User login
An Initiative to Improve 30-Day Readmission Rates Using a Transitions-of-Care Clinic Among a Mixed Urban and Rural Veteran Population
Hospital readmissions are a significant problem in the United States, affecting 15% to 30% of discharges and incurring costs of more than $17 billion annually.1 Timely posthospitalization follow-up visits are critical to ensure the effective transfer of patients to the outpatient setting; such visits reduce readmission rates as well as hospital length of stay and overall health care resource utilization.2-4 Patients who receive inadequate follow-up care (ie, within 4 weeks of discharge) are significantly more likely to be readmitted than those who receive close follow-up care.5
Due to the large clinical and financial consequences associated with hospital readmission, a variety of interventions have been studied, including home visits, telemonitoring, medication management, telephone calls, and postdischarge clinics.6,7 While studies have not shown postdischarge clinics to be universally efficacious in reducing readmission rates, there is increasing evidence of reduced readmission rates in clinics that target high-risk patients (eg, patients with congestive heart failure [CHF]) rather than the total population.2 A study by Hernandez et al that evaluated the relationship between early physician follow-up and 30-day readmissions showed a significantly lower readmission rate among hospitals with higher follow-up rates.8 Similarly, patients with CHF in a large, integrated health system who were seen within 7 days of discharge had an odds ratio (OR) of 0.81 (95% CI, 0.70-0.94) for 30-day readmissions.9
Transitions-of-care clinics (TOCC), designed to provide early postdischarge follow-up to high-risk patients, have been shown to reduce 30-day readmission rates,3,4,10,11 especially in clinics that have same-physician follow-up visits rather than follow-up visits with a community primary care physician (PCP).12 The most pronounced impact of postdischarge follow-up is seen in high-risk patients with high complexity or high severity of disease; however, complex rural patients are less likely to have access to specialty care.13 As a result, since rural residents must travel farther for specialty care, they are seen less frequently than their urban counterparts.14,15
Prior to our TOCC initiative, the Iowa City VA (ICVA) ranked in the fifth quintile of the Veterans Health Administration (VHA)
To meet these challenges, we implemented a TOCC to deliver timely postdischarge care focusing on high-risk and high-complexity patients. To address access-to-care issues of patients living in rural areas within the ICVA, we included virtual follow-up visits as a key component of our intervention.16,17 The aim of this project was to decrease 30-day readmission rates of ICVA patients by 20% within 12 months of implementation.
METHODS
Setting/Study Population
The ICVA serves 184,000 veterans stretched over 50 counties in eastern Iowa, western Illinois, and northern Missouri, with more than 60% of these patients residing in rural areas. Patients were initially eligible for the TOCC if they had an admission diagnosis of CHF and a CAN score > 85 at the time of discharge. The CAN score, developed by the VA to assess the risk of hospital readmission in individual patients, factors in several variables, including demographics, coexisting conditions, vital signs, utilization of services, pharmacy visits, and laboratory results. Patients in the top 5% (95-99) have a readmission rate of 20% at 90 days. Since the CAN is a proprietary tool, it may not be published in full; however, this assessment tool is commonly used and frequently cited in VA research.18-22 The CAN score is expressed as a percentile ranging from 0 (lowest risk) to 99 (highest risk). Patient eligibility was expanded during subsequent Plan-Do-Study-Act (PDSA) cycles, as outlined below. Patient eligibility was expanded during subsequent PDSA cycles (also outlined below). A review by a local institutional review board was obtained, and the study was classified as exempt due to the use of deidentified data. Standards for Quality Improvement Reporting Excellence 2.0 guidelines were used to construct the manuscript.
Magnitude Assessment
The numbers of discharges, readmissions within 30 days, emergency department (ED) visits by all discharged veterans, and veterans discharged with a CHF hospital diagnosis were recorded from February 2017 to February 2018, which were the 12 months immediately preceding the pilot implementation.
Intervention
The primary intervention was referral to the newly formed ICVA TOCC. The multidisciplinary TOCC team consisted of hospitalists, pharmacists, schedulers, and discharge planners/care managers. Patients were identified by the hospitalist team during admission; prior to hospital discharge, these patients were referred to TOCC discharge planners to schedule appropriate follow-up appointments. Virtual follow-up visits were conducted using a patient’s home technology; in cases where a patient lacked adequate technology capabilities (eg, no computer or internet access), the ICVA provided a tablet device with cellular internet capability for temporary use. Specific clinical activities included medication reconciliation by a pharmacist, follow-up of pending laboratory studies, imaging studies, pathology results, medical diagnosis education, counseling regarding dietary restrictions, and contingency planning outside of an ED visit in the event of a change in clinical status. In addition, the TOCC aimed to facilitate a smooth transition of care back to the PCP by arranging follow-up appointments, providing visit summaries, and scheduling consults with specialty care, as appropriate.
Measures
The primary objective measure was the 30-day readmission rate in the ICVA hospital. Secondary measures included the number of VHA ED visits within 30 days of discharge. The main process measures were the number of hospital discharges per month, the number of TOCC referrals, the number of TOCC appointments made, the number of virtual and in-person visits, and the percentage of appointment “no-shows.”
Implementation
The TOCC was piloted from April 2018 to October 2018. During the pilot phase, TOCC enrollment was limited to virtual appointments and to patients with an admission diagnosis of CHF and a CAN score of > 85. The TOCC had staff on-site 2 days a week; this included pharmacists to reconcile medications and hospitalists to address follow-up care needs.
The TOCC clinic was temporarily closed at the end of October 2018 to analyze pilot results. Based on stakeholder feedback, changes made as part of the second PDSA cycle included expanding eligibility criteria to any hospital admission diagnosis and to patients with a CAN score < 85 if the hospitalist team felt the patient was likely to benefit from TOCC follow-up. In addition, on-site clinic staffing was expanded from 2 to 5 days per week to improve access, and the option for an in-person visit was added based on concerns some veterans expressed regarding the use of the technology at home. Finally, a formal resident program was added, and the order set for referrals was simplified. The TOCC was restarted in February 2019, and TOCC metrics were reviewed monthly. By July 2019, we identified issues with TOCC referrals and appointment creation that required additional modifications to the intervention.
A third PDSA cycle was initiated in July 2019 and included major changes, notably the formation of a designated TOCC committee. The committee appointed a dedicated TOCC scheduler whose role was to reduce confusion regarding scheduling, to update the discharge instructions/orders template to lower incidences of “double-booking” that occurred with PCP and TOCC appointments, to modify discharge educational instruction regarding virtual visits and tablet use, to adjust the TOCC-PCP handoff, and to formalize interactions between discharge coordinators and residents to review possible referrals every morning (Appendix Figure 1).
Statistical Analysis
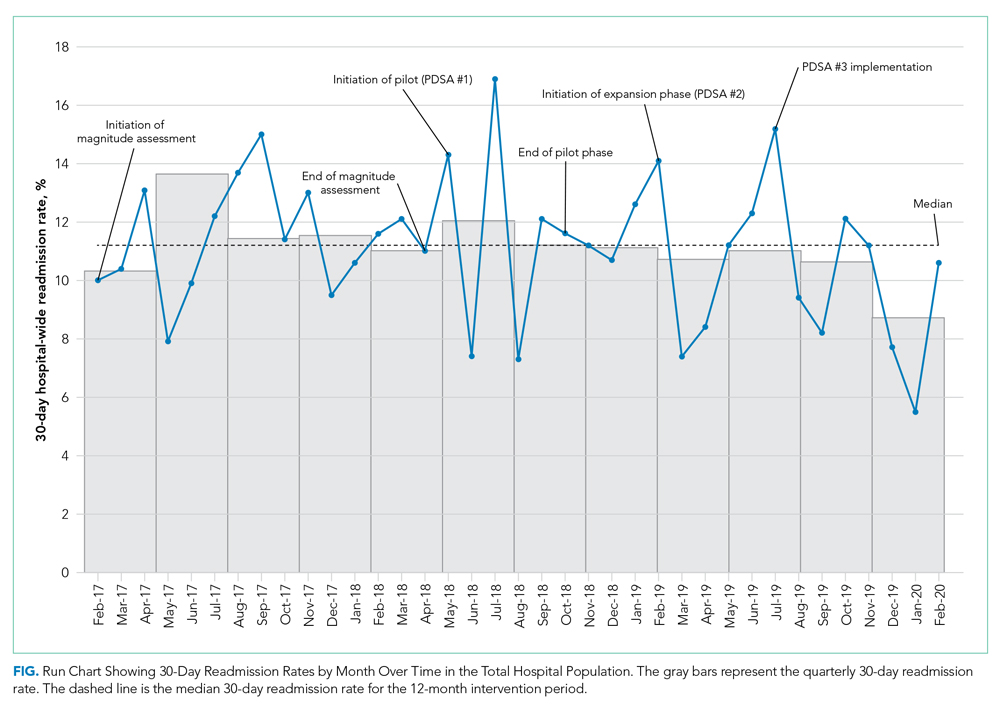
Run charts were constructed by plotting monthly primary outcome values and monthly process metrics (Figure, Appendix Figure 2, Appendix Figure 3). Chi-square tests were used to compare 30-day readmission rates before and after the intervention.
Mean (SD) or counts and percentages were used to describe the distribution of continuous and categorical variables, respectively. Kruskal-Wallis test, t test, or chi-square tests were used, as appropriate, across categories. Generalized linear models with a logistic link function were used to test for differences between patients who kept their appointment at the TOCC and those who did not keep their TOCC appointment (both unadjusted and adjusted for all of the covariates previously mentioned). In addition, generalized linear models were also used to compare outcomes between TOCC patients seen virtually vs those seen in-person (both unadjusted and adjusted for all the covariates previously mentioned). All statistical tests were considered significant at a two-sided P < .05. All analyses were performed using SAS software version 9.4 (SAS Institute Inc).
RESULTS
Magnitude Assessment
During the preimplementation period (February 2017-February 2018), there were 3014 patient discharges from ICVA and 343 readmissions, resulting in a readmission rate of 11.4%. Among patients with a hospital-admission diagnosis of cardiorespiratory disease, which included patients with CHF, there were 381 discharges and 46 readmissions, resulting in a readmission rate of 12.1%.
Primary Outcome
During the pilot phase, which was conducted from April 2018 to October 2018, 142 patients who met inclusion criteria (CHF diagnosis and a CAN score > 85) were discharged from ICVA, and 56 referrals to the TOCC were placed. The readmission rate among the cardiorespiratory cohort of veterans was 9.5%.
During the expansion of the intervention from February 2019 to February 2020, there were 2844 discharges from the ICVA and 291 readmissions, resulting in a readmission rate of 10.2%. However, there was a further decrease in the readmission rate after the third PDSA cycle was initiated in July 2019 (Appendix Figure 1). The readmission rate was 9.2% in the final 6 months of the intervention period, and 7.9% in the final 3 months.
When comparing the 6 months following the third PDSA cycle to the magnitude assessment period, there was a relative readmission reduction of 19.3% (P = .04), and an absolute reduction of 2.2%. If the final 3 months of the intervention period are included, there was an absolute reduction of 3.5% and a relative reduction of 30.7% (P = .01). Notably, before the pilot phase, ICVA was in the fifth quintile for HWR among VA hospitals but improved to the second quintile by the end of the expansion phase.
Process Outcomes
Process metrics for TOCC referrals, the number of patients seen, and the number of virtual and in-person visits over time are shown in Appendix Figure 3. Rates of TOCC referrals and the number of TOCC visits were lower than anticipated during the first 5 months of the intervention. However, TOCC referrals increased significantly after we implemented the previously described changes as part of the third PDSA cycle. As a result, total, virtual, and in-person visits also significantly increased from July 2019 to February 2020. The proportion of patients choosing virtual vs in-person visits fluctuated over time, but virtual visits were generally chosen more often than in-person visits.
Statistical Modeling
Baseline Data
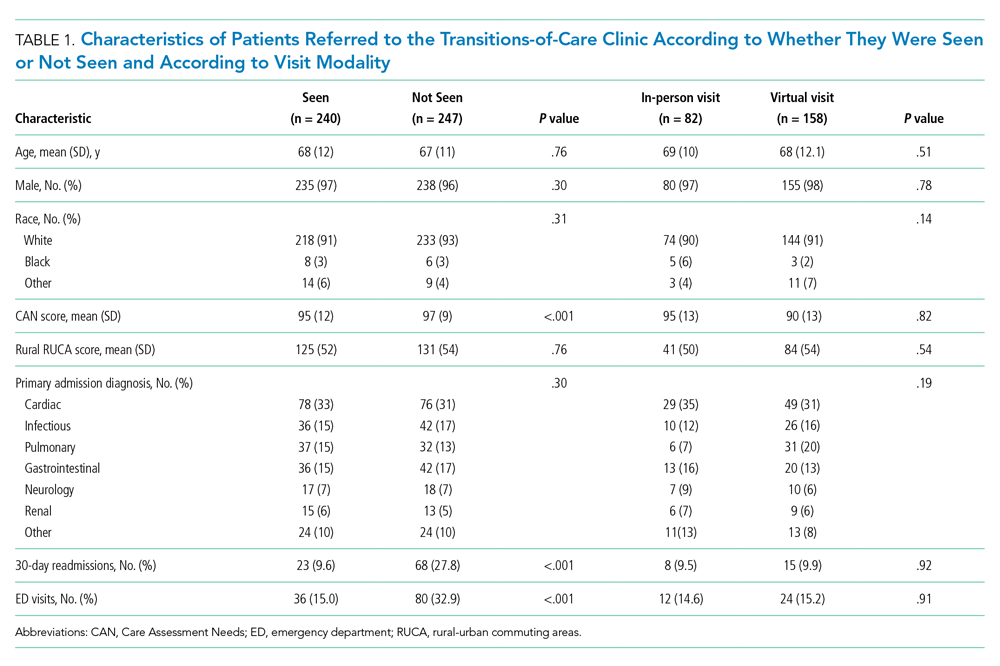
Cohort characteristics are shown in Table 1. The cohort, which reflected the ICVA population, was predominantly male (96%) and White (93%), with a mean age of 67 years. The population was approximately half urban and half rural in composition, and the most common reason for hospital admission was cardiac. Other than a small but statistically significant difference in CAN scores, there were no significant differences between patients who kept their TOCC appointment and those who did not. There were also no differences in baseline characteristics between patients who chose virtual follow-up and patients who chose in-person follow-up, including the proportion of urban and rural patients.
Outcomes
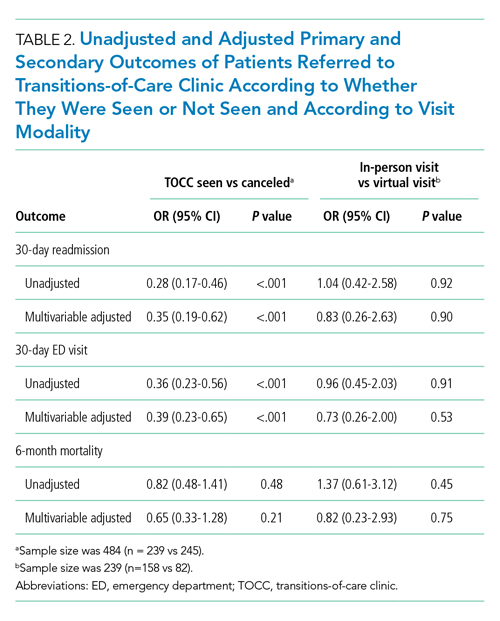
Patients who kept their TOCC appointments had a 30-day readmission rate of 9.6%, which was significantly lower than the 30-day readmission rate of 27% in the group that did not keep their TOCC appointment (P < .001). Similarly, the percentage of patients treated in the ED was 15% in the TOCC group compared to 31.2% in the group that canceled their appointment (P < .001) (Table 1). In the multivariable analysis, patients who were seen in the TOCC group had an OR for 30-day readmission of 0.35 (95% CI, 0.19-0.62, P < .001), and an OR for ED visits of 0.39 (95% CI, 0.23-0.65; P < .001) (Table 2). There was no statistically significant difference in 6-month mortality between the two groups. In the virtual group compared to the in-person group, there were no statistically significant differences in outcomes between the two groups in the unadjusted or adjusted analysis (Table 2).
DISCUSSION
In the expansion phase, eligibility was expanded to include any hospital indication but continued to focus on high-risk patients. Existing literature suggests that providing postdischarge care to all patients, including low- or medium-risk patients, may not be as impactful as enrolling high-risk patients only. For instance, a postdischarge clinic offered to all patients at a VA system in Colorado did not reduce readmission rates compared to PCP follow-up.23 In contrast, a study of more than 10,000 high-risk urban patients demonstrated that postdischarge care resulted in a 9.3% reduction in readmission risk.24 Our data are consistent with the previously published studies, as the average CAN score of patients seen in TOCC was 90, suggesting a high risk of readmission. In the final 12 months of the intervention, 15% of discharged patients were seen at the TOCC clinic, suggesting that targeted intervention within the small subset of high-risk patients was sufficient to achieve our primary aim. Of note, among patients who did not meet the inclusion criteria for TOCC referral (ie, patients not considered high risk [CAN score ≤ 85]), the rate of readmissions was 8.6%.
Most of the available research on the efficacy of postdischarge clinics was conducted in urban environments. Our ICVA population sees a large proportion of rural veterans, who account for just over 50% of the discharge population. In a study of more than 2 million Medicare patients discharged from US hospitals, the 30-day readmission rates and adjusted mortality rates were higher among patients in rural counties, and post–acute care seemed to have a greater impact in rural rather than urban settings.25 Previous studies have demonstrated that virtual visits have the potential to improve readmission rates, especially in patients with CHF26 and in patients at the highest risk for readmission.27 In our study, the pilot phase offered only virtual visits, but we subsequently added an in-person option based on veteran feedback. Interestingly, over the next 12 months, virtual visits were more popular with both urban and rural veterans, and there were no differences in the number of rural patients in the in-person vs the virtual group. These findings suggest factors other than rurality influenced the decision to choose virtual follow-up visits over in-person visits. Future studies should seek to determine the extent to which factors such as age, race, educational level, and socioeconomic circumstances impact veterans’ follow-up decisions. Not only were outcomes among patients who chose virtual visits the same as those of patients who chose in-person visits, but both of these groups had better outcomes compared to the non-TOCC group (Table 2). This finding demonstrating the efficacy of virtual visits among rural and urban patients has taken on increased significance due to the COVID-19 pandemic, as virtual visits offer a safer option, one that minimizes physical contact.
