User login
Prevention of Venous Thromboembolism
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a serious and growing public health problem. In the United States an estimated 900,000 people are affected and more than 100,000 die from VTE or related complications each year. More than half of VTE events occur in association with hospitalization or major surgery; many are thought to be preventable.[1, 2, 3, 4, 5] The Centers for Medicare and Medicaid Services (CMS), Centers for Disease Control and Prevention (CDC), and the Agency for Healthcare Research and Quality (AHRQ),[6, 7, 8, 9] among other organizations, have identified VTE as a potentially preventable never event. Evidence‐based guidelines and resources exist to help support hospital‐acquired venous thromboembolism (HA‐VTE) prevention.[1, 2, 3, 4, 5, 6, 7, 8, 9, 10] Harborview Medical Center, a tertiary referral center with more than 17,000 patients hospitalized annually, many requiring surgery, serves one of the highest‐risk populations for HA‐VTE development. Despite high rates of VTE prophylaxis in accordance with an established institutional guideline,[11, 12] VTE remains the most common hospital‐acquired condition in our institution.
OBJECTIVES
To improve the safety and care of all patients in our medical center and eliminate preventable HA‐VTE events, we set out to: (1) incorporate evidence‐based best practices in VTE prevention and treatment into current practice in alignment with institutional guidelines, (2) standardize the review process for all HA‐VTE events to identify opportunities for improvement, (3) utilize quality improvement (QI) analytics and information technology (IT) to actively improve our processes at the point of care, and (4) share process and outcome performance relating to VTE prevention transparently across our institution
METHODS
To prevent HA‐VTE, we employ a multifactorial strategy that includes designated clinical leadership, active engagement of all care team members, decision support tools embedded in the electronic health record (EHR), QI analytics, and retrospective and prospective reporting that provides ongoing measurement and analysis of the effectiveness of implemented interventions.
Setting/Patients
Harborview Medical Center, a 413‐bed academic tertiary referral center and the only level 1 adult and pediatric trauma and burn center for a 5‐state area, also serves as the primary safety‐net provider in the region. Harborview has centers of excellence in trauma, neurosciences, orthopedic and vascular surgery and rehabilitation, and is the only certified comprehensive stroke center in 5 states. With more than 17,000 admissions annually, including over 6000 trauma cases, HA‐VTE is a disease that spans critical and acute care settings and impacts patients on all clinical services. Harborview serves a population that is at extremely high risk for VTE as well as bleeding, particularly patients who have sustained central nervous system trauma or polytrauma.
Intervention
In 2010, at the request of the Harborview Medical Executive Board and Medical Director, we formed the Harborview VTE Task Force to assess VTE prevention practices across services and identify improvement opportunities for all hospitalized patients. This multidisciplinary team, co‐chaired by a hospitalist and trauma surgeon, includes representatives from trauma/general surgery, orthopedic surgery, hospital medicine, nursing, pharmacy, and QI. Task force members represent critical and acute care as well as the ambulatory setting. Additional stakeholders and local experts including IT directors and analysts, continuity of care nurses, and other clinical service representatives participate on an ad hoc basis.
Since its inception, the VTE Task Force has met monthly to review performance data and develop improvement initiatives. Initially we collaborated with experts across our health system to update an existing institutional VTE prophylaxis guideline to reflect current evidence‐based standards.[1, 3, 4, 5, 12] We met with all clinical services to ensure that the guidelines incorporated departmental best practices. These guidelines were integrated into our Cerner‐based (Cerner Corp., North Kansas City, MO) computerized provider order entry (CPOE) system to support accurate VTE risk assessment and appropriate ordering of prophylaxis.
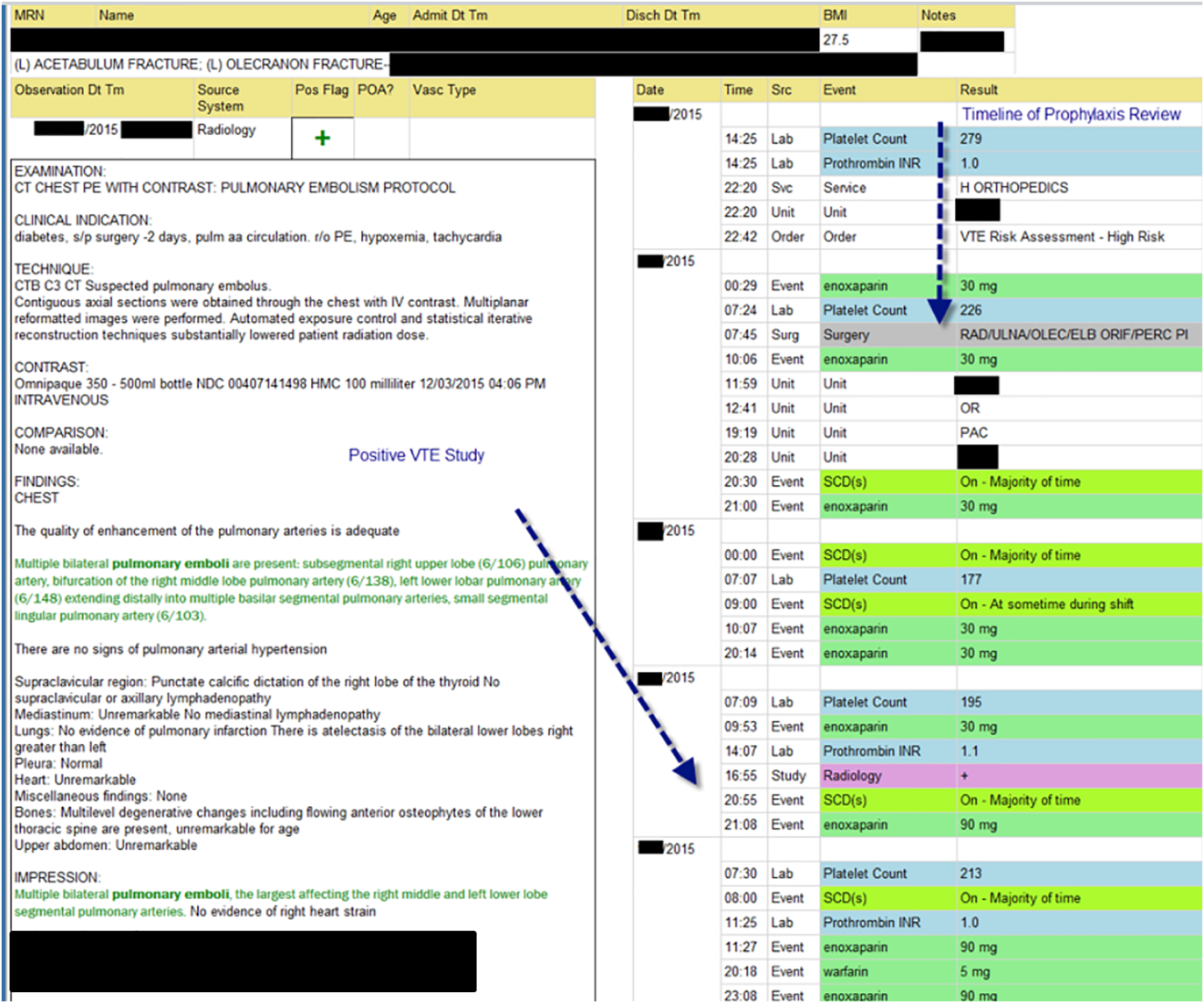
The VTE Task Force collaborated with QI programmers to develop an electronic tool, the Harborview VTE Tool (Figure 1),[13] that allows for efficient, standardized review of all HA‐VTE at monthly meetings. The tool uses word and phrase search capabilities to identify PEs and DVTs from imaging and vascular studies and links those events with pertinent demographic and clinical data from the EHR in a timeline. Information about VTE risk assigned by physicians in the CPOE system is extracted as well as specific VTE prophylaxis and treatment (drug, dose, timing of administration of medications, reason for doses being held, and orders for and application of mechanical prophylaxis). Using the VTE tool, the task force reviews each VTE event to assess the accuracy of VTE risk assignment, the appropriateness of prophylaxis received relative to guidelines, and the adequacy of VTE treatment and follow‐up. This tool has facilitated our review process, decreasing time from >30 minutes of manual chart review per event to several minutes. In recent months, a quality analyst has prescreened all VTEs prior to task force discussion to further improve efficiency. The tool allows the team to assess the case together and reach consensus regarding VTE prevention.
