User login
‘Unexpected’: Breast cancer spreads most during sleep
a discovery the investigators called “striking and unexpected.”
“This has not been shown before [and] we were surprised, indeed,” Nicola Aceto, PhD, professor of molecular oncology, Swiss Federal Institute of Technology Zürich, said in an interview.
The findings carry potential implications for the timing of biopsy and treatment of metastasis-prone cancers, the authors said.
The study was published online in Nature.
Circulating tumor cells (CTCs) are generally believed to shed constantly or following particular events such as surgery or physical activity; however, the processes that regulate tumor cell metastasis and how circadian rhythms may play into tumorigenesis remain unclear.
To better understand these processes, Dr. Aceto and colleagues collected blood samples from 30 women with breast cancer at 4:00 a.m. and 10:00 a.m. – times representing the body’s resting and active phases, respectively.
The researchers observed that more than 78% of all CTCs obtained were from samples taken during the resting phase.
This finding is astounding, Harrison Ball, a PhD candidate, and Sunitha Nagrath, PhD, with the University of Michigan, Ann Arbor, wrote in Nature News & Views .
Dr. Aceto and colleagues also found that CTCs generated at night divide more quickly and therefore have a higher potential to metastasize, compared with those generated during the day, which “are devoid of metastatic ability,” according to the authors, who obtained similar results in a series of mouse models.
The team also observed that key circadian rhythm hormones (such as melatonin, testosterone, and glucocorticoids) regulate CTC generation, and insulin promotes tumor cell proliferation in a time-dependent manner, suggesting a “need for time-controlled approaches for the characterization and treatment of breast cancer,” the authors wrote.
Practice changing?
Dr. Ball and Dr. Nagrath said the time-dependent nature of CTC dynamics could very well transform how cancer patients are assessed and treated.
“The data pointing to CTC proliferation and release during the rest phase suggest that doctors might need to become more conscious of when to administer specific treatments,” they wrote.
Both cautioned, however, that large clinical trials would be needed before any consideration of circadian rhythms is incorporated into standard practice. It’s also unclear whether these results in breast cancer hold true for other tumor types.
Mariana G. Figueiro, PhD, who was not involved in the research, agreed that, if studies confirm more metastatic spread at night, “there is an opportunity to treat patients at strategic times.”
Dr. Figueiro, of the Icahn School of Medicine at Mount Sinai, New York, also saw a potential impact on the timing of blood draws. “I think tightening up on how people do biopsies and bloodwork based on circadian time is important.”
Marleen Meyers, MD, agreed that these findings could have many clinical implications.
“The most obvious is that the time of day [that] treatment is administered may influence efficacy,” said Dr. Meyers, clinical professor of medicine at New York University Langone’s Perlmutter Cancer Center.
But, Dr. Meyers noted, the benefits of treating someone at night would need to be weighed against the downsides of interrupting a person’s normal sleep-wake cycle. “If this finding is clinically important it will be a challenge incorporating this into clinical care,” she said.
The study had no funding reported. Dr. Aceto is a cofounder and member of the board of PAGE Therapeutics, listed as an inventor in patent applications related to circulating tumor cells, a paid consultant for several companies, and a Novartis shareholder. One coauthor is a cofounder of PAGE Therapeutics. All other authors declare no competing interests. Dr. Meyers and Dr. Figueiro reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a discovery the investigators called “striking and unexpected.”
“This has not been shown before [and] we were surprised, indeed,” Nicola Aceto, PhD, professor of molecular oncology, Swiss Federal Institute of Technology Zürich, said in an interview.
The findings carry potential implications for the timing of biopsy and treatment of metastasis-prone cancers, the authors said.
The study was published online in Nature.
Circulating tumor cells (CTCs) are generally believed to shed constantly or following particular events such as surgery or physical activity; however, the processes that regulate tumor cell metastasis and how circadian rhythms may play into tumorigenesis remain unclear.
To better understand these processes, Dr. Aceto and colleagues collected blood samples from 30 women with breast cancer at 4:00 a.m. and 10:00 a.m. – times representing the body’s resting and active phases, respectively.
The researchers observed that more than 78% of all CTCs obtained were from samples taken during the resting phase.
This finding is astounding, Harrison Ball, a PhD candidate, and Sunitha Nagrath, PhD, with the University of Michigan, Ann Arbor, wrote in Nature News & Views .
Dr. Aceto and colleagues also found that CTCs generated at night divide more quickly and therefore have a higher potential to metastasize, compared with those generated during the day, which “are devoid of metastatic ability,” according to the authors, who obtained similar results in a series of mouse models.
The team also observed that key circadian rhythm hormones (such as melatonin, testosterone, and glucocorticoids) regulate CTC generation, and insulin promotes tumor cell proliferation in a time-dependent manner, suggesting a “need for time-controlled approaches for the characterization and treatment of breast cancer,” the authors wrote.
Practice changing?
Dr. Ball and Dr. Nagrath said the time-dependent nature of CTC dynamics could very well transform how cancer patients are assessed and treated.
“The data pointing to CTC proliferation and release during the rest phase suggest that doctors might need to become more conscious of when to administer specific treatments,” they wrote.
Both cautioned, however, that large clinical trials would be needed before any consideration of circadian rhythms is incorporated into standard practice. It’s also unclear whether these results in breast cancer hold true for other tumor types.
Mariana G. Figueiro, PhD, who was not involved in the research, agreed that, if studies confirm more metastatic spread at night, “there is an opportunity to treat patients at strategic times.”
Dr. Figueiro, of the Icahn School of Medicine at Mount Sinai, New York, also saw a potential impact on the timing of blood draws. “I think tightening up on how people do biopsies and bloodwork based on circadian time is important.”
Marleen Meyers, MD, agreed that these findings could have many clinical implications.
“The most obvious is that the time of day [that] treatment is administered may influence efficacy,” said Dr. Meyers, clinical professor of medicine at New York University Langone’s Perlmutter Cancer Center.
But, Dr. Meyers noted, the benefits of treating someone at night would need to be weighed against the downsides of interrupting a person’s normal sleep-wake cycle. “If this finding is clinically important it will be a challenge incorporating this into clinical care,” she said.
The study had no funding reported. Dr. Aceto is a cofounder and member of the board of PAGE Therapeutics, listed as an inventor in patent applications related to circulating tumor cells, a paid consultant for several companies, and a Novartis shareholder. One coauthor is a cofounder of PAGE Therapeutics. All other authors declare no competing interests. Dr. Meyers and Dr. Figueiro reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a discovery the investigators called “striking and unexpected.”
“This has not been shown before [and] we were surprised, indeed,” Nicola Aceto, PhD, professor of molecular oncology, Swiss Federal Institute of Technology Zürich, said in an interview.
The findings carry potential implications for the timing of biopsy and treatment of metastasis-prone cancers, the authors said.
The study was published online in Nature.
Circulating tumor cells (CTCs) are generally believed to shed constantly or following particular events such as surgery or physical activity; however, the processes that regulate tumor cell metastasis and how circadian rhythms may play into tumorigenesis remain unclear.
To better understand these processes, Dr. Aceto and colleagues collected blood samples from 30 women with breast cancer at 4:00 a.m. and 10:00 a.m. – times representing the body’s resting and active phases, respectively.
The researchers observed that more than 78% of all CTCs obtained were from samples taken during the resting phase.
This finding is astounding, Harrison Ball, a PhD candidate, and Sunitha Nagrath, PhD, with the University of Michigan, Ann Arbor, wrote in Nature News & Views .
Dr. Aceto and colleagues also found that CTCs generated at night divide more quickly and therefore have a higher potential to metastasize, compared with those generated during the day, which “are devoid of metastatic ability,” according to the authors, who obtained similar results in a series of mouse models.
The team also observed that key circadian rhythm hormones (such as melatonin, testosterone, and glucocorticoids) regulate CTC generation, and insulin promotes tumor cell proliferation in a time-dependent manner, suggesting a “need for time-controlled approaches for the characterization and treatment of breast cancer,” the authors wrote.
Practice changing?
Dr. Ball and Dr. Nagrath said the time-dependent nature of CTC dynamics could very well transform how cancer patients are assessed and treated.
“The data pointing to CTC proliferation and release during the rest phase suggest that doctors might need to become more conscious of when to administer specific treatments,” they wrote.
Both cautioned, however, that large clinical trials would be needed before any consideration of circadian rhythms is incorporated into standard practice. It’s also unclear whether these results in breast cancer hold true for other tumor types.
Mariana G. Figueiro, PhD, who was not involved in the research, agreed that, if studies confirm more metastatic spread at night, “there is an opportunity to treat patients at strategic times.”
Dr. Figueiro, of the Icahn School of Medicine at Mount Sinai, New York, also saw a potential impact on the timing of blood draws. “I think tightening up on how people do biopsies and bloodwork based on circadian time is important.”
Marleen Meyers, MD, agreed that these findings could have many clinical implications.
“The most obvious is that the time of day [that] treatment is administered may influence efficacy,” said Dr. Meyers, clinical professor of medicine at New York University Langone’s Perlmutter Cancer Center.
But, Dr. Meyers noted, the benefits of treating someone at night would need to be weighed against the downsides of interrupting a person’s normal sleep-wake cycle. “If this finding is clinically important it will be a challenge incorporating this into clinical care,” she said.
The study had no funding reported. Dr. Aceto is a cofounder and member of the board of PAGE Therapeutics, listed as an inventor in patent applications related to circulating tumor cells, a paid consultant for several companies, and a Novartis shareholder. One coauthor is a cofounder of PAGE Therapeutics. All other authors declare no competing interests. Dr. Meyers and Dr. Figueiro reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE
Jury out on low-FODMAP diet for kids
There is scarce evidence to support the use of a FODMAP-lowering diet for children with irritable bowel syndrome (IBS), and there is no evidence to recommend its use for other gastrointestinal (GI) diseases and complaints in children, according to a position paper from the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN).
A low-FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) diet is increasingly being used to treat children with various GI complaints and disorders.
“Awareness of how and when to use the diet is crucial, as a restrictive diet may impact nutritional adequacy and/or promote distorted eating in vulnerable subjects,” the authors note.
Rut Anne Thomassen, department of pediatric medicine, Oslo University Hospital, and an international team of experts conducted a systematic literature review of the evidence on the safety and efficacy of the low-FODMAP diet in children.
The low-FODMAP diet has not been well studied in children, they report.
From 53 publications and registers that they screened, only seven studies (four randomized clinical trials and three interventions without control group or observational studies) were included in their assessment.
In the seven studies, only 111 children received the low-FODMAP diet, while 85 followed a control diet for comparison (a diet described as healthy, usual, or typical American diet for children).
All of the pediatric studies focused on functional abdominal pain disorders. None addressed nonceliac gluten sensitivity, small-intestinal bacterial overgrowth, or inflammatory bowel disease.
From their review, the authors conclude that, at present, there is “insufficient evidence” to routinely recommend the low-FODMAP diet for the treatment of functional GI disorders, nonceliac gluten sensitivity, inflammatory bowel diseases, or small-intestinal bacterial overgrowth in children.
When the low-FODMAP diet is considered for children, the authors recommend a thorough clinical history, physical examination, and assessment of nutritional status and GI symptoms by a multidisciplinary team.
“Ideally, a standardized questionnaire should be used before and following the start of the diet to assess objectively the effect of the low-FODMAP diet,” the authors advise.
