User login
VIDEO: CPX-351 ‘new standard of care’ for older patients with secondary AML
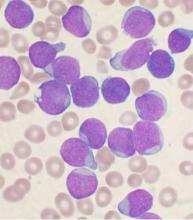
CHICAGO – The investigational drug CPX-351 (Vyxeos) may become the new standard of care for older patients with secondary acute myeloid leukemia (AML), based on data presented at the annual meeting of the American Society of Clinical Oncology.
CPX-351 significantly improved overall survival, event-free survival, and treatment response without an increase in 60-day mortality or in the frequency and severity of adverse events as compared to the standard 7+3 regimen of cytarabine and daunorubicin.
In a video interview, primary investigator Dr. Jeffrey Lancet of H. Lee Moffitt Cancer Center & Research Institute, Tampa, Fla., discusses the data to be presented to the Food and Drug Administration for approval of the drug, and why the liposomal formulation of cytarabine and daunorubicin achieved superior results in these difficult to treat patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @maryjodales
CHICAGO – The investigational drug CPX-351 (Vyxeos) may become the new standard of care for older patients with secondary acute myeloid leukemia (AML), based on data presented at the annual meeting of the American Society of Clinical Oncology.
CPX-351 significantly improved overall survival, event-free survival, and treatment response without an increase in 60-day mortality or in the frequency and severity of adverse events as compared to the standard 7+3 regimen of cytarabine and daunorubicin.
In a video interview, primary investigator Dr. Jeffrey Lancet of H. Lee Moffitt Cancer Center & Research Institute, Tampa, Fla., discusses the data to be presented to the Food and Drug Administration for approval of the drug, and why the liposomal formulation of cytarabine and daunorubicin achieved superior results in these difficult to treat patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @maryjodales
CHICAGO – The investigational drug CPX-351 (Vyxeos) may become the new standard of care for older patients with secondary acute myeloid leukemia (AML), based on data presented at the annual meeting of the American Society of Clinical Oncology.
CPX-351 significantly improved overall survival, event-free survival, and treatment response without an increase in 60-day mortality or in the frequency and severity of adverse events as compared to the standard 7+3 regimen of cytarabine and daunorubicin.
In a video interview, primary investigator Dr. Jeffrey Lancet of H. Lee Moffitt Cancer Center & Research Institute, Tampa, Fla., discusses the data to be presented to the Food and Drug Administration for approval of the drug, and why the liposomal formulation of cytarabine and daunorubicin achieved superior results in these difficult to treat patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @maryjodales
AT THE 2016 ASCO ANNUAL MEETING
Lenalidomide maintenance prolongs overall survival after ASCT
CHICAGO – Lenalidomide maintenance therapy significantly prolonged overall survival after autologous stem cell transplant in newly diagnosed multiple myeloma patients, including patients who had a complete response to ASCT, based on a meta-analysis presented at the annual meeting of the American Society of Clinical Oncology.
While several studies have indicated that lenalidomide maintenance reduces the risk of disease progression or death compared to a control group, none of the individual studies were powered to detect a significant improvement in overall survival, said Dr. Philip L. McCarthy, director of the Blood and Marrow Transplant Center at Roswell Park Cancer Institute, Buffalo, N.Y., who is a co-author of the meta-analysis and reported the results on behalf of Dr. Michel Attal of University Hospital, Toulouse, France.
The researchers found that three randomized controlled trials using lenalidomide post-ASCT (IFM 2005-02, CALGB 100104 [Alliance], GIMEMA RV-209) met the criteria of having patient-level data, a control arm, and primary efficacy data for newly-diagnosed patients with multiple myeloma.
After induction and single (82%) or tandem (18%) ASCT, 55% of patients in the meta-analysis had complete or very good partial responses.
From 2005 to 2009, 605 patients received lenalidomide either 10 mg/day on days 1-21 of a 28-day cycle (GIMEMA) or on days 1-28 of a 28-day cycle (IFM and CALGB); 604 patients were in a control group. With a median follow-up of 6.6 years, 491 patients (41%) had died.
Median overall survival was not reached in the lenolidamide group and was 86 months in the control group (HR = 0.74; 95% CI, 0.62-0.89; log-rank P = .001). Survival was longer in the lenolidamine group as compared to the control group at 5 years (71% vs 66%), 6 years (65% vs 58%), and 7 years (62% vs 50%), reported Dr. McCarthy.
Dr. McCarthy receives research funding from Celgene, the maker of Revlimid (lenalidomide); and is a consultant or advisor to and receives honoraria from Binding Site; Bristol-Myers Squibb; Celgene; Janssen; Karyopharm Therapeutics; and Sanofi. Dr. Attal had no disclosures.
On Twitter @maryjodales
CHICAGO – Lenalidomide maintenance therapy significantly prolonged overall survival after autologous stem cell transplant in newly diagnosed multiple myeloma patients, including patients who had a complete response to ASCT, based on a meta-analysis presented at the annual meeting of the American Society of Clinical Oncology.
While several studies have indicated that lenalidomide maintenance reduces the risk of disease progression or death compared to a control group, none of the individual studies were powered to detect a significant improvement in overall survival, said Dr. Philip L. McCarthy, director of the Blood and Marrow Transplant Center at Roswell Park Cancer Institute, Buffalo, N.Y., who is a co-author of the meta-analysis and reported the results on behalf of Dr. Michel Attal of University Hospital, Toulouse, France.
The researchers found that three randomized controlled trials using lenalidomide post-ASCT (IFM 2005-02, CALGB 100104 [Alliance], GIMEMA RV-209) met the criteria of having patient-level data, a control arm, and primary efficacy data for newly-diagnosed patients with multiple myeloma.
After induction and single (82%) or tandem (18%) ASCT, 55% of patients in the meta-analysis had complete or very good partial responses.
From 2005 to 2009, 605 patients received lenalidomide either 10 mg/day on days 1-21 of a 28-day cycle (GIMEMA) or on days 1-28 of a 28-day cycle (IFM and CALGB); 604 patients were in a control group. With a median follow-up of 6.6 years, 491 patients (41%) had died.
Median overall survival was not reached in the lenolidamide group and was 86 months in the control group (HR = 0.74; 95% CI, 0.62-0.89; log-rank P = .001). Survival was longer in the lenolidamine group as compared to the control group at 5 years (71% vs 66%), 6 years (65% vs 58%), and 7 years (62% vs 50%), reported Dr. McCarthy.
Dr. McCarthy receives research funding from Celgene, the maker of Revlimid (lenalidomide); and is a consultant or advisor to and receives honoraria from Binding Site; Bristol-Myers Squibb; Celgene; Janssen; Karyopharm Therapeutics; and Sanofi. Dr. Attal had no disclosures.
On Twitter @maryjodales
CHICAGO – Lenalidomide maintenance therapy significantly prolonged overall survival after autologous stem cell transplant in newly diagnosed multiple myeloma patients, including patients who had a complete response to ASCT, based on a meta-analysis presented at the annual meeting of the American Society of Clinical Oncology.
While several studies have indicated that lenalidomide maintenance reduces the risk of disease progression or death compared to a control group, none of the individual studies were powered to detect a significant improvement in overall survival, said Dr. Philip L. McCarthy, director of the Blood and Marrow Transplant Center at Roswell Park Cancer Institute, Buffalo, N.Y., who is a co-author of the meta-analysis and reported the results on behalf of Dr. Michel Attal of University Hospital, Toulouse, France.
The researchers found that three randomized controlled trials using lenalidomide post-ASCT (IFM 2005-02, CALGB 100104 [Alliance], GIMEMA RV-209) met the criteria of having patient-level data, a control arm, and primary efficacy data for newly-diagnosed patients with multiple myeloma.
After induction and single (82%) or tandem (18%) ASCT, 55% of patients in the meta-analysis had complete or very good partial responses.
From 2005 to 2009, 605 patients received lenalidomide either 10 mg/day on days 1-21 of a 28-day cycle (GIMEMA) or on days 1-28 of a 28-day cycle (IFM and CALGB); 604 patients were in a control group. With a median follow-up of 6.6 years, 491 patients (41%) had died.
Median overall survival was not reached in the lenolidamide group and was 86 months in the control group (HR = 0.74; 95% CI, 0.62-0.89; log-rank P = .001). Survival was longer in the lenolidamine group as compared to the control group at 5 years (71% vs 66%), 6 years (65% vs 58%), and 7 years (62% vs 50%), reported Dr. McCarthy.
Dr. McCarthy receives research funding from Celgene, the maker of Revlimid (lenalidomide); and is a consultant or advisor to and receives honoraria from Binding Site; Bristol-Myers Squibb; Celgene; Janssen; Karyopharm Therapeutics; and Sanofi. Dr. Attal had no disclosures.
On Twitter @maryjodales
AT ASCO 2016
Key clinical point: Lenalidomide maintenance therapy significantly prolonged overall survival after autologous stem cell transplant in newly diagnosed multiple myeloma patients.
Major finding: Median overall survival was not reached in the lenolidamide group and was 86 months in the control group (HR = 0.74; 95% CI, 0.62-0.89; log-rank P = .001).
