User login
It’s Time to Reconsider Early-morning Testosterone Tests
PRACTICE CHANGER
Early-morning testosterone tests are necessary only for men younger than 45. Because the natural diurnal variation in testosterone levels tends to diminish with age, it is acceptable to test men ages 45 and older before 2 pm.1
STRENGTH OF RECOMMENDATION
B: Based on a retrospective cohort study.1
ILLUSTRATIVE CASE
You are finishing up a visit with a 62-year-old man who has erectile dysfunction (ED), and you want to evaluate for androgen deficiency. It’s already noon. Should you ask him to return for an early-morning visit so you can test his testosterone level?
Increasing public awareness of androgen deficiency has led to more men being tested for testosterone levels. Current Endocrine Society guidelines recommend against routine screening for androgen deficiency in men who do not have symptoms.2 However, for men with classic symptoms of androgen deficiency—such as decreased libido, ED, infertility, depression, osteoporosis, loss of secondary sexual characteristics, or reduced muscle bulk or strength—measurement of total testosterone level is recommended.2
Due to the natural diurnal variation in serum testosterone levels, the guidelines recommend collecting the sample in the early morning.2 This recommendation is based on small observational studies that included mostly men younger than 45, which found a significant difference in testosterone levels between samples drawn early in the morning and in the afternoon.3-5
In recent years, several studies have indicated that this variation declines as men age.4-6 Recently, researchers evaluated the effects of age and time of testing on men’s total testosterone levels.
STUDY SUMMARY
Differences in testosterone levels are significant only in younger men
Welliver et al1 performed a retrospective review of charts from a Minneapolis Veterans Affairs hospital. They identified 2,569 men seen for ED who had total testosterone levels measured between 7 AM and 2 PM in a 15-year period. Men whose total testosterone levels were outside the normal range (> 1,000 or < 50 ng/dL) or who had total testosterone drawn after 2 PM were excluded.
The authors analyzed the results based on age, creating one group for men younger than 40 and five-year age-groups for all other men. Using scatterplot techniques, they separated each age-group into two subgroups based on draw times—7 AM to 9 AM, or 9 AM to 2 PM—and compared the mean total testosterone level for each age and time.
Participants’ mean age was 63. Younger men (< 45) had the largest variation in serum total testosterone, with a large and significant decrease after 9 AM. Only the two youngest groups (ages < 40 and 40 to 44) showed a large decrease in total testosterone in specimens collected after 9 am, compared to those drawn earlier (mean difference, 207 and 149 ng/dL, respectively). This variation was not observed in patients older than 45. Although there was a statistically significant difference between early and later testosterone levels in men ages 70 to 74, the absolute difference—34 ng/dL (452 vs 418 ng/dL)—was unlikely to be clinically significant.
WHAT’S NEW
For older men, later testing will not affect results
This study confirms previous research indicating that the diurnal effect on testosterone levels becomes blunted with increasing age, at least in this group of men with ED. Allowing older men to have their total testosterone levels drawn until 2 PM would allow for greater patient flexibility in draw times, with little change in results.
CAVEATS
Study’s methodology cannot account for several potential confounders
This retrospective study analyzed a single random testosterone measurement from each participant, rather than repeat testosterone levels over the course of a day. However, the study was large (2,569 men) and used mean values, which should at least partially mitigate the effect of having only a single measurement from each participant.
The study measured total testosterone and did not account for potential confounding factors—such as obesity or use of testosterone replacement therapy or androgen deprivation therapy—that could affect sex hormone binding globulin, thus potentially altering total testosterone level. However, the authors estimated that less than 2% of the entire cohort was likely to have unrecognized hormonal manipulation with exogenous gonadotropins.
All of the men in the study were seen for ED, and it is possible that men with ED have more flattening of the diurnal variation than men without ED. However, we are unaware of other data that support this.
Up to 30% of men who have a low early-morning testosterone level may have a normal result when testing is repeated.2,5 Therefore, for all men who have low testosterone level test results, draw a repeat total testosterone level before 9 am to confirm the diagnosis. Also, this study did not evaluate the course of testosterone levels throughout the later afternoon and evening, and it remains unclear whether levels can be drawn even later in the day.
CHALLENGES TO IMPLEMENTATION
Your lab’s policies might require early-morning draws
There will probably be few barriers to implementing this change, unless local laboratory policies are inflexible regarding the timing of testosterone draws.
REFERENCES
1. Welliver RC Jr, Wiser HJ, Brannigan RE, et al. Validity of midday total testosterone levels in older men with erectile dysfunction. J Urol. 2014;192:165-169.
2. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536-2559.
3. Cooke RR, McIntosh JE, McIntosh RP. Circadian variation in serum free and non-SHBG-bound testosterone in normal men: measurements, and simulation using a mass action model. Clin Endocrinol (Oxf). 1993;39:163-171.
4. Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56:1278-1281.
5. Brambilla DJ, Matsumoto AM, Araujo AB, et al. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab. 2009;94:907-913.
6. Crawford ED, Barqawi AB, O’Donnell C, et al. The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007;100:509-513.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(7):418-419.
PRACTICE CHANGER
Early-morning testosterone tests are necessary only for men younger than 45. Because the natural diurnal variation in testosterone levels tends to diminish with age, it is acceptable to test men ages 45 and older before 2 pm.1
STRENGTH OF RECOMMENDATION
B: Based on a retrospective cohort study.1
ILLUSTRATIVE CASE
You are finishing up a visit with a 62-year-old man who has erectile dysfunction (ED), and you want to evaluate for androgen deficiency. It’s already noon. Should you ask him to return for an early-morning visit so you can test his testosterone level?
Increasing public awareness of androgen deficiency has led to more men being tested for testosterone levels. Current Endocrine Society guidelines recommend against routine screening for androgen deficiency in men who do not have symptoms.2 However, for men with classic symptoms of androgen deficiency—such as decreased libido, ED, infertility, depression, osteoporosis, loss of secondary sexual characteristics, or reduced muscle bulk or strength—measurement of total testosterone level is recommended.2
Due to the natural diurnal variation in serum testosterone levels, the guidelines recommend collecting the sample in the early morning.2 This recommendation is based on small observational studies that included mostly men younger than 45, which found a significant difference in testosterone levels between samples drawn early in the morning and in the afternoon.3-5
In recent years, several studies have indicated that this variation declines as men age.4-6 Recently, researchers evaluated the effects of age and time of testing on men’s total testosterone levels.
STUDY SUMMARY
Differences in testosterone levels are significant only in younger men
Welliver et al1 performed a retrospective review of charts from a Minneapolis Veterans Affairs hospital. They identified 2,569 men seen for ED who had total testosterone levels measured between 7 AM and 2 PM in a 15-year period. Men whose total testosterone levels were outside the normal range (> 1,000 or < 50 ng/dL) or who had total testosterone drawn after 2 PM were excluded.
The authors analyzed the results based on age, creating one group for men younger than 40 and five-year age-groups for all other men. Using scatterplot techniques, they separated each age-group into two subgroups based on draw times—7 AM to 9 AM, or 9 AM to 2 PM—and compared the mean total testosterone level for each age and time.
Participants’ mean age was 63. Younger men (< 45) had the largest variation in serum total testosterone, with a large and significant decrease after 9 AM. Only the two youngest groups (ages < 40 and 40 to 44) showed a large decrease in total testosterone in specimens collected after 9 am, compared to those drawn earlier (mean difference, 207 and 149 ng/dL, respectively). This variation was not observed in patients older than 45. Although there was a statistically significant difference between early and later testosterone levels in men ages 70 to 74, the absolute difference—34 ng/dL (452 vs 418 ng/dL)—was unlikely to be clinically significant.
WHAT’S NEW
For older men, later testing will not affect results
This study confirms previous research indicating that the diurnal effect on testosterone levels becomes blunted with increasing age, at least in this group of men with ED. Allowing older men to have their total testosterone levels drawn until 2 PM would allow for greater patient flexibility in draw times, with little change in results.
CAVEATS
Study’s methodology cannot account for several potential confounders
This retrospective study analyzed a single random testosterone measurement from each participant, rather than repeat testosterone levels over the course of a day. However, the study was large (2,569 men) and used mean values, which should at least partially mitigate the effect of having only a single measurement from each participant.
The study measured total testosterone and did not account for potential confounding factors—such as obesity or use of testosterone replacement therapy or androgen deprivation therapy—that could affect sex hormone binding globulin, thus potentially altering total testosterone level. However, the authors estimated that less than 2% of the entire cohort was likely to have unrecognized hormonal manipulation with exogenous gonadotropins.
All of the men in the study were seen for ED, and it is possible that men with ED have more flattening of the diurnal variation than men without ED. However, we are unaware of other data that support this.
Up to 30% of men who have a low early-morning testosterone level may have a normal result when testing is repeated.2,5 Therefore, for all men who have low testosterone level test results, draw a repeat total testosterone level before 9 am to confirm the diagnosis. Also, this study did not evaluate the course of testosterone levels throughout the later afternoon and evening, and it remains unclear whether levels can be drawn even later in the day.
CHALLENGES TO IMPLEMENTATION
Your lab’s policies might require early-morning draws
There will probably be few barriers to implementing this change, unless local laboratory policies are inflexible regarding the timing of testosterone draws.
REFERENCES
1. Welliver RC Jr, Wiser HJ, Brannigan RE, et al. Validity of midday total testosterone levels in older men with erectile dysfunction. J Urol. 2014;192:165-169.
2. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536-2559.
3. Cooke RR, McIntosh JE, McIntosh RP. Circadian variation in serum free and non-SHBG-bound testosterone in normal men: measurements, and simulation using a mass action model. Clin Endocrinol (Oxf). 1993;39:163-171.
4. Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56:1278-1281.
5. Brambilla DJ, Matsumoto AM, Araujo AB, et al. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab. 2009;94:907-913.
6. Crawford ED, Barqawi AB, O’Donnell C, et al. The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007;100:509-513.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(7):418-419.
PRACTICE CHANGER
Early-morning testosterone tests are necessary only for men younger than 45. Because the natural diurnal variation in testosterone levels tends to diminish with age, it is acceptable to test men ages 45 and older before 2 pm.1
STRENGTH OF RECOMMENDATION
B: Based on a retrospective cohort study.1
ILLUSTRATIVE CASE
You are finishing up a visit with a 62-year-old man who has erectile dysfunction (ED), and you want to evaluate for androgen deficiency. It’s already noon. Should you ask him to return for an early-morning visit so you can test his testosterone level?
Increasing public awareness of androgen deficiency has led to more men being tested for testosterone levels. Current Endocrine Society guidelines recommend against routine screening for androgen deficiency in men who do not have symptoms.2 However, for men with classic symptoms of androgen deficiency—such as decreased libido, ED, infertility, depression, osteoporosis, loss of secondary sexual characteristics, or reduced muscle bulk or strength—measurement of total testosterone level is recommended.2
Due to the natural diurnal variation in serum testosterone levels, the guidelines recommend collecting the sample in the early morning.2 This recommendation is based on small observational studies that included mostly men younger than 45, which found a significant difference in testosterone levels between samples drawn early in the morning and in the afternoon.3-5
In recent years, several studies have indicated that this variation declines as men age.4-6 Recently, researchers evaluated the effects of age and time of testing on men’s total testosterone levels.
STUDY SUMMARY
Differences in testosterone levels are significant only in younger men
Welliver et al1 performed a retrospective review of charts from a Minneapolis Veterans Affairs hospital. They identified 2,569 men seen for ED who had total testosterone levels measured between 7 AM and 2 PM in a 15-year period. Men whose total testosterone levels were outside the normal range (> 1,000 or < 50 ng/dL) or who had total testosterone drawn after 2 PM were excluded.
The authors analyzed the results based on age, creating one group for men younger than 40 and five-year age-groups for all other men. Using scatterplot techniques, they separated each age-group into two subgroups based on draw times—7 AM to 9 AM, or 9 AM to 2 PM—and compared the mean total testosterone level for each age and time.
Participants’ mean age was 63. Younger men (< 45) had the largest variation in serum total testosterone, with a large and significant decrease after 9 AM. Only the two youngest groups (ages < 40 and 40 to 44) showed a large decrease in total testosterone in specimens collected after 9 am, compared to those drawn earlier (mean difference, 207 and 149 ng/dL, respectively). This variation was not observed in patients older than 45. Although there was a statistically significant difference between early and later testosterone levels in men ages 70 to 74, the absolute difference—34 ng/dL (452 vs 418 ng/dL)—was unlikely to be clinically significant.
WHAT’S NEW
For older men, later testing will not affect results
This study confirms previous research indicating that the diurnal effect on testosterone levels becomes blunted with increasing age, at least in this group of men with ED. Allowing older men to have their total testosterone levels drawn until 2 PM would allow for greater patient flexibility in draw times, with little change in results.
CAVEATS
Study’s methodology cannot account for several potential confounders
This retrospective study analyzed a single random testosterone measurement from each participant, rather than repeat testosterone levels over the course of a day. However, the study was large (2,569 men) and used mean values, which should at least partially mitigate the effect of having only a single measurement from each participant.
The study measured total testosterone and did not account for potential confounding factors—such as obesity or use of testosterone replacement therapy or androgen deprivation therapy—that could affect sex hormone binding globulin, thus potentially altering total testosterone level. However, the authors estimated that less than 2% of the entire cohort was likely to have unrecognized hormonal manipulation with exogenous gonadotropins.
All of the men in the study were seen for ED, and it is possible that men with ED have more flattening of the diurnal variation than men without ED. However, we are unaware of other data that support this.
Up to 30% of men who have a low early-morning testosterone level may have a normal result when testing is repeated.2,5 Therefore, for all men who have low testosterone level test results, draw a repeat total testosterone level before 9 am to confirm the diagnosis. Also, this study did not evaluate the course of testosterone levels throughout the later afternoon and evening, and it remains unclear whether levels can be drawn even later in the day.
CHALLENGES TO IMPLEMENTATION
Your lab’s policies might require early-morning draws
There will probably be few barriers to implementing this change, unless local laboratory policies are inflexible regarding the timing of testosterone draws.
REFERENCES
1. Welliver RC Jr, Wiser HJ, Brannigan RE, et al. Validity of midday total testosterone levels in older men with erectile dysfunction. J Urol. 2014;192:165-169.
2. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536-2559.
3. Cooke RR, McIntosh JE, McIntosh RP. Circadian variation in serum free and non-SHBG-bound testosterone in normal men: measurements, and simulation using a mass action model. Clin Endocrinol (Oxf). 1993;39:163-171.
4. Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56:1278-1281.
5. Brambilla DJ, Matsumoto AM, Araujo AB, et al. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab. 2009;94:907-913.
6. Crawford ED, Barqawi AB, O’Donnell C, et al. The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007;100:509-513.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(7):418-419.
It’s time to reconsider early-morning testosterone tests
Early-morning testosterone tests are necessary only for men younger than age 45. Because the natural diurnal variation in testosterone levels tends to diminish with age, it is acceptable to test men ages 45 and older before 2 pm.1
Strength of recommendation
B: Based on a retrospective cohort study.
Welliver RC Jr, Wiser HJ, Brannigan RE, et al. Validity of midday total testosterone levels in older men with erectile dysfunction. J Urol. 2014;192:165-169.
Illustrative case
It’s noon, you are finishing up a visit with a 62-year-old man with erectile dysfunction (ED), and you want to evaluate for androgen deficiency. Should you ask him to return for an early-morning visit so you can test his testosterone level?