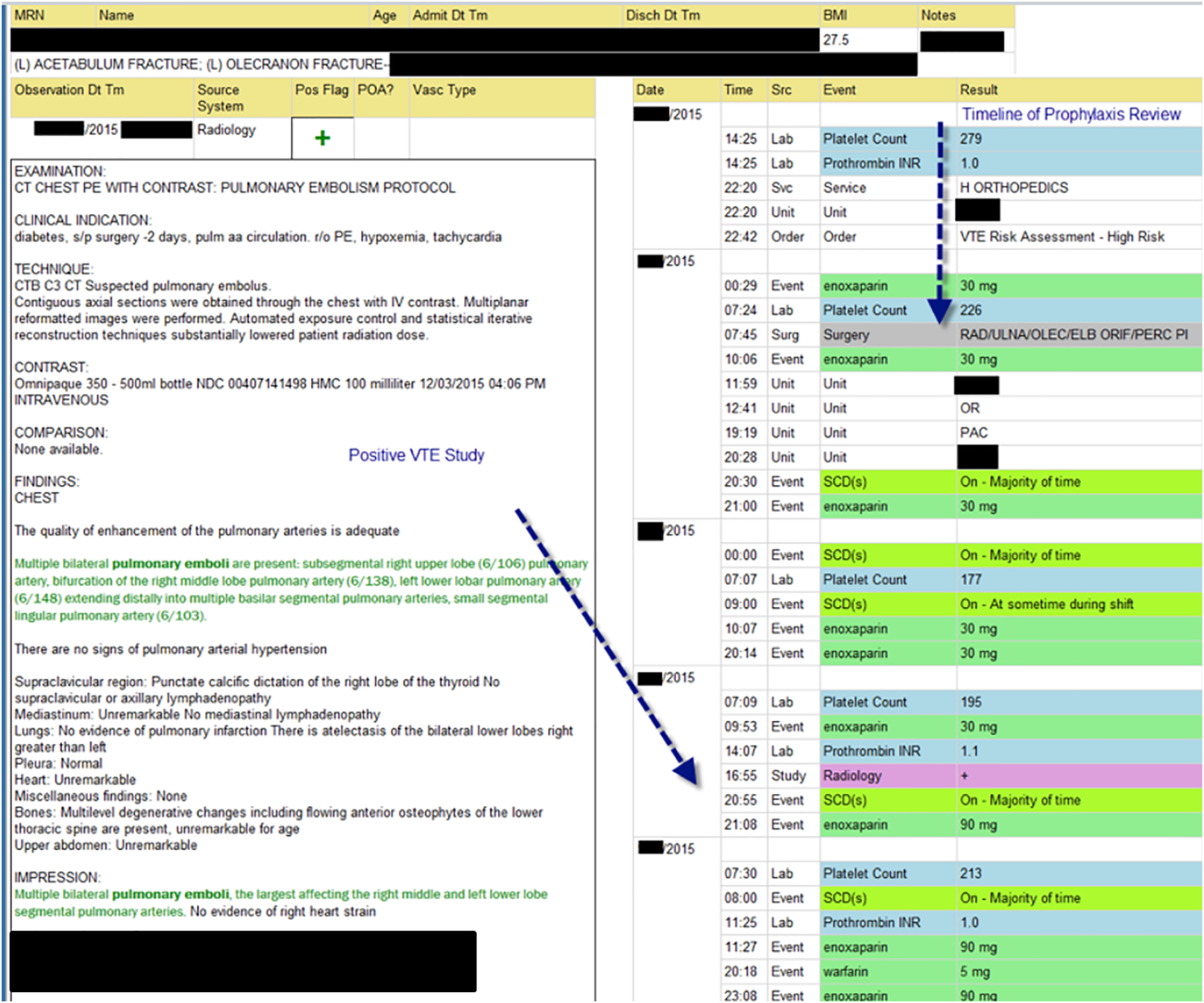
Prompt event reviews allow the task force to provide timely feedback about specific VTE events to physicians, nurses, and pharmacists. Cases with potential opportunities for improvement are referred to a medical center‐wide QI committee for secondary review. Areas of opportunity identified are tracked and trended to direct ongoing system improvement cycles. In 2014, as a result of reviewing patient cases with VTE diagnosed after discharge, we began a similar review process to assess current practice and standardize prophylaxis across care transitions.
