User login
Delivery of Care: The Ethical Imperative in Healthcare
The ethical imperative in healthcare necessitates equitable delivery of care to all individuals, regardless of their socio-economic status or insurance coverage. This principle is rooted in the concept of justice and is crucial to achieving health equity.
As gastroenterologists, despite our various practice settings, we have seen the harmful effects of economic and social disparities on health outcomes. We must therefore ensure that we acknowledge the existence of these disparities, and then begin to provide a framework that allows us to ethically and successfully navigate these complexities for our patients and our affiliated structures.
The following cases illustrate the complexities and ethical dilemmas that gastroenterology and hepatology healthcare professionals encounter in delivering care within the traditional healthcare system.
- Case 1: A 44-year-old male presents to the hospital with intermittent rectal bleeding every few weeks without associated abdominal pain or weight loss and not associated with straining. He has bowel movements every 2-3 days. There is no family history of underlying gastrointestinal disease or associated neoplasm. He is accompanied at the time of the interview by his coworker who offered to drive him to the hospital as he is having personal car trouble. Physical examination reveals normal hemodynamics, abdomen is benign, a digital rectal exam reveals small internal hemorrhoids without pain. Hemoglobin is 10, MCV 85. There is scant blood on the glove. He is uninsured. A GI consult is placed to determine the disposition of the patient. The resident on service suggests outpatient follow-up given low risk of clinical deterioration.
- Case 2: A 28-year-old woman postpartum 6 weeks presents in the office with a history of ulcerative colitis which was diagnosed 2 years prior. She was initially placed on steroid therapy. She underwent a colonoscopy at the time of her diagnosis and was following with a gastroenterologist at which time she was found to have moderate left-sided disease with a modified Mayo score of 9. She complains of urgency and rectal bleeding. She saw a gastroenterologist during her pregnancy and was placed on oral mesalamine, which she remains on at the time of evaluation. Once her physical examination is completed and laboratory values are reviewed, you begin to discuss advanced therapies including biologics as she has failed conventional therapies.
- Case 3: You receive a phone call from an outside hospital about a potential transfer for a 46-year-old male who is an immigrant of unknown citizenship status with fulminant liver failure. He meets all criteria including encephalopathy and coagulopathy. He drinks only socially. His secondary liver workup for extensive disease including ceruloplasmin remains pending. Viral hepatology serologies and autoimmune serologies are negative.
Challenges to the Delivery of Equitable Care
These cases underscore the challenges of delivering equitable care within a system that often fails to address the social determinants of health (SDOH). The disparity in the evaluation and treatment of patients based on insurance status not only affects patient outcomes, but also emphasizes the ethical dilemma of balancing cost with population health management.
The introduction of measures SDOH-1 and SDOH-2 by the Centers for Medicare & Medicaid Services in the 2023 IPPS Final Rule is a step towards requiring hospitals to systematically collect patient-level SDOH data, aiming to establish meaningful collaborations between healthcare providers and community-based organizations for whole-person care.1 The primary goal is to allow ecosystems to collect patient-level social risk factors followed by the creation of meaningful collaboration between healthcare providers and the community-based organizations.
The office settings may or may not implement the SDOH and the current electronic medical record systems. However, from a social history standpoint and certainly from a decision standpoint, the impact of SDOH is realized in all settings.
Interplay of SDOH and Ethical Considerations
The recognition of social determinants of health is crucial for ethical healthcare delivery. In the first case, considering the patient’s identified social determinants of health — including lack of insurance and transportation, combined with the rising incidence of colorectal cancer in individuals under 55 — an argument could be made for admitting the patient under observation for inpatient colonoscopy.
Data have shown disparities in treatment and referrals in emergency care setting for Black patients with rectal bleeding.2 It is imperative that we recognize these existing disparities in diagnosis and outcomes, along with determining SDOH to appropriately come to a final disposition. This approach aligns with the principle of justice and the imperative to deliver equitable care.
In the third case study, we have a patient facing the life-or-death situation of fulminant liver failure. He requires an expeditious decision to be made about transfer candidacy for liver transplant evaluation by the hepatology team.
Impact of Insurance Status on Healthcare Access
Insurance status significantly influences access to healthcare and disparities in treatment outcomes. As seen in case 2 and case 3, our therapies often hinge upon access.
In the inflammatory bowel disease (IBD) case, the therapy that we will choose for our IBD patient may be more influenced by access than efficacy. In a national sample of children with Crohn’s disease, publicly insured children were more likely to receive a biologic within 18 months of diagnosis compared to children with private insurance.3 This would suggest that those with private insurance perhaps experience increased barriers.
In the IBD case that we presented here, we do have a publicly insured woman who will face a potential loss of her Medicaid coverage. Our therapeutic decision will therefore not just rely on risk stratification and individualized approach, but rather the programs that are put in place by our pharmaceutical partners to support a future self-pay patient. This may or may not be favorable to her outcome. This discrepancy points to systemic inequalities in healthcare access and the need for policies that ensure equitable treatment for all, regardless of insurance status.
Conclusion
The delivery of care in healthcare is an ethical imperative that demands equity and justice. The cases discussed above illustrate the complex interplay between socioeconomic factors, insurance status, and the ethical challenges in providing equitable care.
Systematic efforts to address social determinants of health, as mandated by recent CMS measures, along with a commitment to ethical principles, are essential steps toward reducing disparities and ensuring that all individuals receive the care they need. As healthcare expenditures continue to rise, particularly in areas like gastrointestinal health, addressing these ethical and systemic challenges becomes even more critical for the sustainability of the healthcare system and the well-being of the population it serves.
Gastrointestinal healthcare expenditures totaled $119.6 billion in 2018. Annually there were more than 36.8 million ambulatory visits for GI symptoms and 43.4 million ambulatory visits with primary GI diagnosis.4 The use of higher-acuity settings and lack of continuity of care, and the under-recognition and lack of longitudinal framework to follow those families at risk continue to compromise our healthcare system.
Dr. McCutchen is a gastroenterologist at United Digestive, Atlanta, Georgia. She is vice chair of the AGA Research Foundation. Dr. Boules is vice president of global medical and scientific affairs at Ironwood Pharmaceuticals, Cleveland, Ohio.
References
1. www.govinfo.gov/content/pkg/FR-2022-08-10/pdf/2022-16472.pdf.
2. Shields HM et al. Disparities in evaluation of patients with rectal bleeding 40 years and older. Clin Gastroenterol Hepatol. 2014 Apr. doi: 10.1016/j.cgh.2013.07.008.
3. Quiros JA et al. Insurance type influences access to biologics and healthcare utilization in pediatric Crohn’s disease. Crohns Colitis 360. 2021 Aug. doi: 10.1093/crocol/otab057.
4. Peery AF et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: Update 2021. Gastroenterology. 2022 Feb. doi: 10.1053/j.gastro.2021.10.017.
The ethical imperative in healthcare necessitates equitable delivery of care to all individuals, regardless of their socio-economic status or insurance coverage. This principle is rooted in the concept of justice and is crucial to achieving health equity.
As gastroenterologists, despite our various practice settings, we have seen the harmful effects of economic and social disparities on health outcomes. We must therefore ensure that we acknowledge the existence of these disparities, and then begin to provide a framework that allows us to ethically and successfully navigate these complexities for our patients and our affiliated structures.
The following cases illustrate the complexities and ethical dilemmas that gastroenterology and hepatology healthcare professionals encounter in delivering care within the traditional healthcare system.
- Case 1: A 44-year-old male presents to the hospital with intermittent rectal bleeding every few weeks without associated abdominal pain or weight loss and not associated with straining. He has bowel movements every 2-3 days. There is no family history of underlying gastrointestinal disease or associated neoplasm. He is accompanied at the time of the interview by his coworker who offered to drive him to the hospital as he is having personal car trouble. Physical examination reveals normal hemodynamics, abdomen is benign, a digital rectal exam reveals small internal hemorrhoids without pain. Hemoglobin is 10, MCV 85. There is scant blood on the glove. He is uninsured. A GI consult is placed to determine the disposition of the patient. The resident on service suggests outpatient follow-up given low risk of clinical deterioration.
- Case 2: A 28-year-old woman postpartum 6 weeks presents in the office with a history of ulcerative colitis which was diagnosed 2 years prior. She was initially placed on steroid therapy. She underwent a colonoscopy at the time of her diagnosis and was following with a gastroenterologist at which time she was found to have moderate left-sided disease with a modified Mayo score of 9. She complains of urgency and rectal bleeding. She saw a gastroenterologist during her pregnancy and was placed on oral mesalamine, which she remains on at the time of evaluation. Once her physical examination is completed and laboratory values are reviewed, you begin to discuss advanced therapies including biologics as she has failed conventional therapies.
- Case 3: You receive a phone call from an outside hospital about a potential transfer for a 46-year-old male who is an immigrant of unknown citizenship status with fulminant liver failure. He meets all criteria including encephalopathy and coagulopathy. He drinks only socially. His secondary liver workup for extensive disease including ceruloplasmin remains pending. Viral hepatology serologies and autoimmune serologies are negative.
Challenges to the Delivery of Equitable Care
These cases underscore the challenges of delivering equitable care within a system that often fails to address the social determinants of health (SDOH). The disparity in the evaluation and treatment of patients based on insurance status not only affects patient outcomes, but also emphasizes the ethical dilemma of balancing cost with population health management.
The introduction of measures SDOH-1 and SDOH-2 by the Centers for Medicare & Medicaid Services in the 2023 IPPS Final Rule is a step towards requiring hospitals to systematically collect patient-level SDOH data, aiming to establish meaningful collaborations between healthcare providers and community-based organizations for whole-person care.1 The primary goal is to allow ecosystems to collect patient-level social risk factors followed by the creation of meaningful collaboration between healthcare providers and the community-based organizations.
The office settings may or may not implement the SDOH and the current electronic medical record systems. However, from a social history standpoint and certainly from a decision standpoint, the impact of SDOH is realized in all settings.
Interplay of SDOH and Ethical Considerations
The recognition of social determinants of health is crucial for ethical healthcare delivery. In the first case, considering the patient’s identified social determinants of health — including lack of insurance and transportation, combined with the rising incidence of colorectal cancer in individuals under 55 — an argument could be made for admitting the patient under observation for inpatient colonoscopy.
Data have shown disparities in treatment and referrals in emergency care setting for Black patients with rectal bleeding.2 It is imperative that we recognize these existing disparities in diagnosis and outcomes, along with determining SDOH to appropriately come to a final disposition. This approach aligns with the principle of justice and the imperative to deliver equitable care.
In the third case study, we have a patient facing the life-or-death situation of fulminant liver failure. He requires an expeditious decision to be made about transfer candidacy for liver transplant evaluation by the hepatology team.
Impact of Insurance Status on Healthcare Access
Insurance status significantly influences access to healthcare and disparities in treatment outcomes. As seen in case 2 and case 3, our therapies often hinge upon access.
In the inflammatory bowel disease (IBD) case, the therapy that we will choose for our IBD patient may be more influenced by access than efficacy. In a national sample of children with Crohn’s disease, publicly insured children were more likely to receive a biologic within 18 months of diagnosis compared to children with private insurance.3 This would suggest that those with private insurance perhaps experience increased barriers.
In the IBD case that we presented here, we do have a publicly insured woman who will face a potential loss of her Medicaid coverage. Our therapeutic decision will therefore not just rely on risk stratification and individualized approach, but rather the programs that are put in place by our pharmaceutical partners to support a future self-pay patient. This may or may not be favorable to her outcome. This discrepancy points to systemic inequalities in healthcare access and the need for policies that ensure equitable treatment for all, regardless of insurance status.
