User login
How safe is the blackout rage gallon drinking trend?
This discussion was recorded on April 6, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical adviser for Medscape Emergency Medicine. Joining us today is Dr. Lewis Nelson, professor and chair of emergency medicine at Rutgers New Jersey Medical School and a certified medical toxicologist.
Today, we will be discussing an important and disturbing Gen Z trend circulating on social media, known as blackout rage gallon, or BORG.
Welcome, Lewis.
Lewis S. Nelson, MD: Thanks for having me.
Dr. Glatter: Thanks so much for joining us. This trend that’s been circulating on social media is really disturbing. It has elements that focus on binge drinking: Talking about taking a jug; emptying half of it out; and putting one fifth of vodka and some electrolytes, caffeine, or other things too is just incredibly disturbing. Teens and parents are looking at this. I’ll let you jump into the discussion.
Dr. Nelson: You’re totally right, it is disturbing. Binge drinking is a huge problem in this country in general. It’s a particular problem with young people – teenagers and young adults. I don’t think people appreciate the dangers associated with binge drinking, such as the amount of alcohol they consume and some of the unintended consequences of doing that.
To frame things quickly, we think there are probably around six people a day in the United States who die of alcohol poisoning. Alcohol poisoning basically is binge drinking to such an extent that you die of the alcohol itself. You’re not dying of a car crash or doing something that injures you. You’re dying of the alcohol. You’re drinking so much that your breathing slows, it stops, you have heart rhythm disturbances, and so on. It totals about 2,200 people a year in the United States.
Dr. Glatter: That’s alarming. For this trend, their argument is that half of the gallon is water. Therefore, I’m fine. I can drink it over 8-12 hours and it’s not an issue. How would you respond to that?
Dr. Nelson: Well, alcohol is alcohol. It’s all about how much you take in over what time period. I guess, in concept, it could be safer if you do it right. That’s not the way it’s been, so to speak, marketed on the various social media platforms. It’s meant to be a way to protect yourself from having your drink spiked or eating or ingesting contaminants from other people’s mouths when you share glasses or dip cups into communal pots like jungle juice or something.
Clearly, if you’re going to drink a large amount of alcohol over a short or long period of time, you do run the risk of having significant consequences, including bad decision-making if you’re just a little drunk all the way down to that of the complications you described about alcohol poisoning.
Dr. Glatter: There has been a comment made that this could be a form of harm reduction. The point of harm reduction is that we run trials, we validate it, and we test it. This, certainly in my mind, is no form of true harm reduction. I think you would agree.
Dr. Nelson: Many things that are marketed as harm reduction aren’t. There could be some aspects of this that could be considered harm reduction. You may believe – and there’s no reason not to – that protecting your drink is a good idea. If you’re at a bar and you leave your glass open and somebody put something in it, you can be drugged. Drug-facilitated sexual assault, for example, is a big issue. That means you have to leave your glass unattended. If you tend to your glass, it’s probably fine. One of the ways of harm reduction they mention is that by having a cap and having this bottle with you at all times, that can’t happen.
Now, in fairness, by far the drug most commonly associated with sexual assault is alcohol. It’s not gamma-hydroxybutyrate or ketamine. It’s not the other things that people are concerned about. Those happen, but those are small problems in the big picture. It’s drinking too much.
A form of harm reduction that you can comment on perhaps is that you make this drink concoction yourself, so you know what is in there. You can take that bottle, pour out half the water, and fill up the other half with water and nobody’s going to know. More likely, the way they say you should do it is you take your gallon jug, you pour it out, and you fill it up with one fifth of vodka.
One fifth of vodka is the same amount of volume as a bottle of wine. At 750 mL, that’s a huge amount of alcohol. If you measure the number of shots in that bottle, it’s about 17 shots. Even if you drink that over 6 hours, that’s still several shots an hour. That’s a large amount of alcohol. You might do two or three shots once and then not drink for a few hours. To sit and drink two or three shots an hour for 6 hours, that’s just an exceptional amount of alcohol.
They flavorize it and add caffeine, which only adds to the risk. It doesn’t make it in any way safer. With the volume, 1 gal of water or equivalent over a short period of time in and of itself could be a problem. There’s a large amount of mismessaging here. Whether something’s harm reduction, it could flip around to be easily construed or understood as being harmful.
Not to mention, the idea that when you make something safer, one of the unintended consequences of harm reduction is what we call risk compensation. This is best probably described as what’s called the Peltzman effect. The way that we think about airbags and seatbelts is that they’re going to reduce car crash deaths; and they do, but people drive faster and more recklessly because they know they’re safe.
This is a well-described problem in epidemiology: You expect a certain amount of harm reduction through some implemented process, but you don’t meet that because people take increased risks.
Dr. Glatter: Right. The idea of not developing a hangover is common among many teens and 20-somethings, thinking that because there’s hydration there, because half of it is water, it’s just not going to happen. There’s your “harm reduction,” but your judgment’s impaired. It’s day drinking at its best, all day long. Then someone has the idea to get behind the wheel. These are the disastrous consequences that we all fear.
Dr. Nelson: There is a great example, perhaps of an unintended consequence of harm reduction. By putting caffeine in it, depending on how much caffeine you put in, some of these mixtures can have up to 1,000 mg of caffeine. Remember, a cup of coffee is about 1-200 mg, so you’re talking about several cups of coffee. The idea is that you will not be able to sense, as you normally do, how drunk you are. You’re not going to be a sleepy drunk, you’re going to be an awake drunk.
The idea that you’re going to have to drive so you’re going to drink a strong cup of black coffee before you go driving, you’re not going to drive any better. I can assure you that. You’re going to be more awake, perhaps, and not fall asleep at the wheel, but you’re still going to have psychomotor impairment. Your judgment is going to be impaired. There’s nothing good that comes with adding caffeine except that you’re going to be awake.
From a hangover perspective, there are many things that we’ve guessed at or suggested as either prevention or cures for hangovers. I don’t doubt that you’re going to have some volume depletion if you drink a large amount of alcohol. Alcohol’s a diuretic, so you’re going to lose more volume than you bring in.
Hydrating is probably always a good idea, but there is hydrating and then there’s overhydrating. We don’t need volumes like that. If you drink a cup or two of water, you’re probably fine. You don’t need to drink half a gallon of water. That can lead to problems like delusional hyponatremia, and so forth. There’s not any clear benefit to doing it.
If you want to prevent a hangover, one of the ways you might do it is by using vodka. There are nice data that show that clear alcohols typically, particularly vodka, don’t have many of the congeners that make the specific forms of alcohol what they are. Bourbon smells and tastes like bourbon because of these little molecules, these alkalis and ketones and amino acids and things that make it taste and smell the way it does. That’s true for all the other alcohols.
Vodka has the least amount of that. Even wine and beer have those in them, but vodka is basically alcohol mixed with water. It’s probably the least hangover-prone of all the alcohols; but still, if you drink a lot of vodka, you’re going to have a hangover. It’s just a dose-response curve to how much alcohol you drink, to how drunk you get, and to how much of a hangover you’re going to have.
Dr. Glatter: The hangover is really what it’s about because people want to be functional the next day. There are many companies out there that market hangover remedies, but people are using this as the hangover remedy in a way that’s socially accepted. That’s a good point you make.
The question is how do we get the message out to parents and teens? What’s the best way you feel to really sound the alarm here?
Dr. Nelson: These are challenging issues. We face this all the time with all the sorts of social media in particular. Most parents are not as savvy on social media as their kids are. You have to know what your children are doing. You should know what they’re listening to and watching. You do have to pay attention to the media directed at parents that will inform you a little bit about what your kids are doing. You have to talk with your kids and make sure they understand what it is that they’re doing.
We do this with our kids for some things. Hopefully, we talk about drinking, smoking, sex, and other things with our children (like driving if they get to that stage) and make sure they understand what the risks are and how to mitigate those risks. Being an attentive parent is part of it.
Sometimes you need outside messengers to do it. We’d like to believe that these social media companies are able to police themselves – at least they pay lip service to the fact they do. They have warnings that they’ll take things down that aren’t socially appropriate. Whether they do or not, I don’t know, because you keep seeing things about BORG on these media sites. If they are doing it, they’re not doing it efficiently or quickly enough.
Dr. Glatter: There has to be some censorship. These are young persons who are impressionable, who have developing brains, who are looking at this, thinking that if it’s out there on social media, such as TikTok or Instagram, then it’s okay to do so. That message has to be driven home.
Dr. Nelson: That’s a great point, and it’s tough. We know there’s been debate over the liability of social media or what they post, and whether or not they should be held liable like a more conventional media company or not. That’s politics and philosophy, and we’re probably not going to solve it here.
All these things wind up going viral and there’s probably got to be some filter on things that go viral. Maybe they need to have a bit more attentiveness to that when those things start happening. Now, clearly not every one of these is viral. When you think about some of the challenges we’ve seen in the past, such as the Tide Pod challenge and cinnamon challenge, some of these things could be quickly figured out to be dangerous.
I remember that the ice bucket challenge for amyotrophic lateral sclerosis was pretty benign. You pour a bucket of water over your head, and people aren’t really getting hurt. That’s fun and good, and let people go out and do that. That could pass through the filter. When you start to see people drinking excessive amounts of alcohol, it doesn’t take an emergency physician to know that’s not a good thing. Any parent should know that if my kid drinks half a bottle or a bottle of vodka over a short period of time, that just can’t be okay.
Dr. Glatter: It’s a public health issue. That’s what we need to elevate it to because ultimately that’s what it impacts: welfare and safety.
Speaking of buckets, there’s a new bucket challenge, wherein unsuspecting people have a bucket put on their head, can’t breathe, and then pass out. There’s been a number of these reported and actually filmed on social media. Here’s another example of dangerous types of behavior that essentially are a form of assault. Unsuspecting people suffer injuries from young children and teens trying to play pranks.
Again, had there not been this medium, we wouldn’t necessarily see the extent of the injuries. I guess going forward, the next step would be to send a message to colleges that there should be some form of warning if this trend is seen, at least from a public health standpoint.
Dr. Nelson: Education is a necessary thing to do, but it’s almost never the real solution to a problem. We can educate people as best we can that they need to do things right. At some point, we’re going to need to regulate it or manage it somehow.
Whether it’s through a carrot or a stick approach, or whether you want to give people kudos for doing the right thing or punish them for doing something wrong, that’s a tough decision to make and one that is going to be made by a parent or guardian, a school official, or law enforcement. Somehow, we have to figure out how to make this happen.
There’s not going to be a single size that fits all for this. At some level, we have to do something to educate and regulate. The balance between those two things is going to be political and philosophical in nature.
Dr. Glatter: Right, and the element of peer pressure and conformity in this is really part of the element. If we try to remove that aspect of it, then often these trends would go away. That aspect of conformity and peer pressure is instrumental in fueling these trends. Maybe we can make a full gallon of water be the trend without any alcohol in there.
Dr. Nelson: We say water is only water, but as a medical toxicologist, I can tell you that one of the foundations in medical toxicology is that everything is toxic. It’s just the dose that determines the toxicity. Oxygen is toxic, water is toxic. Everything’s toxic if you take enough of it.
We know that whether it’s psychogenic or intentional, polydipsia by drinking excessive amounts of water, especially without electrolytes, is one of the reasons they say you should add electrolytes. That’s all relative as well, because depending on the electrolyte and how much you put in and things like that, that could also become dangerous. Drinking excessive amounts of water like they’re suggesting, which sounds like a good thing to prevent hangover and so on, can in and of itself be a problem too.
Dr. Glatter: Right, and we know that there’s no magic bullet for a hangover. Obviously, abstinence is the only thing that truly works.
Dr. Nelson: Or moderation.
Dr. Glatter: Until research proves further.
Thank you so much. You’ve made some really important points. Thank you for talking about the BORG phenomenon, how it relates to society in general, and what we can do to try to change people’s perception of alcohol and the bigger picture of binge drinking. I really appreciate it.
Dr. Nelson: Thanks, Rob, for having me. It’s an important topic and hopefully we can get a handle on this. I appreciate your time.
Dr. Glatter is an attending physician at Lenox Hill Hospital in New York City and assistant professor of emergency medicine at Hofstra University, Hempstead, N.Y. Dr. Nelson is professor and chair of the department of emergency medicine and chief of the division of medical toxicology at Rutgers New Jersey Medical School, Newark. He is a member of the board of directors of the American Board of Emergency Medicine, the Accreditation Council for Continuing Medical Education, and Association of Academic Chairs in Emergency Medicine and is past-president of the American College of Medical Toxicology. Dr. Glatter and Dr. Nelson disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
This discussion was recorded on April 6, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical adviser for Medscape Emergency Medicine. Joining us today is Dr. Lewis Nelson, professor and chair of emergency medicine at Rutgers New Jersey Medical School and a certified medical toxicologist.
Today, we will be discussing an important and disturbing Gen Z trend circulating on social media, known as blackout rage gallon, or BORG.
Welcome, Lewis.
Lewis S. Nelson, MD: Thanks for having me.
Dr. Glatter: Thanks so much for joining us. This trend that’s been circulating on social media is really disturbing. It has elements that focus on binge drinking: Talking about taking a jug; emptying half of it out; and putting one fifth of vodka and some electrolytes, caffeine, or other things too is just incredibly disturbing. Teens and parents are looking at this. I’ll let you jump into the discussion.
Dr. Nelson: You’re totally right, it is disturbing. Binge drinking is a huge problem in this country in general. It’s a particular problem with young people – teenagers and young adults. I don’t think people appreciate the dangers associated with binge drinking, such as the amount of alcohol they consume and some of the unintended consequences of doing that.
To frame things quickly, we think there are probably around six people a day in the United States who die of alcohol poisoning. Alcohol poisoning basically is binge drinking to such an extent that you die of the alcohol itself. You’re not dying of a car crash or doing something that injures you. You’re dying of the alcohol. You’re drinking so much that your breathing slows, it stops, you have heart rhythm disturbances, and so on. It totals about 2,200 people a year in the United States.
Dr. Glatter: That’s alarming. For this trend, their argument is that half of the gallon is water. Therefore, I’m fine. I can drink it over 8-12 hours and it’s not an issue. How would you respond to that?
Dr. Nelson: Well, alcohol is alcohol. It’s all about how much you take in over what time period. I guess, in concept, it could be safer if you do it right. That’s not the way it’s been, so to speak, marketed on the various social media platforms. It’s meant to be a way to protect yourself from having your drink spiked or eating or ingesting contaminants from other people’s mouths when you share glasses or dip cups into communal pots like jungle juice or something.
Clearly, if you’re going to drink a large amount of alcohol over a short or long period of time, you do run the risk of having significant consequences, including bad decision-making if you’re just a little drunk all the way down to that of the complications you described about alcohol poisoning.
Dr. Glatter: There has been a comment made that this could be a form of harm reduction. The point of harm reduction is that we run trials, we validate it, and we test it. This, certainly in my mind, is no form of true harm reduction. I think you would agree.
Dr. Nelson: Many things that are marketed as harm reduction aren’t. There could be some aspects of this that could be considered harm reduction. You may believe – and there’s no reason not to – that protecting your drink is a good idea. If you’re at a bar and you leave your glass open and somebody put something in it, you can be drugged. Drug-facilitated sexual assault, for example, is a big issue. That means you have to leave your glass unattended. If you tend to your glass, it’s probably fine. One of the ways of harm reduction they mention is that by having a cap and having this bottle with you at all times, that can’t happen.
Now, in fairness, by far the drug most commonly associated with sexual assault is alcohol. It’s not gamma-hydroxybutyrate or ketamine. It’s not the other things that people are concerned about. Those happen, but those are small problems in the big picture. It’s drinking too much.
A form of harm reduction that you can comment on perhaps is that you make this drink concoction yourself, so you know what is in there. You can take that bottle, pour out half the water, and fill up the other half with water and nobody’s going to know. More likely, the way they say you should do it is you take your gallon jug, you pour it out, and you fill it up with one fifth of vodka.
One fifth of vodka is the same amount of volume as a bottle of wine. At 750 mL, that’s a huge amount of alcohol. If you measure the number of shots in that bottle, it’s about 17 shots. Even if you drink that over 6 hours, that’s still several shots an hour. That’s a large amount of alcohol. You might do two or three shots once and then not drink for a few hours. To sit and drink two or three shots an hour for 6 hours, that’s just an exceptional amount of alcohol.
They flavorize it and add caffeine, which only adds to the risk. It doesn’t make it in any way safer. With the volume, 1 gal of water or equivalent over a short period of time in and of itself could be a problem. There’s a large amount of mismessaging here. Whether something’s harm reduction, it could flip around to be easily construed or understood as being harmful.
Not to mention, the idea that when you make something safer, one of the unintended consequences of harm reduction is what we call risk compensation. This is best probably described as what’s called the Peltzman effect. The way that we think about airbags and seatbelts is that they’re going to reduce car crash deaths; and they do, but people drive faster and more recklessly because they know they’re safe.
This is a well-described problem in epidemiology: You expect a certain amount of harm reduction through some implemented process, but you don’t meet that because people take increased risks.
Dr. Glatter: Right. The idea of not developing a hangover is common among many teens and 20-somethings, thinking that because there’s hydration there, because half of it is water, it’s just not going to happen. There’s your “harm reduction,” but your judgment’s impaired. It’s day drinking at its best, all day long. Then someone has the idea to get behind the wheel. These are the disastrous consequences that we all fear.
Dr. Nelson: There is a great example, perhaps of an unintended consequence of harm reduction. By putting caffeine in it, depending on how much caffeine you put in, some of these mixtures can have up to 1,000 mg of caffeine. Remember, a cup of coffee is about 1-200 mg, so you’re talking about several cups of coffee. The idea is that you will not be able to sense, as you normally do, how drunk you are. You’re not going to be a sleepy drunk, you’re going to be an awake drunk.
The idea that you’re going to have to drive so you’re going to drink a strong cup of black coffee before you go driving, you’re not going to drive any better. I can assure you that. You’re going to be more awake, perhaps, and not fall asleep at the wheel, but you’re still going to have psychomotor impairment. Your judgment is going to be impaired. There’s nothing good that comes with adding caffeine except that you’re going to be awake.
From a hangover perspective, there are many things that we’ve guessed at or suggested as either prevention or cures for hangovers. I don’t doubt that you’re going to have some volume depletion if you drink a large amount of alcohol. Alcohol’s a diuretic, so you’re going to lose more volume than you bring in.
Hydrating is probably always a good idea, but there is hydrating and then there’s overhydrating. We don’t need volumes like that. If you drink a cup or two of water, you’re probably fine. You don’t need to drink half a gallon of water. That can lead to problems like delusional hyponatremia, and so forth. There’s not any clear benefit to doing it.
If you want to prevent a hangover, one of the ways you might do it is by using vodka. There are nice data that show that clear alcohols typically, particularly vodka, don’t have many of the congeners that make the specific forms of alcohol what they are. Bourbon smells and tastes like bourbon because of these little molecules, these alkalis and ketones and amino acids and things that make it taste and smell the way it does. That’s true for all the other alcohols.
Vodka has the least amount of that. Even wine and beer have those in them, but vodka is basically alcohol mixed with water. It’s probably the least hangover-prone of all the alcohols; but still, if you drink a lot of vodka, you’re going to have a hangover. It’s just a dose-response curve to how much alcohol you drink, to how drunk you get, and to how much of a hangover you’re going to have.
Dr. Glatter: The hangover is really what it’s about because people want to be functional the next day. There are many companies out there that market hangover remedies, but people are using this as the hangover remedy in a way that’s socially accepted. That’s a good point you make.
The question is how do we get the message out to parents and teens? What’s the best way you feel to really sound the alarm here?
Dr. Nelson: These are challenging issues. We face this all the time with all the sorts of social media in particular. Most parents are not as savvy on social media as their kids are. You have to know what your children are doing. You should know what they’re listening to and watching. You do have to pay attention to the media directed at parents that will inform you a little bit about what your kids are doing. You have to talk with your kids and make sure they understand what it is that they’re doing.
We do this with our kids for some things. Hopefully, we talk about drinking, smoking, sex, and other things with our children (like driving if they get to that stage) and make sure they understand what the risks are and how to mitigate those risks. Being an attentive parent is part of it.
Sometimes you need outside messengers to do it. We’d like to believe that these social media companies are able to police themselves – at least they pay lip service to the fact they do. They have warnings that they’ll take things down that aren’t socially appropriate. Whether they do or not, I don’t know, because you keep seeing things about BORG on these media sites. If they are doing it, they’re not doing it efficiently or quickly enough.
Dr. Glatter: There has to be some censorship. These are young persons who are impressionable, who have developing brains, who are looking at this, thinking that if it’s out there on social media, such as TikTok or Instagram, then it’s okay to do so. That message has to be driven home.
Dr. Nelson: That’s a great point, and it’s tough. We know there’s been debate over the liability of social media or what they post, and whether or not they should be held liable like a more conventional media company or not. That’s politics and philosophy, and we’re probably not going to solve it here.
All these things wind up going viral and there’s probably got to be some filter on things that go viral. Maybe they need to have a bit more attentiveness to that when those things start happening. Now, clearly not every one of these is viral. When you think about some of the challenges we’ve seen in the past, such as the Tide Pod challenge and cinnamon challenge, some of these things could be quickly figured out to be dangerous.
I remember that the ice bucket challenge for amyotrophic lateral sclerosis was pretty benign. You pour a bucket of water over your head, and people aren’t really getting hurt. That’s fun and good, and let people go out and do that. That could pass through the filter. When you start to see people drinking excessive amounts of alcohol, it doesn’t take an emergency physician to know that’s not a good thing. Any parent should know that if my kid drinks half a bottle or a bottle of vodka over a short period of time, that just can’t be okay.
Dr. Glatter: It’s a public health issue. That’s what we need to elevate it to because ultimately that’s what it impacts: welfare and safety.
Speaking of buckets, there’s a new bucket challenge, wherein unsuspecting people have a bucket put on their head, can’t breathe, and then pass out. There’s been a number of these reported and actually filmed on social media. Here’s another example of dangerous types of behavior that essentially are a form of assault. Unsuspecting people suffer injuries from young children and teens trying to play pranks.
Again, had there not been this medium, we wouldn’t necessarily see the extent of the injuries. I guess going forward, the next step would be to send a message to colleges that there should be some form of warning if this trend is seen, at least from a public health standpoint.
Dr. Nelson: Education is a necessary thing to do, but it’s almost never the real solution to a problem. We can educate people as best we can that they need to do things right. At some point, we’re going to need to regulate it or manage it somehow.
Whether it’s through a carrot or a stick approach, or whether you want to give people kudos for doing the right thing or punish them for doing something wrong, that’s a tough decision to make and one that is going to be made by a parent or guardian, a school official, or law enforcement. Somehow, we have to figure out how to make this happen.
There’s not going to be a single size that fits all for this. At some level, we have to do something to educate and regulate. The balance between those two things is going to be political and philosophical in nature.
Dr. Glatter: Right, and the element of peer pressure and conformity in this is really part of the element. If we try to remove that aspect of it, then often these trends would go away. That aspect of conformity and peer pressure is instrumental in fueling these trends. Maybe we can make a full gallon of water be the trend without any alcohol in there.
Dr. Nelson: We say water is only water, but as a medical toxicologist, I can tell you that one of the foundations in medical toxicology is that everything is toxic. It’s just the dose that determines the toxicity. Oxygen is toxic, water is toxic. Everything’s toxic if you take enough of it.
We know that whether it’s psychogenic or intentional, polydipsia by drinking excessive amounts of water, especially without electrolytes, is one of the reasons they say you should add electrolytes. That’s all relative as well, because depending on the electrolyte and how much you put in and things like that, that could also become dangerous. Drinking excessive amounts of water like they’re suggesting, which sounds like a good thing to prevent hangover and so on, can in and of itself be a problem too.
Dr. Glatter: Right, and we know that there’s no magic bullet for a hangover. Obviously, abstinence is the only thing that truly works.
Dr. Nelson: Or moderation.
Dr. Glatter: Until research proves further.
Thank you so much. You’ve made some really important points. Thank you for talking about the BORG phenomenon, how it relates to society in general, and what we can do to try to change people’s perception of alcohol and the bigger picture of binge drinking. I really appreciate it.
Dr. Nelson: Thanks, Rob, for having me. It’s an important topic and hopefully we can get a handle on this. I appreciate your time.
Dr. Glatter is an attending physician at Lenox Hill Hospital in New York City and assistant professor of emergency medicine at Hofstra University, Hempstead, N.Y. Dr. Nelson is professor and chair of the department of emergency medicine and chief of the division of medical toxicology at Rutgers New Jersey Medical School, Newark. He is a member of the board of directors of the American Board of Emergency Medicine, the Accreditation Council for Continuing Medical Education, and Association of Academic Chairs in Emergency Medicine and is past-president of the American College of Medical Toxicology. Dr. Glatter and Dr. Nelson disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
This discussion was recorded on April 6, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical adviser for Medscape Emergency Medicine. Joining us today is Dr. Lewis Nelson, professor and chair of emergency medicine at Rutgers New Jersey Medical School and a certified medical toxicologist.
Today, we will be discussing an important and disturbing Gen Z trend circulating on social media, known as blackout rage gallon, or BORG.
Welcome, Lewis.
Lewis S. Nelson, MD: Thanks for having me.
Dr. Glatter: Thanks so much for joining us. This trend that’s been circulating on social media is really disturbing. It has elements that focus on binge drinking: Talking about taking a jug; emptying half of it out; and putting one fifth of vodka and some electrolytes, caffeine, or other things too is just incredibly disturbing. Teens and parents are looking at this. I’ll let you jump into the discussion.
Dr. Nelson: You’re totally right, it is disturbing. Binge drinking is a huge problem in this country in general. It’s a particular problem with young people – teenagers and young adults. I don’t think people appreciate the dangers associated with binge drinking, such as the amount of alcohol they consume and some of the unintended consequences of doing that.
To frame things quickly, we think there are probably around six people a day in the United States who die of alcohol poisoning. Alcohol poisoning basically is binge drinking to such an extent that you die of the alcohol itself. You’re not dying of a car crash or doing something that injures you. You’re dying of the alcohol. You’re drinking so much that your breathing slows, it stops, you have heart rhythm disturbances, and so on. It totals about 2,200 people a year in the United States.
Dr. Glatter: That’s alarming. For this trend, their argument is that half of the gallon is water. Therefore, I’m fine. I can drink it over 8-12 hours and it’s not an issue. How would you respond to that?
Dr. Nelson: Well, alcohol is alcohol. It’s all about how much you take in over what time period. I guess, in concept, it could be safer if you do it right. That’s not the way it’s been, so to speak, marketed on the various social media platforms. It’s meant to be a way to protect yourself from having your drink spiked or eating or ingesting contaminants from other people’s mouths when you share glasses or dip cups into communal pots like jungle juice or something.
Clearly, if you’re going to drink a large amount of alcohol over a short or long period of time, you do run the risk of having significant consequences, including bad decision-making if you’re just a little drunk all the way down to that of the complications you described about alcohol poisoning.
Dr. Glatter: There has been a comment made that this could be a form of harm reduction. The point of harm reduction is that we run trials, we validate it, and we test it. This, certainly in my mind, is no form of true harm reduction. I think you would agree.
Dr. Nelson: Many things that are marketed as harm reduction aren’t. There could be some aspects of this that could be considered harm reduction. You may believe – and there’s no reason not to – that protecting your drink is a good idea. If you’re at a bar and you leave your glass open and somebody put something in it, you can be drugged. Drug-facilitated sexual assault, for example, is a big issue. That means you have to leave your glass unattended. If you tend to your glass, it’s probably fine. One of the ways of harm reduction they mention is that by having a cap and having this bottle with you at all times, that can’t happen.
Now, in fairness, by far the drug most commonly associated with sexual assault is alcohol. It’s not gamma-hydroxybutyrate or ketamine. It’s not the other things that people are concerned about. Those happen, but those are small problems in the big picture. It’s drinking too much.
A form of harm reduction that you can comment on perhaps is that you make this drink concoction yourself, so you know what is in there. You can take that bottle, pour out half the water, and fill up the other half with water and nobody’s going to know. More likely, the way they say you should do it is you take your gallon jug, you pour it out, and you fill it up with one fifth of vodka.
One fifth of vodka is the same amount of volume as a bottle of wine. At 750 mL, that’s a huge amount of alcohol. If you measure the number of shots in that bottle, it’s about 17 shots. Even if you drink that over 6 hours, that’s still several shots an hour. That’s a large amount of alcohol. You might do two or three shots once and then not drink for a few hours. To sit and drink two or three shots an hour for 6 hours, that’s just an exceptional amount of alcohol.
They flavorize it and add caffeine, which only adds to the risk. It doesn’t make it in any way safer. With the volume, 1 gal of water or equivalent over a short period of time in and of itself could be a problem. There’s a large amount of mismessaging here. Whether something’s harm reduction, it could flip around to be easily construed or understood as being harmful.
Not to mention, the idea that when you make something safer, one of the unintended consequences of harm reduction is what we call risk compensation. This is best probably described as what’s called the Peltzman effect. The way that we think about airbags and seatbelts is that they’re going to reduce car crash deaths; and they do, but people drive faster and more recklessly because they know they’re safe.
This is a well-described problem in epidemiology: You expect a certain amount of harm reduction through some implemented process, but you don’t meet that because people take increased risks.
Dr. Glatter: Right. The idea of not developing a hangover is common among many teens and 20-somethings, thinking that because there’s hydration there, because half of it is water, it’s just not going to happen. There’s your “harm reduction,” but your judgment’s impaired. It’s day drinking at its best, all day long. Then someone has the idea to get behind the wheel. These are the disastrous consequences that we all fear.
Dr. Nelson: There is a great example, perhaps of an unintended consequence of harm reduction. By putting caffeine in it, depending on how much caffeine you put in, some of these mixtures can have up to 1,000 mg of caffeine. Remember, a cup of coffee is about 1-200 mg, so you’re talking about several cups of coffee. The idea is that you will not be able to sense, as you normally do, how drunk you are. You’re not going to be a sleepy drunk, you’re going to be an awake drunk.
The idea that you’re going to have to drive so you’re going to drink a strong cup of black coffee before you go driving, you’re not going to drive any better. I can assure you that. You’re going to be more awake, perhaps, and not fall asleep at the wheel, but you’re still going to have psychomotor impairment. Your judgment is going to be impaired. There’s nothing good that comes with adding caffeine except that you’re going to be awake.
From a hangover perspective, there are many things that we’ve guessed at or suggested as either prevention or cures for hangovers. I don’t doubt that you’re going to have some volume depletion if you drink a large amount of alcohol. Alcohol’s a diuretic, so you’re going to lose more volume than you bring in.
Hydrating is probably always a good idea, but there is hydrating and then there’s overhydrating. We don’t need volumes like that. If you drink a cup or two of water, you’re probably fine. You don’t need to drink half a gallon of water. That can lead to problems like delusional hyponatremia, and so forth. There’s not any clear benefit to doing it.
If you want to prevent a hangover, one of the ways you might do it is by using vodka. There are nice data that show that clear alcohols typically, particularly vodka, don’t have many of the congeners that make the specific forms of alcohol what they are. Bourbon smells and tastes like bourbon because of these little molecules, these alkalis and ketones and amino acids and things that make it taste and smell the way it does. That’s true for all the other alcohols.
Vodka has the least amount of that. Even wine and beer have those in them, but vodka is basically alcohol mixed with water. It’s probably the least hangover-prone of all the alcohols; but still, if you drink a lot of vodka, you’re going to have a hangover. It’s just a dose-response curve to how much alcohol you drink, to how drunk you get, and to how much of a hangover you’re going to have.
Dr. Glatter: The hangover is really what it’s about because people want to be functional the next day. There are many companies out there that market hangover remedies, but people are using this as the hangover remedy in a way that’s socially accepted. That’s a good point you make.
The question is how do we get the message out to parents and teens? What’s the best way you feel to really sound the alarm here?
Dr. Nelson: These are challenging issues. We face this all the time with all the sorts of social media in particular. Most parents are not as savvy on social media as their kids are. You have to know what your children are doing. You should know what they’re listening to and watching. You do have to pay attention to the media directed at parents that will inform you a little bit about what your kids are doing. You have to talk with your kids and make sure they understand what it is that they’re doing.
We do this with our kids for some things. Hopefully, we talk about drinking, smoking, sex, and other things with our children (like driving if they get to that stage) and make sure they understand what the risks are and how to mitigate those risks. Being an attentive parent is part of it.
Sometimes you need outside messengers to do it. We’d like to believe that these social media companies are able to police themselves – at least they pay lip service to the fact they do. They have warnings that they’ll take things down that aren’t socially appropriate. Whether they do or not, I don’t know, because you keep seeing things about BORG on these media sites. If they are doing it, they’re not doing it efficiently or quickly enough.
Dr. Glatter: There has to be some censorship. These are young persons who are impressionable, who have developing brains, who are looking at this, thinking that if it’s out there on social media, such as TikTok or Instagram, then it’s okay to do so. That message has to be driven home.
Dr. Nelson: That’s a great point, and it’s tough. We know there’s been debate over the liability of social media or what they post, and whether or not they should be held liable like a more conventional media company or not. That’s politics and philosophy, and we’re probably not going to solve it here.
All these things wind up going viral and there’s probably got to be some filter on things that go viral. Maybe they need to have a bit more attentiveness to that when those things start happening. Now, clearly not every one of these is viral. When you think about some of the challenges we’ve seen in the past, such as the Tide Pod challenge and cinnamon challenge, some of these things could be quickly figured out to be dangerous.
I remember that the ice bucket challenge for amyotrophic lateral sclerosis was pretty benign. You pour a bucket of water over your head, and people aren’t really getting hurt. That’s fun and good, and let people go out and do that. That could pass through the filter. When you start to see people drinking excessive amounts of alcohol, it doesn’t take an emergency physician to know that’s not a good thing. Any parent should know that if my kid drinks half a bottle or a bottle of vodka over a short period of time, that just can’t be okay.
