User login
Radial Shaft Stress Fracture in a Major League Pitcher
Take-Home Points
- Stress fractures should always be considered when dealing with overuse injuries.
- Radial shaft stress fractures in overhead throwing athletes are rare.
- Stress fractures can occur anywhere increased muscular forces exceed the bone’s ability to remodel.
- Proper imaging is necessary to make the diagnosis of a stress fracture.
- Nonoperative management of radial shaft stress fractures is an effective treatment.
In athletes, the incidence of stress fractures has been reported to be 1.4% to 4.4%.1 Stress fractures of the upper extremity are less common and not as well described as lower extremity stress fractures. Although data is lacking, stress fractures involving the upper extremity appear to account for <6% of all stress fractures.2 Stress fractures of the upper extremity, though rare, are being recognized more often in overhead athletes.3-6 In baseball pitchers, stress fractures most commonly occur in the olecranon but have also been found in the ribs, clavicle, humerus, and ulnar shaft.2,4,7-10 Stress fractures of the radius are a rare cause of forearm pain in athletes, and there are only a few case reports involving overhead athletes.4,11-15 To our knowledge, a stress fracture of the radial shaft has not been reported in a throwing athlete. Currently, there are no reports on stress fractures of the proximal radial shaft.16-18
In this article, we report the case of a radial shaft stress fracture that was causing forearm pain in a Major League Baseball (MLB) pitcher. We also discuss the etiology, diagnosis, and management of stress fractures of the upper extremity of overhead throwing athletes. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 28-year-old right-hand-dominant MLB pitcher presented to the clinic with a 4-week history of right dorsal forearm pain that was refractory to a period of rest and physical therapy modalities. The pain radiated to the wrist and along the dorsal forearm. The pain started after the man attempted to develop a new pitch that required a significant amount of supination. The pain prevented him from pitching competitively. Indomethacin, diclofenac sodium topical gel, and methylprednisolone (Medrol Dosepak) reduced his symptoms only slightly.
Physical examination of the right elbow showed mild range of motion deficits; about 5° of extension and 5° of flexion were lacking. The patient had full pronation and supination. Palpation of the dorsal aspect of the forearm revealed marked tenderness in the area of the proximal radius. There was no tenderness over the posterior olecranon or the ulnar collateral ligament, and a moving valgus stress test was negative. No pain was elicited by resisted extension of the wrist or fingers. Motor innervation from the posterior interosseous nerve, anterior interosseous nerve, and ulnar nerve was intact with 5/5 strength, and there were no sensory deficits in the distribution of the radial, median, or ulnar nerves.
Discussion
Stress fractures account for 0.7% to 20% of sports medicine clinic injuries; <10% of all stress fractures involve the rib or upper extremity.4,6 When the intensity or frequency of physical activity is increased, as with overuse, bone resorption surpasses bone production, locally weakening the bone and making it prone to mechanical failure. Failure is thought to be induced by a combination of contractile muscular forces across damaged bone and increased mechanical loading caused by fatigue of supporting structures.5,6,19 These forces may have contributed to our baseball pitcher’s development of a stress fracture near the insertion of the supinator muscle in his throwing arm.
Given the insidious nature of stress fractures, the evaluating physician must have a high index of suspicion. Early recognition of a stress fracture is important in preventing further injury and allowing for early intervention, which is associated with faster healing.6,20 The clinical history often involves a change in training regimen within the weeks before pain onset. Furthermore, understanding the type of pitches used and the mechanics of each pitch can help with diagnosis. Often, pain increases as the inciting activity continues, and relief comes with rest. In an upper extremity examination, it is important to recall the usual stress fracture locations in throwers—the ribs, clavicle, humerus, ulnar shaft, and most often the olecranon—though the patient’s history often narrows the anatomical region of suspicion.2,4,7-10 Examination begins with inspection of the skin and soft tissues. Range of motion and strength testing results likely are normal throughout the upper extremity.3 Palpation over the suspected injury location often elicits pain and indicates further imaging is needed.6 The tuning fork test or the 3-point fulcrum test may elicit symptoms in occult fractures.3 Completing the assessment is a thorough neurovascular examination.
Insidious forearm pain requires a broad differential, including flexor-pronator mass or distal biceps injury, chronic exertional compartment syndrome, radial tunnel syndrome, intersection syndrome, pronator teres syndrome, anterior interosseous syndrome, thoracic outlet syndrome, musculocutaneous nerve compression, deep vein thrombosis of ulnar vein, and periostitis. Stress fractures distal to the elbow more commonly occur in weight-bearing athletes, though as this case shows it is important to consider stress fractures of the radius and ulna when evaluating forearm pain in a throwing athlete.21
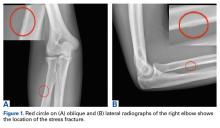
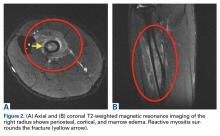
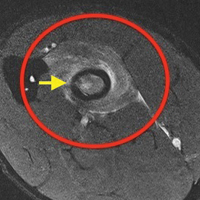
The first imaging examination for a suspected stress fracture is a radiograph, which can be normal in up to 90% of patients, as it initially was in our athlete’s case.22 Often, radiographic evidence takes 2 to 12 weeks to appear.5 Even then, radiographs may be positive in only 50% of cases.19 CT, often regarded as insensitive during the early stages, is useful in visualizing fracture lines in a suspicious location.19,22 Radionuclide uptake scanning is highly sensitive during the early stages of stress injury but is nonspecific and may indicate neoplasm or infection; in addition, up to 46% of abnormal foci are asymptomatic.19 MRI has sensitivity comparable to that of radionuclide scanning but also many advantages, including lack of ionizing radiation, improved spatial resolution, and ability to image bone and soft tissue simultaneously.19 In our patient’s case, the unusual stress fracture location potentially could have hindered identification of the cause of injury. The lesion was just distal to the field of view of a normal elbow MRI and was not detected until a dedicated forearm MRI was examined. Both MRI and CT helped in identifying the stress fracture, and CT was used to follow interval healing.
In baseball players, upper extremity stress fractures are often nonoperatively treated with throwing cessation for 4 to 6 weeks followed by participation in a structured rehabilitation program.4,5 The throwing program that we suggest, and that was used in this case, has 21 stages of progression in duration, distance, and velocity of throwing. The athlete advances from each stage on the basis of symptoms.23 Other issues that may be addressed are vitamin D and calcium status and any flawed throwing mechanics that may have predisposed the athlete to injury. Such mechanics are gradually corrected.
The literature suggests that appropriate nonoperative management of stress fractures allows for return to sport in 8 to 10 weeks. It is important to note that most of the literature on stress fractures involves the lower extremity, and that treatment and time to return to play are therefore better described for such fractures.6 More study and evaluation of upper extremity stress fractures are needed to make return-to-sport predictions more reliable and successful treatment modalities more unified for this patient population. Last, it is imperative that clinical examination and symptoms be correlated with serial imaging when deciding on return to play. Our patient took 12 weeks to return to high-level sport. He progressed pain-free through the throwing program and showed radiographic evidence of healing on follow-up CT.
Conclusion
Radial shaft stress fractures are rare in throwing athletes. However, with a thorough history, a physical examination, and appropriate imaging, the correct diagnosis can be made early on, and proper treatment can be started to facilitate return to sport. To our knowledge, this is the first report of a stress fracture in the radial shaft of a MLB pitcher. Although the radial shaft is an uncommon location for stress fractures, we should keep in mind that they can occur wherever increased muscular forces exceed the ability of native bone to remodel. After diagnosis, the fracture usually heals with nonoperative treatment, and healing is confirmed with follow-up imaging, as was done in our patient’s case. Improved prediction of time to return to play for upper extremity fractures, such as the radial stress fracture described in this article, requires more study.
1. Monteleone GP Jr. Stress fractures in the athlete. Orthop Clin North Am. 1995;26(3):423-432.
2. Iwamoto J, Takeda T. Stress fractures in athletes: review of 196 cases. J Orthop Sci. 2003;8(3):273-278.
3. Miller TL, Kaeding CC. Upper-extremity stress fractures: distribution and causative activities in 70 patients. Orthopedics. 2012;35(9):789-793.
4. Jones GL. Upper extremity stress fractures. Clin Sports Med. 2006;25(1):159-174.
5. Brooks AA. Stress fractures of the upper extremity. Clin Sports Med. 2001;20(3):613-620.
6. Fredericson M, Jennings F, Beaulieu C, Matheson GO. Stress fractures in athletes. Top Magn Reson Imaging. 2006;17(5):309-325.
7. Gurtler R, Pavlov H, Torg JS. Stress fracture of the ipsilateral first rib in a pitcher. Am J Sports Med. 1985;13(4):277-279.
8. Polu KR, Schenck RC Jr, Wirth MA, Greeson J, Cone RO 3rd, Rockwood CA Jr. Stress fracture of the humerus in a collegiate baseball pitcher. A case report. Am J Sports Med. 1999;27(6):813-816.
9. Wu C, Chen Y. Stress fracture of the clavicle in a professional baseball player. J Shoulder Elbow Surg. 1998;7(2):164-167.
10. Schickendantz MS, Ho CP, Koh J. Stress injury of the proximal ulna in professional baseball players. Am J Sports Med. 2002;30(5):737-741.
11. Loosli AR, Leslie M. Stress fractures of the distal radius. A case report. Am J Sports Med. 1991;19(5):523-524.
12. Inagaki H, Inoue G. Stress fracture of the scaphoid combined with the distal radial epiphysiolysis. Br J Sports Med. 1997;31(3):256-257.
13. Read MT. Stress fractures of the distal radius in adolescent gymnasts. Br J Sports Med. 1981;15(4):272-276.
14. Orloff AS, Resnick D. Fatigue fracture of the distal part of the radius in a pool player. Injury. 1986;17(6):418-419.
15. Eisenberg D, Kirchner SG, Green NE. Stress fracture of the distal radius caused by “wheelies.” South Med J. 1986;79(7):918-919.
16. Brukner P. Stress fractures of the upper limb. Sports Med. 1998;26(6):415-424.
17. Farquharson-Roberts MA, Fulford PC. Stress fracture of the radius. J Bone Joint Surg Br. 1980;62(2):194-195.
18. Orloff AS, Resnick D. Fatigue fracture of the distal part of the radius in a pool player. Injury. 1986;17(6):418-419.
19. Anderson MW. Imaging of upper extremity stress fractures in the athlete. Clin Sports Med. 2006;25(3):489-504.
20. Bennell K, Brukner P. Preventing and managing stress fractures in athletes. Phys Ther Sport. 2005;6(4):171-180.
21. Sinha AK, Kaeding CC, Wadley GM. Upper extremity stress fractures in athletes: clinical features of 44 cases. Clin J Sport Med. 1999;9(4):199-202.
22. Matheson GO, Clement DB, McKenzie DC, Taunton JE, Lloyd-Smith DR, MacIntyre JG. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15(1):46-58.
23. Kaplan L, Lesniak B, Baraga M, et al. Throwing program for baseball players. 2009. http://uhealthsportsmedicine.com/documents/UHealth_Throwing_Program.pdf. Accessed May 24, 2016.
Take-Home Points
- Stress fractures should always be considered when dealing with overuse injuries.
- Radial shaft stress fractures in overhead throwing athletes are rare.
- Stress fractures can occur anywhere increased muscular forces exceed the bone’s ability to remodel.
- Proper imaging is necessary to make the diagnosis of a stress fracture.
- Nonoperative management of radial shaft stress fractures is an effective treatment.
In athletes, the incidence of stress fractures has been reported to be 1.4% to 4.4%.1 Stress fractures of the upper extremity are less common and not as well described as lower extremity stress fractures. Although data is lacking, stress fractures involving the upper extremity appear to account for <6% of all stress fractures.2 Stress fractures of the upper extremity, though rare, are being recognized more often in overhead athletes.3-6 In baseball pitchers, stress fractures most commonly occur in the olecranon but have also been found in the ribs, clavicle, humerus, and ulnar shaft.2,4,7-10 Stress fractures of the radius are a rare cause of forearm pain in athletes, and there are only a few case reports involving overhead athletes.4,11-15 To our knowledge, a stress fracture of the radial shaft has not been reported in a throwing athlete. Currently, there are no reports on stress fractures of the proximal radial shaft.16-18
In this article, we report the case of a radial shaft stress fracture that was causing forearm pain in a Major League Baseball (MLB) pitcher. We also discuss the etiology, diagnosis, and management of stress fractures of the upper extremity of overhead throwing athletes. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 28-year-old right-hand-dominant MLB pitcher presented to the clinic with a 4-week history of right dorsal forearm pain that was refractory to a period of rest and physical therapy modalities. The pain radiated to the wrist and along the dorsal forearm. The pain started after the man attempted to develop a new pitch that required a significant amount of supination. The pain prevented him from pitching competitively. Indomethacin, diclofenac sodium topical gel, and methylprednisolone (Medrol Dosepak) reduced his symptoms only slightly.
Physical examination of the right elbow showed mild range of motion deficits; about 5° of extension and 5° of flexion were lacking. The patient had full pronation and supination. Palpation of the dorsal aspect of the forearm revealed marked tenderness in the area of the proximal radius. There was no tenderness over the posterior olecranon or the ulnar collateral ligament, and a moving valgus stress test was negative. No pain was elicited by resisted extension of the wrist or fingers. Motor innervation from the posterior interosseous nerve, anterior interosseous nerve, and ulnar nerve was intact with 5/5 strength, and there were no sensory deficits in the distribution of the radial, median, or ulnar nerves.
Discussion
Stress fractures account for 0.7% to 20% of sports medicine clinic injuries; <10% of all stress fractures involve the rib or upper extremity.4,6 When the intensity or frequency of physical activity is increased, as with overuse, bone resorption surpasses bone production, locally weakening the bone and making it prone to mechanical failure. Failure is thought to be induced by a combination of contractile muscular forces across damaged bone and increased mechanical loading caused by fatigue of supporting structures.5,6,19 These forces may have contributed to our baseball pitcher’s development of a stress fracture near the insertion of the supinator muscle in his throwing arm.
Given the insidious nature of stress fractures, the evaluating physician must have a high index of suspicion. Early recognition of a stress fracture is important in preventing further injury and allowing for early intervention, which is associated with faster healing.6,20 The clinical history often involves a change in training regimen within the weeks before pain onset. Furthermore, understanding the type of pitches used and the mechanics of each pitch can help with diagnosis. Often, pain increases as the inciting activity continues, and relief comes with rest. In an upper extremity examination, it is important to recall the usual stress fracture locations in throwers—the ribs, clavicle, humerus, ulnar shaft, and most often the olecranon—though the patient’s history often narrows the anatomical region of suspicion.2,4,7-10 Examination begins with inspection of the skin and soft tissues. Range of motion and strength testing results likely are normal throughout the upper extremity.3 Palpation over the suspected injury location often elicits pain and indicates further imaging is needed.6 The tuning fork test or the 3-point fulcrum test may elicit symptoms in occult fractures.3 Completing the assessment is a thorough neurovascular examination.
