User login
The Dilemma of the Racist Patient
Medicine is not immune from the pervasive grasp of racism. It spills from other dimensions into the realm of healing and poses challenges to those charged with care of the patient. The literature widely documents racist experiences of patients, and differential treatment and health care disparities based on race.1,2 As a field, medicine is overshadowed by infamous experiments, such as the Tuskegee and Guatemala experiments, and routine studies that demonstrate poor treatment of minority patients.3-5 Although much-needed discussion and research is being done on the unfair treatment of patients, little is written about racist patients and their subsequent effect on health care providers and institutions. Such interactions can cause significant distress to providers, damage the therapeutic physician–patient relationship, and threaten the collegial and structural framework of an institution.6 The silent acquiescence to patients’ racist demands in recent times has become a legal, ethical, and medical dilemma that deserves attention.
No specific example of patient-generated racism is needed because most minority physicians have experienced an overtly racist interaction with a patient. The true incidence of these interactions is unknown because of underreporting secondary to the tendency of physicians to disregard this behavior in the name of “professionalism,” and because reporting of these incidents can sometimes expose how poorly a provider has dealt with the issue and draw admonishment.7 In addition to the overt interactions, numerous examples of subtle racism may exist. Manifestations of such subtleties include failure to cooperate with a history and physical examination, use of hostile language, and aggressive body language. The New York Times gives the example of an Asian female physician tending to a burly, unreceptive, swastika-tattooed patient.8 Such racist interactions are concerning, especially as diversity among newly practicing physicians increases.9
Medical Training
In medical school, students are educated to embody compassion and caring. Their care of patients should rise above the fray of poverty, interpersonal conflict, and prejudice.10 To further this point, medical school curricula have recently introduced standardized patients to teach empathy and simulate difficult encounters in order to help students learn to navigate interactions with aggressive, racist patients. In these scenarios, the patient quickly relinquishes his/her views after an overly understanding student engages the patient in conversation and addresses the source of their angst. Rarely do real-life scenarios play out in such an idealistic manner. The expectation remains, however, that the physician model extreme patience and understanding and honor the patient’s autonomy.
The American Medical Association (AMA), a guiding force in medical education, outlines the patient–physician relationship.10 Such a relationship is a mutually trusting undertaking in which the provider is the patient’s advocate and holds the well-being of the patient supreme. The goal is to alleviate suffering, and it should be done without regard to self-interest.10 The AMA also offers clear instruction to the physician in its code of medical ethics that the physician may not discriminate based on race, color, religion, national origin, sexual orientation, gender identity, or any other basis that would constitute invidious discrimination. With regard to the discriminatory practices of patients, the AMA instructs that “patients who use derogatory language or otherwise act in a prejudicial manner toward physicians, other health care professionals, or others in the health care setting, seriously undermine the integrity of the patient–physician relationship. Such behavior, if unmodified, may constitute sufficient justification for the physician to arrange for the transfer of care.”10 The AMA has also recently launched an online ethics journal, AMA Journal of Ethics, which explores difficult patient interactions and continues to reiterate the supreme role of the physician. When dealing with patients, the anti-discrimination policy is clearly set forth for physicians.
The Dilemma
Anti-discrimination policies for patients are not as clear. Patients are allowed to pick their own provider, and most institutions allow selection based on gender. Most institutions have no guidelines prohibiting provider-selection based on race, and no published hospital policies explicitly restrict racist demands. Although a culture of respect is encouraged through many hospitals’ published slogans and on websites, at the authors’ institution, no published guidelines exist about the behavior of the patient. When no such policies exist, differential treatment of patients’ racist requests ensues and frustration results. Legally, Title VII of the Civil Rights Act of 1964 bars all employers from discriminating with respect to employment conditions or terms on the basis of race, color, religion, sex, or national origin.11 Honoring a patient’s racist demands that results in discrimination of employees is a violation of that law. Reports of hospitals acceding to racist requests have often resulted in upset staff and lawsuits.12-14 Legal language, however, may be foreign in cases of life and death, or scenarios involving significant illness. Physicians in such cases often grant racist requests; for example, a Korean patient underwent life-saving measures only after he was given a non-Japanese provider, and a surgeon granted the wish of a patient’s husband to prohibit African American providers and staff members from entering the operating suite when his wife was undergoing an operation.15 Some would argue that granting a patient’s bigoted request is akin to institutionalized racism.16
The doctor–patient relationship is a powerful cornerstone for medicine. Confidence in the physician results in higher satisfaction for both parties and adherence to the treatment regimen on the part of the patient. Prejudiced interactions threaten the therapeutic alliance between patient and provider. Research has investigated how race plays a role in the doctor–patient relationship. When permitted, patients more often pick a provider of their own race.17 One of 5 African American patients wishes to have an African American provider, and such a desire is often based on a previous negative racist encounter.18 A patient’s perceptions of discrimination in general correlate with preference for same-race providers, highlighting that a patient’s overall experience with discrimination leads them to prefer a same-race physician. Race-concordant relationships (ie, one in which the provider and patient are of the same race) not only show increased satisfaction, but patients also perceive that their interactions with a racially similar physician are more participatory.19-22 In non–English speaking groups, preferences for racially similar physicians are largely based on language similarity, but Latinos feel that Latino physicians are more empathetic to their complaints.23 Such views are felt not only by patients, but also by providers. One of 3 physicians feels that patients receiving care from a physician that is of the patient’s own race is superior to that provided by a race-discordant physician.24 Superior outcomes from race-concordant doctor–patient relationships have led some to argue in favor of granting a patient’s wishes for a provider of similar race because doing so can confer additional health benefits.25
Possible Solutions
The solution to such a complex and uncomfortable issue begins with addressing the problem. Patients who make racist remarks and racist demands should be courteously informed that their behavior is inappropriate and hurtful. Failure to voice such a concern results in passive, tacit approval of racist remarks and can be distressing to other patients and staff members in the vicinity.26 It is unfair for a physician, as the leader of the care team, to ignore such behavior because it places staff members, who spend much more time with the patient, in a potentially abusive situation and leaves them feeling helpless.27 Toward this end, appropriate training, beginning in medical school and continuing in residency, in confronting racist patients is needed to ease the too often felt sense of discomfort among providers.7,26
Medical school, although rightly placing patient comfort at the center of dialogue, too often drowns out the personality of the student in the name of professionalism, which becomes a problem as a young physician struggles to reconcile his or her personality with the newly ingrained teaching to remain professional. This internal conflict can lead to frustration. A necessary prerequisite to beginning dialogue is that the physician recognizes his or her own emotional baggage from prior racially charged events and continues to remain professional. Airing the issue can help establish dialogue that can identify underlying causes of the patient’s misplaced anger. An illness and its subsequent hospitalization can make a patient feel vulnerable and helpless, and in those with poor coping mechanisms, misdirection of emotion is not uncommon.
In more difficult scenarios where attempts at dialogue reach an impasse, an ethics team should be consulted. Most institutions have such help available. Their expertise and experience can help in addressing the needs of the patient judiciously. Some institutions have dedicated multidisciplinary teams to help providers deal with dangerous and difficult patients. The implementation of the teams has reduced confrontation and litigation.28 If the impasse remains despite intervention, the physician should step aside after the patient’s care is transitioned to a provider that satisfies the needs of the patient.
In clinically emergent scenarios, ethics consultation or prolonged discussion may not be feasible. In such cases, the patient’s wishes should be honored and attempts should be made to receive permission for life-saving or limb-saving intervention. At large tertiary care centers, the wishes of the patient can be more easily granted than at an outlying facility or rural clinic. If the patient’s wishes cannot be respected in a life-or-death scenario and the patient continues to refuse care, the principle of patient autonomy dictates that no care can be provided. Much in the same way Jehovah’s Witnesses can refuse transfusion of blood products based upon their belief system, any patient can and should be allowed to freely refuse care from a provider.
Racism is a societal disease that is complex and multilayered, and it can be deeply entrenched in the minds of those afflicted and, thus, difficult to eradicate. The manifestations of bigotry in medical settings are only one example of a mindset that likely exists in multiple aspects of life. Hospitals and clinics can become a place to establish dialogue between racially intolerant patients and their providers, but they are not the venue where firmly held racist views can be expected to be wholly reversed. Having the objective to reverse prejudiced beliefs prior to providing care is discordant to the practice of medicine and can harm a patient if an unnecessary delay ensues. Although hospitals should try to avoid offending staff members, there should be an understanding that appropriate and timely patient care is the primary goal in medicine.29 As we move to a more multicultural society, it is the hope of the authors that these already infrequent racist encounters will continue to diminish, and that medical schools and residency programs will train physicians who are highly understanding and culturally competent.
1. Dimick J, Ruhter J, Sarrazin MV, Birkmeyer JD. Black patients more likely than whites to undergo surgery at low-quality hospitals in segregated regions. Health Aff (Millwood). 2013;32(6):1046-1053.
2. Kelaher MA, Ferdinand AS, Paradies Y. Experiencing racism in health care: the mental health impacts for Victorian Aboriginal communities. Med J Aust. 2014;201(1):44-47.
3. Johnson RL, Roter D, Powe NR, Cooper LA. Patient race/ethnicity and quality of patient-physician communication during medical visits. Am J Public Health. 2004;94(12):2084-2090.
4. US Public Health Service Syphilis Study at Tuskegee. Centers for Disease Control and Prevention website. http://www.cdc.gov/tuskegee. Updated December 30, 2013. Accessed October 27, 2015.
5. Fact Sheet on the 1946-1948 US Public Health Service Sexually Transmitted Diseases (STD) Inoculation Study. US Department of Health and Human Services website. http://www.hhs.gov/1946inoculationstudy/factsheet.html. Accessed October 27, 2015.
6. Inoue M, Tsukano K, Muraoka M, Kaneko F, Okamura H. Psychological impact of verbal abuse and violence by patients on nurses working in psychiatric departments. Psychiatry Clin Neurosci. 2006;60(1):29-36.
7. Jain SH. The racist patient. Ann Intern Med. 2013;158(8):632.
8. Chen PW. When the patient is racist. New York Times. July 25, 2013. http://well.blogs.nytimes.com/2013/07/25/when-the-patient-is-racist/?_php=true&_type=blogs&_php=true&_type=blogs&_r=1. Accessed October 27, 2015.
9. Castillo-Page L. Diversity in Medical Education: Facts & Figures 2012. Washington, DC: Association of American Medical Colleges; 2012:26-32. https://members.aamc.org/eweb/upload/Diversity%20in%20Medical%20Education_Facts%20and%20Figures%202012.pdf. Accessed October 27, 2015.
10. The patient-physician relationship. Opinion 10.015. Code of Medical Ethics. American Medical Association website. http://www.ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/opinion10015.page?. Issued December 2001. Accessed October 27, 2015.
11. Civil Rights Act of 1964, 42 US Code § 2000e (1964). US Government Printing Office website. http://www.gpo.gov/fdsys/pkg/USCODE-2011-title42/html/USCODE-2011-title42-chap21.htm. Accessed October 27, 2015.
12. Some hospitals grant patients’ racist requests. Houston Chronicle. February 23, 2013. http://www.chron.com/life/healthzone/article/Some-hospitals-grant-patients-racist-requests-4302145.php. Accessed October 27, 2015.
13. Prichard O. Three workers sue Abington Hospital over racist incident; supervisors obliged a 2003 demand for only white staff in a delivery. The suits follow a federal ruling. Philadelphia Inquirer. September 16, 2005. http://articles.philly.com/2005-09-16/news/25429798_1_nursing-racial-slur-obstetrical-resident. Accessed October 27, 2015.
14. Nurses told not to touch white patient. WNEM website. http://www.wnem.com/story/22911660/nurses-told-not-to-touch-white-patient. Published July 23, 2013. Updated August 20, 2013. Accessed October 27, 2015.
15. Kipnis K. Quality care and the wounds of diversity. In: Mappes T DD, ed. Biomedical Ethics. 6th ed. Boston, MA: McGraw-Hill; 2006.
16. Moghal N. Allowing patients to choose the ethnicity of attending doctors is institutional racism. BMJ. 2014;348:g265.
17. Saha S, Taggart SH, Komaromy M, Bindman AB. Do patients choose physicians of their own race? Health Aff (Millwood). 2000;19(4):76-83.
18. Malat J, van Ryn M. African-American preference for same-race healthcare providers: the role of healthcare discrimination. Ethnicity Dis. 2005;15(4):740-747.
19. LaVeist TA, Carroll T. Race of physician and satisfaction with care among African-American patients. J Natl Med Assoc. 2002;94(11):937-943.
20. Saha S, Komaromy M, Koepsell TD, Bindman AB. Patient-physician racial concordance and the perceived quality and use of health care. Arch Intern Med. 1999;159(9):997-1004.
21. Cooper-Patrick L, Gallo JJ, Gonzales JJ, et al. Race, gender, and partnership in the patient-physician relationship. JAMA. 1999;282(6):583-589.
22. Cooper LA, Roter DL, Johnson RL, Ford DE, Steinwachs DM, Powe NR. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003;139(11):907-915.
