User login
In the Eye of the Storm
A 37‐year‐old man presented to an ophthalmologist in July 2004 with a history of slowly decreasing vision in both eyes for several weeks. His vision on presentation was 20/400 in the right eye and 20/200 in the left eye. Slit‐lamp examination showed a bilateral anterior uveitis with 360 degrees of posterior synechiae (adhesions) and a dense vitritis (posterior uveitis) that obscured the view of the retina in both eyes. He was diagnosed with panuveitis and started on topical steroid and cycloplegic drops. He was referred to a uveitis specialist for investigation but missed his appointments.
One year later he presented to the emergency room with fever and severe pain in his left eye. On initial assessment he had no complaints of mouth or genital ulcers, recent or remote rashes, joint symptoms, or penile discharge. He denied any prior eye trauma or surgery. He reported that his last sexual encounter had been 8 months prior with a male and that his most recent HIV screen was negative 6 months ago. His family history was negative for autoimmune disorders.
On inspection, he appeared cachectic, lethargic, and very ill. He was febrile and tachycardic; the remainder of his vital signs were normal. There was no lymphadenopathy. His neck was supple with no meningismal signs. There were no heart murmurs, oral ulcers, swollen joints, mucosal eschar, or skin lesions. Respiratory and abdominal examinations were unremarkable.
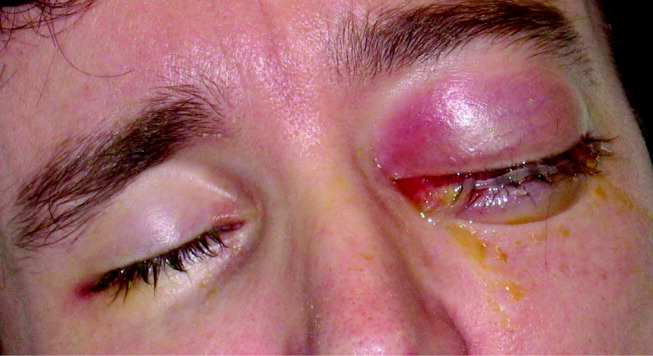
His visual acuity was light perception in right eye and no light perception in the left eye. There was significant eyelid edema, erythema and purulent discharge with mild proptosis of the left eye (Fig. 1). Pupils were 3 mm and fixed, with 360 of posterior synechiae. Intraocular pressure was elevated in the left eye (42 mm Hg, where normal is 20 mm Hg). There was moderate uveitis in both eyes, with a 1‐mm hypopyon in the left eye and forward bowing of the iris (iris bomb). A dense vitritis was present in both eyes, preventing visualization of the retina. B‐scan ultrasound examination showed bilateral retinal detachments, worse in the left eye.

Because of the high intraocular pressure in the left eye, the patient was given topical Cosopt (dorzolamide hydrochloride‐timolol maleate), bromonidine 0.15%, and oral acetazolamide to lower intraocular pressures. He was started on a preliminary treatment of hourly topical prednisolone acetate 1%, atropine 1% 4 times daily, and topical moxifloxacin 0.5%. He was admitted to hospital to investigate the source of his panophthalmitis (suppurative infection of the eye and sclera, extending to involve the orbit).
Blood and urine cultures, HIV, rapid plasma reagin test (RPR), HLA B27, toxoplasmosis serology, and ANA rheumatoid factor were sent. Overnight, he developed classic Janeway lesions on his palms and soles, and both blood and urine cultures grew gram‐positive cocci in clusters. Repeat blood cultures were taken. He was started on IV vancomycin empirically. Ultimately, all 3 blood cultures grew Staphylococcus aureus.
A transesophageal echocardiogram diagnosed endocarditis with a pedunculated mobile mass identified on the posterior mitral valve leaflet. Mild mitral regurgitation was noted. The aortic valve was normal, as were ventricular size and function. Antibiotics were modified to cloxacillin and gentamicin IV 2 days later, once sensitivities were reported.