Dr. Glatter: It’s a public health issue. That’s what we need to elevate it to because ultimately that’s what it impacts: welfare and safety.
Speaking of buckets, there’s a new bucket challenge, wherein unsuspecting people have a bucket put on their head, can’t breathe, and then pass out. There’s been a number of these reported and actually filmed on social media. Here’s another example of dangerous types of behavior that essentially are a form of assault. Unsuspecting people suffer injuries from young children and teens trying to play pranks.
Again, had there not been this medium, we wouldn’t necessarily see the extent of the injuries. I guess going forward, the next step would be to send a message to colleges that there should be some form of warning if this trend is seen, at least from a public health standpoint.
Dr. Nelson: Education is a necessary thing to do, but it’s almost never the real solution to a problem. We can educate people as best we can that they need to do things right. At some point, we’re going to need to regulate it or manage it somehow.
Whether it’s through a carrot or a stick approach, or whether you want to give people kudos for doing the right thing or punish them for doing something wrong, that’s a tough decision to make and one that is going to be made by a parent or guardian, a school official, or law enforcement. Somehow, we have to figure out how to make this happen.
There’s not going to be a single size that fits all for this. At some level, we have to do something to educate and regulate. The balance between those two things is going to be political and philosophical in nature.
Dr. Glatter: Right, and the element of peer pressure and conformity in this is really part of the element. If we try to remove that aspect of it, then often these trends would go away. That aspect of conformity and peer pressure is instrumental in fueling these trends. Maybe we can make a full gallon of water be the trend without any alcohol in there.
Dr. Nelson: We say water is only water, but as a medical toxicologist, I can tell you that one of the foundations in medical toxicology is that everything is toxic. It’s just the dose that determines the toxicity. Oxygen is toxic, water is toxic. Everything’s toxic if you take enough of it.
We know that whether it’s psychogenic or intentional, polydipsia by drinking excessive amounts of water, especially without electrolytes, is one of the reasons they say you should add electrolytes. That’s all relative as well, because depending on the electrolyte and how much you put in and things like that, that could also become dangerous. Drinking excessive amounts of water like they’re suggesting, which sounds like a good thing to prevent hangover and so on, can in and of itself be a problem too.
Dr. Glatter: Right, and we know that there’s no magic bullet for a hangover. Obviously, abstinence is the only thing that truly works.
Dr. Nelson: Or moderation.
Dr. Glatter: Until research proves further.
Thank you so much. You’ve made some really important points. Thank you for talking about the BORG phenomenon, how it relates to society in general, and what we can do to try to change people’s perception of alcohol and the bigger picture of binge drinking. I really appreciate it.
Dr. Nelson: Thanks, Rob, for having me. It’s an important topic and hopefully we can get a handle on this. I appreciate your time.
Dr. Glatter is an attending physician at Lenox Hill Hospital in New York City and assistant professor of emergency medicine at Hofstra University, Hempstead, N.Y. Dr. Nelson is professor and chair of the department of emergency medicine and chief of the division of medical toxicology at Rutgers New Jersey Medical School, Newark. He is a member of the board of directors of the American Board of Emergency Medicine, the Accreditation Council for Continuing Medical Education, and Association of Academic Chairs in Emergency Medicine and is past-president of the American College of Medical Toxicology. Dr. Glatter and Dr. Nelson disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
TOO GOOD TO LAST?
When I first started in my training, I could not wait until the next issue of Emergency Medicine arrived in my mailbox (not yet inbox!). Emergency Medicine provided a service to our community and specialty at a time when no other publication was willing or able to do so. For no cost, no membership requirements, and no strings attached, we all received a monthly treasure trove of contemporary and practical information. The articles within were written by credible authors, in an approachable style, with professional illustrations that focused on key clinical issues that we saw in our everyday clinical practices.
As my career matured, I was asked to write and subsequently oversee a recurring feature on practical aspects of managing poisoning. Unclear if this was going to be well read, I agreed with trepidation. I quickly learned just how widely appreciated this journal was. People around the country wrote to let me know their thoughts on our thoughts (which meant they were reading it at least!). And people around the country offered to submit interesting toxicology cases for publication. For many of these authors, and many of my med tox trainees, this journal represented the first time they saw their name in print.
Clearly, to me at least, despite all the available blogs, podcasts, reddit and subreddit streams, and continuing medical education programs out there, people still loved getting this small but effective educational tool sent to them. And it is certainly sad to me, and likely many, that this wonderful benefactor of high-quality EM knowledge is losing its hard-fought battle against the modern reality of medical publishing.
I rest assured that there are other credible sources of education that we all can access. I know that our authors and readers will miss the journal dearly. But to paraphrase an unknown author: We should not be sad that it’s over, but glad that it happened.
Lewis S. Nelson, MD
Rutgers New Jersey Medical School
When I first started in my training, I could not wait until the next issue of Emergency Medicine arrived in my mailbox (not yet inbox!). Emergency Medicine provided a service to our community and specialty at a time when no other publication was willing or able to do so. For no cost, no membership requirements, and no strings attached, we all received a monthly treasure trove of contemporary and practical information. The articles within were written by credible authors, in an approachable style, with professional illustrations that focused on key clinical issues that we saw in our everyday clinical practices.
As my career matured, I was asked to write and subsequently oversee a recurring feature on practical aspects of managing poisoning. Unclear if this was going to be well read, I agreed with trepidation. I quickly learned just how widely appreciated this journal was. People around the country wrote to let me know their thoughts on our thoughts (which meant they were reading it at least!). And people around the country offered to submit interesting toxicology cases for publication. For many of these authors, and many of my med tox trainees, this journal represented the first time they saw their name in print.
Clearly, to me at least, despite all the available blogs, podcasts, reddit and subreddit streams, and continuing medical education programs out there, people still loved getting this small but effective educational tool sent to them. And it is certainly sad to me, and likely many, that this wonderful benefactor of high-quality EM knowledge is losing its hard-fought battle against the modern reality of medical publishing.
I rest assured that there are other credible sources of education that we all can access. I know that our authors and readers will miss the journal dearly. But to paraphrase an unknown author: We should not be sad that it’s over, but glad that it happened.
Lewis S. Nelson, MD
Rutgers New Jersey Medical School
When I first started in my training, I could not wait until the next issue of Emergency Medicine arrived in my mailbox (not yet inbox!). Emergency Medicine provided a service to our community and specialty at a time when no other publication was willing or able to do so. For no cost, no membership requirements, and no strings attached, we all received a monthly treasure trove of contemporary and practical information. The articles within were written by credible authors, in an approachable style, with professional illustrations that focused on key clinical issues that we saw in our everyday clinical practices.
As my career matured, I was asked to write and subsequently oversee a recurring feature on practical aspects of managing poisoning. Unclear if this was going to be well read, I agreed with trepidation. I quickly learned just how widely appreciated this journal was. People around the country wrote to let me know their thoughts on our thoughts (which meant they were reading it at least!). And people around the country offered to submit interesting toxicology cases for publication. For many of these authors, and many of my med tox trainees, this journal represented the first time they saw their name in print.
Clearly, to me at least, despite all the available blogs, podcasts, reddit and subreddit streams, and continuing medical education programs out there, people still loved getting this small but effective educational tool sent to them. And it is certainly sad to me, and likely many, that this wonderful benefactor of high-quality EM knowledge is losing its hard-fought battle against the modern reality of medical publishing.
I rest assured that there are other credible sources of education that we all can access. I know that our authors and readers will miss the journal dearly. But to paraphrase an unknown author: We should not be sad that it’s over, but glad that it happened.
Lewis S. Nelson, MD
Rutgers New Jersey Medical School
When the Poisoned Risk Poisoning Others: Fatal Sodium Azide Overdose
Case
A 24-year-old man in cardiac arrest was brought to the ED via emergency medical services (EMS). Unfortunately, resuscitation efforts were unsuccessful. Little was known about the patient, but the emergency physician was informed that the patient had ingested sodium azide (NaN3), which he had ordered online. The patient collapsed shortly after ingesting the sodium azide, approximately the same time police officers arrived at the patient’s home.
No specific details were known about the patient’s ingestion. Upon learning of the exposure to sodium azide, a member of the ED staff contacted the local poison control center for information on the proper course of action to ensure staff safety and limit exposure. Shortly thereafter, several of emergency medical technicians and police officers, who had responded to the emergency assistance call for this patient, presented to the ED with concerns of exposure.
What is sodium azide?
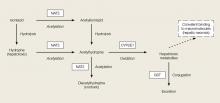
Sodium azide is a colorless, odorless crystalline water-soluble solid that has a pK of 4.8.1 When sodium azide is dissolved in an acid, it liberates hydrazoic acid (HN3), which has a pungent odor, high vapor pressure (484 mm Hg), and a relatively low-boiling point of 37°C (98°F).2
The most common industrial use of sodium azide is as a propellant in air bags. In this capacity, sodium azide rapidly decomposes to nitrogen gas when it reaches a temperature of 300°C (572°F), causing rapid expansion of the air bag. In addition to air bags, sodium azide is used in research laboratories as a preservative and in agriculture as a pesticide. The main nontoxicological concern with all azide agents is the potential for explosion when they react with metals, such as lead, copper, silver, and mercury, to form metal azides that are sensitive to shock.3 An example of the explosive nature of these azides was demonstrated in a report wherein diluted sodium azide was poured down a drain, causing an explosion as a worker was fixing the pipe.4
In addition to industrial and commercial use, sodium azide is occasionally used in suicide attempts because it is rapidly fatal, has no specific antidote, and can be purchased online.3
What is the toxicity of sodium azide?
The lethal dose for both oral and dermal exposure to sodium azide is approximately 10 to 20 mg/kg.3,5 Therefore, ingestion of 700 mg of sodium azide, a volume approximately the size of a penny, is likely to be fatal.3
Sodium azide is primarily a mitochondrial toxin, which binds the electron transport chain, inhibiting oxidative phosphorylation. The resulting reduction in adenosine triphosphate (ATP) production, even in the presence of oxygen, results in metabolic failure.6 This mechanism of action is similar to that of cyanide, although sodium azide causes more pronounced vasodilation due to the in vivo conversion of some azide to the vasodilator nitric oxide.7 Some reports suggest that azide lethality is due to enhanced excitatory transmission from nitric oxide in the central nervous system.8
What are the clinical manifestations of azide poisoning, and what is the treatment?
The early clinical findings of a patient with azide poisoning include hypotension, dizziness, headache, nausea, vomiting, palpitations, tachycardia, dyspnea, and restlessness. Inhalation of hydrazoic acid can also produce wheezing and coughing. The most common effect is hypotension, which can occur within 1 minute of exposure. Following depletion of cellular ATP, anaerobic glycolysis generates lactate and produces acidemia. More severe findings of azide poisoning include seizures, cardiac arrhythmia, loss of consciousness, pulmonary edema, and cardiopulmonary failure.3
Currently, there is no specific antidote for azide poisoning, and treatment mainly consists of supportive care. Cyanide antidote treatments are generally ineffective in reducing azide-related death in animal models.3,8Early aggressive supportive care can improve survival rates.9 Some authors suggest that administration of oral activated charcoal, orogastric lavage, hemodialysis, and plasma exchange reduce azide concentrations, while others believe these treatments have little effect.3,9 More research is needed to identify effective therapeutic measures and to control for dose, time, and patient population.
What are the safety concerns for emergency medical technicians and hospital staff following exposure to sodium azide?
The most probable routes of exposure for prehospital and hospital staff include dermal contact with sodium azide or inhalation of gaseous hydrazoic acid; inhalational exposure is most concerning.1 In one case, hospital-staff members developed headaches, light-headedness, and nausea while treating a patient for azide poisoning; however, staff exposure was not confirmed and no sequelae were evident.10
More objectively, workers at an azide plant exposed to azide concentrations above the occupational exposure limit developed headaches, hypotension, and palpitations.11 Another study found no evidence of kidney, heart, or liver damage after patients were given sodium azide for more than a year during a clinical trial.12 Not unexpectedly, there is little risk of exposure when proper safety precautions are taken.
Emergency response personnel should carefully inspect the scene for the presence of any sodium azide powder, and should also question bystanders and family members to determine if anyone performed mouth-to-mouth resuscitation on the patient. Standard universal precautions, along with attentiveness to one’s surroundings, should be sufficient to prevent dermal exposure. If small amounts of sodium azide residue are found on the patient, his or her clothes should be cautiously removed and placed in a plastic bag to prevent dispersion of particles. If large quantities of sodium azide are present on a patient, the hazardous materials response team should be called, in accordance with institutional and regional protocols. To avoid explosion, every attempt should be made to prevent azide salt (eg, from emesis) from contact with any metal surfaces (eg, oxygen tanks, metal stretcher).13Vomit from patients who have ingested sodium azide can cause liberation of hydrazoic acid, which can escape through the esophagus. A pungent ambient odor may provide a warning, which is particularly concerning in a confined space such as an ambulance. As a precaution, EMS personnel should open windows and maximize ventilation. After the call, EMS and hospital personnel should thoroughly wash their hands with soap and water, and change their uniform if they believe it has been contaminated. There is no risk of delayed exposure following exposure to hydrazoic acid.
During autopsy, medical examiners must exercise caution due to the potential for liberation of hydrazoic acids from the stomach.14Unless it is absolutely necessary, the medical examiner should avoid opening the stomach. If this is unavoidable, the autopsy should occur in a well-ventilated setting with the examiner wearing a supplied air respirator to limit exposure in a high-risk scenario.
Case Conclusion
None of the exposed first responders experienced dizziness, light-headedness, or irritation, and after a period of observation in the ED, they were discharged home without further sequelae. All hospital staff involved in the patient’s care, including those who performed cardiopulmonary resuscitation on the patient and cleaned his room, were advised to use protective equipment when handling the patient and bodily secretions. None of the health care workers developed abnormal clinical findings. Given the hazard in conducting a full postmortem examination, the medical examiner opted to send blood, bile, urine, and vitreous humor out for analysis, but did not conduct a full postmortem examination. Notably, the stomach was not opened, and its contents were not exposed.
1. Compound summary for CID 33557 (sodium azide). National Center for Biotechnology Information. PubChem Compound Database. https://pubchem.ncbi.nlm.nih.gov/compound/sodium_azide. Accessed May 10, 2018.
2. Compound summary for CID 24530 (hydrogen azide). National Center for Biotechnology Information. PubChem Compound Database. https://pubchem.ncbi.nlm.nih.gov/compound/hydrazoic_acid. Accessed May 10, 2018.
3. Chang S, Lamm SH. Human health effects of sodium azide exposure: a literature review and analysis. Int J Toxicol. 2003;22(3):175-186. doi:10.1080/10915810305109.
4. Sodium azide explosion hazard. Washington State Department of Labor & Industries. Division of Occupational Safety and Health. https://www.lni.wa.gov/safety/hazardalerts/SodiumAzide.pdf. August 11, 2011. Accessed May 10, 2018.
5. Safety data sheet: sodium azide. ThermoFischer Scientific. https://www.fishersci.com/store/msds?partNumber=S227I1&productDescription=SODIUM+AZIDE+GRAN+PURIF+1+KG&vendorId=VN00033897&countryCode=US&language=en. Updated January 17, 2018. Accessed May 10, 2018.
6. Bogucka K, Wojtczak L. Effect of sodium azide on oxidation and phosphorylation processes in rat-liver mitochondria. Biochim Biophys Acta. 1966;122(3):381-392. doi:10.1016/0926-6593(66)90031-2.
7. Kruszyna H, Kruszyna R, Smith RP, Wilcox DE. Red blood cells generate nitric oxide from directly acting, nitrogenous vasodilators. Toxicol Appl Pharmacol. 1987;91(3):429-438. doi:10.1016/0041-008x(87)90064-0.
8. Smith RP, Louis CA, Kruszyna R, Kruszyna H. Acute neurotoxicity of sodium azide and nitric oxide. Fundam Appl Toxicol. 1991;17(1):120-127. doi:10.1093/toxsci/17.1.120.
9. Watanabe K, Hirasawa H, Oda S, et al. A case of survival following high-dose sodium azide poisoning. Clin Toxicol (Phila). 2007;45(7):810-811.
10. Abrams J, el-Mallakh RS, Meyer R. Suicidal sodium azide ingestion. Ann Emerg Med. 1987;16(12):1378-1380. doi:10.1016/s0196-0644(87)80423-7
11. Trout D, Esswein EJ, Hales T, Brown K, Solomon G, Miller M. Exposures and health effects: an evaluation of workers at a sodium azide production plant. Am J Ind Med. 1996;30(3):343-350.
12. Black, MM, Zweifach BW, Speer FD. Comparison of hypotensive action of sodium azide in normotensive and hypertensive patients. Exper Biol Med. 1954;85(1):11-16. doi:10.3181/00379727-85-20770.
13. Emergency preparedness and response. Facts about sodium azide. Centers for Disease Control and Prevention. Office of Public Health Preparedness and Response. https://emergency.cdc.gov/agent/sodiumazide/basics/facts.asp. Updated April 10, 2018. Accessed May 10, 2018.
14. Le Blanc-Louvry I, Laburthe-Tolra P, Massol V, et al. Suicidal sodium azide intoxication: An analytical challenge based on a rare case. Forensic Sci Int. 2012;221(1-3):e17-20. doi:10.1016/j.forsciint.2012.04.006.
Case
A 24-year-old man in cardiac arrest was brought to the ED via emergency medical services (EMS). Unfortunately, resuscitation efforts were unsuccessful. Little was known about the patient, but the emergency physician was informed that the patient had ingested sodium azide (NaN3), which he had ordered online. The patient collapsed shortly after ingesting the sodium azide, approximately the same time police officers arrived at the patient’s home.
No specific details were known about the patient’s ingestion. Upon learning of the exposure to sodium azide, a member of the ED staff contacted the local poison control center for information on the proper course of action to ensure staff safety and limit exposure. Shortly thereafter, several of emergency medical technicians and police officers, who had responded to the emergency assistance call for this patient, presented to the ED with concerns of exposure.
What is sodium azide?
Sodium azide is a colorless, odorless crystalline water-soluble solid that has a pK of 4.8.1 When sodium azide is dissolved in an acid, it liberates hydrazoic acid (HN3), which has a pungent odor, high vapor pressure (484 mm Hg), and a relatively low-boiling point of 37°C (98°F).2
The most common industrial use of sodium azide is as a propellant in air bags. In this capacity, sodium azide rapidly decomposes to nitrogen gas when it reaches a temperature of 300°C (572°F), causing rapid expansion of the air bag. In addition to air bags, sodium azide is used in research laboratories as a preservative and in agriculture as a pesticide. The main nontoxicological concern with all azide agents is the potential for explosion when they react with metals, such as lead, copper, silver, and mercury, to form metal azides that are sensitive to shock.3 An example of the explosive nature of these azides was demonstrated in a report wherein diluted sodium azide was poured down a drain, causing an explosion as a worker was fixing the pipe.4
In addition to industrial and commercial use, sodium azide is occasionally used in suicide attempts because it is rapidly fatal, has no specific antidote, and can be purchased online.3
What is the toxicity of sodium azide?
The lethal dose for both oral and dermal exposure to sodium azide is approximately 10 to 20 mg/kg.3,5 Therefore, ingestion of 700 mg of sodium azide, a volume approximately the size of a penny, is likely to be fatal.3
Sodium azide is primarily a mitochondrial toxin, which binds the electron transport chain, inhibiting oxidative phosphorylation. The resulting reduction in adenosine triphosphate (ATP) production, even in the presence of oxygen, results in metabolic failure.6 This mechanism of action is similar to that of cyanide, although sodium azide causes more pronounced vasodilation due to the in vivo conversion of some azide to the vasodilator nitric oxide.7 Some reports suggest that azide lethality is due to enhanced excitatory transmission from nitric oxide in the central nervous system.8
What are the clinical manifestations of azide poisoning, and what is the treatment?
The early clinical findings of a patient with azide poisoning include hypotension, dizziness, headache, nausea, vomiting, palpitations, tachycardia, dyspnea, and restlessness. Inhalation of hydrazoic acid can also produce wheezing and coughing. The most common effect is hypotension, which can occur within 1 minute of exposure. Following depletion of cellular ATP, anaerobic glycolysis generates lactate and produces acidemia. More severe findings of azide poisoning include seizures, cardiac arrhythmia, loss of consciousness, pulmonary edema, and cardiopulmonary failure.3
Currently, there is no specific antidote for azide poisoning, and treatment mainly consists of supportive care. Cyanide antidote treatments are generally ineffective in reducing azide-related death in animal models.3,8Early aggressive supportive care can improve survival rates.9 Some authors suggest that administration of oral activated charcoal, orogastric lavage, hemodialysis, and plasma exchange reduce azide concentrations, while others believe these treatments have little effect.3,9 More research is needed to identify effective therapeutic measures and to control for dose, time, and patient population.
What are the safety concerns for emergency medical technicians and hospital staff following exposure to sodium azide?
The most probable routes of exposure for prehospital and hospital staff include dermal contact with sodium azide or inhalation of gaseous hydrazoic acid; inhalational exposure is most concerning.1 In one case, hospital-staff members developed headaches, light-headedness, and nausea while treating a patient for azide poisoning; however, staff exposure was not confirmed and no sequelae were evident.10
More objectively, workers at an azide plant exposed to azide concentrations above the occupational exposure limit developed headaches, hypotension, and palpitations.11 Another study found no evidence of kidney, heart, or liver damage after patients were given sodium azide for more than a year during a clinical trial.12 Not unexpectedly, there is little risk of exposure when proper safety precautions are taken.
Emergency response personnel should carefully inspect the scene for the presence of any sodium azide powder, and should also question bystanders and family members to determine if anyone performed mouth-to-mouth resuscitation on the patient. Standard universal precautions, along with attentiveness to one’s surroundings, should be sufficient to prevent dermal exposure. If small amounts of sodium azide residue are found on the patient, his or her clothes should be cautiously removed and placed in a plastic bag to prevent dispersion of particles. If large quantities of sodium azide are present on a patient, the hazardous materials response team should be called, in accordance with institutional and regional protocols. To avoid explosion, every attempt should be made to prevent azide salt (eg, from emesis) from contact with any metal surfaces (eg, oxygen tanks, metal stretcher).13Vomit from patients who have ingested sodium azide can cause liberation of hydrazoic acid, which can escape through the esophagus. A pungent ambient odor may provide a warning, which is particularly concerning in a confined space such as an ambulance. As a precaution, EMS personnel should open windows and maximize ventilation. After the call, EMS and hospital personnel should thoroughly wash their hands with soap and water, and change their uniform if they believe it has been contaminated. There is no risk of delayed exposure following exposure to hydrazoic acid.
During autopsy, medical examiners must exercise caution due to the potential for liberation of hydrazoic acids from the stomach.14Unless it is absolutely necessary, the medical examiner should avoid opening the stomach. If this is unavoidable, the autopsy should occur in a well-ventilated setting with the examiner wearing a supplied air respirator to limit exposure in a high-risk scenario.
Case Conclusion
None of the exposed first responders experienced dizziness, light-headedness, or irritation, and after a period of observation in the ED, they were discharged home without further sequelae. All hospital staff involved in the patient’s care, including those who performed cardiopulmonary resuscitation on the patient and cleaned his room, were advised to use protective equipment when handling the patient and bodily secretions. None of the health care workers developed abnormal clinical findings. Given the hazard in conducting a full postmortem examination, the medical examiner opted to send blood, bile, urine, and vitreous humor out for analysis, but did not conduct a full postmortem examination. Notably, the stomach was not opened, and its contents were not exposed.
Case
A 24-year-old man in cardiac arrest was brought to the ED via emergency medical services (EMS). Unfortunately, resuscitation efforts were unsuccessful. Little was known about the patient, but the emergency physician was informed that the patient had ingested sodium azide (NaN3), which he had ordered online. The patient collapsed shortly after ingesting the sodium azide, approximately the same time police officers arrived at the patient’s home.
No specific details were known about the patient’s ingestion. Upon learning of the exposure to sodium azide, a member of the ED staff contacted the local poison control center for information on the proper course of action to ensure staff safety and limit exposure. Shortly thereafter, several of emergency medical technicians and police officers, who had responded to the emergency assistance call for this patient, presented to the ED with concerns of exposure.
What is sodium azide?
Sodium azide is a colorless, odorless crystalline water-soluble solid that has a pK of 4.8.1 When sodium azide is dissolved in an acid, it liberates hydrazoic acid (HN3), which has a pungent odor, high vapor pressure (484 mm Hg), and a relatively low-boiling point of 37°C (98°F).2
The most common industrial use of sodium azide is as a propellant in air bags. In this capacity, sodium azide rapidly decomposes to nitrogen gas when it reaches a temperature of 300°C (572°F), causing rapid expansion of the air bag. In addition to air bags, sodium azide is used in research laboratories as a preservative and in agriculture as a pesticide. The main nontoxicological concern with all azide agents is the potential for explosion when they react with metals, such as lead, copper, silver, and mercury, to form metal azides that are sensitive to shock.3 An example of the explosive nature of these azides was demonstrated in a report wherein diluted sodium azide was poured down a drain, causing an explosion as a worker was fixing the pipe.4
In addition to industrial and commercial use, sodium azide is occasionally used in suicide attempts because it is rapidly fatal, has no specific antidote, and can be purchased online.3
What is the toxicity of sodium azide?
The lethal dose for both oral and dermal exposure to sodium azide is approximately 10 to 20 mg/kg.3,5 Therefore, ingestion of 700 mg of sodium azide, a volume approximately the size of a penny, is likely to be fatal.3
Sodium azide is primarily a mitochondrial toxin, which binds the electron transport chain, inhibiting oxidative phosphorylation. The resulting reduction in adenosine triphosphate (ATP) production, even in the presence of oxygen, results in metabolic failure.6 This mechanism of action is similar to that of cyanide, although sodium azide causes more pronounced vasodilation due to the in vivo conversion of some azide to the vasodilator nitric oxide.7 Some reports suggest that azide lethality is due to enhanced excitatory transmission from nitric oxide in the central nervous system.8
What are the clinical manifestations of azide poisoning, and what is the treatment?
The early clinical findings of a patient with azide poisoning include hypotension, dizziness, headache, nausea, vomiting, palpitations, tachycardia, dyspnea, and restlessness. Inhalation of hydrazoic acid can also produce wheezing and coughing. The most common effect is hypotension, which can occur within 1 minute of exposure. Following depletion of cellular ATP, anaerobic glycolysis generates lactate and produces acidemia. More severe findings of azide poisoning include seizures, cardiac arrhythmia, loss of consciousness, pulmonary edema, and cardiopulmonary failure.3
Currently, there is no specific antidote for azide poisoning, and treatment mainly consists of supportive care. Cyanide antidote treatments are generally ineffective in reducing azide-related death in animal models.3,8Early aggressive supportive care can improve survival rates.9 Some authors suggest that administration of oral activated charcoal, orogastric lavage, hemodialysis, and plasma exchange reduce azide concentrations, while others believe these treatments have little effect.3,9 More research is needed to identify effective therapeutic measures and to control for dose, time, and patient population.
What are the safety concerns for emergency medical technicians and hospital staff following exposure to sodium azide?
The most probable routes of exposure for prehospital and hospital staff include dermal contact with sodium azide or inhalation of gaseous hydrazoic acid; inhalational exposure is most concerning.1 In one case, hospital-staff members developed headaches, light-headedness, and nausea while treating a patient for azide poisoning; however, staff exposure was not confirmed and no sequelae were evident.10
More objectively, workers at an azide plant exposed to azide concentrations above the occupational exposure limit developed headaches, hypotension, and palpitations.11 Another study found no evidence of kidney, heart, or liver damage after patients were given sodium azide for more than a year during a clinical trial.12 Not unexpectedly, there is little risk of exposure when proper safety precautions are taken.
Emergency response personnel should carefully inspect the scene for the presence of any sodium azide powder, and should also question bystanders and family members to determine if anyone performed mouth-to-mouth resuscitation on the patient. Standard universal precautions, along with attentiveness to one’s surroundings, should be sufficient to prevent dermal exposure. If small amounts of sodium azide residue are found on the patient, his or her clothes should be cautiously removed and placed in a plastic bag to prevent dispersion of particles. If large quantities of sodium azide are present on a patient, the hazardous materials response team should be called, in accordance with institutional and regional protocols. To avoid explosion, every attempt should be made to prevent azide salt (eg, from emesis) from contact with any metal surfaces (eg, oxygen tanks, metal stretcher).13Vomit from patients who have ingested sodium azide can cause liberation of hydrazoic acid, which can escape through the esophagus. A pungent ambient odor may provide a warning, which is particularly concerning in a confined space such as an ambulance. As a precaution, EMS personnel should open windows and maximize ventilation. After the call, EMS and hospital personnel should thoroughly wash their hands with soap and water, and change their uniform if they believe it has been contaminated. There is no risk of delayed exposure following exposure to hydrazoic acid.
During autopsy, medical examiners must exercise caution due to the potential for liberation of hydrazoic acids from the stomach.14Unless it is absolutely necessary, the medical examiner should avoid opening the stomach. If this is unavoidable, the autopsy should occur in a well-ventilated setting with the examiner wearing a supplied air respirator to limit exposure in a high-risk scenario.
Case Conclusion
None of the exposed first responders experienced dizziness, light-headedness, or irritation, and after a period of observation in the ED, they were discharged home without further sequelae. All hospital staff involved in the patient’s care, including those who performed cardiopulmonary resuscitation on the patient and cleaned his room, were advised to use protective equipment when handling the patient and bodily secretions. None of the health care workers developed abnormal clinical findings. Given the hazard in conducting a full postmortem examination, the medical examiner opted to send blood, bile, urine, and vitreous humor out for analysis, but did not conduct a full postmortem examination. Notably, the stomach was not opened, and its contents were not exposed.
1. Compound summary for CID 33557 (sodium azide). National Center for Biotechnology Information. PubChem Compound Database. https://pubchem.ncbi.nlm.nih.gov/compound/sodium_azide. Accessed May 10, 2018.
2. Compound summary for CID 24530 (hydrogen azide). National Center for Biotechnology Information. PubChem Compound Database. https://pubchem.ncbi.nlm.nih.gov/compound/hydrazoic_acid. Accessed May 10, 2018.
3. Chang S, Lamm SH. Human health effects of sodium azide exposure: a literature review and analysis. Int J Toxicol. 2003;22(3):175-186. doi:10.1080/10915810305109.
4. Sodium azide explosion hazard. Washington State Department of Labor & Industries. Division of Occupational Safety and Health. https://www.lni.wa.gov/safety/hazardalerts/SodiumAzide.pdf. August 11, 2011. Accessed May 10, 2018.
5. Safety data sheet: sodium azide. ThermoFischer Scientific. https://www.fishersci.com/store/msds?partNumber=S227I1&productDescription=SODIUM+AZIDE+GRAN+PURIF+1+KG&vendorId=VN00033897&countryCode=US&language=en. Updated January 17, 2018. Accessed May 10, 2018.
6. Bogucka K, Wojtczak L. Effect of sodium azide on oxidation and phosphorylation processes in rat-liver mitochondria. Biochim Biophys Acta. 1966;122(3):381-392. doi:10.1016/0926-6593(66)90031-2.
7. Kruszyna H, Kruszyna R, Smith RP, Wilcox DE. Red blood cells generate nitric oxide from directly acting, nitrogenous vasodilators. Toxicol Appl Pharmacol. 1987;91(3):429-438. doi:10.1016/0041-008x(87)90064-0.
8. Smith RP, Louis CA, Kruszyna R, Kruszyna H. Acute neurotoxicity of sodium azide and nitric oxide. Fundam Appl Toxicol. 1991;17(1):120-127. doi:10.1093/toxsci/17.1.120.
9. Watanabe K, Hirasawa H, Oda S, et al. A case of survival following high-dose sodium azide poisoning. Clin Toxicol (Phila). 2007;45(7):810-811.
10. Abrams J, el-Mallakh RS, Meyer R. Suicidal sodium azide ingestion. Ann Emerg Med. 1987;16(12):1378-1380. doi:10.1016/s0196-0644(87)80423-7
11. Trout D, Esswein EJ, Hales T, Brown K, Solomon G, Miller M. Exposures and health effects: an evaluation of workers at a sodium azide production plant. Am J Ind Med. 1996;30(3):343-350.
12. Black, MM, Zweifach BW, Speer FD. Comparison of hypotensive action of sodium azide in normotensive and hypertensive patients. Exper Biol Med. 1954;85(1):11-16. doi:10.3181/00379727-85-20770.
13. Emergency preparedness and response. Facts about sodium azide. Centers for Disease Control and Prevention. Office of Public Health Preparedness and Response. https://emergency.cdc.gov/agent/sodiumazide/basics/facts.asp. Updated April 10, 2018. Accessed May 10, 2018.
14. Le Blanc-Louvry I, Laburthe-Tolra P, Massol V, et al. Suicidal sodium azide intoxication: An analytical challenge based on a rare case. Forensic Sci Int. 2012;221(1-3):e17-20. doi:10.1016/j.forsciint.2012.04.006.
1. Compound summary for CID 33557 (sodium azide). National Center for Biotechnology Information. PubChem Compound Database. https://pubchem.ncbi.nlm.nih.gov/compound/sodium_azide. Accessed May 10, 2018.
2. Compound summary for CID 24530 (hydrogen azide). National Center for Biotechnology Information. PubChem Compound Database. https://pubchem.ncbi.nlm.nih.gov/compound/hydrazoic_acid. Accessed May 10, 2018.
3. Chang S, Lamm SH. Human health effects of sodium azide exposure: a literature review and analysis. Int J Toxicol. 2003;22(3):175-186. doi:10.1080/10915810305109.
4. Sodium azide explosion hazard. Washington State Department of Labor & Industries. Division of Occupational Safety and Health. https://www.lni.wa.gov/safety/hazardalerts/SodiumAzide.pdf. August 11, 2011. Accessed May 10, 2018.
