User login
Upper Extremity DVT in Hospitalized Patients
Increasingly, there is a focus on prevention of hospital‐acquired conditions including venous thromboembolism (VTE). Many studies have evaluated pulmonary embolism (PE) and lower extremity deep vein thrombosis (LEDVT), but despite increasing recognition of upper extremity deep vein thrombosis (UEDVT),[1, 2, 3, 4] less is known about this condition in hospitalized patients.
UEDVTs may be classified as primary, including disorders such as Paget‐Schroetter syndrome or other structural abnormality, or may be idiopathic; the majority are secondary clots.[5] Conventional risk factors for LEDVT including older age and obesity have been found to be less commonly associated,[1, 2, 5, 6, 7] and patients with UEDVT are generally younger, leaner, and a higher proportion are men. They are more likely to have malignancy or history of VTE and have undergone recent surgery or intensive care unit stay.[1, 2, 6] Central venous catheters (CVCs), often used in hospitalized patients, remain among the biggest known risks for UEDVT[1, 2, 3, 7, 8, 9, 10]; concomitant malignancy, VTE history, severe infection, surgery lasting >1 hour, and length of stay (LOS) >10 days confer additional risks with CVCs.[6, 7, 8, 11]
UEDVTs, once thought to be relatively benign, are now recognized to result in complications including PE, progression, recurrence, and post‐thrombotic syndrome.[2, 4, 12, 13] Despite extensive efforts to increase appropriate VTE prophylaxis in inpatients,[14] the role of chemoprophylaxis to prevent UEDVT remains undefined. Current guidelines recommend anticoagulation for treatment and complication prevention,[13, 15] but to date the evidence derives largely from observational studies or is extrapolated from the LEDVT literature.[2, 13]
To improve understanding of UEDVT at our institution, we set out to (1) determine UEDVT incidence in hospitalized patients, (2) describe associated risks and outcomes, and (3) assess management during hospitalization and at discharge.
METHODS
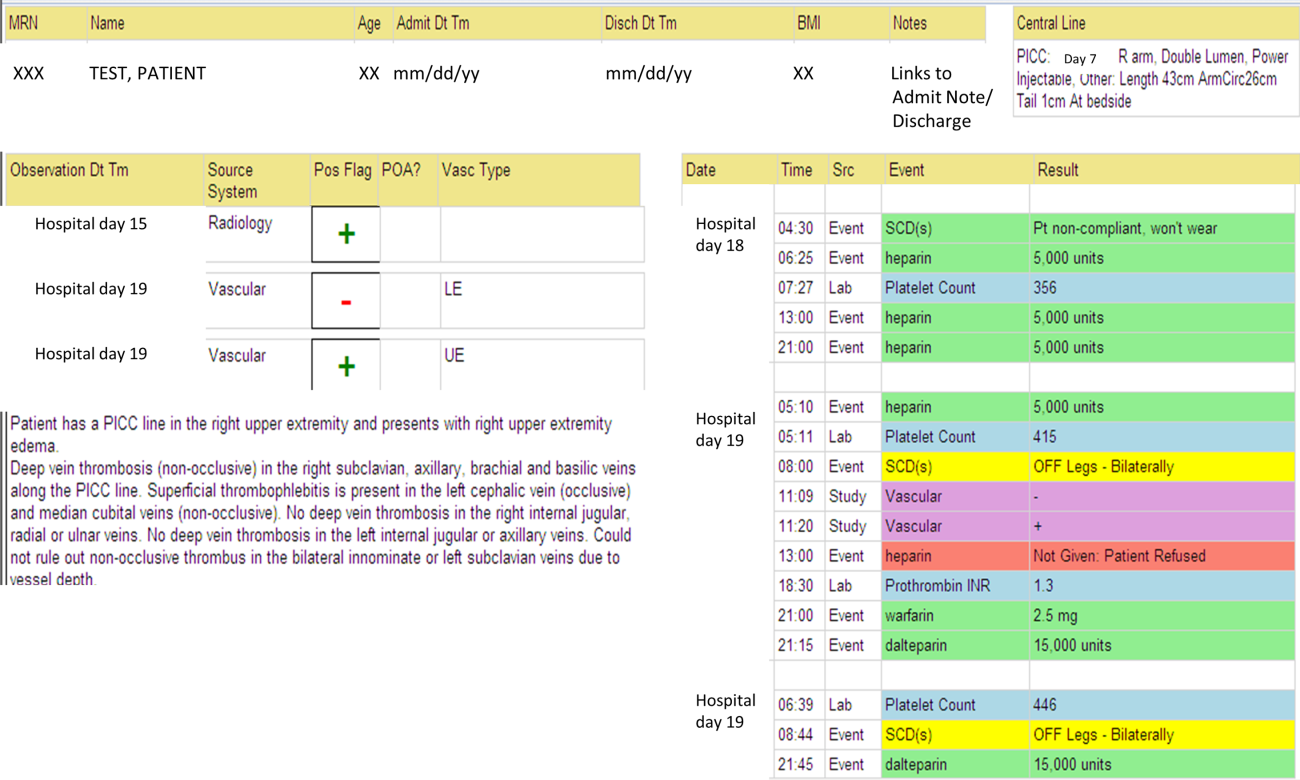
We identified all consecutive adult patients diagnosed with Doppler ultrasound‐confirmed UEDVT during hospitalization at Harborview Medical Center between September 2011 and November 2012. For patients who were readmitted during the study period, the first of their hospitalizations was used to describe associated factors, management, and outcomes. We present characteristics of all other hospitalizations during this time period for comparison. Harborview is a 413‐bed academic tertiary referral center and the only level 1 trauma center in a 5‐state area. Patients with UEDVT were identified using an information technology (IT) tool (the Harborview VTE tool) (Figure 1), which captures VTE events from vascular laboratory and radiology studies using natural language processing. Doppler ultrasound to assess for deep vein thrombosis (DVT) and computed tomographic scans to diagnose PE were ordered by inpatient physicians for symptomatic patients. The reason for obtaining the study is included in the ultrasound reports. We do not routinely screen for UEDVT at our institution. UEDVT included clots in the deep veins of the upper extremities including internal jugular, subclavian, axillary, and brachial veins. Superficial thrombosis and thrombophlebitis were excluded. We previously compared VTE events captured by this tool with administrative billing data and found that all VTE events that were coded were captured with the tool.
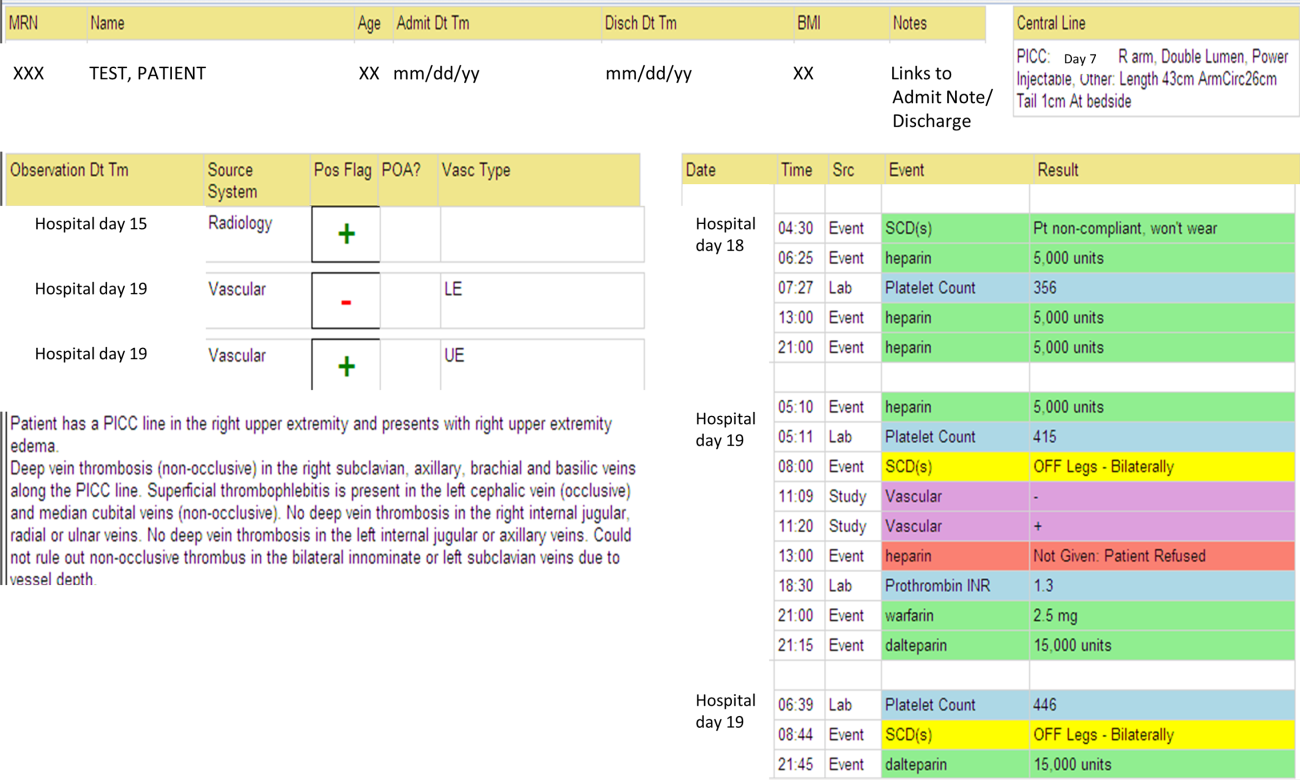
The VTE tool (Figure 1) displays imaging results together with demographic, clinical, and medication data and links this information with admission, discharge, and death summaries as well as CVC insertion procedure notes from the electronic health record (EHR). Additional data, including comorbid conditions, primary reason for hospitalization, past medical history such as prior VTE events, and cause of death (if not available in the admission note or discharge/death summaries), were obtained from EHR abstraction by 1 of the investigators. A 10% random sample of charts was rereviewed by another investigator with complete concordance. Supplementary data about date of CVC insertion if placed at an outside facility, date of CVC removal if applicable, clinical assessments regarding whether a clot was CVC‐associated, and contraindications to therapeutic anticoagulation were also abstracted directly from the EHR. Administrative data were used to identify the case mix index, an indicator of severity of illness.
Pharmacologic VTE prophylaxis included all chemical prophylaxis specified on our institutional guideline, most commonly subcutaneous unfractionated heparin 5000 units every 8 hours or low molecular weight heparin (LMWH), either enoxaparin 40 mg every 12 or 24 hours or dalteparin 5000 units every 24 hours. Mechanical prophylaxis was defined as use of sequential compression devices (SCDs) when pharmacologic prophylaxis was contraindicated. Prophylaxis was considered to be appropriate if it was applied according to our guideline for >90% of hospital days prior to UEDVT diagnosis. Therapeutic anticoagulation included heparin bridging (most commonly continuous heparin infusion, LMWH 1 mg/kg or dalteparin) as well as oral vitamin K antagonists. The VTE tool (Figure 1) allows identification of pharmacologic prophylaxis and therapy that is actually administered (not just ordered) directly from our pharmacy IT system. SCD application (not just ordered SCDs) is electronically integrated into the tool from nursing documentation.
CVCs included internal jugular or subclavian triple lumen catheters, tunneled dialysis catheters, or peripherally inserted central catheters (PICCs), single or double lumen. Criteria used to identify that a UEDVT was CVC‐associated included temporal relationship (CVC was placed prior to clot diagnosis), plausibility (ipsilateral clot), evidence of clot surrounding CVC on ultrasound, and physician designation of association (as documented in progress notes or discharge summary).
Simple percentages of patient characteristics, associated factors, management, and outcomes were calculated using counts as the numerator and number of patients as the denominator. For information about UEDVTs, we used total number of UEDVTs as the denominator. Line days were day counts from insertion until removal if applicable. The CVC placement date was available in our mandated central line placement procedure notes (directly accessed from the VTE tool) for all lines placed at our institution; date of removal (if applicable) was determined from chart abstraction. For the vast majority of patients whose CVCs were placed at outside facilities, date of placement was available in the EHR (often in the admission note or in the ultrasound report/reason for study). If date of line placement at an outside facility was not known, date of admission was used. The University of Washington Human Subjects Board approved this review.
RESULTS
General Characteristics
Fifty inpatients were diagnosed with 76 UEDVTs during 53 hospitalizations. Three patients were admitted twice during the study period. Their first admission is used for the purposes of this review. None of these 3 patients had new UEDVTs diagnosed during their second admission.
The patients' mean age was 49 years (standard deviation [SD] 15.6; range, 2482 years) vs 50.9 years (SD 17.49; range, 18112 years) among all other hospitalizations during this time (Table 1). Seventy percent (35) of patients with UEDVT were men. Sixteen percent (8) of patients with UEDVT had known VTE history, 20% (10) of patients had malignancy, and 22% (11) of patients had stage V chronic kidney disease or were hemodialysis dependent.
| Characteristic | Patients With UEDVT, N=50 | All Hospitalizations, N=23,407a |
|---|---|---|
| ||
| Age, y, mean (range) | 49 (2482) | 51 (18112) |
| Sex, % male (no.) | 70% (35) | 63% (14,746) |
| Case mix index, mean (range) | 4.78 (0.6917.99) | 1.87 (0.1626.34) |
| Length of stay, d, mean (range) | 24.6 (291) | 7.2 (1178) |
| Transfer from outside hospital (no.) | 50% (25) | 25% (5,866) |
| Intensive care unit stay (no.) | 46% (23) | 36% (8,356) |
| Operative procedure (no.) | 46% (23) | 41% (9,706) |
| In‐hospital mortality (no.) | 10% (5) | 4% (842) |
| Discharge to skilled nursing facility or other hospital, n=45 surviving patients (no.) | 62% (28) | 13% (3,095) |
| 30‐day readmission, n=45 surviving patients (no.) | 18% (8) | 5% (1,167) |
Patients diagnosed with UEDVT had complex illness, long LOS, and were often transferred from outside hospitals relative to other hospitalizations during this time period (Table 1). Slightly more required intensive care and underwent surgery. Eighty‐four percent (42) of patients with UEDVT required CVCs during hospitalization. Among patients whose UEDVT was not present on admission, 94% received appropriate VTE prophylaxis prior to UEDVT diagnosis.
In patients with UEDVT, the most common reasons for hospitalization were sepsis/severe infection (43%), cerebral hemorrhage (16%), and trauma (8%). Primary service at diagnosis was medicine 56.9%, surgery 25.5%, and neurosciences 17.6%.
Upper Extremity Deep Vein Thromboses
Fifty patients were diagnosed with 76 UEDVTs during their hospitalizations. In 40% (20) of patients, UEDVTs were present in >1 upper extremity deep vein; concurrent LEDVT was present in 26% (13) and PE in 10% (5). The majority of UEDVTs were found in internal jugular veins, followed by brachial and axillary veins. Seventeen percent were present on admission. Upper extremity swelling was the most common sign/symptom and reason for study. Characteristics of UEDVTs diagnosed are listed in Table 2.
| Characteristic | % UEDVTs (No.), n=76 |
|---|---|
| |
| Anatomic site | |
| Internal jugular | 38% (29) |
| Axillary | 21% (16) |
| Subclavian/axillary | 9% (7) |
| Subclavian | 7% (5) |
| Brachial | 25% (19) |
| Hospital day of diagnosis, d, mean (range) | 9.2 (044) |
| Present on admission | 17% (13) |
| Diagnosed at outside hospital or within 24 hours of transfer | 54% (7) |
| Diagnosed during prior hospitalization at our institution | 15% (2) |
| Diagnosed within 24 hours of admission via our emergency department | 23% (3) |
| Patient‐reported chronic UEDVT | 8% (1) |
| Primary UEDVT/anatomic anomaly | 0% (0) |
| Signs and symptoms (reasons for obtaining study) | |
| Upper extremity swelling | 71% (54) |
| Presence of clot elsewhere (eg, pulmonary embolism) | 9% (7) |
| Inability to place central venous access | 8% (6) |
| Assessment of clot propagation (known clot) | 8% (6) |
| Pain | 3% (2) |
| Patient‐reported history | 1% (1) |
Of the 50 patients diagnosed with UEDVT during hospitalization, 44% (22) were found to have UEDVTs directly associated with a CVC. Forty‐two of the 50 patients had a CVC; 52% (22 of 42) had CVC‐associated UEDVTs. Fifty percent (11) of these CVCs were triple lumen catheters, 32% (7) were PICCs, and 18% (4) were tunneled dialysis lines. Three of 42 patients with CVCs and line‐associated clots were had a malignancy. For patients with CVC‐associated clot, lines were in place for an average of 14.3 days (range, 273 days) prior to UEDVT diagnosis.
Treatment and Management
Seventy‐eight percent (39) of patients with UEDVT received in‐hospital treatment with heparin/LMWH bridging and oral anticoagulation. Of the 45 patients who survived hospitalization, 75% (34) were prescribed anticoagulation for 3+ months at discharge; 23% (10) had documented contraindications to anticoagulation, most commonly recent gastrointestinal or intracranial bleeding. Two percent of patients (1) was not prescribed pharmacologic treatment at discharge and had no contraindications documented. No patients underwent thrombolysis or had superior vena cava filters placed. Sixty‐four percent (14 of 22) of CVCs that were thought to be directly associated with UEDVT were removed at diagnosis.
Outcomes
Five patients (10%) died during hospitalization, none because of VTE or complications thereof. Cause of death included septic shock, cancer, intracranial hemorrhage, heart failure, and recurrent gastrointestinal bleeding. Of the 45 surviving patients, only 38% (17) were discharged to self‐care; more than half (62%[28]) were discharged to skilled nursing facilities, other hospitals, or rehabilitation centers. Eight patients (18%) were readmitted to our institution within 30 days; none for recurrent or new DVT or PE. No additional patients died at our medical center within 30 days of discharge.
DISCUSSION
UEDVT is increasingly recognized in hospitalized patients.[3, 9] At our medical center, 0.2% of symptomatic inpatients were diagnosed with UEDVT over 14 months. These patients were predominantly men with high rates of CVCs, malignancy, VTE history, severe infection, and renal disease. Interestingly, although the literature suggests that some proportion of patients with UEDVT have anatomic abnormalities, such as Paget‐Schroetter syndrome,[15] none of the patients in our study were found to have these anomalies. In our review, hospitalized patients with UEDVT were critically ill, with a long LOS and high morbidity and mortality, suggesting that in addition to just being a complication of hospitalization,[1, 6] UEDVT may be a marker of severe illness.
In our institution, clinical presentation was consistent with what has been described with the majority of patients presenting with upper extremity swelling.[1, 3] The internal jugular veins were the most common anatomic UEDVT site, followed by brachial then axillary veins. In other series including both in‐ and outpatients, subclavian clots were most commonly diagnosed, reflecting in part higher rates of CVC association and CVC location in those studies.[3, 9] Concurrent DVT and PE rates were similar to those reported.[1, 3, 10]
Although many studies have focused on prevention of LEDVT and PE, few trials have specifically targeted UEDVT. Among our patients with UEDVTs that were not present on admission, VTE prophylaxis rates were considerably higher than what has been reported,[1, 6] suggesting that in these critically ill patients' prophylaxis may not prevent symptomatic UEDVT. It is unknown how many UEDVTs were prevented with prophylaxis, as only patients with symptomatic UEDVT were included. Adequacy of prophylaxis at outside hospitals for patients transferred in could not be assessed. Nonetheless, low numbers of UEDVT at a trauma referral center with many high‐risk patients raise the question of whether prophylaxis makes a difference. Additional study is needed to further define the role of chemoprophylaxis to prevent UEDVT in hospitalized patients.
In our inpatient group, 84% required CVCs; 44% of patients were thought to have CVC‐associated UEDVTs. Careful patient selection and attention to potentially modifiable risks, such as insertion site, catheter type, and tip position, may need further examination in this population.[3, 11, 16] Catheter duration was long; focus on removing CVCs when no longer necessary is important. Interestingly, almost 10% in our study underwent diagnostic ultrasound because a new CVC could not be successfully placed suggesting that UEDVT may develop in critically ill patients regardless of CVCs.
In our study, there were high rates of guideline‐recommended pharmacologic treatment; surprisingly the majority of CVCs with associated clot were removed. Guidelines currently support 3 months of anticoagulation for treatment of UEDVT[2, 13, 17]; evidence derives from observational trials or is largely extrapolated from LEDVT literature.[2, 13] Routine CVC removal is not specifically recommended for CVC‐associated UEDVT, particularly if lines remain functional and medically necessary; systemic anticoagulation should be provided.[13]
In our review, no hospitalized patients with UEDVT developed complications or were readmitted to our medical center within 30 days for clot progression, new PE, or post‐thrombotic syndrome, which is lower than rates reported over longer time periods.[2, 6, 10, 12] Ten percent died during hospitalization, all from their primary disease rather than from complications of VTE or VTE treatment, and no additional patients died at our institution within 30 days. Although these rates are lower than have been otherwise reported,[2, 10] the inpatient mortality rate is similar to a recent study that included inpatients; however, all patients who died in that study had cancer and CVCs.[3] In the latter study, 6.4% died within 30 days of discharge.
Limitations
There are several limitations to this study. It was conducted at a single academic referral center with a large and critically ill trauma and neurosciences population, thereby limiting generalizability. This study describes hospitalized patients at a tertiary care center who were diagnosed with UEDVT. For comparison, we obtained information regarding characteristics of hospitalization for all other inpatients during this time frame. Individuals may have had multiple hospitalizations during the study period, but because we were unable to identify information about individuals, direct statistical comparisons could not be made. However, in general, inpatients with UEDVT appeared to be sicker, with prolonged LOS and high in‐hospital mortality relative to other hospitalized patients.