Data source: Meta-analysis of 1,209 patients in three randomized controlled trials using lenalidomide post-ASCT (IFM 2005-02, CALGB 100104 [Alliance], GIMEMA RV-209).
Disclosures: Dr. McCarthy receives research funding from Celgene, the maker of Revlimid (lenalidomide); and is a consultant or advisor to and receives honoraria from Binding Site; Bristol-Myers Squibb; Celgene; Janssen; Karyopharm Therapeutics; and Sanofi. Dr. Attal had no disclosures.
Up-front ASCT superior for fit patients with newly diagnosed myeloma
CHICAGO – Up-front high-dose melphalan and autologous stem cell transplantation (ASCT) was superior to bortezomib-melphalan-prednisone (VMP) for fit patients younger than age 65 years with newly diagnosed multiple myeloma, based on the interim results from a randomized study of the European Myeloma Network.
With more than 1,200 patients, the study is the largest trial yet to show superior progression-free survival for ASCT. Follow-up has been too short to show significance for overall survival.
“Up-front ASCT was associated with a significant improvement in progression-free survival as compared to bortezomib-melphalan-prednisone in the overall patient population, (and the advantage) was retained across prespecified subgroups of patients at low and high risk … (as well as) in a multivariate analysis” of the overall population, Dr. Michele Cavo of the University of Bologna, Italy, reported at the annual meeting of the American Society of Clinical Oncology. “Up-front high-dose melphalan and ASCT continue to be the reference treatment choice for fit patients with newly diagnosed multiple myeloma, even in the novel agent era.”
ASCT was associated with higher rates of grade 3 or more adverse events in nearly every category, however, with the exception of peripheral nephropathy.
For the study, 1,266 patients, stratified by International Staging System stage and FISH (fluorescence in situ hybridization) analysis, were randomized to either VMP (512 pts) or to high-dose melphalan and ASCT (754 pts). At the meeting, Dr. Cavo reported on the study’s interim outcome results in 695 patients who had ASCT and 497 newly diagnosed patients who received VMP.
At 3 years, progression-free survival rates were 66.1% in the ASCT patients and 57.5% in the VMP patients. The median progression-free survival had not yet been reached in the ASCT patients and was 44 months in the bortezomib-melphalan-prednisone patients. (hazard ratio, 95% confidence interval, 0.73 [0.59-0.90]; P = .003).
Stringent complete responses were seen in 18.2% of patients in the VMP group and 17% of the ASCT group; Complete responses occurred in 25.3% of both groups. The main difference observed was in the number of very good partial responses, seen in 30.4% of the VMP group and in 43.2% of the ASCT group. Also, 11.2% of those in the VMP group had less than a partial response while that was the case for only 3.3% of the ASCT group.
Alternatively, the rate of grade 3 or more adverse events was higher in the ASCT group, with 17.5% experiencing febrile neutropenia, 15.6% mucositis, 3.2% sepsis, and 2.6% respiratory infections. The rates of these side effects in the VMP group were 0.2%, 0%, 0%, and 1.7%, respectively. The only grade 3 or more adverse event seen with greater frequency in the VMP group was peripheral neuropathy, seen in 13.8% as compared with 1.6% in the ASCT group.
Dr. Cavo receives honoraria from Amgen, Bristol-Myers Squibb, Celgene, Janssen, and Takeda. He serves as a consultant or advisor to and is on the speakers bureau for Amgen. Celgene, Janssen.
On Twitter @maryjodales
This story was updated on June 10, 2016.
Although novel drug combinations have become a standard of care for newly diagnosed multiple myeloma patients, high-dose melphalan and ASCT improve the depth of remission, regardless of the induction regimen. Four trials comparing differing induction and consolidation regimens to one or more ASCTs now have shown significantly improved progression-free survival. Two of the trials have more than 36 months of follow-up.
Dr. William Bensinger is with the Fred Hutchinson Cancer Research Center and the Swedish Cancer Institute in Seattle.
Although novel drug combinations have become a standard of care for newly diagnosed multiple myeloma patients, high-dose melphalan and ASCT improve the depth of remission, regardless of the induction regimen. Four trials comparing differing induction and consolidation regimens to one or more ASCTs now have shown significantly improved progression-free survival. Two of the trials have more than 36 months of follow-up.
Dr. William Bensinger is with the Fred Hutchinson Cancer Research Center and the Swedish Cancer Institute in Seattle.
Although novel drug combinations have become a standard of care for newly diagnosed multiple myeloma patients, high-dose melphalan and ASCT improve the depth of remission, regardless of the induction regimen. Four trials comparing differing induction and consolidation regimens to one or more ASCTs now have shown significantly improved progression-free survival. Two of the trials have more than 36 months of follow-up.
Dr. William Bensinger is with the Fred Hutchinson Cancer Research Center and the Swedish Cancer Institute in Seattle.
CHICAGO – Up-front high-dose melphalan and autologous stem cell transplantation (ASCT) was superior to bortezomib-melphalan-prednisone (VMP) for fit patients younger than age 65 years with newly diagnosed multiple myeloma, based on the interim results from a randomized study of the European Myeloma Network.
With more than 1,200 patients, the study is the largest trial yet to show superior progression-free survival for ASCT. Follow-up has been too short to show significance for overall survival.
“Up-front ASCT was associated with a significant improvement in progression-free survival as compared to bortezomib-melphalan-prednisone in the overall patient population, (and the advantage) was retained across prespecified subgroups of patients at low and high risk … (as well as) in a multivariate analysis” of the overall population, Dr. Michele Cavo of the University of Bologna, Italy, reported at the annual meeting of the American Society of Clinical Oncology. “Up-front high-dose melphalan and ASCT continue to be the reference treatment choice for fit patients with newly diagnosed multiple myeloma, even in the novel agent era.”
ASCT was associated with higher rates of grade 3 or more adverse events in nearly every category, however, with the exception of peripheral nephropathy.
For the study, 1,266 patients, stratified by International Staging System stage and FISH (fluorescence in situ hybridization) analysis, were randomized to either VMP (512 pts) or to high-dose melphalan and ASCT (754 pts). At the meeting, Dr. Cavo reported on the study’s interim outcome results in 695 patients who had ASCT and 497 newly diagnosed patients who received VMP.
At 3 years, progression-free survival rates were 66.1% in the ASCT patients and 57.5% in the VMP patients. The median progression-free survival had not yet been reached in the ASCT patients and was 44 months in the bortezomib-melphalan-prednisone patients. (hazard ratio, 95% confidence interval, 0.73 [0.59-0.90]; P = .003).
Stringent complete responses were seen in 18.2% of patients in the VMP group and 17% of the ASCT group; Complete responses occurred in 25.3% of both groups. The main difference observed was in the number of very good partial responses, seen in 30.4% of the VMP group and in 43.2% of the ASCT group. Also, 11.2% of those in the VMP group had less than a partial response while that was the case for only 3.3% of the ASCT group.
Alternatively, the rate of grade 3 or more adverse events was higher in the ASCT group, with 17.5% experiencing febrile neutropenia, 15.6% mucositis, 3.2% sepsis, and 2.6% respiratory infections. The rates of these side effects in the VMP group were 0.2%, 0%, 0%, and 1.7%, respectively. The only grade 3 or more adverse event seen with greater frequency in the VMP group was peripheral neuropathy, seen in 13.8% as compared with 1.6% in the ASCT group.
Dr. Cavo receives honoraria from Amgen, Bristol-Myers Squibb, Celgene, Janssen, and Takeda. He serves as a consultant or advisor to and is on the speakers bureau for Amgen. Celgene, Janssen.
On Twitter @maryjodales
This story was updated on June 10, 2016.
CHICAGO – Up-front high-dose melphalan and autologous stem cell transplantation (ASCT) was superior to bortezomib-melphalan-prednisone (VMP) for fit patients younger than age 65 years with newly diagnosed multiple myeloma, based on the interim results from a randomized study of the European Myeloma Network.
With more than 1,200 patients, the study is the largest trial yet to show superior progression-free survival for ASCT. Follow-up has been too short to show significance for overall survival.
“Up-front ASCT was associated with a significant improvement in progression-free survival as compared to bortezomib-melphalan-prednisone in the overall patient population, (and the advantage) was retained across prespecified subgroups of patients at low and high risk … (as well as) in a multivariate analysis” of the overall population, Dr. Michele Cavo of the University of Bologna, Italy, reported at the annual meeting of the American Society of Clinical Oncology. “Up-front high-dose melphalan and ASCT continue to be the reference treatment choice for fit patients with newly diagnosed multiple myeloma, even in the novel agent era.”
ASCT was associated with higher rates of grade 3 or more adverse events in nearly every category, however, with the exception of peripheral nephropathy.
For the study, 1,266 patients, stratified by International Staging System stage and FISH (fluorescence in situ hybridization) analysis, were randomized to either VMP (512 pts) or to high-dose melphalan and ASCT (754 pts). At the meeting, Dr. Cavo reported on the study’s interim outcome results in 695 patients who had ASCT and 497 newly diagnosed patients who received VMP.
