User login
Obesity, psychiatric disorders, and pregnancy
The etiology of premenstrual dysphoric disorder: 5 interwoven pieces
In an age when psychiatry strives to identify the biologic causes of disease, studying endocrine-related mood disorders is particularly intriguing. DSM-5 defines premenstrual dysphoric disorder (PMDD) as a depressive disorder, with a 12-month prevalence ranging from 1.8% to 5.8% among women who menstruate.1-3 Factors that differentiate PMDD from other affective disorders include etiology, duration, and temporal relationship with the menstrual cycle.
PMDD is a disorder of consistent yet intermittent change in mental health and functionality. Therefore, it may be underdiagnosed and consequently undertreated if a psychiatric evaluation does not coincide with symptom occurrence or if patients do not understand that intermittent symptoms are treatable.
This article summarizes what is known about the etiology of PMDD. Although there are several treatments for PMDD, many women experience adverse effects or incomplete effectiveness. Further understanding of this disorder may lead to more efficacious treatments. Additionally, understanding the pathophysiology of PMDD might shed a light on the etiology of other disorders that are temporally related to reproductive life changes, such as pregnancy-, postpartum-, or menopause-related affective dysregulation.
Making the diagnosis
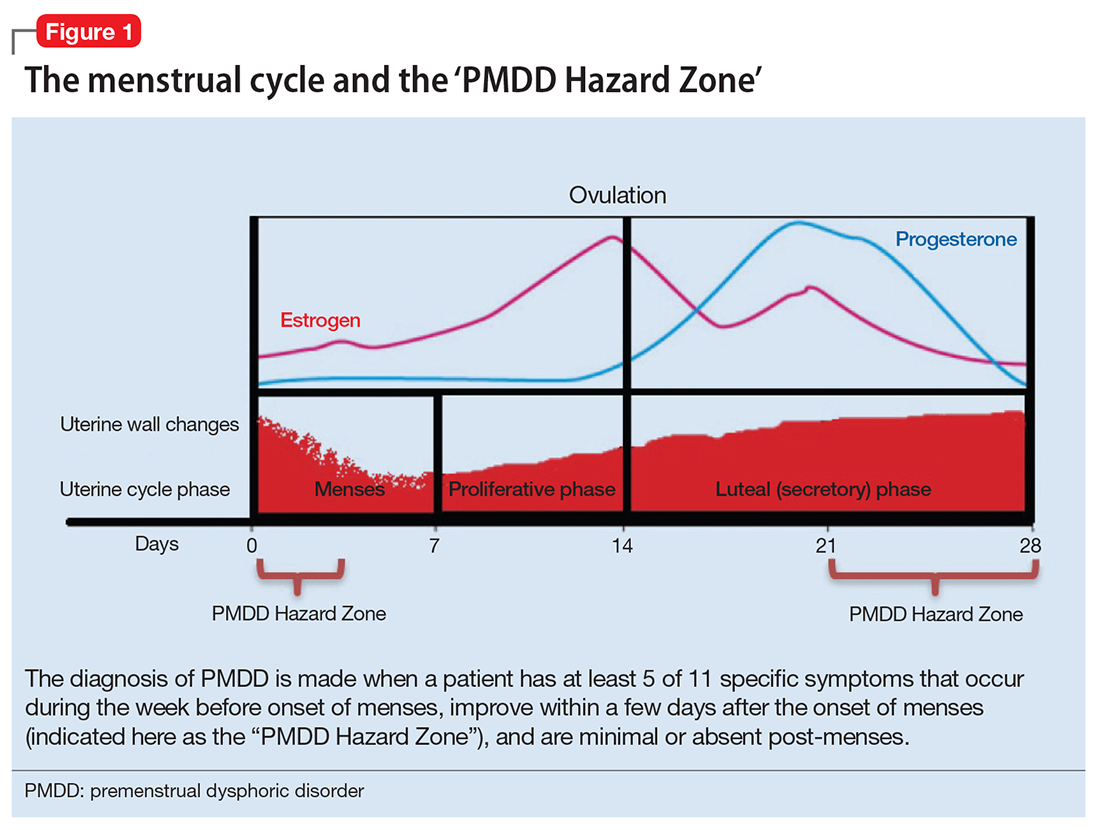
The diagnosis of PMDD is made when a patient has at least 5 of 11 specific symptoms that occur during the week before onset of menses, improve within a few days after the onset of menses (shown as the “PMDD Hazard Zone” in Figure 1), and are minimal or absent post-menses.3 Symptoms should be tracked prospectively for at least 2 menstrual cycles in order to confirm the diagnosis (one must be an affective symptom and another must be a behavioral/cognitive symptom).3
The affective symptoms are:
- lability of affect (eg, sudden sadness, tearfulness, or sensitivity to rejection)
- irritability, anger, or increased interpersonal conflicts
- depressed mood, hopelessness, or self- deprecating thoughts
- anxiety or tension, feeling “keyed up” or “on edge.”
The behavioral/cognitive symptoms are:
- decreased interest in usual activities (eg, work, hobbies, friends, school)
- difficulty concentrating
- lethargy, low energy, easy fatigability
- change in appetite, overeating, food cravings
- hypersomnia or insomnia
- feeling overwhelmed or out of control
- physical symptoms (breast tenderness or swelling, headache, joint or muscle pain, bloating, weight gain).
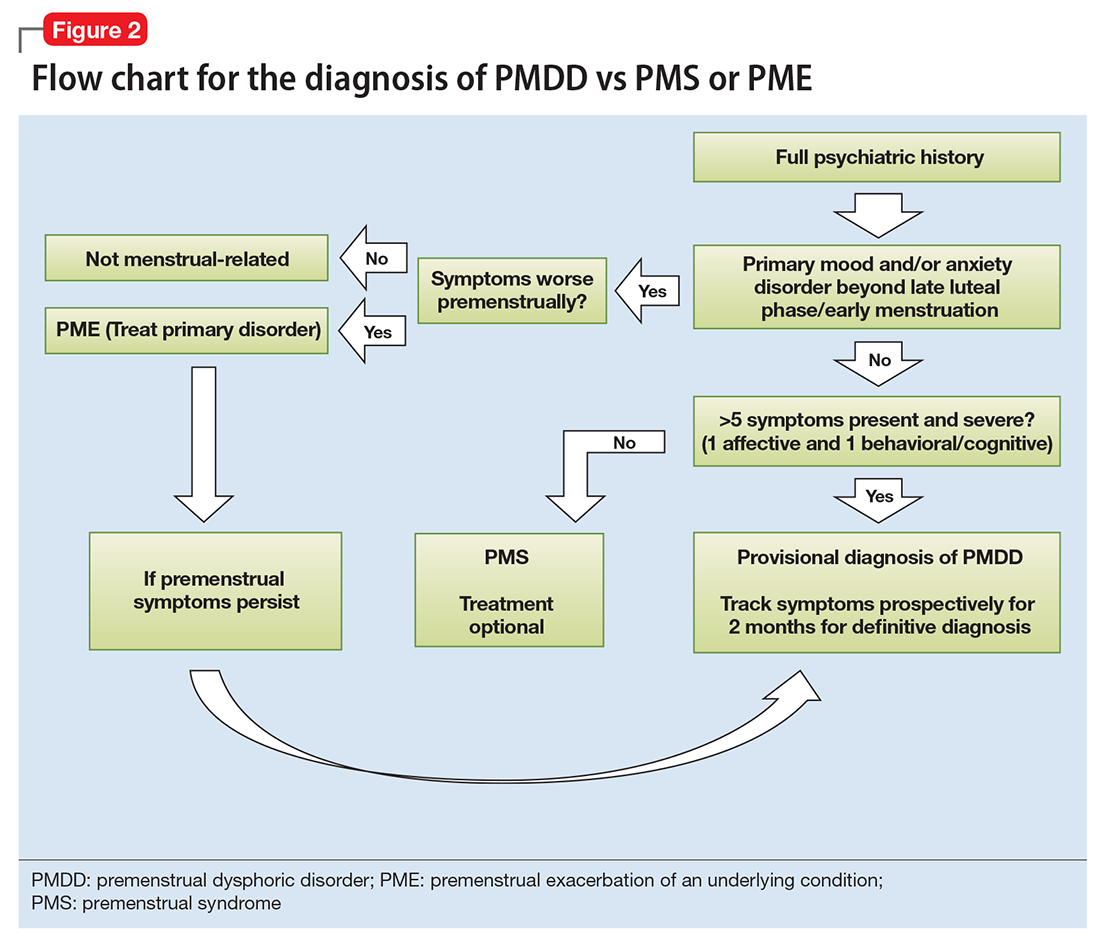
Ruling out premenstrual exacerbation (PME). Perhaps the most common cause for misdiagnosis of PMDD is failing to rule out PME of another underlying or comorbid condition (Figure 2). In many women who have a primary mood or anxiety disorder, the late luteal phase is a vulnerable time. A patient might be coping with untreated anxiety, for example, but the symptoms become unbearable the week before menstruation begins, which is likely when she seeks help. At this stage, a diagnosis of PMDD should be provisional at best. Often, PME is treated by treating the underlying condition. Therefore, a full diagnostic psychiatric interview is important to first rule out other underlying psychiatric disorders. PMDD is diagnosed if the premenstrual symptoms persist for 2 consecutive months after treating the suspected mood or anxiety disorder. Patients can use one of many PMDD daily symptom charts available online. Alternatively, they can use a cycle-tracking mobile phone application to correlate their symptoms with their cycle and share this information with their providers.
Consider these 5 interwoven pieces
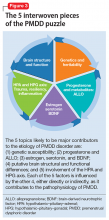
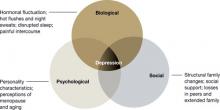
The many variables that contribute to the pathophysiology of PMDD overlap and should be considered connecting pieces in the puzzle that is the etiology of this disorder (Figure 3). In reviewing the literature, we have identified 5 topics likely to be major contributors to this disorder:
- genetic susceptibility
- progesterone and allopregnanolone (ALLO)
- estrogen, serotonin, and brain-derived neurotrophic factor (BDNF)
- putative brain structural and functional differences
- further involvement of the hypothalamic–pituitary–adrenal (HPA) axis and hypothalamic–pituitary–gonadal (HPG) axis: trauma, resiliency, and inflammation.
Genetic susceptibility. PMDD is thought to have a heritability range between 30% to 80%.3 This is demonstrated by family and twin studies4-7 and specific genetic studies.8 The involvement of genetics means an underlying neurobiologic pathophysiology is in place.
Estrogen receptor alpha (ESR1) gene. Huo et al8 found an associated variation in ESR1 in women with PMDD compared with controls. They speculated that because ESR1 is important for arousal, if dysfunctional, this gene could be implicated in somatic as well as affective and cognitive deficits in PMDD patients. In another study, investigators reported a relationship between PMDD and heritable personality traits, as well as a link between these traits and ESR1 polymorphic variants.1 They suggested that personality traits (independent of affective state) might be used to distinguish patients with PMDD from controls.1
Studies on serotonin gene polymorphism and serotonin transporter genotype. Although a study of serotonin gene polymorphism did not find an association between serotonin1A gene polymorphism and PMDD, it did show that the presence of at least 1 C allele was associated with a 2.5-fold increased risk of PMDD.9 Another study did not find an association between the serotonin transporter genotype 5-HTTLPR and PMDD.10 However, it showed lower frontocingulate cortex activation during the luteal phase of PMDD patients compared with controls, suggesting that PMDD is linked to impaired frontocingulate cortex activation induced by emotions during the luteal phase.10
Seasonal affective disorder (SAD) and PMDD have shared clinical features. A polymorphism in the serotonin transporter promoter gene 5-HTTLPR has been associated with SAD. One study found that patients with comorbid SAD and PMDD are genetically more vulnerable to comorbid affective disorders compared with patients who have SAD only.11
Progesterone and ALLO. Chronic exposure to progesterone and ALLO (a main progesterone metabolite) and rapid withdrawal from ovarian hormones may play a role in the etiology of PMDD. Much like alcohol or benzodiazepines, ALLO is a potent positive allosteric modulator of GABAA receptors and has sedative, anesthetic, and anxiolytic properties. In times of acute stress, increased ALLO is known to provide relief.12,13 However, in women with PMDD, this typical ALLO increase might not occur.14
Patients with PMDD have been reported to have decreased levels of ALLO in the luteal phase.15-17 In one study, women with highly symptomatic PMDD had lower levels of ALLO compared with women with less symptomatic PMDD.14 A gonadotropin-releasing hormone challenge study showed the increase in ALLO response was less in PMDD patients compared with controls.17 Luteal-phase ALLO concentrations are reported to be lower in women with premenstrual syndrome (PMS), a milder form of PMDD.14,17
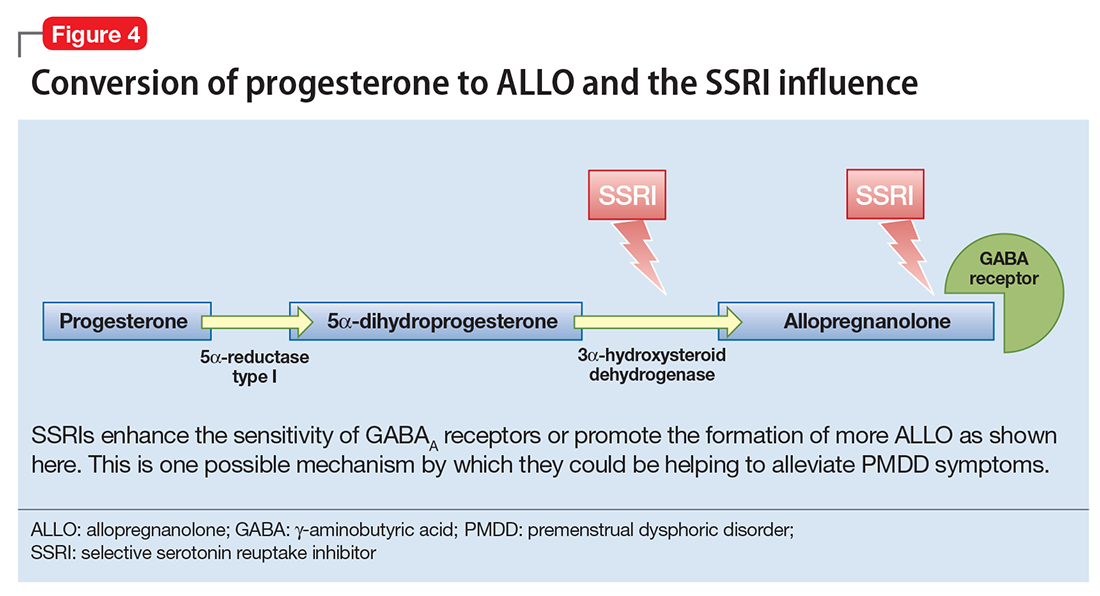
The efficacy of selective serotonin reuptake inhibitors (SSRIs) for treating PMDD could be the result of the interaction of these medications with neuroactive steroids,18 possibly because SSRIs enhance the sensitivity of GABAA receptors or promote the formation of more ALLO (Figure 4).19-21
Estrogen, serotonin, and BDNF. Estrogen affects multiple neurotransmitter systems that regulate mood, cognition, sleep, and eating.22 Studying estrogen in context of PMDD is important because women with PMDD can have low mood, specific food cravings, and impaired cognitive function.
Estrogen–serotonin interactions are thought to be involved in hormone-related mood disorders such as perimenopausal depression and PMDD.23,24 However, the nature of their relationship is not yet fully understood. Ovariectomized animals have shown estrogen-induced changes related to serotonin metabolism, binding, and transmission in the regions of the brain involved in regulation of affect and cognition. Research in menopausal women also has provided some support for this interaction.24
Positron emission tomography studies in humans have found increased cortical serotonin binding modulated by levels of estrogen, similar to those previously seen in rat studies.24-27 One study showed an increased binding potential of serotonin in the cerebral cortex with estrogen treatment. This study further showed an even greater binding potential with estrogen plus progesterone, signaling a synergistic effect of the 2 hormones.28
SSRIs are an effective treatment for the irritability, anxiety, and mood swings of PMDD.29-30 Although the exact mechanism of action is unknown, the serotonergic properties are certainly of primary attention. For some PMDD patients, SSRIs work within hours to days, as opposed to days or weeks for patients with depression or anxiety, which suggests a separate or co-occurring mechanism of action is in place. In a double-blind, placebo-controlled crossover study, researchers administered the serotonin receptor antagonist metergoline to women with PMDD whose symptoms had remitted during treatment with fluoxetine and a group of healthy controls who were not receiving any medication.31 The women with PMDD experienced a return of symptoms 24 hours after treatment with metergoline but not with placebo; the controls experienced no mood changes.31
BDNF is a neurotransmitter linked to estrogen and likely related to PMDD. BDNF is critical for neurogenesis and is expressed in brain regions involved in learning and memory and also affects regulation.32 BDNF levels are increased by serotonergic antidepressants, affected by estradiol, and have cyclicity throughout the menstrual cycle.33-35
Putative brain structural and functional differences. Imaging studies have suggested differences in brain structure in women with PMDD, with a focus on the amygdala and the prefrontal cortex. Women with PMDD have greater gray matter volume in the posterior cerebellum,36 greater gray matter density of hippocampal cortex, and lower gray matter density in the parahippocampal cortex.37
Some studies have shown a functional variability of the amygdala’s response to stress in women with PMDD vs healthy controls.38,39 A proton magnetic resonance spectroscopy (1H-MRS) study of the displays the possibility of an altered GABAergic function in patients with PMDD.40
Patients will PMDD have enhanced dorsolateral prefrontal cortex reactivity when anticipating negative stimuli (but not to the actual exposure) during the luteal phase. A positive correlation between this reactivity and progesterone levels also was observed.41 Some researchers have suggested that prefrontal cortex dysfunction may be a risk factor for PMDD.42
HPA axis and HPG axis: Trauma, resiliency, inflammation. Altered cortisol levels (higher during the luteal phase43 and lower during times of stress14,44) suggest a possibly altered HPA axis in some women with PMDD. However, studies on this topic have been few and inconsistent.
Dysregulation of the HPG axis could cause vasomotor symptoms, sleep dysregulation, and mood symptoms during menopause; women with PMDD can also experience these symptoms. The influence of estrogen and progesterone on mood is also highly dependent on this axis.
Ultimately, the interplay between the HPA axis and the HPG axis is important. One study found that women with PMDD who had high serum ALLO levels (HPG-related) had blunted nocturnal cortisol levels (HPA-related) compared with healthy controls who had low ALLO levels.45
Significant stress and trauma exposure have been associated with PMDD. A study of 3,968 women found a history of trauma and PTSD were independently associated with PMDD.46 Another study of approximately 3,000 women found a strong correlation between abuse and PMS.47 However, a third study found no correlations between PMDD and trauma.48
Patients with a predisposition to PMDD may be more vulnerable to develop a posttraumatic stress-related disorder, perhaps due to decreased biologic resiliency. For example, the startle response (hypervigilance) has been shown to be different in women with PMDD. One study suggested that suboptimal production of premenstrual ALLO may lead to increased arousal and increased stress reactivity to psychosocial or environmental triggers.49
The possible role of inflammation in PMDD deserves further investigation. The luteal phase entails an increase in the production of proinflammatory markers.50,51 A 10-fold increase in progesterone is correlated with a 20% to 23% increase in C-reactive protein levels.52,53 Women with inflammatory diseases (eg, gingivitis or irritable bowel syndrome) show worsening of symptoms prior to menstruation.54-56 One study found increased levels of proinflammatory markers in women with PMDD compared with controls.57
Putting together the 5 pieces of the puzzle
Because PMDD is heritable, it must have an underlying neurobiologic pathophysiology. Brain imaging studies show differences in structure and function in women with PMDD across the menstrual cycle. Conversion of progesterone to ALLO and the GABAergic influence of this metabolite is a topic of interest in current research. Similarly, the role of estrogen and its connection to serotonin and other neurotransmitters such as BDNF have been implicated.
The link between a history of stress, trauma, and PMDD raises the question of biologic resiliency and illness in these patients, as it connects to the HPA and HPG axis and production of inflammatory stress hormones and steroid hormones and their metabolites. PMDD can be conceptualized as variable sensitivity to hormonal response to stress,58 thus contextualizing biochemical and psychological resiliency.
Further research is needed to clarify the possibility of a shared pathophysiology between endocrine-related mood disorders such as postpartum depression (PPD) and PMDD because current research is controversial.59,60 In PPD, women who are exposed to high levels of progesterone and estrogen during pregnancy (just like in the mid-luteal phase) have a sudden drop in these hormones postpartum.
The ‘withdrawal theory.’ The affective symptoms of PMDD resolve almost instantaneously after the start of menstruation. Perhaps this type of immediate relief is akin to substance use disorders and symptoms of withdrawal. It could be that reinstatement of a certain amount of gonadal steroids in the follicular phase of the cycle diminishes a withdrawal-like response to these steroids.
Currently, the main leading theory is that PMDD is a result of “an abnormal response to normal hormonal changes.”61 A new study also has shown that the change in estradiol/progesterone levels (vs the steady state) was associated with PMDD symptoms.62 Thinking of PMDD as a disorder of withdrawal offers an alternative (yet complementary) perspective to the current theory: PMDD may be caused by the absence or diminishing of the above-named hormones and their metabolites in the late luteal phase (in the context of developed “tolerance” during the early- to mid-luteal phase).
Considering the interplay between neurotransmitters and neurosteroids, both a “serotonin withdrawal theory” (caused by a drop in steroid hormones) and a “GABAergic withdrawal theory” (due to the decline in progesterone) could be proposed. This theory would be supported by the fact that SSRIs seem to mitigate symptoms of PMDD as well as the genetic association between PMDD and ESR1. It is more than likely that the “withdrawal” is caused by the interactions between estrogen-serotonin, progesterone-ALLO, and GABA receptors, and the complementary fashion in which progesterone and estrogen influence each other.
1. Miller A, Vo H, Huo L, et al. Estrogen receptor alpha (ESR-1) associations with psychological traits in women with PMDD and controls. J Psychiatr Res. 2010;44(12):788-794.
2. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169(5):465-475.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Wilson CA, Turner CW, Keye WR Jr. Firstborn adolescent daughters and mothers with and without premenstrual syndrome: a comparison. J Adolesc Health. 1991;12(2):130-137.
5. Kendler KS, Silberg JL, Neale MC, et al. Genetic and environmental factors in the aetiology of menstrual, premenstrual and neurotic symptoms: a population-based twin study. Psychol Med. 1992;22(1):85-100.
6. Condon JT. The premenstrual syndrome: a twin study. Br J Psychiatry. 1993;162:481-486.
7. Kendler KS, Karkowski LM, Corey LA, et al. Longitudinal population-based twin study of retrospectively reported premenstrual symptoms and lifetime major depression. Am J Psychiatry. 1998;155(9):1234-1240.
8. Huo L, Straub RE, Roca C, et al. Risk for premenstrual dysphoric disorder is associated with genetic variation in ESR1, the estrogen receptor alpha gene. Biol Psychiatry. 2007;62(8):925-933.
9. Dhingra V, Magnay JL, O’Brien PM, et al. Serotonin receptor 1A C(-1019)G polymorphism associated with premenstrual dysphoric disorder. Obstet Gynecol. 2007;110(4):788-792.
10. Comasco E, Hahn A, Ganger S, et al. Emotional fronto-cingulate cortex activation and brain derived neurotrophic factor polymorphism in premenstrual dysphoric disorder. Hum Brain Mapp. 2014;35(9):4450-4458.
11. Praschak-Rieder N, Willeit M, Winkler D, et al. Role of family history and 5-HTTLPR polymorphism in female seasonal affective disorder patients with and without premenstrual dysphoric disorder. Eur Neuropsychopharmacol. 2002;12(2):129-134.
12. Klatzkin RR, Morrow AL, Light KC, et al. Associations of histories of depression and PMDD diagnosis with allopregnanolone concentrations following the oral administration of micronized progesterone. Psychoneuroendocrinology. 2006;31(10):1208-1219.
13. Crowley SK, Girdler SS. Neurosteroid, GABAergic and hypothalamic pituitary adrenal (HPA) axis regulation: what is the current state of knowledge in humans? Psychopharmacology (Berl). 2014;231(17):3619-3634.
14. Girdler SS, Straneva PA, Light KC, et al. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49(9):788-797.
15. Rapkin AJ, Morgan M, Goldman L, et al. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol. 1997;90(5):709-714.
16. Bicíková M, Dibbelt L, Hill M, et al. Allopregnanolone in women with premenstrual syndrome. Horm Metab Res. 1998;30(4):227-230.
17. Monteleone P, Luisi S, Tonetti A, et al. Allopregnanolone concentrations and premenstrual syndrome. Eur J Endocrinol. 2000;142(3):269-273.
18. Steiner M, Steinberg S, Stewart D, et al. Fluoxetine in the treatment of premenstrual dysphoria. Canadian Fluoxetine/Premenstrual Dysphoria Collaborative Study Group. N Engl J Med. 1995;332(23):1529-1534.
19. Sundström I, Bäckström T. Citalopram increases pregnanolone sensitivity in patients with premenstrual syndrome: an open trial. Psychoneuroendocrinology. 1998;23(1):73-88.
20. Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci U S A. 1999;96(23):13512-13517.
21. Trauger JW, Jiang A, Stearns BA, et al. Kinetics of allopregnanolone formation catalyzed by human 3 alpha-hydroxysteroid dehydrogenase type III (AKR1C2). Biochemistry. 2002;41(45):13451-13459.
22. Shanmugan S, Epperson CN. Estrogen and the prefrontal cortex: towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Hum Brain Mapp. 2014;35(3):847-865.
23. Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry. 1998;44(9):839-850.
24. Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behav Cogn Neurosci Rev. 2005;4(1):43-58.
25. Cyr M, Bossé R, Di Paolo T. Gonadal hormones modulate 5-hydroxytryptamine2A receptors: emphasis on the rat frontal cortex. Neuroscience. 1998;83(3):829-836.
26. Fink G, Sumner BE, Rosie R, et al. Estrogen control of central neurotransmission: effect on mood, mental state, and memory. Cell Mol Neurobiol. 1996;16(3):325-344.
27. Sumner BE, Grant KE, Rosie R, et al. Effects of tamoxifen on serotonin transporter and 5-hydroxytryptamine(2A) receptor binding sites and mRNA levels in the brain of ovariectomized rats with or without acute estradiol replacement. Brain Res Mol Brain Res. 1999;73(1-2):119-128.
28. Moses-Kolko EL, Berga SL, Greer PJ, et al. Widespread increases of cortical serotonin type 2A receptor availability after hormone therapy in euthymic postmenopausal women. Fertil Steril. 2003;80(3):554-559.
29. Su TP, Schmidt PJ, Danaceau MA, et al. Fluoxetine in the treatment of premenstrual dysphoria. Neuropsychopharmacology. 1997;16(5):346-356.
30. Steinberg EM, Cardoso GM, Martinez PE, et al. Rapid response to fluoxetine in women with premenstrual dysphoric disorder. Depress Anxiety. 2012;29(6):531-540.
31. Roca CA, Schmidt PJ, Smith MJ, et al. Effects of metergoline on symptoms in women with premenstrual dysphoric disorder. Am J Psychiatry. 2002;159(11):1876-1881.
32. Gray JD, Milner TA, McEwen BS. Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience. 2013;239:214-227.
33. Carbone DL, Handa RJ. Sex and stress hormone influences on the expression and activity of brain-derived neurotrophic factor. Neuroscience. 2013;239:295-303.
34. Pilar-Cuéllar F, Vidal R, Pazos A. Subchronic treatment with fluoxetine and ketanserin increases hippocampal brain-derived neurotrophic factor, β-catenin and antidepressant-like effects. Br J Pharmacol. 2012;165(4b):1046-1057.
35. Deuschle M, Gilles M, Scharnholz B, et al. Changes of serum concentrations of brain-derived neurotrophic factor (BDNF) during treatment with venlafaxine and mirtazapine: role of medication and response to treatment. Pharmacopsychiatry. 2013;46(2):54-58.
36. Berman SM, London ED, Morgan M, et al. Elevated gray matter volume of the emotional cerebellum in women with premenstrual dysphoric disorder. J Affect Disord. 2013;146(2):266-271.
37. Jeong HG, Ham BJ, Yeo HB, et al. Gray matter abnormalities in patients with premenstrual dysphoric disorder: an optimized voxel-based morphometry. J Affect Disord. 2012;140(3):260-267.
38. Protopopescu X, Tuescher O, Pan H, et al. Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affect Disord. 2008;108(1-2):87-94.
39. Gingnell M, Morell A, Bannbers E, et al. Menstrual cycle effects on amygdala reactivity to emotional stimulation in premenstrual dysphoric disorder. Horm Behav. 2012;62(4):400-406.
40. Epperson CN, Haga K, Mason GF, et al. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59(9):851-858.
41. Gingnell M, Bannbers E, Wikström J, et al. Premenstrual dysphoric disorder and prefrontal reactivity during anticipation of emotional stimuli. Eur Neuropsychopharmacol. 2013;23(11):1474-1483.
42. Baller EB, Wei SM, Kohn PD, et al. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: a multimodal neuroimaging study. Am J Psychiatry. 2013;170(3):305-314.
43. Rasgon N, McGuire M, Tanavoli S, et al. Neuroendocrine response to an intravenous L-tryptophan challenge in women with premenstrual syndrome. Fertil Steril. 2000;73(1):144-149.
44. Huang Y, Zhou R, Wu M, et al. Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress. 2015;18(2):160-168.
45. Segebladh B, Bannbers E, Moby L, et al. Allopregnanolone serum concentrations and diurnal cortisol secretion in women with premenstrual dysphoric disorder. Arch Womens Ment Health. 2013;16(2):131-137.
46. Pilver CE, Levy BR, Libby DJ, et al. Posttraumatic stress disorder and trauma characteristics are correlates of premenstrual dysphoric disorder. Arch Womens Ment Health. 2011;14(5):383-393.
47. Bertone-Johnson ER, Whitcomb BW, Missmer SA, et al. Early life emotional, physical, and sexual abuse and the development of premenstrual syndrome: a longitudinal study. J Womens Health (Larchmt). 2014;23(9):729-739.
48. Segebladh B, Bannbers E, Kask K, et al. Prevalence of violence exposure in women with premenstrual dysphoric disorder in comparison with other gynecological patients and asymptomatic controls. Acta Obstet Gynecol Scand. 2011;90(7):746-752.
49. Kask K, Gulinello M, Bäckström T, et al. Patients with premenstrual dysphoric disorder have increased startle response across both cycle phases and lower levels of prepulse inhibition during the late luteal phase of the menstrual cycle. Neuropsychopharmacology. 2008;33(9):2283-2290.
50. O’Brien SM, Fitzgerald P, Scully P, et al. Impact of gender and menstrual cycle phase on plasma cytokine concentrations. Neuroimmunomodulation. 2007;14(2):84-90.
51. Northoff H, Symons S, Zieker D, et al. Gender- and menstrual phase dependent regulation of inflammatory gene expression in response to aerobic exercise. Exerc Immunol Rev. 2008;14:86-103.
52. Gaskins AJ, Wilchesky M, Mumford SL, et al. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: the BioCycle Study. Am J Epidemiol. 2012;175(5):423-431.
53. Wander K, Brindle E, O’Connor KA. C-reactive protein across the menstrual cycle. Am J Phys Anthropol. 2008;136(2):138-146.
54. Jane ZY, Chang CC, Lin HK, et al. The association between the exacerbation of irritable bowel syndrome and menstrual symptoms in young Taiwanese women. Gastroenterol Nurs. 2011;34(4):277-286.
55. Kane SV, Sable K, Hanauer SB. The menstrual cycle and its effect on inflammatory bowel disease and irritable bowel syndrome: a prevalence study. Am J Gastroenterol. 1998;93(10):1867-1872.
56. Shourie V, Dwarakanath CD, Prashanth GV, et al. The effect of menstrual cycle on periodontal health - a clinical and microbiological study. Oral Health Prev Dent. 2012;10(2):185-192.
57. Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. 2015;17(11):87.
58. Maeng LY, Milad MR. Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Horm Behav. 2015;76:106-117.
59. Lee YJ, Yi SW, Ju DH, et al. Correlation between postpartum depression and premenstrual dysphoric disorder: single center study. Obstet Gynecol Sci. 2015;58(5):353-358.
60. Kepple AL, Lee EE, Haq N, et al. History of postpartum depression in a clinic-based sample of women with premenstrual dysphoric disorder. J Clin Psychiatry. 2016;77(4):e415-e420.
61. Schmidt PJ, Nieman LK, Danaceau MA, et al. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;338(4):209-216.
62. Schmidt PJ, Martinez PE, Nieman LK, et al. Premenstrual dysphoric disorder symptoms following ovarian suppression: Triggered by change in ovarian steroid levels but not continuous stable levels. Am J Psychiatry. [published online April 21, 2017]. doi: 10.1176/appi.ajp.2017.16101113.
In an age when psychiatry strives to identify the biologic causes of disease, studying endocrine-related mood disorders is particularly intriguing. DSM-5 defines premenstrual dysphoric disorder (PMDD) as a depressive disorder, with a 12-month prevalence ranging from 1.8% to 5.8% among women who menstruate.1-3 Factors that differentiate PMDD from other affective disorders include etiology, duration, and temporal relationship with the menstrual cycle.
PMDD is a disorder of consistent yet intermittent change in mental health and functionality. Therefore, it may be underdiagnosed and consequently undertreated if a psychiatric evaluation does not coincide with symptom occurrence or if patients do not understand that intermittent symptoms are treatable.
This article summarizes what is known about the etiology of PMDD. Although there are several treatments for PMDD, many women experience adverse effects or incomplete effectiveness. Further understanding of this disorder may lead to more efficacious treatments. Additionally, understanding the pathophysiology of PMDD might shed a light on the etiology of other disorders that are temporally related to reproductive life changes, such as pregnancy-, postpartum-, or menopause-related affective dysregulation.
Making the diagnosis
The diagnosis of PMDD is made when a patient has at least 5 of 11 specific symptoms that occur during the week before onset of menses, improve within a few days after the onset of menses (shown as the “PMDD Hazard Zone” in Figure 1), and are minimal or absent post-menses.3 Symptoms should be tracked prospectively for at least 2 menstrual cycles in order to confirm the diagnosis (one must be an affective symptom and another must be a behavioral/cognitive symptom).3
The affective symptoms are:
- lability of affect (eg, sudden sadness, tearfulness, or sensitivity to rejection)
- irritability, anger, or increased interpersonal conflicts
- depressed mood, hopelessness, or self- deprecating thoughts
- anxiety or tension, feeling “keyed up” or “on edge.”
The behavioral/cognitive symptoms are:
- decreased interest in usual activities (eg, work, hobbies, friends, school)
- difficulty concentrating
- lethargy, low energy, easy fatigability
- change in appetite, overeating, food cravings
- hypersomnia or insomnia
- feeling overwhelmed or out of control
- physical symptoms (breast tenderness or swelling, headache, joint or muscle pain, bloating, weight gain).
Ruling out premenstrual exacerbation (PME). Perhaps the most common cause for misdiagnosis of PMDD is failing to rule out PME of another underlying or comorbid condition (Figure 2). In many women who have a primary mood or anxiety disorder, the late luteal phase is a vulnerable time. A patient might be coping with untreated anxiety, for example, but the symptoms become unbearable the week before menstruation begins, which is likely when she seeks help. At this stage, a diagnosis of PMDD should be provisional at best. Often, PME is treated by treating the underlying condition. Therefore, a full diagnostic psychiatric interview is important to first rule out other underlying psychiatric disorders. PMDD is diagnosed if the premenstrual symptoms persist for 2 consecutive months after treating the suspected mood or anxiety disorder. Patients can use one of many PMDD daily symptom charts available online. Alternatively, they can use a cycle-tracking mobile phone application to correlate their symptoms with their cycle and share this information with their providers.
