User login
Progressive Cardiomyopathy in a Patient With Elevated Cobalt Ion Levels and Bilateral Metal-on-Metal Hip Arthroplasties
Systemic cobalt toxicity has been reported in the literature after hip arthroplasty revisions for failed ceramic components secondary to third-body abrasive wear of cobalt-chrome (CoCr) components, as well as with metal-on-metal (MOM) hip arthroplasty designs. There have been several cases of systemic cobalt toxicity after revision for fractured ceramic components.1,2 Of these 7 reported cases, all patients had neurologic complaints and 4 patients developed cardiomyopathy secondary to toxic cobalt levels, with 1 case being fatal.1 MOM hip prostheses have also been associated with local and systemic problems secondary to metal debris. Adverse local tissue reactions have been reported to occur in up to 59% of patients, and, in some registries, the failure rate of MOM arthroplasty caused by these soft-tissue reactions is 2 to 3 times that of conventional metal-on-polyethylene design failures.3,4 The occurrence of systemic complications from MOM total hip arthroplasty (THA) wear debris is much less common. There have been 6 cases of systemic cobalt toxicity reported in the literature resulting from MOM total hip prosthesis design.1,2
We present a case of biopsy-confirmed cardiomyopathy secondary to cobalt toxicity from a MOM THA design with subsequent requirement for left ventricular assist device (LVAD) implantation despite prosthesis removal. To our knowledge, this is the first report in the literature of this specific implant design causing systemic cobalt toxicity. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a healthy nondiabetic man age 54 years who presented to our clinic 6 years after undergoing left THA and 5 years after undergoing right THA with the Biomet M2a-Magnum MOM prosthesis at an outside facility. The left-side components placed at the index procedure were a size 50 cup, 44 magnum head, 10 Taperloc stem (Biomet), and +9 neck. The right-side components were a size 52 cup, 46 magnum head, 10 Taperloc stem, and +3 neck. The patient emphasized that he was very happy with his hip prostheses and denied groin or thigh pain. His medical history was significant for exogenous obesity, and he denied any history of alcohol, tobacco, steroid, or recreational drug use.
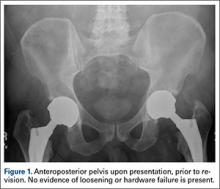
The patient’s review of systems suggested that, approximately 11 months prior to presentation at our facility, he began having difficulty with his activities of daily living secondary to chest pressure with exertion, fatigue, and associated diaphoresis. He complained of decreased sensation in his feet bilaterally but denied any hearing loss, tinnitus, or vision changes. He underwent evaluation of the new-onset chest discomfort with a cardiac stress test that suggested no active cardiac ischemia. An echocardiogram revealed mitral regurgitation, stage II diastolic dysfunction with a left ventricular ejection fraction of 55%. Additionally, during this time period, the patient was being followed by his local orthopedic surgeon for an elevated cobalt level of 120 ppb and a chromium level of 109 ppb. The patient was referred to our clinic for recommendations regarding the elevated metal-ion levels. Upon initial evaluation, the patient denied any hip or groin pain. His physical examination revealed a nonantalgic gait with full range of motion and no signs of instability, tenderness, or masses. The patient was also noted to have no vibratory sensation in his feet bilaterally. The plain radiographs indicated bilateral MOM THA with acetabular inclination levels of 55º on the right and left sides. No cystic changes or other worrisome signs that would suggest implant loosening or failure were present (Figure 1). The serum metal levels were repeated and showed a cobalt level of 189 ppb and a chromium level of 71 ppb. Whole venous blood samples were drawn at our request using trace element tubes and were sent to Medtox Laboratories Inc. for analysis. Other pertinent laboratory values, including hematocrit and thyroid levels, were within normal limits. Because of concerns of systemic toxicity from significantly elevated cobalt and chromium levels, the patient elected to proceed with revision of the MOM components.
During the preoperative medical evaluation, the patient’s cardiac status was a concern, and the etiology of the cardiac dysfunction was unclear. Cardiac magnetic resonance imaging (MRI), which was performed to evaluate the extent and etiology of cardiac dysfunction, showed biventricular dysfunction. To evaluate the underlying myocardial tissue characteristics, delayed contrast imaging was performed and showed diffuse myocardial hyperenhancement of the anterior, lateral, and apical walls, with sparing of the base and midseptum. This type of extensive hyperenhancement is commonly seen with cardiac amyloidosis; however, the blood-pool kinetics during contrast administration is unusual for amyloidosis, as well as the diffuse edema noted on T2-weighted MRI. Importantly, cardiac MRI is very specific in excluding alternative diagnoses, such as postinfarct, infiltrative, acquired, viral, or alcoholic/drugs of abuse etiologies. In the absence of amyloidosis, the only other pattern that would be consistent with symptoms was diffuse, fulminant myocarditis of toxic origin lacking clinical evidence for an infectious origin. The patient’s prior exposure to cobalt was noted. Thus, the hyperenhancement and edema could be strong supportive evidence of cobalt infiltration, despite no reported cases in the literature of cobalt cardiomyopathy found on cardiac MRI.
