User login
Photographic Confirmation of Biopsy Sites Saves Lives
Quality photographic documentation of lesions prior to biopsy can decrease the risk of wrong site surgery, improve patient care, and save lives.
Preventable errors by health care workers are widespread and cause significant morbidity and mortality. Wrong site surgery (WSS) is a preventable error that causes harm through both the direct insult of surgery and propagation of the untreated initial problem. WSS also can cause poor patient outcomes, low morale, malpractice claims, and increased costs to the health care system. The estimated median prevalence of WSS across all specialties is 9 events per 1,000,000 surgical procedures, and an institutional study of 112,500 surgical procedures reported 1 wrong-site event, which involved removing the incorrect skin lesion and not removing the intended lesion.1,2
Though the prevalence is low when examining all specialties together, dermatology is also susceptible to WSS.3 Watson and colleagues demonstrated that 31% of intervention errors were due to WSS and suggested that prebiopsy photography helps decrease errors.4 Thus, the American Academy of Dermatology has emphasized the importance of reducing WSS.5 A study by Nijhawan and colleagues found that 25% of patients receiving Mohs surgery at a private single cancer center could not identify their biopsy location because the duration between biopsy and surgery allowed biopsy sites to heal well, which made finding the lesion difficult.6
Risk factors for WSS include having multiple health care providers (HCPs) living remote from the surgery location involved in the case, being a traveling veteran, receiving care at multiple facilities inside and outside the US Department of Veterans Affairs (VA) system, mislabeling photographs or specimens, and photographs not taken at time of biopsy and too close with no frame of reference to assist in finding the correct site. The VA electronic health record (EHR) is not integrated with outside facility EHRs, and the Office of Community Care (OCC) at the VA is responsible for obtaining copies of outside records. If unsuccessful, the HCP and/or patient must provide the records. Frequently, records are not received or require multiple attempts to be obtained. This mostly affects veterans receiving care at multiple facilities inside and outside the VA system as the lack of or timely receipt of past health records could increase the risk for WSS.
To combat WSS, some institutions have implemented standardized protocols requiring photographic documentation of lesions before biopsy so that the surgeon can properly identify the correct site prior to operating.7 Fortunately, recent advances in technology have made it easier to provide photographic documentation of skin lesions. Highsmith and colleagues highlighted use of smartphones to avoid WSS in dermatology.7 Despite these advances, photographic documentation of lesions is not universal. A study by Rossy and colleagues found that less than half of patients referred for Mohs surgery had clear documentation of the biopsy site with photography, diagram, or measurements, and of those documented, only a small fraction used photographs.8
Photographic documentation is not currently required by the VA, increasing the risk of WSS. About 20% of the ~150 VA dermatology departments nationwide are associated with a dermatology residency program and have implemented photographic documentation of lesions before biopsy. The other 80% of departments may not be using photographic documentation. The following 3 cases experienced by the authors highlight instances of how quality photographic documentation of lesions prior to biopsy can improve patient care and save lives. Then, we propose a photographic documentation protocol for VA dermatology departments to follow based on the photographic standards outlined by the American Society for Dermatologic Surgery.9
Case 1 Presentation
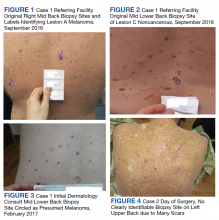
A 36-year-old traveling veteran who relocates frequently and receives care at multiple VA medical centers (VAMCs) presented for excision of a melanoma. The patient had been managed at another VAMC where the lesion was biopsied in September 2016. He presented to the Orlando, Florida, VAMC dermatology clinic 5 months later with the photographs of his biopsy sites along with the biopsy reports. The patient had 6 biopsies labeled A through F. Lesion A at the right mid back was positive for melanoma (Figure 1), whereas lesion C on the mid lower back was not cancerous (Figure 2). On examination of the patient’s back, he had numerous moles and scars. The initial receiving HCP circled and photographed the scar presumed to be the melanoma on the mid lower back (Figure 3).
On the day of surgery, the surgeon routinely checked the biopsy report as well as the photograph from the patient’s most recent HCP visit. The surgeon noted that biopsy A (right mid back) on the pathology report had been identified as the melanoma; however, biopsy C (mid lower back) was circled and presumed to be the melanoma in the recent photograph by the receiving HCP—a nurse practitioner. The surgeon compared the initial photos from the referring VAMC with those from the receiving HCP and subsequently matched biopsy A (melanoma) with the correct location on the right mid back.
This discrepancy was explained to the patient with photographic confirmation, allowing for agreement on the correct site before the surgery. The pathology results of the surgical excision confirmed melanoma in the specimen and clear margins. Thus, the correct site was operated on.
Case 2 Presentation
A veteran aged 86 years with a medical history of a double transplant and long-term immunosuppression leading to numerous skin cancers was referred for surgical excision of a confirmed squamous cell carcinoma (SCC) on the left upper back. On the day of surgery, the biopsy site could not be identified clearly due to numerous preexisting scars (Figure 4). No photograph of the original biopsy site was available. The referring HCP was called to the bedside to assist in identifying the biopsy site but also was unable to clearly identify the site. This was explained to the patient. As 2-person confirmation was unsuccessful, conservative treatment was used with patient consent. The patient has since had subsequent close follow-up to monitor for recurrence, as SCC in transplant patients can display aggressive growth and potential for metastasis.
Case 3 Presentation
A veteran was referred for surgical excision of a nonmelanoma skin cancer. The biopsy was completed well in advance of the anticipated surgery day. On the day of surgery, the site could not be detected as it healed well after the biopsy. Although a clinical photograph was available, it was taken too close-up to find a frame of reference for identifying the location of the biopsy site. The referring HCP was called to the bedside to assist in identification of the biopsy site, but 2-person confirmation was unsuccessful. This was explained to the patient, and with his consent, the HCPs agreed on conservative treatment and close follow-up.
Discussion
To prevent and minimize poor outcomes associated with WSS, the health care team should routinely document the lesion location in detail before the biopsy. Many HCPs believe a preoperative photograph is the best method for documentation. As demonstrated in the third case presentation, photographs must be taken at a distance that includes nearby anatomic landmarks for reference. It is suggested that the providers obtain 2 images, one that is far enough to include landmarks, and one that is close enough to clearly differentiate the targeted lesion from others.10
Although high-resolution digital cameras are preferred, mobile phones also can be used if they provide quality images. As phones with built-in cameras are ubiquitous, they offer a quick and easy method of photographic documentation. St John and colleagues also presented the possibility of having patients keep pictures of the lesion on their phones, as this removes potential privacy concerns and facilitates easy transportation of information between HCPs.10 If it is discovered that a photograph was not taken at the time of biopsy, our practice contacts the patient and asks them to photograph and circle the biopsy site using their mobile phone or camera and bring it to the surgery appointment. We propose a VA protocol for photographic documentation of biopsy sites (Table).
HCPs who are not comfortable with technology may be hesitant to use photographic documentation using a smartphone or camera. Further, HCPs often face time constraints, and taking photographs and uploading them to the EHR could decrease patient contact time. Therefore, photographic documentation presents an opportunity for a team approach to patient-centered care: Nursing and other medical staff can assist with these duties and learn the proper photographic documentation of biopsy sites. Using phone or tablet applications that provide rapid photographic documentation and uploading to the EHR also would facilitate universal use of photographic documentation.