Conclusion
The delivery of care in healthcare is an ethical imperative that demands equity and justice. The cases discussed above illustrate the complex interplay between socioeconomic factors, insurance status, and the ethical challenges in providing equitable care.
Systematic efforts to address social determinants of health, as mandated by recent CMS measures, along with a commitment to ethical principles, are essential steps toward reducing disparities and ensuring that all individuals receive the care they need. As healthcare expenditures continue to rise, particularly in areas like gastrointestinal health, addressing these ethical and systemic challenges becomes even more critical for the sustainability of the healthcare system and the well-being of the population it serves.
Gastrointestinal healthcare expenditures totaled $119.6 billion in 2018. Annually there were more than 36.8 million ambulatory visits for GI symptoms and 43.4 million ambulatory visits with primary GI diagnosis.4 The use of higher-acuity settings and lack of continuity of care, and the under-recognition and lack of longitudinal framework to follow those families at risk continue to compromise our healthcare system.
Dr. McCutchen is a gastroenterologist at United Digestive, Atlanta, Georgia. She is vice chair of the AGA Research Foundation. Dr. Boules is vice president of global medical and scientific affairs at Ironwood Pharmaceuticals, Cleveland, Ohio.
References
1. www.govinfo.gov/content/pkg/FR-2022-08-10/pdf/2022-16472.pdf.
2. Shields HM et al. Disparities in evaluation of patients with rectal bleeding 40 years and older. Clin Gastroenterol Hepatol. 2014 Apr. doi: 10.1016/j.cgh.2013.07.008.
3. Quiros JA et al. Insurance type influences access to biologics and healthcare utilization in pediatric Crohn’s disease. Crohns Colitis 360. 2021 Aug. doi: 10.1093/crocol/otab057.
4. Peery AF et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: Update 2021. Gastroenterology. 2022 Feb. doi: 10.1053/j.gastro.2021.10.017.
The ethical imperative in healthcare necessitates equitable delivery of care to all individuals, regardless of their socio-economic status or insurance coverage. This principle is rooted in the concept of justice and is crucial to achieving health equity.
As gastroenterologists, despite our various practice settings, we have seen the harmful effects of economic and social disparities on health outcomes. We must therefore ensure that we acknowledge the existence of these disparities, and then begin to provide a framework that allows us to ethically and successfully navigate these complexities for our patients and our affiliated structures.
The following cases illustrate the complexities and ethical dilemmas that gastroenterology and hepatology healthcare professionals encounter in delivering care within the traditional healthcare system.
- Case 1: A 44-year-old male presents to the hospital with intermittent rectal bleeding every few weeks without associated abdominal pain or weight loss and not associated with straining. He has bowel movements every 2-3 days. There is no family history of underlying gastrointestinal disease or associated neoplasm. He is accompanied at the time of the interview by his coworker who offered to drive him to the hospital as he is having personal car trouble. Physical examination reveals normal hemodynamics, abdomen is benign, a digital rectal exam reveals small internal hemorrhoids without pain. Hemoglobin is 10, MCV 85. There is scant blood on the glove. He is uninsured. A GI consult is placed to determine the disposition of the patient. The resident on service suggests outpatient follow-up given low risk of clinical deterioration.
- Case 2: A 28-year-old woman postpartum 6 weeks presents in the office with a history of ulcerative colitis which was diagnosed 2 years prior. She was initially placed on steroid therapy. She underwent a colonoscopy at the time of her diagnosis and was following with a gastroenterologist at which time she was found to have moderate left-sided disease with a modified Mayo score of 9. She complains of urgency and rectal bleeding. She saw a gastroenterologist during her pregnancy and was placed on oral mesalamine, which she remains on at the time of evaluation. Once her physical examination is completed and laboratory values are reviewed, you begin to discuss advanced therapies including biologics as she has failed conventional therapies.
- Case 3: You receive a phone call from an outside hospital about a potential transfer for a 46-year-old male who is an immigrant of unknown citizenship status with fulminant liver failure. He meets all criteria including encephalopathy and coagulopathy. He drinks only socially. His secondary liver workup for extensive disease including ceruloplasmin remains pending. Viral hepatology serologies and autoimmune serologies are negative.
Challenges to the Delivery of Equitable Care
These cases underscore the challenges of delivering equitable care within a system that often fails to address the social determinants of health (SDOH). The disparity in the evaluation and treatment of patients based on insurance status not only affects patient outcomes, but also emphasizes the ethical dilemma of balancing cost with population health management.
The introduction of measures SDOH-1 and SDOH-2 by the Centers for Medicare & Medicaid Services in the 2023 IPPS Final Rule is a step towards requiring hospitals to systematically collect patient-level SDOH data, aiming to establish meaningful collaborations between healthcare providers and community-based organizations for whole-person care.1 The primary goal is to allow ecosystems to collect patient-level social risk factors followed by the creation of meaningful collaboration between healthcare providers and the community-based organizations.
The office settings may or may not implement the SDOH and the current electronic medical record systems. However, from a social history standpoint and certainly from a decision standpoint, the impact of SDOH is realized in all settings.
Interplay of SDOH and Ethical Considerations
The recognition of social determinants of health is crucial for ethical healthcare delivery. In the first case, considering the patient’s identified social determinants of health — including lack of insurance and transportation, combined with the rising incidence of colorectal cancer in individuals under 55 — an argument could be made for admitting the patient under observation for inpatient colonoscopy.
Data have shown disparities in treatment and referrals in emergency care setting for Black patients with rectal bleeding.2 It is imperative that we recognize these existing disparities in diagnosis and outcomes, along with determining SDOH to appropriately come to a final disposition. This approach aligns with the principle of justice and the imperative to deliver equitable care.
In the third case study, we have a patient facing the life-or-death situation of fulminant liver failure. He requires an expeditious decision to be made about transfer candidacy for liver transplant evaluation by the hepatology team.
Impact of Insurance Status on Healthcare Access
Insurance status significantly influences access to healthcare and disparities in treatment outcomes. As seen in case 2 and case 3, our therapies often hinge upon access.
In the inflammatory bowel disease (IBD) case, the therapy that we will choose for our IBD patient may be more influenced by access than efficacy. In a national sample of children with Crohn’s disease, publicly insured children were more likely to receive a biologic within 18 months of diagnosis compared to children with private insurance.3 This would suggest that those with private insurance perhaps experience increased barriers.
In the IBD case that we presented here, we do have a publicly insured woman who will face a potential loss of her Medicaid coverage. Our therapeutic decision will therefore not just rely on risk stratification and individualized approach, but rather the programs that are put in place by our pharmaceutical partners to support a future self-pay patient. This may or may not be favorable to her outcome. This discrepancy points to systemic inequalities in healthcare access and the need for policies that ensure equitable treatment for all, regardless of insurance status.
Conclusion
The delivery of care in healthcare is an ethical imperative that demands equity and justice. The cases discussed above illustrate the complex interplay between socioeconomic factors, insurance status, and the ethical challenges in providing equitable care.
Systematic efforts to address social determinants of health, as mandated by recent CMS measures, along with a commitment to ethical principles, are essential steps toward reducing disparities and ensuring that all individuals receive the care they need. As healthcare expenditures continue to rise, particularly in areas like gastrointestinal health, addressing these ethical and systemic challenges becomes even more critical for the sustainability of the healthcare system and the well-being of the population it serves.
Gastrointestinal healthcare expenditures totaled $119.6 billion in 2018. Annually there were more than 36.8 million ambulatory visits for GI symptoms and 43.4 million ambulatory visits with primary GI diagnosis.4 The use of higher-acuity settings and lack of continuity of care, and the under-recognition and lack of longitudinal framework to follow those families at risk continue to compromise our healthcare system.
Dr. McCutchen is a gastroenterologist at United Digestive, Atlanta, Georgia. She is vice chair of the AGA Research Foundation. Dr. Boules is vice president of global medical and scientific affairs at Ironwood Pharmaceuticals, Cleveland, Ohio.
References
1. www.govinfo.gov/content/pkg/FR-2022-08-10/pdf/2022-16472.pdf.
2. Shields HM et al. Disparities in evaluation of patients with rectal bleeding 40 years and older. Clin Gastroenterol Hepatol. 2014 Apr. doi: 10.1016/j.cgh.2013.07.008.
3. Quiros JA et al. Insurance type influences access to biologics and healthcare utilization in pediatric Crohn’s disease. Crohns Colitis 360. 2021 Aug. doi: 10.1093/crocol/otab057.
4. Peery AF et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: Update 2021. Gastroenterology. 2022 Feb. doi: 10.1053/j.gastro.2021.10.017.
Not all abdominal pain is gastrointestinal
A 31-year-old woman presents to the office with a chief complaint of right mid-abdominal pain that began 1 day ago. She says she did not seek medical attention earlier because she had to be at work that morning and she thought the pain would resolve on its own.
She reports no fever, headache, anorexia, nausea, vomiting, malaise, loss of weight, melena, or changes in bowel habits. She describes the pain as sharp, localized to the right side, and radiating to the vulva upon sitting up. She denies any association of pain with current dietary habits or bowel function. She has no recollection of precipitating or alleviating factors, including the use of analgesics to reduce the pain.
On further discussion, she mentions that 1 year ago she began experiencing chronic abdominal pain, which she says is sometimes exacerbated by coughing, by standing for extended periods of time, and during menses, and is alleviated upon lying down.
She has regular menstrual periods, and her last one ended 7 days ago.
Her surgical history includes two uncomplicated cesarean deliveries. She does not use tobacco, alcohol, or illicit substances. She is not aware of any allergies to drugs or foods.
She appears to be in no acute distress and has been sitting quietly thus far. She seems to have positioned her hand on her abdomen over the corresponding area of pain.
On physical examination, vital signs are within normal limits, and she is alert and oriented to person, place, and time. Her sclerae are anicteric, and the pupils are equal, round, and reactive to light.
Cardiovascular and pulmonary examinations are also within normal limits. Examination of the abdomen elicits tenderness and guarding along the lateral border of the rectus abdominis muscle on the right side at the level of umbilicus, with no rebound tenderness or rigidity. The liver and spleen are not enlarged, and no abdominal mass is detected. No skin rash, joint swelling, or peripheral edema is noted. A neurologic examination is normal.
1. With the information provided, which of the following is least likely to be causing her symptoms?
- Chronic mesenteric ischemia
- Peptic ulcer
- Acute cholecystitis
- Slipping rib syndrome
CHRONIC MESENTERIC ISCHEMIA
Chronic mesenteric ischemia is the least likely diagnosis because the patient lacks risk factors for atherosclerosis and because she does not have postprandial pain, which is pathognomonic for chronic mesenteric ischemia. It is thought to be caused by a decrease in blood flow through the splanchnic vessels.1 Symptoms tend to arise after eating because of a postprandial increase in metabolic demands.1 These patients also often have atherosclerotic risk factors such as hypertension, hyperlipidemia, and smoking causing coronary artery disease, or a history of stroke.
The primary symptom is abdominal pain, most often described as achy, crampy, or spastic episodes of pain, usually occurring within 2 hours of eating.2 Weight loss is common, as patients can develop a fear of eating. Postprandial pain may also be associated with nausea, vomiting, and bloating.
