User login
Minimizing Opioids After Joint Operation: Protocol to Decrease Postoperative Opioid Use After Primary Total Knee Arthroplasty
For decades, opioids have been a mainstay in the management of pain after total joint arthroplasty. In the past 10 years, however, opioid prescribing has come under increased scrutiny due to a rise in rates of opioid abuse, pill diversion, and opioid-related deaths.1,2 Opioids are associated with adverse effects, including nausea, vomiting, constipation, apathy, and respiratory depression, all of which influence arthroplasty outcomes and affect the patient experience. Although primary care groups account for nearly half of prescriptions written, orthopedic surgeons have the third highest per capita rate of opioid prescribing of all medical specialties.3,4 This puts orthopedic surgeons, particularly those who perform routine procedures, in an opportune but challenging position to confront this problem through novel pain management strategies.
Approximately 1 million total knee arthroplasties (TKAs) are performed in the US every year, and the US Department of Veterans Affairs (VA) health system performs about 10,000 hip and knee joint replacements.5,6 There is no standardization of opioid prescribing in the postoperative period following these procedures, and studies have reported a wide variation in prescribing habits even within a single institution for a specific surgery.7 Patients who undergo TKA are at particularly high risk of long-term opioid use if they are on continuous opioids at the time of surgery; this is problematic in a VA patient population in which at least 16% of patients are prescribed opioids in a given year.8 Furthermore, veterans are twice as likely as nonveterans to die of an accidental overdose.9 Despite these risks, opioids remain a cornerstone of postoperative pain management both within and outside of the VA.10
In 2018, to limit unnecessary prescribing of opioid pain medication, the total joint service at the VA Portland Health Care System (VAPHCS) in Oregon implemented the Minimizing Opioids after Joint Operation (MOJO) postoperative pain protocol. The goal of the protocol was to reduce opioid use following TKA. The objectives were to provide safe, appropriate analgesia while allowing early mobilization and discharge without a concomitant increase in readmissions or emergency department (ED) visits. The purpose of this retrospective chart review was to compare the efficacy of the MOJO protocol with our historical experience and report our preliminary results.
Methods
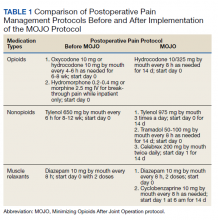
Institutional review board approval was obtained to retrospectively review the medical records of patients who had undergone TKA surgery during 2018 at VAPHCS. The MOJO protocol was composed of several simultaneous changes. The centerpiece of the new protocol was a drastic decrease in routine prescription of postoperative opioids (Table 1). Other changes included instructing patients to reduce the use of preoperative opioid pain medication 6 weeks before surgery with a goal of no opioid consumption, perform daily sets of preoperative exercises, and attend a preoperative consultation/education session with a nurse coordinator to emphasize early recovery and discharge. In patients with chronic use of opioid pain medication (particularly those for whom the medication had been prescribed for other sources of pain, such as lumbar back pain), the goal was daily opioid use of ≤ 30 morphine equivalent doses (MEDs). During the inpatient stay, we stopped prescribing prophylactic pain medication prior to physical therapy (PT).
We encouraged preoperative optimization of muscle strength by giving instructions for 4 to 8 weeks of daily exercises (Appendix). We introduced perioperative adductor canal blocks (at the discretion of the anesthesia team) and transitioned to surgery without a tourniquet. Patients in both groups received intraoperative antibiotics and IV tranexamic acid (TXA); the MOJO group also received topical TXA.
Further patient care optimization included providing patients with a team-based approach, which consisted of nurse coordinators, physician assistants and nurse practitioners, residents, and the attending surgeon. Our team reviews the planned pain management protocol, perioperative expectations, criteria for discharge, and anticipated surgical outcomes with the patient during their preoperative visits. On postoperative day 1, these members round as a team to encourage patients in their immediate postoperative recovery and rehabilitation. During rounds, the team assesses whether the patient meets the criteria for discharge, adjusting the pain management protocol if necessary.
Changes in surgical technique included arthrotomy with electrocautery, minimizing traumatic dissection or resection of the synovial tissue, and intra-articular injection of a cocktail of ropivacaine 5 mg/mL 40 mL, epinephrine 1:1,000 0.5 mL, and methylprednisolone sodium 40 mg diluted with normal saline to a total volume of 120 mL.
The new routine was gradually implemented beginning January 2017 and fully implemented by July 2018. This study compared the first 20 consecutive patients undergoing primary TKA after July 2018 to the last 20 consecutive patients undergoing primary TKA prior to January 2017. Exclusion criteria included bilateral TKA, death before 90 days, and revision as the indication for surgery. The senior attending surgeon performed all surgeries using a standard midline approach. The majority of surgeries were performed using a cemented Vanguard total knee system (Zimmer Biomet); 4 patients in the historical group had a NexGen knee system, cementless monoblock tibial components (Zimmer Biomet); and 1 patient had a Logic knee system (Exactech). Surgical selection criteria for patients did not differ between groups.
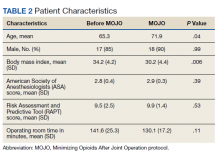
Electronic health records were reviewed and data were abstracted. The data included demographic information (age, gender, body mass index [BMI], diagnosis, and procedure), surgical factors (American Society of Anesthesiologists score, Risk Assessment and Predictive Tool score, operative time, tourniquet time, estimated blood loss), hospital factors (length of stay [LOS], discharge location), postoperative pain scores (measured on postoperative day 1 and on day of discharge), and postdischarge events (90-day complications, telephone calls reporting pain, reoperations, returns to the ED, 90-day readmissions).
The primary outcome was the mean postoperative daily MED during the inpatient stay. Secondary outcomes included pain on postoperative day 1, pain at the time of discharge, LOS, hospital readmissions, and ED visits within 90 days of surgery. Because different opioid pain medications were used by patients postoperatively, all opioids were converted to MED prior to the final analysis. Collected patient data were de-identified prior to analysis.
Power analysis was conducted to determine whether the study had sufficient population size to reject the null hypothesis for the primary outcome measure. Because practitioners controlled postoperative opioid use, a Cohen’s d of 1.0 was used so that a very large effect size was needed to reach clinical significance. Statistical significance was set to 0.05, and patient groups were set at 20 patients each. This yielded an appropriate power of 0.87. Population characteristics were compared between groups using t tests and χ2 tests as appropriate. To analyze the primary outcome, comparisons were made between the 2 cohorts using 2-tailed t tests. Secondary outcomes were compared between groups using t tests or χ2 tests. All statistics were performed using R version 3.5.2. Power analysis was conducted using the package pwr.11 Statistical significance was set at
Results
Forty patients met the inclusion criteria, evenly divided between those undergoing TKA before and after instituting the MOJO protocol (Table 2). A single patient in the MOJO group died and was excluded. A patient who underwent bilateral TKA also was excluded. Both groups reflected the male predominance of the VA patient population. MOJO patients tended to have lower BMIs (34 vs 30, P < .01). All patients indicated for surgery with preoperative opioid use were able to titrate down to their preoperative goal as verified by prescriptions filled at VA pharmacies. Twelve of the patients in the MOJO group received adductor canal blocks.
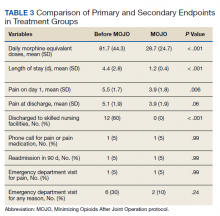
Results of t tests and χ2 tests comparing primary and secondary endpoints are listed in Table 3. Differences between the daily MEDs given in the historical and MOJO groups are shown. There were significant differences between the pre-MOJO and MOJO groups with regard to daily inpatient MEDs (82 mg vs 29 mg, P < .01) and total inpatient MEDs (306 mg vs 32 mg, P < .01). There was less self-reported pain on postoperative day 1 in the MOJO group (5.5 vs 3.9, P < .01), decreased LOS (4.4 days vs 1.2 days, P < .01), a trend toward fewer total ED visits (6 vs 2, P = .24), and fewer discharges to skilled nursing facilities (12 vs 0, P < .01). There were no blood transfusions in either group.
There were no readmissions due to uncontrolled pain. There was 1 readmission for shortness of breath in the MOJO group. The patient was discharged home the following day after ruling out thromboembolic and cardiovascular events. One patient from the control group was readmitted after missing a step on a staircase and falling. The patient sustained a quadriceps tendon rupture and underwent primary suture repair.
Discussion
Our results demonstrate that a multimodal approach to significantly reduce postoperative opioid use in patients with TKA is possible without increasing readmissions or ED visits for pain control. The patients in the MOJO group had a faster recovery, earlier discharge, and less use of postoperative opioid medication. Our approach to postoperative pain management was divided into 2 main categories: patient optimization and surgical optimization.
Patient Selection
Besides the standard evaluation and optimization of patients’ medical conditions, identifying and optimizing at-risk patients before surgery was a critical component of our protocol. Managing postoperative pain in patients with prior opioid use is an intractable challenge in orthopedic surgery. Patients with a history of chronic pain and preoperative use of opioid medications remain at higher risk of postoperative chronic pain and persistent use of opioid medication despite no obvious surgical complications.8 In a sample of > 6,000 veterans who underwent TKA at VA hospitals in 2014, 57% of the patients with daily use of opioids in the 90 days before surgery remained on opioids 1 year after surgery (vs 2 % in patients not on long-term opioids).8 This relationship between pre- and postoperative opioid use also was dose dependent.12
Furthermore, those with high preoperative use may experience worse outcomes relative to the opioid naive population as measured by arthritis-specific pain indices.13 In a well-powered retrospective study of patients who underwent elective orthopedic procedures, preoperative opioid abuse or dependence (determined by the International Classification of Diseases, Ninth Revision diagnosis) increased inpatient mortality, aggregate morbidity, surgical site infection, myocardial infarction, and LOS.14 Preoperative opioid use also has been associated with increased risk of ED visits, readmission, infection, stiffness, and aseptic revision.15 In patients with TKA in the VA specifically, preoperative opioid use (> 3 months in the prior year) was associated with increased revision rates that were even higher than those for patients with diabetes mellitus.16
Patient Education
Based on this evidence, we instruct patients to reduce their preoperative opioid dosing to zero (for patients with joint pain) or < 30 MED (for patients using opioids for other reasons). Although preoperative reduction of opioid use has been shown to improve outcomes after TKA, pain subspecialty recommendations for patients with chronic opioid use recommend considering adjunctive therapies, including transcutaneous electrical nerve stimulation, cognitive behavioral therapy, gabapentin, or ketamine.17,18 Through patient education our team has been successful in decreasing preoperative opioid use without adding other drugs or modalities.
Patient Optimization
Preoperative patient optimization included 4 to 8 weeks of daily sets of physical activity instructions (prehab) to improve the musculoskeletal function. These instructions are given to patients 4 to 8 weeks before surgery and aim to improve the patient’s balance, mobility, and functional ability (Appendix). Meta-analysis has shown that patients who undergo preoperative PT have a small but statistically significant decrease in postoperative pain at 4 weeks, though this does not persist beyond that period.19
We did note a lower BMI in patients in the MOJO group. Though this has the potential to be a confounder, a study of BMI in > 4,000 patients who underwent joint replacement surgery has shown that BMI is not associated with differences in postoperative pain.20
Surgeon and Surgical-Related Variables
Patients in the MOJO group had increased use of adductor canal blocks. A 2017 meta-analysis of 12,530 patients comparing analgesic modalities found that peripheral nerve blocks targeting multiple nerves (eg, femoral/sciatic) decreased pain at rest, decreased opioid consumption, and improved range of motion postoperatively.21 Also, these were found to be superior to single nerve blocks, periarticular infiltration, and epidural blocks.21 However, major nerve and epidural blocks affecting the lower extremity may increase the risk of falls and prolong LOS.22,23 The preferred peripheral block at VAPHCS is a single shot ultrasound-guided adductor canal block before the induction of general or spinal anesthesia. A randomized controlled trial has demonstrated superiority of this block to the femoral nerve block with regard to postoperative quadriceps strength, conferring the theoretical advantage of decreased fall risk and ability to participate in immediate PT.24 Although we are unable to confirm an association between anesthetic modalities and opioid burden, our clinical impression is that blocks were effective at reducing immediate postoperative pain. However, among MOJO patients there were no differences in patients with and without blocks for either pain (4.2 vs 3.8, P = .69) or opioid consumption (28.8 vs 33.0, P = .72) after surgery, though our study was not powered to detect a difference in this restricted subgroup.
Patients who frequently had reported postoperative thigh pain prompted us to make changes in our surgical technique, performing TKA without use of a tourniquet. Tourniquet use has been associated with an increased risk of thigh pain after TKA by multiple authors.25,26 Postoperative thigh pain also is pressure dependent.27 In addition, its use may be associated with a slightly increased risk of thromboembolic events and delayed functional recovery.28,29
Because postoperative hemarthrosis is associated with more pain and reduced joint recovery function, we used topical TXA to reduce postoperative surgical site and joint hematoma. TXA (either oral, IV, or topical) during TKA is used to control postoperative bleeding primarily and decrease the need for transfusion without concomitant increase in thromboembolic events.30,31 Topical TXA may be more effective than IV, particularly in the immediate postoperative period.32 Although pain typically is not an endpoint in studies of TXA, a prospective study of 48 patients showed evidence that its use may be associated with decreased postoperative pain in the first 24 hours after surgery (though not after).33 Finally, the use of intra-articular injection has evolved in our clinical practice, but literature is lacking with regard to its efficacy; more studies are needed to determine its effect relative to no injection. We have not seen any benefits to using
Limitations
This is a nonrandomized retrospective single-institution study. Our study population is composed of mostly males with military experience and is not necessarily a representative sample of the general population eligible for joint arthroplasty. Our primary endpoint (reduction of opioid use postoperatively) also was a cornerstone of our intervention. To account for this, we set a very large effect size in our power analysis and evaluated multiple secondary endpoints to determine whether postoperative pain remained well controlled and complications/readmission minimized with our interventions. Because our intervention was multimodal, our study cannot make conclusions about the effect of a particular component of our treatment strategy. We did not measure or compare functional outcomes between both groups, which offers an opportunity for further research.
These limitations are balanced by several strengths. Our cohort was well controlled with respect to the dose and type of drug used. There is staff dedicated to postoperative telephone follow-up after discharge, and veterans are apt to seek care within the VA health care system, which improves case finding for complications and ED visits. No patients were lost to follow-up. Moreover, our drastic reduction in opioid use is promising enough to warrant reporting, while the broader orthopedic literature explores the relative impact of each variable.
Conclusions
The MOJO protocol has been effective for reducing postoperative opioid use after TKA without compromising effective pain management. The drastic reduction in the postoperative use of opioid pain medications and LOS have contributed to a cultural shift within our department, comprehensive team approach, multimodal pain management, and preoperative patient optimization. Further investigations are required to assess the impact of each intervention on observed outcomes. However, the framework and routines are applicable to other institutions and surgical specialties.
Acknowledgments
The authors recognize Derek Bond, MD, for his help in creating the MOJO acronym.
1. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics Data Brief No. 329. Published November 2018. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db329-h.pdf
2. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics NCHS data brief No. 294. Published December 2017. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db294.pdf
3. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic–prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015;49(3):409-413. doi:10.1016/j.amepre.2015.02.020
4. Guy GP, Zhang K. Opioid prescribing by specialty and volume in the U.S. Am J Prev Med. 2018;55(5):e153-155. doi:10.1016/j.amepre.2018.06.008
5. Kremers HM, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surgery Am. 2015;17:1386-1397. doi:10.2106/JBJS.N.01141
6. Giori NJ, Amanatullah DF, Gupta S, Bowe T, Harris AHS. Risk reduction compared with access to care: quantifying the trade-off of enforcing a body mass index eligibility criterion for joint replacement. J Bone Joint Surg Am. 2018; 4(100):539-545. doi:10.2106/JBJS.17.00120
7. Sabatino MJ, Kunkel ST, Ramkumar DB, Keeney BJ, Jevsevar DS. Excess opioid medication and variation in prescribing patterns following common orthopaedic procedures. J Bone Joint Surg Am. 2018;100(3):180-188. doi:10.2106/JBJS.17.00672
8. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
9. Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321. doi:10.1001/jama.2011.370
10. Hall MJ, Schwartzman A, Zhang J, Liu X. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl Health Stat Report. 2017(102):1-15.