Increasing public awareness of androgen deficiency has led to more men being tested for testosterone levels. Current Endocrine Society guidelines recommend against routine screening for androgen deficiency in men who do not have symptoms.2 However, for men with classic symptoms of androgen deficiency—such as decreased libido, ED, infertility, depression, osteoporosis, loss of secondary sexual characteristics, or reduced muscle bulk or strength—measurement of total testosterone level is recommended.2
Due to the natural diurnal variation in serum testosterone levels, the guidelines recommend collecting the sample in the early morning.2 This recommendation is based on small observational studies that included men mostly younger than 45 years of age that found a significant difference in testosterone levels between samples drawn early in the morning and in the afternoon.3-5
In recent years, several studies have indicated that this variation declines as men age.4-6 Recently, researchers evaluated the effects of age and time of testing on men’s total testosterone levels.
STUDY SUMMARY: Differences in testosterone levels are significant only in younger men
Welliver et al1 performed a retrospective chart review of 2569 men seen at a Minneapolis Veterans Affairs hospital for ED who had total testosterone levels measured between 7 am and 2 pm over a 15-year period. Men whose total testosterone levels were outside the normal range (>1000 or <50 ng/dL) or who had total testosterone drawn after 2 pm were excluded. The authors analyzed the results based on age, creating one group for men ages <40 years and 5-year age groups for all other men. Using scatterplot techniques, they separated each age group into 2 subgroups based on draw times—7 am to 9 am, or 9 am to 2 pm—and compared the mean total testosterone level for each age and time.
The participants’ mean age was 63 years. Younger men (<45 years) had the largest variation in serum total testosterone, with a large and significant decrease after 9 am. Only the youngest 2 groups (ages <40 and 40-44 years) showed a large decrease in total testosterone in specimens collected after 9 am compared to those drawn between 7 am and 9 am (mean difference 207 and 149 ng/dL, respectively). This variation was not observed in patients over age 45. Although there was a statistically significant difference between early and later testosterone levels in men ages 70 to 74 years, the absolute difference—34 ng/dL (452 vs 418 ng/dL)—was unlikely to be clinically significant.
WHAT'S NEW: For older men, later testing will not affect results
This study confirms previous research showing that the diurnal effect on testosterone levels becomes blunted with increasing age, at least in this group of men with ED. Allowing older men to have total testosterone levels drawn until 2 pm would allow for greater patient flexibility in draw times with little change in results.
CAVEATS: Study's methodology cannot account for several potential confounders
This retrospective study analyzed only a single random testosterone level measurement from each participant, rather than repeat testosterone levels over the course of a day. However, the study was large (2569 men) and it used mean values, which should at least partially mitigate the effect of having only a single level from each participant.
The study measured total testosterone and did not account for potential confounding factors—such as obesity or use of testosterone replacement therapy or androgen deprivation therapy—that could affect sexhormone binding globulin, thus potentially altering total testosterone level. However, the authors estimated that less than 2% of the entire cohort were likely to have unrecognized hormonal manipulation with exogenous gonadotropins.
All of the men in the study were seen for ED, and it could be that men with ED have more flattening of the diurnal variation than men without ED; however, we are unaware of other data that support this.
Up to 30% of men who have an early-morning testosterone level that is low may have a normal result when testing is repeated.2,5 Therefore, for all men who have low testosterone level test results, draw a repeat total testosterone level before 9 am to confirm the diagnosis. Also, this study did not evaluate the course of testosterone levels throughout the later afternoon and evening, and it remains unclear whether levels can be drawn even later in the day.
CHALLENGES TO IMPLEMENTATION: Your lab's policies might require early-morning draws
There will probably be few barriers to implementing this change, unless local laboratory policies are inflexible regarding the timing of testosterone draws.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Welliver RC Jr, Wiser HJ, Brannigan RE, et al. Validity of midday total testosterone levels in older men with erectile dysfunction. J Urol. 2014;192:165-169.
2. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536-2559.
3. Cooke RR, McIntosh JE, McIntosh RP. Circadian variation in serum free and non-SHBG-bound testosterone in normal men: measurements, and simulation using a mass action model. Clin Endocrinol (Oxf). 1993;39:163-171.
4. Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56:1278-1281.
5. Brambilla DJ, Matsumoto AM, Araujo AB, et al. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab. 2009;94:907-913.
6. Crawford ED, Barqawi AB, O’Donnell C, et al. The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007;100:509-513.
Early-morning testosterone tests are necessary only for men younger than age 45. Because the natural diurnal variation in testosterone levels tends to diminish with age, it is acceptable to test men ages 45 and older before 2 pm.1
Strength of recommendation
B: Based on a retrospective cohort study.
Welliver RC Jr, Wiser HJ, Brannigan RE, et al. Validity of midday total testosterone levels in older men with erectile dysfunction. J Urol. 2014;192:165-169.
Illustrative case
It’s noon, you are finishing up a visit with a 62-year-old man with erectile dysfunction (ED), and you want to evaluate for androgen deficiency. Should you ask him to return for an early-morning visit so you can test his testosterone level?
Increasing public awareness of androgen deficiency has led to more men being tested for testosterone levels. Current Endocrine Society guidelines recommend against routine screening for androgen deficiency in men who do not have symptoms.2 However, for men with classic symptoms of androgen deficiency—such as decreased libido, ED, infertility, depression, osteoporosis, loss of secondary sexual characteristics, or reduced muscle bulk or strength—measurement of total testosterone level is recommended.2
Due to the natural diurnal variation in serum testosterone levels, the guidelines recommend collecting the sample in the early morning.2 This recommendation is based on small observational studies that included men mostly younger than 45 years of age that found a significant difference in testosterone levels between samples drawn early in the morning and in the afternoon.3-5
In recent years, several studies have indicated that this variation declines as men age.4-6 Recently, researchers evaluated the effects of age and time of testing on men’s total testosterone levels.
STUDY SUMMARY: Differences in testosterone levels are significant only in younger men
Welliver et al1 performed a retrospective chart review of 2569 men seen at a Minneapolis Veterans Affairs hospital for ED who had total testosterone levels measured between 7 am and 2 pm over a 15-year period. Men whose total testosterone levels were outside the normal range (>1000 or <50 ng/dL) or who had total testosterone drawn after 2 pm were excluded. The authors analyzed the results based on age, creating one group for men ages <40 years and 5-year age groups for all other men. Using scatterplot techniques, they separated each age group into 2 subgroups based on draw times—7 am to 9 am, or 9 am to 2 pm—and compared the mean total testosterone level for each age and time.
The participants’ mean age was 63 years. Younger men (<45 years) had the largest variation in serum total testosterone, with a large and significant decrease after 9 am. Only the youngest 2 groups (ages <40 and 40-44 years) showed a large decrease in total testosterone in specimens collected after 9 am compared to those drawn between 7 am and 9 am (mean difference 207 and 149 ng/dL, respectively). This variation was not observed in patients over age 45. Although there was a statistically significant difference between early and later testosterone levels in men ages 70 to 74 years, the absolute difference—34 ng/dL (452 vs 418 ng/dL)—was unlikely to be clinically significant.
WHAT'S NEW: For older men, later testing will not affect results
This study confirms previous research showing that the diurnal effect on testosterone levels becomes blunted with increasing age, at least in this group of men with ED. Allowing older men to have total testosterone levels drawn until 2 pm would allow for greater patient flexibility in draw times with little change in results.
CAVEATS: Study's methodology cannot account for several potential confounders
This retrospective study analyzed only a single random testosterone level measurement from each participant, rather than repeat testosterone levels over the course of a day. However, the study was large (2569 men) and it used mean values, which should at least partially mitigate the effect of having only a single level from each participant.
The study measured total testosterone and did not account for potential confounding factors—such as obesity or use of testosterone replacement therapy or androgen deprivation therapy—that could affect sexhormone binding globulin, thus potentially altering total testosterone level. However, the authors estimated that less than 2% of the entire cohort were likely to have unrecognized hormonal manipulation with exogenous gonadotropins.
All of the men in the study were seen for ED, and it could be that men with ED have more flattening of the diurnal variation than men without ED; however, we are unaware of other data that support this.
Up to 30% of men who have an early-morning testosterone level that is low may have a normal result when testing is repeated.2,5 Therefore, for all men who have low testosterone level test results, draw a repeat total testosterone level before 9 am to confirm the diagnosis. Also, this study did not evaluate the course of testosterone levels throughout the later afternoon and evening, and it remains unclear whether levels can be drawn even later in the day.
CHALLENGES TO IMPLEMENTATION: Your lab's policies might require early-morning draws
There will probably be few barriers to implementing this change, unless local laboratory policies are inflexible regarding the timing of testosterone draws.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Early-morning testosterone tests are necessary only for men younger than age 45. Because the natural diurnal variation in testosterone levels tends to diminish with age, it is acceptable to test men ages 45 and older before 2 pm.1
Strength of recommendation
B: Based on a retrospective cohort study.
Welliver RC Jr, Wiser HJ, Brannigan RE, et al. Validity of midday total testosterone levels in older men with erectile dysfunction. J Urol. 2014;192:165-169.
Illustrative case
It’s noon, you are finishing up a visit with a 62-year-old man with erectile dysfunction (ED), and you want to evaluate for androgen deficiency. Should you ask him to return for an early-morning visit so you can test his testosterone level?
Increasing public awareness of androgen deficiency has led to more men being tested for testosterone levels. Current Endocrine Society guidelines recommend against routine screening for androgen deficiency in men who do not have symptoms.2 However, for men with classic symptoms of androgen deficiency—such as decreased libido, ED, infertility, depression, osteoporosis, loss of secondary sexual characteristics, or reduced muscle bulk or strength—measurement of total testosterone level is recommended.2
Due to the natural diurnal variation in serum testosterone levels, the guidelines recommend collecting the sample in the early morning.2 This recommendation is based on small observational studies that included men mostly younger than 45 years of age that found a significant difference in testosterone levels between samples drawn early in the morning and in the afternoon.3-5
In recent years, several studies have indicated that this variation declines as men age.4-6 Recently, researchers evaluated the effects of age and time of testing on men’s total testosterone levels.
STUDY SUMMARY: Differences in testosterone levels are significant only in younger men
Welliver et al1 performed a retrospective chart review of 2569 men seen at a Minneapolis Veterans Affairs hospital for ED who had total testosterone levels measured between 7 am and 2 pm over a 15-year period. Men whose total testosterone levels were outside the normal range (>1000 or <50 ng/dL) or who had total testosterone drawn after 2 pm were excluded. The authors analyzed the results based on age, creating one group for men ages <40 years and 5-year age groups for all other men. Using scatterplot techniques, they separated each age group into 2 subgroups based on draw times—7 am to 9 am, or 9 am to 2 pm—and compared the mean total testosterone level for each age and time.
The participants’ mean age was 63 years. Younger men (<45 years) had the largest variation in serum total testosterone, with a large and significant decrease after 9 am. Only the youngest 2 groups (ages <40 and 40-44 years) showed a large decrease in total testosterone in specimens collected after 9 am compared to those drawn between 7 am and 9 am (mean difference 207 and 149 ng/dL, respectively). This variation was not observed in patients over age 45. Although there was a statistically significant difference between early and later testosterone levels in men ages 70 to 74 years, the absolute difference—34 ng/dL (452 vs 418 ng/dL)—was unlikely to be clinically significant.
WHAT'S NEW: For older men, later testing will not affect results
This study confirms previous research showing that the diurnal effect on testosterone levels becomes blunted with increasing age, at least in this group of men with ED. Allowing older men to have total testosterone levels drawn until 2 pm would allow for greater patient flexibility in draw times with little change in results.
CAVEATS: Study's methodology cannot account for several potential confounders
This retrospective study analyzed only a single random testosterone level measurement from each participant, rather than repeat testosterone levels over the course of a day. However, the study was large (2569 men) and it used mean values, which should at least partially mitigate the effect of having only a single level from each participant.
The study measured total testosterone and did not account for potential confounding factors—such as obesity or use of testosterone replacement therapy or androgen deprivation therapy—that could affect sexhormone binding globulin, thus potentially altering total testosterone level. However, the authors estimated that less than 2% of the entire cohort were likely to have unrecognized hormonal manipulation with exogenous gonadotropins.
All of the men in the study were seen for ED, and it could be that men with ED have more flattening of the diurnal variation than men without ED; however, we are unaware of other data that support this.
Up to 30% of men who have an early-morning testosterone level that is low may have a normal result when testing is repeated.2,5 Therefore, for all men who have low testosterone level test results, draw a repeat total testosterone level before 9 am to confirm the diagnosis. Also, this study did not evaluate the course of testosterone levels throughout the later afternoon and evening, and it remains unclear whether levels can be drawn even later in the day.
CHALLENGES TO IMPLEMENTATION: Your lab's policies might require early-morning draws
There will probably be few barriers to implementing this change, unless local laboratory policies are inflexible regarding the timing of testosterone draws.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Welliver RC Jr, Wiser HJ, Brannigan RE, et al. Validity of midday total testosterone levels in older men with erectile dysfunction. J Urol. 2014;192:165-169.
2. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536-2559.
3. Cooke RR, McIntosh JE, McIntosh RP. Circadian variation in serum free and non-SHBG-bound testosterone in normal men: measurements, and simulation using a mass action model. Clin Endocrinol (Oxf). 1993;39:163-171.
4. Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56:1278-1281.
5. Brambilla DJ, Matsumoto AM, Araujo AB, et al. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab. 2009;94:907-913.
6. Crawford ED, Barqawi AB, O’Donnell C, et al. The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007;100:509-513.
1. Welliver RC Jr, Wiser HJ, Brannigan RE, et al. Validity of midday total testosterone levels in older men with erectile dysfunction. J Urol. 2014;192:165-169.
2. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536-2559.
3. Cooke RR, McIntosh JE, McIntosh RP. Circadian variation in serum free and non-SHBG-bound testosterone in normal men: measurements, and simulation using a mass action model. Clin Endocrinol (Oxf). 1993;39:163-171.
4. Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56:1278-1281.
5. Brambilla DJ, Matsumoto AM, Araujo AB, et al. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab. 2009;94:907-913.
6. Crawford ED, Barqawi AB, O’Donnell C, et al. The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007;100:509-513.
Copyright © 2015 Family Physicians Inquiries Network. All rights reserved.
A Simple Way to Reduce Catheter-associated UTIs
PRACTICE CHANGER
Ensure that antibiotics are administered to surgical patients when their urinary catheter is removed to reduce the risk for urinary tract infections (UTIs).1
STRENGTH OF RECOMMENDATION
B: Based on a meta-analysis.1
ILLUSTRATIVE CASE
A 49-year-old man was admitted to the hospital for resection of a vertebral mass. He is almost ready for discharge, and soon his urinary catheter will be removed. Should he be given an antibiotic when his catheter is removed to prevent a UTI?
Approximately 15% to 25% of hospitalized patients receive a urinary catheter, typically during the perioperative period.2 UTIs are the most common hospital-acquired infections, and virtually all of these UTIs are caused by instrumentation of the urinary tract, primarily by catheters.2
Although the mortality rate among patients with catheter-associated UTIs (CAUTIs) is just 2.3%, CAUTIs are the leading cause of hospital-acquired bacteremia, which increases morbidity and length of stay.2 The most common pathogens for CAUTIs are Escherichia coli (21.4%), Candida species (21%), and Enterococcus species (14.9%).2Pseudomonas aeruginosa, Klebsiella, and Enterobacter species comprise the bulk of the remainder.2
Support for antibiotic prophylaxis has historically been equivocal
Until now, no data clearly supported routine use of prophylactic antibiotics after urinary catheterization. CDC guidelines published in 2009 outline which patients are appropriate candidates for catheterization but do not recommend routine use of antibiotics to prevent CAUTIs.2 A 2014 Infectious Diseases Society of America practice recommendation, which was published after the study reported on here, states the benefit of antibiotics at the time of catheter removal is an unresolved issue.3
STUDY SUMMARY
Analysis shows prophylactic antibiotics reduce UTIs
Marschall et al1 searched multiple databases for studies published between 1947 and 2012 that evaluated prophylactic use of antibiotics at the time of urinary catheter removal. The endpoint for their analysis was symptomatic UTI, which they defined as bacteriuria plus at least one clinical symptom. Trials were excluded if patients had suprapubic catheters or if antibiotics were started shortly after the catheter was inserted.