Findings on clinical examination are usually less severe than the actual symptoms. Visceral duplex or multidetector computed tomography (CT) is an excellent tool to detect blood flow in potential stenotic vessels.2
PEPTIC ULCER DISEASE
Peptic ulcer disease is not a likely diagnosis in this patient because she has no history of taking nonsteroidal anti-inflammatory drugs (NSAIDs).
A study of US patients between 1997 and 2007 reported an annual incidence of peptic ulcer disease of 0.05% to 0.19% depending on the method of diagnosis.3 Peptic ulcer is thought to result from increased gastric acid secretion with a resultant inflammatory response, leading to erosion and ulceration.
The most common possible catalysts include Helicobacter pylori infection, NSAIDs, smoking, alcohol use, and hypersecretory states such as Zollinger-Ellison syndrome.4–6 Complications include internal bleeding, perforation causing peritonitis, and penetration to adjacent organs.
Pathophysiology
Peptic ulcer is the result of an increase in the normal level of gastric acid and a decrease in the protective ability of the gastric mucosa.7 Cytoprotection may be lost through a decrease in the products of arachidonic acid metabolism (eg, prostaglandins, which have a protective effect) or an increase in leukotriene B4 (LTB4), which has a damaging effect. Prostaglandins are thought not only to protect the normal gastric mucosa, but also to provide an antisecretory effect.
On the other hand, leukotrienes—specifically LTB4 and LTC4—are proinflammatory agents and can damage the gastric mucosa. NSAIDs enhance the production of leukotrienes through the 5-lipoxygenase pathway. The ability of LTB4 to cause degranulation and release of lysosomal enzymes may play a vital role in the inflammatory response to NSAIDs.8–10 LTC4 may promote gastric mucosal damage through a reduction of tissue perfusion resulting from the promotion of vascular stasis.8,11,12
Symptoms help differentiate ulcer type
The classic symptom is burning epigastric pain after meals. Pain that occurs immediately after meals is a classic symptom of gastric ulcer. Pain that occurs 2 to 3 hours after meals and that is relieved by food or antacids is a strong indicator of duodenal ulcer.13 Other symptoms include dyspepsia, bloating, distention, heartburn, and chest discomfort.13
Accurate diagnosis is vital in selecting the proper treatment. Diagnostic tests may include H pylori testing, upper-gastrointestinal endoscopy, and radiography with barium swallow.
CHOLECYSTITIS
In cholecystitis, the primary complaint is pain, usually in the right upper quadrant of the abdomen. Patients describe sudden, sharp, and intense pain that radiates to the back or shoulder. Patients may report pain after heavy meals, and some report nausea and vomiting. Cholecystitis is in the differential diagnosis of this patient because of the anatomic location of her pain.
The diagnosis is confirmed by imaging. Abdominal ultrasonography, technetium-99m hepatic iminodiacetic acid scanning, and CT are the most commonly used studies.14
Cholecystitis can be acute or chronic. Acute cholecystitis is categorized as calculous or acalculous. Calculous cholecystitis is multifactorial, but the primary cause is blockage of the cystic duct by gallstones.15 Other factors include irritants such as lysolecithin (released during bile stasis), which can trigger gallbladder inflammation,15–17 and infection.18
When the cystic duct is blocked, bile builds up inside the gallbladder, causing irritation and inflammation of the walls of the gallbladder.14
Acalculous cholecystitis, which resembles calculous cholecystitis but without the gallstones,19 accounts for 2% to 15% of all cases of acute cholecystitis.19,20 It has been observed in hospitalized critically ill patients, but it can also present in an outpatient setting, most often in elderly men with vascular disease.21 Causes include infection, trauma, and tumor obstruction, resulting in endothelial injury, gallbladder stasis, ischemia, and eventually necrosis.14,20,22,23
SLIPPING RIB SYNDROME
Slipping rib syndrome, also known as Tietze syndrome, is believed to be caused by hypermobile costal cartilage. The affected rib slips behind the rib above on contraction of the abdominal wall. This displacement increases the probability of costal nerve impingement and tissue inflammation producing unilateral, sharp, subcostal and upper-abdominal pain.
In this patient, slipping rib syndrome is a possible diagnosis because of the location of the pain and because the pain described by the patient is highly suggestive of neuropathic pain.
Slipping rib syndrome is diagnosed clinically by a “hooking” maneuver: the clinician hooks his or her fingers at the patient’s subcostal area, reproducing the pain by movement of the ribs anteriorly.24 When this test is performed in our patient the result is negative, ruling out slipping rib syndrome.
THE WORKUP CONTINUES
A complete blood cell count and comprehensive metabolic panel are within normal limits. Abdominal duplex ultrasonography reveals no celiac or mesenteric occlusions, thus ruling out chronic mesenteric ischemia.
Noncontrast CT shows no renal or ureteric stones and no evidence of bleeding in the urinary tract. CT with contrast shows no bowel distention, no evidence of hernia, and a normal appendix and ovaries.
2. After exclusion of the previous choices, which of the following is the most likely cause of her symptoms?
- Anterior cutaneous nerve entrapment syndrome (ACNES)
- Ovarian cyst
- Renal stones
- Appendicitis
- Ventral hernia
- Median arcuate ligament syndrome
ANTERIOR CUTANEOUS NERVE ENTRAPMENT SYNDROME
ACNES is the most likely diagnosis. A study published in 2013 indicated that many cases of functional abdominal pain may actually be undiagnosed cases of chronic abdominal wall pain such as ACNES.25 The condition, first described in 1972,26 is thought to be caused by thoracic cutaneous intercostal nerve entrapment between the abdominal muscles, causing pain at the point of entrapment.
The patient may present with pain that is either acute or chronic. Acute pain is localized more on the right side close to an old scar, or at the outer edge of the rectus abdominis muscle. The pain may vary from dull to burning to sharp; it can radiate horizontally in the upper half of the abdomen or obliquely in the lower half of the abdomen with movements such as twisting and sitting up.27
Despite the acute pain, patients are able to carry on daily functions. The pain may be alleviated by lying down.
The pain may be misdiagnosed as gynecologic or renal. In younger men, the pain may raise concern about hernia, and in older patients, cancer.27 Patients may complain of chronic intermittent pain, usually unilateral, and to a lesser extent bilateral.27
The anatomic location of the pain usually reflects the intercostal nerve involved. The pain is not related to eating or to bowel movements.25 Some patients report exacerbation upon coughing or standing, during menses, and with use of oral contraceptives.28,29 When inquiring about surgical history, it is common to find that the patient has had multiple abdominal surgical procedures.
On examination, the patient has nondistressing pain, with a hand often placed over the painful area.27 On firm palpation, a tender spot of less than 2 cm can be detected.
The diagnosis can be confirmed with a positive Carnett test. The patient lies supine on the examination table with the arms crossed over the chest, then elevates the head or the feet to tense the abdominal muscles.26,27 If doing so reproduces the pain (ie, a positive test), this increases the suspicion of ACNES; if the pain decreases or is not reproducible, an intra-abdominal cause is more likely.
If the pain is difficult to localize, the “pinch test” can be done by using the thumb and index finger to pinch and lift the skin of the abdomen, including the subcutaneous layer of fat, first on one side and then on the other. This helps determine the side with greater pain.27
OVARIAN CYSTS
Ovarian cysts are fluid-filled sacs on the surface of or within the ovary. They are often benign and require no intervention. However, 5% to 10% of US women with a suspicious ovarian mass undergo a surgical procedure, and 13% to 21% of these are found to have a malignancy.30,31
Ovarian cysts are usually painless unless complicated by rupture or bleeding. Patients who present with pain describe it as dull and aching and in the abdomen or pelvis. In rare cases, ovarian cysts can be large enough to cause pain from torsion. Other symptoms may include delayed menses and bleeding outside of the menstrual period.32–34
Ovarian cysts are thought to be caused by hormonal changes during the menstrual cycle. They can be detected during pelvic examination or during pelvic ultrasonography. Cysts that are primarily fluid-filled are generally benign and require no intervention. On the other hand, cysts composed of solid material require intervention.
Treatment depends on several factors, including size and type of cyst, the patient’s age, and whether torsion is present. Treatment can range from observation to medical or surgical management. Laparoscopic surgery is commonly used when surgical treatment is warranted.
RENAL STONES
From 10% to 15% of US adults develop a kidney stone at some time during their life.35 There is no single cause, but one factor that promotes stone formation is a greater amount of crystal-forming substances in the urine, such as calcium, oxalate, and uric acid.36 Most renal stones are calcium oxalate, uric acid, struvite, or cysteine.
Symptoms arise when the stone moves within the urinary tract. Patients present to the emergency room in severe distress, usually with flank pain that radiates to the lower abdomen or groin. The pain is episodic, fluctuates in intensity, and may present with dysuria, frequency, or urgency. It is also associated with nausea and vomiting.37
Renal stones are diagnosed through a series of laboratory and imaging studies. Imaging studies include plain radiography (which can miss small stones), renal sonography, and computed tomography without contrast.
APPENDICITIS
In the United States, the lifetime risk of developing appendicitis is 8.6% in men and 6.7% in women.38 Appendicitis is one of the most common reasons for emergency surgery.
Appendicitis is thought to result from obstruction by fecal matter blocking the opening of the appendix or from a viral infection (eg, with an adenovirus).39,40 The resulting bacterial growth can cause the appendix to become inflamed and purulent.
Patients typically present with umbilical or epigastric pain radiating to the right lower quadrant of the abdomen. Over time, the pain becomes sharper. Certain movements can exacerbate the pain, and lying down may alleviate it. Other symptoms are nausea, vomiting, loss of appetite, and low-grade fever.
Findings on the abdominal examination that help to confirm the diagnosis include rigidity and tenderness, classically localized to a point two-thirds of the way from the umbilicus to the anterior superior iliac spine. Rebound tenderness is usually present. Up to 25% of cases in some series presented atypically, with variable location and findings on physical examination (eg, bowel irregularities, indigestion, flatulence, generalized malaise). In addition to the physical examination, laboratory testing and imaging (ultrasonography, CT) may aid in confirming the diagnosis of appendicitis or any other cause of the pain.38
VENTRAL HERNIA
Ventral hernia is a bulging of abdominal organs or other tissues through a defect of the musculature of the abdominal wall. Ventral hernia is categorized by its location as epigastric, abdominal, or incisional. An open abdominal procedure is the cause in nearly 10% of cases41; the herniation occurs with weakening of the surgical scar.
Ventral hernia is usually detected on physical examination, and patients may present after noting a bulge in the abdominal wall. Symptoms vary. Some patients have no symptoms, while others have mild abdominal discomfort or severe abdominal pain as well as nausea and vomiting. Imaging with CT, ultrasonography, or magnetic resonance imaging helps confirm the diagnosis. Complications of ventral hernia include incarceration and bowel strangulation.
MEDIAN ARCUATE LIGAMENT SYNDROME
Median arcuate ligament syndrome is a challenging diagnosis and a very rare cause of abdominal pain. It is thought to be caused by celiac artery compression by fibroligamentous bands. Pain fluctuates with respiration and is greater during expiration.
Patients may present with recurrent episodes of crampy postprandial pain that cause them to avoid eating, resulting in weight loss. The pain may be associated with nausea, vomiting, and bloating.
