User login
Navigating Ethical and Clinical Considerations Relating to Percutaneous Gastrostomy (PEG) Tubes
Cases
Consults for percutaneous gastrostomy (PEG) tube placement for a patient ...
- With dysphagia after stroke: A 70-year-old female with a history of hypertension presented to the hospital with altered mental status and left-sided weakness. She was previously active and independently living. MRI of the brain revealed a right basal ganglia infarct. As a result, she developed dysphagia. She was evaluated by speech and language pathology and underwent a modified barium swallow. Given concerns for aspiration, the recommendation was made for gastroenterology (GI) consultation to place PEG tube for nutrition and medication administration.
- With advanced dementia: An 85-year-old male with an extensive medical history including advanced dementia was admitted from his nursing home for decreased oral intake. His baseline mental status is awake and alert, but he is nonverbal and does not follow commands. Upon 72-hour calorie count, the nutrition consultants determined that he cannot independently meet his nutrition goals. His family wants “everything done” and are asking about a “feeding tube.” The primary team has now consulted GI for PEG tube placement.
- Who is being discharged to a long-term care facility: A 45-year-old male was admitted to the ICU after a heroin overdose. CPR was initiated in the field and return of spontaneous circulation was obtained after 25 minutes. The patient has minimal brainstem reflexes. He is ventilator dependent. He has no family, and now is status-post tracheostomy placement by two-physician consent. The patient is ready for discharge to a long-term care facility that will not accept patients with nasogastric tubes. GI is consulted for PEG tube placement.
Discussion
Gastroenterologists are often consulted for PEG tube placement. However, This is rooted in the fact that, as one expert wrote, “feeding, unlike any other medical treatment, has a moral and emotional significance derived from culture.”1 Understanding the evidence, ethical considerations, and team dynamic behind PEG tube placement is critical for every gastroenterologist. Herein we review these topics and offer guidelines for having patient-centered conversations involving these fundamental concepts.
First, the gastroenterologist should understand the evidence to debunk myths and clarify truths surrounding PEG tube placement. While PEG tubes may help patients with amyotrophic lateral sclerosis stabilize their weight and can even be prophylactically placed in select patients with head and neck cancer,2,3 they are not always appropriate in patients in early recovery from stroke and have not been shown to improve outcomes in patients with advanced dementia. At least 50% of stroke-related dysphagia resolves within 1-2 weeks, and so the American Heart Association Stroke Council recommends continuing nasogastric tube feeding for 2-3 weeks in patients such as the one presented in case 1 before considering PEG tube placement.4
In situations of advanced dementia such as in case,2 several studies demonstrate that PEG tubes do not reduce or prevent aspiration pneumonia, prevent consequences of malnutrition, prolong life, reduce pressure ulcers, reduce urinary of gastrointestinal tract infections, lead to functional improvement, mitigate decline, or even improve comfort or quality of life for patients or their caregivers.5-7 Despite this evidence, as demonstrated in case,3 it is true that many American skilled nursing facilities will not accept a patient without a PEG if enteral feeding is needed. This restriction may vary by state: One study found that skilled nursing facilities in New York City are much less likely to accept patients with nasogastric feeding tubes than randomly selected skilled nursing facilities throughout the country.6 Nonetheless, gastroenterologists should look to the literature to understand the outcomes of populations of patients after PEG tube placement and use that data to guide decision-making.
Secondly, the five ethical principles that inform all medical decision making – autonomy, beneficence, nonmaleficence, justice, and futility – should also inform the gastroenterologist’s rationale in offering PEG placement.8
Autonomy implies that the medical team has determined who is able to make the decision regarding PEG tube placement for the patient. Beneficence connects the patient’s medical diagnosis and technical parameters of PEG tube placement with his or her goals of care. Nonmaleficence ensures the decision-making party understands the benefits and risks of the procedure, including anticipatory guidance on possible PEG tube management, complications, risks, and need for replacement. Justice incorporates the context of the patient’s life, including family dynamics, religious, cultural, and financial factors. Futility connects the patient’s prognosis with practical aspects of having a PEG tube.
The complexity of PEG placement lies in the fact that these ethical principles are often at odds with each other. For example, case 2 highlights the conflicting principles of autonomy and futility for elderly dementia patients: While PEG tube placements do not improve comfort or quality of life in advanced dementia (futility), the family representing the patient has stated they want everything done for his care, including PEG tube placement (autonomy). Navigating these ethical principles can be difficult, but having a framework to organize the different factors offers sound guidance for the gastroenterologist.
Finally, the gastroenterologist should recognize the roles of the multidisciplinary team members, including the patient and their representatives, regarding PEG tube placement consults. While gastroenterologists can be viewed as the technicians consulted to simply “place the tube,” they must seek to understand the members of the team representing the patient to be stewards of their skill set. Consulting team physicians carry great responsibility in organizing the medical and psychosocial aspects of each patient’s care, and their proper goals to relieve suffering and prevent death may color their judgment regarding who they believe is a candidate for a PEG tube. Nutritionists, speech therapists, and case managers can help provide objective data on the practicality and feasibility of a PEG tube in their patients. The healthcare system may influence the decision to consult heavily, as seen in the rules of the long-term care facility in case.3 While it is the job of the multidisciplinary medical team to explain the evidence and ethical considerations of PEG tube placement in a patient-centered manner, ultimately the decision belongs to the patient and their family or representatives.
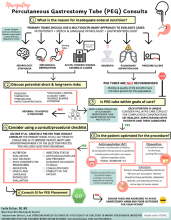
The moral burden of not pursuing PEG placement may supersede the medical advice in many situations. There is an emotionally taxing perception that withholding nutrition via PEG is “starving the patient,” despite literature showing many terminally ill patients do not experience thirst or hunger, and those who do have alleviation of these symptoms with small amounts of food or liquid, not with PEG placement.5 As every patient is unique, PEG tube consultation guidelines created with input from all stakeholders have been utilized to ensure that patients are medically optimized for PEG tube placement and that evidence and ethics-based considerations are evaluated by the multidisciplinary team. An example of such a guideline is shown in Figure 1.
If the gastroenterologist encounters more contentious consultations, there are ways to build consensus to both alleviate patient and family suffering as well as elevate the discussions between teams.
First, identify the type of consult that is repeatedly bringing differing viewpoints and differing ethical principles into play. Second, get representatives from teams together in a neutral environment to understand stakeholders needs. New data suggest, in stroke cases like case 1, there may be dramatic benefit in long-term ability to recover if patients can get early intensive rehabilitation.9 This intense daily rehabilitation is not available within the hospital setting at many locations, and facilitation of discharge may be requested earlier than usually advised tube placement. Third, build a common language for requests and responses between teams. For instance, neurologists can identify and document which patients have less likelihood of early spontaneous recovery, and this can allow gastroenterologists to understand that those patients with little potential for early swallowing recovery can safely be targeted for PEG earlier during the hospital course. Other patients described as having a potential for spontaneous improvement should be given time to recover before an intervention is considered.10 Having a common understanding of goals and a better-informed decision pathway helps each team member feel fulfilled and rewarded, which will ultimately help reduce compassion fatigue and moral burden on providers.
In conclusion, PEG tube placement can be a challenging consultation for gastroenterologists because of the clinical, social, and ethical ramifications at stake for the patient. Even when PEG tube placement is technically feasible, the gastroenterologist should feel empowered to address the evidence-based outcomes of PEG tube placement, discuss the ethical principles of the decision-making process, and communicate with a multidisciplinary team using guidelines as set forth by this paper to best serve the patient.
Dr. Seltzer is based in the Department of Internal Medicine, Mount Sinai Morningside-West, New York City. Dr. Pusateri is based in the Division of Gastroenterology, Hepatology and Nutrition, Ohio State University Wexner Medical Center, Columbus. Dr. Nguyen is based in the Division of Gastroenterology and Center for Esophageal Diseases, Baylor Scott & White Health, Dallas, Texas. Dr. Stein is based in the Division of Gastroenterology, Robert Wood Johnson University Hospital, Rutgers University, New Brunswick, New Jersey. All authors contributed equally to this manuscript, and have no disclosures related to this article.
References
1. Mackie S. Gastroenterol Nurs. 2001 May-Jun;24(3):138-42.
2. Miller RG et al. Neurology. 2009 Oct. doi: 10.1212/WNL.0b013e3181bc0141.
3. Colevas AD et al. J Natl Compr Canc Netw. 2018 May. doi: 10.6004/jnccn.2018.0026.
4. Holloway RG et al. Stroke. 2014 Jun. doi: 10.1161/STR.0000000000000015.
5. Finucane TE et al. JAMA. 1999 Oct. doi: 10.1001/jama.282.14.1365.
6. Burgermaster M et al. Nutr Clin Pract. 2016 Jun. doi: 10.1177/0884533616629636.
7. American Geriatrics Society Ethics C, Clinical P, Models of Care C. J Am Geriatr Soc. 2014 Aug. doi: 10.1111/jgs.12924.