Insidious forearm pain requires a broad differential, including flexor-pronator mass or distal biceps injury, chronic exertional compartment syndrome, radial tunnel syndrome, intersection syndrome, pronator teres syndrome, anterior interosseous syndrome, thoracic outlet syndrome, musculocutaneous nerve compression, deep vein thrombosis of ulnar vein, and periostitis. Stress fractures distal to the elbow more commonly occur in weight-bearing athletes, though as this case shows it is important to consider stress fractures of the radius and ulna when evaluating forearm pain in a throwing athlete.21
The first imaging examination for a suspected stress fracture is a radiograph, which can be normal in up to 90% of patients, as it initially was in our athlete’s case.22 Often, radiographic evidence takes 2 to 12 weeks to appear.5 Even then, radiographs may be positive in only 50% of cases.19 CT, often regarded as insensitive during the early stages, is useful in visualizing fracture lines in a suspicious location.19,22 Radionuclide uptake scanning is highly sensitive during the early stages of stress injury but is nonspecific and may indicate neoplasm or infection; in addition, up to 46% of abnormal foci are asymptomatic.19 MRI has sensitivity comparable to that of radionuclide scanning but also many advantages, including lack of ionizing radiation, improved spatial resolution, and ability to image bone and soft tissue simultaneously.19 In our patient’s case, the unusual stress fracture location potentially could have hindered identification of the cause of injury. The lesion was just distal to the field of view of a normal elbow MRI and was not detected until a dedicated forearm MRI was examined. Both MRI and CT helped in identifying the stress fracture, and CT was used to follow interval healing.
In baseball players, upper extremity stress fractures are often nonoperatively treated with throwing cessation for 4 to 6 weeks followed by participation in a structured rehabilitation program.4,5 The throwing program that we suggest, and that was used in this case, has 21 stages of progression in duration, distance, and velocity of throwing. The athlete advances from each stage on the basis of symptoms.23 Other issues that may be addressed are vitamin D and calcium status and any flawed throwing mechanics that may have predisposed the athlete to injury. Such mechanics are gradually corrected.
The literature suggests that appropriate nonoperative management of stress fractures allows for return to sport in 8 to 10 weeks. It is important to note that most of the literature on stress fractures involves the lower extremity, and that treatment and time to return to play are therefore better described for such fractures.6 More study and evaluation of upper extremity stress fractures are needed to make return-to-sport predictions more reliable and successful treatment modalities more unified for this patient population. Last, it is imperative that clinical examination and symptoms be correlated with serial imaging when deciding on return to play. Our patient took 12 weeks to return to high-level sport. He progressed pain-free through the throwing program and showed radiographic evidence of healing on follow-up CT.
Conclusion
Radial shaft stress fractures are rare in throwing athletes. However, with a thorough history, a physical examination, and appropriate imaging, the correct diagnosis can be made early on, and proper treatment can be started to facilitate return to sport. To our knowledge, this is the first report of a stress fracture in the radial shaft of a MLB pitcher. Although the radial shaft is an uncommon location for stress fractures, we should keep in mind that they can occur wherever increased muscular forces exceed the ability of native bone to remodel. After diagnosis, the fracture usually heals with nonoperative treatment, and healing is confirmed with follow-up imaging, as was done in our patient’s case. Improved prediction of time to return to play for upper extremity fractures, such as the radial stress fracture described in this article, requires more study.
Take-Home Points
- Stress fractures should always be considered when dealing with overuse injuries.
- Radial shaft stress fractures in overhead throwing athletes are rare.
- Stress fractures can occur anywhere increased muscular forces exceed the bone’s ability to remodel.
- Proper imaging is necessary to make the diagnosis of a stress fracture.
- Nonoperative management of radial shaft stress fractures is an effective treatment.
In athletes, the incidence of stress fractures has been reported to be 1.4% to 4.4%.1 Stress fractures of the upper extremity are less common and not as well described as lower extremity stress fractures. Although data is lacking, stress fractures involving the upper extremity appear to account for <6% of all stress fractures.2 Stress fractures of the upper extremity, though rare, are being recognized more often in overhead athletes.3-6 In baseball pitchers, stress fractures most commonly occur in the olecranon but have also been found in the ribs, clavicle, humerus, and ulnar shaft.2,4,7-10 Stress fractures of the radius are a rare cause of forearm pain in athletes, and there are only a few case reports involving overhead athletes.4,11-15 To our knowledge, a stress fracture of the radial shaft has not been reported in a throwing athlete. Currently, there are no reports on stress fractures of the proximal radial shaft.16-18
In this article, we report the case of a radial shaft stress fracture that was causing forearm pain in a Major League Baseball (MLB) pitcher. We also discuss the etiology, diagnosis, and management of stress fractures of the upper extremity of overhead throwing athletes. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 28-year-old right-hand-dominant MLB pitcher presented to the clinic with a 4-week history of right dorsal forearm pain that was refractory to a period of rest and physical therapy modalities. The pain radiated to the wrist and along the dorsal forearm. The pain started after the man attempted to develop a new pitch that required a significant amount of supination. The pain prevented him from pitching competitively. Indomethacin, diclofenac sodium topical gel, and methylprednisolone (Medrol Dosepak) reduced his symptoms only slightly.
Physical examination of the right elbow showed mild range of motion deficits; about 5° of extension and 5° of flexion were lacking. The patient had full pronation and supination. Palpation of the dorsal aspect of the forearm revealed marked tenderness in the area of the proximal radius. There was no tenderness over the posterior olecranon or the ulnar collateral ligament, and a moving valgus stress test was negative. No pain was elicited by resisted extension of the wrist or fingers. Motor innervation from the posterior interosseous nerve, anterior interosseous nerve, and ulnar nerve was intact with 5/5 strength, and there were no sensory deficits in the distribution of the radial, median, or ulnar nerves.
Discussion
Stress fractures account for 0.7% to 20% of sports medicine clinic injuries; <10% of all stress fractures involve the rib or upper extremity.4,6 When the intensity or frequency of physical activity is increased, as with overuse, bone resorption surpasses bone production, locally weakening the bone and making it prone to mechanical failure. Failure is thought to be induced by a combination of contractile muscular forces across damaged bone and increased mechanical loading caused by fatigue of supporting structures.5,6,19 These forces may have contributed to our baseball pitcher’s development of a stress fracture near the insertion of the supinator muscle in his throwing arm.
Given the insidious nature of stress fractures, the evaluating physician must have a high index of suspicion. Early recognition of a stress fracture is important in preventing further injury and allowing for early intervention, which is associated with faster healing.6,20 The clinical history often involves a change in training regimen within the weeks before pain onset. Furthermore, understanding the type of pitches used and the mechanics of each pitch can help with diagnosis. Often, pain increases as the inciting activity continues, and relief comes with rest. In an upper extremity examination, it is important to recall the usual stress fracture locations in throwers—the ribs, clavicle, humerus, ulnar shaft, and most often the olecranon—though the patient’s history often narrows the anatomical region of suspicion.2,4,7-10 Examination begins with inspection of the skin and soft tissues. Range of motion and strength testing results likely are normal throughout the upper extremity.3 Palpation over the suspected injury location often elicits pain and indicates further imaging is needed.6 The tuning fork test or the 3-point fulcrum test may elicit symptoms in occult fractures.3 Completing the assessment is a thorough neurovascular examination.
Insidious forearm pain requires a broad differential, including flexor-pronator mass or distal biceps injury, chronic exertional compartment syndrome, radial tunnel syndrome, intersection syndrome, pronator teres syndrome, anterior interosseous syndrome, thoracic outlet syndrome, musculocutaneous nerve compression, deep vein thrombosis of ulnar vein, and periostitis. Stress fractures distal to the elbow more commonly occur in weight-bearing athletes, though as this case shows it is important to consider stress fractures of the radius and ulna when evaluating forearm pain in a throwing athlete.21
The first imaging examination for a suspected stress fracture is a radiograph, which can be normal in up to 90% of patients, as it initially was in our athlete’s case.22 Often, radiographic evidence takes 2 to 12 weeks to appear.5 Even then, radiographs may be positive in only 50% of cases.19 CT, often regarded as insensitive during the early stages, is useful in visualizing fracture lines in a suspicious location.19,22 Radionuclide uptake scanning is highly sensitive during the early stages of stress injury but is nonspecific and may indicate neoplasm or infection; in addition, up to 46% of abnormal foci are asymptomatic.19 MRI has sensitivity comparable to that of radionuclide scanning but also many advantages, including lack of ionizing radiation, improved spatial resolution, and ability to image bone and soft tissue simultaneously.19 In our patient’s case, the unusual stress fracture location potentially could have hindered identification of the cause of injury. The lesion was just distal to the field of view of a normal elbow MRI and was not detected until a dedicated forearm MRI was examined. Both MRI and CT helped in identifying the stress fracture, and CT was used to follow interval healing.
In baseball players, upper extremity stress fractures are often nonoperatively treated with throwing cessation for 4 to 6 weeks followed by participation in a structured rehabilitation program.4,5 The throwing program that we suggest, and that was used in this case, has 21 stages of progression in duration, distance, and velocity of throwing. The athlete advances from each stage on the basis of symptoms.23 Other issues that may be addressed are vitamin D and calcium status and any flawed throwing mechanics that may have predisposed the athlete to injury. Such mechanics are gradually corrected.
The literature suggests that appropriate nonoperative management of stress fractures allows for return to sport in 8 to 10 weeks. It is important to note that most of the literature on stress fractures involves the lower extremity, and that treatment and time to return to play are therefore better described for such fractures.6 More study and evaluation of upper extremity stress fractures are needed to make return-to-sport predictions more reliable and successful treatment modalities more unified for this patient population. Last, it is imperative that clinical examination and symptoms be correlated with serial imaging when deciding on return to play. Our patient took 12 weeks to return to high-level sport. He progressed pain-free through the throwing program and showed radiographic evidence of healing on follow-up CT.
Conclusion
Radial shaft stress fractures are rare in throwing athletes. However, with a thorough history, a physical examination, and appropriate imaging, the correct diagnosis can be made early on, and proper treatment can be started to facilitate return to sport. To our knowledge, this is the first report of a stress fracture in the radial shaft of a MLB pitcher. Although the radial shaft is an uncommon location for stress fractures, we should keep in mind that they can occur wherever increased muscular forces exceed the ability of native bone to remodel. After diagnosis, the fracture usually heals with nonoperative treatment, and healing is confirmed with follow-up imaging, as was done in our patient’s case. Improved prediction of time to return to play for upper extremity fractures, such as the radial stress fracture described in this article, requires more study.
1. Monteleone GP Jr. Stress fractures in the athlete. Orthop Clin North Am. 1995;26(3):423-432.
2. Iwamoto J, Takeda T. Stress fractures in athletes: review of 196 cases. J Orthop Sci. 2003;8(3):273-278.
3. Miller TL, Kaeding CC. Upper-extremity stress fractures: distribution and causative activities in 70 patients. Orthopedics. 2012;35(9):789-793.
4. Jones GL. Upper extremity stress fractures. Clin Sports Med. 2006;25(1):159-174.
5. Brooks AA. Stress fractures of the upper extremity. Clin Sports Med. 2001;20(3):613-620.
6. Fredericson M, Jennings F, Beaulieu C, Matheson GO. Stress fractures in athletes. Top Magn Reson Imaging. 2006;17(5):309-325.
7. Gurtler R, Pavlov H, Torg JS. Stress fracture of the ipsilateral first rib in a pitcher. Am J Sports Med. 1985;13(4):277-279.
8. Polu KR, Schenck RC Jr, Wirth MA, Greeson J, Cone RO 3rd, Rockwood CA Jr. Stress fracture of the humerus in a collegiate baseball pitcher. A case report. Am J Sports Med. 1999;27(6):813-816.
9. Wu C, Chen Y. Stress fracture of the clavicle in a professional baseball player. J Shoulder Elbow Surg. 1998;7(2):164-167.
10. Schickendantz MS, Ho CP, Koh J. Stress injury of the proximal ulna in professional baseball players. Am J Sports Med. 2002;30(5):737-741.
11. Loosli AR, Leslie M. Stress fractures of the distal radius. A case report. Am J Sports Med. 1991;19(5):523-524.
12. Inagaki H, Inoue G. Stress fracture of the scaphoid combined with the distal radial epiphysiolysis. Br J Sports Med. 1997;31(3):256-257.
13. Read MT. Stress fractures of the distal radius in adolescent gymnasts. Br J Sports Med. 1981;15(4):272-276.
14. Orloff AS, Resnick D. Fatigue fracture of the distal part of the radius in a pool player. Injury. 1986;17(6):418-419.
15. Eisenberg D, Kirchner SG, Green NE. Stress fracture of the distal radius caused by “wheelies.” South Med J. 1986;79(7):918-919.
16. Brukner P. Stress fractures of the upper limb. Sports Med. 1998;26(6):415-424.
17. Farquharson-Roberts MA, Fulford PC. Stress fracture of the radius. J Bone Joint Surg Br. 1980;62(2):194-195.
18. Orloff AS, Resnick D. Fatigue fracture of the distal part of the radius in a pool player. Injury. 1986;17(6):418-419.
19. Anderson MW. Imaging of upper extremity stress fractures in the athlete. Clin Sports Med. 2006;25(3):489-504.
20. Bennell K, Brukner P. Preventing and managing stress fractures in athletes. Phys Ther Sport. 2005;6(4):171-180.
21. Sinha AK, Kaeding CC, Wadley GM. Upper extremity stress fractures in athletes: clinical features of 44 cases. Clin J Sport Med. 1999;9(4):199-202.
22. Matheson GO, Clement DB, McKenzie DC, Taunton JE, Lloyd-Smith DR, MacIntyre JG. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15(1):46-58.
23. Kaplan L, Lesniak B, Baraga M, et al. Throwing program for baseball players. 2009. http://uhealthsportsmedicine.com/documents/UHealth_Throwing_Program.pdf. Accessed May 24, 2016.
1. Monteleone GP Jr. Stress fractures in the athlete. Orthop Clin North Am. 1995;26(3):423-432.
2. Iwamoto J, Takeda T. Stress fractures in athletes: review of 196 cases. J Orthop Sci. 2003;8(3):273-278.
3. Miller TL, Kaeding CC. Upper-extremity stress fractures: distribution and causative activities in 70 patients. Orthopedics. 2012;35(9):789-793.
4. Jones GL. Upper extremity stress fractures. Clin Sports Med. 2006;25(1):159-174.
5. Brooks AA. Stress fractures of the upper extremity. Clin Sports Med. 2001;20(3):613-620.
6. Fredericson M, Jennings F, Beaulieu C, Matheson GO. Stress fractures in athletes. Top Magn Reson Imaging. 2006;17(5):309-325.
7. Gurtler R, Pavlov H, Torg JS. Stress fracture of the ipsilateral first rib in a pitcher. Am J Sports Med. 1985;13(4):277-279.
8. Polu KR, Schenck RC Jr, Wirth MA, Greeson J, Cone RO 3rd, Rockwood CA Jr. Stress fracture of the humerus in a collegiate baseball pitcher. A case report. Am J Sports Med. 1999;27(6):813-816.
9. Wu C, Chen Y. Stress fracture of the clavicle in a professional baseball player. J Shoulder Elbow Surg. 1998;7(2):164-167.
10. Schickendantz MS, Ho CP, Koh J. Stress injury of the proximal ulna in professional baseball players. Am J Sports Med. 2002;30(5):737-741.
11. Loosli AR, Leslie M. Stress fractures of the distal radius. A case report. Am J Sports Med. 1991;19(5):523-524.
12. Inagaki H, Inoue G. Stress fracture of the scaphoid combined with the distal radial epiphysiolysis. Br J Sports Med. 1997;31(3):256-257.
13. Read MT. Stress fractures of the distal radius in adolescent gymnasts. Br J Sports Med. 1981;15(4):272-276.
14. Orloff AS, Resnick D. Fatigue fracture of the distal part of the radius in a pool player. Injury. 1986;17(6):418-419.
15. Eisenberg D, Kirchner SG, Green NE. Stress fracture of the distal radius caused by “wheelies.” South Med J. 1986;79(7):918-919.