If a HCP is uncomfortable or unable to use photography to document lesions, alternative strategies for documenting lesions exist, including diagrams, anatomic landmarks, ultraviolet (UV) fluorescent tattoos, and patient identification of lesions.10 In the diagram method, a HCP marks the lesion location on a diagram of the body preferably with a short description of the lesion’s location and/or characteristics.11 The diagram should be uploaded into the EHR. There are other methods for documenting lesion location relative to anatomic landmarks. Triangulation involves documenting distance between the lesion and 3 distinct anatomic locations.10 UV fluorescent tattooing involves putting UV tattoo dye in the biopsy site and locating the dye using a Wood lamp at the time of surgery. The lamp was used in a single case report of a patient with recurrent basal cell carcinoma.12 Patient identification of lesions by phone applications that allow patients to track their lesion, a phone selfie of the biopsy site, or a direct account of a lesion can be used to confirm lesion location based on the other methods mentioned.10
Patients often are poorly adherent to instructions aimed at reducing the risk of WSS. In a study that asked patients undergoing elective foot or ankle surgery to mark the foot not being operated on, 41% of patients were either partially or nonadherent with this request.13 Educating patients on the importance of lesion self-identification has the potential to improve identification of biopsy location and prevent WSS. Nursing and medical staff can provide patient education while photographing the biopsy site including taking a photograph with the patient’s cell phone for their records.
Due to subsequent morbidity and mortality that can result from WSS, photographic confirmation of biopsy sites is a step that surgeons can take to ensure identification of the correct site prior to surgery. Case 1 provides an example of how photographs taken prior to biopsy can prevent WSS. In a disease such as melanoma, photographs are particularly important, as insufficient treatment can lead to fatal metastases. To increase quality of care, all available photographs should be reviewed, especially in cases where the pathology report does not match the clinical presentation.
If WSS occurs, HCPs may be hesitant to disclose their mistakes due to potential lawsuits, the possibility that disclosure may inadvertently harm the patient, and their relative inexperience in and training regarding disclosure skills.14 Surgeons who perform WSS may receive severe penalties from state licensing boards, including suspension of medical license. Financially, many insurers will not compensate providers for WSS. Also, many incidents of WSS result in a malpractice claim, with about 80% of those cases resulting in a malpractice award.15 However, it is important that HCPs are open with their patients regarding WSS.
As demonstrated in case presentations 2 and 3, having 2-person confirmation and patient confirmation before to surgery is important in preventing WSS for patients who have poor documentation of biopsy sites. In cases where agreement is not achieved, HCPs can consider several other options to help identify lesions. Dermabrasion and alcohol wipes are options.10 Dermabrasion uses friction to expose surgical sights that have healed, scarred, or been hidden by sun damage.10 Alcohol wipes remove surface scale and crust, creating a glisten with tangential lighting that highlights surface irregularities. Anesthesia injection prior to surgery creates a blister at the location of the cancer. This is because skin cancer weakens the attachments between keratinocytes, and as a result, the hydrostatic pressure from the anesthesia favorably blisters the malignancy location.10,16
Dermoscopy is another strategy shown to help identify scar margins.10,17 Under dermoscopy, a scar demonstrates a white-pink homogenous patch with underlying vessels, whereas basal cell carcinoma remnants include blue-gray ovoid nests and globules, telangiectasias, spoke wheel and leaflike structures.17 As a final option, HCPs can perform an additional biopsy of potential cancer locations to find the lesion again.10 If the lesions cannot be identified, HCPs should consider conservative measures or less invasive treatments with close and frequent follow-up.
Conclusions
The cases described here highlight how the lack of proper photographic documentation can prevent the use of curative surgical treatment. In order to reduce WSS and improve quality care, HCPs must continue to take steps and create safeguards to minimize risk. Proper documentation of lesions prior to biopsy provides an effective route to reduce incidence of WSS. If the biopsy site cannot be found, various strategies to properly identify the site can be employed. If WSS occurs, it is important that HCPs provide full disclosure to patients. With a growing emphasis on patient safety measures and advances in technology, HCPs are becoming increasingly cognizant about the most effective ways to optimize patient care, and it is anticipated that this will result in a decrease in morbidity and mortality.
1. Hempel S, Maggard-Gibbons M, Nguyen DK, et al. Wrong-site surgery, retained surgical items, and surgical fires: a systematic review of surgical never events. JAMA Surg. 2015;150(8):796-805. doi:10.1001/jamasurg.2015.0301
2. Knight N, Aucar J. Use of an anatomic marking form as an alternative to the Universal Protocol for Preventing Wrong Site, Wrong Procedure and Wrong Person Surgery. Am J Surg. 2010;200(6):803-809. doi:10.1016/j.amjsurg.2010.06.010
3. Elston DM, Stratman EJ, Miller SJ. Skin biopsy: biopsy issues in specific diseases [published correction appears in J Am Acad Dermatol. 2016 Oct;75(4):854]. J Am Acad Dermatol. 2016;74(1):1-18. doi:10.1016/j.jaad.2015.06.033
4. Watson AJ, Redbord K, Taylor JS, Shippy A, Kostecki J, Swerlick R. Medical error in dermatology practice: development of a classification system to drive priority setting in patient safety efforts. J Am Acad Dermatol. 2013;68(5):729-737. doi:10.1016/j.jaad.2012.10.058
5. Elston DM, Taylor JS, Coldiron B, et al. Patient safety: Part I. Patient safety and the dermatologist. J Am Acad Dermatol. 2009;61(2):179-191. doi:10.1016/j.jaad.2009.04.056
6. Nijhawan RI, Lee EH, Nehal KS. Biopsy site selfies--a quality improvement pilot study to assist with correct surgical site identification. Dermatol Surg. 2015;41(4):499-504. doi:10.1097/DSS.0000000000000305
7. Highsmith JT, Weinstein DA, Highsmith MJ, Etzkorn JR. BIOPSY 1-2-3 in dermatologic surgery: improving smartphone use to avoid wrong-site surgery. Technol Innov. 2016;18(2-3):203-206. doi:10.21300/18.2-3.2016.203
8. Rossy KM, Lawrence N. Difficulty with surgical site identification: what role does it play in dermatology? J Am Acad Dermatol. 2012;67(2):257-261. doi:10.1016/j.jaad.2012.02.034
9. American Society for Dermatologic Surgery. Photographic standards in dermatologic surgery poster. Accessed April 12, 2021. https://www.asds.net/medical-professionals/members-resources/product-details/productname/photographic-standards-poster
10. St John J, Walker J, Goldberg D, Maloney ME. Avoiding Medical Errors in Cutaneous Site Identification: A Best Practices Review. Dermatol Surg. 2016;42(4):477-484. doi:10.1097/DSS.0000000000000683
11. Alam M, Lee A, Ibrahimi OA, et al. A multistep approach to improving biopsy site identification in dermatology: physician, staff, and patient roles based on a Delphi consensus. JAMA Dermatol. 2014;150(5):550-558. doi:10.1001/jamadermatol.2013.9804
12. Chuang GS, Gilchrest BA. Ultraviolet-fluorescent tattoo location of cutaneous biopsy site. Dermatol Surg. 2012;38(3):479-483. doi:10.1111/j.1524-4725.2011.02238.x
13. DiGiovanni CW, Kang L, Manuel J. Patient compliance in avoiding wrong-site surgery. J Bone Joint Surg Am. 2003;85(5):815-819. doi:10.2106/00004623-200305000-00007
14. Gallagher TH. A 62-year-old woman with skin cancer who experienced wrong-site surgery: review of medical error. JAMA. 2009;302(6):669-677. doi:10.1001/jama.2009.1011
15. Mulloy DF, Hughes RG. Wrong-site surgery: a preventable medical error. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Agency for Healthcare Research and Quality (US); 2008:chap 36. Accessed April 23, 2021. https://www.ncbi.nlm.nih.gov/books/NBK2678
16. Zaiac M, Tongdee E, Porges L, Touloei K, Prodanovich S. Anesthetic blister induction to identify biopsy site prior to Mohs surgery. J Drugs Dermatol. 2015;14(5):446-447.
17. Jawed SI, Goldberg LH, Wang SQ. Dermoscopy to identify biopsy sites before Mohs surgery. Dermatol Surg. 2014;40(3):334-337. doi:10.1111/dsu.12422
Quality photographic documentation of lesions prior to biopsy can decrease the risk of wrong site surgery, improve patient care, and save lives.
Quality photographic documentation of lesions prior to biopsy can decrease the risk of wrong site surgery, improve patient care, and save lives.
