User login
Prevention of Venous Thromboembolism
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a serious and growing public health problem. In the United States an estimated 900,000 people are affected and more than 100,000 die from VTE or related complications each year. More than half of VTE events occur in association with hospitalization or major surgery; many are thought to be preventable.[1, 2, 3, 4, 5] The Centers for Medicare and Medicaid Services (CMS), Centers for Disease Control and Prevention (CDC), and the Agency for Healthcare Research and Quality (AHRQ),[6, 7, 8, 9] among other organizations, have identified VTE as a potentially preventable never event. Evidence‐based guidelines and resources exist to help support hospital‐acquired venous thromboembolism (HA‐VTE) prevention.[1, 2, 3, 4, 5, 6, 7, 8, 9, 10] Harborview Medical Center, a tertiary referral center with more than 17,000 patients hospitalized annually, many requiring surgery, serves one of the highest‐risk populations for HA‐VTE development. Despite high rates of VTE prophylaxis in accordance with an established institutional guideline,[11, 12] VTE remains the most common hospital‐acquired condition in our institution.
OBJECTIVES
To improve the safety and care of all patients in our medical center and eliminate preventable HA‐VTE events, we set out to: (1) incorporate evidence‐based best practices in VTE prevention and treatment into current practice in alignment with institutional guidelines, (2) standardize the review process for all HA‐VTE events to identify opportunities for improvement, (3) utilize quality improvement (QI) analytics and information technology (IT) to actively improve our processes at the point of care, and (4) share process and outcome performance relating to VTE prevention transparently across our institution
METHODS
To prevent HA‐VTE, we employ a multifactorial strategy that includes designated clinical leadership, active engagement of all care team members, decision support tools embedded in the electronic health record (EHR), QI analytics, and retrospective and prospective reporting that provides ongoing measurement and analysis of the effectiveness of implemented interventions.
Setting/Patients
Harborview Medical Center, a 413‐bed academic tertiary referral center and the only level 1 adult and pediatric trauma and burn center for a 5‐state area, also serves as the primary safety‐net provider in the region. Harborview has centers of excellence in trauma, neurosciences, orthopedic and vascular surgery and rehabilitation, and is the only certified comprehensive stroke center in 5 states. With more than 17,000 admissions annually, including over 6000 trauma cases, HA‐VTE is a disease that spans critical and acute care settings and impacts patients on all clinical services. Harborview serves a population that is at extremely high risk for VTE as well as bleeding, particularly patients who have sustained central nervous system trauma or polytrauma.
Intervention
In 2010, at the request of the Harborview Medical Executive Board and Medical Director, we formed the Harborview VTE Task Force to assess VTE prevention practices across services and identify improvement opportunities for all hospitalized patients. This multidisciplinary team, co‐chaired by a hospitalist and trauma surgeon, includes representatives from trauma/general surgery, orthopedic surgery, hospital medicine, nursing, pharmacy, and QI. Task force members represent critical and acute care as well as the ambulatory setting. Additional stakeholders and local experts including IT directors and analysts, continuity of care nurses, and other clinical service representatives participate on an ad hoc basis.
Since its inception, the VTE Task Force has met monthly to review performance data and develop improvement initiatives. Initially we collaborated with experts across our health system to update an existing institutional VTE prophylaxis guideline to reflect current evidence‐based standards.[1, 3, 4, 5, 12] We met with all clinical services to ensure that the guidelines incorporated departmental best practices. These guidelines were integrated into our Cerner‐based (Cerner Corp., North Kansas City, MO) computerized provider order entry (CPOE) system to support accurate VTE risk assessment and appropriate ordering of prophylaxis.
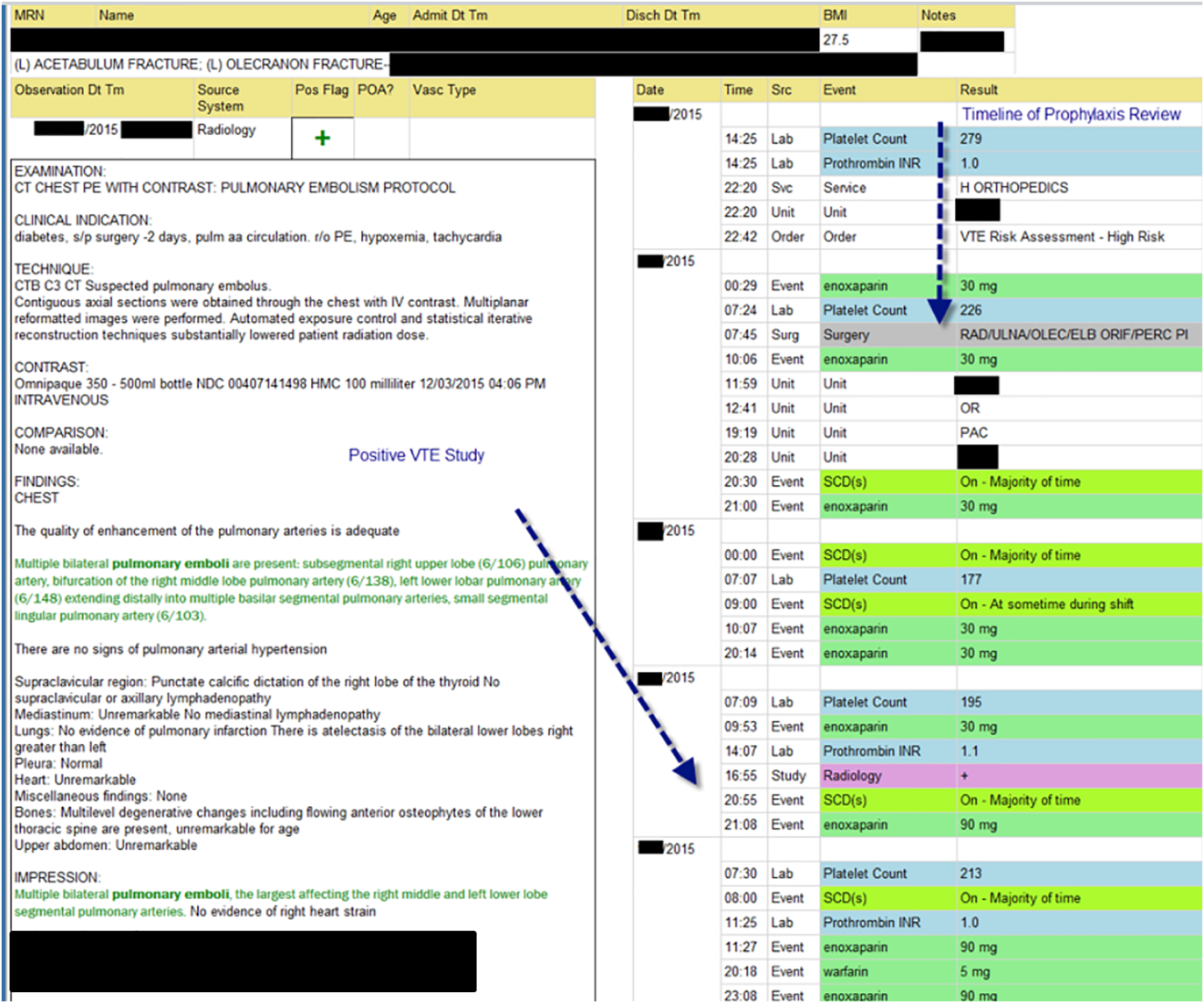
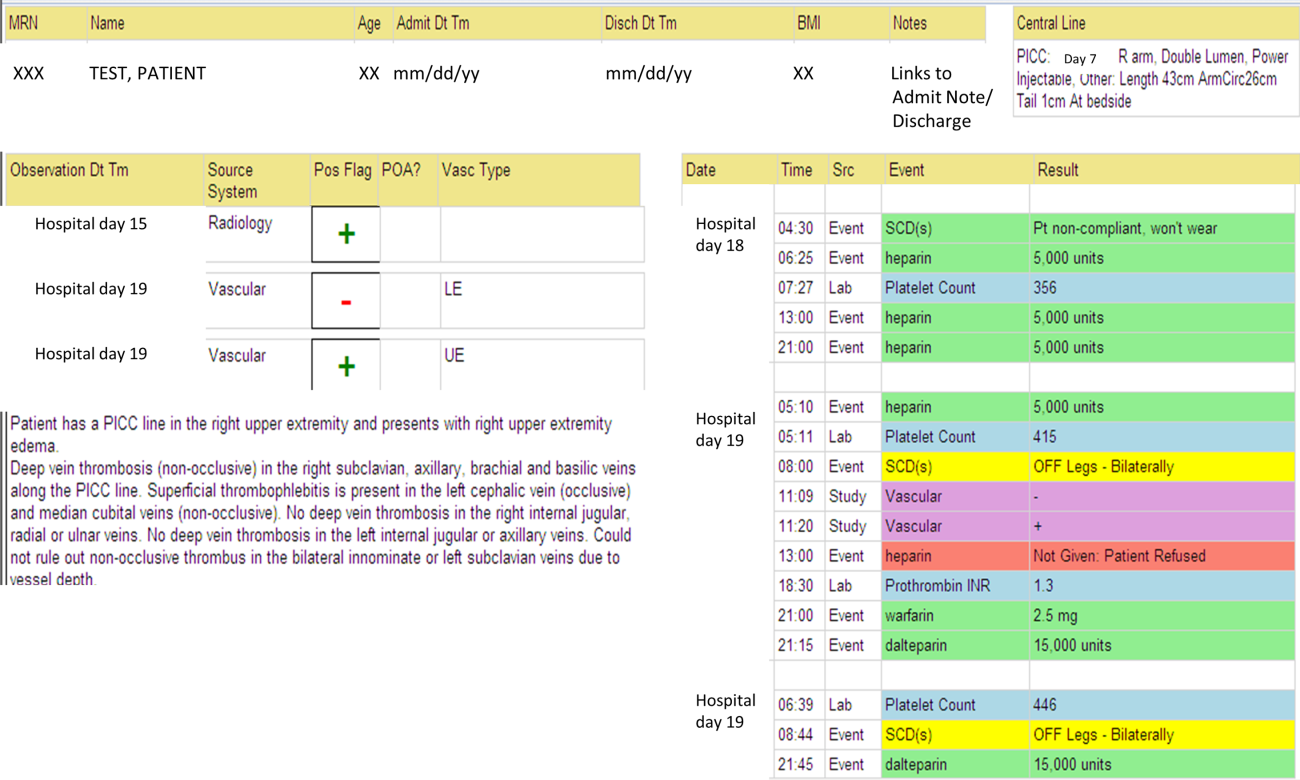
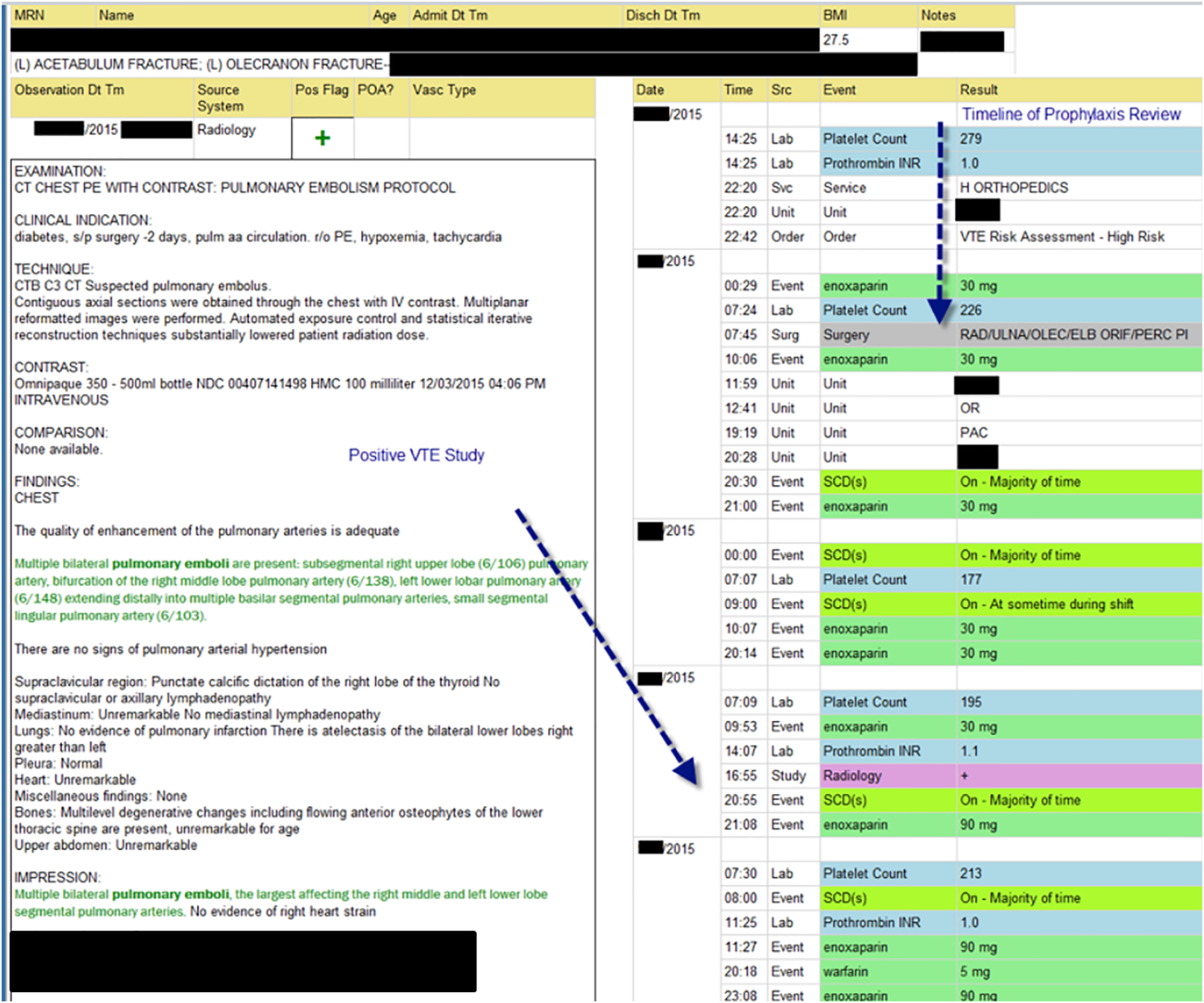
The VTE Task Force collaborated with QI programmers to develop an electronic tool, the Harborview VTE Tool (Figure 1),[13] that allows for efficient, standardized review of all HA‐VTE at monthly meetings. The tool uses word and phrase search capabilities to identify PEs and DVTs from imaging and vascular studies and links those events with pertinent demographic and clinical data from the EHR in a timeline. Information about VTE risk assigned by physicians in the CPOE system is extracted as well as specific VTE prophylaxis and treatment (drug, dose, timing of administration of medications, reason for doses being held, and orders for and application of mechanical prophylaxis). Using the VTE tool, the task force reviews each VTE event to assess the accuracy of VTE risk assignment, the appropriateness of prophylaxis received relative to guidelines, and the adequacy of VTE treatment and follow‐up. This tool has facilitated our review process, decreasing time from >30 minutes of manual chart review per event to several minutes. In recent months, a quality analyst has prescreened all VTEs prior to task force discussion to further improve efficiency. The tool allows the team to assess the case together and reach consensus regarding VTE prevention.