A dietitian should assess the child’s diet to highlight any potential deficiencies, which could be exacerbated by the restrictions of the low-FODMAP diet.
To promote adherence to the diet, potential difficulties, such as how to provide a suitable lunch at school or what to do when the child is staying at a friend’s house, should be addressed.
The authors suggest providing parents with written information about sources of FODMAPs and suitable replacement foods. Offering meal plans can reduce the risk of diet mistakes as well as the risk of offering a diet insufficient in essential nutrients, they say.
‘Useful paper’
“This is a useful paper primarily to outline the paucity of data regarding dietary therapies in children and the importance of doing studies in this population,” Ashwin Ananthakrishnan, MD, MPH, a gastroenterologist with Massachusetts General Hospital and Harvard Medical School, both in Boston, who wasn’t involved in the research, told this news organization.
Samuel Nurko, MD, MPH, director of the Center for Motility and Functional Gastrointestinal Disorders at Boston Children’s Hospital, Massachusetts, noted that some studies have shown that a low-FODMAP diet can be effective in controlling symptoms for both adults and kids.
“The problem in kids is that the trials are very small, and there’s not a lot of them, so the evidence is limited,” said Dr. Nurko, who wasn’t involved in writing the position paper.
That’s not to say that it should not be tried in appropriate cases. “There’s no question that in some patients, taking away the FODMAPs gives them a big improvement in GI symptoms,” Dr. Nurko told this news organization.
“The problem with the low-FODMAP diet is, if you don’t do it right, then you get into trouble with nutritional deficiencies,” he cautioned.
“If you are going to try the low-FODMAP diet, it has to be short, no more than 4-6 weeks, and you need to do a top-down approach. Take FODMAPs out, and then start to reintroduce them. Either kids will respond to the diet, or they won’t. If they don’t, there is no reason to keep them on the diet. It’s a very hard diet to take,” Dr. Nurko said.
No source of funding for the study was disclosed. The authors, Dr. Ananthakrishnan, and Dr. Nurko have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There is scarce evidence to support the use of a FODMAP-lowering diet for children with irritable bowel syndrome (IBS), and there is no evidence to recommend its use for other gastrointestinal (GI) diseases and complaints in children, according to a position paper from the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN).
A low-FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) diet is increasingly being used to treat children with various GI complaints and disorders.
“Awareness of how and when to use the diet is crucial, as a restrictive diet may impact nutritional adequacy and/or promote distorted eating in vulnerable subjects,” the authors note.
Rut Anne Thomassen, department of pediatric medicine, Oslo University Hospital, and an international team of experts conducted a systematic literature review of the evidence on the safety and efficacy of the low-FODMAP diet in children.
The low-FODMAP diet has not been well studied in children, they report.
From 53 publications and registers that they screened, only seven studies (four randomized clinical trials and three interventions without control group or observational studies) were included in their assessment.
In the seven studies, only 111 children received the low-FODMAP diet, while 85 followed a control diet for comparison (a diet described as healthy, usual, or typical American diet for children).
All of the pediatric studies focused on functional abdominal pain disorders. None addressed nonceliac gluten sensitivity, small-intestinal bacterial overgrowth, or inflammatory bowel disease.
From their review, the authors conclude that, at present, there is “insufficient evidence” to routinely recommend the low-FODMAP diet for the treatment of functional GI disorders, nonceliac gluten sensitivity, inflammatory bowel diseases, or small-intestinal bacterial overgrowth in children.
When the low-FODMAP diet is considered for children, the authors recommend a thorough clinical history, physical examination, and assessment of nutritional status and GI symptoms by a multidisciplinary team.
“Ideally, a standardized questionnaire should be used before and following the start of the diet to assess objectively the effect of the low-FODMAP diet,” the authors advise.
A dietitian should assess the child’s diet to highlight any potential deficiencies, which could be exacerbated by the restrictions of the low-FODMAP diet.
To promote adherence to the diet, potential difficulties, such as how to provide a suitable lunch at school or what to do when the child is staying at a friend’s house, should be addressed.
The authors suggest providing parents with written information about sources of FODMAPs and suitable replacement foods. Offering meal plans can reduce the risk of diet mistakes as well as the risk of offering a diet insufficient in essential nutrients, they say.
‘Useful paper’
“This is a useful paper primarily to outline the paucity of data regarding dietary therapies in children and the importance of doing studies in this population,” Ashwin Ananthakrishnan, MD, MPH, a gastroenterologist with Massachusetts General Hospital and Harvard Medical School, both in Boston, who wasn’t involved in the research, told this news organization.
Samuel Nurko, MD, MPH, director of the Center for Motility and Functional Gastrointestinal Disorders at Boston Children’s Hospital, Massachusetts, noted that some studies have shown that a low-FODMAP diet can be effective in controlling symptoms for both adults and kids.
“The problem in kids is that the trials are very small, and there’s not a lot of them, so the evidence is limited,” said Dr. Nurko, who wasn’t involved in writing the position paper.
That’s not to say that it should not be tried in appropriate cases. “There’s no question that in some patients, taking away the FODMAPs gives them a big improvement in GI symptoms,” Dr. Nurko told this news organization.
“The problem with the low-FODMAP diet is, if you don’t do it right, then you get into trouble with nutritional deficiencies,” he cautioned.
“If you are going to try the low-FODMAP diet, it has to be short, no more than 4-6 weeks, and you need to do a top-down approach. Take FODMAPs out, and then start to reintroduce them. Either kids will respond to the diet, or they won’t. If they don’t, there is no reason to keep them on the diet. It’s a very hard diet to take,” Dr. Nurko said.
No source of funding for the study was disclosed. The authors, Dr. Ananthakrishnan, and Dr. Nurko have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There is scarce evidence to support the use of a FODMAP-lowering diet for children with irritable bowel syndrome (IBS), and there is no evidence to recommend its use for other gastrointestinal (GI) diseases and complaints in children, according to a position paper from the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN).
A low-FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) diet is increasingly being used to treat children with various GI complaints and disorders.
“Awareness of how and when to use the diet is crucial, as a restrictive diet may impact nutritional adequacy and/or promote distorted eating in vulnerable subjects,” the authors note.
Rut Anne Thomassen, department of pediatric medicine, Oslo University Hospital, and an international team of experts conducted a systematic literature review of the evidence on the safety and efficacy of the low-FODMAP diet in children.
The low-FODMAP diet has not been well studied in children, they report.
From 53 publications and registers that they screened, only seven studies (four randomized clinical trials and three interventions without control group or observational studies) were included in their assessment.
In the seven studies, only 111 children received the low-FODMAP diet, while 85 followed a control diet for comparison (a diet described as healthy, usual, or typical American diet for children).
All of the pediatric studies focused on functional abdominal pain disorders. None addressed nonceliac gluten sensitivity, small-intestinal bacterial overgrowth, or inflammatory bowel disease.
From their review, the authors conclude that, at present, there is “insufficient evidence” to routinely recommend the low-FODMAP diet for the treatment of functional GI disorders, nonceliac gluten sensitivity, inflammatory bowel diseases, or small-intestinal bacterial overgrowth in children.
When the low-FODMAP diet is considered for children, the authors recommend a thorough clinical history, physical examination, and assessment of nutritional status and GI symptoms by a multidisciplinary team.
“Ideally, a standardized questionnaire should be used before and following the start of the diet to assess objectively the effect of the low-FODMAP diet,” the authors advise.
A dietitian should assess the child’s diet to highlight any potential deficiencies, which could be exacerbated by the restrictions of the low-FODMAP diet.
To promote adherence to the diet, potential difficulties, such as how to provide a suitable lunch at school or what to do when the child is staying at a friend’s house, should be addressed.
The authors suggest providing parents with written information about sources of FODMAPs and suitable replacement foods. Offering meal plans can reduce the risk of diet mistakes as well as the risk of offering a diet insufficient in essential nutrients, they say.
‘Useful paper’
“This is a useful paper primarily to outline the paucity of data regarding dietary therapies in children and the importance of doing studies in this population,” Ashwin Ananthakrishnan, MD, MPH, a gastroenterologist with Massachusetts General Hospital and Harvard Medical School, both in Boston, who wasn’t involved in the research, told this news organization.
Samuel Nurko, MD, MPH, director of the Center for Motility and Functional Gastrointestinal Disorders at Boston Children’s Hospital, Massachusetts, noted that some studies have shown that a low-FODMAP diet can be effective in controlling symptoms for both adults and kids.
“The problem in kids is that the trials are very small, and there’s not a lot of them, so the evidence is limited,” said Dr. Nurko, who wasn’t involved in writing the position paper.
That’s not to say that it should not be tried in appropriate cases. “There’s no question that in some patients, taking away the FODMAPs gives them a big improvement in GI symptoms,” Dr. Nurko told this news organization.
“The problem with the low-FODMAP diet is, if you don’t do it right, then you get into trouble with nutritional deficiencies,” he cautioned.
“If you are going to try the low-FODMAP diet, it has to be short, no more than 4-6 weeks, and you need to do a top-down approach. Take FODMAPs out, and then start to reintroduce them. Either kids will respond to the diet, or they won’t. If they don’t, there is no reason to keep them on the diet. It’s a very hard diet to take,” Dr. Nurko said.
No source of funding for the study was disclosed. The authors, Dr. Ananthakrishnan, and Dr. Nurko have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
$150K: Average industry payment to top 1% of oncologists
A small number of U.S. medical oncologists make more than $100,000 a year in general payments from drug companies, a new study shows.
These high-payment physicians represent just 1% of all U.S. medical oncologists, yet they account for 37% of industry payments. These oncologists often hold important leadership positions, draft treatment guidelines, and sit on journal editorial boards.
The findings highlight a risk for “perceived and real conflict of interest,” corresponding author Christopher Booth, MD, of Queen’s University Cancer Research Center, Kingston, Ont., said in an interview. “Because of the leadership positions they hold, the potential impact of this small group of physicians on oncology practice and policy may be substantial.”
The study was published online in JCO Oncology Practice.
‘We have a problem’
It’s no secret that many oncologists have financial relationships with pharmaceutical companies. They receive payments for research initiatives, but they also receive more general, personal payments in the form of honoraria, consultant fees, gifts, and reimbursement for travel and meals.
Prior studies have shown that these payments are typically modest, but a small subset of medical oncologists receive more than $100,000 annually. Dr. Booth and colleagues wanted to know more about the characteristics of these “high-payment” oncologists.
Using the national Open Payments database, the researchers identified a total of 139 medical oncologists who practice in the United States and who received $100,000 or more in general payments linked to cancer medications in 2018.
In U.S. dollars, the median payment was $154,613, and the total was $24.2 million.
The majority (95%) of high-payment oncologists were active in clinical work, 56% worked in an academic setting, 31% worked at National Cancer Institute–designated cancer centers, and 23% worked at National Comprehensive Cancer Network (NCCN) centers.
Many were based in California (17%), Texas (12%), Florida (10%), and New York (8%).
Most currently hold or have held hospital leadership positions (60%) or faculty appointments (72%) and 21% have held leadership positions in specialty associations in the past 5 years. Nearly one-quarter (24%) have served on journal editorial boards, and 10% have authored clinical practice guidelines in the past 5 years.