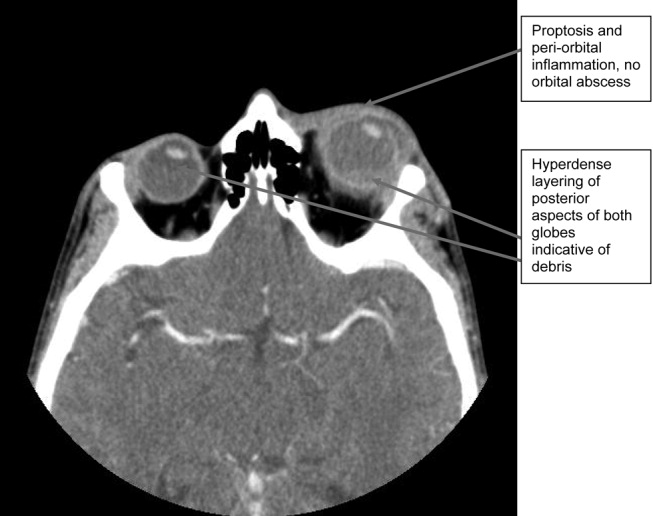
A CT scan of the orbits revealed diffuse orbital inflammation with no evidence of an orbital abscess (Fig. 2). The inflammation and proptosis of the left eye continued to worsen, and a vitreous paracentesis of the left eye was performed for 1.5 mL of dark brown fluid. The aspirated sample was sent for C&S, PCR (for HSV, CMV), acid‐fast stain, and fungal, viral, and mycobacterial cultures. Intravitreal injections of vancomycin and ceftazidime were given. Bacterial cultures showed a heavy intraocular growth of S. aureus, giving the diagnosis of endophthalmitis (bacterial or fungal infection of the vitreous or aqueous humor); all remaining stains and cultures were negative.
Over the next several days, the initial blood work returned with the following abnormal results: CD4 count was 70/L, and HIV serology was positive. The rapid plasma reagin test (RPR) was positive (titer 1:64). The enzyme immunoassay (EIA) and Treponema pallidum particle agglutination (TPPA) were also positive.
A lumbar puncture was performed, and CSF analysis indicated CSF fluid was clear, 2 erythrocytes and 2 leukocytes in the fourth tube, CSF glucose of 2.7 mmol/L (serum glucose 8.2 mmol/L), and CSF total protein of 1100 mg/L. There were no bacteria seen on the gram stain, and a rapid agglutination test for cryptococcal antigen was negative. The CSF RPR titer was 1:2, and the Treponema pallidum particle agglutination assay (TP‐PA) was reactive. The MRI of the brain indicated diffuse white matter disease but no meningeal enhancement. In combination, these results were indicative of neurosyphilis, and penicillin G IV therapy was initiated. He received a total of 14 days of IV therapy, followed by 3 weekly IM doses of benzathine penicillin. He also received a total of 28 days of IV cloxacillin therapy with 5 days of concomitant IV gentamicin for endocarditis treatment.
Over 8 weeks, the patient's panophthalmitis slowly improved. However, he maintained only light perception in the right eye and did not regain any vision in the left eye. He was discharged home to follow‐up with the infectious diseases and ophthalmology departments. The issue of initiating antiretroviral therapy, deferred during hospital admission because of his poor compliance history and the threat of immune reconstitution symptoms, was to be readdressed at this time. He missed both appointments and returned to the emergency room several months later with widespread Kaposi's sarcoma.
