User login
Disposable Navigation for Total Knee Arthroplasty
Total knee arthroplasty (TKA) continues to be a widely utilized treatment option for end-stage knee osteoarthritis, and the number of patients undergoing TKA is projected to continually increase over the next decade.1 Although TKA is highly successful for many patients, studies continue to report that approximately 20% of patients are dissatisfied after undergoing TKA, and nearly 25% of knee revisions are performed for instability or malalignment.2,3 Technological advances have been developed to help improve clinical outcomes and implant survivorship. Over the past decade, computer navigation and intraoperative guides have been introduced to help control surgical variables and overcome the limitations and inaccuracies of traditional mechanical instrumentation. Currently, there are a variety of technologies available to assist surgeons with component alignment, including extramedullary devices, computer-assisted navigation systems (CAS), and patient-specific instrumentation (PSI) that help achieve desired alignment goals.4,5
Computer-assisted navigation tools were introduced in an effort to improve implant alignment and clinical outcomes compared to traditional mechanical guides. Some argue that the use of computer-assisted surgery has a steep learning curve and successful use is dependent on the user’s experience; however, studies have suggested computer-assisted surgery may allow less experienced surgeons to reliably achieve anticipated intraoperative alignment goals with a low complication rate.6,7 Various studies have looked at computer-assisted TKA at short to mid-term follow-up, but few studies have reported long-term outcomes.6-9 de Steiger and colleagues10 recently found that computer-assisted TKA reduced the overall revision rate for aseptic loosening following TKA in patients younger than age 65 years, which suggests benefit of CAS for younger patients. Short-term follow-up has also shown the benefit of CAS TKA in patients with severe extra-articular deformity, where traditional instrumentation cannot be utilized.11 Currently, there is no consensus that computer-assisted TKA leads to improved postoperative patient reported outcomes, because many studies are limited by study design or small cohorts; however, current literature does show an improvement in component alignment as compared to mechanical instrumentation.9,12,13 As future implant and position targets are defined to improve implant survivorship and clinical outcomes in total joint arthroplasty, computer-assisted devices will be useful to help achieve more precise and accurate component positioning.
In addition to CAS devices, some companies have sought to improve TKA surgery by introducing PSI. PSI was introduced to improve component alignment in TKA, with the purported advantages of a shorter surgical time, decrease in the number of instruments needed, and improved clinical outcomes. PSI accuracy remains variable, which may be attributed to the various systems and implant designs in each study.14-17 In addition, advanced preoperative imaging is necessary, which further adds to the overall cost.17 While the recent advancement in technology may provide decreased costs at the time of surgery, the increased cost and time incurred by the patient preoperatively has not resulted in significantly better clinical outcomes.18,19 Additionally, recent work has not shown PSI to have superior accuracy as compared to currently available CAS devices.14 These findings suggest that the additional cost and time incurred by patients may limit the widespread use of PSI.
Although computer navigation has been shown to be more accurate than conventional instrumentation and PSI, the lack of improvement in long-term clinical outcome data has limited its use. In a meta-analysis, Bauwens and colleagues20 suggested that while navigated TKAs have improved component alignment outcomes as compared to conventional surgery, the clinical benefit remains unclear. Less than 5% of surgeons are currently using navigation systems due to the perceived learning curve, cost, additional surgical time, and imaging required to utilize these systems. Certain navigation systems can be seemingly cumbersome, with large consoles, increased number of instruments required, and optical instruments with line-of-sight issues. Recent technological advances have worked to decrease this challenge by using accelerometer- and gyroscope-based electronic components, which combine the accuracy of computer-assisted technology with the ease of use of mechanical guides.
Accelerometer and gyroscope technology systems, such as the iAssist system, are portable devices that do not require a large computer console or navigation arrays. This technology relies on accelerator-based navigation without additional preoperative imaging. A recent study demonstrated the iAssist had reproducible accuracy in component alignment that could be easily incorporated into the operating room without optical trackers.21 The use of portable computer-assisted devices provides a more compact and easily accessible technology that can be used to achieve accurate component alignment without additional large equipment in the operating room.22 These new handheld intraoperative tools have been introduced to place implants according to a preoperative plan in order to minimize failure due to preoperative extra-articular deformity or intraoperative technical miscues.23 Nam and colleagues24 used an accelerometer-based surgical navigation system to perform tibial resections in cadaveric models, and found that the accelerometer-based guide was accurate for tibial resection in both the coronal and sagittal planes. In a prospective randomized controlled trial evaluating 100 patients undergoing a TKA using either an accelerometer-based guide or conventional alignment methods, the authors showed that the accelerometer-based guide decreased outliers in tibial component alignment compared to conventional guides.25 In the accelerometer-based guide cohort, 95.7% of tibial components were within 2° of perpendicular to the tibial mechanical axis, compared to 68.1% in the conventional group (P < .001). These results suggested that portable accelerometer-based navigation allows surgeons to achieve satisfactory tibial component alignment with a decrease in the number of potential outliers.24,25 Similarly, Bugbee and colleagues26 found that accelerometer-based handheld navigation was accurate for tibial coronal and sagittal alignment and no additional surgical time was required compared to conventional techniques.
The relationship between knee alignment and clinical outcomes for TKA remains controversial. Regardless of the surgeon’s alignment preference, it is important to reliably and accurately execute the preoperative plan in a reproducible fashion. Advances in technology that assist with intraoperative component alignment can be useful, and may help decrease the incidence of implant malalignment in clinical practice.
Preoperative Planning and Intraoperative Technique
Preoperative planning is carried out in a manner identical to the use of conventional mechanical guides. Long leg films are taken for evaluation of overall limb alignment, and calibrated lateral images are taken for templating implant sizes. Lines are drawn on the images to determine the difference between the mechanical and anatomic axis of the femur, and a line drawn perpendicular to the mechanical axis is placed to show the expected bone cut. In similar fashion a perpendicular line to the tibial mechanical axis is also drawn to show the expected tibial resection. This preoperative plan allows the surgeon to have an additional intraoperative guide to ensure accuracy of the computer-assisted device.
After standard exposure, the distal femoral or proximal tibial cut can be made based on surgeon preference. The system being demonstrated in the accompanying photos is the KneeAlign 2 system (OrthAlign).
Distal Femoral Cut
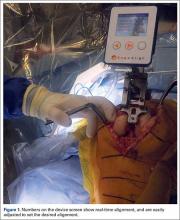
The KneeAlign 2 femoral cutting guide is attached to the distal femur with a central pin that is placed in the middle of the distal femur measured from medial to lateral, and 1 cm anterior to the intercondylar notch. It is important to note that this spot is often more medial than traditionally used for insertion of an intramedullary rod. This central point sets the distal point of the femoral mechanical axis. The device is then held in place with 2 oblique pins, and is solidly fixed to the bone. Using a rotating motion, the femur is rotated around the hip joint. The accelerometer and gyroscope in the unit are able to determine the center of the hip joint from this motion, creating the proximal point of the mechanical axis of the femur. Once the mechanical axis of the femur is determined, varus/valgus and flexion/extension can be adjusted on the guide. One adjustment screw is available for varus/valgus, and a second is available for flexion/extension. Numbers on the device screen show real-time alignment, and are easily adjusted to set the desired alignment (Figure 1). Once alignment is obtained, a mechanical stylus is used to determine depth of resection, and the distal femoral cutting block is pinned. After pinning the block, the 3 pins in the device are removed, and the device is removed from the bone. This leaves only the distal femoral cutting block in place. In experienced hands, this part of the procedure takes less than 3 minutes.
Proximal Tibial Cut
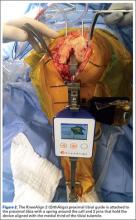
The KneeAlign 2 proximal tibial guide is similar in appearance to a standard mechanical tibial cutting guide. It is attached to the proximal tibia with a spring around the calf and 2 pins that hold the device aligned with the medial third of the tibial tubercle. A stylus is then centered on the anterior cruciate ligament (ACL) footprint, which sets the proximal mechanical axis point of the tibia (Figure 2). An offset number is read off the stylus on the ACL footprint, and this number is matched on the ankle offset portion of the guide. The device has 2 sensors. One sensor is on the chassis of the device, and the other is on a mobile arm. Movements between the 2 are monitored by the accelerometers, allowing for accurate maintenance of alignment position even with motion in the leg. A point is taken from the lateral malleolus and then a second point is taken from the medial malleolus. These points are used to determine the center of the ankle joint, which sets the distal mechanical axis point. Once mechanical axis of the tibia is determined, the tibial cutting guide is pinned in place, and can be adjusted with real-time guidance of the varus/valgus and posterior slope values seen on the device (Figure 3). Cut depth is once again determined with a mechanical stylus.
Limitations
Although these devices have proven to be very accurate, surgeons must continue to recognize that all tools can have errors. With computerized guides of any sort, these errors are usually user errors that cannot be detected by the device. Surgeons need to be able to recognize this and always double-check bone cuts for accuracy. Templating the bone cuts prior to surgery is an effective double-check. In addition, many handheld accelerometer devices do not currently assist with the rotational alignment of the femoral component. This is still performed using the surgeon’s preferred technique, and is a limitation of these systems.
Discussion
Currently, TKA provides satisfactory 10-year results with modern implant designs and survival rates as high as 90% to 95%.27,28 Even with good survival rates, a percentage of patients fail within the first 5 years.3 At a single institution, 50% of revision TKAs were related to instability, malalignment, or failure of fixation that occurred less than 2 years after the index procedure.29 In general, TKA with mechanical instrumentation provides satisfactory pain relief and postoperative knee function; however, studies have consistently shown that the use of advanced technology decreases the risk of implant malalignment, which may decrease early implant failure rates as compared to mechanical and some PSI.13,14,22 While there is a paucity of literature that has shown better clinical outcomes with the use of advanced technology, there are studies supporting the notion that proper limb alignment and component positioning improves implant survivorship.23,30
CAS may have additional advantages if the surgeon chooses to place the TKA in an alignment other than a neutral mechanical axis. Kinematic alignment for TKA has gained increasing popularity, where the target of a neutral mechanical axis alignment is not always the goal.31,32 The reported benefit is a more natural ligament tension with the hope of improving patient satisfaction. One concern with this technique is that it is a departure from the long-held teaching that a TKA aligned to a neutral mechanical axis is necessary for long-term implant survivorship.33,34 In addition, if the goal of surgery is to cut the tibia and femur at a specific varus/valgus cut, standard instrumentation may not allow for this level of accuracy. This in turn increases the risk of having a tibial or femoral cut that is outside the commonly accepted standards of alignment, which may lead to early implant failure. If further research suggests alignment is a variable that differs from patient to patient, the use of precise tools to ensure accuracy of executing the preoperatively templated alignment becomes even more important.
As the number of TKAs continues to rise each year, even a small percentage of malaligned knees that go on to revision surgery will create a large burden on the healthcare system.1,3 Although the short-term clinical benefits of CAS have not shown substantial differences as compared to conventional TKA, the number of knees aligned outside of a desired neutral mechanical axis alignment has been shown in multiple studies to be decreased with the use of advanced technology.7,12,34 Although CAS is an additional cost to a primary TKA, if the orthopedic community can decrease the number of TKA revisions due to malalignment from 6.6% to nearly zero, this may decrease the revision burden and overall cost to the healthcare system.1,3
TKA technology continues to evolve, and we must continue to assess each new advance not only to understand how it works, but also to ensure it addresses a specific clinical problem, and to be aware of the costs associated before incorporating it into routine practice. Some argue that the use of advanced technology requires increased surgical time, which in turn ultimately increases costs; however, one study has documented no increase in surgical time with handheld navigation while maintaining the accuracy of the device.34 In addition, no significant radiographic or clinical differences have been found between handheld navigation and larger console CAS systems, but large console systems have been associated with increased surgical times.22 The use of handheld accelerometer- and gyroscope-based guides has proven to provide reliable coronal and sagittal implant alignment that can easily be incorporated into the operating room. More widespread use of such technology will help decrease alignment outliers for TKA, and future long-term clinical outcome studies will be necessary to assess functional outcomes.
