User login
Implementing a Telehospitalist Program Between Veterans Health Administration Hospitals: Outcomes, Acceptance, and Barriers to Implementation
Healthcare in rural areas faces increasing challenges due to community hospital closures, physician shortages, and a more concentrated population of older adults with higher rates of comorbid conditions than their urban counterparts.1-3 Critical access hospitals (CAHs), which primarily serve rural areas, have fewer clinical capabilities, worse process-of-care measures, and higher mortality rates for some conditions when compared to non-CAHs.4 As such, CAHs are closing at record numbers across the United States,5 resulting in loss of available hospital beds and patient access to timely emergency services,6 which can worsen outcomes, further widening the rural-urban healthcare gap.7,8 Furthermore, this strain on an overwhelmed health system in the most vulnerable areas restricts the ability to respond to healthcare crises like the coronavirus disease 2019 pandemic.9
Providing adequate staff for currently available hospital beds is also a problem in rural areas. Studies demonstrating improved outcomes, decreased length of stay (LOS), and increased quality with hospitalist services have resulted in a high demand for hospitalists nationwide.10-12 Recruiting hospitalists to work in rural areas, however, has become increasingly challenging due to low-patient volumes, financial viability of hospitalist-model adoption, and provider shortages.13,14 Recently, the Veterans Health Administration (VHA) reported a 28% nationwide shortage of hospitalists,15 which disproportionally affects rural VHA hospitals. Staffing difficulties and reliance on intermittent providers were reported by more than 80% of rural and low-complexity VHA facilities.16
Telehospitalist services (THS) can help deliver high-quality care to rural residents locally, decrease travel expenses, support hospital volume, and increase healthcare capacity in response to a pandemic.14,17,18 Only a few studies have described THS (mostly with overnight or cross-coverage models directed to CAHs), and clinical outcomes have been inconsistently reported.17,19-21 Furthermore, no program has been conducted within an integrated health system akin to the VHA. The primary objective of this quality improvement (QI) initiative was to perform a mixed-methods evaluation of THS between VHA hospitals to compare clinical outcomes and patient and staff satisfaction. Secondary outcomes included description of the implementation process, unexpected challenges, and subsequent QI initiatives. These results will expand the knowledge on feasibility of THS and provide implementation guidance.
METHODS
A mixed-methods approach was used to evaluate outcomes of this QI project. Reporting follows the revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0).22
Context
The VHA is the largest integrated healthcare system in the United States, with more than 8 million veterans enrolled, more than 30% of whom reside in a rural area. The VHA comprises more than 1,000 outpatient clinics and 170 acute care VA Medical Centers,23,24 including more than 35 rural and low-complexity hospitals.25 Low-complexity hospitals are those with the lowest volume and levels of patient complexity and minimal or no teaching programs, research, intensive care unit (ICU) beds, and subspecialists. Lack of reimbursement and interstate licensing, often cited as barriers to telemedicine, do not apply to the VHA. The hub site was a large tertiary care (high-complexity) VHA hospital located in Iowa City, Iowa. The spoke site was a low-complexity (10-bed acute inpatient unit with no ICU) rural VA hospital located in Tomah, Wisconsin.
Study Population
The preimplementation cohort for comparison included all patients admitted between January 1, 2018, and January 6, 2019. The postimplementation study cohort included all observation and acute care admissions during the pilot phase (January 7 to May 3, 2019) and sustainability phase (July 15 to December 31, 2019). The postimplementation analysis excluded the time period of May 4 to July 14, 2019, due to an interruption (gap) in THS. The gap period allowed for preliminary data analysis, optimization of the telecommunication system, and the recruitment and training of additional providers who could provide long-term staffing to the service.
Intervention
Preimplementation
Prior to THS implementation, Tomah’s inpatient ward was staffed by one physician per shift, who could be a hospitalist, medical officer of the day (MOD), or an intermittent provider (locum tenens). Hospitalists covering the acute inpatient ward prior to the THS transitioned to cover weekends, nights, and urgent care service shifts.
We visited the spoke site and held information-sharing sessions with key stakeholders (administrators, clinician leaders, nurses, and ancillary staff) prior to kick-off. Recurrent phone meetings addressed anticipated and emerging challenges. Telehospitalist and local providers underwent technology and service training.
Technology and Connectivity
A low-cost technology system using tablet computers provided Health Insurance Portability and Accountability Act–compliant videoconferencing with a telehospitalist at the hub site. An Eko-Core digital stethoscope® with a web-based audio stream was available. Telehospitalists conducted encounters from a private office space with telehealth capabilities. A total of $9,000 was spent on equipment at both sites. Due to connectivity problems and data limits, the tablets were switched to mobile computer-on-wheels workstations and hospital-based Wi-Fi for the sustainability phase.
THS Description
An experienced hub hospitalist, together with an advanced practice provider (APP; nurse practitioner [NP] or physician assistant [PA]), cared for all patients admitted to the 10-bed inpatient unit at the spoke site, Monday through Friday from 8:00 AM to 4:30 PM. The APP had limited or no prior experience in acute inpatient medicine. The telehospitalist worked as a team with the APP. The APP was the main point of contact for nurses, performed physical examinations, and directed patient care to their level of comfort (in a similar manner as a teaching team). The telehospitalist conducted bedside patient rounds, participated in multidisciplinary huddles, and shared clinical documentation and administrative duties with the APP. The telehospitalist was the primary staff for admitted patients and had full access to the electronic health record (EHR). The THS was staffed by 10 hospitalists during the study period. Overnight and weekend cross-coverage and admissions were performed by MODs, who also covered the urgent care and cross-covered other nonmedical units.
Quantitative Evaluation Methods
Workload and Clinical Outcomes
An EHR query identified all patients admitted during the pre- and postimplementation periods. Demographic data, clinical Nosos risk scores,26,27 and top admission diagnoses were reported. Workload was evaluated using the average number of encounters per day and self-reported telehospitalist worksheets, which were cross-referenced with EHR data. Clinical outcomes included LOS, 30-day hospital readmission rate, 30-day standardized mortality (SMR30), in-hospital mortality, and VHA-specific inpatient quality metrics. Independent sample t tests for continuous variables and chi-square tests or Fisher’s exact test (for patient class) for categorical variables were used to compare pre- and postimplementation groups. Statistical process control (SPC) charts evaluated changes over time. All analyses were conducted using Microsoft Excel and R.28
Anonymous surveys were distributed to spoke-site inpatient and administrative staff at 1 month and 12 months postimplementation, assessing satisfaction, technology/connectivity, communication, and challenges (Appendix Figure 1). Satisfaction of the telehospitalist physicians at the hub site was measured 12 months postimplementation by a 26-question survey assessing the same domains, plus quality of care (Appendix Figure 2).
Patient Satisfaction
The VHA Survey of Healthcare Experiences of Patients (SHEP), a version of the Hospital Consumer Assessment of Healthcare Providers and Systems Survey,29,30 was mailed to all patients after discharge. Survey responses concerning inpatient provider care (eg, care coordination, communication, hospital rating, willingness to recommend the hospital) during the pre- and postimplementation phases were compared using a two-sample test of independent proportions. Responses obtained during May and June 2019 were excluded.
Qualitative Evaluation Methods
The authors had full access to, and took full responsibility for, the integrity of the data. The project was evaluated by the University of Iowa Institutional Review Board and the Iowa City VA Research and Development Committee and was determined to be a non–human-subjects QI project.
RESULTS
Quantitative Workload and Clinical Outcomes
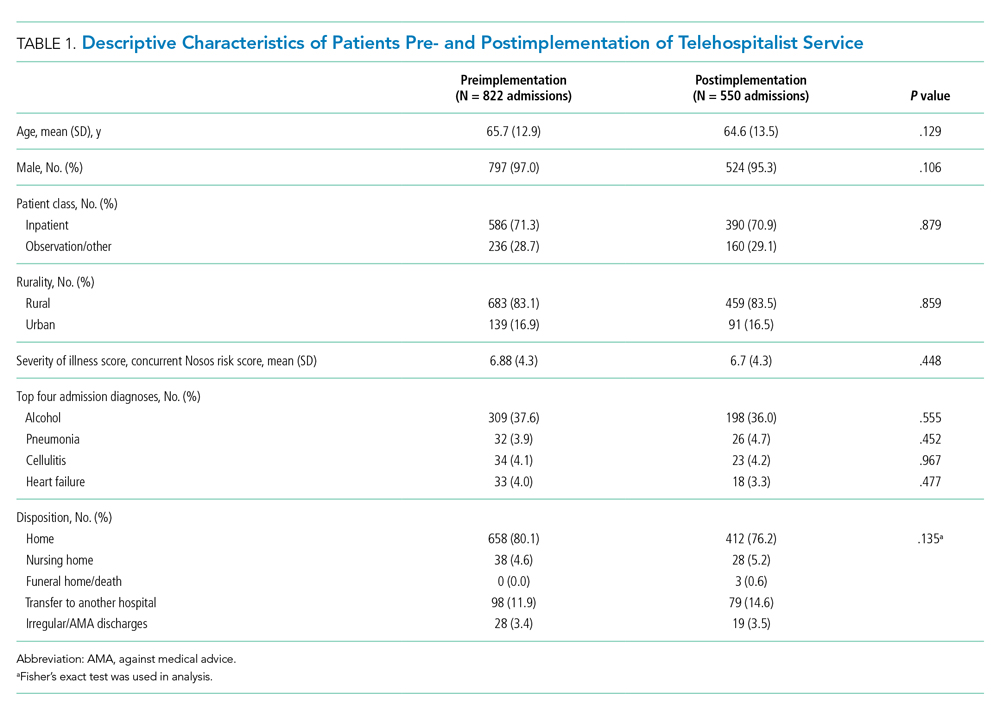
There were 822 admissions during the preimplementation period and 550 admissions during the postimplementation period (253 during the pilot and 297 during sustainability phase). Patient characteristics pre- and postimplementation were not significantly different (Table 1). The median patient age was 65 years; 96% of patients were male, and 83% were rural residents. The most common admission diagnosis was alcohol-related (36%); regarding patient disposition, 78% of admissions were discharged home.
Workload
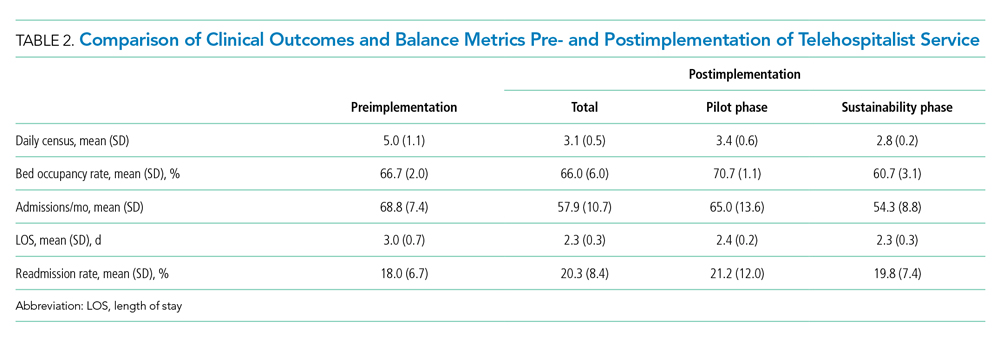
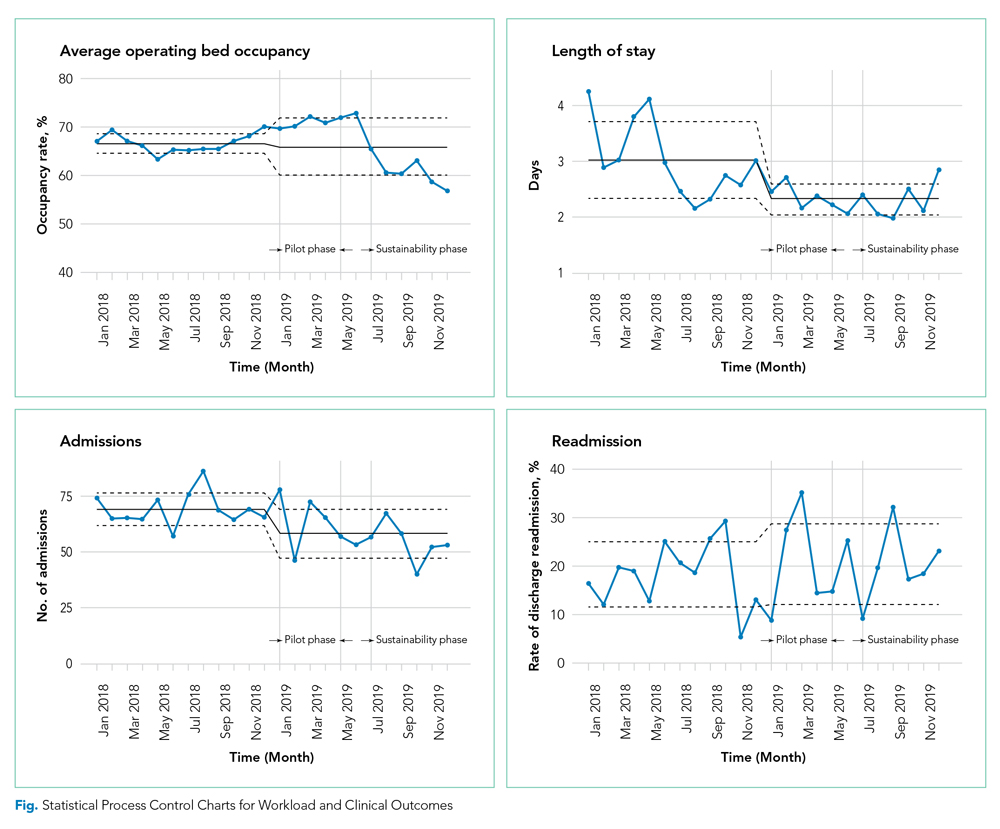
There were 502 patient encounters staffed by the telehospitalist in the pilot phase, with an average of 6.25 encounters per day, and a telehospitalist-reported workload of 7 hours per day. There were 538 patient encounters, with an average of 4.67 encounters per day and a workload of 5.6 hours per day in the sustainability phase. The average daily census decreased from 5.0 (SD, 1.1) patients per day during preimplementation to 3.1 (SD, 0.5) patients per day during postimplementation (Table 2). In some of the months during the study period, admissions decreased below the lower SPC limit, suggesting a significant change (Figure). Adjusted LOS was significantly lower, with 3.0 (SD, 0.7) days vs 2.3 (SD, 0.3) days in the pre- and postimplementation periods, respectively. Bed occupancy rates were significantly lower in the sustainability phase compared with the pilot phase and the preimplementation period. Readmission rates varied, ranging from <10% to >30%, not significantly different but slightly higher in the postimplementation period. Readmission rates for heart failure, chronic obstructive pulmonary disease, and pneumonia remained unchanged; other medical readmissions (mostly alcohol-related) were slightly higher in the postimplementation period.
In-hospital mortality and SMR30 did not change significantly, but there was improvement in the 12-month rolling average of the observed/expected SMR30 from 1.40 to 1.08. Additional VHA-specific quality metrics were monitored and showed either small improvements or no change (data not shown).
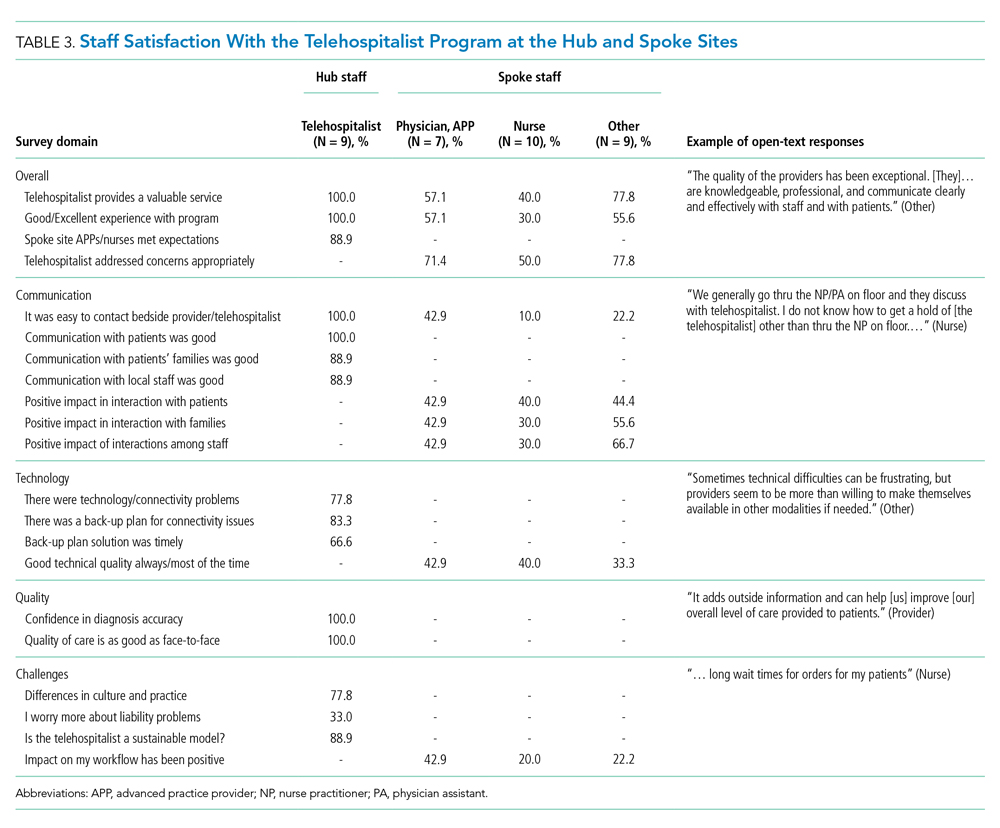
Satisfaction at Hub and Spoke Sites
After sending two reminder communications via email, the telehospitalist satisfaction survey had a total response rate of 90% (9/10). Telehospitalists were satisfied or very satisfied (89%) with the program and the local providers (88.9%), rating their experience as good or excellent (100%) (Table 3). Communication with patients, families, and local staff was noted as being “positive” or “mostly positive.” Telehospitalists reported confidence in the accuracy of their diagnoses and rated the quality of care as being equal to that of a face-to-face encounter. Connectivity problems were prevalent, although most providers were able to resort to a back-up plan. Other challenges included differences in culture and concerns about liability. We received 27 responses from the spoke-site satisfaction survey; the response rate could not be determined because the survey was distributed by the spoke site for anonymity. Of the respondents, 37% identified as nurses, 25.9% as healthcare providers (APPs or physicians), and 33.3% as other staff (eg, social worker, nutritionist, physical therapist, utilization management, administrators); 3.7% did not respond. Among the participants, 88% had personally interacted with the THS. Most providers and other staff perceived THS as valuable (57.1% and 77.8%, respectively) and were satisfied or highly satisfied with THS (57.1% and 55.6%, respectively). On average, nurses provided lower ratings across all survey items than providers and other staff. Challenges noted by all staff included issues with communication, workflow, and technology/connectivity.
Qualitative Strengths
Our process evaluation identified high quality of care and teamwork as contributors to the success of the program. Overall, staff credited perceived improvements in quality of care to the quality of providers staffing the THS, including the local APPs. Noting the telehospitalists’ knowledge base and level of engagement as key attributes, one staff member commented: “I prefer a telehospitalist that really care[s] about patients than some provider that is physically here but does not engage.” Staff perceived improvements in the continuity of care, as well as care processes such as handoffs and transitions of care.
Improvements in teamwork were perceived compared with the previous model of care. Telehospitalists were lauded for their professionalism and communication skills. Overall, nurses felt providers in the THS listened more to their views. In addition, nurse respondents felt they could learn from several providers and said they enjoyed the telehospitalists’ disposition to teach and discuss patient care. The responsiveness of the THS staff was instrumental in building teamwork and acceptance. A bedside interdisciplinary protocol was established for appropriate patients. Local staff felt this was crucial for teamwork and patient satisfaction. Telehospitalists reported high-value in interdisciplinary rounds, facilitating interaction with nurses and ancillary staff. Handoff problems were identified, leading to QI initiatives to mitigate those issues.
Challenges
The survey identified administrative barriers, technical difficulties, workflow constraints, and clinical concerns. The credentialing process was complicated, delaying the onboarding of telehospitalists. Internet connectivity was inconsistent, leading to disruption in video communications; however, during the sustainability phase, updated technology improved communications. The communication workflow was resisted by some nurses, who wanted to phone the telehospitalist directly rather than having the local APP as the first contact. Secure messaging was enabled to allow nurses direct contact during the sustainability phase.
Workload was a concern among telehospitalists and local staff. Telehospitalists perceived the documentation requirements and administrative workload to be two to three times higher than at other hospitals—despite the lower number of encounters. Finally, clinical concerns from spoke-site clinicians included a perceived rise in the acuity of patients (which was not evident by the Nosos score) and delayed decisions to transfer-out patients. These concerns were addressed with educational sessions for telehospitalists during the sustainability phase.
Additional Quality Improvement Projects
The implementation of THS resulted in QI initiatives at the spoke site, including an EHR-integrated handoff tool; a documentation evaluation that led to the elimination of duplicative, inefficient, and error-prone templates; and a revision of the alcohol withdrawal treatment protocol during the sustainability phase to reduce the use of intravenous benzodiazepines. A more comprehensive benzodiazepine-sparing alcohol withdrawal treatment protocol was also developed but was not implemented until after the study period (January 2020).
DISCUSSION
Our pre-post study evaluation found implementation of a THS to be noninferior to face-to-face care, with no significant change in mortality, readmission rate, or patient satisfaction. The significant improvement observed in LOS is consistent with the adoption of hospitalist models in other medical care settings,11 but had not been reported by previous telehospitalist studies. For example, in their retrospective chart review comparing an NP-supported telehospitalist model to locum tenens hospitalists, Boltz et al found no difference in LOS.31 Moreover, as in our study, they found no differences in readmissions, mortality, and patient satisfaction.31 Similarly, Kuperman et al reported unchanged daily census, LOS, and transfer rates from a CAH with their virtual hospitalist program, but a decrease in the percentage of patients transferred-out from the emergency department, suggesting that more patients were treated locally.19
Reduction in LOS is one of the primary measures of efficiency in hospital care31; reducing LOS while maintaining the quality of care lowers hospital costs. The reduction in LOS in our study could be attributed to greater continuity of care, engagement/experience of the telehospitalists, or other factors. This decrease in LOS and slight reduction in admissions resulted in an overall lower daily census during the study period and impacted efficiency. Our study was unable to determine the cause for the reduction in admissions; however, several concurrent events, including the expansion of community-care options for veterans under the MISSION ACT (Maintaining Internal Systems and Strengthening Integrated Outside Networks Act) in June 2019, a nationwide smoking ban at VA facilities (October 2019), and a modification in the alcohol withdrawal treatment protocol might have influenced veterans’ choice of hospital.
Readmission rates were slightly higher, though nonsignificant, in the postimplementation period. Alcohol-related readmissions accounted for most readmissions; some of the protocol changes, such as admitting all patients with alcohol withdrawal to inpatient class instead of admitting some to the observation class, accounted for part of the increase in readmission rates. Readmission rates for other conditions such as chronic obstructive pulmonary disease, chronic heart failure, or pneumonia were not significantly different, suggesting that the reduction in LOS did not result in an unintended increased readmission rate for those conditions.
Rural hospitals are struggling with staffing and finances. Resorting to locum tenens staffing is costly and can result in variable quality of care.32,33 APPs are increasingly taking on hospitalist positions, with 65% of adult hospitalist programs, including half of all VHA hospitals, employing NPs and PAs.34,35 In response to this expanded scope of practice, hospitals employing APPs in hospitalist roles must comply with state and federal laws, which often require that APPs be supervised by or work in collaboration with an on-site or off-site physician. The THS is a great model to support APPs and address staffing and cost challenges in low-volume rural facilities, while maintaining quality of care. Some APP-telehospitalist programs similar to ours have reported cost reductions of up 58% compared to programs that employ locum tenens physicians.31 In our model, we assume that a single telehospitalist hub could provide coverage to two or three spoke sites with APP support, reducing staffing costs.
Hub telehospitalists reported satisfaction with the program, and they perceived the quality of care to be comparable to face-to-face encounters; their responses were consistent with those previously reported in an evaluation of telemedicine acute care by JaKa et al.
This study has several limitations. First, the VHA is an integrated health system, one that serves an older, predominantly male patient population. Also, the lack of reimbursement and interstate licensing restrictions limit generalizability of these results to other CAHs or healthcare systems. Furthermore, the intervention was limited to a single rural site; while this allowed for a detailed evaluation, unique barriers or facilitators might exist that limit its applicability. In addition, QI initiatives implemented by the VHA during the project period might have confounded some of our results. Last, patient satisfaction survey data are overall limited in their ability to fully assess patient’s experience and satisfaction with the program. Further qualitative studies are needed to gain deeper insight into patient perspectives with the THS and whether modality of care delivery influences patients’ care decisions. Future studies should consider a multisite design with one or more hubs and multiple spoke sites.
CONCLUSION
Telehospitalist services are a feasible and safe approach to provide inpatient services and address staffing needs of rural hospitals. To enhance program performance, it is essential to ensure adequate technological quality, clearly delineate and define roles and responsibilities of the care team, and address communication issues or staff concerns in a timely manner.
Acknowledgments
The authors thank the staff, administration, and leadership at the Tomah and Iowa City VA Medical Centers for working with us on this project. They offer special thanks to Kevin Glenn, MD, MS, Ethan Kuperman, MD, MS, FHM, and Jennifer Chapin, MSN, RN, for sharing their expertise, and the telehealth team, including Nathaniel Samuelson, Angela McDowell, and Katrin Metcalf.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
1. O’Connor A, Wellenius G. Rural-urban disparities in the prevalence of diabetes and coronary heart disease. Public Health. 2012;126(10):813-820. https://doi.org/10.1016/j.puhe.2012.05.029
2. Kaufman BG, Thomas SR, Randolph RK, et al. The rising rate of rural hospital closures. J Rural Health. 2016;32(1):35-43. https://doi.org/10.1111/jrh.12128
3. MacDowell M, Glasser M, Fitts M, Nielsen K, Hunsaker M. A national view of rural health workforce issues in the USA. Rural Remote Health. 2010;10(3):1531.
4. Joynt KE, Harris Y, Orav EJ, Jha AK. Quality of care and patient outcomes in critical access rural hospitals. JAMA. 2011;306(1):45-52. https://doi.org/10.1001/jama.2011.902
5. The Chartis Group. Chartis Center for Rural Health. The Rural Health Safety Net Under Pressure: Rural Hospital Vulnerability. Published February 2020. Accessed May 07, 2020. https://www.chartis.com/forum/wp-content/uploads/2020/02/CCRH_Vulnerability-Research_FiNAL-02.14.20.pdf
6. Miller KEM, James HJ, Holmes GM, Van Houtven CH. The effect of rural hospital closures on emergency medical service response and transport times. Health Serv Res. 2020;55(2):288-300. https://doi.org/10.1111/1475-6773.13254
7. Buchmueller TC, Jacobson M, Wold C. How far to the hospital? The effect of hospital closures on access to care. J Health Econ. 2006;25(4):740-761. https://doi.org/10.1016/j.jhealeco.2005.10.006
8. Mohr NM, Harland KK, Shane DM, et al. Rural patients with severe sepsis or septic shock who bypass rural hospitals have increased mortality: an instrumental variables approach. Crit Care Med. 2017;45(1):85-93. https://doi.org/10.1097/ccm.0000000000002026
9. Gutierrez J, Kuperman E, Kaboli PJ. Using telehealth as a tool for rural hospitals in the COVID-19 pandemic response. J Rural Health. 2020;10.1111/jrh.12443. https://doi.org/10.1111/jrh.12443
10. Kisuule F, Howell EE. Hospitalists and their impact on quality, patient safety, and satisfaction. Obstet Gynecol Clin North Am. 2015;42(3):433-446. https://doi.org/10.1016/j.ogc.2015.05.003
11. Peterson MC. A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84(3):248-254. https://doi.org/10.4065/84.3.248
12. Auerbach AD, Wachter RM, Katz P, Showstack J, Baron RB, Goldman L. Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes. Ann Intern Med. 2002;137(11):859-865. https://doi.org/10.7326/0003-4819-137-11-200212030-00006
13. Casey MM, Hung P, Moscovice I, Prasad S. The use of hospitalists by small rural hospitals: results of a national survey. Med Care Res Rev. 2014;71(4):356-366. https://doi.org/10.1177/1077558714533822
14. Sanders RB, Simpson KN, Kazley AS, Giarrizzi DP. New hospital telemedicine services: potential market for a nighttime telehospitalist service. Telemed J E Health. 2014;20(10):902-908. https://doi.org/10.1089/tmj.2013.0344
15. Department of Veterans Affairs. Office of Inspector General. OIG Determination of Veterans Health Administration’s Occupational Staffing Shortages. Published September 30, 2019. Accessed June 15, 2020. https://www.va.gov/oig/pubs/VAOIG-19-00346-241.pdf
16. Gutierrez J, Moeckli J, McAdams N, Kaboli PJ. Perceptions of telehospitalist services to address staffing needs in rural and low complexity hospitals in the Veterans Health Administration. J Rural Health. 2019;36(3):355-359. https://doi.org/10.1111/jrh.12403
17. Eagle Telemedicine. EAGLE TELEMEDICINE NIGHT COVERAGE SOLUTIONS: Why They Work for Hospitals and Physicians. Accessed May 28, 2018. http://www.eagletelemedicine.com/wp-content/uploads/2016/11/EHP_WP_Telenocturnist_FINAL.pdf
18. Gujral J, Antoine C, Chandra S. The role of telehospitalist in COVID-19 response: Hospitalist caring remotely for New York patients explain their role. ACP Hospitalist. 2020; May 2020.