At 3 years, progression-free survival rates were 66.1% in the ASCT patients and 57.5% in the VMP patients. The median progression-free survival had not yet been reached in the ASCT patients and was 44 months in the bortezomib-melphalan-prednisone patients. (hazard ratio, 95% confidence interval, 0.73 [0.59-0.90]; P = .003).
Stringent complete responses were seen in 18.2% of patients in the VMP group and 17% of the ASCT group; Complete responses occurred in 25.3% of both groups. The main difference observed was in the number of very good partial responses, seen in 30.4% of the VMP group and in 43.2% of the ASCT group. Also, 11.2% of those in the VMP group had less than a partial response while that was the case for only 3.3% of the ASCT group.
Alternatively, the rate of grade 3 or more adverse events was higher in the ASCT group, with 17.5% experiencing febrile neutropenia, 15.6% mucositis, 3.2% sepsis, and 2.6% respiratory infections. The rates of these side effects in the VMP group were 0.2%, 0%, 0%, and 1.7%, respectively. The only grade 3 or more adverse event seen with greater frequency in the VMP group was peripheral neuropathy, seen in 13.8% as compared with 1.6% in the ASCT group.
Dr. Cavo receives honoraria from Amgen, Bristol-Myers Squibb, Celgene, Janssen, and Takeda. He serves as a consultant or advisor to and is on the speakers bureau for Amgen. Celgene, Janssen.
On Twitter @maryjodales
This story was updated on June 10, 2016.
AT THE 2016 ASCO ANNUAL MEETING
Key clinical point: Up-front high-dose melphalan and autologous stem cell transplantation was superior to bortezomib-melphalan-prednisone for fit patients under age 65 years with newly diagnosed multiple myeloma.
Major finding: At 3 years of follow-up, progression-free survival rates were 66.1% in the ASCT patients and 57.5% in the bortezomib-melphalan-prednisone patients.
Data source: Interim results from a randomized study of over 1200 patients in the European Myeloma Network.
Disclosures: Dr. Cavo receives honoraria from Amgen, Bristol-Myers Squibb, Celgene, Janssen, and Takeda. He serves as a consultant or advisor to and is on the speakers bureau for Amgen. Celgene, Janssen.
Ocaliva approved for primary biliary cholangitis
Obeticholic acid (Ocaliva) has been granted accelerated approval for use in combination with ursodeoxycholic acid (UDCA) for the treatment of primary biliary cholangitis in adults with an inadequate response to UDCA, and for use as a single therapy in adults unable to tolerate UDCA, the U.S. Food and Drug Administration announced.
Obeticholic acid should not be used in patients with complete biliary obstruction.
Given orally, obeticholic acid binds to the farnesoid X receptor (FXR), a receptor found in cells of the liver and intestine. FXR is a key regulator of bile acid metabolic pathways. Obeticholic acid increases bile flow from the liver and suppresses bile acid production in the liver, thus reducing exposure to toxic levels of bile acids.
Obeticholic acid was shown to reduce levels of alkaline phosphatase, which was used as a surrogate endpoint to predict clinical benefit, including an improvement in transplant-free survival. The FDA’s accelerated approval program allows approval based on a surrogate endpoint that is reasonably likely to predict clinical benefit. The manufacturer will conduct confirmatory clinical trials to examine any improvements in survival, progression to cirrhosis, or other disease-related symptoms.
After 12 months, in a controlled clinical trial with 216 participants, the proportion of participants achieving reductions in levels of alkaline phosphatase was higher among treated participants than in participants given placebo. Pruritus, fatigue, abdominal pain and discomfort, arthralgia, oropharyngeal pain, dizziness, and constipation were the drug’s most common side effects in the study.
Obeticholic acid is manufactured by Intercept Pharmaceuticals.
Obeticholic acid (Ocaliva) has been granted accelerated approval for use in combination with ursodeoxycholic acid (UDCA) for the treatment of primary biliary cholangitis in adults with an inadequate response to UDCA, and for use as a single therapy in adults unable to tolerate UDCA, the U.S. Food and Drug Administration announced.
Obeticholic acid should not be used in patients with complete biliary obstruction.
Given orally, obeticholic acid binds to the farnesoid X receptor (FXR), a receptor found in cells of the liver and intestine. FXR is a key regulator of bile acid metabolic pathways. Obeticholic acid increases bile flow from the liver and suppresses bile acid production in the liver, thus reducing exposure to toxic levels of bile acids.
Obeticholic acid was shown to reduce levels of alkaline phosphatase, which was used as a surrogate endpoint to predict clinical benefit, including an improvement in transplant-free survival. The FDA’s accelerated approval program allows approval based on a surrogate endpoint that is reasonably likely to predict clinical benefit. The manufacturer will conduct confirmatory clinical trials to examine any improvements in survival, progression to cirrhosis, or other disease-related symptoms.
After 12 months, in a controlled clinical trial with 216 participants, the proportion of participants achieving reductions in levels of alkaline phosphatase was higher among treated participants than in participants given placebo. Pruritus, fatigue, abdominal pain and discomfort, arthralgia, oropharyngeal pain, dizziness, and constipation were the drug’s most common side effects in the study.
Obeticholic acid is manufactured by Intercept Pharmaceuticals.
Obeticholic acid (Ocaliva) has been granted accelerated approval for use in combination with ursodeoxycholic acid (UDCA) for the treatment of primary biliary cholangitis in adults with an inadequate response to UDCA, and for use as a single therapy in adults unable to tolerate UDCA, the U.S. Food and Drug Administration announced.
Obeticholic acid should not be used in patients with complete biliary obstruction.
Given orally, obeticholic acid binds to the farnesoid X receptor (FXR), a receptor found in cells of the liver and intestine. FXR is a key regulator of bile acid metabolic pathways. Obeticholic acid increases bile flow from the liver and suppresses bile acid production in the liver, thus reducing exposure to toxic levels of bile acids.
Obeticholic acid was shown to reduce levels of alkaline phosphatase, which was used as a surrogate endpoint to predict clinical benefit, including an improvement in transplant-free survival. The FDA’s accelerated approval program allows approval based on a surrogate endpoint that is reasonably likely to predict clinical benefit. The manufacturer will conduct confirmatory clinical trials to examine any improvements in survival, progression to cirrhosis, or other disease-related symptoms.
After 12 months, in a controlled clinical trial with 216 participants, the proportion of participants achieving reductions in levels of alkaline phosphatase was higher among treated participants than in participants given placebo. Pruritus, fatigue, abdominal pain and discomfort, arthralgia, oropharyngeal pain, dizziness, and constipation were the drug’s most common side effects in the study.
Obeticholic acid is manufactured by Intercept Pharmaceuticals.
Venetoclax achieves responses in CLL refractory to ibrutinib, idelalisib
Venetoclax monotherapy was active, and even elicited minimal residual disease negativity in a proportion of patients with chronic lymphocytic leukemia (CLL) that was resistant or refractory to ibrutinib or idelalisib, Dr. Jeffrey Alan Jones of the Ohio State University Comprehensive Cancer Center, Columbus, and colleagues wrote in an abstract to be presented at the annual meeting of the American Society of Clinical Oncology.
The findings from their ongoing phase II study are the first prospective results to demonstrate efficacy in this poor-prognosis population, the researchers wrote. Earlier results from the study were reported at the American Society of Hematology meeting.
The 54 patients in the study, 25 of them refractory to ibrutinib and 6 to idelalisib, received venetoclax 20 mg daily followed by a 5-week ramp up in dosage to 400 mg daily. Over half of the patients had received more than five prior therapies; 83% did not have IGHV mutations, 20% had absolute lymphocyte counts exceeding 100 x 109, 35% had 17p deletions, and 24% had at least one node that was 10 cm or larger.
Of the patients who had previously received ibrutinib, four discontinued venetoclax because of progressive disease and four others had respiratory failure, multiorgan failure, death of unknown cause, or consent withdrawal. Of those who previously received idelalisib, four halted therapy because of progressive disease and failure to respond. Of 48 evaluable patients, 38 previously received ibrutinib, and 10 previously received idelalisib.
The overall response rate was 61% (23 of 38 patients) for patients refractory to ibrutinib and 50% (5 of 10 patients) for those refractory to idelalisib. A complete response was seen in three patients, all refractory to ibrutinib.
Grade 3/4 adverse events were seen in over 10% of patients and included neutropenia (39%), thrombocytopenia (22%), anemia (20%), leukopenia (13%), and pneumonia (13%). Significant adverse events seen in at least two patients included pneumonia (9%), febrile neutropenia (7%), increased potassium, multiorgan failure, and septic shock (4% each).
The study is sponsored by AbbVie.
Venetoclax monotherapy was active, and even elicited minimal residual disease negativity in a proportion of patients with chronic lymphocytic leukemia (CLL) that was resistant or refractory to ibrutinib or idelalisib, Dr. Jeffrey Alan Jones of the Ohio State University Comprehensive Cancer Center, Columbus, and colleagues wrote in an abstract to be presented at the annual meeting of the American Society of Clinical Oncology.