More specifically, three physicians authored NCCN guidelines, and two authored American Society of Clinical Oncology guidelines during 2016-2021; one guideline was published in 2018 when payments were made.
“Oncology specialty associations, guideline panels, and journal editorial boards should reconsider if it is appropriate for physicians with such large payments to hold these high-profile positions,” Dr. Booth said.
Following publication of the study, some oncologists took to Twitter with reactions, including Manni Mohyuddin, MD (@ManniMD1), from the Huntsman Cancer Institute, University of Utah, Salt Lake City, who wrote: “I recognize that some conflict of interest ‘may’ be unavoidable in order to run trials. But when greater than TWICE the average American household annual salary is taken in payments from industry by those in leadership/editorial roles, we have a problem.”
Weighing in on the results, ASCO CEO Clifford A. Hudis, MD, told this news organization that the “limitations of the study make it difficult to draw conclusions about the scope or potential impact of these payments on care.”
For example, he explained, some payments attributed to individuals may have been made directly to the physicians’ institutions or employers for sponsored research expenses.
Dr. Hudis also noted that the payments examined in the study were made in 2018, whereas the potentially relevant leadership positions could have been attained at a different time.
Furthermore, in 2020, an editorial appeared in Cancer, showing that errors in Open Payments are “fairly common,” Dr. Hudis said. It’s also unclear whether the reported financial relationships were appropriately disclosed and were managed at the time under relevant conflict of interest policies, he said.
“The question left unanswered by this study is whether or not these relationships influence patient care,” said Dr. Hudis. He noted that decisions about care should come from physicians and patients who are informed of the best available, unbiased, peer-reviewed, scientific evidence.
“The potential impact of financial conflicts of interest on this effort is an issue of concern, even if this study does not directly address it,” Dr. Hudis said.
The study had no specific funding. Dr. Booth has disclosed no relevant financial relationships. A complete list of author disclosures is available with the original article. Dr. Hudis has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A small number of U.S. medical oncologists make more than $100,000 a year in general payments from drug companies, a new study shows.
These high-payment physicians represent just 1% of all U.S. medical oncologists, yet they account for 37% of industry payments. These oncologists often hold important leadership positions, draft treatment guidelines, and sit on journal editorial boards.
The findings highlight a risk for “perceived and real conflict of interest,” corresponding author Christopher Booth, MD, of Queen’s University Cancer Research Center, Kingston, Ont., said in an interview. “Because of the leadership positions they hold, the potential impact of this small group of physicians on oncology practice and policy may be substantial.”
The study was published online in JCO Oncology Practice.
‘We have a problem’
It’s no secret that many oncologists have financial relationships with pharmaceutical companies. They receive payments for research initiatives, but they also receive more general, personal payments in the form of honoraria, consultant fees, gifts, and reimbursement for travel and meals.
Prior studies have shown that these payments are typically modest, but a small subset of medical oncologists receive more than $100,000 annually. Dr. Booth and colleagues wanted to know more about the characteristics of these “high-payment” oncologists.
Using the national Open Payments database, the researchers identified a total of 139 medical oncologists who practice in the United States and who received $100,000 or more in general payments linked to cancer medications in 2018.
In U.S. dollars, the median payment was $154,613, and the total was $24.2 million.
The majority (95%) of high-payment oncologists were active in clinical work, 56% worked in an academic setting, 31% worked at National Cancer Institute–designated cancer centers, and 23% worked at National Comprehensive Cancer Network (NCCN) centers.
Many were based in California (17%), Texas (12%), Florida (10%), and New York (8%).
Most currently hold or have held hospital leadership positions (60%) or faculty appointments (72%) and 21% have held leadership positions in specialty associations in the past 5 years. Nearly one-quarter (24%) have served on journal editorial boards, and 10% have authored clinical practice guidelines in the past 5 years.
More specifically, three physicians authored NCCN guidelines, and two authored American Society of Clinical Oncology guidelines during 2016-2021; one guideline was published in 2018 when payments were made.
“Oncology specialty associations, guideline panels, and journal editorial boards should reconsider if it is appropriate for physicians with such large payments to hold these high-profile positions,” Dr. Booth said.
Following publication of the study, some oncologists took to Twitter with reactions, including Manni Mohyuddin, MD (@ManniMD1), from the Huntsman Cancer Institute, University of Utah, Salt Lake City, who wrote: “I recognize that some conflict of interest ‘may’ be unavoidable in order to run trials. But when greater than TWICE the average American household annual salary is taken in payments from industry by those in leadership/editorial roles, we have a problem.”
Weighing in on the results, ASCO CEO Clifford A. Hudis, MD, told this news organization that the “limitations of the study make it difficult to draw conclusions about the scope or potential impact of these payments on care.”
For example, he explained, some payments attributed to individuals may have been made directly to the physicians’ institutions or employers for sponsored research expenses.
Dr. Hudis also noted that the payments examined in the study were made in 2018, whereas the potentially relevant leadership positions could have been attained at a different time.
Furthermore, in 2020, an editorial appeared in Cancer, showing that errors in Open Payments are “fairly common,” Dr. Hudis said. It’s also unclear whether the reported financial relationships were appropriately disclosed and were managed at the time under relevant conflict of interest policies, he said.
“The question left unanswered by this study is whether or not these relationships influence patient care,” said Dr. Hudis. He noted that decisions about care should come from physicians and patients who are informed of the best available, unbiased, peer-reviewed, scientific evidence.
“The potential impact of financial conflicts of interest on this effort is an issue of concern, even if this study does not directly address it,” Dr. Hudis said.
The study had no specific funding. Dr. Booth has disclosed no relevant financial relationships. A complete list of author disclosures is available with the original article. Dr. Hudis has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A small number of U.S. medical oncologists make more than $100,000 a year in general payments from drug companies, a new study shows.
These high-payment physicians represent just 1% of all U.S. medical oncologists, yet they account for 37% of industry payments. These oncologists often hold important leadership positions, draft treatment guidelines, and sit on journal editorial boards.
The findings highlight a risk for “perceived and real conflict of interest,” corresponding author Christopher Booth, MD, of Queen’s University Cancer Research Center, Kingston, Ont., said in an interview. “Because of the leadership positions they hold, the potential impact of this small group of physicians on oncology practice and policy may be substantial.”
The study was published online in JCO Oncology Practice.
‘We have a problem’
It’s no secret that many oncologists have financial relationships with pharmaceutical companies. They receive payments for research initiatives, but they also receive more general, personal payments in the form of honoraria, consultant fees, gifts, and reimbursement for travel and meals.
Prior studies have shown that these payments are typically modest, but a small subset of medical oncologists receive more than $100,000 annually. Dr. Booth and colleagues wanted to know more about the characteristics of these “high-payment” oncologists.
Using the national Open Payments database, the researchers identified a total of 139 medical oncologists who practice in the United States and who received $100,000 or more in general payments linked to cancer medications in 2018.
In U.S. dollars, the median payment was $154,613, and the total was $24.2 million.
The majority (95%) of high-payment oncologists were active in clinical work, 56% worked in an academic setting, 31% worked at National Cancer Institute–designated cancer centers, and 23% worked at National Comprehensive Cancer Network (NCCN) centers.
Many were based in California (17%), Texas (12%), Florida (10%), and New York (8%).
Most currently hold or have held hospital leadership positions (60%) or faculty appointments (72%) and 21% have held leadership positions in specialty associations in the past 5 years. Nearly one-quarter (24%) have served on journal editorial boards, and 10% have authored clinical practice guidelines in the past 5 years.
More specifically, three physicians authored NCCN guidelines, and two authored American Society of Clinical Oncology guidelines during 2016-2021; one guideline was published in 2018 when payments were made.
“Oncology specialty associations, guideline panels, and journal editorial boards should reconsider if it is appropriate for physicians with such large payments to hold these high-profile positions,” Dr. Booth said.
Following publication of the study, some oncologists took to Twitter with reactions, including Manni Mohyuddin, MD (@ManniMD1), from the Huntsman Cancer Institute, University of Utah, Salt Lake City, who wrote: “I recognize that some conflict of interest ‘may’ be unavoidable in order to run trials. But when greater than TWICE the average American household annual salary is taken in payments from industry by those in leadership/editorial roles, we have a problem.”
Weighing in on the results, ASCO CEO Clifford A. Hudis, MD, told this news organization that the “limitations of the study make it difficult to draw conclusions about the scope or potential impact of these payments on care.”
For example, he explained, some payments attributed to individuals may have been made directly to the physicians’ institutions or employers for sponsored research expenses.
Dr. Hudis also noted that the payments examined in the study were made in 2018, whereas the potentially relevant leadership positions could have been attained at a different time.
Furthermore, in 2020, an editorial appeared in Cancer, showing that errors in Open Payments are “fairly common,” Dr. Hudis said. It’s also unclear whether the reported financial relationships were appropriately disclosed and were managed at the time under relevant conflict of interest policies, he said.
“The question left unanswered by this study is whether or not these relationships influence patient care,” said Dr. Hudis. He noted that decisions about care should come from physicians and patients who are informed of the best available, unbiased, peer-reviewed, scientific evidence.
“The potential impact of financial conflicts of interest on this effort is an issue of concern, even if this study does not directly address it,” Dr. Hudis said.
The study had no specific funding. Dr. Booth has disclosed no relevant financial relationships. A complete list of author disclosures is available with the original article. Dr. Hudis has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JCO ONCOLOGY PRACTICE
Class I recall for Medtronic’s HeartWare HVAD batteries
Medtronic is recalling a single lot of HeartWare Ventricular Assist Device (HVAD) System batteries because of welding defects that may cause separation of the two cell battery packs used to power the system, according to an alert on the Food and Drug Administration website.
“The welding defect may cause the battery to malfunction and no longer provide power or prevent the battery from holding a full charge or properly recharging,” the FDA said.
The agency has identified this as a class I recall, the most serious type because of the potential for serious injury or death.
Medtronic reports one death associated with this recall and two complaints in the affected lot.
Back in April, as reported by this news organization, Medtronic alerted providers that patients implanted with the Medtronic HVAD System who develop pump thrombosis could have a welding defect in the internal pump that causes the pump to malfunction.
The batteries from the recalled lot have a model number of 1650DE, were manufactured from April 13 to 19, 2021 and distributed from April 20 to July 19, 2021. The recall affects a total of 429 devices.
On May 5, 2022, Medtronic sent an urgent medical device correction notice to customers asking them to identify and quarantine all affected batteries and notify affected patients. The notice includes a patient template to help communicate directly with patients.
It also includes a customer confirmation form to initiate an exchange. The completed form should be returned to rs.cfqfca@medtronic.com.
Medtronic is replacing the affected batteries with new product and has implemented actions to improve control of the welding process.
The Medtronic HVAD System was approved as a bridge to heart transplantation in 2012. Since then, it’s been fraught with problems.
Earlier in June, the company announced it was stopping all sales of the device and advised physicians to stop implanting it, as reported by this news organization.
Problems related to the Medtronic HVAD System should be reported to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
Medtronic is recalling a single lot of HeartWare Ventricular Assist Device (HVAD) System batteries because of welding defects that may cause separation of the two cell battery packs used to power the system, according to an alert on the Food and Drug Administration website.
“The welding defect may cause the battery to malfunction and no longer provide power or prevent the battery from holding a full charge or properly recharging,” the FDA said.