DISCUSSION
One of the key learning points from this case underlines that panuveitis carries a broad differential including inflammatory and infectious conditions, as well as lymphoma. Systemic infections include tuberculosis, syphilis, and in cases of severe immunosuppression, toxoplasmosis. Cytomegalovirus and candidiasis are less likely as they are not associated with intraocular inflammation. HIV is also on the differential, although it rarely causes severe panuveitis on its own. Inflammatory disorders such as Behcet syndrome, sarcoidosis, and, rarely, lens‐associated uveitis (if presented with a history of lens trauma or surgery) are also included on the differential. A systematic approach to the history and physical examination must be undertaken to narrow the search. A syphilis screen should always be included in the differential when investigating uveitis,1 especially given the resurgence of syphilis since 2000.2
Our patient presents an interesting study as he was coinfected with both syphilis and HIV. The progression of syphilis is far more aggressive in this scenario,3 as there is a higher frequency of initial presentation as secondary syphilis4 and with multiple persisting chancres.5 Secondary‐stage skin lesions are also more aggressive in coinfected patients (nodular or ulcerative lesions with necrotic centers), although the same dermatological presentations can be seen in HIV‐negative patients.6 It has not been definitively established whether HIV‐positive patients develop neurological complications of syphilis more frequently or earlier in disease, but most patients present with early neurosyphilis at the time of diagnosis.7 In keeping with these findings, our patient's initial presentation included both ocular and neurosyphilis as diagnostic features.
An atypical link highlighted by our case is that of endogenous, bacterial endophthalmitis secondary to endocarditis. Although traumatic or surgical complications are the most common causes of endophthalmitis, seeding from an endogenous infective source, although rare, is possible.810 Staphylococcus aureus endocarditis is one of the most common causes of endogenous spread.9 In our patient, his chronic uveitis and decompensated blood‐ocular barrier may have contributed to S. aureus seeding of his eye. As is the case with many patients diagnosed with S. aureus endocarditis, the source of infection was unknown, although several risk factors for S. aureus bacteremia have been documented. These risk factors include hospitalization, dialysis, transplantation, HIV‐positive status, heart disease, cancer, diabetes, and intravenous drug use. In a population‐based surveillance study from 1999 to 2000, 550 invasive isolates of S. aureus were obtained; the relative risk in HIV‐positive patients was 23.7.11 In a similar study, the source of the S. aureus bacteremia/endocarditis was not identified in 26% of patients with underlying medical conditions such as HIV infection.12
This case has demonstrated several intertwined disease presentations in a patient coinfected with multiple organisms. In an immunocompromised patient, Occam's razor does not necessarily hold true, and the possibility of multiple diagnoses must be entertained. Thus, clinicians must maintain a high index of suspicion for atypical presentations of typical diseases if their patients are to survive in the eye of the storm.
- ,.Ocular syphilis.Surv Ophthalmol.1992;37:203.
- ,,, et al.Primary and secondary syphilis—United States, 2003‐2004.MMWR.2006;55:269–273.
- ,,.Update on syphilis—resurgence of an old problem.JAMA.2003;290:1510.
- ,,,,.Altered clinical presentation of early syphilis in patients with human immunodeficiency virus infection.Ann Intern Med.1994;121:94–100.
- ,,, et al.A randomized trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. The Syphilis and HIV Study Group.N Engl J Med.1997;337:307–314.
- ,.Prominent osseous and unusual dermatologic manifestations of early syphilis in two patients with discordant serological statuses for human immunodeficiency virus infection.Clin Infect Dis.1996;23:462–467.
- ,,,,,.Neurosyphilis during the AIDS epidemic, San Francisco, 1985‐1992.J Infect Dis.1998;177:931–940.
- ,,, et al.Nosocomial endophthalmitis survey: Current incidence of infection after intraocular surgery.Ophthalmology.1991;98:227.
- ,,,.Endophthalmitis following open‐globe injuries.Curr Opin Ophthalmol.1998;9:59.
- ,,, et al.Endogenous bacterial endophthalmitis: Report of a ten‐year retrospective study.Ophthalmology.1994;101:832.
- ,,, et al.Population‐based study of the epidemiology of and the risk factors for invasive Staphylococcus aureus infections.J Infect Dis.2003;187:1452–1459.
- ,.Population‐based incidence and characteristics of community‐onset Staphylococcus aureus infections with bacteremia in 4 metropolitan Connecticut areas, 1998.J Infect Dis.2001;184:1029–1034.