The authors analyzed seven studies. Six were randomized controlled trials, of which one was unpublished. The seventh trial was a nonrandomized study that compared outcomes of patients of two surgeons, one of whom used prophylactic antibiotics and one who did not. Five studies enrolled surgical patients exclusively, including two that focused on urology patients. In all of the studies, patients had a urinary catheter in place for fewer than 15 days. The duration of antibiotic treatment varied from a single dose to three days. The antibiotics used included trimethoprim/sulfamethoxazole, nitrofurantoin, ciprofloxacin, and a cephalosporin.
Antibiotic prophylaxis significantly reduced the rate of CAUTIs. The absolute risk reduction was 5.8%; the rate of CAUTIs was 4.7% in the group treated with antibiotics and 10.5% in the control group. The number needed to treat to prevent one CAUTI was 17, with a risk ratio (RR) of .45. The RR varied only slightly (.36) when the researchers repeated their analysis but excluded the unpublished trial and remained at .45 when they analyzed only studies of surgical patients.
The reduction in CAUTIs remained consistent despite varying lengths of antibiotic administration and choice of antimicrobial agents. However, when the authors looked at pooled results just from the two studies that included both surgical and medical patients, they found no decrease in CAUTIs.
WHAT’S NEW
We now have an effective way to reduce CAUTIs
Prophylactic use of antibiotics when a urinary catheter is removed appears to reduce the rate of CAUTIs by more than 50% in surgical patients. The 2009 CDC guidelines on CAUTI prevention emphasize the use of appropriate infection control measures and limiting the duration of urinary catheter use.2 Now there are data showing a reduction in the incidence of CAUTIs when prophylactic antibiotics are given during catheter removal.
Continue for caveats and challenges >>
CAVEATS
Results may not apply to nonsurgical patients
This meta-analysis does not provide enough information to identify which patients are most likely to benefit from antibiotic prophylaxis. Most patients (92%) in this analysis had undergone surgery, but urinary catheterization is common among medically hospitalized patients. Studies of antibiotic prophylaxis at the time of catheter removal in nonsurgical patients are needed to strengthen the recommendation of this practice for all patients.
Some of the studies analyzed may have been biased. The authors determined that most of the studies in their meta-analysis were at high risk for attrition bias because there was potential for systematic differences in withdrawals between the treatment and control groups. In addition, in most studies, the randomization and allocation appeared to be inadequate, which increased the risk for selection bias.
CHALLENGES TO IMPLEMENTATION
Which antibiotics to use—and for how long—remains unclear
Antibiotic choice depends upon institutional policies and local resistance patterns, which complicates making universal recommendations. The optimal duration of treatment also is unknown, although this meta-analysis suggests that prophylaxis for three days or less can reduce CAUTI risk.
Catheters impregnated with antimicrobials or with microbial resistance barriers may be an alternative to administration of antibiotics at catheter removal, but in preliminary studies, these devices have not been shown to reduce the incidence of CAUTIs.4,5 Increasing antimicrobial resistance also complicates the widespread use of prophylaxis.
REFERENCES
1. Marschall J, Carpenter CR, Fowler S, et al; CDC Prevention Epicenters Program. Antibiotic prophylaxis for urinary tract infections after removal of urinary catheter: meta-analysis. BMJ. 2013;346:f3147.
2. Gould CV, Umscheid CA, Agarwal RK, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. www.cdc.gov/hicpac/pdf/cauti/cautiguideline2009final.pdf. Accessed November 12, 2014.
3. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35:464-479.
4. Pickard R, Lam T, Maclennan G, et al. Types of urethral catheter for reducing symptomatic urinary tract infections in hospitalised adults requiring short-term catheterisation: multicentre randomised controlled trial and economic evaluation of antimicrobial- and antiseptic-impregnated urethral catheters (the CATHETER trial). Health Technol Assess. 2012;16:1-197.
5. Pickard R, Lam T, MacLennan G, et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: a multicentre randomised controlled trial. Lancet. 2012;380:1927-1935.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(5):E10-E12.
PRACTICE CHANGER
Ensure that antibiotics are administered to surgical patients when their urinary catheter is removed to reduce the risk for urinary tract infections (UTIs).1
STRENGTH OF RECOMMENDATION
B: Based on a meta-analysis.1
ILLUSTRATIVE CASE
A 49-year-old man was admitted to the hospital for resection of a vertebral mass. He is almost ready for discharge, and soon his urinary catheter will be removed. Should he be given an antibiotic when his catheter is removed to prevent a UTI?
Approximately 15% to 25% of hospitalized patients receive a urinary catheter, typically during the perioperative period.2 UTIs are the most common hospital-acquired infections, and virtually all of these UTIs are caused by instrumentation of the urinary tract, primarily by catheters.2
Although the mortality rate among patients with catheter-associated UTIs (CAUTIs) is just 2.3%, CAUTIs are the leading cause of hospital-acquired bacteremia, which increases morbidity and length of stay.2 The most common pathogens for CAUTIs are Escherichia coli (21.4%), Candida species (21%), and Enterococcus species (14.9%).2Pseudomonas aeruginosa, Klebsiella, and Enterobacter species comprise the bulk of the remainder.2
Support for antibiotic prophylaxis has historically been equivocal
Until now, no data clearly supported routine use of prophylactic antibiotics after urinary catheterization. CDC guidelines published in 2009 outline which patients are appropriate candidates for catheterization but do not recommend routine use of antibiotics to prevent CAUTIs.2 A 2014 Infectious Diseases Society of America practice recommendation, which was published after the study reported on here, states the benefit of antibiotics at the time of catheter removal is an unresolved issue.3
STUDY SUMMARY
Analysis shows prophylactic antibiotics reduce UTIs
Marschall et al1 searched multiple databases for studies published between 1947 and 2012 that evaluated prophylactic use of antibiotics at the time of urinary catheter removal. The endpoint for their analysis was symptomatic UTI, which they defined as bacteriuria plus at least one clinical symptom. Trials were excluded if patients had suprapubic catheters or if antibiotics were started shortly after the catheter was inserted.
The authors analyzed seven studies. Six were randomized controlled trials, of which one was unpublished. The seventh trial was a nonrandomized study that compared outcomes of patients of two surgeons, one of whom used prophylactic antibiotics and one who did not. Five studies enrolled surgical patients exclusively, including two that focused on urology patients. In all of the studies, patients had a urinary catheter in place for fewer than 15 days. The duration of antibiotic treatment varied from a single dose to three days. The antibiotics used included trimethoprim/sulfamethoxazole, nitrofurantoin, ciprofloxacin, and a cephalosporin.
Antibiotic prophylaxis significantly reduced the rate of CAUTIs. The absolute risk reduction was 5.8%; the rate of CAUTIs was 4.7% in the group treated with antibiotics and 10.5% in the control group. The number needed to treat to prevent one CAUTI was 17, with a risk ratio (RR) of .45. The RR varied only slightly (.36) when the researchers repeated their analysis but excluded the unpublished trial and remained at .45 when they analyzed only studies of surgical patients.
The reduction in CAUTIs remained consistent despite varying lengths of antibiotic administration and choice of antimicrobial agents. However, when the authors looked at pooled results just from the two studies that included both surgical and medical patients, they found no decrease in CAUTIs.
WHAT’S NEW
We now have an effective way to reduce CAUTIs
Prophylactic use of antibiotics when a urinary catheter is removed appears to reduce the rate of CAUTIs by more than 50% in surgical patients. The 2009 CDC guidelines on CAUTI prevention emphasize the use of appropriate infection control measures and limiting the duration of urinary catheter use.2 Now there are data showing a reduction in the incidence of CAUTIs when prophylactic antibiotics are given during catheter removal.
Continue for caveats and challenges >>
CAVEATS
Results may not apply to nonsurgical patients
This meta-analysis does not provide enough information to identify which patients are most likely to benefit from antibiotic prophylaxis. Most patients (92%) in this analysis had undergone surgery, but urinary catheterization is common among medically hospitalized patients. Studies of antibiotic prophylaxis at the time of catheter removal in nonsurgical patients are needed to strengthen the recommendation of this practice for all patients.
Some of the studies analyzed may have been biased. The authors determined that most of the studies in their meta-analysis were at high risk for attrition bias because there was potential for systematic differences in withdrawals between the treatment and control groups. In addition, in most studies, the randomization and allocation appeared to be inadequate, which increased the risk for selection bias.
CHALLENGES TO IMPLEMENTATION
Which antibiotics to use—and for how long—remains unclear
Antibiotic choice depends upon institutional policies and local resistance patterns, which complicates making universal recommendations. The optimal duration of treatment also is unknown, although this meta-analysis suggests that prophylaxis for three days or less can reduce CAUTI risk.
Catheters impregnated with antimicrobials or with microbial resistance barriers may be an alternative to administration of antibiotics at catheter removal, but in preliminary studies, these devices have not been shown to reduce the incidence of CAUTIs.4,5 Increasing antimicrobial resistance also complicates the widespread use of prophylaxis.
REFERENCES
1. Marschall J, Carpenter CR, Fowler S, et al; CDC Prevention Epicenters Program. Antibiotic prophylaxis for urinary tract infections after removal of urinary catheter: meta-analysis. BMJ. 2013;346:f3147.
2. Gould CV, Umscheid CA, Agarwal RK, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. www.cdc.gov/hicpac/pdf/cauti/cautiguideline2009final.pdf. Accessed November 12, 2014.
3. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35:464-479.
4. Pickard R, Lam T, Maclennan G, et al. Types of urethral catheter for reducing symptomatic urinary tract infections in hospitalised adults requiring short-term catheterisation: multicentre randomised controlled trial and economic evaluation of antimicrobial- and antiseptic-impregnated urethral catheters (the CATHETER trial). Health Technol Assess. 2012;16:1-197.
5. Pickard R, Lam T, MacLennan G, et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: a multicentre randomised controlled trial. Lancet. 2012;380:1927-1935.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(5):E10-E12.
PRACTICE CHANGER
Ensure that antibiotics are administered to surgical patients when their urinary catheter is removed to reduce the risk for urinary tract infections (UTIs).1
STRENGTH OF RECOMMENDATION
B: Based on a meta-analysis.1
ILLUSTRATIVE CASE
A 49-year-old man was admitted to the hospital for resection of a vertebral mass. He is almost ready for discharge, and soon his urinary catheter will be removed. Should he be given an antibiotic when his catheter is removed to prevent a UTI?
Approximately 15% to 25% of hospitalized patients receive a urinary catheter, typically during the perioperative period.2 UTIs are the most common hospital-acquired infections, and virtually all of these UTIs are caused by instrumentation of the urinary tract, primarily by catheters.2
Although the mortality rate among patients with catheter-associated UTIs (CAUTIs) is just 2.3%, CAUTIs are the leading cause of hospital-acquired bacteremia, which increases morbidity and length of stay.2 The most common pathogens for CAUTIs are Escherichia coli (21.4%), Candida species (21%), and Enterococcus species (14.9%).2Pseudomonas aeruginosa, Klebsiella, and Enterobacter species comprise the bulk of the remainder.2
Support for antibiotic prophylaxis has historically been equivocal
Until now, no data clearly supported routine use of prophylactic antibiotics after urinary catheterization. CDC guidelines published in 2009 outline which patients are appropriate candidates for catheterization but do not recommend routine use of antibiotics to prevent CAUTIs.2 A 2014 Infectious Diseases Society of America practice recommendation, which was published after the study reported on here, states the benefit of antibiotics at the time of catheter removal is an unresolved issue.3
STUDY SUMMARY
Analysis shows prophylactic antibiotics reduce UTIs
Marschall et al1 searched multiple databases for studies published between 1947 and 2012 that evaluated prophylactic use of antibiotics at the time of urinary catheter removal. The endpoint for their analysis was symptomatic UTI, which they defined as bacteriuria plus at least one clinical symptom. Trials were excluded if patients had suprapubic catheters or if antibiotics were started shortly after the catheter was inserted.
The authors analyzed seven studies. Six were randomized controlled trials, of which one was unpublished. The seventh trial was a nonrandomized study that compared outcomes of patients of two surgeons, one of whom used prophylactic antibiotics and one who did not. Five studies enrolled surgical patients exclusively, including two that focused on urology patients. In all of the studies, patients had a urinary catheter in place for fewer than 15 days. The duration of antibiotic treatment varied from a single dose to three days. The antibiotics used included trimethoprim/sulfamethoxazole, nitrofurantoin, ciprofloxacin, and a cephalosporin.
Antibiotic prophylaxis significantly reduced the rate of CAUTIs. The absolute risk reduction was 5.8%; the rate of CAUTIs was 4.7% in the group treated with antibiotics and 10.5% in the control group. The number needed to treat to prevent one CAUTI was 17, with a risk ratio (RR) of .45. The RR varied only slightly (.36) when the researchers repeated their analysis but excluded the unpublished trial and remained at .45 when they analyzed only studies of surgical patients.
The reduction in CAUTIs remained consistent despite varying lengths of antibiotic administration and choice of antimicrobial agents. However, when the authors looked at pooled results just from the two studies that included both surgical and medical patients, they found no decrease in CAUTIs.
WHAT’S NEW
We now have an effective way to reduce CAUTIs
Prophylactic use of antibiotics when a urinary catheter is removed appears to reduce the rate of CAUTIs by more than 50% in surgical patients. The 2009 CDC guidelines on CAUTI prevention emphasize the use of appropriate infection control measures and limiting the duration of urinary catheter use.2 Now there are data showing a reduction in the incidence of CAUTIs when prophylactic antibiotics are given during catheter removal.
Continue for caveats and challenges >>
CAVEATS
Results may not apply to nonsurgical patients
This meta-analysis does not provide enough information to identify which patients are most likely to benefit from antibiotic prophylaxis. Most patients (92%) in this analysis had undergone surgery, but urinary catheterization is common among medically hospitalized patients. Studies of antibiotic prophylaxis at the time of catheter removal in nonsurgical patients are needed to strengthen the recommendation of this practice for all patients.
Some of the studies analyzed may have been biased. The authors determined that most of the studies in their meta-analysis were at high risk for attrition bias because there was potential for systematic differences in withdrawals between the treatment and control groups. In addition, in most studies, the randomization and allocation appeared to be inadequate, which increased the risk for selection bias.
CHALLENGES TO IMPLEMENTATION
Which antibiotics to use—and for how long—remains unclear
Antibiotic choice depends upon institutional policies and local resistance patterns, which complicates making universal recommendations. The optimal duration of treatment also is unknown, although this meta-analysis suggests that prophylaxis for three days or less can reduce CAUTI risk.
Catheters impregnated with antimicrobials or with microbial resistance barriers may be an alternative to administration of antibiotics at catheter removal, but in preliminary studies, these devices have not been shown to reduce the incidence of CAUTIs.4,5 Increasing antimicrobial resistance also complicates the widespread use of prophylaxis.
REFERENCES
1. Marschall J, Carpenter CR, Fowler S, et al; CDC Prevention Epicenters Program. Antibiotic prophylaxis for urinary tract infections after removal of urinary catheter: meta-analysis. BMJ. 2013;346:f3147.
2. Gould CV, Umscheid CA, Agarwal RK, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. www.cdc.gov/hicpac/pdf/cauti/cautiguideline2009final.pdf. Accessed November 12, 2014.
3. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35:464-479.
4. Pickard R, Lam T, Maclennan G, et al. Types of urethral catheter for reducing symptomatic urinary tract infections in hospitalised adults requiring short-term catheterisation: multicentre randomised controlled trial and economic evaluation of antimicrobial- and antiseptic-impregnated urethral catheters (the CATHETER trial). Health Technol Assess. 2012;16:1-197.