Conclusion
Advanced computer based technology offers an additional tool to the surgeon for reliably improving component positioning during TKA. The use of handheld accelerometer- and gyroscope-based guides increases the accuracy of component placement while decreasing the incidence of outliers compared to standard mechanical guides, without the need for a large computer console. Long-term radiographic and patient-reported outcomes are necessary to further validate these devices.
1. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
2. Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28( 8 Suppl):116-119.
4. Sassoon A, Nam D, Nunley R, Barrack R. Systematic review of patient-specific instrumentation in total knee arthroplasty: new but not improved. Clin Orthop Relat Res. 2015;473(1):151-158.
5. Anderson KC, Buehler KC, Markel DC. Computer assisted navigation in total knee arthroplasty: comparison with conventional methods. J Arthroplasty. 2005;20(7 Suppl 3):132-138.
6. Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22(8):1097-1106.
7. Khakha RS, Chowdhry M, Sivaprakasam M, Kheiran A, Chauhan SK. Radiological and functional outcomes in computer assisted total knee arthroplasty between consultants and trainees - a prospective randomized controlled trial. J Arthroplasty. 2015;30(8):1344-1347.
8. Zhu M, Ang CL, Yeo SJ, Lo NN, Chia SL, Chong HC. Minimally invasive computer-assisted total knee arthroplasty compared with conventional total knee arthroplasty: a prospective 9-year follow-up. J Arthroplasty. 2015. [Epub ahead of print]
9. Roberts TD, Clatworthy MG, Frampton CM, Young SW. Does computer assisted navigation improve functional outcomes and implant survivability after total knee arthroplasty? J Arthroplasty. 2015;30(9 Suppl):59-63.
10. de Steiger RN, Liu YL, Graves SE. Computer navigation for total knee arthroplasty reduces revision rate for patients less than sixty-five years of age. J Bone Joint Surg Am. 2015;97(8):635-642.
11. Fehring TK, Mason JB, Moskal J, Pollock DC, Mann J, Williams VJ. When computer-assisted knee replacement is the best alternative. Clin Orthop Relat Res. 2006;452:132-136.
12. Iorio R, Mazza D, Drogo P, et al. Clinical and radiographic outcomes of an accelerometer-based system for the tibial resection in total knee arthroplasty. Int Orthop. 2015;39(3):461-466.
13. Haaker RG, Stockheim M, Kamp M, Proff G, Breitenfelder J, Ottersbach A. Computer-assisted navigation increases precision of component placement in total knee arthroplasty. Clin Orthop Relat Res. 2005;433:152-159.
14. Ollivier M, Tribot-Laspiere Q, Amzallag J, Boisrenoult P, Pujol N, Beaufils P. Abnormal rate of intraoperative and postoperative implant positioning outliers using “MRI-based patient-specific” compared to “computer assisted” instrumentation in total knee replacement. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
15. Nunley RM, Ellison BS, Zhu J, Ruh EL, Howell SM, Barrack RL. Do patient-specific guides improve coronal alignment in total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):895-902.
16. Nunley RM, Ellison BS, Ruh EL, et al. Are patient-specific cutting blocks cost-effective for total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):889-894.
17. Barrack RL, Ruh EL, Williams BM, Ford AD, Foreman K, Nunley RM. Patient specific cutting blocks are currently of no proven value. J Bone Joint Surg Br. 2012;94(11 Suppl A):95-99.
18. Chen JY, Chin PL, Tay DK, Chia SL, Lo NN, Yeo SJ. Functional outcome and quality of life after patient-specific instrumentation in total knee arthroplasty. J Arthroplasty. 2015;30(10):1724-1728.
19. Goyal N, Patel AR, Yaffe MA, Luo MY, Stulberg SD. Does implant design influence the accuracy of patient specific instrumentation in total knee arthroplasty? J Arthroplasty. 2015;30(9):1526-1530.
20. Bauwens K, Matthes G, Wich M, et al. Navigated total knee replacement. A meta-analysis. J Bone Joint Surg Am. 2007;89(2):261-269.
21. Scuderi GR, Fallaha M, Masse V, Lavigne P, Amiot LP, Berthiaume MJ. Total knee arthroplasty with a novel navigation system within the surgical field. Orthop Clin North Am. 2014;45(2):167-173.
22. Goh GS, Liow MH, Lim WS, Tay DK, Yeo SJ, Tan MH. Accelerometer-based navigation is as accurate as optical computer navigation in restoring the joint line and mechanical axis after total knee arthroplasty: a prospective matched study. J Arthroplasty. 2016;31(1):92-97.
23. Berend KR, Lombardi AV Jr. Liberal indications for minimally invasive oxford unicondylar arthroplasty provide rapid functional recovery and pain relief. Surg Technol Int. 2007;16:193-197.
24. Nam D, Jerabek SA, Cross MB, Mayman DJ. Cadaveric analysis of an accelerometer-based portable navigation device for distal femoral cutting block alignment in total knee arthroplasty. Comput Aided Surg. 2012;17(4):205-210.
25. Nam D, Cody EA, Nguyen JT, Figgie MP, Mayman DJ. Extramedullary guides versus portable, accelerometer-based navigation for tibial alignment in total knee arthroplasty: a randomized, controlled trial: winner of the 2013 HAP PAUL award. J Arthroplasty. 2014;29(2):288-294.
26. Bugbee WD, Kermanshahi AY, Munro MM, McCauley JC, Copp SN. Accuracy of a hand-held surgical navigation system for tibial resection in total knee arthroplasty. Knee. 2014;21(6):1225-1228.
27. Schai PA, Thornhill TS, Scott RD. Total knee arthroplasty with the PFC system. Results at a minimum of ten years and survivorship analysis. J Bone Joint Surg Br. 1998;80(5):850-858.
28. Pradhan NR, Gambhir A, Porter ML. Survivorship analysis of 3234 primary knee arthroplasties implanted over a 26-year period: a study of eight different implant designs. Knee. 2006;13(1):7-11.
29. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
30. Fang DM, Ritter MA, Davis KE. Coronal alignment in total knee arthroplasty: just how important is it? J Arthroplasty. 2009;24(6 Suppl):39-43.
31. Cherian JJ, Kapadia BH, Banerjee S, Jauregui JJ, Issa K, Mont MA. Mechanical, anatomical, and kinematic axis in TKA: concepts and practical applications. Curr Rev Musculoskelet Med. 2014;7(2):89-95.
32. Howell SM, Papadopoulos S, Kuznik K, Ghaly LR, Hull ML. Does varus alignment adversely affect implant survival and function six years after kinematically aligned total knee arthroplasty? Int Orthop. 2015;39(11):2117-2124.
33. Ritter MA, Faris PM, Keating EM, Meding JB. Postoperative alignment of total knee replacement. Its effect on survival. Clin Orthop Relat Res. 1994;299:153-156.
34. Huang EH, Copp SN, Bugbee WD. Accuracy of a handheld accelerometer-based navigation system for femoral and tibial resection in total knee arthroplasty. J Arthroplasty. 2015;30(11):1906-1910.
Total knee arthroplasty (TKA) continues to be a widely utilized treatment option for end-stage knee osteoarthritis, and the number of patients undergoing TKA is projected to continually increase over the next decade.1 Although TKA is highly successful for many patients, studies continue to report that approximately 20% of patients are dissatisfied after undergoing TKA, and nearly 25% of knee revisions are performed for instability or malalignment.2,3 Technological advances have been developed to help improve clinical outcomes and implant survivorship. Over the past decade, computer navigation and intraoperative guides have been introduced to help control surgical variables and overcome the limitations and inaccuracies of traditional mechanical instrumentation. Currently, there are a variety of technologies available to assist surgeons with component alignment, including extramedullary devices, computer-assisted navigation systems (CAS), and patient-specific instrumentation (PSI) that help achieve desired alignment goals.4,5
Computer-assisted navigation tools were introduced in an effort to improve implant alignment and clinical outcomes compared to traditional mechanical guides. Some argue that the use of computer-assisted surgery has a steep learning curve and successful use is dependent on the user’s experience; however, studies have suggested computer-assisted surgery may allow less experienced surgeons to reliably achieve anticipated intraoperative alignment goals with a low complication rate.6,7 Various studies have looked at computer-assisted TKA at short to mid-term follow-up, but few studies have reported long-term outcomes.6-9 de Steiger and colleagues10 recently found that computer-assisted TKA reduced the overall revision rate for aseptic loosening following TKA in patients younger than age 65 years, which suggests benefit of CAS for younger patients. Short-term follow-up has also shown the benefit of CAS TKA in patients with severe extra-articular deformity, where traditional instrumentation cannot be utilized.11 Currently, there is no consensus that computer-assisted TKA leads to improved postoperative patient reported outcomes, because many studies are limited by study design or small cohorts; however, current literature does show an improvement in component alignment as compared to mechanical instrumentation.9,12,13 As future implant and position targets are defined to improve implant survivorship and clinical outcomes in total joint arthroplasty, computer-assisted devices will be useful to help achieve more precise and accurate component positioning.
In addition to CAS devices, some companies have sought to improve TKA surgery by introducing PSI. PSI was introduced to improve component alignment in TKA, with the purported advantages of a shorter surgical time, decrease in the number of instruments needed, and improved clinical outcomes. PSI accuracy remains variable, which may be attributed to the various systems and implant designs in each study.14-17 In addition, advanced preoperative imaging is necessary, which further adds to the overall cost.17 While the recent advancement in technology may provide decreased costs at the time of surgery, the increased cost and time incurred by the patient preoperatively has not resulted in significantly better clinical outcomes.18,19 Additionally, recent work has not shown PSI to have superior accuracy as compared to currently available CAS devices.14 These findings suggest that the additional cost and time incurred by patients may limit the widespread use of PSI.
Although computer navigation has been shown to be more accurate than conventional instrumentation and PSI, the lack of improvement in long-term clinical outcome data has limited its use. In a meta-analysis, Bauwens and colleagues20 suggested that while navigated TKAs have improved component alignment outcomes as compared to conventional surgery, the clinical benefit remains unclear. Less than 5% of surgeons are currently using navigation systems due to the perceived learning curve, cost, additional surgical time, and imaging required to utilize these systems. Certain navigation systems can be seemingly cumbersome, with large consoles, increased number of instruments required, and optical instruments with line-of-sight issues. Recent technological advances have worked to decrease this challenge by using accelerometer- and gyroscope-based electronic components, which combine the accuracy of computer-assisted technology with the ease of use of mechanical guides.
Accelerometer and gyroscope technology systems, such as the iAssist system, are portable devices that do not require a large computer console or navigation arrays. This technology relies on accelerator-based navigation without additional preoperative imaging. A recent study demonstrated the iAssist had reproducible accuracy in component alignment that could be easily incorporated into the operating room without optical trackers.21 The use of portable computer-assisted devices provides a more compact and easily accessible technology that can be used to achieve accurate component alignment without additional large equipment in the operating room.22 These new handheld intraoperative tools have been introduced to place implants according to a preoperative plan in order to minimize failure due to preoperative extra-articular deformity or intraoperative technical miscues.23 Nam and colleagues24 used an accelerometer-based surgical navigation system to perform tibial resections in cadaveric models, and found that the accelerometer-based guide was accurate for tibial resection in both the coronal and sagittal planes. In a prospective randomized controlled trial evaluating 100 patients undergoing a TKA using either an accelerometer-based guide or conventional alignment methods, the authors showed that the accelerometer-based guide decreased outliers in tibial component alignment compared to conventional guides.25 In the accelerometer-based guide cohort, 95.7% of tibial components were within 2° of perpendicular to the tibial mechanical axis, compared to 68.1% in the conventional group (P < .001). These results suggested that portable accelerometer-based navigation allows surgeons to achieve satisfactory tibial component alignment with a decrease in the number of potential outliers.24,25 Similarly, Bugbee and colleagues26 found that accelerometer-based handheld navigation was accurate for tibial coronal and sagittal alignment and no additional surgical time was required compared to conventional techniques.