Prompt event reviews allow the task force to provide timely feedback about specific VTE events to physicians, nurses, and pharmacists. Cases with potential opportunities for improvement are referred to a medical center‐wide QI committee for secondary review. Areas of opportunity identified are tracked and trended to direct ongoing system improvement cycles. In 2014, as a result of reviewing patient cases with VTE diagnosed after discharge, we began a similar review process to assess current practice and standardize prophylaxis across care transitions.
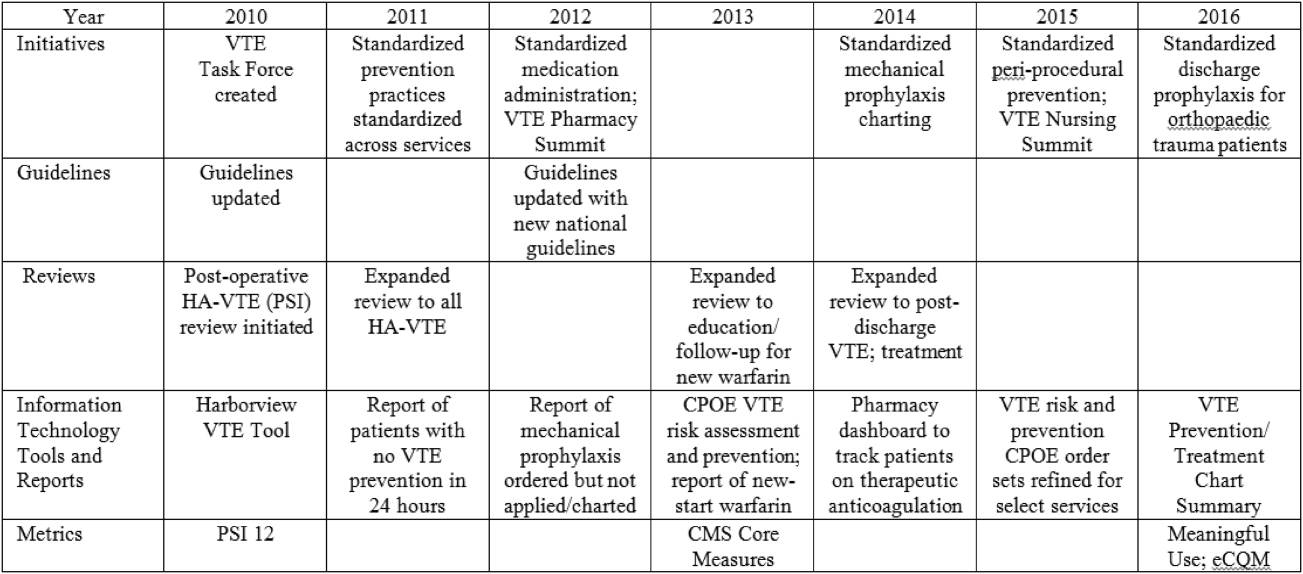
In response to opportunities identified from reviews, the VTE Task Force developed multiple reporting tools that provide real‐time, actionable information to clinicians at the bedside. Daily electronic lists highlight patients who have not received chemical or mechanical prophylaxis in 24 hours and are utilized by nursing, pharmacy, and physician groups. Patients receiving new start vitamin K antagonists or direct oral anticoagulants are identified for pharmacists and discharge care coordinators to support early patient/family education and ensure appropriate follow‐up. Based on input from frontline providers, tools are continually refined to improve their clinical utility. A timeline of initiatives that the Harborview VTE Task Force has championed is outlined in Figure 2.
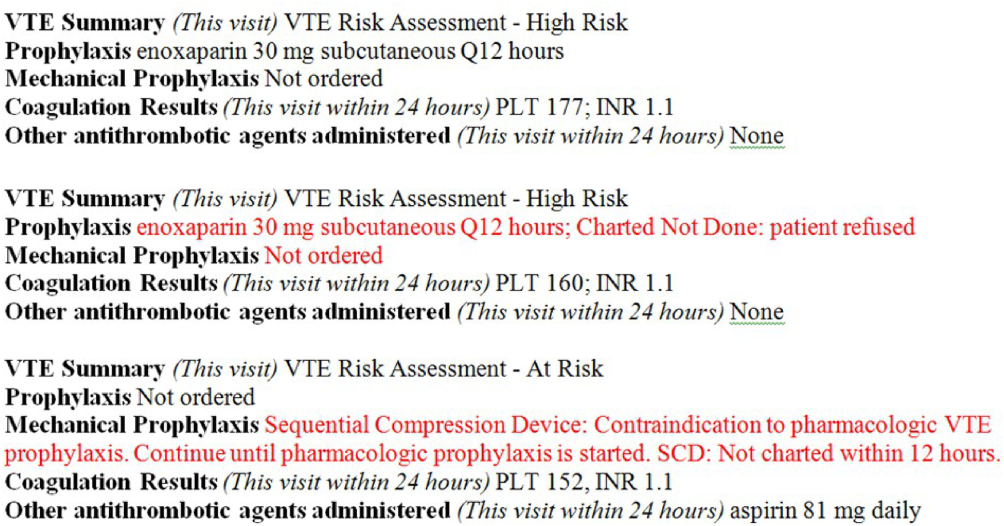
To bring HA‐VTE prevention information to the point of care, we developed a VTE Prevention/Treatment Summary within the EHR (Figure 3). Information about VTE risk assigned by the physician based on guidelines, current prophylaxis orders (pharmacologic/nonpharmacologic) and administration status, therapeutic anticoagulation and pertinent laboratory values are imported into a summary snapshot that can be accessed on demand by any member of the care team from within the patient's chart. The same data elements are being imbedded in resident physician and nursing handoff tools to highlight VTE prevention for all hospitalized patients and ensure optimal prophylaxis at transitions of care.
To emphasize Harborview's commitment to VTE prevention and ensure that care providers across the institution are aware of and engaged in this effort, we utilize our intranet to disseminate information in a fully transparent manner. Both process and outcome measures are available to all physicians and staff at service and unit levels on a Web‐based institutional dashboard. Data are updated monthly by QI analysts and improvement opportunities are highlighted in multiple fora. Descriptions of the quality metrics that are tracked are summarized in Table 1.
| Quality Metric | Description |
|---|---|
| |
| AHRQ PSI 12 | Cases of VTE not present on admission per 1000 surgical discharges with select operating room procedures |
| CMS Core Measure VTE‐1 | Percent of patients without VTE who received VTE prophylaxis on day of or day after arrival to an acute care area, random sample |
| CMS Core Measure VTE‐2 | Percent of patients without VTE who received VTE prophylaxis on day of or day after arrival to an intensive care unit or surgery date, random sample |
| CMS Core Measure VTE 5 | Percent of patients with hospital acquired VTE discharged to home on warfarin who received education and written discharge instructions |
| CMS Core Measure VTE‐6 | Percent of patients with hospital‐acquired VTE who received VTE prophylaxis prior to the event diagnosis |
MEASUREMENTS
Outcomes
Harborview benchmarks performance against hospitals nationally using the CMS Hospital Compare data and with peer academic institutions through Vizient data (Vizient, Irving, TX). To measure the impact of our initiatives, the task force began tracking postoperative VTE rates based on the AHRQ Patient Safety Indicator (PSI) 12 and expanded to include HA‐VTE rates for all hospitalized patients. We also report performance on Core Measure VTE‐6: incidence of potentially preventable VTE.
Process
We monitor VTE prophylaxis compliance based on the CMS Core Measures VTE‐1 and 2, random samples of acute and critical care patients without VTE. Internally, we measure compliance with guideline‐directed therapy for all HA‐VTE cases reviewed by the task force. With the upcoming retirement of the CMS chart‐abstracted measures, we are developing methods to track appropriate VTE prophylaxis provided to all eligible patients and will replace the sampled populations with this more expansive dataset. This approach will provide information for further improvements in VTE prophylaxis and act as an important step for success with the Electronic Clinical Quality Measures under the Meaningful Use program.
RESULTS
Our VTE prevention initiatives have resulted in improved compliance with our institutional guideline‐directed VTE prophylaxis and a decrease in HA‐VTE at our institution.
VTE Core Measures
Since the inception of VTE Core Measures in 2013, our annual performance on VTE‐1: prophylaxis for acute care patients has been above 95% and VTE‐2: prophylaxis for critical care patients has been above 98%. This performance has been consistently above the national mean for both measures (VTE‐1: 91% among Washington state hospitals and 93% nationally; VTE‐2: 95% among Washington state hospitals and 97% nationally). The CMS Hospital Compare current public reporting period is based on information collected from July 2014 through June 2015. Our internal performance for calendar year 2015 was 96% (289 of 302) for VTE‐1 and 98% (235 of 241) for VTE‐2.
Harborview has had zero potentially preventable VTE events (VTE‐6) compared with a reported national average of 4% since the inception of these measures in January 2013.
Guideline‐Directed VTE Prevention: Patients Diagnosed With HA‐VTE
The task force reviews each case to determine if the patient received guideline‐adherent prophylaxis on every day prior to the event. Patients with active bleeding or those with high bleeding risk should have mechanical prophylaxis ordered and applied until pharmacologic prophylaxis is appropriate. Any missed single dose of pharmacologic prophylaxis or missed day of applied mechanical prophylaxis is considered a possible opportunity for improvement, and the case is referred to the appropriate clinical service for additional review.
Since task force launch, the percent of all patients diagnosed with HA‐VTE who received guideline‐directed prophylaxis increased 7% from 86% (105 of 122) in 2012 to 92% (80 of 87) in the first 9 months of 2015. Of events with possible opportunities, most were deemed not to have been preventable. Some trauma patients were ineligible for pharmacologic and mechanical prophylaxis, some were prophylaxed according to the best available evidence, and some had risk factors (for example, active malignancy) only identified after the VTE event. The few remaining events highlighted opportunities regarding standardization of pharmacologic prophylaxis periprocedurally, documentation of application of mechanical prophylaxis, and communication of patient refusal of doses, all ongoing focus areas for improvement.
Reduction in HA‐VTE
Improved VTE prophylaxis has contributed to a 15% reduction in HA‐VTE in all hospitalized patients over 5 years from a rate of 7.5 events/1000 inpatients in 2011 to 6.4/1000 inpatients for the first 9 months of 2015. Among postoperative patients (AHRQ PSI 12), the rate of VTE decreased 21% from 11.7/1000 patients in 2011 to 9.3/1000 patients in the first 9 months of 2015.
Patient/Family Engagement
We further improved our processes to ensure that patients with HA‐VTE who discharge to home receive written discharge instructions for warfarin use (VTE‐5). In 2014, performance on this measure was 91% (51 of 56 eligible patients) and in 2015 performance improved to 96% (78 of 81 eligible patients) compared with a reported national average of 91%. Additionally, 97% (79 of 81) of patients who discharged home on warfarin after HA‐VTE now have outpatient anticoagulation follow‐up arranged prior to hospital discharge. We are developing new initiatives for patient and family education regarding direct oral anticoagulants.
Discussion/Conclusions
With interdisciplinary teamwork and use of QI analytics to drive transparency, we have improved VTE prevention and reduced rates of HA‐VTE. Harborview's HA‐VTE prevention initiative can be duplicated by other organizations given the structured nature of the intervention. The multidisciplinary approach, clinical presence of task force members, and support and engagement of senior clinical leadership have been key elements to our program's success. The existence of a standard institutional guideline based on evidence‐based national guidelines and incorporation of these standards into the EHR is vital. The VTE task force has consistently used QI analytics both for retrospective review and real‐time data feedback. Complete and easy accessibility and transparency of performance at the service and unit level supports accountability. Integration of the task force work into existing institutional QI structures has further led to improvements in patient safety.
Ongoing task force collaboration and communication with frontline providers and clinical departments has been critical to engagement and sustained improvements in VTE prevention and treatment. The work of the VTE task force represents the steadfast commitment of Harborview and our clinical staff to prevent preventable harm. This multidisciplinary effort has served as a model for other QI initiatives across our institution and health system.
Disclosure
Nothing to report.
- , , , et al. Executive Summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐based Clinical Guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , , , . Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(4 suppl):S495–S501.
- , , , et al. Prevention of VTE in orthopedic surgery patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S–e325S.
- , , , et al. Prevention of VTE in nonsurgical patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S–e226S.
- , , , et al; American College of Chest Physicians. Prevention of VTE in nonorthopedic surgical patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S–e277S.
- Centers for Medicare and Medicaid Services. Core measures. Available at: https://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/QualityMeasures/Core‐Measures.html. Accessed September 1, 2016.
- Centers for Disease Control and Prevention. Venous thromboembolism. Available at: http://www.cdc.gov/ncbddd/dvt/index.html. Accessed September 1, 2016.
- . Preventing hospital‐associated venous thromboembolism: a guide for effective quality improvement, 2nd ed. AHRQ Publication No. 16‐0001‐EF. Rockville MD: Agency for Healthcare Research and Quality; 2016.
- . Preventing hospital‐associated venous thromboembolism: a guide for effective quality improvement. Available at: http://www.ahrq.gov/professionals/quality‐patient‐safety/patient‐safety‐resources/resources/vtguide/index.html. Accessed September 1, 2016.
- , . Preventing hospital‐acquired venous‐thromboembolism, a guide for effective quality improvement. Version 3.3. Venous Thromboembolism Quality Improvement Implementation Toolkit. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed September 1, 2016.
- , , , , . Adherence to guideline‐directed venous thromboembolism prophylaxis among medical and surgical inpatients at 33 academic medical centers in the United States. Am J Med Qual. 2010;26(3):174–180.
- UW Medicine guidelines for prevention of venous thromboembolism (VTE) in hospitalized patients. Available at: https://depts.washington.edu/anticoag/home. Accessed June 13, 2016.
- , , , , , . Upper extremity deep vein thrombosis in hospitalized patients: a descriptive study. J Hosp Med. 2014;9(1):48–53.
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a serious and growing public health problem. In the United States an estimated 900,000 people are affected and more than 100,000 die from VTE or related complications each year. More than half of VTE events occur in association with hospitalization or major surgery; many are thought to be preventable.[1, 2, 3, 4, 5] The Centers for Medicare and Medicaid Services (CMS), Centers for Disease Control and Prevention (CDC), and the Agency for Healthcare Research and Quality (AHRQ),[6, 7, 8, 9] among other organizations, have identified VTE as a potentially preventable never event. Evidence‐based guidelines and resources exist to help support hospital‐acquired venous thromboembolism (HA‐VTE) prevention.[1, 2, 3, 4, 5, 6, 7, 8, 9, 10] Harborview Medical Center, a tertiary referral center with more than 17,000 patients hospitalized annually, many requiring surgery, serves one of the highest‐risk populations for HA‐VTE development. Despite high rates of VTE prophylaxis in accordance with an established institutional guideline,[11, 12] VTE remains the most common hospital‐acquired condition in our institution.
