User login
Acute Pancreatitis
Acute pancreatitis accounts for more than 220,000 hospital admissions in the United States annually.1 In the following review, we outline the etiology of acute pancreatitis, discuss its complications, and provide an updated review on its management for the hospitalized patient.
Etiology
Gallstone disease and excess alcohol ingestion are the most common causes of acute pancreatitis in the United States. Gallstones account for roughly 45% of all cases, and the pathogenesis is due to transient obstruction of the pancreatic duct orifice to the flow of pancreatic exocrine secretions.2 Excess alcohol ingestion accounts for approximately 35% of all cases, yet the pathogenesis here is less understood.3 Most theories suggest a direct toxic effect of the ethanol upon the pancreatic parenchyma or its neurovascular supply.4
There are many other less common causes of acute pancreatitis including toxins, drugs, infections, trauma, vascular insults, anatomic abnormalities, and metabolic derangements. Hypertriglyceridemia and hypercalcemia are both implicated in acute pancreatitis. Serum triglyceride levels >1000 mg/dL can precipitate an attack of acute pancreatitis though the pathogenesis is not clearly understood.5 Hypercalcemia is also an uncommon cause of acute pancreatitis, and is thought to result from deposition of calcium in the pancreatic duct and calcium activation of trypsinogen.6
Idiopathic pancreatitis occurs in up to 20% of patients with acute pancreatitis, and by definition, the cause is not established by history, physical examination, routine laboratory tests, or imaging. The majority of idiopathic cases of pancreatitis are thought to have a biliary source. In patients with gallbladder in situ, it is estimated that up to 75% acquire pancreatitis from microlithiasis, or biliary sludge and stone debris, that causes obstruction of the distal common bile and main pancreatic ducts. Conversely, sphincter of Oddi dysfunction (SOD) resulting in transient pancreatic ductal obstruction is felt to be the most common cause in those patients who have undergone a previous cholecystectomy.7
An emerging entity, autoimmune pancreatitis (AIP), is more commonly associated with chronic pancreatitis but may cause episodes of acute pancreatitis or mimic pancreatic carcinoma. Typically, the diagnosis is based on elevated levels of serum gammaglobulin subgroup 4 (IgG4) populations, along with characteristic findings on computed tomography (CT) scan (eg, narrowed or wispy main pancreatic duct and an enlarged pancreatic parenchyma). Core‐needle biopsy may confirm the diagnosis of AIP with lymphoplasmacytic infiltration and dense fibrosis.8 Since AIP can mimic pancreatic cancer, the diagnosis may not be made until the time of surgical resection.
Diagnosis
Along with characteristic symptoms, the diagnosis of acute pancreatitis is often based on elevated serum levels of pancreatic enzymes that are at least twice the normal level. Amylase and lipase are the most frequently used serum markers for acute pancreatitis, though their elevation is not pathognomonic for the presence of disease. These enzymes may not always be significantly elevated during times of acute inflammation, and elevation of the enzymes can come from nonpancreatic origins as well (Table 1). Although there is no gold standard for the diagnosis of acute pancreatitis, using serum lipase (>250 IU/L) in conjunction with amylase (>160 IU/L) improves the overall diagnostic sensitivity from 81% to 94%.9 Isoamylase levels can be used to distinguish among pancreatic, salivary, and macroamylasemia though this is not often used if pancreatitis is suspected clinically. Similarly, serum isolipase can be measured, though this is not readily available.
| Nonpancreatic causes of hyperamylasemia | |
| Abdominal/pelvic | Pancreatic pseudocyst, biliary tract disorders, gastritis, peptic ulcer disease, intestinal obstruction, intestinal infarction, perforation, mesenteric thrombosis, pancreatic cancer, appendicitis, peritonitis, pyelonephritis, renal insufficiency, liver disease, pregnancy, ruptured ectopic pregnancy, aortic aneurysm dissection, prostatic disease, ovarian neoplasm |
| Thoracic | Esophagitis, myocardial infarction, pulmonary embolism, pneumonia, metastatic carcinoma of lung, breast cancer |
| Procedural | Abdominal operations, nonabdominal operations, post‐ERCP |
| Trauma | Brain trauma, burns, and traumatic shock |
| Metabolic | Diabetic ketoacidosis |
| Drugs | Opiate administration, oxyphenbutazone, phenylbutazone, aminosalicylic acid, aspirin, atovaquone, bethanecol, estrogens, lamivudine, meperidine, metoclopramide, ranitidine, thiazides, valproic acid, sulfonamides |
| Other | Parotitis, renal transplantation, alcoholism, human immunodeficiency virus, macroamylasemia |
| Nonpancreatic causes of hyperlipasemia | |
| Abdominal/pelvic | Pancreatic pseudocyst, biliary tract disorders, gastritis, peptic ulcer disease, spontaneous bacterial peritonitis, liver disease, pancreatic carcinoma, intestinal obstruction, ischemia, perforation, appendicitis, celiac disease |
| Thoracic | Esophagitis |
| Drugs | Furosemide, thiazides, metronidazole, valproic acid, bethanecol, oral contraceptives, indomethacin |
| Other | Renal insufficiency, macrolipasemia |
In order to improve the sensitivity and specificity of diagnosis, other tests have been studied to help predict disease presence and severity. Previously, serum tests for trypsin, elastase, phospholipase A2, and carboxylester lipase have all been evaluated but shown to have no significant improvement in diagnostic capability.1014 More recently, trypsinogen (a pancreatic proteinase) has proven to be a useful aid in the accurate diagnosis of acute disease. Trypsinogen undergoes activation into trypsin during acute pancreatic inflammation.3 It is comprised of 2 main isoenzymes (trypsinogen‐1 and trypsinogen‐2) that are secreted into the pancreatic fluid with a small proportion escaping into the circulation.15 Higher concentrations of trypsinogen‐1 are seen in healthy people, while higher concentrations of trypsinogen‐2 are seen in those with acute pancreatitis.16 Urinary trypsinogen‐2 dipstick tests detect acute pancreatitis more accurately than quantitative serum or urinary amylase, with a sensitivity as high as 94%, and a specificity of 95%.17 Studies have shown that in post‐endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis, serum trypsinogen‐2 levels begin to rise as early as 1 hour and peak at 6 hours.17 The Actim Pancreatitis (Medix Biomedica, Kauniainen, Finland) urine test strips measure concentrations of trypsinogen‐2 as low as 50 g/L, but is not a quantitative test and, thus, it does not predict severity. Some studies have advocated the use of urinary trypsinogen‐2 as a screening tool, with a positive result indicating a need for further evaluation of acute pancreatitis.1820 Urinary trypsinogen‐2 is less costly than serum tests, plus may result in additional cost savings with earlier patient discharge. Unfortunately, this test is not widely available for clinical use. Urinary trypsinogen activation peptide (TAP) is another test that has been studied in the diagnosis of acute pancreatitis, but may signify disease severity rather than the presence or absence of disease.21 Currently urinary assays for TAP are not widely available in the United States.
Choosing the Appropriate Imaging Modality
Along with the measurement of pancreatic release enzymes, abdominal imaging is often used, though not always necessary to confirm the diagnosis of acute pancreatitis. Imaging techniques such as CT, magnetic resonance imaging (MRI), and transabdominal ultrasonography may be used to rule out other causes of abdominal pain or elucidate the cause of the pancreatitis itself. Ultrasound may show pancreatic enlargement, diminished echogenicity, and possible adjacent fluid collections.22 In searching for evidence of gallstone pancreatitis, transabdominal ultrasound has a sensitivity of 67% and a specificity of 100%.23 However, it may be insensitive for detecting stones in the distal common bile duct near the ampulla due to acoustic interference from gas within the small bowel.24 Furthermore, ultrasound itself is operator‐dependent.
Contrast‐enhanced CT is the standard mode of imaging for diagnosing acute pancreatitis and provides superior imaging of the pancreas. Unfortunately it is more costly than ultrasound, involves radiation exposure, and requires intravenous contrast medium.25 Findings of acute pancreatitis frequently seen on CT include diffuse or segmental enlargement of the gland, irregular pancreatic contour, obliteration of peripancreatic fat planes, parenchymal heterogeneity, and ill‐defined fluid collections within the pancreas or in the lesser sac and pararenal spaces.26 CT scan may also be used to detect pancreatic necrosis, an important finding for the management and prognosis of this disease.27 Despite this, normal CT findings have been reported in patients with acute pancreatitis, and certain CT findings may be related to disease severity.25
Although MRI is less commonly used in the diagnosis of acute pancreatitis, it may provide a useful alternative to CT, especially in cases of renal failure or intravenous contrast hypersensitivity. When combined with magnetic resonance cholangiopancreatography (MRCP) imaging, MRI may even be able to detect a local area of pancreatic duct disruption.27 MRCP allows for a noninvasive cholangiogram and is frequently used to stratify patients who may benefit from ERCP. It can accurately identify common bile duct stones, with a higher sensitivity for choledocholithiasis than ultrasound or CT.2830 MRCP can also assist in the diagnosis of other disorders of the intrahepatic and extrahepatic biliary tree that may be related to the cause of pancreatitis. Overall, unless a patient has a contraindication, or the goal of the study is to diagnose choledocholithiasis, a contrast‐enhanced CT scan remains the imaging procedure of choice due to improved accessibility, lower cost, ease of performance, and increased sensitivity in the detection of gas bubbles (potentially indicating pancreatic infection).3133 Ordering a CT scan or other imaging at admission is not necessary in the diagnosis of acute pancreatitis if the patient's presentation is classic. At admission, however, a CT scan may be reasonable to exclude other serious causes of abdominal pain, such as a perforated ulcer. Imaging may also be ordered to define the cause of the episode of pancreatitis and to exclude occult malignancy. In addition, CT scan should be strongly considered in patients who do not improve within 2 to 3 days to assess for complications such as pancreatic necrosis, pseudocysts, or other complications.34
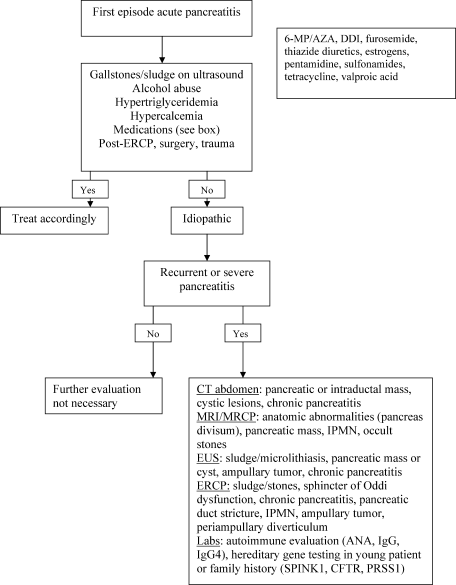
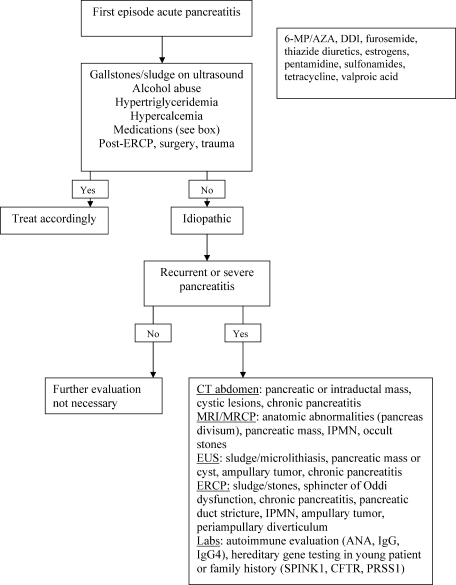
Most recently, endoscopic ultrasound (EUS) has risen to the forefront as a leader in accurate imaging of the pancreas and biliary tree. EUS is more sensitive than transabdominal ultrasound in detecting biliary stones,35 and it has been shown to have equivalent, and in some cases superior, sensitivity to ERCP and MRCP. Because EUS is able to detect smaller stones or sludge, it may have a role in those patients diagnosed with idiopathic pancreatitis.36 Like MRCP, EUS can also help stratify patients into those that are likely to benefit most from ERCP.37 Figure 1 reviews the evaluation of acute pancreatitis.