5. Safety data sheet: sodium azide. ThermoFischer Scientific. https://www.fishersci.com/store/msds?partNumber=S227I1&productDescription=SODIUM+AZIDE+GRAN+PURIF+1+KG&vendorId=VN00033897&countryCode=US&language=en. Updated January 17, 2018. Accessed May 10, 2018.
6. Bogucka K, Wojtczak L. Effect of sodium azide on oxidation and phosphorylation processes in rat-liver mitochondria. Biochim Biophys Acta. 1966;122(3):381-392. doi:10.1016/0926-6593(66)90031-2.
7. Kruszyna H, Kruszyna R, Smith RP, Wilcox DE. Red blood cells generate nitric oxide from directly acting, nitrogenous vasodilators. Toxicol Appl Pharmacol. 1987;91(3):429-438. doi:10.1016/0041-008x(87)90064-0.
8. Smith RP, Louis CA, Kruszyna R, Kruszyna H. Acute neurotoxicity of sodium azide and nitric oxide. Fundam Appl Toxicol. 1991;17(1):120-127. doi:10.1093/toxsci/17.1.120.
9. Watanabe K, Hirasawa H, Oda S, et al. A case of survival following high-dose sodium azide poisoning. Clin Toxicol (Phila). 2007;45(7):810-811.
10. Abrams J, el-Mallakh RS, Meyer R. Suicidal sodium azide ingestion. Ann Emerg Med. 1987;16(12):1378-1380. doi:10.1016/s0196-0644(87)80423-7
11. Trout D, Esswein EJ, Hales T, Brown K, Solomon G, Miller M. Exposures and health effects: an evaluation of workers at a sodium azide production plant. Am J Ind Med. 1996;30(3):343-350.
12. Black, MM, Zweifach BW, Speer FD. Comparison of hypotensive action of sodium azide in normotensive and hypertensive patients. Exper Biol Med. 1954;85(1):11-16. doi:10.3181/00379727-85-20770.
13. Emergency preparedness and response. Facts about sodium azide. Centers for Disease Control and Prevention. Office of Public Health Preparedness and Response. https://emergency.cdc.gov/agent/sodiumazide/basics/facts.asp. Updated April 10, 2018. Accessed May 10, 2018.
14. Le Blanc-Louvry I, Laburthe-Tolra P, Massol V, et al. Suicidal sodium azide intoxication: An analytical challenge based on a rare case. Forensic Sci Int. 2012;221(1-3):e17-20. doi:10.1016/j.forsciint.2012.04.006.
Case Studies in Toxicology: Start Low and Go Slow
Case
A woman in her third decade with no known medical history was dropped off at the waiting area of the ED for evaluation of depressed mental status. Upon arrival, the patient was unresponsive and cyanotic, with a pulse oximetry of 65% on room air. Bag-valve mask (BVM) ventilation rapidly improved oxygen saturation to 90%. The patient’s other vital signs were: heart rate, 141 beats/min; blood pressure (BP), 117/65 mm Hg; and temperature, afebrile.
Upon examination, the patient’s pupils were pinpoint and her ventilatory effort was shallow, leading the emergency physician (EP) to suspect the patient’s depressed mental status was due to an opioid overdose.
The patient was given 2 mg of intravenous (IV) naloxone, after which she became more alert and responsive, with improved respiratory effort. After receiving naloxone, the patient vomited copiously. Pulmonary examination revealed diffuse rales, most prominently at the right lung base, and a cough productive of thick sputum.
During the patient’s course in the ED, she became increasingly hypotensive with systolic BP readings around 70 mm Hg; tachycardia, fluctuating at around 120 beats/min; and persistent hypoxia of 90% saturation on a nonrebreather mask. A chest X-ray demonstrated pulmonary edema with a continuous diaphragm sign suggesting pneumomediastinum. A computed tomography (CT) scan of the chest confirmed pulmonary edema with extensive pneumomediastinum, and the patient was admitted to the intensive care unit (ICU).
What is naloxone and why is it used?
Naloxone is a nonselective, short-acting, pure opioid antagonist that works at the mu, kappa, and sigma receptors, with the highest affinity for the mu receptor. It is a competitive opioid receptor antagonist that has an elimination half-life of approximately 30 minutes. Though naloxone was originally developed to reverse the effects of anesthesia postoperatively,1 today it is more commonly used to treat ventilatory depression in patients whose clinical findings are most likely due to an opioid overdose.
Opioid-dependent individuals who abstain from use for more than a few hours generally develop opioid withdrawal syndrome (OWS). The effects of OWS include mild-to-moderate tachycardia and hypertension, nausea, vomiting, piloerection, rhinorrhea, and agitated behavior. However, when opioid-dependent patients receive naloxone, OWS develops at a much faster rate (ie, seconds after naloxone administration) and is often more severe.
Findings of naloxone-precipitated OWS include pronounced vital sign abnormalities, seizures,pulmonary edema, and cardiac arrhythmias such as ventricular tachycardia.2 These latter findings are primarily due to the sudden release of catecholamines.3 In addition, patients suffer the psychological pangs of withdrawal, including dysphoria and drug craving, which often leads to poor decision-making as they search for additional opioids to alleviate these troubling effects.
What determines response to naloxone and development of OWS?
The severity of precipitated OWS following naloxone administration is determined by both the degree of the patient’s opioid dependency and the dosage and rate at which naloxone is given. The depth of opioid dependence is determined to a large extent by the quantity of opioid regularly used and the frequency of exposure. For example, a patient who takes 30 mg of oxycodone daily will likely demonstrate mild OWS, while one who uses 300 mg daily will demonstrate more severe OWS—whether due to abstinence or naloxone.
In addition, longer exposure time of the patient’s brain to opioids increases the dependency level. Continuous use of extended-release opioids or methadone, which are both of long duration, essentially “bathe” the brain receptors in opioid around the clock, whereas short-acting opioids, such as fentanyl or heroin, cause peaks and troughs in brain concentrations throughout the day. These trough periods reduce dependency, but increase the abuse liability of the opioid. Patients who only use opioids on the weekend, for example, will have minimal or no OWS following naloxone administration, nor will the toddler with an exploratory ingestion of an opioid medication found in the home. It is therefore important to gauge the extent of a patient’s opioid use to improve the safe use of naloxone in the ED.
What is the optimal dosing of naloxone and proper patient management?
It is essential for clinicians to remember that the ultimate goal of naloxone administration in the ED is to reverse ventilatory depression—not to restore a patient to a normal mental status.4 In fact, full awakening, in addition to precipitating OWS, may lead to difficult interpersonal situations in the ED, since such patients often insist on leaving the ED before the effects of naloxone wear off. This situation places the EP in the undesirable position of discharging a patient who may predictably relapse—though unlikely to die—after release.5
Management in the Hospital Setting. Given the advanced medical care environment in a hospital, the approach to opioid overdose patients can be metered. This means providing temporary noninvasive mechanical ventilatory support through BVM or laryngeal mask airways, which allow both oxygenation and ventilation (reducing the patient’s partial pressure of carbon dioxide), prior to giving naloxone.6 Studies on animal models have shown that lowering the partial pressure of carbon dioxide reduces the catecholamine response to naloxone.7
Although recent literature and textbook recommendations regarding naloxone dosages vary,1 the safest initial dose of naloxone in the hospital setting is 0.04 mg (40 mcg) IV, or 0.08 mg (80 mcg) intramuscularly (IM).8 Whether given by IV or IM route, frequent reassessment of the adequacy of spontaneous ventilatory effort and oxygenation are required.
While the rate of opioid reversal is slower when giving lower doses of naloxone, this approach reduces the severity of precipitated OWS. In fact, in most patients who receive low-dose naloxone administration will not awaken but will develop life-sustaining spontaneous ventilation.8
By monitoring of the patient’s ventilatory rate and depth, along with capnometry and pulse oximetry (without providing exogenous oxygen), the EP can identify the need for additional naloxone. Since the half-life of naloxone is shorter than that of many opioids, proper ventilatory monitoring is essential to assess for the waning of naloxone’s effects and return of respiratory depression.
Treatment in the Nonhospital Setting. Emergency medical service (EMS) workers typically, and often by situational necessity, approach opioid overdose patients more aggressively than do EPs in the ED. Although some EMS systems utilize the IV route, most EMS workers, like laypersons, administer an initial naloxone dose of 0.4 mg IM or 2 or 4 mg intranasally (IN). Due to the slower rate of absorption and lower bioavailability (with IN administration), both IM and IN naloxone equate to roughly 0.08 mg IV.
For patients in whom there is no risk for opioid dependence, the initial dose of naloxone is relatively inconsequential, and higher doses can be safely administered. However, for most patients, including those in the ED setting, in whom one cannot be certain of their depth of dependence, the safest approach is to “start low and go slow” with naloxone administration, while providing supportive care.
Case Conclusion
The patient was not opioid-naïve, explaining the catecholamine surge and related cardiovascular dysfunction and pulmonary edema. The pneumomediastinum and pulmonary aspiration were due to the violent retching and vomiting. After being admitted to the ICU, the patient was started on vancomycin and piperacillin/tazobactam for empiric coverage for mediastinal emphysema. She was kept NPO, assessed by cardiothoracic surgery, and treated with gentle fluid hydration.
A repeat CT showed a stable pneumomediastinum. Her hypoxia, tachycardia, and hypotension gradually improved over about 6 hours. The following day, the patient’s mental status normalized, and she discharged herself from the hospital against medical advice.
1. Connors NJ, Nelson LS. The evolution of recommended naloxone dosing for opioid overdose by medical specialty. J Med Toxicol. 2016;12(3):276-281. doi:10.1007/s13181-016-0559-3.
2. Lameijer, H, Azizi N, Ligtenberg JJ, Ter Maaten JC. Ventricular tachycardia after naloxone administration: a drug related complication? Case report and literature review. Drug Saf Case Rep. 2014;1(1):2. doi:10.1007/s40800-014-0002-0.
3. Kienbaum P, Thürauf N, Michel MC, Scherbaum N, Gastpar M, Peters J. Profound increase in epinephrine concentration in plasma and cardiovascular stimulation after mu-opioid receptor blockade in opioid-addicted patients during barbiturate-induced anesthesia for acute detoxification. Anesthesiology. 1998;88(5):1154-1161.
4. Kim HK, Nelson LS. Reducing the harm of opioid overdose with the safe use of naloxone: a pharmacologic review. Expert Opin Drug Saf. 2015;14 (7 ):1137-1146. doi:10.1517/14740338.2015.1037274.
5. Willman MW, Liss DB, Schwarz ES, Mullins ME. Do heroin overdose patients require observation after receiving naloxone? Clin Toxicol (Phila). 2017;55(2):81-87. doi:10.1080/15563650.2016.1253846.
6. Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367(2):146-155. doi:10.1056/NEJMra1202561.
7. Mills CA, Flacke JW, Miller JD, Davis LJ, Bloor BC, Flacke WE. Cardiovascular effects of fentanyl reversal by naloxone at varying arterial carbon dioxide tensions in dogs. Anesth Analg. 1988;67(8):730-736.
8. Kim HK, Nelson LS. Reversal of opioid-induced ventilatory depression using low-dose naloxone (0.04 mg): a case series. J Med Toxicol. 2015;12(1):107-110. doi:10.1007/s13181-015-0499-3.
Case
A woman in her third decade with no known medical history was dropped off at the waiting area of the ED for evaluation of depressed mental status. Upon arrival, the patient was unresponsive and cyanotic, with a pulse oximetry of 65% on room air. Bag-valve mask (BVM) ventilation rapidly improved oxygen saturation to 90%. The patient’s other vital signs were: heart rate, 141 beats/min; blood pressure (BP), 117/65 mm Hg; and temperature, afebrile.
Upon examination, the patient’s pupils were pinpoint and her ventilatory effort was shallow, leading the emergency physician (EP) to suspect the patient’s depressed mental status was due to an opioid overdose.
The patient was given 2 mg of intravenous (IV) naloxone, after which she became more alert and responsive, with improved respiratory effort. After receiving naloxone, the patient vomited copiously. Pulmonary examination revealed diffuse rales, most prominently at the right lung base, and a cough productive of thick sputum.
During the patient’s course in the ED, she became increasingly hypotensive with systolic BP readings around 70 mm Hg; tachycardia, fluctuating at around 120 beats/min; and persistent hypoxia of 90% saturation on a nonrebreather mask. A chest X-ray demonstrated pulmonary edema with a continuous diaphragm sign suggesting pneumomediastinum. A computed tomography (CT) scan of the chest confirmed pulmonary edema with extensive pneumomediastinum, and the patient was admitted to the intensive care unit (ICU).
What is naloxone and why is it used?
Naloxone is a nonselective, short-acting, pure opioid antagonist that works at the mu, kappa, and sigma receptors, with the highest affinity for the mu receptor. It is a competitive opioid receptor antagonist that has an elimination half-life of approximately 30 minutes. Though naloxone was originally developed to reverse the effects of anesthesia postoperatively,1 today it is more commonly used to treat ventilatory depression in patients whose clinical findings are most likely due to an opioid overdose.
Opioid-dependent individuals who abstain from use for more than a few hours generally develop opioid withdrawal syndrome (OWS). The effects of OWS include mild-to-moderate tachycardia and hypertension, nausea, vomiting, piloerection, rhinorrhea, and agitated behavior. However, when opioid-dependent patients receive naloxone, OWS develops at a much faster rate (ie, seconds after naloxone administration) and is often more severe.
Findings of naloxone-precipitated OWS include pronounced vital sign abnormalities, seizures,pulmonary edema, and cardiac arrhythmias such as ventricular tachycardia.2 These latter findings are primarily due to the sudden release of catecholamines.3 In addition, patients suffer the psychological pangs of withdrawal, including dysphoria and drug craving, which often leads to poor decision-making as they search for additional opioids to alleviate these troubling effects.
What determines response to naloxone and development of OWS?
The severity of precipitated OWS following naloxone administration is determined by both the degree of the patient’s opioid dependency and the dosage and rate at which naloxone is given. The depth of opioid dependence is determined to a large extent by the quantity of opioid regularly used and the frequency of exposure. For example, a patient who takes 30 mg of oxycodone daily will likely demonstrate mild OWS, while one who uses 300 mg daily will demonstrate more severe OWS—whether due to abstinence or naloxone.
In addition, longer exposure time of the patient’s brain to opioids increases the dependency level. Continuous use of extended-release opioids or methadone, which are both of long duration, essentially “bathe” the brain receptors in opioid around the clock, whereas short-acting opioids, such as fentanyl or heroin, cause peaks and troughs in brain concentrations throughout the day. These trough periods reduce dependency, but increase the abuse liability of the opioid. Patients who only use opioids on the weekend, for example, will have minimal or no OWS following naloxone administration, nor will the toddler with an exploratory ingestion of an opioid medication found in the home. It is therefore important to gauge the extent of a patient’s opioid use to improve the safe use of naloxone in the ED.
What is the optimal dosing of naloxone and proper patient management?
It is essential for clinicians to remember that the ultimate goal of naloxone administration in the ED is to reverse ventilatory depression—not to restore a patient to a normal mental status.4 In fact, full awakening, in addition to precipitating OWS, may lead to difficult interpersonal situations in the ED, since such patients often insist on leaving the ED before the effects of naloxone wear off. This situation places the EP in the undesirable position of discharging a patient who may predictably relapse—though unlikely to die—after release.5
Management in the Hospital Setting. Given the advanced medical care environment in a hospital, the approach to opioid overdose patients can be metered. This means providing temporary noninvasive mechanical ventilatory support through BVM or laryngeal mask airways, which allow both oxygenation and ventilation (reducing the patient’s partial pressure of carbon dioxide), prior to giving naloxone.6 Studies on animal models have shown that lowering the partial pressure of carbon dioxide reduces the catecholamine response to naloxone.7
Although recent literature and textbook recommendations regarding naloxone dosages vary,1 the safest initial dose of naloxone in the hospital setting is 0.04 mg (40 mcg) IV, or 0.08 mg (80 mcg) intramuscularly (IM).8 Whether given by IV or IM route, frequent reassessment of the adequacy of spontaneous ventilatory effort and oxygenation are required.
While the rate of opioid reversal is slower when giving lower doses of naloxone, this approach reduces the severity of precipitated OWS. In fact, in most patients who receive low-dose naloxone administration will not awaken but will develop life-sustaining spontaneous ventilation.8
By monitoring of the patient’s ventilatory rate and depth, along with capnometry and pulse oximetry (without providing exogenous oxygen), the EP can identify the need for additional naloxone. Since the half-life of naloxone is shorter than that of many opioids, proper ventilatory monitoring is essential to assess for the waning of naloxone’s effects and return of respiratory depression.
Treatment in the Nonhospital Setting. Emergency medical service (EMS) workers typically, and often by situational necessity, approach opioid overdose patients more aggressively than do EPs in the ED. Although some EMS systems utilize the IV route, most EMS workers, like laypersons, administer an initial naloxone dose of 0.4 mg IM or 2 or 4 mg intranasally (IN). Due to the slower rate of absorption and lower bioavailability (with IN administration), both IM and IN naloxone equate to roughly 0.08 mg IV.
For patients in whom there is no risk for opioid dependence, the initial dose of naloxone is relatively inconsequential, and higher doses can be safely administered. However, for most patients, including those in the ED setting, in whom one cannot be certain of their depth of dependence, the safest approach is to “start low and go slow” with naloxone administration, while providing supportive care.
Case Conclusion
The patient was not opioid-naïve, explaining the catecholamine surge and related cardiovascular dysfunction and pulmonary edema. The pneumomediastinum and pulmonary aspiration were due to the violent retching and vomiting. After being admitted to the ICU, the patient was started on vancomycin and piperacillin/tazobactam for empiric coverage for mediastinal emphysema. She was kept NPO, assessed by cardiothoracic surgery, and treated with gentle fluid hydration.
A repeat CT showed a stable pneumomediastinum. Her hypoxia, tachycardia, and hypotension gradually improved over about 6 hours. The following day, the patient’s mental status normalized, and she discharged herself from the hospital against medical advice.
Case
A woman in her third decade with no known medical history was dropped off at the waiting area of the ED for evaluation of depressed mental status. Upon arrival, the patient was unresponsive and cyanotic, with a pulse oximetry of 65% on room air. Bag-valve mask (BVM) ventilation rapidly improved oxygen saturation to 90%. The patient’s other vital signs were: heart rate, 141 beats/min; blood pressure (BP), 117/65 mm Hg; and temperature, afebrile.
Upon examination, the patient’s pupils were pinpoint and her ventilatory effort was shallow, leading the emergency physician (EP) to suspect the patient’s depressed mental status was due to an opioid overdose.
The patient was given 2 mg of intravenous (IV) naloxone, after which she became more alert and responsive, with improved respiratory effort. After receiving naloxone, the patient vomited copiously. Pulmonary examination revealed diffuse rales, most prominently at the right lung base, and a cough productive of thick sputum.
During the patient’s course in the ED, she became increasingly hypotensive with systolic BP readings around 70 mm Hg; tachycardia, fluctuating at around 120 beats/min; and persistent hypoxia of 90% saturation on a nonrebreather mask. A chest X-ray demonstrated pulmonary edema with a continuous diaphragm sign suggesting pneumomediastinum. A computed tomography (CT) scan of the chest confirmed pulmonary edema with extensive pneumomediastinum, and the patient was admitted to the intensive care unit (ICU).
What is naloxone and why is it used?
Naloxone is a nonselective, short-acting, pure opioid antagonist that works at the mu, kappa, and sigma receptors, with the highest affinity for the mu receptor. It is a competitive opioid receptor antagonist that has an elimination half-life of approximately 30 minutes. Though naloxone was originally developed to reverse the effects of anesthesia postoperatively,1 today it is more commonly used to treat ventilatory depression in patients whose clinical findings are most likely due to an opioid overdose.
Opioid-dependent individuals who abstain from use for more than a few hours generally develop opioid withdrawal syndrome (OWS). The effects of OWS include mild-to-moderate tachycardia and hypertension, nausea, vomiting, piloerection, rhinorrhea, and agitated behavior. However, when opioid-dependent patients receive naloxone, OWS develops at a much faster rate (ie, seconds after naloxone administration) and is often more severe.
Findings of naloxone-precipitated OWS include pronounced vital sign abnormalities, seizures,pulmonary edema, and cardiac arrhythmias such as ventricular tachycardia.2 These latter findings are primarily due to the sudden release of catecholamines.3 In addition, patients suffer the psychological pangs of withdrawal, including dysphoria and drug craving, which often leads to poor decision-making as they search for additional opioids to alleviate these troubling effects.
What determines response to naloxone and development of OWS?
The severity of precipitated OWS following naloxone administration is determined by both the degree of the patient’s opioid dependency and the dosage and rate at which naloxone is given. The depth of opioid dependence is determined to a large extent by the quantity of opioid regularly used and the frequency of exposure. For example, a patient who takes 30 mg of oxycodone daily will likely demonstrate mild OWS, while one who uses 300 mg daily will demonstrate more severe OWS—whether due to abstinence or naloxone.
In addition, longer exposure time of the patient’s brain to opioids increases the dependency level. Continuous use of extended-release opioids or methadone, which are both of long duration, essentially “bathe” the brain receptors in opioid around the clock, whereas short-acting opioids, such as fentanyl or heroin, cause peaks and troughs in brain concentrations throughout the day. These trough periods reduce dependency, but increase the abuse liability of the opioid. Patients who only use opioids on the weekend, for example, will have minimal or no OWS following naloxone administration, nor will the toddler with an exploratory ingestion of an opioid medication found in the home. It is therefore important to gauge the extent of a patient’s opioid use to improve the safe use of naloxone in the ED.
What is the optimal dosing of naloxone and proper patient management?
It is essential for clinicians to remember that the ultimate goal of naloxone administration in the ED is to reverse ventilatory depression—not to restore a patient to a normal mental status.4 In fact, full awakening, in addition to precipitating OWS, may lead to difficult interpersonal situations in the ED, since such patients often insist on leaving the ED before the effects of naloxone wear off. This situation places the EP in the undesirable position of discharging a patient who may predictably relapse—though unlikely to die—after release.5
Management in the Hospital Setting. Given the advanced medical care environment in a hospital, the approach to opioid overdose patients can be metered. This means providing temporary noninvasive mechanical ventilatory support through BVM or laryngeal mask airways, which allow both oxygenation and ventilation (reducing the patient’s partial pressure of carbon dioxide), prior to giving naloxone.6 Studies on animal models have shown that lowering the partial pressure of carbon dioxide reduces the catecholamine response to naloxone.7
Although recent literature and textbook recommendations regarding naloxone dosages vary,1 the safest initial dose of naloxone in the hospital setting is 0.04 mg (40 mcg) IV, or 0.08 mg (80 mcg) intramuscularly (IM).8 Whether given by IV or IM route, frequent reassessment of the adequacy of spontaneous ventilatory effort and oxygenation are required.
While the rate of opioid reversal is slower when giving lower doses of naloxone, this approach reduces the severity of precipitated OWS. In fact, in most patients who receive low-dose naloxone administration will not awaken but will develop life-sustaining spontaneous ventilation.8
By monitoring of the patient’s ventilatory rate and depth, along with capnometry and pulse oximetry (without providing exogenous oxygen), the EP can identify the need for additional naloxone. Since the half-life of naloxone is shorter than that of many opioids, proper ventilatory monitoring is essential to assess for the waning of naloxone’s effects and return of respiratory depression.
Treatment in the Nonhospital Setting. Emergency medical service (EMS) workers typically, and often by situational necessity, approach opioid overdose patients more aggressively than do EPs in the ED. Although some EMS systems utilize the IV route, most EMS workers, like laypersons, administer an initial naloxone dose of 0.4 mg IM or 2 or 4 mg intranasally (IN). Due to the slower rate of absorption and lower bioavailability (with IN administration), both IM and IN naloxone equate to roughly 0.08 mg IV.
For patients in whom there is no risk for opioid dependence, the initial dose of naloxone is relatively inconsequential, and higher doses can be safely administered. However, for most patients, including those in the ED setting, in whom one cannot be certain of their depth of dependence, the safest approach is to “start low and go slow” with naloxone administration, while providing supportive care.
Case Conclusion
The patient was not opioid-naïve, explaining the catecholamine surge and related cardiovascular dysfunction and pulmonary edema. The pneumomediastinum and pulmonary aspiration were due to the violent retching and vomiting. After being admitted to the ICU, the patient was started on vancomycin and piperacillin/tazobactam for empiric coverage for mediastinal emphysema. She was kept NPO, assessed by cardiothoracic surgery, and treated with gentle fluid hydration.
A repeat CT showed a stable pneumomediastinum. Her hypoxia, tachycardia, and hypotension gradually improved over about 6 hours. The following day, the patient’s mental status normalized, and she discharged herself from the hospital against medical advice.
1. Connors NJ, Nelson LS. The evolution of recommended naloxone dosing for opioid overdose by medical specialty. J Med Toxicol. 2016;12(3):276-281. doi:10.1007/s13181-016-0559-3.
2. Lameijer, H, Azizi N, Ligtenberg JJ, Ter Maaten JC. Ventricular tachycardia after naloxone administration: a drug related complication? Case report and literature review. Drug Saf Case Rep. 2014;1(1):2. doi:10.1007/s40800-014-0002-0.
3. Kienbaum P, Thürauf N, Michel MC, Scherbaum N, Gastpar M, Peters J. Profound increase in epinephrine concentration in plasma and cardiovascular stimulation after mu-opioid receptor blockade in opioid-addicted patients during barbiturate-induced anesthesia for acute detoxification. Anesthesiology. 1998;88(5):1154-1161.
4. Kim HK, Nelson LS. Reducing the harm of opioid overdose with the safe use of naloxone: a pharmacologic review. Expert Opin Drug Saf. 2015;14 (7 ):1137-1146. doi:10.1517/14740338.2015.1037274.
5. Willman MW, Liss DB, Schwarz ES, Mullins ME. Do heroin overdose patients require observation after receiving naloxone? Clin Toxicol (Phila). 2017;55(2):81-87. doi:10.1080/15563650.2016.1253846.
6. Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367(2):146-155. doi:10.1056/NEJMra1202561.
7. Mills CA, Flacke JW, Miller JD, Davis LJ, Bloor BC, Flacke WE. Cardiovascular effects of fentanyl reversal by naloxone at varying arterial carbon dioxide tensions in dogs. Anesth Analg. 1988;67(8):730-736.
8. Kim HK, Nelson LS. Reversal of opioid-induced ventilatory depression using low-dose naloxone (0.04 mg): a case series. J Med Toxicol. 2015;12(1):107-110. doi:10.1007/s13181-015-0499-3.
1. Connors NJ, Nelson LS. The evolution of recommended naloxone dosing for opioid overdose by medical specialty. J Med Toxicol. 2016;12(3):276-281. doi:10.1007/s13181-016-0559-3.
2. Lameijer, H, Azizi N, Ligtenberg JJ, Ter Maaten JC. Ventricular tachycardia after naloxone administration: a drug related complication? Case report and literature review. Drug Saf Case Rep. 2014;1(1):2. doi:10.1007/s40800-014-0002-0.
3. Kienbaum P, Thürauf N, Michel MC, Scherbaum N, Gastpar M, Peters J. Profound increase in epinephrine concentration in plasma and cardiovascular stimulation after mu-opioid receptor blockade in opioid-addicted patients during barbiturate-induced anesthesia for acute detoxification. Anesthesiology. 1998;88(5):1154-1161.
4. Kim HK, Nelson LS. Reducing the harm of opioid overdose with the safe use of naloxone: a pharmacologic review. Expert Opin Drug Saf. 2015;14 (7 ):1137-1146. doi:10.1517/14740338.2015.1037274.
5. Willman MW, Liss DB, Schwarz ES, Mullins ME. Do heroin overdose patients require observation after receiving naloxone? Clin Toxicol (Phila). 2017;55(2):81-87. doi:10.1080/15563650.2016.1253846.
6. Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367(2):146-155. doi:10.1056/NEJMra1202561.
7. Mills CA, Flacke JW, Miller JD, Davis LJ, Bloor BC, Flacke WE. Cardiovascular effects of fentanyl reversal by naloxone at varying arterial carbon dioxide tensions in dogs. Anesth Analg. 1988;67(8):730-736.
8. Kim HK, Nelson LS. Reversal of opioid-induced ventilatory depression using low-dose naloxone (0.04 mg): a case series. J Med Toxicol. 2015;12(1):107-110. doi:10.1007/s13181-015-0499-3.
Case Studies in Toxicology: DILI Dally
Case
A 50-year-old Hispanic woman with a history of rheumatoid arthritis (RA), for which she was not currently taking medication, was referred to the ED by her primary care physician (PCP) for evaluation of generalized pruritus and jaundice, and an abnormal hepatic function panel.
The patient’s recent history was significant for a positive tuberculosis test (purified protein derivative [PPD], 13 mm), for which she had been on prophylactic medication. Laboratory evaluation taken during the patient’s recent follow-up visit with her PCP revealed the following significant hepatic abnormalities: total bilirubin, 20.0 mg/dL; direct bilirubin, 16.4 mg/dL; international normalized ratio, 2.9; aspartate aminotransferase, greater than 2,000 IU/L; and alanine aminotransferase, greater than 2,000 IU/L. The patient had no history of hepatic disease, and a hepatitis panel obtained in the ED was unremarkable.
Can this be drug-induced liver injury?
Drug-induced liver injury (DILI) accounts for nearly 50% of cases of acute liver failure in the United States.1 According to the National Institutes of Health database of drugs, supplements, and herbal medications acetaminophen is the most common drug associated with hepatotoxicity in the United States, whereas amoxicillin-clavulanate is the most common implicated drug worldwide.1,2 The histological pattern of DILI varies by drug (Table).3
Who is susceptible to drug-induced liver injury?
The factors that help predict DILI include drug pharmacokinetics and metabolism, as well as patient age, sex, and comorbidities. Although some patients are at an increased risk of DILI, it is extraordinarily difficult to accurately predict which patients will develop it. In general, there is a positive correlation between age and risk of developing DILI. For example, in a large US-based tuberculosis study, the incidence of isoniazid (INH)-induced hepatotoxicity was 4.4 per 1,000 patients aged 25 to 34 years. Patients older than age 50 years had a 20.83 per 1,000 incidence of DILI, and women also appear to be at increased risk.4
Pharmacogenetic factors affecting drug metabolism such as the specific cytochrome profile and acetylator status of an individual also influence a patient’s risk of developing DILI. Although our understanding of these issues is growing rapidly, our ability to apply this knowledge to the clinical venue is limited by the available technology, regulatory requirements, and cost.
Case Continuation
A detailed, careful history-taking in the ED revealed that, 2 months prior, the patient had been started on INH, rifampin, and pyridoxine for latent tuberculosis. She had been taking methotrexate for the RA but discontinued it 3 months ago because of the positive PPD. When routine outpatient laboratory testing results demonstrated significant hepatic dysfunction, the patient’s PCP advised her to immediately discontinue her medications and referred her to the ED for further evaluation and management.
By what mechanism does INH cause DILI?
Acute INH-associated hepatitis primarily results from the direct hepatotoxic effects of INH metabolites. Isoniazid is metabolized in the liver via N-acetylation to acetylisoniazid (Figure). Oxidation of this compound in the liver leads to an accumulation of the hepatotoxic metabolites acetylhydrazine and hydrazine.5,6
Is there a role for N-acetylcysteine in INH hepatotoxicity?
No antidote is specifically designed to treat INH-induced hepatotoxicity, and management is largely supportive. Observation for progressive liver failure is indicated and evaluation for liver transplant may become necessary.
N-acetylcysteine (NAC) has a clear role in preventing hepatotoxicity from acetaminophen overdose through its ability to act as a precursor for the synthesis of glutathione—a compound that protects hepatocytes from oxidative damage. In advanced acetaminophen-toxic patients and those with non-acetaminophen toxicity, NAC has nonspecific effects that promote healing through several mechanisms, including anti-inflammatory effect and enhanced hepatic perfusion. Though there are no studies that specifically evaluate the role of NAC in patients with INH-induced hepatotoxicity, it is commonly and appropriately administered for its aforementioned nonspecific effects.8 Common side effects from NAC administration include nausea, vomiting, and diarrhea, which are generally treatable with symptomatic and supportive care.
Case Conclusion
The patient was admitted to the hepatology service for continued clinical care. Although she received NAC, hepatic function testing showed only mild improvement. Additional etiologies of liver failure were investigated, including a computed tomography scan of the abdomen/pelvis and an abdominal ultrasound with Doppler. Both studies were negative for any pathology, and autoimmune laboratory studies were likewise unremarkable.
The patient underwent a liver biopsy, which revealed inflammation and scattered eosinophils suggestive of drug-induced hepatic injury. Her clinical condition continued to deteriorate, and she was transferred to another hospital for transplant evaluation.
1. Lee WM. Drug-induced acute liver failure. Clin Liver Dis. 2013;17(4):575-586, viii. doi:10.1016/j.cld.2013.07.001.
2. National Institutes of Health Web site. LiverTox: Clinical and research information on drug-induced liver injury. https://livertox.nlm.nih.gov/. Updated February 10, 2017. Accessed October 12, 2017.
3. Ansari JA, Sayyed M, Sayeed F. Management of non alcoholic fatty liver diseases and their complications. Int J Pharmacol. 2011;7:579-588. doi:10.3923/ijp.2011.579.588.
4. Fountain FF, Tolley E, Chrisman CR, Self TH. Isoniazid hepatotoxicity associated with treatment of latent tuberculosis infection: a 7-year evaluation from a public health tuberculosis clinic. Chest. 2005;128(1):116-123. doi:10.1378/chest.128.1.116.
5. Hernon CH. Antituberculous medications. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, eds. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2015:787-796.
6. Teixeira RL, Morato RG, Cabello PH, et al. Genetic polymorphisms of NAT2, CYP2E1 and GST enzymes and the occurrence of antituberculosis drug-induced hepatitis in Brazilian TB patients. Mem Inst Oswaldo Cruz. 2011;106(6):716-724.