OBJECTIVES
To improve the safety and care of all patients in our medical center and eliminate preventable HA‐VTE events, we set out to: (1) incorporate evidence‐based best practices in VTE prevention and treatment into current practice in alignment with institutional guidelines, (2) standardize the review process for all HA‐VTE events to identify opportunities for improvement, (3) utilize quality improvement (QI) analytics and information technology (IT) to actively improve our processes at the point of care, and (4) share process and outcome performance relating to VTE prevention transparently across our institution
METHODS
To prevent HA‐VTE, we employ a multifactorial strategy that includes designated clinical leadership, active engagement of all care team members, decision support tools embedded in the electronic health record (EHR), QI analytics, and retrospective and prospective reporting that provides ongoing measurement and analysis of the effectiveness of implemented interventions.
Setting/Patients
Harborview Medical Center, a 413‐bed academic tertiary referral center and the only level 1 adult and pediatric trauma and burn center for a 5‐state area, also serves as the primary safety‐net provider in the region. Harborview has centers of excellence in trauma, neurosciences, orthopedic and vascular surgery and rehabilitation, and is the only certified comprehensive stroke center in 5 states. With more than 17,000 admissions annually, including over 6000 trauma cases, HA‐VTE is a disease that spans critical and acute care settings and impacts patients on all clinical services. Harborview serves a population that is at extremely high risk for VTE as well as bleeding, particularly patients who have sustained central nervous system trauma or polytrauma.
Intervention
In 2010, at the request of the Harborview Medical Executive Board and Medical Director, we formed the Harborview VTE Task Force to assess VTE prevention practices across services and identify improvement opportunities for all hospitalized patients. This multidisciplinary team, co‐chaired by a hospitalist and trauma surgeon, includes representatives from trauma/general surgery, orthopedic surgery, hospital medicine, nursing, pharmacy, and QI. Task force members represent critical and acute care as well as the ambulatory setting. Additional stakeholders and local experts including IT directors and analysts, continuity of care nurses, and other clinical service representatives participate on an ad hoc basis.
Since its inception, the VTE Task Force has met monthly to review performance data and develop improvement initiatives. Initially we collaborated with experts across our health system to update an existing institutional VTE prophylaxis guideline to reflect current evidence‐based standards.[1, 3, 4, 5, 12] We met with all clinical services to ensure that the guidelines incorporated departmental best practices. These guidelines were integrated into our Cerner‐based (Cerner Corp., North Kansas City, MO) computerized provider order entry (CPOE) system to support accurate VTE risk assessment and appropriate ordering of prophylaxis.
The VTE Task Force collaborated with QI programmers to develop an electronic tool, the Harborview VTE Tool (Figure 1),[13] that allows for efficient, standardized review of all HA‐VTE at monthly meetings. The tool uses word and phrase search capabilities to identify PEs and DVTs from imaging and vascular studies and links those events with pertinent demographic and clinical data from the EHR in a timeline. Information about VTE risk assigned by physicians in the CPOE system is extracted as well as specific VTE prophylaxis and treatment (drug, dose, timing of administration of medications, reason for doses being held, and orders for and application of mechanical prophylaxis). Using the VTE tool, the task force reviews each VTE event to assess the accuracy of VTE risk assignment, the appropriateness of prophylaxis received relative to guidelines, and the adequacy of VTE treatment and follow‐up. This tool has facilitated our review process, decreasing time from >30 minutes of manual chart review per event to several minutes. In recent months, a quality analyst has prescreened all VTEs prior to task force discussion to further improve efficiency. The tool allows the team to assess the case together and reach consensus regarding VTE prevention.

Prompt event reviews allow the task force to provide timely feedback about specific VTE events to physicians, nurses, and pharmacists. Cases with potential opportunities for improvement are referred to a medical center‐wide QI committee for secondary review. Areas of opportunity identified are tracked and trended to direct ongoing system improvement cycles. In 2014, as a result of reviewing patient cases with VTE diagnosed after discharge, we began a similar review process to assess current practice and standardize prophylaxis across care transitions.
In response to opportunities identified from reviews, the VTE Task Force developed multiple reporting tools that provide real‐time, actionable information to clinicians at the bedside. Daily electronic lists highlight patients who have not received chemical or mechanical prophylaxis in 24 hours and are utilized by nursing, pharmacy, and physician groups. Patients receiving new start vitamin K antagonists or direct oral anticoagulants are identified for pharmacists and discharge care coordinators to support early patient/family education and ensure appropriate follow‐up. Based on input from frontline providers, tools are continually refined to improve their clinical utility. A timeline of initiatives that the Harborview VTE Task Force has championed is outlined in Figure 2.

To bring HA‐VTE prevention information to the point of care, we developed a VTE Prevention/Treatment Summary within the EHR (Figure 3). Information about VTE risk assigned by the physician based on guidelines, current prophylaxis orders (pharmacologic/nonpharmacologic) and administration status, therapeutic anticoagulation and pertinent laboratory values are imported into a summary snapshot that can be accessed on demand by any member of the care team from within the patient's chart. The same data elements are being imbedded in resident physician and nursing handoff tools to highlight VTE prevention for all hospitalized patients and ensure optimal prophylaxis at transitions of care.

To emphasize Harborview's commitment to VTE prevention and ensure that care providers across the institution are aware of and engaged in this effort, we utilize our intranet to disseminate information in a fully transparent manner. Both process and outcome measures are available to all physicians and staff at service and unit levels on a Web‐based institutional dashboard. Data are updated monthly by QI analysts and improvement opportunities are highlighted in multiple fora. Descriptions of the quality metrics that are tracked are summarized in Table 1.
| Quality Metric | Description |
|---|---|
| |
| AHRQ PSI 12 | Cases of VTE not present on admission per 1000 surgical discharges with select operating room procedures |
| CMS Core Measure VTE‐1 | Percent of patients without VTE who received VTE prophylaxis on day of or day after arrival to an acute care area, random sample |
| CMS Core Measure VTE‐2 | Percent of patients without VTE who received VTE prophylaxis on day of or day after arrival to an intensive care unit or surgery date, random sample |
| CMS Core Measure VTE 5 | Percent of patients with hospital acquired VTE discharged to home on warfarin who received education and written discharge instructions |
| CMS Core Measure VTE‐6 | Percent of patients with hospital‐acquired VTE who received VTE prophylaxis prior to the event diagnosis |
MEASUREMENTS
Outcomes
Harborview benchmarks performance against hospitals nationally using the CMS Hospital Compare data and with peer academic institutions through Vizient data (Vizient, Irving, TX). To measure the impact of our initiatives, the task force began tracking postoperative VTE rates based on the AHRQ Patient Safety Indicator (PSI) 12 and expanded to include HA‐VTE rates for all hospitalized patients. We also report performance on Core Measure VTE‐6: incidence of potentially preventable VTE.
Process
We monitor VTE prophylaxis compliance based on the CMS Core Measures VTE‐1 and 2, random samples of acute and critical care patients without VTE. Internally, we measure compliance with guideline‐directed therapy for all HA‐VTE cases reviewed by the task force. With the upcoming retirement of the CMS chart‐abstracted measures, we are developing methods to track appropriate VTE prophylaxis provided to all eligible patients and will replace the sampled populations with this more expansive dataset. This approach will provide information for further improvements in VTE prophylaxis and act as an important step for success with the Electronic Clinical Quality Measures under the Meaningful Use program.
RESULTS
Our VTE prevention initiatives have resulted in improved compliance with our institutional guideline‐directed VTE prophylaxis and a decrease in HA‐VTE at our institution.
VTE Core Measures
Since the inception of VTE Core Measures in 2013, our annual performance on VTE‐1: prophylaxis for acute care patients has been above 95% and VTE‐2: prophylaxis for critical care patients has been above 98%. This performance has been consistently above the national mean for both measures (VTE‐1: 91% among Washington state hospitals and 93% nationally; VTE‐2: 95% among Washington state hospitals and 97% nationally). The CMS Hospital Compare current public reporting period is based on information collected from July 2014 through June 2015. Our internal performance for calendar year 2015 was 96% (289 of 302) for VTE‐1 and 98% (235 of 241) for VTE‐2.
Harborview has had zero potentially preventable VTE events (VTE‐6) compared with a reported national average of 4% since the inception of these measures in January 2013.
Guideline‐Directed VTE Prevention: Patients Diagnosed With HA‐VTE
The task force reviews each case to determine if the patient received guideline‐adherent prophylaxis on every day prior to the event. Patients with active bleeding or those with high bleeding risk should have mechanical prophylaxis ordered and applied until pharmacologic prophylaxis is appropriate. Any missed single dose of pharmacologic prophylaxis or missed day of applied mechanical prophylaxis is considered a possible opportunity for improvement, and the case is referred to the appropriate clinical service for additional review.
Since task force launch, the percent of all patients diagnosed with HA‐VTE who received guideline‐directed prophylaxis increased 7% from 86% (105 of 122) in 2012 to 92% (80 of 87) in the first 9 months of 2015. Of events with possible opportunities, most were deemed not to have been preventable. Some trauma patients were ineligible for pharmacologic and mechanical prophylaxis, some were prophylaxed according to the best available evidence, and some had risk factors (for example, active malignancy) only identified after the VTE event. The few remaining events highlighted opportunities regarding standardization of pharmacologic prophylaxis periprocedurally, documentation of application of mechanical prophylaxis, and communication of patient refusal of doses, all ongoing focus areas for improvement.
Reduction in HA‐VTE
Improved VTE prophylaxis has contributed to a 15% reduction in HA‐VTE in all hospitalized patients over 5 years from a rate of 7.5 events/1000 inpatients in 2011 to 6.4/1000 inpatients for the first 9 months of 2015. Among postoperative patients (AHRQ PSI 12), the rate of VTE decreased 21% from 11.7/1000 patients in 2011 to 9.3/1000 patients in the first 9 months of 2015.
Patient/Family Engagement
We further improved our processes to ensure that patients with HA‐VTE who discharge to home receive written discharge instructions for warfarin use (VTE‐5). In 2014, performance on this measure was 91% (51 of 56 eligible patients) and in 2015 performance improved to 96% (78 of 81 eligible patients) compared with a reported national average of 91%. Additionally, 97% (79 of 81) of patients who discharged home on warfarin after HA‐VTE now have outpatient anticoagulation follow‐up arranged prior to hospital discharge. We are developing new initiatives for patient and family education regarding direct oral anticoagulants.
Discussion/Conclusions
With interdisciplinary teamwork and use of QI analytics to drive transparency, we have improved VTE prevention and reduced rates of HA‐VTE. Harborview's HA‐VTE prevention initiative can be duplicated by other organizations given the structured nature of the intervention. The multidisciplinary approach, clinical presence of task force members, and support and engagement of senior clinical leadership have been key elements to our program's success. The existence of a standard institutional guideline based on evidence‐based national guidelines and incorporation of these standards into the EHR is vital. The VTE task force has consistently used QI analytics both for retrospective review and real‐time data feedback. Complete and easy accessibility and transparency of performance at the service and unit level supports accountability. Integration of the task force work into existing institutional QI structures has further led to improvements in patient safety.
Ongoing task force collaboration and communication with frontline providers and clinical departments has been critical to engagement and sustained improvements in VTE prevention and treatment. The work of the VTE task force represents the steadfast commitment of Harborview and our clinical staff to prevent preventable harm. This multidisciplinary effort has served as a model for other QI initiatives across our institution and health system.
Disclosure
Nothing to report.
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a serious and growing public health problem. In the United States an estimated 900,000 people are affected and more than 100,000 die from VTE or related complications each year. More than half of VTE events occur in association with hospitalization or major surgery; many are thought to be preventable.[1, 2, 3, 4, 5] The Centers for Medicare and Medicaid Services (CMS), Centers for Disease Control and Prevention (CDC), and the Agency for Healthcare Research and Quality (AHRQ),[6, 7, 8, 9] among other organizations, have identified VTE as a potentially preventable never event. Evidence‐based guidelines and resources exist to help support hospital‐acquired venous thromboembolism (HA‐VTE) prevention.[1, 2, 3, 4, 5, 6, 7, 8, 9, 10] Harborview Medical Center, a tertiary referral center with more than 17,000 patients hospitalized annually, many requiring surgery, serves one of the highest‐risk populations for HA‐VTE development. Despite high rates of VTE prophylaxis in accordance with an established institutional guideline,[11, 12] VTE remains the most common hospital‐acquired condition in our institution.
OBJECTIVES
To improve the safety and care of all patients in our medical center and eliminate preventable HA‐VTE events, we set out to: (1) incorporate evidence‐based best practices in VTE prevention and treatment into current practice in alignment with institutional guidelines, (2) standardize the review process for all HA‐VTE events to identify opportunities for improvement, (3) utilize quality improvement (QI) analytics and information technology (IT) to actively improve our processes at the point of care, and (4) share process and outcome performance relating to VTE prevention transparently across our institution
METHODS
To prevent HA‐VTE, we employ a multifactorial strategy that includes designated clinical leadership, active engagement of all care team members, decision support tools embedded in the electronic health record (EHR), QI analytics, and retrospective and prospective reporting that provides ongoing measurement and analysis of the effectiveness of implemented interventions.
Setting/Patients
Harborview Medical Center, a 413‐bed academic tertiary referral center and the only level 1 adult and pediatric trauma and burn center for a 5‐state area, also serves as the primary safety‐net provider in the region. Harborview has centers of excellence in trauma, neurosciences, orthopedic and vascular surgery and rehabilitation, and is the only certified comprehensive stroke center in 5 states. With more than 17,000 admissions annually, including over 6000 trauma cases, HA‐VTE is a disease that spans critical and acute care settings and impacts patients on all clinical services. Harborview serves a population that is at extremely high risk for VTE as well as bleeding, particularly patients who have sustained central nervous system trauma or polytrauma.
Intervention
In 2010, at the request of the Harborview Medical Executive Board and Medical Director, we formed the Harborview VTE Task Force to assess VTE prevention practices across services and identify improvement opportunities for all hospitalized patients. This multidisciplinary team, co‐chaired by a hospitalist and trauma surgeon, includes representatives from trauma/general surgery, orthopedic surgery, hospital medicine, nursing, pharmacy, and QI. Task force members represent critical and acute care as well as the ambulatory setting. Additional stakeholders and local experts including IT directors and analysts, continuity of care nurses, and other clinical service representatives participate on an ad hoc basis.
