attenuation of anything that is enhancing ... at about 75 keV you have something that looks akin to a 120-keV image that we would normally get." As the energy increases, "you get away from what we would do on a routine clinical basis."
The ATLANTA I study (Assessment of Tissue Characteristics, Lesion Morphology, and Hemodynamics by Angiography With Fractional Flow Reserve, Intravascular Ultrasound and Virtual Histology, and Noninvasive Computed Tomography in Atherosclerotic Plaques) showed that with conventional single-energy CT calcium, plaque is overestimated and lumen size is therefore underestimated (J. Am. Coll. Cardiol. Intv. 2011;4:198-208 [doi:10.1016/j.jcin.2010.10.008]).
The ATLANTA I study tells us that "when we do routine single-energy CT, calcium plaques are significantly overportrayed." In this study, researchers found that calcified plaque volume is 104% greater than its true volume as determined by intravascular ultrasound. As a result, the minimal luminal diameter is underestimated by 21% and the percentage diameter stenosis is overrepresented by 39%. "Lots of false positives [are] caused by calcified plaque in the area," Dr. Earls observed.
The reason for this is that as energy increases, the calcium blooming decreases. This accounts for the enlargement of the lumen.
Noncalcified plaques don’t show as dramatic an effect, though. Soft plaque size does not change much when seen at different energies, Dr. Earls noted. "Ultimately it may be that we use a combination of monochromatic images and material density images, as we approach this going forward. These are both available every time that you do a scan," said Dr. Earls.
Dual-source CT does have limitations, though. There is no retrospective gating; there are limited milliAmpere (electrical charge) presets, and there is no high-resolution mode.
Intelligent Motion Correction
Motion artifacts make it difficult or impossible to evaluate vessels, leaving clinicians to treat the vessels as narrowed by default. A new technology is designed to overcome the problem of motion artifacts in cardiovascular CT.
"The images are acquired in a standard fashion, but instead of reconstructing them in a standard fashion, there is integration of this intelligent motion correction that tracks the motion of the vessel over two or three phases of the cardiac cycle," Dr. Jonathon A. Leipsic said in an interview. "When we acquire the study, we get a couple of phases beyond just the minimal acquisition – which is standard. It sees where the vessel is over a couple of phases and how it looks. Based on the known velocity of the artery, how it appears, and the patient’s known heart rate, [the algorithm] then corrects for the expected motion."
The technology is already changing practice. It is used on Europe and Japan; it is also being used in many centers in the United States. The motion correction algorithm has wide range of applications. "Anywhere there is motion, anyone with a sudden ectopic pace or premature ventricular contraction, anyone with an arrhythmia – all of those patients will benefit from this motion correction," said Dr. Leipsic, chairman of the department of radiology for Providence Health Care and vice chairman of research for the department of radiology at the University of British Columbia, Vancouver.
Importantly, the scan time and radiation dose don’t increase appreciably with the acquisition of vessel velocity data. "It doesn’t change the acquisition. You can just wait and see. If you have a study that has no motion, then you don’t need to use [motion correction]. But if you have a study with motion, then you can use it. You can make that choice after you see the initial data," Dr. Leipsic explained.
Dr. Leipsic was the lead author on one of the first studies to assess the accuracy this of this method (J. Cardiovasc. Comput. Tomography 2012;6:164-71. Epub 2012 Apr 6). In the study, "we looked at a population of convenience patients that happened to be going to the cath lab. We chose very difficult patients – patients undergoing transcatheter valve replacement – who have very high heart rates. We saw a significant improvement in interpretability, overall image quality, and diagnostic accuracy" with this technology. The researchers also noted significant improvements in right coronary evaluation, as well as other coronary territories.
Some have argued that all that is needed to avoid motion artifacts is to rate-control patients. However, "we aggressively rate-controlled patients ... there’s just too much motion," Dr. Leipsic countered. "It’s hard to anticipate some problems – an irregular beat or some irregular rhythm – and having this in your back pocket ... has exciting potential."
Additional trials to assess accuracy are expected, perhaps most prominently the VICTORY trial (Validation of an Intracycle CT Motion Correction Algorithm for Diagnostic Accuracy: A Prospective Multicenter Study). In the study, CCTA will be compared with invasive coronary angiography (ICA) for diagnostic sensitivity and specificity, positive predictive value, negative predictive value, diagnostic accuracy, and positive and negative likelihood ratios.
Coronary segments will be assessed for "significance" of coronary artery luminal diameter obstruction. Individual segments will be graded based on image quality, with the third reader used to achieve consensus. Dr. Leipsic and Dr. James Min are the principal investigators for the study.
For now, the intelligent motion correction algorithm, called SnapShot Freez4e, is available only from GE Healthcare, as part of its Discovery CT750 HD FREEdom Edition, which was granted 510(k) clearance in June. "I think that other companies are going to come up with something similar but I think that each type will be adequately different enough that [these technologies] will require their own validation studies," said Dr. Leipsic.
Siemens is reportedly developing a similar technology.
Dr. Min is a speaker for GE Healthcare. Dr. Budoff receives grant support from HeartFlow. Dr. George has received research support from Toshiba Medical Systems and GE Healthcare, is on the advisory board of GE Healthcare, and is a consultant to ICON Medical Imaging. Dr. Earls is on the speakers bureau for and has research funded by GE Healthcare. Dr. Leipsic is a consultant/speaker for Edwards Lifesciences and GE Healthcare.
New technologies that solve some old problems could make cardiovascular CT the go-to imaging tool to assess both anatomy and function.
Cardiovascular CT imaging has been a welcome tool in patient assessment due to its noninvasive nature, ease, and quickness. Likewise, cardiovascular CT angiography (CCTA) has provided noninvasive information about vessel stenoses, with lower costs and radiation exposure compared with invasive angiography.
Perhaps the biggest criticism about cardiovascular CT is that, while it provides very good anatomic detail, functional information – a crucial piece to the overall picture – is not discernable.
"Historically, coronary CT angiography has been an extremely anatomic method for documenting the severity of coronary artery stenoses or luminal compromise," said Dr. James K. Min, immediate past president of SCCT. This has left CTA open to the criticism that the more useful evaluation of coronary artery disease is not only to document high-grade stenoses – as with coronary CT angiography – but also to determine the physiologic significance of those stenoses.
However, at this year’s meeting of the Society of Cardiovascular Computed Tomography in Baltimore, "we saw the emergence of two robust methods for physiologic assessment of coronary disease by cardiac CT," he said. These are fractional flow reserve CT and CT-based myocardial perfusion imaging.
Evidence regarding these methods was then bolstered at the annual congress of the European Society of Cardiology in Munich, with the presentation of two imaging trials. The DeFACTO study, comparing CT-based FFR with FFR obtained by conventional angiography, did not show diagnostic superiority of CT-based FFR over angiography, but it did enhance diagnoses compared with CT without FFR. The CORE320 trial, assessing the diagnostic accuracy CT-based myocardial perfusion imaging in patients with suspected coronary artery disease, showed that CT-based myocardial perfusion imaging significantly increased the diagnostic accuracy of CTA alone to delineate flow-limiting disease.
Cardiovascular CT also has been hamstrung by a number of technical limitations. Calcium blooming artifacts make it difficult to get an accurate measurement of calcium lesion components and lumen size and motion artifacts also limit the clarity of CT images, leaving some vessels simply unreadable.
Two new technological advances – dual-energy CT and intelligent motion correction using snapshot freeze – are already changing practice, according to leaders at this year’s annual meeting of the SCCT.
CT Fractional Flow Reserve
Fractional flow reserve (FFR) is defined as the maximal blood flow through a diseased artery to the blood flow in the hypothetical case that this artery is normal. CT-based FFR relies on the use of some complicated math and modeling to calculate FFR from CT data and actually traces its roots back to the air flow modeling that goes into designing airplane wings.
All of the data needed can be gleaned from a single-phase static CT image. Geometry can be extracted from CCTA anatomic data. Boundary conditions can also be determined. Resting coronary blood flow can be calculated from myocardial mass. Mean blood pressure can be estimated from brachial artery pressure. Coronary microcirculatory resistance can be derived from morphometry data. Lastly, fluid properties include the viscosity and density of blood. "Putting it all together, we can come out with a calculated assessment of things like coronary flow and FFR ... now, we can do anatomy and function," said Dr. Matthew J. Budoff, director of cardiology at the University of California at Los Angeles School of Medicine in Torrance.
Until now, FFR was determined through invasive coronary angiography, which was the only method for specific determination of coronary artery lesions (lesion-specific ischemia). Values of 0.80 and lower or 0.75 and lower are considered diagnostic of lesion-specific ischemia. "It tells us whether there is a physiologic significance of the lesion," Dr. Budoff said.
At the same time, stenosis seen on CCTA has been an unreliable predictor of lesion-specific ischemia in trials. "Just seeing that anatomical stenosis doesn’t mean it’s functionally significant," said Dr. Budoff. CCTA results in a lot of false positives when it comes to physiologic significance.
FFR estimates throughout the entire coronary tree. "Because it relies on the entire coronary anatomy, it’s actually less sensitive to artifacts and less sensitive to things like calcification than individual segment assessment."
The first trial of FFR-CT was in the Diagnosis of Ischemia-Causing Stenoses Obtained via Noninvasive Fractional Flow Reserve (DISCOVER-FLOW) trial. In the study, the researchers compared the accuracy of CCTA with FFR-CT (invasive FFR served as the reference).
The results showed that the coronary stenoses that cause ischemia can be identified noninvasively with computer analysis of CCTA to construct FFR for specific lesions (Eur. Heart J. Cardiovasc. Imaging 2012 Jul 15 [doi: 10.1093/ehjci/jes130]).
The upshot of this study is that "when we think it’s a high-grade stenosis [with FFR-CT], it is a physiologically significant stenosis," Dr. Budoff said. "So we’ve dramatically improved the ability of CT to correlate with the most invasive and probably the most definitive way, currently, of assessing physiologic significance, and that’s FFR."
CT-based fractional flow reserve (FFR) is now a step closer to clinical use with the results of the Determination of FFR by Anatomic CT Angiography (DeFACTO) trial presented at the ESC meeting and simultaneously published (JAMA 2012 Aug. 26 [doi:10.1001/2012.jama.11274]). The addition of CT-based FFR information to CT alone improved diagnostic accuracy of stenoses, compared with invasive angiography and FFR.
"We’ve dramatically improved the ability of CT to correlate with ... FFR," said Dr. Matthew J. Budoff.
Although FFR-CT plus CT narrowly failed to meet the trial’s primary end point – diagnostic accuracy greater than 70% for the lower bound of the 95% confidence interval, the per-patient performance diagnostic accuracy of FFR-CT plus CT was 73% with a 95% CI of 67%-78%.
Nevertheless, the addition of FFR-CT "demonstrated superior diagnostic performance characteristics, as compared with CT stenosis alone in all patients, in all vessels and also in vessels of intermediate stenosis severity," Dr. Min said during a press conference.
Myocardial Perfusion
"The second method of physiologic assessment of coronary disease, which is emerging, is myocardial perfusion imaging – performing a typical stress test with a CT angiogram," said Dr. Min.
This allows the visualization of perfusion/ischemia at specific lesions identified by CTA, providing functional and anatomic information. The data for perfusion images are extracted from a standard CTA scan.
With CT perfusion, a rest CT angiogram is performed to document the coronary artery stenoses that are within the coronary artery bed and also to look at the rest perfusion of the myocardium to determine whether it’s normal or abnormal, according to Dr. Min.
Before or after the rest CTA, a stress CT would be performed via pharmacologic means using a traditional 64-row cardiac CT.
It’s known that patients who have a normal SPECT myocardial perfusion examination have a very low rate of cardiovascular events over the next year, according to Dr. Richard T. George. "However, those patients with an abnormal nuclear scan actually have a quite high event rate over the next year."
In addition, it’s also known that CCTA has great prognostic value "and probably for a longer period than SPECT does, but SPECT myocardial perfusion imaging probably tells you more about the intermediate time period in the future about the event rate in the patient," he said.
Several studies have looked at the additional value of CT perfusion testing.
One of the first studies that compared CCTA and CT perfusion (CTP) with quantitative coronary angiography and SPECT perfusion showed a sensitivity with CTA/CTP of 88% and a specificity of 91% (Circ. Cardiovasc. Imaging 2009;2:174-82). "This study demonstrates some of the additional value of stress CT myocardial perfusion imaging," said Dr. George, assistant professor of medicine at the Heart and Vascular Institute at Johns Hopkins Hospital in Baltimore.
In another study, researchers assessed the additional value of dipyridamole stress myocardial perfusion by 64-row CT in patients with coronary stents (J. Cardiovasc. Comput. Tomogr. 2011;5:449-58). It is often difficult in many of these patients to assess whether in-stent restenosis is present, said Dr. George. The researchers in this study found that the addition of CT myocardial perfusion imaging to CCTA improved accuracy.
In a study targeting reversible ischemia, researchers assessed CT myocardial perfusion imaging with 320-row detector CT in 50 patients with an intermediate- to high-risk for CAD (Circ. Cardiovasc. Imaging 2012;5:333-40).
"The important part of this study is that they actually looked at reversible ischemia. A lot of our studies with CT perfusion imaging just lump all perfusion deficits together." In it, 40% of patients had an abnormal SPECT scan; 90% of those abnormal scans were reversible ischemia," said Dr. George.
In a per-patient analysis of CT perfusion imaging vs. CTA stenosis greater than 50% in the setting of a territorial SPECT myocardial perfusion deficit, sensitivity was 100% and specificity was 81%. The study shows the effectiveness of CT perfusion for assessing lesion-specific ischemia, Dr. George noted.
"What we’re finding is that CT perfusion or stress testing with CT can be as accurate, if not more accurate, than the conventional stress testing methods that we’ve used until this day," Dr. Min said.
"CT perfusion or stress testing with CT can be as accurate, if not more accurate, than the conventional stress testing method," said Dr. James K. Min.
In the Combined Non-invasive Coronary Angiography and Myocardial Perfusion Imaging Using 320 Detector Computed Tomography (Core320) trial, researchers compared the diagnostic accuracy of CTA plus CT-based myocardial perfusion against ICA plus SPECT myocardial perfusion imaging in 381 patients with suspected or diagnosed coronary artery disease with a clinical indication for coronary angiography.
"When we add perfusion [to CTA alone] we gain power to diagnose flow-limiting stenosis," lead author Dr. Joao A.C. Lima said at the ESC meeting. Together, "they have the same power as invasive angiography and SPECT MPI in defining who are the patients who end up going through revascularization," he said.
The area under the receiver operating characteristic curve (ROC) – an effective method of evaluating the performance of diagnostic tests – was 0.79 for CTA-CTP and 0.81 for ICA-SPECT.
"At this point, the [CT perfusion] technology could be used precisely to find the patients who have flow-limiting disease and, therefore, are going to need revascularization," said Dr. Lima, director of cardiovascular imaging at the Johns Hopkins University in Baltimore.
Dual-Energy CT
Dual-energy CT (also known as dual-source CT) "interleaves sets of high- and low-keV images. The energy alternates between two energies (80 keV to 140 keV) every 0.5 msec," said Dr. James P. Earls. The result is two competing sets that can be used simultaneously. The importance of this dual-energy approach is that with higher energies and a software algorithm, calcium blooming is decreased. This means a more accurate size of calcium in lesions and lumen size.
Back-end software produces a range of monochromatic images (images at a single energy). "In terms of calcium reduction, the monochromatic images are probably more important," said Dr. Earls, codirector of the cardiac CT program at Inova Heart and Vascular Institute in Falls Church, Va.
Monochromatic images range from 40 keV to 140 keV. "At 40 keV you’re very close to the k-edge of iodine, so you have significant attenuation of anything that is enhancing ... at about 75 keV you have something that looks akin to a 120-keV image that we would normally get." As the energy increases, "you get away from what we would do on a routine clinical basis."
The ATLANTA I study (Assessment of Tissue Characteristics, Lesion Morphology, and Hemodynamics by Angiography With Fractional Flow Reserve, Intravascular Ultrasound and Virtual Histology, and Noninvasive Computed Tomography in Atherosclerotic Plaques) showed that with conventional single-energy CT calcium, plaque is overestimated and lumen size is therefore underestimated (J. Am. Coll. Cardiol. Intv. 2011;4:198-208 [doi:10.1016/j.jcin.2010.10.008]).
The ATLANTA I study tells us that "when we do routine single-energy CT, calcium plaques are significantly overportrayed." In this study, researchers found that calcified plaque volume is 104% greater than its true volume as determined by intravascular ultrasound. As a result, the minimal luminal diameter is underestimated by 21% and the percentage diameter stenosis is overrepresented by 39%. "Lots of false positives [are] caused by calcified plaque in the area," Dr. Earls observed.
The reason for this is that as energy increases, the calcium blooming decreases. This accounts for the enlargement of the lumen.
Noncalcified plaques don’t show as dramatic an effect, though. Soft plaque size does not change much when seen at different energies, Dr. Earls noted. "Ultimately it may be that we use a combination of monochromatic images and material density images, as we approach this going forward. These are both available every time that you do a scan," said Dr. Earls.
Dual-source CT does have limitations, though. There is no retrospective gating; there are limited milliAmpere (electrical charge) presets, and there is no high-resolution mode.
