User login
Disposable Navigation for Total Knee Arthroplasty
Total knee arthroplasty (TKA) continues to be a widely utilized treatment option for end-stage knee osteoarthritis, and the number of patients undergoing TKA is projected to continually increase over the next decade.1 Although TKA is highly successful for many patients, studies continue to report that approximately 20% of patients are dissatisfied after undergoing TKA, and nearly 25% of knee revisions are performed for instability or malalignment.2,3 Technological advances have been developed to help improve clinical outcomes and implant survivorship. Over the past decade, computer navigation and intraoperative guides have been introduced to help control surgical variables and overcome the limitations and inaccuracies of traditional mechanical instrumentation. Currently, there are a variety of technologies available to assist surgeons with component alignment, including extramedullary devices, computer-assisted navigation systems (CAS), and patient-specific instrumentation (PSI) that help achieve desired alignment goals.4,5
Computer-assisted navigation tools were introduced in an effort to improve implant alignment and clinical outcomes compared to traditional mechanical guides. Some argue that the use of computer-assisted surgery has a steep learning curve and successful use is dependent on the user’s experience; however, studies have suggested computer-assisted surgery may allow less experienced surgeons to reliably achieve anticipated intraoperative alignment goals with a low complication rate.6,7 Various studies have looked at computer-assisted TKA at short to mid-term follow-up, but few studies have reported long-term outcomes.6-9 de Steiger and colleagues10 recently found that computer-assisted TKA reduced the overall revision rate for aseptic loosening following TKA in patients younger than age 65 years, which suggests benefit of CAS for younger patients. Short-term follow-up has also shown the benefit of CAS TKA in patients with severe extra-articular deformity, where traditional instrumentation cannot be utilized.11 Currently, there is no consensus that computer-assisted TKA leads to improved postoperative patient reported outcomes, because many studies are limited by study design or small cohorts; however, current literature does show an improvement in component alignment as compared to mechanical instrumentation.9,12,13 As future implant and position targets are defined to improve implant survivorship and clinical outcomes in total joint arthroplasty, computer-assisted devices will be useful to help achieve more precise and accurate component positioning.
In addition to CAS devices, some companies have sought to improve TKA surgery by introducing PSI. PSI was introduced to improve component alignment in TKA, with the purported advantages of a shorter surgical time, decrease in the number of instruments needed, and improved clinical outcomes. PSI accuracy remains variable, which may be attributed to the various systems and implant designs in each study.14-17 In addition, advanced preoperative imaging is necessary, which further adds to the overall cost.17 While the recent advancement in technology may provide decreased costs at the time of surgery, the increased cost and time incurred by the patient preoperatively has not resulted in significantly better clinical outcomes.18,19 Additionally, recent work has not shown PSI to have superior accuracy as compared to currently available CAS devices.14 These findings suggest that the additional cost and time incurred by patients may limit the widespread use of PSI.
Although computer navigation has been shown to be more accurate than conventional instrumentation and PSI, the lack of improvement in long-term clinical outcome data has limited its use. In a meta-analysis, Bauwens and colleagues20 suggested that while navigated TKAs have improved component alignment outcomes as compared to conventional surgery, the clinical benefit remains unclear. Less than 5% of surgeons are currently using navigation systems due to the perceived learning curve, cost, additional surgical time, and imaging required to utilize these systems. Certain navigation systems can be seemingly cumbersome, with large consoles, increased number of instruments required, and optical instruments with line-of-sight issues. Recent technological advances have worked to decrease this challenge by using accelerometer- and gyroscope-based electronic components, which combine the accuracy of computer-assisted technology with the ease of use of mechanical guides.
Accelerometer and gyroscope technology systems, such as the iAssist system, are portable devices that do not require a large computer console or navigation arrays. This technology relies on accelerator-based navigation without additional preoperative imaging. A recent study demonstrated the iAssist had reproducible accuracy in component alignment that could be easily incorporated into the operating room without optical trackers.21 The use of portable computer-assisted devices provides a more compact and easily accessible technology that can be used to achieve accurate component alignment without additional large equipment in the operating room.22 These new handheld intraoperative tools have been introduced to place implants according to a preoperative plan in order to minimize failure due to preoperative extra-articular deformity or intraoperative technical miscues.23 Nam and colleagues24 used an accelerometer-based surgical navigation system to perform tibial resections in cadaveric models, and found that the accelerometer-based guide was accurate for tibial resection in both the coronal and sagittal planes. In a prospective randomized controlled trial evaluating 100 patients undergoing a TKA using either an accelerometer-based guide or conventional alignment methods, the authors showed that the accelerometer-based guide decreased outliers in tibial component alignment compared to conventional guides.25 In the accelerometer-based guide cohort, 95.7% of tibial components were within 2° of perpendicular to the tibial mechanical axis, compared to 68.1% in the conventional group (P < .001). These results suggested that portable accelerometer-based navigation allows surgeons to achieve satisfactory tibial component alignment with a decrease in the number of potential outliers.24,25 Similarly, Bugbee and colleagues26 found that accelerometer-based handheld navigation was accurate for tibial coronal and sagittal alignment and no additional surgical time was required compared to conventional techniques.
The relationship between knee alignment and clinical outcomes for TKA remains controversial. Regardless of the surgeon’s alignment preference, it is important to reliably and accurately execute the preoperative plan in a reproducible fashion. Advances in technology that assist with intraoperative component alignment can be useful, and may help decrease the incidence of implant malalignment in clinical practice.
Preoperative Planning and Intraoperative Technique
Preoperative planning is carried out in a manner identical to the use of conventional mechanical guides. Long leg films are taken for evaluation of overall limb alignment, and calibrated lateral images are taken for templating implant sizes. Lines are drawn on the images to determine the difference between the mechanical and anatomic axis of the femur, and a line drawn perpendicular to the mechanical axis is placed to show the expected bone cut. In similar fashion a perpendicular line to the tibial mechanical axis is also drawn to show the expected tibial resection. This preoperative plan allows the surgeon to have an additional intraoperative guide to ensure accuracy of the computer-assisted device.
After standard exposure, the distal femoral or proximal tibial cut can be made based on surgeon preference. The system being demonstrated in the accompanying photos is the KneeAlign 2 system (OrthAlign).
Distal Femoral Cut
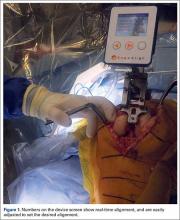
The KneeAlign 2 femoral cutting guide is attached to the distal femur with a central pin that is placed in the middle of the distal femur measured from medial to lateral, and 1 cm anterior to the intercondylar notch. It is important to note that this spot is often more medial than traditionally used for insertion of an intramedullary rod. This central point sets the distal point of the femoral mechanical axis. The device is then held in place with 2 oblique pins, and is solidly fixed to the bone. Using a rotating motion, the femur is rotated around the hip joint. The accelerometer and gyroscope in the unit are able to determine the center of the hip joint from this motion, creating the proximal point of the mechanical axis of the femur. Once the mechanical axis of the femur is determined, varus/valgus and flexion/extension can be adjusted on the guide. One adjustment screw is available for varus/valgus, and a second is available for flexion/extension. Numbers on the device screen show real-time alignment, and are easily adjusted to set the desired alignment (Figure 1). Once alignment is obtained, a mechanical stylus is used to determine depth of resection, and the distal femoral cutting block is pinned. After pinning the block, the 3 pins in the device are removed, and the device is removed from the bone. This leaves only the distal femoral cutting block in place. In experienced hands, this part of the procedure takes less than 3 minutes.
Proximal Tibial Cut
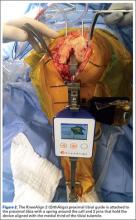
The KneeAlign 2 proximal tibial guide is similar in appearance to a standard mechanical tibial cutting guide. It is attached to the proximal tibia with a spring around the calf and 2 pins that hold the device aligned with the medial third of the tibial tubercle. A stylus is then centered on the anterior cruciate ligament (ACL) footprint, which sets the proximal mechanical axis point of the tibia (Figure 2). An offset number is read off the stylus on the ACL footprint, and this number is matched on the ankle offset portion of the guide. The device has 2 sensors. One sensor is on the chassis of the device, and the other is on a mobile arm. Movements between the 2 are monitored by the accelerometers, allowing for accurate maintenance of alignment position even with motion in the leg. A point is taken from the lateral malleolus and then a second point is taken from the medial malleolus. These points are used to determine the center of the ankle joint, which sets the distal mechanical axis point. Once mechanical axis of the tibia is determined, the tibial cutting guide is pinned in place, and can be adjusted with real-time guidance of the varus/valgus and posterior slope values seen on the device (Figure 3). Cut depth is once again determined with a mechanical stylus.
Limitations
Although these devices have proven to be very accurate, surgeons must continue to recognize that all tools can have errors. With computerized guides of any sort, these errors are usually user errors that cannot be detected by the device. Surgeons need to be able to recognize this and always double-check bone cuts for accuracy. Templating the bone cuts prior to surgery is an effective double-check. In addition, many handheld accelerometer devices do not currently assist with the rotational alignment of the femoral component. This is still performed using the surgeon’s preferred technique, and is a limitation of these systems.
Discussion
Currently, TKA provides satisfactory 10-year results with modern implant designs and survival rates as high as 90% to 95%.27,28 Even with good survival rates, a percentage of patients fail within the first 5 years.3 At a single institution, 50% of revision TKAs were related to instability, malalignment, or failure of fixation that occurred less than 2 years after the index procedure.29 In general, TKA with mechanical instrumentation provides satisfactory pain relief and postoperative knee function; however, studies have consistently shown that the use of advanced technology decreases the risk of implant malalignment, which may decrease early implant failure rates as compared to mechanical and some PSI.13,14,22 While there is a paucity of literature that has shown better clinical outcomes with the use of advanced technology, there are studies supporting the notion that proper limb alignment and component positioning improves implant survivorship.23,30
CAS may have additional advantages if the surgeon chooses to place the TKA in an alignment other than a neutral mechanical axis. Kinematic alignment for TKA has gained increasing popularity, where the target of a neutral mechanical axis alignment is not always the goal.31,32 The reported benefit is a more natural ligament tension with the hope of improving patient satisfaction. One concern with this technique is that it is a departure from the long-held teaching that a TKA aligned to a neutral mechanical axis is necessary for long-term implant survivorship.33,34 In addition, if the goal of surgery is to cut the tibia and femur at a specific varus/valgus cut, standard instrumentation may not allow for this level of accuracy. This in turn increases the risk of having a tibial or femoral cut that is outside the commonly accepted standards of alignment, which may lead to early implant failure. If further research suggests alignment is a variable that differs from patient to patient, the use of precise tools to ensure accuracy of executing the preoperatively templated alignment becomes even more important.
As the number of TKAs continues to rise each year, even a small percentage of malaligned knees that go on to revision surgery will create a large burden on the healthcare system.1,3 Although the short-term clinical benefits of CAS have not shown substantial differences as compared to conventional TKA, the number of knees aligned outside of a desired neutral mechanical axis alignment has been shown in multiple studies to be decreased with the use of advanced technology.7,12,34 Although CAS is an additional cost to a primary TKA, if the orthopedic community can decrease the number of TKA revisions due to malalignment from 6.6% to nearly zero, this may decrease the revision burden and overall cost to the healthcare system.1,3
TKA technology continues to evolve, and we must continue to assess each new advance not only to understand how it works, but also to ensure it addresses a specific clinical problem, and to be aware of the costs associated before incorporating it into routine practice. Some argue that the use of advanced technology requires increased surgical time, which in turn ultimately increases costs; however, one study has documented no increase in surgical time with handheld navigation while maintaining the accuracy of the device.34 In addition, no significant radiographic or clinical differences have been found between handheld navigation and larger console CAS systems, but large console systems have been associated with increased surgical times.22 The use of handheld accelerometer- and gyroscope-based guides has proven to provide reliable coronal and sagittal implant alignment that can easily be incorporated into the operating room. More widespread use of such technology will help decrease alignment outliers for TKA, and future long-term clinical outcome studies will be necessary to assess functional outcomes.
Conclusion
Advanced computer based technology offers an additional tool to the surgeon for reliably improving component positioning during TKA. The use of handheld accelerometer- and gyroscope-based guides increases the accuracy of component placement while decreasing the incidence of outliers compared to standard mechanical guides, without the need for a large computer console. Long-term radiographic and patient-reported outcomes are necessary to further validate these devices.
1. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
2. Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28( 8 Suppl):116-119.
4. Sassoon A, Nam D, Nunley R, Barrack R. Systematic review of patient-specific instrumentation in total knee arthroplasty: new but not improved. Clin Orthop Relat Res. 2015;473(1):151-158.
5. Anderson KC, Buehler KC, Markel DC. Computer assisted navigation in total knee arthroplasty: comparison with conventional methods. J Arthroplasty. 2005;20(7 Suppl 3):132-138.
6. Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22(8):1097-1106.
7. Khakha RS, Chowdhry M, Sivaprakasam M, Kheiran A, Chauhan SK. Radiological and functional outcomes in computer assisted total knee arthroplasty between consultants and trainees - a prospective randomized controlled trial. J Arthroplasty. 2015;30(8):1344-1347.
8. Zhu M, Ang CL, Yeo SJ, Lo NN, Chia SL, Chong HC. Minimally invasive computer-assisted total knee arthroplasty compared with conventional total knee arthroplasty: a prospective 9-year follow-up. J Arthroplasty. 2015. [Epub ahead of print]
9. Roberts TD, Clatworthy MG, Frampton CM, Young SW. Does computer assisted navigation improve functional outcomes and implant survivability after total knee arthroplasty? J Arthroplasty. 2015;30(9 Suppl):59-63.
10. de Steiger RN, Liu YL, Graves SE. Computer navigation for total knee arthroplasty reduces revision rate for patients less than sixty-five years of age. J Bone Joint Surg Am. 2015;97(8):635-642.
11. Fehring TK, Mason JB, Moskal J, Pollock DC, Mann J, Williams VJ. When computer-assisted knee replacement is the best alternative. Clin Orthop Relat Res. 2006;452:132-136.
12. Iorio R, Mazza D, Drogo P, et al. Clinical and radiographic outcomes of an accelerometer-based system for the tibial resection in total knee arthroplasty. Int Orthop. 2015;39(3):461-466.
13. Haaker RG, Stockheim M, Kamp M, Proff G, Breitenfelder J, Ottersbach A. Computer-assisted navigation increases precision of component placement in total knee arthroplasty. Clin Orthop Relat Res. 2005;433:152-159.
14. Ollivier M, Tribot-Laspiere Q, Amzallag J, Boisrenoult P, Pujol N, Beaufils P. Abnormal rate of intraoperative and postoperative implant positioning outliers using “MRI-based patient-specific” compared to “computer assisted” instrumentation in total knee replacement. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
15. Nunley RM, Ellison BS, Zhu J, Ruh EL, Howell SM, Barrack RL. Do patient-specific guides improve coronal alignment in total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):895-902.
16. Nunley RM, Ellison BS, Ruh EL, et al. Are patient-specific cutting blocks cost-effective for total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):889-894.
17. Barrack RL, Ruh EL, Williams BM, Ford AD, Foreman K, Nunley RM. Patient specific cutting blocks are currently of no proven value. J Bone Joint Surg Br. 2012;94(11 Suppl A):95-99.