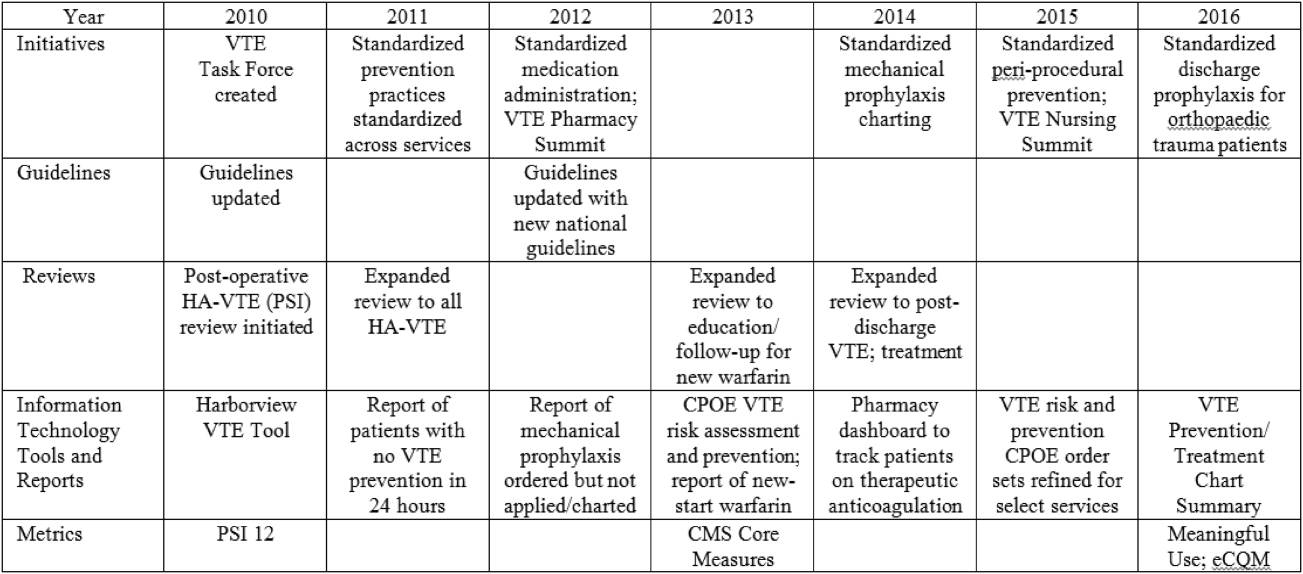
In response to opportunities identified from reviews, the VTE Task Force developed multiple reporting tools that provide real‐time, actionable information to clinicians at the bedside. Daily electronic lists highlight patients who have not received chemical or mechanical prophylaxis in 24 hours and are utilized by nursing, pharmacy, and physician groups. Patients receiving new start vitamin K antagonists or direct oral anticoagulants are identified for pharmacists and discharge care coordinators to support early patient/family education and ensure appropriate follow‐up. Based on input from frontline providers, tools are continually refined to improve their clinical utility. A timeline of initiatives that the Harborview VTE Task Force has championed is outlined in Figure 2.
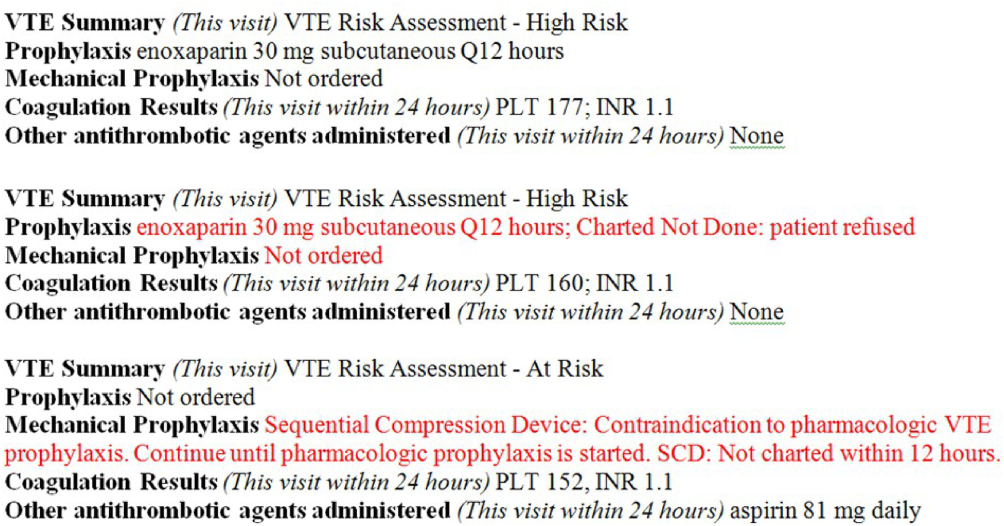
To bring HA‐VTE prevention information to the point of care, we developed a VTE Prevention/Treatment Summary within the EHR (Figure 3). Information about VTE risk assigned by the physician based on guidelines, current prophylaxis orders (pharmacologic/nonpharmacologic) and administration status, therapeutic anticoagulation and pertinent laboratory values are imported into a summary snapshot that can be accessed on demand by any member of the care team from within the patient's chart. The same data elements are being imbedded in resident physician and nursing handoff tools to highlight VTE prevention for all hospitalized patients and ensure optimal prophylaxis at transitions of care.
To emphasize Harborview's commitment to VTE prevention and ensure that care providers across the institution are aware of and engaged in this effort, we utilize our intranet to disseminate information in a fully transparent manner. Both process and outcome measures are available to all physicians and staff at service and unit levels on a Web‐based institutional dashboard. Data are updated monthly by QI analysts and improvement opportunities are highlighted in multiple fora. Descriptions of the quality metrics that are tracked are summarized in Table 1.
| Quality Metric | Description |
|---|---|
| |
| AHRQ PSI 12 | Cases of VTE not present on admission per 1000 surgical discharges with select operating room procedures |
| CMS Core Measure VTE‐1 | Percent of patients without VTE who received VTE prophylaxis on day of or day after arrival to an acute care area, random sample |
| CMS Core Measure VTE‐2 | Percent of patients without VTE who received VTE prophylaxis on day of or day after arrival to an intensive care unit or surgery date, random sample |
| CMS Core Measure VTE 5 | Percent of patients with hospital acquired VTE discharged to home on warfarin who received education and written discharge instructions |
| CMS Core Measure VTE‐6 | Percent of patients with hospital‐acquired VTE who received VTE prophylaxis prior to the event diagnosis |
MEASUREMENTS
Outcomes
Harborview benchmarks performance against hospitals nationally using the CMS Hospital Compare data and with peer academic institutions through Vizient data (Vizient, Irving, TX). To measure the impact of our initiatives, the task force began tracking postoperative VTE rates based on the AHRQ Patient Safety Indicator (PSI) 12 and expanded to include HA‐VTE rates for all hospitalized patients. We also report performance on Core Measure VTE‐6: incidence of potentially preventable VTE.
Process
We monitor VTE prophylaxis compliance based on the CMS Core Measures VTE‐1 and 2, random samples of acute and critical care patients without VTE. Internally, we measure compliance with guideline‐directed therapy for all HA‐VTE cases reviewed by the task force. With the upcoming retirement of the CMS chart‐abstracted measures, we are developing methods to track appropriate VTE prophylaxis provided to all eligible patients and will replace the sampled populations with this more expansive dataset. This approach will provide information for further improvements in VTE prophylaxis and act as an important step for success with the Electronic Clinical Quality Measures under the Meaningful Use program.
RESULTS
Our VTE prevention initiatives have resulted in improved compliance with our institutional guideline‐directed VTE prophylaxis and a decrease in HA‐VTE at our institution.