5. Pickard R, Lam T, MacLennan G, et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: a multicentre randomised controlled trial. Lancet. 2012;380:1927-1935.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(5):E10-E12.
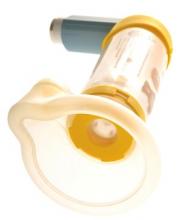
Think Twice About Nebulizers for Asthma Attacks
PRACTICE CHANGER
Stop ordering nebulizers to deliver β-agonists to patients older than 2 who have mild or moderate asthma exacerbations. A metered-dose inhaler (MDI) with a spacer produces the same benefits with fewer adverse effects.1
STRENGTH OF RECOMMENDATION
A: Based on an updated Cochrane meta-analysis of 39 randomized controlled trials (RCTs). 1
ILLUSTRATIVE CASE
A 6-year-old girl with a history of reactive airway disease comes to your office complaining of cough and wheezing. On exam, she has mild retractions, a respiratory rate of 35 breaths/min, and an O2 saturation of 96% on room air. Her lung fields are diffusely wheezy. Her parents would like to keep her out of the hospital. How should you order her albuterol to decrease her wheezing and minimize adverse effects?
Asthma affects nearly 19 million adults and 7 million children in the United States.2 Asthma exacerbations are the third most common reason for hospitalization in children.2,3 Treatment usually requires multiple agents, including inhaled β-agonists. These are most effective when delivered to the peripheral airways, which is a challenge during an asthma exacerbation because of airway swelling and rapid breathing. Two devices have been developed to effectively deliver medication to the peripheral airways: nebulizers and MDIs with a holding chamber (spacer).1
Several studies have demonstrated that for mild to moderate asthma exacerbations, administering a β-agonist via an MDI with a spacer is as effective as using a nebulizer.4,5 Asthma treatment guidelines also state that spacers are either comparable or preferable to nebulizers for β-agonist administration in children and adults.6,7 However, based on our experience, clinicians still frequently order nebulizer treatments for patients with asthma exacerbations, despite several advantages of MDIs with spacers. Notably, they cost less and don’t require maintenance or a power source. Clinicians administered nebulizer therapy at more than 3.6 million emergency department (ED) visits in 2006.8
In this latest Cochrane review, Cates et al1 added four new studies to those included in their earlier Cochrane meta-analysis and evaluated what, if any, effect these studies had on our understanding of nebulizers versus MDIs with spacers.
STUDY SUMMARY
Outcomes with nebulizers are no better than those with spacers
This systematic review and meta-analysis pooled the results of RCTs comparing spacers to nebulizers for administering β-agonists during acute, non–life-threatening asthma exacerbations.1 The authors reviewed studies conducted in EDs, hospitals, and outpatient settings that included children and adults. The primary outcomes were hospital admission rates and duration of hospital stay. Secondary outcomes included time spent in the ED, change in pulse rate, and incidence of tremor.
Cates et al1 analyzed 39 trials that included 1,897 children and 729 adults and were conducted primarily in an ED or outpatient setting. The four new studies added 295 children and 58 adults to the researchers’ earlier meta-analysis. Studies involving adults and children were pooled separately. Most patients received multiple treatments with β-agonists titrated to the individual’s response.
No differences in hospitalizations. Rates of hospital admissions did not differ between patients receiving β-agonists via a spacer compared to a nebulizer in both adults (relative risk [RR] = 0.94) and children (RR = 0.71). Duration of hospital stay did not differ between the two delivery methods in adults (mean difference [MD] = –0.60 d) and children (MD = 0.33 d).
For kids, spacers meant less time in the ED. Duration in the ED was approximately half an hour shorter for children using spacers (MD = –33.48 min). There was no difference observed in adults (MD = 1.75 min). The rate of tremor was lower in children using spacers (RR = 0.64) and was similar in adults (RR = 1.12). The rise in pulse rate was lower in children using spacers
(MD = –5.41% change from baseline) and was similar in adults (MD = –1.23%).
On the next page: What's new and challenges to implementation >>
WHAT’S NEW
Additional evidence that spacers are as effective as nebulizers
This meta-analysis, which included four new studies, should finally dispel the myth that nebulizers deliver β-agonists more effectively than MDIs with spacers. Additionally, in children, spacers are associated with lower rates of adverse effects, including tremor and elevated pulse rate.
CAVEATS
Most studies involving children were open label
Although most of the adult trials in this meta-analysis involved a double-dummy design, which allows for effective participant blinding, most of the studies involving children were open label. This open-label design might have been a source of reporting bias for symptom-related outcomes but should not have affected hospital admission rates or duration of hospital stay.
In the double-dummy studies, adults received both a nebulizer and a spacer, which likely explains the similar time spent in the ED by the treatment and control groups.
CHALLENGES TO IMPLEMENTATION
Old habits are hard to break
Clinicians may think that patients view nebulizers as more potent or more effective than spacers and thus be more likely to order them. Some patients may prefer nebulizers because of convenience or other factors.
REFERENCES
1. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9: CD000052.
2. Barrett ML, Wier LM, Washington R. Trends in pediatric and adult hospital stays for asthma, 2000-2010. HCUP Statistical Brief #169. www.hcup-us.ahrq.gov/reports/stat briefs/sb169-Asthma-Trends-Hospital-Stays.pdf. Accessed June 16, 2014.
3. Pfuntner A, Wier LM, Stocks C. Most frequent conditions in US hospitals, 2011. HCUP Statistical Brief #162. www.hcup-us.ahrq.gov/reports/statbriefs/sb162.pdf. Accessed June 16, 2014.
4. Cates CJ, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2006;(2): CD000052.
5. Turner MO, Patel A, Ginsburg S, et al. Bronchodilator delivery in acute airflow obstruction: a meta-analysis. Arch Intern Med. 1997;157:1736-1744.
6. National Heart, Lung, and Blood Institute Expert Panel Report 3 (EPR3): Guidelines for the diagnosis and management of asthma. www.nhlbi.nih.gov/guidelines/asthma/asth gdln.htm. Accessed June 16, 2014.
7. British Thoracic Society. British guideline of the management of asthma: a national clinical guideline. www.brit-thoracic.org.uk/document-library/clinical-information/asth ma/btssign-guideline-on-the-management-of-asthma/. Accessed June 16, 2014.
8. Pitts SR, Niska RW, Xu J, et al. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. www.cdc.gov/nchs/data/nhsr/nhsr007.pdf. Accessed June 16, 2014.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(6):321-322, 346.
PRACTICE CHANGER
Stop ordering nebulizers to deliver β-agonists to patients older than 2 who have mild or moderate asthma exacerbations. A metered-dose inhaler (MDI) with a spacer produces the same benefits with fewer adverse effects.1
STRENGTH OF RECOMMENDATION
A: Based on an updated Cochrane meta-analysis of 39 randomized controlled trials (RCTs). 1
ILLUSTRATIVE CASE
A 6-year-old girl with a history of reactive airway disease comes to your office complaining of cough and wheezing. On exam, she has mild retractions, a respiratory rate of 35 breaths/min, and an O2 saturation of 96% on room air. Her lung fields are diffusely wheezy. Her parents would like to keep her out of the hospital. How should you order her albuterol to decrease her wheezing and minimize adverse effects?
Asthma affects nearly 19 million adults and 7 million children in the United States.2 Asthma exacerbations are the third most common reason for hospitalization in children.2,3 Treatment usually requires multiple agents, including inhaled β-agonists. These are most effective when delivered to the peripheral airways, which is a challenge during an asthma exacerbation because of airway swelling and rapid breathing. Two devices have been developed to effectively deliver medication to the peripheral airways: nebulizers and MDIs with a holding chamber (spacer).1
Several studies have demonstrated that for mild to moderate asthma exacerbations, administering a β-agonist via an MDI with a spacer is as effective as using a nebulizer.4,5 Asthma treatment guidelines also state that spacers are either comparable or preferable to nebulizers for β-agonist administration in children and adults.6,7 However, based on our experience, clinicians still frequently order nebulizer treatments for patients with asthma exacerbations, despite several advantages of MDIs with spacers. Notably, they cost less and don’t require maintenance or a power source. Clinicians administered nebulizer therapy at more than 3.6 million emergency department (ED) visits in 2006.8
In this latest Cochrane review, Cates et al1 added four new studies to those included in their earlier Cochrane meta-analysis and evaluated what, if any, effect these studies had on our understanding of nebulizers versus MDIs with spacers.
STUDY SUMMARY
Outcomes with nebulizers are no better than those with spacers
This systematic review and meta-analysis pooled the results of RCTs comparing spacers to nebulizers for administering β-agonists during acute, non–life-threatening asthma exacerbations.1 The authors reviewed studies conducted in EDs, hospitals, and outpatient settings that included children and adults. The primary outcomes were hospital admission rates and duration of hospital stay. Secondary outcomes included time spent in the ED, change in pulse rate, and incidence of tremor.
Cates et al1 analyzed 39 trials that included 1,897 children and 729 adults and were conducted primarily in an ED or outpatient setting. The four new studies added 295 children and 58 adults to the researchers’ earlier meta-analysis. Studies involving adults and children were pooled separately. Most patients received multiple treatments with β-agonists titrated to the individual’s response.
No differences in hospitalizations. Rates of hospital admissions did not differ between patients receiving β-agonists via a spacer compared to a nebulizer in both adults (relative risk [RR] = 0.94) and children (RR = 0.71). Duration of hospital stay did not differ between the two delivery methods in adults (mean difference [MD] = –0.60 d) and children (MD = 0.33 d).
For kids, spacers meant less time in the ED. Duration in the ED was approximately half an hour shorter for children using spacers (MD = –33.48 min). There was no difference observed in adults (MD = 1.75 min). The rate of tremor was lower in children using spacers (RR = 0.64) and was similar in adults (RR = 1.12). The rise in pulse rate was lower in children using spacers
(MD = –5.41% change from baseline) and was similar in adults (MD = –1.23%).
On the next page: What's new and challenges to implementation >>
WHAT’S NEW
Additional evidence that spacers are as effective as nebulizers
This meta-analysis, which included four new studies, should finally dispel the myth that nebulizers deliver β-agonists more effectively than MDIs with spacers. Additionally, in children, spacers are associated with lower rates of adverse effects, including tremor and elevated pulse rate.
CAVEATS
Most studies involving children were open label
Although most of the adult trials in this meta-analysis involved a double-dummy design, which allows for effective participant blinding, most of the studies involving children were open label. This open-label design might have been a source of reporting bias for symptom-related outcomes but should not have affected hospital admission rates or duration of hospital stay.
In the double-dummy studies, adults received both a nebulizer and a spacer, which likely explains the similar time spent in the ED by the treatment and control groups.
CHALLENGES TO IMPLEMENTATION
Old habits are hard to break
Clinicians may think that patients view nebulizers as more potent or more effective than spacers and thus be more likely to order them. Some patients may prefer nebulizers because of convenience or other factors.
REFERENCES
1. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9: CD000052.
2. Barrett ML, Wier LM, Washington R. Trends in pediatric and adult hospital stays for asthma, 2000-2010. HCUP Statistical Brief #169. www.hcup-us.ahrq.gov/reports/stat briefs/sb169-Asthma-Trends-Hospital-Stays.pdf. Accessed June 16, 2014.
3. Pfuntner A, Wier LM, Stocks C. Most frequent conditions in US hospitals, 2011. HCUP Statistical Brief #162. www.hcup-us.ahrq.gov/reports/statbriefs/sb162.pdf. Accessed June 16, 2014.
4. Cates CJ, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2006;(2): CD000052.
5. Turner MO, Patel A, Ginsburg S, et al. Bronchodilator delivery in acute airflow obstruction: a meta-analysis. Arch Intern Med. 1997;157:1736-1744.
6. National Heart, Lung, and Blood Institute Expert Panel Report 3 (EPR3): Guidelines for the diagnosis and management of asthma. www.nhlbi.nih.gov/guidelines/asthma/asth gdln.htm. Accessed June 16, 2014.
7. British Thoracic Society. British guideline of the management of asthma: a national clinical guideline. www.brit-thoracic.org.uk/document-library/clinical-information/asth ma/btssign-guideline-on-the-management-of-asthma/. Accessed June 16, 2014.
8. Pitts SR, Niska RW, Xu J, et al. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. www.cdc.gov/nchs/data/nhsr/nhsr007.pdf. Accessed June 16, 2014.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(6):321-322, 346.
PRACTICE CHANGER
Stop ordering nebulizers to deliver β-agonists to patients older than 2 who have mild or moderate asthma exacerbations. A metered-dose inhaler (MDI) with a spacer produces the same benefits with fewer adverse effects.1
STRENGTH OF RECOMMENDATION
A: Based on an updated Cochrane meta-analysis of 39 randomized controlled trials (RCTs). 1
ILLUSTRATIVE CASE
A 6-year-old girl with a history of reactive airway disease comes to your office complaining of cough and wheezing. On exam, she has mild retractions, a respiratory rate of 35 breaths/min, and an O2 saturation of 96% on room air. Her lung fields are diffusely wheezy. Her parents would like to keep her out of the hospital. How should you order her albuterol to decrease her wheezing and minimize adverse effects?
Asthma affects nearly 19 million adults and 7 million children in the United States.2 Asthma exacerbations are the third most common reason for hospitalization in children.2,3 Treatment usually requires multiple agents, including inhaled β-agonists. These are most effective when delivered to the peripheral airways, which is a challenge during an asthma exacerbation because of airway swelling and rapid breathing. Two devices have been developed to effectively deliver medication to the peripheral airways: nebulizers and MDIs with a holding chamber (spacer).1
Several studies have demonstrated that for mild to moderate asthma exacerbations, administering a β-agonist via an MDI with a spacer is as effective as using a nebulizer.4,5 Asthma treatment guidelines also state that spacers are either comparable or preferable to nebulizers for β-agonist administration in children and adults.6,7 However, based on our experience, clinicians still frequently order nebulizer treatments for patients with asthma exacerbations, despite several advantages of MDIs with spacers. Notably, they cost less and don’t require maintenance or a power source. Clinicians administered nebulizer therapy at more than 3.6 million emergency department (ED) visits in 2006.8
In this latest Cochrane review, Cates et al1 added four new studies to those included in their earlier Cochrane meta-analysis and evaluated what, if any, effect these studies had on our understanding of nebulizers versus MDIs with spacers.
STUDY SUMMARY
Outcomes with nebulizers are no better than those with spacers
This systematic review and meta-analysis pooled the results of RCTs comparing spacers to nebulizers for administering β-agonists during acute, non–life-threatening asthma exacerbations.1 The authors reviewed studies conducted in EDs, hospitals, and outpatient settings that included children and adults. The primary outcomes were hospital admission rates and duration of hospital stay. Secondary outcomes included time spent in the ED, change in pulse rate, and incidence of tremor.
Cates et al1 analyzed 39 trials that included 1,897 children and 729 adults and were conducted primarily in an ED or outpatient setting. The four new studies added 295 children and 58 adults to the researchers’ earlier meta-analysis. Studies involving adults and children were pooled separately. Most patients received multiple treatments with β-agonists titrated to the individual’s response.
No differences in hospitalizations. Rates of hospital admissions did not differ between patients receiving β-agonists via a spacer compared to a nebulizer in both adults (relative risk [RR] = 0.94) and children (RR = 0.71). Duration of hospital stay did not differ between the two delivery methods in adults (mean difference [MD] = –0.60 d) and children (MD = 0.33 d).
For kids, spacers meant less time in the ED. Duration in the ED was approximately half an hour shorter for children using spacers (MD = –33.48 min). There was no difference observed in adults (MD = 1.75 min). The rate of tremor was lower in children using spacers (RR = 0.64) and was similar in adults (RR = 1.12). The rise in pulse rate was lower in children using spacers
(MD = –5.41% change from baseline) and was similar in adults (MD = –1.23%).