23. Garcia JA, Paterniti DA, Romano PS, Kravitz RL. Patient p for physician characteristics in university-based primary care clinics. Ethnicity Dis. 2003;13(2):259-267.
24. Padela AI, Schneider SM, He H, Ali Z, Richardson TM. Patient choice of provider type in the emergency department: perceptions and factors relating to accommodation of requests for care providers. Emerg Med J. 2010;27(6):465-469.
25. Paul-Emile K. Patients’ racial p and the medical culture of accommodation. UCLA Law Rev. 2012;60(2):462-504.
26. Selby M. Ethical dilemma: dealing with racist patients. BMJ. 1999;318(7191):1129.
27. Warshafsky RJ. Lack of support for staff to combat racism. BMJ. 2014;348:g1716.
28. Carlson MJ, Baker LH. Difficult, dangerous, and drug seeking: the 3D way to better patient care. Am J Public Health. 1998;88(8):1250-1252.
29. Lane-Fall M. A piece of my mind. Accommodating bigotry. JAMA. 2014;311(2):139-140
Medicine is not immune from the pervasive grasp of racism. It spills from other dimensions into the realm of healing and poses challenges to those charged with care of the patient. The literature widely documents racist experiences of patients, and differential treatment and health care disparities based on race.1,2 As a field, medicine is overshadowed by infamous experiments, such as the Tuskegee and Guatemala experiments, and routine studies that demonstrate poor treatment of minority patients.3-5 Although much-needed discussion and research is being done on the unfair treatment of patients, little is written about racist patients and their subsequent effect on health care providers and institutions. Such interactions can cause significant distress to providers, damage the therapeutic physician–patient relationship, and threaten the collegial and structural framework of an institution.6 The silent acquiescence to patients’ racist demands in recent times has become a legal, ethical, and medical dilemma that deserves attention.
No specific example of patient-generated racism is needed because most minority physicians have experienced an overtly racist interaction with a patient. The true incidence of these interactions is unknown because of underreporting secondary to the tendency of physicians to disregard this behavior in the name of “professionalism,” and because reporting of these incidents can sometimes expose how poorly a provider has dealt with the issue and draw admonishment.7 In addition to the overt interactions, numerous examples of subtle racism may exist. Manifestations of such subtleties include failure to cooperate with a history and physical examination, use of hostile language, and aggressive body language. The New York Times gives the example of an Asian female physician tending to a burly, unreceptive, swastika-tattooed patient.8 Such racist interactions are concerning, especially as diversity among newly practicing physicians increases.9
Medical Training
In medical school, students are educated to embody compassion and caring. Their care of patients should rise above the fray of poverty, interpersonal conflict, and prejudice.10 To further this point, medical school curricula have recently introduced standardized patients to teach empathy and simulate difficult encounters in order to help students learn to navigate interactions with aggressive, racist patients. In these scenarios, the patient quickly relinquishes his/her views after an overly understanding student engages the patient in conversation and addresses the source of their angst. Rarely do real-life scenarios play out in such an idealistic manner. The expectation remains, however, that the physician model extreme patience and understanding and honor the patient’s autonomy.
The American Medical Association (AMA), a guiding force in medical education, outlines the patient–physician relationship.10 Such a relationship is a mutually trusting undertaking in which the provider is the patient’s advocate and holds the well-being of the patient supreme. The goal is to alleviate suffering, and it should be done without regard to self-interest.10 The AMA also offers clear instruction to the physician in its code of medical ethics that the physician may not discriminate based on race, color, religion, national origin, sexual orientation, gender identity, or any other basis that would constitute invidious discrimination. With regard to the discriminatory practices of patients, the AMA instructs that “patients who use derogatory language or otherwise act in a prejudicial manner toward physicians, other health care professionals, or others in the health care setting, seriously undermine the integrity of the patient–physician relationship. Such behavior, if unmodified, may constitute sufficient justification for the physician to arrange for the transfer of care.”10 The AMA has also recently launched an online ethics journal, AMA Journal of Ethics, which explores difficult patient interactions and continues to reiterate the supreme role of the physician. When dealing with patients, the anti-discrimination policy is clearly set forth for physicians.
The Dilemma
Anti-discrimination policies for patients are not as clear. Patients are allowed to pick their own provider, and most institutions allow selection based on gender. Most institutions have no guidelines prohibiting provider-selection based on race, and no published hospital policies explicitly restrict racist demands. Although a culture of respect is encouraged through many hospitals’ published slogans and on websites, at the authors’ institution, no published guidelines exist about the behavior of the patient. When no such policies exist, differential treatment of patients’ racist requests ensues and frustration results. Legally, Title VII of the Civil Rights Act of 1964 bars all employers from discriminating with respect to employment conditions or terms on the basis of race, color, religion, sex, or national origin.11 Honoring a patient’s racist demands that results in discrimination of employees is a violation of that law. Reports of hospitals acceding to racist requests have often resulted in upset staff and lawsuits.12-14 Legal language, however, may be foreign in cases of life and death, or scenarios involving significant illness. Physicians in such cases often grant racist requests; for example, a Korean patient underwent life-saving measures only after he was given a non-Japanese provider, and a surgeon granted the wish of a patient’s husband to prohibit African American providers and staff members from entering the operating suite when his wife was undergoing an operation.15 Some would argue that granting a patient’s bigoted request is akin to institutionalized racism.16
The doctor–patient relationship is a powerful cornerstone for medicine. Confidence in the physician results in higher satisfaction for both parties and adherence to the treatment regimen on the part of the patient. Prejudiced interactions threaten the therapeutic alliance between patient and provider. Research has investigated how race plays a role in the doctor–patient relationship. When permitted, patients more often pick a provider of their own race.17 One of 5 African American patients wishes to have an African American provider, and such a desire is often based on a previous negative racist encounter.18 A patient’s perceptions of discrimination in general correlate with preference for same-race providers, highlighting that a patient’s overall experience with discrimination leads them to prefer a same-race physician. Race-concordant relationships (ie, one in which the provider and patient are of the same race) not only show increased satisfaction, but patients also perceive that their interactions with a racially similar physician are more participatory.19-22 In non–English speaking groups, preferences for racially similar physicians are largely based on language similarity, but Latinos feel that Latino physicians are more empathetic to their complaints.23 Such views are felt not only by patients, but also by providers. One of 3 physicians feels that patients receiving care from a physician that is of the patient’s own race is superior to that provided by a race-discordant physician.24 Superior outcomes from race-concordant doctor–patient relationships have led some to argue in favor of granting a patient’s wishes for a provider of similar race because doing so can confer additional health benefits.25
Possible Solutions
The solution to such a complex and uncomfortable issue begins with addressing the problem. Patients who make racist remarks and racist demands should be courteously informed that their behavior is inappropriate and hurtful. Failure to voice such a concern results in passive, tacit approval of racist remarks and can be distressing to other patients and staff members in the vicinity.26 It is unfair for a physician, as the leader of the care team, to ignore such behavior because it places staff members, who spend much more time with the patient, in a potentially abusive situation and leaves them feeling helpless.27 Toward this end, appropriate training, beginning in medical school and continuing in residency, in confronting racist patients is needed to ease the too often felt sense of discomfort among providers.7,26
Medical school, although rightly placing patient comfort at the center of dialogue, too often drowns out the personality of the student in the name of professionalism, which becomes a problem as a young physician struggles to reconcile his or her personality with the newly ingrained teaching to remain professional. This internal conflict can lead to frustration. A necessary prerequisite to beginning dialogue is that the physician recognizes his or her own emotional baggage from prior racially charged events and continues to remain professional. Airing the issue can help establish dialogue that can identify underlying causes of the patient’s misplaced anger. An illness and its subsequent hospitalization can make a patient feel vulnerable and helpless, and in those with poor coping mechanisms, misdirection of emotion is not uncommon.
In more difficult scenarios where attempts at dialogue reach an impasse, an ethics team should be consulted. Most institutions have such help available. Their expertise and experience can help in addressing the needs of the patient judiciously. Some institutions have dedicated multidisciplinary teams to help providers deal with dangerous and difficult patients. The implementation of the teams has reduced confrontation and litigation.28 If the impasse remains despite intervention, the physician should step aside after the patient’s care is transitioned to a provider that satisfies the needs of the patient.
In clinically emergent scenarios, ethics consultation or prolonged discussion may not be feasible. In such cases, the patient’s wishes should be honored and attempts should be made to receive permission for life-saving or limb-saving intervention. At large tertiary care centers, the wishes of the patient can be more easily granted than at an outlying facility or rural clinic. If the patient’s wishes cannot be respected in a life-or-death scenario and the patient continues to refuse care, the principle of patient autonomy dictates that no care can be provided. Much in the same way Jehovah’s Witnesses can refuse transfusion of blood products based upon their belief system, any patient can and should be allowed to freely refuse care from a provider.
Racism is a societal disease that is complex and multilayered, and it can be deeply entrenched in the minds of those afflicted and, thus, difficult to eradicate. The manifestations of bigotry in medical settings are only one example of a mindset that likely exists in multiple aspects of life. Hospitals and clinics can become a place to establish dialogue between racially intolerant patients and their providers, but they are not the venue where firmly held racist views can be expected to be wholly reversed. Having the objective to reverse prejudiced beliefs prior to providing care is discordant to the practice of medicine and can harm a patient if an unnecessary delay ensues. Although hospitals should try to avoid offending staff members, there should be an understanding that appropriate and timely patient care is the primary goal in medicine.29 As we move to a more multicultural society, it is the hope of the authors that these already infrequent racist encounters will continue to diminish, and that medical schools and residency programs will train physicians who are highly understanding and culturally competent.
Medicine is not immune from the pervasive grasp of racism. It spills from other dimensions into the realm of healing and poses challenges to those charged with care of the patient. The literature widely documents racist experiences of patients, and differential treatment and health care disparities based on race.1,2 As a field, medicine is overshadowed by infamous experiments, such as the Tuskegee and Guatemala experiments, and routine studies that demonstrate poor treatment of minority patients.3-5 Although much-needed discussion and research is being done on the unfair treatment of patients, little is written about racist patients and their subsequent effect on health care providers and institutions. Such interactions can cause significant distress to providers, damage the therapeutic physician–patient relationship, and threaten the collegial and structural framework of an institution.6 The silent acquiescence to patients’ racist demands in recent times has become a legal, ethical, and medical dilemma that deserves attention.
No specific example of patient-generated racism is needed because most minority physicians have experienced an overtly racist interaction with a patient. The true incidence of these interactions is unknown because of underreporting secondary to the tendency of physicians to disregard this behavior in the name of “professionalism,” and because reporting of these incidents can sometimes expose how poorly a provider has dealt with the issue and draw admonishment.7 In addition to the overt interactions, numerous examples of subtle racism may exist. Manifestations of such subtleties include failure to cooperate with a history and physical examination, use of hostile language, and aggressive body language. The New York Times gives the example of an Asian female physician tending to a burly, unreceptive, swastika-tattooed patient.8 Such racist interactions are concerning, especially as diversity among newly practicing physicians increases.9
Medical Training
In medical school, students are educated to embody compassion and caring. Their care of patients should rise above the fray of poverty, interpersonal conflict, and prejudice.10 To further this point, medical school curricula have recently introduced standardized patients to teach empathy and simulate difficult encounters in order to help students learn to navigate interactions with aggressive, racist patients. In these scenarios, the patient quickly relinquishes his/her views after an overly understanding student engages the patient in conversation and addresses the source of their angst. Rarely do real-life scenarios play out in such an idealistic manner. The expectation remains, however, that the physician model extreme patience and understanding and honor the patient’s autonomy.
The American Medical Association (AMA), a guiding force in medical education, outlines the patient–physician relationship.10 Such a relationship is a mutually trusting undertaking in which the provider is the patient’s advocate and holds the well-being of the patient supreme. The goal is to alleviate suffering, and it should be done without regard to self-interest.10 The AMA also offers clear instruction to the physician in its code of medical ethics that the physician may not discriminate based on race, color, religion, national origin, sexual orientation, gender identity, or any other basis that would constitute invidious discrimination. With regard to the discriminatory practices of patients, the AMA instructs that “patients who use derogatory language or otherwise act in a prejudicial manner toward physicians, other health care professionals, or others in the health care setting, seriously undermine the integrity of the patient–physician relationship. Such behavior, if unmodified, may constitute sufficient justification for the physician to arrange for the transfer of care.”10 The AMA has also recently launched an online ethics journal, AMA Journal of Ethics, which explores difficult patient interactions and continues to reiterate the supreme role of the physician. When dealing with patients, the anti-discrimination policy is clearly set forth for physicians.