Only symptomatic UEDVT events were captured, likely underestimating true UEDVT incidence. In addition, we defined UEDVTs as those diagnosed by Doppler ultrasound; therefore theoretically, UEDVTs that were more centrally located or diagnosed using another modality would not be represented here. However, in a prior internal review we found that all VTE events coded in billing data during this time period were identified using our operational definition.
In our study, VTE prophylaxis was administered in accordance with an institutional guideline. We did not have information regarding adequacy of prophylaxis at outside institutions for patients transferred in, and patients admitted through the emergency department likely were not on prophylaxis. Therefore, information about prophylaxis is limited to prophylaxis administered at our medical center for hospitalized patients who had UEDVTs not present on admission.
Information regarding CVC insertion date and CVC type for CVCs placed in our institution is accurate based on our internal reviews. Although we had reasonable capture of information about CVC placement at outside facilities, these data may be incomplete, thereby underestimating potential association of CVCs with UEDVTs identified in our hospitalized patients. Additionally, criteria used to assess association of a CVC with UEDVT may have led to underrepresentation of CVC‐associated UEDVT.
Management of UEDVT in this study was determined by the treating physicians, and patients were only followed for 30 days after discharge. Information about readmission or death within 30 days of discharge was limited to patient contact with our medical center only. Treatment at discharge was determined from the discharge summary. Therefore, compliance with treatment cannot be assessed. Although these factors may limit the nature of the conclusions, data reflect actual practice and experience in hospitalized patients with UEDVT and may be hypothesis generating.
CONCLUSIONS
Among hospitalized patients, UEDVT is increasingly recognized. In our medical center, hospitalized patients diagnosed with UEDVT were more likely to have CVCs, malignancy, renal disease, and severe infection. Many of these patients were transferred critically ill, had prolonged LOS, and had high in‐hospital mortality. Most developed UEDVT despite prophylaxis, and the majority of UEDVTs were treated even in the absence of concurrent LEDVT or PE. As we move toward an era of increasing accountability, with a focus on preventing hospital‐acquired conditions including VTE, additional research is needed to identify modifiable risks, explore opportunities for effective prevention, and optimize outcomes such as prevention of complications or readmissions, particularly in critically ill patients with UEDVT.
Acknowledgements
The authors would like to thank Ronald Pergamit and Kevin Middleton for their extraordinary creativity and expert programming.
- , , , . Upper‐extremity deep vein thrombosis: a prospective registry of 592 patients. Circulation. 2004;110(12):1605–1611.
- , , , et al. Clinical outcome of patients with upper‐extremity deep vein thrombosis: results from the RIETE Registry. Chest. 2008;133(1):143–148.
- , , . The risk factors and clinical outcomes of upper extremity deep vein thrombosis. Vasc Endovascular Surg. 2012;46(2):139–144.
- , , , et al. Upper extremity versus lower extremity deep venous thrombosis. Am J Surg. 1997;174(2):214–217.
- , . Upper‐extremity deep vein thrombosis. Circulation. 2002;106(14):1874–1880.
- , , , . Upper extremity deep vein thrombosis: a community‐based perspective. Am J Med. 2007;120(8):678–684.
- , , , et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947–954.e2.
- , , , , . Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. 2009;4(7):417–422.
- , , , , . Characterization and probability of upper extremity deep venous thrombosis. Ann Vasc Surg. 2004;18(5):552–557.
- , , , et al. Risk factors for mortality in patients with upper extremity and internal jugular deep venous thrombosis. J Vasc Surg. 2005;41(3):476–478.
- , , , et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138(4):803–810.
- , , , et al. The long term clinical course of acute deep vein thrombosis of the arm: prospective cohort study. BMJ. 2004;329(7464):484–485.
- , , , et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e419S–e494S.
- , , , , , . Introduction to the ninth edition: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):48S–52S.
- . Clinical practice. Deep‐vein thrombosis of the upper extremities. N Engl J Med. 2011;364(9):861–869.
- , , , et al. Diagnosis and management of upper extremity deep‐vein thrombosis in adults. Thromb Haemost. 2012;108(6):1097–1108.
- , , . Treatment of upper‐extremity deep vein thrombosis. J Thromb Haemost. 2011;9(10):1924–1930.
Increasingly, there is a focus on prevention of hospital‐acquired conditions including venous thromboembolism (VTE). Many studies have evaluated pulmonary embolism (PE) and lower extremity deep vein thrombosis (LEDVT), but despite increasing recognition of upper extremity deep vein thrombosis (UEDVT),[1, 2, 3, 4] less is known about this condition in hospitalized patients.
UEDVTs may be classified as primary, including disorders such as Paget‐Schroetter syndrome or other structural abnormality, or may be idiopathic; the majority are secondary clots.[5] Conventional risk factors for LEDVT including older age and obesity have been found to be less commonly associated,[1, 2, 5, 6, 7] and patients with UEDVT are generally younger, leaner, and a higher proportion are men. They are more likely to have malignancy or history of VTE and have undergone recent surgery or intensive care unit stay.[1, 2, 6] Central venous catheters (CVCs), often used in hospitalized patients, remain among the biggest known risks for UEDVT[1, 2, 3, 7, 8, 9, 10]; concomitant malignancy, VTE history, severe infection, surgery lasting >1 hour, and length of stay (LOS) >10 days confer additional risks with CVCs.[6, 7, 8, 11]
UEDVTs, once thought to be relatively benign, are now recognized to result in complications including PE, progression, recurrence, and post‐thrombotic syndrome.[2, 4, 12, 13] Despite extensive efforts to increase appropriate VTE prophylaxis in inpatients,[14] the role of chemoprophylaxis to prevent UEDVT remains undefined. Current guidelines recommend anticoagulation for treatment and complication prevention,[13, 15] but to date the evidence derives largely from observational studies or is extrapolated from the LEDVT literature.[2, 13]
To improve understanding of UEDVT at our institution, we set out to (1) determine UEDVT incidence in hospitalized patients, (2) describe associated risks and outcomes, and (3) assess management during hospitalization and at discharge.
METHODS
We identified all consecutive adult patients diagnosed with Doppler ultrasound‐confirmed UEDVT during hospitalization at Harborview Medical Center between September 2011 and November 2012. For patients who were readmitted during the study period, the first of their hospitalizations was used to describe associated factors, management, and outcomes. We present characteristics of all other hospitalizations during this time period for comparison. Harborview is a 413‐bed academic tertiary referral center and the only level 1 trauma center in a 5‐state area. Patients with UEDVT were identified using an information technology (IT) tool (the Harborview VTE tool) (Figure 1), which captures VTE events from vascular laboratory and radiology studies using natural language processing. Doppler ultrasound to assess for deep vein thrombosis (DVT) and computed tomographic scans to diagnose PE were ordered by inpatient physicians for symptomatic patients. The reason for obtaining the study is included in the ultrasound reports. We do not routinely screen for UEDVT at our institution. UEDVT included clots in the deep veins of the upper extremities including internal jugular, subclavian, axillary, and brachial veins. Superficial thrombosis and thrombophlebitis were excluded. We previously compared VTE events captured by this tool with administrative billing data and found that all VTE events that were coded were captured with the tool.

The VTE tool (Figure 1) displays imaging results together with demographic, clinical, and medication data and links this information with admission, discharge, and death summaries as well as CVC insertion procedure notes from the electronic health record (EHR). Additional data, including comorbid conditions, primary reason for hospitalization, past medical history such as prior VTE events, and cause of death (if not available in the admission note or discharge/death summaries), were obtained from EHR abstraction by 1 of the investigators. A 10% random sample of charts was rereviewed by another investigator with complete concordance. Supplementary data about date of CVC insertion if placed at an outside facility, date of CVC removal if applicable, clinical assessments regarding whether a clot was CVC‐associated, and contraindications to therapeutic anticoagulation were also abstracted directly from the EHR. Administrative data were used to identify the case mix index, an indicator of severity of illness.
Pharmacologic VTE prophylaxis included all chemical prophylaxis specified on our institutional guideline, most commonly subcutaneous unfractionated heparin 5000 units every 8 hours or low molecular weight heparin (LMWH), either enoxaparin 40 mg every 12 or 24 hours or dalteparin 5000 units every 24 hours. Mechanical prophylaxis was defined as use of sequential compression devices (SCDs) when pharmacologic prophylaxis was contraindicated. Prophylaxis was considered to be appropriate if it was applied according to our guideline for >90% of hospital days prior to UEDVT diagnosis. Therapeutic anticoagulation included heparin bridging (most commonly continuous heparin infusion, LMWH 1 mg/kg or dalteparin) as well as oral vitamin K antagonists. The VTE tool (Figure 1) allows identification of pharmacologic prophylaxis and therapy that is actually administered (not just ordered) directly from our pharmacy IT system. SCD application (not just ordered SCDs) is electronically integrated into the tool from nursing documentation.
CVCs included internal jugular or subclavian triple lumen catheters, tunneled dialysis catheters, or peripherally inserted central catheters (PICCs), single or double lumen. Criteria used to identify that a UEDVT was CVC‐associated included temporal relationship (CVC was placed prior to clot diagnosis), plausibility (ipsilateral clot), evidence of clot surrounding CVC on ultrasound, and physician designation of association (as documented in progress notes or discharge summary).
Simple percentages of patient characteristics, associated factors, management, and outcomes were calculated using counts as the numerator and number of patients as the denominator. For information about UEDVTs, we used total number of UEDVTs as the denominator. Line days were day counts from insertion until removal if applicable. The CVC placement date was available in our mandated central line placement procedure notes (directly accessed from the VTE tool) for all lines placed at our institution; date of removal (if applicable) was determined from chart abstraction. For the vast majority of patients whose CVCs were placed at outside facilities, date of placement was available in the EHR (often in the admission note or in the ultrasound report/reason for study). If date of line placement at an outside facility was not known, date of admission was used. The University of Washington Human Subjects Board approved this review.
RESULTS
General Characteristics
Fifty inpatients were diagnosed with 76 UEDVTs during 53 hospitalizations. Three patients were admitted twice during the study period. Their first admission is used for the purposes of this review. None of these 3 patients had new UEDVTs diagnosed during their second admission.
The patients' mean age was 49 years (standard deviation [SD] 15.6; range, 2482 years) vs 50.9 years (SD 17.49; range, 18112 years) among all other hospitalizations during this time (Table 1). Seventy percent (35) of patients with UEDVT were men. Sixteen percent (8) of patients with UEDVT had known VTE history, 20% (10) of patients had malignancy, and 22% (11) of patients had stage V chronic kidney disease or were hemodialysis dependent.
| Characteristic | Patients With UEDVT, N=50 | All Hospitalizations, N=23,407a |
|---|---|---|
| ||
| Age, y, mean (range) | 49 (2482) | 51 (18112) |
| Sex, % male (no.) | 70% (35) | 63% (14,746) |
| Case mix index, mean (range) | 4.78 (0.6917.99) | 1.87 (0.1626.34) |
| Length of stay, d, mean (range) | 24.6 (291) | 7.2 (1178) |
| Transfer from outside hospital (no.) | 50% (25) | 25% (5,866) |
| Intensive care unit stay (no.) | 46% (23) | 36% (8,356) |
| Operative procedure (no.) | 46% (23) | 41% (9,706) |
| In‐hospital mortality (no.) | 10% (5) | 4% (842) |
| Discharge to skilled nursing facility or other hospital, n=45 surviving patients (no.) | 62% (28) | 13% (3,095) |
| 30‐day readmission, n=45 surviving patients (no.) | 18% (8) | 5% (1,167) |
Patients diagnosed with UEDVT had complex illness, long LOS, and were often transferred from outside hospitals relative to other hospitalizations during this time period (Table 1). Slightly more required intensive care and underwent surgery. Eighty‐four percent (42) of patients with UEDVT required CVCs during hospitalization. Among patients whose UEDVT was not present on admission, 94% received appropriate VTE prophylaxis prior to UEDVT diagnosis.
In patients with UEDVT, the most common reasons for hospitalization were sepsis/severe infection (43%), cerebral hemorrhage (16%), and trauma (8%). Primary service at diagnosis was medicine 56.9%, surgery 25.5%, and neurosciences 17.6%.
Upper Extremity Deep Vein Thromboses
Fifty patients were diagnosed with 76 UEDVTs during their hospitalizations. In 40% (20) of patients, UEDVTs were present in >1 upper extremity deep vein; concurrent LEDVT was present in 26% (13) and PE in 10% (5). The majority of UEDVTs were found in internal jugular veins, followed by brachial and axillary veins. Seventeen percent were present on admission. Upper extremity swelling was the most common sign/symptom and reason for study. Characteristics of UEDVTs diagnosed are listed in Table 2.
| Characteristic | % UEDVTs (No.), n=76 |
|---|---|
| |
| Anatomic site | |
| Internal jugular | 38% (29) |
| Axillary | 21% (16) |
| Subclavian/axillary | 9% (7) |
| Subclavian | 7% (5) |
| Brachial | 25% (19) |
| Hospital day of diagnosis, d, mean (range) | 9.2 (044) |
| Present on admission | 17% (13) |
| Diagnosed at outside hospital or within 24 hours of transfer | 54% (7) |
| Diagnosed during prior hospitalization at our institution | 15% (2) |
| Diagnosed within 24 hours of admission via our emergency department | 23% (3) |
| Patient‐reported chronic UEDVT | 8% (1) |
| Primary UEDVT/anatomic anomaly | 0% (0) |
| Signs and symptoms (reasons for obtaining study) | |
| Upper extremity swelling | 71% (54) |
| Presence of clot elsewhere (eg, pulmonary embolism) | 9% (7) |
| Inability to place central venous access | 8% (6) |
| Assessment of clot propagation (known clot) | 8% (6) |
| Pain | 3% (2) |
| Patient‐reported history | 1% (1) |
Of the 50 patients diagnosed with UEDVT during hospitalization, 44% (22) were found to have UEDVTs directly associated with a CVC. Forty‐two of the 50 patients had a CVC; 52% (22 of 42) had CVC‐associated UEDVTs. Fifty percent (11) of these CVCs were triple lumen catheters, 32% (7) were PICCs, and 18% (4) were tunneled dialysis lines. Three of 42 patients with CVCs and line‐associated clots were had a malignancy. For patients with CVC‐associated clot, lines were in place for an average of 14.3 days (range, 273 days) prior to UEDVT diagnosis.
Treatment and Management
Seventy‐eight percent (39) of patients with UEDVT received in‐hospital treatment with heparin/LMWH bridging and oral anticoagulation. Of the 45 patients who survived hospitalization, 75% (34) were prescribed anticoagulation for 3+ months at discharge; 23% (10) had documented contraindications to anticoagulation, most commonly recent gastrointestinal or intracranial bleeding. Two percent of patients (1) was not prescribed pharmacologic treatment at discharge and had no contraindications documented. No patients underwent thrombolysis or had superior vena cava filters placed. Sixty‐four percent (14 of 22) of CVCs that were thought to be directly associated with UEDVT were removed at diagnosis.
Outcomes
Five patients (10%) died during hospitalization, none because of VTE or complications thereof. Cause of death included septic shock, cancer, intracranial hemorrhage, heart failure, and recurrent gastrointestinal bleeding. Of the 45 surviving patients, only 38% (17) were discharged to self‐care; more than half (62%[28]) were discharged to skilled nursing facilities, other hospitals, or rehabilitation centers. Eight patients (18%) were readmitted to our institution within 30 days; none for recurrent or new DVT or PE. No additional patients died at our medical center within 30 days of discharge.
DISCUSSION
UEDVT is increasingly recognized in hospitalized patients.[3, 9] At our medical center, 0.2% of symptomatic inpatients were diagnosed with UEDVT over 14 months. These patients were predominantly men with high rates of CVCs, malignancy, VTE history, severe infection, and renal disease. Interestingly, although the literature suggests that some proportion of patients with UEDVT have anatomic abnormalities, such as Paget‐Schroetter syndrome,[15] none of the patients in our study were found to have these anomalies. In our review, hospitalized patients with UEDVT were critically ill, with a long LOS and high morbidity and mortality, suggesting that in addition to just being a complication of hospitalization,[1, 6] UEDVT may be a marker of severe illness.
In our institution, clinical presentation was consistent with what has been described with the majority of patients presenting with upper extremity swelling.[1, 3] The internal jugular veins were the most common anatomic UEDVT site, followed by brachial then axillary veins. In other series including both in‐ and outpatients, subclavian clots were most commonly diagnosed, reflecting in part higher rates of CVC association and CVC location in those studies.[3, 9] Concurrent DVT and PE rates were similar to those reported.[1, 3, 10]
Although many studies have focused on prevention of LEDVT and PE, few trials have specifically targeted UEDVT. Among our patients with UEDVTs that were not present on admission, VTE prophylaxis rates were considerably higher than what has been reported,[1, 6] suggesting that in these critically ill patients' prophylaxis may not prevent symptomatic UEDVT. It is unknown how many UEDVTs were prevented with prophylaxis, as only patients with symptomatic UEDVT were included. Adequacy of prophylaxis at outside hospitals for patients transferred in could not be assessed. Nonetheless, low numbers of UEDVT at a trauma referral center with many high‐risk patients raise the question of whether prophylaxis makes a difference. Additional study is needed to further define the role of chemoprophylaxis to prevent UEDVT in hospitalized patients.
In our inpatient group, 84% required CVCs; 44% of patients were thought to have CVC‐associated UEDVTs. Careful patient selection and attention to potentially modifiable risks, such as insertion site, catheter type, and tip position, may need further examination in this population.[3, 11, 16] Catheter duration was long; focus on removing CVCs when no longer necessary is important. Interestingly, almost 10% in our study underwent diagnostic ultrasound because a new CVC could not be successfully placed suggesting that UEDVT may develop in critically ill patients regardless of CVCs.
In our study, there were high rates of guideline‐recommended pharmacologic treatment; surprisingly the majority of CVCs with associated clot were removed. Guidelines currently support 3 months of anticoagulation for treatment of UEDVT[2, 13, 17]; evidence derives from observational trials or is largely extrapolated from LEDVT literature.[2, 13] Routine CVC removal is not specifically recommended for CVC‐associated UEDVT, particularly if lines remain functional and medically necessary; systemic anticoagulation should be provided.[13]
In our review, no hospitalized patients with UEDVT developed complications or were readmitted to our medical center within 30 days for clot progression, new PE, or post‐thrombotic syndrome, which is lower than rates reported over longer time periods.[2, 6, 10, 12] Ten percent died during hospitalization, all from their primary disease rather than from complications of VTE or VTE treatment, and no additional patients died at our institution within 30 days. Although these rates are lower than have been otherwise reported,[2, 10] the inpatient mortality rate is similar to a recent study that included inpatients; however, all patients who died in that study had cancer and CVCs.[3] In the latter study, 6.4% died within 30 days of discharge.
Limitations
There are several limitations to this study. It was conducted at a single academic referral center with a large and critically ill trauma and neurosciences population, thereby limiting generalizability. This study describes hospitalized patients at a tertiary care center who were diagnosed with UEDVT. For comparison, we obtained information regarding characteristics of hospitalization for all other inpatients during this time frame. Individuals may have had multiple hospitalizations during the study period, but because we were unable to identify information about individuals, direct statistical comparisons could not be made. However, in general, inpatients with UEDVT appeared to be sicker, with prolonged LOS and high in‐hospital mortality relative to other hospitalized patients.
Only symptomatic UEDVT events were captured, likely underestimating true UEDVT incidence. In addition, we defined UEDVTs as those diagnosed by Doppler ultrasound; therefore theoretically, UEDVTs that were more centrally located or diagnosed using another modality would not be represented here. However, in a prior internal review we found that all VTE events coded in billing data during this time period were identified using our operational definition.
In our study, VTE prophylaxis was administered in accordance with an institutional guideline. We did not have information regarding adequacy of prophylaxis at outside institutions for patients transferred in, and patients admitted through the emergency department likely were not on prophylaxis. Therefore, information about prophylaxis is limited to prophylaxis administered at our medical center for hospitalized patients who had UEDVTs not present on admission.
Information regarding CVC insertion date and CVC type for CVCs placed in our institution is accurate based on our internal reviews. Although we had reasonable capture of information about CVC placement at outside facilities, these data may be incomplete, thereby underestimating potential association of CVCs with UEDVTs identified in our hospitalized patients. Additionally, criteria used to assess association of a CVC with UEDVT may have led to underrepresentation of CVC‐associated UEDVT.
Management of UEDVT in this study was determined by the treating physicians, and patients were only followed for 30 days after discharge. Information about readmission or death within 30 days of discharge was limited to patient contact with our medical center only. Treatment at discharge was determined from the discharge summary. Therefore, compliance with treatment cannot be assessed. Although these factors may limit the nature of the conclusions, data reflect actual practice and experience in hospitalized patients with UEDVT and may be hypothesis generating.
CONCLUSIONS
Among hospitalized patients, UEDVT is increasingly recognized. In our medical center, hospitalized patients diagnosed with UEDVT were more likely to have CVCs, malignancy, renal disease, and severe infection. Many of these patients were transferred critically ill, had prolonged LOS, and had high in‐hospital mortality. Most developed UEDVT despite prophylaxis, and the majority of UEDVTs were treated even in the absence of concurrent LEDVT or PE. As we move toward an era of increasing accountability, with a focus on preventing hospital‐acquired conditions including VTE, additional research is needed to identify modifiable risks, explore opportunities for effective prevention, and optimize outcomes such as prevention of complications or readmissions, particularly in critically ill patients with UEDVT.
Acknowledgements
The authors would like to thank Ronald Pergamit and Kevin Middleton for their extraordinary creativity and expert programming.