Intelligent Motion Correction
Motion artifacts make it difficult or impossible to evaluate vessels, leaving clinicians to treat the vessels as narrowed by default. A new technology is designed to overcome the problem of motion artifacts in cardiovascular CT.
"The images are acquired in a standard fashion, but instead of reconstructing them in a standard fashion, there is integration of this intelligent motion correction that tracks the motion of the vessel over two or three phases of the cardiac cycle," Dr. Jonathon A. Leipsic said in an interview. "When we acquire the study, we get a couple of phases beyond just the minimal acquisition – which is standard. It sees where the vessel is over a couple of phases and how it looks. Based on the known velocity of the artery, how it appears, and the patient’s known heart rate, [the algorithm] then corrects for the expected motion."
The technology is already changing practice. It is used on Europe and Japan; it is also being used in many centers in the United States. The motion correction algorithm has wide range of applications. "Anywhere there is motion, anyone with a sudden ectopic pace or premature ventricular contraction, anyone with an arrhythmia – all of those patients will benefit from this motion correction," said Dr. Leipsic, chairman of the department of radiology for Providence Health Care and vice chairman of research for the department of radiology at the University of British Columbia, Vancouver.
Importantly, the scan time and radiation dose don’t increase appreciably with the acquisition of vessel velocity data. "It doesn’t change the acquisition. You can just wait and see. If you have a study that has no motion, then you don’t need to use [motion correction]. But if you have a study with motion, then you can use it. You can make that choice after you see the initial data," Dr. Leipsic explained.
Dr. Leipsic was the lead author on one of the first studies to assess the accuracy this of this method (J. Cardiovasc. Comput. Tomography 2012;6:164-71. Epub 2012 Apr 6). In the study, "we looked at a population of convenience patients that happened to be going to the cath lab. We chose very difficult patients – patients undergoing transcatheter valve replacement – who have very high heart rates. We saw a significant improvement in interpretability, overall image quality, and diagnostic accuracy" with this technology. The researchers also noted significant improvements in right coronary evaluation, as well as other coronary territories.
Some have argued that all that is needed to avoid motion artifacts is to rate-control patients. However, "we aggressively rate-controlled patients ... there’s just too much motion," Dr. Leipsic countered. "It’s hard to anticipate some problems – an irregular beat or some irregular rhythm – and having this in your back pocket ... has exciting potential."
Additional trials to assess accuracy are expected, perhaps most prominently the VICTORY trial (Validation of an Intracycle CT Motion Correction Algorithm for Diagnostic Accuracy: A Prospective Multicenter Study). In the study, CCTA will be compared with invasive coronary angiography (ICA) for diagnostic sensitivity and specificity, positive predictive value, negative predictive value, diagnostic accuracy, and positive and negative likelihood ratios.
Coronary segments will be assessed for "significance" of coronary artery luminal diameter obstruction. Individual segments will be graded based on image quality, with the third reader used to achieve consensus. Dr. Leipsic and Dr. James Min are the principal investigators for the study.
For now, the intelligent motion correction algorithm, called SnapShot Freez4e, is available only from GE Healthcare, as part of its Discovery CT750 HD FREEdom Edition, which was granted 510(k) clearance in June. "I think that other companies are going to come up with something similar but I think that each type will be adequately different enough that [these technologies] will require their own validation studies," said Dr. Leipsic.
Siemens is reportedly developing a similar technology.
Dr. Min is a speaker for GE Healthcare. Dr. Budoff receives grant support from HeartFlow. Dr. George has received research support from Toshiba Medical Systems and GE Healthcare, is on the advisory board of GE Healthcare, and is a consultant to ICON Medical Imaging. Dr. Earls is on the speakers bureau for and has research funded by GE Healthcare. Dr. Leipsic is a consultant/speaker for Edwards Lifesciences and GE Healthcare.
Antimalarial Response in CLE Takes Time
BOSTON – The use of adjunctive therapy and a lot of patience may go a long way to improving the response of cutaneous lupus erythematosus to antimalarial agents, according to Dr. Jennie T. Clarke.
Antimalarials are the go-to treatment for cutaneous lupus erythematosus (CLE), yet roughly a third of patients do not respond, said Dr. Clarke, who spoke at the American Academy of Dermatology’s Summer Academy Meeting.
Findings from some recent studies shed light on how to improve that dismal response rate. In one of these trials involving 128 patients with CLE, researchers found that slightly more than half of patients who initiated treatment with hydroxychloroquine monotherapy were responders (55%) (Arch. Dermatol. 2011;147:1261-7). When quinacrine was added to the treatment regimen of the nonresponders, two-thirds experienced a lessening of their disease. Improvement continued beyond 2 months in 43%.
"With antimalarials remember that patience is important. [These agents] have a slow onset. You have to give the drugs for 2-3 months before assessing for efficacy. Patients need to know this because otherwise they’re going to become frustrated and noncompliant," said Dr. Clarke of the department of dermatology at Pennsylvania State University, Hershey.
The other important lesson from the study is about combination antimalarial therapy. Quinacrine can be added to either hydroxychloroquine or chloroquine to achieve improvement in patients who don’t respond to a single antimalarial agent.
However, roughly a third of patients don’t respond to a combination of antimalarials. Some research in the last 18 months has tried to identify which patients fail antimalarial treatment and why, in order to improve subsequent treatment. Three possible reasons have been identified: dosing and compliance, disease severity, and smoking status.
Bioavailability and clearance of hydroxychloroquine seems to vary by individual, she said. In addition, noncompliance is estimated to be about 10%. In a study of 300 CLE patients, French researchers found that the median blood concentration of hydroxychloroquine correlated with response (Arch. Dermatol. 2012;148:479-84). Specifically, the median blood hydroxychloroquine concentration was significantly higher in patients with complete remission compared with those with either partial remission or treatment failure. Findings from the multivariate analysis showed that complete remission was associated with higher blood hydroxychloroquine concentrations and the absence of discoid lesions.
Concentration and response were correlated with actual rather than ideal body weight dosing. Smoking was not found to be related to concentration, and the subset of 170 patients with discoid lupus erythematosus (DLE) was less responsive to hydroxychloroquine.
Thirty patients (10%) had very low blood hydroxychloroquine concentrations (less than 200 ng/mL) and may be considered nonadherent to the treatment regimen, Dr. Clarke noted. However, additional study is needed to determine the optimal blood concentration of hydroxychloroquine and the impact of toxicity with actual weight-based dosing rather than ideal body weight dosing.
In another study, researchers assessed the clinical and pharmacogenic influences of disease severity on response to blood concentration of hydroxychloroquine (J. Invest. Dermatol. 2011;131:1981-6). They assessed 200 patients with DLE. Slightly more than a third (35%) of patients had not responded to hydroxychloroquine at 6 months. Poor response was associated with disease severity and concomitant systemic lupus erythematosus (SLE). However, response was not associated with the presence of cytochrome P450 genotype or smoking.
It has been long held medical dogma that smoking lessens patients’ response to antimalarial drugs. The impact of smoking in patients with CLE was assessed directly in another paper (Arch. Dermatol. 2012;148:317-22). The researchers included 218 patients with CLE or SLE with skin disease. They found that current smokers had more severe disease and poorer disease-related quality of life. Smokers were also more likely to receive combination antimalarial therapy. Current smokers responded better to antimalarials than past or never smokers. However, smokers responded worse than nonsmokers if antimalarials and immunomodulator/suppressives were required.
"This tells us that antimalarials can be effective for smokers, particularly in those with more mild disease. But we need to remember that smokers who don’t respond are likely going to have poorer outcomes than nonsmokers who don’t respond to antimalarials."
Dr. Clarke reported having no relevant financial conflicts.
BOSTON – The use of adjunctive therapy and a lot of patience may go a long way to improving the response of cutaneous lupus erythematosus to antimalarial agents, according to Dr. Jennie T. Clarke.
Antimalarials are the go-to treatment for cutaneous lupus erythematosus (CLE), yet roughly a third of patients do not respond, said Dr. Clarke, who spoke at the American Academy of Dermatology’s Summer Academy Meeting.
Findings from some recent studies shed light on how to improve that dismal response rate. In one of these trials involving 128 patients with CLE, researchers found that slightly more than half of patients who initiated treatment with hydroxychloroquine monotherapy were responders (55%) (Arch. Dermatol. 2011;147:1261-7). When quinacrine was added to the treatment regimen of the nonresponders, two-thirds experienced a lessening of their disease. Improvement continued beyond 2 months in 43%.
"With antimalarials remember that patience is important. [These agents] have a slow onset. You have to give the drugs for 2-3 months before assessing for efficacy. Patients need to know this because otherwise they’re going to become frustrated and noncompliant," said Dr. Clarke of the department of dermatology at Pennsylvania State University, Hershey.
The other important lesson from the study is about combination antimalarial therapy. Quinacrine can be added to either hydroxychloroquine or chloroquine to achieve improvement in patients who don’t respond to a single antimalarial agent.
However, roughly a third of patients don’t respond to a combination of antimalarials. Some research in the last 18 months has tried to identify which patients fail antimalarial treatment and why, in order to improve subsequent treatment. Three possible reasons have been identified: dosing and compliance, disease severity, and smoking status.
Bioavailability and clearance of hydroxychloroquine seems to vary by individual, she said. In addition, noncompliance is estimated to be about 10%. In a study of 300 CLE patients, French researchers found that the median blood concentration of hydroxychloroquine correlated with response (Arch. Dermatol. 2012;148:479-84). Specifically, the median blood hydroxychloroquine concentration was significantly higher in patients with complete remission compared with those with either partial remission or treatment failure. Findings from the multivariate analysis showed that complete remission was associated with higher blood hydroxychloroquine concentrations and the absence of discoid lesions.
Concentration and response were correlated with actual rather than ideal body weight dosing. Smoking was not found to be related to concentration, and the subset of 170 patients with discoid lupus erythematosus (DLE) was less responsive to hydroxychloroquine.
Thirty patients (10%) had very low blood hydroxychloroquine concentrations (less than 200 ng/mL) and may be considered nonadherent to the treatment regimen, Dr. Clarke noted. However, additional study is needed to determine the optimal blood concentration of hydroxychloroquine and the impact of toxicity with actual weight-based dosing rather than ideal body weight dosing.
In another study, researchers assessed the clinical and pharmacogenic influences of disease severity on response to blood concentration of hydroxychloroquine (J. Invest. Dermatol. 2011;131:1981-6). They assessed 200 patients with DLE. Slightly more than a third (35%) of patients had not responded to hydroxychloroquine at 6 months. Poor response was associated with disease severity and concomitant systemic lupus erythematosus (SLE). However, response was not associated with the presence of cytochrome P450 genotype or smoking.
It has been long held medical dogma that smoking lessens patients’ response to antimalarial drugs. The impact of smoking in patients with CLE was assessed directly in another paper (Arch. Dermatol. 2012;148:317-22). The researchers included 218 patients with CLE or SLE with skin disease. They found that current smokers had more severe disease and poorer disease-related quality of life. Smokers were also more likely to receive combination antimalarial therapy. Current smokers responded better to antimalarials than past or never smokers. However, smokers responded worse than nonsmokers if antimalarials and immunomodulator/suppressives were required.
"This tells us that antimalarials can be effective for smokers, particularly in those with more mild disease. But we need to remember that smokers who don’t respond are likely going to have poorer outcomes than nonsmokers who don’t respond to antimalarials."
Dr. Clarke reported having no relevant financial conflicts.
BOSTON – The use of adjunctive therapy and a lot of patience may go a long way to improving the response of cutaneous lupus erythematosus to antimalarial agents, according to Dr. Jennie T. Clarke.
Antimalarials are the go-to treatment for cutaneous lupus erythematosus (CLE), yet roughly a third of patients do not respond, said Dr. Clarke, who spoke at the American Academy of Dermatology’s Summer Academy Meeting.
Findings from some recent studies shed light on how to improve that dismal response rate. In one of these trials involving 128 patients with CLE, researchers found that slightly more than half of patients who initiated treatment with hydroxychloroquine monotherapy were responders (55%) (Arch. Dermatol. 2011;147:1261-7). When quinacrine was added to the treatment regimen of the nonresponders, two-thirds experienced a lessening of their disease. Improvement continued beyond 2 months in 43%.
"With antimalarials remember that patience is important. [These agents] have a slow onset. You have to give the drugs for 2-3 months before assessing for efficacy. Patients need to know this because otherwise they’re going to become frustrated and noncompliant," said Dr. Clarke of the department of dermatology at Pennsylvania State University, Hershey.
The other important lesson from the study is about combination antimalarial therapy. Quinacrine can be added to either hydroxychloroquine or chloroquine to achieve improvement in patients who don’t respond to a single antimalarial agent.
However, roughly a third of patients don’t respond to a combination of antimalarials. Some research in the last 18 months has tried to identify which patients fail antimalarial treatment and why, in order to improve subsequent treatment. Three possible reasons have been identified: dosing and compliance, disease severity, and smoking status.
Bioavailability and clearance of hydroxychloroquine seems to vary by individual, she said. In addition, noncompliance is estimated to be about 10%. In a study of 300 CLE patients, French researchers found that the median blood concentration of hydroxychloroquine correlated with response (Arch. Dermatol. 2012;148:479-84). Specifically, the median blood hydroxychloroquine concentration was significantly higher in patients with complete remission compared with those with either partial remission or treatment failure. Findings from the multivariate analysis showed that complete remission was associated with higher blood hydroxychloroquine concentrations and the absence of discoid lesions.
Concentration and response were correlated with actual rather than ideal body weight dosing. Smoking was not found to be related to concentration, and the subset of 170 patients with discoid lupus erythematosus (DLE) was less responsive to hydroxychloroquine.
Thirty patients (10%) had very low blood hydroxychloroquine concentrations (less than 200 ng/mL) and may be considered nonadherent to the treatment regimen, Dr. Clarke noted. However, additional study is needed to determine the optimal blood concentration of hydroxychloroquine and the impact of toxicity with actual weight-based dosing rather than ideal body weight dosing.
In another study, researchers assessed the clinical and pharmacogenic influences of disease severity on response to blood concentration of hydroxychloroquine (J. Invest. Dermatol. 2011;131:1981-6). They assessed 200 patients with DLE. Slightly more than a third (35%) of patients had not responded to hydroxychloroquine at 6 months. Poor response was associated with disease severity and concomitant systemic lupus erythematosus (SLE). However, response was not associated with the presence of cytochrome P450 genotype or smoking.
It has been long held medical dogma that smoking lessens patients’ response to antimalarial drugs. The impact of smoking in patients with CLE was assessed directly in another paper (Arch. Dermatol. 2012;148:317-22). The researchers included 218 patients with CLE or SLE with skin disease. They found that current smokers had more severe disease and poorer disease-related quality of life. Smokers were also more likely to receive combination antimalarial therapy. Current smokers responded better to antimalarials than past or never smokers. However, smokers responded worse than nonsmokers if antimalarials and immunomodulator/suppressives were required.
"This tells us that antimalarials can be effective for smokers, particularly in those with more mild disease. But we need to remember that smokers who don’t respond are likely going to have poorer outcomes than nonsmokers who don’t respond to antimalarials."
Dr. Clarke reported having no relevant financial conflicts.
EXPERT ANALYSIS FROM THE AMERICAN ACADEMY OF DERMATOLOGY'S SUMMER ACADEMY MEETING
Psoriasis Severity Linked to Tonsil Size
BOSTON – Patients with psoriasis are almost nine times more likely to have enlarged tonsils, compared with patients without psoriasis, according to the results of a small study.
"Our findings suggest that hypertrophic tonsils may be associated with a pathogenic role in psoriasis," Dr. Marianna Shvartsbeyn and her coinvestigators reported in a poster presented at the American Academy of Dermatology’s Summer Academy Meeting. But it is still too soon to know the clinical implications.
In all, 32 patients with psoriasis and 14 patients with noninflammatory skin conditions (common warts, melanoma, and nonmelanoma skin diseases) were recruited. Patients who previously underwent tonsillectomy were excluded.
Tonsils were examined by one investigator, using a 5-point standardized tonsillar hypertrophy grading scale (adopted from Am. Fam. Physician 2004;69:1147-55).
Tonsils that were entirely within the tonsillar fossa received a grade of 0. Tonsils occupying less than 25% of the lateral dimension of the oropharynx, as measured between the anterior tonsillar pillars, received a grade of 1; tonsils occupying less than 50% of the lateral dimension of the oropharynx were a 2; tonsils occupying less than 75% of the lateral dimension of the oropharynx were a 3; and tonsils occupying 75 % or more of the lateral dimension of the oropharynx received a grade of 4.
Chart reviews were conducted to collect information on patient age, sex, race, social history (tobacco, alcohol, and drug use), diagnosis of skin condition, and the duration/severity of disease, noted Dr. Shvartsbeyn and her colleague of the departments of pathology and dermatology at the New York University.
Patients with psoriasis were found to have had an odds ratio of 8.77 for having enlarged tonsils (grade 2 or greater), compared with healthy controls. Tonsillar size also was significantly larger in patients with psoriasis (mean tonsil grade, 1.78), than in control patients (mean tonsil grade, 0.86); the severity of psoriasis was positively associated with tonsil size, Dr. Shvartsbeyn and her colleagues reported.