The findings from their ongoing phase II study are the first prospective results to demonstrate efficacy in this poor-prognosis population, the researchers wrote. Earlier results from the study were reported at the American Society of Hematology meeting.
The 54 patients in the study, 25 of them refractory to ibrutinib and 6 to idelalisib, received venetoclax 20 mg daily followed by a 5-week ramp up in dosage to 400 mg daily. Over half of the patients had received more than five prior therapies; 83% did not have IGHV mutations, 20% had absolute lymphocyte counts exceeding 100 x 109, 35% had 17p deletions, and 24% had at least one node that was 10 cm or larger.
Of the patients who had previously received ibrutinib, four discontinued venetoclax because of progressive disease and four others had respiratory failure, multiorgan failure, death of unknown cause, or consent withdrawal. Of those who previously received idelalisib, four halted therapy because of progressive disease and failure to respond. Of 48 evaluable patients, 38 previously received ibrutinib, and 10 previously received idelalisib.
The overall response rate was 61% (23 of 38 patients) for patients refractory to ibrutinib and 50% (5 of 10 patients) for those refractory to idelalisib. A complete response was seen in three patients, all refractory to ibrutinib.
Grade 3/4 adverse events were seen in over 10% of patients and included neutropenia (39%), thrombocytopenia (22%), anemia (20%), leukopenia (13%), and pneumonia (13%). Significant adverse events seen in at least two patients included pneumonia (9%), febrile neutropenia (7%), increased potassium, multiorgan failure, and septic shock (4% each).
The study is sponsored by AbbVie.
Venetoclax monotherapy was active, and even elicited minimal residual disease negativity in a proportion of patients with chronic lymphocytic leukemia (CLL) that was resistant or refractory to ibrutinib or idelalisib, Dr. Jeffrey Alan Jones of the Ohio State University Comprehensive Cancer Center, Columbus, and colleagues wrote in an abstract to be presented at the annual meeting of the American Society of Clinical Oncology.
The findings from their ongoing phase II study are the first prospective results to demonstrate efficacy in this poor-prognosis population, the researchers wrote. Earlier results from the study were reported at the American Society of Hematology meeting.
The 54 patients in the study, 25 of them refractory to ibrutinib and 6 to idelalisib, received venetoclax 20 mg daily followed by a 5-week ramp up in dosage to 400 mg daily. Over half of the patients had received more than five prior therapies; 83% did not have IGHV mutations, 20% had absolute lymphocyte counts exceeding 100 x 109, 35% had 17p deletions, and 24% had at least one node that was 10 cm or larger.
Of the patients who had previously received ibrutinib, four discontinued venetoclax because of progressive disease and four others had respiratory failure, multiorgan failure, death of unknown cause, or consent withdrawal. Of those who previously received idelalisib, four halted therapy because of progressive disease and failure to respond. Of 48 evaluable patients, 38 previously received ibrutinib, and 10 previously received idelalisib.
The overall response rate was 61% (23 of 38 patients) for patients refractory to ibrutinib and 50% (5 of 10 patients) for those refractory to idelalisib. A complete response was seen in three patients, all refractory to ibrutinib.
Grade 3/4 adverse events were seen in over 10% of patients and included neutropenia (39%), thrombocytopenia (22%), anemia (20%), leukopenia (13%), and pneumonia (13%). Significant adverse events seen in at least two patients included pneumonia (9%), febrile neutropenia (7%), increased potassium, multiorgan failure, and septic shock (4% each).
The study is sponsored by AbbVie.
FROM ASCO 2016
Key clinical point: Venetoclax can achieve responses in patients who are refractory to ibrutinib.
Major finding: The overall response rate was 61% (23 of 38 patients) for patients refractory to ibrutinib and 50% (5 of 10 patients) for those refractory to idelalisib. A complete response was seen in three patients, all refractory to ibrutinib..
Data source: Data on 54 patients in an ongoing phase II study.
Disclosures: The study is sponsored by AbbVie.
VIDEO: Adding ixazomib to len-dex boosts progression-free survival in multiple myeloma
Adding ixazomib to lenalidomide and dexamethasone was associated with longer progression-free survival and limited additional toxic effects in patients with multiple myeloma, based on the published phase 3 results of the TOURMALINE trial.
The double-blind, placebo-controlled trial included 722 patients who had relapsed, refractory, or relapsed and refractory multiple myeloma and were randomly assigned to receive the oral proteasome inhibitor plus lenalidomide-dexamethasone or placebo plus lenalidomide-dexamethasone (len-dex), according to Dr. Philippe Moreau of University Hospital Hôtel
Dieu, Nantes, France, and his colleagues in the TOURMALINE-MM1 Study Group.
At a median follow-up of nearly 14.7 months, median progression-free survival was 20.6 months in the ixazomib plus len-dex group and 14.7 months in the placebo plus len-dex group, a significant difference for ixazomib with a 0.74 hazard ratio for disease progression or death (P = .01). The benefit was noted for all prespecified patient subgroups, including patients with high-risk cytogenetic abnormalities. The overall rates of response were 78% in the ixazomib plus len-dex group and 72% in the placebo plus len-dex group, and the corresponding rates of complete response plus very good partial response were 48% and 39%, respectively. At a median follow-up of approximately 23 months, the median duration of response was 20.5 months for ixazomib plus len-dex and 15 months for len-dex alone, the researchers reported (N Engl J Med. 2016;374:1621-34. doi: 10.1056/NEJMoa1516282).
The rates of serious adverse events were 47% in the ixazomib plus len-dex group and 49% in the placebo plus len-dex group; the rates of death during the study period were 4% and 6%, respectively.
The results of the trial also were presented at the annual meeting of the American Society of Hematology, where Dr. Shaji Kumar, one the study investigators, discussed the implications of the TOURMALINE results in a video interview.
The study was sponsored by Millennium Pharmaceuticals, the makers of ixazomib (Ninlaro). Dr. Moreau reports receiving fees for serving on advisory boards for Millennium Pharmaceuticals and several other drug companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @maryjodales
Adding ixazomib to lenalidomide and dexamethasone was associated with longer progression-free survival and limited additional toxic effects in patients with multiple myeloma, based on the published phase 3 results of the TOURMALINE trial.
The double-blind, placebo-controlled trial included 722 patients who had relapsed, refractory, or relapsed and refractory multiple myeloma and were randomly assigned to receive the oral proteasome inhibitor plus lenalidomide-dexamethasone or placebo plus lenalidomide-dexamethasone (len-dex), according to Dr. Philippe Moreau of University Hospital Hôtel
Dieu, Nantes, France, and his colleagues in the TOURMALINE-MM1 Study Group.
At a median follow-up of nearly 14.7 months, median progression-free survival was 20.6 months in the ixazomib plus len-dex group and 14.7 months in the placebo plus len-dex group, a significant difference for ixazomib with a 0.74 hazard ratio for disease progression or death (P = .01). The benefit was noted for all prespecified patient subgroups, including patients with high-risk cytogenetic abnormalities. The overall rates of response were 78% in the ixazomib plus len-dex group and 72% in the placebo plus len-dex group, and the corresponding rates of complete response plus very good partial response were 48% and 39%, respectively. At a median follow-up of approximately 23 months, the median duration of response was 20.5 months for ixazomib plus len-dex and 15 months for len-dex alone, the researchers reported (N Engl J Med. 2016;374:1621-34. doi: 10.1056/NEJMoa1516282).
The rates of serious adverse events were 47% in the ixazomib plus len-dex group and 49% in the placebo plus len-dex group; the rates of death during the study period were 4% and 6%, respectively.
The results of the trial also were presented at the annual meeting of the American Society of Hematology, where Dr. Shaji Kumar, one the study investigators, discussed the implications of the TOURMALINE results in a video interview.
The study was sponsored by Millennium Pharmaceuticals, the makers of ixazomib (Ninlaro). Dr. Moreau reports receiving fees for serving on advisory boards for Millennium Pharmaceuticals and several other drug companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @maryjodales
Adding ixazomib to lenalidomide and dexamethasone was associated with longer progression-free survival and limited additional toxic effects in patients with multiple myeloma, based on the published phase 3 results of the TOURMALINE trial.
The double-blind, placebo-controlled trial included 722 patients who had relapsed, refractory, or relapsed and refractory multiple myeloma and were randomly assigned to receive the oral proteasome inhibitor plus lenalidomide-dexamethasone or placebo plus lenalidomide-dexamethasone (len-dex), according to Dr. Philippe Moreau of University Hospital Hôtel
Dieu, Nantes, France, and his colleagues in the TOURMALINE-MM1 Study Group.
At a median follow-up of nearly 14.7 months, median progression-free survival was 20.6 months in the ixazomib plus len-dex group and 14.7 months in the placebo plus len-dex group, a significant difference for ixazomib with a 0.74 hazard ratio for disease progression or death (P = .01). The benefit was noted for all prespecified patient subgroups, including patients with high-risk cytogenetic abnormalities. The overall rates of response were 78% in the ixazomib plus len-dex group and 72% in the placebo plus len-dex group, and the corresponding rates of complete response plus very good partial response were 48% and 39%, respectively. At a median follow-up of approximately 23 months, the median duration of response was 20.5 months for ixazomib plus len-dex and 15 months for len-dex alone, the researchers reported (N Engl J Med. 2016;374:1621-34. doi: 10.1056/NEJMoa1516282).