The Dilemma
Anti-discrimination policies for patients are not as clear. Patients are allowed to pick their own provider, and most institutions allow selection based on gender. Most institutions have no guidelines prohibiting provider-selection based on race, and no published hospital policies explicitly restrict racist demands. Although a culture of respect is encouraged through many hospitals’ published slogans and on websites, at the authors’ institution, no published guidelines exist about the behavior of the patient. When no such policies exist, differential treatment of patients’ racist requests ensues and frustration results. Legally, Title VII of the Civil Rights Act of 1964 bars all employers from discriminating with respect to employment conditions or terms on the basis of race, color, religion, sex, or national origin.11 Honoring a patient’s racist demands that results in discrimination of employees is a violation of that law. Reports of hospitals acceding to racist requests have often resulted in upset staff and lawsuits.12-14 Legal language, however, may be foreign in cases of life and death, or scenarios involving significant illness. Physicians in such cases often grant racist requests; for example, a Korean patient underwent life-saving measures only after he was given a non-Japanese provider, and a surgeon granted the wish of a patient’s husband to prohibit African American providers and staff members from entering the operating suite when his wife was undergoing an operation.15 Some would argue that granting a patient’s bigoted request is akin to institutionalized racism.16
The doctor–patient relationship is a powerful cornerstone for medicine. Confidence in the physician results in higher satisfaction for both parties and adherence to the treatment regimen on the part of the patient. Prejudiced interactions threaten the therapeutic alliance between patient and provider. Research has investigated how race plays a role in the doctor–patient relationship. When permitted, patients more often pick a provider of their own race.17 One of 5 African American patients wishes to have an African American provider, and such a desire is often based on a previous negative racist encounter.18 A patient’s perceptions of discrimination in general correlate with preference for same-race providers, highlighting that a patient’s overall experience with discrimination leads them to prefer a same-race physician. Race-concordant relationships (ie, one in which the provider and patient are of the same race) not only show increased satisfaction, but patients also perceive that their interactions with a racially similar physician are more participatory.19-22 In non–English speaking groups, preferences for racially similar physicians are largely based on language similarity, but Latinos feel that Latino physicians are more empathetic to their complaints.23 Such views are felt not only by patients, but also by providers. One of 3 physicians feels that patients receiving care from a physician that is of the patient’s own race is superior to that provided by a race-discordant physician.24 Superior outcomes from race-concordant doctor–patient relationships have led some to argue in favor of granting a patient’s wishes for a provider of similar race because doing so can confer additional health benefits.25
Possible Solutions
The solution to such a complex and uncomfortable issue begins with addressing the problem. Patients who make racist remarks and racist demands should be courteously informed that their behavior is inappropriate and hurtful. Failure to voice such a concern results in passive, tacit approval of racist remarks and can be distressing to other patients and staff members in the vicinity.26 It is unfair for a physician, as the leader of the care team, to ignore such behavior because it places staff members, who spend much more time with the patient, in a potentially abusive situation and leaves them feeling helpless.27 Toward this end, appropriate training, beginning in medical school and continuing in residency, in confronting racist patients is needed to ease the too often felt sense of discomfort among providers.7,26
Medical school, although rightly placing patient comfort at the center of dialogue, too often drowns out the personality of the student in the name of professionalism, which becomes a problem as a young physician struggles to reconcile his or her personality with the newly ingrained teaching to remain professional. This internal conflict can lead to frustration. A necessary prerequisite to beginning dialogue is that the physician recognizes his or her own emotional baggage from prior racially charged events and continues to remain professional. Airing the issue can help establish dialogue that can identify underlying causes of the patient’s misplaced anger. An illness and its subsequent hospitalization can make a patient feel vulnerable and helpless, and in those with poor coping mechanisms, misdirection of emotion is not uncommon.
In more difficult scenarios where attempts at dialogue reach an impasse, an ethics team should be consulted. Most institutions have such help available. Their expertise and experience can help in addressing the needs of the patient judiciously. Some institutions have dedicated multidisciplinary teams to help providers deal with dangerous and difficult patients. The implementation of the teams has reduced confrontation and litigation.28 If the impasse remains despite intervention, the physician should step aside after the patient’s care is transitioned to a provider that satisfies the needs of the patient.
In clinically emergent scenarios, ethics consultation or prolonged discussion may not be feasible. In such cases, the patient’s wishes should be honored and attempts should be made to receive permission for life-saving or limb-saving intervention. At large tertiary care centers, the wishes of the patient can be more easily granted than at an outlying facility or rural clinic. If the patient’s wishes cannot be respected in a life-or-death scenario and the patient continues to refuse care, the principle of patient autonomy dictates that no care can be provided. Much in the same way Jehovah’s Witnesses can refuse transfusion of blood products based upon their belief system, any patient can and should be allowed to freely refuse care from a provider.
Racism is a societal disease that is complex and multilayered, and it can be deeply entrenched in the minds of those afflicted and, thus, difficult to eradicate. The manifestations of bigotry in medical settings are only one example of a mindset that likely exists in multiple aspects of life. Hospitals and clinics can become a place to establish dialogue between racially intolerant patients and their providers, but they are not the venue where firmly held racist views can be expected to be wholly reversed. Having the objective to reverse prejudiced beliefs prior to providing care is discordant to the practice of medicine and can harm a patient if an unnecessary delay ensues. Although hospitals should try to avoid offending staff members, there should be an understanding that appropriate and timely patient care is the primary goal in medicine.29 As we move to a more multicultural society, it is the hope of the authors that these already infrequent racist encounters will continue to diminish, and that medical schools and residency programs will train physicians who are highly understanding and culturally competent.
1. Dimick J, Ruhter J, Sarrazin MV, Birkmeyer JD. Black patients more likely than whites to undergo surgery at low-quality hospitals in segregated regions. Health Aff (Millwood). 2013;32(6):1046-1053.
2. Kelaher MA, Ferdinand AS, Paradies Y. Experiencing racism in health care: the mental health impacts for Victorian Aboriginal communities. Med J Aust. 2014;201(1):44-47.
3. Johnson RL, Roter D, Powe NR, Cooper LA. Patient race/ethnicity and quality of patient-physician communication during medical visits. Am J Public Health. 2004;94(12):2084-2090.
4. US Public Health Service Syphilis Study at Tuskegee. Centers for Disease Control and Prevention website. http://www.cdc.gov/tuskegee. Updated December 30, 2013. Accessed October 27, 2015.
5. Fact Sheet on the 1946-1948 US Public Health Service Sexually Transmitted Diseases (STD) Inoculation Study. US Department of Health and Human Services website. http://www.hhs.gov/1946inoculationstudy/factsheet.html. Accessed October 27, 2015.
6. Inoue M, Tsukano K, Muraoka M, Kaneko F, Okamura H. Psychological impact of verbal abuse and violence by patients on nurses working in psychiatric departments. Psychiatry Clin Neurosci. 2006;60(1):29-36.
7. Jain SH. The racist patient. Ann Intern Med. 2013;158(8):632.
8. Chen PW. When the patient is racist. New York Times. July 25, 2013. http://well.blogs.nytimes.com/2013/07/25/when-the-patient-is-racist/?_php=true&_type=blogs&_php=true&_type=blogs&_r=1. Accessed October 27, 2015.
9. Castillo-Page L. Diversity in Medical Education: Facts & Figures 2012. Washington, DC: Association of American Medical Colleges; 2012:26-32. https://members.aamc.org/eweb/upload/Diversity%20in%20Medical%20Education_Facts%20and%20Figures%202012.pdf. Accessed October 27, 2015.
10. The patient-physician relationship. Opinion 10.015. Code of Medical Ethics. American Medical Association website. http://www.ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/opinion10015.page?. Issued December 2001. Accessed October 27, 2015.
11. Civil Rights Act of 1964, 42 US Code § 2000e (1964). US Government Printing Office website. http://www.gpo.gov/fdsys/pkg/USCODE-2011-title42/html/USCODE-2011-title42-chap21.htm. Accessed October 27, 2015.
12. Some hospitals grant patients’ racist requests. Houston Chronicle. February 23, 2013. http://www.chron.com/life/healthzone/article/Some-hospitals-grant-patients-racist-requests-4302145.php. Accessed October 27, 2015.
13. Prichard O. Three workers sue Abington Hospital over racist incident; supervisors obliged a 2003 demand for only white staff in a delivery. The suits follow a federal ruling. Philadelphia Inquirer. September 16, 2005. http://articles.philly.com/2005-09-16/news/25429798_1_nursing-racial-slur-obstetrical-resident. Accessed October 27, 2015.
14. Nurses told not to touch white patient. WNEM website. http://www.wnem.com/story/22911660/nurses-told-not-to-touch-white-patient. Published July 23, 2013. Updated August 20, 2013. Accessed October 27, 2015.
15. Kipnis K. Quality care and the wounds of diversity. In: Mappes T DD, ed. Biomedical Ethics. 6th ed. Boston, MA: McGraw-Hill; 2006.
16. Moghal N. Allowing patients to choose the ethnicity of attending doctors is institutional racism. BMJ. 2014;348:g265.
17. Saha S, Taggart SH, Komaromy M, Bindman AB. Do patients choose physicians of their own race? Health Aff (Millwood). 2000;19(4):76-83.
18. Malat J, van Ryn M. African-American preference for same-race healthcare providers: the role of healthcare discrimination. Ethnicity Dis. 2005;15(4):740-747.
19. LaVeist TA, Carroll T. Race of physician and satisfaction with care among African-American patients. J Natl Med Assoc. 2002;94(11):937-943.
20. Saha S, Komaromy M, Koepsell TD, Bindman AB. Patient-physician racial concordance and the perceived quality and use of health care. Arch Intern Med. 1999;159(9):997-1004.
21. Cooper-Patrick L, Gallo JJ, Gonzales JJ, et al. Race, gender, and partnership in the patient-physician relationship. JAMA. 1999;282(6):583-589.
22. Cooper LA, Roter DL, Johnson RL, Ford DE, Steinwachs DM, Powe NR. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003;139(11):907-915.
23. Garcia JA, Paterniti DA, Romano PS, Kravitz RL. Patient p for physician characteristics in university-based primary care clinics. Ethnicity Dis. 2003;13(2):259-267.
24. Padela AI, Schneider SM, He H, Ali Z, Richardson TM. Patient choice of provider type in the emergency department: perceptions and factors relating to accommodation of requests for care providers. Emerg Med J. 2010;27(6):465-469.
25. Paul-Emile K. Patients’ racial p and the medical culture of accommodation. UCLA Law Rev. 2012;60(2):462-504.
26. Selby M. Ethical dilemma: dealing with racist patients. BMJ. 1999;318(7191):1129.
27. Warshafsky RJ. Lack of support for staff to combat racism. BMJ. 2014;348:g1716.
28. Carlson MJ, Baker LH. Difficult, dangerous, and drug seeking: the 3D way to better patient care. Am J Public Health. 1998;88(8):1250-1252.
29. Lane-Fall M. A piece of my mind. Accommodating bigotry. JAMA. 2014;311(2):139-140
1. Dimick J, Ruhter J, Sarrazin MV, Birkmeyer JD. Black patients more likely than whites to undergo surgery at low-quality hospitals in segregated regions. Health Aff (Millwood). 2013;32(6):1046-1053.
2. Kelaher MA, Ferdinand AS, Paradies Y. Experiencing racism in health care: the mental health impacts for Victorian Aboriginal communities. Med J Aust. 2014;201(1):44-47.
3. Johnson RL, Roter D, Powe NR, Cooper LA. Patient race/ethnicity and quality of patient-physician communication during medical visits. Am J Public Health. 2004;94(12):2084-2090.
4. US Public Health Service Syphilis Study at Tuskegee. Centers for Disease Control and Prevention website. http://www.cdc.gov/tuskegee. Updated December 30, 2013. Accessed October 27, 2015.
5. Fact Sheet on the 1946-1948 US Public Health Service Sexually Transmitted Diseases (STD) Inoculation Study. US Department of Health and Human Services website. http://www.hhs.gov/1946inoculationstudy/factsheet.html. Accessed October 27, 2015.
6. Inoue M, Tsukano K, Muraoka M, Kaneko F, Okamura H. Psychological impact of verbal abuse and violence by patients on nurses working in psychiatric departments. Psychiatry Clin Neurosci. 2006;60(1):29-36.
7. Jain SH. The racist patient. Ann Intern Med. 2013;158(8):632.
8. Chen PW. When the patient is racist. New York Times. July 25, 2013. http://well.blogs.nytimes.com/2013/07/25/when-the-patient-is-racist/?_php=true&_type=blogs&_php=true&_type=blogs&_r=1. Accessed October 27, 2015.
9. Castillo-Page L. Diversity in Medical Education: Facts & Figures 2012. Washington, DC: Association of American Medical Colleges; 2012:26-32. https://members.aamc.org/eweb/upload/Diversity%20in%20Medical%20Education_Facts%20and%20Figures%202012.pdf. Accessed October 27, 2015.
10. The patient-physician relationship. Opinion 10.015. Code of Medical Ethics. American Medical Association website. http://www.ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/opinion10015.page?. Issued December 2001. Accessed October 27, 2015.
11. Civil Rights Act of 1964, 42 US Code § 2000e (1964). US Government Printing Office website. http://www.gpo.gov/fdsys/pkg/USCODE-2011-title42/html/USCODE-2011-title42-chap21.htm. Accessed October 27, 2015.