Preventable errors by health care workers are widespread and cause significant morbidity and mortality. Wrong site surgery (WSS) is a preventable error that causes harm through both the direct insult of surgery and propagation of the untreated initial problem. WSS also can cause poor patient outcomes, low morale, malpractice claims, and increased costs to the health care system. The estimated median prevalence of WSS across all specialties is 9 events per 1,000,000 surgical procedures, and an institutional study of 112,500 surgical procedures reported 1 wrong-site event, which involved removing the incorrect skin lesion and not removing the intended lesion.1,2
Though the prevalence is low when examining all specialties together, dermatology is also susceptible to WSS.3 Watson and colleagues demonstrated that 31% of intervention errors were due to WSS and suggested that prebiopsy photography helps decrease errors.4 Thus, the American Academy of Dermatology has emphasized the importance of reducing WSS.5 A study by Nijhawan and colleagues found that 25% of patients receiving Mohs surgery at a private single cancer center could not identify their biopsy location because the duration between biopsy and surgery allowed biopsy sites to heal well, which made finding the lesion difficult.6
Risk factors for WSS include having multiple health care providers (HCPs) living remote from the surgery location involved in the case, being a traveling veteran, receiving care at multiple facilities inside and outside the US Department of Veterans Affairs (VA) system, mislabeling photographs or specimens, and photographs not taken at time of biopsy and too close with no frame of reference to assist in finding the correct site. The VA electronic health record (EHR) is not integrated with outside facility EHRs, and the Office of Community Care (OCC) at the VA is responsible for obtaining copies of outside records. If unsuccessful, the HCP and/or patient must provide the records. Frequently, records are not received or require multiple attempts to be obtained. This mostly affects veterans receiving care at multiple facilities inside and outside the VA system as the lack of or timely receipt of past health records could increase the risk for WSS.
To combat WSS, some institutions have implemented standardized protocols requiring photographic documentation of lesions before biopsy so that the surgeon can properly identify the correct site prior to operating.7 Fortunately, recent advances in technology have made it easier to provide photographic documentation of skin lesions. Highsmith and colleagues highlighted use of smartphones to avoid WSS in dermatology.7 Despite these advances, photographic documentation of lesions is not universal. A study by Rossy and colleagues found that less than half of patients referred for Mohs surgery had clear documentation of the biopsy site with photography, diagram, or measurements, and of those documented, only a small fraction used photographs.8
Photographic documentation is not currently required by the VA, increasing the risk of WSS. About 20% of the ~150 VA dermatology departments nationwide are associated with a dermatology residency program and have implemented photographic documentation of lesions before biopsy. The other 80% of departments may not be using photographic documentation. The following 3 cases experienced by the authors highlight instances of how quality photographic documentation of lesions prior to biopsy can improve patient care and save lives. Then, we propose a photographic documentation protocol for VA dermatology departments to follow based on the photographic standards outlined by the American Society for Dermatologic Surgery.9
Case 1 Presentation
A 36-year-old traveling veteran who relocates frequently and receives care at multiple VA medical centers (VAMCs) presented for excision of a melanoma. The patient had been managed at another VAMC where the lesion was biopsied in September 2016. He presented to the Orlando, Florida, VAMC dermatology clinic 5 months later with the photographs of his biopsy sites along with the biopsy reports. The patient had 6 biopsies labeled A through F. Lesion A at the right mid back was positive for melanoma (Figure 1), whereas lesion C on the mid lower back was not cancerous (Figure 2). On examination of the patient’s back, he had numerous moles and scars. The initial receiving HCP circled and photographed the scar presumed to be the melanoma on the mid lower back (Figure 3).
On the day of surgery, the surgeon routinely checked the biopsy report as well as the photograph from the patient’s most recent HCP visit. The surgeon noted that biopsy A (right mid back) on the pathology report had been identified as the melanoma; however, biopsy C (mid lower back) was circled and presumed to be the melanoma in the recent photograph by the receiving HCP—a nurse practitioner. The surgeon compared the initial photos from the referring VAMC with those from the receiving HCP and subsequently matched biopsy A (melanoma) with the correct location on the right mid back.
This discrepancy was explained to the patient with photographic confirmation, allowing for agreement on the correct site before the surgery. The pathology results of the surgical excision confirmed melanoma in the specimen and clear margins. Thus, the correct site was operated on.
Case 2 Presentation
A veteran aged 86 years with a medical history of a double transplant and long-term immunosuppression leading to numerous skin cancers was referred for surgical excision of a confirmed squamous cell carcinoma (SCC) on the left upper back. On the day of surgery, the biopsy site could not be identified clearly due to numerous preexisting scars (Figure 4). No photograph of the original biopsy site was available. The referring HCP was called to the bedside to assist in identifying the biopsy site but also was unable to clearly identify the site. This was explained to the patient. As 2-person confirmation was unsuccessful, conservative treatment was used with patient consent. The patient has since had subsequent close follow-up to monitor for recurrence, as SCC in transplant patients can display aggressive growth and potential for metastasis.
Case 3 Presentation
A veteran was referred for surgical excision of a nonmelanoma skin cancer. The biopsy was completed well in advance of the anticipated surgery day. On the day of surgery, the site could not be detected as it healed well after the biopsy. Although a clinical photograph was available, it was taken too close-up to find a frame of reference for identifying the location of the biopsy site. The referring HCP was called to the bedside to assist in identification of the biopsy site, but 2-person confirmation was unsuccessful. This was explained to the patient, and with his consent, the HCPs agreed on conservative treatment and close follow-up.
Discussion
To prevent and minimize poor outcomes associated with WSS, the health care team should routinely document the lesion location in detail before the biopsy. Many HCPs believe a preoperative photograph is the best method for documentation. As demonstrated in the third case presentation, photographs must be taken at a distance that includes nearby anatomic landmarks for reference. It is suggested that the providers obtain 2 images, one that is far enough to include landmarks, and one that is close enough to clearly differentiate the targeted lesion from others.10
Although high-resolution digital cameras are preferred, mobile phones also can be used if they provide quality images. As phones with built-in cameras are ubiquitous, they offer a quick and easy method of photographic documentation. St John and colleagues also presented the possibility of having patients keep pictures of the lesion on their phones, as this removes potential privacy concerns and facilitates easy transportation of information between HCPs.10 If it is discovered that a photograph was not taken at the time of biopsy, our practice contacts the patient and asks them to photograph and circle the biopsy site using their mobile phone or camera and bring it to the surgery appointment. We propose a VA protocol for photographic documentation of biopsy sites (Table).
HCPs who are not comfortable with technology may be hesitant to use photographic documentation using a smartphone or camera. Further, HCPs often face time constraints, and taking photographs and uploading them to the EHR could decrease patient contact time. Therefore, photographic documentation presents an opportunity for a team approach to patient-centered care: Nursing and other medical staff can assist with these duties and learn the proper photographic documentation of biopsy sites. Using phone or tablet applications that provide rapid photographic documentation and uploading to the EHR also would facilitate universal use of photographic documentation.
If a HCP is uncomfortable or unable to use photography to document lesions, alternative strategies for documenting lesions exist, including diagrams, anatomic landmarks, ultraviolet (UV) fluorescent tattoos, and patient identification of lesions.10 In the diagram method, a HCP marks the lesion location on a diagram of the body preferably with a short description of the lesion’s location and/or characteristics.11 The diagram should be uploaded into the EHR. There are other methods for documenting lesion location relative to anatomic landmarks. Triangulation involves documenting distance between the lesion and 3 distinct anatomic locations.10 UV fluorescent tattooing involves putting UV tattoo dye in the biopsy site and locating the dye using a Wood lamp at the time of surgery. The lamp was used in a single case report of a patient with recurrent basal cell carcinoma.12 Patient identification of lesions by phone applications that allow patients to track their lesion, a phone selfie of the biopsy site, or a direct account of a lesion can be used to confirm lesion location based on the other methods mentioned.10
Patients often are poorly adherent to instructions aimed at reducing the risk of WSS. In a study that asked patients undergoing elective foot or ankle surgery to mark the foot not being operated on, 41% of patients were either partially or nonadherent with this request.13 Educating patients on the importance of lesion self-identification has the potential to improve identification of biopsy location and prevent WSS. Nursing and medical staff can provide patient education while photographing the biopsy site including taking a photograph with the patient’s cell phone for their records.
