User login
Oropouche Virus
The pediatrician’s first patient of the day was a 15-year-old boy complaining of fever, chills, and profound arthralgias. His exam, including a careful assessment of his joints, yielded no clues, and the pediatrician was ready to diagnose this as a routine viral illness. An additional bit of history provided by the patient’s mother prompted the pediatrician to pause and reconsider.
“A week ago, we returned from a visit to Cuba,” the mother reported. “Could this be Oropouche virus infection?”
Oropouche virus disease is an arboviral disease caused by the Oropouche virus (OROV). It is transmitted to humans through midge or mosquito bites. Although largely unknown to most United States clinicians until recently, this vector-borne virus is not new. The first human Oropouche virus infection was identified in Trinidad and Tobago in 1955 and since then, there have been intermittent outbreaks in the Amazon region. In recent months, though, the epidemiology of Oropouche virus infections has changed. Infections are being identified in new geographic areas, including Cuba. According to the Pan American Health Organization, 506 cases of Oropouche virus infection have been identified in Cuba since May 27, 2024.
Two deaths from Oropouche virus infection have been reported in previously healthy people. Evolving data suggests adverse outcomes associated with vertical transmission during pregnancy. One fetal death and child with congenital anomalies have been reported in Brazil. Additional fetal deaths, miscarriages, and congenital anomalies are under investigation.
Travel-associated cases have been reported in the United States. As of September 10, 2024, 52 Oropouche virus disease cases had been reported from five states in the United States. The Centers for Disease Control and Prevention confirmed that the first 31 of these cases were travelers returning from Cuba. The CDC issued a health advisory on August 16, 2024: Increased Oropouche Virus Activity and Associated Risk to Travelers.
The pediatrician quickly reviewed the signs and symptoms of Oropouche virus infection. Disease typically presents as an abrupt onset of fever, severe headache, chills, myalgia, and arthralgia 3 to 10 days after the bite of infected mosquito. Some patients develop a maculopapular rash that starts on the trunk and spreads to the extremities. Meningitis and encephalitis develop in less than 1 in 20 people. The symptoms of Oropouche virus infection overlap with those of other arboviruses such as dengue, chikungunya, and Zika viruses. The disease can also mimic malaria or rickettsial infection. Approximately 60% of people with Oropouche virus infection experience a recurrence of symptoms within days to weeks of the initial resolution of symptoms.
Testing for Oropouche virus infection is available through the CDC’s Arbovirus Diagnostic Laboratory. In people who are acutely ill, reverse transcription-polymerase chain reaction testing can be used to identify the virus in serum and cerebrospinal fluid. Serologic testing is also available for people who have been symptomatic for at least 6 days.
The pediatrician contacted his local health department to discuss the possibility of Oropouche virus infection. After reviewing the case definition, public health authorities recommended laboratory testing for Oropouche virus, dengue, and Zika virus.
Back in the exam room, the pediatrician provided anticipatory guidance to the patient and his mother. There are no antiviral medications to treat Oropouche virus infection, so the pediatrician recommended supportive care, including acetaminophen for fever and pain. He also advised avoiding aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs) until dengue could be ruled out to reduce the risk of bleeding. After confirming that no one else in the home was sick with similar symptoms, he counseled about prevention strategies.
To date, transmission of Oropouche virus in the United States has not been documented, but vectors potentially capable of transmitting the virus are present in some areas of the United States. When people who are infected with Oropouche are bitten, they can spread the virus through their blood to biting midges or mosquitoes. The insects can then spread the virus to other people. To reduce to potential for local transmission, people who are sick with suspected Oropouche virus infection are advised to avoid biting-midge and mosquito bites for the first week of their illness. Any person who has recently traveled to an area where Oropouche virus transmission is occurring should also avoid insect bites for 3 weeks after returning home to account for the potential incubation period of the virus. This includes wearing an EPA-registered insect repellent.
A suspect case is a patient who has been in an area with documented or suspected OROV circulation* within 2 weeks of initial symptom onset (as patients may experience recurrent symptoms) and the following:
- Abrupt onset of reported fever, headache, and one or more of the following: myalgia, arthralgia, photophobia, retro-orbital/eye pain, or signs and symptoms of neuroinvasive disease (eg, stiff neck, altered mental status, seizures, limb weakness, or cerebrospinal fluid pleocytosis).
- Tested negative for other possible diseases, in particular dengue.†
- Absence of a more likely clinical explanation.
*If concern exists for local transmission in a nonendemic area, consider if the patient shared an exposure location with a person with confirmed OROV infection, lives in an area where travel-related cases have been identified, or has known vector exposure (eg, mosquitoes or biting midges).
†If strong suspicion of OROV disease exists based on the patient’s clinical features and history of travel to an area with virus circulation, do not wait on negative testing before sending specimens to CDC.
Adapted from: Centers for Disease Control and Prevention. Response to Oropouche Virus Disease Cases in U.S. States and Territories in the Americas. Available at: https.//www.cdc.gov/oropouche/media/pdfs/2024/09/response-to-oropouche-virus-disease.pdf
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu)
The pediatrician’s first patient of the day was a 15-year-old boy complaining of fever, chills, and profound arthralgias. His exam, including a careful assessment of his joints, yielded no clues, and the pediatrician was ready to diagnose this as a routine viral illness. An additional bit of history provided by the patient’s mother prompted the pediatrician to pause and reconsider.
“A week ago, we returned from a visit to Cuba,” the mother reported. “Could this be Oropouche virus infection?”
Oropouche virus disease is an arboviral disease caused by the Oropouche virus (OROV). It is transmitted to humans through midge or mosquito bites. Although largely unknown to most United States clinicians until recently, this vector-borne virus is not new. The first human Oropouche virus infection was identified in Trinidad and Tobago in 1955 and since then, there have been intermittent outbreaks in the Amazon region. In recent months, though, the epidemiology of Oropouche virus infections has changed. Infections are being identified in new geographic areas, including Cuba. According to the Pan American Health Organization, 506 cases of Oropouche virus infection have been identified in Cuba since May 27, 2024.
Two deaths from Oropouche virus infection have been reported in previously healthy people. Evolving data suggests adverse outcomes associated with vertical transmission during pregnancy. One fetal death and child with congenital anomalies have been reported in Brazil. Additional fetal deaths, miscarriages, and congenital anomalies are under investigation.
Travel-associated cases have been reported in the United States. As of September 10, 2024, 52 Oropouche virus disease cases had been reported from five states in the United States. The Centers for Disease Control and Prevention confirmed that the first 31 of these cases were travelers returning from Cuba. The CDC issued a health advisory on August 16, 2024: Increased Oropouche Virus Activity and Associated Risk to Travelers.
The pediatrician quickly reviewed the signs and symptoms of Oropouche virus infection. Disease typically presents as an abrupt onset of fever, severe headache, chills, myalgia, and arthralgia 3 to 10 days after the bite of infected mosquito. Some patients develop a maculopapular rash that starts on the trunk and spreads to the extremities. Meningitis and encephalitis develop in less than 1 in 20 people. The symptoms of Oropouche virus infection overlap with those of other arboviruses such as dengue, chikungunya, and Zika viruses. The disease can also mimic malaria or rickettsial infection. Approximately 60% of people with Oropouche virus infection experience a recurrence of symptoms within days to weeks of the initial resolution of symptoms.
Testing for Oropouche virus infection is available through the CDC’s Arbovirus Diagnostic Laboratory. In people who are acutely ill, reverse transcription-polymerase chain reaction testing can be used to identify the virus in serum and cerebrospinal fluid. Serologic testing is also available for people who have been symptomatic for at least 6 days.
The pediatrician contacted his local health department to discuss the possibility of Oropouche virus infection. After reviewing the case definition, public health authorities recommended laboratory testing for Oropouche virus, dengue, and Zika virus.
Back in the exam room, the pediatrician provided anticipatory guidance to the patient and his mother. There are no antiviral medications to treat Oropouche virus infection, so the pediatrician recommended supportive care, including acetaminophen for fever and pain. He also advised avoiding aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs) until dengue could be ruled out to reduce the risk of bleeding. After confirming that no one else in the home was sick with similar symptoms, he counseled about prevention strategies.
To date, transmission of Oropouche virus in the United States has not been documented, but vectors potentially capable of transmitting the virus are present in some areas of the United States. When people who are infected with Oropouche are bitten, they can spread the virus through their blood to biting midges or mosquitoes. The insects can then spread the virus to other people. To reduce to potential for local transmission, people who are sick with suspected Oropouche virus infection are advised to avoid biting-midge and mosquito bites for the first week of their illness. Any person who has recently traveled to an area where Oropouche virus transmission is occurring should also avoid insect bites for 3 weeks after returning home to account for the potential incubation period of the virus. This includes wearing an EPA-registered insect repellent.