19. Kuperman EF, Linson EL, Klefstad K, Perry E, Glenn K. The virtual hospitalist: a single-site implementation bringing hospitalist coverage to critical access hospitals. J Hosp Med. 2018;13(11):759-763. https://doi.org/10.12788/jhm.3061
20. JaKa MM, Dinh JM, Ziegenfuss JY, et al. Patient and care team perspectives of telemedicine in critical access hospitals. J Hosp Med. 2020;15(6):345-348. https://doi.org/10.12788/jhm.3412
21. Khanal S, Burgon J, Leonard S, Griffiths M, Eddowes LA. Recommendations for the improved effectiveness and reporting of telemedicine programs in developing countries: results of a systematic literature review. Telemed J E Health. 2015;21(11):903-915. https://doi.org/10.1089/tmj.2014.0194
22. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
23. VHA Office of Rural Health. ORH 2020-2024 STRATEGIC PLAN. In: U.S. Department of Veterans Affairs, ed 2020. Accessed January 18, 2021 https://www.ruralhealth.va.gov/aboutus/index.asp
24. Veterans Health Administration. About VHA. In: U.S. Department of Veterans Affairs, ed. 2019. Accessed January 18, 2021.https://www.va.gov/health/aboutvha.asp
25. GeoSpatial Outcomes Division. VHA Office of Rural Health. U.S. Department of Veterans Affairs. Rural Veterans Health Care Atlas. 2nd ed - FY-2015. Accessed July 30, 2020. https://www.ruralhealth.va.gov/docs/atlas/CHAPTER_02_RHRI_Pts_treated_at_VAMCs.pdf
26. Wagner TH, Upadhyay A, Cowgill E, et al. Risk adjustment tools for learning health systems: a comparison of DxCG and CMS-HCC V21. Health Serv Res. 2016;51(5):2002-2019. https://doi.org/10.1111/1475-6773.12454
27. Wagner T, Stefos T, Moran E, et al. Technical Report 30: Risk Adjustment: Guide to the V21 and Nosos Risk Score Programs. Updated February 8, 2016. Accessed July 30, 2020. https://www.herc.research.va.gov/include/page.asp?id=technical-report-risk-adjustment
28. The R Foundation. The R Project for Statistical Computing. Accessed August 10, 2020. https://www.R-project.org/
29. Cleary PD, Meterko M, Wright SM, Zaslavsky AM. Are comparisons of patient experiences across hospitals fair? A study in Veterans Health Administration hospitals. Med Care. 2014;52(7):619-625. https://doi.org/10.1097/mlr.0000000000000144
30. Giordano LA, Elliott MN, Goldstein E, Lehrman WG, Spencer PA. Development, implementation, and public reporting of the HCAHPS survey. Med Care Res Rev. 2010;67(1):27-37. doi:10.1177/1077558709341065
31. Boltz M, Cuellar NG, Cole C, Pistorese B. Comparing an on-site nurse practitioner with telemedicine physician support hospitalist programme with a traditional physician hospitalist programme. J Telemed and Telecare. 2019;25(4):213-220. https://doi.org/10.1177%2F1357633X18758744
32. Quinn R. The pros and cons of locum tenens for hospitalists. The Hospitalist. 2012(12). Accessed May 29, 2018. https://www.the-hospitalist.org/hospitalist/article/124988/pros-and-cons-locum-tenens-hospitalists
33. Blumenthal DM, Olenski AR, Tsugawa Y, Jena AB. Association between treatment by locum tenens internal medicine physicians and 30-day mortality among hospitalized Medicare beneficiaries. JAMA. 2017;318(21):2119-2129. https://doi.org/10.1001/jama.2017.17925
34. Butcher L. Nurses as hospitalists | AHA Trustee Services. American Hospital Association. Accessed July 14, 2020 https://trustees.aha.org/articles/1238-nurses-as-hospitalists
35. Kartha A, Restuccia JD, Burgess JF, Jr, et al. Nurse practitioner and physician assistant scope of practice in 118 acute care hospitals. J Hosp Med. 2014;9(10):615-620. https://doi.org/10.1002/jhm.2231
Healthcare in rural areas faces increasing challenges due to community hospital closures, physician shortages, and a more concentrated population of older adults with higher rates of comorbid conditions than their urban counterparts.1-3 Critical access hospitals (CAHs), which primarily serve rural areas, have fewer clinical capabilities, worse process-of-care measures, and higher mortality rates for some conditions when compared to non-CAHs.4 As such, CAHs are closing at record numbers across the United States,5 resulting in loss of available hospital beds and patient access to timely emergency services,6 which can worsen outcomes, further widening the rural-urban healthcare gap.7,8 Furthermore, this strain on an overwhelmed health system in the most vulnerable areas restricts the ability to respond to healthcare crises like the coronavirus disease 2019 pandemic.9
Providing adequate staff for currently available hospital beds is also a problem in rural areas. Studies demonstrating improved outcomes, decreased length of stay (LOS), and increased quality with hospitalist services have resulted in a high demand for hospitalists nationwide.10-12 Recruiting hospitalists to work in rural areas, however, has become increasingly challenging due to low-patient volumes, financial viability of hospitalist-model adoption, and provider shortages.13,14 Recently, the Veterans Health Administration (VHA) reported a 28% nationwide shortage of hospitalists,15 which disproportionally affects rural VHA hospitals. Staffing difficulties and reliance on intermittent providers were reported by more than 80% of rural and low-complexity VHA facilities.16
Telehospitalist services (THS) can help deliver high-quality care to rural residents locally, decrease travel expenses, support hospital volume, and increase healthcare capacity in response to a pandemic.14,17,18 Only a few studies have described THS (mostly with overnight or cross-coverage models directed to CAHs), and clinical outcomes have been inconsistently reported.17,19-21 Furthermore, no program has been conducted within an integrated health system akin to the VHA. The primary objective of this quality improvement (QI) initiative was to perform a mixed-methods evaluation of THS between VHA hospitals to compare clinical outcomes and patient and staff satisfaction. Secondary outcomes included description of the implementation process, unexpected challenges, and subsequent QI initiatives. These results will expand the knowledge on feasibility of THS and provide implementation guidance.
METHODS
A mixed-methods approach was used to evaluate outcomes of this QI project. Reporting follows the revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0).22
Context
The VHA is the largest integrated healthcare system in the United States, with more than 8 million veterans enrolled, more than 30% of whom reside in a rural area. The VHA comprises more than 1,000 outpatient clinics and 170 acute care VA Medical Centers,23,24 including more than 35 rural and low-complexity hospitals.25 Low-complexity hospitals are those with the lowest volume and levels of patient complexity and minimal or no teaching programs, research, intensive care unit (ICU) beds, and subspecialists. Lack of reimbursement and interstate licensing, often cited as barriers to telemedicine, do not apply to the VHA. The hub site was a large tertiary care (high-complexity) VHA hospital located in Iowa City, Iowa. The spoke site was a low-complexity (10-bed acute inpatient unit with no ICU) rural VA hospital located in Tomah, Wisconsin.
Study Population
The preimplementation cohort for comparison included all patients admitted between January 1, 2018, and January 6, 2019. The postimplementation study cohort included all observation and acute care admissions during the pilot phase (January 7 to May 3, 2019) and sustainability phase (July 15 to December 31, 2019). The postimplementation analysis excluded the time period of May 4 to July 14, 2019, due to an interruption (gap) in THS. The gap period allowed for preliminary data analysis, optimization of the telecommunication system, and the recruitment and training of additional providers who could provide long-term staffing to the service.
Intervention
Preimplementation
Prior to THS implementation, Tomah’s inpatient ward was staffed by one physician per shift, who could be a hospitalist, medical officer of the day (MOD), or an intermittent provider (locum tenens). Hospitalists covering the acute inpatient ward prior to the THS transitioned to cover weekends, nights, and urgent care service shifts.
We visited the spoke site and held information-sharing sessions with key stakeholders (administrators, clinician leaders, nurses, and ancillary staff) prior to kick-off. Recurrent phone meetings addressed anticipated and emerging challenges. Telehospitalist and local providers underwent technology and service training.
Technology and Connectivity
A low-cost technology system using tablet computers provided Health Insurance Portability and Accountability Act–compliant videoconferencing with a telehospitalist at the hub site. An Eko-Core digital stethoscope® with a web-based audio stream was available. Telehospitalists conducted encounters from a private office space with telehealth capabilities. A total of $9,000 was spent on equipment at both sites. Due to connectivity problems and data limits, the tablets were switched to mobile computer-on-wheels workstations and hospital-based Wi-Fi for the sustainability phase.
THS Description
An experienced hub hospitalist, together with an advanced practice provider (APP; nurse practitioner [NP] or physician assistant [PA]), cared for all patients admitted to the 10-bed inpatient unit at the spoke site, Monday through Friday from 8:00 AM to 4:30 PM. The APP had limited or no prior experience in acute inpatient medicine. The telehospitalist worked as a team with the APP. The APP was the main point of contact for nurses, performed physical examinations, and directed patient care to their level of comfort (in a similar manner as a teaching team). The telehospitalist conducted bedside patient rounds, participated in multidisciplinary huddles, and shared clinical documentation and administrative duties with the APP. The telehospitalist was the primary staff for admitted patients and had full access to the electronic health record (EHR). The THS was staffed by 10 hospitalists during the study period. Overnight and weekend cross-coverage and admissions were performed by MODs, who also covered the urgent care and cross-covered other nonmedical units.
Quantitative Evaluation Methods
Workload and Clinical Outcomes
An EHR query identified all patients admitted during the pre- and postimplementation periods. Demographic data, clinical Nosos risk scores,26,27 and top admission diagnoses were reported. Workload was evaluated using the average number of encounters per day and self-reported telehospitalist worksheets, which were cross-referenced with EHR data. Clinical outcomes included LOS, 30-day hospital readmission rate, 30-day standardized mortality (SMR30), in-hospital mortality, and VHA-specific inpatient quality metrics. Independent sample t tests for continuous variables and chi-square tests or Fisher’s exact test (for patient class) for categorical variables were used to compare pre- and postimplementation groups. Statistical process control (SPC) charts evaluated changes over time. All analyses were conducted using Microsoft Excel and R.28
Anonymous surveys were distributed to spoke-site inpatient and administrative staff at 1 month and 12 months postimplementation, assessing satisfaction, technology/connectivity, communication, and challenges (Appendix Figure 1). Satisfaction of the telehospitalist physicians at the hub site was measured 12 months postimplementation by a 26-question survey assessing the same domains, plus quality of care (Appendix Figure 2).
Patient Satisfaction
The VHA Survey of Healthcare Experiences of Patients (SHEP), a version of the Hospital Consumer Assessment of Healthcare Providers and Systems Survey,29,30 was mailed to all patients after discharge. Survey responses concerning inpatient provider care (eg, care coordination, communication, hospital rating, willingness to recommend the hospital) during the pre- and postimplementation phases were compared using a two-sample test of independent proportions. Responses obtained during May and June 2019 were excluded.
Qualitative Evaluation Methods
The authors had full access to, and took full responsibility for, the integrity of the data. The project was evaluated by the University of Iowa Institutional Review Board and the Iowa City VA Research and Development Committee and was determined to be a non–human-subjects QI project.
RESULTS
Quantitative Workload and Clinical Outcomes
There were 822 admissions during the preimplementation period and 550 admissions during the postimplementation period (253 during the pilot and 297 during sustainability phase). Patient characteristics pre- and postimplementation were not significantly different (Table 1). The median patient age was 65 years; 96% of patients were male, and 83% were rural residents. The most common admission diagnosis was alcohol-related (36%); regarding patient disposition, 78% of admissions were discharged home.

Workload
There were 502 patient encounters staffed by the telehospitalist in the pilot phase, with an average of 6.25 encounters per day, and a telehospitalist-reported workload of 7 hours per day. There were 538 patient encounters, with an average of 4.67 encounters per day and a workload of 5.6 hours per day in the sustainability phase. The average daily census decreased from 5.0 (SD, 1.1) patients per day during preimplementation to 3.1 (SD, 0.5) patients per day during postimplementation (Table 2). In some of the months during the study period, admissions decreased below the lower SPC limit, suggesting a significant change (Figure). Adjusted LOS was significantly lower, with 3.0 (SD, 0.7) days vs 2.3 (SD, 0.3) days in the pre- and postimplementation periods, respectively. Bed occupancy rates were significantly lower in the sustainability phase compared with the pilot phase and the preimplementation period. Readmission rates varied, ranging from <10% to >30%, not significantly different but slightly higher in the postimplementation period. Readmission rates for heart failure, chronic obstructive pulmonary disease, and pneumonia remained unchanged; other medical readmissions (mostly alcohol-related) were slightly higher in the postimplementation period.

In-hospital mortality and SMR30 did not change significantly, but there was improvement in the 12-month rolling average of the observed/expected SMR30 from 1.40 to 1.08. Additional VHA-specific quality metrics were monitored and showed either small improvements or no change (data not shown).

Satisfaction at Hub and Spoke Sites
After sending two reminder communications via email, the telehospitalist satisfaction survey had a total response rate of 90% (9/10). Telehospitalists were satisfied or very satisfied (89%) with the program and the local providers (88.9%), rating their experience as good or excellent (100%) (Table 3). Communication with patients, families, and local staff was noted as being “positive” or “mostly positive.” Telehospitalists reported confidence in the accuracy of their diagnoses and rated the quality of care as being equal to that of a face-to-face encounter. Connectivity problems were prevalent, although most providers were able to resort to a back-up plan. Other challenges included differences in culture and concerns about liability. We received 27 responses from the spoke-site satisfaction survey; the response rate could not be determined because the survey was distributed by the spoke site for anonymity. Of the respondents, 37% identified as nurses, 25.9% as healthcare providers (APPs or physicians), and 33.3% as other staff (eg, social worker, nutritionist, physical therapist, utilization management, administrators); 3.7% did not respond. Among the participants, 88% had personally interacted with the THS. Most providers and other staff perceived THS as valuable (57.1% and 77.8%, respectively) and were satisfied or highly satisfied with THS (57.1% and 55.6%, respectively). On average, nurses provided lower ratings across all survey items than providers and other staff. Challenges noted by all staff included issues with communication, workflow, and technology/connectivity.

Qualitative Strengths
Our process evaluation identified high quality of care and teamwork as contributors to the success of the program. Overall, staff credited perceived improvements in quality of care to the quality of providers staffing the THS, including the local APPs. Noting the telehospitalists’ knowledge base and level of engagement as key attributes, one staff member commented: “I prefer a telehospitalist that really care[s] about patients than some provider that is physically here but does not engage.” Staff perceived improvements in the continuity of care, as well as care processes such as handoffs and transitions of care.
Improvements in teamwork were perceived compared with the previous model of care. Telehospitalists were lauded for their professionalism and communication skills. Overall, nurses felt providers in the THS listened more to their views. In addition, nurse respondents felt they could learn from several providers and said they enjoyed the telehospitalists’ disposition to teach and discuss patient care. The responsiveness of the THS staff was instrumental in building teamwork and acceptance. A bedside interdisciplinary protocol was established for appropriate patients. Local staff felt this was crucial for teamwork and patient satisfaction. Telehospitalists reported high-value in interdisciplinary rounds, facilitating interaction with nurses and ancillary staff. Handoff problems were identified, leading to QI initiatives to mitigate those issues.
Challenges
The survey identified administrative barriers, technical difficulties, workflow constraints, and clinical concerns. The credentialing process was complicated, delaying the onboarding of telehospitalists. Internet connectivity was inconsistent, leading to disruption in video communications; however, during the sustainability phase, updated technology improved communications. The communication workflow was resisted by some nurses, who wanted to phone the telehospitalist directly rather than having the local APP as the first contact. Secure messaging was enabled to allow nurses direct contact during the sustainability phase.
Workload was a concern among telehospitalists and local staff. Telehospitalists perceived the documentation requirements and administrative workload to be two to three times higher than at other hospitals—despite the lower number of encounters. Finally, clinical concerns from spoke-site clinicians included a perceived rise in the acuity of patients (which was not evident by the Nosos score) and delayed decisions to transfer-out patients. These concerns were addressed with educational sessions for telehospitalists during the sustainability phase.
Additional Quality Improvement Projects
The implementation of THS resulted in QI initiatives at the spoke site, including an EHR-integrated handoff tool; a documentation evaluation that led to the elimination of duplicative, inefficient, and error-prone templates; and a revision of the alcohol withdrawal treatment protocol during the sustainability phase to reduce the use of intravenous benzodiazepines. A more comprehensive benzodiazepine-sparing alcohol withdrawal treatment protocol was also developed but was not implemented until after the study period (January 2020).
DISCUSSION
Our pre-post study evaluation found implementation of a THS to be noninferior to face-to-face care, with no significant change in mortality, readmission rate, or patient satisfaction. The significant improvement observed in LOS is consistent with the adoption of hospitalist models in other medical care settings,11 but had not been reported by previous telehospitalist studies. For example, in their retrospective chart review comparing an NP-supported telehospitalist model to locum tenens hospitalists, Boltz et al found no difference in LOS.31 Moreover, as in our study, they found no differences in readmissions, mortality, and patient satisfaction.31 Similarly, Kuperman et al reported unchanged daily census, LOS, and transfer rates from a CAH with their virtual hospitalist program, but a decrease in the percentage of patients transferred-out from the emergency department, suggesting that more patients were treated locally.19
Reduction in LOS is one of the primary measures of efficiency in hospital care31; reducing LOS while maintaining the quality of care lowers hospital costs. The reduction in LOS in our study could be attributed to greater continuity of care, engagement/experience of the telehospitalists, or other factors. This decrease in LOS and slight reduction in admissions resulted in an overall lower daily census during the study period and impacted efficiency. Our study was unable to determine the cause for the reduction in admissions; however, several concurrent events, including the expansion of community-care options for veterans under the MISSION ACT (Maintaining Internal Systems and Strengthening Integrated Outside Networks Act) in June 2019, a nationwide smoking ban at VA facilities (October 2019), and a modification in the alcohol withdrawal treatment protocol might have influenced veterans’ choice of hospital.
Readmission rates were slightly higher, though nonsignificant, in the postimplementation period. Alcohol-related readmissions accounted for most readmissions; some of the protocol changes, such as admitting all patients with alcohol withdrawal to inpatient class instead of admitting some to the observation class, accounted for part of the increase in readmission rates. Readmission rates for other conditions such as chronic obstructive pulmonary disease, chronic heart failure, or pneumonia were not significantly different, suggesting that the reduction in LOS did not result in an unintended increased readmission rate for those conditions.
Rural hospitals are struggling with staffing and finances. Resorting to locum tenens staffing is costly and can result in variable quality of care.32,33 APPs are increasingly taking on hospitalist positions, with 65% of adult hospitalist programs, including half of all VHA hospitals, employing NPs and PAs.34,35 In response to this expanded scope of practice, hospitals employing APPs in hospitalist roles must comply with state and federal laws, which often require that APPs be supervised by or work in collaboration with an on-site or off-site physician. The THS is a great model to support APPs and address staffing and cost challenges in low-volume rural facilities, while maintaining quality of care. Some APP-telehospitalist programs similar to ours have reported cost reductions of up 58% compared to programs that employ locum tenens physicians.31 In our model, we assume that a single telehospitalist hub could provide coverage to two or three spoke sites with APP support, reducing staffing costs.
Hub telehospitalists reported satisfaction with the program, and they perceived the quality of care to be comparable to face-to-face encounters; their responses were consistent with those previously reported in an evaluation of telemedicine acute care by JaKa et al.
This study has several limitations. First, the VHA is an integrated health system, one that serves an older, predominantly male patient population. Also, the lack of reimbursement and interstate licensing restrictions limit generalizability of these results to other CAHs or healthcare systems. Furthermore, the intervention was limited to a single rural site; while this allowed for a detailed evaluation, unique barriers or facilitators might exist that limit its applicability. In addition, QI initiatives implemented by the VHA during the project period might have confounded some of our results. Last, patient satisfaction survey data are overall limited in their ability to fully assess patient’s experience and satisfaction with the program. Further qualitative studies are needed to gain deeper insight into patient perspectives with the THS and whether modality of care delivery influences patients’ care decisions. Future studies should consider a multisite design with one or more hubs and multiple spoke sites.
CONCLUSION
Telehospitalist services are a feasible and safe approach to provide inpatient services and address staffing needs of rural hospitals. To enhance program performance, it is essential to ensure adequate technological quality, clearly delineate and define roles and responsibilities of the care team, and address communication issues or staff concerns in a timely manner.
Acknowledgments
The authors thank the staff, administration, and leadership at the Tomah and Iowa City VA Medical Centers for working with us on this project. They offer special thanks to Kevin Glenn, MD, MS, Ethan Kuperman, MD, MS, FHM, and Jennifer Chapin, MSN, RN, for sharing their expertise, and the telehealth team, including Nathaniel Samuelson, Angela McDowell, and Katrin Metcalf.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Healthcare in rural areas faces increasing challenges due to community hospital closures, physician shortages, and a more concentrated population of older adults with higher rates of comorbid conditions than their urban counterparts.1-3 Critical access hospitals (CAHs), which primarily serve rural areas, have fewer clinical capabilities, worse process-of-care measures, and higher mortality rates for some conditions when compared to non-CAHs.4 As such, CAHs are closing at record numbers across the United States,5 resulting in loss of available hospital beds and patient access to timely emergency services,6 which can worsen outcomes, further widening the rural-urban healthcare gap.7,8 Furthermore, this strain on an overwhelmed health system in the most vulnerable areas restricts the ability to respond to healthcare crises like the coronavirus disease 2019 pandemic.9
Providing adequate staff for currently available hospital beds is also a problem in rural areas. Studies demonstrating improved outcomes, decreased length of stay (LOS), and increased quality with hospitalist services have resulted in a high demand for hospitalists nationwide.10-12 Recruiting hospitalists to work in rural areas, however, has become increasingly challenging due to low-patient volumes, financial viability of hospitalist-model adoption, and provider shortages.13,14 Recently, the Veterans Health Administration (VHA) reported a 28% nationwide shortage of hospitalists,15 which disproportionally affects rural VHA hospitals. Staffing difficulties and reliance on intermittent providers were reported by more than 80% of rural and low-complexity VHA facilities.16
Telehospitalist services (THS) can help deliver high-quality care to rural residents locally, decrease travel expenses, support hospital volume, and increase healthcare capacity in response to a pandemic.14,17,18 Only a few studies have described THS (mostly with overnight or cross-coverage models directed to CAHs), and clinical outcomes have been inconsistently reported.17,19-21 Furthermore, no program has been conducted within an integrated health system akin to the VHA. The primary objective of this quality improvement (QI) initiative was to perform a mixed-methods evaluation of THS between VHA hospitals to compare clinical outcomes and patient and staff satisfaction. Secondary outcomes included description of the implementation process, unexpected challenges, and subsequent QI initiatives. These results will expand the knowledge on feasibility of THS and provide implementation guidance.
METHODS
A mixed-methods approach was used to evaluate outcomes of this QI project. Reporting follows the revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0).22
Context
The VHA is the largest integrated healthcare system in the United States, with more than 8 million veterans enrolled, more than 30% of whom reside in a rural area. The VHA comprises more than 1,000 outpatient clinics and 170 acute care VA Medical Centers,23,24 including more than 35 rural and low-complexity hospitals.25 Low-complexity hospitals are those with the lowest volume and levels of patient complexity and minimal or no teaching programs, research, intensive care unit (ICU) beds, and subspecialists. Lack of reimbursement and interstate licensing, often cited as barriers to telemedicine, do not apply to the VHA. The hub site was a large tertiary care (high-complexity) VHA hospital located in Iowa City, Iowa. The spoke site was a low-complexity (10-bed acute inpatient unit with no ICU) rural VA hospital located in Tomah, Wisconsin.
Study Population
The preimplementation cohort for comparison included all patients admitted between January 1, 2018, and January 6, 2019. The postimplementation study cohort included all observation and acute care admissions during the pilot phase (January 7 to May 3, 2019) and sustainability phase (July 15 to December 31, 2019). The postimplementation analysis excluded the time period of May 4 to July 14, 2019, due to an interruption (gap) in THS. The gap period allowed for preliminary data analysis, optimization of the telecommunication system, and the recruitment and training of additional providers who could provide long-term staffing to the service.
Intervention
Preimplementation
Prior to THS implementation, Tomah’s inpatient ward was staffed by one physician per shift, who could be a hospitalist, medical officer of the day (MOD), or an intermittent provider (locum tenens). Hospitalists covering the acute inpatient ward prior to the THS transitioned to cover weekends, nights, and urgent care service shifts.
We visited the spoke site and held information-sharing sessions with key stakeholders (administrators, clinician leaders, nurses, and ancillary staff) prior to kick-off. Recurrent phone meetings addressed anticipated and emerging challenges. Telehospitalist and local providers underwent technology and service training.
Technology and Connectivity
A low-cost technology system using tablet computers provided Health Insurance Portability and Accountability Act–compliant videoconferencing with a telehospitalist at the hub site. An Eko-Core digital stethoscope® with a web-based audio stream was available. Telehospitalists conducted encounters from a private office space with telehealth capabilities. A total of $9,000 was spent on equipment at both sites. Due to connectivity problems and data limits, the tablets were switched to mobile computer-on-wheels workstations and hospital-based Wi-Fi for the sustainability phase.
THS Description
An experienced hub hospitalist, together with an advanced practice provider (APP; nurse practitioner [NP] or physician assistant [PA]), cared for all patients admitted to the 10-bed inpatient unit at the spoke site, Monday through Friday from 8:00 AM to 4:30 PM. The APP had limited or no prior experience in acute inpatient medicine. The telehospitalist worked as a team with the APP. The APP was the main point of contact for nurses, performed physical examinations, and directed patient care to their level of comfort (in a similar manner as a teaching team). The telehospitalist conducted bedside patient rounds, participated in multidisciplinary huddles, and shared clinical documentation and administrative duties with the APP. The telehospitalist was the primary staff for admitted patients and had full access to the electronic health record (EHR). The THS was staffed by 10 hospitalists during the study period. Overnight and weekend cross-coverage and admissions were performed by MODs, who also covered the urgent care and cross-covered other nonmedical units.
Quantitative Evaluation Methods
Workload and Clinical Outcomes
An EHR query identified all patients admitted during the pre- and postimplementation periods. Demographic data, clinical Nosos risk scores,26,27 and top admission diagnoses were reported. Workload was evaluated using the average number of encounters per day and self-reported telehospitalist worksheets, which were cross-referenced with EHR data. Clinical outcomes included LOS, 30-day hospital readmission rate, 30-day standardized mortality (SMR30), in-hospital mortality, and VHA-specific inpatient quality metrics. Independent sample t tests for continuous variables and chi-square tests or Fisher’s exact test (for patient class) for categorical variables were used to compare pre- and postimplementation groups. Statistical process control (SPC) charts evaluated changes over time. All analyses were conducted using Microsoft Excel and R.28
Anonymous surveys were distributed to spoke-site inpatient and administrative staff at 1 month and 12 months postimplementation, assessing satisfaction, technology/connectivity, communication, and challenges (Appendix Figure 1). Satisfaction of the telehospitalist physicians at the hub site was measured 12 months postimplementation by a 26-question survey assessing the same domains, plus quality of care (Appendix Figure 2).