On the next page: What's new and challenges to implementation >>
WHAT’S NEW
Additional evidence that spacers are as effective as nebulizers
This meta-analysis, which included four new studies, should finally dispel the myth that nebulizers deliver β-agonists more effectively than MDIs with spacers. Additionally, in children, spacers are associated with lower rates of adverse effects, including tremor and elevated pulse rate.
CAVEATS
Most studies involving children were open label
Although most of the adult trials in this meta-analysis involved a double-dummy design, which allows for effective participant blinding, most of the studies involving children were open label. This open-label design might have been a source of reporting bias for symptom-related outcomes but should not have affected hospital admission rates or duration of hospital stay.
In the double-dummy studies, adults received both a nebulizer and a spacer, which likely explains the similar time spent in the ED by the treatment and control groups.
CHALLENGES TO IMPLEMENTATION
Old habits are hard to break
Clinicians may think that patients view nebulizers as more potent or more effective than spacers and thus be more likely to order them. Some patients may prefer nebulizers because of convenience or other factors.
REFERENCES
1. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9: CD000052.
2. Barrett ML, Wier LM, Washington R. Trends in pediatric and adult hospital stays for asthma, 2000-2010. HCUP Statistical Brief #169. www.hcup-us.ahrq.gov/reports/stat briefs/sb169-Asthma-Trends-Hospital-Stays.pdf. Accessed June 16, 2014.
3. Pfuntner A, Wier LM, Stocks C. Most frequent conditions in US hospitals, 2011. HCUP Statistical Brief #162. www.hcup-us.ahrq.gov/reports/statbriefs/sb162.pdf. Accessed June 16, 2014.
4. Cates CJ, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2006;(2): CD000052.
5. Turner MO, Patel A, Ginsburg S, et al. Bronchodilator delivery in acute airflow obstruction: a meta-analysis. Arch Intern Med. 1997;157:1736-1744.
6. National Heart, Lung, and Blood Institute Expert Panel Report 3 (EPR3): Guidelines for the diagnosis and management of asthma. www.nhlbi.nih.gov/guidelines/asthma/asth gdln.htm. Accessed June 16, 2014.
7. British Thoracic Society. British guideline of the management of asthma: a national clinical guideline. www.brit-thoracic.org.uk/document-library/clinical-information/asth ma/btssign-guideline-on-the-management-of-asthma/. Accessed June 16, 2014.
8. Pitts SR, Niska RW, Xu J, et al. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. www.cdc.gov/nchs/data/nhsr/nhsr007.pdf. Accessed June 16, 2014.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(6):321-322, 346.
Think twice about nebulizers for asthma attacks
Stop ordering nebulizers to deliver beta-agonists to patients over age 2 with mild or moderate asthma exacerbations. A metered-dose inhaler (MDI) with a spacer produces the same benefits with fewer adverse effects.1
Strength of recommendation
A: Based on an updated Cochrane meta-analysis of 39 randomized controlled trials (RCTs).
Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
Illustrative case
A 6-year-old girl with a history of reactive airway disease comes to your office complaining of cough and wheezing. On exam, she has mild retractions, a respiratory rate of 35, and an oxygen saturation of 96% on room air. Her lung fields are diffusely wheezy. Her parents would like to keep her out of the hospital. How should you order her albuterol to decrease her wheezing and minimize adverse effects?
Asthma affects nearly 19 million adults and 7 million children in the United States.2 Asthma exacerbations are the third most common reason for hospitalization in children.2,3 Treatment usually requires multiple agents, including inhaled beta-agonists. These are most effective when delivered to the peripheral airways, which is a challenge during an asthma exacerbation because of airway swelling and rapid breathing. Two devices have been developed to effectively deliver medication to the peripheral airways: nebulizers and MDIs with a holding chamber (spacer).1
Several studies have demonstrated that for mild to moderate asthma exacerbations, administering a beta-agonist via an MDI with a spacer is as effective as using a nebulizer.4,5 Asthma treatment guidelines also state that spacers are either comparable to or preferred over nebulizers for beta-agonist administration in children and adults.6,7 However, based on our experience, physicians still frequently order nebulizer treatments for patients with asthma exacerbations, despite several advantages of MDIs with spacers. Notably, they cost less and don’t require maintenance or a power source. Physicians administered nebulizer therapy at more than 3.6 million emergency department (ED) visits in 2006.8
In this latest Cochrane review, Cates et al1 added 4 new studies to those included in their earlier Cochrane meta-analysis, and looked at what, if any, effect these studies had on our understanding of nebulizers vs MDIs with spacers.
STUDY SUMMARY: Outcomes with nebulizers are no better than those with spacers
This systematic review and meta-analysis pooled the results of RCTs comparing spacers to nebulizers for administering beta-agonists during acute, non-life-threatening asthma exacerbations.1 The authors reviewed studies conducted in EDs, hospitals, and outpatient settings that included children and adults. The primary outcomes were hospital admission rates and duration of hospital stay. Secondary outcomes included time spent in the ED, change in pulse rate, and incidence of tremor.
Cates et al1 analyzed 39 trials that included 1897 children and 729 adults and were conducted primarily in an ED or outpatient setting. The 4 new studies added 295 children and 58 adults to the researchers’ earlier meta-analysis. Studies involving adults and children were pooled separately. Most patients received multiple treatments with beta-agonists titrated to the individual’s response.
No differences in hospitalizations. Rates of hospital admissions did not differ between patients receiving beta-agonists via a spacer compared to a nebulizer in both adults (relative risk [RR]=.94; 95% confidence interval [CI], .61-1.43) and children (RR=.71; 95% CI, .47-1.08). Duration of hospital stay did not differ between the 2 delivery methods in adults (mean difference [MD]=-.60 days; 95% CI, -3.23 to 2.03) and children (MD=.33 days; 95% CI, -.10 to .76).
For kids, spacers meant less time in the ED. Duration in the ED was approximately half an hour shorter for children using spacers (MD=-33.48 minutes; 95% CI, -43.3 to -23.6, P<.001). There was no difference in time spent in the ED observed in adults (MD=1.75 minutes; 95% CI, -23.45 to 26.95). The rate of tremor was lower in children using spacers (RR=.64; 95% CI, .44-.95, P=.027), and was similar in adults (RR=1.12; 95% CI, .66-1.9). The rise in pulse rate was lower in children using spacers (MD=-5.41% change from baseline; 95% CI, -8.34 to -2.48; P<.001), and was similar in adults (MD=-1.23%; 95% CI, -4.06 to 1.60).
WHAT'S NEW: Additional evidence that spacers are as effective as nebulizers
This meta-analysis, which included 4 new studies, should finally dispel the myth that nebulizers deliver beta-agonists more effectively than MDIs with spacers. Additionally, in children, spacers are associated with lower rates of side effects, including tremor and elevated pulse rate.
CAVEATS: Most studies involving children were open label
Although most of the adult trials in this meta-analysis involved a double-dummy design, which allows for effective participant blinding, most of the studies involving children were open label. This open-label design might have been a source of reporting bias for symptom-related outcomes, but should not have affected hospital admission rates or duration of hospital stay.
In the double-dummy studies, adults received both a nebulizer and a spacer, which likely explains the similar time spent in the ED by the treatment and control groups.
CHALLENGES TO IMPLEMENTATION: Old habits are hard to break
Doctors may think that patients view nebulizers as more potent or more effective than spacers and thus be more likely to order them. Some patients may prefer nebulizers because of convenience or other factors.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
2. Barrett ML, Wier LM, Washington R. Trends in pediatric and adult hospital stays for asthma, 2000-2010. HCUP Statistical Brief #169. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb169-Asthma-Trends-Hospital-Stays.pdf. Published January 2014. Accessed March 18, 2014.
3. Pfuntner A, Wier LM, Stocks C. Most frequent conditions in US hospitals, 2011. HCUP Statistical Brief #162. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb162.pdf. Published September 2013. Accessed March 18, 2014.
4. Cates CJ, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2006;(2):CD000052.
5. Turner MO, Patel A, Ginsburg S, et al. Bronchodilator delivery in acute airflow obstruction. A meta-analysis. Arch Intern Med. 1997;157:1736-1744.
6. Expert Panel Report 3 (EPR3): Guidelines for the diagnosis and management of asthma. National Heart, Lung, and Blood Institute Web site. Available at: www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed March 18, 2014.
7. British guideline of the management of asthma: A national clinical guideline. British Thoracic Society Web site. Available at: https://www.brit-thoracic.org.uk/document-library/clinical-information/asthma/btssign-guideline-on-the-management-of-asthma/. Published May 2008. Revised January 2012. Accessed March 15, 2014.
8. Pitts SR, Niska RW, Xu J, et al. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. Available at: http://www.cdc.gov/nchs/data/nhsr/nhsr007.pdf. Accessed May 8, 2014.
Stop ordering nebulizers to deliver beta-agonists to patients over age 2 with mild or moderate asthma exacerbations. A metered-dose inhaler (MDI) with a spacer produces the same benefits with fewer adverse effects.1
Strength of recommendation
A: Based on an updated Cochrane meta-analysis of 39 randomized controlled trials (RCTs).
Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
Illustrative case
A 6-year-old girl with a history of reactive airway disease comes to your office complaining of cough and wheezing. On exam, she has mild retractions, a respiratory rate of 35, and an oxygen saturation of 96% on room air. Her lung fields are diffusely wheezy. Her parents would like to keep her out of the hospital. How should you order her albuterol to decrease her wheezing and minimize adverse effects?
Asthma affects nearly 19 million adults and 7 million children in the United States.2 Asthma exacerbations are the third most common reason for hospitalization in children.2,3 Treatment usually requires multiple agents, including inhaled beta-agonists. These are most effective when delivered to the peripheral airways, which is a challenge during an asthma exacerbation because of airway swelling and rapid breathing. Two devices have been developed to effectively deliver medication to the peripheral airways: nebulizers and MDIs with a holding chamber (spacer).1
Several studies have demonstrated that for mild to moderate asthma exacerbations, administering a beta-agonist via an MDI with a spacer is as effective as using a nebulizer.4,5 Asthma treatment guidelines also state that spacers are either comparable to or preferred over nebulizers for beta-agonist administration in children and adults.6,7 However, based on our experience, physicians still frequently order nebulizer treatments for patients with asthma exacerbations, despite several advantages of MDIs with spacers. Notably, they cost less and don’t require maintenance or a power source. Physicians administered nebulizer therapy at more than 3.6 million emergency department (ED) visits in 2006.8
In this latest Cochrane review, Cates et al1 added 4 new studies to those included in their earlier Cochrane meta-analysis, and looked at what, if any, effect these studies had on our understanding of nebulizers vs MDIs with spacers.
STUDY SUMMARY: Outcomes with nebulizers are no better than those with spacers
This systematic review and meta-analysis pooled the results of RCTs comparing spacers to nebulizers for administering beta-agonists during acute, non-life-threatening asthma exacerbations.1 The authors reviewed studies conducted in EDs, hospitals, and outpatient settings that included children and adults. The primary outcomes were hospital admission rates and duration of hospital stay. Secondary outcomes included time spent in the ED, change in pulse rate, and incidence of tremor.
Cates et al1 analyzed 39 trials that included 1897 children and 729 adults and were conducted primarily in an ED or outpatient setting. The 4 new studies added 295 children and 58 adults to the researchers’ earlier meta-analysis. Studies involving adults and children were pooled separately. Most patients received multiple treatments with beta-agonists titrated to the individual’s response.
No differences in hospitalizations. Rates of hospital admissions did not differ between patients receiving beta-agonists via a spacer compared to a nebulizer in both adults (relative risk [RR]=.94; 95% confidence interval [CI], .61-1.43) and children (RR=.71; 95% CI, .47-1.08). Duration of hospital stay did not differ between the 2 delivery methods in adults (mean difference [MD]=-.60 days; 95% CI, -3.23 to 2.03) and children (MD=.33 days; 95% CI, -.10 to .76).
For kids, spacers meant less time in the ED. Duration in the ED was approximately half an hour shorter for children using spacers (MD=-33.48 minutes; 95% CI, -43.3 to -23.6, P<.001). There was no difference in time spent in the ED observed in adults (MD=1.75 minutes; 95% CI, -23.45 to 26.95). The rate of tremor was lower in children using spacers (RR=.64; 95% CI, .44-.95, P=.027), and was similar in adults (RR=1.12; 95% CI, .66-1.9). The rise in pulse rate was lower in children using spacers (MD=-5.41% change from baseline; 95% CI, -8.34 to -2.48; P<.001), and was similar in adults (MD=-1.23%; 95% CI, -4.06 to 1.60).
WHAT'S NEW: Additional evidence that spacers are as effective as nebulizers
This meta-analysis, which included 4 new studies, should finally dispel the myth that nebulizers deliver beta-agonists more effectively than MDIs with spacers. Additionally, in children, spacers are associated with lower rates of side effects, including tremor and elevated pulse rate.
CAVEATS: Most studies involving children were open label
Although most of the adult trials in this meta-analysis involved a double-dummy design, which allows for effective participant blinding, most of the studies involving children were open label. This open-label design might have been a source of reporting bias for symptom-related outcomes, but should not have affected hospital admission rates or duration of hospital stay.
In the double-dummy studies, adults received both a nebulizer and a spacer, which likely explains the similar time spent in the ED by the treatment and control groups.
CHALLENGES TO IMPLEMENTATION: Old habits are hard to break
Doctors may think that patients view nebulizers as more potent or more effective than spacers and thus be more likely to order them. Some patients may prefer nebulizers because of convenience or other factors.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Stop ordering nebulizers to deliver beta-agonists to patients over age 2 with mild or moderate asthma exacerbations. A metered-dose inhaler (MDI) with a spacer produces the same benefits with fewer adverse effects.1
Strength of recommendation
A: Based on an updated Cochrane meta-analysis of 39 randomized controlled trials (RCTs).
Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
Illustrative case
A 6-year-old girl with a history of reactive airway disease comes to your office complaining of cough and wheezing. On exam, she has mild retractions, a respiratory rate of 35, and an oxygen saturation of 96% on room air. Her lung fields are diffusely wheezy. Her parents would like to keep her out of the hospital. How should you order her albuterol to decrease her wheezing and minimize adverse effects?
Asthma affects nearly 19 million adults and 7 million children in the United States.2 Asthma exacerbations are the third most common reason for hospitalization in children.2,3 Treatment usually requires multiple agents, including inhaled beta-agonists. These are most effective when delivered to the peripheral airways, which is a challenge during an asthma exacerbation because of airway swelling and rapid breathing. Two devices have been developed to effectively deliver medication to the peripheral airways: nebulizers and MDIs with a holding chamber (spacer).1
Several studies have demonstrated that for mild to moderate asthma exacerbations, administering a beta-agonist via an MDI with a spacer is as effective as using a nebulizer.4,5 Asthma treatment guidelines also state that spacers are either comparable to or preferred over nebulizers for beta-agonist administration in children and adults.6,7 However, based on our experience, physicians still frequently order nebulizer treatments for patients with asthma exacerbations, despite several advantages of MDIs with spacers. Notably, they cost less and don’t require maintenance or a power source. Physicians administered nebulizer therapy at more than 3.6 million emergency department (ED) visits in 2006.8
In this latest Cochrane review, Cates et al1 added 4 new studies to those included in their earlier Cochrane meta-analysis, and looked at what, if any, effect these studies had on our understanding of nebulizers vs MDIs with spacers.
STUDY SUMMARY: Outcomes with nebulizers are no better than those with spacers
This systematic review and meta-analysis pooled the results of RCTs comparing spacers to nebulizers for administering beta-agonists during acute, non-life-threatening asthma exacerbations.1 The authors reviewed studies conducted in EDs, hospitals, and outpatient settings that included children and adults. The primary outcomes were hospital admission rates and duration of hospital stay. Secondary outcomes included time spent in the ED, change in pulse rate, and incidence of tremor.