A suspect case is a patient who has been in an area with documented or suspected OROV circulation* within 2 weeks of initial symptom onset (as patients may experience recurrent symptoms) and the following:
- Abrupt onset of reported fever, headache, and one or more of the following: myalgia, arthralgia, photophobia, retro-orbital/eye pain, or signs and symptoms of neuroinvasive disease (eg, stiff neck, altered mental status, seizures, limb weakness, or cerebrospinal fluid pleocytosis).
- Tested negative for other possible diseases, in particular dengue.†
- Absence of a more likely clinical explanation.
*If concern exists for local transmission in a nonendemic area, consider if the patient shared an exposure location with a person with confirmed OROV infection, lives in an area where travel-related cases have been identified, or has known vector exposure (eg, mosquitoes or biting midges).
†If strong suspicion of OROV disease exists based on the patient’s clinical features and history of travel to an area with virus circulation, do not wait on negative testing before sending specimens to CDC.
Adapted from: Centers for Disease Control and Prevention. Response to Oropouche Virus Disease Cases in U.S. States and Territories in the Americas. Available at: https.//www.cdc.gov/oropouche/media/pdfs/2024/09/response-to-oropouche-virus-disease.pdf
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu)
The pediatrician’s first patient of the day was a 15-year-old boy complaining of fever, chills, and profound arthralgias. His exam, including a careful assessment of his joints, yielded no clues, and the pediatrician was ready to diagnose this as a routine viral illness. An additional bit of history provided by the patient’s mother prompted the pediatrician to pause and reconsider.
“A week ago, we returned from a visit to Cuba,” the mother reported. “Could this be Oropouche virus infection?”
Oropouche virus disease is an arboviral disease caused by the Oropouche virus (OROV). It is transmitted to humans through midge or mosquito bites. Although largely unknown to most United States clinicians until recently, this vector-borne virus is not new. The first human Oropouche virus infection was identified in Trinidad and Tobago in 1955 and since then, there have been intermittent outbreaks in the Amazon region. In recent months, though, the epidemiology of Oropouche virus infections has changed. Infections are being identified in new geographic areas, including Cuba. According to the Pan American Health Organization, 506 cases of Oropouche virus infection have been identified in Cuba since May 27, 2024.
Two deaths from Oropouche virus infection have been reported in previously healthy people. Evolving data suggests adverse outcomes associated with vertical transmission during pregnancy. One fetal death and child with congenital anomalies have been reported in Brazil. Additional fetal deaths, miscarriages, and congenital anomalies are under investigation.
Travel-associated cases have been reported in the United States. As of September 10, 2024, 52 Oropouche virus disease cases had been reported from five states in the United States. The Centers for Disease Control and Prevention confirmed that the first 31 of these cases were travelers returning from Cuba. The CDC issued a health advisory on August 16, 2024: Increased Oropouche Virus Activity and Associated Risk to Travelers.
The pediatrician quickly reviewed the signs and symptoms of Oropouche virus infection. Disease typically presents as an abrupt onset of fever, severe headache, chills, myalgia, and arthralgia 3 to 10 days after the bite of infected mosquito. Some patients develop a maculopapular rash that starts on the trunk and spreads to the extremities. Meningitis and encephalitis develop in less than 1 in 20 people. The symptoms of Oropouche virus infection overlap with those of other arboviruses such as dengue, chikungunya, and Zika viruses. The disease can also mimic malaria or rickettsial infection. Approximately 60% of people with Oropouche virus infection experience a recurrence of symptoms within days to weeks of the initial resolution of symptoms.
Testing for Oropouche virus infection is available through the CDC’s Arbovirus Diagnostic Laboratory. In people who are acutely ill, reverse transcription-polymerase chain reaction testing can be used to identify the virus in serum and cerebrospinal fluid. Serologic testing is also available for people who have been symptomatic for at least 6 days.
The pediatrician contacted his local health department to discuss the possibility of Oropouche virus infection. After reviewing the case definition, public health authorities recommended laboratory testing for Oropouche virus, dengue, and Zika virus.
Back in the exam room, the pediatrician provided anticipatory guidance to the patient and his mother. There are no antiviral medications to treat Oropouche virus infection, so the pediatrician recommended supportive care, including acetaminophen for fever and pain. He also advised avoiding aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs) until dengue could be ruled out to reduce the risk of bleeding. After confirming that no one else in the home was sick with similar symptoms, he counseled about prevention strategies.
To date, transmission of Oropouche virus in the United States has not been documented, but vectors potentially capable of transmitting the virus are present in some areas of the United States. When people who are infected with Oropouche are bitten, they can spread the virus through their blood to biting midges or mosquitoes. The insects can then spread the virus to other people. To reduce to potential for local transmission, people who are sick with suspected Oropouche virus infection are advised to avoid biting-midge and mosquito bites for the first week of their illness. Any person who has recently traveled to an area where Oropouche virus transmission is occurring should also avoid insect bites for 3 weeks after returning home to account for the potential incubation period of the virus. This includes wearing an EPA-registered insect repellent.
A suspect case is a patient who has been in an area with documented or suspected OROV circulation* within 2 weeks of initial symptom onset (as patients may experience recurrent symptoms) and the following:
- Abrupt onset of reported fever, headache, and one or more of the following: myalgia, arthralgia, photophobia, retro-orbital/eye pain, or signs and symptoms of neuroinvasive disease (eg, stiff neck, altered mental status, seizures, limb weakness, or cerebrospinal fluid pleocytosis).
- Tested negative for other possible diseases, in particular dengue.†
- Absence of a more likely clinical explanation.
*If concern exists for local transmission in a nonendemic area, consider if the patient shared an exposure location with a person with confirmed OROV infection, lives in an area where travel-related cases have been identified, or has known vector exposure (eg, mosquitoes or biting midges).
†If strong suspicion of OROV disease exists based on the patient’s clinical features and history of travel to an area with virus circulation, do not wait on negative testing before sending specimens to CDC.
Adapted from: Centers for Disease Control and Prevention. Response to Oropouche Virus Disease Cases in U.S. States and Territories in the Americas. Available at: https.//www.cdc.gov/oropouche/media/pdfs/2024/09/response-to-oropouche-virus-disease.pdf
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu)
Highly Pathogenic Avian Influenza (HPAI)
Imagine this: A 15-year-old male presents to an urgent care center with a one-day history of fever, cough, and shortness of breath. He is mildly tachypneic with bilateral scattered crackles on lung exam. A rapid test for COVID-19 and influenza is positive for influenza A — a surprising result in June.
An oxygen saturation of 90% prompts transfer to the emergency department at the local children’s hospital. The emergency medicine fellow is skeptical of the presumptive diagnosis. Influenza in the summer in a boy who had not traveled outside his small hometown in the southeastern United States? A respiratory viral panel also detected influenza A, but the specimen did not type as influenza A H1 or H3. This result prompted the laboratory technician to place a call to the ordering physician. “Does this patient have risk factors for avian flu?” the tech asked.
Highly pathogenic avian influenza (HPAI) A(H5N1) is not a new virus. It was discovered in waterfowl in China in 1996 and has since evolved into multiple clades and subclades, spreading to every continent on the globe except Oceania. It is called highly pathogenic because it kills a large number of the birds that it infects. In 2021, Clade 2.3.4.4b HPAI A(H5N1) viruses emerged in North America, causing large outbreaks in wild birds and farmed poultry populations, including backyard flocks. Sporadic infections have been identified in a diverse group of mammals, including foxes, raccoons, baby goats, bears, and harbor seals. In March of this year, HPAI A(H5N1) was detected for the first time in United States dairy cattle. As we go to press, the United States Department of Agriculture has detected HPAI A(H5N1) in dairy cattle on 36 farms in 9 states.
Human infections are rare, but often severe. Following a 1997 outbreak of HPAI A(H5N1) in Hong Kong, 18 people were infected and 6 died. Since then, more than 900 cases have been reported in humans and approximately half of these have been fatal. The spectrum of disease includes asymptomatic infection and mild disease, as occurred recently in Texas. A dairy farm worker who was exposed to dairy cattle presumed to be infected with HPAI A(H5N1) developed conjunctivitis and no other symptoms. An individual infected in Colorado in 2022 had no symptoms other than fatigue and recovered.