The agency has identified this as a class I recall, the most serious type because of the potential for serious injury or death.
Medtronic reports one death associated with this recall and two complaints in the affected lot.
Back in April, as reported by this news organization, Medtronic alerted providers that patients implanted with the Medtronic HVAD System who develop pump thrombosis could have a welding defect in the internal pump that causes the pump to malfunction.
The batteries from the recalled lot have a model number of 1650DE, were manufactured from April 13 to 19, 2021 and distributed from April 20 to July 19, 2021. The recall affects a total of 429 devices.
On May 5, 2022, Medtronic sent an urgent medical device correction notice to customers asking them to identify and quarantine all affected batteries and notify affected patients. The notice includes a patient template to help communicate directly with patients.
It also includes a customer confirmation form to initiate an exchange. The completed form should be returned to rs.cfqfca@medtronic.com.
Medtronic is replacing the affected batteries with new product and has implemented actions to improve control of the welding process.
The Medtronic HVAD System was approved as a bridge to heart transplantation in 2012. Since then, it’s been fraught with problems.
Earlier in June, the company announced it was stopping all sales of the device and advised physicians to stop implanting it, as reported by this news organization.
Problems related to the Medtronic HVAD System should be reported to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
Medtronic is recalling a single lot of HeartWare Ventricular Assist Device (HVAD) System batteries because of welding defects that may cause separation of the two cell battery packs used to power the system, according to an alert on the Food and Drug Administration website.
“The welding defect may cause the battery to malfunction and no longer provide power or prevent the battery from holding a full charge or properly recharging,” the FDA said.
The agency has identified this as a class I recall, the most serious type because of the potential for serious injury or death.
Medtronic reports one death associated with this recall and two complaints in the affected lot.
Back in April, as reported by this news organization, Medtronic alerted providers that patients implanted with the Medtronic HVAD System who develop pump thrombosis could have a welding defect in the internal pump that causes the pump to malfunction.
The batteries from the recalled lot have a model number of 1650DE, were manufactured from April 13 to 19, 2021 and distributed from April 20 to July 19, 2021. The recall affects a total of 429 devices.
On May 5, 2022, Medtronic sent an urgent medical device correction notice to customers asking them to identify and quarantine all affected batteries and notify affected patients. The notice includes a patient template to help communicate directly with patients.
It also includes a customer confirmation form to initiate an exchange. The completed form should be returned to rs.cfqfca@medtronic.com.
Medtronic is replacing the affected batteries with new product and has implemented actions to improve control of the welding process.
The Medtronic HVAD System was approved as a bridge to heart transplantation in 2012. Since then, it’s been fraught with problems.
Earlier in June, the company announced it was stopping all sales of the device and advised physicians to stop implanting it, as reported by this news organization.
Problems related to the Medtronic HVAD System should be reported to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
FDA okays cancer drugs faster than EMA. But at what cost?
Over the past decade, the U.S. Food and Drug Administration has approved new cancer drugs twice as fast as the European Medicines Agency (EMA), often using accelerated pathways, a new analysis shows.
Between 2010 and 2019, the FDA approved almost all oncology therapies ahead of the EMA. Drugs entered the United States market about 8 months (241 days) before European market authorization.
“The faster FDA approval process potentially provides earlier access to potentially life-prolonging medications for patients with cancer in the United States,” Ali Raza Khaki, MD, department of oncology, Stanford (Calif.) University School of Medicine, told this news organization. “On the surface, this is a good thing. However, it comes with limitations.”
Earlier drug approval often means greater uncertainty about an agent’s benefit – most notably, whether it will improve a patient’s survival or quality of life. Dr. Khaki pointed to a study published in JAMA Internal Medicine, which found that only 19 of 93 (20%) cancer drugs that had been recently approved through the FDA’s accelerated approval pathway demonstrated an improvement in overall survival.
In the new study, published online in JAMA Network Open, Dr. Khaki and colleagues found that among the 89 cancer drugs approved in the United States and Europe between January 2010 and December 2019, the FDA approved 85 (95%) before European authorization and four (5%) after.
The researchers found that the median FDA review time was half that of the EMA’s (200 vs. 426 days). Furthermore, 64 new drug applications (72%) were submitted to the FDA first, compared with 21 (23%) to the EMA.
Of the drugs approved through an accelerated pathway, three were ultimately pulled from the U.S. market, compared with one in Europe.
“These early drug approvals that later lead to withdrawal expose many more patients to toxicity, including financial toxicity, given the high cost of cancer medications,” Dr. Khaki commented.
In addition, 35 oncology therapies (39%) were approved by the FDA before trial results were published, compared with only eight (9%) by the EMA. Although FDA drug labels contain some information about efficacy and toxicity, scientific publications often have much more, including details about study populations and toxicities.
“Without this information, providers may be limited in their knowledge about patient selection, clinical benefit, and optimal toxicity management,” Dr. Khaki said.
Jeff Allen PhD, president and CEO of the nonprofit Friends of Cancer Research, who wasn’t involved in the study, believes that an FDA approval before publication shouldn’t be “particularly concerning.”
“Peer-reviewed publication is an important component of validating and communicating scientific findings, but the processes and time lines for individual journals can be highly variable,” he said. “I don’t think we would want to see a situation where potential beneficial treatments are held up due to unrelated publication processes.”
The author of an invited commentary in JAMA Network Open had a different take on the study findings.
“A tempting interpretation” of this study is that the FDA is a “superior agency for expedited review times that bring cancer drugs to patients earlier,” Kristina Jenei, BSN, MSc, with the University of British Columbia School of Population and Public Health, writes. In addition, the fact that more drugs were pulled from the market after approval in the United States than in Europe could be interpreted to mean that the system is working as it should.
Although the speed of FDA reviews and the number of subsequent approvals have increased over time, the proportion of cancer drugs that improve survival has declined. In addition, because the FDA’s follow-up of postmarketing studies has been “inconsistent,” a substantial number of cancer drugs that were approved through accelerated pathways have remained on the market for years without confirmation of their benefit.
Although regulatory agencies must balance earlier patient access to novel treatments with evidence that the therapies are effective and safe, “faster review times and approvals are not cause for celebration; better patient outcomes are,” Ms. Jenei writes. “In other words, quality over quantity.”
The study was supported by the National Cancer Institute. Dr. Khaki reported stock ownership from Merck and stock ownership from Sanofi outside the submitted work. Dr. Allen and Ms. Jenei have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Over the past decade, the U.S. Food and Drug Administration has approved new cancer drugs twice as fast as the European Medicines Agency (EMA), often using accelerated pathways, a new analysis shows.
Between 2010 and 2019, the FDA approved almost all oncology therapies ahead of the EMA. Drugs entered the United States market about 8 months (241 days) before European market authorization.
“The faster FDA approval process potentially provides earlier access to potentially life-prolonging medications for patients with cancer in the United States,” Ali Raza Khaki, MD, department of oncology, Stanford (Calif.) University School of Medicine, told this news organization. “On the surface, this is a good thing. However, it comes with limitations.”
Earlier drug approval often means greater uncertainty about an agent’s benefit – most notably, whether it will improve a patient’s survival or quality of life. Dr. Khaki pointed to a study published in JAMA Internal Medicine, which found that only 19 of 93 (20%) cancer drugs that had been recently approved through the FDA’s accelerated approval pathway demonstrated an improvement in overall survival.
In the new study, published online in JAMA Network Open, Dr. Khaki and colleagues found that among the 89 cancer drugs approved in the United States and Europe between January 2010 and December 2019, the FDA approved 85 (95%) before European authorization and four (5%) after.
The researchers found that the median FDA review time was half that of the EMA’s (200 vs. 426 days). Furthermore, 64 new drug applications (72%) were submitted to the FDA first, compared with 21 (23%) to the EMA.
Of the drugs approved through an accelerated pathway, three were ultimately pulled from the U.S. market, compared with one in Europe.
“These early drug approvals that later lead to withdrawal expose many more patients to toxicity, including financial toxicity, given the high cost of cancer medications,” Dr. Khaki commented.
In addition, 35 oncology therapies (39%) were approved by the FDA before trial results were published, compared with only eight (9%) by the EMA. Although FDA drug labels contain some information about efficacy and toxicity, scientific publications often have much more, including details about study populations and toxicities.
“Without this information, providers may be limited in their knowledge about patient selection, clinical benefit, and optimal toxicity management,” Dr. Khaki said.
Jeff Allen PhD, president and CEO of the nonprofit Friends of Cancer Research, who wasn’t involved in the study, believes that an FDA approval before publication shouldn’t be “particularly concerning.”
“Peer-reviewed publication is an important component of validating and communicating scientific findings, but the processes and time lines for individual journals can be highly variable,” he said. “I don’t think we would want to see a situation where potential beneficial treatments are held up due to unrelated publication processes.”
The author of an invited commentary in JAMA Network Open had a different take on the study findings.
“A tempting interpretation” of this study is that the FDA is a “superior agency for expedited review times that bring cancer drugs to patients earlier,” Kristina Jenei, BSN, MSc, with the University of British Columbia School of Population and Public Health, writes. In addition, the fact that more drugs were pulled from the market after approval in the United States than in Europe could be interpreted to mean that the system is working as it should.
Although the speed of FDA reviews and the number of subsequent approvals have increased over time, the proportion of cancer drugs that improve survival has declined. In addition, because the FDA’s follow-up of postmarketing studies has been “inconsistent,” a substantial number of cancer drugs that were approved through accelerated pathways have remained on the market for years without confirmation of their benefit.
Although regulatory agencies must balance earlier patient access to novel treatments with evidence that the therapies are effective and safe, “faster review times and approvals are not cause for celebration; better patient outcomes are,” Ms. Jenei writes. “In other words, quality over quantity.”
The study was supported by the National Cancer Institute. Dr. Khaki reported stock ownership from Merck and stock ownership from Sanofi outside the submitted work. Dr. Allen and Ms. Jenei have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Over the past decade, the U.S. Food and Drug Administration has approved new cancer drugs twice as fast as the European Medicines Agency (EMA), often using accelerated pathways, a new analysis shows.
Between 2010 and 2019, the FDA approved almost all oncology therapies ahead of the EMA. Drugs entered the United States market about 8 months (241 days) before European market authorization.
“The faster FDA approval process potentially provides earlier access to potentially life-prolonging medications for patients with cancer in the United States,” Ali Raza Khaki, MD, department of oncology, Stanford (Calif.) University School of Medicine, told this news organization. “On the surface, this is a good thing. However, it comes with limitations.”
Earlier drug approval often means greater uncertainty about an agent’s benefit – most notably, whether it will improve a patient’s survival or quality of life. Dr. Khaki pointed to a study published in JAMA Internal Medicine, which found that only 19 of 93 (20%) cancer drugs that had been recently approved through the FDA’s accelerated approval pathway demonstrated an improvement in overall survival.
In the new study, published online in JAMA Network Open, Dr. Khaki and colleagues found that among the 89 cancer drugs approved in the United States and Europe between January 2010 and December 2019, the FDA approved 85 (95%) before European authorization and four (5%) after.
The researchers found that the median FDA review time was half that of the EMA’s (200 vs. 426 days). Furthermore, 64 new drug applications (72%) were submitted to the FDA first, compared with 21 (23%) to the EMA.
Of the drugs approved through an accelerated pathway, three were ultimately pulled from the U.S. market, compared with one in Europe.