18. Chen JY, Chin PL, Tay DK, Chia SL, Lo NN, Yeo SJ. Functional outcome and quality of life after patient-specific instrumentation in total knee arthroplasty. J Arthroplasty. 2015;30(10):1724-1728.
19. Goyal N, Patel AR, Yaffe MA, Luo MY, Stulberg SD. Does implant design influence the accuracy of patient specific instrumentation in total knee arthroplasty? J Arthroplasty. 2015;30(9):1526-1530.
20. Bauwens K, Matthes G, Wich M, et al. Navigated total knee replacement. A meta-analysis. J Bone Joint Surg Am. 2007;89(2):261-269.
21. Scuderi GR, Fallaha M, Masse V, Lavigne P, Amiot LP, Berthiaume MJ. Total knee arthroplasty with a novel navigation system within the surgical field. Orthop Clin North Am. 2014;45(2):167-173.
22. Goh GS, Liow MH, Lim WS, Tay DK, Yeo SJ, Tan MH. Accelerometer-based navigation is as accurate as optical computer navigation in restoring the joint line and mechanical axis after total knee arthroplasty: a prospective matched study. J Arthroplasty. 2016;31(1):92-97.
23. Berend KR, Lombardi AV Jr. Liberal indications for minimally invasive oxford unicondylar arthroplasty provide rapid functional recovery and pain relief. Surg Technol Int. 2007;16:193-197.
24. Nam D, Jerabek SA, Cross MB, Mayman DJ. Cadaveric analysis of an accelerometer-based portable navigation device for distal femoral cutting block alignment in total knee arthroplasty. Comput Aided Surg. 2012;17(4):205-210.
25. Nam D, Cody EA, Nguyen JT, Figgie MP, Mayman DJ. Extramedullary guides versus portable, accelerometer-based navigation for tibial alignment in total knee arthroplasty: a randomized, controlled trial: winner of the 2013 HAP PAUL award. J Arthroplasty. 2014;29(2):288-294.
26. Bugbee WD, Kermanshahi AY, Munro MM, McCauley JC, Copp SN. Accuracy of a hand-held surgical navigation system for tibial resection in total knee arthroplasty. Knee. 2014;21(6):1225-1228.
27. Schai PA, Thornhill TS, Scott RD. Total knee arthroplasty with the PFC system. Results at a minimum of ten years and survivorship analysis. J Bone Joint Surg Br. 1998;80(5):850-858.
28. Pradhan NR, Gambhir A, Porter ML. Survivorship analysis of 3234 primary knee arthroplasties implanted over a 26-year period: a study of eight different implant designs. Knee. 2006;13(1):7-11.
29. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
30. Fang DM, Ritter MA, Davis KE. Coronal alignment in total knee arthroplasty: just how important is it? J Arthroplasty. 2009;24(6 Suppl):39-43.
31. Cherian JJ, Kapadia BH, Banerjee S, Jauregui JJ, Issa K, Mont MA. Mechanical, anatomical, and kinematic axis in TKA: concepts and practical applications. Curr Rev Musculoskelet Med. 2014;7(2):89-95.
32. Howell SM, Papadopoulos S, Kuznik K, Ghaly LR, Hull ML. Does varus alignment adversely affect implant survival and function six years after kinematically aligned total knee arthroplasty? Int Orthop. 2015;39(11):2117-2124.
33. Ritter MA, Faris PM, Keating EM, Meding JB. Postoperative alignment of total knee replacement. Its effect on survival. Clin Orthop Relat Res. 1994;299:153-156.
34. Huang EH, Copp SN, Bugbee WD. Accuracy of a handheld accelerometer-based navigation system for femoral and tibial resection in total knee arthroplasty. J Arthroplasty. 2015;30(11):1906-1910.
Total knee arthroplasty (TKA) continues to be a widely utilized treatment option for end-stage knee osteoarthritis, and the number of patients undergoing TKA is projected to continually increase over the next decade.1 Although TKA is highly successful for many patients, studies continue to report that approximately 20% of patients are dissatisfied after undergoing TKA, and nearly 25% of knee revisions are performed for instability or malalignment.2,3 Technological advances have been developed to help improve clinical outcomes and implant survivorship. Over the past decade, computer navigation and intraoperative guides have been introduced to help control surgical variables and overcome the limitations and inaccuracies of traditional mechanical instrumentation. Currently, there are a variety of technologies available to assist surgeons with component alignment, including extramedullary devices, computer-assisted navigation systems (CAS), and patient-specific instrumentation (PSI) that help achieve desired alignment goals.4,5
Computer-assisted navigation tools were introduced in an effort to improve implant alignment and clinical outcomes compared to traditional mechanical guides. Some argue that the use of computer-assisted surgery has a steep learning curve and successful use is dependent on the user’s experience; however, studies have suggested computer-assisted surgery may allow less experienced surgeons to reliably achieve anticipated intraoperative alignment goals with a low complication rate.6,7 Various studies have looked at computer-assisted TKA at short to mid-term follow-up, but few studies have reported long-term outcomes.6-9 de Steiger and colleagues10 recently found that computer-assisted TKA reduced the overall revision rate for aseptic loosening following TKA in patients younger than age 65 years, which suggests benefit of CAS for younger patients. Short-term follow-up has also shown the benefit of CAS TKA in patients with severe extra-articular deformity, where traditional instrumentation cannot be utilized.11 Currently, there is no consensus that computer-assisted TKA leads to improved postoperative patient reported outcomes, because many studies are limited by study design or small cohorts; however, current literature does show an improvement in component alignment as compared to mechanical instrumentation.9,12,13 As future implant and position targets are defined to improve implant survivorship and clinical outcomes in total joint arthroplasty, computer-assisted devices will be useful to help achieve more precise and accurate component positioning.
In addition to CAS devices, some companies have sought to improve TKA surgery by introducing PSI. PSI was introduced to improve component alignment in TKA, with the purported advantages of a shorter surgical time, decrease in the number of instruments needed, and improved clinical outcomes. PSI accuracy remains variable, which may be attributed to the various systems and implant designs in each study.14-17 In addition, advanced preoperative imaging is necessary, which further adds to the overall cost.17 While the recent advancement in technology may provide decreased costs at the time of surgery, the increased cost and time incurred by the patient preoperatively has not resulted in significantly better clinical outcomes.18,19 Additionally, recent work has not shown PSI to have superior accuracy as compared to currently available CAS devices.14 These findings suggest that the additional cost and time incurred by patients may limit the widespread use of PSI.
Although computer navigation has been shown to be more accurate than conventional instrumentation and PSI, the lack of improvement in long-term clinical outcome data has limited its use. In a meta-analysis, Bauwens and colleagues20 suggested that while navigated TKAs have improved component alignment outcomes as compared to conventional surgery, the clinical benefit remains unclear. Less than 5% of surgeons are currently using navigation systems due to the perceived learning curve, cost, additional surgical time, and imaging required to utilize these systems. Certain navigation systems can be seemingly cumbersome, with large consoles, increased number of instruments required, and optical instruments with line-of-sight issues. Recent technological advances have worked to decrease this challenge by using accelerometer- and gyroscope-based electronic components, which combine the accuracy of computer-assisted technology with the ease of use of mechanical guides.
Accelerometer and gyroscope technology systems, such as the iAssist system, are portable devices that do not require a large computer console or navigation arrays. This technology relies on accelerator-based navigation without additional preoperative imaging. A recent study demonstrated the iAssist had reproducible accuracy in component alignment that could be easily incorporated into the operating room without optical trackers.21 The use of portable computer-assisted devices provides a more compact and easily accessible technology that can be used to achieve accurate component alignment without additional large equipment in the operating room.22 These new handheld intraoperative tools have been introduced to place implants according to a preoperative plan in order to minimize failure due to preoperative extra-articular deformity or intraoperative technical miscues.23 Nam and colleagues24 used an accelerometer-based surgical navigation system to perform tibial resections in cadaveric models, and found that the accelerometer-based guide was accurate for tibial resection in both the coronal and sagittal planes. In a prospective randomized controlled trial evaluating 100 patients undergoing a TKA using either an accelerometer-based guide or conventional alignment methods, the authors showed that the accelerometer-based guide decreased outliers in tibial component alignment compared to conventional guides.25 In the accelerometer-based guide cohort, 95.7% of tibial components were within 2° of perpendicular to the tibial mechanical axis, compared to 68.1% in the conventional group (P < .001). These results suggested that portable accelerometer-based navigation allows surgeons to achieve satisfactory tibial component alignment with a decrease in the number of potential outliers.24,25 Similarly, Bugbee and colleagues26 found that accelerometer-based handheld navigation was accurate for tibial coronal and sagittal alignment and no additional surgical time was required compared to conventional techniques.
The relationship between knee alignment and clinical outcomes for TKA remains controversial. Regardless of the surgeon’s alignment preference, it is important to reliably and accurately execute the preoperative plan in a reproducible fashion. Advances in technology that assist with intraoperative component alignment can be useful, and may help decrease the incidence of implant malalignment in clinical practice.
Preoperative Planning and Intraoperative Technique
Preoperative planning is carried out in a manner identical to the use of conventional mechanical guides. Long leg films are taken for evaluation of overall limb alignment, and calibrated lateral images are taken for templating implant sizes. Lines are drawn on the images to determine the difference between the mechanical and anatomic axis of the femur, and a line drawn perpendicular to the mechanical axis is placed to show the expected bone cut. In similar fashion a perpendicular line to the tibial mechanical axis is also drawn to show the expected tibial resection. This preoperative plan allows the surgeon to have an additional intraoperative guide to ensure accuracy of the computer-assisted device.
After standard exposure, the distal femoral or proximal tibial cut can be made based on surgeon preference. The system being demonstrated in the accompanying photos is the KneeAlign 2 system (OrthAlign).
Distal Femoral Cut
The KneeAlign 2 femoral cutting guide is attached to the distal femur with a central pin that is placed in the middle of the distal femur measured from medial to lateral, and 1 cm anterior to the intercondylar notch. It is important to note that this spot is often more medial than traditionally used for insertion of an intramedullary rod. This central point sets the distal point of the femoral mechanical axis. The device is then held in place with 2 oblique pins, and is solidly fixed to the bone. Using a rotating motion, the femur is rotated around the hip joint. The accelerometer and gyroscope in the unit are able to determine the center of the hip joint from this motion, creating the proximal point of the mechanical axis of the femur. Once the mechanical axis of the femur is determined, varus/valgus and flexion/extension can be adjusted on the guide. One adjustment screw is available for varus/valgus, and a second is available for flexion/extension. Numbers on the device screen show real-time alignment, and are easily adjusted to set the desired alignment (Figure 1). Once alignment is obtained, a mechanical stylus is used to determine depth of resection, and the distal femoral cutting block is pinned. After pinning the block, the 3 pins in the device are removed, and the device is removed from the bone. This leaves only the distal femoral cutting block in place. In experienced hands, this part of the procedure takes less than 3 minutes.
Proximal Tibial Cut
The KneeAlign 2 proximal tibial guide is similar in appearance to a standard mechanical tibial cutting guide. It is attached to the proximal tibia with a spring around the calf and 2 pins that hold the device aligned with the medial third of the tibial tubercle. A stylus is then centered on the anterior cruciate ligament (ACL) footprint, which sets the proximal mechanical axis point of the tibia (Figure 2). An offset number is read off the stylus on the ACL footprint, and this number is matched on the ankle offset portion of the guide. The device has 2 sensors. One sensor is on the chassis of the device, and the other is on a mobile arm. Movements between the 2 are monitored by the accelerometers, allowing for accurate maintenance of alignment position even with motion in the leg. A point is taken from the lateral malleolus and then a second point is taken from the medial malleolus. These points are used to determine the center of the ankle joint, which sets the distal mechanical axis point. Once mechanical axis of the tibia is determined, the tibial cutting guide is pinned in place, and can be adjusted with real-time guidance of the varus/valgus and posterior slope values seen on the device (Figure 3). Cut depth is once again determined with a mechanical stylus.
Limitations
Although these devices have proven to be very accurate, surgeons must continue to recognize that all tools can have errors. With computerized guides of any sort, these errors are usually user errors that cannot be detected by the device. Surgeons need to be able to recognize this and always double-check bone cuts for accuracy. Templating the bone cuts prior to surgery is an effective double-check. In addition, many handheld accelerometer devices do not currently assist with the rotational alignment of the femoral component. This is still performed using the surgeon’s preferred technique, and is a limitation of these systems.
Discussion
Currently, TKA provides satisfactory 10-year results with modern implant designs and survival rates as high as 90% to 95%.27,28 Even with good survival rates, a percentage of patients fail within the first 5 years.3 At a single institution, 50% of revision TKAs were related to instability, malalignment, or failure of fixation that occurred less than 2 years after the index procedure.29 In general, TKA with mechanical instrumentation provides satisfactory pain relief and postoperative knee function; however, studies have consistently shown that the use of advanced technology decreases the risk of implant malalignment, which may decrease early implant failure rates as compared to mechanical and some PSI.13,14,22 While there is a paucity of literature that has shown better clinical outcomes with the use of advanced technology, there are studies supporting the notion that proper limb alignment and component positioning improves implant survivorship.23,30
CAS may have additional advantages if the surgeon chooses to place the TKA in an alignment other than a neutral mechanical axis. Kinematic alignment for TKA has gained increasing popularity, where the target of a neutral mechanical axis alignment is not always the goal.31,32 The reported benefit is a more natural ligament tension with the hope of improving patient satisfaction. One concern with this technique is that it is a departure from the long-held teaching that a TKA aligned to a neutral mechanical axis is necessary for long-term implant survivorship.33,34 In addition, if the goal of surgery is to cut the tibia and femur at a specific varus/valgus cut, standard instrumentation may not allow for this level of accuracy. This in turn increases the risk of having a tibial or femoral cut that is outside the commonly accepted standards of alignment, which may lead to early implant failure. If further research suggests alignment is a variable that differs from patient to patient, the use of precise tools to ensure accuracy of executing the preoperatively templated alignment becomes even more important.
As the number of TKAs continues to rise each year, even a small percentage of malaligned knees that go on to revision surgery will create a large burden on the healthcare system.1,3 Although the short-term clinical benefits of CAS have not shown substantial differences as compared to conventional TKA, the number of knees aligned outside of a desired neutral mechanical axis alignment has been shown in multiple studies to be decreased with the use of advanced technology.7,12,34 Although CAS is an additional cost to a primary TKA, if the orthopedic community can decrease the number of TKA revisions due to malalignment from 6.6% to nearly zero, this may decrease the revision burden and overall cost to the healthcare system.1,3
TKA technology continues to evolve, and we must continue to assess each new advance not only to understand how it works, but also to ensure it addresses a specific clinical problem, and to be aware of the costs associated before incorporating it into routine practice. Some argue that the use of advanced technology requires increased surgical time, which in turn ultimately increases costs; however, one study has documented no increase in surgical time with handheld navigation while maintaining the accuracy of the device.34 In addition, no significant radiographic or clinical differences have been found between handheld navigation and larger console CAS systems, but large console systems have been associated with increased surgical times.22 The use of handheld accelerometer- and gyroscope-based guides has proven to provide reliable coronal and sagittal implant alignment that can easily be incorporated into the operating room. More widespread use of such technology will help decrease alignment outliers for TKA, and future long-term clinical outcome studies will be necessary to assess functional outcomes.