Limited clinical data have suggested that there is an association between hypertrophic tonsils and inflammatory skin disease. Small studies have shown that among patients with psoriasis, the cutaneous lesions disappeared or improved after tonsillectomy. It is suspected that there may be a genetic predisposition that makes certain patient populations more susceptible, the researchers noted.
Histopathologic studies also point to the possible link between the robust immune response that takes place in the tonsils and the changes in the skin of patients with pustulosis palmaris et plantaris (PPP).
Histologic evaluation of tonsils obtained from patients with PPP has revealed enlargement of the secondary T nodules and atrophy of the lymph follicles, with a decrease in the number of the germinal center cells and fibrosis – changes typically seen in older tonsils. This finding provides indirect evidence of the intensely advanced stage of the immune response within the tonsils.
"Our hypothesis is that in chronic tonsillar hypertrophy, bacterial species that reside in the tonsils are released into the circulation and cause stimulation of T cells. As a result of this constant chronic stimulation, an autoreactive clone may be formed. The auto-clone may produce an antibody attacking the skin and drive inflammatory response. In some individuals, this exaggerated immune response may manifest as psoriasis," the investigators wrote.
And although there is empirical evidence "that tonsillectomy improved skin lesions in patients with psoriasis and pustulosis palmaris et plantaris in small retrospective studies, further studies are needed. ... The observed association needs validation and interventional study is needed to prove causation/contribution," Dr. Shvartsbeyn noted in an interview.
The study was supported by grants from the National Cancer Institute, the National Institute for Allergy and Infectious Diseases, and the National Institute of Dental and Craniofacial Research. The investigators did not report having any conflicts of interest.
BOSTON – Patients with psoriasis are almost nine times more likely to have enlarged tonsils, compared with patients without psoriasis, according to the results of a small study.
"Our findings suggest that hypertrophic tonsils may be associated with a pathogenic role in psoriasis," Dr. Marianna Shvartsbeyn and her coinvestigators reported in a poster presented at the American Academy of Dermatology’s Summer Academy Meeting. But it is still too soon to know the clinical implications.
In all, 32 patients with psoriasis and 14 patients with noninflammatory skin conditions (common warts, melanoma, and nonmelanoma skin diseases) were recruited. Patients who previously underwent tonsillectomy were excluded.
Tonsils were examined by one investigator, using a 5-point standardized tonsillar hypertrophy grading scale (adopted from Am. Fam. Physician 2004;69:1147-55).
Tonsils that were entirely within the tonsillar fossa received a grade of 0. Tonsils occupying less than 25% of the lateral dimension of the oropharynx, as measured between the anterior tonsillar pillars, received a grade of 1; tonsils occupying less than 50% of the lateral dimension of the oropharynx were a 2; tonsils occupying less than 75% of the lateral dimension of the oropharynx were a 3; and tonsils occupying 75 % or more of the lateral dimension of the oropharynx received a grade of 4.
Chart reviews were conducted to collect information on patient age, sex, race, social history (tobacco, alcohol, and drug use), diagnosis of skin condition, and the duration/severity of disease, noted Dr. Shvartsbeyn and her colleague of the departments of pathology and dermatology at the New York University.
Patients with psoriasis were found to have had an odds ratio of 8.77 for having enlarged tonsils (grade 2 or greater), compared with healthy controls. Tonsillar size also was significantly larger in patients with psoriasis (mean tonsil grade, 1.78), than in control patients (mean tonsil grade, 0.86); the severity of psoriasis was positively associated with tonsil size, Dr. Shvartsbeyn and her colleagues reported.
Limited clinical data have suggested that there is an association between hypertrophic tonsils and inflammatory skin disease. Small studies have shown that among patients with psoriasis, the cutaneous lesions disappeared or improved after tonsillectomy. It is suspected that there may be a genetic predisposition that makes certain patient populations more susceptible, the researchers noted.
Histopathologic studies also point to the possible link between the robust immune response that takes place in the tonsils and the changes in the skin of patients with pustulosis palmaris et plantaris (PPP).
Histologic evaluation of tonsils obtained from patients with PPP has revealed enlargement of the secondary T nodules and atrophy of the lymph follicles, with a decrease in the number of the germinal center cells and fibrosis – changes typically seen in older tonsils. This finding provides indirect evidence of the intensely advanced stage of the immune response within the tonsils.
"Our hypothesis is that in chronic tonsillar hypertrophy, bacterial species that reside in the tonsils are released into the circulation and cause stimulation of T cells. As a result of this constant chronic stimulation, an autoreactive clone may be formed. The auto-clone may produce an antibody attacking the skin and drive inflammatory response. In some individuals, this exaggerated immune response may manifest as psoriasis," the investigators wrote.
And although there is empirical evidence "that tonsillectomy improved skin lesions in patients with psoriasis and pustulosis palmaris et plantaris in small retrospective studies, further studies are needed. ... The observed association needs validation and interventional study is needed to prove causation/contribution," Dr. Shvartsbeyn noted in an interview.
The study was supported by grants from the National Cancer Institute, the National Institute for Allergy and Infectious Diseases, and the National Institute of Dental and Craniofacial Research. The investigators did not report having any conflicts of interest.
BOSTON – Patients with psoriasis are almost nine times more likely to have enlarged tonsils, compared with patients without psoriasis, according to the results of a small study.
"Our findings suggest that hypertrophic tonsils may be associated with a pathogenic role in psoriasis," Dr. Marianna Shvartsbeyn and her coinvestigators reported in a poster presented at the American Academy of Dermatology’s Summer Academy Meeting. But it is still too soon to know the clinical implications.
In all, 32 patients with psoriasis and 14 patients with noninflammatory skin conditions (common warts, melanoma, and nonmelanoma skin diseases) were recruited. Patients who previously underwent tonsillectomy were excluded.
Tonsils were examined by one investigator, using a 5-point standardized tonsillar hypertrophy grading scale (adopted from Am. Fam. Physician 2004;69:1147-55).
Tonsils that were entirely within the tonsillar fossa received a grade of 0. Tonsils occupying less than 25% of the lateral dimension of the oropharynx, as measured between the anterior tonsillar pillars, received a grade of 1; tonsils occupying less than 50% of the lateral dimension of the oropharynx were a 2; tonsils occupying less than 75% of the lateral dimension of the oropharynx were a 3; and tonsils occupying 75 % or more of the lateral dimension of the oropharynx received a grade of 4.
Chart reviews were conducted to collect information on patient age, sex, race, social history (tobacco, alcohol, and drug use), diagnosis of skin condition, and the duration/severity of disease, noted Dr. Shvartsbeyn and her colleague of the departments of pathology and dermatology at the New York University.
Patients with psoriasis were found to have had an odds ratio of 8.77 for having enlarged tonsils (grade 2 or greater), compared with healthy controls. Tonsillar size also was significantly larger in patients with psoriasis (mean tonsil grade, 1.78), than in control patients (mean tonsil grade, 0.86); the severity of psoriasis was positively associated with tonsil size, Dr. Shvartsbeyn and her colleagues reported.
Limited clinical data have suggested that there is an association between hypertrophic tonsils and inflammatory skin disease. Small studies have shown that among patients with psoriasis, the cutaneous lesions disappeared or improved after tonsillectomy. It is suspected that there may be a genetic predisposition that makes certain patient populations more susceptible, the researchers noted.
Histopathologic studies also point to the possible link between the robust immune response that takes place in the tonsils and the changes in the skin of patients with pustulosis palmaris et plantaris (PPP).
Histologic evaluation of tonsils obtained from patients with PPP has revealed enlargement of the secondary T nodules and atrophy of the lymph follicles, with a decrease in the number of the germinal center cells and fibrosis – changes typically seen in older tonsils. This finding provides indirect evidence of the intensely advanced stage of the immune response within the tonsils.
"Our hypothesis is that in chronic tonsillar hypertrophy, bacterial species that reside in the tonsils are released into the circulation and cause stimulation of T cells. As a result of this constant chronic stimulation, an autoreactive clone may be formed. The auto-clone may produce an antibody attacking the skin and drive inflammatory response. In some individuals, this exaggerated immune response may manifest as psoriasis," the investigators wrote.
And although there is empirical evidence "that tonsillectomy improved skin lesions in patients with psoriasis and pustulosis palmaris et plantaris in small retrospective studies, further studies are needed. ... The observed association needs validation and interventional study is needed to prove causation/contribution," Dr. Shvartsbeyn noted in an interview.
The study was supported by grants from the National Cancer Institute, the National Institute for Allergy and Infectious Diseases, and the National Institute of Dental and Craniofacial Research. The investigators did not report having any conflicts of interest.
AT THE AMERICAN ACADEMY OF DERMATOLOGY'S SUMMER ACADEMY MEETING
Major Finding: Patients with psoriasis had an odds ratio of 8.77 for having enlarged tonsils (grade 2 or greater), compared with healthy controls.
Data Source: The findings come from a prospective study of 32 patients with psoriasis and 14 patients with noninflammatory skin conditions, who served as controls.
Disclosures: The study was supported by grants from the National Cancer Institute, the National Institute for Allergy and Infectious Diseases, and the National Institute of Dental and Craniofacial Research. The investigators did not report having any conflicts of interest.
FDA Approves Linaclotide for Constipation Conditions
The Food and Drug Administration approved linaclotide on Aug. 30 to treat two conditions: chronic idiopathic constipation and irritable bowel syndrome with constipation in adults.
Linaclotide (Linzess) is administered as a capsule taken once daily on an empty stomach, at least 30 minutes before the first meal of the day. This agent helps relieve constipation by increasing the frequency of bowel movements. In irritable bowel syndrome with constipation (IBS-C), linaclotide has been shown to reduce abdominal pain, according to a statement from the FDA.
The drug is approved with a boxed warning to alert patients and health care professionals that linaclotide should not be used in patients 16 years of age and younger. The most common side effect reported during the clinical studies was diarrhea, the statement said.
According to the FDA, the safety and effectiveness of linaclotide for the management of IBS-C were established in two double-blind studies (Gastroenterology 2011;140:S138 and Gastroenterology 2011;140:S135). A total of 1,604 patients were randomly assigned to take 290 mcg of linaclotide or a placebo for at least 12 weeks. Linaclotide was more effective in reducing abdominal pain and increasing the number of complete spontaneous bowel movements, compared with placebo, in both trials.
The safety and effectiveness of linaclotide for the management of chronic idiopathic constipation also were established in two double-blind studies (N. Engl. J. Med. 2011;365:527-36). A total of 1,272 patients were randomly assigned to take 145 mcg or 290 mcg linaclotide or a placebo for 12 weeks. Patients on linaclotide had more complete spontaneous bowel movements than did those taking the placebo. The 290-mcg dose is not approved for chronic constipation because the data showed that it was no more effective than the 145-mcg dose.
Linzess is marketed by Ironwood Pharmaceuticals Inc.
Linaclotide is currently the only FDA-approved medication indicated for increasing bowel movements and decreasing abdominal pain in men and women with irritable bowel syndrome with constipation (IBS-C). It has been shown to be efficacious in relieving abdominal pain and constipation in patients with IBS-C, and constipation in those with chronic idiopathic constipation (CIC). The drug is a peripherally-acting agent that activates guanylate cyclase-C (GC-C) on intestinal epithelial cells resulting in increased intracellular and extracellular concentrations of cyclic guanosine monophosphate (cGMP).
Relief of constipation symptoms in IBS-C and CIC is believed to be due to an increase in intracellular cGMP resulting in chloride and fluid secretion through the cystic fibrosis transmembrane conductance regulator (CFTR) ion channel and acceleration of colonic transit. Linaclotide’s effect on reducing abdominal pain in IBS-C is thought to be due to increased extracellular cGMP, which has been shown to decrease firing of sensory nerves within the bowel wall in preclinical animal studies.
Patients with CIC who responded to linaclotide had at least three complete spontaneous bowel movements (CSBMs) per week and an increase in one CSBM for at least 9 out of 12 weeks. The 145 mcg and 290 mcg daily doses showed a statistically significant benefit over placebo; the FDA has approved only the lower dose for CIC. The efficacy of linaclotide was sustained throughout the 12 weeks of the trials.
The dose of 290 mcg per day was approved for the treatment of IBS-C, which is usually differentiated from CIC by the presence of predominant abdominal pain associated with constipation. The significant improvement in CSBMs occurred within the first week of treatment. The decrease in abdominal pain was more gradual and appeared to reach its maximum effect at 8 weeks. The significant effect of linaclotide on abdominal pain may be due to an additional independent effect beyond relief of constipation, but further studies are needed to better understand linaclotide’s effect on abdominal pain.
LIN CHANG, M.D., is co-director of the Oppenheimer Family Center for Neurobiology of Stress and director of the Digestive Health and Nutrition Clinic at the University of California, Los Angeles. She is a consultant for Ironwood Pharmaceuticals and Forest Laboratories and has received grant support from Ironwood Pharmaceuticals.
Linaclotide is currently the only FDA-approved medication indicated for increasing bowel movements and decreasing abdominal pain in men and women with irritable bowel syndrome with constipation (IBS-C). It has been shown to be efficacious in relieving abdominal pain and constipation in patients with IBS-C, and constipation in those with chronic idiopathic constipation (CIC). The drug is a peripherally-acting agent that activates guanylate cyclase-C (GC-C) on intestinal epithelial cells resulting in increased intracellular and extracellular concentrations of cyclic guanosine monophosphate (cGMP).
Relief of constipation symptoms in IBS-C and CIC is believed to be due to an increase in intracellular cGMP resulting in chloride and fluid secretion through the cystic fibrosis transmembrane conductance regulator (CFTR) ion channel and acceleration of colonic transit. Linaclotide’s effect on reducing abdominal pain in IBS-C is thought to be due to increased extracellular cGMP, which has been shown to decrease firing of sensory nerves within the bowel wall in preclinical animal studies.
Patients with CIC who responded to linaclotide had at least three complete spontaneous bowel movements (CSBMs) per week and an increase in one CSBM for at least 9 out of 12 weeks. The 145 mcg and 290 mcg daily doses showed a statistically significant benefit over placebo; the FDA has approved only the lower dose for CIC. The efficacy of linaclotide was sustained throughout the 12 weeks of the trials.
The dose of 290 mcg per day was approved for the treatment of IBS-C, which is usually differentiated from CIC by the presence of predominant abdominal pain associated with constipation. The significant improvement in CSBMs occurred within the first week of treatment. The decrease in abdominal pain was more gradual and appeared to reach its maximum effect at 8 weeks. The significant effect of linaclotide on abdominal pain may be due to an additional independent effect beyond relief of constipation, but further studies are needed to better understand linaclotide’s effect on abdominal pain.
LIN CHANG, M.D., is co-director of the Oppenheimer Family Center for Neurobiology of Stress and director of the Digestive Health and Nutrition Clinic at the University of California, Los Angeles. She is a consultant for Ironwood Pharmaceuticals and Forest Laboratories and has received grant support from Ironwood Pharmaceuticals.
Linaclotide is currently the only FDA-approved medication indicated for increasing bowel movements and decreasing abdominal pain in men and women with irritable bowel syndrome with constipation (IBS-C). It has been shown to be efficacious in relieving abdominal pain and constipation in patients with IBS-C, and constipation in those with chronic idiopathic constipation (CIC). The drug is a peripherally-acting agent that activates guanylate cyclase-C (GC-C) on intestinal epithelial cells resulting in increased intracellular and extracellular concentrations of cyclic guanosine monophosphate (cGMP).
Relief of constipation symptoms in IBS-C and CIC is believed to be due to an increase in intracellular cGMP resulting in chloride and fluid secretion through the cystic fibrosis transmembrane conductance regulator (CFTR) ion channel and acceleration of colonic transit. Linaclotide’s effect on reducing abdominal pain in IBS-C is thought to be due to increased extracellular cGMP, which has been shown to decrease firing of sensory nerves within the bowel wall in preclinical animal studies.
Patients with CIC who responded to linaclotide had at least three complete spontaneous bowel movements (CSBMs) per week and an increase in one CSBM for at least 9 out of 12 weeks. The 145 mcg and 290 mcg daily doses showed a statistically significant benefit over placebo; the FDA has approved only the lower dose for CIC. The efficacy of linaclotide was sustained throughout the 12 weeks of the trials.
The dose of 290 mcg per day was approved for the treatment of IBS-C, which is usually differentiated from CIC by the presence of predominant abdominal pain associated with constipation. The significant improvement in CSBMs occurred within the first week of treatment. The decrease in abdominal pain was more gradual and appeared to reach its maximum effect at 8 weeks. The significant effect of linaclotide on abdominal pain may be due to an additional independent effect beyond relief of constipation, but further studies are needed to better understand linaclotide’s effect on abdominal pain.
LIN CHANG, M.D., is co-director of the Oppenheimer Family Center for Neurobiology of Stress and director of the Digestive Health and Nutrition Clinic at the University of California, Los Angeles. She is a consultant for Ironwood Pharmaceuticals and Forest Laboratories and has received grant support from Ironwood Pharmaceuticals.
The Food and Drug Administration approved linaclotide on Aug. 30 to treat two conditions: chronic idiopathic constipation and irritable bowel syndrome with constipation in adults.