11. Champely S. pwr: basic functions for power analysis. R package version 1.2-2; 2018. Accessed January 13, 2021. https://rdrr.io/cran/pwr/
12. Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265. doi:10.1097/j.pain.0000000000000516
13. Smith SR, Bido J, Collins JE, Yang H, Katz JN, Losina E. Impact of preoperative opioid use on total knee arthroplasty outcomes. J Bone Joint Surg Am. 2017;99(10):803-808. doi:10.2106/JBJS.16.01200
14. Menendez ME, Ring D, Bateman BT. Preoperative opioid misuse is associated with increased morbidity and mortality after elective orthopaedic surgery. Clin Orthop Relat Res. 2015;473(7):2402-412. doi:10.1007/s11999-015-4173-5
15. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
16. Ben-Ari A, Chansky H, Rozet I. Preoperative opioid use is associated with early revision after total knee arthroplasty: a study of male patients treated in the Veterans Affairs System. J Bone Joint Surg Am. 2017;99(1):1-9. doi:10.2106/JBJS.16.00167
17. Nguyen L-CL, Sing DC, Bozic KJ. Preoperative reduction of opioid use before total joint arthroplasty. J Arthroplasty. 2016;31(suppl 9):282-287. doi:10.1016/j.arth.2016.01.068
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
19. Wang L, Lee M, Zhang Z, Moodie J, Cheng D, Martin J. Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes? A systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2016;6(2):e009857. doi:10.1136/bmjopen-2015-009857
20. Li W, Ayers DC, Lewis CG, Bowen TR, Allison JJ, Franklin PD. Functional gain and pain relief after total joint replacement according to obesity status. J Bone Joint Surg. 2017;99(14):1183-1189. doi:10.2106/JBJS.16.00960
21. Terkawi AS, Mavridis D, Sessler DI, et al. Pain management modalities after total knee arthroplasty: a network meta-analysis of 170 randomized controlled trials. Anesthesiology. 2017;126(5):923-937. doi:10.1097/ALN.0000000000001607
22. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111(6):1552-1554. doi:10.1213/ANE.0b013e3181fb9507
23. Elkassabany NM, Antosh S, Ahmed M, et al. The risk of falls after total knee arthroplasty with the use of a femoral nerve block versus an adductor canal block. Anest Analg. 2016;122(5):1696-1703. doi:10.1213/ane.0000000000001237
24. Wang D, Yang Y, Li Q, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a meta-analysis of randomized controlled trials. Sci Rep. 2017;7:40721. doi:10.1038/srep40721
25. Liu D, Graham D, Gillies K, Gillies RM. Effects of tourniquet use on quadriceps function and pain in total knee arthroplasty. Knee Surg Relat Res. 2014;26(4):207-213. doi:10.5792/ksrr.2014.26.4.207
26. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
27. Worland RL, Arredondo J, Angles F, Lopez-Jimenez F, Jessup DE. Thigh pain following tourniquet application in simultaneous bilateral total knee replacement arthroplasty. J Arthroplasty. 1997;12(8):848-852. doi:10.1016/s0883-5403(97)90153-4
28. Tai T-W, Lin C-J, Jou I-M, Chang C-W, Lai K-A, Yang C-Y. Tourniquet use in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol, Arthrosc. 2011;19(7):1121-1130. doi:10.1007/s00167-010-1342-7
29. Jiang F-Z, Zhong H-M, Hong Y-C, Zhao G-F. Use of a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Orthop Sci. 2015;20(21):110-123. doi:10.1007/s00776-014-0664-6
30. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585. doi:10.1302/0301-620X.93B12.26989
31. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee. 2013;20(5):300-309. doi:10.1016/j.knee.2013.05.014
32. Wang J, Wang Q, Zhang X, Wang Q. Intra-articular application is more effective than intravenous application of tranexamic acid in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2017;32(11):3385-3389. doi:10.1016/j.arth.2017.06.024
33. Guerreiro JPF, Badaro BS, Balbino JRM, Danieli MV, Queiroz AO, Cataneo DC. Application of tranexamic acid in total knee arthroplasty – prospective randomized trial. J Open Orthop J. 2017;11:1049-1057. doi:10.2174/1874325001711011049
For decades, opioids have been a mainstay in the management of pain after total joint arthroplasty. In the past 10 years, however, opioid prescribing has come under increased scrutiny due to a rise in rates of opioid abuse, pill diversion, and opioid-related deaths.1,2 Opioids are associated with adverse effects, including nausea, vomiting, constipation, apathy, and respiratory depression, all of which influence arthroplasty outcomes and affect the patient experience. Although primary care groups account for nearly half of prescriptions written, orthopedic surgeons have the third highest per capita rate of opioid prescribing of all medical specialties.3,4 This puts orthopedic surgeons, particularly those who perform routine procedures, in an opportune but challenging position to confront this problem through novel pain management strategies.
Approximately 1 million total knee arthroplasties (TKAs) are performed in the US every year, and the US Department of Veterans Affairs (VA) health system performs about 10,000 hip and knee joint replacements.5,6 There is no standardization of opioid prescribing in the postoperative period following these procedures, and studies have reported a wide variation in prescribing habits even within a single institution for a specific surgery.7 Patients who undergo TKA are at particularly high risk of long-term opioid use if they are on continuous opioids at the time of surgery; this is problematic in a VA patient population in which at least 16% of patients are prescribed opioids in a given year.8 Furthermore, veterans are twice as likely as nonveterans to die of an accidental overdose.9 Despite these risks, opioids remain a cornerstone of postoperative pain management both within and outside of the VA.10
In 2018, to limit unnecessary prescribing of opioid pain medication, the total joint service at the VA Portland Health Care System (VAPHCS) in Oregon implemented the Minimizing Opioids after Joint Operation (MOJO) postoperative pain protocol. The goal of the protocol was to reduce opioid use following TKA. The objectives were to provide safe, appropriate analgesia while allowing early mobilization and discharge without a concomitant increase in readmissions or emergency department (ED) visits. The purpose of this retrospective chart review was to compare the efficacy of the MOJO protocol with our historical experience and report our preliminary results.
Methods
Institutional review board approval was obtained to retrospectively review the medical records of patients who had undergone TKA surgery during 2018 at VAPHCS. The MOJO protocol was composed of several simultaneous changes. The centerpiece of the new protocol was a drastic decrease in routine prescription of postoperative opioids (Table 1). Other changes included instructing patients to reduce the use of preoperative opioid pain medication 6 weeks before surgery with a goal of no opioid consumption, perform daily sets of preoperative exercises, and attend a preoperative consultation/education session with a nurse coordinator to emphasize early recovery and discharge. In patients with chronic use of opioid pain medication (particularly those for whom the medication had been prescribed for other sources of pain, such as lumbar back pain), the goal was daily opioid use of ≤ 30 morphine equivalent doses (MEDs). During the inpatient stay, we stopped prescribing prophylactic pain medication prior to physical therapy (PT).
We encouraged preoperative optimization of muscle strength by giving instructions for 4 to 8 weeks of daily exercises (Appendix). We introduced perioperative adductor canal blocks (at the discretion of the anesthesia team) and transitioned to surgery without a tourniquet. Patients in both groups received intraoperative antibiotics and IV tranexamic acid (TXA); the MOJO group also received topical TXA.
Further patient care optimization included providing patients with a team-based approach, which consisted of nurse coordinators, physician assistants and nurse practitioners, residents, and the attending surgeon. Our team reviews the planned pain management protocol, perioperative expectations, criteria for discharge, and anticipated surgical outcomes with the patient during their preoperative visits. On postoperative day 1, these members round as a team to encourage patients in their immediate postoperative recovery and rehabilitation. During rounds, the team assesses whether the patient meets the criteria for discharge, adjusting the pain management protocol if necessary.
Changes in surgical technique included arthrotomy with electrocautery, minimizing traumatic dissection or resection of the synovial tissue, and intra-articular injection of a cocktail of ropivacaine 5 mg/mL 40 mL, epinephrine 1:1,000 0.5 mL, and methylprednisolone sodium 40 mg diluted with normal saline to a total volume of 120 mL.
The new routine was gradually implemented beginning January 2017 and fully implemented by July 2018. This study compared the first 20 consecutive patients undergoing primary TKA after July 2018 to the last 20 consecutive patients undergoing primary TKA prior to January 2017. Exclusion criteria included bilateral TKA, death before 90 days, and revision as the indication for surgery. The senior attending surgeon performed all surgeries using a standard midline approach. The majority of surgeries were performed using a cemented Vanguard total knee system (Zimmer Biomet); 4 patients in the historical group had a NexGen knee system, cementless monoblock tibial components (Zimmer Biomet); and 1 patient had a Logic knee system (Exactech). Surgical selection criteria for patients did not differ between groups.
Electronic health records were reviewed and data were abstracted. The data included demographic information (age, gender, body mass index [BMI], diagnosis, and procedure), surgical factors (American Society of Anesthesiologists score, Risk Assessment and Predictive Tool score, operative time, tourniquet time, estimated blood loss), hospital factors (length of stay [LOS], discharge location), postoperative pain scores (measured on postoperative day 1 and on day of discharge), and postdischarge events (90-day complications, telephone calls reporting pain, reoperations, returns to the ED, 90-day readmissions).
The primary outcome was the mean postoperative daily MED during the inpatient stay. Secondary outcomes included pain on postoperative day 1, pain at the time of discharge, LOS, hospital readmissions, and ED visits within 90 days of surgery. Because different opioid pain medications were used by patients postoperatively, all opioids were converted to MED prior to the final analysis. Collected patient data were de-identified prior to analysis.
Power analysis was conducted to determine whether the study had sufficient population size to reject the null hypothesis for the primary outcome measure. Because practitioners controlled postoperative opioid use, a Cohen’s d of 1.0 was used so that a very large effect size was needed to reach clinical significance. Statistical significance was set to 0.05, and patient groups were set at 20 patients each. This yielded an appropriate power of 0.87. Population characteristics were compared between groups using t tests and χ2 tests as appropriate. To analyze the primary outcome, comparisons were made between the 2 cohorts using 2-tailed t tests. Secondary outcomes were compared between groups using t tests or χ2 tests. All statistics were performed using R version 3.5.2. Power analysis was conducted using the package pwr.11 Statistical significance was set at
Results
Forty patients met the inclusion criteria, evenly divided between those undergoing TKA before and after instituting the MOJO protocol (Table 2). A single patient in the MOJO group died and was excluded. A patient who underwent bilateral TKA also was excluded. Both groups reflected the male predominance of the VA patient population. MOJO patients tended to have lower BMIs (34 vs 30, P < .01). All patients indicated for surgery with preoperative opioid use were able to titrate down to their preoperative goal as verified by prescriptions filled at VA pharmacies. Twelve of the patients in the MOJO group received adductor canal blocks.
Results of t tests and χ2 tests comparing primary and secondary endpoints are listed in Table 3. Differences between the daily MEDs given in the historical and MOJO groups are shown. There were significant differences between the pre-MOJO and MOJO groups with regard to daily inpatient MEDs (82 mg vs 29 mg, P < .01) and total inpatient MEDs (306 mg vs 32 mg, P < .01). There was less self-reported pain on postoperative day 1 in the MOJO group (5.5 vs 3.9, P < .01), decreased LOS (4.4 days vs 1.2 days, P < .01), a trend toward fewer total ED visits (6 vs 2, P = .24), and fewer discharges to skilled nursing facilities (12 vs 0, P < .01). There were no blood transfusions in either group.
There were no readmissions due to uncontrolled pain. There was 1 readmission for shortness of breath in the MOJO group. The patient was discharged home the following day after ruling out thromboembolic and cardiovascular events. One patient from the control group was readmitted after missing a step on a staircase and falling. The patient sustained a quadriceps tendon rupture and underwent primary suture repair.
Discussion
Our results demonstrate that a multimodal approach to significantly reduce postoperative opioid use in patients with TKA is possible without increasing readmissions or ED visits for pain control. The patients in the MOJO group had a faster recovery, earlier discharge, and less use of postoperative opioid medication. Our approach to postoperative pain management was divided into 2 main categories: patient optimization and surgical optimization.
Patient Selection
Besides the standard evaluation and optimization of patients’ medical conditions, identifying and optimizing at-risk patients before surgery was a critical component of our protocol. Managing postoperative pain in patients with prior opioid use is an intractable challenge in orthopedic surgery. Patients with a history of chronic pain and preoperative use of opioid medications remain at higher risk of postoperative chronic pain and persistent use of opioid medication despite no obvious surgical complications.8 In a sample of > 6,000 veterans who underwent TKA at VA hospitals in 2014, 57% of the patients with daily use of opioids in the 90 days before surgery remained on opioids 1 year after surgery (vs 2 % in patients not on long-term opioids).8 This relationship between pre- and postoperative opioid use also was dose dependent.12
Furthermore, those with high preoperative use may experience worse outcomes relative to the opioid naive population as measured by arthritis-specific pain indices.13 In a well-powered retrospective study of patients who underwent elective orthopedic procedures, preoperative opioid abuse or dependence (determined by the International Classification of Diseases, Ninth Revision diagnosis) increased inpatient mortality, aggregate morbidity, surgical site infection, myocardial infarction, and LOS.14 Preoperative opioid use also has been associated with increased risk of ED visits, readmission, infection, stiffness, and aseptic revision.15 In patients with TKA in the VA specifically, preoperative opioid use (> 3 months in the prior year) was associated with increased revision rates that were even higher than those for patients with diabetes mellitus.16
Patient Education
Based on this evidence, we instruct patients to reduce their preoperative opioid dosing to zero (for patients with joint pain) or < 30 MED (for patients using opioids for other reasons). Although preoperative reduction of opioid use has been shown to improve outcomes after TKA, pain subspecialty recommendations for patients with chronic opioid use recommend considering adjunctive therapies, including transcutaneous electrical nerve stimulation, cognitive behavioral therapy, gabapentin, or ketamine.17,18 Through patient education our team has been successful in decreasing preoperative opioid use without adding other drugs or modalities.
Patient Optimization
Preoperative patient optimization included 4 to 8 weeks of daily sets of physical activity instructions (prehab) to improve the musculoskeletal function. These instructions are given to patients 4 to 8 weeks before surgery and aim to improve the patient’s balance, mobility, and functional ability (Appendix). Meta-analysis has shown that patients who undergo preoperative PT have a small but statistically significant decrease in postoperative pain at 4 weeks, though this does not persist beyond that period.19
We did note a lower BMI in patients in the MOJO group. Though this has the potential to be a confounder, a study of BMI in > 4,000 patients who underwent joint replacement surgery has shown that BMI is not associated with differences in postoperative pain.20
Surgeon and Surgical-Related Variables
Patients in the MOJO group had increased use of adductor canal blocks. A 2017 meta-analysis of 12,530 patients comparing analgesic modalities found that peripheral nerve blocks targeting multiple nerves (eg, femoral/sciatic) decreased pain at rest, decreased opioid consumption, and improved range of motion postoperatively.21 Also, these were found to be superior to single nerve blocks, periarticular infiltration, and epidural blocks.21 However, major nerve and epidural blocks affecting the lower extremity may increase the risk of falls and prolong LOS.22,23 The preferred peripheral block at VAPHCS is a single shot ultrasound-guided adductor canal block before the induction of general or spinal anesthesia. A randomized controlled trial has demonstrated superiority of this block to the femoral nerve block with regard to postoperative quadriceps strength, conferring the theoretical advantage of decreased fall risk and ability to participate in immediate PT.24 Although we are unable to confirm an association between anesthetic modalities and opioid burden, our clinical impression is that blocks were effective at reducing immediate postoperative pain. However, among MOJO patients there were no differences in patients with and without blocks for either pain (4.2 vs 3.8, P = .69) or opioid consumption (28.8 vs 33.0, P = .72) after surgery, though our study was not powered to detect a difference in this restricted subgroup.