The diagnosis is confirmed by duplex ultrasonography, angiography, or magnetic resonance angiography. Treatment is surgical division of the fibroligamentous band and crus, and this is often done laparascopically. In patients with severe persistent celiac artery stenosis, angioplasty and stenting may be considered.2
CASE CONTINUED
Before the physical examination, our patient identifies the location of her pain. A Carnett test is performed, as for ACNES: the patient is placed in the supine position and is instructed to cross both arms over her chest. In an effort to promote muscle tension, she is asked to elevate her head off the examination table, as if performing a mini sit-up, and as she does this, pressure is applied to the identified tender area. The pain is easily reproduced, further confirming involvement of the abdominal wall rather than the viscera. After this, electromyography shows abnormal findings. The patient is then referred to the pain management clinic for a diagnostic nerve block.
3. Which of the following is the first-line treatment of ACNES?
- Local injection of anesthetic
- Surgical neurectomy
LOCAL INJECTION OF ANESTHETIC
Local injection of anesthetic is the first-line treatment of ACNES.
Since ACNES is underdiagnosed, the patient may be less likely to be familiar with it. He or she should receive a detailed explanation of the condition and its management; this will help achieve a successful outcome.
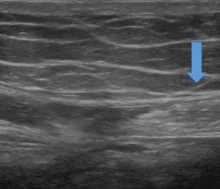
Local anesthetic injection is used for both diagnosis and treatment; 2% lidocaine (or an equivalent) or dehydrated (absolute) alcohol or both can eliminate the pain caused by ACNES. The injection is commonly done under ultrasonographic guidance (Figure 1).42
Complete pain relief may be achieved with a single injection, but some patients require up to five injections.
The adjuvant use of corticosteroids in ACNES to reduce inflammation is controversial.
If anesthetic injections bring only minimal pain relief or if the patient has nerve entrapment in a scar, then surgical neurectomy is an option.43 The procedure is performed under local anesthesia, as the patient’s response aids in identifying the specific nerve or nerves involved.
RETURNING TO THE PATIENT
After a long discussion with our patient about ACNES and the treatment options, she agrees to undergo nerve block in the hope of relieving her pain. She receives a 0.5-mL injection of 2% lidocaine subcutaneously, and within minutes she reports relief of pain. She cannot believe that with a simple injection her pain was relieved. We advise her to return if her pain recurs or if new symptoms arise.
KEEP ACNES IN MIND
ACNES is one of the most commonly misdiagnosed conditions of patients presenting to the outpatient clinic with acute or chronic abdominal pain. This is because the focus is directed to intra-abdominal causes. But if ACNES is kept in consideration from the beginning of the patient encounter, extensive testing, time, and patient anxiety may be reduced significantly. A simple physical examination and the Carnett test aid in raising suspicion of ACNES. If ACNES is confirmed, ultrasonographically guided local anesthetic injection is both diagnostic and therapeutic.
- American Gastroenterological Association Medical Position Statement: Guidelines On Intestinal Ischemia. Gastroenterology 2000; 118:951–953.
- Bobadilla JL. Mesenteric ischemia. Surg Clin North Am 2013; 93:925–940.
- Sung JJ, Kuipers EJ, El-Serag HB. Systematic review: the global incidence and prevalence of peptic ulcer disease. Aliment Pharmacol Ther 2009; 29:938–946.
- Najm WI. Peptic ulcer disease. Prim Care 2011; 38:383–394.
- Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet 2009; 374:1449–1461.
- Chan FK, Leung WK. Peptic-ulcer disease. Lancet 2002; 360:933–941.
- Bright-Asare P, Habte T, Yirgou B, Benjamin J. Prostaglandins, H2-receptor antagonists and peptic ulcer disease. Drugs 1988; 35(suppl 3):1–9.
- Hudson N, Balsitis M, Everitt S, Hawkey CJ. Enhanced gastric mucosal leukotriene B4 synthesis in patients taking non-steroidal anti-inflammatory drugs. Gut 1993; 34:742–747.
- Ford-Hutchinson AW, Bray MA, Doig MV, Shipley ME, Smith MJ. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature 1980; 286:264–265.
- Bokoch GM, Reed PW. Effect of various lipoxygenase metabolites of arachidonic acid on degranulation of polymorphonuclear leukocytes. J Biol Chem 1981; 256:5317–5320.
- Whittle BJ, Oren-Wolman N, Guth PH. Gastric vasoconstrictor actions of leukotriene C4, PGF2 alpha, and thromboxane mimetic U-46619 on rat submucosal microcirculation in vivo. Am J Physiol 1985; 248:G580–G586.
- Pihan G, Rogers C, Szabo S. Vascular injury in acute gastric mucosal damage. Mediatory role of leukotrienes. Dig Dis Sci 1988; 33:625–632.
- Ramakrishnan K, Salinas RC. Peptic ulcer disease. Am Fam Physician 2007; 76:1005–1012.
- Parmet S, Lynm C, Glass RM. JAMA patient page. Acute cholecystitis. JAMA 2003; 289:124.
- Roslyn JJ, DenBesten L, Thompson JE Jr, Silverman BF. Roles of lithogenic bile and cystic duct occlusion in the pathogenesis of acute cholecystitis. Am J Surg 1980; 140:126–130.
- Kaminski DL. Arachidonic acid metabolites in hepatobiliary physiology and disease. Gastroenterology 1989; 97:781–792.
- Jivegård L, Thornell E, Svanvik J. Pathophysiology of acute obstructive cholecystitis: implications for non-operative management. Br J Surg 1987; 74:1084–1086.
- Csendes A, Burdiles P, Maluenda F, Diaz JC, Csendes P, Mitru N. Simultaneous bacteriologic assessment of bile from gallbladder and common bile duct in control subjects and patients with gallstones and common duct stones. Arch Surg 1996; 131:389–394.
- Barie PS, Fischer E. Acute acalculous cholecystitis. J Am Coll Surg 1995; 180:232–244.
- Shapiro MJ, Luchtefeld WB, Kurzweil S, Kaminski DL, Durham RM, Mazuski JE. Acute acalculous cholecystitis in the critically ill. Am Surg 1994; 60:335–339.
- Savoca PE, Longo WE, Zucker KA, McMillen MM, Modlin IM. The increasing prevalence of acalculous cholecystitis in outpatients. Results of a 7-year study. Ann Surg 1990; 211:433–437.
- Gofrit O, Eid A, Pikarsky A, Lebensart PD, Pizov G, Rivkind A. Cholesterol embolisation causing chronic acalculous cholecystitis. Eur J Surg 1996; 162:243–245.
- McChesney JA, Northup PG, Bickston SJ. Acute acalculous cholecystitis associated with systemic sepsis and visceral arterial hypoperfusion: a case series and review of pathophysiology. Dig Dis Sci 2003; 48:1960–1967.
- Aeschlimann A, Kahn MF. Tietze’s syndrome: a critical review. Clin Exp Rheumatol 1990; 8:407–412.
- van Assen T, de Jager-Kievit JW, Scheltinga MR, Roumen RM. Chronic abdominal wall pain misdiagnosed as functional abdominal pain. J Am Board Fam Med 2013; 26:738–744.
- Akhnikh S, de Korte N, de Winter P. Anterior cutaneous nerve entrapment syndrome (ACNES): the forgotten diagnosis. Eur J Pediatr 2014; 173:445–449.
- Applegate WV. Abdominal cutaneous nerve entrapment syndrome (ACNES): a commonly overlooked cause of abdominal pain. Perm J 2002; 6:20–27.
- Grover M. UNC Center for Functional GI & Motility Disorders. Chronic abdominal wall pain: a missed diagnosis. www.med.unc.edu/ibs/files/educational-gi-handouts/Chronic%20Abdominal%20Pain.pdf. Accessed September 9, 2015.
- Greenbaum D, Dawson F, Watson R. Chronic abdominal wall pain (CAWP): a common but frequently overlooked disorder. Poster presented at the World Congress of Gastroenterology, Sydney, Australia, August 26–31, 1990.
- National Institutes of Health Consensus Development Conference Statement. Ovarian cancer: screening, treatment, and follow-up. Gynecol Oncol 1994; 55:S4–S14.
- Koonings PP, Campbell K, Mishell DR Jr, Grimes DA. Relative frequency of primary ovarian neoplasms: a 10-year review. Obstet Gynecol 1989; 74:921–926.
- Givens V, Mitchell GE, Harraway-Smith C, Reddy A, Maness DL. Diagnosis and management of adnexal masses. Am Fam Physician 2009; 80:815–820.
- Goff BA, Mandel L, Muntz HG, Melancon CH. Ovarian carcinoma diagnosis. Cancer 2000; 89:2068–2075.
- Friedman GD, Skilling JS, Udaltsova NV, Smith LH. Early symptoms of ovarian cancer: a case-control study without recall bias. Fam Pract 2005; 22:548–553.
- Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney Int 2003; 63:1817–1823.
- Worcester EM, Coe FL. Clinical practice. Calcium kidney stones. N Engl J Med 2010; 363:954–963.
- Miller NL, Lingeman JE. Management of kidney stones. BMJ 2007; 334:468–472.
- Lewis SR, Mahony PJ, Simpson J. Appendicitis. BMJ 2011; 343:d5976.
- Lamps LW. Infectious causes of appendicitis. Infect Dis Clin North Am 2010; 24:995–1018.
- Reif RM. Viral appendicitis. Hum Pathol 1981; 12:193–196.
- Akkary E, Panait L, Roberts K, Duffy A, Bell R. Sutureless laparoscopic ventral hernia repair in obese patients. JSLS 2011; 15:154–159.
- Boelens OB, Scheltinga MR, Houterman S, Roumen RM. Randomized clinical trial of trigger point infiltration with lidocaine to diagnose anterior cutaneous nerve entrapment syndrome. Br J Surg 2013; 100:217–221.
- Boelens OB, Scheltinga MR, Houterman S, Roumen RM. Management of anterior cutaneous nerve entrapment syndrome in a cohort of 139 patients. Ann Surg 2011; 254:1054–1058.
A 31-year-old woman presents to the office with a chief complaint of right mid-abdominal pain that began 1 day ago. She says she did not seek medical attention earlier because she had to be at work that morning and she thought the pain would resolve on its own.
She reports no fever, headache, anorexia, nausea, vomiting, malaise, loss of weight, melena, or changes in bowel habits. She describes the pain as sharp, localized to the right side, and radiating to the vulva upon sitting up. She denies any association of pain with current dietary habits or bowel function. She has no recollection of precipitating or alleviating factors, including the use of analgesics to reduce the pain.
On further discussion, she mentions that 1 year ago she began experiencing chronic abdominal pain, which she says is sometimes exacerbated by coughing, by standing for extended periods of time, and during menses, and is alleviated upon lying down.
She has regular menstrual periods, and her last one ended 7 days ago.
Her surgical history includes two uncomplicated cesarean deliveries. She does not use tobacco, alcohol, or illicit substances. She is not aware of any allergies to drugs or foods.
She appears to be in no acute distress and has been sitting quietly thus far. She seems to have positioned her hand on her abdomen over the corresponding area of pain.
On physical examination, vital signs are within normal limits, and she is alert and oriented to person, place, and time. Her sclerae are anicteric, and the pupils are equal, round, and reactive to light.
Cardiovascular and pulmonary examinations are also within normal limits. Examination of the abdomen elicits tenderness and guarding along the lateral border of the rectus abdominis muscle on the right side at the level of umbilicus, with no rebound tenderness or rigidity. The liver and spleen are not enlarged, and no abdominal mass is detected. No skin rash, joint swelling, or peripheral edema is noted. A neurologic examination is normal.
