User login
Octreotide Scan for Carcinoids
Bronchopulmonary carcinoids are relatively rare endocrine tumors. They can present with Cushing's syndrome secondary to ectopic adrenocorticotropic hormone (ACTH) secretion. They were first described in 1957, and by 1990, only 72 cases had been reported in the literature worldwide.1 The largest series reported had 7 patients seen over a 16‐year period.2 Curative resection is possible only after adequate localization of the ectopic source. In this article, we describe a case illustrating the role of octreotide scanning in the management of bronchopulmonary carcinoid.
Case
A 23‐year‐old male presented with features of Cushing's syndrome. He had a 2‐year history of abdominal striae associated with progressive fatigue, adiposity, mood swings, a 10‐kg weight gain over 6 months, and recent onset of recurrent stones in his left kidney. His past medical history was significant for delayed puberty and juvenile rheumatoid arthritis. Family history also was remarkable for rheumatoid arthritis. Physical examination of the patient at the time of presentation revealed elevated blood pressure (150‐180 mm Hg systolic and 90‐120 mm Hg diastolic) and classic cushingoid features including moon facies and abdominal and axillary striae.
Blood work performed at this time revealed elevated morning and afternoon cortisol of 841 and 918 nmol/L (30.5 and 33.3 g/dL), respectively. Thyroid‐stimulating hormone was low at 0.17 mU/L (normal 0.35‐5.5 mU/L), and free triiodothyronine (T3) and thyroxine (T4) were normal. ACTH was elevated at 70.21 pmol/L (normal 1.98‐11.6 pmol/L). Cortisol levels failed to suppress in response to our dexamethasone suppression test, as shown by the absence of suppression of urinary and serum cortisol despite administration of 0.5 mg of dexamethasone intramuscularly every 6 hours for 2 days, followed by high‐dose dexamethasone (2.0 mg) every 6 hours for 2 additional days. The chest radiograph was normal. Computed tomography (CT) could not confirm a mass but suggested a possible 1.5‐cm lesion in the superior segment of the right lower lobe of the lung. The liver, spleen, pancreas, kidneys, and adrenals were normal. There was no lymphadenopathy.
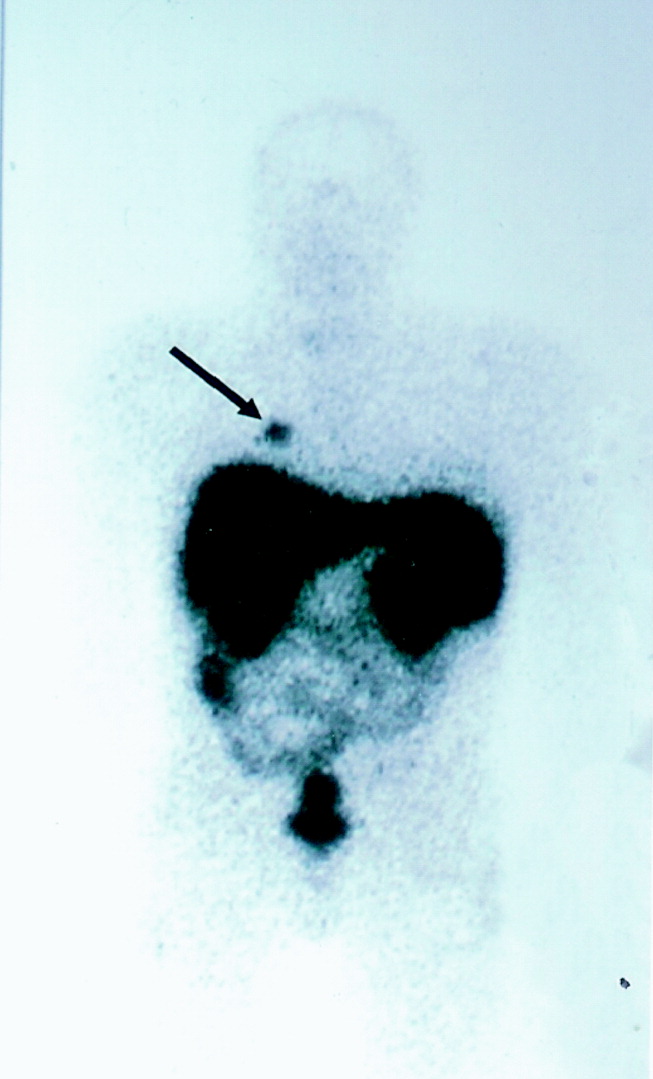
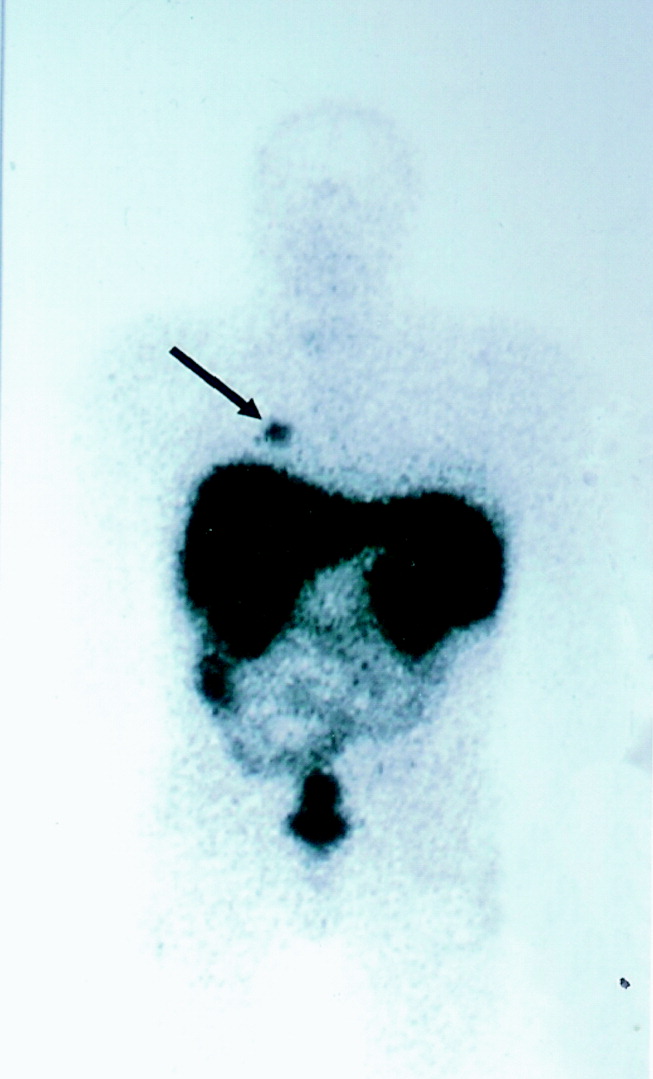
Octreotide scanning was done following intravenous administration of indium 111 Octreotide at a dose of 119 MBq. It showed a solitary focus in the superior segment of the right lower lobe, confirming the neuroendocrine nature of the suspicious lesion initially suspected on CT scan (Fig. 1). No other foci were found. The patient was diagnosed with ectopic adrenocorticotropic hormone (ACTH) secretion secondary to a bronchopulmonary carcinoid in the superior segment of the right lower lobe.