8. Beauchamp TL. Principlism in Bioethics. In: Serna P, eds. Bioethical Decision Making and Argumentation. International Library of Ethics, Law, and the New Medicine, vol 70. Springer; Cham. 2016 Sept:1-16. doi: 10.1007/978-3-319-43419-3_1.
9. Powers WJ et al. Stroke. 2019 Oct. doi: 10.1161/STR.0000000000000211.
10. Galovic M et al. JAMA Neurol. 2019 May. doi: 10.1001/jamaneurol.2018.4858.
Cases
Consults for percutaneous gastrostomy (PEG) tube placement for a patient ...
- With dysphagia after stroke: A 70-year-old female with a history of hypertension presented to the hospital with altered mental status and left-sided weakness. She was previously active and independently living. MRI of the brain revealed a right basal ganglia infarct. As a result, she developed dysphagia. She was evaluated by speech and language pathology and underwent a modified barium swallow. Given concerns for aspiration, the recommendation was made for gastroenterology (GI) consultation to place PEG tube for nutrition and medication administration.
- With advanced dementia: An 85-year-old male with an extensive medical history including advanced dementia was admitted from his nursing home for decreased oral intake. His baseline mental status is awake and alert, but he is nonverbal and does not follow commands. Upon 72-hour calorie count, the nutrition consultants determined that he cannot independently meet his nutrition goals. His family wants “everything done” and are asking about a “feeding tube.” The primary team has now consulted GI for PEG tube placement.
- Who is being discharged to a long-term care facility: A 45-year-old male was admitted to the ICU after a heroin overdose. CPR was initiated in the field and return of spontaneous circulation was obtained after 25 minutes. The patient has minimal brainstem reflexes. He is ventilator dependent. He has no family, and now is status-post tracheostomy placement by two-physician consent. The patient is ready for discharge to a long-term care facility that will not accept patients with nasogastric tubes. GI is consulted for PEG tube placement.
Discussion
Gastroenterologists are often consulted for PEG tube placement. However, This is rooted in the fact that, as one expert wrote, “feeding, unlike any other medical treatment, has a moral and emotional significance derived from culture.”1 Understanding the evidence, ethical considerations, and team dynamic behind PEG tube placement is critical for every gastroenterologist. Herein we review these topics and offer guidelines for having patient-centered conversations involving these fundamental concepts.
First, the gastroenterologist should understand the evidence to debunk myths and clarify truths surrounding PEG tube placement. While PEG tubes may help patients with amyotrophic lateral sclerosis stabilize their weight and can even be prophylactically placed in select patients with head and neck cancer,2,3 they are not always appropriate in patients in early recovery from stroke and have not been shown to improve outcomes in patients with advanced dementia. At least 50% of stroke-related dysphagia resolves within 1-2 weeks, and so the American Heart Association Stroke Council recommends continuing nasogastric tube feeding for 2-3 weeks in patients such as the one presented in case 1 before considering PEG tube placement.4
In situations of advanced dementia such as in case,2 several studies demonstrate that PEG tubes do not reduce or prevent aspiration pneumonia, prevent consequences of malnutrition, prolong life, reduce pressure ulcers, reduce urinary of gastrointestinal tract infections, lead to functional improvement, mitigate decline, or even improve comfort or quality of life for patients or their caregivers.5-7 Despite this evidence, as demonstrated in case,3 it is true that many American skilled nursing facilities will not accept a patient without a PEG if enteral feeding is needed. This restriction may vary by state: One study found that skilled nursing facilities in New York City are much less likely to accept patients with nasogastric feeding tubes than randomly selected skilled nursing facilities throughout the country.6 Nonetheless, gastroenterologists should look to the literature to understand the outcomes of populations of patients after PEG tube placement and use that data to guide decision-making.
Secondly, the five ethical principles that inform all medical decision making – autonomy, beneficence, nonmaleficence, justice, and futility – should also inform the gastroenterologist’s rationale in offering PEG placement.8
Autonomy implies that the medical team has determined who is able to make the decision regarding PEG tube placement for the patient. Beneficence connects the patient’s medical diagnosis and technical parameters of PEG tube placement with his or her goals of care. Nonmaleficence ensures the decision-making party understands the benefits and risks of the procedure, including anticipatory guidance on possible PEG tube management, complications, risks, and need for replacement. Justice incorporates the context of the patient’s life, including family dynamics, religious, cultural, and financial factors. Futility connects the patient’s prognosis with practical aspects of having a PEG tube.
The complexity of PEG placement lies in the fact that these ethical principles are often at odds with each other. For example, case 2 highlights the conflicting principles of autonomy and futility for elderly dementia patients: While PEG tube placements do not improve comfort or quality of life in advanced dementia (futility), the family representing the patient has stated they want everything done for his care, including PEG tube placement (autonomy). Navigating these ethical principles can be difficult, but having a framework to organize the different factors offers sound guidance for the gastroenterologist.
Finally, the gastroenterologist should recognize the roles of the multidisciplinary team members, including the patient and their representatives, regarding PEG tube placement consults. While gastroenterologists can be viewed as the technicians consulted to simply “place the tube,” they must seek to understand the members of the team representing the patient to be stewards of their skill set. Consulting team physicians carry great responsibility in organizing the medical and psychosocial aspects of each patient’s care, and their proper goals to relieve suffering and prevent death may color their judgment regarding who they believe is a candidate for a PEG tube. Nutritionists, speech therapists, and case managers can help provide objective data on the practicality and feasibility of a PEG tube in their patients. The healthcare system may influence the decision to consult heavily, as seen in the rules of the long-term care facility in case.3 While it is the job of the multidisciplinary medical team to explain the evidence and ethical considerations of PEG tube placement in a patient-centered manner, ultimately the decision belongs to the patient and their family or representatives.
The moral burden of not pursuing PEG placement may supersede the medical advice in many situations. There is an emotionally taxing perception that withholding nutrition via PEG is “starving the patient,” despite literature showing many terminally ill patients do not experience thirst or hunger, and those who do have alleviation of these symptoms with small amounts of food or liquid, not with PEG placement.5 As every patient is unique, PEG tube consultation guidelines created with input from all stakeholders have been utilized to ensure that patients are medically optimized for PEG tube placement and that evidence and ethics-based considerations are evaluated by the multidisciplinary team. An example of such a guideline is shown in Figure 1.
If the gastroenterologist encounters more contentious consultations, there are ways to build consensus to both alleviate patient and family suffering as well as elevate the discussions between teams.
First, identify the type of consult that is repeatedly bringing differing viewpoints and differing ethical principles into play. Second, get representatives from teams together in a neutral environment to understand stakeholders needs. New data suggest, in stroke cases like case 1, there may be dramatic benefit in long-term ability to recover if patients can get early intensive rehabilitation.9 This intense daily rehabilitation is not available within the hospital setting at many locations, and facilitation of discharge may be requested earlier than usually advised tube placement. Third, build a common language for requests and responses between teams. For instance, neurologists can identify and document which patients have less likelihood of early spontaneous recovery, and this can allow gastroenterologists to understand that those patients with little potential for early swallowing recovery can safely be targeted for PEG earlier during the hospital course. Other patients described as having a potential for spontaneous improvement should be given time to recover before an intervention is considered.10 Having a common understanding of goals and a better-informed decision pathway helps each team member feel fulfilled and rewarded, which will ultimately help reduce compassion fatigue and moral burden on providers.
In conclusion, PEG tube placement can be a challenging consultation for gastroenterologists because of the clinical, social, and ethical ramifications at stake for the patient. Even when PEG tube placement is technically feasible, the gastroenterologist should feel empowered to address the evidence-based outcomes of PEG tube placement, discuss the ethical principles of the decision-making process, and communicate with a multidisciplinary team using guidelines as set forth by this paper to best serve the patient.
Dr. Seltzer is based in the Department of Internal Medicine, Mount Sinai Morningside-West, New York City. Dr. Pusateri is based in the Division of Gastroenterology, Hepatology and Nutrition, Ohio State University Wexner Medical Center, Columbus. Dr. Nguyen is based in the Division of Gastroenterology and Center for Esophageal Diseases, Baylor Scott & White Health, Dallas, Texas. Dr. Stein is based in the Division of Gastroenterology, Robert Wood Johnson University Hospital, Rutgers University, New Brunswick, New Jersey. All authors contributed equally to this manuscript, and have no disclosures related to this article.
References
1. Mackie S. Gastroenterol Nurs. 2001 May-Jun;24(3):138-42.
2. Miller RG et al. Neurology. 2009 Oct. doi: 10.1212/WNL.0b013e3181bc0141.
3. Colevas AD et al. J Natl Compr Canc Netw. 2018 May. doi: 10.6004/jnccn.2018.0026.
4. Holloway RG et al. Stroke. 2014 Jun. doi: 10.1161/STR.0000000000000015.
5. Finucane TE et al. JAMA. 1999 Oct. doi: 10.1001/jama.282.14.1365.
6. Burgermaster M et al. Nutr Clin Pract. 2016 Jun. doi: 10.1177/0884533616629636.
7. American Geriatrics Society Ethics C, Clinical P, Models of Care C. J Am Geriatr Soc. 2014 Aug. doi: 10.1111/jgs.12924.