A 37‐year‐old man presented to an ophthalmologist in July 2004 with a history of slowly decreasing vision in both eyes for several weeks. His vision on presentation was 20/400 in the right eye and 20/200 in the left eye. Slit‐lamp examination showed a bilateral anterior uveitis with 360 degrees of posterior synechiae (adhesions) and a dense vitritis (posterior uveitis) that obscured the view of the retina in both eyes. He was diagnosed with panuveitis and started on topical steroid and cycloplegic drops. He was referred to a uveitis specialist for investigation but missed his appointments.
One year later he presented to the emergency room with fever and severe pain in his left eye. On initial assessment he had no complaints of mouth or genital ulcers, recent or remote rashes, joint symptoms, or penile discharge. He denied any prior eye trauma or surgery. He reported that his last sexual encounter had been 8 months prior with a male and that his most recent HIV screen was negative 6 months ago. His family history was negative for autoimmune disorders.
On inspection, he appeared cachectic, lethargic, and very ill. He was febrile and tachycardic; the remainder of his vital signs were normal. There was no lymphadenopathy. His neck was supple with no meningismal signs. There were no heart murmurs, oral ulcers, swollen joints, mucosal eschar, or skin lesions. Respiratory and abdominal examinations were unremarkable.
His visual acuity was light perception in right eye and no light perception in the left eye. There was significant eyelid edema, erythema and purulent discharge with mild proptosis of the left eye (Fig. 1). Pupils were 3 mm and fixed, with 360 of posterior synechiae. Intraocular pressure was elevated in the left eye (42 mm Hg, where normal is 20 mm Hg). There was moderate uveitis in both eyes, with a 1‐mm hypopyon in the left eye and forward bowing of the iris (iris bomb). A dense vitritis was present in both eyes, preventing visualization of the retina. B‐scan ultrasound examination showed bilateral retinal detachments, worse in the left eye.

Because of the high intraocular pressure in the left eye, the patient was given topical Cosopt (dorzolamide hydrochloride‐timolol maleate), bromonidine 0.15%, and oral acetazolamide to lower intraocular pressures. He was started on a preliminary treatment of hourly topical prednisolone acetate 1%, atropine 1% 4 times daily, and topical moxifloxacin 0.5%. He was admitted to hospital to investigate the source of his panophthalmitis (suppurative infection of the eye and sclera, extending to involve the orbit).
Blood and urine cultures, HIV, rapid plasma reagin test (RPR), HLA B27, toxoplasmosis serology, and ANA rheumatoid factor were sent. Overnight, he developed classic Janeway lesions on his palms and soles, and both blood and urine cultures grew gram‐positive cocci in clusters. Repeat blood cultures were taken. He was started on IV vancomycin empirically. Ultimately, all 3 blood cultures grew Staphylococcus aureus.
A transesophageal echocardiogram diagnosed endocarditis with a pedunculated mobile mass identified on the posterior mitral valve leaflet. Mild mitral regurgitation was noted. The aortic valve was normal, as were ventricular size and function. Antibiotics were modified to cloxacillin and gentamicin IV 2 days later, once sensitivities were reported.
A CT scan of the orbits revealed diffuse orbital inflammation with no evidence of an orbital abscess (Fig. 2). The inflammation and proptosis of the left eye continued to worsen, and a vitreous paracentesis of the left eye was performed for 1.5 mL of dark brown fluid. The aspirated sample was sent for C&S, PCR (for HSV, CMV), acid‐fast stain, and fungal, viral, and mycobacterial cultures. Intravitreal injections of vancomycin and ceftazidime were given. Bacterial cultures showed a heavy intraocular growth of S. aureus, giving the diagnosis of endophthalmitis (bacterial or fungal infection of the vitreous or aqueous humor); all remaining stains and cultures were negative.

Over the next several days, the initial blood work returned with the following abnormal results: CD4 count was 70/L, and HIV serology was positive. The rapid plasma reagin test (RPR) was positive (titer 1:64). The enzyme immunoassay (EIA) and Treponema pallidum particle agglutination (TPPA) were also positive.