Human-to-human transmission was not identified with either of these cases, although very limited, non-sustained transmission has been observed in the past, usually in family members of infected people after prolonged close exposure.
Right now, most people in the United States are not at risk for HPAI A(H5N1) infection.
Careful history taking with our illustrative and hypothetical case revealed exposure to farm animals but in a state without known cases of HPAI A(H5N1) in dairy cattle. State health department officials nevertheless agreed with further testing of the patient. Some influenza diagnostic tests cleared by the US Food and Drug Administration (FDA) can detect some novel influenza A viruses such as HPAI A(H5N1) but cannot distinguish between infection with seasonal influenza A or novel influenza A viruses. Molecular assays may give an “influenza A untypeable” result, as in our case. The CDC urges further testing on these untypeable specimens at local or state public health laboratories. When HPAI A(H5N1) is suspected, a negative result on a commercially available test is not considered sufficient to exclude the possibility of infection.
Our patient was admitted to the hospital and droplet, contact, and airborne precautions were instituted along with antiviral treatment with oseltamivir. Preliminary analysis of HPAI A(H5N1) viruses predicts susceptibility to currently available antivirals. The admitting physician confirmed that the boy had received influenza vaccine in the preceding season but, unfortunately, seasonal vaccines do not protect against HPAI A(H5N1) infection.
Advice for Clinicians
Given the recent media attention and public health focus on HPAI A(H5N1), frontline clinicians may start receiving questions from patients and families and perhaps requests for testing. At this point, testing is generally recommended only for individuals with risk factors or known exposures. Healthcare providers with questions about testing are encouraged to reach out to their local or state health departments.
Public health authorities have provided recommendations for protection from HPAI. These include avoiding unprotected exposures to sick or dead wild birds, poultry, other domesticated birds, and wild or domesticated animals (including cattle). People should avoid unprotected contact with animals with suspected or confirmed HPAI A(H5N1)-virus infection or products from these animals, including raw or unpasteurized milk and raw milk products.
We can, however, reassure families that the commercial milk supply is safe. In late April, the FDA reported that HPAI viral fragments were found in one of five retail milk samples by polymerase chain reaction testing. Additional testing did not detect any live, infectious virus, indicating the effectiveness of pasteurization at inactivating the virus. Of importance to pediatricians and others pediatric clinicians, limited sampling of retail powdered infant formula and powdered milk products marketed as toddler formula revealed no viral fragments or viable virus.
The million-dollar question is whether HPAI A(H5N1) could start a new pandemic. To date, the virus has not acquired the mutations that would make it easily transmissible from person to person. If that changes and the virus does start spreading more widely, candidate vaccines that could protect against HPAI A(H5N1) have been developed and are part of the national stockpile. Let’s hope we don’t need them.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the American Academy of Pediatrics’ Committee on Infectious Diseases and the physician lead for Red Book Online. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu.)
Imagine this: A 15-year-old male presents to an urgent care center with a one-day history of fever, cough, and shortness of breath. He is mildly tachypneic with bilateral scattered crackles on lung exam. A rapid test for COVID-19 and influenza is positive for influenza A — a surprising result in June.
An oxygen saturation of 90% prompts transfer to the emergency department at the local children’s hospital. The emergency medicine fellow is skeptical of the presumptive diagnosis. Influenza in the summer in a boy who had not traveled outside his small hometown in the southeastern United States? A respiratory viral panel also detected influenza A, but the specimen did not type as influenza A H1 or H3. This result prompted the laboratory technician to place a call to the ordering physician. “Does this patient have risk factors for avian flu?” the tech asked.
Highly pathogenic avian influenza (HPAI) A(H5N1) is not a new virus. It was discovered in waterfowl in China in 1996 and has since evolved into multiple clades and subclades, spreading to every continent on the globe except Oceania. It is called highly pathogenic because it kills a large number of the birds that it infects. In 2021, Clade 2.3.4.4b HPAI A(H5N1) viruses emerged in North America, causing large outbreaks in wild birds and farmed poultry populations, including backyard flocks. Sporadic infections have been identified in a diverse group of mammals, including foxes, raccoons, baby goats, bears, and harbor seals. In March of this year, HPAI A(H5N1) was detected for the first time in United States dairy cattle. As we go to press, the United States Department of Agriculture has detected HPAI A(H5N1) in dairy cattle on 36 farms in 9 states.
Human infections are rare, but often severe. Following a 1997 outbreak of HPAI A(H5N1) in Hong Kong, 18 people were infected and 6 died. Since then, more than 900 cases have been reported in humans and approximately half of these have been fatal. The spectrum of disease includes asymptomatic infection and mild disease, as occurred recently in Texas. A dairy farm worker who was exposed to dairy cattle presumed to be infected with HPAI A(H5N1) developed conjunctivitis and no other symptoms. An individual infected in Colorado in 2022 had no symptoms other than fatigue and recovered.
Human-to-human transmission was not identified with either of these cases, although very limited, non-sustained transmission has been observed in the past, usually in family members of infected people after prolonged close exposure.
Right now, most people in the United States are not at risk for HPAI A(H5N1) infection.
Careful history taking with our illustrative and hypothetical case revealed exposure to farm animals but in a state without known cases of HPAI A(H5N1) in dairy cattle. State health department officials nevertheless agreed with further testing of the patient. Some influenza diagnostic tests cleared by the US Food and Drug Administration (FDA) can detect some novel influenza A viruses such as HPAI A(H5N1) but cannot distinguish between infection with seasonal influenza A or novel influenza A viruses. Molecular assays may give an “influenza A untypeable” result, as in our case. The CDC urges further testing on these untypeable specimens at local or state public health laboratories. When HPAI A(H5N1) is suspected, a negative result on a commercially available test is not considered sufficient to exclude the possibility of infection.
Our patient was admitted to the hospital and droplet, contact, and airborne precautions were instituted along with antiviral treatment with oseltamivir. Preliminary analysis of HPAI A(H5N1) viruses predicts susceptibility to currently available antivirals. The admitting physician confirmed that the boy had received influenza vaccine in the preceding season but, unfortunately, seasonal vaccines do not protect against HPAI A(H5N1) infection.
Advice for Clinicians
Given the recent media attention and public health focus on HPAI A(H5N1), frontline clinicians may start receiving questions from patients and families and perhaps requests for testing. At this point, testing is generally recommended only for individuals with risk factors or known exposures. Healthcare providers with questions about testing are encouraged to reach out to their local or state health departments.
Public health authorities have provided recommendations for protection from HPAI. These include avoiding unprotected exposures to sick or dead wild birds, poultry, other domesticated birds, and wild or domesticated animals (including cattle). People should avoid unprotected contact with animals with suspected or confirmed HPAI A(H5N1)-virus infection or products from these animals, including raw or unpasteurized milk and raw milk products.
We can, however, reassure families that the commercial milk supply is safe. In late April, the FDA reported that HPAI viral fragments were found in one of five retail milk samples by polymerase chain reaction testing. Additional testing did not detect any live, infectious virus, indicating the effectiveness of pasteurization at inactivating the virus. Of importance to pediatricians and others pediatric clinicians, limited sampling of retail powdered infant formula and powdered milk products marketed as toddler formula revealed no viral fragments or viable virus.
The million-dollar question is whether HPAI A(H5N1) could start a new pandemic. To date, the virus has not acquired the mutations that would make it easily transmissible from person to person. If that changes and the virus does start spreading more widely, candidate vaccines that could protect against HPAI A(H5N1) have been developed and are part of the national stockpile. Let’s hope we don’t need them.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the American Academy of Pediatrics’ Committee on Infectious Diseases and the physician lead for Red Book Online. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu.)
Imagine this: A 15-year-old male presents to an urgent care center with a one-day history of fever, cough, and shortness of breath. He is mildly tachypneic with bilateral scattered crackles on lung exam. A rapid test for COVID-19 and influenza is positive for influenza A — a surprising result in June.
An oxygen saturation of 90% prompts transfer to the emergency department at the local children’s hospital. The emergency medicine fellow is skeptical of the presumptive diagnosis. Influenza in the summer in a boy who had not traveled outside his small hometown in the southeastern United States? A respiratory viral panel also detected influenza A, but the specimen did not type as influenza A H1 or H3. This result prompted the laboratory technician to place a call to the ordering physician. “Does this patient have risk factors for avian flu?” the tech asked.