Due to subsequent morbidity and mortality that can result from WSS, photographic confirmation of biopsy sites is a step that surgeons can take to ensure identification of the correct site prior to surgery. Case 1 provides an example of how photographs taken prior to biopsy can prevent WSS. In a disease such as melanoma, photographs are particularly important, as insufficient treatment can lead to fatal metastases. To increase quality of care, all available photographs should be reviewed, especially in cases where the pathology report does not match the clinical presentation.
If WSS occurs, HCPs may be hesitant to disclose their mistakes due to potential lawsuits, the possibility that disclosure may inadvertently harm the patient, and their relative inexperience in and training regarding disclosure skills.14 Surgeons who perform WSS may receive severe penalties from state licensing boards, including suspension of medical license. Financially, many insurers will not compensate providers for WSS. Also, many incidents of WSS result in a malpractice claim, with about 80% of those cases resulting in a malpractice award.15 However, it is important that HCPs are open with their patients regarding WSS.
As demonstrated in case presentations 2 and 3, having 2-person confirmation and patient confirmation before to surgery is important in preventing WSS for patients who have poor documentation of biopsy sites. In cases where agreement is not achieved, HCPs can consider several other options to help identify lesions. Dermabrasion and alcohol wipes are options.10 Dermabrasion uses friction to expose surgical sights that have healed, scarred, or been hidden by sun damage.10 Alcohol wipes remove surface scale and crust, creating a glisten with tangential lighting that highlights surface irregularities. Anesthesia injection prior to surgery creates a blister at the location of the cancer. This is because skin cancer weakens the attachments between keratinocytes, and as a result, the hydrostatic pressure from the anesthesia favorably blisters the malignancy location.10,16
Dermoscopy is another strategy shown to help identify scar margins.10,17 Under dermoscopy, a scar demonstrates a white-pink homogenous patch with underlying vessels, whereas basal cell carcinoma remnants include blue-gray ovoid nests and globules, telangiectasias, spoke wheel and leaflike structures.17 As a final option, HCPs can perform an additional biopsy of potential cancer locations to find the lesion again.10 If the lesions cannot be identified, HCPs should consider conservative measures or less invasive treatments with close and frequent follow-up.
Conclusions
The cases described here highlight how the lack of proper photographic documentation can prevent the use of curative surgical treatment. In order to reduce WSS and improve quality care, HCPs must continue to take steps and create safeguards to minimize risk. Proper documentation of lesions prior to biopsy provides an effective route to reduce incidence of WSS. If the biopsy site cannot be found, various strategies to properly identify the site can be employed. If WSS occurs, it is important that HCPs provide full disclosure to patients. With a growing emphasis on patient safety measures and advances in technology, HCPs are becoming increasingly cognizant about the most effective ways to optimize patient care, and it is anticipated that this will result in a decrease in morbidity and mortality.
Preventable errors by health care workers are widespread and cause significant morbidity and mortality. Wrong site surgery (WSS) is a preventable error that causes harm through both the direct insult of surgery and propagation of the untreated initial problem. WSS also can cause poor patient outcomes, low morale, malpractice claims, and increased costs to the health care system. The estimated median prevalence of WSS across all specialties is 9 events per 1,000,000 surgical procedures, and an institutional study of 112,500 surgical procedures reported 1 wrong-site event, which involved removing the incorrect skin lesion and not removing the intended lesion.1,2
Though the prevalence is low when examining all specialties together, dermatology is also susceptible to WSS.3 Watson and colleagues demonstrated that 31% of intervention errors were due to WSS and suggested that prebiopsy photography helps decrease errors.4 Thus, the American Academy of Dermatology has emphasized the importance of reducing WSS.5 A study by Nijhawan and colleagues found that 25% of patients receiving Mohs surgery at a private single cancer center could not identify their biopsy location because the duration between biopsy and surgery allowed biopsy sites to heal well, which made finding the lesion difficult.6
Risk factors for WSS include having multiple health care providers (HCPs) living remote from the surgery location involved in the case, being a traveling veteran, receiving care at multiple facilities inside and outside the US Department of Veterans Affairs (VA) system, mislabeling photographs or specimens, and photographs not taken at time of biopsy and too close with no frame of reference to assist in finding the correct site. The VA electronic health record (EHR) is not integrated with outside facility EHRs, and the Office of Community Care (OCC) at the VA is responsible for obtaining copies of outside records. If unsuccessful, the HCP and/or patient must provide the records. Frequently, records are not received or require multiple attempts to be obtained. This mostly affects veterans receiving care at multiple facilities inside and outside the VA system as the lack of or timely receipt of past health records could increase the risk for WSS.
To combat WSS, some institutions have implemented standardized protocols requiring photographic documentation of lesions before biopsy so that the surgeon can properly identify the correct site prior to operating.7 Fortunately, recent advances in technology have made it easier to provide photographic documentation of skin lesions. Highsmith and colleagues highlighted use of smartphones to avoid WSS in dermatology.7 Despite these advances, photographic documentation of lesions is not universal. A study by Rossy and colleagues found that less than half of patients referred for Mohs surgery had clear documentation of the biopsy site with photography, diagram, or measurements, and of those documented, only a small fraction used photographs.8
Photographic documentation is not currently required by the VA, increasing the risk of WSS. About 20% of the ~150 VA dermatology departments nationwide are associated with a dermatology residency program and have implemented photographic documentation of lesions before biopsy. The other 80% of departments may not be using photographic documentation. The following 3 cases experienced by the authors highlight instances of how quality photographic documentation of lesions prior to biopsy can improve patient care and save lives. Then, we propose a photographic documentation protocol for VA dermatology departments to follow based on the photographic standards outlined by the American Society for Dermatologic Surgery.9
Case 1 Presentation
A 36-year-old traveling veteran who relocates frequently and receives care at multiple VA medical centers (VAMCs) presented for excision of a melanoma. The patient had been managed at another VAMC where the lesion was biopsied in September 2016. He presented to the Orlando, Florida, VAMC dermatology clinic 5 months later with the photographs of his biopsy sites along with the biopsy reports. The patient had 6 biopsies labeled A through F. Lesion A at the right mid back was positive for melanoma (Figure 1), whereas lesion C on the mid lower back was not cancerous (Figure 2). On examination of the patient’s back, he had numerous moles and scars. The initial receiving HCP circled and photographed the scar presumed to be the melanoma on the mid lower back (Figure 3).
On the day of surgery, the surgeon routinely checked the biopsy report as well as the photograph from the patient’s most recent HCP visit. The surgeon noted that biopsy A (right mid back) on the pathology report had been identified as the melanoma; however, biopsy C (mid lower back) was circled and presumed to be the melanoma in the recent photograph by the receiving HCP—a nurse practitioner. The surgeon compared the initial photos from the referring VAMC with those from the receiving HCP and subsequently matched biopsy A (melanoma) with the correct location on the right mid back.
This discrepancy was explained to the patient with photographic confirmation, allowing for agreement on the correct site before the surgery. The pathology results of the surgical excision confirmed melanoma in the specimen and clear margins. Thus, the correct site was operated on.
Case 2 Presentation
A veteran aged 86 years with a medical history of a double transplant and long-term immunosuppression leading to numerous skin cancers was referred for surgical excision of a confirmed squamous cell carcinoma (SCC) on the left upper back. On the day of surgery, the biopsy site could not be identified clearly due to numerous preexisting scars (Figure 4). No photograph of the original biopsy site was available. The referring HCP was called to the bedside to assist in identifying the biopsy site but also was unable to clearly identify the site. This was explained to the patient. As 2-person confirmation was unsuccessful, conservative treatment was used with patient consent. The patient has since had subsequent close follow-up to monitor for recurrence, as SCC in transplant patients can display aggressive growth and potential for metastasis.
