User login
Association Between LDL-C and Androgenetic Alopecia Among Female Patients in a Specialty Alopecia Clinic
To the Editor:
Female pattern hair loss (FPHL), or androgenetic alopecia (AGA), is the most common form of alopecia worldwide and is characterized by a reduction of hair follicles spent in the anagen phase of growth as well as progressive terminal hair loss.1 It is caused by an excessive response to androgens and leads to the characteristic distribution of hair loss in both sexes. Studies have shown a notable association between AGA and markers of metabolic syndrome such as dyslipidemia, insulin resistance, and obesity in age- and sex-matched controls.2,3 However, research describing the relationship between AGA severity and these markers is scarce.
To understand the relationship between FPHL severity and abnormal cholesterol levels, we performed a retrospective chart review of patients diagnosed with FPHL at a specialty alopecia clinic from June 2022 to December 2022. Patient age and age at onset of FPHL were collected. The severity of FPHL was measured using the Sinclair scale (score range, 1–5) and unidentifiable patient photographs. Laboratory values were collected; abnormal cholesterol was defined by the American Heart Association as having a low-density lipoprotein cholesterol (LDL-C) level of 100 mg/dL or higher.4 Finally, data on medication use were noted to understand patient treatment status (Table).
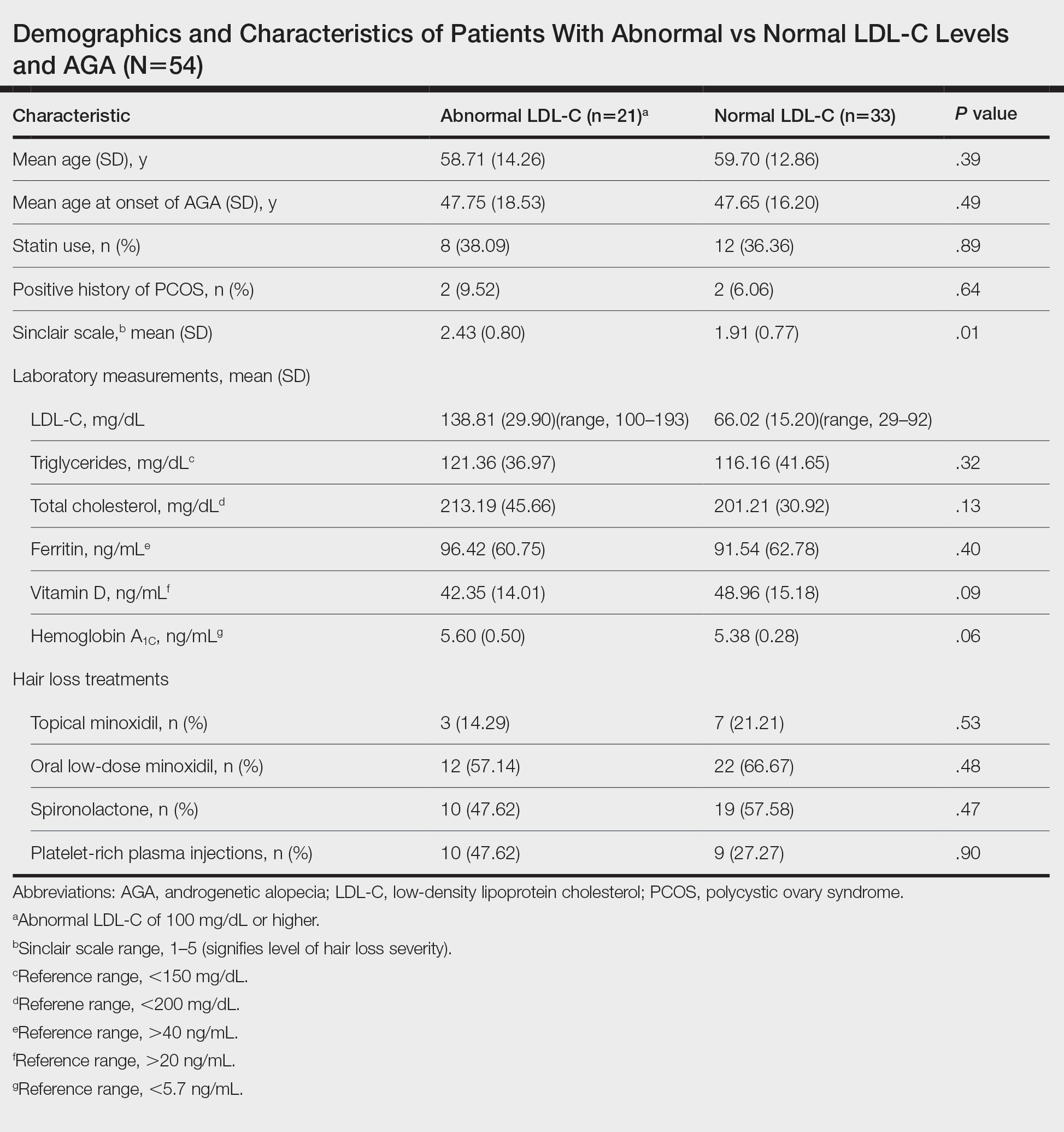
We identified 54 female patients with FPHL with an average age of 59 years (range, 34–80 years). Thirty-three females (61.11%) had a normal LDL-C level and 21 (38.89%) had an abnormal level. The mean (SD) LDL-C level was 66.02 (15.20) mg/dL (range, 29–92 mg/dL) in the group with normal levels and 138.81 (29.90) mg/dL (range, 100–193 mg/dL) in the group with abnormal levels. Patients with abnormal LDL-C had significantly higher Sinclair scale scores compared to those with normal levels (2.43 vs 1.91; P=.01). There were no significant differences in patient age (58.71 vs 59.70 years; P=.39), age at onset of AGA (47.75 vs 47.65 years; P=.49), history of polycystic ovary syndrome (9.52% vs 6.06%; P=.64), or statin use (38.09% vs 36.36%; P=.89) between patients with abnormal and normal LDL-C levels, respectively. There also were no significant differences in ferritin (96.42 vs 91.54 ng/mL; P=.40), vitamin D (42.35 vs 48.96 ng/mL; P=.09), or hemoglobin A1c levels (5.60 ng/mL vs 5.38 ng/mL; P=.06)—variables that could have confounded this relationship. Triglycerides were within reference range in both groups (121.36 vs 116.16 mg/dL; P=.32), while total cholesterol was mildly elevated in both groups but not significantly different (213.19 vs 201.21 mg/dL; P=.13). Use of hair loss treatments such as topical minoxidil (14.29% vs 21.21%; P=.53), oral low-dose minoxidil (57.14% vs 66.67%; P=.48), oral spironolactone (47.62% vs 57.58%; P=.47), and platelet-rich plasma injections (47.62% vs 27.27%; P=.90) were not significantly different across both groups.