8. Beauchamp TL. Principlism in Bioethics. In: Serna P, eds. Bioethical Decision Making and Argumentation. International Library of Ethics, Law, and the New Medicine, vol 70. Springer; Cham. 2016 Sept:1-16. doi: 10.1007/978-3-319-43419-3_1.
9. Powers WJ et al. Stroke. 2019 Oct. doi: 10.1161/STR.0000000000000211.
10. Galovic M et al. JAMA Neurol. 2019 May. doi: 10.1001/jamaneurol.2018.4858.
Cases
Consults for percutaneous gastrostomy (PEG) tube placement for a patient ...
- With dysphagia after stroke: A 70-year-old female with a history of hypertension presented to the hospital with altered mental status and left-sided weakness. She was previously active and independently living. MRI of the brain revealed a right basal ganglia infarct. As a result, she developed dysphagia. She was evaluated by speech and language pathology and underwent a modified barium swallow. Given concerns for aspiration, the recommendation was made for gastroenterology (GI) consultation to place PEG tube for nutrition and medication administration.
- With advanced dementia: An 85-year-old male with an extensive medical history including advanced dementia was admitted from his nursing home for decreased oral intake. His baseline mental status is awake and alert, but he is nonverbal and does not follow commands. Upon 72-hour calorie count, the nutrition consultants determined that he cannot independently meet his nutrition goals. His family wants “everything done” and are asking about a “feeding tube.” The primary team has now consulted GI for PEG tube placement.
- Who is being discharged to a long-term care facility: A 45-year-old male was admitted to the ICU after a heroin overdose. CPR was initiated in the field and return of spontaneous circulation was obtained after 25 minutes. The patient has minimal brainstem reflexes. He is ventilator dependent. He has no family, and now is status-post tracheostomy placement by two-physician consent. The patient is ready for discharge to a long-term care facility that will not accept patients with nasogastric tubes. GI is consulted for PEG tube placement.
Discussion
Gastroenterologists are often consulted for PEG tube placement. However, This is rooted in the fact that, as one expert wrote, “feeding, unlike any other medical treatment, has a moral and emotional significance derived from culture.”1 Understanding the evidence, ethical considerations, and team dynamic behind PEG tube placement is critical for every gastroenterologist. Herein we review these topics and offer guidelines for having patient-centered conversations involving these fundamental concepts.
First, the gastroenterologist should understand the evidence to debunk myths and clarify truths surrounding PEG tube placement. While PEG tubes may help patients with amyotrophic lateral sclerosis stabilize their weight and can even be prophylactically placed in select patients with head and neck cancer,2,3 they are not always appropriate in patients in early recovery from stroke and have not been shown to improve outcomes in patients with advanced dementia. At least 50% of stroke-related dysphagia resolves within 1-2 weeks, and so the American Heart Association Stroke Council recommends continuing nasogastric tube feeding for 2-3 weeks in patients such as the one presented in case 1 before considering PEG tube placement.4
In situations of advanced dementia such as in case,2 several studies demonstrate that PEG tubes do not reduce or prevent aspiration pneumonia, prevent consequences of malnutrition, prolong life, reduce pressure ulcers, reduce urinary of gastrointestinal tract infections, lead to functional improvement, mitigate decline, or even improve comfort or quality of life for patients or their caregivers.5-7 Despite this evidence, as demonstrated in case,3 it is true that many American skilled nursing facilities will not accept a patient without a PEG if enteral feeding is needed. This restriction may vary by state: One study found that skilled nursing facilities in New York City are much less likely to accept patients with nasogastric feeding tubes than randomly selected skilled nursing facilities throughout the country.6 Nonetheless, gastroenterologists should look to the literature to understand the outcomes of populations of patients after PEG tube placement and use that data to guide decision-making.
Secondly, the five ethical principles that inform all medical decision making – autonomy, beneficence, nonmaleficence, justice, and futility – should also inform the gastroenterologist’s rationale in offering PEG placement.8
Autonomy implies that the medical team has determined who is able to make the decision regarding PEG tube placement for the patient. Beneficence connects the patient’s medical diagnosis and technical parameters of PEG tube placement with his or her goals of care. Nonmaleficence ensures the decision-making party understands the benefits and risks of the procedure, including anticipatory guidance on possible PEG tube management, complications, risks, and need for replacement. Justice incorporates the context of the patient’s life, including family dynamics, religious, cultural, and financial factors. Futility connects the patient’s prognosis with practical aspects of having a PEG tube.
The complexity of PEG placement lies in the fact that these ethical principles are often at odds with each other. For example, case 2 highlights the conflicting principles of autonomy and futility for elderly dementia patients: While PEG tube placements do not improve comfort or quality of life in advanced dementia (futility), the family representing the patient has stated they want everything done for his care, including PEG tube placement (autonomy). Navigating these ethical principles can be difficult, but having a framework to organize the different factors offers sound guidance for the gastroenterologist.
Finally, the gastroenterologist should recognize the roles of the multidisciplinary team members, including the patient and their representatives, regarding PEG tube placement consults. While gastroenterologists can be viewed as the technicians consulted to simply “place the tube,” they must seek to understand the members of the team representing the patient to be stewards of their skill set. Consulting team physicians carry great responsibility in organizing the medical and psychosocial aspects of each patient’s care, and their proper goals to relieve suffering and prevent death may color their judgment regarding who they believe is a candidate for a PEG tube. Nutritionists, speech therapists, and case managers can help provide objective data on the practicality and feasibility of a PEG tube in their patients. The healthcare system may influence the decision to consult heavily, as seen in the rules of the long-term care facility in case.3 While it is the job of the multidisciplinary medical team to explain the evidence and ethical considerations of PEG tube placement in a patient-centered manner, ultimately the decision belongs to the patient and their family or representatives.
The moral burden of not pursuing PEG placement may supersede the medical advice in many situations. There is an emotionally taxing perception that withholding nutrition via PEG is “starving the patient,” despite literature showing many terminally ill patients do not experience thirst or hunger, and those who do have alleviation of these symptoms with small amounts of food or liquid, not with PEG placement.5 As every patient is unique, PEG tube consultation guidelines created with input from all stakeholders have been utilized to ensure that patients are medically optimized for PEG tube placement and that evidence and ethics-based considerations are evaluated by the multidisciplinary team. An example of such a guideline is shown in Figure 1.
If the gastroenterologist encounters more contentious consultations, there are ways to build consensus to both alleviate patient and family suffering as well as elevate the discussions between teams.
First, identify the type of consult that is repeatedly bringing differing viewpoints and differing ethical principles into play. Second, get representatives from teams together in a neutral environment to understand stakeholders needs. New data suggest, in stroke cases like case 1, there may be dramatic benefit in long-term ability to recover if patients can get early intensive rehabilitation.9 This intense daily rehabilitation is not available within the hospital setting at many locations, and facilitation of discharge may be requested earlier than usually advised tube placement. Third, build a common language for requests and responses between teams. For instance, neurologists can identify and document which patients have less likelihood of early spontaneous recovery, and this can allow gastroenterologists to understand that those patients with little potential for early swallowing recovery can safely be targeted for PEG earlier during the hospital course. Other patients described as having a potential for spontaneous improvement should be given time to recover before an intervention is considered.10 Having a common understanding of goals and a better-informed decision pathway helps each team member feel fulfilled and rewarded, which will ultimately help reduce compassion fatigue and moral burden on providers.
In conclusion, PEG tube placement can be a challenging consultation for gastroenterologists because of the clinical, social, and ethical ramifications at stake for the patient. Even when PEG tube placement is technically feasible, the gastroenterologist should feel empowered to address the evidence-based outcomes of PEG tube placement, discuss the ethical principles of the decision-making process, and communicate with a multidisciplinary team using guidelines as set forth by this paper to best serve the patient.
Dr. Seltzer is based in the Department of Internal Medicine, Mount Sinai Morningside-West, New York City. Dr. Pusateri is based in the Division of Gastroenterology, Hepatology and Nutrition, Ohio State University Wexner Medical Center, Columbus. Dr. Nguyen is based in the Division of Gastroenterology and Center for Esophageal Diseases, Baylor Scott & White Health, Dallas, Texas. Dr. Stein is based in the Division of Gastroenterology, Robert Wood Johnson University Hospital, Rutgers University, New Brunswick, New Jersey. All authors contributed equally to this manuscript, and have no disclosures related to this article.
References
1. Mackie S. Gastroenterol Nurs. 2001 May-Jun;24(3):138-42.
2. Miller RG et al. Neurology. 2009 Oct. doi: 10.1212/WNL.0b013e3181bc0141.
3. Colevas AD et al. J Natl Compr Canc Netw. 2018 May. doi: 10.6004/jnccn.2018.0026.
4. Holloway RG et al. Stroke. 2014 Jun. doi: 10.1161/STR.0000000000000015.
5. Finucane TE et al. JAMA. 1999 Oct. doi: 10.1001/jama.282.14.1365.
6. Burgermaster M et al. Nutr Clin Pract. 2016 Jun. doi: 10.1177/0884533616629636.
7. American Geriatrics Society Ethics C, Clinical P, Models of Care C. J Am Geriatr Soc. 2014 Aug. doi: 10.1111/jgs.12924.