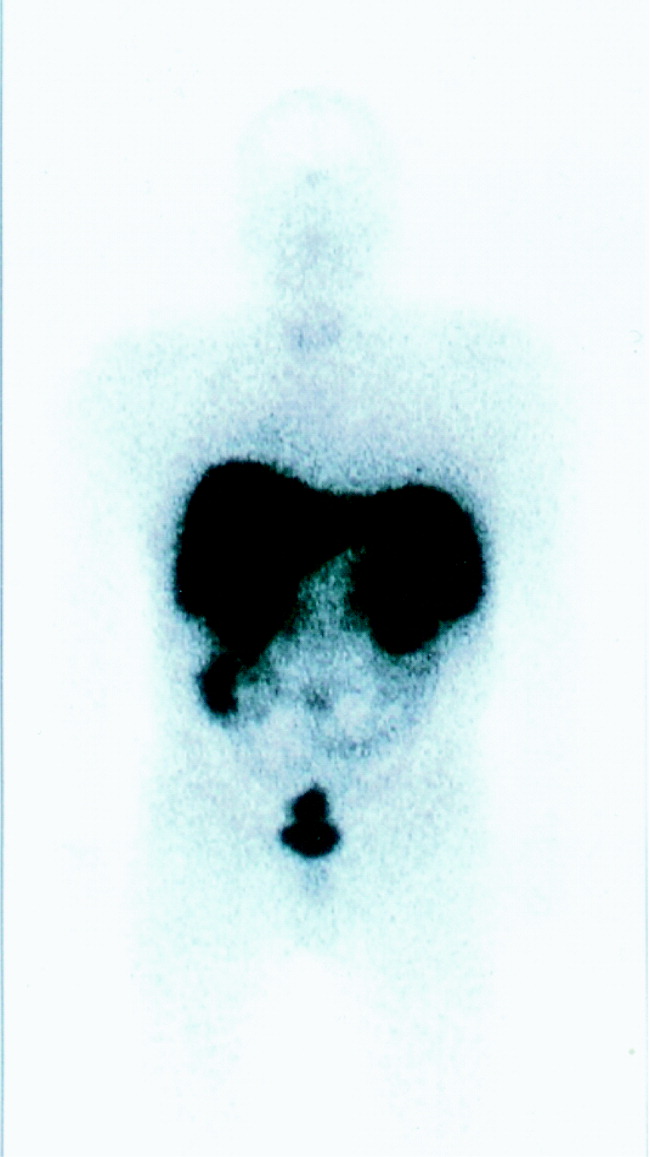
The patient was brought to the operating room for resection. Intraoperative bronchoscopy revealed no evidence of endotracheal lesions. At thoracotomy the mass was difficult to appreciate on palpation. On the basis of what preoperative imaging showed, the patient underwent a right lower lobe superior segmentectomy. Local nodes dissected at the time of the operation were negative for malignancy. To confirm adequate surgical resection, postoperative ACTH levels and octreotide scanning were performed. The ACTH level was 1.21 pmol/L (normal). A second octreotide scan showed no evidence of residual tumor (Fig. 2). The patient's blood pressure normalized, and his cushingoid features had declined by his first follow‐up visit. The final pathology confirmed carcinoid tumor, and the tumor stained with ACTH.
DISCUSSION
Ectopic ACTH secretion is responsible for 10%‐15% of the cases of Cushing's syndrome.3 Sources of the ectopic ACTH include small cell carcinoma of the lung, bronchopulmonary carcinoid, islet cell tumors, medullary carcinoma of the thyroid, and pheochromocytoma. The diagnosis of Cushing's syndrome is established by the nonsuppressibility of the serum and urinary cortisol levels. The etiology may be ACTH dependent (eg, Cushing's disease or ectopic ACTH syndrome) or ACTH independent (eg, adrenal tumor). A pituitary source is usually excluded by lack of cortisol suppression with high‐dose dexamethasone, which supports a diagnosis of ectopic ACTH. The CRH‐stimulation test can also be used to differentiate patients with Cushing's disease from those with ectopic ACTH secretion. Typically, 1 g/kg of intravenous CRH is administered to the patient, which elicits a rise in plasma ACTH or cortisol levels in a patient with Cushing's disease. However, only 5% of patients with ectopic ACTH secretion will demonstrate a plasma response,18, 19 thereby helping these 2 groups of patients. Following this differentiation, the source of ACTH can sometimes be located with traditional investigations including computed tomography of the thorax and abdomen. Finally, some authors have also advocated the use of bilateral petrosal sinus catheterization for diagnosing Cushing's syndrome if this diagnosis remains uncertain. Performed since 1982, this procedure involves the simultaneous sampling of petrosal sinuses and peripheral veins for ACTH levels both prior to and following administration of CRH. A diagnosis of ectopic ACTH secretion is strongly suggested by lack of a gradient between central and peripheral ACTH levels.