A lumbar puncture was performed, and CSF analysis indicated CSF fluid was clear, 2 erythrocytes and 2 leukocytes in the fourth tube, CSF glucose of 2.7 mmol/L (serum glucose 8.2 mmol/L), and CSF total protein of 1100 mg/L. There were no bacteria seen on the gram stain, and a rapid agglutination test for cryptococcal antigen was negative. The CSF RPR titer was 1:2, and the Treponema pallidum particle agglutination assay (TP‐PA) was reactive. The MRI of the brain indicated diffuse white matter disease but no meningeal enhancement. In combination, these results were indicative of neurosyphilis, and penicillin G IV therapy was initiated. He received a total of 14 days of IV therapy, followed by 3 weekly IM doses of benzathine penicillin. He also received a total of 28 days of IV cloxacillin therapy with 5 days of concomitant IV gentamicin for endocarditis treatment.
Over 8 weeks, the patient's panophthalmitis slowly improved. However, he maintained only light perception in the right eye and did not regain any vision in the left eye. He was discharged home to follow‐up with the infectious diseases and ophthalmology departments. The issue of initiating antiretroviral therapy, deferred during hospital admission because of his poor compliance history and the threat of immune reconstitution symptoms, was to be readdressed at this time. He missed both appointments and returned to the emergency room several months later with widespread Kaposi's sarcoma.
DISCUSSION
One of the key learning points from this case underlines that panuveitis carries a broad differential including inflammatory and infectious conditions, as well as lymphoma. Systemic infections include tuberculosis, syphilis, and in cases of severe immunosuppression, toxoplasmosis. Cytomegalovirus and candidiasis are less likely as they are not associated with intraocular inflammation. HIV is also on the differential, although it rarely causes severe panuveitis on its own. Inflammatory disorders such as Behcet syndrome, sarcoidosis, and, rarely, lens‐associated uveitis (if presented with a history of lens trauma or surgery) are also included on the differential. A systematic approach to the history and physical examination must be undertaken to narrow the search. A syphilis screen should always be included in the differential when investigating uveitis,1 especially given the resurgence of syphilis since 2000.2
Our patient presents an interesting study as he was coinfected with both syphilis and HIV. The progression of syphilis is far more aggressive in this scenario,3 as there is a higher frequency of initial presentation as secondary syphilis4 and with multiple persisting chancres.5 Secondary‐stage skin lesions are also more aggressive in coinfected patients (nodular or ulcerative lesions with necrotic centers), although the same dermatological presentations can be seen in HIV‐negative patients.6 It has not been definitively established whether HIV‐positive patients develop neurological complications of syphilis more frequently or earlier in disease, but most patients present with early neurosyphilis at the time of diagnosis.7 In keeping with these findings, our patient's initial presentation included both ocular and neurosyphilis as diagnostic features.
An atypical link highlighted by our case is that of endogenous, bacterial endophthalmitis secondary to endocarditis. Although traumatic or surgical complications are the most common causes of endophthalmitis, seeding from an endogenous infective source, although rare, is possible.810 Staphylococcus aureus endocarditis is one of the most common causes of endogenous spread.9 In our patient, his chronic uveitis and decompensated blood‐ocular barrier may have contributed to S. aureus seeding of his eye. As is the case with many patients diagnosed with S. aureus endocarditis, the source of infection was unknown, although several risk factors for S. aureus bacteremia have been documented. These risk factors include hospitalization, dialysis, transplantation, HIV‐positive status, heart disease, cancer, diabetes, and intravenous drug use. In a population‐based surveillance study from 1999 to 2000, 550 invasive isolates of S. aureus were obtained; the relative risk in HIV‐positive patients was 23.7.11 In a similar study, the source of the S. aureus bacteremia/endocarditis was not identified in 26% of patients with underlying medical conditions such as HIV infection.12
This case has demonstrated several intertwined disease presentations in a patient coinfected with multiple organisms. In an immunocompromised patient, Occam's razor does not necessarily hold true, and the possibility of multiple diagnoses must be entertained. Thus, clinicians must maintain a high index of suspicion for atypical presentations of typical diseases if their patients are to survive in the eye of the storm.