16. Brukner P. Stress fractures of the upper limb. Sports Med. 1998;26(6):415-424.
17. Farquharson-Roberts MA, Fulford PC. Stress fracture of the radius. J Bone Joint Surg Br. 1980;62(2):194-195.
18. Orloff AS, Resnick D. Fatigue fracture of the distal part of the radius in a pool player. Injury. 1986;17(6):418-419.
19. Anderson MW. Imaging of upper extremity stress fractures in the athlete. Clin Sports Med. 2006;25(3):489-504.
20. Bennell K, Brukner P. Preventing and managing stress fractures in athletes. Phys Ther Sport. 2005;6(4):171-180.
21. Sinha AK, Kaeding CC, Wadley GM. Upper extremity stress fractures in athletes: clinical features of 44 cases. Clin J Sport Med. 1999;9(4):199-202.
22. Matheson GO, Clement DB, McKenzie DC, Taunton JE, Lloyd-Smith DR, MacIntyre JG. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15(1):46-58.
23. Kaplan L, Lesniak B, Baraga M, et al. Throwing program for baseball players. 2009. http://uhealthsportsmedicine.com/documents/UHealth_Throwing_Program.pdf. Accessed May 24, 2016.
Systematic Review of Novel Synovial Fluid Markers and Polymerase Chain Reaction in the Diagnosis of Prosthetic Joint Infection
Take-Home Points
- Novel synovial markers and PCR have the potential to improve the detection of PJIs.
- 10Difficult-to-detect infections of prosthetic joints pose a diagnostic problem to surgeons and can lead to suboptimal outcomes.
- AD is a highly sensitive and specific synovial fluid marker for detecting PJIs.
- AD has shown promising results in detecting low virulence organisms.
- Studies are needed to determine how to best incorporate novel synovial markers and PCR to current diagnostic criteria in order to improve diagnostic accuracy.
Approximately 7 million Americans are living with a hip or knee replacement.1 According to projections, primary hip arthroplasties will increase by 174% and knee arthroplasties by 673% by 2030. Revision arthroplasties are projected to increase by 137% for hips and 601% for knees during the same time period.2 Infection and aseptic loosening are the most common causes of implant failure.3 The literature shows that infection is the most common cause of failure within 2 years after surgery and that aseptic loosening is the most common cause for late revision.3
Recent studies suggest that prosthetic joint infection (PJI) may be underreported because of difficulty making a diagnosis and that cases of aseptic loosening may in fact be attributable to infections with low-virulence organisms.2,3 These findings have led to new efforts to develop uniform criteria for diagnosing PJIs. In 2011, the Musculoskeletal Infection Society (MSIS) offered a new definition for PJI diagnosis, based on clinical and laboratory criteria, to increase the accuracy of PJI diagnosis.4 The MSIS committee acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-virulence organisms, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. Reports of PJI cases misdiagnosed as aseptic loosening suggest that current screening and diagnostic tools are not sensitive enough to detect all infections and that PJI is likely underdiagnosed.
According to MSIS criteria, the diagnosis of PJI can be made when there is a sinus tract communicating with the prosthesis, when a pathogen is isolated by culture from 2 or more separate tissue or fluid samples obtained from the affected prosthetic joint, or when 4 of 6 criteria are met. The 6 criteria are (1) elevated serum erythrocyte sedimentation rate (ESR) (>30 mm/hour) and elevated C-reactive protein (CRP) level (>10 mg/L); (2) elevated synovial white blood cell (WBC) count (1100-4000 cells/μL); (3) elevated synovial polymorphonuclear leukocytes (>64%); (4) purulence in affected joint; (5) isolation of a microorganism in a culture of periprosthetic tissue or fluid; and (6) more than 5 neutrophils per high-power field in 5 high-power fields observed.
In this review article, we discuss recently developed novel synovial biomarkers and polymerase chain reaction (PCR) technologies that may help increase the sensitivity and specificity of diagnostic guidelines for PJI.
Methods
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses), we performed a systematic review of specific synovial fluid markers and PCR used in PJI diagnosis. In May 2016, we searched the PubMed database for these criteria: ((((((PCR[Text Word]) OR IL-6[Text Word]) OR leukocyte esterase[Text Word]) OR alpha defensin[Text Word]) AND ((“infection/diagnosis”[MeSH Terms] OR “infection/surgery”[MeSH Terms])))) AND (prosthetic joint infection[MeSH Terms] OR periprosthetic joint infection[MeSH Terms]).
We included patients who had undergone total hip, knee, or shoulder arthroplasty (THA, TKA, TSA). Index tests were PCR and the synovial fluid markers α-defensin (AD), interleukin 6 (IL-6), and leukocyte esterase (LE). Reference tests included joint fluid/serum analysis or tissue analysis (ESR/CRP level, cell count, culture, frozen section), which defined the MSIS criteria for PJI. Primary outcomes of interest were sensitivity and specificity, and secondary outcomes of interest included positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (+LR), and negative likelihood ratio (–LR). Randomized controlled trials and controlled cohort studies in humans published within the past 10 years were included.
Results
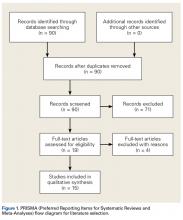
Our full-text review yielded 15 papers that met our study inclusion criteria (Figure 1).
α-Defensin
One of the novel synovial biomarkers that has shown significant promise in diagnosing PJIs, even with difficult-to-detect organisms, is AD.
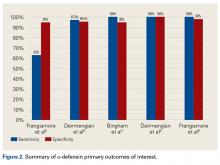
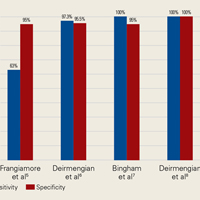
AD has shown even more impressive results as a biomarker for PJI in the hip and knee, where infection with low virulence organism is less common. In 2014, Deirmengian and colleagues6 conducted a prospective clinical study of 149 patients who underwent revision THA or TKA for aseptic loosening (n = 112) or PJI (n = 37) as defined by MSIS criteria. Aseptic loosening was diagnosed when there was no identifiable reason for pain, and MSIS criteria were not met. Synovial fluid aspirates were collected before or during surgery. AD correctly identified 143 of the 149 patients with confirmed infection with sensitivity of 97.3% (95% confidence interval [CI], 85.8%-99.6%) and specificity of 95.5% (95% CI, 89.9%-98.5%). Similarly, Bingham and colleagues7 conducted a retrospective clinical study of 61 assays done on 57 patients who underwent revision arthroplasty for PJI as defined by MSIS criteria. Synovial fluid aspirates were collected before or during surgery. AD correctly identified all 19 PJIs with sensitivity of 100% (95% CI, 79%-100%) and specificity of 95% (95% CI, 83%-99%). Sensitivity and specificity of the AD assay more accurately predicted infection than synovial cell count or serum ESR/CRP level did.
These results are supported by another prospective study by Deirmengian and colleagues8 differentiating aseptic failures and PJIs in THA or TKA. The sensitivity and specificity of AD in diagnosing PJI were 100% (95% CI, 85.05%-100%).
In a prospective study of 102 patients who underwent revision THA or TKA secondary to aseptic loosening or PJI, Frangiamore and colleagues9 also demonstrated the value of AD as a diagnostic for PJI in primary and revision hip and knee arthroplasty.
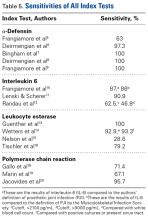
Table 1 and Figure 2 provide a concise review of the findings of each study.
Interleukin 6
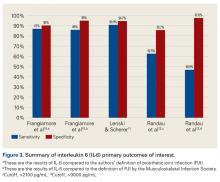
Another synovial fluid biomarker that has shown promise in PJI diagnosis is IL-6. In 2015, Frangiamore and colleagues10 conducted a prospective clinical study of 32 patients who underwent revision TSA. Synovial fluid aspiration was obtained before or during surgery. MSIS criteria were used to establish the diagnosis of PJI. IL-6 had sensitivity of 87% and specificity of 90%, with +LR of 8.45 and –LR of 0.15 in predicting PJI. Synovial fluid IL-6 had strong associations with frozen section histology and growth of P acnes. Frangiamore and colleagues10 recommended an ideal IL-6 cutoff of 359.1 pg/mL and reported that, though not as accurate as AD, synovial fluid IL-6 levels can help predict positive cultures in patients who undergo revision TSA.
Lenski and Scherer11 conducted another retrospective clinical study of the diagnostic value of IL-6 in PJI.
Randau and colleagues12 conducted a prospective clinical study of 120 patients who presented with painful THA or TKA and underwent revision for PJI, aseptic failure, or aseptic revision without signs of infection or loosening. Synovial fluid aspirate was collected before or during surgery.
Table 2 and Figure 3 provide a concise review of the findings of each study.
Leukocyte Esterase
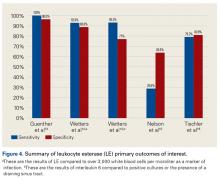
LE strips are an inexpensive screening tool for PJI, according to some studies. In a prospective clinical study of 364 endoprosthetic joint (hip, knee, shoulder) interventions, Guenther and colleagues13 collected synovial fluid before surgery. Samples were tested with graded LE strips using PJI criteria set by the authors. Results were correlated with preoperative synovial fluid aspirations, serum CRP level, serum WBC count, and intraoperative histopathologic and microbiological findings. Whereas 293 (93.31%) of the 314 aseptic cases had negative test strip readings, 100% of the 50 infected cases were positive. LE had sensitivity of 100%, specificity of 96.5%, PPV of 82%, and NPV of 100%.
Wetters et al14 performed a prospective clinical study on 223 patients who underwent TKAs and THAs for suspected PJI based on having criteria defined by the authors of the study. Synovial fluid samples were collected either preoperatively or intraoperatively.
Other authors have reported different findings that LE is an unreliable marker in PJI diagnosis. In one prospective clinical study of 85 patients who underwent primary or revision TSA, synovial fluid was collected during surgery.15 According to MSIS criteria, only 5 positive LE results predicted PJI among 21 primary and revision patients with positive cultures. Of the 7 revision patients who met the MSIS criteria for PJI, only 2 had a positive LE test. LE had sensitivity of 28.6%, specificity of 63.6%, PPV of 28.6%, and NPV of 87.5%. Six of the 7 revision patients grew P acnes. These results showed that LE was unreliable in detecting shoulder PJI.15
In another prospective clinical study, Tischler and colleagues16 enrolled 189 patients who underwent revision TKA or THA for aseptic failure or PJI as defined by the MSIS criteria. Synovial fluid was collected intraoperatively.
Table 3 and Figure 4 provide a concise review of the findings of each study.
Polymerase Chain Reaction
Studies have found that PCR analysis of synovial fluid is effective in detecting bacteria on the surface of implants removed during revision arthroplasties. Comparison of the 16S rRNA gene sequences of bacterial genomes showed a diverse range of bacterial species within biofilms on the surface of clinical and subclinical infections.17 These findings, along with those of other studies, suggest that PCR analysis of synovial fluid is useful in diagnosing PJI and identifying organisms and their sensitivities to antibiotics.
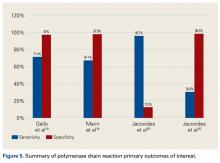
Gallo and colleagues18 performed a prospective clinical study on 115 patients who underwent revision TKAs or THAs. Synovial fluid was collected intraoperatively. PCR assays targeting the 16S rDNA were carried out on 101 patients. PJIs were classified based on criteria of the authors of this study, of which there were 42. The sensitivity, specificity, PPV, NPV, +LR, and -LR for PCR were 71.4% (95% CI, 61.5%-75.5%), 97% (95% CI, 91.7%-99.1%), 92.6% (95% CI, 79.8%-97.9%), 86.5% (95% CI, 81.8%-88.4%), 23.6 (95% CI, 5.9%-93.8%), and 0.29 (95% CI, 0.17%-0.49%), respectively. Of note the most common organism detected in 42 PJIs was coagulase-negative Staphylococcus.
Marin and colleagues19 conducted a prospective study of 122 patients who underwent arthroplasty for suspected infection or aseptic loosening as defined by the authors’ clinicohistopathologic criteria. Synovial fluid and biopsy specimens were collected during surgery, and 40 patients met the infection criteria. The authors concluded that 16S PCR is more specific and has better PPV than culture does as one positive 16S PCR resulted in a specificity and PPV of PJI of 96.3% and 91.7%, respectively. However, they noted that culture was more sensitive in diagnosing PJI.
Jacovides and colleagues20 conducted a prospective study on 82 patients undergoing primary TKA, revision TKA, and revision THA.
The low PCR sensitivities reported in the literature were explained in a review by Hartley and Harris.21 They wrote that BR 16S rDNA and sequencing of PJI samples inherently have low sensitivity because of the contamination that can occur from the PCR reagents themselves or from sample mishandling. Techniques that address contaminant (extraneous DNA) removal, such as ultraviolet irradiation and DNase treatment, reduce Taq DNA polymerase activity, which reduces PCR sensitivity.
Table 4 and Figure 5 provide a concise review of the findings of each study.
Discussion
Although there is no gold standard for the diagnosis of PJIs, several clinical and laboratory criteria guidelines are currently used to help clinicians diagnose infections of prosthetic joints. However, despite standardization of diagnostic criteria, PJI continue to be a diagnostic challenge.
AD is a highly sensitive and specific synovial fluid biomarker in detecting common PJIs.
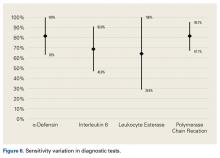
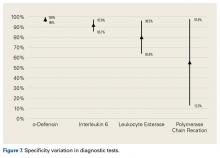
In summary, 5 AD studies5-9 had sensitivity ranging from 63% to 100% and specificity ranging from 95% to 100%; 3 IL-6 studies10-12 had sensitivity ranging from 46.8% to 90.9% and specificity ranging from 85.7% to 97.6%; 4 LE studies13-16 had sensitivity ranging from 28.6% to 100% and specificity ranging from 63.6% to 96.5%; and 3 PCR studies18-20 had sensitivity ranging from 67.1% to 95.7% and specificity ranging from 12.3% to 97.8%. Sensitivity and specificity were consistently higher for AD than for IL-6, LE, and PCR, though there was significant overlap, heterogeneity, and variation across all the included studies.
Although the overall incidence of PJI is low, infected revisions remain a substantial financial burden to hospitals, as annual costs of infected revisions is estimated to exceed $1.62 billion by 2020.25 The usefulness of novel biomarkers and PCR in diagnosing PJI can be found in their ability to diagnose infections and facilitate appropriate early treatment. Several of these tests are readily available commercially and have the potential to be cost-effective diagnostic tools. The price to perform an AD test from Synovasure TM (Zimmer Biomet) ranges from $93 to $143. LE also provides an economic option for diagnosing PJI, as LE strips are commercially available for the cost of about 25 cents. PCR has also become an economic option, as costs can average $15.50 per sample extraction or PCR assay and $42.50 per amplicon sequence as reported in a study by Vandercam and colleagues.26 Future studies are needed to determine a diagnostic algorithm which incorporates these novel synovial markers to improve diagnostic accuracy of PJI in the most cost effective manner.
The current literature supports that AD can potentially be used to screen for PJI. Our findings suggest novel synovial fluid biomarkers may become of significant diagnostic use when combined with current laboratory and clinical diagnostic criteria. We recommend use of AD in cases in which pain, stiffness, and poor TJA outcome cannot be explained by errors in surgical technique, and infection is suspected despite MSIS criteria not being met.