Carcinoid tumors account for 5% of lung tumors, and only a minority of these secrete ACTH. Only 1% of cases of Cushing's syndrome are accounted for by bronchial carcinoids,4 and as of 2004, only 100 cases had been reviewed in the literature worldwide.23 Pathologically, carcinoids tumors represent a low‐grade neuroendocrine malignancy arising from enterochromaffin or Kulchitsky cells, which are in the mucosa of the bronchi. There is a single line of derivation between bronchial carcinoid and small cell lung carcinoma, which was first demonstrated by Arrigoni.5
It is known that most carcinoid and other types of neuroendocrine tumors express somatostatin receptors,7, 11 and as such, a number of authors have recently described the ability to localize tumors of this type with radiolabeled somatostatin analogues.7, 1116 The sensitivity of somatostatin‐analogue scanning has been well described in the workup of gastropancreatic neuroendocrine malignancies.3, 17 although some false‐positive results do occur and have been attributed to inflammatory conditions such as sarcoidosis. Some work has been documented with this technique in other neuroendocrine malignancies.11, 14 Specifically, this technique was used by Rodriguez et al.12 to intraoperatively scan a patient's resection bed following primary removal of a bronchial carcinoid. This scan was able to identify residual disease despite gross tumor‐free margins of the primary resection specimen and thus enable complete removal of the disease.
In the past, authors have suggested that somatostatin‐analogue scanning is a useful tool in the localization of ectopic ACTH sources only after traditional modalities like CT have yielded equivocal results.3, 9, 10 Indeed, many studies have demonstrated the usefulness of octreotide scanning in localizing tumors with ectopic ACTH secretion. However, 2 recent studies have raised doubts about the clinical utility of octreotide scanning.20, 21 The study by Torpy et al. reported a significantly high false‐positive rate with octreotide scans. However, they also had false positives with conventional imaging in their series. Perhaps the best synthesis of the literature on the subject comes from Pacak et al., who looked at 17 patients with ectopic ACTH syndrome.22 They demonstrated that low‐dose octreotide scanning (L‐OCT) worked just as well as CT and better than MRI in visualizing ACTH‐secreting tumors. Moreover, they demonstrated that L‐OCT highlighted involvement of lymph nodes that was missed by CT and MRI and identified 2 abdominal lesions missed by conventional imaging. Finally, high‐dose octreotide scanning (H‐OCT) was able to pick up an intrathoracic ACTH‐secreting tumor that was not seen on CT, MRI, or L‐OCT. Although in the article the authors advocated L‐OCT as complimentary to CT and MRI, they did acknowledge that it provided additional diagnostic information, at least in their series. They advocated the use of all 3 modalities in order to provide the most comprehensive information on the location and extent of a tumor.
In this case report, we document the use of pre‐ and postoperative octreotide scanning in a patient whose CT scan was equivocal and for whom adequate surgical excision of an indistinct lesion was questionable. The use of octreotide scanning also permitted a limited resection, allowing preservation of lung parenchyma. Furthermore, it allowed us to avoid petrosal sinus catheterization. We propose that octreotide scanning can be a very important and informative test in the management of carcinoid tumors. In situations when conventional imaging is not conclusive, octreotide scanning may be of help in determining the source of ectopic ACTH syndrome. Certainly, CT scanning, currently the modality of first choice, is presently more practical and cost effective. However, octreotide scanning has been shown to be at least as sensitive in localizing ectopic ACTH‐secreting tumors and often can provide additional diagnostic information that can influence surgical management. Somatostatin‐analogue scanning, if performed initially, can guide a diagnostician about where to perform further imaging, so that limited but complete resections of this rare but curable tumor can be planned. Somatostatin‐analogue scanning also may have a role intraoperatively in ensuring complete resection despite pathologically clear tumor margins of the primary specimen, as well as an effective modality in following patients after their primary surgery for disease recurrence. These points all support the idea that octreotide scanning should play a vital and perhaps more central role in the diagnostic workup for ectopic ACTH‐secreting tumors.
CONCLUSIONS
Accurate localization of an ectopic source of ACTH in Cushing's syndrome is important for surgical cure. Octreotide scanning has been shown to be an excellent modality for both the diagnosis and the follow‐up of neuroendocrine tumors. Although computed tomography scanning of the chest and abdomen is currently used as the initial adjuncts in an attempt to localize such tumors, in the case we have presented, in which the initial CT scan was equivocal, subsequent octreotide scanning provided excellent localization of the ectopic ACTH source. We also believe that postoperative surveillance with octreotide scanning offers an excellent means of detecting residual or metastatic tumor. Indeed, somatostatin‐analogue scanning is a very useful modality for the detection, perioperative planning, and postoperative follow‐up of ectopic ACTH‐secreting tumors and neuroendocrine tumors in general and should be considered in surgical workups of such malignancies.
- ,,, et al.Management of the ectopic ACTH syndrome due to thoracic carcinoids.Ann Thorac Surg.1990;50(1):52–57.
- ,,,,,.Bronchopulmonary cacinoid tumors associated with Cushing's syndrome: a more aggressive variant of the typical carcinoid.J Thorac Cardiovasc Surg.1997;114:367–375.
- ,,,,.Ectopic ACTH secretion due to a bronchopulmonary carcinoid localized by somatostatin receptor scintigraphy.Clin Investig.1994;72:887–891.
- .Diagnostic evaluation of Cushing's syndrome.Endocrinol Metab Clin North Am.1988;17:445–472.
- ,,.Atypical carcinoid tumors of the lung.J Thorac Cardiovasc Surg.1972;64:413–421.
- ,,, et al.Somatostatin receptor scintigraphy. In:Freeman LM, ed.Nuclear Medicine Annual 1995.New York:Raven Press,1995:1–21.
- ,,.The role of somatostatin and its analogues in the diagnosis and treatment of tumors.Endocr Rev.1991;12:450–482.
- ,,.Somatostatin analogue treatment of neuroendocrine tumours.Postgrad Med J.1996;72:403–408.