The relationship between knee alignment and clinical outcomes for TKA remains controversial. Regardless of the surgeon’s alignment preference, it is important to reliably and accurately execute the preoperative plan in a reproducible fashion. Advances in technology that assist with intraoperative component alignment can be useful, and may help decrease the incidence of implant malalignment in clinical practice.
Preoperative Planning and Intraoperative Technique
Preoperative planning is carried out in a manner identical to the use of conventional mechanical guides. Long leg films are taken for evaluation of overall limb alignment, and calibrated lateral images are taken for templating implant sizes. Lines are drawn on the images to determine the difference between the mechanical and anatomic axis of the femur, and a line drawn perpendicular to the mechanical axis is placed to show the expected bone cut. In similar fashion a perpendicular line to the tibial mechanical axis is also drawn to show the expected tibial resection. This preoperative plan allows the surgeon to have an additional intraoperative guide to ensure accuracy of the computer-assisted device.
After standard exposure, the distal femoral or proximal tibial cut can be made based on surgeon preference. The system being demonstrated in the accompanying photos is the KneeAlign 2 system (OrthAlign).
Distal Femoral Cut
The KneeAlign 2 femoral cutting guide is attached to the distal femur with a central pin that is placed in the middle of the distal femur measured from medial to lateral, and 1 cm anterior to the intercondylar notch. It is important to note that this spot is often more medial than traditionally used for insertion of an intramedullary rod. This central point sets the distal point of the femoral mechanical axis. The device is then held in place with 2 oblique pins, and is solidly fixed to the bone. Using a rotating motion, the femur is rotated around the hip joint. The accelerometer and gyroscope in the unit are able to determine the center of the hip joint from this motion, creating the proximal point of the mechanical axis of the femur. Once the mechanical axis of the femur is determined, varus/valgus and flexion/extension can be adjusted on the guide. One adjustment screw is available for varus/valgus, and a second is available for flexion/extension. Numbers on the device screen show real-time alignment, and are easily adjusted to set the desired alignment (Figure 1). Once alignment is obtained, a mechanical stylus is used to determine depth of resection, and the distal femoral cutting block is pinned. After pinning the block, the 3 pins in the device are removed, and the device is removed from the bone. This leaves only the distal femoral cutting block in place. In experienced hands, this part of the procedure takes less than 3 minutes.
Proximal Tibial Cut
The KneeAlign 2 proximal tibial guide is similar in appearance to a standard mechanical tibial cutting guide. It is attached to the proximal tibia with a spring around the calf and 2 pins that hold the device aligned with the medial third of the tibial tubercle. A stylus is then centered on the anterior cruciate ligament (ACL) footprint, which sets the proximal mechanical axis point of the tibia (Figure 2). An offset number is read off the stylus on the ACL footprint, and this number is matched on the ankle offset portion of the guide. The device has 2 sensors. One sensor is on the chassis of the device, and the other is on a mobile arm. Movements between the 2 are monitored by the accelerometers, allowing for accurate maintenance of alignment position even with motion in the leg. A point is taken from the lateral malleolus and then a second point is taken from the medial malleolus. These points are used to determine the center of the ankle joint, which sets the distal mechanical axis point. Once mechanical axis of the tibia is determined, the tibial cutting guide is pinned in place, and can be adjusted with real-time guidance of the varus/valgus and posterior slope values seen on the device (Figure 3). Cut depth is once again determined with a mechanical stylus.
Limitations
Although these devices have proven to be very accurate, surgeons must continue to recognize that all tools can have errors. With computerized guides of any sort, these errors are usually user errors that cannot be detected by the device. Surgeons need to be able to recognize this and always double-check bone cuts for accuracy. Templating the bone cuts prior to surgery is an effective double-check. In addition, many handheld accelerometer devices do not currently assist with the rotational alignment of the femoral component. This is still performed using the surgeon’s preferred technique, and is a limitation of these systems.
Discussion
Currently, TKA provides satisfactory 10-year results with modern implant designs and survival rates as high as 90% to 95%.27,28 Even with good survival rates, a percentage of patients fail within the first 5 years.3 At a single institution, 50% of revision TKAs were related to instability, malalignment, or failure of fixation that occurred less than 2 years after the index procedure.29 In general, TKA with mechanical instrumentation provides satisfactory pain relief and postoperative knee function; however, studies have consistently shown that the use of advanced technology decreases the risk of implant malalignment, which may decrease early implant failure rates as compared to mechanical and some PSI.13,14,22 While there is a paucity of literature that has shown better clinical outcomes with the use of advanced technology, there are studies supporting the notion that proper limb alignment and component positioning improves implant survivorship.23,30
CAS may have additional advantages if the surgeon chooses to place the TKA in an alignment other than a neutral mechanical axis. Kinematic alignment for TKA has gained increasing popularity, where the target of a neutral mechanical axis alignment is not always the goal.31,32 The reported benefit is a more natural ligament tension with the hope of improving patient satisfaction. One concern with this technique is that it is a departure from the long-held teaching that a TKA aligned to a neutral mechanical axis is necessary for long-term implant survivorship.33,34 In addition, if the goal of surgery is to cut the tibia and femur at a specific varus/valgus cut, standard instrumentation may not allow for this level of accuracy. This in turn increases the risk of having a tibial or femoral cut that is outside the commonly accepted standards of alignment, which may lead to early implant failure. If further research suggests alignment is a variable that differs from patient to patient, the use of precise tools to ensure accuracy of executing the preoperatively templated alignment becomes even more important.
As the number of TKAs continues to rise each year, even a small percentage of malaligned knees that go on to revision surgery will create a large burden on the healthcare system.1,3 Although the short-term clinical benefits of CAS have not shown substantial differences as compared to conventional TKA, the number of knees aligned outside of a desired neutral mechanical axis alignment has been shown in multiple studies to be decreased with the use of advanced technology.7,12,34 Although CAS is an additional cost to a primary TKA, if the orthopedic community can decrease the number of TKA revisions due to malalignment from 6.6% to nearly zero, this may decrease the revision burden and overall cost to the healthcare system.1,3
TKA technology continues to evolve, and we must continue to assess each new advance not only to understand how it works, but also to ensure it addresses a specific clinical problem, and to be aware of the costs associated before incorporating it into routine practice. Some argue that the use of advanced technology requires increased surgical time, which in turn ultimately increases costs; however, one study has documented no increase in surgical time with handheld navigation while maintaining the accuracy of the device.34 In addition, no significant radiographic or clinical differences have been found between handheld navigation and larger console CAS systems, but large console systems have been associated with increased surgical times.22 The use of handheld accelerometer- and gyroscope-based guides has proven to provide reliable coronal and sagittal implant alignment that can easily be incorporated into the operating room. More widespread use of such technology will help decrease alignment outliers for TKA, and future long-term clinical outcome studies will be necessary to assess functional outcomes.
Conclusion
Advanced computer based technology offers an additional tool to the surgeon for reliably improving component positioning during TKA. The use of handheld accelerometer- and gyroscope-based guides increases the accuracy of component placement while decreasing the incidence of outliers compared to standard mechanical guides, without the need for a large computer console. Long-term radiographic and patient-reported outcomes are necessary to further validate these devices.
Total knee arthroplasty (TKA) continues to be a widely utilized treatment option for end-stage knee osteoarthritis, and the number of patients undergoing TKA is projected to continually increase over the next decade.1 Although TKA is highly successful for many patients, studies continue to report that approximately 20% of patients are dissatisfied after undergoing TKA, and nearly 25% of knee revisions are performed for instability or malalignment.2,3 Technological advances have been developed to help improve clinical outcomes and implant survivorship. Over the past decade, computer navigation and intraoperative guides have been introduced to help control surgical variables and overcome the limitations and inaccuracies of traditional mechanical instrumentation. Currently, there are a variety of technologies available to assist surgeons with component alignment, including extramedullary devices, computer-assisted navigation systems (CAS), and patient-specific instrumentation (PSI) that help achieve desired alignment goals.4,5
Computer-assisted navigation tools were introduced in an effort to improve implant alignment and clinical outcomes compared to traditional mechanical guides. Some argue that the use of computer-assisted surgery has a steep learning curve and successful use is dependent on the user’s experience; however, studies have suggested computer-assisted surgery may allow less experienced surgeons to reliably achieve anticipated intraoperative alignment goals with a low complication rate.6,7 Various studies have looked at computer-assisted TKA at short to mid-term follow-up, but few studies have reported long-term outcomes.6-9 de Steiger and colleagues10 recently found that computer-assisted TKA reduced the overall revision rate for aseptic loosening following TKA in patients younger than age 65 years, which suggests benefit of CAS for younger patients. Short-term follow-up has also shown the benefit of CAS TKA in patients with severe extra-articular deformity, where traditional instrumentation cannot be utilized.11 Currently, there is no consensus that computer-assisted TKA leads to improved postoperative patient reported outcomes, because many studies are limited by study design or small cohorts; however, current literature does show an improvement in component alignment as compared to mechanical instrumentation.9,12,13 As future implant and position targets are defined to improve implant survivorship and clinical outcomes in total joint arthroplasty, computer-assisted devices will be useful to help achieve more precise and accurate component positioning.
In addition to CAS devices, some companies have sought to improve TKA surgery by introducing PSI. PSI was introduced to improve component alignment in TKA, with the purported advantages of a shorter surgical time, decrease in the number of instruments needed, and improved clinical outcomes. PSI accuracy remains variable, which may be attributed to the various systems and implant designs in each study.14-17 In addition, advanced preoperative imaging is necessary, which further adds to the overall cost.17 While the recent advancement in technology may provide decreased costs at the time of surgery, the increased cost and time incurred by the patient preoperatively has not resulted in significantly better clinical outcomes.18,19 Additionally, recent work has not shown PSI to have superior accuracy as compared to currently available CAS devices.14 These findings suggest that the additional cost and time incurred by patients may limit the widespread use of PSI.
Although computer navigation has been shown to be more accurate than conventional instrumentation and PSI, the lack of improvement in long-term clinical outcome data has limited its use. In a meta-analysis, Bauwens and colleagues20 suggested that while navigated TKAs have improved component alignment outcomes as compared to conventional surgery, the clinical benefit remains unclear. Less than 5% of surgeons are currently using navigation systems due to the perceived learning curve, cost, additional surgical time, and imaging required to utilize these systems. Certain navigation systems can be seemingly cumbersome, with large consoles, increased number of instruments required, and optical instruments with line-of-sight issues. Recent technological advances have worked to decrease this challenge by using accelerometer- and gyroscope-based electronic components, which combine the accuracy of computer-assisted technology with the ease of use of mechanical guides.
Accelerometer and gyroscope technology systems, such as the iAssist system, are portable devices that do not require a large computer console or navigation arrays. This technology relies on accelerator-based navigation without additional preoperative imaging. A recent study demonstrated the iAssist had reproducible accuracy in component alignment that could be easily incorporated into the operating room without optical trackers.21 The use of portable computer-assisted devices provides a more compact and easily accessible technology that can be used to achieve accurate component alignment without additional large equipment in the operating room.22 These new handheld intraoperative tools have been introduced to place implants according to a preoperative plan in order to minimize failure due to preoperative extra-articular deformity or intraoperative technical miscues.23 Nam and colleagues24 used an accelerometer-based surgical navigation system to perform tibial resections in cadaveric models, and found that the accelerometer-based guide was accurate for tibial resection in both the coronal and sagittal planes. In a prospective randomized controlled trial evaluating 100 patients undergoing a TKA using either an accelerometer-based guide or conventional alignment methods, the authors showed that the accelerometer-based guide decreased outliers in tibial component alignment compared to conventional guides.25 In the accelerometer-based guide cohort, 95.7% of tibial components were within 2° of perpendicular to the tibial mechanical axis, compared to 68.1% in the conventional group (P < .001). These results suggested that portable accelerometer-based navigation allows surgeons to achieve satisfactory tibial component alignment with a decrease in the number of potential outliers.24,25 Similarly, Bugbee and colleagues26 found that accelerometer-based handheld navigation was accurate for tibial coronal and sagittal alignment and no additional surgical time was required compared to conventional techniques.