Highly pathogenic avian influenza (HPAI) A(H5N1) is not a new virus. It was discovered in waterfowl in China in 1996 and has since evolved into multiple clades and subclades, spreading to every continent on the globe except Oceania. It is called highly pathogenic because it kills a large number of the birds that it infects. In 2021, Clade 2.3.4.4b HPAI A(H5N1) viruses emerged in North America, causing large outbreaks in wild birds and farmed poultry populations, including backyard flocks. Sporadic infections have been identified in a diverse group of mammals, including foxes, raccoons, baby goats, bears, and harbor seals. In March of this year, HPAI A(H5N1) was detected for the first time in United States dairy cattle. As we go to press, the United States Department of Agriculture has detected HPAI A(H5N1) in dairy cattle on 36 farms in 9 states.
Human infections are rare, but often severe. Following a 1997 outbreak of HPAI A(H5N1) in Hong Kong, 18 people were infected and 6 died. Since then, more than 900 cases have been reported in humans and approximately half of these have been fatal. The spectrum of disease includes asymptomatic infection and mild disease, as occurred recently in Texas. A dairy farm worker who was exposed to dairy cattle presumed to be infected with HPAI A(H5N1) developed conjunctivitis and no other symptoms. An individual infected in Colorado in 2022 had no symptoms other than fatigue and recovered.
Human-to-human transmission was not identified with either of these cases, although very limited, non-sustained transmission has been observed in the past, usually in family members of infected people after prolonged close exposure.
Right now, most people in the United States are not at risk for HPAI A(H5N1) infection.
Careful history taking with our illustrative and hypothetical case revealed exposure to farm animals but in a state without known cases of HPAI A(H5N1) in dairy cattle. State health department officials nevertheless agreed with further testing of the patient. Some influenza diagnostic tests cleared by the US Food and Drug Administration (FDA) can detect some novel influenza A viruses such as HPAI A(H5N1) but cannot distinguish between infection with seasonal influenza A or novel influenza A viruses. Molecular assays may give an “influenza A untypeable” result, as in our case. The CDC urges further testing on these untypeable specimens at local or state public health laboratories. When HPAI A(H5N1) is suspected, a negative result on a commercially available test is not considered sufficient to exclude the possibility of infection.
Our patient was admitted to the hospital and droplet, contact, and airborne precautions were instituted along with antiviral treatment with oseltamivir. Preliminary analysis of HPAI A(H5N1) viruses predicts susceptibility to currently available antivirals. The admitting physician confirmed that the boy had received influenza vaccine in the preceding season but, unfortunately, seasonal vaccines do not protect against HPAI A(H5N1) infection.
Advice for Clinicians
Given the recent media attention and public health focus on HPAI A(H5N1), frontline clinicians may start receiving questions from patients and families and perhaps requests for testing. At this point, testing is generally recommended only for individuals with risk factors or known exposures. Healthcare providers with questions about testing are encouraged to reach out to their local or state health departments.
Public health authorities have provided recommendations for protection from HPAI. These include avoiding unprotected exposures to sick or dead wild birds, poultry, other domesticated birds, and wild or domesticated animals (including cattle). People should avoid unprotected contact with animals with suspected or confirmed HPAI A(H5N1)-virus infection or products from these animals, including raw or unpasteurized milk and raw milk products.
We can, however, reassure families that the commercial milk supply is safe. In late April, the FDA reported that HPAI viral fragments were found in one of five retail milk samples by polymerase chain reaction testing. Additional testing did not detect any live, infectious virus, indicating the effectiveness of pasteurization at inactivating the virus. Of importance to pediatricians and others pediatric clinicians, limited sampling of retail powdered infant formula and powdered milk products marketed as toddler formula revealed no viral fragments or viable virus.
The million-dollar question is whether HPAI A(H5N1) could start a new pandemic. To date, the virus has not acquired the mutations that would make it easily transmissible from person to person. If that changes and the virus does start spreading more widely, candidate vaccines that could protect against HPAI A(H5N1) have been developed and are part of the national stockpile. Let’s hope we don’t need them.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the American Academy of Pediatrics’ Committee on Infectious Diseases and the physician lead for Red Book Online. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at pdnews@mdedge.com. (Also kristina.bryant@louisville.edu.)
Infection prevention
Not a long ago, I received a call from a friend working in a local pediatric clinic. One of her partners had just seen a young child with an unusual rash. The diagnosis? Crusted scabies.
Sarcoptes scabiei var. hominis, the mite that causes typical scabies, also causes crusted or Norwegian scabies. These terms refer to severe infestations that occur in individuals who are immune compromised or debilitated. The rash is characterized by vesicles and thick crusts and may or may not be itchy. Because patients with crusted scabies can be infested with as many as 2 million mites, transmission from very brief skin-to-skin contact is possible, and outbreaks have occurred in health care facilities and other institutional settings.
That was the reason for my friend’s call. “What do we do for the doctors and nurses in the clinic who saw the patient?” she wanted to know.
“Everyone wore gloves, right?” I asked. There was silence on the other end of the phone.
After a quick consultation with our health department, every health care provider (HCP) who touched the patient without gloves was treated preemptively with topical permethrin. None went on to develop scabies. The experience prompted me to think about the challenges of infection prevention in ambulatory care.
Both the American Academy of Pediatrics (AAP Committee on Infectious Diseases, “Infection prevention and control in pediatric ambulatory settings,” Pediatrics 2007;20[3]:650-65) and the Centers for Disease Control and Prevention (Guide to Infection Prevention for Outpatient Settings: Minimum Expectations for Safe Care) have published recommendations for infection prevention in outpatient settings. Both organizations emphasize the importance of standard precautions. According to the CDC, standard precautions “are the minimum infection prevention practices that apply to all patient care, regardless of suspected or confirmed infection status of the patient, in any setting where health care is delivered.” They are designed to protect HCPs, as well as prevent us from spreading infections among patients. Standard precautions include:
• Hand hygiene.
• Use of personal protective equipment (gloves, gowns, masks).
• Safe injection practices.
• Safe handling of potentially contaminated equipment or surfaces in the patient environment.
• Respiratory hygiene/cough etiquette.
Some of these elements are likely second nature to office-based pediatricians. Hands must be cleaned before and after every patient encounter or an encounter with the patient’s immediate environment. “Cover your cough” signs have become ubiquitous in ambulatory care waiting rooms, even as we acknowledge the difficulties associated with expecting toddlers to wear masks or use a tissue to contain their coughs and sneezes.
Other elements of standard precautions may receive increased attention because the consequences of noncompliance are perceived to be dangerous or severe. For example, we know that failure to reliably employ safe injection practices (see table) has resulted in transmission of blood-borne pathogens, including hepatitis B and C, in ambulatory settings.
In my experience, the use of personal protective equipment (PPE) in the ambulatory setting is the element of standard precautions that is the least understood and perhaps the most underutilized. It’s certainly easier in the inpatient setting, where we use transmission-based precautions, and colorful isolation signs instruct us to put on gown and gloves when we visit the patient with viral gastroenteritis, or gown, gloves, and mask for the child with acute viral respiratory tract infection. In the office, we expect the HCP to anticipate what kind of contact with blood or body fluids is likely and choose PPE accordingly.
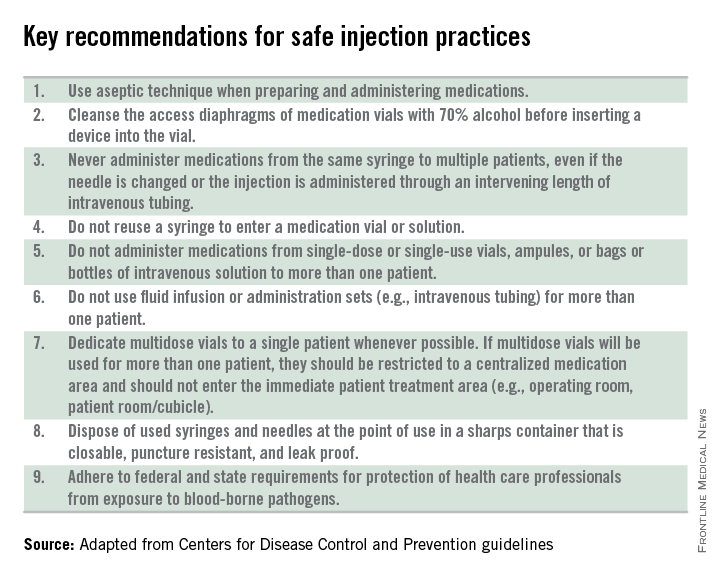
Of course, anticipation can be tricky. Gowns, for example, are only required during procedures or activities when contact with blood and body fluids is likely. In routine office-based care, these sorts of procedures are uncommon. Incision and drainage of an abscess is one example of a procedure that might warrant protection of one’s clothing with a gown. Conversely, the need for a mask might arise several times a day, as these are worn to protect the mouth, nose, and eyes “during procedures that are likely to generate splashes or sprays of blood or other body fluids.” Examination of a coughing patient is a common “procedure” likely to results in sprays of saliva. Use of a mask can protect the examiner from potential exposures to Bordetella pertussis, Mycoplasma pneumoniae, and a host of respiratory viruses.