A 37‐year‐old man presented to an ophthalmologist in July 2004 with a history of slowly decreasing vision in both eyes for several weeks. His vision on presentation was 20/400 in the right eye and 20/200 in the left eye. Slit‐lamp examination showed a bilateral anterior uveitis with 360 degrees of posterior synechiae (adhesions) and a dense vitritis (posterior uveitis) that obscured the view of the retina in both eyes. He was diagnosed with panuveitis and started on topical steroid and cycloplegic drops. He was referred to a uveitis specialist for investigation but missed his appointments.
One year later he presented to the emergency room with fever and severe pain in his left eye. On initial assessment he had no complaints of mouth or genital ulcers, recent or remote rashes, joint symptoms, or penile discharge. He denied any prior eye trauma or surgery. He reported that his last sexual encounter had been 8 months prior with a male and that his most recent HIV screen was negative 6 months ago. His family history was negative for autoimmune disorders.
On inspection, he appeared cachectic, lethargic, and very ill. He was febrile and tachycardic; the remainder of his vital signs were normal. There was no lymphadenopathy. His neck was supple with no meningismal signs. There were no heart murmurs, oral ulcers, swollen joints, mucosal eschar, or skin lesions. Respiratory and abdominal examinations were unremarkable.
His visual acuity was light perception in right eye and no light perception in the left eye. There was significant eyelid edema, erythema and purulent discharge with mild proptosis of the left eye (Fig. 1). Pupils were 3 mm and fixed, with 360 of posterior synechiae. Intraocular pressure was elevated in the left eye (42 mm Hg, where normal is 20 mm Hg). There was moderate uveitis in both eyes, with a 1‐mm hypopyon in the left eye and forward bowing of the iris (iris bomb). A dense vitritis was present in both eyes, preventing visualization of the retina. B‐scan ultrasound examination showed bilateral retinal detachments, worse in the left eye.

Because of the high intraocular pressure in the left eye, the patient was given topical Cosopt (dorzolamide hydrochloride‐timolol maleate), bromonidine 0.15%, and oral acetazolamide to lower intraocular pressures. He was started on a preliminary treatment of hourly topical prednisolone acetate 1%, atropine 1% 4 times daily, and topical moxifloxacin 0.5%. He was admitted to hospital to investigate the source of his panophthalmitis (suppurative infection of the eye and sclera, extending to involve the orbit).
Blood and urine cultures, HIV, rapid plasma reagin test (RPR), HLA B27, toxoplasmosis serology, and ANA rheumatoid factor were sent. Overnight, he developed classic Janeway lesions on his palms and soles, and both blood and urine cultures grew gram‐positive cocci in clusters. Repeat blood cultures were taken. He was started on IV vancomycin empirically. Ultimately, all 3 blood cultures grew Staphylococcus aureus.
A transesophageal echocardiogram diagnosed endocarditis with a pedunculated mobile mass identified on the posterior mitral valve leaflet. Mild mitral regurgitation was noted. The aortic valve was normal, as were ventricular size and function. Antibiotics were modified to cloxacillin and gentamicin IV 2 days later, once sensitivities were reported.
A CT scan of the orbits revealed diffuse orbital inflammation with no evidence of an orbital abscess (Fig. 2). The inflammation and proptosis of the left eye continued to worsen, and a vitreous paracentesis of the left eye was performed for 1.5 mL of dark brown fluid. The aspirated sample was sent for C&S, PCR (for HSV, CMV), acid‐fast stain, and fungal, viral, and mycobacterial cultures. Intravitreal injections of vancomycin and ceftazidime were given. Bacterial cultures showed a heavy intraocular growth of S. aureus, giving the diagnosis of endophthalmitis (bacterial or fungal infection of the vitreous or aqueous humor); all remaining stains and cultures were negative.