The relationship between knee alignment and clinical outcomes for TKA remains controversial. Regardless of the surgeon’s alignment preference, it is important to reliably and accurately execute the preoperative plan in a reproducible fashion. Advances in technology that assist with intraoperative component alignment can be useful, and may help decrease the incidence of implant malalignment in clinical practice.
Preoperative Planning and Intraoperative Technique
Preoperative planning is carried out in a manner identical to the use of conventional mechanical guides. Long leg films are taken for evaluation of overall limb alignment, and calibrated lateral images are taken for templating implant sizes. Lines are drawn on the images to determine the difference between the mechanical and anatomic axis of the femur, and a line drawn perpendicular to the mechanical axis is placed to show the expected bone cut. In similar fashion a perpendicular line to the tibial mechanical axis is also drawn to show the expected tibial resection. This preoperative plan allows the surgeon to have an additional intraoperative guide to ensure accuracy of the computer-assisted device.
After standard exposure, the distal femoral or proximal tibial cut can be made based on surgeon preference. The system being demonstrated in the accompanying photos is the KneeAlign 2 system (OrthAlign).
Distal Femoral Cut
The KneeAlign 2 femoral cutting guide is attached to the distal femur with a central pin that is placed in the middle of the distal femur measured from medial to lateral, and 1 cm anterior to the intercondylar notch. It is important to note that this spot is often more medial than traditionally used for insertion of an intramedullary rod. This central point sets the distal point of the femoral mechanical axis. The device is then held in place with 2 oblique pins, and is solidly fixed to the bone. Using a rotating motion, the femur is rotated around the hip joint. The accelerometer and gyroscope in the unit are able to determine the center of the hip joint from this motion, creating the proximal point of the mechanical axis of the femur. Once the mechanical axis of the femur is determined, varus/valgus and flexion/extension can be adjusted on the guide. One adjustment screw is available for varus/valgus, and a second is available for flexion/extension. Numbers on the device screen show real-time alignment, and are easily adjusted to set the desired alignment (Figure 1). Once alignment is obtained, a mechanical stylus is used to determine depth of resection, and the distal femoral cutting block is pinned. After pinning the block, the 3 pins in the device are removed, and the device is removed from the bone. This leaves only the distal femoral cutting block in place. In experienced hands, this part of the procedure takes less than 3 minutes.
Proximal Tibial Cut
The KneeAlign 2 proximal tibial guide is similar in appearance to a standard mechanical tibial cutting guide. It is attached to the proximal tibia with a spring around the calf and 2 pins that hold the device aligned with the medial third of the tibial tubercle. A stylus is then centered on the anterior cruciate ligament (ACL) footprint, which sets the proximal mechanical axis point of the tibia (Figure 2). An offset number is read off the stylus on the ACL footprint, and this number is matched on the ankle offset portion of the guide. The device has 2 sensors. One sensor is on the chassis of the device, and the other is on a mobile arm. Movements between the 2 are monitored by the accelerometers, allowing for accurate maintenance of alignment position even with motion in the leg. A point is taken from the lateral malleolus and then a second point is taken from the medial malleolus. These points are used to determine the center of the ankle joint, which sets the distal mechanical axis point. Once mechanical axis of the tibia is determined, the tibial cutting guide is pinned in place, and can be adjusted with real-time guidance of the varus/valgus and posterior slope values seen on the device (Figure 3). Cut depth is once again determined with a mechanical stylus.
Limitations
Although these devices have proven to be very accurate, surgeons must continue to recognize that all tools can have errors. With computerized guides of any sort, these errors are usually user errors that cannot be detected by the device. Surgeons need to be able to recognize this and always double-check bone cuts for accuracy. Templating the bone cuts prior to surgery is an effective double-check. In addition, many handheld accelerometer devices do not currently assist with the rotational alignment of the femoral component. This is still performed using the surgeon’s preferred technique, and is a limitation of these systems.
Discussion
Currently, TKA provides satisfactory 10-year results with modern implant designs and survival rates as high as 90% to 95%.27,28 Even with good survival rates, a percentage of patients fail within the first 5 years.3 At a single institution, 50% of revision TKAs were related to instability, malalignment, or failure of fixation that occurred less than 2 years after the index procedure.29 In general, TKA with mechanical instrumentation provides satisfactory pain relief and postoperative knee function; however, studies have consistently shown that the use of advanced technology decreases the risk of implant malalignment, which may decrease early implant failure rates as compared to mechanical and some PSI.13,14,22 While there is a paucity of literature that has shown better clinical outcomes with the use of advanced technology, there are studies supporting the notion that proper limb alignment and component positioning improves implant survivorship.23,30
CAS may have additional advantages if the surgeon chooses to place the TKA in an alignment other than a neutral mechanical axis. Kinematic alignment for TKA has gained increasing popularity, where the target of a neutral mechanical axis alignment is not always the goal.31,32 The reported benefit is a more natural ligament tension with the hope of improving patient satisfaction. One concern with this technique is that it is a departure from the long-held teaching that a TKA aligned to a neutral mechanical axis is necessary for long-term implant survivorship.33,34 In addition, if the goal of surgery is to cut the tibia and femur at a specific varus/valgus cut, standard instrumentation may not allow for this level of accuracy. This in turn increases the risk of having a tibial or femoral cut that is outside the commonly accepted standards of alignment, which may lead to early implant failure. If further research suggests alignment is a variable that differs from patient to patient, the use of precise tools to ensure accuracy of executing the preoperatively templated alignment becomes even more important.
As the number of TKAs continues to rise each year, even a small percentage of malaligned knees that go on to revision surgery will create a large burden on the healthcare system.1,3 Although the short-term clinical benefits of CAS have not shown substantial differences as compared to conventional TKA, the number of knees aligned outside of a desired neutral mechanical axis alignment has been shown in multiple studies to be decreased with the use of advanced technology.7,12,34 Although CAS is an additional cost to a primary TKA, if the orthopedic community can decrease the number of TKA revisions due to malalignment from 6.6% to nearly zero, this may decrease the revision burden and overall cost to the healthcare system.1,3
TKA technology continues to evolve, and we must continue to assess each new advance not only to understand how it works, but also to ensure it addresses a specific clinical problem, and to be aware of the costs associated before incorporating it into routine practice. Some argue that the use of advanced technology requires increased surgical time, which in turn ultimately increases costs; however, one study has documented no increase in surgical time with handheld navigation while maintaining the accuracy of the device.34 In addition, no significant radiographic or clinical differences have been found between handheld navigation and larger console CAS systems, but large console systems have been associated with increased surgical times.22 The use of handheld accelerometer- and gyroscope-based guides has proven to provide reliable coronal and sagittal implant alignment that can easily be incorporated into the operating room. More widespread use of such technology will help decrease alignment outliers for TKA, and future long-term clinical outcome studies will be necessary to assess functional outcomes.
Conclusion
Advanced computer based technology offers an additional tool to the surgeon for reliably improving component positioning during TKA. The use of handheld accelerometer- and gyroscope-based guides increases the accuracy of component placement while decreasing the incidence of outliers compared to standard mechanical guides, without the need for a large computer console. Long-term radiographic and patient-reported outcomes are necessary to further validate these devices.
1. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
2. Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28( 8 Suppl):116-119.
4. Sassoon A, Nam D, Nunley R, Barrack R. Systematic review of patient-specific instrumentation in total knee arthroplasty: new but not improved. Clin Orthop Relat Res. 2015;473(1):151-158.
5. Anderson KC, Buehler KC, Markel DC. Computer assisted navigation in total knee arthroplasty: comparison with conventional methods. J Arthroplasty. 2005;20(7 Suppl 3):132-138.
6. Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22(8):1097-1106.
7. Khakha RS, Chowdhry M, Sivaprakasam M, Kheiran A, Chauhan SK. Radiological and functional outcomes in computer assisted total knee arthroplasty between consultants and trainees - a prospective randomized controlled trial. J Arthroplasty. 2015;30(8):1344-1347.
8. Zhu M, Ang CL, Yeo SJ, Lo NN, Chia SL, Chong HC. Minimally invasive computer-assisted total knee arthroplasty compared with conventional total knee arthroplasty: a prospective 9-year follow-up. J Arthroplasty. 2015. [Epub ahead of print]
9. Roberts TD, Clatworthy MG, Frampton CM, Young SW. Does computer assisted navigation improve functional outcomes and implant survivability after total knee arthroplasty? J Arthroplasty. 2015;30(9 Suppl):59-63.
10. de Steiger RN, Liu YL, Graves SE. Computer navigation for total knee arthroplasty reduces revision rate for patients less than sixty-five years of age. J Bone Joint Surg Am. 2015;97(8):635-642.
11. Fehring TK, Mason JB, Moskal J, Pollock DC, Mann J, Williams VJ. When computer-assisted knee replacement is the best alternative. Clin Orthop Relat Res. 2006;452:132-136.
12. Iorio R, Mazza D, Drogo P, et al. Clinical and radiographic outcomes of an accelerometer-based system for the tibial resection in total knee arthroplasty. Int Orthop. 2015;39(3):461-466.
13. Haaker RG, Stockheim M, Kamp M, Proff G, Breitenfelder J, Ottersbach A. Computer-assisted navigation increases precision of component placement in total knee arthroplasty. Clin Orthop Relat Res. 2005;433:152-159.
14. Ollivier M, Tribot-Laspiere Q, Amzallag J, Boisrenoult P, Pujol N, Beaufils P. Abnormal rate of intraoperative and postoperative implant positioning outliers using “MRI-based patient-specific” compared to “computer assisted” instrumentation in total knee replacement. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
15. Nunley RM, Ellison BS, Zhu J, Ruh EL, Howell SM, Barrack RL. Do patient-specific guides improve coronal alignment in total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):895-902.
16. Nunley RM, Ellison BS, Ruh EL, et al. Are patient-specific cutting blocks cost-effective for total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):889-894.
17. Barrack RL, Ruh EL, Williams BM, Ford AD, Foreman K, Nunley RM. Patient specific cutting blocks are currently of no proven value. J Bone Joint Surg Br. 2012;94(11 Suppl A):95-99.
18. Chen JY, Chin PL, Tay DK, Chia SL, Lo NN, Yeo SJ. Functional outcome and quality of life after patient-specific instrumentation in total knee arthroplasty. J Arthroplasty. 2015;30(10):1724-1728.
19. Goyal N, Patel AR, Yaffe MA, Luo MY, Stulberg SD. Does implant design influence the accuracy of patient specific instrumentation in total knee arthroplasty? J Arthroplasty. 2015;30(9):1526-1530.
20. Bauwens K, Matthes G, Wich M, et al. Navigated total knee replacement. A meta-analysis. J Bone Joint Surg Am. 2007;89(2):261-269.
21. Scuderi GR, Fallaha M, Masse V, Lavigne P, Amiot LP, Berthiaume MJ. Total knee arthroplasty with a novel navigation system within the surgical field. Orthop Clin North Am. 2014;45(2):167-173.
22. Goh GS, Liow MH, Lim WS, Tay DK, Yeo SJ, Tan MH. Accelerometer-based navigation is as accurate as optical computer navigation in restoring the joint line and mechanical axis after total knee arthroplasty: a prospective matched study. J Arthroplasty. 2016;31(1):92-97.