Conclusion
Advanced computer based technology offers an additional tool to the surgeon for reliably improving component positioning during TKA. The use of handheld accelerometer- and gyroscope-based guides increases the accuracy of component placement while decreasing the incidence of outliers compared to standard mechanical guides, without the need for a large computer console. Long-term radiographic and patient-reported outcomes are necessary to further validate these devices.
Total knee arthroplasty (TKA) continues to be a widely utilized treatment option for end-stage knee osteoarthritis, and the number of patients undergoing TKA is projected to continually increase over the next decade.1 Although TKA is highly successful for many patients, studies continue to report that approximately 20% of patients are dissatisfied after undergoing TKA, and nearly 25% of knee revisions are performed for instability or malalignment.2,3 Technological advances have been developed to help improve clinical outcomes and implant survivorship. Over the past decade, computer navigation and intraoperative guides have been introduced to help control surgical variables and overcome the limitations and inaccuracies of traditional mechanical instrumentation. Currently, there are a variety of technologies available to assist surgeons with component alignment, including extramedullary devices, computer-assisted navigation systems (CAS), and patient-specific instrumentation (PSI) that help achieve desired alignment goals.4,5
Computer-assisted navigation tools were introduced in an effort to improve implant alignment and clinical outcomes compared to traditional mechanical guides. Some argue that the use of computer-assisted surgery has a steep learning curve and successful use is dependent on the user’s experience; however, studies have suggested computer-assisted surgery may allow less experienced surgeons to reliably achieve anticipated intraoperative alignment goals with a low complication rate.6,7 Various studies have looked at computer-assisted TKA at short to mid-term follow-up, but few studies have reported long-term outcomes.6-9 de Steiger and colleagues10 recently found that computer-assisted TKA reduced the overall revision rate for aseptic loosening following TKA in patients younger than age 65 years, which suggests benefit of CAS for younger patients. Short-term follow-up has also shown the benefit of CAS TKA in patients with severe extra-articular deformity, where traditional instrumentation cannot be utilized.11 Currently, there is no consensus that computer-assisted TKA leads to improved postoperative patient reported outcomes, because many studies are limited by study design or small cohorts; however, current literature does show an improvement in component alignment as compared to mechanical instrumentation.9,12,13 As future implant and position targets are defined to improve implant survivorship and clinical outcomes in total joint arthroplasty, computer-assisted devices will be useful to help achieve more precise and accurate component positioning.
In addition to CAS devices, some companies have sought to improve TKA surgery by introducing PSI. PSI was introduced to improve component alignment in TKA, with the purported advantages of a shorter surgical time, decrease in the number of instruments needed, and improved clinical outcomes. PSI accuracy remains variable, which may be attributed to the various systems and implant designs in each study.14-17 In addition, advanced preoperative imaging is necessary, which further adds to the overall cost.17 While the recent advancement in technology may provide decreased costs at the time of surgery, the increased cost and time incurred by the patient preoperatively has not resulted in significantly better clinical outcomes.18,19 Additionally, recent work has not shown PSI to have superior accuracy as compared to currently available CAS devices.14 These findings suggest that the additional cost and time incurred by patients may limit the widespread use of PSI.
Although computer navigation has been shown to be more accurate than conventional instrumentation and PSI, the lack of improvement in long-term clinical outcome data has limited its use. In a meta-analysis, Bauwens and colleagues20 suggested that while navigated TKAs have improved component alignment outcomes as compared to conventional surgery, the clinical benefit remains unclear. Less than 5% of surgeons are currently using navigation systems due to the perceived learning curve, cost, additional surgical time, and imaging required to utilize these systems. Certain navigation systems can be seemingly cumbersome, with large consoles, increased number of instruments required, and optical instruments with line-of-sight issues. Recent technological advances have worked to decrease this challenge by using accelerometer- and gyroscope-based electronic components, which combine the accuracy of computer-assisted technology with the ease of use of mechanical guides.
Accelerometer and gyroscope technology systems, such as the iAssist system, are portable devices that do not require a large computer console or navigation arrays. This technology relies on accelerator-based navigation without additional preoperative imaging. A recent study demonstrated the iAssist had reproducible accuracy in component alignment that could be easily incorporated into the operating room without optical trackers.21 The use of portable computer-assisted devices provides a more compact and easily accessible technology that can be used to achieve accurate component alignment without additional large equipment in the operating room.22 These new handheld intraoperative tools have been introduced to place implants according to a preoperative plan in order to minimize failure due to preoperative extra-articular deformity or intraoperative technical miscues.23 Nam and colleagues24 used an accelerometer-based surgical navigation system to perform tibial resections in cadaveric models, and found that the accelerometer-based guide was accurate for tibial resection in both the coronal and sagittal planes. In a prospective randomized controlled trial evaluating 100 patients undergoing a TKA using either an accelerometer-based guide or conventional alignment methods, the authors showed that the accelerometer-based guide decreased outliers in tibial component alignment compared to conventional guides.25 In the accelerometer-based guide cohort, 95.7% of tibial components were within 2° of perpendicular to the tibial mechanical axis, compared to 68.1% in the conventional group (P < .001). These results suggested that portable accelerometer-based navigation allows surgeons to achieve satisfactory tibial component alignment with a decrease in the number of potential outliers.24,25 Similarly, Bugbee and colleagues26 found that accelerometer-based handheld navigation was accurate for tibial coronal and sagittal alignment and no additional surgical time was required compared to conventional techniques.
The relationship between knee alignment and clinical outcomes for TKA remains controversial. Regardless of the surgeon’s alignment preference, it is important to reliably and accurately execute the preoperative plan in a reproducible fashion. Advances in technology that assist with intraoperative component alignment can be useful, and may help decrease the incidence of implant malalignment in clinical practice.
Preoperative Planning and Intraoperative Technique
Preoperative planning is carried out in a manner identical to the use of conventional mechanical guides. Long leg films are taken for evaluation of overall limb alignment, and calibrated lateral images are taken for templating implant sizes. Lines are drawn on the images to determine the difference between the mechanical and anatomic axis of the femur, and a line drawn perpendicular to the mechanical axis is placed to show the expected bone cut. In similar fashion a perpendicular line to the tibial mechanical axis is also drawn to show the expected tibial resection. This preoperative plan allows the surgeon to have an additional intraoperative guide to ensure accuracy of the computer-assisted device.
After standard exposure, the distal femoral or proximal tibial cut can be made based on surgeon preference. The system being demonstrated in the accompanying photos is the KneeAlign 2 system (OrthAlign).
Distal Femoral Cut
The KneeAlign 2 femoral cutting guide is attached to the distal femur with a central pin that is placed in the middle of the distal femur measured from medial to lateral, and 1 cm anterior to the intercondylar notch. It is important to note that this spot is often more medial than traditionally used for insertion of an intramedullary rod. This central point sets the distal point of the femoral mechanical axis. The device is then held in place with 2 oblique pins, and is solidly fixed to the bone. Using a rotating motion, the femur is rotated around the hip joint. The accelerometer and gyroscope in the unit are able to determine the center of the hip joint from this motion, creating the proximal point of the mechanical axis of the femur. Once the mechanical axis of the femur is determined, varus/valgus and flexion/extension can be adjusted on the guide. One adjustment screw is available for varus/valgus, and a second is available for flexion/extension. Numbers on the device screen show real-time alignment, and are easily adjusted to set the desired alignment (Figure 1). Once alignment is obtained, a mechanical stylus is used to determine depth of resection, and the distal femoral cutting block is pinned. After pinning the block, the 3 pins in the device are removed, and the device is removed from the bone. This leaves only the distal femoral cutting block in place. In experienced hands, this part of the procedure takes less than 3 minutes.
Proximal Tibial Cut
The KneeAlign 2 proximal tibial guide is similar in appearance to a standard mechanical tibial cutting guide. It is attached to the proximal tibia with a spring around the calf and 2 pins that hold the device aligned with the medial third of the tibial tubercle. A stylus is then centered on the anterior cruciate ligament (ACL) footprint, which sets the proximal mechanical axis point of the tibia (Figure 2). An offset number is read off the stylus on the ACL footprint, and this number is matched on the ankle offset portion of the guide. The device has 2 sensors. One sensor is on the chassis of the device, and the other is on a mobile arm. Movements between the 2 are monitored by the accelerometers, allowing for accurate maintenance of alignment position even with motion in the leg. A point is taken from the lateral malleolus and then a second point is taken from the medial malleolus. These points are used to determine the center of the ankle joint, which sets the distal mechanical axis point. Once mechanical axis of the tibia is determined, the tibial cutting guide is pinned in place, and can be adjusted with real-time guidance of the varus/valgus and posterior slope values seen on the device (Figure 3). Cut depth is once again determined with a mechanical stylus.
Limitations
Although these devices have proven to be very accurate, surgeons must continue to recognize that all tools can have errors. With computerized guides of any sort, these errors are usually user errors that cannot be detected by the device. Surgeons need to be able to recognize this and always double-check bone cuts for accuracy. Templating the bone cuts prior to surgery is an effective double-check. In addition, many handheld accelerometer devices do not currently assist with the rotational alignment of the femoral component. This is still performed using the surgeon’s preferred technique, and is a limitation of these systems.
Discussion
Currently, TKA provides satisfactory 10-year results with modern implant designs and survival rates as high as 90% to 95%.27,28 Even with good survival rates, a percentage of patients fail within the first 5 years.3 At a single institution, 50% of revision TKAs were related to instability, malalignment, or failure of fixation that occurred less than 2 years after the index procedure.29 In general, TKA with mechanical instrumentation provides satisfactory pain relief and postoperative knee function; however, studies have consistently shown that the use of advanced technology decreases the risk of implant malalignment, which may decrease early implant failure rates as compared to mechanical and some PSI.13,14,22 While there is a paucity of literature that has shown better clinical outcomes with the use of advanced technology, there are studies supporting the notion that proper limb alignment and component positioning improves implant survivorship.23,30
CAS may have additional advantages if the surgeon chooses to place the TKA in an alignment other than a neutral mechanical axis. Kinematic alignment for TKA has gained increasing popularity, where the target of a neutral mechanical axis alignment is not always the goal.31,32 The reported benefit is a more natural ligament tension with the hope of improving patient satisfaction. One concern with this technique is that it is a departure from the long-held teaching that a TKA aligned to a neutral mechanical axis is necessary for long-term implant survivorship.33,34 In addition, if the goal of surgery is to cut the tibia and femur at a specific varus/valgus cut, standard instrumentation may not allow for this level of accuracy. This in turn increases the risk of having a tibial or femoral cut that is outside the commonly accepted standards of alignment, which may lead to early implant failure. If further research suggests alignment is a variable that differs from patient to patient, the use of precise tools to ensure accuracy of executing the preoperatively templated alignment becomes even more important.
As the number of TKAs continues to rise each year, even a small percentage of malaligned knees that go on to revision surgery will create a large burden on the healthcare system.1,3 Although the short-term clinical benefits of CAS have not shown substantial differences as compared to conventional TKA, the number of knees aligned outside of a desired neutral mechanical axis alignment has been shown in multiple studies to be decreased with the use of advanced technology.7,12,34 Although CAS is an additional cost to a primary TKA, if the orthopedic community can decrease the number of TKA revisions due to malalignment from 6.6% to nearly zero, this may decrease the revision burden and overall cost to the healthcare system.1,3
TKA technology continues to evolve, and we must continue to assess each new advance not only to understand how it works, but also to ensure it addresses a specific clinical problem, and to be aware of the costs associated before incorporating it into routine practice. Some argue that the use of advanced technology requires increased surgical time, which in turn ultimately increases costs; however, one study has documented no increase in surgical time with handheld navigation while maintaining the accuracy of the device.34 In addition, no significant radiographic or clinical differences have been found between handheld navigation and larger console CAS systems, but large console systems have been associated with increased surgical times.22 The use of handheld accelerometer- and gyroscope-based guides has proven to provide reliable coronal and sagittal implant alignment that can easily be incorporated into the operating room. More widespread use of such technology will help decrease alignment outliers for TKA, and future long-term clinical outcome studies will be necessary to assess functional outcomes.
Conclusion
Advanced computer based technology offers an additional tool to the surgeon for reliably improving component positioning during TKA. The use of handheld accelerometer- and gyroscope-based guides increases the accuracy of component placement while decreasing the incidence of outliers compared to standard mechanical guides, without the need for a large computer console. Long-term radiographic and patient-reported outcomes are necessary to further validate these devices.
1. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
2. Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28( 8 Suppl):116-119.
4. Sassoon A, Nam D, Nunley R, Barrack R. Systematic review of patient-specific instrumentation in total knee arthroplasty: new but not improved. Clin Orthop Relat Res. 2015;473(1):151-158.
5. Anderson KC, Buehler KC, Markel DC. Computer assisted navigation in total knee arthroplasty: comparison with conventional methods. J Arthroplasty. 2005;20(7 Suppl 3):132-138.
6. Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22(8):1097-1106.
7. Khakha RS, Chowdhry M, Sivaprakasam M, Kheiran A, Chauhan SK. Radiological and functional outcomes in computer assisted total knee arthroplasty between consultants and trainees - a prospective randomized controlled trial. J Arthroplasty. 2015;30(8):1344-1347.
8. Zhu M, Ang CL, Yeo SJ, Lo NN, Chia SL, Chong HC. Minimally invasive computer-assisted total knee arthroplasty compared with conventional total knee arthroplasty: a prospective 9-year follow-up. J Arthroplasty. 2015. [Epub ahead of print]
9. Roberts TD, Clatworthy MG, Frampton CM, Young SW. Does computer assisted navigation improve functional outcomes and implant survivability after total knee arthroplasty? J Arthroplasty. 2015;30(9 Suppl):59-63.
10. de Steiger RN, Liu YL, Graves SE. Computer navigation for total knee arthroplasty reduces revision rate for patients less than sixty-five years of age. J Bone Joint Surg Am. 2015;97(8):635-642.
11. Fehring TK, Mason JB, Moskal J, Pollock DC, Mann J, Williams VJ. When computer-assisted knee replacement is the best alternative. Clin Orthop Relat Res. 2006;452:132-136.
12. Iorio R, Mazza D, Drogo P, et al. Clinical and radiographic outcomes of an accelerometer-based system for the tibial resection in total knee arthroplasty. Int Orthop. 2015;39(3):461-466.
13. Haaker RG, Stockheim M, Kamp M, Proff G, Breitenfelder J, Ottersbach A. Computer-assisted navigation increases precision of component placement in total knee arthroplasty. Clin Orthop Relat Res. 2005;433:152-159.
14. Ollivier M, Tribot-Laspiere Q, Amzallag J, Boisrenoult P, Pujol N, Beaufils P. Abnormal rate of intraoperative and postoperative implant positioning outliers using “MRI-based patient-specific” compared to “computer assisted” instrumentation in total knee replacement. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
15. Nunley RM, Ellison BS, Zhu J, Ruh EL, Howell SM, Barrack RL. Do patient-specific guides improve coronal alignment in total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):895-902.
16. Nunley RM, Ellison BS, Ruh EL, et al. Are patient-specific cutting blocks cost-effective for total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):889-894.
17. Barrack RL, Ruh EL, Williams BM, Ford AD, Foreman K, Nunley RM. Patient specific cutting blocks are currently of no proven value. J Bone Joint Surg Br. 2012;94(11 Suppl A):95-99.