Prognosis
For the majority of patients with acute pancreatitis, the clinical course is mild and self‐limiting. In approximately 20% to 25% of patients, however, it is severe and associated with organ failure and significant morbidity and mortality.38, 39 Determining the severity of acute pancreatitis is critical, as patients at high‐risk for severe disease require closer monitoring and possible intervention. Several validated scoring systems are available that aim to predict the severity of acute pancreatitis including Ranson's criteria, the Imrie scoring system, the Acute Physiology and Chronic Health Evaluation (APACHE II) scale, and the CT Severity Index (CTSI) (Table 2).4043
| Ranson's Criteria | |
|---|---|
| |
| At admission or diagnosis | |
| Age | >55 years |
| WBC | >16,000/mm3 |
| Blood glucose | >200 mg/dL |
| Lactate dehydrogenase | >350 IU/L |
| AST | >250 IU/L |
| Within 48 hours after presentation | |
| Hematocrit decrease | >10% |
| Blood urea nitrogen increase | >5 mg/dL |
| Serum calcium | <8 mg/dL |
| Base deficit | >4 mEq/L |
| Fluid sequestration | >6 L |
| PaO2 | <60 mmHg |
| Scoring | 1 point for each criterion |
| APACHE II Scale | |
| Equation includes these factors: age, rectal temperature, mean arterial pressure, heart rate, PaO2, arterial pH, serum potassium, sodium, creatinine, hematocrit, WBC count, Glasgow coma scale score, chronic health status | |
| Scoring calculation available at |
|
| CT Severity Index (Balthazar Score) | |
| Grade of pancreatitis on CT | |
| A | Normal pancreas (0 points) |
| B | Pancreatic enlargement (1 point) |
| C | Pancreatic enlargement with peripancreatic inflammation (2 points) |
| D | Extrapancreatic changes plus 1 fluid collection (3 points) |
| E | More than 1 fluid collection (4 points) |
| Necrosis score | |
| None | 0 points |
| One‐third | 2 points |
| >One‐third but less than one‐half | 4 points |
| >One‐half | 6 points |
| Scoring | CT grade plus necrosis score |
| Imrie Scoring System | |
| Age | >55 years |
| WBC | >15,000/mm3 |
| Blood glucose | >180 mg/dL (absence of diabetes) |
| Lactate dehydrogenase | >600 IU/L |
| AST or ALT | >100 IU/L |
| Serum calcium | <8 mg/dl |
| PaO2 | <60 mm Hg |
| Serum albumin | <3.2 g/dL |
| Serum urea | >45 mg/dL |
| Scoring | 1 point for each criterion met after 48 hours of admission |
| Atlanta Criteria | |
| Ranson's score | 3 |
| APACHE II score | 8 |
| Presence of 1 or more organ failures: | |
| Shock | Blood pressure of <90 mmHg |
| Pulmonary insufficiency | PaO2<60 mmHg |
| Renal failure | Creatinine level >2 mg/dL after hydration |
| Gastrointestinal bleeding | Estimated >500‐mL blood loss/24 hours |
| Disseminated intravascular coagulation | Thrombocytopenia, hypofibrinogenemia, fibrin split products |
| Severe hypocalcemia | Calcium level 7.5 mg/dL |
| Presence of 1 or more local complications | |
| Pancreatic necrosis | |
| Pancreatic abscess | |
| Pancreatic pseudocyst | |
| Scoring | Severe pancreatitis indicated by any positive factor listed |
In 1992, the Atlanta Classification of acute pancreatitis was developed to provide a rational approach in predicting disease severity, thus allowing for comparison between clinical trials. It defines severe acute pancreatitis (SAP) on the basis of standard clinical manifestations, a Ranson's score 3, an APACHE II score 8, and evidence of organ failure and intrapancreatic pathological findings.44 Serum markers such as C‐reactive protein (CRP), interleukin‐6, and phospholipase A2 have all been studied to predict severity; however, only CRP is widely available. A cutoff level of 150 mg/L at 48 hours distinguishes mild disease from SAP.45 Clinical findings such as thirst, poor urine output, progressive tachycardia, tachypnea, hypoxemia, confusion, and a lack of improvement in symptoms within the first 48 hours are warning signs of impending severe disease, and thus warrant consideration of admission to an intensive care unit (ICU).34
Natural History and Complications
Despite initial aggressive intensive care treatment, 30% to 50% of patients with SAP do not respond promptly to ICU treatment and develop persistent multisystem organ failure.39 Severe organ failure in the first week of onset of acute pancreatitis is closely linked to the development of pancreatic infection occurring within 2 weeks of the initiation of symptoms.46 Early multiorgan dysfunction triggers additional mechanisms that render bacterial translocation into clinically manifested sepsis and septic shock.39 In most studied series, infection (including bacteremia, fungemia, and pancreatic abscess) remains the leading cause of death in patients with acute pancreatitis, accounting for up to 80% of fatal cases.4749 While sepsis is the more frequent cause of death in patients surviving beyond 7 days, death occurring early in the course of disease is more likely to be from respiratory complications such as pulmonary edema.50
In the spectrum of acute pancreatitis, ongoing pancreatic injury can lead to pancreatic necrosis, fluid collections, pseudocyst formation, and pancreatic duct disruption (Figures 24).51 In patients hospitalized with acute pancreatitis, up to 57% will have peripancreatic fluid collections that are initially ill‐defined.44, 52 Typically, these fluid collections may be managed conservatively; however, if they continue to enlarge, cause persistent abdominal pain, become infected, or compress adjacent organs, they may require further intervention.53 Ductal disruption may be diagnosed when fluid collections have high levels of pancreatic amylase, and their presence may lead to the formation of pseudocysts, persistent ascites, or pleural effusions.54 Pancreatic pseudocysts usually require 4 weeks for complete formation, and they classically contain fluid only without significant solid debris.55 Formation typically occurs as a result of limited pancreatic necrosis causing a pancreatic duct leak with subsequent organization, or from areas of necrosis that liquefy over time.56 Both pancreatic pseudocysts and necrotic pancreatic tissue may become infected leading to abscess formation.51
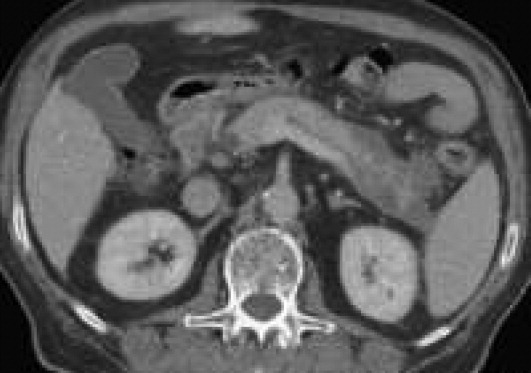
Pancreatic necrosis is defined as diffuse or focal areas of nonviable pancreatic parenchyma, and it is seen in approximately 20% of patients with acute pancreatitis.44, 57 While pseudocyst formation takes approximately 1 month to occur, pancreatic necrosis can occur within the first few days of initial symptoms and is associated with an increase in complications leading to an increased risk of morbidity and mortality.58 More than 80% of deaths in acute pancreatitis are associated with the presence of pancreatic necrosis.39 Patients at highest risk for complications are those with necrosis involving more than 50% of the gland based on MRI or contrast‐enhanced CT scan.59, 60
Patients with pancreatic infection may have infected necrosis, pancreatic abscess, and/or infected pseudocysts.39 The microbes most frequently involved are gram‐negative organisms including Escherichia coli, Enterococcus, and Klebsiella.61 Recently, gram‐positive bacteria have been implicated in pancreatic infection.62 Fungal infection with Candida species is seen in up to 15% of patients with infected necrosis and is associated with more serious systemic complications.63 The use of prophylactic antibiotics may increase the risk of fungal infection. It may be challenging to distinguish between infected and sterile pancreatic necrosis; hence, needle aspiration under EUS or radiologic guidance may be required.61, 64
Management
Supportive Care and Nutrition
The majority (80%) of cases of acute pancreatitis respond well to supportive care with fluid replacement, pain control, and controlled initiation of regular food intake.39 Aggressive intravenous fluid resuscitation is needed to overcome hypovolemia caused by intravascular fluid loss.65 Currently there is a paucity of data to support clinical recommendations regarding rate of fluid resuscitation, but previous studies have suggested a rate of at least 250 to 300 mL/hour for the first 48 hours if fluid status permits.65, 66 Typically, a diet is reintroduced when abdominal tenderness improves and appetite returns.34 Traditionally patients are started on a clear liquid diet and advanced either to a full‐liquid or lowfat diet as toleratedthough there is little data on this subject.67 A recent study randomized 121 subjects to initiate either a clear liquid diet or a lowfat solid diet once recovering from acute pancreatitis and found that the lowfat solid diet was as safe as the clear liquid diet and resulted in improved caloric intake.68
In patients with SAP or complicated disease, nutritional support is critical. In an effort to achieve pancreatic rest, total parenteral nutrition (TPN) has historically been used as the primary means of nutritional support in those patients who require it. TPN, however, carries significant risks of infection and metabolic disturbance,69 and recent studies have shown that enteral nutrition may improve outcomes by decreasing the rates of infection, need for surgical intervention, hospital length of stay, and overall total cost of care.7074 Research has shown that enteral nutrition prevents intestinal atrophy and improves the barrier function of the gut mucosa.75 Typically enteral feeds are given via the nasojejunal (NJ) route, though some data suggest that nasogastric (NG) feeding is also acceptable.76, 77 Despite good intentions by physicians to provide postpyloric feeding, often NJ tubes migrate back into the stomach, yet anecdotal reports showed patients continued to tolerate enteral feeding, prompting further studies. One randomized controlled trial of 49 patients showed NG feeds to be as good as NJ feeds in patients with SAP, plus they were less costly and easier to perform.78 Similarly, this was demonstrated in 16 patients receiving NJ feeds and 15 patients receiving NG feeds with no worsening of SAP in either group.77 In the 2 previous studies, patients with objective evidence of SAP were included and semielemental feeds were started within 24 to 72 hours after onset of pain. Presumably, NG feeds were given over oral feeds as semielemental feeds are not palatable. These are small studies and further research is needed comparing NG to NJ feeds. However, patients who have severe acute pancreatitis with prolonged pain and significant pancreatic necrosis on imaging may benefit from a trial of NJ feeds before advancing to oral feeds.79 TPN may be necessary in those patients who do not tolerate enteral feeding, or do not reach an adequate infusion rate within 2 to 4 days.80
When utilizing enteral feeding, the question of semielemental formula vs. polymeric formula frequently arises. Semielemental formulas seem to pose the advantage of less pancreatic stimulation while not requiring the presence of pancreatic enzymes for absorption.81, 82 Studies, however, have not uniformly supported this hypothesis.83
Antibiotics
Antibiotics do not have a role in mild acute pancreatitis. In SAP, the role of antibiotics is more controversial. Pancreatic or peripancreatic infection develops in a significant number of patients with acute pancreatitis and is associated with substantial morbidity and mortality, particularly in patients with pancreatic necrosis.84 Prophylactic antibiotics have been attempted to reduce infectious complications, but their role in SAP is not entirely clear. Two recent meta‐analyses showed that antibiotic prophylaxis had no significant effect on infection of pancreatic necrosis and mortality, though this did contradict earlier meta‐analyses.8587 Current American College of Gastroenterology guidelines recommend against the use of prophylactic antibiotics to prevent pancreatic infection.88 Though prophylactic antibiotics are not recommended, antibiotics may be given empirically for fever, leukocytosis, and/or sepsis while a possible infectious source is investigated, including fine needle aspiration of pancreatic necrosis.88 Imipenem, meropenem, and a combination of a quinolone and metronidazole have adequate penetration into pancreatic necrotic material and are the antibiotics of choice. Use of antibiotics may increase the risk of resistant organisms and possibly fungal infections.
Endoscopy
Urgent endoscopic therapy for acute pancreatitis is only indicated in gallstone, or biliary pancreatitis. Approximately 5% of patients with symptomatic gallstones will develop acute biliary pancreatitis.89 The risk of a recurrent attack is approximately 30% to 50% if definitive therapy is not sought.90, 91 Multiple studies have demonstrated that ERCP significantly reduces morbidity and mortality in acute biliary pancreatitis.92 Urgent ERCP (within 48 hours of symptom onset) should be considered in cases of cholangitis, or in the setting of severe symptoms of disease with ongoing biliary obstruction. Elective ERCP is indicated in patients with jaundice and imaging studies demonstrating choledocholithiasis, as well as those surgical patients with abnormal intraoperative cholangiography. ERCP should also be considered for suspected pancreatic duct disruption and for biliary sphincterotomy as primary therapy in poor operative candidates, or as temporary therapy during pregnancy.93 ERCP may also have a role in recurrent idiopathic acute pancreatitis if pancreas divisum or SOD is suspected. Sphincter of Oddi manometry may be performed, and if a diagnosis is confirmed, endoscopic sphincterotomy should be performed.94 For pancreas divisum, minor sphincterotomy and/or pancreatic duct stent may be performed.95 ERCP typically does not have a role in those patients with a single attack of acute pancreatitis, as significant complications may occur due to the ERCP itself. EUS, however, can be considered in a single attack of idiopathic pancreatitis in order to further investigate possible causes of the disease.7
Cholecystectomy
Cholecystectomy is indicated for appropriate operative candidates with resolving gallstone pancreatitis. Recurrent pancreatitis can be seen in up to 30% of patients if cholecystectomy is not performed.96, 97 Based on the American Gastroenterological Association (AGA) guidelines, definitive surgical management should be performed in the same hospitalization if possible, but no later than 2 to 4 weeks after discharge.98 In most patients with mild gallstone pancreatitis and no evidence of cholangitis, routine ERCP prior to cholecystectomy is not indicated, as long as pancreatitis is resolving and liver function abnormalities have normalized.88 As mentioned previously, for patients who are not candidates for surgery, endoscopic sphincterotomy should be considered. Cholecystectomy may also be indicated for those with 2 or more episodes of idiopathic pancreatitis, particularly if biliary pancreatitis is suspected.
Failure to Improve
In patients who fail to improve, contrast‐enhanced CT scan should be performed to evaluate for fluid collections, pancreatic necrosis, or other complications that may require intervention. Antibiotic therapy may need to be considered, and in any patient without rapid improvement, nutritional support should be addressed.34 The diagnosis of infected necrosis is typically made by fine‐needle aspiration of the necrotic area under EUS, CT, or transabdominal ultrasound guidance.64
Indications for Drainage of Pseudocysts
The indications for drainage of pancreatic pseudocysts are limited, but drainage is typically performed in those patients that are symptomatic, including abdominal pain, weight loss, gastric outlet obstruction, obstructive jaundice, pancreatic duct leakage, or infectious complications.55 Depending on the location of the pseudocyst and whether it communicates with the pancreatic duct, pseudocysts may be drained by transpapillary means (endoprosthesis placed in the pancreatic duct), or by transmural means (percutaneous, surgical, or endoscopic cyst‐gastrostomy, or endoscopic cyst‐duodenostomy).55 Prior to drainage the pseudocyst wall needs to be mature, which may require up to 4 to 6 weeks. Pancreatic duct leaks may occur as a result of acute or chronic pancreatitis, and they can arise from the head, tail, or body of the gland. Fluid may ultimately track into the mediastinum or peritoneum causing effusions or ascites.55 Treatment for such pancreatic duct leaks includes transpapillary therapy to cross, or bridge, the disrupted duct.
Management of Pancreatic Necrosis
Sterile pancreatic necrosis is typically managed conservatively without drainage. Generally, CT scans are repeated every 7 to 10 days to assess the necrosis and to evaluate for further complications.32 Patients who are clinically unstable with fever, tachycardia, leukocytosis, or organ failure may require percutaneous sampling to evaluate for infected necrosis.33 If the pancreatic tissue is sterile, the patient is determined to have sterile necrosis. If the patient with sterile necrosis is clinically unstable then prophylactic antibiotics may be indicated. If the pancreatic tissue is infected, the patient is deemed to have infected necrosis and treatment with antibiotics and necrosectomy is often indicated, especially in those with a poor clinical state. The antibiotic chosen should have adequate penetration into the necrotic material, such as imipenem, meropenem, or a combination of quinolone and metronidazole.99
It may be challenging to distinguish between sterile and infected pancreatic necrosis. A CT scan is unable to differentiate them with certainty; though, intrapancreatic, retroperitoneal, or lesser sac gas may indicate infection.31 In addition, inducing infection within a previously sterile collection is a potential risk of percutaneous sampling. As a result, sampling should not be performed unless completely indicated.31
In patients with sterile pancreatic necrosis who are symptomatic with refractory abdominal pain, gastric outlet obstruction, or failure to thrive at 4 or more weeks following the onset of acute pancreatitis, drainage and/or debridement is usually indicated. Pancreatic necrosectomy for sterile pancreatic necrosis may be accomplished endoscopically, or more traditionally by a surgical approach.55 Although endoscopic drainage is less invasive, it is technically difficult and has a higher rate of complication in the hands of inexperienced operators.100 Careful selection and evaluation of patients undergoing endoscopic drainage procedures is necessary. Bleeding, perforation, infection, pancreatitis, aspiration, stent migration, and pancreatic ductal damage are all possible complications during the drainage of necrotic pancreatic fluid collections.55 If pancreatic necrosis is infected, surgical necrosectomy should be performed as this is the gold standard for infected necrosis when debridement is necessary.55 Figure 5 reviews the management of acute pancreatitis.
Conclusion
Acute pancreatitis is a common disease frequently caused by choledocholithiasis or excess alcohol ingestion. In idiopathic acute pancreatitis, microlithiasis and SOD should be considered. Though CT scan remains the imaging modality of choice, newer methods such as MRCP and EUS may help to provide additional and improved diagnostic information.
The management of acute pancreatitis is frequently challenging, and severity scales help to predict the likelihood of complications, determine necessary interventions, and guide the appropriate level of care. Nutrition is critical in patients with SAP, and enteral feeding is clearly preferred over TPN. Currently, prophylactic antibiotics do not appear to have a role in SAP. Finally, though not always straightforward, recommendations do exist to guide the management of many of the complications of acute pancreatitis, such as pseudocyst formation and necrotizing disease. A multidisciplinary approach should be used in managing patients with severe disease, and the primary inpatient physician should not hesitate to involve specialists, including gastroenterologists, radiologists, and surgeons.
- ,.2005 National hospital discharge survey.Adv Data.2007;385:1–19.
- ,.Gallstones.BMJ.2007;335:295–299.
- ,.Acute pancreatitis.N Engl J Med.1994;330:1198–1210.
- ,.Pathobiology of alcoholic pancreatitis.Pancreatology.2007;7:105–114.
- .Hyperlipidemic pancreatitis.Gastroenterol Clin North Am.1990;19:783–791.
- ,,,,,.Acute hypercalcemia causes acute pancreatitis and ectopic trypsinogen activation in the rat.Gastroenterology.1995;109:239–246.
- ,,.Role of endoscopic evaluation in idiopathic pancreatitis: a systematic review.Gastrointest Endosc.2006;63:1037–1045.
- ,.Autoimmune pancreatitis: a review.World J Gastroenterol.2007;13:6327–6332.
- ,,, et al.Acute pancreatitis and normoamylasemia. not an uncommon combination.Ann Surg.1989;210:614–620.
- .Normal amylase levels in the presentation of acute pancreatitis.Am J Emerg Med.1994;12:21–24.
- .Diagnostic standards for acute pancreatitis.World J Surg.1997;21:136–142.
- .Support of the diagnosis of pancreatitis by enzyme tests — old problems, new techniques.Clinica Chimica Acta.1997;257:85–98.
- ,,,.What is the best biochemical test to diagnose acute pancreatitis? A prospective clinical study.Mayo Clin Proc.1996;71:1138–1144.
- .The value of clinical laboratory studies in acute pancreatitis.Arch Pathol Lab Med.1991;115:325–326.
- ,.Anionic and cationic trypsinogens (trypsins) in mammalian pancreas.Enzyme.1972/73;14:116–130.
- ,,,,,.Time‐resolved immunofluorometric assays for trypsinogen‐1 and 2 in serum reveal preferential elevation of trypsinogen‐2 in pancreatitis.J Lab Clin Med.1990;115:712–718.
- ,,, et al.Rapid measurement of urinary trypsinogen‐2 as a screening test for acute pancreatitis.N Engl J Med.1997;336:1788–1793.
- ,,,,.Rapid urinary trypsinogen‐2 test strip in the diagnosis of acute pancreatitis.Pancreas.2005;30:243–247.
- ,,, et al.Clinical value of rapid urine trypsinogen‐2 test strip, urinary trypsinogen activation peptide, and serum and urinary activation peptide of carboxypeptidase B in acute pancreatitis.World J Gastroenterol.2005;11:7261–7265.
- ,,,,.Use of the urinary trypsinogen‐2 dip stick test in early diagnosis of pancreatitis after endoscopic retrograde cholangiopancreatography.Surg Endosc.2007;21:1312–1315.
- ,,, et al.Early prediction of severity in acute pancreatitis by urinary trypsinogen activation peptide: a multicentre study.Lancet.2000;355:1955–1960.
- ,.The ultrasonic findings in inflammatory pancreatic disease.Semin Ultrasound.1980;1:178.