Cates et al1 analyzed 39 trials that included 1897 children and 729 adults and were conducted primarily in an ED or outpatient setting. The 4 new studies added 295 children and 58 adults to the researchers’ earlier meta-analysis. Studies involving adults and children were pooled separately. Most patients received multiple treatments with beta-agonists titrated to the individual’s response.
No differences in hospitalizations. Rates of hospital admissions did not differ between patients receiving beta-agonists via a spacer compared to a nebulizer in both adults (relative risk [RR]=.94; 95% confidence interval [CI], .61-1.43) and children (RR=.71; 95% CI, .47-1.08). Duration of hospital stay did not differ between the 2 delivery methods in adults (mean difference [MD]=-.60 days; 95% CI, -3.23 to 2.03) and children (MD=.33 days; 95% CI, -.10 to .76).
For kids, spacers meant less time in the ED. Duration in the ED was approximately half an hour shorter for children using spacers (MD=-33.48 minutes; 95% CI, -43.3 to -23.6, P<.001). There was no difference in time spent in the ED observed in adults (MD=1.75 minutes; 95% CI, -23.45 to 26.95). The rate of tremor was lower in children using spacers (RR=.64; 95% CI, .44-.95, P=.027), and was similar in adults (RR=1.12; 95% CI, .66-1.9). The rise in pulse rate was lower in children using spacers (MD=-5.41% change from baseline; 95% CI, -8.34 to -2.48; P<.001), and was similar in adults (MD=-1.23%; 95% CI, -4.06 to 1.60).
WHAT'S NEW: Additional evidence that spacers are as effective as nebulizers
This meta-analysis, which included 4 new studies, should finally dispel the myth that nebulizers deliver beta-agonists more effectively than MDIs with spacers. Additionally, in children, spacers are associated with lower rates of side effects, including tremor and elevated pulse rate.
CAVEATS: Most studies involving children were open label
Although most of the adult trials in this meta-analysis involved a double-dummy design, which allows for effective participant blinding, most of the studies involving children were open label. This open-label design might have been a source of reporting bias for symptom-related outcomes, but should not have affected hospital admission rates or duration of hospital stay.
In the double-dummy studies, adults received both a nebulizer and a spacer, which likely explains the similar time spent in the ED by the treatment and control groups.
CHALLENGES TO IMPLEMENTATION: Old habits are hard to break
Doctors may think that patients view nebulizers as more potent or more effective than spacers and thus be more likely to order them. Some patients may prefer nebulizers because of convenience or other factors.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
2. Barrett ML, Wier LM, Washington R. Trends in pediatric and adult hospital stays for asthma, 2000-2010. HCUP Statistical Brief #169. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb169-Asthma-Trends-Hospital-Stays.pdf. Published January 2014. Accessed March 18, 2014.
3. Pfuntner A, Wier LM, Stocks C. Most frequent conditions in US hospitals, 2011. HCUP Statistical Brief #162. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb162.pdf. Published September 2013. Accessed March 18, 2014.
4. Cates CJ, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2006;(2):CD000052.
5. Turner MO, Patel A, Ginsburg S, et al. Bronchodilator delivery in acute airflow obstruction. A meta-analysis. Arch Intern Med. 1997;157:1736-1744.
6. Expert Panel Report 3 (EPR3): Guidelines for the diagnosis and management of asthma. National Heart, Lung, and Blood Institute Web site. Available at: www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed March 18, 2014.
7. British guideline of the management of asthma: A national clinical guideline. British Thoracic Society Web site. Available at: https://www.brit-thoracic.org.uk/document-library/clinical-information/asthma/btssign-guideline-on-the-management-of-asthma/. Published May 2008. Revised January 2012. Accessed March 15, 2014.
8. Pitts SR, Niska RW, Xu J, et al. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. Available at: http://www.cdc.gov/nchs/data/nhsr/nhsr007.pdf. Accessed May 8, 2014.
1. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
2. Barrett ML, Wier LM, Washington R. Trends in pediatric and adult hospital stays for asthma, 2000-2010. HCUP Statistical Brief #169. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb169-Asthma-Trends-Hospital-Stays.pdf. Published January 2014. Accessed March 18, 2014.
3. Pfuntner A, Wier LM, Stocks C. Most frequent conditions in US hospitals, 2011. HCUP Statistical Brief #162. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb162.pdf. Published September 2013. Accessed March 18, 2014.
4. Cates CJ, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2006;(2):CD000052.
5. Turner MO, Patel A, Ginsburg S, et al. Bronchodilator delivery in acute airflow obstruction. A meta-analysis. Arch Intern Med. 1997;157:1736-1744.
6. Expert Panel Report 3 (EPR3): Guidelines for the diagnosis and management of asthma. National Heart, Lung, and Blood Institute Web site. Available at: www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed March 18, 2014.
7. British guideline of the management of asthma: A national clinical guideline. British Thoracic Society Web site. Available at: https://www.brit-thoracic.org.uk/document-library/clinical-information/asthma/btssign-guideline-on-the-management-of-asthma/. Published May 2008. Revised January 2012. Accessed March 15, 2014.
8. Pitts SR, Niska RW, Xu J, et al. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. Available at: http://www.cdc.gov/nchs/data/nhsr/nhsr007.pdf. Accessed May 8, 2014.
Copyright © 2014 Family Physicians Inquiries Network. All rights reserved.
A simple way to reduce catheter-associated UTIs
Ensure that antibiotics are administered to surgical patients when their urinary catheter is removed to reduce the risk of urinary tract infections (UTIs).1
Strength of recommendation
B: Based on a meta-analysis.
Marschall J, Carpenter CR, Fowler S, et al; CDC Prevention Epicenters Program. Antibiotic prophylaxis for urinary tract infections after removal of urinary catheter: meta-analysis. BMJ. 2013;346:f3147.
Illustrative case
A 49-year-old man was admitted to the hospital for resection of a vertebral mass. He is almost ready for discharge, and his urinary catheter soon will be removed. Should he be given an antibiotic when his catheter is removed to prevent a UTI?
Approximately 15% to 25% of hospitalized patients receive a urinary catheter, typically during the perioperative period.2 UTIs are the most common hospital-acquired infections, and virtually all of these UTIs are caused by instrumentation of the urinary tract, primarily by catheters.2 Although the mortality rate among patients with catheter-associated UTIs (CAUTIs) is just 2.3%, CAUTIs are the leading cause of hospital-acquired bacteremia, which increases morbidity and length of stay.2 The most common pathogens for CAUTIs are Escherichia coli (21.4%), Candida species (21%), and Enterococcus species (14.9%).2Pseudomonas aeruginosa, Klebsiella, and Enterobacter species make up the bulk of the remainder.2
Support for antibiotic prophylaxis has historically been equivocal
Until now, no data clearly supported routine use of prophylactic antibiotics after urinary catheterization. Centers for Disease Control and Prevention (CDC) guidelines published in 2009 outline which patients are appropriate for catheterization, but do not recommend routine use of antibiotics to prevent CAUTIs.2 The 2014 Infectious Diseases Society of America guidelines, which came out before the study reported on here was published, state the benefit of antibiotics at the time of catheter removal is an unresolved issue.3
STUDY SUMMARY: Meta-analysis shows prophylactic antibiotics reduce UTI ris
Marschall et al1 searched multiple databases for studies published between 1947 and 2012 that evaluated prophylactic use of antibiotics at the time of urinary catheter removal. The endpoint for their analysis was symptomatic UTI, which they defined as bacteriuria plus at least one clinical symptom. Trials were excluded if patients had suprapubic catheters or if antibiotics were started shortly after the catheter was inserted.
The authors analyzed 7 studies. Six were randomized controlled trials, of which one was unpublished. The seventh trial was a nonrandomized study that compared outcomes of patients of 2 surgeons, one of whom used prophylactic antibiotics and one who did not. Five studies enrolled surgical patients exclusively, including 2 that focused on urology patients. In all of the studies, patients had a urinary catheter in place for fewer than 15 days. The duration of antibiotic treatment varied from a single dose to 3 days. The antibiotics used included trimethoprim/sulfamethoxazole, nitrofurantoin, ciprofloxacin, and a cephalosporin.
Antibiotic prophylaxis significantly reduced the rate of CAUTIs. The absolute risk reduction was 5.8%; the rate of CAUTIs was 4.7% in the group treated with antibiotics vs 10.5% in the control group. The number needed to treat to prevent one CAUTI was 17 (95% confidence interval [CI], 12-30), with a risk ratio (RR) of .45 (95% CI, .28-.72). The RR varied only slightly (.36) when the researchers repeated their analysis but excluded the unpublished trial, and remained at .45 when they analyzed only studies of surgical patients.
The reduction in CAUTIs remained consistent despite varying lengths of antibiotic administration and choice of antimicrobial agents. However, when the authors looked at pooled results just from the 2 studies that included both surgical and medical patients, they found no decrease in CAUTIs.
WHAT'S NEW: We now have an effective way to reduce CAUTIs
Prophylactic use of antibiotics when a urinary catheter is removed appears to reduce the rate of CAUTIs by more than 50% in surgical patients. The 2009 CDC guidelines on CAUTI prevention emphasize the use of appropriate infection control measures and limiting the duration of urinary catheter use.2 Now there are data showing a reduction in the incidence of CAUTIs when prophylactic antibiotics are given during catheter removal.
CAVEATS: Results may not apply to nonsurgical patients
This meta-analysis does not provide enough information to identify which patients are most likely to benefit from antibiotic prophylaxis. Most patients (92%) in this analysis had undergone surgery, but urinary catheterization is common among medically hospitalized patients. Studies of antibiotic prophylaxis at the time of catheter removal in nonsurgical patients are needed to strengthen the recommendation of this practice for all patients.
Some of the studies analyzed may have been biased. The authors determined that most of the studies in their meta-analysis were at high risk of attrition bias because there was potential for systematic differences in withdrawals between the treatment and control groups. In addition, in most studies, the randomization and allocation appeared to be inadequate, which increased the risk for selection bias.
CHALLENGES TO IMPLEMENTATION: Which antibiotics to use, and for how long, remains unclear
Antibiotic choice depends upon institutional policies and local resistance patterns, which complicates making universal recommendations. The optimal duration of treatment also is unknown, although this meta-analysis suggests that prophylaxis for 3 days or less can reduce CAUTI risk.
Catheters impregnated with antimicrobials or with microbial resistance barriers may be an alternative to administering antibiotics at catheter removal, but in preliminary studies, these devices have not been shown to reduce the incidence of CAUTIs.4,5 Increasing antimicrobial resistance also complicates the widespread use of prophylaxis.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Marschall J, Carpenter CR, Fowler S, et al; CDC Prevention Epicenters Program. Antibiotic prophylaxis for urinary tract infections after removal of urinary catheter: meta-analysis. BMJ. 2013;346:f3147.
2. Gould CV, Umscheid CA, Agarwal RK, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. Available at: http://www.cdc.gov/hicpac/pdf/cauti/cautiguideline2009final.pdf. Accessed April 15, 2014.
3. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35:464-479.
4. Pickard R, Lam T, Maclennan G, et al. Types of urethral catheter for reducing symptomatic urinary tract infections in hospitalised adults requiring short-term catheterisation: multicentre randomised controlled trial and economic evaluation of antimicrobial- and antiseptic-impregnated urethral catheters (the CATHETER trial). Health Technol Assess. 2012;16:1-197.
5. Pickard R, Lam T, MacLennan G, et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: a multicentre randomised controlled trial. Lancet. 2012;380:1927-1935.
Ensure that antibiotics are administered to surgical patients when their urinary catheter is removed to reduce the risk of urinary tract infections (UTIs).1
Strength of recommendation
B: Based on a meta-analysis.
Marschall J, Carpenter CR, Fowler S, et al; CDC Prevention Epicenters Program. Antibiotic prophylaxis for urinary tract infections after removal of urinary catheter: meta-analysis. BMJ. 2013;346:f3147.
Illustrative case
A 49-year-old man was admitted to the hospital for resection of a vertebral mass. He is almost ready for discharge, and his urinary catheter soon will be removed. Should he be given an antibiotic when his catheter is removed to prevent a UTI?
Approximately 15% to 25% of hospitalized patients receive a urinary catheter, typically during the perioperative period.2 UTIs are the most common hospital-acquired infections, and virtually all of these UTIs are caused by instrumentation of the urinary tract, primarily by catheters.2 Although the mortality rate among patients with catheter-associated UTIs (CAUTIs) is just 2.3%, CAUTIs are the leading cause of hospital-acquired bacteremia, which increases morbidity and length of stay.2 The most common pathogens for CAUTIs are Escherichia coli (21.4%), Candida species (21%), and Enterococcus species (14.9%).2Pseudomonas aeruginosa, Klebsiella, and Enterobacter species make up the bulk of the remainder.2
Support for antibiotic prophylaxis has historically been equivocal
Until now, no data clearly supported routine use of prophylactic antibiotics after urinary catheterization. Centers for Disease Control and Prevention (CDC) guidelines published in 2009 outline which patients are appropriate for catheterization, but do not recommend routine use of antibiotics to prevent CAUTIs.2 The 2014 Infectious Diseases Society of America guidelines, which came out before the study reported on here was published, state the benefit of antibiotics at the time of catheter removal is an unresolved issue.3
STUDY SUMMARY: Meta-analysis shows prophylactic antibiotics reduce UTI ris
Marschall et al1 searched multiple databases for studies published between 1947 and 2012 that evaluated prophylactic use of antibiotics at the time of urinary catheter removal. The endpoint for their analysis was symptomatic UTI, which they defined as bacteriuria plus at least one clinical symptom. Trials were excluded if patients had suprapubic catheters or if antibiotics were started shortly after the catheter was inserted.
The authors analyzed 7 studies. Six were randomized controlled trials, of which one was unpublished. The seventh trial was a nonrandomized study that compared outcomes of patients of 2 surgeons, one of whom used prophylactic antibiotics and one who did not. Five studies enrolled surgical patients exclusively, including 2 that focused on urology patients. In all of the studies, patients had a urinary catheter in place for fewer than 15 days. The duration of antibiotic treatment varied from a single dose to 3 days. The antibiotics used included trimethoprim/sulfamethoxazole, nitrofurantoin, ciprofloxacin, and a cephalosporin.
Antibiotic prophylaxis significantly reduced the rate of CAUTIs. The absolute risk reduction was 5.8%; the rate of CAUTIs was 4.7% in the group treated with antibiotics vs 10.5% in the control group. The number needed to treat to prevent one CAUTI was 17 (95% confidence interval [CI], 12-30), with a risk ratio (RR) of .45 (95% CI, .28-.72). The RR varied only slightly (.36) when the researchers repeated their analysis but excluded the unpublished trial, and remained at .45 when they analyzed only studies of surgical patients.
The reduction in CAUTIs remained consistent despite varying lengths of antibiotic administration and choice of antimicrobial agents. However, when the authors looked at pooled results just from the 2 studies that included both surgical and medical patients, they found no decrease in CAUTIs.
WHAT'S NEW: We now have an effective way to reduce CAUTIs
Prophylactic use of antibiotics when a urinary catheter is removed appears to reduce the rate of CAUTIs by more than 50% in surgical patients. The 2009 CDC guidelines on CAUTI prevention emphasize the use of appropriate infection control measures and limiting the duration of urinary catheter use.2 Now there are data showing a reduction in the incidence of CAUTIs when prophylactic antibiotics are given during catheter removal.