The rates of serious adverse events were 47% in the ixazomib plus len-dex group and 49% in the placebo plus len-dex group; the rates of death during the study period were 4% and 6%, respectively.
The results of the trial also were presented at the annual meeting of the American Society of Hematology, where Dr. Shaji Kumar, one the study investigators, discussed the implications of the TOURMALINE results in a video interview.
The study was sponsored by Millennium Pharmaceuticals, the makers of ixazomib (Ninlaro). Dr. Moreau reports receiving fees for serving on advisory boards for Millennium Pharmaceuticals and several other drug companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @maryjodales
FROM NEJM
Key clinical point: Adding ixazomib to lenalidomide and dexamethasone was associated with a longer progression-free survival and limited additional toxic effects in patients with multiple myeloma.
Major finding: At a median follow-up of nearly 14.7 months, median progression-free survival was 20.6 months in the ixazomib plus len-dex group and 14.7 months in the placebo plus len-dex group.
Data source: Phase III results on 722 patients in the TOURMALINE trial.
Disclosures: The study was sponsored by Millennium Pharmaceuticals, the makers of ixazomib (Ninlaro). Dr. Moreau reports receiving fees for serving on advisory boards for Millennium Pharmaceuticals and several other drug companies.
New single-tube assay detects one CLL cell in 1 million leukocytes
Chronic lymphocytic leukemia cells can be identified at levels as low as 0.0010% with a newly developed and validated single-tube assay, Dr. Andy C. Rawstro of St. James’s Institute of Oncology, Leeds, England, and his colleagues said in a European Research Initiative on CLL (ERIC) project report in Leukemia.
The high-throughput sequencing assay consists of a core panel of six markers – CD19, CD20, CD5, CD43, CD79b and CD81 – with a component specification independent of instrument and reagents. The new assay eliminates the need to distribute the blood sample across multiple tubes, which can impair sensitivity in cases with poor cellularity. The assay can be locally revalidated using normal peripheral blood and can be used to investigate new markers.
The new assay was validated in a multicenter study of 128 samples from 108 patients with CLL or monoclonal B-cell lymphocytosis, studied either at diagnosis or after FCR-based (fludarabine, cyclophosphamide, rituximab) treatment and compared with peripheral blood samples from healthy young women. In a parallel analysis, the assay compared with flow cytometry results at the 0.010% level, the minimal residual disease threshold defined in the 2008 International Workshop on CLL guidelines. The new assay, however, also was able to detect disease at the 0.0010% (one CLL cell in 1 million leukocytes) level.
The ability to detect disease below the levels that can be assessed by flow cytometry may prove to be a valuable resource to improve quantification of minimal residual disease and evaluate treatment response in CLL. Using minimal residual disease as a surrogate measure for treatment effectiveness would avoid the need for prolonged observation times in assessing new therapies, the researchers said (Leukemia 2016;30:929-36).
Dr. Rawstro disclosed receiving honoraria from Celgene, Abbvie, Gilead, Roche, and GSK. He receives royalty payments from BD Biosciences (Intrasure reagent) and study reagents. He is a consultant for Gilead and Biogen Idec.
On Twitter @maryjodales
Chronic lymphocytic leukemia cells can be identified at levels as low as 0.0010% with a newly developed and validated single-tube assay, Dr. Andy C. Rawstro of St. James’s Institute of Oncology, Leeds, England, and his colleagues said in a European Research Initiative on CLL (ERIC) project report in Leukemia.
The high-throughput sequencing assay consists of a core panel of six markers – CD19, CD20, CD5, CD43, CD79b and CD81 – with a component specification independent of instrument and reagents. The new assay eliminates the need to distribute the blood sample across multiple tubes, which can impair sensitivity in cases with poor cellularity. The assay can be locally revalidated using normal peripheral blood and can be used to investigate new markers.
The new assay was validated in a multicenter study of 128 samples from 108 patients with CLL or monoclonal B-cell lymphocytosis, studied either at diagnosis or after FCR-based (fludarabine, cyclophosphamide, rituximab) treatment and compared with peripheral blood samples from healthy young women. In a parallel analysis, the assay compared with flow cytometry results at the 0.010% level, the minimal residual disease threshold defined in the 2008 International Workshop on CLL guidelines. The new assay, however, also was able to detect disease at the 0.0010% (one CLL cell in 1 million leukocytes) level.
The ability to detect disease below the levels that can be assessed by flow cytometry may prove to be a valuable resource to improve quantification of minimal residual disease and evaluate treatment response in CLL. Using minimal residual disease as a surrogate measure for treatment effectiveness would avoid the need for prolonged observation times in assessing new therapies, the researchers said (Leukemia 2016;30:929-36).
Dr. Rawstro disclosed receiving honoraria from Celgene, Abbvie, Gilead, Roche, and GSK. He receives royalty payments from BD Biosciences (Intrasure reagent) and study reagents. He is a consultant for Gilead and Biogen Idec.
On Twitter @maryjodales
Chronic lymphocytic leukemia cells can be identified at levels as low as 0.0010% with a newly developed and validated single-tube assay, Dr. Andy C. Rawstro of St. James’s Institute of Oncology, Leeds, England, and his colleagues said in a European Research Initiative on CLL (ERIC) project report in Leukemia.
The high-throughput sequencing assay consists of a core panel of six markers – CD19, CD20, CD5, CD43, CD79b and CD81 – with a component specification independent of instrument and reagents. The new assay eliminates the need to distribute the blood sample across multiple tubes, which can impair sensitivity in cases with poor cellularity. The assay can be locally revalidated using normal peripheral blood and can be used to investigate new markers.
The new assay was validated in a multicenter study of 128 samples from 108 patients with CLL or monoclonal B-cell lymphocytosis, studied either at diagnosis or after FCR-based (fludarabine, cyclophosphamide, rituximab) treatment and compared with peripheral blood samples from healthy young women. In a parallel analysis, the assay compared with flow cytometry results at the 0.010% level, the minimal residual disease threshold defined in the 2008 International Workshop on CLL guidelines. The new assay, however, also was able to detect disease at the 0.0010% (one CLL cell in 1 million leukocytes) level.
The ability to detect disease below the levels that can be assessed by flow cytometry may prove to be a valuable resource to improve quantification of minimal residual disease and evaluate treatment response in CLL. Using minimal residual disease as a surrogate measure for treatment effectiveness would avoid the need for prolonged observation times in assessing new therapies, the researchers said (Leukemia 2016;30:929-36).
Dr. Rawstro disclosed receiving honoraria from Celgene, Abbvie, Gilead, Roche, and GSK. He receives royalty payments from BD Biosciences (Intrasure reagent) and study reagents. He is a consultant for Gilead and Biogen Idec.
On Twitter @maryjodales
FROM LEUKEMIA
Key clinical point: Chronic lymphocytic leukemia cells can be identified at levels as low as 0.0010% with a newly developed and validated single-tube assay.
Major finding: In a parallel analysis, the assay compared with flow cytometry results at the 0.010% level, and also was able to detect disease at the 0.0010% (one CLL cell in 1 million leukocytes) level.
Data source: The new assay was validated in a multicenter study of 128 samples from 108 patients with CLL or monoclonal B-cell lymphocytosis.
Disclosures: Dr. Rawstro disclosed receiving honoraria from Celgene, Abbvie, Gilead, Roche, and GSK. He receives royalty payments from BD Biosciences (Intrasure reagent) and study reagents. He is a consultant for Gilead and Biogen Idec.
In severe hemophilia B, rIX-FP prophylaxis gets good results with less frequent dosing
Recombinant fusion protein linking coagulation factor IX with albumin (rIX-FP) could result in a “paradigm shift” in prophylaxis regimens for patients with hemophilia B, according to researchers reporting results from a phase III trial.
At dosing intervals of up to 14 days, rIX-FP was a safe and effective factor IX (FIX) replacement product for preventing and treating bleeding episodes in an open-label study of 63 previously treated adolescents and adults with severe hemophilia B.
Compared to standard FIX products, rIX-FP is more active, has a longer half-life, and has better clearance. A study is now underway to see whether a 21-day prophylaxis regimen provides even better results than the 14-day regimen, reported Dr. Elena Santagostino of Istituto di Ricovero e Cura a Carattere Scientifico Ca’ Granda Foundation, Maggiore Hospital Policlinico, Milan, and her associates in the PROLONG-9FP Investigators Study Group.
For prophylaxis, standard FIX products are administered two times per week. Patients are at increased risk of bleeding when their FIX activity is low, particularly just before their next scheduled dose. With the prolonged FIX activity of rIX-FP, the dosing is less frequent and that could potentially improve adherence, the researchers wrote (Blood. 2016;127[14]:1761-9).
In addition, rIX-FP was associated with a reduced risk of spontaneous and trauma-related bleeding episodes.