8. Beauchamp TL. Principlism in Bioethics. In: Serna P, eds. Bioethical Decision Making and Argumentation. International Library of Ethics, Law, and the New Medicine, vol 70. Springer; Cham. 2016 Sept:1-16. doi: 10.1007/978-3-319-43419-3_1.
9. Powers WJ et al. Stroke. 2019 Oct. doi: 10.1161/STR.0000000000000211.
10. Galovic M et al. JAMA Neurol. 2019 May. doi: 10.1001/jamaneurol.2018.4858.
Delivery of Care: The Ethical Imperative in Healthcare
The ethical imperative in healthcare necessitates equitable delivery of care to all individuals, regardless of their socio-economic status or insurance coverage. This principle is rooted in the concept of justice and is crucial to achieving health equity.
As gastroenterologists, despite our various practice settings, we have seen the harmful effects of economic and social disparities on health outcomes. We must therefore ensure that we acknowledge the existence of these disparities, and then begin to provide a framework that allows us to ethically and successfully navigate these complexities for our patients and our affiliated structures.
The following cases illustrate the complexities and ethical dilemmas that gastroenterology and hepatology healthcare professionals encounter in delivering care within the traditional healthcare system.
- Case 1: A 44-year-old male presents to the hospital with intermittent rectal bleeding every few weeks without associated abdominal pain or weight loss and not associated with straining. He has bowel movements every 2-3 days. There is no family history of underlying gastrointestinal disease or associated neoplasm. He is accompanied at the time of the interview by his coworker who offered to drive him to the hospital as he is having personal car trouble. Physical examination reveals normal hemodynamics, abdomen is benign, a digital rectal exam reveals small internal hemorrhoids without pain. Hemoglobin is 10, MCV 85. There is scant blood on the glove. He is uninsured. A GI consult is placed to determine the disposition of the patient. The resident on service suggests outpatient follow-up given low risk of clinical deterioration.
- Case 2: A 28-year-old woman postpartum 6 weeks presents in the office with a history of ulcerative colitis which was diagnosed 2 years prior. She was initially placed on steroid therapy. She underwent a colonoscopy at the time of her diagnosis and was following with a gastroenterologist at which time she was found to have moderate left-sided disease with a modified Mayo score of 9. She complains of urgency and rectal bleeding. She saw a gastroenterologist during her pregnancy and was placed on oral mesalamine, which she remains on at the time of evaluation. Once her physical examination is completed and laboratory values are reviewed, you begin to discuss advanced therapies including biologics as she has failed conventional therapies.
- Case 3: You receive a phone call from an outside hospital about a potential transfer for a 46-year-old male who is an immigrant of unknown citizenship status with fulminant liver failure. He meets all criteria including encephalopathy and coagulopathy. He drinks only socially. His secondary liver workup for extensive disease including ceruloplasmin remains pending. Viral hepatology serologies and autoimmune serologies are negative.
Challenges to the Delivery of Equitable Care
These cases underscore the challenges of delivering equitable care within a system that often fails to address the social determinants of health (SDOH). The disparity in the evaluation and treatment of patients based on insurance status not only affects patient outcomes, but also emphasizes the ethical dilemma of balancing cost with population health management.
The introduction of measures SDOH-1 and SDOH-2 by the Centers for Medicare & Medicaid Services in the 2023 IPPS Final Rule is a step towards requiring hospitals to systematically collect patient-level SDOH data, aiming to establish meaningful collaborations between healthcare providers and community-based organizations for whole-person care.1 The primary goal is to allow ecosystems to collect patient-level social risk factors followed by the creation of meaningful collaboration between healthcare providers and the community-based organizations.
The office settings may or may not implement the SDOH and the current electronic medical record systems. However, from a social history standpoint and certainly from a decision standpoint, the impact of SDOH is realized in all settings.
Interplay of SDOH and Ethical Considerations
The recognition of social determinants of health is crucial for ethical healthcare delivery. In the first case, considering the patient’s identified social determinants of health — including lack of insurance and transportation, combined with the rising incidence of colorectal cancer in individuals under 55 — an argument could be made for admitting the patient under observation for inpatient colonoscopy.
Data have shown disparities in treatment and referrals in emergency care setting for Black patients with rectal bleeding.2 It is imperative that we recognize these existing disparities in diagnosis and outcomes, along with determining SDOH to appropriately come to a final disposition. This approach aligns with the principle of justice and the imperative to deliver equitable care.
In the third case study, we have a patient facing the life-or-death situation of fulminant liver failure. He requires an expeditious decision to be made about transfer candidacy for liver transplant evaluation by the hepatology team.
Impact of Insurance Status on Healthcare Access
Insurance status significantly influences access to healthcare and disparities in treatment outcomes. As seen in case 2 and case 3, our therapies often hinge upon access.
In the inflammatory bowel disease (IBD) case, the therapy that we will choose for our IBD patient may be more influenced by access than efficacy. In a national sample of children with Crohn’s disease, publicly insured children were more likely to receive a biologic within 18 months of diagnosis compared to children with private insurance.3 This would suggest that those with private insurance perhaps experience increased barriers.
In the IBD case that we presented here, we do have a publicly insured woman who will face a potential loss of her Medicaid coverage. Our therapeutic decision will therefore not just rely on risk stratification and individualized approach, but rather the programs that are put in place by our pharmaceutical partners to support a future self-pay patient. This may or may not be favorable to her outcome. This discrepancy points to systemic inequalities in healthcare access and the need for policies that ensure equitable treatment for all, regardless of insurance status.
Conclusion
The delivery of care in healthcare is an ethical imperative that demands equity and justice. The cases discussed above illustrate the complex interplay between socioeconomic factors, insurance status, and the ethical challenges in providing equitable care.
Systematic efforts to address social determinants of health, as mandated by recent CMS measures, along with a commitment to ethical principles, are essential steps toward reducing disparities and ensuring that all individuals receive the care they need. As healthcare expenditures continue to rise, particularly in areas like gastrointestinal health, addressing these ethical and systemic challenges becomes even more critical for the sustainability of the healthcare system and the well-being of the population it serves.
Gastrointestinal healthcare expenditures totaled $119.6 billion in 2018. Annually there were more than 36.8 million ambulatory visits for GI symptoms and 43.4 million ambulatory visits with primary GI diagnosis.4 The use of higher-acuity settings and lack of continuity of care, and the under-recognition and lack of longitudinal framework to follow those families at risk continue to compromise our healthcare system.
Dr. McCutchen is a gastroenterologist at United Digestive, Atlanta, Georgia. She is vice chair of the AGA Research Foundation. Dr. Boules is vice president of global medical and scientific affairs at Ironwood Pharmaceuticals, Cleveland, Ohio.
References
1. www.govinfo.gov/content/pkg/FR-2022-08-10/pdf/2022-16472.pdf.
2. Shields HM et al. Disparities in evaluation of patients with rectal bleeding 40 years and older. Clin Gastroenterol Hepatol. 2014 Apr. doi: 10.1016/j.cgh.2013.07.008.
3. Quiros JA et al. Insurance type influences access to biologics and healthcare utilization in pediatric Crohn’s disease. Crohns Colitis 360. 2021 Aug. doi: 10.1093/crocol/otab057.
4. Peery AF et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: Update 2021. Gastroenterology. 2022 Feb. doi: 10.1053/j.gastro.2021.10.017.
The ethical imperative in healthcare necessitates equitable delivery of care to all individuals, regardless of their socio-economic status or insurance coverage. This principle is rooted in the concept of justice and is crucial to achieving health equity.
As gastroenterologists, despite our various practice settings, we have seen the harmful effects of economic and social disparities on health outcomes. We must therefore ensure that we acknowledge the existence of these disparities, and then begin to provide a framework that allows us to ethically and successfully navigate these complexities for our patients and our affiliated structures.
The following cases illustrate the complexities and ethical dilemmas that gastroenterology and hepatology healthcare professionals encounter in delivering care within the traditional healthcare system.
- Case 1: A 44-year-old male presents to the hospital with intermittent rectal bleeding every few weeks without associated abdominal pain or weight loss and not associated with straining. He has bowel movements every 2-3 days. There is no family history of underlying gastrointestinal disease or associated neoplasm. He is accompanied at the time of the interview by his coworker who offered to drive him to the hospital as he is having personal car trouble. Physical examination reveals normal hemodynamics, abdomen is benign, a digital rectal exam reveals small internal hemorrhoids without pain. Hemoglobin is 10, MCV 85. There is scant blood on the glove. He is uninsured. A GI consult is placed to determine the disposition of the patient. The resident on service suggests outpatient follow-up given low risk of clinical deterioration.