12. Some hospitals grant patients’ racist requests. Houston Chronicle. February 23, 2013. http://www.chron.com/life/healthzone/article/Some-hospitals-grant-patients-racist-requests-4302145.php. Accessed October 27, 2015.
13. Prichard O. Three workers sue Abington Hospital over racist incident; supervisors obliged a 2003 demand for only white staff in a delivery. The suits follow a federal ruling. Philadelphia Inquirer. September 16, 2005. http://articles.philly.com/2005-09-16/news/25429798_1_nursing-racial-slur-obstetrical-resident. Accessed October 27, 2015.
14. Nurses told not to touch white patient. WNEM website. http://www.wnem.com/story/22911660/nurses-told-not-to-touch-white-patient. Published July 23, 2013. Updated August 20, 2013. Accessed October 27, 2015.
15. Kipnis K. Quality care and the wounds of diversity. In: Mappes T DD, ed. Biomedical Ethics. 6th ed. Boston, MA: McGraw-Hill; 2006.
16. Moghal N. Allowing patients to choose the ethnicity of attending doctors is institutional racism. BMJ. 2014;348:g265.
17. Saha S, Taggart SH, Komaromy M, Bindman AB. Do patients choose physicians of their own race? Health Aff (Millwood). 2000;19(4):76-83.
18. Malat J, van Ryn M. African-American preference for same-race healthcare providers: the role of healthcare discrimination. Ethnicity Dis. 2005;15(4):740-747.
19. LaVeist TA, Carroll T. Race of physician and satisfaction with care among African-American patients. J Natl Med Assoc. 2002;94(11):937-943.
20. Saha S, Komaromy M, Koepsell TD, Bindman AB. Patient-physician racial concordance and the perceived quality and use of health care. Arch Intern Med. 1999;159(9):997-1004.
21. Cooper-Patrick L, Gallo JJ, Gonzales JJ, et al. Race, gender, and partnership in the patient-physician relationship. JAMA. 1999;282(6):583-589.
22. Cooper LA, Roter DL, Johnson RL, Ford DE, Steinwachs DM, Powe NR. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003;139(11):907-915.
23. Garcia JA, Paterniti DA, Romano PS, Kravitz RL. Patient p for physician characteristics in university-based primary care clinics. Ethnicity Dis. 2003;13(2):259-267.
24. Padela AI, Schneider SM, He H, Ali Z, Richardson TM. Patient choice of provider type in the emergency department: perceptions and factors relating to accommodation of requests for care providers. Emerg Med J. 2010;27(6):465-469.
25. Paul-Emile K. Patients’ racial p and the medical culture of accommodation. UCLA Law Rev. 2012;60(2):462-504.
26. Selby M. Ethical dilemma: dealing with racist patients. BMJ. 1999;318(7191):1129.
27. Warshafsky RJ. Lack of support for staff to combat racism. BMJ. 2014;348:g1716.
28. Carlson MJ, Baker LH. Difficult, dangerous, and drug seeking: the 3D way to better patient care. Am J Public Health. 1998;88(8):1250-1252.
29. Lane-Fall M. A piece of my mind. Accommodating bigotry. JAMA. 2014;311(2):139-140
Incidence and Functional Outcomes of Malunion of Nonoperatively Treated Humeral Shaft Fractures
Humeral shaft fractures account for about 1% of all fractures.1 With the exception of the few absolute indications for surgical intervention, such as the presence of an open fracture, the current teaching on treatment of these fractures is that the majority can be successfully managed nonoperatively.1-3 These conservative measures consist of bandages, abduction splints, U-casts, hanging arm casts, and, most commonly, functional bracing, which is considered the gold standard for treatment of humeral shaft fractures by many authors.1-3 One of the most often cited disadvantages of nonoperative management over surgical treatment is the higher incidence of residual deformity, the most common of which is varus angulation.4
The incidence of malunion (>20° of angulation in any plane or shortening of ≥2.5 cm) after nonoperative treatment varies in the literature from 0% to 13%,2,4-9 with a recent literature review documenting a mean incidence of 4.4% within the frontal plane and 2% within the sagittal plane across all studies.2 As reported initially by Sarmiento and colleagues3,9 and echoed by other authors,2,5,8 angular deformity of less than 20° is thought to be both cosmetically and functionally acceptable. Whether angular deformities or malunion of more than 20° actually leads to functional limitations is unknown. Although some observational reports suggest that the degree of radiographic malalignment does not necessarily correlate with functional outcome,8 no studies have specifically evaluated patient outcomes of humeral shaft fracture malunions.
We conducted a study to determine the overall incidence and long-term clinical and functional outcomes of patients with malunion after nonoperative management of humeral shaft fractures. Long-term outcomes were assessed with current symptoms, physical examination findings, need for subsequent operative intervention, DASH (Disabilities of the Arm, Shoulder, and Hand) scores, and a self-reported questionnaire. We hypothesized that patients who develop a malunion after nonoperative treatment of a closed humeral shaft fracture will have satisfactory functional outcomes based on subjective reports, physical examination findings, and DASH scores.
Methods
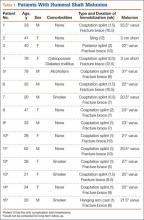
After obtaining institutional review board approval for the study, we selected patients from a retrospective medical record review of all those 18 years or older with a humeral shaft fracture managed nonoperatively at our institution between January 1, 2001, and June 30, 2012, with a minimum 1-year follow-up. We identified 156 patients with nonoperatively managed midshaft humerus fractures. Study exclusion criteria included fracture associated with a tumor (3 patients), ipsilateral upper extremity injury (9), open/ballistic injury (18), nonunion (9), underlying cognitive disability or psychiatric illness (4), and insufficient follow-up to clinical or radiographic healing (22). Ninety-one patients were eligible for study inclusion. Radiographs at time of final clinical visit were reviewed to assess for evidence of malunion at the fracture site, as defined by previously reported criteria3 (>20° angulation in anterior/posterior or varus/valgus plane of motion or shortening of ≥2.5 cm). Fifteen patients met all the inclusion criteria for further evaluation.
Medical records were retrospectively reviewed for information on age at injury, sex, comorbidities (eg, diabetes, osteoporosis, smoking), body mass index, type and duration of immobilization, complications, return to work, cosmetic perception, time to final clinical follow-up, and symptoms at final clinical follow-up. Incidence of potential risk factors associated with malunion—obesity, noncompliance, and comorbidities such as smoking and diabetes—was compared between the 15 patients with malunion and the other study patients, who healed without malunion.
For long-term postoperative follow-up, patients were contacted to be seen in clinic to complete an updated physical examination, self-reported questionnaire, and the DASH form. Physical examination included measurements of range of motion (ROM) and strength involving the shoulder, elbow, and forearm, with ROM reported as the difference between the injured and contralateral upper extremities. Neurovascular status and focal tenderness to palpation were also assessed on examination. When in-person examination was not possible, the questionnaire and DASH form were completed over the telephone. The self-reported questionnaire asked for information on smoking status, pain, functional limitations, cosmetic perception, satisfaction, and whether or not the patient would still opt for nonoperative management if presented with the same injury again. Pain and satisfaction were measured on numerical scales: Pain scores ranged from 0 (no pain) to 10 (worst possible pain), and satisfaction scores ranged from 1 (not satisfied) to 5 (very satisfied). Data are presented as mean values.
Results
Of the 91 study-eligible patients, 15 (16%) met the radiographic criteria for the diagnosis of malunion. Retrospective data were available for all 15 patients from time of injury to final clinical follow-up (mean, 19 weeks; range, 7-53 weeks). Mean age at injury was 39 years (range, 20-79 years). Additional demographics are listed in Table 1. Incidence of potential risk factors, such as body mass index (26.5 vs 25.4), smoking (33% vs 33%), and diabetes (0% vs 8%), was not significantly different between the malunion and healed-without-malunion groups, respectively. Furthermore, all malunion patients were compliant with their treatment protocol.
Radiographs were assessed at time of final follow-up to confirm healing and to document malunion. Varus malunion was found in 13 patients (mean, 24°; range, 20.5°-35.5°), and shortening was documented in the other 2 patients (mean, 4 cm; range, 3-5 cm). Patients were immobilized a mean of 10 weeks (range, 6-13 weeks). Initial fracture management consisted of coaptation splinting for 1 to 2 weeks (12 patients), hanging arm cast for 1 week (1 patient), and posterior splint for 1 week (1 patient). Patients were then transitioned to Sarmiento fracture bracing for the duration of their treatment (range, 5-12 months). One patient, followed initially at an outside institution, was managed in a sling throughout the duration of treatment (12 weeks) (Table 1). All 15 patients were neurovascularly intact at time of final clinical examination, with return of full upper extremity ROM in all but 3 patients. Only 1 of these 3 patients reported residual pain and functional limitations 4 months after injury (Table 2). Twelve patients were evaluated for return to work, with all successfully returning to work without restrictions at time of final follow-up. The 1 minor complication noted during the treatment period involved medial-sided elbow skin breakdown from brace wear, which resolved with local wound care. No patient required or requested surgical intervention for their residual malunion.
Of the 15 patients, 8 (53%) were reached for in-person examination (6 patients) or telephone interview (2 patients) for follow-up assessment by means of DASH form and self-reported questionnaire a mean of 47 months (range, 12-99 months) after initial injury. The 6 patients who had a physical examination were neurovascularly intact, lacked focal tenderness to palpation, and demonstrated full (5/5) strength within the deltoid, biceps, triceps, pronator, and supinator musculature. Each patient had equal ROM compared with the contralateral uninjured extremity on shoulder forward flexion and abduction, elbow flexion and extension, and forearm pronation and supination. Three patients (50%) had mild residual loss of ROM, with 2 demonstrating decreased shoulder external rotation of 10° and 15°, respectively, and 1 demonstrating decreased shoulder internal rotation of 10°.
Mean DASH score was 10.4 (range, 0-49.2). Evaluation of the self-reported questionnaire revealed a mean pain score of 1.1 (range, 0-7), with only 2 patients reporting any ongoing pain. In addition, 2 patients reported functional limitations, both related to overhead activities. However, 6 (75%) of the 8 patients reported noticeable cosmetic deformity, most commonly varus angulation (4 patients), as well as palpable bony prominence (2) and muscle atrophy (1). The majority of patients were satisfied with the outcome of their treatment (mean, 4; range, 2-5), with 6 patients reporting being satisfied or very satisfied, and all 6 indicating they would undergo nonoperative management again if presented with the same injury. Two patients reported being dissatisfied with their outcome, 1 because of cosmetic appearance and 1 because of cosmetic appearance and functional limitations. Both patients indicated they would choose operative management if presented with the same injury. There was no apparent relationship between outcome and degree of residual deformity, as both patients with varus angulation of more than 30° reported no residual pain or functional limitation and were very satisfied with the outcome of their treatment (Table 2).
Of the 7 patients who could not be reached for final follow-up, 2 on initial contact expressed overall satisfaction with their outcome and denied functional limitations. However, both asked to complete the study at a later date. Subsequently, these 2 patients could not be reached to complete the formal follow-up.
Discussion
Humeral shaft fractures are usually managed nonoperatively. One of the most commonly cited disadvantages of nonoperative management is its higher incidence of residual angular deformity, up to 13% in previous studies.4 Our study found a slightly higher incidence, 16%, on review of 91 nonoperatively managed humeral shaft fractures treated over an 11.5 year period. Although previous studies have reported acceptable functional and cosmetic outcomes with residual angular deformity of less than 20°,2,3,5,8,9 only observational reports have suggested acceptable function in patients with a documented malunion.8
To our knowledge, ours is the first study to correlate malunion with functional parameters and subjective patient-reported outcomes. We found that malunion was not associated with significant pain or functional limitation after nonoperative management of humeral shaft fractures. Furthermore, 75% of patients were satisfied or very satisfied with the outcome of their treatment and indicated they would undergo nonoperative management if presented with the same injury again. However, 75% of patients reported a noticeable cosmetic deformity, and one-third of these patients cited it as a major reason for dissatisfaction with their overall outcome. Regarding function, all patients returned to full strength and ROM of the affected extremity, aside from small losses of internal or external shoulder rotation on the magnitude of 10° to 15° in 50% of those patients tested. In addition, 75% of patients returned to regular activity without functional limitations; the other 25% reported trouble with overhead activities. There were no significant complications during the treatment or follow-up period, once the fracture had healed.
The major limitation of this study was its small patient population. (Obtaining a larger series of patients with malunion after nonoperative treatment of humeral shaft fractures likely would require a multicenter study.) Some of our study findings, such as lack of correlation between degree of malunion and subsequent functional or subjective outcomes, would require a larger sample size for verification and more definitive conclusions. Another limitation is that the study was not designed to evaluate the cause of malunion. Therefore, we cannot draw any definitive conclusions regarding what may have contributed to the development of malunion in our study population. However, all our malunion patients were compliant with their treatment protocol, and they showed no significant difference in incidence of potential risk factors (eg, obesity, comorbidities) compared with the patients who healed without malunion.