The findings came from a study of patients who had FIX activity levels of 2% or less and were assigned to one of two groups. The first group received routine prophylaxis once every 7 days for 26 weeks; they were then placed on either a 7-, 10-, or 14-day prophylaxis regimen for a mean of 50, 38, or 51 weeks, respectively. The second group received on-demand treatment of bleeding episodes for 26 weeks and were then switched to a 7-day prophylaxis regimen for a mean of 45 weeks. Only patients who were previously receiving on-demand treatment were eligible for group 2 assignment.
Patients self-administered rIX-FP for routine prophylaxis and on-demand treatment of bleeding episodes; all home administrations were recorded in an electronic diary. A second dose of rIX-FP was administered at least 24 hours after the first injection, if needed, to achieve hemostasis. Efficacy and safety assessments were performed at study sites on a monthly basis.
The study design controlled for the variability of bleeding frequency within the hemophilia B patient population, as group 2 patients started rIX-FP treatment on-demand and continued on 7-day prophylaxis. During the on-demand phase, rIX-FP controlled 98.6% of bleeding episodes, and 93.6% of bleeds were controlled with one infusion.
Once the group was switched to 7-day prophylaxis, the median annualized spontaneous bleeding rate dropped to 0.0 and all target joints resolved.(P less than .0001). No patient developed an inhibitor, and no safety concerns were identified.
The annualized bleeding rate decreased slightly in group 2 patients during on-demand treatment with rIX-FP, compared with the rate in the 12-month period before they entered the study. The researchers speculated that on-demand treatment with rIX-FP “might provide, to a certain extent, some protection against a subsequent bleeding episode. Therefore, patients with a severe bleeding phenotype treated on-demand might experience fewer bleeding episodes if switched to rIX-FP. Furthermore, such on-demand patients who require more than one infusion per month may have the benefit of a reduction of ABR by administering a similar number of infusions according to a 14-day prophylaxis regimen with rIX-FP.”
The sample size was small, however, and differences of care in the clinical study could have affected the results.
Compared to other FIX products, rIX-FP had an extended terminal half-life of 102 hours, 4.3-fold longer than seen with the patients’ previous FIX treatment. Other pharmacokinetic measures such as area under the curve (7176 h × IU/dL), clearance (0.77 mL/h per kg), and incremental recovery (1.27 IU/dL per IU/kg) also were improved. Patients maintained a mean trough of 20 and 12 IU/dL FIX activity on prophylaxis with rIX-FP 40 IU/kg every 7 days and 75 IU/kg every 14 days, respectively. Rather than half-life alone, a slower clearance may be a factor in the success of prophylaxis regimens, the researchers said.
The principal investigators received research support from CSL Behring, which sponsored the study and was responsible for trial operations and data analysis.
On Twitter @maryjodales
Recombinant fusion protein linking coagulation factor IX with albumin (rIX-FP) could result in a “paradigm shift” in prophylaxis regimens for patients with hemophilia B, according to researchers reporting results from a phase III trial.
At dosing intervals of up to 14 days, rIX-FP was a safe and effective factor IX (FIX) replacement product for preventing and treating bleeding episodes in an open-label study of 63 previously treated adolescents and adults with severe hemophilia B.
Compared to standard FIX products, rIX-FP is more active, has a longer half-life, and has better clearance. A study is now underway to see whether a 21-day prophylaxis regimen provides even better results than the 14-day regimen, reported Dr. Elena Santagostino of Istituto di Ricovero e Cura a Carattere Scientifico Ca’ Granda Foundation, Maggiore Hospital Policlinico, Milan, and her associates in the PROLONG-9FP Investigators Study Group.
For prophylaxis, standard FIX products are administered two times per week. Patients are at increased risk of bleeding when their FIX activity is low, particularly just before their next scheduled dose. With the prolonged FIX activity of rIX-FP, the dosing is less frequent and that could potentially improve adherence, the researchers wrote (Blood. 2016;127[14]:1761-9).
In addition, rIX-FP was associated with a reduced risk of spontaneous and trauma-related bleeding episodes.
The findings came from a study of patients who had FIX activity levels of 2% or less and were assigned to one of two groups. The first group received routine prophylaxis once every 7 days for 26 weeks; they were then placed on either a 7-, 10-, or 14-day prophylaxis regimen for a mean of 50, 38, or 51 weeks, respectively. The second group received on-demand treatment of bleeding episodes for 26 weeks and were then switched to a 7-day prophylaxis regimen for a mean of 45 weeks. Only patients who were previously receiving on-demand treatment were eligible for group 2 assignment.
Patients self-administered rIX-FP for routine prophylaxis and on-demand treatment of bleeding episodes; all home administrations were recorded in an electronic diary. A second dose of rIX-FP was administered at least 24 hours after the first injection, if needed, to achieve hemostasis. Efficacy and safety assessments were performed at study sites on a monthly basis.
The study design controlled for the variability of bleeding frequency within the hemophilia B patient population, as group 2 patients started rIX-FP treatment on-demand and continued on 7-day prophylaxis. During the on-demand phase, rIX-FP controlled 98.6% of bleeding episodes, and 93.6% of bleeds were controlled with one infusion.
Once the group was switched to 7-day prophylaxis, the median annualized spontaneous bleeding rate dropped to 0.0 and all target joints resolved.(P less than .0001). No patient developed an inhibitor, and no safety concerns were identified.
The annualized bleeding rate decreased slightly in group 2 patients during on-demand treatment with rIX-FP, compared with the rate in the 12-month period before they entered the study. The researchers speculated that on-demand treatment with rIX-FP “might provide, to a certain extent, some protection against a subsequent bleeding episode. Therefore, patients with a severe bleeding phenotype treated on-demand might experience fewer bleeding episodes if switched to rIX-FP. Furthermore, such on-demand patients who require more than one infusion per month may have the benefit of a reduction of ABR by administering a similar number of infusions according to a 14-day prophylaxis regimen with rIX-FP.”
The sample size was small, however, and differences of care in the clinical study could have affected the results.
Compared to other FIX products, rIX-FP had an extended terminal half-life of 102 hours, 4.3-fold longer than seen with the patients’ previous FIX treatment. Other pharmacokinetic measures such as area under the curve (7176 h × IU/dL), clearance (0.77 mL/h per kg), and incremental recovery (1.27 IU/dL per IU/kg) also were improved. Patients maintained a mean trough of 20 and 12 IU/dL FIX activity on prophylaxis with rIX-FP 40 IU/kg every 7 days and 75 IU/kg every 14 days, respectively. Rather than half-life alone, a slower clearance may be a factor in the success of prophylaxis regimens, the researchers said.
The principal investigators received research support from CSL Behring, which sponsored the study and was responsible for trial operations and data analysis.
On Twitter @maryjodales
Recombinant fusion protein linking coagulation factor IX with albumin (rIX-FP) could result in a “paradigm shift” in prophylaxis regimens for patients with hemophilia B, according to researchers reporting results from a phase III trial.
At dosing intervals of up to 14 days, rIX-FP was a safe and effective factor IX (FIX) replacement product for preventing and treating bleeding episodes in an open-label study of 63 previously treated adolescents and adults with severe hemophilia B.
Compared to standard FIX products, rIX-FP is more active, has a longer half-life, and has better clearance. A study is now underway to see whether a 21-day prophylaxis regimen provides even better results than the 14-day regimen, reported Dr. Elena Santagostino of Istituto di Ricovero e Cura a Carattere Scientifico Ca’ Granda Foundation, Maggiore Hospital Policlinico, Milan, and her associates in the PROLONG-9FP Investigators Study Group.
For prophylaxis, standard FIX products are administered two times per week. Patients are at increased risk of bleeding when their FIX activity is low, particularly just before their next scheduled dose. With the prolonged FIX activity of rIX-FP, the dosing is less frequent and that could potentially improve adherence, the researchers wrote (Blood. 2016;127[14]:1761-9).
In addition, rIX-FP was associated with a reduced risk of spontaneous and trauma-related bleeding episodes.
The findings came from a study of patients who had FIX activity levels of 2% or less and were assigned to one of two groups. The first group received routine prophylaxis once every 7 days for 26 weeks; they were then placed on either a 7-, 10-, or 14-day prophylaxis regimen for a mean of 50, 38, or 51 weeks, respectively. The second group received on-demand treatment of bleeding episodes for 26 weeks and were then switched to a 7-day prophylaxis regimen for a mean of 45 weeks. Only patients who were previously receiving on-demand treatment were eligible for group 2 assignment.
Patients self-administered rIX-FP for routine prophylaxis and on-demand treatment of bleeding episodes; all home administrations were recorded in an electronic diary. A second dose of rIX-FP was administered at least 24 hours after the first injection, if needed, to achieve hemostasis. Efficacy and safety assessments were performed at study sites on a monthly basis.
The study design controlled for the variability of bleeding frequency within the hemophilia B patient population, as group 2 patients started rIX-FP treatment on-demand and continued on 7-day prophylaxis. During the on-demand phase, rIX-FP controlled 98.6% of bleeding episodes, and 93.6% of bleeds were controlled with one infusion.