- ,,,,,.The urgent diagnosis of gallstones in acute pancreatitis: a prospective study of three methods.Br J Surg.1984;71:230–233.
- ,,.Gallstone pancreatitis: a community teaching hospital experience.J Clin Gastroenterol.2001;33:41–44.
- ,,,.Diagnostic imaging of acute pancreatitis: prospective study using CT and sonography.AJR Am J Roentgenol.1981;137:497–502.
- ,,,.Value of contrast‐enhanced computerized tomography in the early diagnosis and prognosis of acute pancreatitis: a prospective study of 202 patients.Am J Surg.1988;155:457–466.
- ,,, et al.Computed tomography and magnetic resonance imaging in the assessment of acute pancreatitis.Gastroenterology.2004;126:715–723.
- ,,, et al.The role of magnetic resonance cholangiography in the management of patients with gallstone pancreatitis.Ann Surg.2005;241:119–124.
- ,,.Magnetic resonance imaging of acute pancreatitis: the pancreatogram.JOP.2004;5:48–50.
- ,,.Magnetic resonance cholangiopancreatography.N Engl J Med.1999;341(4):258–264.
- ,,,,.Imaging and percutaneous management of acute complicated pancreatitis.Cardiovasc Intervent Radiol.2004;27:567–580.
- ,,.State‐of‐the‐art imaging of acute pancreatitis.JBR‐BTR.2003;86:193–208.
- ,,,.Acute necrotizing pancreatitis: Role of CT‐guided percutaneous catheter drainage.Abdom Imaging.2007;32:351–361.
- .Acute pancreatitis.N Engl J Med.2006;354:2142–2150.
- ,,, et al.Detection of choledocholithiasis by EUS in acute pancreatitis: a prospective evaluation in 100 consecutive patients.Gastrointest Endosc.2001;54:325–330.
- ,.Endoscopic ultrasonography in the evaluation of idiopathic acute pancreatitis.Br J Surg.2000;87:1650–1655.
- ,; theCalgary Advanced Therapeutic Endoscopy Center (ATEC) Study Group.Noninvasive vs. selective invasive biliary imaging for acute biliary pancreatitis: an economic evaluation by using decision tree analysis.Gastrointest Endosc.2005;61:86–97.
- ,.Prognostic factors in acute pancreatitis.J Clin Gastroenterol.2002;34:167–176.
- ,.Severe acute pancreatitis: clinical course and management.World J Gastroenterol.2007;13:5043–5051.
- .Etiological and prognostic factors in human acute pancreatitis: a review.Am J Gastroenterol.1982;77:633–638.
- ,,,,.Prognostic factors in acute pancreatitis.Gut.1984;25:1340–1346.
- ,,,,.APACHE‐acute physiology and chronic health evaluation: a physiologically based classification system.Crit Care Med.1981;9:591–597.
- ,,,.Acute pancreatitis: value of CT in establishing prognosis.Radiology.1990;174:331–336.
- .A clinically based classification system for acute pancreatitis. summary of the international symposium on acute pancreatitis, Atlanta, GA, September 11 through 13, 1992.Arch Surg.1993;128:586–590.
- ,,, et al.Diagnosis, objective assessment of severity, and management of acute pancreatitis. Santorini Consensus Conference.Int J Pancreatol.1999;25:195–210.
- ,,,.Role of early multisystem organ failure as major risk factor for pancreatic infections and death in severe acute pancreatitis.Clin Gastroenterol Hepatol.2006;4:1053–1061.
- ,.Lethal pancreatitis.Am J Gastroenterol.1983;78:810–814.
- ,,,.A randomized multicenter clinical trial of antibiotic prophylaxis of septic complications in acute necrotizing pancreatitis with imipenem.Surg Gynecol Obstet.1993;176:480–483.
- ,.Prevention, diagnosis, and treatment of pancreatic abscess.Surgery.1977;82:99–106.
- ,,,.Death due to acute pancreatitis. a retrospective analysis of 405 autopsy cases.Dig Dis Sci.1985;30:1005–1018.
- .Endoscopic drainage of pancreatic fluid collections and pancreatic necrosis.Gastrointest Endosc Clin North Am.2003;13:743–764.
- ,,, et al.Early prediction of acute pancreatitis: prospective study comparing computed tomography scans, Ranson, Glascow, Acute Physiology and Chronic Health Evaluation II scores, and various serum markers.World J Surg.2002;26:612–619.
- ,,, et al.ASGE guideline: the role of endoscopy in the diagnosis and the management of cystic lesions and inflammatory fluid collections of the pancreas.Gastrointest Endosc.2005;61:363–370.
- .Endoscopic therapy of complete and partial pancreatic duct disruptions.Gastrointest Endosc Clin N Am.1998;8:39–53.
- .Treatment of pancreatic pseudocysts, pancreatic necrosis, and pancreatic duct leaks.Gastrointest Endosc Clin North Am.2007;17:559–579.
- .Pathology of severe acute pancreatitis. In: Bradley EL III, ed.Acute Pancreatitis: Diagnosis and Therapy.New York, NY:Raven Press;1994:35–46.
- ,,,,,.Identification of pancreas necrosis in severe acute pancreatitis: imaging procedures versus clinical staging.Gut.1986;27:1035–1042.
- ,,,,.Results of surgical treatment of necrotizing pancreatitis.World J Surg.1985;9:972–979.
- ,,,,.Management of sterile necrosis in instances of severe acute pancreatitis.J Am Coll Surg.1995;181:279–288.
- ,,,.Prognostic factors in sterile pancreatic necrosis.Gastroenterology.1992;103:1636–1640.
- ,,,.Bacterial contamination of pancreatic necrosis. A prospective clinical study.Gastroenterology.1986;91:433–438.
- ,,.Surgical treatment of necrotizing pancreatitis by necrosectomy and closed lavage: changing patient characteristics and outcome in a 19‐year, single‐center series.Surgery.2005;138:28–39.
- ,,,,,.Characteristics of infection with Candida species in patients with necrotizing pancreatitis.World J Surg.2002;26:372–376.
- ,,,,,.CT‐guided aspiration of suspected pancreatic infection: bacteriology and clinical outcome.Int J Pancreatol.1995;18:265–270.
- .Initial management of acute pancreatitis: critical issues during the first 72 hours.Am J Gastroenterol.2004;99:2489–2494.
- ,,,.Fluid resuscitation in acute pancreatitis.Clin Gastroenterol Hepatol.2008;6:1070–1076.
- ,,, et al.ESPEN guidelines on nutrition in acute pancreatitis.Clin Nutr.2002;21:173–183.
- ,,,,,.A prospective, randomized trial of clear liquids versus low‐fat solid diet as the initial meal in mild acute pancreatitis.Clin Gastroenterol Hepatol.2007;5:946–951.
- ,,,,.Total parenteral nutrition in severe acute pancreatitis.J Am Coll Nutr.1991;10:156–162.
- ,,.Enteral versus parenteral nutrition for acute pancreatitis.Cochrane Database Syst Rev.2003; (1):CD002837.
- ,.Meta‐analysis of parenteral nutrition versus enteral nutrition in patients with acute pancreatitis.BMJ.2004;328:1407.
- ,,.Current management of acute pancreatitis.Nat Clin Pract Gastroenterol Hepatol.2005;2:473–483.
- ,,, et al.Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis.Gut.1998;42:431–435.
- ,,,,.Enteral nutrition is superior to parenteral nutrition in severe acute pancreatitis: Results of a randomized prospective trial.Br J Surg.1997;84:1665–1669.
- ,,.Nutrition in patients with acute pancreatitis.Curr Opin Crit Care.2001;7:251–256.
- ,,,,.Early nasogastric enteral nutrition for severe acute pancreatitis: a systematic review.World J Gastroenterol.2007;13:5253–5260.
- ,,,,.Early enteral nutrition in severe acute pancreatitis: a prospective randomized controlled trial comparing nasojejunal and nasogastric routes.J Clin Gastroenterol.2006;40:431–434.
- ,,, et al.A randomized study of early nasogastric versus nasojejunal feeding in severe acute pancreatitis.Am J Gastroenterol.2005;100:432–439.
- ,,,.Nutrition support in acute pancreatitis: a systematic review of the literature.JPEN J Parenter Enteral Nutr.2006;30:143–156.
- ,.Issues of nutritional support for the patient with acute pancreatitis.Semin Gastrointest Dis.2002;13:154–160.
- ,,,.Effect of continuous jejunal perfusion of elemental and complex nutritional solutions on pancreatic enzyme secretion in human subjects.Gut.1978;19:194–198.
- ,,, et al.Efficiency of enteral nitrogen support in surgical patients: small peptides v non‐degraded proteins.Gut.1990;31:1277–1283.
- ,,, et al.Semi‐elemental formula or polymeric formula: Is there a better choice for enteral nutrition in acute pancreatitis? randomized comparative study.JPEN J Parenter Enteral Nutr.2006;30:1–5.
- ,,, et al.IAP guidelines for the surgical management of acute pancreatitis.Pancreatology.2002;2:565–573.
- ,,, et al.Randomized controlled trials of antibiotic prophylaxis in severe acute pancreatitis: relationship between methodological quality and outcome.Pancreatology.2007;7:531–538.
- ,,,.Prophylactic antibiotics cannot reduce infected pancreatic necrosis and mortality in acute necrotizing pancreatitis: evidence from a meta‐analysis of randomized controlled trials.Am J Gastroenterol.2008;103:104–110.
- ,.Prophylactic antibiotic administration reduces sepsis and mortality in acute necrotizing pancreatitis: a meta‐analysis.Pancreas.2001;22:28–31.
- ,,Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis.Am J Gastroenterol.2006;101:2379–2400.
- ,.Acute biliary pancreatitis.Ann Ital Chir.1995;66:197–202.
- .The timing of biliary surgery in acute pancreatitis.Ann Surg.1979;189:654–663.
- ,,,.Acute biliary pancreatitis. The roles of laparoscopic cholecystectomy and endoscopic retrograde cholangiopancreatography.Surg Endosc.1995;9:392–396.
- ,.Metaanalysis of randomized controlled trials of endoscopic retrograde cholangiography and endoscopic sphincterotomy for the treatment of acute biliary pancreatitis.Am J Gastroenterol.1999;94:3211–3214.
- ,.Endoscopic management of acute pancreatitis.Gastrointest Endosc Clin N Am.2007;17:307–322.
- ,.Review of idiopathic pancreatitis.World J Gastroenterol.2007;13:6296–6313.
- ,,,,.Does endoscopic therapy favorably affect the outcome of patients who have recurrent acute pancreatitis and pancreas divisum?Pancreas.2007;34:21–45.
- .The timing of cholecystectomy in patients with gallstone pancreatitis. A retrospective analysis of 89 patients.Acta Chir Scand.1978;144:487–490.
- ,,, et al.Recurrence of acute gallstone pancreatitis and relationship with cholecystectomy or endoscopic sphincterotomy.Am J Gastroenterol.2004;99:2417–2423.
- American Gastroenterological Association (AGA) Institute on “Management of Acute Pancreatitis” Clinical Practice and Economics Committee, AGA Institute Governing Board.AGA institute medical position statement on acute pancreatitis.Gastroenterology.2007;132:2019–2021.
- ,;AGA Institute Clinical Practice and Economics Committee, AGA Institute Governing Board. AGA institute technical review on acute pancreatitis.Gastroenterology.2007;132:2022–2044.
- .Endoscopic management of pancreatic necrosis: not for the uncommitted.Gastrointest Endosc.2005;62:101–104.
- ,,,.Serum amylase and lipase in the evaluation of acute abdominal pain.Am Surg.1996;62:1028–1033.
Acute pancreatitis accounts for more than 220,000 hospital admissions in the United States annually.1 In the following review, we outline the etiology of acute pancreatitis, discuss its complications, and provide an updated review on its management for the hospitalized patient.
Etiology
Gallstone disease and excess alcohol ingestion are the most common causes of acute pancreatitis in the United States. Gallstones account for roughly 45% of all cases, and the pathogenesis is due to transient obstruction of the pancreatic duct orifice to the flow of pancreatic exocrine secretions.2 Excess alcohol ingestion accounts for approximately 35% of all cases, yet the pathogenesis here is less understood.3 Most theories suggest a direct toxic effect of the ethanol upon the pancreatic parenchyma or its neurovascular supply.4
There are many other less common causes of acute pancreatitis including toxins, drugs, infections, trauma, vascular insults, anatomic abnormalities, and metabolic derangements. Hypertriglyceridemia and hypercalcemia are both implicated in acute pancreatitis. Serum triglyceride levels >1000 mg/dL can precipitate an attack of acute pancreatitis though the pathogenesis is not clearly understood.5 Hypercalcemia is also an uncommon cause of acute pancreatitis, and is thought to result from deposition of calcium in the pancreatic duct and calcium activation of trypsinogen.6
Idiopathic pancreatitis occurs in up to 20% of patients with acute pancreatitis, and by definition, the cause is not established by history, physical examination, routine laboratory tests, or imaging. The majority of idiopathic cases of pancreatitis are thought to have a biliary source. In patients with gallbladder in situ, it is estimated that up to 75% acquire pancreatitis from microlithiasis, or biliary sludge and stone debris, that causes obstruction of the distal common bile and main pancreatic ducts. Conversely, sphincter of Oddi dysfunction (SOD) resulting in transient pancreatic ductal obstruction is felt to be the most common cause in those patients who have undergone a previous cholecystectomy.7
An emerging entity, autoimmune pancreatitis (AIP), is more commonly associated with chronic pancreatitis but may cause episodes of acute pancreatitis or mimic pancreatic carcinoma. Typically, the diagnosis is based on elevated levels of serum gammaglobulin subgroup 4 (IgG4) populations, along with characteristic findings on computed tomography (CT) scan (eg, narrowed or wispy main pancreatic duct and an enlarged pancreatic parenchyma). Core‐needle biopsy may confirm the diagnosis of AIP with lymphoplasmacytic infiltration and dense fibrosis.8 Since AIP can mimic pancreatic cancer, the diagnosis may not be made until the time of surgical resection.
Diagnosis
Along with characteristic symptoms, the diagnosis of acute pancreatitis is often based on elevated serum levels of pancreatic enzymes that are at least twice the normal level. Amylase and lipase are the most frequently used serum markers for acute pancreatitis, though their elevation is not pathognomonic for the presence of disease. These enzymes may not always be significantly elevated during times of acute inflammation, and elevation of the enzymes can come from nonpancreatic origins as well (Table 1). Although there is no gold standard for the diagnosis of acute pancreatitis, using serum lipase (>250 IU/L) in conjunction with amylase (>160 IU/L) improves the overall diagnostic sensitivity from 81% to 94%.9 Isoamylase levels can be used to distinguish among pancreatic, salivary, and macroamylasemia though this is not often used if pancreatitis is suspected clinically. Similarly, serum isolipase can be measured, though this is not readily available.
| Nonpancreatic causes of hyperamylasemia | |
| Abdominal/pelvic | Pancreatic pseudocyst, biliary tract disorders, gastritis, peptic ulcer disease, intestinal obstruction, intestinal infarction, perforation, mesenteric thrombosis, pancreatic cancer, appendicitis, peritonitis, pyelonephritis, renal insufficiency, liver disease, pregnancy, ruptured ectopic pregnancy, aortic aneurysm dissection, prostatic disease, ovarian neoplasm |
| Thoracic | Esophagitis, myocardial infarction, pulmonary embolism, pneumonia, metastatic carcinoma of lung, breast cancer |
| Procedural | Abdominal operations, nonabdominal operations, post‐ERCP |
| Trauma | Brain trauma, burns, and traumatic shock |
| Metabolic | Diabetic ketoacidosis |
| Drugs | Opiate administration, oxyphenbutazone, phenylbutazone, aminosalicylic acid, aspirin, atovaquone, bethanecol, estrogens, lamivudine, meperidine, metoclopramide, ranitidine, thiazides, valproic acid, sulfonamides |
| Other | Parotitis, renal transplantation, alcoholism, human immunodeficiency virus, macroamylasemia |
| Nonpancreatic causes of hyperlipasemia | |
| Abdominal/pelvic | Pancreatic pseudocyst, biliary tract disorders, gastritis, peptic ulcer disease, spontaneous bacterial peritonitis, liver disease, pancreatic carcinoma, intestinal obstruction, ischemia, perforation, appendicitis, celiac disease |
| Thoracic | Esophagitis |
| Drugs | Furosemide, thiazides, metronidazole, valproic acid, bethanecol, oral contraceptives, indomethacin |
| Other | Renal insufficiency, macrolipasemia |
In order to improve the sensitivity and specificity of diagnosis, other tests have been studied to help predict disease presence and severity. Previously, serum tests for trypsin, elastase, phospholipase A2, and carboxylester lipase have all been evaluated but shown to have no significant improvement in diagnostic capability.1014 More recently, trypsinogen (a pancreatic proteinase) has proven to be a useful aid in the accurate diagnosis of acute disease. Trypsinogen undergoes activation into trypsin during acute pancreatic inflammation.3 It is comprised of 2 main isoenzymes (trypsinogen‐1 and trypsinogen‐2) that are secreted into the pancreatic fluid with a small proportion escaping into the circulation.15 Higher concentrations of trypsinogen‐1 are seen in healthy people, while higher concentrations of trypsinogen‐2 are seen in those with acute pancreatitis.16 Urinary trypsinogen‐2 dipstick tests detect acute pancreatitis more accurately than quantitative serum or urinary amylase, with a sensitivity as high as 94%, and a specificity of 95%.17 Studies have shown that in post‐endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis, serum trypsinogen‐2 levels begin to rise as early as 1 hour and peak at 6 hours.17 The Actim Pancreatitis (Medix Biomedica, Kauniainen, Finland) urine test strips measure concentrations of trypsinogen‐2 as low as 50 g/L, but is not a quantitative test and, thus, it does not predict severity. Some studies have advocated the use of urinary trypsinogen‐2 as a screening tool, with a positive result indicating a need for further evaluation of acute pancreatitis.1820 Urinary trypsinogen‐2 is less costly than serum tests, plus may result in additional cost savings with earlier patient discharge. Unfortunately, this test is not widely available for clinical use. Urinary trypsinogen activation peptide (TAP) is another test that has been studied in the diagnosis of acute pancreatitis, but may signify disease severity rather than the presence or absence of disease.21 Currently urinary assays for TAP are not widely available in the United States.
