User login
Efficacy, Safety, and Tolerability of Halobetasol Propionate 0.01%–Tazarotene 0.045% Lotion for Moderate to Severe Plaque Psoriasis in the Hispanic Population: Post Hoc Analysis
Psoriasis is a common chronic inflammatory disease affecting a diverse patient population, yet epidemiological and clinical data related to psoriasis in patients with skin of color are sparse. The Hispanic ethnic group includes a broad range of skin types and cultures. Prevalence of psoriasis in a Hispanic population has been reported as lower than in a white population1; however, these data may be influenced by the finding that Hispanic patients are less likely to see a dermatologist when they have skin problems.2 In addition, socioeconomic disparities and cultural variations among racial/ethnic groups may contribute to differences in access to care and thresholds for seeking care,3 leading to a tendency for more severe disease in skin of color and Hispanic ethnic groups.4,5 Greater impairments in health-related quality of life have been reported in patients with skin of color and Hispanic racial/ethnic groups compared to white patients, independent of psoriasis severity.4,6 Postinflammatory pigment alteration at the sites of resolving lesions, a common clinical feature in skin of color, may contribute to the impact of psoriasis on quality of life in patients with skin of color. Psoriasis in darker skin types also can present diagnostic challenges due to overlapping features with other papulosquamous disorders and less conspicuous erythema.7
We present a post hoc analysis of the treatment of moderate to severe psoriasis with a novel fixed-combination halobetasol propionate (HP) 0.01%–tazarotene (TAZ) 0.045% lotion in a Hispanic patient population. Historically, clinical trials for psoriasis have enrolled low proportions of Hispanic patients and other patients with skin of color; in this analysis, the Hispanic population (115/418) represented 28% of the total study population and provided valuable insights.
Methods
Study Design
Two phase 3 randomized controlled trials were conducted to demonstrate the efficacy and safety of HP/TAZ lotion. Patients with a clinical diagnosis of moderate or severe localized psoriasis (N=418) were randomized to receive HP/TAZ lotion or vehicle (2:1 ratio) once daily for 8 weeks with a 4-week posttreatment follow-up.8,9 A post hoc analysis was conducted on data of the self-identified Hispanic population.
Assessments
Efficacy assessments included treatment success (at least a 2-grade improvement from baseline in the investigator global assessment [IGA] and a score of clear or almost clear) and impact on individual signs of psoriasis (at least a 2-grade improvement in erythema, plaque elevation, and scaling) at the target lesion. In addition, reduction in body surface area (BSA) was recorded, and an IGA×BSA score was calculated by multiplying IGA by BSA at each timepoint for each individual patient. A clinically meaningful improvement in disease severity (percentage of patients achieving a 75% reduction in IGA×BSA [IGA×BSA-75]) also was calculated.
Information on reported and observed adverse events (AEs) was obtained at each visit. The safety population included 112 participants (76 in the HP/TAZ group and 36 in the vehicle group).
Statistical Analysis
The statistical and analytical plan is detailed elsewhere9 and relevant to this post hoc analysis. No statistical analysis was carried out to compare data in the Hispanic population with either the overall study population or the non-Hispanic population.
Results
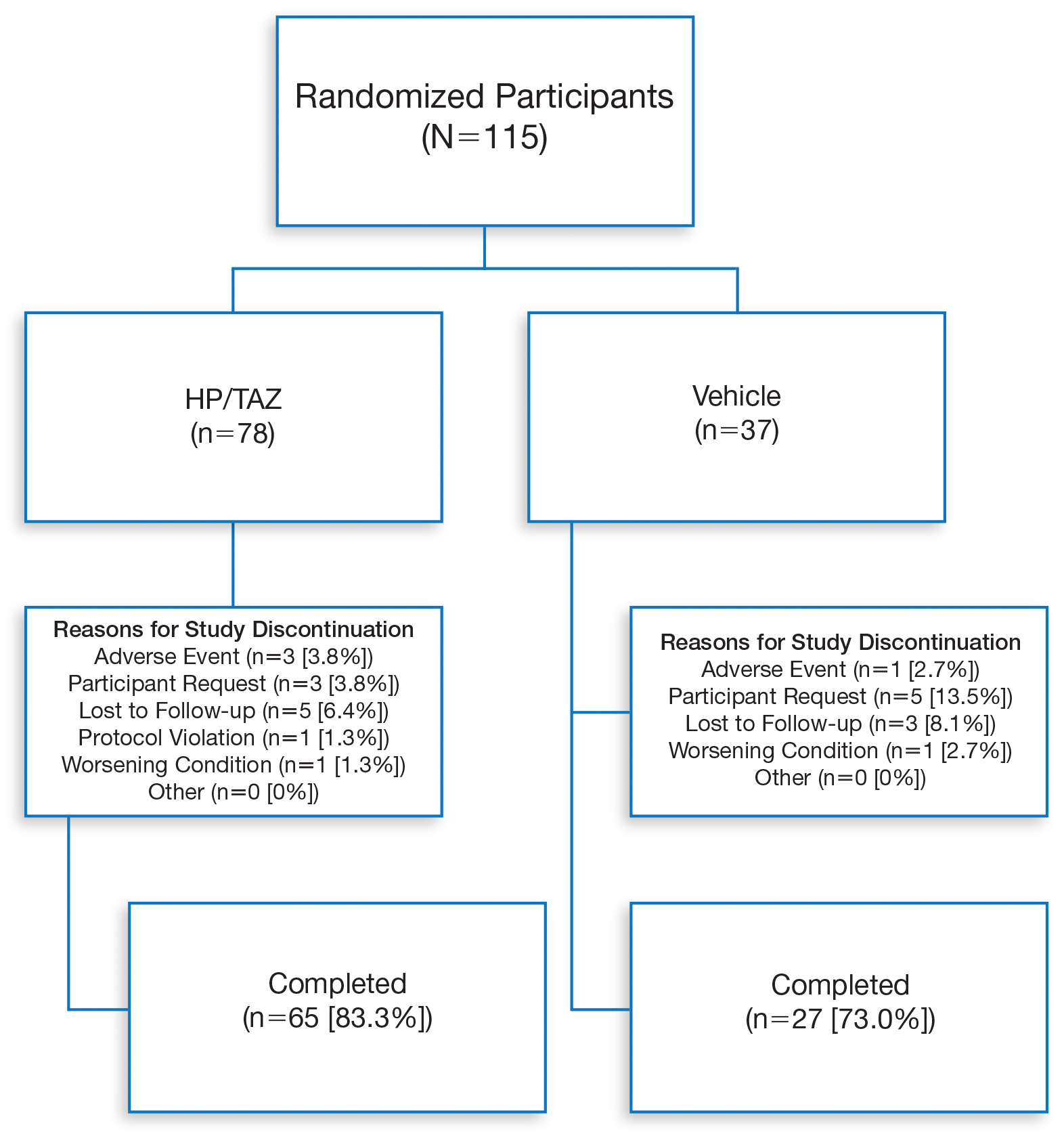
Overall, 115 Hispanic patients (27.5%) were enrolled (eFigure). Patients had a mean (standard deviation [SD]) age of 46.7 (13.12) years, and more than two-thirds were male (n=80, 69.6%).