“These early drug approvals that later lead to withdrawal expose many more patients to toxicity, including financial toxicity, given the high cost of cancer medications,” Dr. Khaki commented.
In addition, 35 oncology therapies (39%) were approved by the FDA before trial results were published, compared with only eight (9%) by the EMA. Although FDA drug labels contain some information about efficacy and toxicity, scientific publications often have much more, including details about study populations and toxicities.
“Without this information, providers may be limited in their knowledge about patient selection, clinical benefit, and optimal toxicity management,” Dr. Khaki said.
Jeff Allen PhD, president and CEO of the nonprofit Friends of Cancer Research, who wasn’t involved in the study, believes that an FDA approval before publication shouldn’t be “particularly concerning.”
“Peer-reviewed publication is an important component of validating and communicating scientific findings, but the processes and time lines for individual journals can be highly variable,” he said. “I don’t think we would want to see a situation where potential beneficial treatments are held up due to unrelated publication processes.”
The author of an invited commentary in JAMA Network Open had a different take on the study findings.
“A tempting interpretation” of this study is that the FDA is a “superior agency for expedited review times that bring cancer drugs to patients earlier,” Kristina Jenei, BSN, MSc, with the University of British Columbia School of Population and Public Health, writes. In addition, the fact that more drugs were pulled from the market after approval in the United States than in Europe could be interpreted to mean that the system is working as it should.
Although the speed of FDA reviews and the number of subsequent approvals have increased over time, the proportion of cancer drugs that improve survival has declined. In addition, because the FDA’s follow-up of postmarketing studies has been “inconsistent,” a substantial number of cancer drugs that were approved through accelerated pathways have remained on the market for years without confirmation of their benefit.
Although regulatory agencies must balance earlier patient access to novel treatments with evidence that the therapies are effective and safe, “faster review times and approvals are not cause for celebration; better patient outcomes are,” Ms. Jenei writes. “In other words, quality over quantity.”
The study was supported by the National Cancer Institute. Dr. Khaki reported stock ownership from Merck and stock ownership from Sanofi outside the submitted work. Dr. Allen and Ms. Jenei have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
FDA approves risankizumab (Skyrizi) for Crohn’s disease
The U.S. Food and Drug Administration – making it the first specific anti–interleukin-23 monoclonal antibody indicated for Crohn’s disease.
The safety and efficacy of risankizumab in Crohn’s disease is supported by data from two induction clinical trials (ADVANCE and MOTIVATE) and one maintenance clinical trial (FORTIFY).
Results of the three studies were presented at the annual scientific meeting of the American College of Gastroenterology in 2021.
“In both the induction and maintenance clinical trials, a significantly greater number of adult patients saw few or no symptoms and a meaningful reduction of visible signs of intestinal inflammation, compared to placebo,” Marla Dubinsky, MD, gastroenterologist with the Mount Sinai Health System and codirector of the IBD Center at Mount Sinai, New York, said in a news release from AbbVie.
“This approval provides health care professionals with a greatly needed additional option for treating the disruptive symptoms of Crohn’s disease,” Dr. Dubinsky said.
For the treatment of Crohn’s disease, risankizumab is dosed at 600 mg administered by intravenous infusion over at least 1 hour at week 0, 4, and 8, followed by 360 mg self-administered by subcutaneous injection at week 12, and every 8 weeks thereafter.
Risankizumab is already approved in the United States for the treatment of adults with active psoriatic arthritis and moderate to severe plaque psoriasis.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration – making it the first specific anti–interleukin-23 monoclonal antibody indicated for Crohn’s disease.
The safety and efficacy of risankizumab in Crohn’s disease is supported by data from two induction clinical trials (ADVANCE and MOTIVATE) and one maintenance clinical trial (FORTIFY).
Results of the three studies were presented at the annual scientific meeting of the American College of Gastroenterology in 2021.
“In both the induction and maintenance clinical trials, a significantly greater number of adult patients saw few or no symptoms and a meaningful reduction of visible signs of intestinal inflammation, compared to placebo,” Marla Dubinsky, MD, gastroenterologist with the Mount Sinai Health System and codirector of the IBD Center at Mount Sinai, New York, said in a news release from AbbVie.
“This approval provides health care professionals with a greatly needed additional option for treating the disruptive symptoms of Crohn’s disease,” Dr. Dubinsky said.
For the treatment of Crohn’s disease, risankizumab is dosed at 600 mg administered by intravenous infusion over at least 1 hour at week 0, 4, and 8, followed by 360 mg self-administered by subcutaneous injection at week 12, and every 8 weeks thereafter.
Risankizumab is already approved in the United States for the treatment of adults with active psoriatic arthritis and moderate to severe plaque psoriasis.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration – making it the first specific anti–interleukin-23 monoclonal antibody indicated for Crohn’s disease.
The safety and efficacy of risankizumab in Crohn’s disease is supported by data from two induction clinical trials (ADVANCE and MOTIVATE) and one maintenance clinical trial (FORTIFY).
Results of the three studies were presented at the annual scientific meeting of the American College of Gastroenterology in 2021.
“In both the induction and maintenance clinical trials, a significantly greater number of adult patients saw few or no symptoms and a meaningful reduction of visible signs of intestinal inflammation, compared to placebo,” Marla Dubinsky, MD, gastroenterologist with the Mount Sinai Health System and codirector of the IBD Center at Mount Sinai, New York, said in a news release from AbbVie.
“This approval provides health care professionals with a greatly needed additional option for treating the disruptive symptoms of Crohn’s disease,” Dr. Dubinsky said.
For the treatment of Crohn’s disease, risankizumab is dosed at 600 mg administered by intravenous infusion over at least 1 hour at week 0, 4, and 8, followed by 360 mg self-administered by subcutaneous injection at week 12, and every 8 weeks thereafter.
Risankizumab is already approved in the United States for the treatment of adults with active psoriatic arthritis and moderate to severe plaque psoriasis.
A version of this article first appeared on Medscape.com.
Snoring may lead to a sedentary lifestyle
“People who snore are also likely to have sleep apnea, but those who snore and don’t have sleep apnea are a largely understudied group,” senior author Michael Grandner, PhD, told this news organization.
“We found that even just snoring alone can impact health and well-being,” said Dr. Grandner, director of the sleep and health research program at the University of Arizona, Tucson.
The findings were presented at the annual meeting of the Associated Professional Sleep Societies.
A viscous cycle
Frequent snoring can signal sleep-disordered breathing, which is associated with a myriad of comorbidities, including increased risk for cardiovascular disease.
Prior studies have shown that sleep-disordered breathing is associated with less physical activity, but few studies have examined this at the population level or in relation to primary snoring.
Dr. Grandner and colleagues evaluated the relationship between snoring frequency and minutes of sedentary activity using 3 years’ worth of data from the National Health and Nutrition Examination Survey. Participants reported snoring frequency and sedentary activity.
After adjusting for sex, age, race, education level, and marital status, adults who were frequent snorers (5+ nights per week) spent about 36 more minutes per day sedentary, compared with peers who reported never snoring.
In addition, those individuals who were determined to be at increased risk of having sleep apnea had about 54 more minutes per day of sedentary time in the adjusted model.
“Snoring is very common, and it doesn’t just affect the nighttime,” said Dr. Grandner.
Snoring can lead to “more tiredness and less energy, which can impact everything from mood to stress to – as we saw – activity level,” he noted.
Commenting on the results for this news organization, Raman Malhotra, MD, of the Washington University Sleep Center in St. Louis, said this study clearly demonstrates how people who snore and people who are at risk for sleep apnea are more sedentary.
This could explain the “vicious cycle” that these patients suffer from, inasmuch as having obesity can lead to sleep apnea, and having sleep apnea can lead to further sedentary lifestyle and weight gain, owing to lack of energy and feeling tired, Dr. Malhotra told this news organization.
“It is important to intervene and treat the sleep disorder to hopefully make people more active,” he added.
The study had no specific funding. Dr. Grandner and Dr. Malhotra disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“People who snore are also likely to have sleep apnea, but those who snore and don’t have sleep apnea are a largely understudied group,” senior author Michael Grandner, PhD, told this news organization.
“We found that even just snoring alone can impact health and well-being,” said Dr. Grandner, director of the sleep and health research program at the University of Arizona, Tucson.
The findings were presented at the annual meeting of the Associated Professional Sleep Societies.
A viscous cycle
Frequent snoring can signal sleep-disordered breathing, which is associated with a myriad of comorbidities, including increased risk for cardiovascular disease.
Prior studies have shown that sleep-disordered breathing is associated with less physical activity, but few studies have examined this at the population level or in relation to primary snoring.
Dr. Grandner and colleagues evaluated the relationship between snoring frequency and minutes of sedentary activity using 3 years’ worth of data from the National Health and Nutrition Examination Survey. Participants reported snoring frequency and sedentary activity.
After adjusting for sex, age, race, education level, and marital status, adults who were frequent snorers (5+ nights per week) spent about 36 more minutes per day sedentary, compared with peers who reported never snoring.
In addition, those individuals who were determined to be at increased risk of having sleep apnea had about 54 more minutes per day of sedentary time in the adjusted model.
“Snoring is very common, and it doesn’t just affect the nighttime,” said Dr. Grandner.
Snoring can lead to “more tiredness and less energy, which can impact everything from mood to stress to – as we saw – activity level,” he noted.
Commenting on the results for this news organization, Raman Malhotra, MD, of the Washington University Sleep Center in St. Louis, said this study clearly demonstrates how people who snore and people who are at risk for sleep apnea are more sedentary.
This could explain the “vicious cycle” that these patients suffer from, inasmuch as having obesity can lead to sleep apnea, and having sleep apnea can lead to further sedentary lifestyle and weight gain, owing to lack of energy and feeling tired, Dr. Malhotra told this news organization.
“It is important to intervene and treat the sleep disorder to hopefully make people more active,” he added.
The study had no specific funding. Dr. Grandner and Dr. Malhotra disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“People who snore are also likely to have sleep apnea, but those who snore and don’t have sleep apnea are a largely understudied group,” senior author Michael Grandner, PhD, told this news organization.
“We found that even just snoring alone can impact health and well-being,” said Dr. Grandner, director of the sleep and health research program at the University of Arizona, Tucson.
The findings were presented at the annual meeting of the Associated Professional Sleep Societies.
A viscous cycle
Frequent snoring can signal sleep-disordered breathing, which is associated with a myriad of comorbidities, including increased risk for cardiovascular disease.
Prior studies have shown that sleep-disordered breathing is associated with less physical activity, but few studies have examined this at the population level or in relation to primary snoring.
Dr. Grandner and colleagues evaluated the relationship between snoring frequency and minutes of sedentary activity using 3 years’ worth of data from the National Health and Nutrition Examination Survey. Participants reported snoring frequency and sedentary activity.
After adjusting for sex, age, race, education level, and marital status, adults who were frequent snorers (5+ nights per week) spent about 36 more minutes per day sedentary, compared with peers who reported never snoring.
In addition, those individuals who were determined to be at increased risk of having sleep apnea had about 54 more minutes per day of sedentary time in the adjusted model.
“Snoring is very common, and it doesn’t just affect the nighttime,” said Dr. Grandner.
Snoring can lead to “more tiredness and less energy, which can impact everything from mood to stress to – as we saw – activity level,” he noted.