18. Chen JY, Chin PL, Tay DK, Chia SL, Lo NN, Yeo SJ. Functional outcome and quality of life after patient-specific instrumentation in total knee arthroplasty. J Arthroplasty. 2015;30(10):1724-1728.
19. Goyal N, Patel AR, Yaffe MA, Luo MY, Stulberg SD. Does implant design influence the accuracy of patient specific instrumentation in total knee arthroplasty? J Arthroplasty. 2015;30(9):1526-1530.
20. Bauwens K, Matthes G, Wich M, et al. Navigated total knee replacement. A meta-analysis. J Bone Joint Surg Am. 2007;89(2):261-269.
21. Scuderi GR, Fallaha M, Masse V, Lavigne P, Amiot LP, Berthiaume MJ. Total knee arthroplasty with a novel navigation system within the surgical field. Orthop Clin North Am. 2014;45(2):167-173.
22. Goh GS, Liow MH, Lim WS, Tay DK, Yeo SJ, Tan MH. Accelerometer-based navigation is as accurate as optical computer navigation in restoring the joint line and mechanical axis after total knee arthroplasty: a prospective matched study. J Arthroplasty. 2016;31(1):92-97.
23. Berend KR, Lombardi AV Jr. Liberal indications for minimally invasive oxford unicondylar arthroplasty provide rapid functional recovery and pain relief. Surg Technol Int. 2007;16:193-197.
24. Nam D, Jerabek SA, Cross MB, Mayman DJ. Cadaveric analysis of an accelerometer-based portable navigation device for distal femoral cutting block alignment in total knee arthroplasty. Comput Aided Surg. 2012;17(4):205-210.
25. Nam D, Cody EA, Nguyen JT, Figgie MP, Mayman DJ. Extramedullary guides versus portable, accelerometer-based navigation for tibial alignment in total knee arthroplasty: a randomized, controlled trial: winner of the 2013 HAP PAUL award. J Arthroplasty. 2014;29(2):288-294.
26. Bugbee WD, Kermanshahi AY, Munro MM, McCauley JC, Copp SN. Accuracy of a hand-held surgical navigation system for tibial resection in total knee arthroplasty. Knee. 2014;21(6):1225-1228.
27. Schai PA, Thornhill TS, Scott RD. Total knee arthroplasty with the PFC system. Results at a minimum of ten years and survivorship analysis. J Bone Joint Surg Br. 1998;80(5):850-858.
28. Pradhan NR, Gambhir A, Porter ML. Survivorship analysis of 3234 primary knee arthroplasties implanted over a 26-year period: a study of eight different implant designs. Knee. 2006;13(1):7-11.
29. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
30. Fang DM, Ritter MA, Davis KE. Coronal alignment in total knee arthroplasty: just how important is it? J Arthroplasty. 2009;24(6 Suppl):39-43.
31. Cherian JJ, Kapadia BH, Banerjee S, Jauregui JJ, Issa K, Mont MA. Mechanical, anatomical, and kinematic axis in TKA: concepts and practical applications. Curr Rev Musculoskelet Med. 2014;7(2):89-95.
32. Howell SM, Papadopoulos S, Kuznik K, Ghaly LR, Hull ML. Does varus alignment adversely affect implant survival and function six years after kinematically aligned total knee arthroplasty? Int Orthop. 2015;39(11):2117-2124.
33. Ritter MA, Faris PM, Keating EM, Meding JB. Postoperative alignment of total knee replacement. Its effect on survival. Clin Orthop Relat Res. 1994;299:153-156.
34. Huang EH, Copp SN, Bugbee WD. Accuracy of a handheld accelerometer-based navigation system for femoral and tibial resection in total knee arthroplasty. J Arthroplasty. 2015;30(11):1906-1910.
1. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
2. Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28( 8 Suppl):116-119.
4. Sassoon A, Nam D, Nunley R, Barrack R. Systematic review of patient-specific instrumentation in total knee arthroplasty: new but not improved. Clin Orthop Relat Res. 2015;473(1):151-158.
5. Anderson KC, Buehler KC, Markel DC. Computer assisted navigation in total knee arthroplasty: comparison with conventional methods. J Arthroplasty. 2005;20(7 Suppl 3):132-138.
6. Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22(8):1097-1106.
7. Khakha RS, Chowdhry M, Sivaprakasam M, Kheiran A, Chauhan SK. Radiological and functional outcomes in computer assisted total knee arthroplasty between consultants and trainees - a prospective randomized controlled trial. J Arthroplasty. 2015;30(8):1344-1347.
8. Zhu M, Ang CL, Yeo SJ, Lo NN, Chia SL, Chong HC. Minimally invasive computer-assisted total knee arthroplasty compared with conventional total knee arthroplasty: a prospective 9-year follow-up. J Arthroplasty. 2015. [Epub ahead of print]
9. Roberts TD, Clatworthy MG, Frampton CM, Young SW. Does computer assisted navigation improve functional outcomes and implant survivability after total knee arthroplasty? J Arthroplasty. 2015;30(9 Suppl):59-63.
10. de Steiger RN, Liu YL, Graves SE. Computer navigation for total knee arthroplasty reduces revision rate for patients less than sixty-five years of age. J Bone Joint Surg Am. 2015;97(8):635-642.
11. Fehring TK, Mason JB, Moskal J, Pollock DC, Mann J, Williams VJ. When computer-assisted knee replacement is the best alternative. Clin Orthop Relat Res. 2006;452:132-136.
12. Iorio R, Mazza D, Drogo P, et al. Clinical and radiographic outcomes of an accelerometer-based system for the tibial resection in total knee arthroplasty. Int Orthop. 2015;39(3):461-466.
13. Haaker RG, Stockheim M, Kamp M, Proff G, Breitenfelder J, Ottersbach A. Computer-assisted navigation increases precision of component placement in total knee arthroplasty. Clin Orthop Relat Res. 2005;433:152-159.
14. Ollivier M, Tribot-Laspiere Q, Amzallag J, Boisrenoult P, Pujol N, Beaufils P. Abnormal rate of intraoperative and postoperative implant positioning outliers using “MRI-based patient-specific” compared to “computer assisted” instrumentation in total knee replacement. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
15. Nunley RM, Ellison BS, Zhu J, Ruh EL, Howell SM, Barrack RL. Do patient-specific guides improve coronal alignment in total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):895-902.
16. Nunley RM, Ellison BS, Ruh EL, et al. Are patient-specific cutting blocks cost-effective for total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):889-894.
17. Barrack RL, Ruh EL, Williams BM, Ford AD, Foreman K, Nunley RM. Patient specific cutting blocks are currently of no proven value. J Bone Joint Surg Br. 2012;94(11 Suppl A):95-99.
18. Chen JY, Chin PL, Tay DK, Chia SL, Lo NN, Yeo SJ. Functional outcome and quality of life after patient-specific instrumentation in total knee arthroplasty. J Arthroplasty. 2015;30(10):1724-1728.
19. Goyal N, Patel AR, Yaffe MA, Luo MY, Stulberg SD. Does implant design influence the accuracy of patient specific instrumentation in total knee arthroplasty? J Arthroplasty. 2015;30(9):1526-1530.
20. Bauwens K, Matthes G, Wich M, et al. Navigated total knee replacement. A meta-analysis. J Bone Joint Surg Am. 2007;89(2):261-269.
21. Scuderi GR, Fallaha M, Masse V, Lavigne P, Amiot LP, Berthiaume MJ. Total knee arthroplasty with a novel navigation system within the surgical field. Orthop Clin North Am. 2014;45(2):167-173.
22. Goh GS, Liow MH, Lim WS, Tay DK, Yeo SJ, Tan MH. Accelerometer-based navigation is as accurate as optical computer navigation in restoring the joint line and mechanical axis after total knee arthroplasty: a prospective matched study. J Arthroplasty. 2016;31(1):92-97.
23. Berend KR, Lombardi AV Jr. Liberal indications for minimally invasive oxford unicondylar arthroplasty provide rapid functional recovery and pain relief. Surg Technol Int. 2007;16:193-197.
24. Nam D, Jerabek SA, Cross MB, Mayman DJ. Cadaveric analysis of an accelerometer-based portable navigation device for distal femoral cutting block alignment in total knee arthroplasty. Comput Aided Surg. 2012;17(4):205-210.
25. Nam D, Cody EA, Nguyen JT, Figgie MP, Mayman DJ. Extramedullary guides versus portable, accelerometer-based navigation for tibial alignment in total knee arthroplasty: a randomized, controlled trial: winner of the 2013 HAP PAUL award. J Arthroplasty. 2014;29(2):288-294.
26. Bugbee WD, Kermanshahi AY, Munro MM, McCauley JC, Copp SN. Accuracy of a hand-held surgical navigation system for tibial resection in total knee arthroplasty. Knee. 2014;21(6):1225-1228.
27. Schai PA, Thornhill TS, Scott RD. Total knee arthroplasty with the PFC system. Results at a minimum of ten years and survivorship analysis. J Bone Joint Surg Br. 1998;80(5):850-858.
28. Pradhan NR, Gambhir A, Porter ML. Survivorship analysis of 3234 primary knee arthroplasties implanted over a 26-year period: a study of eight different implant designs. Knee. 2006;13(1):7-11.
29. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
30. Fang DM, Ritter MA, Davis KE. Coronal alignment in total knee arthroplasty: just how important is it? J Arthroplasty. 2009;24(6 Suppl):39-43.
31. Cherian JJ, Kapadia BH, Banerjee S, Jauregui JJ, Issa K, Mont MA. Mechanical, anatomical, and kinematic axis in TKA: concepts and practical applications. Curr Rev Musculoskelet Med. 2014;7(2):89-95.
32. Howell SM, Papadopoulos S, Kuznik K, Ghaly LR, Hull ML. Does varus alignment adversely affect implant survival and function six years after kinematically aligned total knee arthroplasty? Int Orthop. 2015;39(11):2117-2124.
33. Ritter MA, Faris PM, Keating EM, Meding JB. Postoperative alignment of total knee replacement. Its effect on survival. Clin Orthop Relat Res. 1994;299:153-156.
34. Huang EH, Copp SN, Bugbee WD. Accuracy of a handheld accelerometer-based navigation system for femoral and tibial resection in total knee arthroplasty. J Arthroplasty. 2015;30(11):1906-1910.
Targeting a New Safe Zone: A Step in the Development of Patient-Specific Component Positioning for Total Hip Arthroplasty
Postoperative dislocation remains a common complication of primary total hip arthroplasties (THAs), affecting less than 1% to more than 10% in reported series.1,2 In large datasets for modern implants, the incidence of dislocation is 2% to 4%.3,4 Given that more than 200,000 THAs are performed in the United States each year,5 these low percentages represent a large number of patients. The multiplex patient variables that affect THA stability include age, sex, body mass index (BMI), and comorbid conditions.6-8 Surgical approach, restoration of leg length and femoral offset, femoral head size, and component positioning are also important surgical factors that can increase or decrease the incidence of dislocation.3,8,9 In particular, appropriate acetabular component orientation is crucial; surgeons can control this factor and thereby limit the occurrence of dislocation.10 Furthermore, acetabular malpositioning can increase the risk of liner fractures and accelerate bearing-surface wear.11-14
To minimize the risk of postoperative dislocation, surgeons traditionally have targeted the Lewinnek safe zone, with its mean (SD) inclination of 40° (10°) and mean (SD) anteversion of 15° (10°), for acetabular component orientation.15 However, the applicability of this target zone to preventing hip instability using modern implant designs, components, and surgical techniques remains unknown. Achieving acetabular orientation based on maximizing range of motion (ROM) before impingement may be optimal, with anteversion from 20° to 30° and inclination from 40° to 45°.16,17 Furthermore, mean (SD) native acetabular anteversion ranges from 21.3° (6.2°) for men to 24.6° (6.6°) for women.18 Placing THA acetabular components near the native range for anteversion may best provide impingement-free ROM and thus optimize THA stability,16,19 but this has not been proved in a clinical study.
Early dislocation is typically classified as occurring within 6 months after surgery,9 with almost 80% of dislocations occurring within 3 months after surgery.10 Surgeon-specific factors, such as acetabular component positioning, are thought to have a predominant effect on dislocations in the early postoperative period.10 Computer-assisted surgery (CAS), such as imageless navigation, is more accurate than conventional methods for acetabular component placement,20-23 but the clinical relevance of improving accuracy for acetabular component placement has not been shown with respect to altering patient outcomes.23
We conducted a study in a large single-surgeon patient cohort to determine the incidence of early postoperative dislocation with target anteversion increased to 25°, approximating mean native acetabular anteversion.16,19 In addition, we sought to determine the accuracy of imageless navigation in achieving target acetabular component placement.
Materials and Methods
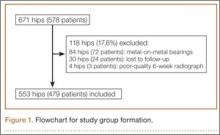
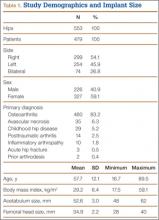
After obtaining institutional review board approval for this retrospective clinical study, we reviewed 671 consecutive cases of primary THA performed by a single surgeon using an imageless CAS system (AchieveCAS; Smith & Nephew, Memphis, Tennessee) between July 2006 and October 2012. THAs were excluded if a metal-on-metal bearing surface was used, if an adequate 6-week postoperative supine anteroposterior (AP) pelvis radiograph was unavailable, or if 6-month clinical follow-up findings were not available (Figure 1). The quality of AP radiographs was deemed poor if they were not centered on the symphysis pubis and if the sacrococcygeal joint was not centered over the symphysis pubis. After exclusion criteria were applied, 553 arthroplasties (479 patients) with a mean (SD) follow-up of 2.4 (1.4) years remained. Perioperative demographic data and component sizes are listed in Table 1.
During surgery, the anterior pelvic plane, defined by the anterior-superior iliac spines and pubic tubercle, was registered with the CAS system with the patient in the supine position. THA was performed with the patient in the lateral decubitus position using a posterolateral technique. For all patients, the surgeon used a hemispherical acetabular component (R3 Acetabular System; Smith & Nephew); bearings that were either metal on highly cross-linked polyethylene (XLPE) or Oxinium (Smith & Nephew) on XLPE; and neutral XLPE acetabular inserts. The goals for acetabular inclination and anteversion were 40° and 25°, respectively, with ±10° each for the target zone. The CAS system was used to adjust target anteversion for sagittal pelvic tilt.24 Uncemented femoral components were used for all patients, and the goal for femoral component anteversion was 15°. Transosseous repair of the posterior capsule and short external rotators was performed after component implantation.25
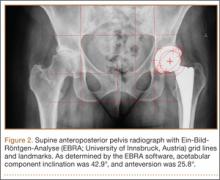
On each 6-week postoperative radiograph, acetabular orientation was measured with Ein-Bild-Röntgen-Analyse (EBRA; University of Innsbruck, Austria) software, which provides a validated method for measuring acetabular inclination and anteversion on supine AP pelvis radiographs.10,26 Pelvic boundaries were delineated with grid lines defining pelvic position. Reference points around the projections of the prosthetic femoral head, the hemispherical cup, and the rim of the cup were marked (Figure 2). EBRA calculated radiographic inclination and anteversion of the acetabular component based on the spatial position of the cup center in relation to the plane of the radiograph and the pelvic position.26
Charts were reviewed to identify patients with early postoperative dislocations, as well as dislocation timing, recurrence, and other characteristics. We defined early dislocation as instability occurring within 6 months after surgery. Revision surgery for instability was also identified.