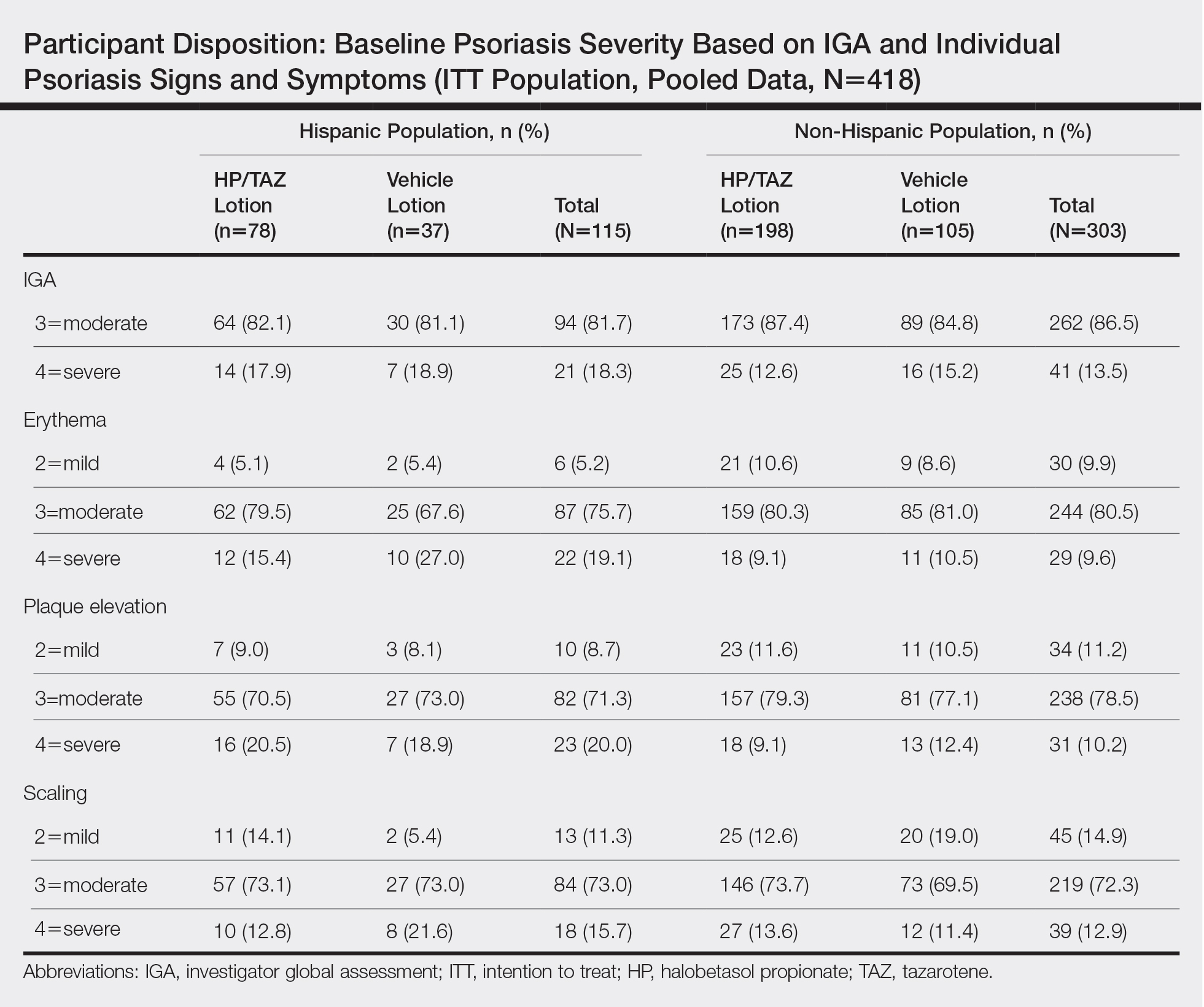
Overall completion rates (80.0%) for Hispanic patients were similar to those in the overall study population, though there were more discontinuations in the vehicle group. The main reasons for treatment discontinuation among Hispanic patients were participant request (n=8, 7.0%), lost to follow-up (n=8, 7.0%), and AEs (n=4, 3.5%). Hispanic patients in this study had more severe disease—18.3% (n=21) had an IGA score of 4 compared to 13.5% (n=41) of non-Hispanic patients—and more severe erythema (19.1% vs 9.6%), plaque elevation (20.0% vs 10.2%), and scaling (15.7% vs 12.9%) compared to the non-Hispanic populations (Table).
Efficacy of HP/TAZ lotion in Hispanic patients was similar to the overall study populations,9 though maintenance of effect posttreatment appeared to be better. The incidence of treatment-related AEs also was lower.
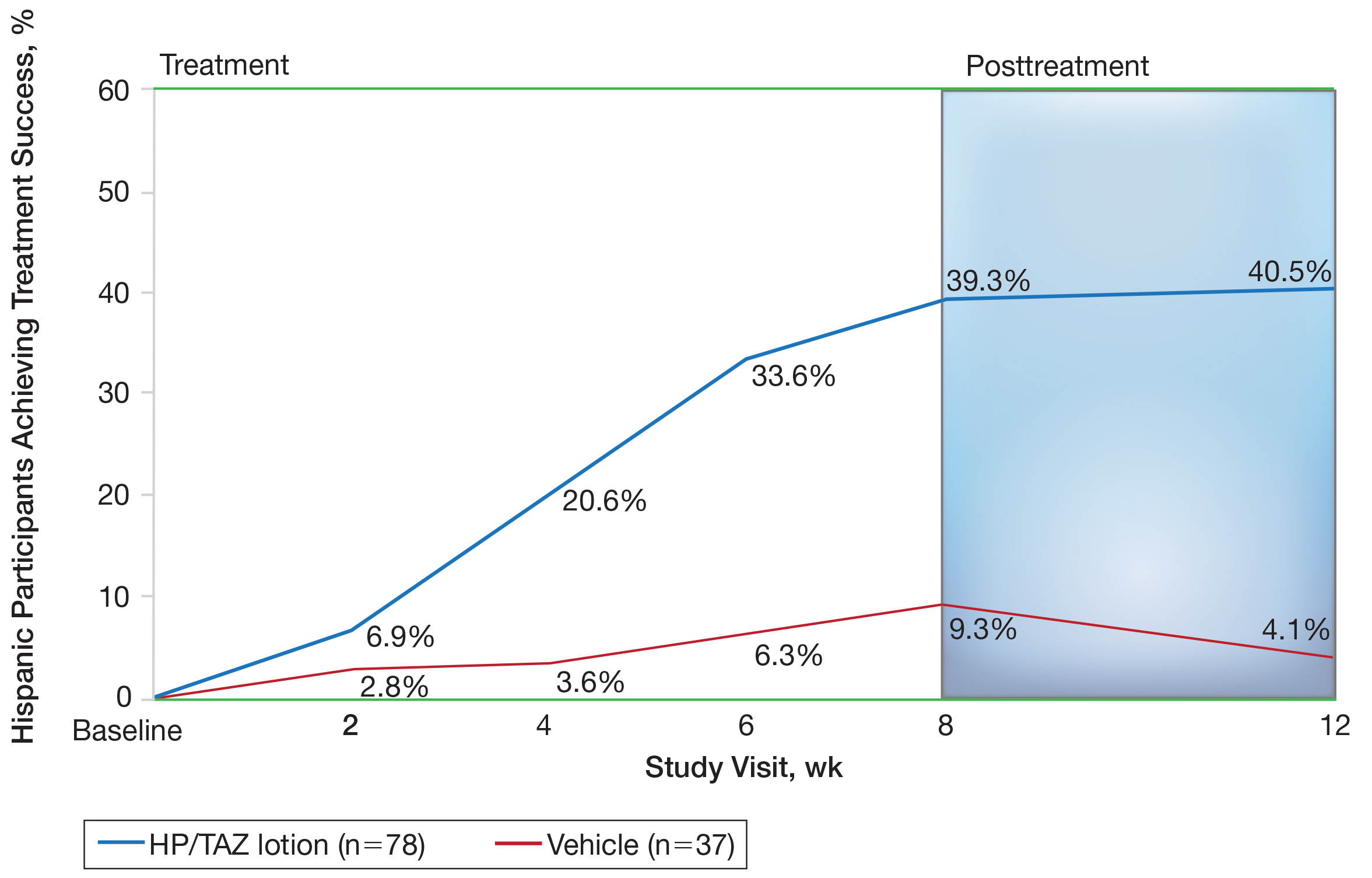
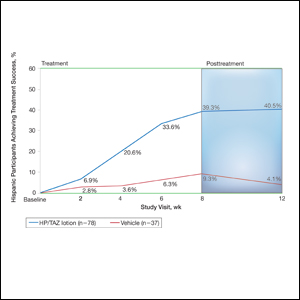
Halobetasol propionate 0.01%–TAZ 0.045% lotion demonstrated statistically significant superiority based on treatment success compared to vehicle as early as week 4 (P=.034). By week 8, 39.3% of participants treated with HP/TAZ lotion achieved treatment success compared to 9.3% of participants in the vehicle group (P=.002)(Figure 1). Treatment success was maintained over the 4-week posttreatment period, whereby 40.5% of the HP/TAZ-treated participants were treatment successes at week 12 compared to only 4.1% of participants in the vehicle group (P<.001).