Consider these 5 interwoven pieces
The many variables that contribute to the pathophysiology of PMDD overlap and should be considered connecting pieces in the puzzle that is the etiology of this disorder (Figure 3). In reviewing the literature, we have identified 5 topics likely to be major contributors to this disorder:
- genetic susceptibility
- progesterone and allopregnanolone (ALLO)
- estrogen, serotonin, and brain-derived neurotrophic factor (BDNF)
- putative brain structural and functional differences
- further involvement of the hypothalamic–pituitary–adrenal (HPA) axis and hypothalamic–pituitary–gonadal (HPG) axis: trauma, resiliency, and inflammation.
Genetic susceptibility. PMDD is thought to have a heritability range between 30% to 80%.3 This is demonstrated by family and twin studies4-7 and specific genetic studies.8 The involvement of genetics means an underlying neurobiologic pathophysiology is in place.
Estrogen receptor alpha (ESR1) gene. Huo et al8 found an associated variation in ESR1 in women with PMDD compared with controls. They speculated that because ESR1 is important for arousal, if dysfunctional, this gene could be implicated in somatic as well as affective and cognitive deficits in PMDD patients. In another study, investigators reported a relationship between PMDD and heritable personality traits, as well as a link between these traits and ESR1 polymorphic variants.1 They suggested that personality traits (independent of affective state) might be used to distinguish patients with PMDD from controls.1
Studies on serotonin gene polymorphism and serotonin transporter genotype. Although a study of serotonin gene polymorphism did not find an association between serotonin1A gene polymorphism and PMDD, it did show that the presence of at least 1 C allele was associated with a 2.5-fold increased risk of PMDD.9 Another study did not find an association between the serotonin transporter genotype 5-HTTLPR and PMDD.10 However, it showed lower frontocingulate cortex activation during the luteal phase of PMDD patients compared with controls, suggesting that PMDD is linked to impaired frontocingulate cortex activation induced by emotions during the luteal phase.10
Seasonal affective disorder (SAD) and PMDD have shared clinical features. A polymorphism in the serotonin transporter promoter gene 5-HTTLPR has been associated with SAD. One study found that patients with comorbid SAD and PMDD are genetically more vulnerable to comorbid affective disorders compared with patients who have SAD only.11
Progesterone and ALLO. Chronic exposure to progesterone and ALLO (a main progesterone metabolite) and rapid withdrawal from ovarian hormones may play a role in the etiology of PMDD. Much like alcohol or benzodiazepines, ALLO is a potent positive allosteric modulator of GABAA receptors and has sedative, anesthetic, and anxiolytic properties. In times of acute stress, increased ALLO is known to provide relief.12,13 However, in women with PMDD, this typical ALLO increase might not occur.14
Patients with PMDD have been reported to have decreased levels of ALLO in the luteal phase.15-17 In one study, women with highly symptomatic PMDD had lower levels of ALLO compared with women with less symptomatic PMDD.14 A gonadotropin-releasing hormone challenge study showed the increase in ALLO response was less in PMDD patients compared with controls.17 Luteal-phase ALLO concentrations are reported to be lower in women with premenstrual syndrome (PMS), a milder form of PMDD.14,17
The efficacy of selective serotonin reuptake inhibitors (SSRIs) for treating PMDD could be the result of the interaction of these medications with neuroactive steroids,18 possibly because SSRIs enhance the sensitivity of GABAA receptors or promote the formation of more ALLO (Figure 4).19-21
Estrogen, serotonin, and BDNF. Estrogen affects multiple neurotransmitter systems that regulate mood, cognition, sleep, and eating.22 Studying estrogen in context of PMDD is important because women with PMDD can have low mood, specific food cravings, and impaired cognitive function.
Estrogen–serotonin interactions are thought to be involved in hormone-related mood disorders such as perimenopausal depression and PMDD.23,24 However, the nature of their relationship is not yet fully understood. Ovariectomized animals have shown estrogen-induced changes related to serotonin metabolism, binding, and transmission in the regions of the brain involved in regulation of affect and cognition. Research in menopausal women also has provided some support for this interaction.24
Positron emission tomography studies in humans have found increased cortical serotonin binding modulated by levels of estrogen, similar to those previously seen in rat studies.24-27 One study showed an increased binding potential of serotonin in the cerebral cortex with estrogen treatment. This study further showed an even greater binding potential with estrogen plus progesterone, signaling a synergistic effect of the 2 hormones.28
SSRIs are an effective treatment for the irritability, anxiety, and mood swings of PMDD.29-30 Although the exact mechanism of action is unknown, the serotonergic properties are certainly of primary attention. For some PMDD patients, SSRIs work within hours to days, as opposed to days or weeks for patients with depression or anxiety, which suggests a separate or co-occurring mechanism of action is in place. In a double-blind, placebo-controlled crossover study, researchers administered the serotonin receptor antagonist metergoline to women with PMDD whose symptoms had remitted during treatment with fluoxetine and a group of healthy controls who were not receiving any medication.31 The women with PMDD experienced a return of symptoms 24 hours after treatment with metergoline but not with placebo; the controls experienced no mood changes.31
BDNF is a neurotransmitter linked to estrogen and likely related to PMDD. BDNF is critical for neurogenesis and is expressed in brain regions involved in learning and memory and also affects regulation.32 BDNF levels are increased by serotonergic antidepressants, affected by estradiol, and have cyclicity throughout the menstrual cycle.33-35
Putative brain structural and functional differences. Imaging studies have suggested differences in brain structure in women with PMDD, with a focus on the amygdala and the prefrontal cortex. Women with PMDD have greater gray matter volume in the posterior cerebellum,36 greater gray matter density of hippocampal cortex, and lower gray matter density in the parahippocampal cortex.37
Some studies have shown a functional variability of the amygdala’s response to stress in women with PMDD vs healthy controls.38,39 A proton magnetic resonance spectroscopy (1H-MRS) study of the displays the possibility of an altered GABAergic function in patients with PMDD.40
Patients will PMDD have enhanced dorsolateral prefrontal cortex reactivity when anticipating negative stimuli (but not to the actual exposure) during the luteal phase. A positive correlation between this reactivity and progesterone levels also was observed.41 Some researchers have suggested that prefrontal cortex dysfunction may be a risk factor for PMDD.42
HPA axis and HPG axis: Trauma, resiliency, inflammation. Altered cortisol levels (higher during the luteal phase43 and lower during times of stress14,44) suggest a possibly altered HPA axis in some women with PMDD. However, studies on this topic have been few and inconsistent.
Dysregulation of the HPG axis could cause vasomotor symptoms, sleep dysregulation, and mood symptoms during menopause; women with PMDD can also experience these symptoms. The influence of estrogen and progesterone on mood is also highly dependent on this axis.
Ultimately, the interplay between the HPA axis and the HPG axis is important. One study found that women with PMDD who had high serum ALLO levels (HPG-related) had blunted nocturnal cortisol levels (HPA-related) compared with healthy controls who had low ALLO levels.45
Significant stress and trauma exposure have been associated with PMDD. A study of 3,968 women found a history of trauma and PTSD were independently associated with PMDD.46 Another study of approximately 3,000 women found a strong correlation between abuse and PMS.47 However, a third study found no correlations between PMDD and trauma.48
Patients with a predisposition to PMDD may be more vulnerable to develop a posttraumatic stress-related disorder, perhaps due to decreased biologic resiliency. For example, the startle response (hypervigilance) has been shown to be different in women with PMDD. One study suggested that suboptimal production of premenstrual ALLO may lead to increased arousal and increased stress reactivity to psychosocial or environmental triggers.49
The possible role of inflammation in PMDD deserves further investigation. The luteal phase entails an increase in the production of proinflammatory markers.50,51 A 10-fold increase in progesterone is correlated with a 20% to 23% increase in C-reactive protein levels.52,53 Women with inflammatory diseases (eg, gingivitis or irritable bowel syndrome) show worsening of symptoms prior to menstruation.54-56 One study found increased levels of proinflammatory markers in women with PMDD compared with controls.57
Putting together the 5 pieces of the puzzle
Because PMDD is heritable, it must have an underlying neurobiologic pathophysiology. Brain imaging studies show differences in structure and function in women with PMDD across the menstrual cycle. Conversion of progesterone to ALLO and the GABAergic influence of this metabolite is a topic of interest in current research. Similarly, the role of estrogen and its connection to serotonin and other neurotransmitters such as BDNF have been implicated.
The link between a history of stress, trauma, and PMDD raises the question of biologic resiliency and illness in these patients, as it connects to the HPA and HPG axis and production of inflammatory stress hormones and steroid hormones and their metabolites. PMDD can be conceptualized as variable sensitivity to hormonal response to stress,58 thus contextualizing biochemical and psychological resiliency.
Further research is needed to clarify the possibility of a shared pathophysiology between endocrine-related mood disorders such as postpartum depression (PPD) and PMDD because current research is controversial.59,60 In PPD, women who are exposed to high levels of progesterone and estrogen during pregnancy (just like in the mid-luteal phase) have a sudden drop in these hormones postpartum.
The ‘withdrawal theory.’ The affective symptoms of PMDD resolve almost instantaneously after the start of menstruation. Perhaps this type of immediate relief is akin to substance use disorders and symptoms of withdrawal. It could be that reinstatement of a certain amount of gonadal steroids in the follicular phase of the cycle diminishes a withdrawal-like response to these steroids.
Currently, the main leading theory is that PMDD is a result of “an abnormal response to normal hormonal changes.”61 A new study also has shown that the change in estradiol/progesterone levels (vs the steady state) was associated with PMDD symptoms.62 Thinking of PMDD as a disorder of withdrawal offers an alternative (yet complementary) perspective to the current theory: PMDD may be caused by the absence or diminishing of the above-named hormones and their metabolites in the late luteal phase (in the context of developed “tolerance” during the early- to mid-luteal phase).
Considering the interplay between neurotransmitters and neurosteroids, both a “serotonin withdrawal theory” (caused by a drop in steroid hormones) and a “GABAergic withdrawal theory” (due to the decline in progesterone) could be proposed. This theory would be supported by the fact that SSRIs seem to mitigate symptoms of PMDD as well as the genetic association between PMDD and ESR1. It is more than likely that the “withdrawal” is caused by the interactions between estrogen-serotonin, progesterone-ALLO, and GABA receptors, and the complementary fashion in which progesterone and estrogen influence each other.
In an age when psychiatry strives to identify the biologic causes of disease, studying endocrine-related mood disorders is particularly intriguing. DSM-5 defines premenstrual dysphoric disorder (PMDD) as a depressive disorder, with a 12-month prevalence ranging from 1.8% to 5.8% among women who menstruate.1-3 Factors that differentiate PMDD from other affective disorders include etiology, duration, and temporal relationship with the menstrual cycle.
PMDD is a disorder of consistent yet intermittent change in mental health and functionality. Therefore, it may be underdiagnosed and consequently undertreated if a psychiatric evaluation does not coincide with symptom occurrence or if patients do not understand that intermittent symptoms are treatable.
This article summarizes what is known about the etiology of PMDD. Although there are several treatments for PMDD, many women experience adverse effects or incomplete effectiveness. Further understanding of this disorder may lead to more efficacious treatments. Additionally, understanding the pathophysiology of PMDD might shed a light on the etiology of other disorders that are temporally related to reproductive life changes, such as pregnancy-, postpartum-, or menopause-related affective dysregulation.
Making the diagnosis
The diagnosis of PMDD is made when a patient has at least 5 of 11 specific symptoms that occur during the week before onset of menses, improve within a few days after the onset of menses (shown as the “PMDD Hazard Zone” in Figure 1), and are minimal or absent post-menses.3 Symptoms should be tracked prospectively for at least 2 menstrual cycles in order to confirm the diagnosis (one must be an affective symptom and another must be a behavioral/cognitive symptom).3
The affective symptoms are:
- lability of affect (eg, sudden sadness, tearfulness, or sensitivity to rejection)
- irritability, anger, or increased interpersonal conflicts
- depressed mood, hopelessness, or self- deprecating thoughts
- anxiety or tension, feeling “keyed up” or “on edge.”
The behavioral/cognitive symptoms are:
- decreased interest in usual activities (eg, work, hobbies, friends, school)
- difficulty concentrating
- lethargy, low energy, easy fatigability
- change in appetite, overeating, food cravings
- hypersomnia or insomnia
- feeling overwhelmed or out of control
- physical symptoms (breast tenderness or swelling, headache, joint or muscle pain, bloating, weight gain).
Ruling out premenstrual exacerbation (PME). Perhaps the most common cause for misdiagnosis of PMDD is failing to rule out PME of another underlying or comorbid condition (Figure 2). In many women who have a primary mood or anxiety disorder, the late luteal phase is a vulnerable time. A patient might be coping with untreated anxiety, for example, but the symptoms become unbearable the week before menstruation begins, which is likely when she seeks help. At this stage, a diagnosis of PMDD should be provisional at best. Often, PME is treated by treating the underlying condition. Therefore, a full diagnostic psychiatric interview is important to first rule out other underlying psychiatric disorders. PMDD is diagnosed if the premenstrual symptoms persist for 2 consecutive months after treating the suspected mood or anxiety disorder. Patients can use one of many PMDD daily symptom charts available online. Alternatively, they can use a cycle-tracking mobile phone application to correlate their symptoms with their cycle and share this information with their providers.
Consider these 5 interwoven pieces
The many variables that contribute to the pathophysiology of PMDD overlap and should be considered connecting pieces in the puzzle that is the etiology of this disorder (Figure 3). In reviewing the literature, we have identified 5 topics likely to be major contributors to this disorder:
- genetic susceptibility
- progesterone and allopregnanolone (ALLO)
- estrogen, serotonin, and brain-derived neurotrophic factor (BDNF)
- putative brain structural and functional differences
- further involvement of the hypothalamic–pituitary–adrenal (HPA) axis and hypothalamic–pituitary–gonadal (HPG) axis: trauma, resiliency, and inflammation.
Genetic susceptibility. PMDD is thought to have a heritability range between 30% to 80%.3 This is demonstrated by family and twin studies4-7 and specific genetic studies.8 The involvement of genetics means an underlying neurobiologic pathophysiology is in place.
Estrogen receptor alpha (ESR1) gene. Huo et al8 found an associated variation in ESR1 in women with PMDD compared with controls. They speculated that because ESR1 is important for arousal, if dysfunctional, this gene could be implicated in somatic as well as affective and cognitive deficits in PMDD patients. In another study, investigators reported a relationship between PMDD and heritable personality traits, as well as a link between these traits and ESR1 polymorphic variants.1 They suggested that personality traits (independent of affective state) might be used to distinguish patients with PMDD from controls.1
Studies on serotonin gene polymorphism and serotonin transporter genotype. Although a study of serotonin gene polymorphism did not find an association between serotonin1A gene polymorphism and PMDD, it did show that the presence of at least 1 C allele was associated with a 2.5-fold increased risk of PMDD.9 Another study did not find an association between the serotonin transporter genotype 5-HTTLPR and PMDD.10 However, it showed lower frontocingulate cortex activation during the luteal phase of PMDD patients compared with controls, suggesting that PMDD is linked to impaired frontocingulate cortex activation induced by emotions during the luteal phase.10
Seasonal affective disorder (SAD) and PMDD have shared clinical features. A polymorphism in the serotonin transporter promoter gene 5-HTTLPR has been associated with SAD. One study found that patients with comorbid SAD and PMDD are genetically more vulnerable to comorbid affective disorders compared with patients who have SAD only.11
Progesterone and ALLO. Chronic exposure to progesterone and ALLO (a main progesterone metabolite) and rapid withdrawal from ovarian hormones may play a role in the etiology of PMDD. Much like alcohol or benzodiazepines, ALLO is a potent positive allosteric modulator of GABAA receptors and has sedative, anesthetic, and anxiolytic properties. In times of acute stress, increased ALLO is known to provide relief.12,13 However, in women with PMDD, this typical ALLO increase might not occur.14
Patients with PMDD have been reported to have decreased levels of ALLO in the luteal phase.15-17 In one study, women with highly symptomatic PMDD had lower levels of ALLO compared with women with less symptomatic PMDD.14 A gonadotropin-releasing hormone challenge study showed the increase in ALLO response was less in PMDD patients compared with controls.17 Luteal-phase ALLO concentrations are reported to be lower in women with premenstrual syndrome (PMS), a milder form of PMDD.14,17
The efficacy of selective serotonin reuptake inhibitors (SSRIs) for treating PMDD could be the result of the interaction of these medications with neuroactive steroids,18 possibly because SSRIs enhance the sensitivity of GABAA receptors or promote the formation of more ALLO (Figure 4).19-21
Estrogen, serotonin, and BDNF. Estrogen affects multiple neurotransmitter systems that regulate mood, cognition, sleep, and eating.22 Studying estrogen in context of PMDD is important because women with PMDD can have low mood, specific food cravings, and impaired cognitive function.
Estrogen–serotonin interactions are thought to be involved in hormone-related mood disorders such as perimenopausal depression and PMDD.23,24 However, the nature of their relationship is not yet fully understood. Ovariectomized animals have shown estrogen-induced changes related to serotonin metabolism, binding, and transmission in the regions of the brain involved in regulation of affect and cognition. Research in menopausal women also has provided some support for this interaction.24
Positron emission tomography studies in humans have found increased cortical serotonin binding modulated by levels of estrogen, similar to those previously seen in rat studies.24-27 One study showed an increased binding potential of serotonin in the cerebral cortex with estrogen treatment. This study further showed an even greater binding potential with estrogen plus progesterone, signaling a synergistic effect of the 2 hormones.28
SSRIs are an effective treatment for the irritability, anxiety, and mood swings of PMDD.29-30 Although the exact mechanism of action is unknown, the serotonergic properties are certainly of primary attention. For some PMDD patients, SSRIs work within hours to days, as opposed to days or weeks for patients with depression or anxiety, which suggests a separate or co-occurring mechanism of action is in place. In a double-blind, placebo-controlled crossover study, researchers administered the serotonin receptor antagonist metergoline to women with PMDD whose symptoms had remitted during treatment with fluoxetine and a group of healthy controls who were not receiving any medication.31 The women with PMDD experienced a return of symptoms 24 hours after treatment with metergoline but not with placebo; the controls experienced no mood changes.31
BDNF is a neurotransmitter linked to estrogen and likely related to PMDD. BDNF is critical for neurogenesis and is expressed in brain regions involved in learning and memory and also affects regulation.32 BDNF levels are increased by serotonergic antidepressants, affected by estradiol, and have cyclicity throughout the menstrual cycle.33-35
Putative brain structural and functional differences. Imaging studies have suggested differences in brain structure in women with PMDD, with a focus on the amygdala and the prefrontal cortex. Women with PMDD have greater gray matter volume in the posterior cerebellum,36 greater gray matter density of hippocampal cortex, and lower gray matter density in the parahippocampal cortex.37
Some studies have shown a functional variability of the amygdala’s response to stress in women with PMDD vs healthy controls.38,39 A proton magnetic resonance spectroscopy (1H-MRS) study of the displays the possibility of an altered GABAergic function in patients with PMDD.40
Patients will PMDD have enhanced dorsolateral prefrontal cortex reactivity when anticipating negative stimuli (but not to the actual exposure) during the luteal phase. A positive correlation between this reactivity and progesterone levels also was observed.41 Some researchers have suggested that prefrontal cortex dysfunction may be a risk factor for PMDD.42
HPA axis and HPG axis: Trauma, resiliency, inflammation. Altered cortisol levels (higher during the luteal phase43 and lower during times of stress14,44) suggest a possibly altered HPA axis in some women with PMDD. However, studies on this topic have been few and inconsistent.
Dysregulation of the HPG axis could cause vasomotor symptoms, sleep dysregulation, and mood symptoms during menopause; women with PMDD can also experience these symptoms. The influence of estrogen and progesterone on mood is also highly dependent on this axis.
Ultimately, the interplay between the HPA axis and the HPG axis is important. One study found that women with PMDD who had high serum ALLO levels (HPG-related) had blunted nocturnal cortisol levels (HPA-related) compared with healthy controls who had low ALLO levels.45
Significant stress and trauma exposure have been associated with PMDD. A study of 3,968 women found a history of trauma and PTSD were independently associated with PMDD.46 Another study of approximately 3,000 women found a strong correlation between abuse and PMS.47 However, a third study found no correlations between PMDD and trauma.48
Patients with a predisposition to PMDD may be more vulnerable to develop a posttraumatic stress-related disorder, perhaps due to decreased biologic resiliency. For example, the startle response (hypervigilance) has been shown to be different in women with PMDD. One study suggested that suboptimal production of premenstrual ALLO may lead to increased arousal and increased stress reactivity to psychosocial or environmental triggers.49
The possible role of inflammation in PMDD deserves further investigation. The luteal phase entails an increase in the production of proinflammatory markers.50,51 A 10-fold increase in progesterone is correlated with a 20% to 23% increase in C-reactive protein levels.52,53 Women with inflammatory diseases (eg, gingivitis or irritable bowel syndrome) show worsening of symptoms prior to menstruation.54-56 One study found increased levels of proinflammatory markers in women with PMDD compared with controls.57
Putting together the 5 pieces of the puzzle
Because PMDD is heritable, it must have an underlying neurobiologic pathophysiology. Brain imaging studies show differences in structure and function in women with PMDD across the menstrual cycle. Conversion of progesterone to ALLO and the GABAergic influence of this metabolite is a topic of interest in current research. Similarly, the role of estrogen and its connection to serotonin and other neurotransmitters such as BDNF have been implicated.
The link between a history of stress, trauma, and PMDD raises the question of biologic resiliency and illness in these patients, as it connects to the HPA and HPG axis and production of inflammatory stress hormones and steroid hormones and their metabolites. PMDD can be conceptualized as variable sensitivity to hormonal response to stress,58 thus contextualizing biochemical and psychological resiliency.
Further research is needed to clarify the possibility of a shared pathophysiology between endocrine-related mood disorders such as postpartum depression (PPD) and PMDD because current research is controversial.59,60 In PPD, women who are exposed to high levels of progesterone and estrogen during pregnancy (just like in the mid-luteal phase) have a sudden drop in these hormones postpartum.
The ‘withdrawal theory.’ The affective symptoms of PMDD resolve almost instantaneously after the start of menstruation. Perhaps this type of immediate relief is akin to substance use disorders and symptoms of withdrawal. It could be that reinstatement of a certain amount of gonadal steroids in the follicular phase of the cycle diminishes a withdrawal-like response to these steroids.
Currently, the main leading theory is that PMDD is a result of “an abnormal response to normal hormonal changes.”61 A new study also has shown that the change in estradiol/progesterone levels (vs the steady state) was associated with PMDD symptoms.62 Thinking of PMDD as a disorder of withdrawal offers an alternative (yet complementary) perspective to the current theory: PMDD may be caused by the absence or diminishing of the above-named hormones and their metabolites in the late luteal phase (in the context of developed “tolerance” during the early- to mid-luteal phase).
Considering the interplay between neurotransmitters and neurosteroids, both a “serotonin withdrawal theory” (caused by a drop in steroid hormones) and a “GABAergic withdrawal theory” (due to the decline in progesterone) could be proposed. This theory would be supported by the fact that SSRIs seem to mitigate symptoms of PMDD as well as the genetic association between PMDD and ESR1. It is more than likely that the “withdrawal” is caused by the interactions between estrogen-serotonin, progesterone-ALLO, and GABA receptors, and the complementary fashion in which progesterone and estrogen influence each other.
1. Miller A, Vo H, Huo L, et al. Estrogen receptor alpha (ESR-1) associations with psychological traits in women with PMDD and controls. J Psychiatr Res. 2010;44(12):788-794.
2. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169(5):465-475.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Wilson CA, Turner CW, Keye WR Jr. Firstborn adolescent daughters and mothers with and without premenstrual syndrome: a comparison. J Adolesc Health. 1991;12(2):130-137.
5. Kendler KS, Silberg JL, Neale MC, et al. Genetic and environmental factors in the aetiology of menstrual, premenstrual and neurotic symptoms: a population-based twin study. Psychol Med. 1992;22(1):85-100.
6. Condon JT. The premenstrual syndrome: a twin study. Br J Psychiatry. 1993;162:481-486.
7. Kendler KS, Karkowski LM, Corey LA, et al. Longitudinal population-based twin study of retrospectively reported premenstrual symptoms and lifetime major depression. Am J Psychiatry. 1998;155(9):1234-1240.
8. Huo L, Straub RE, Roca C, et al. Risk for premenstrual dysphoric disorder is associated with genetic variation in ESR1, the estrogen receptor alpha gene. Biol Psychiatry. 2007;62(8):925-933.
9. Dhingra V, Magnay JL, O’Brien PM, et al. Serotonin receptor 1A C(-1019)G polymorphism associated with premenstrual dysphoric disorder. Obstet Gynecol. 2007;110(4):788-792.
10. Comasco E, Hahn A, Ganger S, et al. Emotional fronto-cingulate cortex activation and brain derived neurotrophic factor polymorphism in premenstrual dysphoric disorder. Hum Brain Mapp. 2014;35(9):4450-4458.
11. Praschak-Rieder N, Willeit M, Winkler D, et al. Role of family history and 5-HTTLPR polymorphism in female seasonal affective disorder patients with and without premenstrual dysphoric disorder. Eur Neuropsychopharmacol. 2002;12(2):129-134.
12. Klatzkin RR, Morrow AL, Light KC, et al. Associations of histories of depression and PMDD diagnosis with allopregnanolone concentrations following the oral administration of micronized progesterone. Psychoneuroendocrinology. 2006;31(10):1208-1219.
13. Crowley SK, Girdler SS. Neurosteroid, GABAergic and hypothalamic pituitary adrenal (HPA) axis regulation: what is the current state of knowledge in humans? Psychopharmacology (Berl). 2014;231(17):3619-3634.
14. Girdler SS, Straneva PA, Light KC, et al. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49(9):788-797.
15. Rapkin AJ, Morgan M, Goldman L, et al. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol. 1997;90(5):709-714.
16. Bicíková M, Dibbelt L, Hill M, et al. Allopregnanolone in women with premenstrual syndrome. Horm Metab Res. 1998;30(4):227-230.
17. Monteleone P, Luisi S, Tonetti A, et al. Allopregnanolone concentrations and premenstrual syndrome. Eur J Endocrinol. 2000;142(3):269-273.
18. Steiner M, Steinberg S, Stewart D, et al. Fluoxetine in the treatment of premenstrual dysphoria. Canadian Fluoxetine/Premenstrual Dysphoria Collaborative Study Group. N Engl J Med. 1995;332(23):1529-1534.
19. Sundström I, Bäckström T. Citalopram increases pregnanolone sensitivity in patients with premenstrual syndrome: an open trial. Psychoneuroendocrinology. 1998;23(1):73-88.
20. Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci U S A. 1999;96(23):13512-13517.
21. Trauger JW, Jiang A, Stearns BA, et al. Kinetics of allopregnanolone formation catalyzed by human 3 alpha-hydroxysteroid dehydrogenase type III (AKR1C2). Biochemistry. 2002;41(45):13451-13459.
22. Shanmugan S, Epperson CN. Estrogen and the prefrontal cortex: towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Hum Brain Mapp. 2014;35(3):847-865.
23. Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry. 1998;44(9):839-850.
24. Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behav Cogn Neurosci Rev. 2005;4(1):43-58.
25. Cyr M, Bossé R, Di Paolo T. Gonadal hormones modulate 5-hydroxytryptamine2A receptors: emphasis on the rat frontal cortex. Neuroscience. 1998;83(3):829-836.
26. Fink G, Sumner BE, Rosie R, et al. Estrogen control of central neurotransmission: effect on mood, mental state, and memory. Cell Mol Neurobiol. 1996;16(3):325-344.
27. Sumner BE, Grant KE, Rosie R, et al. Effects of tamoxifen on serotonin transporter and 5-hydroxytryptamine(2A) receptor binding sites and mRNA levels in the brain of ovariectomized rats with or without acute estradiol replacement. Brain Res Mol Brain Res. 1999;73(1-2):119-128.
28. Moses-Kolko EL, Berga SL, Greer PJ, et al. Widespread increases of cortical serotonin type 2A receptor availability after hormone therapy in euthymic postmenopausal women. Fertil Steril. 2003;80(3):554-559.
29. Su TP, Schmidt PJ, Danaceau MA, et al. Fluoxetine in the treatment of premenstrual dysphoria. Neuropsychopharmacology. 1997;16(5):346-356.
30. Steinberg EM, Cardoso GM, Martinez PE, et al. Rapid response to fluoxetine in women with premenstrual dysphoric disorder. Depress Anxiety. 2012;29(6):531-540.
31. Roca CA, Schmidt PJ, Smith MJ, et al. Effects of metergoline on symptoms in women with premenstrual dysphoric disorder. Am J Psychiatry. 2002;159(11):1876-1881.
32. Gray JD, Milner TA, McEwen BS. Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience. 2013;239:214-227.
33. Carbone DL, Handa RJ. Sex and stress hormone influences on the expression and activity of brain-derived neurotrophic factor. Neuroscience. 2013;239:295-303.
34. Pilar-Cuéllar F, Vidal R, Pazos A. Subchronic treatment with fluoxetine and ketanserin increases hippocampal brain-derived neurotrophic factor, β-catenin and antidepressant-like effects. Br J Pharmacol. 2012;165(4b):1046-1057.
35. Deuschle M, Gilles M, Scharnholz B, et al. Changes of serum concentrations of brain-derived neurotrophic factor (BDNF) during treatment with venlafaxine and mirtazapine: role of medication and response to treatment. Pharmacopsychiatry. 2013;46(2):54-58.
36. Berman SM, London ED, Morgan M, et al. Elevated gray matter volume of the emotional cerebellum in women with premenstrual dysphoric disorder. J Affect Disord. 2013;146(2):266-271.
37. Jeong HG, Ham BJ, Yeo HB, et al. Gray matter abnormalities in patients with premenstrual dysphoric disorder: an optimized voxel-based morphometry. J Affect Disord. 2012;140(3):260-267.
38. Protopopescu X, Tuescher O, Pan H, et al. Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affect Disord. 2008;108(1-2):87-94.
39. Gingnell M, Morell A, Bannbers E, et al. Menstrual cycle effects on amygdala reactivity to emotional stimulation in premenstrual dysphoric disorder. Horm Behav. 2012;62(4):400-406.
40. Epperson CN, Haga K, Mason GF, et al. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59(9):851-858.
41. Gingnell M, Bannbers E, Wikström J, et al. Premenstrual dysphoric disorder and prefrontal reactivity during anticipation of emotional stimuli. Eur Neuropsychopharmacol. 2013;23(11):1474-1483.
42. Baller EB, Wei SM, Kohn PD, et al. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: a multimodal neuroimaging study. Am J Psychiatry. 2013;170(3):305-314.
43. Rasgon N, McGuire M, Tanavoli S, et al. Neuroendocrine response to an intravenous L-tryptophan challenge in women with premenstrual syndrome. Fertil Steril. 2000;73(1):144-149.
44. Huang Y, Zhou R, Wu M, et al. Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress. 2015;18(2):160-168.
45. Segebladh B, Bannbers E, Moby L, et al. Allopregnanolone serum concentrations and diurnal cortisol secretion in women with premenstrual dysphoric disorder. Arch Womens Ment Health. 2013;16(2):131-137.
46. Pilver CE, Levy BR, Libby DJ, et al. Posttraumatic stress disorder and trauma characteristics are correlates of premenstrual dysphoric disorder. Arch Womens Ment Health. 2011;14(5):383-393.
47. Bertone-Johnson ER, Whitcomb BW, Missmer SA, et al. Early life emotional, physical, and sexual abuse and the development of premenstrual syndrome: a longitudinal study. J Womens Health (Larchmt). 2014;23(9):729-739.
48. Segebladh B, Bannbers E, Kask K, et al. Prevalence of violence exposure in women with premenstrual dysphoric disorder in comparison with other gynecological patients and asymptomatic controls. Acta Obstet Gynecol Scand. 2011;90(7):746-752.
49. Kask K, Gulinello M, Bäckström T, et al. Patients with premenstrual dysphoric disorder have increased startle response across both cycle phases and lower levels of prepulse inhibition during the late luteal phase of the menstrual cycle. Neuropsychopharmacology. 2008;33(9):2283-2290.
50. O’Brien SM, Fitzgerald P, Scully P, et al. Impact of gender and menstrual cycle phase on plasma cytokine concentrations. Neuroimmunomodulation. 2007;14(2):84-90.
51. Northoff H, Symons S, Zieker D, et al. Gender- and menstrual phase dependent regulation of inflammatory gene expression in response to aerobic exercise. Exerc Immunol Rev. 2008;14:86-103.
52. Gaskins AJ, Wilchesky M, Mumford SL, et al. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: the BioCycle Study. Am J Epidemiol. 2012;175(5):423-431.
53. Wander K, Brindle E, O’Connor KA. C-reactive protein across the menstrual cycle. Am J Phys Anthropol. 2008;136(2):138-146.
54. Jane ZY, Chang CC, Lin HK, et al. The association between the exacerbation of irritable bowel syndrome and menstrual symptoms in young Taiwanese women. Gastroenterol Nurs. 2011;34(4):277-286.
55. Kane SV, Sable K, Hanauer SB. The menstrual cycle and its effect on inflammatory bowel disease and irritable bowel syndrome: a prevalence study. Am J Gastroenterol. 1998;93(10):1867-1872.
56. Shourie V, Dwarakanath CD, Prashanth GV, et al. The effect of menstrual cycle on periodontal health - a clinical and microbiological study. Oral Health Prev Dent. 2012;10(2):185-192.
57. Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. 2015;17(11):87.
58. Maeng LY, Milad MR. Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Horm Behav. 2015;76:106-117.
59. Lee YJ, Yi SW, Ju DH, et al. Correlation between postpartum depression and premenstrual dysphoric disorder: single center study. Obstet Gynecol Sci. 2015;58(5):353-358.
60. Kepple AL, Lee EE, Haq N, et al. History of postpartum depression in a clinic-based sample of women with premenstrual dysphoric disorder. J Clin Psychiatry. 2016;77(4):e415-e420.
61. Schmidt PJ, Nieman LK, Danaceau MA, et al. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;338(4):209-216.
62. Schmidt PJ, Martinez PE, Nieman LK, et al. Premenstrual dysphoric disorder symptoms following ovarian suppression: Triggered by change in ovarian steroid levels but not continuous stable levels. Am J Psychiatry. [published online April 21, 2017]. doi: 10.1176/appi.ajp.2017.16101113.
1. Miller A, Vo H, Huo L, et al. Estrogen receptor alpha (ESR-1) associations with psychological traits in women with PMDD and controls. J Psychiatr Res. 2010;44(12):788-794.
2. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169(5):465-475.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Wilson CA, Turner CW, Keye WR Jr. Firstborn adolescent daughters and mothers with and without premenstrual syndrome: a comparison. J Adolesc Health. 1991;12(2):130-137.
5. Kendler KS, Silberg JL, Neale MC, et al. Genetic and environmental factors in the aetiology of menstrual, premenstrual and neurotic symptoms: a population-based twin study. Psychol Med. 1992;22(1):85-100.