Choosing the Appropriate Imaging Modality
Along with the measurement of pancreatic release enzymes, abdominal imaging is often used, though not always necessary to confirm the diagnosis of acute pancreatitis. Imaging techniques such as CT, magnetic resonance imaging (MRI), and transabdominal ultrasonography may be used to rule out other causes of abdominal pain or elucidate the cause of the pancreatitis itself. Ultrasound may show pancreatic enlargement, diminished echogenicity, and possible adjacent fluid collections.22 In searching for evidence of gallstone pancreatitis, transabdominal ultrasound has a sensitivity of 67% and a specificity of 100%.23 However, it may be insensitive for detecting stones in the distal common bile duct near the ampulla due to acoustic interference from gas within the small bowel.24 Furthermore, ultrasound itself is operator‐dependent.
Contrast‐enhanced CT is the standard mode of imaging for diagnosing acute pancreatitis and provides superior imaging of the pancreas. Unfortunately it is more costly than ultrasound, involves radiation exposure, and requires intravenous contrast medium.25 Findings of acute pancreatitis frequently seen on CT include diffuse or segmental enlargement of the gland, irregular pancreatic contour, obliteration of peripancreatic fat planes, parenchymal heterogeneity, and ill‐defined fluid collections within the pancreas or in the lesser sac and pararenal spaces.26 CT scan may also be used to detect pancreatic necrosis, an important finding for the management and prognosis of this disease.27 Despite this, normal CT findings have been reported in patients with acute pancreatitis, and certain CT findings may be related to disease severity.25
Although MRI is less commonly used in the diagnosis of acute pancreatitis, it may provide a useful alternative to CT, especially in cases of renal failure or intravenous contrast hypersensitivity. When combined with magnetic resonance cholangiopancreatography (MRCP) imaging, MRI may even be able to detect a local area of pancreatic duct disruption.27 MRCP allows for a noninvasive cholangiogram and is frequently used to stratify patients who may benefit from ERCP. It can accurately identify common bile duct stones, with a higher sensitivity for choledocholithiasis than ultrasound or CT.2830 MRCP can also assist in the diagnosis of other disorders of the intrahepatic and extrahepatic biliary tree that may be related to the cause of pancreatitis. Overall, unless a patient has a contraindication, or the goal of the study is to diagnose choledocholithiasis, a contrast‐enhanced CT scan remains the imaging procedure of choice due to improved accessibility, lower cost, ease of performance, and increased sensitivity in the detection of gas bubbles (potentially indicating pancreatic infection).3133 Ordering a CT scan or other imaging at admission is not necessary in the diagnosis of acute pancreatitis if the patient's presentation is classic. At admission, however, a CT scan may be reasonable to exclude other serious causes of abdominal pain, such as a perforated ulcer. Imaging may also be ordered to define the cause of the episode of pancreatitis and to exclude occult malignancy. In addition, CT scan should be strongly considered in patients who do not improve within 2 to 3 days to assess for complications such as pancreatic necrosis, pseudocysts, or other complications.34
Most recently, endoscopic ultrasound (EUS) has risen to the forefront as a leader in accurate imaging of the pancreas and biliary tree. EUS is more sensitive than transabdominal ultrasound in detecting biliary stones,35 and it has been shown to have equivalent, and in some cases superior, sensitivity to ERCP and MRCP. Because EUS is able to detect smaller stones or sludge, it may have a role in those patients diagnosed with idiopathic pancreatitis.36 Like MRCP, EUS can also help stratify patients into those that are likely to benefit most from ERCP.37 Figure 1 reviews the evaluation of acute pancreatitis.

Prognosis
For the majority of patients with acute pancreatitis, the clinical course is mild and self‐limiting. In approximately 20% to 25% of patients, however, it is severe and associated with organ failure and significant morbidity and mortality.38, 39 Determining the severity of acute pancreatitis is critical, as patients at high‐risk for severe disease require closer monitoring and possible intervention. Several validated scoring systems are available that aim to predict the severity of acute pancreatitis including Ranson's criteria, the Imrie scoring system, the Acute Physiology and Chronic Health Evaluation (APACHE II) scale, and the CT Severity Index (CTSI) (Table 2).4043
| Ranson's Criteria | |
|---|---|
| |
| At admission or diagnosis | |
| Age | >55 years |
| WBC | >16,000/mm3 |
| Blood glucose | >200 mg/dL |
| Lactate dehydrogenase | >350 IU/L |
| AST | >250 IU/L |
| Within 48 hours after presentation | |
| Hematocrit decrease | >10% |
| Blood urea nitrogen increase | >5 mg/dL |
| Serum calcium | <8 mg/dL |
| Base deficit | >4 mEq/L |
| Fluid sequestration | >6 L |
| PaO2 | <60 mmHg |
| Scoring | 1 point for each criterion |
| APACHE II Scale | |
| Equation includes these factors: age, rectal temperature, mean arterial pressure, heart rate, PaO2, arterial pH, serum potassium, sodium, creatinine, hematocrit, WBC count, Glasgow coma scale score, chronic health status | |
| Scoring calculation available at |
|
| CT Severity Index (Balthazar Score) | |
| Grade of pancreatitis on CT | |
| A | Normal pancreas (0 points) |
| B | Pancreatic enlargement (1 point) |
| C | Pancreatic enlargement with peripancreatic inflammation (2 points) |
| D | Extrapancreatic changes plus 1 fluid collection (3 points) |
| E | More than 1 fluid collection (4 points) |
| Necrosis score | |
| None | 0 points |
| One‐third | 2 points |
| >One‐third but less than one‐half | 4 points |
| >One‐half | 6 points |
| Scoring | CT grade plus necrosis score |
| Imrie Scoring System | |
| Age | >55 years |
| WBC | >15,000/mm3 |
| Blood glucose | >180 mg/dL (absence of diabetes) |
| Lactate dehydrogenase | >600 IU/L |
| AST or ALT | >100 IU/L |
| Serum calcium | <8 mg/dl |
| PaO2 | <60 mm Hg |
| Serum albumin | <3.2 g/dL |
| Serum urea | >45 mg/dL |
| Scoring | 1 point for each criterion met after 48 hours of admission |
| Atlanta Criteria | |
| Ranson's score | 3 |
| APACHE II score | 8 |
| Presence of 1 or more organ failures: | |
| Shock | Blood pressure of <90 mmHg |
| Pulmonary insufficiency | PaO2<60 mmHg |
| Renal failure | Creatinine level >2 mg/dL after hydration |
| Gastrointestinal bleeding | Estimated >500‐mL blood loss/24 hours |
| Disseminated intravascular coagulation | Thrombocytopenia, hypofibrinogenemia, fibrin split products |
| Severe hypocalcemia | Calcium level 7.5 mg/dL |
| Presence of 1 or more local complications | |
| Pancreatic necrosis | |
| Pancreatic abscess | |
| Pancreatic pseudocyst | |
| Scoring | Severe pancreatitis indicated by any positive factor listed |
In 1992, the Atlanta Classification of acute pancreatitis was developed to provide a rational approach in predicting disease severity, thus allowing for comparison between clinical trials. It defines severe acute pancreatitis (SAP) on the basis of standard clinical manifestations, a Ranson's score 3, an APACHE II score 8, and evidence of organ failure and intrapancreatic pathological findings.44 Serum markers such as C‐reactive protein (CRP), interleukin‐6, and phospholipase A2 have all been studied to predict severity; however, only CRP is widely available. A cutoff level of 150 mg/L at 48 hours distinguishes mild disease from SAP.45 Clinical findings such as thirst, poor urine output, progressive tachycardia, tachypnea, hypoxemia, confusion, and a lack of improvement in symptoms within the first 48 hours are warning signs of impending severe disease, and thus warrant consideration of admission to an intensive care unit (ICU).34
Natural History and Complications
Despite initial aggressive intensive care treatment, 30% to 50% of patients with SAP do not respond promptly to ICU treatment and develop persistent multisystem organ failure.39 Severe organ failure in the first week of onset of acute pancreatitis is closely linked to the development of pancreatic infection occurring within 2 weeks of the initiation of symptoms.46 Early multiorgan dysfunction triggers additional mechanisms that render bacterial translocation into clinically manifested sepsis and septic shock.39 In most studied series, infection (including bacteremia, fungemia, and pancreatic abscess) remains the leading cause of death in patients with acute pancreatitis, accounting for up to 80% of fatal cases.4749 While sepsis is the more frequent cause of death in patients surviving beyond 7 days, death occurring early in the course of disease is more likely to be from respiratory complications such as pulmonary edema.50
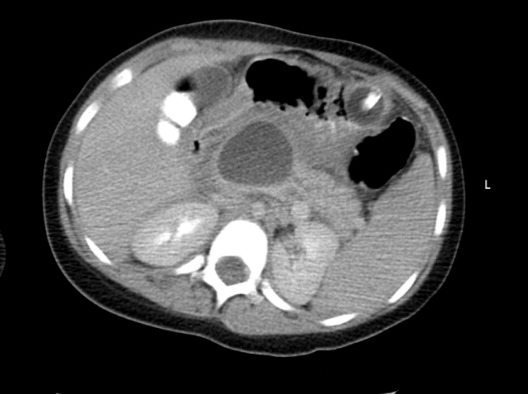
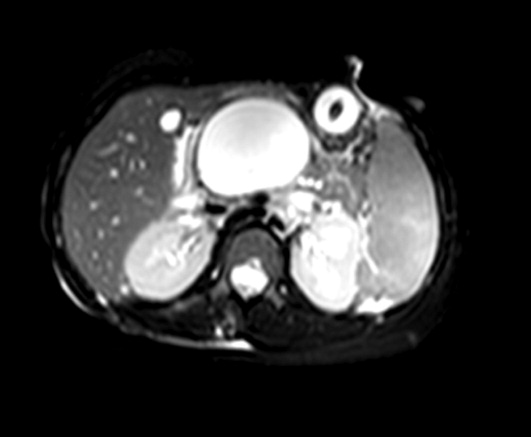
In the spectrum of acute pancreatitis, ongoing pancreatic injury can lead to pancreatic necrosis, fluid collections, pseudocyst formation, and pancreatic duct disruption (Figures 24).51 In patients hospitalized with acute pancreatitis, up to 57% will have peripancreatic fluid collections that are initially ill‐defined.44, 52 Typically, these fluid collections may be managed conservatively; however, if they continue to enlarge, cause persistent abdominal pain, become infected, or compress adjacent organs, they may require further intervention.53 Ductal disruption may be diagnosed when fluid collections have high levels of pancreatic amylase, and their presence may lead to the formation of pseudocysts, persistent ascites, or pleural effusions.54 Pancreatic pseudocysts usually require 4 weeks for complete formation, and they classically contain fluid only without significant solid debris.55 Formation typically occurs as a result of limited pancreatic necrosis causing a pancreatic duct leak with subsequent organization, or from areas of necrosis that liquefy over time.56 Both pancreatic pseudocysts and necrotic pancreatic tissue may become infected leading to abscess formation.51



Pancreatic necrosis is defined as diffuse or focal areas of nonviable pancreatic parenchyma, and it is seen in approximately 20% of patients with acute pancreatitis.44, 57 While pseudocyst formation takes approximately 1 month to occur, pancreatic necrosis can occur within the first few days of initial symptoms and is associated with an increase in complications leading to an increased risk of morbidity and mortality.58 More than 80% of deaths in acute pancreatitis are associated with the presence of pancreatic necrosis.39 Patients at highest risk for complications are those with necrosis involving more than 50% of the gland based on MRI or contrast‐enhanced CT scan.59, 60
Patients with pancreatic infection may have infected necrosis, pancreatic abscess, and/or infected pseudocysts.39 The microbes most frequently involved are gram‐negative organisms including Escherichia coli, Enterococcus, and Klebsiella.61 Recently, gram‐positive bacteria have been implicated in pancreatic infection.62 Fungal infection with Candida species is seen in up to 15% of patients with infected necrosis and is associated with more serious systemic complications.63 The use of prophylactic antibiotics may increase the risk of fungal infection. It may be challenging to distinguish between infected and sterile pancreatic necrosis; hence, needle aspiration under EUS or radiologic guidance may be required.61, 64
Management
Supportive Care and Nutrition
The majority (80%) of cases of acute pancreatitis respond well to supportive care with fluid replacement, pain control, and controlled initiation of regular food intake.39 Aggressive intravenous fluid resuscitation is needed to overcome hypovolemia caused by intravascular fluid loss.65 Currently there is a paucity of data to support clinical recommendations regarding rate of fluid resuscitation, but previous studies have suggested a rate of at least 250 to 300 mL/hour for the first 48 hours if fluid status permits.65, 66 Typically, a diet is reintroduced when abdominal tenderness improves and appetite returns.34 Traditionally patients are started on a clear liquid diet and advanced either to a full‐liquid or lowfat diet as toleratedthough there is little data on this subject.67 A recent study randomized 121 subjects to initiate either a clear liquid diet or a lowfat solid diet once recovering from acute pancreatitis and found that the lowfat solid diet was as safe as the clear liquid diet and resulted in improved caloric intake.68
In patients with SAP or complicated disease, nutritional support is critical. In an effort to achieve pancreatic rest, total parenteral nutrition (TPN) has historically been used as the primary means of nutritional support in those patients who require it. TPN, however, carries significant risks of infection and metabolic disturbance,69 and recent studies have shown that enteral nutrition may improve outcomes by decreasing the rates of infection, need for surgical intervention, hospital length of stay, and overall total cost of care.7074 Research has shown that enteral nutrition prevents intestinal atrophy and improves the barrier function of the gut mucosa.75 Typically enteral feeds are given via the nasojejunal (NJ) route, though some data suggest that nasogastric (NG) feeding is also acceptable.76, 77 Despite good intentions by physicians to provide postpyloric feeding, often NJ tubes migrate back into the stomach, yet anecdotal reports showed patients continued to tolerate enteral feeding, prompting further studies. One randomized controlled trial of 49 patients showed NG feeds to be as good as NJ feeds in patients with SAP, plus they were less costly and easier to perform.78 Similarly, this was demonstrated in 16 patients receiving NJ feeds and 15 patients receiving NG feeds with no worsening of SAP in either group.77 In the 2 previous studies, patients with objective evidence of SAP were included and semielemental feeds were started within 24 to 72 hours after onset of pain. Presumably, NG feeds were given over oral feeds as semielemental feeds are not palatable. These are small studies and further research is needed comparing NG to NJ feeds. However, patients who have severe acute pancreatitis with prolonged pain and significant pancreatic necrosis on imaging may benefit from a trial of NJ feeds before advancing to oral feeds.79 TPN may be necessary in those patients who do not tolerate enteral feeding, or do not reach an adequate infusion rate within 2 to 4 days.80
When utilizing enteral feeding, the question of semielemental formula vs. polymeric formula frequently arises. Semielemental formulas seem to pose the advantage of less pancreatic stimulation while not requiring the presence of pancreatic enzymes for absorption.81, 82 Studies, however, have not uniformly supported this hypothesis.83
Antibiotics
Antibiotics do not have a role in mild acute pancreatitis. In SAP, the role of antibiotics is more controversial. Pancreatic or peripancreatic infection develops in a significant number of patients with acute pancreatitis and is associated with substantial morbidity and mortality, particularly in patients with pancreatic necrosis.84 Prophylactic antibiotics have been attempted to reduce infectious complications, but their role in SAP is not entirely clear. Two recent meta‐analyses showed that antibiotic prophylaxis had no significant effect on infection of pancreatic necrosis and mortality, though this did contradict earlier meta‐analyses.8587 Current American College of Gastroenterology guidelines recommend against the use of prophylactic antibiotics to prevent pancreatic infection.88 Though prophylactic antibiotics are not recommended, antibiotics may be given empirically for fever, leukocytosis, and/or sepsis while a possible infectious source is investigated, including fine needle aspiration of pancreatic necrosis.88 Imipenem, meropenem, and a combination of a quinolone and metronidazole have adequate penetration into pancreatic necrotic material and are the antibiotics of choice. Use of antibiotics may increase the risk of resistant organisms and possibly fungal infections.