VTE Core Measures
Since the inception of VTE Core Measures in 2013, our annual performance on VTE‐1: prophylaxis for acute care patients has been above 95% and VTE‐2: prophylaxis for critical care patients has been above 98%. This performance has been consistently above the national mean for both measures (VTE‐1: 91% among Washington state hospitals and 93% nationally; VTE‐2: 95% among Washington state hospitals and 97% nationally). The CMS Hospital Compare current public reporting period is based on information collected from July 2014 through June 2015. Our internal performance for calendar year 2015 was 96% (289 of 302) for VTE‐1 and 98% (235 of 241) for VTE‐2.
Harborview has had zero potentially preventable VTE events (VTE‐6) compared with a reported national average of 4% since the inception of these measures in January 2013.
Guideline‐Directed VTE Prevention: Patients Diagnosed With HA‐VTE
The task force reviews each case to determine if the patient received guideline‐adherent prophylaxis on every day prior to the event. Patients with active bleeding or those with high bleeding risk should have mechanical prophylaxis ordered and applied until pharmacologic prophylaxis is appropriate. Any missed single dose of pharmacologic prophylaxis or missed day of applied mechanical prophylaxis is considered a possible opportunity for improvement, and the case is referred to the appropriate clinical service for additional review.
Since task force launch, the percent of all patients diagnosed with HA‐VTE who received guideline‐directed prophylaxis increased 7% from 86% (105 of 122) in 2012 to 92% (80 of 87) in the first 9 months of 2015. Of events with possible opportunities, most were deemed not to have been preventable. Some trauma patients were ineligible for pharmacologic and mechanical prophylaxis, some were prophylaxed according to the best available evidence, and some had risk factors (for example, active malignancy) only identified after the VTE event. The few remaining events highlighted opportunities regarding standardization of pharmacologic prophylaxis periprocedurally, documentation of application of mechanical prophylaxis, and communication of patient refusal of doses, all ongoing focus areas for improvement.
Reduction in HA‐VTE
Improved VTE prophylaxis has contributed to a 15% reduction in HA‐VTE in all hospitalized patients over 5 years from a rate of 7.5 events/1000 inpatients in 2011 to 6.4/1000 inpatients for the first 9 months of 2015. Among postoperative patients (AHRQ PSI 12), the rate of VTE decreased 21% from 11.7/1000 patients in 2011 to 9.3/1000 patients in the first 9 months of 2015.
Patient/Family Engagement
We further improved our processes to ensure that patients with HA‐VTE who discharge to home receive written discharge instructions for warfarin use (VTE‐5). In 2014, performance on this measure was 91% (51 of 56 eligible patients) and in 2015 performance improved to 96% (78 of 81 eligible patients) compared with a reported national average of 91%. Additionally, 97% (79 of 81) of patients who discharged home on warfarin after HA‐VTE now have outpatient anticoagulation follow‐up arranged prior to hospital discharge. We are developing new initiatives for patient and family education regarding direct oral anticoagulants.
Discussion/Conclusions
With interdisciplinary teamwork and use of QI analytics to drive transparency, we have improved VTE prevention and reduced rates of HA‐VTE. Harborview's HA‐VTE prevention initiative can be duplicated by other organizations given the structured nature of the intervention. The multidisciplinary approach, clinical presence of task force members, and support and engagement of senior clinical leadership have been key elements to our program's success. The existence of a standard institutional guideline based on evidence‐based national guidelines and incorporation of these standards into the EHR is vital. The VTE task force has consistently used QI analytics both for retrospective review and real‐time data feedback. Complete and easy accessibility and transparency of performance at the service and unit level supports accountability. Integration of the task force work into existing institutional QI structures has further led to improvements in patient safety.
Ongoing task force collaboration and communication with frontline providers and clinical departments has been critical to engagement and sustained improvements in VTE prevention and treatment. The work of the VTE task force represents the steadfast commitment of Harborview and our clinical staff to prevent preventable harm. This multidisciplinary effort has served as a model for other QI initiatives across our institution and health system.
Disclosure
Nothing to report.
- , , , et al. Executive Summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐based Clinical Guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , , , . Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(4 suppl):S495–S501.
- , , , et al. Prevention of VTE in orthopedic surgery patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S–e325S.
- , , , et al. Prevention of VTE in nonsurgical patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S–e226S.
- , , , et al; American College of Chest Physicians. Prevention of VTE in nonorthopedic surgical patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S–e277S.
- Centers for Medicare and Medicaid Services. Core measures. Available at: https://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/QualityMeasures/Core‐Measures.html. Accessed September 1, 2016.
- Centers for Disease Control and Prevention. Venous thromboembolism. Available at: http://www.cdc.gov/ncbddd/dvt/index.html. Accessed September 1, 2016.
- . Preventing hospital‐associated venous thromboembolism: a guide for effective quality improvement, 2nd ed. AHRQ Publication No. 16‐0001‐EF. Rockville MD: Agency for Healthcare Research and Quality; 2016.
- . Preventing hospital‐associated venous thromboembolism: a guide for effective quality improvement. Available at: http://www.ahrq.gov/professionals/quality‐patient‐safety/patient‐safety‐resources/resources/vtguide/index.html. Accessed September 1, 2016.
- , . Preventing hospital‐acquired venous‐thromboembolism, a guide for effective quality improvement. Version 3.3. Venous Thromboembolism Quality Improvement Implementation Toolkit. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed September 1, 2016.
- , , , , . Adherence to guideline‐directed venous thromboembolism prophylaxis among medical and surgical inpatients at 33 academic medical centers in the United States. Am J Med Qual. 2010;26(3):174–180.
- UW Medicine guidelines for prevention of venous thromboembolism (VTE) in hospitalized patients. Available at: https://depts.washington.edu/anticoag/home. Accessed June 13, 2016.
- , , , , , . Upper extremity deep vein thrombosis in hospitalized patients: a descriptive study. J Hosp Med. 2014;9(1):48–53.
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a serious and growing public health problem. In the United States an estimated 900,000 people are affected and more than 100,000 die from VTE or related complications each year. More than half of VTE events occur in association with hospitalization or major surgery; many are thought to be preventable.[1, 2, 3, 4, 5] The Centers for Medicare and Medicaid Services (CMS), Centers for Disease Control and Prevention (CDC), and the Agency for Healthcare Research and Quality (AHRQ),[6, 7, 8, 9] among other organizations, have identified VTE as a potentially preventable never event. Evidence‐based guidelines and resources exist to help support hospital‐acquired venous thromboembolism (HA‐VTE) prevention.[1, 2, 3, 4, 5, 6, 7, 8, 9, 10] Harborview Medical Center, a tertiary referral center with more than 17,000 patients hospitalized annually, many requiring surgery, serves one of the highest‐risk populations for HA‐VTE development. Despite high rates of VTE prophylaxis in accordance with an established institutional guideline,[11, 12] VTE remains the most common hospital‐acquired condition in our institution.