6. Condon JT. The premenstrual syndrome: a twin study. Br J Psychiatry. 1993;162:481-486.
7. Kendler KS, Karkowski LM, Corey LA, et al. Longitudinal population-based twin study of retrospectively reported premenstrual symptoms and lifetime major depression. Am J Psychiatry. 1998;155(9):1234-1240.
8. Huo L, Straub RE, Roca C, et al. Risk for premenstrual dysphoric disorder is associated with genetic variation in ESR1, the estrogen receptor alpha gene. Biol Psychiatry. 2007;62(8):925-933.
9. Dhingra V, Magnay JL, O’Brien PM, et al. Serotonin receptor 1A C(-1019)G polymorphism associated with premenstrual dysphoric disorder. Obstet Gynecol. 2007;110(4):788-792.
10. Comasco E, Hahn A, Ganger S, et al. Emotional fronto-cingulate cortex activation and brain derived neurotrophic factor polymorphism in premenstrual dysphoric disorder. Hum Brain Mapp. 2014;35(9):4450-4458.
11. Praschak-Rieder N, Willeit M, Winkler D, et al. Role of family history and 5-HTTLPR polymorphism in female seasonal affective disorder patients with and without premenstrual dysphoric disorder. Eur Neuropsychopharmacol. 2002;12(2):129-134.
12. Klatzkin RR, Morrow AL, Light KC, et al. Associations of histories of depression and PMDD diagnosis with allopregnanolone concentrations following the oral administration of micronized progesterone. Psychoneuroendocrinology. 2006;31(10):1208-1219.
13. Crowley SK, Girdler SS. Neurosteroid, GABAergic and hypothalamic pituitary adrenal (HPA) axis regulation: what is the current state of knowledge in humans? Psychopharmacology (Berl). 2014;231(17):3619-3634.
14. Girdler SS, Straneva PA, Light KC, et al. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49(9):788-797.
15. Rapkin AJ, Morgan M, Goldman L, et al. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol. 1997;90(5):709-714.
16. Bicíková M, Dibbelt L, Hill M, et al. Allopregnanolone in women with premenstrual syndrome. Horm Metab Res. 1998;30(4):227-230.
17. Monteleone P, Luisi S, Tonetti A, et al. Allopregnanolone concentrations and premenstrual syndrome. Eur J Endocrinol. 2000;142(3):269-273.
18. Steiner M, Steinberg S, Stewart D, et al. Fluoxetine in the treatment of premenstrual dysphoria. Canadian Fluoxetine/Premenstrual Dysphoria Collaborative Study Group. N Engl J Med. 1995;332(23):1529-1534.
19. Sundström I, Bäckström T. Citalopram increases pregnanolone sensitivity in patients with premenstrual syndrome: an open trial. Psychoneuroendocrinology. 1998;23(1):73-88.
20. Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci U S A. 1999;96(23):13512-13517.
21. Trauger JW, Jiang A, Stearns BA, et al. Kinetics of allopregnanolone formation catalyzed by human 3 alpha-hydroxysteroid dehydrogenase type III (AKR1C2). Biochemistry. 2002;41(45):13451-13459.
22. Shanmugan S, Epperson CN. Estrogen and the prefrontal cortex: towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Hum Brain Mapp. 2014;35(3):847-865.
23. Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry. 1998;44(9):839-850.
24. Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behav Cogn Neurosci Rev. 2005;4(1):43-58.
25. Cyr M, Bossé R, Di Paolo T. Gonadal hormones modulate 5-hydroxytryptamine2A receptors: emphasis on the rat frontal cortex. Neuroscience. 1998;83(3):829-836.
26. Fink G, Sumner BE, Rosie R, et al. Estrogen control of central neurotransmission: effect on mood, mental state, and memory. Cell Mol Neurobiol. 1996;16(3):325-344.
27. Sumner BE, Grant KE, Rosie R, et al. Effects of tamoxifen on serotonin transporter and 5-hydroxytryptamine(2A) receptor binding sites and mRNA levels in the brain of ovariectomized rats with or without acute estradiol replacement. Brain Res Mol Brain Res. 1999;73(1-2):119-128.
28. Moses-Kolko EL, Berga SL, Greer PJ, et al. Widespread increases of cortical serotonin type 2A receptor availability after hormone therapy in euthymic postmenopausal women. Fertil Steril. 2003;80(3):554-559.
29. Su TP, Schmidt PJ, Danaceau MA, et al. Fluoxetine in the treatment of premenstrual dysphoria. Neuropsychopharmacology. 1997;16(5):346-356.
30. Steinberg EM, Cardoso GM, Martinez PE, et al. Rapid response to fluoxetine in women with premenstrual dysphoric disorder. Depress Anxiety. 2012;29(6):531-540.
31. Roca CA, Schmidt PJ, Smith MJ, et al. Effects of metergoline on symptoms in women with premenstrual dysphoric disorder. Am J Psychiatry. 2002;159(11):1876-1881.
32. Gray JD, Milner TA, McEwen BS. Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience. 2013;239:214-227.
33. Carbone DL, Handa RJ. Sex and stress hormone influences on the expression and activity of brain-derived neurotrophic factor. Neuroscience. 2013;239:295-303.
34. Pilar-Cuéllar F, Vidal R, Pazos A. Subchronic treatment with fluoxetine and ketanserin increases hippocampal brain-derived neurotrophic factor, β-catenin and antidepressant-like effects. Br J Pharmacol. 2012;165(4b):1046-1057.
35. Deuschle M, Gilles M, Scharnholz B, et al. Changes of serum concentrations of brain-derived neurotrophic factor (BDNF) during treatment with venlafaxine and mirtazapine: role of medication and response to treatment. Pharmacopsychiatry. 2013;46(2):54-58.
36. Berman SM, London ED, Morgan M, et al. Elevated gray matter volume of the emotional cerebellum in women with premenstrual dysphoric disorder. J Affect Disord. 2013;146(2):266-271.
37. Jeong HG, Ham BJ, Yeo HB, et al. Gray matter abnormalities in patients with premenstrual dysphoric disorder: an optimized voxel-based morphometry. J Affect Disord. 2012;140(3):260-267.
38. Protopopescu X, Tuescher O, Pan H, et al. Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affect Disord. 2008;108(1-2):87-94.
39. Gingnell M, Morell A, Bannbers E, et al. Menstrual cycle effects on amygdala reactivity to emotional stimulation in premenstrual dysphoric disorder. Horm Behav. 2012;62(4):400-406.
40. Epperson CN, Haga K, Mason GF, et al. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59(9):851-858.
41. Gingnell M, Bannbers E, Wikström J, et al. Premenstrual dysphoric disorder and prefrontal reactivity during anticipation of emotional stimuli. Eur Neuropsychopharmacol. 2013;23(11):1474-1483.
42. Baller EB, Wei SM, Kohn PD, et al. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: a multimodal neuroimaging study. Am J Psychiatry. 2013;170(3):305-314.
43. Rasgon N, McGuire M, Tanavoli S, et al. Neuroendocrine response to an intravenous L-tryptophan challenge in women with premenstrual syndrome. Fertil Steril. 2000;73(1):144-149.
44. Huang Y, Zhou R, Wu M, et al. Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress. 2015;18(2):160-168.
45. Segebladh B, Bannbers E, Moby L, et al. Allopregnanolone serum concentrations and diurnal cortisol secretion in women with premenstrual dysphoric disorder. Arch Womens Ment Health. 2013;16(2):131-137.
46. Pilver CE, Levy BR, Libby DJ, et al. Posttraumatic stress disorder and trauma characteristics are correlates of premenstrual dysphoric disorder. Arch Womens Ment Health. 2011;14(5):383-393.
47. Bertone-Johnson ER, Whitcomb BW, Missmer SA, et al. Early life emotional, physical, and sexual abuse and the development of premenstrual syndrome: a longitudinal study. J Womens Health (Larchmt). 2014;23(9):729-739.
48. Segebladh B, Bannbers E, Kask K, et al. Prevalence of violence exposure in women with premenstrual dysphoric disorder in comparison with other gynecological patients and asymptomatic controls. Acta Obstet Gynecol Scand. 2011;90(7):746-752.
49. Kask K, Gulinello M, Bäckström T, et al. Patients with premenstrual dysphoric disorder have increased startle response across both cycle phases and lower levels of prepulse inhibition during the late luteal phase of the menstrual cycle. Neuropsychopharmacology. 2008;33(9):2283-2290.
50. O’Brien SM, Fitzgerald P, Scully P, et al. Impact of gender and menstrual cycle phase on plasma cytokine concentrations. Neuroimmunomodulation. 2007;14(2):84-90.
51. Northoff H, Symons S, Zieker D, et al. Gender- and menstrual phase dependent regulation of inflammatory gene expression in response to aerobic exercise. Exerc Immunol Rev. 2008;14:86-103.
52. Gaskins AJ, Wilchesky M, Mumford SL, et al. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: the BioCycle Study. Am J Epidemiol. 2012;175(5):423-431.
53. Wander K, Brindle E, O’Connor KA. C-reactive protein across the menstrual cycle. Am J Phys Anthropol. 2008;136(2):138-146.
54. Jane ZY, Chang CC, Lin HK, et al. The association between the exacerbation of irritable bowel syndrome and menstrual symptoms in young Taiwanese women. Gastroenterol Nurs. 2011;34(4):277-286.
55. Kane SV, Sable K, Hanauer SB. The menstrual cycle and its effect on inflammatory bowel disease and irritable bowel syndrome: a prevalence study. Am J Gastroenterol. 1998;93(10):1867-1872.
56. Shourie V, Dwarakanath CD, Prashanth GV, et al. The effect of menstrual cycle on periodontal health - a clinical and microbiological study. Oral Health Prev Dent. 2012;10(2):185-192.
57. Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. 2015;17(11):87.
58. Maeng LY, Milad MR. Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Horm Behav. 2015;76:106-117.
59. Lee YJ, Yi SW, Ju DH, et al. Correlation between postpartum depression and premenstrual dysphoric disorder: single center study. Obstet Gynecol Sci. 2015;58(5):353-358.
60. Kepple AL, Lee EE, Haq N, et al. History of postpartum depression in a clinic-based sample of women with premenstrual dysphoric disorder. J Clin Psychiatry. 2016;77(4):e415-e420.
61. Schmidt PJ, Nieman LK, Danaceau MA, et al. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;338(4):209-216.
62. Schmidt PJ, Martinez PE, Nieman LK, et al. Premenstrual dysphoric disorder symptoms following ovarian suppression: Triggered by change in ovarian steroid levels but not continuous stable levels. Am J Psychiatry. [published online April 21, 2017]. doi: 10.1176/appi.ajp.2017.16101113.
Pregnant and nursing patients benefit from ‘ambitious’ changes to drug labeling for safety
In December 2014, the FDA issued draft guidance for sweeping changes to labeling of pharmaceutical treatments in regard to pregnancy and lactation information. These changes are now in effect for use in practice.1 The undertaking has been years in the making, and is truly ambitious.
The outdated system of letter categories (A, B, C, D, X) falls short of clinical needs in several ways:
- the quality and volume of data can be lacking
- comparative risk is not described
- using letters can led to oversimplification or, in some cases, exaggeration of risk and safety (Box).
Other drawbacks include infrequent updating of information and omission of information about baseline rates of reproductive-related adverse events, to provide a more meaningful context for risk assessment.
A note before we continue discussion of labeling: Recognize that pregnancy itself is inherently risky; poor outcomes are, regrettably, not uncommon. The rate of birth defects in the United States is approximately 3%, and obstetric complications, such as prematurity, are common.2,3
New system described
The new labeling content has been described in the FDA’s Pregnancy and Lactation Labeling Rule (also called the “final rule”), issued in December 2014. For each medication, there will be subsections in the labeling:
- Pregnancy
- Lactation
- Females and Males of Reproductive Potential.
In addition, FDA instructions now state that labeling:
- must be updated when new information becomes available
- needs to include evaluation of human data that becomes available mainly after the drug is approved
- needs to include information about the background rates of adverse events related to reproduction.
Labeling in pregnancy. As an example, the “Pregnancy” section of every label contains 3 subsections, all of great clinical importance. First is information about pregnancy exposure registries, with a listing of scientifically acceptable registries (if a registry is available for that drug) and contact information; this section focuses on the high value of data that are systematically and prospectively collected. The second summarizes risk associated with the drug during pregnancy, based on available human, animal, and pharmacologic data. Third is a discussion of clinical considerations.
Need for appropriate controls. Psychiatric disorders increase the risk of pregnancy complications, and often are associated with variables that might increase the risk of a poor pregnancy outcome. For example, a patient who has a psychiatric disorder might be less likely to seek prenatal care, take a prenatal vitamin, and sleep and eat well; she also might use alcohol, tobacco, or other substances of abuse.
The medical literature on the reproductive safety of psychotropic medications is fraught with confounding variables other than the medications themselves. These include variables that, taken alone, might confer a poorer outcome on the fetus or newborn of a pregnant or lactating woman who has a psychiatric illness (to the extent that she uses psychotropics during a pregnancy), compared with what would be seen in (1) a healthy woman who is not taking such medication or (2) the general population.
On the new labels, detailed statements on human data include information from clinical trials, pregnancy exposure registries, and epidemiologic studies. Labels are also to include:
- incidence of adverse events
- effect of dosage
- effect of duration of exposure
- effect of gestational timing of exposure.
The labels emphasize quantifying risk relative to the risk of the same outcome in infants born to women who have not been exposed to the particular drug, but who have the disease or condition for which the drug is indicated (ie, appropriate controls).
Clinical considerations are to include information on the following related to the specific medication (when that information is known):
- more information for prescribers, to further risk-benefit counseling
- disease-associated maternal-fetal risks
- dosage adjustments during pregnancy and postpartum
- maternal adverse reactions
- fetal and neonatal adverse reactions
- labor and delivery.
Clearly, this overdue shift in providing information regarding reproductive safety has the potential to inform clinicians and patients in a meaningful way about the risks and benefits of specific treatments during pregnancy and lactation. Translating that information into practice is daunting, however.
Important aspects of implementation
Pregnancy exposure registries will play a crucial role. For most medications, no systematic registry has been established; to do so, rigorous methodology is required to acquire prospective data and account for confounding variables.4 Appropriate control groups also are required to yield data that are useful and interpretable. Primary outcomes require verification, such as review of medical records. Last, registries must be well-conducted and therefore adequately funded, yet labeling changes have not been accompanied by funding requirements set forth by regulators to pharmaceutical manufacturers.
Labeling must be updated continually. Furthermore, it is unclear who will review data for precision and comprehensiveness.
Data need to be understandable to health care providers across disciplines and to patients with varying levels of education for the label to have a meaningful impact on clinical care.
As noted, there is no mandate for funding the meticulous pharmacovigilance required to provide definitive data for labeling. It is unclear if the potential benefits of the new labeling can be reaped without adequate financing of the pharmacovigilance mechanisms required to inform patients adequately.
Role of pregnancy registries
Over the past 2 decades, pregnancy registries have emerged as a rapid, systematic means of collecting important reproductive safety data on the risk for major malformations after prenatal exposure to a medication or a class of medications.5,6 Such registries enhance the rigor of available cohort studies and other analyses of reproductive safety data that have been derived from large administrative databases.
NPRAA and NPRAD. Recently, the National Pregnancy Registry for Atypical Antipsychotics (NPRAA) and the National Pregnancy Registry for Antidepressants (NPRAD) were established in an effort to obtain reproductive safety data about fetal exposure to second-generation antipsychotics (SGAs) and to newer antidepressants.7 Based at Massachusetts General Hospital in Boston, NPRAA and NPRAD systematically and prospectively evaluate the risk of malformations among infants who have been exposed in utero to an SGA or an antidepressant.
The structure of both registries are the same, modeled after the North American Antiepileptic Drug Registry.5,8 Data are collected prospectively from pregnant women, age 18 to 45, by means of 3 telephone interviews conducted proximate to enrollment, at 7 months’ gestation, and at 2 or 3 months’ postpartum.
Participants include (1) pregnant women who have a history of fetal exposure to an SGA or an antidepressant, or both, and (2) a comparison group of non-exposed pregnant women who have a history of a psychiatric illness. Authorization for release of medical records is obtained for obstetric care, labor and delivery, and neonatal care (≤6 months of age).
Information on the presence of major malformations is abstracted from the medical record, along with other data on neonatal and maternal health outcomes. Identified cases of a congenital malformation are sent to a dysmorphologist, who has been blinded to drug exposure, for final adjudication. Release of findings is dictated by a governing Scientific Advisory Board.
Results so far. Results are available from the NPRAA.9 As of December 2014, 487 women were enrolled: 353 who used an SGA and 134 comparison women. Medical records were obtained for 82.2% of participants. A total of 303 women completed the study and were eligible for inclusion in the analysis. Findings include:
- Of 214 live births with first-trimester exposure to an SGA, 3 major malformations were confirmed. In the control group (n = 89), 1 major malformation was confirmed
- The absolute risk of a major malformation was 1.4% for an exposed infant and 1.1% for an unexposed infant
- The odds ratio for a major malformation, comparing exposed infants with unexposed infants, was 1.25 (95% CI, 0.13–12.19).
It is reasonable, therefore, to conclude that, as a class, SGAs are not major teratogens. Although the confidence intervals around the odds ratio estimate remain wide, with the probability for change over the course of the study, it is unlikely that risk will rise to the level of known major teratogens, such as valproate and thalidomide.10,11
Help with decision-making
Given recent FDA guidance about the importance of pregnancy registries (www.fda.gov/pregnancyregistries), such carefully collected data might help clinicians and patients make informed choices about treatment. Future efforts of NPRAA and NPRAD will focus on sustaining growth in enrollment of participants so that the reproductive safety of SGAs and newer antidepressants can be delineated more clearly.
Last, you can refer potential participants to NPRAA and NPRAD by calling 1-866-961-2388. More information is available at www.womensmentalhealth.org.
1. U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research (CDER); Center for Biologic Evaluation and Research (CBER). Pregnancy, lactation, and reproductive potential: labeling for human prescription drug and biological products—content and format: guidance for industry. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM425398.pdf. Published December 2014. Accessed June 7, 2016.
2. Centers for Disease Control and Prevention. Birth defects. http://www.cdc.gov/ncbddd/birthdefects/facts.html. Updated September 21, 2005. Accessed June 7, 2016.
3. Centers for Disease Control and Prevention. Preterm birth. http://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth.htm. Updated December 4, 2015. Accessed June 7, 2016.
4. U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research (CDER); Center for Biologic Evaluation and Research (CBER). Guidance for industry: establishing pregnancy exposure registries. http://www.fda.gov/downloads/ScienceResearch/SpecialTopics/WomensHealthResearch/UCM133332.pdf. Published August 2002. Accessed June 7, 2016.
5. Holmes LB, Wyszynski DF. North American antiepileptic drug pregnancy registry. Epilepsia. 2004;45(11):1465.
6. Tomson T, Battino D, Craig J, et al; ILAE Commission on Therapeutic Strategies. Pregnancy registries: differences, similarities, and possible harmonization. Epilepsia. 2010;51(5):909-915.
7. Cohen LS, Viguera AC, McInerney KA, et al. Establishment of the National Pregnancy Registry for Atypical Antipsychotics. J Clin Psychiatry. 2015;76(7):986-989.
8. Holmes LB, Wyszynski DF, Lieberman E. The AED (antiepileptic drug) pregnancy registry: a 6-year experience. Arch Neurol. 2004;61(5):673-678.
9. Cohen LS, Viguera AC, McInerney KA, et al. Reproductive safety of second-generation antipsychotics: current data from the Massachusetts General Hospital National Pregnancy Registry for Atypical Antipsychotics. Am J Psychiatry. 2016;173(3):263-270.
10. McBride WG. Thalidomide and congenital abnormalities. Lancet. 1961;2(7216):1358.
11. Wyszynski DF, Nambisan M, Surve T, et al; Antiepileptic Drug Pregnancy Registry. Increased rate of major malformations in offspring exposed to valproate during pregnancy. Neurology. 2005;64(6):961-965.
In December 2014, the FDA issued draft guidance for sweeping changes to labeling of pharmaceutical treatments in regard to pregnancy and lactation information. These changes are now in effect for use in practice.1 The undertaking has been years in the making, and is truly ambitious.
The outdated system of letter categories (A, B, C, D, X) falls short of clinical needs in several ways:
- the quality and volume of data can be lacking
- comparative risk is not described
- using letters can led to oversimplification or, in some cases, exaggeration of risk and safety (Box).
Other drawbacks include infrequent updating of information and omission of information about baseline rates of reproductive-related adverse events, to provide a more meaningful context for risk assessment.
A note before we continue discussion of labeling: Recognize that pregnancy itself is inherently risky; poor outcomes are, regrettably, not uncommon. The rate of birth defects in the United States is approximately 3%, and obstetric complications, such as prematurity, are common.2,3
New system described
The new labeling content has been described in the FDA’s Pregnancy and Lactation Labeling Rule (also called the “final rule”), issued in December 2014. For each medication, there will be subsections in the labeling:
- Pregnancy
- Lactation
- Females and Males of Reproductive Potential.
In addition, FDA instructions now state that labeling:
- must be updated when new information becomes available
- needs to include evaluation of human data that becomes available mainly after the drug is approved
- needs to include information about the background rates of adverse events related to reproduction.
Labeling in pregnancy. As an example, the “Pregnancy” section of every label contains 3 subsections, all of great clinical importance. First is information about pregnancy exposure registries, with a listing of scientifically acceptable registries (if a registry is available for that drug) and contact information; this section focuses on the high value of data that are systematically and prospectively collected. The second summarizes risk associated with the drug during pregnancy, based on available human, animal, and pharmacologic data. Third is a discussion of clinical considerations.
Need for appropriate controls. Psychiatric disorders increase the risk of pregnancy complications, and often are associated with variables that might increase the risk of a poor pregnancy outcome. For example, a patient who has a psychiatric disorder might be less likely to seek prenatal care, take a prenatal vitamin, and sleep and eat well; she also might use alcohol, tobacco, or other substances of abuse.
The medical literature on the reproductive safety of psychotropic medications is fraught with confounding variables other than the medications themselves. These include variables that, taken alone, might confer a poorer outcome on the fetus or newborn of a pregnant or lactating woman who has a psychiatric illness (to the extent that she uses psychotropics during a pregnancy), compared with what would be seen in (1) a healthy woman who is not taking such medication or (2) the general population.
On the new labels, detailed statements on human data include information from clinical trials, pregnancy exposure registries, and epidemiologic studies. Labels are also to include:
- incidence of adverse events
- effect of dosage
- effect of duration of exposure
- effect of gestational timing of exposure.
The labels emphasize quantifying risk relative to the risk of the same outcome in infants born to women who have not been exposed to the particular drug, but who have the disease or condition for which the drug is indicated (ie, appropriate controls).
Clinical considerations are to include information on the following related to the specific medication (when that information is known):
- more information for prescribers, to further risk-benefit counseling
- disease-associated maternal-fetal risks
- dosage adjustments during pregnancy and postpartum
- maternal adverse reactions
- fetal and neonatal adverse reactions
- labor and delivery.
Clearly, this overdue shift in providing information regarding reproductive safety has the potential to inform clinicians and patients in a meaningful way about the risks and benefits of specific treatments during pregnancy and lactation. Translating that information into practice is daunting, however.
Important aspects of implementation
Pregnancy exposure registries will play a crucial role. For most medications, no systematic registry has been established; to do so, rigorous methodology is required to acquire prospective data and account for confounding variables.4 Appropriate control groups also are required to yield data that are useful and interpretable. Primary outcomes require verification, such as review of medical records. Last, registries must be well-conducted and therefore adequately funded, yet labeling changes have not been accompanied by funding requirements set forth by regulators to pharmaceutical manufacturers.
Labeling must be updated continually. Furthermore, it is unclear who will review data for precision and comprehensiveness.
Data need to be understandable to health care providers across disciplines and to patients with varying levels of education for the label to have a meaningful impact on clinical care.
As noted, there is no mandate for funding the meticulous pharmacovigilance required to provide definitive data for labeling. It is unclear if the potential benefits of the new labeling can be reaped without adequate financing of the pharmacovigilance mechanisms required to inform patients adequately.
Role of pregnancy registries
Over the past 2 decades, pregnancy registries have emerged as a rapid, systematic means of collecting important reproductive safety data on the risk for major malformations after prenatal exposure to a medication or a class of medications.5,6 Such registries enhance the rigor of available cohort studies and other analyses of reproductive safety data that have been derived from large administrative databases.
NPRAA and NPRAD. Recently, the National Pregnancy Registry for Atypical Antipsychotics (NPRAA) and the National Pregnancy Registry for Antidepressants (NPRAD) were established in an effort to obtain reproductive safety data about fetal exposure to second-generation antipsychotics (SGAs) and to newer antidepressants.7 Based at Massachusetts General Hospital in Boston, NPRAA and NPRAD systematically and prospectively evaluate the risk of malformations among infants who have been exposed in utero to an SGA or an antidepressant.
The structure of both registries are the same, modeled after the North American Antiepileptic Drug Registry.5,8 Data are collected prospectively from pregnant women, age 18 to 45, by means of 3 telephone interviews conducted proximate to enrollment, at 7 months’ gestation, and at 2 or 3 months’ postpartum.
Participants include (1) pregnant women who have a history of fetal exposure to an SGA or an antidepressant, or both, and (2) a comparison group of non-exposed pregnant women who have a history of a psychiatric illness. Authorization for release of medical records is obtained for obstetric care, labor and delivery, and neonatal care (≤6 months of age).
Information on the presence of major malformations is abstracted from the medical record, along with other data on neonatal and maternal health outcomes. Identified cases of a congenital malformation are sent to a dysmorphologist, who has been blinded to drug exposure, for final adjudication. Release of findings is dictated by a governing Scientific Advisory Board.
Results so far. Results are available from the NPRAA.9 As of December 2014, 487 women were enrolled: 353 who used an SGA and 134 comparison women. Medical records were obtained for 82.2% of participants. A total of 303 women completed the study and were eligible for inclusion in the analysis. Findings include:
- Of 214 live births with first-trimester exposure to an SGA, 3 major malformations were confirmed. In the control group (n = 89), 1 major malformation was confirmed
- The absolute risk of a major malformation was 1.4% for an exposed infant and 1.1% for an unexposed infant
- The odds ratio for a major malformation, comparing exposed infants with unexposed infants, was 1.25 (95% CI, 0.13–12.19).
It is reasonable, therefore, to conclude that, as a class, SGAs are not major teratogens. Although the confidence intervals around the odds ratio estimate remain wide, with the probability for change over the course of the study, it is unlikely that risk will rise to the level of known major teratogens, such as valproate and thalidomide.10,11
Help with decision-making
Given recent FDA guidance about the importance of pregnancy registries (www.fda.gov/pregnancyregistries), such carefully collected data might help clinicians and patients make informed choices about treatment. Future efforts of NPRAA and NPRAD will focus on sustaining growth in enrollment of participants so that the reproductive safety of SGAs and newer antidepressants can be delineated more clearly.
Last, you can refer potential participants to NPRAA and NPRAD by calling 1-866-961-2388. More information is available at www.womensmentalhealth.org.
In December 2014, the FDA issued draft guidance for sweeping changes to labeling of pharmaceutical treatments in regard to pregnancy and lactation information. These changes are now in effect for use in practice.1 The undertaking has been years in the making, and is truly ambitious.
The outdated system of letter categories (A, B, C, D, X) falls short of clinical needs in several ways:
- the quality and volume of data can be lacking
- comparative risk is not described
- using letters can led to oversimplification or, in some cases, exaggeration of risk and safety (Box).
Other drawbacks include infrequent updating of information and omission of information about baseline rates of reproductive-related adverse events, to provide a more meaningful context for risk assessment.
A note before we continue discussion of labeling: Recognize that pregnancy itself is inherently risky; poor outcomes are, regrettably, not uncommon. The rate of birth defects in the United States is approximately 3%, and obstetric complications, such as prematurity, are common.2,3
New system described
The new labeling content has been described in the FDA’s Pregnancy and Lactation Labeling Rule (also called the “final rule”), issued in December 2014. For each medication, there will be subsections in the labeling:
- Pregnancy
- Lactation
- Females and Males of Reproductive Potential.
In addition, FDA instructions now state that labeling:
- must be updated when new information becomes available
- needs to include evaluation of human data that becomes available mainly after the drug is approved
- needs to include information about the background rates of adverse events related to reproduction.
Labeling in pregnancy. As an example, the “Pregnancy” section of every label contains 3 subsections, all of great clinical importance. First is information about pregnancy exposure registries, with a listing of scientifically acceptable registries (if a registry is available for that drug) and contact information; this section focuses on the high value of data that are systematically and prospectively collected. The second summarizes risk associated with the drug during pregnancy, based on available human, animal, and pharmacologic data. Third is a discussion of clinical considerations.
Need for appropriate controls. Psychiatric disorders increase the risk of pregnancy complications, and often are associated with variables that might increase the risk of a poor pregnancy outcome. For example, a patient who has a psychiatric disorder might be less likely to seek prenatal care, take a prenatal vitamin, and sleep and eat well; she also might use alcohol, tobacco, or other substances of abuse.
The medical literature on the reproductive safety of psychotropic medications is fraught with confounding variables other than the medications themselves. These include variables that, taken alone, might confer a poorer outcome on the fetus or newborn of a pregnant or lactating woman who has a psychiatric illness (to the extent that she uses psychotropics during a pregnancy), compared with what would be seen in (1) a healthy woman who is not taking such medication or (2) the general population.
On the new labels, detailed statements on human data include information from clinical trials, pregnancy exposure registries, and epidemiologic studies. Labels are also to include:
- incidence of adverse events
- effect of dosage
- effect of duration of exposure
- effect of gestational timing of exposure.
The labels emphasize quantifying risk relative to the risk of the same outcome in infants born to women who have not been exposed to the particular drug, but who have the disease or condition for which the drug is indicated (ie, appropriate controls).
Clinical considerations are to include information on the following related to the specific medication (when that information is known):
- more information for prescribers, to further risk-benefit counseling
- disease-associated maternal-fetal risks
- dosage adjustments during pregnancy and postpartum
- maternal adverse reactions
- fetal and neonatal adverse reactions
- labor and delivery.
Clearly, this overdue shift in providing information regarding reproductive safety has the potential to inform clinicians and patients in a meaningful way about the risks and benefits of specific treatments during pregnancy and lactation. Translating that information into practice is daunting, however.
Important aspects of implementation
Pregnancy exposure registries will play a crucial role. For most medications, no systematic registry has been established; to do so, rigorous methodology is required to acquire prospective data and account for confounding variables.4 Appropriate control groups also are required to yield data that are useful and interpretable. Primary outcomes require verification, such as review of medical records. Last, registries must be well-conducted and therefore adequately funded, yet labeling changes have not been accompanied by funding requirements set forth by regulators to pharmaceutical manufacturers.
Labeling must be updated continually. Furthermore, it is unclear who will review data for precision and comprehensiveness.
Data need to be understandable to health care providers across disciplines and to patients with varying levels of education for the label to have a meaningful impact on clinical care.
As noted, there is no mandate for funding the meticulous pharmacovigilance required to provide definitive data for labeling. It is unclear if the potential benefits of the new labeling can be reaped without adequate financing of the pharmacovigilance mechanisms required to inform patients adequately.
Role of pregnancy registries
Over the past 2 decades, pregnancy registries have emerged as a rapid, systematic means of collecting important reproductive safety data on the risk for major malformations after prenatal exposure to a medication or a class of medications.5,6 Such registries enhance the rigor of available cohort studies and other analyses of reproductive safety data that have been derived from large administrative databases.
NPRAA and NPRAD. Recently, the National Pregnancy Registry for Atypical Antipsychotics (NPRAA) and the National Pregnancy Registry for Antidepressants (NPRAD) were established in an effort to obtain reproductive safety data about fetal exposure to second-generation antipsychotics (SGAs) and to newer antidepressants.7 Based at Massachusetts General Hospital in Boston, NPRAA and NPRAD systematically and prospectively evaluate the risk of malformations among infants who have been exposed in utero to an SGA or an antidepressant.
The structure of both registries are the same, modeled after the North American Antiepileptic Drug Registry.5,8 Data are collected prospectively from pregnant women, age 18 to 45, by means of 3 telephone interviews conducted proximate to enrollment, at 7 months’ gestation, and at 2 or 3 months’ postpartum.
Participants include (1) pregnant women who have a history of fetal exposure to an SGA or an antidepressant, or both, and (2) a comparison group of non-exposed pregnant women who have a history of a psychiatric illness. Authorization for release of medical records is obtained for obstetric care, labor and delivery, and neonatal care (≤6 months of age).
Information on the presence of major malformations is abstracted from the medical record, along with other data on neonatal and maternal health outcomes. Identified cases of a congenital malformation are sent to a dysmorphologist, who has been blinded to drug exposure, for final adjudication. Release of findings is dictated by a governing Scientific Advisory Board.
Results so far. Results are available from the NPRAA.9 As of December 2014, 487 women were enrolled: 353 who used an SGA and 134 comparison women. Medical records were obtained for 82.2% of participants. A total of 303 women completed the study and were eligible for inclusion in the analysis. Findings include:
- Of 214 live births with first-trimester exposure to an SGA, 3 major malformations were confirmed. In the control group (n = 89), 1 major malformation was confirmed
- The absolute risk of a major malformation was 1.4% for an exposed infant and 1.1% for an unexposed infant
- The odds ratio for a major malformation, comparing exposed infants with unexposed infants, was 1.25 (95% CI, 0.13–12.19).
It is reasonable, therefore, to conclude that, as a class, SGAs are not major teratogens. Although the confidence intervals around the odds ratio estimate remain wide, with the probability for change over the course of the study, it is unlikely that risk will rise to the level of known major teratogens, such as valproate and thalidomide.10,11
Help with decision-making
Given recent FDA guidance about the importance of pregnancy registries (www.fda.gov/pregnancyregistries), such carefully collected data might help clinicians and patients make informed choices about treatment. Future efforts of NPRAA and NPRAD will focus on sustaining growth in enrollment of participants so that the reproductive safety of SGAs and newer antidepressants can be delineated more clearly.
Last, you can refer potential participants to NPRAA and NPRAD by calling 1-866-961-2388. More information is available at www.womensmentalhealth.org.
1. U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research (CDER); Center for Biologic Evaluation and Research (CBER). Pregnancy, lactation, and reproductive potential: labeling for human prescription drug and biological products—content and format: guidance for industry. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM425398.pdf. Published December 2014. Accessed June 7, 2016.
2. Centers for Disease Control and Prevention. Birth defects. http://www.cdc.gov/ncbddd/birthdefects/facts.html. Updated September 21, 2005. Accessed June 7, 2016.
3. Centers for Disease Control and Prevention. Preterm birth. http://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth.htm. Updated December 4, 2015. Accessed June 7, 2016.
4. U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research (CDER); Center for Biologic Evaluation and Research (CBER). Guidance for industry: establishing pregnancy exposure registries. http://www.fda.gov/downloads/ScienceResearch/SpecialTopics/WomensHealthResearch/UCM133332.pdf. Published August 2002. Accessed June 7, 2016.
5. Holmes LB, Wyszynski DF. North American antiepileptic drug pregnancy registry. Epilepsia. 2004;45(11):1465.
6. Tomson T, Battino D, Craig J, et al; ILAE Commission on Therapeutic Strategies. Pregnancy registries: differences, similarities, and possible harmonization. Epilepsia. 2010;51(5):909-915.