Linaclotide (Linzess) is administered as a capsule taken once daily on an empty stomach, at least 30 minutes before the first meal of the day. This agent helps relieve constipation by increasing the frequency of bowel movements. In irritable bowel syndrome with constipation (IBS-C), linaclotide has been shown to reduce abdominal pain, according to a statement from the FDA.
The drug is approved with a boxed warning to alert patients and health care professionals that linaclotide should not be used in patients 16 years of age and younger. The most common side effect reported during the clinical studies was diarrhea, the statement said.
According to the FDA, the safety and effectiveness of linaclotide for the management of IBS-C were established in two double-blind studies (Gastroenterology 2011;140:S138 and Gastroenterology 2011;140:S135). A total of 1,604 patients were randomly assigned to take 290 mcg of linaclotide or a placebo for at least 12 weeks. Linaclotide was more effective in reducing abdominal pain and increasing the number of complete spontaneous bowel movements, compared with placebo, in both trials.
The safety and effectiveness of linaclotide for the management of chronic idiopathic constipation also were established in two double-blind studies (N. Engl. J. Med. 2011;365:527-36). A total of 1,272 patients were randomly assigned to take 145 mcg or 290 mcg linaclotide or a placebo for 12 weeks. Patients on linaclotide had more complete spontaneous bowel movements than did those taking the placebo. The 290-mcg dose is not approved for chronic constipation because the data showed that it was no more effective than the 145-mcg dose.
Linzess is marketed by Ironwood Pharmaceuticals Inc.
The Food and Drug Administration approved linaclotide on Aug. 30 to treat two conditions: chronic idiopathic constipation and irritable bowel syndrome with constipation in adults.
Linaclotide (Linzess) is administered as a capsule taken once daily on an empty stomach, at least 30 minutes before the first meal of the day. This agent helps relieve constipation by increasing the frequency of bowel movements. In irritable bowel syndrome with constipation (IBS-C), linaclotide has been shown to reduce abdominal pain, according to a statement from the FDA.
The drug is approved with a boxed warning to alert patients and health care professionals that linaclotide should not be used in patients 16 years of age and younger. The most common side effect reported during the clinical studies was diarrhea, the statement said.
According to the FDA, the safety and effectiveness of linaclotide for the management of IBS-C were established in two double-blind studies (Gastroenterology 2011;140:S138 and Gastroenterology 2011;140:S135). A total of 1,604 patients were randomly assigned to take 290 mcg of linaclotide or a placebo for at least 12 weeks. Linaclotide was more effective in reducing abdominal pain and increasing the number of complete spontaneous bowel movements, compared with placebo, in both trials.
The safety and effectiveness of linaclotide for the management of chronic idiopathic constipation also were established in two double-blind studies (N. Engl. J. Med. 2011;365:527-36). A total of 1,272 patients were randomly assigned to take 145 mcg or 290 mcg linaclotide or a placebo for 12 weeks. Patients on linaclotide had more complete spontaneous bowel movements than did those taking the placebo. The 290-mcg dose is not approved for chronic constipation because the data showed that it was no more effective than the 145-mcg dose.
Linzess is marketed by Ironwood Pharmaceuticals Inc.
FAME 2 Favorable PCI Results Driven by Revascularization
The use of fractional flow reserve to guide percutaneous coronary intervention, along with best medical management, sharply reduced the need for urgent revascularization in patients with stable coronary artery disease and at least one physiologically significant lesion.
However, FFR-guided percutaneous coronary intervention (PCI) had little effect on deaths or myocardial infarctions, when compared with best medical management alone, according to the results of the FAME 2 (Fractional Flow Reserve vs. Angiography for Multivessel Evaluation 2) trial, which was conducted at 28 sites in Europe and North America and halted early.
The percentage of patients who had an MI, death, or urgent revascularization (the combined primary end point) was significantly lower in the PCI group than in the medical therapy group (4.3% vs.12.7%; hazard ratio with PCI, 0.32). This difference was driven by a 13-fold increase in the need for urgent revascularization in the medical-therapy group. Notably, the rate of death from any cause and the rate of MI did not differ significantly between the PCI group and the medical therapy group.
Importantly, patient recruitment was stopped on Jan. 15, 2012, at the recommendation of an independent data and safety monitoring board because of the highly significant difference in the incidence rates of the primary end point between the PCI and medical-therapy groups.
The results of the study were released in the New England Journal of Medicine on Aug. 28 to coincide with the presentation of the study at the annual congress of the European Society of Cardiology (N. Engl. J. Med. 2012 Aug. 28 [doi:10.1056/NEJMoa1205361]).
A total of 1,220 patients out a planned 1,832 were enrolled. Of those, 888 patients had at least one stenosis with an FFR of 0.80 or less: 447 patients were randomly assigned to FFR-guided PCI plus the best available medical therapy, and 441 patients to the best available medical therapy alone. The 332 patients with angiographically significant stenoses, but none with an FFR of 0.80 or less were enrolled in the registry and received the best available medical therapy alone. The mean duration of follow-up was 212 days.
Patients in stable condition who were appropriate candidates for PCI and who had angiographically assessed one-, two-, or three-vessel coronary artery disease suitable for PCI were included in the trial.
All patients were prescribed aspirin at a dose of 80-325 mg daily, metoprolol at a dose of 50 mg-200 mg daily (or any other beta-blocker), lisinopril (at least 5 mg daily, or another ACE inhibitor or an angiotensin receptor blocker)and atorvastatin (20-80 mg daily, or another statin).
All PCI patients were treated with second-generation drug-eluting stents.
Among the 56 patients who underwent urgent revascularization, the procedure was triggered by a MI in 12 patients (21%), by unstable angina accompanied by evidence of ischemia on ECG in 15 patients (27%), and by unstable angina diagnosed on the basis of clinical features in 29 patients (52%).
Patients in the PCI group were 86% less likely to undergo any revascularization and 83% less likely to undergo or nonurgent revascularization than were those in the medical therapy group.
The researchers identified several factors that may explain the differences between results in the present study and those in previous trials involving patients with stable coronary disease. "First, in previous trials in which various revascularization methods were compared with the best available medical therapy, patient enrollment was based primarily on angiographic findings, with or without noninvasive documentation of ischemia. It is likely that a sizable proportion of the patients had only limited ischemia," wrote lead investigator Dr. Bernard B. De Bruyne and his coinvestigators. Dr. De Bruyne is the codirector of the Cardiovascular Center at OLV Hospital in Aalst, Belgium.
"Even in the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial, in which noninvasive testing was performed in 85% of the patients, less than one-third of the patients had more than 10% ischemia on myocardial perfusion imaging. In daily clinical practice, less than half of patients undergo noninvasive stress testing before elective PCI. In the current trial, all the patients who underwent randomization had at least one functionally significant stenosis," they observed (N. Engl. J. Med. 2007;356:1503-16).
Second, PCI was performed only in lesions with an FFR of 0.80 or less. "This FFR-guided approach is associated with a better clinical outcome than that with PCI performed on the basis of angiographic results alone. These features probably explain the similarity of event rates between patients who were treated with PCI plus the best available medical therapy and patients with equivalent baseline characteristics but no functionally significant lesions who were enrolled in the registry and treated with the best available medical therapy alone," according to the investigators.
Third, second-generation drug-eluting stents were used in PCIs. This strategy is associated with a low number of repeat revascularizations. Finally, the primary end point included urgent revascularization, a component that was not included in the primary end point of previous trials.
The study was sponsored by St. Jude Medical, which makes the two pressure wires used in the trial. The company was involved in the collection and source verification of the data but not in the conduct of the trial. Dr. De Bruyne reported receiving consulting/honorarium and travel fees from St. Jude Medical. Most of the investigators had significant financial relationships with St. Jude Medical, as well as with other device and pharmaceutical companies. One author is an employee of St. Jude Medical.
The recommendation to terminate FAME 2 by the independent data and safety monitoring board at 7 months’ follow-up was based solely on one highly significant treatment difference. That difference was in the end point of urgent revascularization, which was performed in 49 patients in the group that received the best available medical therapy alone vs. 7 patients in the group that underwent percutaneous coronary intervention and also received the best available medical therapy, Dr. William E. Boden wrote in an editorial accompanying the FAME 2 report (N. Engl. J. Med. 2012 Aug. 28 [doi:10.1056/NEJMe1208620]).
"There were very few ‘hard’ events overall, with only four deaths (three in the medical-therapy group and one in the PCI group) and 29 myocardial infarctions (14 in the medical-therapy group and 15 in the PCI group). Of note, the definition of urgent revascularization was largely a clinical one and did not require evidence of ischemia or positive cardiac biomarkers in all patients; 29 of the 56 unplanned revascularizations (52%) were classified solely on the basis of clinical features, whereas in an exploratory subgroup analysis of the remaining 27 patients, there were fewer revascularizations triggered by a myocardial infarction or electrocardiographic evidence of ischemia in the PCI group than in the medical-therapy group," wrote Dr. Boden.
"Clearly, FFR [fractional flow reserve] holds potential promise for a more targeted approach to PCI that might be more clinically effective and cost effective than visually directed PCI for all angiographically significant stenoses," he continued. "Unfortunately, the early termination of the FAME 2 trial before full enrollment and follow-up were achieved, the neutral effects on the rate of death or myocardial infarction, and the lack of a significant, sustained treatment effect on the reduction of angina beyond 6 months leave more questions than answers."
The FAME 2 and COURAGE [Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation] trials are remarkably similar and showed that PCI reduced only the need for revascularization; in the COURAGE trial, there was a significant 40% reduction, whereas "neither the FAME 2 trial (with a mean 7 months of follow-up) nor the COURAGE trial (with a mean 55 months of follow-up) showed a benefit from PCI with respect to a reduction in the rate of death or myocardial infarction. The FAME 2 trial sought to establish the scientific basis for an FFR-guided PCI strategy for all functionally significant stenoses, but the results make this prospect somewhat unappealing," he wrote. Dr. Boden was the lead author of the COURAGE trial.
Current practice guidelines advocate the selective use of FFR to guide PCI decision making regarding borderline visual lesions (approximately 50%-70% stenosis). "It seems likely that the more routine use of FFR for all angiographically-significant stenoses would add considerable time, cost, and complexity to each PCI procedure and might also increase the risk of catheter-related complications such as coronary dissection and perforation," Dr. Boden pointed out.
Some of the uncertainty arising from FAME 2 may be resolved with the results of the ongoing ISCHEMIA (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches) trial. The study is designed and powered to evaluate the long-term superiority of revascularization plus the best available medical therapy as compared with the best available medical therapy alone with respect to cardiovascular death or MI in patients with stable coronary artery disease and moderate-to-severe myocardial ischemia documented by means of noninvasive measures. "Until the results of ISCHEMIA are available, the case for a more durable clinical benefit of PCI beyond relief of angina or a reduction in the rate of subsequent revascularization is likely to remain both elusive and illusory," concluded Dr. Boden, who is a coprincipal investigator of ISCHEMIA.
Dr. Boden is the chief of medicine at the Albany Stratton Veterans Affairs Medical Center and vice chair of the department of medicine at Albany (N.Y.) Medical Center. He reported that he is a paid consultant for Arbor Pharmaceuticals and is a speaker for Abbott Laboratories and Gilead Sciences.
The recommendation to terminate FAME 2 by the independent data and safety monitoring board at 7 months’ follow-up was based solely on one highly significant treatment difference. That difference was in the end point of urgent revascularization, which was performed in 49 patients in the group that received the best available medical therapy alone vs. 7 patients in the group that underwent percutaneous coronary intervention and also received the best available medical therapy, Dr. William E. Boden wrote in an editorial accompanying the FAME 2 report (N. Engl. J. Med. 2012 Aug. 28 [doi:10.1056/NEJMe1208620]).
"There were very few ‘hard’ events overall, with only four deaths (three in the medical-therapy group and one in the PCI group) and 29 myocardial infarctions (14 in the medical-therapy group and 15 in the PCI group). Of note, the definition of urgent revascularization was largely a clinical one and did not require evidence of ischemia or positive cardiac biomarkers in all patients; 29 of the 56 unplanned revascularizations (52%) were classified solely on the basis of clinical features, whereas in an exploratory subgroup analysis of the remaining 27 patients, there were fewer revascularizations triggered by a myocardial infarction or electrocardiographic evidence of ischemia in the PCI group than in the medical-therapy group," wrote Dr. Boden.
"Clearly, FFR [fractional flow reserve] holds potential promise for a more targeted approach to PCI that might be more clinically effective and cost effective than visually directed PCI for all angiographically significant stenoses," he continued. "Unfortunately, the early termination of the FAME 2 trial before full enrollment and follow-up were achieved, the neutral effects on the rate of death or myocardial infarction, and the lack of a significant, sustained treatment effect on the reduction of angina beyond 6 months leave more questions than answers."
The FAME 2 and COURAGE [Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation] trials are remarkably similar and showed that PCI reduced only the need for revascularization; in the COURAGE trial, there was a significant 40% reduction, whereas "neither the FAME 2 trial (with a mean 7 months of follow-up) nor the COURAGE trial (with a mean 55 months of follow-up) showed a benefit from PCI with respect to a reduction in the rate of death or myocardial infarction. The FAME 2 trial sought to establish the scientific basis for an FFR-guided PCI strategy for all functionally significant stenoses, but the results make this prospect somewhat unappealing," he wrote. Dr. Boden was the lead author of the COURAGE trial.
Current practice guidelines advocate the selective use of FFR to guide PCI decision making regarding borderline visual lesions (approximately 50%-70% stenosis). "It seems likely that the more routine use of FFR for all angiographically-significant stenoses would add considerable time, cost, and complexity to each PCI procedure and might also increase the risk of catheter-related complications such as coronary dissection and perforation," Dr. Boden pointed out.
Some of the uncertainty arising from FAME 2 may be resolved with the results of the ongoing ISCHEMIA (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches) trial. The study is designed and powered to evaluate the long-term superiority of revascularization plus the best available medical therapy as compared with the best available medical therapy alone with respect to cardiovascular death or MI in patients with stable coronary artery disease and moderate-to-severe myocardial ischemia documented by means of noninvasive measures. "Until the results of ISCHEMIA are available, the case for a more durable clinical benefit of PCI beyond relief of angina or a reduction in the rate of subsequent revascularization is likely to remain both elusive and illusory," concluded Dr. Boden, who is a coprincipal investigator of ISCHEMIA.
Dr. Boden is the chief of medicine at the Albany Stratton Veterans Affairs Medical Center and vice chair of the department of medicine at Albany (N.Y.) Medical Center. He reported that he is a paid consultant for Arbor Pharmaceuticals and is a speaker for Abbott Laboratories and Gilead Sciences.
The recommendation to terminate FAME 2 by the independent data and safety monitoring board at 7 months’ follow-up was based solely on one highly significant treatment difference. That difference was in the end point of urgent revascularization, which was performed in 49 patients in the group that received the best available medical therapy alone vs. 7 patients in the group that underwent percutaneous coronary intervention and also received the best available medical therapy, Dr. William E. Boden wrote in an editorial accompanying the FAME 2 report (N. Engl. J. Med. 2012 Aug. 28 [doi:10.1056/NEJMe1208620]).
"There were very few ‘hard’ events overall, with only four deaths (three in the medical-therapy group and one in the PCI group) and 29 myocardial infarctions (14 in the medical-therapy group and 15 in the PCI group). Of note, the definition of urgent revascularization was largely a clinical one and did not require evidence of ischemia or positive cardiac biomarkers in all patients; 29 of the 56 unplanned revascularizations (52%) were classified solely on the basis of clinical features, whereas in an exploratory subgroup analysis of the remaining 27 patients, there were fewer revascularizations triggered by a myocardial infarction or electrocardiographic evidence of ischemia in the PCI group than in the medical-therapy group," wrote Dr. Boden.
"Clearly, FFR [fractional flow reserve] holds potential promise for a more targeted approach to PCI that might be more clinically effective and cost effective than visually directed PCI for all angiographically significant stenoses," he continued. "Unfortunately, the early termination of the FAME 2 trial before full enrollment and follow-up were achieved, the neutral effects on the rate of death or myocardial infarction, and the lack of a significant, sustained treatment effect on the reduction of angina beyond 6 months leave more questions than answers."
The FAME 2 and COURAGE [Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation] trials are remarkably similar and showed that PCI reduced only the need for revascularization; in the COURAGE trial, there was a significant 40% reduction, whereas "neither the FAME 2 trial (with a mean 7 months of follow-up) nor the COURAGE trial (with a mean 55 months of follow-up) showed a benefit from PCI with respect to a reduction in the rate of death or myocardial infarction. The FAME 2 trial sought to establish the scientific basis for an FFR-guided PCI strategy for all functionally significant stenoses, but the results make this prospect somewhat unappealing," he wrote. Dr. Boden was the lead author of the COURAGE trial.
Current practice guidelines advocate the selective use of FFR to guide PCI decision making regarding borderline visual lesions (approximately 50%-70% stenosis). "It seems likely that the more routine use of FFR for all angiographically-significant stenoses would add considerable time, cost, and complexity to each PCI procedure and might also increase the risk of catheter-related complications such as coronary dissection and perforation," Dr. Boden pointed out.