While the AAP has been careful to point out that gloves are not needed for the routine care of well children, they should be used when “there is the potential to contact blood, body fluids, mucous membranes, nonintact skin, or potentially infectious material.” In our world, potentially infectious material might include a cluster of vesicles thought to be herpes simplex, the honey-crusted lesions of impetigo, or the weeping, crusted rash of Norwegian scabies.
My own office had a powerful reminder about the importance of standard precautions last year when we were referred a young infant with recurrent fevers and a mostly dry, peeling rash. As we learned in medical school, the mucocutanous lesions of congenital syphilis can be highly contagious. In accordance with AAP recommendations, all HCPs who examined this child without the protection of gloves underwent serologic testing for syphilis. Fortunately, there were no transmissions!
Published data about infectious disease exposures and the transmission of infectious diseases in the outpatient setting, either from patients to health care workers or among patients, are largely limited to outbreak or case reports. A 1991 review identified 53 reports of infectious disease transmission in outpatient settings between 1961 and 1990 (JAMA 1991;265(18): 2377-81). Transmission occurred in medical and dental offices, clinics, emergency departments, ophthalmology offices, and alternative care settings that included chiropractic clinics and an acupuncture practice. A variety of pathogens were involved, including measles, adenovirus, hepatitis B, atypical mycobacteria, and Streptococcus pyogenes. The authors concluded that many of the outbreaks and episodes of transmission could have been prevented “if existing infection control guidelines,” including what we now consider standard precautions, had been utilized. Many reports published in the intervening 25 years have come to similar conclusions.
So why don’t HCPs yet follow standard precautions, including appropriate use of PPE? The reasons are complex and multifactorial. We’re all busy and lack of time is a common complaint. Gowns, gloves, masks, and alcohol hand gel aren’t always readily available. Some HCPs may not be knowledgeable about the elements of standard precautions while others may not understand the risks to themselves and their patients associated with nonadherence. Finally, some organizations have not established clear expectations related to infection prevention and compliance with AAP and CDC recommendations.
Several years ago, at the very beginning of the H1N1 influenza epidemic, a colleague of mine working in a pediatric practice saw a patient complaining of fever, lethargy, and myalgia. Not surprisingly, the patient’s rapid influenza test was positive. My colleague recalls that she was handed the result before she ever walked into the room – without any PPE – to see the patient.
“This was different than my usual routine at the hospital,” she told me. The expectation at the hospital was gown, gloves, and masks for any patient with influenza or influenzalike illness. At the office though, there was no such expectation, and providers did not routinely wear masks, even when seeing patients with respiratory symptoms. My colleague wasn’t reckless or rebellious. She was simply conforming to the culture in that office, and following the behavioral cues of more senior physicians in the practice. Subsequently, she developed severe influenza infection requiring a prolonged hospital stay.
It’s time to change the culture. As a first step, perform a quick audit in the office, using the AAP’s “Infection prevention and control in pediatric ambulatory settings” as a guide.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
Not a long ago, I received a call from a friend working in a local pediatric clinic. One of her partners had just seen a young child with an unusual rash. The diagnosis? Crusted scabies.
Sarcoptes scabiei var. hominis, the mite that causes typical scabies, also causes crusted or Norwegian scabies. These terms refer to severe infestations that occur in individuals who are immune compromised or debilitated. The rash is characterized by vesicles and thick crusts and may or may not be itchy. Because patients with crusted scabies can be infested with as many as 2 million mites, transmission from very brief skin-to-skin contact is possible, and outbreaks have occurred in health care facilities and other institutional settings.
That was the reason for my friend’s call. “What do we do for the doctors and nurses in the clinic who saw the patient?” she wanted to know.
“Everyone wore gloves, right?” I asked. There was silence on the other end of the phone.
After a quick consultation with our health department, every health care provider (HCP) who touched the patient without gloves was treated preemptively with topical permethrin. None went on to develop scabies. The experience prompted me to think about the challenges of infection prevention in ambulatory care.
Both the American Academy of Pediatrics (AAP Committee on Infectious Diseases, “Infection prevention and control in pediatric ambulatory settings,” Pediatrics 2007;20[3]:650-65) and the Centers for Disease Control and Prevention (Guide to Infection Prevention for Outpatient Settings: Minimum Expectations for Safe Care) have published recommendations for infection prevention in outpatient settings. Both organizations emphasize the importance of standard precautions. According to the CDC, standard precautions “are the minimum infection prevention practices that apply to all patient care, regardless of suspected or confirmed infection status of the patient, in any setting where health care is delivered.” They are designed to protect HCPs, as well as prevent us from spreading infections among patients. Standard precautions include:
• Hand hygiene.
• Use of personal protective equipment (gloves, gowns, masks).
• Safe injection practices.
• Safe handling of potentially contaminated equipment or surfaces in the patient environment.
• Respiratory hygiene/cough etiquette.
Some of these elements are likely second nature to office-based pediatricians. Hands must be cleaned before and after every patient encounter or an encounter with the patient’s immediate environment. “Cover your cough” signs have become ubiquitous in ambulatory care waiting rooms, even as we acknowledge the difficulties associated with expecting toddlers to wear masks or use a tissue to contain their coughs and sneezes.
Other elements of standard precautions may receive increased attention because the consequences of noncompliance are perceived to be dangerous or severe. For example, we know that failure to reliably employ safe injection practices (see table) has resulted in transmission of blood-borne pathogens, including hepatitis B and C, in ambulatory settings.
In my experience, the use of personal protective equipment (PPE) in the ambulatory setting is the element of standard precautions that is the least understood and perhaps the most underutilized. It’s certainly easier in the inpatient setting, where we use transmission-based precautions, and colorful isolation signs instruct us to put on gown and gloves when we visit the patient with viral gastroenteritis, or gown, gloves, and mask for the child with acute viral respiratory tract infection. In the office, we expect the HCP to anticipate what kind of contact with blood or body fluids is likely and choose PPE accordingly.

Of course, anticipation can be tricky. Gowns, for example, are only required during procedures or activities when contact with blood and body fluids is likely. In routine office-based care, these sorts of procedures are uncommon. Incision and drainage of an abscess is one example of a procedure that might warrant protection of one’s clothing with a gown. Conversely, the need for a mask might arise several times a day, as these are worn to protect the mouth, nose, and eyes “during procedures that are likely to generate splashes or sprays of blood or other body fluids.” Examination of a coughing patient is a common “procedure” likely to results in sprays of saliva. Use of a mask can protect the examiner from potential exposures to Bordetella pertussis, Mycoplasma pneumoniae, and a host of respiratory viruses.
While the AAP has been careful to point out that gloves are not needed for the routine care of well children, they should be used when “there is the potential to contact blood, body fluids, mucous membranes, nonintact skin, or potentially infectious material.” In our world, potentially infectious material might include a cluster of vesicles thought to be herpes simplex, the honey-crusted lesions of impetigo, or the weeping, crusted rash of Norwegian scabies.
My own office had a powerful reminder about the importance of standard precautions last year when we were referred a young infant with recurrent fevers and a mostly dry, peeling rash. As we learned in medical school, the mucocutanous lesions of congenital syphilis can be highly contagious. In accordance with AAP recommendations, all HCPs who examined this child without the protection of gloves underwent serologic testing for syphilis. Fortunately, there were no transmissions!
Published data about infectious disease exposures and the transmission of infectious diseases in the outpatient setting, either from patients to health care workers or among patients, are largely limited to outbreak or case reports. A 1991 review identified 53 reports of infectious disease transmission in outpatient settings between 1961 and 1990 (JAMA 1991;265(18): 2377-81). Transmission occurred in medical and dental offices, clinics, emergency departments, ophthalmology offices, and alternative care settings that included chiropractic clinics and an acupuncture practice. A variety of pathogens were involved, including measles, adenovirus, hepatitis B, atypical mycobacteria, and Streptococcus pyogenes. The authors concluded that many of the outbreaks and episodes of transmission could have been prevented “if existing infection control guidelines,” including what we now consider standard precautions, had been utilized. Many reports published in the intervening 25 years have come to similar conclusions.