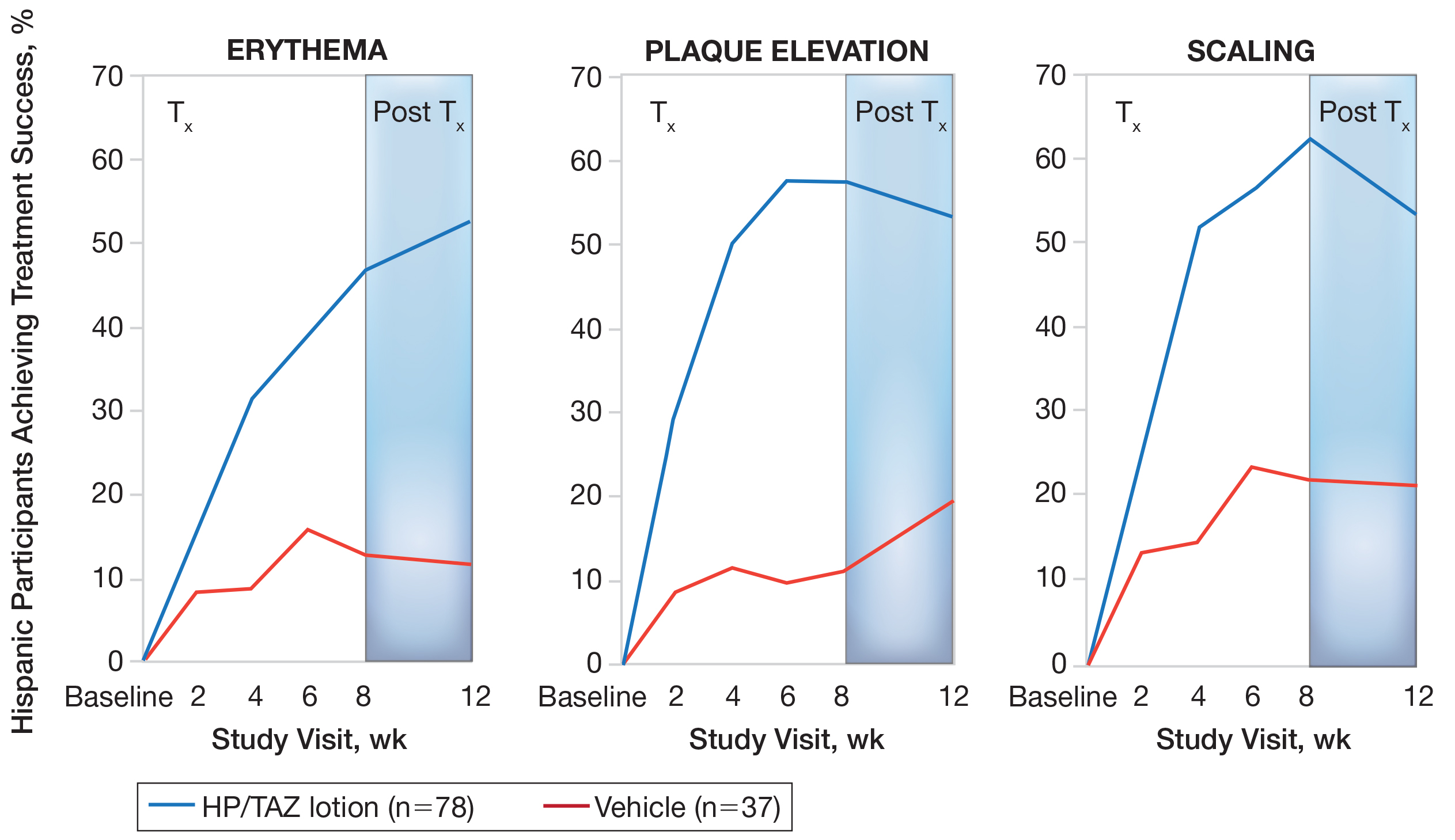
Improvements in psoriasis signs and symptoms at the target lesion were statistically significant compared to vehicle from week 2 (plaque elevation, P=.018) or week 4 (erythema, P=.004; scaling, P<.001)(Figure 2). By week 8, 46.8%, 58.1%, and 63.2% of participants showed at least a 2-grade improvement from baseline and were therefore treatment successes for erythema, plaque elevation, and scaling, respectively (all statistically significant [P<.001] compared to vehicle). The number of participants who achieved at least a 2-grade improvement in erythema with HP/TAZ lotion increased posttreatment from 46.8% to 53.0%.
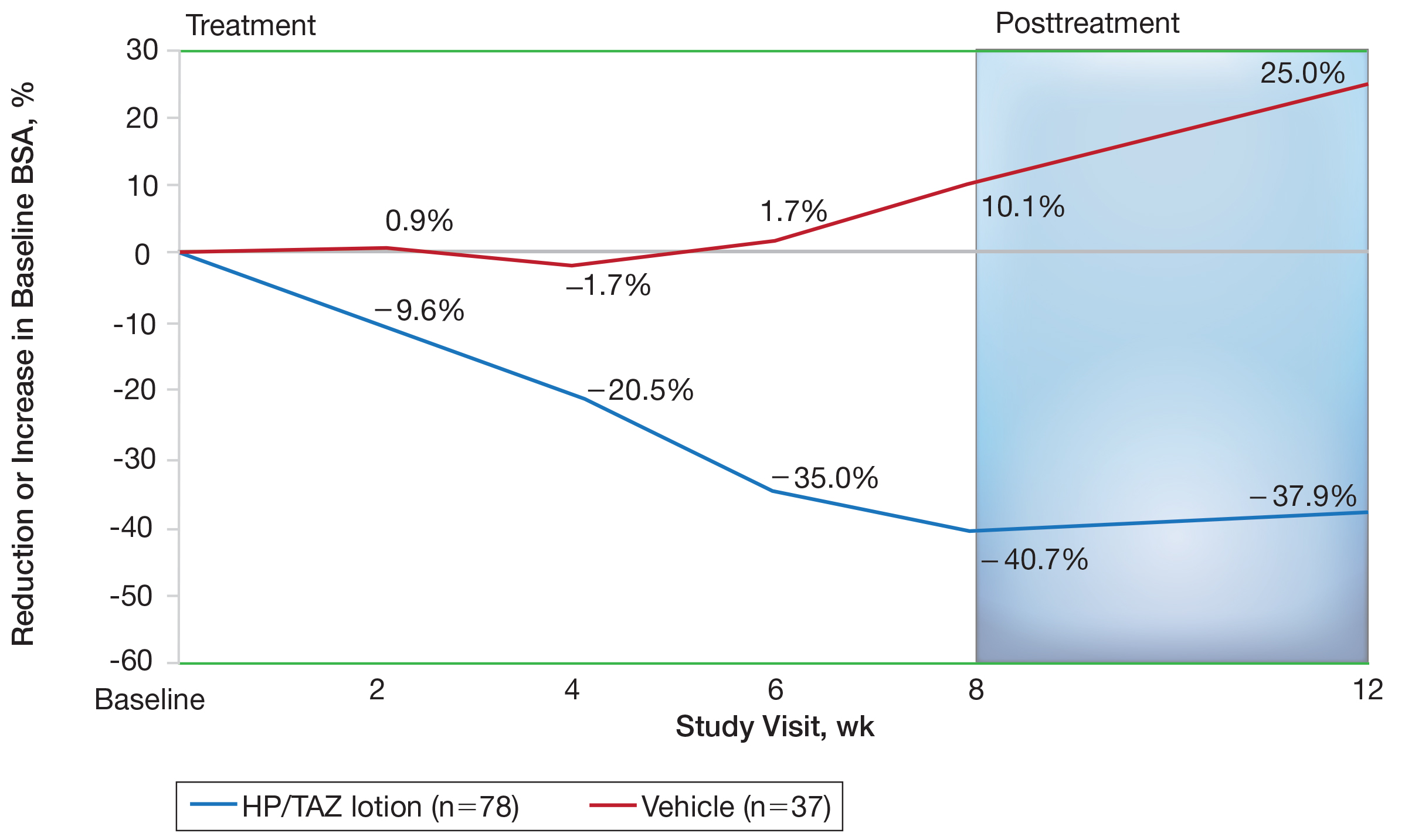
Mean (SD) baseline BSA was 6.2 (3.07), and the mean (SD) size of the target lesion was 36.3 (21.85) cm2. Overall, BSA also was significantly reduced in participants treated with HP/TAZ lotion compared to vehicle. At week 8, the mean percentage change from baseline was —40.7% compared to an increase (+10.1%) in the vehicle group (P=.002)(Figure 3). Improvements in BSA were maintained posttreatment, whereas in the vehicle group, mean (SD) BSA had increased to 6.1 (4.64).