Commenting on the results for this news organization, Raman Malhotra, MD, of the Washington University Sleep Center in St. Louis, said this study clearly demonstrates how people who snore and people who are at risk for sleep apnea are more sedentary.
This could explain the “vicious cycle” that these patients suffer from, inasmuch as having obesity can lead to sleep apnea, and having sleep apnea can lead to further sedentary lifestyle and weight gain, owing to lack of energy and feeling tired, Dr. Malhotra told this news organization.
“It is important to intervene and treat the sleep disorder to hopefully make people more active,” he added.
The study had no specific funding. Dr. Grandner and Dr. Malhotra disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SLEEP 2022
Can too much sleep raise the risk of cancer?
The findings reveal that sleeping 10-plus hours may increase a woman’s risk of getting cancer and both men and women’s risk of dying from cancer.
The researchers say their findings may help refine sleep recommendations in Japan, which currently advise working, middle-aged adults to sleep “as long as they can.”
Based on the new findings, a sleep duration of 6-8 hours for men and 6-9 hours for women “may be the safest” regarding cancer incidence and mortality risk among Japanese adults, the authors conclude.
The findings were published online in the International Journal of Cancer.
The literature on sleep time and cancer risk is mixed. A trio of meta-analyses conducted between 2016 and 2019 found that long sleep duration, but not short, was associated with a slightly elevated risk of all cancer mortality in Asians.
A separate meta-analysis conducted in 2018 found that both short and long sleep durations were not related to cancer incidence. But in the stratified analysis, shorter sleep time was associated with 36% increased cancer risk among Asians.
To investigate further, the researchers pooled data from six population-based cohorts that included 271,694 adults – 126,930 men and 144,764 women – with 40,751 total incident cancer cases and 18,323 total cancer deaths during a follow-up lasting about 5.9 million person-years.
In the multivariable analysis, longer sleep duration was not associated with total cancer incidence in men. In women, however, sleeping 10 or more hours vs. 7 was associated with a 19% increased risk of cancer.
In addition, sleeping 10 or more hours was associated with an increased risk of dying from cancer in women (hazard ratio, 1.44) and men (HR, 1.18).
Sleeping for 5 hours or fewer, compared with 7, was not associated with cancer incidence and mortality. However, among postmenopausal women, shorter sleep durations did increase the risk of dying from cancer (HR, 1.15).
The authors highlight several strengths of the analysis, including a large sample size as well as stratification of the results by body mass index and menopause status, which has rarely been done in previous studies.
Limitations include self-reported sleep durations and lack of data on sleep quality. The researchers note that the mechanism by which sleep time may influence cancer incidence and mortality is unclear but likely to be complex and cancer site specific.
It’s also possible that reverse causation could explain associations between sleep duration and cancer occurrence and mortality – with pain from cancer, for instance, impairing sleep duration and quality. However, the sensitivity analysis found no evidence of reverse causality or other confounding factors.
Based on these findings, the researchers say sleep duration “may be an important variable to include in cancer incidence and mortality risk prediction models.”
The study had no specific funding. The authors declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The findings reveal that sleeping 10-plus hours may increase a woman’s risk of getting cancer and both men and women’s risk of dying from cancer.
The researchers say their findings may help refine sleep recommendations in Japan, which currently advise working, middle-aged adults to sleep “as long as they can.”
Based on the new findings, a sleep duration of 6-8 hours for men and 6-9 hours for women “may be the safest” regarding cancer incidence and mortality risk among Japanese adults, the authors conclude.
The findings were published online in the International Journal of Cancer.
The literature on sleep time and cancer risk is mixed. A trio of meta-analyses conducted between 2016 and 2019 found that long sleep duration, but not short, was associated with a slightly elevated risk of all cancer mortality in Asians.
A separate meta-analysis conducted in 2018 found that both short and long sleep durations were not related to cancer incidence. But in the stratified analysis, shorter sleep time was associated with 36% increased cancer risk among Asians.
To investigate further, the researchers pooled data from six population-based cohorts that included 271,694 adults – 126,930 men and 144,764 women – with 40,751 total incident cancer cases and 18,323 total cancer deaths during a follow-up lasting about 5.9 million person-years.
In the multivariable analysis, longer sleep duration was not associated with total cancer incidence in men. In women, however, sleeping 10 or more hours vs. 7 was associated with a 19% increased risk of cancer.
In addition, sleeping 10 or more hours was associated with an increased risk of dying from cancer in women (hazard ratio, 1.44) and men (HR, 1.18).
Sleeping for 5 hours or fewer, compared with 7, was not associated with cancer incidence and mortality. However, among postmenopausal women, shorter sleep durations did increase the risk of dying from cancer (HR, 1.15).
The authors highlight several strengths of the analysis, including a large sample size as well as stratification of the results by body mass index and menopause status, which has rarely been done in previous studies.
Limitations include self-reported sleep durations and lack of data on sleep quality. The researchers note that the mechanism by which sleep time may influence cancer incidence and mortality is unclear but likely to be complex and cancer site specific.
It’s also possible that reverse causation could explain associations between sleep duration and cancer occurrence and mortality – with pain from cancer, for instance, impairing sleep duration and quality. However, the sensitivity analysis found no evidence of reverse causality or other confounding factors.
Based on these findings, the researchers say sleep duration “may be an important variable to include in cancer incidence and mortality risk prediction models.”
The study had no specific funding. The authors declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The findings reveal that sleeping 10-plus hours may increase a woman’s risk of getting cancer and both men and women’s risk of dying from cancer.
The researchers say their findings may help refine sleep recommendations in Japan, which currently advise working, middle-aged adults to sleep “as long as they can.”
Based on the new findings, a sleep duration of 6-8 hours for men and 6-9 hours for women “may be the safest” regarding cancer incidence and mortality risk among Japanese adults, the authors conclude.
The findings were published online in the International Journal of Cancer.
The literature on sleep time and cancer risk is mixed. A trio of meta-analyses conducted between 2016 and 2019 found that long sleep duration, but not short, was associated with a slightly elevated risk of all cancer mortality in Asians.
A separate meta-analysis conducted in 2018 found that both short and long sleep durations were not related to cancer incidence. But in the stratified analysis, shorter sleep time was associated with 36% increased cancer risk among Asians.
To investigate further, the researchers pooled data from six population-based cohorts that included 271,694 adults – 126,930 men and 144,764 women – with 40,751 total incident cancer cases and 18,323 total cancer deaths during a follow-up lasting about 5.9 million person-years.
In the multivariable analysis, longer sleep duration was not associated with total cancer incidence in men. In women, however, sleeping 10 or more hours vs. 7 was associated with a 19% increased risk of cancer.
In addition, sleeping 10 or more hours was associated with an increased risk of dying from cancer in women (hazard ratio, 1.44) and men (HR, 1.18).
Sleeping for 5 hours or fewer, compared with 7, was not associated with cancer incidence and mortality. However, among postmenopausal women, shorter sleep durations did increase the risk of dying from cancer (HR, 1.15).
The authors highlight several strengths of the analysis, including a large sample size as well as stratification of the results by body mass index and menopause status, which has rarely been done in previous studies.
Limitations include self-reported sleep durations and lack of data on sleep quality. The researchers note that the mechanism by which sleep time may influence cancer incidence and mortality is unclear but likely to be complex and cancer site specific.
It’s also possible that reverse causation could explain associations between sleep duration and cancer occurrence and mortality – with pain from cancer, for instance, impairing sleep duration and quality. However, the sensitivity analysis found no evidence of reverse causality or other confounding factors.
Based on these findings, the researchers say sleep duration “may be an important variable to include in cancer incidence and mortality risk prediction models.”
The study had no specific funding. The authors declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM THE INTERNATIONAL JOURNAL OF CANCER
‘Forever chemicals’ linked to hypertension in middle-aged women
In a large, prospective study, researchers found an association between higher blood levels of PFAS and increased risk of hypertension in middle-aged women. Women in the highest tertile of overall PFAS concentrations had a 71% increased risk of developing hypertension.
“Our findings suggest that long-term cumulative exposure, even before midlife, may increase the risk of high blood pressure, and therefore, the benefit of reducing the population exposure to PFAS and potential prevention of high blood pressure and other health conditions would be enormous,” Sung Kyun Park, ScD, MPH, University of Michigan School of Public Health, Ann Arbor, said in an interview.
The study was published online in Hypertension.
Everywhere and forever
“PFAS are forever chemicals as well as everywhere chemicals,” Dr. Park noted.
Possible sources of PFAS exposure run the gamut from nonstick cookware, food wrappers, and waterproof fabrics to cosmetics and drinking water. They have been detected in the blood of most people and have been linked to a variety of health concerns.
“A few studies showed an association between PFAS and hypertension, but those were cross-sectional and examined prevalence of hypertension. It was unclear whether PFAS are associated with the development (incidence) of hypertension,” Dr. Park explained.
For their study, the researchers examined the association between serum concentrations of PFAS and risks of incident hypertension in 1,058 initially normotensive women participating in the Study of Women’s Health Across the Nation-Multi-Pollutant Study (SWAN-MPS). They were followed annually between 1999 and 2017.
During 11,722 person-years of follow-up, 470 of the women developed hypertension, at a rate of 40.1 cases per 1,000 person-years. Hypertension was defined as blood pressure of at least 140 mm Hg systolic or at least 90 mm Hg diastolic or receiving antihypertensive treatment.
Women in the highest tertile of baseline serum concentration of perfluorooctane sulfonate (PFOS) had a 42% higher risk of developing hypertension, compared with peers in the lowest tertile (adjusted hazard ratio, 1.42; 95% confidence interval, 1.19-1.68; P trend = .01).
Similar results were found for perfluorooctanoate (PFOA) and 2-N-ethyl-perfluorooctane sulfonamido acetate (EtFOSAA), with 47% (aHR, 1.47; 95% CI, 1.24-1.75; P trend = .01) and 42% (aHR, 1.42; 95% CI, 1.19-1.70; P trend = .01) higher risks of incident hypertension, comparing the highest to the lowest tertiles.
The risks persisted after adjusting for various factors, including race, study site, education, financial strain, smoking status, alcohol use, total calorie intake, and menopausal status.
In the PFAS “mixture” analysis, women in the highest tertile of overall PFAS concentrations were 71% more likely to develop hypertension during follow-up, compared with women in the lowest tertile (aHR, 1.71; 95% CI, 1.15-2.54; P trend = .008).
“These findings suggest that PFAS might be an underappreciated contributing factor to women’s cardiovascular disease risk,” the researchers write.
They caution that the study only included middle-aged women and that it is unclear whether the findings hold for middle-aged men.
“This is an important question, but the answer is that we do not know,” Dr. Park told this news organization.
“Women become more susceptible to metabolic changes and hypertension risk during the menopausal transition. Our findings suggest that PFAS may play a role in the development of hypertension in women during this critical life stage,” Dr. Park said.
The researchers say more research is needed to confirm and expand the findings and to find ways to reduce PFAS exposure.
“If confirmed in future studies, these findings suggest that understanding human exposure to PFAS and developing effective strategies to reduce PFAS exposure may help prevent the development of hypertension and thereby reduce the global burden of CVD,” the researchers write.