Our quality improvement analysis included a statistical comparison of patients seen vs those not seen at the TOCC. Patients who were referred to the TOCC but chose not to keep their appointment were similar to those seen in TOCC in terms of age, CAN score, rurality, and hospital diagnosis, but readmission rates were substantially higher in this group even after adjustments for covariates (Table 2). Evaluating causality in interventions aimed to reduce hospital readmission rates is complicated.28 Our findings add greater plausibility to the utility of TOCC in accounting for at least a portion of the reported decrease in ICVA 30-day readmissions.
Our study has several strengths, including an observation period longer than 2 years, a large population of discharged veterans within an integrated healthcare system, and a large proportion of patients living in rural areas. Another strength of our study is the innovative nature of the intervention, which features a multidisciplinary team and the option of virtual or in-person visits. Nevertheless, this study also has several important limitations. As a single-center study, our findings may not be generalizable to other institutions, especially those outside the VHA system. Similarly, our study population reflected that of the ICVA, which may limit generalizability to a more diverse population. While we attempted to account in our statistical modeling for baseline differences between referred patients seen by the TOCC and those referred but not seen, we cannot exclude residual confounding between the groups. Specifically, the comparison of patients who did and did not choose TOCC follow-up introduces the possibility of selection bias. Future randomized/controlled studies will need to evaluate whether TOCC is more effective than the standard of care to reduce readmissions. Finally, since the analysis period following the final PDSA cycle was compressed due to the onset of the COVID-19 pandemic in the United States, no data are available regarding the sustained impacts of changes made during this cycle.
CONCLUSION
A multidisciplinary TOCC within the ICVA, featuring both virtual and in-person visits, reduced 30-day readmission rates by 19.3%; this approach to care was especially effective in patients with CHF. Virtual visits were the follow-up mode of choice for both urban and rural veterans, and there was no difference in outcomes between these two follow-up options. Future studies will focus on additional quality metrics, including cost-effectiveness and patient satisfaction.
1. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. https://doi.org/10.1056/nejmsa0803563
2. Doctoroff L. Postdischarge clinics and hospitalists: a review of the evidence and existing models. J Hosp Med. 2017;12(6):467-471. https://doi.org/10.12788/jhm.2750
3. Koehler BE, Richter KM, Youngblood L, et al. Reduction of 30-day postdischarge hospital readmission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med. 2009;4(4):211-218. https://doi.org/10.1002/jhm.427
4. Abrashkin KA, Cho HJ, Torgalkar S, Markoff B. Improving transitions of care from hospital to home: what works? Mt Sinai J Med. 2012;79(5):535-544. https://doi.org/10.1002/msj.21332
5. Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5(7):392-397. https://doi.org/10.1002/jhm.666
6. Greysen SR, Harrison JD, Kripalani S, et al. Understanding patient-centred readmission factors: a multi-site, mixed-methods study. BMJ Qual Saf. 2017;26(1):33-41. https://doi.org/10.1136/bmjqs-2015-004570
7. Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. https://doi.org/10.7326/0003-4819-155-8-201110180-00008
8. Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716-1722. https://doi.org/10.1001/jama.2010.533
9. Lee KK, Yang J, Hernandez AF, Steimle AE, Go AS. Post-discharge follow-up characteristics associated with 30-day readmission after heart failure hospitalization. Med Care. 2016;54(4):365-372. https://doi.org/10.1097/mlr.0000000000000492
10. Balaban RB, Williams MV. Improving care transitions: hospitalists partnering with primary care. J Hosp Med. 2010;5(7):375-377. https://doi.org/10.1002/jhm.824
11. Rodrigues CR, Harrington AR, Murdock N, et al. Effect of pharmacy-supported transition-of-care interventions on 30-day readmissions: a systematic review and meta-analysis. Ann Pharmacother. 2017;51(10):866-889. https://doi.org/10.1177/1060028017712725
12. van Walraven C, Taljaard M, Etchells E, et al. The independent association of provider and information continuity on outcomes after hospital discharge: implications for hospitalists. J Hosp Med. 2010;5(7):398-405. https://doi.org/10.1002/jhm.716
13. Gruca TS, Pyo TH, Nelson GC. Providing cardiology care in rural areas through vsiting consultant clinics. J Am Heart Assoc. 2016;5(7):e002909. https://doi.org/10.1161/jaha.115.002909
14. Chan L, Hart LG, Goodman DC. Geographic access to health care for rural Medicare beneficiaries. J Rural Health. 2006;22(2):140-146. https://doi.org/10.1111/j.1748-0361.2006.00022.x
15. Burke RE, Jones CD, Coleman EA, Falvey JR, Stevens-Lapsley JE, Ginde AA. Use of post-acute care after hospital discharge in urban and rural hospitals. Am J Accountable Care. 2017;5(1):16-22.
16. Jetty A, Moore MA, Coffman M, Petterson S, Bazemore A. Rural family physicians are twice as likely to use telehealth as urban family physicians. Telemed J E Health. 2018;24(4):268-276. https://doi.org/10.1089/tmj.2017.0161
17. Harrison PL, Hara PA, Pope JE, Young MC, Rula EY. The impact of postdischarge telephonic follow-up on hospital readmissions. Popul Health Manag. 2011;14(1):27-32. https://doi.org/10.1089/pop.2009.0076
18. Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368-373. https://doi.org/10.1097/mlr.0b013e31827da95a
19. Spece LJ, Donovan LM, Griffith MF, et al. Initiating low-value inhaled corticosteroids in an inception cohort with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2020;17(5):589-595. https://doi.org/10.1513/annalsats.201911-854oc
20. Osborne TF, Suarez P, Edwards D, Hernandez-Boussard T, Curtin C. Patient electronic health records score for preoperative risk assessment before total knee arthroplasty. JB JS Open Access. 2020;5(2):e0061. https://doi.org/10.2106/jbjs.oa.19.00061
21. Levy C, Ersek M, Scott W, et al. Life-sustaining treatment decisions initiative: early implementation results of a national Veterans Affairs program to honor veterans’ care preferences. J Gen Intern Med. 2020;35(6):1803-1812. https://doi.org/10.1007/s11606-020-05697-2
22. Ibrahim SA. High-risk patients and utilization of primary care in the US Veterans Affairs health system. JAMA Netw Open. 2020;3(6):e209518. https://doi.org/10.1001/jamanetworkopen.2020.9518
23. Burke RE, Whitfield E, Prochazka AV. Effect of a hospitalist-run postdischarge clinic on outcomes. J Hosp Med. 2014;9(1):7-12. https://doi.org/10.1002/jhm.2099
24. Jenq GY, Doyle MM, Belton BM, Herrin J, Horwitz LI. Quasi-experimental evaluation of the effectiveness of a large-scale readmission reduction program. JAMA Intern Med. 2016;176(5):681-690. https://doi.org/10.1001/jamainternmed.2016.0833
25. Kosar CM, Loomer L, Ferdows NB, Trivedi AN, Panagiotou OA, Rahman M. Assessment of rural-urban differences in postacute care utilization and outcomes among older US adults. JAMA Netw Open. 2020;3(1):e1918738. https://doi.org/10.1001/jamanetworkopen.2019.18738
26. Pandor A, Thokala P, Gomersall T, et al. Home telemonitoring or structured telephone support programmes after recent discharge in patients with heart failure: systematic review and economic evaluation. Health Technol Assess. 2013;17(32):1-207, v-vi. https://doi.org/10.3310/hta17320
27. Low LL, Tan SY, Ng MJM, et al. Applying the integrated practice unit concept to a modified virtual ward model of care for patients at highest risk of readmission: a randomized controlled trial. PloS One. 2017;12(1):e0168757. https://doi.org/10.1371/journal.pone.0168757
28. McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):1796-1803. https://doi.org/10.1161/circulationaha.114.010270
Hospital readmissions are a significant problem in the United States, affecting 15% to 30% of discharges and incurring costs of more than $17 billion annually.1 Timely posthospitalization follow-up visits are critical to ensure the effective transfer of patients to the outpatient setting; such visits reduce readmission rates as well as hospital length of stay and overall health care resource utilization.2-4 Patients who receive inadequate follow-up care (ie, within 4 weeks of discharge) are significantly more likely to be readmitted than those who receive close follow-up care.5
Due to the large clinical and financial consequences associated with hospital readmission, a variety of interventions have been studied, including home visits, telemonitoring, medication management, telephone calls, and postdischarge clinics.6,7 While studies have not shown postdischarge clinics to be universally efficacious in reducing readmission rates, there is increasing evidence of reduced readmission rates in clinics that target high-risk patients (eg, patients with congestive heart failure [CHF]) rather than the total population.2 A study by Hernandez et al that evaluated the relationship between early physician follow-up and 30-day readmissions showed a significantly lower readmission rate among hospitals with higher follow-up rates.8 Similarly, patients with CHF in a large, integrated health system who were seen within 7 days of discharge had an odds ratio (OR) of 0.81 (95% CI, 0.70-0.94) for 30-day readmissions.9
Transitions-of-care clinics (TOCC), designed to provide early postdischarge follow-up to high-risk patients, have been shown to reduce 30-day readmission rates,3,4,10,11 especially in clinics that have same-physician follow-up visits rather than follow-up visits with a community primary care physician (PCP).12 The most pronounced impact of postdischarge follow-up is seen in high-risk patients with high complexity or high severity of disease; however, complex rural patients are less likely to have access to specialty care.13 As a result, since rural residents must travel farther for specialty care, they are seen less frequently than their urban counterparts.14,15
Prior to our TOCC initiative, the Iowa City VA (ICVA) ranked in the fifth quintile of the Veterans Health Administration (VHA)
To meet these challenges, we implemented a TOCC to deliver timely postdischarge care focusing on high-risk and high-complexity patients. To address access-to-care issues of patients living in rural areas within the ICVA, we included virtual follow-up visits as a key component of our intervention.16,17 The aim of this project was to decrease 30-day readmission rates of ICVA patients by 20% within 12 months of implementation.
METHODS
Setting/Study Population
The ICVA serves 184,000 veterans stretched over 50 counties in eastern Iowa, western Illinois, and northern Missouri, with more than 60% of these patients residing in rural areas. Patients were initially eligible for the TOCC if they had an admission diagnosis of CHF and a CAN score > 85 at the time of discharge. The CAN score, developed by the VA to assess the risk of hospital readmission in individual patients, factors in several variables, including demographics, coexisting conditions, vital signs, utilization of services, pharmacy visits, and laboratory results. Patients in the top 5% (95-99) have a readmission rate of 20% at 90 days. Since the CAN is a proprietary tool, it may not be published in full; however, this assessment tool is commonly used and frequently cited in VA research.18-22 The CAN score is expressed as a percentile ranging from 0 (lowest risk) to 99 (highest risk). Patient eligibility was expanded during subsequent Plan-Do-Study-Act (PDSA) cycles, as outlined below. Patient eligibility was expanded during subsequent PDSA cycles (also outlined below). A review by a local institutional review board was obtained, and the study was classified as exempt due to the use of deidentified data. Standards for Quality Improvement Reporting Excellence 2.0 guidelines were used to construct the manuscript.
Magnitude Assessment
The numbers of discharges, readmissions within 30 days, emergency department (ED) visits by all discharged veterans, and veterans discharged with a CHF hospital diagnosis were recorded from February 2017 to February 2018, which were the 12 months immediately preceding the pilot implementation.
Intervention
The primary intervention was referral to the newly formed ICVA TOCC. The multidisciplinary TOCC team consisted of hospitalists, pharmacists, schedulers, and discharge planners/care managers. Patients were identified by the hospitalist team during admission; prior to hospital discharge, these patients were referred to TOCC discharge planners to schedule appropriate follow-up appointments. Virtual follow-up visits were conducted using a patient’s home technology; in cases where a patient lacked adequate technology capabilities (eg, no computer or internet access), the ICVA provided a tablet device with cellular internet capability for temporary use. Specific clinical activities included medication reconciliation by a pharmacist, follow-up of pending laboratory studies, imaging studies, pathology results, medical diagnosis education, counseling regarding dietary restrictions, and contingency planning outside of an ED visit in the event of a change in clinical status. In addition, the TOCC aimed to facilitate a smooth transition of care back to the PCP by arranging follow-up appointments, providing visit summaries, and scheduling consults with specialty care, as appropriate.
Measures
The primary objective measure was the 30-day readmission rate in the ICVA hospital. Secondary measures included the number of VHA ED visits within 30 days of discharge. The main process measures were the number of hospital discharges per month, the number of TOCC referrals, the number of TOCC appointments made, the number of virtual and in-person visits, and the percentage of appointment “no-shows.”
Implementation
The TOCC was piloted from April 2018 to October 2018. During the pilot phase, TOCC enrollment was limited to virtual appointments and to patients with an admission diagnosis of CHF and a CAN score of > 85. The TOCC had staff on-site 2 days a week; this included pharmacists to reconcile medications and hospitalists to address follow-up care needs.
The TOCC clinic was temporarily closed at the end of October 2018 to analyze pilot results. Based on stakeholder feedback, changes made as part of the second PDSA cycle included expanding eligibility criteria to any hospital admission diagnosis and to patients with a CAN score < 85 if the hospitalist team felt the patient was likely to benefit from TOCC follow-up. In addition, on-site clinic staffing was expanded from 2 to 5 days per week to improve access, and the option for an in-person visit was added based on concerns some veterans expressed regarding the use of the technology at home. Finally, a formal resident program was added, and the order set for referrals was simplified. The TOCC was restarted in February 2019, and TOCC metrics were reviewed monthly. By July 2019, we identified issues with TOCC referrals and appointment creation that required additional modifications to the intervention.
A third PDSA cycle was initiated in July 2019 and included major changes, notably the formation of a designated TOCC committee. The committee appointed a dedicated TOCC scheduler whose role was to reduce confusion regarding scheduling, to update the discharge instructions/orders template to lower incidences of “double-booking” that occurred with PCP and TOCC appointments, to modify discharge educational instruction regarding virtual visits and tablet use, to adjust the TOCC-PCP handoff, and to formalize interactions between discharge coordinators and residents to review possible referrals every morning (Appendix Figure 1).
Statistical Analysis
Run charts were constructed by plotting monthly primary outcome values and monthly process metrics (Figure, Appendix Figure 2, Appendix Figure 3). Chi-square tests were used to compare 30-day readmission rates before and after the intervention.
Mean (SD) or counts and percentages were used to describe the distribution of continuous and categorical variables, respectively. Kruskal-Wallis test, t test, or chi-square tests were used, as appropriate, across categories. Generalized linear models with a logistic link function were used to test for differences between patients who kept their appointment at the TOCC and those who did not keep their TOCC appointment (both unadjusted and adjusted for all of the covariates previously mentioned). In addition, generalized linear models were also used to compare outcomes between TOCC patients seen virtually vs those seen in-person (both unadjusted and adjusted for all the covariates previously mentioned). All statistical tests were considered significant at a two-sided P < .05. All analyses were performed using SAS software version 9.4 (SAS Institute Inc).
RESULTS
Magnitude Assessment
During the preimplementation period (February 2017-February 2018), there were 3014 patient discharges from ICVA and 343 readmissions, resulting in a readmission rate of 11.4%. Among patients with a hospital-admission diagnosis of cardiorespiratory disease, which included patients with CHF, there were 381 discharges and 46 readmissions, resulting in a readmission rate of 12.1%.
Primary Outcome
During the pilot phase, which was conducted from April 2018 to October 2018, 142 patients who met inclusion criteria (CHF diagnosis and a CAN score > 85) were discharged from ICVA, and 56 referrals to the TOCC were placed. The readmission rate among the cardiorespiratory cohort of veterans was 9.5%.
During the expansion of the intervention from February 2019 to February 2020, there were 2844 discharges from the ICVA and 291 readmissions, resulting in a readmission rate of 10.2%. However, there was a further decrease in the readmission rate after the third PDSA cycle was initiated in July 2019 (Appendix Figure 1). The readmission rate was 9.2% in the final 6 months of the intervention period, and 7.9% in the final 3 months.
When comparing the 6 months following the third PDSA cycle to the magnitude assessment period, there was a relative readmission reduction of 19.3% (P = .04), and an absolute reduction of 2.2%. If the final 3 months of the intervention period are included, there was an absolute reduction of 3.5% and a relative reduction of 30.7% (P = .01). Notably, before the pilot phase, ICVA was in the fifth quintile for HWR among VA hospitals but improved to the second quintile by the end of the expansion phase.
Process Outcomes
Process metrics for TOCC referrals, the number of patients seen, and the number of virtual and in-person visits over time are shown in Appendix Figure 3. Rates of TOCC referrals and the number of TOCC visits were lower than anticipated during the first 5 months of the intervention. However, TOCC referrals increased significantly after we implemented the previously described changes as part of the third PDSA cycle. As a result, total, virtual, and in-person visits also significantly increased from July 2019 to February 2020. The proportion of patients choosing virtual vs in-person visits fluctuated over time, but virtual visits were generally chosen more often than in-person visits.
Statistical Modeling
Baseline Data
Cohort characteristics are shown in Table 1. The cohort, which reflected the ICVA population, was predominantly male (96%) and White (93%), with a mean age of 67 years. The population was approximately half urban and half rural in composition, and the most common reason for hospital admission was cardiac. Other than a small but statistically significant difference in CAN scores, there were no significant differences between patients who kept their TOCC appointment and those who did not. There were also no differences in baseline characteristics between patients who chose virtual follow-up and patients who chose in-person follow-up, including the proportion of urban and rural patients.
Outcomes
Patients who kept their TOCC appointments had a 30-day readmission rate of 9.6%, which was significantly lower than the 30-day readmission rate of 27% in the group that did not keep their TOCC appointment (P < .001). Similarly, the percentage of patients treated in the ED was 15% in the TOCC group compared to 31.2% in the group that canceled their appointment (P < .001) (Table 1). In the multivariable analysis, patients who were seen in the TOCC group had an OR for 30-day readmission of 0.35 (95% CI, 0.19-0.62, P < .001), and an OR for ED visits of 0.39 (95% CI, 0.23-0.65; P < .001) (Table 2). There was no statistically significant difference in 6-month mortality between the two groups. In the virtual group compared to the in-person group, there were no statistically significant differences in outcomes between the two groups in the unadjusted or adjusted analysis (Table 2).
DISCUSSION
In the expansion phase, eligibility was expanded to include any hospital indication but continued to focus on high-risk patients. Existing literature suggests that providing postdischarge care to all patients, including low- or medium-risk patients, may not be as impactful as enrolling high-risk patients only. For instance, a postdischarge clinic offered to all patients at a VA system in Colorado did not reduce readmission rates compared to PCP follow-up.23 In contrast, a study of more than 10,000 high-risk urban patients demonstrated that postdischarge care resulted in a 9.3% reduction in readmission risk.24 Our data are consistent with the previously published studies, as the average CAN score of patients seen in TOCC was 90, suggesting a high risk of readmission. In the final 12 months of the intervention, 15% of discharged patients were seen at the TOCC clinic, suggesting that targeted intervention within the small subset of high-risk patients was sufficient to achieve our primary aim. Of note, among patients who did not meet the inclusion criteria for TOCC referral (ie, patients not considered high risk [CAN score ≤ 85]), the rate of readmissions was 8.6%.