Over the next several days, the initial blood work returned with the following abnormal results: CD4 count was 70/L, and HIV serology was positive. The rapid plasma reagin test (RPR) was positive (titer 1:64). The enzyme immunoassay (EIA) and Treponema pallidum particle agglutination (TPPA) were also positive.
A lumbar puncture was performed, and CSF analysis indicated CSF fluid was clear, 2 erythrocytes and 2 leukocytes in the fourth tube, CSF glucose of 2.7 mmol/L (serum glucose 8.2 mmol/L), and CSF total protein of 1100 mg/L. There were no bacteria seen on the gram stain, and a rapid agglutination test for cryptococcal antigen was negative. The CSF RPR titer was 1:2, and the Treponema pallidum particle agglutination assay (TP‐PA) was reactive. The MRI of the brain indicated diffuse white matter disease but no meningeal enhancement. In combination, these results were indicative of neurosyphilis, and penicillin G IV therapy was initiated. He received a total of 14 days of IV therapy, followed by 3 weekly IM doses of benzathine penicillin. He also received a total of 28 days of IV cloxacillin therapy with 5 days of concomitant IV gentamicin for endocarditis treatment.
Over 8 weeks, the patient's panophthalmitis slowly improved. However, he maintained only light perception in the right eye and did not regain any vision in the left eye. He was discharged home to follow‐up with the infectious diseases and ophthalmology departments. The issue of initiating antiretroviral therapy, deferred during hospital admission because of his poor compliance history and the threat of immune reconstitution symptoms, was to be readdressed at this time. He missed both appointments and returned to the emergency room several months later with widespread Kaposi's sarcoma.
DISCUSSION
One of the key learning points from this case underlines that panuveitis carries a broad differential including inflammatory and infectious conditions, as well as lymphoma. Systemic infections include tuberculosis, syphilis, and in cases of severe immunosuppression, toxoplasmosis. Cytomegalovirus and candidiasis are less likely as they are not associated with intraocular inflammation. HIV is also on the differential, although it rarely causes severe panuveitis on its own. Inflammatory disorders such as Behcet syndrome, sarcoidosis, and, rarely, lens‐associated uveitis (if presented with a history of lens trauma or surgery) are also included on the differential. A systematic approach to the history and physical examination must be undertaken to narrow the search. A syphilis screen should always be included in the differential when investigating uveitis,1 especially given the resurgence of syphilis since 2000.2
Our patient presents an interesting study as he was coinfected with both syphilis and HIV. The progression of syphilis is far more aggressive in this scenario,3 as there is a higher frequency of initial presentation as secondary syphilis4 and with multiple persisting chancres.5 Secondary‐stage skin lesions are also more aggressive in coinfected patients (nodular or ulcerative lesions with necrotic centers), although the same dermatological presentations can be seen in HIV‐negative patients.6 It has not been definitively established whether HIV‐positive patients develop neurological complications of syphilis more frequently or earlier in disease, but most patients present with early neurosyphilis at the time of diagnosis.7 In keeping with these findings, our patient's initial presentation included both ocular and neurosyphilis as diagnostic features.
An atypical link highlighted by our case is that of endogenous, bacterial endophthalmitis secondary to endocarditis. Although traumatic or surgical complications are the most common causes of endophthalmitis, seeding from an endogenous infective source, although rare, is possible.810 Staphylococcus aureus endocarditis is one of the most common causes of endogenous spread.9 In our patient, his chronic uveitis and decompensated blood‐ocular barrier may have contributed to S. aureus seeding of his eye. As is the case with many patients diagnosed with S. aureus endocarditis, the source of infection was unknown, although several risk factors for S. aureus bacteremia have been documented. These risk factors include hospitalization, dialysis, transplantation, HIV‐positive status, heart disease, cancer, diabetes, and intravenous drug use. In a population‐based surveillance study from 1999 to 2000, 550 invasive isolates of S. aureus were obtained; the relative risk in HIV‐positive patients was 23.7.11 In a similar study, the source of the S. aureus bacteremia/endocarditis was not identified in 26% of patients with underlying medical conditions such as HIV infection.12
This case has demonstrated several intertwined disease presentations in a patient coinfected with multiple organisms. In an immunocompromised patient, Occam's razor does not necessarily hold true, and the possibility of multiple diagnoses must be entertained. Thus, clinicians must maintain a high index of suspicion for atypical presentations of typical diseases if their patients are to survive in the eye of the storm.