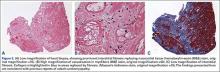
Additional workup was initiated, and cardiac catheterization showed that the patient continued to decompensate, with worsening global left ventricular dysfunction with an ejection fraction of 30% without evidence of coronary artery disease. Also, he was noted to have mild renal impairment with a blood urea nitrogen level of 31 mg/dL and a creatinine level of 1.7 mg/dL. The etiology of the renal impairment was unknown and had not been established, according to the patient and his wife. The renal impairment was not thought to be caused by the elevated metal ions levels but likely resulted from prerenal azotemia secondary to decreased cardiac output. During catheterization, an endomyocardial biopsy was performed and the tissue sent to the Mayo Clinic pathology department for analysis. The sample showed myocyte hypertrophy and interstitial fibrosis with scattered myofibers containing large cytoplasmic vacuoles. Also present was karyomegaly consistent with myocyte hypertrophy (Figures 2A, 2B). Trichrome stain confirmed replacement of myofibers by collagen (Figure 2C). Electron microscopy performed on a paraffin block showed reduced contractile elements, vacuolar spaces, and increased lipofuscin. The findings were very consistent with, but not specific for, cardiomyopathy from cobalt toxicity. No evidence of an inflammatory infiltrate was identified. The diagnosis was cobalt cardiomyopathy based on biopsy, presentation, cobalt levels, and intraoperative findings.
The patient was admitted to the cardiac intensive care unit preoperatively and optimized with inotropic agents. A multidisciplinary consultation with the cardiology and anesthesia departments was obtained. Both recommended cardiac anesthesia with intraoperative Swan-Ganz catheter and transesophageal echo monitoring. Assuming that the patient remained hemodynamically stable with limited blood loss and the first hip was timely performed, the cardiology department recommended a single surgery, because fewer risks and complications could be expected than from a staged procedure. Subsequently, surgery was performed on the left hip via a conservative anterior approach on the fracture table. The patient remained stable with limited blood loss. During the same operating room time, revision of the right hip was performed using an anterior approach. The intraoperative findings showed evidence of pseudotumors in the adjacent soft tissues and abundant brown, creamy fluid upon entering the joint capsule, consistent with a metallic appearance. Both hips showed similar prosthetic findings. There was no significant visible wear of the large diameter metal heads or gross abnormality of the acetabular components. The trunnion area on both femoral implants was abnormal, revealing a black coating suggestive of marked corrosion. The components were all well fixed, without visible damage, and, because of his fragile cardiac status, the patient’s acetabular components were not revised. The trunnions were cleaned and the femoral heads were revised to active articulation dual-mobility metal-on-polyethylene constructs using 28-mm Biolox Option ceramic (CeramTec). The tissue specimens from the operation showed chronic inflammation with areas of fibroconnective tissue and bland fibrinoid necrosis with extensive brown pigment-laden macrophage reaction. The intraoperative cultures were negative.
The patient tolerated the surgery without complication, and his postoperative period was without incident. Nine months after surgery, the patient’s cobalt and chromium levels had declined to 16 ppb and 32 ppb, respectively (normal, <1 ppb). However, his cardiac status continued to worsen with significant shortness of breath and bilateral lower extremity edema despite diuresis. Follow-up cardiac MRI indicated progressive left and right dysfunction with ejection fractions of 23% and 25%, respectively. After progressive heart-failure symptoms, the patient was admitted to the hospital for severe congestive heart failure and underwent implantation of a HeartWare LVAD with tricuspid valve repair using an Edwards annuloplasty ring. He has since had a cardiac transplant and is doing well.
Discussion
To our knowledge, this is the first reported case of cardiomyopathy in a patient with elevated cobalt ion levels and a Biomet M2a-Magnum hip prosthesis. This is also the first reported case of cardiac MRI–defined cobalt cardiomyopathy. The cobalt levels seen in this patient were similar to those of other cases with systemic cobalt toxicity from a MOM hip construct. Mao and colleagues5 reported 2 cases of systemic cobalt toxicity in 2 patients with articular surface replacement hip prostheses.One patient presented with mild groin pain, neurologic symptoms, and a cobalt level of 410 ppb 5 years after her index procedure. The other patient presented with cardiac and neurologic symptoms but no hip complaints. The patient’s cobalt levels ranged from 185 ppb to 210 ppb. Both patients improved after their revision surgery, and their cobalt levels decreased. The 2 patients in Tower’s report6 were 49-year-old men who had articular surface replacement implants (DePuy). One patient who presented with progressive hip pain 11 months postoperatively developed neurologic symptoms and cardiomyopathy, with cobalt levels of 83 ppb before revision surgery 43 months after his index procedure. The other patient presented with hip pain and vertigo, headaches, fatigue, and dyspnea. He underwent hip revision 40 months postoperatively and required closed reduction under sedation for dislocation. Finally, and most recently, Allen and colleagues2 reported a 59-year-old woman with a cobalt level of 287 ppb whose symptoms did not resolve after implantation of an LVAD or cardiac transplantation but only after removal of her bilateral hip prosthesis. Our case is most similar to this report but significantly adds to the literature in 2 distinct manners: (1) Biomet M2a-Magnum has not been implicated in cobalt toxicity; and (2) this is the first reported use of dedicated cardiac MRI to noninvasively define underlying cardiac pathology.