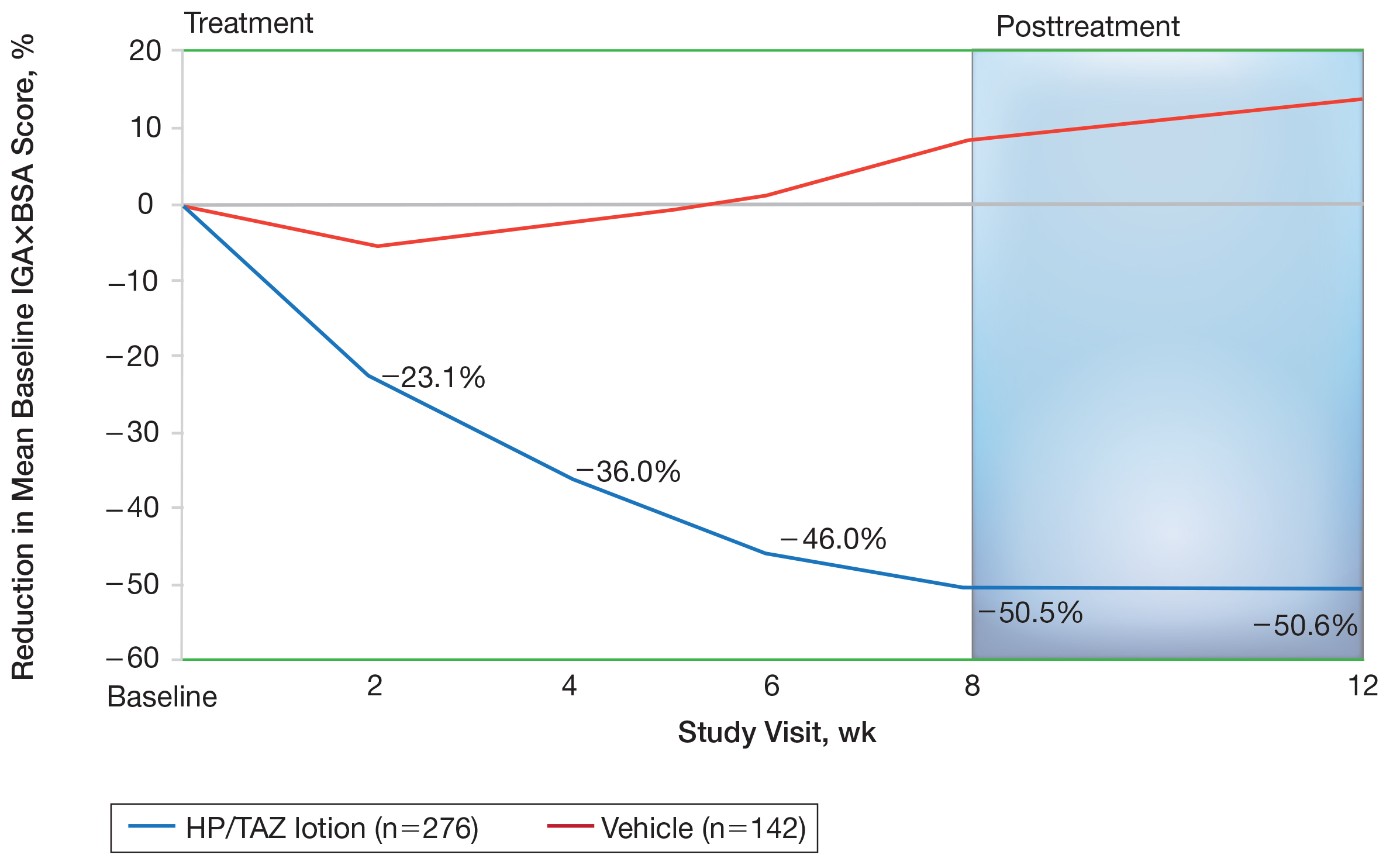
Halobetasol propionate 0.01%–TAZ 0.045% lotion achieved a 50.5% reduction from baseline IGA×BSA by week 8 compared to an 8.5% increase with vehicle (P<.001)(Figure 4). Differences in treatment groups were significant from week 2 (P=.016). Efficacy was maintained posttreatment, with a 50.6% reduction from baseline IGA×BSA at week 12 compared to an increase of 13.6% in the vehicle group (P<.001). Again, although results were similar to the overall study population at week 8 (50.5% vs 51.9%), maintenance of effect was better posttreatment (50.6% vs 46.6%).10
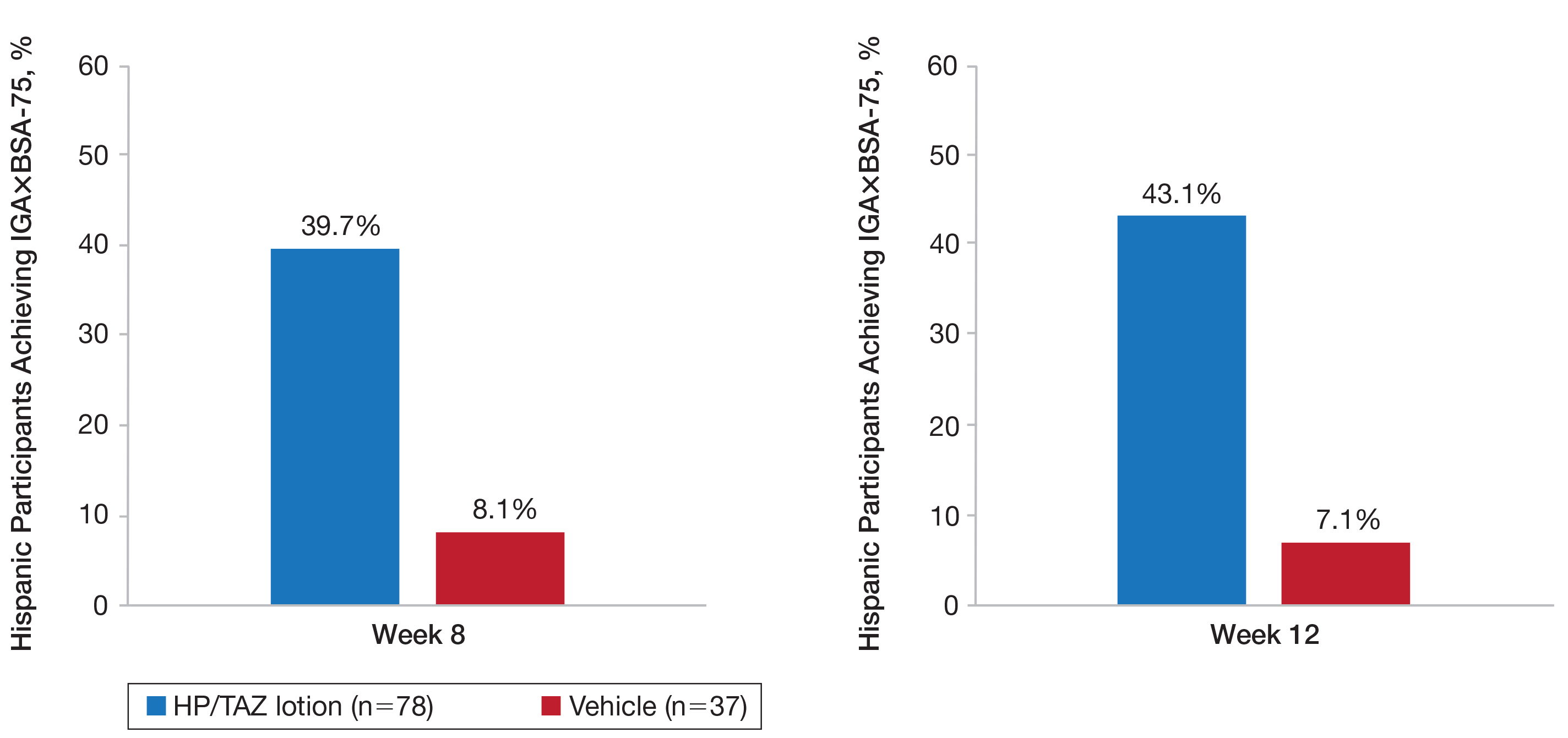
A clinically meaningful effect (IGA×BSA-75) was achieved in 39.7% of Hispanic participants treated with HP/TAZ lotion compared to 8.1% of participants treated with vehicle (P<.001) at week 8. The benefits were significantly different from week 4 and more participants maintained a clinically meaningful effect posttreatment (43.1% vs 7.1%, P<.001)(Figure 5).