Conclusion
Malunion after nonoperative management of humeral shaft fractures does not appear to result in significant pain, dissatisfaction, or functional limitation as measured on physical examination and with validated objective outcome measures in the majority of patients. Furthermore, no patients in this study required surgical intervention for any residual limitations or complications after malunion. The majority of patients reported a noticeable cosmetic deformity, which left a small subset of patients dissatisfied. Overall, our study findings can be used to help counsel patients before and during nonoperative management—particularly patients who appear to be healing with some malunion. Our findings suggest that operative intervention to prevent malunion is not necessary, as it likely would not result in any overall improvement in patient function or satisfaction, but patients should be counseled regarding the high likelihood of cosmetic deformity, which may or may not be bothersome.
1. Rockwood CA, Green DP, Bucholz RW, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010.
2. Papasoulis E, Drosos GI, Ververidis AN, Verettas DA. Functional bracing of humeral shaft fractures. A review of clinical studies. Injury. 2010;41(7):e21-e27.
3. Sarmiento A, Latta LL. Functional fracture bracing. J Am Acad Orthop Surg. 1999;7(1):66-75.
4. Denard A Jr, Richards JE, Obremskey WT, Tucker MC, Floyd M, Herzog GA. Outcome of nonoperative vs operative treatment of humeral shaft fractures: a retrospective study of 213 patients. Orthopedics. 2010;33(8).
5. Fjalestad T, Strømsøe K, Salvesen P, Rostad B. Functional results of braced humeral diaphyseal fractures: why do 38% lose external rotation of the shoulder? Arch Orthop Trauma Surg. 2000;120(5-6):281-285.
6. Koch PP, Gross DF, Gerber C. The results of functional (Sarmiento) bracing of humeral shaft fractures. J Shoulder Elbow Surg. 2002;11(2):143-150.
7. Ozkurt B, Altay M, Aktekin CN, Toprak A, Tabak Y. The role of functional bracing in the treatment of humeral shaft fractures [in Turkish]. Acta Orthop Traumatol Turc. 2007;41(1):15-20.
8. Rutgers M, Ring D. Treatment of diaphyseal fractures of the humerus using a functional brace. J Orthop Trauma. 2006;20(9):597-601.
9. Sarmiento A, Kinman PB, Galvin EG, Schmitt RH, Phillips JG. Functional bracing of fractures of the shaft of the humerus. J Bone Joint Surg Am. 1977;59(5):596-601.
Humeral shaft fractures account for about 1% of all fractures.1 With the exception of the few absolute indications for surgical intervention, such as the presence of an open fracture, the current teaching on treatment of these fractures is that the majority can be successfully managed nonoperatively.1-3 These conservative measures consist of bandages, abduction splints, U-casts, hanging arm casts, and, most commonly, functional bracing, which is considered the gold standard for treatment of humeral shaft fractures by many authors.1-3 One of the most often cited disadvantages of nonoperative management over surgical treatment is the higher incidence of residual deformity, the most common of which is varus angulation.4
The incidence of malunion (>20° of angulation in any plane or shortening of ≥2.5 cm) after nonoperative treatment varies in the literature from 0% to 13%,2,4-9 with a recent literature review documenting a mean incidence of 4.4% within the frontal plane and 2% within the sagittal plane across all studies.2 As reported initially by Sarmiento and colleagues3,9 and echoed by other authors,2,5,8 angular deformity of less than 20° is thought to be both cosmetically and functionally acceptable. Whether angular deformities or malunion of more than 20° actually leads to functional limitations is unknown. Although some observational reports suggest that the degree of radiographic malalignment does not necessarily correlate with functional outcome,8 no studies have specifically evaluated patient outcomes of humeral shaft fracture malunions.
We conducted a study to determine the overall incidence and long-term clinical and functional outcomes of patients with malunion after nonoperative management of humeral shaft fractures. Long-term outcomes were assessed with current symptoms, physical examination findings, need for subsequent operative intervention, DASH (Disabilities of the Arm, Shoulder, and Hand) scores, and a self-reported questionnaire. We hypothesized that patients who develop a malunion after nonoperative treatment of a closed humeral shaft fracture will have satisfactory functional outcomes based on subjective reports, physical examination findings, and DASH scores.
Methods
After obtaining institutional review board approval for the study, we selected patients from a retrospective medical record review of all those 18 years or older with a humeral shaft fracture managed nonoperatively at our institution between January 1, 2001, and June 30, 2012, with a minimum 1-year follow-up. We identified 156 patients with nonoperatively managed midshaft humerus fractures. Study exclusion criteria included fracture associated with a tumor (3 patients), ipsilateral upper extremity injury (9), open/ballistic injury (18), nonunion (9), underlying cognitive disability or psychiatric illness (4), and insufficient follow-up to clinical or radiographic healing (22). Ninety-one patients were eligible for study inclusion. Radiographs at time of final clinical visit were reviewed to assess for evidence of malunion at the fracture site, as defined by previously reported criteria3 (>20° angulation in anterior/posterior or varus/valgus plane of motion or shortening of ≥2.5 cm). Fifteen patients met all the inclusion criteria for further evaluation.
Medical records were retrospectively reviewed for information on age at injury, sex, comorbidities (eg, diabetes, osteoporosis, smoking), body mass index, type and duration of immobilization, complications, return to work, cosmetic perception, time to final clinical follow-up, and symptoms at final clinical follow-up. Incidence of potential risk factors associated with malunion—obesity, noncompliance, and comorbidities such as smoking and diabetes—was compared between the 15 patients with malunion and the other study patients, who healed without malunion.
For long-term postoperative follow-up, patients were contacted to be seen in clinic to complete an updated physical examination, self-reported questionnaire, and the DASH form. Physical examination included measurements of range of motion (ROM) and strength involving the shoulder, elbow, and forearm, with ROM reported as the difference between the injured and contralateral upper extremities. Neurovascular status and focal tenderness to palpation were also assessed on examination. When in-person examination was not possible, the questionnaire and DASH form were completed over the telephone. The self-reported questionnaire asked for information on smoking status, pain, functional limitations, cosmetic perception, satisfaction, and whether or not the patient would still opt for nonoperative management if presented with the same injury again. Pain and satisfaction were measured on numerical scales: Pain scores ranged from 0 (no pain) to 10 (worst possible pain), and satisfaction scores ranged from 1 (not satisfied) to 5 (very satisfied). Data are presented as mean values.
Results
Of the 91 study-eligible patients, 15 (16%) met the radiographic criteria for the diagnosis of malunion. Retrospective data were available for all 15 patients from time of injury to final clinical follow-up (mean, 19 weeks; range, 7-53 weeks). Mean age at injury was 39 years (range, 20-79 years). Additional demographics are listed in Table 1. Incidence of potential risk factors, such as body mass index (26.5 vs 25.4), smoking (33% vs 33%), and diabetes (0% vs 8%), was not significantly different between the malunion and healed-without-malunion groups, respectively. Furthermore, all malunion patients were compliant with their treatment protocol.
Radiographs were assessed at time of final follow-up to confirm healing and to document malunion. Varus malunion was found in 13 patients (mean, 24°; range, 20.5°-35.5°), and shortening was documented in the other 2 patients (mean, 4 cm; range, 3-5 cm). Patients were immobilized a mean of 10 weeks (range, 6-13 weeks). Initial fracture management consisted of coaptation splinting for 1 to 2 weeks (12 patients), hanging arm cast for 1 week (1 patient), and posterior splint for 1 week (1 patient). Patients were then transitioned to Sarmiento fracture bracing for the duration of their treatment (range, 5-12 months). One patient, followed initially at an outside institution, was managed in a sling throughout the duration of treatment (12 weeks) (Table 1). All 15 patients were neurovascularly intact at time of final clinical examination, with return of full upper extremity ROM in all but 3 patients. Only 1 of these 3 patients reported residual pain and functional limitations 4 months after injury (Table 2). Twelve patients were evaluated for return to work, with all successfully returning to work without restrictions at time of final follow-up. The 1 minor complication noted during the treatment period involved medial-sided elbow skin breakdown from brace wear, which resolved with local wound care. No patient required or requested surgical intervention for their residual malunion.
Of the 15 patients, 8 (53%) were reached for in-person examination (6 patients) or telephone interview (2 patients) for follow-up assessment by means of DASH form and self-reported questionnaire a mean of 47 months (range, 12-99 months) after initial injury. The 6 patients who had a physical examination were neurovascularly intact, lacked focal tenderness to palpation, and demonstrated full (5/5) strength within the deltoid, biceps, triceps, pronator, and supinator musculature. Each patient had equal ROM compared with the contralateral uninjured extremity on shoulder forward flexion and abduction, elbow flexion and extension, and forearm pronation and supination. Three patients (50%) had mild residual loss of ROM, with 2 demonstrating decreased shoulder external rotation of 10° and 15°, respectively, and 1 demonstrating decreased shoulder internal rotation of 10°.
Mean DASH score was 10.4 (range, 0-49.2). Evaluation of the self-reported questionnaire revealed a mean pain score of 1.1 (range, 0-7), with only 2 patients reporting any ongoing pain. In addition, 2 patients reported functional limitations, both related to overhead activities. However, 6 (75%) of the 8 patients reported noticeable cosmetic deformity, most commonly varus angulation (4 patients), as well as palpable bony prominence (2) and muscle atrophy (1). The majority of patients were satisfied with the outcome of their treatment (mean, 4; range, 2-5), with 6 patients reporting being satisfied or very satisfied, and all 6 indicating they would undergo nonoperative management again if presented with the same injury. Two patients reported being dissatisfied with their outcome, 1 because of cosmetic appearance and 1 because of cosmetic appearance and functional limitations. Both patients indicated they would choose operative management if presented with the same injury. There was no apparent relationship between outcome and degree of residual deformity, as both patients with varus angulation of more than 30° reported no residual pain or functional limitation and were very satisfied with the outcome of their treatment (Table 2).
Of the 7 patients who could not be reached for final follow-up, 2 on initial contact expressed overall satisfaction with their outcome and denied functional limitations. However, both asked to complete the study at a later date. Subsequently, these 2 patients could not be reached to complete the formal follow-up.
Discussion
Humeral shaft fractures are usually managed nonoperatively. One of the most commonly cited disadvantages of nonoperative management is its higher incidence of residual angular deformity, up to 13% in previous studies.4 Our study found a slightly higher incidence, 16%, on review of 91 nonoperatively managed humeral shaft fractures treated over an 11.5 year period. Although previous studies have reported acceptable functional and cosmetic outcomes with residual angular deformity of less than 20°,2,3,5,8,9 only observational reports have suggested acceptable function in patients with a documented malunion.8
To our knowledge, ours is the first study to correlate malunion with functional parameters and subjective patient-reported outcomes. We found that malunion was not associated with significant pain or functional limitation after nonoperative management of humeral shaft fractures. Furthermore, 75% of patients were satisfied or very satisfied with the outcome of their treatment and indicated they would undergo nonoperative management if presented with the same injury again. However, 75% of patients reported a noticeable cosmetic deformity, and one-third of these patients cited it as a major reason for dissatisfaction with their overall outcome. Regarding function, all patients returned to full strength and ROM of the affected extremity, aside from small losses of internal or external shoulder rotation on the magnitude of 10° to 15° in 50% of those patients tested. In addition, 75% of patients returned to regular activity without functional limitations; the other 25% reported trouble with overhead activities. There were no significant complications during the treatment or follow-up period, once the fracture had healed.
The major limitation of this study was its small patient population. (Obtaining a larger series of patients with malunion after nonoperative treatment of humeral shaft fractures likely would require a multicenter study.) Some of our study findings, such as lack of correlation between degree of malunion and subsequent functional or subjective outcomes, would require a larger sample size for verification and more definitive conclusions. Another limitation is that the study was not designed to evaluate the cause of malunion. Therefore, we cannot draw any definitive conclusions regarding what may have contributed to the development of malunion in our study population. However, all our malunion patients were compliant with their treatment protocol, and they showed no significant difference in incidence of potential risk factors (eg, obesity, comorbidities) compared with the patients who healed without malunion.
Conclusion
Malunion after nonoperative management of humeral shaft fractures does not appear to result in significant pain, dissatisfaction, or functional limitation as measured on physical examination and with validated objective outcome measures in the majority of patients. Furthermore, no patients in this study required surgical intervention for any residual limitations or complications after malunion. The majority of patients reported a noticeable cosmetic deformity, which left a small subset of patients dissatisfied. Overall, our study findings can be used to help counsel patients before and during nonoperative management—particularly patients who appear to be healing with some malunion. Our findings suggest that operative intervention to prevent malunion is not necessary, as it likely would not result in any overall improvement in patient function or satisfaction, but patients should be counseled regarding the high likelihood of cosmetic deformity, which may or may not be bothersome.
