Article
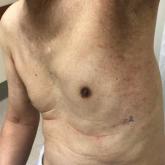
Thalidomide Analogue Drug Eruption Along the Lines of Blaschko
- Author:
- Alexander J. Jafari, MD, MPH
- Garrett L. Vick, MD
- Laura C. Williams, MD
Lenalidomide is a thalidomide analogue used to treat various hematologic malignancies, including non-Hodgkin lymphoma, myelodysplastic syndrome,...