‘The more we learn, the worse it gets’
This is an “interesting” study and shows that “the more we learn about PFAS, the worse it seems to get,” Ankur Shah, MD, division of kidney disease and hypertension, Warren Alpert Medical School of Brown University, Providence, R.I., said in an interview.
“This multisite, multiracial and multiethnic, community-based longitudinal study establishes an association between PFAS and hypertension,” said Dr. Shah, who wasn’t involved in the study.
“This adds to a growing literature base of associations of PFAS with illnesses, including malignancy, thyroid disorders, diabetes, ulcerative colitis, hyperlipidemia, and pregnancy-induced hypertension,” he noted.
Dr. Shah also noted that the authors adjusted for race and ethnicity, study site, education, financial strain, smoking status, environmental tobacco smoke, alcohol consumption, total calorie intake, and menopausal status “and still found a strong association.”
“Still to be determined are both whether PFAS are the causative agent or if there is an unmeasured/unadjusted for entity which has resulted in both increased PFAS exposure and hypertension, as well as if PFAS are causative, if reduction in PFAS exposure would be result in blood pressure reduction,” Dr. Shah added.
The study had no sources of funding. Dr. Park and Dr. Shah have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a large, prospective study, researchers found an association between higher blood levels of PFAS and increased risk of hypertension in middle-aged women. Women in the highest tertile of overall PFAS concentrations had a 71% increased risk of developing hypertension.
“Our findings suggest that long-term cumulative exposure, even before midlife, may increase the risk of high blood pressure, and therefore, the benefit of reducing the population exposure to PFAS and potential prevention of high blood pressure and other health conditions would be enormous,” Sung Kyun Park, ScD, MPH, University of Michigan School of Public Health, Ann Arbor, said in an interview.
The study was published online in Hypertension.
Everywhere and forever
“PFAS are forever chemicals as well as everywhere chemicals,” Dr. Park noted.
Possible sources of PFAS exposure run the gamut from nonstick cookware, food wrappers, and waterproof fabrics to cosmetics and drinking water. They have been detected in the blood of most people and have been linked to a variety of health concerns.
“A few studies showed an association between PFAS and hypertension, but those were cross-sectional and examined prevalence of hypertension. It was unclear whether PFAS are associated with the development (incidence) of hypertension,” Dr. Park explained.
For their study, the researchers examined the association between serum concentrations of PFAS and risks of incident hypertension in 1,058 initially normotensive women participating in the Study of Women’s Health Across the Nation-Multi-Pollutant Study (SWAN-MPS). They were followed annually between 1999 and 2017.
During 11,722 person-years of follow-up, 470 of the women developed hypertension, at a rate of 40.1 cases per 1,000 person-years. Hypertension was defined as blood pressure of at least 140 mm Hg systolic or at least 90 mm Hg diastolic or receiving antihypertensive treatment.
Women in the highest tertile of baseline serum concentration of perfluorooctane sulfonate (PFOS) had a 42% higher risk of developing hypertension, compared with peers in the lowest tertile (adjusted hazard ratio, 1.42; 95% confidence interval, 1.19-1.68; P trend = .01).
Similar results were found for perfluorooctanoate (PFOA) and 2-N-ethyl-perfluorooctane sulfonamido acetate (EtFOSAA), with 47% (aHR, 1.47; 95% CI, 1.24-1.75; P trend = .01) and 42% (aHR, 1.42; 95% CI, 1.19-1.70; P trend = .01) higher risks of incident hypertension, comparing the highest to the lowest tertiles.
The risks persisted after adjusting for various factors, including race, study site, education, financial strain, smoking status, alcohol use, total calorie intake, and menopausal status.
In the PFAS “mixture” analysis, women in the highest tertile of overall PFAS concentrations were 71% more likely to develop hypertension during follow-up, compared with women in the lowest tertile (aHR, 1.71; 95% CI, 1.15-2.54; P trend = .008).
“These findings suggest that PFAS might be an underappreciated contributing factor to women’s cardiovascular disease risk,” the researchers write.
They caution that the study only included middle-aged women and that it is unclear whether the findings hold for middle-aged men.
“This is an important question, but the answer is that we do not know,” Dr. Park told this news organization.
“Women become more susceptible to metabolic changes and hypertension risk during the menopausal transition. Our findings suggest that PFAS may play a role in the development of hypertension in women during this critical life stage,” Dr. Park said.
The researchers say more research is needed to confirm and expand the findings and to find ways to reduce PFAS exposure.
“If confirmed in future studies, these findings suggest that understanding human exposure to PFAS and developing effective strategies to reduce PFAS exposure may help prevent the development of hypertension and thereby reduce the global burden of CVD,” the researchers write.
‘The more we learn, the worse it gets’
This is an “interesting” study and shows that “the more we learn about PFAS, the worse it seems to get,” Ankur Shah, MD, division of kidney disease and hypertension, Warren Alpert Medical School of Brown University, Providence, R.I., said in an interview.
“This multisite, multiracial and multiethnic, community-based longitudinal study establishes an association between PFAS and hypertension,” said Dr. Shah, who wasn’t involved in the study.
“This adds to a growing literature base of associations of PFAS with illnesses, including malignancy, thyroid disorders, diabetes, ulcerative colitis, hyperlipidemia, and pregnancy-induced hypertension,” he noted.
Dr. Shah also noted that the authors adjusted for race and ethnicity, study site, education, financial strain, smoking status, environmental tobacco smoke, alcohol consumption, total calorie intake, and menopausal status “and still found a strong association.”
“Still to be determined are both whether PFAS are the causative agent or if there is an unmeasured/unadjusted for entity which has resulted in both increased PFAS exposure and hypertension, as well as if PFAS are causative, if reduction in PFAS exposure would be result in blood pressure reduction,” Dr. Shah added.
The study had no sources of funding. Dr. Park and Dr. Shah have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a large, prospective study, researchers found an association between higher blood levels of PFAS and increased risk of hypertension in middle-aged women. Women in the highest tertile of overall PFAS concentrations had a 71% increased risk of developing hypertension.
“Our findings suggest that long-term cumulative exposure, even before midlife, may increase the risk of high blood pressure, and therefore, the benefit of reducing the population exposure to PFAS and potential prevention of high blood pressure and other health conditions would be enormous,” Sung Kyun Park, ScD, MPH, University of Michigan School of Public Health, Ann Arbor, said in an interview.
The study was published online in Hypertension.
Everywhere and forever
“PFAS are forever chemicals as well as everywhere chemicals,” Dr. Park noted.
Possible sources of PFAS exposure run the gamut from nonstick cookware, food wrappers, and waterproof fabrics to cosmetics and drinking water. They have been detected in the blood of most people and have been linked to a variety of health concerns.
“A few studies showed an association between PFAS and hypertension, but those were cross-sectional and examined prevalence of hypertension. It was unclear whether PFAS are associated with the development (incidence) of hypertension,” Dr. Park explained.
For their study, the researchers examined the association between serum concentrations of PFAS and risks of incident hypertension in 1,058 initially normotensive women participating in the Study of Women’s Health Across the Nation-Multi-Pollutant Study (SWAN-MPS). They were followed annually between 1999 and 2017.
During 11,722 person-years of follow-up, 470 of the women developed hypertension, at a rate of 40.1 cases per 1,000 person-years. Hypertension was defined as blood pressure of at least 140 mm Hg systolic or at least 90 mm Hg diastolic or receiving antihypertensive treatment.
Women in the highest tertile of baseline serum concentration of perfluorooctane sulfonate (PFOS) had a 42% higher risk of developing hypertension, compared with peers in the lowest tertile (adjusted hazard ratio, 1.42; 95% confidence interval, 1.19-1.68; P trend = .01).
Similar results were found for perfluorooctanoate (PFOA) and 2-N-ethyl-perfluorooctane sulfonamido acetate (EtFOSAA), with 47% (aHR, 1.47; 95% CI, 1.24-1.75; P trend = .01) and 42% (aHR, 1.42; 95% CI, 1.19-1.70; P trend = .01) higher risks of incident hypertension, comparing the highest to the lowest tertiles.
The risks persisted after adjusting for various factors, including race, study site, education, financial strain, smoking status, alcohol use, total calorie intake, and menopausal status.
In the PFAS “mixture” analysis, women in the highest tertile of overall PFAS concentrations were 71% more likely to develop hypertension during follow-up, compared with women in the lowest tertile (aHR, 1.71; 95% CI, 1.15-2.54; P trend = .008).
“These findings suggest that PFAS might be an underappreciated contributing factor to women’s cardiovascular disease risk,” the researchers write.
They caution that the study only included middle-aged women and that it is unclear whether the findings hold for middle-aged men.
“This is an important question, but the answer is that we do not know,” Dr. Park told this news organization.
“Women become more susceptible to metabolic changes and hypertension risk during the menopausal transition. Our findings suggest that PFAS may play a role in the development of hypertension in women during this critical life stage,” Dr. Park said.
The researchers say more research is needed to confirm and expand the findings and to find ways to reduce PFAS exposure.
“If confirmed in future studies, these findings suggest that understanding human exposure to PFAS and developing effective strategies to reduce PFAS exposure may help prevent the development of hypertension and thereby reduce the global burden of CVD,” the researchers write.
‘The more we learn, the worse it gets’
This is an “interesting” study and shows that “the more we learn about PFAS, the worse it seems to get,” Ankur Shah, MD, division of kidney disease and hypertension, Warren Alpert Medical School of Brown University, Providence, R.I., said in an interview.
“This multisite, multiracial and multiethnic, community-based longitudinal study establishes an association between PFAS and hypertension,” said Dr. Shah, who wasn’t involved in the study.
“This adds to a growing literature base of associations of PFAS with illnesses, including malignancy, thyroid disorders, diabetes, ulcerative colitis, hyperlipidemia, and pregnancy-induced hypertension,” he noted.
Dr. Shah also noted that the authors adjusted for race and ethnicity, study site, education, financial strain, smoking status, environmental tobacco smoke, alcohol consumption, total calorie intake, and menopausal status “and still found a strong association.”
“Still to be determined are both whether PFAS are the causative agent or if there is an unmeasured/unadjusted for entity which has resulted in both increased PFAS exposure and hypertension, as well as if PFAS are causative, if reduction in PFAS exposure would be result in blood pressure reduction,” Dr. Shah added.
The study had no sources of funding. Dr. Park and Dr. Shah have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM HYPERTENSION
A ‘crisis’ of suicidal thoughts, attempts in transgender youth
Transgender youth are significantly more likely to consider suicide and attempt it, compared with their cisgender peers, new research shows.
In a large population-based study, investigators found the increased risk of suicidality is partly because of bullying and cyberbullying experienced by transgender teens.
The findings are “extremely concerning and should be a wake-up call,” Ian Colman, PhD, with the University of Ottawa School of Epidemiology and Public Health, said in an interview.
Young people who are exploring their sexual identities may suffer from depression and anxiety, both about the reactions of their peers and families, as well as their own sense of self.
“These youth are highly marginalized and stigmatized in many corners of our society, and these findings highlight just how distressing these experiences can be,” Dr. Colman said.
The study was published online in the Canadian Medical Association Journal.
Sevenfold increased risk of attempted suicide
The risk of suicidal thoughts and actions is not well studied in transgender and nonbinary youth.
To expand the evidence base, the researchers analyzed data for 6,800 adolescents aged 15-17 years from the 2019 Canadian Health Survey on Children and Youth.