The cardiac manifestations secondary to systemic cobalt toxicity in this patient represent a frightening consequence of MOM prosthetic wear. The effects of cobalt toxicity on cardiac tissues were first described in a series of alcoholic patients from Manchester in 1900;7 however, it was not until 1967, in a series of patients in Quebec, that cobalt was found to be the inciting factor. In the modern era, hip arthroplasty techniques resulting in excessive cobalt and chromium wear have demonstrated the same findings of myocyte hypertrophy, interstitial fibrosis, and scattered myofibers containing large cytoplasmic inclusions.8,9 The patient presented here has pathologic findings consistent with previous cases of cobalt cardiomyopathy; however, in the other cases of cardiomyopathy due to MOM total hip components, the patients’ cardiac conditions improved after the prostheses were revised and the cobalt levels began to diminish.5,6In our case, the patient has sustained permanent damage to his myocardium and a progressive decline in his cardiac status, which is a deviation from reported cases as of 2014.
While there is no guideline to unequivocally diagnose cobalt cardiomyopathy, the constellation of findings, including pathologic, biologic, blood levels, imaging, and surgical, all uniformly indicate a unifying diagnosis. The lack of improvement after prosthetic device removal supports a diagnosis of permanent myocardial damage, which is consistent with cardiomyopathy of advanced toxic etiology.
Conclusion
This case presents a patient with bilateral MOM THAs, acetabular cup inclinations of greater than 55º, renal impairment, and cobalt levels greater than 60 ppb, with occult cardiac failure leading to LVAD implantation as a prelude to cardiac transplantation in order to avoid certain death. These factors have been shown, in prior case reports, to be associated with cardiac damage that may be reversible.6 However; it is important for orthopedic surgeons to recognize that certain hip prostheses can be associated or lead to irreversible cardiac damage.
1. Zywiel MG, Brandt JM, Overgaard CB, Cheung AC, Turgeon TR, Syed KA. Fatal cardiomyopathy after revision total hip replacement for fracture of a ceramic liner. Bone Joint J. 2013;95(1):31-37.
2. Allen LA, Ambardekar AV, Devaraj KM, Maleszewski JJ, Wolfel EE. Clinical problem-solving. Missing elements of the history. N Engl J Med. 2014;370(6):559-566.
3. Hart AJ, Satchihananda K, Liddle AD, et al. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: a case-control study using three-dimensional tomography and magnetic resonance imaging. J Bone Joint Surg Am. 2012;94(4);317-325.
4. Kwon MK, Jacobs JJ, MacDonald SJ, Potter HG, Fehring TK, Lombardi AV. Evidence-based understanding of management perils for metal-on-metal hip arthroplasty patients. J Arthroplasty. 2012;27(8 suppl):20-25.
5. Mao X, Wong AA, Crawford RW. Cobalt toxicity- -an emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194(12):649-651.
6. Tower SS. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92(17):2847-2851.
7. Morin Y, Daniel P. Quebec beer-drinkers’ cardiomyopathy: etiological considerations. Can Med Assoc J. 1967;97(15):926-928.
8. Gilbert C, Cheung A, Butany J, et al. Hip pain and heart failure: the missing link. Can J Cardiol. 2013;29(5):639.e1-e2.
9. Seghizzi P, D’Adda F, Borleri D, Barbic F, Mosconi G. Cobalt myocardiopathy. A critical review of literature. Sci Total Environ. 1994;150(1-3):105-109.
Systemic cobalt toxicity has been reported in the literature after hip arthroplasty revisions for failed ceramic components secondary to third-body abrasive wear of cobalt-chrome (CoCr) components, as well as with metal-on-metal (MOM) hip arthroplasty designs. There have been several cases of systemic cobalt toxicity after revision for fractured ceramic components.1,2 Of these 7 reported cases, all patients had neurologic complaints and 4 patients developed cardiomyopathy secondary to toxic cobalt levels, with 1 case being fatal.1 MOM hip prostheses have also been associated with local and systemic problems secondary to metal debris. Adverse local tissue reactions have been reported to occur in up to 59% of patients, and, in some registries, the failure rate of MOM arthroplasty caused by these soft-tissue reactions is 2 to 3 times that of conventional metal-on-polyethylene design failures.3,4 The occurrence of systemic complications from MOM total hip arthroplasty (THA) wear debris is much less common. There have been 6 cases of systemic cobalt toxicity reported in the literature resulting from MOM total hip prosthesis design.1,2
We present a case of biopsy-confirmed cardiomyopathy secondary to cobalt toxicity from a MOM THA design with subsequent requirement for left ventricular assist device (LVAD) implantation despite prosthesis removal. To our knowledge, this is the first report in the literature of this specific implant design causing systemic cobalt toxicity. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a healthy nondiabetic man age 54 years who presented to our clinic 6 years after undergoing left THA and 5 years after undergoing right THA with the Biomet M2a-Magnum MOM prosthesis at an outside facility. The left-side components placed at the index procedure were a size 50 cup, 44 magnum head, 10 Taperloc stem (Biomet), and +9 neck. The right-side components were a size 52 cup, 46 magnum head, 10 Taperloc stem, and +3 neck. The patient emphasized that he was very happy with his hip prostheses and denied groin or thigh pain. His medical history was significant for exogenous obesity, and he denied any history of alcohol, tobacco, steroid, or recreational drug use.