For the statistical analysis, orientation error was defined as the absolute value of the difference between target orientation (40° inclination, 25° anteversion) and radiographic measurements. Repeated-measures multiple regression with the generalized estimating equations approach was used to identify baseline patient characteristics (age, sex, BMI, primary diagnosis, laterality) associated with component positioning outside of our targeted ranges for inclination and anteversion. Fisher exact tests were used to examine the relationship between dislocation and component placement in either the Lewinnek safe zone or our targeted zone. All tests were 2-sided with a significance level of .05. All analyses were performed with SAS for Windows 9.3 (SAS Institute, Cary, North Carolina).
Results
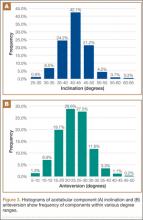
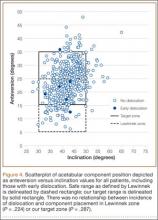
Mean (SD) acetabular inclination was 42.2° (4.9°) (range, 27.6°-65.0°), with a mean (SD) orientation error of 4.2° (3.4°) (Figure 3A). Mean (SD) anteversion was 23.9° (6.5°) (range, 6.2°-48.0°), with a mean (SD) orientation error of 5.2° (4.1°) (Figure 3B). Components were placed outside the Lewinnek safe zone for inclination or anteversion in 46.5% of cases and outside the target zone in 17.7% of cases (Figure 4). Variation in acetabular anteversion alone accounted for 67.3% of target zone outliers (Table 2). Only 0.9% of components were placed outside the target ranges for both inclination and anteversion.
Regression analysis was performed separately for inclination and anteversion to determine the risk factors for placing the acetabular component outside the target orientation ranges. Only higher BMI was associated with malposition with respect to inclination (hazard ratio [HR], 1.059; 95% confidence interval [CI], 1.011-1.111; P = .017). Of obese patients with inclination outside the target range, 90.9% had an inclination angle of more than 50°. Associations between inclination outside the target range and age (P = .769), sex (P = .217), preoperative diagnosis (P > .99), and laterality (P = .106) were statistically insignificant. Only female sex was associated with position of the acetabular component outside the target range for anteversion (HR, 1.871; 95% CI, 1.061-3.299; P = .030). Of female patients with anteversion outside the target range, 70.0% had anteversion of less than 15°. Associations between anteversion outside the target range and age (P = .762), BMI (P = .583), preoperative diagnosis (P > .99), and laterality (P = .235) were statistically insignificant.
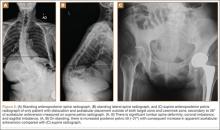
Six THAs (1.1%) in 6 patients experienced dislocation within 6 months after surgery (Table 3); mean (SD) time of dislocation was 58.3 (13.8) days after surgery. There was no relationship between dislocation incidence and component placement in the Lewinnek zone (P = .224) or our target zone (P = .287). Of the dislocation cases, 50% involved female patients, and 50% involved right hips. Mean (SD) age of these patients was 53.3 (7.6) years. Mean (SD) BMI was 25.4 (0.9) kg/m2. Osteoarthritis was the primary diagnosis for all patients with early dislocation; 32- or 36-mm femoral heads were used in these cases. Two patients had acetabular components placed outside of our target zone. One patient, who had abnormal pelvic obliquity and sagittal tilt from scoliosis (Figures 5A, 5B), had an acetabular component placed outside both the target zone and the Lewinnek safe zone. Mean (SD) acetabular inclination was 39.8° (3.6°), and mean (SD) anteversion was 21.8° (7.3°) (Figure 5C). Two dislocations resulted from trauma, 1 dislocation was related to hyperlaxity, 1 patient had cerebral palsy, and 1 patient had no evident predisposing risk factors. Three patients (0.54%) had multiple episodes of instability requiring revision during the follow-up period.
Discussion
To our knowledge, this study represents the largest cohort of primary THAs performed with an imageless navigation system. Our results showed that increasing targeted acetabular anteversion to 25° using a posterolateral surgical approach and modern implants resulted in a 1.1% incidence of early dislocation and a 0.54% incidence of recurrent instability requiring reoperation. Of the patients with a dislocation, only 1 did not experience trauma and did not have a risk factor for dislocation. Only 1 patient with a dislocation had acetabular components positioned outside both the target zone and the Lewinnek safe zone. The acetabular component was placed within the target zone in 82.3% of cases in which the imageless navigation system was used. In our cohort, BMI was the only risk factor for placement of the acetabular component outside our target range for inclination, and sex was associated with components outside the target range for anteversion.
Early dislocation after THA is often related to improper implant orientation, inadequate restoration of offset and myofascial tension, and decreased femoral head–neck ratio.8 Although dislocation rates in the literature vary widely,1,2 Medicare data suggest that the rate for the first 6 months after surgery can be as high as 4.21%.3,4 Although use of femoral heads with a diameter of 32 mm or larger may decrease this rate to 2.14%,3 accurate acetabular component orientation helps prevent postoperative dislocation.10 Using an imageless navigation system to target 25° of anteversion and 40° of inclination resulted in an early-dislocation rate about 49% less than the rate in a Medicare population treated with similar, modern implants.3
Callanan and colleagues11 found that freehand techniques were inaccurate for acetabular positioning in up to 50% of cases, and several studies have demonstrated that imageless navigation systems were more accurate than conventional guides.20,21,27-29 Higher BMI has been implicated as a risk factor for acetabular malpositioning in several studies of the accuracy of freehand techniques11 and imageless navigation techniques.23,30 Soft-tissue impediment to the component insertion handle poses a risk of increased inclination and inadequate anteversion, regardless of method used (conventional, CAS). When the acetabular component is placed freehand in obese patients, it is difficult to judge the position of the pelvis on the operating room table. For imageless navigation, a larger amount of adipose tissue over bony landmarks may limit the accuracy of anterior pelvic plane registration.30 Sex typically is not cited as a risk factor for inaccurate acetabular component positioning. We speculate that omitted-variable bias may explain the observed association between female sex and anteversion. For example, changes in postoperative pelvic tilt alter apparent anteversion on plain radiographs,31-34 but preoperative and postoperative sagittal pelvic tilt was not recorded in this study.
The proper position of the acetabular component has been debated.15,16,35,36 Although it is generally agreed that inclination of 40° ± 10° balances ROM, stability, and bearing-surface wear,12,13,15,16 proposed targets for anteversion vary widely, from 0° to 40°.35,36 Patel and colleagues16 formulated computer models based on cadaveric specimens to determine that THA impingement was minimized when the acetabular component was placed to match the native anteversion of the acetabulum.In their study model, 20° of anteversion paralleled native acetabular orientation. Tohtz and colleagues18 reviewed computed tomography scans of 144 female hips and 192 male hips and found that mean (SD) anteversion was 24.6° (6.6°) for women and 21.3° (6.2°) for men. Whether native anatomy is a valid reference for acetabular anteversion is controversial,19 and definitive recommendations for target anteversion cannot be made, as the effect of acetabular anteversion on the wear of various bearing materials is unknown.14 Yet, as with inclination, ideal anteversion is likely a compromise between maximizing impingement-free ROM and minimizing wear.
The present study had several limitations. A single-surgeon patient series was reviewed retrospectively, and there was no control group. We determined the incidence only of early dislocation, and 5.3% of THAs that were not metal-on-metal were either lost to follow-up or had inadequate radiographs. However, of the patients excluded for inadequate radiographs, none had an early dislocation. The effects of our surgical techniques on long-term outcomes, bearing wear, and dislocation are unknown. We were not able to comment on the direction of dislocation for any of the 6 patients with early dislocation, as all dislocations were reduced at facilities other than our hospital. Therefore, we cannot determine whether increasing acetabular anteversion resulted in a larger number of anterior versus posterior dislocations.15
We did not use CAS to place any of the femoral components. Therefore, we could not accurately target combined anteversion, defined as the sum of acetabular and femoral version, which may be an important determinant of THA stability.28 Although restoration of femoral offset and leg length is important in preventing THA dislocation,8 the CAS techniques used did not influence these parameters, and they were not measured.
As an imageless navigation system was used, there were no preoperative axial images, which could have been used to assess native acetabular orientation. This limited our assessment with respect to matching each patient’s natural anteversion. Imageless navigation, which references only the anterior pelvic plane, may not be reliable in patients with excessive sagittal pelvic tilt.37 Furthermore, changes in the functional position of the pelvis from supine to sitting to standing were not accounted for, and changes in sagittal tilt between these positions can be significant.38 Changes in sagittal pelvic tilt affect measurement of acetabular anteversion on plain radiographs, with anterior tilt reducing apparent anteversion and posterior tilt increasing it.32,34 Although postoperative computed tomography is the gold standard for assessing acetabular component orientation, EBRA significantly reduces errors of measurement on plain radiographs.10 Some variability in measured anteversion may be explained by our surgical technique. In particular, if the cup was uncovered anteriorly, additional anteversion was usually accepted during surgery to minimize anterior impingement and limit the risk of iliopsoas tendonitis.16,39
Our study results suggested that increasing target acetabular anteversion to 25° may reduce the incidence of early postoperative instability relative to rates reported in the literature. Despite the higher accuracy of component placement with an imageless navigation system, dislocations occurred in patients with acetabular components positioned in our target zone and in the historical safe zone. These dislocations support the notion that there likely is no absolute safe range for acetabular component positioning, as THA stability depends on many factors. Ideal targets for implant orientation for acetabulum and femur may be patient-specific.16,19 Investigators should prospectively evaluate patient-specific THA component positioning and determine its effect on postoperative dislocation and bearing-surface wear. As specific implant targets are further defined, tools that are more precise and accurate than conventional techniques will be needed to achieve goal component positioning. Our study results confirmed that imageless navigation is an accurate method for achieving acetabular orientation targets.
1. Kwon MS, Kuskowski M, Mulhall KJ, Macaulay W, Brown TE, Saleh KJ. Does surgical approach affect total hip arthroplasty dislocation rates? Clin Orthop. 2006;(447):34-38.
2. Sierra RJ, Raposo JM, Trousdale RT, Cabanela ME. Dislocation of primary THA done through a posterolateral approach in the elderly. Clin Orthop. 2005;(441):262-267.
3. Malkani AL, Ong KL, Lau E, Kurtz SM, Justice BJ, Manley MT. Early- and late-term dislocation risk after primary hip arthroplasty in the Medicare population. J Arthroplasty. 2010;25(6 suppl):21-25.
4. Berry DJ, von Knoch M, Schleck CD, Harmsen WS. Effect of femoral head diameter and operative approach on risk of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2005;87(11):2456-2463.
5. Nho SJ, Kymes SM, Callaghan JJ, Felson DT. The burden of hip osteoarthritis in the United States: epidemiologic and economic considerations. J Am Acad Orthop Surg. 2013;21(suppl 1):S1-S6.
6. Sadr Azodi O, Adami J, Lindstrom D, Eriksson KO, Wladis A, Bellocco R. High body mass index is associated with increased risk of implant dislocation following primary total hip replacement: 2,106 patients followed for up to 8 years. Acta Orthop. 2008;79(1):141-147.
7. Conroy JL, Whitehouse SL, Graves SE, Pratt NL, Ryan P, Crawford RW. Risk factors for revision for early dislocation in total hip arthroplasty. J Arthroplasty. 2008;23(6):867-872.
8. Morrey BF. Difficult complications after hip joint replacement. Dislocation. Clin Orthop. 1997;(344):179-187.
9. Ho KW, Whitwell GS, Young SK. Reducing the rate of early primary hip dislocation by combining a change in surgical technique and an increase in femoral head diameter to 36 mm. Arch Orthop Trauma Surg. 2012;132(7):1031-1036.
10. Biedermann R, Tonin A, Krismer M, Rachbauer F, Eibl G, Stockl B. Reducing the risk of dislocation after total hip arthroplasty: the effect of orientation of the acetabular component. J Bone Joint Surg Br. 2005;87(6):762-769.
11. Callanan MC, Jarrett B, Bragdon CR, et al. The John Charnley Award: risk factors for cup malpositioning: quality improvement through a joint registry at a tertiary hospital. Clin Orthop. 2011;469(2):319-329.
12. Gallo J, Havranek V, Zapletalova J. Risk factors for accelerated polyethylene wear and osteolysis in ABG I total hip arthroplasty. Int Orthop. 2010;34(1):19-26.
13. Leslie IJ, Williams S, Isaac G, Ingham E, Fisher J. High cup angle and microseparation increase the wear of hip surface replacements. Clin Orthop. 2009;467(9):2259-2265.
14. Esposito CI, Walter WL, Roques A, et al. Wear in alumina-on-alumina ceramic total hip replacements: a retrieval analysis of edge loading. J Bone Joint Surg Br. 2012;94(7):901-907.
15. Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60(2):217-220.
16. Patel AB, Wagle RR, Usrey MM, Thompson MT, Incavo SJ, Noble PC. Guidelines for implant placement to minimize impingement during activities of daily living after total hip arthroplasty. J Arthroplasty. 2010;25(8):1275-1281.e1.
17. Widmer KH, Zurfluh B. Compliant positioning of total hip components for optimal range of motion. J Orthop Res. 2004;22(4):815-821.
18. Tohtz SW, Sassy D, Matziolis G, Preininger B, Perka C, Hasart O. CT evaluation of native acetabular orientation and localization: sex-specific data comparison on 336 hip joints. Technol Health Care. 2010;18(2):129-136.
19. Merle C, Grammatopoulos G, Waldstein W, et al. Comparison of native anatomy with recommended safe component orientation in total hip arthroplasty for primary osteoarthritis. J Bone Joint Surg Am. 2013;95(22):e172.
20. Nogler M, Kessler O, Prassl A, et al. Reduced variability of acetabular cup positioning with use of an imageless navigation system. Clin Orthop. 2004;(426):159-163.
21. Wixson RL, MacDonald MA. Total hip arthroplasty through a minimal posterior approach using imageless computer-assisted hip navigation. J Arthroplasty. 2005;20(7 suppl 3):51-56.
22. Jolles BM, Genoud P, Hoffmeyer P. Computer-assisted cup placement techniques in total hip arthroplasty improve accuracy of placement. Clin Orthop. 2004;(426):174-179.
23. Lass R, Kubista B, Olischar B, Frantal S, Windhager R, Giurea A. Total hip arthroplasty using imageless computer-assisted hip navigation: a prospective randomized study. J Arthroplasty. 2014;29(4):786-791.
24. Babisch JW, Layher F, Amiot LP. The rationale for tilt-adjusted acetabular cup navigation. J Bone Joint Surg Am. 2008;90(2):357-365.
25. Pellicci PM, Bostrom M, Poss R. Posterior approach to total hip replacement using enhanced posterior soft tissue repair. Clin Orthop. 1998;(355):224-228.
26. Krismer M, Bauer R, Tschupik J, Mayrhofer P. EBRA: a method to measure migration of acetabular components. J Biomech. 1995;28(10):1225-1236.
27. Parratte S, Argenson JN. Validation and usefulness of a computer-assisted cup-positioning system in total hip arthroplasty. A prospective, randomized, controlled study. J Bone Joint Surg Am. 2007;89(3):494-499.