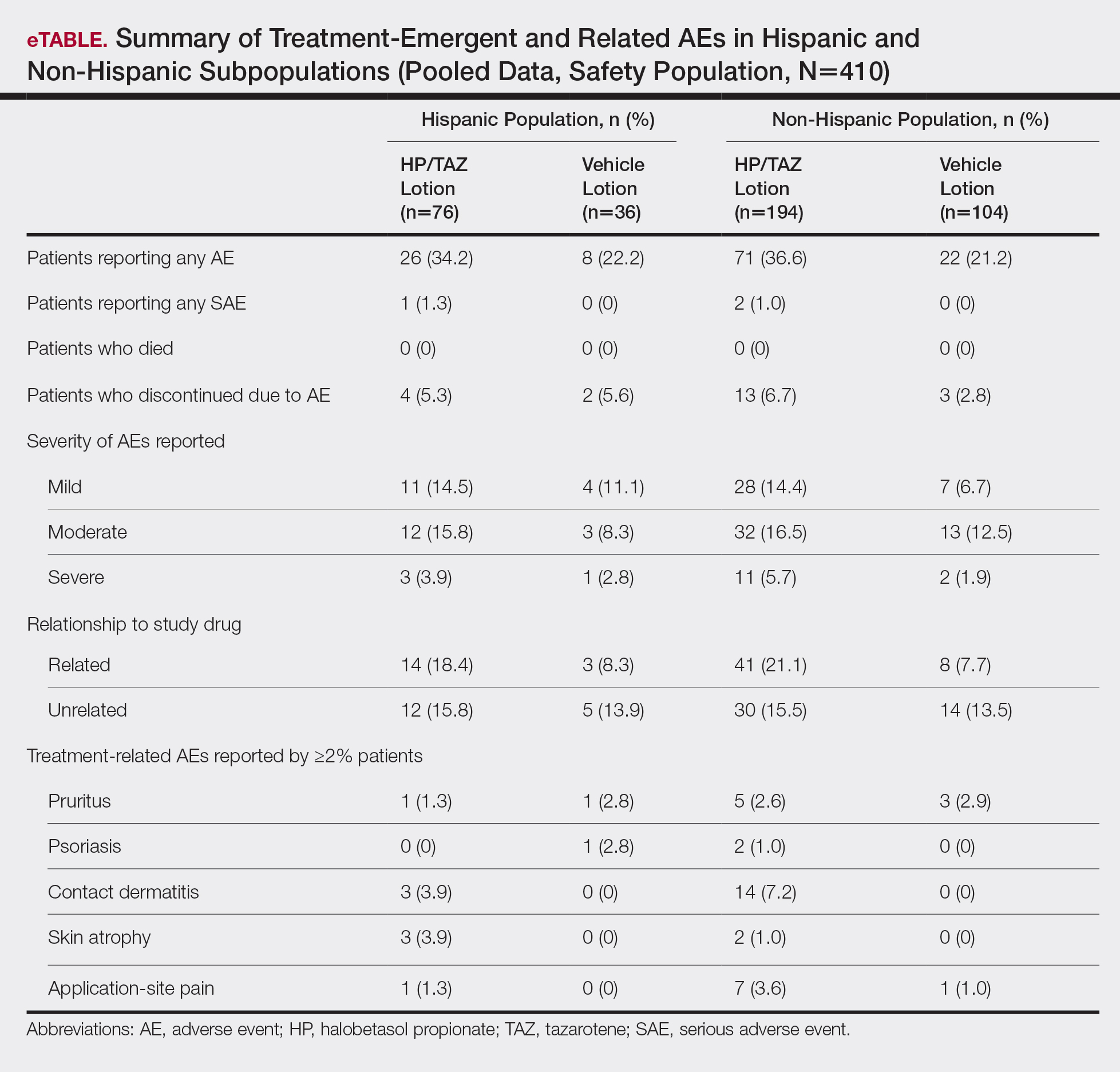
For Hispanic participants overall, 34 participants reported AEs: 26 (34.2%) treated with HP/TAZ lotion and 8 (22.2%) treated with vehicle (eTable). There was 1 (1.3%) serious AE in the HP/TAZ group. Most of the AEs were mild or moderate, with approximately half being related to study treatment. The most common treatment-related AEs in Hispanic participants treated with HP/TAZ lotion were contact dermatitis (n=3, 3.9%) and skin atrophy (n=3, 3.9%) compared to contact dermatitis (n=14, 7.2%) and application-site pain (n=7, 3.6%) in the non-Hispanic population. Pruritus was the most common AE in Hispanic participants treated with vehicle.
Comment
The large number of Hispanic patients in the 2 phase 3 trials8,9 allowed for this valuable subgroup analysis on the topical treatment of Hispanic patients with plaque psoriasis. Validation of observed differences in maintenance of effect and tolerability warrant further study. Prior clinical studies in psoriasis have tended to enroll a small proportion of Hispanic patients without any post hoc analysis. For example, in a pooled analysis of 4 phase 3 trials with secukinumab, Hispanic patients accounted for only 16% of the overall population.11 In our analysis, the Hispanic cohort represented 28% of the overall study population of 2 phase 3 studies investigating the efficacy, safety, and tolerability of HP/TAZ lotion in patients with moderate to severe psoriasis.8,9 In addition, proportionately more Hispanic patients had severe disease (IGA of 4) or severe signs and symptoms of psoriasis (erythema, plaque elevation, and scaling) than the non-Hispanic population. This finding supports other studies that have suggested Hispanic patients with psoriasis tend to have more severe disease but also may reflect thresholds for seeking care.3-5
Halobetasol propionate 0.01%–TAZ 0.045% lotion was significantly more effective than vehicle for all efficacy assessments. In general, efficacy results with HP/TAZ lotion were similar to those reported in the overall phase 3 study populations over the 8-week treatment period. The only noticeable difference was in the posttreatment period. In the overall study population, efficacy was maintained over the 4-week posttreatment period in the HP/TAZ group. In the Hispanic subpopulation, there appeared to be continued improvement in the number of participants achieving treatment success (IGA and erythema), clinically meaningful success, and further reductions in BSA. Although there is a paucity of studies evaluating psoriasis therapies in Hispanic populations, data on etanercept and secukinumab have been published.6,11
Onset of effect also is an important aspect of treatment. In patients with skin of color, including patients of Hispanic ethnicity and higher Fitzpatrick skin phototypes, early clearance of lesions may help limit the severity and duration of postinflammatory pigment alteration. Improvements in IGA×BSA scores were significant compared to vehicle from week 2 (P=.016), and a clinically meaningful improvement with HP/TAZ lotion (IGA×BSA-75) was seen by week 4 (P=.024).
Halobetasol propionate 0.01%–TAZ 0.045% lotion was well tolerated, both in the 2 phase 3 studies and in the post hoc analysis of the Hispanic subpopulation. The incidence of skin atrophy (n=3, 3.9%) was more common vs the non-Hispanic population (n=2, 1.0%). Other common AEs—contact dermatitis, pruritus, and application-site pain—were more common in the non-Hispanic population.