Patients who frequently had reported postoperative thigh pain prompted us to make changes in our surgical technique, performing TKA without use of a tourniquet. Tourniquet use has been associated with an increased risk of thigh pain after TKA by multiple authors.25,26 Postoperative thigh pain also is pressure dependent.27 In addition, its use may be associated with a slightly increased risk of thromboembolic events and delayed functional recovery.28,29
Because postoperative hemarthrosis is associated with more pain and reduced joint recovery function, we used topical TXA to reduce postoperative surgical site and joint hematoma. TXA (either oral, IV, or topical) during TKA is used to control postoperative bleeding primarily and decrease the need for transfusion without concomitant increase in thromboembolic events.30,31 Topical TXA may be more effective than IV, particularly in the immediate postoperative period.32 Although pain typically is not an endpoint in studies of TXA, a prospective study of 48 patients showed evidence that its use may be associated with decreased postoperative pain in the first 24 hours after surgery (though not after).33 Finally, the use of intra-articular injection has evolved in our clinical practice, but literature is lacking with regard to its efficacy; more studies are needed to determine its effect relative to no injection. We have not seen any benefits to using
Limitations
This is a nonrandomized retrospective single-institution study. Our study population is composed of mostly males with military experience and is not necessarily a representative sample of the general population eligible for joint arthroplasty. Our primary endpoint (reduction of opioid use postoperatively) also was a cornerstone of our intervention. To account for this, we set a very large effect size in our power analysis and evaluated multiple secondary endpoints to determine whether postoperative pain remained well controlled and complications/readmission minimized with our interventions. Because our intervention was multimodal, our study cannot make conclusions about the effect of a particular component of our treatment strategy. We did not measure or compare functional outcomes between both groups, which offers an opportunity for further research.
These limitations are balanced by several strengths. Our cohort was well controlled with respect to the dose and type of drug used. There is staff dedicated to postoperative telephone follow-up after discharge, and veterans are apt to seek care within the VA health care system, which improves case finding for complications and ED visits. No patients were lost to follow-up. Moreover, our drastic reduction in opioid use is promising enough to warrant reporting, while the broader orthopedic literature explores the relative impact of each variable.
Conclusions
The MOJO protocol has been effective for reducing postoperative opioid use after TKA without compromising effective pain management. The drastic reduction in the postoperative use of opioid pain medications and LOS have contributed to a cultural shift within our department, comprehensive team approach, multimodal pain management, and preoperative patient optimization. Further investigations are required to assess the impact of each intervention on observed outcomes. However, the framework and routines are applicable to other institutions and surgical specialties.
Acknowledgments
The authors recognize Derek Bond, MD, for his help in creating the MOJO acronym.
For decades, opioids have been a mainstay in the management of pain after total joint arthroplasty. In the past 10 years, however, opioid prescribing has come under increased scrutiny due to a rise in rates of opioid abuse, pill diversion, and opioid-related deaths.1,2 Opioids are associated with adverse effects, including nausea, vomiting, constipation, apathy, and respiratory depression, all of which influence arthroplasty outcomes and affect the patient experience. Although primary care groups account for nearly half of prescriptions written, orthopedic surgeons have the third highest per capita rate of opioid prescribing of all medical specialties.3,4 This puts orthopedic surgeons, particularly those who perform routine procedures, in an opportune but challenging position to confront this problem through novel pain management strategies.
Approximately 1 million total knee arthroplasties (TKAs) are performed in the US every year, and the US Department of Veterans Affairs (VA) health system performs about 10,000 hip and knee joint replacements.5,6 There is no standardization of opioid prescribing in the postoperative period following these procedures, and studies have reported a wide variation in prescribing habits even within a single institution for a specific surgery.7 Patients who undergo TKA are at particularly high risk of long-term opioid use if they are on continuous opioids at the time of surgery; this is problematic in a VA patient population in which at least 16% of patients are prescribed opioids in a given year.8 Furthermore, veterans are twice as likely as nonveterans to die of an accidental overdose.9 Despite these risks, opioids remain a cornerstone of postoperative pain management both within and outside of the VA.10
In 2018, to limit unnecessary prescribing of opioid pain medication, the total joint service at the VA Portland Health Care System (VAPHCS) in Oregon implemented the Minimizing Opioids after Joint Operation (MOJO) postoperative pain protocol. The goal of the protocol was to reduce opioid use following TKA. The objectives were to provide safe, appropriate analgesia while allowing early mobilization and discharge without a concomitant increase in readmissions or emergency department (ED) visits. The purpose of this retrospective chart review was to compare the efficacy of the MOJO protocol with our historical experience and report our preliminary results.
Methods
Institutional review board approval was obtained to retrospectively review the medical records of patients who had undergone TKA surgery during 2018 at VAPHCS. The MOJO protocol was composed of several simultaneous changes. The centerpiece of the new protocol was a drastic decrease in routine prescription of postoperative opioids (Table 1). Other changes included instructing patients to reduce the use of preoperative opioid pain medication 6 weeks before surgery with a goal of no opioid consumption, perform daily sets of preoperative exercises, and attend a preoperative consultation/education session with a nurse coordinator to emphasize early recovery and discharge. In patients with chronic use of opioid pain medication (particularly those for whom the medication had been prescribed for other sources of pain, such as lumbar back pain), the goal was daily opioid use of ≤ 30 morphine equivalent doses (MEDs). During the inpatient stay, we stopped prescribing prophylactic pain medication prior to physical therapy (PT).
We encouraged preoperative optimization of muscle strength by giving instructions for 4 to 8 weeks of daily exercises (Appendix). We introduced perioperative adductor canal blocks (at the discretion of the anesthesia team) and transitioned to surgery without a tourniquet. Patients in both groups received intraoperative antibiotics and IV tranexamic acid (TXA); the MOJO group also received topical TXA.
Further patient care optimization included providing patients with a team-based approach, which consisted of nurse coordinators, physician assistants and nurse practitioners, residents, and the attending surgeon. Our team reviews the planned pain management protocol, perioperative expectations, criteria for discharge, and anticipated surgical outcomes with the patient during their preoperative visits. On postoperative day 1, these members round as a team to encourage patients in their immediate postoperative recovery and rehabilitation. During rounds, the team assesses whether the patient meets the criteria for discharge, adjusting the pain management protocol if necessary.
Changes in surgical technique included arthrotomy with electrocautery, minimizing traumatic dissection or resection of the synovial tissue, and intra-articular injection of a cocktail of ropivacaine 5 mg/mL 40 mL, epinephrine 1:1,000 0.5 mL, and methylprednisolone sodium 40 mg diluted with normal saline to a total volume of 120 mL.
The new routine was gradually implemented beginning January 2017 and fully implemented by July 2018. This study compared the first 20 consecutive patients undergoing primary TKA after July 2018 to the last 20 consecutive patients undergoing primary TKA prior to January 2017. Exclusion criteria included bilateral TKA, death before 90 days, and revision as the indication for surgery. The senior attending surgeon performed all surgeries using a standard midline approach. The majority of surgeries were performed using a cemented Vanguard total knee system (Zimmer Biomet); 4 patients in the historical group had a NexGen knee system, cementless monoblock tibial components (Zimmer Biomet); and 1 patient had a Logic knee system (Exactech). Surgical selection criteria for patients did not differ between groups.
Electronic health records were reviewed and data were abstracted. The data included demographic information (age, gender, body mass index [BMI], diagnosis, and procedure), surgical factors (American Society of Anesthesiologists score, Risk Assessment and Predictive Tool score, operative time, tourniquet time, estimated blood loss), hospital factors (length of stay [LOS], discharge location), postoperative pain scores (measured on postoperative day 1 and on day of discharge), and postdischarge events (90-day complications, telephone calls reporting pain, reoperations, returns to the ED, 90-day readmissions).
The primary outcome was the mean postoperative daily MED during the inpatient stay. Secondary outcomes included pain on postoperative day 1, pain at the time of discharge, LOS, hospital readmissions, and ED visits within 90 days of surgery. Because different opioid pain medications were used by patients postoperatively, all opioids were converted to MED prior to the final analysis. Collected patient data were de-identified prior to analysis.
Power analysis was conducted to determine whether the study had sufficient population size to reject the null hypothesis for the primary outcome measure. Because practitioners controlled postoperative opioid use, a Cohen’s d of 1.0 was used so that a very large effect size was needed to reach clinical significance. Statistical significance was set to 0.05, and patient groups were set at 20 patients each. This yielded an appropriate power of 0.87. Population characteristics were compared between groups using t tests and χ2 tests as appropriate. To analyze the primary outcome, comparisons were made between the 2 cohorts using 2-tailed t tests. Secondary outcomes were compared between groups using t tests or χ2 tests. All statistics were performed using R version 3.5.2. Power analysis was conducted using the package pwr.11 Statistical significance was set at
Results
Forty patients met the inclusion criteria, evenly divided between those undergoing TKA before and after instituting the MOJO protocol (Table 2). A single patient in the MOJO group died and was excluded. A patient who underwent bilateral TKA also was excluded. Both groups reflected the male predominance of the VA patient population. MOJO patients tended to have lower BMIs (34 vs 30, P < .01). All patients indicated for surgery with preoperative opioid use were able to titrate down to their preoperative goal as verified by prescriptions filled at VA pharmacies. Twelve of the patients in the MOJO group received adductor canal blocks.
Results of t tests and χ2 tests comparing primary and secondary endpoints are listed in Table 3. Differences between the daily MEDs given in the historical and MOJO groups are shown. There were significant differences between the pre-MOJO and MOJO groups with regard to daily inpatient MEDs (82 mg vs 29 mg, P < .01) and total inpatient MEDs (306 mg vs 32 mg, P < .01). There was less self-reported pain on postoperative day 1 in the MOJO group (5.5 vs 3.9, P < .01), decreased LOS (4.4 days vs 1.2 days, P < .01), a trend toward fewer total ED visits (6 vs 2, P = .24), and fewer discharges to skilled nursing facilities (12 vs 0, P < .01). There were no blood transfusions in either group.
There were no readmissions due to uncontrolled pain. There was 1 readmission for shortness of breath in the MOJO group. The patient was discharged home the following day after ruling out thromboembolic and cardiovascular events. One patient from the control group was readmitted after missing a step on a staircase and falling. The patient sustained a quadriceps tendon rupture and underwent primary suture repair.
Discussion
Our results demonstrate that a multimodal approach to significantly reduce postoperative opioid use in patients with TKA is possible without increasing readmissions or ED visits for pain control. The patients in the MOJO group had a faster recovery, earlier discharge, and less use of postoperative opioid medication. Our approach to postoperative pain management was divided into 2 main categories: patient optimization and surgical optimization.
Patient Selection
Besides the standard evaluation and optimization of patients’ medical conditions, identifying and optimizing at-risk patients before surgery was a critical component of our protocol. Managing postoperative pain in patients with prior opioid use is an intractable challenge in orthopedic surgery. Patients with a history of chronic pain and preoperative use of opioid medications remain at higher risk of postoperative chronic pain and persistent use of opioid medication despite no obvious surgical complications.8 In a sample of > 6,000 veterans who underwent TKA at VA hospitals in 2014, 57% of the patients with daily use of opioids in the 90 days before surgery remained on opioids 1 year after surgery (vs 2 % in patients not on long-term opioids).8 This relationship between pre- and postoperative opioid use also was dose dependent.12
Furthermore, those with high preoperative use may experience worse outcomes relative to the opioid naive population as measured by arthritis-specific pain indices.13 In a well-powered retrospective study of patients who underwent elective orthopedic procedures, preoperative opioid abuse or dependence (determined by the International Classification of Diseases, Ninth Revision diagnosis) increased inpatient mortality, aggregate morbidity, surgical site infection, myocardial infarction, and LOS.14 Preoperative opioid use also has been associated with increased risk of ED visits, readmission, infection, stiffness, and aseptic revision.15 In patients with TKA in the VA specifically, preoperative opioid use (> 3 months in the prior year) was associated with increased revision rates that were even higher than those for patients with diabetes mellitus.16
Patient Education
Based on this evidence, we instruct patients to reduce their preoperative opioid dosing to zero (for patients with joint pain) or < 30 MED (for patients using opioids for other reasons). Although preoperative reduction of opioid use has been shown to improve outcomes after TKA, pain subspecialty recommendations for patients with chronic opioid use recommend considering adjunctive therapies, including transcutaneous electrical nerve stimulation, cognitive behavioral therapy, gabapentin, or ketamine.17,18 Through patient education our team has been successful in decreasing preoperative opioid use without adding other drugs or modalities.
Patient Optimization
Preoperative patient optimization included 4 to 8 weeks of daily sets of physical activity instructions (prehab) to improve the musculoskeletal function. These instructions are given to patients 4 to 8 weeks before surgery and aim to improve the patient’s balance, mobility, and functional ability (Appendix). Meta-analysis has shown that patients who undergo preoperative PT have a small but statistically significant decrease in postoperative pain at 4 weeks, though this does not persist beyond that period.19
We did note a lower BMI in patients in the MOJO group. Though this has the potential to be a confounder, a study of BMI in > 4,000 patients who underwent joint replacement surgery has shown that BMI is not associated with differences in postoperative pain.20
Surgeon and Surgical-Related Variables
Patients in the MOJO group had increased use of adductor canal blocks. A 2017 meta-analysis of 12,530 patients comparing analgesic modalities found that peripheral nerve blocks targeting multiple nerves (eg, femoral/sciatic) decreased pain at rest, decreased opioid consumption, and improved range of motion postoperatively.21 Also, these were found to be superior to single nerve blocks, periarticular infiltration, and epidural blocks.21 However, major nerve and epidural blocks affecting the lower extremity may increase the risk of falls and prolong LOS.22,23 The preferred peripheral block at VAPHCS is a single shot ultrasound-guided adductor canal block before the induction of general or spinal anesthesia. A randomized controlled trial has demonstrated superiority of this block to the femoral nerve block with regard to postoperative quadriceps strength, conferring the theoretical advantage of decreased fall risk and ability to participate in immediate PT.24 Although we are unable to confirm an association between anesthetic modalities and opioid burden, our clinical impression is that blocks were effective at reducing immediate postoperative pain. However, among MOJO patients there were no differences in patients with and without blocks for either pain (4.2 vs 3.8, P = .69) or opioid consumption (28.8 vs 33.0, P = .72) after surgery, though our study was not powered to detect a difference in this restricted subgroup.
Patients who frequently had reported postoperative thigh pain prompted us to make changes in our surgical technique, performing TKA without use of a tourniquet. Tourniquet use has been associated with an increased risk of thigh pain after TKA by multiple authors.25,26 Postoperative thigh pain also is pressure dependent.27 In addition, its use may be associated with a slightly increased risk of thromboembolic events and delayed functional recovery.28,29
Because postoperative hemarthrosis is associated with more pain and reduced joint recovery function, we used topical TXA to reduce postoperative surgical site and joint hematoma. TXA (either oral, IV, or topical) during TKA is used to control postoperative bleeding primarily and decrease the need for transfusion without concomitant increase in thromboembolic events.30,31 Topical TXA may be more effective than IV, particularly in the immediate postoperative period.32 Although pain typically is not an endpoint in studies of TXA, a prospective study of 48 patients showed evidence that its use may be associated with decreased postoperative pain in the first 24 hours after surgery (though not after).33 Finally, the use of intra-articular injection has evolved in our clinical practice, but literature is lacking with regard to its efficacy; more studies are needed to determine its effect relative to no injection. We have not seen any benefits to using
Limitations
This is a nonrandomized retrospective single-institution study. Our study population is composed of mostly males with military experience and is not necessarily a representative sample of the general population eligible for joint arthroplasty. Our primary endpoint (reduction of opioid use postoperatively) also was a cornerstone of our intervention. To account for this, we set a very large effect size in our power analysis and evaluated multiple secondary endpoints to determine whether postoperative pain remained well controlled and complications/readmission minimized with our interventions. Because our intervention was multimodal, our study cannot make conclusions about the effect of a particular component of our treatment strategy. We did not measure or compare functional outcomes between both groups, which offers an opportunity for further research.
These limitations are balanced by several strengths. Our cohort was well controlled with respect to the dose and type of drug used. There is staff dedicated to postoperative telephone follow-up after discharge, and veterans are apt to seek care within the VA health care system, which improves case finding for complications and ED visits. No patients were lost to follow-up. Moreover, our drastic reduction in opioid use is promising enough to warrant reporting, while the broader orthopedic literature explores the relative impact of each variable.
Conclusions
The MOJO protocol has been effective for reducing postoperative opioid use after TKA without compromising effective pain management. The drastic reduction in the postoperative use of opioid pain medications and LOS have contributed to a cultural shift within our department, comprehensive team approach, multimodal pain management, and preoperative patient optimization. Further investigations are required to assess the impact of each intervention on observed outcomes. However, the framework and routines are applicable to other institutions and surgical specialties.
Acknowledgments
The authors recognize Derek Bond, MD, for his help in creating the MOJO acronym.
1. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics Data Brief No. 329. Published November 2018. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db329-h.pdf
2. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics NCHS data brief No. 294. Published December 2017. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db294.pdf
3. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic–prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015;49(3):409-413. doi:10.1016/j.amepre.2015.02.020
4. Guy GP, Zhang K. Opioid prescribing by specialty and volume in the U.S. Am J Prev Med. 2018;55(5):e153-155. doi:10.1016/j.amepre.2018.06.008
5. Kremers HM, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surgery Am. 2015;17:1386-1397. doi:10.2106/JBJS.N.01141
6. Giori NJ, Amanatullah DF, Gupta S, Bowe T, Harris AHS. Risk reduction compared with access to care: quantifying the trade-off of enforcing a body mass index eligibility criterion for joint replacement. J Bone Joint Surg Am. 2018; 4(100):539-545. doi:10.2106/JBJS.17.00120
7. Sabatino MJ, Kunkel ST, Ramkumar DB, Keeney BJ, Jevsevar DS. Excess opioid medication and variation in prescribing patterns following common orthopaedic procedures. J Bone Joint Surg Am. 2018;100(3):180-188. doi:10.2106/JBJS.17.00672
8. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
9. Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321. doi:10.1001/jama.2011.370
10. Hall MJ, Schwartzman A, Zhang J, Liu X. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl Health Stat Report. 2017(102):1-15.
11. Champely S. pwr: basic functions for power analysis. R package version 1.2-2; 2018. Accessed January 13, 2021. https://rdrr.io/cran/pwr/
12. Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265. doi:10.1097/j.pain.0000000000000516
13. Smith SR, Bido J, Collins JE, Yang H, Katz JN, Losina E. Impact of preoperative opioid use on total knee arthroplasty outcomes. J Bone Joint Surg Am. 2017;99(10):803-808. doi:10.2106/JBJS.16.01200
14. Menendez ME, Ring D, Bateman BT. Preoperative opioid misuse is associated with increased morbidity and mortality after elective orthopaedic surgery. Clin Orthop Relat Res. 2015;473(7):2402-412. doi:10.1007/s11999-015-4173-5
15. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
16. Ben-Ari A, Chansky H, Rozet I. Preoperative opioid use is associated with early revision after total knee arthroplasty: a study of male patients treated in the Veterans Affairs System. J Bone Joint Surg Am. 2017;99(1):1-9. doi:10.2106/JBJS.16.00167
17. Nguyen L-CL, Sing DC, Bozic KJ. Preoperative reduction of opioid use before total joint arthroplasty. J Arthroplasty. 2016;31(suppl 9):282-287. doi:10.1016/j.arth.2016.01.068
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
19. Wang L, Lee M, Zhang Z, Moodie J, Cheng D, Martin J. Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes? A systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2016;6(2):e009857. doi:10.1136/bmjopen-2015-009857
20. Li W, Ayers DC, Lewis CG, Bowen TR, Allison JJ, Franklin PD. Functional gain and pain relief after total joint replacement according to obesity status. J Bone Joint Surg. 2017;99(14):1183-1189. doi:10.2106/JBJS.16.00960
21. Terkawi AS, Mavridis D, Sessler DI, et al. Pain management modalities after total knee arthroplasty: a network meta-analysis of 170 randomized controlled trials. Anesthesiology. 2017;126(5):923-937. doi:10.1097/ALN.0000000000001607
22. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111(6):1552-1554. doi:10.1213/ANE.0b013e3181fb9507
23. Elkassabany NM, Antosh S, Ahmed M, et al. The risk of falls after total knee arthroplasty with the use of a femoral nerve block versus an adductor canal block. Anest Analg. 2016;122(5):1696-1703. doi:10.1213/ane.0000000000001237
24. Wang D, Yang Y, Li Q, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a meta-analysis of randomized controlled trials. Sci Rep. 2017;7:40721. doi:10.1038/srep40721
25. Liu D, Graham D, Gillies K, Gillies RM. Effects of tourniquet use on quadriceps function and pain in total knee arthroplasty. Knee Surg Relat Res. 2014;26(4):207-213. doi:10.5792/ksrr.2014.26.4.207
26. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
27. Worland RL, Arredondo J, Angles F, Lopez-Jimenez F, Jessup DE. Thigh pain following tourniquet application in simultaneous bilateral total knee replacement arthroplasty. J Arthroplasty. 1997;12(8):848-852. doi:10.1016/s0883-5403(97)90153-4
28. Tai T-W, Lin C-J, Jou I-M, Chang C-W, Lai K-A, Yang C-Y. Tourniquet use in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol, Arthrosc. 2011;19(7):1121-1130. doi:10.1007/s00167-010-1342-7
29. Jiang F-Z, Zhong H-M, Hong Y-C, Zhao G-F. Use of a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Orthop Sci. 2015;20(21):110-123. doi:10.1007/s00776-014-0664-6
30. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585. doi:10.1302/0301-620X.93B12.26989
31. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee. 2013;20(5):300-309. doi:10.1016/j.knee.2013.05.014
32. Wang J, Wang Q, Zhang X, Wang Q. Intra-articular application is more effective than intravenous application of tranexamic acid in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2017;32(11):3385-3389. doi:10.1016/j.arth.2017.06.024
33. Guerreiro JPF, Badaro BS, Balbino JRM, Danieli MV, Queiroz AO, Cataneo DC. Application of tranexamic acid in total knee arthroplasty – prospective randomized trial. J Open Orthop J. 2017;11:1049-1057. doi:10.2174/1874325001711011049
1. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics Data Brief No. 329. Published November 2018. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db329-h.pdf
2. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics NCHS data brief No. 294. Published December 2017. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db294.pdf
3. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic–prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015;49(3):409-413. doi:10.1016/j.amepre.2015.02.020
4. Guy GP, Zhang K. Opioid prescribing by specialty and volume in the U.S. Am J Prev Med. 2018;55(5):e153-155. doi:10.1016/j.amepre.2018.06.008
5. Kremers HM, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surgery Am. 2015;17:1386-1397. doi:10.2106/JBJS.N.01141
6. Giori NJ, Amanatullah DF, Gupta S, Bowe T, Harris AHS. Risk reduction compared with access to care: quantifying the trade-off of enforcing a body mass index eligibility criterion for joint replacement. J Bone Joint Surg Am. 2018; 4(100):539-545. doi:10.2106/JBJS.17.00120
7. Sabatino MJ, Kunkel ST, Ramkumar DB, Keeney BJ, Jevsevar DS. Excess opioid medication and variation in prescribing patterns following common orthopaedic procedures. J Bone Joint Surg Am. 2018;100(3):180-188. doi:10.2106/JBJS.17.00672
8. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
9. Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321. doi:10.1001/jama.2011.370
10. Hall MJ, Schwartzman A, Zhang J, Liu X. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl Health Stat Report. 2017(102):1-15.
11. Champely S. pwr: basic functions for power analysis. R package version 1.2-2; 2018. Accessed January 13, 2021. https://rdrr.io/cran/pwr/
12. Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265. doi:10.1097/j.pain.0000000000000516
13. Smith SR, Bido J, Collins JE, Yang H, Katz JN, Losina E. Impact of preoperative opioid use on total knee arthroplasty outcomes. J Bone Joint Surg Am. 2017;99(10):803-808. doi:10.2106/JBJS.16.01200
14. Menendez ME, Ring D, Bateman BT. Preoperative opioid misuse is associated with increased morbidity and mortality after elective orthopaedic surgery. Clin Orthop Relat Res. 2015;473(7):2402-412. doi:10.1007/s11999-015-4173-5
15. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
16. Ben-Ari A, Chansky H, Rozet I. Preoperative opioid use is associated with early revision after total knee arthroplasty: a study of male patients treated in the Veterans Affairs System. J Bone Joint Surg Am. 2017;99(1):1-9. doi:10.2106/JBJS.16.00167
17. Nguyen L-CL, Sing DC, Bozic KJ. Preoperative reduction of opioid use before total joint arthroplasty. J Arthroplasty. 2016;31(suppl 9):282-287. doi:10.1016/j.arth.2016.01.068
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
19. Wang L, Lee M, Zhang Z, Moodie J, Cheng D, Martin J. Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes? A systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2016;6(2):e009857. doi:10.1136/bmjopen-2015-009857
20. Li W, Ayers DC, Lewis CG, Bowen TR, Allison JJ, Franklin PD. Functional gain and pain relief after total joint replacement according to obesity status. J Bone Joint Surg. 2017;99(14):1183-1189. doi:10.2106/JBJS.16.00960
21. Terkawi AS, Mavridis D, Sessler DI, et al. Pain management modalities after total knee arthroplasty: a network meta-analysis of 170 randomized controlled trials. Anesthesiology. 2017;126(5):923-937. doi:10.1097/ALN.0000000000001607
22. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111(6):1552-1554. doi:10.1213/ANE.0b013e3181fb9507
23. Elkassabany NM, Antosh S, Ahmed M, et al. The risk of falls after total knee arthroplasty with the use of a femoral nerve block versus an adductor canal block. Anest Analg. 2016;122(5):1696-1703. doi:10.1213/ane.0000000000001237
24. Wang D, Yang Y, Li Q, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a meta-analysis of randomized controlled trials. Sci Rep. 2017;7:40721. doi:10.1038/srep40721
25. Liu D, Graham D, Gillies K, Gillies RM. Effects of tourniquet use on quadriceps function and pain in total knee arthroplasty. Knee Surg Relat Res. 2014;26(4):207-213. doi:10.5792/ksrr.2014.26.4.207
26. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
27. Worland RL, Arredondo J, Angles F, Lopez-Jimenez F, Jessup DE. Thigh pain following tourniquet application in simultaneous bilateral total knee replacement arthroplasty. J Arthroplasty. 1997;12(8):848-852. doi:10.1016/s0883-5403(97)90153-4
28. Tai T-W, Lin C-J, Jou I-M, Chang C-W, Lai K-A, Yang C-Y. Tourniquet use in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol, Arthrosc. 2011;19(7):1121-1130. doi:10.1007/s00167-010-1342-7
29. Jiang F-Z, Zhong H-M, Hong Y-C, Zhao G-F. Use of a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Orthop Sci. 2015;20(21):110-123. doi:10.1007/s00776-014-0664-6
30. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585. doi:10.1302/0301-620X.93B12.26989
31. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee. 2013;20(5):300-309. doi:10.1016/j.knee.2013.05.014
32. Wang J, Wang Q, Zhang X, Wang Q. Intra-articular application is more effective than intravenous application of tranexamic acid in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2017;32(11):3385-3389. doi:10.1016/j.arth.2017.06.024
33. Guerreiro JPF, Badaro BS, Balbino JRM, Danieli MV, Queiroz AO, Cataneo DC. Application of tranexamic acid in total knee arthroplasty – prospective randomized trial. J Open Orthop J. 2017;11:1049-1057. doi:10.2174/1874325001711011049
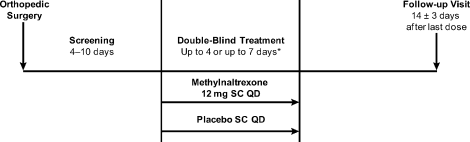
Methylnaltrexone for Acute OIC
The management of postoperative pain is essential to perioperative care, and adequate postoperative analgesia has been associated with several key clinical benefits, including fewer postoperative complications, earlier patient ambulation, reduced costs due to shorter hospital stays, and improved rehabilitation.1, 2 While opioids have long been central to postoperative analgesia, they have been associated with various adverse effects, including sedation, dizziness, nausea, vomiting, constipation, dependence, tolerance, and respiratory depression.2, 3 Constipation, one of the most common adverse effects resulting from opioid therapy, can be debilitating. Indeed, opioid effects on gut motility can occur even after a single dose.3 The consequences of opioid‐induced constipation (OIC) may be severe enough to warrant a dosage reduction of the opioid; however, this may lead to compromised analgesia, which can hinder recovery.4, 5 Thus, effective treatment of OIC is an important clinical consideration in patients undergoing pain management with opioids. Unfortunately, laxatives and other treatment strategies can have unpredictable or suboptimal results for many patients with OIC; therefore, other options are needed for the treatment of OIC.6, 7
Opioid receptor agonists cause constipation by adversely altering many aspects of intestinal function, including fluid dynamics, gastric emptying, propulsive motor activity, and transit time.3 Opioid receptors are widely distributed in the central nervous system and throughout the intestinal system. The mechanism of OIC may have both peripherally and centrally mediated components.8 Nonselective opioid receptor antagonists block the undesired effects on the gut, but because they cross the blood‐brain barrier, they also interfere with analgesia and may lead to symptoms of withdrawal. Methylnaltrexone is a selective, peripherally acting mu‐opioid receptor antagonist,9 formed by the addition of a methyl group to the amine ring of the mu‐opioid receptor antagonist naltrexone. The resulting quarternary amine has greater polarity, lower lipid solubility, and restricted ability to cross the blood‐brain barrier.10 Thus, methylnaltrexone was designed to decrease the peripheral adverse effects of opioids without interfering with centrally mediated analgesia.
Investigations of methylnaltrexone effects in healthy volunteers showed that methylnaltrexone attenuated morphine‐induced delays in gastric emptying and oral‐cecal transit without affecting analgesia.1113 Further studies of methylnaltrexone for the treatment of constipation due to methadone use demonstrated rapid laxation response.1416 Two randomized, double‐blind, placebo‐controlled studies of methylnaltrexone in 288 patients with advanced illness and OIC showed that methylnaltrexone rapidly induced laxation without compromising analgesia.17, 18 Methylnaltrexone is currently approved for the treatment of OIC in patients with advanced illness who are receiving palliative care, when response to laxative therapy has not been sufficient.19
Recently, the use of methylnaltrexone for the treatment of OIC in patients with chronic, nonmalignant pain was assessed in a randomized, double‐blind, placebo‐controlled trial of more than 400 patients. Investigators found that methylnaltrexone induced laxation and was generally well tolerated (Blonsky et al., 28th Annual Scientific Meeting of the American Pain Society, May 7‐9, 2009, San Diego, CA; Duerden et al., 29th Annual Scientific Meeting of the American Pain Society, May 6‐10, 2010, Baltimore, MD), supporting the safety and efficacy of methylnaltrexone in the setting of OIC resulting from chronic opioid treatment. The present study aimed to assess the activity of methylnaltrexone in patients receiving mu‐agonist opioid analgesics during rehabilitation, following an orthopedic surgical procedure, who were experiencing acute OIC.
METHODS
Patients
Patients who had undergone orthopedic procedures within 4 to 10 days were screened for eligibility. Adults aged 18 years or older were considered eligible if they were acutely constipated, were receiving mu‐agonist opioid analgesics, and were expected to require daily opioid analgesics for at least 7 days following randomization. Acute constipation was defined as having no bowel movement for at least 48 hours prior to randomization, difficulty in having a spontaneous bowel movement (straining or sensation of incomplete evacuation or hard, lumpy stools), or the inability to have a spontaneous bowel movement. Exclusion criteria included fecal impaction, mechanical bowel obstruction, constipation not attributed to postprocedure opioid use, calculated creatinine clearance less than 50 mL/min, and corrected QT interval greater than 500 msec on a 12‐lead screening electrocardiogram (ECG). Patients with a known hypersensitivity to methylnaltrexone, naltrexone, or naloxone, who were pregnant or lactating, who had a history of alcohol or drug abuse within the past 2 years, or who had a spinal cord injury or gastrointestinal ostomy were also excluded. Any laxatives, enemas, and/or promotility agents being used must have been discontinued at least 48 hours prior to first dose of study medication and were not permitted during the study, but stool softener use was permitted if it had been administered at least 24 hours prior to screening and a stable dose was maintained throughout the study.
Study Design
This randomized, double‐blind, placebo‐controlled, parallel‐group, hypothesis‐generating phase 2 study was conducted from October 2007 to January 2009 at 16 US hospitals and rehabilitation facilities in accordance with the International Conference on Harmonisation Good Clinical Practice Guidelines and the Declaration of Helsinki, and was approved by the Institutional Review Board and/or Independent Ethics Committee at each of the participating investigational centers. All patients provided written informed consent prior to study participation.