So why don’t HCPs yet follow standard precautions, including appropriate use of PPE? The reasons are complex and multifactorial. We’re all busy and lack of time is a common complaint. Gowns, gloves, masks, and alcohol hand gel aren’t always readily available. Some HCPs may not be knowledgeable about the elements of standard precautions while others may not understand the risks to themselves and their patients associated with nonadherence. Finally, some organizations have not established clear expectations related to infection prevention and compliance with AAP and CDC recommendations.
Several years ago, at the very beginning of the H1N1 influenza epidemic, a colleague of mine working in a pediatric practice saw a patient complaining of fever, lethargy, and myalgia. Not surprisingly, the patient’s rapid influenza test was positive. My colleague recalls that she was handed the result before she ever walked into the room – without any PPE – to see the patient.
“This was different than my usual routine at the hospital,” she told me. The expectation at the hospital was gown, gloves, and masks for any patient with influenza or influenzalike illness. At the office though, there was no such expectation, and providers did not routinely wear masks, even when seeing patients with respiratory symptoms. My colleague wasn’t reckless or rebellious. She was simply conforming to the culture in that office, and following the behavioral cues of more senior physicians in the practice. Subsequently, she developed severe influenza infection requiring a prolonged hospital stay.
It’s time to change the culture. As a first step, perform a quick audit in the office, using the AAP’s “Infection prevention and control in pediatric ambulatory settings” as a guide.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
Not a long ago, I received a call from a friend working in a local pediatric clinic. One of her partners had just seen a young child with an unusual rash. The diagnosis? Crusted scabies.
Sarcoptes scabiei var. hominis, the mite that causes typical scabies, also causes crusted or Norwegian scabies. These terms refer to severe infestations that occur in individuals who are immune compromised or debilitated. The rash is characterized by vesicles and thick crusts and may or may not be itchy. Because patients with crusted scabies can be infested with as many as 2 million mites, transmission from very brief skin-to-skin contact is possible, and outbreaks have occurred in health care facilities and other institutional settings.
That was the reason for my friend’s call. “What do we do for the doctors and nurses in the clinic who saw the patient?” she wanted to know.
“Everyone wore gloves, right?” I asked. There was silence on the other end of the phone.
After a quick consultation with our health department, every health care provider (HCP) who touched the patient without gloves was treated preemptively with topical permethrin. None went on to develop scabies. The experience prompted me to think about the challenges of infection prevention in ambulatory care.
Both the American Academy of Pediatrics (AAP Committee on Infectious Diseases, “Infection prevention and control in pediatric ambulatory settings,” Pediatrics 2007;20[3]:650-65) and the Centers for Disease Control and Prevention (Guide to Infection Prevention for Outpatient Settings: Minimum Expectations for Safe Care) have published recommendations for infection prevention in outpatient settings. Both organizations emphasize the importance of standard precautions. According to the CDC, standard precautions “are the minimum infection prevention practices that apply to all patient care, regardless of suspected or confirmed infection status of the patient, in any setting where health care is delivered.” They are designed to protect HCPs, as well as prevent us from spreading infections among patients. Standard precautions include:
• Hand hygiene.
• Use of personal protective equipment (gloves, gowns, masks).
• Safe injection practices.
• Safe handling of potentially contaminated equipment or surfaces in the patient environment.
• Respiratory hygiene/cough etiquette.
Some of these elements are likely second nature to office-based pediatricians. Hands must be cleaned before and after every patient encounter or an encounter with the patient’s immediate environment. “Cover your cough” signs have become ubiquitous in ambulatory care waiting rooms, even as we acknowledge the difficulties associated with expecting toddlers to wear masks or use a tissue to contain their coughs and sneezes.
Other elements of standard precautions may receive increased attention because the consequences of noncompliance are perceived to be dangerous or severe. For example, we know that failure to reliably employ safe injection practices (see table) has resulted in transmission of blood-borne pathogens, including hepatitis B and C, in ambulatory settings.
In my experience, the use of personal protective equipment (PPE) in the ambulatory setting is the element of standard precautions that is the least understood and perhaps the most underutilized. It’s certainly easier in the inpatient setting, where we use transmission-based precautions, and colorful isolation signs instruct us to put on gown and gloves when we visit the patient with viral gastroenteritis, or gown, gloves, and mask for the child with acute viral respiratory tract infection. In the office, we expect the HCP to anticipate what kind of contact with blood or body fluids is likely and choose PPE accordingly.

Of course, anticipation can be tricky. Gowns, for example, are only required during procedures or activities when contact with blood and body fluids is likely. In routine office-based care, these sorts of procedures are uncommon. Incision and drainage of an abscess is one example of a procedure that might warrant protection of one’s clothing with a gown. Conversely, the need for a mask might arise several times a day, as these are worn to protect the mouth, nose, and eyes “during procedures that are likely to generate splashes or sprays of blood or other body fluids.” Examination of a coughing patient is a common “procedure” likely to results in sprays of saliva. Use of a mask can protect the examiner from potential exposures to Bordetella pertussis, Mycoplasma pneumoniae, and a host of respiratory viruses.
While the AAP has been careful to point out that gloves are not needed for the routine care of well children, they should be used when “there is the potential to contact blood, body fluids, mucous membranes, nonintact skin, or potentially infectious material.” In our world, potentially infectious material might include a cluster of vesicles thought to be herpes simplex, the honey-crusted lesions of impetigo, or the weeping, crusted rash of Norwegian scabies.
My own office had a powerful reminder about the importance of standard precautions last year when we were referred a young infant with recurrent fevers and a mostly dry, peeling rash. As we learned in medical school, the mucocutanous lesions of congenital syphilis can be highly contagious. In accordance with AAP recommendations, all HCPs who examined this child without the protection of gloves underwent serologic testing for syphilis. Fortunately, there were no transmissions!
Published data about infectious disease exposures and the transmission of infectious diseases in the outpatient setting, either from patients to health care workers or among patients, are largely limited to outbreak or case reports. A 1991 review identified 53 reports of infectious disease transmission in outpatient settings between 1961 and 1990 (JAMA 1991;265(18): 2377-81). Transmission occurred in medical and dental offices, clinics, emergency departments, ophthalmology offices, and alternative care settings that included chiropractic clinics and an acupuncture practice. A variety of pathogens were involved, including measles, adenovirus, hepatitis B, atypical mycobacteria, and Streptococcus pyogenes. The authors concluded that many of the outbreaks and episodes of transmission could have been prevented “if existing infection control guidelines,” including what we now consider standard precautions, had been utilized. Many reports published in the intervening 25 years have come to similar conclusions.
So why don’t HCPs yet follow standard precautions, including appropriate use of PPE? The reasons are complex and multifactorial. We’re all busy and lack of time is a common complaint. Gowns, gloves, masks, and alcohol hand gel aren’t always readily available. Some HCPs may not be knowledgeable about the elements of standard precautions while others may not understand the risks to themselves and their patients associated with nonadherence. Finally, some organizations have not established clear expectations related to infection prevention and compliance with AAP and CDC recommendations.
Several years ago, at the very beginning of the H1N1 influenza epidemic, a colleague of mine working in a pediatric practice saw a patient complaining of fever, lethargy, and myalgia. Not surprisingly, the patient’s rapid influenza test was positive. My colleague recalls that she was handed the result before she ever walked into the room – without any PPE – to see the patient.
“This was different than my usual routine at the hospital,” she told me. The expectation at the hospital was gown, gloves, and masks for any patient with influenza or influenzalike illness. At the office though, there was no such expectation, and providers did not routinely wear masks, even when seeing patients with respiratory symptoms. My colleague wasn’t reckless or rebellious. She was simply conforming to the culture in that office, and following the behavioral cues of more senior physicians in the practice. Subsequently, she developed severe influenza infection requiring a prolonged hospital stay.
It’s time to change the culture. As a first step, perform a quick audit in the office, using the AAP’s “Infection prevention and control in pediatric ambulatory settings” as a guide.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
Food recalls highlight risk of listeriosis
Recently, after a long day at the hospital, I stopped at the grocery store to pick up something for a quick dinner. I drifted to the frozen food case in the organic food section, but pulled up short when I saw empty shelves. A paper sign announced that Amy’s Kitchen, a manufacturer of organic and natural frozen foods, had become the latest company to recall its products because of concern about Listeria monocytogenes contamination.