A limitation of our analysis was that it was a post hoc analysis of the Hispanic participants. The phase 3 studies were not designed to specifically study the impact of treatment on ethnicity/race, though the number of Hispanic participants enrolled in the 2 studies was relatively high. The absence of Fitzpatrick skin phototypes in this data set is another limitation of this study.
Conclusion
Halobetasol propionate 0.01%–TAZ 0.045% lotion was associated with significant, rapid, and sustained reductions in disease severity in a Hispanic population with moderate to severe psoriasis that continued to show improvement posttreatment with good tolerability and safety.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of the manuscript. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Yan D, Afifi L, Jeon C, et al. A cross-sectional study of the distribution of psoriasis subtypes in different ethno-racial groups. Dermatol Online J. 2018;24. pii:13030/qt5z21q4k2.
- Abrouk M, Lee K, Brodsky M, et al. Ethnicity affects the presenting severity of psoriasis. J Am Acad Dermatol. 2017;77:180-182.
- Shah SK, Arthur A, Yang YC, et al. A retrospective study to investigate racial and ethnic variations in the treatment of psoriasis with etanercept. J Drugs Dermatol. 2011;10:866-872.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Blauvelt A, Green LJ, Lebwohl MG, et al. Efficacy of a once-daily fixed combination halobetasol (0.01%) and tazarotene (0.045%) lotion in the treatment of localized moderate-to-severe plaque psoriasis. J Drugs Dermatol. 2019;18:297-299.
- Adsit S, Zaldivar ER, Sofen H, et al. Secukinumab is efficacious and safe in Hispanic patients with moderate-to-severe plaque psoriasis: pooled analysis of four phase 3 trials. Adv Ther. 2017;34:1327-1339.
Psoriasis is a common chronic inflammatory disease affecting a diverse patient population, yet epidemiological and clinical data related to psoriasis in patients with skin of color are sparse. The Hispanic ethnic group includes a broad range of skin types and cultures. Prevalence of psoriasis in a Hispanic population has been reported as lower than in a white population1; however, these data may be influenced by the finding that Hispanic patients are less likely to see a dermatologist when they have skin problems.2 In addition, socioeconomic disparities and cultural variations among racial/ethnic groups may contribute to differences in access to care and thresholds for seeking care,3 leading to a tendency for more severe disease in skin of color and Hispanic ethnic groups.4,5 Greater impairments in health-related quality of life have been reported in patients with skin of color and Hispanic racial/ethnic groups compared to white patients, independent of psoriasis severity.4,6 Postinflammatory pigment alteration at the sites of resolving lesions, a common clinical feature in skin of color, may contribute to the impact of psoriasis on quality of life in patients with skin of color. Psoriasis in darker skin types also can present diagnostic challenges due to overlapping features with other papulosquamous disorders and less conspicuous erythema.7
We present a post hoc analysis of the treatment of moderate to severe psoriasis with a novel fixed-combination halobetasol propionate (HP) 0.01%–tazarotene (TAZ) 0.045% lotion in a Hispanic patient population. Historically, clinical trials for psoriasis have enrolled low proportions of Hispanic patients and other patients with skin of color; in this analysis, the Hispanic population (115/418) represented 28% of the total study population and provided valuable insights.
Methods
Study Design
Two phase 3 randomized controlled trials were conducted to demonstrate the efficacy and safety of HP/TAZ lotion. Patients with a clinical diagnosis of moderate or severe localized psoriasis (N=418) were randomized to receive HP/TAZ lotion or vehicle (2:1 ratio) once daily for 8 weeks with a 4-week posttreatment follow-up.8,9 A post hoc analysis was conducted on data of the self-identified Hispanic population.
Assessments
Efficacy assessments included treatment success (at least a 2-grade improvement from baseline in the investigator global assessment [IGA] and a score of clear or almost clear) and impact on individual signs of psoriasis (at least a 2-grade improvement in erythema, plaque elevation, and scaling) at the target lesion. In addition, reduction in body surface area (BSA) was recorded, and an IGA×BSA score was calculated by multiplying IGA by BSA at each timepoint for each individual patient. A clinically meaningful improvement in disease severity (percentage of patients achieving a 75% reduction in IGA×BSA [IGA×BSA-75]) also was calculated.
Information on reported and observed adverse events (AEs) was obtained at each visit. The safety population included 112 participants (76 in the HP/TAZ group and 36 in the vehicle group).
Statistical Analysis
The statistical and analytical plan is detailed elsewhere9 and relevant to this post hoc analysis. No statistical analysis was carried out to compare data in the Hispanic population with either the overall study population or the non-Hispanic population.
Results
Overall, 115 Hispanic patients (27.5%) were enrolled (eFigure). Patients had a mean (standard deviation [SD]) age of 46.7 (13.12) years, and more than two-thirds were male (n=80, 69.6%).
Overall completion rates (80.0%) for Hispanic patients were similar to those in the overall study population, though there were more discontinuations in the vehicle group. The main reasons for treatment discontinuation among Hispanic patients were participant request (n=8, 7.0%), lost to follow-up (n=8, 7.0%), and AEs (n=4, 3.5%). Hispanic patients in this study had more severe disease—18.3% (n=21) had an IGA score of 4 compared to 13.5% (n=41) of non-Hispanic patients—and more severe erythema (19.1% vs 9.6%), plaque elevation (20.0% vs 10.2%), and scaling (15.7% vs 12.9%) compared to the non-Hispanic populations (Table).
Efficacy of HP/TAZ lotion in Hispanic patients was similar to the overall study populations,9 though maintenance of effect posttreatment appeared to be better. The incidence of treatment-related AEs also was lower.
Halobetasol propionate 0.01%–TAZ 0.045% lotion demonstrated statistically significant superiority based on treatment success compared to vehicle as early as week 4 (P=.034). By week 8, 39.3% of participants treated with HP/TAZ lotion achieved treatment success compared to 9.3% of participants in the vehicle group (P=.002)(Figure 1). Treatment success was maintained over the 4-week posttreatment period, whereby 40.5% of the HP/TAZ-treated participants were treatment successes at week 12 compared to only 4.1% of participants in the vehicle group (P<.001).
Improvements in psoriasis signs and symptoms at the target lesion were statistically significant compared to vehicle from week 2 (plaque elevation, P=.018) or week 4 (erythema, P=.004; scaling, P<.001)(Figure 2). By week 8, 46.8%, 58.1%, and 63.2% of participants showed at least a 2-grade improvement from baseline and were therefore treatment successes for erythema, plaque elevation, and scaling, respectively (all statistically significant [P<.001] compared to vehicle). The number of participants who achieved at least a 2-grade improvement in erythema with HP/TAZ lotion increased posttreatment from 46.8% to 53.0%.
Mean (SD) baseline BSA was 6.2 (3.07), and the mean (SD) size of the target lesion was 36.3 (21.85) cm2. Overall, BSA also was significantly reduced in participants treated with HP/TAZ lotion compared to vehicle. At week 8, the mean percentage change from baseline was —40.7% compared to an increase (+10.1%) in the vehicle group (P=.002)(Figure 3). Improvements in BSA were maintained posttreatment, whereas in the vehicle group, mean (SD) BSA had increased to 6.1 (4.64).
Halobetasol propionate 0.01%–TAZ 0.045% lotion achieved a 50.5% reduction from baseline IGA×BSA by week 8 compared to an 8.5% increase with vehicle (P<.001)(Figure 4). Differences in treatment groups were significant from week 2 (P=.016). Efficacy was maintained posttreatment, with a 50.6% reduction from baseline IGA×BSA at week 12 compared to an increase of 13.6% in the vehicle group (P<.001). Again, although results were similar to the overall study population at week 8 (50.5% vs 51.9%), maintenance of effect was better posttreatment (50.6% vs 46.6%).10
A clinically meaningful effect (IGA×BSA-75) was achieved in 39.7% of Hispanic participants treated with HP/TAZ lotion compared to 8.1% of participants treated with vehicle (P<.001) at week 8. The benefits were significantly different from week 4 and more participants maintained a clinically meaningful effect posttreatment (43.1% vs 7.1%, P<.001)(Figure 5).