Once the group was switched to 7-day prophylaxis, the median annualized spontaneous bleeding rate dropped to 0.0 and all target joints resolved.(P less than .0001). No patient developed an inhibitor, and no safety concerns were identified.
The annualized bleeding rate decreased slightly in group 2 patients during on-demand treatment with rIX-FP, compared with the rate in the 12-month period before they entered the study. The researchers speculated that on-demand treatment with rIX-FP “might provide, to a certain extent, some protection against a subsequent bleeding episode. Therefore, patients with a severe bleeding phenotype treated on-demand might experience fewer bleeding episodes if switched to rIX-FP. Furthermore, such on-demand patients who require more than one infusion per month may have the benefit of a reduction of ABR by administering a similar number of infusions according to a 14-day prophylaxis regimen with rIX-FP.”
The sample size was small, however, and differences of care in the clinical study could have affected the results.
Compared to other FIX products, rIX-FP had an extended terminal half-life of 102 hours, 4.3-fold longer than seen with the patients’ previous FIX treatment. Other pharmacokinetic measures such as area under the curve (7176 h × IU/dL), clearance (0.77 mL/h per kg), and incremental recovery (1.27 IU/dL per IU/kg) also were improved. Patients maintained a mean trough of 20 and 12 IU/dL FIX activity on prophylaxis with rIX-FP 40 IU/kg every 7 days and 75 IU/kg every 14 days, respectively. Rather than half-life alone, a slower clearance may be a factor in the success of prophylaxis regimens, the researchers said.
The principal investigators received research support from CSL Behring, which sponsored the study and was responsible for trial operations and data analysis.
On Twitter @maryjodales
FROM BLOOD
Key clinical point: Weekly and 14-day prophylaxis regimens with rIX-FP were well tolerated and provided low bleeding rates and target joint improvement.
Major finding: On 7-day prophylaxis, the median annualized spontaneous bleeding rate dropped to 0.0 and all target joints resolved (P less than .0001).
Data source: An open-label study of 63 previously treated adolescents and adults with severe hemophilia B.
Disclosures: The principal investigators received research support from CSL Behring, which sponsored the study and was responsible for trial operations and data analysis.
Prelabor cesarean delivery linked to increased risk of childhood ALL
An increased risk of acute lymphoblastic leukemia (ALL) was seen in young children born by prelabor cesarean delivery, in a pooled analysis of 13 case-control studies from nine countries.
The odds ratio was significant at 1.23 for an association between prelabor cesarean delivery and ALL (P = .018); there was not a significant association between ALL and all indications of cesarean delivery nor was there an association with emergency cesarean delivery. Further, the risk for childhood AML was not associated with cesarean delivery, prelabor cesarean delivery, or emergency cesarean delivery, reported Erin L. Marcotte, Ph.D., of the University of Minnesota, Minneapolis, and her associates.
The association between ALL and prelabor cesarean delivery is based on 13 case-control studies from the Childhood Leukemia International Consortium. Birth delivery method was known for 97%-99% of 8,780 ALL cases, 1,332 AML cases, and 23,459 controls in those studies. In four of the studies, the indications for cesarean delivery were known for 1,061 of 4,313 ALL cases, 138 of 664 AML cases, and 1,401 of 5,884 controls. The multivariable logistic regression models used for the analysis were adjusted for birth weight, sex, age, ethnic origin, parental education, maternal age, and study.
If the association proves to be causal, “maladaptive immune activation due to an absence of stress response before birth in children born by prelabor caesarean delivery could be considered as a potential mechanism,” the researchers wrote (Lancet Haematol. 2016;3[4]:e176–e185).
ALL involves genetic and developmental aberrations that are probably modified by exposure and response to infectious agents. Early exposure to a variety of infections seems to decrease risk, and a vigorous response to infections (quantified by physician visits for infections) increases risk. During vaginal birth, the newborn is exposed to commensal bacteria that modulate immune development, Joseph Weimels, Ph.D., of the University of California at San Francisco, and Xiaomei Ma, Ph.D., of the Yale School of Public Health, New Haven, Conn., wrote in an editorial published in the same issue of The Lancet Haematology. Children delivered vaginally have different gut microbiomes and T-cell reactivity persisting up to age 2 years compared with children born by cesarean, they wrote.
The National Cancer Institute funded the study. The researchers had no relevant disclosures.
On Twitter @maryjodales
An increased risk of acute lymphoblastic leukemia (ALL) was seen in young children born by prelabor cesarean delivery, in a pooled analysis of 13 case-control studies from nine countries.
The odds ratio was significant at 1.23 for an association between prelabor cesarean delivery and ALL (P = .018); there was not a significant association between ALL and all indications of cesarean delivery nor was there an association with emergency cesarean delivery. Further, the risk for childhood AML was not associated with cesarean delivery, prelabor cesarean delivery, or emergency cesarean delivery, reported Erin L. Marcotte, Ph.D., of the University of Minnesota, Minneapolis, and her associates.
The association between ALL and prelabor cesarean delivery is based on 13 case-control studies from the Childhood Leukemia International Consortium. Birth delivery method was known for 97%-99% of 8,780 ALL cases, 1,332 AML cases, and 23,459 controls in those studies. In four of the studies, the indications for cesarean delivery were known for 1,061 of 4,313 ALL cases, 138 of 664 AML cases, and 1,401 of 5,884 controls. The multivariable logistic regression models used for the analysis were adjusted for birth weight, sex, age, ethnic origin, parental education, maternal age, and study.
If the association proves to be causal, “maladaptive immune activation due to an absence of stress response before birth in children born by prelabor caesarean delivery could be considered as a potential mechanism,” the researchers wrote (Lancet Haematol. 2016;3[4]:e176–e185).
ALL involves genetic and developmental aberrations that are probably modified by exposure and response to infectious agents. Early exposure to a variety of infections seems to decrease risk, and a vigorous response to infections (quantified by physician visits for infections) increases risk. During vaginal birth, the newborn is exposed to commensal bacteria that modulate immune development, Joseph Weimels, Ph.D., of the University of California at San Francisco, and Xiaomei Ma, Ph.D., of the Yale School of Public Health, New Haven, Conn., wrote in an editorial published in the same issue of The Lancet Haematology. Children delivered vaginally have different gut microbiomes and T-cell reactivity persisting up to age 2 years compared with children born by cesarean, they wrote.
The National Cancer Institute funded the study. The researchers had no relevant disclosures.
On Twitter @maryjodales
An increased risk of acute lymphoblastic leukemia (ALL) was seen in young children born by prelabor cesarean delivery, in a pooled analysis of 13 case-control studies from nine countries.
The odds ratio was significant at 1.23 for an association between prelabor cesarean delivery and ALL (P = .018); there was not a significant association between ALL and all indications of cesarean delivery nor was there an association with emergency cesarean delivery. Further, the risk for childhood AML was not associated with cesarean delivery, prelabor cesarean delivery, or emergency cesarean delivery, reported Erin L. Marcotte, Ph.D., of the University of Minnesota, Minneapolis, and her associates.
The association between ALL and prelabor cesarean delivery is based on 13 case-control studies from the Childhood Leukemia International Consortium. Birth delivery method was known for 97%-99% of 8,780 ALL cases, 1,332 AML cases, and 23,459 controls in those studies. In four of the studies, the indications for cesarean delivery were known for 1,061 of 4,313 ALL cases, 138 of 664 AML cases, and 1,401 of 5,884 controls. The multivariable logistic regression models used for the analysis were adjusted for birth weight, sex, age, ethnic origin, parental education, maternal age, and study.
If the association proves to be causal, “maladaptive immune activation due to an absence of stress response before birth in children born by prelabor caesarean delivery could be considered as a potential mechanism,” the researchers wrote (Lancet Haematol. 2016;3[4]:e176–e185).
ALL involves genetic and developmental aberrations that are probably modified by exposure and response to infectious agents. Early exposure to a variety of infections seems to decrease risk, and a vigorous response to infections (quantified by physician visits for infections) increases risk. During vaginal birth, the newborn is exposed to commensal bacteria that modulate immune development, Joseph Weimels, Ph.D., of the University of California at San Francisco, and Xiaomei Ma, Ph.D., of the Yale School of Public Health, New Haven, Conn., wrote in an editorial published in the same issue of The Lancet Haematology. Children delivered vaginally have different gut microbiomes and T-cell reactivity persisting up to age 2 years compared with children born by cesarean, they wrote.
The National Cancer Institute funded the study. The researchers had no relevant disclosures.
On Twitter @maryjodales
THE LANCET HAEMATOLOGY
Key clinical point: Prelabor cesarean delivery was associated with an increased risk of childhood ALL, but not AML.
Major finding: The odds ratio was significant at 1.23 for an association between prelabor cesarean delivery and ALL (P = .018).
Data source: Thirteen case-control studies from the Childhood Leukemia International Consortium.
Disclosures: The National Cancer Institute funded the study. The researchers had no relevant disclosures.
In newly diagnosed CLL, mutation tests are advised
Patients with newly diagnosed chronic lymphocytic leukemia should standardly undergo immunoglobulin heavy-chain variable region gene (IGHV) mutation status and interphase fluorescence in situ hybridization (FISH) tests, based on the results of a meta-analysis published in Blood.