Case 3 Presentation
A veteran was referred for surgical excision of a nonmelanoma skin cancer. The biopsy was completed well in advance of the anticipated surgery day. On the day of surgery, the site could not be detected as it healed well after the biopsy. Although a clinical photograph was available, it was taken too close-up to find a frame of reference for identifying the location of the biopsy site. The referring HCP was called to the bedside to assist in identification of the biopsy site, but 2-person confirmation was unsuccessful. This was explained to the patient, and with his consent, the HCPs agreed on conservative treatment and close follow-up.
Discussion
To prevent and minimize poor outcomes associated with WSS, the health care team should routinely document the lesion location in detail before the biopsy. Many HCPs believe a preoperative photograph is the best method for documentation. As demonstrated in the third case presentation, photographs must be taken at a distance that includes nearby anatomic landmarks for reference. It is suggested that the providers obtain 2 images, one that is far enough to include landmarks, and one that is close enough to clearly differentiate the targeted lesion from others.10
Although high-resolution digital cameras are preferred, mobile phones also can be used if they provide quality images. As phones with built-in cameras are ubiquitous, they offer a quick and easy method of photographic documentation. St John and colleagues also presented the possibility of having patients keep pictures of the lesion on their phones, as this removes potential privacy concerns and facilitates easy transportation of information between HCPs.10 If it is discovered that a photograph was not taken at the time of biopsy, our practice contacts the patient and asks them to photograph and circle the biopsy site using their mobile phone or camera and bring it to the surgery appointment. We propose a VA protocol for photographic documentation of biopsy sites (Table).
HCPs who are not comfortable with technology may be hesitant to use photographic documentation using a smartphone or camera. Further, HCPs often face time constraints, and taking photographs and uploading them to the EHR could decrease patient contact time. Therefore, photographic documentation presents an opportunity for a team approach to patient-centered care: Nursing and other medical staff can assist with these duties and learn the proper photographic documentation of biopsy sites. Using phone or tablet applications that provide rapid photographic documentation and uploading to the EHR also would facilitate universal use of photographic documentation.
If a HCP is uncomfortable or unable to use photography to document lesions, alternative strategies for documenting lesions exist, including diagrams, anatomic landmarks, ultraviolet (UV) fluorescent tattoos, and patient identification of lesions.10 In the diagram method, a HCP marks the lesion location on a diagram of the body preferably with a short description of the lesion’s location and/or characteristics.11 The diagram should be uploaded into the EHR. There are other methods for documenting lesion location relative to anatomic landmarks. Triangulation involves documenting distance between the lesion and 3 distinct anatomic locations.10 UV fluorescent tattooing involves putting UV tattoo dye in the biopsy site and locating the dye using a Wood lamp at the time of surgery. The lamp was used in a single case report of a patient with recurrent basal cell carcinoma.12 Patient identification of lesions by phone applications that allow patients to track their lesion, a phone selfie of the biopsy site, or a direct account of a lesion can be used to confirm lesion location based on the other methods mentioned.10
Patients often are poorly adherent to instructions aimed at reducing the risk of WSS. In a study that asked patients undergoing elective foot or ankle surgery to mark the foot not being operated on, 41% of patients were either partially or nonadherent with this request.13 Educating patients on the importance of lesion self-identification has the potential to improve identification of biopsy location and prevent WSS. Nursing and medical staff can provide patient education while photographing the biopsy site including taking a photograph with the patient’s cell phone for their records.
Due to subsequent morbidity and mortality that can result from WSS, photographic confirmation of biopsy sites is a step that surgeons can take to ensure identification of the correct site prior to surgery. Case 1 provides an example of how photographs taken prior to biopsy can prevent WSS. In a disease such as melanoma, photographs are particularly important, as insufficient treatment can lead to fatal metastases. To increase quality of care, all available photographs should be reviewed, especially in cases where the pathology report does not match the clinical presentation.
If WSS occurs, HCPs may be hesitant to disclose their mistakes due to potential lawsuits, the possibility that disclosure may inadvertently harm the patient, and their relative inexperience in and training regarding disclosure skills.14 Surgeons who perform WSS may receive severe penalties from state licensing boards, including suspension of medical license. Financially, many insurers will not compensate providers for WSS. Also, many incidents of WSS result in a malpractice claim, with about 80% of those cases resulting in a malpractice award.15 However, it is important that HCPs are open with their patients regarding WSS.
As demonstrated in case presentations 2 and 3, having 2-person confirmation and patient confirmation before to surgery is important in preventing WSS for patients who have poor documentation of biopsy sites. In cases where agreement is not achieved, HCPs can consider several other options to help identify lesions. Dermabrasion and alcohol wipes are options.10 Dermabrasion uses friction to expose surgical sights that have healed, scarred, or been hidden by sun damage.10 Alcohol wipes remove surface scale and crust, creating a glisten with tangential lighting that highlights surface irregularities. Anesthesia injection prior to surgery creates a blister at the location of the cancer. This is because skin cancer weakens the attachments between keratinocytes, and as a result, the hydrostatic pressure from the anesthesia favorably blisters the malignancy location.10,16
Dermoscopy is another strategy shown to help identify scar margins.10,17 Under dermoscopy, a scar demonstrates a white-pink homogenous patch with underlying vessels, whereas basal cell carcinoma remnants include blue-gray ovoid nests and globules, telangiectasias, spoke wheel and leaflike structures.17 As a final option, HCPs can perform an additional biopsy of potential cancer locations to find the lesion again.10 If the lesions cannot be identified, HCPs should consider conservative measures or less invasive treatments with close and frequent follow-up.
Conclusions
The cases described here highlight how the lack of proper photographic documentation can prevent the use of curative surgical treatment. In order to reduce WSS and improve quality care, HCPs must continue to take steps and create safeguards to minimize risk. Proper documentation of lesions prior to biopsy provides an effective route to reduce incidence of WSS. If the biopsy site cannot be found, various strategies to properly identify the site can be employed. If WSS occurs, it is important that HCPs provide full disclosure to patients. With a growing emphasis on patient safety measures and advances in technology, HCPs are becoming increasingly cognizant about the most effective ways to optimize patient care, and it is anticipated that this will result in a decrease in morbidity and mortality.
