Article
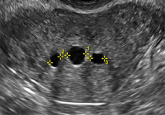
How gynecologic procedures and pharmacologic treatments can affect the uterus
- Author:
- Michelle Stalnaker Ozcan, MD
- Andrew M. Kaunitz, MD
Understanding and identifying possible uterine changes caused by endometrial ablation and tamoxifen use can be important for subsequent treatment...
Article

Imaging the suspected ovarian malignancy: 14 cases
- Author:
- Michelle Stalnaker Ozcan, MD
- Andrew M. Kaunitz, MD
The presented cases demonstrate transvaginal ultrasonography, as well as intraoperative imaging, of cystadenoma, low malignant potential tumors,...
News

“Cogwheel” and other signs of hydrosalpinx and pelvic inclusion cysts
- Author:
- Michelle Stalnaker Ozcan, MD
- Andrew M. Kaunitz, MD
These types of cysts do not require further imaging if diagnosis is certain. When can you be confident with the final diagnosis? These authors...
Article
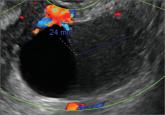
Imaging the endometrioma and mature cystic teratoma
- Author:
- Michelle Stalnaker Ozcan, MD
- Andrew M. Kaunitz, MD
A 25-year-old patient presents with pelvic pain and dyspareunia. A 19-year-old patient with a history of ovarian cystectomy for dermoid cyst...
News
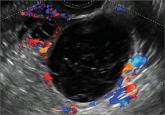
Telltale sonographic features of simple and hemorrhagic cysts
- Author:
- Michelle Stalnaker Ozcan, MD
- Andrew M. Kaunitz, MD
Myriad sonographic features characterize cystic adnexal pathology. Here, three cases of benign, resolving cysts, including when to follow-up.
Article
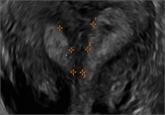
Congenital uterine anomalies: A resource of diagnostic images, Part 2
- Author:
- Michelle Stalnaker Ozcan, MD
- Andrew M. Kaunitz, MD
The following images demonstrate the ability of 3D sonography to identify the unicornuate, bicornuate, didelphic, and DES-exposed uterus