Endoscopy
Urgent endoscopic therapy for acute pancreatitis is only indicated in gallstone, or biliary pancreatitis. Approximately 5% of patients with symptomatic gallstones will develop acute biliary pancreatitis.89 The risk of a recurrent attack is approximately 30% to 50% if definitive therapy is not sought.90, 91 Multiple studies have demonstrated that ERCP significantly reduces morbidity and mortality in acute biliary pancreatitis.92 Urgent ERCP (within 48 hours of symptom onset) should be considered in cases of cholangitis, or in the setting of severe symptoms of disease with ongoing biliary obstruction. Elective ERCP is indicated in patients with jaundice and imaging studies demonstrating choledocholithiasis, as well as those surgical patients with abnormal intraoperative cholangiography. ERCP should also be considered for suspected pancreatic duct disruption and for biliary sphincterotomy as primary therapy in poor operative candidates, or as temporary therapy during pregnancy.93 ERCP may also have a role in recurrent idiopathic acute pancreatitis if pancreas divisum or SOD is suspected. Sphincter of Oddi manometry may be performed, and if a diagnosis is confirmed, endoscopic sphincterotomy should be performed.94 For pancreas divisum, minor sphincterotomy and/or pancreatic duct stent may be performed.95 ERCP typically does not have a role in those patients with a single attack of acute pancreatitis, as significant complications may occur due to the ERCP itself. EUS, however, can be considered in a single attack of idiopathic pancreatitis in order to further investigate possible causes of the disease.7
Cholecystectomy
Cholecystectomy is indicated for appropriate operative candidates with resolving gallstone pancreatitis. Recurrent pancreatitis can be seen in up to 30% of patients if cholecystectomy is not performed.96, 97 Based on the American Gastroenterological Association (AGA) guidelines, definitive surgical management should be performed in the same hospitalization if possible, but no later than 2 to 4 weeks after discharge.98 In most patients with mild gallstone pancreatitis and no evidence of cholangitis, routine ERCP prior to cholecystectomy is not indicated, as long as pancreatitis is resolving and liver function abnormalities have normalized.88 As mentioned previously, for patients who are not candidates for surgery, endoscopic sphincterotomy should be considered. Cholecystectomy may also be indicated for those with 2 or more episodes of idiopathic pancreatitis, particularly if biliary pancreatitis is suspected.
Failure to Improve
In patients who fail to improve, contrast‐enhanced CT scan should be performed to evaluate for fluid collections, pancreatic necrosis, or other complications that may require intervention. Antibiotic therapy may need to be considered, and in any patient without rapid improvement, nutritional support should be addressed.34 The diagnosis of infected necrosis is typically made by fine‐needle aspiration of the necrotic area under EUS, CT, or transabdominal ultrasound guidance.64
Indications for Drainage of Pseudocysts
The indications for drainage of pancreatic pseudocysts are limited, but drainage is typically performed in those patients that are symptomatic, including abdominal pain, weight loss, gastric outlet obstruction, obstructive jaundice, pancreatic duct leakage, or infectious complications.55 Depending on the location of the pseudocyst and whether it communicates with the pancreatic duct, pseudocysts may be drained by transpapillary means (endoprosthesis placed in the pancreatic duct), or by transmural means (percutaneous, surgical, or endoscopic cyst‐gastrostomy, or endoscopic cyst‐duodenostomy).55 Prior to drainage the pseudocyst wall needs to be mature, which may require up to 4 to 6 weeks. Pancreatic duct leaks may occur as a result of acute or chronic pancreatitis, and they can arise from the head, tail, or body of the gland. Fluid may ultimately track into the mediastinum or peritoneum causing effusions or ascites.55 Treatment for such pancreatic duct leaks includes transpapillary therapy to cross, or bridge, the disrupted duct.
Management of Pancreatic Necrosis
Sterile pancreatic necrosis is typically managed conservatively without drainage. Generally, CT scans are repeated every 7 to 10 days to assess the necrosis and to evaluate for further complications.32 Patients who are clinically unstable with fever, tachycardia, leukocytosis, or organ failure may require percutaneous sampling to evaluate for infected necrosis.33 If the pancreatic tissue is sterile, the patient is determined to have sterile necrosis. If the patient with sterile necrosis is clinically unstable then prophylactic antibiotics may be indicated. If the pancreatic tissue is infected, the patient is deemed to have infected necrosis and treatment with antibiotics and necrosectomy is often indicated, especially in those with a poor clinical state. The antibiotic chosen should have adequate penetration into the necrotic material, such as imipenem, meropenem, or a combination of quinolone and metronidazole.99
It may be challenging to distinguish between sterile and infected pancreatic necrosis. A CT scan is unable to differentiate them with certainty; though, intrapancreatic, retroperitoneal, or lesser sac gas may indicate infection.31 In addition, inducing infection within a previously sterile collection is a potential risk of percutaneous sampling. As a result, sampling should not be performed unless completely indicated.31
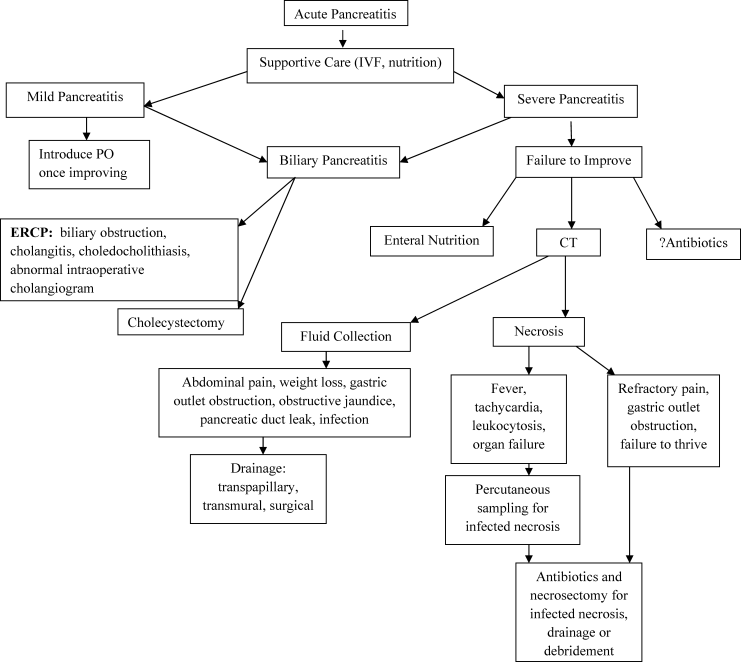
In patients with sterile pancreatic necrosis who are symptomatic with refractory abdominal pain, gastric outlet obstruction, or failure to thrive at 4 or more weeks following the onset of acute pancreatitis, drainage and/or debridement is usually indicated. Pancreatic necrosectomy for sterile pancreatic necrosis may be accomplished endoscopically, or more traditionally by a surgical approach.55 Although endoscopic drainage is less invasive, it is technically difficult and has a higher rate of complication in the hands of inexperienced operators.100 Careful selection and evaluation of patients undergoing endoscopic drainage procedures is necessary. Bleeding, perforation, infection, pancreatitis, aspiration, stent migration, and pancreatic ductal damage are all possible complications during the drainage of necrotic pancreatic fluid collections.55 If pancreatic necrosis is infected, surgical necrosectomy should be performed as this is the gold standard for infected necrosis when debridement is necessary.55 Figure 5 reviews the management of acute pancreatitis.

Conclusion
Acute pancreatitis is a common disease frequently caused by choledocholithiasis or excess alcohol ingestion. In idiopathic acute pancreatitis, microlithiasis and SOD should be considered. Though CT scan remains the imaging modality of choice, newer methods such as MRCP and EUS may help to provide additional and improved diagnostic information.
The management of acute pancreatitis is frequently challenging, and severity scales help to predict the likelihood of complications, determine necessary interventions, and guide the appropriate level of care. Nutrition is critical in patients with SAP, and enteral feeding is clearly preferred over TPN. Currently, prophylactic antibiotics do not appear to have a role in SAP. Finally, though not always straightforward, recommendations do exist to guide the management of many of the complications of acute pancreatitis, such as pseudocyst formation and necrotizing disease. A multidisciplinary approach should be used in managing patients with severe disease, and the primary inpatient physician should not hesitate to involve specialists, including gastroenterologists, radiologists, and surgeons.
Acute pancreatitis accounts for more than 220,000 hospital admissions in the United States annually.1 In the following review, we outline the etiology of acute pancreatitis, discuss its complications, and provide an updated review on its management for the hospitalized patient.
Etiology
Gallstone disease and excess alcohol ingestion are the most common causes of acute pancreatitis in the United States. Gallstones account for roughly 45% of all cases, and the pathogenesis is due to transient obstruction of the pancreatic duct orifice to the flow of pancreatic exocrine secretions.2 Excess alcohol ingestion accounts for approximately 35% of all cases, yet the pathogenesis here is less understood.3 Most theories suggest a direct toxic effect of the ethanol upon the pancreatic parenchyma or its neurovascular supply.4
There are many other less common causes of acute pancreatitis including toxins, drugs, infections, trauma, vascular insults, anatomic abnormalities, and metabolic derangements. Hypertriglyceridemia and hypercalcemia are both implicated in acute pancreatitis. Serum triglyceride levels >1000 mg/dL can precipitate an attack of acute pancreatitis though the pathogenesis is not clearly understood.5 Hypercalcemia is also an uncommon cause of acute pancreatitis, and is thought to result from deposition of calcium in the pancreatic duct and calcium activation of trypsinogen.6
Idiopathic pancreatitis occurs in up to 20% of patients with acute pancreatitis, and by definition, the cause is not established by history, physical examination, routine laboratory tests, or imaging. The majority of idiopathic cases of pancreatitis are thought to have a biliary source. In patients with gallbladder in situ, it is estimated that up to 75% acquire pancreatitis from microlithiasis, or biliary sludge and stone debris, that causes obstruction of the distal common bile and main pancreatic ducts. Conversely, sphincter of Oddi dysfunction (SOD) resulting in transient pancreatic ductal obstruction is felt to be the most common cause in those patients who have undergone a previous cholecystectomy.7
An emerging entity, autoimmune pancreatitis (AIP), is more commonly associated with chronic pancreatitis but may cause episodes of acute pancreatitis or mimic pancreatic carcinoma. Typically, the diagnosis is based on elevated levels of serum gammaglobulin subgroup 4 (IgG4) populations, along with characteristic findings on computed tomography (CT) scan (eg, narrowed or wispy main pancreatic duct and an enlarged pancreatic parenchyma). Core‐needle biopsy may confirm the diagnosis of AIP with lymphoplasmacytic infiltration and dense fibrosis.8 Since AIP can mimic pancreatic cancer, the diagnosis may not be made until the time of surgical resection.
Diagnosis
Along with characteristic symptoms, the diagnosis of acute pancreatitis is often based on elevated serum levels of pancreatic enzymes that are at least twice the normal level. Amylase and lipase are the most frequently used serum markers for acute pancreatitis, though their elevation is not pathognomonic for the presence of disease. These enzymes may not always be significantly elevated during times of acute inflammation, and elevation of the enzymes can come from nonpancreatic origins as well (Table 1). Although there is no gold standard for the diagnosis of acute pancreatitis, using serum lipase (>250 IU/L) in conjunction with amylase (>160 IU/L) improves the overall diagnostic sensitivity from 81% to 94%.9 Isoamylase levels can be used to distinguish among pancreatic, salivary, and macroamylasemia though this is not often used if pancreatitis is suspected clinically. Similarly, serum isolipase can be measured, though this is not readily available.
| Nonpancreatic causes of hyperamylasemia | |
| Abdominal/pelvic | Pancreatic pseudocyst, biliary tract disorders, gastritis, peptic ulcer disease, intestinal obstruction, intestinal infarction, perforation, mesenteric thrombosis, pancreatic cancer, appendicitis, peritonitis, pyelonephritis, renal insufficiency, liver disease, pregnancy, ruptured ectopic pregnancy, aortic aneurysm dissection, prostatic disease, ovarian neoplasm |
| Thoracic | Esophagitis, myocardial infarction, pulmonary embolism, pneumonia, metastatic carcinoma of lung, breast cancer |
| Procedural | Abdominal operations, nonabdominal operations, post‐ERCP |
| Trauma | Brain trauma, burns, and traumatic shock |
| Metabolic | Diabetic ketoacidosis |
| Drugs | Opiate administration, oxyphenbutazone, phenylbutazone, aminosalicylic acid, aspirin, atovaquone, bethanecol, estrogens, lamivudine, meperidine, metoclopramide, ranitidine, thiazides, valproic acid, sulfonamides |
| Other | Parotitis, renal transplantation, alcoholism, human immunodeficiency virus, macroamylasemia |
| Nonpancreatic causes of hyperlipasemia | |
| Abdominal/pelvic | Pancreatic pseudocyst, biliary tract disorders, gastritis, peptic ulcer disease, spontaneous bacterial peritonitis, liver disease, pancreatic carcinoma, intestinal obstruction, ischemia, perforation, appendicitis, celiac disease |
| Thoracic | Esophagitis |
| Drugs | Furosemide, thiazides, metronidazole, valproic acid, bethanecol, oral contraceptives, indomethacin |
| Other | Renal insufficiency, macrolipasemia |
In order to improve the sensitivity and specificity of diagnosis, other tests have been studied to help predict disease presence and severity. Previously, serum tests for trypsin, elastase, phospholipase A2, and carboxylester lipase have all been evaluated but shown to have no significant improvement in diagnostic capability.1014 More recently, trypsinogen (a pancreatic proteinase) has proven to be a useful aid in the accurate diagnosis of acute disease. Trypsinogen undergoes activation into trypsin during acute pancreatic inflammation.3 It is comprised of 2 main isoenzymes (trypsinogen‐1 and trypsinogen‐2) that are secreted into the pancreatic fluid with a small proportion escaping into the circulation.15 Higher concentrations of trypsinogen‐1 are seen in healthy people, while higher concentrations of trypsinogen‐2 are seen in those with acute pancreatitis.16 Urinary trypsinogen‐2 dipstick tests detect acute pancreatitis more accurately than quantitative serum or urinary amylase, with a sensitivity as high as 94%, and a specificity of 95%.17 Studies have shown that in post‐endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis, serum trypsinogen‐2 levels begin to rise as early as 1 hour and peak at 6 hours.17 The Actim Pancreatitis (Medix Biomedica, Kauniainen, Finland) urine test strips measure concentrations of trypsinogen‐2 as low as 50 g/L, but is not a quantitative test and, thus, it does not predict severity. Some studies have advocated the use of urinary trypsinogen‐2 as a screening tool, with a positive result indicating a need for further evaluation of acute pancreatitis.1820 Urinary trypsinogen‐2 is less costly than serum tests, plus may result in additional cost savings with earlier patient discharge. Unfortunately, this test is not widely available for clinical use. Urinary trypsinogen activation peptide (TAP) is another test that has been studied in the diagnosis of acute pancreatitis, but may signify disease severity rather than the presence or absence of disease.21 Currently urinary assays for TAP are not widely available in the United States.
Choosing the Appropriate Imaging Modality
Along with the measurement of pancreatic release enzymes, abdominal imaging is often used, though not always necessary to confirm the diagnosis of acute pancreatitis. Imaging techniques such as CT, magnetic resonance imaging (MRI), and transabdominal ultrasonography may be used to rule out other causes of abdominal pain or elucidate the cause of the pancreatitis itself. Ultrasound may show pancreatic enlargement, diminished echogenicity, and possible adjacent fluid collections.22 In searching for evidence of gallstone pancreatitis, transabdominal ultrasound has a sensitivity of 67% and a specificity of 100%.23 However, it may be insensitive for detecting stones in the distal common bile duct near the ampulla due to acoustic interference from gas within the small bowel.24 Furthermore, ultrasound itself is operator‐dependent.