OBJECTIVES
To improve the safety and care of all patients in our medical center and eliminate preventable HA‐VTE events, we set out to: (1) incorporate evidence‐based best practices in VTE prevention and treatment into current practice in alignment with institutional guidelines, (2) standardize the review process for all HA‐VTE events to identify opportunities for improvement, (3) utilize quality improvement (QI) analytics and information technology (IT) to actively improve our processes at the point of care, and (4) share process and outcome performance relating to VTE prevention transparently across our institution
METHODS
To prevent HA‐VTE, we employ a multifactorial strategy that includes designated clinical leadership, active engagement of all care team members, decision support tools embedded in the electronic health record (EHR), QI analytics, and retrospective and prospective reporting that provides ongoing measurement and analysis of the effectiveness of implemented interventions.
Setting/Patients
Harborview Medical Center, a 413‐bed academic tertiary referral center and the only level 1 adult and pediatric trauma and burn center for a 5‐state area, also serves as the primary safety‐net provider in the region. Harborview has centers of excellence in trauma, neurosciences, orthopedic and vascular surgery and rehabilitation, and is the only certified comprehensive stroke center in 5 states. With more than 17,000 admissions annually, including over 6000 trauma cases, HA‐VTE is a disease that spans critical and acute care settings and impacts patients on all clinical services. Harborview serves a population that is at extremely high risk for VTE as well as bleeding, particularly patients who have sustained central nervous system trauma or polytrauma.
Intervention
In 2010, at the request of the Harborview Medical Executive Board and Medical Director, we formed the Harborview VTE Task Force to assess VTE prevention practices across services and identify improvement opportunities for all hospitalized patients. This multidisciplinary team, co‐chaired by a hospitalist and trauma surgeon, includes representatives from trauma/general surgery, orthopedic surgery, hospital medicine, nursing, pharmacy, and QI. Task force members represent critical and acute care as well as the ambulatory setting. Additional stakeholders and local experts including IT directors and analysts, continuity of care nurses, and other clinical service representatives participate on an ad hoc basis.
Since its inception, the VTE Task Force has met monthly to review performance data and develop improvement initiatives. Initially we collaborated with experts across our health system to update an existing institutional VTE prophylaxis guideline to reflect current evidence‐based standards.[1, 3, 4, 5, 12] We met with all clinical services to ensure that the guidelines incorporated departmental best practices. These guidelines were integrated into our Cerner‐based (Cerner Corp., North Kansas City, MO) computerized provider order entry (CPOE) system to support accurate VTE risk assessment and appropriate ordering of prophylaxis.
The VTE Task Force collaborated with QI programmers to develop an electronic tool, the Harborview VTE Tool (Figure 1),[13] that allows for efficient, standardized review of all HA‐VTE at monthly meetings. The tool uses word and phrase search capabilities to identify PEs and DVTs from imaging and vascular studies and links those events with pertinent demographic and clinical data from the EHR in a timeline. Information about VTE risk assigned by physicians in the CPOE system is extracted as well as specific VTE prophylaxis and treatment (drug, dose, timing of administration of medications, reason for doses being held, and orders for and application of mechanical prophylaxis). Using the VTE tool, the task force reviews each VTE event to assess the accuracy of VTE risk assignment, the appropriateness of prophylaxis received relative to guidelines, and the adequacy of VTE treatment and follow‐up. This tool has facilitated our review process, decreasing time from >30 minutes of manual chart review per event to several minutes. In recent months, a quality analyst has prescreened all VTEs prior to task force discussion to further improve efficiency. The tool allows the team to assess the case together and reach consensus regarding VTE prevention.

Prompt event reviews allow the task force to provide timely feedback about specific VTE events to physicians, nurses, and pharmacists. Cases with potential opportunities for improvement are referred to a medical center‐wide QI committee for secondary review. Areas of opportunity identified are tracked and trended to direct ongoing system improvement cycles. In 2014, as a result of reviewing patient cases with VTE diagnosed after discharge, we began a similar review process to assess current practice and standardize prophylaxis across care transitions.
In response to opportunities identified from reviews, the VTE Task Force developed multiple reporting tools that provide real‐time, actionable information to clinicians at the bedside. Daily electronic lists highlight patients who have not received chemical or mechanical prophylaxis in 24 hours and are utilized by nursing, pharmacy, and physician groups. Patients receiving new start vitamin K antagonists or direct oral anticoagulants are identified for pharmacists and discharge care coordinators to support early patient/family education and ensure appropriate follow‐up. Based on input from frontline providers, tools are continually refined to improve their clinical utility. A timeline of initiatives that the Harborview VTE Task Force has championed is outlined in Figure 2.

To bring HA‐VTE prevention information to the point of care, we developed a VTE Prevention/Treatment Summary within the EHR (Figure 3). Information about VTE risk assigned by the physician based on guidelines, current prophylaxis orders (pharmacologic/nonpharmacologic) and administration status, therapeutic anticoagulation and pertinent laboratory values are imported into a summary snapshot that can be accessed on demand by any member of the care team from within the patient's chart. The same data elements are being imbedded in resident physician and nursing handoff tools to highlight VTE prevention for all hospitalized patients and ensure optimal prophylaxis at transitions of care.

To emphasize Harborview's commitment to VTE prevention and ensure that care providers across the institution are aware of and engaged in this effort, we utilize our intranet to disseminate information in a fully transparent manner. Both process and outcome measures are available to all physicians and staff at service and unit levels on a Web‐based institutional dashboard. Data are updated monthly by QI analysts and improvement opportunities are highlighted in multiple fora. Descriptions of the quality metrics that are tracked are summarized in Table 1.
| Quality Metric | Description |
|---|---|
| |
| AHRQ PSI 12 | Cases of VTE not present on admission per 1000 surgical discharges with select operating room procedures |
| CMS Core Measure VTE‐1 | Percent of patients without VTE who received VTE prophylaxis on day of or day after arrival to an acute care area, random sample |
| CMS Core Measure VTE‐2 | Percent of patients without VTE who received VTE prophylaxis on day of or day after arrival to an intensive care unit or surgery date, random sample |
| CMS Core Measure VTE 5 | Percent of patients with hospital acquired VTE discharged to home on warfarin who received education and written discharge instructions |
| CMS Core Measure VTE‐6 | Percent of patients with hospital‐acquired VTE who received VTE prophylaxis prior to the event diagnosis |
MEASUREMENTS
Outcomes
Harborview benchmarks performance against hospitals nationally using the CMS Hospital Compare data and with peer academic institutions through Vizient data (Vizient, Irving, TX). To measure the impact of our initiatives, the task force began tracking postoperative VTE rates based on the AHRQ Patient Safety Indicator (PSI) 12 and expanded to include HA‐VTE rates for all hospitalized patients. We also report performance on Core Measure VTE‐6: incidence of potentially preventable VTE.
Process
We monitor VTE prophylaxis compliance based on the CMS Core Measures VTE‐1 and 2, random samples of acute and critical care patients without VTE. Internally, we measure compliance with guideline‐directed therapy for all HA‐VTE cases reviewed by the task force. With the upcoming retirement of the CMS chart‐abstracted measures, we are developing methods to track appropriate VTE prophylaxis provided to all eligible patients and will replace the sampled populations with this more expansive dataset. This approach will provide information for further improvements in VTE prophylaxis and act as an important step for success with the Electronic Clinical Quality Measures under the Meaningful Use program.