For Hispanic participants overall, 34 participants reported AEs: 26 (34.2%) treated with HP/TAZ lotion and 8 (22.2%) treated with vehicle (eTable). There was 1 (1.3%) serious AE in the HP/TAZ group. Most of the AEs were mild or moderate, with approximately half being related to study treatment. The most common treatment-related AEs in Hispanic participants treated with HP/TAZ lotion were contact dermatitis (n=3, 3.9%) and skin atrophy (n=3, 3.9%) compared to contact dermatitis (n=14, 7.2%) and application-site pain (n=7, 3.6%) in the non-Hispanic population. Pruritus was the most common AE in Hispanic participants treated with vehicle.
Comment
The large number of Hispanic patients in the 2 phase 3 trials8,9 allowed for this valuable subgroup analysis on the topical treatment of Hispanic patients with plaque psoriasis. Validation of observed differences in maintenance of effect and tolerability warrant further study. Prior clinical studies in psoriasis have tended to enroll a small proportion of Hispanic patients without any post hoc analysis. For example, in a pooled analysis of 4 phase 3 trials with secukinumab, Hispanic patients accounted for only 16% of the overall population.11 In our analysis, the Hispanic cohort represented 28% of the overall study population of 2 phase 3 studies investigating the efficacy, safety, and tolerability of HP/TAZ lotion in patients with moderate to severe psoriasis.8,9 In addition, proportionately more Hispanic patients had severe disease (IGA of 4) or severe signs and symptoms of psoriasis (erythema, plaque elevation, and scaling) than the non-Hispanic population. This finding supports other studies that have suggested Hispanic patients with psoriasis tend to have more severe disease but also may reflect thresholds for seeking care.3-5
Halobetasol propionate 0.01%–TAZ 0.045% lotion was significantly more effective than vehicle for all efficacy assessments. In general, efficacy results with HP/TAZ lotion were similar to those reported in the overall phase 3 study populations over the 8-week treatment period. The only noticeable difference was in the posttreatment period. In the overall study population, efficacy was maintained over the 4-week posttreatment period in the HP/TAZ group. In the Hispanic subpopulation, there appeared to be continued improvement in the number of participants achieving treatment success (IGA and erythema), clinically meaningful success, and further reductions in BSA. Although there is a paucity of studies evaluating psoriasis therapies in Hispanic populations, data on etanercept and secukinumab have been published.6,11
Onset of effect also is an important aspect of treatment. In patients with skin of color, including patients of Hispanic ethnicity and higher Fitzpatrick skin phototypes, early clearance of lesions may help limit the severity and duration of postinflammatory pigment alteration. Improvements in IGA×BSA scores were significant compared to vehicle from week 2 (P=.016), and a clinically meaningful improvement with HP/TAZ lotion (IGA×BSA-75) was seen by week 4 (P=.024).
Halobetasol propionate 0.01%–TAZ 0.045% lotion was well tolerated, both in the 2 phase 3 studies and in the post hoc analysis of the Hispanic subpopulation. The incidence of skin atrophy (n=3, 3.9%) was more common vs the non-Hispanic population (n=2, 1.0%). Other common AEs—contact dermatitis, pruritus, and application-site pain—were more common in the non-Hispanic population.
A limitation of our analysis was that it was a post hoc analysis of the Hispanic participants. The phase 3 studies were not designed to specifically study the impact of treatment on ethnicity/race, though the number of Hispanic participants enrolled in the 2 studies was relatively high. The absence of Fitzpatrick skin phototypes in this data set is another limitation of this study.
Conclusion
Halobetasol propionate 0.01%–TAZ 0.045% lotion was associated with significant, rapid, and sustained reductions in disease severity in a Hispanic population with moderate to severe psoriasis that continued to show improvement posttreatment with good tolerability and safety.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of the manuscript. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
Psoriasis is a common chronic inflammatory disease affecting a diverse patient population, yet epidemiological and clinical data related to psoriasis in patients with skin of color are sparse. The Hispanic ethnic group includes a broad range of skin types and cultures. Prevalence of psoriasis in a Hispanic population has been reported as lower than in a white population1; however, these data may be influenced by the finding that Hispanic patients are less likely to see a dermatologist when they have skin problems.2 In addition, socioeconomic disparities and cultural variations among racial/ethnic groups may contribute to differences in access to care and thresholds for seeking care,3 leading to a tendency for more severe disease in skin of color and Hispanic ethnic groups.4,5 Greater impairments in health-related quality of life have been reported in patients with skin of color and Hispanic racial/ethnic groups compared to white patients, independent of psoriasis severity.4,6 Postinflammatory pigment alteration at the sites of resolving lesions, a common clinical feature in skin of color, may contribute to the impact of psoriasis on quality of life in patients with skin of color. Psoriasis in darker skin types also can present diagnostic challenges due to overlapping features with other papulosquamous disorders and less conspicuous erythema.7
We present a post hoc analysis of the treatment of moderate to severe psoriasis with a novel fixed-combination halobetasol propionate (HP) 0.01%–tazarotene (TAZ) 0.045% lotion in a Hispanic patient population. Historically, clinical trials for psoriasis have enrolled low proportions of Hispanic patients and other patients with skin of color; in this analysis, the Hispanic population (115/418) represented 28% of the total study population and provided valuable insights.
Methods
Study Design
Two phase 3 randomized controlled trials were conducted to demonstrate the efficacy and safety of HP/TAZ lotion. Patients with a clinical diagnosis of moderate or severe localized psoriasis (N=418) were randomized to receive HP/TAZ lotion or vehicle (2:1 ratio) once daily for 8 weeks with a 4-week posttreatment follow-up.8,9 A post hoc analysis was conducted on data of the self-identified Hispanic population.
Assessments
Efficacy assessments included treatment success (at least a 2-grade improvement from baseline in the investigator global assessment [IGA] and a score of clear or almost clear) and impact on individual signs of psoriasis (at least a 2-grade improvement in erythema, plaque elevation, and scaling) at the target lesion. In addition, reduction in body surface area (BSA) was recorded, and an IGA×BSA score was calculated by multiplying IGA by BSA at each timepoint for each individual patient. A clinically meaningful improvement in disease severity (percentage of patients achieving a 75% reduction in IGA×BSA [IGA×BSA-75]) also was calculated.
Information on reported and observed adverse events (AEs) was obtained at each visit. The safety population included 112 participants (76 in the HP/TAZ group and 36 in the vehicle group).
Statistical Analysis
The statistical and analytical plan is detailed elsewhere9 and relevant to this post hoc analysis. No statistical analysis was carried out to compare data in the Hispanic population with either the overall study population or the non-Hispanic population.
Results
Overall, 115 Hispanic patients (27.5%) were enrolled (eFigure). Patients had a mean (standard deviation [SD]) age of 46.7 (13.12) years, and more than two-thirds were male (n=80, 69.6%).
Overall completion rates (80.0%) for Hispanic patients were similar to those in the overall study population, though there were more discontinuations in the vehicle group. The main reasons for treatment discontinuation among Hispanic patients were participant request (n=8, 7.0%), lost to follow-up (n=8, 7.0%), and AEs (n=4, 3.5%). Hispanic patients in this study had more severe disease—18.3% (n=21) had an IGA score of 4 compared to 13.5% (n=41) of non-Hispanic patients—and more severe erythema (19.1% vs 9.6%), plaque elevation (20.0% vs 10.2%), and scaling (15.7% vs 12.9%) compared to the non-Hispanic populations (Table).
Efficacy of HP/TAZ lotion in Hispanic patients was similar to the overall study populations,9 though maintenance of effect posttreatment appeared to be better. The incidence of treatment-related AEs also was lower.
Halobetasol propionate 0.01%–TAZ 0.045% lotion demonstrated statistically significant superiority based on treatment success compared to vehicle as early as week 4 (P=.034). By week 8, 39.3% of participants treated with HP/TAZ lotion achieved treatment success compared to 9.3% of participants in the vehicle group (P=.002)(Figure 1). Treatment success was maintained over the 4-week posttreatment period, whereby 40.5% of the HP/TAZ-treated participants were treatment successes at week 12 compared to only 4.1% of participants in the vehicle group (P<.001).