Eligible patients were randomized by interactive voice response system in a 1:1 ratio to receive once‐daily subcutaneous (SC) injections of either 12 mg methylnaltrexone or placebo (Figure 1). The chosen 12‐mg unit dosing corresponds to approximately 0.15 mg/kg (assuming an 80‐kg patient) and was found to be both efficacious and well tolerated in the treatment of OIC in prior studies, including studies in advanced‐illness patients17, 18 and in patients with chronic, nonmalignant pain (Blonsky et al., 28th Annual Scientific Meeting of the American Pain Society, May 7‐9, 2009, San Diego, CA; Duerden et al., 29th Annual Scientific Meeting of the American Pain Society, May 6‐10, 2010, Baltimore, MD.20 The first dose of study medication was administered on the day of randomization or on the next calendar day. Once enrolled, the patient received once‐daily doses of methylnaltrexone for up to 4 or 7 days. Dosing continued until the patient received the maximum number of doses allowed, no longer needed opioid medication, or was discharged from the medical facility. Each patient completed a follow‐up safety visit at 14 3 days following the last dose.
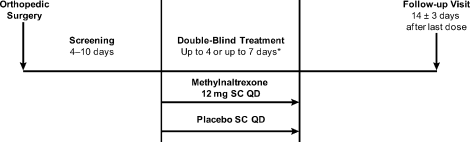
Evaluations
All efficacy variables were considered exploratory and included the occurrence of laxation within 2 and 4 hours of the first dose of study drug, time to laxation, and a questionnaire assessing patient global satisfaction. Patients recorded the date, time, and assessment of each bowel movement in diaries.
Safety variables included adverse events (AEs), serious AEs (SAEs), clinical laboratory parameters, physical examinations, vital signs, ECGs, concomitant medications, Objective and Subjective Opioid Withdrawal Scales (OOWS and SOWS),21 and Numeric Rating Scales for Pain ([NRSP] 0 = no pain, 10 = worst pain possible).
Statistical Analysis
Enrolled patients were defined as all patients who consented to participate in the study. Both the modified intent‐to‐treat (mITT) population and the safety population were defined as all patients who were randomized and received at least 1 injection of study drug. All study results are based on the mITT population.
Categorical variables were summarized using frequency and percentage, while descriptive statistics for continuous variables included sample size, mean, median, standard deviation, and minimum and maximum values. All inferential statistical tests were 2‐tailed and used a tolerance for nominal type I error (alpha, ) of 0.05. There was no correction for multiplicity and no imputations were performed to account for missing data.
Fisher's exact test was used for comparisons between the proportion of patients with laxation within 2 hours and 4 hours of the first dose in the methylnaltrexone group versus the placebo group. The time to first laxation analysis was performed using the log‐rank test and Kaplan‐Meier method.
RESULTS
Patient Populations
The flow of patients through the study is summarized in Figure 2. A total of 51 patients were enrolled. Of these, 33 received at least 1 dose of study treatment following double‐blind randomization and comprised both the mITT and safety populations. Seventeen of these patients were enrolled under the original protocol and could receive study drug for up to 7 days, while 16 patients enrolled under a subsequent protocol revision could receive study drug for up to 4 days. This change from a 7‐day to a 4‐day treatment protocol allowed for the capture of more study patients in view of the time pressures of short lengths of stay in postoperative settings. In total, 31 patients received at least 2 doses, and 26 patients received at least 4 doses of study drug. A total of 27 patients completed the study. Baseline demographics and prestudy surgical procedures were similar in both treatment groups (Table 1).
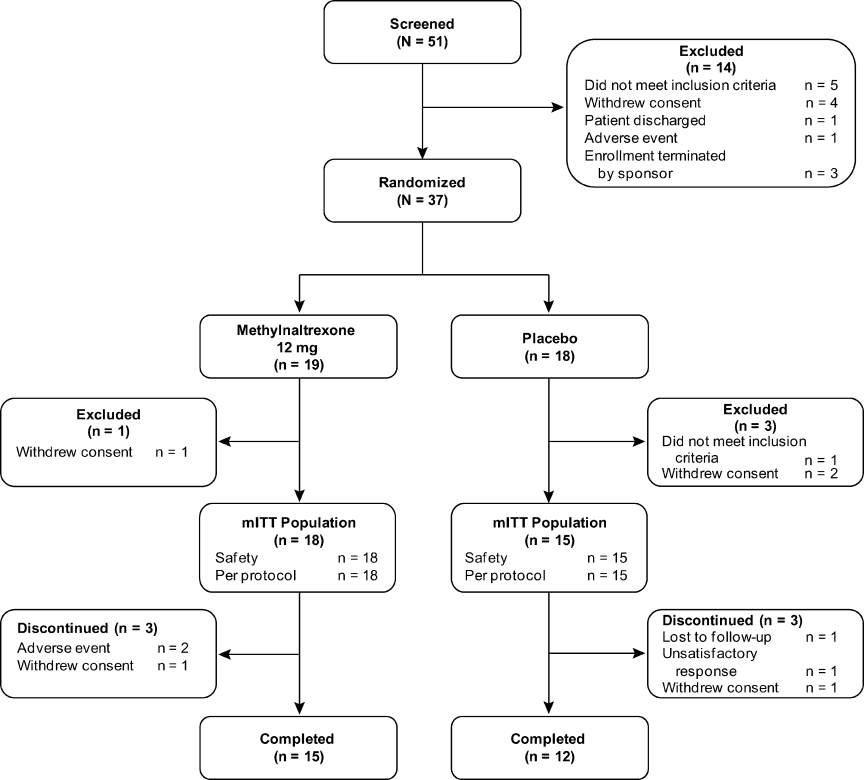
| Characteristic | Methylnaltrexone (n = 18) | Placebo (n = 15) |
|---|---|---|
| ||
| Mean age, yr (SD) | 64.2 (9.0) | 65.2 (11.6) |
| Mean weight, kg (SD) | 92.5 (22.5) | 91.0 (20.2) |
| Mean BMI, kg/m2 (SD) | 32.3 (7.2) | 34.2 (6.41) |
| Sex, n (%) | ||
| Female | 11 (61.1) | 11 (73.3) |
| Male | 7 (38.9) | 4 (26.7) |
| Race, n (%) | ||
| White | 14 (77.8) | 10 (66.7) |
| Black | 4 (22.2) | 5 (33.3) |
| Type of surgery, n (%) | ||
| Total knee replacement | 8 (44.4) | 7 (46.7) |
| Total hip replacement | 6 (33.3) | 6 (40.0) |
| Spinal fusion | 2 (11.1) | 0 |
| Fracture reduction | 2 (11.1) | 2 (13.3) |
| Median opioid use,* mg (range) | 28.00 (6.75‐168.01) | 25.00 (9.00‐75.00) |
| Median time from surgery to study drug administration, days (range) | 4 (3‐6) | 4 (3‐6) |
Efficacy
A significantly greater percentage of patients had a bowel movement within 2 hours (P = 0.021) and 4 hours (P = 0.046) of the first dose of methylnaltrexone compared with patients who received placebo (Figure 3). Within 2 hours, 6 patients (33.3%; 95% confidence interval [CI], 13.34‐59.01) who received methylnaltrexone achieved laxation, while laxation did not occur in any patient who received placebo. By 4 hours posttreatment, 7 patients (38.9%; 95% CI, 17.30‐64.25) in the methylnaltrexone group achieved laxation compared with only 1 patient (6.7%; 95% CI, 0.17‐31.95) on placebo. Three patients in each treatment group received rescue laxatives.
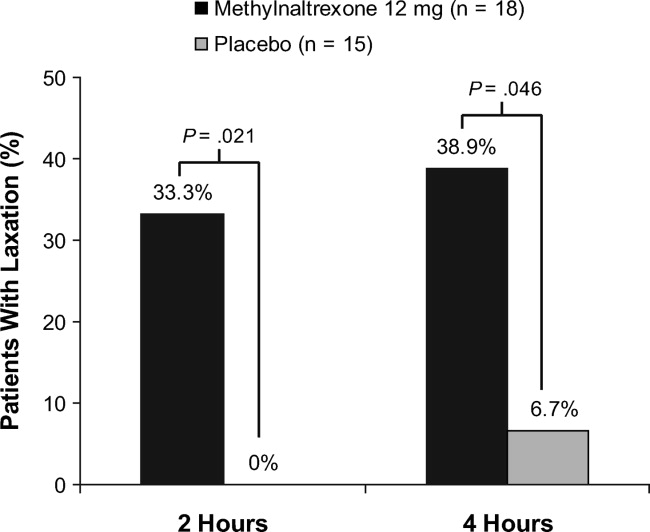
The time to first laxation (Figure 4) was significantly shorter in patients who received methylnaltrexone compared with those in the placebo group. Patients on methylnaltrexone achieved laxation in a median time of 15.8 hours, compared with a median time of 50.9 hours for patients in the placebo group (P = 0.02, log‐rank test). The median time to laxation was less than 1 hour in the 7 methylnaltrexone‐treated patients who experienced laxation within 4 hours following the first dose. Of the remaining 11 methylnaltrexone‐treated patients, one experienced no laxation after 6 doses, and the median time to laxation for the others was 29.9 hours (not shown in figure).
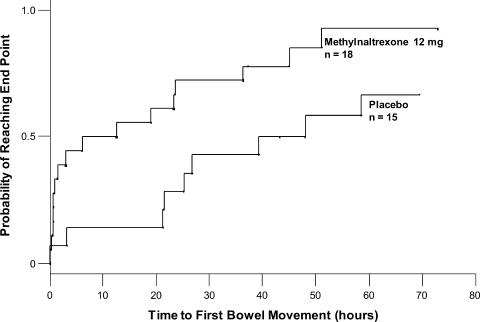
Analysis of the Global Satisfaction With Treatment Scale revealed that more patients expressed overall treatment satisfaction (defined as very satisfied, satisfied, or minimally satisfied) with methylnaltrexone assessed 4 hours ( 30 minutes) after the first dose, compared with patients on placebo (83.3% vs 60.0%, respectively). At the study endpoint, overall treatment satisfaction with methylnaltrexone remained high (83.3%), whereas satisfaction with placebo was 53.3%. Additionally, no patients in the methylnaltrexone group expressed any dissatisfaction with treatment (defined as minimally dissatisfied, dissatisfied, or very dissatisfied) at endpoint, compared with 26.7% of patients in the placebo group who expressed some degree of dissatisfaction.
Safety
Overall AE rates were similar between treatment groups (Table 2), with at least 1 treatment‐emergent AE reported in 6 patients (33.3%) in the methylnaltrexone group and 4 patients (26.7%) in the placebo group. The most common AEs reported during the study were classified as gastrointestinal in nature; 3 (nausea, abdominal pain, and diarrhea) were considered by the investigator to be possibly related to study medication. Two patients receiving methylnaltrexone discontinued the study because of AEs (one with moderate constipation, one with mild diarrhea) compared with none of the placebo group patients. No treatment‐emergent SAEs or deaths were reported during this study. Analysis of clinical laboratory parameters, vital signs, and ECGs revealed no safety signals and showed no pattern of concern related to methylnaltrexone exposure.
| Adverse Event* | Methylnaltrexone 12 mg (n = 18) n (%) | Placebo (n = 15) n (%) |
|---|---|---|
| ||
| Any | 6 (33.3) | 4 (26.7) |
| Anemia | 1 (5.6) | 0 |
| Gastrointestinal disorders | 3 (16.7) | 1 (6.7) |
| Abdominal discomfort | 0 | 1 (6.7) |
| Abdominal distension | 1 (5.6) | 0 |
| Abdominal pain | 1 (5.6) | 0 |
| Abdominal tenderness | 1 (5.6) | 0 |
| Constipation | 1 (5.6) | 0 |
| Diarrhea | 1 (5.6) | 0 |
| Nausea | 1 (5.6) | 0 |
| Headache | 1 (5.6) | 0 |
| Hypotension | 1 (5.6) | 0 |
| Joint swelling | 0 | 1 (6.7) |
| Peripheral edema | 0 | 2 (13.3) |
| Procedural pain | 0 | 1 (6.7) |
| Skin ulcer | 0 | 1 (6.7) |
| Somnolence | 0 | 1 (6.7) |
| Urinary tract infection | 1 (5.6) | 0 |
| Wound infection | 1 (5.6) | 0 |
Pain and Opioid Withdrawal
Results from the SOWS and OOWS measures indicated that signs and symptoms of withdrawal did not increase over time in patients treated with methylnaltrexone, and no discernable differences were found between study groups. Pain was assessed using a numeric rating scale ranging from 0 to 10, with higher scores indicating greater severity. Baseline pain scores were not significantly different between treatment groups, with a mean of 5.7 2.7 for placebo, and 5.4 3.0 for the methylnaltrexone group. At 1 day postdose, mean pain scores did not increase from baseline in the placebo (0.9 2.33) or methylnaltrexone group (0.5 2.5), and no significant between‐group differences were found. Similar results were observed at the end of the study. Thus, pain did not appear to increase in patients treated with methylnaltrexone, and changes in pain scores were indistinguishable between the 2 treatment groups.
DISCUSSION
This pilot study suggests that methylnaltrexone actively induces laxation and is generally well tolerated in patients receiving mu‐opioid analgesia, following orthopedic surgery, who develop OIC acutely. It was the first study, to our knowledge, to investigate the efficacy of methylnaltrexone for the treatment of OIC in an acute postoperative setting. The protocol amendment changing the duration of treatment from 7 days to 4 days did not materially affect the results of the study. The response to methylnaltrexone was rapid, with 33.3% experiencing laxation within 2 hours. The median time to laxation was nearly 1.5 days shorter in patients treated with methylnaltrexone compared with those receiving placebo. Correspondingly, overall patient satisfaction was high in the methylnaltrexone group. Efficacy was attained without diminishing opioid analgesia, and without inducing signs or symptoms of opioid withdrawal. The incidence of AEs was similar between groups, and no treatment‐emergent SAEs were reported in this study.
Previous clinical trials investigated the safety and efficacy of methylnaltrexone for the treatment of OIC in patients with advanced illness and with chronic, nonmalignant pain. The present study extends those findings to a population of patients experiencing acute OIC following orthopedic surgery. Previous studies showed that approximately 48% to 62% of advanced‐illness patients experienced laxation within 4 hours of receiving SC methylnaltrexone,17, 18 compared with 38.9% of acute OIC patients in this study. In a clinical trial of patients with chronic, nonmalignant pain, 34.2% of patients experienced laxation within 4 hours of SC methylnaltrexone injection (Blonsky et al., 28th Annual Scientific Meeting of the American Pain Society, May 7‐9, 2009, San Diego, CA). The differences in laxation response between these trials may be attributable to differences in the patient populations or to methodologic differences between the studies.
Similar to findings demonstrated in a clinical study evaluating methylnaltrexone for OIC in a different patient population, those with advanced illness,22 this study supports the premise that future laxation response with prolonged use is most likely to occur when a laxation response was achieved after the first or second initial administrations of methylnaltrexone. In contradistinction, if laxation does not occur with these early doses, continued methylnaltrexone dosing is less likely to produce a response later.
This study has some limitations that must be considered. First, as this was a hypothesis‐generating study, all efficacy parameters investigated were exploratory in nature. The results reported herein warrant careful consideration, owing to a small sample size that may limit their generalizability, prior to replication in a more rigorously designed study with prespecified efficacy endpoints. Likewise, the assessment of health outcome parameters is limited. Another limitation is the small sample size utilized in this study, potentially resulting in a type II error.
Subcutaneous administration potentially offers a considerable benefit over oral therapies for OIC in this patient population post‐orthopedic surgery. Nausea and vomiting can occur as a consequence of anesthesia and of postoperative opioid analgesia, and may compromise adequate dosing of oral medications prescribed to treat OIC. Subcutaneous delivery of methylnaltrexone may circumvent this potential drawback while providing potentially rapid, effective treatment for OIC. Once‐daily dosing may also help to minimize caregiver burden and patient discomfort by preventing the need for more frequent or unpleasant treatments for OIC, such as enemas.
This study provides an initial positive signal for a broader, albeit off‐label use for methylnaltrexonethat being for the treatment of acute constipation that occurs as a consequence of postoperative opioid‐mediated analgesia in patients following orthopedic procedures. Adequate treatment of OIC, even in the acute postoperative setting, is likely to lead to better overall pain management and improved patient outcomes. Additionally, effective management of acute OIC is likely to be cost‐effective in terms of reducing the duration of hospital stays, reducing the need for nursing resources and the time spent administering rescue treatments for OIC (eg, enemas), and avoiding returns to an acute setting (eg, the emergency department) for treatment. The results presented herein suggest that methylnaltrexone may be effective and have a good safety profile in the treatment of acute OIC following orthopedic surgery. Validation of these results in larger well‐controlled trials would be welcome.