7. Mitchell JR, Thorgeirsson UP, Black M, et al. Increased incidence of isoniazid hepatitis in rapid acetylators: possible relation to hydranize metabolites. Clin Pharmacol Ther. 1975;18(1):70-79.
8. Lee WM, Hynan LS, Rossaro L, et al; Acute Liver Failure Study Group. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137(3):856-864. doi:10.1053/j.gastro.2009.06.006.
Case
A 50-year-old Hispanic woman with a history of rheumatoid arthritis (RA), for which she was not currently taking medication, was referred to the ED by her primary care physician (PCP) for evaluation of generalized pruritus and jaundice, and an abnormal hepatic function panel.
The patient’s recent history was significant for a positive tuberculosis test (purified protein derivative [PPD], 13 mm), for which she had been on prophylactic medication. Laboratory evaluation taken during the patient’s recent follow-up visit with her PCP revealed the following significant hepatic abnormalities: total bilirubin, 20.0 mg/dL; direct bilirubin, 16.4 mg/dL; international normalized ratio, 2.9; aspartate aminotransferase, greater than 2,000 IU/L; and alanine aminotransferase, greater than 2,000 IU/L. The patient had no history of hepatic disease, and a hepatitis panel obtained in the ED was unremarkable.
Can this be drug-induced liver injury?
Drug-induced liver injury (DILI) accounts for nearly 50% of cases of acute liver failure in the United States.1 According to the National Institutes of Health database of drugs, supplements, and herbal medications acetaminophen is the most common drug associated with hepatotoxicity in the United States, whereas amoxicillin-clavulanate is the most common implicated drug worldwide.1,2 The histological pattern of DILI varies by drug (Table).3
Who is susceptible to drug-induced liver injury?
The factors that help predict DILI include drug pharmacokinetics and metabolism, as well as patient age, sex, and comorbidities. Although some patients are at an increased risk of DILI, it is extraordinarily difficult to accurately predict which patients will develop it. In general, there is a positive correlation between age and risk of developing DILI. For example, in a large US-based tuberculosis study, the incidence of isoniazid (INH)-induced hepatotoxicity was 4.4 per 1,000 patients aged 25 to 34 years. Patients older than age 50 years had a 20.83 per 1,000 incidence of DILI, and women also appear to be at increased risk.4
Pharmacogenetic factors affecting drug metabolism such as the specific cytochrome profile and acetylator status of an individual also influence a patient’s risk of developing DILI. Although our understanding of these issues is growing rapidly, our ability to apply this knowledge to the clinical venue is limited by the available technology, regulatory requirements, and cost.
Case Continuation
A detailed, careful history-taking in the ED revealed that, 2 months prior, the patient had been started on INH, rifampin, and pyridoxine for latent tuberculosis. She had been taking methotrexate for the RA but discontinued it 3 months ago because of the positive PPD. When routine outpatient laboratory testing results demonstrated significant hepatic dysfunction, the patient’s PCP advised her to immediately discontinue her medications and referred her to the ED for further evaluation and management.
By what mechanism does INH cause DILI?
Acute INH-associated hepatitis primarily results from the direct hepatotoxic effects of INH metabolites. Isoniazid is metabolized in the liver via N-acetylation to acetylisoniazid (Figure). Oxidation of this compound in the liver leads to an accumulation of the hepatotoxic metabolites acetylhydrazine and hydrazine.5,6
Is there a role for N-acetylcysteine in INH hepatotoxicity?
No antidote is specifically designed to treat INH-induced hepatotoxicity, and management is largely supportive. Observation for progressive liver failure is indicated and evaluation for liver transplant may become necessary.
N-acetylcysteine (NAC) has a clear role in preventing hepatotoxicity from acetaminophen overdose through its ability to act as a precursor for the synthesis of glutathione—a compound that protects hepatocytes from oxidative damage. In advanced acetaminophen-toxic patients and those with non-acetaminophen toxicity, NAC has nonspecific effects that promote healing through several mechanisms, including anti-inflammatory effect and enhanced hepatic perfusion. Though there are no studies that specifically evaluate the role of NAC in patients with INH-induced hepatotoxicity, it is commonly and appropriately administered for its aforementioned nonspecific effects.8 Common side effects from NAC administration include nausea, vomiting, and diarrhea, which are generally treatable with symptomatic and supportive care.
Case Conclusion
The patient was admitted to the hepatology service for continued clinical care. Although she received NAC, hepatic function testing showed only mild improvement. Additional etiologies of liver failure were investigated, including a computed tomography scan of the abdomen/pelvis and an abdominal ultrasound with Doppler. Both studies were negative for any pathology, and autoimmune laboratory studies were likewise unremarkable.
The patient underwent a liver biopsy, which revealed inflammation and scattered eosinophils suggestive of drug-induced hepatic injury. Her clinical condition continued to deteriorate, and she was transferred to another hospital for transplant evaluation.
Case
A 50-year-old Hispanic woman with a history of rheumatoid arthritis (RA), for which she was not currently taking medication, was referred to the ED by her primary care physician (PCP) for evaluation of generalized pruritus and jaundice, and an abnormal hepatic function panel.
The patient’s recent history was significant for a positive tuberculosis test (purified protein derivative [PPD], 13 mm), for which she had been on prophylactic medication. Laboratory evaluation taken during the patient’s recent follow-up visit with her PCP revealed the following significant hepatic abnormalities: total bilirubin, 20.0 mg/dL; direct bilirubin, 16.4 mg/dL; international normalized ratio, 2.9; aspartate aminotransferase, greater than 2,000 IU/L; and alanine aminotransferase, greater than 2,000 IU/L. The patient had no history of hepatic disease, and a hepatitis panel obtained in the ED was unremarkable.
Can this be drug-induced liver injury?
Drug-induced liver injury (DILI) accounts for nearly 50% of cases of acute liver failure in the United States.1 According to the National Institutes of Health database of drugs, supplements, and herbal medications acetaminophen is the most common drug associated with hepatotoxicity in the United States, whereas amoxicillin-clavulanate is the most common implicated drug worldwide.1,2 The histological pattern of DILI varies by drug (Table).3
Who is susceptible to drug-induced liver injury?
The factors that help predict DILI include drug pharmacokinetics and metabolism, as well as patient age, sex, and comorbidities. Although some patients are at an increased risk of DILI, it is extraordinarily difficult to accurately predict which patients will develop it. In general, there is a positive correlation between age and risk of developing DILI. For example, in a large US-based tuberculosis study, the incidence of isoniazid (INH)-induced hepatotoxicity was 4.4 per 1,000 patients aged 25 to 34 years. Patients older than age 50 years had a 20.83 per 1,000 incidence of DILI, and women also appear to be at increased risk.4
Pharmacogenetic factors affecting drug metabolism such as the specific cytochrome profile and acetylator status of an individual also influence a patient’s risk of developing DILI. Although our understanding of these issues is growing rapidly, our ability to apply this knowledge to the clinical venue is limited by the available technology, regulatory requirements, and cost.
Case Continuation
A detailed, careful history-taking in the ED revealed that, 2 months prior, the patient had been started on INH, rifampin, and pyridoxine for latent tuberculosis. She had been taking methotrexate for the RA but discontinued it 3 months ago because of the positive PPD. When routine outpatient laboratory testing results demonstrated significant hepatic dysfunction, the patient’s PCP advised her to immediately discontinue her medications and referred her to the ED for further evaluation and management.
By what mechanism does INH cause DILI?
Acute INH-associated hepatitis primarily results from the direct hepatotoxic effects of INH metabolites. Isoniazid is metabolized in the liver via N-acetylation to acetylisoniazid (Figure). Oxidation of this compound in the liver leads to an accumulation of the hepatotoxic metabolites acetylhydrazine and hydrazine.5,6
Is there a role for N-acetylcysteine in INH hepatotoxicity?
No antidote is specifically designed to treat INH-induced hepatotoxicity, and management is largely supportive. Observation for progressive liver failure is indicated and evaluation for liver transplant may become necessary.
N-acetylcysteine (NAC) has a clear role in preventing hepatotoxicity from acetaminophen overdose through its ability to act as a precursor for the synthesis of glutathione—a compound that protects hepatocytes from oxidative damage. In advanced acetaminophen-toxic patients and those with non-acetaminophen toxicity, NAC has nonspecific effects that promote healing through several mechanisms, including anti-inflammatory effect and enhanced hepatic perfusion. Though there are no studies that specifically evaluate the role of NAC in patients with INH-induced hepatotoxicity, it is commonly and appropriately administered for its aforementioned nonspecific effects.8 Common side effects from NAC administration include nausea, vomiting, and diarrhea, which are generally treatable with symptomatic and supportive care.
Case Conclusion
The patient was admitted to the hepatology service for continued clinical care. Although she received NAC, hepatic function testing showed only mild improvement. Additional etiologies of liver failure were investigated, including a computed tomography scan of the abdomen/pelvis and an abdominal ultrasound with Doppler. Both studies were negative for any pathology, and autoimmune laboratory studies were likewise unremarkable.
The patient underwent a liver biopsy, which revealed inflammation and scattered eosinophils suggestive of drug-induced hepatic injury. Her clinical condition continued to deteriorate, and she was transferred to another hospital for transplant evaluation.
1. Lee WM. Drug-induced acute liver failure. Clin Liver Dis. 2013;17(4):575-586, viii. doi:10.1016/j.cld.2013.07.001.
2. National Institutes of Health Web site. LiverTox: Clinical and research information on drug-induced liver injury. https://livertox.nlm.nih.gov/. Updated February 10, 2017. Accessed October 12, 2017.
3. Ansari JA, Sayyed M, Sayeed F. Management of non alcoholic fatty liver diseases and their complications. Int J Pharmacol. 2011;7:579-588. doi:10.3923/ijp.2011.579.588.
4. Fountain FF, Tolley E, Chrisman CR, Self TH. Isoniazid hepatotoxicity associated with treatment of latent tuberculosis infection: a 7-year evaluation from a public health tuberculosis clinic. Chest. 2005;128(1):116-123. doi:10.1378/chest.128.1.116.
5. Hernon CH. Antituberculous medications. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, eds. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2015:787-796.
6. Teixeira RL, Morato RG, Cabello PH, et al. Genetic polymorphisms of NAT2, CYP2E1 and GST enzymes and the occurrence of antituberculosis drug-induced hepatitis in Brazilian TB patients. Mem Inst Oswaldo Cruz. 2011;106(6):716-724.
7. Mitchell JR, Thorgeirsson UP, Black M, et al. Increased incidence of isoniazid hepatitis in rapid acetylators: possible relation to hydranize metabolites. Clin Pharmacol Ther. 1975;18(1):70-79.
8. Lee WM, Hynan LS, Rossaro L, et al; Acute Liver Failure Study Group. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137(3):856-864. doi:10.1053/j.gastro.2009.06.006.
1. Lee WM. Drug-induced acute liver failure. Clin Liver Dis. 2013;17(4):575-586, viii. doi:10.1016/j.cld.2013.07.001.
2. National Institutes of Health Web site. LiverTox: Clinical and research information on drug-induced liver injury. https://livertox.nlm.nih.gov/. Updated February 10, 2017. Accessed October 12, 2017.
3. Ansari JA, Sayyed M, Sayeed F. Management of non alcoholic fatty liver diseases and their complications. Int J Pharmacol. 2011;7:579-588. doi:10.3923/ijp.2011.579.588.
4. Fountain FF, Tolley E, Chrisman CR, Self TH. Isoniazid hepatotoxicity associated with treatment of latent tuberculosis infection: a 7-year evaluation from a public health tuberculosis clinic. Chest. 2005;128(1):116-123. doi:10.1378/chest.128.1.116.
5. Hernon CH. Antituberculous medications. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, eds. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2015:787-796.
6. Teixeira RL, Morato RG, Cabello PH, et al. Genetic polymorphisms of NAT2, CYP2E1 and GST enzymes and the occurrence of antituberculosis drug-induced hepatitis in Brazilian TB patients. Mem Inst Oswaldo Cruz. 2011;106(6):716-724.
7. Mitchell JR, Thorgeirsson UP, Black M, et al. Increased incidence of isoniazid hepatitis in rapid acetylators: possible relation to hydranize metabolites. Clin Pharmacol Ther. 1975;18(1):70-79.
8. Lee WM, Hynan LS, Rossaro L, et al; Acute Liver Failure Study Group. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137(3):856-864. doi:10.1053/j.gastro.2009.06.006.
Don’t Always Rush to Rally Renal
Case
An otherwise healthy 20-month-old boy presented to the ED for evaluation after his father witnessed the child ingest a model race car fuel additive. According to the patient’s father, the boy was playing with several closed bottles that were stored in the garage, when he witnessed the boy open up and take a sip of a pink-colored fuel additive, which the father believed to contain 100% methanol. The patient’s father further noted that immediately after drinking the fluid, the patient spat and drooled, and had one episode of nonbloody emesis prior to arrival at the ED.
Initial vital signs at presentation were: blood pressure, 84/54 mm Hg; heart rate, 97 beats/min; respiratory rate, 24 breaths/min; and temperature 98°F. Oxygen saturation was 99% on room air. Physical examination was notable for mild erythema in the posterior oropharynx. Otherwise, the patient was acting appropriately for his age and in no acute distress. Laboratory studies were within normal limits, except for the following: serum anion gap, 18 mEq/L (reference range for children < 3 years old, 10-14 mEq/L); serum bicarbonate, 19 mmol/L (reference range for children 12-24 months, 17-25 mmol/L); and serum creatinine, 2.8 mg/dL (reference range for children 12 to 24 months, 0.2-0.5 mg/dL). A repeat creatinine test taken after bolus of fluid administration was 2.4 mg/dL. A renal ultrasound, performed to investigate the cause of the renal failure, was unremarkable.
What toxic exposures are of concern based on the clinical history?
The history of exposure to a liquid stored in a garage raises the likelihood of exposure to an automobile-related item such as diethylene glycol, ethylene glycol (EG), and methanol.
Diethylene Glycol. Diethylene glycol is an ingredient in brake and power steering fluids, and has toxic properties qualitatively similar to EG.
Ethylene Glycol. A clear, colorless, odorless fluid with a sweet taste, EG is an ingredient in radiator antifreeze, refrigerant fluid, coolants, and pesticides. Like methylene, EG reaches peak plasma concentration within 1 to 4 hours, but toxic clinical findings do not occur for 3 to 6 hours.1
Methanol. Methanol is a clear, colorless, alcohol found in antifreeze, windshield washer fluid, and race car fuel.2 Although methanol reaches peak plasma concentration in about 30 to 60 minutes, signs of systemic toxicity (ie, metabolic acidosis) typically take 6 to 12 hours to manifest.1
In both EG and methanol, there is a delay in toxic clinical findings because the parent compounds are not toxic in their initial form; rather, major toxicity is derived from their metabolites: formic acid and oxalic acid, respectively.
Other Toxins. Many other potentially toxic liquids are associated with a homeowner’s occupation or avocational interests. These include painting supplies (eg, industrial paints containing lead), gardening materials (eg, pesticides containing organophosphates), fuels (eg, gasoline, polychlorinated biphenyls in coolant, and lubricants), and cleaning supplies (eg, caustics, detergents, and air freshener).
Case Continuation
Since the patient’s elevated anion gap raised concerns for methanol or EG exposure, he was given fomepizole and transferred to a tertiary care children’s hospital for further management and possible hemodialysis. Upon arrival at the receiving hospital, the patient’s vital signs and physical examination remained unchanged. Repeat laboratory studies were notable for a creatinine level of 0.3 mg/dL. The patient’s father was instructed to retrieve the implicated bottle from home. An inspection of the bottle’s ingredients was notable for nitromethane, castor oil, and methanol.
What is nitromethane and what are its uses?
Nitromethane, the simplest nitro compound, is a colorless, viscous, lipid-soluble fluid.3 The polarity of nitromethane permits its use as a stabilizer in a number of chemical solvents, such as dry cleaning fluid, degreasers, and "super glue."4,5 Nitromethane is also commonly added to model-engine and drag-race fuels, which also contain methanol and castor oil.3 In this capacity, nitromethane functions as an oxygen carrier, allowing more efficient fuel use in combustion cylinders (compared to gasoline), thereby increasing the horsepower of the vehicle.6 It is therefore commonly added to fuel for drag racers, radio-controlled cars, and model aircrafts.4 In the small concentrations typically inadvertently ingested, the clinical effects of nitromethane itself are inconsequential.
What is the differential for creatinine elevation?
Creatinine itself is a normal breakdown product of muscle metabolism produced by spontaneous conversion from creatine and is found at a fairly constant serum level in proportion to muscle mass.7 Thus, as people age and muscle mass decreases, their baseline creatinine levels decrease proportionally.
Elimination. The majority of creatinine (85%-90%) is filtered and excreted by the kidneys, with the remaining 10% to 15% secreted by the tubules, allowing creatinine to be a surrogate measure of the glomerular filtration rate.7 Exogenous sources of creatine or creatinine include meat and creatine supplements, the latter of which are used as an "energy source" to enhance athletic performance.
Etiology. The etiology for an elevated serum creatinine concentration includes renal failure, both acute and chronic; volume depletion; hemorrhage (low blood volume); and medications, including diuretics, angiotensin converting enzyme inhibitors, angiotensin-receptor blockers, nonsteroidal anti-inflammatory drugs, and certain antibiotics. These etiologies can also be categorized as processes that increase creatinine production, decrease elimination (H2 antagonist and trimethoprim both inhibit the cation secretory pump in the tubules), or interfere with the creatinine assay (ketones, keto acids, lipemia, hemolysis, cephalosporins).7
Because creatinine is filtered so efficiently by the kidney, neither exogenous nor endogenous creatinine sources are expected to increase serum creatinine in the absence of renal dysfunction. However, transient elevation may occur in body builders who use extreme doses of creatine. Patients with rhabdomyolysis often develop elevated creatinine concentrations, but nearly always in the setting of myoglobinuric renal failure.
Jaffe Reaction and Enzymatic Methods. Serum creatinine can be measured using either the Jaffe reaction or the enzymatic method. In the Jaffe reaction, creatinine reacts with alkaline sodium picrate to form a red-orange chromophore, which absorbs light in the range of 470 to 550 nanometers on spectroscopy.6,8,9 The active methylene group on nitromethane also reacts with alkaline sodium picrate to form a chromophore which absorbs light in the same wavelength range.10 Thus, serum creatinine measurements via the Jaffe reaction are falsely elevated due to the cross-reactivity between nitromethane and alkaline sodium picrate. In some reported cases, there is a 20-fold increase in the measured serum creatinine in the presence of nitromethane; renal function, however, remains normal.5
This false reading seen in the Jaffe reaction can be avoided by utilizing the enzymatic method of creatinine measurement, a three-step process that ultimately produces hydrogen peroxide, which is measured and accurately correlates with serum creatinine—even in the presence of nitromethane.8 This distinction explains the dramatically different creatinine concentrations measured at the two institutions in this case.
Case Conclusion
The patient was monitored overnight at the children’s hospital. Repeat laboratory studies in the morning showed a normal creatinine level of 0.3 mg/dL and a negative methanol level. The patient was discharged home in the care of his father, who was instructed to follow-up with his son’s pediatrician. The father also received counseling on safe storage practices for dangerous chemicals.
1. Kruse JA. Methanol and ethylene glycol intoxication. Crit Care Clin. 2012;28(4):661-711. doi:10.1016/j.ccc.2012.07.002.
2. McMahon DM, Winstead S, Weant KA. Toxic alcohol ingestions: focus on ethylene glycol and methanol. Adv Emerg Nurs J. 2009;31(3):206-213. doi:10.1097/TME.0b013e3181ad8be8.
3. Cook MD, Clark RF. Creatinine elevation associated with nitromethane exposure: a marker of potential methanol toxicity. J Emerg Med. 2007;33(3):249-253. doi:10.1016/j.jemermed.2007.02.015.
4. Markofsky SB. Nitro compounds, aliphatic. In: Elvers B, ed. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA; 2000. doi:10.1002/14356007.a17_401. [digital]
5. Mullins ME, Hammett-Stabler CA. Intoxication with nitromethane-containing fuels: don’t be "fueled" by the creatinine. J Toxicol Clin Toxicol. 1998;36(4):
315-320.
6. Ngo AS, Rowley F, Olson KR. Case files of the California poison control system, San Francisco division: blue thunder ingestion: methanol, nitromethane, and elevated creatinine. J Med Toxicol. 2010;6(1):67-71. doi:10.1007/s13181-010-0042-5.
7. Samra M, Abcar AC. False estimates of elevated creatinine. Perm J. 2012;16(2):51-52.
8. Booth C, Naidoo D, Rosenberg A, Kainer G. Elevated creatinine after ingestion of model aviation fuel: interference with the Jaffe reaction by nitromethane. J Paediatr Child Health. 1999;35(5):503-504.
9. de Lelis Medeiros de Morais C, Gomes de Lima KM. Determination and analytical validation of creatinine content in serum using image analysis by multivariate transfer calibration procedures. Anal Meth. 2015;7:6904-6910. doi:10.1039/C5AY01369K.
10. Killorn E, Lim RK, Rieder M. Apparent elevated creatinine after ingestion of nitromethane: interference with the Jaffe reaction. Ther Drug Monit. 2011;33(1):1-2. doi:10.1097/FTD.0b013e3181fe7e52.
Case
An otherwise healthy 20-month-old boy presented to the ED for evaluation after his father witnessed the child ingest a model race car fuel additive. According to the patient’s father, the boy was playing with several closed bottles that were stored in the garage, when he witnessed the boy open up and take a sip of a pink-colored fuel additive, which the father believed to contain 100% methanol. The patient’s father further noted that immediately after drinking the fluid, the patient spat and drooled, and had one episode of nonbloody emesis prior to arrival at the ED.
Initial vital signs at presentation were: blood pressure, 84/54 mm Hg; heart rate, 97 beats/min; respiratory rate, 24 breaths/min; and temperature 98°F. Oxygen saturation was 99% on room air. Physical examination was notable for mild erythema in the posterior oropharynx. Otherwise, the patient was acting appropriately for his age and in no acute distress. Laboratory studies were within normal limits, except for the following: serum anion gap, 18 mEq/L (reference range for children < 3 years old, 10-14 mEq/L); serum bicarbonate, 19 mmol/L (reference range for children 12-24 months, 17-25 mmol/L); and serum creatinine, 2.8 mg/dL (reference range for children 12 to 24 months, 0.2-0.5 mg/dL). A repeat creatinine test taken after bolus of fluid administration was 2.4 mg/dL. A renal ultrasound, performed to investigate the cause of the renal failure, was unremarkable.
What toxic exposures are of concern based on the clinical history?
The history of exposure to a liquid stored in a garage raises the likelihood of exposure to an automobile-related item such as diethylene glycol, ethylene glycol (EG), and methanol.
Diethylene Glycol. Diethylene glycol is an ingredient in brake and power steering fluids, and has toxic properties qualitatively similar to EG.
Ethylene Glycol. A clear, colorless, odorless fluid with a sweet taste, EG is an ingredient in radiator antifreeze, refrigerant fluid, coolants, and pesticides. Like methylene, EG reaches peak plasma concentration within 1 to 4 hours, but toxic clinical findings do not occur for 3 to 6 hours.1
Methanol. Methanol is a clear, colorless, alcohol found in antifreeze, windshield washer fluid, and race car fuel.2 Although methanol reaches peak plasma concentration in about 30 to 60 minutes, signs of systemic toxicity (ie, metabolic acidosis) typically take 6 to 12 hours to manifest.1
In both EG and methanol, there is a delay in toxic clinical findings because the parent compounds are not toxic in their initial form; rather, major toxicity is derived from their metabolites: formic acid and oxalic acid, respectively.
Other Toxins. Many other potentially toxic liquids are associated with a homeowner’s occupation or avocational interests. These include painting supplies (eg, industrial paints containing lead), gardening materials (eg, pesticides containing organophosphates), fuels (eg, gasoline, polychlorinated biphenyls in coolant, and lubricants), and cleaning supplies (eg, caustics, detergents, and air freshener).
Case Continuation
Since the patient’s elevated anion gap raised concerns for methanol or EG exposure, he was given fomepizole and transferred to a tertiary care children’s hospital for further management and possible hemodialysis. Upon arrival at the receiving hospital, the patient’s vital signs and physical examination remained unchanged. Repeat laboratory studies were notable for a creatinine level of 0.3 mg/dL. The patient’s father was instructed to retrieve the implicated bottle from home. An inspection of the bottle’s ingredients was notable for nitromethane, castor oil, and methanol.
What is nitromethane and what are its uses?
Nitromethane, the simplest nitro compound, is a colorless, viscous, lipid-soluble fluid.3 The polarity of nitromethane permits its use as a stabilizer in a number of chemical solvents, such as dry cleaning fluid, degreasers, and "super glue."4,5 Nitromethane is also commonly added to model-engine and drag-race fuels, which also contain methanol and castor oil.3 In this capacity, nitromethane functions as an oxygen carrier, allowing more efficient fuel use in combustion cylinders (compared to gasoline), thereby increasing the horsepower of the vehicle.6 It is therefore commonly added to fuel for drag racers, radio-controlled cars, and model aircrafts.4 In the small concentrations typically inadvertently ingested, the clinical effects of nitromethane itself are inconsequential.
What is the differential for creatinine elevation?
Creatinine itself is a normal breakdown product of muscle metabolism produced by spontaneous conversion from creatine and is found at a fairly constant serum level in proportion to muscle mass.7 Thus, as people age and muscle mass decreases, their baseline creatinine levels decrease proportionally.
Elimination. The majority of creatinine (85%-90%) is filtered and excreted by the kidneys, with the remaining 10% to 15% secreted by the tubules, allowing creatinine to be a surrogate measure of the glomerular filtration rate.7 Exogenous sources of creatine or creatinine include meat and creatine supplements, the latter of which are used as an "energy source" to enhance athletic performance.
Etiology. The etiology for an elevated serum creatinine concentration includes renal failure, both acute and chronic; volume depletion; hemorrhage (low blood volume); and medications, including diuretics, angiotensin converting enzyme inhibitors, angiotensin-receptor blockers, nonsteroidal anti-inflammatory drugs, and certain antibiotics. These etiologies can also be categorized as processes that increase creatinine production, decrease elimination (H2 antagonist and trimethoprim both inhibit the cation secretory pump in the tubules), or interfere with the creatinine assay (ketones, keto acids, lipemia, hemolysis, cephalosporins).7
Because creatinine is filtered so efficiently by the kidney, neither exogenous nor endogenous creatinine sources are expected to increase serum creatinine in the absence of renal dysfunction. However, transient elevation may occur in body builders who use extreme doses of creatine. Patients with rhabdomyolysis often develop elevated creatinine concentrations, but nearly always in the setting of myoglobinuric renal failure.
Jaffe Reaction and Enzymatic Methods. Serum creatinine can be measured using either the Jaffe reaction or the enzymatic method. In the Jaffe reaction, creatinine reacts with alkaline sodium picrate to form a red-orange chromophore, which absorbs light in the range of 470 to 550 nanometers on spectroscopy.6,8,9 The active methylene group on nitromethane also reacts with alkaline sodium picrate to form a chromophore which absorbs light in the same wavelength range.10 Thus, serum creatinine measurements via the Jaffe reaction are falsely elevated due to the cross-reactivity between nitromethane and alkaline sodium picrate. In some reported cases, there is a 20-fold increase in the measured serum creatinine in the presence of nitromethane; renal function, however, remains normal.5
This false reading seen in the Jaffe reaction can be avoided by utilizing the enzymatic method of creatinine measurement, a three-step process that ultimately produces hydrogen peroxide, which is measured and accurately correlates with serum creatinine—even in the presence of nitromethane.8 This distinction explains the dramatically different creatinine concentrations measured at the two institutions in this case.
Case Conclusion
The patient was monitored overnight at the children’s hospital. Repeat laboratory studies in the morning showed a normal creatinine level of 0.3 mg/dL and a negative methanol level. The patient was discharged home in the care of his father, who was instructed to follow-up with his son’s pediatrician. The father also received counseling on safe storage practices for dangerous chemicals.
Case
An otherwise healthy 20-month-old boy presented to the ED for evaluation after his father witnessed the child ingest a model race car fuel additive. According to the patient’s father, the boy was playing with several closed bottles that were stored in the garage, when he witnessed the boy open up and take a sip of a pink-colored fuel additive, which the father believed to contain 100% methanol. The patient’s father further noted that immediately after drinking the fluid, the patient spat and drooled, and had one episode of nonbloody emesis prior to arrival at the ED.
Initial vital signs at presentation were: blood pressure, 84/54 mm Hg; heart rate, 97 beats/min; respiratory rate, 24 breaths/min; and temperature 98°F. Oxygen saturation was 99% on room air. Physical examination was notable for mild erythema in the posterior oropharynx. Otherwise, the patient was acting appropriately for his age and in no acute distress. Laboratory studies were within normal limits, except for the following: serum anion gap, 18 mEq/L (reference range for children < 3 years old, 10-14 mEq/L); serum bicarbonate, 19 mmol/L (reference range for children 12-24 months, 17-25 mmol/L); and serum creatinine, 2.8 mg/dL (reference range for children 12 to 24 months, 0.2-0.5 mg/dL). A repeat creatinine test taken after bolus of fluid administration was 2.4 mg/dL. A renal ultrasound, performed to investigate the cause of the renal failure, was unremarkable.
What toxic exposures are of concern based on the clinical history?
The history of exposure to a liquid stored in a garage raises the likelihood of exposure to an automobile-related item such as diethylene glycol, ethylene glycol (EG), and methanol.
Diethylene Glycol. Diethylene glycol is an ingredient in brake and power steering fluids, and has toxic properties qualitatively similar to EG.
Ethylene Glycol. A clear, colorless, odorless fluid with a sweet taste, EG is an ingredient in radiator antifreeze, refrigerant fluid, coolants, and pesticides. Like methylene, EG reaches peak plasma concentration within 1 to 4 hours, but toxic clinical findings do not occur for 3 to 6 hours.1
Methanol. Methanol is a clear, colorless, alcohol found in antifreeze, windshield washer fluid, and race car fuel.2 Although methanol reaches peak plasma concentration in about 30 to 60 minutes, signs of systemic toxicity (ie, metabolic acidosis) typically take 6 to 12 hours to manifest.1
In both EG and methanol, there is a delay in toxic clinical findings because the parent compounds are not toxic in their initial form; rather, major toxicity is derived from their metabolites: formic acid and oxalic acid, respectively.
Other Toxins. Many other potentially toxic liquids are associated with a homeowner’s occupation or avocational interests. These include painting supplies (eg, industrial paints containing lead), gardening materials (eg, pesticides containing organophosphates), fuels (eg, gasoline, polychlorinated biphenyls in coolant, and lubricants), and cleaning supplies (eg, caustics, detergents, and air freshener).
Case Continuation
Since the patient’s elevated anion gap raised concerns for methanol or EG exposure, he was given fomepizole and transferred to a tertiary care children’s hospital for further management and possible hemodialysis. Upon arrival at the receiving hospital, the patient’s vital signs and physical examination remained unchanged. Repeat laboratory studies were notable for a creatinine level of 0.3 mg/dL. The patient’s father was instructed to retrieve the implicated bottle from home. An inspection of the bottle’s ingredients was notable for nitromethane, castor oil, and methanol.
What is nitromethane and what are its uses?
Nitromethane, the simplest nitro compound, is a colorless, viscous, lipid-soluble fluid.3 The polarity of nitromethane permits its use as a stabilizer in a number of chemical solvents, such as dry cleaning fluid, degreasers, and "super glue."4,5 Nitromethane is also commonly added to model-engine and drag-race fuels, which also contain methanol and castor oil.3 In this capacity, nitromethane functions as an oxygen carrier, allowing more efficient fuel use in combustion cylinders (compared to gasoline), thereby increasing the horsepower of the vehicle.6 It is therefore commonly added to fuel for drag racers, radio-controlled cars, and model aircrafts.4 In the small concentrations typically inadvertently ingested, the clinical effects of nitromethane itself are inconsequential.
What is the differential for creatinine elevation?
Creatinine itself is a normal breakdown product of muscle metabolism produced by spontaneous conversion from creatine and is found at a fairly constant serum level in proportion to muscle mass.7 Thus, as people age and muscle mass decreases, their baseline creatinine levels decrease proportionally.
Elimination. The majority of creatinine (85%-90%) is filtered and excreted by the kidneys, with the remaining 10% to 15% secreted by the tubules, allowing creatinine to be a surrogate measure of the glomerular filtration rate.7 Exogenous sources of creatine or creatinine include meat and creatine supplements, the latter of which are used as an "energy source" to enhance athletic performance.
Etiology. The etiology for an elevated serum creatinine concentration includes renal failure, both acute and chronic; volume depletion; hemorrhage (low blood volume); and medications, including diuretics, angiotensin converting enzyme inhibitors, angiotensin-receptor blockers, nonsteroidal anti-inflammatory drugs, and certain antibiotics. These etiologies can also be categorized as processes that increase creatinine production, decrease elimination (H2 antagonist and trimethoprim both inhibit the cation secretory pump in the tubules), or interfere with the creatinine assay (ketones, keto acids, lipemia, hemolysis, cephalosporins).7
Because creatinine is filtered so efficiently by the kidney, neither exogenous nor endogenous creatinine sources are expected to increase serum creatinine in the absence of renal dysfunction. However, transient elevation may occur in body builders who use extreme doses of creatine. Patients with rhabdomyolysis often develop elevated creatinine concentrations, but nearly always in the setting of myoglobinuric renal failure.