Since its inception, the VTE Task Force has met monthly to review performance data and develop improvement initiatives. Initially we collaborated with experts across our health system to update an existing institutional VTE prophylaxis guideline to reflect current evidence‐based standards.[1, 3, 4, 5, 12] We met with all clinical services to ensure that the guidelines incorporated departmental best practices. These guidelines were integrated into our Cerner‐based (Cerner Corp., North Kansas City, MO) computerized provider order entry (CPOE) system to support accurate VTE risk assessment and appropriate ordering of prophylaxis.
The VTE Task Force collaborated with QI programmers to develop an electronic tool, the Harborview VTE Tool (Figure 1),[13] that allows for efficient, standardized review of all HA‐VTE at monthly meetings. The tool uses word and phrase search capabilities to identify PEs and DVTs from imaging and vascular studies and links those events with pertinent demographic and clinical data from the EHR in a timeline. Information about VTE risk assigned by physicians in the CPOE system is extracted as well as specific VTE prophylaxis and treatment (drug, dose, timing of administration of medications, reason for doses being held, and orders for and application of mechanical prophylaxis). Using the VTE tool, the task force reviews each VTE event to assess the accuracy of VTE risk assignment, the appropriateness of prophylaxis received relative to guidelines, and the adequacy of VTE treatment and follow‐up. This tool has facilitated our review process, decreasing time from >30 minutes of manual chart review per event to several minutes. In recent months, a quality analyst has prescreened all VTEs prior to task force discussion to further improve efficiency. The tool allows the team to assess the case together and reach consensus regarding VTE prevention.

Prompt event reviews allow the task force to provide timely feedback about specific VTE events to physicians, nurses, and pharmacists. Cases with potential opportunities for improvement are referred to a medical center‐wide QI committee for secondary review. Areas of opportunity identified are tracked and trended to direct ongoing system improvement cycles. In 2014, as a result of reviewing patient cases with VTE diagnosed after discharge, we began a similar review process to assess current practice and standardize prophylaxis across care transitions.
In response to opportunities identified from reviews, the VTE Task Force developed multiple reporting tools that provide real‐time, actionable information to clinicians at the bedside. Daily electronic lists highlight patients who have not received chemical or mechanical prophylaxis in 24 hours and are utilized by nursing, pharmacy, and physician groups. Patients receiving new start vitamin K antagonists or direct oral anticoagulants are identified for pharmacists and discharge care coordinators to support early patient/family education and ensure appropriate follow‐up. Based on input from frontline providers, tools are continually refined to improve their clinical utility. A timeline of initiatives that the Harborview VTE Task Force has championed is outlined in Figure 2.

To bring HA‐VTE prevention information to the point of care, we developed a VTE Prevention/Treatment Summary within the EHR (Figure 3). Information about VTE risk assigned by the physician based on guidelines, current prophylaxis orders (pharmacologic/nonpharmacologic) and administration status, therapeutic anticoagulation and pertinent laboratory values are imported into a summary snapshot that can be accessed on demand by any member of the care team from within the patient's chart. The same data elements are being imbedded in resident physician and nursing handoff tools to highlight VTE prevention for all hospitalized patients and ensure optimal prophylaxis at transitions of care.

To emphasize Harborview's commitment to VTE prevention and ensure that care providers across the institution are aware of and engaged in this effort, we utilize our intranet to disseminate information in a fully transparent manner. Both process and outcome measures are available to all physicians and staff at service and unit levels on a Web‐based institutional dashboard. Data are updated monthly by QI analysts and improvement opportunities are highlighted in multiple fora. Descriptions of the quality metrics that are tracked are summarized in Table 1.
| Quality Metric | Description |
|---|---|
| |
| AHRQ PSI 12 | Cases of VTE not present on admission per 1000 surgical discharges with select operating room procedures |
| CMS Core Measure VTE‐1 | Percent of patients without VTE who received VTE prophylaxis on day of or day after arrival to an acute care area, random sample |
| CMS Core Measure VTE‐2 | Percent of patients without VTE who received VTE prophylaxis on day of or day after arrival to an intensive care unit or surgery date, random sample |
| CMS Core Measure VTE 5 | Percent of patients with hospital acquired VTE discharged to home on warfarin who received education and written discharge instructions |
| CMS Core Measure VTE‐6 | Percent of patients with hospital‐acquired VTE who received VTE prophylaxis prior to the event diagnosis |
MEASUREMENTS
Outcomes
Harborview benchmarks performance against hospitals nationally using the CMS Hospital Compare data and with peer academic institutions through Vizient data (Vizient, Irving, TX). To measure the impact of our initiatives, the task force began tracking postoperative VTE rates based on the AHRQ Patient Safety Indicator (PSI) 12 and expanded to include HA‐VTE rates for all hospitalized patients. We also report performance on Core Measure VTE‐6: incidence of potentially preventable VTE.
Process
We monitor VTE prophylaxis compliance based on the CMS Core Measures VTE‐1 and 2, random samples of acute and critical care patients without VTE. Internally, we measure compliance with guideline‐directed therapy for all HA‐VTE cases reviewed by the task force. With the upcoming retirement of the CMS chart‐abstracted measures, we are developing methods to track appropriate VTE prophylaxis provided to all eligible patients and will replace the sampled populations with this more expansive dataset. This approach will provide information for further improvements in VTE prophylaxis and act as an important step for success with the Electronic Clinical Quality Measures under the Meaningful Use program.
RESULTS
Our VTE prevention initiatives have resulted in improved compliance with our institutional guideline‐directed VTE prophylaxis and a decrease in HA‐VTE at our institution.
VTE Core Measures
Since the inception of VTE Core Measures in 2013, our annual performance on VTE‐1: prophylaxis for acute care patients has been above 95% and VTE‐2: prophylaxis for critical care patients has been above 98%. This performance has been consistently above the national mean for both measures (VTE‐1: 91% among Washington state hospitals and 93% nationally; VTE‐2: 95% among Washington state hospitals and 97% nationally). The CMS Hospital Compare current public reporting period is based on information collected from July 2014 through June 2015. Our internal performance for calendar year 2015 was 96% (289 of 302) for VTE‐1 and 98% (235 of 241) for VTE‐2.
Harborview has had zero potentially preventable VTE events (VTE‐6) compared with a reported national average of 4% since the inception of these measures in January 2013.
Guideline‐Directed VTE Prevention: Patients Diagnosed With HA‐VTE
The task force reviews each case to determine if the patient received guideline‐adherent prophylaxis on every day prior to the event. Patients with active bleeding or those with high bleeding risk should have mechanical prophylaxis ordered and applied until pharmacologic prophylaxis is appropriate. Any missed single dose of pharmacologic prophylaxis or missed day of applied mechanical prophylaxis is considered a possible opportunity for improvement, and the case is referred to the appropriate clinical service for additional review.
Since task force launch, the percent of all patients diagnosed with HA‐VTE who received guideline‐directed prophylaxis increased 7% from 86% (105 of 122) in 2012 to 92% (80 of 87) in the first 9 months of 2015. Of events with possible opportunities, most were deemed not to have been preventable. Some trauma patients were ineligible for pharmacologic and mechanical prophylaxis, some were prophylaxed according to the best available evidence, and some had risk factors (for example, active malignancy) only identified after the VTE event. The few remaining events highlighted opportunities regarding standardization of pharmacologic prophylaxis periprocedurally, documentation of application of mechanical prophylaxis, and communication of patient refusal of doses, all ongoing focus areas for improvement.
Reduction in HA‐VTE
Improved VTE prophylaxis has contributed to a 15% reduction in HA‐VTE in all hospitalized patients over 5 years from a rate of 7.5 events/1000 inpatients in 2011 to 6.4/1000 inpatients for the first 9 months of 2015. Among postoperative patients (AHRQ PSI 12), the rate of VTE decreased 21% from 11.7/1000 patients in 2011 to 9.3/1000 patients in the first 9 months of 2015.
Patient/Family Engagement
We further improved our processes to ensure that patients with HA‐VTE who discharge to home receive written discharge instructions for warfarin use (VTE‐5). In 2014, performance on this measure was 91% (51 of 56 eligible patients) and in 2015 performance improved to 96% (78 of 81 eligible patients) compared with a reported national average of 91%. Additionally, 97% (79 of 81) of patients who discharged home on warfarin after HA‐VTE now have outpatient anticoagulation follow‐up arranged prior to hospital discharge. We are developing new initiatives for patient and family education regarding direct oral anticoagulants.
Discussion/Conclusions
With interdisciplinary teamwork and use of QI analytics to drive transparency, we have improved VTE prevention and reduced rates of HA‐VTE. Harborview's HA‐VTE prevention initiative can be duplicated by other organizations given the structured nature of the intervention. The multidisciplinary approach, clinical presence of task force members, and support and engagement of senior clinical leadership have been key elements to our program's success. The existence of a standard institutional guideline based on evidence‐based national guidelines and incorporation of these standards into the EHR is vital. The VTE task force has consistently used QI analytics both for retrospective review and real‐time data feedback. Complete and easy accessibility and transparency of performance at the service and unit level supports accountability. Integration of the task force work into existing institutional QI structures has further led to improvements in patient safety.
Ongoing task force collaboration and communication with frontline providers and clinical departments has been critical to engagement and sustained improvements in VTE prevention and treatment. The work of the VTE task force represents the steadfast commitment of Harborview and our clinical staff to prevent preventable harm. This multidisciplinary effort has served as a model for other QI initiatives across our institution and health system.
Disclosure
Nothing to report.
- , , , et al. Executive Summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐based Clinical Guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , , , . Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(4 suppl):S495–S501.
- , , , et al. Prevention of VTE in orthopedic surgery patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S–e325S.
- , , , et al. Prevention of VTE in nonsurgical patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S–e226S.
- , , , et al; American College of Chest Physicians. Prevention of VTE in nonorthopedic surgical patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S–e277S.
- Centers for Medicare and Medicaid Services. Core measures. Available at: https://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/QualityMeasures/Core‐Measures.html. Accessed September 1, 2016.
- Centers for Disease Control and Prevention. Venous thromboembolism. Available at: http://www.cdc.gov/ncbddd/dvt/index.html. Accessed September 1, 2016.
- . Preventing hospital‐associated venous thromboembolism: a guide for effective quality improvement, 2nd ed. AHRQ Publication No. 16‐0001‐EF. Rockville MD: Agency for Healthcare Research and Quality; 2016.
- . Preventing hospital‐associated venous thromboembolism: a guide for effective quality improvement. Available at: http://www.ahrq.gov/professionals/quality‐patient‐safety/patient‐safety‐resources/resources/vtguide/index.html. Accessed September 1, 2016.
- , . Preventing hospital‐acquired venous‐thromboembolism, a guide for effective quality improvement. Version 3.3. Venous Thromboembolism Quality Improvement Implementation Toolkit. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed September 1, 2016.
- , , , , . Adherence to guideline‐directed venous thromboembolism prophylaxis among medical and surgical inpatients at 33 academic medical centers in the United States. Am J Med Qual. 2010;26(3):174–180.
- UW Medicine guidelines for prevention of venous thromboembolism (VTE) in hospitalized patients. Available at: https://depts.washington.edu/anticoag/home. Accessed June 13, 2016.
- , , , , , . Upper extremity deep vein thrombosis in hospitalized patients: a descriptive study. J Hosp Med. 2014;9(1):48–53.
- , , , et al. Executive Summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐based Clinical Guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , , , . Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(4 suppl):S495–S501.
- , , , et al. Prevention of VTE in orthopedic surgery patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S–e325S.
- , , , et al. Prevention of VTE in nonsurgical patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S–e226S.
- , , , et al; American College of Chest Physicians. Prevention of VTE in nonorthopedic surgical patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S–e277S.
- Centers for Medicare and Medicaid Services. Core measures. Available at: https://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/QualityMeasures/Core‐Measures.html. Accessed September 1, 2016.
- Centers for Disease Control and Prevention. Venous thromboembolism. Available at: http://www.cdc.gov/ncbddd/dvt/index.html. Accessed September 1, 2016.
- . Preventing hospital‐associated venous thromboembolism: a guide for effective quality improvement, 2nd ed. AHRQ Publication No. 16‐0001‐EF. Rockville MD: Agency for Healthcare Research and Quality; 2016.
- . Preventing hospital‐associated venous thromboembolism: a guide for effective quality improvement. Available at: http://www.ahrq.gov/professionals/quality‐patient‐safety/patient‐safety‐resources/resources/vtguide/index.html. Accessed September 1, 2016.
- , . Preventing hospital‐acquired venous‐thromboembolism, a guide for effective quality improvement. Version 3.3. Venous Thromboembolism Quality Improvement Implementation Toolkit. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed September 1, 2016.
- , , , , . Adherence to guideline‐directed venous thromboembolism prophylaxis among medical and surgical inpatients at 33 academic medical centers in the United States. Am J Med Qual. 2010;26(3):174–180.
- UW Medicine guidelines for prevention of venous thromboembolism (VTE) in hospitalized patients. Available at: https://depts.washington.edu/anticoag/home. Accessed June 13, 2016.
- , , , , , . Upper extremity deep vein thrombosis in hospitalized patients: a descriptive study. J Hosp Med. 2014;9(1):48–53.
© 2016 Society of Hospital Medicine
Upper Extremity DVT in Hospitalized Patients
Increasingly, there is a focus on prevention of hospital‐acquired conditions including venous thromboembolism (VTE). Many studies have evaluated pulmonary embolism (PE) and lower extremity deep vein thrombosis (LEDVT), but despite increasing recognition of upper extremity deep vein thrombosis (UEDVT),[1, 2, 3, 4] less is known about this condition in hospitalized patients.
UEDVTs may be classified as primary, including disorders such as Paget‐Schroetter syndrome or other structural abnormality, or may be idiopathic; the majority are secondary clots.[5] Conventional risk factors for LEDVT including older age and obesity have been found to be less commonly associated,[1, 2, 5, 6, 7] and patients with UEDVT are generally younger, leaner, and a higher proportion are men. They are more likely to have malignancy or history of VTE and have undergone recent surgery or intensive care unit stay.[1, 2, 6] Central venous catheters (CVCs), often used in hospitalized patients, remain among the biggest known risks for UEDVT[1, 2, 3, 7, 8, 9, 10]; concomitant malignancy, VTE history, severe infection, surgery lasting >1 hour, and length of stay (LOS) >10 days confer additional risks with CVCs.[6, 7, 8, 11]
UEDVTs, once thought to be relatively benign, are now recognized to result in complications including PE, progression, recurrence, and post‐thrombotic syndrome.[2, 4, 12, 13] Despite extensive efforts to increase appropriate VTE prophylaxis in inpatients,[14] the role of chemoprophylaxis to prevent UEDVT remains undefined. Current guidelines recommend anticoagulation for treatment and complication prevention,[13, 15] but to date the evidence derives largely from observational studies or is extrapolated from the LEDVT literature.[2, 13]
To improve understanding of UEDVT at our institution, we set out to (1) determine UEDVT incidence in hospitalized patients, (2) describe associated risks and outcomes, and (3) assess management during hospitalization and at discharge.