- ,,, et al.Bronchial carcinoid associated with Cushing's syndrome.J Cardiovasc Surg (Torino).1995;36:511–514.
- ,,, et al.Somatostatin analogs for the localization and preoperative treatment of an adrenocorticotropin‐secreting bronchial carcinoid tumor.J Clin Endocrinol Metab.1994;78(1):20–24.
- ,,, et al.In vitro detection of somatostatin receptors in human tumors.Digestion.1993;54(suppl.):68–71.
- ,,, et al.Intraoperative detection of a bronchial carcinoid with a radiolabeled somatostatin analog.Chest.2002;121:985–988.
- ,,, et al.Localization of endocrine‐related tumours with a radioiodinated analogue of somatostatin.Lancet.1989;1:242–244.
- ,,, et al.Somatostatin receptor scintigraphy with [111In‐DTPA‐D‐Phe1]‐octreotide and [123I‐Tyr3]‐octreotide: the Rotterdam experience with more that 1,000 patients.Eur J Nucl Med.1993;20:716–731.
- ,,, et al.Comparison of somatostatin analog and meta‐iodobenzylguanidine radionuclides in the diagnosis and localization of advanced neuroendocrine tumors.J Clin Endocr Metab.2001;86:895–902.
- .MIBG and radiolabeled octreotide in neuroendocrine tumors.Q J Nucl Med.1995;39(suppl 1‐4):137–139.
- ,,, et al.Recent advances in radiological and radionuclide imaging and therapy of neuroendocrine tumours.Eur J Endocrinol2004;151:15–27.
- ,,, et al.Cushing's syndrome in children and adolescents. Presentation, diagnosis, and therapy.N Engl J Med.1994;331:629–636.
- ,,, et al.A simplified morning ovine corticotropin‐releasing hormone stimulation test for the differential diagnosis of adrenocorticotropin‐dependent Cushing's syndrome.J Clin Endocrinol Metab.1993;77:1308–1312.
- ,,, et al.Lack of utility of 111In‐pentreotide scintigraphy in localizing ectopic ACTH producing tumors: follow‐up of 18 patients.J Clin Endocrinol Metab.1999;84:1186–1192.
- ,,, et al.Usefulness of somatostatin receptor scintigraphy in patients with occult ectopic adrenocorticotropin syndrome.J Clin Endocrinol Metab.1999;84:1193–1202.
- ,,,,,.The role of [18F]fluorodeoxyglucose positron emission tomography and [111In]‐diethylenetriaminepentaacetate‐D‐Phe‐pentetreotide scintigraphy in the localization of ectopic adrenocorticotropin‐secreting tumors causing Cushing's syndrome.J Clin Endocrin Metab.2004;89:2214–2221.
- ,,, et al.Cushing's syndrome induced by bronchopulmonary carcinoid tumours: a review of 98 cases and our experience of two cases.Chir Ita.2004;56(1):63–70.
Bronchopulmonary carcinoids are relatively rare endocrine tumors. They can present with Cushing's syndrome secondary to ectopic adrenocorticotropic hormone (ACTH) secretion. They were first described in 1957, and by 1990, only 72 cases had been reported in the literature worldwide.1 The largest series reported had 7 patients seen over a 16‐year period.2 Curative resection is possible only after adequate localization of the ectopic source. In this article, we describe a case illustrating the role of octreotide scanning in the management of bronchopulmonary carcinoid.
Case
A 23‐year‐old male presented with features of Cushing's syndrome. He had a 2‐year history of abdominal striae associated with progressive fatigue, adiposity, mood swings, a 10‐kg weight gain over 6 months, and recent onset of recurrent stones in his left kidney. His past medical history was significant for delayed puberty and juvenile rheumatoid arthritis. Family history also was remarkable for rheumatoid arthritis. Physical examination of the patient at the time of presentation revealed elevated blood pressure (150‐180 mm Hg systolic and 90‐120 mm Hg diastolic) and classic cushingoid features including moon facies and abdominal and axillary striae.
Blood work performed at this time revealed elevated morning and afternoon cortisol of 841 and 918 nmol/L (30.5 and 33.3 g/dL), respectively. Thyroid‐stimulating hormone was low at 0.17 mU/L (normal 0.35‐5.5 mU/L), and free triiodothyronine (T3) and thyroxine (T4) were normal. ACTH was elevated at 70.21 pmol/L (normal 1.98‐11.6 pmol/L). Cortisol levels failed to suppress in response to our dexamethasone suppression test, as shown by the absence of suppression of urinary and serum cortisol despite administration of 0.5 mg of dexamethasone intramuscularly every 6 hours for 2 days, followed by high‐dose dexamethasone (2.0 mg) every 6 hours for 2 additional days. The chest radiograph was normal. Computed tomography (CT) could not confirm a mass but suggested a possible 1.5‐cm lesion in the superior segment of the right lower lobe of the lung. The liver, spleen, pancreas, kidneys, and adrenals were normal. There was no lymphadenopathy.
Octreotide scanning was done following intravenous administration of indium 111 Octreotide at a dose of 119 MBq. It showed a solitary focus in the superior segment of the right lower lobe, confirming the neuroendocrine nature of the suspicious lesion initially suspected on CT scan (Fig. 1). No other foci were found. The patient was diagnosed with ectopic adrenocorticotropic hormone (ACTH) secretion secondary to a bronchopulmonary carcinoid in the superior segment of the right lower lobe.

The patient was brought to the operating room for resection. Intraoperative bronchoscopy revealed no evidence of endotracheal lesions. At thoracotomy the mass was difficult to appreciate on palpation. On the basis of what preoperative imaging showed, the patient underwent a right lower lobe superior segmentectomy. Local nodes dissected at the time of the operation were negative for malignancy. To confirm adequate surgical resection, postoperative ACTH levels and octreotide scanning were performed. The ACTH level was 1.21 pmol/L (normal). A second octreotide scan showed no evidence of residual tumor (Fig. 2). The patient's blood pressure normalized, and his cushingoid features had declined by his first follow‐up visit. The final pathology confirmed carcinoid tumor, and the tumor stained with ACTH.