Increasingly, there is a focus on prevention of hospital‐acquired conditions including venous thromboembolism (VTE). Many studies have evaluated pulmonary embolism (PE) and lower extremity deep vein thrombosis (LEDVT), but despite increasing recognition of upper extremity deep vein thrombosis (UEDVT),[1, 2, 3, 4] less is known about this condition in hospitalized patients.
UEDVTs may be classified as primary, including disorders such as Paget‐Schroetter syndrome or other structural abnormality, or may be idiopathic; the majority are secondary clots.[5] Conventional risk factors for LEDVT including older age and obesity have been found to be less commonly associated,[1, 2, 5, 6, 7] and patients with UEDVT are generally younger, leaner, and a higher proportion are men. They are more likely to have malignancy or history of VTE and have undergone recent surgery or intensive care unit stay.[1, 2, 6] Central venous catheters (CVCs), often used in hospitalized patients, remain among the biggest known risks for UEDVT[1, 2, 3, 7, 8, 9, 10]; concomitant malignancy, VTE history, severe infection, surgery lasting >1 hour, and length of stay (LOS) >10 days confer additional risks with CVCs.[6, 7, 8, 11]
UEDVTs, once thought to be relatively benign, are now recognized to result in complications including PE, progression, recurrence, and post‐thrombotic syndrome.[2, 4, 12, 13] Despite extensive efforts to increase appropriate VTE prophylaxis in inpatients,[14] the role of chemoprophylaxis to prevent UEDVT remains undefined. Current guidelines recommend anticoagulation for treatment and complication prevention,[13, 15] but to date the evidence derives largely from observational studies or is extrapolated from the LEDVT literature.[2, 13]
To improve understanding of UEDVT at our institution, we set out to (1) determine UEDVT incidence in hospitalized patients, (2) describe associated risks and outcomes, and (3) assess management during hospitalization and at discharge.
METHODS
We identified all consecutive adult patients diagnosed with Doppler ultrasound‐confirmed UEDVT during hospitalization at Harborview Medical Center between September 2011 and November 2012. For patients who were readmitted during the study period, the first of their hospitalizations was used to describe associated factors, management, and outcomes. We present characteristics of all other hospitalizations during this time period for comparison. Harborview is a 413‐bed academic tertiary referral center and the only level 1 trauma center in a 5‐state area. Patients with UEDVT were identified using an information technology (IT) tool (the Harborview VTE tool) (Figure 1), which captures VTE events from vascular laboratory and radiology studies using natural language processing. Doppler ultrasound to assess for deep vein thrombosis (DVT) and computed tomographic scans to diagnose PE were ordered by inpatient physicians for symptomatic patients. The reason for obtaining the study is included in the ultrasound reports. We do not routinely screen for UEDVT at our institution. UEDVT included clots in the deep veins of the upper extremities including internal jugular, subclavian, axillary, and brachial veins. Superficial thrombosis and thrombophlebitis were excluded. We previously compared VTE events captured by this tool with administrative billing data and found that all VTE events that were coded were captured with the tool.

The VTE tool (Figure 1) displays imaging results together with demographic, clinical, and medication data and links this information with admission, discharge, and death summaries as well as CVC insertion procedure notes from the electronic health record (EHR). Additional data, including comorbid conditions, primary reason for hospitalization, past medical history such as prior VTE events, and cause of death (if not available in the admission note or discharge/death summaries), were obtained from EHR abstraction by 1 of the investigators. A 10% random sample of charts was rereviewed by another investigator with complete concordance. Supplementary data about date of CVC insertion if placed at an outside facility, date of CVC removal if applicable, clinical assessments regarding whether a clot was CVC‐associated, and contraindications to therapeutic anticoagulation were also abstracted directly from the EHR. Administrative data were used to identify the case mix index, an indicator of severity of illness.
Pharmacologic VTE prophylaxis included all chemical prophylaxis specified on our institutional guideline, most commonly subcutaneous unfractionated heparin 5000 units every 8 hours or low molecular weight heparin (LMWH), either enoxaparin 40 mg every 12 or 24 hours or dalteparin 5000 units every 24 hours. Mechanical prophylaxis was defined as use of sequential compression devices (SCDs) when pharmacologic prophylaxis was contraindicated. Prophylaxis was considered to be appropriate if it was applied according to our guideline for >90% of hospital days prior to UEDVT diagnosis. Therapeutic anticoagulation included heparin bridging (most commonly continuous heparin infusion, LMWH 1 mg/kg or dalteparin) as well as oral vitamin K antagonists. The VTE tool (Figure 1) allows identification of pharmacologic prophylaxis and therapy that is actually administered (not just ordered) directly from our pharmacy IT system. SCD application (not just ordered SCDs) is electronically integrated into the tool from nursing documentation.
CVCs included internal jugular or subclavian triple lumen catheters, tunneled dialysis catheters, or peripherally inserted central catheters (PICCs), single or double lumen. Criteria used to identify that a UEDVT was CVC‐associated included temporal relationship (CVC was placed prior to clot diagnosis), plausibility (ipsilateral clot), evidence of clot surrounding CVC on ultrasound, and physician designation of association (as documented in progress notes or discharge summary).
Simple percentages of patient characteristics, associated factors, management, and outcomes were calculated using counts as the numerator and number of patients as the denominator. For information about UEDVTs, we used total number of UEDVTs as the denominator. Line days were day counts from insertion until removal if applicable. The CVC placement date was available in our mandated central line placement procedure notes (directly accessed from the VTE tool) for all lines placed at our institution; date of removal (if applicable) was determined from chart abstraction. For the vast majority of patients whose CVCs were placed at outside facilities, date of placement was available in the EHR (often in the admission note or in the ultrasound report/reason for study). If date of line placement at an outside facility was not known, date of admission was used. The University of Washington Human Subjects Board approved this review.
RESULTS
General Characteristics
Fifty inpatients were diagnosed with 76 UEDVTs during 53 hospitalizations. Three patients were admitted twice during the study period. Their first admission is used for the purposes of this review. None of these 3 patients had new UEDVTs diagnosed during their second admission.
The patients' mean age was 49 years (standard deviation [SD] 15.6; range, 2482 years) vs 50.9 years (SD 17.49; range, 18112 years) among all other hospitalizations during this time (Table 1). Seventy percent (35) of patients with UEDVT were men. Sixteen percent (8) of patients with UEDVT had known VTE history, 20% (10) of patients had malignancy, and 22% (11) of patients had stage V chronic kidney disease or were hemodialysis dependent.
| Characteristic | Patients With UEDVT, N=50 | All Hospitalizations, N=23,407a |
|---|---|---|
| ||
| Age, y, mean (range) | 49 (2482) | 51 (18112) |
| Sex, % male (no.) | 70% (35) | 63% (14,746) |
| Case mix index, mean (range) | 4.78 (0.6917.99) | 1.87 (0.1626.34) |
| Length of stay, d, mean (range) | 24.6 (291) | 7.2 (1178) |
| Transfer from outside hospital (no.) | 50% (25) | 25% (5,866) |
| Intensive care unit stay (no.) | 46% (23) | 36% (8,356) |
| Operative procedure (no.) | 46% (23) | 41% (9,706) |
| In‐hospital mortality (no.) | 10% (5) | 4% (842) |
| Discharge to skilled nursing facility or other hospital, n=45 surviving patients (no.) | 62% (28) | 13% (3,095) |
| 30‐day readmission, n=45 surviving patients (no.) | 18% (8) | 5% (1,167) |
Patients diagnosed with UEDVT had complex illness, long LOS, and were often transferred from outside hospitals relative to other hospitalizations during this time period (Table 1). Slightly more required intensive care and underwent surgery. Eighty‐four percent (42) of patients with UEDVT required CVCs during hospitalization. Among patients whose UEDVT was not present on admission, 94% received appropriate VTE prophylaxis prior to UEDVT diagnosis.
In patients with UEDVT, the most common reasons for hospitalization were sepsis/severe infection (43%), cerebral hemorrhage (16%), and trauma (8%). Primary service at diagnosis was medicine 56.9%, surgery 25.5%, and neurosciences 17.6%.
Upper Extremity Deep Vein Thromboses
Fifty patients were diagnosed with 76 UEDVTs during their hospitalizations. In 40% (20) of patients, UEDVTs were present in >1 upper extremity deep vein; concurrent LEDVT was present in 26% (13) and PE in 10% (5). The majority of UEDVTs were found in internal jugular veins, followed by brachial and axillary veins. Seventeen percent were present on admission. Upper extremity swelling was the most common sign/symptom and reason for study. Characteristics of UEDVTs diagnosed are listed in Table 2.
| Characteristic | % UEDVTs (No.), n=76 |
|---|---|
| |
| Anatomic site | |
| Internal jugular | 38% (29) |
| Axillary | 21% (16) |
| Subclavian/axillary | 9% (7) |
| Subclavian | 7% (5) |
| Brachial | 25% (19) |
| Hospital day of diagnosis, d, mean (range) | 9.2 (044) |
| Present on admission | 17% (13) |
| Diagnosed at outside hospital or within 24 hours of transfer | 54% (7) |
| Diagnosed during prior hospitalization at our institution | 15% (2) |
| Diagnosed within 24 hours of admission via our emergency department | 23% (3) |
| Patient‐reported chronic UEDVT | 8% (1) |
| Primary UEDVT/anatomic anomaly | 0% (0) |
| Signs and symptoms (reasons for obtaining study) | |
| Upper extremity swelling | 71% (54) |
| Presence of clot elsewhere (eg, pulmonary embolism) | 9% (7) |
| Inability to place central venous access | 8% (6) |
| Assessment of clot propagation (known clot) | 8% (6) |
| Pain | 3% (2) |
| Patient‐reported history | 1% (1) |
Of the 50 patients diagnosed with UEDVT during hospitalization, 44% (22) were found to have UEDVTs directly associated with a CVC. Forty‐two of the 50 patients had a CVC; 52% (22 of 42) had CVC‐associated UEDVTs. Fifty percent (11) of these CVCs were triple lumen catheters, 32% (7) were PICCs, and 18% (4) were tunneled dialysis lines. Three of 42 patients with CVCs and line‐associated clots were had a malignancy. For patients with CVC‐associated clot, lines were in place for an average of 14.3 days (range, 273 days) prior to UEDVT diagnosis.
Treatment and Management
Seventy‐eight percent (39) of patients with UEDVT received in‐hospital treatment with heparin/LMWH bridging and oral anticoagulation. Of the 45 patients who survived hospitalization, 75% (34) were prescribed anticoagulation for 3+ months at discharge; 23% (10) had documented contraindications to anticoagulation, most commonly recent gastrointestinal or intracranial bleeding. Two percent of patients (1) was not prescribed pharmacologic treatment at discharge and had no contraindications documented. No patients underwent thrombolysis or had superior vena cava filters placed. Sixty‐four percent (14 of 22) of CVCs that were thought to be directly associated with UEDVT were removed at diagnosis.
Outcomes
Five patients (10%) died during hospitalization, none because of VTE or complications thereof. Cause of death included septic shock, cancer, intracranial hemorrhage, heart failure, and recurrent gastrointestinal bleeding. Of the 45 surviving patients, only 38% (17) were discharged to self‐care; more than half (62%[28]) were discharged to skilled nursing facilities, other hospitals, or rehabilitation centers. Eight patients (18%) were readmitted to our institution within 30 days; none for recurrent or new DVT or PE. No additional patients died at our medical center within 30 days of discharge.
DISCUSSION
UEDVT is increasingly recognized in hospitalized patients.[3, 9] At our medical center, 0.2% of symptomatic inpatients were diagnosed with UEDVT over 14 months. These patients were predominantly men with high rates of CVCs, malignancy, VTE history, severe infection, and renal disease. Interestingly, although the literature suggests that some proportion of patients with UEDVT have anatomic abnormalities, such as Paget‐Schroetter syndrome,[15] none of the patients in our study were found to have these anomalies. In our review, hospitalized patients with UEDVT were critically ill, with a long LOS and high morbidity and mortality, suggesting that in addition to just being a complication of hospitalization,[1, 6] UEDVT may be a marker of severe illness.
In our institution, clinical presentation was consistent with what has been described with the majority of patients presenting with upper extremity swelling.[1, 3] The internal jugular veins were the most common anatomic UEDVT site, followed by brachial then axillary veins. In other series including both in‐ and outpatients, subclavian clots were most commonly diagnosed, reflecting in part higher rates of CVC association and CVC location in those studies.[3, 9] Concurrent DVT and PE rates were similar to those reported.[1, 3, 10]
Although many studies have focused on prevention of LEDVT and PE, few trials have specifically targeted UEDVT. Among our patients with UEDVTs that were not present on admission, VTE prophylaxis rates were considerably higher than what has been reported,[1, 6] suggesting that in these critically ill patients' prophylaxis may not prevent symptomatic UEDVT. It is unknown how many UEDVTs were prevented with prophylaxis, as only patients with symptomatic UEDVT were included. Adequacy of prophylaxis at outside hospitals for patients transferred in could not be assessed. Nonetheless, low numbers of UEDVT at a trauma referral center with many high‐risk patients raise the question of whether prophylaxis makes a difference. Additional study is needed to further define the role of chemoprophylaxis to prevent UEDVT in hospitalized patients.
In our inpatient group, 84% required CVCs; 44% of patients were thought to have CVC‐associated UEDVTs. Careful patient selection and attention to potentially modifiable risks, such as insertion site, catheter type, and tip position, may need further examination in this population.[3, 11, 16] Catheter duration was long; focus on removing CVCs when no longer necessary is important. Interestingly, almost 10% in our study underwent diagnostic ultrasound because a new CVC could not be successfully placed suggesting that UEDVT may develop in critically ill patients regardless of CVCs.
In our study, there were high rates of guideline‐recommended pharmacologic treatment; surprisingly the majority of CVCs with associated clot were removed. Guidelines currently support 3 months of anticoagulation for treatment of UEDVT[2, 13, 17]; evidence derives from observational trials or is largely extrapolated from LEDVT literature.[2, 13] Routine CVC removal is not specifically recommended for CVC‐associated UEDVT, particularly if lines remain functional and medically necessary; systemic anticoagulation should be provided.[13]
In our review, no hospitalized patients with UEDVT developed complications or were readmitted to our medical center within 30 days for clot progression, new PE, or post‐thrombotic syndrome, which is lower than rates reported over longer time periods.[2, 6, 10, 12] Ten percent died during hospitalization, all from their primary disease rather than from complications of VTE or VTE treatment, and no additional patients died at our institution within 30 days. Although these rates are lower than have been otherwise reported,[2, 10] the inpatient mortality rate is similar to a recent study that included inpatients; however, all patients who died in that study had cancer and CVCs.[3] In the latter study, 6.4% died within 30 days of discharge.
Limitations
There are several limitations to this study. It was conducted at a single academic referral center with a large and critically ill trauma and neurosciences population, thereby limiting generalizability. This study describes hospitalized patients at a tertiary care center who were diagnosed with UEDVT. For comparison, we obtained information regarding characteristics of hospitalization for all other inpatients during this time frame. Individuals may have had multiple hospitalizations during the study period, but because we were unable to identify information about individuals, direct statistical comparisons could not be made. However, in general, inpatients with UEDVT appeared to be sicker, with prolonged LOS and high in‐hospital mortality relative to other hospitalized patients.
Only symptomatic UEDVT events were captured, likely underestimating true UEDVT incidence. In addition, we defined UEDVTs as those diagnosed by Doppler ultrasound; therefore theoretically, UEDVTs that were more centrally located or diagnosed using another modality would not be represented here. However, in a prior internal review we found that all VTE events coded in billing data during this time period were identified using our operational definition.
In our study, VTE prophylaxis was administered in accordance with an institutional guideline. We did not have information regarding adequacy of prophylaxis at outside institutions for patients transferred in, and patients admitted through the emergency department likely were not on prophylaxis. Therefore, information about prophylaxis is limited to prophylaxis administered at our medical center for hospitalized patients who had UEDVTs not present on admission.
Information regarding CVC insertion date and CVC type for CVCs placed in our institution is accurate based on our internal reviews. Although we had reasonable capture of information about CVC placement at outside facilities, these data may be incomplete, thereby underestimating potential association of CVCs with UEDVTs identified in our hospitalized patients. Additionally, criteria used to assess association of a CVC with UEDVT may have led to underrepresentation of CVC‐associated UEDVT.
Management of UEDVT in this study was determined by the treating physicians, and patients were only followed for 30 days after discharge. Information about readmission or death within 30 days of discharge was limited to patient contact with our medical center only. Treatment at discharge was determined from the discharge summary. Therefore, compliance with treatment cannot be assessed. Although these factors may limit the nature of the conclusions, data reflect actual practice and experience in hospitalized patients with UEDVT and may be hypothesis generating.
CONCLUSIONS
Among hospitalized patients, UEDVT is increasingly recognized. In our medical center, hospitalized patients diagnosed with UEDVT were more likely to have CVCs, malignancy, renal disease, and severe infection. Many of these patients were transferred critically ill, had prolonged LOS, and had high in‐hospital mortality. Most developed UEDVT despite prophylaxis, and the majority of UEDVTs were treated even in the absence of concurrent LEDVT or PE. As we move toward an era of increasing accountability, with a focus on preventing hospital‐acquired conditions including VTE, additional research is needed to identify modifiable risks, explore opportunities for effective prevention, and optimize outcomes such as prevention of complications or readmissions, particularly in critically ill patients with UEDVT.
Acknowledgements
The authors would like to thank Ronald Pergamit and Kevin Middleton for their extraordinary creativity and expert programming.
- , , , . Upper‐extremity deep vein thrombosis: a prospective registry of 592 patients. Circulation. 2004;110(12):1605–1611.
- , , , et al. Clinical outcome of patients with upper‐extremity deep vein thrombosis: results from the RIETE Registry. Chest. 2008;133(1):143–148.
- , , . The risk factors and clinical outcomes of upper extremity deep vein thrombosis. Vasc Endovascular Surg. 2012;46(2):139–144.
- , , , et al. Upper extremity versus lower extremity deep venous thrombosis. Am J Surg. 1997;174(2):214–217.
- , . Upper‐extremity deep vein thrombosis. Circulation. 2002;106(14):1874–1880.
- , , , . Upper extremity deep vein thrombosis: a community‐based perspective. Am J Med. 2007;120(8):678–684.
- , , , et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947–954.e2.
- , , , , . Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. 2009;4(7):417–422.
- , , , , . Characterization and probability of upper extremity deep venous thrombosis. Ann Vasc Surg. 2004;18(5):552–557.
- , , , et al. Risk factors for mortality in patients with upper extremity and internal jugular deep venous thrombosis. J Vasc Surg. 2005;41(3):476–478.
- , , , et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138(4):803–810.
- , , , et al. The long term clinical course of acute deep vein thrombosis of the arm: prospective cohort study. BMJ. 2004;329(7464):484–485.
- , , , et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e419S–e494S.
- , , , , , . Introduction to the ninth edition: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):48S–52S.
- . Clinical practice. Deep‐vein thrombosis of the upper extremities. N Engl J Med. 2011;364(9):861–869.
- , , , et al. Diagnosis and management of upper extremity deep‐vein thrombosis in adults. Thromb Haemost. 2012;108(6):1097–1108.
- , , . Treatment of upper‐extremity deep vein thrombosis. J Thromb Haemost. 2011;9(10):1924–1930.
- , , , . Upper‐extremity deep vein thrombosis: a prospective registry of 592 patients. Circulation. 2004;110(12):1605–1611.
- , , , et al. Clinical outcome of patients with upper‐extremity deep vein thrombosis: results from the RIETE Registry. Chest. 2008;133(1):143–148.
- , , . The risk factors and clinical outcomes of upper extremity deep vein thrombosis. Vasc Endovascular Surg. 2012;46(2):139–144.
- , , , et al. Upper extremity versus lower extremity deep venous thrombosis. Am J Surg. 1997;174(2):214–217.
- , . Upper‐extremity deep vein thrombosis. Circulation. 2002;106(14):1874–1880.
- , , , . Upper extremity deep vein thrombosis: a community‐based perspective. Am J Med. 2007;120(8):678–684.
- , , , et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947–954.e2.
- , , , , . Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. 2009;4(7):417–422.
- , , , , . Characterization and probability of upper extremity deep venous thrombosis. Ann Vasc Surg. 2004;18(5):552–557.
- , , , et al. Risk factors for mortality in patients with upper extremity and internal jugular deep venous thrombosis. J Vasc Surg. 2005;41(3):476–478.
- , , , et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138(4):803–810.
- , , , et al. The long term clinical course of acute deep vein thrombosis of the arm: prospective cohort study. BMJ. 2004;329(7464):484–485.
- , , , et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e419S–e494S.
- , , , , , . Introduction to the ninth edition: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):48S–52S.
- . Clinical practice. Deep‐vein thrombosis of the upper extremities. N Engl J Med. 2011;364(9):861–869.
- , , , et al. Diagnosis and management of upper extremity deep‐vein thrombosis in adults. Thromb Haemost. 2012;108(6):1097–1108.
- , , . Treatment of upper‐extremity deep vein thrombosis. J Thromb Haemost. 2011;9(10):1924–1930.