1. With the information provided, which of the following is least likely to be causing her symptoms?
- Chronic mesenteric ischemia
- Peptic ulcer
- Acute cholecystitis
- Slipping rib syndrome
CHRONIC MESENTERIC ISCHEMIA
Chronic mesenteric ischemia is the least likely diagnosis because the patient lacks risk factors for atherosclerosis and because she does not have postprandial pain, which is pathognomonic for chronic mesenteric ischemia. It is thought to be caused by a decrease in blood flow through the splanchnic vessels.1 Symptoms tend to arise after eating because of a postprandial increase in metabolic demands.1 These patients also often have atherosclerotic risk factors such as hypertension, hyperlipidemia, and smoking causing coronary artery disease, or a history of stroke.
The primary symptom is abdominal pain, most often described as achy, crampy, or spastic episodes of pain, usually occurring within 2 hours of eating.2 Weight loss is common, as patients can develop a fear of eating. Postprandial pain may also be associated with nausea, vomiting, and bloating.
Findings on clinical examination are usually less severe than the actual symptoms. Visceral duplex or multidetector computed tomography (CT) is an excellent tool to detect blood flow in potential stenotic vessels.2
PEPTIC ULCER DISEASE
Peptic ulcer disease is not a likely diagnosis in this patient because she has no history of taking nonsteroidal anti-inflammatory drugs (NSAIDs).
A study of US patients between 1997 and 2007 reported an annual incidence of peptic ulcer disease of 0.05% to 0.19% depending on the method of diagnosis.3 Peptic ulcer is thought to result from increased gastric acid secretion with a resultant inflammatory response, leading to erosion and ulceration.
The most common possible catalysts include Helicobacter pylori infection, NSAIDs, smoking, alcohol use, and hypersecretory states such as Zollinger-Ellison syndrome.4–6 Complications include internal bleeding, perforation causing peritonitis, and penetration to adjacent organs.
Pathophysiology
Peptic ulcer is the result of an increase in the normal level of gastric acid and a decrease in the protective ability of the gastric mucosa.7 Cytoprotection may be lost through a decrease in the products of arachidonic acid metabolism (eg, prostaglandins, which have a protective effect) or an increase in leukotriene B4 (LTB4), which has a damaging effect. Prostaglandins are thought not only to protect the normal gastric mucosa, but also to provide an antisecretory effect.
On the other hand, leukotrienes—specifically LTB4 and LTC4—are proinflammatory agents and can damage the gastric mucosa. NSAIDs enhance the production of leukotrienes through the 5-lipoxygenase pathway. The ability of LTB4 to cause degranulation and release of lysosomal enzymes may play a vital role in the inflammatory response to NSAIDs.8–10 LTC4 may promote gastric mucosal damage through a reduction of tissue perfusion resulting from the promotion of vascular stasis.8,11,12
Symptoms help differentiate ulcer type
The classic symptom is burning epigastric pain after meals. Pain that occurs immediately after meals is a classic symptom of gastric ulcer. Pain that occurs 2 to 3 hours after meals and that is relieved by food or antacids is a strong indicator of duodenal ulcer.13 Other symptoms include dyspepsia, bloating, distention, heartburn, and chest discomfort.13
Accurate diagnosis is vital in selecting the proper treatment. Diagnostic tests may include H pylori testing, upper-gastrointestinal endoscopy, and radiography with barium swallow.
CHOLECYSTITIS
In cholecystitis, the primary complaint is pain, usually in the right upper quadrant of the abdomen. Patients describe sudden, sharp, and intense pain that radiates to the back or shoulder. Patients may report pain after heavy meals, and some report nausea and vomiting. Cholecystitis is in the differential diagnosis of this patient because of the anatomic location of her pain.
The diagnosis is confirmed by imaging. Abdominal ultrasonography, technetium-99m hepatic iminodiacetic acid scanning, and CT are the most commonly used studies.14
Cholecystitis can be acute or chronic. Acute cholecystitis is categorized as calculous or acalculous. Calculous cholecystitis is multifactorial, but the primary cause is blockage of the cystic duct by gallstones.15 Other factors include irritants such as lysolecithin (released during bile stasis), which can trigger gallbladder inflammation,15–17 and infection.18
When the cystic duct is blocked, bile builds up inside the gallbladder, causing irritation and inflammation of the walls of the gallbladder.14
Acalculous cholecystitis, which resembles calculous cholecystitis but without the gallstones,19 accounts for 2% to 15% of all cases of acute cholecystitis.19,20 It has been observed in hospitalized critically ill patients, but it can also present in an outpatient setting, most often in elderly men with vascular disease.21 Causes include infection, trauma, and tumor obstruction, resulting in endothelial injury, gallbladder stasis, ischemia, and eventually necrosis.14,20,22,23
SLIPPING RIB SYNDROME
Slipping rib syndrome, also known as Tietze syndrome, is believed to be caused by hypermobile costal cartilage. The affected rib slips behind the rib above on contraction of the abdominal wall. This displacement increases the probability of costal nerve impingement and tissue inflammation producing unilateral, sharp, subcostal and upper-abdominal pain.
In this patient, slipping rib syndrome is a possible diagnosis because of the location of the pain and because the pain described by the patient is highly suggestive of neuropathic pain.
Slipping rib syndrome is diagnosed clinically by a “hooking” maneuver: the clinician hooks his or her fingers at the patient’s subcostal area, reproducing the pain by movement of the ribs anteriorly.24 When this test is performed in our patient the result is negative, ruling out slipping rib syndrome.
THE WORKUP CONTINUES
A complete blood cell count and comprehensive metabolic panel are within normal limits. Abdominal duplex ultrasonography reveals no celiac or mesenteric occlusions, thus ruling out chronic mesenteric ischemia.
Noncontrast CT shows no renal or ureteric stones and no evidence of bleeding in the urinary tract. CT with contrast shows no bowel distention, no evidence of hernia, and a normal appendix and ovaries.
2. After exclusion of the previous choices, which of the following is the most likely cause of her symptoms?
- Anterior cutaneous nerve entrapment syndrome (ACNES)
- Ovarian cyst
- Renal stones
- Appendicitis
- Ventral hernia
- Median arcuate ligament syndrome
ANTERIOR CUTANEOUS NERVE ENTRAPMENT SYNDROME
ACNES is the most likely diagnosis. A study published in 2013 indicated that many cases of functional abdominal pain may actually be undiagnosed cases of chronic abdominal wall pain such as ACNES.25 The condition, first described in 1972,26 is thought to be caused by thoracic cutaneous intercostal nerve entrapment between the abdominal muscles, causing pain at the point of entrapment.
The patient may present with pain that is either acute or chronic. Acute pain is localized more on the right side close to an old scar, or at the outer edge of the rectus abdominis muscle. The pain may vary from dull to burning to sharp; it can radiate horizontally in the upper half of the abdomen or obliquely in the lower half of the abdomen with movements such as twisting and sitting up.27
Despite the acute pain, patients are able to carry on daily functions. The pain may be alleviated by lying down.
The pain may be misdiagnosed as gynecologic or renal. In younger men, the pain may raise concern about hernia, and in older patients, cancer.27 Patients may complain of chronic intermittent pain, usually unilateral, and to a lesser extent bilateral.27
The anatomic location of the pain usually reflects the intercostal nerve involved. The pain is not related to eating or to bowel movements.25 Some patients report exacerbation upon coughing or standing, during menses, and with use of oral contraceptives.28,29 When inquiring about surgical history, it is common to find that the patient has had multiple abdominal surgical procedures.
On examination, the patient has nondistressing pain, with a hand often placed over the painful area.27 On firm palpation, a tender spot of less than 2 cm can be detected.
The diagnosis can be confirmed with a positive Carnett test. The patient lies supine on the examination table with the arms crossed over the chest, then elevates the head or the feet to tense the abdominal muscles.26,27 If doing so reproduces the pain (ie, a positive test), this increases the suspicion of ACNES; if the pain decreases or is not reproducible, an intra-abdominal cause is more likely.
If the pain is difficult to localize, the “pinch test” can be done by using the thumb and index finger to pinch and lift the skin of the abdomen, including the subcutaneous layer of fat, first on one side and then on the other. This helps determine the side with greater pain.27
OVARIAN CYSTS
Ovarian cysts are fluid-filled sacs on the surface of or within the ovary. They are often benign and require no intervention. However, 5% to 10% of US women with a suspicious ovarian mass undergo a surgical procedure, and 13% to 21% of these are found to have a malignancy.30,31
Ovarian cysts are usually painless unless complicated by rupture or bleeding. Patients who present with pain describe it as dull and aching and in the abdomen or pelvis. In rare cases, ovarian cysts can be large enough to cause pain from torsion. Other symptoms may include delayed menses and bleeding outside of the menstrual period.32–34
Ovarian cysts are thought to be caused by hormonal changes during the menstrual cycle. They can be detected during pelvic examination or during pelvic ultrasonography. Cysts that are primarily fluid-filled are generally benign and require no intervention. On the other hand, cysts composed of solid material require intervention.
Treatment depends on several factors, including size and type of cyst, the patient’s age, and whether torsion is present. Treatment can range from observation to medical or surgical management. Laparoscopic surgery is commonly used when surgical treatment is warranted.
RENAL STONES
From 10% to 15% of US adults develop a kidney stone at some time during their life.35 There is no single cause, but one factor that promotes stone formation is a greater amount of crystal-forming substances in the urine, such as calcium, oxalate, and uric acid.36 Most renal stones are calcium oxalate, uric acid, struvite, or cysteine.
Symptoms arise when the stone moves within the urinary tract. Patients present to the emergency room in severe distress, usually with flank pain that radiates to the lower abdomen or groin. The pain is episodic, fluctuates in intensity, and may present with dysuria, frequency, or urgency. It is also associated with nausea and vomiting.37
Renal stones are diagnosed through a series of laboratory and imaging studies. Imaging studies include plain radiography (which can miss small stones), renal sonography, and computed tomography without contrast.
APPENDICITIS
In the United States, the lifetime risk of developing appendicitis is 8.6% in men and 6.7% in women.38 Appendicitis is one of the most common reasons for emergency surgery.
Appendicitis is thought to result from obstruction by fecal matter blocking the opening of the appendix or from a viral infection (eg, with an adenovirus).39,40 The resulting bacterial growth can cause the appendix to become inflamed and purulent.
Patients typically present with umbilical or epigastric pain radiating to the right lower quadrant of the abdomen. Over time, the pain becomes sharper. Certain movements can exacerbate the pain, and lying down may alleviate it. Other symptoms are nausea, vomiting, loss of appetite, and low-grade fever.
Findings on the abdominal examination that help to confirm the diagnosis include rigidity and tenderness, classically localized to a point two-thirds of the way from the umbilicus to the anterior superior iliac spine. Rebound tenderness is usually present. Up to 25% of cases in some series presented atypically, with variable location and findings on physical examination (eg, bowel irregularities, indigestion, flatulence, generalized malaise). In addition to the physical examination, laboratory testing and imaging (ultrasonography, CT) may aid in confirming the diagnosis of appendicitis or any other cause of the pain.38
VENTRAL HERNIA
Ventral hernia is a bulging of abdominal organs or other tissues through a defect of the musculature of the abdominal wall. Ventral hernia is categorized by its location as epigastric, abdominal, or incisional. An open abdominal procedure is the cause in nearly 10% of cases41; the herniation occurs with weakening of the surgical scar.