Humeral shaft fractures account for about 1% of all fractures.1 With the exception of the few absolute indications for surgical intervention, such as the presence of an open fracture, the current teaching on treatment of these fractures is that the majority can be successfully managed nonoperatively.1-3 These conservative measures consist of bandages, abduction splints, U-casts, hanging arm casts, and, most commonly, functional bracing, which is considered the gold standard for treatment of humeral shaft fractures by many authors.1-3 One of the most often cited disadvantages of nonoperative management over surgical treatment is the higher incidence of residual deformity, the most common of which is varus angulation.4
The incidence of malunion (>20° of angulation in any plane or shortening of ≥2.5 cm) after nonoperative treatment varies in the literature from 0% to 13%,2,4-9 with a recent literature review documenting a mean incidence of 4.4% within the frontal plane and 2% within the sagittal plane across all studies.2 As reported initially by Sarmiento and colleagues3,9 and echoed by other authors,2,5,8 angular deformity of less than 20° is thought to be both cosmetically and functionally acceptable. Whether angular deformities or malunion of more than 20° actually leads to functional limitations is unknown. Although some observational reports suggest that the degree of radiographic malalignment does not necessarily correlate with functional outcome,8 no studies have specifically evaluated patient outcomes of humeral shaft fracture malunions.
We conducted a study to determine the overall incidence and long-term clinical and functional outcomes of patients with malunion after nonoperative management of humeral shaft fractures. Long-term outcomes were assessed with current symptoms, physical examination findings, need for subsequent operative intervention, DASH (Disabilities of the Arm, Shoulder, and Hand) scores, and a self-reported questionnaire. We hypothesized that patients who develop a malunion after nonoperative treatment of a closed humeral shaft fracture will have satisfactory functional outcomes based on subjective reports, physical examination findings, and DASH scores.
Methods
After obtaining institutional review board approval for the study, we selected patients from a retrospective medical record review of all those 18 years or older with a humeral shaft fracture managed nonoperatively at our institution between January 1, 2001, and June 30, 2012, with a minimum 1-year follow-up. We identified 156 patients with nonoperatively managed midshaft humerus fractures. Study exclusion criteria included fracture associated with a tumor (3 patients), ipsilateral upper extremity injury (9), open/ballistic injury (18), nonunion (9), underlying cognitive disability or psychiatric illness (4), and insufficient follow-up to clinical or radiographic healing (22). Ninety-one patients were eligible for study inclusion. Radiographs at time of final clinical visit were reviewed to assess for evidence of malunion at the fracture site, as defined by previously reported criteria3 (>20° angulation in anterior/posterior or varus/valgus plane of motion or shortening of ≥2.5 cm). Fifteen patients met all the inclusion criteria for further evaluation.
Medical records were retrospectively reviewed for information on age at injury, sex, comorbidities (eg, diabetes, osteoporosis, smoking), body mass index, type and duration of immobilization, complications, return to work, cosmetic perception, time to final clinical follow-up, and symptoms at final clinical follow-up. Incidence of potential risk factors associated with malunion—obesity, noncompliance, and comorbidities such as smoking and diabetes—was compared between the 15 patients with malunion and the other study patients, who healed without malunion.
For long-term postoperative follow-up, patients were contacted to be seen in clinic to complete an updated physical examination, self-reported questionnaire, and the DASH form. Physical examination included measurements of range of motion (ROM) and strength involving the shoulder, elbow, and forearm, with ROM reported as the difference between the injured and contralateral upper extremities. Neurovascular status and focal tenderness to palpation were also assessed on examination. When in-person examination was not possible, the questionnaire and DASH form were completed over the telephone. The self-reported questionnaire asked for information on smoking status, pain, functional limitations, cosmetic perception, satisfaction, and whether or not the patient would still opt for nonoperative management if presented with the same injury again. Pain and satisfaction were measured on numerical scales: Pain scores ranged from 0 (no pain) to 10 (worst possible pain), and satisfaction scores ranged from 1 (not satisfied) to 5 (very satisfied). Data are presented as mean values.
Results
Of the 91 study-eligible patients, 15 (16%) met the radiographic criteria for the diagnosis of malunion. Retrospective data were available for all 15 patients from time of injury to final clinical follow-up (mean, 19 weeks; range, 7-53 weeks). Mean age at injury was 39 years (range, 20-79 years). Additional demographics are listed in Table 1. Incidence of potential risk factors, such as body mass index (26.5 vs 25.4), smoking (33% vs 33%), and diabetes (0% vs 8%), was not significantly different between the malunion and healed-without-malunion groups, respectively. Furthermore, all malunion patients were compliant with their treatment protocol.
Radiographs were assessed at time of final follow-up to confirm healing and to document malunion. Varus malunion was found in 13 patients (mean, 24°; range, 20.5°-35.5°), and shortening was documented in the other 2 patients (mean, 4 cm; range, 3-5 cm). Patients were immobilized a mean of 10 weeks (range, 6-13 weeks). Initial fracture management consisted of coaptation splinting for 1 to 2 weeks (12 patients), hanging arm cast for 1 week (1 patient), and posterior splint for 1 week (1 patient). Patients were then transitioned to Sarmiento fracture bracing for the duration of their treatment (range, 5-12 months). One patient, followed initially at an outside institution, was managed in a sling throughout the duration of treatment (12 weeks) (Table 1). All 15 patients were neurovascularly intact at time of final clinical examination, with return of full upper extremity ROM in all but 3 patients. Only 1 of these 3 patients reported residual pain and functional limitations 4 months after injury (Table 2). Twelve patients were evaluated for return to work, with all successfully returning to work without restrictions at time of final follow-up. The 1 minor complication noted during the treatment period involved medial-sided elbow skin breakdown from brace wear, which resolved with local wound care. No patient required or requested surgical intervention for their residual malunion.
Of the 15 patients, 8 (53%) were reached for in-person examination (6 patients) or telephone interview (2 patients) for follow-up assessment by means of DASH form and self-reported questionnaire a mean of 47 months (range, 12-99 months) after initial injury. The 6 patients who had a physical examination were neurovascularly intact, lacked focal tenderness to palpation, and demonstrated full (5/5) strength within the deltoid, biceps, triceps, pronator, and supinator musculature. Each patient had equal ROM compared with the contralateral uninjured extremity on shoulder forward flexion and abduction, elbow flexion and extension, and forearm pronation and supination. Three patients (50%) had mild residual loss of ROM, with 2 demonstrating decreased shoulder external rotation of 10° and 15°, respectively, and 1 demonstrating decreased shoulder internal rotation of 10°.
Mean DASH score was 10.4 (range, 0-49.2). Evaluation of the self-reported questionnaire revealed a mean pain score of 1.1 (range, 0-7), with only 2 patients reporting any ongoing pain. In addition, 2 patients reported functional limitations, both related to overhead activities. However, 6 (75%) of the 8 patients reported noticeable cosmetic deformity, most commonly varus angulation (4 patients), as well as palpable bony prominence (2) and muscle atrophy (1). The majority of patients were satisfied with the outcome of their treatment (mean, 4; range, 2-5), with 6 patients reporting being satisfied or very satisfied, and all 6 indicating they would undergo nonoperative management again if presented with the same injury. Two patients reported being dissatisfied with their outcome, 1 because of cosmetic appearance and 1 because of cosmetic appearance and functional limitations. Both patients indicated they would choose operative management if presented with the same injury. There was no apparent relationship between outcome and degree of residual deformity, as both patients with varus angulation of more than 30° reported no residual pain or functional limitation and were very satisfied with the outcome of their treatment (Table 2).
Of the 7 patients who could not be reached for final follow-up, 2 on initial contact expressed overall satisfaction with their outcome and denied functional limitations. However, both asked to complete the study at a later date. Subsequently, these 2 patients could not be reached to complete the formal follow-up.
Discussion
Humeral shaft fractures are usually managed nonoperatively. One of the most commonly cited disadvantages of nonoperative management is its higher incidence of residual angular deformity, up to 13% in previous studies.4 Our study found a slightly higher incidence, 16%, on review of 91 nonoperatively managed humeral shaft fractures treated over an 11.5 year period. Although previous studies have reported acceptable functional and cosmetic outcomes with residual angular deformity of less than 20°,2,3,5,8,9 only observational reports have suggested acceptable function in patients with a documented malunion.8
To our knowledge, ours is the first study to correlate malunion with functional parameters and subjective patient-reported outcomes. We found that malunion was not associated with significant pain or functional limitation after nonoperative management of humeral shaft fractures. Furthermore, 75% of patients were satisfied or very satisfied with the outcome of their treatment and indicated they would undergo nonoperative management if presented with the same injury again. However, 75% of patients reported a noticeable cosmetic deformity, and one-third of these patients cited it as a major reason for dissatisfaction with their overall outcome. Regarding function, all patients returned to full strength and ROM of the affected extremity, aside from small losses of internal or external shoulder rotation on the magnitude of 10° to 15° in 50% of those patients tested. In addition, 75% of patients returned to regular activity without functional limitations; the other 25% reported trouble with overhead activities. There were no significant complications during the treatment or follow-up period, once the fracture had healed.
The major limitation of this study was its small patient population. (Obtaining a larger series of patients with malunion after nonoperative treatment of humeral shaft fractures likely would require a multicenter study.) Some of our study findings, such as lack of correlation between degree of malunion and subsequent functional or subjective outcomes, would require a larger sample size for verification and more definitive conclusions. Another limitation is that the study was not designed to evaluate the cause of malunion. Therefore, we cannot draw any definitive conclusions regarding what may have contributed to the development of malunion in our study population. However, all our malunion patients were compliant with their treatment protocol, and they showed no significant difference in incidence of potential risk factors (eg, obesity, comorbidities) compared with the patients who healed without malunion.
Conclusion
Malunion after nonoperative management of humeral shaft fractures does not appear to result in significant pain, dissatisfaction, or functional limitation as measured on physical examination and with validated objective outcome measures in the majority of patients. Furthermore, no patients in this study required surgical intervention for any residual limitations or complications after malunion. The majority of patients reported a noticeable cosmetic deformity, which left a small subset of patients dissatisfied. Overall, our study findings can be used to help counsel patients before and during nonoperative management—particularly patients who appear to be healing with some malunion. Our findings suggest that operative intervention to prevent malunion is not necessary, as it likely would not result in any overall improvement in patient function or satisfaction, but patients should be counseled regarding the high likelihood of cosmetic deformity, which may or may not be bothersome.
1. Rockwood CA, Green DP, Bucholz RW, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010.
2. Papasoulis E, Drosos GI, Ververidis AN, Verettas DA. Functional bracing of humeral shaft fractures. A review of clinical studies. Injury. 2010;41(7):e21-e27.
3. Sarmiento A, Latta LL. Functional fracture bracing. J Am Acad Orthop Surg. 1999;7(1):66-75.
4. Denard A Jr, Richards JE, Obremskey WT, Tucker MC, Floyd M, Herzog GA. Outcome of nonoperative vs operative treatment of humeral shaft fractures: a retrospective study of 213 patients. Orthopedics. 2010;33(8).
5. Fjalestad T, Strømsøe K, Salvesen P, Rostad B. Functional results of braced humeral diaphyseal fractures: why do 38% lose external rotation of the shoulder? Arch Orthop Trauma Surg. 2000;120(5-6):281-285.
6. Koch PP, Gross DF, Gerber C. The results of functional (Sarmiento) bracing of humeral shaft fractures. J Shoulder Elbow Surg. 2002;11(2):143-150.
7. Ozkurt B, Altay M, Aktekin CN, Toprak A, Tabak Y. The role of functional bracing in the treatment of humeral shaft fractures [in Turkish]. Acta Orthop Traumatol Turc. 2007;41(1):15-20.
8. Rutgers M, Ring D. Treatment of diaphyseal fractures of the humerus using a functional brace. J Orthop Trauma. 2006;20(9):597-601.
9. Sarmiento A, Kinman PB, Galvin EG, Schmitt RH, Phillips JG. Functional bracing of fractures of the shaft of the humerus. J Bone Joint Surg Am. 1977;59(5):596-601.
1. Rockwood CA, Green DP, Bucholz RW, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010.
2. Papasoulis E, Drosos GI, Ververidis AN, Verettas DA. Functional bracing of humeral shaft fractures. A review of clinical studies. Injury. 2010;41(7):e21-e27.
3. Sarmiento A, Latta LL. Functional fracture bracing. J Am Acad Orthop Surg. 1999;7(1):66-75.
4. Denard A Jr, Richards JE, Obremskey WT, Tucker MC, Floyd M, Herzog GA. Outcome of nonoperative vs operative treatment of humeral shaft fractures: a retrospective study of 213 patients. Orthopedics. 2010;33(8).
5. Fjalestad T, Strømsøe K, Salvesen P, Rostad B. Functional results of braced humeral diaphyseal fractures: why do 38% lose external rotation of the shoulder? Arch Orthop Trauma Surg. 2000;120(5-6):281-285.
6. Koch PP, Gross DF, Gerber C. The results of functional (Sarmiento) bracing of humeral shaft fractures. J Shoulder Elbow Surg. 2002;11(2):143-150.
7. Ozkurt B, Altay M, Aktekin CN, Toprak A, Tabak Y. The role of functional bracing in the treatment of humeral shaft fractures [in Turkish]. Acta Orthop Traumatol Turc. 2007;41(1):15-20.