- ,.Ocular syphilis.Surv Ophthalmol.1992;37:203.
- ,,, et al.Primary and secondary syphilis—United States, 2003‐2004.MMWR.2006;55:269–273.
- ,,.Update on syphilis—resurgence of an old problem.JAMA.2003;290:1510.
- ,,,,.Altered clinical presentation of early syphilis in patients with human immunodeficiency virus infection.Ann Intern Med.1994;121:94–100.
- ,,, et al.A randomized trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. The Syphilis and HIV Study Group.N Engl J Med.1997;337:307–314.
- ,.Prominent osseous and unusual dermatologic manifestations of early syphilis in two patients with discordant serological statuses for human immunodeficiency virus infection.Clin Infect Dis.1996;23:462–467.
- ,,,,,.Neurosyphilis during the AIDS epidemic, San Francisco, 1985‐1992.J Infect Dis.1998;177:931–940.
- ,,, et al.Nosocomial endophthalmitis survey: Current incidence of infection after intraocular surgery.Ophthalmology.1991;98:227.
- ,,,.Endophthalmitis following open‐globe injuries.Curr Opin Ophthalmol.1998;9:59.
- ,,, et al.Endogenous bacterial endophthalmitis: Report of a ten‐year retrospective study.Ophthalmology.1994;101:832.
- ,,, et al.Population‐based study of the epidemiology of and the risk factors for invasive Staphylococcus aureus infections.J Infect Dis.2003;187:1452–1459.
- ,.Population‐based incidence and characteristics of community‐onset Staphylococcus aureus infections with bacteremia in 4 metropolitan Connecticut areas, 1998.J Infect Dis.2001;184:1029–1034.
- ,.Ocular syphilis.Surv Ophthalmol.1992;37:203.
- ,,, et al.Primary and secondary syphilis—United States, 2003‐2004.MMWR.2006;55:269–273.
- ,,.Update on syphilis—resurgence of an old problem.JAMA.2003;290:1510.
- ,,,,.Altered clinical presentation of early syphilis in patients with human immunodeficiency virus infection.Ann Intern Med.1994;121:94–100.
- ,,, et al.A randomized trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. The Syphilis and HIV Study Group.N Engl J Med.1997;337:307–314.
- ,.Prominent osseous and unusual dermatologic manifestations of early syphilis in two patients with discordant serological statuses for human immunodeficiency virus infection.Clin Infect Dis.1996;23:462–467.
- ,,,,,.Neurosyphilis during the AIDS epidemic, San Francisco, 1985‐1992.J Infect Dis.1998;177:931–940.
- ,,, et al.Nosocomial endophthalmitis survey: Current incidence of infection after intraocular surgery.Ophthalmology.1991;98:227.
- ,,,.Endophthalmitis following open‐globe injuries.Curr Opin Ophthalmol.1998;9:59.
- ,,, et al.Endogenous bacterial endophthalmitis: Report of a ten‐year retrospective study.Ophthalmology.1994;101:832.
- ,,, et al.Population‐based study of the epidemiology of and the risk factors for invasive Staphylococcus aureus infections.J Infect Dis.2003;187:1452–1459.
- ,.Population‐based incidence and characteristics of community‐onset Staphylococcus aureus infections with bacteremia in 4 metropolitan Connecticut areas, 1998.J Infect Dis.2001;184:1029–1034.