The data suggest a significant (P<.05) association between abnormal LDL-C and hair loss severity in FPHL patients. Our study was limited by its small sample size and lack of causality; however, it coincides with and reiterates the findings established in the literature. The mechanism of the association between hyperlipidemia and AGA is not well understood but is thought to stem from the homology between cholesterol and androgens. Increased cholesterol release from dermal adipocytes and subsequent absorption into hair follicle cell populations may increase hair follicle steroidogenesis, thereby accelerating the anagen-catagen transition and inducing AGA. Alternatively, impaired cholesterol homeostasis may disrupt normal hair follicle cycling by interrupting signaling pathways in follicle proliferation and differentiation.5 Adequate control and monitoring of LDL-C levels may be important, particularly in patients with more severe FPHL.
- Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11:E9860. doi:10.5812/ijem.9860
- El Sayed MH, Abdallah MA, Aly DG, et al. Association of metabolic syndrome with female pattern hair loss in women: a case-control study. Int J Dermatol. 2016;55:1131-1137. doi:10.1111/ijd.13303
- Kim MW, Shin IS, Yoon HS, et al. Lipid profile in patients with androgenetic alopecia: a meta-analysis. J Eur Acad Dermatol Venereol. 2017;31:942-951. doi:10.1111/jdv.14000
- Birtcher KK, Ballantyne CM. Cardiology patient page. measurement of cholesterol: a patient perspective. Circulation. 2004;110:E296-E297. doi:10.1161/01.CIR.0000141564.89465.4E
- Palmer MA, Blakeborough L, Harries M, et al. Cholesterol homeostasis: links to hair follicle biology and hair disorders. Exp Dermatol. 2020;29:299-311. doi:10.1111/exd.13993
To the Editor:
Female pattern hair loss (FPHL), or androgenetic alopecia (AGA), is the most common form of alopecia worldwide and is characterized by a reduction of hair follicles spent in the anagen phase of growth as well as progressive terminal hair loss.1 It is caused by an excessive response to androgens and leads to the characteristic distribution of hair loss in both sexes. Studies have shown a notable association between AGA and markers of metabolic syndrome such as dyslipidemia, insulin resistance, and obesity in age- and sex-matched controls.2,3 However, research describing the relationship between AGA severity and these markers is scarce.