Ventral hernia is usually detected on physical examination, and patients may present after noting a bulge in the abdominal wall. Symptoms vary. Some patients have no symptoms, while others have mild abdominal discomfort or severe abdominal pain as well as nausea and vomiting. Imaging with CT, ultrasonography, or magnetic resonance imaging helps confirm the diagnosis. Complications of ventral hernia include incarceration and bowel strangulation.
MEDIAN ARCUATE LIGAMENT SYNDROME
Median arcuate ligament syndrome is a challenging diagnosis and a very rare cause of abdominal pain. It is thought to be caused by celiac artery compression by fibroligamentous bands. Pain fluctuates with respiration and is greater during expiration.
Patients may present with recurrent episodes of crampy postprandial pain that cause them to avoid eating, resulting in weight loss. The pain may be associated with nausea, vomiting, and bloating.
The diagnosis is confirmed by duplex ultrasonography, angiography, or magnetic resonance angiography. Treatment is surgical division of the fibroligamentous band and crus, and this is often done laparascopically. In patients with severe persistent celiac artery stenosis, angioplasty and stenting may be considered.2
CASE CONTINUED
Before the physical examination, our patient identifies the location of her pain. A Carnett test is performed, as for ACNES: the patient is placed in the supine position and is instructed to cross both arms over her chest. In an effort to promote muscle tension, she is asked to elevate her head off the examination table, as if performing a mini sit-up, and as she does this, pressure is applied to the identified tender area. The pain is easily reproduced, further confirming involvement of the abdominal wall rather than the viscera. After this, electromyography shows abnormal findings. The patient is then referred to the pain management clinic for a diagnostic nerve block.
3. Which of the following is the first-line treatment of ACNES?
- Local injection of anesthetic
- Surgical neurectomy
LOCAL INJECTION OF ANESTHETIC
Local injection of anesthetic is the first-line treatment of ACNES.
Since ACNES is underdiagnosed, the patient may be less likely to be familiar with it. He or she should receive a detailed explanation of the condition and its management; this will help achieve a successful outcome.
Local anesthetic injection is used for both diagnosis and treatment; 2% lidocaine (or an equivalent) or dehydrated (absolute) alcohol or both can eliminate the pain caused by ACNES. The injection is commonly done under ultrasonographic guidance (Figure 1).42
Complete pain relief may be achieved with a single injection, but some patients require up to five injections.
The adjuvant use of corticosteroids in ACNES to reduce inflammation is controversial.
If anesthetic injections bring only minimal pain relief or if the patient has nerve entrapment in a scar, then surgical neurectomy is an option.43 The procedure is performed under local anesthesia, as the patient’s response aids in identifying the specific nerve or nerves involved.
RETURNING TO THE PATIENT
After a long discussion with our patient about ACNES and the treatment options, she agrees to undergo nerve block in the hope of relieving her pain. She receives a 0.5-mL injection of 2% lidocaine subcutaneously, and within minutes she reports relief of pain. She cannot believe that with a simple injection her pain was relieved. We advise her to return if her pain recurs or if new symptoms arise.
KEEP ACNES IN MIND
ACNES is one of the most commonly misdiagnosed conditions of patients presenting to the outpatient clinic with acute or chronic abdominal pain. This is because the focus is directed to intra-abdominal causes. But if ACNES is kept in consideration from the beginning of the patient encounter, extensive testing, time, and patient anxiety may be reduced significantly. A simple physical examination and the Carnett test aid in raising suspicion of ACNES. If ACNES is confirmed, ultrasonographically guided local anesthetic injection is both diagnostic and therapeutic.
A 31-year-old woman presents to the office with a chief complaint of right mid-abdominal pain that began 1 day ago. She says she did not seek medical attention earlier because she had to be at work that morning and she thought the pain would resolve on its own.
She reports no fever, headache, anorexia, nausea, vomiting, malaise, loss of weight, melena, or changes in bowel habits. She describes the pain as sharp, localized to the right side, and radiating to the vulva upon sitting up. She denies any association of pain with current dietary habits or bowel function. She has no recollection of precipitating or alleviating factors, including the use of analgesics to reduce the pain.
On further discussion, she mentions that 1 year ago she began experiencing chronic abdominal pain, which she says is sometimes exacerbated by coughing, by standing for extended periods of time, and during menses, and is alleviated upon lying down.
She has regular menstrual periods, and her last one ended 7 days ago.
Her surgical history includes two uncomplicated cesarean deliveries. She does not use tobacco, alcohol, or illicit substances. She is not aware of any allergies to drugs or foods.
She appears to be in no acute distress and has been sitting quietly thus far. She seems to have positioned her hand on her abdomen over the corresponding area of pain.
On physical examination, vital signs are within normal limits, and she is alert and oriented to person, place, and time. Her sclerae are anicteric, and the pupils are equal, round, and reactive to light.
Cardiovascular and pulmonary examinations are also within normal limits. Examination of the abdomen elicits tenderness and guarding along the lateral border of the rectus abdominis muscle on the right side at the level of umbilicus, with no rebound tenderness or rigidity. The liver and spleen are not enlarged, and no abdominal mass is detected. No skin rash, joint swelling, or peripheral edema is noted. A neurologic examination is normal.
1. With the information provided, which of the following is least likely to be causing her symptoms?
- Chronic mesenteric ischemia
- Peptic ulcer
- Acute cholecystitis
- Slipping rib syndrome
CHRONIC MESENTERIC ISCHEMIA
Chronic mesenteric ischemia is the least likely diagnosis because the patient lacks risk factors for atherosclerosis and because she does not have postprandial pain, which is pathognomonic for chronic mesenteric ischemia. It is thought to be caused by a decrease in blood flow through the splanchnic vessels.1 Symptoms tend to arise after eating because of a postprandial increase in metabolic demands.1 These patients also often have atherosclerotic risk factors such as hypertension, hyperlipidemia, and smoking causing coronary artery disease, or a history of stroke.
The primary symptom is abdominal pain, most often described as achy, crampy, or spastic episodes of pain, usually occurring within 2 hours of eating.2 Weight loss is common, as patients can develop a fear of eating. Postprandial pain may also be associated with nausea, vomiting, and bloating.
Findings on clinical examination are usually less severe than the actual symptoms. Visceral duplex or multidetector computed tomography (CT) is an excellent tool to detect blood flow in potential stenotic vessels.2
PEPTIC ULCER DISEASE
Peptic ulcer disease is not a likely diagnosis in this patient because she has no history of taking nonsteroidal anti-inflammatory drugs (NSAIDs).
A study of US patients between 1997 and 2007 reported an annual incidence of peptic ulcer disease of 0.05% to 0.19% depending on the method of diagnosis.3 Peptic ulcer is thought to result from increased gastric acid secretion with a resultant inflammatory response, leading to erosion and ulceration.
The most common possible catalysts include Helicobacter pylori infection, NSAIDs, smoking, alcohol use, and hypersecretory states such as Zollinger-Ellison syndrome.4–6 Complications include internal bleeding, perforation causing peritonitis, and penetration to adjacent organs.
Pathophysiology
Peptic ulcer is the result of an increase in the normal level of gastric acid and a decrease in the protective ability of the gastric mucosa.7 Cytoprotection may be lost through a decrease in the products of arachidonic acid metabolism (eg, prostaglandins, which have a protective effect) or an increase in leukotriene B4 (LTB4), which has a damaging effect. Prostaglandins are thought not only to protect the normal gastric mucosa, but also to provide an antisecretory effect.
On the other hand, leukotrienes—specifically LTB4 and LTC4—are proinflammatory agents and can damage the gastric mucosa. NSAIDs enhance the production of leukotrienes through the 5-lipoxygenase pathway. The ability of LTB4 to cause degranulation and release of lysosomal enzymes may play a vital role in the inflammatory response to NSAIDs.8–10 LTC4 may promote gastric mucosal damage through a reduction of tissue perfusion resulting from the promotion of vascular stasis.8,11,12
Symptoms help differentiate ulcer type
The classic symptom is burning epigastric pain after meals. Pain that occurs immediately after meals is a classic symptom of gastric ulcer. Pain that occurs 2 to 3 hours after meals and that is relieved by food or antacids is a strong indicator of duodenal ulcer.13 Other symptoms include dyspepsia, bloating, distention, heartburn, and chest discomfort.13
Accurate diagnosis is vital in selecting the proper treatment. Diagnostic tests may include H pylori testing, upper-gastrointestinal endoscopy, and radiography with barium swallow.
CHOLECYSTITIS
In cholecystitis, the primary complaint is pain, usually in the right upper quadrant of the abdomen. Patients describe sudden, sharp, and intense pain that radiates to the back or shoulder. Patients may report pain after heavy meals, and some report nausea and vomiting. Cholecystitis is in the differential diagnosis of this patient because of the anatomic location of her pain.
The diagnosis is confirmed by imaging. Abdominal ultrasonography, technetium-99m hepatic iminodiacetic acid scanning, and CT are the most commonly used studies.14
Cholecystitis can be acute or chronic. Acute cholecystitis is categorized as calculous or acalculous. Calculous cholecystitis is multifactorial, but the primary cause is blockage of the cystic duct by gallstones.15 Other factors include irritants such as lysolecithin (released during bile stasis), which can trigger gallbladder inflammation,15–17 and infection.18
When the cystic duct is blocked, bile builds up inside the gallbladder, causing irritation and inflammation of the walls of the gallbladder.14
Acalculous cholecystitis, which resembles calculous cholecystitis but without the gallstones,19 accounts for 2% to 15% of all cases of acute cholecystitis.19,20 It has been observed in hospitalized critically ill patients, but it can also present in an outpatient setting, most often in elderly men with vascular disease.21 Causes include infection, trauma, and tumor obstruction, resulting in endothelial injury, gallbladder stasis, ischemia, and eventually necrosis.14,20,22,23
SLIPPING RIB SYNDROME
Slipping rib syndrome, also known as Tietze syndrome, is believed to be caused by hypermobile costal cartilage. The affected rib slips behind the rib above on contraction of the abdominal wall. This displacement increases the probability of costal nerve impingement and tissue inflammation producing unilateral, sharp, subcostal and upper-abdominal pain.
In this patient, slipping rib syndrome is a possible diagnosis because of the location of the pain and because the pain described by the patient is highly suggestive of neuropathic pain.
Slipping rib syndrome is diagnosed clinically by a “hooking” maneuver: the clinician hooks his or her fingers at the patient’s subcostal area, reproducing the pain by movement of the ribs anteriorly.24 When this test is performed in our patient the result is negative, ruling out slipping rib syndrome.
THE WORKUP CONTINUES
A complete blood cell count and comprehensive metabolic panel are within normal limits. Abdominal duplex ultrasonography reveals no celiac or mesenteric occlusions, thus ruling out chronic mesenteric ischemia.
Noncontrast CT shows no renal or ureteric stones and no evidence of bleeding in the urinary tract. CT with contrast shows no bowel distention, no evidence of hernia, and a normal appendix and ovaries.
2. After exclusion of the previous choices, which of the following is the most likely cause of her symptoms?
- Anterior cutaneous nerve entrapment syndrome (ACNES)
- Ovarian cyst
- Renal stones
- Appendicitis
- Ventral hernia
- Median arcuate ligament syndrome
ANTERIOR CUTANEOUS NERVE ENTRAPMENT SYNDROME
ACNES is the most likely diagnosis. A study published in 2013 indicated that many cases of functional abdominal pain may actually be undiagnosed cases of chronic abdominal wall pain such as ACNES.25 The condition, first described in 1972,26 is thought to be caused by thoracic cutaneous intercostal nerve entrapment between the abdominal muscles, causing pain at the point of entrapment.