METHODS
We identified all consecutive adult patients diagnosed with Doppler ultrasound‐confirmed UEDVT during hospitalization at Harborview Medical Center between September 2011 and November 2012. For patients who were readmitted during the study period, the first of their hospitalizations was used to describe associated factors, management, and outcomes. We present characteristics of all other hospitalizations during this time period for comparison. Harborview is a 413‐bed academic tertiary referral center and the only level 1 trauma center in a 5‐state area. Patients with UEDVT were identified using an information technology (IT) tool (the Harborview VTE tool) (Figure 1), which captures VTE events from vascular laboratory and radiology studies using natural language processing. Doppler ultrasound to assess for deep vein thrombosis (DVT) and computed tomographic scans to diagnose PE were ordered by inpatient physicians for symptomatic patients. The reason for obtaining the study is included in the ultrasound reports. We do not routinely screen for UEDVT at our institution. UEDVT included clots in the deep veins of the upper extremities including internal jugular, subclavian, axillary, and brachial veins. Superficial thrombosis and thrombophlebitis were excluded. We previously compared VTE events captured by this tool with administrative billing data and found that all VTE events that were coded were captured with the tool.
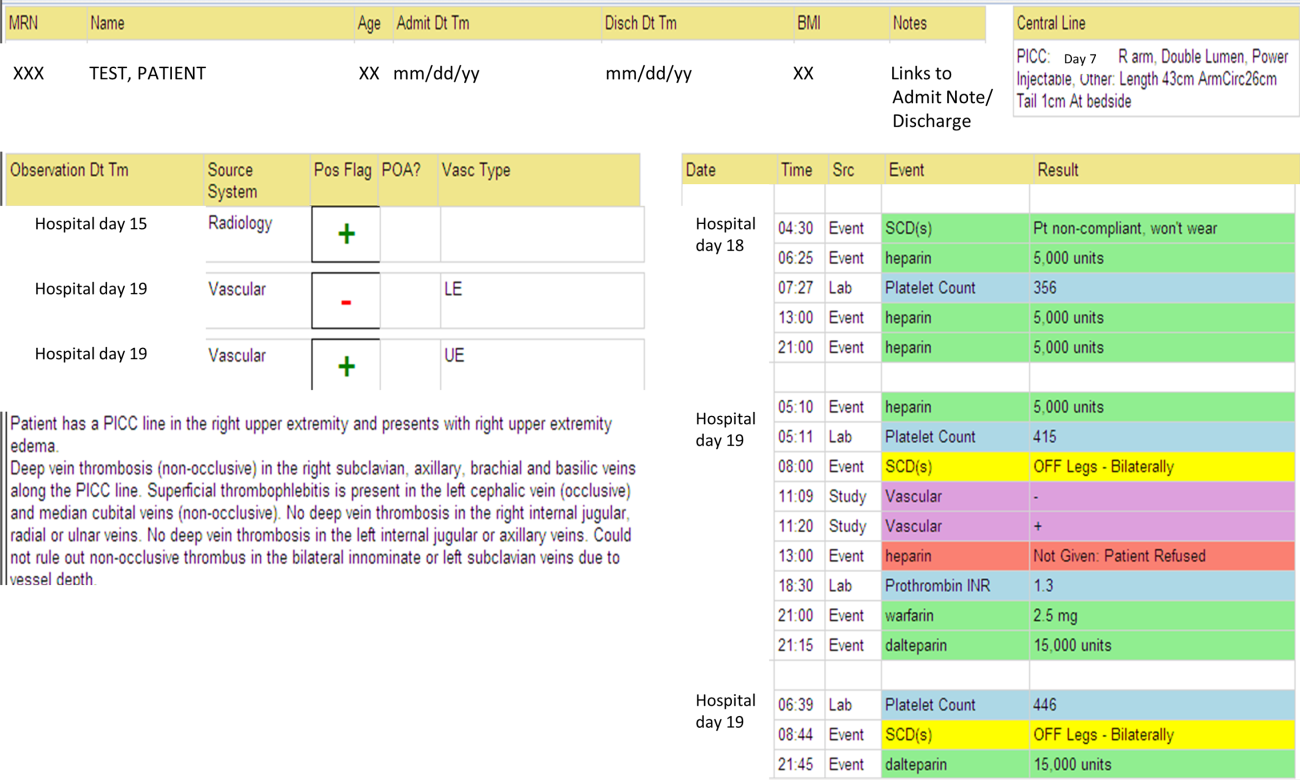
The VTE tool (Figure 1) displays imaging results together with demographic, clinical, and medication data and links this information with admission, discharge, and death summaries as well as CVC insertion procedure notes from the electronic health record (EHR). Additional data, including comorbid conditions, primary reason for hospitalization, past medical history such as prior VTE events, and cause of death (if not available in the admission note or discharge/death summaries), were obtained from EHR abstraction by 1 of the investigators. A 10% random sample of charts was rereviewed by another investigator with complete concordance. Supplementary data about date of CVC insertion if placed at an outside facility, date of CVC removal if applicable, clinical assessments regarding whether a clot was CVC‐associated, and contraindications to therapeutic anticoagulation were also abstracted directly from the EHR. Administrative data were used to identify the case mix index, an indicator of severity of illness.
Pharmacologic VTE prophylaxis included all chemical prophylaxis specified on our institutional guideline, most commonly subcutaneous unfractionated heparin 5000 units every 8 hours or low molecular weight heparin (LMWH), either enoxaparin 40 mg every 12 or 24 hours or dalteparin 5000 units every 24 hours. Mechanical prophylaxis was defined as use of sequential compression devices (SCDs) when pharmacologic prophylaxis was contraindicated. Prophylaxis was considered to be appropriate if it was applied according to our guideline for >90% of hospital days prior to UEDVT diagnosis. Therapeutic anticoagulation included heparin bridging (most commonly continuous heparin infusion, LMWH 1 mg/kg or dalteparin) as well as oral vitamin K antagonists. The VTE tool (Figure 1) allows identification of pharmacologic prophylaxis and therapy that is actually administered (not just ordered) directly from our pharmacy IT system. SCD application (not just ordered SCDs) is electronically integrated into the tool from nursing documentation.
CVCs included internal jugular or subclavian triple lumen catheters, tunneled dialysis catheters, or peripherally inserted central catheters (PICCs), single or double lumen. Criteria used to identify that a UEDVT was CVC‐associated included temporal relationship (CVC was placed prior to clot diagnosis), plausibility (ipsilateral clot), evidence of clot surrounding CVC on ultrasound, and physician designation of association (as documented in progress notes or discharge summary).
Simple percentages of patient characteristics, associated factors, management, and outcomes were calculated using counts as the numerator and number of patients as the denominator. For information about UEDVTs, we used total number of UEDVTs as the denominator. Line days were day counts from insertion until removal if applicable. The CVC placement date was available in our mandated central line placement procedure notes (directly accessed from the VTE tool) for all lines placed at our institution; date of removal (if applicable) was determined from chart abstraction. For the vast majority of patients whose CVCs were placed at outside facilities, date of placement was available in the EHR (often in the admission note or in the ultrasound report/reason for study). If date of line placement at an outside facility was not known, date of admission was used. The University of Washington Human Subjects Board approved this review.
RESULTS
General Characteristics
Fifty inpatients were diagnosed with 76 UEDVTs during 53 hospitalizations. Three patients were admitted twice during the study period. Their first admission is used for the purposes of this review. None of these 3 patients had new UEDVTs diagnosed during their second admission.
The patients' mean age was 49 years (standard deviation [SD] 15.6; range, 2482 years) vs 50.9 years (SD 17.49; range, 18112 years) among all other hospitalizations during this time (Table 1). Seventy percent (35) of patients with UEDVT were men. Sixteen percent (8) of patients with UEDVT had known VTE history, 20% (10) of patients had malignancy, and 22% (11) of patients had stage V chronic kidney disease or were hemodialysis dependent.
| Characteristic | Patients With UEDVT, N=50 | All Hospitalizations, N=23,407a |
|---|---|---|
| ||
| Age, y, mean (range) | 49 (2482) | 51 (18112) |
| Sex, % male (no.) | 70% (35) | 63% (14,746) |
| Case mix index, mean (range) | 4.78 (0.6917.99) | 1.87 (0.1626.34) |
| Length of stay, d, mean (range) | 24.6 (291) | 7.2 (1178) |
| Transfer from outside hospital (no.) | 50% (25) | 25% (5,866) |
| Intensive care unit stay (no.) | 46% (23) | 36% (8,356) |
| Operative procedure (no.) | 46% (23) | 41% (9,706) |
| In‐hospital mortality (no.) | 10% (5) | 4% (842) |
| Discharge to skilled nursing facility or other hospital, n=45 surviving patients (no.) | 62% (28) | 13% (3,095) |
| 30‐day readmission, n=45 surviving patients (no.) | 18% (8) | 5% (1,167) |
Patients diagnosed with UEDVT had complex illness, long LOS, and were often transferred from outside hospitals relative to other hospitalizations during this time period (Table 1). Slightly more required intensive care and underwent surgery. Eighty‐four percent (42) of patients with UEDVT required CVCs during hospitalization. Among patients whose UEDVT was not present on admission, 94% received appropriate VTE prophylaxis prior to UEDVT diagnosis.
In patients with UEDVT, the most common reasons for hospitalization were sepsis/severe infection (43%), cerebral hemorrhage (16%), and trauma (8%). Primary service at diagnosis was medicine 56.9%, surgery 25.5%, and neurosciences 17.6%.
Upper Extremity Deep Vein Thromboses
Fifty patients were diagnosed with 76 UEDVTs during their hospitalizations. In 40% (20) of patients, UEDVTs were present in >1 upper extremity deep vein; concurrent LEDVT was present in 26% (13) and PE in 10% (5). The majority of UEDVTs were found in internal jugular veins, followed by brachial and axillary veins. Seventeen percent were present on admission. Upper extremity swelling was the most common sign/symptom and reason for study. Characteristics of UEDVTs diagnosed are listed in Table 2.
| Characteristic | % UEDVTs (No.), n=76 |
|---|---|
| |
| Anatomic site | |
| Internal jugular | 38% (29) |
| Axillary | 21% (16) |
| Subclavian/axillary | 9% (7) |
| Subclavian | 7% (5) |
| Brachial | 25% (19) |
| Hospital day of diagnosis, d, mean (range) | 9.2 (044) |
| Present on admission | 17% (13) |
| Diagnosed at outside hospital or within 24 hours of transfer | 54% (7) |
| Diagnosed during prior hospitalization at our institution | 15% (2) |
| Diagnosed within 24 hours of admission via our emergency department | 23% (3) |
| Patient‐reported chronic UEDVT | 8% (1) |
| Primary UEDVT/anatomic anomaly | 0% (0) |
| Signs and symptoms (reasons for obtaining study) | |
| Upper extremity swelling | 71% (54) |
| Presence of clot elsewhere (eg, pulmonary embolism) | 9% (7) |
| Inability to place central venous access | 8% (6) |
| Assessment of clot propagation (known clot) | 8% (6) |
| Pain | 3% (2) |
| Patient‐reported history | 1% (1) |
Of the 50 patients diagnosed with UEDVT during hospitalization, 44% (22) were found to have UEDVTs directly associated with a CVC. Forty‐two of the 50 patients had a CVC; 52% (22 of 42) had CVC‐associated UEDVTs. Fifty percent (11) of these CVCs were triple lumen catheters, 32% (7) were PICCs, and 18% (4) were tunneled dialysis lines. Three of 42 patients with CVCs and line‐associated clots were had a malignancy. For patients with CVC‐associated clot, lines were in place for an average of 14.3 days (range, 273 days) prior to UEDVT diagnosis.
Treatment and Management
Seventy‐eight percent (39) of patients with UEDVT received in‐hospital treatment with heparin/LMWH bridging and oral anticoagulation. Of the 45 patients who survived hospitalization, 75% (34) were prescribed anticoagulation for 3+ months at discharge; 23% (10) had documented contraindications to anticoagulation, most commonly recent gastrointestinal or intracranial bleeding. Two percent of patients (1) was not prescribed pharmacologic treatment at discharge and had no contraindications documented. No patients underwent thrombolysis or had superior vena cava filters placed. Sixty‐four percent (14 of 22) of CVCs that were thought to be directly associated with UEDVT were removed at diagnosis.
Outcomes
Five patients (10%) died during hospitalization, none because of VTE or complications thereof. Cause of death included septic shock, cancer, intracranial hemorrhage, heart failure, and recurrent gastrointestinal bleeding. Of the 45 surviving patients, only 38% (17) were discharged to self‐care; more than half (62%[28]) were discharged to skilled nursing facilities, other hospitals, or rehabilitation centers. Eight patients (18%) were readmitted to our institution within 30 days; none for recurrent or new DVT or PE. No additional patients died at our medical center within 30 days of discharge.
DISCUSSION
UEDVT is increasingly recognized in hospitalized patients.[3, 9] At our medical center, 0.2% of symptomatic inpatients were diagnosed with UEDVT over 14 months. These patients were predominantly men with high rates of CVCs, malignancy, VTE history, severe infection, and renal disease. Interestingly, although the literature suggests that some proportion of patients with UEDVT have anatomic abnormalities, such as Paget‐Schroetter syndrome,[15] none of the patients in our study were found to have these anomalies. In our review, hospitalized patients with UEDVT were critically ill, with a long LOS and high morbidity and mortality, suggesting that in addition to just being a complication of hospitalization,[1, 6] UEDVT may be a marker of severe illness.
In our institution, clinical presentation was consistent with what has been described with the majority of patients presenting with upper extremity swelling.[1, 3] The internal jugular veins were the most common anatomic UEDVT site, followed by brachial then axillary veins. In other series including both in‐ and outpatients, subclavian clots were most commonly diagnosed, reflecting in part higher rates of CVC association and CVC location in those studies.[3, 9] Concurrent DVT and PE rates were similar to those reported.[1, 3, 10]
Although many studies have focused on prevention of LEDVT and PE, few trials have specifically targeted UEDVT. Among our patients with UEDVTs that were not present on admission, VTE prophylaxis rates were considerably higher than what has been reported,[1, 6] suggesting that in these critically ill patients' prophylaxis may not prevent symptomatic UEDVT. It is unknown how many UEDVTs were prevented with prophylaxis, as only patients with symptomatic UEDVT were included. Adequacy of prophylaxis at outside hospitals for patients transferred in could not be assessed. Nonetheless, low numbers of UEDVT at a trauma referral center with many high‐risk patients raise the question of whether prophylaxis makes a difference. Additional study is needed to further define the role of chemoprophylaxis to prevent UEDVT in hospitalized patients.
In our inpatient group, 84% required CVCs; 44% of patients were thought to have CVC‐associated UEDVTs. Careful patient selection and attention to potentially modifiable risks, such as insertion site, catheter type, and tip position, may need further examination in this population.[3, 11, 16] Catheter duration was long; focus on removing CVCs when no longer necessary is important. Interestingly, almost 10% in our study underwent diagnostic ultrasound because a new CVC could not be successfully placed suggesting that UEDVT may develop in critically ill patients regardless of CVCs.