- Case 2: A 28-year-old woman postpartum 6 weeks presents in the office with a history of ulcerative colitis which was diagnosed 2 years prior. She was initially placed on steroid therapy. She underwent a colonoscopy at the time of her diagnosis and was following with a gastroenterologist at which time she was found to have moderate left-sided disease with a modified Mayo score of 9. She complains of urgency and rectal bleeding. She saw a gastroenterologist during her pregnancy and was placed on oral mesalamine, which she remains on at the time of evaluation. Once her physical examination is completed and laboratory values are reviewed, you begin to discuss advanced therapies including biologics as she has failed conventional therapies.
- Case 3: You receive a phone call from an outside hospital about a potential transfer for a 46-year-old male who is an immigrant of unknown citizenship status with fulminant liver failure. He meets all criteria including encephalopathy and coagulopathy. He drinks only socially. His secondary liver workup for extensive disease including ceruloplasmin remains pending. Viral hepatology serologies and autoimmune serologies are negative.
Challenges to the Delivery of Equitable Care
These cases underscore the challenges of delivering equitable care within a system that often fails to address the social determinants of health (SDOH). The disparity in the evaluation and treatment of patients based on insurance status not only affects patient outcomes, but also emphasizes the ethical dilemma of balancing cost with population health management.
The introduction of measures SDOH-1 and SDOH-2 by the Centers for Medicare & Medicaid Services in the 2023 IPPS Final Rule is a step towards requiring hospitals to systematically collect patient-level SDOH data, aiming to establish meaningful collaborations between healthcare providers and community-based organizations for whole-person care.1 The primary goal is to allow ecosystems to collect patient-level social risk factors followed by the creation of meaningful collaboration between healthcare providers and the community-based organizations.
The office settings may or may not implement the SDOH and the current electronic medical record systems. However, from a social history standpoint and certainly from a decision standpoint, the impact of SDOH is realized in all settings.
Interplay of SDOH and Ethical Considerations
The recognition of social determinants of health is crucial for ethical healthcare delivery. In the first case, considering the patient’s identified social determinants of health — including lack of insurance and transportation, combined with the rising incidence of colorectal cancer in individuals under 55 — an argument could be made for admitting the patient under observation for inpatient colonoscopy.
Data have shown disparities in treatment and referrals in emergency care setting for Black patients with rectal bleeding.2 It is imperative that we recognize these existing disparities in diagnosis and outcomes, along with determining SDOH to appropriately come to a final disposition. This approach aligns with the principle of justice and the imperative to deliver equitable care.
In the third case study, we have a patient facing the life-or-death situation of fulminant liver failure. He requires an expeditious decision to be made about transfer candidacy for liver transplant evaluation by the hepatology team.
Impact of Insurance Status on Healthcare Access
Insurance status significantly influences access to healthcare and disparities in treatment outcomes. As seen in case 2 and case 3, our therapies often hinge upon access.
In the inflammatory bowel disease (IBD) case, the therapy that we will choose for our IBD patient may be more influenced by access than efficacy. In a national sample of children with Crohn’s disease, publicly insured children were more likely to receive a biologic within 18 months of diagnosis compared to children with private insurance.3 This would suggest that those with private insurance perhaps experience increased barriers.
In the IBD case that we presented here, we do have a publicly insured woman who will face a potential loss of her Medicaid coverage. Our therapeutic decision will therefore not just rely on risk stratification and individualized approach, but rather the programs that are put in place by our pharmaceutical partners to support a future self-pay patient. This may or may not be favorable to her outcome. This discrepancy points to systemic inequalities in healthcare access and the need for policies that ensure equitable treatment for all, regardless of insurance status.
Conclusion
The delivery of care in healthcare is an ethical imperative that demands equity and justice. The cases discussed above illustrate the complex interplay between socioeconomic factors, insurance status, and the ethical challenges in providing equitable care.
Systematic efforts to address social determinants of health, as mandated by recent CMS measures, along with a commitment to ethical principles, are essential steps toward reducing disparities and ensuring that all individuals receive the care they need. As healthcare expenditures continue to rise, particularly in areas like gastrointestinal health, addressing these ethical and systemic challenges becomes even more critical for the sustainability of the healthcare system and the well-being of the population it serves.
Gastrointestinal healthcare expenditures totaled $119.6 billion in 2018. Annually there were more than 36.8 million ambulatory visits for GI symptoms and 43.4 million ambulatory visits with primary GI diagnosis.4 The use of higher-acuity settings and lack of continuity of care, and the under-recognition and lack of longitudinal framework to follow those families at risk continue to compromise our healthcare system.
Dr. McCutchen is a gastroenterologist at United Digestive, Atlanta, Georgia. She is vice chair of the AGA Research Foundation. Dr. Boules is vice president of global medical and scientific affairs at Ironwood Pharmaceuticals, Cleveland, Ohio.
References
1. www.govinfo.gov/content/pkg/FR-2022-08-10/pdf/2022-16472.pdf.
2. Shields HM et al. Disparities in evaluation of patients with rectal bleeding 40 years and older. Clin Gastroenterol Hepatol. 2014 Apr. doi: 10.1016/j.cgh.2013.07.008.
3. Quiros JA et al. Insurance type influences access to biologics and healthcare utilization in pediatric Crohn’s disease. Crohns Colitis 360. 2021 Aug. doi: 10.1093/crocol/otab057.
4. Peery AF et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: Update 2021. Gastroenterology. 2022 Feb. doi: 10.1053/j.gastro.2021.10.017.
The ethical imperative in healthcare necessitates equitable delivery of care to all individuals, regardless of their socio-economic status or insurance coverage. This principle is rooted in the concept of justice and is crucial to achieving health equity.
As gastroenterologists, despite our various practice settings, we have seen the harmful effects of economic and social disparities on health outcomes. We must therefore ensure that we acknowledge the existence of these disparities, and then begin to provide a framework that allows us to ethically and successfully navigate these complexities for our patients and our affiliated structures.
The following cases illustrate the complexities and ethical dilemmas that gastroenterology and hepatology healthcare professionals encounter in delivering care within the traditional healthcare system.
- Case 1: A 44-year-old male presents to the hospital with intermittent rectal bleeding every few weeks without associated abdominal pain or weight loss and not associated with straining. He has bowel movements every 2-3 days. There is no family history of underlying gastrointestinal disease or associated neoplasm. He is accompanied at the time of the interview by his coworker who offered to drive him to the hospital as he is having personal car trouble. Physical examination reveals normal hemodynamics, abdomen is benign, a digital rectal exam reveals small internal hemorrhoids without pain. Hemoglobin is 10, MCV 85. There is scant blood on the glove. He is uninsured. A GI consult is placed to determine the disposition of the patient. The resident on service suggests outpatient follow-up given low risk of clinical deterioration.
- Case 2: A 28-year-old woman postpartum 6 weeks presents in the office with a history of ulcerative colitis which was diagnosed 2 years prior. She was initially placed on steroid therapy. She underwent a colonoscopy at the time of her diagnosis and was following with a gastroenterologist at which time she was found to have moderate left-sided disease with a modified Mayo score of 9. She complains of urgency and rectal bleeding. She saw a gastroenterologist during her pregnancy and was placed on oral mesalamine, which she remains on at the time of evaluation. Once her physical examination is completed and laboratory values are reviewed, you begin to discuss advanced therapies including biologics as she has failed conventional therapies.
- Case 3: You receive a phone call from an outside hospital about a potential transfer for a 46-year-old male who is an immigrant of unknown citizenship status with fulminant liver failure. He meets all criteria including encephalopathy and coagulopathy. He drinks only socially. His secondary liver workup for extensive disease including ceruloplasmin remains pending. Viral hepatology serologies and autoimmune serologies are negative.
Challenges to the Delivery of Equitable Care
These cases underscore the challenges of delivering equitable care within a system that often fails to address the social determinants of health (SDOH). The disparity in the evaluation and treatment of patients based on insurance status not only affects patient outcomes, but also emphasizes the ethical dilemma of balancing cost with population health management.
The introduction of measures SDOH-1 and SDOH-2 by the Centers for Medicare & Medicaid Services in the 2023 IPPS Final Rule is a step towards requiring hospitals to systematically collect patient-level SDOH data, aiming to establish meaningful collaborations between healthcare providers and community-based organizations for whole-person care.1 The primary goal is to allow ecosystems to collect patient-level social risk factors followed by the creation of meaningful collaboration between healthcare providers and the community-based organizations.
The office settings may or may not implement the SDOH and the current electronic medical record systems. However, from a social history standpoint and certainly from a decision standpoint, the impact of SDOH is realized in all settings.
Interplay of SDOH and Ethical Considerations
The recognition of social determinants of health is crucial for ethical healthcare delivery. In the first case, considering the patient’s identified social determinants of health — including lack of insurance and transportation, combined with the rising incidence of colorectal cancer in individuals under 55 — an argument could be made for admitting the patient under observation for inpatient colonoscopy.
Data have shown disparities in treatment and referrals in emergency care setting for Black patients with rectal bleeding.2 It is imperative that we recognize these existing disparities in diagnosis and outcomes, along with determining SDOH to appropriately come to a final disposition. This approach aligns with the principle of justice and the imperative to deliver equitable care.
In the third case study, we have a patient facing the life-or-death situation of fulminant liver failure. He requires an expeditious decision to be made about transfer candidacy for liver transplant evaluation by the hepatology team.