Patient Satisfaction
The VHA Survey of Healthcare Experiences of Patients (SHEP), a version of the Hospital Consumer Assessment of Healthcare Providers and Systems Survey,29,30 was mailed to all patients after discharge. Survey responses concerning inpatient provider care (eg, care coordination, communication, hospital rating, willingness to recommend the hospital) during the pre- and postimplementation phases were compared using a two-sample test of independent proportions. Responses obtained during May and June 2019 were excluded.
Qualitative Evaluation Methods
The authors had full access to, and took full responsibility for, the integrity of the data. The project was evaluated by the University of Iowa Institutional Review Board and the Iowa City VA Research and Development Committee and was determined to be a non–human-subjects QI project.
RESULTS
Quantitative Workload and Clinical Outcomes
There were 822 admissions during the preimplementation period and 550 admissions during the postimplementation period (253 during the pilot and 297 during sustainability phase). Patient characteristics pre- and postimplementation were not significantly different (Table 1). The median patient age was 65 years; 96% of patients were male, and 83% were rural residents. The most common admission diagnosis was alcohol-related (36%); regarding patient disposition, 78% of admissions were discharged home.

Workload
There were 502 patient encounters staffed by the telehospitalist in the pilot phase, with an average of 6.25 encounters per day, and a telehospitalist-reported workload of 7 hours per day. There were 538 patient encounters, with an average of 4.67 encounters per day and a workload of 5.6 hours per day in the sustainability phase. The average daily census decreased from 5.0 (SD, 1.1) patients per day during preimplementation to 3.1 (SD, 0.5) patients per day during postimplementation (Table 2). In some of the months during the study period, admissions decreased below the lower SPC limit, suggesting a significant change (Figure). Adjusted LOS was significantly lower, with 3.0 (SD, 0.7) days vs 2.3 (SD, 0.3) days in the pre- and postimplementation periods, respectively. Bed occupancy rates were significantly lower in the sustainability phase compared with the pilot phase and the preimplementation period. Readmission rates varied, ranging from <10% to >30%, not significantly different but slightly higher in the postimplementation period. Readmission rates for heart failure, chronic obstructive pulmonary disease, and pneumonia remained unchanged; other medical readmissions (mostly alcohol-related) were slightly higher in the postimplementation period.

In-hospital mortality and SMR30 did not change significantly, but there was improvement in the 12-month rolling average of the observed/expected SMR30 from 1.40 to 1.08. Additional VHA-specific quality metrics were monitored and showed either small improvements or no change (data not shown).

Satisfaction at Hub and Spoke Sites
After sending two reminder communications via email, the telehospitalist satisfaction survey had a total response rate of 90% (9/10). Telehospitalists were satisfied or very satisfied (89%) with the program and the local providers (88.9%), rating their experience as good or excellent (100%) (Table 3). Communication with patients, families, and local staff was noted as being “positive” or “mostly positive.” Telehospitalists reported confidence in the accuracy of their diagnoses and rated the quality of care as being equal to that of a face-to-face encounter. Connectivity problems were prevalent, although most providers were able to resort to a back-up plan. Other challenges included differences in culture and concerns about liability. We received 27 responses from the spoke-site satisfaction survey; the response rate could not be determined because the survey was distributed by the spoke site for anonymity. Of the respondents, 37% identified as nurses, 25.9% as healthcare providers (APPs or physicians), and 33.3% as other staff (eg, social worker, nutritionist, physical therapist, utilization management, administrators); 3.7% did not respond. Among the participants, 88% had personally interacted with the THS. Most providers and other staff perceived THS as valuable (57.1% and 77.8%, respectively) and were satisfied or highly satisfied with THS (57.1% and 55.6%, respectively). On average, nurses provided lower ratings across all survey items than providers and other staff. Challenges noted by all staff included issues with communication, workflow, and technology/connectivity.

Qualitative Strengths
Our process evaluation identified high quality of care and teamwork as contributors to the success of the program. Overall, staff credited perceived improvements in quality of care to the quality of providers staffing the THS, including the local APPs. Noting the telehospitalists’ knowledge base and level of engagement as key attributes, one staff member commented: “I prefer a telehospitalist that really care[s] about patients than some provider that is physically here but does not engage.” Staff perceived improvements in the continuity of care, as well as care processes such as handoffs and transitions of care.
Improvements in teamwork were perceived compared with the previous model of care. Telehospitalists were lauded for their professionalism and communication skills. Overall, nurses felt providers in the THS listened more to their views. In addition, nurse respondents felt they could learn from several providers and said they enjoyed the telehospitalists’ disposition to teach and discuss patient care. The responsiveness of the THS staff was instrumental in building teamwork and acceptance. A bedside interdisciplinary protocol was established for appropriate patients. Local staff felt this was crucial for teamwork and patient satisfaction. Telehospitalists reported high-value in interdisciplinary rounds, facilitating interaction with nurses and ancillary staff. Handoff problems were identified, leading to QI initiatives to mitigate those issues.
Challenges
The survey identified administrative barriers, technical difficulties, workflow constraints, and clinical concerns. The credentialing process was complicated, delaying the onboarding of telehospitalists. Internet connectivity was inconsistent, leading to disruption in video communications; however, during the sustainability phase, updated technology improved communications. The communication workflow was resisted by some nurses, who wanted to phone the telehospitalist directly rather than having the local APP as the first contact. Secure messaging was enabled to allow nurses direct contact during the sustainability phase.
Workload was a concern among telehospitalists and local staff. Telehospitalists perceived the documentation requirements and administrative workload to be two to three times higher than at other hospitals—despite the lower number of encounters. Finally, clinical concerns from spoke-site clinicians included a perceived rise in the acuity of patients (which was not evident by the Nosos score) and delayed decisions to transfer-out patients. These concerns were addressed with educational sessions for telehospitalists during the sustainability phase.
Additional Quality Improvement Projects
The implementation of THS resulted in QI initiatives at the spoke site, including an EHR-integrated handoff tool; a documentation evaluation that led to the elimination of duplicative, inefficient, and error-prone templates; and a revision of the alcohol withdrawal treatment protocol during the sustainability phase to reduce the use of intravenous benzodiazepines. A more comprehensive benzodiazepine-sparing alcohol withdrawal treatment protocol was also developed but was not implemented until after the study period (January 2020).
DISCUSSION
Our pre-post study evaluation found implementation of a THS to be noninferior to face-to-face care, with no significant change in mortality, readmission rate, or patient satisfaction. The significant improvement observed in LOS is consistent with the adoption of hospitalist models in other medical care settings,11 but had not been reported by previous telehospitalist studies. For example, in their retrospective chart review comparing an NP-supported telehospitalist model to locum tenens hospitalists, Boltz et al found no difference in LOS.31 Moreover, as in our study, they found no differences in readmissions, mortality, and patient satisfaction.31 Similarly, Kuperman et al reported unchanged daily census, LOS, and transfer rates from a CAH with their virtual hospitalist program, but a decrease in the percentage of patients transferred-out from the emergency department, suggesting that more patients were treated locally.19
Reduction in LOS is one of the primary measures of efficiency in hospital care31; reducing LOS while maintaining the quality of care lowers hospital costs. The reduction in LOS in our study could be attributed to greater continuity of care, engagement/experience of the telehospitalists, or other factors. This decrease in LOS and slight reduction in admissions resulted in an overall lower daily census during the study period and impacted efficiency. Our study was unable to determine the cause for the reduction in admissions; however, several concurrent events, including the expansion of community-care options for veterans under the MISSION ACT (Maintaining Internal Systems and Strengthening Integrated Outside Networks Act) in June 2019, a nationwide smoking ban at VA facilities (October 2019), and a modification in the alcohol withdrawal treatment protocol might have influenced veterans’ choice of hospital.
Readmission rates were slightly higher, though nonsignificant, in the postimplementation period. Alcohol-related readmissions accounted for most readmissions; some of the protocol changes, such as admitting all patients with alcohol withdrawal to inpatient class instead of admitting some to the observation class, accounted for part of the increase in readmission rates. Readmission rates for other conditions such as chronic obstructive pulmonary disease, chronic heart failure, or pneumonia were not significantly different, suggesting that the reduction in LOS did not result in an unintended increased readmission rate for those conditions.
Rural hospitals are struggling with staffing and finances. Resorting to locum tenens staffing is costly and can result in variable quality of care.32,33 APPs are increasingly taking on hospitalist positions, with 65% of adult hospitalist programs, including half of all VHA hospitals, employing NPs and PAs.34,35 In response to this expanded scope of practice, hospitals employing APPs in hospitalist roles must comply with state and federal laws, which often require that APPs be supervised by or work in collaboration with an on-site or off-site physician. The THS is a great model to support APPs and address staffing and cost challenges in low-volume rural facilities, while maintaining quality of care. Some APP-telehospitalist programs similar to ours have reported cost reductions of up 58% compared to programs that employ locum tenens physicians.31 In our model, we assume that a single telehospitalist hub could provide coverage to two or three spoke sites with APP support, reducing staffing costs.
Hub telehospitalists reported satisfaction with the program, and they perceived the quality of care to be comparable to face-to-face encounters; their responses were consistent with those previously reported in an evaluation of telemedicine acute care by JaKa et al.
This study has several limitations. First, the VHA is an integrated health system, one that serves an older, predominantly male patient population. Also, the lack of reimbursement and interstate licensing restrictions limit generalizability of these results to other CAHs or healthcare systems. Furthermore, the intervention was limited to a single rural site; while this allowed for a detailed evaluation, unique barriers or facilitators might exist that limit its applicability. In addition, QI initiatives implemented by the VHA during the project period might have confounded some of our results. Last, patient satisfaction survey data are overall limited in their ability to fully assess patient’s experience and satisfaction with the program. Further qualitative studies are needed to gain deeper insight into patient perspectives with the THS and whether modality of care delivery influences patients’ care decisions. Future studies should consider a multisite design with one or more hubs and multiple spoke sites.
CONCLUSION
Telehospitalist services are a feasible and safe approach to provide inpatient services and address staffing needs of rural hospitals. To enhance program performance, it is essential to ensure adequate technological quality, clearly delineate and define roles and responsibilities of the care team, and address communication issues or staff concerns in a timely manner.
Acknowledgments
The authors thank the staff, administration, and leadership at the Tomah and Iowa City VA Medical Centers for working with us on this project. They offer special thanks to Kevin Glenn, MD, MS, Ethan Kuperman, MD, MS, FHM, and Jennifer Chapin, MSN, RN, for sharing their expertise, and the telehealth team, including Nathaniel Samuelson, Angela McDowell, and Katrin Metcalf.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
1. O’Connor A, Wellenius G. Rural-urban disparities in the prevalence of diabetes and coronary heart disease. Public Health. 2012;126(10):813-820. https://doi.org/10.1016/j.puhe.2012.05.029
2. Kaufman BG, Thomas SR, Randolph RK, et al. The rising rate of rural hospital closures. J Rural Health. 2016;32(1):35-43. https://doi.org/10.1111/jrh.12128
3. MacDowell M, Glasser M, Fitts M, Nielsen K, Hunsaker M. A national view of rural health workforce issues in the USA. Rural Remote Health. 2010;10(3):1531.
4. Joynt KE, Harris Y, Orav EJ, Jha AK. Quality of care and patient outcomes in critical access rural hospitals. JAMA. 2011;306(1):45-52. https://doi.org/10.1001/jama.2011.902
5. The Chartis Group. Chartis Center for Rural Health. The Rural Health Safety Net Under Pressure: Rural Hospital Vulnerability. Published February 2020. Accessed May 07, 2020. https://www.chartis.com/forum/wp-content/uploads/2020/02/CCRH_Vulnerability-Research_FiNAL-02.14.20.pdf
6. Miller KEM, James HJ, Holmes GM, Van Houtven CH. The effect of rural hospital closures on emergency medical service response and transport times. Health Serv Res. 2020;55(2):288-300. https://doi.org/10.1111/1475-6773.13254
7. Buchmueller TC, Jacobson M, Wold C. How far to the hospital? The effect of hospital closures on access to care. J Health Econ. 2006;25(4):740-761. https://doi.org/10.1016/j.jhealeco.2005.10.006
8. Mohr NM, Harland KK, Shane DM, et al. Rural patients with severe sepsis or septic shock who bypass rural hospitals have increased mortality: an instrumental variables approach. Crit Care Med. 2017;45(1):85-93. https://doi.org/10.1097/ccm.0000000000002026
9. Gutierrez J, Kuperman E, Kaboli PJ. Using telehealth as a tool for rural hospitals in the COVID-19 pandemic response. J Rural Health. 2020;10.1111/jrh.12443. https://doi.org/10.1111/jrh.12443
10. Kisuule F, Howell EE. Hospitalists and their impact on quality, patient safety, and satisfaction. Obstet Gynecol Clin North Am. 2015;42(3):433-446. https://doi.org/10.1016/j.ogc.2015.05.003
11. Peterson MC. A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84(3):248-254. https://doi.org/10.4065/84.3.248
12. Auerbach AD, Wachter RM, Katz P, Showstack J, Baron RB, Goldman L. Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes. Ann Intern Med. 2002;137(11):859-865. https://doi.org/10.7326/0003-4819-137-11-200212030-00006
13. Casey MM, Hung P, Moscovice I, Prasad S. The use of hospitalists by small rural hospitals: results of a national survey. Med Care Res Rev. 2014;71(4):356-366. https://doi.org/10.1177/1077558714533822
14. Sanders RB, Simpson KN, Kazley AS, Giarrizzi DP. New hospital telemedicine services: potential market for a nighttime telehospitalist service. Telemed J E Health. 2014;20(10):902-908. https://doi.org/10.1089/tmj.2013.0344
15. Department of Veterans Affairs. Office of Inspector General. OIG Determination of Veterans Health Administration’s Occupational Staffing Shortages. Published September 30, 2019. Accessed June 15, 2020. https://www.va.gov/oig/pubs/VAOIG-19-00346-241.pdf
16. Gutierrez J, Moeckli J, McAdams N, Kaboli PJ. Perceptions of telehospitalist services to address staffing needs in rural and low complexity hospitals in the Veterans Health Administration. J Rural Health. 2019;36(3):355-359. https://doi.org/10.1111/jrh.12403
17. Eagle Telemedicine. EAGLE TELEMEDICINE NIGHT COVERAGE SOLUTIONS: Why They Work for Hospitals and Physicians. Accessed May 28, 2018. http://www.eagletelemedicine.com/wp-content/uploads/2016/11/EHP_WP_Telenocturnist_FINAL.pdf
18. Gujral J, Antoine C, Chandra S. The role of telehospitalist in COVID-19 response: Hospitalist caring remotely for New York patients explain their role. ACP Hospitalist. 2020; May 2020.
19. Kuperman EF, Linson EL, Klefstad K, Perry E, Glenn K. The virtual hospitalist: a single-site implementation bringing hospitalist coverage to critical access hospitals. J Hosp Med. 2018;13(11):759-763. https://doi.org/10.12788/jhm.3061
20. JaKa MM, Dinh JM, Ziegenfuss JY, et al. Patient and care team perspectives of telemedicine in critical access hospitals. J Hosp Med. 2020;15(6):345-348. https://doi.org/10.12788/jhm.3412
21. Khanal S, Burgon J, Leonard S, Griffiths M, Eddowes LA. Recommendations for the improved effectiveness and reporting of telemedicine programs in developing countries: results of a systematic literature review. Telemed J E Health. 2015;21(11):903-915. https://doi.org/10.1089/tmj.2014.0194
22. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
23. VHA Office of Rural Health. ORH 2020-2024 STRATEGIC PLAN. In: U.S. Department of Veterans Affairs, ed 2020. Accessed January 18, 2021 https://www.ruralhealth.va.gov/aboutus/index.asp
24. Veterans Health Administration. About VHA. In: U.S. Department of Veterans Affairs, ed. 2019. Accessed January 18, 2021.https://www.va.gov/health/aboutvha.asp
25. GeoSpatial Outcomes Division. VHA Office of Rural Health. U.S. Department of Veterans Affairs. Rural Veterans Health Care Atlas. 2nd ed - FY-2015. Accessed July 30, 2020. https://www.ruralhealth.va.gov/docs/atlas/CHAPTER_02_RHRI_Pts_treated_at_VAMCs.pdf
26. Wagner TH, Upadhyay A, Cowgill E, et al. Risk adjustment tools for learning health systems: a comparison of DxCG and CMS-HCC V21. Health Serv Res. 2016;51(5):2002-2019. https://doi.org/10.1111/1475-6773.12454
27. Wagner T, Stefos T, Moran E, et al. Technical Report 30: Risk Adjustment: Guide to the V21 and Nosos Risk Score Programs. Updated February 8, 2016. Accessed July 30, 2020. https://www.herc.research.va.gov/include/page.asp?id=technical-report-risk-adjustment
28. The R Foundation. The R Project for Statistical Computing. Accessed August 10, 2020. https://www.R-project.org/
29. Cleary PD, Meterko M, Wright SM, Zaslavsky AM. Are comparisons of patient experiences across hospitals fair? A study in Veterans Health Administration hospitals. Med Care. 2014;52(7):619-625. https://doi.org/10.1097/mlr.0000000000000144
30. Giordano LA, Elliott MN, Goldstein E, Lehrman WG, Spencer PA. Development, implementation, and public reporting of the HCAHPS survey. Med Care Res Rev. 2010;67(1):27-37. doi:10.1177/1077558709341065
31. Boltz M, Cuellar NG, Cole C, Pistorese B. Comparing an on-site nurse practitioner with telemedicine physician support hospitalist programme with a traditional physician hospitalist programme. J Telemed and Telecare. 2019;25(4):213-220. https://doi.org/10.1177%2F1357633X18758744
32. Quinn R. The pros and cons of locum tenens for hospitalists. The Hospitalist. 2012(12). Accessed May 29, 2018. https://www.the-hospitalist.org/hospitalist/article/124988/pros-and-cons-locum-tenens-hospitalists
33. Blumenthal DM, Olenski AR, Tsugawa Y, Jena AB. Association between treatment by locum tenens internal medicine physicians and 30-day mortality among hospitalized Medicare beneficiaries. JAMA. 2017;318(21):2119-2129. https://doi.org/10.1001/jama.2017.17925
34. Butcher L. Nurses as hospitalists | AHA Trustee Services. American Hospital Association. Accessed July 14, 2020 https://trustees.aha.org/articles/1238-nurses-as-hospitalists
35. Kartha A, Restuccia JD, Burgess JF, Jr, et al. Nurse practitioner and physician assistant scope of practice in 118 acute care hospitals. J Hosp Med. 2014;9(10):615-620. https://doi.org/10.1002/jhm.2231
1. O’Connor A, Wellenius G. Rural-urban disparities in the prevalence of diabetes and coronary heart disease. Public Health. 2012;126(10):813-820. https://doi.org/10.1016/j.puhe.2012.05.029
2. Kaufman BG, Thomas SR, Randolph RK, et al. The rising rate of rural hospital closures. J Rural Health. 2016;32(1):35-43. https://doi.org/10.1111/jrh.12128
3. MacDowell M, Glasser M, Fitts M, Nielsen K, Hunsaker M. A national view of rural health workforce issues in the USA. Rural Remote Health. 2010;10(3):1531.
4. Joynt KE, Harris Y, Orav EJ, Jha AK. Quality of care and patient outcomes in critical access rural hospitals. JAMA. 2011;306(1):45-52. https://doi.org/10.1001/jama.2011.902
5. The Chartis Group. Chartis Center for Rural Health. The Rural Health Safety Net Under Pressure: Rural Hospital Vulnerability. Published February 2020. Accessed May 07, 2020. https://www.chartis.com/forum/wp-content/uploads/2020/02/CCRH_Vulnerability-Research_FiNAL-02.14.20.pdf
6. Miller KEM, James HJ, Holmes GM, Van Houtven CH. The effect of rural hospital closures on emergency medical service response and transport times. Health Serv Res. 2020;55(2):288-300. https://doi.org/10.1111/1475-6773.13254
7. Buchmueller TC, Jacobson M, Wold C. How far to the hospital? The effect of hospital closures on access to care. J Health Econ. 2006;25(4):740-761. https://doi.org/10.1016/j.jhealeco.2005.10.006
8. Mohr NM, Harland KK, Shane DM, et al. Rural patients with severe sepsis or septic shock who bypass rural hospitals have increased mortality: an instrumental variables approach. Crit Care Med. 2017;45(1):85-93. https://doi.org/10.1097/ccm.0000000000002026
9. Gutierrez J, Kuperman E, Kaboli PJ. Using telehealth as a tool for rural hospitals in the COVID-19 pandemic response. J Rural Health. 2020;10.1111/jrh.12443. https://doi.org/10.1111/jrh.12443
10. Kisuule F, Howell EE. Hospitalists and their impact on quality, patient safety, and satisfaction. Obstet Gynecol Clin North Am. 2015;42(3):433-446. https://doi.org/10.1016/j.ogc.2015.05.003
11. Peterson MC. A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84(3):248-254. https://doi.org/10.4065/84.3.248
12. Auerbach AD, Wachter RM, Katz P, Showstack J, Baron RB, Goldman L. Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes. Ann Intern Med. 2002;137(11):859-865. https://doi.org/10.7326/0003-4819-137-11-200212030-00006
13. Casey MM, Hung P, Moscovice I, Prasad S. The use of hospitalists by small rural hospitals: results of a national survey. Med Care Res Rev. 2014;71(4):356-366. https://doi.org/10.1177/1077558714533822
14. Sanders RB, Simpson KN, Kazley AS, Giarrizzi DP. New hospital telemedicine services: potential market for a nighttime telehospitalist service. Telemed J E Health. 2014;20(10):902-908. https://doi.org/10.1089/tmj.2013.0344
15. Department of Veterans Affairs. Office of Inspector General. OIG Determination of Veterans Health Administration’s Occupational Staffing Shortages. Published September 30, 2019. Accessed June 15, 2020. https://www.va.gov/oig/pubs/VAOIG-19-00346-241.pdf
16. Gutierrez J, Moeckli J, McAdams N, Kaboli PJ. Perceptions of telehospitalist services to address staffing needs in rural and low complexity hospitals in the Veterans Health Administration. J Rural Health. 2019;36(3):355-359. https://doi.org/10.1111/jrh.12403
17. Eagle Telemedicine. EAGLE TELEMEDICINE NIGHT COVERAGE SOLUTIONS: Why They Work for Hospitals and Physicians. Accessed May 28, 2018. http://www.eagletelemedicine.com/wp-content/uploads/2016/11/EHP_WP_Telenocturnist_FINAL.pdf
18. Gujral J, Antoine C, Chandra S. The role of telehospitalist in COVID-19 response: Hospitalist caring remotely for New York patients explain their role. ACP Hospitalist. 2020; May 2020.
19. Kuperman EF, Linson EL, Klefstad K, Perry E, Glenn K. The virtual hospitalist: a single-site implementation bringing hospitalist coverage to critical access hospitals. J Hosp Med. 2018;13(11):759-763. https://doi.org/10.12788/jhm.3061
20. JaKa MM, Dinh JM, Ziegenfuss JY, et al. Patient and care team perspectives of telemedicine in critical access hospitals. J Hosp Med. 2020;15(6):345-348. https://doi.org/10.12788/jhm.3412
21. Khanal S, Burgon J, Leonard S, Griffiths M, Eddowes LA. Recommendations for the improved effectiveness and reporting of telemedicine programs in developing countries: results of a systematic literature review. Telemed J E Health. 2015;21(11):903-915. https://doi.org/10.1089/tmj.2014.0194
22. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
23. VHA Office of Rural Health. ORH 2020-2024 STRATEGIC PLAN. In: U.S. Department of Veterans Affairs, ed 2020. Accessed January 18, 2021 https://www.ruralhealth.va.gov/aboutus/index.asp
24. Veterans Health Administration. About VHA. In: U.S. Department of Veterans Affairs, ed. 2019. Accessed January 18, 2021.https://www.va.gov/health/aboutvha.asp
25. GeoSpatial Outcomes Division. VHA Office of Rural Health. U.S. Department of Veterans Affairs. Rural Veterans Health Care Atlas. 2nd ed - FY-2015. Accessed July 30, 2020. https://www.ruralhealth.va.gov/docs/atlas/CHAPTER_02_RHRI_Pts_treated_at_VAMCs.pdf
26. Wagner TH, Upadhyay A, Cowgill E, et al. Risk adjustment tools for learning health systems: a comparison of DxCG and CMS-HCC V21. Health Serv Res. 2016;51(5):2002-2019. https://doi.org/10.1111/1475-6773.12454
27. Wagner T, Stefos T, Moran E, et al. Technical Report 30: Risk Adjustment: Guide to the V21 and Nosos Risk Score Programs. Updated February 8, 2016. Accessed July 30, 2020. https://www.herc.research.va.gov/include/page.asp?id=technical-report-risk-adjustment
28. The R Foundation. The R Project for Statistical Computing. Accessed August 10, 2020. https://www.R-project.org/
29. Cleary PD, Meterko M, Wright SM, Zaslavsky AM. Are comparisons of patient experiences across hospitals fair? A study in Veterans Health Administration hospitals. Med Care. 2014;52(7):619-625. https://doi.org/10.1097/mlr.0000000000000144
30. Giordano LA, Elliott MN, Goldstein E, Lehrman WG, Spencer PA. Development, implementation, and public reporting of the HCAHPS survey. Med Care Res Rev. 2010;67(1):27-37. doi:10.1177/1077558709341065
31. Boltz M, Cuellar NG, Cole C, Pistorese B. Comparing an on-site nurse practitioner with telemedicine physician support hospitalist programme with a traditional physician hospitalist programme. J Telemed and Telecare. 2019;25(4):213-220. https://doi.org/10.1177%2F1357633X18758744
32. Quinn R. The pros and cons of locum tenens for hospitalists. The Hospitalist. 2012(12). Accessed May 29, 2018. https://www.the-hospitalist.org/hospitalist/article/124988/pros-and-cons-locum-tenens-hospitalists
33. Blumenthal DM, Olenski AR, Tsugawa Y, Jena AB. Association between treatment by locum tenens internal medicine physicians and 30-day mortality among hospitalized Medicare beneficiaries. JAMA. 2017;318(21):2119-2129. https://doi.org/10.1001/jama.2017.17925
34. Butcher L. Nurses as hospitalists | AHA Trustee Services. American Hospital Association. Accessed July 14, 2020 https://trustees.aha.org/articles/1238-nurses-as-hospitalists
35. Kartha A, Restuccia JD, Burgess JF, Jr, et al. Nurse practitioner and physician assistant scope of practice in 118 acute care hospitals. J Hosp Med. 2014;9(10):615-620. https://doi.org/10.1002/jhm.2231
© 2021 Society of Hospital Medicine
UGIB vs. LGIB
Gastrointestinal bleeding (GIB) is a frequent reason for acute hospitalization, with estimated rates of hospitalization at 375 per 100,000 per year in the United States.1 GIB is not a specific disease but rather a diverse set of conditions that lead to the clinical manifestations associated with bleeding into the gastrointestinal tract. One of the most commonly used organizing frameworks in gastrointestinal bleeding is the differentiation between upper gastrointestinal bleeding (UGIB) and lower gastrointestinal bleeding (LGIB). There are important differences in the etiologies between the 2 sources. For example, acid‐related disease is a common etiology in UGIB but does not occur in LGIB. While some aspects of the acute management are shared between UGIB and LGIB, important differences exist in the management, including initial endoscopy and medication choice. There have been few direct comparisons of rates, resource use, and clinical outcomes between UGIB and LGIB.