In our study, there were high rates of guideline‐recommended pharmacologic treatment; surprisingly the majority of CVCs with associated clot were removed. Guidelines currently support 3 months of anticoagulation for treatment of UEDVT[2, 13, 17]; evidence derives from observational trials or is largely extrapolated from LEDVT literature.[2, 13] Routine CVC removal is not specifically recommended for CVC‐associated UEDVT, particularly if lines remain functional and medically necessary; systemic anticoagulation should be provided.[13]
In our review, no hospitalized patients with UEDVT developed complications or were readmitted to our medical center within 30 days for clot progression, new PE, or post‐thrombotic syndrome, which is lower than rates reported over longer time periods.[2, 6, 10, 12] Ten percent died during hospitalization, all from their primary disease rather than from complications of VTE or VTE treatment, and no additional patients died at our institution within 30 days. Although these rates are lower than have been otherwise reported,[2, 10] the inpatient mortality rate is similar to a recent study that included inpatients; however, all patients who died in that study had cancer and CVCs.[3] In the latter study, 6.4% died within 30 days of discharge.
Limitations
There are several limitations to this study. It was conducted at a single academic referral center with a large and critically ill trauma and neurosciences population, thereby limiting generalizability. This study describes hospitalized patients at a tertiary care center who were diagnosed with UEDVT. For comparison, we obtained information regarding characteristics of hospitalization for all other inpatients during this time frame. Individuals may have had multiple hospitalizations during the study period, but because we were unable to identify information about individuals, direct statistical comparisons could not be made. However, in general, inpatients with UEDVT appeared to be sicker, with prolonged LOS and high in‐hospital mortality relative to other hospitalized patients.
Only symptomatic UEDVT events were captured, likely underestimating true UEDVT incidence. In addition, we defined UEDVTs as those diagnosed by Doppler ultrasound; therefore theoretically, UEDVTs that were more centrally located or diagnosed using another modality would not be represented here. However, in a prior internal review we found that all VTE events coded in billing data during this time period were identified using our operational definition.
In our study, VTE prophylaxis was administered in accordance with an institutional guideline. We did not have information regarding adequacy of prophylaxis at outside institutions for patients transferred in, and patients admitted through the emergency department likely were not on prophylaxis. Therefore, information about prophylaxis is limited to prophylaxis administered at our medical center for hospitalized patients who had UEDVTs not present on admission.
Information regarding CVC insertion date and CVC type for CVCs placed in our institution is accurate based on our internal reviews. Although we had reasonable capture of information about CVC placement at outside facilities, these data may be incomplete, thereby underestimating potential association of CVCs with UEDVTs identified in our hospitalized patients. Additionally, criteria used to assess association of a CVC with UEDVT may have led to underrepresentation of CVC‐associated UEDVT.
Management of UEDVT in this study was determined by the treating physicians, and patients were only followed for 30 days after discharge. Information about readmission or death within 30 days of discharge was limited to patient contact with our medical center only. Treatment at discharge was determined from the discharge summary. Therefore, compliance with treatment cannot be assessed. Although these factors may limit the nature of the conclusions, data reflect actual practice and experience in hospitalized patients with UEDVT and may be hypothesis generating.
CONCLUSIONS
Among hospitalized patients, UEDVT is increasingly recognized. In our medical center, hospitalized patients diagnosed with UEDVT were more likely to have CVCs, malignancy, renal disease, and severe infection. Many of these patients were transferred critically ill, had prolonged LOS, and had high in‐hospital mortality. Most developed UEDVT despite prophylaxis, and the majority of UEDVTs were treated even in the absence of concurrent LEDVT or PE. As we move toward an era of increasing accountability, with a focus on preventing hospital‐acquired conditions including VTE, additional research is needed to identify modifiable risks, explore opportunities for effective prevention, and optimize outcomes such as prevention of complications or readmissions, particularly in critically ill patients with UEDVT.
Acknowledgements
The authors would like to thank Ronald Pergamit and Kevin Middleton for their extraordinary creativity and expert programming.
- , , , . Upper‐extremity deep vein thrombosis: a prospective registry of 592 patients. Circulation. 2004;110(12):1605–1611.
- , , , et al. Clinical outcome of patients with upper‐extremity deep vein thrombosis: results from the RIETE Registry. Chest. 2008;133(1):143–148.
- , , . The risk factors and clinical outcomes of upper extremity deep vein thrombosis. Vasc Endovascular Surg. 2012;46(2):139–144.
- , , , et al. Upper extremity versus lower extremity deep venous thrombosis. Am J Surg. 1997;174(2):214–217.
- , . Upper‐extremity deep vein thrombosis. Circulation. 2002;106(14):1874–1880.
- , , , . Upper extremity deep vein thrombosis: a community‐based perspective. Am J Med. 2007;120(8):678–684.
- , , , et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947–954.e2.
- , , , , . Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. 2009;4(7):417–422.
- , , , , . Characterization and probability of upper extremity deep venous thrombosis. Ann Vasc Surg. 2004;18(5):552–557.
- , , , et al. Risk factors for mortality in patients with upper extremity and internal jugular deep venous thrombosis. J Vasc Surg. 2005;41(3):476–478.
- , , , et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138(4):803–810.
- , , , et al. The long term clinical course of acute deep vein thrombosis of the arm: prospective cohort study. BMJ. 2004;329(7464):484–485.
- , , , et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e419S–e494S.
- , , , , , . Introduction to the ninth edition: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):48S–52S.
- . Clinical practice. Deep‐vein thrombosis of the upper extremities. N Engl J Med. 2011;364(9):861–869.
- , , , et al. Diagnosis and management of upper extremity deep‐vein thrombosis in adults. Thromb Haemost. 2012;108(6):1097–1108.
- , , . Treatment of upper‐extremity deep vein thrombosis. J Thromb Haemost. 2011;9(10):1924–1930.
Increasingly, there is a focus on prevention of hospital‐acquired conditions including venous thromboembolism (VTE). Many studies have evaluated pulmonary embolism (PE) and lower extremity deep vein thrombosis (LEDVT), but despite increasing recognition of upper extremity deep vein thrombosis (UEDVT),[1, 2, 3, 4] less is known about this condition in hospitalized patients.
UEDVTs may be classified as primary, including disorders such as Paget‐Schroetter syndrome or other structural abnormality, or may be idiopathic; the majority are secondary clots.[5] Conventional risk factors for LEDVT including older age and obesity have been found to be less commonly associated,[1, 2, 5, 6, 7] and patients with UEDVT are generally younger, leaner, and a higher proportion are men. They are more likely to have malignancy or history of VTE and have undergone recent surgery or intensive care unit stay.[1, 2, 6] Central venous catheters (CVCs), often used in hospitalized patients, remain among the biggest known risks for UEDVT[1, 2, 3, 7, 8, 9, 10]; concomitant malignancy, VTE history, severe infection, surgery lasting >1 hour, and length of stay (LOS) >10 days confer additional risks with CVCs.[6, 7, 8, 11]
UEDVTs, once thought to be relatively benign, are now recognized to result in complications including PE, progression, recurrence, and post‐thrombotic syndrome.[2, 4, 12, 13] Despite extensive efforts to increase appropriate VTE prophylaxis in inpatients,[14] the role of chemoprophylaxis to prevent UEDVT remains undefined. Current guidelines recommend anticoagulation for treatment and complication prevention,[13, 15] but to date the evidence derives largely from observational studies or is extrapolated from the LEDVT literature.[2, 13]
To improve understanding of UEDVT at our institution, we set out to (1) determine UEDVT incidence in hospitalized patients, (2) describe associated risks and outcomes, and (3) assess management during hospitalization and at discharge.
METHODS
We identified all consecutive adult patients diagnosed with Doppler ultrasound‐confirmed UEDVT during hospitalization at Harborview Medical Center between September 2011 and November 2012. For patients who were readmitted during the study period, the first of their hospitalizations was used to describe associated factors, management, and outcomes. We present characteristics of all other hospitalizations during this time period for comparison. Harborview is a 413‐bed academic tertiary referral center and the only level 1 trauma center in a 5‐state area. Patients with UEDVT were identified using an information technology (IT) tool (the Harborview VTE tool) (Figure 1), which captures VTE events from vascular laboratory and radiology studies using natural language processing. Doppler ultrasound to assess for deep vein thrombosis (DVT) and computed tomographic scans to diagnose PE were ordered by inpatient physicians for symptomatic patients. The reason for obtaining the study is included in the ultrasound reports. We do not routinely screen for UEDVT at our institution. UEDVT included clots in the deep veins of the upper extremities including internal jugular, subclavian, axillary, and brachial veins. Superficial thrombosis and thrombophlebitis were excluded. We previously compared VTE events captured by this tool with administrative billing data and found that all VTE events that were coded were captured with the tool.

The VTE tool (Figure 1) displays imaging results together with demographic, clinical, and medication data and links this information with admission, discharge, and death summaries as well as CVC insertion procedure notes from the electronic health record (EHR). Additional data, including comorbid conditions, primary reason for hospitalization, past medical history such as prior VTE events, and cause of death (if not available in the admission note or discharge/death summaries), were obtained from EHR abstraction by 1 of the investigators. A 10% random sample of charts was rereviewed by another investigator with complete concordance. Supplementary data about date of CVC insertion if placed at an outside facility, date of CVC removal if applicable, clinical assessments regarding whether a clot was CVC‐associated, and contraindications to therapeutic anticoagulation were also abstracted directly from the EHR. Administrative data were used to identify the case mix index, an indicator of severity of illness.
Pharmacologic VTE prophylaxis included all chemical prophylaxis specified on our institutional guideline, most commonly subcutaneous unfractionated heparin 5000 units every 8 hours or low molecular weight heparin (LMWH), either enoxaparin 40 mg every 12 or 24 hours or dalteparin 5000 units every 24 hours. Mechanical prophylaxis was defined as use of sequential compression devices (SCDs) when pharmacologic prophylaxis was contraindicated. Prophylaxis was considered to be appropriate if it was applied according to our guideline for >90% of hospital days prior to UEDVT diagnosis. Therapeutic anticoagulation included heparin bridging (most commonly continuous heparin infusion, LMWH 1 mg/kg or dalteparin) as well as oral vitamin K antagonists. The VTE tool (Figure 1) allows identification of pharmacologic prophylaxis and therapy that is actually administered (not just ordered) directly from our pharmacy IT system. SCD application (not just ordered SCDs) is electronically integrated into the tool from nursing documentation.
CVCs included internal jugular or subclavian triple lumen catheters, tunneled dialysis catheters, or peripherally inserted central catheters (PICCs), single or double lumen. Criteria used to identify that a UEDVT was CVC‐associated included temporal relationship (CVC was placed prior to clot diagnosis), plausibility (ipsilateral clot), evidence of clot surrounding CVC on ultrasound, and physician designation of association (as documented in progress notes or discharge summary).
Simple percentages of patient characteristics, associated factors, management, and outcomes were calculated using counts as the numerator and number of patients as the denominator. For information about UEDVTs, we used total number of UEDVTs as the denominator. Line days were day counts from insertion until removal if applicable. The CVC placement date was available in our mandated central line placement procedure notes (directly accessed from the VTE tool) for all lines placed at our institution; date of removal (if applicable) was determined from chart abstraction. For the vast majority of patients whose CVCs were placed at outside facilities, date of placement was available in the EHR (often in the admission note or in the ultrasound report/reason for study). If date of line placement at an outside facility was not known, date of admission was used. The University of Washington Human Subjects Board approved this review.
RESULTS
General Characteristics
Fifty inpatients were diagnosed with 76 UEDVTs during 53 hospitalizations. Three patients were admitted twice during the study period. Their first admission is used for the purposes of this review. None of these 3 patients had new UEDVTs diagnosed during their second admission.
The patients' mean age was 49 years (standard deviation [SD] 15.6; range, 2482 years) vs 50.9 years (SD 17.49; range, 18112 years) among all other hospitalizations during this time (Table 1). Seventy percent (35) of patients with UEDVT were men. Sixteen percent (8) of patients with UEDVT had known VTE history, 20% (10) of patients had malignancy, and 22% (11) of patients had stage V chronic kidney disease or were hemodialysis dependent.
| Characteristic | Patients With UEDVT, N=50 | All Hospitalizations, N=23,407a |
|---|---|---|
| ||
| Age, y, mean (range) | 49 (2482) | 51 (18112) |
| Sex, % male (no.) | 70% (35) | 63% (14,746) |
| Case mix index, mean (range) | 4.78 (0.6917.99) | 1.87 (0.1626.34) |
| Length of stay, d, mean (range) | 24.6 (291) | 7.2 (1178) |
| Transfer from outside hospital (no.) | 50% (25) | 25% (5,866) |
| Intensive care unit stay (no.) | 46% (23) | 36% (8,356) |
| Operative procedure (no.) | 46% (23) | 41% (9,706) |
| In‐hospital mortality (no.) | 10% (5) | 4% (842) |
| Discharge to skilled nursing facility or other hospital, n=45 surviving patients (no.) | 62% (28) | 13% (3,095) |
| 30‐day readmission, n=45 surviving patients (no.) | 18% (8) | 5% (1,167) |
Patients diagnosed with UEDVT had complex illness, long LOS, and were often transferred from outside hospitals relative to other hospitalizations during this time period (Table 1). Slightly more required intensive care and underwent surgery. Eighty‐four percent (42) of patients with UEDVT required CVCs during hospitalization. Among patients whose UEDVT was not present on admission, 94% received appropriate VTE prophylaxis prior to UEDVT diagnosis.
In patients with UEDVT, the most common reasons for hospitalization were sepsis/severe infection (43%), cerebral hemorrhage (16%), and trauma (8%). Primary service at diagnosis was medicine 56.9%, surgery 25.5%, and neurosciences 17.6%.