To understand the relationship between FPHL severity and abnormal cholesterol levels, we performed a retrospective chart review of patients diagnosed with FPHL at a specialty alopecia clinic from June 2022 to December 2022. Patient age and age at onset of FPHL were collected. The severity of FPHL was measured using the Sinclair scale (score range, 1–5) and unidentifiable patient photographs. Laboratory values were collected; abnormal cholesterol was defined by the American Heart Association as having a low-density lipoprotein cholesterol (LDL-C) level of 100 mg/dL or higher.4 Finally, data on medication use were noted to understand patient treatment status (Table).

We identified 54 female patients with FPHL with an average age of 59 years (range, 34–80 years). Thirty-three females (61.11%) had a normal LDL-C level and 21 (38.89%) had an abnormal level. The mean (SD) LDL-C level was 66.02 (15.20) mg/dL (range, 29–92 mg/dL) in the group with normal levels and 138.81 (29.90) mg/dL (range, 100–193 mg/dL) in the group with abnormal levels. Patients with abnormal LDL-C had significantly higher Sinclair scale scores compared to those with normal levels (2.43 vs 1.91; P=.01). There were no significant differences in patient age (58.71 vs 59.70 years; P=.39), age at onset of AGA (47.75 vs 47.65 years; P=.49), history of polycystic ovary syndrome (9.52% vs 6.06%; P=.64), or statin use (38.09% vs 36.36%; P=.89) between patients with abnormal and normal LDL-C levels, respectively. There also were no significant differences in ferritin (96.42 vs 91.54 ng/mL; P=.40), vitamin D (42.35 vs 48.96 ng/mL; P=.09), or hemoglobin A1c levels (5.60 ng/mL vs 5.38 ng/mL; P=.06)—variables that could have confounded this relationship. Triglycerides were within reference range in both groups (121.36 vs 116.16 mg/dL; P=.32), while total cholesterol was mildly elevated in both groups but not significantly different (213.19 vs 201.21 mg/dL; P=.13). Use of hair loss treatments such as topical minoxidil (14.29% vs 21.21%; P=.53), oral low-dose minoxidil (57.14% vs 66.67%; P=.48), oral spironolactone (47.62% vs 57.58%; P=.47), and platelet-rich plasma injections (47.62% vs 27.27%; P=.90) were not significantly different across both groups.
The data suggest a significant (P<.05) association between abnormal LDL-C and hair loss severity in FPHL patients. Our study was limited by its small sample size and lack of causality; however, it coincides with and reiterates the findings established in the literature. The mechanism of the association between hyperlipidemia and AGA is not well understood but is thought to stem from the homology between cholesterol and androgens. Increased cholesterol release from dermal adipocytes and subsequent absorption into hair follicle cell populations may increase hair follicle steroidogenesis, thereby accelerating the anagen-catagen transition and inducing AGA. Alternatively, impaired cholesterol homeostasis may disrupt normal hair follicle cycling by interrupting signaling pathways in follicle proliferation and differentiation.5 Adequate control and monitoring of LDL-C levels may be important, particularly in patients with more severe FPHL.
To the Editor:
Female pattern hair loss (FPHL), or androgenetic alopecia (AGA), is the most common form of alopecia worldwide and is characterized by a reduction of hair follicles spent in the anagen phase of growth as well as progressive terminal hair loss.1 It is caused by an excessive response to androgens and leads to the characteristic distribution of hair loss in both sexes. Studies have shown a notable association between AGA and markers of metabolic syndrome such as dyslipidemia, insulin resistance, and obesity in age- and sex-matched controls.2,3 However, research describing the relationship between AGA severity and these markers is scarce.
To understand the relationship between FPHL severity and abnormal cholesterol levels, we performed a retrospective chart review of patients diagnosed with FPHL at a specialty alopecia clinic from June 2022 to December 2022. Patient age and age at onset of FPHL were collected. The severity of FPHL was measured using the Sinclair scale (score range, 1–5) and unidentifiable patient photographs. Laboratory values were collected; abnormal cholesterol was defined by the American Heart Association as having a low-density lipoprotein cholesterol (LDL-C) level of 100 mg/dL or higher.4 Finally, data on medication use were noted to understand patient treatment status (Table).