Historically, rates of UGIB have been reported to exceed those of LGIB by 2‐fold to 8‐fold.25 Protocols, clinical practice guidelines, and policy decisions reflect this emphasis on UGIB.68 Among 9 guidelines hosted by National Guideline Clearinghouse addressing GIB, 6 were focused on UGIB, 2 on both UGIB and LGIB, and only 1 on LGIB.9 There are several reasons to believe that these relative incidence rates may not be accurate. First, recent advances in therapy and prevention of UGIB, such as the treatment of Helicobacter pylori infection; proton pump inhibitors (PPIs); and selective cyclooxygenase‐2 (COX‐2) inhibitors, may have affected the epidemiology of gastrointestinal bleeding.1016 Among these therapies, only COX‐2 inhibitors may also reduce the incidence of LGIB.14, 1618 Therefore, these advances may result in a disproportionate drop in UGIB relative to LGIB. In addition, known risk factors for both LGIB and UGIB, including advancing age and renal failure, are increasing in the general population.5, 19, 20 Finally, given the recent increased recommendations for aspirin therapy and systemic anticoagulation, exposure to aspirin and warfarin have increased, both risk factors for LGIB and UGIB.2124 Indeed, recent studies in the epidemiology of UGIB do suggest a changing pattern of etiologies of UGIB reflecting these advances.25 One study examining rates of both UGIB and LGIB demonstrate a decrease in hospitalizations overall for GIB driven by a reduction in UGIB while at the same time reporting an increase in the incidence of hospitalization for LGIB.1
In addition to a changing epidemiology, a second reason for a potential underestimation of LGIB incidence is one of methodology. There are well‐recognized limitations with using purely administrative data due to difficulties in accurately identifying patients with LGIB.26
Studies using large administrative databases may not accurately identify LGIB because of the poor sensitivity and specificity of International Classification of Diseases, Ninth revision, Clinical Modification (ICD‐9) codes for LGIB.5 While there are standard methods of identifying patients with UGIB using ICD‐9 codes,19 there is not an accepted standard for LGIB. Thus, estimates using only ICD‐9 codes may overidentify or underidentify patients with LGIB. Prior studies that have most accurately identified patients with LGIB used a 2‐step method to address this issue. The initial ICD‐9 identification included a high sensitivity/low specificity approach. These identified patient charts undergo chart review to confirm the presence of an LGIB.5 This method is labor intensive and cannot be done using administrative databases. No direct comparison of UGIB to LGIB among hospitalized patients using this 2‐step method has been done recently.
The current emphasis on UGIB as seen in the published guidelines could also be supported if patients with UGIB had greater resource utilization or worse clinical outcomes. Limited direct comparisons for these outcomes are available. However, 1 administrative database study reported similar mortality rates for UGIB (2.7%) and LGIB (2.9%) in 2006.1 No direct comparisons of other clinical outcomes or resource use outcomes are available. Therefore, the emphasis on UGIB in publications and guidelines is best supported by the incidence rates that are, as has already been discussed, problematic.
We conducted a retrospective cohort study to examine the incidences of UGIB and LGIB among patients admitted to an academic medical center over 2 years using methods designed to optimally identify patients with either UGIB or LGIB. Our study also examined differences in clinical outcomes and resource utilization between subjects with UGIB and LGIB to examine the relative severity of these 2 clinical entities. These results may be useful in determining the need to reconsider clinical approaches as well as protocols and guidelines among patients with gastrointestinal bleeding.
Patients and Methods
Patients
This retrospective cohort study evaluated all patients who were admitted with GIB to a large urban academic medical center from July 1, 2001 to June 30, 2003 and who consented to a larger study examining the effects of hospitalists on patient care. Subjects unable to provide consent due to death or lack of decisional capacity were consented via proxy. To identify patients with GIB, all patients were screened for a primary or secondary diagnosis of GIB using ICD 9 codes. These codes were selected for a very high sensitivity threshold to assure that all potential subjects with GIB were identified. All subjects identified using these codes underwent chart abstraction to determine if they met criteria for GIB. These inclusion criteria required documentation in any portion of the chart (including emergency department [ED] clinician documentation, admission note, nursing intake note, etc.) of signs or symptoms of GI hemorrhage upon admission, including: hematemesis, coffee ground emesis, gastrooccult‐positive emesis, melena, hematochezia, maroon stools, and hemoccult‐positive stools interpreted by the treating physician team as an acute GIB. Subjects identified using the ICD‐9 codes and confirmed to have an acute GIB by chart review were included in the study and underwent additional chart abstraction and administrative data analysis.
ICD‐9 codes for GIB included: esophageal varices with hemorrhage (456.0, 456.20), Mallory‐Weiss syndrome (530.7), gastric ulcer with hemorrhage (531.00531.61), duodenal ulcer with hemorrhage (532.00532.61), peptic ulcer, site unspecified, with hemorrhage (533.00533.61), gastrojejunal ulcer with hemorrhage (534.00534.61), gastritis with hemorrhage (535.61), angiodysplasia of stomach/duodenum with hemorrhage (537.83), hematemesis (578.0578.9), diverticular disease (562.00562.9), other disorders of the intestine (569.00569.9), congenital anomalies of the digestive system (751.00), proctocolitis (556.00), hemorrhoids (455.00455.6), nondysenteric colitis (006.2), noninfectious gastroenteritis and colitis (558.0558.9), salmonella gastroenteritis (003.3), malignant neoplasm of colon (153), familial adenomatous polyposis (211.3), and gastric varices (456.8).
Data
Trained research assistants performed chart abstraction with validation by the principal investigators (PIs) of the first 15 charts to ensure accuracy. Subsequently, research assistants consulted with PIs with any questions during abstracting with final decisions being made by PIs. Detailed chart abstraction collected admission medication lists as obtained by the admitting physician team, including the use of PPIs, histamine‐2 (H‐2) blockers, COX‐2 inhibitors, and medications known to increase the risk of GIB, such as nonselective NSAIDs (nsNSAIDs), aspirin, and other anticoagulants. Other clinical data including risk factors, comorbid illnesses, laboratory tests, and vital signs were also abstracted from subjects' charts.
The source (UGIB vs. LGIB) and etiology (peptic ulcer disease [PUD], varices, diverticula, etc.) of bleeding were assessed using endoscopic reports as the primary source. When no clear source was identified on endoscopy or no endoscopy was done, the abstracter would review all progress notes, discharge summaries, and other diagnostic test results such as angiography in order to identify the source of bleeding (UGIB vs. LGIB). Endoscopic reports that identified a patient as having a UGIB or LGIB but no confirmed etiology were classified as undetermined etiology unless review of the other clinical documentation provided a specific etiology.
Tachycardia was defined as pulse greater than 100 beats per minute. Orthostasis was defined by either a drop in systolic blood pressure of 20 mmHg or an increase in pulse of 10 beats per minute. Hospital administrative databases were utilized to obtain resource utilization (ie, length of stay [LOS], total cost of care, intensive care transfers), Charlson comorbidity index,27 30‐day readmission rate, and in‐hospital mortality. Hospital costs were determined using TSI cost accounting software (Transition Systems Incorporated [now Eclypsis Corporation], Boston, MA), a validated system to assess actual direct and indirect costs of care.
Statistical Analysis
Descriptive statistics (means and proportions) were calculated by location of GIB for all variables describing patient characteristics, clinical presentation, clinical outcomes, and resource utilization. Differences in age and Charlson comorbidity index by GIB location were evaluated using t tests. Differences in gender, race, and medication use were evaluated using chi‐squared tests of independence.
We fit generalized linear models to investigate differences by location of bleed for those variables measuring clinical outcomes (inpatient mortality, intensive care unit [ICU] transfer, emergency surgery, 30‐day readmission, change in hemoglobin) and those variables measuring resource outcomes (total cost, LOS, number of procedures, number of correct scopes, repeat scope indicator, incorrect scope indicator, number of red blood cell [RBC] transfusions). The repeat scope indicator was used to denote a repeat scope (either esophagogastroduodenoscopy [EGD] or colonoscopy) and the incorrect scope indicator was used to denote when the initial scope was negative and a follow‐up scope from the other direction was positive (negative EGD followed by positive colonoscopy or negative colonoscopy followed by positive EGD). For each variable we fit 2 regression models, the first model (unadjusted effect) only included location of bleed as a covariate. The second model (adjusted effect) included location of bleed, age, gender, race (black/not black) and Charlson comorbidity index as covariates. Binary outcomes were modeled using logistic regressions. For continuous variables, we determined the distribution and link of the outcome variable using residual diagnostics and by comparing the log likelihood and information criteria of competing models. All analyses were performed using STATA SE Version 9.0 (StataCorp, College Station, TX)
This study was approved by the University of Chicago Institutional Review Board.
Results
During the 2 years of observation, a total of 7741 subjects were admitted to the internal medicine service and enrolled in the hospitalist study. Of these, 1014 had a primary or secondary ICD‐9 code that may be consistent with UGIB or LGIB and underwent chart review to determine if they had an acute GIB. Out of 1014 subjects, 647 were determined not to have an acute GI hemorrhage and were excluded from the remaining analyses; 367 of the 1104 subjects identified by ICD‐9 codes were found to have a clinical presentation consistent with GIB and were included in this study. A total of 180 of these 367 had UGIB and 187 had LGIB. The mean age was 62.4 years, 56.7% were female, 82.6% were African American, 12.7% were Caucasian, and the mean Charlson index was 1.5. (Table 1) Among baseline characteristics, both gender and age were statistically associated with a difference in rates of upper vs. lower source bleeding, with LGIB patients more likely to be female (P = 0.01) and older (P < 0.001). Etiologies of UGIB include erosive disease, peptic ulcer disease, variceal bleeding, arteriovenous malformation, and malignancy. Etiologies of LGIB include: diverticulosis, colitis, arteriovenous malformation, cancer, ischemic colitis, polyp, hemorrhoidal bleed, ulcer, inflammatory bowel disease, other, and not determined (Table 2).
| Upper and Lower GI Bleeding (n = 367) | Upper GI Bleeding (n = 180) | Lower GI Bleeding (n = 187) | P Value | |
|---|---|---|---|---|
| ||||
| Age (years), mean (SD) | 62.4 (18.0) | 58.6 (18.2) | 66.0 (17.1) | <0.001 |
| Female gender (%) | 56.7 | 50.0 | 63.1 | 0.01 |
| Race (%) | ||||
| African American | 82.6 | 85.3 | 80.1 | 0.43 |
| White | 12.7 | 10.7 | 14.5 | |
| Other | 4.7 | 4.0 | 5.4 | |
| Charlson comorbidity index, mean (SD) | 1.5 (1.5) | 1.6 (1.6) | 1.4 (1.5) | 0.44 |
| Lower GI Bleed (n = 187) | Upper GI Bleed (n = 180) | ||||
|---|---|---|---|---|---|
| Etiology | Frequency | Percent of Total (%) | Etiology | Frequency | Percent of Total (%) |
| |||||
| Diverticulosis | 76 | 41 | Erosive disease | 86 | 48 |
| Not identified | 38 | 20 | Peptic ulcer | 51 | 28 |
| Colitis, NOS | 14 | 7 | Not identified | 26 | 14 |
| AVM | 13 | 7 | Mallory Weiss | 17 | 9 |
| Cancer | 11 | 6 | Varices | 8 | 4 |
| Ischemic colitis | 9 | 5 | AVMs | 5 | 3 |
| Polyp | 9 | 5 | Mass/cancer | 5 | 3 |
| Hemorrhoid | 8 | 4 | |||
| Ulcer | 5 | 3 | |||
| Other | 3 | 1 | |||
| IBD | 1 | <1 | |||
Baseline use of medications known to be associated with either increased or decreased risk of GIB was common. Approximately one‐third of subjects with both LGIB and UGIB used aspirin and 10% used warfarin. LGIB subjects were less likely to use an nsNSAID (P < 0.001), but more likely to use a proton pump inhibitor (PPI) (P = 0.06) (Table 3).
| Upper and Lower GI Bleeding (%) (n = 367) | Upper GI Bleeding (%) (n = 180) | Lower GI Bleeding (%) (n = 187) | P Value* | |
|---|---|---|---|---|
| ||||
| Aspirin | 34.9 | 31.8 | 37.4 | 0.28 |
| nsNSAID | 12.9 | 20.8 | 6.4 | < 0.001 |
| COX‐2 selective inhibitor | 8.2 | 6.5 | 9.6 | 0.29 |
| Warfarin | 10.9 | 8.4 | 12.8 | 0.19 |
| PPI | 24.3 | 19.5 | 28.3 | 0.06 |
| nsNSAID + PPI | 1.8 | 1.3 | 2.1 | 0.56 |
| COX‐2 + PPI | 2.9 | 1.3 | 4.3 | 0.11 |
Key initial clinical presentation findings included vital sign abnormalities and admission hemoglobin levels. While hypotension was not common (4.7%), resting tachycardia (37%) and orthostasis (16%) were seen frequently. Subjects with LGIB were significantly less likely than those with UGIB to present with orthostasis (8.8% vs. 21.0%, respectively; P = 0.006) and resting tachycardia (32.3% vs. 42.5%, respectively; P = 0.04). Subjects with LGIB had a higher admission hemoglobin than those with UGIB (10.7 vs. 9.7, respectively; P < 0.001) (Table 4).
| Clinical Finding | Upper and Lower GI Bleeding (n = 367) | Upper GI Bleeding (n = 180) | Lower GI Bleeding (n = 187) | P Value* |
|---|---|---|---|---|
| ||||
| Hypotension (%) | 4.7 | 5.7 | 3.8 | 0.39 |
| Resting tachycardia (%) | 37.3 | 42.5 | 32.3 | 0.04 |
| Orthostatic hypotension (%) | 16.2 | 21.0 | 8.8 | 0.006 |
| Admission hemoglobin (g/dL), mean (SD) | 10.2 (2.6) | 9.7 (2.7) | 10.7 (2.5) | <0.001 |
We also examined several clinical outcomes. When comparing LGIB to UGIB patients for these clinical outcomes using bivariate and multivariate statistics, there was no difference for in‐hospital mortality (1.1% vs. 1.1%), transfer to ICU (16.0% vs. 13.9%), 30‐day readmission (5.9% vs.7.8%), number of red blood cell (RBC) transfusions (2.7 vs. 2.4), or need for GI surgery (1.1% vs. 0.0%). The mean drop in hemoglobin was greater among subjects with LGIB compared to UGIB (1.9 g/dL vs. 1.5 g/dL, respectively) by both bivariate (P = 0.01) and multivariate (P = 0.003) analyses (Table 5).
| Upper GI Bleeding (n = 180) | Lower GI Bleeding (n = 187) | Bivariate P Value | Multivariate P Value | |
|---|---|---|---|---|
| ||||
| In‐hospital mortality (%)* | 1.1 | 1.1 | 0.97 | 0.74 |
| Transfer to ICU (%)* | 13.9 | 16.0 | 0.56 | 0.44 |
| Drop in hemoglobin (g/dL), mean (SD) | 1.5 (1.5) | 1.9 (1.6) | 0.01 | 0.003 |
| Packed RBC transfusions required (units), mean (SD)* | 2.4 (2.9) | 2.7 (3.7) | 0.36 | 0.33 |
| Surgery for GI bleeding (%) | 0.0% | 1.1 | ||
| 30‐day readmission rate (%)* | 7.8 | 5.9 | 0.49 | 0.45 |
Mean costs were $11,892 for LGIB and $14,301 for UGIB and median costs were $7,890 for LGIB and $9,548 for UGIB, but were not statistically different. LOS was also similar between subjects with LGIB (5.1 days) and UGIB (5.7 days). In bivariate and multivariate analyses, UGIB subjects had a similar mean number of endoscopic procedures (1.3) compared to LGIB subjects (1.2). Thirteen percent of subjects with UGIB required a second EGD while only 8% of subjects with LGIB required 2 colonoscopies. In addition, 29% of subjects with LGIB received an EGD while only 16% of subjects with an UGIB received a colonoscopy (P = 0.001) (Table 6).
| Upper GI Bleeding (n = 180) | Lower GI Bleeding (n = 187) | Bivariate P Value | Multivariate P Value | |
|---|---|---|---|---|
| ||||
| Cost ($), mean (SD)* | 14,301 (17,196) | 11,892 (13,100) | 0.13 | 0.21 |
| Cost ($), median | $9,548 | $7,890 | ||
| Length of stay (days), mean (SD)* | 5.7 (7.0) | 5.1 (5.3) | 0.37 | 0.72 |
| Number of endoscopies/ patient, mean (SD) | 1.3 (0.5) | 1.2 (0.9) | 0.18 | 0.20 |
Conclusions
This study represents one of the largest direct comparisons of LGIB to UGIB not based on administrative databases. The most striking finding was the nearly equal rates of LGIB and UGIB. There are 2 likely explanations for this surprising result. First, there may be methodological reasons that we identified a greater proportion of true LGIBs; our study used a highly sensitive search strategy of ICD‐9 coding with confirmatory chart abstraction to ensure that as many LGIB and UGIB cases would be identified as possible while also excluding cases not meeting accepted criteria for GIB. The second possibility is that there is an actual change in epidemiology of GIB. Known risk factors for LGIB are increasing such as advancing age, increased use of chronic aspirin therapy, and renal disease. At the same time, significant advances in the treatment and prevention of UGIB have been made. Recent studies have demonstrated similar trends in admissions for upper and lower GI complications, suggesting that there may be a changing epidemiology due primarily to reductions in upper GI complications.1, 16
Either explanation would have implications for the care of patients with GIB. Clinical decision‐making based on prior literature would support that in ambiguous clinical situations and initial evaluation for an UGIB is appropriate. Most risk stratification literature and clinical guidelines focus on UGIB. If rates of LGIB and UGIB are similar, then existing clinical decision protocols may need to be reevaluated to incorporate the higher likelihood of LGIB. This reevaluation would be less important if the clinical outcomes or resource utilization of UGIB was significantly greater than that for LGIB, but we did not find this was the case. Similarly, if the ability to distinguish between LGIB and UGIB were robust on clinical signs and symptoms, then a reevaluation would be less important. However, we found fairly similar numbers of patients initially receiving evaluation for UGIB then being evaluated for LGIB as we found patients initially receiving evaluation for LGIB then being evaluated for UGIB. This suggests the potential benefit of clinical decision protocols that could better distinguish between UGIB and LGIB and account for the potentially higher incidence of LGIB than previously thought.
In addition to affecting the attention paid to LGIB for acute management, a changed understanding of incidence could also affect the attention paid to prevention of LGIB. Of the recent nonendoscopic advances in the treatment and prevention of GIB, only the use of COX‐2s (when used in place of traditional nsNSAIDs) reduces the risk of both LGIB and UGIB;14, 1618 H .pylori treatment and PPIs only prevent UGIB. Therefore, if the clinical and financial burdens of LGIB are similar to those seen in UGIB, more attention may need to be focused on preventing LGIB.
Baseline medication use was notable primarily for the similarities between UGIB and LGIB. Agents known to affect the rates of GIB were common in both groups. Over one‐third of the population was using aspirin and 10% were taking warfarin. Over 20% of subjects were taking an nsNSAID or a COX‐2 inhibitor. Almost one‐quarter of subjects were taking a PPI, agents known to decrease rates of UGIB and potentially increase LGIB through the risk of C. difficile colitis. Notably, the only statistically significant difference in baseline medication use between subjects with UGIB and LGIB was the more than 3‐fold higher use of nsNSAIDs in patients with UGIB as compared to LGIB. While current guidelines are not clear and consistent about which populations of at‐risk patients should receive GI prophylaxis,2830 these results suggest that patients admitted with GIB are very likely to be taking medications which impact the risk of GIB.
In terms of disease severity, the clinical presentation at admission suggests a greater degree of hemodynamic instability among subjects with UGIB. Rates of orthostatic hypotension and resting tachycardia are higher in UGIB subjects, as well as having a lower mean hemoglobin levels at presentation. However, despite the more severe clinical presentation, clinical outcomes did not differ significantly between the 2 bleeding sources. Thus, the most relevant clinical outcomes suggest that the severity of both LGIB and UGIB are similar. This similarity again suggest that the clinical burden of LGIB is not significantly different than UGIB.
Our results concerning resource utilization demonstrate a similar pattern. While the point estimates for costs and LOS suggest that UGIB may be associated with higher resource utilization, these differences were not significant in either bivariate or multivariate analyses. Those subjects with UGIB did receive more total endoscopic procedures than subjects with LGIB. More interesting though was that 24% of all subjects received an endoscopy of the opposite site (LGIB with EGD and UGIB with colonoscopy). These results suggest that the site of bleeding is not clear in a significant proportion of patients who present with GIB. These additional endoscopies are associated with increased risk, costs, LOS, and discomfort to patients. Improving our ability to accurately predict the source (upper vs. lower) of bleeding would allow us to reduce the number of these excess endoscopies. Additionally, it is interesting that despite the almost universal use of endoscopies, 20% of LGIB and 14% of UGIB subjects could not have a specific etiology identified during endoscopy or subsequent workup.
There are some important limitations to this study. While the sample size is among the largest of its type involving chart abstraction, it may be underpowered to detect some differences. Additionally, our results are from a single urban academic medical center with a patient population that is predominantly African American, which may limit generalizability. This study required consent and therefore only examines a subset of patients admitted to the medical center with GIB, which could potentially introduce bias into the sample. However, it is not clear why there would be systematic differences in subjects who choose to consent vs. those who decide not to consent that would affect the results of this study in substantive ways.
Despite significant efforts at identifying all subjects with GIB admitted during this time period, there were potential methodological reasons that may have resulted in some cases being missed. Only subjects admitted to a medicine service were approached for consent. All subjects in this medical center with GIB are admitted to a medicine service. We captured all subjects who were initially admitted to a medicine service as well as those admitted initially to an ICU and then transferred to the floor at any point prior to discharge. It is possible, though, that a subject would be admitted to an ICU for GIB and die prior to being transferred to the floor. While it is the impression of the director of the ICU that this would be a very unusual event, as most of the patients would be discharged to the floor prior to death (personal communication), given the very low mortality rate seen in this study, small numbers of missed events could have a significant impact on the interpretation of in‐hospital mortality results. It is also important to note that this medical center did not have the ability to perform endoscopy prior to admission for patients with GIB at the time of the study; all patients who presented with GIB would have been admitted and identified for this study. Finally, we were unable to routinely identify the rationale for obtaining an endoscopic exam. We assumed that all endoscopic exams were done for the purpose of evaluating and/or treating the GIB for which the subject was admitted. It is possible that some subjects had additional endoscopies for other reasons, which would have led to our overestimating the rates of additional endoscopies for GIB.
This study highlights the similarities between LGIB and UGIB rather than the differences. There were few significant differences between the 2 bleeding sources in terms of incidence, clinical outcomes, and resource utilization. In fact, the study also suggests that determining the source of bleeding may not be clear, given the high rates of opposite site endoscopies. While this study did reveal several similarities between UGIB and LGIB, it also highlights the need to identify improved strategies to improve the sensitivity and specificity of identification of LGIB compared to UGIB, both for clinical purposes and for research. The value of such improved clinical algorithms have the potential to improve both the cost and outcomes of care, while better algorithms for separating UGIB and LGIB using administrative data might help produce more precise estimates of costs and clinical outcomes, and aid in the development of risk stratification models.
- ,.Hospitalizations for Gastrointestinal Bleeding in 1998 and 2006. HCUP Statistical Brief #65, December 2008.Rockville, MD:Agency for Healthcare Research and Quality.
- ,.Causes and outcome of upper and lower gastrointestinal bleeding: The Grady Hospital Experience.South Med J.1999;92(1):44–50.
- ,,, et al.Acute upper gastrointestinal haemorrhage in west of Scotland: case ascertainment study.BMJ.1997;315:510–540.
- ,.A cost‐effective approach to the patient with peptic ulcer bleeding.Surg Clin North Am.1996;76:83–103.
- .Epidemiology and outcome of patients hospitalized with acute lower gastrointestinal hemorrhage: a population based study.Am J Gastroenterol.1997;92:419–424.
- ,,.Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding.Ann Int Med.2003;139:843–857.
- ,.Incremental value of upper endoscopy for triage of patients with acute non‐variceal upper‐GI hemorrhage.Gastrointest Endosc2004;60:9–14.
- ,,, et al.Upper gastrointestinal hemorrhage clinical guideline‐determining the optimal hospital length of stay.Am J Med.1996;100:313–322.
- National Guideline Clearinghouse. Available at: http://www.guideline.gov. Accessed August2009.
- ,,, et al.Prevention of ulcer recurrence after eradication of Helicobacter pylori: a prospective long‐term follow‐up study.Gastroenterology.1997;113:1082–1086.
- ,,, et al.Treatment of Helicobacter pylori in patients with duodenal ulcer hemorrhage‐a long‐term randomized, controlled study.Am J Gastroenterol.2000;95:2225–2232.
- ,,, et al.Preventing recurrent upper gastrointestinal bleeding in patients with Helicobacter pylori infection who are taking low‐dose aspirin or naproxen.N Engl J Med.2001;344:967–973.
- ,,, et al.Lansoprazole for the prevention of recurrences of ulcer complications from long‐term low‐dose aspirin use.N Engl J Med.2002;346:2033–2038.
- ,,, et al.Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group.N Engl J Med.2000;343:1520–1528.
- ,,, et al.Gastrointestinal toxicity with celecoxib vs nonsteroidal anti‐inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long‐Term Arthritis Safety Study.JAMA.2000;284:1247–1255.
- ,,, et al.Time trends and clinical impact of upper and lower gastrointestinal complications. Digestive Disease Week National Meeting,2008. San Diego, CA, May 17–22.
- ,,, et al.Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo.Clin Gastroenterol Hepatol.2005;3:133–141.
- ,,, et al.Serious lower gastrointestinal clinical events with nonselective NSAID or Coxib use.Gastroenterology.2003;124:288–292.
- ,,, et al.Risk factors for upper gastrointestinal bleeding among end‐stage renal disease patients.Kidney Int.2003;64:1455–1461.
- ,,, et al.Risk factors for hospitalized bleeding among older patients.J Am Geriatr Soc.2001;49:126–133.
- Institute for Clinical Systems Improvement (ICSI). Preventive services in adults. Bloomington, MN: Institute for Clinical Systems Improvement (ICSI).2005. Available at http://www.isci.org/guidelines_and_more/guidelines_order_sets_protocol/for_patients_families/preventive_services_for_adults_for_patients_families_.html. Accessed Month year.
- .NSAID‐associated deaths: the rise and fall of NSAID‐associated GI mortality.Am J Gastroenterol.2005;100:1694–1695.
- ,.Effects of very low doses of daily long‐term aspirin therapy on gastric, duodenal, and rectal prostaglandins on mucosal injury in healthy humans.Gastroenterology. 199;117:17–25.
- ,,, et al.A nationwide study of mortality associated with hospital admission due to severe gastrointestinal events and those associated with nonsteroidal antiinflammatory drug use.Am J Gastroenterol.2005;100:1685–1693.
- ,,, et al.Acute upper GI bleeding: did anything change?Am J Gastroenterol.2003;98:1494–1499.
- ,.Lower intestinal bleeding.Best Pract Res Clin Gastroenterol.2001;15:135–153.
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis.1987;40:373–383.
- AGS Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons.J Am Geriatr Soc.2002;50(6 suppl):S205–S224.
- ,,, et al.Pain in Osteoarthritis, Rheumatoid Arthritis and Juvenile Chronic Arthritis.2nd ed.Clinical practice guideline no. 2.Glenview, IL:American Pain Society (APS);2002:179.
- American College of Rheumatology Subcommittee on Osteoarthritis Guidelines.Recommendations for the Medical Management of Osteoarthritis of the Hip and Knee.Arthritis Rheum.2000;43:1905–1915.
Gastrointestinal bleeding (GIB) is a frequent reason for acute hospitalization, with estimated rates of hospitalization at 375 per 100,000 per year in the United States.1 GIB is not a specific disease but rather a diverse set of conditions that lead to the clinical manifestations associated with bleeding into the gastrointestinal tract. One of the most commonly used organizing frameworks in gastrointestinal bleeding is the differentiation between upper gastrointestinal bleeding (UGIB) and lower gastrointestinal bleeding (LGIB). There are important differences in the etiologies between the 2 sources. For example, acid‐related disease is a common etiology in UGIB but does not occur in LGIB. While some aspects of the acute management are shared between UGIB and LGIB, important differences exist in the management, including initial endoscopy and medication choice. There have been few direct comparisons of rates, resource use, and clinical outcomes between UGIB and LGIB.