RESULTS
Our VTE prevention initiatives have resulted in improved compliance with our institutional guideline‐directed VTE prophylaxis and a decrease in HA‐VTE at our institution.
VTE Core Measures
Since the inception of VTE Core Measures in 2013, our annual performance on VTE‐1: prophylaxis for acute care patients has been above 95% and VTE‐2: prophylaxis for critical care patients has been above 98%. This performance has been consistently above the national mean for both measures (VTE‐1: 91% among Washington state hospitals and 93% nationally; VTE‐2: 95% among Washington state hospitals and 97% nationally). The CMS Hospital Compare current public reporting period is based on information collected from July 2014 through June 2015. Our internal performance for calendar year 2015 was 96% (289 of 302) for VTE‐1 and 98% (235 of 241) for VTE‐2.
Harborview has had zero potentially preventable VTE events (VTE‐6) compared with a reported national average of 4% since the inception of these measures in January 2013.
Guideline‐Directed VTE Prevention: Patients Diagnosed With HA‐VTE
The task force reviews each case to determine if the patient received guideline‐adherent prophylaxis on every day prior to the event. Patients with active bleeding or those with high bleeding risk should have mechanical prophylaxis ordered and applied until pharmacologic prophylaxis is appropriate. Any missed single dose of pharmacologic prophylaxis or missed day of applied mechanical prophylaxis is considered a possible opportunity for improvement, and the case is referred to the appropriate clinical service for additional review.
Since task force launch, the percent of all patients diagnosed with HA‐VTE who received guideline‐directed prophylaxis increased 7% from 86% (105 of 122) in 2012 to 92% (80 of 87) in the first 9 months of 2015. Of events with possible opportunities, most were deemed not to have been preventable. Some trauma patients were ineligible for pharmacologic and mechanical prophylaxis, some were prophylaxed according to the best available evidence, and some had risk factors (for example, active malignancy) only identified after the VTE event. The few remaining events highlighted opportunities regarding standardization of pharmacologic prophylaxis periprocedurally, documentation of application of mechanical prophylaxis, and communication of patient refusal of doses, all ongoing focus areas for improvement.
Reduction in HA‐VTE
Improved VTE prophylaxis has contributed to a 15% reduction in HA‐VTE in all hospitalized patients over 5 years from a rate of 7.5 events/1000 inpatients in 2011 to 6.4/1000 inpatients for the first 9 months of 2015. Among postoperative patients (AHRQ PSI 12), the rate of VTE decreased 21% from 11.7/1000 patients in 2011 to 9.3/1000 patients in the first 9 months of 2015.
Patient/Family Engagement
We further improved our processes to ensure that patients with HA‐VTE who discharge to home receive written discharge instructions for warfarin use (VTE‐5). In 2014, performance on this measure was 91% (51 of 56 eligible patients) and in 2015 performance improved to 96% (78 of 81 eligible patients) compared with a reported national average of 91%. Additionally, 97% (79 of 81) of patients who discharged home on warfarin after HA‐VTE now have outpatient anticoagulation follow‐up arranged prior to hospital discharge. We are developing new initiatives for patient and family education regarding direct oral anticoagulants.
Discussion/Conclusions
With interdisciplinary teamwork and use of QI analytics to drive transparency, we have improved VTE prevention and reduced rates of HA‐VTE. Harborview's HA‐VTE prevention initiative can be duplicated by other organizations given the structured nature of the intervention. The multidisciplinary approach, clinical presence of task force members, and support and engagement of senior clinical leadership have been key elements to our program's success. The existence of a standard institutional guideline based on evidence‐based national guidelines and incorporation of these standards into the EHR is vital. The VTE task force has consistently used QI analytics both for retrospective review and real‐time data feedback. Complete and easy accessibility and transparency of performance at the service and unit level supports accountability. Integration of the task force work into existing institutional QI structures has further led to improvements in patient safety.
Ongoing task force collaboration and communication with frontline providers and clinical departments has been critical to engagement and sustained improvements in VTE prevention and treatment. The work of the VTE task force represents the steadfast commitment of Harborview and our clinical staff to prevent preventable harm. This multidisciplinary effort has served as a model for other QI initiatives across our institution and health system.
Disclosure
Nothing to report.
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a serious and growing public health problem. In the United States an estimated 900,000 people are affected and more than 100,000 die from VTE or related complications each year. More than half of VTE events occur in association with hospitalization or major surgery; many are thought to be preventable.[1, 2, 3, 4, 5] The Centers for Medicare and Medicaid Services (CMS), Centers for Disease Control and Prevention (CDC), and the Agency for Healthcare Research and Quality (AHRQ),[6, 7, 8, 9] among other organizations, have identified VTE as a potentially preventable never event. Evidence‐based guidelines and resources exist to help support hospital‐acquired venous thromboembolism (HA‐VTE) prevention.[1, 2, 3, 4, 5, 6, 7, 8, 9, 10] Harborview Medical Center, a tertiary referral center with more than 17,000 patients hospitalized annually, many requiring surgery, serves one of the highest‐risk populations for HA‐VTE development. Despite high rates of VTE prophylaxis in accordance with an established institutional guideline,[11, 12] VTE remains the most common hospital‐acquired condition in our institution.
OBJECTIVES
To improve the safety and care of all patients in our medical center and eliminate preventable HA‐VTE events, we set out to: (1) incorporate evidence‐based best practices in VTE prevention and treatment into current practice in alignment with institutional guidelines, (2) standardize the review process for all HA‐VTE events to identify opportunities for improvement, (3) utilize quality improvement (QI) analytics and information technology (IT) to actively improve our processes at the point of care, and (4) share process and outcome performance relating to VTE prevention transparently across our institution
METHODS
To prevent HA‐VTE, we employ a multifactorial strategy that includes designated clinical leadership, active engagement of all care team members, decision support tools embedded in the electronic health record (EHR), QI analytics, and retrospective and prospective reporting that provides ongoing measurement and analysis of the effectiveness of implemented interventions.
Setting/Patients
Harborview Medical Center, a 413‐bed academic tertiary referral center and the only level 1 adult and pediatric trauma and burn center for a 5‐state area, also serves as the primary safety‐net provider in the region. Harborview has centers of excellence in trauma, neurosciences, orthopedic and vascular surgery and rehabilitation, and is the only certified comprehensive stroke center in 5 states. With more than 17,000 admissions annually, including over 6000 trauma cases, HA‐VTE is a disease that spans critical and acute care settings and impacts patients on all clinical services. Harborview serves a population that is at extremely high risk for VTE as well as bleeding, particularly patients who have sustained central nervous system trauma or polytrauma.