Acknowledgements
The authors thank the patients and clinical personnel involved in this study; John Charity, NP, for data collection and management, and John H. Simmons, MD, of Peloton Advantage, LLC, for assistance with manuscript preparation, which was funded by Pfizer Inc.
In addition to the authors, the following investigators participated in this trial: David Nathan Feldman, MD, Holy Name Hospital, Teaneck, NJ; Sam Hakki, MD, Bay Pines VA Healthcare System, Bay Pines, FL; Forrest A. Hanke, MD, Trover Health System, Madisonville, KY; William H. Horton, Jr, MD, Palmetto Clinical Research, Greenville, SC; M. Jay Jazayeri, MD, Pacific Hospital of Long Beach, Long Beach, CA; John F. Peppin, DO, The Pain Treatment Center of the Bluegrass, Lexington, KY; Bruce Pomeranz, MD, Kessler Institute for Rehabilitation, Saddle Brook, NJ, and Chester, NJ; Alan C. Schwartz, MD, Helping Hands Medical Associates, Santa Ana, CA; Michael J. Skyhar, MD, CORE Orthopaedic Medical Center, Encinitas, CA; Lex A. Simpson, MD, CORE Orthopaedic Medical Center, Encinitas, CA; James Slover, MD, New York University Hospital for Joint Disease, New York, NY; Dilip Tapadiya, MD, Fountain Valley Regional Hospital, Fountain Valley, CA; Stanley J. Waters, MD, PhD, Americana Orthopedics, Boise, ID.
- ,.Postoperative pain management.Chest Surg Clin N Am.1997;7:773–799.
- ,.Strategies for effective postoperative pain management.Minerva Anestesiol.2006;72:145–150.
- ,,.Are peripheral opioid antagonists the solution to opioid side effects?Anesth Analg.2004;98:116–122.
- ,.Neuroplasticity—an important factor in acute and chronic pain.Swiss Med Wkly.2002;132:273–278.
- ,,,,.The burden of acute postoperative pain and the potential role of the COX‐2‐specific inhibitors.Rheumatology (Oxford).2003;42(suppl 3):iii40–iii52.
- .Incidence, prevalence, and management of opioid bowel dysfunction.Am J Surg.2001;182(suppl 5A):11S–18S.
- ,.Management of common opioid‐induced adverse effects.Am Fam Physician.2006;74:1347–1354.
- ,.Antagonism of gastrointestinal opioid effects.Reg Anesth Pain Med.2000;25:639–642.
- .Methylnaltrexone mechanisms of action and effects on opioid bowel dysfunction and other opioid adverse effects.Ann Pharmacother.2007;41:984–993.
- ,.Methylnaltrexone: investigation of clinical applications.Drug Dev Res.2000;50:133–141.
- ,,,.Opioid‐induced delay in gastric emptying: a peripheral mechanism in humans.Anesthesiology.1997;87:765–770.
- ,,, et al.Effects of enteric‐coated methylnaltrexone in preventing opioid‐induced delay in oral‐cecal transit time.Clin Pharmacol Ther.2000;67:398–404.
- ,,,,,.Methylnaltrexone prevents morphine‐induced delay in oral‐cecal transit time without affecting analgesia: a double‐blind randomized placebo‐controlled trial.Clin Pharmacol Ther.1996;59:469–475.
- ,,,,,.Effects of intravenous methylnaltrexone on opioid‐induced gut motility and transit time changes in subjects receiving chronic methadone therapy: a pilot study.Pain.1999;83:631–635.
- ,,, et al.Methylnaltrexone for reversal of constipation due to chronic methadone use: a randomized controlled trial.JAMA.2000;283:367–372.
- ,.Oral methylnaltrexone for opioid‐induced constipation.JAMA.2000;284:1383–1384.
- ,,, et al.Methylnaltrexone for opioid‐induced constipation in advanced illness.N Engl J Med.2008;328:2332–2343.
- ,,, et al.Methylnaltrexone for treatment of opioid‐induced constipation in advanced illness patients.J Support Oncol.2009;7:39–46.
- Relistor [package insert].Philadelphia, PA, and Tarrytown, NY:Wyeth Pharmaceuticals Inc and Progenics Pharmaceuticals;2009.
- ,,, et al.Subcutaneous methylnaltrexone for treatment of opioid‐induced constipation in patients with chronic, nonmalignant pain: a randomized controlled study.J Pain.2011;12:554–562.
- ,,,,,.Two new rating scales for opiate withdrawal.Am J Drug Alcohol Abuse.1987;13:293–308.
- ,,, et al.Methylnaltrexone treatment of opioid‐induced constipation in patients with advanced illness.J Pain Symptom Manage.2009;38:683–690.
The management of postoperative pain is essential to perioperative care, and adequate postoperative analgesia has been associated with several key clinical benefits, including fewer postoperative complications, earlier patient ambulation, reduced costs due to shorter hospital stays, and improved rehabilitation.1, 2 While opioids have long been central to postoperative analgesia, they have been associated with various adverse effects, including sedation, dizziness, nausea, vomiting, constipation, dependence, tolerance, and respiratory depression.2, 3 Constipation, one of the most common adverse effects resulting from opioid therapy, can be debilitating. Indeed, opioid effects on gut motility can occur even after a single dose.3 The consequences of opioid‐induced constipation (OIC) may be severe enough to warrant a dosage reduction of the opioid; however, this may lead to compromised analgesia, which can hinder recovery.4, 5 Thus, effective treatment of OIC is an important clinical consideration in patients undergoing pain management with opioids. Unfortunately, laxatives and other treatment strategies can have unpredictable or suboptimal results for many patients with OIC; therefore, other options are needed for the treatment of OIC.6, 7
Opioid receptor agonists cause constipation by adversely altering many aspects of intestinal function, including fluid dynamics, gastric emptying, propulsive motor activity, and transit time.3 Opioid receptors are widely distributed in the central nervous system and throughout the intestinal system. The mechanism of OIC may have both peripherally and centrally mediated components.8 Nonselective opioid receptor antagonists block the undesired effects on the gut, but because they cross the blood‐brain barrier, they also interfere with analgesia and may lead to symptoms of withdrawal. Methylnaltrexone is a selective, peripherally acting mu‐opioid receptor antagonist,9 formed by the addition of a methyl group to the amine ring of the mu‐opioid receptor antagonist naltrexone. The resulting quarternary amine has greater polarity, lower lipid solubility, and restricted ability to cross the blood‐brain barrier.10 Thus, methylnaltrexone was designed to decrease the peripheral adverse effects of opioids without interfering with centrally mediated analgesia.
Investigations of methylnaltrexone effects in healthy volunteers showed that methylnaltrexone attenuated morphine‐induced delays in gastric emptying and oral‐cecal transit without affecting analgesia.1113 Further studies of methylnaltrexone for the treatment of constipation due to methadone use demonstrated rapid laxation response.1416 Two randomized, double‐blind, placebo‐controlled studies of methylnaltrexone in 288 patients with advanced illness and OIC showed that methylnaltrexone rapidly induced laxation without compromising analgesia.17, 18 Methylnaltrexone is currently approved for the treatment of OIC in patients with advanced illness who are receiving palliative care, when response to laxative therapy has not been sufficient.19
Recently, the use of methylnaltrexone for the treatment of OIC in patients with chronic, nonmalignant pain was assessed in a randomized, double‐blind, placebo‐controlled trial of more than 400 patients. Investigators found that methylnaltrexone induced laxation and was generally well tolerated (Blonsky et al., 28th Annual Scientific Meeting of the American Pain Society, May 7‐9, 2009, San Diego, CA; Duerden et al., 29th Annual Scientific Meeting of the American Pain Society, May 6‐10, 2010, Baltimore, MD), supporting the safety and efficacy of methylnaltrexone in the setting of OIC resulting from chronic opioid treatment. The present study aimed to assess the activity of methylnaltrexone in patients receiving mu‐agonist opioid analgesics during rehabilitation, following an orthopedic surgical procedure, who were experiencing acute OIC.
METHODS
Patients
Patients who had undergone orthopedic procedures within 4 to 10 days were screened for eligibility. Adults aged 18 years or older were considered eligible if they were acutely constipated, were receiving mu‐agonist opioid analgesics, and were expected to require daily opioid analgesics for at least 7 days following randomization. Acute constipation was defined as having no bowel movement for at least 48 hours prior to randomization, difficulty in having a spontaneous bowel movement (straining or sensation of incomplete evacuation or hard, lumpy stools), or the inability to have a spontaneous bowel movement. Exclusion criteria included fecal impaction, mechanical bowel obstruction, constipation not attributed to postprocedure opioid use, calculated creatinine clearance less than 50 mL/min, and corrected QT interval greater than 500 msec on a 12‐lead screening electrocardiogram (ECG). Patients with a known hypersensitivity to methylnaltrexone, naltrexone, or naloxone, who were pregnant or lactating, who had a history of alcohol or drug abuse within the past 2 years, or who had a spinal cord injury or gastrointestinal ostomy were also excluded. Any laxatives, enemas, and/or promotility agents being used must have been discontinued at least 48 hours prior to first dose of study medication and were not permitted during the study, but stool softener use was permitted if it had been administered at least 24 hours prior to screening and a stable dose was maintained throughout the study.
Study Design
This randomized, double‐blind, placebo‐controlled, parallel‐group, hypothesis‐generating phase 2 study was conducted from October 2007 to January 2009 at 16 US hospitals and rehabilitation facilities in accordance with the International Conference on Harmonisation Good Clinical Practice Guidelines and the Declaration of Helsinki, and was approved by the Institutional Review Board and/or Independent Ethics Committee at each of the participating investigational centers. All patients provided written informed consent prior to study participation.
Eligible patients were randomized by interactive voice response system in a 1:1 ratio to receive once‐daily subcutaneous (SC) injections of either 12 mg methylnaltrexone or placebo (Figure 1). The chosen 12‐mg unit dosing corresponds to approximately 0.15 mg/kg (assuming an 80‐kg patient) and was found to be both efficacious and well tolerated in the treatment of OIC in prior studies, including studies in advanced‐illness patients17, 18 and in patients with chronic, nonmalignant pain (Blonsky et al., 28th Annual Scientific Meeting of the American Pain Society, May 7‐9, 2009, San Diego, CA; Duerden et al., 29th Annual Scientific Meeting of the American Pain Society, May 6‐10, 2010, Baltimore, MD.20 The first dose of study medication was administered on the day of randomization or on the next calendar day. Once enrolled, the patient received once‐daily doses of methylnaltrexone for up to 4 or 7 days. Dosing continued until the patient received the maximum number of doses allowed, no longer needed opioid medication, or was discharged from the medical facility. Each patient completed a follow‐up safety visit at 14 3 days following the last dose.

Evaluations
All efficacy variables were considered exploratory and included the occurrence of laxation within 2 and 4 hours of the first dose of study drug, time to laxation, and a questionnaire assessing patient global satisfaction. Patients recorded the date, time, and assessment of each bowel movement in diaries.
Safety variables included adverse events (AEs), serious AEs (SAEs), clinical laboratory parameters, physical examinations, vital signs, ECGs, concomitant medications, Objective and Subjective Opioid Withdrawal Scales (OOWS and SOWS),21 and Numeric Rating Scales for Pain ([NRSP] 0 = no pain, 10 = worst pain possible).
Statistical Analysis
Enrolled patients were defined as all patients who consented to participate in the study. Both the modified intent‐to‐treat (mITT) population and the safety population were defined as all patients who were randomized and received at least 1 injection of study drug. All study results are based on the mITT population.
Categorical variables were summarized using frequency and percentage, while descriptive statistics for continuous variables included sample size, mean, median, standard deviation, and minimum and maximum values. All inferential statistical tests were 2‐tailed and used a tolerance for nominal type I error (alpha, ) of 0.05. There was no correction for multiplicity and no imputations were performed to account for missing data.
Fisher's exact test was used for comparisons between the proportion of patients with laxation within 2 hours and 4 hours of the first dose in the methylnaltrexone group versus the placebo group. The time to first laxation analysis was performed using the log‐rank test and Kaplan‐Meier method.
RESULTS
Patient Populations
The flow of patients through the study is summarized in Figure 2. A total of 51 patients were enrolled. Of these, 33 received at least 1 dose of study treatment following double‐blind randomization and comprised both the mITT and safety populations. Seventeen of these patients were enrolled under the original protocol and could receive study drug for up to 7 days, while 16 patients enrolled under a subsequent protocol revision could receive study drug for up to 4 days. This change from a 7‐day to a 4‐day treatment protocol allowed for the capture of more study patients in view of the time pressures of short lengths of stay in postoperative settings. In total, 31 patients received at least 2 doses, and 26 patients received at least 4 doses of study drug. A total of 27 patients completed the study. Baseline demographics and prestudy surgical procedures were similar in both treatment groups (Table 1).

| Characteristic | Methylnaltrexone (n = 18) | Placebo (n = 15) |
|---|---|---|
| ||
| Mean age, yr (SD) | 64.2 (9.0) | 65.2 (11.6) |
| Mean weight, kg (SD) | 92.5 (22.5) | 91.0 (20.2) |
| Mean BMI, kg/m2 (SD) | 32.3 (7.2) | 34.2 (6.41) |
| Sex, n (%) | ||
| Female | 11 (61.1) | 11 (73.3) |
| Male | 7 (38.9) | 4 (26.7) |
| Race, n (%) | ||
| White | 14 (77.8) | 10 (66.7) |
| Black | 4 (22.2) | 5 (33.3) |
| Type of surgery, n (%) | ||
| Total knee replacement | 8 (44.4) | 7 (46.7) |
| Total hip replacement | 6 (33.3) | 6 (40.0) |
| Spinal fusion | 2 (11.1) | 0 |
| Fracture reduction | 2 (11.1) | 2 (13.3) |
| Median opioid use,* mg (range) | 28.00 (6.75‐168.01) | 25.00 (9.00‐75.00) |
| Median time from surgery to study drug administration, days (range) | 4 (3‐6) | 4 (3‐6) |
Efficacy
A significantly greater percentage of patients had a bowel movement within 2 hours (P = 0.021) and 4 hours (P = 0.046) of the first dose of methylnaltrexone compared with patients who received placebo (Figure 3). Within 2 hours, 6 patients (33.3%; 95% confidence interval [CI], 13.34‐59.01) who received methylnaltrexone achieved laxation, while laxation did not occur in any patient who received placebo. By 4 hours posttreatment, 7 patients (38.9%; 95% CI, 17.30‐64.25) in the methylnaltrexone group achieved laxation compared with only 1 patient (6.7%; 95% CI, 0.17‐31.95) on placebo. Three patients in each treatment group received rescue laxatives.

The time to first laxation (Figure 4) was significantly shorter in patients who received methylnaltrexone compared with those in the placebo group. Patients on methylnaltrexone achieved laxation in a median time of 15.8 hours, compared with a median time of 50.9 hours for patients in the placebo group (P = 0.02, log‐rank test). The median time to laxation was less than 1 hour in the 7 methylnaltrexone‐treated patients who experienced laxation within 4 hours following the first dose. Of the remaining 11 methylnaltrexone‐treated patients, one experienced no laxation after 6 doses, and the median time to laxation for the others was 29.9 hours (not shown in figure).

Analysis of the Global Satisfaction With Treatment Scale revealed that more patients expressed overall treatment satisfaction (defined as very satisfied, satisfied, or minimally satisfied) with methylnaltrexone assessed 4 hours ( 30 minutes) after the first dose, compared with patients on placebo (83.3% vs 60.0%, respectively). At the study endpoint, overall treatment satisfaction with methylnaltrexone remained high (83.3%), whereas satisfaction with placebo was 53.3%. Additionally, no patients in the methylnaltrexone group expressed any dissatisfaction with treatment (defined as minimally dissatisfied, dissatisfied, or very dissatisfied) at endpoint, compared with 26.7% of patients in the placebo group who expressed some degree of dissatisfaction.