Historically, rates of UGIB have been reported to exceed those of LGIB by 2‐fold to 8‐fold.25 Protocols, clinical practice guidelines, and policy decisions reflect this emphasis on UGIB.68 Among 9 guidelines hosted by National Guideline Clearinghouse addressing GIB, 6 were focused on UGIB, 2 on both UGIB and LGIB, and only 1 on LGIB.9 There are several reasons to believe that these relative incidence rates may not be accurate. First, recent advances in therapy and prevention of UGIB, such as the treatment of Helicobacter pylori infection; proton pump inhibitors (PPIs); and selective cyclooxygenase‐2 (COX‐2) inhibitors, may have affected the epidemiology of gastrointestinal bleeding.1016 Among these therapies, only COX‐2 inhibitors may also reduce the incidence of LGIB.14, 1618 Therefore, these advances may result in a disproportionate drop in UGIB relative to LGIB. In addition, known risk factors for both LGIB and UGIB, including advancing age and renal failure, are increasing in the general population.5, 19, 20 Finally, given the recent increased recommendations for aspirin therapy and systemic anticoagulation, exposure to aspirin and warfarin have increased, both risk factors for LGIB and UGIB.2124 Indeed, recent studies in the epidemiology of UGIB do suggest a changing pattern of etiologies of UGIB reflecting these advances.25 One study examining rates of both UGIB and LGIB demonstrate a decrease in hospitalizations overall for GIB driven by a reduction in UGIB while at the same time reporting an increase in the incidence of hospitalization for LGIB.1
In addition to a changing epidemiology, a second reason for a potential underestimation of LGIB incidence is one of methodology. There are well‐recognized limitations with using purely administrative data due to difficulties in accurately identifying patients with LGIB.26
Studies using large administrative databases may not accurately identify LGIB because of the poor sensitivity and specificity of International Classification of Diseases, Ninth revision, Clinical Modification (ICD‐9) codes for LGIB.5 While there are standard methods of identifying patients with UGIB using ICD‐9 codes,19 there is not an accepted standard for LGIB. Thus, estimates using only ICD‐9 codes may overidentify or underidentify patients with LGIB. Prior studies that have most accurately identified patients with LGIB used a 2‐step method to address this issue. The initial ICD‐9 identification included a high sensitivity/low specificity approach. These identified patient charts undergo chart review to confirm the presence of an LGIB.5 This method is labor intensive and cannot be done using administrative databases. No direct comparison of UGIB to LGIB among hospitalized patients using this 2‐step method has been done recently.
The current emphasis on UGIB as seen in the published guidelines could also be supported if patients with UGIB had greater resource utilization or worse clinical outcomes. Limited direct comparisons for these outcomes are available. However, 1 administrative database study reported similar mortality rates for UGIB (2.7%) and LGIB (2.9%) in 2006.1 No direct comparisons of other clinical outcomes or resource use outcomes are available. Therefore, the emphasis on UGIB in publications and guidelines is best supported by the incidence rates that are, as has already been discussed, problematic.
We conducted a retrospective cohort study to examine the incidences of UGIB and LGIB among patients admitted to an academic medical center over 2 years using methods designed to optimally identify patients with either UGIB or LGIB. Our study also examined differences in clinical outcomes and resource utilization between subjects with UGIB and LGIB to examine the relative severity of these 2 clinical entities. These results may be useful in determining the need to reconsider clinical approaches as well as protocols and guidelines among patients with gastrointestinal bleeding.
Patients and Methods
Patients
This retrospective cohort study evaluated all patients who were admitted with GIB to a large urban academic medical center from July 1, 2001 to June 30, 2003 and who consented to a larger study examining the effects of hospitalists on patient care. Subjects unable to provide consent due to death or lack of decisional capacity were consented via proxy. To identify patients with GIB, all patients were screened for a primary or secondary diagnosis of GIB using ICD 9 codes. These codes were selected for a very high sensitivity threshold to assure that all potential subjects with GIB were identified. All subjects identified using these codes underwent chart abstraction to determine if they met criteria for GIB. These inclusion criteria required documentation in any portion of the chart (including emergency department [ED] clinician documentation, admission note, nursing intake note, etc.) of signs or symptoms of GI hemorrhage upon admission, including: hematemesis, coffee ground emesis, gastrooccult‐positive emesis, melena, hematochezia, maroon stools, and hemoccult‐positive stools interpreted by the treating physician team as an acute GIB. Subjects identified using the ICD‐9 codes and confirmed to have an acute GIB by chart review were included in the study and underwent additional chart abstraction and administrative data analysis.
ICD‐9 codes for GIB included: esophageal varices with hemorrhage (456.0, 456.20), Mallory‐Weiss syndrome (530.7), gastric ulcer with hemorrhage (531.00531.61), duodenal ulcer with hemorrhage (532.00532.61), peptic ulcer, site unspecified, with hemorrhage (533.00533.61), gastrojejunal ulcer with hemorrhage (534.00534.61), gastritis with hemorrhage (535.61), angiodysplasia of stomach/duodenum with hemorrhage (537.83), hematemesis (578.0578.9), diverticular disease (562.00562.9), other disorders of the intestine (569.00569.9), congenital anomalies of the digestive system (751.00), proctocolitis (556.00), hemorrhoids (455.00455.6), nondysenteric colitis (006.2), noninfectious gastroenteritis and colitis (558.0558.9), salmonella gastroenteritis (003.3), malignant neoplasm of colon (153), familial adenomatous polyposis (211.3), and gastric varices (456.8).
Data
Trained research assistants performed chart abstraction with validation by the principal investigators (PIs) of the first 15 charts to ensure accuracy. Subsequently, research assistants consulted with PIs with any questions during abstracting with final decisions being made by PIs. Detailed chart abstraction collected admission medication lists as obtained by the admitting physician team, including the use of PPIs, histamine‐2 (H‐2) blockers, COX‐2 inhibitors, and medications known to increase the risk of GIB, such as nonselective NSAIDs (nsNSAIDs), aspirin, and other anticoagulants. Other clinical data including risk factors, comorbid illnesses, laboratory tests, and vital signs were also abstracted from subjects' charts.
The source (UGIB vs. LGIB) and etiology (peptic ulcer disease [PUD], varices, diverticula, etc.) of bleeding were assessed using endoscopic reports as the primary source. When no clear source was identified on endoscopy or no endoscopy was done, the abstracter would review all progress notes, discharge summaries, and other diagnostic test results such as angiography in order to identify the source of bleeding (UGIB vs. LGIB). Endoscopic reports that identified a patient as having a UGIB or LGIB but no confirmed etiology were classified as undetermined etiology unless review of the other clinical documentation provided a specific etiology.
Tachycardia was defined as pulse greater than 100 beats per minute. Orthostasis was defined by either a drop in systolic blood pressure of 20 mmHg or an increase in pulse of 10 beats per minute. Hospital administrative databases were utilized to obtain resource utilization (ie, length of stay [LOS], total cost of care, intensive care transfers), Charlson comorbidity index,27 30‐day readmission rate, and in‐hospital mortality. Hospital costs were determined using TSI cost accounting software (Transition Systems Incorporated [now Eclypsis Corporation], Boston, MA), a validated system to assess actual direct and indirect costs of care.
Statistical Analysis
Descriptive statistics (means and proportions) were calculated by location of GIB for all variables describing patient characteristics, clinical presentation, clinical outcomes, and resource utilization. Differences in age and Charlson comorbidity index by GIB location were evaluated using t tests. Differences in gender, race, and medication use were evaluated using chi‐squared tests of independence.
We fit generalized linear models to investigate differences by location of bleed for those variables measuring clinical outcomes (inpatient mortality, intensive care unit [ICU] transfer, emergency surgery, 30‐day readmission, change in hemoglobin) and those variables measuring resource outcomes (total cost, LOS, number of procedures, number of correct scopes, repeat scope indicator, incorrect scope indicator, number of red blood cell [RBC] transfusions). The repeat scope indicator was used to denote a repeat scope (either esophagogastroduodenoscopy [EGD] or colonoscopy) and the incorrect scope indicator was used to denote when the initial scope was negative and a follow‐up scope from the other direction was positive (negative EGD followed by positive colonoscopy or negative colonoscopy followed by positive EGD). For each variable we fit 2 regression models, the first model (unadjusted effect) only included location of bleed as a covariate. The second model (adjusted effect) included location of bleed, age, gender, race (black/not black) and Charlson comorbidity index as covariates. Binary outcomes were modeled using logistic regressions. For continuous variables, we determined the distribution and link of the outcome variable using residual diagnostics and by comparing the log likelihood and information criteria of competing models. All analyses were performed using STATA SE Version 9.0 (StataCorp, College Station, TX)
This study was approved by the University of Chicago Institutional Review Board.
Results
During the 2 years of observation, a total of 7741 subjects were admitted to the internal medicine service and enrolled in the hospitalist study. Of these, 1014 had a primary or secondary ICD‐9 code that may be consistent with UGIB or LGIB and underwent chart review to determine if they had an acute GIB. Out of 1014 subjects, 647 were determined not to have an acute GI hemorrhage and were excluded from the remaining analyses; 367 of the 1104 subjects identified by ICD‐9 codes were found to have a clinical presentation consistent with GIB and were included in this study. A total of 180 of these 367 had UGIB and 187 had LGIB. The mean age was 62.4 years, 56.7% were female, 82.6% were African American, 12.7% were Caucasian, and the mean Charlson index was 1.5. (Table 1) Among baseline characteristics, both gender and age were statistically associated with a difference in rates of upper vs. lower source bleeding, with LGIB patients more likely to be female (P = 0.01) and older (P < 0.001). Etiologies of UGIB include erosive disease, peptic ulcer disease, variceal bleeding, arteriovenous malformation, and malignancy. Etiologies of LGIB include: diverticulosis, colitis, arteriovenous malformation, cancer, ischemic colitis, polyp, hemorrhoidal bleed, ulcer, inflammatory bowel disease, other, and not determined (Table 2).
| Upper and Lower GI Bleeding (n = 367) | Upper GI Bleeding (n = 180) | Lower GI Bleeding (n = 187) | P Value | |
|---|---|---|---|---|
| ||||
| Age (years), mean (SD) | 62.4 (18.0) | 58.6 (18.2) | 66.0 (17.1) | <0.001 |
| Female gender (%) | 56.7 | 50.0 | 63.1 | 0.01 |
| Race (%) | ||||
| African American | 82.6 | 85.3 | 80.1 | 0.43 |
| White | 12.7 | 10.7 | 14.5 | |
| Other | 4.7 | 4.0 | 5.4 | |
| Charlson comorbidity index, mean (SD) | 1.5 (1.5) | 1.6 (1.6) | 1.4 (1.5) | 0.44 |
| Lower GI Bleed (n = 187) | Upper GI Bleed (n = 180) | ||||
|---|---|---|---|---|---|
| Etiology | Frequency | Percent of Total (%) | Etiology | Frequency | Percent of Total (%) |
| |||||
| Diverticulosis | 76 | 41 | Erosive disease | 86 | 48 |
| Not identified | 38 | 20 | Peptic ulcer | 51 | 28 |
| Colitis, NOS | 14 | 7 | Not identified | 26 | 14 |
| AVM | 13 | 7 | Mallory Weiss | 17 | 9 |
| Cancer | 11 | 6 | Varices | 8 | 4 |
| Ischemic colitis | 9 | 5 | AVMs | 5 | 3 |
| Polyp | 9 | 5 | Mass/cancer | 5 | 3 |
| Hemorrhoid | 8 | 4 | |||
| Ulcer | 5 | 3 | |||
| Other | 3 | 1 | |||
| IBD | 1 | <1 | |||
Baseline use of medications known to be associated with either increased or decreased risk of GIB was common. Approximately one‐third of subjects with both LGIB and UGIB used aspirin and 10% used warfarin. LGIB subjects were less likely to use an nsNSAID (P < 0.001), but more likely to use a proton pump inhibitor (PPI) (P = 0.06) (Table 3).
| Upper and Lower GI Bleeding (%) (n = 367) | Upper GI Bleeding (%) (n = 180) | Lower GI Bleeding (%) (n = 187) | P Value* | |
|---|---|---|---|---|
| ||||
| Aspirin | 34.9 | 31.8 | 37.4 | 0.28 |
| nsNSAID | 12.9 | 20.8 | 6.4 | < 0.001 |
| COX‐2 selective inhibitor | 8.2 | 6.5 | 9.6 | 0.29 |
| Warfarin | 10.9 | 8.4 | 12.8 | 0.19 |
| PPI | 24.3 | 19.5 | 28.3 | 0.06 |
| nsNSAID + PPI | 1.8 | 1.3 | 2.1 | 0.56 |
| COX‐2 + PPI | 2.9 | 1.3 | 4.3 | 0.11 |
Key initial clinical presentation findings included vital sign abnormalities and admission hemoglobin levels. While hypotension was not common (4.7%), resting tachycardia (37%) and orthostasis (16%) were seen frequently. Subjects with LGIB were significantly less likely than those with UGIB to present with orthostasis (8.8% vs. 21.0%, respectively; P = 0.006) and resting tachycardia (32.3% vs. 42.5%, respectively; P = 0.04). Subjects with LGIB had a higher admission hemoglobin than those with UGIB (10.7 vs. 9.7, respectively; P < 0.001) (Table 4).
| Clinical Finding | Upper and Lower GI Bleeding (n = 367) | Upper GI Bleeding (n = 180) | Lower GI Bleeding (n = 187) | P Value* |
|---|---|---|---|---|
| ||||
| Hypotension (%) | 4.7 | 5.7 | 3.8 | 0.39 |
| Resting tachycardia (%) | 37.3 | 42.5 | 32.3 | 0.04 |
| Orthostatic hypotension (%) | 16.2 | 21.0 | 8.8 | 0.006 |
| Admission hemoglobin (g/dL), mean (SD) | 10.2 (2.6) | 9.7 (2.7) | 10.7 (2.5) | <0.001 |
We also examined several clinical outcomes. When comparing LGIB to UGIB patients for these clinical outcomes using bivariate and multivariate statistics, there was no difference for in‐hospital mortality (1.1% vs. 1.1%), transfer to ICU (16.0% vs. 13.9%), 30‐day readmission (5.9% vs.7.8%), number of red blood cell (RBC) transfusions (2.7 vs. 2.4), or need for GI surgery (1.1% vs. 0.0%). The mean drop in hemoglobin was greater among subjects with LGIB compared to UGIB (1.9 g/dL vs. 1.5 g/dL, respectively) by both bivariate (P = 0.01) and multivariate (P = 0.003) analyses (Table 5).
| Upper GI Bleeding (n = 180) | Lower GI Bleeding (n = 187) | Bivariate P Value | Multivariate P Value | |
|---|---|---|---|---|
| ||||
| In‐hospital mortality (%)* | 1.1 | 1.1 | 0.97 | 0.74 |
| Transfer to ICU (%)* | 13.9 | 16.0 | 0.56 | 0.44 |
| Drop in hemoglobin (g/dL), mean (SD) | 1.5 (1.5) | 1.9 (1.6) | 0.01 | 0.003 |
| Packed RBC transfusions required (units), mean (SD)* | 2.4 (2.9) | 2.7 (3.7) | 0.36 | 0.33 |
| Surgery for GI bleeding (%) | 0.0% | 1.1 | ||
| 30‐day readmission rate (%)* | 7.8 | 5.9 | 0.49 | 0.45 |
Mean costs were $11,892 for LGIB and $14,301 for UGIB and median costs were $7,890 for LGIB and $9,548 for UGIB, but were not statistically different. LOS was also similar between subjects with LGIB (5.1 days) and UGIB (5.7 days). In bivariate and multivariate analyses, UGIB subjects had a similar mean number of endoscopic procedures (1.3) compared to LGIB subjects (1.2). Thirteen percent of subjects with UGIB required a second EGD while only 8% of subjects with LGIB required 2 colonoscopies. In addition, 29% of subjects with LGIB received an EGD while only 16% of subjects with an UGIB received a colonoscopy (P = 0.001) (Table 6).
| Upper GI Bleeding (n = 180) | Lower GI Bleeding (n = 187) | Bivariate P Value | Multivariate P Value | |
|---|---|---|---|---|
| ||||
| Cost ($), mean (SD)* | 14,301 (17,196) | 11,892 (13,100) | 0.13 | 0.21 |
| Cost ($), median | $9,548 | $7,890 | ||
| Length of stay (days), mean (SD)* | 5.7 (7.0) | 5.1 (5.3) | 0.37 | 0.72 |
| Number of endoscopies/ patient, mean (SD) | 1.3 (0.5) | 1.2 (0.9) | 0.18 | 0.20 |
Conclusions
This study represents one of the largest direct comparisons of LGIB to UGIB not based on administrative databases. The most striking finding was the nearly equal rates of LGIB and UGIB. There are 2 likely explanations for this surprising result. First, there may be methodological reasons that we identified a greater proportion of true LGIBs; our study used a highly sensitive search strategy of ICD‐9 coding with confirmatory chart abstraction to ensure that as many LGIB and UGIB cases would be identified as possible while also excluding cases not meeting accepted criteria for GIB. The second possibility is that there is an actual change in epidemiology of GIB. Known risk factors for LGIB are increasing such as advancing age, increased use of chronic aspirin therapy, and renal disease. At the same time, significant advances in the treatment and prevention of UGIB have been made. Recent studies have demonstrated similar trends in admissions for upper and lower GI complications, suggesting that there may be a changing epidemiology due primarily to reductions in upper GI complications.1, 16
Either explanation would have implications for the care of patients with GIB. Clinical decision‐making based on prior literature would support that in ambiguous clinical situations and initial evaluation for an UGIB is appropriate. Most risk stratification literature and clinical guidelines focus on UGIB. If rates of LGIB and UGIB are similar, then existing clinical decision protocols may need to be reevaluated to incorporate the higher likelihood of LGIB. This reevaluation would be less important if the clinical outcomes or resource utilization of UGIB was significantly greater than that for LGIB, but we did not find this was the case. Similarly, if the ability to distinguish between LGIB and UGIB were robust on clinical signs and symptoms, then a reevaluation would be less important. However, we found fairly similar numbers of patients initially receiving evaluation for UGIB then being evaluated for LGIB as we found patients initially receiving evaluation for LGIB then being evaluated for UGIB. This suggests the potential benefit of clinical decision protocols that could better distinguish between UGIB and LGIB and account for the potentially higher incidence of LGIB than previously thought.
In addition to affecting the attention paid to LGIB for acute management, a changed understanding of incidence could also affect the attention paid to prevention of LGIB. Of the recent nonendoscopic advances in the treatment and prevention of GIB, only the use of COX‐2s (when used in place of traditional nsNSAIDs) reduces the risk of both LGIB and UGIB;14, 1618 H .pylori treatment and PPIs only prevent UGIB. Therefore, if the clinical and financial burdens of LGIB are similar to those seen in UGIB, more attention may need to be focused on preventing LGIB.
Baseline medication use was notable primarily for the similarities between UGIB and LGIB. Agents known to affect the rates of GIB were common in both groups. Over one‐third of the population was using aspirin and 10% were taking warfarin. Over 20% of subjects were taking an nsNSAID or a COX‐2 inhibitor. Almost one‐quarter of subjects were taking a PPI, agents known to decrease rates of UGIB and potentially increase LGIB through the risk of C. difficile colitis. Notably, the only statistically significant difference in baseline medication use between subjects with UGIB and LGIB was the more than 3‐fold higher use of nsNSAIDs in patients with UGIB as compared to LGIB. While current guidelines are not clear and consistent about which populations of at‐risk patients should receive GI prophylaxis,2830 these results suggest that patients admitted with GIB are very likely to be taking medications which impact the risk of GIB.
In terms of disease severity, the clinical presentation at admission suggests a greater degree of hemodynamic instability among subjects with UGIB. Rates of orthostatic hypotension and resting tachycardia are higher in UGIB subjects, as well as having a lower mean hemoglobin levels at presentation. However, despite the more severe clinical presentation, clinical outcomes did not differ significantly between the 2 bleeding sources. Thus, the most relevant clinical outcomes suggest that the severity of both LGIB and UGIB are similar. This similarity again suggest that the clinical burden of LGIB is not significantly different than UGIB.
Our results concerning resource utilization demonstrate a similar pattern. While the point estimates for costs and LOS suggest that UGIB may be associated with higher resource utilization, these differences were not significant in either bivariate or multivariate analyses. Those subjects with UGIB did receive more total endoscopic procedures than subjects with LGIB. More interesting though was that 24% of all subjects received an endoscopy of the opposite site (LGIB with EGD and UGIB with colonoscopy). These results suggest that the site of bleeding is not clear in a significant proportion of patients who present with GIB. These additional endoscopies are associated with increased risk, costs, LOS, and discomfort to patients. Improving our ability to accurately predict the source (upper vs. lower) of bleeding would allow us to reduce the number of these excess endoscopies. Additionally, it is interesting that despite the almost universal use of endoscopies, 20% of LGIB and 14% of UGIB subjects could not have a specific etiology identified during endoscopy or subsequent workup.
There are some important limitations to this study. While the sample size is among the largest of its type involving chart abstraction, it may be underpowered to detect some differences. Additionally, our results are from a single urban academic medical center with a patient population that is predominantly African American, which may limit generalizability. This study required consent and therefore only examines a subset of patients admitted to the medical center with GIB, which could potentially introduce bias into the sample. However, it is not clear why there would be systematic differences in subjects who choose to consent vs. those who decide not to consent that would affect the results of this study in substantive ways.
Despite significant efforts at identifying all subjects with GIB admitted during this time period, there were potential methodological reasons that may have resulted in some cases being missed. Only subjects admitted to a medicine service were approached for consent. All subjects in this medical center with GIB are admitted to a medicine service. We captured all subjects who were initially admitted to a medicine service as well as those admitted initially to an ICU and then transferred to the floor at any point prior to discharge. It is possible, though, that a subject would be admitted to an ICU for GIB and die prior to being transferred to the floor. While it is the impression of the director of the ICU that this would be a very unusual event, as most of the patients would be discharged to the floor prior to death (personal communication), given the very low mortality rate seen in this study, small numbers of missed events could have a significant impact on the interpretation of in‐hospital mortality results. It is also important to note that this medical center did not have the ability to perform endoscopy prior to admission for patients with GIB at the time of the study; all patients who presented with GIB would have been admitted and identified for this study. Finally, we were unable to routinely identify the rationale for obtaining an endoscopic exam. We assumed that all endoscopic exams were done for the purpose of evaluating and/or treating the GIB for which the subject was admitted. It is possible that some subjects had additional endoscopies for other reasons, which would have led to our overestimating the rates of additional endoscopies for GIB.
This study highlights the similarities between LGIB and UGIB rather than the differences. There were few significant differences between the 2 bleeding sources in terms of incidence, clinical outcomes, and resource utilization. In fact, the study also suggests that determining the source of bleeding may not be clear, given the high rates of opposite site endoscopies. While this study did reveal several similarities between UGIB and LGIB, it also highlights the need to identify improved strategies to improve the sensitivity and specificity of identification of LGIB compared to UGIB, both for clinical purposes and for research. The value of such improved clinical algorithms have the potential to improve both the cost and outcomes of care, while better algorithms for separating UGIB and LGIB using administrative data might help produce more precise estimates of costs and clinical outcomes, and aid in the development of risk stratification models.
Gastrointestinal bleeding (GIB) is a frequent reason for acute hospitalization, with estimated rates of hospitalization at 375 per 100,000 per year in the United States.1 GIB is not a specific disease but rather a diverse set of conditions that lead to the clinical manifestations associated with bleeding into the gastrointestinal tract. One of the most commonly used organizing frameworks in gastrointestinal bleeding is the differentiation between upper gastrointestinal bleeding (UGIB) and lower gastrointestinal bleeding (LGIB). There are important differences in the etiologies between the 2 sources. For example, acid‐related disease is a common etiology in UGIB but does not occur in LGIB. While some aspects of the acute management are shared between UGIB and LGIB, important differences exist in the management, including initial endoscopy and medication choice. There have been few direct comparisons of rates, resource use, and clinical outcomes between UGIB and LGIB.
Historically, rates of UGIB have been reported to exceed those of LGIB by 2‐fold to 8‐fold.25 Protocols, clinical practice guidelines, and policy decisions reflect this emphasis on UGIB.68 Among 9 guidelines hosted by National Guideline Clearinghouse addressing GIB, 6 were focused on UGIB, 2 on both UGIB and LGIB, and only 1 on LGIB.9 There are several reasons to believe that these relative incidence rates may not be accurate. First, recent advances in therapy and prevention of UGIB, such as the treatment of Helicobacter pylori infection; proton pump inhibitors (PPIs); and selective cyclooxygenase‐2 (COX‐2) inhibitors, may have affected the epidemiology of gastrointestinal bleeding.1016 Among these therapies, only COX‐2 inhibitors may also reduce the incidence of LGIB.14, 1618 Therefore, these advances may result in a disproportionate drop in UGIB relative to LGIB. In addition, known risk factors for both LGIB and UGIB, including advancing age and renal failure, are increasing in the general population.5, 19, 20 Finally, given the recent increased recommendations for aspirin therapy and systemic anticoagulation, exposure to aspirin and warfarin have increased, both risk factors for LGIB and UGIB.2124 Indeed, recent studies in the epidemiology of UGIB do suggest a changing pattern of etiologies of UGIB reflecting these advances.25 One study examining rates of both UGIB and LGIB demonstrate a decrease in hospitalizations overall for GIB driven by a reduction in UGIB while at the same time reporting an increase in the incidence of hospitalization for LGIB.1
In addition to a changing epidemiology, a second reason for a potential underestimation of LGIB incidence is one of methodology. There are well‐recognized limitations with using purely administrative data due to difficulties in accurately identifying patients with LGIB.26
Studies using large administrative databases may not accurately identify LGIB because of the poor sensitivity and specificity of International Classification of Diseases, Ninth revision, Clinical Modification (ICD‐9) codes for LGIB.5 While there are standard methods of identifying patients with UGIB using ICD‐9 codes,19 there is not an accepted standard for LGIB. Thus, estimates using only ICD‐9 codes may overidentify or underidentify patients with LGIB. Prior studies that have most accurately identified patients with LGIB used a 2‐step method to address this issue. The initial ICD‐9 identification included a high sensitivity/low specificity approach. These identified patient charts undergo chart review to confirm the presence of an LGIB.5 This method is labor intensive and cannot be done using administrative databases. No direct comparison of UGIB to LGIB among hospitalized patients using this 2‐step method has been done recently.
The current emphasis on UGIB as seen in the published guidelines could also be supported if patients with UGIB had greater resource utilization or worse clinical outcomes. Limited direct comparisons for these outcomes are available. However, 1 administrative database study reported similar mortality rates for UGIB (2.7%) and LGIB (2.9%) in 2006.1 No direct comparisons of other clinical outcomes or resource use outcomes are available. Therefore, the emphasis on UGIB in publications and guidelines is best supported by the incidence rates that are, as has already been discussed, problematic.
We conducted a retrospective cohort study to examine the incidences of UGIB and LGIB among patients admitted to an academic medical center over 2 years using methods designed to optimally identify patients with either UGIB or LGIB. Our study also examined differences in clinical outcomes and resource utilization between subjects with UGIB and LGIB to examine the relative severity of these 2 clinical entities. These results may be useful in determining the need to reconsider clinical approaches as well as protocols and guidelines among patients with gastrointestinal bleeding.
Patients and Methods
Patients
This retrospective cohort study evaluated all patients who were admitted with GIB to a large urban academic medical center from July 1, 2001 to June 30, 2003 and who consented to a larger study examining the effects of hospitalists on patient care. Subjects unable to provide consent due to death or lack of decisional capacity were consented via proxy. To identify patients with GIB, all patients were screened for a primary or secondary diagnosis of GIB using ICD 9 codes. These codes were selected for a very high sensitivity threshold to assure that all potential subjects with GIB were identified. All subjects identified using these codes underwent chart abstraction to determine if they met criteria for GIB. These inclusion criteria required documentation in any portion of the chart (including emergency department [ED] clinician documentation, admission note, nursing intake note, etc.) of signs or symptoms of GI hemorrhage upon admission, including: hematemesis, coffee ground emesis, gastrooccult‐positive emesis, melena, hematochezia, maroon stools, and hemoccult‐positive stools interpreted by the treating physician team as an acute GIB. Subjects identified using the ICD‐9 codes and confirmed to have an acute GIB by chart review were included in the study and underwent additional chart abstraction and administrative data analysis.
ICD‐9 codes for GIB included: esophageal varices with hemorrhage (456.0, 456.20), Mallory‐Weiss syndrome (530.7), gastric ulcer with hemorrhage (531.00531.61), duodenal ulcer with hemorrhage (532.00532.61), peptic ulcer, site unspecified, with hemorrhage (533.00533.61), gastrojejunal ulcer with hemorrhage (534.00534.61), gastritis with hemorrhage (535.61), angiodysplasia of stomach/duodenum with hemorrhage (537.83), hematemesis (578.0578.9), diverticular disease (562.00562.9), other disorders of the intestine (569.00569.9), congenital anomalies of the digestive system (751.00), proctocolitis (556.00), hemorrhoids (455.00455.6), nondysenteric colitis (006.2), noninfectious gastroenteritis and colitis (558.0558.9), salmonella gastroenteritis (003.3), malignant neoplasm of colon (153), familial adenomatous polyposis (211.3), and gastric varices (456.8).