Most of the available research on the efficacy of postdischarge clinics was conducted in urban environments. Our ICVA population sees a large proportion of rural veterans, who account for just over 50% of the discharge population. In a study of more than 2 million Medicare patients discharged from US hospitals, the 30-day readmission rates and adjusted mortality rates were higher among patients in rural counties, and post–acute care seemed to have a greater impact in rural rather than urban settings.25 Previous studies have demonstrated that virtual visits have the potential to improve readmission rates, especially in patients with CHF26 and in patients at the highest risk for readmission.27 In our study, the pilot phase offered only virtual visits, but we subsequently added an in-person option based on veteran feedback. Interestingly, over the next 12 months, virtual visits were more popular with both urban and rural veterans, and there were no differences in the number of rural patients in the in-person vs the virtual group. These findings suggest factors other than rurality influenced the decision to choose virtual follow-up visits over in-person visits. Future studies should seek to determine the extent to which factors such as age, race, educational level, and socioeconomic circumstances impact veterans’ follow-up decisions. Not only were outcomes among patients who chose virtual visits the same as those of patients who chose in-person visits, but both of these groups had better outcomes compared to the non-TOCC group (Table 2). This finding demonstrating the efficacy of virtual visits among rural and urban patients has taken on increased significance due to the COVID-19 pandemic, as virtual visits offer a safer option, one that minimizes physical contact.
Our quality improvement analysis included a statistical comparison of patients seen vs those not seen at the TOCC. Patients who were referred to the TOCC but chose not to keep their appointment were similar to those seen in TOCC in terms of age, CAN score, rurality, and hospital diagnosis, but readmission rates were substantially higher in this group even after adjustments for covariates (Table 2). Evaluating causality in interventions aimed to reduce hospital readmission rates is complicated.28 Our findings add greater plausibility to the utility of TOCC in accounting for at least a portion of the reported decrease in ICVA 30-day readmissions.
Our study has several strengths, including an observation period longer than 2 years, a large population of discharged veterans within an integrated healthcare system, and a large proportion of patients living in rural areas. Another strength of our study is the innovative nature of the intervention, which features a multidisciplinary team and the option of virtual or in-person visits. Nevertheless, this study also has several important limitations. As a single-center study, our findings may not be generalizable to other institutions, especially those outside the VHA system. Similarly, our study population reflected that of the ICVA, which may limit generalizability to a more diverse population. While we attempted to account in our statistical modeling for baseline differences between referred patients seen by the TOCC and those referred but not seen, we cannot exclude residual confounding between the groups. Specifically, the comparison of patients who did and did not choose TOCC follow-up introduces the possibility of selection bias. Future randomized/controlled studies will need to evaluate whether TOCC is more effective than the standard of care to reduce readmissions. Finally, since the analysis period following the final PDSA cycle was compressed due to the onset of the COVID-19 pandemic in the United States, no data are available regarding the sustained impacts of changes made during this cycle.
CONCLUSION
A multidisciplinary TOCC within the ICVA, featuring both virtual and in-person visits, reduced 30-day readmission rates by 19.3%; this approach to care was especially effective in patients with CHF. Virtual visits were the follow-up mode of choice for both urban and rural veterans, and there was no difference in outcomes between these two follow-up options. Future studies will focus on additional quality metrics, including cost-effectiveness and patient satisfaction.
Hospital readmissions are a significant problem in the United States, affecting 15% to 30% of discharges and incurring costs of more than $17 billion annually.1 Timely posthospitalization follow-up visits are critical to ensure the effective transfer of patients to the outpatient setting; such visits reduce readmission rates as well as hospital length of stay and overall health care resource utilization.2-4 Patients who receive inadequate follow-up care (ie, within 4 weeks of discharge) are significantly more likely to be readmitted than those who receive close follow-up care.5
Due to the large clinical and financial consequences associated with hospital readmission, a variety of interventions have been studied, including home visits, telemonitoring, medication management, telephone calls, and postdischarge clinics.6,7 While studies have not shown postdischarge clinics to be universally efficacious in reducing readmission rates, there is increasing evidence of reduced readmission rates in clinics that target high-risk patients (eg, patients with congestive heart failure [CHF]) rather than the total population.2 A study by Hernandez et al that evaluated the relationship between early physician follow-up and 30-day readmissions showed a significantly lower readmission rate among hospitals with higher follow-up rates.8 Similarly, patients with CHF in a large, integrated health system who were seen within 7 days of discharge had an odds ratio (OR) of 0.81 (95% CI, 0.70-0.94) for 30-day readmissions.9
Transitions-of-care clinics (TOCC), designed to provide early postdischarge follow-up to high-risk patients, have been shown to reduce 30-day readmission rates,3,4,10,11 especially in clinics that have same-physician follow-up visits rather than follow-up visits with a community primary care physician (PCP).12 The most pronounced impact of postdischarge follow-up is seen in high-risk patients with high complexity or high severity of disease; however, complex rural patients are less likely to have access to specialty care.13 As a result, since rural residents must travel farther for specialty care, they are seen less frequently than their urban counterparts.14,15
Prior to our TOCC initiative, the Iowa City VA (ICVA) ranked in the fifth quintile of the Veterans Health Administration (VHA)
To meet these challenges, we implemented a TOCC to deliver timely postdischarge care focusing on high-risk and high-complexity patients. To address access-to-care issues of patients living in rural areas within the ICVA, we included virtual follow-up visits as a key component of our intervention.16,17 The aim of this project was to decrease 30-day readmission rates of ICVA patients by 20% within 12 months of implementation.
METHODS
Setting/Study Population
The ICVA serves 184,000 veterans stretched over 50 counties in eastern Iowa, western Illinois, and northern Missouri, with more than 60% of these patients residing in rural areas. Patients were initially eligible for the TOCC if they had an admission diagnosis of CHF and a CAN score > 85 at the time of discharge. The CAN score, developed by the VA to assess the risk of hospital readmission in individual patients, factors in several variables, including demographics, coexisting conditions, vital signs, utilization of services, pharmacy visits, and laboratory results. Patients in the top 5% (95-99) have a readmission rate of 20% at 90 days. Since the CAN is a proprietary tool, it may not be published in full; however, this assessment tool is commonly used and frequently cited in VA research.18-22 The CAN score is expressed as a percentile ranging from 0 (lowest risk) to 99 (highest risk). Patient eligibility was expanded during subsequent Plan-Do-Study-Act (PDSA) cycles, as outlined below. Patient eligibility was expanded during subsequent PDSA cycles (also outlined below). A review by a local institutional review board was obtained, and the study was classified as exempt due to the use of deidentified data. Standards for Quality Improvement Reporting Excellence 2.0 guidelines were used to construct the manuscript.
Magnitude Assessment
The numbers of discharges, readmissions within 30 days, emergency department (ED) visits by all discharged veterans, and veterans discharged with a CHF hospital diagnosis were recorded from February 2017 to February 2018, which were the 12 months immediately preceding the pilot implementation.
Intervention
The primary intervention was referral to the newly formed ICVA TOCC. The multidisciplinary TOCC team consisted of hospitalists, pharmacists, schedulers, and discharge planners/care managers. Patients were identified by the hospitalist team during admission; prior to hospital discharge, these patients were referred to TOCC discharge planners to schedule appropriate follow-up appointments. Virtual follow-up visits were conducted using a patient’s home technology; in cases where a patient lacked adequate technology capabilities (eg, no computer or internet access), the ICVA provided a tablet device with cellular internet capability for temporary use. Specific clinical activities included medication reconciliation by a pharmacist, follow-up of pending laboratory studies, imaging studies, pathology results, medical diagnosis education, counseling regarding dietary restrictions, and contingency planning outside of an ED visit in the event of a change in clinical status. In addition, the TOCC aimed to facilitate a smooth transition of care back to the PCP by arranging follow-up appointments, providing visit summaries, and scheduling consults with specialty care, as appropriate.
Measures
The primary objective measure was the 30-day readmission rate in the ICVA hospital. Secondary measures included the number of VHA ED visits within 30 days of discharge. The main process measures were the number of hospital discharges per month, the number of TOCC referrals, the number of TOCC appointments made, the number of virtual and in-person visits, and the percentage of appointment “no-shows.”
Implementation
The TOCC was piloted from April 2018 to October 2018. During the pilot phase, TOCC enrollment was limited to virtual appointments and to patients with an admission diagnosis of CHF and a CAN score of > 85. The TOCC had staff on-site 2 days a week; this included pharmacists to reconcile medications and hospitalists to address follow-up care needs.
The TOCC clinic was temporarily closed at the end of October 2018 to analyze pilot results. Based on stakeholder feedback, changes made as part of the second PDSA cycle included expanding eligibility criteria to any hospital admission diagnosis and to patients with a CAN score < 85 if the hospitalist team felt the patient was likely to benefit from TOCC follow-up. In addition, on-site clinic staffing was expanded from 2 to 5 days per week to improve access, and the option for an in-person visit was added based on concerns some veterans expressed regarding the use of the technology at home. Finally, a formal resident program was added, and the order set for referrals was simplified. The TOCC was restarted in February 2019, and TOCC metrics were reviewed monthly. By July 2019, we identified issues with TOCC referrals and appointment creation that required additional modifications to the intervention.
A third PDSA cycle was initiated in July 2019 and included major changes, notably the formation of a designated TOCC committee. The committee appointed a dedicated TOCC scheduler whose role was to reduce confusion regarding scheduling, to update the discharge instructions/orders template to lower incidences of “double-booking” that occurred with PCP and TOCC appointments, to modify discharge educational instruction regarding virtual visits and tablet use, to adjust the TOCC-PCP handoff, and to formalize interactions between discharge coordinators and residents to review possible referrals every morning (Appendix Figure 1).
Statistical Analysis
Run charts were constructed by plotting monthly primary outcome values and monthly process metrics (Figure, Appendix Figure 2, Appendix Figure 3). Chi-square tests were used to compare 30-day readmission rates before and after the intervention.
Mean (SD) or counts and percentages were used to describe the distribution of continuous and categorical variables, respectively. Kruskal-Wallis test, t test, or chi-square tests were used, as appropriate, across categories. Generalized linear models with a logistic link function were used to test for differences between patients who kept their appointment at the TOCC and those who did not keep their TOCC appointment (both unadjusted and adjusted for all of the covariates previously mentioned). In addition, generalized linear models were also used to compare outcomes between TOCC patients seen virtually vs those seen in-person (both unadjusted and adjusted for all the covariates previously mentioned). All statistical tests were considered significant at a two-sided P < .05. All analyses were performed using SAS software version 9.4 (SAS Institute Inc).
RESULTS
Magnitude Assessment
During the preimplementation period (February 2017-February 2018), there were 3014 patient discharges from ICVA and 343 readmissions, resulting in a readmission rate of 11.4%. Among patients with a hospital-admission diagnosis of cardiorespiratory disease, which included patients with CHF, there were 381 discharges and 46 readmissions, resulting in a readmission rate of 12.1%.
Primary Outcome
During the pilot phase, which was conducted from April 2018 to October 2018, 142 patients who met inclusion criteria (CHF diagnosis and a CAN score > 85) were discharged from ICVA, and 56 referrals to the TOCC were placed. The readmission rate among the cardiorespiratory cohort of veterans was 9.5%.
During the expansion of the intervention from February 2019 to February 2020, there were 2844 discharges from the ICVA and 291 readmissions, resulting in a readmission rate of 10.2%. However, there was a further decrease in the readmission rate after the third PDSA cycle was initiated in July 2019 (Appendix Figure 1). The readmission rate was 9.2% in the final 6 months of the intervention period, and 7.9% in the final 3 months.
When comparing the 6 months following the third PDSA cycle to the magnitude assessment period, there was a relative readmission reduction of 19.3% (P = .04), and an absolute reduction of 2.2%. If the final 3 months of the intervention period are included, there was an absolute reduction of 3.5% and a relative reduction of 30.7% (P = .01). Notably, before the pilot phase, ICVA was in the fifth quintile for HWR among VA hospitals but improved to the second quintile by the end of the expansion phase.
Process Outcomes
Process metrics for TOCC referrals, the number of patients seen, and the number of virtual and in-person visits over time are shown in Appendix Figure 3. Rates of TOCC referrals and the number of TOCC visits were lower than anticipated during the first 5 months of the intervention. However, TOCC referrals increased significantly after we implemented the previously described changes as part of the third PDSA cycle. As a result, total, virtual, and in-person visits also significantly increased from July 2019 to February 2020. The proportion of patients choosing virtual vs in-person visits fluctuated over time, but virtual visits were generally chosen more often than in-person visits.
Statistical Modeling
Baseline Data
Cohort characteristics are shown in Table 1. The cohort, which reflected the ICVA population, was predominantly male (96%) and White (93%), with a mean age of 67 years. The population was approximately half urban and half rural in composition, and the most common reason for hospital admission was cardiac. Other than a small but statistically significant difference in CAN scores, there were no significant differences between patients who kept their TOCC appointment and those who did not. There were also no differences in baseline characteristics between patients who chose virtual follow-up and patients who chose in-person follow-up, including the proportion of urban and rural patients.
Outcomes
Patients who kept their TOCC appointments had a 30-day readmission rate of 9.6%, which was significantly lower than the 30-day readmission rate of 27% in the group that did not keep their TOCC appointment (P < .001). Similarly, the percentage of patients treated in the ED was 15% in the TOCC group compared to 31.2% in the group that canceled their appointment (P < .001) (Table 1). In the multivariable analysis, patients who were seen in the TOCC group had an OR for 30-day readmission of 0.35 (95% CI, 0.19-0.62, P < .001), and an OR for ED visits of 0.39 (95% CI, 0.23-0.65; P < .001) (Table 2). There was no statistically significant difference in 6-month mortality between the two groups. In the virtual group compared to the in-person group, there were no statistically significant differences in outcomes between the two groups in the unadjusted or adjusted analysis (Table 2).
DISCUSSION
In the expansion phase, eligibility was expanded to include any hospital indication but continued to focus on high-risk patients. Existing literature suggests that providing postdischarge care to all patients, including low- or medium-risk patients, may not be as impactful as enrolling high-risk patients only. For instance, a postdischarge clinic offered to all patients at a VA system in Colorado did not reduce readmission rates compared to PCP follow-up.23 In contrast, a study of more than 10,000 high-risk urban patients demonstrated that postdischarge care resulted in a 9.3% reduction in readmission risk.24 Our data are consistent with the previously published studies, as the average CAN score of patients seen in TOCC was 90, suggesting a high risk of readmission. In the final 12 months of the intervention, 15% of discharged patients were seen at the TOCC clinic, suggesting that targeted intervention within the small subset of high-risk patients was sufficient to achieve our primary aim. Of note, among patients who did not meet the inclusion criteria for TOCC referral (ie, patients not considered high risk [CAN score ≤ 85]), the rate of readmissions was 8.6%.
Most of the available research on the efficacy of postdischarge clinics was conducted in urban environments. Our ICVA population sees a large proportion of rural veterans, who account for just over 50% of the discharge population. In a study of more than 2 million Medicare patients discharged from US hospitals, the 30-day readmission rates and adjusted mortality rates were higher among patients in rural counties, and post–acute care seemed to have a greater impact in rural rather than urban settings.25 Previous studies have demonstrated that virtual visits have the potential to improve readmission rates, especially in patients with CHF26 and in patients at the highest risk for readmission.27 In our study, the pilot phase offered only virtual visits, but we subsequently added an in-person option based on veteran feedback. Interestingly, over the next 12 months, virtual visits were more popular with both urban and rural veterans, and there were no differences in the number of rural patients in the in-person vs the virtual group. These findings suggest factors other than rurality influenced the decision to choose virtual follow-up visits over in-person visits. Future studies should seek to determine the extent to which factors such as age, race, educational level, and socioeconomic circumstances impact veterans’ follow-up decisions. Not only were outcomes among patients who chose virtual visits the same as those of patients who chose in-person visits, but both of these groups had better outcomes compared to the non-TOCC group (Table 2). This finding demonstrating the efficacy of virtual visits among rural and urban patients has taken on increased significance due to the COVID-19 pandemic, as virtual visits offer a safer option, one that minimizes physical contact.
Our quality improvement analysis included a statistical comparison of patients seen vs those not seen at the TOCC. Patients who were referred to the TOCC but chose not to keep their appointment were similar to those seen in TOCC in terms of age, CAN score, rurality, and hospital diagnosis, but readmission rates were substantially higher in this group even after adjustments for covariates (Table 2). Evaluating causality in interventions aimed to reduce hospital readmission rates is complicated.28 Our findings add greater plausibility to the utility of TOCC in accounting for at least a portion of the reported decrease in ICVA 30-day readmissions.
Our study has several strengths, including an observation period longer than 2 years, a large population of discharged veterans within an integrated healthcare system, and a large proportion of patients living in rural areas. Another strength of our study is the innovative nature of the intervention, which features a multidisciplinary team and the option of virtual or in-person visits. Nevertheless, this study also has several important limitations. As a single-center study, our findings may not be generalizable to other institutions, especially those outside the VHA system. Similarly, our study population reflected that of the ICVA, which may limit generalizability to a more diverse population. While we attempted to account in our statistical modeling for baseline differences between referred patients seen by the TOCC and those referred but not seen, we cannot exclude residual confounding between the groups. Specifically, the comparison of patients who did and did not choose TOCC follow-up introduces the possibility of selection bias. Future randomized/controlled studies will need to evaluate whether TOCC is more effective than the standard of care to reduce readmissions. Finally, since the analysis period following the final PDSA cycle was compressed due to the onset of the COVID-19 pandemic in the United States, no data are available regarding the sustained impacts of changes made during this cycle.
CONCLUSION
A multidisciplinary TOCC within the ICVA, featuring both virtual and in-person visits, reduced 30-day readmission rates by 19.3%; this approach to care was especially effective in patients with CHF. Virtual visits were the follow-up mode of choice for both urban and rural veterans, and there was no difference in outcomes between these two follow-up options. Future studies will focus on additional quality metrics, including cost-effectiveness and patient satisfaction.
1. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. https://doi.org/10.1056/nejmsa0803563
2. Doctoroff L. Postdischarge clinics and hospitalists: a review of the evidence and existing models. J Hosp Med. 2017;12(6):467-471. https://doi.org/10.12788/jhm.2750
3. Koehler BE, Richter KM, Youngblood L, et al. Reduction of 30-day postdischarge hospital readmission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med. 2009;4(4):211-218. https://doi.org/10.1002/jhm.427
4. Abrashkin KA, Cho HJ, Torgalkar S, Markoff B. Improving transitions of care from hospital to home: what works? Mt Sinai J Med. 2012;79(5):535-544. https://doi.org/10.1002/msj.21332
5. Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5(7):392-397. https://doi.org/10.1002/jhm.666
6. Greysen SR, Harrison JD, Kripalani S, et al. Understanding patient-centred readmission factors: a multi-site, mixed-methods study. BMJ Qual Saf. 2017;26(1):33-41. https://doi.org/10.1136/bmjqs-2015-004570
7. Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. https://doi.org/10.7326/0003-4819-155-8-201110180-00008
8. Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716-1722. https://doi.org/10.1001/jama.2010.533
9. Lee KK, Yang J, Hernandez AF, Steimle AE, Go AS. Post-discharge follow-up characteristics associated with 30-day readmission after heart failure hospitalization. Med Care. 2016;54(4):365-372. https://doi.org/10.1097/mlr.0000000000000492
10. Balaban RB, Williams MV. Improving care transitions: hospitalists partnering with primary care. J Hosp Med. 2010;5(7):375-377. https://doi.org/10.1002/jhm.824
11. Rodrigues CR, Harrington AR, Murdock N, et al. Effect of pharmacy-supported transition-of-care interventions on 30-day readmissions: a systematic review and meta-analysis. Ann Pharmacother. 2017;51(10):866-889. https://doi.org/10.1177/1060028017712725
12. van Walraven C, Taljaard M, Etchells E, et al. The independent association of provider and information continuity on outcomes after hospital discharge: implications for hospitalists. J Hosp Med. 2010;5(7):398-405. https://doi.org/10.1002/jhm.716
13. Gruca TS, Pyo TH, Nelson GC. Providing cardiology care in rural areas through vsiting consultant clinics. J Am Heart Assoc. 2016;5(7):e002909. https://doi.org/10.1161/jaha.115.002909
14. Chan L, Hart LG, Goodman DC. Geographic access to health care for rural Medicare beneficiaries. J Rural Health. 2006;22(2):140-146. https://doi.org/10.1111/j.1748-0361.2006.00022.x
15. Burke RE, Jones CD, Coleman EA, Falvey JR, Stevens-Lapsley JE, Ginde AA. Use of post-acute care after hospital discharge in urban and rural hospitals. Am J Accountable Care. 2017;5(1):16-22.
16. Jetty A, Moore MA, Coffman M, Petterson S, Bazemore A. Rural family physicians are twice as likely to use telehealth as urban family physicians. Telemed J E Health. 2018;24(4):268-276. https://doi.org/10.1089/tmj.2017.0161
17. Harrison PL, Hara PA, Pope JE, Young MC, Rula EY. The impact of postdischarge telephonic follow-up on hospital readmissions. Popul Health Manag. 2011;14(1):27-32. https://doi.org/10.1089/pop.2009.0076
18. Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368-373. https://doi.org/10.1097/mlr.0b013e31827da95a
19. Spece LJ, Donovan LM, Griffith MF, et al. Initiating low-value inhaled corticosteroids in an inception cohort with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2020;17(5):589-595. https://doi.org/10.1513/annalsats.201911-854oc
20. Osborne TF, Suarez P, Edwards D, Hernandez-Boussard T, Curtin C. Patient electronic health records score for preoperative risk assessment before total knee arthroplasty. JB JS Open Access. 2020;5(2):e0061. https://doi.org/10.2106/jbjs.oa.19.00061
21. Levy C, Ersek M, Scott W, et al. Life-sustaining treatment decisions initiative: early implementation results of a national Veterans Affairs program to honor veterans’ care preferences. J Gen Intern Med. 2020;35(6):1803-1812. https://doi.org/10.1007/s11606-020-05697-2
22. Ibrahim SA. High-risk patients and utilization of primary care in the US Veterans Affairs health system. JAMA Netw Open. 2020;3(6):e209518. https://doi.org/10.1001/jamanetworkopen.2020.9518
23. Burke RE, Whitfield E, Prochazka AV. Effect of a hospitalist-run postdischarge clinic on outcomes. J Hosp Med. 2014;9(1):7-12. https://doi.org/10.1002/jhm.2099
24. Jenq GY, Doyle MM, Belton BM, Herrin J, Horwitz LI. Quasi-experimental evaluation of the effectiveness of a large-scale readmission reduction program. JAMA Intern Med. 2016;176(5):681-690. https://doi.org/10.1001/jamainternmed.2016.0833
25. Kosar CM, Loomer L, Ferdows NB, Trivedi AN, Panagiotou OA, Rahman M. Assessment of rural-urban differences in postacute care utilization and outcomes among older US adults. JAMA Netw Open. 2020;3(1):e1918738. https://doi.org/10.1001/jamanetworkopen.2019.18738
26. Pandor A, Thokala P, Gomersall T, et al. Home telemonitoring or structured telephone support programmes after recent discharge in patients with heart failure: systematic review and economic evaluation. Health Technol Assess. 2013;17(32):1-207, v-vi. https://doi.org/10.3310/hta17320
27. Low LL, Tan SY, Ng MJM, et al. Applying the integrated practice unit concept to a modified virtual ward model of care for patients at highest risk of readmission: a randomized controlled trial. PloS One. 2017;12(1):e0168757. https://doi.org/10.1371/journal.pone.0168757
28. McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):1796-1803. https://doi.org/10.1161/circulationaha.114.010270
1. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. https://doi.org/10.1056/nejmsa0803563
2. Doctoroff L. Postdischarge clinics and hospitalists: a review of the evidence and existing models. J Hosp Med. 2017;12(6):467-471. https://doi.org/10.12788/jhm.2750
3. Koehler BE, Richter KM, Youngblood L, et al. Reduction of 30-day postdischarge hospital readmission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med. 2009;4(4):211-218. https://doi.org/10.1002/jhm.427
4. Abrashkin KA, Cho HJ, Torgalkar S, Markoff B. Improving transitions of care from hospital to home: what works? Mt Sinai J Med. 2012;79(5):535-544. https://doi.org/10.1002/msj.21332
5. Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5(7):392-397. https://doi.org/10.1002/jhm.666
6. Greysen SR, Harrison JD, Kripalani S, et al. Understanding patient-centred readmission factors: a multi-site, mixed-methods study. BMJ Qual Saf. 2017;26(1):33-41. https://doi.org/10.1136/bmjqs-2015-004570
7. Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. https://doi.org/10.7326/0003-4819-155-8-201110180-00008
8. Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716-1722. https://doi.org/10.1001/jama.2010.533
9. Lee KK, Yang J, Hernandez AF, Steimle AE, Go AS. Post-discharge follow-up characteristics associated with 30-day readmission after heart failure hospitalization. Med Care. 2016;54(4):365-372. https://doi.org/10.1097/mlr.0000000000000492
10. Balaban RB, Williams MV. Improving care transitions: hospitalists partnering with primary care. J Hosp Med. 2010;5(7):375-377. https://doi.org/10.1002/jhm.824
11. Rodrigues CR, Harrington AR, Murdock N, et al. Effect of pharmacy-supported transition-of-care interventions on 30-day readmissions: a systematic review and meta-analysis. Ann Pharmacother. 2017;51(10):866-889. https://doi.org/10.1177/1060028017712725
12. van Walraven C, Taljaard M, Etchells E, et al. The independent association of provider and information continuity on outcomes after hospital discharge: implications for hospitalists. J Hosp Med. 2010;5(7):398-405. https://doi.org/10.1002/jhm.716
13. Gruca TS, Pyo TH, Nelson GC. Providing cardiology care in rural areas through vsiting consultant clinics. J Am Heart Assoc. 2016;5(7):e002909. https://doi.org/10.1161/jaha.115.002909
14. Chan L, Hart LG, Goodman DC. Geographic access to health care for rural Medicare beneficiaries. J Rural Health. 2006;22(2):140-146. https://doi.org/10.1111/j.1748-0361.2006.00022.x
15. Burke RE, Jones CD, Coleman EA, Falvey JR, Stevens-Lapsley JE, Ginde AA. Use of post-acute care after hospital discharge in urban and rural hospitals. Am J Accountable Care. 2017;5(1):16-22.
16. Jetty A, Moore MA, Coffman M, Petterson S, Bazemore A. Rural family physicians are twice as likely to use telehealth as urban family physicians. Telemed J E Health. 2018;24(4):268-276. https://doi.org/10.1089/tmj.2017.0161
17. Harrison PL, Hara PA, Pope JE, Young MC, Rula EY. The impact of postdischarge telephonic follow-up on hospital readmissions. Popul Health Manag. 2011;14(1):27-32. https://doi.org/10.1089/pop.2009.0076
18. Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368-373. https://doi.org/10.1097/mlr.0b013e31827da95a
19. Spece LJ, Donovan LM, Griffith MF, et al. Initiating low-value inhaled corticosteroids in an inception cohort with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2020;17(5):589-595. https://doi.org/10.1513/annalsats.201911-854oc
20. Osborne TF, Suarez P, Edwards D, Hernandez-Boussard T, Curtin C. Patient electronic health records score for preoperative risk assessment before total knee arthroplasty. JB JS Open Access. 2020;5(2):e0061. https://doi.org/10.2106/jbjs.oa.19.00061
21. Levy C, Ersek M, Scott W, et al. Life-sustaining treatment decisions initiative: early implementation results of a national Veterans Affairs program to honor veterans’ care preferences. J Gen Intern Med. 2020;35(6):1803-1812. https://doi.org/10.1007/s11606-020-05697-2
22. Ibrahim SA. High-risk patients and utilization of primary care in the US Veterans Affairs health system. JAMA Netw Open. 2020;3(6):e209518. https://doi.org/10.1001/jamanetworkopen.2020.9518
23. Burke RE, Whitfield E, Prochazka AV. Effect of a hospitalist-run postdischarge clinic on outcomes. J Hosp Med. 2014;9(1):7-12. https://doi.org/10.1002/jhm.2099
24. Jenq GY, Doyle MM, Belton BM, Herrin J, Horwitz LI. Quasi-experimental evaluation of the effectiveness of a large-scale readmission reduction program. JAMA Intern Med. 2016;176(5):681-690. https://doi.org/10.1001/jamainternmed.2016.0833
25. Kosar CM, Loomer L, Ferdows NB, Trivedi AN, Panagiotou OA, Rahman M. Assessment of rural-urban differences in postacute care utilization and outcomes among older US adults. JAMA Netw Open. 2020;3(1):e1918738. https://doi.org/10.1001/jamanetworkopen.2019.18738
26. Pandor A, Thokala P, Gomersall T, et al. Home telemonitoring or structured telephone support programmes after recent discharge in patients with heart failure: systematic review and economic evaluation. Health Technol Assess. 2013;17(32):1-207, v-vi. https://doi.org/10.3310/hta17320
27. Low LL, Tan SY, Ng MJM, et al. Applying the integrated practice unit concept to a modified virtual ward model of care for patients at highest risk of readmission: a randomized controlled trial. PloS One. 2017;12(1):e0168757. https://doi.org/10.1371/journal.pone.0168757
28. McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):1796-1803. https://doi.org/10.1161/circulationaha.114.010270
© 2021 Society of Hospital Medicine
Implementing a Telehospitalist Program Between Veterans Health Administration Hospitals: Outcomes, Acceptance, and Barriers to Implementation
Healthcare in rural areas faces increasing challenges due to community hospital closures, physician shortages, and a more concentrated population of older adults with higher rates of comorbid conditions than their urban counterparts.1-3 Critical access hospitals (CAHs), which primarily serve rural areas, have fewer clinical capabilities, worse process-of-care measures, and higher mortality rates for some conditions when compared to non-CAHs.4 As such, CAHs are closing at record numbers across the United States,5 resulting in loss of available hospital beds and patient access to timely emergency services,6 which can worsen outcomes, further widening the rural-urban healthcare gap.7,8 Furthermore, this strain on an overwhelmed health system in the most vulnerable areas restricts the ability to respond to healthcare crises like the coronavirus disease 2019 pandemic.9
Providing adequate staff for currently available hospital beds is also a problem in rural areas. Studies demonstrating improved outcomes, decreased length of stay (LOS), and increased quality with hospitalist services have resulted in a high demand for hospitalists nationwide.10-12 Recruiting hospitalists to work in rural areas, however, has become increasingly challenging due to low-patient volumes, financial viability of hospitalist-model adoption, and provider shortages.13,14 Recently, the Veterans Health Administration (VHA) reported a 28% nationwide shortage of hospitalists,15 which disproportionally affects rural VHA hospitals. Staffing difficulties and reliance on intermittent providers were reported by more than 80% of rural and low-complexity VHA facilities.16
Telehospitalist services (THS) can help deliver high-quality care to rural residents locally, decrease travel expenses, support hospital volume, and increase healthcare capacity in response to a pandemic.14,17,18 Only a few studies have described THS (mostly with overnight or cross-coverage models directed to CAHs), and clinical outcomes have been inconsistently reported.17,19-21 Furthermore, no program has been conducted within an integrated health system akin to the VHA. The primary objective of this quality improvement (QI) initiative was to perform a mixed-methods evaluation of THS between VHA hospitals to compare clinical outcomes and patient and staff satisfaction. Secondary outcomes included description of the implementation process, unexpected challenges, and subsequent QI initiatives. These results will expand the knowledge on feasibility of THS and provide implementation guidance.
METHODS
A mixed-methods approach was used to evaluate outcomes of this QI project. Reporting follows the revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0).22
Context
The VHA is the largest integrated healthcare system in the United States, with more than 8 million veterans enrolled, more than 30% of whom reside in a rural area. The VHA comprises more than 1,000 outpatient clinics and 170 acute care VA Medical Centers,23,24 including more than 35 rural and low-complexity hospitals.25 Low-complexity hospitals are those with the lowest volume and levels of patient complexity and minimal or no teaching programs, research, intensive care unit (ICU) beds, and subspecialists. Lack of reimbursement and interstate licensing, often cited as barriers to telemedicine, do not apply to the VHA. The hub site was a large tertiary care (high-complexity) VHA hospital located in Iowa City, Iowa. The spoke site was a low-complexity (10-bed acute inpatient unit with no ICU) rural VA hospital located in Tomah, Wisconsin.
Study Population
The preimplementation cohort for comparison included all patients admitted between January 1, 2018, and January 6, 2019. The postimplementation study cohort included all observation and acute care admissions during the pilot phase (January 7 to May 3, 2019) and sustainability phase (July 15 to December 31, 2019). The postimplementation analysis excluded the time period of May 4 to July 14, 2019, due to an interruption (gap) in THS. The gap period allowed for preliminary data analysis, optimization of the telecommunication system, and the recruitment and training of additional providers who could provide long-term staffing to the service.
Intervention
Preimplementation
Prior to THS implementation, Tomah’s inpatient ward was staffed by one physician per shift, who could be a hospitalist, medical officer of the day (MOD), or an intermittent provider (locum tenens). Hospitalists covering the acute inpatient ward prior to the THS transitioned to cover weekends, nights, and urgent care service shifts.
We visited the spoke site and held information-sharing sessions with key stakeholders (administrators, clinician leaders, nurses, and ancillary staff) prior to kick-off. Recurrent phone meetings addressed anticipated and emerging challenges. Telehospitalist and local providers underwent technology and service training.
Technology and Connectivity
A low-cost technology system using tablet computers provided Health Insurance Portability and Accountability Act–compliant videoconferencing with a telehospitalist at the hub site. An Eko-Core digital stethoscope® with a web-based audio stream was available. Telehospitalists conducted encounters from a private office space with telehealth capabilities. A total of $9,000 was spent on equipment at both sites. Due to connectivity problems and data limits, the tablets were switched to mobile computer-on-wheels workstations and hospital-based Wi-Fi for the sustainability phase.
THS Description
An experienced hub hospitalist, together with an advanced practice provider (APP; nurse practitioner [NP] or physician assistant [PA]), cared for all patients admitted to the 10-bed inpatient unit at the spoke site, Monday through Friday from 8:00 AM to 4:30 PM. The APP had limited or no prior experience in acute inpatient medicine. The telehospitalist worked as a team with the APP. The APP was the main point of contact for nurses, performed physical examinations, and directed patient care to their level of comfort (in a similar manner as a teaching team). The telehospitalist conducted bedside patient rounds, participated in multidisciplinary huddles, and shared clinical documentation and administrative duties with the APP. The telehospitalist was the primary staff for admitted patients and had full access to the electronic health record (EHR). The THS was staffed by 10 hospitalists during the study period. Overnight and weekend cross-coverage and admissions were performed by MODs, who also covered the urgent care and cross-covered other nonmedical units.
Quantitative Evaluation Methods
Workload and Clinical Outcomes
An EHR query identified all patients admitted during the pre- and postimplementation periods. Demographic data, clinical Nosos risk scores,26,27 and top admission diagnoses were reported. Workload was evaluated using the average number of encounters per day and self-reported telehospitalist worksheets, which were cross-referenced with EHR data. Clinical outcomes included LOS, 30-day hospital readmission rate, 30-day standardized mortality (SMR30), in-hospital mortality, and VHA-specific inpatient quality metrics. Independent sample t tests for continuous variables and chi-square tests or Fisher’s exact test (for patient class) for categorical variables were used to compare pre- and postimplementation groups. Statistical process control (SPC) charts evaluated changes over time. All analyses were conducted using Microsoft Excel and R.28
Anonymous surveys were distributed to spoke-site inpatient and administrative staff at 1 month and 12 months postimplementation, assessing satisfaction, technology/connectivity, communication, and challenges (Appendix Figure 1). Satisfaction of the telehospitalist physicians at the hub site was measured 12 months postimplementation by a 26-question survey assessing the same domains, plus quality of care (Appendix Figure 2).
Patient Satisfaction
The VHA Survey of Healthcare Experiences of Patients (SHEP), a version of the Hospital Consumer Assessment of Healthcare Providers and Systems Survey,29,30 was mailed to all patients after discharge. Survey responses concerning inpatient provider care (eg, care coordination, communication, hospital rating, willingness to recommend the hospital) during the pre- and postimplementation phases were compared using a two-sample test of independent proportions. Responses obtained during May and June 2019 were excluded.
Qualitative Evaluation Methods
The authors had full access to, and took full responsibility for, the integrity of the data. The project was evaluated by the University of Iowa Institutional Review Board and the Iowa City VA Research and Development Committee and was determined to be a non–human-subjects QI project.
RESULTS
Quantitative Workload and Clinical Outcomes
There were 822 admissions during the preimplementation period and 550 admissions during the postimplementation period (253 during the pilot and 297 during sustainability phase). Patient characteristics pre- and postimplementation were not significantly different (Table 1). The median patient age was 65 years; 96% of patients were male, and 83% were rural residents. The most common admission diagnosis was alcohol-related (36%); regarding patient disposition, 78% of admissions were discharged home.
Workload
There were 502 patient encounters staffed by the telehospitalist in the pilot phase, with an average of 6.25 encounters per day, and a telehospitalist-reported workload of 7 hours per day. There were 538 patient encounters, with an average of 4.67 encounters per day and a workload of 5.6 hours per day in the sustainability phase. The average daily census decreased from 5.0 (SD, 1.1) patients per day during preimplementation to 3.1 (SD, 0.5) patients per day during postimplementation (Table 2). In some of the months during the study period, admissions decreased below the lower SPC limit, suggesting a significant change (Figure). Adjusted LOS was significantly lower, with 3.0 (SD, 0.7) days vs 2.3 (SD, 0.3) days in the pre- and postimplementation periods, respectively. Bed occupancy rates were significantly lower in the sustainability phase compared with the pilot phase and the preimplementation period. Readmission rates varied, ranging from <10% to >30%, not significantly different but slightly higher in the postimplementation period. Readmission rates for heart failure, chronic obstructive pulmonary disease, and pneumonia remained unchanged; other medical readmissions (mostly alcohol-related) were slightly higher in the postimplementation period.
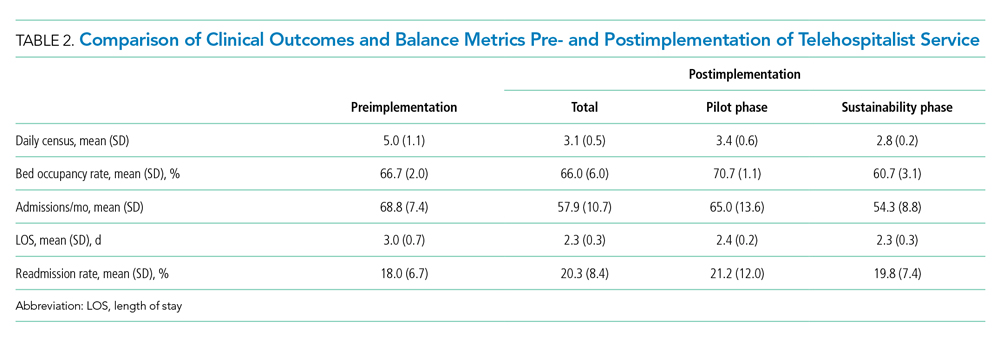
In-hospital mortality and SMR30 did not change significantly, but there was improvement in the 12-month rolling average of the observed/expected SMR30 from 1.40 to 1.08. Additional VHA-specific quality metrics were monitored and showed either small improvements or no change (data not shown).
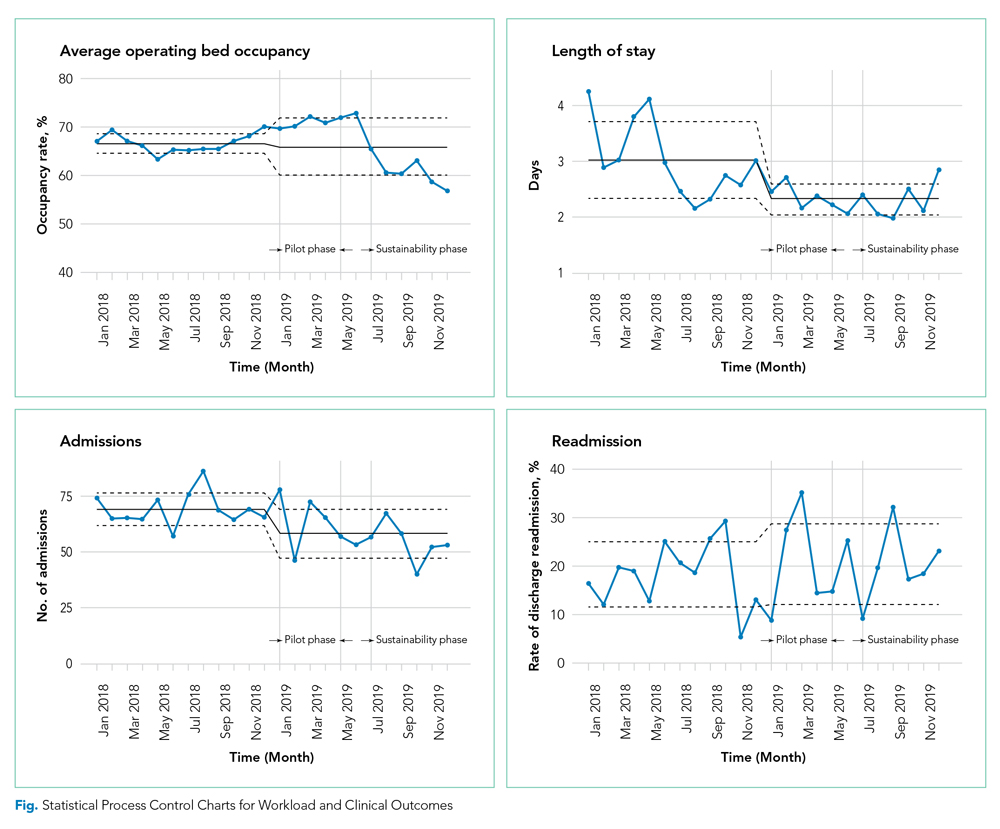
Satisfaction at Hub and Spoke Sites
After sending two reminder communications via email, the telehospitalist satisfaction survey had a total response rate of 90% (9/10). Telehospitalists were satisfied or very satisfied (89%) with the program and the local providers (88.9%), rating their experience as good or excellent (100%) (Table 3). Communication with patients, families, and local staff was noted as being “positive” or “mostly positive.” Telehospitalists reported confidence in the accuracy of their diagnoses and rated the quality of care as being equal to that of a face-to-face encounter. Connectivity problems were prevalent, although most providers were able to resort to a back-up plan. Other challenges included differences in culture and concerns about liability. We received 27 responses from the spoke-site satisfaction survey; the response rate could not be determined because the survey was distributed by the spoke site for anonymity. Of the respondents, 37% identified as nurses, 25.9% as healthcare providers (APPs or physicians), and 33.3% as other staff (eg, social worker, nutritionist, physical therapist, utilization management, administrators); 3.7% did not respond. Among the participants, 88% had personally interacted with the THS. Most providers and other staff perceived THS as valuable (57.1% and 77.8%, respectively) and were satisfied or highly satisfied with THS (57.1% and 55.6%, respectively). On average, nurses provided lower ratings across all survey items than providers and other staff. Challenges noted by all staff included issues with communication, workflow, and technology/connectivity.
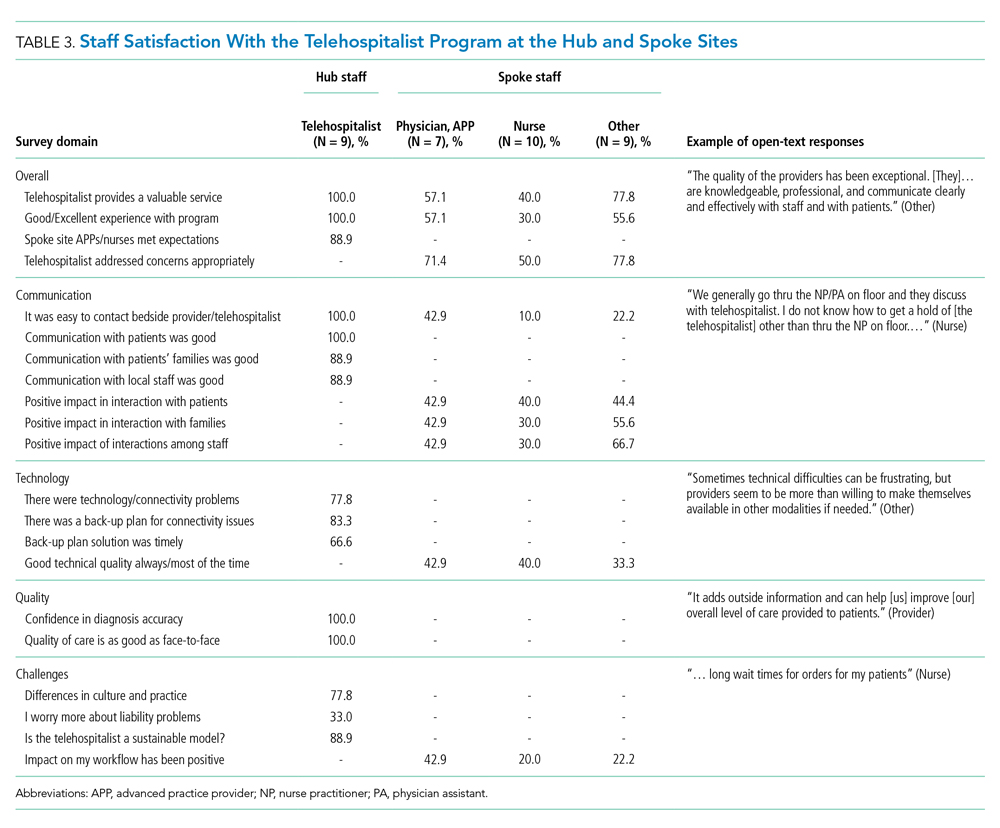
Qualitative Strengths
Our process evaluation identified high quality of care and teamwork as contributors to the success of the program. Overall, staff credited perceived improvements in quality of care to the quality of providers staffing the THS, including the local APPs. Noting the telehospitalists’ knowledge base and level of engagement as key attributes, one staff member commented: “I prefer a telehospitalist that really care[s] about patients than some provider that is physically here but does not engage.” Staff perceived improvements in the continuity of care, as well as care processes such as handoffs and transitions of care.
Improvements in teamwork were perceived compared with the previous model of care. Telehospitalists were lauded for their professionalism and communication skills. Overall, nurses felt providers in the THS listened more to their views. In addition, nurse respondents felt they could learn from several providers and said they enjoyed the telehospitalists’ disposition to teach and discuss patient care. The responsiveness of the THS staff was instrumental in building teamwork and acceptance. A bedside interdisciplinary protocol was established for appropriate patients. Local staff felt this was crucial for teamwork and patient satisfaction. Telehospitalists reported high-value in interdisciplinary rounds, facilitating interaction with nurses and ancillary staff. Handoff problems were identified, leading to QI initiatives to mitigate those issues.
Challenges
The survey identified administrative barriers, technical difficulties, workflow constraints, and clinical concerns. The credentialing process was complicated, delaying the onboarding of telehospitalists. Internet connectivity was inconsistent, leading to disruption in video communications; however, during the sustainability phase, updated technology improved communications. The communication workflow was resisted by some nurses, who wanted to phone the telehospitalist directly rather than having the local APP as the first contact. Secure messaging was enabled to allow nurses direct contact during the sustainability phase.
Workload was a concern among telehospitalists and local staff. Telehospitalists perceived the documentation requirements and administrative workload to be two to three times higher than at other hospitals—despite the lower number of encounters. Finally, clinical concerns from spoke-site clinicians included a perceived rise in the acuity of patients (which was not evident by the Nosos score) and delayed decisions to transfer-out patients. These concerns were addressed with educational sessions for telehospitalists during the sustainability phase.
Additional Quality Improvement Projects
The implementation of THS resulted in QI initiatives at the spoke site, including an EHR-integrated handoff tool; a documentation evaluation that led to the elimination of duplicative, inefficient, and error-prone templates; and a revision of the alcohol withdrawal treatment protocol during the sustainability phase to reduce the use of intravenous benzodiazepines. A more comprehensive benzodiazepine-sparing alcohol withdrawal treatment protocol was also developed but was not implemented until after the study period (January 2020).
DISCUSSION
Our pre-post study evaluation found implementation of a THS to be noninferior to face-to-face care, with no significant change in mortality, readmission rate, or patient satisfaction. The significant improvement observed in LOS is consistent with the adoption of hospitalist models in other medical care settings,11 but had not been reported by previous telehospitalist studies. For example, in their retrospective chart review comparing an NP-supported telehospitalist model to locum tenens hospitalists, Boltz et al found no difference in LOS.31 Moreover, as in our study, they found no differences in readmissions, mortality, and patient satisfaction.31 Similarly, Kuperman et al reported unchanged daily census, LOS, and transfer rates from a CAH with their virtual hospitalist program, but a decrease in the percentage of patients transferred-out from the emergency department, suggesting that more patients were treated locally.19
Reduction in LOS is one of the primary measures of efficiency in hospital care31; reducing LOS while maintaining the quality of care lowers hospital costs. The reduction in LOS in our study could be attributed to greater continuity of care, engagement/experience of the telehospitalists, or other factors. This decrease in LOS and slight reduction in admissions resulted in an overall lower daily census during the study period and impacted efficiency. Our study was unable to determine the cause for the reduction in admissions; however, several concurrent events, including the expansion of community-care options for veterans under the MISSION ACT (Maintaining Internal Systems and Strengthening Integrated Outside Networks Act) in June 2019, a nationwide smoking ban at VA facilities (October 2019), and a modification in the alcohol withdrawal treatment protocol might have influenced veterans’ choice of hospital.
Readmission rates were slightly higher, though nonsignificant, in the postimplementation period. Alcohol-related readmissions accounted for most readmissions; some of the protocol changes, such as admitting all patients with alcohol withdrawal to inpatient class instead of admitting some to the observation class, accounted for part of the increase in readmission rates. Readmission rates for other conditions such as chronic obstructive pulmonary disease, chronic heart failure, or pneumonia were not significantly different, suggesting that the reduction in LOS did not result in an unintended increased readmission rate for those conditions.
Rural hospitals are struggling with staffing and finances. Resorting to locum tenens staffing is costly and can result in variable quality of care.32,33 APPs are increasingly taking on hospitalist positions, with 65% of adult hospitalist programs, including half of all VHA hospitals, employing NPs and PAs.34,35 In response to this expanded scope of practice, hospitals employing APPs in hospitalist roles must comply with state and federal laws, which often require that APPs be supervised by or work in collaboration with an on-site or off-site physician. The THS is a great model to support APPs and address staffing and cost challenges in low-volume rural facilities, while maintaining quality of care. Some APP-telehospitalist programs similar to ours have reported cost reductions of up 58% compared to programs that employ locum tenens physicians.31 In our model, we assume that a single telehospitalist hub could provide coverage to two or three spoke sites with APP support, reducing staffing costs.
Hub telehospitalists reported satisfaction with the program, and they perceived the quality of care to be comparable to face-to-face encounters; their responses were consistent with those previously reported in an evaluation of telemedicine acute care by JaKa et al.
This study has several limitations. First, the VHA is an integrated health system, one that serves an older, predominantly male patient population. Also, the lack of reimbursement and interstate licensing restrictions limit generalizability of these results to other CAHs or healthcare systems. Furthermore, the intervention was limited to a single rural site; while this allowed for a detailed evaluation, unique barriers or facilitators might exist that limit its applicability. In addition, QI initiatives implemented by the VHA during the project period might have confounded some of our results. Last, patient satisfaction survey data are overall limited in their ability to fully assess patient’s experience and satisfaction with the program. Further qualitative studies are needed to gain deeper insight into patient perspectives with the THS and whether modality of care delivery influences patients’ care decisions. Future studies should consider a multisite design with one or more hubs and multiple spoke sites.
CONCLUSION
Telehospitalist services are a feasible and safe approach to provide inpatient services and address staffing needs of rural hospitals. To enhance program performance, it is essential to ensure adequate technological quality, clearly delineate and define roles and responsibilities of the care team, and address communication issues or staff concerns in a timely manner.
Acknowledgments
The authors thank the staff, administration, and leadership at the Tomah and Iowa City VA Medical Centers for working with us on this project. They offer special thanks to Kevin Glenn, MD, MS, Ethan Kuperman, MD, MS, FHM, and Jennifer Chapin, MSN, RN, for sharing their expertise, and the telehealth team, including Nathaniel Samuelson, Angela McDowell, and Katrin Metcalf.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
1. O’Connor A, Wellenius G. Rural-urban disparities in the prevalence of diabetes and coronary heart disease. Public Health. 2012;126(10):813-820. https://doi.org/10.1016/j.puhe.2012.05.029
2. Kaufman BG, Thomas SR, Randolph RK, et al. The rising rate of rural hospital closures. J Rural Health. 2016;32(1):35-43. https://doi.org/10.1111/jrh.12128
3. MacDowell M, Glasser M, Fitts M, Nielsen K, Hunsaker M. A national view of rural health workforce issues in the USA. Rural Remote Health. 2010;10(3):1531.
4. Joynt KE, Harris Y, Orav EJ, Jha AK. Quality of care and patient outcomes in critical access rural hospitals. JAMA. 2011;306(1):45-52. https://doi.org/10.1001/jama.2011.902
5. The Chartis Group. Chartis Center for Rural Health. The Rural Health Safety Net Under Pressure: Rural Hospital Vulnerability. Published February 2020. Accessed May 07, 2020. https://www.chartis.com/forum/wp-content/uploads/2020/02/CCRH_Vulnerability-Research_FiNAL-02.14.20.pdf
6. Miller KEM, James HJ, Holmes GM, Van Houtven CH. The effect of rural hospital closures on emergency medical service response and transport times. Health Serv Res. 2020;55(2):288-300. https://doi.org/10.1111/1475-6773.13254
7. Buchmueller TC, Jacobson M, Wold C. How far to the hospital? The effect of hospital closures on access to care. J Health Econ. 2006;25(4):740-761. https://doi.org/10.1016/j.jhealeco.2005.10.006
8. Mohr NM, Harland KK, Shane DM, et al. Rural patients with severe sepsis or septic shock who bypass rural hospitals have increased mortality: an instrumental variables approach. Crit Care Med. 2017;45(1):85-93. https://doi.org/10.1097/ccm.0000000000002026
9. Gutierrez J, Kuperman E, Kaboli PJ. Using telehealth as a tool for rural hospitals in the COVID-19 pandemic response. J Rural Health. 2020;10.1111/jrh.12443. https://doi.org/10.1111/jrh.12443
10. Kisuule F, Howell EE. Hospitalists and their impact on quality, patient safety, and satisfaction. Obstet Gynecol Clin North Am. 2015;42(3):433-446. https://doi.org/10.1016/j.ogc.2015.05.003
11. Peterson MC. A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84(3):248-254. https://doi.org/10.4065/84.3.248
12. Auerbach AD, Wachter RM, Katz P, Showstack J, Baron RB, Goldman L. Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes. Ann Intern Med. 2002;137(11):859-865. https://doi.org/10.7326/0003-4819-137-11-200212030-00006
13. Casey MM, Hung P, Moscovice I, Prasad S. The use of hospitalists by small rural hospitals: results of a national survey. Med Care Res Rev. 2014;71(4):356-366. https://doi.org/10.1177/1077558714533822
14. Sanders RB, Simpson KN, Kazley AS, Giarrizzi DP. New hospital telemedicine services: potential market for a nighttime telehospitalist service. Telemed J E Health. 2014;20(10):902-908. https://doi.org/10.1089/tmj.2013.0344
15. Department of Veterans Affairs. Office of Inspector General. OIG Determination of Veterans Health Administration’s Occupational Staffing Shortages. Published September 30, 2019. Accessed June 15, 2020. https://www.va.gov/oig/pubs/VAOIG-19-00346-241.pdf
16. Gutierrez J, Moeckli J, McAdams N, Kaboli PJ. Perceptions of telehospitalist services to address staffing needs in rural and low complexity hospitals in the Veterans Health Administration. J Rural Health. 2019;36(3):355-359. https://doi.org/10.1111/jrh.12403
17. Eagle Telemedicine. EAGLE TELEMEDICINE NIGHT COVERAGE SOLUTIONS: Why They Work for Hospitals and Physicians. Accessed May 28, 2018. http://www.eagletelemedicine.com/wp-content/uploads/2016/11/EHP_WP_Telenocturnist_FINAL.pdf
18. Gujral J, Antoine C, Chandra S. The role of telehospitalist in COVID-19 response: Hospitalist caring remotely for New York patients explain their role. ACP Hospitalist. 2020; May 2020.
19. Kuperman EF, Linson EL, Klefstad K, Perry E, Glenn K. The virtual hospitalist: a single-site implementation bringing hospitalist coverage to critical access hospitals. J Hosp Med. 2018;13(11):759-763. https://doi.org/10.12788/jhm.3061
20. JaKa MM, Dinh JM, Ziegenfuss JY, et al. Patient and care team perspectives of telemedicine in critical access hospitals. J Hosp Med. 2020;15(6):345-348. https://doi.org/10.12788/jhm.3412
21. Khanal S, Burgon J, Leonard S, Griffiths M, Eddowes LA. Recommendations for the improved effectiveness and reporting of telemedicine programs in developing countries: results of a systematic literature review. Telemed J E Health. 2015;21(11):903-915. https://doi.org/10.1089/tmj.2014.0194
22. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
23. VHA Office of Rural Health. ORH 2020-2024 STRATEGIC PLAN. In: U.S. Department of Veterans Affairs, ed 2020. Accessed January 18, 2021 https://www.ruralhealth.va.gov/aboutus/index.asp
24. Veterans Health Administration. About VHA. In: U.S. Department of Veterans Affairs, ed. 2019. Accessed January 18, 2021.https://www.va.gov/health/aboutvha.asp
25. GeoSpatial Outcomes Division. VHA Office of Rural Health. U.S. Department of Veterans Affairs. Rural Veterans Health Care Atlas. 2nd ed - FY-2015. Accessed July 30, 2020. https://www.ruralhealth.va.gov/docs/atlas/CHAPTER_02_RHRI_Pts_treated_at_VAMCs.pdf
26. Wagner TH, Upadhyay A, Cowgill E, et al. Risk adjustment tools for learning health systems: a comparison of DxCG and CMS-HCC V21. Health Serv Res. 2016;51(5):2002-2019. https://doi.org/10.1111/1475-6773.12454
27. Wagner T, Stefos T, Moran E, et al. Technical Report 30: Risk Adjustment: Guide to the V21 and Nosos Risk Score Programs. Updated February 8, 2016. Accessed July 30, 2020. https://www.herc.research.va.gov/include/page.asp?id=technical-report-risk-adjustment
28. The R Foundation. The R Project for Statistical Computing. Accessed August 10, 2020. https://www.R-project.org/
29. Cleary PD, Meterko M, Wright SM, Zaslavsky AM. Are comparisons of patient experiences across hospitals fair? A study in Veterans Health Administration hospitals. Med Care. 2014;52(7):619-625. https://doi.org/10.1097/mlr.0000000000000144
30. Giordano LA, Elliott MN, Goldstein E, Lehrman WG, Spencer PA. Development, implementation, and public reporting of the HCAHPS survey. Med Care Res Rev. 2010;67(1):27-37. doi:10.1177/1077558709341065
31. Boltz M, Cuellar NG, Cole C, Pistorese B. Comparing an on-site nurse practitioner with telemedicine physician support hospitalist programme with a traditional physician hospitalist programme. J Telemed and Telecare. 2019;25(4):213-220. https://doi.org/10.1177%2F1357633X18758744
32. Quinn R. The pros and cons of locum tenens for hospitalists. The Hospitalist. 2012(12). Accessed May 29, 2018. https://www.the-hospitalist.org/hospitalist/article/124988/pros-and-cons-locum-tenens-hospitalists
33. Blumenthal DM, Olenski AR, Tsugawa Y, Jena AB. Association between treatment by locum tenens internal medicine physicians and 30-day mortality among hospitalized Medicare beneficiaries. JAMA. 2017;318(21):2119-2129. https://doi.org/10.1001/jama.2017.17925
34. Butcher L. Nurses as hospitalists | AHA Trustee Services. American Hospital Association. Accessed July 14, 2020 https://trustees.aha.org/articles/1238-nurses-as-hospitalists
35. Kartha A, Restuccia JD, Burgess JF, Jr, et al. Nurse practitioner and physician assistant scope of practice in 118 acute care hospitals. J Hosp Med. 2014;9(10):615-620. https://doi.org/10.1002/jhm.2231
Healthcare in rural areas faces increasing challenges due to community hospital closures, physician shortages, and a more concentrated population of older adults with higher rates of comorbid conditions than their urban counterparts.1-3 Critical access hospitals (CAHs), which primarily serve rural areas, have fewer clinical capabilities, worse process-of-care measures, and higher mortality rates for some conditions when compared to non-CAHs.4 As such, CAHs are closing at record numbers across the United States,5 resulting in loss of available hospital beds and patient access to timely emergency services,6 which can worsen outcomes, further widening the rural-urban healthcare gap.7,8 Furthermore, this strain on an overwhelmed health system in the most vulnerable areas restricts the ability to respond to healthcare crises like the coronavirus disease 2019 pandemic.9
Providing adequate staff for currently available hospital beds is also a problem in rural areas. Studies demonstrating improved outcomes, decreased length of stay (LOS), and increased quality with hospitalist services have resulted in a high demand for hospitalists nationwide.10-12 Recruiting hospitalists to work in rural areas, however, has become increasingly challenging due to low-patient volumes, financial viability of hospitalist-model adoption, and provider shortages.13,14 Recently, the Veterans Health Administration (VHA) reported a 28% nationwide shortage of hospitalists,15 which disproportionally affects rural VHA hospitals. Staffing difficulties and reliance on intermittent providers were reported by more than 80% of rural and low-complexity VHA facilities.16
Telehospitalist services (THS) can help deliver high-quality care to rural residents locally, decrease travel expenses, support hospital volume, and increase healthcare capacity in response to a pandemic.14,17,18 Only a few studies have described THS (mostly with overnight or cross-coverage models directed to CAHs), and clinical outcomes have been inconsistently reported.17,19-21 Furthermore, no program has been conducted within an integrated health system akin to the VHA. The primary objective of this quality improvement (QI) initiative was to perform a mixed-methods evaluation of THS between VHA hospitals to compare clinical outcomes and patient and staff satisfaction. Secondary outcomes included description of the implementation process, unexpected challenges, and subsequent QI initiatives. These results will expand the knowledge on feasibility of THS and provide implementation guidance.
METHODS
A mixed-methods approach was used to evaluate outcomes of this QI project. Reporting follows the revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0).22
Context
The VHA is the largest integrated healthcare system in the United States, with more than 8 million veterans enrolled, more than 30% of whom reside in a rural area. The VHA comprises more than 1,000 outpatient clinics and 170 acute care VA Medical Centers,23,24 including more than 35 rural and low-complexity hospitals.25 Low-complexity hospitals are those with the lowest volume and levels of patient complexity and minimal or no teaching programs, research, intensive care unit (ICU) beds, and subspecialists. Lack of reimbursement and interstate licensing, often cited as barriers to telemedicine, do not apply to the VHA. The hub site was a large tertiary care (high-complexity) VHA hospital located in Iowa City, Iowa. The spoke site was a low-complexity (10-bed acute inpatient unit with no ICU) rural VA hospital located in Tomah, Wisconsin.
Study Population
The preimplementation cohort for comparison included all patients admitted between January 1, 2018, and January 6, 2019. The postimplementation study cohort included all observation and acute care admissions during the pilot phase (January 7 to May 3, 2019) and sustainability phase (July 15 to December 31, 2019). The postimplementation analysis excluded the time period of May 4 to July 14, 2019, due to an interruption (gap) in THS. The gap period allowed for preliminary data analysis, optimization of the telecommunication system, and the recruitment and training of additional providers who could provide long-term staffing to the service.
Intervention
Preimplementation
Prior to THS implementation, Tomah’s inpatient ward was staffed by one physician per shift, who could be a hospitalist, medical officer of the day (MOD), or an intermittent provider (locum tenens). Hospitalists covering the acute inpatient ward prior to the THS transitioned to cover weekends, nights, and urgent care service shifts.
We visited the spoke site and held information-sharing sessions with key stakeholders (administrators, clinician leaders, nurses, and ancillary staff) prior to kick-off. Recurrent phone meetings addressed anticipated and emerging challenges. Telehospitalist and local providers underwent technology and service training.
Technology and Connectivity
A low-cost technology system using tablet computers provided Health Insurance Portability and Accountability Act–compliant videoconferencing with a telehospitalist at the hub site. An Eko-Core digital stethoscope® with a web-based audio stream was available. Telehospitalists conducted encounters from a private office space with telehealth capabilities. A total of $9,000 was spent on equipment at both sites. Due to connectivity problems and data limits, the tablets were switched to mobile computer-on-wheels workstations and hospital-based Wi-Fi for the sustainability phase.
THS Description
An experienced hub hospitalist, together with an advanced practice provider (APP; nurse practitioner [NP] or physician assistant [PA]), cared for all patients admitted to the 10-bed inpatient unit at the spoke site, Monday through Friday from 8:00 AM to 4:30 PM. The APP had limited or no prior experience in acute inpatient medicine. The telehospitalist worked as a team with the APP. The APP was the main point of contact for nurses, performed physical examinations, and directed patient care to their level of comfort (in a similar manner as a teaching team). The telehospitalist conducted bedside patient rounds, participated in multidisciplinary huddles, and shared clinical documentation and administrative duties with the APP. The telehospitalist was the primary staff for admitted patients and had full access to the electronic health record (EHR). The THS was staffed by 10 hospitalists during the study period. Overnight and weekend cross-coverage and admissions were performed by MODs, who also covered the urgent care and cross-covered other nonmedical units.
Quantitative Evaluation Methods
Workload and Clinical Outcomes
An EHR query identified all patients admitted during the pre- and postimplementation periods. Demographic data, clinical Nosos risk scores,26,27 and top admission diagnoses were reported. Workload was evaluated using the average number of encounters per day and self-reported telehospitalist worksheets, which were cross-referenced with EHR data. Clinical outcomes included LOS, 30-day hospital readmission rate, 30-day standardized mortality (SMR30), in-hospital mortality, and VHA-specific inpatient quality metrics. Independent sample t tests for continuous variables and chi-square tests or Fisher’s exact test (for patient class) for categorical variables were used to compare pre- and postimplementation groups. Statistical process control (SPC) charts evaluated changes over time. All analyses were conducted using Microsoft Excel and R.28
Anonymous surveys were distributed to spoke-site inpatient and administrative staff at 1 month and 12 months postimplementation, assessing satisfaction, technology/connectivity, communication, and challenges (Appendix Figure 1). Satisfaction of the telehospitalist physicians at the hub site was measured 12 months postimplementation by a 26-question survey assessing the same domains, plus quality of care (Appendix Figure 2).
Patient Satisfaction
The VHA Survey of Healthcare Experiences of Patients (SHEP), a version of the Hospital Consumer Assessment of Healthcare Providers and Systems Survey,29,30 was mailed to all patients after discharge. Survey responses concerning inpatient provider care (eg, care coordination, communication, hospital rating, willingness to recommend the hospital) during the pre- and postimplementation phases were compared using a two-sample test of independent proportions. Responses obtained during May and June 2019 were excluded.
Qualitative Evaluation Methods
The authors had full access to, and took full responsibility for, the integrity of the data. The project was evaluated by the University of Iowa Institutional Review Board and the Iowa City VA Research and Development Committee and was determined to be a non–human-subjects QI project.
RESULTS
Quantitative Workload and Clinical Outcomes
There were 822 admissions during the preimplementation period and 550 admissions during the postimplementation period (253 during the pilot and 297 during sustainability phase). Patient characteristics pre- and postimplementation were not significantly different (Table 1). The median patient age was 65 years; 96% of patients were male, and 83% were rural residents. The most common admission diagnosis was alcohol-related (36%); regarding patient disposition, 78% of admissions were discharged home.

Workload
There were 502 patient encounters staffed by the telehospitalist in the pilot phase, with an average of 6.25 encounters per day, and a telehospitalist-reported workload of 7 hours per day. There were 538 patient encounters, with an average of 4.67 encounters per day and a workload of 5.6 hours per day in the sustainability phase. The average daily census decreased from 5.0 (SD, 1.1) patients per day during preimplementation to 3.1 (SD, 0.5) patients per day during postimplementation (Table 2). In some of the months during the study period, admissions decreased below the lower SPC limit, suggesting a significant change (Figure). Adjusted LOS was significantly lower, with 3.0 (SD, 0.7) days vs 2.3 (SD, 0.3) days in the pre- and postimplementation periods, respectively. Bed occupancy rates were significantly lower in the sustainability phase compared with the pilot phase and the preimplementation period. Readmission rates varied, ranging from <10% to >30%, not significantly different but slightly higher in the postimplementation period. Readmission rates for heart failure, chronic obstructive pulmonary disease, and pneumonia remained unchanged; other medical readmissions (mostly alcohol-related) were slightly higher in the postimplementation period.

In-hospital mortality and SMR30 did not change significantly, but there was improvement in the 12-month rolling average of the observed/expected SMR30 from 1.40 to 1.08. Additional VHA-specific quality metrics were monitored and showed either small improvements or no change (data not shown).

Satisfaction at Hub and Spoke Sites
After sending two reminder communications via email, the telehospitalist satisfaction survey had a total response rate of 90% (9/10). Telehospitalists were satisfied or very satisfied (89%) with the program and the local providers (88.9%), rating their experience as good or excellent (100%) (Table 3). Communication with patients, families, and local staff was noted as being “positive” or “mostly positive.” Telehospitalists reported confidence in the accuracy of their diagnoses and rated the quality of care as being equal to that of a face-to-face encounter. Connectivity problems were prevalent, although most providers were able to resort to a back-up plan. Other challenges included differences in culture and concerns about liability. We received 27 responses from the spoke-site satisfaction survey; the response rate could not be determined because the survey was distributed by the spoke site for anonymity. Of the respondents, 37% identified as nurses, 25.9% as healthcare providers (APPs or physicians), and 33.3% as other staff (eg, social worker, nutritionist, physical therapist, utilization management, administrators); 3.7% did not respond. Among the participants, 88% had personally interacted with the THS. Most providers and other staff perceived THS as valuable (57.1% and 77.8%, respectively) and were satisfied or highly satisfied with THS (57.1% and 55.6%, respectively). On average, nurses provided lower ratings across all survey items than providers and other staff. Challenges noted by all staff included issues with communication, workflow, and technology/connectivity.

Qualitative Strengths
Our process evaluation identified high quality of care and teamwork as contributors to the success of the program. Overall, staff credited perceived improvements in quality of care to the quality of providers staffing the THS, including the local APPs. Noting the telehospitalists’ knowledge base and level of engagement as key attributes, one staff member commented: “I prefer a telehospitalist that really care[s] about patients than some provider that is physically here but does not engage.” Staff perceived improvements in the continuity of care, as well as care processes such as handoffs and transitions of care.
Improvements in teamwork were perceived compared with the previous model of care. Telehospitalists were lauded for their professionalism and communication skills. Overall, nurses felt providers in the THS listened more to their views. In addition, nurse respondents felt they could learn from several providers and said they enjoyed the telehospitalists’ disposition to teach and discuss patient care. The responsiveness of the THS staff was instrumental in building teamwork and acceptance. A bedside interdisciplinary protocol was established for appropriate patients. Local staff felt this was crucial for teamwork and patient satisfaction. Telehospitalists reported high-value in interdisciplinary rounds, facilitating interaction with nurses and ancillary staff. Handoff problems were identified, leading to QI initiatives to mitigate those issues.
Challenges
The survey identified administrative barriers, technical difficulties, workflow constraints, and clinical concerns. The credentialing process was complicated, delaying the onboarding of telehospitalists. Internet connectivity was inconsistent, leading to disruption in video communications; however, during the sustainability phase, updated technology improved communications. The communication workflow was resisted by some nurses, who wanted to phone the telehospitalist directly rather than having the local APP as the first contact. Secure messaging was enabled to allow nurses direct contact during the sustainability phase.
Workload was a concern among telehospitalists and local staff. Telehospitalists perceived the documentation requirements and administrative workload to be two to three times higher than at other hospitals—despite the lower number of encounters. Finally, clinical concerns from spoke-site clinicians included a perceived rise in the acuity of patients (which was not evident by the Nosos score) and delayed decisions to transfer-out patients. These concerns were addressed with educational sessions for telehospitalists during the sustainability phase.
Additional Quality Improvement Projects
The implementation of THS resulted in QI initiatives at the spoke site, including an EHR-integrated handoff tool; a documentation evaluation that led to the elimination of duplicative, inefficient, and error-prone templates; and a revision of the alcohol withdrawal treatment protocol during the sustainability phase to reduce the use of intravenous benzodiazepines. A more comprehensive benzodiazepine-sparing alcohol withdrawal treatment protocol was also developed but was not implemented until after the study period (January 2020).
DISCUSSION
Our pre-post study evaluation found implementation of a THS to be noninferior to face-to-face care, with no significant change in mortality, readmission rate, or patient satisfaction. The significant improvement observed in LOS is consistent with the adoption of hospitalist models in other medical care settings,11 but had not been reported by previous telehospitalist studies. For example, in their retrospective chart review comparing an NP-supported telehospitalist model to locum tenens hospitalists, Boltz et al found no difference in LOS.31 Moreover, as in our study, they found no differences in readmissions, mortality, and patient satisfaction.31 Similarly, Kuperman et al reported unchanged daily census, LOS, and transfer rates from a CAH with their virtual hospitalist program, but a decrease in the percentage of patients transferred-out from the emergency department, suggesting that more patients were treated locally.19
Reduction in LOS is one of the primary measures of efficiency in hospital care31; reducing LOS while maintaining the quality of care lowers hospital costs. The reduction in LOS in our study could be attributed to greater continuity of care, engagement/experience of the telehospitalists, or other factors. This decrease in LOS and slight reduction in admissions resulted in an overall lower daily census during the study period and impacted efficiency. Our study was unable to determine the cause for the reduction in admissions; however, several concurrent events, including the expansion of community-care options for veterans under the MISSION ACT (Maintaining Internal Systems and Strengthening Integrated Outside Networks Act) in June 2019, a nationwide smoking ban at VA facilities (October 2019), and a modification in the alcohol withdrawal treatment protocol might have influenced veterans’ choice of hospital.
Readmission rates were slightly higher, though nonsignificant, in the postimplementation period. Alcohol-related readmissions accounted for most readmissions; some of the protocol changes, such as admitting all patients with alcohol withdrawal to inpatient class instead of admitting some to the observation class, accounted for part of the increase in readmission rates. Readmission rates for other conditions such as chronic obstructive pulmonary disease, chronic heart failure, or pneumonia were not significantly different, suggesting that the reduction in LOS did not result in an unintended increased readmission rate for those conditions.
Rural hospitals are struggling with staffing and finances. Resorting to locum tenens staffing is costly and can result in variable quality of care.32,33 APPs are increasingly taking on hospitalist positions, with 65% of adult hospitalist programs, including half of all VHA hospitals, employing NPs and PAs.34,35 In response to this expanded scope of practice, hospitals employing APPs in hospitalist roles must comply with state and federal laws, which often require that APPs be supervised by or work in collaboration with an on-site or off-site physician. The THS is a great model to support APPs and address staffing and cost challenges in low-volume rural facilities, while maintaining quality of care. Some APP-telehospitalist programs similar to ours have reported cost reductions of up 58% compared to programs that employ locum tenens physicians.31 In our model, we assume that a single telehospitalist hub could provide coverage to two or three spoke sites with APP support, reducing staffing costs.
Hub telehospitalists reported satisfaction with the program, and they perceived the quality of care to be comparable to face-to-face encounters; their responses were consistent with those previously reported in an evaluation of telemedicine acute care by JaKa et al.
This study has several limitations. First, the VHA is an integrated health system, one that serves an older, predominantly male patient population. Also, the lack of reimbursement and interstate licensing restrictions limit generalizability of these results to other CAHs or healthcare systems. Furthermore, the intervention was limited to a single rural site; while this allowed for a detailed evaluation, unique barriers or facilitators might exist that limit its applicability. In addition, QI initiatives implemented by the VHA during the project period might have confounded some of our results. Last, patient satisfaction survey data are overall limited in their ability to fully assess patient’s experience and satisfaction with the program. Further qualitative studies are needed to gain deeper insight into patient perspectives with the THS and whether modality of care delivery influences patients’ care decisions. Future studies should consider a multisite design with one or more hubs and multiple spoke sites.
CONCLUSION
Telehospitalist services are a feasible and safe approach to provide inpatient services and address staffing needs of rural hospitals. To enhance program performance, it is essential to ensure adequate technological quality, clearly delineate and define roles and responsibilities of the care team, and address communication issues or staff concerns in a timely manner.
Acknowledgments
The authors thank the staff, administration, and leadership at the Tomah and Iowa City VA Medical Centers for working with us on this project. They offer special thanks to Kevin Glenn, MD, MS, Ethan Kuperman, MD, MS, FHM, and Jennifer Chapin, MSN, RN, for sharing their expertise, and the telehealth team, including Nathaniel Samuelson, Angela McDowell, and Katrin Metcalf.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Healthcare in rural areas faces increasing challenges due to community hospital closures, physician shortages, and a more concentrated population of older adults with higher rates of comorbid conditions than their urban counterparts.1-3 Critical access hospitals (CAHs), which primarily serve rural areas, have fewer clinical capabilities, worse process-of-care measures, and higher mortality rates for some conditions when compared to non-CAHs.4 As such, CAHs are closing at record numbers across the United States,5 resulting in loss of available hospital beds and patient access to timely emergency services,6 which can worsen outcomes, further widening the rural-urban healthcare gap.7,8 Furthermore, this strain on an overwhelmed health system in the most vulnerable areas restricts the ability to respond to healthcare crises like the coronavirus disease 2019 pandemic.9
Providing adequate staff for currently available hospital beds is also a problem in rural areas. Studies demonstrating improved outcomes, decreased length of stay (LOS), and increased quality with hospitalist services have resulted in a high demand for hospitalists nationwide.10-12 Recruiting hospitalists to work in rural areas, however, has become increasingly challenging due to low-patient volumes, financial viability of hospitalist-model adoption, and provider shortages.13,14 Recently, the Veterans Health Administration (VHA) reported a 28% nationwide shortage of hospitalists,15 which disproportionally affects rural VHA hospitals. Staffing difficulties and reliance on intermittent providers were reported by more than 80% of rural and low-complexity VHA facilities.16
Telehospitalist services (THS) can help deliver high-quality care to rural residents locally, decrease travel expenses, support hospital volume, and increase healthcare capacity in response to a pandemic.14,17,18 Only a few studies have described THS (mostly with overnight or cross-coverage models directed to CAHs), and clinical outcomes have been inconsistently reported.17,19-21 Furthermore, no program has been conducted within an integrated health system akin to the VHA. The primary objective of this quality improvement (QI) initiative was to perform a mixed-methods evaluation of THS between VHA hospitals to compare clinical outcomes and patient and staff satisfaction. Secondary outcomes included description of the implementation process, unexpected challenges, and subsequent QI initiatives. These results will expand the knowledge on feasibility of THS and provide implementation guidance.
METHODS
A mixed-methods approach was used to evaluate outcomes of this QI project. Reporting follows the revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0).22
Context
The VHA is the largest integrated healthcare system in the United States, with more than 8 million veterans enrolled, more than 30% of whom reside in a rural area. The VHA comprises more than 1,000 outpatient clinics and 170 acute care VA Medical Centers,23,24 including more than 35 rural and low-complexity hospitals.25 Low-complexity hospitals are those with the lowest volume and levels of patient complexity and minimal or no teaching programs, research, intensive care unit (ICU) beds, and subspecialists. Lack of reimbursement and interstate licensing, often cited as barriers to telemedicine, do not apply to the VHA. The hub site was a large tertiary care (high-complexity) VHA hospital located in Iowa City, Iowa. The spoke site was a low-complexity (10-bed acute inpatient unit with no ICU) rural VA hospital located in Tomah, Wisconsin.
Study Population
The preimplementation cohort for comparison included all patients admitted between January 1, 2018, and January 6, 2019. The postimplementation study cohort included all observation and acute care admissions during the pilot phase (January 7 to May 3, 2019) and sustainability phase (July 15 to December 31, 2019). The postimplementation analysis excluded the time period of May 4 to July 14, 2019, due to an interruption (gap) in THS. The gap period allowed for preliminary data analysis, optimization of the telecommunication system, and the recruitment and training of additional providers who could provide long-term staffing to the service.
Intervention
Preimplementation
Prior to THS implementation, Tomah’s inpatient ward was staffed by one physician per shift, who could be a hospitalist, medical officer of the day (MOD), or an intermittent provider (locum tenens). Hospitalists covering the acute inpatient ward prior to the THS transitioned to cover weekends, nights, and urgent care service shifts.
We visited the spoke site and held information-sharing sessions with key stakeholders (administrators, clinician leaders, nurses, and ancillary staff) prior to kick-off. Recurrent phone meetings addressed anticipated and emerging challenges. Telehospitalist and local providers underwent technology and service training.
Technology and Connectivity
A low-cost technology system using tablet computers provided Health Insurance Portability and Accountability Act–compliant videoconferencing with a telehospitalist at the hub site. An Eko-Core digital stethoscope® with a web-based audio stream was available. Telehospitalists conducted encounters from a private office space with telehealth capabilities. A total of $9,000 was spent on equipment at both sites. Due to connectivity problems and data limits, the tablets were switched to mobile computer-on-wheels workstations and hospital-based Wi-Fi for the sustainability phase.
THS Description
An experienced hub hospitalist, together with an advanced practice provider (APP; nurse practitioner [NP] or physician assistant [PA]), cared for all patients admitted to the 10-bed inpatient unit at the spoke site, Monday through Friday from 8:00 AM to 4:30 PM. The APP had limited or no prior experience in acute inpatient medicine. The telehospitalist worked as a team with the APP. The APP was the main point of contact for nurses, performed physical examinations, and directed patient care to their level of comfort (in a similar manner as a teaching team). The telehospitalist conducted bedside patient rounds, participated in multidisciplinary huddles, and shared clinical documentation and administrative duties with the APP. The telehospitalist was the primary staff for admitted patients and had full access to the electronic health record (EHR). The THS was staffed by 10 hospitalists during the study period. Overnight and weekend cross-coverage and admissions were performed by MODs, who also covered the urgent care and cross-covered other nonmedical units.
Quantitative Evaluation Methods
Workload and Clinical Outcomes
An EHR query identified all patients admitted during the pre- and postimplementation periods. Demographic data, clinical Nosos risk scores,26,27 and top admission diagnoses were reported. Workload was evaluated using the average number of encounters per day and self-reported telehospitalist worksheets, which were cross-referenced with EHR data. Clinical outcomes included LOS, 30-day hospital readmission rate, 30-day standardized mortality (SMR30), in-hospital mortality, and VHA-specific inpatient quality metrics. Independent sample t tests for continuous variables and chi-square tests or Fisher’s exact test (for patient class) for categorical variables were used to compare pre- and postimplementation groups. Statistical process control (SPC) charts evaluated changes over time. All analyses were conducted using Microsoft Excel and R.28
Anonymous surveys were distributed to spoke-site inpatient and administrative staff at 1 month and 12 months postimplementation, assessing satisfaction, technology/connectivity, communication, and challenges (Appendix Figure 1). Satisfaction of the telehospitalist physicians at the hub site was measured 12 months postimplementation by a 26-question survey assessing the same domains, plus quality of care (Appendix Figure 2).
Patient Satisfaction
The VHA Survey of Healthcare Experiences of Patients (SHEP), a version of the Hospital Consumer Assessment of Healthcare Providers and Systems Survey,29,30 was mailed to all patients after discharge. Survey responses concerning inpatient provider care (eg, care coordination, communication, hospital rating, willingness to recommend the hospital) during the pre- and postimplementation phases were compared using a two-sample test of independent proportions. Responses obtained during May and June 2019 were excluded.
Qualitative Evaluation Methods
The authors had full access to, and took full responsibility for, the integrity of the data. The project was evaluated by the University of Iowa Institutional Review Board and the Iowa City VA Research and Development Committee and was determined to be a non–human-subjects QI project.
RESULTS
Quantitative Workload and Clinical Outcomes
There were 822 admissions during the preimplementation period and 550 admissions during the postimplementation period (253 during the pilot and 297 during sustainability phase). Patient characteristics pre- and postimplementation were not significantly different (Table 1). The median patient age was 65 years; 96% of patients were male, and 83% were rural residents. The most common admission diagnosis was alcohol-related (36%); regarding patient disposition, 78% of admissions were discharged home.

Workload
There were 502 patient encounters staffed by the telehospitalist in the pilot phase, with an average of 6.25 encounters per day, and a telehospitalist-reported workload of 7 hours per day. There were 538 patient encounters, with an average of 4.67 encounters per day and a workload of 5.6 hours per day in the sustainability phase. The average daily census decreased from 5.0 (SD, 1.1) patients per day during preimplementation to 3.1 (SD, 0.5) patients per day during postimplementation (Table 2). In some of the months during the study period, admissions decreased below the lower SPC limit, suggesting a significant change (Figure). Adjusted LOS was significantly lower, with 3.0 (SD, 0.7) days vs 2.3 (SD, 0.3) days in the pre- and postimplementation periods, respectively. Bed occupancy rates were significantly lower in the sustainability phase compared with the pilot phase and the preimplementation period. Readmission rates varied, ranging from <10% to >30%, not significantly different but slightly higher in the postimplementation period. Readmission rates for heart failure, chronic obstructive pulmonary disease, and pneumonia remained unchanged; other medical readmissions (mostly alcohol-related) were slightly higher in the postimplementation period.

In-hospital mortality and SMR30 did not change significantly, but there was improvement in the 12-month rolling average of the observed/expected SMR30 from 1.40 to 1.08. Additional VHA-specific quality metrics were monitored and showed either small improvements or no change (data not shown).

Satisfaction at Hub and Spoke Sites
After sending two reminder communications via email, the telehospitalist satisfaction survey had a total response rate of 90% (9/10). Telehospitalists were satisfied or very satisfied (89%) with the program and the local providers (88.9%), rating their experience as good or excellent (100%) (Table 3). Communication with patients, families, and local staff was noted as being “positive” or “mostly positive.” Telehospitalists reported confidence in the accuracy of their diagnoses and rated the quality of care as being equal to that of a face-to-face encounter. Connectivity problems were prevalent, although most providers were able to resort to a back-up plan. Other challenges included differences in culture and concerns about liability. We received 27 responses from the spoke-site satisfaction survey; the response rate could not be determined because the survey was distributed by the spoke site for anonymity. Of the respondents, 37% identified as nurses, 25.9% as healthcare providers (APPs or physicians), and 33.3% as other staff (eg, social worker, nutritionist, physical therapist, utilization management, administrators); 3.7% did not respond. Among the participants, 88% had personally interacted with the THS. Most providers and other staff perceived THS as valuable (57.1% and 77.8%, respectively) and were satisfied or highly satisfied with THS (57.1% and 55.6%, respectively). On average, nurses provided lower ratings across all survey items than providers and other staff. Challenges noted by all staff included issues with communication, workflow, and technology/connectivity.

Qualitative Strengths
Our process evaluation identified high quality of care and teamwork as contributors to the success of the program. Overall, staff credited perceived improvements in quality of care to the quality of providers staffing the THS, including the local APPs. Noting the telehospitalists’ knowledge base and level of engagement as key attributes, one staff member commented: “I prefer a telehospitalist that really care[s] about patients than some provider that is physically here but does not engage.” Staff perceived improvements in the continuity of care, as well as care processes such as handoffs and transitions of care.
Improvements in teamwork were perceived compared with the previous model of care. Telehospitalists were lauded for their professionalism and communication skills. Overall, nurses felt providers in the THS listened more to their views. In addition, nurse respondents felt they could learn from several providers and said they enjoyed the telehospitalists’ disposition to teach and discuss patient care. The responsiveness of the THS staff was instrumental in building teamwork and acceptance. A bedside interdisciplinary protocol was established for appropriate patients. Local staff felt this was crucial for teamwork and patient satisfaction. Telehospitalists reported high-value in interdisciplinary rounds, facilitating interaction with nurses and ancillary staff. Handoff problems were identified, leading to QI initiatives to mitigate those issues.
Challenges
The survey identified administrative barriers, technical difficulties, workflow constraints, and clinical concerns. The credentialing process was complicated, delaying the onboarding of telehospitalists. Internet connectivity was inconsistent, leading to disruption in video communications; however, during the sustainability phase, updated technology improved communications. The communication workflow was resisted by some nurses, who wanted to phone the telehospitalist directly rather than having the local APP as the first contact. Secure messaging was enabled to allow nurses direct contact during the sustainability phase.
Workload was a concern among telehospitalists and local staff. Telehospitalists perceived the documentation requirements and administrative workload to be two to three times higher than at other hospitals—despite the lower number of encounters. Finally, clinical concerns from spoke-site clinicians included a perceived rise in the acuity of patients (which was not evident by the Nosos score) and delayed decisions to transfer-out patients. These concerns were addressed with educational sessions for telehospitalists during the sustainability phase.
Additional Quality Improvement Projects
The implementation of THS resulted in QI initiatives at the spoke site, including an EHR-integrated handoff tool; a documentation evaluation that led to the elimination of duplicative, inefficient, and error-prone templates; and a revision of the alcohol withdrawal treatment protocol during the sustainability phase to reduce the use of intravenous benzodiazepines. A more comprehensive benzodiazepine-sparing alcohol withdrawal treatment protocol was also developed but was not implemented until after the study period (January 2020).
DISCUSSION
Our pre-post study evaluation found implementation of a THS to be noninferior to face-to-face care, with no significant change in mortality, readmission rate, or patient satisfaction. The significant improvement observed in LOS is consistent with the adoption of hospitalist models in other medical care settings,11 but had not been reported by previous telehospitalist studies. For example, in their retrospective chart review comparing an NP-supported telehospitalist model to locum tenens hospitalists, Boltz et al found no difference in LOS.31 Moreover, as in our study, they found no differences in readmissions, mortality, and patient satisfaction.31 Similarly, Kuperman et al reported unchanged daily census, LOS, and transfer rates from a CAH with their virtual hospitalist program, but a decrease in the percentage of patients transferred-out from the emergency department, suggesting that more patients were treated locally.19
Reduction in LOS is one of the primary measures of efficiency in hospital care31; reducing LOS while maintaining the quality of care lowers hospital costs. The reduction in LOS in our study could be attributed to greater continuity of care, engagement/experience of the telehospitalists, or other factors. This decrease in LOS and slight reduction in admissions resulted in an overall lower daily census during the study period and impacted efficiency. Our study was unable to determine the cause for the reduction in admissions; however, several concurrent events, including the expansion of community-care options for veterans under the MISSION ACT (Maintaining Internal Systems and Strengthening Integrated Outside Networks Act) in June 2019, a nationwide smoking ban at VA facilities (October 2019), and a modification in the alcohol withdrawal treatment protocol might have influenced veterans’ choice of hospital.
Readmission rates were slightly higher, though nonsignificant, in the postimplementation period. Alcohol-related readmissions accounted for most readmissions; some of the protocol changes, such as admitting all patients with alcohol withdrawal to inpatient class instead of admitting some to the observation class, accounted for part of the increase in readmission rates. Readmission rates for other conditions such as chronic obstructive pulmonary disease, chronic heart failure, or pneumonia were not significantly different, suggesting that the reduction in LOS did not result in an unintended increased readmission rate for those conditions.