28. Dorr LD, Malik A, Wan Z, Long WT, Harris M. Precision and bias of imageless computer navigation and surgeon estimates for acetabular component position. Clin Orthop. 2007;(465):92-99.
29. Najarian BC, Kilgore JE, Markel DC. Evaluation of component positioning in primary total hip arthroplasty using an imageless navigation device compared with traditional methods. J Arthroplasty. 2009;24(1):15-21.
30. Hohmann E, Bryant A, Tetsworth K. Anterior pelvic soft tissue thickness influences acetabular cup positioning with imageless navigation. J Arthroplasty. 2012;27(6):945-952.
31. Nguyen AD, Shultz SJ. Sex differences in clinical measures of lower extremity alignment. J Orthop Sports Phys Ther. 2007;37(7):389-398.
32. Malik A, Wan Z, Jaramaz B, Bowman G, Dorr LD. A validation model for measurement of acetabular component position. J Arthroplasty. 2010;25(5):812-819.
33. Tannast M, Murphy SB, Langlotz F, Anderson SE, Siebenrock KA. Estimation of pelvic tilt on anteroposterior X-rays—a comparison of six parameters. Skeletal Radiol. 2006;35(3):149-155.
34. Parratte S, Pagnano MW, Coleman-Wood K, Kaufman KR, Berry DJ. The 2008 Frank Stinchfield Award: variation in postoperative pelvic tilt may confound the accuracy of hip navigation systems. Clin Orthop. 2009;467(1):43-49.
35. McCollum DE, Gray WJ. Dislocation after total hip arthroplasty. Causes and prevention. Clin Orthop. 1990;(261):159-170.
36. Kummer FJ, Shah S, Iyer S, DiCesare PE. The effect of acetabular cup orientations on limiting hip rotation. J Arthroplasty. 1999;14(4):509-513.
37. Lin F, Lim D, Wixson RL, Milos S, Hendrix RW, Makhsous M. Limitations of imageless computer-assisted navigation for total hip arthroplasty. J Arthroplasty. 2011;26(4):596-605.
38. Lazennec JY, Riwan A, Gravez F, et al. Hip spine relationships: application to total hip arthroplasty. Hip Int. 2007;17(suppl 5):S91-S104.
39. Trousdale RT, Cabanela ME, Berry DJ. Anterior iliopsoas impingement after total hip arthroplasty. J Arthroplasty. 1995;10(4):546-549.
Postoperative dislocation remains a common complication of primary total hip arthroplasties (THAs), affecting less than 1% to more than 10% in reported series.1,2 In large datasets for modern implants, the incidence of dislocation is 2% to 4%.3,4 Given that more than 200,000 THAs are performed in the United States each year,5 these low percentages represent a large number of patients. The multiplex patient variables that affect THA stability include age, sex, body mass index (BMI), and comorbid conditions.6-8 Surgical approach, restoration of leg length and femoral offset, femoral head size, and component positioning are also important surgical factors that can increase or decrease the incidence of dislocation.3,8,9 In particular, appropriate acetabular component orientation is crucial; surgeons can control this factor and thereby limit the occurrence of dislocation.10 Furthermore, acetabular malpositioning can increase the risk of liner fractures and accelerate bearing-surface wear.11-14
To minimize the risk of postoperative dislocation, surgeons traditionally have targeted the Lewinnek safe zone, with its mean (SD) inclination of 40° (10°) and mean (SD) anteversion of 15° (10°), for acetabular component orientation.15 However, the applicability of this target zone to preventing hip instability using modern implant designs, components, and surgical techniques remains unknown. Achieving acetabular orientation based on maximizing range of motion (ROM) before impingement may be optimal, with anteversion from 20° to 30° and inclination from 40° to 45°.16,17 Furthermore, mean (SD) native acetabular anteversion ranges from 21.3° (6.2°) for men to 24.6° (6.6°) for women.18 Placing THA acetabular components near the native range for anteversion may best provide impingement-free ROM and thus optimize THA stability,16,19 but this has not been proved in a clinical study.
Early dislocation is typically classified as occurring within 6 months after surgery,9 with almost 80% of dislocations occurring within 3 months after surgery.10 Surgeon-specific factors, such as acetabular component positioning, are thought to have a predominant effect on dislocations in the early postoperative period.10 Computer-assisted surgery (CAS), such as imageless navigation, is more accurate than conventional methods for acetabular component placement,20-23 but the clinical relevance of improving accuracy for acetabular component placement has not been shown with respect to altering patient outcomes.23
We conducted a study in a large single-surgeon patient cohort to determine the incidence of early postoperative dislocation with target anteversion increased to 25°, approximating mean native acetabular anteversion.16,19 In addition, we sought to determine the accuracy of imageless navigation in achieving target acetabular component placement.
Materials and Methods
After obtaining institutional review board approval for this retrospective clinical study, we reviewed 671 consecutive cases of primary THA performed by a single surgeon using an imageless CAS system (AchieveCAS; Smith & Nephew, Memphis, Tennessee) between July 2006 and October 2012. THAs were excluded if a metal-on-metal bearing surface was used, if an adequate 6-week postoperative supine anteroposterior (AP) pelvis radiograph was unavailable, or if 6-month clinical follow-up findings were not available (Figure 1). The quality of AP radiographs was deemed poor if they were not centered on the symphysis pubis and if the sacrococcygeal joint was not centered over the symphysis pubis. After exclusion criteria were applied, 553 arthroplasties (479 patients) with a mean (SD) follow-up of 2.4 (1.4) years remained. Perioperative demographic data and component sizes are listed in Table 1.
During surgery, the anterior pelvic plane, defined by the anterior-superior iliac spines and pubic tubercle, was registered with the CAS system with the patient in the supine position. THA was performed with the patient in the lateral decubitus position using a posterolateral technique. For all patients, the surgeon used a hemispherical acetabular component (R3 Acetabular System; Smith & Nephew); bearings that were either metal on highly cross-linked polyethylene (XLPE) or Oxinium (Smith & Nephew) on XLPE; and neutral XLPE acetabular inserts. The goals for acetabular inclination and anteversion were 40° and 25°, respectively, with ±10° each for the target zone. The CAS system was used to adjust target anteversion for sagittal pelvic tilt.24 Uncemented femoral components were used for all patients, and the goal for femoral component anteversion was 15°. Transosseous repair of the posterior capsule and short external rotators was performed after component implantation.25
On each 6-week postoperative radiograph, acetabular orientation was measured with Ein-Bild-Röntgen-Analyse (EBRA; University of Innsbruck, Austria) software, which provides a validated method for measuring acetabular inclination and anteversion on supine AP pelvis radiographs.10,26 Pelvic boundaries were delineated with grid lines defining pelvic position. Reference points around the projections of the prosthetic femoral head, the hemispherical cup, and the rim of the cup were marked (Figure 2). EBRA calculated radiographic inclination and anteversion of the acetabular component based on the spatial position of the cup center in relation to the plane of the radiograph and the pelvic position.26
Charts were reviewed to identify patients with early postoperative dislocations, as well as dislocation timing, recurrence, and other characteristics. We defined early dislocation as instability occurring within 6 months after surgery. Revision surgery for instability was also identified.
For the statistical analysis, orientation error was defined as the absolute value of the difference between target orientation (40° inclination, 25° anteversion) and radiographic measurements. Repeated-measures multiple regression with the generalized estimating equations approach was used to identify baseline patient characteristics (age, sex, BMI, primary diagnosis, laterality) associated with component positioning outside of our targeted ranges for inclination and anteversion. Fisher exact tests were used to examine the relationship between dislocation and component placement in either the Lewinnek safe zone or our targeted zone. All tests were 2-sided with a significance level of .05. All analyses were performed with SAS for Windows 9.3 (SAS Institute, Cary, North Carolina).
Results
Mean (SD) acetabular inclination was 42.2° (4.9°) (range, 27.6°-65.0°), with a mean (SD) orientation error of 4.2° (3.4°) (Figure 3A). Mean (SD) anteversion was 23.9° (6.5°) (range, 6.2°-48.0°), with a mean (SD) orientation error of 5.2° (4.1°) (Figure 3B). Components were placed outside the Lewinnek safe zone for inclination or anteversion in 46.5% of cases and outside the target zone in 17.7% of cases (Figure 4). Variation in acetabular anteversion alone accounted for 67.3% of target zone outliers (Table 2). Only 0.9% of components were placed outside the target ranges for both inclination and anteversion.
Regression analysis was performed separately for inclination and anteversion to determine the risk factors for placing the acetabular component outside the target orientation ranges. Only higher BMI was associated with malposition with respect to inclination (hazard ratio [HR], 1.059; 95% confidence interval [CI], 1.011-1.111; P = .017). Of obese patients with inclination outside the target range, 90.9% had an inclination angle of more than 50°. Associations between inclination outside the target range and age (P = .769), sex (P = .217), preoperative diagnosis (P > .99), and laterality (P = .106) were statistically insignificant. Only female sex was associated with position of the acetabular component outside the target range for anteversion (HR, 1.871; 95% CI, 1.061-3.299; P = .030). Of female patients with anteversion outside the target range, 70.0% had anteversion of less than 15°. Associations between anteversion outside the target range and age (P = .762), BMI (P = .583), preoperative diagnosis (P > .99), and laterality (P = .235) were statistically insignificant.
Six THAs (1.1%) in 6 patients experienced dislocation within 6 months after surgery (Table 3); mean (SD) time of dislocation was 58.3 (13.8) days after surgery. There was no relationship between dislocation incidence and component placement in the Lewinnek zone (P = .224) or our target zone (P = .287). Of the dislocation cases, 50% involved female patients, and 50% involved right hips. Mean (SD) age of these patients was 53.3 (7.6) years. Mean (SD) BMI was 25.4 (0.9) kg/m2. Osteoarthritis was the primary diagnosis for all patients with early dislocation; 32- or 36-mm femoral heads were used in these cases. Two patients had acetabular components placed outside of our target zone. One patient, who had abnormal pelvic obliquity and sagittal tilt from scoliosis (Figures 5A, 5B), had an acetabular component placed outside both the target zone and the Lewinnek safe zone. Mean (SD) acetabular inclination was 39.8° (3.6°), and mean (SD) anteversion was 21.8° (7.3°) (Figure 5C). Two dislocations resulted from trauma, 1 dislocation was related to hyperlaxity, 1 patient had cerebral palsy, and 1 patient had no evident predisposing risk factors. Three patients (0.54%) had multiple episodes of instability requiring revision during the follow-up period.
Discussion
To our knowledge, this study represents the largest cohort of primary THAs performed with an imageless navigation system. Our results showed that increasing targeted acetabular anteversion to 25° using a posterolateral surgical approach and modern implants resulted in a 1.1% incidence of early dislocation and a 0.54% incidence of recurrent instability requiring reoperation. Of the patients with a dislocation, only 1 did not experience trauma and did not have a risk factor for dislocation. Only 1 patient with a dislocation had acetabular components positioned outside both the target zone and the Lewinnek safe zone. The acetabular component was placed within the target zone in 82.3% of cases in which the imageless navigation system was used. In our cohort, BMI was the only risk factor for placement of the acetabular component outside our target range for inclination, and sex was associated with components outside the target range for anteversion.
Early dislocation after THA is often related to improper implant orientation, inadequate restoration of offset and myofascial tension, and decreased femoral head–neck ratio.8 Although dislocation rates in the literature vary widely,1,2 Medicare data suggest that the rate for the first 6 months after surgery can be as high as 4.21%.3,4 Although use of femoral heads with a diameter of 32 mm or larger may decrease this rate to 2.14%,3 accurate acetabular component orientation helps prevent postoperative dislocation.10 Using an imageless navigation system to target 25° of anteversion and 40° of inclination resulted in an early-dislocation rate about 49% less than the rate in a Medicare population treated with similar, modern implants.3
Callanan and colleagues11 found that freehand techniques were inaccurate for acetabular positioning in up to 50% of cases, and several studies have demonstrated that imageless navigation systems were more accurate than conventional guides.20,21,27-29 Higher BMI has been implicated as a risk factor for acetabular malpositioning in several studies of the accuracy of freehand techniques11 and imageless navigation techniques.23,30 Soft-tissue impediment to the component insertion handle poses a risk of increased inclination and inadequate anteversion, regardless of method used (conventional, CAS). When the acetabular component is placed freehand in obese patients, it is difficult to judge the position of the pelvis on the operating room table. For imageless navigation, a larger amount of adipose tissue over bony landmarks may limit the accuracy of anterior pelvic plane registration.30 Sex typically is not cited as a risk factor for inaccurate acetabular component positioning. We speculate that omitted-variable bias may explain the observed association between female sex and anteversion. For example, changes in postoperative pelvic tilt alter apparent anteversion on plain radiographs,31-34 but preoperative and postoperative sagittal pelvic tilt was not recorded in this study.
The proper position of the acetabular component has been debated.15,16,35,36 Although it is generally agreed that inclination of 40° ± 10° balances ROM, stability, and bearing-surface wear,12,13,15,16 proposed targets for anteversion vary widely, from 0° to 40°.35,36 Patel and colleagues16 formulated computer models based on cadaveric specimens to determine that THA impingement was minimized when the acetabular component was placed to match the native anteversion of the acetabulum.In their study model, 20° of anteversion paralleled native acetabular orientation. Tohtz and colleagues18 reviewed computed tomography scans of 144 female hips and 192 male hips and found that mean (SD) anteversion was 24.6° (6.6°) for women and 21.3° (6.2°) for men. Whether native anatomy is a valid reference for acetabular anteversion is controversial,19 and definitive recommendations for target anteversion cannot be made, as the effect of acetabular anteversion on the wear of various bearing materials is unknown.14 Yet, as with inclination, ideal anteversion is likely a compromise between maximizing impingement-free ROM and minimizing wear.
The present study had several limitations. A single-surgeon patient series was reviewed retrospectively, and there was no control group. We determined the incidence only of early dislocation, and 5.3% of THAs that were not metal-on-metal were either lost to follow-up or had inadequate radiographs. However, of the patients excluded for inadequate radiographs, none had an early dislocation. The effects of our surgical techniques on long-term outcomes, bearing wear, and dislocation are unknown. We were not able to comment on the direction of dislocation for any of the 6 patients with early dislocation, as all dislocations were reduced at facilities other than our hospital. Therefore, we cannot determine whether increasing acetabular anteversion resulted in a larger number of anterior versus posterior dislocations.15
We did not use CAS to place any of the femoral components. Therefore, we could not accurately target combined anteversion, defined as the sum of acetabular and femoral version, which may be an important determinant of THA stability.28 Although restoration of femoral offset and leg length is important in preventing THA dislocation,8 the CAS techniques used did not influence these parameters, and they were not measured.