1. Hempel S, Maggard-Gibbons M, Nguyen DK, et al. Wrong-site surgery, retained surgical items, and surgical fires: a systematic review of surgical never events. JAMA Surg. 2015;150(8):796-805. doi:10.1001/jamasurg.2015.0301
2. Knight N, Aucar J. Use of an anatomic marking form as an alternative to the Universal Protocol for Preventing Wrong Site, Wrong Procedure and Wrong Person Surgery. Am J Surg. 2010;200(6):803-809. doi:10.1016/j.amjsurg.2010.06.010
3. Elston DM, Stratman EJ, Miller SJ. Skin biopsy: biopsy issues in specific diseases [published correction appears in J Am Acad Dermatol. 2016 Oct;75(4):854]. J Am Acad Dermatol. 2016;74(1):1-18. doi:10.1016/j.jaad.2015.06.033
4. Watson AJ, Redbord K, Taylor JS, Shippy A, Kostecki J, Swerlick R. Medical error in dermatology practice: development of a classification system to drive priority setting in patient safety efforts. J Am Acad Dermatol. 2013;68(5):729-737. doi:10.1016/j.jaad.2012.10.058
5. Elston DM, Taylor JS, Coldiron B, et al. Patient safety: Part I. Patient safety and the dermatologist. J Am Acad Dermatol. 2009;61(2):179-191. doi:10.1016/j.jaad.2009.04.056
6. Nijhawan RI, Lee EH, Nehal KS. Biopsy site selfies--a quality improvement pilot study to assist with correct surgical site identification. Dermatol Surg. 2015;41(4):499-504. doi:10.1097/DSS.0000000000000305
7. Highsmith JT, Weinstein DA, Highsmith MJ, Etzkorn JR. BIOPSY 1-2-3 in dermatologic surgery: improving smartphone use to avoid wrong-site surgery. Technol Innov. 2016;18(2-3):203-206. doi:10.21300/18.2-3.2016.203
8. Rossy KM, Lawrence N. Difficulty with surgical site identification: what role does it play in dermatology? J Am Acad Dermatol. 2012;67(2):257-261. doi:10.1016/j.jaad.2012.02.034
9. American Society for Dermatologic Surgery. Photographic standards in dermatologic surgery poster. Accessed April 12, 2021. https://www.asds.net/medical-professionals/members-resources/product-details/productname/photographic-standards-poster
10. St John J, Walker J, Goldberg D, Maloney ME. Avoiding Medical Errors in Cutaneous Site Identification: A Best Practices Review. Dermatol Surg. 2016;42(4):477-484. doi:10.1097/DSS.0000000000000683
11. Alam M, Lee A, Ibrahimi OA, et al. A multistep approach to improving biopsy site identification in dermatology: physician, staff, and patient roles based on a Delphi consensus. JAMA Dermatol. 2014;150(5):550-558. doi:10.1001/jamadermatol.2013.9804
12. Chuang GS, Gilchrest BA. Ultraviolet-fluorescent tattoo location of cutaneous biopsy site. Dermatol Surg. 2012;38(3):479-483. doi:10.1111/j.1524-4725.2011.02238.x
13. DiGiovanni CW, Kang L, Manuel J. Patient compliance in avoiding wrong-site surgery. J Bone Joint Surg Am. 2003;85(5):815-819. doi:10.2106/00004623-200305000-00007
14. Gallagher TH. A 62-year-old woman with skin cancer who experienced wrong-site surgery: review of medical error. JAMA. 2009;302(6):669-677. doi:10.1001/jama.2009.1011
15. Mulloy DF, Hughes RG. Wrong-site surgery: a preventable medical error. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Agency for Healthcare Research and Quality (US); 2008:chap 36. Accessed April 23, 2021. https://www.ncbi.nlm.nih.gov/books/NBK2678
16. Zaiac M, Tongdee E, Porges L, Touloei K, Prodanovich S. Anesthetic blister induction to identify biopsy site prior to Mohs surgery. J Drugs Dermatol. 2015;14(5):446-447.
17. Jawed SI, Goldberg LH, Wang SQ. Dermoscopy to identify biopsy sites before Mohs surgery. Dermatol Surg. 2014;40(3):334-337. doi:10.1111/dsu.12422
1. Hempel S, Maggard-Gibbons M, Nguyen DK, et al. Wrong-site surgery, retained surgical items, and surgical fires: a systematic review of surgical never events. JAMA Surg. 2015;150(8):796-805. doi:10.1001/jamasurg.2015.0301
2. Knight N, Aucar J. Use of an anatomic marking form as an alternative to the Universal Protocol for Preventing Wrong Site, Wrong Procedure and Wrong Person Surgery. Am J Surg. 2010;200(6):803-809. doi:10.1016/j.amjsurg.2010.06.010
3. Elston DM, Stratman EJ, Miller SJ. Skin biopsy: biopsy issues in specific diseases [published correction appears in J Am Acad Dermatol. 2016 Oct;75(4):854]. J Am Acad Dermatol. 2016;74(1):1-18. doi:10.1016/j.jaad.2015.06.033
4. Watson AJ, Redbord K, Taylor JS, Shippy A, Kostecki J, Swerlick R. Medical error in dermatology practice: development of a classification system to drive priority setting in patient safety efforts. J Am Acad Dermatol. 2013;68(5):729-737. doi:10.1016/j.jaad.2012.10.058
5. Elston DM, Taylor JS, Coldiron B, et al. Patient safety: Part I. Patient safety and the dermatologist. J Am Acad Dermatol. 2009;61(2):179-191. doi:10.1016/j.jaad.2009.04.056
6. Nijhawan RI, Lee EH, Nehal KS. Biopsy site selfies--a quality improvement pilot study to assist with correct surgical site identification. Dermatol Surg. 2015;41(4):499-504. doi:10.1097/DSS.0000000000000305
7. Highsmith JT, Weinstein DA, Highsmith MJ, Etzkorn JR. BIOPSY 1-2-3 in dermatologic surgery: improving smartphone use to avoid wrong-site surgery. Technol Innov. 2016;18(2-3):203-206. doi:10.21300/18.2-3.2016.203
8. Rossy KM, Lawrence N. Difficulty with surgical site identification: what role does it play in dermatology? J Am Acad Dermatol. 2012;67(2):257-261. doi:10.1016/j.jaad.2012.02.034
9. American Society for Dermatologic Surgery. Photographic standards in dermatologic surgery poster. Accessed April 12, 2021. https://www.asds.net/medical-professionals/members-resources/product-details/productname/photographic-standards-poster
10. St John J, Walker J, Goldberg D, Maloney ME. Avoiding Medical Errors in Cutaneous Site Identification: A Best Practices Review. Dermatol Surg. 2016;42(4):477-484. doi:10.1097/DSS.0000000000000683
11. Alam M, Lee A, Ibrahimi OA, et al. A multistep approach to improving biopsy site identification in dermatology: physician, staff, and patient roles based on a Delphi consensus. JAMA Dermatol. 2014;150(5):550-558. doi:10.1001/jamadermatol.2013.9804
12. Chuang GS, Gilchrest BA. Ultraviolet-fluorescent tattoo location of cutaneous biopsy site. Dermatol Surg. 2012;38(3):479-483. doi:10.1111/j.1524-4725.2011.02238.x
13. DiGiovanni CW, Kang L, Manuel J. Patient compliance in avoiding wrong-site surgery. J Bone Joint Surg Am. 2003;85(5):815-819. doi:10.2106/00004623-200305000-00007
14. Gallagher TH. A 62-year-old woman with skin cancer who experienced wrong-site surgery: review of medical error. JAMA. 2009;302(6):669-677. doi:10.1001/jama.2009.1011
15. Mulloy DF, Hughes RG. Wrong-site surgery: a preventable medical error. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Agency for Healthcare Research and Quality (US); 2008:chap 36. Accessed April 23, 2021. https://www.ncbi.nlm.nih.gov/books/NBK2678
16. Zaiac M, Tongdee E, Porges L, Touloei K, Prodanovich S. Anesthetic blister induction to identify biopsy site prior to Mohs surgery. J Drugs Dermatol. 2015;14(5):446-447.
17. Jawed SI, Goldberg LH, Wang SQ. Dermoscopy to identify biopsy sites before Mohs surgery. Dermatol Surg. 2014;40(3):334-337. doi:10.1111/dsu.12422
Radiographic Changes of Osteomyelitis in a Patient With Periungual Lichen Planus
To the Editor:
A 60-year-old woman presented for evaluation of a 1-year history of left hallux nail plate dystrophy and proximal nail fold inflammation. Her medical history included Cushing disease with associated uncontrolled diabetes mellitus (DM) and a remote history of cutaneous lichen planus (LP) that resolved 15 years prior to presentation. She noted improvement during intravenous courses of antibiotics for other infections.