The studies reviewed in this manuscript were limited in that none presented level I evidence (12 had level II evidence, and 3 had level III evidence), and there was significant heterogeneity (some studies used their own diagnostic standard, and others used the MSIS criteria). Larger scale prospective studies comparing serum ESR/CRP level and synovial fluid analysis to novel synovial markers are needed.
Am J Orthop. 2017;46(4):190-198. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-1397.
2. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
3. Sharkey PF, Lichstein PM, Shen C, Tokarski AT, Parvizi J. Why are total knee arthroplasties failing today—has anything changed after 10 years? J Arthroplasty. 2014;29(9):1774-1778.
4. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
5. Frangiamore SJ, Saleh A, Grosso MJ, et al. α-Defensin as a predictor of periprosthetic shoulder infection. J Shoulder Elbow Surg. 2015;24(7):1021-1027.
6. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(17):1439-1445.
7. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009.
8. Deirmengian C, Kardos K, Kilmartin P, et al. The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clin Orthop Relat Res. 2015;473(1):198-203.
9. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
10. Frangiamore SJ, Saleh A, Kovac MF, et al. Synovial fluid interleukin-6 as a predictor of periprosthetic shoulder infection. J Bone Joint Surg Am. 2015;97(1):63-70.
11. Lenski M, Scherer MA. Synovial IL-6 as inflammatory marker in periprosthetic joint infections. J Arthroplasty. 2014;29(6):1105-1109.
12. Randau TM, Friedrich MJ, Wimmer MD, et al. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One. 2014;9(2):e89045.
13. Guenther D, Kokenge T, Jacobs O, et al. Excluding infections in arthroplasty using leucocyte esterase test. Int Orthop. 2014;38(11):2385-2390.
14. Wetters NG, Berend KR, Lombardi AV, Morris MJ, Tucker TL, Della Valle CJ. Leukocyte esterase reagent strips for the rapid diagnosis of periprosthetic joint infection. J Arthroplasty. 2012;27(8 suppl):8-11.
15. Nelson GN, Paxton ES, Narzikul A, Williams G, Lazarus MD, Abboud JA. Leukocyte esterase in the diagnosis of shoulder periprosthetic joint infection. J Shoulder Elbow Surg. 2015;24(9):1421-1426.
16. Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: matched for Musculoskeletal Infection Society criteria. J Bone Joint Surg Am. 2014;96(22):1917-1920.
17. Dempsey KE, Riggio MP, Lennon A, et al. Identification of bacteria on the surface of clinically infected and non-infected prosthetic hip joints removed during revision arthroplasties by 16S rRNA gene sequencing and by microbiological culture. Arthritis Res Ther. 2007;9(3):R46.
18. Gallo J, Kolar M, Dendis M, et al. Culture and PCR analysis of joint fluid in the diagnosis of prosthetic joint infection. New Microbiol. 2008;31(1):97-104.
19. Marin M, Garcia-Lechuz JM, Alonso P, et al. Role of universal 16S rRNA gene PCR and sequencing in diagnosis of prosthetic joint infection. J Clin Microbiol. 2012;50(3):583-589.
20. Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, Parvizi J. Successful identification of pathogens by polymerase chain reaction (PCR)-based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2012;94(24):2247-2254.
21. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
22. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
23. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
24. Hunt RW, Bond MJ, Pater GD. Psychological responses to cancer: a case for cancer support groups. Community Health Stud. 1990;14(1):35-38.
25. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
26. Vandercam B, Jeumont S, Cornu O, et al. Amplification-based DNA analysis in the diagnosis of prosthetic joint infection. J Mol Diagn. 2008;10(6):537-543.
Take-Home Points
- Novel synovial markers and PCR have the potential to improve the detection of PJIs.
- 10Difficult-to-detect infections of prosthetic joints pose a diagnostic problem to surgeons and can lead to suboptimal outcomes.
- AD is a highly sensitive and specific synovial fluid marker for detecting PJIs.
- AD has shown promising results in detecting low virulence organisms.
- Studies are needed to determine how to best incorporate novel synovial markers and PCR to current diagnostic criteria in order to improve diagnostic accuracy.
Approximately 7 million Americans are living with a hip or knee replacement.1 According to projections, primary hip arthroplasties will increase by 174% and knee arthroplasties by 673% by 2030. Revision arthroplasties are projected to increase by 137% for hips and 601% for knees during the same time period.2 Infection and aseptic loosening are the most common causes of implant failure.3 The literature shows that infection is the most common cause of failure within 2 years after surgery and that aseptic loosening is the most common cause for late revision.3
Recent studies suggest that prosthetic joint infection (PJI) may be underreported because of difficulty making a diagnosis and that cases of aseptic loosening may in fact be attributable to infections with low-virulence organisms.2,3 These findings have led to new efforts to develop uniform criteria for diagnosing PJIs. In 2011, the Musculoskeletal Infection Society (MSIS) offered a new definition for PJI diagnosis, based on clinical and laboratory criteria, to increase the accuracy of PJI diagnosis.4 The MSIS committee acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-virulence organisms, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. Reports of PJI cases misdiagnosed as aseptic loosening suggest that current screening and diagnostic tools are not sensitive enough to detect all infections and that PJI is likely underdiagnosed.
According to MSIS criteria, the diagnosis of PJI can be made when there is a sinus tract communicating with the prosthesis, when a pathogen is isolated by culture from 2 or more separate tissue or fluid samples obtained from the affected prosthetic joint, or when 4 of 6 criteria are met. The 6 criteria are (1) elevated serum erythrocyte sedimentation rate (ESR) (>30 mm/hour) and elevated C-reactive protein (CRP) level (>10 mg/L); (2) elevated synovial white blood cell (WBC) count (1100-4000 cells/μL); (3) elevated synovial polymorphonuclear leukocytes (>64%); (4) purulence in affected joint; (5) isolation of a microorganism in a culture of periprosthetic tissue or fluid; and (6) more than 5 neutrophils per high-power field in 5 high-power fields observed.
In this review article, we discuss recently developed novel synovial biomarkers and polymerase chain reaction (PCR) technologies that may help increase the sensitivity and specificity of diagnostic guidelines for PJI.
Methods
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses), we performed a systematic review of specific synovial fluid markers and PCR used in PJI diagnosis. In May 2016, we searched the PubMed database for these criteria: ((((((PCR[Text Word]) OR IL-6[Text Word]) OR leukocyte esterase[Text Word]) OR alpha defensin[Text Word]) AND ((“infection/diagnosis”[MeSH Terms] OR “infection/surgery”[MeSH Terms])))) AND (prosthetic joint infection[MeSH Terms] OR periprosthetic joint infection[MeSH Terms]).
We included patients who had undergone total hip, knee, or shoulder arthroplasty (THA, TKA, TSA). Index tests were PCR and the synovial fluid markers α-defensin (AD), interleukin 6 (IL-6), and leukocyte esterase (LE). Reference tests included joint fluid/serum analysis or tissue analysis (ESR/CRP level, cell count, culture, frozen section), which defined the MSIS criteria for PJI. Primary outcomes of interest were sensitivity and specificity, and secondary outcomes of interest included positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (+LR), and negative likelihood ratio (–LR). Randomized controlled trials and controlled cohort studies in humans published within the past 10 years were included.
Results
Our full-text review yielded 15 papers that met our study inclusion criteria (Figure 1).
α-Defensin
One of the novel synovial biomarkers that has shown significant promise in diagnosing PJIs, even with difficult-to-detect organisms, is AD.
AD has shown even more impressive results as a biomarker for PJI in the hip and knee, where infection with low virulence organism is less common. In 2014, Deirmengian and colleagues6 conducted a prospective clinical study of 149 patients who underwent revision THA or TKA for aseptic loosening (n = 112) or PJI (n = 37) as defined by MSIS criteria. Aseptic loosening was diagnosed when there was no identifiable reason for pain, and MSIS criteria were not met. Synovial fluid aspirates were collected before or during surgery. AD correctly identified 143 of the 149 patients with confirmed infection with sensitivity of 97.3% (95% confidence interval [CI], 85.8%-99.6%) and specificity of 95.5% (95% CI, 89.9%-98.5%). Similarly, Bingham and colleagues7 conducted a retrospective clinical study of 61 assays done on 57 patients who underwent revision arthroplasty for PJI as defined by MSIS criteria. Synovial fluid aspirates were collected before or during surgery. AD correctly identified all 19 PJIs with sensitivity of 100% (95% CI, 79%-100%) and specificity of 95% (95% CI, 83%-99%). Sensitivity and specificity of the AD assay more accurately predicted infection than synovial cell count or serum ESR/CRP level did.
These results are supported by another prospective study by Deirmengian and colleagues8 differentiating aseptic failures and PJIs in THA or TKA. The sensitivity and specificity of AD in diagnosing PJI were 100% (95% CI, 85.05%-100%).
In a prospective study of 102 patients who underwent revision THA or TKA secondary to aseptic loosening or PJI, Frangiamore and colleagues9 also demonstrated the value of AD as a diagnostic for PJI in primary and revision hip and knee arthroplasty.
Table 1 and Figure 2 provide a concise review of the findings of each study.
Interleukin 6
Another synovial fluid biomarker that has shown promise in PJI diagnosis is IL-6. In 2015, Frangiamore and colleagues10 conducted a prospective clinical study of 32 patients who underwent revision TSA. Synovial fluid aspiration was obtained before or during surgery. MSIS criteria were used to establish the diagnosis of PJI. IL-6 had sensitivity of 87% and specificity of 90%, with +LR of 8.45 and –LR of 0.15 in predicting PJI. Synovial fluid IL-6 had strong associations with frozen section histology and growth of P acnes. Frangiamore and colleagues10 recommended an ideal IL-6 cutoff of 359.1 pg/mL and reported that, though not as accurate as AD, synovial fluid IL-6 levels can help predict positive cultures in patients who undergo revision TSA.
Lenski and Scherer11 conducted another retrospective clinical study of the diagnostic value of IL-6 in PJI.
Randau and colleagues12 conducted a prospective clinical study of 120 patients who presented with painful THA or TKA and underwent revision for PJI, aseptic failure, or aseptic revision without signs of infection or loosening. Synovial fluid aspirate was collected before or during surgery.
Table 2 and Figure 3 provide a concise review of the findings of each study.
Leukocyte Esterase
LE strips are an inexpensive screening tool for PJI, according to some studies. In a prospective clinical study of 364 endoprosthetic joint (hip, knee, shoulder) interventions, Guenther and colleagues13 collected synovial fluid before surgery. Samples were tested with graded LE strips using PJI criteria set by the authors. Results were correlated with preoperative synovial fluid aspirations, serum CRP level, serum WBC count, and intraoperative histopathologic and microbiological findings. Whereas 293 (93.31%) of the 314 aseptic cases had negative test strip readings, 100% of the 50 infected cases were positive. LE had sensitivity of 100%, specificity of 96.5%, PPV of 82%, and NPV of 100%.
Wetters et al14 performed a prospective clinical study on 223 patients who underwent TKAs and THAs for suspected PJI based on having criteria defined by the authors of the study. Synovial fluid samples were collected either preoperatively or intraoperatively.
Other authors have reported different findings that LE is an unreliable marker in PJI diagnosis. In one prospective clinical study of 85 patients who underwent primary or revision TSA, synovial fluid was collected during surgery.15 According to MSIS criteria, only 5 positive LE results predicted PJI among 21 primary and revision patients with positive cultures. Of the 7 revision patients who met the MSIS criteria for PJI, only 2 had a positive LE test. LE had sensitivity of 28.6%, specificity of 63.6%, PPV of 28.6%, and NPV of 87.5%. Six of the 7 revision patients grew P acnes. These results showed that LE was unreliable in detecting shoulder PJI.15
In another prospective clinical study, Tischler and colleagues16 enrolled 189 patients who underwent revision TKA or THA for aseptic failure or PJI as defined by the MSIS criteria. Synovial fluid was collected intraoperatively.
Table 3 and Figure 4 provide a concise review of the findings of each study.
Polymerase Chain Reaction
Studies have found that PCR analysis of synovial fluid is effective in detecting bacteria on the surface of implants removed during revision arthroplasties. Comparison of the 16S rRNA gene sequences of bacterial genomes showed a diverse range of bacterial species within biofilms on the surface of clinical and subclinical infections.17 These findings, along with those of other studies, suggest that PCR analysis of synovial fluid is useful in diagnosing PJI and identifying organisms and their sensitivities to antibiotics.
Gallo and colleagues18 performed a prospective clinical study on 115 patients who underwent revision TKAs or THAs. Synovial fluid was collected intraoperatively. PCR assays targeting the 16S rDNA were carried out on 101 patients. PJIs were classified based on criteria of the authors of this study, of which there were 42. The sensitivity, specificity, PPV, NPV, +LR, and -LR for PCR were 71.4% (95% CI, 61.5%-75.5%), 97% (95% CI, 91.7%-99.1%), 92.6% (95% CI, 79.8%-97.9%), 86.5% (95% CI, 81.8%-88.4%), 23.6 (95% CI, 5.9%-93.8%), and 0.29 (95% CI, 0.17%-0.49%), respectively. Of note the most common organism detected in 42 PJIs was coagulase-negative Staphylococcus.
Marin and colleagues19 conducted a prospective study of 122 patients who underwent arthroplasty for suspected infection or aseptic loosening as defined by the authors’ clinicohistopathologic criteria. Synovial fluid and biopsy specimens were collected during surgery, and 40 patients met the infection criteria. The authors concluded that 16S PCR is more specific and has better PPV than culture does as one positive 16S PCR resulted in a specificity and PPV of PJI of 96.3% and 91.7%, respectively. However, they noted that culture was more sensitive in diagnosing PJI.
Jacovides and colleagues20 conducted a prospective study on 82 patients undergoing primary TKA, revision TKA, and revision THA.
The low PCR sensitivities reported in the literature were explained in a review by Hartley and Harris.21 They wrote that BR 16S rDNA and sequencing of PJI samples inherently have low sensitivity because of the contamination that can occur from the PCR reagents themselves or from sample mishandling. Techniques that address contaminant (extraneous DNA) removal, such as ultraviolet irradiation and DNase treatment, reduce Taq DNA polymerase activity, which reduces PCR sensitivity.
Table 4 and Figure 5 provide a concise review of the findings of each study.
Discussion
Although there is no gold standard for the diagnosis of PJIs, several clinical and laboratory criteria guidelines are currently used to help clinicians diagnose infections of prosthetic joints. However, despite standardization of diagnostic criteria, PJI continue to be a diagnostic challenge.
AD is a highly sensitive and specific synovial fluid biomarker in detecting common PJIs.
In summary, 5 AD studies5-9 had sensitivity ranging from 63% to 100% and specificity ranging from 95% to 100%; 3 IL-6 studies10-12 had sensitivity ranging from 46.8% to 90.9% and specificity ranging from 85.7% to 97.6%; 4 LE studies13-16 had sensitivity ranging from 28.6% to 100% and specificity ranging from 63.6% to 96.5%; and 3 PCR studies18-20 had sensitivity ranging from 67.1% to 95.7% and specificity ranging from 12.3% to 97.8%. Sensitivity and specificity were consistently higher for AD than for IL-6, LE, and PCR, though there was significant overlap, heterogeneity, and variation across all the included studies.
