Article
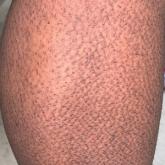
Rippled Macules and Papules on the Legs
- Author:
- Kieu Oanh Nguyen, MD
- Kristopher M. Peters, DO
A 34-year-old woman presented to our dermatology clinic with an intensely pruritic rash on the legs of 2 years’ duration. The pruritus had waxed...
Article
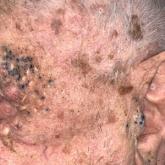
Periorbital and Tragal Cutaneous Lesions
- Author:
- Alexander Mounts, DO, MA
- Kristopher M. Peters, DO
A 91-year-old White man with no personal or family history of skin cancer presented to the dermatology clinic for a total-body skin examination. A...
Article
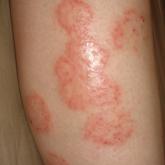
Primary Cutaneous Cryptococcosis in an Immunocompetent Iraq War Veteran
- Author:
- James V. Twede, MD
- Kristopher M. Peters, DO
Disseminated cryptococcosis is not commonly seen as a primary cutaneous infection in immunocompetent hosts. When encountered, primary cutaneous...