23. Berend KR, Lombardi AV Jr. Liberal indications for minimally invasive oxford unicondylar arthroplasty provide rapid functional recovery and pain relief. Surg Technol Int. 2007;16:193-197.
24. Nam D, Jerabek SA, Cross MB, Mayman DJ. Cadaveric analysis of an accelerometer-based portable navigation device for distal femoral cutting block alignment in total knee arthroplasty. Comput Aided Surg. 2012;17(4):205-210.
25. Nam D, Cody EA, Nguyen JT, Figgie MP, Mayman DJ. Extramedullary guides versus portable, accelerometer-based navigation for tibial alignment in total knee arthroplasty: a randomized, controlled trial: winner of the 2013 HAP PAUL award. J Arthroplasty. 2014;29(2):288-294.
26. Bugbee WD, Kermanshahi AY, Munro MM, McCauley JC, Copp SN. Accuracy of a hand-held surgical navigation system for tibial resection in total knee arthroplasty. Knee. 2014;21(6):1225-1228.
27. Schai PA, Thornhill TS, Scott RD. Total knee arthroplasty with the PFC system. Results at a minimum of ten years and survivorship analysis. J Bone Joint Surg Br. 1998;80(5):850-858.
28. Pradhan NR, Gambhir A, Porter ML. Survivorship analysis of 3234 primary knee arthroplasties implanted over a 26-year period: a study of eight different implant designs. Knee. 2006;13(1):7-11.
29. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
30. Fang DM, Ritter MA, Davis KE. Coronal alignment in total knee arthroplasty: just how important is it? J Arthroplasty. 2009;24(6 Suppl):39-43.
31. Cherian JJ, Kapadia BH, Banerjee S, Jauregui JJ, Issa K, Mont MA. Mechanical, anatomical, and kinematic axis in TKA: concepts and practical applications. Curr Rev Musculoskelet Med. 2014;7(2):89-95.
32. Howell SM, Papadopoulos S, Kuznik K, Ghaly LR, Hull ML. Does varus alignment adversely affect implant survival and function six years after kinematically aligned total knee arthroplasty? Int Orthop. 2015;39(11):2117-2124.
33. Ritter MA, Faris PM, Keating EM, Meding JB. Postoperative alignment of total knee replacement. Its effect on survival. Clin Orthop Relat Res. 1994;299:153-156.
34. Huang EH, Copp SN, Bugbee WD. Accuracy of a handheld accelerometer-based navigation system for femoral and tibial resection in total knee arthroplasty. J Arthroplasty. 2015;30(11):1906-1910.
1. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
2. Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28( 8 Suppl):116-119.
4. Sassoon A, Nam D, Nunley R, Barrack R. Systematic review of patient-specific instrumentation in total knee arthroplasty: new but not improved. Clin Orthop Relat Res. 2015;473(1):151-158.
5. Anderson KC, Buehler KC, Markel DC. Computer assisted navigation in total knee arthroplasty: comparison with conventional methods. J Arthroplasty. 2005;20(7 Suppl 3):132-138.
6. Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22(8):1097-1106.
7. Khakha RS, Chowdhry M, Sivaprakasam M, Kheiran A, Chauhan SK. Radiological and functional outcomes in computer assisted total knee arthroplasty between consultants and trainees - a prospective randomized controlled trial. J Arthroplasty. 2015;30(8):1344-1347.
8. Zhu M, Ang CL, Yeo SJ, Lo NN, Chia SL, Chong HC. Minimally invasive computer-assisted total knee arthroplasty compared with conventional total knee arthroplasty: a prospective 9-year follow-up. J Arthroplasty. 2015. [Epub ahead of print]
9. Roberts TD, Clatworthy MG, Frampton CM, Young SW. Does computer assisted navigation improve functional outcomes and implant survivability after total knee arthroplasty? J Arthroplasty. 2015;30(9 Suppl):59-63.
10. de Steiger RN, Liu YL, Graves SE. Computer navigation for total knee arthroplasty reduces revision rate for patients less than sixty-five years of age. J Bone Joint Surg Am. 2015;97(8):635-642.
11. Fehring TK, Mason JB, Moskal J, Pollock DC, Mann J, Williams VJ. When computer-assisted knee replacement is the best alternative. Clin Orthop Relat Res. 2006;452:132-136.
12. Iorio R, Mazza D, Drogo P, et al. Clinical and radiographic outcomes of an accelerometer-based system for the tibial resection in total knee arthroplasty. Int Orthop. 2015;39(3):461-466.
13. Haaker RG, Stockheim M, Kamp M, Proff G, Breitenfelder J, Ottersbach A. Computer-assisted navigation increases precision of component placement in total knee arthroplasty. Clin Orthop Relat Res. 2005;433:152-159.
14. Ollivier M, Tribot-Laspiere Q, Amzallag J, Boisrenoult P, Pujol N, Beaufils P. Abnormal rate of intraoperative and postoperative implant positioning outliers using “MRI-based patient-specific” compared to “computer assisted” instrumentation in total knee replacement. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
15. Nunley RM, Ellison BS, Zhu J, Ruh EL, Howell SM, Barrack RL. Do patient-specific guides improve coronal alignment in total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):895-902.
16. Nunley RM, Ellison BS, Ruh EL, et al. Are patient-specific cutting blocks cost-effective for total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):889-894.
17. Barrack RL, Ruh EL, Williams BM, Ford AD, Foreman K, Nunley RM. Patient specific cutting blocks are currently of no proven value. J Bone Joint Surg Br. 2012;94(11 Suppl A):95-99.
18. Chen JY, Chin PL, Tay DK, Chia SL, Lo NN, Yeo SJ. Functional outcome and quality of life after patient-specific instrumentation in total knee arthroplasty. J Arthroplasty. 2015;30(10):1724-1728.
19. Goyal N, Patel AR, Yaffe MA, Luo MY, Stulberg SD. Does implant design influence the accuracy of patient specific instrumentation in total knee arthroplasty? J Arthroplasty. 2015;30(9):1526-1530.
20. Bauwens K, Matthes G, Wich M, et al. Navigated total knee replacement. A meta-analysis. J Bone Joint Surg Am. 2007;89(2):261-269.
21. Scuderi GR, Fallaha M, Masse V, Lavigne P, Amiot LP, Berthiaume MJ. Total knee arthroplasty with a novel navigation system within the surgical field. Orthop Clin North Am. 2014;45(2):167-173.
22. Goh GS, Liow MH, Lim WS, Tay DK, Yeo SJ, Tan MH. Accelerometer-based navigation is as accurate as optical computer navigation in restoring the joint line and mechanical axis after total knee arthroplasty: a prospective matched study. J Arthroplasty. 2016;31(1):92-97.
23. Berend KR, Lombardi AV Jr. Liberal indications for minimally invasive oxford unicondylar arthroplasty provide rapid functional recovery and pain relief. Surg Technol Int. 2007;16:193-197.
24. Nam D, Jerabek SA, Cross MB, Mayman DJ. Cadaveric analysis of an accelerometer-based portable navigation device for distal femoral cutting block alignment in total knee arthroplasty. Comput Aided Surg. 2012;17(4):205-210.
25. Nam D, Cody EA, Nguyen JT, Figgie MP, Mayman DJ. Extramedullary guides versus portable, accelerometer-based navigation for tibial alignment in total knee arthroplasty: a randomized, controlled trial: winner of the 2013 HAP PAUL award. J Arthroplasty. 2014;29(2):288-294.
26. Bugbee WD, Kermanshahi AY, Munro MM, McCauley JC, Copp SN. Accuracy of a hand-held surgical navigation system for tibial resection in total knee arthroplasty. Knee. 2014;21(6):1225-1228.
27. Schai PA, Thornhill TS, Scott RD. Total knee arthroplasty with the PFC system. Results at a minimum of ten years and survivorship analysis. J Bone Joint Surg Br. 1998;80(5):850-858.
28. Pradhan NR, Gambhir A, Porter ML. Survivorship analysis of 3234 primary knee arthroplasties implanted over a 26-year period: a study of eight different implant designs. Knee. 2006;13(1):7-11.
29. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
30. Fang DM, Ritter MA, Davis KE. Coronal alignment in total knee arthroplasty: just how important is it? J Arthroplasty. 2009;24(6 Suppl):39-43.
31. Cherian JJ, Kapadia BH, Banerjee S, Jauregui JJ, Issa K, Mont MA. Mechanical, anatomical, and kinematic axis in TKA: concepts and practical applications. Curr Rev Musculoskelet Med. 2014;7(2):89-95.
32. Howell SM, Papadopoulos S, Kuznik K, Ghaly LR, Hull ML. Does varus alignment adversely affect implant survival and function six years after kinematically aligned total knee arthroplasty? Int Orthop. 2015;39(11):2117-2124.
33. Ritter MA, Faris PM, Keating EM, Meding JB. Postoperative alignment of total knee replacement. Its effect on survival. Clin Orthop Relat Res. 1994;299:153-156.
34. Huang EH, Copp SN, Bugbee WD. Accuracy of a handheld accelerometer-based navigation system for femoral and tibial resection in total knee arthroplasty. J Arthroplasty. 2015;30(11):1906-1910.
Commentary to "CDC Will Soon Issue Guidelines for the Prevention of Surgical Site Infection"
Analyzing the Guidelines: It Can't All Be Level I
The demand for total joint arthroplasty continues to rise, resulting in a steady increase in the number of primary total hip and knee replacements every year. Unfortunately, as these numbers rise, so will the number of periprosthetic joint infections (PJIs). The economic burden and patient morbidity associated with PJI has resulted in the creation of multiple orthopedic societies and committees focused on formulating “best practice” guidelines in order to reduce the rates of PJI as much as possible.
The new guidelines for surgical site infection (SSI) prevention by the Centers for Disease Control and Prevention (CDC) recently forced the orthopedic community to critically analyze the current literature. Dr. Javad Parvizi’s editorial elegantly notes that many areas of infection prevention and treatment are not well evaluated, and many of our day-to-day practices are based on low levels of evidence. Level I studies continue to be a costly and time-consuming challenge due to the already very low SSI rate, and, in order to show an improvement in this rate, thousands of patients are required for study. This makes a multicenter approach necessary to ensure adequate power, and a multicenter study often requires significant resources and funding outlets. These requirements have resulted in many of our practice recommendations being based on retrospective reviews, which have inherent methodological limitations. The retrospective nature of these studies lacks the experimental design necessary to confidently make treatment recommendations; however, they do allow us to look at what strategies have been tried, and in essence, how well they worked. Although level III and IV studies do not allow us to compare treatments head to head, they do give us some insights into viable treatment strategies and should not be completely disregarded. The results of retrospective studies allow us to design prospective experiments based on what we have observed as successful treatment modalities in particular patient cohorts.
An alternative approach for evaluating new and existing treatment strategies is through basic science translational research. Future advancements in PJI diagnosis and treatment will likely be founded upon translational research efforts from clinician scientists testing treatment protocols both on the benchtop and in animal models. The most glaring knowledge gaps in PJI should be identified through the combined efforts of the CDC, the Musculoskeletal Infection Society, the American Academy of Orthopaedic Surgeons, and the Orthopaedic Research Society. Coordinated efforts should be made and strategies executed to systematically fund translational projects that answer these questions. Translational studies will be able to safely and methodically evaluate new and even established treatment protocols for PJI in a cost-effective manner.
We have made great strides in the prevention and treatment of PJI over the past 2 decades. When working together as a cohesive profession, we will undoubtedly continue to advance our knowledge base and improve treatment recommendations for our patients.
Analyzing the Guidelines: It Can't All Be Level I
The demand for total joint arthroplasty continues to rise, resulting in a steady increase in the number of primary total hip and knee replacements every year. Unfortunately, as these numbers rise, so will the number of periprosthetic joint infections (PJIs). The economic burden and patient morbidity associated with PJI has resulted in the creation of multiple orthopedic societies and committees focused on formulating “best practice” guidelines in order to reduce the rates of PJI as much as possible.