Contrast‐enhanced CT is the standard mode of imaging for diagnosing acute pancreatitis and provides superior imaging of the pancreas. Unfortunately it is more costly than ultrasound, involves radiation exposure, and requires intravenous contrast medium.25 Findings of acute pancreatitis frequently seen on CT include diffuse or segmental enlargement of the gland, irregular pancreatic contour, obliteration of peripancreatic fat planes, parenchymal heterogeneity, and ill‐defined fluid collections within the pancreas or in the lesser sac and pararenal spaces.26 CT scan may also be used to detect pancreatic necrosis, an important finding for the management and prognosis of this disease.27 Despite this, normal CT findings have been reported in patients with acute pancreatitis, and certain CT findings may be related to disease severity.25
Although MRI is less commonly used in the diagnosis of acute pancreatitis, it may provide a useful alternative to CT, especially in cases of renal failure or intravenous contrast hypersensitivity. When combined with magnetic resonance cholangiopancreatography (MRCP) imaging, MRI may even be able to detect a local area of pancreatic duct disruption.27 MRCP allows for a noninvasive cholangiogram and is frequently used to stratify patients who may benefit from ERCP. It can accurately identify common bile duct stones, with a higher sensitivity for choledocholithiasis than ultrasound or CT.2830 MRCP can also assist in the diagnosis of other disorders of the intrahepatic and extrahepatic biliary tree that may be related to the cause of pancreatitis. Overall, unless a patient has a contraindication, or the goal of the study is to diagnose choledocholithiasis, a contrast‐enhanced CT scan remains the imaging procedure of choice due to improved accessibility, lower cost, ease of performance, and increased sensitivity in the detection of gas bubbles (potentially indicating pancreatic infection).3133 Ordering a CT scan or other imaging at admission is not necessary in the diagnosis of acute pancreatitis if the patient's presentation is classic. At admission, however, a CT scan may be reasonable to exclude other serious causes of abdominal pain, such as a perforated ulcer. Imaging may also be ordered to define the cause of the episode of pancreatitis and to exclude occult malignancy. In addition, CT scan should be strongly considered in patients who do not improve within 2 to 3 days to assess for complications such as pancreatic necrosis, pseudocysts, or other complications.34
Most recently, endoscopic ultrasound (EUS) has risen to the forefront as a leader in accurate imaging of the pancreas and biliary tree. EUS is more sensitive than transabdominal ultrasound in detecting biliary stones,35 and it has been shown to have equivalent, and in some cases superior, sensitivity to ERCP and MRCP. Because EUS is able to detect smaller stones or sludge, it may have a role in those patients diagnosed with idiopathic pancreatitis.36 Like MRCP, EUS can also help stratify patients into those that are likely to benefit most from ERCP.37 Figure 1 reviews the evaluation of acute pancreatitis.

Prognosis
For the majority of patients with acute pancreatitis, the clinical course is mild and self‐limiting. In approximately 20% to 25% of patients, however, it is severe and associated with organ failure and significant morbidity and mortality.38, 39 Determining the severity of acute pancreatitis is critical, as patients at high‐risk for severe disease require closer monitoring and possible intervention. Several validated scoring systems are available that aim to predict the severity of acute pancreatitis including Ranson's criteria, the Imrie scoring system, the Acute Physiology and Chronic Health Evaluation (APACHE II) scale, and the CT Severity Index (CTSI) (Table 2).4043
| Ranson's Criteria | |
|---|---|
| |
| At admission or diagnosis | |
| Age | >55 years |
| WBC | >16,000/mm3 |
| Blood glucose | >200 mg/dL |
| Lactate dehydrogenase | >350 IU/L |
| AST | >250 IU/L |
| Within 48 hours after presentation | |
| Hematocrit decrease | >10% |
| Blood urea nitrogen increase | >5 mg/dL |
| Serum calcium | <8 mg/dL |
| Base deficit | >4 mEq/L |
| Fluid sequestration | >6 L |
| PaO2 | <60 mmHg |
| Scoring | 1 point for each criterion |
| APACHE II Scale | |
| Equation includes these factors: age, rectal temperature, mean arterial pressure, heart rate, PaO2, arterial pH, serum potassium, sodium, creatinine, hematocrit, WBC count, Glasgow coma scale score, chronic health status | |
| Scoring calculation available at |
|
| CT Severity Index (Balthazar Score) | |
| Grade of pancreatitis on CT | |
| A | Normal pancreas (0 points) |
| B | Pancreatic enlargement (1 point) |
| C | Pancreatic enlargement with peripancreatic inflammation (2 points) |
| D | Extrapancreatic changes plus 1 fluid collection (3 points) |
| E | More than 1 fluid collection (4 points) |
| Necrosis score | |
| None | 0 points |
| One‐third | 2 points |
| >One‐third but less than one‐half | 4 points |
| >One‐half | 6 points |
| Scoring | CT grade plus necrosis score |
| Imrie Scoring System | |
| Age | >55 years |
| WBC | >15,000/mm3 |
| Blood glucose | >180 mg/dL (absence of diabetes) |
| Lactate dehydrogenase | >600 IU/L |
| AST or ALT | >100 IU/L |
| Serum calcium | <8 mg/dl |
| PaO2 | <60 mm Hg |
| Serum albumin | <3.2 g/dL |
| Serum urea | >45 mg/dL |
| Scoring | 1 point for each criterion met after 48 hours of admission |
| Atlanta Criteria | |
| Ranson's score | 3 |
| APACHE II score | 8 |
| Presence of 1 or more organ failures: | |
| Shock | Blood pressure of <90 mmHg |
| Pulmonary insufficiency | PaO2<60 mmHg |
| Renal failure | Creatinine level >2 mg/dL after hydration |
| Gastrointestinal bleeding | Estimated >500‐mL blood loss/24 hours |
| Disseminated intravascular coagulation | Thrombocytopenia, hypofibrinogenemia, fibrin split products |
| Severe hypocalcemia | Calcium level 7.5 mg/dL |
| Presence of 1 or more local complications | |
| Pancreatic necrosis | |
| Pancreatic abscess | |
| Pancreatic pseudocyst | |
| Scoring | Severe pancreatitis indicated by any positive factor listed |
In 1992, the Atlanta Classification of acute pancreatitis was developed to provide a rational approach in predicting disease severity, thus allowing for comparison between clinical trials. It defines severe acute pancreatitis (SAP) on the basis of standard clinical manifestations, a Ranson's score 3, an APACHE II score 8, and evidence of organ failure and intrapancreatic pathological findings.44 Serum markers such as C‐reactive protein (CRP), interleukin‐6, and phospholipase A2 have all been studied to predict severity; however, only CRP is widely available. A cutoff level of 150 mg/L at 48 hours distinguishes mild disease from SAP.45 Clinical findings such as thirst, poor urine output, progressive tachycardia, tachypnea, hypoxemia, confusion, and a lack of improvement in symptoms within the first 48 hours are warning signs of impending severe disease, and thus warrant consideration of admission to an intensive care unit (ICU).34
Natural History and Complications
Despite initial aggressive intensive care treatment, 30% to 50% of patients with SAP do not respond promptly to ICU treatment and develop persistent multisystem organ failure.39 Severe organ failure in the first week of onset of acute pancreatitis is closely linked to the development of pancreatic infection occurring within 2 weeks of the initiation of symptoms.46 Early multiorgan dysfunction triggers additional mechanisms that render bacterial translocation into clinically manifested sepsis and septic shock.39 In most studied series, infection (including bacteremia, fungemia, and pancreatic abscess) remains the leading cause of death in patients with acute pancreatitis, accounting for up to 80% of fatal cases.4749 While sepsis is the more frequent cause of death in patients surviving beyond 7 days, death occurring early in the course of disease is more likely to be from respiratory complications such as pulmonary edema.50
In the spectrum of acute pancreatitis, ongoing pancreatic injury can lead to pancreatic necrosis, fluid collections, pseudocyst formation, and pancreatic duct disruption (Figures 24).51 In patients hospitalized with acute pancreatitis, up to 57% will have peripancreatic fluid collections that are initially ill‐defined.44, 52 Typically, these fluid collections may be managed conservatively; however, if they continue to enlarge, cause persistent abdominal pain, become infected, or compress adjacent organs, they may require further intervention.53 Ductal disruption may be diagnosed when fluid collections have high levels of pancreatic amylase, and their presence may lead to the formation of pseudocysts, persistent ascites, or pleural effusions.54 Pancreatic pseudocysts usually require 4 weeks for complete formation, and they classically contain fluid only without significant solid debris.55 Formation typically occurs as a result of limited pancreatic necrosis causing a pancreatic duct leak with subsequent organization, or from areas of necrosis that liquefy over time.56 Both pancreatic pseudocysts and necrotic pancreatic tissue may become infected leading to abscess formation.51



Pancreatic necrosis is defined as diffuse or focal areas of nonviable pancreatic parenchyma, and it is seen in approximately 20% of patients with acute pancreatitis.44, 57 While pseudocyst formation takes approximately 1 month to occur, pancreatic necrosis can occur within the first few days of initial symptoms and is associated with an increase in complications leading to an increased risk of morbidity and mortality.58 More than 80% of deaths in acute pancreatitis are associated with the presence of pancreatic necrosis.39 Patients at highest risk for complications are those with necrosis involving more than 50% of the gland based on MRI or contrast‐enhanced CT scan.59, 60
Patients with pancreatic infection may have infected necrosis, pancreatic abscess, and/or infected pseudocysts.39 The microbes most frequently involved are gram‐negative organisms including Escherichia coli, Enterococcus, and Klebsiella.61 Recently, gram‐positive bacteria have been implicated in pancreatic infection.62 Fungal infection with Candida species is seen in up to 15% of patients with infected necrosis and is associated with more serious systemic complications.63 The use of prophylactic antibiotics may increase the risk of fungal infection. It may be challenging to distinguish between infected and sterile pancreatic necrosis; hence, needle aspiration under EUS or radiologic guidance may be required.61, 64
Management
Supportive Care and Nutrition
The majority (80%) of cases of acute pancreatitis respond well to supportive care with fluid replacement, pain control, and controlled initiation of regular food intake.39 Aggressive intravenous fluid resuscitation is needed to overcome hypovolemia caused by intravascular fluid loss.65 Currently there is a paucity of data to support clinical recommendations regarding rate of fluid resuscitation, but previous studies have suggested a rate of at least 250 to 300 mL/hour for the first 48 hours if fluid status permits.65, 66 Typically, a diet is reintroduced when abdominal tenderness improves and appetite returns.34 Traditionally patients are started on a clear liquid diet and advanced either to a full‐liquid or lowfat diet as toleratedthough there is little data on this subject.67 A recent study randomized 121 subjects to initiate either a clear liquid diet or a lowfat solid diet once recovering from acute pancreatitis and found that the lowfat solid diet was as safe as the clear liquid diet and resulted in improved caloric intake.68
In patients with SAP or complicated disease, nutritional support is critical. In an effort to achieve pancreatic rest, total parenteral nutrition (TPN) has historically been used as the primary means of nutritional support in those patients who require it. TPN, however, carries significant risks of infection and metabolic disturbance,69 and recent studies have shown that enteral nutrition may improve outcomes by decreasing the rates of infection, need for surgical intervention, hospital length of stay, and overall total cost of care.7074 Research has shown that enteral nutrition prevents intestinal atrophy and improves the barrier function of the gut mucosa.75 Typically enteral feeds are given via the nasojejunal (NJ) route, though some data suggest that nasogastric (NG) feeding is also acceptable.76, 77 Despite good intentions by physicians to provide postpyloric feeding, often NJ tubes migrate back into the stomach, yet anecdotal reports showed patients continued to tolerate enteral feeding, prompting further studies. One randomized controlled trial of 49 patients showed NG feeds to be as good as NJ feeds in patients with SAP, plus they were less costly and easier to perform.78 Similarly, this was demonstrated in 16 patients receiving NJ feeds and 15 patients receiving NG feeds with no worsening of SAP in either group.77 In the 2 previous studies, patients with objective evidence of SAP were included and semielemental feeds were started within 24 to 72 hours after onset of pain. Presumably, NG feeds were given over oral feeds as semielemental feeds are not palatable. These are small studies and further research is needed comparing NG to NJ feeds. However, patients who have severe acute pancreatitis with prolonged pain and significant pancreatic necrosis on imaging may benefit from a trial of NJ feeds before advancing to oral feeds.79 TPN may be necessary in those patients who do not tolerate enteral feeding, or do not reach an adequate infusion rate within 2 to 4 days.80
When utilizing enteral feeding, the question of semielemental formula vs. polymeric formula frequently arises. Semielemental formulas seem to pose the advantage of less pancreatic stimulation while not requiring the presence of pancreatic enzymes for absorption.81, 82 Studies, however, have not uniformly supported this hypothesis.83
Antibiotics
Antibiotics do not have a role in mild acute pancreatitis. In SAP, the role of antibiotics is more controversial. Pancreatic or peripancreatic infection develops in a significant number of patients with acute pancreatitis and is associated with substantial morbidity and mortality, particularly in patients with pancreatic necrosis.84 Prophylactic antibiotics have been attempted to reduce infectious complications, but their role in SAP is not entirely clear. Two recent meta‐analyses showed that antibiotic prophylaxis had no significant effect on infection of pancreatic necrosis and mortality, though this did contradict earlier meta‐analyses.8587 Current American College of Gastroenterology guidelines recommend against the use of prophylactic antibiotics to prevent pancreatic infection.88 Though prophylactic antibiotics are not recommended, antibiotics may be given empirically for fever, leukocytosis, and/or sepsis while a possible infectious source is investigated, including fine needle aspiration of pancreatic necrosis.88 Imipenem, meropenem, and a combination of a quinolone and metronidazole have adequate penetration into pancreatic necrotic material and are the antibiotics of choice. Use of antibiotics may increase the risk of resistant organisms and possibly fungal infections.
Endoscopy
Urgent endoscopic therapy for acute pancreatitis is only indicated in gallstone, or biliary pancreatitis. Approximately 5% of patients with symptomatic gallstones will develop acute biliary pancreatitis.89 The risk of a recurrent attack is approximately 30% to 50% if definitive therapy is not sought.90, 91 Multiple studies have demonstrated that ERCP significantly reduces morbidity and mortality in acute biliary pancreatitis.92 Urgent ERCP (within 48 hours of symptom onset) should be considered in cases of cholangitis, or in the setting of severe symptoms of disease with ongoing biliary obstruction. Elective ERCP is indicated in patients with jaundice and imaging studies demonstrating choledocholithiasis, as well as those surgical patients with abnormal intraoperative cholangiography. ERCP should also be considered for suspected pancreatic duct disruption and for biliary sphincterotomy as primary therapy in poor operative candidates, or as temporary therapy during pregnancy.93 ERCP may also have a role in recurrent idiopathic acute pancreatitis if pancreas divisum or SOD is suspected. Sphincter of Oddi manometry may be performed, and if a diagnosis is confirmed, endoscopic sphincterotomy should be performed.94 For pancreas divisum, minor sphincterotomy and/or pancreatic duct stent may be performed.95 ERCP typically does not have a role in those patients with a single attack of acute pancreatitis, as significant complications may occur due to the ERCP itself. EUS, however, can be considered in a single attack of idiopathic pancreatitis in order to further investigate possible causes of the disease.7
Cholecystectomy
Cholecystectomy is indicated for appropriate operative candidates with resolving gallstone pancreatitis. Recurrent pancreatitis can be seen in up to 30% of patients if cholecystectomy is not performed.96, 97 Based on the American Gastroenterological Association (AGA) guidelines, definitive surgical management should be performed in the same hospitalization if possible, but no later than 2 to 4 weeks after discharge.98 In most patients with mild gallstone pancreatitis and no evidence of cholangitis, routine ERCP prior to cholecystectomy is not indicated, as long as pancreatitis is resolving and liver function abnormalities have normalized.88 As mentioned previously, for patients who are not candidates for surgery, endoscopic sphincterotomy should be considered. Cholecystectomy may also be indicated for those with 2 or more episodes of idiopathic pancreatitis, particularly if biliary pancreatitis is suspected.