The patient may present with pain that is either acute or chronic. Acute pain is localized more on the right side close to an old scar, or at the outer edge of the rectus abdominis muscle. The pain may vary from dull to burning to sharp; it can radiate horizontally in the upper half of the abdomen or obliquely in the lower half of the abdomen with movements such as twisting and sitting up.27
Despite the acute pain, patients are able to carry on daily functions. The pain may be alleviated by lying down.
The pain may be misdiagnosed as gynecologic or renal. In younger men, the pain may raise concern about hernia, and in older patients, cancer.27 Patients may complain of chronic intermittent pain, usually unilateral, and to a lesser extent bilateral.27
The anatomic location of the pain usually reflects the intercostal nerve involved. The pain is not related to eating or to bowel movements.25 Some patients report exacerbation upon coughing or standing, during menses, and with use of oral contraceptives.28,29 When inquiring about surgical history, it is common to find that the patient has had multiple abdominal surgical procedures.
On examination, the patient has nondistressing pain, with a hand often placed over the painful area.27 On firm palpation, a tender spot of less than 2 cm can be detected.
The diagnosis can be confirmed with a positive Carnett test. The patient lies supine on the examination table with the arms crossed over the chest, then elevates the head or the feet to tense the abdominal muscles.26,27 If doing so reproduces the pain (ie, a positive test), this increases the suspicion of ACNES; if the pain decreases or is not reproducible, an intra-abdominal cause is more likely.
If the pain is difficult to localize, the “pinch test” can be done by using the thumb and index finger to pinch and lift the skin of the abdomen, including the subcutaneous layer of fat, first on one side and then on the other. This helps determine the side with greater pain.27
OVARIAN CYSTS
Ovarian cysts are fluid-filled sacs on the surface of or within the ovary. They are often benign and require no intervention. However, 5% to 10% of US women with a suspicious ovarian mass undergo a surgical procedure, and 13% to 21% of these are found to have a malignancy.30,31
Ovarian cysts are usually painless unless complicated by rupture or bleeding. Patients who present with pain describe it as dull and aching and in the abdomen or pelvis. In rare cases, ovarian cysts can be large enough to cause pain from torsion. Other symptoms may include delayed menses and bleeding outside of the menstrual period.32–34
Ovarian cysts are thought to be caused by hormonal changes during the menstrual cycle. They can be detected during pelvic examination or during pelvic ultrasonography. Cysts that are primarily fluid-filled are generally benign and require no intervention. On the other hand, cysts composed of solid material require intervention.
Treatment depends on several factors, including size and type of cyst, the patient’s age, and whether torsion is present. Treatment can range from observation to medical or surgical management. Laparoscopic surgery is commonly used when surgical treatment is warranted.
RENAL STONES
From 10% to 15% of US adults develop a kidney stone at some time during their life.35 There is no single cause, but one factor that promotes stone formation is a greater amount of crystal-forming substances in the urine, such as calcium, oxalate, and uric acid.36 Most renal stones are calcium oxalate, uric acid, struvite, or cysteine.
Symptoms arise when the stone moves within the urinary tract. Patients present to the emergency room in severe distress, usually with flank pain that radiates to the lower abdomen or groin. The pain is episodic, fluctuates in intensity, and may present with dysuria, frequency, or urgency. It is also associated with nausea and vomiting.37
Renal stones are diagnosed through a series of laboratory and imaging studies. Imaging studies include plain radiography (which can miss small stones), renal sonography, and computed tomography without contrast.
APPENDICITIS
In the United States, the lifetime risk of developing appendicitis is 8.6% in men and 6.7% in women.38 Appendicitis is one of the most common reasons for emergency surgery.
Appendicitis is thought to result from obstruction by fecal matter blocking the opening of the appendix or from a viral infection (eg, with an adenovirus).39,40 The resulting bacterial growth can cause the appendix to become inflamed and purulent.
Patients typically present with umbilical or epigastric pain radiating to the right lower quadrant of the abdomen. Over time, the pain becomes sharper. Certain movements can exacerbate the pain, and lying down may alleviate it. Other symptoms are nausea, vomiting, loss of appetite, and low-grade fever.
Findings on the abdominal examination that help to confirm the diagnosis include rigidity and tenderness, classically localized to a point two-thirds of the way from the umbilicus to the anterior superior iliac spine. Rebound tenderness is usually present. Up to 25% of cases in some series presented atypically, with variable location and findings on physical examination (eg, bowel irregularities, indigestion, flatulence, generalized malaise). In addition to the physical examination, laboratory testing and imaging (ultrasonography, CT) may aid in confirming the diagnosis of appendicitis or any other cause of the pain.38
VENTRAL HERNIA
Ventral hernia is a bulging of abdominal organs or other tissues through a defect of the musculature of the abdominal wall. Ventral hernia is categorized by its location as epigastric, abdominal, or incisional. An open abdominal procedure is the cause in nearly 10% of cases41; the herniation occurs with weakening of the surgical scar.
Ventral hernia is usually detected on physical examination, and patients may present after noting a bulge in the abdominal wall. Symptoms vary. Some patients have no symptoms, while others have mild abdominal discomfort or severe abdominal pain as well as nausea and vomiting. Imaging with CT, ultrasonography, or magnetic resonance imaging helps confirm the diagnosis. Complications of ventral hernia include incarceration and bowel strangulation.
MEDIAN ARCUATE LIGAMENT SYNDROME
Median arcuate ligament syndrome is a challenging diagnosis and a very rare cause of abdominal pain. It is thought to be caused by celiac artery compression by fibroligamentous bands. Pain fluctuates with respiration and is greater during expiration.
Patients may present with recurrent episodes of crampy postprandial pain that cause them to avoid eating, resulting in weight loss. The pain may be associated with nausea, vomiting, and bloating.
The diagnosis is confirmed by duplex ultrasonography, angiography, or magnetic resonance angiography. Treatment is surgical division of the fibroligamentous band and crus, and this is often done laparascopically. In patients with severe persistent celiac artery stenosis, angioplasty and stenting may be considered.2
CASE CONTINUED
Before the physical examination, our patient identifies the location of her pain. A Carnett test is performed, as for ACNES: the patient is placed in the supine position and is instructed to cross both arms over her chest. In an effort to promote muscle tension, she is asked to elevate her head off the examination table, as if performing a mini sit-up, and as she does this, pressure is applied to the identified tender area. The pain is easily reproduced, further confirming involvement of the abdominal wall rather than the viscera. After this, electromyography shows abnormal findings. The patient is then referred to the pain management clinic for a diagnostic nerve block.
3. Which of the following is the first-line treatment of ACNES?
- Local injection of anesthetic
- Surgical neurectomy
LOCAL INJECTION OF ANESTHETIC
Local injection of anesthetic is the first-line treatment of ACNES.
Since ACNES is underdiagnosed, the patient may be less likely to be familiar with it. He or she should receive a detailed explanation of the condition and its management; this will help achieve a successful outcome.
Local anesthetic injection is used for both diagnosis and treatment; 2% lidocaine (or an equivalent) or dehydrated (absolute) alcohol or both can eliminate the pain caused by ACNES. The injection is commonly done under ultrasonographic guidance (Figure 1).42
Complete pain relief may be achieved with a single injection, but some patients require up to five injections.
The adjuvant use of corticosteroids in ACNES to reduce inflammation is controversial.
If anesthetic injections bring only minimal pain relief or if the patient has nerve entrapment in a scar, then surgical neurectomy is an option.43 The procedure is performed under local anesthesia, as the patient’s response aids in identifying the specific nerve or nerves involved.
RETURNING TO THE PATIENT
After a long discussion with our patient about ACNES and the treatment options, she agrees to undergo nerve block in the hope of relieving her pain. She receives a 0.5-mL injection of 2% lidocaine subcutaneously, and within minutes she reports relief of pain. She cannot believe that with a simple injection her pain was relieved. We advise her to return if her pain recurs or if new symptoms arise.
KEEP ACNES IN MIND
ACNES is one of the most commonly misdiagnosed conditions of patients presenting to the outpatient clinic with acute or chronic abdominal pain. This is because the focus is directed to intra-abdominal causes. But if ACNES is kept in consideration from the beginning of the patient encounter, extensive testing, time, and patient anxiety may be reduced significantly. A simple physical examination and the Carnett test aid in raising suspicion of ACNES. If ACNES is confirmed, ultrasonographically guided local anesthetic injection is both diagnostic and therapeutic.
- American Gastroenterological Association Medical Position Statement: Guidelines On Intestinal Ischemia. Gastroenterology 2000; 118:951–953.
- Bobadilla JL. Mesenteric ischemia. Surg Clin North Am 2013; 93:925–940.
- Sung JJ, Kuipers EJ, El-Serag HB. Systematic review: the global incidence and prevalence of peptic ulcer disease. Aliment Pharmacol Ther 2009; 29:938–946.
- Najm WI. Peptic ulcer disease. Prim Care 2011; 38:383–394.
- Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet 2009; 374:1449–1461.
- Chan FK, Leung WK. Peptic-ulcer disease. Lancet 2002; 360:933–941.
- Bright-Asare P, Habte T, Yirgou B, Benjamin J. Prostaglandins, H2-receptor antagonists and peptic ulcer disease. Drugs 1988; 35(suppl 3):1–9.
- Hudson N, Balsitis M, Everitt S, Hawkey CJ. Enhanced gastric mucosal leukotriene B4 synthesis in patients taking non-steroidal anti-inflammatory drugs. Gut 1993; 34:742–747.
- Ford-Hutchinson AW, Bray MA, Doig MV, Shipley ME, Smith MJ. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature 1980; 286:264–265.
- Bokoch GM, Reed PW. Effect of various lipoxygenase metabolites of arachidonic acid on degranulation of polymorphonuclear leukocytes. J Biol Chem 1981; 256:5317–5320.
- Whittle BJ, Oren-Wolman N, Guth PH. Gastric vasoconstrictor actions of leukotriene C4, PGF2 alpha, and thromboxane mimetic U-46619 on rat submucosal microcirculation in vivo. Am J Physiol 1985; 248:G580–G586.
- Pihan G, Rogers C, Szabo S. Vascular injury in acute gastric mucosal damage. Mediatory role of leukotrienes. Dig Dis Sci 1988; 33:625–632.
- Ramakrishnan K, Salinas RC. Peptic ulcer disease. Am Fam Physician 2007; 76:1005–1012.
- Parmet S, Lynm C, Glass RM. JAMA patient page. Acute cholecystitis. JAMA 2003; 289:124.
- Roslyn JJ, DenBesten L, Thompson JE Jr, Silverman BF. Roles of lithogenic bile and cystic duct occlusion in the pathogenesis of acute cholecystitis. Am J Surg 1980; 140:126–130.
- Kaminski DL. Arachidonic acid metabolites in hepatobiliary physiology and disease. Gastroenterology 1989; 97:781–792.
- Jivegård L, Thornell E, Svanvik J. Pathophysiology of acute obstructive cholecystitis: implications for non-operative management. Br J Surg 1987; 74:1084–1086.
- Csendes A, Burdiles P, Maluenda F, Diaz JC, Csendes P, Mitru N. Simultaneous bacteriologic assessment of bile from gallbladder and common bile duct in control subjects and patients with gallstones and common duct stones. Arch Surg 1996; 131:389–394.