Data
Trained research assistants performed chart abstraction with validation by the principal investigators (PIs) of the first 15 charts to ensure accuracy. Subsequently, research assistants consulted with PIs with any questions during abstracting with final decisions being made by PIs. Detailed chart abstraction collected admission medication lists as obtained by the admitting physician team, including the use of PPIs, histamine‐2 (H‐2) blockers, COX‐2 inhibitors, and medications known to increase the risk of GIB, such as nonselective NSAIDs (nsNSAIDs), aspirin, and other anticoagulants. Other clinical data including risk factors, comorbid illnesses, laboratory tests, and vital signs were also abstracted from subjects' charts.
The source (UGIB vs. LGIB) and etiology (peptic ulcer disease [PUD], varices, diverticula, etc.) of bleeding were assessed using endoscopic reports as the primary source. When no clear source was identified on endoscopy or no endoscopy was done, the abstracter would review all progress notes, discharge summaries, and other diagnostic test results such as angiography in order to identify the source of bleeding (UGIB vs. LGIB). Endoscopic reports that identified a patient as having a UGIB or LGIB but no confirmed etiology were classified as undetermined etiology unless review of the other clinical documentation provided a specific etiology.
Tachycardia was defined as pulse greater than 100 beats per minute. Orthostasis was defined by either a drop in systolic blood pressure of 20 mmHg or an increase in pulse of 10 beats per minute. Hospital administrative databases were utilized to obtain resource utilization (ie, length of stay [LOS], total cost of care, intensive care transfers), Charlson comorbidity index,27 30‐day readmission rate, and in‐hospital mortality. Hospital costs were determined using TSI cost accounting software (Transition Systems Incorporated [now Eclypsis Corporation], Boston, MA), a validated system to assess actual direct and indirect costs of care.
Statistical Analysis
Descriptive statistics (means and proportions) were calculated by location of GIB for all variables describing patient characteristics, clinical presentation, clinical outcomes, and resource utilization. Differences in age and Charlson comorbidity index by GIB location were evaluated using t tests. Differences in gender, race, and medication use were evaluated using chi‐squared tests of independence.
We fit generalized linear models to investigate differences by location of bleed for those variables measuring clinical outcomes (inpatient mortality, intensive care unit [ICU] transfer, emergency surgery, 30‐day readmission, change in hemoglobin) and those variables measuring resource outcomes (total cost, LOS, number of procedures, number of correct scopes, repeat scope indicator, incorrect scope indicator, number of red blood cell [RBC] transfusions). The repeat scope indicator was used to denote a repeat scope (either esophagogastroduodenoscopy [EGD] or colonoscopy) and the incorrect scope indicator was used to denote when the initial scope was negative and a follow‐up scope from the other direction was positive (negative EGD followed by positive colonoscopy or negative colonoscopy followed by positive EGD). For each variable we fit 2 regression models, the first model (unadjusted effect) only included location of bleed as a covariate. The second model (adjusted effect) included location of bleed, age, gender, race (black/not black) and Charlson comorbidity index as covariates. Binary outcomes were modeled using logistic regressions. For continuous variables, we determined the distribution and link of the outcome variable using residual diagnostics and by comparing the log likelihood and information criteria of competing models. All analyses were performed using STATA SE Version 9.0 (StataCorp, College Station, TX)
This study was approved by the University of Chicago Institutional Review Board.
Results
During the 2 years of observation, a total of 7741 subjects were admitted to the internal medicine service and enrolled in the hospitalist study. Of these, 1014 had a primary or secondary ICD‐9 code that may be consistent with UGIB or LGIB and underwent chart review to determine if they had an acute GIB. Out of 1014 subjects, 647 were determined not to have an acute GI hemorrhage and were excluded from the remaining analyses; 367 of the 1104 subjects identified by ICD‐9 codes were found to have a clinical presentation consistent with GIB and were included in this study. A total of 180 of these 367 had UGIB and 187 had LGIB. The mean age was 62.4 years, 56.7% were female, 82.6% were African American, 12.7% were Caucasian, and the mean Charlson index was 1.5. (Table 1) Among baseline characteristics, both gender and age were statistically associated with a difference in rates of upper vs. lower source bleeding, with LGIB patients more likely to be female (P = 0.01) and older (P < 0.001). Etiologies of UGIB include erosive disease, peptic ulcer disease, variceal bleeding, arteriovenous malformation, and malignancy. Etiologies of LGIB include: diverticulosis, colitis, arteriovenous malformation, cancer, ischemic colitis, polyp, hemorrhoidal bleed, ulcer, inflammatory bowel disease, other, and not determined (Table 2).
| Upper and Lower GI Bleeding (n = 367) | Upper GI Bleeding (n = 180) | Lower GI Bleeding (n = 187) | P Value | |
|---|---|---|---|---|
| ||||
| Age (years), mean (SD) | 62.4 (18.0) | 58.6 (18.2) | 66.0 (17.1) | <0.001 |
| Female gender (%) | 56.7 | 50.0 | 63.1 | 0.01 |
| Race (%) | ||||
| African American | 82.6 | 85.3 | 80.1 | 0.43 |
| White | 12.7 | 10.7 | 14.5 | |
| Other | 4.7 | 4.0 | 5.4 | |
| Charlson comorbidity index, mean (SD) | 1.5 (1.5) | 1.6 (1.6) | 1.4 (1.5) | 0.44 |
| Lower GI Bleed (n = 187) | Upper GI Bleed (n = 180) | ||||
|---|---|---|---|---|---|
| Etiology | Frequency | Percent of Total (%) | Etiology | Frequency | Percent of Total (%) |
| |||||
| Diverticulosis | 76 | 41 | Erosive disease | 86 | 48 |
| Not identified | 38 | 20 | Peptic ulcer | 51 | 28 |
| Colitis, NOS | 14 | 7 | Not identified | 26 | 14 |
| AVM | 13 | 7 | Mallory Weiss | 17 | 9 |
| Cancer | 11 | 6 | Varices | 8 | 4 |
| Ischemic colitis | 9 | 5 | AVMs | 5 | 3 |
| Polyp | 9 | 5 | Mass/cancer | 5 | 3 |
| Hemorrhoid | 8 | 4 | |||
| Ulcer | 5 | 3 | |||
| Other | 3 | 1 | |||
| IBD | 1 | <1 | |||
Baseline use of medications known to be associated with either increased or decreased risk of GIB was common. Approximately one‐third of subjects with both LGIB and UGIB used aspirin and 10% used warfarin. LGIB subjects were less likely to use an nsNSAID (P < 0.001), but more likely to use a proton pump inhibitor (PPI) (P = 0.06) (Table 3).
| Upper and Lower GI Bleeding (%) (n = 367) | Upper GI Bleeding (%) (n = 180) | Lower GI Bleeding (%) (n = 187) | P Value* | |
|---|---|---|---|---|
| ||||
| Aspirin | 34.9 | 31.8 | 37.4 | 0.28 |
| nsNSAID | 12.9 | 20.8 | 6.4 | < 0.001 |
| COX‐2 selective inhibitor | 8.2 | 6.5 | 9.6 | 0.29 |
| Warfarin | 10.9 | 8.4 | 12.8 | 0.19 |
| PPI | 24.3 | 19.5 | 28.3 | 0.06 |
| nsNSAID + PPI | 1.8 | 1.3 | 2.1 | 0.56 |
| COX‐2 + PPI | 2.9 | 1.3 | 4.3 | 0.11 |
Key initial clinical presentation findings included vital sign abnormalities and admission hemoglobin levels. While hypotension was not common (4.7%), resting tachycardia (37%) and orthostasis (16%) were seen frequently. Subjects with LGIB were significantly less likely than those with UGIB to present with orthostasis (8.8% vs. 21.0%, respectively; P = 0.006) and resting tachycardia (32.3% vs. 42.5%, respectively; P = 0.04). Subjects with LGIB had a higher admission hemoglobin than those with UGIB (10.7 vs. 9.7, respectively; P < 0.001) (Table 4).
| Clinical Finding | Upper and Lower GI Bleeding (n = 367) | Upper GI Bleeding (n = 180) | Lower GI Bleeding (n = 187) | P Value* |
|---|---|---|---|---|
| ||||
| Hypotension (%) | 4.7 | 5.7 | 3.8 | 0.39 |
| Resting tachycardia (%) | 37.3 | 42.5 | 32.3 | 0.04 |
| Orthostatic hypotension (%) | 16.2 | 21.0 | 8.8 | 0.006 |
| Admission hemoglobin (g/dL), mean (SD) | 10.2 (2.6) | 9.7 (2.7) | 10.7 (2.5) | <0.001 |
We also examined several clinical outcomes. When comparing LGIB to UGIB patients for these clinical outcomes using bivariate and multivariate statistics, there was no difference for in‐hospital mortality (1.1% vs. 1.1%), transfer to ICU (16.0% vs. 13.9%), 30‐day readmission (5.9% vs.7.8%), number of red blood cell (RBC) transfusions (2.7 vs. 2.4), or need for GI surgery (1.1% vs. 0.0%). The mean drop in hemoglobin was greater among subjects with LGIB compared to UGIB (1.9 g/dL vs. 1.5 g/dL, respectively) by both bivariate (P = 0.01) and multivariate (P = 0.003) analyses (Table 5).
| Upper GI Bleeding (n = 180) | Lower GI Bleeding (n = 187) | Bivariate P Value | Multivariate P Value | |
|---|---|---|---|---|
| ||||
| In‐hospital mortality (%)* | 1.1 | 1.1 | 0.97 | 0.74 |
| Transfer to ICU (%)* | 13.9 | 16.0 | 0.56 | 0.44 |
| Drop in hemoglobin (g/dL), mean (SD) | 1.5 (1.5) | 1.9 (1.6) | 0.01 | 0.003 |
| Packed RBC transfusions required (units), mean (SD)* | 2.4 (2.9) | 2.7 (3.7) | 0.36 | 0.33 |
| Surgery for GI bleeding (%) | 0.0% | 1.1 | ||
| 30‐day readmission rate (%)* | 7.8 | 5.9 | 0.49 | 0.45 |
Mean costs were $11,892 for LGIB and $14,301 for UGIB and median costs were $7,890 for LGIB and $9,548 for UGIB, but were not statistically different. LOS was also similar between subjects with LGIB (5.1 days) and UGIB (5.7 days). In bivariate and multivariate analyses, UGIB subjects had a similar mean number of endoscopic procedures (1.3) compared to LGIB subjects (1.2). Thirteen percent of subjects with UGIB required a second EGD while only 8% of subjects with LGIB required 2 colonoscopies. In addition, 29% of subjects with LGIB received an EGD while only 16% of subjects with an UGIB received a colonoscopy (P = 0.001) (Table 6).
| Upper GI Bleeding (n = 180) | Lower GI Bleeding (n = 187) | Bivariate P Value | Multivariate P Value | |
|---|---|---|---|---|
| ||||
| Cost ($), mean (SD)* | 14,301 (17,196) | 11,892 (13,100) | 0.13 | 0.21 |
| Cost ($), median | $9,548 | $7,890 | ||
| Length of stay (days), mean (SD)* | 5.7 (7.0) | 5.1 (5.3) | 0.37 | 0.72 |
| Number of endoscopies/ patient, mean (SD) | 1.3 (0.5) | 1.2 (0.9) | 0.18 | 0.20 |
Conclusions
This study represents one of the largest direct comparisons of LGIB to UGIB not based on administrative databases. The most striking finding was the nearly equal rates of LGIB and UGIB. There are 2 likely explanations for this surprising result. First, there may be methodological reasons that we identified a greater proportion of true LGIBs; our study used a highly sensitive search strategy of ICD‐9 coding with confirmatory chart abstraction to ensure that as many LGIB and UGIB cases would be identified as possible while also excluding cases not meeting accepted criteria for GIB. The second possibility is that there is an actual change in epidemiology of GIB. Known risk factors for LGIB are increasing such as advancing age, increased use of chronic aspirin therapy, and renal disease. At the same time, significant advances in the treatment and prevention of UGIB have been made. Recent studies have demonstrated similar trends in admissions for upper and lower GI complications, suggesting that there may be a changing epidemiology due primarily to reductions in upper GI complications.1, 16
Either explanation would have implications for the care of patients with GIB. Clinical decision‐making based on prior literature would support that in ambiguous clinical situations and initial evaluation for an UGIB is appropriate. Most risk stratification literature and clinical guidelines focus on UGIB. If rates of LGIB and UGIB are similar, then existing clinical decision protocols may need to be reevaluated to incorporate the higher likelihood of LGIB. This reevaluation would be less important if the clinical outcomes or resource utilization of UGIB was significantly greater than that for LGIB, but we did not find this was the case. Similarly, if the ability to distinguish between LGIB and UGIB were robust on clinical signs and symptoms, then a reevaluation would be less important. However, we found fairly similar numbers of patients initially receiving evaluation for UGIB then being evaluated for LGIB as we found patients initially receiving evaluation for LGIB then being evaluated for UGIB. This suggests the potential benefit of clinical decision protocols that could better distinguish between UGIB and LGIB and account for the potentially higher incidence of LGIB than previously thought.
In addition to affecting the attention paid to LGIB for acute management, a changed understanding of incidence could also affect the attention paid to prevention of LGIB. Of the recent nonendoscopic advances in the treatment and prevention of GIB, only the use of COX‐2s (when used in place of traditional nsNSAIDs) reduces the risk of both LGIB and UGIB;14, 1618 H .pylori treatment and PPIs only prevent UGIB. Therefore, if the clinical and financial burdens of LGIB are similar to those seen in UGIB, more attention may need to be focused on preventing LGIB.
Baseline medication use was notable primarily for the similarities between UGIB and LGIB. Agents known to affect the rates of GIB were common in both groups. Over one‐third of the population was using aspirin and 10% were taking warfarin. Over 20% of subjects were taking an nsNSAID or a COX‐2 inhibitor. Almost one‐quarter of subjects were taking a PPI, agents known to decrease rates of UGIB and potentially increase LGIB through the risk of C. difficile colitis. Notably, the only statistically significant difference in baseline medication use between subjects with UGIB and LGIB was the more than 3‐fold higher use of nsNSAIDs in patients with UGIB as compared to LGIB. While current guidelines are not clear and consistent about which populations of at‐risk patients should receive GI prophylaxis,2830 these results suggest that patients admitted with GIB are very likely to be taking medications which impact the risk of GIB.
In terms of disease severity, the clinical presentation at admission suggests a greater degree of hemodynamic instability among subjects with UGIB. Rates of orthostatic hypotension and resting tachycardia are higher in UGIB subjects, as well as having a lower mean hemoglobin levels at presentation. However, despite the more severe clinical presentation, clinical outcomes did not differ significantly between the 2 bleeding sources. Thus, the most relevant clinical outcomes suggest that the severity of both LGIB and UGIB are similar. This similarity again suggest that the clinical burden of LGIB is not significantly different than UGIB.
Our results concerning resource utilization demonstrate a similar pattern. While the point estimates for costs and LOS suggest that UGIB may be associated with higher resource utilization, these differences were not significant in either bivariate or multivariate analyses. Those subjects with UGIB did receive more total endoscopic procedures than subjects with LGIB. More interesting though was that 24% of all subjects received an endoscopy of the opposite site (LGIB with EGD and UGIB with colonoscopy). These results suggest that the site of bleeding is not clear in a significant proportion of patients who present with GIB. These additional endoscopies are associated with increased risk, costs, LOS, and discomfort to patients. Improving our ability to accurately predict the source (upper vs. lower) of bleeding would allow us to reduce the number of these excess endoscopies. Additionally, it is interesting that despite the almost universal use of endoscopies, 20% of LGIB and 14% of UGIB subjects could not have a specific etiology identified during endoscopy or subsequent workup.
There are some important limitations to this study. While the sample size is among the largest of its type involving chart abstraction, it may be underpowered to detect some differences. Additionally, our results are from a single urban academic medical center with a patient population that is predominantly African American, which may limit generalizability. This study required consent and therefore only examines a subset of patients admitted to the medical center with GIB, which could potentially introduce bias into the sample. However, it is not clear why there would be systematic differences in subjects who choose to consent vs. those who decide not to consent that would affect the results of this study in substantive ways.
Despite significant efforts at identifying all subjects with GIB admitted during this time period, there were potential methodological reasons that may have resulted in some cases being missed. Only subjects admitted to a medicine service were approached for consent. All subjects in this medical center with GIB are admitted to a medicine service. We captured all subjects who were initially admitted to a medicine service as well as those admitted initially to an ICU and then transferred to the floor at any point prior to discharge. It is possible, though, that a subject would be admitted to an ICU for GIB and die prior to being transferred to the floor. While it is the impression of the director of the ICU that this would be a very unusual event, as most of the patients would be discharged to the floor prior to death (personal communication), given the very low mortality rate seen in this study, small numbers of missed events could have a significant impact on the interpretation of in‐hospital mortality results. It is also important to note that this medical center did not have the ability to perform endoscopy prior to admission for patients with GIB at the time of the study; all patients who presented with GIB would have been admitted and identified for this study. Finally, we were unable to routinely identify the rationale for obtaining an endoscopic exam. We assumed that all endoscopic exams were done for the purpose of evaluating and/or treating the GIB for which the subject was admitted. It is possible that some subjects had additional endoscopies for other reasons, which would have led to our overestimating the rates of additional endoscopies for GIB.
This study highlights the similarities between LGIB and UGIB rather than the differences. There were few significant differences between the 2 bleeding sources in terms of incidence, clinical outcomes, and resource utilization. In fact, the study also suggests that determining the source of bleeding may not be clear, given the high rates of opposite site endoscopies. While this study did reveal several similarities between UGIB and LGIB, it also highlights the need to identify improved strategies to improve the sensitivity and specificity of identification of LGIB compared to UGIB, both for clinical purposes and for research. The value of such improved clinical algorithms have the potential to improve both the cost and outcomes of care, while better algorithms for separating UGIB and LGIB using administrative data might help produce more precise estimates of costs and clinical outcomes, and aid in the development of risk stratification models.
- ,.Hospitalizations for Gastrointestinal Bleeding in 1998 and 2006. HCUP Statistical Brief #65, December 2008.Rockville, MD:Agency for Healthcare Research and Quality.
- ,.Causes and outcome of upper and lower gastrointestinal bleeding: The Grady Hospital Experience.South Med J.1999;92(1):44–50.
- ,,, et al.Acute upper gastrointestinal haemorrhage in west of Scotland: case ascertainment study.BMJ.1997;315:510–540.
- ,.A cost‐effective approach to the patient with peptic ulcer bleeding.Surg Clin North Am.1996;76:83–103.
- .Epidemiology and outcome of patients hospitalized with acute lower gastrointestinal hemorrhage: a population based study.Am J Gastroenterol.1997;92:419–424.
- ,,.Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding.Ann Int Med.2003;139:843–857.
- ,.Incremental value of upper endoscopy for triage of patients with acute non‐variceal upper‐GI hemorrhage.Gastrointest Endosc2004;60:9–14.
- ,,, et al.Upper gastrointestinal hemorrhage clinical guideline‐determining the optimal hospital length of stay.Am J Med.1996;100:313–322.
- National Guideline Clearinghouse. Available at: http://www.guideline.gov. Accessed August2009.
- ,,, et al.Prevention of ulcer recurrence after eradication of Helicobacter pylori: a prospective long‐term follow‐up study.Gastroenterology.1997;113:1082–1086.
- ,,, et al.Treatment of Helicobacter pylori in patients with duodenal ulcer hemorrhage‐a long‐term randomized, controlled study.Am J Gastroenterol.2000;95:2225–2232.
- ,,, et al.Preventing recurrent upper gastrointestinal bleeding in patients with Helicobacter pylori infection who are taking low‐dose aspirin or naproxen.N Engl J Med.2001;344:967–973.
- ,,, et al.Lansoprazole for the prevention of recurrences of ulcer complications from long‐term low‐dose aspirin use.N Engl J Med.2002;346:2033–2038.
- ,,, et al.Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group.N Engl J Med.2000;343:1520–1528.
- ,,, et al.Gastrointestinal toxicity with celecoxib vs nonsteroidal anti‐inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long‐Term Arthritis Safety Study.JAMA.2000;284:1247–1255.
- ,,, et al.Time trends and clinical impact of upper and lower gastrointestinal complications. Digestive Disease Week National Meeting,2008. San Diego, CA, May 17–22.
- ,,, et al.Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo.Clin Gastroenterol Hepatol.2005;3:133–141.
- ,,, et al.Serious lower gastrointestinal clinical events with nonselective NSAID or Coxib use.Gastroenterology.2003;124:288–292.
- ,,, et al.Risk factors for upper gastrointestinal bleeding among end‐stage renal disease patients.Kidney Int.2003;64:1455–1461.
- ,,, et al.Risk factors for hospitalized bleeding among older patients.J Am Geriatr Soc.2001;49:126–133.
- Institute for Clinical Systems Improvement (ICSI). Preventive services in adults. Bloomington, MN: Institute for Clinical Systems Improvement (ICSI).2005. Available at http://www.isci.org/guidelines_and_more/guidelines_order_sets_protocol/for_patients_families/preventive_services_for_adults_for_patients_families_.html. Accessed Month year.
- .NSAID‐associated deaths: the rise and fall of NSAID‐associated GI mortality.Am J Gastroenterol.2005;100:1694–1695.
- ,.Effects of very low doses of daily long‐term aspirin therapy on gastric, duodenal, and rectal prostaglandins on mucosal injury in healthy humans.Gastroenterology. 199;117:17–25.
- ,,, et al.A nationwide study of mortality associated with hospital admission due to severe gastrointestinal events and those associated with nonsteroidal antiinflammatory drug use.Am J Gastroenterol.2005;100:1685–1693.
- ,,, et al.Acute upper GI bleeding: did anything change?Am J Gastroenterol.2003;98:1494–1499.
- ,.Lower intestinal bleeding.Best Pract Res Clin Gastroenterol.2001;15:135–153.
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis.1987;40:373–383.
- AGS Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons.J Am Geriatr Soc.2002;50(6 suppl):S205–S224.
- ,,, et al.Pain in Osteoarthritis, Rheumatoid Arthritis and Juvenile Chronic Arthritis.2nd ed.Clinical practice guideline no. 2.Glenview, IL:American Pain Society (APS);2002:179.
- American College of Rheumatology Subcommittee on Osteoarthritis Guidelines.Recommendations for the Medical Management of Osteoarthritis of the Hip and Knee.Arthritis Rheum.2000;43:1905–1915.
- ,.Hospitalizations for Gastrointestinal Bleeding in 1998 and 2006. HCUP Statistical Brief #65, December 2008.Rockville, MD:Agency for Healthcare Research and Quality.
- ,.Causes and outcome of upper and lower gastrointestinal bleeding: The Grady Hospital Experience.South Med J.1999;92(1):44–50.
- ,,, et al.Acute upper gastrointestinal haemorrhage in west of Scotland: case ascertainment study.BMJ.1997;315:510–540.
- ,.A cost‐effective approach to the patient with peptic ulcer bleeding.Surg Clin North Am.1996;76:83–103.
- .Epidemiology and outcome of patients hospitalized with acute lower gastrointestinal hemorrhage: a population based study.Am J Gastroenterol.1997;92:419–424.
- ,,.Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding.Ann Int Med.2003;139:843–857.
- ,.Incremental value of upper endoscopy for triage of patients with acute non‐variceal upper‐GI hemorrhage.Gastrointest Endosc2004;60:9–14.
- ,,, et al.Upper gastrointestinal hemorrhage clinical guideline‐determining the optimal hospital length of stay.Am J Med.1996;100:313–322.
- National Guideline Clearinghouse. Available at: http://www.guideline.gov. Accessed August2009.
- ,,, et al.Prevention of ulcer recurrence after eradication of Helicobacter pylori: a prospective long‐term follow‐up study.Gastroenterology.1997;113:1082–1086.
- ,,, et al.Treatment of Helicobacter pylori in patients with duodenal ulcer hemorrhage‐a long‐term randomized, controlled study.Am J Gastroenterol.2000;95:2225–2232.
- ,,, et al.Preventing recurrent upper gastrointestinal bleeding in patients with Helicobacter pylori infection who are taking low‐dose aspirin or naproxen.N Engl J Med.2001;344:967–973.
- ,,, et al.Lansoprazole for the prevention of recurrences of ulcer complications from long‐term low‐dose aspirin use.N Engl J Med.2002;346:2033–2038.
- ,,, et al.Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group.N Engl J Med.2000;343:1520–1528.
- ,,, et al.Gastrointestinal toxicity with celecoxib vs nonsteroidal anti‐inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long‐Term Arthritis Safety Study.JAMA.2000;284:1247–1255.
- ,,, et al.Time trends and clinical impact of upper and lower gastrointestinal complications. Digestive Disease Week National Meeting,2008. San Diego, CA, May 17–22.
- ,,, et al.Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo.Clin Gastroenterol Hepatol.2005;3:133–141.
- ,,, et al.Serious lower gastrointestinal clinical events with nonselective NSAID or Coxib use.Gastroenterology.2003;124:288–292.
- ,,, et al.Risk factors for upper gastrointestinal bleeding among end‐stage renal disease patients.Kidney Int.2003;64:1455–1461.
- ,,, et al.Risk factors for hospitalized bleeding among older patients.J Am Geriatr Soc.2001;49:126–133.
- Institute for Clinical Systems Improvement (ICSI). Preventive services in adults. Bloomington, MN: Institute for Clinical Systems Improvement (ICSI).2005. Available at http://www.isci.org/guidelines_and_more/guidelines_order_sets_protocol/for_patients_families/preventive_services_for_adults_for_patients_families_.html. Accessed Month year.
- .NSAID‐associated deaths: the rise and fall of NSAID‐associated GI mortality.Am J Gastroenterol.2005;100:1694–1695.
- ,.Effects of very low doses of daily long‐term aspirin therapy on gastric, duodenal, and rectal prostaglandins on mucosal injury in healthy humans.Gastroenterology. 199;117:17–25.
- ,,, et al.A nationwide study of mortality associated with hospital admission due to severe gastrointestinal events and those associated with nonsteroidal antiinflammatory drug use.Am J Gastroenterol.2005;100:1685–1693.
- ,,, et al.Acute upper GI bleeding: did anything change?Am J Gastroenterol.2003;98:1494–1499.
- ,.Lower intestinal bleeding.Best Pract Res Clin Gastroenterol.2001;15:135–153.
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis.1987;40:373–383.
- AGS Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons.J Am Geriatr Soc.2002;50(6 suppl):S205–S224.
- ,,, et al.Pain in Osteoarthritis, Rheumatoid Arthritis and Juvenile Chronic Arthritis.2nd ed.Clinical practice guideline no. 2.Glenview, IL:American Pain Society (APS);2002:179.
- American College of Rheumatology Subcommittee on Osteoarthritis Guidelines.Recommendations for the Medical Management of Osteoarthritis of the Hip and Knee.Arthritis Rheum.2000;43:1905–1915.
Copyright © 2010 Society of Hospital Medicine
Continuing Medical Education Program in
If you wish to receive credit for this activity, which begins on the next page, please refer to the website: www. blackwellpublishing.com/cme.
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to www.blackwellpublishing.com/cme.
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which begins on the next page, please refer to the website: www. blackwellpublishing.com/cme.
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to www.blackwellpublishing.com/cme.
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which begins on the next page, please refer to the website: www. blackwellpublishing.com/cme.
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to www.blackwellpublishing.com/cme.
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
New Therapies for UGH
Upper gastrointestinal hemorrhage (UGH) is a common cause of acute admission for hospitalization.13 However, recent advances in our understanding of erosive disease (ED) and peptic ulcer disease (PUD), 2 of the most common etiologies of UGH, have led to effective strategies to reduce the risk of UGH. Successful implementation of these strategies, such as treatment of Helicobacter pylori (H. pylori) and the use of proton pump inhibitors (PPIs) and selective cyclooxygenase‐2 inhibitors (COX‐2s) in place of traditional nonselective nonsteroidal antiinflammatory drugs (NSAIDs), may be able to significantly reduce rates of UGH caused by ED and PUD.47
Prior to these preventive treatments, PUD and ED, both acid‐related disorders, were the most common causes of UGH requiring admission to the hospital, accounting for 62% and 14% of all UGHs, respectively.2 Given the widespread treatment of H. pylori and use of PPIs and COX‐2s, we might expect that the distribution of etiologies of UGH may have changed. However, there are limited data on the distribution of etiologies of UGH in the era of effective preventive therapy.8 If the distribution of etiologies causing patients to present with UGH has fundamentally changed with these new treatments, established strategies of managing acute UGH may need to be reevaluated. Given that well‐established guidelines exist and that many hospitals use a protocol‐driven management strategy to decide on the need for admission and/or intensive care unit (ICU) admission, changes in the distribution of etiologies since the widespread use of these new pharmacologic approaches may affect the appropriateness of these protocols.9, 10 For example, if the eradication of H. pylori has dramatically reduced the proportion of UGH caused by PUD, then risk stratification studies developed when PUD was far more common may need to be revisited. This would be particularly important if bleeding from PUD was of significantly different severity than bleeding from other causes.