Impact of Insurance Status on Healthcare Access
Insurance status significantly influences access to healthcare and disparities in treatment outcomes. As seen in case 2 and case 3, our therapies often hinge upon access.
In the inflammatory bowel disease (IBD) case, the therapy that we will choose for our IBD patient may be more influenced by access than efficacy. In a national sample of children with Crohn’s disease, publicly insured children were more likely to receive a biologic within 18 months of diagnosis compared to children with private insurance.3 This would suggest that those with private insurance perhaps experience increased barriers.
In the IBD case that we presented here, we do have a publicly insured woman who will face a potential loss of her Medicaid coverage. Our therapeutic decision will therefore not just rely on risk stratification and individualized approach, but rather the programs that are put in place by our pharmaceutical partners to support a future self-pay patient. This may or may not be favorable to her outcome. This discrepancy points to systemic inequalities in healthcare access and the need for policies that ensure equitable treatment for all, regardless of insurance status.
Conclusion
The delivery of care in healthcare is an ethical imperative that demands equity and justice. The cases discussed above illustrate the complex interplay between socioeconomic factors, insurance status, and the ethical challenges in providing equitable care.
Systematic efforts to address social determinants of health, as mandated by recent CMS measures, along with a commitment to ethical principles, are essential steps toward reducing disparities and ensuring that all individuals receive the care they need. As healthcare expenditures continue to rise, particularly in areas like gastrointestinal health, addressing these ethical and systemic challenges becomes even more critical for the sustainability of the healthcare system and the well-being of the population it serves.
Gastrointestinal healthcare expenditures totaled $119.6 billion in 2018. Annually there were more than 36.8 million ambulatory visits for GI symptoms and 43.4 million ambulatory visits with primary GI diagnosis.4 The use of higher-acuity settings and lack of continuity of care, and the under-recognition and lack of longitudinal framework to follow those families at risk continue to compromise our healthcare system.
Dr. McCutchen is a gastroenterologist at United Digestive, Atlanta, Georgia. She is vice chair of the AGA Research Foundation. Dr. Boules is vice president of global medical and scientific affairs at Ironwood Pharmaceuticals, Cleveland, Ohio.
References
1. www.govinfo.gov/content/pkg/FR-2022-08-10/pdf/2022-16472.pdf.
2. Shields HM et al. Disparities in evaluation of patients with rectal bleeding 40 years and older. Clin Gastroenterol Hepatol. 2014 Apr. doi: 10.1016/j.cgh.2013.07.008.
3. Quiros JA et al. Insurance type influences access to biologics and healthcare utilization in pediatric Crohn’s disease. Crohns Colitis 360. 2021 Aug. doi: 10.1093/crocol/otab057.
4. Peery AF et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: Update 2021. Gastroenterology. 2022 Feb. doi: 10.1053/j.gastro.2021.10.017.
The good, bad, and ugly of direct-to-consumer advertising
Case 1: A 48-year-old female with a 10-year history of left-sided Ulcerative Colitis (UC) has been well controlled on an injectable biologic for 5 years. Her last colonoscopy one year ago was normal without erythema or friability. She presents for an interim visit due to an increase in stool frequency from 2 to 4 bowel movements a day. She denies urgency, nocturnal bowel movements, or blood in her stool. She is concerned about a disease flare and wonders if she is on the right medication. She just saw a TV ad for a new oral UC medication and wants to switch because she prefers an oral medication to an injectable one. Physical exam was normal and in-office flexible sigmoidoscopy demonstrates no change in her colon appearance. You advise her to stay on the biologic because she is still considered well-controlled. She insists on being switched to the new oral medicine. When you probe her more, she says that the TV ad she saw shows people getting the medicine leading normal lives, which has enormous appeal to her.
Case 2: A 52-year-old healthy male is referred for colonoscopy evaluation. He reports no change in bowel habits with rare blood in his stool and thinks his uncle had colon cancer at age 48. He is anxious and not very receptive to having a procedure. He recently saw a TV advertisement promoting non-colonoscopy-based colon cancer screening. You recommend a colonoscopy based on his family history, but he insists on stool-based screening.
Case 3: A 32-year-old female with moderately to well-controlled IBD asks you to review a new “multi-omic” profile of her gut microbiome that she saw advertised on social media. The test report she provides contains a snapshot of her microbiome including abundances of single species and predicted functions for these bacteria from a single stool sample collected and submitted 6 months ago. You counsel her on the role of the gut microbiome in IBD and explain that currently there is not enough knowledge or technology to incorporate these test results into clinical care yet. The patient is frustrated and wants to know why you’re “behind the times.”
These cases may sound familiar to a practicing gastroenterologist. The platform driving all three of these clinical encounters, direct-to-consumer advertising (DTCA), is a legal mechanism by which a commercial entity can communicate directly with the consumer about a medicine or device, bypassing a health care professional.
In the 1960s, Congress granted the Food and Drug Administration regulatory authority over prescription drug labeling and advertising. This included ensuring that ads (1) were not false or misleading, (2) presented a “fair balance” of drug risks and benefits, (3) included facts “material” to a drug’s advertised uses, and (4) included a “brief summary” of all risks described in the drug’s labeling.
Direct-to-consumer advertising increased dramatically in the late 1990s after the FDA eased regulations around risk information by requiring ads to include only “major risks” and provide resources directing consumers to full risk information.
In 2022, the top 10 pharmaceutical ad spenders combined for a total of about $1.7 billion in TV ad spend, with the two top categories being inflammation and diabetes.
The role of the FDA in regulating DTCA of at-home tests is still evolving and largely depends on the intended use of the test results and the health claims used to market the test (that is, whether the test is designed to simply return general information, as in case 3 where DTCA regulations may not apply, or is marketed with specific medical claims or diagnostic interpretations, as in case 2 with clear applications for DTCA regulations.).
It has both potential benefits and potential risks. DTCA can serve to increase disease awareness (e.g., the need for colon cancer screening). It may also prompt patients who might otherwise disregard “red flag” signs and symptoms to seek medical evaluation (e.g., rectal bleeding). DTCA can also alert healthcare providers to new treatment options for diseases within their scope of practice and encourage them to expand their armamentarium.
In bioethics terms, DTCA can be beneficial in facilitating patient autonomy and promoting justice. For example, DTCA can “even the playing field” by ensuring that patients have equitable access to information about available treatments regardless of their socioeconomic status. In doing so, it can empower patients and allow them to have more meaningful discussions with their health care providers and make more informed decisions. In addition, patients may be introduced to alternative testing modalities (i.e., stool-based CRC screening) that, while not necessarily the best screening modality given individual risk (as in Case 2), may offer benefit with greater acceptance compared to inaction (i.e., no screening). Last, the idea of direct-to-consumer “omics” profiling has empowered patients as “citizen scientists” and led to the advent of “biohacking” among the lay population. In doing so, it has challenged the previous bounds of patient autonomy in healthcare by broadening the types of personal health data available to individuals, even when the clinical utility of this data may not yet be clear.
On the flip side, it is undeniable that DTCA of medical products is driven by commercial interests. Branded drugs are primarily, if not exclusively, promoted in DTCA, but these drugs may not always align with standard of care treatment recommendations. A study published in February 2023 in JAMA found that drugs with lower added clinical benefit and higher total sales were associated with higher DTCA spending.
With patients entering medical encounters with preconceived notions of what drugs they want to be prescribed based on media exposure, the ability of health care providers to provide sound medical advice regarding treatment may be circumvented. A patient’s preferred therapy based on exposure to DTCA may sharply contrast with their provider’s recommendation based on their experience and expertise and knowledge of the patient’s unique clinical history.
Unreasonable expectations
An additional potential downside of DTCA is that it can instill unreasonable expectations on the part of patients that the advertised product will benefit them. While DTCA is required to be fair and balanced in reporting benefits and risks, it is difficult to meaningfully address nuanced clinical risks in a brief TV ad and patients may come away with a skewed view of the risk-benefit equation. Furthermore, social media advertising and associated formats may not provide the same level of digestible information as other forms of media and are targeted to individuals likely to identify with the product. Finally, as stated above, only branded products (vs. generics) are advertised. Branded products are generally more costly, and where less expensive and equally effective therapies exist societal costs need to be considered. This can lead to inequities in distributive justice which is the societal allocation of resources. The more the healthcare market is driven towards higher costs in one segment, the less resources are available in another. This may affect regions where healthcare resources are limited.
Shared decision-making
Returning to the 3 cases above, in case 1 the UC patient’s awareness of new treatment options has prompted a shared decision-making discussion. She has a renewed interest in exploring a different route of medication administration because of DTCA. In spite of the patient seemingly well-controlled on her current IBD therapy and minor fluctuations in symptoms that might otherwise be a reason to observe more closely, the patient sees this as a reason to change her treatment based on her impression from the DTCA.
Regarding case 2, disease awareness and CRC screening acceptance is itself a positive outcome. Although commercially driven, the outcome/benefit to society leads to a decrease in disease burden and is a ready alternative with established benefits compared to no screening.