DISCUSSION
Ectopic ACTH secretion is responsible for 10%‐15% of the cases of Cushing's syndrome.3 Sources of the ectopic ACTH include small cell carcinoma of the lung, bronchopulmonary carcinoid, islet cell tumors, medullary carcinoma of the thyroid, and pheochromocytoma. The diagnosis of Cushing's syndrome is established by the nonsuppressibility of the serum and urinary cortisol levels. The etiology may be ACTH dependent (eg, Cushing's disease or ectopic ACTH syndrome) or ACTH independent (eg, adrenal tumor). A pituitary source is usually excluded by lack of cortisol suppression with high‐dose dexamethasone, which supports a diagnosis of ectopic ACTH. The CRH‐stimulation test can also be used to differentiate patients with Cushing's disease from those with ectopic ACTH secretion. Typically, 1 g/kg of intravenous CRH is administered to the patient, which elicits a rise in plasma ACTH or cortisol levels in a patient with Cushing's disease. However, only 5% of patients with ectopic ACTH secretion will demonstrate a plasma response,18, 19 thereby helping these 2 groups of patients. Following this differentiation, the source of ACTH can sometimes be located with traditional investigations including computed tomography of the thorax and abdomen. Finally, some authors have also advocated the use of bilateral petrosal sinus catheterization for diagnosing Cushing's syndrome if this diagnosis remains uncertain. Performed since 1982, this procedure involves the simultaneous sampling of petrosal sinuses and peripheral veins for ACTH levels both prior to and following administration of CRH. A diagnosis of ectopic ACTH secretion is strongly suggested by lack of a gradient between central and peripheral ACTH levels.
Carcinoid tumors account for 5% of lung tumors, and only a minority of these secrete ACTH. Only 1% of cases of Cushing's syndrome are accounted for by bronchial carcinoids,4 and as of 2004, only 100 cases had been reviewed in the literature worldwide.23 Pathologically, carcinoids tumors represent a low‐grade neuroendocrine malignancy arising from enterochromaffin or Kulchitsky cells, which are in the mucosa of the bronchi. There is a single line of derivation between bronchial carcinoid and small cell lung carcinoma, which was first demonstrated by Arrigoni.5
It is known that most carcinoid and other types of neuroendocrine tumors express somatostatin receptors,7, 11 and as such, a number of authors have recently described the ability to localize tumors of this type with radiolabeled somatostatin analogues.7, 1116 The sensitivity of somatostatin‐analogue scanning has been well described in the workup of gastropancreatic neuroendocrine malignancies.3, 17 although some false‐positive results do occur and have been attributed to inflammatory conditions such as sarcoidosis. Some work has been documented with this technique in other neuroendocrine malignancies.11, 14 Specifically, this technique was used by Rodriguez et al.12 to intraoperatively scan a patient's resection bed following primary removal of a bronchial carcinoid. This scan was able to identify residual disease despite gross tumor‐free margins of the primary resection specimen and thus enable complete removal of the disease.
In the past, authors have suggested that somatostatin‐analogue scanning is a useful tool in the localization of ectopic ACTH sources only after traditional modalities like CT have yielded equivocal results.3, 9, 10 Indeed, many studies have demonstrated the usefulness of octreotide scanning in localizing tumors with ectopic ACTH secretion. However, 2 recent studies have raised doubts about the clinical utility of octreotide scanning.20, 21 The study by Torpy et al. reported a significantly high false‐positive rate with octreotide scans. However, they also had false positives with conventional imaging in their series. Perhaps the best synthesis of the literature on the subject comes from Pacak et al., who looked at 17 patients with ectopic ACTH syndrome.22 They demonstrated that low‐dose octreotide scanning (L‐OCT) worked just as well as CT and better than MRI in visualizing ACTH‐secreting tumors. Moreover, they demonstrated that L‐OCT highlighted involvement of lymph nodes that was missed by CT and MRI and identified 2 abdominal lesions missed by conventional imaging. Finally, high‐dose octreotide scanning (H‐OCT) was able to pick up an intrathoracic ACTH‐secreting tumor that was not seen on CT, MRI, or L‐OCT. Although in the article the authors advocated L‐OCT as complimentary to CT and MRI, they did acknowledge that it provided additional diagnostic information, at least in their series. They advocated the use of all 3 modalities in order to provide the most comprehensive information on the location and extent of a tumor.
In this case report, we document the use of pre‐ and postoperative octreotide scanning in a patient whose CT scan was equivocal and for whom adequate surgical excision of an indistinct lesion was questionable. The use of octreotide scanning also permitted a limited resection, allowing preservation of lung parenchyma. Furthermore, it allowed us to avoid petrosal sinus catheterization. We propose that octreotide scanning can be a very important and informative test in the management of carcinoid tumors. In situations when conventional imaging is not conclusive, octreotide scanning may be of help in determining the source of ectopic ACTH syndrome. Certainly, CT scanning, currently the modality of first choice, is presently more practical and cost effective. However, octreotide scanning has been shown to be at least as sensitive in localizing ectopic ACTH‐secreting tumors and often can provide additional diagnostic information that can influence surgical management. Somatostatin‐analogue scanning, if performed initially, can guide a diagnostician about where to perform further imaging, so that limited but complete resections of this rare but curable tumor can be planned. Somatostatin‐analogue scanning also may have a role intraoperatively in ensuring complete resection despite pathologically clear tumor margins of the primary specimen, as well as an effective modality in following patients after their primary surgery for disease recurrence. These points all support the idea that octreotide scanning should play a vital and perhaps more central role in the diagnostic workup for ectopic ACTH‐secreting tumors.
CONCLUSIONS
Accurate localization of an ectopic source of ACTH in Cushing's syndrome is important for surgical cure. Octreotide scanning has been shown to be an excellent modality for both the diagnosis and the follow‐up of neuroendocrine tumors. Although computed tomography scanning of the chest and abdomen is currently used as the initial adjuncts in an attempt to localize such tumors, in the case we have presented, in which the initial CT scan was equivocal, subsequent octreotide scanning provided excellent localization of the ectopic ACTH source. We also believe that postoperative surveillance with octreotide scanning offers an excellent means of detecting residual or metastatic tumor. Indeed, somatostatin‐analogue scanning is a very useful modality for the detection, perioperative planning, and postoperative follow‐up of ectopic ACTH‐secreting tumors and neuroendocrine tumors in general and should be considered in surgical workups of such malignancies.