According to information posted on the Food and Drug Administration website, this facultative, anaerobic gram-positive bacillus has been the impetus behind 10 national recalls of food products between April 1 and May 8, 2015 alone. Implicated food products have ranged from gourmet ice cream to soybean sprouts to frozen vegetables. Unlike some other bacterial causes of food-borne illness, Listeria organisms can thrive at cold temperatures. Historically, outbreaks of disease have been linked to a variety of foods, including raw produce, contaminated ready-to-eat foods such as deli meats and prepared salads, and unpasteurized milk and milk products.
Clinical manifestations of listeriosis range from febrile gastroenteritis to bacteremia and meningitis, with severe disease seen primarily in immunocompromised individuals and adults 65 and older.
Pregnant women are especially susceptible, with incidence rates 13 times higher than in the general population. Probably as a result of food choices, Hispanic women are disproportionately affected, with rates up to 24 times higher. Maternal infection may be asymptomatic or may manifest with flulike symptoms that include fever, myalgias, headache, and backache, with or without a preceding diarrhea illness. Even mild maternal illness may result in adverse pregnancy outcomes such as fetal loss, premature labor, and severe neonatal infection.
While medical students and residents are still taught to think of Listeria infection as one of the “big three” causes of neonatal sepsis along with group B streptococcus and Escherichia coli, many pediatricians have never seen a case of this rare, but potentially devastating disease. As with group B streptococcus, both early-onset and late-onset disease occur. Sepsis is the most common presentation of disease in the first week of life, while meningitis predominates in late-onset disease. Pneumonia and myocarditis are occasionally seen. Granulomatosis infantisepticum is an uncommon manifestation of severe, disseminated Listeria infection. Granuloma can occur in nearly every organ, although involvement of the liver and skin is most common.
In 2002, investigators from the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists surveyed more than 400 pregnant women from across the United States about their knowledge of the transmission, risk factors, symptoms, and prevention of listeriosis (Infect. Dis. Obstet. Gyn. 2005;13:11-15). A year later, the Minnesota Department of Health surveyed an additional 286 pregnant women from their state using the same survey instrument.
More than 80% of survey respondents had never heard of the disease, and knowledge about prevention strategies was therefore predictably limited. Only 33% of respondents in the national survey and 17% of respondents in the Minnesota survey knew, for example, that infection could be prevented by avoiding delicatessen meats and soft cheeses. Investigators concluded that “timely and appropriate education” of pregnant women about listeriosis could reduce cases of perinatal infection.
Data from the CDC suggest we have more work to do. The Listeria Initiative is an enhanced national surveillance system that collects laboratory, clinical, and food exposure data about listeriosis cases in the United States. Between 2009 and 2011, 14% of the 1,651 invasive Listeria infections reported were classified as pregnancy associated. Morbidity and mortality were significant, with 40 fetal losses and 6 neonatal deaths (MMWR 2013;62:448-52).
The CDC offers some common sense tips for preventing listeriosis and other food-borne illness. Raw fruits and vegetables should be thoroughly rinsed with tap water and dried with a clean cloth or paper towel before being eaten or cooked. Even foods that are typically peeled first should be washed, and firm produce, such as cantaloupe, should be scrubbed with a produce brush to reduce surface contamination. Uncooked meats and poultry should never come in contact with other food. Hands, knives, cutting boards, and other food preparation surfaces should be washed thoroughly after uncooked food is handled.
Pregnant women and others at increased risk for listeriosis should not eat hot dogs or deli meats unless they are cooked to steaming. Soft cheeses, including feta, brie, Camembert, queso blanco, or anything blue veined, should be avoided unless the label clearly states that the product has been made with pasteurized milk. Even then, it might not be safe. Pasteurized Mexican-style cheeses, such as queso fresco, have been linked to Listeria infections, likely as a result of contamination during the cheese-making process.
Physicians should be prepared to field calls from concerned parents who believe their children may have consumed a product potentially contaminated with Listeria. In general, someone who has eaten a recalled food product but has no symptoms doesn’t need a laboratory evaluation or treatment. Screening blood cultures is not indicated, and routine tests such as a complete blood count are unlikely to be helpful. Instead, patients should be counseled about the symptoms of listeriosis and undergo prompt evaluation if any develop within 2 months of exposure. The typical interval between exposure and the development of symptoms is 1 day to 3 weeks, but may be as long as 70 days.
Although Listeria infection may result in gastrointestinal symptoms, stool cultures are not recommended for diagnosis. According to the CDC, ingestion of food contaminated with Listeria occurs frequently because the organisms are commonly found in the environment. Although uncommon, intermittent fecal carriage and shedding have been observed in asymptomatic individuals.
Back at the grocery, I sighed and resigned myself to a grilled cheese sandwich for dinner. I turned and saw another woman in the aisle stop and read the sign on the freezer case.
“It’s a little scary,” she said with a sigh. “It seems like there is another recall every week, and I’m wondering what’s safe to eat.”
The parents of our patients may have similar questions. Although the Food and Drug Administration offers detailed guidance for food manufacturers about reducing Listeria contamination, perfect compliance wouldn’t eliminate the risk for consumers because L. monocytogenes is widespread in the environment. The organisms are found in water, soil, sewage, and decaying vegetation, and can be isolated from a variety of animals. Fresh fruits and vegetables are “healthy” choices as long as they are handled and prepared appropriately. Conversely, unpasteurized milk and milk products can never be considered safe.
That’s food for thought.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
Recently, after a long day at the hospital, I stopped at the grocery store to pick up something for a quick dinner. I drifted to the frozen food case in the organic food section, but pulled up short when I saw empty shelves. A paper sign announced that Amy’s Kitchen, a manufacturer of organic and natural frozen foods, had become the latest company to recall its products because of concern about Listeria monocytogenes contamination.
According to information posted on the Food and Drug Administration website, this facultative, anaerobic gram-positive bacillus has been the impetus behind 10 national recalls of food products between April 1 and May 8, 2015 alone. Implicated food products have ranged from gourmet ice cream to soybean sprouts to frozen vegetables. Unlike some other bacterial causes of food-borne illness, Listeria organisms can thrive at cold temperatures. Historically, outbreaks of disease have been linked to a variety of foods, including raw produce, contaminated ready-to-eat foods such as deli meats and prepared salads, and unpasteurized milk and milk products.
Clinical manifestations of listeriosis range from febrile gastroenteritis to bacteremia and meningitis, with severe disease seen primarily in immunocompromised individuals and adults 65 and older.
Pregnant women are especially susceptible, with incidence rates 13 times higher than in the general population. Probably as a result of food choices, Hispanic women are disproportionately affected, with rates up to 24 times higher. Maternal infection may be asymptomatic or may manifest with flulike symptoms that include fever, myalgias, headache, and backache, with or without a preceding diarrhea illness. Even mild maternal illness may result in adverse pregnancy outcomes such as fetal loss, premature labor, and severe neonatal infection.
While medical students and residents are still taught to think of Listeria infection as one of the “big three” causes of neonatal sepsis along with group B streptococcus and Escherichia coli, many pediatricians have never seen a case of this rare, but potentially devastating disease. As with group B streptococcus, both early-onset and late-onset disease occur. Sepsis is the most common presentation of disease in the first week of life, while meningitis predominates in late-onset disease. Pneumonia and myocarditis are occasionally seen. Granulomatosis infantisepticum is an uncommon manifestation of severe, disseminated Listeria infection. Granuloma can occur in nearly every organ, although involvement of the liver and skin is most common.
In 2002, investigators from the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists surveyed more than 400 pregnant women from across the United States about their knowledge of the transmission, risk factors, symptoms, and prevention of listeriosis (Infect. Dis. Obstet. Gyn. 2005;13:11-15). A year later, the Minnesota Department of Health surveyed an additional 286 pregnant women from their state using the same survey instrument.
More than 80% of survey respondents had never heard of the disease, and knowledge about prevention strategies was therefore predictably limited. Only 33% of respondents in the national survey and 17% of respondents in the Minnesota survey knew, for example, that infection could be prevented by avoiding delicatessen meats and soft cheeses. Investigators concluded that “timely and appropriate education” of pregnant women about listeriosis could reduce cases of perinatal infection.
Data from the CDC suggest we have more work to do. The Listeria Initiative is an enhanced national surveillance system that collects laboratory, clinical, and food exposure data about listeriosis cases in the United States. Between 2009 and 2011, 14% of the 1,651 invasive Listeria infections reported were classified as pregnancy associated. Morbidity and mortality were significant, with 40 fetal losses and 6 neonatal deaths (MMWR 2013;62:448-52).