7. Cohen LS, Viguera AC, McInerney KA, et al. Establishment of the National Pregnancy Registry for Atypical Antipsychotics. J Clin Psychiatry. 2015;76(7):986-989.
8. Holmes LB, Wyszynski DF, Lieberman E. The AED (antiepileptic drug) pregnancy registry: a 6-year experience. Arch Neurol. 2004;61(5):673-678.
9. Cohen LS, Viguera AC, McInerney KA, et al. Reproductive safety of second-generation antipsychotics: current data from the Massachusetts General Hospital National Pregnancy Registry for Atypical Antipsychotics. Am J Psychiatry. 2016;173(3):263-270.
10. McBride WG. Thalidomide and congenital abnormalities. Lancet. 1961;2(7216):1358.
11. Wyszynski DF, Nambisan M, Surve T, et al; Antiepileptic Drug Pregnancy Registry. Increased rate of major malformations in offspring exposed to valproate during pregnancy. Neurology. 2005;64(6):961-965.
1. U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research (CDER); Center for Biologic Evaluation and Research (CBER). Pregnancy, lactation, and reproductive potential: labeling for human prescription drug and biological products—content and format: guidance for industry. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM425398.pdf. Published December 2014. Accessed June 7, 2016.
2. Centers for Disease Control and Prevention. Birth defects. http://www.cdc.gov/ncbddd/birthdefects/facts.html. Updated September 21, 2005. Accessed June 7, 2016.
3. Centers for Disease Control and Prevention. Preterm birth. http://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth.htm. Updated December 4, 2015. Accessed June 7, 2016.
4. U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research (CDER); Center for Biologic Evaluation and Research (CBER). Guidance for industry: establishing pregnancy exposure registries. http://www.fda.gov/downloads/ScienceResearch/SpecialTopics/WomensHealthResearch/UCM133332.pdf. Published August 2002. Accessed June 7, 2016.
5. Holmes LB, Wyszynski DF. North American antiepileptic drug pregnancy registry. Epilepsia. 2004;45(11):1465.
6. Tomson T, Battino D, Craig J, et al; ILAE Commission on Therapeutic Strategies. Pregnancy registries: differences, similarities, and possible harmonization. Epilepsia. 2010;51(5):909-915.
7. Cohen LS, Viguera AC, McInerney KA, et al. Establishment of the National Pregnancy Registry for Atypical Antipsychotics. J Clin Psychiatry. 2015;76(7):986-989.
8. Holmes LB, Wyszynski DF, Lieberman E. The AED (antiepileptic drug) pregnancy registry: a 6-year experience. Arch Neurol. 2004;61(5):673-678.
9. Cohen LS, Viguera AC, McInerney KA, et al. Reproductive safety of second-generation antipsychotics: current data from the Massachusetts General Hospital National Pregnancy Registry for Atypical Antipsychotics. Am J Psychiatry. 2016;173(3):263-270.
10. McBride WG. Thalidomide and congenital abnormalities. Lancet. 1961;2(7216):1358.
11. Wyszynski DF, Nambisan M, Surve T, et al; Antiepileptic Drug Pregnancy Registry. Increased rate of major malformations in offspring exposed to valproate during pregnancy. Neurology. 2005;64(6):961-965.
Awareness and management of obstetrical complications of depression
When a patient who has a preexisting medical illness seeks prenatal care, the obstetrician asks herself (himself) 2 questions:
• What impact will the illness have on the pregnancy?
• What impact will the pregnancy have on the illness?
Depression is both a pregnancy-associated and pregnancy-independent illness, which, in the setting of a pregnant woman who has a depressive disorder, makes these questions particularly difficult to answer. In such a case, coordination of care with a mental health provider is essential.
Awareness of the obstetrical complications associated with depression during pregnancy, as well as their implications for the future health of the mother–infant dyad, is important for the entire care team. This article reviews the associations and interconnectedness of depression with complications of pregnancy, childbirth, and the neonatal period.
Diagnosis of depression during prenatal care
The American College of Obstetricians and Gynecologists (ACOG) states that evidence is insufficient to support a recommendation for universal screening for depression among prenatal patients, although such screening should be considered.1 There is considerable variability among obstetrical providers regarding the practice of depression screening; tools to be used if such screening is done; and screening frequency through the pregnancy.
Discernment of depression is difficult. Many somatic symptoms of depression overlap with common prenatal complaints and, consequentially, can be overlooked. Among a sample of 700 pregnant women, for example, 56% complained of lack of energy; 19%, of insomnia; and 19%, of appetite changes.2 Weight change, of course, is universal.
The 10-question self-rating Edinburgh Postnatal Depression Scale has been validated for use during pregnancy and postnatally. This screening instrument can be helpful for differentiating purely physical complaints from mental distress due to depressive symptoms.2,3
When an obstetrical provider suspects a depressive disorder, or one has been diagnosed, she (he) faces the problem of what to do with that information. Women of low socioeconomic status and victims of domestic violence are at increased risk of depression during pregnancy, but barriers to appropriate referral can seem nearly insurmountable because they lack insurance and social support.4-9
In addition, within the setting of numerous tasks that need attending during the relatively short prenatal period, it is common for women newly given a diagnosis of depression to fail to follow up on a referral to a mental health provider.
Although most providers will “check in” with a depressed or at-risk patient at each prenatal visit about her mood, any effort at follow-up can be overshadowed by tangible physical concerns, such as preterm contractions, fetal growth restriction, and coordination of routine testing that has been delayed because of scant prenatal care. All these physical concerns and circumstances of care are associated with maternal depression, as we will discuss.
Preterm labor and birth
Preterm labor is defined as uterine contractions that lead to cervical change before 37 weeks gestational age. Preterm labor increases the risk of preterm birth; preterm labor precedes 50% of preterm births. Preterm birth is the leading cause of neonatal mortality in the United States, and rates of morbidity and mortality increase as gestational age decreases.10 Common neonatal complications related to prematurity are shown in the Figure.11
Women who suffer from depression have an increased risk of preterm labor and preterm birth, as many studies of treated and untreated depressed pregnant women have shown.12-20 The causative mechanism is unknown; it has been proposed that the increase in maternal cortisol production associated with depression and distress triggers overproduction of placental cortisol releasing hormone, which is thought to be involved in initiation of parturition.21,22 Depression also is associated with other risk factors for preterm birth, such as low socioeconomic status, substance use, and smoking.
Intrauterine growth restriction
Women who have depression during pregnancy have an increased risk of intrauterine growth restriction (IUGR), which leads to delivery of an infant who is small for gestational age (SGA) or of low birth weight (LBW) (weighing <2,500 g at birth), or both.23 Again, the basis of the association between depression and IUGR and SGA is unknown; it is theorized that increased levels of cortisol and catecholamines associated with maternal distress might, by increasing blood pressure and inducing vasoconstriction, cause placental hypoperfusion.24,25
It also is possible that the association of depression with other risk factors for IUGR, such as smoking, substance use, obesity, and poor prenatal care, puts the infants of depressed women at risk of growth restriction.26 Several large-scale studies showed that the association between LBW and depression is lost when smoking and substance use are accounted for; other studies, however, found a persistent association in untreated depressed women when smokers, substance users, and drinkers were excluded.17,26,27
IUGR infants are at increased risk of iatrogenic prematurity and stillbirth. Fetuses that weigh <10th percentile for their gestational age are delivered no later than 40 weeks; delivery can be indicated as early as 32 weeks, depending on the results of other antenatal tests. Women who have a growth-restricted infant have a higher risk of cesarean delivery because growth-restricted infants often have less reserve and poorer tolerance of labor.
Preeclampsia and eclampsia
Preeclampsia is defined as blood pressure >140/90 mm HG on at least 2 occasions, with proteinuria, that occurs later than the twentieth week of pregnancy in women who did not have hypertension or renal dysfunction at baseline. Preeclampsia is a progressive disease that can cause severe maternal morbidity, including renal failure, stroke, hepatic rupture, pulmonary edema, and heart failure.
Eclampsia refers to onset of seizures in the setting of preeclampsia. These 2 hypertensive disorders are the third leading world wide cause of maternal mortality.28
Depressed women have an elevated risk of preeclampsia. The association between preeclampsia and depression might be caused by the presence of increased levels of inflammatory mediators29,30; other comorbidities, such as increased body mass index, also might be involved, but the risk for preeclampsia in depressed women still is increased after controlling for obesity.31
The presence of preeclampsia is responsible for a high percentage of iatrogenic preterm births, because the cure for the disorder is delivery—even at early or previable gestational age. Complication rates for mother and infant are high.
The presence of preeclampsia is a significant risk factor for intrauterine fetal demise. Treating the mother after delivery involves administration of IV magnesium for 24 hours; often, the mother is separated from her infant for a day after birth.
Impact on prenatal care
Depression increases odds that women will have fewer prenatal visits.32 During pregnancy, women typically initiate prenatal care during the first trimester, when pregnancy-dating ultrasonography and early screening tests for chromosomal abnormalities are performed. Prenatal visits occur monthly until the third trimester, then every 2 weeks between 32 and 36 weeks’ gestation, increasing to weekly after 36 weeks’ gestation.
The increased number of visits in late pregnancy allows for early detection and treatment of hypertensive disorders; assesses fetal well-being; and decreases the risks of morbidity and mortality for mother and fetus.33 Because women who suffer from depression are at increased risk of an array of adverse pregnancy outcomes, the importance of regular and timely prenatal care cannot be understated.
In addition, the prenatal visit gives the obstetrician the opportunity to connect women with other specialists for management of any unmet medical needs. One study showed that, when women have adequate prenatal care (measured by the number of visits), the association between preterm birth and self-reported maternal depression was eliminated.34
Substance use
Substance use and depression often co-exist.35,36 Unlike screening for depression, screening for substance use is universal during prenatal care. Studies have shown that women who screen positive for depression are at higher risk of a number of comorbidities, including substance use.37,38 Conversely, women who use substances are more likely to screen positive for depression.
Evidence suggests that best practice might be to screen for depression in any woman who has a positive drug screen, if a provider is not routinely screening their general patient population.39 Substance use in pregnancy is associated with a number of poor outcomes, including placental abruption (cocaine use); dysmorphic facies and congenital anomalies (alcohol); and neonatal abstinence syndrome (heroin).
Antidepressants in pregnancy
A full discussion of the risks and benefits associated with pharmacotherapy for depression in pregnancy is beyond the scope of this article. Generally, antidepressant use is fraught with concerns over teratogenicity and adverse fetal outcomes. Although ACOG states that (1) pharmacotherapy for depression should be individualized and (2) most selective serotonin reuptake inhibitors (SSRIs) are not considered major teratogenic agents, many obstetricians and patients feel uncomfortable using these medications in pregnancy.40 Often, pre-pregnancy antidepressants are discontinued in the first trimester; one large population-based study found that only 0.9% of women who had depression filled their antidepressant prescription consistently throughout their pregnancy.41
It is unclear whether antidepressant use in pregnancy contributes to the risk of preterm birth seen in women who have depression. In a large population-based study, use of antidepressants in the second trimester was associated with preterm delivery but severe depression was not.18 A recent meta-analysis revealed an increased risk of preterm birth in women who used an antidepressant, compared with healthy women and untreated depressed women.42
Research limits, unanswered questions. Regrettably, it is difficult to untangle risk factors for preterm birth among depressed women without randomized controlled studies that are not ethically feasible. It cannot be said with certainty whether antidepressant pharmacotherapy is associated with a higher risk of preterm birth than depression alone.
Likewise, it is difficult to clarify the extent to which antidepressants contribute to infant growth restriction, if at all. Two recent meta-analyses concluded that exposure to antidepressants is associated with a statistically significant risk of LBW.42,43 However, increased severity of depressive symptoms generally is associated with exposure to antidepressants during pregnancy, and a randomized controlled trial is, again, impossible to conduct for ethical reasons.
Whereas a plausible biological mechanism associating IUGR, SGA, and LBW with depression exists, the same cannot be said for antidepressants. In one study, exposure to maternal depression altered the expression of certain placental genes but exposure to SSRIs did not cause further changes. This suggests that, on a cellular level, placental function might differ in depressed women.44 Although antidepressants do cross the placenta, it remains to be seen whether fetal growth is impacted as a result. One study found decreased fetal head circumference in infants who had been exposed to antidepressants during pregnancy, but no increased risk for having a SGA or LWB infant.45
Obstetrical management and mental health implications
Treated or not, women who suffer depression are a high-risk group when it comes to preterm birth and a host of other pregnancy comorbidities. Women with serious complications of pregnancy often are hospitalized for observation, and can undergo a prolonged stay when close proximity to medical services or a surgical suite is required.
For example, hospitalization until delivery is the standard of care for women who have preterm premature rupture of membranes or preeclampsia before 34 weeks’ gestation. Prolonged inpatient admissions and associated restriction of activity is profoundly deleterious on mood, with depression and anxiety significantly correlated with length of stay.46,47 Given the associations between depression and preterm birth, it might be reasonable to consider screening antenatal inpatients at risk of preterm birth for depression on a regular basis, so that treatment can be initiated if needed.
Depression during pregnancy is relatively common; an estimated 12.7% of pregnant women are affected at some time between conception and birth.48 Not only does depression appear to have deleterious effects on pregnancy outcomes, it also plays a pivotal role in the qualitative experience of pregnancy for the mother.
Bottom Line
Awareness of obstetrical complications associated with depression in pregnancy is important for the entire care team, including the psychiatrist and obstetrician. Depression not only appears to have deleterious effects on pregnancy outcomes, it also plays a pivotal role in the qualitative experience of pregnancy for the mother. Antidepressant use generally is fraught with concerns over teratogenicity and adverse fetal outcomes.
Related Resources
• Freeman MP. Some SSRIs are better than others for pregnant women (audio interview). Current Psychiatry. 2014;13(7). http://www.currentpsychiatry.com/specialty-focus/practice-trends/article/some-ssris-are-better-thanothers-for-pregnant-women/e3adb4704e25492f3e15331fc1cc058d.html.
• Freeman MP, Joffe H, Cohen LS. Postpartum depression: Help patients find the right treatment. Current Psychiatry. 2012;11(11):14-16,19-21.
Disclosures
Dr. Habecker reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Freeman is a member of the advisory board of JDS Therapeutics, Sunovion Pharmaceuticals, Inc., and Takeda Pharmaceutical Co. She receives research grant support from Takeda Pharmaceutical Co.
1. American College of Obstetricians and Gynecologists. Committee on Obstetric Practice. Committee opinion no. 630. 2015;125:1268-1271.
2. Apter G, Devouche E, Garez V, et al. Pregnancy, somatic complaints and depression: a French population-based study. Eur J Obstet Gynecol Reprod Biol. 2013;171(1):35-39.
3. Murray D, Cox JL. Screening for depression during pregnancy with the Edinburgh Depression Scale (EDDS). J Reprod Infant Psychol. 1990;8(2):99-107.
4. Gotlib IH, Whiffen VE, Mount JH, et al. Prevalence rates and demographic characteristics associated with depression in pregnancy and the postpartum. J Consult Clin Psychol. 1989;57(2):269-274.
5. Melville JL, Gavin A, Guo Y, et al. Depressive disorders during pregnancy: prevalence and risk factors in a large urban sample. Obstet Gynecol. 2010;116(5):1064-1070.
6. Leddy M, Haaga D, Gray J, et al. Postpartum mental health screening and diagnosis by obstetrician-gynecologists. J Psychosom Obstet Gynaecol. 2011;32(1):27-34.
7. McFarlane J, Maddoux J, Cesario S, et al. Effect of abuse during pregnancy on maternal and child safety and functioning for 24 months after delivery. Obstet Gynecol. 2014;123(4):839-847.
8. Vesga-López O, Bianco C, Keyes K, et al. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65(7):805-815.
9. Farr SL, Bitsko RH, Hayes DK, et al. Mental health and access to services among US women of reproductive age. Am J Obstet Gynecol. 2010;203(6):542.e1-e542.e9. doi: 10.1016/j.ajog.2010.07.007.
10. Committee on Practice Bulletins—Obstetrics; The American College of Obstetricians and Gynecologists. Practice bulletin no. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120(4):964-973.
11. Stoll BJ, Hansen NI, Bell EF, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443-456.
12. Steer RA, Scholl TO, Hediger ML, et al. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol. 1992;45(10):1093-1099.
13. Goldenberg RL, Cliver SP, Mulvihill FX, et al. Medical, psychosocial, and behavioral risk factors do not explain the increased risk for low birth weight among black women. Am J Obstet Gynecol. 1996;175(5):1317-1324.
14. Orr ST, James SA, Blackmore Prince C. Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore, Maryland. Am J Epidemiol. 2002;156(9):797-802.
15. Dayan J, Creveuil C, Marks MN, et al. Prenatal depression, prenatal anxiety, and spontaneous preterm birth: a prospective cohort study among women with early and regular care. Psychosom Med. 2006;68(6):938-946.
16. Goedhart G, Snijders AC, Hesselink AE, et al. Maternal depressive symptoms in relation to perinatal mortality and morbidity: results from a large multiethnic cohort study. Psychosom Med. 2010;72(8):769-776.
17. Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012-1024.
18. Hayes RM, Wu P, Shelton RC, et al. Maternal antidepressant use and adverse outcomes: a cohort study of 228,876 pregnancies [published online April 30, 2012]. Am J Obstet Gynecol. 2012;207(1):49.e1-49.e9. doi: 10.1016/j. ajog.2012.04.028.
19. McDonagh MS, Matthews A, Phillipi C, et al. Depression drug treatment outcomes in pregnancy and the postpartum period: a systematic review and meta-analysis. Obstet Gynecol. 2014;124(3):526-534.
20. Sahingöz M, Yuksel G, Karsidag C, et al. Birth weight and preterm birth in babies of pregnant women with major depression in relation to treatment with antidepressants. J Clin Psychopharmacol. 2014;34(2):226-229.
When a patient who has a preexisting medical illness seeks prenatal care, the obstetrician asks herself (himself) 2 questions:
• What impact will the illness have on the pregnancy?
• What impact will the pregnancy have on the illness?
Depression is both a pregnancy-associated and pregnancy-independent illness, which, in the setting of a pregnant woman who has a depressive disorder, makes these questions particularly difficult to answer. In such a case, coordination of care with a mental health provider is essential.
Awareness of the obstetrical complications associated with depression during pregnancy, as well as their implications for the future health of the mother–infant dyad, is important for the entire care team. This article reviews the associations and interconnectedness of depression with complications of pregnancy, childbirth, and the neonatal period.
Diagnosis of depression during prenatal care
The American College of Obstetricians and Gynecologists (ACOG) states that evidence is insufficient to support a recommendation for universal screening for depression among prenatal patients, although such screening should be considered.1 There is considerable variability among obstetrical providers regarding the practice of depression screening; tools to be used if such screening is done; and screening frequency through the pregnancy.
Discernment of depression is difficult. Many somatic symptoms of depression overlap with common prenatal complaints and, consequentially, can be overlooked. Among a sample of 700 pregnant women, for example, 56% complained of lack of energy; 19%, of insomnia; and 19%, of appetite changes.2 Weight change, of course, is universal.
The 10-question self-rating Edinburgh Postnatal Depression Scale has been validated for use during pregnancy and postnatally. This screening instrument can be helpful for differentiating purely physical complaints from mental distress due to depressive symptoms.2,3
When an obstetrical provider suspects a depressive disorder, or one has been diagnosed, she (he) faces the problem of what to do with that information. Women of low socioeconomic status and victims of domestic violence are at increased risk of depression during pregnancy, but barriers to appropriate referral can seem nearly insurmountable because they lack insurance and social support.4-9
In addition, within the setting of numerous tasks that need attending during the relatively short prenatal period, it is common for women newly given a diagnosis of depression to fail to follow up on a referral to a mental health provider.
Although most providers will “check in” with a depressed or at-risk patient at each prenatal visit about her mood, any effort at follow-up can be overshadowed by tangible physical concerns, such as preterm contractions, fetal growth restriction, and coordination of routine testing that has been delayed because of scant prenatal care. All these physical concerns and circumstances of care are associated with maternal depression, as we will discuss.
Preterm labor and birth
Preterm labor is defined as uterine contractions that lead to cervical change before 37 weeks gestational age. Preterm labor increases the risk of preterm birth; preterm labor precedes 50% of preterm births. Preterm birth is the leading cause of neonatal mortality in the United States, and rates of morbidity and mortality increase as gestational age decreases.10 Common neonatal complications related to prematurity are shown in the Figure.11
Women who suffer from depression have an increased risk of preterm labor and preterm birth, as many studies of treated and untreated depressed pregnant women have shown.12-20 The causative mechanism is unknown; it has been proposed that the increase in maternal cortisol production associated with depression and distress triggers overproduction of placental cortisol releasing hormone, which is thought to be involved in initiation of parturition.21,22 Depression also is associated with other risk factors for preterm birth, such as low socioeconomic status, substance use, and smoking.
Intrauterine growth restriction
Women who have depression during pregnancy have an increased risk of intrauterine growth restriction (IUGR), which leads to delivery of an infant who is small for gestational age (SGA) or of low birth weight (LBW) (weighing <2,500 g at birth), or both.23 Again, the basis of the association between depression and IUGR and SGA is unknown; it is theorized that increased levels of cortisol and catecholamines associated with maternal distress might, by increasing blood pressure and inducing vasoconstriction, cause placental hypoperfusion.24,25
It also is possible that the association of depression with other risk factors for IUGR, such as smoking, substance use, obesity, and poor prenatal care, puts the infants of depressed women at risk of growth restriction.26 Several large-scale studies showed that the association between LBW and depression is lost when smoking and substance use are accounted for; other studies, however, found a persistent association in untreated depressed women when smokers, substance users, and drinkers were excluded.17,26,27
IUGR infants are at increased risk of iatrogenic prematurity and stillbirth. Fetuses that weigh <10th percentile for their gestational age are delivered no later than 40 weeks; delivery can be indicated as early as 32 weeks, depending on the results of other antenatal tests. Women who have a growth-restricted infant have a higher risk of cesarean delivery because growth-restricted infants often have less reserve and poorer tolerance of labor.
Preeclampsia and eclampsia
Preeclampsia is defined as blood pressure >140/90 mm HG on at least 2 occasions, with proteinuria, that occurs later than the twentieth week of pregnancy in women who did not have hypertension or renal dysfunction at baseline. Preeclampsia is a progressive disease that can cause severe maternal morbidity, including renal failure, stroke, hepatic rupture, pulmonary edema, and heart failure.
Eclampsia refers to onset of seizures in the setting of preeclampsia. These 2 hypertensive disorders are the third leading world wide cause of maternal mortality.28
Depressed women have an elevated risk of preeclampsia. The association between preeclampsia and depression might be caused by the presence of increased levels of inflammatory mediators29,30; other comorbidities, such as increased body mass index, also might be involved, but the risk for preeclampsia in depressed women still is increased after controlling for obesity.31
The presence of preeclampsia is responsible for a high percentage of iatrogenic preterm births, because the cure for the disorder is delivery—even at early or previable gestational age. Complication rates for mother and infant are high.
The presence of preeclampsia is a significant risk factor for intrauterine fetal demise. Treating the mother after delivery involves administration of IV magnesium for 24 hours; often, the mother is separated from her infant for a day after birth.
Impact on prenatal care
Depression increases odds that women will have fewer prenatal visits.32 During pregnancy, women typically initiate prenatal care during the first trimester, when pregnancy-dating ultrasonography and early screening tests for chromosomal abnormalities are performed. Prenatal visits occur monthly until the third trimester, then every 2 weeks between 32 and 36 weeks’ gestation, increasing to weekly after 36 weeks’ gestation.
The increased number of visits in late pregnancy allows for early detection and treatment of hypertensive disorders; assesses fetal well-being; and decreases the risks of morbidity and mortality for mother and fetus.33 Because women who suffer from depression are at increased risk of an array of adverse pregnancy outcomes, the importance of regular and timely prenatal care cannot be understated.
In addition, the prenatal visit gives the obstetrician the opportunity to connect women with other specialists for management of any unmet medical needs. One study showed that, when women have adequate prenatal care (measured by the number of visits), the association between preterm birth and self-reported maternal depression was eliminated.34
Substance use
Substance use and depression often co-exist.35,36 Unlike screening for depression, screening for substance use is universal during prenatal care. Studies have shown that women who screen positive for depression are at higher risk of a number of comorbidities, including substance use.37,38 Conversely, women who use substances are more likely to screen positive for depression.
Evidence suggests that best practice might be to screen for depression in any woman who has a positive drug screen, if a provider is not routinely screening their general patient population.39 Substance use in pregnancy is associated with a number of poor outcomes, including placental abruption (cocaine use); dysmorphic facies and congenital anomalies (alcohol); and neonatal abstinence syndrome (heroin).
Antidepressants in pregnancy
A full discussion of the risks and benefits associated with pharmacotherapy for depression in pregnancy is beyond the scope of this article. Generally, antidepressant use is fraught with concerns over teratogenicity and adverse fetal outcomes. Although ACOG states that (1) pharmacotherapy for depression should be individualized and (2) most selective serotonin reuptake inhibitors (SSRIs) are not considered major teratogenic agents, many obstetricians and patients feel uncomfortable using these medications in pregnancy.40 Often, pre-pregnancy antidepressants are discontinued in the first trimester; one large population-based study found that only 0.9% of women who had depression filled their antidepressant prescription consistently throughout their pregnancy.41
It is unclear whether antidepressant use in pregnancy contributes to the risk of preterm birth seen in women who have depression. In a large population-based study, use of antidepressants in the second trimester was associated with preterm delivery but severe depression was not.18 A recent meta-analysis revealed an increased risk of preterm birth in women who used an antidepressant, compared with healthy women and untreated depressed women.42
Research limits, unanswered questions. Regrettably, it is difficult to untangle risk factors for preterm birth among depressed women without randomized controlled studies that are not ethically feasible. It cannot be said with certainty whether antidepressant pharmacotherapy is associated with a higher risk of preterm birth than depression alone.
Likewise, it is difficult to clarify the extent to which antidepressants contribute to infant growth restriction, if at all. Two recent meta-analyses concluded that exposure to antidepressants is associated with a statistically significant risk of LBW.42,43 However, increased severity of depressive symptoms generally is associated with exposure to antidepressants during pregnancy, and a randomized controlled trial is, again, impossible to conduct for ethical reasons.
Whereas a plausible biological mechanism associating IUGR, SGA, and LBW with depression exists, the same cannot be said for antidepressants. In one study, exposure to maternal depression altered the expression of certain placental genes but exposure to SSRIs did not cause further changes. This suggests that, on a cellular level, placental function might differ in depressed women.44 Although antidepressants do cross the placenta, it remains to be seen whether fetal growth is impacted as a result. One study found decreased fetal head circumference in infants who had been exposed to antidepressants during pregnancy, but no increased risk for having a SGA or LWB infant.45
Obstetrical management and mental health implications
Treated or not, women who suffer depression are a high-risk group when it comes to preterm birth and a host of other pregnancy comorbidities. Women with serious complications of pregnancy often are hospitalized for observation, and can undergo a prolonged stay when close proximity to medical services or a surgical suite is required.
For example, hospitalization until delivery is the standard of care for women who have preterm premature rupture of membranes or preeclampsia before 34 weeks’ gestation. Prolonged inpatient admissions and associated restriction of activity is profoundly deleterious on mood, with depression and anxiety significantly correlated with length of stay.46,47 Given the associations between depression and preterm birth, it might be reasonable to consider screening antenatal inpatients at risk of preterm birth for depression on a regular basis, so that treatment can be initiated if needed.
Depression during pregnancy is relatively common; an estimated 12.7% of pregnant women are affected at some time between conception and birth.48 Not only does depression appear to have deleterious effects on pregnancy outcomes, it also plays a pivotal role in the qualitative experience of pregnancy for the mother.
Bottom Line
Awareness of obstetrical complications associated with depression in pregnancy is important for the entire care team, including the psychiatrist and obstetrician. Depression not only appears to have deleterious effects on pregnancy outcomes, it also plays a pivotal role in the qualitative experience of pregnancy for the mother. Antidepressant use generally is fraught with concerns over teratogenicity and adverse fetal outcomes.
Related Resources
• Freeman MP. Some SSRIs are better than others for pregnant women (audio interview). Current Psychiatry. 2014;13(7). http://www.currentpsychiatry.com/specialty-focus/practice-trends/article/some-ssris-are-better-thanothers-for-pregnant-women/e3adb4704e25492f3e15331fc1cc058d.html.
• Freeman MP, Joffe H, Cohen LS. Postpartum depression: Help patients find the right treatment. Current Psychiatry. 2012;11(11):14-16,19-21.
Disclosures
Dr. Habecker reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Freeman is a member of the advisory board of JDS Therapeutics, Sunovion Pharmaceuticals, Inc., and Takeda Pharmaceutical Co. She receives research grant support from Takeda Pharmaceutical Co.
When a patient who has a preexisting medical illness seeks prenatal care, the obstetrician asks herself (himself) 2 questions:
• What impact will the illness have on the pregnancy?
• What impact will the pregnancy have on the illness?
Depression is both a pregnancy-associated and pregnancy-independent illness, which, in the setting of a pregnant woman who has a depressive disorder, makes these questions particularly difficult to answer. In such a case, coordination of care with a mental health provider is essential.
Awareness of the obstetrical complications associated with depression during pregnancy, as well as their implications for the future health of the mother–infant dyad, is important for the entire care team. This article reviews the associations and interconnectedness of depression with complications of pregnancy, childbirth, and the neonatal period.
Diagnosis of depression during prenatal care
The American College of Obstetricians and Gynecologists (ACOG) states that evidence is insufficient to support a recommendation for universal screening for depression among prenatal patients, although such screening should be considered.1 There is considerable variability among obstetrical providers regarding the practice of depression screening; tools to be used if such screening is done; and screening frequency through the pregnancy.
Discernment of depression is difficult. Many somatic symptoms of depression overlap with common prenatal complaints and, consequentially, can be overlooked. Among a sample of 700 pregnant women, for example, 56% complained of lack of energy; 19%, of insomnia; and 19%, of appetite changes.2 Weight change, of course, is universal.
The 10-question self-rating Edinburgh Postnatal Depression Scale has been validated for use during pregnancy and postnatally. This screening instrument can be helpful for differentiating purely physical complaints from mental distress due to depressive symptoms.2,3
When an obstetrical provider suspects a depressive disorder, or one has been diagnosed, she (he) faces the problem of what to do with that information. Women of low socioeconomic status and victims of domestic violence are at increased risk of depression during pregnancy, but barriers to appropriate referral can seem nearly insurmountable because they lack insurance and social support.4-9
In addition, within the setting of numerous tasks that need attending during the relatively short prenatal period, it is common for women newly given a diagnosis of depression to fail to follow up on a referral to a mental health provider.
Although most providers will “check in” with a depressed or at-risk patient at each prenatal visit about her mood, any effort at follow-up can be overshadowed by tangible physical concerns, such as preterm contractions, fetal growth restriction, and coordination of routine testing that has been delayed because of scant prenatal care. All these physical concerns and circumstances of care are associated with maternal depression, as we will discuss.
Preterm labor and birth
Preterm labor is defined as uterine contractions that lead to cervical change before 37 weeks gestational age. Preterm labor increases the risk of preterm birth; preterm labor precedes 50% of preterm births. Preterm birth is the leading cause of neonatal mortality in the United States, and rates of morbidity and mortality increase as gestational age decreases.10 Common neonatal complications related to prematurity are shown in the Figure.11
Women who suffer from depression have an increased risk of preterm labor and preterm birth, as many studies of treated and untreated depressed pregnant women have shown.12-20 The causative mechanism is unknown; it has been proposed that the increase in maternal cortisol production associated with depression and distress triggers overproduction of placental cortisol releasing hormone, which is thought to be involved in initiation of parturition.21,22 Depression also is associated with other risk factors for preterm birth, such as low socioeconomic status, substance use, and smoking.
Intrauterine growth restriction
Women who have depression during pregnancy have an increased risk of intrauterine growth restriction (IUGR), which leads to delivery of an infant who is small for gestational age (SGA) or of low birth weight (LBW) (weighing <2,500 g at birth), or both.23 Again, the basis of the association between depression and IUGR and SGA is unknown; it is theorized that increased levels of cortisol and catecholamines associated with maternal distress might, by increasing blood pressure and inducing vasoconstriction, cause placental hypoperfusion.24,25
It also is possible that the association of depression with other risk factors for IUGR, such as smoking, substance use, obesity, and poor prenatal care, puts the infants of depressed women at risk of growth restriction.26 Several large-scale studies showed that the association between LBW and depression is lost when smoking and substance use are accounted for; other studies, however, found a persistent association in untreated depressed women when smokers, substance users, and drinkers were excluded.17,26,27
IUGR infants are at increased risk of iatrogenic prematurity and stillbirth. Fetuses that weigh <10th percentile for their gestational age are delivered no later than 40 weeks; delivery can be indicated as early as 32 weeks, depending on the results of other antenatal tests. Women who have a growth-restricted infant have a higher risk of cesarean delivery because growth-restricted infants often have less reserve and poorer tolerance of labor.
Preeclampsia and eclampsia
Preeclampsia is defined as blood pressure >140/90 mm HG on at least 2 occasions, with proteinuria, that occurs later than the twentieth week of pregnancy in women who did not have hypertension or renal dysfunction at baseline. Preeclampsia is a progressive disease that can cause severe maternal morbidity, including renal failure, stroke, hepatic rupture, pulmonary edema, and heart failure.
Eclampsia refers to onset of seizures in the setting of preeclampsia. These 2 hypertensive disorders are the third leading world wide cause of maternal mortality.28
Depressed women have an elevated risk of preeclampsia. The association between preeclampsia and depression might be caused by the presence of increased levels of inflammatory mediators29,30; other comorbidities, such as increased body mass index, also might be involved, but the risk for preeclampsia in depressed women still is increased after controlling for obesity.31
The presence of preeclampsia is responsible for a high percentage of iatrogenic preterm births, because the cure for the disorder is delivery—even at early or previable gestational age. Complication rates for mother and infant are high.
The presence of preeclampsia is a significant risk factor for intrauterine fetal demise. Treating the mother after delivery involves administration of IV magnesium for 24 hours; often, the mother is separated from her infant for a day after birth.
Impact on prenatal care
Depression increases odds that women will have fewer prenatal visits.32 During pregnancy, women typically initiate prenatal care during the first trimester, when pregnancy-dating ultrasonography and early screening tests for chromosomal abnormalities are performed. Prenatal visits occur monthly until the third trimester, then every 2 weeks between 32 and 36 weeks’ gestation, increasing to weekly after 36 weeks’ gestation.
The increased number of visits in late pregnancy allows for early detection and treatment of hypertensive disorders; assesses fetal well-being; and decreases the risks of morbidity and mortality for mother and fetus.33 Because women who suffer from depression are at increased risk of an array of adverse pregnancy outcomes, the importance of regular and timely prenatal care cannot be understated.
In addition, the prenatal visit gives the obstetrician the opportunity to connect women with other specialists for management of any unmet medical needs. One study showed that, when women have adequate prenatal care (measured by the number of visits), the association between preterm birth and self-reported maternal depression was eliminated.34
Substance use
Substance use and depression often co-exist.35,36 Unlike screening for depression, screening for substance use is universal during prenatal care. Studies have shown that women who screen positive for depression are at higher risk of a number of comorbidities, including substance use.37,38 Conversely, women who use substances are more likely to screen positive for depression.