Safety
Overall AE rates were similar between treatment groups (Table 2), with at least 1 treatment‐emergent AE reported in 6 patients (33.3%) in the methylnaltrexone group and 4 patients (26.7%) in the placebo group. The most common AEs reported during the study were classified as gastrointestinal in nature; 3 (nausea, abdominal pain, and diarrhea) were considered by the investigator to be possibly related to study medication. Two patients receiving methylnaltrexone discontinued the study because of AEs (one with moderate constipation, one with mild diarrhea) compared with none of the placebo group patients. No treatment‐emergent SAEs or deaths were reported during this study. Analysis of clinical laboratory parameters, vital signs, and ECGs revealed no safety signals and showed no pattern of concern related to methylnaltrexone exposure.
| Adverse Event* | Methylnaltrexone 12 mg (n = 18) n (%) | Placebo (n = 15) n (%) |
|---|---|---|
| ||
| Any | 6 (33.3) | 4 (26.7) |
| Anemia | 1 (5.6) | 0 |
| Gastrointestinal disorders | 3 (16.7) | 1 (6.7) |
| Abdominal discomfort | 0 | 1 (6.7) |
| Abdominal distension | 1 (5.6) | 0 |
| Abdominal pain | 1 (5.6) | 0 |
| Abdominal tenderness | 1 (5.6) | 0 |
| Constipation | 1 (5.6) | 0 |
| Diarrhea | 1 (5.6) | 0 |
| Nausea | 1 (5.6) | 0 |
| Headache | 1 (5.6) | 0 |
| Hypotension | 1 (5.6) | 0 |
| Joint swelling | 0 | 1 (6.7) |
| Peripheral edema | 0 | 2 (13.3) |
| Procedural pain | 0 | 1 (6.7) |
| Skin ulcer | 0 | 1 (6.7) |
| Somnolence | 0 | 1 (6.7) |
| Urinary tract infection | 1 (5.6) | 0 |
| Wound infection | 1 (5.6) | 0 |
Pain and Opioid Withdrawal
Results from the SOWS and OOWS measures indicated that signs and symptoms of withdrawal did not increase over time in patients treated with methylnaltrexone, and no discernable differences were found between study groups. Pain was assessed using a numeric rating scale ranging from 0 to 10, with higher scores indicating greater severity. Baseline pain scores were not significantly different between treatment groups, with a mean of 5.7 2.7 for placebo, and 5.4 3.0 for the methylnaltrexone group. At 1 day postdose, mean pain scores did not increase from baseline in the placebo (0.9 2.33) or methylnaltrexone group (0.5 2.5), and no significant between‐group differences were found. Similar results were observed at the end of the study. Thus, pain did not appear to increase in patients treated with methylnaltrexone, and changes in pain scores were indistinguishable between the 2 treatment groups.
DISCUSSION
This pilot study suggests that methylnaltrexone actively induces laxation and is generally well tolerated in patients receiving mu‐opioid analgesia, following orthopedic surgery, who develop OIC acutely. It was the first study, to our knowledge, to investigate the efficacy of methylnaltrexone for the treatment of OIC in an acute postoperative setting. The protocol amendment changing the duration of treatment from 7 days to 4 days did not materially affect the results of the study. The response to methylnaltrexone was rapid, with 33.3% experiencing laxation within 2 hours. The median time to laxation was nearly 1.5 days shorter in patients treated with methylnaltrexone compared with those receiving placebo. Correspondingly, overall patient satisfaction was high in the methylnaltrexone group. Efficacy was attained without diminishing opioid analgesia, and without inducing signs or symptoms of opioid withdrawal. The incidence of AEs was similar between groups, and no treatment‐emergent SAEs were reported in this study.
Previous clinical trials investigated the safety and efficacy of methylnaltrexone for the treatment of OIC in patients with advanced illness and with chronic, nonmalignant pain. The present study extends those findings to a population of patients experiencing acute OIC following orthopedic surgery. Previous studies showed that approximately 48% to 62% of advanced‐illness patients experienced laxation within 4 hours of receiving SC methylnaltrexone,17, 18 compared with 38.9% of acute OIC patients in this study. In a clinical trial of patients with chronic, nonmalignant pain, 34.2% of patients experienced laxation within 4 hours of SC methylnaltrexone injection (Blonsky et al., 28th Annual Scientific Meeting of the American Pain Society, May 7‐9, 2009, San Diego, CA). The differences in laxation response between these trials may be attributable to differences in the patient populations or to methodologic differences between the studies.
Similar to findings demonstrated in a clinical study evaluating methylnaltrexone for OIC in a different patient population, those with advanced illness,22 this study supports the premise that future laxation response with prolonged use is most likely to occur when a laxation response was achieved after the first or second initial administrations of methylnaltrexone. In contradistinction, if laxation does not occur with these early doses, continued methylnaltrexone dosing is less likely to produce a response later.
This study has some limitations that must be considered. First, as this was a hypothesis‐generating study, all efficacy parameters investigated were exploratory in nature. The results reported herein warrant careful consideration, owing to a small sample size that may limit their generalizability, prior to replication in a more rigorously designed study with prespecified efficacy endpoints. Likewise, the assessment of health outcome parameters is limited. Another limitation is the small sample size utilized in this study, potentially resulting in a type II error.
Subcutaneous administration potentially offers a considerable benefit over oral therapies for OIC in this patient population post‐orthopedic surgery. Nausea and vomiting can occur as a consequence of anesthesia and of postoperative opioid analgesia, and may compromise adequate dosing of oral medications prescribed to treat OIC. Subcutaneous delivery of methylnaltrexone may circumvent this potential drawback while providing potentially rapid, effective treatment for OIC. Once‐daily dosing may also help to minimize caregiver burden and patient discomfort by preventing the need for more frequent or unpleasant treatments for OIC, such as enemas.
This study provides an initial positive signal for a broader, albeit off‐label use for methylnaltrexonethat being for the treatment of acute constipation that occurs as a consequence of postoperative opioid‐mediated analgesia in patients following orthopedic procedures. Adequate treatment of OIC, even in the acute postoperative setting, is likely to lead to better overall pain management and improved patient outcomes. Additionally, effective management of acute OIC is likely to be cost‐effective in terms of reducing the duration of hospital stays, reducing the need for nursing resources and the time spent administering rescue treatments for OIC (eg, enemas), and avoiding returns to an acute setting (eg, the emergency department) for treatment. The results presented herein suggest that methylnaltrexone may be effective and have a good safety profile in the treatment of acute OIC following orthopedic surgery. Validation of these results in larger well‐controlled trials would be welcome.
Acknowledgements
The authors thank the patients and clinical personnel involved in this study; John Charity, NP, for data collection and management, and John H. Simmons, MD, of Peloton Advantage, LLC, for assistance with manuscript preparation, which was funded by Pfizer Inc.
In addition to the authors, the following investigators participated in this trial: David Nathan Feldman, MD, Holy Name Hospital, Teaneck, NJ; Sam Hakki, MD, Bay Pines VA Healthcare System, Bay Pines, FL; Forrest A. Hanke, MD, Trover Health System, Madisonville, KY; William H. Horton, Jr, MD, Palmetto Clinical Research, Greenville, SC; M. Jay Jazayeri, MD, Pacific Hospital of Long Beach, Long Beach, CA; John F. Peppin, DO, The Pain Treatment Center of the Bluegrass, Lexington, KY; Bruce Pomeranz, MD, Kessler Institute for Rehabilitation, Saddle Brook, NJ, and Chester, NJ; Alan C. Schwartz, MD, Helping Hands Medical Associates, Santa Ana, CA; Michael J. Skyhar, MD, CORE Orthopaedic Medical Center, Encinitas, CA; Lex A. Simpson, MD, CORE Orthopaedic Medical Center, Encinitas, CA; James Slover, MD, New York University Hospital for Joint Disease, New York, NY; Dilip Tapadiya, MD, Fountain Valley Regional Hospital, Fountain Valley, CA; Stanley J. Waters, MD, PhD, Americana Orthopedics, Boise, ID.
The management of postoperative pain is essential to perioperative care, and adequate postoperative analgesia has been associated with several key clinical benefits, including fewer postoperative complications, earlier patient ambulation, reduced costs due to shorter hospital stays, and improved rehabilitation.1, 2 While opioids have long been central to postoperative analgesia, they have been associated with various adverse effects, including sedation, dizziness, nausea, vomiting, constipation, dependence, tolerance, and respiratory depression.2, 3 Constipation, one of the most common adverse effects resulting from opioid therapy, can be debilitating. Indeed, opioid effects on gut motility can occur even after a single dose.3 The consequences of opioid‐induced constipation (OIC) may be severe enough to warrant a dosage reduction of the opioid; however, this may lead to compromised analgesia, which can hinder recovery.4, 5 Thus, effective treatment of OIC is an important clinical consideration in patients undergoing pain management with opioids. Unfortunately, laxatives and other treatment strategies can have unpredictable or suboptimal results for many patients with OIC; therefore, other options are needed for the treatment of OIC.6, 7
Opioid receptor agonists cause constipation by adversely altering many aspects of intestinal function, including fluid dynamics, gastric emptying, propulsive motor activity, and transit time.3 Opioid receptors are widely distributed in the central nervous system and throughout the intestinal system. The mechanism of OIC may have both peripherally and centrally mediated components.8 Nonselective opioid receptor antagonists block the undesired effects on the gut, but because they cross the blood‐brain barrier, they also interfere with analgesia and may lead to symptoms of withdrawal. Methylnaltrexone is a selective, peripherally acting mu‐opioid receptor antagonist,9 formed by the addition of a methyl group to the amine ring of the mu‐opioid receptor antagonist naltrexone. The resulting quarternary amine has greater polarity, lower lipid solubility, and restricted ability to cross the blood‐brain barrier.10 Thus, methylnaltrexone was designed to decrease the peripheral adverse effects of opioids without interfering with centrally mediated analgesia.
Investigations of methylnaltrexone effects in healthy volunteers showed that methylnaltrexone attenuated morphine‐induced delays in gastric emptying and oral‐cecal transit without affecting analgesia.1113 Further studies of methylnaltrexone for the treatment of constipation due to methadone use demonstrated rapid laxation response.1416 Two randomized, double‐blind, placebo‐controlled studies of methylnaltrexone in 288 patients with advanced illness and OIC showed that methylnaltrexone rapidly induced laxation without compromising analgesia.17, 18 Methylnaltrexone is currently approved for the treatment of OIC in patients with advanced illness who are receiving palliative care, when response to laxative therapy has not been sufficient.19
Recently, the use of methylnaltrexone for the treatment of OIC in patients with chronic, nonmalignant pain was assessed in a randomized, double‐blind, placebo‐controlled trial of more than 400 patients. Investigators found that methylnaltrexone induced laxation and was generally well tolerated (Blonsky et al., 28th Annual Scientific Meeting of the American Pain Society, May 7‐9, 2009, San Diego, CA; Duerden et al., 29th Annual Scientific Meeting of the American Pain Society, May 6‐10, 2010, Baltimore, MD), supporting the safety and efficacy of methylnaltrexone in the setting of OIC resulting from chronic opioid treatment. The present study aimed to assess the activity of methylnaltrexone in patients receiving mu‐agonist opioid analgesics during rehabilitation, following an orthopedic surgical procedure, who were experiencing acute OIC.
METHODS
Patients
Patients who had undergone orthopedic procedures within 4 to 10 days were screened for eligibility. Adults aged 18 years or older were considered eligible if they were acutely constipated, were receiving mu‐agonist opioid analgesics, and were expected to require daily opioid analgesics for at least 7 days following randomization. Acute constipation was defined as having no bowel movement for at least 48 hours prior to randomization, difficulty in having a spontaneous bowel movement (straining or sensation of incomplete evacuation or hard, lumpy stools), or the inability to have a spontaneous bowel movement. Exclusion criteria included fecal impaction, mechanical bowel obstruction, constipation not attributed to postprocedure opioid use, calculated creatinine clearance less than 50 mL/min, and corrected QT interval greater than 500 msec on a 12‐lead screening electrocardiogram (ECG). Patients with a known hypersensitivity to methylnaltrexone, naltrexone, or naloxone, who were pregnant or lactating, who had a history of alcohol or drug abuse within the past 2 years, or who had a spinal cord injury or gastrointestinal ostomy were also excluded. Any laxatives, enemas, and/or promotility agents being used must have been discontinued at least 48 hours prior to first dose of study medication and were not permitted during the study, but stool softener use was permitted if it had been administered at least 24 hours prior to screening and a stable dose was maintained throughout the study.
Study Design
This randomized, double‐blind, placebo‐controlled, parallel‐group, hypothesis‐generating phase 2 study was conducted from October 2007 to January 2009 at 16 US hospitals and rehabilitation facilities in accordance with the International Conference on Harmonisation Good Clinical Practice Guidelines and the Declaration of Helsinki, and was approved by the Institutional Review Board and/or Independent Ethics Committee at each of the participating investigational centers. All patients provided written informed consent prior to study participation.
Eligible patients were randomized by interactive voice response system in a 1:1 ratio to receive once‐daily subcutaneous (SC) injections of either 12 mg methylnaltrexone or placebo (Figure 1). The chosen 12‐mg unit dosing corresponds to approximately 0.15 mg/kg (assuming an 80‐kg patient) and was found to be both efficacious and well tolerated in the treatment of OIC in prior studies, including studies in advanced‐illness patients17, 18 and in patients with chronic, nonmalignant pain (Blonsky et al., 28th Annual Scientific Meeting of the American Pain Society, May 7‐9, 2009, San Diego, CA; Duerden et al., 29th Annual Scientific Meeting of the American Pain Society, May 6‐10, 2010, Baltimore, MD.20 The first dose of study medication was administered on the day of randomization or on the next calendar day. Once enrolled, the patient received once‐daily doses of methylnaltrexone for up to 4 or 7 days. Dosing continued until the patient received the maximum number of doses allowed, no longer needed opioid medication, or was discharged from the medical facility. Each patient completed a follow‐up safety visit at 14 3 days following the last dose.

Evaluations
All efficacy variables were considered exploratory and included the occurrence of laxation within 2 and 4 hours of the first dose of study drug, time to laxation, and a questionnaire assessing patient global satisfaction. Patients recorded the date, time, and assessment of each bowel movement in diaries.
Safety variables included adverse events (AEs), serious AEs (SAEs), clinical laboratory parameters, physical examinations, vital signs, ECGs, concomitant medications, Objective and Subjective Opioid Withdrawal Scales (OOWS and SOWS),21 and Numeric Rating Scales for Pain ([NRSP] 0 = no pain, 10 = worst pain possible).
Statistical Analysis
Enrolled patients were defined as all patients who consented to participate in the study. Both the modified intent‐to‐treat (mITT) population and the safety population were defined as all patients who were randomized and received at least 1 injection of study drug. All study results are based on the mITT population.
Categorical variables were summarized using frequency and percentage, while descriptive statistics for continuous variables included sample size, mean, median, standard deviation, and minimum and maximum values. All inferential statistical tests were 2‐tailed and used a tolerance for nominal type I error (alpha, ) of 0.05. There was no correction for multiplicity and no imputations were performed to account for missing data.
Fisher's exact test was used for comparisons between the proportion of patients with laxation within 2 hours and 4 hours of the first dose in the methylnaltrexone group versus the placebo group. The time to first laxation analysis was performed using the log‐rank test and Kaplan‐Meier method.
RESULTS
Patient Populations
The flow of patients through the study is summarized in Figure 2. A total of 51 patients were enrolled. Of these, 33 received at least 1 dose of study treatment following double‐blind randomization and comprised both the mITT and safety populations. Seventeen of these patients were enrolled under the original protocol and could receive study drug for up to 7 days, while 16 patients enrolled under a subsequent protocol revision could receive study drug for up to 4 days. This change from a 7‐day to a 4‐day treatment protocol allowed for the capture of more study patients in view of the time pressures of short lengths of stay in postoperative settings. In total, 31 patients received at least 2 doses, and 26 patients received at least 4 doses of study drug. A total of 27 patients completed the study. Baseline demographics and prestudy surgical procedures were similar in both treatment groups (Table 1).