Although the overall incidence of PJI is low, infected revisions remain a substantial financial burden to hospitals, as annual costs of infected revisions is estimated to exceed $1.62 billion by 2020.25 The usefulness of novel biomarkers and PCR in diagnosing PJI can be found in their ability to diagnose infections and facilitate appropriate early treatment. Several of these tests are readily available commercially and have the potential to be cost-effective diagnostic tools. The price to perform an AD test from Synovasure TM (Zimmer Biomet) ranges from $93 to $143. LE also provides an economic option for diagnosing PJI, as LE strips are commercially available for the cost of about 25 cents. PCR has also become an economic option, as costs can average $15.50 per sample extraction or PCR assay and $42.50 per amplicon sequence as reported in a study by Vandercam and colleagues.26 Future studies are needed to determine a diagnostic algorithm which incorporates these novel synovial markers to improve diagnostic accuracy of PJI in the most cost effective manner.
The current literature supports that AD can potentially be used to screen for PJI. Our findings suggest novel synovial fluid biomarkers may become of significant diagnostic use when combined with current laboratory and clinical diagnostic criteria. We recommend use of AD in cases in which pain, stiffness, and poor TJA outcome cannot be explained by errors in surgical technique, and infection is suspected despite MSIS criteria not being met.
The studies reviewed in this manuscript were limited in that none presented level I evidence (12 had level II evidence, and 3 had level III evidence), and there was significant heterogeneity (some studies used their own diagnostic standard, and others used the MSIS criteria). Larger scale prospective studies comparing serum ESR/CRP level and synovial fluid analysis to novel synovial markers are needed.
Am J Orthop. 2017;46(4):190-198. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Novel synovial markers and PCR have the potential to improve the detection of PJIs.
- 10Difficult-to-detect infections of prosthetic joints pose a diagnostic problem to surgeons and can lead to suboptimal outcomes.
- AD is a highly sensitive and specific synovial fluid marker for detecting PJIs.
- AD has shown promising results in detecting low virulence organisms.
- Studies are needed to determine how to best incorporate novel synovial markers and PCR to current diagnostic criteria in order to improve diagnostic accuracy.
Approximately 7 million Americans are living with a hip or knee replacement.1 According to projections, primary hip arthroplasties will increase by 174% and knee arthroplasties by 673% by 2030. Revision arthroplasties are projected to increase by 137% for hips and 601% for knees during the same time period.2 Infection and aseptic loosening are the most common causes of implant failure.3 The literature shows that infection is the most common cause of failure within 2 years after surgery and that aseptic loosening is the most common cause for late revision.3
Recent studies suggest that prosthetic joint infection (PJI) may be underreported because of difficulty making a diagnosis and that cases of aseptic loosening may in fact be attributable to infections with low-virulence organisms.2,3 These findings have led to new efforts to develop uniform criteria for diagnosing PJIs. In 2011, the Musculoskeletal Infection Society (MSIS) offered a new definition for PJI diagnosis, based on clinical and laboratory criteria, to increase the accuracy of PJI diagnosis.4 The MSIS committee acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-virulence organisms, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. Reports of PJI cases misdiagnosed as aseptic loosening suggest that current screening and diagnostic tools are not sensitive enough to detect all infections and that PJI is likely underdiagnosed.
According to MSIS criteria, the diagnosis of PJI can be made when there is a sinus tract communicating with the prosthesis, when a pathogen is isolated by culture from 2 or more separate tissue or fluid samples obtained from the affected prosthetic joint, or when 4 of 6 criteria are met. The 6 criteria are (1) elevated serum erythrocyte sedimentation rate (ESR) (>30 mm/hour) and elevated C-reactive protein (CRP) level (>10 mg/L); (2) elevated synovial white blood cell (WBC) count (1100-4000 cells/μL); (3) elevated synovial polymorphonuclear leukocytes (>64%); (4) purulence in affected joint; (5) isolation of a microorganism in a culture of periprosthetic tissue or fluid; and (6) more than 5 neutrophils per high-power field in 5 high-power fields observed.
In this review article, we discuss recently developed novel synovial biomarkers and polymerase chain reaction (PCR) technologies that may help increase the sensitivity and specificity of diagnostic guidelines for PJI.
Methods
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses), we performed a systematic review of specific synovial fluid markers and PCR used in PJI diagnosis. In May 2016, we searched the PubMed database for these criteria: ((((((PCR[Text Word]) OR IL-6[Text Word]) OR leukocyte esterase[Text Word]) OR alpha defensin[Text Word]) AND ((“infection/diagnosis”[MeSH Terms] OR “infection/surgery”[MeSH Terms])))) AND (prosthetic joint infection[MeSH Terms] OR periprosthetic joint infection[MeSH Terms]).
We included patients who had undergone total hip, knee, or shoulder arthroplasty (THA, TKA, TSA). Index tests were PCR and the synovial fluid markers α-defensin (AD), interleukin 6 (IL-6), and leukocyte esterase (LE). Reference tests included joint fluid/serum analysis or tissue analysis (ESR/CRP level, cell count, culture, frozen section), which defined the MSIS criteria for PJI. Primary outcomes of interest were sensitivity and specificity, and secondary outcomes of interest included positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (+LR), and negative likelihood ratio (–LR). Randomized controlled trials and controlled cohort studies in humans published within the past 10 years were included.
Results
Our full-text review yielded 15 papers that met our study inclusion criteria (Figure 1).
α-Defensin
One of the novel synovial biomarkers that has shown significant promise in diagnosing PJIs, even with difficult-to-detect organisms, is AD.
AD has shown even more impressive results as a biomarker for PJI in the hip and knee, where infection with low virulence organism is less common. In 2014, Deirmengian and colleagues6 conducted a prospective clinical study of 149 patients who underwent revision THA or TKA for aseptic loosening (n = 112) or PJI (n = 37) as defined by MSIS criteria. Aseptic loosening was diagnosed when there was no identifiable reason for pain, and MSIS criteria were not met. Synovial fluid aspirates were collected before or during surgery. AD correctly identified 143 of the 149 patients with confirmed infection with sensitivity of 97.3% (95% confidence interval [CI], 85.8%-99.6%) and specificity of 95.5% (95% CI, 89.9%-98.5%). Similarly, Bingham and colleagues7 conducted a retrospective clinical study of 61 assays done on 57 patients who underwent revision arthroplasty for PJI as defined by MSIS criteria. Synovial fluid aspirates were collected before or during surgery. AD correctly identified all 19 PJIs with sensitivity of 100% (95% CI, 79%-100%) and specificity of 95% (95% CI, 83%-99%). Sensitivity and specificity of the AD assay more accurately predicted infection than synovial cell count or serum ESR/CRP level did.
These results are supported by another prospective study by Deirmengian and colleagues8 differentiating aseptic failures and PJIs in THA or TKA. The sensitivity and specificity of AD in diagnosing PJI were 100% (95% CI, 85.05%-100%).
In a prospective study of 102 patients who underwent revision THA or TKA secondary to aseptic loosening or PJI, Frangiamore and colleagues9 also demonstrated the value of AD as a diagnostic for PJI in primary and revision hip and knee arthroplasty.
Table 1 and Figure 2 provide a concise review of the findings of each study.
Interleukin 6
Another synovial fluid biomarker that has shown promise in PJI diagnosis is IL-6. In 2015, Frangiamore and colleagues10 conducted a prospective clinical study of 32 patients who underwent revision TSA. Synovial fluid aspiration was obtained before or during surgery. MSIS criteria were used to establish the diagnosis of PJI. IL-6 had sensitivity of 87% and specificity of 90%, with +LR of 8.45 and –LR of 0.15 in predicting PJI. Synovial fluid IL-6 had strong associations with frozen section histology and growth of P acnes. Frangiamore and colleagues10 recommended an ideal IL-6 cutoff of 359.1 pg/mL and reported that, though not as accurate as AD, synovial fluid IL-6 levels can help predict positive cultures in patients who undergo revision TSA.
Lenski and Scherer11 conducted another retrospective clinical study of the diagnostic value of IL-6 in PJI.
Randau and colleagues12 conducted a prospective clinical study of 120 patients who presented with painful THA or TKA and underwent revision for PJI, aseptic failure, or aseptic revision without signs of infection or loosening. Synovial fluid aspirate was collected before or during surgery.
Table 2 and Figure 3 provide a concise review of the findings of each study.
Leukocyte Esterase
LE strips are an inexpensive screening tool for PJI, according to some studies. In a prospective clinical study of 364 endoprosthetic joint (hip, knee, shoulder) interventions, Guenther and colleagues13 collected synovial fluid before surgery. Samples were tested with graded LE strips using PJI criteria set by the authors. Results were correlated with preoperative synovial fluid aspirations, serum CRP level, serum WBC count, and intraoperative histopathologic and microbiological findings. Whereas 293 (93.31%) of the 314 aseptic cases had negative test strip readings, 100% of the 50 infected cases were positive. LE had sensitivity of 100%, specificity of 96.5%, PPV of 82%, and NPV of 100%.
Wetters et al14 performed a prospective clinical study on 223 patients who underwent TKAs and THAs for suspected PJI based on having criteria defined by the authors of the study. Synovial fluid samples were collected either preoperatively or intraoperatively.
Other authors have reported different findings that LE is an unreliable marker in PJI diagnosis. In one prospective clinical study of 85 patients who underwent primary or revision TSA, synovial fluid was collected during surgery.15 According to MSIS criteria, only 5 positive LE results predicted PJI among 21 primary and revision patients with positive cultures. Of the 7 revision patients who met the MSIS criteria for PJI, only 2 had a positive LE test. LE had sensitivity of 28.6%, specificity of 63.6%, PPV of 28.6%, and NPV of 87.5%. Six of the 7 revision patients grew P acnes. These results showed that LE was unreliable in detecting shoulder PJI.15
In another prospective clinical study, Tischler and colleagues16 enrolled 189 patients who underwent revision TKA or THA for aseptic failure or PJI as defined by the MSIS criteria. Synovial fluid was collected intraoperatively.
Table 3 and Figure 4 provide a concise review of the findings of each study.
Polymerase Chain Reaction
Studies have found that PCR analysis of synovial fluid is effective in detecting bacteria on the surface of implants removed during revision arthroplasties. Comparison of the 16S rRNA gene sequences of bacterial genomes showed a diverse range of bacterial species within biofilms on the surface of clinical and subclinical infections.17 These findings, along with those of other studies, suggest that PCR analysis of synovial fluid is useful in diagnosing PJI and identifying organisms and their sensitivities to antibiotics.
Gallo and colleagues18 performed a prospective clinical study on 115 patients who underwent revision TKAs or THAs. Synovial fluid was collected intraoperatively. PCR assays targeting the 16S rDNA were carried out on 101 patients. PJIs were classified based on criteria of the authors of this study, of which there were 42. The sensitivity, specificity, PPV, NPV, +LR, and -LR for PCR were 71.4% (95% CI, 61.5%-75.5%), 97% (95% CI, 91.7%-99.1%), 92.6% (95% CI, 79.8%-97.9%), 86.5% (95% CI, 81.8%-88.4%), 23.6 (95% CI, 5.9%-93.8%), and 0.29 (95% CI, 0.17%-0.49%), respectively. Of note the most common organism detected in 42 PJIs was coagulase-negative Staphylococcus.
Marin and colleagues19 conducted a prospective study of 122 patients who underwent arthroplasty for suspected infection or aseptic loosening as defined by the authors’ clinicohistopathologic criteria. Synovial fluid and biopsy specimens were collected during surgery, and 40 patients met the infection criteria. The authors concluded that 16S PCR is more specific and has better PPV than culture does as one positive 16S PCR resulted in a specificity and PPV of PJI of 96.3% and 91.7%, respectively. However, they noted that culture was more sensitive in diagnosing PJI.
Jacovides and colleagues20 conducted a prospective study on 82 patients undergoing primary TKA, revision TKA, and revision THA.
The low PCR sensitivities reported in the literature were explained in a review by Hartley and Harris.21 They wrote that BR 16S rDNA and sequencing of PJI samples inherently have low sensitivity because of the contamination that can occur from the PCR reagents themselves or from sample mishandling. Techniques that address contaminant (extraneous DNA) removal, such as ultraviolet irradiation and DNase treatment, reduce Taq DNA polymerase activity, which reduces PCR sensitivity.
Table 4 and Figure 5 provide a concise review of the findings of each study.
Discussion
Although there is no gold standard for the diagnosis of PJIs, several clinical and laboratory criteria guidelines are currently used to help clinicians diagnose infections of prosthetic joints. However, despite standardization of diagnostic criteria, PJI continue to be a diagnostic challenge.
AD is a highly sensitive and specific synovial fluid biomarker in detecting common PJIs.
In summary, 5 AD studies5-9 had sensitivity ranging from 63% to 100% and specificity ranging from 95% to 100%; 3 IL-6 studies10-12 had sensitivity ranging from 46.8% to 90.9% and specificity ranging from 85.7% to 97.6%; 4 LE studies13-16 had sensitivity ranging from 28.6% to 100% and specificity ranging from 63.6% to 96.5%; and 3 PCR studies18-20 had sensitivity ranging from 67.1% to 95.7% and specificity ranging from 12.3% to 97.8%. Sensitivity and specificity were consistently higher for AD than for IL-6, LE, and PCR, though there was significant overlap, heterogeneity, and variation across all the included studies.
Although the overall incidence of PJI is low, infected revisions remain a substantial financial burden to hospitals, as annual costs of infected revisions is estimated to exceed $1.62 billion by 2020.25 The usefulness of novel biomarkers and PCR in diagnosing PJI can be found in their ability to diagnose infections and facilitate appropriate early treatment. Several of these tests are readily available commercially and have the potential to be cost-effective diagnostic tools. The price to perform an AD test from Synovasure TM (Zimmer Biomet) ranges from $93 to $143. LE also provides an economic option for diagnosing PJI, as LE strips are commercially available for the cost of about 25 cents. PCR has also become an economic option, as costs can average $15.50 per sample extraction or PCR assay and $42.50 per amplicon sequence as reported in a study by Vandercam and colleagues.26 Future studies are needed to determine a diagnostic algorithm which incorporates these novel synovial markers to improve diagnostic accuracy of PJI in the most cost effective manner.
The current literature supports that AD can potentially be used to screen for PJI. Our findings suggest novel synovial fluid biomarkers may become of significant diagnostic use when combined with current laboratory and clinical diagnostic criteria. We recommend use of AD in cases in which pain, stiffness, and poor TJA outcome cannot be explained by errors in surgical technique, and infection is suspected despite MSIS criteria not being met.
The studies reviewed in this manuscript were limited in that none presented level I evidence (12 had level II evidence, and 3 had level III evidence), and there was significant heterogeneity (some studies used their own diagnostic standard, and others used the MSIS criteria). Larger scale prospective studies comparing serum ESR/CRP level and synovial fluid analysis to novel synovial markers are needed.
Am J Orthop. 2017;46(4):190-198. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-1397.
2. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
3. Sharkey PF, Lichstein PM, Shen C, Tokarski AT, Parvizi J. Why are total knee arthroplasties failing today—has anything changed after 10 years? J Arthroplasty. 2014;29(9):1774-1778.
4. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
5. Frangiamore SJ, Saleh A, Grosso MJ, et al. α-Defensin as a predictor of periprosthetic shoulder infection. J Shoulder Elbow Surg. 2015;24(7):1021-1027.
6. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(17):1439-1445.
7. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009.
8. Deirmengian C, Kardos K, Kilmartin P, et al. The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clin Orthop Relat Res. 2015;473(1):198-203.
9. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
10. Frangiamore SJ, Saleh A, Kovac MF, et al. Synovial fluid interleukin-6 as a predictor of periprosthetic shoulder infection. J Bone Joint Surg Am. 2015;97(1):63-70.
11. Lenski M, Scherer MA. Synovial IL-6 as inflammatory marker in periprosthetic joint infections. J Arthroplasty. 2014;29(6):1105-1109.
12. Randau TM, Friedrich MJ, Wimmer MD, et al. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One. 2014;9(2):e89045.