Jaffe Reaction and Enzymatic Methods. Serum creatinine can be measured using either the Jaffe reaction or the enzymatic method. In the Jaffe reaction, creatinine reacts with alkaline sodium picrate to form a red-orange chromophore, which absorbs light in the range of 470 to 550 nanometers on spectroscopy.6,8,9 The active methylene group on nitromethane also reacts with alkaline sodium picrate to form a chromophore which absorbs light in the same wavelength range.10 Thus, serum creatinine measurements via the Jaffe reaction are falsely elevated due to the cross-reactivity between nitromethane and alkaline sodium picrate. In some reported cases, there is a 20-fold increase in the measured serum creatinine in the presence of nitromethane; renal function, however, remains normal.5
This false reading seen in the Jaffe reaction can be avoided by utilizing the enzymatic method of creatinine measurement, a three-step process that ultimately produces hydrogen peroxide, which is measured and accurately correlates with serum creatinine—even in the presence of nitromethane.8 This distinction explains the dramatically different creatinine concentrations measured at the two institutions in this case.
Case Conclusion
The patient was monitored overnight at the children’s hospital. Repeat laboratory studies in the morning showed a normal creatinine level of 0.3 mg/dL and a negative methanol level. The patient was discharged home in the care of his father, who was instructed to follow-up with his son’s pediatrician. The father also received counseling on safe storage practices for dangerous chemicals.
1. Kruse JA. Methanol and ethylene glycol intoxication. Crit Care Clin. 2012;28(4):661-711. doi:10.1016/j.ccc.2012.07.002.
2. McMahon DM, Winstead S, Weant KA. Toxic alcohol ingestions: focus on ethylene glycol and methanol. Adv Emerg Nurs J. 2009;31(3):206-213. doi:10.1097/TME.0b013e3181ad8be8.
3. Cook MD, Clark RF. Creatinine elevation associated with nitromethane exposure: a marker of potential methanol toxicity. J Emerg Med. 2007;33(3):249-253. doi:10.1016/j.jemermed.2007.02.015.
4. Markofsky SB. Nitro compounds, aliphatic. In: Elvers B, ed. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA; 2000. doi:10.1002/14356007.a17_401. [digital]
5. Mullins ME, Hammett-Stabler CA. Intoxication with nitromethane-containing fuels: don’t be "fueled" by the creatinine. J Toxicol Clin Toxicol. 1998;36(4):
315-320.
6. Ngo AS, Rowley F, Olson KR. Case files of the California poison control system, San Francisco division: blue thunder ingestion: methanol, nitromethane, and elevated creatinine. J Med Toxicol. 2010;6(1):67-71. doi:10.1007/s13181-010-0042-5.
7. Samra M, Abcar AC. False estimates of elevated creatinine. Perm J. 2012;16(2):51-52.
8. Booth C, Naidoo D, Rosenberg A, Kainer G. Elevated creatinine after ingestion of model aviation fuel: interference with the Jaffe reaction by nitromethane. J Paediatr Child Health. 1999;35(5):503-504.
9. de Lelis Medeiros de Morais C, Gomes de Lima KM. Determination and analytical validation of creatinine content in serum using image analysis by multivariate transfer calibration procedures. Anal Meth. 2015;7:6904-6910. doi:10.1039/C5AY01369K.
10. Killorn E, Lim RK, Rieder M. Apparent elevated creatinine after ingestion of nitromethane: interference with the Jaffe reaction. Ther Drug Monit. 2011;33(1):1-2. doi:10.1097/FTD.0b013e3181fe7e52.
1. Kruse JA. Methanol and ethylene glycol intoxication. Crit Care Clin. 2012;28(4):661-711. doi:10.1016/j.ccc.2012.07.002.
2. McMahon DM, Winstead S, Weant KA. Toxic alcohol ingestions: focus on ethylene glycol and methanol. Adv Emerg Nurs J. 2009;31(3):206-213. doi:10.1097/TME.0b013e3181ad8be8.
3. Cook MD, Clark RF. Creatinine elevation associated with nitromethane exposure: a marker of potential methanol toxicity. J Emerg Med. 2007;33(3):249-253. doi:10.1016/j.jemermed.2007.02.015.
4. Markofsky SB. Nitro compounds, aliphatic. In: Elvers B, ed. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA; 2000. doi:10.1002/14356007.a17_401. [digital]
5. Mullins ME, Hammett-Stabler CA. Intoxication with nitromethane-containing fuels: don’t be "fueled" by the creatinine. J Toxicol Clin Toxicol. 1998;36(4):
315-320.
6. Ngo AS, Rowley F, Olson KR. Case files of the California poison control system, San Francisco division: blue thunder ingestion: methanol, nitromethane, and elevated creatinine. J Med Toxicol. 2010;6(1):67-71. doi:10.1007/s13181-010-0042-5.
7. Samra M, Abcar AC. False estimates of elevated creatinine. Perm J. 2012;16(2):51-52.
8. Booth C, Naidoo D, Rosenberg A, Kainer G. Elevated creatinine after ingestion of model aviation fuel: interference with the Jaffe reaction by nitromethane. J Paediatr Child Health. 1999;35(5):503-504.
9. de Lelis Medeiros de Morais C, Gomes de Lima KM. Determination and analytical validation of creatinine content in serum using image analysis by multivariate transfer calibration procedures. Anal Meth. 2015;7:6904-6910. doi:10.1039/C5AY01369K.
10. Killorn E, Lim RK, Rieder M. Apparent elevated creatinine after ingestion of nitromethane: interference with the Jaffe reaction. Ther Drug Monit. 2011;33(1):1-2. doi:10.1097/FTD.0b013e3181fe7e52.
Case Studies in Toxicology: An Unlikely Cause of Paralysis
Case
An Asian man in his third decade, with a medical history of hypertension and hyperlipidemia, and who had recently been involved in a motor vehicle collision (MVC), presented to the ED with a chief complaint of severe bilateral upper and lower extremity weakness. The patient noted that the weakness had begun the previous evening and became progressively worse throughout the night, to the point that he was unable to move any of his extremities on the morning of presentation.
Upon arrival at the ED, the patient was awake, alert, and oriented to self, time, and place; he also spoke in full sentences without distress. He denied fever, chills, difficulty breathing, or preceding viral illness. The patient stated that he was not taking any medications and denied a history of alcohol, tobacco, or drug abuse.
Initial vital signs at presentation were: blood pressure, 141/50 mm Hg; heart rate, 90 beats/min; respiratory rate, 16 breaths/min; and temperature, 97.4°F. Oxygen saturation was 100% on room air. On physical examination, the patient was in no acute distress and had a normal mental status. His pupils were normally reactive and his other cranial nerves were normal. Muscle strength in the upper and lower extremities was 1/5 with 1+ reflexes bilaterally, and there was no sensory deficit. The patient was placed on continuous cardiac monitoring with pulse oximetry.
What is the differential diagnosis for acute extremity weakness or paralysis?
The differential diagnosis for acute symmetrical extremity weakness or paralysis is broad and includes conditions of neurological, inflammatory, and toxic/metabolic etiologies.1 Neurological diagnoses to consider include acute stroke, specifically of the anterior cerebral or middle cerebral artery territories; Guillain-Barré syndrome; myasthenia gravis; spinal cord compression; and tick paralysis. Acute ischemic or hemorrhagic stroke most frequently presents with unilateral upper or lower extremity weakness accompanied by garbled speech and sensory deficits. Patients who have suffered a brainstem or cerebellar stroke commonly present with alterations of consciousness, visual changes, and ataxia. Posterior circulation strokes are also characterized by crossed neurological deficits, such as motor deficits on one side of the body and sensory deficits on the other.
Spinal Cord Pathology. Signs and symptoms of spinal cord compression or inflammation vary widely depending on the level affected. Motor and sensory findings of spinal cord pathology include muscle weakness, spasticity, hyper- or hyporeflexia, and a discrete level below which sensation is absent or reduced.
Guillain-Barré Syndrome. Patients who have Guillain-Barré syndrome (a disease of the myelin sheaths of the peripheral nerves) often present with complaints of numbness or paresthesias in the extremities.2 The condition is characterized by progressive symmetric muscle weakness accompanied by absent or depressed deep tendon reflexes and is typically associated with a recent exposure to an infectious agent such as a viral upper respiratory infection, bacterial infection, or vaccine.
Myasthenia Gravis. Myasthenia gravis is a disease of the neuromuscular junction. It presents with weakness in any muscle group, and the muscles are easily fatigued by repetitive use.
Toxic Exposures. Toxins, such as botulinum, ixovotoxin, nicotine, succinylcholine, and tetrodotoxin, are prominent, though less common, causes of muscular weakness or paralysis. Botulinum toxin acts at the neuromuscular junction. Patients with botulism typically present with a gastrointestinal prodrome of nausea, vomiting, and diarrhea followed by cranial nerve dysfunction and descending muscle weakness.3
Tetrodotoxin, nicotine, and curare-like paralytics act at the motor end plate of the neuromuscular junction to produce neuromuscular blockade with subsequent muscular weakness or paralysis. Similarly, ixovotoxin, the toxin responsible for tick paralysis, causes ascending flaccid paralysis by decreasing the release of acetylcholine at the neuromuscular junction.3
Metabolic and Endocrine Disorders. Conditions such as hypokalemia, hypomagnesemia, and periodic paralysis can also present with neurological complaints such as generalized weakness and paresthesias. Of note, it is important to differentiate true neuromuscular weakness from weakness secondary to limited effort.
Case Continuation
Because of the patient’s history of an MVC, cervical cord compression was considered concerning enough to require exclusion through magnetic resonance imaging (MRI) of the cervical spine. However, upon arrival at the MRI suite, the patient became severely tachypneic and tachycardic, and was unable to tolerate lying flat. He was intubated for impending respiratory failure. Laboratory results from blood drawn prior to transport to MRI were reported immediately after the resuscitation and were notable for the following: potassium, <1.5 mEq/L; bicarbonate, 20 mEq/L; creatine kinase, 889 U/L; ethanol, not detected.
What is hypokalemic periodic paralysis?
Hypokalemic periodic paralysis (HypoKPP) is a syndrome of episodic muscle weakness with concomitant hypokalemia. Familial forms of HypoKPP have been attributed to mutations in genes coding for either calcium or sodium channels.
The nonfamilial form of HypoKPP is attributed to hyperthyroidism and is most often seen in Asian men in the second and third decades of life. The disorder is characterized by acute onset hypokalemia and extremity paralysis with simultaneous hyperthyroid state. It is believed that hypokalemia occurs as a result of intracellular shift of potassium from thyroid-induced hormone sensitization of the Na+/K+-ATPase rather than a depletion of total body potassium. Acute episodes of paralysis are triggered by high-carbohydrate meals, alcohol consumption, emotional stress, and infection. Paralysis can last from 3 to 96 hours and is accompanied by decreased or absent deep tendon reflexes with normal sensation and mental status.
In the nonfamilial form of HypoKPP, signs of thyrotoxicosis are often present and include tachycardia, moist skin, and hyperthermia, but it may be difficult to specifically recognize this etiology given the patient’s grave clinical condition.4 Similar to many significant metabolic and electrolyte disturbances, complications of HypoKPP include dysrhythmia, respiratory failure, and sometimes death.5
How should HypoKPP be managed in the ED?
Management of HypoKPP begins with careful assessment of the patient’s airway, breathing, and circulation. Once the patient is stabilized, management of consequential effects of hypokalemia, such as respiratory distress and muscular paralysis, should focus on correcting the electrolyte and endocrine derangements.
Propranolol. If the patient exhibits signs of thyrotoxicosis, initial treatment includes propranolol, a nonselective beta-blocker, which both prevents the intracellular shift of potassium and assists in correcting the underlying hyperthyroid and hypermetabolic state. Although there is no standard propranolol dosing protocol for HypoKPP, some authors suggest that an aggressive dose of 2 mg intravenously (IV) every 10 minutes can shorten the patient’s episode of paralysis to 6 hours.6
Potassium Chloride. Administration of potassium chloride to raise the serum potassium to life-sustaining concentrations should be done cautiously through IV infusion of standard doses.7 In correcting hypokalemia with potassium, care should be taken to avoid overcorrection, which may subsequently result in rebound hyperkalemia as the total body potassium redistributes. Lower doses of potassium (ie, <50 mEq per dose), are preferred to achieve adequate repletion while avoiding rebound hyperkalemia.8
Case Conclusion
The results of thyroid studies that had been added on to the original set of laboratory studies revealed profound hyperthyroidism, with an essentially absent concentration of thyroid-stimulating hormone.
1. Morchi RS. Weakness. In: Rosen P, ed. Rosen’s Emergency Medicine. 8th ed. Philadelphia, PA: Elsevier; 2014:124-128.
2. McGillicuddy DC, Walker O, Shapiro NI, Edlow JA. Guillain-Barré syndrome in the emergency department. Ann Emerg Med. 2006;47(4):390-393. doi:10.1016/j.annemergmed.2005.05.008.
3. Rao RB. Neurological principles. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, eds. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2015:315-323.
4. Lam L, Nair RJ, Tingle L. Thyrotoxic periodic paralysis. Proc (Bayl Univ Med Cent). 2006;19(2):126-129.
5. Li X, Yao S, Xiang Y, et al. The clinical and genetic features in a cohort of mainland Chinese patients with thyrotoxic periodic paralysis. BMC Neurol. 2015;15:38. doi:10.1186/s12883-015-0290-8.
6. Birkhahn RH, Gaeta TJ, Melniker L. Thyrotoxic periodic paralysis and intravenous propranolol in the emergency setting. J Emerg Med. 2000;18(2):199-202.
7. Lu KC, Hsu YJ, Chiu JS, Hsu YD, Lin SH. Effects of potassium supplementation on the recovery of thyrotoxic periodic paralysis. Am J Emerg Med. 2004;22(7):544-547.
8. Tassone H, Moulin A, Henderson SO. The pitfalls of potassium replacement in thyrotoxic periodic paralysis: a case report and review of the literature. J Emerg Med. 2004;26(2):157-161. doi:10.1016/j.jemermed.2003.05.004.
Case
An Asian man in his third decade, with a medical history of hypertension and hyperlipidemia, and who had recently been involved in a motor vehicle collision (MVC), presented to the ED with a chief complaint of severe bilateral upper and lower extremity weakness. The patient noted that the weakness had begun the previous evening and became progressively worse throughout the night, to the point that he was unable to move any of his extremities on the morning of presentation.
Upon arrival at the ED, the patient was awake, alert, and oriented to self, time, and place; he also spoke in full sentences without distress. He denied fever, chills, difficulty breathing, or preceding viral illness. The patient stated that he was not taking any medications and denied a history of alcohol, tobacco, or drug abuse.
Initial vital signs at presentation were: blood pressure, 141/50 mm Hg; heart rate, 90 beats/min; respiratory rate, 16 breaths/min; and temperature, 97.4°F. Oxygen saturation was 100% on room air. On physical examination, the patient was in no acute distress and had a normal mental status. His pupils were normally reactive and his other cranial nerves were normal. Muscle strength in the upper and lower extremities was 1/5 with 1+ reflexes bilaterally, and there was no sensory deficit. The patient was placed on continuous cardiac monitoring with pulse oximetry.
What is the differential diagnosis for acute extremity weakness or paralysis?
The differential diagnosis for acute symmetrical extremity weakness or paralysis is broad and includes conditions of neurological, inflammatory, and toxic/metabolic etiologies.1 Neurological diagnoses to consider include acute stroke, specifically of the anterior cerebral or middle cerebral artery territories; Guillain-Barré syndrome; myasthenia gravis; spinal cord compression; and tick paralysis. Acute ischemic or hemorrhagic stroke most frequently presents with unilateral upper or lower extremity weakness accompanied by garbled speech and sensory deficits. Patients who have suffered a brainstem or cerebellar stroke commonly present with alterations of consciousness, visual changes, and ataxia. Posterior circulation strokes are also characterized by crossed neurological deficits, such as motor deficits on one side of the body and sensory deficits on the other.
Spinal Cord Pathology. Signs and symptoms of spinal cord compression or inflammation vary widely depending on the level affected. Motor and sensory findings of spinal cord pathology include muscle weakness, spasticity, hyper- or hyporeflexia, and a discrete level below which sensation is absent or reduced.
Guillain-Barré Syndrome. Patients who have Guillain-Barré syndrome (a disease of the myelin sheaths of the peripheral nerves) often present with complaints of numbness or paresthesias in the extremities.2 The condition is characterized by progressive symmetric muscle weakness accompanied by absent or depressed deep tendon reflexes and is typically associated with a recent exposure to an infectious agent such as a viral upper respiratory infection, bacterial infection, or vaccine.
Myasthenia Gravis. Myasthenia gravis is a disease of the neuromuscular junction. It presents with weakness in any muscle group, and the muscles are easily fatigued by repetitive use.
Toxic Exposures. Toxins, such as botulinum, ixovotoxin, nicotine, succinylcholine, and tetrodotoxin, are prominent, though less common, causes of muscular weakness or paralysis. Botulinum toxin acts at the neuromuscular junction. Patients with botulism typically present with a gastrointestinal prodrome of nausea, vomiting, and diarrhea followed by cranial nerve dysfunction and descending muscle weakness.3
Tetrodotoxin, nicotine, and curare-like paralytics act at the motor end plate of the neuromuscular junction to produce neuromuscular blockade with subsequent muscular weakness or paralysis. Similarly, ixovotoxin, the toxin responsible for tick paralysis, causes ascending flaccid paralysis by decreasing the release of acetylcholine at the neuromuscular junction.3
Metabolic and Endocrine Disorders. Conditions such as hypokalemia, hypomagnesemia, and periodic paralysis can also present with neurological complaints such as generalized weakness and paresthesias. Of note, it is important to differentiate true neuromuscular weakness from weakness secondary to limited effort.
Case Continuation
Because of the patient’s history of an MVC, cervical cord compression was considered concerning enough to require exclusion through magnetic resonance imaging (MRI) of the cervical spine. However, upon arrival at the MRI suite, the patient became severely tachypneic and tachycardic, and was unable to tolerate lying flat. He was intubated for impending respiratory failure. Laboratory results from blood drawn prior to transport to MRI were reported immediately after the resuscitation and were notable for the following: potassium, <1.5 mEq/L; bicarbonate, 20 mEq/L; creatine kinase, 889 U/L; ethanol, not detected.
What is hypokalemic periodic paralysis?
Hypokalemic periodic paralysis (HypoKPP) is a syndrome of episodic muscle weakness with concomitant hypokalemia. Familial forms of HypoKPP have been attributed to mutations in genes coding for either calcium or sodium channels.
The nonfamilial form of HypoKPP is attributed to hyperthyroidism and is most often seen in Asian men in the second and third decades of life. The disorder is characterized by acute onset hypokalemia and extremity paralysis with simultaneous hyperthyroid state. It is believed that hypokalemia occurs as a result of intracellular shift of potassium from thyroid-induced hormone sensitization of the Na+/K+-ATPase rather than a depletion of total body potassium. Acute episodes of paralysis are triggered by high-carbohydrate meals, alcohol consumption, emotional stress, and infection. Paralysis can last from 3 to 96 hours and is accompanied by decreased or absent deep tendon reflexes with normal sensation and mental status.
In the nonfamilial form of HypoKPP, signs of thyrotoxicosis are often present and include tachycardia, moist skin, and hyperthermia, but it may be difficult to specifically recognize this etiology given the patient’s grave clinical condition.4 Similar to many significant metabolic and electrolyte disturbances, complications of HypoKPP include dysrhythmia, respiratory failure, and sometimes death.5
How should HypoKPP be managed in the ED?
Management of HypoKPP begins with careful assessment of the patient’s airway, breathing, and circulation. Once the patient is stabilized, management of consequential effects of hypokalemia, such as respiratory distress and muscular paralysis, should focus on correcting the electrolyte and endocrine derangements.
Propranolol. If the patient exhibits signs of thyrotoxicosis, initial treatment includes propranolol, a nonselective beta-blocker, which both prevents the intracellular shift of potassium and assists in correcting the underlying hyperthyroid and hypermetabolic state. Although there is no standard propranolol dosing protocol for HypoKPP, some authors suggest that an aggressive dose of 2 mg intravenously (IV) every 10 minutes can shorten the patient’s episode of paralysis to 6 hours.6
Potassium Chloride. Administration of potassium chloride to raise the serum potassium to life-sustaining concentrations should be done cautiously through IV infusion of standard doses.7 In correcting hypokalemia with potassium, care should be taken to avoid overcorrection, which may subsequently result in rebound hyperkalemia as the total body potassium redistributes. Lower doses of potassium (ie, <50 mEq per dose), are preferred to achieve adequate repletion while avoiding rebound hyperkalemia.8
Case Conclusion
The results of thyroid studies that had been added on to the original set of laboratory studies revealed profound hyperthyroidism, with an essentially absent concentration of thyroid-stimulating hormone.
Case
An Asian man in his third decade, with a medical history of hypertension and hyperlipidemia, and who had recently been involved in a motor vehicle collision (MVC), presented to the ED with a chief complaint of severe bilateral upper and lower extremity weakness. The patient noted that the weakness had begun the previous evening and became progressively worse throughout the night, to the point that he was unable to move any of his extremities on the morning of presentation.
Upon arrival at the ED, the patient was awake, alert, and oriented to self, time, and place; he also spoke in full sentences without distress. He denied fever, chills, difficulty breathing, or preceding viral illness. The patient stated that he was not taking any medications and denied a history of alcohol, tobacco, or drug abuse.
Initial vital signs at presentation were: blood pressure, 141/50 mm Hg; heart rate, 90 beats/min; respiratory rate, 16 breaths/min; and temperature, 97.4°F. Oxygen saturation was 100% on room air. On physical examination, the patient was in no acute distress and had a normal mental status. His pupils were normally reactive and his other cranial nerves were normal. Muscle strength in the upper and lower extremities was 1/5 with 1+ reflexes bilaterally, and there was no sensory deficit. The patient was placed on continuous cardiac monitoring with pulse oximetry.
What is the differential diagnosis for acute extremity weakness or paralysis?
The differential diagnosis for acute symmetrical extremity weakness or paralysis is broad and includes conditions of neurological, inflammatory, and toxic/metabolic etiologies.1 Neurological diagnoses to consider include acute stroke, specifically of the anterior cerebral or middle cerebral artery territories; Guillain-Barré syndrome; myasthenia gravis; spinal cord compression; and tick paralysis. Acute ischemic or hemorrhagic stroke most frequently presents with unilateral upper or lower extremity weakness accompanied by garbled speech and sensory deficits. Patients who have suffered a brainstem or cerebellar stroke commonly present with alterations of consciousness, visual changes, and ataxia. Posterior circulation strokes are also characterized by crossed neurological deficits, such as motor deficits on one side of the body and sensory deficits on the other.
Spinal Cord Pathology. Signs and symptoms of spinal cord compression or inflammation vary widely depending on the level affected. Motor and sensory findings of spinal cord pathology include muscle weakness, spasticity, hyper- or hyporeflexia, and a discrete level below which sensation is absent or reduced.
Guillain-Barré Syndrome. Patients who have Guillain-Barré syndrome (a disease of the myelin sheaths of the peripheral nerves) often present with complaints of numbness or paresthesias in the extremities.2 The condition is characterized by progressive symmetric muscle weakness accompanied by absent or depressed deep tendon reflexes and is typically associated with a recent exposure to an infectious agent such as a viral upper respiratory infection, bacterial infection, or vaccine.
Myasthenia Gravis. Myasthenia gravis is a disease of the neuromuscular junction. It presents with weakness in any muscle group, and the muscles are easily fatigued by repetitive use.
Toxic Exposures. Toxins, such as botulinum, ixovotoxin, nicotine, succinylcholine, and tetrodotoxin, are prominent, though less common, causes of muscular weakness or paralysis. Botulinum toxin acts at the neuromuscular junction. Patients with botulism typically present with a gastrointestinal prodrome of nausea, vomiting, and diarrhea followed by cranial nerve dysfunction and descending muscle weakness.3
Tetrodotoxin, nicotine, and curare-like paralytics act at the motor end plate of the neuromuscular junction to produce neuromuscular blockade with subsequent muscular weakness or paralysis. Similarly, ixovotoxin, the toxin responsible for tick paralysis, causes ascending flaccid paralysis by decreasing the release of acetylcholine at the neuromuscular junction.3
Metabolic and Endocrine Disorders. Conditions such as hypokalemia, hypomagnesemia, and periodic paralysis can also present with neurological complaints such as generalized weakness and paresthesias. Of note, it is important to differentiate true neuromuscular weakness from weakness secondary to limited effort.
Case Continuation
Because of the patient’s history of an MVC, cervical cord compression was considered concerning enough to require exclusion through magnetic resonance imaging (MRI) of the cervical spine. However, upon arrival at the MRI suite, the patient became severely tachypneic and tachycardic, and was unable to tolerate lying flat. He was intubated for impending respiratory failure. Laboratory results from blood drawn prior to transport to MRI were reported immediately after the resuscitation and were notable for the following: potassium, <1.5 mEq/L; bicarbonate, 20 mEq/L; creatine kinase, 889 U/L; ethanol, not detected.
What is hypokalemic periodic paralysis?
Hypokalemic periodic paralysis (HypoKPP) is a syndrome of episodic muscle weakness with concomitant hypokalemia. Familial forms of HypoKPP have been attributed to mutations in genes coding for either calcium or sodium channels.
The nonfamilial form of HypoKPP is attributed to hyperthyroidism and is most often seen in Asian men in the second and third decades of life. The disorder is characterized by acute onset hypokalemia and extremity paralysis with simultaneous hyperthyroid state. It is believed that hypokalemia occurs as a result of intracellular shift of potassium from thyroid-induced hormone sensitization of the Na+/K+-ATPase rather than a depletion of total body potassium. Acute episodes of paralysis are triggered by high-carbohydrate meals, alcohol consumption, emotional stress, and infection. Paralysis can last from 3 to 96 hours and is accompanied by decreased or absent deep tendon reflexes with normal sensation and mental status.
In the nonfamilial form of HypoKPP, signs of thyrotoxicosis are often present and include tachycardia, moist skin, and hyperthermia, but it may be difficult to specifically recognize this etiology given the patient’s grave clinical condition.4 Similar to many significant metabolic and electrolyte disturbances, complications of HypoKPP include dysrhythmia, respiratory failure, and sometimes death.5
How should HypoKPP be managed in the ED?
Management of HypoKPP begins with careful assessment of the patient’s airway, breathing, and circulation. Once the patient is stabilized, management of consequential effects of hypokalemia, such as respiratory distress and muscular paralysis, should focus on correcting the electrolyte and endocrine derangements.
Propranolol. If the patient exhibits signs of thyrotoxicosis, initial treatment includes propranolol, a nonselective beta-blocker, which both prevents the intracellular shift of potassium and assists in correcting the underlying hyperthyroid and hypermetabolic state. Although there is no standard propranolol dosing protocol for HypoKPP, some authors suggest that an aggressive dose of 2 mg intravenously (IV) every 10 minutes can shorten the patient’s episode of paralysis to 6 hours.6
Potassium Chloride. Administration of potassium chloride to raise the serum potassium to life-sustaining concentrations should be done cautiously through IV infusion of standard doses.7 In correcting hypokalemia with potassium, care should be taken to avoid overcorrection, which may subsequently result in rebound hyperkalemia as the total body potassium redistributes. Lower doses of potassium (ie, <50 mEq per dose), are preferred to achieve adequate repletion while avoiding rebound hyperkalemia.8
Case Conclusion
The results of thyroid studies that had been added on to the original set of laboratory studies revealed profound hyperthyroidism, with an essentially absent concentration of thyroid-stimulating hormone.
1. Morchi RS. Weakness. In: Rosen P, ed. Rosen’s Emergency Medicine. 8th ed. Philadelphia, PA: Elsevier; 2014:124-128.
2. McGillicuddy DC, Walker O, Shapiro NI, Edlow JA. Guillain-Barré syndrome in the emergency department. Ann Emerg Med. 2006;47(4):390-393. doi:10.1016/j.annemergmed.2005.05.008.
3. Rao RB. Neurological principles. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, eds. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2015:315-323.
4. Lam L, Nair RJ, Tingle L. Thyrotoxic periodic paralysis. Proc (Bayl Univ Med Cent). 2006;19(2):126-129.
5. Li X, Yao S, Xiang Y, et al. The clinical and genetic features in a cohort of mainland Chinese patients with thyrotoxic periodic paralysis. BMC Neurol. 2015;15:38. doi:10.1186/s12883-015-0290-8.
6. Birkhahn RH, Gaeta TJ, Melniker L. Thyrotoxic periodic paralysis and intravenous propranolol in the emergency setting. J Emerg Med. 2000;18(2):199-202.
7. Lu KC, Hsu YJ, Chiu JS, Hsu YD, Lin SH. Effects of potassium supplementation on the recovery of thyrotoxic periodic paralysis. Am J Emerg Med. 2004;22(7):544-547.
8. Tassone H, Moulin A, Henderson SO. The pitfalls of potassium replacement in thyrotoxic periodic paralysis: a case report and review of the literature. J Emerg Med. 2004;26(2):157-161. doi:10.1016/j.jemermed.2003.05.004.
1. Morchi RS. Weakness. In: Rosen P, ed. Rosen’s Emergency Medicine. 8th ed. Philadelphia, PA: Elsevier; 2014:124-128.
2. McGillicuddy DC, Walker O, Shapiro NI, Edlow JA. Guillain-Barré syndrome in the emergency department. Ann Emerg Med. 2006;47(4):390-393. doi:10.1016/j.annemergmed.2005.05.008.
3. Rao RB. Neurological principles. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, eds. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2015:315-323.
4. Lam L, Nair RJ, Tingle L. Thyrotoxic periodic paralysis. Proc (Bayl Univ Med Cent). 2006;19(2):126-129.
5. Li X, Yao S, Xiang Y, et al. The clinical and genetic features in a cohort of mainland Chinese patients with thyrotoxic periodic paralysis. BMC Neurol. 2015;15:38. doi:10.1186/s12883-015-0290-8.
6. Birkhahn RH, Gaeta TJ, Melniker L. Thyrotoxic periodic paralysis and intravenous propranolol in the emergency setting. J Emerg Med. 2000;18(2):199-202.
7. Lu KC, Hsu YJ, Chiu JS, Hsu YD, Lin SH. Effects of potassium supplementation on the recovery of thyrotoxic periodic paralysis. Am J Emerg Med. 2004;22(7):544-547.
8. Tassone H, Moulin A, Henderson SO. The pitfalls of potassium replacement in thyrotoxic periodic paralysis: a case report and review of the literature. J Emerg Med. 2004;26(2):157-161. doi:10.1016/j.jemermed.2003.05.004.
Case Studies in Toxicology: Angioedema Post-tPA: Hemorrhage Is Not the Only Risk Factor
Case
A 49-year-old man with a history of hypertension, for which he was taking aspirin, carvedilol, hydralazine, and nifedipine, presented to the ED with complaints of left-sided weakness that started 3 hours before he came to the ED. Initial vital signs were: blood pressure, 158/90 mm Hg; heart rate, 74 beats/min; respiratory rate, 18 breaths/min; and temperature, 98°F. Oxygen saturation was 100% on room air, and a finger-stick glucose test was 106 mg/dL.
Physical examination revealed slowed speech with mild dysarthria, mild left facial droop, 2/5 strength in all muscle groups in the left upper and lower extremities, and decreased sensation to light touch on the left side. The patient also had left-sided sensory neglect and an abnormal gait, and dragged his left foot on the floor when walking. The rest of his examination was normal.
The stroke team was activated, and the patient was immediately transferred to the ED radiology department for imaging studies. A noncontrast head computed tomography (CT) was negative for any acute intracranial hemorrhage or cerebral edema. A CT angiogram (CTA) also was performed, which revealed atherosclerosis but no arterial occlusion. Based on these findings and the existing protocol, the patient received an intravenous (IV) bolus of tissue plasminogen activator (tPA). Approximately 17 minutes after tPA administration, the patient developed left-sided upper and lower lip swelling. There was no voice change, tongue swelling, or uvular deviation.
What is the differential diagnosis of swelling of the lip?
The differential diagnoses for lip swelling includes trauma, allergic reaction, and angioedema (hereditary, or angiotensin converting enzyme inhibitor [ACEI]-induced). The patient in this case denied any trauma to the lip, and no bleeding was noted from the lip; however, his entire left lip (upper and lower) was swollen. He was not taking any ACEIs or angiotensin-receptor blockers (ARBs). He also denied a family history of angioedema or any prior similar episodes. The patient further denied exposure to any new medications, foods, or other substances and had no respiratory distress, urticaria, or other findings consistent with an allergy.
What are the common adverse effects of tPA?
The only US Food and Drug-approved pharmacological treatment for ischemic stroke is tPA (also known as IV rtPA). Tissue plasminogen activator hydrolyzes plasminogen to plasmin, which exerts a fibrinolytic effect. Based on the ability of tPA to lyse thrombus, it is also a standard therapy for hemodynamically unstable patients with confirmed pulmonary embolism, as well as for patients with myocardial infarction in whom percutaneous intervention is contraindicated or unavailable. Despite the beneficial effects of tPA, significant adverse effects are associated with the drug. For example, thrombolysis may result in conversion of an ischemic stroke into a hemorrhagic event, resulting in generalized bleeding from mucosal surfaces.
The increase in plasmin may play a role in the development of angioedema by activating the kinin pathway, leading to the formation of the vasodilator bradykinin (Figure). Plasmin also activates the complement system and leads to the production of anaphylatoxins C3a, C4a, and C5a, which also cause mast cell degranulation and histamine release.1
When does post-tPA angioedema occur?
In the few published case reports available, tPA-induced angioedema was shown to typically occur in the stroke distribution (which was attributed to the left-sided swelling in this patient).2 Following tPA administration, the onset of angioedema reportedly varies from as early as 10 to 15 minutes from initiation until about 1 hour postinfusion. The short half-life of tPA (approximately 7 minutes)2 limits the outer- time window for the initial development of angioedema, but progression can continue well beyond this timeframe.
What is the treatment for tPA-induced angioedema?
The first priority of acute management of angioedema is discontinuation of the inciting substance, if possible—in this case, the tPA infusion.3 Assessment and maintenance of a patent airway are of utmost concern. Patients with posterior oropharyngeal effects or who are progressing should be admitted to an intensive care unit (ICU) for observation.4-6
Endotracheal Intubation. Providers should have a low threshold for endotracheal intubation, which should ideally be performed in any patient at risk for airway compromise.4 Due to the extensive airway swelling that can occur in the setting of angioedema, airway intervention should optimally be performed by an available clinician with the most skill and experience in this area. It is wise to be prepared to utilize advanced airway techniques, if available, including fiberoptic laryngoscopy or potentially cricothyrotomy.