Evidence suggests that best practice might be to screen for depression in any woman who has a positive drug screen, if a provider is not routinely screening their general patient population.39 Substance use in pregnancy is associated with a number of poor outcomes, including placental abruption (cocaine use); dysmorphic facies and congenital anomalies (alcohol); and neonatal abstinence syndrome (heroin).
Antidepressants in pregnancy
A full discussion of the risks and benefits associated with pharmacotherapy for depression in pregnancy is beyond the scope of this article. Generally, antidepressant use is fraught with concerns over teratogenicity and adverse fetal outcomes. Although ACOG states that (1) pharmacotherapy for depression should be individualized and (2) most selective serotonin reuptake inhibitors (SSRIs) are not considered major teratogenic agents, many obstetricians and patients feel uncomfortable using these medications in pregnancy.40 Often, pre-pregnancy antidepressants are discontinued in the first trimester; one large population-based study found that only 0.9% of women who had depression filled their antidepressant prescription consistently throughout their pregnancy.41
It is unclear whether antidepressant use in pregnancy contributes to the risk of preterm birth seen in women who have depression. In a large population-based study, use of antidepressants in the second trimester was associated with preterm delivery but severe depression was not.18 A recent meta-analysis revealed an increased risk of preterm birth in women who used an antidepressant, compared with healthy women and untreated depressed women.42
Research limits, unanswered questions. Regrettably, it is difficult to untangle risk factors for preterm birth among depressed women without randomized controlled studies that are not ethically feasible. It cannot be said with certainty whether antidepressant pharmacotherapy is associated with a higher risk of preterm birth than depression alone.
Likewise, it is difficult to clarify the extent to which antidepressants contribute to infant growth restriction, if at all. Two recent meta-analyses concluded that exposure to antidepressants is associated with a statistically significant risk of LBW.42,43 However, increased severity of depressive symptoms generally is associated with exposure to antidepressants during pregnancy, and a randomized controlled trial is, again, impossible to conduct for ethical reasons.
Whereas a plausible biological mechanism associating IUGR, SGA, and LBW with depression exists, the same cannot be said for antidepressants. In one study, exposure to maternal depression altered the expression of certain placental genes but exposure to SSRIs did not cause further changes. This suggests that, on a cellular level, placental function might differ in depressed women.44 Although antidepressants do cross the placenta, it remains to be seen whether fetal growth is impacted as a result. One study found decreased fetal head circumference in infants who had been exposed to antidepressants during pregnancy, but no increased risk for having a SGA or LWB infant.45
Obstetrical management and mental health implications
Treated or not, women who suffer depression are a high-risk group when it comes to preterm birth and a host of other pregnancy comorbidities. Women with serious complications of pregnancy often are hospitalized for observation, and can undergo a prolonged stay when close proximity to medical services or a surgical suite is required.
For example, hospitalization until delivery is the standard of care for women who have preterm premature rupture of membranes or preeclampsia before 34 weeks’ gestation. Prolonged inpatient admissions and associated restriction of activity is profoundly deleterious on mood, with depression and anxiety significantly correlated with length of stay.46,47 Given the associations between depression and preterm birth, it might be reasonable to consider screening antenatal inpatients at risk of preterm birth for depression on a regular basis, so that treatment can be initiated if needed.
Depression during pregnancy is relatively common; an estimated 12.7% of pregnant women are affected at some time between conception and birth.48 Not only does depression appear to have deleterious effects on pregnancy outcomes, it also plays a pivotal role in the qualitative experience of pregnancy for the mother.
Bottom Line
Awareness of obstetrical complications associated with depression in pregnancy is important for the entire care team, including the psychiatrist and obstetrician. Depression not only appears to have deleterious effects on pregnancy outcomes, it also plays a pivotal role in the qualitative experience of pregnancy for the mother. Antidepressant use generally is fraught with concerns over teratogenicity and adverse fetal outcomes.
Related Resources
• Freeman MP. Some SSRIs are better than others for pregnant women (audio interview). Current Psychiatry. 2014;13(7). http://www.currentpsychiatry.com/specialty-focus/practice-trends/article/some-ssris-are-better-thanothers-for-pregnant-women/e3adb4704e25492f3e15331fc1cc058d.html.
• Freeman MP, Joffe H, Cohen LS. Postpartum depression: Help patients find the right treatment. Current Psychiatry. 2012;11(11):14-16,19-21.
Disclosures
Dr. Habecker reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Freeman is a member of the advisory board of JDS Therapeutics, Sunovion Pharmaceuticals, Inc., and Takeda Pharmaceutical Co. She receives research grant support from Takeda Pharmaceutical Co.
1. American College of Obstetricians and Gynecologists. Committee on Obstetric Practice. Committee opinion no. 630. 2015;125:1268-1271.
2. Apter G, Devouche E, Garez V, et al. Pregnancy, somatic complaints and depression: a French population-based study. Eur J Obstet Gynecol Reprod Biol. 2013;171(1):35-39.
3. Murray D, Cox JL. Screening for depression during pregnancy with the Edinburgh Depression Scale (EDDS). J Reprod Infant Psychol. 1990;8(2):99-107.
4. Gotlib IH, Whiffen VE, Mount JH, et al. Prevalence rates and demographic characteristics associated with depression in pregnancy and the postpartum. J Consult Clin Psychol. 1989;57(2):269-274.
5. Melville JL, Gavin A, Guo Y, et al. Depressive disorders during pregnancy: prevalence and risk factors in a large urban sample. Obstet Gynecol. 2010;116(5):1064-1070.
6. Leddy M, Haaga D, Gray J, et al. Postpartum mental health screening and diagnosis by obstetrician-gynecologists. J Psychosom Obstet Gynaecol. 2011;32(1):27-34.
7. McFarlane J, Maddoux J, Cesario S, et al. Effect of abuse during pregnancy on maternal and child safety and functioning for 24 months after delivery. Obstet Gynecol. 2014;123(4):839-847.
8. Vesga-López O, Bianco C, Keyes K, et al. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65(7):805-815.
9. Farr SL, Bitsko RH, Hayes DK, et al. Mental health and access to services among US women of reproductive age. Am J Obstet Gynecol. 2010;203(6):542.e1-e542.e9. doi: 10.1016/j.ajog.2010.07.007.
10. Committee on Practice Bulletins—Obstetrics; The American College of Obstetricians and Gynecologists. Practice bulletin no. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120(4):964-973.
11. Stoll BJ, Hansen NI, Bell EF, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443-456.
12. Steer RA, Scholl TO, Hediger ML, et al. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol. 1992;45(10):1093-1099.
13. Goldenberg RL, Cliver SP, Mulvihill FX, et al. Medical, psychosocial, and behavioral risk factors do not explain the increased risk for low birth weight among black women. Am J Obstet Gynecol. 1996;175(5):1317-1324.
14. Orr ST, James SA, Blackmore Prince C. Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore, Maryland. Am J Epidemiol. 2002;156(9):797-802.
15. Dayan J, Creveuil C, Marks MN, et al. Prenatal depression, prenatal anxiety, and spontaneous preterm birth: a prospective cohort study among women with early and regular care. Psychosom Med. 2006;68(6):938-946.
16. Goedhart G, Snijders AC, Hesselink AE, et al. Maternal depressive symptoms in relation to perinatal mortality and morbidity: results from a large multiethnic cohort study. Psychosom Med. 2010;72(8):769-776.
17. Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012-1024.
18. Hayes RM, Wu P, Shelton RC, et al. Maternal antidepressant use and adverse outcomes: a cohort study of 228,876 pregnancies [published online April 30, 2012]. Am J Obstet Gynecol. 2012;207(1):49.e1-49.e9. doi: 10.1016/j. ajog.2012.04.028.
19. McDonagh MS, Matthews A, Phillipi C, et al. Depression drug treatment outcomes in pregnancy and the postpartum period: a systematic review and meta-analysis. Obstet Gynecol. 2014;124(3):526-534.
20. Sahingöz M, Yuksel G, Karsidag C, et al. Birth weight and preterm birth in babies of pregnant women with major depression in relation to treatment with antidepressants. J Clin Psychopharmacol. 2014;34(2):226-229.
1. American College of Obstetricians and Gynecologists. Committee on Obstetric Practice. Committee opinion no. 630. 2015;125:1268-1271.
2. Apter G, Devouche E, Garez V, et al. Pregnancy, somatic complaints and depression: a French population-based study. Eur J Obstet Gynecol Reprod Biol. 2013;171(1):35-39.
3. Murray D, Cox JL. Screening for depression during pregnancy with the Edinburgh Depression Scale (EDDS). J Reprod Infant Psychol. 1990;8(2):99-107.
4. Gotlib IH, Whiffen VE, Mount JH, et al. Prevalence rates and demographic characteristics associated with depression in pregnancy and the postpartum. J Consult Clin Psychol. 1989;57(2):269-274.
5. Melville JL, Gavin A, Guo Y, et al. Depressive disorders during pregnancy: prevalence and risk factors in a large urban sample. Obstet Gynecol. 2010;116(5):1064-1070.
6. Leddy M, Haaga D, Gray J, et al. Postpartum mental health screening and diagnosis by obstetrician-gynecologists. J Psychosom Obstet Gynaecol. 2011;32(1):27-34.
7. McFarlane J, Maddoux J, Cesario S, et al. Effect of abuse during pregnancy on maternal and child safety and functioning for 24 months after delivery. Obstet Gynecol. 2014;123(4):839-847.
8. Vesga-López O, Bianco C, Keyes K, et al. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65(7):805-815.
9. Farr SL, Bitsko RH, Hayes DK, et al. Mental health and access to services among US women of reproductive age. Am J Obstet Gynecol. 2010;203(6):542.e1-e542.e9. doi: 10.1016/j.ajog.2010.07.007.
10. Committee on Practice Bulletins—Obstetrics; The American College of Obstetricians and Gynecologists. Practice bulletin no. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120(4):964-973.
11. Stoll BJ, Hansen NI, Bell EF, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443-456.
12. Steer RA, Scholl TO, Hediger ML, et al. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol. 1992;45(10):1093-1099.
13. Goldenberg RL, Cliver SP, Mulvihill FX, et al. Medical, psychosocial, and behavioral risk factors do not explain the increased risk for low birth weight among black women. Am J Obstet Gynecol. 1996;175(5):1317-1324.
14. Orr ST, James SA, Blackmore Prince C. Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore, Maryland. Am J Epidemiol. 2002;156(9):797-802.
15. Dayan J, Creveuil C, Marks MN, et al. Prenatal depression, prenatal anxiety, and spontaneous preterm birth: a prospective cohort study among women with early and regular care. Psychosom Med. 2006;68(6):938-946.
16. Goedhart G, Snijders AC, Hesselink AE, et al. Maternal depressive symptoms in relation to perinatal mortality and morbidity: results from a large multiethnic cohort study. Psychosom Med. 2010;72(8):769-776.
17. Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012-1024.
18. Hayes RM, Wu P, Shelton RC, et al. Maternal antidepressant use and adverse outcomes: a cohort study of 228,876 pregnancies [published online April 30, 2012]. Am J Obstet Gynecol. 2012;207(1):49.e1-49.e9. doi: 10.1016/j. ajog.2012.04.028.
19. McDonagh MS, Matthews A, Phillipi C, et al. Depression drug treatment outcomes in pregnancy and the postpartum period: a systematic review and meta-analysis. Obstet Gynecol. 2014;124(3):526-534.
20. Sahingöz M, Yuksel G, Karsidag C, et al. Birth weight and preterm birth in babies of pregnant women with major depression in relation to treatment with antidepressants. J Clin Psychopharmacol. 2014;34(2):226-229.
Beware of methylmercury during pregnancy!
Dr. Henry A. Nasrallah is correct that wild salmon is a good choice for pregnant women who want to boost intake of omega-3 fatty acids (Current Psychiatry, Comments & Controversies, December 2014; pg 33 [http://bit.ly/1wQoXdP]). The main concern about fish intake during pregnancy is exposure to methylmercury, and much of this concern is derived from the tragic results of epic mercury poisonings of food sources in the past.
The FDA advises that pregnant women and children avoid eating shark, tilefish, king mackerel, and swordfish because these fish have a relatively high level of mercury.1 Fish that are low in methyl-mercury include salmon and canned light tuna. (More information is available at http://www.fda.gov/Food/ResourcesForYou/HealthEducators/ucm083324.htm.)
Although wild fish tend to be higher in omega-3 fatty acids than farm-raised fish, farmed fish can be an excellent source of omega-3 fatty acids. This is analogous to eating farm-produced livestock vs free-range, grass-fed livestock: Animals in their natural environment eat healthier and have more omega-3 fatty acids, whereas farmed livestock generally eat cheap and less healthy feed. Because wild fish can be pricey, it’s important that women understand that farm-raised fish are a good source of protein and other nutrients such as omega-3 fatty acids.
Research has been inconclusive regarding the antidepressant benefits of omega-3 fatty acids, with some, but not all, studies demonstrating an add-on benefit of omega-3 fatty acid supplements for mood disorders. However, several epidemiological studies have reported that the low quality of dietary intake of omega-3 fatty acids is associated with psychiatric illness, and fish and seafood are sources of essential fatty acids and other nutrients.2
1. Food safety for moms-to-be: while you’re pregnant–methylmercury. U.S. Food and Drug Administration. http://www.fda.gov/Food/ ResourcesForYou/HealthEducators/ucm083324. htm. Updated October 30, 2014. Accessed January 5, 2015.
2. Quirk SE, Williams LJ, O’Neil A, et al. The association between diet quality, dietary patterns and depression in adults: a systematic review. BMC Psychiatry. 2013;13:175.
Dr. Henry A. Nasrallah is correct that wild salmon is a good choice for pregnant women who want to boost intake of omega-3 fatty acids (Current Psychiatry, Comments & Controversies, December 2014; pg 33 [http://bit.ly/1wQoXdP]). The main concern about fish intake during pregnancy is exposure to methylmercury, and much of this concern is derived from the tragic results of epic mercury poisonings of food sources in the past.
The FDA advises that pregnant women and children avoid eating shark, tilefish, king mackerel, and swordfish because these fish have a relatively high level of mercury.1 Fish that are low in methyl-mercury include salmon and canned light tuna. (More information is available at http://www.fda.gov/Food/ResourcesForYou/HealthEducators/ucm083324.htm.)
Although wild fish tend to be higher in omega-3 fatty acids than farm-raised fish, farmed fish can be an excellent source of omega-3 fatty acids. This is analogous to eating farm-produced livestock vs free-range, grass-fed livestock: Animals in their natural environment eat healthier and have more omega-3 fatty acids, whereas farmed livestock generally eat cheap and less healthy feed. Because wild fish can be pricey, it’s important that women understand that farm-raised fish are a good source of protein and other nutrients such as omega-3 fatty acids.
Research has been inconclusive regarding the antidepressant benefits of omega-3 fatty acids, with some, but not all, studies demonstrating an add-on benefit of omega-3 fatty acid supplements for mood disorders. However, several epidemiological studies have reported that the low quality of dietary intake of omega-3 fatty acids is associated with psychiatric illness, and fish and seafood are sources of essential fatty acids and other nutrients.2
Dr. Henry A. Nasrallah is correct that wild salmon is a good choice for pregnant women who want to boost intake of omega-3 fatty acids (Current Psychiatry, Comments & Controversies, December 2014; pg 33 [http://bit.ly/1wQoXdP]). The main concern about fish intake during pregnancy is exposure to methylmercury, and much of this concern is derived from the tragic results of epic mercury poisonings of food sources in the past.
The FDA advises that pregnant women and children avoid eating shark, tilefish, king mackerel, and swordfish because these fish have a relatively high level of mercury.1 Fish that are low in methyl-mercury include salmon and canned light tuna. (More information is available at http://www.fda.gov/Food/ResourcesForYou/HealthEducators/ucm083324.htm.)
Although wild fish tend to be higher in omega-3 fatty acids than farm-raised fish, farmed fish can be an excellent source of omega-3 fatty acids. This is analogous to eating farm-produced livestock vs free-range, grass-fed livestock: Animals in their natural environment eat healthier and have more omega-3 fatty acids, whereas farmed livestock generally eat cheap and less healthy feed. Because wild fish can be pricey, it’s important that women understand that farm-raised fish are a good source of protein and other nutrients such as omega-3 fatty acids.
Research has been inconclusive regarding the antidepressant benefits of omega-3 fatty acids, with some, but not all, studies demonstrating an add-on benefit of omega-3 fatty acid supplements for mood disorders. However, several epidemiological studies have reported that the low quality of dietary intake of omega-3 fatty acids is associated with psychiatric illness, and fish and seafood are sources of essential fatty acids and other nutrients.2
1. Food safety for moms-to-be: while you’re pregnant–methylmercury. U.S. Food and Drug Administration. http://www.fda.gov/Food/ ResourcesForYou/HealthEducators/ucm083324. htm. Updated October 30, 2014. Accessed January 5, 2015.
2. Quirk SE, Williams LJ, O’Neil A, et al. The association between diet quality, dietary patterns and depression in adults: a systematic review. BMC Psychiatry. 2013;13:175.
1. Food safety for moms-to-be: while you’re pregnant–methylmercury. U.S. Food and Drug Administration. http://www.fda.gov/Food/ ResourcesForYou/HealthEducators/ucm083324. htm. Updated October 30, 2014. Accessed January 5, 2015.
2. Quirk SE, Williams LJ, O’Neil A, et al. The association between diet quality, dietary patterns and depression in adults: a systematic review. BMC Psychiatry. 2013;13:175.
Some SSRIs are better than others for pregnant women
An open-label trial of escitalopram for PPD: Considerations for research
Challenges in recruiting women to postpartum depression (PPD) antidepressant treatment trials, which we encountered when conducting a trial of escitalopram, contribute to the limited body of knowledge about PPD treatment. Here we discuss results from a preliminary trial of escitalopram for PPD, and challenges of research in this area.
Escitalopram, the S-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with high selectivity and potency that is FDA-approved for treating major depressive disorder (MDD) and generalized anxiety disorder. An agent with antidepressant and anxiolytic effects is particularly desirable for PPD because anxiety is more common in postpartum major depressive episodes than non-postpartum MDD.1 Anxiety and depressive disorders commonly are comorbid in postpartum women.2
We conducted an open-label trial of escitalopram for women with PPD and anxiety. We initially attempted to recruit 20 women.
Methods
Patients received 8 weeks of treatment with escitalopram, 10 to 20 mg/d (flexible dose). After completing the initial phone screen, patients had 5 follow-up visits, once every 2 weeks for 8 weeks. The institutional review board at Massachusetts General Hospital approved this study and we obtained written informed consent from all patients at the first visit. Twelve patients completed the phone screen and 7 eligible patients were enrolled in the study over 32 months. Reasons for ineligibility included having a history of psychosis, onset of symptoms >3 months postpartum, or presenting >6 months after onset. Others declined to participate because of concern about the time commitment or because they pursued nonpharmacologic treatments after the evaluation visit. One patient was lost to follow-up. Three patients completed the study. The study was halted because of the slow pace of recruitment.
Patient selection. Patients were screened for a major depressive episode with postpartum onset within 3 months of childbirth; depressive symptoms may have developed during pregnancy and worsened postpartum to meet criteria for MDD. Women were eligible for the study if they:
- were age 18 to 45
- experienced a major depressive episode with symptoms developing within 3 months of childbirth
- presented within 6 months of childbirth
- had a Montgomery-Åsberg Depression Rating Scale (MADRS) score >15
- had a Beck Anxiety Inventory (BAI) score >10.
Patients who were pregnant or breast-feeding were excluded from the study per an agreement with the sponsor. In addition, women were excluded if they had taken any psychotropic medication within 2 weeks of enrollment; had active suicidal ideation, homicidal ideation, or presence of psychotic symptoms; had chronic depression or dysthymia; had chronic or treatment-resistant anxiety disorders; had a history of mania or hypomania; or had active alcohol or substance abuse within the past year.
Treatment. Patients received escitalopram, 10 mg/d, after the baseline visit. At the investigator’s discretion, the dose could be increased to 20 mg/d or lowered to 5 mg/d if side effects occurred.
Measures. At the first visit, patients were assessed with the Mini-International Neuropsychiatric Interview to verify MDD and exclude diagnoses that would determine ineligibility. MADRS and Edinburgh Postnatal Depression Scale (EPDS) were used at each visit to measure depressive symptoms.3,4 The BAI was completed at each visit to measure anxiety symptoms. Obsessions and compulsions were measured with the Yale-Brown Obsessive Compulsive Scale (Y-BOCS)5 at baseline, and at all following visits if the patient scored >8 at baseline. The Clinical Global Impression Scales for severity and improvement were completed at each visit.6
Results
Of 7 patients enrolled, 3 completed the study, 2 were ineligible after the baseline visit, and 2 did not participate after the baseline visit (1 selected to pursue psychotherapy, and 1 was lost to follow-up).
Two of 3 patients responded to escitalopram (≥50% decrease on MADRS), and both were remitters (MADRS score <7). All 3 patients were responders on EPDS and BAI. One patient had Y-BOCS >8 at baseline (Total Y-BOCS score of 9, and final Y-BOCS score of 8) (Table).
Table
Symptom rating scale scores at baseline and study end
| Baseline (Visit 1) | Final (Visit 5) | |||||
|---|---|---|---|---|---|---|
| Patient | MADRS | BAI | EPDS | MADRS | BAI | EPDS |
| Ms. A | 21 | 18 | 22 | 12 | 0 | 0 |
| Ms. B | 28 | 28 | 19 | 4 | 5 | 2 |
| Ms. C | 37 | 6 | 19 | 6 | 2 | 0 |
| BAI: Beck Anxiety Inventory; EPDS: Edinburgh Postnatal Depression Scale; MADRS: Montgomery-Åsberg Depression Rating Scale | ||||||
Discussion
Patients who stayed in treatment improved during the course of this study. Recruitment was difficult; we were able to recruit only 7 patients out of a projected 20 for the screening visit. We solicited feedback from local obstetrics health care providers and social workers on recruitment and attractiveness of the study as part of our routine collaboration with obstetrical services that screen for PPD. Primary reasons patients were not referred were that they were breast-feeding or they stated they would prefer to receive treatment from their primary care doctor. Recruitment difficulty in this study was in stark contrast to other recent studies completed at our center. For example, we have successfully recruited for menopausal depression and premenstrual dysphoric disorder treatment studies, and have completed large naturalistic studies of women with unipolar depression and bipolar disorder across pregnancy and postpartum. We suspect that many patients who were eligible for the study preferred to seek care from an obstetrician or primary care doctor with whom they already had a therapeutic alliance, and we also suspect that many women with PPD do not seek treatment at all, which is consistent with findings from other research groups.
Lessons learned from PPD research include:
- Including women who are breast-feeding is important because many women choose to breast-feed and suffer from PPD. Because antidepressant use during breast-feeding has been closely studied, it is appropriate to include potential research participants who are breast-feeding as long as they receive adequate information and are able to provide informed consent.
- Participants in PPD studies may require accommodations that take into account their role as a new mother, such as on-site childcare, home visits, or other strategies.
- Because of recruitment challenges in postpartum patients, multisite trials may be required to include adequate numbers of participants.
Related Resource
- Freeman MP, Joffe H, Cohen LS. Postpartum depression: Help patients find the right treatment. Current Psychiatry. 2012;11(11):14-21.
Drug Brand Names
- Citalopram • Celexa
- Escitalopram • Lexapro
Disclosures
Dr. Freeman has received grant or research support from Eli Lilly and Company, Forest Laboratories, and GlaxoSmithKline, is on the advisory boards of Otsuka and Takeda/Lundbeck, and is a consultant for PamLab LLC.
Dr. Joffe has received grant or research support from Cephalon/Teva, and is a consultant to Noven and Sunovion.
Dr. Cohen has received research support from AstraZeneca, Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Forest Laboratories, GlaxoSmithKline, National Institute of Mental Health, National Institute on Aging, National Institutes of Health, Ortho-McNeil Janssen, and Pfizer and has served on an advisory board for PamLab LLC.
This study was funded as an investigator-initiated trial by Forest Pharmaceuticals.
1. Bernstein IH, Rush AJ, Yonkers K, et al. Symptom features of postpartum depression: are they distinct? Depress Anxiety. 2008;25(1):20-26.
2. Wenzel A, Haugen EN, Jackson LC, et al. Anxiety symptoms and disorders at eight weeks postpartum. J Anxiety Disord. 2005;19(3):295-311.
3. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
4. Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389.
5. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006-1011.
6. Guy W. ECDEU assessment manual for psychopharmacology. Rockville MD: US Department of Health and Human Services; 1976. Department of Health, Education, and Welfare Publication (ADM) 76–338.
Challenges in recruiting women to postpartum depression (PPD) antidepressant treatment trials, which we encountered when conducting a trial of escitalopram, contribute to the limited body of knowledge about PPD treatment. Here we discuss results from a preliminary trial of escitalopram for PPD, and challenges of research in this area.
Escitalopram, the S-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with high selectivity and potency that is FDA-approved for treating major depressive disorder (MDD) and generalized anxiety disorder. An agent with antidepressant and anxiolytic effects is particularly desirable for PPD because anxiety is more common in postpartum major depressive episodes than non-postpartum MDD.1 Anxiety and depressive disorders commonly are comorbid in postpartum women.2
We conducted an open-label trial of escitalopram for women with PPD and anxiety. We initially attempted to recruit 20 women.
Methods
Patients received 8 weeks of treatment with escitalopram, 10 to 20 mg/d (flexible dose). After completing the initial phone screen, patients had 5 follow-up visits, once every 2 weeks for 8 weeks. The institutional review board at Massachusetts General Hospital approved this study and we obtained written informed consent from all patients at the first visit. Twelve patients completed the phone screen and 7 eligible patients were enrolled in the study over 32 months. Reasons for ineligibility included having a history of psychosis, onset of symptoms >3 months postpartum, or presenting >6 months after onset. Others declined to participate because of concern about the time commitment or because they pursued nonpharmacologic treatments after the evaluation visit. One patient was lost to follow-up. Three patients completed the study. The study was halted because of the slow pace of recruitment.
Patient selection. Patients were screened for a major depressive episode with postpartum onset within 3 months of childbirth; depressive symptoms may have developed during pregnancy and worsened postpartum to meet criteria for MDD. Women were eligible for the study if they:
- were age 18 to 45
- experienced a major depressive episode with symptoms developing within 3 months of childbirth
- presented within 6 months of childbirth
- had a Montgomery-Åsberg Depression Rating Scale (MADRS) score >15
- had a Beck Anxiety Inventory (BAI) score >10.
Patients who were pregnant or breast-feeding were excluded from the study per an agreement with the sponsor. In addition, women were excluded if they had taken any psychotropic medication within 2 weeks of enrollment; had active suicidal ideation, homicidal ideation, or presence of psychotic symptoms; had chronic depression or dysthymia; had chronic or treatment-resistant anxiety disorders; had a history of mania or hypomania; or had active alcohol or substance abuse within the past year.
Treatment. Patients received escitalopram, 10 mg/d, after the baseline visit. At the investigator’s discretion, the dose could be increased to 20 mg/d or lowered to 5 mg/d if side effects occurred.
Measures. At the first visit, patients were assessed with the Mini-International Neuropsychiatric Interview to verify MDD and exclude diagnoses that would determine ineligibility. MADRS and Edinburgh Postnatal Depression Scale (EPDS) were used at each visit to measure depressive symptoms.3,4 The BAI was completed at each visit to measure anxiety symptoms. Obsessions and compulsions were measured with the Yale-Brown Obsessive Compulsive Scale (Y-BOCS)5 at baseline, and at all following visits if the patient scored >8 at baseline. The Clinical Global Impression Scales for severity and improvement were completed at each visit.6
Results
Of 7 patients enrolled, 3 completed the study, 2 were ineligible after the baseline visit, and 2 did not participate after the baseline visit (1 selected to pursue psychotherapy, and 1 was lost to follow-up).
Two of 3 patients responded to escitalopram (≥50% decrease on MADRS), and both were remitters (MADRS score <7). All 3 patients were responders on EPDS and BAI. One patient had Y-BOCS >8 at baseline (Total Y-BOCS score of 9, and final Y-BOCS score of 8) (Table).
Table
Symptom rating scale scores at baseline and study end
| Baseline (Visit 1) | Final (Visit 5) | |||||
|---|---|---|---|---|---|---|
| Patient | MADRS | BAI | EPDS | MADRS | BAI | EPDS |
| Ms. A | 21 | 18 | 22 | 12 | 0 | 0 |
| Ms. B | 28 | 28 | 19 | 4 | 5 | 2 |
| Ms. C | 37 | 6 | 19 | 6 | 2 | 0 |
| BAI: Beck Anxiety Inventory; EPDS: Edinburgh Postnatal Depression Scale; MADRS: Montgomery-Åsberg Depression Rating Scale | ||||||
Discussion
Patients who stayed in treatment improved during the course of this study. Recruitment was difficult; we were able to recruit only 7 patients out of a projected 20 for the screening visit. We solicited feedback from local obstetrics health care providers and social workers on recruitment and attractiveness of the study as part of our routine collaboration with obstetrical services that screen for PPD. Primary reasons patients were not referred were that they were breast-feeding or they stated they would prefer to receive treatment from their primary care doctor. Recruitment difficulty in this study was in stark contrast to other recent studies completed at our center. For example, we have successfully recruited for menopausal depression and premenstrual dysphoric disorder treatment studies, and have completed large naturalistic studies of women with unipolar depression and bipolar disorder across pregnancy and postpartum. We suspect that many patients who were eligible for the study preferred to seek care from an obstetrician or primary care doctor with whom they already had a therapeutic alliance, and we also suspect that many women with PPD do not seek treatment at all, which is consistent with findings from other research groups.
Lessons learned from PPD research include:
- Including women who are breast-feeding is important because many women choose to breast-feed and suffer from PPD. Because antidepressant use during breast-feeding has been closely studied, it is appropriate to include potential research participants who are breast-feeding as long as they receive adequate information and are able to provide informed consent.
- Participants in PPD studies may require accommodations that take into account their role as a new mother, such as on-site childcare, home visits, or other strategies.
- Because of recruitment challenges in postpartum patients, multisite trials may be required to include adequate numbers of participants.
Related Resource
- Freeman MP, Joffe H, Cohen LS. Postpartum depression: Help patients find the right treatment. Current Psychiatry. 2012;11(11):14-21.
Drug Brand Names
- Citalopram • Celexa
- Escitalopram • Lexapro
Disclosures
Dr. Freeman has received grant or research support from Eli Lilly and Company, Forest Laboratories, and GlaxoSmithKline, is on the advisory boards of Otsuka and Takeda/Lundbeck, and is a consultant for PamLab LLC.
Dr. Joffe has received grant or research support from Cephalon/Teva, and is a consultant to Noven and Sunovion.
Dr. Cohen has received research support from AstraZeneca, Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Forest Laboratories, GlaxoSmithKline, National Institute of Mental Health, National Institute on Aging, National Institutes of Health, Ortho-McNeil Janssen, and Pfizer and has served on an advisory board for PamLab LLC.
This study was funded as an investigator-initiated trial by Forest Pharmaceuticals.
Challenges in recruiting women to postpartum depression (PPD) antidepressant treatment trials, which we encountered when conducting a trial of escitalopram, contribute to the limited body of knowledge about PPD treatment. Here we discuss results from a preliminary trial of escitalopram for PPD, and challenges of research in this area.
Escitalopram, the S-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with high selectivity and potency that is FDA-approved for treating major depressive disorder (MDD) and generalized anxiety disorder. An agent with antidepressant and anxiolytic effects is particularly desirable for PPD because anxiety is more common in postpartum major depressive episodes than non-postpartum MDD.1 Anxiety and depressive disorders commonly are comorbid in postpartum women.2
We conducted an open-label trial of escitalopram for women with PPD and anxiety. We initially attempted to recruit 20 women.
Methods
Patients received 8 weeks of treatment with escitalopram, 10 to 20 mg/d (flexible dose). After completing the initial phone screen, patients had 5 follow-up visits, once every 2 weeks for 8 weeks. The institutional review board at Massachusetts General Hospital approved this study and we obtained written informed consent from all patients at the first visit. Twelve patients completed the phone screen and 7 eligible patients were enrolled in the study over 32 months. Reasons for ineligibility included having a history of psychosis, onset of symptoms >3 months postpartum, or presenting >6 months after onset. Others declined to participate because of concern about the time commitment or because they pursued nonpharmacologic treatments after the evaluation visit. One patient was lost to follow-up. Three patients completed the study. The study was halted because of the slow pace of recruitment.
Patient selection. Patients were screened for a major depressive episode with postpartum onset within 3 months of childbirth; depressive symptoms may have developed during pregnancy and worsened postpartum to meet criteria for MDD. Women were eligible for the study if they:
- were age 18 to 45
- experienced a major depressive episode with symptoms developing within 3 months of childbirth
- presented within 6 months of childbirth
- had a Montgomery-Åsberg Depression Rating Scale (MADRS) score >15
- had a Beck Anxiety Inventory (BAI) score >10.
Patients who were pregnant or breast-feeding were excluded from the study per an agreement with the sponsor. In addition, women were excluded if they had taken any psychotropic medication within 2 weeks of enrollment; had active suicidal ideation, homicidal ideation, or presence of psychotic symptoms; had chronic depression or dysthymia; had chronic or treatment-resistant anxiety disorders; had a history of mania or hypomania; or had active alcohol or substance abuse within the past year.
Treatment. Patients received escitalopram, 10 mg/d, after the baseline visit. At the investigator’s discretion, the dose could be increased to 20 mg/d or lowered to 5 mg/d if side effects occurred.
Measures. At the first visit, patients were assessed with the Mini-International Neuropsychiatric Interview to verify MDD and exclude diagnoses that would determine ineligibility. MADRS and Edinburgh Postnatal Depression Scale (EPDS) were used at each visit to measure depressive symptoms.3,4 The BAI was completed at each visit to measure anxiety symptoms. Obsessions and compulsions were measured with the Yale-Brown Obsessive Compulsive Scale (Y-BOCS)5 at baseline, and at all following visits if the patient scored >8 at baseline. The Clinical Global Impression Scales for severity and improvement were completed at each visit.6
Results
Of 7 patients enrolled, 3 completed the study, 2 were ineligible after the baseline visit, and 2 did not participate after the baseline visit (1 selected to pursue psychotherapy, and 1 was lost to follow-up).
Two of 3 patients responded to escitalopram (≥50% decrease on MADRS), and both were remitters (MADRS score <7). All 3 patients were responders on EPDS and BAI. One patient had Y-BOCS >8 at baseline (Total Y-BOCS score of 9, and final Y-BOCS score of 8) (Table).
Table
Symptom rating scale scores at baseline and study end
| Baseline (Visit 1) | Final (Visit 5) | |||||
|---|---|---|---|---|---|---|
| Patient | MADRS | BAI | EPDS | MADRS | BAI | EPDS |
| Ms. A | 21 | 18 | 22 | 12 | 0 | 0 |
| Ms. B | 28 | 28 | 19 | 4 | 5 | 2 |
| Ms. C | 37 | 6 | 19 | 6 | 2 | 0 |
| BAI: Beck Anxiety Inventory; EPDS: Edinburgh Postnatal Depression Scale; MADRS: Montgomery-Åsberg Depression Rating Scale | ||||||
Discussion
Patients who stayed in treatment improved during the course of this study. Recruitment was difficult; we were able to recruit only 7 patients out of a projected 20 for the screening visit. We solicited feedback from local obstetrics health care providers and social workers on recruitment and attractiveness of the study as part of our routine collaboration with obstetrical services that screen for PPD. Primary reasons patients were not referred were that they were breast-feeding or they stated they would prefer to receive treatment from their primary care doctor. Recruitment difficulty in this study was in stark contrast to other recent studies completed at our center. For example, we have successfully recruited for menopausal depression and premenstrual dysphoric disorder treatment studies, and have completed large naturalistic studies of women with unipolar depression and bipolar disorder across pregnancy and postpartum. We suspect that many patients who were eligible for the study preferred to seek care from an obstetrician or primary care doctor with whom they already had a therapeutic alliance, and we also suspect that many women with PPD do not seek treatment at all, which is consistent with findings from other research groups.