As an imageless navigation system was used, there were no preoperative axial images, which could have been used to assess native acetabular orientation. This limited our assessment with respect to matching each patient’s natural anteversion. Imageless navigation, which references only the anterior pelvic plane, may not be reliable in patients with excessive sagittal pelvic tilt.37 Furthermore, changes in the functional position of the pelvis from supine to sitting to standing were not accounted for, and changes in sagittal tilt between these positions can be significant.38 Changes in sagittal pelvic tilt affect measurement of acetabular anteversion on plain radiographs, with anterior tilt reducing apparent anteversion and posterior tilt increasing it.32,34 Although postoperative computed tomography is the gold standard for assessing acetabular component orientation, EBRA significantly reduces errors of measurement on plain radiographs.10 Some variability in measured anteversion may be explained by our surgical technique. In particular, if the cup was uncovered anteriorly, additional anteversion was usually accepted during surgery to minimize anterior impingement and limit the risk of iliopsoas tendonitis.16,39
Our study results suggested that increasing target acetabular anteversion to 25° may reduce the incidence of early postoperative instability relative to rates reported in the literature. Despite the higher accuracy of component placement with an imageless navigation system, dislocations occurred in patients with acetabular components positioned in our target zone and in the historical safe zone. These dislocations support the notion that there likely is no absolute safe range for acetabular component positioning, as THA stability depends on many factors. Ideal targets for implant orientation for acetabulum and femur may be patient-specific.16,19 Investigators should prospectively evaluate patient-specific THA component positioning and determine its effect on postoperative dislocation and bearing-surface wear. As specific implant targets are further defined, tools that are more precise and accurate than conventional techniques will be needed to achieve goal component positioning. Our study results confirmed that imageless navigation is an accurate method for achieving acetabular orientation targets.
Postoperative dislocation remains a common complication of primary total hip arthroplasties (THAs), affecting less than 1% to more than 10% in reported series.1,2 In large datasets for modern implants, the incidence of dislocation is 2% to 4%.3,4 Given that more than 200,000 THAs are performed in the United States each year,5 these low percentages represent a large number of patients. The multiplex patient variables that affect THA stability include age, sex, body mass index (BMI), and comorbid conditions.6-8 Surgical approach, restoration of leg length and femoral offset, femoral head size, and component positioning are also important surgical factors that can increase or decrease the incidence of dislocation.3,8,9 In particular, appropriate acetabular component orientation is crucial; surgeons can control this factor and thereby limit the occurrence of dislocation.10 Furthermore, acetabular malpositioning can increase the risk of liner fractures and accelerate bearing-surface wear.11-14
To minimize the risk of postoperative dislocation, surgeons traditionally have targeted the Lewinnek safe zone, with its mean (SD) inclination of 40° (10°) and mean (SD) anteversion of 15° (10°), for acetabular component orientation.15 However, the applicability of this target zone to preventing hip instability using modern implant designs, components, and surgical techniques remains unknown. Achieving acetabular orientation based on maximizing range of motion (ROM) before impingement may be optimal, with anteversion from 20° to 30° and inclination from 40° to 45°.16,17 Furthermore, mean (SD) native acetabular anteversion ranges from 21.3° (6.2°) for men to 24.6° (6.6°) for women.18 Placing THA acetabular components near the native range for anteversion may best provide impingement-free ROM and thus optimize THA stability,16,19 but this has not been proved in a clinical study.
Early dislocation is typically classified as occurring within 6 months after surgery,9 with almost 80% of dislocations occurring within 3 months after surgery.10 Surgeon-specific factors, such as acetabular component positioning, are thought to have a predominant effect on dislocations in the early postoperative period.10 Computer-assisted surgery (CAS), such as imageless navigation, is more accurate than conventional methods for acetabular component placement,20-23 but the clinical relevance of improving accuracy for acetabular component placement has not been shown with respect to altering patient outcomes.23
We conducted a study in a large single-surgeon patient cohort to determine the incidence of early postoperative dislocation with target anteversion increased to 25°, approximating mean native acetabular anteversion.16,19 In addition, we sought to determine the accuracy of imageless navigation in achieving target acetabular component placement.
Materials and Methods
After obtaining institutional review board approval for this retrospective clinical study, we reviewed 671 consecutive cases of primary THA performed by a single surgeon using an imageless CAS system (AchieveCAS; Smith & Nephew, Memphis, Tennessee) between July 2006 and October 2012. THAs were excluded if a metal-on-metal bearing surface was used, if an adequate 6-week postoperative supine anteroposterior (AP) pelvis radiograph was unavailable, or if 6-month clinical follow-up findings were not available (Figure 1). The quality of AP radiographs was deemed poor if they were not centered on the symphysis pubis and if the sacrococcygeal joint was not centered over the symphysis pubis. After exclusion criteria were applied, 553 arthroplasties (479 patients) with a mean (SD) follow-up of 2.4 (1.4) years remained. Perioperative demographic data and component sizes are listed in Table 1.
During surgery, the anterior pelvic plane, defined by the anterior-superior iliac spines and pubic tubercle, was registered with the CAS system with the patient in the supine position. THA was performed with the patient in the lateral decubitus position using a posterolateral technique. For all patients, the surgeon used a hemispherical acetabular component (R3 Acetabular System; Smith & Nephew); bearings that were either metal on highly cross-linked polyethylene (XLPE) or Oxinium (Smith & Nephew) on XLPE; and neutral XLPE acetabular inserts. The goals for acetabular inclination and anteversion were 40° and 25°, respectively, with ±10° each for the target zone. The CAS system was used to adjust target anteversion for sagittal pelvic tilt.24 Uncemented femoral components were used for all patients, and the goal for femoral component anteversion was 15°. Transosseous repair of the posterior capsule and short external rotators was performed after component implantation.25
On each 6-week postoperative radiograph, acetabular orientation was measured with Ein-Bild-Röntgen-Analyse (EBRA; University of Innsbruck, Austria) software, which provides a validated method for measuring acetabular inclination and anteversion on supine AP pelvis radiographs.10,26 Pelvic boundaries were delineated with grid lines defining pelvic position. Reference points around the projections of the prosthetic femoral head, the hemispherical cup, and the rim of the cup were marked (Figure 2). EBRA calculated radiographic inclination and anteversion of the acetabular component based on the spatial position of the cup center in relation to the plane of the radiograph and the pelvic position.26
Charts were reviewed to identify patients with early postoperative dislocations, as well as dislocation timing, recurrence, and other characteristics. We defined early dislocation as instability occurring within 6 months after surgery. Revision surgery for instability was also identified.
For the statistical analysis, orientation error was defined as the absolute value of the difference between target orientation (40° inclination, 25° anteversion) and radiographic measurements. Repeated-measures multiple regression with the generalized estimating equations approach was used to identify baseline patient characteristics (age, sex, BMI, primary diagnosis, laterality) associated with component positioning outside of our targeted ranges for inclination and anteversion. Fisher exact tests were used to examine the relationship between dislocation and component placement in either the Lewinnek safe zone or our targeted zone. All tests were 2-sided with a significance level of .05. All analyses were performed with SAS for Windows 9.3 (SAS Institute, Cary, North Carolina).
Results
Mean (SD) acetabular inclination was 42.2° (4.9°) (range, 27.6°-65.0°), with a mean (SD) orientation error of 4.2° (3.4°) (Figure 3A). Mean (SD) anteversion was 23.9° (6.5°) (range, 6.2°-48.0°), with a mean (SD) orientation error of 5.2° (4.1°) (Figure 3B). Components were placed outside the Lewinnek safe zone for inclination or anteversion in 46.5% of cases and outside the target zone in 17.7% of cases (Figure 4). Variation in acetabular anteversion alone accounted for 67.3% of target zone outliers (Table 2). Only 0.9% of components were placed outside the target ranges for both inclination and anteversion.
Regression analysis was performed separately for inclination and anteversion to determine the risk factors for placing the acetabular component outside the target orientation ranges. Only higher BMI was associated with malposition with respect to inclination (hazard ratio [HR], 1.059; 95% confidence interval [CI], 1.011-1.111; P = .017). Of obese patients with inclination outside the target range, 90.9% had an inclination angle of more than 50°. Associations between inclination outside the target range and age (P = .769), sex (P = .217), preoperative diagnosis (P > .99), and laterality (P = .106) were statistically insignificant. Only female sex was associated with position of the acetabular component outside the target range for anteversion (HR, 1.871; 95% CI, 1.061-3.299; P = .030). Of female patients with anteversion outside the target range, 70.0% had anteversion of less than 15°. Associations between anteversion outside the target range and age (P = .762), BMI (P = .583), preoperative diagnosis (P > .99), and laterality (P = .235) were statistically insignificant.
Six THAs (1.1%) in 6 patients experienced dislocation within 6 months after surgery (Table 3); mean (SD) time of dislocation was 58.3 (13.8) days after surgery. There was no relationship between dislocation incidence and component placement in the Lewinnek zone (P = .224) or our target zone (P = .287). Of the dislocation cases, 50% involved female patients, and 50% involved right hips. Mean (SD) age of these patients was 53.3 (7.6) years. Mean (SD) BMI was 25.4 (0.9) kg/m2. Osteoarthritis was the primary diagnosis for all patients with early dislocation; 32- or 36-mm femoral heads were used in these cases. Two patients had acetabular components placed outside of our target zone. One patient, who had abnormal pelvic obliquity and sagittal tilt from scoliosis (Figures 5A, 5B), had an acetabular component placed outside both the target zone and the Lewinnek safe zone. Mean (SD) acetabular inclination was 39.8° (3.6°), and mean (SD) anteversion was 21.8° (7.3°) (Figure 5C). Two dislocations resulted from trauma, 1 dislocation was related to hyperlaxity, 1 patient had cerebral palsy, and 1 patient had no evident predisposing risk factors. Three patients (0.54%) had multiple episodes of instability requiring revision during the follow-up period.
Discussion
To our knowledge, this study represents the largest cohort of primary THAs performed with an imageless navigation system. Our results showed that increasing targeted acetabular anteversion to 25° using a posterolateral surgical approach and modern implants resulted in a 1.1% incidence of early dislocation and a 0.54% incidence of recurrent instability requiring reoperation. Of the patients with a dislocation, only 1 did not experience trauma and did not have a risk factor for dislocation. Only 1 patient with a dislocation had acetabular components positioned outside both the target zone and the Lewinnek safe zone. The acetabular component was placed within the target zone in 82.3% of cases in which the imageless navigation system was used. In our cohort, BMI was the only risk factor for placement of the acetabular component outside our target range for inclination, and sex was associated with components outside the target range for anteversion.
Early dislocation after THA is often related to improper implant orientation, inadequate restoration of offset and myofascial tension, and decreased femoral head–neck ratio.8 Although dislocation rates in the literature vary widely,1,2 Medicare data suggest that the rate for the first 6 months after surgery can be as high as 4.21%.3,4 Although use of femoral heads with a diameter of 32 mm or larger may decrease this rate to 2.14%,3 accurate acetabular component orientation helps prevent postoperative dislocation.10 Using an imageless navigation system to target 25° of anteversion and 40° of inclination resulted in an early-dislocation rate about 49% less than the rate in a Medicare population treated with similar, modern implants.3
Callanan and colleagues11 found that freehand techniques were inaccurate for acetabular positioning in up to 50% of cases, and several studies have demonstrated that imageless navigation systems were more accurate than conventional guides.20,21,27-29 Higher BMI has been implicated as a risk factor for acetabular malpositioning in several studies of the accuracy of freehand techniques11 and imageless navigation techniques.23,30 Soft-tissue impediment to the component insertion handle poses a risk of increased inclination and inadequate anteversion, regardless of method used (conventional, CAS). When the acetabular component is placed freehand in obese patients, it is difficult to judge the position of the pelvis on the operating room table. For imageless navigation, a larger amount of adipose tissue over bony landmarks may limit the accuracy of anterior pelvic plane registration.30 Sex typically is not cited as a risk factor for inaccurate acetabular component positioning. We speculate that omitted-variable bias may explain the observed association between female sex and anteversion. For example, changes in postoperative pelvic tilt alter apparent anteversion on plain radiographs,31-34 but preoperative and postoperative sagittal pelvic tilt was not recorded in this study.
The proper position of the acetabular component has been debated.15,16,35,36 Although it is generally agreed that inclination of 40° ± 10° balances ROM, stability, and bearing-surface wear,12,13,15,16 proposed targets for anteversion vary widely, from 0° to 40°.35,36 Patel and colleagues16 formulated computer models based on cadaveric specimens to determine that THA impingement was minimized when the acetabular component was placed to match the native anteversion of the acetabulum.In their study model, 20° of anteversion paralleled native acetabular orientation. Tohtz and colleagues18 reviewed computed tomography scans of 144 female hips and 192 male hips and found that mean (SD) anteversion was 24.6° (6.6°) for women and 21.3° (6.2°) for men. Whether native anatomy is a valid reference for acetabular anteversion is controversial,19 and definitive recommendations for target anteversion cannot be made, as the effect of acetabular anteversion on the wear of various bearing materials is unknown.14 Yet, as with inclination, ideal anteversion is likely a compromise between maximizing impingement-free ROM and minimizing wear.
The present study had several limitations. A single-surgeon patient series was reviewed retrospectively, and there was no control group. We determined the incidence only of early dislocation, and 5.3% of THAs that were not metal-on-metal were either lost to follow-up or had inadequate radiographs. However, of the patients excluded for inadequate radiographs, none had an early dislocation. The effects of our surgical techniques on long-term outcomes, bearing wear, and dislocation are unknown. We were not able to comment on the direction of dislocation for any of the 6 patients with early dislocation, as all dislocations were reduced at facilities other than our hospital. Therefore, we cannot determine whether increasing acetabular anteversion resulted in a larger number of anterior versus posterior dislocations.15
We did not use CAS to place any of the femoral components. Therefore, we could not accurately target combined anteversion, defined as the sum of acetabular and femoral version, which may be an important determinant of THA stability.28 Although restoration of femoral offset and leg length is important in preventing THA dislocation,8 the CAS techniques used did not influence these parameters, and they were not measured.
As an imageless navigation system was used, there were no preoperative axial images, which could have been used to assess native acetabular orientation. This limited our assessment with respect to matching each patient’s natural anteversion. Imageless navigation, which references only the anterior pelvic plane, may not be reliable in patients with excessive sagittal pelvic tilt.37 Furthermore, changes in the functional position of the pelvis from supine to sitting to standing were not accounted for, and changes in sagittal tilt between these positions can be significant.38 Changes in sagittal pelvic tilt affect measurement of acetabular anteversion on plain radiographs, with anterior tilt reducing apparent anteversion and posterior tilt increasing it.32,34 Although postoperative computed tomography is the gold standard for assessing acetabular component orientation, EBRA significantly reduces errors of measurement on plain radiographs.10 Some variability in measured anteversion may be explained by our surgical technique. In particular, if the cup was uncovered anteriorly, additional anteversion was usually accepted during surgery to minimize anterior impingement and limit the risk of iliopsoas tendonitis.16,39
Our study results suggested that increasing target acetabular anteversion to 25° may reduce the incidence of early postoperative instability relative to rates reported in the literature. Despite the higher accuracy of component placement with an imageless navigation system, dislocations occurred in patients with acetabular components positioned in our target zone and in the historical safe zone. These dislocations support the notion that there likely is no absolute safe range for acetabular component positioning, as THA stability depends on many factors. Ideal targets for implant orientation for acetabulum and femur may be patient-specific.16,19 Investigators should prospectively evaluate patient-specific THA component positioning and determine its effect on postoperative dislocation and bearing-surface wear. As specific implant targets are further defined, tools that are more precise and accurate than conventional techniques will be needed to achieve goal component positioning. Our study results confirmed that imageless navigation is an accurate method for achieving acetabular orientation targets.
1. Kwon MS, Kuskowski M, Mulhall KJ, Macaulay W, Brown TE, Saleh KJ. Does surgical approach affect total hip arthroplasty dislocation rates? Clin Orthop. 2006;(447):34-38.