Histamine Agonists. Standard therapy for patients who develop angioedema should include histamine antagonists, such as diphenhydramine (H1 antagonist) and famotidine (H2 antagonist) along with corticosteroids. Although these therapies are unlikely to be helpful in the treatment of tPA-induced angioedema, the difficulty in excluding allergic angioedema and the low risk of adverse effects associated with these medications support their use.
Fresh Frozen Plasma. Fresh frozen plasma (FFP) should be considered for patients who have a history of hereditary angioedema. Fresh frozen plasma contains enzymes that degrade bradykinin. Although FFP has been used successfully in the treatment of ACEI-induced angioedema, its use (or benefit) in tPA-related cases is not clear.
Icatibant. A selective bradykinin B2-receptor antagonist, icatibant has been used to treat patients with ACEI-induced angioedema because of its effects on bradykinin receptors. Comparison of the efficacy of icatibant to the prevailing treatment strategy of diphenhydramine, famotidine, and methylprednisolone found a shorter time to symptom relief with icatibant.7 However, icatibant is extremely expensive ($23,000/30 mg). As previously mentioned, based on its similar mechanism of action, lower cost, and safety profile, FFP can be given (off label) in this situation.
Case Conclusion
The patient was given diphenhydramine, famotidine, and methylprednisolone, but did not show any improvement. His upper/lower lip swelling continued to worsen, and 30 minutes after the onset of angioedema, he was unable to open his mouth more than 1 cm.
Multiple attempts to perform awake fiberoptic intubation failed due to inadequate sedation; however, intubation was successfully performed following light sedation. The patient self-extubated in the ICU on hospital day 3, and the angioedema had progressively decreased. Angioedema and weakness completely resolved by hospital day 4, and he was discharged home on hospital day 7.
1. Molinaro G, Gervais N, Adam A. Biochemical basis of angioedema associated with recombinant tissue plasminogen activator treatment: an in vitro experimental approach. Stroke. 2002;33(6):1712-1716.
2. Madden B, Chebl RB. Hemi orolingual angioedema after tPA administration for acute ischemic stroke. West J Emerg Med. 2015;16(1):175-177. doi:10.5811/westjem.2014.12.24210.
3. Hill MD, Lye T, Moss H, et al. Hemi-orolingual angioedema and ACE inhibition after alteplase treatment of stroke. Neurology. 2003;60(9):1525-1527.
4. Temiño VM, Peebles RS Jr. The spectrum and treatment of angioedema. Am J Med. 2008;121(4):282-286. doi:10.1016/j.amjmed.2007.09.024.
5. Hill MD, Barber PA, Takahashi J, Demchuk AM, Feasby TE, Buchan AM. Anaphylactoid reactions and angioedema during alteplase treatment of acute ischemic stroke. CMAJ. 2000;162(9):1281-1284.
6. Maertins M, Wold R, Swider M. Angioedema after administration of tPA for ischemic stroke: case report. Air Med J. 2011;30(5):276-278. doi:10.1016/j.amj.2010.12.011.
7. Baş M, Greve J, Stelter K, et al. A randomized trial of icatibant in ACE-inhibitor-induced angioedema. N Engl J Med. 2015;372(5):418-425. doi:10.1056/NEJMoa1312524.
Case
A 49-year-old man with a history of hypertension, for which he was taking aspirin, carvedilol, hydralazine, and nifedipine, presented to the ED with complaints of left-sided weakness that started 3 hours before he came to the ED. Initial vital signs were: blood pressure, 158/90 mm Hg; heart rate, 74 beats/min; respiratory rate, 18 breaths/min; and temperature, 98°F. Oxygen saturation was 100% on room air, and a finger-stick glucose test was 106 mg/dL.
Physical examination revealed slowed speech with mild dysarthria, mild left facial droop, 2/5 strength in all muscle groups in the left upper and lower extremities, and decreased sensation to light touch on the left side. The patient also had left-sided sensory neglect and an abnormal gait, and dragged his left foot on the floor when walking. The rest of his examination was normal.
The stroke team was activated, and the patient was immediately transferred to the ED radiology department for imaging studies. A noncontrast head computed tomography (CT) was negative for any acute intracranial hemorrhage or cerebral edema. A CT angiogram (CTA) also was performed, which revealed atherosclerosis but no arterial occlusion. Based on these findings and the existing protocol, the patient received an intravenous (IV) bolus of tissue plasminogen activator (tPA). Approximately 17 minutes after tPA administration, the patient developed left-sided upper and lower lip swelling. There was no voice change, tongue swelling, or uvular deviation.
What is the differential diagnosis of swelling of the lip?
The differential diagnoses for lip swelling includes trauma, allergic reaction, and angioedema (hereditary, or angiotensin converting enzyme inhibitor [ACEI]-induced). The patient in this case denied any trauma to the lip, and no bleeding was noted from the lip; however, his entire left lip (upper and lower) was swollen. He was not taking any ACEIs or angiotensin-receptor blockers (ARBs). He also denied a family history of angioedema or any prior similar episodes. The patient further denied exposure to any new medications, foods, or other substances and had no respiratory distress, urticaria, or other findings consistent with an allergy.
What are the common adverse effects of tPA?
The only US Food and Drug-approved pharmacological treatment for ischemic stroke is tPA (also known as IV rtPA). Tissue plasminogen activator hydrolyzes plasminogen to plasmin, which exerts a fibrinolytic effect. Based on the ability of tPA to lyse thrombus, it is also a standard therapy for hemodynamically unstable patients with confirmed pulmonary embolism, as well as for patients with myocardial infarction in whom percutaneous intervention is contraindicated or unavailable. Despite the beneficial effects of tPA, significant adverse effects are associated with the drug. For example, thrombolysis may result in conversion of an ischemic stroke into a hemorrhagic event, resulting in generalized bleeding from mucosal surfaces.
The increase in plasmin may play a role in the development of angioedema by activating the kinin pathway, leading to the formation of the vasodilator bradykinin (Figure). Plasmin also activates the complement system and leads to the production of anaphylatoxins C3a, C4a, and C5a, which also cause mast cell degranulation and histamine release.1
When does post-tPA angioedema occur?
In the few published case reports available, tPA-induced angioedema was shown to typically occur in the stroke distribution (which was attributed to the left-sided swelling in this patient).2 Following tPA administration, the onset of angioedema reportedly varies from as early as 10 to 15 minutes from initiation until about 1 hour postinfusion. The short half-life of tPA (approximately 7 minutes)2 limits the outer- time window for the initial development of angioedema, but progression can continue well beyond this timeframe.
What is the treatment for tPA-induced angioedema?
The first priority of acute management of angioedema is discontinuation of the inciting substance, if possible—in this case, the tPA infusion.3 Assessment and maintenance of a patent airway are of utmost concern. Patients with posterior oropharyngeal effects or who are progressing should be admitted to an intensive care unit (ICU) for observation.4-6
Endotracheal Intubation. Providers should have a low threshold for endotracheal intubation, which should ideally be performed in any patient at risk for airway compromise.4 Due to the extensive airway swelling that can occur in the setting of angioedema, airway intervention should optimally be performed by an available clinician with the most skill and experience in this area. It is wise to be prepared to utilize advanced airway techniques, if available, including fiberoptic laryngoscopy or potentially cricothyrotomy.
Histamine Agonists. Standard therapy for patients who develop angioedema should include histamine antagonists, such as diphenhydramine (H1 antagonist) and famotidine (H2 antagonist) along with corticosteroids. Although these therapies are unlikely to be helpful in the treatment of tPA-induced angioedema, the difficulty in excluding allergic angioedema and the low risk of adverse effects associated with these medications support their use.
Fresh Frozen Plasma. Fresh frozen plasma (FFP) should be considered for patients who have a history of hereditary angioedema. Fresh frozen plasma contains enzymes that degrade bradykinin. Although FFP has been used successfully in the treatment of ACEI-induced angioedema, its use (or benefit) in tPA-related cases is not clear.
Icatibant. A selective bradykinin B2-receptor antagonist, icatibant has been used to treat patients with ACEI-induced angioedema because of its effects on bradykinin receptors. Comparison of the efficacy of icatibant to the prevailing treatment strategy of diphenhydramine, famotidine, and methylprednisolone found a shorter time to symptom relief with icatibant.7 However, icatibant is extremely expensive ($23,000/30 mg). As previously mentioned, based on its similar mechanism of action, lower cost, and safety profile, FFP can be given (off label) in this situation.
Case Conclusion
The patient was given diphenhydramine, famotidine, and methylprednisolone, but did not show any improvement. His upper/lower lip swelling continued to worsen, and 30 minutes after the onset of angioedema, he was unable to open his mouth more than 1 cm.
Multiple attempts to perform awake fiberoptic intubation failed due to inadequate sedation; however, intubation was successfully performed following light sedation. The patient self-extubated in the ICU on hospital day 3, and the angioedema had progressively decreased. Angioedema and weakness completely resolved by hospital day 4, and he was discharged home on hospital day 7.
Case
A 49-year-old man with a history of hypertension, for which he was taking aspirin, carvedilol, hydralazine, and nifedipine, presented to the ED with complaints of left-sided weakness that started 3 hours before he came to the ED. Initial vital signs were: blood pressure, 158/90 mm Hg; heart rate, 74 beats/min; respiratory rate, 18 breaths/min; and temperature, 98°F. Oxygen saturation was 100% on room air, and a finger-stick glucose test was 106 mg/dL.
Physical examination revealed slowed speech with mild dysarthria, mild left facial droop, 2/5 strength in all muscle groups in the left upper and lower extremities, and decreased sensation to light touch on the left side. The patient also had left-sided sensory neglect and an abnormal gait, and dragged his left foot on the floor when walking. The rest of his examination was normal.
The stroke team was activated, and the patient was immediately transferred to the ED radiology department for imaging studies. A noncontrast head computed tomography (CT) was negative for any acute intracranial hemorrhage or cerebral edema. A CT angiogram (CTA) also was performed, which revealed atherosclerosis but no arterial occlusion. Based on these findings and the existing protocol, the patient received an intravenous (IV) bolus of tissue plasminogen activator (tPA). Approximately 17 minutes after tPA administration, the patient developed left-sided upper and lower lip swelling. There was no voice change, tongue swelling, or uvular deviation.
What is the differential diagnosis of swelling of the lip?
The differential diagnoses for lip swelling includes trauma, allergic reaction, and angioedema (hereditary, or angiotensin converting enzyme inhibitor [ACEI]-induced). The patient in this case denied any trauma to the lip, and no bleeding was noted from the lip; however, his entire left lip (upper and lower) was swollen. He was not taking any ACEIs or angiotensin-receptor blockers (ARBs). He also denied a family history of angioedema or any prior similar episodes. The patient further denied exposure to any new medications, foods, or other substances and had no respiratory distress, urticaria, or other findings consistent with an allergy.
What are the common adverse effects of tPA?
The only US Food and Drug-approved pharmacological treatment for ischemic stroke is tPA (also known as IV rtPA). Tissue plasminogen activator hydrolyzes plasminogen to plasmin, which exerts a fibrinolytic effect. Based on the ability of tPA to lyse thrombus, it is also a standard therapy for hemodynamically unstable patients with confirmed pulmonary embolism, as well as for patients with myocardial infarction in whom percutaneous intervention is contraindicated or unavailable. Despite the beneficial effects of tPA, significant adverse effects are associated with the drug. For example, thrombolysis may result in conversion of an ischemic stroke into a hemorrhagic event, resulting in generalized bleeding from mucosal surfaces.
The increase in plasmin may play a role in the development of angioedema by activating the kinin pathway, leading to the formation of the vasodilator bradykinin (Figure). Plasmin also activates the complement system and leads to the production of anaphylatoxins C3a, C4a, and C5a, which also cause mast cell degranulation and histamine release.1
When does post-tPA angioedema occur?
In the few published case reports available, tPA-induced angioedema was shown to typically occur in the stroke distribution (which was attributed to the left-sided swelling in this patient).2 Following tPA administration, the onset of angioedema reportedly varies from as early as 10 to 15 minutes from initiation until about 1 hour postinfusion. The short half-life of tPA (approximately 7 minutes)2 limits the outer- time window for the initial development of angioedema, but progression can continue well beyond this timeframe.
What is the treatment for tPA-induced angioedema?
The first priority of acute management of angioedema is discontinuation of the inciting substance, if possible—in this case, the tPA infusion.3 Assessment and maintenance of a patent airway are of utmost concern. Patients with posterior oropharyngeal effects or who are progressing should be admitted to an intensive care unit (ICU) for observation.4-6
Endotracheal Intubation. Providers should have a low threshold for endotracheal intubation, which should ideally be performed in any patient at risk for airway compromise.4 Due to the extensive airway swelling that can occur in the setting of angioedema, airway intervention should optimally be performed by an available clinician with the most skill and experience in this area. It is wise to be prepared to utilize advanced airway techniques, if available, including fiberoptic laryngoscopy or potentially cricothyrotomy.
Histamine Agonists. Standard therapy for patients who develop angioedema should include histamine antagonists, such as diphenhydramine (H1 antagonist) and famotidine (H2 antagonist) along with corticosteroids. Although these therapies are unlikely to be helpful in the treatment of tPA-induced angioedema, the difficulty in excluding allergic angioedema and the low risk of adverse effects associated with these medications support their use.
Fresh Frozen Plasma. Fresh frozen plasma (FFP) should be considered for patients who have a history of hereditary angioedema. Fresh frozen plasma contains enzymes that degrade bradykinin. Although FFP has been used successfully in the treatment of ACEI-induced angioedema, its use (or benefit) in tPA-related cases is not clear.
Icatibant. A selective bradykinin B2-receptor antagonist, icatibant has been used to treat patients with ACEI-induced angioedema because of its effects on bradykinin receptors. Comparison of the efficacy of icatibant to the prevailing treatment strategy of diphenhydramine, famotidine, and methylprednisolone found a shorter time to symptom relief with icatibant.7 However, icatibant is extremely expensive ($23,000/30 mg). As previously mentioned, based on its similar mechanism of action, lower cost, and safety profile, FFP can be given (off label) in this situation.
Case Conclusion
The patient was given diphenhydramine, famotidine, and methylprednisolone, but did not show any improvement. His upper/lower lip swelling continued to worsen, and 30 minutes after the onset of angioedema, he was unable to open his mouth more than 1 cm.
Multiple attempts to perform awake fiberoptic intubation failed due to inadequate sedation; however, intubation was successfully performed following light sedation. The patient self-extubated in the ICU on hospital day 3, and the angioedema had progressively decreased. Angioedema and weakness completely resolved by hospital day 4, and he was discharged home on hospital day 7.
1. Molinaro G, Gervais N, Adam A. Biochemical basis of angioedema associated with recombinant tissue plasminogen activator treatment: an in vitro experimental approach. Stroke. 2002;33(6):1712-1716.
2. Madden B, Chebl RB. Hemi orolingual angioedema after tPA administration for acute ischemic stroke. West J Emerg Med. 2015;16(1):175-177. doi:10.5811/westjem.2014.12.24210.
3. Hill MD, Lye T, Moss H, et al. Hemi-orolingual angioedema and ACE inhibition after alteplase treatment of stroke. Neurology. 2003;60(9):1525-1527.
4. Temiño VM, Peebles RS Jr. The spectrum and treatment of angioedema. Am J Med. 2008;121(4):282-286. doi:10.1016/j.amjmed.2007.09.024.
5. Hill MD, Barber PA, Takahashi J, Demchuk AM, Feasby TE, Buchan AM. Anaphylactoid reactions and angioedema during alteplase treatment of acute ischemic stroke. CMAJ. 2000;162(9):1281-1284.
6. Maertins M, Wold R, Swider M. Angioedema after administration of tPA for ischemic stroke: case report. Air Med J. 2011;30(5):276-278. doi:10.1016/j.amj.2010.12.011.
7. Baş M, Greve J, Stelter K, et al. A randomized trial of icatibant in ACE-inhibitor-induced angioedema. N Engl J Med. 2015;372(5):418-425. doi:10.1056/NEJMoa1312524.
1. Molinaro G, Gervais N, Adam A. Biochemical basis of angioedema associated with recombinant tissue plasminogen activator treatment: an in vitro experimental approach. Stroke. 2002;33(6):1712-1716.
2. Madden B, Chebl RB. Hemi orolingual angioedema after tPA administration for acute ischemic stroke. West J Emerg Med. 2015;16(1):175-177. doi:10.5811/westjem.2014.12.24210.
3. Hill MD, Lye T, Moss H, et al. Hemi-orolingual angioedema and ACE inhibition after alteplase treatment of stroke. Neurology. 2003;60(9):1525-1527.
4. Temiño VM, Peebles RS Jr. The spectrum and treatment of angioedema. Am J Med. 2008;121(4):282-286. doi:10.1016/j.amjmed.2007.09.024.
5. Hill MD, Barber PA, Takahashi J, Demchuk AM, Feasby TE, Buchan AM. Anaphylactoid reactions and angioedema during alteplase treatment of acute ischemic stroke. CMAJ. 2000;162(9):1281-1284.
6. Maertins M, Wold R, Swider M. Angioedema after administration of tPA for ischemic stroke: case report. Air Med J. 2011;30(5):276-278. doi:10.1016/j.amj.2010.12.011.
7. Baş M, Greve J, Stelter K, et al. A randomized trial of icatibant in ACE-inhibitor-induced angioedema. N Engl J Med. 2015;372(5):418-425. doi:10.1056/NEJMoa1312524.
Case Studies in Toxicology: Drink the Water, but Don’t Eat the Paint
Case
A 2-year-old boy and his mother were referred to the ED by the child’s pediatrician after a routine venous blood lead level (BLL) taken at the boy’s recent well visit revealed an elevated lead level of 52 mcg/dL (normal range, <5 mcg/dL). The child’s mother reported that although her son had always been a picky eater, he had recently been complaining of abdominal pain.
The patient’s well-child visits had been normal until his recent 2-year checkup, at which time his pediatrician noticed some speech delay. On further history taking, the emergency physician (EP) learned the patient and his mother resided in an older home (built in the 1950s) that was in disrepair. The mother asked the EP if the elevation in the child’s BLL could be due to the drinking water in their town.
What are the most likely sources of environmental lead exposure?
In 2016, the topic of lead poisoning grabbed national attention when a pediatrician in Flint, Michigan detected an abrupt doubling of the number of children with elevated lead levels in her practice.1 Upon further investigation, it was discovered that these kids had one thing in common: the source of their drinking water. The City of Flint had recently switched the source of its potable water from Lake Huron to the Flint River. The lower quality water, which was not properly treated with an anticorrosive agent such as orthophosphate, led to widespread pipe corrosion and lead contamination. This finding resulted in a cascade of water testing by other municipalities and school systems, many of which identified lead concentrations above the currently accepted drinking water standard of 15 parts per billion (ppb).
Thousands of children each year are identified to have elevated BLLs, based on the Centers for Disease Control and Prevention definition of a “level of concern” as more than 5 mcg/dL.2 The majority of these exposures stem from environmental exposure to lead paint dust in the home, but drinking water normally contributes as a low-level, constant, “basal” exposure. While lead-contaminated drinking water is not acceptable, it is unlikely to generate many ED visits. However, there are a variety of other lead sources that may prompt children to present to the ED with acute or subacute lead poisoning.
Lead is a heavy metal whose physical properties indicate its common uses. It provides durability and opacity to pigments, which is why it is found in oil paint, house paint used before 1976, and on paint for large outdoor structures, where it is still used. Lead is also found in the pigments used in cosmetics, stained glass, and painted pottery, and as an adulterant in highly colored foodstuffs such as imported turmeric.3
The physicochemical characteristics of lead make it an ideal component of solder. Many plumbing pipes in use today are not lead, but join one another using lead solder at the joints, sites that are vulnerable to corrosion. The heavy molecular weight of lead makes it a useful component of bullets and munitions.
Tetraethyl lead was used as an “anti-knock” agent to smooth out the combustion of heterogenous compounds in automotive fuel before it was removed in the mid-1970s.4 Prior to its removal, leaded gasoline was the largest source of air, soil, and groundwater contamination leading to environmental exposures.4 At present, the most common source of environmental lead exposure among young children is through peeling paint in deteriorating residential buildings. Hazardous occupational lead exposures arise from work involving munitions, reclamation and salvage, painting, welding, and numerous other settings—particularly sites where industrial hygiene is suboptimal. Lead from these sites can be inadvertently transported home on clothing or shoes, raising the exposure risk for children in the household.4
What are the health effects of lead exposure?
Like most heavy metals, lead is toxic to many organ systems in the body. The signs and symptoms of lead poisoning vary depending on the patient’s BLL and age (Table 1).5 The most common clinical effect of lead in the adult population is hypertension.6 Additional renal effects include a Fanconi-type syndrome with glycosuria and proteinuria. Lead can cause a peripheral neuropathy that is predominantly motor, classically causing foot or wrist drop. Abdominal pain from lead exposure is sometimes termed “lead colic” due to its intermittent and often severe nature. Abnormalities in urate metabolism cause a gouty arthritis referred to as “saturnine gout.” 6
The young pediatric central nervous system (CNS) is much more vulnerable to the effects of lead than the adult CNS. Even low-level lead exposure to the developing brain causes deficits in intelligence quotient, attention, impulse control, and other neurocognitive functions that are largely irreversible.7
Children with an elevated BLL may also develop constipation, anorexia, pallor, and pica.8 The development of geophagia (subtype of pica in which one craves and ingests nonfood clay or soil-like materials), represents a “chicken-or-egg” phenomena as it both causes and results from lead poisoning.
Lead impairs multiple steps of the heme synthesis pathway, causing microcytic anemia with basophilic stippling. Lead-induced anemia exacerbates pica as anemic patients are more likely to eat leaded paint chips and other lead-containing materials such as pottery.8 Of note, leaded white paint is reported to have a pleasant taste due to the sweet-tasting lead acetate used as a pigment.
The most dramatic and consequential manifestation of lead poisoning is lead encephalopathy. This can occur at any age, but manifests in children at much lower BLLs than in adults. Patients can be altered or obtunded, have convulsive activity, and may develop cerebral edema. Encephalopathy is a life-threatening emergency and must be recognized and treated immediately. Lead encephalopathy should be suspected in any young child with hand-to-mouth behavior who has any of the above environmental risk factors.4 The findings of anemia or the other diagnostic signs described below are too unreliable and take too long to be truly helpful in making the diagnosis.
How is the diagnosis of lead poisoning made?
The gold standard for the diagnosis of lead poisoning is the measurement of BLL. However, the turnaround time for this test is usually at least 24 hours, but may take up to several days. As such, adjunctive testing can accelerate obtaining a diagnosis. A complete blood count (CBC) to evaluate for microcytic anemia may demonstrate a characteristic pattern of basophilic stippling.9 A protoporphyrin level—either a free erythrocyte protoporphyrin (FEP) or a zinc protoporphyrin level—will be elevated, a result of heme synthesis disruption.9 Urinalysis may demonstrate glycosuria or proteinuria.6 Hypertension is often present, even in pediatric patients.
An abdominal radiograph is essential in children to determine whether a lead foreign body, such as a paint chip, is present in the intestinal lumen. Long bone films may demonstrate “lead lines” at the metaphysis, which in fact do not reflect lead itself but abnormal calcium deposition in growing bone due to lead’s interference with bone remodeling. A computed tomography (CT) scan of the brain in patients with encephalopathy will often demonstrate cerebral edema.6
Of note, capillary BLLs taken via finger-stick can be falsely elevated due contamination during collection (eg, the presence of lead dust on the skin). However, this screening method is often used by clinicians in the pediatric primary care setting because of its feasibility. Elevated BLLs from capillary testing should always be followed by a BLL obtained by venipuncture.2
Case Continuation
The patient’s mother was counseled on sources of lead contamination. She was informed that although drinking water may contribute some amount to an elevated BLL, the most likely source of contamination is still lead paint found in older homes such as the one in which she and her son resided.
Diagnostic studies to support the diagnosis of lead poisoning were performed. A CBC revealed a hemoglobin of 9.8 g/dL with a mean corpuscular volume of 68 fL. A microscopic smear of blood demonstrated basophilic stippling of red blood cells. An FEP level was 386 mcg/dL. An abdominal radiograph demonstrated small radiopacities throughout the large intestine, without obstruction, which was suggestive of ingested lead paint chips.
What is the best management approach to patients with suspected lead poisoning?
The first-line treatment for patients with lead poisoning is removal from the exposure source, which first and foremost requires identification of the hazard through careful history taking and scene investigation by the local health department. This will avoid recurrent visits following successful chelation for repeat exposure to an unidentified source. Relocation to another dwelling will often be required for patients with presumed exposure until the hazard can be identified and abated.
Patients who have ingested or have embedded leaded foreign bodies will require removal via whole bowel irrigation or surgical means.
Following decontamination, chelation is required for children with a BLL more than 45 mcg/dL, and adults with CNS symptomatology and a BLL more than 70 mcg/dL. Table 2 provides guidelines for chelation therapy based on BLL.5
There are three chelating agents commonly used to reduce the body lead burden (Table 2).5 The most common, owing largely to it being the only agent used orally, is succimer (or dimercaptosuccinic acid, DMSA). The second agent is calcium disodium edetate (CaNa2EDTA), which is given intravenously. In patients with encephalopathy, EDTA should be given after the first dose of the third agent, British anti-Lewisite (BAL; 2,3-dimercaptopropanol), in order to prevent redistribution of lead from the peripheral compartment into the CNS.10 However, BAL is the most difficult of the three agents to administer as it is suspended in peanut oil and is given via intramuscular injection every 4 hours.
Unfortunately, while chelation therapy is highly beneficial for patients with severe lead poisoning, it has not been demonstrated to positively impact children who already have developed neurocognitive sequelae associated with lower level lead exposure.11 This highlights the importance of prevention.
Work-up and Management in the ED
The patient with lead poisoning, while an unusual presentation in the ED, requires specialized management to minimize sequelae of exposure. Careful attention to history is vital. When in doubt, the EP should consult with her or his regional poison control center (800-222-1222) or with a medical toxicologist or other expert.
There are several scenarios in which a patient may present to the ED with lead toxicity. The following scenarios, along with their respective clinical approach strategies, represent three of the most common presentations.
Scenario 1: The Pediatric Patient With Elevated Venous Blood Lead Levels
The EP should employ the following clinical approach when evaluating and managing the pediatric patient with normal mental status whose routine screening reveals a BLL sufficiently elevated to warrant evaluation or admission—perhaps to discontinue exposure or initiate chelation therapy.
- Obtain a history, including possible lead sources; perform a complete physical examination; and obtain a repeat BLL, CBC with microscopic examination, and renal function test.
- Obtain an abdominal film to look for radiopacities, including paint chips or larger ingested foreign bodies.
- If radiopaque foreign bodies are present on abdominal radiograph, whole bowel irrigation with polyethylene glycol solution given via a nasogastric tube at 250 to 500 cc/h for a pediatric patient (1 to 2 L/h for adult patients) should be given until no residual foreign bodies remain.
- Obtain a radiograph of the long bone, which may demonstrate metaphyseal enhancement in the pediatric patient, suggesting long-term exposure.
- Ensure local or state health departments are contacted to arrange for environmental inspection of the home. This is essential prior to discharge to the home environment.
- Begin chelation therapy according to the BLL (Table 2).
Scenario 2: Adult Patients Presenting With Signs and Symptoms of Lead Toxicity
The adult patient who presents to the ED with complaints suggestive of lead poisoning and whose history is indicative of lead exposure should be evaluated and managed as follows:
- Obtain a thorough history, including the occupation and hobbies of the patient and all family members.
- Obtain vital signs to evaluate for hypertension; repeat BLL, CBC with smear, and serum creatinine test. Perform a physical examination to evaluate for lead lines.
- Obtain radiographic images, which may demonstrate a leaded foreign body, such as in the patient with prior history of gunshot wounds.
- If the BLL is sufficiently elevated or clinical findings are sufficiently severe, admit for chelation.
Scenario 3: The Pediatric or Adult Patient Presenting With Altered Mental Status
The patient presenting with altered mental status of unclear etiology—regardless of age—and in whom lead encephalopathy is a possible cause, should be worked-up and managed as follows:
- Obtain BLL, CBC, FEP levels. Consider radiographic imaging to assess for ingested or embedded foreign bodies.
- If abnormalities in the above laboratory studies are consistent with lead poisoning, initiate chelation immediately—prior to receiving repeat BLL result.
- Obtain a CT scan of the head to assess for cerebral edema.
- Provide supportive care for encephalopathy, including airway control and management of increased intracranial pressure.
Case Conclusion
The patient was admitted to the hospital for whole bowel irrigation and chelation therapy with succimer. The local health department conducted an investigation of the home and found multiple areas of peeling lead paint and lead dust, and ordered remediation of the property before it could be re-occupied by the family. A test of the home’s drinking water found no elevation above the 15 ppb standard.
The patient was discharged from the hospital in the care of his mother. They were relocated to a lead-free home, with follow-up by the pediatrician for ongoing monitoring of the BLL and developmental milestones.
1. Hanna-Attisha M, LaChance J, Sadler RC, Champney Schnepp A. Elevated blood lead levels in children associated with the flint drinking water crisis: A spatial analysis of risk and public health response. Am J Public Health. 2016;106(2):283-290. doi:0.2105/AJPH.2015.303003.
2. Centers for Disease Control and Prevention Advisory Committee on Childhood Lead Poisoning Prevention. Low level lead exposure harms children: a renewed call for primary prevention. January 4, 2012. Available at https://www.cdc.gov/nceh/lead/acclpp/final_document_030712.pdf. Accessed February 27, 2017.
3. Food and Drug Administration. Spices USA Inc. issues alert on elevated levels of lead in ground turmeric. http://www.fda.gov/safety/recalls/ucm523561.htm, September 26, 2016. Accessed February 27, 2017.
4. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Toxic substances portal: lead. US Department of Health and Human Services Web site. Available at https://www.atsdr.cdc.gov/ToxProfiles/TP.asp?id=96&tid=22. Updated January 21, 2015. Accessed February 27, 2017.
5. Calello DP, Henretig FM. Lead. In: Goldfrank LG, Flomenbaum NE, Lewin NA, Howland MA, Hoffman RS, Nelson LS (eds.). Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2014:1219-1234.
6. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Environmental health and medicine education: lead toxicity. https://www.atsdr.cdc.gov/csem/csem.asp?csem=7&po=10. Updated August 26, 2016. Accessed February 27, 2017.
7. Canfield RL, Henderson Jr CR, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. New Engl J Med. 2003;348:1517-1526.
8. Kathuria P, Rowden AK. Lead toxicity. Medscape Web site. Available at http://emedicine.medscape.com/article/1174752-clinical. Updated January 31, 2017. Accessed February 27, 2017.
9. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Environmental health and medicine education. Lead toxicity: what tests can assist with diagnosis of lead toxicity? https://www.atsdr.cdc.gov/csem/csem.asp?csem=7&po=12. Updated August 25, 2016. Accessed February 27, 2017.
10. Chisholm JJ Jr. The use of chelating agents in the treatment of acute and chronic lead intoxication in childhood. J Pediatr. 1968;73(1):1-38.
11. Rogan WJ, Dietrich KN, Ware JH, et al; Treatment of Lead-Exposed Children Trial Group. The effect of chelation therapy with succimer on neuropsychological development in children exposed to lead. N Engl J Med. 2001;344(19):1421-1426.
Case
A 2-year-old boy and his mother were referred to the ED by the child’s pediatrician after a routine venous blood lead level (BLL) taken at the boy’s recent well visit revealed an elevated lead level of 52 mcg/dL (normal range, <5 mcg/dL). The child’s mother reported that although her son had always been a picky eater, he had recently been complaining of abdominal pain.
The patient’s well-child visits had been normal until his recent 2-year checkup, at which time his pediatrician noticed some speech delay. On further history taking, the emergency physician (EP) learned the patient and his mother resided in an older home (built in the 1950s) that was in disrepair. The mother asked the EP if the elevation in the child’s BLL could be due to the drinking water in their town.
What are the most likely sources of environmental lead exposure?
In 2016, the topic of lead poisoning grabbed national attention when a pediatrician in Flint, Michigan detected an abrupt doubling of the number of children with elevated lead levels in her practice.1 Upon further investigation, it was discovered that these kids had one thing in common: the source of their drinking water. The City of Flint had recently switched the source of its potable water from Lake Huron to the Flint River. The lower quality water, which was not properly treated with an anticorrosive agent such as orthophosphate, led to widespread pipe corrosion and lead contamination. This finding resulted in a cascade of water testing by other municipalities and school systems, many of which identified lead concentrations above the currently accepted drinking water standard of 15 parts per billion (ppb).
Thousands of children each year are identified to have elevated BLLs, based on the Centers for Disease Control and Prevention definition of a “level of concern” as more than 5 mcg/dL.2 The majority of these exposures stem from environmental exposure to lead paint dust in the home, but drinking water normally contributes as a low-level, constant, “basal” exposure. While lead-contaminated drinking water is not acceptable, it is unlikely to generate many ED visits. However, there are a variety of other lead sources that may prompt children to present to the ED with acute or subacute lead poisoning.
Lead is a heavy metal whose physical properties indicate its common uses. It provides durability and opacity to pigments, which is why it is found in oil paint, house paint used before 1976, and on paint for large outdoor structures, where it is still used. Lead is also found in the pigments used in cosmetics, stained glass, and painted pottery, and as an adulterant in highly colored foodstuffs such as imported turmeric.3
The physicochemical characteristics of lead make it an ideal component of solder. Many plumbing pipes in use today are not lead, but join one another using lead solder at the joints, sites that are vulnerable to corrosion. The heavy molecular weight of lead makes it a useful component of bullets and munitions.