Lessons learned from PPD research include:
- Including women who are breast-feeding is important because many women choose to breast-feed and suffer from PPD. Because antidepressant use during breast-feeding has been closely studied, it is appropriate to include potential research participants who are breast-feeding as long as they receive adequate information and are able to provide informed consent.
- Participants in PPD studies may require accommodations that take into account their role as a new mother, such as on-site childcare, home visits, or other strategies.
- Because of recruitment challenges in postpartum patients, multisite trials may be required to include adequate numbers of participants.
Related Resource
- Freeman MP, Joffe H, Cohen LS. Postpartum depression: Help patients find the right treatment. Current Psychiatry. 2012;11(11):14-21.
Drug Brand Names
- Citalopram • Celexa
- Escitalopram • Lexapro
Disclosures
Dr. Freeman has received grant or research support from Eli Lilly and Company, Forest Laboratories, and GlaxoSmithKline, is on the advisory boards of Otsuka and Takeda/Lundbeck, and is a consultant for PamLab LLC.
Dr. Joffe has received grant or research support from Cephalon/Teva, and is a consultant to Noven and Sunovion.
Dr. Cohen has received research support from AstraZeneca, Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Forest Laboratories, GlaxoSmithKline, National Institute of Mental Health, National Institute on Aging, National Institutes of Health, Ortho-McNeil Janssen, and Pfizer and has served on an advisory board for PamLab LLC.
This study was funded as an investigator-initiated trial by Forest Pharmaceuticals.
1. Bernstein IH, Rush AJ, Yonkers K, et al. Symptom features of postpartum depression: are they distinct? Depress Anxiety. 2008;25(1):20-26.
2. Wenzel A, Haugen EN, Jackson LC, et al. Anxiety symptoms and disorders at eight weeks postpartum. J Anxiety Disord. 2005;19(3):295-311.
3. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
4. Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389.
5. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006-1011.
6. Guy W. ECDEU assessment manual for psychopharmacology. Rockville MD: US Department of Health and Human Services; 1976. Department of Health, Education, and Welfare Publication (ADM) 76–338.
1. Bernstein IH, Rush AJ, Yonkers K, et al. Symptom features of postpartum depression: are they distinct? Depress Anxiety. 2008;25(1):20-26.
2. Wenzel A, Haugen EN, Jackson LC, et al. Anxiety symptoms and disorders at eight weeks postpartum. J Anxiety Disord. 2005;19(3):295-311.
3. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
4. Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389.
5. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006-1011.
6. Guy W. ECDEU assessment manual for psychopharmacology. Rockville MD: US Department of Health and Human Services; 1976. Department of Health, Education, and Welfare Publication (ADM) 76–338.
Postpartum depression: Help patients find the right treatment
Discuss this article at www.facebook.com/CurrentPsychiatry
Postpartum depression (PPD)—emergence of a major depressive episode after childbirth—has broad negative consequences for the mother, baby, and other family members. The time of onset after delivery for a depressive episode to be considered postpartum is debatable, but the DSM-IV-TR specifier states that onset within 4 weeks of childbirth is considered postpartum. PPD can impact many aspects of child development, including mother-infant attachment, cognitive development, and behavior.1-3
An estimated 10% of women who have given birth experience PPD.4,5 The risk of PPD is particularly high among women who have had previous episodes of PPD or major depressive disorder (MDD). Other risk factors include stressful life events, depression and/or anxiety during pregnancy, family history of PPD, and obstetrical complications.6-8 Anxiety disorders are common in postpartum women, and anxiety symptoms often are prominent in PPD.9
Despite the prevalence of PPD and its serious consequences, few studies have addressed antidepressant treatment. In this article we discuss screening and treating PPD and considerations for breast-feeding mothers. Click here for results of an open-label trial of escitalopram for PPD we conducted in which patient recruitment was challenging.
Screening for PPD: A good start
Initiatives by state governments and health care providers have led to programs in which universal screening for PPD has been implemented. Screening provides a mechanism for early detection and intervention. The Edinburgh Postnatal Depression Scale10 is a self-rated, 10-item scale developed for the postpartum setting, and its use increases identification of PPD at postpartum obstetrics visits.11 Other screening tools such as the Patient Health Questionnaire-9 also are commonly used. Despite the success of screening programs in attempting the feasibility of screening, it is unclear if the identification of women who may be experiencing PPD increases their engagement in treatment. Studies have demonstrated that even when depressive symptoms suggesting a PPD episode are identified in the postpartum period, many women still do not receive treatment.12,13 Studies of PPD screening programs have not demonstrated that screening itself improves treatment engagement or improves outcomes.12,13
Psychotherapy: An effective option
Psychotherapy is an important first-line option for PPD, particularly because of considerations of medication exposure during breast-feeding and many women are reluctant to take antidepressants while breast-feeding.16 Interpersonal psychotherapy and cognitive-behavioral therapy (CBT) have been most studied for PPD, and both appear effective for prevention and acute treatment of PPD.17-20 Although psychotherapy alone may be sufficient for some women, for others, medication may be an important first-line treatment, depending on symptom severity, access to psychotherapy, and personal preference.
Evidence for antidepressants
Randomization to placebo is rare in PPD trials. Most trials have used open-label designs because placebo arms pose ethical dilemmas considering the impact of PPD on a mother and her baby. In a randomized study of sertraline or nortriptyline for PPD, both drugs were similarly efficacious.22 In another study comparing paroxetine monotherapy and paroxetine plus CBT for PPD, both groups experienced significant improvement in depression and anxiety symptoms, with no difference between groups at endpoint.23 Open-label trials have suggested antidepressants’ efficacy, although some studies have included small sample sizes (Table 1).20-27
Table 1
Antidepressants for PPD: Summary of the evidence
| Study | Design and size | Medication | Results |
|---|---|---|---|
| Appleby et al, 199720 | 12-week, placebo-controlled, N = 87 | Fluoxetine | Patients taking fluoxetine showed greater improvement than those taking placebo |
| Yonkers et al, 200821 | 8-week, placebo-controlled, N = 70 | Paroxetine | Both groups improved over time, but patients taking paroxetine had greater improvement in overall clinical severity |
| Wisner et al, 200622 | 8-week, RCT, N = 109 | Sertraline vs nortriptyline | Proportion of women who responded or remitted did not differ between those taking sertraline or nortriptyline |
| Misri et al, 200423 | 12-week, RCT, N = 35 | Paroxetine monotherapy vs paroxetine + CBT | Both groups showed significant improvement in mood and anxiety symptoms |
| Stowe et al, 199524 | 8-week, open-label, N = 21 | Sertraline | 20 patients experienced >50% reduction in SIGH-D score |
| Cohen et al, 199725 | Open-label, N = 15 | Venlafaxine | 12 patients achieved remission |
| Suri et al, 200126 | 8-week, open-label, N = 6 | Fluvoxamine | 4 patients became euthymic, with HDRS scores ranging from 2 to 5 |
| Nonacs et al, 200527 | 8-week, open-label, N = 8 | Bupropion | 6 patients had ≥50% decrease in HDRS score from baseline; 3 achieved remission |
| CBT: cognitive-behavioral therapy; HDRS: Hamilton Depression Rating Scale; PPD: postpartum depression; RCT: randomized controlled trial; SIGH-D: Structured Interview Guide for the Hamilton Depression Rating Scale | |||
Breast-feeding considerations
From a nutritional standpoint, breast-feeding is optimal for a newborn. However, for some women, breast-feeding is difficult and stressful, and new mothers may experience this difficulty as failure. Some women prefer not to breast-feed, and others may prefer to formula feed if they require pharmacotherapy, particularly if the medication has not been well studied in breast-feeding patients. Some women may decline to take medications if they are breast-feeding out of concern for the baby’s exposure via breast milk and prefer to try nonpharmacologic approaches first. Many mothers with PPD need to be reassured that stopping breast-feeding may be exactly what is needed if the experience is contributing to their PPD or making them uncomfortable accepting pharmacotherapy when indicated. Maternal mental health is more important than breast-feeding to the health and wellness of the mother-baby dyad.
Table 2
Considerations for antidepressant use during breast-feeding
| Drug(s) | Comments |
|---|---|
| Fluoxetine | Because of long half-life, may be more likely to be detected in infant serum, especially at higher doses. Reasonable for use during breast-feeding if a woman has had a good previous response to the drug or used it during pregnancy |
| Sertraline | Reports of low levels of exposure. Relatively large amount of data available |
| Citalopram, escitalopram | Less systematic study of mother-infant pairs compared with sertraline and paroxetine. Low levels of exposure to infant via breast-feeding observed |
| Paroxetine | Consistent reports of low levels of exposure and has been relatively well studied without reported adverse events. Use limited by commonly experienced withdrawal symptoms; may be more sedating than other SSRIs |
| Bupropion | Paucity of systematic study in newborns of nursing mothers; a few case reports in older infants demonstrated low levels of exposure via breast-feeding. May help women who smoke to quit or to maintain abstinence from smoking. Reasonable to use if a woman had good previous response. One case report of possible infant seizure; no other reported adverse events |
| Venlafaxine, desvenlafaxine | Higher levels of desvenlafaxine than venlafaxine found in breast milk. No adverse events reported. Patients may experience withdrawal with discontinuation or missed doses |
| Tricyclic antidepressants | Considered reasonable for breast-feeding mothers if use is clinically warranted; few adverse effects in babies and generally low levels of exposure reported |
| Mirtazapine, nefazodone, MAOIs, duloxetine | Systematic human data not available for breast-feeding patients. May be reasonable if a woman previously has responded best to 1 of these; advise patients that data are not available to guide decisions |
| MAOIs: monoamine oxidase inhibitors; SSRIs: selective serotonin reuptake inhibitors Source: References 29-31 | |
28,29
The psychiatrist’s role
PPD has great public health significance because it affects a large number of women and their families. Screening during obstetrical visits or in other settings may increase identification of women who are suffering from PPD. In order for this screening to lead to meaningful changes, women must receive timely and expert evaluations for PPD and treatment that is efficacious and accessible.
Diagnosis and treatment: 4 pearls
Verify the diagnosis. Many women who present with postpartum depressive symptoms may have previously unrecognized bipolar disorder, and many women presenting with a primary complaint of anxiety have PPD.33,34
Discuss breast-feeding. This topic is important in assessing the risks and benefits of antidepressants in postpartum women, but many women also experience breast-feeding as a topic with emotional valence of its own and may need support with infant feeding.
Meet the patient where she is. Patient preferences strongly influence PPD treatment decisions. Women with similar clinical presentations may have strong preferences for different treatments.
Make treatment accessible. Postpartum women may find it challenging to engage in treatment. Treatment plans need to be feasible for women who are depressed while caring for a newborn. On-site childcare, home visits, Internet communication, and other accommodations that may facilitate treatment should be considered at a systems level.
Related Resources
- American College of Obstetricians and Gynecologists. Screening for depression during and after pregnancy. www.acog.org/Resources_And_Publications/Committee_Opinions/Committee_on_Obstetric_Practice/Screening_for_Depression_During_and_After_Pregnancy.
- Meltzer-Brody S. New insights into perinatal depression: pathogenesis and treatment during pregnancy and postpartum. Dialogues Clin Neurosci. 2011;13(1):89-100.
- Dennis CL, Stewart DE. Treatment of postpartum depression, part 1: a critical review of biological interventions. J Clin Psychiatry. 2004;65(9):1242-1251.
- Dennis CL. Treatment of postpartum depression, part 2: a critical review of nonbiological interventions. J Clin Psychiatry. 2004;65(9):1252-1265.
- Cohen LS, Wang B, Nonacs R, et al. Treatment of mood disorders during pregnancy and postpartum. Psychiatr Clin North Am. 2010;33(2):273-293.
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Desvenlafaxine • Pristiq
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
Dr. Joffe has received grant or research support from Cephalon/Teva, and is a consultant to Noven and Sunovion.
Dr. Cohen has received research support from AstraZeneca, Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Forest Laboratories, GlaxoSmithKline, National Institute of Mental Health, National Institute on Aging, National Institutes of Health, Ortho-McNeil Janssen, and Pfizer and has served on an advisory board for PamLab LLC.
1. Cicchetti D, Rogosch FA, Toth SL. Maternal depressive disorder and contextual risk: contributions to the development of attachment insecurity and behavior problems in toddlerhood. Dev Psychopathol. 1998;10(2):283-300.
2. Murray L, Fiori-Cowley A, Hooper R, et al. The impact of postnatal depression and associated adversity on early mother-infant interactions and later infant outcome. Child Dev. 1996;67(5):2512-2526.
3. Sharp D, Hay DF, Pawlby S, et al. The impact of postnatal depression on boys’ intellectual development. J Child Psychol Psychiatry. 1995;36(8):1315-1336.
4. Altshuler LL, Hendrick V, Cohen LS. Course of mood and anxiety disorders during pregnancy and the postpartum period. J Clin Psychiatry. 1998;59(suppl 2):29-33.
5. Pariser SF. Women and mood disorders. Menarche to menopause. Ann Clin Psychiatry. 1993;5(4):249-254.
6. Dennis CL, Janssen PA, Singer J. Identifying women at-risk for postpartum depression in the immediate postpartum period. Acta Psychiatr Scand. 2004;110(5):338-346.
7. Chaudron LH, Klein MH, Remington P, et al. Predictors, prodromes and incidence of postpartum depression. J Psychosom Obstet Gynaecol. 2001;22(2):103-112.
8. Heron J, O’Connor TG, Evans J, et al. ALSPAC Study Team. The course of anxiety and depression through pregnancy and the postpartum in a community sample. J Affect Disord. 2004;80(1):65-73.
9. Wenzel A, Haugen EN, Jackson LC, et al. Anxiety symptoms and disorders at eight weeks postpartum. J Anxiety Disord. 2005;19(3):295-311.
10. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
11. Evins GG, Theofrastous JP, Galvin SL. Postpartum depression: a comparison of screening and routine clinical evaluation. Am J Obstet Gynecol. 2000;182(5):1080-1082.
12. Flynn HA, O’Mahen HA, Massey L, et al. The impact of a brief obstetrics clinic-based intervention on treatment use for perinatal depression. J Womens Health (Larchmt). 2006;15(10):1195-1204.
13. Yonkers KA, Smith MV, Lin H, et al. Depression screening of perinatal women: an evaluation of the healthy start depression initiative. Psychiatr Serv. 2009;60(3):322-328.
14. van Schaik DJ, Klijn AF, van Hout HP, et al. Patients’ p in the treatment of depressive disorder in primary care. Gen Hosp Psychiatry. 2004;26(3):184-189.
15. Boath E, Bradley E, Henshaw C. Women’s views of antidepressants in the treatment of postnatal depression. J Psychosom Obstet Gynaecol. 2004;25(3-4):221-233.
16. Pearlstein TB, Zlotnick C, Battle CL, et al. Patient choice of treatment for postpartum depression: a pilot study. Arch Womens Ment Health. 2006;9(6):303-308.
17. Zlotnick C, Johnson SL, Miller IW, et al. Postpartum depression in women receiving public assistance: pilot study of an interpersonal-therapy-oriented group intervention. Am J Psychiatry. 2001;158(4):638-640.
18. Klier CM, Muzik M, Rosenblum KL, et al. Interpersonal psychotherapy adapted for the group setting in the treatment of postpartum depression. J Psychother Pract Res. 2001;10(2):124-131.
19. Stuart S, O’Hara MW, Gorman LL. The prevention and psychotherapeutic treatment of postpartum depression. Arch Womens Ment Health. 2003;6(suppl 2):S57-S69.
20. Appleby L, Warner R, Whitton A, et al. A controlled study of fluoxetine and cognitive-behavioural counselling in the treatment of postnatal depression. BMJ. 1997;314(7085):932-936.
21. Yonkers KA, Lin H, Howell HB, et al. Pharmacologic treatment of postpartum women with new-onset major depressive disorder: a randomized controlled trial with paroxetine. J Clin Psychiatry. 2008;69(4):659-665.
22. Wisner KL, Hanusa BH, Perel JM, et al. Postpartum depression: a randomized trial of sertraline versus nortriptyline. J Clin Psychopharmacol. 2006;(4)26:353-360.
23. Misri S, Reebye P, Corral M, et al. The use of paroxetine and cognitive-behavioral therapy in postpartum depression and anxiety: a randomized controlled trial. J Clin Psychiatry. 2004;65(9):1236-1241.
24. Stowe ZN, Casarella J, Landry J, et al. Sertraline in the treatment of women with postpartum major depression. Depression. 1995;3(1-2):49-55.
25. Cohen LS, Viguera AC, Bouffard SM, et al. Venlafaxine in the treatment of postpartum depression. J Clin Psychiatry. 2001;62(8):592-596.
26. Suri R, Burt VK, Altshuler LL, et al. Fluvoxamine for postpartum depression. Am J Psychiatry. 2001;158(10):1739-1740.
27. Nonacs RM, Soares CN, Viguera AC, et al. Bupropion SR for the treatment of postpartum depression: a pilot study. Int J Neuropsychopharmacol. 2005;8(3):445-449.
28. Burt VK, Suri R, Altshuler L, et al. The use of psychotropic medications during breast-feeding. Am J Psychiatry. 2001;158(7):1001-1009.
29. Weissman AM, Levy BT, Hartz AJ, et al. Pooled analysis of antidepressant levels in lactating mothers, breast milk, and nursing infants. Am J Psychiatry. 2004;161(6):1066-1078.
30. Newport DJ, Ritchie JC, Knight BT, et al. Venlafaxine in human breast milk and nursing infant plasma: determination of exposure. J Clin Psychiatry. 2009;70(9):1304-1310.
31. Chaudron LH, Schoenecker CJ. Bupropion and breastfeeding: a case of a possible infant seizure. J Clin Psychiatry. 2004;65(6):881-882.
32. Hendrick V, Stowe ZN, Altshuler LL, et al. Fluoxetine and norfluoxetine concentrations in nursing infants and breast milk. Biol Psychiatry. 2001;50(10):775-782.
33. Sharma V, Khan M. Identification of bipolar disorder in women with postpartum depression. Bipolar Disord. 2010;12(3):335-340.
34. Austin MP, Hadzi-Pavlovic D, Priest SR, et al. Depressive and anxiety disorders in the postpartum period: how prevalent are they and can we improve their detection? Arch Womens Ment Health. 2010;13(5):395-401.
Discuss this article at www.facebook.com/CurrentPsychiatry
Postpartum depression (PPD)—emergence of a major depressive episode after childbirth—has broad negative consequences for the mother, baby, and other family members. The time of onset after delivery for a depressive episode to be considered postpartum is debatable, but the DSM-IV-TR specifier states that onset within 4 weeks of childbirth is considered postpartum. PPD can impact many aspects of child development, including mother-infant attachment, cognitive development, and behavior.1-3
An estimated 10% of women who have given birth experience PPD.4,5 The risk of PPD is particularly high among women who have had previous episodes of PPD or major depressive disorder (MDD). Other risk factors include stressful life events, depression and/or anxiety during pregnancy, family history of PPD, and obstetrical complications.6-8 Anxiety disorders are common in postpartum women, and anxiety symptoms often are prominent in PPD.9
Despite the prevalence of PPD and its serious consequences, few studies have addressed antidepressant treatment. In this article we discuss screening and treating PPD and considerations for breast-feeding mothers. Click here for results of an open-label trial of escitalopram for PPD we conducted in which patient recruitment was challenging.
Screening for PPD: A good start
Initiatives by state governments and health care providers have led to programs in which universal screening for PPD has been implemented. Screening provides a mechanism for early detection and intervention. The Edinburgh Postnatal Depression Scale10 is a self-rated, 10-item scale developed for the postpartum setting, and its use increases identification of PPD at postpartum obstetrics visits.11 Other screening tools such as the Patient Health Questionnaire-9 also are commonly used. Despite the success of screening programs in attempting the feasibility of screening, it is unclear if the identification of women who may be experiencing PPD increases their engagement in treatment. Studies have demonstrated that even when depressive symptoms suggesting a PPD episode are identified in the postpartum period, many women still do not receive treatment.12,13 Studies of PPD screening programs have not demonstrated that screening itself improves treatment engagement or improves outcomes.12,13
Psychotherapy: An effective option
Psychotherapy is an important first-line option for PPD, particularly because of considerations of medication exposure during breast-feeding and many women are reluctant to take antidepressants while breast-feeding.16 Interpersonal psychotherapy and cognitive-behavioral therapy (CBT) have been most studied for PPD, and both appear effective for prevention and acute treatment of PPD.17-20 Although psychotherapy alone may be sufficient for some women, for others, medication may be an important first-line treatment, depending on symptom severity, access to psychotherapy, and personal preference.
Evidence for antidepressants
Randomization to placebo is rare in PPD trials. Most trials have used open-label designs because placebo arms pose ethical dilemmas considering the impact of PPD on a mother and her baby. In a randomized study of sertraline or nortriptyline for PPD, both drugs were similarly efficacious.22 In another study comparing paroxetine monotherapy and paroxetine plus CBT for PPD, both groups experienced significant improvement in depression and anxiety symptoms, with no difference between groups at endpoint.23 Open-label trials have suggested antidepressants’ efficacy, although some studies have included small sample sizes (Table 1).20-27
Table 1
Antidepressants for PPD: Summary of the evidence
| Study | Design and size | Medication | Results |
|---|---|---|---|
| Appleby et al, 199720 | 12-week, placebo-controlled, N = 87 | Fluoxetine | Patients taking fluoxetine showed greater improvement than those taking placebo |
| Yonkers et al, 200821 | 8-week, placebo-controlled, N = 70 | Paroxetine | Both groups improved over time, but patients taking paroxetine had greater improvement in overall clinical severity |
| Wisner et al, 200622 | 8-week, RCT, N = 109 | Sertraline vs nortriptyline | Proportion of women who responded or remitted did not differ between those taking sertraline or nortriptyline |
| Misri et al, 200423 | 12-week, RCT, N = 35 | Paroxetine monotherapy vs paroxetine + CBT | Both groups showed significant improvement in mood and anxiety symptoms |
| Stowe et al, 199524 | 8-week, open-label, N = 21 | Sertraline | 20 patients experienced >50% reduction in SIGH-D score |
| Cohen et al, 199725 | Open-label, N = 15 | Venlafaxine | 12 patients achieved remission |
| Suri et al, 200126 | 8-week, open-label, N = 6 | Fluvoxamine | 4 patients became euthymic, with HDRS scores ranging from 2 to 5 |
| Nonacs et al, 200527 | 8-week, open-label, N = 8 | Bupropion | 6 patients had ≥50% decrease in HDRS score from baseline; 3 achieved remission |
| CBT: cognitive-behavioral therapy; HDRS: Hamilton Depression Rating Scale; PPD: postpartum depression; RCT: randomized controlled trial; SIGH-D: Structured Interview Guide for the Hamilton Depression Rating Scale | |||
Breast-feeding considerations
From a nutritional standpoint, breast-feeding is optimal for a newborn. However, for some women, breast-feeding is difficult and stressful, and new mothers may experience this difficulty as failure. Some women prefer not to breast-feed, and others may prefer to formula feed if they require pharmacotherapy, particularly if the medication has not been well studied in breast-feeding patients. Some women may decline to take medications if they are breast-feeding out of concern for the baby’s exposure via breast milk and prefer to try nonpharmacologic approaches first. Many mothers with PPD need to be reassured that stopping breast-feeding may be exactly what is needed if the experience is contributing to their PPD or making them uncomfortable accepting pharmacotherapy when indicated. Maternal mental health is more important than breast-feeding to the health and wellness of the mother-baby dyad.
Table 2
Considerations for antidepressant use during breast-feeding
| Drug(s) | Comments |
|---|---|
| Fluoxetine | Because of long half-life, may be more likely to be detected in infant serum, especially at higher doses. Reasonable for use during breast-feeding if a woman has had a good previous response to the drug or used it during pregnancy |
| Sertraline | Reports of low levels of exposure. Relatively large amount of data available |
| Citalopram, escitalopram | Less systematic study of mother-infant pairs compared with sertraline and paroxetine. Low levels of exposure to infant via breast-feeding observed |
| Paroxetine | Consistent reports of low levels of exposure and has been relatively well studied without reported adverse events. Use limited by commonly experienced withdrawal symptoms; may be more sedating than other SSRIs |
| Bupropion | Paucity of systematic study in newborns of nursing mothers; a few case reports in older infants demonstrated low levels of exposure via breast-feeding. May help women who smoke to quit or to maintain abstinence from smoking. Reasonable to use if a woman had good previous response. One case report of possible infant seizure; no other reported adverse events |
| Venlafaxine, desvenlafaxine | Higher levels of desvenlafaxine than venlafaxine found in breast milk. No adverse events reported. Patients may experience withdrawal with discontinuation or missed doses |
| Tricyclic antidepressants | Considered reasonable for breast-feeding mothers if use is clinically warranted; few adverse effects in babies and generally low levels of exposure reported |
| Mirtazapine, nefazodone, MAOIs, duloxetine | Systematic human data not available for breast-feeding patients. May be reasonable if a woman previously has responded best to 1 of these; advise patients that data are not available to guide decisions |
| MAOIs: monoamine oxidase inhibitors; SSRIs: selective serotonin reuptake inhibitors Source: References 29-31 | |
28,29
The psychiatrist’s role
PPD has great public health significance because it affects a large number of women and their families. Screening during obstetrical visits or in other settings may increase identification of women who are suffering from PPD. In order for this screening to lead to meaningful changes, women must receive timely and expert evaluations for PPD and treatment that is efficacious and accessible.
Diagnosis and treatment: 4 pearls
Verify the diagnosis. Many women who present with postpartum depressive symptoms may have previously unrecognized bipolar disorder, and many women presenting with a primary complaint of anxiety have PPD.33,34
Discuss breast-feeding. This topic is important in assessing the risks and benefits of antidepressants in postpartum women, but many women also experience breast-feeding as a topic with emotional valence of its own and may need support with infant feeding.
Meet the patient where she is. Patient preferences strongly influence PPD treatment decisions. Women with similar clinical presentations may have strong preferences for different treatments.
Make treatment accessible. Postpartum women may find it challenging to engage in treatment. Treatment plans need to be feasible for women who are depressed while caring for a newborn. On-site childcare, home visits, Internet communication, and other accommodations that may facilitate treatment should be considered at a systems level.
Related Resources
- American College of Obstetricians and Gynecologists. Screening for depression during and after pregnancy. www.acog.org/Resources_And_Publications/Committee_Opinions/Committee_on_Obstetric_Practice/Screening_for_Depression_During_and_After_Pregnancy.
- Meltzer-Brody S. New insights into perinatal depression: pathogenesis and treatment during pregnancy and postpartum. Dialogues Clin Neurosci. 2011;13(1):89-100.
- Dennis CL, Stewart DE. Treatment of postpartum depression, part 1: a critical review of biological interventions. J Clin Psychiatry. 2004;65(9):1242-1251.
- Dennis CL. Treatment of postpartum depression, part 2: a critical review of nonbiological interventions. J Clin Psychiatry. 2004;65(9):1252-1265.
- Cohen LS, Wang B, Nonacs R, et al. Treatment of mood disorders during pregnancy and postpartum. Psychiatr Clin North Am. 2010;33(2):273-293.
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Desvenlafaxine • Pristiq
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
Dr. Joffe has received grant or research support from Cephalon/Teva, and is a consultant to Noven and Sunovion.
Dr. Cohen has received research support from AstraZeneca, Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Forest Laboratories, GlaxoSmithKline, National Institute of Mental Health, National Institute on Aging, National Institutes of Health, Ortho-McNeil Janssen, and Pfizer and has served on an advisory board for PamLab LLC.
Discuss this article at www.facebook.com/CurrentPsychiatry
Postpartum depression (PPD)—emergence of a major depressive episode after childbirth—has broad negative consequences for the mother, baby, and other family members. The time of onset after delivery for a depressive episode to be considered postpartum is debatable, but the DSM-IV-TR specifier states that onset within 4 weeks of childbirth is considered postpartum. PPD can impact many aspects of child development, including mother-infant attachment, cognitive development, and behavior.1-3
An estimated 10% of women who have given birth experience PPD.4,5 The risk of PPD is particularly high among women who have had previous episodes of PPD or major depressive disorder (MDD). Other risk factors include stressful life events, depression and/or anxiety during pregnancy, family history of PPD, and obstetrical complications.6-8 Anxiety disorders are common in postpartum women, and anxiety symptoms often are prominent in PPD.9
Despite the prevalence of PPD and its serious consequences, few studies have addressed antidepressant treatment. In this article we discuss screening and treating PPD and considerations for breast-feeding mothers. Click here for results of an open-label trial of escitalopram for PPD we conducted in which patient recruitment was challenging.
Screening for PPD: A good start
Initiatives by state governments and health care providers have led to programs in which universal screening for PPD has been implemented. Screening provides a mechanism for early detection and intervention. The Edinburgh Postnatal Depression Scale10 is a self-rated, 10-item scale developed for the postpartum setting, and its use increases identification of PPD at postpartum obstetrics visits.11 Other screening tools such as the Patient Health Questionnaire-9 also are commonly used. Despite the success of screening programs in attempting the feasibility of screening, it is unclear if the identification of women who may be experiencing PPD increases their engagement in treatment. Studies have demonstrated that even when depressive symptoms suggesting a PPD episode are identified in the postpartum period, many women still do not receive treatment.12,13 Studies of PPD screening programs have not demonstrated that screening itself improves treatment engagement or improves outcomes.12,13
Psychotherapy: An effective option
Psychotherapy is an important first-line option for PPD, particularly because of considerations of medication exposure during breast-feeding and many women are reluctant to take antidepressants while breast-feeding.16 Interpersonal psychotherapy and cognitive-behavioral therapy (CBT) have been most studied for PPD, and both appear effective for prevention and acute treatment of PPD.17-20 Although psychotherapy alone may be sufficient for some women, for others, medication may be an important first-line treatment, depending on symptom severity, access to psychotherapy, and personal preference.
Evidence for antidepressants
Randomization to placebo is rare in PPD trials. Most trials have used open-label designs because placebo arms pose ethical dilemmas considering the impact of PPD on a mother and her baby. In a randomized study of sertraline or nortriptyline for PPD, both drugs were similarly efficacious.22 In another study comparing paroxetine monotherapy and paroxetine plus CBT for PPD, both groups experienced significant improvement in depression and anxiety symptoms, with no difference between groups at endpoint.23 Open-label trials have suggested antidepressants’ efficacy, although some studies have included small sample sizes (Table 1).20-27
Table 1
Antidepressants for PPD: Summary of the evidence
| Study | Design and size | Medication | Results |
|---|---|---|---|
| Appleby et al, 199720 | 12-week, placebo-controlled, N = 87 | Fluoxetine | Patients taking fluoxetine showed greater improvement than those taking placebo |
| Yonkers et al, 200821 | 8-week, placebo-controlled, N = 70 | Paroxetine | Both groups improved over time, but patients taking paroxetine had greater improvement in overall clinical severity |
| Wisner et al, 200622 | 8-week, RCT, N = 109 | Sertraline vs nortriptyline | Proportion of women who responded or remitted did not differ between those taking sertraline or nortriptyline |
| Misri et al, 200423 | 12-week, RCT, N = 35 | Paroxetine monotherapy vs paroxetine + CBT | Both groups showed significant improvement in mood and anxiety symptoms |
| Stowe et al, 199524 | 8-week, open-label, N = 21 | Sertraline | 20 patients experienced >50% reduction in SIGH-D score |
| Cohen et al, 199725 | Open-label, N = 15 | Venlafaxine | 12 patients achieved remission |
| Suri et al, 200126 | 8-week, open-label, N = 6 | Fluvoxamine | 4 patients became euthymic, with HDRS scores ranging from 2 to 5 |
| Nonacs et al, 200527 | 8-week, open-label, N = 8 | Bupropion | 6 patients had ≥50% decrease in HDRS score from baseline; 3 achieved remission |
| CBT: cognitive-behavioral therapy; HDRS: Hamilton Depression Rating Scale; PPD: postpartum depression; RCT: randomized controlled trial; SIGH-D: Structured Interview Guide for the Hamilton Depression Rating Scale | |||
Breast-feeding considerations
From a nutritional standpoint, breast-feeding is optimal for a newborn. However, for some women, breast-feeding is difficult and stressful, and new mothers may experience this difficulty as failure. Some women prefer not to breast-feed, and others may prefer to formula feed if they require pharmacotherapy, particularly if the medication has not been well studied in breast-feeding patients. Some women may decline to take medications if they are breast-feeding out of concern for the baby’s exposure via breast milk and prefer to try nonpharmacologic approaches first. Many mothers with PPD need to be reassured that stopping breast-feeding may be exactly what is needed if the experience is contributing to their PPD or making them uncomfortable accepting pharmacotherapy when indicated. Maternal mental health is more important than breast-feeding to the health and wellness of the mother-baby dyad.
Table 2
Considerations for antidepressant use during breast-feeding
| Drug(s) | Comments |
|---|---|
| Fluoxetine | Because of long half-life, may be more likely to be detected in infant serum, especially at higher doses. Reasonable for use during breast-feeding if a woman has had a good previous response to the drug or used it during pregnancy |
| Sertraline | Reports of low levels of exposure. Relatively large amount of data available |
| Citalopram, escitalopram | Less systematic study of mother-infant pairs compared with sertraline and paroxetine. Low levels of exposure to infant via breast-feeding observed |
| Paroxetine | Consistent reports of low levels of exposure and has been relatively well studied without reported adverse events. Use limited by commonly experienced withdrawal symptoms; may be more sedating than other SSRIs |
| Bupropion | Paucity of systematic study in newborns of nursing mothers; a few case reports in older infants demonstrated low levels of exposure via breast-feeding. May help women who smoke to quit or to maintain abstinence from smoking. Reasonable to use if a woman had good previous response. One case report of possible infant seizure; no other reported adverse events |
| Venlafaxine, desvenlafaxine | Higher levels of desvenlafaxine than venlafaxine found in breast milk. No adverse events reported. Patients may experience withdrawal with discontinuation or missed doses |
| Tricyclic antidepressants | Considered reasonable for breast-feeding mothers if use is clinically warranted; few adverse effects in babies and generally low levels of exposure reported |
| Mirtazapine, nefazodone, MAOIs, duloxetine | Systematic human data not available for breast-feeding patients. May be reasonable if a woman previously has responded best to 1 of these; advise patients that data are not available to guide decisions |
| MAOIs: monoamine oxidase inhibitors; SSRIs: selective serotonin reuptake inhibitors Source: References 29-31 | |
28,29
The psychiatrist’s role
PPD has great public health significance because it affects a large number of women and their families. Screening during obstetrical visits or in other settings may increase identification of women who are suffering from PPD. In order for this screening to lead to meaningful changes, women must receive timely and expert evaluations for PPD and treatment that is efficacious and accessible.