Upper Extremity Deep Vein Thromboses
Fifty patients were diagnosed with 76 UEDVTs during their hospitalizations. In 40% (20) of patients, UEDVTs were present in >1 upper extremity deep vein; concurrent LEDVT was present in 26% (13) and PE in 10% (5). The majority of UEDVTs were found in internal jugular veins, followed by brachial and axillary veins. Seventeen percent were present on admission. Upper extremity swelling was the most common sign/symptom and reason for study. Characteristics of UEDVTs diagnosed are listed in Table 2.
| Characteristic | % UEDVTs (No.), n=76 |
|---|---|
| |
| Anatomic site | |
| Internal jugular | 38% (29) |
| Axillary | 21% (16) |
| Subclavian/axillary | 9% (7) |
| Subclavian | 7% (5) |
| Brachial | 25% (19) |
| Hospital day of diagnosis, d, mean (range) | 9.2 (044) |
| Present on admission | 17% (13) |
| Diagnosed at outside hospital or within 24 hours of transfer | 54% (7) |
| Diagnosed during prior hospitalization at our institution | 15% (2) |
| Diagnosed within 24 hours of admission via our emergency department | 23% (3) |
| Patient‐reported chronic UEDVT | 8% (1) |
| Primary UEDVT/anatomic anomaly | 0% (0) |
| Signs and symptoms (reasons for obtaining study) | |
| Upper extremity swelling | 71% (54) |
| Presence of clot elsewhere (eg, pulmonary embolism) | 9% (7) |
| Inability to place central venous access | 8% (6) |
| Assessment of clot propagation (known clot) | 8% (6) |
| Pain | 3% (2) |
| Patient‐reported history | 1% (1) |
Of the 50 patients diagnosed with UEDVT during hospitalization, 44% (22) were found to have UEDVTs directly associated with a CVC. Forty‐two of the 50 patients had a CVC; 52% (22 of 42) had CVC‐associated UEDVTs. Fifty percent (11) of these CVCs were triple lumen catheters, 32% (7) were PICCs, and 18% (4) were tunneled dialysis lines. Three of 42 patients with CVCs and line‐associated clots were had a malignancy. For patients with CVC‐associated clot, lines were in place for an average of 14.3 days (range, 273 days) prior to UEDVT diagnosis.
Treatment and Management
Seventy‐eight percent (39) of patients with UEDVT received in‐hospital treatment with heparin/LMWH bridging and oral anticoagulation. Of the 45 patients who survived hospitalization, 75% (34) were prescribed anticoagulation for 3+ months at discharge; 23% (10) had documented contraindications to anticoagulation, most commonly recent gastrointestinal or intracranial bleeding. Two percent of patients (1) was not prescribed pharmacologic treatment at discharge and had no contraindications documented. No patients underwent thrombolysis or had superior vena cava filters placed. Sixty‐four percent (14 of 22) of CVCs that were thought to be directly associated with UEDVT were removed at diagnosis.
Outcomes
Five patients (10%) died during hospitalization, none because of VTE or complications thereof. Cause of death included septic shock, cancer, intracranial hemorrhage, heart failure, and recurrent gastrointestinal bleeding. Of the 45 surviving patients, only 38% (17) were discharged to self‐care; more than half (62%[28]) were discharged to skilled nursing facilities, other hospitals, or rehabilitation centers. Eight patients (18%) were readmitted to our institution within 30 days; none for recurrent or new DVT or PE. No additional patients died at our medical center within 30 days of discharge.
DISCUSSION
UEDVT is increasingly recognized in hospitalized patients.[3, 9] At our medical center, 0.2% of symptomatic inpatients were diagnosed with UEDVT over 14 months. These patients were predominantly men with high rates of CVCs, malignancy, VTE history, severe infection, and renal disease. Interestingly, although the literature suggests that some proportion of patients with UEDVT have anatomic abnormalities, such as Paget‐Schroetter syndrome,[15] none of the patients in our study were found to have these anomalies. In our review, hospitalized patients with UEDVT were critically ill, with a long LOS and high morbidity and mortality, suggesting that in addition to just being a complication of hospitalization,[1, 6] UEDVT may be a marker of severe illness.
In our institution, clinical presentation was consistent with what has been described with the majority of patients presenting with upper extremity swelling.[1, 3] The internal jugular veins were the most common anatomic UEDVT site, followed by brachial then axillary veins. In other series including both in‐ and outpatients, subclavian clots were most commonly diagnosed, reflecting in part higher rates of CVC association and CVC location in those studies.[3, 9] Concurrent DVT and PE rates were similar to those reported.[1, 3, 10]
Although many studies have focused on prevention of LEDVT and PE, few trials have specifically targeted UEDVT. Among our patients with UEDVTs that were not present on admission, VTE prophylaxis rates were considerably higher than what has been reported,[1, 6] suggesting that in these critically ill patients' prophylaxis may not prevent symptomatic UEDVT. It is unknown how many UEDVTs were prevented with prophylaxis, as only patients with symptomatic UEDVT were included. Adequacy of prophylaxis at outside hospitals for patients transferred in could not be assessed. Nonetheless, low numbers of UEDVT at a trauma referral center with many high‐risk patients raise the question of whether prophylaxis makes a difference. Additional study is needed to further define the role of chemoprophylaxis to prevent UEDVT in hospitalized patients.
In our inpatient group, 84% required CVCs; 44% of patients were thought to have CVC‐associated UEDVTs. Careful patient selection and attention to potentially modifiable risks, such as insertion site, catheter type, and tip position, may need further examination in this population.[3, 11, 16] Catheter duration was long; focus on removing CVCs when no longer necessary is important. Interestingly, almost 10% in our study underwent diagnostic ultrasound because a new CVC could not be successfully placed suggesting that UEDVT may develop in critically ill patients regardless of CVCs.
In our study, there were high rates of guideline‐recommended pharmacologic treatment; surprisingly the majority of CVCs with associated clot were removed. Guidelines currently support 3 months of anticoagulation for treatment of UEDVT[2, 13, 17]; evidence derives from observational trials or is largely extrapolated from LEDVT literature.[2, 13] Routine CVC removal is not specifically recommended for CVC‐associated UEDVT, particularly if lines remain functional and medically necessary; systemic anticoagulation should be provided.[13]
In our review, no hospitalized patients with UEDVT developed complications or were readmitted to our medical center within 30 days for clot progression, new PE, or post‐thrombotic syndrome, which is lower than rates reported over longer time periods.[2, 6, 10, 12] Ten percent died during hospitalization, all from their primary disease rather than from complications of VTE or VTE treatment, and no additional patients died at our institution within 30 days. Although these rates are lower than have been otherwise reported,[2, 10] the inpatient mortality rate is similar to a recent study that included inpatients; however, all patients who died in that study had cancer and CVCs.[3] In the latter study, 6.4% died within 30 days of discharge.
Limitations
There are several limitations to this study. It was conducted at a single academic referral center with a large and critically ill trauma and neurosciences population, thereby limiting generalizability. This study describes hospitalized patients at a tertiary care center who were diagnosed with UEDVT. For comparison, we obtained information regarding characteristics of hospitalization for all other inpatients during this time frame. Individuals may have had multiple hospitalizations during the study period, but because we were unable to identify information about individuals, direct statistical comparisons could not be made. However, in general, inpatients with UEDVT appeared to be sicker, with prolonged LOS and high in‐hospital mortality relative to other hospitalized patients.
Only symptomatic UEDVT events were captured, likely underestimating true UEDVT incidence. In addition, we defined UEDVTs as those diagnosed by Doppler ultrasound; therefore theoretically, UEDVTs that were more centrally located or diagnosed using another modality would not be represented here. However, in a prior internal review we found that all VTE events coded in billing data during this time period were identified using our operational definition.
In our study, VTE prophylaxis was administered in accordance with an institutional guideline. We did not have information regarding adequacy of prophylaxis at outside institutions for patients transferred in, and patients admitted through the emergency department likely were not on prophylaxis. Therefore, information about prophylaxis is limited to prophylaxis administered at our medical center for hospitalized patients who had UEDVTs not present on admission.
Information regarding CVC insertion date and CVC type for CVCs placed in our institution is accurate based on our internal reviews. Although we had reasonable capture of information about CVC placement at outside facilities, these data may be incomplete, thereby underestimating potential association of CVCs with UEDVTs identified in our hospitalized patients. Additionally, criteria used to assess association of a CVC with UEDVT may have led to underrepresentation of CVC‐associated UEDVT.
Management of UEDVT in this study was determined by the treating physicians, and patients were only followed for 30 days after discharge. Information about readmission or death within 30 days of discharge was limited to patient contact with our medical center only. Treatment at discharge was determined from the discharge summary. Therefore, compliance with treatment cannot be assessed. Although these factors may limit the nature of the conclusions, data reflect actual practice and experience in hospitalized patients with UEDVT and may be hypothesis generating.
CONCLUSIONS
Among hospitalized patients, UEDVT is increasingly recognized. In our medical center, hospitalized patients diagnosed with UEDVT were more likely to have CVCs, malignancy, renal disease, and severe infection. Many of these patients were transferred critically ill, had prolonged LOS, and had high in‐hospital mortality. Most developed UEDVT despite prophylaxis, and the majority of UEDVTs were treated even in the absence of concurrent LEDVT or PE. As we move toward an era of increasing accountability, with a focus on preventing hospital‐acquired conditions including VTE, additional research is needed to identify modifiable risks, explore opportunities for effective prevention, and optimize outcomes such as prevention of complications or readmissions, particularly in critically ill patients with UEDVT.
Acknowledgements
The authors would like to thank Ronald Pergamit and Kevin Middleton for their extraordinary creativity and expert programming.
Increasingly, there is a focus on prevention of hospital‐acquired conditions including venous thromboembolism (VTE). Many studies have evaluated pulmonary embolism (PE) and lower extremity deep vein thrombosis (LEDVT), but despite increasing recognition of upper extremity deep vein thrombosis (UEDVT),[1, 2, 3, 4] less is known about this condition in hospitalized patients.
UEDVTs may be classified as primary, including disorders such as Paget‐Schroetter syndrome or other structural abnormality, or may be idiopathic; the majority are secondary clots.[5] Conventional risk factors for LEDVT including older age and obesity have been found to be less commonly associated,[1, 2, 5, 6, 7] and patients with UEDVT are generally younger, leaner, and a higher proportion are men. They are more likely to have malignancy or history of VTE and have undergone recent surgery or intensive care unit stay.[1, 2, 6] Central venous catheters (CVCs), often used in hospitalized patients, remain among the biggest known risks for UEDVT[1, 2, 3, 7, 8, 9, 10]; concomitant malignancy, VTE history, severe infection, surgery lasting >1 hour, and length of stay (LOS) >10 days confer additional risks with CVCs.[6, 7, 8, 11]
UEDVTs, once thought to be relatively benign, are now recognized to result in complications including PE, progression, recurrence, and post‐thrombotic syndrome.[2, 4, 12, 13] Despite extensive efforts to increase appropriate VTE prophylaxis in inpatients,[14] the role of chemoprophylaxis to prevent UEDVT remains undefined. Current guidelines recommend anticoagulation for treatment and complication prevention,[13, 15] but to date the evidence derives largely from observational studies or is extrapolated from the LEDVT literature.[2, 13]
To improve understanding of UEDVT at our institution, we set out to (1) determine UEDVT incidence in hospitalized patients, (2) describe associated risks and outcomes, and (3) assess management during hospitalization and at discharge.
METHODS
We identified all consecutive adult patients diagnosed with Doppler ultrasound‐confirmed UEDVT during hospitalization at Harborview Medical Center between September 2011 and November 2012. For patients who were readmitted during the study period, the first of their hospitalizations was used to describe associated factors, management, and outcomes. We present characteristics of all other hospitalizations during this time period for comparison. Harborview is a 413‐bed academic tertiary referral center and the only level 1 trauma center in a 5‐state area. Patients with UEDVT were identified using an information technology (IT) tool (the Harborview VTE tool) (Figure 1), which captures VTE events from vascular laboratory and radiology studies using natural language processing. Doppler ultrasound to assess for deep vein thrombosis (DVT) and computed tomographic scans to diagnose PE were ordered by inpatient physicians for symptomatic patients. The reason for obtaining the study is included in the ultrasound reports. We do not routinely screen for UEDVT at our institution. UEDVT included clots in the deep veins of the upper extremities including internal jugular, subclavian, axillary, and brachial veins. Superficial thrombosis and thrombophlebitis were excluded. We previously compared VTE events captured by this tool with administrative billing data and found that all VTE events that were coded were captured with the tool.

The VTE tool (Figure 1) displays imaging results together with demographic, clinical, and medication data and links this information with admission, discharge, and death summaries as well as CVC insertion procedure notes from the electronic health record (EHR). Additional data, including comorbid conditions, primary reason for hospitalization, past medical history such as prior VTE events, and cause of death (if not available in the admission note or discharge/death summaries), were obtained from EHR abstraction by 1 of the investigators. A 10% random sample of charts was rereviewed by another investigator with complete concordance. Supplementary data about date of CVC insertion if placed at an outside facility, date of CVC removal if applicable, clinical assessments regarding whether a clot was CVC‐associated, and contraindications to therapeutic anticoagulation were also abstracted directly from the EHR. Administrative data were used to identify the case mix index, an indicator of severity of illness.
Pharmacologic VTE prophylaxis included all chemical prophylaxis specified on our institutional guideline, most commonly subcutaneous unfractionated heparin 5000 units every 8 hours or low molecular weight heparin (LMWH), either enoxaparin 40 mg every 12 or 24 hours or dalteparin 5000 units every 24 hours. Mechanical prophylaxis was defined as use of sequential compression devices (SCDs) when pharmacologic prophylaxis was contraindicated. Prophylaxis was considered to be appropriate if it was applied according to our guideline for >90% of hospital days prior to UEDVT diagnosis. Therapeutic anticoagulation included heparin bridging (most commonly continuous heparin infusion, LMWH 1 mg/kg or dalteparin) as well as oral vitamin K antagonists. The VTE tool (Figure 1) allows identification of pharmacologic prophylaxis and therapy that is actually administered (not just ordered) directly from our pharmacy IT system. SCD application (not just ordered SCDs) is electronically integrated into the tool from nursing documentation.
CVCs included internal jugular or subclavian triple lumen catheters, tunneled dialysis catheters, or peripherally inserted central catheters (PICCs), single or double lumen. Criteria used to identify that a UEDVT was CVC‐associated included temporal relationship (CVC was placed prior to clot diagnosis), plausibility (ipsilateral clot), evidence of clot surrounding CVC on ultrasound, and physician designation of association (as documented in progress notes or discharge summary).
Simple percentages of patient characteristics, associated factors, management, and outcomes were calculated using counts as the numerator and number of patients as the denominator. For information about UEDVTs, we used total number of UEDVTs as the denominator. Line days were day counts from insertion until removal if applicable. The CVC placement date was available in our mandated central line placement procedure notes (directly accessed from the VTE tool) for all lines placed at our institution; date of removal (if applicable) was determined from chart abstraction. For the vast majority of patients whose CVCs were placed at outside facilities, date of placement was available in the EHR (often in the admission note or in the ultrasound report/reason for study). If date of line placement at an outside facility was not known, date of admission was used. The University of Washington Human Subjects Board approved this review.
RESULTS
General Characteristics
Fifty inpatients were diagnosed with 76 UEDVTs during 53 hospitalizations. Three patients were admitted twice during the study period. Their first admission is used for the purposes of this review. None of these 3 patients had new UEDVTs diagnosed during their second admission.