CAVEATS: Results may not apply to nonsurgical patients
This meta-analysis does not provide enough information to identify which patients are most likely to benefit from antibiotic prophylaxis. Most patients (92%) in this analysis had undergone surgery, but urinary catheterization is common among medically hospitalized patients. Studies of antibiotic prophylaxis at the time of catheter removal in nonsurgical patients are needed to strengthen the recommendation of this practice for all patients.
Some of the studies analyzed may have been biased. The authors determined that most of the studies in their meta-analysis were at high risk of attrition bias because there was potential for systematic differences in withdrawals between the treatment and control groups. In addition, in most studies, the randomization and allocation appeared to be inadequate, which increased the risk for selection bias.
CHALLENGES TO IMPLEMENTATION: Which antibiotics to use, and for how long, remains unclear
Antibiotic choice depends upon institutional policies and local resistance patterns, which complicates making universal recommendations. The optimal duration of treatment also is unknown, although this meta-analysis suggests that prophylaxis for 3 days or less can reduce CAUTI risk.
Catheters impregnated with antimicrobials or with microbial resistance barriers may be an alternative to administering antibiotics at catheter removal, but in preliminary studies, these devices have not been shown to reduce the incidence of CAUTIs.4,5 Increasing antimicrobial resistance also complicates the widespread use of prophylaxis.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Ensure that antibiotics are administered to surgical patients when their urinary catheter is removed to reduce the risk of urinary tract infections (UTIs).1
Strength of recommendation
B: Based on a meta-analysis.
Marschall J, Carpenter CR, Fowler S, et al; CDC Prevention Epicenters Program. Antibiotic prophylaxis for urinary tract infections after removal of urinary catheter: meta-analysis. BMJ. 2013;346:f3147.
Illustrative case
A 49-year-old man was admitted to the hospital for resection of a vertebral mass. He is almost ready for discharge, and his urinary catheter soon will be removed. Should he be given an antibiotic when his catheter is removed to prevent a UTI?
Approximately 15% to 25% of hospitalized patients receive a urinary catheter, typically during the perioperative period.2 UTIs are the most common hospital-acquired infections, and virtually all of these UTIs are caused by instrumentation of the urinary tract, primarily by catheters.2 Although the mortality rate among patients with catheter-associated UTIs (CAUTIs) is just 2.3%, CAUTIs are the leading cause of hospital-acquired bacteremia, which increases morbidity and length of stay.2 The most common pathogens for CAUTIs are Escherichia coli (21.4%), Candida species (21%), and Enterococcus species (14.9%).2Pseudomonas aeruginosa, Klebsiella, and Enterobacter species make up the bulk of the remainder.2
Support for antibiotic prophylaxis has historically been equivocal
Until now, no data clearly supported routine use of prophylactic antibiotics after urinary catheterization. Centers for Disease Control and Prevention (CDC) guidelines published in 2009 outline which patients are appropriate for catheterization, but do not recommend routine use of antibiotics to prevent CAUTIs.2 The 2014 Infectious Diseases Society of America guidelines, which came out before the study reported on here was published, state the benefit of antibiotics at the time of catheter removal is an unresolved issue.3
STUDY SUMMARY: Meta-analysis shows prophylactic antibiotics reduce UTI ris
Marschall et al1 searched multiple databases for studies published between 1947 and 2012 that evaluated prophylactic use of antibiotics at the time of urinary catheter removal. The endpoint for their analysis was symptomatic UTI, which they defined as bacteriuria plus at least one clinical symptom. Trials were excluded if patients had suprapubic catheters or if antibiotics were started shortly after the catheter was inserted.
The authors analyzed 7 studies. Six were randomized controlled trials, of which one was unpublished. The seventh trial was a nonrandomized study that compared outcomes of patients of 2 surgeons, one of whom used prophylactic antibiotics and one who did not. Five studies enrolled surgical patients exclusively, including 2 that focused on urology patients. In all of the studies, patients had a urinary catheter in place for fewer than 15 days. The duration of antibiotic treatment varied from a single dose to 3 days. The antibiotics used included trimethoprim/sulfamethoxazole, nitrofurantoin, ciprofloxacin, and a cephalosporin.
Antibiotic prophylaxis significantly reduced the rate of CAUTIs. The absolute risk reduction was 5.8%; the rate of CAUTIs was 4.7% in the group treated with antibiotics vs 10.5% in the control group. The number needed to treat to prevent one CAUTI was 17 (95% confidence interval [CI], 12-30), with a risk ratio (RR) of .45 (95% CI, .28-.72). The RR varied only slightly (.36) when the researchers repeated their analysis but excluded the unpublished trial, and remained at .45 when they analyzed only studies of surgical patients.
The reduction in CAUTIs remained consistent despite varying lengths of antibiotic administration and choice of antimicrobial agents. However, when the authors looked at pooled results just from the 2 studies that included both surgical and medical patients, they found no decrease in CAUTIs.
WHAT'S NEW: We now have an effective way to reduce CAUTIs
Prophylactic use of antibiotics when a urinary catheter is removed appears to reduce the rate of CAUTIs by more than 50% in surgical patients. The 2009 CDC guidelines on CAUTI prevention emphasize the use of appropriate infection control measures and limiting the duration of urinary catheter use.2 Now there are data showing a reduction in the incidence of CAUTIs when prophylactic antibiotics are given during catheter removal.
CAVEATS: Results may not apply to nonsurgical patients
This meta-analysis does not provide enough information to identify which patients are most likely to benefit from antibiotic prophylaxis. Most patients (92%) in this analysis had undergone surgery, but urinary catheterization is common among medically hospitalized patients. Studies of antibiotic prophylaxis at the time of catheter removal in nonsurgical patients are needed to strengthen the recommendation of this practice for all patients.
Some of the studies analyzed may have been biased. The authors determined that most of the studies in their meta-analysis were at high risk of attrition bias because there was potential for systematic differences in withdrawals between the treatment and control groups. In addition, in most studies, the randomization and allocation appeared to be inadequate, which increased the risk for selection bias.
CHALLENGES TO IMPLEMENTATION: Which antibiotics to use, and for how long, remains unclear
Antibiotic choice depends upon institutional policies and local resistance patterns, which complicates making universal recommendations. The optimal duration of treatment also is unknown, although this meta-analysis suggests that prophylaxis for 3 days or less can reduce CAUTI risk.
Catheters impregnated with antimicrobials or with microbial resistance barriers may be an alternative to administering antibiotics at catheter removal, but in preliminary studies, these devices have not been shown to reduce the incidence of CAUTIs.4,5 Increasing antimicrobial resistance also complicates the widespread use of prophylaxis.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Marschall J, Carpenter CR, Fowler S, et al; CDC Prevention Epicenters Program. Antibiotic prophylaxis for urinary tract infections after removal of urinary catheter: meta-analysis. BMJ. 2013;346:f3147.
2. Gould CV, Umscheid CA, Agarwal RK, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. Available at: http://www.cdc.gov/hicpac/pdf/cauti/cautiguideline2009final.pdf. Accessed April 15, 2014.
3. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35:464-479.
4. Pickard R, Lam T, Maclennan G, et al. Types of urethral catheter for reducing symptomatic urinary tract infections in hospitalised adults requiring short-term catheterisation: multicentre randomised controlled trial and economic evaluation of antimicrobial- and antiseptic-impregnated urethral catheters (the CATHETER trial). Health Technol Assess. 2012;16:1-197.
5. Pickard R, Lam T, MacLennan G, et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: a multicentre randomised controlled trial. Lancet. 2012;380:1927-1935.
1. Marschall J, Carpenter CR, Fowler S, et al; CDC Prevention Epicenters Program. Antibiotic prophylaxis for urinary tract infections after removal of urinary catheter: meta-analysis. BMJ. 2013;346:f3147.
2. Gould CV, Umscheid CA, Agarwal RK, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. Available at: http://www.cdc.gov/hicpac/pdf/cauti/cautiguideline2009final.pdf. Accessed April 15, 2014.
3. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35:464-479.
4. Pickard R, Lam T, Maclennan G, et al. Types of urethral catheter for reducing symptomatic urinary tract infections in hospitalised adults requiring short-term catheterisation: multicentre randomised controlled trial and economic evaluation of antimicrobial- and antiseptic-impregnated urethral catheters (the CATHETER trial). Health Technol Assess. 2012;16:1-197.
5. Pickard R, Lam T, MacLennan G, et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: a multicentre randomised controlled trial. Lancet. 2012;380:1927-1935.
Copyright © 2014 Family Physicians Inquiries Network. All rights reserved.
Low-dose penicillin for recurrent cellulitis?
Prescribe low-dose penicillin to patients with recurrent leg cellulitis to decrease the frequency of recurrent episodes.1
Strength of recommendation
B: Based on a single blinded randomized controlled trial (RCT).
Thomas KS, Crook AM, Nunn AJ, et al. Penicillin to prevent recurrent leg cellulitis. N Engl J Med. 2013; 368:1695-1703.
Illustrative case
An obese 50-year-old man presents with cellulitis of his right lower leg. He has a history of chronic venous insufficiency and has had 2 previous episodes of leg cellulitis in the past year. Should you initiate prophylactic antibiotics?
The incidence of cellulitis is 24.6 in 1000 person-years, according to a US population-based study of insurance claims for the years 1997 to 2002—the most recent data available.2 The lower extremities are most commonly affected, accounting for 70% to 80% of cases.3 Risk factors associated with recurrence include venous insufficiency, lymphedema, overweight, skin breakdown, and leg edema, which are often difficult to modify.4,5
Keeping well hydrated to avoid skin breakdown, elevating affected extremities to decrease edema, wearing compression stockings, and treating tinea pedis can help reduce the incidence of recurrent cellulitis.6,7 Prophylactic antibiotics can help, as well.
Which drug? What dose and duration?
These questions aren’t easily answered, as recommendations vary among specialty groups. The Infectious Diseases Society of America recommends benzathine penicillin (1.2 million units/month IM), erythromycin 250 mg PO BID, penicillin V 1 g PO BID, or nasal mupirocin BID for 5 days per month for “frequent” cellulitis, but “frequent” is not clearly defined.6,8-11 The British Lymphology Society (BLS)’s first-line recommendation for patients with ≥2 episodes of cellulitis per year is penicillin V 250 mg BID (or penicillin V 500 mg BID for patients with a BMI ≥33) for one year, then penicillin V 250 mg daily for an additional year. The BLS suggests lifelong antibiotic prophylaxis if cellulitis recurs after 2 years of prophylaxis.6
Consensus is lacking as to the optimal duration of prophylactic antibiotics. Kremer et al11 found that prophylactic erythromycin for 18 months reduced recurrent cellulitis; Thomas et al12 showed an insignificant reduction in cellulitis recurrence with 6 months of low-dose penicillin in patients who’d had one prior episode. The study detailed in this PURL provides additional evidence about the use of prophylactic antibiotics for recurrent lower extremity cellulitis and tests the efficacy of a particular dose and duration.
STUDY SUMMARY: Low-dose penicillin reduces recurrence rate
This double-blind RCT compared penicillin with placebo for the prevention of recurrent leg cellulitis. To be included in the study, patients had to have had at least 2 episodes of cellulitis within the previous 3 years, one of which occurred in the preceding 6 months. Participants (N=274) were recruited at hospitals in the United Kingdom and Ireland. Baseline characteristics included obesity (mean BMI, 35), mean age late 50s, and 3 to 4 prior episodes of cellulitis. About 25% of the participants had a history of venous insufficiency, as well.
The study had 2 phases—one for prophylaxis, the other for follow-up. During the prophylaxis phase, which lasted 12 months, patients received either penicillin 250 mg PO BID or placebo. Participants were followed for up to 3 years. They received phone calls every 3 months during the prophylaxis phase and every 6 months during the follow-up phase to assess adverse events, use of health care services, and recurrence of cellulitis.
Protection diminishes after prophylaxis ends
The primary outcome was the time from randomization to recurrence of cellulitis: Median times to recurrent cellulitis were 626 days for the penicillin group and 532 days for patients on placebo. Recurrence rates were 45% lower in those who received penicillin (hazard ratio=0.55; 95% confidence interval, 0.35-0.86; number needed to treat=5; P=.01) during the prophylaxis phase, but there was no difference in incidence in the follow-up phase.
Secondary outcomes measured were the proportions of patients with recurrent cellulitis in both the prophylaxis and follow-up phases, new leg edema or ulceration, duration of hospital admission for cellulitis, cost-effectiveness, and adverse drug effects/events of interest. The penicillin group had fewer episodes of recurrent cellulitis (119 vs 164; P=.02). The percentage of patients with new edema or ulceration between the 2 groups (40% penicillin vs 48% placebo; P=.46) and difference in cost-effectiveness between the 2 groups were not significant. Mean duration of hospitalization was 10 days (penicillin) and 9.2 days (placebo). There was no significant difference in the number of participants who experienced one or more adverse events (37 for those taking penicillin vs 48 for the placebo group; P=.50), including nausea, diarrhea, vulvovaginitis/thrush, rash, and death. There were 8 deaths in the penicillin group and 3 in the placebo group, although none was considered study related.
WHAT'S NEW?: Evidence that low-dose penicillin is effective
This trial provides strong evidence that a lower dose of penicillin than is currently recommended by the IDSA (250 mg vs 1 g BID) is effective in reducing leg cellulitis recurrence. It also shows that 12 months of prophylaxis significantly reduces the risk of recurrent leg cellulitis, but that the effect may diminish when the penicillin is stopped.
CAVEATS: Questions about dose and duration remain
Participant characteristics predictive of prophylaxis failure in this study included BMI≥33 and ≥3 previous episodes of cellulitis. It could be that patients with higher BMIs need a higher dose of penicillin. And we still don’t know whether prophylactic treatment for longer than 12 months would provide continued benefit, what the optimal time period for prophylactic antibiotics should be, and whether the higher recommended dose of penicillin would be more effective than the low dose that was used in this study. Antibiotic resistance associated with long-term penicillin use is a concern, as well.
challenges to implementation
Even when we know that patients are likely to benefit, we are often hesitant to prescribe long-term antibiotics because of reasonable fears of resistance and adverse effects.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Thomas KS, Crook AM, Nunn AJ, et al. Penicillin to prevent recurrent leg cellulitis. N Engl J Med. 2013; 368:1695-1703.
2. Ellis Simonsen SM, Van Orman ER, Hatch BE, et al. Cellulitis incidence in a defined population. Epidemiol Infect 2006;134:293-299.
3. Hirschmann Raugi GJ. Lower limb cellulitis and its mimics. Part I: Lower Limb Cellulitis. J Am Acad Dermatol. 2012;67:163e1-163e12.
4. Dupuy A, Benchikhi H, Roujeau JC, et al. Risk factors for erysipelas of the leg(cellulitis): case-control study. BMJ 1999;18:1591-1594.
5. McNamara DR, Tleyjah IM, Berbari EF, et al. A predictive model of recurrent lower extremity cellulitis in a population-based cohort. Arch Int Med. 2007;167:709-715.
6. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373-1406.
7. Consensus document on the management of cellulitis in lymphoedema. British Lymphology Society, 2013. Available at: http://www.lymphoedema.org/Menu3/Revised%20Cellulitis%20Consensus%202013.pdf. Accessed January 2, 2014.
8. Babb RR, Spittell JA Jr, Martin WJ, et al. Prophylaxis of recurrent lymphangitis complicating lymphedema. JAMA. 1966;195:871-873.
9. Sjoblom AC, Eriksson B, Jorup-Ronstrom C, et al. Antibiotic prophylaxis in recurrent erysipelas. Infection 1993;21:390-393.
10. Wang JH, Liu YC, Cheng DL, et al. Role of benzathine penicillin G in prophylaxis for recurrent streptococcal cellulitis of the the lower legs. Clin Infect Dis. 1997;25:685-689.
11. Kremer M, Zuckerman R, Avraham Z, et al. Long-term antimicrobial therapy in the prevention of recurrent soft-tissue infections. J Infect. 1991;22:37-40.