The patient’s review of systems suggested that, approximately 11 months prior to presentation at our facility, he began having difficulty with his activities of daily living secondary to chest pressure with exertion, fatigue, and associated diaphoresis. He complained of decreased sensation in his feet bilaterally but denied any hearing loss, tinnitus, or vision changes. He underwent evaluation of the new-onset chest discomfort with a cardiac stress test that suggested no active cardiac ischemia. An echocardiogram revealed mitral regurgitation, stage II diastolic dysfunction with a left ventricular ejection fraction of 55%. Additionally, during this time period, the patient was being followed by his local orthopedic surgeon for an elevated cobalt level of 120 ppb and a chromium level of 109 ppb. The patient was referred to our clinic for recommendations regarding the elevated metal-ion levels. Upon initial evaluation, the patient denied any hip or groin pain. His physical examination revealed a nonantalgic gait with full range of motion and no signs of instability, tenderness, or masses. The patient was also noted to have no vibratory sensation in his feet bilaterally. The plain radiographs indicated bilateral MOM THA with acetabular inclination levels of 55º on the right and left sides. No cystic changes or other worrisome signs that would suggest implant loosening or failure were present (Figure 1). The serum metal levels were repeated and showed a cobalt level of 189 ppb and a chromium level of 71 ppb. Whole venous blood samples were drawn at our request using trace element tubes and were sent to Medtox Laboratories Inc. for analysis. Other pertinent laboratory values, including hematocrit and thyroid levels, were within normal limits. Because of concerns of systemic toxicity from significantly elevated cobalt and chromium levels, the patient elected to proceed with revision of the MOM components.
During the preoperative medical evaluation, the patient’s cardiac status was a concern, and the etiology of the cardiac dysfunction was unclear. Cardiac magnetic resonance imaging (MRI), which was performed to evaluate the extent and etiology of cardiac dysfunction, showed biventricular dysfunction. To evaluate the underlying myocardial tissue characteristics, delayed contrast imaging was performed and showed diffuse myocardial hyperenhancement of the anterior, lateral, and apical walls, with sparing of the base and midseptum. This type of extensive hyperenhancement is commonly seen with cardiac amyloidosis; however, the blood-pool kinetics during contrast administration is unusual for amyloidosis, as well as the diffuse edema noted on T2-weighted MRI. Importantly, cardiac MRI is very specific in excluding alternative diagnoses, such as postinfarct, infiltrative, acquired, viral, or alcoholic/drugs of abuse etiologies. In the absence of amyloidosis, the only other pattern that would be consistent with symptoms was diffuse, fulminant myocarditis of toxic origin lacking clinical evidence for an infectious origin. The patient’s prior exposure to cobalt was noted. Thus, the hyperenhancement and edema could be strong supportive evidence of cobalt infiltration, despite no reported cases in the literature of cobalt cardiomyopathy found on cardiac MRI.
Additional workup was initiated, and cardiac catheterization showed that the patient continued to decompensate, with worsening global left ventricular dysfunction with an ejection fraction of 30% without evidence of coronary artery disease. Also, he was noted to have mild renal impairment with a blood urea nitrogen level of 31 mg/dL and a creatinine level of 1.7 mg/dL. The etiology of the renal impairment was unknown and had not been established, according to the patient and his wife. The renal impairment was not thought to be caused by the elevated metal ions levels but likely resulted from prerenal azotemia secondary to decreased cardiac output. During catheterization, an endomyocardial biopsy was performed and the tissue sent to the Mayo Clinic pathology department for analysis. The sample showed myocyte hypertrophy and interstitial fibrosis with scattered myofibers containing large cytoplasmic vacuoles. Also present was karyomegaly consistent with myocyte hypertrophy (Figures 2A, 2B). Trichrome stain confirmed replacement of myofibers by collagen (Figure 2C). Electron microscopy performed on a paraffin block showed reduced contractile elements, vacuolar spaces, and increased lipofuscin. The findings were very consistent with, but not specific for, cardiomyopathy from cobalt toxicity. No evidence of an inflammatory infiltrate was identified. The diagnosis was cobalt cardiomyopathy based on biopsy, presentation, cobalt levels, and intraoperative findings.