The sample included 1,130 (16.5%) adolescents who identified as having some degree of same-gender attraction, 265 (4.3%) who were unsure of their attraction (“questioning”), and 50 (0.6%) who were transgender, meaning they identified as being of a gender different from that assigned at birth.
Overall, 980 (14.0%) adolescents reported having thoughts of suicide in the prior year, and 480 (6.8%) had attempted suicide in their life.
Transgender youth were five times more likely to think about suicide and more than seven times more likely to have ever attempted suicide than cisgender, heterosexual peers.
Among cisgender adolescents, girls who were attracted to girls had 3.6 times the risk of suicidal ideation and 3.3 times the risk of having ever attempted suicide, compared with their heterosexual peers.
Teens attracted to multiple genders had more than twice the risk of suicidal ideation and suicide attempt. Youth who were questioning their sexual orientation had twice the risk of having attempted suicide in their lifetime.
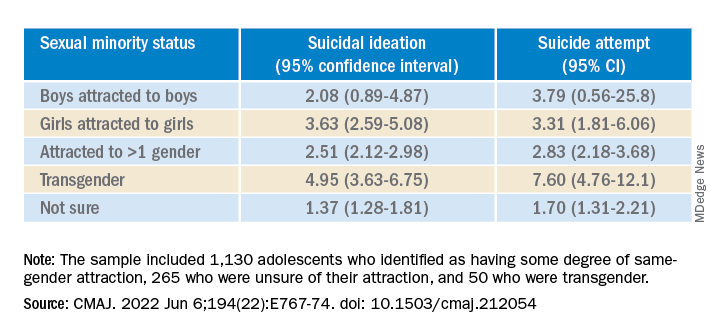
A crisis – with reason for hope
“This is a crisis, and it shows just how much more needs to be done to support transgender young people,” co-author Fae Johnstone, MSW, executive director, Wisdom2Action, who is a trans woman herself, said in the news release.
“Suicide prevention programs specifically targeted to transgender, nonbinary, and sexual minority adolescents, as well as gender-affirming care for transgender adolescents, may help reduce the burden of suicidality among this group,” Ms. Johnstone added.
“The most important thing that parents, teachers, and health care providers can do is to be supportive of these youth,” Dr. Colman told this news organization.
“Providing a safe place where gender and sexual minorities can explore and express themselves is crucial. The first step is to listen and to be compassionate,” Dr. Colman added.
Reached for comment, Jess Ting, MD, director of surgery at the Mount Sinai Center for Transgender Medicine and Surgery, New York, said the data from this study on suicidal thoughts and actions among sexual minority and transgender adolescents “mirror what we see and what we know” about suicidality in trans and nonbinary adults.
“The reasons for this are complex, and it’s hard for someone who doesn’t have a lived experience as a trans or nonbinary person to understand the reasons for suicidality,” he told this news organization.
“But we also know that there are higher rates of anxiety and depression and self-image issues and posttraumatic stress disorder, not to mention outside factors – marginalization, discrimination, violence, abuse. When you add up all these intrinsic and extrinsic factors, it’s not hard to believe that there is a high rate of suicidality,” Dr. Ting said.
“There have been studies that have shown that in children who are supported in their gender identity, the rates of depression and anxiety decreased to almost the same levels as non-trans and nonbinary children, so I think that gives cause for hope,” Dr. Ting added.
The study was funded in part by the Research Council of Norway through its Centres of Excellence funding scheme and by a Frederick Banting and Charles Best Canada Graduate Scholarship Doctoral Award. Ms. Johnstone reports consulting fees from Spectrum Waterloo and volunteer participation with the Youth Suicide Prevention Leadership Committee of Ontario. No other competing interests were declared. Dr. Ting has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Transgender youth are significantly more likely to consider suicide and attempt it, compared with their cisgender peers, new research shows.
In a large population-based study, investigators found the increased risk of suicidality is partly because of bullying and cyberbullying experienced by transgender teens.
The findings are “extremely concerning and should be a wake-up call,” Ian Colman, PhD, with the University of Ottawa School of Epidemiology and Public Health, said in an interview.
Young people who are exploring their sexual identities may suffer from depression and anxiety, both about the reactions of their peers and families, as well as their own sense of self.
“These youth are highly marginalized and stigmatized in many corners of our society, and these findings highlight just how distressing these experiences can be,” Dr. Colman said.
The study was published online in the Canadian Medical Association Journal.
Sevenfold increased risk of attempted suicide
The risk of suicidal thoughts and actions is not well studied in transgender and nonbinary youth.
To expand the evidence base, the researchers analyzed data for 6,800 adolescents aged 15-17 years from the 2019 Canadian Health Survey on Children and Youth.
The sample included 1,130 (16.5%) adolescents who identified as having some degree of same-gender attraction, 265 (4.3%) who were unsure of their attraction (“questioning”), and 50 (0.6%) who were transgender, meaning they identified as being of a gender different from that assigned at birth.
Overall, 980 (14.0%) adolescents reported having thoughts of suicide in the prior year, and 480 (6.8%) had attempted suicide in their life.
Transgender youth were five times more likely to think about suicide and more than seven times more likely to have ever attempted suicide than cisgender, heterosexual peers.
Among cisgender adolescents, girls who were attracted to girls had 3.6 times the risk of suicidal ideation and 3.3 times the risk of having ever attempted suicide, compared with their heterosexual peers.
Teens attracted to multiple genders had more than twice the risk of suicidal ideation and suicide attempt. Youth who were questioning their sexual orientation had twice the risk of having attempted suicide in their lifetime.

A crisis – with reason for hope
“This is a crisis, and it shows just how much more needs to be done to support transgender young people,” co-author Fae Johnstone, MSW, executive director, Wisdom2Action, who is a trans woman herself, said in the news release.
“Suicide prevention programs specifically targeted to transgender, nonbinary, and sexual minority adolescents, as well as gender-affirming care for transgender adolescents, may help reduce the burden of suicidality among this group,” Ms. Johnstone added.
“The most important thing that parents, teachers, and health care providers can do is to be supportive of these youth,” Dr. Colman told this news organization.
“Providing a safe place where gender and sexual minorities can explore and express themselves is crucial. The first step is to listen and to be compassionate,” Dr. Colman added.
Reached for comment, Jess Ting, MD, director of surgery at the Mount Sinai Center for Transgender Medicine and Surgery, New York, said the data from this study on suicidal thoughts and actions among sexual minority and transgender adolescents “mirror what we see and what we know” about suicidality in trans and nonbinary adults.
“The reasons for this are complex, and it’s hard for someone who doesn’t have a lived experience as a trans or nonbinary person to understand the reasons for suicidality,” he told this news organization.
“But we also know that there are higher rates of anxiety and depression and self-image issues and posttraumatic stress disorder, not to mention outside factors – marginalization, discrimination, violence, abuse. When you add up all these intrinsic and extrinsic factors, it’s not hard to believe that there is a high rate of suicidality,” Dr. Ting said.
“There have been studies that have shown that in children who are supported in their gender identity, the rates of depression and anxiety decreased to almost the same levels as non-trans and nonbinary children, so I think that gives cause for hope,” Dr. Ting added.
The study was funded in part by the Research Council of Norway through its Centres of Excellence funding scheme and by a Frederick Banting and Charles Best Canada Graduate Scholarship Doctoral Award. Ms. Johnstone reports consulting fees from Spectrum Waterloo and volunteer participation with the Youth Suicide Prevention Leadership Committee of Ontario. No other competing interests were declared. Dr. Ting has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Transgender youth are significantly more likely to consider suicide and attempt it, compared with their cisgender peers, new research shows.
In a large population-based study, investigators found the increased risk of suicidality is partly because of bullying and cyberbullying experienced by transgender teens.
The findings are “extremely concerning and should be a wake-up call,” Ian Colman, PhD, with the University of Ottawa School of Epidemiology and Public Health, said in an interview.
Young people who are exploring their sexual identities may suffer from depression and anxiety, both about the reactions of their peers and families, as well as their own sense of self.
“These youth are highly marginalized and stigmatized in many corners of our society, and these findings highlight just how distressing these experiences can be,” Dr. Colman said.
The study was published online in the Canadian Medical Association Journal.
Sevenfold increased risk of attempted suicide
The risk of suicidal thoughts and actions is not well studied in transgender and nonbinary youth.
To expand the evidence base, the researchers analyzed data for 6,800 adolescents aged 15-17 years from the 2019 Canadian Health Survey on Children and Youth.
The sample included 1,130 (16.5%) adolescents who identified as having some degree of same-gender attraction, 265 (4.3%) who were unsure of their attraction (“questioning”), and 50 (0.6%) who were transgender, meaning they identified as being of a gender different from that assigned at birth.
Overall, 980 (14.0%) adolescents reported having thoughts of suicide in the prior year, and 480 (6.8%) had attempted suicide in their life.
Transgender youth were five times more likely to think about suicide and more than seven times more likely to have ever attempted suicide than cisgender, heterosexual peers.
Among cisgender adolescents, girls who were attracted to girls had 3.6 times the risk of suicidal ideation and 3.3 times the risk of having ever attempted suicide, compared with their heterosexual peers.
Teens attracted to multiple genders had more than twice the risk of suicidal ideation and suicide attempt. Youth who were questioning their sexual orientation had twice the risk of having attempted suicide in their lifetime.

A crisis – with reason for hope
“This is a crisis, and it shows just how much more needs to be done to support transgender young people,” co-author Fae Johnstone, MSW, executive director, Wisdom2Action, who is a trans woman herself, said in the news release.
“Suicide prevention programs specifically targeted to transgender, nonbinary, and sexual minority adolescents, as well as gender-affirming care for transgender adolescents, may help reduce the burden of suicidality among this group,” Ms. Johnstone added.
“The most important thing that parents, teachers, and health care providers can do is to be supportive of these youth,” Dr. Colman told this news organization.
“Providing a safe place where gender and sexual minorities can explore and express themselves is crucial. The first step is to listen and to be compassionate,” Dr. Colman added.
Reached for comment, Jess Ting, MD, director of surgery at the Mount Sinai Center for Transgender Medicine and Surgery, New York, said the data from this study on suicidal thoughts and actions among sexual minority and transgender adolescents “mirror what we see and what we know” about suicidality in trans and nonbinary adults.
“The reasons for this are complex, and it’s hard for someone who doesn’t have a lived experience as a trans or nonbinary person to understand the reasons for suicidality,” he told this news organization.
“But we also know that there are higher rates of anxiety and depression and self-image issues and posttraumatic stress disorder, not to mention outside factors – marginalization, discrimination, violence, abuse. When you add up all these intrinsic and extrinsic factors, it’s not hard to believe that there is a high rate of suicidality,” Dr. Ting said.
“There have been studies that have shown that in children who are supported in their gender identity, the rates of depression and anxiety decreased to almost the same levels as non-trans and nonbinary children, so I think that gives cause for hope,” Dr. Ting added.
The study was funded in part by the Research Council of Norway through its Centres of Excellence funding scheme and by a Frederick Banting and Charles Best Canada Graduate Scholarship Doctoral Award. Ms. Johnstone reports consulting fees from Spectrum Waterloo and volunteer participation with the Youth Suicide Prevention Leadership Committee of Ontario. No other competing interests were declared. Dr. Ting has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL