Article
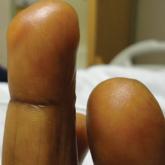
Acral Cutaneous Metastasis From a Primary Breast Carcinoma Following Chemotherapy With Bevacizumab and Paclitaxel
- Author:
- Patrick Armstrong, MD
- Meghan M. Woody, MD, MPH
- Jason S. Reichenberg, MD
- Alde Carlo P. Gavino, MD
Acral cutaneous metastasis from internal malignancy is rare, and the pathogenesis is not completely understood. We present the case of a 54-year-...
Article

Metastatic Vulvovaginal Crohn Disease in the Setting of Well-Controlled Intestinal Disease
- Author:
- Meghan M. Woody, MD, MPH
- Alex C. Holliday, MD
- Alde Carlo P. Gavino, MD
- Abby McReynolds, PA-C
- Anthony C. Soldano, MD
The cutaneous manifestations of Crohn disease (CD) are varied and include pyoderma gangrenosum, erythema nodosum, and metastatic CD (MCD). The...