The patient was admitted to the cardiac intensive care unit preoperatively and optimized with inotropic agents. A multidisciplinary consultation with the cardiology and anesthesia departments was obtained. Both recommended cardiac anesthesia with intraoperative Swan-Ganz catheter and transesophageal echo monitoring. Assuming that the patient remained hemodynamically stable with limited blood loss and the first hip was timely performed, the cardiology department recommended a single surgery, because fewer risks and complications could be expected than from a staged procedure. Subsequently, surgery was performed on the left hip via a conservative anterior approach on the fracture table. The patient remained stable with limited blood loss. During the same operating room time, revision of the right hip was performed using an anterior approach. The intraoperative findings showed evidence of pseudotumors in the adjacent soft tissues and abundant brown, creamy fluid upon entering the joint capsule, consistent with a metallic appearance. Both hips showed similar prosthetic findings. There was no significant visible wear of the large diameter metal heads or gross abnormality of the acetabular components. The trunnion area on both femoral implants was abnormal, revealing a black coating suggestive of marked corrosion. The components were all well fixed, without visible damage, and, because of his fragile cardiac status, the patient’s acetabular components were not revised. The trunnions were cleaned and the femoral heads were revised to active articulation dual-mobility metal-on-polyethylene constructs using 28-mm Biolox Option ceramic (CeramTec). The tissue specimens from the operation showed chronic inflammation with areas of fibroconnective tissue and bland fibrinoid necrosis with extensive brown pigment-laden macrophage reaction. The intraoperative cultures were negative.
The patient tolerated the surgery without complication, and his postoperative period was without incident. Nine months after surgery, the patient’s cobalt and chromium levels had declined to 16 ppb and 32 ppb, respectively (normal, <1 ppb). However, his cardiac status continued to worsen with significant shortness of breath and bilateral lower extremity edema despite diuresis. Follow-up cardiac MRI indicated progressive left and right dysfunction with ejection fractions of 23% and 25%, respectively. After progressive heart-failure symptoms, the patient was admitted to the hospital for severe congestive heart failure and underwent implantation of a HeartWare LVAD with tricuspid valve repair using an Edwards annuloplasty ring. He has since had a cardiac transplant and is doing well.
Discussion
To our knowledge, this is the first reported case of cardiomyopathy in a patient with elevated cobalt ion levels and a Biomet M2a-Magnum hip prosthesis. This is also the first reported case of cardiac MRI–defined cobalt cardiomyopathy. The cobalt levels seen in this patient were similar to those of other cases with systemic cobalt toxicity from a MOM hip construct. Mao and colleagues5 reported 2 cases of systemic cobalt toxicity in 2 patients with articular surface replacement hip prostheses.One patient presented with mild groin pain, neurologic symptoms, and a cobalt level of 410 ppb 5 years after her index procedure. The other patient presented with cardiac and neurologic symptoms but no hip complaints. The patient’s cobalt levels ranged from 185 ppb to 210 ppb. Both patients improved after their revision surgery, and their cobalt levels decreased. The 2 patients in Tower’s report6 were 49-year-old men who had articular surface replacement implants (DePuy). One patient who presented with progressive hip pain 11 months postoperatively developed neurologic symptoms and cardiomyopathy, with cobalt levels of 83 ppb before revision surgery 43 months after his index procedure. The other patient presented with hip pain and vertigo, headaches, fatigue, and dyspnea. He underwent hip revision 40 months postoperatively and required closed reduction under sedation for dislocation. Finally, and most recently, Allen and colleagues2 reported a 59-year-old woman with a cobalt level of 287 ppb whose symptoms did not resolve after implantation of an LVAD or cardiac transplantation but only after removal of her bilateral hip prosthesis. Our case is most similar to this report but significantly adds to the literature in 2 distinct manners: (1) Biomet M2a-Magnum has not been implicated in cobalt toxicity; and (2) this is the first reported use of dedicated cardiac MRI to noninvasively define underlying cardiac pathology.
The cardiac manifestations secondary to systemic cobalt toxicity in this patient represent a frightening consequence of MOM prosthetic wear. The effects of cobalt toxicity on cardiac tissues were first described in a series of alcoholic patients from Manchester in 1900;7 however, it was not until 1967, in a series of patients in Quebec, that cobalt was found to be the inciting factor. In the modern era, hip arthroplasty techniques resulting in excessive cobalt and chromium wear have demonstrated the same findings of myocyte hypertrophy, interstitial fibrosis, and scattered myofibers containing large cytoplasmic inclusions.8,9 The patient presented here has pathologic findings consistent with previous cases of cobalt cardiomyopathy; however, in the other cases of cardiomyopathy due to MOM total hip components, the patients’ cardiac conditions improved after the prostheses were revised and the cobalt levels began to diminish.5,6In our case, the patient has sustained permanent damage to his myocardium and a progressive decline in his cardiac status, which is a deviation from reported cases as of 2014.
While there is no guideline to unequivocally diagnose cobalt cardiomyopathy, the constellation of findings, including pathologic, biologic, blood levels, imaging, and surgical, all uniformly indicate a unifying diagnosis. The lack of improvement after prosthetic device removal supports a diagnosis of permanent myocardial damage, which is consistent with cardiomyopathy of advanced toxic etiology.
Conclusion
This case presents a patient with bilateral MOM THAs, acetabular cup inclinations of greater than 55º, renal impairment, and cobalt levels greater than 60 ppb, with occult cardiac failure leading to LVAD implantation as a prelude to cardiac transplantation in order to avoid certain death. These factors have been shown, in prior case reports, to be associated with cardiac damage that may be reversible.6 However; it is important for orthopedic surgeons to recognize that certain hip prostheses can be associated or lead to irreversible cardiac damage.
