Article

Is NPH associated with fewer adverse events than analog basal insulin for adults with T2D?
- Author:
- Corey Lyon, DO
- Swetha Iruku, MD
- Molly Hoss, MD
- Kristen Desanto, MSLS, MS, RD, AHIP
EVIDENCE-BASED ANSWER: NO. Insulin glargine may lead to less patient-reported, symptomatic, and nocturnal hypoglycemia, although overall, there...
Article
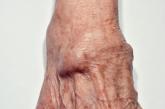
What is the best treatment for wrist ganglion cysts?
- Author:
- Corey Lyon, DO
- Stephanie V. Eldred, MD
- Kristen Desanto, MSLS, MS, RD, AHIP
EVIDENCE-BASED ANSWER: Open surgical excision of wrist ganglion cysts is associated with a lower recurrence rate than aspiration with or without...
Article
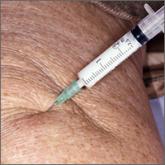
How do hyaluronic acid and corticosteroid injections compare for knee OA relief?
- Author:
- Corey Lyon, DO
- Emily Spencer, MD
- Jack Spittler, MD
- Kristen Desanto, MSLS, MS, RD, AHIP
EVIDENCE-BASED ANSWER: Inconsistent evidence shows a small amount of pain relief early (one week to 3 months) with corticosteroid (CS) injections...