Rural hospitals are struggling with staffing and finances. Resorting to locum tenens staffing is costly and can result in variable quality of care.32,33 APPs are increasingly taking on hospitalist positions, with 65% of adult hospitalist programs, including half of all VHA hospitals, employing NPs and PAs.34,35 In response to this expanded scope of practice, hospitals employing APPs in hospitalist roles must comply with state and federal laws, which often require that APPs be supervised by or work in collaboration with an on-site or off-site physician. The THS is a great model to support APPs and address staffing and cost challenges in low-volume rural facilities, while maintaining quality of care. Some APP-telehospitalist programs similar to ours have reported cost reductions of up 58% compared to programs that employ locum tenens physicians.31 In our model, we assume that a single telehospitalist hub could provide coverage to two or three spoke sites with APP support, reducing staffing costs.
Hub telehospitalists reported satisfaction with the program, and they perceived the quality of care to be comparable to face-to-face encounters; their responses were consistent with those previously reported in an evaluation of telemedicine acute care by JaKa et al.
This study has several limitations. First, the VHA is an integrated health system, one that serves an older, predominantly male patient population. Also, the lack of reimbursement and interstate licensing restrictions limit generalizability of these results to other CAHs or healthcare systems. Furthermore, the intervention was limited to a single rural site; while this allowed for a detailed evaluation, unique barriers or facilitators might exist that limit its applicability. In addition, QI initiatives implemented by the VHA during the project period might have confounded some of our results. Last, patient satisfaction survey data are overall limited in their ability to fully assess patient’s experience and satisfaction with the program. Further qualitative studies are needed to gain deeper insight into patient perspectives with the THS and whether modality of care delivery influences patients’ care decisions. Future studies should consider a multisite design with one or more hubs and multiple spoke sites.
CONCLUSION
Telehospitalist services are a feasible and safe approach to provide inpatient services and address staffing needs of rural hospitals. To enhance program performance, it is essential to ensure adequate technological quality, clearly delineate and define roles and responsibilities of the care team, and address communication issues or staff concerns in a timely manner.
Acknowledgments
The authors thank the staff, administration, and leadership at the Tomah and Iowa City VA Medical Centers for working with us on this project. They offer special thanks to Kevin Glenn, MD, MS, Ethan Kuperman, MD, MS, FHM, and Jennifer Chapin, MSN, RN, for sharing their expertise, and the telehealth team, including Nathaniel Samuelson, Angela McDowell, and Katrin Metcalf.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
1. O’Connor A, Wellenius G. Rural-urban disparities in the prevalence of diabetes and coronary heart disease. Public Health. 2012;126(10):813-820. https://doi.org/10.1016/j.puhe.2012.05.029
2. Kaufman BG, Thomas SR, Randolph RK, et al. The rising rate of rural hospital closures. J Rural Health. 2016;32(1):35-43. https://doi.org/10.1111/jrh.12128
3. MacDowell M, Glasser M, Fitts M, Nielsen K, Hunsaker M. A national view of rural health workforce issues in the USA. Rural Remote Health. 2010;10(3):1531.
4. Joynt KE, Harris Y, Orav EJ, Jha AK. Quality of care and patient outcomes in critical access rural hospitals. JAMA. 2011;306(1):45-52. https://doi.org/10.1001/jama.2011.902
5. The Chartis Group. Chartis Center for Rural Health. The Rural Health Safety Net Under Pressure: Rural Hospital Vulnerability. Published February 2020. Accessed May 07, 2020. https://www.chartis.com/forum/wp-content/uploads/2020/02/CCRH_Vulnerability-Research_FiNAL-02.14.20.pdf
6. Miller KEM, James HJ, Holmes GM, Van Houtven CH. The effect of rural hospital closures on emergency medical service response and transport times. Health Serv Res. 2020;55(2):288-300. https://doi.org/10.1111/1475-6773.13254
7. Buchmueller TC, Jacobson M, Wold C. How far to the hospital? The effect of hospital closures on access to care. J Health Econ. 2006;25(4):740-761. https://doi.org/10.1016/j.jhealeco.2005.10.006
8. Mohr NM, Harland KK, Shane DM, et al. Rural patients with severe sepsis or septic shock who bypass rural hospitals have increased mortality: an instrumental variables approach. Crit Care Med. 2017;45(1):85-93. https://doi.org/10.1097/ccm.0000000000002026
9. Gutierrez J, Kuperman E, Kaboli PJ. Using telehealth as a tool for rural hospitals in the COVID-19 pandemic response. J Rural Health. 2020;10.1111/jrh.12443. https://doi.org/10.1111/jrh.12443
10. Kisuule F, Howell EE. Hospitalists and their impact on quality, patient safety, and satisfaction. Obstet Gynecol Clin North Am. 2015;42(3):433-446. https://doi.org/10.1016/j.ogc.2015.05.003
11. Peterson MC. A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84(3):248-254. https://doi.org/10.4065/84.3.248
12. Auerbach AD, Wachter RM, Katz P, Showstack J, Baron RB, Goldman L. Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes. Ann Intern Med. 2002;137(11):859-865. https://doi.org/10.7326/0003-4819-137-11-200212030-00006
13. Casey MM, Hung P, Moscovice I, Prasad S. The use of hospitalists by small rural hospitals: results of a national survey. Med Care Res Rev. 2014;71(4):356-366. https://doi.org/10.1177/1077558714533822
14. Sanders RB, Simpson KN, Kazley AS, Giarrizzi DP. New hospital telemedicine services: potential market for a nighttime telehospitalist service. Telemed J E Health. 2014;20(10):902-908. https://doi.org/10.1089/tmj.2013.0344
15. Department of Veterans Affairs. Office of Inspector General. OIG Determination of Veterans Health Administration’s Occupational Staffing Shortages. Published September 30, 2019. Accessed June 15, 2020. https://www.va.gov/oig/pubs/VAOIG-19-00346-241.pdf
16. Gutierrez J, Moeckli J, McAdams N, Kaboli PJ. Perceptions of telehospitalist services to address staffing needs in rural and low complexity hospitals in the Veterans Health Administration. J Rural Health. 2019;36(3):355-359. https://doi.org/10.1111/jrh.12403
17. Eagle Telemedicine. EAGLE TELEMEDICINE NIGHT COVERAGE SOLUTIONS: Why They Work for Hospitals and Physicians. Accessed May 28, 2018. http://www.eagletelemedicine.com/wp-content/uploads/2016/11/EHP_WP_Telenocturnist_FINAL.pdf
18. Gujral J, Antoine C, Chandra S. The role of telehospitalist in COVID-19 response: Hospitalist caring remotely for New York patients explain their role. ACP Hospitalist. 2020; May 2020.
19. Kuperman EF, Linson EL, Klefstad K, Perry E, Glenn K. The virtual hospitalist: a single-site implementation bringing hospitalist coverage to critical access hospitals. J Hosp Med. 2018;13(11):759-763. https://doi.org/10.12788/jhm.3061
20. JaKa MM, Dinh JM, Ziegenfuss JY, et al. Patient and care team perspectives of telemedicine in critical access hospitals. J Hosp Med. 2020;15(6):345-348. https://doi.org/10.12788/jhm.3412
21. Khanal S, Burgon J, Leonard S, Griffiths M, Eddowes LA. Recommendations for the improved effectiveness and reporting of telemedicine programs in developing countries: results of a systematic literature review. Telemed J E Health. 2015;21(11):903-915. https://doi.org/10.1089/tmj.2014.0194
22. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
23. VHA Office of Rural Health. ORH 2020-2024 STRATEGIC PLAN. In: U.S. Department of Veterans Affairs, ed 2020. Accessed January 18, 2021 https://www.ruralhealth.va.gov/aboutus/index.asp
24. Veterans Health Administration. About VHA. In: U.S. Department of Veterans Affairs, ed. 2019. Accessed January 18, 2021.https://www.va.gov/health/aboutvha.asp
25. GeoSpatial Outcomes Division. VHA Office of Rural Health. U.S. Department of Veterans Affairs. Rural Veterans Health Care Atlas. 2nd ed - FY-2015. Accessed July 30, 2020. https://www.ruralhealth.va.gov/docs/atlas/CHAPTER_02_RHRI_Pts_treated_at_VAMCs.pdf
26. Wagner TH, Upadhyay A, Cowgill E, et al. Risk adjustment tools for learning health systems: a comparison of DxCG and CMS-HCC V21. Health Serv Res. 2016;51(5):2002-2019. https://doi.org/10.1111/1475-6773.12454
27. Wagner T, Stefos T, Moran E, et al. Technical Report 30: Risk Adjustment: Guide to the V21 and Nosos Risk Score Programs. Updated February 8, 2016. Accessed July 30, 2020. https://www.herc.research.va.gov/include/page.asp?id=technical-report-risk-adjustment
28. The R Foundation. The R Project for Statistical Computing. Accessed August 10, 2020. https://www.R-project.org/
29. Cleary PD, Meterko M, Wright SM, Zaslavsky AM. Are comparisons of patient experiences across hospitals fair? A study in Veterans Health Administration hospitals. Med Care. 2014;52(7):619-625. https://doi.org/10.1097/mlr.0000000000000144
30. Giordano LA, Elliott MN, Goldstein E, Lehrman WG, Spencer PA. Development, implementation, and public reporting of the HCAHPS survey. Med Care Res Rev. 2010;67(1):27-37. doi:10.1177/1077558709341065
31. Boltz M, Cuellar NG, Cole C, Pistorese B. Comparing an on-site nurse practitioner with telemedicine physician support hospitalist programme with a traditional physician hospitalist programme. J Telemed and Telecare. 2019;25(4):213-220. https://doi.org/10.1177%2F1357633X18758744
32. Quinn R. The pros and cons of locum tenens for hospitalists. The Hospitalist. 2012(12). Accessed May 29, 2018. https://www.the-hospitalist.org/hospitalist/article/124988/pros-and-cons-locum-tenens-hospitalists
33. Blumenthal DM, Olenski AR, Tsugawa Y, Jena AB. Association between treatment by locum tenens internal medicine physicians and 30-day mortality among hospitalized Medicare beneficiaries. JAMA. 2017;318(21):2119-2129. https://doi.org/10.1001/jama.2017.17925
34. Butcher L. Nurses as hospitalists | AHA Trustee Services. American Hospital Association. Accessed July 14, 2020 https://trustees.aha.org/articles/1238-nurses-as-hospitalists
35. Kartha A, Restuccia JD, Burgess JF, Jr, et al. Nurse practitioner and physician assistant scope of practice in 118 acute care hospitals. J Hosp Med. 2014;9(10):615-620. https://doi.org/10.1002/jhm.2231
1. O’Connor A, Wellenius G. Rural-urban disparities in the prevalence of diabetes and coronary heart disease. Public Health. 2012;126(10):813-820. https://doi.org/10.1016/j.puhe.2012.05.029
2. Kaufman BG, Thomas SR, Randolph RK, et al. The rising rate of rural hospital closures. J Rural Health. 2016;32(1):35-43. https://doi.org/10.1111/jrh.12128
3. MacDowell M, Glasser M, Fitts M, Nielsen K, Hunsaker M. A national view of rural health workforce issues in the USA. Rural Remote Health. 2010;10(3):1531.
4. Joynt KE, Harris Y, Orav EJ, Jha AK. Quality of care and patient outcomes in critical access rural hospitals. JAMA. 2011;306(1):45-52. https://doi.org/10.1001/jama.2011.902
5. The Chartis Group. Chartis Center for Rural Health. The Rural Health Safety Net Under Pressure: Rural Hospital Vulnerability. Published February 2020. Accessed May 07, 2020. https://www.chartis.com/forum/wp-content/uploads/2020/02/CCRH_Vulnerability-Research_FiNAL-02.14.20.pdf
6. Miller KEM, James HJ, Holmes GM, Van Houtven CH. The effect of rural hospital closures on emergency medical service response and transport times. Health Serv Res. 2020;55(2):288-300. https://doi.org/10.1111/1475-6773.13254
7. Buchmueller TC, Jacobson M, Wold C. How far to the hospital? The effect of hospital closures on access to care. J Health Econ. 2006;25(4):740-761. https://doi.org/10.1016/j.jhealeco.2005.10.006
8. Mohr NM, Harland KK, Shane DM, et al. Rural patients with severe sepsis or septic shock who bypass rural hospitals have increased mortality: an instrumental variables approach. Crit Care Med. 2017;45(1):85-93. https://doi.org/10.1097/ccm.0000000000002026
9. Gutierrez J, Kuperman E, Kaboli PJ. Using telehealth as a tool for rural hospitals in the COVID-19 pandemic response. J Rural Health. 2020;10.1111/jrh.12443. https://doi.org/10.1111/jrh.12443
10. Kisuule F, Howell EE. Hospitalists and their impact on quality, patient safety, and satisfaction. Obstet Gynecol Clin North Am. 2015;42(3):433-446. https://doi.org/10.1016/j.ogc.2015.05.003
11. Peterson MC. A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84(3):248-254. https://doi.org/10.4065/84.3.248
12. Auerbach AD, Wachter RM, Katz P, Showstack J, Baron RB, Goldman L. Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes. Ann Intern Med. 2002;137(11):859-865. https://doi.org/10.7326/0003-4819-137-11-200212030-00006
13. Casey MM, Hung P, Moscovice I, Prasad S. The use of hospitalists by small rural hospitals: results of a national survey. Med Care Res Rev. 2014;71(4):356-366. https://doi.org/10.1177/1077558714533822
14. Sanders RB, Simpson KN, Kazley AS, Giarrizzi DP. New hospital telemedicine services: potential market for a nighttime telehospitalist service. Telemed J E Health. 2014;20(10):902-908. https://doi.org/10.1089/tmj.2013.0344
15. Department of Veterans Affairs. Office of Inspector General. OIG Determination of Veterans Health Administration’s Occupational Staffing Shortages. Published September 30, 2019. Accessed June 15, 2020. https://www.va.gov/oig/pubs/VAOIG-19-00346-241.pdf
16. Gutierrez J, Moeckli J, McAdams N, Kaboli PJ. Perceptions of telehospitalist services to address staffing needs in rural and low complexity hospitals in the Veterans Health Administration. J Rural Health. 2019;36(3):355-359. https://doi.org/10.1111/jrh.12403
17. Eagle Telemedicine. EAGLE TELEMEDICINE NIGHT COVERAGE SOLUTIONS: Why They Work for Hospitals and Physicians. Accessed May 28, 2018. http://www.eagletelemedicine.com/wp-content/uploads/2016/11/EHP_WP_Telenocturnist_FINAL.pdf
18. Gujral J, Antoine C, Chandra S. The role of telehospitalist in COVID-19 response: Hospitalist caring remotely for New York patients explain their role. ACP Hospitalist. 2020; May 2020.
19. Kuperman EF, Linson EL, Klefstad K, Perry E, Glenn K. The virtual hospitalist: a single-site implementation bringing hospitalist coverage to critical access hospitals. J Hosp Med. 2018;13(11):759-763. https://doi.org/10.12788/jhm.3061
20. JaKa MM, Dinh JM, Ziegenfuss JY, et al. Patient and care team perspectives of telemedicine in critical access hospitals. J Hosp Med. 2020;15(6):345-348. https://doi.org/10.12788/jhm.3412
21. Khanal S, Burgon J, Leonard S, Griffiths M, Eddowes LA. Recommendations for the improved effectiveness and reporting of telemedicine programs in developing countries: results of a systematic literature review. Telemed J E Health. 2015;21(11):903-915. https://doi.org/10.1089/tmj.2014.0194
22. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
23. VHA Office of Rural Health. ORH 2020-2024 STRATEGIC PLAN. In: U.S. Department of Veterans Affairs, ed 2020. Accessed January 18, 2021 https://www.ruralhealth.va.gov/aboutus/index.asp
24. Veterans Health Administration. About VHA. In: U.S. Department of Veterans Affairs, ed. 2019. Accessed January 18, 2021.https://www.va.gov/health/aboutvha.asp
25. GeoSpatial Outcomes Division. VHA Office of Rural Health. U.S. Department of Veterans Affairs. Rural Veterans Health Care Atlas. 2nd ed - FY-2015. Accessed July 30, 2020. https://www.ruralhealth.va.gov/docs/atlas/CHAPTER_02_RHRI_Pts_treated_at_VAMCs.pdf
26. Wagner TH, Upadhyay A, Cowgill E, et al. Risk adjustment tools for learning health systems: a comparison of DxCG and CMS-HCC V21. Health Serv Res. 2016;51(5):2002-2019. https://doi.org/10.1111/1475-6773.12454
27. Wagner T, Stefos T, Moran E, et al. Technical Report 30: Risk Adjustment: Guide to the V21 and Nosos Risk Score Programs. Updated February 8, 2016. Accessed July 30, 2020. https://www.herc.research.va.gov/include/page.asp?id=technical-report-risk-adjustment
28. The R Foundation. The R Project for Statistical Computing. Accessed August 10, 2020. https://www.R-project.org/
29. Cleary PD, Meterko M, Wright SM, Zaslavsky AM. Are comparisons of patient experiences across hospitals fair? A study in Veterans Health Administration hospitals. Med Care. 2014;52(7):619-625. https://doi.org/10.1097/mlr.0000000000000144
30. Giordano LA, Elliott MN, Goldstein E, Lehrman WG, Spencer PA. Development, implementation, and public reporting of the HCAHPS survey. Med Care Res Rev. 2010;67(1):27-37. doi:10.1177/1077558709341065
31. Boltz M, Cuellar NG, Cole C, Pistorese B. Comparing an on-site nurse practitioner with telemedicine physician support hospitalist programme with a traditional physician hospitalist programme. J Telemed and Telecare. 2019;25(4):213-220. https://doi.org/10.1177%2F1357633X18758744
32. Quinn R. The pros and cons of locum tenens for hospitalists. The Hospitalist. 2012(12). Accessed May 29, 2018. https://www.the-hospitalist.org/hospitalist/article/124988/pros-and-cons-locum-tenens-hospitalists
33. Blumenthal DM, Olenski AR, Tsugawa Y, Jena AB. Association between treatment by locum tenens internal medicine physicians and 30-day mortality among hospitalized Medicare beneficiaries. JAMA. 2017;318(21):2119-2129. https://doi.org/10.1001/jama.2017.17925
34. Butcher L. Nurses as hospitalists | AHA Trustee Services. American Hospital Association. Accessed July 14, 2020 https://trustees.aha.org/articles/1238-nurses-as-hospitalists
35. Kartha A, Restuccia JD, Burgess JF, Jr, et al. Nurse practitioner and physician assistant scope of practice in 118 acute care hospitals. J Hosp Med. 2014;9(10):615-620. https://doi.org/10.1002/jhm.2231
© 2021 Society of Hospital Medicine