Failure to Improve
In patients who fail to improve, contrast‐enhanced CT scan should be performed to evaluate for fluid collections, pancreatic necrosis, or other complications that may require intervention. Antibiotic therapy may need to be considered, and in any patient without rapid improvement, nutritional support should be addressed.34 The diagnosis of infected necrosis is typically made by fine‐needle aspiration of the necrotic area under EUS, CT, or transabdominal ultrasound guidance.64
Indications for Drainage of Pseudocysts
The indications for drainage of pancreatic pseudocysts are limited, but drainage is typically performed in those patients that are symptomatic, including abdominal pain, weight loss, gastric outlet obstruction, obstructive jaundice, pancreatic duct leakage, or infectious complications.55 Depending on the location of the pseudocyst and whether it communicates with the pancreatic duct, pseudocysts may be drained by transpapillary means (endoprosthesis placed in the pancreatic duct), or by transmural means (percutaneous, surgical, or endoscopic cyst‐gastrostomy, or endoscopic cyst‐duodenostomy).55 Prior to drainage the pseudocyst wall needs to be mature, which may require up to 4 to 6 weeks. Pancreatic duct leaks may occur as a result of acute or chronic pancreatitis, and they can arise from the head, tail, or body of the gland. Fluid may ultimately track into the mediastinum or peritoneum causing effusions or ascites.55 Treatment for such pancreatic duct leaks includes transpapillary therapy to cross, or bridge, the disrupted duct.
Management of Pancreatic Necrosis
Sterile pancreatic necrosis is typically managed conservatively without drainage. Generally, CT scans are repeated every 7 to 10 days to assess the necrosis and to evaluate for further complications.32 Patients who are clinically unstable with fever, tachycardia, leukocytosis, or organ failure may require percutaneous sampling to evaluate for infected necrosis.33 If the pancreatic tissue is sterile, the patient is determined to have sterile necrosis. If the patient with sterile necrosis is clinically unstable then prophylactic antibiotics may be indicated. If the pancreatic tissue is infected, the patient is deemed to have infected necrosis and treatment with antibiotics and necrosectomy is often indicated, especially in those with a poor clinical state. The antibiotic chosen should have adequate penetration into the necrotic material, such as imipenem, meropenem, or a combination of quinolone and metronidazole.99
It may be challenging to distinguish between sterile and infected pancreatic necrosis. A CT scan is unable to differentiate them with certainty; though, intrapancreatic, retroperitoneal, or lesser sac gas may indicate infection.31 In addition, inducing infection within a previously sterile collection is a potential risk of percutaneous sampling. As a result, sampling should not be performed unless completely indicated.31
In patients with sterile pancreatic necrosis who are symptomatic with refractory abdominal pain, gastric outlet obstruction, or failure to thrive at 4 or more weeks following the onset of acute pancreatitis, drainage and/or debridement is usually indicated. Pancreatic necrosectomy for sterile pancreatic necrosis may be accomplished endoscopically, or more traditionally by a surgical approach.55 Although endoscopic drainage is less invasive, it is technically difficult and has a higher rate of complication in the hands of inexperienced operators.100 Careful selection and evaluation of patients undergoing endoscopic drainage procedures is necessary. Bleeding, perforation, infection, pancreatitis, aspiration, stent migration, and pancreatic ductal damage are all possible complications during the drainage of necrotic pancreatic fluid collections.55 If pancreatic necrosis is infected, surgical necrosectomy should be performed as this is the gold standard for infected necrosis when debridement is necessary.55 Figure 5 reviews the management of acute pancreatitis.

Conclusion
Acute pancreatitis is a common disease frequently caused by choledocholithiasis or excess alcohol ingestion. In idiopathic acute pancreatitis, microlithiasis and SOD should be considered. Though CT scan remains the imaging modality of choice, newer methods such as MRCP and EUS may help to provide additional and improved diagnostic information.
The management of acute pancreatitis is frequently challenging, and severity scales help to predict the likelihood of complications, determine necessary interventions, and guide the appropriate level of care. Nutrition is critical in patients with SAP, and enteral feeding is clearly preferred over TPN. Currently, prophylactic antibiotics do not appear to have a role in SAP. Finally, though not always straightforward, recommendations do exist to guide the management of many of the complications of acute pancreatitis, such as pseudocyst formation and necrotizing disease. A multidisciplinary approach should be used in managing patients with severe disease, and the primary inpatient physician should not hesitate to involve specialists, including gastroenterologists, radiologists, and surgeons.
- ,.2005 National hospital discharge survey.Adv Data.2007;385:1–19.
- ,.Gallstones.BMJ.2007;335:295–299.
- ,.Acute pancreatitis.N Engl J Med.1994;330:1198–1210.
- ,.Pathobiology of alcoholic pancreatitis.Pancreatology.2007;7:105–114.
- .Hyperlipidemic pancreatitis.Gastroenterol Clin North Am.1990;19:783–791.
- ,,,,,.Acute hypercalcemia causes acute pancreatitis and ectopic trypsinogen activation in the rat.Gastroenterology.1995;109:239–246.
- ,,.Role of endoscopic evaluation in idiopathic pancreatitis: a systematic review.Gastrointest Endosc.2006;63:1037–1045.
- ,.Autoimmune pancreatitis: a review.World J Gastroenterol.2007;13:6327–6332.
- ,,, et al.Acute pancreatitis and normoamylasemia. not an uncommon combination.Ann Surg.1989;210:614–620.
- .Normal amylase levels in the presentation of acute pancreatitis.Am J Emerg Med.1994;12:21–24.
- .Diagnostic standards for acute pancreatitis.World J Surg.1997;21:136–142.
- .Support of the diagnosis of pancreatitis by enzyme tests — old problems, new techniques.Clinica Chimica Acta.1997;257:85–98.
- ,,,.What is the best biochemical test to diagnose acute pancreatitis? A prospective clinical study.Mayo Clin Proc.1996;71:1138–1144.
- .The value of clinical laboratory studies in acute pancreatitis.Arch Pathol Lab Med.1991;115:325–326.
- ,.Anionic and cationic trypsinogens (trypsins) in mammalian pancreas.Enzyme.1972/73;14:116–130.
- ,,,,,.Time‐resolved immunofluorometric assays for trypsinogen‐1 and 2 in serum reveal preferential elevation of trypsinogen‐2 in pancreatitis.J Lab Clin Med.1990;115:712–718.
- ,,, et al.Rapid measurement of urinary trypsinogen‐2 as a screening test for acute pancreatitis.N Engl J Med.1997;336:1788–1793.
- ,,,,.Rapid urinary trypsinogen‐2 test strip in the diagnosis of acute pancreatitis.Pancreas.2005;30:243–247.
- ,,, et al.Clinical value of rapid urine trypsinogen‐2 test strip, urinary trypsinogen activation peptide, and serum and urinary activation peptide of carboxypeptidase B in acute pancreatitis.World J Gastroenterol.2005;11:7261–7265.
- ,,,,.Use of the urinary trypsinogen‐2 dip stick test in early diagnosis of pancreatitis after endoscopic retrograde cholangiopancreatography.Surg Endosc.2007;21:1312–1315.
- ,,, et al.Early prediction of severity in acute pancreatitis by urinary trypsinogen activation peptide: a multicentre study.Lancet.2000;355:1955–1960.
- ,.The ultrasonic findings in inflammatory pancreatic disease.Semin Ultrasound.1980;1:178.
- ,,,,,.The urgent diagnosis of gallstones in acute pancreatitis: a prospective study of three methods.Br J Surg.1984;71:230–233.
- ,,.Gallstone pancreatitis: a community teaching hospital experience.J Clin Gastroenterol.2001;33:41–44.
- ,,,.Diagnostic imaging of acute pancreatitis: prospective study using CT and sonography.AJR Am J Roentgenol.1981;137:497–502.
- ,,,.Value of contrast‐enhanced computerized tomography in the early diagnosis and prognosis of acute pancreatitis: a prospective study of 202 patients.Am J Surg.1988;155:457–466.
- ,,, et al.Computed tomography and magnetic resonance imaging in the assessment of acute pancreatitis.Gastroenterology.2004;126:715–723.
- ,,, et al.The role of magnetic resonance cholangiography in the management of patients with gallstone pancreatitis.Ann Surg.2005;241:119–124.
- ,,.Magnetic resonance imaging of acute pancreatitis: the pancreatogram.JOP.2004;5:48–50.
- ,,.Magnetic resonance cholangiopancreatography.N Engl J Med.1999;341(4):258–264.
- ,,,,.Imaging and percutaneous management of acute complicated pancreatitis.Cardiovasc Intervent Radiol.2004;27:567–580.
- ,,.State‐of‐the‐art imaging of acute pancreatitis.JBR‐BTR.2003;86:193–208.
- ,,,.Acute necrotizing pancreatitis: Role of CT‐guided percutaneous catheter drainage.Abdom Imaging.2007;32:351–361.
- .Acute pancreatitis.N Engl J Med.2006;354:2142–2150.
- ,,, et al.Detection of choledocholithiasis by EUS in acute pancreatitis: a prospective evaluation in 100 consecutive patients.Gastrointest Endosc.2001;54:325–330.
- ,.Endoscopic ultrasonography in the evaluation of idiopathic acute pancreatitis.Br J Surg.2000;87:1650–1655.
- ,; theCalgary Advanced Therapeutic Endoscopy Center (ATEC) Study Group.Noninvasive vs. selective invasive biliary imaging for acute biliary pancreatitis: an economic evaluation by using decision tree analysis.Gastrointest Endosc.2005;61:86–97.
- ,.Prognostic factors in acute pancreatitis.J Clin Gastroenterol.2002;34:167–176.
- ,.Severe acute pancreatitis: clinical course and management.World J Gastroenterol.2007;13:5043–5051.
- .Etiological and prognostic factors in human acute pancreatitis: a review.Am J Gastroenterol.1982;77:633–638.
- ,,,,.Prognostic factors in acute pancreatitis.Gut.1984;25:1340–1346.
- ,,,,.APACHE‐acute physiology and chronic health evaluation: a physiologically based classification system.Crit Care Med.1981;9:591–597.
- ,,,.Acute pancreatitis: value of CT in establishing prognosis.Radiology.1990;174:331–336.
- .A clinically based classification system for acute pancreatitis. summary of the international symposium on acute pancreatitis, Atlanta, GA, September 11 through 13, 1992.Arch Surg.1993;128:586–590.
- ,,, et al.Diagnosis, objective assessment of severity, and management of acute pancreatitis. Santorini Consensus Conference.Int J Pancreatol.1999;25:195–210.
- ,,,.Role of early multisystem organ failure as major risk factor for pancreatic infections and death in severe acute pancreatitis.Clin Gastroenterol Hepatol.2006;4:1053–1061.
- ,.Lethal pancreatitis.Am J Gastroenterol.1983;78:810–814.
- ,,,.A randomized multicenter clinical trial of antibiotic prophylaxis of septic complications in acute necrotizing pancreatitis with imipenem.Surg Gynecol Obstet.1993;176:480–483.
- ,.Prevention, diagnosis, and treatment of pancreatic abscess.Surgery.1977;82:99–106.
- ,,,.Death due to acute pancreatitis. a retrospective analysis of 405 autopsy cases.Dig Dis Sci.1985;30:1005–1018.
- .Endoscopic drainage of pancreatic fluid collections and pancreatic necrosis.Gastrointest Endosc Clin North Am.2003;13:743–764.
- ,,, et al.Early prediction of acute pancreatitis: prospective study comparing computed tomography scans, Ranson, Glascow, Acute Physiology and Chronic Health Evaluation II scores, and various serum markers.World J Surg.2002;26:612–619.
- ,,, et al.ASGE guideline: the role of endoscopy in the diagnosis and the management of cystic lesions and inflammatory fluid collections of the pancreas.Gastrointest Endosc.2005;61:363–370.
- .Endoscopic therapy of complete and partial pancreatic duct disruptions.Gastrointest Endosc Clin N Am.1998;8:39–53.
- .Treatment of pancreatic pseudocysts, pancreatic necrosis, and pancreatic duct leaks.Gastrointest Endosc Clin North Am.2007;17:559–579.
- .Pathology of severe acute pancreatitis. In: Bradley EL III, ed.Acute Pancreatitis: Diagnosis and Therapy.New York, NY:Raven Press;1994:35–46.
- ,,,,,.Identification of pancreas necrosis in severe acute pancreatitis: imaging procedures versus clinical staging.Gut.1986;27:1035–1042.
- ,,,,.Results of surgical treatment of necrotizing pancreatitis.World J Surg.1985;9:972–979.
- ,,,,.Management of sterile necrosis in instances of severe acute pancreatitis.J Am Coll Surg.1995;181:279–288.
- ,,,.Prognostic factors in sterile pancreatic necrosis.Gastroenterology.1992;103:1636–1640.
- ,,,.Bacterial contamination of pancreatic necrosis. A prospective clinical study.Gastroenterology.1986;91:433–438.
- ,,.Surgical treatment of necrotizing pancreatitis by necrosectomy and closed lavage: changing patient characteristics and outcome in a 19‐year, single‐center series.Surgery.2005;138:28–39.
- ,,,,,.Characteristics of infection with Candida species in patients with necrotizing pancreatitis.World J Surg.2002;26:372–376.
- ,,,,,.CT‐guided aspiration of suspected pancreatic infection: bacteriology and clinical outcome.Int J Pancreatol.1995;18:265–270.
- .Initial management of acute pancreatitis: critical issues during the first 72 hours.Am J Gastroenterol.2004;99:2489–2494.
- ,,,.Fluid resuscitation in acute pancreatitis.Clin Gastroenterol Hepatol.2008;6:1070–1076.
- ,,, et al.ESPEN guidelines on nutrition in acute pancreatitis.Clin Nutr.2002;21:173–183.
- ,,,,,.A prospective, randomized trial of clear liquids versus low‐fat solid diet as the initial meal in mild acute pancreatitis.Clin Gastroenterol Hepatol.2007;5:946–951.
- ,,,,.Total parenteral nutrition in severe acute pancreatitis.J Am Coll Nutr.1991;10:156–162.
- ,,.Enteral versus parenteral nutrition for acute pancreatitis.Cochrane Database Syst Rev.2003; (1):CD002837.
- ,.Meta‐analysis of parenteral nutrition versus enteral nutrition in patients with acute pancreatitis.BMJ.2004;328:1407.
- ,,.Current management of acute pancreatitis.Nat Clin Pract Gastroenterol Hepatol.2005;2:473–483.
- ,,, et al.Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis.Gut.1998;42:431–435.
- ,,,,.Enteral nutrition is superior to parenteral nutrition in severe acute pancreatitis: Results of a randomized prospective trial.Br J Surg.1997;84:1665–1669.
- ,,.Nutrition in patients with acute pancreatitis.Curr Opin Crit Care.2001;7:251–256.
- ,,,,.Early nasogastric enteral nutrition for severe acute pancreatitis: a systematic review.World J Gastroenterol.2007;13:5253–5260.
- ,,,,.Early enteral nutrition in severe acute pancreatitis: a prospective randomized controlled trial comparing nasojejunal and nasogastric routes.J Clin Gastroenterol.2006;40:431–434.
- ,,, et al.A randomized study of early nasogastric versus nasojejunal feeding in severe acute pancreatitis.Am J Gastroenterol.2005;100:432–439.
- ,,,.Nutrition support in acute pancreatitis: a systematic review of the literature.JPEN J Parenter Enteral Nutr.2006;30:143–156.
- ,.Issues of nutritional support for the patient with acute pancreatitis.Semin Gastrointest Dis.2002;13:154–160.
- ,,,.Effect of continuous jejunal perfusion of elemental and complex nutritional solutions on pancreatic enzyme secretion in human subjects.Gut.1978;19:194–198.
- ,,, et al.Efficiency of enteral nitrogen support in surgical patients: small peptides v non‐degraded proteins.Gut.1990;31:1277–1283.
- ,,, et al.Semi‐elemental formula or polymeric formula: Is there a better choice for enteral nutrition in acute pancreatitis? randomized comparative study.JPEN J Parenter Enteral Nutr.2006;30:1–5.
- ,,, et al.IAP guidelines for the surgical management of acute pancreatitis.Pancreatology.2002;2:565–573.
- ,,, et al.Randomized controlled trials of antibiotic prophylaxis in severe acute pancreatitis: relationship between methodological quality and outcome.Pancreatology.2007;7:531–538.
- ,,,.Prophylactic antibiotics cannot reduce infected pancreatic necrosis and mortality in acute necrotizing pancreatitis: evidence from a meta‐analysis of randomized controlled trials.Am J Gastroenterol.2008;103:104–110.
- ,.Prophylactic antibiotic administration reduces sepsis and mortality in acute necrotizing pancreatitis: a meta‐analysis.Pancreas.2001;22:28–31.
- ,,Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis.Am J Gastroenterol.2006;101:2379–2400.
- ,.Acute biliary pancreatitis.Ann Ital Chir.1995;66:197–202.
- .The timing of biliary surgery in acute pancreatitis.Ann Surg.1979;189:654–663.