Some of the uncertainty arising from FAME 2 may be resolved with the results of the ongoing ISCHEMIA (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches) trial. The study is designed and powered to evaluate the long-term superiority of revascularization plus the best available medical therapy as compared with the best available medical therapy alone with respect to cardiovascular death or MI in patients with stable coronary artery disease and moderate-to-severe myocardial ischemia documented by means of noninvasive measures. "Until the results of ISCHEMIA are available, the case for a more durable clinical benefit of PCI beyond relief of angina or a reduction in the rate of subsequent revascularization is likely to remain both elusive and illusory," concluded Dr. Boden, who is a coprincipal investigator of ISCHEMIA.
Dr. Boden is the chief of medicine at the Albany Stratton Veterans Affairs Medical Center and vice chair of the department of medicine at Albany (N.Y.) Medical Center. He reported that he is a paid consultant for Arbor Pharmaceuticals and is a speaker for Abbott Laboratories and Gilead Sciences.
The use of fractional flow reserve to guide percutaneous coronary intervention, along with best medical management, sharply reduced the need for urgent revascularization in patients with stable coronary artery disease and at least one physiologically significant lesion.
However, FFR-guided percutaneous coronary intervention (PCI) had little effect on deaths or myocardial infarctions, when compared with best medical management alone, according to the results of the FAME 2 (Fractional Flow Reserve vs. Angiography for Multivessel Evaluation 2) trial, which was conducted at 28 sites in Europe and North America and halted early.
The percentage of patients who had an MI, death, or urgent revascularization (the combined primary end point) was significantly lower in the PCI group than in the medical therapy group (4.3% vs.12.7%; hazard ratio with PCI, 0.32). This difference was driven by a 13-fold increase in the need for urgent revascularization in the medical-therapy group. Notably, the rate of death from any cause and the rate of MI did not differ significantly between the PCI group and the medical therapy group.
Importantly, patient recruitment was stopped on Jan. 15, 2012, at the recommendation of an independent data and safety monitoring board because of the highly significant difference in the incidence rates of the primary end point between the PCI and medical-therapy groups.
The results of the study were released in the New England Journal of Medicine on Aug. 28 to coincide with the presentation of the study at the annual congress of the European Society of Cardiology (N. Engl. J. Med. 2012 Aug. 28 [doi:10.1056/NEJMoa1205361]).
A total of 1,220 patients out a planned 1,832 were enrolled. Of those, 888 patients had at least one stenosis with an FFR of 0.80 or less: 447 patients were randomly assigned to FFR-guided PCI plus the best available medical therapy, and 441 patients to the best available medical therapy alone. The 332 patients with angiographically significant stenoses, but none with an FFR of 0.80 or less were enrolled in the registry and received the best available medical therapy alone. The mean duration of follow-up was 212 days.
Patients in stable condition who were appropriate candidates for PCI and who had angiographically assessed one-, two-, or three-vessel coronary artery disease suitable for PCI were included in the trial.
All patients were prescribed aspirin at a dose of 80-325 mg daily, metoprolol at a dose of 50 mg-200 mg daily (or any other beta-blocker), lisinopril (at least 5 mg daily, or another ACE inhibitor or an angiotensin receptor blocker)and atorvastatin (20-80 mg daily, or another statin).
All PCI patients were treated with second-generation drug-eluting stents.
Among the 56 patients who underwent urgent revascularization, the procedure was triggered by a MI in 12 patients (21%), by unstable angina accompanied by evidence of ischemia on ECG in 15 patients (27%), and by unstable angina diagnosed on the basis of clinical features in 29 patients (52%).
Patients in the PCI group were 86% less likely to undergo any revascularization and 83% less likely to undergo or nonurgent revascularization than were those in the medical therapy group.
The researchers identified several factors that may explain the differences between results in the present study and those in previous trials involving patients with stable coronary disease. "First, in previous trials in which various revascularization methods were compared with the best available medical therapy, patient enrollment was based primarily on angiographic findings, with or without noninvasive documentation of ischemia. It is likely that a sizable proportion of the patients had only limited ischemia," wrote lead investigator Dr. Bernard B. De Bruyne and his coinvestigators. Dr. De Bruyne is the codirector of the Cardiovascular Center at OLV Hospital in Aalst, Belgium.
"Even in the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial, in which noninvasive testing was performed in 85% of the patients, less than one-third of the patients had more than 10% ischemia on myocardial perfusion imaging. In daily clinical practice, less than half of patients undergo noninvasive stress testing before elective PCI. In the current trial, all the patients who underwent randomization had at least one functionally significant stenosis," they observed (N. Engl. J. Med. 2007;356:1503-16).
Second, PCI was performed only in lesions with an FFR of 0.80 or less. "This FFR-guided approach is associated with a better clinical outcome than that with PCI performed on the basis of angiographic results alone. These features probably explain the similarity of event rates between patients who were treated with PCI plus the best available medical therapy and patients with equivalent baseline characteristics but no functionally significant lesions who were enrolled in the registry and treated with the best available medical therapy alone," according to the investigators.
Third, second-generation drug-eluting stents were used in PCIs. This strategy is associated with a low number of repeat revascularizations. Finally, the primary end point included urgent revascularization, a component that was not included in the primary end point of previous trials.
The study was sponsored by St. Jude Medical, which makes the two pressure wires used in the trial. The company was involved in the collection and source verification of the data but not in the conduct of the trial. Dr. De Bruyne reported receiving consulting/honorarium and travel fees from St. Jude Medical. Most of the investigators had significant financial relationships with St. Jude Medical, as well as with other device and pharmaceutical companies. One author is an employee of St. Jude Medical.
The use of fractional flow reserve to guide percutaneous coronary intervention, along with best medical management, sharply reduced the need for urgent revascularization in patients with stable coronary artery disease and at least one physiologically significant lesion.
However, FFR-guided percutaneous coronary intervention (PCI) had little effect on deaths or myocardial infarctions, when compared with best medical management alone, according to the results of the FAME 2 (Fractional Flow Reserve vs. Angiography for Multivessel Evaluation 2) trial, which was conducted at 28 sites in Europe and North America and halted early.
The percentage of patients who had an MI, death, or urgent revascularization (the combined primary end point) was significantly lower in the PCI group than in the medical therapy group (4.3% vs.12.7%; hazard ratio with PCI, 0.32). This difference was driven by a 13-fold increase in the need for urgent revascularization in the medical-therapy group. Notably, the rate of death from any cause and the rate of MI did not differ significantly between the PCI group and the medical therapy group.
Importantly, patient recruitment was stopped on Jan. 15, 2012, at the recommendation of an independent data and safety monitoring board because of the highly significant difference in the incidence rates of the primary end point between the PCI and medical-therapy groups.
The results of the study were released in the New England Journal of Medicine on Aug. 28 to coincide with the presentation of the study at the annual congress of the European Society of Cardiology (N. Engl. J. Med. 2012 Aug. 28 [doi:10.1056/NEJMoa1205361]).
A total of 1,220 patients out a planned 1,832 were enrolled. Of those, 888 patients had at least one stenosis with an FFR of 0.80 or less: 447 patients were randomly assigned to FFR-guided PCI plus the best available medical therapy, and 441 patients to the best available medical therapy alone. The 332 patients with angiographically significant stenoses, but none with an FFR of 0.80 or less were enrolled in the registry and received the best available medical therapy alone. The mean duration of follow-up was 212 days.
Patients in stable condition who were appropriate candidates for PCI and who had angiographically assessed one-, two-, or three-vessel coronary artery disease suitable for PCI were included in the trial.
All patients were prescribed aspirin at a dose of 80-325 mg daily, metoprolol at a dose of 50 mg-200 mg daily (or any other beta-blocker), lisinopril (at least 5 mg daily, or another ACE inhibitor or an angiotensin receptor blocker)and atorvastatin (20-80 mg daily, or another statin).
All PCI patients were treated with second-generation drug-eluting stents.
Among the 56 patients who underwent urgent revascularization, the procedure was triggered by a MI in 12 patients (21%), by unstable angina accompanied by evidence of ischemia on ECG in 15 patients (27%), and by unstable angina diagnosed on the basis of clinical features in 29 patients (52%).
Patients in the PCI group were 86% less likely to undergo any revascularization and 83% less likely to undergo or nonurgent revascularization than were those in the medical therapy group.
The researchers identified several factors that may explain the differences between results in the present study and those in previous trials involving patients with stable coronary disease. "First, in previous trials in which various revascularization methods were compared with the best available medical therapy, patient enrollment was based primarily on angiographic findings, with or without noninvasive documentation of ischemia. It is likely that a sizable proportion of the patients had only limited ischemia," wrote lead investigator Dr. Bernard B. De Bruyne and his coinvestigators. Dr. De Bruyne is the codirector of the Cardiovascular Center at OLV Hospital in Aalst, Belgium.
"Even in the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial, in which noninvasive testing was performed in 85% of the patients, less than one-third of the patients had more than 10% ischemia on myocardial perfusion imaging. In daily clinical practice, less than half of patients undergo noninvasive stress testing before elective PCI. In the current trial, all the patients who underwent randomization had at least one functionally significant stenosis," they observed (N. Engl. J. Med. 2007;356:1503-16).
Second, PCI was performed only in lesions with an FFR of 0.80 or less. "This FFR-guided approach is associated with a better clinical outcome than that with PCI performed on the basis of angiographic results alone. These features probably explain the similarity of event rates between patients who were treated with PCI plus the best available medical therapy and patients with equivalent baseline characteristics but no functionally significant lesions who were enrolled in the registry and treated with the best available medical therapy alone," according to the investigators.
Third, second-generation drug-eluting stents were used in PCIs. This strategy is associated with a low number of repeat revascularizations. Finally, the primary end point included urgent revascularization, a component that was not included in the primary end point of previous trials.
The study was sponsored by St. Jude Medical, which makes the two pressure wires used in the trial. The company was involved in the collection and source verification of the data but not in the conduct of the trial. Dr. De Bruyne reported receiving consulting/honorarium and travel fees from St. Jude Medical. Most of the investigators had significant financial relationships with St. Jude Medical, as well as with other device and pharmaceutical companies. One author is an employee of St. Jude Medical.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Major Finding: The percentage of patients who had an MI, death, or urgent revascularization (the combined primary end point) was significantly lower in patients who underwent FFR-guided PCI than in patients who received best medical management alone (4.3% vs.12.7%; hazard ratio with PCI, 0.32). This difference was driven by a 13-fold increase in the need for urgent revascularization in the medical therapy group.
Data Source: Data are from the FAME 2 trial, in 1,220 stable patients who had angiographically assessed coronary artery disease and were appropriate candidates for PCI. The trial was stopped early because of a highly significant difference in the primary outcome.
Disclosures: The study was sponsored by St. Jude Medical. Dr. De Bruyne reported receiving consulting/honorarium and travel fees from St. Jude Medical. Most of the investigators had significant financial relationships with St. Jude Medical, as well as with other device and pharmaceutical companies. One author is an employee of St. Jude Medical.
DeFACTO Propels CT Fractional Flow Reserve Closer to Clinical Practice
The addition of CT-based fractional flow reserve information to CT alone improved the diagnostic accuracy of stenoses, allowing noninvasive assessment of the physiologic consequences of lesions, according to the long-awaited results of the Determination of Fractional Flow Reserve by Anatomic Computed Tomographic Angiography (DeFACTO) study.
However, CT fractional flow reserve (FFR-CT) plus CT narrowly failed to meet the trial’s primary end point – diagnostic accuracy greater than 70% for the lower bound of the 95% confidence interval. Per-patient performance diagnostic accuracy of FFR-CT plus CT was 73% with a 95% CI of 67%-78%.
Nevertheless, the addition of FFR-CT "demonstrated superior diagnostic performance characteristics, as compared with CT stenosis alone, in all patients, in all vessels, and also in vessels of intermediate stenosis severity," lead author Dr. James K. Min said during a press conference.
The results of the study were released in JAMA on Aug. 26th to coincide with the presentation of the study at the European Society of Cardiology meeting (JAMA 2012;308 [doi: 10.1001/2012.jama.11274]).
Fractional flow reserve (FFR) is currently assessed during invasive coronary angiography (ICA) to determine whether a coronary stenosis results in ischemia, and is the currently accepted reference standard for determining lesion-specific ischemia. FFR is the ratio of the mean coronary pressure distal to a coronary stenosis to the mean aortic pressure during maximal coronary blood flow. This value describes coronary flow still attainable despite the presence of a stenotic lesion.
While CT angiography has long been used to accurately and noninvasively assess the anatomic severity of stenoses, the technique has been criticized because it does not yield functional information about the hemodynamic effect of lesions.
Noninvasive calculation of FFR from CT "is a novel method that applies computational fluid dynamics to determine the physiologic significance of CAD [coronary artery disease]. Fractional flow reserve from CT enables calculation of rest and hyperemic pressure fields in coronary arteries without additional imaging, modification of CT acquisition protocols, or administration of medications," the investigators wrote.
"Taken together, these study results suggest the potential of FFR-CT as a promising noninvasive method for identification of individuals with ischemia. The present study findings can be considered proof of concept of the feasibility of this novel technology."
A total of 252 patients were included in the final analysis of the DeFACTO study. These patients had CAD and underwent clinically indicated ICA after CT with no intervening coronary event. Patients were not eligible if they had a history of coronary artery bypass graft (CABG) surgery or percutaneous coronary intervention. About 77% of patients had experienced angina within the past month.
Among 615 study vessels, 271 had less than 30% stenosis and 101 had at least 90% stenosis. In all, 407 vessels were directly assessed by both FFR and FFR-CT.
Computed tomographic angiography was performed on 64- or greater detector scanners with prospective or retrospective electrocardiographic gating.
The investigators evaluated CTs for maximal patient-, vessel-, and segment-based diameter stenosis (characterized as 0%, 1%-29%, 30%-49%, or 50% or larger).
Per-patient and per-vessel CAD stenosis were the maximal stenoses identified in all segments or in all segments within a vessel distribution, respectively. Vessel distributions were categorized for the left anterior descending, left circumflex, and right coronary artery. Computed tomographic angiograms (CTAs) were judged as excellent, good, adequate, or nondiagnostic.
Selective ICA was performed by standard protocol, and FFR was performed at the time of ICA. Fractional flow reserve was considered diagnostic of ischemia at a threshold of 0.80 or less. Computation of FFR-CT was performed in blinded fashion by the FFR-CT core laboratory at HeartFlow, the study’s sponsor.
Per-patient diagnostic accuracy for FFR-CT plus CT was 73%. By comparison, diagnostic accuracy of CT alone was 64%.
FFR-CT also demonstrated greater discriminatory power than CT alone for vessels directly assessed by invasive FFR. For these vessels, the diagnostic sensitivity and specificity of FFR-CT alone were 80% and 61%, respectively.
Importantly, the researchers performed a secondary analysis of patients with an intermediate stenosis ranging from 30% to 70%, "wherein the clinical utility of FFR-CT would be most commonly expected for use." Diagnostic accuracy (73% for FFR-CT and 57% for CT), sensitivity (82% and 37%, respectively), positive predictive value (54% and 34%) and negative predictive value (88% and 68%) were greater for FFR-CT than for CT, though specificity was similar at 66%.
This intermediate group is an important patient population. "We know that patients with 30%-70% stenosis – even though they don’t look high-risk anatomically – actually, some of them experience ischemia and physiologic consequences of their coronary artery disease," said Dr. Min, director of cardiac imaging research and co-director of cardiac imaging at the Cedars-Sinai Heart Institute in Los Angeles.
High sensitivity/low specificity among patients with intermediate stenoses suggests "a low false-negative rate if assessments by FFR-CT were used to identify ischemia causing intermediate lesions, with negligible effects on reductions of false positive results. In this regard, the use of FFR-CT may significantly advance clinical assessment of patients without conventional measures of anatomic high-grade coronary stenosis, largely by proper identification of a significantly greater proportion of patients with manifest ischemia rather than as a safeguard to further invasive evaluation," the researchers noted.
They also pointed out that the prespecified primary end point for FFR-CT – a lower bound of the 95% confidence interval greater than 70% – "represents a 15% increase over traditional noninvasive histologic imaging methods, including myocardial perfusion imaging by SPECT or stress echocardiography," Dr. Min said.
Dr. Min and several of his coauthors reported significant financial relationships with GE Healthcare and Philips Medical, as well as other medical imaging/pharmaceutical companies. Dr. Jason H. Cole reported a grant for research support from HeartFlow. Dr. John Mancini reported a grant to his institution from HeartFlow. This study was funded by HeartFlow.
"Technologies that provide both a highly sensitive anatomic evaluation for obstructive disease and a highly specific physiologic evaluation for ischemia represent the ‘Holy Grail’ for noninvasive imaging for CAD," Dr. Manesh R. Patel wrote in an accompanying editorial (JAMA 2012 Aug. 26 [doi: 10.1001/2012.jama.11383]).
One possible investigational approach is the combination of anatomic analysis using CT and functional analysis using fractional flow reserve based on CT data (FFR-CT).
The DeFACTO investigators "raise the bar by comparing this diagnostic technology with a reference standard of both invasive angiography and invasive FFR. This change in reference standard may in part explain some of the accuracy findings. So how should these findings be considered with regard to current clinical evaluation for chest pain?" asked Dr. Patel.