Systemic cobalt toxicity has been reported in the literature after hip arthroplasty revisions for failed ceramic components secondary to third-body abrasive wear of cobalt-chrome (CoCr) components, as well as with metal-on-metal (MOM) hip arthroplasty designs. There have been several cases of systemic cobalt toxicity after revision for fractured ceramic components.1,2 Of these 7 reported cases, all patients had neurologic complaints and 4 patients developed cardiomyopathy secondary to toxic cobalt levels, with 1 case being fatal.1 MOM hip prostheses have also been associated with local and systemic problems secondary to metal debris. Adverse local tissue reactions have been reported to occur in up to 59% of patients, and, in some registries, the failure rate of MOM arthroplasty caused by these soft-tissue reactions is 2 to 3 times that of conventional metal-on-polyethylene design failures.3,4 The occurrence of systemic complications from MOM total hip arthroplasty (THA) wear debris is much less common. There have been 6 cases of systemic cobalt toxicity reported in the literature resulting from MOM total hip prosthesis design.1,2
We present a case of biopsy-confirmed cardiomyopathy secondary to cobalt toxicity from a MOM THA design with subsequent requirement for left ventricular assist device (LVAD) implantation despite prosthesis removal. To our knowledge, this is the first report in the literature of this specific implant design causing systemic cobalt toxicity. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a healthy nondiabetic man age 54 years who presented to our clinic 6 years after undergoing left THA and 5 years after undergoing right THA with the Biomet M2a-Magnum MOM prosthesis at an outside facility. The left-side components placed at the index procedure were a size 50 cup, 44 magnum head, 10 Taperloc stem (Biomet), and +9 neck. The right-side components were a size 52 cup, 46 magnum head, 10 Taperloc stem, and +3 neck. The patient emphasized that he was very happy with his hip prostheses and denied groin or thigh pain. His medical history was significant for exogenous obesity, and he denied any history of alcohol, tobacco, steroid, or recreational drug use.
The patient’s review of systems suggested that, approximately 11 months prior to presentation at our facility, he began having difficulty with his activities of daily living secondary to chest pressure with exertion, fatigue, and associated diaphoresis. He complained of decreased sensation in his feet bilaterally but denied any hearing loss, tinnitus, or vision changes. He underwent evaluation of the new-onset chest discomfort with a cardiac stress test that suggested no active cardiac ischemia. An echocardiogram revealed mitral regurgitation, stage II diastolic dysfunction with a left ventricular ejection fraction of 55%. Additionally, during this time period, the patient was being followed by his local orthopedic surgeon for an elevated cobalt level of 120 ppb and a chromium level of 109 ppb. The patient was referred to our clinic for recommendations regarding the elevated metal-ion levels. Upon initial evaluation, the patient denied any hip or groin pain. His physical examination revealed a nonantalgic gait with full range of motion and no signs of instability, tenderness, or masses. The patient was also noted to have no vibratory sensation in his feet bilaterally. The plain radiographs indicated bilateral MOM THA with acetabular inclination levels of 55º on the right and left sides. No cystic changes or other worrisome signs that would suggest implant loosening or failure were present (Figure 1). The serum metal levels were repeated and showed a cobalt level of 189 ppb and a chromium level of 71 ppb. Whole venous blood samples were drawn at our request using trace element tubes and were sent to Medtox Laboratories Inc. for analysis. Other pertinent laboratory values, including hematocrit and thyroid levels, were within normal limits. Because of concerns of systemic toxicity from significantly elevated cobalt and chromium levels, the patient elected to proceed with revision of the MOM components.
During the preoperative medical evaluation, the patient’s cardiac status was a concern, and the etiology of the cardiac dysfunction was unclear. Cardiac magnetic resonance imaging (MRI), which was performed to evaluate the extent and etiology of cardiac dysfunction, showed biventricular dysfunction. To evaluate the underlying myocardial tissue characteristics, delayed contrast imaging was performed and showed diffuse myocardial hyperenhancement of the anterior, lateral, and apical walls, with sparing of the base and midseptum. This type of extensive hyperenhancement is commonly seen with cardiac amyloidosis; however, the blood-pool kinetics during contrast administration is unusual for amyloidosis, as well as the diffuse edema noted on T2-weighted MRI. Importantly, cardiac MRI is very specific in excluding alternative diagnoses, such as postinfarct, infiltrative, acquired, viral, or alcoholic/drugs of abuse etiologies. In the absence of amyloidosis, the only other pattern that would be consistent with symptoms was diffuse, fulminant myocarditis of toxic origin lacking clinical evidence for an infectious origin. The patient’s prior exposure to cobalt was noted. Thus, the hyperenhancement and edema could be strong supportive evidence of cobalt infiltration, despite no reported cases in the literature of cobalt cardiomyopathy found on cardiac MRI.