The new guidelines for surgical site infection (SSI) prevention by the Centers for Disease Control and Prevention (CDC) recently forced the orthopedic community to critically analyze the current literature. Dr. Javad Parvizi’s editorial elegantly notes that many areas of infection prevention and treatment are not well evaluated, and many of our day-to-day practices are based on low levels of evidence. Level I studies continue to be a costly and time-consuming challenge due to the already very low SSI rate, and, in order to show an improvement in this rate, thousands of patients are required for study. This makes a multicenter approach necessary to ensure adequate power, and a multicenter study often requires significant resources and funding outlets. These requirements have resulted in many of our practice recommendations being based on retrospective reviews, which have inherent methodological limitations. The retrospective nature of these studies lacks the experimental design necessary to confidently make treatment recommendations; however, they do allow us to look at what strategies have been tried, and in essence, how well they worked. Although level III and IV studies do not allow us to compare treatments head to head, they do give us some insights into viable treatment strategies and should not be completely disregarded. The results of retrospective studies allow us to design prospective experiments based on what we have observed as successful treatment modalities in particular patient cohorts.
An alternative approach for evaluating new and existing treatment strategies is through basic science translational research. Future advancements in PJI diagnosis and treatment will likely be founded upon translational research efforts from clinician scientists testing treatment protocols both on the benchtop and in animal models. The most glaring knowledge gaps in PJI should be identified through the combined efforts of the CDC, the Musculoskeletal Infection Society, the American Academy of Orthopaedic Surgeons, and the Orthopaedic Research Society. Coordinated efforts should be made and strategies executed to systematically fund translational projects that answer these questions. Translational studies will be able to safely and methodically evaluate new and even established treatment protocols for PJI in a cost-effective manner.
We have made great strides in the prevention and treatment of PJI over the past 2 decades. When working together as a cohesive profession, we will undoubtedly continue to advance our knowledge base and improve treatment recommendations for our patients.
Analyzing the Guidelines: It Can't All Be Level I
The demand for total joint arthroplasty continues to rise, resulting in a steady increase in the number of primary total hip and knee replacements every year. Unfortunately, as these numbers rise, so will the number of periprosthetic joint infections (PJIs). The economic burden and patient morbidity associated with PJI has resulted in the creation of multiple orthopedic societies and committees focused on formulating “best practice” guidelines in order to reduce the rates of PJI as much as possible.
The new guidelines for surgical site infection (SSI) prevention by the Centers for Disease Control and Prevention (CDC) recently forced the orthopedic community to critically analyze the current literature. Dr. Javad Parvizi’s editorial elegantly notes that many areas of infection prevention and treatment are not well evaluated, and many of our day-to-day practices are based on low levels of evidence. Level I studies continue to be a costly and time-consuming challenge due to the already very low SSI rate, and, in order to show an improvement in this rate, thousands of patients are required for study. This makes a multicenter approach necessary to ensure adequate power, and a multicenter study often requires significant resources and funding outlets. These requirements have resulted in many of our practice recommendations being based on retrospective reviews, which have inherent methodological limitations. The retrospective nature of these studies lacks the experimental design necessary to confidently make treatment recommendations; however, they do allow us to look at what strategies have been tried, and in essence, how well they worked. Although level III and IV studies do not allow us to compare treatments head to head, they do give us some insights into viable treatment strategies and should not be completely disregarded. The results of retrospective studies allow us to design prospective experiments based on what we have observed as successful treatment modalities in particular patient cohorts.
An alternative approach for evaluating new and existing treatment strategies is through basic science translational research. Future advancements in PJI diagnosis and treatment will likely be founded upon translational research efforts from clinician scientists testing treatment protocols both on the benchtop and in animal models. The most glaring knowledge gaps in PJI should be identified through the combined efforts of the CDC, the Musculoskeletal Infection Society, the American Academy of Orthopaedic Surgeons, and the Orthopaedic Research Society. Coordinated efforts should be made and strategies executed to systematically fund translational projects that answer these questions. Translational studies will be able to safely and methodically evaluate new and even established treatment protocols for PJI in a cost-effective manner.
We have made great strides in the prevention and treatment of PJI over the past 2 decades. When working together as a cohesive profession, we will undoubtedly continue to advance our knowledge base and improve treatment recommendations for our patients.
Nanotechnology: Why Should We Care?
The orthopedic community is increasingly deluged with advancements in the basic sciences. With each step, we must evaluate the necessity of new information and the relevance of these topics for clinical practice. Since the late 1990s, the promise of nanotechnology to effect significant changes in the medical field has been heralded. However, in this coming decade, we as a profession will see unprecedented advances in the movement of this technology “from the bench to the bedside.” Not unlike many other basic science advancements in our field, nanotechnology is poorly understood among clinicians and residents. As the use of biologics and drug delivery systems expands in orthopedics, nanoparticle-based devices will become more prevalent and have a momentous impact on the way we treat and diagnose orthopedic patients.
A nanoparticle is generally defined as a particle in which at least 1 dimension is between 1 to 100 nanometers and has material properties consistent with quantum mechanics.1 Nanomaterials can be composed of organic and inorganic chemical elements that enable basic chemical processes to create more complex systems. Individual nanoparticle units can be synthesized to form nanostructures, including nanotubes, nanoscaffolds, nanofibers, and even nanodiamonds.2-4 Nanoparticles at this scale display unique optical, chemical, and physical properties that can be manipulated to create specific end-use applications. Such uses may include glass fabrication, optical probes, television screens, drug delivery, gene delivery, and multiplex diagnostic assays.5-7 By crossing disciplines of physics, engineering, and medical sciences, we can create novel technology that includes nanomanufacturing, targeted drug delivery, nanorobotics in conjunction with artificial intelligence, and point-of-care diagnostics.7-9
The field of orthopedics has benefited from nanotechnologic advances, such as new therapeutics and implant-related technology. Nanotubes are hollow nanosized cylinders that are commonly created from titania, silica, or carbon-based substrates. They have garnered significant interest for their high tensile and shear strength, favorable microstructure for bony ingrowth, and their capacity to hold antibiotics or growth factors, such as bone morphogenic proteins (BMPs).10 The current local delivery limitations of BMPs via a collagen sponge have the potential to be maximized and better controlled with a nanotechnology-based approach. The size, internal structure, and shape of the nanoparticle can be manipulated to control the release of these growth factors, and certain nanoparticles can be dual-layered, allowing for release of multiple growth factors at once or in succession.11,12 A more powerful and targeted delivery system of these types of growth factors may result in improved or more robust outcomes, and further research is warranted.
It is possible that carbon-based nanotubes can be categorized as a biomedical implant secondary to their mechanical properties.13 Their strength and ability to be augmented with osteogenic materials has made them an attractive area of research as alternative implant surfaces and stand-alone implants. Nanotubes are capable of acting as a scaffold for antibiotic-loaded, carbon-based nanodiamonds for localized treatment of periprosthetic infection, and research has been directed toward controlled release of the nanodiamond-antibiotic construct from these scaffolds or hydrogels.4,14 Technologies like this may allow the clinician to treat periprosthetic infections locally and minimize the use of systemic antibiotics. The perfection of this type of delivery system may augment the role of antibiotic-laden cement and improve our treatment success rates, even in traditionally hard-to-treat organisms.
Nanoscaffolds and nanofibers are created from nanosized polymers and rendered into a 3-dimensional structure that can be loaded with biologic particles or acting as a scaffold/template for tissue or bone ingrowth. Nanofibers created using biodegradable substrates such as poly(lactic-co-glycolic acid) (PLGA) and chitosan have been extensively studied for their delayed-release properties and biocompatibility.15 These scaffolds are often soaked or loaded with chondrogenic, osteogenic, or antibacterial agents, and have been evaluated in both in vitro and in vivo studies with promising results.15,16 They have been an exciting area of research in tissue engineering, and have been accepted as an adjunct in tendon-repair treatments and local bone regeneration.3,17 As this technology is perfected, the potential to treat more effectively massive rotator cuff tears or tears with poor tissue integrity will dramatically improve and expand the indications for rotator cuff repair.
Augmentation of implant surfaces with nanomaterials that improve osseointegration, or that act as antimicrobial agents have also been a focus of research in hopes of decreasing the rates of aseptic failure and periprosthetic infection in arthroplasty procedures. Nanocrystalline surfaces made of hydroxyapatite and cobalt chromium have been evaluated for their enhanced osteoconductive properties, and may replace standard surfaces.18-20 Recent work evaluating nanoparticle-antibiotic constructs that have been covalently bound to implant surfaces for delayed release of antibiotics during the perioperative period has shown promise, and may allow a more targeted and localized treatment strategy for periprosthetic infection.21,22
Major limitations regarding successful clinical implementation of nanotechnology include both cost and regulatory processes. Currently, pharmaceutical companies estimate that, on average, successful clinical trials from phase 1 to completion for new drugs can cost hundreds of millions of dollars.23 Such high costs result partially from the laborious and capital-intensive process of conducting clinical trials that meet US Food and Drug Administration (FDA) requirements. These regulations would apply to both surface-coated implants and nanoparticle-based drug delivery systems. These types of implants would not be expedited into the market secondary to their drug delivery component and would likely require lengthy clinical studies. Implant companies may be reluctant to invest millions of dollars in multiple FDA trials when they have lucrative implants on the market.
Other limitations include the particles’ complex 3-dimensional structure, which can present challenges for mass production. Producing large quantities of nanoparticles at a consistent quality may be a major limitation to the more unique and target-based nanotherapies. Recent concerns with the toxicity profile of nanotechnology-based medicines have resulted in more intense scrutiny of the nanotechnology safety profile.24,25 Currently, nanoparticle technology is evaluated case by case with each technology requiring its own toxicology and safety profile testing if it is intended for human use. These tests can be cost-prohibitive and require extensive private and government capital for successful market entry. Despite these limitations, nanotechnology will impact the next generation of orthopedic surgeons. Current estimates project the nanomedicine market to be worth $177.6 billion by 2019.26
Advances in nanobased orthopedic technologies have expanded dramatically in the past decade, and we, as the treating physicians, must make educated decisions on how and when to use nanoparticle-based therapies and treatment options. Nanotechnology’s basic science is confusing and often burdensome, but contemporary review articles may be helpful in keeping the orthopedic resident and clinician current with advancements.10,27,28 The more we educate ourselves about evolving nanotechnologies, the less reluctance we will have when evaluating new diagnostic and therapeutic treatment modalities.
1. Hewakuruppu YL, Dombrovsky LA, Chen C, et al. Plasmonic “pump-probe” method to study semi-transparent nanofluids. Appl Opt. 2013;52(24):6041-6050.
2. Balasundaram G, Webster TJ. An overview of nano-polymers for orthopedic applications. Macromol Biosci. 2007;7(5):635-642.
3. Zhang Z, Hu J, Ma PX. Nanofiber-based delivery of bioactive agents and stem cells to bone sites. Adv Drug Deliv Rev. 2012;64(12):1129-1141.
4. Mochalin VN, Shenderova O, Ho D, Gogotsi Y. The properties and applications of nanodiamonds. Nat Nanotechnol. 2012;7(1):11-23.
5. Kneipp J, Kneipp H, Rice WL, Kneipp K. Optical probes for biological applications based on surface-enhanced Raman scattering from indocyanine green on gold nanoparticles. Anal Chem. 2005;77(8):2381-2385.
6. Wang L, O’Donoghue MB, Tan W. Nanoparticles for multiplex diagnostics and imaging. Nanomedicine (Lond). 2006;1(4):413-426.
7. Krebs MD, Salter E, Chen E, Sutter KA, Alsberg E. Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis. J Biomed Mater Res A. 2010;92(3):1131-1138.