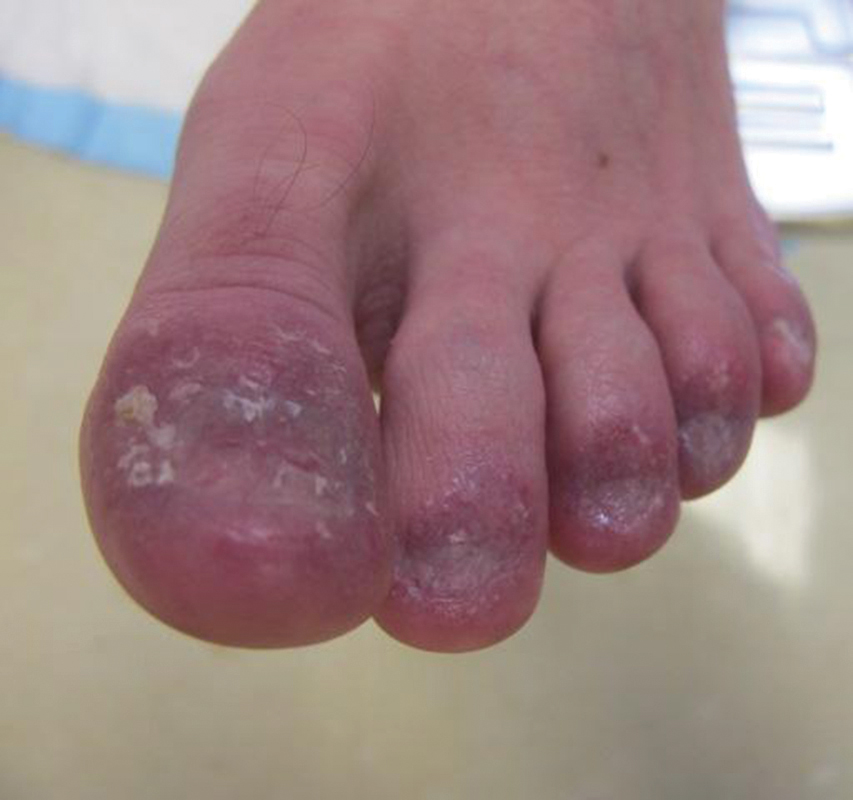
Examination of the left hallux revealed onycholysis, loss of the nail plate, and a yellow fibrinous base alongside erosion, erythema, and edema of the proximal toenail fold (Figure 1). The left second toe pad was markedly tender to palpation with scant exudate expressed from underneath the nail bed. Two biopsies of the hallux were performed. The proximal nail fold specimen revealed mild epidermal hyperplasia, and the nail bed demonstrated a nonspecific ulcer that was negative for acid-fast bacilli and fungi.
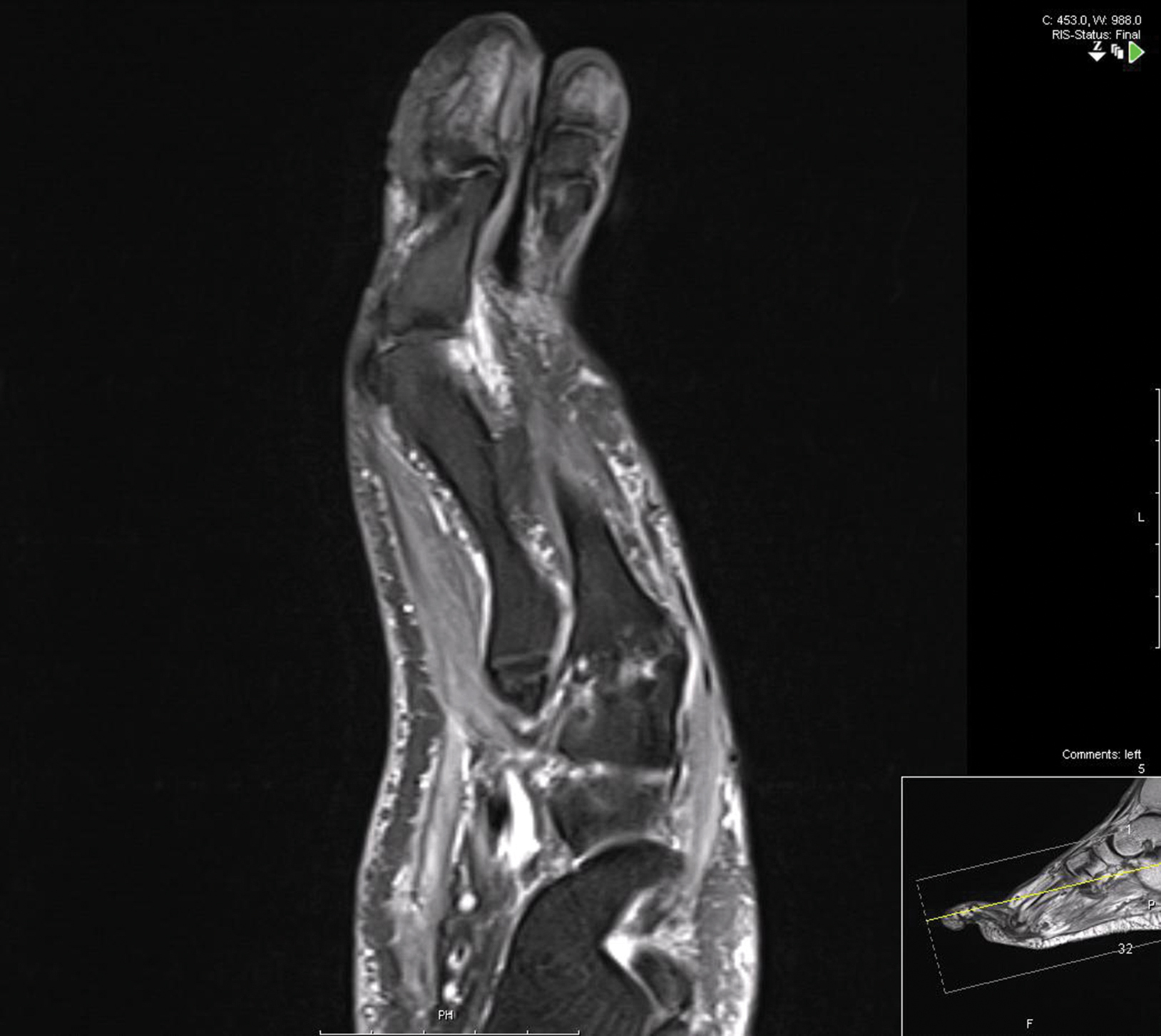
Treatment over 2 months with cephalexin yielded improvement in both erythema and edema. Initial and repeat nail plate cultures grew ampicillin- and penicillin-sensitive Enterococcus faecalis. Magnetic resonance imaging was performed to evaluate for osteomyelitis because of lack of resolution. Results demonstrated osteomyelitis of the distal tuft of the left hallux and the distal phalanx of the second toe (Figure 2). Vascular surgery evaluation revealed no evidence of large vessel arterial insufficiency. She was started on amoxicillin for superficial Enterococcus and ciprofloxacin for underlying enteric bacilli. The persistence of infection was attributed to microvascular disease secondary to the patient's associated DM. Months later, due to suspected worsening of osteomyelitis, she underwent treatment with oral fluconazole to cover potential fungal co-infection and intravenous vancomycin and piperacillin-tazobactam for broad-spectrum antibacterial coverage. She was eventually transitioned to antimicrobial agents including amoxicillin-clavulanate potassium and topical mupirocin with improvement in periungual erythema and edema.
On subsequent dermatologic evaluation after 1 month, she presented with pterygium and loss of all nail plates on the left foot. The nail bed now had a violaceous color and was studded with milia. The clinical findings were suggestive of LP, consistent with her history of LP. In light of these new findings, both topical corticosteroids and retinoids were utilized for treatment without remarkable benefit. The patient declined further management with systemic medications.
We report a case of nail LP associated with underlying radiographic osteomyelitis. Erosive nail LP has been associated with underlying osteomyelitis of the phalanx.1 Our patient developed these manifestations in the setting of Cushing disease, a unique finding given that many report improvement of LP with systemic corticosteroids.2,3 Tacrolimus, a calcineurin inhibitor, has been used in oral or topical formulations for lower extremity ulcers caused by LP as well as nail LP.1,4 Long-term prognosis of nail LP is poor, with high relapse rates and permanent damage to the nail unit.2 It is important to be aware that LP of the nail unit may cause radiographic changes of osteomyelitis that are not infectious in nature.