13. Guenther D, Kokenge T, Jacobs O, et al. Excluding infections in arthroplasty using leucocyte esterase test. Int Orthop. 2014;38(11):2385-2390.
14. Wetters NG, Berend KR, Lombardi AV, Morris MJ, Tucker TL, Della Valle CJ. Leukocyte esterase reagent strips for the rapid diagnosis of periprosthetic joint infection. J Arthroplasty. 2012;27(8 suppl):8-11.
15. Nelson GN, Paxton ES, Narzikul A, Williams G, Lazarus MD, Abboud JA. Leukocyte esterase in the diagnosis of shoulder periprosthetic joint infection. J Shoulder Elbow Surg. 2015;24(9):1421-1426.
16. Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: matched for Musculoskeletal Infection Society criteria. J Bone Joint Surg Am. 2014;96(22):1917-1920.
17. Dempsey KE, Riggio MP, Lennon A, et al. Identification of bacteria on the surface of clinically infected and non-infected prosthetic hip joints removed during revision arthroplasties by 16S rRNA gene sequencing and by microbiological culture. Arthritis Res Ther. 2007;9(3):R46.
18. Gallo J, Kolar M, Dendis M, et al. Culture and PCR analysis of joint fluid in the diagnosis of prosthetic joint infection. New Microbiol. 2008;31(1):97-104.
19. Marin M, Garcia-Lechuz JM, Alonso P, et al. Role of universal 16S rRNA gene PCR and sequencing in diagnosis of prosthetic joint infection. J Clin Microbiol. 2012;50(3):583-589.
20. Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, Parvizi J. Successful identification of pathogens by polymerase chain reaction (PCR)-based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2012;94(24):2247-2254.
21. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
22. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
23. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
24. Hunt RW, Bond MJ, Pater GD. Psychological responses to cancer: a case for cancer support groups. Community Health Stud. 1990;14(1):35-38.
25. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
26. Vandercam B, Jeumont S, Cornu O, et al. Amplification-based DNA analysis in the diagnosis of prosthetic joint infection. J Mol Diagn. 2008;10(6):537-543.
1. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-1397.
2. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
3. Sharkey PF, Lichstein PM, Shen C, Tokarski AT, Parvizi J. Why are total knee arthroplasties failing today—has anything changed after 10 years? J Arthroplasty. 2014;29(9):1774-1778.
4. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
5. Frangiamore SJ, Saleh A, Grosso MJ, et al. α-Defensin as a predictor of periprosthetic shoulder infection. J Shoulder Elbow Surg. 2015;24(7):1021-1027.
6. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(17):1439-1445.
7. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009.
8. Deirmengian C, Kardos K, Kilmartin P, et al. The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clin Orthop Relat Res. 2015;473(1):198-203.
9. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
10. Frangiamore SJ, Saleh A, Kovac MF, et al. Synovial fluid interleukin-6 as a predictor of periprosthetic shoulder infection. J Bone Joint Surg Am. 2015;97(1):63-70.
11. Lenski M, Scherer MA. Synovial IL-6 as inflammatory marker in periprosthetic joint infections. J Arthroplasty. 2014;29(6):1105-1109.
12. Randau TM, Friedrich MJ, Wimmer MD, et al. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One. 2014;9(2):e89045.
13. Guenther D, Kokenge T, Jacobs O, et al. Excluding infections in arthroplasty using leucocyte esterase test. Int Orthop. 2014;38(11):2385-2390.
14. Wetters NG, Berend KR, Lombardi AV, Morris MJ, Tucker TL, Della Valle CJ. Leukocyte esterase reagent strips for the rapid diagnosis of periprosthetic joint infection. J Arthroplasty. 2012;27(8 suppl):8-11.
15. Nelson GN, Paxton ES, Narzikul A, Williams G, Lazarus MD, Abboud JA. Leukocyte esterase in the diagnosis of shoulder periprosthetic joint infection. J Shoulder Elbow Surg. 2015;24(9):1421-1426.
16. Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: matched for Musculoskeletal Infection Society criteria. J Bone Joint Surg Am. 2014;96(22):1917-1920.
17. Dempsey KE, Riggio MP, Lennon A, et al. Identification of bacteria on the surface of clinically infected and non-infected prosthetic hip joints removed during revision arthroplasties by 16S rRNA gene sequencing and by microbiological culture. Arthritis Res Ther. 2007;9(3):R46.
18. Gallo J, Kolar M, Dendis M, et al. Culture and PCR analysis of joint fluid in the diagnosis of prosthetic joint infection. New Microbiol. 2008;31(1):97-104.
19. Marin M, Garcia-Lechuz JM, Alonso P, et al. Role of universal 16S rRNA gene PCR and sequencing in diagnosis of prosthetic joint infection. J Clin Microbiol. 2012;50(3):583-589.
20. Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, Parvizi J. Successful identification of pathogens by polymerase chain reaction (PCR)-based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2012;94(24):2247-2254.
21. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
22. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
23. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
24. Hunt RW, Bond MJ, Pater GD. Psychological responses to cancer: a case for cancer support groups. Community Health Stud. 1990;14(1):35-38.
25. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
26. Vandercam B, Jeumont S, Cornu O, et al. Amplification-based DNA analysis in the diagnosis of prosthetic joint infection. J Mol Diagn. 2008;10(6):537-543.
Difficult-to-Detect Low-Grade Infections Responsible for Poor Outcomes in Total Knee Arthroplasty
Take-Home Points
- Despite standardization of diagnostic criteria by the MSIS for the diagnosis of PJI, some low-grade inflections create a diagnostic challenge for clinicians.
- P acnes infection following TJA can be present despite patients having normal serum inflammatory marker levels and synovial fluid aspirations.
- Patients with a PJI with low virulence organisms can present with painful, arthrofibrotic joints that do not appear to be clinically infected.
- Biopsy for pathology and culture can aid in the diagnosis of suspected PJI in patients who fail to meet MSIS criteria.
- If detected and accurately diagnosed, PJI with P acnes can be successfully eradicated with IV antibiotics and 2-stage revision arthroplasty with a good functional outcome.
Total joint arthroplasty (TJA) is a routinely performed, highly efficacious procedure for patients with degenerative osteoarthritis.1,2 In the United States in 2003, more than 450,000 total knee arthroplasties (TKAs) were performed, and this number is projected to increase by more than 673% by 2030, as America’s population continues to age.3 With the increase in primary TJAs has come an increase in revision TJAs. The most common cause of revision TJA is infection (25.2%), which has a rate of 1% to 4% after primary TJA.1,4 Despite advancements in implant technology, preoperative preventive strategies, perioperative techniques, and postoperative management, a recent meta-analysis of patient follow-up data revealed that 15% to 20% of patients remained dissatisfied after TJA, despite having technically well-placed implants.5,6
Recent studies have suggested that prosthetic joint infection (PJI) may be underreported because of the difficulty in diagnosis, which may be one of the reasons why patients remain dissatisfied after TJA.7 As a result, new efforts have been made to develop uniform criteria for PJI diagnosis.8 In 2011, the Musculoskeletal Infection Society (MSIS) developed a new definition for the PJI diagnosis, based on clinical and laboratory criteria, in order to increase diagnostic accuracy. However, MSIS acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-grade infections, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. The biofilm-forming bacteria Propionibacterium acnes and Staphylococcus epidermidis are 2 such low-virulence organisms—once commonly considered contaminants but now recognized as potential pathogens for postoperative joint infections.9 In a review performed at a major orthopedic hospital, Bjerke-Kroll and colleagues10 found that the rate of PJI with P acnes has been increasing linearly over the past 14 years. According to reports in the literature,11-13P acnes has been isolated in 2% to 4% of all cases of PJI, and Zappe and colleagues13 found a P acnes PJI rate of 6% in a retrospective analysis performed at their institution. Given the high rate of P acnes colonization of the axilla, this organism is now increasingly recognized as a cause of infection after shoulder surgery, as found in a case series of 10 patients with P acnes PJI after total shoulder arthroplasty (TSA).14 However, there is still limited data on the role of P acnes in lower extremity PJI.
Although patients with P acnes PJI can present with overt signs of infection, more often they lack systemic or local signs of infection, making the diagnosis difficult.15 Surgeons may not consider PJI as a cause of TJA failure in patients who do not meet diagnostic criteria.7 In a case series of patients with P acnes PJI after TSA, Millett and colleagues14 concluded that erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level are not always reliable indicators of infection with low-virulence organisms. Eighty percent of patients in their study had normal ESR and CRP level before surgery. Zappe and colleagues13 reported on P acnes PJI diagnoses in 4 total hip arthroplasties (THAs), 3 TKAs, and 1 TSA. Of the 8 patients, 6 (75%) had borderline elevated CRP levels, and 4 (50%) had normal synovial fluid analysis and cultures from joint aspirations. In a study using electron microscopy and fluorescence in situ hybridization (FISH) labeling, Stoodley and colleagues16 found, in 8 polyethylene liners removed from culture-negative THA patients for aseptic loosening, extensive biofilm colonization with S epidermidis.
Reports of PJI cases misdiagnosed as aseptic loosening also suggest that screening and diagnostic tools are not sensitive enough to detect all infections and that PJI likely is underdiagnosed. In a prospective cohort study, Portillo and colleagues17 categorized patients who were undergoing revision surgery after TJA by cause of failure: aseptic loosening, mechanical failure, or PJI based on current MSIS guidelines. Intraoperative cultures were taken during the revisions. P acnes was isolated in 2 (3%) of the 63 cases classified as PJI and in 12 (19%) of the 63 classified as aseptic loosening. Tsukayama and colleagues18 reported an 11% rate of positive intraoperative cultures for P acnes during revision surgery in cases that the operating surgeon considered aseptic, based on white blood cell (WBC) count, ESR, and CRP level. Rasouli and colleagues19 used an Ibis biosensor to perform polymerase chain reaction (PCR) on synovial fluid from 44 patients who underwent aseptic revision of TKA failures. The authors detected a pathogen in 17 (38%) of the 44 presumed aseptic patients and concluded some aseptic loosening cases are actually chronic low-grade organism PJIs not diagnosed according to current PJI criteria.
In this article, we present the case of a patient with a stiff, painful knee after TKA and with ESR, CRP level, and synovial fluid analysis within normal limits. Open biopsy for cultures showed P acnes PJI, which was successfully treated with 2-stage revision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 69-year-old man with a past medical history of hypertension underwent left primary TKA in 2012. In 2014, he presented to our office complaining of chronic left knee pain and stiffness that had developed insidiously over the first 3 months after surgery and never improved, despite rigorous physical therapy (Table).
Despite not meeting MSIS diagnostic criteria, the patient elected to undergo open biopsy for synovial culture as a last resort. During surgery, there was no purulence in the joint, and frozen section showed <5 neutrophils per high-power field. All cultures from 5 separate synovial tissue samples grew P acnes, confirming the PJI diagnosis. Cultures turned positive after being incubated an average of 12.2 days (range, 10-14 days). Sensitivities showed the organism was responsive to oxacillin. The risks and benefits of 2-stage revision surgery were discussed with the patient at the next office visit, and he decided on 2-stage revision. On November 4, 2014, he underwent open synovectomy, irrigation and débridement with iodine and Dakin solution, hardware removal, and cement antibiotic spacer placement without complication (Figures 3A, 3B).
Just before stage 2 revision on January 6, 2015, preoperative inflammatory markers were within normal limits. During surgery, additional cultures were taken from synovial tissue. At 15 days, these cultures showed no growth, confirming eradication of the infection. The patient underwent reimplantation without complication and had an uneventful postoperative course with no wound-healing issues (Figures 4A, 4B).
Discussion
Because PJIs with low-virulence organisms can present with normal levels of inflammatory markers and negative fluid analysis and culture from joint aspirations, they pose a diagnostic challenge for arthroplasty surgeons. In this case report, there was a low index of suspicion for PJI based on radiographic, physical examination, and laboratory findings. Our patient did not meet MSIS diagnostic criteria for PJI before undergoing open biopsy. Initial cultures from joint aspiration of synovial fluid were negative, and inflammatory markers were within normal limits. However, all 5 synovial tissue biopsy specimens that were cultured confirmed a low-grade periprosthetic infection with P acnes—likely the reason for the poor outcome. This case supports Zappe and colleagues13 and Millett and colleagues,14 who found that a subset of patients with a low-grade organism PJI had normal to mildly elevated inflammatory markers and negative fluid analysis and cultures from joint aspirations.
Hardware-involved orthopedic infections are often caused by bacteria that form a biofilm, which can be difficult to culture. Biofilm matrix binds cells into aggregates, which grow only a single colony on culture media, decreasing positive yield. Therefore, synovial fluid cultures are often negative, because of the low number of planktonic cells removed by aspirate. Using FISH and PCR, Stoodley and colleagues16 found biofilm on hardware removed for “culture-negative aseptic loosening.” This is especially important for low-grade organism infections that lack a strong inflammatory response in the joint and that may be missed with traditional screening. This may be one reason our patient’s synovial fluid cultures and inflammatory markers were negative.
Another reason these low-grade infections can be missed is that P acnes is notoriously difficult to culture—it may take up to 15 days to grow in a special medium.20 Intraoperative cultures may be read as false-negative if not incubated the right amount of time. In many hospitals, aerobic and anaerobic cultures are discarded if there is no growth after 3 to 5 days. In our patient’s case, the earliest that cultures turned positive was on day 10—which is consistent with other reports, including one by Butler-Wu and colleagues,15 who suggested a minimum incubation of 13 days for optimal recovery of organisms. Our case highlights the importance of lengthening incubation to allow for growth of low-virulent organisms. Given the different types of management used for PJI and aseptic loosening, it is imperative that surgeons take cultures during revision TJA and that cultures are held up to 14 days to allow enough time for low-virulence organisms to grow.
Fortunately, PJI with low-virulence organisms can be treated successfully. Treating P acnes PJI with exchange arthroplasty and IV antibiotics has documented success rates as high as 92%.21 Again, we emphasize the importance of obtaining intraoperative cultures to determine antibiotic sensitivities, which can guide treatment. Our patient’s infection was eradicated with 2-stage revision and IV antibiotics, and his symptoms, ROM, and function improved significantly.
Diagnosing PJI after TJA can be challenging, as there is no definitive test that is sensitive, specific, rapid, and minimally invasive. Researchers have looked for novel serum or synovial fluid biomarkers that may be elevated in PJI. Synovial interleukin 6 (IL-6) and synovial α-defensin show great promise. In 2 separate studies, elevated IL-6 levels strongly correlated with infection.22,23 Jacovides and colleagues23 found that a synovial IL-6 level higher than 4270 pg/mL had a 100% positive predictive value and a 91% negative predictive value for diagnosing PJI. In some trials, synovial α-defensin has shown up to 100% sensitivity and specificity for PJI diagnosis. Most notably, in a trial by Frangiamore and colleagues,24 α-defensin levels were elevated to statistically significant levels in P acnes PJI, indicating this test may help in diagnosing PJI with low-virulence organisms. Finally, PCR has also shown promise in detecting low-grade joint infections. PCR uses 16 primers that allow not only for the identification of pan-genomic bacterial markers, specific bacterial organisms, and Candida, but also for the presence of antibiotic resistance markers. Use of pan-genomic PCR also allows for detection of a wider variety of pathogens, including organisms commonly missed by conventional culture methods.25Early intervention can significantly improve outcomes in PJI. Therefore, we recommend maintaining a high index of suspicion for low-virulence PJI in patients with chronic pain and decreased functionality after TJA with well-placed implants, despite their not meeting current MSIS diagnostic criteria for PJI. As new microbiological tools for detecting PJI with low-grade organisms are developed, use of these technologies can be incorporated into the diagnosis algorithm. Screening tools more sensitive in detecting low-grade organisms can help avoid the morbidity associated with interoperative synovial biopsies for culture and can allow for more efficient surgical planning. These tools, along with increased clinical awareness of potential PJIs, ultimately will lead to earlier detection, accurate diagnosis, and optimal treatment.