It’s important to put the findings on the performance of CT angiography into context, he wrote. "Several recent multicenter studies have reported diagnostic performance of CT angiography to have high sensitivity (i.e., between 85%-95%) compared with conventional invasive angiography for stenoses of 50% or greater." The high sensitivity of CTA has been used to triage low-risk patients in acute settings.
"However, in stable intermediate-risk patients, for whom a higher degree of specificity (low rate of false positive results) may be desirable to reduce referrals for invasive angiography, concerns exist about the specificity of CT angiography," Dr. Patel noted. In the present study, CT angiography had a sensitivity of 84% but a specificity of only 42% with the more rigorous reference standard.
"It is in this context that FFR-CT represents a novel and important innovation, with the possibility not only to diagnose but also to help direct invasive treatment. The current ... multicenter report by Min et al. confirms a high sensitivity (90%) but demonstrates modest specificity (54%), albeit better than CTA alone," he wrote.
"At first glance, readers of the study may consider FFR-CT technology to be limited based on the results presented. However, this would be a naive conclusion, likely based on the published diagnostic performance of noninvasive tests compared only with invasive angiography," Dr. Patel warned. By comparing existing noninvasive imaging technologies with invasive angiography plus FFR, it is highly likely that the published diagnostic performance would be reduced. "In fact, in clinical practice, the sole use of invasive angiography for lesion evaluation has decreased. Additionally, in real-world practice, the current noninvasive technologies used for diagnosis and risk stratification in stable elective patients prior to invasive angiography do not perform at the published diagnostic levels, as evidenced by the low rates of obstructive CAD at elective catheterization. Hence, the current report describes an important noninvasive technology that may improve existing care and has the potential to outperform established noninvasive technologies," according to Dr. Patel.
DR. PATEL is the cardiology section leader in the peripheral vascular program at Duke University in Durham, N.C., and is assistant director of the cardiac catheterization laboratory. Dr. Patel reports consultancy for Bayer, Jansen, Baxter, and Otsuka, and grants from Johnson & Johnson and AstraZeneca.
"Technologies that provide both a highly sensitive anatomic evaluation for obstructive disease and a highly specific physiologic evaluation for ischemia represent the ‘Holy Grail’ for noninvasive imaging for CAD," Dr. Manesh R. Patel wrote in an accompanying editorial (JAMA 2012 Aug. 26 [doi: 10.1001/2012.jama.11383]).
One possible investigational approach is the combination of anatomic analysis using CT and functional analysis using fractional flow reserve based on CT data (FFR-CT).
The DeFACTO investigators "raise the bar by comparing this diagnostic technology with a reference standard of both invasive angiography and invasive FFR. This change in reference standard may in part explain some of the accuracy findings. So how should these findings be considered with regard to current clinical evaluation for chest pain?" asked Dr. Patel.
It’s important to put the findings on the performance of CT angiography into context, he wrote. "Several recent multicenter studies have reported diagnostic performance of CT angiography to have high sensitivity (i.e., between 85%-95%) compared with conventional invasive angiography for stenoses of 50% or greater." The high sensitivity of CTA has been used to triage low-risk patients in acute settings.
"However, in stable intermediate-risk patients, for whom a higher degree of specificity (low rate of false positive results) may be desirable to reduce referrals for invasive angiography, concerns exist about the specificity of CT angiography," Dr. Patel noted. In the present study, CT angiography had a sensitivity of 84% but a specificity of only 42% with the more rigorous reference standard.
"It is in this context that FFR-CT represents a novel and important innovation, with the possibility not only to diagnose but also to help direct invasive treatment. The current ... multicenter report by Min et al. confirms a high sensitivity (90%) but demonstrates modest specificity (54%), albeit better than CTA alone," he wrote.
"At first glance, readers of the study may consider FFR-CT technology to be limited based on the results presented. However, this would be a naive conclusion, likely based on the published diagnostic performance of noninvasive tests compared only with invasive angiography," Dr. Patel warned. By comparing existing noninvasive imaging technologies with invasive angiography plus FFR, it is highly likely that the published diagnostic performance would be reduced. "In fact, in clinical practice, the sole use of invasive angiography for lesion evaluation has decreased. Additionally, in real-world practice, the current noninvasive technologies used for diagnosis and risk stratification in stable elective patients prior to invasive angiography do not perform at the published diagnostic levels, as evidenced by the low rates of obstructive CAD at elective catheterization. Hence, the current report describes an important noninvasive technology that may improve existing care and has the potential to outperform established noninvasive technologies," according to Dr. Patel.
DR. PATEL is the cardiology section leader in the peripheral vascular program at Duke University in Durham, N.C., and is assistant director of the cardiac catheterization laboratory. Dr. Patel reports consultancy for Bayer, Jansen, Baxter, and Otsuka, and grants from Johnson & Johnson and AstraZeneca.
"Technologies that provide both a highly sensitive anatomic evaluation for obstructive disease and a highly specific physiologic evaluation for ischemia represent the ‘Holy Grail’ for noninvasive imaging for CAD," Dr. Manesh R. Patel wrote in an accompanying editorial (JAMA 2012 Aug. 26 [doi: 10.1001/2012.jama.11383]).
One possible investigational approach is the combination of anatomic analysis using CT and functional analysis using fractional flow reserve based on CT data (FFR-CT).
The DeFACTO investigators "raise the bar by comparing this diagnostic technology with a reference standard of both invasive angiography and invasive FFR. This change in reference standard may in part explain some of the accuracy findings. So how should these findings be considered with regard to current clinical evaluation for chest pain?" asked Dr. Patel.
It’s important to put the findings on the performance of CT angiography into context, he wrote. "Several recent multicenter studies have reported diagnostic performance of CT angiography to have high sensitivity (i.e., between 85%-95%) compared with conventional invasive angiography for stenoses of 50% or greater." The high sensitivity of CTA has been used to triage low-risk patients in acute settings.
"However, in stable intermediate-risk patients, for whom a higher degree of specificity (low rate of false positive results) may be desirable to reduce referrals for invasive angiography, concerns exist about the specificity of CT angiography," Dr. Patel noted. In the present study, CT angiography had a sensitivity of 84% but a specificity of only 42% with the more rigorous reference standard.
"It is in this context that FFR-CT represents a novel and important innovation, with the possibility not only to diagnose but also to help direct invasive treatment. The current ... multicenter report by Min et al. confirms a high sensitivity (90%) but demonstrates modest specificity (54%), albeit better than CTA alone," he wrote.
"At first glance, readers of the study may consider FFR-CT technology to be limited based on the results presented. However, this would be a naive conclusion, likely based on the published diagnostic performance of noninvasive tests compared only with invasive angiography," Dr. Patel warned. By comparing existing noninvasive imaging technologies with invasive angiography plus FFR, it is highly likely that the published diagnostic performance would be reduced. "In fact, in clinical practice, the sole use of invasive angiography for lesion evaluation has decreased. Additionally, in real-world practice, the current noninvasive technologies used for diagnosis and risk stratification in stable elective patients prior to invasive angiography do not perform at the published diagnostic levels, as evidenced by the low rates of obstructive CAD at elective catheterization. Hence, the current report describes an important noninvasive technology that may improve existing care and has the potential to outperform established noninvasive technologies," according to Dr. Patel.
DR. PATEL is the cardiology section leader in the peripheral vascular program at Duke University in Durham, N.C., and is assistant director of the cardiac catheterization laboratory. Dr. Patel reports consultancy for Bayer, Jansen, Baxter, and Otsuka, and grants from Johnson & Johnson and AstraZeneca.
The addition of CT-based fractional flow reserve information to CT alone improved the diagnostic accuracy of stenoses, allowing noninvasive assessment of the physiologic consequences of lesions, according to the long-awaited results of the Determination of Fractional Flow Reserve by Anatomic Computed Tomographic Angiography (DeFACTO) study.
However, CT fractional flow reserve (FFR-CT) plus CT narrowly failed to meet the trial’s primary end point – diagnostic accuracy greater than 70% for the lower bound of the 95% confidence interval. Per-patient performance diagnostic accuracy of FFR-CT plus CT was 73% with a 95% CI of 67%-78%.
Nevertheless, the addition of FFR-CT "demonstrated superior diagnostic performance characteristics, as compared with CT stenosis alone, in all patients, in all vessels, and also in vessels of intermediate stenosis severity," lead author Dr. James K. Min said during a press conference.
The results of the study were released in JAMA on Aug. 26th to coincide with the presentation of the study at the European Society of Cardiology meeting (JAMA 2012;308 [doi: 10.1001/2012.jama.11274]).
Fractional flow reserve (FFR) is currently assessed during invasive coronary angiography (ICA) to determine whether a coronary stenosis results in ischemia, and is the currently accepted reference standard for determining lesion-specific ischemia. FFR is the ratio of the mean coronary pressure distal to a coronary stenosis to the mean aortic pressure during maximal coronary blood flow. This value describes coronary flow still attainable despite the presence of a stenotic lesion.
While CT angiography has long been used to accurately and noninvasively assess the anatomic severity of stenoses, the technique has been criticized because it does not yield functional information about the hemodynamic effect of lesions.
Noninvasive calculation of FFR from CT "is a novel method that applies computational fluid dynamics to determine the physiologic significance of CAD [coronary artery disease]. Fractional flow reserve from CT enables calculation of rest and hyperemic pressure fields in coronary arteries without additional imaging, modification of CT acquisition protocols, or administration of medications," the investigators wrote.
"Taken together, these study results suggest the potential of FFR-CT as a promising noninvasive method for identification of individuals with ischemia. The present study findings can be considered proof of concept of the feasibility of this novel technology."
A total of 252 patients were included in the final analysis of the DeFACTO study. These patients had CAD and underwent clinically indicated ICA after CT with no intervening coronary event. Patients were not eligible if they had a history of coronary artery bypass graft (CABG) surgery or percutaneous coronary intervention. About 77% of patients had experienced angina within the past month.
Among 615 study vessels, 271 had less than 30% stenosis and 101 had at least 90% stenosis. In all, 407 vessels were directly assessed by both FFR and FFR-CT.
Computed tomographic angiography was performed on 64- or greater detector scanners with prospective or retrospective electrocardiographic gating.
The investigators evaluated CTs for maximal patient-, vessel-, and segment-based diameter stenosis (characterized as 0%, 1%-29%, 30%-49%, or 50% or larger).
Per-patient and per-vessel CAD stenosis were the maximal stenoses identified in all segments or in all segments within a vessel distribution, respectively. Vessel distributions were categorized for the left anterior descending, left circumflex, and right coronary artery. Computed tomographic angiograms (CTAs) were judged as excellent, good, adequate, or nondiagnostic.
Selective ICA was performed by standard protocol, and FFR was performed at the time of ICA. Fractional flow reserve was considered diagnostic of ischemia at a threshold of 0.80 or less. Computation of FFR-CT was performed in blinded fashion by the FFR-CT core laboratory at HeartFlow, the study’s sponsor.
Per-patient diagnostic accuracy for FFR-CT plus CT was 73%. By comparison, diagnostic accuracy of CT alone was 64%.
FFR-CT also demonstrated greater discriminatory power than CT alone for vessels directly assessed by invasive FFR. For these vessels, the diagnostic sensitivity and specificity of FFR-CT alone were 80% and 61%, respectively.
Importantly, the researchers performed a secondary analysis of patients with an intermediate stenosis ranging from 30% to 70%, "wherein the clinical utility of FFR-CT would be most commonly expected for use." Diagnostic accuracy (73% for FFR-CT and 57% for CT), sensitivity (82% and 37%, respectively), positive predictive value (54% and 34%) and negative predictive value (88% and 68%) were greater for FFR-CT than for CT, though specificity was similar at 66%.
This intermediate group is an important patient population. "We know that patients with 30%-70% stenosis – even though they don’t look high-risk anatomically – actually, some of them experience ischemia and physiologic consequences of their coronary artery disease," said Dr. Min, director of cardiac imaging research and co-director of cardiac imaging at the Cedars-Sinai Heart Institute in Los Angeles.
High sensitivity/low specificity among patients with intermediate stenoses suggests "a low false-negative rate if assessments by FFR-CT were used to identify ischemia causing intermediate lesions, with negligible effects on reductions of false positive results. In this regard, the use of FFR-CT may significantly advance clinical assessment of patients without conventional measures of anatomic high-grade coronary stenosis, largely by proper identification of a significantly greater proportion of patients with manifest ischemia rather than as a safeguard to further invasive evaluation," the researchers noted.
They also pointed out that the prespecified primary end point for FFR-CT – a lower bound of the 95% confidence interval greater than 70% – "represents a 15% increase over traditional noninvasive histologic imaging methods, including myocardial perfusion imaging by SPECT or stress echocardiography," Dr. Min said.
Dr. Min and several of his coauthors reported significant financial relationships with GE Healthcare and Philips Medical, as well as other medical imaging/pharmaceutical companies. Dr. Jason H. Cole reported a grant for research support from HeartFlow. Dr. John Mancini reported a grant to his institution from HeartFlow. This study was funded by HeartFlow.
The addition of CT-based fractional flow reserve information to CT alone improved the diagnostic accuracy of stenoses, allowing noninvasive assessment of the physiologic consequences of lesions, according to the long-awaited results of the Determination of Fractional Flow Reserve by Anatomic Computed Tomographic Angiography (DeFACTO) study.
However, CT fractional flow reserve (FFR-CT) plus CT narrowly failed to meet the trial’s primary end point – diagnostic accuracy greater than 70% for the lower bound of the 95% confidence interval. Per-patient performance diagnostic accuracy of FFR-CT plus CT was 73% with a 95% CI of 67%-78%.
Nevertheless, the addition of FFR-CT "demonstrated superior diagnostic performance characteristics, as compared with CT stenosis alone, in all patients, in all vessels, and also in vessels of intermediate stenosis severity," lead author Dr. James K. Min said during a press conference.
The results of the study were released in JAMA on Aug. 26th to coincide with the presentation of the study at the European Society of Cardiology meeting (JAMA 2012;308 [doi: 10.1001/2012.jama.11274]).
Fractional flow reserve (FFR) is currently assessed during invasive coronary angiography (ICA) to determine whether a coronary stenosis results in ischemia, and is the currently accepted reference standard for determining lesion-specific ischemia. FFR is the ratio of the mean coronary pressure distal to a coronary stenosis to the mean aortic pressure during maximal coronary blood flow. This value describes coronary flow still attainable despite the presence of a stenotic lesion.
While CT angiography has long been used to accurately and noninvasively assess the anatomic severity of stenoses, the technique has been criticized because it does not yield functional information about the hemodynamic effect of lesions.
Noninvasive calculation of FFR from CT "is a novel method that applies computational fluid dynamics to determine the physiologic significance of CAD [coronary artery disease]. Fractional flow reserve from CT enables calculation of rest and hyperemic pressure fields in coronary arteries without additional imaging, modification of CT acquisition protocols, or administration of medications," the investigators wrote.
"Taken together, these study results suggest the potential of FFR-CT as a promising noninvasive method for identification of individuals with ischemia. The present study findings can be considered proof of concept of the feasibility of this novel technology."
A total of 252 patients were included in the final analysis of the DeFACTO study. These patients had CAD and underwent clinically indicated ICA after CT with no intervening coronary event. Patients were not eligible if they had a history of coronary artery bypass graft (CABG) surgery or percutaneous coronary intervention. About 77% of patients had experienced angina within the past month.
Among 615 study vessels, 271 had less than 30% stenosis and 101 had at least 90% stenosis. In all, 407 vessels were directly assessed by both FFR and FFR-CT.
Computed tomographic angiography was performed on 64- or greater detector scanners with prospective or retrospective electrocardiographic gating.
The investigators evaluated CTs for maximal patient-, vessel-, and segment-based diameter stenosis (characterized as 0%, 1%-29%, 30%-49%, or 50% or larger).
Per-patient and per-vessel CAD stenosis were the maximal stenoses identified in all segments or in all segments within a vessel distribution, respectively. Vessel distributions were categorized for the left anterior descending, left circumflex, and right coronary artery. Computed tomographic angiograms (CTAs) were judged as excellent, good, adequate, or nondiagnostic.
Selective ICA was performed by standard protocol, and FFR was performed at the time of ICA. Fractional flow reserve was considered diagnostic of ischemia at a threshold of 0.80 or less. Computation of FFR-CT was performed in blinded fashion by the FFR-CT core laboratory at HeartFlow, the study’s sponsor.
Per-patient diagnostic accuracy for FFR-CT plus CT was 73%. By comparison, diagnostic accuracy of CT alone was 64%.
FFR-CT also demonstrated greater discriminatory power than CT alone for vessels directly assessed by invasive FFR. For these vessels, the diagnostic sensitivity and specificity of FFR-CT alone were 80% and 61%, respectively.
Importantly, the researchers performed a secondary analysis of patients with an intermediate stenosis ranging from 30% to 70%, "wherein the clinical utility of FFR-CT would be most commonly expected for use." Diagnostic accuracy (73% for FFR-CT and 57% for CT), sensitivity (82% and 37%, respectively), positive predictive value (54% and 34%) and negative predictive value (88% and 68%) were greater for FFR-CT than for CT, though specificity was similar at 66%.
This intermediate group is an important patient population. "We know that patients with 30%-70% stenosis – even though they don’t look high-risk anatomically – actually, some of them experience ischemia and physiologic consequences of their coronary artery disease," said Dr. Min, director of cardiac imaging research and co-director of cardiac imaging at the Cedars-Sinai Heart Institute in Los Angeles.