8. Rutgers M, Ring D. Treatment of diaphyseal fractures of the humerus using a functional brace. J Orthop Trauma. 2006;20(9):597-601.
9. Sarmiento A, Kinman PB, Galvin EG, Schmitt RH, Phillips JG. Functional bracing of fractures of the shaft of the humerus. J Bone Joint Surg Am. 1977;59(5):596-601.
Well-Leg Positioning on a Fracture Table: Using a Pillow Sling
The development of acute compartment syndrome in lower legs placed in the lithotomy position is a rare complication reported within various surgical subspecialties, including general surgery, gynecology, and urology.1-5 Although it is reported in arthroscopic knee cases, the more frequent occurrence in orthopedics, based on available case reports, appears to involve the well (uninjured or contralateral) leg placed in the hemilithotomy position on the fracture table.6-9
Prior studies have found significantly elevated lower leg compartment pressures in legs placed in the lithotomy position. Chase and colleagues10 measured the anterior compartment pressures in 16 limbs placed in the lithotomy position. They found minor elevations after initial lithotomy positioning, but gradual increases over time, with an average elevation to 30 mm Hg and maximum of 70 mm Hg. Similarly, Meyer and colleagues11 recorded the lower leg pressures in 8 healthy volunteers positioned on a fracture table. Changing from the supine position to the lithotomy position significantly increased the intramuscular pressure in the anterior compartment (from 11.6 to 19.4 mm Hg) and in the lateral compartment (from 13.0 to 25.8 mm Hg).
Along with increased intramuscular pressures, local hypotension occurs in lower legs placed in the lithotomy position. Mean diastolic blood pressure in the ankle was 63.9 mm Hg in the leg placed in the supine position as opposed to 34.6 mm Hg in the same leg placed in the lithotomy position.10 This finding is not unexpected, given that local arteriolar pressure decreases by 0.78 mm Hg for every 1.0 cm of elevation.12-14 Furthermore, some “kinking” of either femoral vessels at the hip or popliteal vessels at the knee may also occur.15
For prevention of these problems, the well leg can be placed in a position of slight hip extension and full knee extension on the fracture table—the so-called scissored position. This position is commonly achieved with an additional traction boot and support bar connected to the well leg. However, this additional setup can make positioning the C-arm machine difficult; there is obstruction by the additional support bars and the leg itself. In addition, the uninjured extremity may be placed into positions that cause unnecessarily high stresses across the joints and can potentially lead to iatrogenic injury and pain.
Risk of fracture in the well leg results from the C-arm machine abutting the well leg when swinging through to obtain a lateral image. This problem is overcome by securing the well leg to the fracture table’s longitudinal support bar using a pillow sling, thereby reducing the risks of compartment syndrome, allowing the uninjured limb to be in a relaxed position, and allowing good fluoroscopic images to easily be obtained. This brief report is an introduction to this positioning method.
Surgical Technique
The patient is intubated and anesthetized on the hospital bed before being transferred to the fracture table. On the fracture table, the operative leg is placed in a boot traction device in the standard fashion. The perineal post is then inserted, and the patient is pulled caudally on the bed so that the post is appropriately positioned for countertraction.
With an assistant holding the well leg, the distal flat-top table extension is removed. With a calf or foot sequential compression device still in place, a pillow enclosed in a pillowcase is wrapped around the lower leg and ankle in a U-shaped fashion using the longitudinal length of the pillow (Figure 1). The pillow-wrapped leg is then placed against the side (not the top) of the table’s support bar and secured in place using a 6-in self-adherent compression bandage (eg, Coban; 3M, St. Paul, Minnesota), wrapped circumferentially around both the pillow and the support post (Figures 2A, 2B). Although an Ace wrap may be more readily accessible, we have found it to slowly loosen and/or migrate, thus potentially changing the leg position throughout the case.
As shown in Figure 3, the C-arm machine can then be positioned in an oblique fashion relative to the bed with an unobstructed view of the hip. The C-arm can also be repositioned perpendicular to the injured limb, and unobstructed images can be obtained of the entire length of the femur. This quick and efficient setup of the well leg allows for an optimal amount of relaxed hip and knee extension, and limb adduction to midline along the table’s support bar, to permit lateral fluoroscopic imaging of the injured limb without overlap of the well leg or interference with C-arm positioning.
Results
For more than 2 years, Dr. Mir has used the pillow-sling technique for placement of the well leg in the scissored position on the fracture table in all patients. Between September 2010 and January 2013, he applied the technique 93 times, with the procedures listed as follows with their Current Procedural Terminology (CPT) codes: 14 cases of percutaneous fixation of femoral neck fracture (CPT 27235), 8 cases of treatment of intertrochanteric or subtrochanteric fracture with plate/screw type implant (CPT 27244), 34 cases of treatment of intertrochanteric or subtrochanteric fracture with intramedullary implant (CPT 27245), and 37 cases of treatment of femoral shaft fracture with intramedullary implant (CPT 27506).
With respect to compartment syndrome, there were no intraoperative or postoperative complications. Furthermore, no patients complained of pain in the well leg immediately after surgery or at subsequent follow-ups. No difficulty was encountered with intraoperative C-arm imaging of the injured limb at the hip or along the length of the femur in the lateral or anteroposterior planes. The well leg did not have to be repositioned in any cases to achieve adequate imaging of the hip and femur.
Discussion
Although rare, acute compartment syndrome remains a potential yet avoidable complication of the lithotomy position. Some surgeons avoid this setup of the well leg on the fracture table and instead use a scissored position for the uninjured limb.
In this report, we presented a safe and efficient technique for placing the well leg in a scissored position on the fracture table using a pillow and a self-adherent compression bandage. We did not compare the pillow-sling with other well-leg positioning techniques but instead described a reproducible technique that we have used effectively and successfully, even with multiple morbidly obese patients who met the weight limits for the fracture table.
In addition, even with consistent use of this pillow-sling technique at our high-volume trauma center, there have been no complications, such as compartment syndrome, well-leg pain, or difficulty in intraoperative imaging of the injured limb. The pillow-sling is a safe and expedient alternative technique for well-leg positioning on the fracture table, and it can be easily reproduced by other surgeons.
1. Leff RG, Shapiro SR. Lower extremity complications of the lithotomy position: prevention and management. J Urol. 1979;122(1):138-139.
2. Lydon JC, Spielman FJ. Bilateral compartment syndrome following prolonged surgery in the lithotomy position. Anesthesiology. 1984;60(3):236-238.
3. Kubiak R, Wilcox DT, Spitz L, Kiely EM. Neurovascular morbidity from the lithotomy position. J Pediatr Surg. 1998;33(12):1808-1810.
4. Cohen SA, Hurt WG. Compartment syndrome associated with lithotomy position and intermittent compression stockings. Obstet Gynecol. 2001;97(5 pt 2):832-833.
5. Moses TA, Kreder KJ, Thrasher JB. Compartment syndrome: an unusual complication of the lithotomy position. Urology. 1994;43(5):746-747.
6. Chung JH, Ahn KR, Park JH, et al. Lower leg compartment syndrome following prolonged orthopedic surgery in the lithotomy position –A case report–. Korean J Anesthesiol. 2010;59(suppl):S49-S52.
7. Tan V, Pepe MD, Glaser DL, Seldes RM, Heppenstall RB, Esterhai JL Jr. Well-leg compartment pressures during hemilithotomy position for fracture fixation. J Orthop Trauma. 2000;14(3):157-161.
8. Anglen J, Banovetz J. Compartment syndrome in the well leg resulting from fracture-table positioning. Clin Orthop. 1994;(301):239-242.
9. Mathews PV, Perry JJ, Murray PC. Compartment syndrome of the well leg as a result of the hemilithotomy position: a report of two cases and review of literature. J Orthop Trauma. 2001;15(8):580-583.
10. Chase J, Harford F, Pinzur MS, Zussman M. Intraoperative lower extremity compartment pressures in lithotomy-positioned patients. Dis Colon Rectum. 2000;43(5):678-680.
11. Meyer RS, White KK, Smith JM, Groppo ER, Mubarak SJ, Hargens AR. Intramuscular and blood pressures in legs positioned in the hemilithotomy position: clarification of risk factors for well-leg acute compartment syndrome. J Bone Joint Surg Am. 2002;84(10):1829-1835.
12. Enderby GE. Postural ischaemia and blood-pressure. Lancet. 1954;266(6804):185-187.
13. Matsen FA 3rd, Mayo KA, Krugmire RB Jr, Sheridan GW, Kraft GH. A model compartmental syndrome in man with particular reference to the quantification of nerve function. J Bone Joint Surg Am. 1977;59(5):648-653.
14. Peters P, Baker SR, Leopold PW, Taub NA, Burnand KG. Compartment syndrome following prolonged pelvic surgery. Br J Surg. 1994;81(8):1128-1131.
15. Gershuni DH, Yaru NC, Hargens AR, Lieber RL, O’Hara RC, Akeson WH. Ankle and knee position as a factor modifying intracompartmental pressure in the human leg. J Bone Joint Surg Am. 1984;66(9):1415-1420.
The development of acute compartment syndrome in lower legs placed in the lithotomy position is a rare complication reported within various surgical subspecialties, including general surgery, gynecology, and urology.1-5 Although it is reported in arthroscopic knee cases, the more frequent occurrence in orthopedics, based on available case reports, appears to involve the well (uninjured or contralateral) leg placed in the hemilithotomy position on the fracture table.6-9
Prior studies have found significantly elevated lower leg compartment pressures in legs placed in the lithotomy position. Chase and colleagues10 measured the anterior compartment pressures in 16 limbs placed in the lithotomy position. They found minor elevations after initial lithotomy positioning, but gradual increases over time, with an average elevation to 30 mm Hg and maximum of 70 mm Hg. Similarly, Meyer and colleagues11 recorded the lower leg pressures in 8 healthy volunteers positioned on a fracture table. Changing from the supine position to the lithotomy position significantly increased the intramuscular pressure in the anterior compartment (from 11.6 to 19.4 mm Hg) and in the lateral compartment (from 13.0 to 25.8 mm Hg).
Along with increased intramuscular pressures, local hypotension occurs in lower legs placed in the lithotomy position. Mean diastolic blood pressure in the ankle was 63.9 mm Hg in the leg placed in the supine position as opposed to 34.6 mm Hg in the same leg placed in the lithotomy position.10 This finding is not unexpected, given that local arteriolar pressure decreases by 0.78 mm Hg for every 1.0 cm of elevation.12-14 Furthermore, some “kinking” of either femoral vessels at the hip or popliteal vessels at the knee may also occur.15
For prevention of these problems, the well leg can be placed in a position of slight hip extension and full knee extension on the fracture table—the so-called scissored position. This position is commonly achieved with an additional traction boot and support bar connected to the well leg. However, this additional setup can make positioning the C-arm machine difficult; there is obstruction by the additional support bars and the leg itself. In addition, the uninjured extremity may be placed into positions that cause unnecessarily high stresses across the joints and can potentially lead to iatrogenic injury and pain.
Risk of fracture in the well leg results from the C-arm machine abutting the well leg when swinging through to obtain a lateral image. This problem is overcome by securing the well leg to the fracture table’s longitudinal support bar using a pillow sling, thereby reducing the risks of compartment syndrome, allowing the uninjured limb to be in a relaxed position, and allowing good fluoroscopic images to easily be obtained. This brief report is an introduction to this positioning method.
Surgical Technique
The patient is intubated and anesthetized on the hospital bed before being transferred to the fracture table. On the fracture table, the operative leg is placed in a boot traction device in the standard fashion. The perineal post is then inserted, and the patient is pulled caudally on the bed so that the post is appropriately positioned for countertraction.
With an assistant holding the well leg, the distal flat-top table extension is removed. With a calf or foot sequential compression device still in place, a pillow enclosed in a pillowcase is wrapped around the lower leg and ankle in a U-shaped fashion using the longitudinal length of the pillow (Figure 1). The pillow-wrapped leg is then placed against the side (not the top) of the table’s support bar and secured in place using a 6-in self-adherent compression bandage (eg, Coban; 3M, St. Paul, Minnesota), wrapped circumferentially around both the pillow and the support post (Figures 2A, 2B). Although an Ace wrap may be more readily accessible, we have found it to slowly loosen and/or migrate, thus potentially changing the leg position throughout the case.
As shown in Figure 3, the C-arm machine can then be positioned in an oblique fashion relative to the bed with an unobstructed view of the hip. The C-arm can also be repositioned perpendicular to the injured limb, and unobstructed images can be obtained of the entire length of the femur. This quick and efficient setup of the well leg allows for an optimal amount of relaxed hip and knee extension, and limb adduction to midline along the table’s support bar, to permit lateral fluoroscopic imaging of the injured limb without overlap of the well leg or interference with C-arm positioning.
Results
For more than 2 years, Dr. Mir has used the pillow-sling technique for placement of the well leg in the scissored position on the fracture table in all patients. Between September 2010 and January 2013, he applied the technique 93 times, with the procedures listed as follows with their Current Procedural Terminology (CPT) codes: 14 cases of percutaneous fixation of femoral neck fracture (CPT 27235), 8 cases of treatment of intertrochanteric or subtrochanteric fracture with plate/screw type implant (CPT 27244), 34 cases of treatment of intertrochanteric or subtrochanteric fracture with intramedullary implant (CPT 27245), and 37 cases of treatment of femoral shaft fracture with intramedullary implant (CPT 27506).