Regarding the proactive “omics-curious” IBD patient in case 3, despite the patient’s disappointment with the DCTA-promoted test’s limited clinical utility at this time, the patient may be communicating a general openness to novel approaches to treating IBD and to advancing her understanding of her disease.
So where does that leave you as a clinician?
As of today, if you live in the U.S., DTCA is a reality. As you navigate day-to-day patient interactions, it is important to keep in mind that your first obligation is to your patients and their best medical interests. Having a well-informed and engaged patient is a positive effect of DTCA over the past 30 years despite the challenging discussions you sometimes are forced to have. In many cases, patients are more self-aware of and engaged in their health and are acting on it due to direct acquisition of information from DTCA platforms. As a clinician, you have an ethical obligation to educate your patients and manage expectations, even when those expectations may be formed on the basis of DTCA with inherent conflicts of interest for promoting a product. Moreover, though certain products may be trendy or promoted on a popular science basis, the underlying technology (i.e. stool-based screening or metagenomics) and/or resultant data are likely not well understood by the typical lay patient, such that it may be difficult for a patient to comprehend how the product may not be particularly informative or inappropriate with respect to their personal medical history without additional counseling. Despite the potentially awkward clinician-patient dynamic precipitated by DTCA, these moments do offer an opportunity for you to gain rapport with your patients by taking the time to fill in the gaps of their understanding of their treatment plans, alternatives, individual risk factors and/or disease while gaining a greater appreciation for what they may personally prioritize with respect to their own health. Ultimately, as we transition further toward precision medicine approaches in healthcare, shared interest in individualized health decisions, at least partially informed by DTCA, is a positive outcome.
Dr. Sloan is a chief medical officer at Abivax. He is a member of the AGA Ethics Committee for COI. David A. Drew, PhD is assistant professor of medicine, Harvard Medical School, Boston. He is director of the Massachusetts General Hospital Biobanking, Clinical & Translational Epidemiology Unit, Boston. He is a member of the AGA Ethics Committee.
Case 1: A 48-year-old female with a 10-year history of left-sided Ulcerative Colitis (UC) has been well controlled on an injectable biologic for 5 years. Her last colonoscopy one year ago was normal without erythema or friability. She presents for an interim visit due to an increase in stool frequency from 2 to 4 bowel movements a day. She denies urgency, nocturnal bowel movements, or blood in her stool. She is concerned about a disease flare and wonders if she is on the right medication. She just saw a TV ad for a new oral UC medication and wants to switch because she prefers an oral medication to an injectable one. Physical exam was normal and in-office flexible sigmoidoscopy demonstrates no change in her colon appearance. You advise her to stay on the biologic because she is still considered well-controlled. She insists on being switched to the new oral medicine. When you probe her more, she says that the TV ad she saw shows people getting the medicine leading normal lives, which has enormous appeal to her.
Case 2: A 52-year-old healthy male is referred for colonoscopy evaluation. He reports no change in bowel habits with rare blood in his stool and thinks his uncle had colon cancer at age 48. He is anxious and not very receptive to having a procedure. He recently saw a TV advertisement promoting non-colonoscopy-based colon cancer screening. You recommend a colonoscopy based on his family history, but he insists on stool-based screening.
Case 3: A 32-year-old female with moderately to well-controlled IBD asks you to review a new “multi-omic” profile of her gut microbiome that she saw advertised on social media. The test report she provides contains a snapshot of her microbiome including abundances of single species and predicted functions for these bacteria from a single stool sample collected and submitted 6 months ago. You counsel her on the role of the gut microbiome in IBD and explain that currently there is not enough knowledge or technology to incorporate these test results into clinical care yet. The patient is frustrated and wants to know why you’re “behind the times.”
These cases may sound familiar to a practicing gastroenterologist. The platform driving all three of these clinical encounters, direct-to-consumer advertising (DTCA), is a legal mechanism by which a commercial entity can communicate directly with the consumer about a medicine or device, bypassing a health care professional.
In the 1960s, Congress granted the Food and Drug Administration regulatory authority over prescription drug labeling and advertising. This included ensuring that ads (1) were not false or misleading, (2) presented a “fair balance” of drug risks and benefits, (3) included facts “material” to a drug’s advertised uses, and (4) included a “brief summary” of all risks described in the drug’s labeling.
Direct-to-consumer advertising increased dramatically in the late 1990s after the FDA eased regulations around risk information by requiring ads to include only “major risks” and provide resources directing consumers to full risk information.
In 2022, the top 10 pharmaceutical ad spenders combined for a total of about $1.7 billion in TV ad spend, with the two top categories being inflammation and diabetes.
The role of the FDA in regulating DTCA of at-home tests is still evolving and largely depends on the intended use of the test results and the health claims used to market the test (that is, whether the test is designed to simply return general information, as in case 3 where DTCA regulations may not apply, or is marketed with specific medical claims or diagnostic interpretations, as in case 2 with clear applications for DTCA regulations.).
It has both potential benefits and potential risks. DTCA can serve to increase disease awareness (e.g., the need for colon cancer screening). It may also prompt patients who might otherwise disregard “red flag” signs and symptoms to seek medical evaluation (e.g., rectal bleeding). DTCA can also alert healthcare providers to new treatment options for diseases within their scope of practice and encourage them to expand their armamentarium.
In bioethics terms, DTCA can be beneficial in facilitating patient autonomy and promoting justice. For example, DTCA can “even the playing field” by ensuring that patients have equitable access to information about available treatments regardless of their socioeconomic status. In doing so, it can empower patients and allow them to have more meaningful discussions with their health care providers and make more informed decisions. In addition, patients may be introduced to alternative testing modalities (i.e., stool-based CRC screening) that, while not necessarily the best screening modality given individual risk (as in Case 2), may offer benefit with greater acceptance compared to inaction (i.e., no screening). Last, the idea of direct-to-consumer “omics” profiling has empowered patients as “citizen scientists” and led to the advent of “biohacking” among the lay population. In doing so, it has challenged the previous bounds of patient autonomy in healthcare by broadening the types of personal health data available to individuals, even when the clinical utility of this data may not yet be clear.
On the flip side, it is undeniable that DTCA of medical products is driven by commercial interests. Branded drugs are primarily, if not exclusively, promoted in DTCA, but these drugs may not always align with standard of care treatment recommendations. A study published in February 2023 in JAMA found that drugs with lower added clinical benefit and higher total sales were associated with higher DTCA spending.
With patients entering medical encounters with preconceived notions of what drugs they want to be prescribed based on media exposure, the ability of health care providers to provide sound medical advice regarding treatment may be circumvented. A patient’s preferred therapy based on exposure to DTCA may sharply contrast with their provider’s recommendation based on their experience and expertise and knowledge of the patient’s unique clinical history.
Unreasonable expectations
An additional potential downside of DTCA is that it can instill unreasonable expectations on the part of patients that the advertised product will benefit them. While DTCA is required to be fair and balanced in reporting benefits and risks, it is difficult to meaningfully address nuanced clinical risks in a brief TV ad and patients may come away with a skewed view of the risk-benefit equation. Furthermore, social media advertising and associated formats may not provide the same level of digestible information as other forms of media and are targeted to individuals likely to identify with the product. Finally, as stated above, only branded products (vs. generics) are advertised. Branded products are generally more costly, and where less expensive and equally effective therapies exist societal costs need to be considered. This can lead to inequities in distributive justice which is the societal allocation of resources. The more the healthcare market is driven towards higher costs in one segment, the less resources are available in another. This may affect regions where healthcare resources are limited.
Shared decision-making
Returning to the 3 cases above, in case 1 the UC patient’s awareness of new treatment options has prompted a shared decision-making discussion. She has a renewed interest in exploring a different route of medication administration because of DTCA. In spite of the patient seemingly well-controlled on her current IBD therapy and minor fluctuations in symptoms that might otherwise be a reason to observe more closely, the patient sees this as a reason to change her treatment based on her impression from the DTCA.
Regarding case 2, disease awareness and CRC screening acceptance is itself a positive outcome. Although commercially driven, the outcome/benefit to society leads to a decrease in disease burden and is a ready alternative with established benefits compared to no screening.
Regarding the proactive “omics-curious” IBD patient in case 3, despite the patient’s disappointment with the DCTA-promoted test’s limited clinical utility at this time, the patient may be communicating a general openness to novel approaches to treating IBD and to advancing her understanding of her disease.
So where does that leave you as a clinician?
As of today, if you live in the U.S., DTCA is a reality. As you navigate day-to-day patient interactions, it is important to keep in mind that your first obligation is to your patients and their best medical interests. Having a well-informed and engaged patient is a positive effect of DTCA over the past 30 years despite the challenging discussions you sometimes are forced to have. In many cases, patients are more self-aware of and engaged in their health and are acting on it due to direct acquisition of information from DTCA platforms. As a clinician, you have an ethical obligation to educate your patients and manage expectations, even when those expectations may be formed on the basis of DTCA with inherent conflicts of interest for promoting a product. Moreover, though certain products may be trendy or promoted on a popular science basis, the underlying technology (i.e. stool-based screening or metagenomics) and/or resultant data are likely not well understood by the typical lay patient, such that it may be difficult for a patient to comprehend how the product may not be particularly informative or inappropriate with respect to their personal medical history without additional counseling. Despite the potentially awkward clinician-patient dynamic precipitated by DTCA, these moments do offer an opportunity for you to gain rapport with your patients by taking the time to fill in the gaps of their understanding of their treatment plans, alternatives, individual risk factors and/or disease while gaining a greater appreciation for what they may personally prioritize with respect to their own health. Ultimately, as we transition further toward precision medicine approaches in healthcare, shared interest in individualized health decisions, at least partially informed by DTCA, is a positive outcome.