High sensitivity/low specificity among patients with intermediate stenoses suggests "a low false-negative rate if assessments by FFR-CT were used to identify ischemia causing intermediate lesions, with negligible effects on reductions of false positive results. In this regard, the use of FFR-CT may significantly advance clinical assessment of patients without conventional measures of anatomic high-grade coronary stenosis, largely by proper identification of a significantly greater proportion of patients with manifest ischemia rather than as a safeguard to further invasive evaluation," the researchers noted.
They also pointed out that the prespecified primary end point for FFR-CT – a lower bound of the 95% confidence interval greater than 70% – "represents a 15% increase over traditional noninvasive histologic imaging methods, including myocardial perfusion imaging by SPECT or stress echocardiography," Dr. Min said.
Dr. Min and several of his coauthors reported significant financial relationships with GE Healthcare and Philips Medical, as well as other medical imaging/pharmaceutical companies. Dr. Jason H. Cole reported a grant for research support from HeartFlow. Dr. John Mancini reported a grant to his institution from HeartFlow. This study was funded by HeartFlow.
FROM JAMA
Major Finding: CT fractional flow reserve (FFR-CT) plus CT had a per-patient performance diagnostic accuracy of 73% with a 95% confidence interval of 67%-78% – narrowly failing to meet the trial’s primary end point of diagnostic accuracy greater than 70% for the lower bound of the 95% confidence interval.
Data Source: DeFACTO was a multicenter prospective study of 252 patients with CAD, who underwent clinically indicated invasive coronary angiography after CT.
Disclosures: Dr. Min and several of his coauthors reported significant financial relationships with GE Healthcare and Philips Medical, as well as other medical imaging/pharmaceutical companies. Dr. Jason H. Cole reported a grant for research support from HeartFlow. Dr. John Mancini reported a grant to his institution from HeartFlow. This study was funded by HeartFlow.
Derm Research Unsettles Ped's Treatment of Acne
BOSTON – Pediatricians treat preadolescent acne nearly as often as dermatologists but appear to be less comfortable with the use of topical retinoids, according to the results of a study of almost 55 million pediatric acne visits.
"This study identifies a significant knowledge gap among pediatricians, in terms of treatment of acne based on age of the patient. This is especially important in the preadolescent age group, since pediatricians treat acne in this population nearly as much as dermatologists," Dr. Laura F. Sandoval wrote in a poster presented at the American Academy of Dermatology’s Summer Academy Meeting.
Treatment by physicians in different specialties differed markedly. The younger the child, the more likely a pediatrician treated the acne; most (75.6%) neonatal or infantile acne was managed by a pediatrician. However, the older the child, the more likely a dermatologist treated the acne; slightly more than two-thirds (67.1%) of adolescent acne was managed by a dermatologist. Dermatologists and pediatricians almost equally managed preadolescent acne – 38.4% and 34.2%, respectively.
NAMCS (National Ambulatory Medical Care Survey) data were collected for outpatient visits of children receiving a diagnosis of acne vulgaris during 1993-2009. Patient visits were stratified by age groups: younger than 1 year (neonatal or infantile acne), 1-6 years (mid-childhood acne), 7-11 years (preadolescent acne), and 12-18 years (adolescent acne). Medications prescribed for each age group were compared across physician specialties.
There were almost 55 million estimated visits for patients aged 18 years and younger with a diagnosis of acne. Adolescent acne accounted for most of these visits (91.4%), followed by preadolescent visits (4.8%), mid-childhood visits (0.9%) and neonatal or infantile acne visits (3.0%).
Treatment of preadolescent and adolescent acne differed substantially between dermatologists and pediatricians/primary care physicians (PCPs), with prescribing differences being most pronounced in the preadolescent population. Topical retinoids were prescribed mainly by dermatologists in this age group, while oral antibiotics were preferred by pediatricians/PCPs.
"Comedonal acne is the most common type of acne in preadolescents and thus warrants the use of topical retinoids. Most PCPs have minimal dermatologic education and may be unaware of the benefits of retinoids," wrote Dr. Sandoval of the Center for Dermatology Research at Wake Forest University in Winston-Salem, N.C., and her coauthors.
The most common treatment for preadolescent acne across all specialties was adapalene (14.4%), followed by benzoyl peroxide [BPO] (12.8%), tretinoin (12.5%), minocycline (10.4%), and a combination of BPO/erythromycin (8.1%). The most common treatment for adolescent acne was tretinoin (19.5%), followed by isotretinoin (18.1%), minocycline (16.9%), BPO (16.1%) and adapalene (14.1%).
Isotretinoin was the only medication commonly prescribed in adolescents but not in preadolescents, by both dermatologists and pediatricians/PCPs.
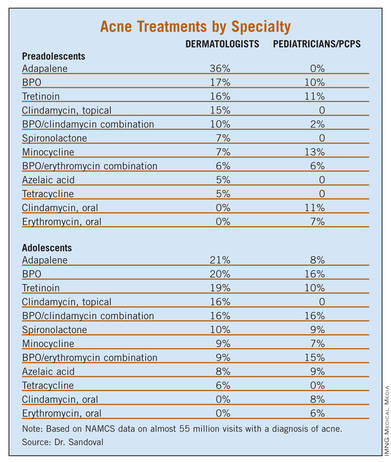
This could be because severe acne is typically rare in young children, according to Dr. Sandoval and her colleagues. And, severity is not recorded by the NAMCS, making it difficult to determine whether preadolescent children had severe enough acne to warrant the use of isotretinoin.
However, when topicals, BPO, and antibiotics fail, isotretinoin should be considered, the researchers noted. Also, isotretinoin should be considered in preadolescents when scarring is a concern.
While dermatologists prescribed isotretinoin and topical retinoids more frequently for adolescent acne than did pediatricians/PCPs, "Hesitancy to prescribe isotretinoin by PCPs may be due to strict requirements of federal monitoring programs, the need for monitoring blood work, and/or safety concerns," the authors noted.
Although tetracycline is the only Food and Drug Administration–approved drug for use in children aged 8 years and older, the data showed it was prescribed to an estimated 120,000 patients younger than 8 years, including children as young as 3 years. This practice was seen among both dermatologists and pediatricians. In all cases, it was used in conjunction with tretinoin, BPO, and/or topical clindamycin.
Minocycline is only FDA approved for use in patients 12 years and older. However, it was prescribed more often in younger patients than are doxycycline and tetracycline by both dermatologists and pediatricians/PCPs.
"All specialties recognize that off-label prescribing is necessary given the very limited range of treatment that is currently FDA-approved for preadolescent patients," the researchers wrote. "PCPs may have outdated concerns in regards to the efficacy and tolerability of retinoids, which is not supported by newer retinoid formulations."
The Center for Dermatology Research at Wake Forest is supported by an educational grant from Galderma. Principal investigator, Dr. Steven R. Feldman, reported significant financial relationships with several pharmaceutical companies, including Galderma. Dr. Sandoval and her other coauthors reported no conflicts of interest.
BOSTON – Pediatricians treat preadolescent acne nearly as often as dermatologists but appear to be less comfortable with the use of topical retinoids, according to the results of a study of almost 55 million pediatric acne visits.
"This study identifies a significant knowledge gap among pediatricians, in terms of treatment of acne based on age of the patient. This is especially important in the preadolescent age group, since pediatricians treat acne in this population nearly as much as dermatologists," Dr. Laura F. Sandoval wrote in a poster presented at the American Academy of Dermatology’s Summer Academy Meeting.
Treatment by physicians in different specialties differed markedly. The younger the child, the more likely a pediatrician treated the acne; most (75.6%) neonatal or infantile acne was managed by a pediatrician. However, the older the child, the more likely a dermatologist treated the acne; slightly more than two-thirds (67.1%) of adolescent acne was managed by a dermatologist. Dermatologists and pediatricians almost equally managed preadolescent acne – 38.4% and 34.2%, respectively.
NAMCS (National Ambulatory Medical Care Survey) data were collected for outpatient visits of children receiving a diagnosis of acne vulgaris during 1993-2009. Patient visits were stratified by age groups: younger than 1 year (neonatal or infantile acne), 1-6 years (mid-childhood acne), 7-11 years (preadolescent acne), and 12-18 years (adolescent acne). Medications prescribed for each age group were compared across physician specialties.
There were almost 55 million estimated visits for patients aged 18 years and younger with a diagnosis of acne. Adolescent acne accounted for most of these visits (91.4%), followed by preadolescent visits (4.8%), mid-childhood visits (0.9%) and neonatal or infantile acne visits (3.0%).
Treatment of preadolescent and adolescent acne differed substantially between dermatologists and pediatricians/primary care physicians (PCPs), with prescribing differences being most pronounced in the preadolescent population. Topical retinoids were prescribed mainly by dermatologists in this age group, while oral antibiotics were preferred by pediatricians/PCPs.
"Comedonal acne is the most common type of acne in preadolescents and thus warrants the use of topical retinoids. Most PCPs have minimal dermatologic education and may be unaware of the benefits of retinoids," wrote Dr. Sandoval of the Center for Dermatology Research at Wake Forest University in Winston-Salem, N.C., and her coauthors.
The most common treatment for preadolescent acne across all specialties was adapalene (14.4%), followed by benzoyl peroxide [BPO] (12.8%), tretinoin (12.5%), minocycline (10.4%), and a combination of BPO/erythromycin (8.1%). The most common treatment for adolescent acne was tretinoin (19.5%), followed by isotretinoin (18.1%), minocycline (16.9%), BPO (16.1%) and adapalene (14.1%).
Isotretinoin was the only medication commonly prescribed in adolescents but not in preadolescents, by both dermatologists and pediatricians/PCPs.

This could be because severe acne is typically rare in young children, according to Dr. Sandoval and her colleagues. And, severity is not recorded by the NAMCS, making it difficult to determine whether preadolescent children had severe enough acne to warrant the use of isotretinoin.
However, when topicals, BPO, and antibiotics fail, isotretinoin should be considered, the researchers noted. Also, isotretinoin should be considered in preadolescents when scarring is a concern.
While dermatologists prescribed isotretinoin and topical retinoids more frequently for adolescent acne than did pediatricians/PCPs, "Hesitancy to prescribe isotretinoin by PCPs may be due to strict requirements of federal monitoring programs, the need for monitoring blood work, and/or safety concerns," the authors noted.
Although tetracycline is the only Food and Drug Administration–approved drug for use in children aged 8 years and older, the data showed it was prescribed to an estimated 120,000 patients younger than 8 years, including children as young as 3 years. This practice was seen among both dermatologists and pediatricians. In all cases, it was used in conjunction with tretinoin, BPO, and/or topical clindamycin.
Minocycline is only FDA approved for use in patients 12 years and older. However, it was prescribed more often in younger patients than are doxycycline and tetracycline by both dermatologists and pediatricians/PCPs.
"All specialties recognize that off-label prescribing is necessary given the very limited range of treatment that is currently FDA-approved for preadolescent patients," the researchers wrote. "PCPs may have outdated concerns in regards to the efficacy and tolerability of retinoids, which is not supported by newer retinoid formulations."
The Center for Dermatology Research at Wake Forest is supported by an educational grant from Galderma. Principal investigator, Dr. Steven R. Feldman, reported significant financial relationships with several pharmaceutical companies, including Galderma. Dr. Sandoval and her other coauthors reported no conflicts of interest.
BOSTON – Pediatricians treat preadolescent acne nearly as often as dermatologists but appear to be less comfortable with the use of topical retinoids, according to the results of a study of almost 55 million pediatric acne visits.
"This study identifies a significant knowledge gap among pediatricians, in terms of treatment of acne based on age of the patient. This is especially important in the preadolescent age group, since pediatricians treat acne in this population nearly as much as dermatologists," Dr. Laura F. Sandoval wrote in a poster presented at the American Academy of Dermatology’s Summer Academy Meeting.
Treatment by physicians in different specialties differed markedly. The younger the child, the more likely a pediatrician treated the acne; most (75.6%) neonatal or infantile acne was managed by a pediatrician. However, the older the child, the more likely a dermatologist treated the acne; slightly more than two-thirds (67.1%) of adolescent acne was managed by a dermatologist. Dermatologists and pediatricians almost equally managed preadolescent acne – 38.4% and 34.2%, respectively.
NAMCS (National Ambulatory Medical Care Survey) data were collected for outpatient visits of children receiving a diagnosis of acne vulgaris during 1993-2009. Patient visits were stratified by age groups: younger than 1 year (neonatal or infantile acne), 1-6 years (mid-childhood acne), 7-11 years (preadolescent acne), and 12-18 years (adolescent acne). Medications prescribed for each age group were compared across physician specialties.
There were almost 55 million estimated visits for patients aged 18 years and younger with a diagnosis of acne. Adolescent acne accounted for most of these visits (91.4%), followed by preadolescent visits (4.8%), mid-childhood visits (0.9%) and neonatal or infantile acne visits (3.0%).
Treatment of preadolescent and adolescent acne differed substantially between dermatologists and pediatricians/primary care physicians (PCPs), with prescribing differences being most pronounced in the preadolescent population. Topical retinoids were prescribed mainly by dermatologists in this age group, while oral antibiotics were preferred by pediatricians/PCPs.
"Comedonal acne is the most common type of acne in preadolescents and thus warrants the use of topical retinoids. Most PCPs have minimal dermatologic education and may be unaware of the benefits of retinoids," wrote Dr. Sandoval of the Center for Dermatology Research at Wake Forest University in Winston-Salem, N.C., and her coauthors.
The most common treatment for preadolescent acne across all specialties was adapalene (14.4%), followed by benzoyl peroxide [BPO] (12.8%), tretinoin (12.5%), minocycline (10.4%), and a combination of BPO/erythromycin (8.1%). The most common treatment for adolescent acne was tretinoin (19.5%), followed by isotretinoin (18.1%), minocycline (16.9%), BPO (16.1%) and adapalene (14.1%).
Isotretinoin was the only medication commonly prescribed in adolescents but not in preadolescents, by both dermatologists and pediatricians/PCPs.

This could be because severe acne is typically rare in young children, according to Dr. Sandoval and her colleagues. And, severity is not recorded by the NAMCS, making it difficult to determine whether preadolescent children had severe enough acne to warrant the use of isotretinoin.
However, when topicals, BPO, and antibiotics fail, isotretinoin should be considered, the researchers noted. Also, isotretinoin should be considered in preadolescents when scarring is a concern.
While dermatologists prescribed isotretinoin and topical retinoids more frequently for adolescent acne than did pediatricians/PCPs, "Hesitancy to prescribe isotretinoin by PCPs may be due to strict requirements of federal monitoring programs, the need for monitoring blood work, and/or safety concerns," the authors noted.
Although tetracycline is the only Food and Drug Administration–approved drug for use in children aged 8 years and older, the data showed it was prescribed to an estimated 120,000 patients younger than 8 years, including children as young as 3 years. This practice was seen among both dermatologists and pediatricians. In all cases, it was used in conjunction with tretinoin, BPO, and/or topical clindamycin.
Minocycline is only FDA approved for use in patients 12 years and older. However, it was prescribed more often in younger patients than are doxycycline and tetracycline by both dermatologists and pediatricians/PCPs.
"All specialties recognize that off-label prescribing is necessary given the very limited range of treatment that is currently FDA-approved for preadolescent patients," the researchers wrote. "PCPs may have outdated concerns in regards to the efficacy and tolerability of retinoids, which is not supported by newer retinoid formulations."
The Center for Dermatology Research at Wake Forest is supported by an educational grant from Galderma. Principal investigator, Dr. Steven R. Feldman, reported significant financial relationships with several pharmaceutical companies, including Galderma. Dr. Sandoval and her other coauthors reported no conflicts of interest.
AT THE AMERICAN ACADEMY OF DERMATOLOGY'S SUMMER ACADEMY MEETING
Major Finding: Most (75.6%) neonatal and infantile acne was managed by a pediatrician, while most (67.1%) adolescent acne was managed by a dermatologist.
Data Source: NAMCS data was collected for outpatient visits by children receiving a diagnosis of acne vulgaris from 1993 to 2009.
Disclosures: The Center for Dermatology Research at Wake Forest is supported by an educational grant from Galderma. Principal investigator, Dr. Steven R. Feldman, reported significant financial relationships with several pharmaceutical companies, including Galderma. Dr. Sandoval and her other coauthors reported no conflicts of interest.
Role of DNRs in Elderly Patients’ Outcomes Analyzed
SAN FRANCISCO – Elderly patients with preexisting Do Not Resuscitate directives appear to be less likely to pursue rescue from complications following emergency surgery than similar patients without such orders, according to an analysis of data from the National Surgical Quality Improvement Program.
When patients with preoperative DNRs were propensity-matched with non-DNR patients, major complication rates were similar – 42% for the DNR group and 41% for the non-DNR group. However, 37% of DNR patients died, compared with 22% of non-DNR patients, Dr. John E. Scarborough reported at the meeting. The investigators adjusted for baseline differences in level of illness to create a propensity-matched cohort of 1,053 patients in each group.