Survey of Hospitalist Supervision
In 2003, the Accreditation Council for Graduate Medical Education (ACGME) announced the first in a series of guidelines related to the regulation and oversight of residency training.1 The initial iteration specifically focused on the total and consecutive numbers of duty hours worked by trainees. These limitations began a new era of shift work in internal medicine residency training. With decreases in housestaff admitting capacity, clinical work has frequently been offloaded to non‐teaching or attending‐only services, increasing the demand for hospitalists to fill the void in physician‐staffed care in the hospital.2, 3 Since the implementation of the 2003 ACGME guidelines and a growing focus on patient safety, there has been increased study of, and call for, oversight of trainees in medicine; among these was the 2008 Institute of Medicine report,4 calling for 24/7 attending‐level supervision. The updated ACGME requirements,5 effective July 1, 2011, mandate enhanced on‐site supervision of trainee physicians. These new regulations not only define varying levels of supervision for trainees, including direct supervision with the physical presence of a supervisor and the degree of availability of said supervisor, they also describe ensuring the quality of supervision provided.5 While continuous attending‐level supervision is not yet mandated, many residency programs look to their academic hospitalists to fill the supervisory void, particularly at night. However, what specific roles hospitalists play in the nighttime supervision of trainees or the impact of this supervision remains unclear. To date, no study has examined a broad sample of hospitalist programs in teaching hospitals and the types of resident oversight they provide. We aimed to describe the current state of academic hospitalists in the clinical supervision of housestaff, specifically during the overnight period, and hospitalist perceptions of how the new ACGME requirements would impact traineehospitalist interactions.
METHODS
The Housestaff Oversight Subcommittee, a working group of the Society of General Internal Medicine (SGIM) Academic Hospitalist Task Force, surveyed a sample of academic hospitalist program leaders to assess the current status of trainee supervision performed by hospitalists. Programs were considered academic if they were located in the primary hospital of a residency that participates in the National Resident Matching Program for Internal Medicine. To obtain a broad geographic spectrum of academic hospitalist programs, all programs, both university and community‐based, in 4 states and 2 metropolitan regions were sampled: Washington, Oregon, Texas, Maryland, and the Philadelphia and Chicago metropolitan areas. Hospitalist program leaders were identified by members of the Taskforce using individual program websites and by querying departmental leadership at eligible teaching hospitals. Respondents were contacted by e‐mail for participation. None of the authors of the manuscript were participants in the survey.
The survey was developed by consensus of the working group after reviewing the salient literature and included additional questions queried to internal medicine program directors.6 The 19‐item SurveyMonkey instrument included questions about hospitalists' role in trainees' education and evaluation. A Likert‐type scale was used to assess perceptions regarding the impact of on‐site hospitalist supervision on trainee autonomy and hospitalist workload (1 = strongly disagree to 5 = strongly agree). Descriptive statistics were performed and, where appropriate, t test and Fisher's exact test were performed to identify associations between program characteristics and perceptions. Stata SE was used (STATA Corp, College Station, TX) for all statistical analysis.
RESULTS
The survey was sent to 47 individuals identified as likely hospitalist program leaders and completed by 41 individuals (87%). However, 7 respondents turned out not to be program leaders and were therefore excluded, resulting in a 72% (34/47) survey response rate.
The programs for which we did not obtain responses were similar to respondent programs, and did not include a larger proportion of community‐based programs or overrepresent a specific geographic region. Twenty‐five (73%) of the 34 hospitalist program leaders were male, with an average age of 44.3 years, and an average of 12 years post‐residency training (range, 530 years). They reported leading groups with an average of 18 full‐time equivalent (FTE) faculty (range, 350 persons).
Relationship of Hospitalist Programs With the Residency Program
The majority (32/34, 94%) of respondents describe their program as having traditional housestaffhospitalist interactions on an attending‐covered housestaff teaching service. Other hospitalists' clinical roles included: attending on uncovered (non‐housestaff services; 29/34, 85%); nighttime coverage (24/34, 70%); attending on consult services with housestaff (24/34, 70%). All respondents reported that hospitalist faculty are expected to participate in housestaff teaching or to fulfill other educational roles within the residency training program. These educational roles include participating in didactics or educational conferences, and serving as advisors. Additionally, the faculty of 30 (88%) programs have a formal evaluative role over the housestaff they supervise on teaching services (eg, members of formal housestaff evaluation committee). Finally, 28 (82%) programs have faculty who play administrative roles in the residency programs, such as involvement in program leadership or recruitment. Although 63% of the corresponding internal medicine residency programs have a formal housestaff supervision policy, only 43% of program leaders stated that their hospitalists receive formal faculty development on how to provide this supervision to resident trainees. Instead, the majority of hospitalist programs were described as having teaching expectations in the absence of a formal policy.
Twenty‐one programs (21/34, 61%) described having an attending hospitalist physician on‐site overnight to provide ongoing patient care or admit new patients. Of those with on‐site attending coverage, a minority of programs (8/21, 38%) reported having a formal defined supervisory role of housestaff trainees for hospitalists during the overnight period. In these 8 programs, this defined role included a requirement for housestaff to present newly admitted patients or contact hospitalists with questions regarding patient management. Twenty‐four percent (5/21) of the programs with nighttime coverage stated that the role of the nocturnal attending was only to cover the non‐teaching services, without housestaff interaction or supervision. The remainder of programs (8/21, 38%) describe only informal interactions between housestaff and hospitalist faculty, without clearly defined expectations for supervision.
Perceptions of New Regulations and Night Work
Hospitalist leaders viewed increased supervision of housestaff both positively and negatively. Leaders were asked their level of agreement with the potential impact of increased hospitalist nighttime supervision. Of respondents, 85% (27/32) agreed that formal overnight supervision by an attending hospitalist would improve patient safety, and 60% (20/33) agreed that formal overnight supervision would improve traineehospitalist relationships. In addition, 60% (20/33) of respondents felt that nighttime supervision of housestaff by faculty hospitalists would improve resident education. However, approximately 40% (13/33) expressed concern that increased on‐site hospitalist supervision would hamper resident decision‐making autonomy, and 75% (25/33) agreed that a formal housestaff supervisory role would increase hospitalist work load. The perception of increased workload was influenced by a hospitalist program's current supervisory role. Hospitalists programs providing formal nighttime supervision for housestaff, compared to those with informal or poorly defined faculty roles, were less likely to perceive these new regulations as resulting in an increase in hospitalist workload (3.72 vs 4.42; P = 0.02). In addition, hospitalist programs with a formal nighttime role were more likely to identify lack of specific parameters for attending‐level contact as a barrier to residents not contacting their supervisors during the overnight period (2.54 vs 3.54; P = 0.03). No differences in perception of the regulations were noted for those hospitalist programs which had existing faculty development on clinical supervision.
DISCUSSION
This study provides important information about how academic hospitalists currently contribute to the supervision of internal medicine residents. While academic hospitalist groups frequently have faculty providing clinical care on‐site at night, and often hospitalists provide overnight supervision of internal medicine trainees, formal supervision of trainees is not uniform, and few hospitalists groups have a mechanism to provide training or faculty development on how to effectively supervise resident trainees. Hospitalist leaders expressed concerns that creating additional formal overnight supervisory responsibilities may add to an already burdened overnight hospitalist. Formalizing this supervisory role, including explicit role definitions and faculty training for trainee supervision, is necessary.
Though our sample size is small, we captured a diverse geographic range of both university and community‐based academic hospitalist programs by surveying group leaders in several distinct regions. We are unable to comment on differences between responding and non‐responding hospitalist programs, but there does not appear to be a systematic difference between these groups.
Our findings are consistent with work describing a lack of structured conceptual frameworks in effectively supervising trainees,7, 8 and also, at times, nebulous expectations for hospitalist faculty. We found that the existence of a formal supervisory policy within the associated residency program, as well as defined roles for hospitalists, increases the likelihood of positive perceptions of the new ACGME supervisory recommendations. However, the existence of these requirements does not mean that all programs are capable of following them. While additional discussion is required to best delineate a formal overnight hospitalist role in trainee supervision, clearly defining expectations for both faculty and trainees, and their interactions, may alleviate the struggles that exist in programs with ill‐defined roles for hospitalist faculty supervision. While faculty duty hours standards do not exist, additional duties of nighttime coverage for hospitalists suggests that close attention should be paid to burn‐out.9 Faculty development on nighttime supervision and teaching may help maximize both learning and patient care efficiency, and provide a framework for this often unstructured educational time.
Acknowledgements
The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service (REA 05‐129, CDA 07‐022). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
- ,,.New requirements for resident duty hours.JAMA.2002;288:1112–1114.
- ,,,,.Cost implications of reduced work hours and workloads for resident physicians.N Engl J Med.2009;360:2202–2215.
- Why have working hour restrictions apparently not improved patient safety?BMJ.2011;342:d1200.
- Ulmer C, Wolman DM, Johns MME, eds.Resident Duty Hours: Enhancing Sleep, Supervision, and Safety.Washington, DC:National Academies Press;2008.
- ,,;for the ACGME Duty Hour Task Force.The new recommendations on duty hours from the ACGME Task Force.N Engl J Med.2010;363.
- Association of Program Directors in Internal Medicine (APDIM) Survey 2009. Available at: http://www.im.org/toolbox/surveys/SurveyDataand Reports/APDIMSurveyData/Documents/2009_APDIM_summary_web. pdf. Accessed on July 30, 2012.
- ,,,,.Clinical oversight: conceptualizing the relationship between supervision and safety.J Gen Intern Med.2007;22(8):1080–1085.
- ,,, et al.Strategies for effective on‐call supervision for internal medicine residents: the SUPERB/SAFETY model.J Grad Med Educ.2010;2(1):46–52.
- ,,,,,.Career satisfaction and burn‐out in academic hospital medicine.Arch Intern Med.2011;171(8):782–785.
In 2003, the Accreditation Council for Graduate Medical Education (ACGME) announced the first in a series of guidelines related to the regulation and oversight of residency training.1 The initial iteration specifically focused on the total and consecutive numbers of duty hours worked by trainees. These limitations began a new era of shift work in internal medicine residency training. With decreases in housestaff admitting capacity, clinical work has frequently been offloaded to non‐teaching or attending‐only services, increasing the demand for hospitalists to fill the void in physician‐staffed care in the hospital.2, 3 Since the implementation of the 2003 ACGME guidelines and a growing focus on patient safety, there has been increased study of, and call for, oversight of trainees in medicine; among these was the 2008 Institute of Medicine report,4 calling for 24/7 attending‐level supervision. The updated ACGME requirements,5 effective July 1, 2011, mandate enhanced on‐site supervision of trainee physicians. These new regulations not only define varying levels of supervision for trainees, including direct supervision with the physical presence of a supervisor and the degree of availability of said supervisor, they also describe ensuring the quality of supervision provided.5 While continuous attending‐level supervision is not yet mandated, many residency programs look to their academic hospitalists to fill the supervisory void, particularly at night. However, what specific roles hospitalists play in the nighttime supervision of trainees or the impact of this supervision remains unclear. To date, no study has examined a broad sample of hospitalist programs in teaching hospitals and the types of resident oversight they provide. We aimed to describe the current state of academic hospitalists in the clinical supervision of housestaff, specifically during the overnight period, and hospitalist perceptions of how the new ACGME requirements would impact traineehospitalist interactions.
METHODS
The Housestaff Oversight Subcommittee, a working group of the Society of General Internal Medicine (SGIM) Academic Hospitalist Task Force, surveyed a sample of academic hospitalist program leaders to assess the current status of trainee supervision performed by hospitalists. Programs were considered academic if they were located in the primary hospital of a residency that participates in the National Resident Matching Program for Internal Medicine. To obtain a broad geographic spectrum of academic hospitalist programs, all programs, both university and community‐based, in 4 states and 2 metropolitan regions were sampled: Washington, Oregon, Texas, Maryland, and the Philadelphia and Chicago metropolitan areas. Hospitalist program leaders were identified by members of the Taskforce using individual program websites and by querying departmental leadership at eligible teaching hospitals. Respondents were contacted by e‐mail for participation. None of the authors of the manuscript were participants in the survey.
The survey was developed by consensus of the working group after reviewing the salient literature and included additional questions queried to internal medicine program directors.6 The 19‐item SurveyMonkey instrument included questions about hospitalists' role in trainees' education and evaluation. A Likert‐type scale was used to assess perceptions regarding the impact of on‐site hospitalist supervision on trainee autonomy and hospitalist workload (1 = strongly disagree to 5 = strongly agree). Descriptive statistics were performed and, where appropriate, t test and Fisher's exact test were performed to identify associations between program characteristics and perceptions. Stata SE was used (STATA Corp, College Station, TX) for all statistical analysis.
RESULTS
The survey was sent to 47 individuals identified as likely hospitalist program leaders and completed by 41 individuals (87%). However, 7 respondents turned out not to be program leaders and were therefore excluded, resulting in a 72% (34/47) survey response rate.
The programs for which we did not obtain responses were similar to respondent programs, and did not include a larger proportion of community‐based programs or overrepresent a specific geographic region. Twenty‐five (73%) of the 34 hospitalist program leaders were male, with an average age of 44.3 years, and an average of 12 years post‐residency training (range, 530 years). They reported leading groups with an average of 18 full‐time equivalent (FTE) faculty (range, 350 persons).
Relationship of Hospitalist Programs With the Residency Program
The majority (32/34, 94%) of respondents describe their program as having traditional housestaffhospitalist interactions on an attending‐covered housestaff teaching service. Other hospitalists' clinical roles included: attending on uncovered (non‐housestaff services; 29/34, 85%); nighttime coverage (24/34, 70%); attending on consult services with housestaff (24/34, 70%). All respondents reported that hospitalist faculty are expected to participate in housestaff teaching or to fulfill other educational roles within the residency training program. These educational roles include participating in didactics or educational conferences, and serving as advisors. Additionally, the faculty of 30 (88%) programs have a formal evaluative role over the housestaff they supervise on teaching services (eg, members of formal housestaff evaluation committee). Finally, 28 (82%) programs have faculty who play administrative roles in the residency programs, such as involvement in program leadership or recruitment. Although 63% of the corresponding internal medicine residency programs have a formal housestaff supervision policy, only 43% of program leaders stated that their hospitalists receive formal faculty development on how to provide this supervision to resident trainees. Instead, the majority of hospitalist programs were described as having teaching expectations in the absence of a formal policy.
Twenty‐one programs (21/34, 61%) described having an attending hospitalist physician on‐site overnight to provide ongoing patient care or admit new patients. Of those with on‐site attending coverage, a minority of programs (8/21, 38%) reported having a formal defined supervisory role of housestaff trainees for hospitalists during the overnight period. In these 8 programs, this defined role included a requirement for housestaff to present newly admitted patients or contact hospitalists with questions regarding patient management. Twenty‐four percent (5/21) of the programs with nighttime coverage stated that the role of the nocturnal attending was only to cover the non‐teaching services, without housestaff interaction or supervision. The remainder of programs (8/21, 38%) describe only informal interactions between housestaff and hospitalist faculty, without clearly defined expectations for supervision.
Perceptions of New Regulations and Night Work
Hospitalist leaders viewed increased supervision of housestaff both positively and negatively. Leaders were asked their level of agreement with the potential impact of increased hospitalist nighttime supervision. Of respondents, 85% (27/32) agreed that formal overnight supervision by an attending hospitalist would improve patient safety, and 60% (20/33) agreed that formal overnight supervision would improve traineehospitalist relationships. In addition, 60% (20/33) of respondents felt that nighttime supervision of housestaff by faculty hospitalists would improve resident education. However, approximately 40% (13/33) expressed concern that increased on‐site hospitalist supervision would hamper resident decision‐making autonomy, and 75% (25/33) agreed that a formal housestaff supervisory role would increase hospitalist work load. The perception of increased workload was influenced by a hospitalist program's current supervisory role. Hospitalists programs providing formal nighttime supervision for housestaff, compared to those with informal or poorly defined faculty roles, were less likely to perceive these new regulations as resulting in an increase in hospitalist workload (3.72 vs 4.42; P = 0.02). In addition, hospitalist programs with a formal nighttime role were more likely to identify lack of specific parameters for attending‐level contact as a barrier to residents not contacting their supervisors during the overnight period (2.54 vs 3.54; P = 0.03). No differences in perception of the regulations were noted for those hospitalist programs which had existing faculty development on clinical supervision.
DISCUSSION
This study provides important information about how academic hospitalists currently contribute to the supervision of internal medicine residents. While academic hospitalist groups frequently have faculty providing clinical care on‐site at night, and often hospitalists provide overnight supervision of internal medicine trainees, formal supervision of trainees is not uniform, and few hospitalists groups have a mechanism to provide training or faculty development on how to effectively supervise resident trainees. Hospitalist leaders expressed concerns that creating additional formal overnight supervisory responsibilities may add to an already burdened overnight hospitalist. Formalizing this supervisory role, including explicit role definitions and faculty training for trainee supervision, is necessary.
Though our sample size is small, we captured a diverse geographic range of both university and community‐based academic hospitalist programs by surveying group leaders in several distinct regions. We are unable to comment on differences between responding and non‐responding hospitalist programs, but there does not appear to be a systematic difference between these groups.
Our findings are consistent with work describing a lack of structured conceptual frameworks in effectively supervising trainees,7, 8 and also, at times, nebulous expectations for hospitalist faculty. We found that the existence of a formal supervisory policy within the associated residency program, as well as defined roles for hospitalists, increases the likelihood of positive perceptions of the new ACGME supervisory recommendations. However, the existence of these requirements does not mean that all programs are capable of following them. While additional discussion is required to best delineate a formal overnight hospitalist role in trainee supervision, clearly defining expectations for both faculty and trainees, and their interactions, may alleviate the struggles that exist in programs with ill‐defined roles for hospitalist faculty supervision. While faculty duty hours standards do not exist, additional duties of nighttime coverage for hospitalists suggests that close attention should be paid to burn‐out.9 Faculty development on nighttime supervision and teaching may help maximize both learning and patient care efficiency, and provide a framework for this often unstructured educational time.
Acknowledgements
The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service (REA 05‐129, CDA 07‐022). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
In 2003, the Accreditation Council for Graduate Medical Education (ACGME) announced the first in a series of guidelines related to the regulation and oversight of residency training.1 The initial iteration specifically focused on the total and consecutive numbers of duty hours worked by trainees. These limitations began a new era of shift work in internal medicine residency training. With decreases in housestaff admitting capacity, clinical work has frequently been offloaded to non‐teaching or attending‐only services, increasing the demand for hospitalists to fill the void in physician‐staffed care in the hospital.2, 3 Since the implementation of the 2003 ACGME guidelines and a growing focus on patient safety, there has been increased study of, and call for, oversight of trainees in medicine; among these was the 2008 Institute of Medicine report,4 calling for 24/7 attending‐level supervision. The updated ACGME requirements,5 effective July 1, 2011, mandate enhanced on‐site supervision of trainee physicians. These new regulations not only define varying levels of supervision for trainees, including direct supervision with the physical presence of a supervisor and the degree of availability of said supervisor, they also describe ensuring the quality of supervision provided.5 While continuous attending‐level supervision is not yet mandated, many residency programs look to their academic hospitalists to fill the supervisory void, particularly at night. However, what specific roles hospitalists play in the nighttime supervision of trainees or the impact of this supervision remains unclear. To date, no study has examined a broad sample of hospitalist programs in teaching hospitals and the types of resident oversight they provide. We aimed to describe the current state of academic hospitalists in the clinical supervision of housestaff, specifically during the overnight period, and hospitalist perceptions of how the new ACGME requirements would impact traineehospitalist interactions.
METHODS
The Housestaff Oversight Subcommittee, a working group of the Society of General Internal Medicine (SGIM) Academic Hospitalist Task Force, surveyed a sample of academic hospitalist program leaders to assess the current status of trainee supervision performed by hospitalists. Programs were considered academic if they were located in the primary hospital of a residency that participates in the National Resident Matching Program for Internal Medicine. To obtain a broad geographic spectrum of academic hospitalist programs, all programs, both university and community‐based, in 4 states and 2 metropolitan regions were sampled: Washington, Oregon, Texas, Maryland, and the Philadelphia and Chicago metropolitan areas. Hospitalist program leaders were identified by members of the Taskforce using individual program websites and by querying departmental leadership at eligible teaching hospitals. Respondents were contacted by e‐mail for participation. None of the authors of the manuscript were participants in the survey.
The survey was developed by consensus of the working group after reviewing the salient literature and included additional questions queried to internal medicine program directors.6 The 19‐item SurveyMonkey instrument included questions about hospitalists' role in trainees' education and evaluation. A Likert‐type scale was used to assess perceptions regarding the impact of on‐site hospitalist supervision on trainee autonomy and hospitalist workload (1 = strongly disagree to 5 = strongly agree). Descriptive statistics were performed and, where appropriate, t test and Fisher's exact test were performed to identify associations between program characteristics and perceptions. Stata SE was used (STATA Corp, College Station, TX) for all statistical analysis.
RESULTS
The survey was sent to 47 individuals identified as likely hospitalist program leaders and completed by 41 individuals (87%). However, 7 respondents turned out not to be program leaders and were therefore excluded, resulting in a 72% (34/47) survey response rate.
The programs for which we did not obtain responses were similar to respondent programs, and did not include a larger proportion of community‐based programs or overrepresent a specific geographic region. Twenty‐five (73%) of the 34 hospitalist program leaders were male, with an average age of 44.3 years, and an average of 12 years post‐residency training (range, 530 years). They reported leading groups with an average of 18 full‐time equivalent (FTE) faculty (range, 350 persons).
Relationship of Hospitalist Programs With the Residency Program
The majority (32/34, 94%) of respondents describe their program as having traditional housestaffhospitalist interactions on an attending‐covered housestaff teaching service. Other hospitalists' clinical roles included: attending on uncovered (non‐housestaff services; 29/34, 85%); nighttime coverage (24/34, 70%); attending on consult services with housestaff (24/34, 70%). All respondents reported that hospitalist faculty are expected to participate in housestaff teaching or to fulfill other educational roles within the residency training program. These educational roles include participating in didactics or educational conferences, and serving as advisors. Additionally, the faculty of 30 (88%) programs have a formal evaluative role over the housestaff they supervise on teaching services (eg, members of formal housestaff evaluation committee). Finally, 28 (82%) programs have faculty who play administrative roles in the residency programs, such as involvement in program leadership or recruitment. Although 63% of the corresponding internal medicine residency programs have a formal housestaff supervision policy, only 43% of program leaders stated that their hospitalists receive formal faculty development on how to provide this supervision to resident trainees. Instead, the majority of hospitalist programs were described as having teaching expectations in the absence of a formal policy.