Additional workup was initiated, and cardiac catheterization showed that the patient continued to decompensate, with worsening global left ventricular dysfunction with an ejection fraction of 30% without evidence of coronary artery disease. Also, he was noted to have mild renal impairment with a blood urea nitrogen level of 31 mg/dL and a creatinine level of 1.7 mg/dL. The etiology of the renal impairment was unknown and had not been established, according to the patient and his wife. The renal impairment was not thought to be caused by the elevated metal ions levels but likely resulted from prerenal azotemia secondary to decreased cardiac output. During catheterization, an endomyocardial biopsy was performed and the tissue sent to the Mayo Clinic pathology department for analysis. The sample showed myocyte hypertrophy and interstitial fibrosis with scattered myofibers containing large cytoplasmic vacuoles. Also present was karyomegaly consistent with myocyte hypertrophy (Figures 2A, 2B). Trichrome stain confirmed replacement of myofibers by collagen (Figure 2C). Electron microscopy performed on a paraffin block showed reduced contractile elements, vacuolar spaces, and increased lipofuscin. The findings were very consistent with, but not specific for, cardiomyopathy from cobalt toxicity. No evidence of an inflammatory infiltrate was identified. The diagnosis was cobalt cardiomyopathy based on biopsy, presentation, cobalt levels, and intraoperative findings.
The patient was admitted to the cardiac intensive care unit preoperatively and optimized with inotropic agents. A multidisciplinary consultation with the cardiology and anesthesia departments was obtained. Both recommended cardiac anesthesia with intraoperative Swan-Ganz catheter and transesophageal echo monitoring. Assuming that the patient remained hemodynamically stable with limited blood loss and the first hip was timely performed, the cardiology department recommended a single surgery, because fewer risks and complications could be expected than from a staged procedure. Subsequently, surgery was performed on the left hip via a conservative anterior approach on the fracture table. The patient remained stable with limited blood loss. During the same operating room time, revision of the right hip was performed using an anterior approach. The intraoperative findings showed evidence of pseudotumors in the adjacent soft tissues and abundant brown, creamy fluid upon entering the joint capsule, consistent with a metallic appearance. Both hips showed similar prosthetic findings. There was no significant visible wear of the large diameter metal heads or gross abnormality of the acetabular components. The trunnion area on both femoral implants was abnormal, revealing a black coating suggestive of marked corrosion. The components were all well fixed, without visible damage, and, because of his fragile cardiac status, the patient’s acetabular components were not revised. The trunnions were cleaned and the femoral heads were revised to active articulation dual-mobility metal-on-polyethylene constructs using 28-mm Biolox Option ceramic (CeramTec). The tissue specimens from the operation showed chronic inflammation with areas of fibroconnective tissue and bland fibrinoid necrosis with extensive brown pigment-laden macrophage reaction. The intraoperative cultures were negative.
The patient tolerated the surgery without complication, and his postoperative period was without incident. Nine months after surgery, the patient’s cobalt and chromium levels had declined to 16 ppb and 32 ppb, respectively (normal, <1 ppb). However, his cardiac status continued to worsen with significant shortness of breath and bilateral lower extremity edema despite diuresis. Follow-up cardiac MRI indicated progressive left and right dysfunction with ejection fractions of 23% and 25%, respectively. After progressive heart-failure symptoms, the patient was admitted to the hospital for severe congestive heart failure and underwent implantation of a HeartWare LVAD with tricuspid valve repair using an Edwards annuloplasty ring. He has since had a cardiac transplant and is doing well.
Discussion
To our knowledge, this is the first reported case of cardiomyopathy in a patient with elevated cobalt ion levels and a Biomet M2a-Magnum hip prosthesis. This is also the first reported case of cardiac MRI–defined cobalt cardiomyopathy. The cobalt levels seen in this patient were similar to those of other cases with systemic cobalt toxicity from a MOM hip construct. Mao and colleagues5 reported 2 cases of systemic cobalt toxicity in 2 patients with articular surface replacement hip prostheses.One patient presented with mild groin pain, neurologic symptoms, and a cobalt level of 410 ppb 5 years after her index procedure. The other patient presented with cardiac and neurologic symptoms but no hip complaints. The patient’s cobalt levels ranged from 185 ppb to 210 ppb. Both patients improved after their revision surgery, and their cobalt levels decreased. The 2 patients in Tower’s report6 were 49-year-old men who had articular surface replacement implants (DePuy). One patient who presented with progressive hip pain 11 months postoperatively developed neurologic symptoms and cardiomyopathy, with cobalt levels of 83 ppb before revision surgery 43 months after his index procedure. The other patient presented with hip pain and vertigo, headaches, fatigue, and dyspnea. He underwent hip revision 40 months postoperatively and required closed reduction under sedation for dislocation. Finally, and most recently, Allen and colleagues2 reported a 59-year-old woman with a cobalt level of 287 ppb whose symptoms did not resolve after implantation of an LVAD or cardiac transplantation but only after removal of her bilateral hip prosthesis. Our case is most similar to this report but significantly adds to the literature in 2 distinct manners: (1) Biomet M2a-Magnum has not been implicated in cobalt toxicity; and (2) this is the first reported use of dedicated cardiac MRI to noninvasively define underlying cardiac pathology.