Intervention
In 2010, at the request of the Harborview Medical Executive Board and Medical Director, we formed the Harborview VTE Task Force to assess VTE prevention practices across services and identify improvement opportunities for all hospitalized patients. This multidisciplinary team, co‐chaired by a hospitalist and trauma surgeon, includes representatives from trauma/general surgery, orthopedic surgery, hospital medicine, nursing, pharmacy, and QI. Task force members represent critical and acute care as well as the ambulatory setting. Additional stakeholders and local experts including IT directors and analysts, continuity of care nurses, and other clinical service representatives participate on an ad hoc basis.
Since its inception, the VTE Task Force has met monthly to review performance data and develop improvement initiatives. Initially we collaborated with experts across our health system to update an existing institutional VTE prophylaxis guideline to reflect current evidence‐based standards.[1, 3, 4, 5, 12] We met with all clinical services to ensure that the guidelines incorporated departmental best practices. These guidelines were integrated into our Cerner‐based (Cerner Corp., North Kansas City, MO) computerized provider order entry (CPOE) system to support accurate VTE risk assessment and appropriate ordering of prophylaxis.
The VTE Task Force collaborated with QI programmers to develop an electronic tool, the Harborview VTE Tool (Figure 1),[13] that allows for efficient, standardized review of all HA‐VTE at monthly meetings. The tool uses word and phrase search capabilities to identify PEs and DVTs from imaging and vascular studies and links those events with pertinent demographic and clinical data from the EHR in a timeline. Information about VTE risk assigned by physicians in the CPOE system is extracted as well as specific VTE prophylaxis and treatment (drug, dose, timing of administration of medications, reason for doses being held, and orders for and application of mechanical prophylaxis). Using the VTE tool, the task force reviews each VTE event to assess the accuracy of VTE risk assignment, the appropriateness of prophylaxis received relative to guidelines, and the adequacy of VTE treatment and follow‐up. This tool has facilitated our review process, decreasing time from >30 minutes of manual chart review per event to several minutes. In recent months, a quality analyst has prescreened all VTEs prior to task force discussion to further improve efficiency. The tool allows the team to assess the case together and reach consensus regarding VTE prevention.

Prompt event reviews allow the task force to provide timely feedback about specific VTE events to physicians, nurses, and pharmacists. Cases with potential opportunities for improvement are referred to a medical center‐wide QI committee for secondary review. Areas of opportunity identified are tracked and trended to direct ongoing system improvement cycles. In 2014, as a result of reviewing patient cases with VTE diagnosed after discharge, we began a similar review process to assess current practice and standardize prophylaxis across care transitions.
In response to opportunities identified from reviews, the VTE Task Force developed multiple reporting tools that provide real‐time, actionable information to clinicians at the bedside. Daily electronic lists highlight patients who have not received chemical or mechanical prophylaxis in 24 hours and are utilized by nursing, pharmacy, and physician groups. Patients receiving new start vitamin K antagonists or direct oral anticoagulants are identified for pharmacists and discharge care coordinators to support early patient/family education and ensure appropriate follow‐up. Based on input from frontline providers, tools are continually refined to improve their clinical utility. A timeline of initiatives that the Harborview VTE Task Force has championed is outlined in Figure 2.

To bring HA‐VTE prevention information to the point of care, we developed a VTE Prevention/Treatment Summary within the EHR (Figure 3). Information about VTE risk assigned by the physician based on guidelines, current prophylaxis orders (pharmacologic/nonpharmacologic) and administration status, therapeutic anticoagulation and pertinent laboratory values are imported into a summary snapshot that can be accessed on demand by any member of the care team from within the patient's chart. The same data elements are being imbedded in resident physician and nursing handoff tools to highlight VTE prevention for all hospitalized patients and ensure optimal prophylaxis at transitions of care.

To emphasize Harborview's commitment to VTE prevention and ensure that care providers across the institution are aware of and engaged in this effort, we utilize our intranet to disseminate information in a fully transparent manner. Both process and outcome measures are available to all physicians and staff at service and unit levels on a Web‐based institutional dashboard. Data are updated monthly by QI analysts and improvement opportunities are highlighted in multiple fora. Descriptions of the quality metrics that are tracked are summarized in Table 1.
| Quality Metric | Description |
|---|---|
| |
| AHRQ PSI 12 | Cases of VTE not present on admission per 1000 surgical discharges with select operating room procedures |
| CMS Core Measure VTE‐1 | Percent of patients without VTE who received VTE prophylaxis on day of or day after arrival to an acute care area, random sample |
| CMS Core Measure VTE‐2 | Percent of patients without VTE who received VTE prophylaxis on day of or day after arrival to an intensive care unit or surgery date, random sample |
| CMS Core Measure VTE 5 | Percent of patients with hospital acquired VTE discharged to home on warfarin who received education and written discharge instructions |
| CMS Core Measure VTE‐6 | Percent of patients with hospital‐acquired VTE who received VTE prophylaxis prior to the event diagnosis |
MEASUREMENTS
Outcomes
Harborview benchmarks performance against hospitals nationally using the CMS Hospital Compare data and with peer academic institutions through Vizient data (Vizient, Irving, TX). To measure the impact of our initiatives, the task force began tracking postoperative VTE rates based on the AHRQ Patient Safety Indicator (PSI) 12 and expanded to include HA‐VTE rates for all hospitalized patients. We also report performance on Core Measure VTE‐6: incidence of potentially preventable VTE.
Process
We monitor VTE prophylaxis compliance based on the CMS Core Measures VTE‐1 and 2, random samples of acute and critical care patients without VTE. Internally, we measure compliance with guideline‐directed therapy for all HA‐VTE cases reviewed by the task force. With the upcoming retirement of the CMS chart‐abstracted measures, we are developing methods to track appropriate VTE prophylaxis provided to all eligible patients and will replace the sampled populations with this more expansive dataset. This approach will provide information for further improvements in VTE prophylaxis and act as an important step for success with the Electronic Clinical Quality Measures under the Meaningful Use program.
RESULTS
Our VTE prevention initiatives have resulted in improved compliance with our institutional guideline‐directed VTE prophylaxis and a decrease in HA‐VTE at our institution.
VTE Core Measures
Since the inception of VTE Core Measures in 2013, our annual performance on VTE‐1: prophylaxis for acute care patients has been above 95% and VTE‐2: prophylaxis for critical care patients has been above 98%. This performance has been consistently above the national mean for both measures (VTE‐1: 91% among Washington state hospitals and 93% nationally; VTE‐2: 95% among Washington state hospitals and 97% nationally). The CMS Hospital Compare current public reporting period is based on information collected from July 2014 through June 2015. Our internal performance for calendar year 2015 was 96% (289 of 302) for VTE‐1 and 98% (235 of 241) for VTE‐2.
Harborview has had zero potentially preventable VTE events (VTE‐6) compared with a reported national average of 4% since the inception of these measures in January 2013.