"While we called this outcome failure-to-rescue, we believe that term to be misleading. The term implies that rescue from complications is attempted but is unsuccessful. ... We had no reason to believe that the DNR patients in this well-matched cohort were any less capable of being rescued than non-DNR patients," Dr. Scarborough said. "Instead, we believe that the DNR patients in the matched cohort were less likely than non-DNR patients to pursue rescue from complications."
This conclusion is supported by the finding that DNR patients were significantly less likely to undergo reoperation within 30 days of the index procedure (odds ratio, 0.67).
The authors used participant files from the National Surgical Quality Improvement Program (NSQIP) for 2005-2010, involving medical records for 25,558 patients. These patients were at least 65 years old and underwent an emergency operation for one of 10 common surgical diagnoses. The primary predictor variable was preoperative DNR status, which was defined as "an order signed or cosigned by an attending physician in the 30 days prior to surgery ... regardless of whether the DNR order was subsequently rescinded immediately before the index operation." Other predictor variables included patient demographics, chronic comorbid disease burden, acute physical condition at presentation, and complexity of the emergency operation.
Outcome variables included the 30-day postoperative mortality rate and the 30-day major complication rate – organ/space surgical site infection, wound dehiscence, deep vein thrombosis, pulmonary embolism, pneumonia, reintubation, ventilator use longer than 48 hours, cardiac arrest, myocardial infarction, sepsis, shock, coma longer than 24 hours, prosthetic/graft failure, and bleeding. The failure-to-rescue rate was defined as the mortality rate among patients who had one or more major complications.
A total of 1,061 patients had DNR orders, and 24,497 patients did not. The overall 30-day mortality rate for patients with a DNR order was 37% (395/1,061). The overall 30-day morbidity for patients with a DNR was 42% (446/1,061).
Patients with DNR orders were older: 22% were at least 90 years of age, compared with 5% of the non-DNR patients. They were also sicker, with significantly greater rates of non–independent functional status, cognitive dysfunction, known malignancy, congestive heart failure, chronic obstructive pulmonary disease, American Society of Anesthesiologists physical status class 4, preoperative hypoalbuminemia, and septic shock.
"Although the DNR patients were sicker, we did not find any overt evidence that they were treated less aggressively than non-DNR patients in the preoperative period," said Dr. Scarborough, of the department of surgery at Duke University in Durham, N.C. There was no significant difference between DNR and non-DNR patients in terms of preoperative mechanical ventilation (6% vs. 5%, respectively); and DNR patients were significantly more likely to receive a preoperative transfusion.
There was also no indication that DNR patients were treated less aggressively in the operating room. Operative time was significantly longer for DNR patients, and DNR patients underwent procedures at least as complex as, if not more than, procedures for non-DNR patients.
Invited discussant Dr. Ronnie A. Rosenthal asked how these data could be used to improve the way families are counseled before operations involving elderly patients with DNRs. Dr. Rosenthal is surgeon-in-chief at the VA Connecticut Healthcare System in New Haven.
"What we hope this study provides is a more reliable and sturdy resource for surgeons to counsel such patients than merely explaining to them what the average outcomes are," said Dr. Scarborough. He noted that the oncology literature suggests that patients who better understand their prognosis are in a better position to evaluate whether they want to pursue more aggressive treatment or treatments that have a lot of side effects.
Dr. Norman Estes, chair of the surgery department at the University of Illinois in Peoria, questioned how much of a role surgeons should play in advance planning. "I think that sometimes the advance directive creates a self-fulfilling prophecy for the patient."
Dr. Scarborough noted that advance directives need to be signed by the attending physician. "As to whether the surgeon should be more engaged in the conversation, I guess I would say that it depends on the surgeon. This is a very delicate conversation and obviously one that requires a fair amount of time," he said. Other physicians – such as geriatricians and palliative care physicians – are often more skilled at handling these conversations.
However, it is important for surgeons to have a greater understanding of the patient’s intent with regard to DNR directives, he concluded.
The authors reported that they had no financial disclosures.
Dealing with surgical illness in patients with a DNR advanced directive presents both ethical and clinical challenges. In my experience many patients create such advanced directives out of a desire to not have a prolonged attempt at rescuing them from complications of surgery with little chance of either survival or more importantly, meaningful survival. In spite of these wishes, many are willing to have treatment that runs the risk of creating just such a situation.
A typical example is an elderly patient with a DNR advanced directive, living independently with a reasonable quality of life who presents with a large AAA needing treatment to prevent rupture. If postoperative complications occur requiring a prolonged stay in the ICU on the ventilator, pressers etc.both the surgeon and the next of kin or health care proxy can find themselves in a difficult moral dilemma of doing everything possible to give the patient the best chance of a successful outcome while at the same time not violating the spirit of their advanced directive.
| Dr. Frank Pomposelli |
The crux of such a dilemma is being able to identify the point when recovery is no longer likely, which is difficult. When a patient is a DNR, it may well be that surgeons and the family will err on the side of withdrawing support sooner than in a patient without such a directive, which may partially explain the findings of this study. It that sense, it may be a self fulfilling prophecy but is that really such a bad thing? I personally think it is not.
Doing what's best for the patient, which includes, respecting their wishes includes knowing when to stop.
Frank Pomposelli, M.D., is Chairman of Surgery, St. Elizabeth’s Medical Center, Boston, Mass. He is also an associate medical editor of Vascular Specialist.
Dealing with surgical illness in patients with a DNR advanced directive presents both ethical and clinical challenges. In my experience many patients create such advanced directives out of a desire to not have a prolonged attempt at rescuing them from complications of surgery with little chance of either survival or more importantly, meaningful survival. In spite of these wishes, many are willing to have treatment that runs the risk of creating just such a situation.
A typical example is an elderly patient with a DNR advanced directive, living independently with a reasonable quality of life who presents with a large AAA needing treatment to prevent rupture. If postoperative complications occur requiring a prolonged stay in the ICU on the ventilator, pressers etc.both the surgeon and the next of kin or health care proxy can find themselves in a difficult moral dilemma of doing everything possible to give the patient the best chance of a successful outcome while at the same time not violating the spirit of their advanced directive.
| Dr. Frank Pomposelli |
The crux of such a dilemma is being able to identify the point when recovery is no longer likely, which is difficult. When a patient is a DNR, it may well be that surgeons and the family will err on the side of withdrawing support sooner than in a patient without such a directive, which may partially explain the findings of this study. It that sense, it may be a self fulfilling prophecy but is that really such a bad thing? I personally think it is not.
Doing what's best for the patient, which includes, respecting their wishes includes knowing when to stop.
Frank Pomposelli, M.D., is Chairman of Surgery, St. Elizabeth’s Medical Center, Boston, Mass. He is also an associate medical editor of Vascular Specialist.
Dealing with surgical illness in patients with a DNR advanced directive presents both ethical and clinical challenges. In my experience many patients create such advanced directives out of a desire to not have a prolonged attempt at rescuing them from complications of surgery with little chance of either survival or more importantly, meaningful survival. In spite of these wishes, many are willing to have treatment that runs the risk of creating just such a situation.
A typical example is an elderly patient with a DNR advanced directive, living independently with a reasonable quality of life who presents with a large AAA needing treatment to prevent rupture. If postoperative complications occur requiring a prolonged stay in the ICU on the ventilator, pressers etc.both the surgeon and the next of kin or health care proxy can find themselves in a difficult moral dilemma of doing everything possible to give the patient the best chance of a successful outcome while at the same time not violating the spirit of their advanced directive.
| Dr. Frank Pomposelli |
The crux of such a dilemma is being able to identify the point when recovery is no longer likely, which is difficult. When a patient is a DNR, it may well be that surgeons and the family will err on the side of withdrawing support sooner than in a patient without such a directive, which may partially explain the findings of this study. It that sense, it may be a self fulfilling prophecy but is that really such a bad thing? I personally think it is not.
Doing what's best for the patient, which includes, respecting their wishes includes knowing when to stop.
Frank Pomposelli, M.D., is Chairman of Surgery, St. Elizabeth’s Medical Center, Boston, Mass. He is also an associate medical editor of Vascular Specialist.
SAN FRANCISCO – Elderly patients with preexisting Do Not Resuscitate directives appear to be less likely to pursue rescue from complications following emergency surgery than similar patients without such orders, according to an analysis of data from the National Surgical Quality Improvement Program.
When patients with preoperative DNRs were propensity-matched with non-DNR patients, major complication rates were similar – 42% for the DNR group and 41% for the non-DNR group. However, 37% of DNR patients died, compared with 22% of non-DNR patients, Dr. John E. Scarborough reported at the meeting. The investigators adjusted for baseline differences in level of illness to create a propensity-matched cohort of 1,053 patients in each group.
"While we called this outcome failure-to-rescue, we believe that term to be misleading. The term implies that rescue from complications is attempted but is unsuccessful. ... We had no reason to believe that the DNR patients in this well-matched cohort were any less capable of being rescued than non-DNR patients," Dr. Scarborough said. "Instead, we believe that the DNR patients in the matched cohort were less likely than non-DNR patients to pursue rescue from complications."
This conclusion is supported by the finding that DNR patients were significantly less likely to undergo reoperation within 30 days of the index procedure (odds ratio, 0.67).
The authors used participant files from the National Surgical Quality Improvement Program (NSQIP) for 2005-2010, involving medical records for 25,558 patients. These patients were at least 65 years old and underwent an emergency operation for one of 10 common surgical diagnoses. The primary predictor variable was preoperative DNR status, which was defined as "an order signed or cosigned by an attending physician in the 30 days prior to surgery ... regardless of whether the DNR order was subsequently rescinded immediately before the index operation." Other predictor variables included patient demographics, chronic comorbid disease burden, acute physical condition at presentation, and complexity of the emergency operation.
Outcome variables included the 30-day postoperative mortality rate and the 30-day major complication rate – organ/space surgical site infection, wound dehiscence, deep vein thrombosis, pulmonary embolism, pneumonia, reintubation, ventilator use longer than 48 hours, cardiac arrest, myocardial infarction, sepsis, shock, coma longer than 24 hours, prosthetic/graft failure, and bleeding. The failure-to-rescue rate was defined as the mortality rate among patients who had one or more major complications.
A total of 1,061 patients had DNR orders, and 24,497 patients did not. The overall 30-day mortality rate for patients with a DNR order was 37% (395/1,061). The overall 30-day morbidity for patients with a DNR was 42% (446/1,061).
Patients with DNR orders were older: 22% were at least 90 years of age, compared with 5% of the non-DNR patients. They were also sicker, with significantly greater rates of non–independent functional status, cognitive dysfunction, known malignancy, congestive heart failure, chronic obstructive pulmonary disease, American Society of Anesthesiologists physical status class 4, preoperative hypoalbuminemia, and septic shock.
"Although the DNR patients were sicker, we did not find any overt evidence that they were treated less aggressively than non-DNR patients in the preoperative period," said Dr. Scarborough, of the department of surgery at Duke University in Durham, N.C. There was no significant difference between DNR and non-DNR patients in terms of preoperative mechanical ventilation (6% vs. 5%, respectively); and DNR patients were significantly more likely to receive a preoperative transfusion.
There was also no indication that DNR patients were treated less aggressively in the operating room. Operative time was significantly longer for DNR patients, and DNR patients underwent procedures at least as complex as, if not more than, procedures for non-DNR patients.
Invited discussant Dr. Ronnie A. Rosenthal asked how these data could be used to improve the way families are counseled before operations involving elderly patients with DNRs. Dr. Rosenthal is surgeon-in-chief at the VA Connecticut Healthcare System in New Haven.
"What we hope this study provides is a more reliable and sturdy resource for surgeons to counsel such patients than merely explaining to them what the average outcomes are," said Dr. Scarborough. He noted that the oncology literature suggests that patients who better understand their prognosis are in a better position to evaluate whether they want to pursue more aggressive treatment or treatments that have a lot of side effects.
Dr. Norman Estes, chair of the surgery department at the University of Illinois in Peoria, questioned how much of a role surgeons should play in advance planning. "I think that sometimes the advance directive creates a self-fulfilling prophecy for the patient."
Dr. Scarborough noted that advance directives need to be signed by the attending physician. "As to whether the surgeon should be more engaged in the conversation, I guess I would say that it depends on the surgeon. This is a very delicate conversation and obviously one that requires a fair amount of time," he said. Other physicians – such as geriatricians and palliative care physicians – are often more skilled at handling these conversations.
However, it is important for surgeons to have a greater understanding of the patient’s intent with regard to DNR directives, he concluded.
The authors reported that they had no financial disclosures.
SAN FRANCISCO – Elderly patients with preexisting Do Not Resuscitate directives appear to be less likely to pursue rescue from complications following emergency surgery than similar patients without such orders, according to an analysis of data from the National Surgical Quality Improvement Program.
When patients with preoperative DNRs were propensity-matched with non-DNR patients, major complication rates were similar – 42% for the DNR group and 41% for the non-DNR group. However, 37% of DNR patients died, compared with 22% of non-DNR patients, Dr. John E. Scarborough reported at the meeting. The investigators adjusted for baseline differences in level of illness to create a propensity-matched cohort of 1,053 patients in each group.
"While we called this outcome failure-to-rescue, we believe that term to be misleading. The term implies that rescue from complications is attempted but is unsuccessful. ... We had no reason to believe that the DNR patients in this well-matched cohort were any less capable of being rescued than non-DNR patients," Dr. Scarborough said. "Instead, we believe that the DNR patients in the matched cohort were less likely than non-DNR patients to pursue rescue from complications."
This conclusion is supported by the finding that DNR patients were significantly less likely to undergo reoperation within 30 days of the index procedure (odds ratio, 0.67).
The authors used participant files from the National Surgical Quality Improvement Program (NSQIP) for 2005-2010, involving medical records for 25,558 patients. These patients were at least 65 years old and underwent an emergency operation for one of 10 common surgical diagnoses. The primary predictor variable was preoperative DNR status, which was defined as "an order signed or cosigned by an attending physician in the 30 days prior to surgery ... regardless of whether the DNR order was subsequently rescinded immediately before the index operation." Other predictor variables included patient demographics, chronic comorbid disease burden, acute physical condition at presentation, and complexity of the emergency operation.
Outcome variables included the 30-day postoperative mortality rate and the 30-day major complication rate – organ/space surgical site infection, wound dehiscence, deep vein thrombosis, pulmonary embolism, pneumonia, reintubation, ventilator use longer than 48 hours, cardiac arrest, myocardial infarction, sepsis, shock, coma longer than 24 hours, prosthetic/graft failure, and bleeding. The failure-to-rescue rate was defined as the mortality rate among patients who had one or more major complications.
A total of 1,061 patients had DNR orders, and 24,497 patients did not. The overall 30-day mortality rate for patients with a DNR order was 37% (395/1,061). The overall 30-day morbidity for patients with a DNR was 42% (446/1,061).
Patients with DNR orders were older: 22% were at least 90 years of age, compared with 5% of the non-DNR patients. They were also sicker, with significantly greater rates of non–independent functional status, cognitive dysfunction, known malignancy, congestive heart failure, chronic obstructive pulmonary disease, American Society of Anesthesiologists physical status class 4, preoperative hypoalbuminemia, and septic shock.
"Although the DNR patients were sicker, we did not find any overt evidence that they were treated less aggressively than non-DNR patients in the preoperative period," said Dr. Scarborough, of the department of surgery at Duke University in Durham, N.C. There was no significant difference between DNR and non-DNR patients in terms of preoperative mechanical ventilation (6% vs. 5%, respectively); and DNR patients were significantly more likely to receive a preoperative transfusion.
There was also no indication that DNR patients were treated less aggressively in the operating room. Operative time was significantly longer for DNR patients, and DNR patients underwent procedures at least as complex as, if not more than, procedures for non-DNR patients.
Invited discussant Dr. Ronnie A. Rosenthal asked how these data could be used to improve the way families are counseled before operations involving elderly patients with DNRs. Dr. Rosenthal is surgeon-in-chief at the VA Connecticut Healthcare System in New Haven.
"What we hope this study provides is a more reliable and sturdy resource for surgeons to counsel such patients than merely explaining to them what the average outcomes are," said Dr. Scarborough. He noted that the oncology literature suggests that patients who better understand their prognosis are in a better position to evaluate whether they want to pursue more aggressive treatment or treatments that have a lot of side effects.
Dr. Norman Estes, chair of the surgery department at the University of Illinois in Peoria, questioned how much of a role surgeons should play in advance planning. "I think that sometimes the advance directive creates a self-fulfilling prophecy for the patient."
Dr. Scarborough noted that advance directives need to be signed by the attending physician. "As to whether the surgeon should be more engaged in the conversation, I guess I would say that it depends on the surgeon. This is a very delicate conversation and obviously one that requires a fair amount of time," he said. Other physicians – such as geriatricians and palliative care physicians – are often more skilled at handling these conversations.
However, it is important for surgeons to have a greater understanding of the patient’s intent with regard to DNR directives, he concluded.
The authors reported that they had no financial disclosures.
FROM THE ANNUAL MEETING OF THE AMERICAN SURGICAL ASSOCIATION
Major Finding: Elderly patients with a DNR order were two times more likely to die in the postoperative period (OR, 2.07) than were matched controls without a DNR.
Data Source: The findings come from an analysis of data from the National Surgical Quality Improvement Program, involving medical records for 25,558 patients.
Disclosures: The authors reported that they had no financial disclosures.