While patients with H. pylori‐related UGH from PUD should be treated for H. pylori eradication, several important questions remain surrounding the use of newer therapeutics that may mitigate the risk of UGH in some patients. It is unclear what proportion of patients admitted with UGH in this new era developed bleeding despite using preventive therapy. These treatment failures are known to occur, but it is not well known how much of the burden of UGH today is due to this breakthrough bleeding.5, 6, 11, 12 Contrastingly, there are also patients who are admitted with UGH who are not on preventive treatment. Current guidelines suggest that high‐risk patients requiring NSAIDs be given COX‐2s or traditional NSAIDs with a PPI.1315 However, there is significant disagreement between these national guidelines about what constitutes a high‐risk profile.1315 For example, some guidelines recommend that elderly patients requiring NSAIDs should be on a PPI while others do not make that recommendation. Similarly, while prior UGH is a well‐recognized risk factor for future bleeding risk even without NSAIDs, current guidelines do not provide guidance toward the use of preventive therapy in these patients. If there are few patients who present with UGH related to acid disease that are not on a preventive therapy, then these unanswered questions or conflicts within current guidelines become less important. However, if a large portion of UGH is due to acid‐related disease in patients not on preventive therapy, then these unanswered questions may become important for future research.
In contrast to previous studies, the current study examines the distribution of etiologies of UGH in the era of widespread use of effective preventive therapy for ED and PUD in 2 U.S. academic medical centers. Prior studies were done before the advent of new therapeutics and did not compare different sites, which may be important.16, 17
PATIENTS AND METHODS
Patients
Consecutive patients admitted with UGH were identified at 2 academic medical centers as part of a larger observational study examining the impact of hospitalist physicians on the care of acute medical patients.18 The sample was selected from the 12,091 consecutive general medical patients admitted from July 2001 to June 2003 with UGH identified by International Classification of Diseases, Ninth revision, Clinical Modification (ICD‐9 CM) codes from administrative data and confirmed by chart abstraction. ICD‐9 CM codes for UGH included: esophageal varices with hemorrhage (456.0 and 456.20), Mallory‐Weiss syndrome (530.7), gastric ulcer with hemorrhage (531.00‐531.61), duodenal ulcer with hemorrhage (532.00‐532.61), peptic ulcer, site unspecified, with hemorrhage (533.00‐533.61), gastrojejunal ulcer with hemorrhage (534.00‐534.61), gastritis with hemorrhage (535.61), angiodysplasia of stomach/duodenum with hemorrhage (537.83), and hematemesis (578.0 and 578.9).19 Finally, the admission diagnoses for all patients in the larger cohort were reviewed and any with gastrointestinal hemorrhage were screened for possible inclusion to account for any missed ICD‐9 codes. Subjects were then included in this analysis if they had observed hematemesis, nasogastric (NG) tube aspirate with gross or hemoccult blood, or history of hematemesis, bloody diarrhea, or melena upon chart review.
Data
The inpatient medical records were abstracted by trained researchers. Etiologies of UGH were assessed by esophagogastroduodenoscopy (EGD) report, which listed findings and etiologies as assessed by the endoscopist. Multiple etiologies were allowed if more than 1 source of bleeding was identified. Prior medical history and preadmission medication use were obtained from 3 sources: (1) the emergency department medical record; (2) nursing admission documentation; and (3) the admission history and physical documentation. Risk factors and preadmission medication use were considered present if documented in any of the 3 sources. Relevant past medical history included known risk factors for UGH, including: end‐stage renal disease, alcohol abuse, prior history of UGH, and steroid use. Prior H. pylori status/testing could not reliably be obtained from these data sources. Preadmission medication use of interest included aspirin, NSAIDS, anticoagulants, antiplatelet agents, as well as PPIs and COX‐2s. Demographics, including age, race, and gender, were obtained from administrative databases.
We defined subjects as at‐risk if they had any of the following risk factors: prior UGH (at any time), use of an NSAID (traditional or selective COX‐2), or use of an aspirin prior to admission. Patients taking COX‐2s were included for 2 reasons. First, while COX‐2 inhibitors are associated with a lower risk of UGH than traditional NSAIDs, it is likely that they still lead to an increased risk of UGH compared to placebo. Second, if a patient required NSAIDs of some type (traditional or selective), preadmission use of a COX‐2 rather than a traditional NSAID may reflect the intention of decreasing the risk of UGH compared to using traditional NSAIDs. In order to use the most conservative estimate of potential missed opportunities for prevention, preadmission use of a PPI or COX‐2 was considered preventive therapy. All preadmission medication use was obtained from chart review. Therefore, duration of and purpose for medication use were not available.
Development of the abstraction tool was performed by the authors. Testing of the tool was performed on a learning set of 20 charts at each center. All additional abstractors were trained with a learning set of at least 20 charts to assure uniform abstraction techniques.
Analysis
For each risk factor and etiology, we calculated the proportion of patients with the risk factor or etiology both overall and by site. Differences in risk factors between sites were assessed using chi‐square tests of association. Differences in etiologies between sites were assessed using unadjusted odds ratios (ORs) as well as ORs from logistic regression models controlling for age, gender, and race (black versus not black). Center 1 was the urban center and center 2 was the rural site.
This study was approved by the Institutional Review Board at the University of Iowa Carver College of Medicine and the University of Chicago.
RESULTS
From the entire cohort of 12,091 admitted to the 2 inpatient medical services, 227 (1.9%) patients were identified as having UGH; 138 (61%) were from center 1, where 87% of patients were black and 89 (39%) were from center 2, where 89% of patients were white. Overall, the mean age was 59 years, 45% were female, and 41% were white (Table 1).
| Characteristic | Total (n = 227) | Center 1 (n = 138) | Center 2 (n = 89) | P Value Center 1 versus 2 |
|---|---|---|---|---|
| ||||
| Mean age (years) | 58.6 | 59.5 | 57.1 | 0.317 |
| % Female | 44.5 | 48.6 | 38.2 | 0.126 |
| % White | 41.2 | 10.2 | 88.8 | <0.001 |
| % African American | 54.0 | 86.9 | 3.4 | <0.001 |
| % Other | 4.9 | 2.9 | 7.9 | <0.001 |
The most common etiologies of UGH were ED (44%), PUD (33%), and varices (17%) in the overall population. These same 3 etiologies were also the most common in both of the medical centers, although there were significant differences in the rates of etiologies between the 2 centers. ED was more common among subjects from center 1 (59%) than from center 2 (19%) (P < 0.001), while variceal bleeding was more common among subjects from center 2 (34%) than from center 1 (6.5%) (P = 0.009) (Table 2).
| Etiology | All n = 227 (%) | Center 1 n = 138 (%) | Center 2 n = 89 (%) | Unadjusted OR (95% CI): Center 1 versus 2 | P Value for Unadjusted OR | Adjusted* OR (95% CI): Center 1 versus 2 | P Value (for Adjusted OR) |
|---|---|---|---|---|---|---|---|
| |||||||
| ED | 43.6 | 59.4 | 19.1 | 6.20 (3.3111.62) | <0.001 | 7.10 (2.4820.31) | <0.001 |
| PUD | 33.0 | 37.0 | 27.0 | 1.59 (0.892.84) | 0.119 | 1.33 (0.483.67) | 0.578 |
| Varices | 17.2 | 6.5 | 33.7 | 0.14 (0.060.31) | <0.001 | 0.12 (0.030.60) | 0.009 |
| AVM | 5.3 | 2.9 | 9.0 | 0.30 (0.091.04) | 0.057 | 0.21 (0.031.69) | 0.141 |
| Mallory Weiss Tear | 4.9 | 4.4 | 5.6 | 0.76 (0.232.58) | 0.664 | 0.34 (0.024.85) | 0.425 |
| Cancer/masses | 2.6 | 2.9 | 2.3 | 1.30 (0.237.24) | 0.766 | 0.62 (0.0312.12) | 0.751 |
In multivariate logistic regression analyses, only age and site remained independent predictors of etiologies. Advancing age was associated with a higher risk of arteriovenous malformations (AVMs) with the odds of AVMs increasing 6% for every additional year of life (P = 0.007). Site was associated with both ED and variceal bleeding. Patients from center 1 were significantly more likely to have UGH caused by ED, with an OR = 7.10 (P < 0.001), compared to subjects from center 2. However, subjects from center 1 had a significantly lower OR (OR = 0.12) than those subjects at center 2 (P = 0.009) of having UGH caused by a variceal bleed (Table 2).
Risk factors for UGH were common among the patients, including use of aspirin (25.1%), NSAIDs (22.9%), COX‐2s (4.9%), or prior history of UGH (43%). Additionally, 6.6% of patients were taking both an NSAID and aspirin. Differences between the 2 sites were seen only in aspirin use, with 34.8% of patients in the center 1 population using aspirin compared to 10.1% in center 2 (P < 0.001) (Table 3).
| Risk Factor | All (%) | Center 1 (%) | Center 2 (%) | P Value |
|---|---|---|---|---|
| ||||
| Previous UGH | 42.7 | 41.3 | 45.2 | 0.586 |
| NSAID use | 22.9 | 21.7 | 24.7 | 0.602 |
| ASA use | 25.1 | 34.8 | 10.1 | <0.001 |
| NSAID + ASA | 6.6 | 6.5 | 6.7 | 0.948 |
| COX‐2 use | 4.9 | 6.5 | 2.3 | 0.143 |
| PPI use | 18.5 | 18.1 | 19.1 | 0.852 |
Among the overall population, 68.7% of patients had identifiable risk factors (prior history of UGH or preadmission use of aspirin, NSAIDs, or COX‐2s). Of all subjects, 18.5% were on PPIs and 4.9% were taking COX‐2s while 21.1% of at risk subjects were on PPIs and 6.5% of these subjects were on a COX‐2.
Finally, we examined the effects of variations in preadmission medication use between the sites on the etiologies of UGH. None of the site‐based differences in etiologies could be explained by differences in preadmission medication patterns.
DISCUSSION
Despite the emergence of effective therapies for lowering the risk of ED and PUD, these remain the most common etiologies of UGH in our cohort of patients. In a dramatic change from historically reported patterns, ED was more common than PUD. In prior studies, PUD accounted for almost two‐thirds of all UGH.2 While some of the newer therapeutics such as PPIs and COX‐2s reduce the risk for acid‐related bleeding of all types, H. pylori eradication is effective primarily for PUD. Therefore, it may be that widespread testing and treatment of H. pylori have dramatically decreased rates of PUD. Unfortunately, this study does not allow us to directly evaluate the effect of H. pylori treatment on the changing epidemiology of UGH, as that would require a population‐based study.
While decreasing rates of PUD could explain a portion of the change in the distribution of etiologies, increasing rates of ED could also be playing a role. Prior studies have suggested that African Americans and the elderly are more susceptible to ED, particularly in the setting of NSAIDs and/or aspirin use, and less susceptible to cirrhosis.13, 16, 17, 2023 Our finding of a higher rate of ED and lower rates of cirrhosis in center 1 with a higher proportion of African Americans and greater aspirin use is consistent with these prior findings. However, in multivariate analyses, neither race nor preadmission medication use patterns explained the differences in etiologies seen. This suggests that some other factors must play a role in the differences between the 2 centers studied. These results emphasize the importance of local site characteristics in the interpretation and implementation of national guidelines and recommendations. This finding may be particularly important in diseases and clinical presentations that rely on protocol‐driven pathways, such as UGH. Current recommendations on implementing clinical pathways derived from national guidelines emphasize the fact that national development and local implementation optimization is probably the best approach for effective pathway utilization.24
It is important to understand why ED and PUD, for which we now have effective pharmacologic therapies, continue to account for such a large percentage of the burden of UGH. In this study, we found that a majority of subjects were known to have significant risk factors for UGH (aspirin use, NSAID use, COX‐2s, or prior UGH) and only 31% of the subjects could not have been identified as at‐risk prior to admission. PPIs or COX‐2s should not be used universally as preventive therapy, and they are not completely effective at preventing UGH in at‐risk patients. In this study, two‐thirds of patients with risk factors were not on preventive therapy, but almost one‐third of patients with risk factors had bleeding despite being on preventive therapy. A better understanding of why these treatment failures (bleeding despite preventive therapy) occur may be helpful in our future ability to prevent UGH. This study was not designed to determine if the two‐thirds of patients not taking preventive therapy were being treated consistent with established guidelines. However, current guidelines have significant variation in recommendations as to which patients are at high enough risk to warrant preventive therapy,1315 and there is no consensus as to which patients are at high enough risk to warrant preventive therapy. Our data suggest that additional studies will be required to determine the optimal recommendations for preventive therapy among at‐risk patients.
There are several limitations to this study. First, it only included 2 academic institutions. However, these institutions represented very different patient populations. Second, the study design is not a population‐based study. This limitation prevents us from addressing questions such as the effectiveness or cost‐effectiveness of interventions to prevent admission for UGH. Although we analyzed preadmission PPI or COX‐2 use in at‐risk patients as preventive therapy, we are unable to determine the actual intent of the physician in prescribing these drugs. Finally, although the mechanisms by which PPIs and COX‐2 affect the risk of UGH are fundamentally different and should not be considered equivalent choices, we chose to analyze either option as representing a preventive strategy in order to provide the most conservative estimate possible of preventive therapy utilization rates. However, our assumptions would generally overestimate the use of preventive therapy (as opposed to PPI use for symptom control), as we assumed all potentially preventive therapy was intended as such.
This study highlights several unanswered questions that may be important in the management of UGH. First, identifying factors that affect local patters of UGH may better inform local implementation of nationally developed guidelines. Second, a more complete understanding of the impact positive and negative risk factors for UGH have on specific patient populations may allow for a more consistent targeted approach to using preventive therapy in at‐risk patients.
Finally, and perhaps most importantly, is to determine if the change in distribution of etiologies is in fact related to a decline in bleeding related to PUD. In addition to this being a marker of the success of the H. pylori story, it may have important implications on our understanding of the acute management of UGH. If PUD is of a different severity than other common causes of UGH, such as ED, current risk stratification prediction models may need to be revalidated. For example, if UGH secondary to PUD results in greater morbidity and mortality than UGH secondary to ED, our current models identifying who requires ICU admission, urgent endoscopy, and other therapeutic interventions may result in overutilization of these resource intensive interventions. However, if larger studies do not confirm this decline in PUD it suggests the need for additional studies to identify why PUD remains so prevalent despite the major advances in treatment and prevention of PUD through H. pylori identification and eradication.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Int Med.2002;137(11):866–874.
- .Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage: a population‐based study.Am J Gastroenterol.1995;90(2):206–210.
- ,,, et al.Epidemiology and course of acute upper gastro‐intestinal haemorrhage in four French geographical areas.Eur J Gastroenterol Hepatol.2000;12:175–181.
- ,,, et al.Prevention of ulcer recurrence after eradication of Helicobacter pylore: a prospective long‐term follow‐up study.Gastroenterology.1997;113:1082–1086.
- ,,, et al.Treatment of Helicobacter pylore in patients with duodenal ulcer hemorrhage‐a long‐term randomized, controlled study.Am J Gasterenterol.2000;95:2225–2232.
- ,,, et al.Preventing recurrent upper gastrointestinal bleeding in patients with Helicobacter pylori infection who are taking low‐dose aspirin or naproxen.N Engl J Med.2001;344:967–973.
- ,,, et al.Lansoprazole for the prevention of recurrences of ulcer complications from long‐term low‐dose aspirin use.N Engl J Med.2002;346:2033–2038.
- ,,, et al.Acute upper GI bleeding: did anything change?: time trend analysis of incidence and outcome of acute upper GI bleeding between 1993/1994 and 2000.Am J Gastroenterol.2003;98:1494–1499.
- ,,, et al.Upper gastrointestinal hemorrhage clinical guideline‐determining the optimal length of stay.Am J Med.1996;100:313–322.
- ,,.Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding.Ann Intern Med.2003;139:843–857.
- ,,, et al.Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group.N Engl J Med.2000;343:1520–1528.
- ,, et al.Gastrointestinal toxicity with celecoxib vs nonsteroidal anti‐inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long‐term Arthritis Safety Study.JAMA.2000;284:1247–1255.
- AGS Panel on Persistent Pain in Older Persons.The management of persistent pain in older persons.J Am Geriatr Soc.2002;50(6 Suppl):S205–S224.
- ,,, et al.Pain in osteoarthritis, rheumatoid arthritis and juvenile chronic arthritis.2nd ed.Clinical practice guideline no. 2.Glenview, IL:American Pain Society (APS);2002:179 p.
- .Recommendations for the medical management of osteoarthritis of the hip and knee.Arthritis Rheum.2000;43:1905–1915.
- ,,, et al.Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom.BMJ.1995;311:222–226.
- ,,, et al.Risk factors for hospitalized gastrointestinal bleeding among older persons.J Am Geriatr Soc.2001;49:126–133.
- ,,, et al.Effects of inpatient experience on outcomes and costs in a multicenter trial of academic hospitalists.Society of General Internal Medicine Annual Meeting2005.
- ,,,,,.Early endoscopy in upper gastrointestinal hemorrhage: association with recurrent bleeding, surgery, and length of hospital stay.Gastrointest Endosc.1999;49(2):145–152.
- ,,, et al.A comparison of the spectrum of chronic hepatitis C virus between Caucasians and African Americans.Clin Gastroenterol Hepatol.2004;2:469–473.
- ,,, et al.Gastroesophageal reflux among different racial groups in the United States.Gastroenterology.2004;126:1692–1699.
- ,,,.Risk factors for erosive reflux esophagitis: a case‐control study.Am J Gastroenterol.2001;96:41–46.
- ,.Upper gastrointestinal toxicity of nonsteroidal anti‐inflammatory drugs in African‐American and Hispanic elderly patients.Ethn Dis.2003;13:528–533.
- ,.Evidence‐based quality improvement: the state of the science.Health Aff (Millwood).2005;24(1):138–150.
Upper gastrointestinal hemorrhage (UGH) is a common cause of acute admission for hospitalization.13 However, recent advances in our understanding of erosive disease (ED) and peptic ulcer disease (PUD), 2 of the most common etiologies of UGH, have led to effective strategies to reduce the risk of UGH. Successful implementation of these strategies, such as treatment of Helicobacter pylori (H. pylori) and the use of proton pump inhibitors (PPIs) and selective cyclooxygenase‐2 inhibitors (COX‐2s) in place of traditional nonselective nonsteroidal antiinflammatory drugs (NSAIDs), may be able to significantly reduce rates of UGH caused by ED and PUD.47
Prior to these preventive treatments, PUD and ED, both acid‐related disorders, were the most common causes of UGH requiring admission to the hospital, accounting for 62% and 14% of all UGHs, respectively.2 Given the widespread treatment of H. pylori and use of PPIs and COX‐2s, we might expect that the distribution of etiologies of UGH may have changed. However, there are limited data on the distribution of etiologies of UGH in the era of effective preventive therapy.8 If the distribution of etiologies causing patients to present with UGH has fundamentally changed with these new treatments, established strategies of managing acute UGH may need to be reevaluated. Given that well‐established guidelines exist and that many hospitals use a protocol‐driven management strategy to decide on the need for admission and/or intensive care unit (ICU) admission, changes in the distribution of etiologies since the widespread use of these new pharmacologic approaches may affect the appropriateness of these protocols.9, 10 For example, if the eradication of H. pylori has dramatically reduced the proportion of UGH caused by PUD, then risk stratification studies developed when PUD was far more common may need to be revisited. This would be particularly important if bleeding from PUD was of significantly different severity than bleeding from other causes.
While patients with H. pylori‐related UGH from PUD should be treated for H. pylori eradication, several important questions remain surrounding the use of newer therapeutics that may mitigate the risk of UGH in some patients. It is unclear what proportion of patients admitted with UGH in this new era developed bleeding despite using preventive therapy. These treatment failures are known to occur, but it is not well known how much of the burden of UGH today is due to this breakthrough bleeding.5, 6, 11, 12 Contrastingly, there are also patients who are admitted with UGH who are not on preventive treatment. Current guidelines suggest that high‐risk patients requiring NSAIDs be given COX‐2s or traditional NSAIDs with a PPI.1315 However, there is significant disagreement between these national guidelines about what constitutes a high‐risk profile.1315 For example, some guidelines recommend that elderly patients requiring NSAIDs should be on a PPI while others do not make that recommendation. Similarly, while prior UGH is a well‐recognized risk factor for future bleeding risk even without NSAIDs, current guidelines do not provide guidance toward the use of preventive therapy in these patients. If there are few patients who present with UGH related to acid disease that are not on a preventive therapy, then these unanswered questions or conflicts within current guidelines become less important. However, if a large portion of UGH is due to acid‐related disease in patients not on preventive therapy, then these unanswered questions may become important for future research.
In contrast to previous studies, the current study examines the distribution of etiologies of UGH in the era of widespread use of effective preventive therapy for ED and PUD in 2 U.S. academic medical centers. Prior studies were done before the advent of new therapeutics and did not compare different sites, which may be important.16, 17
PATIENTS AND METHODS
Patients
Consecutive patients admitted with UGH were identified at 2 academic medical centers as part of a larger observational study examining the impact of hospitalist physicians on the care of acute medical patients.18 The sample was selected from the 12,091 consecutive general medical patients admitted from July 2001 to June 2003 with UGH identified by International Classification of Diseases, Ninth revision, Clinical Modification (ICD‐9 CM) codes from administrative data and confirmed by chart abstraction. ICD‐9 CM codes for UGH included: esophageal varices with hemorrhage (456.0 and 456.20), Mallory‐Weiss syndrome (530.7), gastric ulcer with hemorrhage (531.00‐531.61), duodenal ulcer with hemorrhage (532.00‐532.61), peptic ulcer, site unspecified, with hemorrhage (533.00‐533.61), gastrojejunal ulcer with hemorrhage (534.00‐534.61), gastritis with hemorrhage (535.61), angiodysplasia of stomach/duodenum with hemorrhage (537.83), and hematemesis (578.0 and 578.9).19 Finally, the admission diagnoses for all patients in the larger cohort were reviewed and any with gastrointestinal hemorrhage were screened for possible inclusion to account for any missed ICD‐9 codes. Subjects were then included in this analysis if they had observed hematemesis, nasogastric (NG) tube aspirate with gross or hemoccult blood, or history of hematemesis, bloody diarrhea, or melena upon chart review.
Data
The inpatient medical records were abstracted by trained researchers. Etiologies of UGH were assessed by esophagogastroduodenoscopy (EGD) report, which listed findings and etiologies as assessed by the endoscopist. Multiple etiologies were allowed if more than 1 source of bleeding was identified. Prior medical history and preadmission medication use were obtained from 3 sources: (1) the emergency department medical record; (2) nursing admission documentation; and (3) the admission history and physical documentation. Risk factors and preadmission medication use were considered present if documented in any of the 3 sources. Relevant past medical history included known risk factors for UGH, including: end‐stage renal disease, alcohol abuse, prior history of UGH, and steroid use. Prior H. pylori status/testing could not reliably be obtained from these data sources. Preadmission medication use of interest included aspirin, NSAIDS, anticoagulants, antiplatelet agents, as well as PPIs and COX‐2s. Demographics, including age, race, and gender, were obtained from administrative databases.
We defined subjects as at‐risk if they had any of the following risk factors: prior UGH (at any time), use of an NSAID (traditional or selective COX‐2), or use of an aspirin prior to admission. Patients taking COX‐2s were included for 2 reasons. First, while COX‐2 inhibitors are associated with a lower risk of UGH than traditional NSAIDs, it is likely that they still lead to an increased risk of UGH compared to placebo. Second, if a patient required NSAIDs of some type (traditional or selective), preadmission use of a COX‐2 rather than a traditional NSAID may reflect the intention of decreasing the risk of UGH compared to using traditional NSAIDs. In order to use the most conservative estimate of potential missed opportunities for prevention, preadmission use of a PPI or COX‐2 was considered preventive therapy. All preadmission medication use was obtained from chart review. Therefore, duration of and purpose for medication use were not available.
Development of the abstraction tool was performed by the authors. Testing of the tool was performed on a learning set of 20 charts at each center. All additional abstractors were trained with a learning set of at least 20 charts to assure uniform abstraction techniques.
Analysis
For each risk factor and etiology, we calculated the proportion of patients with the risk factor or etiology both overall and by site. Differences in risk factors between sites were assessed using chi‐square tests of association. Differences in etiologies between sites were assessed using unadjusted odds ratios (ORs) as well as ORs from logistic regression models controlling for age, gender, and race (black versus not black). Center 1 was the urban center and center 2 was the rural site.
This study was approved by the Institutional Review Board at the University of Iowa Carver College of Medicine and the University of Chicago.
RESULTS
From the entire cohort of 12,091 admitted to the 2 inpatient medical services, 227 (1.9%) patients were identified as having UGH; 138 (61%) were from center 1, where 87% of patients were black and 89 (39%) were from center 2, where 89% of patients were white. Overall, the mean age was 59 years, 45% were female, and 41% were white (Table 1).
| Characteristic | Total (n = 227) | Center 1 (n = 138) | Center 2 (n = 89) | P Value Center 1 versus 2 |
|---|---|---|---|---|
| ||||
| Mean age (years) | 58.6 | 59.5 | 57.1 | 0.317 |
| % Female | 44.5 | 48.6 | 38.2 | 0.126 |
| % White | 41.2 | 10.2 | 88.8 | <0.001 |
| % African American | 54.0 | 86.9 | 3.4 | <0.001 |
| % Other | 4.9 | 2.9 | 7.9 | <0.001 |
The most common etiologies of UGH were ED (44%), PUD (33%), and varices (17%) in the overall population. These same 3 etiologies were also the most common in both of the medical centers, although there were significant differences in the rates of etiologies between the 2 centers. ED was more common among subjects from center 1 (59%) than from center 2 (19%) (P < 0.001), while variceal bleeding was more common among subjects from center 2 (34%) than from center 1 (6.5%) (P = 0.009) (Table 2).
| Etiology | All n = 227 (%) | Center 1 n = 138 (%) | Center 2 n = 89 (%) | Unadjusted OR (95% CI): Center 1 versus 2 | P Value for Unadjusted OR | Adjusted* OR (95% CI): Center 1 versus 2 | P Value (for Adjusted OR) |
|---|---|---|---|---|---|---|---|
| |||||||
| ED | 43.6 | 59.4 | 19.1 | 6.20 (3.3111.62) | <0.001 | 7.10 (2.4820.31) | <0.001 |
| PUD | 33.0 | 37.0 | 27.0 | 1.59 (0.892.84) | 0.119 | 1.33 (0.483.67) | 0.578 |
| Varices | 17.2 | 6.5 | 33.7 | 0.14 (0.060.31) | <0.001 | 0.12 (0.030.60) | 0.009 |
| AVM | 5.3 | 2.9 | 9.0 | 0.30 (0.091.04) | 0.057 | 0.21 (0.031.69) | 0.141 |
| Mallory Weiss Tear | 4.9 | 4.4 | 5.6 | 0.76 (0.232.58) | 0.664 | 0.34 (0.024.85) | 0.425 |
| Cancer/masses | 2.6 | 2.9 | 2.3 | 1.30 (0.237.24) | 0.766 | 0.62 (0.0312.12) | 0.751 |
In multivariate logistic regression analyses, only age and site remained independent predictors of etiologies. Advancing age was associated with a higher risk of arteriovenous malformations (AVMs) with the odds of AVMs increasing 6% for every additional year of life (P = 0.007). Site was associated with both ED and variceal bleeding. Patients from center 1 were significantly more likely to have UGH caused by ED, with an OR = 7.10 (P < 0.001), compared to subjects from center 2. However, subjects from center 1 had a significantly lower OR (OR = 0.12) than those subjects at center 2 (P = 0.009) of having UGH caused by a variceal bleed (Table 2).