Guideline‐Directed VTE Prevention: Patients Diagnosed With HA‐VTE
The task force reviews each case to determine if the patient received guideline‐adherent prophylaxis on every day prior to the event. Patients with active bleeding or those with high bleeding risk should have mechanical prophylaxis ordered and applied until pharmacologic prophylaxis is appropriate. Any missed single dose of pharmacologic prophylaxis or missed day of applied mechanical prophylaxis is considered a possible opportunity for improvement, and the case is referred to the appropriate clinical service for additional review.
Since task force launch, the percent of all patients diagnosed with HA‐VTE who received guideline‐directed prophylaxis increased 7% from 86% (105 of 122) in 2012 to 92% (80 of 87) in the first 9 months of 2015. Of events with possible opportunities, most were deemed not to have been preventable. Some trauma patients were ineligible for pharmacologic and mechanical prophylaxis, some were prophylaxed according to the best available evidence, and some had risk factors (for example, active malignancy) only identified after the VTE event. The few remaining events highlighted opportunities regarding standardization of pharmacologic prophylaxis periprocedurally, documentation of application of mechanical prophylaxis, and communication of patient refusal of doses, all ongoing focus areas for improvement.
Reduction in HA‐VTE
Improved VTE prophylaxis has contributed to a 15% reduction in HA‐VTE in all hospitalized patients over 5 years from a rate of 7.5 events/1000 inpatients in 2011 to 6.4/1000 inpatients for the first 9 months of 2015. Among postoperative patients (AHRQ PSI 12), the rate of VTE decreased 21% from 11.7/1000 patients in 2011 to 9.3/1000 patients in the first 9 months of 2015.
Patient/Family Engagement
We further improved our processes to ensure that patients with HA‐VTE who discharge to home receive written discharge instructions for warfarin use (VTE‐5). In 2014, performance on this measure was 91% (51 of 56 eligible patients) and in 2015 performance improved to 96% (78 of 81 eligible patients) compared with a reported national average of 91%. Additionally, 97% (79 of 81) of patients who discharged home on warfarin after HA‐VTE now have outpatient anticoagulation follow‐up arranged prior to hospital discharge. We are developing new initiatives for patient and family education regarding direct oral anticoagulants.
Discussion/Conclusions
With interdisciplinary teamwork and use of QI analytics to drive transparency, we have improved VTE prevention and reduced rates of HA‐VTE. Harborview's HA‐VTE prevention initiative can be duplicated by other organizations given the structured nature of the intervention. The multidisciplinary approach, clinical presence of task force members, and support and engagement of senior clinical leadership have been key elements to our program's success. The existence of a standard institutional guideline based on evidence‐based national guidelines and incorporation of these standards into the EHR is vital. The VTE task force has consistently used QI analytics both for retrospective review and real‐time data feedback. Complete and easy accessibility and transparency of performance at the service and unit level supports accountability. Integration of the task force work into existing institutional QI structures has further led to improvements in patient safety.
Ongoing task force collaboration and communication with frontline providers and clinical departments has been critical to engagement and sustained improvements in VTE prevention and treatment. The work of the VTE task force represents the steadfast commitment of Harborview and our clinical staff to prevent preventable harm. This multidisciplinary effort has served as a model for other QI initiatives across our institution and health system.
Disclosure
Nothing to report.
- , , , et al. Executive Summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐based Clinical Guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , , , . Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(4 suppl):S495–S501.
- , , , et al. Prevention of VTE in orthopedic surgery patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S–e325S.
- , , , et al. Prevention of VTE in nonsurgical patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S–e226S.
- , , , et al; American College of Chest Physicians. Prevention of VTE in nonorthopedic surgical patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S–e277S.
- Centers for Medicare and Medicaid Services. Core measures. Available at: https://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/QualityMeasures/Core‐Measures.html. Accessed September 1, 2016.
- Centers for Disease Control and Prevention. Venous thromboembolism. Available at: http://www.cdc.gov/ncbddd/dvt/index.html. Accessed September 1, 2016.
- . Preventing hospital‐associated venous thromboembolism: a guide for effective quality improvement, 2nd ed. AHRQ Publication No. 16‐0001‐EF. Rockville MD: Agency for Healthcare Research and Quality; 2016.
- . Preventing hospital‐associated venous thromboembolism: a guide for effective quality improvement. Available at: http://www.ahrq.gov/professionals/quality‐patient‐safety/patient‐safety‐resources/resources/vtguide/index.html. Accessed September 1, 2016.
- , . Preventing hospital‐acquired venous‐thromboembolism, a guide for effective quality improvement. Version 3.3. Venous Thromboembolism Quality Improvement Implementation Toolkit. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed September 1, 2016.
- , , , , . Adherence to guideline‐directed venous thromboembolism prophylaxis among medical and surgical inpatients at 33 academic medical centers in the United States. Am J Med Qual. 2010;26(3):174–180.
- UW Medicine guidelines for prevention of venous thromboembolism (VTE) in hospitalized patients. Available at: https://depts.washington.edu/anticoag/home. Accessed June 13, 2016.
- , , , , , . Upper extremity deep vein thrombosis in hospitalized patients: a descriptive study. J Hosp Med. 2014;9(1):48–53.
- , , , et al. Executive Summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐based Clinical Guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , , , . Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(4 suppl):S495–S501.
- , , , et al. Prevention of VTE in orthopedic surgery patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S–e325S.
- , , , et al. Prevention of VTE in nonsurgical patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S–e226S.
- , , , et al; American College of Chest Physicians. Prevention of VTE in nonorthopedic surgical patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S–e277S.
- Centers for Medicare and Medicaid Services. Core measures. Available at: https://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/QualityMeasures/Core‐Measures.html. Accessed September 1, 2016.
- Centers for Disease Control and Prevention. Venous thromboembolism. Available at: http://www.cdc.gov/ncbddd/dvt/index.html. Accessed September 1, 2016.
- . Preventing hospital‐associated venous thromboembolism: a guide for effective quality improvement, 2nd ed. AHRQ Publication No. 16‐0001‐EF. Rockville MD: Agency for Healthcare Research and Quality; 2016.
- . Preventing hospital‐associated venous thromboembolism: a guide for effective quality improvement. Available at: http://www.ahrq.gov/professionals/quality‐patient‐safety/patient‐safety‐resources/resources/vtguide/index.html. Accessed September 1, 2016.
- , . Preventing hospital‐acquired venous‐thromboembolism, a guide for effective quality improvement. Version 3.3. Venous Thromboembolism Quality Improvement Implementation Toolkit. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed September 1, 2016.
- , , , , . Adherence to guideline‐directed venous thromboembolism prophylaxis among medical and surgical inpatients at 33 academic medical centers in the United States. Am J Med Qual. 2010;26(3):174–180.
- UW Medicine guidelines for prevention of venous thromboembolism (VTE) in hospitalized patients. Available at: https://depts.washington.edu/anticoag/home. Accessed June 13, 2016.
- , , , , , . Upper extremity deep vein thrombosis in hospitalized patients: a descriptive study. J Hosp Med. 2014;9(1):48–53.
© 2016 Society of Hospital Medicine