The CDC offers some common sense tips for preventing listeriosis and other food-borne illness. Raw fruits and vegetables should be thoroughly rinsed with tap water and dried with a clean cloth or paper towel before being eaten or cooked. Even foods that are typically peeled first should be washed, and firm produce, such as cantaloupe, should be scrubbed with a produce brush to reduce surface contamination. Uncooked meats and poultry should never come in contact with other food. Hands, knives, cutting boards, and other food preparation surfaces should be washed thoroughly after uncooked food is handled.
Pregnant women and others at increased risk for listeriosis should not eat hot dogs or deli meats unless they are cooked to steaming. Soft cheeses, including feta, brie, Camembert, queso blanco, or anything blue veined, should be avoided unless the label clearly states that the product has been made with pasteurized milk. Even then, it might not be safe. Pasteurized Mexican-style cheeses, such as queso fresco, have been linked to Listeria infections, likely as a result of contamination during the cheese-making process.
Physicians should be prepared to field calls from concerned parents who believe their children may have consumed a product potentially contaminated with Listeria. In general, someone who has eaten a recalled food product but has no symptoms doesn’t need a laboratory evaluation or treatment. Screening blood cultures is not indicated, and routine tests such as a complete blood count are unlikely to be helpful. Instead, patients should be counseled about the symptoms of listeriosis and undergo prompt evaluation if any develop within 2 months of exposure. The typical interval between exposure and the development of symptoms is 1 day to 3 weeks, but may be as long as 70 days.
Although Listeria infection may result in gastrointestinal symptoms, stool cultures are not recommended for diagnosis. According to the CDC, ingestion of food contaminated with Listeria occurs frequently because the organisms are commonly found in the environment. Although uncommon, intermittent fecal carriage and shedding have been observed in asymptomatic individuals.
Back at the grocery, I sighed and resigned myself to a grilled cheese sandwich for dinner. I turned and saw another woman in the aisle stop and read the sign on the freezer case.
“It’s a little scary,” she said with a sigh. “It seems like there is another recall every week, and I’m wondering what’s safe to eat.”
The parents of our patients may have similar questions. Although the Food and Drug Administration offers detailed guidance for food manufacturers about reducing Listeria contamination, perfect compliance wouldn’t eliminate the risk for consumers because L. monocytogenes is widespread in the environment. The organisms are found in water, soil, sewage, and decaying vegetation, and can be isolated from a variety of animals. Fresh fruits and vegetables are “healthy” choices as long as they are handled and prepared appropriately. Conversely, unpasteurized milk and milk products can never be considered safe.
That’s food for thought.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
Recently, after a long day at the hospital, I stopped at the grocery store to pick up something for a quick dinner. I drifted to the frozen food case in the organic food section, but pulled up short when I saw empty shelves. A paper sign announced that Amy’s Kitchen, a manufacturer of organic and natural frozen foods, had become the latest company to recall its products because of concern about Listeria monocytogenes contamination.
According to information posted on the Food and Drug Administration website, this facultative, anaerobic gram-positive bacillus has been the impetus behind 10 national recalls of food products between April 1 and May 8, 2015 alone. Implicated food products have ranged from gourmet ice cream to soybean sprouts to frozen vegetables. Unlike some other bacterial causes of food-borne illness, Listeria organisms can thrive at cold temperatures. Historically, outbreaks of disease have been linked to a variety of foods, including raw produce, contaminated ready-to-eat foods such as deli meats and prepared salads, and unpasteurized milk and milk products.
Clinical manifestations of listeriosis range from febrile gastroenteritis to bacteremia and meningitis, with severe disease seen primarily in immunocompromised individuals and adults 65 and older.
Pregnant women are especially susceptible, with incidence rates 13 times higher than in the general population. Probably as a result of food choices, Hispanic women are disproportionately affected, with rates up to 24 times higher. Maternal infection may be asymptomatic or may manifest with flulike symptoms that include fever, myalgias, headache, and backache, with or without a preceding diarrhea illness. Even mild maternal illness may result in adverse pregnancy outcomes such as fetal loss, premature labor, and severe neonatal infection.
While medical students and residents are still taught to think of Listeria infection as one of the “big three” causes of neonatal sepsis along with group B streptococcus and Escherichia coli, many pediatricians have never seen a case of this rare, but potentially devastating disease. As with group B streptococcus, both early-onset and late-onset disease occur. Sepsis is the most common presentation of disease in the first week of life, while meningitis predominates in late-onset disease. Pneumonia and myocarditis are occasionally seen. Granulomatosis infantisepticum is an uncommon manifestation of severe, disseminated Listeria infection. Granuloma can occur in nearly every organ, although involvement of the liver and skin is most common.
In 2002, investigators from the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists surveyed more than 400 pregnant women from across the United States about their knowledge of the transmission, risk factors, symptoms, and prevention of listeriosis (Infect. Dis. Obstet. Gyn. 2005;13:11-15). A year later, the Minnesota Department of Health surveyed an additional 286 pregnant women from their state using the same survey instrument.
More than 80% of survey respondents had never heard of the disease, and knowledge about prevention strategies was therefore predictably limited. Only 33% of respondents in the national survey and 17% of respondents in the Minnesota survey knew, for example, that infection could be prevented by avoiding delicatessen meats and soft cheeses. Investigators concluded that “timely and appropriate education” of pregnant women about listeriosis could reduce cases of perinatal infection.
Data from the CDC suggest we have more work to do. The Listeria Initiative is an enhanced national surveillance system that collects laboratory, clinical, and food exposure data about listeriosis cases in the United States. Between 2009 and 2011, 14% of the 1,651 invasive Listeria infections reported were classified as pregnancy associated. Morbidity and mortality were significant, with 40 fetal losses and 6 neonatal deaths (MMWR 2013;62:448-52).
The CDC offers some common sense tips for preventing listeriosis and other food-borne illness. Raw fruits and vegetables should be thoroughly rinsed with tap water and dried with a clean cloth or paper towel before being eaten or cooked. Even foods that are typically peeled first should be washed, and firm produce, such as cantaloupe, should be scrubbed with a produce brush to reduce surface contamination. Uncooked meats and poultry should never come in contact with other food. Hands, knives, cutting boards, and other food preparation surfaces should be washed thoroughly after uncooked food is handled.
Pregnant women and others at increased risk for listeriosis should not eat hot dogs or deli meats unless they are cooked to steaming. Soft cheeses, including feta, brie, Camembert, queso blanco, or anything blue veined, should be avoided unless the label clearly states that the product has been made with pasteurized milk. Even then, it might not be safe. Pasteurized Mexican-style cheeses, such as queso fresco, have been linked to Listeria infections, likely as a result of contamination during the cheese-making process.
Physicians should be prepared to field calls from concerned parents who believe their children may have consumed a product potentially contaminated with Listeria. In general, someone who has eaten a recalled food product but has no symptoms doesn’t need a laboratory evaluation or treatment. Screening blood cultures is not indicated, and routine tests such as a complete blood count are unlikely to be helpful. Instead, patients should be counseled about the symptoms of listeriosis and undergo prompt evaluation if any develop within 2 months of exposure. The typical interval between exposure and the development of symptoms is 1 day to 3 weeks, but may be as long as 70 days.
Although Listeria infection may result in gastrointestinal symptoms, stool cultures are not recommended for diagnosis. According to the CDC, ingestion of food contaminated with Listeria occurs frequently because the organisms are commonly found in the environment. Although uncommon, intermittent fecal carriage and shedding have been observed in asymptomatic individuals.
Back at the grocery, I sighed and resigned myself to a grilled cheese sandwich for dinner. I turned and saw another woman in the aisle stop and read the sign on the freezer case.
“It’s a little scary,” she said with a sigh. “It seems like there is another recall every week, and I’m wondering what’s safe to eat.”
The parents of our patients may have similar questions. Although the Food and Drug Administration offers detailed guidance for food manufacturers about reducing Listeria contamination, perfect compliance wouldn’t eliminate the risk for consumers because L. monocytogenes is widespread in the environment. The organisms are found in water, soil, sewage, and decaying vegetation, and can be isolated from a variety of animals. Fresh fruits and vegetables are “healthy” choices as long as they are handled and prepared appropriately. Conversely, unpasteurized milk and milk products can never be considered safe.
That’s food for thought.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.