8. Myers FB, Lee LP. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip. 2008;8(12):2015-2031.
9. Sacha GM, Varona P. Artificial intelligence in nanotechnology. Nanotechnology. 2013;24(45):452002.
10. Ganguly DY, Shahbazian R, Shokuhfar T. Recent advances in nanotubes for orthopedic implants. J Nanotech Smart Mater. 2014;1:1-10.
11. Srivastava S, Kotov NA. Composite Layer-by-Layer (LBL) assembly with inorganic nanoparticles and nanowires. Acc Chem Res. 2008;41(12):1831-1841.
12. Panda HS, Srivastava R, Bahadur D. Shape and size control of nano dispersed Mg/Al layered double hydroxide. J Nanosci Nanotechnol. 2008;8(8):4218-4223.
13. Wang X, Li Q, Xie J, et al. Fabrication of ultralong and electrically uniform single-walled carbon nanotubes on clean substrates. Nano Lett. 2009;9(9):3137-3141.
14. Zhu Y, Li J, Li W, et al. The biocompatibility of nanodiamonds and their application in drug delivery systems. Theranostics. 2012;2(3):302-312.
15. Wu L, Ding J. In vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2004;25(2):5821-5830.
16. Wu X, Rabkin-Aikawa E, Guleserian KJ, et al. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2004;287(2):H480-H487.
17. Xia W, Liu W, Cui L, et al. Tissue engineering of cartilage with the use of chitosan-gelatin complex scaffolds. J Biomed Mater Res B Appl Biomater. 2004;71(2):373-380.
18. Laurencin CT, Kumbar SG, Nukavarapu SP. Nanotechnology and orthopedics: a personal perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(1):6-10.
19. Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;25(19):4731-4739.
20. Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21(17):1803-1810.
21. Stewart S, Barr S, Engiles J, et al. Vancomycin-modified implant surface inhibits biofilm formation and supports bone-healing in an infected osteotomy model in sheep: a proof-of-concept study. J Bone Joint Surg Am. 2012;94(15):1406-1415.
22. Hickok NJ, Shapiro IM. Immobilized antibiotics to prevent orthopaedic implant infections. Adv Drug Deliv Rev. 2012;64(12):1165-1176.
23. DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22(2):151-185.
24. Vines T, Faunce T. Assessing the safety and cost-effectiveness of early nanodrugs. J Law Med. 2009;16(5):822-845.
25. Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622-627.
26. Nanomedicine Market (Neurology, Cardiovascular, Anti-Inflammatory, Anti-Infective, and Oncology Applications): Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013-2019. Transparency Market Research website. http://www.transparencymarketresearch.com/nanomedicine-market.html. Published August 1, 2014. Accessed January 20, 2015.
27. Sullivan MP, McHale KJ, Parvizi J, Mehta S. Nanotechnology: current concepts in orthopaedic surgery and future directions. Bone Joint J. 2014;96-B(5):569-573.
28. Pleshko N, Grande DA, Myers KR. Nanotechnology in orthopaedics. J Am Acad Orthop Surg. 2012;20(1):60-62.
The orthopedic community is increasingly deluged with advancements in the basic sciences. With each step, we must evaluate the necessity of new information and the relevance of these topics for clinical practice. Since the late 1990s, the promise of nanotechnology to effect significant changes in the medical field has been heralded. However, in this coming decade, we as a profession will see unprecedented advances in the movement of this technology “from the bench to the bedside.” Not unlike many other basic science advancements in our field, nanotechnology is poorly understood among clinicians and residents. As the use of biologics and drug delivery systems expands in orthopedics, nanoparticle-based devices will become more prevalent and have a momentous impact on the way we treat and diagnose orthopedic patients.
A nanoparticle is generally defined as a particle in which at least 1 dimension is between 1 to 100 nanometers and has material properties consistent with quantum mechanics.1 Nanomaterials can be composed of organic and inorganic chemical elements that enable basic chemical processes to create more complex systems. Individual nanoparticle units can be synthesized to form nanostructures, including nanotubes, nanoscaffolds, nanofibers, and even nanodiamonds.2-4 Nanoparticles at this scale display unique optical, chemical, and physical properties that can be manipulated to create specific end-use applications. Such uses may include glass fabrication, optical probes, television screens, drug delivery, gene delivery, and multiplex diagnostic assays.5-7 By crossing disciplines of physics, engineering, and medical sciences, we can create novel technology that includes nanomanufacturing, targeted drug delivery, nanorobotics in conjunction with artificial intelligence, and point-of-care diagnostics.7-9
The field of orthopedics has benefited from nanotechnologic advances, such as new therapeutics and implant-related technology. Nanotubes are hollow nanosized cylinders that are commonly created from titania, silica, or carbon-based substrates. They have garnered significant interest for their high tensile and shear strength, favorable microstructure for bony ingrowth, and their capacity to hold antibiotics or growth factors, such as bone morphogenic proteins (BMPs).10 The current local delivery limitations of BMPs via a collagen sponge have the potential to be maximized and better controlled with a nanotechnology-based approach. The size, internal structure, and shape of the nanoparticle can be manipulated to control the release of these growth factors, and certain nanoparticles can be dual-layered, allowing for release of multiple growth factors at once or in succession.11,12 A more powerful and targeted delivery system of these types of growth factors may result in improved or more robust outcomes, and further research is warranted.
It is possible that carbon-based nanotubes can be categorized as a biomedical implant secondary to their mechanical properties.13 Their strength and ability to be augmented with osteogenic materials has made them an attractive area of research as alternative implant surfaces and stand-alone implants. Nanotubes are capable of acting as a scaffold for antibiotic-loaded, carbon-based nanodiamonds for localized treatment of periprosthetic infection, and research has been directed toward controlled release of the nanodiamond-antibiotic construct from these scaffolds or hydrogels.4,14 Technologies like this may allow the clinician to treat periprosthetic infections locally and minimize the use of systemic antibiotics. The perfection of this type of delivery system may augment the role of antibiotic-laden cement and improve our treatment success rates, even in traditionally hard-to-treat organisms.
Nanoscaffolds and nanofibers are created from nanosized polymers and rendered into a 3-dimensional structure that can be loaded with biologic particles or acting as a scaffold/template for tissue or bone ingrowth. Nanofibers created using biodegradable substrates such as poly(lactic-co-glycolic acid) (PLGA) and chitosan have been extensively studied for their delayed-release properties and biocompatibility.15 These scaffolds are often soaked or loaded with chondrogenic, osteogenic, or antibacterial agents, and have been evaluated in both in vitro and in vivo studies with promising results.15,16 They have been an exciting area of research in tissue engineering, and have been accepted as an adjunct in tendon-repair treatments and local bone regeneration.3,17 As this technology is perfected, the potential to treat more effectively massive rotator cuff tears or tears with poor tissue integrity will dramatically improve and expand the indications for rotator cuff repair.
Augmentation of implant surfaces with nanomaterials that improve osseointegration, or that act as antimicrobial agents have also been a focus of research in hopes of decreasing the rates of aseptic failure and periprosthetic infection in arthroplasty procedures. Nanocrystalline surfaces made of hydroxyapatite and cobalt chromium have been evaluated for their enhanced osteoconductive properties, and may replace standard surfaces.18-20 Recent work evaluating nanoparticle-antibiotic constructs that have been covalently bound to implant surfaces for delayed release of antibiotics during the perioperative period has shown promise, and may allow a more targeted and localized treatment strategy for periprosthetic infection.21,22
Major limitations regarding successful clinical implementation of nanotechnology include both cost and regulatory processes. Currently, pharmaceutical companies estimate that, on average, successful clinical trials from phase 1 to completion for new drugs can cost hundreds of millions of dollars.23 Such high costs result partially from the laborious and capital-intensive process of conducting clinical trials that meet US Food and Drug Administration (FDA) requirements. These regulations would apply to both surface-coated implants and nanoparticle-based drug delivery systems. These types of implants would not be expedited into the market secondary to their drug delivery component and would likely require lengthy clinical studies. Implant companies may be reluctant to invest millions of dollars in multiple FDA trials when they have lucrative implants on the market.
Other limitations include the particles’ complex 3-dimensional structure, which can present challenges for mass production. Producing large quantities of nanoparticles at a consistent quality may be a major limitation to the more unique and target-based nanotherapies. Recent concerns with the toxicity profile of nanotechnology-based medicines have resulted in more intense scrutiny of the nanotechnology safety profile.24,25 Currently, nanoparticle technology is evaluated case by case with each technology requiring its own toxicology and safety profile testing if it is intended for human use. These tests can be cost-prohibitive and require extensive private and government capital for successful market entry. Despite these limitations, nanotechnology will impact the next generation of orthopedic surgeons. Current estimates project the nanomedicine market to be worth $177.6 billion by 2019.26
Advances in nanobased orthopedic technologies have expanded dramatically in the past decade, and we, as the treating physicians, must make educated decisions on how and when to use nanoparticle-based therapies and treatment options. Nanotechnology’s basic science is confusing and often burdensome, but contemporary review articles may be helpful in keeping the orthopedic resident and clinician current with advancements.10,27,28 The more we educate ourselves about evolving nanotechnologies, the less reluctance we will have when evaluating new diagnostic and therapeutic treatment modalities.
The orthopedic community is increasingly deluged with advancements in the basic sciences. With each step, we must evaluate the necessity of new information and the relevance of these topics for clinical practice. Since the late 1990s, the promise of nanotechnology to effect significant changes in the medical field has been heralded. However, in this coming decade, we as a profession will see unprecedented advances in the movement of this technology “from the bench to the bedside.” Not unlike many other basic science advancements in our field, nanotechnology is poorly understood among clinicians and residents. As the use of biologics and drug delivery systems expands in orthopedics, nanoparticle-based devices will become more prevalent and have a momentous impact on the way we treat and diagnose orthopedic patients.
A nanoparticle is generally defined as a particle in which at least 1 dimension is between 1 to 100 nanometers and has material properties consistent with quantum mechanics.1 Nanomaterials can be composed of organic and inorganic chemical elements that enable basic chemical processes to create more complex systems. Individual nanoparticle units can be synthesized to form nanostructures, including nanotubes, nanoscaffolds, nanofibers, and even nanodiamonds.2-4 Nanoparticles at this scale display unique optical, chemical, and physical properties that can be manipulated to create specific end-use applications. Such uses may include glass fabrication, optical probes, television screens, drug delivery, gene delivery, and multiplex diagnostic assays.5-7 By crossing disciplines of physics, engineering, and medical sciences, we can create novel technology that includes nanomanufacturing, targeted drug delivery, nanorobotics in conjunction with artificial intelligence, and point-of-care diagnostics.7-9
The field of orthopedics has benefited from nanotechnologic advances, such as new therapeutics and implant-related technology. Nanotubes are hollow nanosized cylinders that are commonly created from titania, silica, or carbon-based substrates. They have garnered significant interest for their high tensile and shear strength, favorable microstructure for bony ingrowth, and their capacity to hold antibiotics or growth factors, such as bone morphogenic proteins (BMPs).10 The current local delivery limitations of BMPs via a collagen sponge have the potential to be maximized and better controlled with a nanotechnology-based approach. The size, internal structure, and shape of the nanoparticle can be manipulated to control the release of these growth factors, and certain nanoparticles can be dual-layered, allowing for release of multiple growth factors at once or in succession.11,12 A more powerful and targeted delivery system of these types of growth factors may result in improved or more robust outcomes, and further research is warranted.
It is possible that carbon-based nanotubes can be categorized as a biomedical implant secondary to their mechanical properties.13 Their strength and ability to be augmented with osteogenic materials has made them an attractive area of research as alternative implant surfaces and stand-alone implants. Nanotubes are capable of acting as a scaffold for antibiotic-loaded, carbon-based nanodiamonds for localized treatment of periprosthetic infection, and research has been directed toward controlled release of the nanodiamond-antibiotic construct from these scaffolds or hydrogels.4,14 Technologies like this may allow the clinician to treat periprosthetic infections locally and minimize the use of systemic antibiotics. The perfection of this type of delivery system may augment the role of antibiotic-laden cement and improve our treatment success rates, even in traditionally hard-to-treat organisms.