| Characteristic | Methylnaltrexone (n = 18) | Placebo (n = 15) |
|---|---|---|
| ||
| Mean age, yr (SD) | 64.2 (9.0) | 65.2 (11.6) |
| Mean weight, kg (SD) | 92.5 (22.5) | 91.0 (20.2) |
| Mean BMI, kg/m2 (SD) | 32.3 (7.2) | 34.2 (6.41) |
| Sex, n (%) | ||
| Female | 11 (61.1) | 11 (73.3) |
| Male | 7 (38.9) | 4 (26.7) |
| Race, n (%) | ||
| White | 14 (77.8) | 10 (66.7) |
| Black | 4 (22.2) | 5 (33.3) |
| Type of surgery, n (%) | ||
| Total knee replacement | 8 (44.4) | 7 (46.7) |
| Total hip replacement | 6 (33.3) | 6 (40.0) |
| Spinal fusion | 2 (11.1) | 0 |
| Fracture reduction | 2 (11.1) | 2 (13.3) |
| Median opioid use,* mg (range) | 28.00 (6.75‐168.01) | 25.00 (9.00‐75.00) |
| Median time from surgery to study drug administration, days (range) | 4 (3‐6) | 4 (3‐6) |
Efficacy
A significantly greater percentage of patients had a bowel movement within 2 hours (P = 0.021) and 4 hours (P = 0.046) of the first dose of methylnaltrexone compared with patients who received placebo (Figure 3). Within 2 hours, 6 patients (33.3%; 95% confidence interval [CI], 13.34‐59.01) who received methylnaltrexone achieved laxation, while laxation did not occur in any patient who received placebo. By 4 hours posttreatment, 7 patients (38.9%; 95% CI, 17.30‐64.25) in the methylnaltrexone group achieved laxation compared with only 1 patient (6.7%; 95% CI, 0.17‐31.95) on placebo. Three patients in each treatment group received rescue laxatives.

The time to first laxation (Figure 4) was significantly shorter in patients who received methylnaltrexone compared with those in the placebo group. Patients on methylnaltrexone achieved laxation in a median time of 15.8 hours, compared with a median time of 50.9 hours for patients in the placebo group (P = 0.02, log‐rank test). The median time to laxation was less than 1 hour in the 7 methylnaltrexone‐treated patients who experienced laxation within 4 hours following the first dose. Of the remaining 11 methylnaltrexone‐treated patients, one experienced no laxation after 6 doses, and the median time to laxation for the others was 29.9 hours (not shown in figure).

Analysis of the Global Satisfaction With Treatment Scale revealed that more patients expressed overall treatment satisfaction (defined as very satisfied, satisfied, or minimally satisfied) with methylnaltrexone assessed 4 hours ( 30 minutes) after the first dose, compared with patients on placebo (83.3% vs 60.0%, respectively). At the study endpoint, overall treatment satisfaction with methylnaltrexone remained high (83.3%), whereas satisfaction with placebo was 53.3%. Additionally, no patients in the methylnaltrexone group expressed any dissatisfaction with treatment (defined as minimally dissatisfied, dissatisfied, or very dissatisfied) at endpoint, compared with 26.7% of patients in the placebo group who expressed some degree of dissatisfaction.
Safety
Overall AE rates were similar between treatment groups (Table 2), with at least 1 treatment‐emergent AE reported in 6 patients (33.3%) in the methylnaltrexone group and 4 patients (26.7%) in the placebo group. The most common AEs reported during the study were classified as gastrointestinal in nature; 3 (nausea, abdominal pain, and diarrhea) were considered by the investigator to be possibly related to study medication. Two patients receiving methylnaltrexone discontinued the study because of AEs (one with moderate constipation, one with mild diarrhea) compared with none of the placebo group patients. No treatment‐emergent SAEs or deaths were reported during this study. Analysis of clinical laboratory parameters, vital signs, and ECGs revealed no safety signals and showed no pattern of concern related to methylnaltrexone exposure.
| Adverse Event* | Methylnaltrexone 12 mg (n = 18) n (%) | Placebo (n = 15) n (%) |
|---|---|---|
| ||
| Any | 6 (33.3) | 4 (26.7) |
| Anemia | 1 (5.6) | 0 |
| Gastrointestinal disorders | 3 (16.7) | 1 (6.7) |
| Abdominal discomfort | 0 | 1 (6.7) |
| Abdominal distension | 1 (5.6) | 0 |
| Abdominal pain | 1 (5.6) | 0 |
| Abdominal tenderness | 1 (5.6) | 0 |
| Constipation | 1 (5.6) | 0 |
| Diarrhea | 1 (5.6) | 0 |
| Nausea | 1 (5.6) | 0 |
| Headache | 1 (5.6) | 0 |
| Hypotension | 1 (5.6) | 0 |
| Joint swelling | 0 | 1 (6.7) |
| Peripheral edema | 0 | 2 (13.3) |
| Procedural pain | 0 | 1 (6.7) |
| Skin ulcer | 0 | 1 (6.7) |
| Somnolence | 0 | 1 (6.7) |
| Urinary tract infection | 1 (5.6) | 0 |
| Wound infection | 1 (5.6) | 0 |
Pain and Opioid Withdrawal
Results from the SOWS and OOWS measures indicated that signs and symptoms of withdrawal did not increase over time in patients treated with methylnaltrexone, and no discernable differences were found between study groups. Pain was assessed using a numeric rating scale ranging from 0 to 10, with higher scores indicating greater severity. Baseline pain scores were not significantly different between treatment groups, with a mean of 5.7 2.7 for placebo, and 5.4 3.0 for the methylnaltrexone group. At 1 day postdose, mean pain scores did not increase from baseline in the placebo (0.9 2.33) or methylnaltrexone group (0.5 2.5), and no significant between‐group differences were found. Similar results were observed at the end of the study. Thus, pain did not appear to increase in patients treated with methylnaltrexone, and changes in pain scores were indistinguishable between the 2 treatment groups.
DISCUSSION
This pilot study suggests that methylnaltrexone actively induces laxation and is generally well tolerated in patients receiving mu‐opioid analgesia, following orthopedic surgery, who develop OIC acutely. It was the first study, to our knowledge, to investigate the efficacy of methylnaltrexone for the treatment of OIC in an acute postoperative setting. The protocol amendment changing the duration of treatment from 7 days to 4 days did not materially affect the results of the study. The response to methylnaltrexone was rapid, with 33.3% experiencing laxation within 2 hours. The median time to laxation was nearly 1.5 days shorter in patients treated with methylnaltrexone compared with those receiving placebo. Correspondingly, overall patient satisfaction was high in the methylnaltrexone group. Efficacy was attained without diminishing opioid analgesia, and without inducing signs or symptoms of opioid withdrawal. The incidence of AEs was similar between groups, and no treatment‐emergent SAEs were reported in this study.
Previous clinical trials investigated the safety and efficacy of methylnaltrexone for the treatment of OIC in patients with advanced illness and with chronic, nonmalignant pain. The present study extends those findings to a population of patients experiencing acute OIC following orthopedic surgery. Previous studies showed that approximately 48% to 62% of advanced‐illness patients experienced laxation within 4 hours of receiving SC methylnaltrexone,17, 18 compared with 38.9% of acute OIC patients in this study. In a clinical trial of patients with chronic, nonmalignant pain, 34.2% of patients experienced laxation within 4 hours of SC methylnaltrexone injection (Blonsky et al., 28th Annual Scientific Meeting of the American Pain Society, May 7‐9, 2009, San Diego, CA). The differences in laxation response between these trials may be attributable to differences in the patient populations or to methodologic differences between the studies.
Similar to findings demonstrated in a clinical study evaluating methylnaltrexone for OIC in a different patient population, those with advanced illness,22 this study supports the premise that future laxation response with prolonged use is most likely to occur when a laxation response was achieved after the first or second initial administrations of methylnaltrexone. In contradistinction, if laxation does not occur with these early doses, continued methylnaltrexone dosing is less likely to produce a response later.
This study has some limitations that must be considered. First, as this was a hypothesis‐generating study, all efficacy parameters investigated were exploratory in nature. The results reported herein warrant careful consideration, owing to a small sample size that may limit their generalizability, prior to replication in a more rigorously designed study with prespecified efficacy endpoints. Likewise, the assessment of health outcome parameters is limited. Another limitation is the small sample size utilized in this study, potentially resulting in a type II error.
Subcutaneous administration potentially offers a considerable benefit over oral therapies for OIC in this patient population post‐orthopedic surgery. Nausea and vomiting can occur as a consequence of anesthesia and of postoperative opioid analgesia, and may compromise adequate dosing of oral medications prescribed to treat OIC. Subcutaneous delivery of methylnaltrexone may circumvent this potential drawback while providing potentially rapid, effective treatment for OIC. Once‐daily dosing may also help to minimize caregiver burden and patient discomfort by preventing the need for more frequent or unpleasant treatments for OIC, such as enemas.
This study provides an initial positive signal for a broader, albeit off‐label use for methylnaltrexonethat being for the treatment of acute constipation that occurs as a consequence of postoperative opioid‐mediated analgesia in patients following orthopedic procedures. Adequate treatment of OIC, even in the acute postoperative setting, is likely to lead to better overall pain management and improved patient outcomes. Additionally, effective management of acute OIC is likely to be cost‐effective in terms of reducing the duration of hospital stays, reducing the need for nursing resources and the time spent administering rescue treatments for OIC (eg, enemas), and avoiding returns to an acute setting (eg, the emergency department) for treatment. The results presented herein suggest that methylnaltrexone may be effective and have a good safety profile in the treatment of acute OIC following orthopedic surgery. Validation of these results in larger well‐controlled trials would be welcome.
Acknowledgements
The authors thank the patients and clinical personnel involved in this study; John Charity, NP, for data collection and management, and John H. Simmons, MD, of Peloton Advantage, LLC, for assistance with manuscript preparation, which was funded by Pfizer Inc.
In addition to the authors, the following investigators participated in this trial: David Nathan Feldman, MD, Holy Name Hospital, Teaneck, NJ; Sam Hakki, MD, Bay Pines VA Healthcare System, Bay Pines, FL; Forrest A. Hanke, MD, Trover Health System, Madisonville, KY; William H. Horton, Jr, MD, Palmetto Clinical Research, Greenville, SC; M. Jay Jazayeri, MD, Pacific Hospital of Long Beach, Long Beach, CA; John F. Peppin, DO, The Pain Treatment Center of the Bluegrass, Lexington, KY; Bruce Pomeranz, MD, Kessler Institute for Rehabilitation, Saddle Brook, NJ, and Chester, NJ; Alan C. Schwartz, MD, Helping Hands Medical Associates, Santa Ana, CA; Michael J. Skyhar, MD, CORE Orthopaedic Medical Center, Encinitas, CA; Lex A. Simpson, MD, CORE Orthopaedic Medical Center, Encinitas, CA; James Slover, MD, New York University Hospital for Joint Disease, New York, NY; Dilip Tapadiya, MD, Fountain Valley Regional Hospital, Fountain Valley, CA; Stanley J. Waters, MD, PhD, Americana Orthopedics, Boise, ID.
- ,.Postoperative pain management.Chest Surg Clin N Am.1997;7:773–799.
- ,.Strategies for effective postoperative pain management.Minerva Anestesiol.2006;72:145–150.
- ,,.Are peripheral opioid antagonists the solution to opioid side effects?Anesth Analg.2004;98:116–122.
- ,.Neuroplasticity—an important factor in acute and chronic pain.Swiss Med Wkly.2002;132:273–278.
- ,,,,.The burden of acute postoperative pain and the potential role of the COX‐2‐specific inhibitors.Rheumatology (Oxford).2003;42(suppl 3):iii40–iii52.
- .Incidence, prevalence, and management of opioid bowel dysfunction.Am J Surg.2001;182(suppl 5A):11S–18S.
- ,.Management of common opioid‐induced adverse effects.Am Fam Physician.2006;74:1347–1354.
- ,.Antagonism of gastrointestinal opioid effects.Reg Anesth Pain Med.2000;25:639–642.
- .Methylnaltrexone mechanisms of action and effects on opioid bowel dysfunction and other opioid adverse effects.Ann Pharmacother.2007;41:984–993.
- ,.Methylnaltrexone: investigation of clinical applications.Drug Dev Res.2000;50:133–141.
- ,,,.Opioid‐induced delay in gastric emptying: a peripheral mechanism in humans.Anesthesiology.1997;87:765–770.
- ,,, et al.Effects of enteric‐coated methylnaltrexone in preventing opioid‐induced delay in oral‐cecal transit time.Clin Pharmacol Ther.2000;67:398–404.
- ,,,,,.Methylnaltrexone prevents morphine‐induced delay in oral‐cecal transit time without affecting analgesia: a double‐blind randomized placebo‐controlled trial.Clin Pharmacol Ther.1996;59:469–475.
- ,,,,,.Effects of intravenous methylnaltrexone on opioid‐induced gut motility and transit time changes in subjects receiving chronic methadone therapy: a pilot study.Pain.1999;83:631–635.
- ,,, et al.Methylnaltrexone for reversal of constipation due to chronic methadone use: a randomized controlled trial.JAMA.2000;283:367–372.
- ,.Oral methylnaltrexone for opioid‐induced constipation.JAMA.2000;284:1383–1384.
- ,,, et al.Methylnaltrexone for opioid‐induced constipation in advanced illness.N Engl J Med.2008;328:2332–2343.
- ,,, et al.Methylnaltrexone for treatment of opioid‐induced constipation in advanced illness patients.J Support Oncol.2009;7:39–46.
- Relistor [package insert].Philadelphia, PA, and Tarrytown, NY:Wyeth Pharmaceuticals Inc and Progenics Pharmaceuticals;2009.
- ,,, et al.Subcutaneous methylnaltrexone for treatment of opioid‐induced constipation in patients with chronic, nonmalignant pain: a randomized controlled study.J Pain.2011;12:554–562.
- ,,,,,.Two new rating scales for opiate withdrawal.Am J Drug Alcohol Abuse.1987;13:293–308.
- ,,, et al.Methylnaltrexone treatment of opioid‐induced constipation in patients with advanced illness.J Pain Symptom Manage.2009;38:683–690.
- ,.Postoperative pain management.Chest Surg Clin N Am.1997;7:773–799.
- ,.Strategies for effective postoperative pain management.Minerva Anestesiol.2006;72:145–150.
- ,,.Are peripheral opioid antagonists the solution to opioid side effects?Anesth Analg.2004;98:116–122.
- ,.Neuroplasticity—an important factor in acute and chronic pain.Swiss Med Wkly.2002;132:273–278.
- ,,,,.The burden of acute postoperative pain and the potential role of the COX‐2‐specific inhibitors.Rheumatology (Oxford).2003;42(suppl 3):iii40–iii52.
- .Incidence, prevalence, and management of opioid bowel dysfunction.Am J Surg.2001;182(suppl 5A):11S–18S.
- ,.Management of common opioid‐induced adverse effects.Am Fam Physician.2006;74:1347–1354.
- ,.Antagonism of gastrointestinal opioid effects.Reg Anesth Pain Med.2000;25:639–642.
- .Methylnaltrexone mechanisms of action and effects on opioid bowel dysfunction and other opioid adverse effects.Ann Pharmacother.2007;41:984–993.
- ,.Methylnaltrexone: investigation of clinical applications.Drug Dev Res.2000;50:133–141.
- ,,,.Opioid‐induced delay in gastric emptying: a peripheral mechanism in humans.Anesthesiology.1997;87:765–770.
- ,,, et al.Effects of enteric‐coated methylnaltrexone in preventing opioid‐induced delay in oral‐cecal transit time.Clin Pharmacol Ther.2000;67:398–404.
- ,,,,,.Methylnaltrexone prevents morphine‐induced delay in oral‐cecal transit time without affecting analgesia: a double‐blind randomized placebo‐controlled trial.Clin Pharmacol Ther.1996;59:469–475.
- ,,,,,.Effects of intravenous methylnaltrexone on opioid‐induced gut motility and transit time changes in subjects receiving chronic methadone therapy: a pilot study.Pain.1999;83:631–635.
- ,,, et al.Methylnaltrexone for reversal of constipation due to chronic methadone use: a randomized controlled trial.JAMA.2000;283:367–372.
- ,.Oral methylnaltrexone for opioid‐induced constipation.JAMA.2000;284:1383–1384.
- ,,, et al.Methylnaltrexone for opioid‐induced constipation in advanced illness.N Engl J Med.2008;328:2332–2343.
- ,,, et al.Methylnaltrexone for treatment of opioid‐induced constipation in advanced illness patients.J Support Oncol.2009;7:39–46.
- Relistor [package insert].Philadelphia, PA, and Tarrytown, NY:Wyeth Pharmaceuticals Inc and Progenics Pharmaceuticals;2009.
- ,,, et al.Subcutaneous methylnaltrexone for treatment of opioid‐induced constipation in patients with chronic, nonmalignant pain: a randomized controlled study.J Pain.2011;12:554–562.
- ,,,,,.Two new rating scales for opiate withdrawal.Am J Drug Alcohol Abuse.1987;13:293–308.
- ,,, et al.Methylnaltrexone treatment of opioid‐induced constipation in patients with advanced illness.J Pain Symptom Manage.2009;38:683–690.
Copyright © 2011 Society of Hospital Medicine