Twenty‐one programs (21/34, 61%) described having an attending hospitalist physician on‐site overnight to provide ongoing patient care or admit new patients. Of those with on‐site attending coverage, a minority of programs (8/21, 38%) reported having a formal defined supervisory role of housestaff trainees for hospitalists during the overnight period. In these 8 programs, this defined role included a requirement for housestaff to present newly admitted patients or contact hospitalists with questions regarding patient management. Twenty‐four percent (5/21) of the programs with nighttime coverage stated that the role of the nocturnal attending was only to cover the non‐teaching services, without housestaff interaction or supervision. The remainder of programs (8/21, 38%) describe only informal interactions between housestaff and hospitalist faculty, without clearly defined expectations for supervision.
Perceptions of New Regulations and Night Work
Hospitalist leaders viewed increased supervision of housestaff both positively and negatively. Leaders were asked their level of agreement with the potential impact of increased hospitalist nighttime supervision. Of respondents, 85% (27/32) agreed that formal overnight supervision by an attending hospitalist would improve patient safety, and 60% (20/33) agreed that formal overnight supervision would improve traineehospitalist relationships. In addition, 60% (20/33) of respondents felt that nighttime supervision of housestaff by faculty hospitalists would improve resident education. However, approximately 40% (13/33) expressed concern that increased on‐site hospitalist supervision would hamper resident decision‐making autonomy, and 75% (25/33) agreed that a formal housestaff supervisory role would increase hospitalist work load. The perception of increased workload was influenced by a hospitalist program's current supervisory role. Hospitalists programs providing formal nighttime supervision for housestaff, compared to those with informal or poorly defined faculty roles, were less likely to perceive these new regulations as resulting in an increase in hospitalist workload (3.72 vs 4.42; P = 0.02). In addition, hospitalist programs with a formal nighttime role were more likely to identify lack of specific parameters for attending‐level contact as a barrier to residents not contacting their supervisors during the overnight period (2.54 vs 3.54; P = 0.03). No differences in perception of the regulations were noted for those hospitalist programs which had existing faculty development on clinical supervision.
DISCUSSION
This study provides important information about how academic hospitalists currently contribute to the supervision of internal medicine residents. While academic hospitalist groups frequently have faculty providing clinical care on‐site at night, and often hospitalists provide overnight supervision of internal medicine trainees, formal supervision of trainees is not uniform, and few hospitalists groups have a mechanism to provide training or faculty development on how to effectively supervise resident trainees. Hospitalist leaders expressed concerns that creating additional formal overnight supervisory responsibilities may add to an already burdened overnight hospitalist. Formalizing this supervisory role, including explicit role definitions and faculty training for trainee supervision, is necessary.
Though our sample size is small, we captured a diverse geographic range of both university and community‐based academic hospitalist programs by surveying group leaders in several distinct regions. We are unable to comment on differences between responding and non‐responding hospitalist programs, but there does not appear to be a systematic difference between these groups.
Our findings are consistent with work describing a lack of structured conceptual frameworks in effectively supervising trainees,7, 8 and also, at times, nebulous expectations for hospitalist faculty. We found that the existence of a formal supervisory policy within the associated residency program, as well as defined roles for hospitalists, increases the likelihood of positive perceptions of the new ACGME supervisory recommendations. However, the existence of these requirements does not mean that all programs are capable of following them. While additional discussion is required to best delineate a formal overnight hospitalist role in trainee supervision, clearly defining expectations for both faculty and trainees, and their interactions, may alleviate the struggles that exist in programs with ill‐defined roles for hospitalist faculty supervision. While faculty duty hours standards do not exist, additional duties of nighttime coverage for hospitalists suggests that close attention should be paid to burn‐out.9 Faculty development on nighttime supervision and teaching may help maximize both learning and patient care efficiency, and provide a framework for this often unstructured educational time.
Acknowledgements
The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service (REA 05‐129, CDA 07‐022). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
- ,,.New requirements for resident duty hours.JAMA.2002;288:1112–1114.
- ,,,,.Cost implications of reduced work hours and workloads for resident physicians.N Engl J Med.2009;360:2202–2215.
- Why have working hour restrictions apparently not improved patient safety?BMJ.2011;342:d1200.
- Ulmer C, Wolman DM, Johns MME, eds.Resident Duty Hours: Enhancing Sleep, Supervision, and Safety.Washington, DC:National Academies Press;2008.
- ,,;for the ACGME Duty Hour Task Force.The new recommendations on duty hours from the ACGME Task Force.N Engl J Med.2010;363.
- Association of Program Directors in Internal Medicine (APDIM) Survey 2009. Available at: http://www.im.org/toolbox/surveys/SurveyDataand Reports/APDIMSurveyData/Documents/2009_APDIM_summary_web. pdf. Accessed on July 30, 2012.
- ,,,,.Clinical oversight: conceptualizing the relationship between supervision and safety.J Gen Intern Med.2007;22(8):1080–1085.
- ,,, et al.Strategies for effective on‐call supervision for internal medicine residents: the SUPERB/SAFETY model.J Grad Med Educ.2010;2(1):46–52.
- ,,,,,.Career satisfaction and burn‐out in academic hospital medicine.Arch Intern Med.2011;171(8):782–785.
- ,,.New requirements for resident duty hours.JAMA.2002;288:1112–1114.
- ,,,,.Cost implications of reduced work hours and workloads for resident physicians.N Engl J Med.2009;360:2202–2215.
- Why have working hour restrictions apparently not improved patient safety?BMJ.2011;342:d1200.
- Ulmer C, Wolman DM, Johns MME, eds.Resident Duty Hours: Enhancing Sleep, Supervision, and Safety.Washington, DC:National Academies Press;2008.
- ,,;for the ACGME Duty Hour Task Force.The new recommendations on duty hours from the ACGME Task Force.N Engl J Med.2010;363.
- Association of Program Directors in Internal Medicine (APDIM) Survey 2009. Available at: http://www.im.org/toolbox/surveys/SurveyDataand Reports/APDIMSurveyData/Documents/2009_APDIM_summary_web. pdf. Accessed on July 30, 2012.
- ,,,,.Clinical oversight: conceptualizing the relationship between supervision and safety.J Gen Intern Med.2007;22(8):1080–1085.
- ,,, et al.Strategies for effective on‐call supervision for internal medicine residents: the SUPERB/SAFETY model.J Grad Med Educ.2010;2(1):46–52.
- ,,,,,.Career satisfaction and burn‐out in academic hospital medicine.Arch Intern Med.2011;171(8):782–785.
Thromboembolism Prophylaxis in Liver Disease
Venous thromboembolism (VTE) is a major cause of morbidity and mortality in hospitalized patients.13 Major efforts are underway to increase appropriate VTE prophylaxis (VTEP)4 and adherence to VTEP guidelines are increasingly used as a quality of care measure. National 2008 VTEP guidelines suggest that all medical patients ill enough to require hospitalization, particularly those requiring admission to the Intensive Care Unit (ICU), have at least a moderate risk of developing VTE and prophylaxis is recommended.4 Hospitalized patients with end‐stage liver disease (ESLD), despite their coagulopathy, are known to be at risk for VTE48 and may be VTEP candidates.
Based on available literature, it is unknown whether pharmacologic VTEP should be utilized in acutely ill, hospitalized patients with ESLD, particularly in those admitted with variceal bleeding. These patients are at high risk for rebleeding, with the highest risk in the first 5 days.9 Early rebleeding, defined as recurrent bleeding within 6 weeks of initial bleed, declined from 47% in the 1980s to 13% by 2000 because of increased early endoscopic intervention and use of medications to prevent rebleeding.911 In multicenter cohort studies, D'Amico and De Franchis12 reported that 13% of patients with variceal bleeding had uncontrolled bleeding, rebleeding, or death within 5 days of admission while Bahmba et al.13 reported a 16% rate of rebleeding within 5 days. We are unaware of prior reports regarding the safety of VTEP in this high‐risk group of patients.
Objective
We sought to describe rebleeding in a series of 22 patients with ESLD admitted with variceal bleeding who received pharmacologic VTEP.
Methods
We identified all patients 18 years and older with upper gastrointestinal bleeding admitted to Harborview Medical Center, a 400‐bed urban county teaching hospital in Seattle, Washington, between January 1, 2003 and December 31, 2005 (Figure 1), just prior to medical center‐wide implementation of a VTEP guideline. Potential cases were identified using administrative data based on 8 discharge diagnoses (Supporting Information Appendix 1) and 10 procedure codes (Supporting Information Appendix 2).14 Inpatient pharmacy data indicating continuous octreotide infusion were used to refine the sample. At our institution, it is a standard of care to initiate octreotide in patients admitted with variceal bleeding. We excluded patients who did not have ESLD (defined as evidence of cirrhosis and associated complications including but not limited to ascites, encephalopathy, variceal bleeding, portal hypertension) documented in their problem list or past medical history and those with no variceal bleeding based on medical record review. We identified cases receiving pharmacologic VTEP, either subcutaneous unfractionated heparin (UFH) or low molecular weight heparin (LMWH), during hospitalization from pharmacy records.
We obtained demographic and clinical data from administrative billing systems, electronic and paper medical records, and inpatient pharmacy databases and verified transfusion data from the Puget Sound Blood Center. We abstracted esophagogastroduodenoscopy (EGD) findings indicating high risk of rebleeding including variceal grade and stigmata of recent bleeding such as red spots or wales.15, 16 Data were abstracted by the first 3 authors (AS, MS, KJ) and reviewed again by 2 authors (AS, KJ) blinded to the others' abstractions.
We calculated Model for ESLD (MELD) scores on admission. These scores correlate with 3 month mortality in ESLD.17 We tabulated 5 factors shown in some studies to predict bleeding including high International Normalized Ratio (INR) (>1.5), low hematocrit (<25%), low platelet count (<100,000 per microliter), active bleeding at EGD, and transfusion of four or more units of red cells within 24 hours of admission.1013
We defined rebleeding as a decrease in hematocrit of greater than 5 percentage points compared with postresuscitation hematocrit, transfusion of additional red cells more than 48 hours after initial resuscitation, repeat unscheduled EGD, or return to the ICU for therapies related to rebleeding.18 The University of Washington Human Subjects Board approved this study.
Results
Of 224 patients initially identified, 36 received pharmacologic VTEP. We excluded 14 who did not have ESLD (n = 1) or did not have a variceal bleed (n = 13). The remaining 22 patients form the sample described in Figure 1.
The median age of patients was 52 years (range 42‐85) and 77% were men (Table 1). Twenty‐one of 22 patients (95%) were initially admitted to the ICU; median length of stay was 8 days (range 4‐30). Median MELD score on admission was 15 (range 825). On EGD, the number of variceal columns ranged from 1 to 4; 17 patients (77%) had at least 3. A total of 15 patients (68%) had stigmata of recent bleeding and 16 (72%) underwent banding (range 16 bands). All patients had at least 1 bleeding risk factor (Table 1) of which the most common factors observed were initial transfusion of 4 or more units of red cells (50%, n = 11), INR > 1.5 (45%, n = 10), and hematocrit < 25% (45%, n = 10).
| Parameter | Range | Median Value/% | Interquartile Range | Mean | Standard Deviation |
|---|---|---|---|---|---|
| |||||
| Age (years) | 4285 | 52 | 4758 | 53 | 9 |
| Sex (men) | 17 | 77% | |||
| MELD scores | 825 | 14.5 | 1120 | 15 | 5 |
| Initial ICU admission | 21 | 95% | |||
| Hospital length of stay (days) | 430 | 8 | 9.9 | 6.7 | |
| Initial INR | 1.12.4 | 1.5 | 1.42.0 | 1.7 | 0.4 |
| Initial hematocrit (%) | 1444 | 26 | 2232 | 27 | 8 |
| Initial platelets (thousand/L) | 43494 | 131 | 83159 | 147 | 98 |
| EGD results | |||||
| Grade 1 | 3 | 14% | |||
| Grade 2 | 6 | 27% | |||
| Grade 3 | 12 | 55% | |||
| Grade 4 | 1 | 5% | |||
| Stigmata of recent bleeding | 15 | 68% | |||
| Number of risk factors for rebleeding* | |||||
| 0 | 0 | 0% | |||
| 1 | 9 | 41% | |||
| 2 | 7 | 32% | |||
| 3 | 5 | 23% | |||
| 4 | 1 | 4% | |||
| Initial transfusion red blood cells | |||||
| None | 2 | 9% | |||
| 13 units | 9 | 41% | |||
| 4+ units | 11 | 50% | |||
| Initial transfusion frozen plasma | |||||
| None | 10 | 45% | |||
| 14 units | 3 | 14% | |||
| 58 units | 6 | 27% | |||
| 9+ units | 4 | 18% | |||
| Initial transfusion platelets | |||||
| None | 13 | 59% | |||
| 14 units | 4 | 18% | |||
| 5+ units | 5 | 23% | |||
A total of 12 patients (55%) received 5000 units of UFH every 8 hours, 8 (36%) received 5000 units UFH every 12 hours, and 2 (9%) received LMWH. VTEP was initiated as early as day of admission and as late as day 19. Median VTEP start date was hospital day 4. Median duration of of VTEP was 5 days.
Only 1 patient (4.5%) rebled after VTEP initiation. The patient received UFH every 8 hours starting on hospital day 6, and rebleeding occurred on day 9. Repeat EGD showed ulcers at banding sites. The patient was restarted on VTEP on hospital day 13 without recurrence of rebleeding. This patient had a MELD score of 24, initial INR >2, hematocrit <25%, had grade 3 varices and stigmata of recent bleeding on EGD, and received 4 units of packed red cells. These values are similar to those of the cohort as a whole (Table 1). This patient also was diagnosed with DVT while receiving VTEP on hospital day 15. This patient's coagulopathy was in the setting of terminal illness; the patient expired on hospital day 25.
One additional patient rebled prior to VTEP initiation on day 3 with repeat EGD showing a bleeding varix. This patient was nevertheless started on VTEP 4 days after rebleeding. Despite use of VTEP, this patient was diagnosed with DVT on hospital day 9 (and may well have had the DVT at the time of VTEP initiation). The patient was transitioned to therapeutic dose heparin which was tolerated without recurrence of rebleeding.
There were no other confirmed cases of DVT in this series. One additional patient underwent angiogram that showed no pulmonary embolism; 2 other patients underwent lower extremity ultrasounds that were negative for DVT.
Discussion
At our medical center, only a few inpatients with ESLD admitted with variceal bleed received VTEP. These patients were seemingly at high risk for bleeding and rebleeding given high MELD scores, variceal bleeding, and presence of at least one clinical factor suggesting bleeding risk, and in several cases 3 or more such factors.13, 18 Despite this, only 1 patient rebled while receiving VTEP. We captured rebleeding rates only during the index hospitalization. We therefore may underestimate early rebleeding rates.1013 Nevertheless, our inpatient data included complete coverage of the earliest period after the index bleeds and the period during which patients were exposed to VTEP, which should be the time of highest rebleeding risk related to VTEP exposure. Interestingly the patient who rebled while on VTEP was also diagnosed with VTE while on VTEP. Two patients (9%) in our sample were diagnosed with VTE.
This case series is limited by its small sample size, retrospective nature, single center observation, and perhaps especially by possible selection bias. We were unable to specifically quantify rebleeding risk. Several authors have identified individual factors associated with rebleeding,1013 these were tabulated for patients in this case series (Table 1) and all patients had at least 1 of these factors. Concurrent infection and hepatic vein pressure gradient have been shown to predict rebleeding;9, 19 we were unable to identify these factors in our data.
There was considerable variability in this case series in timing of VTEP initiation relative to initial bleed. We were unable to characterize provider or patient characteristics that may have influenced the decision to initiate VTEP and timing. The sample size was also too small to comment upon factors associated with choice of UFH versus LMWH and any potential differences in rebleeding risk between the 2. We also did not look at outcomes postindex hospitalization so we can not comment on the extended risk of rebleeding with VTEP after discharge. However, the risk of rebleeding is highest within the first 96 hours13 and all patients in this series were hospitalized at least 4 days. Nonetheless, we captured all patients with ESLD and variceal bleeding exposed to VTEP at a large center over a three‐year period and found rebleeding rates less than what might be expected.
Conclusions
Our observations suggest that some inpatients with ESLD and variceal bleeding may tolerate pharmacologic VTEP. In this small group of patients, VTEP was associated with an unexpectedly low incidence of rebleeding. While this case series does not support broad use of VTEP in this population, the lower‐than‐expected rates of rebleeding suggest that further study of the safety and effectiveness of pharmacologic VTEP in inpatient populations with ESLD may be warranted, particularly given the recommendations of recent national VTE prophylaxis guidelines.4
- ,,, et al.Validation of a model to predict adverse outcomes in patients with pulmonary embolism.Eur Heart J.2006;27(4):476–481.
- .The epidemiology of venous thromboembolism.Circulation.2003;107(23 Suppl 1):I4–I8.
- ,,,,.The prevalence of risk factors for venous thromboembolism among hospital patients.Arch Intern Med.1992;152(8):1660–1664.
- ,,, et al.Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. 8th Edition.Chest.2008;133(6 Suppl):381S–453S.
- ,,, et al.Coagulopathy does not fully protect hospitalized cirrhosis patients from peripheral venous thromboembolism.Am J Gastroenterol.2006;101(7):1524–1528; quiz 680.
- ,,,.Coagulation disorders in liver disease.Semin Liver Dis.2002;22(1):83–96.
- ,,,,.Deep vein thrombosis and pulmonary embolism in cirrhosis patients.Dig Dis Sci.2008;53(11):3012–3017.
- ,,,,,.Risk of venous thromboembolism in patients with liver disease: a nationwide population‐based case‐control study.Am J Gastroenterol.2009;104(1):96–101.
- ,.Non‐invasive diagnosis of cirrhosis and the natural history of its complications.Best Pract Res Clin Gastroenterol.2007;21(1):3–18.
- ,,,et al.Improved patient survival after acute variceal bleeding: a multicenter, cohort study.Am J Gastroenterol.2003;98(3):653–659.
- ,,,,,.Improved survival after variceal bleeding in patients with cirrhosis over the past two decades.Hepatology.2004;40(3):652–659.
- ,.Upper digestive bleeding in cirrhosis. Post‐therapeutic outcome and prognostic indicators.Hepatology.2003;38(3):599–612.
- ,,,,,.Predictors of early re‐bleeding and mortality after acute variceal haemorrhage in patients with cirrhosis.Gut.2008;57(6):814–820.
- ,,,,,.Use of hospital administrative data to assess quality improvement initiatives.J Gen Intern Med.2007;22(Supplement).
- ,.UK guidelines on the management of variceal haemorrhage in cirrhotic patients.Gut.2000,year="2000"2000;46(90003):iii1–15.
- ,,,,,.Prognostic significance of the white nipple sign in variceal bleeding.Gastrointest Endosc.1991;37(1):51–55.
- ,,, et al.A model to predict survival in patients with end‐stage liver disease.Hepatology.2001;33(2):464–470.
- .Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension.J Hepatol.2005;43(1):167–176.
- ,,, et al.Antibiotic prophylaxis after endoscopic therapy prevents rebleeding in acute variceal hemorrhage: a randomized trial.Hepatology.2004;39(3):746–753.
Venous thromboembolism (VTE) is a major cause of morbidity and mortality in hospitalized patients.13 Major efforts are underway to increase appropriate VTE prophylaxis (VTEP)4 and adherence to VTEP guidelines are increasingly used as a quality of care measure. National 2008 VTEP guidelines suggest that all medical patients ill enough to require hospitalization, particularly those requiring admission to the Intensive Care Unit (ICU), have at least a moderate risk of developing VTE and prophylaxis is recommended.4 Hospitalized patients with end‐stage liver disease (ESLD), despite their coagulopathy, are known to be at risk for VTE48 and may be VTEP candidates.
Based on available literature, it is unknown whether pharmacologic VTEP should be utilized in acutely ill, hospitalized patients with ESLD, particularly in those admitted with variceal bleeding. These patients are at high risk for rebleeding, with the highest risk in the first 5 days.9 Early rebleeding, defined as recurrent bleeding within 6 weeks of initial bleed, declined from 47% in the 1980s to 13% by 2000 because of increased early endoscopic intervention and use of medications to prevent rebleeding.911 In multicenter cohort studies, D'Amico and De Franchis12 reported that 13% of patients with variceal bleeding had uncontrolled bleeding, rebleeding, or death within 5 days of admission while Bahmba et al.13 reported a 16% rate of rebleeding within 5 days. We are unaware of prior reports regarding the safety of VTEP in this high‐risk group of patients.
Objective
We sought to describe rebleeding in a series of 22 patients with ESLD admitted with variceal bleeding who received pharmacologic VTEP.
Methods
We identified all patients 18 years and older with upper gastrointestinal bleeding admitted to Harborview Medical Center, a 400‐bed urban county teaching hospital in Seattle, Washington, between January 1, 2003 and December 31, 2005 (Figure 1), just prior to medical center‐wide implementation of a VTEP guideline. Potential cases were identified using administrative data based on 8 discharge diagnoses (Supporting Information Appendix 1) and 10 procedure codes (Supporting Information Appendix 2).14 Inpatient pharmacy data indicating continuous octreotide infusion were used to refine the sample. At our institution, it is a standard of care to initiate octreotide in patients admitted with variceal bleeding. We excluded patients who did not have ESLD (defined as evidence of cirrhosis and associated complications including but not limited to ascites, encephalopathy, variceal bleeding, portal hypertension) documented in their problem list or past medical history and those with no variceal bleeding based on medical record review. We identified cases receiving pharmacologic VTEP, either subcutaneous unfractionated heparin (UFH) or low molecular weight heparin (LMWH), during hospitalization from pharmacy records.