Nanoscaffolds and nanofibers are created from nanosized polymers and rendered into a 3-dimensional structure that can be loaded with biologic particles or acting as a scaffold/template for tissue or bone ingrowth. Nanofibers created using biodegradable substrates such as poly(lactic-co-glycolic acid) (PLGA) and chitosan have been extensively studied for their delayed-release properties and biocompatibility.15 These scaffolds are often soaked or loaded with chondrogenic, osteogenic, or antibacterial agents, and have been evaluated in both in vitro and in vivo studies with promising results.15,16 They have been an exciting area of research in tissue engineering, and have been accepted as an adjunct in tendon-repair treatments and local bone regeneration.3,17 As this technology is perfected, the potential to treat more effectively massive rotator cuff tears or tears with poor tissue integrity will dramatically improve and expand the indications for rotator cuff repair.
Augmentation of implant surfaces with nanomaterials that improve osseointegration, or that act as antimicrobial agents have also been a focus of research in hopes of decreasing the rates of aseptic failure and periprosthetic infection in arthroplasty procedures. Nanocrystalline surfaces made of hydroxyapatite and cobalt chromium have been evaluated for their enhanced osteoconductive properties, and may replace standard surfaces.18-20 Recent work evaluating nanoparticle-antibiotic constructs that have been covalently bound to implant surfaces for delayed release of antibiotics during the perioperative period has shown promise, and may allow a more targeted and localized treatment strategy for periprosthetic infection.21,22
Major limitations regarding successful clinical implementation of nanotechnology include both cost and regulatory processes. Currently, pharmaceutical companies estimate that, on average, successful clinical trials from phase 1 to completion for new drugs can cost hundreds of millions of dollars.23 Such high costs result partially from the laborious and capital-intensive process of conducting clinical trials that meet US Food and Drug Administration (FDA) requirements. These regulations would apply to both surface-coated implants and nanoparticle-based drug delivery systems. These types of implants would not be expedited into the market secondary to their drug delivery component and would likely require lengthy clinical studies. Implant companies may be reluctant to invest millions of dollars in multiple FDA trials when they have lucrative implants on the market.
Other limitations include the particles’ complex 3-dimensional structure, which can present challenges for mass production. Producing large quantities of nanoparticles at a consistent quality may be a major limitation to the more unique and target-based nanotherapies. Recent concerns with the toxicity profile of nanotechnology-based medicines have resulted in more intense scrutiny of the nanotechnology safety profile.24,25 Currently, nanoparticle technology is evaluated case by case with each technology requiring its own toxicology and safety profile testing if it is intended for human use. These tests can be cost-prohibitive and require extensive private and government capital for successful market entry. Despite these limitations, nanotechnology will impact the next generation of orthopedic surgeons. Current estimates project the nanomedicine market to be worth $177.6 billion by 2019.26
Advances in nanobased orthopedic technologies have expanded dramatically in the past decade, and we, as the treating physicians, must make educated decisions on how and when to use nanoparticle-based therapies and treatment options. Nanotechnology’s basic science is confusing and often burdensome, but contemporary review articles may be helpful in keeping the orthopedic resident and clinician current with advancements.10,27,28 The more we educate ourselves about evolving nanotechnologies, the less reluctance we will have when evaluating new diagnostic and therapeutic treatment modalities.
1. Hewakuruppu YL, Dombrovsky LA, Chen C, et al. Plasmonic “pump-probe” method to study semi-transparent nanofluids. Appl Opt. 2013;52(24):6041-6050.
2. Balasundaram G, Webster TJ. An overview of nano-polymers for orthopedic applications. Macromol Biosci. 2007;7(5):635-642.
3. Zhang Z, Hu J, Ma PX. Nanofiber-based delivery of bioactive agents and stem cells to bone sites. Adv Drug Deliv Rev. 2012;64(12):1129-1141.
4. Mochalin VN, Shenderova O, Ho D, Gogotsi Y. The properties and applications of nanodiamonds. Nat Nanotechnol. 2012;7(1):11-23.
5. Kneipp J, Kneipp H, Rice WL, Kneipp K. Optical probes for biological applications based on surface-enhanced Raman scattering from indocyanine green on gold nanoparticles. Anal Chem. 2005;77(8):2381-2385.
6. Wang L, O’Donoghue MB, Tan W. Nanoparticles for multiplex diagnostics and imaging. Nanomedicine (Lond). 2006;1(4):413-426.
7. Krebs MD, Salter E, Chen E, Sutter KA, Alsberg E. Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis. J Biomed Mater Res A. 2010;92(3):1131-1138.
8. Myers FB, Lee LP. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip. 2008;8(12):2015-2031.
9. Sacha GM, Varona P. Artificial intelligence in nanotechnology. Nanotechnology. 2013;24(45):452002.
10. Ganguly DY, Shahbazian R, Shokuhfar T. Recent advances in nanotubes for orthopedic implants. J Nanotech Smart Mater. 2014;1:1-10.
11. Srivastava S, Kotov NA. Composite Layer-by-Layer (LBL) assembly with inorganic nanoparticles and nanowires. Acc Chem Res. 2008;41(12):1831-1841.
12. Panda HS, Srivastava R, Bahadur D. Shape and size control of nano dispersed Mg/Al layered double hydroxide. J Nanosci Nanotechnol. 2008;8(8):4218-4223.
13. Wang X, Li Q, Xie J, et al. Fabrication of ultralong and electrically uniform single-walled carbon nanotubes on clean substrates. Nano Lett. 2009;9(9):3137-3141.
14. Zhu Y, Li J, Li W, et al. The biocompatibility of nanodiamonds and their application in drug delivery systems. Theranostics. 2012;2(3):302-312.
15. Wu L, Ding J. In vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2004;25(2):5821-5830.
16. Wu X, Rabkin-Aikawa E, Guleserian KJ, et al. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2004;287(2):H480-H487.
17. Xia W, Liu W, Cui L, et al. Tissue engineering of cartilage with the use of chitosan-gelatin complex scaffolds. J Biomed Mater Res B Appl Biomater. 2004;71(2):373-380.
18. Laurencin CT, Kumbar SG, Nukavarapu SP. Nanotechnology and orthopedics: a personal perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(1):6-10.
19. Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;25(19):4731-4739.
20. Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21(17):1803-1810.
21. Stewart S, Barr S, Engiles J, et al. Vancomycin-modified implant surface inhibits biofilm formation and supports bone-healing in an infected osteotomy model in sheep: a proof-of-concept study. J Bone Joint Surg Am. 2012;94(15):1406-1415.
22. Hickok NJ, Shapiro IM. Immobilized antibiotics to prevent orthopaedic implant infections. Adv Drug Deliv Rev. 2012;64(12):1165-1176.
23. DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22(2):151-185.
24. Vines T, Faunce T. Assessing the safety and cost-effectiveness of early nanodrugs. J Law Med. 2009;16(5):822-845.
25. Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622-627.
26. Nanomedicine Market (Neurology, Cardiovascular, Anti-Inflammatory, Anti-Infective, and Oncology Applications): Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013-2019. Transparency Market Research website. http://www.transparencymarketresearch.com/nanomedicine-market.html. Published August 1, 2014. Accessed January 20, 2015.
27. Sullivan MP, McHale KJ, Parvizi J, Mehta S. Nanotechnology: current concepts in orthopaedic surgery and future directions. Bone Joint J. 2014;96-B(5):569-573.
28. Pleshko N, Grande DA, Myers KR. Nanotechnology in orthopaedics. J Am Acad Orthop Surg. 2012;20(1):60-62.
1. Hewakuruppu YL, Dombrovsky LA, Chen C, et al. Plasmonic “pump-probe” method to study semi-transparent nanofluids. Appl Opt. 2013;52(24):6041-6050.
2. Balasundaram G, Webster TJ. An overview of nano-polymers for orthopedic applications. Macromol Biosci. 2007;7(5):635-642.
3. Zhang Z, Hu J, Ma PX. Nanofiber-based delivery of bioactive agents and stem cells to bone sites. Adv Drug Deliv Rev. 2012;64(12):1129-1141.
4. Mochalin VN, Shenderova O, Ho D, Gogotsi Y. The properties and applications of nanodiamonds. Nat Nanotechnol. 2012;7(1):11-23.
5. Kneipp J, Kneipp H, Rice WL, Kneipp K. Optical probes for biological applications based on surface-enhanced Raman scattering from indocyanine green on gold nanoparticles. Anal Chem. 2005;77(8):2381-2385.
6. Wang L, O’Donoghue MB, Tan W. Nanoparticles for multiplex diagnostics and imaging. Nanomedicine (Lond). 2006;1(4):413-426.
7. Krebs MD, Salter E, Chen E, Sutter KA, Alsberg E. Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis. J Biomed Mater Res A. 2010;92(3):1131-1138.
8. Myers FB, Lee LP. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip. 2008;8(12):2015-2031.
9. Sacha GM, Varona P. Artificial intelligence in nanotechnology. Nanotechnology. 2013;24(45):452002.
10. Ganguly DY, Shahbazian R, Shokuhfar T. Recent advances in nanotubes for orthopedic implants. J Nanotech Smart Mater. 2014;1:1-10.
11. Srivastava S, Kotov NA. Composite Layer-by-Layer (LBL) assembly with inorganic nanoparticles and nanowires. Acc Chem Res. 2008;41(12):1831-1841.
12. Panda HS, Srivastava R, Bahadur D. Shape and size control of nano dispersed Mg/Al layered double hydroxide. J Nanosci Nanotechnol. 2008;8(8):4218-4223.
13. Wang X, Li Q, Xie J, et al. Fabrication of ultralong and electrically uniform single-walled carbon nanotubes on clean substrates. Nano Lett. 2009;9(9):3137-3141.
14. Zhu Y, Li J, Li W, et al. The biocompatibility of nanodiamonds and their application in drug delivery systems. Theranostics. 2012;2(3):302-312.
15. Wu L, Ding J. In vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2004;25(2):5821-5830.
16. Wu X, Rabkin-Aikawa E, Guleserian KJ, et al. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2004;287(2):H480-H487.
17. Xia W, Liu W, Cui L, et al. Tissue engineering of cartilage with the use of chitosan-gelatin complex scaffolds. J Biomed Mater Res B Appl Biomater. 2004;71(2):373-380.
18. Laurencin CT, Kumbar SG, Nukavarapu SP. Nanotechnology and orthopedics: a personal perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(1):6-10.
19. Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;25(19):4731-4739.
20. Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21(17):1803-1810.
21. Stewart S, Barr S, Engiles J, et al. Vancomycin-modified implant surface inhibits biofilm formation and supports bone-healing in an infected osteotomy model in sheep: a proof-of-concept study. J Bone Joint Surg Am. 2012;94(15):1406-1415.
22. Hickok NJ, Shapiro IM. Immobilized antibiotics to prevent orthopaedic implant infections. Adv Drug Deliv Rev. 2012;64(12):1165-1176.
23. DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22(2):151-185.
24. Vines T, Faunce T. Assessing the safety and cost-effectiveness of early nanodrugs. J Law Med. 2009;16(5):822-845.
25. Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622-627.
26. Nanomedicine Market (Neurology, Cardiovascular, Anti-Inflammatory, Anti-Infective, and Oncology Applications): Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013-2019. Transparency Market Research website. http://www.transparencymarketresearch.com/nanomedicine-market.html. Published August 1, 2014. Accessed January 20, 2015.
27. Sullivan MP, McHale KJ, Parvizi J, Mehta S. Nanotechnology: current concepts in orthopaedic surgery and future directions. Bone Joint J. 2014;96-B(5):569-573.
28. Pleshko N, Grande DA, Myers KR. Nanotechnology in orthopaedics. J Am Acad Orthop Surg. 2012;20(1):60-62.