Diagnosis and treatment: 4 pearls
Verify the diagnosis. Many women who present with postpartum depressive symptoms may have previously unrecognized bipolar disorder, and many women presenting with a primary complaint of anxiety have PPD.33,34
Discuss breast-feeding. This topic is important in assessing the risks and benefits of antidepressants in postpartum women, but many women also experience breast-feeding as a topic with emotional valence of its own and may need support with infant feeding.
Meet the patient where she is. Patient preferences strongly influence PPD treatment decisions. Women with similar clinical presentations may have strong preferences for different treatments.
Make treatment accessible. Postpartum women may find it challenging to engage in treatment. Treatment plans need to be feasible for women who are depressed while caring for a newborn. On-site childcare, home visits, Internet communication, and other accommodations that may facilitate treatment should be considered at a systems level.
Related Resources
- American College of Obstetricians and Gynecologists. Screening for depression during and after pregnancy. www.acog.org/Resources_And_Publications/Committee_Opinions/Committee_on_Obstetric_Practice/Screening_for_Depression_During_and_After_Pregnancy.
- Meltzer-Brody S. New insights into perinatal depression: pathogenesis and treatment during pregnancy and postpartum. Dialogues Clin Neurosci. 2011;13(1):89-100.
- Dennis CL, Stewart DE. Treatment of postpartum depression, part 1: a critical review of biological interventions. J Clin Psychiatry. 2004;65(9):1242-1251.
- Dennis CL. Treatment of postpartum depression, part 2: a critical review of nonbiological interventions. J Clin Psychiatry. 2004;65(9):1252-1265.
- Cohen LS, Wang B, Nonacs R, et al. Treatment of mood disorders during pregnancy and postpartum. Psychiatr Clin North Am. 2010;33(2):273-293.
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Desvenlafaxine • Pristiq
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
Dr. Joffe has received grant or research support from Cephalon/Teva, and is a consultant to Noven and Sunovion.
Dr. Cohen has received research support from AstraZeneca, Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Forest Laboratories, GlaxoSmithKline, National Institute of Mental Health, National Institute on Aging, National Institutes of Health, Ortho-McNeil Janssen, and Pfizer and has served on an advisory board for PamLab LLC.
1. Cicchetti D, Rogosch FA, Toth SL. Maternal depressive disorder and contextual risk: contributions to the development of attachment insecurity and behavior problems in toddlerhood. Dev Psychopathol. 1998;10(2):283-300.
2. Murray L, Fiori-Cowley A, Hooper R, et al. The impact of postnatal depression and associated adversity on early mother-infant interactions and later infant outcome. Child Dev. 1996;67(5):2512-2526.
3. Sharp D, Hay DF, Pawlby S, et al. The impact of postnatal depression on boys’ intellectual development. J Child Psychol Psychiatry. 1995;36(8):1315-1336.
4. Altshuler LL, Hendrick V, Cohen LS. Course of mood and anxiety disorders during pregnancy and the postpartum period. J Clin Psychiatry. 1998;59(suppl 2):29-33.
5. Pariser SF. Women and mood disorders. Menarche to menopause. Ann Clin Psychiatry. 1993;5(4):249-254.
6. Dennis CL, Janssen PA, Singer J. Identifying women at-risk for postpartum depression in the immediate postpartum period. Acta Psychiatr Scand. 2004;110(5):338-346.
7. Chaudron LH, Klein MH, Remington P, et al. Predictors, prodromes and incidence of postpartum depression. J Psychosom Obstet Gynaecol. 2001;22(2):103-112.
8. Heron J, O’Connor TG, Evans J, et al. ALSPAC Study Team. The course of anxiety and depression through pregnancy and the postpartum in a community sample. J Affect Disord. 2004;80(1):65-73.
9. Wenzel A, Haugen EN, Jackson LC, et al. Anxiety symptoms and disorders at eight weeks postpartum. J Anxiety Disord. 2005;19(3):295-311.
10. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
11. Evins GG, Theofrastous JP, Galvin SL. Postpartum depression: a comparison of screening and routine clinical evaluation. Am J Obstet Gynecol. 2000;182(5):1080-1082.
12. Flynn HA, O’Mahen HA, Massey L, et al. The impact of a brief obstetrics clinic-based intervention on treatment use for perinatal depression. J Womens Health (Larchmt). 2006;15(10):1195-1204.
13. Yonkers KA, Smith MV, Lin H, et al. Depression screening of perinatal women: an evaluation of the healthy start depression initiative. Psychiatr Serv. 2009;60(3):322-328.
14. van Schaik DJ, Klijn AF, van Hout HP, et al. Patients’ p in the treatment of depressive disorder in primary care. Gen Hosp Psychiatry. 2004;26(3):184-189.
15. Boath E, Bradley E, Henshaw C. Women’s views of antidepressants in the treatment of postnatal depression. J Psychosom Obstet Gynaecol. 2004;25(3-4):221-233.
16. Pearlstein TB, Zlotnick C, Battle CL, et al. Patient choice of treatment for postpartum depression: a pilot study. Arch Womens Ment Health. 2006;9(6):303-308.
17. Zlotnick C, Johnson SL, Miller IW, et al. Postpartum depression in women receiving public assistance: pilot study of an interpersonal-therapy-oriented group intervention. Am J Psychiatry. 2001;158(4):638-640.
18. Klier CM, Muzik M, Rosenblum KL, et al. Interpersonal psychotherapy adapted for the group setting in the treatment of postpartum depression. J Psychother Pract Res. 2001;10(2):124-131.
19. Stuart S, O’Hara MW, Gorman LL. The prevention and psychotherapeutic treatment of postpartum depression. Arch Womens Ment Health. 2003;6(suppl 2):S57-S69.
20. Appleby L, Warner R, Whitton A, et al. A controlled study of fluoxetine and cognitive-behavioural counselling in the treatment of postnatal depression. BMJ. 1997;314(7085):932-936.
21. Yonkers KA, Lin H, Howell HB, et al. Pharmacologic treatment of postpartum women with new-onset major depressive disorder: a randomized controlled trial with paroxetine. J Clin Psychiatry. 2008;69(4):659-665.
22. Wisner KL, Hanusa BH, Perel JM, et al. Postpartum depression: a randomized trial of sertraline versus nortriptyline. J Clin Psychopharmacol. 2006;(4)26:353-360.
23. Misri S, Reebye P, Corral M, et al. The use of paroxetine and cognitive-behavioral therapy in postpartum depression and anxiety: a randomized controlled trial. J Clin Psychiatry. 2004;65(9):1236-1241.
24. Stowe ZN, Casarella J, Landry J, et al. Sertraline in the treatment of women with postpartum major depression. Depression. 1995;3(1-2):49-55.
25. Cohen LS, Viguera AC, Bouffard SM, et al. Venlafaxine in the treatment of postpartum depression. J Clin Psychiatry. 2001;62(8):592-596.
26. Suri R, Burt VK, Altshuler LL, et al. Fluvoxamine for postpartum depression. Am J Psychiatry. 2001;158(10):1739-1740.
27. Nonacs RM, Soares CN, Viguera AC, et al. Bupropion SR for the treatment of postpartum depression: a pilot study. Int J Neuropsychopharmacol. 2005;8(3):445-449.
28. Burt VK, Suri R, Altshuler L, et al. The use of psychotropic medications during breast-feeding. Am J Psychiatry. 2001;158(7):1001-1009.
29. Weissman AM, Levy BT, Hartz AJ, et al. Pooled analysis of antidepressant levels in lactating mothers, breast milk, and nursing infants. Am J Psychiatry. 2004;161(6):1066-1078.
30. Newport DJ, Ritchie JC, Knight BT, et al. Venlafaxine in human breast milk and nursing infant plasma: determination of exposure. J Clin Psychiatry. 2009;70(9):1304-1310.
31. Chaudron LH, Schoenecker CJ. Bupropion and breastfeeding: a case of a possible infant seizure. J Clin Psychiatry. 2004;65(6):881-882.
32. Hendrick V, Stowe ZN, Altshuler LL, et al. Fluoxetine and norfluoxetine concentrations in nursing infants and breast milk. Biol Psychiatry. 2001;50(10):775-782.
33. Sharma V, Khan M. Identification of bipolar disorder in women with postpartum depression. Bipolar Disord. 2010;12(3):335-340.
34. Austin MP, Hadzi-Pavlovic D, Priest SR, et al. Depressive and anxiety disorders in the postpartum period: how prevalent are they and can we improve their detection? Arch Womens Ment Health. 2010;13(5):395-401.
1. Cicchetti D, Rogosch FA, Toth SL. Maternal depressive disorder and contextual risk: contributions to the development of attachment insecurity and behavior problems in toddlerhood. Dev Psychopathol. 1998;10(2):283-300.
2. Murray L, Fiori-Cowley A, Hooper R, et al. The impact of postnatal depression and associated adversity on early mother-infant interactions and later infant outcome. Child Dev. 1996;67(5):2512-2526.
3. Sharp D, Hay DF, Pawlby S, et al. The impact of postnatal depression on boys’ intellectual development. J Child Psychol Psychiatry. 1995;36(8):1315-1336.
4. Altshuler LL, Hendrick V, Cohen LS. Course of mood and anxiety disorders during pregnancy and the postpartum period. J Clin Psychiatry. 1998;59(suppl 2):29-33.
5. Pariser SF. Women and mood disorders. Menarche to menopause. Ann Clin Psychiatry. 1993;5(4):249-254.
6. Dennis CL, Janssen PA, Singer J. Identifying women at-risk for postpartum depression in the immediate postpartum period. Acta Psychiatr Scand. 2004;110(5):338-346.
7. Chaudron LH, Klein MH, Remington P, et al. Predictors, prodromes and incidence of postpartum depression. J Psychosom Obstet Gynaecol. 2001;22(2):103-112.
8. Heron J, O’Connor TG, Evans J, et al. ALSPAC Study Team. The course of anxiety and depression through pregnancy and the postpartum in a community sample. J Affect Disord. 2004;80(1):65-73.
9. Wenzel A, Haugen EN, Jackson LC, et al. Anxiety symptoms and disorders at eight weeks postpartum. J Anxiety Disord. 2005;19(3):295-311.
10. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
11. Evins GG, Theofrastous JP, Galvin SL. Postpartum depression: a comparison of screening and routine clinical evaluation. Am J Obstet Gynecol. 2000;182(5):1080-1082.
12. Flynn HA, O’Mahen HA, Massey L, et al. The impact of a brief obstetrics clinic-based intervention on treatment use for perinatal depression. J Womens Health (Larchmt). 2006;15(10):1195-1204.
13. Yonkers KA, Smith MV, Lin H, et al. Depression screening of perinatal women: an evaluation of the healthy start depression initiative. Psychiatr Serv. 2009;60(3):322-328.
14. van Schaik DJ, Klijn AF, van Hout HP, et al. Patients’ p in the treatment of depressive disorder in primary care. Gen Hosp Psychiatry. 2004;26(3):184-189.
15. Boath E, Bradley E, Henshaw C. Women’s views of antidepressants in the treatment of postnatal depression. J Psychosom Obstet Gynaecol. 2004;25(3-4):221-233.
16. Pearlstein TB, Zlotnick C, Battle CL, et al. Patient choice of treatment for postpartum depression: a pilot study. Arch Womens Ment Health. 2006;9(6):303-308.
17. Zlotnick C, Johnson SL, Miller IW, et al. Postpartum depression in women receiving public assistance: pilot study of an interpersonal-therapy-oriented group intervention. Am J Psychiatry. 2001;158(4):638-640.
18. Klier CM, Muzik M, Rosenblum KL, et al. Interpersonal psychotherapy adapted for the group setting in the treatment of postpartum depression. J Psychother Pract Res. 2001;10(2):124-131.
19. Stuart S, O’Hara MW, Gorman LL. The prevention and psychotherapeutic treatment of postpartum depression. Arch Womens Ment Health. 2003;6(suppl 2):S57-S69.
20. Appleby L, Warner R, Whitton A, et al. A controlled study of fluoxetine and cognitive-behavioural counselling in the treatment of postnatal depression. BMJ. 1997;314(7085):932-936.
21. Yonkers KA, Lin H, Howell HB, et al. Pharmacologic treatment of postpartum women with new-onset major depressive disorder: a randomized controlled trial with paroxetine. J Clin Psychiatry. 2008;69(4):659-665.
22. Wisner KL, Hanusa BH, Perel JM, et al. Postpartum depression: a randomized trial of sertraline versus nortriptyline. J Clin Psychopharmacol. 2006;(4)26:353-360.
23. Misri S, Reebye P, Corral M, et al. The use of paroxetine and cognitive-behavioral therapy in postpartum depression and anxiety: a randomized controlled trial. J Clin Psychiatry. 2004;65(9):1236-1241.
24. Stowe ZN, Casarella J, Landry J, et al. Sertraline in the treatment of women with postpartum major depression. Depression. 1995;3(1-2):49-55.
25. Cohen LS, Viguera AC, Bouffard SM, et al. Venlafaxine in the treatment of postpartum depression. J Clin Psychiatry. 2001;62(8):592-596.
26. Suri R, Burt VK, Altshuler LL, et al. Fluvoxamine for postpartum depression. Am J Psychiatry. 2001;158(10):1739-1740.
27. Nonacs RM, Soares CN, Viguera AC, et al. Bupropion SR for the treatment of postpartum depression: a pilot study. Int J Neuropsychopharmacol. 2005;8(3):445-449.
28. Burt VK, Suri R, Altshuler L, et al. The use of psychotropic medications during breast-feeding. Am J Psychiatry. 2001;158(7):1001-1009.
29. Weissman AM, Levy BT, Hartz AJ, et al. Pooled analysis of antidepressant levels in lactating mothers, breast milk, and nursing infants. Am J Psychiatry. 2004;161(6):1066-1078.
30. Newport DJ, Ritchie JC, Knight BT, et al. Venlafaxine in human breast milk and nursing infant plasma: determination of exposure. J Clin Psychiatry. 2009;70(9):1304-1310.
31. Chaudron LH, Schoenecker CJ. Bupropion and breastfeeding: a case of a possible infant seizure. J Clin Psychiatry. 2004;65(6):881-882.
32. Hendrick V, Stowe ZN, Altshuler LL, et al. Fluoxetine and norfluoxetine concentrations in nursing infants and breast milk. Biol Psychiatry. 2001;50(10):775-782.
33. Sharma V, Khan M. Identification of bipolar disorder in women with postpartum depression. Bipolar Disord. 2010;12(3):335-340.
34. Austin MP, Hadzi-Pavlovic D, Priest SR, et al. Depressive and anxiety disorders in the postpartum period: how prevalent are they and can we improve their detection? Arch Womens Ment Health. 2010;13(5):395-401.
Perimenopausal depression: Covering mood and vasomotor symptoms
Symptoms of perimenopausal depression are not inherently different from those of depression diagnosed at any other time in life, but they present in a unique context:
- Hormonal fluctuations may persist for a long duration.
- Women experiencing hormonal fluctuations may be vulnerable to mood problems.
- Psychosocial/psychodynamic stressors often complicate this life transition.
Managing perimenopausal depression has become more complicated since the Women’s Health Initiative (WHI) studies found fewer benefits and greater risks with hormone replacement therapy (HRT) than had been perceived. This article discusses the clinical presentation of perimenopausal depression, its risk factors, and treatment options in post-WHI psychiatric practice.
Who is at risk?
Perimenopausal depression is diagnosed when onset of major depressive disorder (MDD) is associated with menstrual cycle irregularity and/or somatic symptoms of the menopausal transition.1 Diagnosis is based on the overall clinical picture, and treatment requires a thoughtful exploration of the complex relationship between hormonal function and mood regulation.
Presentation. For many women, perimenopause is characterized by mild to severe vasomotor, cognitive, and mood symptoms (Table 1). Thus, in your workup of depression in midlife women, document somatic symptoms—such as hot flushes, vaginal dryness, and incontinence—and affective/behavioral symp toms such as mood and sleep disturbances.
Table 1
Vasomotor, cognitive, and mood symptoms of perimenopause
| Vasomotor | Cognitive and mood |
|---|---|
| Hot flushes | Decreased concentration |
| Sweating | Anxiety |
| Heart palpitations | Irritability |
| Painful intercourse | Mood lability |
| Vaginal dryness and discomfort | Memory difficulty |
| Sleep disruption | |
| Headache |
Explore psychiatric and medical histories of your patient and her close relatives. Ask about depression, dysthymia, hypomania, or mood fluctuations around hormonal events such as menses, pregnancy, postpartum, or starting/stopping oral contraceptives. In the differential diagnosis, consider:
- Is low mood temporally connected with hot flushes and disturbed sleep?
- Is low mood secondary to stressful life events?
- Does the patient have another medical illness (such as thyroid disorder) with symptoms similar to depression?
- Is low mood secondary to anxiety or another psychiatric disorder?
Screening. Menopause is considered to have been reached after 12 months of amenorrhea not due to another cause. Median ages for this transition in the United States are 47.5 for perimenopause and 51 for menopause, with an average of 8 years between regular cycles and amenorrhea.2 Therefore, begin talking with women about perimenopausal symptoms when they turn 40.
Evidence supports screening perimenopausal women for depressive symptoms even when their primary complaints are vasomotor. The Greene Climacteric Scale3 is convenient for quantifying and monitoring perimenopausal symptoms. It includes depressive symptoms plus physical and cognitive markers. The Quick Inventory of Depressive Symptomatology—Self Report (QIDS-SR)4 questionnaire:
- takes minutes to complete
- is easy to score
- quantitates the number and severity of depressive symptoms (see Related Resources).
Psychosocial factors can predict depression at any time in life, but some are specific to the menopausal transition (Table 2).5 The “empty nest syndrome,” for example, is often used to explain depressive symptoms in midlife mothers, but no evidence links mood lability with the maturation and departure of children. What may be more stressing for women is supporting adolescents/young adults in their exit to independence while caring for aging parents.
Table 2
Risk factors for depression in women
| Predictive over lifetime | High risk during menopausal transition |
|---|---|
| History of depression | History of PMS, perinatal depression, mood symptoms associated with contraceptives |
| Family history of affective disorders | Premature or surgical menopause |
| Insomnia | Lengthy menopausal transition (≥27 months) |
| Reduced physical activities | Persistent and/or severe vasomotor symptoms |
| Weight gain | Negative attitudes toward menopause and aging |
| Less education | |
| Perceived lower economic status | |
| Perceived lower social support | |
| Perceived lower health status | |
| Smoking | |
| Stressful life events | |
| History of trauma | |
| Marital dissatisfaction | |
| PMS: Premenstrual syndrome | |
Sociocultural beliefs about sexuality and menopause may play a role in how your patient experiences and reports her symptoms. In some cultures, menopause elevates a woman’s social status and is associated with increased respect and authority. In others, such as Western societies that emphasize youth and beauty, women may view menopause and its physical changes in a negative light.6
Therefore, give careful attention to the psychosocial context of menopause to your patient and the social resources available to her. Questions to ask include:
- Has your lifestyle changed recently?
- Have your husband, family members, or close friends noticed any changes in your functioning?
- Is there anyone in your life that you feel comfortable confiding in?
Explaining the complexity of this life transition may ease her anxiety by normalizing her experience, helping her understand her symptoms, and validating her distress.
What might be the cause?
Although the exact pathophysiology of perimenopausal depression is unknown, hormonal changes,7 general health, the experience of menopause,8 and the psychosocial context2 likely work together to increase vulnerability for depressive symptoms (Figure).
Figure Biopsychosocial milieu of depression during perimenopauseHormonal fluctuation. The estrogen withdrawal theory7 explains depressive symptoms as resulting from a sustained decline in ovarian estrogen in tandem with spiking secretions of follicle-stimulating hormone by the pituitary. The finding that women with surgical menopause have a higher incidence of depressive symptoms than women with natural menopause supports this hypothesis.
Mood disorders occur across various female reproductive events, and increased risk appears to be associated with fluctuating gonadal hormones. Thus, declining estrogen may be less causative of perimenopausal depression than extreme fluctuations in estradiol activity.9,10
Estrogen interacts with dopamine, norepinephrine, beta-endorphin, and serotonin metabolism. In particular, estrogen facilitates serotonin delivery to neurons across the brain. These findings—and the success of selective serotonin reuptake inhibitors (SSRIs) in treating mood disorders—support the theory that fluctuating estrogen affects the serotonergic system and may cause depressive symptoms.
‘Domino theory.’ Others have hypothesized that depressive symptoms are the secondhand result of somatic symptoms of perimenopause. In a “domino effect,” hot flushes and night sweats disrupt women’s sleep, bringing fatigue and impaired daytime concentration, which lead to irritability and feelings of being overwhelmed.8
This theory, which incorporates perimenopausal hormone changes, is supported by elevated levels of depression in women who report frequent and intense vasomotor symptoms persisting >27 months.2
The psychosocial theory suggests that depression results from increased stress or adverse events.2 Midlife women with depressive symptoms report many possible sources of stress:
- demanding jobs
- family responsibilities
- dual demands of career and family
- little time for self
- poverty or employment stressors
- not enough sleep
- changing social relationships.
Negative interpretations of aging or the menopausal transition also have been implicated in cross-cultural studies.6 The predictive nature of psychosocial issues for depression during perimenopause supports this theory.
Evidence-based treatment
HRT. Research and clinical reports suggest that estrogen may have antidepressant effects, either alone or as an adjunct to antidepressant medication.11 Before the WHI studies, expert consensus guidelines on treating depression in women recommended HRT as first-line treatment for patients experiencing a first lifetime onset of mild to moderate depression during perimenopause.12 WHI findings since 2002 that associated HRT with increased risk of stroke, deep vein thrombosis, and pulmonary embolism—without clear protection against coronary heart disease or cognitive decline—have left HRT a controversial option for treating perimenopausal depression. In the WHI trials:
- 10,739 postmenopausal women age 50 to 79 without a uterus received unopposed conjugated equine estrogens, 0.625 mg/d, or placebo for an average 6.8 years.13
- 16,608 postmenopausal women age 50 to 79 with an intact uterus received combination HRT (conjugated equine estrogens, 0.625 mg/d, plus 2.5 mg of medroxyprogesterone), or placebo for an average 5.6 years.14
The study using combination HRT found increased risks of breast cancer, ischemic stroke, blood clots, and coronary heart disease.15 A follow-up study showed that vasomotor symptoms returned in more than one-half the women after they stopped using combination HRT.15
A companion WHI trial found that estrogen, 0.625 mg/d—given unopposed or with a progestin—did not prevent cognitive decline in women age 65 to 79 and may have been associated with a slightly greater risk of probable dementia.16,17
The FDA recommends that women who want to use HRT to control menopausal symptoms use the lowest effective dose for the shortest time necessary.18
Antidepressants. SSRIs may be more useful than estrogen for producing MDD remission in perimenopausal women.19 SSRIs and other psychotropics may reduce perimenopausal vasomotor symptoms in addition to addressing depressive symptoms (Table 3). When choosing antidepressant therapy, consider the patient’s dominant presenting perimenopausal symptoms and side effects associated with treatment.20
Table 3
Nonhormone medications for perimenopausal depression: Evidence-based dosages and target symptoms
| Medication | Dosage effective for perimenopausal depression | Symptoms assessed |
|---|---|---|
| SSRIs | ||
| Citaloprama | 40 to 60 mg | Depressive and vasomotor |
| Escitalopramb,c | 5 to 20 mg | Depressive and vasomotor |
| Fluoxetined | 20 to 40 mg | Depressive and vasomotor |
| Paroxetinee,f | 12.5 or 25 mg | Depressive and vasomotor |
| Sertralineg | 100 mg | Depressive and vasomotor |
| Other antidepressants | ||
| Duloxetineh | 60 to 120 mg | Depressive and vasomotor |
| Venlafaxinei | 75 to 225 mg | Depressive and vasomotor |
| Mirtazapinej | 30 to 60 mg | Severe depressive symptoms; used as an adjunct to estrogen |
| Hypnotics | ||
| Eszopiclonek | 3 mg | Depressive and vasomotor; insomnia |
| Zolpideml | 5 to 10 mg | Insomnia |
| Anticonvulsant | ||
| Gabapentinm | 300 to 900 mg | Vasomotor |
| SSRIs: selective serotonin reuptake inhibitors | ||
| Source: Reference Citations | ||
Nonpharmacologic interventions are viable options for women who are reluctant to begin HRT or psychotropics.
Psychotherapy. Interpersonal psychotherapy (IPT) and cognitive-behavioral therapy (CBT) have been recommended to address psychosocial elements of perimenopausal mood lability.21 For women with climacteric depression, IPT focuses on role transitions, loss, and interpersonal support, whereas CBT focuses on identifying and altering negative thoughts and beliefs.
Although no randomized trials have examined psychotherapies for perimenopausal depression, a pilot open trial provided group CBT—psychoeducation, group discussion, and coping skills training—to 30 women with climacteric symptoms. Anxiety, depression, partnership relations, overall sexuality, hot flushes, and cardiac complaints improved significantly, based on pre- and post-intervention surveys. Sexual satisfaction and the stressfulness of menopausal symptoms did not change.22
Integrative medicine. Plant-based substances and herbal remedies such as phytoestrogens, red-clover isoflavones, black cohosh, and evening primrose oil have been included in a few research investigations, and the evidence is equivocal. Because of potential interactions between alternative therapies and medications, inquire about their use. Although a comprehensive review of integrative medicine for perimenopausal symptoms is beyond the scope of this article, see suggested readings (Box).
- Albertazzi P. Non-estrogenic approaches for the treatment of climacteric symptoms. Climacteric 2007;10(suppl 2):115-20.
- Blair YA, Gold EB, Zhang G, et al. Use of complementary and alternative medicine during the menopause transition: longitudinal results from the Study of Women’s Health Across the Nation. Menopause 2008;15:32-43.
- Freeman MP, Helgason C, Hill RA. Selected integrative medicine treatments for depression: considerations for women. J Am Med Womens Assoc 2004;59(3):216-24.
- Mischoulon D. Update and critique of natural remedies as antidepressant treatments. Psychiatr Clin North Am 2007;30:51-68.
- Thachil AF, Mohan R, Bhugra D. The evidence base of complementary and alternative therapies in depression. J Affect Disord 2007;97:23-35.
- Tremblay A, Sheeran L, Aranda SK. Psychoeducational interventions to alleviate hot flashes: a systematic review. J North Am Menopause Soc 2008;15:193-202.
Clinical recommendations
Explore options with your patient; discuss side effects, risks, and expected minimum duration of treatment. Antidepressants, hormonal therapies, psychotherapy, and complementary and alternative treatments each might have a role in managing perimenopausal depression. A patient’s preferences, psychiatric history, and depression severity help determine which options to consider and in what order. How she responded to past treatments also can help you individualize a plan.
HRT may be appropriate for women who express a preference for HRT, have responded well to past hormone therapy, and have no personal history or high-risk factors for breast cancer. Based on the WHI findings, we consider a history of breast cancer in the patient or a first- or second-degree relative a contraindication to HRT.
Estrogen can be used alone or with an antidepressant. Studies support 17β-estradiol, 0.1 to 0.3 mg/d, for 8 to 12 weeks.11,23 Concomitant progesterone may be indicated to offset the effects of unopposed estrogen in women with an intact uterus. This option calls for an informed discussion with the patient about risks and benefits.
No data support long-term use of estrogen for recurrent or chronic depression. Because HRT’s risks and benefits vary with the length of exposure, individualize the extended use of estrogen solely to augment treatment for depression. Because vasomotor symptoms may recur when HRT is discontinued,15 we recommend that women make an informed decision in consultation with a gynecologist or primary care physician.
Antidepressants that have serotonergic activity—such as SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs)—appear most promising for treating comorbid depressive and vasomotor symptoms. If a patient has had a good response to an antidepressant in the past, consider starting with that medication.
Common antidepressant side effects are difficult to assess in perimenopausal patients with MDD because the symptoms attributed to antidepressant side effects—such as low libido, sleep disturbance, and weight changes—also can be caused by mood disorders and hormonal changes. Therefore, inquire about these symptoms when you initiate antidepressant therapy and at follow-up assessments.
Psychotherapy. We recommend that all women who present with perimenopausal depression receive information about psychotherapy. Psychotherapy alone often is adequate for mild depression, and adding psychotherapy to antidepressant treatment usually enhances recovery from moderate and severe depression episodes. In addition, patients who engage in psychotherapy for depression may have a lower rate of relapse.24
Individual psychotherapy can help patients with perimenopausal depression:
- accept this life transition
- recognize the benefits of menopause, such as no need for contraception
- develop awareness of personal potential in the years ahead.
Because depression often occurs in an interpersonal context, consider including family members in psychotherapy to improve the patient’s interpersonal support.
Integrative therapies. A full evaluation and consideration of standard treatment options is indicated for all women with MDD. Integrative medicine appeals to many patients but has not been sufficiently studied for perimenopausal depression. Supplemental omega-3 fatty acids and folate are reasonable adjuncts to the treatment of MDD25-27 and deserve study in perimenopausal MDD.
- Greene Climacteric Scale. www.menopausematters.co.uk/greenescale.php.
- Quick Inventory of Depressive Symptomatology. www.idsqids.org.
- National Center for Complementary and Alternative Medicine. National Institutes of Health. http://nccam.nih.gov.
Drug brand names
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Estradiol • various
- Eszopiclone • Lunesta
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Medroxyprogesterone • Provera
- Mirtazapine • Remeron
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
- Zolpidem • Ambien
Disclosures
Dr. Brandon and Dr. Shivakumar report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Freeman receives research support from GlaxoSmithKline, Forest Pharmaceuticals, and Eli Lilly and Company. She was associate professor of psychiatry at the University of Texas Southwestern Medical Center at Dallas when this article was written and is now on the faculty at Harvard Medical School and Massachusetts General Hospital, Boston.
1. Schmidt PJ, Rubinow DR. Reproductive ageing, sex steroids and depression. J Br Menopause Soc 2006;12(4):178-85.
2. Rasgon N, Shelton S, Halbreich U. Perimenopausal mental disorders: epidemiology and phenomenology. CNS Spectr 2005;10(6):471-8.
3. Greene JG. Constructing a standard climacteric scale. Maturitas 1998;29(1):25-31.
4. Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003;54(5):573-83.Erratum in: Biol Psychiatry. 2003;54(5):585.
5. Feld J, Halbreich U, Karkun S. The association of perimenopausal mood disorders with other reproductive-related disorders. CNS Spectr 2005;10(6):461-70.
6. Avis NE, Stellato R, Crawford S, et al. Is there a menopausal syndrome? Menopausal status and symptoms across racial/ethnic groups. Soc Sci Med 2001;52:345-56.
7. Campbell S, Whitehead M. Oestrogen therapy and the menopause syndrome. Clin Obstet Gynecol 1977;4:31-47.
8. Schmidt PJ, Rubinow DR. Menopause-related affective disorders: a justification for further study. Am J Psychiatry 1991;48:844-52.
9. Soares CN. Menopausal transition and depression: who is at risk and how to treat it? Expert Rev Neurother 2007;7(10):1285-93.
10. Prior JC. The complex endocrinology of menopausal transition. Endocrinol Rev 1998;19:397-428.
11. Rasgon N, Altshuler LL, Fairbanks LA, et al. Estrogen replacement therapy in the treatment of major depressive disorder in perimenopausal women. J Clin Psychiatry 2002;63(suppl):45-8.
12. Altshuler LL, Cohen LS, Moline ML, et al. The Expert Consensus Guideline Series. Treatment of depression in women. Postgrad Med 2001 Mar;(Spec No):1-107.
13. The Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA 2004;291:1701-12.
14. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321-33.
15. Ockene JK, Barad DH, Cochrane BB, et al. Symptom experience after discontinuing use of estrogen plus progestin. JAMA 2005;294:183-93.
16. Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 2004;291:2947-58.
17. Shumaker SA, Legault C, Rapp SR. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women. The Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 2003;289:2651-62.
18. FDA approves new labeling and provides new advice to postmenopausal women who use or who are considering using estrogen and estrogen with progestin [FDA Fact Sheet January 8, 2003]. Available at: http://www.fda.gov/oc/factsheets/WHI.html. Accessed September 11, 2008.
19. Soares CN, Arsenio H, Joffe H, et al. Escitalopram versus ethinyl estradiol and norethindrone acetate for symptomatic peri- and postmenopausal women: impact on depression, vasomotor symptoms, sleep, and quality of life. Menopause 2006;13(5):780-6.
20. Cohen LS, Soares CN, Joffe H. Diagnosis and management of mood disorders during the menopausal transition. Am J Med 2005;118(suppl 12B):93-7.
21. Kahn DA, Moline ML, Ross RW, et al. Depression during the transition to menopause: a guide for patients and families. Postgrad Med 2001 Mar;(Spec No):110-1.
22. Alder J, Besken KE, Armbruster U, et al. Cognitive-behavioural group intervention for climacteric syndrome. Psychother Psychosom 2006;75(5):298-303.
23. Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry 2001;58(6):529-34.
24. Otto MW, Smits JA, Reese HE. Combined psychotherapy and pharmacotherapy for mood and anxiety disorders in adults: review and analysis. Clinical Psychology: Science and Practice 2005;12:72-86.
25. Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomized, placebo controlled trial. J Affect Disord 2000;60:121-30.
26. Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry [American Psychiatric Association subcommittee report]. J Clin Psychiatry 2006;67:1954-67.
27. Otto MW, Church TS, Craft LL, et al. Exercise for mood and anxiety disorders. J Clin Psychiatry 2007;68:669-76.
Symptoms of perimenopausal depression are not inherently different from those of depression diagnosed at any other time in life, but they present in a unique context:
- Hormonal fluctuations may persist for a long duration.
- Women experiencing hormonal fluctuations may be vulnerable to mood problems.
- Psychosocial/psychodynamic stressors often complicate this life transition.
Managing perimenopausal depression has become more complicated since the Women’s Health Initiative (WHI) studies found fewer benefits and greater risks with hormone replacement therapy (HRT) than had been perceived. This article discusses the clinical presentation of perimenopausal depression, its risk factors, and treatment options in post-WHI psychiatric practice.
Who is at risk?
Perimenopausal depression is diagnosed when onset of major depressive disorder (MDD) is associated with menstrual cycle irregularity and/or somatic symptoms of the menopausal transition.1 Diagnosis is based on the overall clinical picture, and treatment requires a thoughtful exploration of the complex relationship between hormonal function and mood regulation.
Presentation. For many women, perimenopause is characterized by mild to severe vasomotor, cognitive, and mood symptoms (Table 1). Thus, in your workup of depression in midlife women, document somatic symptoms—such as hot flushes, vaginal dryness, and incontinence—and affective/behavioral symp toms such as mood and sleep disturbances.
Table 1
Vasomotor, cognitive, and mood symptoms of perimenopause
| Vasomotor | Cognitive and mood |
|---|---|
| Hot flushes | Decreased concentration |
| Sweating | Anxiety |
| Heart palpitations | Irritability |
| Painful intercourse | Mood lability |
| Vaginal dryness and discomfort | Memory difficulty |
| Sleep disruption | |
| Headache |
Explore psychiatric and medical histories of your patient and her close relatives. Ask about depression, dysthymia, hypomania, or mood fluctuations around hormonal events such as menses, pregnancy, postpartum, or starting/stopping oral contraceptives. In the differential diagnosis, consider:
- Is low mood temporally connected with hot flushes and disturbed sleep?
- Is low mood secondary to stressful life events?
- Does the patient have another medical illness (such as thyroid disorder) with symptoms similar to depression?
- Is low mood secondary to anxiety or another psychiatric disorder?