We obtained demographic and clinical data from administrative billing systems, electronic and paper medical records, and inpatient pharmacy databases and verified transfusion data from the Puget Sound Blood Center. We abstracted esophagogastroduodenoscopy (EGD) findings indicating high risk of rebleeding including variceal grade and stigmata of recent bleeding such as red spots or wales.15, 16 Data were abstracted by the first 3 authors (AS, MS, KJ) and reviewed again by 2 authors (AS, KJ) blinded to the others' abstractions.
We calculated Model for ESLD (MELD) scores on admission. These scores correlate with 3 month mortality in ESLD.17 We tabulated 5 factors shown in some studies to predict bleeding including high International Normalized Ratio (INR) (>1.5), low hematocrit (<25%), low platelet count (<100,000 per microliter), active bleeding at EGD, and transfusion of four or more units of red cells within 24 hours of admission.1013
We defined rebleeding as a decrease in hematocrit of greater than 5 percentage points compared with postresuscitation hematocrit, transfusion of additional red cells more than 48 hours after initial resuscitation, repeat unscheduled EGD, or return to the ICU for therapies related to rebleeding.18 The University of Washington Human Subjects Board approved this study.
Results
Of 224 patients initially identified, 36 received pharmacologic VTEP. We excluded 14 who did not have ESLD (n = 1) or did not have a variceal bleed (n = 13). The remaining 22 patients form the sample described in Figure 1.
The median age of patients was 52 years (range 42‐85) and 77% were men (Table 1). Twenty‐one of 22 patients (95%) were initially admitted to the ICU; median length of stay was 8 days (range 4‐30). Median MELD score on admission was 15 (range 825). On EGD, the number of variceal columns ranged from 1 to 4; 17 patients (77%) had at least 3. A total of 15 patients (68%) had stigmata of recent bleeding and 16 (72%) underwent banding (range 16 bands). All patients had at least 1 bleeding risk factor (Table 1) of which the most common factors observed were initial transfusion of 4 or more units of red cells (50%, n = 11), INR > 1.5 (45%, n = 10), and hematocrit < 25% (45%, n = 10).
| Parameter | Range | Median Value/% | Interquartile Range | Mean | Standard Deviation |
|---|---|---|---|---|---|
| |||||
| Age (years) | 4285 | 52 | 4758 | 53 | 9 |
| Sex (men) | 17 | 77% | |||
| MELD scores | 825 | 14.5 | 1120 | 15 | 5 |
| Initial ICU admission | 21 | 95% | |||
| Hospital length of stay (days) | 430 | 8 | 9.9 | 6.7 | |
| Initial INR | 1.12.4 | 1.5 | 1.42.0 | 1.7 | 0.4 |
| Initial hematocrit (%) | 1444 | 26 | 2232 | 27 | 8 |
| Initial platelets (thousand/L) | 43494 | 131 | 83159 | 147 | 98 |
| EGD results | |||||
| Grade 1 | 3 | 14% | |||
| Grade 2 | 6 | 27% | |||
| Grade 3 | 12 | 55% | |||
| Grade 4 | 1 | 5% | |||
| Stigmata of recent bleeding | 15 | 68% | |||
| Number of risk factors for rebleeding* | |||||
| 0 | 0 | 0% | |||
| 1 | 9 | 41% | |||
| 2 | 7 | 32% | |||
| 3 | 5 | 23% | |||
| 4 | 1 | 4% | |||
| Initial transfusion red blood cells | |||||
| None | 2 | 9% | |||
| 13 units | 9 | 41% | |||
| 4+ units | 11 | 50% | |||
| Initial transfusion frozen plasma | |||||
| None | 10 | 45% | |||
| 14 units | 3 | 14% | |||
| 58 units | 6 | 27% | |||
| 9+ units | 4 | 18% | |||
| Initial transfusion platelets | |||||
| None | 13 | 59% | |||
| 14 units | 4 | 18% | |||
| 5+ units | 5 | 23% | |||
A total of 12 patients (55%) received 5000 units of UFH every 8 hours, 8 (36%) received 5000 units UFH every 12 hours, and 2 (9%) received LMWH. VTEP was initiated as early as day of admission and as late as day 19. Median VTEP start date was hospital day 4. Median duration of of VTEP was 5 days.
Only 1 patient (4.5%) rebled after VTEP initiation. The patient received UFH every 8 hours starting on hospital day 6, and rebleeding occurred on day 9. Repeat EGD showed ulcers at banding sites. The patient was restarted on VTEP on hospital day 13 without recurrence of rebleeding. This patient had a MELD score of 24, initial INR >2, hematocrit <25%, had grade 3 varices and stigmata of recent bleeding on EGD, and received 4 units of packed red cells. These values are similar to those of the cohort as a whole (Table 1). This patient also was diagnosed with DVT while receiving VTEP on hospital day 15. This patient's coagulopathy was in the setting of terminal illness; the patient expired on hospital day 25.
One additional patient rebled prior to VTEP initiation on day 3 with repeat EGD showing a bleeding varix. This patient was nevertheless started on VTEP 4 days after rebleeding. Despite use of VTEP, this patient was diagnosed with DVT on hospital day 9 (and may well have had the DVT at the time of VTEP initiation). The patient was transitioned to therapeutic dose heparin which was tolerated without recurrence of rebleeding.
There were no other confirmed cases of DVT in this series. One additional patient underwent angiogram that showed no pulmonary embolism; 2 other patients underwent lower extremity ultrasounds that were negative for DVT.
Discussion
At our medical center, only a few inpatients with ESLD admitted with variceal bleed received VTEP. These patients were seemingly at high risk for bleeding and rebleeding given high MELD scores, variceal bleeding, and presence of at least one clinical factor suggesting bleeding risk, and in several cases 3 or more such factors.13, 18 Despite this, only 1 patient rebled while receiving VTEP. We captured rebleeding rates only during the index hospitalization. We therefore may underestimate early rebleeding rates.1013 Nevertheless, our inpatient data included complete coverage of the earliest period after the index bleeds and the period during which patients were exposed to VTEP, which should be the time of highest rebleeding risk related to VTEP exposure. Interestingly the patient who rebled while on VTEP was also diagnosed with VTE while on VTEP. Two patients (9%) in our sample were diagnosed with VTE.
This case series is limited by its small sample size, retrospective nature, single center observation, and perhaps especially by possible selection bias. We were unable to specifically quantify rebleeding risk. Several authors have identified individual factors associated with rebleeding,1013 these were tabulated for patients in this case series (Table 1) and all patients had at least 1 of these factors. Concurrent infection and hepatic vein pressure gradient have been shown to predict rebleeding;9, 19 we were unable to identify these factors in our data.
There was considerable variability in this case series in timing of VTEP initiation relative to initial bleed. We were unable to characterize provider or patient characteristics that may have influenced the decision to initiate VTEP and timing. The sample size was also too small to comment upon factors associated with choice of UFH versus LMWH and any potential differences in rebleeding risk between the 2. We also did not look at outcomes postindex hospitalization so we can not comment on the extended risk of rebleeding with VTEP after discharge. However, the risk of rebleeding is highest within the first 96 hours13 and all patients in this series were hospitalized at least 4 days. Nonetheless, we captured all patients with ESLD and variceal bleeding exposed to VTEP at a large center over a three‐year period and found rebleeding rates less than what might be expected.
Conclusions
Our observations suggest that some inpatients with ESLD and variceal bleeding may tolerate pharmacologic VTEP. In this small group of patients, VTEP was associated with an unexpectedly low incidence of rebleeding. While this case series does not support broad use of VTEP in this population, the lower‐than‐expected rates of rebleeding suggest that further study of the safety and effectiveness of pharmacologic VTEP in inpatient populations with ESLD may be warranted, particularly given the recommendations of recent national VTE prophylaxis guidelines.4
Venous thromboembolism (VTE) is a major cause of morbidity and mortality in hospitalized patients.13 Major efforts are underway to increase appropriate VTE prophylaxis (VTEP)4 and adherence to VTEP guidelines are increasingly used as a quality of care measure. National 2008 VTEP guidelines suggest that all medical patients ill enough to require hospitalization, particularly those requiring admission to the Intensive Care Unit (ICU), have at least a moderate risk of developing VTE and prophylaxis is recommended.4 Hospitalized patients with end‐stage liver disease (ESLD), despite their coagulopathy, are known to be at risk for VTE48 and may be VTEP candidates.
Based on available literature, it is unknown whether pharmacologic VTEP should be utilized in acutely ill, hospitalized patients with ESLD, particularly in those admitted with variceal bleeding. These patients are at high risk for rebleeding, with the highest risk in the first 5 days.9 Early rebleeding, defined as recurrent bleeding within 6 weeks of initial bleed, declined from 47% in the 1980s to 13% by 2000 because of increased early endoscopic intervention and use of medications to prevent rebleeding.911 In multicenter cohort studies, D'Amico and De Franchis12 reported that 13% of patients with variceal bleeding had uncontrolled bleeding, rebleeding, or death within 5 days of admission while Bahmba et al.13 reported a 16% rate of rebleeding within 5 days. We are unaware of prior reports regarding the safety of VTEP in this high‐risk group of patients.
Objective
We sought to describe rebleeding in a series of 22 patients with ESLD admitted with variceal bleeding who received pharmacologic VTEP.
Methods
We identified all patients 18 years and older with upper gastrointestinal bleeding admitted to Harborview Medical Center, a 400‐bed urban county teaching hospital in Seattle, Washington, between January 1, 2003 and December 31, 2005 (Figure 1), just prior to medical center‐wide implementation of a VTEP guideline. Potential cases were identified using administrative data based on 8 discharge diagnoses (Supporting Information Appendix 1) and 10 procedure codes (Supporting Information Appendix 2).14 Inpatient pharmacy data indicating continuous octreotide infusion were used to refine the sample. At our institution, it is a standard of care to initiate octreotide in patients admitted with variceal bleeding. We excluded patients who did not have ESLD (defined as evidence of cirrhosis and associated complications including but not limited to ascites, encephalopathy, variceal bleeding, portal hypertension) documented in their problem list or past medical history and those with no variceal bleeding based on medical record review. We identified cases receiving pharmacologic VTEP, either subcutaneous unfractionated heparin (UFH) or low molecular weight heparin (LMWH), during hospitalization from pharmacy records.

We obtained demographic and clinical data from administrative billing systems, electronic and paper medical records, and inpatient pharmacy databases and verified transfusion data from the Puget Sound Blood Center. We abstracted esophagogastroduodenoscopy (EGD) findings indicating high risk of rebleeding including variceal grade and stigmata of recent bleeding such as red spots or wales.15, 16 Data were abstracted by the first 3 authors (AS, MS, KJ) and reviewed again by 2 authors (AS, KJ) blinded to the others' abstractions.
We calculated Model for ESLD (MELD) scores on admission. These scores correlate with 3 month mortality in ESLD.17 We tabulated 5 factors shown in some studies to predict bleeding including high International Normalized Ratio (INR) (>1.5), low hematocrit (<25%), low platelet count (<100,000 per microliter), active bleeding at EGD, and transfusion of four or more units of red cells within 24 hours of admission.1013
We defined rebleeding as a decrease in hematocrit of greater than 5 percentage points compared with postresuscitation hematocrit, transfusion of additional red cells more than 48 hours after initial resuscitation, repeat unscheduled EGD, or return to the ICU for therapies related to rebleeding.18 The University of Washington Human Subjects Board approved this study.
Results
Of 224 patients initially identified, 36 received pharmacologic VTEP. We excluded 14 who did not have ESLD (n = 1) or did not have a variceal bleed (n = 13). The remaining 22 patients form the sample described in Figure 1.
The median age of patients was 52 years (range 42‐85) and 77% were men (Table 1). Twenty‐one of 22 patients (95%) were initially admitted to the ICU; median length of stay was 8 days (range 4‐30). Median MELD score on admission was 15 (range 825). On EGD, the number of variceal columns ranged from 1 to 4; 17 patients (77%) had at least 3. A total of 15 patients (68%) had stigmata of recent bleeding and 16 (72%) underwent banding (range 16 bands). All patients had at least 1 bleeding risk factor (Table 1) of which the most common factors observed were initial transfusion of 4 or more units of red cells (50%, n = 11), INR > 1.5 (45%, n = 10), and hematocrit < 25% (45%, n = 10).
| Parameter | Range | Median Value/% | Interquartile Range | Mean | Standard Deviation |
|---|---|---|---|---|---|
| |||||
| Age (years) | 4285 | 52 | 4758 | 53 | 9 |
| Sex (men) | 17 | 77% | |||
| MELD scores | 825 | 14.5 | 1120 | 15 | 5 |
| Initial ICU admission | 21 | 95% | |||
| Hospital length of stay (days) | 430 | 8 | 9.9 | 6.7 | |
| Initial INR | 1.12.4 | 1.5 | 1.42.0 | 1.7 | 0.4 |
| Initial hematocrit (%) | 1444 | 26 | 2232 | 27 | 8 |
| Initial platelets (thousand/L) | 43494 | 131 | 83159 | 147 | 98 |
| EGD results | |||||
| Grade 1 | 3 | 14% | |||
| Grade 2 | 6 | 27% | |||
| Grade 3 | 12 | 55% | |||
| Grade 4 | 1 | 5% | |||
| Stigmata of recent bleeding | 15 | 68% | |||
| Number of risk factors for rebleeding* | |||||
| 0 | 0 | 0% | |||
| 1 | 9 | 41% | |||
| 2 | 7 | 32% | |||
| 3 | 5 | 23% | |||
| 4 | 1 | 4% | |||
| Initial transfusion red blood cells | |||||
| None | 2 | 9% | |||
| 13 units | 9 | 41% | |||
| 4+ units | 11 | 50% | |||
| Initial transfusion frozen plasma | |||||
| None | 10 | 45% | |||
| 14 units | 3 | 14% | |||
| 58 units | 6 | 27% | |||
| 9+ units | 4 | 18% | |||
| Initial transfusion platelets | |||||
| None | 13 | 59% | |||
| 14 units | 4 | 18% | |||
| 5+ units | 5 | 23% | |||
A total of 12 patients (55%) received 5000 units of UFH every 8 hours, 8 (36%) received 5000 units UFH every 12 hours, and 2 (9%) received LMWH. VTEP was initiated as early as day of admission and as late as day 19. Median VTEP start date was hospital day 4. Median duration of of VTEP was 5 days.
Only 1 patient (4.5%) rebled after VTEP initiation. The patient received UFH every 8 hours starting on hospital day 6, and rebleeding occurred on day 9. Repeat EGD showed ulcers at banding sites. The patient was restarted on VTEP on hospital day 13 without recurrence of rebleeding. This patient had a MELD score of 24, initial INR >2, hematocrit <25%, had grade 3 varices and stigmata of recent bleeding on EGD, and received 4 units of packed red cells. These values are similar to those of the cohort as a whole (Table 1). This patient also was diagnosed with DVT while receiving VTEP on hospital day 15. This patient's coagulopathy was in the setting of terminal illness; the patient expired on hospital day 25.
One additional patient rebled prior to VTEP initiation on day 3 with repeat EGD showing a bleeding varix. This patient was nevertheless started on VTEP 4 days after rebleeding. Despite use of VTEP, this patient was diagnosed with DVT on hospital day 9 (and may well have had the DVT at the time of VTEP initiation). The patient was transitioned to therapeutic dose heparin which was tolerated without recurrence of rebleeding.
There were no other confirmed cases of DVT in this series. One additional patient underwent angiogram that showed no pulmonary embolism; 2 other patients underwent lower extremity ultrasounds that were negative for DVT.
Discussion
At our medical center, only a few inpatients with ESLD admitted with variceal bleed received VTEP. These patients were seemingly at high risk for bleeding and rebleeding given high MELD scores, variceal bleeding, and presence of at least one clinical factor suggesting bleeding risk, and in several cases 3 or more such factors.13, 18 Despite this, only 1 patient rebled while receiving VTEP. We captured rebleeding rates only during the index hospitalization. We therefore may underestimate early rebleeding rates.1013 Nevertheless, our inpatient data included complete coverage of the earliest period after the index bleeds and the period during which patients were exposed to VTEP, which should be the time of highest rebleeding risk related to VTEP exposure. Interestingly the patient who rebled while on VTEP was also diagnosed with VTE while on VTEP. Two patients (9%) in our sample were diagnosed with VTE.
This case series is limited by its small sample size, retrospective nature, single center observation, and perhaps especially by possible selection bias. We were unable to specifically quantify rebleeding risk. Several authors have identified individual factors associated with rebleeding,1013 these were tabulated for patients in this case series (Table 1) and all patients had at least 1 of these factors. Concurrent infection and hepatic vein pressure gradient have been shown to predict rebleeding;9, 19 we were unable to identify these factors in our data.
There was considerable variability in this case series in timing of VTEP initiation relative to initial bleed. We were unable to characterize provider or patient characteristics that may have influenced the decision to initiate VTEP and timing. The sample size was also too small to comment upon factors associated with choice of UFH versus LMWH and any potential differences in rebleeding risk between the 2. We also did not look at outcomes postindex hospitalization so we can not comment on the extended risk of rebleeding with VTEP after discharge. However, the risk of rebleeding is highest within the first 96 hours13 and all patients in this series were hospitalized at least 4 days. Nonetheless, we captured all patients with ESLD and variceal bleeding exposed to VTEP at a large center over a three‐year period and found rebleeding rates less than what might be expected.
Conclusions
Our observations suggest that some inpatients with ESLD and variceal bleeding may tolerate pharmacologic VTEP. In this small group of patients, VTEP was associated with an unexpectedly low incidence of rebleeding. While this case series does not support broad use of VTEP in this population, the lower‐than‐expected rates of rebleeding suggest that further study of the safety and effectiveness of pharmacologic VTEP in inpatient populations with ESLD may be warranted, particularly given the recommendations of recent national VTE prophylaxis guidelines.4
- ,,, et al.Validation of a model to predict adverse outcomes in patients with pulmonary embolism.Eur Heart J.2006;27(4):476–481.
- .The epidemiology of venous thromboembolism.Circulation.2003;107(23 Suppl 1):I4–I8.
- ,,,,.The prevalence of risk factors for venous thromboembolism among hospital patients.Arch Intern Med.1992;152(8):1660–1664.
- ,,, et al.Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. 8th Edition.Chest.2008;133(6 Suppl):381S–453S.
- ,,, et al.Coagulopathy does not fully protect hospitalized cirrhosis patients from peripheral venous thromboembolism.Am J Gastroenterol.2006;101(7):1524–1528; quiz 680.
- ,,,.Coagulation disorders in liver disease.Semin Liver Dis.2002;22(1):83–96.
- ,,,,.Deep vein thrombosis and pulmonary embolism in cirrhosis patients.Dig Dis Sci.2008;53(11):3012–3017.
- ,,,,,.Risk of venous thromboembolism in patients with liver disease: a nationwide population‐based case‐control study.Am J Gastroenterol.2009;104(1):96–101.
- ,.Non‐invasive diagnosis of cirrhosis and the natural history of its complications.Best Pract Res Clin Gastroenterol.2007;21(1):3–18.
- ,,,et al.Improved patient survival after acute variceal bleeding: a multicenter, cohort study.Am J Gastroenterol.2003;98(3):653–659.
- ,,,,,.Improved survival after variceal bleeding in patients with cirrhosis over the past two decades.Hepatology.2004;40(3):652–659.
- ,.Upper digestive bleeding in cirrhosis. Post‐therapeutic outcome and prognostic indicators.Hepatology.2003;38(3):599–612.
- ,,,,,.Predictors of early re‐bleeding and mortality after acute variceal haemorrhage in patients with cirrhosis.Gut.2008;57(6):814–820.
- ,,,,,.Use of hospital administrative data to assess quality improvement initiatives.J Gen Intern Med.2007;22(Supplement).
- ,.UK guidelines on the management of variceal haemorrhage in cirrhotic patients.Gut.2000,year="2000"2000;46(90003):iii1–15.
- ,,,,,.Prognostic significance of the white nipple sign in variceal bleeding.Gastrointest Endosc.1991;37(1):51–55.
- ,,, et al.A model to predict survival in patients with end‐stage liver disease.Hepatology.2001;33(2):464–470.
- .Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension.J Hepatol.2005;43(1):167–176.
- ,,, et al.Antibiotic prophylaxis after endoscopic therapy prevents rebleeding in acute variceal hemorrhage: a randomized trial.Hepatology.2004;39(3):746–753.
- ,,, et al.Validation of a model to predict adverse outcomes in patients with pulmonary embolism.Eur Heart J.2006;27(4):476–481.
- .The epidemiology of venous thromboembolism.Circulation.2003;107(23 Suppl 1):I4–I8.
- ,,,,.The prevalence of risk factors for venous thromboembolism among hospital patients.Arch Intern Med.1992;152(8):1660–1664.
- ,,, et al.Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. 8th Edition.Chest.2008;133(6 Suppl):381S–453S.
- ,,, et al.Coagulopathy does not fully protect hospitalized cirrhosis patients from peripheral venous thromboembolism.Am J Gastroenterol.2006;101(7):1524–1528; quiz 680.
- ,,,.Coagulation disorders in liver disease.Semin Liver Dis.2002;22(1):83–96.
- ,,,,.Deep vein thrombosis and pulmonary embolism in cirrhosis patients.Dig Dis Sci.2008;53(11):3012–3017.