The cardiac manifestations secondary to systemic cobalt toxicity in this patient represent a frightening consequence of MOM prosthetic wear. The effects of cobalt toxicity on cardiac tissues were first described in a series of alcoholic patients from Manchester in 1900;7 however, it was not until 1967, in a series of patients in Quebec, that cobalt was found to be the inciting factor. In the modern era, hip arthroplasty techniques resulting in excessive cobalt and chromium wear have demonstrated the same findings of myocyte hypertrophy, interstitial fibrosis, and scattered myofibers containing large cytoplasmic inclusions.8,9 The patient presented here has pathologic findings consistent with previous cases of cobalt cardiomyopathy; however, in the other cases of cardiomyopathy due to MOM total hip components, the patients’ cardiac conditions improved after the prostheses were revised and the cobalt levels began to diminish.5,6In our case, the patient has sustained permanent damage to his myocardium and a progressive decline in his cardiac status, which is a deviation from reported cases as of 2014.
While there is no guideline to unequivocally diagnose cobalt cardiomyopathy, the constellation of findings, including pathologic, biologic, blood levels, imaging, and surgical, all uniformly indicate a unifying diagnosis. The lack of improvement after prosthetic device removal supports a diagnosis of permanent myocardial damage, which is consistent with cardiomyopathy of advanced toxic etiology.
Conclusion
This case presents a patient with bilateral MOM THAs, acetabular cup inclinations of greater than 55º, renal impairment, and cobalt levels greater than 60 ppb, with occult cardiac failure leading to LVAD implantation as a prelude to cardiac transplantation in order to avoid certain death. These factors have been shown, in prior case reports, to be associated with cardiac damage that may be reversible.6 However; it is important for orthopedic surgeons to recognize that certain hip prostheses can be associated or lead to irreversible cardiac damage.
1. Zywiel MG, Brandt JM, Overgaard CB, Cheung AC, Turgeon TR, Syed KA. Fatal cardiomyopathy after revision total hip replacement for fracture of a ceramic liner. Bone Joint J. 2013;95(1):31-37.
2. Allen LA, Ambardekar AV, Devaraj KM, Maleszewski JJ, Wolfel EE. Clinical problem-solving. Missing elements of the history. N Engl J Med. 2014;370(6):559-566.
3. Hart AJ, Satchihananda K, Liddle AD, et al. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: a case-control study using three-dimensional tomography and magnetic resonance imaging. J Bone Joint Surg Am. 2012;94(4);317-325.
4. Kwon MK, Jacobs JJ, MacDonald SJ, Potter HG, Fehring TK, Lombardi AV. Evidence-based understanding of management perils for metal-on-metal hip arthroplasty patients. J Arthroplasty. 2012;27(8 suppl):20-25.
5. Mao X, Wong AA, Crawford RW. Cobalt toxicity- -an emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194(12):649-651.
6. Tower SS. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92(17):2847-2851.
7. Morin Y, Daniel P. Quebec beer-drinkers’ cardiomyopathy: etiological considerations. Can Med Assoc J. 1967;97(15):926-928.
8. Gilbert C, Cheung A, Butany J, et al. Hip pain and heart failure: the missing link. Can J Cardiol. 2013;29(5):639.e1-e2.
9. Seghizzi P, D’Adda F, Borleri D, Barbic F, Mosconi G. Cobalt myocardiopathy. A critical review of literature. Sci Total Environ. 1994;150(1-3):105-109.
1. Zywiel MG, Brandt JM, Overgaard CB, Cheung AC, Turgeon TR, Syed KA. Fatal cardiomyopathy after revision total hip replacement for fracture of a ceramic liner. Bone Joint J. 2013;95(1):31-37.
2. Allen LA, Ambardekar AV, Devaraj KM, Maleszewski JJ, Wolfel EE. Clinical problem-solving. Missing elements of the history. N Engl J Med. 2014;370(6):559-566.
3. Hart AJ, Satchihananda K, Liddle AD, et al. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: a case-control study using three-dimensional tomography and magnetic resonance imaging. J Bone Joint Surg Am. 2012;94(4);317-325.
4. Kwon MK, Jacobs JJ, MacDonald SJ, Potter HG, Fehring TK, Lombardi AV. Evidence-based understanding of management perils for metal-on-metal hip arthroplasty patients. J Arthroplasty. 2012;27(8 suppl):20-25.
5. Mao X, Wong AA, Crawford RW. Cobalt toxicity- -an emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194(12):649-651.
6. Tower SS. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92(17):2847-2851.
7. Morin Y, Daniel P. Quebec beer-drinkers’ cardiomyopathy: etiological considerations. Can Med Assoc J. 1967;97(15):926-928.
8. Gilbert C, Cheung A, Butany J, et al. Hip pain and heart failure: the missing link. Can J Cardiol. 2013;29(5):639.e1-e2.
9. Seghizzi P, D’Adda F, Borleri D, Barbic F, Mosconi G. Cobalt myocardiopathy. A critical review of literature. Sci Total Environ. 1994;150(1-3):105-109.