Bronchopulmonary carcinoids are relatively rare endocrine tumors. They can present with Cushing's syndrome secondary to ectopic adrenocorticotropic hormone (ACTH) secretion. They were first described in 1957, and by 1990, only 72 cases had been reported in the literature worldwide.1 The largest series reported had 7 patients seen over a 16‐year period.2 Curative resection is possible only after adequate localization of the ectopic source. In this article, we describe a case illustrating the role of octreotide scanning in the management of bronchopulmonary carcinoid.
Case
A 23‐year‐old male presented with features of Cushing's syndrome. He had a 2‐year history of abdominal striae associated with progressive fatigue, adiposity, mood swings, a 10‐kg weight gain over 6 months, and recent onset of recurrent stones in his left kidney. His past medical history was significant for delayed puberty and juvenile rheumatoid arthritis. Family history also was remarkable for rheumatoid arthritis. Physical examination of the patient at the time of presentation revealed elevated blood pressure (150‐180 mm Hg systolic and 90‐120 mm Hg diastolic) and classic cushingoid features including moon facies and abdominal and axillary striae.
Blood work performed at this time revealed elevated morning and afternoon cortisol of 841 and 918 nmol/L (30.5 and 33.3 g/dL), respectively. Thyroid‐stimulating hormone was low at 0.17 mU/L (normal 0.35‐5.5 mU/L), and free triiodothyronine (T3) and thyroxine (T4) were normal. ACTH was elevated at 70.21 pmol/L (normal 1.98‐11.6 pmol/L). Cortisol levels failed to suppress in response to our dexamethasone suppression test, as shown by the absence of suppression of urinary and serum cortisol despite administration of 0.5 mg of dexamethasone intramuscularly every 6 hours for 2 days, followed by high‐dose dexamethasone (2.0 mg) every 6 hours for 2 additional days. The chest radiograph was normal. Computed tomography (CT) could not confirm a mass but suggested a possible 1.5‐cm lesion in the superior segment of the right lower lobe of the lung. The liver, spleen, pancreas, kidneys, and adrenals were normal. There was no lymphadenopathy.
Octreotide scanning was done following intravenous administration of indium 111 Octreotide at a dose of 119 MBq. It showed a solitary focus in the superior segment of the right lower lobe, confirming the neuroendocrine nature of the suspicious lesion initially suspected on CT scan (Fig. 1). No other foci were found. The patient was diagnosed with ectopic adrenocorticotropic hormone (ACTH) secretion secondary to a bronchopulmonary carcinoid in the superior segment of the right lower lobe.

The patient was brought to the operating room for resection. Intraoperative bronchoscopy revealed no evidence of endotracheal lesions. At thoracotomy the mass was difficult to appreciate on palpation. On the basis of what preoperative imaging showed, the patient underwent a right lower lobe superior segmentectomy. Local nodes dissected at the time of the operation were negative for malignancy. To confirm adequate surgical resection, postoperative ACTH levels and octreotide scanning were performed. The ACTH level was 1.21 pmol/L (normal). A second octreotide scan showed no evidence of residual tumor (Fig. 2). The patient's blood pressure normalized, and his cushingoid features had declined by his first follow‐up visit. The final pathology confirmed carcinoid tumor, and the tumor stained with ACTH.

DISCUSSION
Ectopic ACTH secretion is responsible for 10%‐15% of the cases of Cushing's syndrome.3 Sources of the ectopic ACTH include small cell carcinoma of the lung, bronchopulmonary carcinoid, islet cell tumors, medullary carcinoma of the thyroid, and pheochromocytoma. The diagnosis of Cushing's syndrome is established by the nonsuppressibility of the serum and urinary cortisol levels. The etiology may be ACTH dependent (eg, Cushing's disease or ectopic ACTH syndrome) or ACTH independent (eg, adrenal tumor). A pituitary source is usually excluded by lack of cortisol suppression with high‐dose dexamethasone, which supports a diagnosis of ectopic ACTH. The CRH‐stimulation test can also be used to differentiate patients with Cushing's disease from those with ectopic ACTH secretion. Typically, 1 g/kg of intravenous CRH is administered to the patient, which elicits a rise in plasma ACTH or cortisol levels in a patient with Cushing's disease. However, only 5% of patients with ectopic ACTH secretion will demonstrate a plasma response,18, 19 thereby helping these 2 groups of patients. Following this differentiation, the source of ACTH can sometimes be located with traditional investigations including computed tomography of the thorax and abdomen. Finally, some authors have also advocated the use of bilateral petrosal sinus catheterization for diagnosing Cushing's syndrome if this diagnosis remains uncertain. Performed since 1982, this procedure involves the simultaneous sampling of petrosal sinuses and peripheral veins for ACTH levels both prior to and following administration of CRH. A diagnosis of ectopic ACTH secretion is strongly suggested by lack of a gradient between central and peripheral ACTH levels.
Carcinoid tumors account for 5% of lung tumors, and only a minority of these secrete ACTH. Only 1% of cases of Cushing's syndrome are accounted for by bronchial carcinoids,4 and as of 2004, only 100 cases had been reviewed in the literature worldwide.23 Pathologically, carcinoids tumors represent a low‐grade neuroendocrine malignancy arising from enterochromaffin or Kulchitsky cells, which are in the mucosa of the bronchi. There is a single line of derivation between bronchial carcinoid and small cell lung carcinoma, which was first demonstrated by Arrigoni.5
It is known that most carcinoid and other types of neuroendocrine tumors express somatostatin receptors,7, 11 and as such, a number of authors have recently described the ability to localize tumors of this type with radiolabeled somatostatin analogues.7, 1116 The sensitivity of somatostatin‐analogue scanning has been well described in the workup of gastropancreatic neuroendocrine malignancies.3, 17 although some false‐positive results do occur and have been attributed to inflammatory conditions such as sarcoidosis. Some work has been documented with this technique in other neuroendocrine malignancies.11, 14 Specifically, this technique was used by Rodriguez et al.12 to intraoperatively scan a patient's resection bed following primary removal of a bronchial carcinoid. This scan was able to identify residual disease despite gross tumor‐free margins of the primary resection specimen and thus enable complete removal of the disease.