Am J Orthop. 2017;46(3):E148-E153. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
2. Kamath AF, Ong KL, Lau E, et al. Quantifying the burden of revision total joint arthroplasty for periprosthetic infection. J Arthroplasty. 2015;30(9):1492-1497.
3. Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144-151.
4. Zmistowski B, Restrepo C, Huang R, Hozack WJ, Parvizi J. Periprosthetic joint infection diagnosis: a complete understanding of white blood cell count and differential. J Arthroplasty. 2012;27(9):1589-1593.
5. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
6. Djahani O, Rainer S, Pietsch M, Hofmann S. Systematic analysis of painful total knee prosthesis, a diagnostic algorithm. Arch Bone Jt Surg. 2013;1(2):48-52.
7. Parvizi J, Suh DH, Jafari SM, Mullan A, Purtill JJ. Aseptic loosening of total hip arthroplasty: infection always should be ruled out. Clin Orthop Relat Res. 2011;469(5):1401-1405.
8. Della Valle C, Parvizi J, Bauer TW, et al. Diagnosis of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18(12):760-770.
9. Dramis A, Aldlyami E, Grimer RJ, Dunlop DJ, O’Connell N, Elliott T. What is the significance of a positive Propionibacterium acnes culture around a joint replacement? Int Orthop. 2009;33(3):829-833.
10. Bjerke-Kroll BT, Christ AB, Mclawhorn AS, Sculco PK, Jules-Elysée KM, Sculco TP. Periprosthetic joint infections treated with two-stage revision over 14 years: an evolving microbiology profile. J Arthroplasty. 2014;29(5):877-882.
11. Pandey R, Berendt AR, Athanasou NA. Histological and microbiological findings in non-infected and infected revision arthroplasty tissues. The OSIRIS Collaborative Study Group. Oxford Skeletal Infection Research and Intervention Service. Arch Orthop Trauma Surg. 2000;120(10):570-574.
12. Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81(10):1434-1445.
13. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
14. Millett PJ, Yen YM, Price CS, Horan MP, van der Meijden OA, Elser F. Propionibacterium acnes infection as an occult cause of postoperative shoulder pain: a case series. Clin Orthop Relat Res. 2011;469(10):2824-2830.
15. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
16. Stoodley P, Ehrlich GD, Sedghizadeh PP, et al. Orthopaedic biofilm infections. Curr Orthop Pract. 2011;22(6):558-563.
17. Portillo ME, Salvadó M, Alier A, et al. Prosthesis failure within 2 years of implantation is highly predictive of infection. Clin Orthop Relat Res. 2013;471(11):3672-3678.
18. Tsukayama DT, Strada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78(4):512-523.
19. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
20. Schäfer P, Fink B, Sandow D, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis. 2008;47(11):1403-1409.
21. Zeller V, Ghorbani A, Strady C, Leonard P, Mamoudy P, Desplaces N. Propionibacterium acnes: an agent of prosthetic joint infection and colonization. J Infect. 2007;55(2):119-124.
22. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472(11):3254-3262.
23. Jacovides CL, Parvizi J, Adeli B, Jung KA. Molecular markers for diagnosis of periprosthetic joint infection. J Arthroplasty. 2011;26(6 suppl):99-103.e1.
24. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
25. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
Take-Home Points
- Despite standardization of diagnostic criteria by the MSIS for the diagnosis of PJI, some low-grade inflections create a diagnostic challenge for clinicians.
- P acnes infection following TJA can be present despite patients having normal serum inflammatory marker levels and synovial fluid aspirations.
- Patients with a PJI with low virulence organisms can present with painful, arthrofibrotic joints that do not appear to be clinically infected.
- Biopsy for pathology and culture can aid in the diagnosis of suspected PJI in patients who fail to meet MSIS criteria.
- If detected and accurately diagnosed, PJI with P acnes can be successfully eradicated with IV antibiotics and 2-stage revision arthroplasty with a good functional outcome.
Total joint arthroplasty (TJA) is a routinely performed, highly efficacious procedure for patients with degenerative osteoarthritis.1,2 In the United States in 2003, more than 450,000 total knee arthroplasties (TKAs) were performed, and this number is projected to increase by more than 673% by 2030, as America’s population continues to age.3 With the increase in primary TJAs has come an increase in revision TJAs. The most common cause of revision TJA is infection (25.2%), which has a rate of 1% to 4% after primary TJA.1,4 Despite advancements in implant technology, preoperative preventive strategies, perioperative techniques, and postoperative management, a recent meta-analysis of patient follow-up data revealed that 15% to 20% of patients remained dissatisfied after TJA, despite having technically well-placed implants.5,6
Recent studies have suggested that prosthetic joint infection (PJI) may be underreported because of the difficulty in diagnosis, which may be one of the reasons why patients remain dissatisfied after TJA.7 As a result, new efforts have been made to develop uniform criteria for PJI diagnosis.8 In 2011, the Musculoskeletal Infection Society (MSIS) developed a new definition for the PJI diagnosis, based on clinical and laboratory criteria, in order to increase diagnostic accuracy. However, MSIS acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-grade infections, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. The biofilm-forming bacteria Propionibacterium acnes and Staphylococcus epidermidis are 2 such low-virulence organisms—once commonly considered contaminants but now recognized as potential pathogens for postoperative joint infections.9 In a review performed at a major orthopedic hospital, Bjerke-Kroll and colleagues10 found that the rate of PJI with P acnes has been increasing linearly over the past 14 years. According to reports in the literature,11-13P acnes has been isolated in 2% to 4% of all cases of PJI, and Zappe and colleagues13 found a P acnes PJI rate of 6% in a retrospective analysis performed at their institution. Given the high rate of P acnes colonization of the axilla, this organism is now increasingly recognized as a cause of infection after shoulder surgery, as found in a case series of 10 patients with P acnes PJI after total shoulder arthroplasty (TSA).14 However, there is still limited data on the role of P acnes in lower extremity PJI.
Although patients with P acnes PJI can present with overt signs of infection, more often they lack systemic or local signs of infection, making the diagnosis difficult.15 Surgeons may not consider PJI as a cause of TJA failure in patients who do not meet diagnostic criteria.7 In a case series of patients with P acnes PJI after TSA, Millett and colleagues14 concluded that erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level are not always reliable indicators of infection with low-virulence organisms. Eighty percent of patients in their study had normal ESR and CRP level before surgery. Zappe and colleagues13 reported on P acnes PJI diagnoses in 4 total hip arthroplasties (THAs), 3 TKAs, and 1 TSA. Of the 8 patients, 6 (75%) had borderline elevated CRP levels, and 4 (50%) had normal synovial fluid analysis and cultures from joint aspirations. In a study using electron microscopy and fluorescence in situ hybridization (FISH) labeling, Stoodley and colleagues16 found, in 8 polyethylene liners removed from culture-negative THA patients for aseptic loosening, extensive biofilm colonization with S epidermidis.
Reports of PJI cases misdiagnosed as aseptic loosening also suggest that screening and diagnostic tools are not sensitive enough to detect all infections and that PJI likely is underdiagnosed. In a prospective cohort study, Portillo and colleagues17 categorized patients who were undergoing revision surgery after TJA by cause of failure: aseptic loosening, mechanical failure, or PJI based on current MSIS guidelines. Intraoperative cultures were taken during the revisions. P acnes was isolated in 2 (3%) of the 63 cases classified as PJI and in 12 (19%) of the 63 classified as aseptic loosening. Tsukayama and colleagues18 reported an 11% rate of positive intraoperative cultures for P acnes during revision surgery in cases that the operating surgeon considered aseptic, based on white blood cell (WBC) count, ESR, and CRP level. Rasouli and colleagues19 used an Ibis biosensor to perform polymerase chain reaction (PCR) on synovial fluid from 44 patients who underwent aseptic revision of TKA failures. The authors detected a pathogen in 17 (38%) of the 44 presumed aseptic patients and concluded some aseptic loosening cases are actually chronic low-grade organism PJIs not diagnosed according to current PJI criteria.
In this article, we present the case of a patient with a stiff, painful knee after TKA and with ESR, CRP level, and synovial fluid analysis within normal limits. Open biopsy for cultures showed P acnes PJI, which was successfully treated with 2-stage revision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 69-year-old man with a past medical history of hypertension underwent left primary TKA in 2012. In 2014, he presented to our office complaining of chronic left knee pain and stiffness that had developed insidiously over the first 3 months after surgery and never improved, despite rigorous physical therapy (Table).
Despite not meeting MSIS diagnostic criteria, the patient elected to undergo open biopsy for synovial culture as a last resort. During surgery, there was no purulence in the joint, and frozen section showed <5 neutrophils per high-power field. All cultures from 5 separate synovial tissue samples grew P acnes, confirming the PJI diagnosis. Cultures turned positive after being incubated an average of 12.2 days (range, 10-14 days). Sensitivities showed the organism was responsive to oxacillin. The risks and benefits of 2-stage revision surgery were discussed with the patient at the next office visit, and he decided on 2-stage revision. On November 4, 2014, he underwent open synovectomy, irrigation and débridement with iodine and Dakin solution, hardware removal, and cement antibiotic spacer placement without complication (Figures 3A, 3B).
Just before stage 2 revision on January 6, 2015, preoperative inflammatory markers were within normal limits. During surgery, additional cultures were taken from synovial tissue. At 15 days, these cultures showed no growth, confirming eradication of the infection. The patient underwent reimplantation without complication and had an uneventful postoperative course with no wound-healing issues (Figures 4A, 4B).
Discussion
Because PJIs with low-virulence organisms can present with normal levels of inflammatory markers and negative fluid analysis and culture from joint aspirations, they pose a diagnostic challenge for arthroplasty surgeons. In this case report, there was a low index of suspicion for PJI based on radiographic, physical examination, and laboratory findings. Our patient did not meet MSIS diagnostic criteria for PJI before undergoing open biopsy. Initial cultures from joint aspiration of synovial fluid were negative, and inflammatory markers were within normal limits. However, all 5 synovial tissue biopsy specimens that were cultured confirmed a low-grade periprosthetic infection with P acnes—likely the reason for the poor outcome. This case supports Zappe and colleagues13 and Millett and colleagues,14 who found that a subset of patients with a low-grade organism PJI had normal to mildly elevated inflammatory markers and negative fluid analysis and cultures from joint aspirations.
Hardware-involved orthopedic infections are often caused by bacteria that form a biofilm, which can be difficult to culture. Biofilm matrix binds cells into aggregates, which grow only a single colony on culture media, decreasing positive yield. Therefore, synovial fluid cultures are often negative, because of the low number of planktonic cells removed by aspirate. Using FISH and PCR, Stoodley and colleagues16 found biofilm on hardware removed for “culture-negative aseptic loosening.” This is especially important for low-grade organism infections that lack a strong inflammatory response in the joint and that may be missed with traditional screening. This may be one reason our patient’s synovial fluid cultures and inflammatory markers were negative.
Another reason these low-grade infections can be missed is that P acnes is notoriously difficult to culture—it may take up to 15 days to grow in a special medium.20 Intraoperative cultures may be read as false-negative if not incubated the right amount of time. In many hospitals, aerobic and anaerobic cultures are discarded if there is no growth after 3 to 5 days. In our patient’s case, the earliest that cultures turned positive was on day 10—which is consistent with other reports, including one by Butler-Wu and colleagues,15 who suggested a minimum incubation of 13 days for optimal recovery of organisms. Our case highlights the importance of lengthening incubation to allow for growth of low-virulent organisms. Given the different types of management used for PJI and aseptic loosening, it is imperative that surgeons take cultures during revision TJA and that cultures are held up to 14 days to allow enough time for low-virulence organisms to grow.
Fortunately, PJI with low-virulence organisms can be treated successfully. Treating P acnes PJI with exchange arthroplasty and IV antibiotics has documented success rates as high as 92%.21 Again, we emphasize the importance of obtaining intraoperative cultures to determine antibiotic sensitivities, which can guide treatment. Our patient’s infection was eradicated with 2-stage revision and IV antibiotics, and his symptoms, ROM, and function improved significantly.
Diagnosing PJI after TJA can be challenging, as there is no definitive test that is sensitive, specific, rapid, and minimally invasive. Researchers have looked for novel serum or synovial fluid biomarkers that may be elevated in PJI. Synovial interleukin 6 (IL-6) and synovial α-defensin show great promise. In 2 separate studies, elevated IL-6 levels strongly correlated with infection.22,23 Jacovides and colleagues23 found that a synovial IL-6 level higher than 4270 pg/mL had a 100% positive predictive value and a 91% negative predictive value for diagnosing PJI. In some trials, synovial α-defensin has shown up to 100% sensitivity and specificity for PJI diagnosis. Most notably, in a trial by Frangiamore and colleagues,24 α-defensin levels were elevated to statistically significant levels in P acnes PJI, indicating this test may help in diagnosing PJI with low-virulence organisms. Finally, PCR has also shown promise in detecting low-grade joint infections. PCR uses 16 primers that allow not only for the identification of pan-genomic bacterial markers, specific bacterial organisms, and Candida, but also for the presence of antibiotic resistance markers. Use of pan-genomic PCR also allows for detection of a wider variety of pathogens, including organisms commonly missed by conventional culture methods.25Early intervention can significantly improve outcomes in PJI. Therefore, we recommend maintaining a high index of suspicion for low-virulence PJI in patients with chronic pain and decreased functionality after TJA with well-placed implants, despite their not meeting current MSIS diagnostic criteria for PJI. As new microbiological tools for detecting PJI with low-grade organisms are developed, use of these technologies can be incorporated into the diagnosis algorithm. Screening tools more sensitive in detecting low-grade organisms can help avoid the morbidity associated with interoperative synovial biopsies for culture and can allow for more efficient surgical planning. These tools, along with increased clinical awareness of potential PJIs, ultimately will lead to earlier detection, accurate diagnosis, and optimal treatment.
Am J Orthop. 2017;46(3):E148-E153. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Despite standardization of diagnostic criteria by the MSIS for the diagnosis of PJI, some low-grade inflections create a diagnostic challenge for clinicians.
- P acnes infection following TJA can be present despite patients having normal serum inflammatory marker levels and synovial fluid aspirations.
- Patients with a PJI with low virulence organisms can present with painful, arthrofibrotic joints that do not appear to be clinically infected.
- Biopsy for pathology and culture can aid in the diagnosis of suspected PJI in patients who fail to meet MSIS criteria.
- If detected and accurately diagnosed, PJI with P acnes can be successfully eradicated with IV antibiotics and 2-stage revision arthroplasty with a good functional outcome.