12. Thomas K, Crook A, Foster K, et al. Prophylactic antibiotics for the prevention of cellulitis (erysipelas) of the leg: results of the UK Dermatology Clinical Trials Network’s PATCH II trial team. Br J Dermatol. 2012;166:169-178.
Prescribe low-dose penicillin to patients with recurrent leg cellulitis to decrease the frequency of recurrent episodes.1
Strength of recommendation
B: Based on a single blinded randomized controlled trial (RCT).
Thomas KS, Crook AM, Nunn AJ, et al. Penicillin to prevent recurrent leg cellulitis. N Engl J Med. 2013; 368:1695-1703.
Illustrative case
An obese 50-year-old man presents with cellulitis of his right lower leg. He has a history of chronic venous insufficiency and has had 2 previous episodes of leg cellulitis in the past year. Should you initiate prophylactic antibiotics?
The incidence of cellulitis is 24.6 in 1000 person-years, according to a US population-based study of insurance claims for the years 1997 to 2002—the most recent data available.2 The lower extremities are most commonly affected, accounting for 70% to 80% of cases.3 Risk factors associated with recurrence include venous insufficiency, lymphedema, overweight, skin breakdown, and leg edema, which are often difficult to modify.4,5
Keeping well hydrated to avoid skin breakdown, elevating affected extremities to decrease edema, wearing compression stockings, and treating tinea pedis can help reduce the incidence of recurrent cellulitis.6,7 Prophylactic antibiotics can help, as well.
Which drug? What dose and duration?
These questions aren’t easily answered, as recommendations vary among specialty groups. The Infectious Diseases Society of America recommends benzathine penicillin (1.2 million units/month IM), erythromycin 250 mg PO BID, penicillin V 1 g PO BID, or nasal mupirocin BID for 5 days per month for “frequent” cellulitis, but “frequent” is not clearly defined.6,8-11 The British Lymphology Society (BLS)’s first-line recommendation for patients with ≥2 episodes of cellulitis per year is penicillin V 250 mg BID (or penicillin V 500 mg BID for patients with a BMI ≥33) for one year, then penicillin V 250 mg daily for an additional year. The BLS suggests lifelong antibiotic prophylaxis if cellulitis recurs after 2 years of prophylaxis.6
Consensus is lacking as to the optimal duration of prophylactic antibiotics. Kremer et al11 found that prophylactic erythromycin for 18 months reduced recurrent cellulitis; Thomas et al12 showed an insignificant reduction in cellulitis recurrence with 6 months of low-dose penicillin in patients who’d had one prior episode. The study detailed in this PURL provides additional evidence about the use of prophylactic antibiotics for recurrent lower extremity cellulitis and tests the efficacy of a particular dose and duration.
STUDY SUMMARY: Low-dose penicillin reduces recurrence rate
This double-blind RCT compared penicillin with placebo for the prevention of recurrent leg cellulitis. To be included in the study, patients had to have had at least 2 episodes of cellulitis within the previous 3 years, one of which occurred in the preceding 6 months. Participants (N=274) were recruited at hospitals in the United Kingdom and Ireland. Baseline characteristics included obesity (mean BMI, 35), mean age late 50s, and 3 to 4 prior episodes of cellulitis. About 25% of the participants had a history of venous insufficiency, as well.
The study had 2 phases—one for prophylaxis, the other for follow-up. During the prophylaxis phase, which lasted 12 months, patients received either penicillin 250 mg PO BID or placebo. Participants were followed for up to 3 years. They received phone calls every 3 months during the prophylaxis phase and every 6 months during the follow-up phase to assess adverse events, use of health care services, and recurrence of cellulitis.
Protection diminishes after prophylaxis ends
The primary outcome was the time from randomization to recurrence of cellulitis: Median times to recurrent cellulitis were 626 days for the penicillin group and 532 days for patients on placebo. Recurrence rates were 45% lower in those who received penicillin (hazard ratio=0.55; 95% confidence interval, 0.35-0.86; number needed to treat=5; P=.01) during the prophylaxis phase, but there was no difference in incidence in the follow-up phase.
Secondary outcomes measured were the proportions of patients with recurrent cellulitis in both the prophylaxis and follow-up phases, new leg edema or ulceration, duration of hospital admission for cellulitis, cost-effectiveness, and adverse drug effects/events of interest. The penicillin group had fewer episodes of recurrent cellulitis (119 vs 164; P=.02). The percentage of patients with new edema or ulceration between the 2 groups (40% penicillin vs 48% placebo; P=.46) and difference in cost-effectiveness between the 2 groups were not significant. Mean duration of hospitalization was 10 days (penicillin) and 9.2 days (placebo). There was no significant difference in the number of participants who experienced one or more adverse events (37 for those taking penicillin vs 48 for the placebo group; P=.50), including nausea, diarrhea, vulvovaginitis/thrush, rash, and death. There were 8 deaths in the penicillin group and 3 in the placebo group, although none was considered study related.
WHAT'S NEW?: Evidence that low-dose penicillin is effective
This trial provides strong evidence that a lower dose of penicillin than is currently recommended by the IDSA (250 mg vs 1 g BID) is effective in reducing leg cellulitis recurrence. It also shows that 12 months of prophylaxis significantly reduces the risk of recurrent leg cellulitis, but that the effect may diminish when the penicillin is stopped.
CAVEATS: Questions about dose and duration remain
Participant characteristics predictive of prophylaxis failure in this study included BMI≥33 and ≥3 previous episodes of cellulitis. It could be that patients with higher BMIs need a higher dose of penicillin. And we still don’t know whether prophylactic treatment for longer than 12 months would provide continued benefit, what the optimal time period for prophylactic antibiotics should be, and whether the higher recommended dose of penicillin would be more effective than the low dose that was used in this study. Antibiotic resistance associated with long-term penicillin use is a concern, as well.
challenges to implementation
Even when we know that patients are likely to benefit, we are often hesitant to prescribe long-term antibiotics because of reasonable fears of resistance and adverse effects.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Prescribe low-dose penicillin to patients with recurrent leg cellulitis to decrease the frequency of recurrent episodes.1
Strength of recommendation
B: Based on a single blinded randomized controlled trial (RCT).
Thomas KS, Crook AM, Nunn AJ, et al. Penicillin to prevent recurrent leg cellulitis. N Engl J Med. 2013; 368:1695-1703.
Illustrative case
An obese 50-year-old man presents with cellulitis of his right lower leg. He has a history of chronic venous insufficiency and has had 2 previous episodes of leg cellulitis in the past year. Should you initiate prophylactic antibiotics?
The incidence of cellulitis is 24.6 in 1000 person-years, according to a US population-based study of insurance claims for the years 1997 to 2002—the most recent data available.2 The lower extremities are most commonly affected, accounting for 70% to 80% of cases.3 Risk factors associated with recurrence include venous insufficiency, lymphedema, overweight, skin breakdown, and leg edema, which are often difficult to modify.4,5
Keeping well hydrated to avoid skin breakdown, elevating affected extremities to decrease edema, wearing compression stockings, and treating tinea pedis can help reduce the incidence of recurrent cellulitis.6,7 Prophylactic antibiotics can help, as well.
Which drug? What dose and duration?
These questions aren’t easily answered, as recommendations vary among specialty groups. The Infectious Diseases Society of America recommends benzathine penicillin (1.2 million units/month IM), erythromycin 250 mg PO BID, penicillin V 1 g PO BID, or nasal mupirocin BID for 5 days per month for “frequent” cellulitis, but “frequent” is not clearly defined.6,8-11 The British Lymphology Society (BLS)’s first-line recommendation for patients with ≥2 episodes of cellulitis per year is penicillin V 250 mg BID (or penicillin V 500 mg BID for patients with a BMI ≥33) for one year, then penicillin V 250 mg daily for an additional year. The BLS suggests lifelong antibiotic prophylaxis if cellulitis recurs after 2 years of prophylaxis.6
Consensus is lacking as to the optimal duration of prophylactic antibiotics. Kremer et al11 found that prophylactic erythromycin for 18 months reduced recurrent cellulitis; Thomas et al12 showed an insignificant reduction in cellulitis recurrence with 6 months of low-dose penicillin in patients who’d had one prior episode. The study detailed in this PURL provides additional evidence about the use of prophylactic antibiotics for recurrent lower extremity cellulitis and tests the efficacy of a particular dose and duration.
STUDY SUMMARY: Low-dose penicillin reduces recurrence rate
This double-blind RCT compared penicillin with placebo for the prevention of recurrent leg cellulitis. To be included in the study, patients had to have had at least 2 episodes of cellulitis within the previous 3 years, one of which occurred in the preceding 6 months. Participants (N=274) were recruited at hospitals in the United Kingdom and Ireland. Baseline characteristics included obesity (mean BMI, 35), mean age late 50s, and 3 to 4 prior episodes of cellulitis. About 25% of the participants had a history of venous insufficiency, as well.
The study had 2 phases—one for prophylaxis, the other for follow-up. During the prophylaxis phase, which lasted 12 months, patients received either penicillin 250 mg PO BID or placebo. Participants were followed for up to 3 years. They received phone calls every 3 months during the prophylaxis phase and every 6 months during the follow-up phase to assess adverse events, use of health care services, and recurrence of cellulitis.
Protection diminishes after prophylaxis ends
The primary outcome was the time from randomization to recurrence of cellulitis: Median times to recurrent cellulitis were 626 days for the penicillin group and 532 days for patients on placebo. Recurrence rates were 45% lower in those who received penicillin (hazard ratio=0.55; 95% confidence interval, 0.35-0.86; number needed to treat=5; P=.01) during the prophylaxis phase, but there was no difference in incidence in the follow-up phase.
Secondary outcomes measured were the proportions of patients with recurrent cellulitis in both the prophylaxis and follow-up phases, new leg edema or ulceration, duration of hospital admission for cellulitis, cost-effectiveness, and adverse drug effects/events of interest. The penicillin group had fewer episodes of recurrent cellulitis (119 vs 164; P=.02). The percentage of patients with new edema or ulceration between the 2 groups (40% penicillin vs 48% placebo; P=.46) and difference in cost-effectiveness between the 2 groups were not significant. Mean duration of hospitalization was 10 days (penicillin) and 9.2 days (placebo). There was no significant difference in the number of participants who experienced one or more adverse events (37 for those taking penicillin vs 48 for the placebo group; P=.50), including nausea, diarrhea, vulvovaginitis/thrush, rash, and death. There were 8 deaths in the penicillin group and 3 in the placebo group, although none was considered study related.
WHAT'S NEW?: Evidence that low-dose penicillin is effective
This trial provides strong evidence that a lower dose of penicillin than is currently recommended by the IDSA (250 mg vs 1 g BID) is effective in reducing leg cellulitis recurrence. It also shows that 12 months of prophylaxis significantly reduces the risk of recurrent leg cellulitis, but that the effect may diminish when the penicillin is stopped.
CAVEATS: Questions about dose and duration remain
Participant characteristics predictive of prophylaxis failure in this study included BMI≥33 and ≥3 previous episodes of cellulitis. It could be that patients with higher BMIs need a higher dose of penicillin. And we still don’t know whether prophylactic treatment for longer than 12 months would provide continued benefit, what the optimal time period for prophylactic antibiotics should be, and whether the higher recommended dose of penicillin would be more effective than the low dose that was used in this study. Antibiotic resistance associated with long-term penicillin use is a concern, as well.
challenges to implementation
Even when we know that patients are likely to benefit, we are often hesitant to prescribe long-term antibiotics because of reasonable fears of resistance and adverse effects.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Thomas KS, Crook AM, Nunn AJ, et al. Penicillin to prevent recurrent leg cellulitis. N Engl J Med. 2013; 368:1695-1703.
2. Ellis Simonsen SM, Van Orman ER, Hatch BE, et al. Cellulitis incidence in a defined population. Epidemiol Infect 2006;134:293-299.
3. Hirschmann Raugi GJ. Lower limb cellulitis and its mimics. Part I: Lower Limb Cellulitis. J Am Acad Dermatol. 2012;67:163e1-163e12.
4. Dupuy A, Benchikhi H, Roujeau JC, et al. Risk factors for erysipelas of the leg(cellulitis): case-control study. BMJ 1999;18:1591-1594.
5. McNamara DR, Tleyjah IM, Berbari EF, et al. A predictive model of recurrent lower extremity cellulitis in a population-based cohort. Arch Int Med. 2007;167:709-715.
6. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373-1406.
7. Consensus document on the management of cellulitis in lymphoedema. British Lymphology Society, 2013. Available at: http://www.lymphoedema.org/Menu3/Revised%20Cellulitis%20Consensus%202013.pdf. Accessed January 2, 2014.
8. Babb RR, Spittell JA Jr, Martin WJ, et al. Prophylaxis of recurrent lymphangitis complicating lymphedema. JAMA. 1966;195:871-873.
9. Sjoblom AC, Eriksson B, Jorup-Ronstrom C, et al. Antibiotic prophylaxis in recurrent erysipelas. Infection 1993;21:390-393.
10. Wang JH, Liu YC, Cheng DL, et al. Role of benzathine penicillin G in prophylaxis for recurrent streptococcal cellulitis of the the lower legs. Clin Infect Dis. 1997;25:685-689.
11. Kremer M, Zuckerman R, Avraham Z, et al. Long-term antimicrobial therapy in the prevention of recurrent soft-tissue infections. J Infect. 1991;22:37-40.
12. Thomas K, Crook A, Foster K, et al. Prophylactic antibiotics for the prevention of cellulitis (erysipelas) of the leg: results of the UK Dermatology Clinical Trials Network’s PATCH II trial team. Br J Dermatol. 2012;166:169-178.
1. Thomas KS, Crook AM, Nunn AJ, et al. Penicillin to prevent recurrent leg cellulitis. N Engl J Med. 2013; 368:1695-1703.
2. Ellis Simonsen SM, Van Orman ER, Hatch BE, et al. Cellulitis incidence in a defined population. Epidemiol Infect 2006;134:293-299.
3. Hirschmann Raugi GJ. Lower limb cellulitis and its mimics. Part I: Lower Limb Cellulitis. J Am Acad Dermatol. 2012;67:163e1-163e12.
4. Dupuy A, Benchikhi H, Roujeau JC, et al. Risk factors for erysipelas of the leg(cellulitis): case-control study. BMJ 1999;18:1591-1594.
5. McNamara DR, Tleyjah IM, Berbari EF, et al. A predictive model of recurrent lower extremity cellulitis in a population-based cohort. Arch Int Med. 2007;167:709-715.
6. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373-1406.
7. Consensus document on the management of cellulitis in lymphoedema. British Lymphology Society, 2013. Available at: http://www.lymphoedema.org/Menu3/Revised%20Cellulitis%20Consensus%202013.pdf. Accessed January 2, 2014.
8. Babb RR, Spittell JA Jr, Martin WJ, et al. Prophylaxis of recurrent lymphangitis complicating lymphedema. JAMA. 1966;195:871-873.
9. Sjoblom AC, Eriksson B, Jorup-Ronstrom C, et al. Antibiotic prophylaxis in recurrent erysipelas. Infection 1993;21:390-393.
10. Wang JH, Liu YC, Cheng DL, et al. Role of benzathine penicillin G in prophylaxis for recurrent streptococcal cellulitis of the the lower legs. Clin Infect Dis. 1997;25:685-689.
11. Kremer M, Zuckerman R, Avraham Z, et al. Long-term antimicrobial therapy in the prevention of recurrent soft-tissue infections. J Infect. 1991;22:37-40.
12. Thomas K, Crook A, Foster K, et al. Prophylactic antibiotics for the prevention of cellulitis (erysipelas) of the leg: results of the UK Dermatology Clinical Trials Network’s PATCH II trial team. Br J Dermatol. 2012;166:169-178.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.