- ,,,,,.Risk of venous thromboembolism in patients with liver disease: a nationwide population‐based case‐control study.Am J Gastroenterol.2009;104(1):96–101.
- ,.Non‐invasive diagnosis of cirrhosis and the natural history of its complications.Best Pract Res Clin Gastroenterol.2007;21(1):3–18.
- ,,,et al.Improved patient survival after acute variceal bleeding: a multicenter, cohort study.Am J Gastroenterol.2003;98(3):653–659.
- ,,,,,.Improved survival after variceal bleeding in patients with cirrhosis over the past two decades.Hepatology.2004;40(3):652–659.
- ,.Upper digestive bleeding in cirrhosis. Post‐therapeutic outcome and prognostic indicators.Hepatology.2003;38(3):599–612.
- ,,,,,.Predictors of early re‐bleeding and mortality after acute variceal haemorrhage in patients with cirrhosis.Gut.2008;57(6):814–820.
- ,,,,,.Use of hospital administrative data to assess quality improvement initiatives.J Gen Intern Med.2007;22(Supplement).
- ,.UK guidelines on the management of variceal haemorrhage in cirrhotic patients.Gut.2000,year="2000"2000;46(90003):iii1–15.
- ,,,,,.Prognostic significance of the white nipple sign in variceal bleeding.Gastrointest Endosc.1991;37(1):51–55.
- ,,, et al.A model to predict survival in patients with end‐stage liver disease.Hepatology.2001;33(2):464–470.
- .Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension.J Hepatol.2005;43(1):167–176.
- ,,, et al.Antibiotic prophylaxis after endoscopic therapy prevents rebleeding in acute variceal hemorrhage: a randomized trial.Hepatology.2004;39(3):746–753.
In the Literature
Literature at a Glance
- Postoperative prophylactic LMWH should be considered for arthroscopic knee surgery patients.
- Individualized therapy is required for DVT prophylaxis in the neurosurgical patient.
- SMART-COP is a reasonable screening tool for ICU admission.
- Mediterranean and low-carb diets are safe and effective alternatives to low-fat diets.
- Admissions for ACS in both smokers and non-smokers decrease after implementation of smoking ban.
- Beta-blockers should be continued whenever possible in patients hospitalized for heart failure with LVSD.
- Non-invasive ventilation improved dyspnea, but did not improve short-term mortality rates in patients with acute cardiogenic pulmonary edema.
- COX-2 inhibitors should be used with caution in patients with increased cerebrovascular disease risk.
LMWH after Arthroscopic Knee Surgery May Prevent VTE Compared to Graduated Compression Stockings
Clinical question: Does low molecular weight heparin (LMWH) prevent venous thromboembolism (VTE) compared to compression stockings without increasing bleeding complications in arthroscopic knee surgery?
Background: Knee arthroscopy is a common orthopedic surgery and postoperative venous thromboprophylaxis is not routinely recommended.
Study design: Randomized, controlled trial with blinding of the investigators.
Setting: Single orthopedic clinic in Italy, with followup at a university hospital.
Synopsis: 1,761 consecutive patients undergoing knee arthroscopy were randomly assigned to full-length graduated compression stockings (CS) for seven days postoperatively, subcutaneous LMWH (nadoparin 3800 units daily) for seven or 14 days postoperatively. The primary outcome of asymptomatic proximal deep venous thrombosis (DVT), symptomatic VTE, and all-cause mortality within three months of surgery was higher with CS (3.2%) than with LMWH for seven or 14 days (0.9% in each group) (P=0.005). There was no significant difference in bleeding events between groups.
The study was underpowered to detect differences in bleeding risk. Furthermore, almost half the events making up the primary outcome were distal DVTs of uncertain clinical significance. Notably, the 14-day LMWH group was discontinued early because of unspecified safety concerns related to longer exposure to LMWH.
Bottom line: Postoperative prophylactic LMWH for seven days may prevent some thromboses after knee surgery and should be considered in higher-risk patients.
Citation: Camporese G, Bernardi E, Prandoni P, et al. Low-molecular-weight heparin versus compression stockings for thrombophylaxis after knee arthroscopy. Ann Intern Med. 2008;14(9):73-82.
Heparins and Compression Devices are Effective in Preventing VTE in a Mixed Neurosurgical Population
Clinical question: What is the efficacy and safety of LMWH, unfractionated heparin, and mechanical devices in preventing VTE in neurosurgical patients?
Background: Neurosurgical patients are at high risk for VTE, but concerns remain regarding the risk of bleeding complications with the use of LMWH or unfractionated heparin (UFH).
Study design: Meta-analysis of 18 randomized trials and 12 cohort studies.
Setting: Patients undergoing spinal surgery or craniotomy.
Synopsis: Among all patients, the pooled DVT rate was 15.5/100. Use of sequential compression devices (SCD) significantly reduced the risk of DVT compared with placebo (relative risk [RR] 0.41, 95% confidence interval [CI] 0.21-0.78). Subcutaneous LMWH was associated with a significantly reduced risk of DVT compared with CS (RR 0.60, 95% CI 0.44-0.81). No other head-to-head comparisons were associated with significant reductions in VTE risk. After adjusting for potential risk factors for DVT and study design, use of heparins or SCDs was associated with a lower risk of DVT. Intracranial hemorrhage (ICH), minor bleeding, major bleeding, or death was not statistically different between any of the groups, although, after adjustment, LMWH was associated with a slightly increased risk of ICH.
The quality of included studies varied considerably and inter-rater agreement on study quality was low, raising the possibility of study selection bias. Potential publication bias was not addressed. Bleeding complications were rare, so the estimates of risk may be imprecise.
Bottom line: Individualized therapy is required for DVT prophylaxis in the neurosurgical patient; SCDs reduce VTE risk and both pharmacologic and mechanical prophylaxis may be indicated in patients with increased VTE risk.
Citation: Collen JF, Jackson JL, Shorr AF, Moores LK. Prevention of venous thromboembolism in neurosurgery: A metaanalysis. Chest. 2008;13(4):237-249.
SMART-COP Predicts Need for ICU Care in CAP
Clinical question: Can a clinical tool predict the need for critical care in community acquired pneumonia (CAP)?
Background: Clinical tools predicting 30-day mortality in community acquired pneumonia (CAP) exist, but do not accurately identify who will require intensive care unit-level care, such as intensive respiratory or vasosuppressor support (IRVS).
Study design: Prospective multi-center observational study.
Setting: Six hospitals in Australia participating in the Australian Community Acquired Pneumonia Study (ACAPS).
Synopsis: Multivariate analysis of a dataset of 882 episodes of CAP identified eight factors that were associated with the need for IRVS, summarized by the mnemonic “SMART-COP” (Systolic blood pressure, Multilobar chest radiography involvement, low Albumin level, high Respiratory rate, Tachycardia, Confusion, poor Oxygenation, and low arterial pH). Assigning one point for five factors and two points for three factors (systolic blood pressure, poor oxygenation, and low arterial pH) a SMART-COP score >3 identified 92.3% (95% CI 84.8-96.9%) of patients who required IRVS, including 84% who did not initially require ICU care. Specificity was 62.3% (CI 58.8-65.7%). Test characteristics for predicting IRVS were superior to existing prediction rules (PSI and CURB-65).
Most patients were drawn from large, urban teaching hospitals in Australia, so the results may not be generalizable. The authors also presented a modification of SMART-COP, using pulse oximetry rather than blood gas results; this may be even more useful in the pre-hospital setting.
Bottom line: SMART-COP is a reasonable screening tool for predicting need for ICU-level care in patients admitted with CAP.
Citation: Charles PGP, Wolfe, R, Whitby, M, et. al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis. 2008;47(3):375-384.
Mediterranean and Low-Carbohydrate Diets are Effective for Weight Loss
Clinical question: Are dietary intervention with low-fat, Mediterranean or low-carbohydrate diets effective?
Background: Obesity is a growing, worldwide problem. Past trials comparing the effectiveness and safety of various dietary interventions have been limited by short follow up and high dropout rates.
Study design: Prospective randomized trial.
Setting: Employees of a research center in Israel.
Synopsis: 322 subjects (average BMI 31) were randomized to a low-fat/restricted-calorie, Mediterranean/ restricted-calorie, or a low-carbohydrate/non-restricted calorie diet. Diet adherence was 84.6% at two years and all groups lost significant amounts of weight. The Mediterranean and low-carbohydrate diets showed similar aver∆age weight loss of 4.4 kg and 4.7 kg, respectively. The low-fat diet group on average lost 2.9 kg. Diabetic patients had improved glycemic control and lower insulin levels with the Mediterranean diet. Subjects assigned to the low-carbohydrate diet had the greatest improvement in lipid profile (20% relative decrease of total cholesterol to HDL ratio).
The trial took place at a single site (a scientific research center in Israel) and included only 14% women, so its generalization is uncertain. The study was based on self-reported dietary intake and may be subject to reporting bias.
Bottom line: Mediterranean and low-carbohydrate diets are safe and effective alternatives to low-fat diets with favorable effects on glycemic control in diabetics and lipid metabolism, respectively.
Citation: Shai I, Schwarz-fuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. NEJM. 2008;359(3):229-241.
Admissions for Acute Coronary Syndrome Decreased after Implementation of Smoke-free Legislation
Clinical question: Is there a reduction in admissions for acute coronary syndrome (ACS) after enactment of smoke-free legislation?
Background: Multiple, small, retrospective studies have shown a decrease in ACS after implementation of smoke-free legislation.
Study design: Prospective observational multi-center cohort study.
Setting: Nine hospitals in Scotland.
Synopsis: Data was collected on all patients admitted with ACS 10 months before and after implementation of smoke-free legislation, which prohibited smoking in all enclosed public and work places in Scotland. After the smoking ban, the number of ACS admissions fell by 17% (95% CI 16-18) in Scotland as a whole, compared with a 4% reduction in England during the same period (England does not have similar smoke-free legislation). Among smokers, former smokers and non-smokers, the number of ACS admissions decreased by 14% (95% CI 12-16), 19% (95% CI 17-21), and 21% (95% CI 18-24), respectively. Among non-smokers, self-reported exposure to second-hand smoke decreased significantly; these reductions were confirmed by measured reductions in serum cotinine levels, even among those who never smoked.
Results were limited by the observational nature of the study, although the authors did attempt to carefully match comparison cohorts by season and geography. Also, secular trends other than legislation may have reduced prevalent smoking in Scotland during the study period.
Bottom line: Admissions for ACS for both smokers and non-smokers decreased after implementation of smoke-free legislation.
Citation: Pell JP, Haw S, Cobbe S, et al. Smoke-free legislation and hospitalizations for acute coronary syndrome. NEJM. 2008;359(5):482-491.
Continuation of Beta-blockers in Patients Hospitalized for Heart Failure Improves Mortality
Clinical question: Does the withdrawal or continuation of beta-blockers in patients hospitalized with decompensated heart failure have any effect on clinical outcomes?
Background: Previous clinical trials have demonstrated mortality benefit with the use of beta-blockers in patients with symptomatic chronic heart failure and left ventricular systolic dysfunction (LVSD), however, controversy exists whether to continue these medications in acute decompensated heart failure.
Study design: Prospective cohort analysis from the OPTIMIZE-HF registry (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure).
Setting: 91 academic and community hospitals in the United States.
Synopsis: Data was analyzed on 2,373 registry patients with documented LVSD (EF <40) eligible for beta-blocker therapy. During hospitalization, 1,350 patients were continued on beta-blockers, 79 had therapy withdrawn, 303 were not started, and 632 had beta-blockade initiated. Compared with no beta-blocker treatment, adjusted hazard ratio (HR) for death at 60 and 90 days following discharge was lower in patients who were continued on beta-blockade (HR 0.60, 95% CI 0.37–0.99). Compared with continuation of beta-blockade, withdrawal of beta-blockade increased the risk of death (HR 2.3, 95% CI 1.2–4.6).
Results were limited by the observational nature of the study and short follow up. The reason for discontinuation or not starting beta-blockade was not captured in the database, so it is possible sicker patients had beta-blockers discontinued during hospitalization (although the authors attempted to control for this).
Bottom line: Beta-blockers should be continued whenever possible in patients hospitalized for heart failure with LVSD.
Citation: Fonarow GC, Abraham WT, Albert NM, et al. Influence of beta-blocker continuation or withdrawal on outcomes in patients hospitalized with heart failure. J Am Coll Cardiol. 2008;52(3):190-199.
Non-invasive Ventilation Does Not Improve Short-term Mortality in Acute Cardiogenic Pulmonary Edema
Clinical question: Does non-invasive ventilation reduce mortality in patients with acute cardiogenic pulmonary edema and are there differences in outcome between use of continuous positive airway pressure (CPAP) or non-invasive positive pressure ventilation (NIPPV)?
Background: In patients with acute cardiogenic pulmonary edema, noninvasive ventilation improves physiologic variables and symptoms, decreases rates of invasive ventilation, and may improve mortality.
Study design: Randomized multi center controlled trial.
Setting: 26 district and regional hospitals in the United Kingdom.
Synopsis: 1,156 patients admitted with acute cardiogenic pulmonary edema between July 2003 and April 2007 were randomized to standard oxygen therapy, versus CPAP or NIPPV. There were no significant differences in seven- or 30-day mortality rates between the standard oxygen therapy versus noninvasive ventilation. Mortality at seven days was 9.8% in the standard oxygen group versus 9.5% in the noninvasive ventilation group (P=0.87); 30-day mortality was 16% in the standard oxygen group and 15% in the non-invasive ventilation group (P=0.64). There were no major differences in treatment outcome with NIPPV compared to CPAP.
Although mortality was not decreased, non-invasive ventilation did improve dyspnea and tachycardia within one hour of therapy.
Bottom line: In patients admitted with acute cardiogenic pulmonary edema, noninvasive ventilation improved dyspnea and some physiological parameters, but did not improve short-term mortality rates.
Citation: Gray A, Goodacre S, Newby D, Masson M, Sampson F, Nicholl J. Noninvasive ventilation in acute cardiogenic pulmonary edema. NEJM. 2008;359(2):142-151.
Cyclooxygenase 2 Inhibitors May Increase the Risk of Ischemic Stroke
Clinical question: Do NSAIDs and COX-2 inhibitors increase the risk of ischemic or hemorrhagic stroke?
Background: Selected cyclooxygenase 2 (COX-2) inhibitors have been shown to increase cardiovascular morbidity in a dose-dependent manner and are now used with caution in patients at risk for cardiovascular disease. Little is known about the safety of these medications and non-aspirin, non-steroidal anti-inflammatory drugs (NSAIDS) in those at risk for cerebrovascular disease.
Study design: Retrospective observational cohort study.
Setting: Tennessee Medicaid Program enrollees.
Synopsis: Data was collected from the medical records of 336,906 subjects. Non-users had a baseline stroke rate of 4.51 strokes/1000 person-years. The rate increased to 5.15/1,000 person-years and 5.95/1,000 person-years for rofecoxib and valdecoxib, respectively. Celecoxib and other NSAIDs did not significantly increase the risk of stroke. Analysis of new users of rofexocib and valdecoxib yielded a similarly increased risk of stroke. Most strokes were ischemic.
Limitations include the ready availability of NSAIDs raising the possibility that some patients classified as non-users were actually users of NSAIDs. Other potential confounders may not have been measured and, therefore, not available for analysis.
Bottom line: COX-2 inhibitors should be used with caution in patients with increased cerebrovascular disease risk.
Citation: Roumie CL, Mitchel EF, Kaltenback L, Arbogast PG, Gideon P Griffen MR. Nonaspirin NSAIDs, cyclooxygenase 2 inhibitors, and the risk for stroke. Stroke. 2008;39:1037-2045.
Literature at a Glance
- Postoperative prophylactic LMWH should be considered for arthroscopic knee surgery patients.
- Individualized therapy is required for DVT prophylaxis in the neurosurgical patient.
- SMART-COP is a reasonable screening tool for ICU admission.
- Mediterranean and low-carb diets are safe and effective alternatives to low-fat diets.
- Admissions for ACS in both smokers and non-smokers decrease after implementation of smoking ban.
- Beta-blockers should be continued whenever possible in patients hospitalized for heart failure with LVSD.
- Non-invasive ventilation improved dyspnea, but did not improve short-term mortality rates in patients with acute cardiogenic pulmonary edema.
- COX-2 inhibitors should be used with caution in patients with increased cerebrovascular disease risk.
LMWH after Arthroscopic Knee Surgery May Prevent VTE Compared to Graduated Compression Stockings
Clinical question: Does low molecular weight heparin (LMWH) prevent venous thromboembolism (VTE) compared to compression stockings without increasing bleeding complications in arthroscopic knee surgery?
Background: Knee arthroscopy is a common orthopedic surgery and postoperative venous thromboprophylaxis is not routinely recommended.
Study design: Randomized, controlled trial with blinding of the investigators.
Setting: Single orthopedic clinic in Italy, with followup at a university hospital.
Synopsis: 1,761 consecutive patients undergoing knee arthroscopy were randomly assigned to full-length graduated compression stockings (CS) for seven days postoperatively, subcutaneous LMWH (nadoparin 3800 units daily) for seven or 14 days postoperatively. The primary outcome of asymptomatic proximal deep venous thrombosis (DVT), symptomatic VTE, and all-cause mortality within three months of surgery was higher with CS (3.2%) than with LMWH for seven or 14 days (0.9% in each group) (P=0.005). There was no significant difference in bleeding events between groups.
The study was underpowered to detect differences in bleeding risk. Furthermore, almost half the events making up the primary outcome were distal DVTs of uncertain clinical significance. Notably, the 14-day LMWH group was discontinued early because of unspecified safety concerns related to longer exposure to LMWH.
Bottom line: Postoperative prophylactic LMWH for seven days may prevent some thromboses after knee surgery and should be considered in higher-risk patients.
Citation: Camporese G, Bernardi E, Prandoni P, et al. Low-molecular-weight heparin versus compression stockings for thrombophylaxis after knee arthroscopy. Ann Intern Med. 2008;14(9):73-82.
Heparins and Compression Devices are Effective in Preventing VTE in a Mixed Neurosurgical Population
Clinical question: What is the efficacy and safety of LMWH, unfractionated heparin, and mechanical devices in preventing VTE in neurosurgical patients?
Background: Neurosurgical patients are at high risk for VTE, but concerns remain regarding the risk of bleeding complications with the use of LMWH or unfractionated heparin (UFH).
Study design: Meta-analysis of 18 randomized trials and 12 cohort studies.
Setting: Patients undergoing spinal surgery or craniotomy.
Synopsis: Among all patients, the pooled DVT rate was 15.5/100. Use of sequential compression devices (SCD) significantly reduced the risk of DVT compared with placebo (relative risk [RR] 0.41, 95% confidence interval [CI] 0.21-0.78). Subcutaneous LMWH was associated with a significantly reduced risk of DVT compared with CS (RR 0.60, 95% CI 0.44-0.81). No other head-to-head comparisons were associated with significant reductions in VTE risk. After adjusting for potential risk factors for DVT and study design, use of heparins or SCDs was associated with a lower risk of DVT. Intracranial hemorrhage (ICH), minor bleeding, major bleeding, or death was not statistically different between any of the groups, although, after adjustment, LMWH was associated with a slightly increased risk of ICH.
The quality of included studies varied considerably and inter-rater agreement on study quality was low, raising the possibility of study selection bias. Potential publication bias was not addressed. Bleeding complications were rare, so the estimates of risk may be imprecise.
Bottom line: Individualized therapy is required for DVT prophylaxis in the neurosurgical patient; SCDs reduce VTE risk and both pharmacologic and mechanical prophylaxis may be indicated in patients with increased VTE risk.
Citation: Collen JF, Jackson JL, Shorr AF, Moores LK. Prevention of venous thromboembolism in neurosurgery: A metaanalysis. Chest. 2008;13(4):237-249.
SMART-COP Predicts Need for ICU Care in CAP
Clinical question: Can a clinical tool predict the need for critical care in community acquired pneumonia (CAP)?
Background: Clinical tools predicting 30-day mortality in community acquired pneumonia (CAP) exist, but do not accurately identify who will require intensive care unit-level care, such as intensive respiratory or vasosuppressor support (IRVS).
Study design: Prospective multi-center observational study.
Setting: Six hospitals in Australia participating in the Australian Community Acquired Pneumonia Study (ACAPS).
Synopsis: Multivariate analysis of a dataset of 882 episodes of CAP identified eight factors that were associated with the need for IRVS, summarized by the mnemonic “SMART-COP” (Systolic blood pressure, Multilobar chest radiography involvement, low Albumin level, high Respiratory rate, Tachycardia, Confusion, poor Oxygenation, and low arterial pH). Assigning one point for five factors and two points for three factors (systolic blood pressure, poor oxygenation, and low arterial pH) a SMART-COP score >3 identified 92.3% (95% CI 84.8-96.9%) of patients who required IRVS, including 84% who did not initially require ICU care. Specificity was 62.3% (CI 58.8-65.7%). Test characteristics for predicting IRVS were superior to existing prediction rules (PSI and CURB-65).
Most patients were drawn from large, urban teaching hospitals in Australia, so the results may not be generalizable. The authors also presented a modification of SMART-COP, using pulse oximetry rather than blood gas results; this may be even more useful in the pre-hospital setting.
Bottom line: SMART-COP is a reasonable screening tool for predicting need for ICU-level care in patients admitted with CAP.
Citation: Charles PGP, Wolfe, R, Whitby, M, et. al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis. 2008;47(3):375-384.
Mediterranean and Low-Carbohydrate Diets are Effective for Weight Loss
Clinical question: Are dietary intervention with low-fat, Mediterranean or low-carbohydrate diets effective?
Background: Obesity is a growing, worldwide problem. Past trials comparing the effectiveness and safety of various dietary interventions have been limited by short follow up and high dropout rates.