The patients' mean age was 49 years (standard deviation [SD] 15.6; range, 2482 years) vs 50.9 years (SD 17.49; range, 18112 years) among all other hospitalizations during this time (Table 1). Seventy percent (35) of patients with UEDVT were men. Sixteen percent (8) of patients with UEDVT had known VTE history, 20% (10) of patients had malignancy, and 22% (11) of patients had stage V chronic kidney disease or were hemodialysis dependent.
| Characteristic | Patients With UEDVT, N=50 | All Hospitalizations, N=23,407a |
|---|---|---|
| ||
| Age, y, mean (range) | 49 (2482) | 51 (18112) |
| Sex, % male (no.) | 70% (35) | 63% (14,746) |
| Case mix index, mean (range) | 4.78 (0.6917.99) | 1.87 (0.1626.34) |
| Length of stay, d, mean (range) | 24.6 (291) | 7.2 (1178) |
| Transfer from outside hospital (no.) | 50% (25) | 25% (5,866) |
| Intensive care unit stay (no.) | 46% (23) | 36% (8,356) |
| Operative procedure (no.) | 46% (23) | 41% (9,706) |
| In‐hospital mortality (no.) | 10% (5) | 4% (842) |
| Discharge to skilled nursing facility or other hospital, n=45 surviving patients (no.) | 62% (28) | 13% (3,095) |
| 30‐day readmission, n=45 surviving patients (no.) | 18% (8) | 5% (1,167) |
Patients diagnosed with UEDVT had complex illness, long LOS, and were often transferred from outside hospitals relative to other hospitalizations during this time period (Table 1). Slightly more required intensive care and underwent surgery. Eighty‐four percent (42) of patients with UEDVT required CVCs during hospitalization. Among patients whose UEDVT was not present on admission, 94% received appropriate VTE prophylaxis prior to UEDVT diagnosis.
In patients with UEDVT, the most common reasons for hospitalization were sepsis/severe infection (43%), cerebral hemorrhage (16%), and trauma (8%). Primary service at diagnosis was medicine 56.9%, surgery 25.5%, and neurosciences 17.6%.
Upper Extremity Deep Vein Thromboses
Fifty patients were diagnosed with 76 UEDVTs during their hospitalizations. In 40% (20) of patients, UEDVTs were present in >1 upper extremity deep vein; concurrent LEDVT was present in 26% (13) and PE in 10% (5). The majority of UEDVTs were found in internal jugular veins, followed by brachial and axillary veins. Seventeen percent were present on admission. Upper extremity swelling was the most common sign/symptom and reason for study. Characteristics of UEDVTs diagnosed are listed in Table 2.
| Characteristic | % UEDVTs (No.), n=76 |
|---|---|
| |
| Anatomic site | |
| Internal jugular | 38% (29) |
| Axillary | 21% (16) |
| Subclavian/axillary | 9% (7) |
| Subclavian | 7% (5) |
| Brachial | 25% (19) |
| Hospital day of diagnosis, d, mean (range) | 9.2 (044) |
| Present on admission | 17% (13) |
| Diagnosed at outside hospital or within 24 hours of transfer | 54% (7) |
| Diagnosed during prior hospitalization at our institution | 15% (2) |
| Diagnosed within 24 hours of admission via our emergency department | 23% (3) |
| Patient‐reported chronic UEDVT | 8% (1) |
| Primary UEDVT/anatomic anomaly | 0% (0) |
| Signs and symptoms (reasons for obtaining study) | |
| Upper extremity swelling | 71% (54) |
| Presence of clot elsewhere (eg, pulmonary embolism) | 9% (7) |
| Inability to place central venous access | 8% (6) |
| Assessment of clot propagation (known clot) | 8% (6) |
| Pain | 3% (2) |
| Patient‐reported history | 1% (1) |
Of the 50 patients diagnosed with UEDVT during hospitalization, 44% (22) were found to have UEDVTs directly associated with a CVC. Forty‐two of the 50 patients had a CVC; 52% (22 of 42) had CVC‐associated UEDVTs. Fifty percent (11) of these CVCs were triple lumen catheters, 32% (7) were PICCs, and 18% (4) were tunneled dialysis lines. Three of 42 patients with CVCs and line‐associated clots were had a malignancy. For patients with CVC‐associated clot, lines were in place for an average of 14.3 days (range, 273 days) prior to UEDVT diagnosis.
Treatment and Management
Seventy‐eight percent (39) of patients with UEDVT received in‐hospital treatment with heparin/LMWH bridging and oral anticoagulation. Of the 45 patients who survived hospitalization, 75% (34) were prescribed anticoagulation for 3+ months at discharge; 23% (10) had documented contraindications to anticoagulation, most commonly recent gastrointestinal or intracranial bleeding. Two percent of patients (1) was not prescribed pharmacologic treatment at discharge and had no contraindications documented. No patients underwent thrombolysis or had superior vena cava filters placed. Sixty‐four percent (14 of 22) of CVCs that were thought to be directly associated with UEDVT were removed at diagnosis.
Outcomes
Five patients (10%) died during hospitalization, none because of VTE or complications thereof. Cause of death included septic shock, cancer, intracranial hemorrhage, heart failure, and recurrent gastrointestinal bleeding. Of the 45 surviving patients, only 38% (17) were discharged to self‐care; more than half (62%[28]) were discharged to skilled nursing facilities, other hospitals, or rehabilitation centers. Eight patients (18%) were readmitted to our institution within 30 days; none for recurrent or new DVT or PE. No additional patients died at our medical center within 30 days of discharge.
DISCUSSION
UEDVT is increasingly recognized in hospitalized patients.[3, 9] At our medical center, 0.2% of symptomatic inpatients were diagnosed with UEDVT over 14 months. These patients were predominantly men with high rates of CVCs, malignancy, VTE history, severe infection, and renal disease. Interestingly, although the literature suggests that some proportion of patients with UEDVT have anatomic abnormalities, such as Paget‐Schroetter syndrome,[15] none of the patients in our study were found to have these anomalies. In our review, hospitalized patients with UEDVT were critically ill, with a long LOS and high morbidity and mortality, suggesting that in addition to just being a complication of hospitalization,[1, 6] UEDVT may be a marker of severe illness.
In our institution, clinical presentation was consistent with what has been described with the majority of patients presenting with upper extremity swelling.[1, 3] The internal jugular veins were the most common anatomic UEDVT site, followed by brachial then axillary veins. In other series including both in‐ and outpatients, subclavian clots were most commonly diagnosed, reflecting in part higher rates of CVC association and CVC location in those studies.[3, 9] Concurrent DVT and PE rates were similar to those reported.[1, 3, 10]
Although many studies have focused on prevention of LEDVT and PE, few trials have specifically targeted UEDVT. Among our patients with UEDVTs that were not present on admission, VTE prophylaxis rates were considerably higher than what has been reported,[1, 6] suggesting that in these critically ill patients' prophylaxis may not prevent symptomatic UEDVT. It is unknown how many UEDVTs were prevented with prophylaxis, as only patients with symptomatic UEDVT were included. Adequacy of prophylaxis at outside hospitals for patients transferred in could not be assessed. Nonetheless, low numbers of UEDVT at a trauma referral center with many high‐risk patients raise the question of whether prophylaxis makes a difference. Additional study is needed to further define the role of chemoprophylaxis to prevent UEDVT in hospitalized patients.
In our inpatient group, 84% required CVCs; 44% of patients were thought to have CVC‐associated UEDVTs. Careful patient selection and attention to potentially modifiable risks, such as insertion site, catheter type, and tip position, may need further examination in this population.[3, 11, 16] Catheter duration was long; focus on removing CVCs when no longer necessary is important. Interestingly, almost 10% in our study underwent diagnostic ultrasound because a new CVC could not be successfully placed suggesting that UEDVT may develop in critically ill patients regardless of CVCs.
In our study, there were high rates of guideline‐recommended pharmacologic treatment; surprisingly the majority of CVCs with associated clot were removed. Guidelines currently support 3 months of anticoagulation for treatment of UEDVT[2, 13, 17]; evidence derives from observational trials or is largely extrapolated from LEDVT literature.[2, 13] Routine CVC removal is not specifically recommended for CVC‐associated UEDVT, particularly if lines remain functional and medically necessary; systemic anticoagulation should be provided.[13]
In our review, no hospitalized patients with UEDVT developed complications or were readmitted to our medical center within 30 days for clot progression, new PE, or post‐thrombotic syndrome, which is lower than rates reported over longer time periods.[2, 6, 10, 12] Ten percent died during hospitalization, all from their primary disease rather than from complications of VTE or VTE treatment, and no additional patients died at our institution within 30 days. Although these rates are lower than have been otherwise reported,[2, 10] the inpatient mortality rate is similar to a recent study that included inpatients; however, all patients who died in that study had cancer and CVCs.[3] In the latter study, 6.4% died within 30 days of discharge.
Limitations
There are several limitations to this study. It was conducted at a single academic referral center with a large and critically ill trauma and neurosciences population, thereby limiting generalizability. This study describes hospitalized patients at a tertiary care center who were diagnosed with UEDVT. For comparison, we obtained information regarding characteristics of hospitalization for all other inpatients during this time frame. Individuals may have had multiple hospitalizations during the study period, but because we were unable to identify information about individuals, direct statistical comparisons could not be made. However, in general, inpatients with UEDVT appeared to be sicker, with prolonged LOS and high in‐hospital mortality relative to other hospitalized patients.
Only symptomatic UEDVT events were captured, likely underestimating true UEDVT incidence. In addition, we defined UEDVTs as those diagnosed by Doppler ultrasound; therefore theoretically, UEDVTs that were more centrally located or diagnosed using another modality would not be represented here. However, in a prior internal review we found that all VTE events coded in billing data during this time period were identified using our operational definition.
In our study, VTE prophylaxis was administered in accordance with an institutional guideline. We did not have information regarding adequacy of prophylaxis at outside institutions for patients transferred in, and patients admitted through the emergency department likely were not on prophylaxis. Therefore, information about prophylaxis is limited to prophylaxis administered at our medical center for hospitalized patients who had UEDVTs not present on admission.
Information regarding CVC insertion date and CVC type for CVCs placed in our institution is accurate based on our internal reviews. Although we had reasonable capture of information about CVC placement at outside facilities, these data may be incomplete, thereby underestimating potential association of CVCs with UEDVTs identified in our hospitalized patients. Additionally, criteria used to assess association of a CVC with UEDVT may have led to underrepresentation of CVC‐associated UEDVT.
Management of UEDVT in this study was determined by the treating physicians, and patients were only followed for 30 days after discharge. Information about readmission or death within 30 days of discharge was limited to patient contact with our medical center only. Treatment at discharge was determined from the discharge summary. Therefore, compliance with treatment cannot be assessed. Although these factors may limit the nature of the conclusions, data reflect actual practice and experience in hospitalized patients with UEDVT and may be hypothesis generating.
CONCLUSIONS
Among hospitalized patients, UEDVT is increasingly recognized. In our medical center, hospitalized patients diagnosed with UEDVT were more likely to have CVCs, malignancy, renal disease, and severe infection. Many of these patients were transferred critically ill, had prolonged LOS, and had high in‐hospital mortality. Most developed UEDVT despite prophylaxis, and the majority of UEDVTs were treated even in the absence of concurrent LEDVT or PE. As we move toward an era of increasing accountability, with a focus on preventing hospital‐acquired conditions including VTE, additional research is needed to identify modifiable risks, explore opportunities for effective prevention, and optimize outcomes such as prevention of complications or readmissions, particularly in critically ill patients with UEDVT.
Acknowledgements
The authors would like to thank Ronald Pergamit and Kevin Middleton for their extraordinary creativity and expert programming.
- , , , . Upper‐extremity deep vein thrombosis: a prospective registry of 592 patients. Circulation. 2004;110(12):1605–1611.
- , , , et al. Clinical outcome of patients with upper‐extremity deep vein thrombosis: results from the RIETE Registry. Chest. 2008;133(1):143–148.
- , , . The risk factors and clinical outcomes of upper extremity deep vein thrombosis. Vasc Endovascular Surg. 2012;46(2):139–144.
- , , , et al. Upper extremity versus lower extremity deep venous thrombosis. Am J Surg. 1997;174(2):214–217.
- , . Upper‐extremity deep vein thrombosis. Circulation. 2002;106(14):1874–1880.
- , , , . Upper extremity deep vein thrombosis: a community‐based perspective. Am J Med. 2007;120(8):678–684.
- , , , et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947–954.e2.
- , , , , . Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. 2009;4(7):417–422.
- , , , , . Characterization and probability of upper extremity deep venous thrombosis. Ann Vasc Surg. 2004;18(5):552–557.
- , , , et al. Risk factors for mortality in patients with upper extremity and internal jugular deep venous thrombosis. J Vasc Surg. 2005;41(3):476–478.
- , , , et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138(4):803–810.
- , , , et al. The long term clinical course of acute deep vein thrombosis of the arm: prospective cohort study. BMJ. 2004;329(7464):484–485.
- , , , et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e419S–e494S.
- , , , , , . Introduction to the ninth edition: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):48S–52S.
- . Clinical practice. Deep‐vein thrombosis of the upper extremities. N Engl J Med. 2011;364(9):861–869.
- , , , et al. Diagnosis and management of upper extremity deep‐vein thrombosis in adults. Thromb Haemost. 2012;108(6):1097–1108.
- , , . Treatment of upper‐extremity deep vein thrombosis. J Thromb Haemost. 2011;9(10):1924–1930.
- , , , . Upper‐extremity deep vein thrombosis: a prospective registry of 592 patients. Circulation. 2004;110(12):1605–1611.
- , , , et al. Clinical outcome of patients with upper‐extremity deep vein thrombosis: results from the RIETE Registry. Chest. 2008;133(1):143–148.
- , , . The risk factors and clinical outcomes of upper extremity deep vein thrombosis. Vasc Endovascular Surg. 2012;46(2):139–144.
- , , , et al. Upper extremity versus lower extremity deep venous thrombosis. Am J Surg. 1997;174(2):214–217.
- , . Upper‐extremity deep vein thrombosis. Circulation. 2002;106(14):1874–1880.
- , , , . Upper extremity deep vein thrombosis: a community‐based perspective. Am J Med. 2007;120(8):678–684.
- , , , et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947–954.e2.
- , , , , . Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. 2009;4(7):417–422.
- , , , , . Characterization and probability of upper extremity deep venous thrombosis. Ann Vasc Surg. 2004;18(5):552–557.
- , , , et al. Risk factors for mortality in patients with upper extremity and internal jugular deep venous thrombosis. J Vasc Surg. 2005;41(3):476–478.
- , , , et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138(4):803–810.
- , , , et al. The long term clinical course of acute deep vein thrombosis of the arm: prospective cohort study. BMJ. 2004;329(7464):484–485.
- , , , et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e419S–e494S.
- , , , , , . Introduction to the ninth edition: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):48S–52S.
- . Clinical practice. Deep‐vein thrombosis of the upper extremities. N Engl J Med. 2011;364(9):861–869.
- , , , et al. Diagnosis and management of upper extremity deep‐vein thrombosis in adults. Thromb Haemost. 2012;108(6):1097–1108.
- , , . Treatment of upper‐extremity deep vein thrombosis. J Thromb Haemost. 2011;9(10):1924–1930.