- Miller S. The effect of tacrolimus on lower extremity ulcers: a case study and review of the literature. Ostomy Wound Manage. 2008;54:36-42.
- Goettmann S, Zaraa I, Moulonguet I. Nail lichen planus: epidemiological, clinical, pathological, therapeutic and prognosis study of 67 cases. Eur Acad Dermatol Venereol. 2012;26:1304-1309.
- Piraccini BM, Saccani E, Starace M, et al. Nail lichen planus: response to treatment and long term follow-up. Eur J Dermatol. 2010;20:489-496.
- Ujiie H, Shibaki A, Akiyama M, et al. Successful treatment of nail lichen planus with topical tacrolimus. Acta Derm Venereol. 2010;90:218-219.
To the Editor:
A 60-year-old woman presented for evaluation of a 1-year history of left hallux nail plate dystrophy and proximal nail fold inflammation. Her medical history included Cushing disease with associated uncontrolled diabetes mellitus (DM) and a remote history of cutaneous lichen planus (LP) that resolved 15 years prior to presentation. She noted improvement during intravenous courses of antibiotics for other infections.
Examination of the left hallux revealed onycholysis, loss of the nail plate, and a yellow fibrinous base alongside erosion, erythema, and edema of the proximal toenail fold (Figure 1). The left second toe pad was markedly tender to palpation with scant exudate expressed from underneath the nail bed. Two biopsies of the hallux were performed. The proximal nail fold specimen revealed mild epidermal hyperplasia, and the nail bed demonstrated a nonspecific ulcer that was negative for acid-fast bacilli and fungi.
Treatment over 2 months with cephalexin yielded improvement in both erythema and edema. Initial and repeat nail plate cultures grew ampicillin- and penicillin-sensitive Enterococcus faecalis. Magnetic resonance imaging was performed to evaluate for osteomyelitis because of lack of resolution. Results demonstrated osteomyelitis of the distal tuft of the left hallux and the distal phalanx of the second toe (Figure 2). Vascular surgery evaluation revealed no evidence of large vessel arterial insufficiency. She was started on amoxicillin for superficial Enterococcus and ciprofloxacin for underlying enteric bacilli. The persistence of infection was attributed to microvascular disease secondary to the patient's associated DM. Months later, due to suspected worsening of osteomyelitis, she underwent treatment with oral fluconazole to cover potential fungal co-infection and intravenous vancomycin and piperacillin-tazobactam for broad-spectrum antibacterial coverage. She was eventually transitioned to antimicrobial agents including amoxicillin-clavulanate potassium and topical mupirocin with improvement in periungual erythema and edema.
On subsequent dermatologic evaluation after 1 month, she presented with pterygium and loss of all nail plates on the left foot. The nail bed now had a violaceous color and was studded with milia. The clinical findings were suggestive of LP, consistent with her history of LP. In light of these new findings, both topical corticosteroids and retinoids were utilized for treatment without remarkable benefit. The patient declined further management with systemic medications.
We report a case of nail LP associated with underlying radiographic osteomyelitis. Erosive nail LP has been associated with underlying osteomyelitis of the phalanx.1 Our patient developed these manifestations in the setting of Cushing disease, a unique finding given that many report improvement of LP with systemic corticosteroids.2,3 Tacrolimus, a calcineurin inhibitor, has been used in oral or topical formulations for lower extremity ulcers caused by LP as well as nail LP.1,4 Long-term prognosis of nail LP is poor, with high relapse rates and permanent damage to the nail unit.2 It is important to be aware that LP of the nail unit may cause radiographic changes of osteomyelitis that are not infectious in nature.
To the Editor:
A 60-year-old woman presented for evaluation of a 1-year history of left hallux nail plate dystrophy and proximal nail fold inflammation. Her medical history included Cushing disease with associated uncontrolled diabetes mellitus (DM) and a remote history of cutaneous lichen planus (LP) that resolved 15 years prior to presentation. She noted improvement during intravenous courses of antibiotics for other infections.
Examination of the left hallux revealed onycholysis, loss of the nail plate, and a yellow fibrinous base alongside erosion, erythema, and edema of the proximal toenail fold (Figure 1). The left second toe pad was markedly tender to palpation with scant exudate expressed from underneath the nail bed. Two biopsies of the hallux were performed. The proximal nail fold specimen revealed mild epidermal hyperplasia, and the nail bed demonstrated a nonspecific ulcer that was negative for acid-fast bacilli and fungi.
Treatment over 2 months with cephalexin yielded improvement in both erythema and edema. Initial and repeat nail plate cultures grew ampicillin- and penicillin-sensitive Enterococcus faecalis. Magnetic resonance imaging was performed to evaluate for osteomyelitis because of lack of resolution. Results demonstrated osteomyelitis of the distal tuft of the left hallux and the distal phalanx of the second toe (Figure 2). Vascular surgery evaluation revealed no evidence of large vessel arterial insufficiency. She was started on amoxicillin for superficial Enterococcus and ciprofloxacin for underlying enteric bacilli. The persistence of infection was attributed to microvascular disease secondary to the patient's associated DM. Months later, due to suspected worsening of osteomyelitis, she underwent treatment with oral fluconazole to cover potential fungal co-infection and intravenous vancomycin and piperacillin-tazobactam for broad-spectrum antibacterial coverage. She was eventually transitioned to antimicrobial agents including amoxicillin-clavulanate potassium and topical mupirocin with improvement in periungual erythema and edema.
On subsequent dermatologic evaluation after 1 month, she presented with pterygium and loss of all nail plates on the left foot. The nail bed now had a violaceous color and was studded with milia. The clinical findings were suggestive of LP, consistent with her history of LP. In light of these new findings, both topical corticosteroids and retinoids were utilized for treatment without remarkable benefit. The patient declined further management with systemic medications.
We report a case of nail LP associated with underlying radiographic osteomyelitis. Erosive nail LP has been associated with underlying osteomyelitis of the phalanx.1 Our patient developed these manifestations in the setting of Cushing disease, a unique finding given that many report improvement of LP with systemic corticosteroids.2,3 Tacrolimus, a calcineurin inhibitor, has been used in oral or topical formulations for lower extremity ulcers caused by LP as well as nail LP.1,4 Long-term prognosis of nail LP is poor, with high relapse rates and permanent damage to the nail unit.2 It is important to be aware that LP of the nail unit may cause radiographic changes of osteomyelitis that are not infectious in nature.
- Miller S. The effect of tacrolimus on lower extremity ulcers: a case study and review of the literature. Ostomy Wound Manage. 2008;54:36-42.
- Goettmann S, Zaraa I, Moulonguet I. Nail lichen planus: epidemiological, clinical, pathological, therapeutic and prognosis study of 67 cases. Eur Acad Dermatol Venereol. 2012;26:1304-1309.
- Piraccini BM, Saccani E, Starace M, et al. Nail lichen planus: response to treatment and long term follow-up. Eur J Dermatol. 2010;20:489-496.
- Ujiie H, Shibaki A, Akiyama M, et al. Successful treatment of nail lichen planus with topical tacrolimus. Acta Derm Venereol. 2010;90:218-219.
- Miller S. The effect of tacrolimus on lower extremity ulcers: a case study and review of the literature. Ostomy Wound Manage. 2008;54:36-42.
- Goettmann S, Zaraa I, Moulonguet I. Nail lichen planus: epidemiological, clinical, pathological, therapeutic and prognosis study of 67 cases. Eur Acad Dermatol Venereol. 2012;26:1304-1309.
- Piraccini BM, Saccani E, Starace M, et al. Nail lichen planus: response to treatment and long term follow-up. Eur J Dermatol. 2010;20:489-496.
- Ujiie H, Shibaki A, Akiyama M, et al. Successful treatment of nail lichen planus with topical tacrolimus. Acta Derm Venereol. 2010;90:218-219.
Practice Points
- Lichen planus (LP) is an inflammatory mucocutaneous disorder with variable presentations.
- With extensive nail involvement, nail LP may impart radiographic findings suggestive of osteomyelitis.