2. Sierra RJ, Raposo JM, Trousdale RT, Cabanela ME. Dislocation of primary THA done through a posterolateral approach in the elderly. Clin Orthop. 2005;(441):262-267.
3. Malkani AL, Ong KL, Lau E, Kurtz SM, Justice BJ, Manley MT. Early- and late-term dislocation risk after primary hip arthroplasty in the Medicare population. J Arthroplasty. 2010;25(6 suppl):21-25.
4. Berry DJ, von Knoch M, Schleck CD, Harmsen WS. Effect of femoral head diameter and operative approach on risk of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2005;87(11):2456-2463.
5. Nho SJ, Kymes SM, Callaghan JJ, Felson DT. The burden of hip osteoarthritis in the United States: epidemiologic and economic considerations. J Am Acad Orthop Surg. 2013;21(suppl 1):S1-S6.
6. Sadr Azodi O, Adami J, Lindstrom D, Eriksson KO, Wladis A, Bellocco R. High body mass index is associated with increased risk of implant dislocation following primary total hip replacement: 2,106 patients followed for up to 8 years. Acta Orthop. 2008;79(1):141-147.
7. Conroy JL, Whitehouse SL, Graves SE, Pratt NL, Ryan P, Crawford RW. Risk factors for revision for early dislocation in total hip arthroplasty. J Arthroplasty. 2008;23(6):867-872.
8. Morrey BF. Difficult complications after hip joint replacement. Dislocation. Clin Orthop. 1997;(344):179-187.
9. Ho KW, Whitwell GS, Young SK. Reducing the rate of early primary hip dislocation by combining a change in surgical technique and an increase in femoral head diameter to 36 mm. Arch Orthop Trauma Surg. 2012;132(7):1031-1036.
10. Biedermann R, Tonin A, Krismer M, Rachbauer F, Eibl G, Stockl B. Reducing the risk of dislocation after total hip arthroplasty: the effect of orientation of the acetabular component. J Bone Joint Surg Br. 2005;87(6):762-769.
11. Callanan MC, Jarrett B, Bragdon CR, et al. The John Charnley Award: risk factors for cup malpositioning: quality improvement through a joint registry at a tertiary hospital. Clin Orthop. 2011;469(2):319-329.
12. Gallo J, Havranek V, Zapletalova J. Risk factors for accelerated polyethylene wear and osteolysis in ABG I total hip arthroplasty. Int Orthop. 2010;34(1):19-26.
13. Leslie IJ, Williams S, Isaac G, Ingham E, Fisher J. High cup angle and microseparation increase the wear of hip surface replacements. Clin Orthop. 2009;467(9):2259-2265.
14. Esposito CI, Walter WL, Roques A, et al. Wear in alumina-on-alumina ceramic total hip replacements: a retrieval analysis of edge loading. J Bone Joint Surg Br. 2012;94(7):901-907.
15. Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60(2):217-220.
16. Patel AB, Wagle RR, Usrey MM, Thompson MT, Incavo SJ, Noble PC. Guidelines for implant placement to minimize impingement during activities of daily living after total hip arthroplasty. J Arthroplasty. 2010;25(8):1275-1281.e1.
17. Widmer KH, Zurfluh B. Compliant positioning of total hip components for optimal range of motion. J Orthop Res. 2004;22(4):815-821.
18. Tohtz SW, Sassy D, Matziolis G, Preininger B, Perka C, Hasart O. CT evaluation of native acetabular orientation and localization: sex-specific data comparison on 336 hip joints. Technol Health Care. 2010;18(2):129-136.
19. Merle C, Grammatopoulos G, Waldstein W, et al. Comparison of native anatomy with recommended safe component orientation in total hip arthroplasty for primary osteoarthritis. J Bone Joint Surg Am. 2013;95(22):e172.
20. Nogler M, Kessler O, Prassl A, et al. Reduced variability of acetabular cup positioning with use of an imageless navigation system. Clin Orthop. 2004;(426):159-163.
21. Wixson RL, MacDonald MA. Total hip arthroplasty through a minimal posterior approach using imageless computer-assisted hip navigation. J Arthroplasty. 2005;20(7 suppl 3):51-56.
22. Jolles BM, Genoud P, Hoffmeyer P. Computer-assisted cup placement techniques in total hip arthroplasty improve accuracy of placement. Clin Orthop. 2004;(426):174-179.
23. Lass R, Kubista B, Olischar B, Frantal S, Windhager R, Giurea A. Total hip arthroplasty using imageless computer-assisted hip navigation: a prospective randomized study. J Arthroplasty. 2014;29(4):786-791.
24. Babisch JW, Layher F, Amiot LP. The rationale for tilt-adjusted acetabular cup navigation. J Bone Joint Surg Am. 2008;90(2):357-365.
25. Pellicci PM, Bostrom M, Poss R. Posterior approach to total hip replacement using enhanced posterior soft tissue repair. Clin Orthop. 1998;(355):224-228.
26. Krismer M, Bauer R, Tschupik J, Mayrhofer P. EBRA: a method to measure migration of acetabular components. J Biomech. 1995;28(10):1225-1236.
27. Parratte S, Argenson JN. Validation and usefulness of a computer-assisted cup-positioning system in total hip arthroplasty. A prospective, randomized, controlled study. J Bone Joint Surg Am. 2007;89(3):494-499.
28. Dorr LD, Malik A, Wan Z, Long WT, Harris M. Precision and bias of imageless computer navigation and surgeon estimates for acetabular component position. Clin Orthop. 2007;(465):92-99.
29. Najarian BC, Kilgore JE, Markel DC. Evaluation of component positioning in primary total hip arthroplasty using an imageless navigation device compared with traditional methods. J Arthroplasty. 2009;24(1):15-21.
30. Hohmann E, Bryant A, Tetsworth K. Anterior pelvic soft tissue thickness influences acetabular cup positioning with imageless navigation. J Arthroplasty. 2012;27(6):945-952.
31. Nguyen AD, Shultz SJ. Sex differences in clinical measures of lower extremity alignment. J Orthop Sports Phys Ther. 2007;37(7):389-398.
32. Malik A, Wan Z, Jaramaz B, Bowman G, Dorr LD. A validation model for measurement of acetabular component position. J Arthroplasty. 2010;25(5):812-819.
33. Tannast M, Murphy SB, Langlotz F, Anderson SE, Siebenrock KA. Estimation of pelvic tilt on anteroposterior X-rays—a comparison of six parameters. Skeletal Radiol. 2006;35(3):149-155.
34. Parratte S, Pagnano MW, Coleman-Wood K, Kaufman KR, Berry DJ. The 2008 Frank Stinchfield Award: variation in postoperative pelvic tilt may confound the accuracy of hip navigation systems. Clin Orthop. 2009;467(1):43-49.
35. McCollum DE, Gray WJ. Dislocation after total hip arthroplasty. Causes and prevention. Clin Orthop. 1990;(261):159-170.
36. Kummer FJ, Shah S, Iyer S, DiCesare PE. The effect of acetabular cup orientations on limiting hip rotation. J Arthroplasty. 1999;14(4):509-513.
37. Lin F, Lim D, Wixson RL, Milos S, Hendrix RW, Makhsous M. Limitations of imageless computer-assisted navigation for total hip arthroplasty. J Arthroplasty. 2011;26(4):596-605.
38. Lazennec JY, Riwan A, Gravez F, et al. Hip spine relationships: application to total hip arthroplasty. Hip Int. 2007;17(suppl 5):S91-S104.
39. Trousdale RT, Cabanela ME, Berry DJ. Anterior iliopsoas impingement after total hip arthroplasty. J Arthroplasty. 1995;10(4):546-549.
1. Kwon MS, Kuskowski M, Mulhall KJ, Macaulay W, Brown TE, Saleh KJ. Does surgical approach affect total hip arthroplasty dislocation rates? Clin Orthop. 2006;(447):34-38.
2. Sierra RJ, Raposo JM, Trousdale RT, Cabanela ME. Dislocation of primary THA done through a posterolateral approach in the elderly. Clin Orthop. 2005;(441):262-267.
3. Malkani AL, Ong KL, Lau E, Kurtz SM, Justice BJ, Manley MT. Early- and late-term dislocation risk after primary hip arthroplasty in the Medicare population. J Arthroplasty. 2010;25(6 suppl):21-25.
4. Berry DJ, von Knoch M, Schleck CD, Harmsen WS. Effect of femoral head diameter and operative approach on risk of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2005;87(11):2456-2463.
5. Nho SJ, Kymes SM, Callaghan JJ, Felson DT. The burden of hip osteoarthritis in the United States: epidemiologic and economic considerations. J Am Acad Orthop Surg. 2013;21(suppl 1):S1-S6.
6. Sadr Azodi O, Adami J, Lindstrom D, Eriksson KO, Wladis A, Bellocco R. High body mass index is associated with increased risk of implant dislocation following primary total hip replacement: 2,106 patients followed for up to 8 years. Acta Orthop. 2008;79(1):141-147.
7. Conroy JL, Whitehouse SL, Graves SE, Pratt NL, Ryan P, Crawford RW. Risk factors for revision for early dislocation in total hip arthroplasty. J Arthroplasty. 2008;23(6):867-872.
8. Morrey BF. Difficult complications after hip joint replacement. Dislocation. Clin Orthop. 1997;(344):179-187.
9. Ho KW, Whitwell GS, Young SK. Reducing the rate of early primary hip dislocation by combining a change in surgical technique and an increase in femoral head diameter to 36 mm. Arch Orthop Trauma Surg. 2012;132(7):1031-1036.
10. Biedermann R, Tonin A, Krismer M, Rachbauer F, Eibl G, Stockl B. Reducing the risk of dislocation after total hip arthroplasty: the effect of orientation of the acetabular component. J Bone Joint Surg Br. 2005;87(6):762-769.
11. Callanan MC, Jarrett B, Bragdon CR, et al. The John Charnley Award: risk factors for cup malpositioning: quality improvement through a joint registry at a tertiary hospital. Clin Orthop. 2011;469(2):319-329.
12. Gallo J, Havranek V, Zapletalova J. Risk factors for accelerated polyethylene wear and osteolysis in ABG I total hip arthroplasty. Int Orthop. 2010;34(1):19-26.
13. Leslie IJ, Williams S, Isaac G, Ingham E, Fisher J. High cup angle and microseparation increase the wear of hip surface replacements. Clin Orthop. 2009;467(9):2259-2265.
14. Esposito CI, Walter WL, Roques A, et al. Wear in alumina-on-alumina ceramic total hip replacements: a retrieval analysis of edge loading. J Bone Joint Surg Br. 2012;94(7):901-907.
15. Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60(2):217-220.
16. Patel AB, Wagle RR, Usrey MM, Thompson MT, Incavo SJ, Noble PC. Guidelines for implant placement to minimize impingement during activities of daily living after total hip arthroplasty. J Arthroplasty. 2010;25(8):1275-1281.e1.
17. Widmer KH, Zurfluh B. Compliant positioning of total hip components for optimal range of motion. J Orthop Res. 2004;22(4):815-821.
18. Tohtz SW, Sassy D, Matziolis G, Preininger B, Perka C, Hasart O. CT evaluation of native acetabular orientation and localization: sex-specific data comparison on 336 hip joints. Technol Health Care. 2010;18(2):129-136.
19. Merle C, Grammatopoulos G, Waldstein W, et al. Comparison of native anatomy with recommended safe component orientation in total hip arthroplasty for primary osteoarthritis. J Bone Joint Surg Am. 2013;95(22):e172.
20. Nogler M, Kessler O, Prassl A, et al. Reduced variability of acetabular cup positioning with use of an imageless navigation system. Clin Orthop. 2004;(426):159-163.
21. Wixson RL, MacDonald MA. Total hip arthroplasty through a minimal posterior approach using imageless computer-assisted hip navigation. J Arthroplasty. 2005;20(7 suppl 3):51-56.
22. Jolles BM, Genoud P, Hoffmeyer P. Computer-assisted cup placement techniques in total hip arthroplasty improve accuracy of placement. Clin Orthop. 2004;(426):174-179.
23. Lass R, Kubista B, Olischar B, Frantal S, Windhager R, Giurea A. Total hip arthroplasty using imageless computer-assisted hip navigation: a prospective randomized study. J Arthroplasty. 2014;29(4):786-791.
24. Babisch JW, Layher F, Amiot LP. The rationale for tilt-adjusted acetabular cup navigation. J Bone Joint Surg Am. 2008;90(2):357-365.
25. Pellicci PM, Bostrom M, Poss R. Posterior approach to total hip replacement using enhanced posterior soft tissue repair. Clin Orthop. 1998;(355):224-228.
26. Krismer M, Bauer R, Tschupik J, Mayrhofer P. EBRA: a method to measure migration of acetabular components. J Biomech. 1995;28(10):1225-1236.
27. Parratte S, Argenson JN. Validation and usefulness of a computer-assisted cup-positioning system in total hip arthroplasty. A prospective, randomized, controlled study. J Bone Joint Surg Am. 2007;89(3):494-499.
28. Dorr LD, Malik A, Wan Z, Long WT, Harris M. Precision and bias of imageless computer navigation and surgeon estimates for acetabular component position. Clin Orthop. 2007;(465):92-99.
29. Najarian BC, Kilgore JE, Markel DC. Evaluation of component positioning in primary total hip arthroplasty using an imageless navigation device compared with traditional methods. J Arthroplasty. 2009;24(1):15-21.
30. Hohmann E, Bryant A, Tetsworth K. Anterior pelvic soft tissue thickness influences acetabular cup positioning with imageless navigation. J Arthroplasty. 2012;27(6):945-952.
31. Nguyen AD, Shultz SJ. Sex differences in clinical measures of lower extremity alignment. J Orthop Sports Phys Ther. 2007;37(7):389-398.
32. Malik A, Wan Z, Jaramaz B, Bowman G, Dorr LD. A validation model for measurement of acetabular component position. J Arthroplasty. 2010;25(5):812-819.
33. Tannast M, Murphy SB, Langlotz F, Anderson SE, Siebenrock KA. Estimation of pelvic tilt on anteroposterior X-rays—a comparison of six parameters. Skeletal Radiol. 2006;35(3):149-155.
34. Parratte S, Pagnano MW, Coleman-Wood K, Kaufman KR, Berry DJ. The 2008 Frank Stinchfield Award: variation in postoperative pelvic tilt may confound the accuracy of hip navigation systems. Clin Orthop. 2009;467(1):43-49.
35. McCollum DE, Gray WJ. Dislocation after total hip arthroplasty. Causes and prevention. Clin Orthop. 1990;(261):159-170.
36. Kummer FJ, Shah S, Iyer S, DiCesare PE. The effect of acetabular cup orientations on limiting hip rotation. J Arthroplasty. 1999;14(4):509-513.
37. Lin F, Lim D, Wixson RL, Milos S, Hendrix RW, Makhsous M. Limitations of imageless computer-assisted navigation for total hip arthroplasty. J Arthroplasty. 2011;26(4):596-605.
38. Lazennec JY, Riwan A, Gravez F, et al. Hip spine relationships: application to total hip arthroplasty. Hip Int. 2007;17(suppl 5):S91-S104.
39. Trousdale RT, Cabanela ME, Berry DJ. Anterior iliopsoas impingement after total hip arthroplasty. J Arthroplasty. 1995;10(4):546-549.