With respect to compartment syndrome, there were no intraoperative or postoperative complications. Furthermore, no patients complained of pain in the well leg immediately after surgery or at subsequent follow-ups. No difficulty was encountered with intraoperative C-arm imaging of the injured limb at the hip or along the length of the femur in the lateral or anteroposterior planes. The well leg did not have to be repositioned in any cases to achieve adequate imaging of the hip and femur.
Discussion
Although rare, acute compartment syndrome remains a potential yet avoidable complication of the lithotomy position. Some surgeons avoid this setup of the well leg on the fracture table and instead use a scissored position for the uninjured limb.
In this report, we presented a safe and efficient technique for placing the well leg in a scissored position on the fracture table using a pillow and a self-adherent compression bandage. We did not compare the pillow-sling with other well-leg positioning techniques but instead described a reproducible technique that we have used effectively and successfully, even with multiple morbidly obese patients who met the weight limits for the fracture table.
In addition, even with consistent use of this pillow-sling technique at our high-volume trauma center, there have been no complications, such as compartment syndrome, well-leg pain, or difficulty in intraoperative imaging of the injured limb. The pillow-sling is a safe and expedient alternative technique for well-leg positioning on the fracture table, and it can be easily reproduced by other surgeons.
The development of acute compartment syndrome in lower legs placed in the lithotomy position is a rare complication reported within various surgical subspecialties, including general surgery, gynecology, and urology.1-5 Although it is reported in arthroscopic knee cases, the more frequent occurrence in orthopedics, based on available case reports, appears to involve the well (uninjured or contralateral) leg placed in the hemilithotomy position on the fracture table.6-9
Prior studies have found significantly elevated lower leg compartment pressures in legs placed in the lithotomy position. Chase and colleagues10 measured the anterior compartment pressures in 16 limbs placed in the lithotomy position. They found minor elevations after initial lithotomy positioning, but gradual increases over time, with an average elevation to 30 mm Hg and maximum of 70 mm Hg. Similarly, Meyer and colleagues11 recorded the lower leg pressures in 8 healthy volunteers positioned on a fracture table. Changing from the supine position to the lithotomy position significantly increased the intramuscular pressure in the anterior compartment (from 11.6 to 19.4 mm Hg) and in the lateral compartment (from 13.0 to 25.8 mm Hg).
Along with increased intramuscular pressures, local hypotension occurs in lower legs placed in the lithotomy position. Mean diastolic blood pressure in the ankle was 63.9 mm Hg in the leg placed in the supine position as opposed to 34.6 mm Hg in the same leg placed in the lithotomy position.10 This finding is not unexpected, given that local arteriolar pressure decreases by 0.78 mm Hg for every 1.0 cm of elevation.12-14 Furthermore, some “kinking” of either femoral vessels at the hip or popliteal vessels at the knee may also occur.15
For prevention of these problems, the well leg can be placed in a position of slight hip extension and full knee extension on the fracture table—the so-called scissored position. This position is commonly achieved with an additional traction boot and support bar connected to the well leg. However, this additional setup can make positioning the C-arm machine difficult; there is obstruction by the additional support bars and the leg itself. In addition, the uninjured extremity may be placed into positions that cause unnecessarily high stresses across the joints and can potentially lead to iatrogenic injury and pain.
Risk of fracture in the well leg results from the C-arm machine abutting the well leg when swinging through to obtain a lateral image. This problem is overcome by securing the well leg to the fracture table’s longitudinal support bar using a pillow sling, thereby reducing the risks of compartment syndrome, allowing the uninjured limb to be in a relaxed position, and allowing good fluoroscopic images to easily be obtained. This brief report is an introduction to this positioning method.
Surgical Technique
The patient is intubated and anesthetized on the hospital bed before being transferred to the fracture table. On the fracture table, the operative leg is placed in a boot traction device in the standard fashion. The perineal post is then inserted, and the patient is pulled caudally on the bed so that the post is appropriately positioned for countertraction.
With an assistant holding the well leg, the distal flat-top table extension is removed. With a calf or foot sequential compression device still in place, a pillow enclosed in a pillowcase is wrapped around the lower leg and ankle in a U-shaped fashion using the longitudinal length of the pillow (Figure 1). The pillow-wrapped leg is then placed against the side (not the top) of the table’s support bar and secured in place using a 6-in self-adherent compression bandage (eg, Coban; 3M, St. Paul, Minnesota), wrapped circumferentially around both the pillow and the support post (Figures 2A, 2B). Although an Ace wrap may be more readily accessible, we have found it to slowly loosen and/or migrate, thus potentially changing the leg position throughout the case.
As shown in Figure 3, the C-arm machine can then be positioned in an oblique fashion relative to the bed with an unobstructed view of the hip. The C-arm can also be repositioned perpendicular to the injured limb, and unobstructed images can be obtained of the entire length of the femur. This quick and efficient setup of the well leg allows for an optimal amount of relaxed hip and knee extension, and limb adduction to midline along the table’s support bar, to permit lateral fluoroscopic imaging of the injured limb without overlap of the well leg or interference with C-arm positioning.
Results
For more than 2 years, Dr. Mir has used the pillow-sling technique for placement of the well leg in the scissored position on the fracture table in all patients. Between September 2010 and January 2013, he applied the technique 93 times, with the procedures listed as follows with their Current Procedural Terminology (CPT) codes: 14 cases of percutaneous fixation of femoral neck fracture (CPT 27235), 8 cases of treatment of intertrochanteric or subtrochanteric fracture with plate/screw type implant (CPT 27244), 34 cases of treatment of intertrochanteric or subtrochanteric fracture with intramedullary implant (CPT 27245), and 37 cases of treatment of femoral shaft fracture with intramedullary implant (CPT 27506).
With respect to compartment syndrome, there were no intraoperative or postoperative complications. Furthermore, no patients complained of pain in the well leg immediately after surgery or at subsequent follow-ups. No difficulty was encountered with intraoperative C-arm imaging of the injured limb at the hip or along the length of the femur in the lateral or anteroposterior planes. The well leg did not have to be repositioned in any cases to achieve adequate imaging of the hip and femur.
Discussion
Although rare, acute compartment syndrome remains a potential yet avoidable complication of the lithotomy position. Some surgeons avoid this setup of the well leg on the fracture table and instead use a scissored position for the uninjured limb.
In this report, we presented a safe and efficient technique for placing the well leg in a scissored position on the fracture table using a pillow and a self-adherent compression bandage. We did not compare the pillow-sling with other well-leg positioning techniques but instead described a reproducible technique that we have used effectively and successfully, even with multiple morbidly obese patients who met the weight limits for the fracture table.
In addition, even with consistent use of this pillow-sling technique at our high-volume trauma center, there have been no complications, such as compartment syndrome, well-leg pain, or difficulty in intraoperative imaging of the injured limb. The pillow-sling is a safe and expedient alternative technique for well-leg positioning on the fracture table, and it can be easily reproduced by other surgeons.
1. Leff RG, Shapiro SR. Lower extremity complications of the lithotomy position: prevention and management. J Urol. 1979;122(1):138-139.
2. Lydon JC, Spielman FJ. Bilateral compartment syndrome following prolonged surgery in the lithotomy position. Anesthesiology. 1984;60(3):236-238.
3. Kubiak R, Wilcox DT, Spitz L, Kiely EM. Neurovascular morbidity from the lithotomy position. J Pediatr Surg. 1998;33(12):1808-1810.
4. Cohen SA, Hurt WG. Compartment syndrome associated with lithotomy position and intermittent compression stockings. Obstet Gynecol. 2001;97(5 pt 2):832-833.
5. Moses TA, Kreder KJ, Thrasher JB. Compartment syndrome: an unusual complication of the lithotomy position. Urology. 1994;43(5):746-747.
6. Chung JH, Ahn KR, Park JH, et al. Lower leg compartment syndrome following prolonged orthopedic surgery in the lithotomy position –A case report–. Korean J Anesthesiol. 2010;59(suppl):S49-S52.
7. Tan V, Pepe MD, Glaser DL, Seldes RM, Heppenstall RB, Esterhai JL Jr. Well-leg compartment pressures during hemilithotomy position for fracture fixation. J Orthop Trauma. 2000;14(3):157-161.
8. Anglen J, Banovetz J. Compartment syndrome in the well leg resulting from fracture-table positioning. Clin Orthop. 1994;(301):239-242.
9. Mathews PV, Perry JJ, Murray PC. Compartment syndrome of the well leg as a result of the hemilithotomy position: a report of two cases and review of literature. J Orthop Trauma. 2001;15(8):580-583.
10. Chase J, Harford F, Pinzur MS, Zussman M. Intraoperative lower extremity compartment pressures in lithotomy-positioned patients. Dis Colon Rectum. 2000;43(5):678-680.
11. Meyer RS, White KK, Smith JM, Groppo ER, Mubarak SJ, Hargens AR. Intramuscular and blood pressures in legs positioned in the hemilithotomy position: clarification of risk factors for well-leg acute compartment syndrome. J Bone Joint Surg Am. 2002;84(10):1829-1835.
12. Enderby GE. Postural ischaemia and blood-pressure. Lancet. 1954;266(6804):185-187.
13. Matsen FA 3rd, Mayo KA, Krugmire RB Jr, Sheridan GW, Kraft GH. A model compartmental syndrome in man with particular reference to the quantification of nerve function. J Bone Joint Surg Am. 1977;59(5):648-653.
14. Peters P, Baker SR, Leopold PW, Taub NA, Burnand KG. Compartment syndrome following prolonged pelvic surgery. Br J Surg. 1994;81(8):1128-1131.
15. Gershuni DH, Yaru NC, Hargens AR, Lieber RL, O’Hara RC, Akeson WH. Ankle and knee position as a factor modifying intracompartmental pressure in the human leg. J Bone Joint Surg Am. 1984;66(9):1415-1420.
1. Leff RG, Shapiro SR. Lower extremity complications of the lithotomy position: prevention and management. J Urol. 1979;122(1):138-139.
2. Lydon JC, Spielman FJ. Bilateral compartment syndrome following prolonged surgery in the lithotomy position. Anesthesiology. 1984;60(3):236-238.
3. Kubiak R, Wilcox DT, Spitz L, Kiely EM. Neurovascular morbidity from the lithotomy position. J Pediatr Surg. 1998;33(12):1808-1810.
4. Cohen SA, Hurt WG. Compartment syndrome associated with lithotomy position and intermittent compression stockings. Obstet Gynecol. 2001;97(5 pt 2):832-833.
5. Moses TA, Kreder KJ, Thrasher JB. Compartment syndrome: an unusual complication of the lithotomy position. Urology. 1994;43(5):746-747.
6. Chung JH, Ahn KR, Park JH, et al. Lower leg compartment syndrome following prolonged orthopedic surgery in the lithotomy position –A case report–. Korean J Anesthesiol. 2010;59(suppl):S49-S52.
7. Tan V, Pepe MD, Glaser DL, Seldes RM, Heppenstall RB, Esterhai JL Jr. Well-leg compartment pressures during hemilithotomy position for fracture fixation. J Orthop Trauma. 2000;14(3):157-161.
8. Anglen J, Banovetz J. Compartment syndrome in the well leg resulting from fracture-table positioning. Clin Orthop. 1994;(301):239-242.
9. Mathews PV, Perry JJ, Murray PC. Compartment syndrome of the well leg as a result of the hemilithotomy position: a report of two cases and review of literature. J Orthop Trauma. 2001;15(8):580-583.
10. Chase J, Harford F, Pinzur MS, Zussman M. Intraoperative lower extremity compartment pressures in lithotomy-positioned patients. Dis Colon Rectum. 2000;43(5):678-680.
11. Meyer RS, White KK, Smith JM, Groppo ER, Mubarak SJ, Hargens AR. Intramuscular and blood pressures in legs positioned in the hemilithotomy position: clarification of risk factors for well-leg acute compartment syndrome. J Bone Joint Surg Am. 2002;84(10):1829-1835.
12. Enderby GE. Postural ischaemia and blood-pressure. Lancet. 1954;266(6804):185-187.
13. Matsen FA 3rd, Mayo KA, Krugmire RB Jr, Sheridan GW, Kraft GH. A model compartmental syndrome in man with particular reference to the quantification of nerve function. J Bone Joint Surg Am. 1977;59(5):648-653.
14. Peters P, Baker SR, Leopold PW, Taub NA, Burnand KG. Compartment syndrome following prolonged pelvic surgery. Br J Surg. 1994;81(8):1128-1131.
15. Gershuni DH, Yaru NC, Hargens AR, Lieber RL, O’Hara RC, Akeson WH. Ankle and knee position as a factor modifying intracompartmental pressure in the human leg. J Bone Joint Surg Am. 1984;66(9):1415-1420.