Dr. Sloan is a chief medical officer at Abivax. He is a member of the AGA Ethics Committee for COI. David A. Drew, PhD is assistant professor of medicine, Harvard Medical School, Boston. He is director of the Massachusetts General Hospital Biobanking, Clinical & Translational Epidemiology Unit, Boston. He is a member of the AGA Ethics Committee.
Case 1: A 48-year-old female with a 10-year history of left-sided Ulcerative Colitis (UC) has been well controlled on an injectable biologic for 5 years. Her last colonoscopy one year ago was normal without erythema or friability. She presents for an interim visit due to an increase in stool frequency from 2 to 4 bowel movements a day. She denies urgency, nocturnal bowel movements, or blood in her stool. She is concerned about a disease flare and wonders if she is on the right medication. She just saw a TV ad for a new oral UC medication and wants to switch because she prefers an oral medication to an injectable one. Physical exam was normal and in-office flexible sigmoidoscopy demonstrates no change in her colon appearance. You advise her to stay on the biologic because she is still considered well-controlled. She insists on being switched to the new oral medicine. When you probe her more, she says that the TV ad she saw shows people getting the medicine leading normal lives, which has enormous appeal to her.
Case 2: A 52-year-old healthy male is referred for colonoscopy evaluation. He reports no change in bowel habits with rare blood in his stool and thinks his uncle had colon cancer at age 48. He is anxious and not very receptive to having a procedure. He recently saw a TV advertisement promoting non-colonoscopy-based colon cancer screening. You recommend a colonoscopy based on his family history, but he insists on stool-based screening.
Case 3: A 32-year-old female with moderately to well-controlled IBD asks you to review a new “multi-omic” profile of her gut microbiome that she saw advertised on social media. The test report she provides contains a snapshot of her microbiome including abundances of single species and predicted functions for these bacteria from a single stool sample collected and submitted 6 months ago. You counsel her on the role of the gut microbiome in IBD and explain that currently there is not enough knowledge or technology to incorporate these test results into clinical care yet. The patient is frustrated and wants to know why you’re “behind the times.”
These cases may sound familiar to a practicing gastroenterologist. The platform driving all three of these clinical encounters, direct-to-consumer advertising (DTCA), is a legal mechanism by which a commercial entity can communicate directly with the consumer about a medicine or device, bypassing a health care professional.
In the 1960s, Congress granted the Food and Drug Administration regulatory authority over prescription drug labeling and advertising. This included ensuring that ads (1) were not false or misleading, (2) presented a “fair balance” of drug risks and benefits, (3) included facts “material” to a drug’s advertised uses, and (4) included a “brief summary” of all risks described in the drug’s labeling.
Direct-to-consumer advertising increased dramatically in the late 1990s after the FDA eased regulations around risk information by requiring ads to include only “major risks” and provide resources directing consumers to full risk information.
In 2022, the top 10 pharmaceutical ad spenders combined for a total of about $1.7 billion in TV ad spend, with the two top categories being inflammation and diabetes.
The role of the FDA in regulating DTCA of at-home tests is still evolving and largely depends on the intended use of the test results and the health claims used to market the test (that is, whether the test is designed to simply return general information, as in case 3 where DTCA regulations may not apply, or is marketed with specific medical claims or diagnostic interpretations, as in case 2 with clear applications for DTCA regulations.).
It has both potential benefits and potential risks. DTCA can serve to increase disease awareness (e.g., the need for colon cancer screening). It may also prompt patients who might otherwise disregard “red flag” signs and symptoms to seek medical evaluation (e.g., rectal bleeding). DTCA can also alert healthcare providers to new treatment options for diseases within their scope of practice and encourage them to expand their armamentarium.
In bioethics terms, DTCA can be beneficial in facilitating patient autonomy and promoting justice. For example, DTCA can “even the playing field” by ensuring that patients have equitable access to information about available treatments regardless of their socioeconomic status. In doing so, it can empower patients and allow them to have more meaningful discussions with their health care providers and make more informed decisions. In addition, patients may be introduced to alternative testing modalities (i.e., stool-based CRC screening) that, while not necessarily the best screening modality given individual risk (as in Case 2), may offer benefit with greater acceptance compared to inaction (i.e., no screening). Last, the idea of direct-to-consumer “omics” profiling has empowered patients as “citizen scientists” and led to the advent of “biohacking” among the lay population. In doing so, it has challenged the previous bounds of patient autonomy in healthcare by broadening the types of personal health data available to individuals, even when the clinical utility of this data may not yet be clear.
On the flip side, it is undeniable that DTCA of medical products is driven by commercial interests. Branded drugs are primarily, if not exclusively, promoted in DTCA, but these drugs may not always align with standard of care treatment recommendations. A study published in February 2023 in JAMA found that drugs with lower added clinical benefit and higher total sales were associated with higher DTCA spending.
With patients entering medical encounters with preconceived notions of what drugs they want to be prescribed based on media exposure, the ability of health care providers to provide sound medical advice regarding treatment may be circumvented. A patient’s preferred therapy based on exposure to DTCA may sharply contrast with their provider’s recommendation based on their experience and expertise and knowledge of the patient’s unique clinical history.
Unreasonable expectations
An additional potential downside of DTCA is that it can instill unreasonable expectations on the part of patients that the advertised product will benefit them. While DTCA is required to be fair and balanced in reporting benefits and risks, it is difficult to meaningfully address nuanced clinical risks in a brief TV ad and patients may come away with a skewed view of the risk-benefit equation. Furthermore, social media advertising and associated formats may not provide the same level of digestible information as other forms of media and are targeted to individuals likely to identify with the product. Finally, as stated above, only branded products (vs. generics) are advertised. Branded products are generally more costly, and where less expensive and equally effective therapies exist societal costs need to be considered. This can lead to inequities in distributive justice which is the societal allocation of resources. The more the healthcare market is driven towards higher costs in one segment, the less resources are available in another. This may affect regions where healthcare resources are limited.
Shared decision-making
Returning to the 3 cases above, in case 1 the UC patient’s awareness of new treatment options has prompted a shared decision-making discussion. She has a renewed interest in exploring a different route of medication administration because of DTCA. In spite of the patient seemingly well-controlled on her current IBD therapy and minor fluctuations in symptoms that might otherwise be a reason to observe more closely, the patient sees this as a reason to change her treatment based on her impression from the DTCA.
Regarding case 2, disease awareness and CRC screening acceptance is itself a positive outcome. Although commercially driven, the outcome/benefit to society leads to a decrease in disease burden and is a ready alternative with established benefits compared to no screening.
Regarding the proactive “omics-curious” IBD patient in case 3, despite the patient’s disappointment with the DCTA-promoted test’s limited clinical utility at this time, the patient may be communicating a general openness to novel approaches to treating IBD and to advancing her understanding of her disease.
So where does that leave you as a clinician?
As of today, if you live in the U.S., DTCA is a reality. As you navigate day-to-day patient interactions, it is important to keep in mind that your first obligation is to your patients and their best medical interests. Having a well-informed and engaged patient is a positive effect of DTCA over the past 30 years despite the challenging discussions you sometimes are forced to have. In many cases, patients are more self-aware of and engaged in their health and are acting on it due to direct acquisition of information from DTCA platforms. As a clinician, you have an ethical obligation to educate your patients and manage expectations, even when those expectations may be formed on the basis of DTCA with inherent conflicts of interest for promoting a product. Moreover, though certain products may be trendy or promoted on a popular science basis, the underlying technology (i.e. stool-based screening or metagenomics) and/or resultant data are likely not well understood by the typical lay patient, such that it may be difficult for a patient to comprehend how the product may not be particularly informative or inappropriate with respect to their personal medical history without additional counseling. Despite the potentially awkward clinician-patient dynamic precipitated by DTCA, these moments do offer an opportunity for you to gain rapport with your patients by taking the time to fill in the gaps of their understanding of their treatment plans, alternatives, individual risk factors and/or disease while gaining a greater appreciation for what they may personally prioritize with respect to their own health. Ultimately, as we transition further toward precision medicine approaches in healthcare, shared interest in individualized health decisions, at least partially informed by DTCA, is a positive outcome.
Dr. Sloan is a chief medical officer at Abivax. He is a member of the AGA Ethics Committee for COI. David A. Drew, PhD is assistant professor of medicine, Harvard Medical School, Boston. He is director of the Massachusetts General Hospital Biobanking, Clinical & Translational Epidemiology Unit, Boston. He is a member of the AGA Ethics Committee.