Total joint arthroplasty (TJA) is a routinely performed, highly efficacious procedure for patients with degenerative osteoarthritis.1,2 In the United States in 2003, more than 450,000 total knee arthroplasties (TKAs) were performed, and this number is projected to increase by more than 673% by 2030, as America’s population continues to age.3 With the increase in primary TJAs has come an increase in revision TJAs. The most common cause of revision TJA is infection (25.2%), which has a rate of 1% to 4% after primary TJA.1,4 Despite advancements in implant technology, preoperative preventive strategies, perioperative techniques, and postoperative management, a recent meta-analysis of patient follow-up data revealed that 15% to 20% of patients remained dissatisfied after TJA, despite having technically well-placed implants.5,6
Recent studies have suggested that prosthetic joint infection (PJI) may be underreported because of the difficulty in diagnosis, which may be one of the reasons why patients remain dissatisfied after TJA.7 As a result, new efforts have been made to develop uniform criteria for PJI diagnosis.8 In 2011, the Musculoskeletal Infection Society (MSIS) developed a new definition for the PJI diagnosis, based on clinical and laboratory criteria, in order to increase diagnostic accuracy. However, MSIS acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-grade infections, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. The biofilm-forming bacteria Propionibacterium acnes and Staphylococcus epidermidis are 2 such low-virulence organisms—once commonly considered contaminants but now recognized as potential pathogens for postoperative joint infections.9 In a review performed at a major orthopedic hospital, Bjerke-Kroll and colleagues10 found that the rate of PJI with P acnes has been increasing linearly over the past 14 years. According to reports in the literature,11-13P acnes has been isolated in 2% to 4% of all cases of PJI, and Zappe and colleagues13 found a P acnes PJI rate of 6% in a retrospective analysis performed at their institution. Given the high rate of P acnes colonization of the axilla, this organism is now increasingly recognized as a cause of infection after shoulder surgery, as found in a case series of 10 patients with P acnes PJI after total shoulder arthroplasty (TSA).14 However, there is still limited data on the role of P acnes in lower extremity PJI.
Although patients with P acnes PJI can present with overt signs of infection, more often they lack systemic or local signs of infection, making the diagnosis difficult.15 Surgeons may not consider PJI as a cause of TJA failure in patients who do not meet diagnostic criteria.7 In a case series of patients with P acnes PJI after TSA, Millett and colleagues14 concluded that erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level are not always reliable indicators of infection with low-virulence organisms. Eighty percent of patients in their study had normal ESR and CRP level before surgery. Zappe and colleagues13 reported on P acnes PJI diagnoses in 4 total hip arthroplasties (THAs), 3 TKAs, and 1 TSA. Of the 8 patients, 6 (75%) had borderline elevated CRP levels, and 4 (50%) had normal synovial fluid analysis and cultures from joint aspirations. In a study using electron microscopy and fluorescence in situ hybridization (FISH) labeling, Stoodley and colleagues16 found, in 8 polyethylene liners removed from culture-negative THA patients for aseptic loosening, extensive biofilm colonization with S epidermidis.
Reports of PJI cases misdiagnosed as aseptic loosening also suggest that screening and diagnostic tools are not sensitive enough to detect all infections and that PJI likely is underdiagnosed. In a prospective cohort study, Portillo and colleagues17 categorized patients who were undergoing revision surgery after TJA by cause of failure: aseptic loosening, mechanical failure, or PJI based on current MSIS guidelines. Intraoperative cultures were taken during the revisions. P acnes was isolated in 2 (3%) of the 63 cases classified as PJI and in 12 (19%) of the 63 classified as aseptic loosening. Tsukayama and colleagues18 reported an 11% rate of positive intraoperative cultures for P acnes during revision surgery in cases that the operating surgeon considered aseptic, based on white blood cell (WBC) count, ESR, and CRP level. Rasouli and colleagues19 used an Ibis biosensor to perform polymerase chain reaction (PCR) on synovial fluid from 44 patients who underwent aseptic revision of TKA failures. The authors detected a pathogen in 17 (38%) of the 44 presumed aseptic patients and concluded some aseptic loosening cases are actually chronic low-grade organism PJIs not diagnosed according to current PJI criteria.
In this article, we present the case of a patient with a stiff, painful knee after TKA and with ESR, CRP level, and synovial fluid analysis within normal limits. Open biopsy for cultures showed P acnes PJI, which was successfully treated with 2-stage revision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 69-year-old man with a past medical history of hypertension underwent left primary TKA in 2012. In 2014, he presented to our office complaining of chronic left knee pain and stiffness that had developed insidiously over the first 3 months after surgery and never improved, despite rigorous physical therapy (Table).
Despite not meeting MSIS diagnostic criteria, the patient elected to undergo open biopsy for synovial culture as a last resort. During surgery, there was no purulence in the joint, and frozen section showed <5 neutrophils per high-power field. All cultures from 5 separate synovial tissue samples grew P acnes, confirming the PJI diagnosis. Cultures turned positive after being incubated an average of 12.2 days (range, 10-14 days). Sensitivities showed the organism was responsive to oxacillin. The risks and benefits of 2-stage revision surgery were discussed with the patient at the next office visit, and he decided on 2-stage revision. On November 4, 2014, he underwent open synovectomy, irrigation and débridement with iodine and Dakin solution, hardware removal, and cement antibiotic spacer placement without complication (Figures 3A, 3B).
Just before stage 2 revision on January 6, 2015, preoperative inflammatory markers were within normal limits. During surgery, additional cultures were taken from synovial tissue. At 15 days, these cultures showed no growth, confirming eradication of the infection. The patient underwent reimplantation without complication and had an uneventful postoperative course with no wound-healing issues (Figures 4A, 4B).
Discussion
Because PJIs with low-virulence organisms can present with normal levels of inflammatory markers and negative fluid analysis and culture from joint aspirations, they pose a diagnostic challenge for arthroplasty surgeons. In this case report, there was a low index of suspicion for PJI based on radiographic, physical examination, and laboratory findings. Our patient did not meet MSIS diagnostic criteria for PJI before undergoing open biopsy. Initial cultures from joint aspiration of synovial fluid were negative, and inflammatory markers were within normal limits. However, all 5 synovial tissue biopsy specimens that were cultured confirmed a low-grade periprosthetic infection with P acnes—likely the reason for the poor outcome. This case supports Zappe and colleagues13 and Millett and colleagues,14 who found that a subset of patients with a low-grade organism PJI had normal to mildly elevated inflammatory markers and negative fluid analysis and cultures from joint aspirations.
Hardware-involved orthopedic infections are often caused by bacteria that form a biofilm, which can be difficult to culture. Biofilm matrix binds cells into aggregates, which grow only a single colony on culture media, decreasing positive yield. Therefore, synovial fluid cultures are often negative, because of the low number of planktonic cells removed by aspirate. Using FISH and PCR, Stoodley and colleagues16 found biofilm on hardware removed for “culture-negative aseptic loosening.” This is especially important for low-grade organism infections that lack a strong inflammatory response in the joint and that may be missed with traditional screening. This may be one reason our patient’s synovial fluid cultures and inflammatory markers were negative.
Another reason these low-grade infections can be missed is that P acnes is notoriously difficult to culture—it may take up to 15 days to grow in a special medium.20 Intraoperative cultures may be read as false-negative if not incubated the right amount of time. In many hospitals, aerobic and anaerobic cultures are discarded if there is no growth after 3 to 5 days. In our patient’s case, the earliest that cultures turned positive was on day 10—which is consistent with other reports, including one by Butler-Wu and colleagues,15 who suggested a minimum incubation of 13 days for optimal recovery of organisms. Our case highlights the importance of lengthening incubation to allow for growth of low-virulent organisms. Given the different types of management used for PJI and aseptic loosening, it is imperative that surgeons take cultures during revision TJA and that cultures are held up to 14 days to allow enough time for low-virulence organisms to grow.
Fortunately, PJI with low-virulence organisms can be treated successfully. Treating P acnes PJI with exchange arthroplasty and IV antibiotics has documented success rates as high as 92%.21 Again, we emphasize the importance of obtaining intraoperative cultures to determine antibiotic sensitivities, which can guide treatment. Our patient’s infection was eradicated with 2-stage revision and IV antibiotics, and his symptoms, ROM, and function improved significantly.
Diagnosing PJI after TJA can be challenging, as there is no definitive test that is sensitive, specific, rapid, and minimally invasive. Researchers have looked for novel serum or synovial fluid biomarkers that may be elevated in PJI. Synovial interleukin 6 (IL-6) and synovial α-defensin show great promise. In 2 separate studies, elevated IL-6 levels strongly correlated with infection.22,23 Jacovides and colleagues23 found that a synovial IL-6 level higher than 4270 pg/mL had a 100% positive predictive value and a 91% negative predictive value for diagnosing PJI. In some trials, synovial α-defensin has shown up to 100% sensitivity and specificity for PJI diagnosis. Most notably, in a trial by Frangiamore and colleagues,24 α-defensin levels were elevated to statistically significant levels in P acnes PJI, indicating this test may help in diagnosing PJI with low-virulence organisms. Finally, PCR has also shown promise in detecting low-grade joint infections. PCR uses 16 primers that allow not only for the identification of pan-genomic bacterial markers, specific bacterial organisms, and Candida, but also for the presence of antibiotic resistance markers. Use of pan-genomic PCR also allows for detection of a wider variety of pathogens, including organisms commonly missed by conventional culture methods.25Early intervention can significantly improve outcomes in PJI. Therefore, we recommend maintaining a high index of suspicion for low-virulence PJI in patients with chronic pain and decreased functionality after TJA with well-placed implants, despite their not meeting current MSIS diagnostic criteria for PJI. As new microbiological tools for detecting PJI with low-grade organisms are developed, use of these technologies can be incorporated into the diagnosis algorithm. Screening tools more sensitive in detecting low-grade organisms can help avoid the morbidity associated with interoperative synovial biopsies for culture and can allow for more efficient surgical planning. These tools, along with increased clinical awareness of potential PJIs, ultimately will lead to earlier detection, accurate diagnosis, and optimal treatment.
Am J Orthop. 2017;46(3):E148-E153. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
2. Kamath AF, Ong KL, Lau E, et al. Quantifying the burden of revision total joint arthroplasty for periprosthetic infection. J Arthroplasty. 2015;30(9):1492-1497.
3. Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144-151.
4. Zmistowski B, Restrepo C, Huang R, Hozack WJ, Parvizi J. Periprosthetic joint infection diagnosis: a complete understanding of white blood cell count and differential. J Arthroplasty. 2012;27(9):1589-1593.
5. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
6. Djahani O, Rainer S, Pietsch M, Hofmann S. Systematic analysis of painful total knee prosthesis, a diagnostic algorithm. Arch Bone Jt Surg. 2013;1(2):48-52.
7. Parvizi J, Suh DH, Jafari SM, Mullan A, Purtill JJ. Aseptic loosening of total hip arthroplasty: infection always should be ruled out. Clin Orthop Relat Res. 2011;469(5):1401-1405.
8. Della Valle C, Parvizi J, Bauer TW, et al. Diagnosis of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18(12):760-770.
9. Dramis A, Aldlyami E, Grimer RJ, Dunlop DJ, O’Connell N, Elliott T. What is the significance of a positive Propionibacterium acnes culture around a joint replacement? Int Orthop. 2009;33(3):829-833.
10. Bjerke-Kroll BT, Christ AB, Mclawhorn AS, Sculco PK, Jules-Elysée KM, Sculco TP. Periprosthetic joint infections treated with two-stage revision over 14 years: an evolving microbiology profile. J Arthroplasty. 2014;29(5):877-882.
11. Pandey R, Berendt AR, Athanasou NA. Histological and microbiological findings in non-infected and infected revision arthroplasty tissues. The OSIRIS Collaborative Study Group. Oxford Skeletal Infection Research and Intervention Service. Arch Orthop Trauma Surg. 2000;120(10):570-574.
12. Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81(10):1434-1445.
13. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
14. Millett PJ, Yen YM, Price CS, Horan MP, van der Meijden OA, Elser F. Propionibacterium acnes infection as an occult cause of postoperative shoulder pain: a case series. Clin Orthop Relat Res. 2011;469(10):2824-2830.
15. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
16. Stoodley P, Ehrlich GD, Sedghizadeh PP, et al. Orthopaedic biofilm infections. Curr Orthop Pract. 2011;22(6):558-563.
17. Portillo ME, Salvadó M, Alier A, et al. Prosthesis failure within 2 years of implantation is highly predictive of infection. Clin Orthop Relat Res. 2013;471(11):3672-3678.
18. Tsukayama DT, Strada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78(4):512-523.
19. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
20. Schäfer P, Fink B, Sandow D, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis. 2008;47(11):1403-1409.
21. Zeller V, Ghorbani A, Strady C, Leonard P, Mamoudy P, Desplaces N. Propionibacterium acnes: an agent of prosthetic joint infection and colonization. J Infect. 2007;55(2):119-124.
22. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472(11):3254-3262.
23. Jacovides CL, Parvizi J, Adeli B, Jung KA. Molecular markers for diagnosis of periprosthetic joint infection. J Arthroplasty. 2011;26(6 suppl):99-103.e1.
24. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
25. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
1. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
2. Kamath AF, Ong KL, Lau E, et al. Quantifying the burden of revision total joint arthroplasty for periprosthetic infection. J Arthroplasty. 2015;30(9):1492-1497.
3. Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144-151.
4. Zmistowski B, Restrepo C, Huang R, Hozack WJ, Parvizi J. Periprosthetic joint infection diagnosis: a complete understanding of white blood cell count and differential. J Arthroplasty. 2012;27(9):1589-1593.
5. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
6. Djahani O, Rainer S, Pietsch M, Hofmann S. Systematic analysis of painful total knee prosthesis, a diagnostic algorithm. Arch Bone Jt Surg. 2013;1(2):48-52.
7. Parvizi J, Suh DH, Jafari SM, Mullan A, Purtill JJ. Aseptic loosening of total hip arthroplasty: infection always should be ruled out. Clin Orthop Relat Res. 2011;469(5):1401-1405.
8. Della Valle C, Parvizi J, Bauer TW, et al. Diagnosis of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18(12):760-770.
9. Dramis A, Aldlyami E, Grimer RJ, Dunlop DJ, O’Connell N, Elliott T. What is the significance of a positive Propionibacterium acnes culture around a joint replacement? Int Orthop. 2009;33(3):829-833.
10. Bjerke-Kroll BT, Christ AB, Mclawhorn AS, Sculco PK, Jules-Elysée KM, Sculco TP. Periprosthetic joint infections treated with two-stage revision over 14 years: an evolving microbiology profile. J Arthroplasty. 2014;29(5):877-882.
11. Pandey R, Berendt AR, Athanasou NA. Histological and microbiological findings in non-infected and infected revision arthroplasty tissues. The OSIRIS Collaborative Study Group. Oxford Skeletal Infection Research and Intervention Service. Arch Orthop Trauma Surg. 2000;120(10):570-574.
12. Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81(10):1434-1445.
13. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
14. Millett PJ, Yen YM, Price CS, Horan MP, van der Meijden OA, Elser F. Propionibacterium acnes infection as an occult cause of postoperative shoulder pain: a case series. Clin Orthop Relat Res. 2011;469(10):2824-2830.
15. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
16. Stoodley P, Ehrlich GD, Sedghizadeh PP, et al. Orthopaedic biofilm infections. Curr Orthop Pract. 2011;22(6):558-563.
17. Portillo ME, Salvadó M, Alier A, et al. Prosthesis failure within 2 years of implantation is highly predictive of infection. Clin Orthop Relat Res. 2013;471(11):3672-3678.
18. Tsukayama DT, Strada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78(4):512-523.
19. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
20. Schäfer P, Fink B, Sandow D, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis. 2008;47(11):1403-1409.
21. Zeller V, Ghorbani A, Strady C, Leonard P, Mamoudy P, Desplaces N. Propionibacterium acnes: an agent of prosthetic joint infection and colonization. J Infect. 2007;55(2):119-124.
22. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472(11):3254-3262.
23. Jacovides CL, Parvizi J, Adeli B, Jung KA. Molecular markers for diagnosis of periprosthetic joint infection. J Arthroplasty. 2011;26(6 suppl):99-103.e1.
24. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
25. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.