Tetraethyl lead was used as an “anti-knock” agent to smooth out the combustion of heterogenous compounds in automotive fuel before it was removed in the mid-1970s.4 Prior to its removal, leaded gasoline was the largest source of air, soil, and groundwater contamination leading to environmental exposures.4 At present, the most common source of environmental lead exposure among young children is through peeling paint in deteriorating residential buildings. Hazardous occupational lead exposures arise from work involving munitions, reclamation and salvage, painting, welding, and numerous other settings—particularly sites where industrial hygiene is suboptimal. Lead from these sites can be inadvertently transported home on clothing or shoes, raising the exposure risk for children in the household.4
What are the health effects of lead exposure?
Like most heavy metals, lead is toxic to many organ systems in the body. The signs and symptoms of lead poisoning vary depending on the patient’s BLL and age (Table 1).5 The most common clinical effect of lead in the adult population is hypertension.6 Additional renal effects include a Fanconi-type syndrome with glycosuria and proteinuria. Lead can cause a peripheral neuropathy that is predominantly motor, classically causing foot or wrist drop. Abdominal pain from lead exposure is sometimes termed “lead colic” due to its intermittent and often severe nature. Abnormalities in urate metabolism cause a gouty arthritis referred to as “saturnine gout.” 6
The young pediatric central nervous system (CNS) is much more vulnerable to the effects of lead than the adult CNS. Even low-level lead exposure to the developing brain causes deficits in intelligence quotient, attention, impulse control, and other neurocognitive functions that are largely irreversible.7
Children with an elevated BLL may also develop constipation, anorexia, pallor, and pica.8 The development of geophagia (subtype of pica in which one craves and ingests nonfood clay or soil-like materials), represents a “chicken-or-egg” phenomena as it both causes and results from lead poisoning.
Lead impairs multiple steps of the heme synthesis pathway, causing microcytic anemia with basophilic stippling. Lead-induced anemia exacerbates pica as anemic patients are more likely to eat leaded paint chips and other lead-containing materials such as pottery.8 Of note, leaded white paint is reported to have a pleasant taste due to the sweet-tasting lead acetate used as a pigment.
The most dramatic and consequential manifestation of lead poisoning is lead encephalopathy. This can occur at any age, but manifests in children at much lower BLLs than in adults. Patients can be altered or obtunded, have convulsive activity, and may develop cerebral edema. Encephalopathy is a life-threatening emergency and must be recognized and treated immediately. Lead encephalopathy should be suspected in any young child with hand-to-mouth behavior who has any of the above environmental risk factors.4 The findings of anemia or the other diagnostic signs described below are too unreliable and take too long to be truly helpful in making the diagnosis.
How is the diagnosis of lead poisoning made?
The gold standard for the diagnosis of lead poisoning is the measurement of BLL. However, the turnaround time for this test is usually at least 24 hours, but may take up to several days. As such, adjunctive testing can accelerate obtaining a diagnosis. A complete blood count (CBC) to evaluate for microcytic anemia may demonstrate a characteristic pattern of basophilic stippling.9 A protoporphyrin level—either a free erythrocyte protoporphyrin (FEP) or a zinc protoporphyrin level—will be elevated, a result of heme synthesis disruption.9 Urinalysis may demonstrate glycosuria or proteinuria.6 Hypertension is often present, even in pediatric patients.
An abdominal radiograph is essential in children to determine whether a lead foreign body, such as a paint chip, is present in the intestinal lumen. Long bone films may demonstrate “lead lines” at the metaphysis, which in fact do not reflect lead itself but abnormal calcium deposition in growing bone due to lead’s interference with bone remodeling. A computed tomography (CT) scan of the brain in patients with encephalopathy will often demonstrate cerebral edema.6
Of note, capillary BLLs taken via finger-stick can be falsely elevated due contamination during collection (eg, the presence of lead dust on the skin). However, this screening method is often used by clinicians in the pediatric primary care setting because of its feasibility. Elevated BLLs from capillary testing should always be followed by a BLL obtained by venipuncture.2
Case Continuation
The patient’s mother was counseled on sources of lead contamination. She was informed that although drinking water may contribute some amount to an elevated BLL, the most likely source of contamination is still lead paint found in older homes such as the one in which she and her son resided.
Diagnostic studies to support the diagnosis of lead poisoning were performed. A CBC revealed a hemoglobin of 9.8 g/dL with a mean corpuscular volume of 68 fL. A microscopic smear of blood demonstrated basophilic stippling of red blood cells. An FEP level was 386 mcg/dL. An abdominal radiograph demonstrated small radiopacities throughout the large intestine, without obstruction, which was suggestive of ingested lead paint chips.
What is the best management approach to patients with suspected lead poisoning?
The first-line treatment for patients with lead poisoning is removal from the exposure source, which first and foremost requires identification of the hazard through careful history taking and scene investigation by the local health department. This will avoid recurrent visits following successful chelation for repeat exposure to an unidentified source. Relocation to another dwelling will often be required for patients with presumed exposure until the hazard can be identified and abated.
Patients who have ingested or have embedded leaded foreign bodies will require removal via whole bowel irrigation or surgical means.
Following decontamination, chelation is required for children with a BLL more than 45 mcg/dL, and adults with CNS symptomatology and a BLL more than 70 mcg/dL. Table 2 provides guidelines for chelation therapy based on BLL.5
There are three chelating agents commonly used to reduce the body lead burden (Table 2).5 The most common, owing largely to it being the only agent used orally, is succimer (or dimercaptosuccinic acid, DMSA). The second agent is calcium disodium edetate (CaNa2EDTA), which is given intravenously. In patients with encephalopathy, EDTA should be given after the first dose of the third agent, British anti-Lewisite (BAL; 2,3-dimercaptopropanol), in order to prevent redistribution of lead from the peripheral compartment into the CNS.10 However, BAL is the most difficult of the three agents to administer as it is suspended in peanut oil and is given via intramuscular injection every 4 hours.
Unfortunately, while chelation therapy is highly beneficial for patients with severe lead poisoning, it has not been demonstrated to positively impact children who already have developed neurocognitive sequelae associated with lower level lead exposure.11 This highlights the importance of prevention.
Work-up and Management in the ED
The patient with lead poisoning, while an unusual presentation in the ED, requires specialized management to minimize sequelae of exposure. Careful attention to history is vital. When in doubt, the EP should consult with her or his regional poison control center (800-222-1222) or with a medical toxicologist or other expert.
There are several scenarios in which a patient may present to the ED with lead toxicity. The following scenarios, along with their respective clinical approach strategies, represent three of the most common presentations.
Scenario 1: The Pediatric Patient With Elevated Venous Blood Lead Levels
The EP should employ the following clinical approach when evaluating and managing the pediatric patient with normal mental status whose routine screening reveals a BLL sufficiently elevated to warrant evaluation or admission—perhaps to discontinue exposure or initiate chelation therapy.
- Obtain a history, including possible lead sources; perform a complete physical examination; and obtain a repeat BLL, CBC with microscopic examination, and renal function test.
- Obtain an abdominal film to look for radiopacities, including paint chips or larger ingested foreign bodies.
- If radiopaque foreign bodies are present on abdominal radiograph, whole bowel irrigation with polyethylene glycol solution given via a nasogastric tube at 250 to 500 cc/h for a pediatric patient (1 to 2 L/h for adult patients) should be given until no residual foreign bodies remain.
- Obtain a radiograph of the long bone, which may demonstrate metaphyseal enhancement in the pediatric patient, suggesting long-term exposure.
- Ensure local or state health departments are contacted to arrange for environmental inspection of the home. This is essential prior to discharge to the home environment.
- Begin chelation therapy according to the BLL (Table 2).
Scenario 2: Adult Patients Presenting With Signs and Symptoms of Lead Toxicity
The adult patient who presents to the ED with complaints suggestive of lead poisoning and whose history is indicative of lead exposure should be evaluated and managed as follows:
- Obtain a thorough history, including the occupation and hobbies of the patient and all family members.
- Obtain vital signs to evaluate for hypertension; repeat BLL, CBC with smear, and serum creatinine test. Perform a physical examination to evaluate for lead lines.
- Obtain radiographic images, which may demonstrate a leaded foreign body, such as in the patient with prior history of gunshot wounds.
- If the BLL is sufficiently elevated or clinical findings are sufficiently severe, admit for chelation.
Scenario 3: The Pediatric or Adult Patient Presenting With Altered Mental Status
The patient presenting with altered mental status of unclear etiology—regardless of age—and in whom lead encephalopathy is a possible cause, should be worked-up and managed as follows:
- Obtain BLL, CBC, FEP levels. Consider radiographic imaging to assess for ingested or embedded foreign bodies.
- If abnormalities in the above laboratory studies are consistent with lead poisoning, initiate chelation immediately—prior to receiving repeat BLL result.
- Obtain a CT scan of the head to assess for cerebral edema.
- Provide supportive care for encephalopathy, including airway control and management of increased intracranial pressure.
Case Conclusion
The patient was admitted to the hospital for whole bowel irrigation and chelation therapy with succimer. The local health department conducted an investigation of the home and found multiple areas of peeling lead paint and lead dust, and ordered remediation of the property before it could be re-occupied by the family. A test of the home’s drinking water found no elevation above the 15 ppb standard.
The patient was discharged from the hospital in the care of his mother. They were relocated to a lead-free home, with follow-up by the pediatrician for ongoing monitoring of the BLL and developmental milestones.
Case
A 2-year-old boy and his mother were referred to the ED by the child’s pediatrician after a routine venous blood lead level (BLL) taken at the boy’s recent well visit revealed an elevated lead level of 52 mcg/dL (normal range, <5 mcg/dL). The child’s mother reported that although her son had always been a picky eater, he had recently been complaining of abdominal pain.
The patient’s well-child visits had been normal until his recent 2-year checkup, at which time his pediatrician noticed some speech delay. On further history taking, the emergency physician (EP) learned the patient and his mother resided in an older home (built in the 1950s) that was in disrepair. The mother asked the EP if the elevation in the child’s BLL could be due to the drinking water in their town.
What are the most likely sources of environmental lead exposure?
In 2016, the topic of lead poisoning grabbed national attention when a pediatrician in Flint, Michigan detected an abrupt doubling of the number of children with elevated lead levels in her practice.1 Upon further investigation, it was discovered that these kids had one thing in common: the source of their drinking water. The City of Flint had recently switched the source of its potable water from Lake Huron to the Flint River. The lower quality water, which was not properly treated with an anticorrosive agent such as orthophosphate, led to widespread pipe corrosion and lead contamination. This finding resulted in a cascade of water testing by other municipalities and school systems, many of which identified lead concentrations above the currently accepted drinking water standard of 15 parts per billion (ppb).
Thousands of children each year are identified to have elevated BLLs, based on the Centers for Disease Control and Prevention definition of a “level of concern” as more than 5 mcg/dL.2 The majority of these exposures stem from environmental exposure to lead paint dust in the home, but drinking water normally contributes as a low-level, constant, “basal” exposure. While lead-contaminated drinking water is not acceptable, it is unlikely to generate many ED visits. However, there are a variety of other lead sources that may prompt children to present to the ED with acute or subacute lead poisoning.
Lead is a heavy metal whose physical properties indicate its common uses. It provides durability and opacity to pigments, which is why it is found in oil paint, house paint used before 1976, and on paint for large outdoor structures, where it is still used. Lead is also found in the pigments used in cosmetics, stained glass, and painted pottery, and as an adulterant in highly colored foodstuffs such as imported turmeric.3
The physicochemical characteristics of lead make it an ideal component of solder. Many plumbing pipes in use today are not lead, but join one another using lead solder at the joints, sites that are vulnerable to corrosion. The heavy molecular weight of lead makes it a useful component of bullets and munitions.
Tetraethyl lead was used as an “anti-knock” agent to smooth out the combustion of heterogenous compounds in automotive fuel before it was removed in the mid-1970s.4 Prior to its removal, leaded gasoline was the largest source of air, soil, and groundwater contamination leading to environmental exposures.4 At present, the most common source of environmental lead exposure among young children is through peeling paint in deteriorating residential buildings. Hazardous occupational lead exposures arise from work involving munitions, reclamation and salvage, painting, welding, and numerous other settings—particularly sites where industrial hygiene is suboptimal. Lead from these sites can be inadvertently transported home on clothing or shoes, raising the exposure risk for children in the household.4
What are the health effects of lead exposure?
Like most heavy metals, lead is toxic to many organ systems in the body. The signs and symptoms of lead poisoning vary depending on the patient’s BLL and age (Table 1).5 The most common clinical effect of lead in the adult population is hypertension.6 Additional renal effects include a Fanconi-type syndrome with glycosuria and proteinuria. Lead can cause a peripheral neuropathy that is predominantly motor, classically causing foot or wrist drop. Abdominal pain from lead exposure is sometimes termed “lead colic” due to its intermittent and often severe nature. Abnormalities in urate metabolism cause a gouty arthritis referred to as “saturnine gout.” 6
The young pediatric central nervous system (CNS) is much more vulnerable to the effects of lead than the adult CNS. Even low-level lead exposure to the developing brain causes deficits in intelligence quotient, attention, impulse control, and other neurocognitive functions that are largely irreversible.7
Children with an elevated BLL may also develop constipation, anorexia, pallor, and pica.8 The development of geophagia (subtype of pica in which one craves and ingests nonfood clay or soil-like materials), represents a “chicken-or-egg” phenomena as it both causes and results from lead poisoning.
Lead impairs multiple steps of the heme synthesis pathway, causing microcytic anemia with basophilic stippling. Lead-induced anemia exacerbates pica as anemic patients are more likely to eat leaded paint chips and other lead-containing materials such as pottery.8 Of note, leaded white paint is reported to have a pleasant taste due to the sweet-tasting lead acetate used as a pigment.
The most dramatic and consequential manifestation of lead poisoning is lead encephalopathy. This can occur at any age, but manifests in children at much lower BLLs than in adults. Patients can be altered or obtunded, have convulsive activity, and may develop cerebral edema. Encephalopathy is a life-threatening emergency and must be recognized and treated immediately. Lead encephalopathy should be suspected in any young child with hand-to-mouth behavior who has any of the above environmental risk factors.4 The findings of anemia or the other diagnostic signs described below are too unreliable and take too long to be truly helpful in making the diagnosis.
How is the diagnosis of lead poisoning made?
The gold standard for the diagnosis of lead poisoning is the measurement of BLL. However, the turnaround time for this test is usually at least 24 hours, but may take up to several days. As such, adjunctive testing can accelerate obtaining a diagnosis. A complete blood count (CBC) to evaluate for microcytic anemia may demonstrate a characteristic pattern of basophilic stippling.9 A protoporphyrin level—either a free erythrocyte protoporphyrin (FEP) or a zinc protoporphyrin level—will be elevated, a result of heme synthesis disruption.9 Urinalysis may demonstrate glycosuria or proteinuria.6 Hypertension is often present, even in pediatric patients.
An abdominal radiograph is essential in children to determine whether a lead foreign body, such as a paint chip, is present in the intestinal lumen. Long bone films may demonstrate “lead lines” at the metaphysis, which in fact do not reflect lead itself but abnormal calcium deposition in growing bone due to lead’s interference with bone remodeling. A computed tomography (CT) scan of the brain in patients with encephalopathy will often demonstrate cerebral edema.6
Of note, capillary BLLs taken via finger-stick can be falsely elevated due contamination during collection (eg, the presence of lead dust on the skin). However, this screening method is often used by clinicians in the pediatric primary care setting because of its feasibility. Elevated BLLs from capillary testing should always be followed by a BLL obtained by venipuncture.2
Case Continuation
The patient’s mother was counseled on sources of lead contamination. She was informed that although drinking water may contribute some amount to an elevated BLL, the most likely source of contamination is still lead paint found in older homes such as the one in which she and her son resided.
Diagnostic studies to support the diagnosis of lead poisoning were performed. A CBC revealed a hemoglobin of 9.8 g/dL with a mean corpuscular volume of 68 fL. A microscopic smear of blood demonstrated basophilic stippling of red blood cells. An FEP level was 386 mcg/dL. An abdominal radiograph demonstrated small radiopacities throughout the large intestine, without obstruction, which was suggestive of ingested lead paint chips.
What is the best management approach to patients with suspected lead poisoning?
The first-line treatment for patients with lead poisoning is removal from the exposure source, which first and foremost requires identification of the hazard through careful history taking and scene investigation by the local health department. This will avoid recurrent visits following successful chelation for repeat exposure to an unidentified source. Relocation to another dwelling will often be required for patients with presumed exposure until the hazard can be identified and abated.
Patients who have ingested or have embedded leaded foreign bodies will require removal via whole bowel irrigation or surgical means.
Following decontamination, chelation is required for children with a BLL more than 45 mcg/dL, and adults with CNS symptomatology and a BLL more than 70 mcg/dL. Table 2 provides guidelines for chelation therapy based on BLL.5
There are three chelating agents commonly used to reduce the body lead burden (Table 2).5 The most common, owing largely to it being the only agent used orally, is succimer (or dimercaptosuccinic acid, DMSA). The second agent is calcium disodium edetate (CaNa2EDTA), which is given intravenously. In patients with encephalopathy, EDTA should be given after the first dose of the third agent, British anti-Lewisite (BAL; 2,3-dimercaptopropanol), in order to prevent redistribution of lead from the peripheral compartment into the CNS.10 However, BAL is the most difficult of the three agents to administer as it is suspended in peanut oil and is given via intramuscular injection every 4 hours.
Unfortunately, while chelation therapy is highly beneficial for patients with severe lead poisoning, it has not been demonstrated to positively impact children who already have developed neurocognitive sequelae associated with lower level lead exposure.11 This highlights the importance of prevention.
Work-up and Management in the ED
The patient with lead poisoning, while an unusual presentation in the ED, requires specialized management to minimize sequelae of exposure. Careful attention to history is vital. When in doubt, the EP should consult with her or his regional poison control center (800-222-1222) or with a medical toxicologist or other expert.
There are several scenarios in which a patient may present to the ED with lead toxicity. The following scenarios, along with their respective clinical approach strategies, represent three of the most common presentations.
Scenario 1: The Pediatric Patient With Elevated Venous Blood Lead Levels
The EP should employ the following clinical approach when evaluating and managing the pediatric patient with normal mental status whose routine screening reveals a BLL sufficiently elevated to warrant evaluation or admission—perhaps to discontinue exposure or initiate chelation therapy.
- Obtain a history, including possible lead sources; perform a complete physical examination; and obtain a repeat BLL, CBC with microscopic examination, and renal function test.
- Obtain an abdominal film to look for radiopacities, including paint chips or larger ingested foreign bodies.
- If radiopaque foreign bodies are present on abdominal radiograph, whole bowel irrigation with polyethylene glycol solution given via a nasogastric tube at 250 to 500 cc/h for a pediatric patient (1 to 2 L/h for adult patients) should be given until no residual foreign bodies remain.
- Obtain a radiograph of the long bone, which may demonstrate metaphyseal enhancement in the pediatric patient, suggesting long-term exposure.
- Ensure local or state health departments are contacted to arrange for environmental inspection of the home. This is essential prior to discharge to the home environment.
- Begin chelation therapy according to the BLL (Table 2).
Scenario 2: Adult Patients Presenting With Signs and Symptoms of Lead Toxicity
The adult patient who presents to the ED with complaints suggestive of lead poisoning and whose history is indicative of lead exposure should be evaluated and managed as follows:
- Obtain a thorough history, including the occupation and hobbies of the patient and all family members.
- Obtain vital signs to evaluate for hypertension; repeat BLL, CBC with smear, and serum creatinine test. Perform a physical examination to evaluate for lead lines.
- Obtain radiographic images, which may demonstrate a leaded foreign body, such as in the patient with prior history of gunshot wounds.
- If the BLL is sufficiently elevated or clinical findings are sufficiently severe, admit for chelation.
Scenario 3: The Pediatric or Adult Patient Presenting With Altered Mental Status
The patient presenting with altered mental status of unclear etiology—regardless of age—and in whom lead encephalopathy is a possible cause, should be worked-up and managed as follows:
- Obtain BLL, CBC, FEP levels. Consider radiographic imaging to assess for ingested or embedded foreign bodies.
- If abnormalities in the above laboratory studies are consistent with lead poisoning, initiate chelation immediately—prior to receiving repeat BLL result.
- Obtain a CT scan of the head to assess for cerebral edema.
- Provide supportive care for encephalopathy, including airway control and management of increased intracranial pressure.
Case Conclusion
The patient was admitted to the hospital for whole bowel irrigation and chelation therapy with succimer. The local health department conducted an investigation of the home and found multiple areas of peeling lead paint and lead dust, and ordered remediation of the property before it could be re-occupied by the family. A test of the home’s drinking water found no elevation above the 15 ppb standard.
The patient was discharged from the hospital in the care of his mother. They were relocated to a lead-free home, with follow-up by the pediatrician for ongoing monitoring of the BLL and developmental milestones.
1. Hanna-Attisha M, LaChance J, Sadler RC, Champney Schnepp A. Elevated blood lead levels in children associated with the flint drinking water crisis: A spatial analysis of risk and public health response. Am J Public Health. 2016;106(2):283-290. doi:0.2105/AJPH.2015.303003.
2. Centers for Disease Control and Prevention Advisory Committee on Childhood Lead Poisoning Prevention. Low level lead exposure harms children: a renewed call for primary prevention. January 4, 2012. Available at https://www.cdc.gov/nceh/lead/acclpp/final_document_030712.pdf. Accessed February 27, 2017.
3. Food and Drug Administration. Spices USA Inc. issues alert on elevated levels of lead in ground turmeric. http://www.fda.gov/safety/recalls/ucm523561.htm, September 26, 2016. Accessed February 27, 2017.
4. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Toxic substances portal: lead. US Department of Health and Human Services Web site. Available at https://www.atsdr.cdc.gov/ToxProfiles/TP.asp?id=96&tid=22. Updated January 21, 2015. Accessed February 27, 2017.
5. Calello DP, Henretig FM. Lead. In: Goldfrank LG, Flomenbaum NE, Lewin NA, Howland MA, Hoffman RS, Nelson LS (eds.). Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2014:1219-1234.
6. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Environmental health and medicine education: lead toxicity. https://www.atsdr.cdc.gov/csem/csem.asp?csem=7&po=10. Updated August 26, 2016. Accessed February 27, 2017.
7. Canfield RL, Henderson Jr CR, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. New Engl J Med. 2003;348:1517-1526.
8. Kathuria P, Rowden AK. Lead toxicity. Medscape Web site. Available at http://emedicine.medscape.com/article/1174752-clinical. Updated January 31, 2017. Accessed February 27, 2017.
9. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Environmental health and medicine education. Lead toxicity: what tests can assist with diagnosis of lead toxicity? https://www.atsdr.cdc.gov/csem/csem.asp?csem=7&po=12. Updated August 25, 2016. Accessed February 27, 2017.
10. Chisholm JJ Jr. The use of chelating agents in the treatment of acute and chronic lead intoxication in childhood. J Pediatr. 1968;73(1):1-38.
11. Rogan WJ, Dietrich KN, Ware JH, et al; Treatment of Lead-Exposed Children Trial Group. The effect of chelation therapy with succimer on neuropsychological development in children exposed to lead. N Engl J Med. 2001;344(19):1421-1426.
1. Hanna-Attisha M, LaChance J, Sadler RC, Champney Schnepp A. Elevated blood lead levels in children associated with the flint drinking water crisis: A spatial analysis of risk and public health response. Am J Public Health. 2016;106(2):283-290. doi:0.2105/AJPH.2015.303003.
2. Centers for Disease Control and Prevention Advisory Committee on Childhood Lead Poisoning Prevention. Low level lead exposure harms children: a renewed call for primary prevention. January 4, 2012. Available at https://www.cdc.gov/nceh/lead/acclpp/final_document_030712.pdf. Accessed February 27, 2017.
3. Food and Drug Administration. Spices USA Inc. issues alert on elevated levels of lead in ground turmeric. http://www.fda.gov/safety/recalls/ucm523561.htm, September 26, 2016. Accessed February 27, 2017.
4. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Toxic substances portal: lead. US Department of Health and Human Services Web site. Available at https://www.atsdr.cdc.gov/ToxProfiles/TP.asp?id=96&tid=22. Updated January 21, 2015. Accessed February 27, 2017.
5. Calello DP, Henretig FM. Lead. In: Goldfrank LG, Flomenbaum NE, Lewin NA, Howland MA, Hoffman RS, Nelson LS (eds.). Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2014:1219-1234.
6. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Environmental health and medicine education: lead toxicity. https://www.atsdr.cdc.gov/csem/csem.asp?csem=7&po=10. Updated August 26, 2016. Accessed February 27, 2017.
7. Canfield RL, Henderson Jr CR, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. New Engl J Med. 2003;348:1517-1526.
8. Kathuria P, Rowden AK. Lead toxicity. Medscape Web site. Available at http://emedicine.medscape.com/article/1174752-clinical. Updated January 31, 2017. Accessed February 27, 2017.
9. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Environmental health and medicine education. Lead toxicity: what tests can assist with diagnosis of lead toxicity? https://www.atsdr.cdc.gov/csem/csem.asp?csem=7&po=12. Updated August 25, 2016. Accessed February 27, 2017.
10. Chisholm JJ Jr. The use of chelating agents in the treatment of acute and chronic lead intoxication in childhood. J Pediatr. 1968;73(1):1-38.
11. Rogan WJ, Dietrich KN, Ware JH, et al; Treatment of Lead-Exposed Children Trial Group. The effect of chelation therapy with succimer on neuropsychological development in children exposed to lead. N Engl J Med. 2001;344(19):1421-1426.
The New Opioid Epidemic: Prescriptions, Synthetics, and Street Drugs
Opioid misuse, which often is the result of a prescription written for a very painful condition, has created an epidemic of opioid abuse, addiction, and fatalities across the United States. To reduce the risks from prescribed opioids, regulators and public health authorities have implemented intensive risk mitigation programs, prescription-monitoring programs, and prescribing guidelines.
Clinicians have been encouraged to manage acute and chronic pain more comprehensively. Concurrently, pharmaceutical companies have introduced tamper-resistant formulations, also known as abuse-deterrent formulations, intended to limit manipulation of the contents for insufflation or injection. Although some of these formulations have made tampering difficult, overall they have not effectively reduced inappropriate use or abuse.
All of these interventions have resulted in a reduction in the availability of affordable, commercially available pharmaceutical opioids (Table 1). Simultaneously, other prescription opioid users have found that the analgesic or euphoric effects of their prescription opioids were no longer sufficient, due to opioid tolerance and hyperalgesia. Both of these forces are driving opioid users to seek more potent opioid products and higher doses to achieve the desired psychoactive and pain-relieving effects.
For these reasons, many opioid users turned to less expensive, readily available, illicitly produced heroin and potent synthetic opioids—mainly fentanyl derivatives. The increased use of heroin and synthetic opioids has resulted in a sharp rise in overdoses and deaths, which continue to be a daily presentation in EDs throughout the country.
This review describes the emergence of the new synthetic opioids, and the steps emergency physicians (EPs) can take to identify and manage ED patients who have been exposed to these agents.
Case
A 34-year-old woman with a history of opioid-use disorder was found unresponsive by a family member who immediately called emergency medical services (EMS). Upon arrival, the emergency medical technicians noted the patient’s agonal respiration and pinpoint pupils. They immediately provided assisted ventilations via a bag-valve-mask (BVM) and administered 2 mg of intranasal naloxone prior to transport. The patient remained unresponsive, with no improvement in her respiratory status.
Upon arrival at the ED, the patient was still comatose, and her pupils remained pinpoint. Vital signs at presentation were: heart rate, 48 beats/min; blood pressure, 70/40 mm Hg; agonal respiration; and temperature, 98.2°F. Oxygen saturation was 86% while receiving assisted ventilation through BVM. An intravenous (IV) line was established.
What is the differential diagnosis of this toxidrome in the current era of emerging drugs of abuse?
The differential diagnosis of a patient with pinpoint pupils and respiratory depression who does not respond to naloxone typically includes overdose with gamma-hydroxybutyrate, clonidine, or the combined use of sedative-hypnotic agents with ethanol (organophosphate exposure and pontine strokes are two other causes). Naloxone administration may help diagnose opioids as a cause, and, in the past, a lack of response to naloxone was used to help exclude opioids as a cause. However, opioid poisoning should no longer be excluded from consideration in the differential diagnosis when patients are nonresponsive to naloxone. Patients who combine the use of opioids with another sedative hypnotic or who develop hypoxic encephalopathy following opioid overdose may not respond to naloxone with arousal. Most important, the emergence of ultra-potent synthetic opioid use raises the possibility that a patient may appear to be resistant to naloxone due to the extreme potency of these drugs, but may respond to extremely large doses of naloxone. These new opioids pose a grave public health threat and have already resulted in hundreds, if not thousands, of deaths.1
What are novel synthetic opioids?
Unlike heroin, which requires harvesting of plant-derived opium, the novel synthetic opioids are synthesized in laboratories, primarily in China, and shipped to the United States through commercial channels (eg, US Postal Service).2,3 Over the past few years, novel synthetic opioids have been supplementing or replacing heroin sold on the illicit market.1 Most of these novel synthetic opioids are fentanyl analogs (Table 2) that are purchased in bulk on the “Darknet”—an area hidden deep in the Internet (not discoverable by the common major search engines) that allows users to engage in questionable, even illegal, activities utilizing nontraceable currencies such as Bitcoin.4
At the local level, dealers may seek to attract heroin users by adulterating, or even replacing, heroin with fentanyl or novel synthetic opioids, marketing it as a “high-quality” heroin offering more rapid, intense effects. These fentanyl analogs are often hundreds of times more potent than fentanyl, and therefore thousands of times more potent than heroin. Only a miniscule amount increases the perceived potency of the “heroin,” allowing dealers to increase their profit margins.
Selling and using novel synthetic opioids leave little room for error, and small dosing miscalculations have resulted in profound overdoses and deaths. Obviously, the quality control, contents, and dose uniformity of illicitly traded products are poor, adding to the risks of use. In some cases, the novel synthetic opioids are pressed into tablets and marketed as diverted prescription opioids or benzodiazepines. In many, if not most, circumstances, intermediary dealers, as well as users, may be unaware of the product’s contents.5,6 Carfentanil, used as a large-animal tranquilizer, is reportedly 10,000 times more potent than morphine and has recently been implicated in a cluster of deaths of opioid users in the Midwest.7,8 Other synthetic opioids coming to market were initially developed for laboratory research, including W18, which was identified in Canada; and U47700, an opioid identified on autopsy of the musician Prince3,9 (Table 2).
Novel synthetic opioids can be identified only by specific, specialized assays not available in clinical settings. Because their molecular structures differ substantially from morphine, these compounds skirt identification by standard urine “opiate” drug screens. With the exception of fentanyl, pharmacokinetic data for the use of the majority of these agents in humans is unknown.
How are patients who present to EDs with an opioid toxidrome managed in practice today?
Classic teaching for the management of opioid-induced respiratory depression in adults is to provide ventilatory support (ie, BVM or intubation) or administer a low dose of naloxone (0.04 mg IV every 2-5 minutes, up to 2 mg) until adequate respirations are restored. This approach is reasonable for patients exposed to heroin or fentanyl, and provides safer reversal in the ED than administration of a large bolus dose of 0.4 or 2 mg naloxone in opioid-dependent patients.
However, patients exposed to novel synthetic opioids may ultimately require higher than usual doses of naloxone to achieve reversal—reportedly IV doses as high as 6 to 10 mg or more.10 It is not yet fully understood if the need for high-dose naloxone is due to the binding affinity of the opioid or the relatively high dose of opioid administered.
Because the clinical effects of the novel synthetic opioids are generally indistinguishable from those of other opioids, providing respiratory support in the ED remains a critical intervention while awaiting the effect of titrated doses of naloxone. Of concern, though, is that these opioids are so potent that they may cause immediate respiratory arrest, resulting in a more rapid progression to cardiac arrest, limiting the ability to administer rescue breathing or antidote.
In the “bystander” setting, administration of a larger initial dose of naloxone may be reasonable, given the lack of advanced medical supportive care. However, the ability to provide larger doses in these settings is hampered by the accessibility of the antidote. In addition, prehospital-care providers need to consider the possibility of precipitating opioid withdrawal in patients with opioid dependence, which itself can carry significant consequences (eg, aspiration, agitated delirium), as well as the subsequent uncooperativeness of the victim, who may attempt to leave the scene and self-administer an additional dose of opioid or develop recurrent respiratory depression when the naloxone wanes. Since many patients with life-threatening opioid intoxication will suffer long-term consequences if reversal is delayed, the risk of administering high-dose naloxone in the bystander setting generally is worthwhile. However, the risks and benefits of naloxone must still be thoughtfully considered by prehospital-care providers who can provide alternative supportive therapies.
In the ED, the EP must decide whether to intubate the patient directly or first give a brief trial of low-dose naloxone. If a trial of naloxone is unsuccessful at reversing the respiratory depression, dose escalation can be tried while supporting oxygenation and ventilation noninvasively. Administration of naloxone postintubation is not usually necessary or even desired, since respiratory depression, the primary mechanism of death, has been addressed.
Are any special precautions required for health care workers?
Some of the ultra-potent synthetic opioids are available as powders or sprays that can be inadvertently absorbed through the skin (after dissolution in skin moisture) or inhaled.8 The safety of health care providers and law enforcement personnel who may be exposed to synthetic opioids in this manner is currently unknown, though some law enforcement and public health agencies have published warnings in an effort to be proactively cautious.8
While it is highly unlikely that the handling of body fluids of opioid-intoxicated patients poses any health threats, universal safety precautions of wearing disposable gloves should be utilized. As noted, contact with the actual substances may be more concerning, particularly when airborne; in such situations, a particulate mask should also be utilized. Although fentanyl in liquid formulation can slowly enter the skin transdermally (eg, fentanyl patch), there are very limited data to either support or refute the ability of the newer potent opioids to do so. Until more data on these opioid analogs become available, those entering grossly contaminated areas, in which dermal or inhalational exposure is high, should employ a higher level of personal safety precautions.11 In addition, naloxone should be readily available.