We identified 54 female patients with FPHL with an average age of 59 years (range, 34–80 years). Thirty-three females (61.11%) had a normal LDL-C level and 21 (38.89%) had an abnormal level. The mean (SD) LDL-C level was 66.02 (15.20) mg/dL (range, 29–92 mg/dL) in the group with normal levels and 138.81 (29.90) mg/dL (range, 100–193 mg/dL) in the group with abnormal levels. Patients with abnormal LDL-C had significantly higher Sinclair scale scores compared to those with normal levels (2.43 vs 1.91; P=.01). There were no significant differences in patient age (58.71 vs 59.70 years; P=.39), age at onset of AGA (47.75 vs 47.65 years; P=.49), history of polycystic ovary syndrome (9.52% vs 6.06%; P=.64), or statin use (38.09% vs 36.36%; P=.89) between patients with abnormal and normal LDL-C levels, respectively. There also were no significant differences in ferritin (96.42 vs 91.54 ng/mL; P=.40), vitamin D (42.35 vs 48.96 ng/mL; P=.09), or hemoglobin A1c levels (5.60 ng/mL vs 5.38 ng/mL; P=.06)—variables that could have confounded this relationship. Triglycerides were within reference range in both groups (121.36 vs 116.16 mg/dL; P=.32), while total cholesterol was mildly elevated in both groups but not significantly different (213.19 vs 201.21 mg/dL; P=.13). Use of hair loss treatments such as topical minoxidil (14.29% vs 21.21%; P=.53), oral low-dose minoxidil (57.14% vs 66.67%; P=.48), oral spironolactone (47.62% vs 57.58%; P=.47), and platelet-rich plasma injections (47.62% vs 27.27%; P=.90) were not significantly different across both groups.
The data suggest a significant (P<.05) association between abnormal LDL-C and hair loss severity in FPHL patients. Our study was limited by its small sample size and lack of causality; however, it coincides with and reiterates the findings established in the literature. The mechanism of the association between hyperlipidemia and AGA is not well understood but is thought to stem from the homology between cholesterol and androgens. Increased cholesterol release from dermal adipocytes and subsequent absorption into hair follicle cell populations may increase hair follicle steroidogenesis, thereby accelerating the anagen-catagen transition and inducing AGA. Alternatively, impaired cholesterol homeostasis may disrupt normal hair follicle cycling by interrupting signaling pathways in follicle proliferation and differentiation.5 Adequate control and monitoring of LDL-C levels may be important, particularly in patients with more severe FPHL.
- Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11:E9860. doi:10.5812/ijem.9860
- El Sayed MH, Abdallah MA, Aly DG, et al. Association of metabolic syndrome with female pattern hair loss in women: a case-control study. Int J Dermatol. 2016;55:1131-1137. doi:10.1111/ijd.13303
- Kim MW, Shin IS, Yoon HS, et al. Lipid profile in patients with androgenetic alopecia: a meta-analysis. J Eur Acad Dermatol Venereol. 2017;31:942-951. doi:10.1111/jdv.14000
- Birtcher KK, Ballantyne CM. Cardiology patient page. measurement of cholesterol: a patient perspective. Circulation. 2004;110:E296-E297. doi:10.1161/01.CIR.0000141564.89465.4E
- Palmer MA, Blakeborough L, Harries M, et al. Cholesterol homeostasis: links to hair follicle biology and hair disorders. Exp Dermatol. 2020;29:299-311. doi:10.1111/exd.13993
- Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11:E9860. doi:10.5812/ijem.9860
- El Sayed MH, Abdallah MA, Aly DG, et al. Association of metabolic syndrome with female pattern hair loss in women: a case-control study. Int J Dermatol. 2016;55:1131-1137. doi:10.1111/ijd.13303
- Kim MW, Shin IS, Yoon HS, et al. Lipid profile in patients with androgenetic alopecia: a meta-analysis. J Eur Acad Dermatol Venereol. 2017;31:942-951. doi:10.1111/jdv.14000
- Birtcher KK, Ballantyne CM. Cardiology patient page. measurement of cholesterol: a patient perspective. Circulation. 2004;110:E296-E297. doi:10.1161/01.CIR.0000141564.89465.4E
- Palmer MA, Blakeborough L, Harries M, et al. Cholesterol homeostasis: links to hair follicle biology and hair disorders. Exp Dermatol. 2020;29:299-311. doi:10.1111/exd.13993
Practice Points
- Associations have been shown between hair loss and markers of bad health such as insulin resistance and high cholesterol. Research has not yet shown the relationship between hair loss severity and these markers, particularly cholesterol.