Risk factors for UGH were common among the patients, including use of aspirin (25.1%), NSAIDs (22.9%), COX‐2s (4.9%), or prior history of UGH (43%). Additionally, 6.6% of patients were taking both an NSAID and aspirin. Differences between the 2 sites were seen only in aspirin use, with 34.8% of patients in the center 1 population using aspirin compared to 10.1% in center 2 (P < 0.001) (Table 3).
| Risk Factor | All (%) | Center 1 (%) | Center 2 (%) | P Value |
|---|---|---|---|---|
| ||||
| Previous UGH | 42.7 | 41.3 | 45.2 | 0.586 |
| NSAID use | 22.9 | 21.7 | 24.7 | 0.602 |
| ASA use | 25.1 | 34.8 | 10.1 | <0.001 |
| NSAID + ASA | 6.6 | 6.5 | 6.7 | 0.948 |
| COX‐2 use | 4.9 | 6.5 | 2.3 | 0.143 |
| PPI use | 18.5 | 18.1 | 19.1 | 0.852 |
Among the overall population, 68.7% of patients had identifiable risk factors (prior history of UGH or preadmission use of aspirin, NSAIDs, or COX‐2s). Of all subjects, 18.5% were on PPIs and 4.9% were taking COX‐2s while 21.1% of at risk subjects were on PPIs and 6.5% of these subjects were on a COX‐2.
Finally, we examined the effects of variations in preadmission medication use between the sites on the etiologies of UGH. None of the site‐based differences in etiologies could be explained by differences in preadmission medication patterns.
DISCUSSION
Despite the emergence of effective therapies for lowering the risk of ED and PUD, these remain the most common etiologies of UGH in our cohort of patients. In a dramatic change from historically reported patterns, ED was more common than PUD. In prior studies, PUD accounted for almost two‐thirds of all UGH.2 While some of the newer therapeutics such as PPIs and COX‐2s reduce the risk for acid‐related bleeding of all types, H. pylori eradication is effective primarily for PUD. Therefore, it may be that widespread testing and treatment of H. pylori have dramatically decreased rates of PUD. Unfortunately, this study does not allow us to directly evaluate the effect of H. pylori treatment on the changing epidemiology of UGH, as that would require a population‐based study.
While decreasing rates of PUD could explain a portion of the change in the distribution of etiologies, increasing rates of ED could also be playing a role. Prior studies have suggested that African Americans and the elderly are more susceptible to ED, particularly in the setting of NSAIDs and/or aspirin use, and less susceptible to cirrhosis.13, 16, 17, 2023 Our finding of a higher rate of ED and lower rates of cirrhosis in center 1 with a higher proportion of African Americans and greater aspirin use is consistent with these prior findings. However, in multivariate analyses, neither race nor preadmission medication use patterns explained the differences in etiologies seen. This suggests that some other factors must play a role in the differences between the 2 centers studied. These results emphasize the importance of local site characteristics in the interpretation and implementation of national guidelines and recommendations. This finding may be particularly important in diseases and clinical presentations that rely on protocol‐driven pathways, such as UGH. Current recommendations on implementing clinical pathways derived from national guidelines emphasize the fact that national development and local implementation optimization is probably the best approach for effective pathway utilization.24
It is important to understand why ED and PUD, for which we now have effective pharmacologic therapies, continue to account for such a large percentage of the burden of UGH. In this study, we found that a majority of subjects were known to have significant risk factors for UGH (aspirin use, NSAID use, COX‐2s, or prior UGH) and only 31% of the subjects could not have been identified as at‐risk prior to admission. PPIs or COX‐2s should not be used universally as preventive therapy, and they are not completely effective at preventing UGH in at‐risk patients. In this study, two‐thirds of patients with risk factors were not on preventive therapy, but almost one‐third of patients with risk factors had bleeding despite being on preventive therapy. A better understanding of why these treatment failures (bleeding despite preventive therapy) occur may be helpful in our future ability to prevent UGH. This study was not designed to determine if the two‐thirds of patients not taking preventive therapy were being treated consistent with established guidelines. However, current guidelines have significant variation in recommendations as to which patients are at high enough risk to warrant preventive therapy,1315 and there is no consensus as to which patients are at high enough risk to warrant preventive therapy. Our data suggest that additional studies will be required to determine the optimal recommendations for preventive therapy among at‐risk patients.
There are several limitations to this study. First, it only included 2 academic institutions. However, these institutions represented very different patient populations. Second, the study design is not a population‐based study. This limitation prevents us from addressing questions such as the effectiveness or cost‐effectiveness of interventions to prevent admission for UGH. Although we analyzed preadmission PPI or COX‐2 use in at‐risk patients as preventive therapy, we are unable to determine the actual intent of the physician in prescribing these drugs. Finally, although the mechanisms by which PPIs and COX‐2 affect the risk of UGH are fundamentally different and should not be considered equivalent choices, we chose to analyze either option as representing a preventive strategy in order to provide the most conservative estimate possible of preventive therapy utilization rates. However, our assumptions would generally overestimate the use of preventive therapy (as opposed to PPI use for symptom control), as we assumed all potentially preventive therapy was intended as such.
This study highlights several unanswered questions that may be important in the management of UGH. First, identifying factors that affect local patters of UGH may better inform local implementation of nationally developed guidelines. Second, a more complete understanding of the impact positive and negative risk factors for UGH have on specific patient populations may allow for a more consistent targeted approach to using preventive therapy in at‐risk patients.
Finally, and perhaps most importantly, is to determine if the change in distribution of etiologies is in fact related to a decline in bleeding related to PUD. In addition to this being a marker of the success of the H. pylori story, it may have important implications on our understanding of the acute management of UGH. If PUD is of a different severity than other common causes of UGH, such as ED, current risk stratification prediction models may need to be revalidated. For example, if UGH secondary to PUD results in greater morbidity and mortality than UGH secondary to ED, our current models identifying who requires ICU admission, urgent endoscopy, and other therapeutic interventions may result in overutilization of these resource intensive interventions. However, if larger studies do not confirm this decline in PUD it suggests the need for additional studies to identify why PUD remains so prevalent despite the major advances in treatment and prevention of PUD through H. pylori identification and eradication.
Upper gastrointestinal hemorrhage (UGH) is a common cause of acute admission for hospitalization.13 However, recent advances in our understanding of erosive disease (ED) and peptic ulcer disease (PUD), 2 of the most common etiologies of UGH, have led to effective strategies to reduce the risk of UGH. Successful implementation of these strategies, such as treatment of Helicobacter pylori (H. pylori) and the use of proton pump inhibitors (PPIs) and selective cyclooxygenase‐2 inhibitors (COX‐2s) in place of traditional nonselective nonsteroidal antiinflammatory drugs (NSAIDs), may be able to significantly reduce rates of UGH caused by ED and PUD.47
Prior to these preventive treatments, PUD and ED, both acid‐related disorders, were the most common causes of UGH requiring admission to the hospital, accounting for 62% and 14% of all UGHs, respectively.2 Given the widespread treatment of H. pylori and use of PPIs and COX‐2s, we might expect that the distribution of etiologies of UGH may have changed. However, there are limited data on the distribution of etiologies of UGH in the era of effective preventive therapy.8 If the distribution of etiologies causing patients to present with UGH has fundamentally changed with these new treatments, established strategies of managing acute UGH may need to be reevaluated. Given that well‐established guidelines exist and that many hospitals use a protocol‐driven management strategy to decide on the need for admission and/or intensive care unit (ICU) admission, changes in the distribution of etiologies since the widespread use of these new pharmacologic approaches may affect the appropriateness of these protocols.9, 10 For example, if the eradication of H. pylori has dramatically reduced the proportion of UGH caused by PUD, then risk stratification studies developed when PUD was far more common may need to be revisited. This would be particularly important if bleeding from PUD was of significantly different severity than bleeding from other causes.
While patients with H. pylori‐related UGH from PUD should be treated for H. pylori eradication, several important questions remain surrounding the use of newer therapeutics that may mitigate the risk of UGH in some patients. It is unclear what proportion of patients admitted with UGH in this new era developed bleeding despite using preventive therapy. These treatment failures are known to occur, but it is not well known how much of the burden of UGH today is due to this breakthrough bleeding.5, 6, 11, 12 Contrastingly, there are also patients who are admitted with UGH who are not on preventive treatment. Current guidelines suggest that high‐risk patients requiring NSAIDs be given COX‐2s or traditional NSAIDs with a PPI.1315 However, there is significant disagreement between these national guidelines about what constitutes a high‐risk profile.1315 For example, some guidelines recommend that elderly patients requiring NSAIDs should be on a PPI while others do not make that recommendation. Similarly, while prior UGH is a well‐recognized risk factor for future bleeding risk even without NSAIDs, current guidelines do not provide guidance toward the use of preventive therapy in these patients. If there are few patients who present with UGH related to acid disease that are not on a preventive therapy, then these unanswered questions or conflicts within current guidelines become less important. However, if a large portion of UGH is due to acid‐related disease in patients not on preventive therapy, then these unanswered questions may become important for future research.
In contrast to previous studies, the current study examines the distribution of etiologies of UGH in the era of widespread use of effective preventive therapy for ED and PUD in 2 U.S. academic medical centers. Prior studies were done before the advent of new therapeutics and did not compare different sites, which may be important.16, 17
PATIENTS AND METHODS
Patients
Consecutive patients admitted with UGH were identified at 2 academic medical centers as part of a larger observational study examining the impact of hospitalist physicians on the care of acute medical patients.18 The sample was selected from the 12,091 consecutive general medical patients admitted from July 2001 to June 2003 with UGH identified by International Classification of Diseases, Ninth revision, Clinical Modification (ICD‐9 CM) codes from administrative data and confirmed by chart abstraction. ICD‐9 CM codes for UGH included: esophageal varices with hemorrhage (456.0 and 456.20), Mallory‐Weiss syndrome (530.7), gastric ulcer with hemorrhage (531.00‐531.61), duodenal ulcer with hemorrhage (532.00‐532.61), peptic ulcer, site unspecified, with hemorrhage (533.00‐533.61), gastrojejunal ulcer with hemorrhage (534.00‐534.61), gastritis with hemorrhage (535.61), angiodysplasia of stomach/duodenum with hemorrhage (537.83), and hematemesis (578.0 and 578.9).19 Finally, the admission diagnoses for all patients in the larger cohort were reviewed and any with gastrointestinal hemorrhage were screened for possible inclusion to account for any missed ICD‐9 codes. Subjects were then included in this analysis if they had observed hematemesis, nasogastric (NG) tube aspirate with gross or hemoccult blood, or history of hematemesis, bloody diarrhea, or melena upon chart review.
Data
The inpatient medical records were abstracted by trained researchers. Etiologies of UGH were assessed by esophagogastroduodenoscopy (EGD) report, which listed findings and etiologies as assessed by the endoscopist. Multiple etiologies were allowed if more than 1 source of bleeding was identified. Prior medical history and preadmission medication use were obtained from 3 sources: (1) the emergency department medical record; (2) nursing admission documentation; and (3) the admission history and physical documentation. Risk factors and preadmission medication use were considered present if documented in any of the 3 sources. Relevant past medical history included known risk factors for UGH, including: end‐stage renal disease, alcohol abuse, prior history of UGH, and steroid use. Prior H. pylori status/testing could not reliably be obtained from these data sources. Preadmission medication use of interest included aspirin, NSAIDS, anticoagulants, antiplatelet agents, as well as PPIs and COX‐2s. Demographics, including age, race, and gender, were obtained from administrative databases.
We defined subjects as at‐risk if they had any of the following risk factors: prior UGH (at any time), use of an NSAID (traditional or selective COX‐2), or use of an aspirin prior to admission. Patients taking COX‐2s were included for 2 reasons. First, while COX‐2 inhibitors are associated with a lower risk of UGH than traditional NSAIDs, it is likely that they still lead to an increased risk of UGH compared to placebo. Second, if a patient required NSAIDs of some type (traditional or selective), preadmission use of a COX‐2 rather than a traditional NSAID may reflect the intention of decreasing the risk of UGH compared to using traditional NSAIDs. In order to use the most conservative estimate of potential missed opportunities for prevention, preadmission use of a PPI or COX‐2 was considered preventive therapy. All preadmission medication use was obtained from chart review. Therefore, duration of and purpose for medication use were not available.
Development of the abstraction tool was performed by the authors. Testing of the tool was performed on a learning set of 20 charts at each center. All additional abstractors were trained with a learning set of at least 20 charts to assure uniform abstraction techniques.
Analysis
For each risk factor and etiology, we calculated the proportion of patients with the risk factor or etiology both overall and by site. Differences in risk factors between sites were assessed using chi‐square tests of association. Differences in etiologies between sites were assessed using unadjusted odds ratios (ORs) as well as ORs from logistic regression models controlling for age, gender, and race (black versus not black). Center 1 was the urban center and center 2 was the rural site.
This study was approved by the Institutional Review Board at the University of Iowa Carver College of Medicine and the University of Chicago.
RESULTS
From the entire cohort of 12,091 admitted to the 2 inpatient medical services, 227 (1.9%) patients were identified as having UGH; 138 (61%) were from center 1, where 87% of patients were black and 89 (39%) were from center 2, where 89% of patients were white. Overall, the mean age was 59 years, 45% were female, and 41% were white (Table 1).
| Characteristic | Total (n = 227) | Center 1 (n = 138) | Center 2 (n = 89) | P Value Center 1 versus 2 |
|---|---|---|---|---|
| ||||
| Mean age (years) | 58.6 | 59.5 | 57.1 | 0.317 |
| % Female | 44.5 | 48.6 | 38.2 | 0.126 |
| % White | 41.2 | 10.2 | 88.8 | <0.001 |
| % African American | 54.0 | 86.9 | 3.4 | <0.001 |
| % Other | 4.9 | 2.9 | 7.9 | <0.001 |
The most common etiologies of UGH were ED (44%), PUD (33%), and varices (17%) in the overall population. These same 3 etiologies were also the most common in both of the medical centers, although there were significant differences in the rates of etiologies between the 2 centers. ED was more common among subjects from center 1 (59%) than from center 2 (19%) (P < 0.001), while variceal bleeding was more common among subjects from center 2 (34%) than from center 1 (6.5%) (P = 0.009) (Table 2).
| Etiology | All n = 227 (%) | Center 1 n = 138 (%) | Center 2 n = 89 (%) | Unadjusted OR (95% CI): Center 1 versus 2 | P Value for Unadjusted OR | Adjusted* OR (95% CI): Center 1 versus 2 | P Value (for Adjusted OR) |
|---|---|---|---|---|---|---|---|
| |||||||
| ED | 43.6 | 59.4 | 19.1 | 6.20 (3.3111.62) | <0.001 | 7.10 (2.4820.31) | <0.001 |
| PUD | 33.0 | 37.0 | 27.0 | 1.59 (0.892.84) | 0.119 | 1.33 (0.483.67) | 0.578 |
| Varices | 17.2 | 6.5 | 33.7 | 0.14 (0.060.31) | <0.001 | 0.12 (0.030.60) | 0.009 |
| AVM | 5.3 | 2.9 | 9.0 | 0.30 (0.091.04) | 0.057 | 0.21 (0.031.69) | 0.141 |
| Mallory Weiss Tear | 4.9 | 4.4 | 5.6 | 0.76 (0.232.58) | 0.664 | 0.34 (0.024.85) | 0.425 |
| Cancer/masses | 2.6 | 2.9 | 2.3 | 1.30 (0.237.24) | 0.766 | 0.62 (0.0312.12) | 0.751 |
In multivariate logistic regression analyses, only age and site remained independent predictors of etiologies. Advancing age was associated with a higher risk of arteriovenous malformations (AVMs) with the odds of AVMs increasing 6% for every additional year of life (P = 0.007). Site was associated with both ED and variceal bleeding. Patients from center 1 were significantly more likely to have UGH caused by ED, with an OR = 7.10 (P < 0.001), compared to subjects from center 2. However, subjects from center 1 had a significantly lower OR (OR = 0.12) than those subjects at center 2 (P = 0.009) of having UGH caused by a variceal bleed (Table 2).
Risk factors for UGH were common among the patients, including use of aspirin (25.1%), NSAIDs (22.9%), COX‐2s (4.9%), or prior history of UGH (43%). Additionally, 6.6% of patients were taking both an NSAID and aspirin. Differences between the 2 sites were seen only in aspirin use, with 34.8% of patients in the center 1 population using aspirin compared to 10.1% in center 2 (P < 0.001) (Table 3).
| Risk Factor | All (%) | Center 1 (%) | Center 2 (%) | P Value |
|---|---|---|---|---|
| ||||
| Previous UGH | 42.7 | 41.3 | 45.2 | 0.586 |
| NSAID use | 22.9 | 21.7 | 24.7 | 0.602 |
| ASA use | 25.1 | 34.8 | 10.1 | <0.001 |
| NSAID + ASA | 6.6 | 6.5 | 6.7 | 0.948 |
| COX‐2 use | 4.9 | 6.5 | 2.3 | 0.143 |
| PPI use | 18.5 | 18.1 | 19.1 | 0.852 |
Among the overall population, 68.7% of patients had identifiable risk factors (prior history of UGH or preadmission use of aspirin, NSAIDs, or COX‐2s). Of all subjects, 18.5% were on PPIs and 4.9% were taking COX‐2s while 21.1% of at risk subjects were on PPIs and 6.5% of these subjects were on a COX‐2.
Finally, we examined the effects of variations in preadmission medication use between the sites on the etiologies of UGH. None of the site‐based differences in etiologies could be explained by differences in preadmission medication patterns.
DISCUSSION
Despite the emergence of effective therapies for lowering the risk of ED and PUD, these remain the most common etiologies of UGH in our cohort of patients. In a dramatic change from historically reported patterns, ED was more common than PUD. In prior studies, PUD accounted for almost two‐thirds of all UGH.2 While some of the newer therapeutics such as PPIs and COX‐2s reduce the risk for acid‐related bleeding of all types, H. pylori eradication is effective primarily for PUD. Therefore, it may be that widespread testing and treatment of H. pylori have dramatically decreased rates of PUD. Unfortunately, this study does not allow us to directly evaluate the effect of H. pylori treatment on the changing epidemiology of UGH, as that would require a population‐based study.
While decreasing rates of PUD could explain a portion of the change in the distribution of etiologies, increasing rates of ED could also be playing a role. Prior studies have suggested that African Americans and the elderly are more susceptible to ED, particularly in the setting of NSAIDs and/or aspirin use, and less susceptible to cirrhosis.13, 16, 17, 2023 Our finding of a higher rate of ED and lower rates of cirrhosis in center 1 with a higher proportion of African Americans and greater aspirin use is consistent with these prior findings. However, in multivariate analyses, neither race nor preadmission medication use patterns explained the differences in etiologies seen. This suggests that some other factors must play a role in the differences between the 2 centers studied. These results emphasize the importance of local site characteristics in the interpretation and implementation of national guidelines and recommendations. This finding may be particularly important in diseases and clinical presentations that rely on protocol‐driven pathways, such as UGH. Current recommendations on implementing clinical pathways derived from national guidelines emphasize the fact that national development and local implementation optimization is probably the best approach for effective pathway utilization.24
It is important to understand why ED and PUD, for which we now have effective pharmacologic therapies, continue to account for such a large percentage of the burden of UGH. In this study, we found that a majority of subjects were known to have significant risk factors for UGH (aspirin use, NSAID use, COX‐2s, or prior UGH) and only 31% of the subjects could not have been identified as at‐risk prior to admission. PPIs or COX‐2s should not be used universally as preventive therapy, and they are not completely effective at preventing UGH in at‐risk patients. In this study, two‐thirds of patients with risk factors were not on preventive therapy, but almost one‐third of patients with risk factors had bleeding despite being on preventive therapy. A better understanding of why these treatment failures (bleeding despite preventive therapy) occur may be helpful in our future ability to prevent UGH. This study was not designed to determine if the two‐thirds of patients not taking preventive therapy were being treated consistent with established guidelines. However, current guidelines have significant variation in recommendations as to which patients are at high enough risk to warrant preventive therapy,1315 and there is no consensus as to which patients are at high enough risk to warrant preventive therapy. Our data suggest that additional studies will be required to determine the optimal recommendations for preventive therapy among at‐risk patients.
There are several limitations to this study. First, it only included 2 academic institutions. However, these institutions represented very different patient populations. Second, the study design is not a population‐based study. This limitation prevents us from addressing questions such as the effectiveness or cost‐effectiveness of interventions to prevent admission for UGH. Although we analyzed preadmission PPI or COX‐2 use in at‐risk patients as preventive therapy, we are unable to determine the actual intent of the physician in prescribing these drugs. Finally, although the mechanisms by which PPIs and COX‐2 affect the risk of UGH are fundamentally different and should not be considered equivalent choices, we chose to analyze either option as representing a preventive strategy in order to provide the most conservative estimate possible of preventive therapy utilization rates. However, our assumptions would generally overestimate the use of preventive therapy (as opposed to PPI use for symptom control), as we assumed all potentially preventive therapy was intended as such.
This study highlights several unanswered questions that may be important in the management of UGH. First, identifying factors that affect local patters of UGH may better inform local implementation of nationally developed guidelines. Second, a more complete understanding of the impact positive and negative risk factors for UGH have on specific patient populations may allow for a more consistent targeted approach to using preventive therapy in at‐risk patients.
Finally, and perhaps most importantly, is to determine if the change in distribution of etiologies is in fact related to a decline in bleeding related to PUD. In addition to this being a marker of the success of the H. pylori story, it may have important implications on our understanding of the acute management of UGH. If PUD is of a different severity than other common causes of UGH, such as ED, current risk stratification prediction models may need to be revalidated. For example, if UGH secondary to PUD results in greater morbidity and mortality than UGH secondary to ED, our current models identifying who requires ICU admission, urgent endoscopy, and other therapeutic interventions may result in overutilization of these resource intensive interventions. However, if larger studies do not confirm this decline in PUD it suggests the need for additional studies to identify why PUD remains so prevalent despite the major advances in treatment and prevention of PUD through H. pylori identification and eradication.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Int Med.2002;137(11):866–874.
- .Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage: a population‐based study.Am J Gastroenterol.1995;90(2):206–210.
- ,,, et al.Epidemiology and course of acute upper gastro‐intestinal haemorrhage in four French geographical areas.Eur J Gastroenterol Hepatol.2000;12:175–181.
- ,,, et al.Prevention of ulcer recurrence after eradication of Helicobacter pylore: a prospective long‐term follow‐up study.Gastroenterology.1997;113:1082–1086.
- ,,, et al.Treatment of Helicobacter pylore in patients with duodenal ulcer hemorrhage‐a long‐term randomized, controlled study.Am J Gasterenterol.2000;95:2225–2232.
- ,,, et al.Preventing recurrent upper gastrointestinal bleeding in patients with Helicobacter pylori infection who are taking low‐dose aspirin or naproxen.N Engl J Med.2001;344:967–973.
- ,,, et al.Lansoprazole for the prevention of recurrences of ulcer complications from long‐term low‐dose aspirin use.N Engl J Med.2002;346:2033–2038.
- ,,, et al.Acute upper GI bleeding: did anything change?: time trend analysis of incidence and outcome of acute upper GI bleeding between 1993/1994 and 2000.Am J Gastroenterol.2003;98:1494–1499.
- ,,, et al.Upper gastrointestinal hemorrhage clinical guideline‐determining the optimal length of stay.Am J Med.1996;100:313–322.
- ,,.Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding.Ann Intern Med.2003;139:843–857.
- ,,, et al.Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group.N Engl J Med.2000;343:1520–1528.
- ,, et al.Gastrointestinal toxicity with celecoxib vs nonsteroidal anti‐inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long‐term Arthritis Safety Study.JAMA.2000;284:1247–1255.
- AGS Panel on Persistent Pain in Older Persons.The management of persistent pain in older persons.J Am Geriatr Soc.2002;50(6 Suppl):S205–S224.
- ,,, et al.Pain in osteoarthritis, rheumatoid arthritis and juvenile chronic arthritis.2nd ed.Clinical practice guideline no. 2.Glenview, IL:American Pain Society (APS);2002:179 p.
- .Recommendations for the medical management of osteoarthritis of the hip and knee.Arthritis Rheum.2000;43:1905–1915.
- ,,, et al.Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom.BMJ.1995;311:222–226.
- ,,, et al.Risk factors for hospitalized gastrointestinal bleeding among older persons.J Am Geriatr Soc.2001;49:126–133.
- ,,, et al.Effects of inpatient experience on outcomes and costs in a multicenter trial of academic hospitalists.Society of General Internal Medicine Annual Meeting2005.
- ,,,,,.Early endoscopy in upper gastrointestinal hemorrhage: association with recurrent bleeding, surgery, and length of hospital stay.Gastrointest Endosc.1999;49(2):145–152.
- ,,, et al.A comparison of the spectrum of chronic hepatitis C virus between Caucasians and African Americans.Clin Gastroenterol Hepatol.2004;2:469–473.
- ,,, et al.Gastroesophageal reflux among different racial groups in the United States.Gastroenterology.2004;126:1692–1699.
- ,,,.Risk factors for erosive reflux esophagitis: a case‐control study.Am J Gastroenterol.2001;96:41–46.
- ,.Upper gastrointestinal toxicity of nonsteroidal anti‐inflammatory drugs in African‐American and Hispanic elderly patients.Ethn Dis.2003;13:528–533.
- ,.Evidence‐based quality improvement: the state of the science.Health Aff (Millwood).2005;24(1):138–150.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Int Med.2002;137(11):866–874.
- .Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage: a population‐based study.Am J Gastroenterol.1995;90(2):206–210.
- ,,, et al.Epidemiology and course of acute upper gastro‐intestinal haemorrhage in four French geographical areas.Eur J Gastroenterol Hepatol.2000;12:175–181.
- ,,, et al.Prevention of ulcer recurrence after eradication of Helicobacter pylore: a prospective long‐term follow‐up study.Gastroenterology.1997;113:1082–1086.
- ,,, et al.Treatment of Helicobacter pylore in patients with duodenal ulcer hemorrhage‐a long‐term randomized, controlled study.Am J Gasterenterol.2000;95:2225–2232.
- ,,, et al.Preventing recurrent upper gastrointestinal bleeding in patients with Helicobacter pylori infection who are taking low‐dose aspirin or naproxen.N Engl J Med.2001;344:967–973.
- ,,, et al.Lansoprazole for the prevention of recurrences of ulcer complications from long‐term low‐dose aspirin use.N Engl J Med.2002;346:2033–2038.
- ,,, et al.Acute upper GI bleeding: did anything change?: time trend analysis of incidence and outcome of acute upper GI bleeding between 1993/1994 and 2000.Am J Gastroenterol.2003;98:1494–1499.
- ,,, et al.Upper gastrointestinal hemorrhage clinical guideline‐determining the optimal length of stay.Am J Med.1996;100:313–322.
- ,,.Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding.Ann Intern Med.2003;139:843–857.
- ,,, et al.Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group.N Engl J Med.2000;343:1520–1528.
- ,, et al.Gastrointestinal toxicity with celecoxib vs nonsteroidal anti‐inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long‐term Arthritis Safety Study.JAMA.2000;284:1247–1255.
- AGS Panel on Persistent Pain in Older Persons.The management of persistent pain in older persons.J Am Geriatr Soc.2002;50(6 Suppl):S205–S224.
- ,,, et al.Pain in osteoarthritis, rheumatoid arthritis and juvenile chronic arthritis.2nd ed.Clinical practice guideline no. 2.Glenview, IL:American Pain Society (APS);2002:179 p.
- .Recommendations for the medical management of osteoarthritis of the hip and knee.Arthritis Rheum.2000;43:1905–1915.
- ,,, et al.Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom.BMJ.1995;311:222–226.
- ,,, et al.Risk factors for hospitalized gastrointestinal bleeding among older persons.J Am Geriatr Soc.2001;49:126–133.
- ,,, et al.Effects of inpatient experience on outcomes and costs in a multicenter trial of academic hospitalists.Society of General Internal Medicine Annual Meeting2005.
- ,,,,,.Early endoscopy in upper gastrointestinal hemorrhage: association with recurrent bleeding, surgery, and length of hospital stay.Gastrointest Endosc.1999;49(2):145–152.
- ,,, et al.A comparison of the spectrum of chronic hepatitis C virus between Caucasians and African Americans.Clin Gastroenterol Hepatol.2004;2:469–473.
- ,,, et al.Gastroesophageal reflux among different racial groups in the United States.Gastroenterology.2004;126:1692–1699.
- ,,,.Risk factors for erosive reflux esophagitis: a case‐control study.Am J Gastroenterol.2001;96:41–46.
- ,.Upper gastrointestinal toxicity of nonsteroidal anti‐inflammatory drugs in African‐American and Hispanic elderly patients.Ethn Dis.2003;13:528–533.
- ,.Evidence‐based quality improvement: the state of the science.Health Aff (Millwood).2005;24(1):138–150.
Copyright © 2009 Society of Hospital Medicine