How can we detect novel opioid use?
As noted, there is no ability to specifically detect the use of novel potent opioids in the clinical setting (eg, hospital laboratory); therefore, clinicians must maintain a high level of suspicion and provide care empirically. The ability to make a specific diagnosis is further clouded because a patient who has used a synthetic opioid may have also used certain prescription opioids or heroin, which can be detected by standard testing.
Blood and urine samples obtained early in care and sent to specialized laboratories may provide specific identification. Such testing is typically only done by reference laboratories, health departments, or law enforcement agencies. The information obtained from these analyses may help to understand the epidemiology of novel opioid abuse, prevent others from succumbing to addiction, and determine the cause of related deaths.
Which patients can be safely discharged from the ED after an opioid overdose?
Patients who survive reversal of an opioid overdose, whether from a conventional or novel opioid, are at extremely high risk of subsequent death from continued use, as well as from the initial exposure to a long-acting opioid that outlasts the reversal effects of naloxone. Such patients should undergo a sufficient observation period after the last dose of naloxone has been administered to allow its effects to dissipate. This is likely at least 2 hours, but may be longer in certain individuals. Attempts at establishing a link for the patient to long-term treatment or (where available) providing a naloxone rescue kit and training to patients and their families are worthwhile. Although some data support releasing responsive patients after a short, but safe interval after naloxone administration, the changing landscape of opioid use should prompt reconsideration of such practices.12
To whom should suspected opioid overdose patients be reported?
While most EPs are familiar with the management of patients with opioid-induced respiratory depression, atypical cases (eg, patients less responsive to naloxone, those who suffer cardiac arrest) or clusters of suspected cases should always be reported to a regional poison control center (PCC) or health department. The PCC is typically engaged in surveillance and works cooperatively with area EDs and public health officials to track and notify physicians of emerging trends. The epidemiological data derived from reports from a variety of hospitals allow health officials to effectively engage resources for public warnings, facilitate forensic identification of circulating products, and determine any unique clinical information that can then be broadly disseminated.
Case Conclusion
The patient was supported with BVM ventilations. Despite additional titrated IV naloxone (up to a total of 4 mg) the patient was nonresponsive and unarousable. She was intubated, and awoke several hours later. She fully recovered and subsequently was referred to both a harm-reduction and an opioid detoxification program. Analysis of her blood and urine, available several weeks later, confirmed an exposure to U47700.
1. Centers for Disease Control and Prevention. Health Alert Network. Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. https://emergency.cdc.gov/han/han00384.asp. Updated October 26, 2015. Accessed January 10, 2017.
2. MacQuarrie B. Synthetic opioids are getting into US by mail. Boston Globe. December 27, 2016. http://www.bostonglobe.com/metro/2016/12/26/synthetic-opioids-slipping-into-via-mail-security-experts-say/23TCEuIES8aEQYAWWHKCiI/story.html. Accessed January 10, 2017.
3. Lucyk SN, Nelson LS. Novel synthetic opioids: an opioid epidemic within an opioid epidemic. Ann Emerg Med. 2017;69(1):91-93. doi:10.1016/j.annemergmed.2016.08.445.
4. Mounteney J, Bo A, Oteo A; OteoEuropean Monitoring Centre for Drugs and Drug Addiction project group. The Internet and Drug Markets. Publications Office of the European Union, Luxembourg, Luxembourg; 2016:1-136. http://www.emcdda.europa.eu/system/files/publications/2155/TDXD16001ENN_FINAL. pdf. doi:10.2810/324608. Accessed January 17, 2017.
5. Associated Press. ‘Norco’ fentanyl overdose deaths rise to 14; problem spreads to Bay Area. Los Angeles Times. April 26, 2016. http://www.latimes.com/local/lanow/la-me-ln-norco-fentanyl-overdose-deaths-rise-to-14-problem-spreads-to-bay-area-20160426-story.html.
6. Centers for Disease Control and Prevention. Health Alert Network. Influx of fentanyl-laced counterfeit pills and toxic fentanyl-related compounds further increases risk of fentanyl-related overdose and fatalities. https://emergency.cdc.gov/han/han00395.asp. Accessed January 10, 2017.
7. Sandy E. Cleveland Scene. 236 heroin overdoses in Akron in 3 weeks; heroin being cut with elephant sedative. http://www.clevescene.com/scene-and-heard/archives/2016/07/14/akron-police-chief-heroin-being-cut-with-elephant-sedative-88-overdoses-since-july-5. Accessed January 10, 2017.
8. DEA issues carfentanil warning to police and public [news release]. Washington, DC: United States Drug Enforcement Administration; September 22, 2016. https://www.dea.gov/divisions/hq/2016/hq092216.shtml. Accessed January 10, 2017.
9. Armenian P, Olson A, Anaya A, Kurtz A, Ruegner R, Gerona RR. Fentanyl and a novel synthetic opioid U-47700 masquerading as street “Norco” in Central California: a case report. Ann Emerg Med. 2017;69(1):87-90. doi:10.1016/j.annemergmed.2016.06.014.
10. Schumann H, Erickson T, Thompson TM, Zautcke JL, Denton JS. Fentanyl epidemic in Chicago, Illinois and surrounding Cook County. Clin Toxicol (Phila). 2008;46(6):501-506. doi:10.1080/15563650701877374.
11. George AV, Lu JJ, Pisano MV, Metz J, Erickson TB. Carfentanil—an ultra potent opioid. Am J Emerg Med. 2010;28(4):530-532. doi:10.1016/j.ajem.2010.03.003.
12. Kolinsky D, Keim SM, Cohn BG, Schwarz ES, Yealy DM. Is a prehospital treat and release protocol for opioid overdose safe? J Emerg Med. 2017;52(1):52-58. doi:10.1016/j.jemermed.2016.09.015.
Opioid misuse, which often is the result of a prescription written for a very painful condition, has created an epidemic of opioid abuse, addiction, and fatalities across the United States. To reduce the risks from prescribed opioids, regulators and public health authorities have implemented intensive risk mitigation programs, prescription-monitoring programs, and prescribing guidelines.
Clinicians have been encouraged to manage acute and chronic pain more comprehensively. Concurrently, pharmaceutical companies have introduced tamper-resistant formulations, also known as abuse-deterrent formulations, intended to limit manipulation of the contents for insufflation or injection. Although some of these formulations have made tampering difficult, overall they have not effectively reduced inappropriate use or abuse.
All of these interventions have resulted in a reduction in the availability of affordable, commercially available pharmaceutical opioids (Table 1). Simultaneously, other prescription opioid users have found that the analgesic or euphoric effects of their prescription opioids were no longer sufficient, due to opioid tolerance and hyperalgesia. Both of these forces are driving opioid users to seek more potent opioid products and higher doses to achieve the desired psychoactive and pain-relieving effects.
For these reasons, many opioid users turned to less expensive, readily available, illicitly produced heroin and potent synthetic opioids—mainly fentanyl derivatives. The increased use of heroin and synthetic opioids has resulted in a sharp rise in overdoses and deaths, which continue to be a daily presentation in EDs throughout the country.
This review describes the emergence of the new synthetic opioids, and the steps emergency physicians (EPs) can take to identify and manage ED patients who have been exposed to these agents.
Case
A 34-year-old woman with a history of opioid-use disorder was found unresponsive by a family member who immediately called emergency medical services (EMS). Upon arrival, the emergency medical technicians noted the patient’s agonal respiration and pinpoint pupils. They immediately provided assisted ventilations via a bag-valve-mask (BVM) and administered 2 mg of intranasal naloxone prior to transport. The patient remained unresponsive, with no improvement in her respiratory status.
Upon arrival at the ED, the patient was still comatose, and her pupils remained pinpoint. Vital signs at presentation were: heart rate, 48 beats/min; blood pressure, 70/40 mm Hg; agonal respiration; and temperature, 98.2°F. Oxygen saturation was 86% while receiving assisted ventilation through BVM. An intravenous (IV) line was established.
What is the differential diagnosis of this toxidrome in the current era of emerging drugs of abuse?
The differential diagnosis of a patient with pinpoint pupils and respiratory depression who does not respond to naloxone typically includes overdose with gamma-hydroxybutyrate, clonidine, or the combined use of sedative-hypnotic agents with ethanol (organophosphate exposure and pontine strokes are two other causes). Naloxone administration may help diagnose opioids as a cause, and, in the past, a lack of response to naloxone was used to help exclude opioids as a cause. However, opioid poisoning should no longer be excluded from consideration in the differential diagnosis when patients are nonresponsive to naloxone. Patients who combine the use of opioids with another sedative hypnotic or who develop hypoxic encephalopathy following opioid overdose may not respond to naloxone with arousal. Most important, the emergence of ultra-potent synthetic opioid use raises the possibility that a patient may appear to be resistant to naloxone due to the extreme potency of these drugs, but may respond to extremely large doses of naloxone. These new opioids pose a grave public health threat and have already resulted in hundreds, if not thousands, of deaths.1
What are novel synthetic opioids?
Unlike heroin, which requires harvesting of plant-derived opium, the novel synthetic opioids are synthesized in laboratories, primarily in China, and shipped to the United States through commercial channels (eg, US Postal Service).2,3 Over the past few years, novel synthetic opioids have been supplementing or replacing heroin sold on the illicit market.1 Most of these novel synthetic opioids are fentanyl analogs (Table 2) that are purchased in bulk on the “Darknet”—an area hidden deep in the Internet (not discoverable by the common major search engines) that allows users to engage in questionable, even illegal, activities utilizing nontraceable currencies such as Bitcoin.4
At the local level, dealers may seek to attract heroin users by adulterating, or even replacing, heroin with fentanyl or novel synthetic opioids, marketing it as a “high-quality” heroin offering more rapid, intense effects. These fentanyl analogs are often hundreds of times more potent than fentanyl, and therefore thousands of times more potent than heroin. Only a miniscule amount increases the perceived potency of the “heroin,” allowing dealers to increase their profit margins.
Selling and using novel synthetic opioids leave little room for error, and small dosing miscalculations have resulted in profound overdoses and deaths. Obviously, the quality control, contents, and dose uniformity of illicitly traded products are poor, adding to the risks of use. In some cases, the novel synthetic opioids are pressed into tablets and marketed as diverted prescription opioids or benzodiazepines. In many, if not most, circumstances, intermediary dealers, as well as users, may be unaware of the product’s contents.5,6 Carfentanil, used as a large-animal tranquilizer, is reportedly 10,000 times more potent than morphine and has recently been implicated in a cluster of deaths of opioid users in the Midwest.7,8 Other synthetic opioids coming to market were initially developed for laboratory research, including W18, which was identified in Canada; and U47700, an opioid identified on autopsy of the musician Prince3,9 (Table 2).
Novel synthetic opioids can be identified only by specific, specialized assays not available in clinical settings. Because their molecular structures differ substantially from morphine, these compounds skirt identification by standard urine “opiate” drug screens. With the exception of fentanyl, pharmacokinetic data for the use of the majority of these agents in humans is unknown.
How are patients who present to EDs with an opioid toxidrome managed in practice today?
Classic teaching for the management of opioid-induced respiratory depression in adults is to provide ventilatory support (ie, BVM or intubation) or administer a low dose of naloxone (0.04 mg IV every 2-5 minutes, up to 2 mg) until adequate respirations are restored. This approach is reasonable for patients exposed to heroin or fentanyl, and provides safer reversal in the ED than administration of a large bolus dose of 0.4 or 2 mg naloxone in opioid-dependent patients.
However, patients exposed to novel synthetic opioids may ultimately require higher than usual doses of naloxone to achieve reversal—reportedly IV doses as high as 6 to 10 mg or more.10 It is not yet fully understood if the need for high-dose naloxone is due to the binding affinity of the opioid or the relatively high dose of opioid administered.
Because the clinical effects of the novel synthetic opioids are generally indistinguishable from those of other opioids, providing respiratory support in the ED remains a critical intervention while awaiting the effect of titrated doses of naloxone. Of concern, though, is that these opioids are so potent that they may cause immediate respiratory arrest, resulting in a more rapid progression to cardiac arrest, limiting the ability to administer rescue breathing or antidote.
In the “bystander” setting, administration of a larger initial dose of naloxone may be reasonable, given the lack of advanced medical supportive care. However, the ability to provide larger doses in these settings is hampered by the accessibility of the antidote. In addition, prehospital-care providers need to consider the possibility of precipitating opioid withdrawal in patients with opioid dependence, which itself can carry significant consequences (eg, aspiration, agitated delirium), as well as the subsequent uncooperativeness of the victim, who may attempt to leave the scene and self-administer an additional dose of opioid or develop recurrent respiratory depression when the naloxone wanes. Since many patients with life-threatening opioid intoxication will suffer long-term consequences if reversal is delayed, the risk of administering high-dose naloxone in the bystander setting generally is worthwhile. However, the risks and benefits of naloxone must still be thoughtfully considered by prehospital-care providers who can provide alternative supportive therapies.
In the ED, the EP must decide whether to intubate the patient directly or first give a brief trial of low-dose naloxone. If a trial of naloxone is unsuccessful at reversing the respiratory depression, dose escalation can be tried while supporting oxygenation and ventilation noninvasively. Administration of naloxone postintubation is not usually necessary or even desired, since respiratory depression, the primary mechanism of death, has been addressed.
Are any special precautions required for health care workers?
Some of the ultra-potent synthetic opioids are available as powders or sprays that can be inadvertently absorbed through the skin (after dissolution in skin moisture) or inhaled.8 The safety of health care providers and law enforcement personnel who may be exposed to synthetic opioids in this manner is currently unknown, though some law enforcement and public health agencies have published warnings in an effort to be proactively cautious.8
While it is highly unlikely that the handling of body fluids of opioid-intoxicated patients poses any health threats, universal safety precautions of wearing disposable gloves should be utilized. As noted, contact with the actual substances may be more concerning, particularly when airborne; in such situations, a particulate mask should also be utilized. Although fentanyl in liquid formulation can slowly enter the skin transdermally (eg, fentanyl patch), there are very limited data to either support or refute the ability of the newer potent opioids to do so. Until more data on these opioid analogs become available, those entering grossly contaminated areas, in which dermal or inhalational exposure is high, should employ a higher level of personal safety precautions.11 In addition, naloxone should be readily available.
How can we detect novel opioid use?
As noted, there is no ability to specifically detect the use of novel potent opioids in the clinical setting (eg, hospital laboratory); therefore, clinicians must maintain a high level of suspicion and provide care empirically. The ability to make a specific diagnosis is further clouded because a patient who has used a synthetic opioid may have also used certain prescription opioids or heroin, which can be detected by standard testing.
Blood and urine samples obtained early in care and sent to specialized laboratories may provide specific identification. Such testing is typically only done by reference laboratories, health departments, or law enforcement agencies. The information obtained from these analyses may help to understand the epidemiology of novel opioid abuse, prevent others from succumbing to addiction, and determine the cause of related deaths.
Which patients can be safely discharged from the ED after an opioid overdose?
Patients who survive reversal of an opioid overdose, whether from a conventional or novel opioid, are at extremely high risk of subsequent death from continued use, as well as from the initial exposure to a long-acting opioid that outlasts the reversal effects of naloxone. Such patients should undergo a sufficient observation period after the last dose of naloxone has been administered to allow its effects to dissipate. This is likely at least 2 hours, but may be longer in certain individuals. Attempts at establishing a link for the patient to long-term treatment or (where available) providing a naloxone rescue kit and training to patients and their families are worthwhile. Although some data support releasing responsive patients after a short, but safe interval after naloxone administration, the changing landscape of opioid use should prompt reconsideration of such practices.12
To whom should suspected opioid overdose patients be reported?
While most EPs are familiar with the management of patients with opioid-induced respiratory depression, atypical cases (eg, patients less responsive to naloxone, those who suffer cardiac arrest) or clusters of suspected cases should always be reported to a regional poison control center (PCC) or health department. The PCC is typically engaged in surveillance and works cooperatively with area EDs and public health officials to track and notify physicians of emerging trends. The epidemiological data derived from reports from a variety of hospitals allow health officials to effectively engage resources for public warnings, facilitate forensic identification of circulating products, and determine any unique clinical information that can then be broadly disseminated.
Case Conclusion
The patient was supported with BVM ventilations. Despite additional titrated IV naloxone (up to a total of 4 mg) the patient was nonresponsive and unarousable. She was intubated, and awoke several hours later. She fully recovered and subsequently was referred to both a harm-reduction and an opioid detoxification program. Analysis of her blood and urine, available several weeks later, confirmed an exposure to U47700.
Opioid misuse, which often is the result of a prescription written for a very painful condition, has created an epidemic of opioid abuse, addiction, and fatalities across the United States. To reduce the risks from prescribed opioids, regulators and public health authorities have implemented intensive risk mitigation programs, prescription-monitoring programs, and prescribing guidelines.
Clinicians have been encouraged to manage acute and chronic pain more comprehensively. Concurrently, pharmaceutical companies have introduced tamper-resistant formulations, also known as abuse-deterrent formulations, intended to limit manipulation of the contents for insufflation or injection. Although some of these formulations have made tampering difficult, overall they have not effectively reduced inappropriate use or abuse.
All of these interventions have resulted in a reduction in the availability of affordable, commercially available pharmaceutical opioids (Table 1). Simultaneously, other prescription opioid users have found that the analgesic or euphoric effects of their prescription opioids were no longer sufficient, due to opioid tolerance and hyperalgesia. Both of these forces are driving opioid users to seek more potent opioid products and higher doses to achieve the desired psychoactive and pain-relieving effects.
For these reasons, many opioid users turned to less expensive, readily available, illicitly produced heroin and potent synthetic opioids—mainly fentanyl derivatives. The increased use of heroin and synthetic opioids has resulted in a sharp rise in overdoses and deaths, which continue to be a daily presentation in EDs throughout the country.
This review describes the emergence of the new synthetic opioids, and the steps emergency physicians (EPs) can take to identify and manage ED patients who have been exposed to these agents.
Case
A 34-year-old woman with a history of opioid-use disorder was found unresponsive by a family member who immediately called emergency medical services (EMS). Upon arrival, the emergency medical technicians noted the patient’s agonal respiration and pinpoint pupils. They immediately provided assisted ventilations via a bag-valve-mask (BVM) and administered 2 mg of intranasal naloxone prior to transport. The patient remained unresponsive, with no improvement in her respiratory status.
Upon arrival at the ED, the patient was still comatose, and her pupils remained pinpoint. Vital signs at presentation were: heart rate, 48 beats/min; blood pressure, 70/40 mm Hg; agonal respiration; and temperature, 98.2°F. Oxygen saturation was 86% while receiving assisted ventilation through BVM. An intravenous (IV) line was established.
What is the differential diagnosis of this toxidrome in the current era of emerging drugs of abuse?
The differential diagnosis of a patient with pinpoint pupils and respiratory depression who does not respond to naloxone typically includes overdose with gamma-hydroxybutyrate, clonidine, or the combined use of sedative-hypnotic agents with ethanol (organophosphate exposure and pontine strokes are two other causes). Naloxone administration may help diagnose opioids as a cause, and, in the past, a lack of response to naloxone was used to help exclude opioids as a cause. However, opioid poisoning should no longer be excluded from consideration in the differential diagnosis when patients are nonresponsive to naloxone. Patients who combine the use of opioids with another sedative hypnotic or who develop hypoxic encephalopathy following opioid overdose may not respond to naloxone with arousal. Most important, the emergence of ultra-potent synthetic opioid use raises the possibility that a patient may appear to be resistant to naloxone due to the extreme potency of these drugs, but may respond to extremely large doses of naloxone. These new opioids pose a grave public health threat and have already resulted in hundreds, if not thousands, of deaths.1
What are novel synthetic opioids?
Unlike heroin, which requires harvesting of plant-derived opium, the novel synthetic opioids are synthesized in laboratories, primarily in China, and shipped to the United States through commercial channels (eg, US Postal Service).2,3 Over the past few years, novel synthetic opioids have been supplementing or replacing heroin sold on the illicit market.1 Most of these novel synthetic opioids are fentanyl analogs (Table 2) that are purchased in bulk on the “Darknet”—an area hidden deep in the Internet (not discoverable by the common major search engines) that allows users to engage in questionable, even illegal, activities utilizing nontraceable currencies such as Bitcoin.4
At the local level, dealers may seek to attract heroin users by adulterating, or even replacing, heroin with fentanyl or novel synthetic opioids, marketing it as a “high-quality” heroin offering more rapid, intense effects. These fentanyl analogs are often hundreds of times more potent than fentanyl, and therefore thousands of times more potent than heroin. Only a miniscule amount increases the perceived potency of the “heroin,” allowing dealers to increase their profit margins.
Selling and using novel synthetic opioids leave little room for error, and small dosing miscalculations have resulted in profound overdoses and deaths. Obviously, the quality control, contents, and dose uniformity of illicitly traded products are poor, adding to the risks of use. In some cases, the novel synthetic opioids are pressed into tablets and marketed as diverted prescription opioids or benzodiazepines. In many, if not most, circumstances, intermediary dealers, as well as users, may be unaware of the product’s contents.5,6 Carfentanil, used as a large-animal tranquilizer, is reportedly 10,000 times more potent than morphine and has recently been implicated in a cluster of deaths of opioid users in the Midwest.7,8 Other synthetic opioids coming to market were initially developed for laboratory research, including W18, which was identified in Canada; and U47700, an opioid identified on autopsy of the musician Prince3,9 (Table 2).
Novel synthetic opioids can be identified only by specific, specialized assays not available in clinical settings. Because their molecular structures differ substantially from morphine, these compounds skirt identification by standard urine “opiate” drug screens. With the exception of fentanyl, pharmacokinetic data for the use of the majority of these agents in humans is unknown.
How are patients who present to EDs with an opioid toxidrome managed in practice today?
Classic teaching for the management of opioid-induced respiratory depression in adults is to provide ventilatory support (ie, BVM or intubation) or administer a low dose of naloxone (0.04 mg IV every 2-5 minutes, up to 2 mg) until adequate respirations are restored. This approach is reasonable for patients exposed to heroin or fentanyl, and provides safer reversal in the ED than administration of a large bolus dose of 0.4 or 2 mg naloxone in opioid-dependent patients.
However, patients exposed to novel synthetic opioids may ultimately require higher than usual doses of naloxone to achieve reversal—reportedly IV doses as high as 6 to 10 mg or more.10 It is not yet fully understood if the need for high-dose naloxone is due to the binding affinity of the opioid or the relatively high dose of opioid administered.
Because the clinical effects of the novel synthetic opioids are generally indistinguishable from those of other opioids, providing respiratory support in the ED remains a critical intervention while awaiting the effect of titrated doses of naloxone. Of concern, though, is that these opioids are so potent that they may cause immediate respiratory arrest, resulting in a more rapid progression to cardiac arrest, limiting the ability to administer rescue breathing or antidote.
In the “bystander” setting, administration of a larger initial dose of naloxone may be reasonable, given the lack of advanced medical supportive care. However, the ability to provide larger doses in these settings is hampered by the accessibility of the antidote. In addition, prehospital-care providers need to consider the possibility of precipitating opioid withdrawal in patients with opioid dependence, which itself can carry significant consequences (eg, aspiration, agitated delirium), as well as the subsequent uncooperativeness of the victim, who may attempt to leave the scene and self-administer an additional dose of opioid or develop recurrent respiratory depression when the naloxone wanes. Since many patients with life-threatening opioid intoxication will suffer long-term consequences if reversal is delayed, the risk of administering high-dose naloxone in the bystander setting generally is worthwhile. However, the risks and benefits of naloxone must still be thoughtfully considered by prehospital-care providers who can provide alternative supportive therapies.
In the ED, the EP must decide whether to intubate the patient directly or first give a brief trial of low-dose naloxone. If a trial of naloxone is unsuccessful at reversing the respiratory depression, dose escalation can be tried while supporting oxygenation and ventilation noninvasively. Administration of naloxone postintubation is not usually necessary or even desired, since respiratory depression, the primary mechanism of death, has been addressed.
Are any special precautions required for health care workers?
Some of the ultra-potent synthetic opioids are available as powders or sprays that can be inadvertently absorbed through the skin (after dissolution in skin moisture) or inhaled.8 The safety of health care providers and law enforcement personnel who may be exposed to synthetic opioids in this manner is currently unknown, though some law enforcement and public health agencies have published warnings in an effort to be proactively cautious.8
While it is highly unlikely that the handling of body fluids of opioid-intoxicated patients poses any health threats, universal safety precautions of wearing disposable gloves should be utilized. As noted, contact with the actual substances may be more concerning, particularly when airborne; in such situations, a particulate mask should also be utilized. Although fentanyl in liquid formulation can slowly enter the skin transdermally (eg, fentanyl patch), there are very limited data to either support or refute the ability of the newer potent opioids to do so. Until more data on these opioid analogs become available, those entering grossly contaminated areas, in which dermal or inhalational exposure is high, should employ a higher level of personal safety precautions.11 In addition, naloxone should be readily available.
How can we detect novel opioid use?
As noted, there is no ability to specifically detect the use of novel potent opioids in the clinical setting (eg, hospital laboratory); therefore, clinicians must maintain a high level of suspicion and provide care empirically. The ability to make a specific diagnosis is further clouded because a patient who has used a synthetic opioid may have also used certain prescription opioids or heroin, which can be detected by standard testing.
Blood and urine samples obtained early in care and sent to specialized laboratories may provide specific identification. Such testing is typically only done by reference laboratories, health departments, or law enforcement agencies. The information obtained from these analyses may help to understand the epidemiology of novel opioid abuse, prevent others from succumbing to addiction, and determine the cause of related deaths.
Which patients can be safely discharged from the ED after an opioid overdose?
Patients who survive reversal of an opioid overdose, whether from a conventional or novel opioid, are at extremely high risk of subsequent death from continued use, as well as from the initial exposure to a long-acting opioid that outlasts the reversal effects of naloxone. Such patients should undergo a sufficient observation period after the last dose of naloxone has been administered to allow its effects to dissipate. This is likely at least 2 hours, but may be longer in certain individuals. Attempts at establishing a link for the patient to long-term treatment or (where available) providing a naloxone rescue kit and training to patients and their families are worthwhile. Although some data support releasing responsive patients after a short, but safe interval after naloxone administration, the changing landscape of opioid use should prompt reconsideration of such practices.12
To whom should suspected opioid overdose patients be reported?
While most EPs are familiar with the management of patients with opioid-induced respiratory depression, atypical cases (eg, patients less responsive to naloxone, those who suffer cardiac arrest) or clusters of suspected cases should always be reported to a regional poison control center (PCC) or health department. The PCC is typically engaged in surveillance and works cooperatively with area EDs and public health officials to track and notify physicians of emerging trends. The epidemiological data derived from reports from a variety of hospitals allow health officials to effectively engage resources for public warnings, facilitate forensic identification of circulating products, and determine any unique clinical information that can then be broadly disseminated.
Case Conclusion
The patient was supported with BVM ventilations. Despite additional titrated IV naloxone (up to a total of 4 mg) the patient was nonresponsive and unarousable. She was intubated, and awoke several hours later. She fully recovered and subsequently was referred to both a harm-reduction and an opioid detoxification program. Analysis of her blood and urine, available several weeks later, confirmed an exposure to U47700.
1. Centers for Disease Control and Prevention. Health Alert Network. Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. https://emergency.cdc.gov/han/han00384.asp. Updated October 26, 2015. Accessed January 10, 2017.
2. MacQuarrie B. Synthetic opioids are getting into US by mail. Boston Globe. December 27, 2016. http://www.bostonglobe.com/metro/2016/12/26/synthetic-opioids-slipping-into-via-mail-security-experts-say/23TCEuIES8aEQYAWWHKCiI/story.html. Accessed January 10, 2017.
3. Lucyk SN, Nelson LS. Novel synthetic opioids: an opioid epidemic within an opioid epidemic. Ann Emerg Med. 2017;69(1):91-93. doi:10.1016/j.annemergmed.2016.08.445.
4. Mounteney J, Bo A, Oteo A; OteoEuropean Monitoring Centre for Drugs and Drug Addiction project group. The Internet and Drug Markets. Publications Office of the European Union, Luxembourg, Luxembourg; 2016:1-136. http://www.emcdda.europa.eu/system/files/publications/2155/TDXD16001ENN_FINAL. pdf. doi:10.2810/324608. Accessed January 17, 2017.
5. Associated Press. ‘Norco’ fentanyl overdose deaths rise to 14; problem spreads to Bay Area. Los Angeles Times. April 26, 2016. http://www.latimes.com/local/lanow/la-me-ln-norco-fentanyl-overdose-deaths-rise-to-14-problem-spreads-to-bay-area-20160426-story.html.
6. Centers for Disease Control and Prevention. Health Alert Network. Influx of fentanyl-laced counterfeit pills and toxic fentanyl-related compounds further increases risk of fentanyl-related overdose and fatalities. https://emergency.cdc.gov/han/han00395.asp. Accessed January 10, 2017.
7. Sandy E. Cleveland Scene. 236 heroin overdoses in Akron in 3 weeks; heroin being cut with elephant sedative. http://www.clevescene.com/scene-and-heard/archives/2016/07/14/akron-police-chief-heroin-being-cut-with-elephant-sedative-88-overdoses-since-july-5. Accessed January 10, 2017.
8. DEA issues carfentanil warning to police and public [news release]. Washington, DC: United States Drug Enforcement Administration; September 22, 2016. https://www.dea.gov/divisions/hq/2016/hq092216.shtml. Accessed January 10, 2017.
9. Armenian P, Olson A, Anaya A, Kurtz A, Ruegner R, Gerona RR. Fentanyl and a novel synthetic opioid U-47700 masquerading as street “Norco” in Central California: a case report. Ann Emerg Med. 2017;69(1):87-90. doi:10.1016/j.annemergmed.2016.06.014.
10. Schumann H, Erickson T, Thompson TM, Zautcke JL, Denton JS. Fentanyl epidemic in Chicago, Illinois and surrounding Cook County. Clin Toxicol (Phila). 2008;46(6):501-506. doi:10.1080/15563650701877374.
11. George AV, Lu JJ, Pisano MV, Metz J, Erickson TB. Carfentanil—an ultra potent opioid. Am J Emerg Med. 2010;28(4):530-532. doi:10.1016/j.ajem.2010.03.003.
12. Kolinsky D, Keim SM, Cohn BG, Schwarz ES, Yealy DM. Is a prehospital treat and release protocol for opioid overdose safe? J Emerg Med. 2017;52(1):52-58. doi:10.1016/j.jemermed.2016.09.015.
1. Centers for Disease Control and Prevention. Health Alert Network. Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. https://emergency.cdc.gov/han/han00384.asp. Updated October 26, 2015. Accessed January 10, 2017.
2. MacQuarrie B. Synthetic opioids are getting into US by mail. Boston Globe. December 27, 2016. http://www.bostonglobe.com/metro/2016/12/26/synthetic-opioids-slipping-into-via-mail-security-experts-say/23TCEuIES8aEQYAWWHKCiI/story.html. Accessed January 10, 2017.
3. Lucyk SN, Nelson LS. Novel synthetic opioids: an opioid epidemic within an opioid epidemic. Ann Emerg Med. 2017;69(1):91-93. doi:10.1016/j.annemergmed.2016.08.445.
4. Mounteney J, Bo A, Oteo A; OteoEuropean Monitoring Centre for Drugs and Drug Addiction project group. The Internet and Drug Markets. Publications Office of the European Union, Luxembourg, Luxembourg; 2016:1-136. http://www.emcdda.europa.eu/system/files/publications/2155/TDXD16001ENN_FINAL. pdf. doi:10.2810/324608. Accessed January 17, 2017.
5. Associated Press. ‘Norco’ fentanyl overdose deaths rise to 14; problem spreads to Bay Area. Los Angeles Times. April 26, 2016. http://www.latimes.com/local/lanow/la-me-ln-norco-fentanyl-overdose-deaths-rise-to-14-problem-spreads-to-bay-area-20160426-story.html.
6. Centers for Disease Control and Prevention. Health Alert Network. Influx of fentanyl-laced counterfeit pills and toxic fentanyl-related compounds further increases risk of fentanyl-related overdose and fatalities. https://emergency.cdc.gov/han/han00395.asp. Accessed January 10, 2017.
7. Sandy E. Cleveland Scene. 236 heroin overdoses in Akron in 3 weeks; heroin being cut with elephant sedative. http://www.clevescene.com/scene-and-heard/archives/2016/07/14/akron-police-chief-heroin-being-cut-with-elephant-sedative-88-overdoses-since-july-5. Accessed January 10, 2017.
8. DEA issues carfentanil warning to police and public [news release]. Washington, DC: United States Drug Enforcement Administration; September 22, 2016. https://www.dea.gov/divisions/hq/2016/hq092216.shtml. Accessed January 10, 2017.
9. Armenian P, Olson A, Anaya A, Kurtz A, Ruegner R, Gerona RR. Fentanyl and a novel synthetic opioid U-47700 masquerading as street “Norco” in Central California: a case report. Ann Emerg Med. 2017;69(1):87-90. doi:10.1016/j.annemergmed.2016.06.014.
10. Schumann H, Erickson T, Thompson TM, Zautcke JL, Denton JS. Fentanyl epidemic in Chicago, Illinois and surrounding Cook County. Clin Toxicol (Phila). 2008;46(6):501-506. doi:10.1080/15563650701877374.
11. George AV, Lu JJ, Pisano MV, Metz J, Erickson TB. Carfentanil—an ultra potent opioid. Am J Emerg Med. 2010;28(4):530-532. doi:10.1016/j.ajem.2010.03.003.
12. Kolinsky D, Keim SM, Cohn BG, Schwarz ES, Yealy DM. Is a prehospital treat and release protocol for opioid overdose safe? J Emerg Med. 2017;52(1):52-58. doi:10.1016/j.jemermed.2016.09.015.