In the past, authors have suggested that somatostatin‐analogue scanning is a useful tool in the localization of ectopic ACTH sources only after traditional modalities like CT have yielded equivocal results.3, 9, 10 Indeed, many studies have demonstrated the usefulness of octreotide scanning in localizing tumors with ectopic ACTH secretion. However, 2 recent studies have raised doubts about the clinical utility of octreotide scanning.20, 21 The study by Torpy et al. reported a significantly high false‐positive rate with octreotide scans. However, they also had false positives with conventional imaging in their series. Perhaps the best synthesis of the literature on the subject comes from Pacak et al., who looked at 17 patients with ectopic ACTH syndrome.22 They demonstrated that low‐dose octreotide scanning (L‐OCT) worked just as well as CT and better than MRI in visualizing ACTH‐secreting tumors. Moreover, they demonstrated that L‐OCT highlighted involvement of lymph nodes that was missed by CT and MRI and identified 2 abdominal lesions missed by conventional imaging. Finally, high‐dose octreotide scanning (H‐OCT) was able to pick up an intrathoracic ACTH‐secreting tumor that was not seen on CT, MRI, or L‐OCT. Although in the article the authors advocated L‐OCT as complimentary to CT and MRI, they did acknowledge that it provided additional diagnostic information, at least in their series. They advocated the use of all 3 modalities in order to provide the most comprehensive information on the location and extent of a tumor.
In this case report, we document the use of pre‐ and postoperative octreotide scanning in a patient whose CT scan was equivocal and for whom adequate surgical excision of an indistinct lesion was questionable. The use of octreotide scanning also permitted a limited resection, allowing preservation of lung parenchyma. Furthermore, it allowed us to avoid petrosal sinus catheterization. We propose that octreotide scanning can be a very important and informative test in the management of carcinoid tumors. In situations when conventional imaging is not conclusive, octreotide scanning may be of help in determining the source of ectopic ACTH syndrome. Certainly, CT scanning, currently the modality of first choice, is presently more practical and cost effective. However, octreotide scanning has been shown to be at least as sensitive in localizing ectopic ACTH‐secreting tumors and often can provide additional diagnostic information that can influence surgical management. Somatostatin‐analogue scanning, if performed initially, can guide a diagnostician about where to perform further imaging, so that limited but complete resections of this rare but curable tumor can be planned. Somatostatin‐analogue scanning also may have a role intraoperatively in ensuring complete resection despite pathologically clear tumor margins of the primary specimen, as well as an effective modality in following patients after their primary surgery for disease recurrence. These points all support the idea that octreotide scanning should play a vital and perhaps more central role in the diagnostic workup for ectopic ACTH‐secreting tumors.
CONCLUSIONS
Accurate localization of an ectopic source of ACTH in Cushing's syndrome is important for surgical cure. Octreotide scanning has been shown to be an excellent modality for both the diagnosis and the follow‐up of neuroendocrine tumors. Although computed tomography scanning of the chest and abdomen is currently used as the initial adjuncts in an attempt to localize such tumors, in the case we have presented, in which the initial CT scan was equivocal, subsequent octreotide scanning provided excellent localization of the ectopic ACTH source. We also believe that postoperative surveillance with octreotide scanning offers an excellent means of detecting residual or metastatic tumor. Indeed, somatostatin‐analogue scanning is a very useful modality for the detection, perioperative planning, and postoperative follow‐up of ectopic ACTH‐secreting tumors and neuroendocrine tumors in general and should be considered in surgical workups of such malignancies.
- ,,, et al.Management of the ectopic ACTH syndrome due to thoracic carcinoids.Ann Thorac Surg.1990;50(1):52–57.
- ,,,,,.Bronchopulmonary cacinoid tumors associated with Cushing's syndrome: a more aggressive variant of the typical carcinoid.J Thorac Cardiovasc Surg.1997;114:367–375.
- ,,,,.Ectopic ACTH secretion due to a bronchopulmonary carcinoid localized by somatostatin receptor scintigraphy.Clin Investig.1994;72:887–891.
- .Diagnostic evaluation of Cushing's syndrome.Endocrinol Metab Clin North Am.1988;17:445–472.
- ,,.Atypical carcinoid tumors of the lung.J Thorac Cardiovasc Surg.1972;64:413–421.
- ,,, et al.Somatostatin receptor scintigraphy. In:Freeman LM, ed.Nuclear Medicine Annual 1995.New York:Raven Press,1995:1–21.
- ,,.The role of somatostatin and its analogues in the diagnosis and treatment of tumors.Endocr Rev.1991;12:450–482.
- ,,.Somatostatin analogue treatment of neuroendocrine tumours.Postgrad Med J.1996;72:403–408.
- ,,, et al.Bronchial carcinoid associated with Cushing's syndrome.J Cardiovasc Surg (Torino).1995;36:511–514.
- ,,, et al.Somatostatin analogs for the localization and preoperative treatment of an adrenocorticotropin‐secreting bronchial carcinoid tumor.J Clin Endocrinol Metab.1994;78(1):20–24.
- ,,, et al.In vitro detection of somatostatin receptors in human tumors.Digestion.1993;54(suppl.):68–71.
- ,,, et al.Intraoperative detection of a bronchial carcinoid with a radiolabeled somatostatin analog.Chest.2002;121:985–988.
- ,,, et al.Localization of endocrine‐related tumours with a radioiodinated analogue of somatostatin.Lancet.1989;1:242–244.