Improvements in psoriasis signs and symptoms at the target lesion were statistically significant compared to vehicle from week 2 (plaque elevation, P=.018) or week 4 (erythema, P=.004; scaling, P<.001)(Figure 2). By week 8, 46.8%, 58.1%, and 63.2% of participants showed at least a 2-grade improvement from baseline and were therefore treatment successes for erythema, plaque elevation, and scaling, respectively (all statistically significant [P<.001] compared to vehicle). The number of participants who achieved at least a 2-grade improvement in erythema with HP/TAZ lotion increased posttreatment from 46.8% to 53.0%.
Mean (SD) baseline BSA was 6.2 (3.07), and the mean (SD) size of the target lesion was 36.3 (21.85) cm2. Overall, BSA also was significantly reduced in participants treated with HP/TAZ lotion compared to vehicle. At week 8, the mean percentage change from baseline was —40.7% compared to an increase (+10.1%) in the vehicle group (P=.002)(Figure 3). Improvements in BSA were maintained posttreatment, whereas in the vehicle group, mean (SD) BSA had increased to 6.1 (4.64).
Halobetasol propionate 0.01%–TAZ 0.045% lotion achieved a 50.5% reduction from baseline IGA×BSA by week 8 compared to an 8.5% increase with vehicle (P<.001)(Figure 4). Differences in treatment groups were significant from week 2 (P=.016). Efficacy was maintained posttreatment, with a 50.6% reduction from baseline IGA×BSA at week 12 compared to an increase of 13.6% in the vehicle group (P<.001). Again, although results were similar to the overall study population at week 8 (50.5% vs 51.9%), maintenance of effect was better posttreatment (50.6% vs 46.6%).10
A clinically meaningful effect (IGA×BSA-75) was achieved in 39.7% of Hispanic participants treated with HP/TAZ lotion compared to 8.1% of participants treated with vehicle (P<.001) at week 8. The benefits were significantly different from week 4 and more participants maintained a clinically meaningful effect posttreatment (43.1% vs 7.1%, P<.001)(Figure 5).
For Hispanic participants overall, 34 participants reported AEs: 26 (34.2%) treated with HP/TAZ lotion and 8 (22.2%) treated with vehicle (eTable). There was 1 (1.3%) serious AE in the HP/TAZ group. Most of the AEs were mild or moderate, with approximately half being related to study treatment. The most common treatment-related AEs in Hispanic participants treated with HP/TAZ lotion were contact dermatitis (n=3, 3.9%) and skin atrophy (n=3, 3.9%) compared to contact dermatitis (n=14, 7.2%) and application-site pain (n=7, 3.6%) in the non-Hispanic population. Pruritus was the most common AE in Hispanic participants treated with vehicle.
Comment
The large number of Hispanic patients in the 2 phase 3 trials8,9 allowed for this valuable subgroup analysis on the topical treatment of Hispanic patients with plaque psoriasis. Validation of observed differences in maintenance of effect and tolerability warrant further study. Prior clinical studies in psoriasis have tended to enroll a small proportion of Hispanic patients without any post hoc analysis. For example, in a pooled analysis of 4 phase 3 trials with secukinumab, Hispanic patients accounted for only 16% of the overall population.11 In our analysis, the Hispanic cohort represented 28% of the overall study population of 2 phase 3 studies investigating the efficacy, safety, and tolerability of HP/TAZ lotion in patients with moderate to severe psoriasis.8,9 In addition, proportionately more Hispanic patients had severe disease (IGA of 4) or severe signs and symptoms of psoriasis (erythema, plaque elevation, and scaling) than the non-Hispanic population. This finding supports other studies that have suggested Hispanic patients with psoriasis tend to have more severe disease but also may reflect thresholds for seeking care.3-5
Halobetasol propionate 0.01%–TAZ 0.045% lotion was significantly more effective than vehicle for all efficacy assessments. In general, efficacy results with HP/TAZ lotion were similar to those reported in the overall phase 3 study populations over the 8-week treatment period. The only noticeable difference was in the posttreatment period. In the overall study population, efficacy was maintained over the 4-week posttreatment period in the HP/TAZ group. In the Hispanic subpopulation, there appeared to be continued improvement in the number of participants achieving treatment success (IGA and erythema), clinically meaningful success, and further reductions in BSA. Although there is a paucity of studies evaluating psoriasis therapies in Hispanic populations, data on etanercept and secukinumab have been published.6,11
Onset of effect also is an important aspect of treatment. In patients with skin of color, including patients of Hispanic ethnicity and higher Fitzpatrick skin phototypes, early clearance of lesions may help limit the severity and duration of postinflammatory pigment alteration. Improvements in IGA×BSA scores were significant compared to vehicle from week 2 (P=.016), and a clinically meaningful improvement with HP/TAZ lotion (IGA×BSA-75) was seen by week 4 (P=.024).
Halobetasol propionate 0.01%–TAZ 0.045% lotion was well tolerated, both in the 2 phase 3 studies and in the post hoc analysis of the Hispanic subpopulation. The incidence of skin atrophy (n=3, 3.9%) was more common vs the non-Hispanic population (n=2, 1.0%). Other common AEs—contact dermatitis, pruritus, and application-site pain—were more common in the non-Hispanic population.
A limitation of our analysis was that it was a post hoc analysis of the Hispanic participants. The phase 3 studies were not designed to specifically study the impact of treatment on ethnicity/race, though the number of Hispanic participants enrolled in the 2 studies was relatively high. The absence of Fitzpatrick skin phototypes in this data set is another limitation of this study.
Conclusion
Halobetasol propionate 0.01%–TAZ 0.045% lotion was associated with significant, rapid, and sustained reductions in disease severity in a Hispanic population with moderate to severe psoriasis that continued to show improvement posttreatment with good tolerability and safety.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of the manuscript. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Yan D, Afifi L, Jeon C, et al. A cross-sectional study of the distribution of psoriasis subtypes in different ethno-racial groups. Dermatol Online J. 2018;24. pii:13030/qt5z21q4k2.
- Abrouk M, Lee K, Brodsky M, et al. Ethnicity affects the presenting severity of psoriasis. J Am Acad Dermatol. 2017;77:180-182.
- Shah SK, Arthur A, Yang YC, et al. A retrospective study to investigate racial and ethnic variations in the treatment of psoriasis with etanercept. J Drugs Dermatol. 2011;10:866-872.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Blauvelt A, Green LJ, Lebwohl MG, et al. Efficacy of a once-daily fixed combination halobetasol (0.01%) and tazarotene (0.045%) lotion in the treatment of localized moderate-to-severe plaque psoriasis. J Drugs Dermatol. 2019;18:297-299.
- Adsit S, Zaldivar ER, Sofen H, et al. Secukinumab is efficacious and safe in Hispanic patients with moderate-to-severe plaque psoriasis: pooled analysis of four phase 3 trials. Adv Ther. 2017;34:1327-1339.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Yan D, Afifi L, Jeon C, et al. A cross-sectional study of the distribution of psoriasis subtypes in different ethno-racial groups. Dermatol Online J. 2018;24. pii:13030/qt5z21q4k2.
- Abrouk M, Lee K, Brodsky M, et al. Ethnicity affects the presenting severity of psoriasis. J Am Acad Dermatol. 2017;77:180-182.
- Shah SK, Arthur A, Yang YC, et al. A retrospective study to investigate racial and ethnic variations in the treatment of psoriasis with etanercept. J Drugs Dermatol. 2011;10:866-872.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Blauvelt A, Green LJ, Lebwohl MG, et al. Efficacy of a once-daily fixed combination halobetasol (0.01%) and tazarotene (0.045%) lotion in the treatment of localized moderate-to-severe plaque psoriasis. J Drugs Dermatol. 2019;18:297-299.
- Adsit S, Zaldivar ER, Sofen H, et al. Secukinumab is efficacious and safe in Hispanic patients with moderate-to-severe plaque psoriasis: pooled analysis of four phase 3 trials. Adv Ther. 2017;34:1327-1339.
Practice Points
- Although psoriasis is a common inflammatory disease, data in the Hispanic population are sparse and disease may be more severe.
- A recent clinical investigation with halobetasol propionate 0.01%–tazarotene 0.045% lotion included a number of Hispanic patients, affording an ideal opportunity to provide important data on this population.
- This fixed-combination therapy was associated with significant, rapid, and sustained reductions in disease severity in a Hispanic population with moderate to severe psoriasis that continued to show improvement posttreatment with good tolerability and safety.