- Barie PS, Fischer E. Acute acalculous cholecystitis. J Am Coll Surg 1995; 180:232–244.
- Shapiro MJ, Luchtefeld WB, Kurzweil S, Kaminski DL, Durham RM, Mazuski JE. Acute acalculous cholecystitis in the critically ill. Am Surg 1994; 60:335–339.
- Savoca PE, Longo WE, Zucker KA, McMillen MM, Modlin IM. The increasing prevalence of acalculous cholecystitis in outpatients. Results of a 7-year study. Ann Surg 1990; 211:433–437.
- Gofrit O, Eid A, Pikarsky A, Lebensart PD, Pizov G, Rivkind A. Cholesterol embolisation causing chronic acalculous cholecystitis. Eur J Surg 1996; 162:243–245.
- McChesney JA, Northup PG, Bickston SJ. Acute acalculous cholecystitis associated with systemic sepsis and visceral arterial hypoperfusion: a case series and review of pathophysiology. Dig Dis Sci 2003; 48:1960–1967.
- Aeschlimann A, Kahn MF. Tietze’s syndrome: a critical review. Clin Exp Rheumatol 1990; 8:407–412.
- van Assen T, de Jager-Kievit JW, Scheltinga MR, Roumen RM. Chronic abdominal wall pain misdiagnosed as functional abdominal pain. J Am Board Fam Med 2013; 26:738–744.
- Akhnikh S, de Korte N, de Winter P. Anterior cutaneous nerve entrapment syndrome (ACNES): the forgotten diagnosis. Eur J Pediatr 2014; 173:445–449.
- Applegate WV. Abdominal cutaneous nerve entrapment syndrome (ACNES): a commonly overlooked cause of abdominal pain. Perm J 2002; 6:20–27.
- Grover M. UNC Center for Functional GI & Motility Disorders. Chronic abdominal wall pain: a missed diagnosis. www.med.unc.edu/ibs/files/educational-gi-handouts/Chronic%20Abdominal%20Pain.pdf. Accessed September 9, 2015.
- Greenbaum D, Dawson F, Watson R. Chronic abdominal wall pain (CAWP): a common but frequently overlooked disorder. Poster presented at the World Congress of Gastroenterology, Sydney, Australia, August 26–31, 1990.
- National Institutes of Health Consensus Development Conference Statement. Ovarian cancer: screening, treatment, and follow-up. Gynecol Oncol 1994; 55:S4–S14.
- Koonings PP, Campbell K, Mishell DR Jr, Grimes DA. Relative frequency of primary ovarian neoplasms: a 10-year review. Obstet Gynecol 1989; 74:921–926.
- Givens V, Mitchell GE, Harraway-Smith C, Reddy A, Maness DL. Diagnosis and management of adnexal masses. Am Fam Physician 2009; 80:815–820.
- Goff BA, Mandel L, Muntz HG, Melancon CH. Ovarian carcinoma diagnosis. Cancer 2000; 89:2068–2075.
- Friedman GD, Skilling JS, Udaltsova NV, Smith LH. Early symptoms of ovarian cancer: a case-control study without recall bias. Fam Pract 2005; 22:548–553.
- Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney Int 2003; 63:1817–1823.
- Worcester EM, Coe FL. Clinical practice. Calcium kidney stones. N Engl J Med 2010; 363:954–963.
- Miller NL, Lingeman JE. Management of kidney stones. BMJ 2007; 334:468–472.
- Lewis SR, Mahony PJ, Simpson J. Appendicitis. BMJ 2011; 343:d5976.
- Lamps LW. Infectious causes of appendicitis. Infect Dis Clin North Am 2010; 24:995–1018.
- Reif RM. Viral appendicitis. Hum Pathol 1981; 12:193–196.
- Akkary E, Panait L, Roberts K, Duffy A, Bell R. Sutureless laparoscopic ventral hernia repair in obese patients. JSLS 2011; 15:154–159.
- Boelens OB, Scheltinga MR, Houterman S, Roumen RM. Randomized clinical trial of trigger point infiltration with lidocaine to diagnose anterior cutaneous nerve entrapment syndrome. Br J Surg 2013; 100:217–221.
- Boelens OB, Scheltinga MR, Houterman S, Roumen RM. Management of anterior cutaneous nerve entrapment syndrome in a cohort of 139 patients. Ann Surg 2011; 254:1054–1058.
- American Gastroenterological Association Medical Position Statement: Guidelines On Intestinal Ischemia. Gastroenterology 2000; 118:951–953.
- Bobadilla JL. Mesenteric ischemia. Surg Clin North Am 2013; 93:925–940.
- Sung JJ, Kuipers EJ, El-Serag HB. Systematic review: the global incidence and prevalence of peptic ulcer disease. Aliment Pharmacol Ther 2009; 29:938–946.
- Najm WI. Peptic ulcer disease. Prim Care 2011; 38:383–394.
- Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet 2009; 374:1449–1461.
- Chan FK, Leung WK. Peptic-ulcer disease. Lancet 2002; 360:933–941.
- Bright-Asare P, Habte T, Yirgou B, Benjamin J. Prostaglandins, H2-receptor antagonists and peptic ulcer disease. Drugs 1988; 35(suppl 3):1–9.
- Hudson N, Balsitis M, Everitt S, Hawkey CJ. Enhanced gastric mucosal leukotriene B4 synthesis in patients taking non-steroidal anti-inflammatory drugs. Gut 1993; 34:742–747.
- Ford-Hutchinson AW, Bray MA, Doig MV, Shipley ME, Smith MJ. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature 1980; 286:264–265.
- Bokoch GM, Reed PW. Effect of various lipoxygenase metabolites of arachidonic acid on degranulation of polymorphonuclear leukocytes. J Biol Chem 1981; 256:5317–5320.
- Whittle BJ, Oren-Wolman N, Guth PH. Gastric vasoconstrictor actions of leukotriene C4, PGF2 alpha, and thromboxane mimetic U-46619 on rat submucosal microcirculation in vivo. Am J Physiol 1985; 248:G580–G586.
- Pihan G, Rogers C, Szabo S. Vascular injury in acute gastric mucosal damage. Mediatory role of leukotrienes. Dig Dis Sci 1988; 33:625–632.
- Ramakrishnan K, Salinas RC. Peptic ulcer disease. Am Fam Physician 2007; 76:1005–1012.
- Parmet S, Lynm C, Glass RM. JAMA patient page. Acute cholecystitis. JAMA 2003; 289:124.
- Roslyn JJ, DenBesten L, Thompson JE Jr, Silverman BF. Roles of lithogenic bile and cystic duct occlusion in the pathogenesis of acute cholecystitis. Am J Surg 1980; 140:126–130.
- Kaminski DL. Arachidonic acid metabolites in hepatobiliary physiology and disease. Gastroenterology 1989; 97:781–792.
- Jivegård L, Thornell E, Svanvik J. Pathophysiology of acute obstructive cholecystitis: implications for non-operative management. Br J Surg 1987; 74:1084–1086.
- Csendes A, Burdiles P, Maluenda F, Diaz JC, Csendes P, Mitru N. Simultaneous bacteriologic assessment of bile from gallbladder and common bile duct in control subjects and patients with gallstones and common duct stones. Arch Surg 1996; 131:389–394.
- Barie PS, Fischer E. Acute acalculous cholecystitis. J Am Coll Surg 1995; 180:232–244.
- Shapiro MJ, Luchtefeld WB, Kurzweil S, Kaminski DL, Durham RM, Mazuski JE. Acute acalculous cholecystitis in the critically ill. Am Surg 1994; 60:335–339.
- Savoca PE, Longo WE, Zucker KA, McMillen MM, Modlin IM. The increasing prevalence of acalculous cholecystitis in outpatients. Results of a 7-year study. Ann Surg 1990; 211:433–437.
- Gofrit O, Eid A, Pikarsky A, Lebensart PD, Pizov G, Rivkind A. Cholesterol embolisation causing chronic acalculous cholecystitis. Eur J Surg 1996; 162:243–245.
- McChesney JA, Northup PG, Bickston SJ. Acute acalculous cholecystitis associated with systemic sepsis and visceral arterial hypoperfusion: a case series and review of pathophysiology. Dig Dis Sci 2003; 48:1960–1967.
- Aeschlimann A, Kahn MF. Tietze’s syndrome: a critical review. Clin Exp Rheumatol 1990; 8:407–412.
- van Assen T, de Jager-Kievit JW, Scheltinga MR, Roumen RM. Chronic abdominal wall pain misdiagnosed as functional abdominal pain. J Am Board Fam Med 2013; 26:738–744.
- Akhnikh S, de Korte N, de Winter P. Anterior cutaneous nerve entrapment syndrome (ACNES): the forgotten diagnosis. Eur J Pediatr 2014; 173:445–449.
- Applegate WV. Abdominal cutaneous nerve entrapment syndrome (ACNES): a commonly overlooked cause of abdominal pain. Perm J 2002; 6:20–27.
- Grover M. UNC Center for Functional GI & Motility Disorders. Chronic abdominal wall pain: a missed diagnosis. www.med.unc.edu/ibs/files/educational-gi-handouts/Chronic%20Abdominal%20Pain.pdf. Accessed September 9, 2015.
- Greenbaum D, Dawson F, Watson R. Chronic abdominal wall pain (CAWP): a common but frequently overlooked disorder. Poster presented at the World Congress of Gastroenterology, Sydney, Australia, August 26–31, 1990.
- National Institutes of Health Consensus Development Conference Statement. Ovarian cancer: screening, treatment, and follow-up. Gynecol Oncol 1994; 55:S4–S14.
- Koonings PP, Campbell K, Mishell DR Jr, Grimes DA. Relative frequency of primary ovarian neoplasms: a 10-year review. Obstet Gynecol 1989; 74:921–926.
- Givens V, Mitchell GE, Harraway-Smith C, Reddy A, Maness DL. Diagnosis and management of adnexal masses. Am Fam Physician 2009; 80:815–820.
- Goff BA, Mandel L, Muntz HG, Melancon CH. Ovarian carcinoma diagnosis. Cancer 2000; 89:2068–2075.
- Friedman GD, Skilling JS, Udaltsova NV, Smith LH. Early symptoms of ovarian cancer: a case-control study without recall bias. Fam Pract 2005; 22:548–553.
- Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney Int 2003; 63:1817–1823.
- Worcester EM, Coe FL. Clinical practice. Calcium kidney stones. N Engl J Med 2010; 363:954–963.
- Miller NL, Lingeman JE. Management of kidney stones. BMJ 2007; 334:468–472.
- Lewis SR, Mahony PJ, Simpson J. Appendicitis. BMJ 2011; 343:d5976.
- Lamps LW. Infectious causes of appendicitis. Infect Dis Clin North Am 2010; 24:995–1018.
- Reif RM. Viral appendicitis. Hum Pathol 1981; 12:193–196.
- Akkary E, Panait L, Roberts K, Duffy A, Bell R. Sutureless laparoscopic ventral hernia repair in obese patients. JSLS 2011; 15:154–159.
- Boelens OB, Scheltinga MR, Houterman S, Roumen RM. Randomized clinical trial of trigger point infiltration with lidocaine to diagnose anterior cutaneous nerve entrapment syndrome. Br J Surg 2013; 100:217–221.
- Boelens OB, Scheltinga MR, Houterman S, Roumen RM. Management of anterior cutaneous nerve entrapment syndrome in a cohort of 139 patients. Ann Surg 2011; 254:1054–1058.