- ,,, et al.Somatostatin receptor scintigraphy with [111In‐DTPA‐D‐Phe1]‐octreotide and [123I‐Tyr3]‐octreotide: the Rotterdam experience with more that 1,000 patients.Eur J Nucl Med.1993;20:716–731.
- ,,, et al.Comparison of somatostatin analog and meta‐iodobenzylguanidine radionuclides in the diagnosis and localization of advanced neuroendocrine tumors.J Clin Endocr Metab.2001;86:895–902.
- .MIBG and radiolabeled octreotide in neuroendocrine tumors.Q J Nucl Med.1995;39(suppl 1‐4):137–139.
- ,,, et al.Recent advances in radiological and radionuclide imaging and therapy of neuroendocrine tumours.Eur J Endocrinol2004;151:15–27.
- ,,, et al.Cushing's syndrome in children and adolescents. Presentation, diagnosis, and therapy.N Engl J Med.1994;331:629–636.
- ,,, et al.A simplified morning ovine corticotropin‐releasing hormone stimulation test for the differential diagnosis of adrenocorticotropin‐dependent Cushing's syndrome.J Clin Endocrinol Metab.1993;77:1308–1312.
- ,,, et al.Lack of utility of 111In‐pentreotide scintigraphy in localizing ectopic ACTH producing tumors: follow‐up of 18 patients.J Clin Endocrinol Metab.1999;84:1186–1192.
- ,,, et al.Usefulness of somatostatin receptor scintigraphy in patients with occult ectopic adrenocorticotropin syndrome.J Clin Endocrinol Metab.1999;84:1193–1202.
- ,,,,,.The role of [18F]fluorodeoxyglucose positron emission tomography and [111In]‐diethylenetriaminepentaacetate‐D‐Phe‐pentetreotide scintigraphy in the localization of ectopic adrenocorticotropin‐secreting tumors causing Cushing's syndrome.J Clin Endocrin Metab.2004;89:2214–2221.
- ,,, et al.Cushing's syndrome induced by bronchopulmonary carcinoid tumours: a review of 98 cases and our experience of two cases.Chir Ita.2004;56(1):63–70.
- ,,, et al.Management of the ectopic ACTH syndrome due to thoracic carcinoids.Ann Thorac Surg.1990;50(1):52–57.
- ,,,,,.Bronchopulmonary cacinoid tumors associated with Cushing's syndrome: a more aggressive variant of the typical carcinoid.J Thorac Cardiovasc Surg.1997;114:367–375.
- ,,,,.Ectopic ACTH secretion due to a bronchopulmonary carcinoid localized by somatostatin receptor scintigraphy.Clin Investig.1994;72:887–891.
- .Diagnostic evaluation of Cushing's syndrome.Endocrinol Metab Clin North Am.1988;17:445–472.
- ,,.Atypical carcinoid tumors of the lung.J Thorac Cardiovasc Surg.1972;64:413–421.
- ,,, et al.Somatostatin receptor scintigraphy. In:Freeman LM, ed.Nuclear Medicine Annual 1995.New York:Raven Press,1995:1–21.
- ,,.The role of somatostatin and its analogues in the diagnosis and treatment of tumors.Endocr Rev.1991;12:450–482.
- ,,.Somatostatin analogue treatment of neuroendocrine tumours.Postgrad Med J.1996;72:403–408.
- ,,, et al.Bronchial carcinoid associated with Cushing's syndrome.J Cardiovasc Surg (Torino).1995;36:511–514.
- ,,, et al.Somatostatin analogs for the localization and preoperative treatment of an adrenocorticotropin‐secreting bronchial carcinoid tumor.J Clin Endocrinol Metab.1994;78(1):20–24.
- ,,, et al.In vitro detection of somatostatin receptors in human tumors.Digestion.1993;54(suppl.):68–71.
- ,,, et al.Intraoperative detection of a bronchial carcinoid with a radiolabeled somatostatin analog.Chest.2002;121:985–988.
- ,,, et al.Localization of endocrine‐related tumours with a radioiodinated analogue of somatostatin.Lancet.1989;1:242–244.
- ,,, et al.Somatostatin receptor scintigraphy with [111In‐DTPA‐D‐Phe1]‐octreotide and [123I‐Tyr3]‐octreotide: the Rotterdam experience with more that 1,000 patients.Eur J Nucl Med.1993;20:716–731.
- ,,, et al.Comparison of somatostatin analog and meta‐iodobenzylguanidine radionuclides in the diagnosis and localization of advanced neuroendocrine tumors.J Clin Endocr Metab.2001;86:895–902.
- .MIBG and radiolabeled octreotide in neuroendocrine tumors.Q J Nucl Med.1995;39(suppl 1‐4):137–139.
- ,,, et al.Recent advances in radiological and radionuclide imaging and therapy of neuroendocrine tumours.Eur J Endocrinol2004;151:15–27.
- ,,, et al.Cushing's syndrome in children and adolescents. Presentation, diagnosis, and therapy.N Engl J Med.1994;331:629–636.
- ,,, et al.A simplified morning ovine corticotropin‐releasing hormone stimulation test for the differential diagnosis of adrenocorticotropin‐dependent Cushing's syndrome.J Clin Endocrinol Metab.1993;77:1308–1312.
- ,,, et al.Lack of utility of 111In‐pentreotide scintigraphy in localizing ectopic ACTH producing tumors: follow‐up of 18 patients.J Clin Endocrinol Metab.1999;84:1186–1192.
- ,,, et al.Usefulness of somatostatin receptor scintigraphy in patients with occult ectopic adrenocorticotropin syndrome.J Clin Endocrinol Metab.1999;84:1193–1202.
- ,,,,,.The role of [18F]fluorodeoxyglucose positron emission tomography and [111In]‐diethylenetriaminepentaacetate‐D‐Phe‐pentetreotide scintigraphy in the localization of ectopic adrenocorticotropin‐secreting tumors causing Cushing's syndrome.J Clin Endocrin Metab.2004;89:2214–2221.
- ,,, et al.Cushing's syndrome induced by bronchopulmonary carcinoid tumours: a review of 98 cases and our experience of two cases.Chir Ita.2004;56(1):63–70.