“This change will help define the minimal standard initial prognostic evaluation for patients with CLL and help facilitate use of the powerful, recently developed, integrated prognostic indices, all of which are dependent on these 2 variables,” wrote Dr. Sameer A. Parikh of Mayo Clinic, Rochester, Minn., and associates.
IGHV and FISH have prognostic value independent of clinical stage in patients with newly diagnosed and previously untreated CLL, they said (Blood. 2016;127[14]:1752-60). Better understanding of the patient’s risk of disease progression at diagnosis can guide counseling and follow-up intervals, and could potentially influence the decision to treat high-risk patients on early intervention protocols.
IGHV and FISH also appear to provide additional information on progression-free and overall survival.
The researchers cautioned, however, that the results of these tests should not be used to initiate CLL-specific therapy. Only patients who meet indications for therapy based on the 2008 International Workshop on Chronic Lymphocytic Leukemia guidelines should receive treatment.
Further, they noted, the median age of patients included in studies that they analyzed was 64 years; the median age of patients with CLL is 72 years. The prognostic abilities of IGHV mutation and FISH may differ in these older individuals with CLL.
The researchers analyzed 31 studies that met the criteria for inclusion – full-length publications that included at least 200 patients and reported on the prognostic value of IGHV and/or FISH for predicting progression-free or overall survival in patients with newly diagnosed CLL.
They found that the median progression-free survival (range, about 1-5 years) was significantly shorter for patients with unmutated IGHV genes, than was the median progression-free survival (range, about 9-19 years) for those with mutated IGHV genes. Similarly, the median overall survival was significantly shorter for patients with unmutated IGHV (range, about 3-10 years) than for those with mutated IGHV (range, about 18-26 years).
For patients with high-risk FISH (including del17p13 and del11q23), the median progression-free survival was significantly shorter (range, about 0.1-5 years) than for those with low/intermediate-risk FISH (including del13q, normal, and trisomy 12; range, about 1.5-22 years). Median overall survival also significantly differed, ranging from about 3-10 years for patients with high-risk FISH and from about 7.5-20.5 years for those with low/intermediate-risk FISH.
In multivariable analyses, the hazard ratio for high-risk FISH ranged from 1.3 to 4.7 for progression-free survival and from 0.9 to 8.2 for overall survival. In studies reporting the results of multivariable analysis, high-risk FISH remained an independent predictor of progression-free survival in 8 of 17 studies and of overall survival in 10 of 14 studies, including in 10 of 13 studies adjusting for the prognostic impact of IGHV.
In multivariable analyses, IGHV remained an independent predictor of progression-free survival in 15 of 18 studies, including 12 of 15 studies adjusting for the prognostic impact of FISH. IGHV remained an independent predictor of overall survival in 11 of 15 studies reporting the results of multivariable analysis, including 10 of 14 studies adjusting for the prognostic impact of FISH.
Patients with newly diagnosed chronic lymphocytic leukemia should standardly undergo immunoglobulin heavy-chain variable region gene (IGHV) mutation status and interphase fluorescence in situ hybridization (FISH) tests, based on the results of a meta-analysis published in Blood.
“This change will help define the minimal standard initial prognostic evaluation for patients with CLL and help facilitate use of the powerful, recently developed, integrated prognostic indices, all of which are dependent on these 2 variables,” wrote Dr. Sameer A. Parikh of Mayo Clinic, Rochester, Minn., and associates.
IGHV and FISH have prognostic value independent of clinical stage in patients with newly diagnosed and previously untreated CLL, they said (Blood. 2016;127[14]:1752-60). Better understanding of the patient’s risk of disease progression at diagnosis can guide counseling and follow-up intervals, and could potentially influence the decision to treat high-risk patients on early intervention protocols.
IGHV and FISH also appear to provide additional information on progression-free and overall survival.
The researchers cautioned, however, that the results of these tests should not be used to initiate CLL-specific therapy. Only patients who meet indications for therapy based on the 2008 International Workshop on Chronic Lymphocytic Leukemia guidelines should receive treatment.
Further, they noted, the median age of patients included in studies that they analyzed was 64 years; the median age of patients with CLL is 72 years. The prognostic abilities of IGHV mutation and FISH may differ in these older individuals with CLL.
The researchers analyzed 31 studies that met the criteria for inclusion – full-length publications that included at least 200 patients and reported on the prognostic value of IGHV and/or FISH for predicting progression-free or overall survival in patients with newly diagnosed CLL.
They found that the median progression-free survival (range, about 1-5 years) was significantly shorter for patients with unmutated IGHV genes, than was the median progression-free survival (range, about 9-19 years) for those with mutated IGHV genes. Similarly, the median overall survival was significantly shorter for patients with unmutated IGHV (range, about 3-10 years) than for those with mutated IGHV (range, about 18-26 years).
For patients with high-risk FISH (including del17p13 and del11q23), the median progression-free survival was significantly shorter (range, about 0.1-5 years) than for those with low/intermediate-risk FISH (including del13q, normal, and trisomy 12; range, about 1.5-22 years). Median overall survival also significantly differed, ranging from about 3-10 years for patients with high-risk FISH and from about 7.5-20.5 years for those with low/intermediate-risk FISH.
In multivariable analyses, the hazard ratio for high-risk FISH ranged from 1.3 to 4.7 for progression-free survival and from 0.9 to 8.2 for overall survival. In studies reporting the results of multivariable analysis, high-risk FISH remained an independent predictor of progression-free survival in 8 of 17 studies and of overall survival in 10 of 14 studies, including in 10 of 13 studies adjusting for the prognostic impact of IGHV.
In multivariable analyses, IGHV remained an independent predictor of progression-free survival in 15 of 18 studies, including 12 of 15 studies adjusting for the prognostic impact of FISH. IGHV remained an independent predictor of overall survival in 11 of 15 studies reporting the results of multivariable analysis, including 10 of 14 studies adjusting for the prognostic impact of FISH.
Patients with newly diagnosed chronic lymphocytic leukemia should standardly undergo immunoglobulin heavy-chain variable region gene (IGHV) mutation status and interphase fluorescence in situ hybridization (FISH) tests, based on the results of a meta-analysis published in Blood.
“This change will help define the minimal standard initial prognostic evaluation for patients with CLL and help facilitate use of the powerful, recently developed, integrated prognostic indices, all of which are dependent on these 2 variables,” wrote Dr. Sameer A. Parikh of Mayo Clinic, Rochester, Minn., and associates.
IGHV and FISH have prognostic value independent of clinical stage in patients with newly diagnosed and previously untreated CLL, they said (Blood. 2016;127[14]:1752-60). Better understanding of the patient’s risk of disease progression at diagnosis can guide counseling and follow-up intervals, and could potentially influence the decision to treat high-risk patients on early intervention protocols.
IGHV and FISH also appear to provide additional information on progression-free and overall survival.
The researchers cautioned, however, that the results of these tests should not be used to initiate CLL-specific therapy. Only patients who meet indications for therapy based on the 2008 International Workshop on Chronic Lymphocytic Leukemia guidelines should receive treatment.
Further, they noted, the median age of patients included in studies that they analyzed was 64 years; the median age of patients with CLL is 72 years. The prognostic abilities of IGHV mutation and FISH may differ in these older individuals with CLL.
The researchers analyzed 31 studies that met the criteria for inclusion – full-length publications that included at least 200 patients and reported on the prognostic value of IGHV and/or FISH for predicting progression-free or overall survival in patients with newly diagnosed CLL.
They found that the median progression-free survival (range, about 1-5 years) was significantly shorter for patients with unmutated IGHV genes, than was the median progression-free survival (range, about 9-19 years) for those with mutated IGHV genes. Similarly, the median overall survival was significantly shorter for patients with unmutated IGHV (range, about 3-10 years) than for those with mutated IGHV (range, about 18-26 years).
For patients with high-risk FISH (including del17p13 and del11q23), the median progression-free survival was significantly shorter (range, about 0.1-5 years) than for those with low/intermediate-risk FISH (including del13q, normal, and trisomy 12; range, about 1.5-22 years). Median overall survival also significantly differed, ranging from about 3-10 years for patients with high-risk FISH and from about 7.5-20.5 years for those with low/intermediate-risk FISH.
In multivariable analyses, the hazard ratio for high-risk FISH ranged from 1.3 to 4.7 for progression-free survival and from 0.9 to 8.2 for overall survival. In studies reporting the results of multivariable analysis, high-risk FISH remained an independent predictor of progression-free survival in 8 of 17 studies and of overall survival in 10 of 14 studies, including in 10 of 13 studies adjusting for the prognostic impact of IGHV.
In multivariable analyses, IGHV remained an independent predictor of progression-free survival in 15 of 18 studies, including 12 of 15 studies adjusting for the prognostic impact of FISH. IGHV remained an independent predictor of overall survival in 11 of 15 studies reporting the results of multivariable analysis, including 10 of 14 studies adjusting for the prognostic impact of FISH.
FROM BLOOD