- ,,,.Acute biliary pancreatitis. The roles of laparoscopic cholecystectomy and endoscopic retrograde cholangiopancreatography.Surg Endosc.1995;9:392–396.
- ,.Metaanalysis of randomized controlled trials of endoscopic retrograde cholangiography and endoscopic sphincterotomy for the treatment of acute biliary pancreatitis.Am J Gastroenterol.1999;94:3211–3214.
- ,.Endoscopic management of acute pancreatitis.Gastrointest Endosc Clin N Am.2007;17:307–322.
- ,.Review of idiopathic pancreatitis.World J Gastroenterol.2007;13:6296–6313.
- ,,,,.Does endoscopic therapy favorably affect the outcome of patients who have recurrent acute pancreatitis and pancreas divisum?Pancreas.2007;34:21–45.
- .The timing of cholecystectomy in patients with gallstone pancreatitis. A retrospective analysis of 89 patients.Acta Chir Scand.1978;144:487–490.
- ,,, et al.Recurrence of acute gallstone pancreatitis and relationship with cholecystectomy or endoscopic sphincterotomy.Am J Gastroenterol.2004;99:2417–2423.
- American Gastroenterological Association (AGA) Institute on “Management of Acute Pancreatitis” Clinical Practice and Economics Committee, AGA Institute Governing Board.AGA institute medical position statement on acute pancreatitis.Gastroenterology.2007;132:2019–2021.
- ,;AGA Institute Clinical Practice and Economics Committee, AGA Institute Governing Board. AGA institute technical review on acute pancreatitis.Gastroenterology.2007;132:2022–2044.
- .Endoscopic management of pancreatic necrosis: not for the uncommitted.Gastrointest Endosc.2005;62:101–104.
- ,,,.Serum amylase and lipase in the evaluation of acute abdominal pain.Am Surg.1996;62:1028–1033.
- ,.2005 National hospital discharge survey.Adv Data.2007;385:1–19.
- ,.Gallstones.BMJ.2007;335:295–299.
- ,.Acute pancreatitis.N Engl J Med.1994;330:1198–1210.
- ,.Pathobiology of alcoholic pancreatitis.Pancreatology.2007;7:105–114.
- .Hyperlipidemic pancreatitis.Gastroenterol Clin North Am.1990;19:783–791.
- ,,,,,.Acute hypercalcemia causes acute pancreatitis and ectopic trypsinogen activation in the rat.Gastroenterology.1995;109:239–246.
- ,,.Role of endoscopic evaluation in idiopathic pancreatitis: a systematic review.Gastrointest Endosc.2006;63:1037–1045.
- ,.Autoimmune pancreatitis: a review.World J Gastroenterol.2007;13:6327–6332.
- ,,, et al.Acute pancreatitis and normoamylasemia. not an uncommon combination.Ann Surg.1989;210:614–620.
- .Normal amylase levels in the presentation of acute pancreatitis.Am J Emerg Med.1994;12:21–24.
- .Diagnostic standards for acute pancreatitis.World J Surg.1997;21:136–142.
- .Support of the diagnosis of pancreatitis by enzyme tests — old problems, new techniques.Clinica Chimica Acta.1997;257:85–98.
- ,,,.What is the best biochemical test to diagnose acute pancreatitis? A prospective clinical study.Mayo Clin Proc.1996;71:1138–1144.
- .The value of clinical laboratory studies in acute pancreatitis.Arch Pathol Lab Med.1991;115:325–326.
- ,.Anionic and cationic trypsinogens (trypsins) in mammalian pancreas.Enzyme.1972/73;14:116–130.
- ,,,,,.Time‐resolved immunofluorometric assays for trypsinogen‐1 and 2 in serum reveal preferential elevation of trypsinogen‐2 in pancreatitis.J Lab Clin Med.1990;115:712–718.
- ,,, et al.Rapid measurement of urinary trypsinogen‐2 as a screening test for acute pancreatitis.N Engl J Med.1997;336:1788–1793.
- ,,,,.Rapid urinary trypsinogen‐2 test strip in the diagnosis of acute pancreatitis.Pancreas.2005;30:243–247.
- ,,, et al.Clinical value of rapid urine trypsinogen‐2 test strip, urinary trypsinogen activation peptide, and serum and urinary activation peptide of carboxypeptidase B in acute pancreatitis.World J Gastroenterol.2005;11:7261–7265.
- ,,,,.Use of the urinary trypsinogen‐2 dip stick test in early diagnosis of pancreatitis after endoscopic retrograde cholangiopancreatography.Surg Endosc.2007;21:1312–1315.
- ,,, et al.Early prediction of severity in acute pancreatitis by urinary trypsinogen activation peptide: a multicentre study.Lancet.2000;355:1955–1960.
- ,.The ultrasonic findings in inflammatory pancreatic disease.Semin Ultrasound.1980;1:178.
- ,,,,,.The urgent diagnosis of gallstones in acute pancreatitis: a prospective study of three methods.Br J Surg.1984;71:230–233.
- ,,.Gallstone pancreatitis: a community teaching hospital experience.J Clin Gastroenterol.2001;33:41–44.
- ,,,.Diagnostic imaging of acute pancreatitis: prospective study using CT and sonography.AJR Am J Roentgenol.1981;137:497–502.
- ,,,.Value of contrast‐enhanced computerized tomography in the early diagnosis and prognosis of acute pancreatitis: a prospective study of 202 patients.Am J Surg.1988;155:457–466.
- ,,, et al.Computed tomography and magnetic resonance imaging in the assessment of acute pancreatitis.Gastroenterology.2004;126:715–723.
- ,,, et al.The role of magnetic resonance cholangiography in the management of patients with gallstone pancreatitis.Ann Surg.2005;241:119–124.
- ,,.Magnetic resonance imaging of acute pancreatitis: the pancreatogram.JOP.2004;5:48–50.
- ,,.Magnetic resonance cholangiopancreatography.N Engl J Med.1999;341(4):258–264.
- ,,,,.Imaging and percutaneous management of acute complicated pancreatitis.Cardiovasc Intervent Radiol.2004;27:567–580.
- ,,.State‐of‐the‐art imaging of acute pancreatitis.JBR‐BTR.2003;86:193–208.
- ,,,.Acute necrotizing pancreatitis: Role of CT‐guided percutaneous catheter drainage.Abdom Imaging.2007;32:351–361.
- .Acute pancreatitis.N Engl J Med.2006;354:2142–2150.
- ,,, et al.Detection of choledocholithiasis by EUS in acute pancreatitis: a prospective evaluation in 100 consecutive patients.Gastrointest Endosc.2001;54:325–330.
- ,.Endoscopic ultrasonography in the evaluation of idiopathic acute pancreatitis.Br J Surg.2000;87:1650–1655.
- ,; theCalgary Advanced Therapeutic Endoscopy Center (ATEC) Study Group.Noninvasive vs. selective invasive biliary imaging for acute biliary pancreatitis: an economic evaluation by using decision tree analysis.Gastrointest Endosc.2005;61:86–97.
- ,.Prognostic factors in acute pancreatitis.J Clin Gastroenterol.2002;34:167–176.
- ,.Severe acute pancreatitis: clinical course and management.World J Gastroenterol.2007;13:5043–5051.
- .Etiological and prognostic factors in human acute pancreatitis: a review.Am J Gastroenterol.1982;77:633–638.
- ,,,,.Prognostic factors in acute pancreatitis.Gut.1984;25:1340–1346.
- ,,,,.APACHE‐acute physiology and chronic health evaluation: a physiologically based classification system.Crit Care Med.1981;9:591–597.
- ,,,.Acute pancreatitis: value of CT in establishing prognosis.Radiology.1990;174:331–336.
- .A clinically based classification system for acute pancreatitis. summary of the international symposium on acute pancreatitis, Atlanta, GA, September 11 through 13, 1992.Arch Surg.1993;128:586–590.
- ,,, et al.Diagnosis, objective assessment of severity, and management of acute pancreatitis. Santorini Consensus Conference.Int J Pancreatol.1999;25:195–210.
- ,,,.Role of early multisystem organ failure as major risk factor for pancreatic infections and death in severe acute pancreatitis.Clin Gastroenterol Hepatol.2006;4:1053–1061.
- ,.Lethal pancreatitis.Am J Gastroenterol.1983;78:810–814.
- ,,,.A randomized multicenter clinical trial of antibiotic prophylaxis of septic complications in acute necrotizing pancreatitis with imipenem.Surg Gynecol Obstet.1993;176:480–483.
- ,.Prevention, diagnosis, and treatment of pancreatic abscess.Surgery.1977;82:99–106.
- ,,,.Death due to acute pancreatitis. a retrospective analysis of 405 autopsy cases.Dig Dis Sci.1985;30:1005–1018.
- .Endoscopic drainage of pancreatic fluid collections and pancreatic necrosis.Gastrointest Endosc Clin North Am.2003;13:743–764.
- ,,, et al.Early prediction of acute pancreatitis: prospective study comparing computed tomography scans, Ranson, Glascow, Acute Physiology and Chronic Health Evaluation II scores, and various serum markers.World J Surg.2002;26:612–619.
- ,,, et al.ASGE guideline: the role of endoscopy in the diagnosis and the management of cystic lesions and inflammatory fluid collections of the pancreas.Gastrointest Endosc.2005;61:363–370.
- .Endoscopic therapy of complete and partial pancreatic duct disruptions.Gastrointest Endosc Clin N Am.1998;8:39–53.
- .Treatment of pancreatic pseudocysts, pancreatic necrosis, and pancreatic duct leaks.Gastrointest Endosc Clin North Am.2007;17:559–579.
- .Pathology of severe acute pancreatitis. In: Bradley EL III, ed.Acute Pancreatitis: Diagnosis and Therapy.New York, NY:Raven Press;1994:35–46.
- ,,,,,.Identification of pancreas necrosis in severe acute pancreatitis: imaging procedures versus clinical staging.Gut.1986;27:1035–1042.
- ,,,,.Results of surgical treatment of necrotizing pancreatitis.World J Surg.1985;9:972–979.
- ,,,,.Management of sterile necrosis in instances of severe acute pancreatitis.J Am Coll Surg.1995;181:279–288.
- ,,,.Prognostic factors in sterile pancreatic necrosis.Gastroenterology.1992;103:1636–1640.
- ,,,.Bacterial contamination of pancreatic necrosis. A prospective clinical study.Gastroenterology.1986;91:433–438.
- ,,.Surgical treatment of necrotizing pancreatitis by necrosectomy and closed lavage: changing patient characteristics and outcome in a 19‐year, single‐center series.Surgery.2005;138:28–39.
- ,,,,,.Characteristics of infection with Candida species in patients with necrotizing pancreatitis.World J Surg.2002;26:372–376.
- ,,,,,.CT‐guided aspiration of suspected pancreatic infection: bacteriology and clinical outcome.Int J Pancreatol.1995;18:265–270.
- .Initial management of acute pancreatitis: critical issues during the first 72 hours.Am J Gastroenterol.2004;99:2489–2494.
- ,,,.Fluid resuscitation in acute pancreatitis.Clin Gastroenterol Hepatol.2008;6:1070–1076.
- ,,, et al.ESPEN guidelines on nutrition in acute pancreatitis.Clin Nutr.2002;21:173–183.
- ,,,,,.A prospective, randomized trial of clear liquids versus low‐fat solid diet as the initial meal in mild acute pancreatitis.Clin Gastroenterol Hepatol.2007;5:946–951.
- ,,,,.Total parenteral nutrition in severe acute pancreatitis.J Am Coll Nutr.1991;10:156–162.
- ,,.Enteral versus parenteral nutrition for acute pancreatitis.Cochrane Database Syst Rev.2003; (1):CD002837.
- ,.Meta‐analysis of parenteral nutrition versus enteral nutrition in patients with acute pancreatitis.BMJ.2004;328:1407.
- ,,.Current management of acute pancreatitis.Nat Clin Pract Gastroenterol Hepatol.2005;2:473–483.
- ,,, et al.Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis.Gut.1998;42:431–435.
- ,,,,.Enteral nutrition is superior to parenteral nutrition in severe acute pancreatitis: Results of a randomized prospective trial.Br J Surg.1997;84:1665–1669.
- ,,.Nutrition in patients with acute pancreatitis.Curr Opin Crit Care.2001;7:251–256.
- ,,,,.Early nasogastric enteral nutrition for severe acute pancreatitis: a systematic review.World J Gastroenterol.2007;13:5253–5260.
- ,,,,.Early enteral nutrition in severe acute pancreatitis: a prospective randomized controlled trial comparing nasojejunal and nasogastric routes.J Clin Gastroenterol.2006;40:431–434.
- ,,, et al.A randomized study of early nasogastric versus nasojejunal feeding in severe acute pancreatitis.Am J Gastroenterol.2005;100:432–439.
- ,,,.Nutrition support in acute pancreatitis: a systematic review of the literature.JPEN J Parenter Enteral Nutr.2006;30:143–156.
- ,.Issues of nutritional support for the patient with acute pancreatitis.Semin Gastrointest Dis.2002;13:154–160.
- ,,,.Effect of continuous jejunal perfusion of elemental and complex nutritional solutions on pancreatic enzyme secretion in human subjects.Gut.1978;19:194–198.
- ,,, et al.Efficiency of enteral nitrogen support in surgical patients: small peptides v non‐degraded proteins.Gut.1990;31:1277–1283.
- ,,, et al.Semi‐elemental formula or polymeric formula: Is there a better choice for enteral nutrition in acute pancreatitis? randomized comparative study.JPEN J Parenter Enteral Nutr.2006;30:1–5.
- ,,, et al.IAP guidelines for the surgical management of acute pancreatitis.Pancreatology.2002;2:565–573.
- ,,, et al.Randomized controlled trials of antibiotic prophylaxis in severe acute pancreatitis: relationship between methodological quality and outcome.Pancreatology.2007;7:531–538.
- ,,,.Prophylactic antibiotics cannot reduce infected pancreatic necrosis and mortality in acute necrotizing pancreatitis: evidence from a meta‐analysis of randomized controlled trials.Am J Gastroenterol.2008;103:104–110.
- ,.Prophylactic antibiotic administration reduces sepsis and mortality in acute necrotizing pancreatitis: a meta‐analysis.Pancreas.2001;22:28–31.
- ,,Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis.Am J Gastroenterol.2006;101:2379–2400.
- ,.Acute biliary pancreatitis.Ann Ital Chir.1995;66:197–202.
- .The timing of biliary surgery in acute pancreatitis.Ann Surg.1979;189:654–663.
- ,,,.Acute biliary pancreatitis. The roles of laparoscopic cholecystectomy and endoscopic retrograde cholangiopancreatography.Surg Endosc.1995;9:392–396.
- ,.Metaanalysis of randomized controlled trials of endoscopic retrograde cholangiography and endoscopic sphincterotomy for the treatment of acute biliary pancreatitis.Am J Gastroenterol.1999;94:3211–3214.
- ,.Endoscopic management of acute pancreatitis.Gastrointest Endosc Clin N Am.2007;17:307–322.
- ,.Review of idiopathic pancreatitis.World J Gastroenterol.2007;13:6296–6313.
- ,,,,.Does endoscopic therapy favorably affect the outcome of patients who have recurrent acute pancreatitis and pancreas divisum?Pancreas.2007;34:21–45.
- .The timing of cholecystectomy in patients with gallstone pancreatitis. A retrospective analysis of 89 patients.Acta Chir Scand.1978;144:487–490.
- ,,, et al.Recurrence of acute gallstone pancreatitis and relationship with cholecystectomy or endoscopic sphincterotomy.Am J Gastroenterol.2004;99:2417–2423.
- American Gastroenterological Association (AGA) Institute on “Management of Acute Pancreatitis” Clinical Practice and Economics Committee, AGA Institute Governing Board.AGA institute medical position statement on acute pancreatitis.Gastroenterology.2007;132:2019–2021.
- ,;AGA Institute Clinical Practice and Economics Committee, AGA Institute Governing Board. AGA institute technical review on acute pancreatitis.Gastroenterology.2007;132:2022–2044.
- .Endoscopic management of pancreatic necrosis: not for the uncommitted.Gastrointest Endosc.2005;62:101–104.
- ,,,.Serum amylase and lipase in the evaluation of acute abdominal pain.Am Surg.1996;62:1028–1033.
Continuing Medical Education Program in
If you wish to receive credit for this activity, which begins on the next page, please refer to the website: www. blackwellpublishing.com/cme.
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to www.blackwellpublishing.com/cme.
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which begins on the next page, please refer to the website: www. blackwellpublishing.com/cme.
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to www.blackwellpublishing.com/cme.
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which begins on the next page, please refer to the website: www. blackwellpublishing.com/cme.
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to www.blackwellpublishing.com/cme.
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.