Screening. Menopause is considered to have been reached after 12 months of amenorrhea not due to another cause. Median ages for this transition in the United States are 47.5 for perimenopause and 51 for menopause, with an average of 8 years between regular cycles and amenorrhea.2 Therefore, begin talking with women about perimenopausal symptoms when they turn 40.
Evidence supports screening perimenopausal women for depressive symptoms even when their primary complaints are vasomotor. The Greene Climacteric Scale3 is convenient for quantifying and monitoring perimenopausal symptoms. It includes depressive symptoms plus physical and cognitive markers. The Quick Inventory of Depressive Symptomatology—Self Report (QIDS-SR)4 questionnaire:
- takes minutes to complete
- is easy to score
- quantitates the number and severity of depressive symptoms (see Related Resources).
Psychosocial factors can predict depression at any time in life, but some are specific to the menopausal transition (Table 2).5 The “empty nest syndrome,” for example, is often used to explain depressive symptoms in midlife mothers, but no evidence links mood lability with the maturation and departure of children. What may be more stressing for women is supporting adolescents/young adults in their exit to independence while caring for aging parents.
Table 2
Risk factors for depression in women
| Predictive over lifetime | High risk during menopausal transition |
|---|---|
| History of depression | History of PMS, perinatal depression, mood symptoms associated with contraceptives |
| Family history of affective disorders | Premature or surgical menopause |
| Insomnia | Lengthy menopausal transition (≥27 months) |
| Reduced physical activities | Persistent and/or severe vasomotor symptoms |
| Weight gain | Negative attitudes toward menopause and aging |
| Less education | |
| Perceived lower economic status | |
| Perceived lower social support | |
| Perceived lower health status | |
| Smoking | |
| Stressful life events | |
| History of trauma | |
| Marital dissatisfaction | |
| PMS: Premenstrual syndrome | |
Sociocultural beliefs about sexuality and menopause may play a role in how your patient experiences and reports her symptoms. In some cultures, menopause elevates a woman’s social status and is associated with increased respect and authority. In others, such as Western societies that emphasize youth and beauty, women may view menopause and its physical changes in a negative light.6
Therefore, give careful attention to the psychosocial context of menopause to your patient and the social resources available to her. Questions to ask include:
- Has your lifestyle changed recently?
- Have your husband, family members, or close friends noticed any changes in your functioning?
- Is there anyone in your life that you feel comfortable confiding in?
Explaining the complexity of this life transition may ease her anxiety by normalizing her experience, helping her understand her symptoms, and validating her distress.
What might be the cause?
Although the exact pathophysiology of perimenopausal depression is unknown, hormonal changes,7 general health, the experience of menopause,8 and the psychosocial context2 likely work together to increase vulnerability for depressive symptoms (Figure).
Figure Biopsychosocial milieu of depression during perimenopauseHormonal fluctuation. The estrogen withdrawal theory7 explains depressive symptoms as resulting from a sustained decline in ovarian estrogen in tandem with spiking secretions of follicle-stimulating hormone by the pituitary. The finding that women with surgical menopause have a higher incidence of depressive symptoms than women with natural menopause supports this hypothesis.
Mood disorders occur across various female reproductive events, and increased risk appears to be associated with fluctuating gonadal hormones. Thus, declining estrogen may be less causative of perimenopausal depression than extreme fluctuations in estradiol activity.9,10
Estrogen interacts with dopamine, norepinephrine, beta-endorphin, and serotonin metabolism. In particular, estrogen facilitates serotonin delivery to neurons across the brain. These findings—and the success of selective serotonin reuptake inhibitors (SSRIs) in treating mood disorders—support the theory that fluctuating estrogen affects the serotonergic system and may cause depressive symptoms.
‘Domino theory.’ Others have hypothesized that depressive symptoms are the secondhand result of somatic symptoms of perimenopause. In a “domino effect,” hot flushes and night sweats disrupt women’s sleep, bringing fatigue and impaired daytime concentration, which lead to irritability and feelings of being overwhelmed.8
This theory, which incorporates perimenopausal hormone changes, is supported by elevated levels of depression in women who report frequent and intense vasomotor symptoms persisting >27 months.2
The psychosocial theory suggests that depression results from increased stress or adverse events.2 Midlife women with depressive symptoms report many possible sources of stress:
- demanding jobs
- family responsibilities
- dual demands of career and family
- little time for self
- poverty or employment stressors
- not enough sleep
- changing social relationships.
Negative interpretations of aging or the menopausal transition also have been implicated in cross-cultural studies.6 The predictive nature of psychosocial issues for depression during perimenopause supports this theory.
Evidence-based treatment
HRT. Research and clinical reports suggest that estrogen may have antidepressant effects, either alone or as an adjunct to antidepressant medication.11 Before the WHI studies, expert consensus guidelines on treating depression in women recommended HRT as first-line treatment for patients experiencing a first lifetime onset of mild to moderate depression during perimenopause.12 WHI findings since 2002 that associated HRT with increased risk of stroke, deep vein thrombosis, and pulmonary embolism—without clear protection against coronary heart disease or cognitive decline—have left HRT a controversial option for treating perimenopausal depression. In the WHI trials:
- 10,739 postmenopausal women age 50 to 79 without a uterus received unopposed conjugated equine estrogens, 0.625 mg/d, or placebo for an average 6.8 years.13
- 16,608 postmenopausal women age 50 to 79 with an intact uterus received combination HRT (conjugated equine estrogens, 0.625 mg/d, plus 2.5 mg of medroxyprogesterone), or placebo for an average 5.6 years.14
The study using combination HRT found increased risks of breast cancer, ischemic stroke, blood clots, and coronary heart disease.15 A follow-up study showed that vasomotor symptoms returned in more than one-half the women after they stopped using combination HRT.15
A companion WHI trial found that estrogen, 0.625 mg/d—given unopposed or with a progestin—did not prevent cognitive decline in women age 65 to 79 and may have been associated with a slightly greater risk of probable dementia.16,17
The FDA recommends that women who want to use HRT to control menopausal symptoms use the lowest effective dose for the shortest time necessary.18
Antidepressants. SSRIs may be more useful than estrogen for producing MDD remission in perimenopausal women.19 SSRIs and other psychotropics may reduce perimenopausal vasomotor symptoms in addition to addressing depressive symptoms (Table 3). When choosing antidepressant therapy, consider the patient’s dominant presenting perimenopausal symptoms and side effects associated with treatment.20
Table 3
Nonhormone medications for perimenopausal depression: Evidence-based dosages and target symptoms
| Medication | Dosage effective for perimenopausal depression | Symptoms assessed |
|---|---|---|
| SSRIs | ||
| Citaloprama | 40 to 60 mg | Depressive and vasomotor |
| Escitalopramb,c | 5 to 20 mg | Depressive and vasomotor |
| Fluoxetined | 20 to 40 mg | Depressive and vasomotor |
| Paroxetinee,f | 12.5 or 25 mg | Depressive and vasomotor |
| Sertralineg | 100 mg | Depressive and vasomotor |
| Other antidepressants | ||
| Duloxetineh | 60 to 120 mg | Depressive and vasomotor |
| Venlafaxinei | 75 to 225 mg | Depressive and vasomotor |
| Mirtazapinej | 30 to 60 mg | Severe depressive symptoms; used as an adjunct to estrogen |
| Hypnotics | ||
| Eszopiclonek | 3 mg | Depressive and vasomotor; insomnia |
| Zolpideml | 5 to 10 mg | Insomnia |
| Anticonvulsant | ||
| Gabapentinm | 300 to 900 mg | Vasomotor |
| SSRIs: selective serotonin reuptake inhibitors | ||
| Source: Reference Citations | ||
Nonpharmacologic interventions are viable options for women who are reluctant to begin HRT or psychotropics.
Psychotherapy. Interpersonal psychotherapy (IPT) and cognitive-behavioral therapy (CBT) have been recommended to address psychosocial elements of perimenopausal mood lability.21 For women with climacteric depression, IPT focuses on role transitions, loss, and interpersonal support, whereas CBT focuses on identifying and altering negative thoughts and beliefs.
Although no randomized trials have examined psychotherapies for perimenopausal depression, a pilot open trial provided group CBT—psychoeducation, group discussion, and coping skills training—to 30 women with climacteric symptoms. Anxiety, depression, partnership relations, overall sexuality, hot flushes, and cardiac complaints improved significantly, based on pre- and post-intervention surveys. Sexual satisfaction and the stressfulness of menopausal symptoms did not change.22
Integrative medicine. Plant-based substances and herbal remedies such as phytoestrogens, red-clover isoflavones, black cohosh, and evening primrose oil have been included in a few research investigations, and the evidence is equivocal. Because of potential interactions between alternative therapies and medications, inquire about their use. Although a comprehensive review of integrative medicine for perimenopausal symptoms is beyond the scope of this article, see suggested readings (Box).
- Albertazzi P. Non-estrogenic approaches for the treatment of climacteric symptoms. Climacteric 2007;10(suppl 2):115-20.
- Blair YA, Gold EB, Zhang G, et al. Use of complementary and alternative medicine during the menopause transition: longitudinal results from the Study of Women’s Health Across the Nation. Menopause 2008;15:32-43.
- Freeman MP, Helgason C, Hill RA. Selected integrative medicine treatments for depression: considerations for women. J Am Med Womens Assoc 2004;59(3):216-24.
- Mischoulon D. Update and critique of natural remedies as antidepressant treatments. Psychiatr Clin North Am 2007;30:51-68.
- Thachil AF, Mohan R, Bhugra D. The evidence base of complementary and alternative therapies in depression. J Affect Disord 2007;97:23-35.
- Tremblay A, Sheeran L, Aranda SK. Psychoeducational interventions to alleviate hot flashes: a systematic review. J North Am Menopause Soc 2008;15:193-202.
Clinical recommendations
Explore options with your patient; discuss side effects, risks, and expected minimum duration of treatment. Antidepressants, hormonal therapies, psychotherapy, and complementary and alternative treatments each might have a role in managing perimenopausal depression. A patient’s preferences, psychiatric history, and depression severity help determine which options to consider and in what order. How she responded to past treatments also can help you individualize a plan.
HRT may be appropriate for women who express a preference for HRT, have responded well to past hormone therapy, and have no personal history or high-risk factors for breast cancer. Based on the WHI findings, we consider a history of breast cancer in the patient or a first- or second-degree relative a contraindication to HRT.
Estrogen can be used alone or with an antidepressant. Studies support 17β-estradiol, 0.1 to 0.3 mg/d, for 8 to 12 weeks.11,23 Concomitant progesterone may be indicated to offset the effects of unopposed estrogen in women with an intact uterus. This option calls for an informed discussion with the patient about risks and benefits.
No data support long-term use of estrogen for recurrent or chronic depression. Because HRT’s risks and benefits vary with the length of exposure, individualize the extended use of estrogen solely to augment treatment for depression. Because vasomotor symptoms may recur when HRT is discontinued,15 we recommend that women make an informed decision in consultation with a gynecologist or primary care physician.
Antidepressants that have serotonergic activity—such as SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs)—appear most promising for treating comorbid depressive and vasomotor symptoms. If a patient has had a good response to an antidepressant in the past, consider starting with that medication.
Common antidepressant side effects are difficult to assess in perimenopausal patients with MDD because the symptoms attributed to antidepressant side effects—such as low libido, sleep disturbance, and weight changes—also can be caused by mood disorders and hormonal changes. Therefore, inquire about these symptoms when you initiate antidepressant therapy and at follow-up assessments.
Psychotherapy. We recommend that all women who present with perimenopausal depression receive information about psychotherapy. Psychotherapy alone often is adequate for mild depression, and adding psychotherapy to antidepressant treatment usually enhances recovery from moderate and severe depression episodes. In addition, patients who engage in psychotherapy for depression may have a lower rate of relapse.24
Individual psychotherapy can help patients with perimenopausal depression:
- accept this life transition
- recognize the benefits of menopause, such as no need for contraception
- develop awareness of personal potential in the years ahead.
Because depression often occurs in an interpersonal context, consider including family members in psychotherapy to improve the patient’s interpersonal support.
Integrative therapies. A full evaluation and consideration of standard treatment options is indicated for all women with MDD. Integrative medicine appeals to many patients but has not been sufficiently studied for perimenopausal depression. Supplemental omega-3 fatty acids and folate are reasonable adjuncts to the treatment of MDD25-27 and deserve study in perimenopausal MDD.
- Greene Climacteric Scale. www.menopausematters.co.uk/greenescale.php.
- Quick Inventory of Depressive Symptomatology. www.idsqids.org.
- National Center for Complementary and Alternative Medicine. National Institutes of Health. http://nccam.nih.gov.
Drug brand names
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Estradiol • various
- Eszopiclone • Lunesta
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Medroxyprogesterone • Provera
- Mirtazapine • Remeron
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
- Zolpidem • Ambien
Disclosures
Dr. Brandon and Dr. Shivakumar report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Freeman receives research support from GlaxoSmithKline, Forest Pharmaceuticals, and Eli Lilly and Company. She was associate professor of psychiatry at the University of Texas Southwestern Medical Center at Dallas when this article was written and is now on the faculty at Harvard Medical School and Massachusetts General Hospital, Boston.
Symptoms of perimenopausal depression are not inherently different from those of depression diagnosed at any other time in life, but they present in a unique context:
- Hormonal fluctuations may persist for a long duration.
- Women experiencing hormonal fluctuations may be vulnerable to mood problems.
- Psychosocial/psychodynamic stressors often complicate this life transition.
Managing perimenopausal depression has become more complicated since the Women’s Health Initiative (WHI) studies found fewer benefits and greater risks with hormone replacement therapy (HRT) than had been perceived. This article discusses the clinical presentation of perimenopausal depression, its risk factors, and treatment options in post-WHI psychiatric practice.
Who is at risk?
Perimenopausal depression is diagnosed when onset of major depressive disorder (MDD) is associated with menstrual cycle irregularity and/or somatic symptoms of the menopausal transition.1 Diagnosis is based on the overall clinical picture, and treatment requires a thoughtful exploration of the complex relationship between hormonal function and mood regulation.
Presentation. For many women, perimenopause is characterized by mild to severe vasomotor, cognitive, and mood symptoms (Table 1). Thus, in your workup of depression in midlife women, document somatic symptoms—such as hot flushes, vaginal dryness, and incontinence—and affective/behavioral symp toms such as mood and sleep disturbances.
Table 1
Vasomotor, cognitive, and mood symptoms of perimenopause
| Vasomotor | Cognitive and mood |
|---|---|
| Hot flushes | Decreased concentration |
| Sweating | Anxiety |
| Heart palpitations | Irritability |
| Painful intercourse | Mood lability |
| Vaginal dryness and discomfort | Memory difficulty |
| Sleep disruption | |
| Headache |
Explore psychiatric and medical histories of your patient and her close relatives. Ask about depression, dysthymia, hypomania, or mood fluctuations around hormonal events such as menses, pregnancy, postpartum, or starting/stopping oral contraceptives. In the differential diagnosis, consider:
- Is low mood temporally connected with hot flushes and disturbed sleep?
- Is low mood secondary to stressful life events?
- Does the patient have another medical illness (such as thyroid disorder) with symptoms similar to depression?
- Is low mood secondary to anxiety or another psychiatric disorder?
Screening. Menopause is considered to have been reached after 12 months of amenorrhea not due to another cause. Median ages for this transition in the United States are 47.5 for perimenopause and 51 for menopause, with an average of 8 years between regular cycles and amenorrhea.2 Therefore, begin talking with women about perimenopausal symptoms when they turn 40.
Evidence supports screening perimenopausal women for depressive symptoms even when their primary complaints are vasomotor. The Greene Climacteric Scale3 is convenient for quantifying and monitoring perimenopausal symptoms. It includes depressive symptoms plus physical and cognitive markers. The Quick Inventory of Depressive Symptomatology—Self Report (QIDS-SR)4 questionnaire:
- takes minutes to complete
- is easy to score
- quantitates the number and severity of depressive symptoms (see Related Resources).
Psychosocial factors can predict depression at any time in life, but some are specific to the menopausal transition (Table 2).5 The “empty nest syndrome,” for example, is often used to explain depressive symptoms in midlife mothers, but no evidence links mood lability with the maturation and departure of children. What may be more stressing for women is supporting adolescents/young adults in their exit to independence while caring for aging parents.
Table 2
Risk factors for depression in women
| Predictive over lifetime | High risk during menopausal transition |
|---|---|
| History of depression | History of PMS, perinatal depression, mood symptoms associated with contraceptives |
| Family history of affective disorders | Premature or surgical menopause |
| Insomnia | Lengthy menopausal transition (≥27 months) |
| Reduced physical activities | Persistent and/or severe vasomotor symptoms |
| Weight gain | Negative attitudes toward menopause and aging |
| Less education | |
| Perceived lower economic status | |
| Perceived lower social support | |
| Perceived lower health status | |
| Smoking | |
| Stressful life events | |
| History of trauma | |
| Marital dissatisfaction | |
| PMS: Premenstrual syndrome | |
Sociocultural beliefs about sexuality and menopause may play a role in how your patient experiences and reports her symptoms. In some cultures, menopause elevates a woman’s social status and is associated with increased respect and authority. In others, such as Western societies that emphasize youth and beauty, women may view menopause and its physical changes in a negative light.6
Therefore, give careful attention to the psychosocial context of menopause to your patient and the social resources available to her. Questions to ask include:
- Has your lifestyle changed recently?
- Have your husband, family members, or close friends noticed any changes in your functioning?
- Is there anyone in your life that you feel comfortable confiding in?
Explaining the complexity of this life transition may ease her anxiety by normalizing her experience, helping her understand her symptoms, and validating her distress.
What might be the cause?
Although the exact pathophysiology of perimenopausal depression is unknown, hormonal changes,7 general health, the experience of menopause,8 and the psychosocial context2 likely work together to increase vulnerability for depressive symptoms (Figure).
Figure Biopsychosocial milieu of depression during perimenopauseHormonal fluctuation. The estrogen withdrawal theory7 explains depressive symptoms as resulting from a sustained decline in ovarian estrogen in tandem with spiking secretions of follicle-stimulating hormone by the pituitary. The finding that women with surgical menopause have a higher incidence of depressive symptoms than women with natural menopause supports this hypothesis.
Mood disorders occur across various female reproductive events, and increased risk appears to be associated with fluctuating gonadal hormones. Thus, declining estrogen may be less causative of perimenopausal depression than extreme fluctuations in estradiol activity.9,10
Estrogen interacts with dopamine, norepinephrine, beta-endorphin, and serotonin metabolism. In particular, estrogen facilitates serotonin delivery to neurons across the brain. These findings—and the success of selective serotonin reuptake inhibitors (SSRIs) in treating mood disorders—support the theory that fluctuating estrogen affects the serotonergic system and may cause depressive symptoms.
‘Domino theory.’ Others have hypothesized that depressive symptoms are the secondhand result of somatic symptoms of perimenopause. In a “domino effect,” hot flushes and night sweats disrupt women’s sleep, bringing fatigue and impaired daytime concentration, which lead to irritability and feelings of being overwhelmed.8
This theory, which incorporates perimenopausal hormone changes, is supported by elevated levels of depression in women who report frequent and intense vasomotor symptoms persisting >27 months.2
The psychosocial theory suggests that depression results from increased stress or adverse events.2 Midlife women with depressive symptoms report many possible sources of stress:
- demanding jobs
- family responsibilities
- dual demands of career and family
- little time for self
- poverty or employment stressors
- not enough sleep
- changing social relationships.
Negative interpretations of aging or the menopausal transition also have been implicated in cross-cultural studies.6 The predictive nature of psychosocial issues for depression during perimenopause supports this theory.
Evidence-based treatment
HRT. Research and clinical reports suggest that estrogen may have antidepressant effects, either alone or as an adjunct to antidepressant medication.11 Before the WHI studies, expert consensus guidelines on treating depression in women recommended HRT as first-line treatment for patients experiencing a first lifetime onset of mild to moderate depression during perimenopause.12 WHI findings since 2002 that associated HRT with increased risk of stroke, deep vein thrombosis, and pulmonary embolism—without clear protection against coronary heart disease or cognitive decline—have left HRT a controversial option for treating perimenopausal depression. In the WHI trials:
- 10,739 postmenopausal women age 50 to 79 without a uterus received unopposed conjugated equine estrogens, 0.625 mg/d, or placebo for an average 6.8 years.13
- 16,608 postmenopausal women age 50 to 79 with an intact uterus received combination HRT (conjugated equine estrogens, 0.625 mg/d, plus 2.5 mg of medroxyprogesterone), or placebo for an average 5.6 years.14
The study using combination HRT found increased risks of breast cancer, ischemic stroke, blood clots, and coronary heart disease.15 A follow-up study showed that vasomotor symptoms returned in more than one-half the women after they stopped using combination HRT.15
A companion WHI trial found that estrogen, 0.625 mg/d—given unopposed or with a progestin—did not prevent cognitive decline in women age 65 to 79 and may have been associated with a slightly greater risk of probable dementia.16,17
The FDA recommends that women who want to use HRT to control menopausal symptoms use the lowest effective dose for the shortest time necessary.18
Antidepressants. SSRIs may be more useful than estrogen for producing MDD remission in perimenopausal women.19 SSRIs and other psychotropics may reduce perimenopausal vasomotor symptoms in addition to addressing depressive symptoms (Table 3). When choosing antidepressant therapy, consider the patient’s dominant presenting perimenopausal symptoms and side effects associated with treatment.20
Table 3
Nonhormone medications for perimenopausal depression: Evidence-based dosages and target symptoms
| Medication | Dosage effective for perimenopausal depression | Symptoms assessed |
|---|---|---|
| SSRIs | ||
| Citaloprama | 40 to 60 mg | Depressive and vasomotor |
| Escitalopramb,c | 5 to 20 mg | Depressive and vasomotor |
| Fluoxetined | 20 to 40 mg | Depressive and vasomotor |
| Paroxetinee,f | 12.5 or 25 mg | Depressive and vasomotor |
| Sertralineg | 100 mg | Depressive and vasomotor |
| Other antidepressants | ||
| Duloxetineh | 60 to 120 mg | Depressive and vasomotor |
| Venlafaxinei | 75 to 225 mg | Depressive and vasomotor |
| Mirtazapinej | 30 to 60 mg | Severe depressive symptoms; used as an adjunct to estrogen |
| Hypnotics | ||
| Eszopiclonek | 3 mg | Depressive and vasomotor; insomnia |
| Zolpideml | 5 to 10 mg | Insomnia |
| Anticonvulsant | ||
| Gabapentinm | 300 to 900 mg | Vasomotor |
| SSRIs: selective serotonin reuptake inhibitors | ||
| Source: Reference Citations | ||
Nonpharmacologic interventions are viable options for women who are reluctant to begin HRT or psychotropics.
Psychotherapy. Interpersonal psychotherapy (IPT) and cognitive-behavioral therapy (CBT) have been recommended to address psychosocial elements of perimenopausal mood lability.21 For women with climacteric depression, IPT focuses on role transitions, loss, and interpersonal support, whereas CBT focuses on identifying and altering negative thoughts and beliefs.
Although no randomized trials have examined psychotherapies for perimenopausal depression, a pilot open trial provided group CBT—psychoeducation, group discussion, and coping skills training—to 30 women with climacteric symptoms. Anxiety, depression, partnership relations, overall sexuality, hot flushes, and cardiac complaints improved significantly, based on pre- and post-intervention surveys. Sexual satisfaction and the stressfulness of menopausal symptoms did not change.22
Integrative medicine. Plant-based substances and herbal remedies such as phytoestrogens, red-clover isoflavones, black cohosh, and evening primrose oil have been included in a few research investigations, and the evidence is equivocal. Because of potential interactions between alternative therapies and medications, inquire about their use. Although a comprehensive review of integrative medicine for perimenopausal symptoms is beyond the scope of this article, see suggested readings (Box).
- Albertazzi P. Non-estrogenic approaches for the treatment of climacteric symptoms. Climacteric 2007;10(suppl 2):115-20.
- Blair YA, Gold EB, Zhang G, et al. Use of complementary and alternative medicine during the menopause transition: longitudinal results from the Study of Women’s Health Across the Nation. Menopause 2008;15:32-43.
- Freeman MP, Helgason C, Hill RA. Selected integrative medicine treatments for depression: considerations for women. J Am Med Womens Assoc 2004;59(3):216-24.
- Mischoulon D. Update and critique of natural remedies as antidepressant treatments. Psychiatr Clin North Am 2007;30:51-68.
- Thachil AF, Mohan R, Bhugra D. The evidence base of complementary and alternative therapies in depression. J Affect Disord 2007;97:23-35.
- Tremblay A, Sheeran L, Aranda SK. Psychoeducational interventions to alleviate hot flashes: a systematic review. J North Am Menopause Soc 2008;15:193-202.
Clinical recommendations
Explore options with your patient; discuss side effects, risks, and expected minimum duration of treatment. Antidepressants, hormonal therapies, psychotherapy, and complementary and alternative treatments each might have a role in managing perimenopausal depression. A patient’s preferences, psychiatric history, and depression severity help determine which options to consider and in what order. How she responded to past treatments also can help you individualize a plan.
HRT may be appropriate for women who express a preference for HRT, have responded well to past hormone therapy, and have no personal history or high-risk factors for breast cancer. Based on the WHI findings, we consider a history of breast cancer in the patient or a first- or second-degree relative a contraindication to HRT.
Estrogen can be used alone or with an antidepressant. Studies support 17β-estradiol, 0.1 to 0.3 mg/d, for 8 to 12 weeks.11,23 Concomitant progesterone may be indicated to offset the effects of unopposed estrogen in women with an intact uterus. This option calls for an informed discussion with the patient about risks and benefits.
No data support long-term use of estrogen for recurrent or chronic depression. Because HRT’s risks and benefits vary with the length of exposure, individualize the extended use of estrogen solely to augment treatment for depression. Because vasomotor symptoms may recur when HRT is discontinued,15 we recommend that women make an informed decision in consultation with a gynecologist or primary care physician.
Antidepressants that have serotonergic activity—such as SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs)—appear most promising for treating comorbid depressive and vasomotor symptoms. If a patient has had a good response to an antidepressant in the past, consider starting with that medication.
Common antidepressant side effects are difficult to assess in perimenopausal patients with MDD because the symptoms attributed to antidepressant side effects—such as low libido, sleep disturbance, and weight changes—also can be caused by mood disorders and hormonal changes. Therefore, inquire about these symptoms when you initiate antidepressant therapy and at follow-up assessments.
Psychotherapy. We recommend that all women who present with perimenopausal depression receive information about psychotherapy. Psychotherapy alone often is adequate for mild depression, and adding psychotherapy to antidepressant treatment usually enhances recovery from moderate and severe depression episodes. In addition, patients who engage in psychotherapy for depression may have a lower rate of relapse.24
Individual psychotherapy can help patients with perimenopausal depression:
- accept this life transition
- recognize the benefits of menopause, such as no need for contraception
- develop awareness of personal potential in the years ahead.
Because depression often occurs in an interpersonal context, consider including family members in psychotherapy to improve the patient’s interpersonal support.
Integrative therapies. A full evaluation and consideration of standard treatment options is indicated for all women with MDD. Integrative medicine appeals to many patients but has not been sufficiently studied for perimenopausal depression. Supplemental omega-3 fatty acids and folate are reasonable adjuncts to the treatment of MDD25-27 and deserve study in perimenopausal MDD.
- Greene Climacteric Scale. www.menopausematters.co.uk/greenescale.php.
- Quick Inventory of Depressive Symptomatology. www.idsqids.org.
- National Center for Complementary and Alternative Medicine. National Institutes of Health. http://nccam.nih.gov.
Drug brand names
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Estradiol • various
- Eszopiclone • Lunesta
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Medroxyprogesterone • Provera
- Mirtazapine • Remeron
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
- Zolpidem • Ambien
Disclosures
Dr. Brandon and Dr. Shivakumar report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Freeman receives research support from GlaxoSmithKline, Forest Pharmaceuticals, and Eli Lilly and Company. She was associate professor of psychiatry at the University of Texas Southwestern Medical Center at Dallas when this article was written and is now on the faculty at Harvard Medical School and Massachusetts General Hospital, Boston.
1. Schmidt PJ, Rubinow DR. Reproductive ageing, sex steroids and depression. J Br Menopause Soc 2006;12(4):178-85.
2. Rasgon N, Shelton S, Halbreich U. Perimenopausal mental disorders: epidemiology and phenomenology. CNS Spectr 2005;10(6):471-8.
3. Greene JG. Constructing a standard climacteric scale. Maturitas 1998;29(1):25-31.
4. Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003;54(5):573-83.Erratum in: Biol Psychiatry. 2003;54(5):585.
5. Feld J, Halbreich U, Karkun S. The association of perimenopausal mood disorders with other reproductive-related disorders. CNS Spectr 2005;10(6):461-70.
6. Avis NE, Stellato R, Crawford S, et al. Is there a menopausal syndrome? Menopausal status and symptoms across racial/ethnic groups. Soc Sci Med 2001;52:345-56.
7. Campbell S, Whitehead M. Oestrogen therapy and the menopause syndrome. Clin Obstet Gynecol 1977;4:31-47.
8. Schmidt PJ, Rubinow DR. Menopause-related affective disorders: a justification for further study. Am J Psychiatry 1991;48:844-52.
9. Soares CN. Menopausal transition and depression: who is at risk and how to treat it? Expert Rev Neurother 2007;7(10):1285-93.
10. Prior JC. The complex endocrinology of menopausal transition. Endocrinol Rev 1998;19:397-428.
11. Rasgon N, Altshuler LL, Fairbanks LA, et al. Estrogen replacement therapy in the treatment of major depressive disorder in perimenopausal women. J Clin Psychiatry 2002;63(suppl):45-8.
12. Altshuler LL, Cohen LS, Moline ML, et al. The Expert Consensus Guideline Series. Treatment of depression in women. Postgrad Med 2001 Mar;(Spec No):1-107.
13. The Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA 2004;291:1701-12.
14. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321-33.
15. Ockene JK, Barad DH, Cochrane BB, et al. Symptom experience after discontinuing use of estrogen plus progestin. JAMA 2005;294:183-93.
16. Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 2004;291:2947-58.
17. Shumaker SA, Legault C, Rapp SR. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women. The Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 2003;289:2651-62.
18. FDA approves new labeling and provides new advice to postmenopausal women who use or who are considering using estrogen and estrogen with progestin [FDA Fact Sheet January 8, 2003]. Available at: http://www.fda.gov/oc/factsheets/WHI.html. Accessed September 11, 2008.
19. Soares CN, Arsenio H, Joffe H, et al. Escitalopram versus ethinyl estradiol and norethindrone acetate for symptomatic peri- and postmenopausal women: impact on depression, vasomotor symptoms, sleep, and quality of life. Menopause 2006;13(5):780-6.
20. Cohen LS, Soares CN, Joffe H. Diagnosis and management of mood disorders during the menopausal transition. Am J Med 2005;118(suppl 12B):93-7.
21. Kahn DA, Moline ML, Ross RW, et al. Depression during the transition to menopause: a guide for patients and families. Postgrad Med 2001 Mar;(Spec No):110-1.
22. Alder J, Besken KE, Armbruster U, et al. Cognitive-behavioural group intervention for climacteric syndrome. Psychother Psychosom 2006;75(5):298-303.
23. Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry 2001;58(6):529-34.
24. Otto MW, Smits JA, Reese HE. Combined psychotherapy and pharmacotherapy for mood and anxiety disorders in adults: review and analysis. Clinical Psychology: Science and Practice 2005;12:72-86.
25. Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomized, placebo controlled trial. J Affect Disord 2000;60:121-30.
26. Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry [American Psychiatric Association subcommittee report]. J Clin Psychiatry 2006;67:1954-67.
27. Otto MW, Church TS, Craft LL, et al. Exercise for mood and anxiety disorders. J Clin Psychiatry 2007;68:669-76.
1. Schmidt PJ, Rubinow DR. Reproductive ageing, sex steroids and depression. J Br Menopause Soc 2006;12(4):178-85.
2. Rasgon N, Shelton S, Halbreich U. Perimenopausal mental disorders: epidemiology and phenomenology. CNS Spectr 2005;10(6):471-8.
3. Greene JG. Constructing a standard climacteric scale. Maturitas 1998;29(1):25-31.
4. Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003;54(5):573-83.Erratum in: Biol Psychiatry. 2003;54(5):585.
5. Feld J, Halbreich U, Karkun S. The association of perimenopausal mood disorders with other reproductive-related disorders. CNS Spectr 2005;10(6):461-70.
6. Avis NE, Stellato R, Crawford S, et al. Is there a menopausal syndrome? Menopausal status and symptoms across racial/ethnic groups. Soc Sci Med 2001;52:345-56.
7. Campbell S, Whitehead M. Oestrogen therapy and the menopause syndrome. Clin Obstet Gynecol 1977;4:31-47.
8. Schmidt PJ, Rubinow DR. Menopause-related affective disorders: a justification for further study. Am J Psychiatry 1991;48:844-52.
9. Soares CN. Menopausal transition and depression: who is at risk and how to treat it? Expert Rev Neurother 2007;7(10):1285-93.
10. Prior JC. The complex endocrinology of menopausal transition. Endocrinol Rev 1998;19:397-428.
11. Rasgon N, Altshuler LL, Fairbanks LA, et al. Estrogen replacement therapy in the treatment of major depressive disorder in perimenopausal women. J Clin Psychiatry 2002;63(suppl):45-8.
12. Altshuler LL, Cohen LS, Moline ML, et al. The Expert Consensus Guideline Series. Treatment of depression in women. Postgrad Med 2001 Mar;(Spec No):1-107.
13. The Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA 2004;291:1701-12.
14. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321-33.
15. Ockene JK, Barad DH, Cochrane BB, et al. Symptom experience after discontinuing use of estrogen plus progestin. JAMA 2005;294:183-93.
16. Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 2004;291:2947-58.
17. Shumaker SA, Legault C, Rapp SR. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women. The Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 2003;289:2651-62.
18. FDA approves new labeling and provides new advice to postmenopausal women who use or who are considering using estrogen and estrogen with progestin [FDA Fact Sheet January 8, 2003]. Available at: http://www.fda.gov/oc/factsheets/WHI.html. Accessed September 11, 2008.
19. Soares CN, Arsenio H, Joffe H, et al. Escitalopram versus ethinyl estradiol and norethindrone acetate for symptomatic peri- and postmenopausal women: impact on depression, vasomotor symptoms, sleep, and quality of life. Menopause 2006;13(5):780-6.
20. Cohen LS, Soares CN, Joffe H. Diagnosis and management of mood disorders during the menopausal transition. Am J Med 2005;118(suppl 12B):93-7.
21. Kahn DA, Moline ML, Ross RW, et al. Depression during the transition to menopause: a guide for patients and families. Postgrad Med 2001 Mar;(Spec No):110-1.
22. Alder J, Besken KE, Armbruster U, et al. Cognitive-behavioural group intervention for climacteric syndrome. Psychother Psychosom 2006;75(5):298-303.
23. Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry 2001;58(6):529-34.
24. Otto MW, Smits JA, Reese HE. Combined psychotherapy and pharmacotherapy for mood and anxiety disorders in adults: review and analysis. Clinical Psychology: Science and Practice 2005;12:72-86.
25. Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomized, placebo controlled trial. J Affect Disord 2000;60:121-30.
26. Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry [American Psychiatric Association subcommittee report]. J Clin Psychiatry 2006;67:1954-67.
27. Otto MW, Church TS, Craft LL, et al. Exercise for mood and anxiety disorders. J Clin Psychiatry 2007;68:669-76.