Study design: Prospective randomized trial.
Setting: Employees of a research center in Israel.
Synopsis: 322 subjects (average BMI 31) were randomized to a low-fat/restricted-calorie, Mediterranean/ restricted-calorie, or a low-carbohydrate/non-restricted calorie diet. Diet adherence was 84.6% at two years and all groups lost significant amounts of weight. The Mediterranean and low-carbohydrate diets showed similar aver∆age weight loss of 4.4 kg and 4.7 kg, respectively. The low-fat diet group on average lost 2.9 kg. Diabetic patients had improved glycemic control and lower insulin levels with the Mediterranean diet. Subjects assigned to the low-carbohydrate diet had the greatest improvement in lipid profile (20% relative decrease of total cholesterol to HDL ratio).
The trial took place at a single site (a scientific research center in Israel) and included only 14% women, so its generalization is uncertain. The study was based on self-reported dietary intake and may be subject to reporting bias.
Bottom line: Mediterranean and low-carbohydrate diets are safe and effective alternatives to low-fat diets with favorable effects on glycemic control in diabetics and lipid metabolism, respectively.
Citation: Shai I, Schwarz-fuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. NEJM. 2008;359(3):229-241.
Admissions for Acute Coronary Syndrome Decreased after Implementation of Smoke-free Legislation
Clinical question: Is there a reduction in admissions for acute coronary syndrome (ACS) after enactment of smoke-free legislation?
Background: Multiple, small, retrospective studies have shown a decrease in ACS after implementation of smoke-free legislation.
Study design: Prospective observational multi-center cohort study.
Setting: Nine hospitals in Scotland.
Synopsis: Data was collected on all patients admitted with ACS 10 months before and after implementation of smoke-free legislation, which prohibited smoking in all enclosed public and work places in Scotland. After the smoking ban, the number of ACS admissions fell by 17% (95% CI 16-18) in Scotland as a whole, compared with a 4% reduction in England during the same period (England does not have similar smoke-free legislation). Among smokers, former smokers and non-smokers, the number of ACS admissions decreased by 14% (95% CI 12-16), 19% (95% CI 17-21), and 21% (95% CI 18-24), respectively. Among non-smokers, self-reported exposure to second-hand smoke decreased significantly; these reductions were confirmed by measured reductions in serum cotinine levels, even among those who never smoked.
Results were limited by the observational nature of the study, although the authors did attempt to carefully match comparison cohorts by season and geography. Also, secular trends other than legislation may have reduced prevalent smoking in Scotland during the study period.
Bottom line: Admissions for ACS for both smokers and non-smokers decreased after implementation of smoke-free legislation.
Citation: Pell JP, Haw S, Cobbe S, et al. Smoke-free legislation and hospitalizations for acute coronary syndrome. NEJM. 2008;359(5):482-491.
Continuation of Beta-blockers in Patients Hospitalized for Heart Failure Improves Mortality
Clinical question: Does the withdrawal or continuation of beta-blockers in patients hospitalized with decompensated heart failure have any effect on clinical outcomes?
Background: Previous clinical trials have demonstrated mortality benefit with the use of beta-blockers in patients with symptomatic chronic heart failure and left ventricular systolic dysfunction (LVSD), however, controversy exists whether to continue these medications in acute decompensated heart failure.
Study design: Prospective cohort analysis from the OPTIMIZE-HF registry (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure).
Setting: 91 academic and community hospitals in the United States.
Synopsis: Data was analyzed on 2,373 registry patients with documented LVSD (EF <40) eligible for beta-blocker therapy. During hospitalization, 1,350 patients were continued on beta-blockers, 79 had therapy withdrawn, 303 were not started, and 632 had beta-blockade initiated. Compared with no beta-blocker treatment, adjusted hazard ratio (HR) for death at 60 and 90 days following discharge was lower in patients who were continued on beta-blockade (HR 0.60, 95% CI 0.37–0.99). Compared with continuation of beta-blockade, withdrawal of beta-blockade increased the risk of death (HR 2.3, 95% CI 1.2–4.6).
Results were limited by the observational nature of the study and short follow up. The reason for discontinuation or not starting beta-blockade was not captured in the database, so it is possible sicker patients had beta-blockers discontinued during hospitalization (although the authors attempted to control for this).
Bottom line: Beta-blockers should be continued whenever possible in patients hospitalized for heart failure with LVSD.
Citation: Fonarow GC, Abraham WT, Albert NM, et al. Influence of beta-blocker continuation or withdrawal on outcomes in patients hospitalized with heart failure. J Am Coll Cardiol. 2008;52(3):190-199.
Non-invasive Ventilation Does Not Improve Short-term Mortality in Acute Cardiogenic Pulmonary Edema
Clinical question: Does non-invasive ventilation reduce mortality in patients with acute cardiogenic pulmonary edema and are there differences in outcome between use of continuous positive airway pressure (CPAP) or non-invasive positive pressure ventilation (NIPPV)?
Background: In patients with acute cardiogenic pulmonary edema, noninvasive ventilation improves physiologic variables and symptoms, decreases rates of invasive ventilation, and may improve mortality.
Study design: Randomized multi center controlled trial.
Setting: 26 district and regional hospitals in the United Kingdom.
Synopsis: 1,156 patients admitted with acute cardiogenic pulmonary edema between July 2003 and April 2007 were randomized to standard oxygen therapy, versus CPAP or NIPPV. There were no significant differences in seven- or 30-day mortality rates between the standard oxygen therapy versus noninvasive ventilation. Mortality at seven days was 9.8% in the standard oxygen group versus 9.5% in the noninvasive ventilation group (P=0.87); 30-day mortality was 16% in the standard oxygen group and 15% in the non-invasive ventilation group (P=0.64). There were no major differences in treatment outcome with NIPPV compared to CPAP.
Although mortality was not decreased, non-invasive ventilation did improve dyspnea and tachycardia within one hour of therapy.
Bottom line: In patients admitted with acute cardiogenic pulmonary edema, noninvasive ventilation improved dyspnea and some physiological parameters, but did not improve short-term mortality rates.
Citation: Gray A, Goodacre S, Newby D, Masson M, Sampson F, Nicholl J. Noninvasive ventilation in acute cardiogenic pulmonary edema. NEJM. 2008;359(2):142-151.
Cyclooxygenase 2 Inhibitors May Increase the Risk of Ischemic Stroke
Clinical question: Do NSAIDs and COX-2 inhibitors increase the risk of ischemic or hemorrhagic stroke?
Background: Selected cyclooxygenase 2 (COX-2) inhibitors have been shown to increase cardiovascular morbidity in a dose-dependent manner and are now used with caution in patients at risk for cardiovascular disease. Little is known about the safety of these medications and non-aspirin, non-steroidal anti-inflammatory drugs (NSAIDS) in those at risk for cerebrovascular disease.
Study design: Retrospective observational cohort study.
Setting: Tennessee Medicaid Program enrollees.
Synopsis: Data was collected from the medical records of 336,906 subjects. Non-users had a baseline stroke rate of 4.51 strokes/1000 person-years. The rate increased to 5.15/1,000 person-years and 5.95/1,000 person-years for rofecoxib and valdecoxib, respectively. Celecoxib and other NSAIDs did not significantly increase the risk of stroke. Analysis of new users of rofexocib and valdecoxib yielded a similarly increased risk of stroke. Most strokes were ischemic.
Limitations include the ready availability of NSAIDs raising the possibility that some patients classified as non-users were actually users of NSAIDs. Other potential confounders may not have been measured and, therefore, not available for analysis.
Bottom line: COX-2 inhibitors should be used with caution in patients with increased cerebrovascular disease risk.
Citation: Roumie CL, Mitchel EF, Kaltenback L, Arbogast PG, Gideon P Griffen MR. Nonaspirin NSAIDs, cyclooxygenase 2 inhibitors, and the risk for stroke. Stroke. 2008;39:1037-2045.
Literature at a Glance
- Postoperative prophylactic LMWH should be considered for arthroscopic knee surgery patients.
- Individualized therapy is required for DVT prophylaxis in the neurosurgical patient.
- SMART-COP is a reasonable screening tool for ICU admission.
- Mediterranean and low-carb diets are safe and effective alternatives to low-fat diets.
- Admissions for ACS in both smokers and non-smokers decrease after implementation of smoking ban.
- Beta-blockers should be continued whenever possible in patients hospitalized for heart failure with LVSD.
- Non-invasive ventilation improved dyspnea, but did not improve short-term mortality rates in patients with acute cardiogenic pulmonary edema.
- COX-2 inhibitors should be used with caution in patients with increased cerebrovascular disease risk.
LMWH after Arthroscopic Knee Surgery May Prevent VTE Compared to Graduated Compression Stockings
Clinical question: Does low molecular weight heparin (LMWH) prevent venous thromboembolism (VTE) compared to compression stockings without increasing bleeding complications in arthroscopic knee surgery?
Background: Knee arthroscopy is a common orthopedic surgery and postoperative venous thromboprophylaxis is not routinely recommended.
Study design: Randomized, controlled trial with blinding of the investigators.
Setting: Single orthopedic clinic in Italy, with followup at a university hospital.
Synopsis: 1,761 consecutive patients undergoing knee arthroscopy were randomly assigned to full-length graduated compression stockings (CS) for seven days postoperatively, subcutaneous LMWH (nadoparin 3800 units daily) for seven or 14 days postoperatively. The primary outcome of asymptomatic proximal deep venous thrombosis (DVT), symptomatic VTE, and all-cause mortality within three months of surgery was higher with CS (3.2%) than with LMWH for seven or 14 days (0.9% in each group) (P=0.005). There was no significant difference in bleeding events between groups.
The study was underpowered to detect differences in bleeding risk. Furthermore, almost half the events making up the primary outcome were distal DVTs of uncertain clinical significance. Notably, the 14-day LMWH group was discontinued early because of unspecified safety concerns related to longer exposure to LMWH.
Bottom line: Postoperative prophylactic LMWH for seven days may prevent some thromboses after knee surgery and should be considered in higher-risk patients.
Citation: Camporese G, Bernardi E, Prandoni P, et al. Low-molecular-weight heparin versus compression stockings for thrombophylaxis after knee arthroscopy. Ann Intern Med. 2008;14(9):73-82.
Heparins and Compression Devices are Effective in Preventing VTE in a Mixed Neurosurgical Population
Clinical question: What is the efficacy and safety of LMWH, unfractionated heparin, and mechanical devices in preventing VTE in neurosurgical patients?
Background: Neurosurgical patients are at high risk for VTE, but concerns remain regarding the risk of bleeding complications with the use of LMWH or unfractionated heparin (UFH).
Study design: Meta-analysis of 18 randomized trials and 12 cohort studies.
Setting: Patients undergoing spinal surgery or craniotomy.
Synopsis: Among all patients, the pooled DVT rate was 15.5/100. Use of sequential compression devices (SCD) significantly reduced the risk of DVT compared with placebo (relative risk [RR] 0.41, 95% confidence interval [CI] 0.21-0.78). Subcutaneous LMWH was associated with a significantly reduced risk of DVT compared with CS (RR 0.60, 95% CI 0.44-0.81). No other head-to-head comparisons were associated with significant reductions in VTE risk. After adjusting for potential risk factors for DVT and study design, use of heparins or SCDs was associated with a lower risk of DVT. Intracranial hemorrhage (ICH), minor bleeding, major bleeding, or death was not statistically different between any of the groups, although, after adjustment, LMWH was associated with a slightly increased risk of ICH.
The quality of included studies varied considerably and inter-rater agreement on study quality was low, raising the possibility of study selection bias. Potential publication bias was not addressed. Bleeding complications were rare, so the estimates of risk may be imprecise.
Bottom line: Individualized therapy is required for DVT prophylaxis in the neurosurgical patient; SCDs reduce VTE risk and both pharmacologic and mechanical prophylaxis may be indicated in patients with increased VTE risk.
Citation: Collen JF, Jackson JL, Shorr AF, Moores LK. Prevention of venous thromboembolism in neurosurgery: A metaanalysis. Chest. 2008;13(4):237-249.
SMART-COP Predicts Need for ICU Care in CAP
Clinical question: Can a clinical tool predict the need for critical care in community acquired pneumonia (CAP)?
Background: Clinical tools predicting 30-day mortality in community acquired pneumonia (CAP) exist, but do not accurately identify who will require intensive care unit-level care, such as intensive respiratory or vasosuppressor support (IRVS).
Study design: Prospective multi-center observational study.
Setting: Six hospitals in Australia participating in the Australian Community Acquired Pneumonia Study (ACAPS).
Synopsis: Multivariate analysis of a dataset of 882 episodes of CAP identified eight factors that were associated with the need for IRVS, summarized by the mnemonic “SMART-COP” (Systolic blood pressure, Multilobar chest radiography involvement, low Albumin level, high Respiratory rate, Tachycardia, Confusion, poor Oxygenation, and low arterial pH). Assigning one point for five factors and two points for three factors (systolic blood pressure, poor oxygenation, and low arterial pH) a SMART-COP score >3 identified 92.3% (95% CI 84.8-96.9%) of patients who required IRVS, including 84% who did not initially require ICU care. Specificity was 62.3% (CI 58.8-65.7%). Test characteristics for predicting IRVS were superior to existing prediction rules (PSI and CURB-65).
Most patients were drawn from large, urban teaching hospitals in Australia, so the results may not be generalizable. The authors also presented a modification of SMART-COP, using pulse oximetry rather than blood gas results; this may be even more useful in the pre-hospital setting.
Bottom line: SMART-COP is a reasonable screening tool for predicting need for ICU-level care in patients admitted with CAP.
Citation: Charles PGP, Wolfe, R, Whitby, M, et. al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis. 2008;47(3):375-384.
Mediterranean and Low-Carbohydrate Diets are Effective for Weight Loss
Clinical question: Are dietary intervention with low-fat, Mediterranean or low-carbohydrate diets effective?
Background: Obesity is a growing, worldwide problem. Past trials comparing the effectiveness and safety of various dietary interventions have been limited by short follow up and high dropout rates.
Study design: Prospective randomized trial.
Setting: Employees of a research center in Israel.
Synopsis: 322 subjects (average BMI 31) were randomized to a low-fat/restricted-calorie, Mediterranean/ restricted-calorie, or a low-carbohydrate/non-restricted calorie diet. Diet adherence was 84.6% at two years and all groups lost significant amounts of weight. The Mediterranean and low-carbohydrate diets showed similar aver∆age weight loss of 4.4 kg and 4.7 kg, respectively. The low-fat diet group on average lost 2.9 kg. Diabetic patients had improved glycemic control and lower insulin levels with the Mediterranean diet. Subjects assigned to the low-carbohydrate diet had the greatest improvement in lipid profile (20% relative decrease of total cholesterol to HDL ratio).
The trial took place at a single site (a scientific research center in Israel) and included only 14% women, so its generalization is uncertain. The study was based on self-reported dietary intake and may be subject to reporting bias.
Bottom line: Mediterranean and low-carbohydrate diets are safe and effective alternatives to low-fat diets with favorable effects on glycemic control in diabetics and lipid metabolism, respectively.
Citation: Shai I, Schwarz-fuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. NEJM. 2008;359(3):229-241.
Admissions for Acute Coronary Syndrome Decreased after Implementation of Smoke-free Legislation
Clinical question: Is there a reduction in admissions for acute coronary syndrome (ACS) after enactment of smoke-free legislation?
Background: Multiple, small, retrospective studies have shown a decrease in ACS after implementation of smoke-free legislation.
Study design: Prospective observational multi-center cohort study.
Setting: Nine hospitals in Scotland.
Synopsis: Data was collected on all patients admitted with ACS 10 months before and after implementation of smoke-free legislation, which prohibited smoking in all enclosed public and work places in Scotland. After the smoking ban, the number of ACS admissions fell by 17% (95% CI 16-18) in Scotland as a whole, compared with a 4% reduction in England during the same period (England does not have similar smoke-free legislation). Among smokers, former smokers and non-smokers, the number of ACS admissions decreased by 14% (95% CI 12-16), 19% (95% CI 17-21), and 21% (95% CI 18-24), respectively. Among non-smokers, self-reported exposure to second-hand smoke decreased significantly; these reductions were confirmed by measured reductions in serum cotinine levels, even among those who never smoked.
Results were limited by the observational nature of the study, although the authors did attempt to carefully match comparison cohorts by season and geography. Also, secular trends other than legislation may have reduced prevalent smoking in Scotland during the study period.
Bottom line: Admissions for ACS for both smokers and non-smokers decreased after implementation of smoke-free legislation.
Citation: Pell JP, Haw S, Cobbe S, et al. Smoke-free legislation and hospitalizations for acute coronary syndrome. NEJM. 2008;359(5):482-491.
Continuation of Beta-blockers in Patients Hospitalized for Heart Failure Improves Mortality
Clinical question: Does the withdrawal or continuation of beta-blockers in patients hospitalized with decompensated heart failure have any effect on clinical outcomes?
Background: Previous clinical trials have demonstrated mortality benefit with the use of beta-blockers in patients with symptomatic chronic heart failure and left ventricular systolic dysfunction (LVSD), however, controversy exists whether to continue these medications in acute decompensated heart failure.
Study design: Prospective cohort analysis from the OPTIMIZE-HF registry (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure).
Setting: 91 academic and community hospitals in the United States.
Synopsis: Data was analyzed on 2,373 registry patients with documented LVSD (EF <40) eligible for beta-blocker therapy. During hospitalization, 1,350 patients were continued on beta-blockers, 79 had therapy withdrawn, 303 were not started, and 632 had beta-blockade initiated. Compared with no beta-blocker treatment, adjusted hazard ratio (HR) for death at 60 and 90 days following discharge was lower in patients who were continued on beta-blockade (HR 0.60, 95% CI 0.37–0.99). Compared with continuation of beta-blockade, withdrawal of beta-blockade increased the risk of death (HR 2.3, 95% CI 1.2–4.6).
Results were limited by the observational nature of the study and short follow up. The reason for discontinuation or not starting beta-blockade was not captured in the database, so it is possible sicker patients had beta-blockers discontinued during hospitalization (although the authors attempted to control for this).
Bottom line: Beta-blockers should be continued whenever possible in patients hospitalized for heart failure with LVSD.
Citation: Fonarow GC, Abraham WT, Albert NM, et al. Influence of beta-blocker continuation or withdrawal on outcomes in patients hospitalized with heart failure. J Am Coll Cardiol. 2008;52(3):190-199.
Non-invasive Ventilation Does Not Improve Short-term Mortality in Acute Cardiogenic Pulmonary Edema
Clinical question: Does non-invasive ventilation reduce mortality in patients with acute cardiogenic pulmonary edema and are there differences in outcome between use of continuous positive airway pressure (CPAP) or non-invasive positive pressure ventilation (NIPPV)?
Background: In patients with acute cardiogenic pulmonary edema, noninvasive ventilation improves physiologic variables and symptoms, decreases rates of invasive ventilation, and may improve mortality.
Study design: Randomized multi center controlled trial.
Setting: 26 district and regional hospitals in the United Kingdom.
Synopsis: 1,156 patients admitted with acute cardiogenic pulmonary edema between July 2003 and April 2007 were randomized to standard oxygen therapy, versus CPAP or NIPPV. There were no significant differences in seven- or 30-day mortality rates between the standard oxygen therapy versus noninvasive ventilation. Mortality at seven days was 9.8% in the standard oxygen group versus 9.5% in the noninvasive ventilation group (P=0.87); 30-day mortality was 16% in the standard oxygen group and 15% in the non-invasive ventilation group (P=0.64). There were no major differences in treatment outcome with NIPPV compared to CPAP.
Although mortality was not decreased, non-invasive ventilation did improve dyspnea and tachycardia within one hour of therapy.
Bottom line: In patients admitted with acute cardiogenic pulmonary edema, noninvasive ventilation improved dyspnea and some physiological parameters, but did not improve short-term mortality rates.
Citation: Gray A, Goodacre S, Newby D, Masson M, Sampson F, Nicholl J. Noninvasive ventilation in acute cardiogenic pulmonary edema. NEJM. 2008;359(2):142-151.
Cyclooxygenase 2 Inhibitors May Increase the Risk of Ischemic Stroke
Clinical question: Do NSAIDs and COX-2 inhibitors increase the risk of ischemic or hemorrhagic stroke?
Background: Selected cyclooxygenase 2 (COX-2) inhibitors have been shown to increase cardiovascular morbidity in a dose-dependent manner and are now used with caution in patients at risk for cardiovascular disease. Little is known about the safety of these medications and non-aspirin, non-steroidal anti-inflammatory drugs (NSAIDS) in those at risk for cerebrovascular disease.
Study design: Retrospective observational cohort study.
Setting: Tennessee Medicaid Program enrollees.
Synopsis: Data was collected from the medical records of 336,906 subjects. Non-users had a baseline stroke rate of 4.51 strokes/1000 person-years. The rate increased to 5.15/1,000 person-years and 5.95/1,000 person-years for rofecoxib and valdecoxib, respectively. Celecoxib and other NSAIDs did not significantly increase the risk of stroke. Analysis of new users of rofexocib and valdecoxib yielded a similarly increased risk of stroke. Most strokes were ischemic.
Limitations include the ready availability of NSAIDs raising the possibility that some patients classified as non-users were actually users of NSAIDs. Other potential confounders may not have been measured and, therefore, not available for analysis.
Bottom line: COX-2 inhibitors should be used with caution in patients with increased cerebrovascular disease risk.
Citation: Roumie CL, Mitchel EF, Kaltenback L, Arbogast PG, Gideon P Griffen MR. Nonaspirin NSAIDs, cyclooxygenase 2 inhibitors, and the risk for stroke. Stroke. 2008;39:1037-2045.